Abstract
Acquired Immunodeficiency Syndrome (AIDS), which chiefly originatesfroma retrovirus named Human Immunodeficiency Virus (HIV), has impacted about 70 million people worldwide. Even though several advances have been made in the field of antiretroviral combination therapy, HIV is still responsible for a considerable number of deaths in Africa. The current antiretroviral therapies have achieved success in providing instant HIV suppression but with countless undesirable adverse effects. Presently, the biodiversity of the plant kingdom is being explored by several researchers for the discovery of potent anti-HIV drugs with different mechanisms of action. The primary challenge is to afford a treatment that is free from any sort of risk of drug resistance and serious side effects. Hence, there is a strong demand to evaluate drugs derived from plants as well as their derivatives. Several plants, such as Andrographis paniculata, Dioscorea bulbifera, Aegle marmelos, Wistaria floribunda, Lindera chunii, Xanthoceras sorbifolia and others have displayed significant anti-HIV activity. Here, weattempt to summarize the main results, which focus on the structures of most potent plant-based natural products having anti-HIV activity along with their mechanisms of action and IC50 values, structure-activity-relationships and important key findings.
Keywords: AIDS, anti-HIV, natural products, MOAs
1. Introduction
Acquired immunodeficiency syndrome (AIDS) is a disease of the cell-mediated immune system or T-lymphocytes of the human body. In AIDS, the count of helper T cells isreduced, which directly stimulates the production of antibodies from B-cells. Consequently, the body’s natural defense system against AIDS infection isdestroyed [1]. According tothe World Health Organization (WHO), it is estimated that about 75 million individuals have been infected from the human immunodeficiency virus (HIV), and about 37 million people are still under the fighting stage. The prevalence of HIV is expected to increase significantly due to illiteracy, non-hygienic living conditions, unsafe sexual relationships and lack of awareness [2]. Initially, the first human retrovirus was founded at the National Cancer Institute, in the USA by Robert Galloand his colleagues, after being first discovered in 1981 among homosexuals. In 1983, Professor Luc Montagnier and co-workers later discovered the AIDS virus at the Institute Pasteur, in Paris [3]. In 1986, the International Committee on Viral Nomenclature were the first to officially name the AIDS virus the human immunodeficiency virus (HIV) [4]. Currently, Africans worldwide are stricken by this illness more than any other race [5].
1.1. The HIV Structure
The motor agent for AIDS is an animal retrovirus named HIV that is ready to replicate and integrate its infectious DNA into the host cell’s healthy DNA. It is an animal virus that chiefly attacks the body’s helper T cells [6,7]. The virus is spherically shaped, having a diameter of around 90–120 nm. Its genetic material generally contains a single standard RNA fiber metameric into two similar fibers and is connected with an enzyme called reverse transcriptase (RT). The viral coating contains a lipid bilayer that is derived from the membrane of the host cells and spikes of glycoprotein that are like projecting knob. It consists of two protein coats, as depicted in Figure 1 [8,9]. Internally, the virus contains a protein layer (the matrix), which consists of the necessary proteins and nuclear material. The virus also contains an enzyme known as a protease that disintegrates the viral polyproteins to form new functional proteins. The role of reverse transcriptase is to catalyse the conversion of the viral RNA into viral DNA and integrase, which allows the entry of viral DNA into the host nucleus [10,11].
Figure 1.
Structure of human immunodeficiency virus (HIV) virus [10]. Image was originally published within Open Access license.
1.2. Replication Cycle of HIV
The complete HIV replication cycle is represented in Figure 2. After the entrance of the virus into the body of an individual, the virus invades the body cells through CCRS or CXCR4 receptors shown on the top of the macrophages, known as T-lymphocytes, dendritic cells and monocytes [6,7]. After entering the host cell, the virus binds with chemokine receptors and interacts with cell membrane proteins. The virus then releases and utilizes its reverse transcriptase (RT) enzyme for the synthesis of viral DNA from its viral genome i.e., HIV RNA. This conversion allows the virus to enter into the host cell nucleus, where the enzyme integrase releases and perform integration of its viral DNA into the host cell’s DNA [8,9,10,11]. Newly formed HIV proteins and viral RNA shifts towards the cell membrane and reuniteswith immature HIV. The new immature (non-infectious) virus then buds off from the host cells, which inturn initiates the release of the protease enzyme from the viruses that cause the breakdown of long-chain polypeptides of immature viruses. The newly formed small protein particles make the new mature viruses that enter into the new host cells for spreading the infection [12,13].
Figure 2.
The HIV replication cycle [13]. Image was originally published within Open Access license.
1.3. Diagnosis
The level of HIV infection is diagnosed from the blood plasma of the host through their viral RNA mass estimation. The infection has been associated with the period of acute symptoms viz; lymphadenopathy, fever, weight loss, lethargy, general malaise, pharyngitis, rashes, nausea, headache, myalgias and meningitis, etc. [2,6]. During acute HIV infection, the viral RNA is at the highest levels in the blood plasma. It is estimated that the amount and characteristics of the virus indicate its pathogenesis and replication. Hence, the clinical details and infection progression depend on the host characteristics, along with the viral genotype [14,15,16]. ELISA and Western Blotting were the two main tests employed for the diagnosis of AIDS in the past. ELISA is used for the detection and measurement of the antibodies that are produced against a specific pathogen [17], while Western Blotting was employed for confirmation of ELISA positive tests. It is used to check the specific proteins in the blood sample. The samples go through the protein denaturation and then gel electrophoresis. The combined effect of both tests is found to be 99% accurate. Nowadays, various advantageous alternatives are available in place of Western Blotting.Among the advantages associated with such alternatives is less time-consuming testing [18].
1.4. Present Therapy for HIV/AIDS
The knowledge of HIV has beenmade public since the 1980s. However, there is currently no availability of efficacious therapy or vaccine for the entire destruction of the virus [2]. Current AIDS treatments have many drawbacks, e.g., complex and tedious treatment protocols, requiring expertise from medical practitioners, solid motivation and patient’s commitment. Antiretroviral therapy (ART) is only about twenty years old, meaning that further approaches are still in progress. The usage of certain medications can slow the progress of the disease without the patient necessarily being promised total recovery. However, with the development of new entities and immune modulators, it is now feasible to fight this deadly disease [19,20,21]. The drugs provide a meaningful advancement in mitigation, control, cure, and prevention. With the establishment of highly active antiretroviral therapy (HAART) and anti-retroviral agents in 1996 decreased the mortality and morbidity of AIDS has been observed. Antiretroviral therapy is presently prescribed for all adult patients living with HIV [2]. Many types of combination approaches such as the use of nucleoside reverse transcriptase inhibitors, fusion inhibitors, non-nucleoside reverse transcriptase inhibitors, integrase inhibitors, protease inhibitors, together with immunomodulators have been prescribed to achieve a proficient therapeutic response [3,6,9,10,11]. Due to the lack of non-accessible effective regimens, it has been noted that the objective of therapy is to sturdily and maximally prohibit viral replication so that the individual can achieve and maintain an adequate immune response against the potential viral pathogens. The higher the abolition of viral replication, the lower the incidence of development of the drug-resistant virus. The minimization in the mortality and morbidity of the disorder has turned it from a lethal syndrome to a chronic and controllable situation [5,19,21]. It is now advised that all HIV positive individuals with the perceptible virus, disregarding their count of CD4 cells, should be recommended with ART quickly after diagnosis, to avoid further progression [20].
1.5. Drawbacks of Current Anti-Retroviral Therapy
Even though it is impressive to deal with all the symptomatic and asymptomatic HIV infected persons, no long-lasting clinical outcomes have been illustrated in asymptomatic patients with acceptable immune competency [22]. Arguments in contrast to early remedies in asymptomatic patients involve the dangerous side effects of anti-HIV drugs, their toxicity and destructive effect of anti-HIV drugs on quality of life, the possibility of drug resistance restricting future treatment opportunities, big cost, drug interactions, the limited capability of available regimens, failure of treatment [23,24,25]. The right time to start anti-HIV therapy remainsuncertain. The antiviral drugs that act on the HIV also affect the host cells; they may harm the host cell’s nuclear material along with the HIV genome. With nucleoside reverse transcriptase inhibitors, toxicity is primarily due to the partial provision of cellular DNA polymerase. Neutropenia and Anaemia are extremely critical and dose-dependent adverse effects. Moreover, to date, there is no vaccine or cure for HIV infection, and the efficacy of antiretroviral therapy consist of a combination of two or three antiviral agents, targeting different steps of the virus replication cycle, can be compromised by the selection of strains resistant to one or multiple drug classes and current treatment-associated toxicity. However, these drugs have only limited or transient clinical benefit due to their noxious side effects and the emergence of viral variants resistant to HIV-1 inhibitors. Unfortunately, their use is limited due to the speedy emergence of resistant viral strains and to the severe toxic side effects. Hence, new natural products can be considered as novel leads for the development of new effective and selective anti-AIDS agents [24,25,26].
2. Plants with Anti-HIV Potential
Presently, strategies available to combat AIDS are restricted by the evolution of multidrug resistance. That is why novel targets and new efficacious drugs are required for achieving the goal of an entire eradication of AIDS. Also, infected cells persist and constitutea basic barrier to the elimination of HIV-1. For the past 10 years, the mechanism by which the virus persists hasnecessitated anovel pathway in the discovery of new drug compounds that work efficaciously against HIV without activating the T cells of the immune system [27,28]. To attain this goal, it has been recommended by the WHO that ethnomedicines and various other natural constituents should be systematically tested to combat HIV [29,30]. Interestingly, in the 1990s, natural products with their mechanisms against HIV-1 enzymes like reverse transcriptase, integrase, protease and some fusion inhibitors were discovered. The natural drugs have chemical diversity with higher hit rates in high throughput screening and high capability to reach the target site [31,32,33]. Several alkaloids, flavonoids, coumarins, terpenoids, and polyphenolic compounds, aswell as known therapeutic agents having an array of biological activities like anticancer, analgesics, anti-inflammatory and exert anti-HIV activity extracted from various plants, were found [34,35,36,37,38,39,40,41,42,43,44]. These became the sources of inspiration for many research activities, e.g., the anti-HIV potential of components of Dioscorea bulbifera [45], Euphorbia sikkimensis [46], Culendula officinalis [47], Sceletium tortuosum [48], Brazilian propolis, Kadsura lancilimba, Lithocarpus litseifolius, and Ocimum labiatum [49,50,51,52].
Taken together, the present review highlights the discovery of plant-based molecules during the last few decades that have been used in the management of HIV. A detailed account of plants according to their mechanism of action and activity of secondary metabolites has been discussed. In addition to the structures of most potent phytochemicals, mechanistic insights revealed during the biological evaluation, IC50 values and important key findings have also been presented. The detailed mechanisms of this action and structure-activity-relationships of some of the compound classes remain to be further investigated. This assemblage will be of great help for the scientific community working towards the development of anti-HIV drugs. In this review, the natural medicinal plants are described in two categories:
Plants according to their mechanism of action.
Plants according to the activity of secondary metabolites.
2.1. Natural Plants According to Their Mechanism of Action
Therapeutic agents of natural origin may be an encouraging alternative solution for the treatment of several disorders and conditions [53,54,55,56,57,58,59]. In anti-HIV research, attention is chiefly paid tocompounds which interfere with several steps involved in the HIV replication process. For example, almost all the anti-HIV drugs act against the viral proteins represented by the viral protease, integrase, and reverse transcriptase [60]. Anti-HIV drugs can be classified into several groups according to their action on the life cycle of HIV [61]. Hence, different drugs act on these different steps of replication and inhibit the further expansion of the virus into the body. A group of researchers reported the activities of HIV-PR inhibitors from different plants primarily divided into the following categories [62,63,64,65,66,67,68,69,70,71]:
-
(a)
Fusion inhibitors (FI)
-
(b)
Reverse transcriptase inhibitors (RTI)
-
(c)
Integrase inhibitors (ITI)
-
(d)
Protease inhibitors (PRI)
-
(e)
Immunomodulators
-
(f)
Antioxidants
2.1.1. Fusion Inhibitors
Fusion inhibitors are also known as Entry inhibitors. These are mainly CCR5 co-receptor antagonists which inhibit the binding of HIV surface glycoproteins with the host cell’s receptor [72]. Infection primarily starts with the binding of the viral gp120 to the CD4 cell receptor expressed on the surface of T cells, macrophages, and some monocytes. This results in the conformational change which further stimulates the interaction of secondary gp120 with co-receptor CCR5 [73]. FIs prevent the entry of the virus into the host cell by inhibiting the fusion of virus particles with the membrane of the host cell, which is the early first step of virus replication [74].
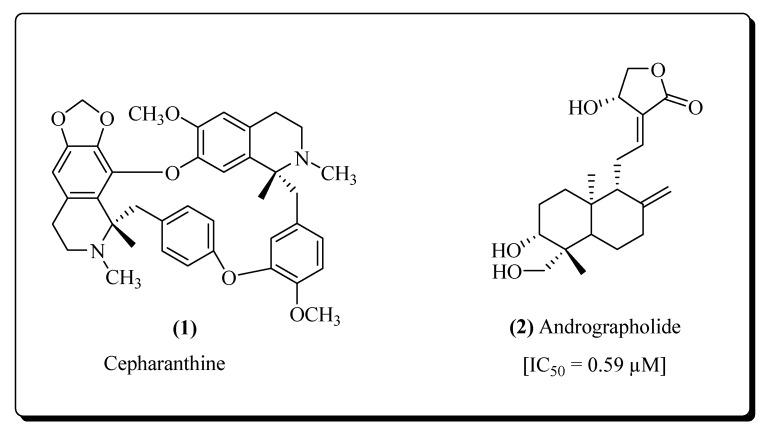
Phytoconstituents from some plants, like Listeria ovate, Cymbidium hybrid, Hippeastrum hybrid, Epipactis helleborine and Urtica dioica possessing the activities of fusion inhibitors and act against the HIV-1 and HIV-2 [75,76]. Matsuda et al. reported an alkaloid Cepharanthine (1) isolated from Stephania cepharantha having anti-HIV and anti-tumour potential without exerting any type of serious toxic effects. This compound modifies the plasma membrane fluidity and prevents viral cell fusion [77]. A diterpene lactone named Andrographolide (2) shown in Figure 3 was obtained from the herb Andrographis paniculata and possesses HIV-1 fusion inhibition propertiesevaluated in vitro using AZT (Zidovudine) as a positive control [78,79,80,81,82]. Several other derivatives have been derived synthetically to exert more potent anti-HIV properties [83,84].
Figure 3.
Structures of fusion inhibitors.
2.1.2. Plant Extracts as Reverse Transcriptase Inhibitors
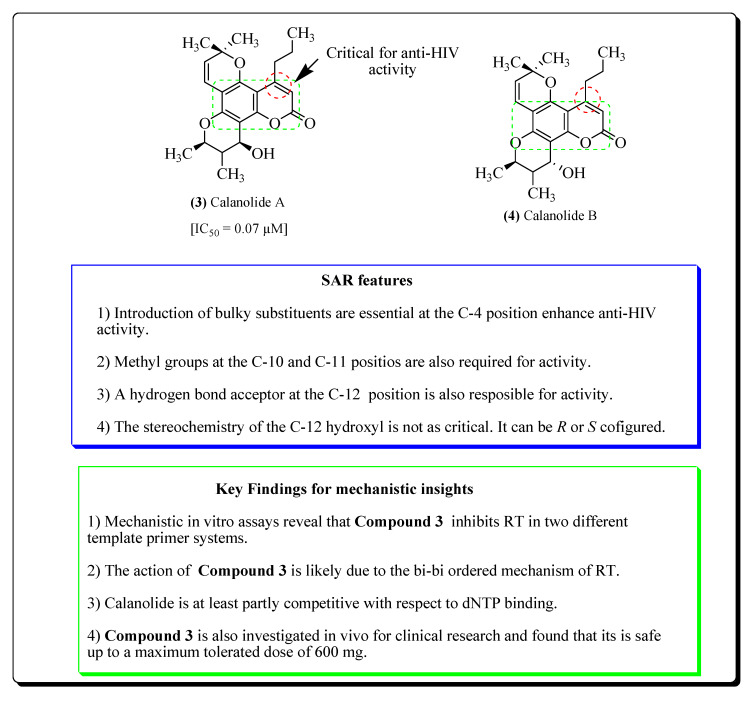
The HIV virus utilizes the reverse transcriptase enzyme for the conversion of its viral RNA into DNA. RT inhibitors mainly act upon this enzyme and prohibit one of the essential steps of viral replication [85,86]. Several natural products have been isolated from plants are available in theliterature, which have been screened for their activity against RT [66]. The plants which tested positively for reverse transcriptase inhibition include; Culendula officinalis, Acacia mellifera, Uvaria angolensis, Hypericum scruglii, Spaganium stoloniferum, Calophyllum brasiliense, Maytenus buchanani, Prunus Africana, Vernonia jugalis, Maytenus senegalensis, Melia azedarach, Calophyllum inophyllum, Lomatium suksdorfii, Coriandrum sativum, Chrysanthemum morifolium and Swertia franchetiana [47,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93]. Capryl aldehyde and methyl-n-nonyl ketone obtained from Houttuyniu cordata directly inhibit the RT enzyme [66]. Calanolides A (3) and B (4) [89] have been obtained from the plant Calophyllum inophyllum. The introduction of bulky groups has been shown to be essential at the C-4 position to enhance anti-HIV activity. The stereochemistry of the C-12 hydroxyl (R or S configured) is not, however, as critical for activity. Methyl groups at the C-10 and C-11positions were also shown to be required for activity. Hydrogen bond acceptors at C-12 were also shown to be responsible for the activity, both in calanolides and inophyllums. In vitro assay results revealed that (+)-Calanolide-A inhibits RT in two diverse template primer systems. The action of (+)-Calanolide-A is possible due to the bi-bi prearranged mechanism of RT. Calanolide is at least partially competitive about dNTP binding. Structure-activity-relationships and important key findings of Calanolides are shown in Figure 4.
Figure 4.
Structure-activity-relationships and important key findings of some potent reverse transcriptase (RT) inhibitors.
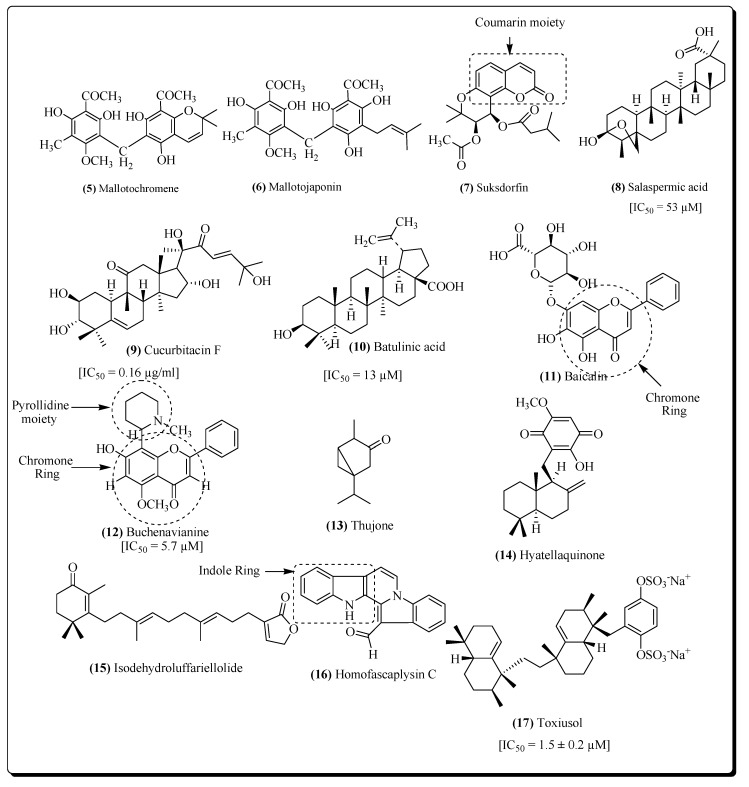
Some naphthoquinones, e.g., Michellamines A, B and C, isolated from the plant Ancistrocladus korupensis, exhibited inhibitory activity against the HIV-RT enzyme [94]. The compound Acetogenin protolichensterinic acid is a RT inhibitory agent, obtained from Cetraria islandica [95]. The compounds Mallotochromene (5) and Mallotojaponin (6) (Figure 5) from Mallotus japonicas have shown strong inhibition against HIV-RT [96]. Nigranoic acid, from Schisandra lancifolia, acted effectively on the RT of HIV [97]. A few more examples of plants showing HIV-RT inhibitory properties are given in Table 1.
Figure 5.
Structure of some potent reverse transcriptase inhibitors.
Table 1.
Plant-based reverse transcriptase inhibitors.
| Compound Class | Plant Species | Chemical Constituents | Reference |
|---|---|---|---|
| Terpenoid | Excoecaria agallocha | Phorbol | [98] |
| Terpenoid | Trypterygium wilfordii | Salaspermic acid | [99] |
| Terpenoid | Euphorbia myrsinites | 15-O-acetyl-3-O-butanoyl-5-O-propionyl-7-O-nicotinoylmyrsinol | [100] |
| Terpenoid | Polyalthia suberosa | Suberosol | [101] |
| Terpenoid | Andrographis paniculata | Dehydroandrographolide succinic acid monoester | [102] |
| Terpenoid | Glycyrrhiza radix | Glycyrrhizin | [103] |
| Terpenoid | Cowania Mexicana | Cucurbitacin F | [104] |
| Terpenoid | Tripterygium wilfordii | Tripterifordin | [105] |
| Terpenoid | Maprounea Africana | 1 β-hydroxymaprounic acid 3-p-hydroxybenzoate | [106] |
| Terpenoid | Szigium claviforum | Betulinic acid, platonic acid | [107] |
| Terpenoid | Houttuynia cordata | Lauryl aldehyde, capryl aldehyde | [108] |
| Flavonoid | Chrysanthemum morifolium | Acacetin-7-O-β-galactopyranoside | [92] |
| Flavonoid | Scutellaria baicalensis | Baicalin | [109] |
| Flavonoid | Buchenavia capitata | Buchenavianine | [110] |
| Flavonoid | Kummerolvia striata | Apigenin-7-O-β-D-glucopyranoside | [111] |
| Coumarin | Calophyllum inophyllum | Inophyllums | [112] |
| Coumarin | Coriandrum sativum | Coriandrin | [91] |
| Coumarin | Lomatium suksdorfii | Suksdorfin | [90] |
| Coumarin | Aegle marmelous | Imperatorin, xanthotoxol, xanthotoxin | [113,114] |
| Tannin | Euphorbia jolkini | Putranjivain A | [115] |
| Tannin | Cornus officinalis | Cornusin A | [116] |
| Tannin | Mallotus repandus | Repandusinic acid | [117] |
| Tannin | Hyssop officinalis | Caffeic acid | [118] |
| Polysaccharide | Thuja occidentalis | Thujone | [119] |
| Polysaccharide | Prunella vulgar | Sulfated polysaccharide | [120] |
| Polysaccharide | Viola yedoensis | Sulfonated polysaccharide | [121] |
| Xanthone | Tripterospermum lenceolaum | 1,3,5,6-tetrahydroxyxanthone, | [122] |
| 3,4,5,6-tetrahydroxyxanthone | |||
| Lignan | Haplophyllum ptilostylum | Ptilostin | [123] |
| Lignan | Schisandra chinensis | Gomisin J | [124] |
| Lignan | Ipomoea cairica | Arctigenin, trachelogenin | [125] |
| Marine origin | Hyatella intestinalis | Hyatellaquinone | [126] |
| Marine origin | Fascaplysinopis reticulate | Fascaplysin, isodehydroluffariellolide, | [127] |
| Homofascaplysin C | |||
| - | Toxiclona toxius | Toxiusol | [128] |
| - | Plakortis sp. | Plakinidine A | [129] |
| - | Kelletia kelletii | Kelletinin 1 | [130] |
| - | Buccinulum corneum | Kelletinin A | [131] |
| - | Maprounea Africana | 1β-hydroxyaleuritolic acid 3-p-hydroxybenzoate | [132] |
The compounds obtained from different plants, showing anti-HIV RT activity show the presence of certain pharmacophores which are essential for the therapeutic activity. These pharmacophoresinclude; coumarin, chromone, indole moiety and steroidal nucleous in the compounds, e.g., suksdorfin (7) [90], salaspermic acid (8) [99], cucurbitacin F (9) [104], batulinic acid (10) [107], baicalin (11) [109], buchenavianine (12) [110], thujone (13) [117], hyatellaquinone (14) [126], isodehyroluffariellolide (15) [127], homofascaplysin-C (16) [127], toxiusol (17) [128] reperesented in Figure 5.
2.1.3. Plants Exhibiting Integrase Inhibition
The insertion of HIV DNA into the DNA of the host cell is generally catalyzed by the integrase enzyme of HIV. The reaction proceeds in two phases; the first phase is the processing phase and the second phase includes strand transfer [133]. Various active components have been separated from the plant Dioscorea bulbifera and exhibited several therapeutic properties such as: anticancer, antibacterial, analgesic, and antidiabetic [134,135,136,137,138]. Chaniad et al. isolated seven different compoundsfrom D. bulbifera which showed anti-HIV properties [45]. These include; allantoin (18), 5,7,4′-trihydroxy-2-styrylchromone,2,4,3′,5′-tetrahydroxybibenzyl, quercetin-3-O-β-D-galacto-pyranoside, 2,4,6,7-tetrahydroxy-9,10-dihydrophenanthrene, quercetin-3-O-β-D-glucopyranoside (19), and myricetin (20) (Figure 6). The results indicate that compound 20 had the best binding affinity within the active site of the integrase enzyme, forming strong interactions with amino acids. Moreover, significant activity is due to the presence of the galloyl, catechol, and sugar moieties which are responsible for the potential actions. In another study, Panthong et al. revealed that Albizia procera is a medicinal plant that has been used in antiretroviral therapy [139,140]. Catechin (21), suramin and protocatechuic acid (22) were shown to be the components identified from the plant extract and were considered to act on the integrase enzyme of HIV, thus prohibiting viral replication [139]. Compound 21 interacted with Thr66, Gly148, and Glu152 in the core domain of the enzyme, whereas compound 22 interacted with Thr66, His67, Glu152, Asn155, and Lys159. Some ribosome-inactivating proteins are considered to act on the integrase enzyme [141]. It was observed that compound 20, having a galloyl moiety, possessed the most potent activity due to its strong binding with amino acids of the integrase enzyme. In compound 19, the catechol group was partly responsible for the activity. Since compound 19 contains sugar moiety as well, which increases the solubility of the molecule, this enhances its activity. A ribosome-inactivating protein (RIP) named MAP30, which has been extracted from Momordica charantia, has been reported to act against HIV and cancer [142,143]. Zhao et al. discovered another RIP trichosanthin, from the roots of Trichosanthes kirilowii, which showed inhibitory activity against the integrase enzyme [144]. A variety of plant RIPs including agrostin, saporin, R-momorcharin, gelonin, α-momorchain, trichosanthin and luffin have also exhibited an inhibitory effect on HIV replication [145].
Figure 6.
Structure-activity-relationships of naturally occurring integrase inhibitors.
2.1.4. Plants Containing Protease Inhibitors
Protease is a viral enzyme that acts at the last step of replication of the virus. It causes the breakdown of long polypeptides and proteins into the small functional proteins that are generally infectious [146,147,148,149]. Hence, protease is another target for the antiretroviral therapy and by inhibiting this enzyme the viral replication can be prohibited. Mostly, drugs act on this enzyme preferentially [147,149,150]. From the Camellia japonica pericarp, the plant components camelliatannins A, F and H have been reported that exhibited potent anti-HIV PR inhibitory properties [148]. Several Korean therapeutic plants, e.g., Viburnum furcatum, IIex cornuta, Berberis amurensis, Lonicera japonica, Chloranthus glaber, Geranium nepalense, Lindera sericea, Wistaria floribunda, Smilax china, Hibiscus hamabo, Lingustrum lucidum, Zanthoxylum piperitum, Styrax obassia, Viola mandshurica, Schisandra nigra, and Cocculus trilobus have also been reported potent activities against protease [71]. From the plant stems of Stauntonia obovatifoliola, various components that act against HIV protease have been identified, e.g., lupenone (23) [151], 3-O-acetyloleanolic acid (24) [152], resinone (25) [153], lupeol (26) [154] and mesenbryanthemoidgenic acid (27) (Figure 7) [155]. Moreover, the therapeutic compounds like oleanolic acid (28), dihydromyricetin, epigallocatechin gallate, myricetin [156,157] and epiafzelechin [158] have been extracted from the wood of Xanthoceras sorbifolia and have the potential for the treatment of AIDS [68,156].
Figure 7.
Compounds exhibiting protease inhibitory activity.
2.1.5. Plants Containing Immunomodulators
Immunomodulators are the agents that stimulate the cellular and humoral immune system against any pathogenic infection [159,160,161]. The dendritic cells of the immune system act as antigen representing cells and move along with antigens into the lymph nodes from the tissues. They represent the antigen to the T cells and the T cells then initiate an immune response. T cells stimulate the B cells for the production of antibodies that bind with the antigen and the T cells activate killer T cells which attack the pathogens [162]. There are several classes of naturally occurring compounds that exhibit immunomodulatory properties, e.g., alkaloids, tannins, terpenoids, coumarins, glycosides, flavonoids, polysaccharides, lignans, etc [163,164]. Among alkaloids, berberine (29) from Hydrasti Canadensis [159,165], sinomenine (30) from Sinomenium acutum [159,166], piperine (31) from Piper longum [159,167], and tetrandrine from Stephania tetrandra [168] have shown immunomodulatory properties in HIV. Among glycosides, aucubin from Plantago major [169], isorhamnetin-3-O-glucoside from Urtica dioica [170], and mangiferin from Mangifera indica [171] have exhibited immune-stimulatory properties in HIV. Among phenols, ellagic acid (32) from Punica granatum [172], curcumin from Curcuma longa [173], ferulic acid (33), vanilic acid (34) (Figure 8) [159] were shown to be immunostimulators in HIV. Chlorogenic acid from Plantago major [169], also expressed effective immunomodulatory potential in AIDS [159]. Within tannins, chebulagic acid and corilagin from Terminalia chebula [174] and punicalagin [175] acted as immunomodulatory agents. Among flavonoids, centaurein from Bidens pilosa [176] and apigenin 7-O-β-D-neohesperidoside, orientin, vitexin and apigenin 7-O-β-D-galactoside from Jatropha curcas [177] have exhibited the effective immunomodulatory action against HIV. From saponins, asiaticoside obtained from Centella asiatica [178] and glycyrrhizin from the roots of Glycyrrhiza glabra [179] have shown significant immunomodulatory activities.
Figure 8.
Plant-based Immunomodulators.
2.1.6. Plants with Antioxidant Potential
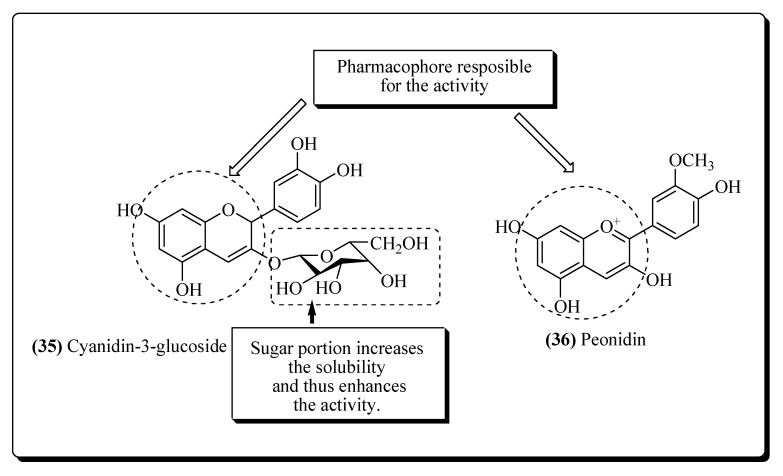
In AIDS, many reactive oxygen species (ROS) have been produced due to the alteration in the levels of antioxidant enzymes [180]. This further leads to the damage of DNA and lipid peroxidation [181]. ROS can also stimulate the nuclear factor kappa B (NF-κB factor) which helps in the transcription of HIV and thus promote its replication [182]. Antioxidants are agents that reduce the levels of ROS and protect cellular DNA. N-Acetylcysteine is reported to acts as an antioxidant and inturn used in themanagement of HIV infection [183]. Various other antioxidants like selenium, lipoic acid, vitamin C, β-carotene and vitamin E have been utilized for the same purpose [184,185]. The antioxidants Cyanidin-3-glucoside (35) and peonidin (36), which are obtained from blackberries, have also been shown to slow down AIDS infection (Figure 9) [159,186].
Figure 9.
Plant-based antioxidants compounds possessing anti-HIV potential.
2.2. Classification of Plants According to Their Secondary Metabolites
Secondary metabolites are the main active compounds in plants that are mainly responsible for therapeutic effects. They are generally obtained from the primary metabolites such as carbohydrates, proteins, amino acids, etc. [187,188,189,190]. Plant-based secondary metabolites mainly include alkaloids, glycosides, coumarins, terpenoids, lignans, tannins and flavonoids, etc. [191,192,193].
2.2.1. Alkaloids
Alkaloids are the basic nitrogen-containing secondary metabolites in plants, active against many pathogens, including HIV. Buchapine is a quinolone alkaloid obtained from Eodia roxburghiana, which has shown activity against HIV [194]. From the roots of Tripterygium hypoglaucum, various alkaloidal compounds have been isolated, e.g., hypoglaumine B, triptonine A (37) and B [159], which exhibited anti-HIV potential and have potential for antiretroviral therapy [195]. Nitidine is another alkaloid that was isolated from plant roots of Toddalia asiatica, and has shown efficacy against HIV [196]. From the plant Symplocos setchuensis, the alkaloid harman (38) and another compound matairesinoside (39) were isolated andshowed potential for antiretroviral therapy due to their anti-HIV potential. Compound 39 acts on the viral replication enzymes, thus inhibiting HIV replication [197]. Another aromatic alkaloid polycitone A, from marine source Polycitor sp., exhibited potential activity against the reverse transcriptase of HIV. Hence, it efficiently inhibits HIV replication. Several other marine sponges have acted against the virus as well as other bacterial diseases [198]. The alkaloid 1-methoxy canthionone was reported from Leitneria floridana, and exhibited anti-HIV property [199]. Papaverine was obtained from Papaver sominiferum, and inhibited HIV replication [39]. Norisoboldine and corydine are two alkaloids obtained from the leaves of Croton echinocarpus, showing anti-HIV activity [200]. Table 2 summarizes plant-based alkaloids possessing anti-HIV activity.
Table 2.
Alkaloidal compounds as anti-HIV agents.
| Plant Species | Parts Used | Chemical Constituents | References |
|---|---|---|---|
| Ancistrocladus korupensis | Leaves | Michellamine A, B and C | [201] |
| Stephania cepharantha | Roots | Cepharantine | [202] |
| Murraya siamensis | Roots, leaves | Siamenol | [203] |
| Clausena excavate | Leaves | O-Methylmukonal, clauszoline and 3-formyl-2,7-dimethoxy-carbazole | [204] |
| Drymaria diandra | Leaves | Canthin-4-one drymaritin | [205] |
| Glycosmis montana | Twigs, leaves | (E)-3-(3-hydroxymethyl-2-butenyl)-7-(3-methyl-2-butenyl)-1H-indole | [206] |
| Aniba sp. | Stems | Anibamine | [207] |
| Zanthoxylum ailanthoides | Root bark | Decarine, ϒ-fagarine and tembamide | [208] |
| Nelumbo nucifera | Leaves | Coclaurine, norcoclaurine, reticuline | [209] |
| Pericampylus glaucus | Leaves | Norruffscine, 8-oxotetrahydro-palmatine | [210] |
| Begonia nantoensis | Rhizomes | Indole-3-carboxylic acid | [211] |
| Leucojum vernum | Bulbs | Lycorine, homolycorine | [212] |
| Epinetrum villosum | Root bark | Cycleanine | [213] |
| Argemone mexicana | Roots | 6-acetonyldihydrochelerythrine, nuciferine | [214] |
| Monanchora sp. | Stems | Crambescidin 826, fromiamycalin and crambescidin 800 | [215] |
| Manadomanzamines A and B | [216] | ||
| Acanthostrongylophora sp. | - | Hernandonine, lindechunine,7-oxohernangerine and laurolistine | [217] |
| Roots | |||
| Lindera chunii | |||
| Artemisia caruifolia | Stems | [218] |
Several alkaloidal compounds, e.g., michellamine A (40) [201], siamenol (41) [203], decarine (42) [208], reticuline (43), norcoclaurine (44) [209], indole-3-carboxylic acid (45) [211], lycorine (46) [212], homolycorine (47) [212], cycleanine (48), 6-acetonyldihydrochelerythrine (49) [214] and hernandonine (50) [217] have revealed significant HIV inhibitory potential (Figure 10 and Figure 11).
Figure 10.
Alkaloidal compounds possessing anti-HIV activity.
Figure 11.
More alkaloidal compounds possessing anti-HIV activity.
2.2.2. Terpenoids
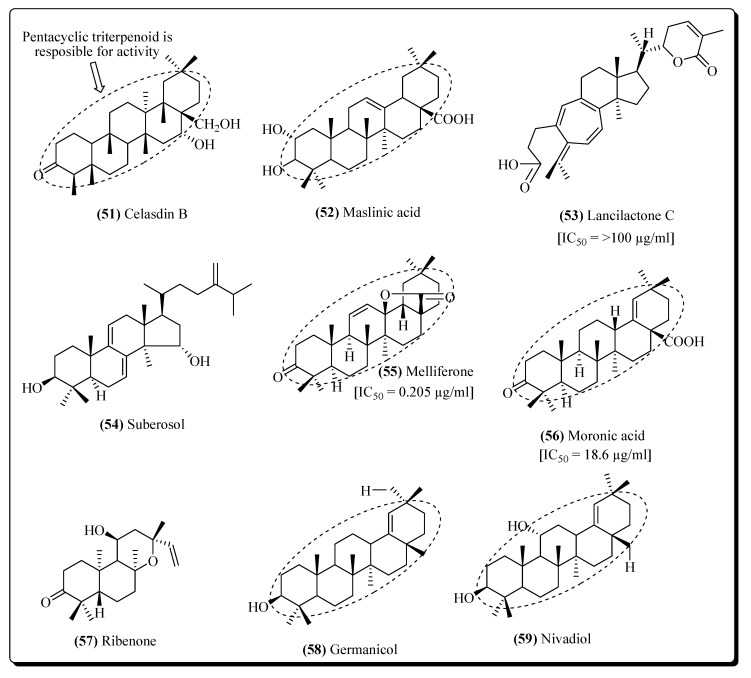
Terpenoids are the secondary metabolites that are derived from the isoprene unit (C5H8). Terpenoids are the most abundant plant-based secondary metabolites and several compounds from this class have been derived from plants and found useful for their therapeutic potential [210,219,220]. Examples of terpenoids that have exhibited inhibition of HIV replication include betulinic acid, oleanolic acid, and platanic acid from Syzigium claviflorum leaves [221]. Celasdin B (51), is a triterpene from Celastrus hindsii (Celastraceae), reported inhibiting the HIV replication [222]. Prostratin from Homalanthus nutans (Euphorbiaceae) has also expressed significant anti-HIV activities [223]. From the stem bark of Garcinia speciosa, some anti-HIV therapeutic constituents have been isolated viz; garcisaterpenes A and C and theprotostanes. These compounds have been found to inhibit the activity of HIV reverse transcriptase and thus stop the HIV life cycle [224]. Maslinic acid (52), a terpenoid compound obtained from Geum japonicum also acts against the HIV protease enzyme [225]. From the stems and roots of plant Kadsura lancilimba, another triterpene lancilactone C (53) has been isolated whch is used to restrict the viral replication [226]. Oleanolic acid is the main terpenoid isolated from many plant species including Xanthoceras sorbifolia (Sapindaceae). The compound is known to inhibit HIV replication and play an important role in the treatment of AIDS [227]. Suberosol (54) is a lanostane type triterpenoid from the leaves of Polyalthia suberosa (Annonaceae) known toact through the same mechanism [228]. Another phorbol diester from Croton tiglium (Euphorbiaceae), 12-O-tetradecanoylphorbol-13-acetate, exhibited anti-HIV activity [223]. A Brazilian alga isolated from Dictyota pfaffii, from which an active diterpene component 8,10,18-trihydroxy-2,6-dolabelladiene has been isolated and has shown inhibitory activity of HIV reverse transcriptase [229,230]. A butenolide triterpene known as 3-epi-litsenolide has been articulated significant anti-HIV activity and was extracted from Litsea verticilla [231]. The alga Dictyota menstrualis is an important source for various diterpenes that exhibit HIV reverse transcriptase inhibition potential [232]. From the roots and rhizomes of plant Clausena excavate, a limonoid terpene named clausenolide-1-ethyl ether has shown potential for antiretroviral therapy [233]. Glycyrrhizin from the Glycyrrhiza glabra roots is another saponin terpenoid that showed anti-HIV activity by inhibiting the viral life cycle [234]. Oleanolic acid is a potent anti-HIV compound and is widely distributed in various plants including the leaves of Rosa woodsii, the leaves of Syzygium claviflorum, the aerial parts of Ternstromia gymnanthera, and the whole plants Hyptis capitata and Phoradendron juniperinum [227]. 12-Deoxyphorbol-13-phenylacetate, a phorbol ester from Euphorbia poissonii, has been reported for possessing anti-tumour activity and recently, it has potential for antiretroviral therapy because of its anti-HIV activity [235]. Pedilstatin [13-O-acetyl-12-O-(2′-Z-4′-Eoctadienoyl)-4-α-deoxyphorbol] is another phorbol ester from Pedilanthus sp., possessing anticancer and anti-HIV properties [236]. Some other plant species containing terpenoid based compounds with efficient anti-HIV activity have been summarized in Table 3.
Table 3.
Terpenoids act as Anti-HIV agents.
| Plant Species | Parts Used | Chemical Constituents | References |
|---|---|---|---|
| Excoecaria acerifolia | Roots | Agallochin J, ribenone, angustanoic acid B | [237,238,239] |
| Propolis | Roots | Melliferone, moronic acid, betulonic acid | [49,240] |
| Homalanthus nutans | Leaves | Prostratin | [241,242] |
| Cassine xylocarpa | Stem | Germanicol, nivadiol | [243] |
| Glycyrrhiza uralensis | Roots | Galacturonic acid, xylose, uralsaponin C | [244] |
| Daphne gnidium | Aerial parts | Daphnetoxin, gniditrin, gnidicin | [245] |
| Euphorbia micractina | Roots | Lanthyrane diterpenoids | [246] |
| Kaempferia pulchra | Rhizomes | Kaempulchraol A, C, E | [247] |
| Picrasama javanica | Bark | Picrajavanicin A, javanicin B, picrasin A | [248] |
| Schisandra lancifolia | Leaves, stem | Lancifodilactone F | [249] |
| Stellera chamaejasme | Roots | Stelleralide D, gnidimacrin | [250] |
| Lindera strychnifolia | Roots | Lindenanolides E, G and F | [251] |
| Daphne acutiloba | Roots | Wikstroelide M | [252] |
| Annona squamosa | Leaves | 16-β,17-dihydroxy-entkauran-19-oic acid | [253] |
| Cimicifuga racemosa | Rhizomes | Actein | [254] |
| Schisandra sphaerandra | Leaves | Nigranoic acid | [255] |
| Allanthus altissima | Roots | Shinjulactone B | [256] |
| Panax ginseng | Roots | Isodehydroprotopanaxatriol | [257] |
| Garcinia hanburyi | Stem, roots | 3-acetoxyalphitolic acid, 2-acetoxyalphitolic acid | [258] |
| 8-methoxyingol-7,12-diacetate-3-phenylacetate | |||
| Euphorbia officinarum | Leaves | Dihydrocucurbitacin F | [259] |
| Forskolin, 1-deoxyforskolin | |||
| Hemsleya jinfushanensis | Tubers | 28-hydroxy-3-oxo-lup-20(29)-en-3-O-al | [260] |
| Coleus forskohlii | Roots | Betulonic acid | [261] |
| Microtropis fokienensis | Stem | 25-hydroxy-3-oxoolean-12-en-28-oic acid | [262] |
| Betula platyphylla | Roots | Capilliposide B | [263] |
| Amoora rohituka | Stem bark | Ganoderic acid D | [264] |
| Ganoderiol F | |||
| Lysimachia capillipes | Roots | Impatienside A, bivittoside D | [265] |
| Ganoderma lucidum | Stem, Leaves | 25-methoxyhispidol A | [266] |
| Ganoderma amboinense | Stem | 23,24-dihydrocucurbitacin B | [267] |
| Holothuria impatiens | - | Dichapetalin A | [268] |
| Poncirus trifoliate | Fruits | Acutissimatriterpene A, B, E | [269] |
| Trichosanthes kirilowii | Roots | Celastrol | [270] |
| Dichapetalum gelonioides | Stem bark | 3α,7α-dideacetylkhivorin | [271] |
| Phyllanthus acutissima | Aerial parts | Nimbolide | [272] |
| Celastrus orbiculatus | Bark | Gedunin, 1 α–hydroxy-1,2-dihydrogedunin | [273] |
| Khaya senegalensis | Roots | 6α-tigloyloxychaparrinone | [274] |
| Azadirachta indica | Flowers | [275] | |
| Xylocarpus granatum | Roots | [276] | |
| Ailanthus integrifolia | [277] |
Many of the terpenoid compounds, e.g., melliferone (55), whoseanti-HIV potentialhas been evaluated inanti-HIV assays towards T cell line H9, and compared with the positive control AZT has been shown in Figure 12 and Figure 13. Melliferone exhibited an IC50 value of 0.205 µg/mL [49], moronic acid (56), [49], ribenone (57) [237], germanicol (58) [243], nivadiol (59) [243], wikstroelide M (60) [252], shinjulactone B (61) [256], ganoderic acid D (62) [266], ganoderiol F (63) [267] gedunin (64) [276] and 1-α–hydroxy-1,2-dihydrogedunin (65) [276] also exhibited anti-HIV activities.
Figure 12.
Potent terpenoids against HIV.
Figure 13.
More potent terpenoids against HIV.
2.2.3. Flavonoids
Flavonoids are well-known phytoconstituents reported to exhibit several antiviral and antioxidant properties [278]. Flavonoids like quercetin 3-O-(2-galloyl)-L-arbinopyranose and gallate ester from Acer okamotoanum (Aceraceae), exhibited significant activity against integrase of HIV [279]. Xanthohumol (66), an important flavonoid from Humulus lupulus, has shown anti-HIV activity [280]. The flavonoid moiety (4H-chromen-4-one) is known to be mainly responsible for the therapeutic activity, while glycosidic portion attached to the flavonoid enhances the solubility of the compounds and thus boosts its therapeutic activity. Two flavonoids 6,8-diprenylkaempferol and 6,8-diprenylaromadendrin isolated from Monotes africanus have expressed potential activity against the AIDS virus [281]. Another anti-HIV biflavonoid named wikstrol B (67) (Figure 14) has been isolated from Wikstroemia indica (Thymelaeaceae) roots [282]. Baicalin is a flavonoid compound that inhibits HIV replication and is derived from Scutellaria baicalensis [282]. From the twigs and leaves of the medicinal plant Rhus succedanea, various anti-HIV flavonoids (robustaflavone, biflavonoids, and hinokiflavone) have been reported to act on the polymerase of the reverse transcriptase of HIV-1 [283,284]. 2-methoxy-3-methyl-4,6-dihydroxy-5-(3′-hydroxy)-cinnamoylbenzaldehyde, a chalcone flavonoid that has been extracted from Desmos sp. roots and exhibited strong activity against HIV-1 [285]. Another chalcone, Hydroxypanduratin A, from the rhizomes of Boesenbergia pandurata depicted its action on the HIV protease enzyme [286].
Figure 14.
Flavanoids with anti-HIV properties.
Several naturally obtained flavonoids, e.g., chrysin, epigallocatechin gallate (68) and quercetin (69) have been reported to show potent inhibitory activities against the replication of HIV [276,277]. The flavonoids Thalassiolin A, B and C from the grass Thalassia testudinum acted against HIV integrase, which inturn inhibited the life cycle of HIV-1. Thalassiolin A was found to be the most potent compound which inhibits the terminal cleavage [287,288,289]. Some biflavonoids, e.g., 2″,3″-dihydroochnaflavone 7″-O-methylether and ochnaflavone 7″-O-methyl ether from Ochna integerrima, have shown moderate to weak anti-HIV activities [290,291]. Taxifolin (70), also known as dihydroquercetin, is mostly found the stems of Juglans mandshurica, and expressed strong inhibitory activity on the reverse transcriptase enzyme of HIV and thus plays a role in the prevention of HIV replication [292]. From Chrysanthemum morifolium flowers, two important flavonoids apigenin-7-O-β-D-(4′-caffeoyl)glucuronide and glucuronide have been isolated, which exhibited significant activity against the integrase of HIV-1 [293]. Mentha longifolia is another plant whose methanolic extracts are used for the isolation of several therapeutic flavonoids those were found to be active through the same mechanism [294]. Compound 70 was demonstrated to inhibitthe activity of HIV-1 replication. Several other flavonoids such as flemiphyllin, formosanatin C (71), euchretin I (72) and quercetin are reported to inhibit the HIV replication and obtained from the alcoholic extracts of Euchresta formosana [295]. Many important flavonoids such as epicatechin-3-O-gallate and epicatechin have extracted from Detarium microcarpum, have shown anti-HIV potential [296]. 4′-methylepigallocatechin-3′-O-β–glucopyranoside, and 4′-methylepigallocatechin-5-O-β-gluco-pyranoside from Maytenus senegalensis shown anti-HIV potential [297]. Kaempferol (73) (Figure 14), a tetrahydroxyflavonol was isolated from Rosa damascene and showed inhibitory activity on the protease enzyme [298,299].
2.2.4. Coumarins
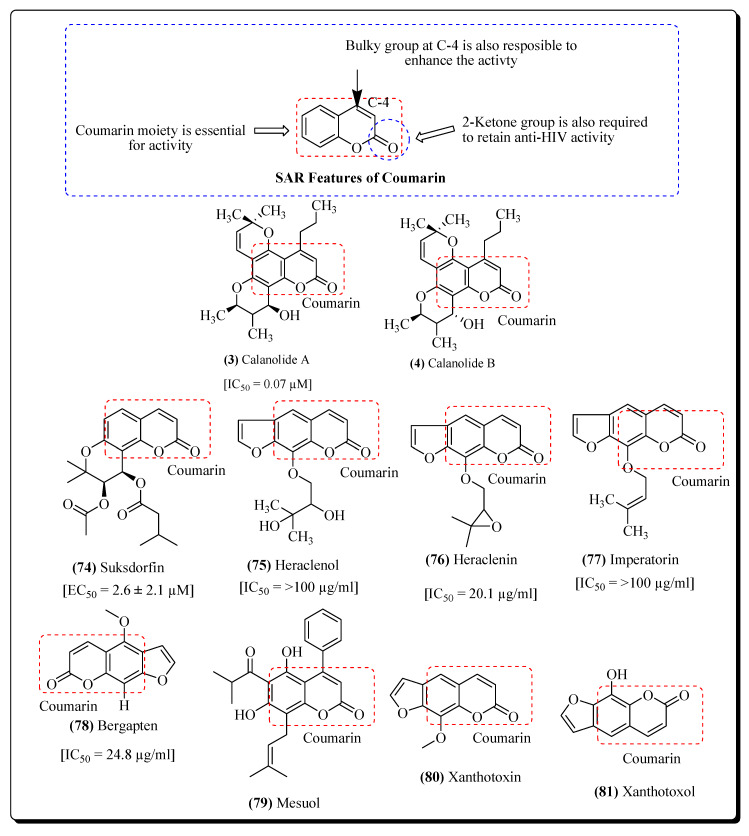
Calanolides are a group of coumarins that act as non-nucleoside reverse transcriptase inhibitors and are derived from plants of the genus Calophyllum (Clusiaceae) [300]. The coumarin (+)-Calanolide A has already been subjected to in vivo studies and up to phase II clinical trials in healthy, HIV-negative subjects. These studies revealthat (+)-calanolide A has a favourable safety profile in humans as well as in animals [301,302], while calanolide B alongwith its derivative known as 7,8-dihydrocalanolide B from the plant Calophyllum lanigerum, showedsignificant anti-HIV potential based on cytopathogenic results of HIV on the cells of the host [300]. Another coumarin named suksdorfin (74) [303,304] isolated from the fruits of Lomatium suksdorfii belonging to the family Apiaceae, which has expressed inhibitory property on the HIV replication [303]. The compounds Cordatolides A and B from Calophyllum cordato-oblongum, were similar in structureto the Calanolides and were found to inhibit the replication of the HIV [300]. The coumarin skeleton is essential for anti-HIV activity (Figure 15). Other coumarins like heraclenol (75) and heraclenin (76) exhibit IC50 value of 20.1 µg/mL against H9 lymphocytes, while imperatorin (77) from the roots of Ferula sumbul falls under the same therapeutic category [305]. Bulky groups at the C-4 are also required to retain the anti-HIV activity, which is present in the prototype of a molecule like (+)-Calanolide-A. (+)-Calanolide-A is the most potent compound when compared with Cordatolide A (less active and devoid of the bulky group at the C-4 position). Several furanocoumarins (e.g., bergapten (78) and psolaren) from the roots of Prangos tschimganica, have exhibited significant activities against the HIV virus [306]. Mesuol (79) is another coumarin (from the category 4-phenylcoumarin) reported to inhibit the replication of HIV-1through the prohibition of the reverse transcription and phosphorylation of HIV [307]. A semisynthetic derivative of calanolide (known as oxocalanolide) was alsoreported to act efficiently against HIV [308]. Various furanocoumarins (e.g., imperatorin, xanthotoxin and xanthotoxol) have been extracted from the Aegle marmelos fruits [121,122]. The stem, roots, fruits, leaves, seeds and bark of the A. marmelous showed variable antiviral effects and have played an important role in Ayurvedic medicine. Imperatorin (77) is reported to exhibit about 60% inhibition of HIV-RT. The absence of a prenyl group resulted in the observed weak activity. This is exemplified in the cases of other furanocoumarins xanthotoxin (80) and xanthotoxol (81), shown in Figure 15 [309,310].
Figure 15.
Coumarins with significant Anti-HIV potential.
2.2.5. Proteins
Proteins are the amino acid-containing plant components that usually contain ribosome-inactivating proteins as well as lectins [311]. A plant protein called MAP30 from Momordica charantia is known to possess anticancer potential along with anti-HIV properties [312]. Various plant ribosome-inactivating proteins have been identified for their anti-HIV activities. Trichosanthin is a ribosome-inactivating protein isolated from Trichosanthes kirilowii that has shown anti-HIV activity [313]. Various plant ribosome-inactivating proteins have been identified for their anti-HIV activities, e.g., an anti-HIV ribosome-inactivating protein balsamin has been extracted from Momordica balsamina [314]. Pf-gp6 is another protein reported from Perilla frutescens which has exhibited an inhibitory action on HIV replication [315]. Some ribosome-inactivating proteins known as Pokeweed antiviral proteins have been separated from a pokeweed plant (Phytolacca americana) and have expressed efficient anti-HIV activities [316]. A list of plant proteins has been given in Table 4, along with their botanical sources.
Table 4.
Proteins containing plants used in HIV.
| Plant Species | Parts Used | Proteins | References |
|---|---|---|---|
| Allium ascalonicum | Bulbs | Ascalin | [317] |
| Chrysanthemum coronarium | Seeds | Chrysancorin | [318] |
| Ginkgo biloba | Seeds | Ginkbilobin | [319] |
| Arachis hypogaea | Seeds | Hypogin | [320] |
| Lyophyllum shimeji | Fruit bodies | Lyophyllin | [321] |
| Panax quinquefolium | Roots | Quinqueginsin | [322] |
| Flammulina velutipes | Fruit bodies | Velutin | [323] |
| Tricholoma giganteum | Fruit bodies | Laccase protein | [324] |
| Castanea mollisima | Seeds | Mollisin | [325] |
| Treculia obovoidea | Bark | Treculavirin | [326] |
| Vigna sesquipedalis | Seeds | Ground bean lectin | [327] |
| Delandia unbellata | Seeds | Delandin | [328] |
| Dorstenia contrajerva | Leaves | Contrajervin | [326] |
| Vigna angularis | Seeds | Angularin | [329] |
| Castanopsis chinensis | Seeds | Castanopsis thaumatin protein | [330] |
| Vigna unguiculata | Seeds | Cowpea α protein | [331] |
| Phaseolus vulgaris | Seeds | A homodimeric lectin | [332] |
| Actinidia chinensis | Fruits | Kiwi fruit thaumatin protein | [333] |
| Lentinus edodes | Fruit bodies | Lentin | [334] |
| Allium tuberosum | Shoots | A mannose-binding lectin | [335] |
| Phaseolus vulgaris | Seeds | Phasein A | [336] |
| Lilium brownie | Bulbs | Lilin | [337] |
| Vicia faba | Seeds | A trypsin-chymotrypsin | [338] |
| Inhibitor peptide | |||
| Vigna unguiculata | Seeds | Unguilin | [339] |
| Panax notoginseng | Roots | A xylanase | [340] |
| Phaseolus vulgaris | Seeds | Vulgin | [341] |
| Cicer arietinum | Seeds | Chickpea cyclophilin-like protein | [342] |
| α–Basrubrin | |||
| Basella rubra | Seeds | Rice bean peptide | [343] |
| Delandia unbellata | Seeds | [344] |
2.2.6. Tannins
Tannins are mainly categorized into gallotannins and ellagitannins. While gallotannins are hydrolysable and contain gallic acid polyesters ellagitannins are non-hydrolyzable, so-called condensed tannins conatining hexahydroxydiphenic acids, i.e., flavan-3-ol (proanthocyanidins) moieties [345,346]. Corilagin (82) and Geraniin (83) (Figure 16), from roots of Phyllanthus amarus, are two ellagitannins that possess anti-HIV activities [347]. Besides, a proanthocyanidin compound from the plant Cupressus sempervirens, exerted anti-HIV properties [348]. Catechins, the polyphenols that are obtained from green tea, and theaflavins (e.g., compound 84) isolated from black tea, possess antiviral activity. Theaflavins and their derivatives are potent inhibitors of HIV replication [349]. Compounds 82 and 83 blocked the interaction of HIV-1 gp120 with its primary cellular receptor CD4. Besides, the observed results showed that compound 83 exhibited inhibitory effects on HIV, not only in vitro but also in vivo. Compound 84 inhibited HIV-1 entry into target cells by blocking the HIV-1 envelope glycoprotein-mediated membrane fusion. The ability of this compound to block the formation of the gp41 six-helix bundle was determined using Fluorescence native polyacrylamide gel electrophoresis, while detection of the binding of gp120 to CD4 was done by ELISA. Molecular docking analyses suggested that compound 84 may bind with to the highly conserved hydrophobic pocket on the surface of the central trimeric coiled-coil of gp41.
Figure 16.
Tannins with anti-HIV properties.
2.2.7. Lignans
Several extensive reports on plant-based lignans which have shown strong activities against viral diseases, including AIDS, exist [350]. Several lignans like anolignan A (85) and anolignan B, alongwith dibenzylbutadiene lignans have been isolated from Anogeissus acuminate and have exhibited significant activity against HIV [351]. From Phyllanthus myrtifolius (Euphorbiaceae), phyllamyricin D (86) and phyllamyricin F (87) (Figure 17) were isolated and shown to possess inhibitory activity against the HIV-RT enzyme [352]. The benzoaryl moiety was proven to be essential for the anti-HIV activity of lignans. This group is responsible for inhibiting HIV replication. Gomisin is another example of lignan isolated from Kadsura interior and showed potent inhibitory activity against the RT enzyme of HIV [353]. From Arnebia euchroma, some caffeic acid isomers have been evaluated but have only expressed weak activities against HIV replication [354]. The compound 2-hydroxy-2 (3′,4′-dihydroxyphenyl)-methyl-3-(3″,4″-dimethoxyphenyl) methyl γ–butyrolactone is a dibenzylbutyrolactone type lignan from Phenax angustifolius with established anti-HIV activity [355]. From Schisandra rubriflora fruits, other dibenzocyclooctadiene type lignans (rubrisandrin A and rubrisandrin B) have been isolated having anti-HIV activities [356].
Figure 17.
Lignans possessing anti-HIV activities.
2.2.8. Miscellaneous Plant-Based Anti-HIV Agents
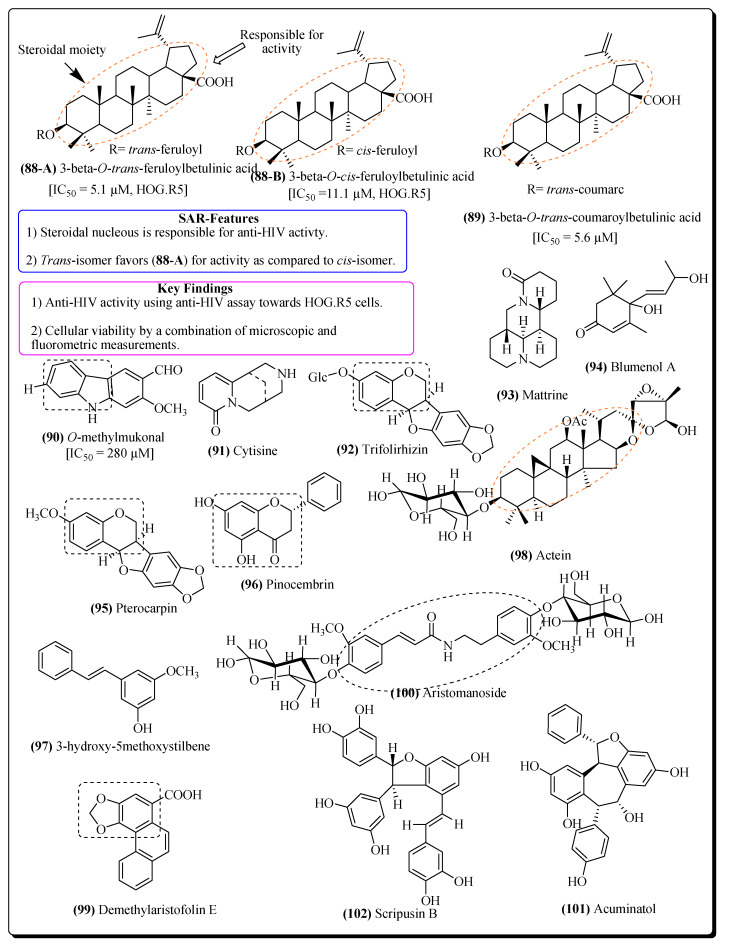
Numerous plants have been evaluated for their anti-HIV activities and are being used in antiretroviral therapy for AIDS [1,2]. Various phenolic compounds isolated from plants such as Quercus pedunculata, Terminalia horrida, Phyllanthus emblica and Rumex cyprius have been identified for their anti-HIV activities [357,358]. From the leaves and twigs of plant Strychnos vanprukii, various betulinic acid derivatives, such as 3-β-O-cis-feruloylbetulinic acid (88-B), 3-β-O-trans-feruloylbetulinic acid (88-A), ursolic acid and 3-β-O-trans-coumaroylbetulinic acid (89) have exhibited potential against HIV [359]. Compounds 88-A, 88-B and 89 have been evaluated for anti-HIV activities against HOG.R5 cells in the anti-HIV assay. Compound 88-A showed significant inhibition against HIV-1 replication. The trans-isomer (88-A) showed a more favourable activity when compared with the cis-isomer (88-B) (Figure 18). The compounds shown in Figure 18 exhibited significant potential against HIV due to the presence of the pharmacophore/heterocyclic moieties, such as chromone, indole, steroidal nucleus, benzodioxole, quinolizine, etc. These compounds also demonstrated various therapeutic properties, e.g., anti-inflammatory, anti-cancer, antiviral, antioxidant and immunomodulatory properties [360]. The constituents of Cinnamomum zylanicum bark have shown anti-inflammatory [361], anti-cancer, antiviral, antioxidant and immunomodulatory properties [362]. The ingenol compounds from Euphorbia ingens have exhibited anti-HIV activities [363], apart from their anti-inflammatory and immunomodulatory potentials [364,365]. Oldenlandia affinis is a medicinal plant from which various cyclotides have been isolated and tested for their activities against HIV [366,367]. Plectranthus barbatus has also shown diverse antiviral, antibacterial and antifungal properties along with antioxidant and anti-inflammatory effects [368,369]. From Clausena excavate some therapeutic constituents like O-methylmukonal (90), 3-formyl-2,7-dimethoxycarbazole, limonoids, and clausenidin have been reported for their anti-HIV properties [370,371]. Several antiviral components like tectorigenin, cytisine (91), formononetin, trifolirhizin (92), mattrine (93), blumenol A (94), pterocarpin (95), 30,40,5-trihydroxyisoflavone, euchretin and 5,7-dihydroxy-3-(2-hydroxy-4-methoxy-phenyl)-chromen-4-one have been isolated from Euchresta formosana and exhibited anti-HIV activities [372,373,374,375].
Figure 18.
Other plant-based compounds with anti-HIV activities.
Extracts from Alepidea amatymbica, have shown efficient anti-HIV activities, as well as inhibitory effect on HIV replication [376]. Artemisinin from the plant Artemisia annua, has established antimalarial and anti-HIV activities [377]. Rosmarinic acid is a polyphenolic compound from the plant Prunella vulgaris, usedfor the treatment of isolated HIV [378]. From Polygonum glabrum, various bioactive constituents with antiretroviral activities have been reported, e.g., (-)-2-methoxy-2-butenolide-3-cinnamate, pinocembrin (96), 3-hydroxy-5-methoxystilbene (97), sitosterol-3-O-β-D-glucopyranoside, and pinocembrin-5-methyl ether [379]. Actein (98) from the rhizomes of Cimicifuga racemosa, possessed a significant activity against HIV [380]. Chrysoeriol from Eurya ciliate is known for its anti-HIV activity [381]. Several constituents such as demethylaristofolin E (99), aristofolin, denitroaristolochic acid, aristolochic acid, aristomanoside (100), N-p-coumaroyltyramine, p-hydroxybenzoic acid, etc. have been isolated for their anti-HIV potential from the stem bark of Aristolochia manshuriensis [382,383,384,385,386]. Malaferin A, from Malania oleifera, was also tested for its antiviral property [387]. Diptoindonesin D, Acuminatol (101), Shoreaphenol, Hopeahainol, and Vaticanol B from Vatica mangachapoi have shown positive effects in the management of antiretroviral therapy [388,389,390,391]. Cararosinol C and D, maackin and scirpusin B (102) from Caragana rosea have been evaluated for their anti-HIV effects [392]. Structures of some important constituents obtained from plants effective in HIV therapy are represented in Figure 18. A list of other plants having anti-HIV potential has been listed in Table 5.
Table 5.
Assortments of other plant species have been given in Table 5.
| Plant Species | Family | Parts Used | References |
|---|---|---|---|
| Khaya grandifoliola | Meliaceae | Leaves | [393] |
| Diospyros mespiliformis | Ebenaceae | Bark | [394] |
| Alternanthera brasiliana | Amaranthaceae | Roots | [395] |
| Ricinus communis | Euphorbiaceae | Leaves | [396] |
| Butea monosperma | Fabaceae | Roots | [397] |
| Prosopis glandulosa | Fabaceae | Leaves | [398] |
| Sophora tonkinensis | Fabaceae | Roots | [399] |
| Gunnera magellanica | Gunneraceae | Stem | [400] |
| Swertia franchetiana | Gentianaceae | Roots | [401] |
| Curcuma longa | Zingiberaceae | Rhizomes | [402] |
| Stewartia koreana | Theaceae | Leaves | [403] |
| Cissus quadrangularis | Vitaceae | Stems | [404] |
| Withania somnifera | Solanaceae | Roots | [405] |
| Ailanthus altissima | Simaroubaceae | Stem bark | [406] |
| Toddalia asiatica | Rutaceae | Roots | [407] |
| Oldenlandia herbacea | Rubiaceae | Roots | [408] |
| Aloe vera | Xanthorrhoeaceae | Leaves | [409] |
| Urtica dioica | Urticaceae | Rhizomes | [410] |
| Rheum tanguticum | Polygonaceae | Leaves | [411] |
| Saccharum officinarum | Poaceae | Stems | [412] |
| Ochna integerrima | Ochnaceae | Leaves | [413] |
| Nelumbo nucifera | Nelumbonaceae | Leaves | [414] |
| Aglaia lawii | Meliaceae | Leaves | [415] |
| Fritillaria cirrhosa | Liliaceae | Rhizomes | [416] |
| Magnolia biondii | Magnoliaceae | Flower buds | [417] |
| Lythrum salicaria | Lythraceae | Leaves | [418] |
| Reseda lutea | Resedaceae | Whole plant | [419] |
| Hypericum perforatum | Hypericaceae | Leaves | [420] |
| Trigonostemon thyrsoideus | Euphorbiaceae | Stems | [421] |
| Hemsleya endecaphylla | Cucurbitaceae | Tubers | [422] |
| Garcinia kingaensis | Clusiaceae | Stem bark | [423] |
| Woodwardia unigemmata | Blechnaceae | Rhizomes | [424] |
| Berberis holstii | Berberidaceae | Roots, leaves | [425] |
| Foeniculum vulgare | Apiaceae | Fruits | [406] |
| Alepidea amatymbica | Apiaceae | Roots | [426] |
| Stachytarpheta jamaicensis | Verbenaceae | Whole plant | [427] |
| Schisandra sphaerandra | Schisandraceae | Stems | [428] |
| Alpinia galangal | Zingiberaceae | Roots | [429] |
| Zanthoxylum chalybeum | Rutaceae | Root bark | [430] |
| Berchemia berchemiifolia | Rhamnaceae | Bark | [431] |
| Scoparia dulcis | Plantaginaceae | Leaves | [432] |
| Phyllanthus myrtifolius | Phyllanthaceae | Fruits | [433] |
| Arundina graminifolia | Orchidaceae | Whole plant | [434] |
| Ximenia Americana | Olacaceae | Stem bark | [435] |
3. Conclusions
Plants are known to exhibit a huge repertoire of bioactive metabolites [436]. A significant number of reports on the capability of natural compounds with potential as anti-HIV agents have appeared during the last few decades. This review article presents the rational approaches for the design of therapeutic potential candidates as anti-HIV agents. Even though there have been many extensive achievements in the field of HIV chemotherapy, there remains a great demand for novel lead compounds for anti-HIV drug discovery and drug development. Numerous plant species have been evaluated for their inhibitory activities on the essential HIV enzymes such as RT, protease, and integrase, which play an important role in HIV replication. Several secondary metabolites have been extracted from the various parts of plantsthat act as potent anti-HIV agents via different mechanisms of action. Therapeutically active compounds from plants can also aid as necessary leads for the discovery and development of novel and more potent compounds that can be derived synthetically. For instance, synthetic ingenol compounds have been derived based on naturally occurring compound Ingenol and a variety of synthetic derivatives have been evolved from the naturally occurring compound Artemisinin, which exhibits significant anti-HIV activitiesof potential scaffolds from them for the complete eradication of HIV/AIDS. A recent review has attempted to show themost successful medical therapeutics derived from natural products, including those studied in the field of HIV/AIDS [437]. Besides, computer-aided (virtual) [438] and large-scale in vitro screening [439] approaches have recently been carried out on natural compound libraries to identify natural products with anti-HIV properties. Novel therapeutic approaches have been attempted, including searching for new HIV-1 latency-reversing agents, i.e., compounds not only capable of HIV suppression but also eliminating HIV reservoirs [440,441].
Acknowledgments
Authors are thankful to B. S. Guman, Vice-chancellor of Punjabi University Patiala for their encouragement. The authors are also thankful to Er. S. K. Punj, Chairman, Sri Sai Group of Institutes and Smt. Tripta Punj, Managing Director, Sri Sai Group of Institutes for their constant moral support.
Author Contributions
Conceptualization, G.K.G., F.N.-K. and D.K.; methodology, R.K., P.S. and D.K.; software, data curation, R.K., P.S., F.N.-K. and D.K.; writing—original draft preparation, R.K., P.S., and D.K., writing—review and editing, R.K., P.S., G.K.G., F.N.-K., and D.K.; visualization, X.X.; supervision, F.N.-K., and D.K.; project administration, F.N.-K., G.K.G., and D.K.; funding acquisition, F.N.-K. All authors have read and agreed to the published version of the manuscript.
Funding
F.N.-K. would also like to acknowledge an equipent subsidy and return fellowship from the Alexander von Humboldt Foundation, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sabde S., Bodiwala H.S., Karmase A., Deshpande P.J., Kaur A., Ahmed N., Chauthe S.K., Brahmbhatt K.G., Phadke R.U., Mitra D., et al. Anti-HIV activity of Indian medicinal plants. J. Nat. Med. 2011;65:662–669. doi: 10.1007/s11418-011-0513-2. [DOI] [PubMed] [Google Scholar]
- 2.Salehi B., Kumar N.V.A., Sener B., Sharifi-Rad M., Kılıç M., Mahady G.B., Vlaisavljevic S., Iriti M., Kobarfard F., Setzer W.N., et al. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018;19:1459. doi: 10.3390/ijms19051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds C., de Koning C.B., Pelly S.C., Otterlo W.A.L., Bode M.L. In search of a treatment for HIV—Current therapies and the role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) Chem. Soc. Rev. 2012;41:4657–4670. doi: 10.1039/c2cs35058k. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S., Tyagi A.K. Curcumin and its analogues: A potential natural compound against HIV 1 infection and AIDS. Food Funct. 2015;6:3412–3419. doi: 10.1039/C5FO00485C. [DOI] [PubMed] [Google Scholar]
- 5.Kharsany A.B., Karim Q.A. HIV infection and AIDS in sub-saharan Africa: Current status, challenges and opportunities. Open AIDS J. 2016;10:34–48. doi: 10.2174/1874613601610010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moir S., Chun T.-W., Fauci A.S. Pathogenic mechanisms of HIV disease. Annu. Rev. Pathol. Mech. Dis. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 7.Deeks S.G., Overbaugh J., Phillips A., Buchbinder S. HIV infection. Nat. Rev. Dis. Prim. 2015;1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 8.Freed E.O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015;13:484–496. doi: 10.1038/nrmicro3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman A., Cherepanov P. The structural biology of HIV-1: Mechanistic and therapeutic insights. Nat. Rev. Microbiol. 2012;10:279–290. doi: 10.1038/nrmicro2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner B.G., Summers M.F. Structural biology of HIV. J. Mol. Biol. 1999;285:1–32. doi: 10.1006/jmbi.1998.2354. [DOI] [PubMed] [Google Scholar]
- 11.Goodsell D.S. Illustrations of the HIV life cycle. Curr. Top. Microbiol. Immunol. 2015;389:243–252. doi: 10.1007/82_2015_437. [DOI] [PubMed] [Google Scholar]
- 12.Mailler E., Bernacchi S., Marquet R., Paillart J.C., Vivet-Boudou V., Smyth R.P. The life-cycle of the HIV-1 Gag–RNA complex. Viruses. 2016;8:248. doi: 10.3390/v8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundquist W.I., Kräusslich H.-G. HIV-1assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012;2:a006924. doi: 10.1101/cshperspect.a006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harden V.A., Fauci A. AIDS at 30: A History. Potomac Books, Inc.; Lincoln, NE, USA: 2012. [Google Scholar]
- 15.Lewis J.M., Macpherson P., Adams E.R., Ochodo E., Sanda A., Taegtmeyer M. Field accuracy of fourth-generation rapid diagnostic tests for acute HIV-1: A systematic review. AIDS. 2015;29:2465–2471. doi: 10.1097/QAD.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander T.S. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin. Vaccine Immunol. 2016;23:249–253. doi: 10.1128/CVI.00053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colebunders R., Francis H., Duma M.-M., Groen G.V.D., Lebughe I., Kapita H., Quinn T.C., Heyward W.L., Piot P. HIV-l infection in HIV-l enzyme-linked immunoassay seronegative patients in Kinshasa, Zaire. Int. J. STD AIDS. 1990;1:330–334. doi: 10.1177/095646249000100505. [DOI] [PubMed] [Google Scholar]
- 18.Feng X., Wangc J., Gaod Z., Tiana Y., Zhanga L., Chene H., Zhangb T., Xiaof L., Yaoa J., Xinga W., et al. An alternative strategy to Western Blot as a confirmatory diagnostic test for HIV Infection. J. Clin. Virol. 2017;88:8–11. doi: 10.1016/j.jcv.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Auvert B., Taljaard D., Lagarde E., Sobngwi-Tambekou J., Sitta R., Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: The ANRS 1265 trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Günthard H.F., Saag M.S., Benson C.A., Del Rio C., Eron J.J., Gallant J.E., Hoy J.F., Mugavero M.J., Sax P.E., Thompson M.A. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society—USA panel. JAMA. 2016;316:191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey R.C., Moses S., Parker C.B., Agot K., Maclean I., Krieger J.N., Williams C.F., Campbell R.T., Ndinya-Achola J.O. Male circumcision for HIV prevention in young men in Kisumu, Kenya: A randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 22.Gravatt L.A.H., Leibrand C.R., Patel S., McRae M. New drugs in the pipeline for the treatment of HIV: A review. Curr. Infect. Dis. Rep. 2017;19:42. doi: 10.1007/s11908-017-0601-x. [DOI] [PubMed] [Google Scholar]
- 23.Sendagire H., Easterbrook P.J., Nankya I., Arts E., Thomas D., Reynolds S.J. The challenge of HIV-1 antiretroviral resistance in Africa in the era of HAART. AIDS Rev. 2009;11:59–70. [PMC free article] [PubMed] [Google Scholar]
- 24.Ramana L.N., Anand A.R., Sethuraman S., Krishnan U.M. Targeting strategies for delivery of anti-HIV drugs. J. Control. Release. 2014;192:271–283. doi: 10.1016/j.jconrel.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu D.-Y., Wu H.Y., Yarla N.S., Xu B., Ding J., Lu T.R. HAART in HIV/AIDS treatments: Future trends. Infect. Disord. Drug Targ. 2018;18:15–22. doi: 10.2174/1871526517666170505122800. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Chan W.K. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv. Drug Deliv. Rev. 1999;39:81–103. doi: 10.1016/S0169-409X(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 27.Cohen M.S., Chen Y.Q., McCauley M., Gamble T., Hosseinipour M.C., Kumarasamy N., Hakim J.G., Kumwenda J., Grinsztejn B., Pilotto J.H. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharifi-Rad J. Herbal antibiotics: Moving back into the mainstream as an alternative for “superbugs”. Cell. Mol. Biol. 2016;62:1–2. [PubMed] [Google Scholar]
- 29.WHO In vitro screening of traditional medicines for anti-HIV activity: Memorandum from a WHO meeting. Bull. World Health Organ. 1989;87:613–618. [PMC free article] [PubMed] [Google Scholar]
- 30.WHO . Report of a Who Informal Consultation on Traditional Medicine and AIDS: In Vitro Screening for Anti-HIV Activity. WHO; Geneva, Switzerland: 1989. [Google Scholar]
- 31.Cos P., Maes L., Berghe D.V., Hermans N., Pieters L., Vlietinck A. Plant substances as anti-HIV agents selected according to their putative mechanism of action. J. Nat. Prod. 2004;67:284–293. doi: 10.1021/np034016p. [DOI] [PubMed] [Google Scholar]
- 32.Kumar D., Sharma P., Singh H., Nepali K., Gupta G.K., Jain S.K., Ntie-Kang F. The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Adv. 2017;7:36977–36999. doi: 10.1039/C7RA05441F. [DOI] [Google Scholar]
- 33.Kumar D., Jain S.K. A comprehensive review of N-heterocycles as cytotoxic agents. Curr. Med. Chem. 2016;23:4338–4394. doi: 10.2174/0929867323666160809093930. [DOI] [PubMed] [Google Scholar]
- 34.Cos P., Maes L., Vlietinck A., Pieters L. Plant-derived leading compounds for chemotherapy of human immunodefiency virus (HIV) infection—An Update (1998–2007) Planta Med. 2008;74:1323–1337. doi: 10.1055/s-2008-1081314. [DOI] [PubMed] [Google Scholar]
- 35.Nepali K., Sharma S., Kumar D., Budiraja A., Dhar K.L. Anticancer hybrids-a patent survey. Recent Pat. Anticancer Drug Discov. 2014;9:303–339. doi: 10.2174/1574892809666140520150459. [DOI] [PubMed] [Google Scholar]
- 36.Kumar D., Bedi P.M.S. Anti-Inflammatory Agents: Some recent advances. Indian Drug. 2009;46:675–681. [Google Scholar]
- 37.Sharma P., Sharma R., Rao H.S., Kumar D. Phytochemistry and medicinal attributes of Alstonia scholaris: A review. Int. J. Pharm. Sci. Res. 2015;6:505–513. [Google Scholar]
- 38.Kumar D., Nepali K., Bedi P.M.S., Kumar S., Malik F., Jain S. 4,6-diaryl pyrimidones as constrained chalcone analogues: Design, synthesis and evaluation as anti-proliferative agents. Anticancer Agents Med. Chem. 2015;15:793–803. doi: 10.2174/1871520615666150318101436. [DOI] [PubMed] [Google Scholar]
- 39.Kurapati K.R.V., Atluri V.S., Samikkannu T., Garcia G., Nair M.P.N. Natural products as anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): A brief overview. Front. Microbiol. 2016;6:1444. doi: 10.3389/fmicb.2015.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar D., Singh O., Nepali K., Bedi P.M.S., Qayum A., Singh S., Jain S.K. Naphthoflavones as anti-proliferative agents: Design, synthesis and biological evaluation. Anticancer Agents Med. Chem. 2016;16:881–890. doi: 10.2174/1871520616666160204113536. [DOI] [PubMed] [Google Scholar]
- 41.Kumar D., Malik F., Bedi P.M.S., Jain S. 2,4-diarylpyrano[3,2-c]chromen-5(4H)-ones as coumarin-chalcone conjugates: Design, synthesis and biological evaluation as apoptosis inducing agents. Chem. Pharm. Bull. 2016;64:399–409. doi: 10.1248/cpb.c15-00958. [DOI] [PubMed] [Google Scholar]
- 42.Kumar D., Singh G., Sharma P., Qayum A., Mahajan G., Mintoo M.J. 4-aryl/heteroaryl-4H-fused pyrans as anti-proliferative agents: Design, synthesis and biological evaluation. Anticancer Agents Med. Chem. 2018;18:57–73. doi: 10.2174/1871520617666170918143911. [DOI] [PubMed] [Google Scholar]
- 43.Kumar D., Sharma P., Nepali K., Mahajan G., Mintoo M.J., Singh A., Singh G., Mondhe D.M., Singh G., Jain S.K., et al. Antitumour, acute toxicity and molecular modeling studies of 4-(pyridin-4-yl)-6-(thiophen-2-yl) pyrimidin-2(1H)-one against Ehrlich ascites carcinoma and sarcoma-180. Heliyon. 2018;4:e00661. doi: 10.1016/j.heliyon.2018.e00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzman J.D., Gupta A., Bucar F., Gibbons S., Bhakta S. Anti-mycobacterials from natural sources: Ancient times, antibiotic era and novel scaffolds. Front. Biosci. 2012;17:1861–1881. doi: 10.2741/4024. [DOI] [PubMed] [Google Scholar]
- 45.Chaniad P., Wattanapiromsakul C., Pianwanit S., Tewtrakul S. Anti-HIV-1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharmaceut. Biol. 2016;54:1077–1085. doi: 10.3109/13880209.2015.1103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang C., Luo P., Zhao Y., Hong J., Morris-Natschke S.L., Xu J., Chen C.H., Lee K.H., Gu Q. Carolignans from the aerial parts of Euphorbia sikkimensis and their anti-HIV activity. J. Nat. Prod. 2016;79:578–583. doi: 10.1021/acs.jnatprod.5b01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalvatchev Z., Walder R., Garzaro D. Anti-HIV activity of extracts from Culendula officinalis flowers. Biomed. Pharmacother. 1997;51:176–180. doi: 10.1016/S0753-3322(97)85587-4. [DOI] [PubMed] [Google Scholar]
- 48.Kapewangolo P., Tawha T., Nawinda T., Knott M., Hans R. Scelectium tortuosum demonstrates in vitro anti-HIV and free radical scavenging activity. S. Afr. J. Bot. 2016;106:140–143. doi: 10.1016/j.sajb.2016.06.009. [DOI] [Google Scholar]
- 49.Ito J., Chang F.R., Wang H.K., Park Y.K., Ikegaki M., Kilgore N., Lee K.H. Anti-AIDS agents. 48. Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001;64:1278–1281. doi: 10.1021/np010211x. [DOI] [PubMed] [Google Scholar]
- 50.Cassels B.K., Asencio M. Anti-HIV activity of natural triterpenoids and hemisynthetic derivatives 2004–2009. Phytochem. Rev. 2011;10:545–564. doi: 10.1007/s11101-010-9172-2. [DOI] [Google Scholar]
- 51.Cheng Y.B., Liu F.J., Wang C.H., Hwang T.L., Tsai Y.F., Yen C.H., Wang H.C., Tseng Y.H., Chien C.T., Chen Y.M.A., et al. bioactive triterpenoids from the leaves and twigs of Lithocarpus litseifolius and L. corneus. Planta Med. 2018;84:49–58. doi: 10.1055/s-0043-113826. [DOI] [PubMed] [Google Scholar]
- 52.Kapewangolo P., Kandawa-Schulz M., Meyer D. Anti-HIV activity of Ocimum labiatum extract and isolated pheophytin-A. Molecules. 2017;22:1763. doi: 10.3390/molecules22111763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharifi-Rad M., Varoni E.M., Salehi B., Sharifi-Rad J., Matthews K.R., Ayatollahi S.A., Kobarfard F., Ibrahim S.A., Mnayer D., Zakaria Z.A. Plants of the genus Zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules. 2017;22:2145. doi: 10.3390/molecules22122145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharifi-Rad J., Salehi B., Schnitzler P., Ayatollahi S., Kobarfard F., Fathi M., Eisazadeh M., Sharifi-Rad M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantiaroyleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Biol. 2017;63:42–47. doi: 10.14715/cmb/2017.63.8.10. [DOI] [PubMed] [Google Scholar]
- 55.Salehi B., Zucca P., Sharifi-Rad M., Pezzani R., Rajabi S., Setzer W., Varoni E., Iriti M., Kobarfard F., Sharifi-Rad J. Phytotherapeutics in cancer invasion and metastasis. Phytother. Res. 2018;32:1425–1449. doi: 10.1002/ptr.6087. [DOI] [PubMed] [Google Scholar]
- 56.Sharifi-Rad J., Hoseini-Alfatemi S., Sharifi-Rad M., Miri A. Phytochemical screening and antibacterial activity of different parts of the Prosopis farcta extracts against methicillin-resistant Staphylococcus aureus (MRSA) Min. Biotecnol. 2014;26:287–293. [Google Scholar]
- 57.Sharifi-Rad M., Tayeboon G., Sharifi-Rad J., Iriti M., Varoni E., Razazi S. Inhibitory activity on type 2 diabetes and hypertension key-enzymes, and antioxidant capacity of Veronica persica phenolic-rich extracts. Cell. Mol. Biol. 2016;62:80–85. [PubMed] [Google Scholar]
- 58.Sharifi-Rad J., Mnayer D., Tabanelli G., Stojanovic’-Radic’ Z., Sharifi-Rad M., Yousaf Z., Vallone L., Setzer W., Iriti M. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 2016;62:57–68. [PubMed] [Google Scholar]
- 59.Bagheri G., Mirzaei M., Mehrabi R., Sharifi-Rad J. Cytotoxic and antioxidant activities of Alstonia scholaris, Alstonia venenata and Moringa oleifera plants from India. Jundishapur J. Nat. Pharm. Prod. 2016;11:e31129. doi: 10.17795/jjnpp-31129. [DOI] [Google Scholar]
- 60.Farnsworth N.R. The role of ethnopharmacology in drug development. In: Chadwick D.J., Marsh J., editors. Bioactive Compounds from Plants. John Wiley & Sons; New York, NY, USA: 1990. pp. 2–21. [DOI] [PubMed] [Google Scholar]
- 61.Clercq E.D. Antiviral therapy for human immunodeficiency virus infections. Clin. Microbiol. Rev. 1995;8:200–239. doi: 10.1128/CMR.8.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanco J.L., Whitlock G., Milinkovic A., Moyle G. HIV integrase inhibitors: A new era in the treatment of HIV. Expert Opin. Pharmacother. 2015;16:1313–1324. doi: 10.1517/14656566.2015.1044436. [DOI] [PubMed] [Google Scholar]
- 63.Andreola M.L., Soultrait V.R.D., Fournier M., Parissi V., Desjobert C., Litvak S. HIV-1 integrase and RNase H activities as therapeutic targets. Expert Opin. Ther. Targets. 2002;6:433–446. doi: 10.1517/14728222.6.4.433. [DOI] [PubMed] [Google Scholar]
- 64.Kanyara J.N., Njagi E.N.M. Anti-HIV-1 activities in extracts from some medicinal plants as assessed in an in vitro biochemical HIV-1 reverse transcriptase assay. Phytother. Res. 2005;19:287–290. doi: 10.1002/ptr.1536. [DOI] [PubMed] [Google Scholar]
- 65.Painter G., Almond M., Mao S., Liotta D. Biochemical and mechanistic basis for the activity of nucleoside analogue inhibitors of HIV reverse transcriptase. Curr. Top. Med. Chem. 2004;4:1035–1044. doi: 10.2174/1568026043388358. [DOI] [PubMed] [Google Scholar]
- 66.Ng T.B., Huang B., Fong W.P., Yeung H.W. Anti-Human Immunodeficiency Virus (Anti-HIV) natural products with special emphasis on hiv reverse transcriptase inhibitors. Life Sci. 1997;61:933–949. doi: 10.1016/S0024-3205(97)00245-2. [DOI] [PubMed] [Google Scholar]
- 67.Deng X., Zhang Y., Jiang F., Chen R., Peng P., Wen B., Liang J. The Chinese herb-derived Sparstolonin B suppresses HIV-1 transcription. Virol. J. 2015;12:108. doi: 10.1186/s12985-015-0339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma C.M., Nakamura N., Hattori M., Kakuda H., Qiao J.C., Yu H.L. Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. J. Nat. Prod. 2000;63:238–242. doi: 10.1021/np9902441. [DOI] [PubMed] [Google Scholar]
- 69.Konvalinka J., Kräusslich H.-G., Müller B. Retroviral proteases and their roles in virion maturation. Virology. 2015;479–480:403–417. doi: 10.1016/j.virol.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Wei Y., Ma C.-M., Chen D.-Y., Hattori M. Anti-HIV-1 protease triterpenoids from Stauntonia obovatifoliola Hayata subsp. intermedia. Phytochemistry. 2008;69:1875–1879. doi: 10.1016/j.phytochem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Park J.C., Hur J.M., Park J.G., Hatano T., Yoshida T., Miyashiro H., Min B.S., Hattori M. Inhibitory effects of korean medicinal plants and camelliatannin H from Camellia japonica on human immunodeficiency virus type 1 protease. Phytother. Res. 2002;16:422–426. doi: 10.1002/ptr.919. [DOI] [PubMed] [Google Scholar]
- 72.Burke B.P., Boyd M.P., Impey H., Breton L.R., Bartlett J.S., Symonds G.P., Hütter G. CCR5 as a natural and modulated target for inhibition of HIV. Viruses. 2014;6:54–68. doi: 10.3390/v6010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang S., Zhao Q., Debnath A.K. Peptide and Non-peptide HIV Fusion Inhibitors. Curr. Pharm. Des. 2002;8:563–580. doi: 10.2174/1381612024607180. [DOI] [PubMed] [Google Scholar]
- 74.Quinones-Mateu M.E., Schols D. Virus-inhibitory peptide: A natural HIV entry inhibitor in search for a formal target in the viral genome. AIDS. 2011;25:1663–1664. doi: 10.1097/QAD.0b013e32834a36ea. [DOI] [PubMed] [Google Scholar]
- 75.Balzarini J., Neyts J., Schols D., Hosoya M., Damme E.V., Peumans W., Clercq E.D. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antivir. Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 76.Vlietinck A.J., Bruyne T.D., Apers S., Pieters L.A. Plant-Derived Leading Compounds for Chemotherapy of Human Immunodeficiency Virus (HIV) Infection. Planta Med. 1998;64:97–109. doi: 10.1055/s-2006-957384. [DOI] [PubMed] [Google Scholar]
- 77.Matsuda K., Hattori S., Komizu Y., Kariya R., Ueoka R., Okada S. Cepharanthine inhibited HIV-1 cell-cell transmission and cell-free infection via modification of cell membrane fluditiy. Bioorg. Med. Chem. Lett. 2014;24:2115–2117. doi: 10.1016/j.bmcl.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 78.Uttekar M.M., Das T., Pawar R.S., Bhandari B., Menon V., Nutan, Gupta S.K., Bhat S.V. Anti-HIV activity of semisynthetic derivatives of andrographolide and computational study of HIV-1 gp120 protein binding. Eur. J. Med. Chem. 2012;56:368–374. doi: 10.1016/j.ejmech.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 79.Kumar R.A., Sridevi K., Kumar N.V., Nanduri S., Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004;92:291–295. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Adams J.D., Lien E.J. Traditional Chinese Medicine: Scientific Basis for Its Use. The Royal Society of Chemistry; London, UK: 2013. [Google Scholar]
- 81.Chao W.-W., Lin B.-F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian) Chin. Med. 2010;5:17. doi: 10.1186/1749-8546-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varma A., Padh H., Shrivastava N. Andrographolide: A new plant-derived antineoplastic entity on horizon. Evid. Based Complement. Alternat. Med. 2011;2011:815390. doi: 10.1093/ecam/nep135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jayakumar T., Hsieh T.Y., Lee J.J., Sheu J.R. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid. Based Complement. Alternat. Med. 2013;2013:846740. doi: 10.1155/2013/846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C.K.L., Clark R.J., Harvey P.J., Rosengren K.J., Cemazar M., Craik D.J. The role of conserved Glu residue on cyclotide stability and activity: A structural and functional study of Kalata B12, a naturally occurring Glu to Asp mutant. Biochemistry. 2011;50:4077–4086. doi: 10.1021/bi2004153. [DOI] [PubMed] [Google Scholar]
- 85.Mfopa A.N., Coron A., Eloh K., Tramontano E., Frau A., Boyom F.F., Caboni P., Tocco G. Uvaria angolensis as a promising source of inhibitors of HIV-1 RT-associated RNA-dependent DNA polymerase and RNase H functions. Nat. Prod. Res. 2018;32:640–647. doi: 10.1080/14786419.2017.1332615. [DOI] [PubMed] [Google Scholar]
- 86.Sanna C., Scognamiglio M., Fiorentino A., Corona A., Graziani V., Caredda A., Cortis P., Montisci M., Ceresola E.R., Canducci F., et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE. 2018;13:e0195168. doi: 10.1371/journal.pone.0195168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang Q., Yu F., Cui X., Duan J., Wu Q., Nagarkatti P., Fan D. Sparstolonin B suppresses lipopolysachharide-induced inflammation in human umbilical vein endothelial cells. Arch. Pharm. Res. 2013;36:890–896. doi: 10.1007/s12272-013-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huerta-Reyes M., Basualdo M.D.C., Abe F., Jimenez-Estrada M., Soler C., Reyes-Chilpa R. HIV-1 inhibitory compounds from Calophyllum brasiliense Leaves. Biol. Pharm. Bull. 2004;27:1471–1475. doi: 10.1248/bpb.27.1471. [DOI] [PubMed] [Google Scholar]
- 89.Matthee G., Wright A.D., Konig G.M. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 1999;65:493–506. doi: 10.1055/s-1999-14004. [DOI] [PubMed] [Google Scholar]
- 90.Lee T.T.-Y., Kashiwada Y., Huang L., Snider J., Cosentino M., Lee K.-H. Suksdorfin: An Anti-HIV principle from Lomutium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides. Bioorg. Med. Chem. 1994;2:1051–1056. doi: 10.1016/S0968-0896(00)82054-4. [DOI] [PubMed] [Google Scholar]
- 91.Hudson J.B., Graham E.A., Harris L., Ashwwd-Smith M.J. The unusual Uva-dependent antiviral properties of the furoisocoumarin, coriandrin. Photochem. Photobiol. 1993;57:491–496. doi: 10.1111/j.1751-1097.1993.tb02324.x. [DOI] [PubMed] [Google Scholar]
- 92.Hu C.Q., Chen K., Shi Q., Kilkuskie R.E., Cheng Y.C., Lee K.H. Anti-aids agents, 10. Acacetin-7-O-β-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. J. Nat. Prod. 1994;57:42–51. doi: 10.1021/np50103a006. [DOI] [PubMed] [Google Scholar]
- 93.Wang J.N., Hou C.Y., Liu Y.L., Lin L.Z., Gil R.R., Cordell G.A. Swertifrancheside, an HIV-reverse transcriptase inhibitor and the first flavone-xanthone dimer from Swertia francheitiana. J. Nat. Prod. 1994;57:211–217. doi: 10.1021/np50104a003. [DOI] [PubMed] [Google Scholar]
- 94.Boyd M.R., Hallock Y.F., Cardellina J.H., Manfredi K.P., Blunt J.W., McMahon J.B., Buckheit R.W., Jr., Bringmann G., Schiiffer J.M., Cragg G.M., et al. Anti-HIV michellamines from Ancistrocladus korupensis. J. Med. Chem. 1994;37:1740–1745. doi: 10.1021/jm00038a003. [DOI] [PubMed] [Google Scholar]
- 95.Ingolfsdottir K., Hjalmarsdottir M.A., Sigurdsson A., Gudjonsdottir G.A., Brynjolfsdottir A., Steingrimsson O. In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob. Agents Chemother. 1997;41:215–217. doi: 10.1128/AAC.41.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakane H., Arisawa M., Fujita A., Koshimura S., Ono K. Inhibition of HIV reverse transcriptase activity by some phloroglucinol derivatives. FEBS Lett. 1991;286:83–85. doi: 10.1016/0014-5793(91)80946-Z. [DOI] [PubMed] [Google Scholar]
- 97.Xiao W.L., Tian R.R., Pu J.X., Li X., Wu L., Lu Y., Li S.H., Li R.T., Zheng Y.T., Zheng Q.T., et al. Triterpenoids from Schisandra lancifolia with anti-HIV-1 activitiy. J. Nat. Prod. 2006;69:277–279. doi: 10.1021/np0503303. [DOI] [PubMed] [Google Scholar]
- 98.Erickson K.L., Beutler J.A., Cardellina J.H., McMahon J.B., Newman D.J., Boyd M.R. A novel phorbol ester from Excoecaria agallocha. J. Nat. Prod. 1995;58:769–772. doi: 10.1021/np50119a020. [DOI] [PubMed] [Google Scholar]
- 99.Chen K., Shi Q., Kashiwada Y., Zhang D.C., Hu C.Q., Jin J.Q., Nozaki H., Kilkuskie R.E., Tramontano E., Mcphail D.R., et al. Anti-AIDS agents, 6. Salaspermic acid, an anti-HIV principle from Tripterygium wilfordii, and the structure activity correlation with its related compounds. J. Nat. Prod. 1992;55:340–346. doi: 10.1021/np50081a010. [DOI] [PubMed] [Google Scholar]
- 100.Oksuz S., Gurek F., Gil R.R., Pengsuparp T., Pezzuto J.M., Cordell G.A. 4 diterpene esters from Euphorbia myrsinites. Phytochemistry. 1995;38:1457–1462. doi: 10.1016/0031-9422(94)00806-5. [DOI] [PubMed] [Google Scholar]
- 101.Chinsembu K.C., Hedimbi M. A survey of plants with anti-HIV active compounds and their modes of action. Med. J. Zambia. 2009;36:178–186. [Google Scholar]
- 102.Chang R.S., Ding L., Chen G.Q., Pan Q.C., Zhao J.L., Smith K.M. Dehydroandrographolide succinic acid monoester as an inhibitor against the human immunodeficiency virus. Proc. Sot. Exp. Biol. Med. 1991;197:59–66. doi: 10.3181/00379727-197-43225. [DOI] [PubMed] [Google Scholar]
- 103.Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., Clercq E.D., Nakashima H., Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antivir. Res. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- 104.Konoshima T., Kashiwada Y., Takasaki M., Kozuka M., Yasuda I., Cosentino L.M., Lee K.H. Cucurbitacin F derivatives, anti-HIV principles from Cowania mexicana. Bioorg. Med. Chem. Lett. 1994;4:1323–1326. doi: 10.1016/S0960-894X(01)80354-1. [DOI] [Google Scholar]
- 105.Chen K., Shi Q.A., Fujioka T., Zhang D.C., Hu C.Q., Jin J.Q., Kilkuskie R.E., Lee K.H. Anti-aids agents, 4. Tripterifordin, a novel anti-HIV principle from Tripterygium wilfordii: Isolation and structural elucidation. J. Nat. Prod. 1992;55:88–92. doi: 10.1021/np50079a013. [DOI] [PubMed] [Google Scholar]
- 106.Pengsuparp T., Cai L., Fong H.H., Kinghorn A.D., Pezzuto J.M., Wani M.C., Wall M.E. Pentacyclic trirepenes derived from Maprounea africana are potent inhibitors of HIV-1 reverse transcriptase. J. Nat. Prod. 1994;57:415–418. doi: 10.1021/np50105a017. [DOI] [PubMed] [Google Scholar]
- 107.Fujioka T., Kashiwada Y., Kilkuskie R.E., Cosentino L.M., Ballas L.M., Jiang J.B., Janzen W.P., Chen I.S., Lee K.H. Anti-aids agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 108.Hayashi K., Kamiya M., Hayashi T. Virucidal effects of steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995;61:237–241. doi: 10.1055/s-2006-958063. [DOI] [PubMed] [Google Scholar]
- 109.Li B.Q., Fu T., Yan Y.D., Baylor N.W., Ruscetti F.W., Kung H.F. Inhibition of HIV infection by baicalin-a flavonoid compound purified from Chinese herbal medicine. Cell. Mol. Biol. Res. 1993;39:119–124. [PubMed] [Google Scholar]
- 110.Beutler J.A., Cardellina J.H., McMahon J.B., Boyd M.R., Cragg G.M. Anti-HIV and cytotoxic alkaloids from Buchenavia capitata. J. Nat. Prod. 1992;55:207–213. doi: 10.1021/np50080a008. [DOI] [PubMed] [Google Scholar]
- 111.Tang X., Chen H., Zhang X., Quan K., Sun M. Screening anti-HIV Chinese material mediea with HIV and equine infectious anemic virus reverse transcriptase. J. Trad. Chin. Med. 1994;14:10–13. [PubMed] [Google Scholar]
- 112.Patil A.D., Freyer A.J., Eggleston D.S., Haltiwanger R.C., Bean M.F., Taylor P.B., Caranfa M.J., Breen A.L., Bartus H.R., Johnson R.K. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993;36:4131–4138. doi: 10.1021/jm00078a001. [DOI] [PubMed] [Google Scholar]
- 113.Sharma B.R., Rattan R.K., Sharma P. Marmeline, an alkaloid, and other components of unripe fruits of Aegle marmelos. Phytochemistry. 1981;20:2606–2607. doi: 10.1016/0031-9422(81)83112-3. [DOI] [Google Scholar]
- 114.Harkar S., Razdan T.K., Waight E.S. Steroids, chromone and coumarins from Angelica officinalis. Phytochemistry. 1984;23:419–426. doi: 10.1016/S0031-9422(00)80344-1. [DOI] [Google Scholar]
- 115.Cheng H.Y., Lin T.C., Yang C.M., Wang K.C., Lin L.T., Lin C.C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chemother. 2004;53:577–583. doi: 10.1093/jac/dkh136. [DOI] [PubMed] [Google Scholar]
- 116.Hatano T., Ogawa N., Kira R., Yasuhara T., Okuda T. Tannins of cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolysable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. (Tokyo) 1989;37:2083–2090. doi: 10.1248/cpb.37.2083. [DOI] [PubMed] [Google Scholar]
- 117.Ogata T., Higuchi H., Mochida S., Matsumoto H., Kato A., Endo T., Kaji A., Kaji H. HIV-1 reverse transcriptase inhibitor from Phyllanthus niruri. AIDS Res. Hum. Retrovir. 1992;8:1937–1944. doi: 10.1089/aid.1992.8.1937. [DOI] [PubMed] [Google Scholar]
- 118.Kreis W., Kaplan M.H., Freeman J., Sun D.K., Sarin P.S. Inhibition of HIV replication by Hyssop officinalis extracts. Antivir. Res. 1990;14:323–337. doi: 10.1016/0166-3542(90)90051-8. [DOI] [PubMed] [Google Scholar]
- 119.Naser B., Bodinet C., Tegtmeier M., Lindequist U. Thuja occidentalis (Arbor vitae): A review of its pharmaceutical, pharmacological and clinical properties. Evid. Based Complement. Altern. Med. 2005;2:69–78. doi: 10.1093/ecam/neh065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tabba H.D., Chang R.S., Smith K.M. Isolation, purification, and partial characterization of Prunellin, an Anti-HIV component from aqueous extracts of Prunella vulgaris. Antivir. Res. 1989;11:263–273. doi: 10.1016/0166-3542(89)90036-3. [DOI] [PubMed] [Google Scholar]
- 121.Ngan F., Chang R.S., Tabba H.D., Smith K.M. Isolation, purification and partial characterization of an active anti-HIV compound from the Chinese medicinal herb Viola yedoensis. Antivir. Res. 1988;10:107–116. doi: 10.1016/0166-3542(88)90019-8. [DOI] [PubMed] [Google Scholar]
- 122.Wang J.P., Raung S.L., Lin C.N., Teng C.M. Inhibitory effect of norathyriol, a xanthone from Tripterospermum lanceolatum, on cutaneous plasma extravasation. Eur. J. Pharmacol. 1994;251:35–42. doi: 10.1016/0014-2999(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 123.Ulubelen A., Gil R.R., Cordell G.A., Mericli A.H., Mericli F. Prenylated lignans from Haplophyllum ptilostylum. Phytochemistry. 1995;39:417–422. doi: 10.1016/0031-9422(94)00841-G. [DOI] [Google Scholar]
- 124.Fujihashi T., Hara H., Sakata T., Mori K., Higuchi H., Tanaka A., Kaji H., Kaji A. Anti-human immunodeficiency virus (HIV) activities of halogenated gomisin J derivatives, new nonnucleoside inhibitors of HIV type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1995;39:2000–2007. doi: 10.1128/AAC.39.9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schröder H.C., Merz H., Steffen R., Müller W.E.G. Differential in vitro Anti-HIV Activity of Natural Lignans. Z. Naturforsch. 1990;45c:1215–1221. doi: 10.1515/znc-1990-11-1222. [DOI] [PubMed] [Google Scholar]
- 126.Talpir R., Rudi A., Kashman Y., Hizi A. Three new sesquiterpene hydroquinones from marine origin. Tetrahedron. 1994;50:4179–4184. doi: 10.1016/S0040-4020(01)86712-0. [DOI] [Google Scholar]
- 127.Jimenez C., Quinoa E., Adamczeski M., Hunter L.M., Crews P. Novel sponge-derived amino acids. 12. Tryptophan-derived pigments and accompanying sesterterpenes from Fascapilsinopsis reticulata. J. Org. Chem. 1991;56:3403–3410. doi: 10.1021/jo00010a041. [DOI] [Google Scholar]
- 128.Loya S., Tal R., Hizi A., Issacs S., Kashman Y., Loya Y. Hexprenoid hydroquinones, novel inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. J. Nat. Prod. 1993;56:2120–2125. doi: 10.1021/np50102a014. [DOI] [PubMed] [Google Scholar]
- 129.Inman W.D., Johnson M.O., Crews P. Novel marine sponge alkaloids. 1. Plakinidine A and B, anthelmintic active alkaloids from a Plakortis sponge. J. Am. Chem. Soc. 1990;112:1–4. doi: 10.1021/ja00157a001. [DOI] [Google Scholar]
- 130.Tymiak A.A., Rinehart K.L., Jr. Structurees of kelletinins 1 and 2, antibacterial metabolites of the marine mollusk Kelletia kelletii. J. Am. Chem. Soc. 1983;105:7396–7401. doi: 10.1021/ja00363a030. [DOI] [Google Scholar]
- 131.Silvestri I., Albonici L., Ciotti M., Lombardi M.P., Sinibaldi P., Manzari V., Orlando P., Carretta F., Strazzullo G., Grippo P. Antimitotic and antiviral activities of Kelletinin A in HTLV-1 infected MT2 cells. Experientia. 1995;51:1076–1080. doi: 10.1007/BF01946920. [DOI] [PubMed] [Google Scholar]
- 132.Chaudhuri S.K., Fullas F., Brown D.M., Wani M.C., Wall M.E., Cai L., Mar W., Lee S.K., Luo Y., Zaw K., et al. Isolatioon and structural elucidation of pentacyclic triterpenoids from Maprounea africana. J. Nat. Prod. 1995;58:1–9. doi: 10.1021/np50115a001. [DOI] [PubMed] [Google Scholar]
- 133.Pani A., Marongiu M.E. Anti-HIV integrase drugs how far from the shelf. Curr. Pharm. Des. 2000;6:569–584. doi: 10.2174/1381612003400759. [DOI] [PubMed] [Google Scholar]
- 134.Ghosh S., Ahire M., Patil S., Jabgunde A., Dusane M.B., Joshi B.N., Pardesi K., Jachak S., Dhavale D.D., Chopade B.A. Antidiabetic activity of Gnidia glauca and Dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid. Based Complement. Altern. Med. 2012;2012:929051. doi: 10.1155/2012/929051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J.M., Ji L.L., Branford-White C.J., Wang Z.Y., Shen K.K., Liu H., Wang Z.T. Antitumor activity of Dioscorea bulbifera L. rhizome in vivo. Fitoterapia. 2012;83:388–394. doi: 10.1016/j.fitote.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Teponno R.B., Tapondjou A.L., Gatsing D., Djoukeng J.D., AbouMansour E., Tabacchi R., Tane P., Stoekli-Evans H., Lontsi D. Bafoudiosbulbins A, and B, two anti-salmonellal clerodane diterpenoids from Dioscorea bulbifera L. var sativa. Phytochemistry. 2006;67:1957–1963. doi: 10.1016/j.phytochem.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 137.Mbiantcha M., Kamanyi A., Teponno R.B., Barreca M.L., Villa L., Monforte P., Chimirri A. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evid. Based Complement. Alternat. Med. 2011;2011:912935. doi: 10.1155/2011/912935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmed Z., Chishti M.Z., Johri R.K., Bhagat A., Gupta K.K., Ram G. Antihyperglycemic and antidyslipidemic activity of aqueous extract of Dioscorea bulbifera tubers. Diabetol. Croat. 2009;38:63–72. [Google Scholar]
- 139.Panthong P., Bunluepuech K., Boonnak N., Chaniad P., Pianwanit S., Wattanapiromsakul C., Tewtrakul S. Anti-HIV-1 integrase activity and molecular docking of compounds from Albizia procera bark. Pharm. Biol. 2015;53:1861–1866. doi: 10.3109/13880209.2015.1014568. [DOI] [PubMed] [Google Scholar]
- 140.Kokila K., Priyadharshini S.D., Sujatha V. Phytopharmacological properties of Albizia species: A review. Int. J. Pharm. Pharm. Sci. 2013;5:70–73. [Google Scholar]
- 141.Yadav S.K., Batra J.K. Mechanism of anti-HIV activity of ribosome inactivating protein, saporin. Protein Pept. Lett. 2015;22:497–503. doi: 10.2174/0929866522666150428120701. [DOI] [PubMed] [Google Scholar]
- 142.Puri M., Kaur I., Kanwar R.K., Gupta R.C., Chauhan A., Kanwar J.R. Ribosome inactivating proteins (RIPs) from Momordica charantia for anti viral therapy. Curr. Mol. Med. 2009;9:1080–1094. doi: 10.2174/156652409789839071. [DOI] [PubMed] [Google Scholar]
- 143.Sun Y., Huang P.L., Li J.J., Huang Y.Q., Zhang L., Huang P.L., Lee-Huang S. Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis with Kaposi’s sarcoma-associated virus. Biochem. Biophys. Res. Commun. 2001;287:983–994. doi: 10.1006/bbrc.2001.5689. [DOI] [PubMed] [Google Scholar]
- 144.Zhao W., Feng D., Sun S., Han T., Sui S. The anti-viral protein of trichosanthin penetrates into human immunodeficiency virus type 1. Acta. Biochim. Biophys. Sin. 2010:91–97. doi: 10.1093/abbs/gmp111. [DOI] [PubMed] [Google Scholar]
- 145.Au T.K., Collins R.A., Lam T.L., Ng T.B., Fong W.P., Wan D.C.C. The plant ribosome inactivating proteins luffin and saporin are potent inhibitors of HIV-1 integrase. FEBS Lett. 2000;471:169–172. doi: 10.1016/S0014-5793(00)01389-2. [DOI] [PubMed] [Google Scholar]
- 146.Kohl N.E., Emini E.A., Schiief W.A. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. USA. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katoch I., Ikawa Y., Yoshinaka Y. Retrovirus protease characterized as a dimeric aspartic proteinase. J. Virol. 1989;63:2226–2232. doi: 10.1128/JVI.63.5.2226-2232.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han L., Hatano T., Yoshida T., Okuda T. Tannins of Theaceous plants. V. Camelliatannins F, G and H, three new tannins from Camellia japonica L. Chem. Pharm. Bull. 1994;42:1399–1409. doi: 10.1248/cpb.42.1399. [DOI] [PubMed] [Google Scholar]
- 149.Mcquade T.J., Tomasselli A.G., Liu L. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990;247:454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- 150.Meek T.D., Dayton B.D., Metcalf B.W. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc. Natl. Acad. Sci. USA. 1989;86:1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dantanarayana A.P., Kumar N.S., Muthukuda P.M., Wazzeer M.I.M. A lupine derivative and the 13C NMR chemical shifts of some lupanols from Pleurostylia opposita. Phytochemistry. 1982;21:2065–2068. doi: 10.1016/0031-9422(82)83044-6. [DOI] [Google Scholar]
- 152.Ikuta A. The triterpenes from Stauntonia hexaphylla callus tissues and their biosynthetic significance. J. Nat. Prod. 1989;52:623–628. doi: 10.1021/np50063a024. [DOI] [Google Scholar]
- 153.Pech G.G., Brito W.F., Mena G.J., Quijano L. Constituents of Acacia cedilloi and Acacia gaumeri. Revised structure and complete NMR assignments of resinone. Z. Naturforsch. 2002;57c:773–776. doi: 10.1515/znc-2002-9-1002. [DOI] [PubMed] [Google Scholar]
- 154.Wenkert E., Baddeley G.V., Burfit I.R., Moreno L.N. Carbon-13 nuclear magnetic resonance spectroscopy of naturally –occuring substances. LVII. Triterpenes related to lupine and hopane. Org. Magn. Reson. 1978;11:343–377. doi: 10.1002/mrc.1270110705. [DOI] [Google Scholar]
- 155.Ikuta A., Itokawa H. Triterpenoids of Akebia quinata callus tissue. Phytochemistry. 1986;25:1625–1628. doi: 10.1016/S0031-9422(00)81222-4. [DOI] [Google Scholar]
- 156.Seo S., Tomita Y., Tori K. Carbon-13 NMR spectra of urs-12-enes and application to structural assignments of components of Isodon japonicus hara tissue cultures. Tetrahedron Lett. 1975;16:7–10. doi: 10.1016/S0040-4039(00)71763-1. [DOI] [Google Scholar]
- 157.Hakkinen S.H., Karenlampi S.O., Heinonen I.M., Mykkanen H.M., Torronen A.R. Content of flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999;47:2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 158.Liang S., Tian J.-M., Feng Y., Liu X.-H., Xiong Z., Zhang W.-D. Flavonoids from Daphne aurantiaca and their inhibitory activities against nitric oxide production. Chem. Pharm. Bull. 2011;59:653–656. doi: 10.1248/cpb.59.653. [DOI] [PubMed] [Google Scholar]
- 159.Venkatalakshmi P., Vadivel V., Brindha P. Role of phytochemicals as immunomodulatory agents: A review. Int. J. Green Pharm. 2016;10:1–18. [Google Scholar]
- 160.Sell S. Immunology Immunopathology and Immunity. Elsevier Science Publishing Company, Inc.; New York, NY, USA: 1987. Immunomodulation; pp. 655–683. [Google Scholar]
- 161.Agarwal S.S., Singh V.K. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part-I: Medicinal plants. Proc. Indian Nation Sci. Acad. B. 1999;65:179–204. [Google Scholar]
- 162.Holtmeier W., Kabelitz D. Gammadelta T cells link innate and adaptive immune responses. Chem. Immunol. Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- 163.Harborne J.B. Phytochemical Methods. Chapman and Hall, Ltd.; London, UK: 1973. pp. 149–188. [Google Scholar]
- 164.Okwu D.E. Phytochemicals and vitamin content of indigenous spices of South Eastern. Nig. J. Sust. Agric. Environ. 2004;6:30–37. [Google Scholar]
- 165.Li F., Wang H.D., Lu D.X., Wang Y.P., Qi R.B., Fu Y.M., Li C.J. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta. Pharmacol. Sin. 2006;27:1199–1205. doi: 10.1111/j.1745-7254.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 166.Mark W., Schneeberger S., Seiler R., Stroka D.M., Amberger A., Offner F., Candinas D., Margreiter R. Sinomenine blocks tissue remodeling in a rat model of chronic cardiac allograft rejection. Transplantation. 2003;75:940–945. doi: 10.1097/01.TP.0000056610.22062.03. [DOI] [PubMed] [Google Scholar]
- 167.Sunila E.S., Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Ethnopharmacol. 2004;90:339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 168.Lai J.H., Ho L.J., Kwan C.Y., Chang D.M., Lee T.C. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: Potential immunosuppressants in transplantation immunology. Transplantation. 1999;68:1383–1392. doi: 10.1097/00007890-199911150-00027. [DOI] [PubMed] [Google Scholar]
- 169.Chiang L.C., Ng L.T., Chiang W., Chang M.Y., Lin C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003;69:600–604. doi: 10.1055/s-2003-41113. [DOI] [PubMed] [Google Scholar]
- 170.Akbay P., Basaran A.A., Undeger U., Basaran N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother. Res. 2003;17:34–37. doi: 10.1002/ptr.1068. [DOI] [PubMed] [Google Scholar]
- 171.Garcia D., Leiro J., Delgado R., Sanmartín M.L., Ubeira F.M. Mangifera indica L. extract (Vimang) and mangiferin modulate mouse humoral immune responses. Phytother. Res. 2003;17:1182–1187. doi: 10.1002/ptr.1338. [DOI] [PubMed] [Google Scholar]
- 172.Seeram N.P., Adams L.S., Henning S.M., Niu Y., Zhang Y., Nair M.G., Heber D. In vitro anti-proliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Ranjan D., Johnston T.D., Wu G., Elliott L., Bondada S., Nagabhushan M. Curcumin blocks cyclosporine A-resistant CD28 costimulatory pathway of human T-cell proliferation. J. Surg. Res. 1998;77:174–178. doi: 10.1006/jsre.1998.5374. [DOI] [PubMed] [Google Scholar]
- 174.Reddy D.B., Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009;381:112–117. doi: 10.1016/j.bbrc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 175.Lee S.I., Kim B.S., Kim K.S., Lee S., Shin K.S., Lim J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem. Biophys. Res. Commun. 2008;371:799–803. doi: 10.1016/j.bbrc.2008.04.150. [DOI] [PubMed] [Google Scholar]
- 176.Chang S.L., Chiang Y.M., Chang C.L., Yeh H.H., Shyur L.F., Kuo Y.H. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-gamma expression. J. Ethnopharmacol. 2007;112:232–236. doi: 10.1016/j.jep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 177.Abd-Alla H.I., Moharram F.A., Gaara A.H., El-Safty M.M. Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cellmediated immune response in chicks. Z. Naturforsch. C. 2009;64:495–501. doi: 10.1515/znc-2009-7-805. [DOI] [PubMed] [Google Scholar]
- 178.Punturee K., Wild C.P., Kasinrerk W., Vinitketkumnuen U. Immunomodulatory activities of Centella asiatica and Rhinacanthus nasutus extracts. Asian Pac. J. Cancer Prev. 2005;6:396–400. [PubMed] [Google Scholar]
- 179.Ablise M., Leininger-Muller B., Wong C.D., Siest G., Loppinet V., Visvikis S. Synthesis and in vitro antioxidant activity of glycyrrhetinic acid derivatives tested with the cytochrome P450/NADPH system. Chem. Pharm. Bull. (Tokyo) 2004;52:1436–1439. doi: 10.1248/cpb.52.1436. [DOI] [PubMed] [Google Scholar]
- 180.Pace G.W., Leaf C.D. The role of oxidative stress in HIV disease. Free Radic. Biol. Med. 1995;19:523–528. doi: 10.1016/0891-5849(95)00047-2. [DOI] [PubMed] [Google Scholar]
- 181.Olinski R., Gackowski D., Foksinski M., Rozalski R., Roszkowski K., Jaruga P. Oxidative DNA damage: Assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic. Biol. Med. 2002;33:192–200. doi: 10.1016/S0891-5849(02)00878-X. [DOI] [PubMed] [Google Scholar]
- 182.Torre D., Pugliese A., Speranza F. Role of nitric oxide in HIV-1 infection: Friend or foe? Lancet Infect. Dis. 2002;2:273–280. doi: 10.1016/S1473-3099(02)00262-1. [DOI] [PubMed] [Google Scholar]
- 183.Kinscherf R., Fischbach T., Mihm S., Roth S., HohenhausSievert E., Weiss C., Edler L., Bartsch P., Droge W. Effect of glutathione depletion and oral N-acetyl-cysteine treatment on CD4+ and CD8+ cells. FASEB J. 1994;8:448–451. doi: 10.1096/fasebj.8.6.7909525. [DOI] [PubMed] [Google Scholar]
- 184.Sappey C., Legrand-Poels S., Best-Belpomme M., Favier A., Rentier B., Piette J. Stimulation of glutathione peroxidase activity decreases HIV type 1 activation after oxidative stress. AIDS Res. Hum. Retrovir. 1994;10:1451–1461. doi: 10.1089/aid.1994.10.1451. [DOI] [PubMed] [Google Scholar]
- 185.Bailly F., Cotelle P. Anti-HIV activities of natural antioxidant caffeic acid derivatives: Toward an antiviral supplementation Diet. Curr. Med. Chem. 2005;12:1811–1818. doi: 10.2174/0929867054367239. [DOI] [PubMed] [Google Scholar]
- 186.Rechner A.R., Kroner C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005;116:327–334. doi: 10.1016/j.thromres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 187.Tanahashi T. Diversity of secondary metabolites from some medicinal plants and cultivated lichen mycobionts. Yakugaku Zasshi. 2017;137:1443–1482. doi: 10.1248/yakushi.17-00147. [DOI] [PubMed] [Google Scholar]
- 188.Shitan N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016;80:1283–1293. doi: 10.1080/09168451.2016.1151344. [DOI] [PubMed] [Google Scholar]
- 189.Connor S.E.O. Engineering of secondary metabolism. Annu. Rev. Genet. 2015;49:5.1–5.2. doi: 10.1146/annurev-genet-120213-092053. [DOI] [PubMed] [Google Scholar]
- 190.Musilova L., Ridl J., Polivkova M., Macek T., Uhlik O. Effects of secondary plant metabolites on microbial populations: Changes in community structure and metabolic activity in contaminated environments. Int. J. Mol. Sci. 2016;17:1205. doi: 10.3390/ijms17081205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh I.P., Bodiwala H.S. Recent advances in anti-HIV natural products. Nat. Prod. Rep. 2010;27:1781–1800. doi: 10.1039/c0np00025f. [DOI] [PubMed] [Google Scholar]
- 192.Yu D., Morris-Natschke S.L., Lee K.-H. New developments in natural products-based anti-AIDS research. Med. Res. Rev. 2007;27:108–132. doi: 10.1002/med.20075. [DOI] [PubMed] [Google Scholar]
- 193.Asres K., Seyoum A., Veeresham C., Bucar F., Gibbons S. Naturally derived anti-HIV agents. Phytother. Res. 2005;19:557–581. doi: 10.1002/ptr.1629. [DOI] [PubMed] [Google Scholar]
- 194.McCormick J.L., Mckee T.C., Cardellina J.H., Boyd M.R. HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J. Nat. Prod. 1996;59:469–471. doi: 10.1021/np960250m. [DOI] [PubMed] [Google Scholar]
- 195.Duan H., Takaishi Y., Imakura Y., Jia Y., Li D., Cosentino L.M. Sesquiterpene alkaloids from Tripterygium hypoglaucum and Tripterygium wilfordii: A new class of potent Anti-HIV agents. J. Nat. Prod. 2000;63:357–361. doi: 10.1021/np990281s. [DOI] [PubMed] [Google Scholar]
- 196.Tan G.T., Pezzuto J.M., Kinghorn A.D., Hughes S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991;54:143–154. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- 197.Ishida J., Wang H.-K., Oyama M., Cosentino M.L., Hu C.-Q., Lee K.H. Anti-AIDS agents. 46.1 anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its Derivatives. J. Nat. Prod. 2001;64:958–960. doi: 10.1021/np0101189. [DOI] [PubMed] [Google Scholar]
- 198.Loya S., Rudi A., Kashman Y., Hizi A. Polycitone A, a novel and potent general inhibitor of retroviral reverse transcriptases and cellular DNA polymerases. Biochem. J. 1999;344:85–92. doi: 10.1042/bj3440085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xu H.X., Wan M., Dong H., But P.P., Foo L.Y. Inhibitory activity of flavonoids and tannins against HIV-1 protease. Biol. Pharm. Bull. 2000;23:1072–1076. doi: 10.1248/bpb.23.1072. [DOI] [PubMed] [Google Scholar]
- 200.Ravanelli N., Santos K.P., Motta L.B., Lago J.H.G., Furlan C.M. Alkaloids from Croton echinocarpus Baill: Anti-HIV potential. S. Afr.J. Bot. 2015;102:153–156. doi: 10.1016/j.sajb.2015.06.011. [DOI] [Google Scholar]
- 201.White E.L., Chao W.R., Ross L.J., Borhani D.W., Hobbs P.D., Upender V., Dawson M.I. Michellamine alkaloids inhibit protein kinase C. Arch. Biochem. Biophys. 1999;365:25–30. doi: 10.1006/abbi.1999.1145. [DOI] [PubMed] [Google Scholar]
- 202.Kondo Y., Imai Y., Hojo H., Hashimoto Y., Nozoe S. Selective inhibition of T cell dependent immune responses by bisbenzylisoquinoline alkaloids in vivo. Int. J. Immunopharmacol. 1992;14:1181–1186. doi: 10.1016/0192-0561(92)90053-N. [DOI] [PubMed] [Google Scholar]
- 203.Meragelman K.M., McKee T.C., Boyd M.R. Siamenol, a new carbazole alkaloid from Murraya siamensis. J. Nat. Prod. 2000;63:427–428. doi: 10.1021/np990570g. [DOI] [PubMed] [Google Scholar]
- 204.Kongkathip B., Kongkathip N., Sunthitikawinsakul A., Napaswat C., Yoosook C. Anti-HIV-1 constituents from Clausena excavata: Part II. Carbazoles and a pyranocoumarin. Phytother. Res. 2005;19:728–731. doi: 10.1002/ptr.1738. [DOI] [PubMed] [Google Scholar]
- 205.Hsieh P.W., Chang F.R., Lee K.H., Hwang T.L., Chang S.M., Wu Y.C. A new anti-HIV alkaloid, drymaritin, and a new C-glycoside flavonoid, diandroflavone, from Drymaria diandra. J. Nat. Prod. 2004;67:1175–1177. doi: 10.1021/np0400196. [DOI] [PubMed] [Google Scholar]
- 206.Wang J., Zheng Y., Efferth T., Wang R., Shen Y., Hao X. Indole and carbazole alkaloids from Glycosmis montana with weak anti-HIV and cytotoxic activities. Phytochemistry. 2005;66:697–701. doi: 10.1016/j.phytochem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 207.Jayasuriya H., Herath K.B., Ondeyka J.G., Polishook J.D., Bills G.F., Dombrowski A.W., Springer M.S., Siciliano S., Malkowitz L., Sanchez M., et al. Isolation and structure of antagonists of chemokine receptor (CCR5) J. Nat. Prod. 2004;67:1036–1038. doi: 10.1021/np049974l. [DOI] [PubMed] [Google Scholar]
- 208.Cheng M.J., Lee K.H., Tsai I.L., Chen I.S. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailantoides. Bioorg. Med. Chem. 2005;13:5915–5920. doi: 10.1016/j.bmc.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 209.Stadler R., Kutchan T.M., Zenk M.H. (S)-norcoclaurine is the central intermediate in benzylisoquinoline alkaloid biosynthesis. Phytochemistry. 1989;28:1083–1086. doi: 10.1016/0031-9422(89)80187-6. [DOI] [Google Scholar]
- 210.Yan M.H., Cheng P., Jiang Z.Y., Ma Y.B., Zhang X.M., Zhang F.X., Yang L.M., Zheng Y.T., Chen J.J. Periglaucines A-D, Anti-HBV and HIV-1 alkaloid from Pericampylus glaucus. J. Nat. Prod. 2008;71:760–763. doi: 10.1021/np070479+. [DOI] [PubMed] [Google Scholar]
- 211.Wu P.L., Lin F.W., Wu T.S., Kuoh C.S., Lee K.H., Lee S.J. Cytotoxic and anti-HIV principles from the rhizomes of Begonia nantoensis. Chem. Pharm. Bull. 2004;52:345–349. doi: 10.1248/cpb.52.345. [DOI] [PubMed] [Google Scholar]
- 212.Szlavik L., Gyuris A., Minarovits J., Forgo P., Molnarand J., Hohmann J. Alkaloids from Leucojum vernum and antiretroviral activity of amaryllidaceae alkaloids. Planta Med. 2004;70:871–873. doi: 10.1055/s-2004-827239. [DOI] [PubMed] [Google Scholar]
- 213.Otshudi A.L., Apers S., Pieters L., Claeys M., Pannecouque C., Clercq E.D., Zeebroeck A.V., Lauwers S., Frederich M., Foriers A. Biologically active bisbenzylisoquinoline alkaloids from the root bark of Epinetrum villosum. J. Ethnopharmacol. 2005;31:89–94. doi: 10.1016/j.jep.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 214.Chang Y.C., Hsieh P.W., Chang F.R., Wu R.R., Liaw C.C., Lee K.H., Wu Y.C. Two new protopines argemexicaines A and B and the anti-HIV alkaloid 6-acetonyldihydrochelerythrine from formosan Argemone mexicana. Planta Med. 2003;69:148–152. doi: 10.1055/s-2003-37710. [DOI] [PubMed] [Google Scholar]
- 215.Hua H.M., Peng J., Dunbar D.C., Schinazi R.F., de Andrews A.G.C., Cuevas C., Garcia-Fernandez L.F., Kellyf M., Hamanna M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron. 2007;63:11179–11188. doi: 10.1016/j.tet.2007.08.005. [DOI] [Google Scholar]
- 216.Peng J., Hu J.F., Kazi A.B. Manadomanzamines A and B: A novel alkaloid ring system with potent activity against mycobacteria and HIV-1. J. Am. Chem. Soc. 2003;125:13382–13386. doi: 10.1021/ja030087z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang C.F., Nakamura N., Tewtrakul S. Sesquiterpenes and alkaloids from Lindera chunii and their inhibitory activities against HIV-1 integrase. Chem. Pharm. Bull. 2002;50:1195–1200. doi: 10.1248/cpb.50.1195. [DOI] [PubMed] [Google Scholar]
- 218.Ma C.M., Nakamura N., Hattori M. Inhibitory effects on HIV1 protease of tri-p-coumaroylspermidine from Artemisia caruifolia and related amides. Chem. Pharm. Bull. 2001;49:915–917. doi: 10.1248/cpb.49.915. [DOI] [PubMed] [Google Scholar]
- 219.Kiyama R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. Eur. J. Pharmacol. 2017;815:405–415. doi: 10.1016/j.ejphar.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 220.Kuo R.Y., Qian K., Susan L., Natschke M., Lee K.H. Plant-derived triterpenoids and analogues as antitumor and anti-HIV agents. Nat. Prod. Rep. 2009;26:1321–1344. doi: 10.1039/b810774m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Min B.S., Jung H.J., Lee J.S., Kim Y.H., Bok S.H., Ma C.M. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med. 1999;65:374–375. doi: 10.1055/s-2006-960792. [DOI] [PubMed] [Google Scholar]
- 222.Yao-Haur K., Li-Ming Y.K. Antitumour and anti-AIDS triterpenes from Celastrus hindsii. Phytochemistry. 1997;44:1275–1281. doi: 10.1016/S0031-9422(96)00719-4. [DOI] [PubMed] [Google Scholar]
- 223.El-Mekkawy S., Meselhy M.R., Nakamura N., Hattori M., Kawahata T., Otake T. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry. 2000;53:457–464. doi: 10.1016/S0031-9422(99)00556-7. [DOI] [PubMed] [Google Scholar]
- 224.Rukachaisirikul V., Pailee P., Hiranrat A., Tuchinda P., Yoosook C., Kasisit J. Anti-HIV-1 protostane triterpenes and digeranylbenzophenone from trunk bark and stems of Garcinia speciosa. Planta Med. 2003;69:1141–1146. doi: 10.1055/s-2003-818006. [DOI] [PubMed] [Google Scholar]
- 225.Xu H.-X., Zeng F.-Q., Wan M., Sim K.-Y. Anti-HIV triterpene acids from Geum japonicum. J. Nat. Prod. 1996;59:643–645. doi: 10.1021/np960165e. [DOI] [PubMed] [Google Scholar]
- 226.Chen D.-F., Zhang S.-X., Wang H.-K., Zhang S.-Y., Sun Q.-Z., Cosentino L.M. Novel anti-HIV lancilactone C and related triterpenes from Kadsura lancilimba. J. Nat. Prod. 1999;62:94–97. doi: 10.1021/np980291d. [DOI] [PubMed] [Google Scholar]
- 227.Nakamura N. Inhibitory effects of some traditional medicines on proliferation of HIV-1 and its protease. Yakugaku Zasshi. 2004;124:519–529. doi: 10.1248/yakushi.124.519. [DOI] [PubMed] [Google Scholar]
- 228.Li H.-Y., Sun N.-J., Kashiwada Y., Sun L., Snider J.V., Cosentino L.M. Anti-AIDS agents, 9. Suberosol, a new C-31 lanostane-type triterpene and Anti-HIV principle from Polyalthia suberosa. J. Nat. Prod. 1993;56:1130–1133. doi: 10.1021/np50097a017. [DOI] [PubMed] [Google Scholar]
- 229.Barbosa J.P., Pereira R.C., Abrantes J.L., DosSantos C.C.C., Rebello M.A., Frugulhetti I.C.P.P. In vitro antiviral diterpenes from the Brazil brown alga Dictyota pfaffii. Planta Med. 2004;70:856–860. doi: 10.1055/s-2004-827235. [DOI] [PubMed] [Google Scholar]
- 230.Cirne-Santos C.C., Teixeira V.L., Castello-Branco L.R., Frugulheti I.C.P.P., Bou Habid D.C. Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta Med. 2006;72:295–299. doi: 10.1055/s-2005-916209. [DOI] [PubMed] [Google Scholar]
- 231.Zhang H.J., Huang N.V., Cuong N.M., Soejarto D.D., Pezzuto J.M., Fong H.H.S. Sesquiterpenes and butenolides, natural anti-HIV constituents from Litsea verticillata. Planta Med. 2005;71:452–457. doi: 10.1055/s-2005-864142. [DOI] [PubMed] [Google Scholar]
- 232.Pereira H.S., Leao-Ferreira L.R., Moussatché N., Teieira V.L., da Cavalcanti D.N., Costa L.J. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on HIV-1 reverse transcriptase. Planta Med. 2005;71:1019–1024. doi: 10.1055/s-2005-873113. [DOI] [PubMed] [Google Scholar]
- 233.Sunthitikawinsakul A., Kongkathip N., Kongkathip B. Anti-HIV-1 limonoid: First isolation from Clausena excavata. Phytother. Res. 2003;17:1101–1103. doi: 10.1002/ptr.1381. [DOI] [PubMed] [Google Scholar]
- 234.Ito M., Nakashima H., Baba M., Pauwels R., De Clercq E., Shigeta S., Yamamoto N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)] Antivir. Res. 1987;7:127–137. doi: 10.1016/0166-3542(87)90001-5. [DOI] [PubMed] [Google Scholar]
- 235.Bocklandt S., Blumberg P.M., Hamer D.H. Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate. Antivir. Res. 2003;59:89–98. doi: 10.1016/S0166-3542(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 236.Pettit G.R., Ducki S., Tan R. Isolation and structure of pedilstatin from a republic of Maldives Pedilanthus sp. J. Nat. Prod. 2002;65:1262–1265. doi: 10.1021/np020115b. [DOI] [PubMed] [Google Scholar]
- 237.Huang S.Z., Zhang X., Ma Q.Y., Zheng Y.T., Xu F.Q., Peng H., Dai H.F., Zhou J., Zhao Y.X. Terpenoids and their anti-HIV activities from Excoecaria acerifolia. Fitoterapia. 2013;91:224–230. doi: 10.1016/j.fitote.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 238.Anjaneyulu A.S.R., Rao V.L., Sreedhar K. Agallochins, New isopimarane diterpenoids from Excoecaria agallocha. J. Nat. Prod. Res. 2003;17:27–32. doi: 10.1080/1057563021000027975. [DOI] [PubMed] [Google Scholar]
- 239.Konishi T., Azuma M., Itoga R., Kiyosawa S., Fujiwara Y., Shimada Y. Three new labdane-type diterpenes from wood, Excoecaria agallocha. Chem. Pharm. Bull. 1996;44:229–231. doi: 10.1248/cpb.44.229. [DOI] [Google Scholar]
- 240.Gangloff A.R., Judge T.M., Helquist P. Light-induced, iodine-catalyzed aerobic oxidation of unsaturated tertiary amines. J. Org. Chem. 1990;55:3679–3682. doi: 10.1021/jo00298a061. [DOI] [Google Scholar]
- 241.Sun I.-C., Kashiwada Y., Morris-Natschke S.L., Lee K.-H. Plant-derived terpenoids and analogues as anti-HIV Agents. Curr. Top. Med. Chem. 2003;3:155–169. doi: 10.2174/1568026033392435. [DOI] [PubMed] [Google Scholar]
- 242.Gustafson K.R., Cardellina J.H., McMahon J.B., Gulakowski R.J., Ishitoya J., Szallasi Z., Lewin N.E., Blumberg P.M., Weislow O.S., Beutler J.A., et al. A Nonpromoting phorbol from the Samoan medicinal plant Homalanthus nutans inhibits cell killing by HIV1. J. Med. Chem. 1992;35:1978–1986. doi: 10.1021/jm00089a006. [DOI] [PubMed] [Google Scholar]
- 243.Osorio A.A., Munoz A., Romero D.T., Bedoya L.M., Perestelo N.R., Jimenez I.A., Alcami J., Bazzocchi I.L. Olean-18-ene triterpenoids from Celastraceae species inhibit HIV replication targeting NF-Kb and Sp1 dependent transcription. Eur. J. Med. Chem. 2012;52:295–303. doi: 10.1016/j.ejmech.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 244.Song W., Si L., Ji S., Wang H., Fang X.-M., Yu L.-Y., Li R.-Y., Liang L.-N., Zhou D., Ye M. Uralsaponins M−Y, Antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014;77:1632–1643. doi: 10.1021/np500253m. [DOI] [PubMed] [Google Scholar]
- 245.Vidal V., Potterat O., Louvel S., Hamy F., Mojarrab M., Sanglier J.-J., Klimkait T., Hamburger M. Library-based discovery and characterization of daphnane diterpenes as potent and selective HIV inhibitors in Daphne gnidium. J. Nat. Prod. 2012;75:414–419. doi: 10.1021/np200855d. [DOI] [PubMed] [Google Scholar]
- 246.Tian Y., Xu W., Zhu C., Lin S., Li Y., Xiong L., Wang S., Wang L., Yang Y., Guo Y., et al. Lathyrane diterpenoids from the roots of Euphorbia micractina and their biological activities. J. Nat. Prod. 2011;74:1221–1229. doi: 10.1021/np2001489. [DOI] [PubMed] [Google Scholar]
- 247.Win N.N., Ito T., Matsui T., Aimaiti S., Kodama T., Ngwe H., Okamoto Y., Tanaka M., Asakawa Y., Abe I., et al. Isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their Vpr inhibitory activity. Bioorg. Med. Chem. Lett. 2016;26:1789–1793. doi: 10.1016/j.bmcl.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 248.Win N.N., Ngwe H., Abe I., Morita H. Naturally occurring Vpr inhibitors from medicinal plants of Myanmar. J. Nat. Med. 2017;71:579–589. doi: 10.1007/s11418-017-1104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xiao W.-L., Li R.-T., Li S.-H., Li X.-L., Sun H.-D., Zheng Y.T., Wang R.R., Lu Y., Wang C., Zheng Q.T. Lancifodilactone F: A novel nortriterpenoid possessing a unique skeleton from Schisandra lancifolia and its anti-HIV activity. Org. Lett. 2005;7:1263–1266. doi: 10.1021/ol0473187. [DOI] [PubMed] [Google Scholar]
- 250.Yan M., Lu Y., Chen C.H., Zhao Y., Lee K.H., Chen D.F. Stelleralides D−J and anti-HIV daphnane diterpenes from Stellera chamaejasme. J. Nat. Prod. 2015;78:2712–2718. doi: 10.1021/acs.jnatprod.5b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Takeda K., Horibe I., Minato H. Components of the root of Lindera strychnifolia vill. Part XIV. Sesquiterpene lactones from the root of Lindera strychnifolia vill. J. Chem. Soc. C. 1968;1:569–572. doi: 10.1039/j39680000569. [DOI] [PubMed] [Google Scholar]
- 252.Zhang X., Huang S.Z., Gu W.G., Yang L.M., Chen H., Zheng C.B., Zhao Y.X., Wan D.C.C., Zheng Y.T. Wikstroelide M potently inhibits HIV replication by targeting reverse transcriptase and integrase nuclear translocation. Chin. J. Nat. Med. 2014;12:0186–0193. doi: 10.1016/S1875-5364(14)60031-5. [DOI] [PubMed] [Google Scholar]
- 253.Wu Y.C., Hung Y.C., Chang F.R., Cosentino M., Wang H.K., Lee K.H. Identification of ent-16β, 17 dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J. Nat. Prod. 1996;59:635–637. doi: 10.1021/np960416j. [DOI] [PubMed] [Google Scholar]
- 254.Singh I.P., Bharate S.B., Bhutani K.K. Anti-HIV natural products. Curr. Sci. 2005;89:269–290. [Google Scholar]
- 255.Sun H.D., Qiu S.X., Lin L.Z., Wang Z.Y., Lin Z.W., Pengsuparp T., Pezzuto J.M., Fong H.H., Cordell G.A., Farnsworth N.R. Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J. Nat. Prod. 1996;59:525–527. doi: 10.1021/np960149h. [DOI] [PubMed] [Google Scholar]
- 256.Okano M., Fukamiya N., Tagahara K., Cosentino M., Lee T.T.-Y., Morris-Natschke S., Lee K.-H. Anti-AIDS agents 25. Anti-HIV activity of quassinoids. Bioorg. Med. Chem. Lett. 1996;6:701–706. doi: 10.1016/0960-894X(96)00096-0. [DOI] [Google Scholar]
- 257.Wei Y., Ma C.M., Hattori M. Anti-HIV protease triterpenoids from the acid hydrolysate of Panax ginseng. Phytochem. Lett. 2009;2:63–66. doi: 10.1016/j.phytol.2008.12.001. [DOI] [Google Scholar]
- 258.Reutrakul V., Anantachoke N., Pohmakotr M., Jaipetch T., Yoosook C., Kasisit J., Napaswa C., Panthong A., Santisuk T., Prabpai S., et al. Anti-HIV-1 and anti-inflammatory lupanes from the leaves, twigs and resin of Garcinia hanburyi. Planta Med. 2010;76:368–371. doi: 10.1055/s-0029-1186193. [DOI] [PubMed] [Google Scholar]
- 259.Daoubi M., Marquez N., Mazoir N., Benharref A., Hernandez-Galan R., Munoz E., Collado I.E. Isolation of new phenylacetylingol derivatives that reactivate HIV-1 latency and a novel spirotriterpenoid from Euphorbia officinarum latex. Bioorg. Med. Chem. 2007;15:4577–4584. doi: 10.1016/j.bmc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 260.Tian R.R., Chen J.C., Zhang G.H., Qiu M.H., Wang Y.H., Du L., Shen X., Liu N.F., Zheng Y.T. Cucurbitane triterpenoids from Hemsleya penxianensis. Chin. J. Nat. Med. 2008;6:214–218. doi: 10.3724/SP.J.1009.2008.00214. [DOI] [Google Scholar]
- 261.Bodiwala H.S., Sabde S., Mitra D., Bhutani K.K., Singh I.P. Anti-HIV diterpenes from Coleus forskohlii. Nat. Prod. Commun. 2009;4:1173–1175. doi: 10.1177/1934578X0900400902. [DOI] [PubMed] [Google Scholar]
- 262.Chen I.H., Du Y.-C., Lu M.C., Lin A.S., Hsieh P.W., Wu C.C., Chen S.L., Yen H.F., Chang F.R., Wu Y.C. Lupane-type triterpenoids from Microtropis fokienensis and Perrottetia arisanensis and the apoptotic effect of 28-hydroxy-3-oxo-lup-20(29)-en-30-al. J. Nat. Prod. 2008;71:1352–1357. doi: 10.1021/np800093a. [DOI] [PubMed] [Google Scholar]
- 263.Kashiwada Y., Sekiya M., Yamazaki K., Ikeshiro Y., Fujioka T., Yamagishi T., Kitagawa S., Takaishi Y. Triterpenoids from the floral spikes of Betula platyphylla var. japonica and their reversing activity against multidrug-resistant cancer cells. J. Nat. Prod. 2007;70:623–627. doi: 10.1021/np060631s. [DOI] [PubMed] [Google Scholar]
- 264.Ramachandran C., Nair P.K., Alamo A., Cochrane C.B., Escalon E., Melnick S.J. Anticancer effects of amooranin in human colon carcinoma cell line in vitro and in nude mice xenografts. Int. J. Cancer. 2006;119:2443–2454. doi: 10.1002/ijc.22174. [DOI] [PubMed] [Google Scholar]
- 265.Tian J.-K., Xu L.Z., Zou Z.M., Yang S.L. Three Novel Triterpenoid Saponins from Lysimachia capillipes and Their Cytotoxic Activities. Chem. Pharm. Bull. 2006;54:567–569. doi: 10.1248/cpb.54.567. [DOI] [PubMed] [Google Scholar]
- 266.Yue Q.X., Cao Z.W., Guan S.H., Liu X.H., Tao L., Wu W.Y., Li Y.X., Yang P.Y., Liu X., Guo D.A. Proteomics characterization of the cytotoxicity mechanism of ganodeic acid D and computer-automated estimation of the possible drug target network. Mol. Cell Proteom. 2008;7:949–961. doi: 10.1074/mcp.M700259-MCP200. [DOI] [PubMed] [Google Scholar]
- 267.Chang U.M., Li C.H., Lin L.I., Huang C.P., Kan L.S., Lin S.B. Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006;79:1129–1139. doi: 10.1016/j.lfs.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 268.Sun P., Liu B.S., Yi Y.H., Li L., Gui M., Tang H.F., Zhang D.Z., Zhang S.L. A new cytotoxic lansotane-type triterpene glycoside from the sea cucumber Holothuria impatiens. Chem. Biodivers. 2007;4:450–457. doi: 10.1002/cbdv.200790037. [DOI] [PubMed] [Google Scholar]
- 269.Feng T., Wang R.R., Cai X.H., Zheng Y.T., Luo X.D. Anti-human immunodeficiency virus-1 constituents of the bark of Poncirus trifoliate. Chem. Pharm. Bull. (Tokyo) 2010;58:971–975. doi: 10.1248/cpb.58.971. [DOI] [PubMed] [Google Scholar]
- 270.Yang L., Wu S., Zhang Q., Liuand F., Wu P. 23, 24-Dihyrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria dependent apoptosis in human breast cancer cells (Bcap37) Cancer Lett. 2007;256:267–278. doi: 10.1016/j.canlet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 271.Fang L., Ito A., Chai H.B., Mi Q., Jones W.P., Madulid D.R., Oliveros M.B., Gao Q., Orjala J., Farnsworth N.R., et al. Cytotoxic constituents from the stem bark of Dichapetalum gelonioides collected in the Philippines. J. Nat. Prod. 2006;69:332–337. doi: 10.1021/np058083q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tuchinda P., Kornsakulkarn J., Pohmakotr M., Kongsaeree P., Prabpai S., Yoosook C., Kasisit J., Napaswad C., Sophasan S., Reutrakul V. Dichapetalin-type triterpenoids and lignans from the aerial parts of Phyllanthus acutissima. J. Nat. Prod. 2008;71:655–663. doi: 10.1021/np7007347. [DOI] [PubMed] [Google Scholar]
- 273.Lee J.H., Koo T.H., Yoon H., Jung H.S., Jin H.Z., Lee K., Hong Y.S., Lee J.J. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinine methide triterpenoid. Biochem. Pharmacol. 2006;72:1311–1321. doi: 10.1016/j.bcp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 274.Zhang H., Wang X., Chen F., Androulakis X.M., Wargovich M.J. Anticancer activity of limonoid from Khaya senegalensis. Phytother. Res. 2007;21:731–734. doi: 10.1002/ptr.2148. [DOI] [PubMed] [Google Scholar]
- 275.Awah F.M., Uzoegwu P.N., Ifeonu P. In vitro anti-HIV and immunomodulatory potentials of Azadirachta indica (Meliaceae) leaf extract. Afr. J. Pharm. Pharmacol. 2011;5:1353–1359. doi: 10.5897/AJPP11.173. [DOI] [Google Scholar]
- 276.Uddin S.J., Nahar L., Shilpi J.A., Shoeb M., Borkowski T., Gibbons S., Middleton M., Byres M., Sarker S.D. Gedunin, a limonoid from Xylocarpus granatuum, inhibits the growth of CaCo-2 colon cancer cell line in vitro. Phytother Res. 2007;21:757–761. doi: 10.1002/ptr.2159. [DOI] [PubMed] [Google Scholar]
- 277.Seida A.A., Kinghorn A.D., Cordell G.A., Farnsworth N.R. Potential anticancer agents. IX. Isolation of a new simaroubolide, 6alpha-tigloyloxychaparrinone, from Ailanthus integrifolia ssp. Calyciina (Simaroubaceae) Lloydia. 1978;41:584–587. [PubMed] [Google Scholar]
- 278.Orhan D.D., Ozcelik B., Ozgen S., Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010;165:496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 279.Kim H.J., Woo E.-R., Shin C.-G., Park H. A new flavonol glycoside gallate ester from Acer okamotoanum and its inhibitory activity againsthuman immunodeficiency virus-1 (HIV-1) integrase. J. Nat. Prod. 1998;61:145–148. doi: 10.1021/np970171q. [DOI] [PubMed] [Google Scholar]
- 280.Wang Q., Ding Z.-H., Liu J.-K., Zheng Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004;64:189–194. doi: 10.1016/S0166-3542(04)00201-3. [DOI] [PubMed] [Google Scholar]
- 281.Meragelman K.M., Mckee T.C., Boyd M.R. Anti-HIV prenylated flavonoids from Monotes africanus 1. J. Nat. Prod. 2001;64:546–548. doi: 10.1021/np0005457. [DOI] [PubMed] [Google Scholar]
- 282.Hu K., Kobayashi H., Dong A., Iwasaki S., Yao X. Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med. 2000;66:564–567. doi: 10.1055/s-2000-8601. [DOI] [PubMed] [Google Scholar]
- 283.Ohtake N., Nakai Y., Yamamoto M., Sakakibara I., Takeda S., Amagaya S. Separation and isolation methods for analysis of the active principles of Sho-saiko-to (SST) oriental medicine. J. Chromatogr. B. 2004;812:135–148. doi: 10.1016/S1570-0232(04)00547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lin Y.-M., Anderson H., Flavin M.T., Pai Y.-H.S., Mata-Greenwood E., Pengsuparp T. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J. Nat. Prod. 1997;60:884–888. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- 285.Wu J.H., Wang X.H., Yi Y.H., Lee K.H. Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg. Med. Chem. Lett. 2003;13:1813–1815. doi: 10.1016/S0960-894X(03)00197-5. [DOI] [PubMed] [Google Scholar]
- 286.Cheenpracha S., Karalai C., Ponglimanont C., Subhadhirasakul S., Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006;14:1710–1714. doi: 10.1016/j.bmc.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 287.Critchfield J.W., Coligan J.E., Folks T.M., Butera S.T. Casein kinase II is a selective target of HIV-1 transcriptional inhibitors. Proc. Natl. Acad. Sci. USA. 1997;94:6110–6115. doi: 10.1073/pnas.94.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Harada S., Haneda E., Maekawa T., Morikawa Y., Funayama S., Nagata N. Casein kinase II (CK-II)-mediated stimulation of HIV-1 reverse transcriptase activity and characterization of selective inhibitors in vitro. Biol. Pharm. Bull. 1999;22:1122–1126. doi: 10.1248/bpb.22.1122. [DOI] [PubMed] [Google Scholar]
- 289.Rowley D.C., Hansen M.S.T., Rhodes D., Sotriffer C.A., Ni H., McCammon J.A. Thalassiolins A-C: New marine-derived inhibitors of HIV cDNA integrase. Bioorg. Med. Chem. 2002;10:3619–3625. doi: 10.1016/S0968-0896(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 290.Alves C.N., Pinheiro J.C., de Camargo A.J., Souza A.J., da Carvalho R.B., Silva A.B.F. A quantum chemical and statistical study of flavonoid compounds with anti-HIV activity. J. Mol. Struct. (Theochem) 1999;491:123–131. doi: 10.1016/S0166-1280(99)00114-1. [DOI] [Google Scholar]
- 291.Alves C.N., Pinheiro J.C., Camargo A.J., Ferreira M.M.C., da Romero R.A.F., Silva A.B.F. A multiple linear regression and partial least squares study of flavonoid compounds with anti-HIV activity. J. Mol. Struct. (Theochem) 2001;541:81–88. doi: 10.1016/S0166-1280(00)00755-7. [DOI] [Google Scholar]
- 292.Min B.S., Lee H.K., Lee S.M. Anti-human immunodeficiency virus-type 1 activity of constituents from Juglans mandshurica. Arch. Pharm. Res. 2002;25:441–445. doi: 10.1007/BF02976598. [DOI] [PubMed] [Google Scholar]
- 293.Lee-Huang S., Zhang L., Huang P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1 infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003;307:1029–1037. doi: 10.1016/S0006-291X(03)01292-0. [DOI] [PubMed] [Google Scholar]
- 294.Amzazi S., Ghoulami S., Bakri Y. Human immunodeficiency virus type 1 inhibitory activity of Mentha longifolia. Therapie. 2003;58:531–534. doi: 10.2515/therapie:2003086. [DOI] [PubMed] [Google Scholar]
- 295.Lo W.L., Wu C.C., Chang F.R. Antiplatelet and anti-HIV constituents from Euchresta formosana. Nat. Prod. Res. 2003;17:91–97. doi: 10.1080/1478641031000103669. [DOI] [PubMed] [Google Scholar]
- 296.Mahmood N., Pizza C., Aquino R. Inhibition of HIV infection by flavonoids. Antivir. Res. 1993;22:189–199. doi: 10.1016/0166-3542(93)90095-Z. [DOI] [PubMed] [Google Scholar]
- 297.Hussein G., Miyashiro H., Nakamura N. Inhibitory effects of Sudanese plant extracts on HIV-1 replication and HIV-1 protease. Phytother Res. 1999;13:31–36. doi: 10.1002/(SICI)1099-1573(199902)13:1<31::AID-PTR381>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 298.Mahmood N., Piacente S., Pizza C. The anti-HIV activity and mechanisms of action of pure compounds isolated from Rosa damascena. Biochem. Biophys. Res. Commun. 1996;229:73–79. doi: 10.1006/bbrc.1996.1759. [DOI] [PubMed] [Google Scholar]
- 299.Boskabady M.H., Shafei M.N., Saberi Z., Amini S. Pharmacological Effects of Rosa Damascena. Iran. J. Basic Med. Sci. 2011;14:295–307. [PMC free article] [PubMed] [Google Scholar]
- 300.Dharmaratne H.R.W., Tan G.T., Marasinghe G.P.K., Pezzuto J.M. Inhibition of HIV-1 reverse transcriptase and HIV-1 replication by Calophyllum coumarins and xanthones. Planta Med. 2002;68:86–87. doi: 10.1055/s-2002-20058. [DOI] [PubMed] [Google Scholar]
- 301.Zembower D.E., Liao S., Flavin M.T., Xu Z.Q., Stup T.L., Buckheit R.W., Khilevich A., Mar A.A., Sheinkman A.K. Structural analogues of the calanolide anti-HIV agents. Modification of the trans-10,11-dimethyldihydropyran-12-ol ring (ring C) J. Med. Chem. 1997;40:1005–1017. doi: 10.1021/jm960355m. [DOI] [PubMed] [Google Scholar]
- 302.Creagh T., Ruckle J.L., Tolbert D.T., Giltner J., Eiznhamer D.A., Dutta B., Flavin M.T., Xu Z.Q. Safety and pharmacokinetics of single doses of (+)-calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virusnegative human subjects. Antimicrob. Agents Chemother. 2001;45:1379–1386. doi: 10.1128/AAC.45.5.1379-1386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kostova I., Raleva S., Genova P., Argirova R. Structure activity relationships of synthetic coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006;2006:68274. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yu D., Suzuki M., Xie L., Morris-Natschke S.L., Lee K.-H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 2003;23:322–345. doi: 10.1002/med.10034. [DOI] [PubMed] [Google Scholar]
- 305.Zhou P., Takaishi Y., Duan H., Chen B., Honda G., Itoh M. Coumarins and bicoumarin from Ferula sumbul: Anti-HIV activity and inhibition of cytokine release. Phytochemistry. 2000;53:689–697. doi: 10.1016/S0031-9422(99)00554-3. [DOI] [PubMed] [Google Scholar]
- 306.Shikishima Y., Takaishi Y., Honda G., Ito M., Takfda Y., Kodzhimatov O.K., Ashurmetov O., Lee K.H. Chemical constituents of Prangos tschiniganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem. Pharm. Bull. (Tokyo) 2001;49:877–880. doi: 10.1248/cpb.49.877. [DOI] [PubMed] [Google Scholar]
- 307.Marquez N., Sancho R., Bedoya L.M., Alcami J., Lopez-Perez J.L., San F.A. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-kB pathway. Antivir. Res. 2005;66:137–145. doi: 10.1016/j.antiviral.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 308.Xu Z.Q., Kern E.R., Westbrook L., Allen L.B., Buckheit R.W., Jr., Tseng C.K., Jenta T., Flavin M.T. Plant-derived and semi-synthetic calanolide compounds with in vitro activity against both human immunodeficiency virus type 1 and human cytomegalovirus. Antivir. Chem. Chemother. 2000;11:23–29. doi: 10.1177/095632020001100102. [DOI] [PubMed] [Google Scholar]
- 309.Mishra B.B., Singh D.D., Kishore N., Tiwari V.K., Tripathi V. Antifungal constituents isolated from the seeds of Aegle marmelos. Phytochemistry. 2010;71:230–234. doi: 10.1016/j.phytochem.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 310.Maity P., Hansda D., Bandyopadhyay U., Mishra D.K. Biological activities of crude extracts and chemical constituents of Bael, Aegle marmelous (L.) Corr. Ind. J. Exp. Biol. 2009;47:849–861. [PubMed] [Google Scholar]
- 311.O’Keefe B.R. Biologically active proteins from natural product extracts. J. Nat. Prod. 2001;64:1373–1381. doi: 10.1021/np0103362. [DOI] [PubMed] [Google Scholar]
- 312.Lee-Huang S., Huang P.L., Bourinbaiar A.S., Chen H.C., Kung H.F. Inhibition of the integrase of human immunodeficiency virus (HIV) type-1 by anti-HIV plant proteins MAP30 and GAP31. Proc. Natl. Acad. Sci. USA. 1995;92:8818–8822. doi: 10.1073/pnas.92.19.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.McGrath M.S., Hwang K.M., Caldwell S.E., Gaston I., Luk K.C., Wu P. GLQ223—An inhibitor of human immunodeficiency virus replication in acutely and chronically infected-cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA. 1989;86:2844–2848. doi: 10.1073/pnas.86.8.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kaur I., Puri M., Ahmed Z., Blanchet F.P., Mangeat B., Piguet V. Inhibition of HIV-1 Replication by Balsamin, a Ribosome Inactivating Protein of Momordica balsamina. PLoS ONE. 2013;8:e73780. doi: 10.1371/journal.pone.0073780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Kawahata T., Otake T., Mori H. A novel substance purified from Perilla frutescens Britton inhibits an early stage of HIV-1 replication without blocking viral adsorption. Antivir. Chem. Chemother. 2002;13:283–288. doi: 10.1177/095632020201300503. [DOI] [PubMed] [Google Scholar]
- 316.Irvin J.D., Uckun F.M. Pokeweed antiviral protein: Ribosome inactivation and therapeutic applications. Pharmacol. Ther. 1992;55:279–302. doi: 10.1016/0163-7258(92)90053-3. [DOI] [PubMed] [Google Scholar]
- 317.Wang H.X., Ng T.B. Ascalin, a new anti-fungal peptide with human immunodeficiency virus type 1 reverse transcriptase-inhibiting activity from shallot bulbs. Peptides. 2002;23:1025–1029. doi: 10.1016/S0196-9781(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 318.Wang H., Ye X.Y., Ng T.B. Purification of chrysancorin, a novel antifungal protein with mitogenic activity from garland chrysanthemum seeds. Biol. Chem. 2001;382:947–951. doi: 10.1515/BC.2001.118. [DOI] [PubMed] [Google Scholar]
- 319.Wang H., Ng T.B. Ginkbilobin, a novel antifungal protein from Ginkgo biloba seeds with sequence similarity to embryo-abundant protein. Biochem. Biophys. Res. Commun. 2002;279:407–411. doi: 10.1006/bbrc.2000.3929. [DOI] [PubMed] [Google Scholar]
- 320.Ye X.Y., Ng T.B. Hypogin, a novel antifungal peptide from peanuts with sequence similarity to peanut allergen. J. Pept. Res. 2001;57:330–336. doi: 10.1046/j.1397-002X.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- 321.Lam S.K., Ng T.B. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001;393:271–280. doi: 10.1006/abbi.2001.2506. [DOI] [PubMed] [Google Scholar]
- 322.Wang H.X., Ng T.B. Quinqueginsin, a novel protein with anti-human immunodeficiency virus, antifungal, ribonuclease and cell-free translation-inhibitory activities from American ginseng roots. Biochem. Biophys. Res. Commun. 2000;269:203–208. doi: 10.1006/bbrc.2000.2114. [DOI] [PubMed] [Google Scholar]
- 323.Wang H., Ng T.B. Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci. 2001;68:2151–2158. doi: 10.1016/S0024-3205(01)01023-2. [DOI] [PubMed] [Google Scholar]
- 324.Wang H.X., Ng T.B. Purification of a novel low-molecular mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochem. Biophys. Res. Commun. 2004;315:450–454. doi: 10.1016/j.bbrc.2004.01.064. [DOI] [PubMed] [Google Scholar]
- 325.Chu K.T., Ng T.B. Mollisin, an antifungal protein from the chestnut Castanea mollissima. Planta Med. 2003;69:809–813. doi: 10.1055/s-2003-43216. [DOI] [PubMed] [Google Scholar]
- 326.Bokesch H.R., Charan R.D., Meragelman K.M. Isolation and characterization of anti-HIV peptides from Dorstenia contrajerva and Treculia obovoidea. FEBS Lett. 2004;567:287–290. doi: 10.1016/j.febslet.2004.04.085. [DOI] [PubMed] [Google Scholar]
- 327.Wong J.H., Ng T.B. Purification of a trypsin-stable lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activity. Biochem. Biophys. Res. Commun. 2003;301:545–550. doi: 10.1016/S0006-291X(02)03080-2. [DOI] [PubMed] [Google Scholar]
- 328.Ye X.Y., Ng T.B. Delandin, a chitinase-like protein with antifungal, HIV-1 reverse transcriptase inhibitory and mitogenic activities from the rice bean Delandia umbellata. Protein Expr. Purif. 2002;24:524–529. doi: 10.1006/prep.2001.1596. [DOI] [PubMed] [Google Scholar]
- 329.Ye X.Y., Ng T.B. Purification of angularin, a novel antifungal peptide from adzuki beans. J. Pept. Sci. 2002;8:101–106. doi: 10.1002/psc.372. [DOI] [PubMed] [Google Scholar]
- 330.Chu K.T., Ng T.B. Isolation of a large thaumatin-like antifungal protein from seeds of the Kweilin chestnut Castanopsis chinensis. Biochem. Biophys. Res. Commun. 2003;301:364–370. doi: 10.1016/S0006-291X(02)02998-4. [DOI] [PubMed] [Google Scholar]
- 331.Ye X.Y., Wang H.X., Ng T.B. Structurally dissimilar proteins with antiviral and antifungal potency from cowpea (Vigna unguiculata) seeds. Life Sci. 2000;67:3199–3207. doi: 10.1016/S0024-3205(00)00905-X. [DOI] [PubMed] [Google Scholar]
- 332.Ye X.Y., Ng T.B., Tsang P.W., Wang J. Isolation of a homodimeric lectin with antifungal and antiviral activities from red kidney bean (Phaseolus vulgaris) seeds. J. Protein Chem. 2001;20:367–375. doi: 10.1023/A:1012276619686. [DOI] [PubMed] [Google Scholar]
- 333.Wang H., Ng T.B. Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochemistry. 2002;61:1–6. doi: 10.1016/S0031-9422(02)00144-9. [DOI] [PubMed] [Google Scholar]
- 334.Ngai P.H., Ng T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003;73:3363–3374. doi: 10.1016/j.lfs.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 335.Lam Y.W., Ng T.B. A monomeric mannose-binding lectin from inner shoots of the edible chive (Allium tuberosum) J. Protein Chem. 2001;20:361–366. doi: 10.1023/A:1012224602848. [DOI] [PubMed] [Google Scholar]
- 336.Ye X.Y., Ng T.B. A new antifungal protein and a chitinase with prominent macrophage-stimulating activity from seeds of Phaseolus vulgaris cv. pinto. Biochem. Biophys. Res. Commun. 2002;290:813–819. doi: 10.1006/bbrc.2001.6240. [DOI] [PubMed] [Google Scholar]
- 337.Wang H., Ng T.B. Isolation of lilin, a novel arginine- and glutamate-rich protein with potent antifungal and mitogenic activities from lily bulbs. Life Sci. 2002;70:1075–1084. doi: 10.1016/S0024-3205(01)01472-2. [DOI] [PubMed] [Google Scholar]
- 338.Ye X.Y., Ng T.B. A new peptidic protease inhibitor from Vicia faba seeds exhibit antifungal, HIV-1 reverse transcriptase inhibiting and mitogenic activities. J. Pept. Sci. 2002;8:656–662. doi: 10.1002/psc.425. [DOI] [PubMed] [Google Scholar]
- 339.Ye X.Y., Ng T.B. Isolation of unguilin, a cyclophilin-like protein with anti-mitogenic, antiviral, and antifungal activities, from black-eyed pea. J. Protein Chem. 2001;20:353–359. doi: 10.1023/A:1012272518778. [DOI] [PubMed] [Google Scholar]
- 340.Lam S.K., Ng T.B. A xylanase from roots of sanchi ginseng (Panax notoginseng) with inhibitory effects on human immunodeficiency virus-1 reverse transcriptase. Life Sci. 2002;70:3049–3058. doi: 10.1016/S0024-3205(02)01557-6. [DOI] [PubMed] [Google Scholar]
- 341.Ye X.Y., Ng T.B. Isolation of vulgin, a new antifungal polypeptide with mitogenic activity from the pinto bean. J. Pept. Sci. 2003;9:114–119. doi: 10.1002/psc.436. [DOI] [PubMed] [Google Scholar]
- 342.Ye X.Y., Ng T.B. Isolation of a new cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci. 2002;70:1129–1138. doi: 10.1016/S0024-3205(01)01473-4. [DOI] [PubMed] [Google Scholar]
- 343.Wang H., Ng T.B. Novel antifungal peptides from Ceylon spinach seeds. Biochem. Biophys. Res. Commun. 2001;288:765–770. doi: 10.1006/bbrc.2001.5822. [DOI] [PubMed] [Google Scholar]
- 344.Ye X.Y., Ng T.B. A new antifungal peptide from rice beans. J. Pept. Res. 2002;60:81–87. doi: 10.1034/j.1399-3011.2002.20962.x. [DOI] [PubMed] [Google Scholar]
- 345.DeBruyne T., Pieters L., Deelstra H., Vlietinck A. Condensed vegetable tannins: Biodiversity in structure and biological activities. Biochem. Syst. Ecol. 1999;27:445–459. [Google Scholar]
- 346.Van Khanbabaee K., Ree T. Tannins: Classification and definition. Nat. Prod. Rep. 2001;18:641–649. doi: 10.1039/b101061l. [DOI] [PubMed] [Google Scholar]
- 347.Notka F., Meier G., Wagner R. Concerted inhibitory activities of Phyllanthus amarus on HIV replication in vitro and ex vivo. Antivir. Res. 2004;64:93–102. doi: 10.1016/S0166-3542(04)00129-9. [DOI] [PubMed] [Google Scholar]
- 348.Amouroux P., Jean D., Lamaison J.L. Antiviral activity in vitro of Cupressus sempervirens on two human retroviruses HIV and HTLV. Phytother Res. 1998;12:367–368. doi: 10.1002/(SICI)1099-1573(199808)12:5<367::AID-PTR301>3.0.CO;2-N. [DOI] [Google Scholar]
- 349.Liu S., Lu H., Zhao Q., He Y., Niu J., Debnath A.K., Wu S., Jiang S. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochem. Biophys. Acta. 2005;1723:270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 350.Charlton J.L. Antiviral activity of lignans. J. Nat. Prod. 1998;61:1447–1451. doi: 10.1021/np980136z. [DOI] [PubMed] [Google Scholar]
- 351.Rimando A.M., Pezzuto J.M., Farnsworth N.R., Santisuk T., Reutrakul V., Kawanishi K. New lignans from Anogeissus acuminata with HIV1 reverse transcriptase inhibitory activity. J. Nat. Prod. 1994;57:896–904. doi: 10.1021/np50109a004. [DOI] [PubMed] [Google Scholar]
- 352.Lee S.S., Lin M.T., Liu C.L., Lin Y.Y., Liu K.C.S.C. Six lignans from Phyllanthus myrtifolius. J. Nat. Prod. 1996;59:1061–1065. doi: 10.1021/np960322+. [DOI] [PubMed] [Google Scholar]
- 353.Chen D.F., Zhang S.X., Xie L., Xie J.X., Chen K., Kashiwada Y. Anti-AIDS agents–XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg. Med. Chem. 1997;5:1715–1723. doi: 10.1016/S0968-0896(97)00118-1. [DOI] [PubMed] [Google Scholar]
- 354.Kashiwada Y., Nishizawa M., Yamagishi T., Tanaka T., Nonaka G., Cosentino L.M., Lee K.-H. Anti-AIDS agents 18. Sodium and potassium salts of caffeic acid tetramers from Arnebia euchroma as anti-HIV agents. J. Nat. Prod. 1995;58:392–400. doi: 10.1021/np50117a007. [DOI] [PubMed] [Google Scholar]
- 355.Piccinelli A.L., Mahmood N., Mora G., Poveda L., De Simone F., Rastrelli L. Anti-HIV activity of dibenzylbutyrolactone-type lignans from Phenax species endemic in Costa Rica. J. Pharm. Pharmacol. 2005;57:1109–1115. doi: 10.1211/jpp.57.9.0006. [DOI] [PubMed] [Google Scholar]
- 356.Chen M., Kilgore N., Lee K.H., Chen D.F. Rubrisandrins A and B, lignans and related anti-HIV compounds from Schisandra rubriflora. J. Nat. Prod. 2006;69:1697–1701. doi: 10.1021/np060239e. [DOI] [PubMed] [Google Scholar]
- 357.Khan M.T.H., Ather A. Potentials of phenolic molecules of natural origin and their derivatives as anti-HIV agents. Biotechnol. Annu. Rev. 2007;13:223–264. doi: 10.1016/S1387-2656(07)13009-X. [DOI] [PubMed] [Google Scholar]
- 358.El-Mekkawy S., Meselhy M.R., Kusumoto I.T., Kadota S., Hattori M., Namba T. Inhibitory effects of Egyptian folk medicines on human immunodeficiency virus (HIV) reverse transcriptase. Chem. Pharm. Bull. 1995;43:641–648. doi: 10.1248/cpb.43.641. [DOI] [PubMed] [Google Scholar]
- 359.Chien N.Q., Hung N.V., Santarsiero B.D., Mesecar A.D., Cuong N.M., Soejarto D.D., Pezzuto J.M., Fong H.H.S., Tan G.T. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J. Nat. Prod. 2004;67:994–998. doi: 10.1021/np030469i. [DOI] [PubMed] [Google Scholar]
- 360.Connell B.J., Chang S.-Y., Prakash E., Yousfi R., Mohan V., Posch W., Wilflingseder D., Moog C., Kodama E.N., Clayette P., et al. A Cinnamon-derived procyanidin compound displays anti-HIV-1 activity by blocking heparan sulfate- and co-receptor- binding sites on gp120 and reverses T cell exhaustion via impeding Tim-3 and PD-1 upregulation. PLoS ONE. 2016;11:e0165386. doi: 10.1371/journal.pone.0165386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lin W.L., Guu S.Y., Tsai C.C., Prakash E., Viswaraman M., Chen H.B., Chang C.F. Derivation of cinnamon blocks leukocyte attachment by interacting with sialosides. PLoS ONE. 2015;10:e0130389. doi: 10.1371/journal.pone.0130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gruenwald J., Freder J., Armbruester N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010;50:822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 363.Hong K.J., Lee H.S., Kim Y.S., Kim S.S. Ingenol protects human T cells from HIV-1 infection. Public Health Res. Perspect. 2011;2:109–114. doi: 10.1016/j.phrp.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Hasler C.M., Acs G., Blumberg P.M. Specific binding to protein kinase C by ingenol and its induction of biological responses. Cancer Res. 1992;52:202–208. [PubMed] [Google Scholar]
- 365.Sorg B., Hecker E., Zur C.D., Ingenols I.I. On the chemistry of ingenol ii. Esters of ingenol and delta7,8-isoingenol. Z Naturforsch. 1982;37b:748–756. doi: 10.1515/znb-1982-0615. [DOI] [Google Scholar]
- 366.Ireland D.C., Wang C.K.L., Wilson J.A., Gustafson K.R., Craik D.J. Cyclotides as natural anti-HIV agents. Biopolymers. 2008;90:51–60. doi: 10.1002/bip.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ireland D.C., Clark R.J., Daly N.L., Craik D.J. Isolation, Sequencing, and Structure-Activity Relationships of Cyclotides. J. Nat. Prod. 2010;73:1610–1622. doi: 10.1021/np1000413. [DOI] [PubMed] [Google Scholar]
- 368.Kapewangolo P., Hussein A.A., Meyer D. Inhibition of HIV-1 enzymes, antioxidant and anti-inflammatory activities of Plectranthus barbatus. J. Ethnopharmacol. 2013;149:184–190. doi: 10.1016/j.jep.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 369.Lukhoba C.W., Simmonds M.S.J., Paton A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006;103:1–24. doi: 10.1016/j.jep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 370.Sunthitikawinsakul A., Kongkathip N., Kongkathip B., Phonnakhu S., Daly J.W., Spande T.F., Nimit Y., Rochanaruangrai S. Coumarins and carbazoles from Clausena excavata exhibited antimycobacterial and antifungal activities. Planta Med. 2003;69:155–157. doi: 10.1055/s-2003-37716. [DOI] [PubMed] [Google Scholar]
- 371.Ruangrungsi N., Ariyaprayoon J., Lange G.L., Organ M.G. Three new carbazole alkaloids isolated from Murraya siamensis. J. Nat. Prod. 1990;53:946–952. doi: 10.1021/np50070a025. [DOI] [Google Scholar]
- 372.Prinsloo G., Marokane C.K., Street R.A. Anti-HIV activity of Southern African plants: Current developments, phytochemistry and future research. J. Ethnopharmacol. 2018;210:133–155. doi: 10.1016/j.jep.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lo W.L., Chang F.R., Liaw C.C., Wu Y.C. Cytotoxic coumaronochromones from the roots of Euchresta formosana. Planta Med. 2002;68:146–151. doi: 10.1055/s-2002-20248. [DOI] [PubMed] [Google Scholar]
- 374.Nageswara R.K., Srimannarayana G. Flemiphyllin, an isoflavone from stems of Flemingia macrophylla. Phytochemistry. 1984;23:927–929. [Google Scholar]
- 375.Mizuno M., Tamura K.I., Tanaka T., Iinuma M. Three prenylflavanones from Euchresta japonica. Phytochemistry. 1988;27:1831–1834. doi: 10.1016/0031-9422(88)80454-0. [DOI] [Google Scholar]
- 376.Wintola O.A., Afolayan A.J. Alepidea amatymbica Eckl. & Zeyh.: A review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Alternat. Med. 2014;2014:284517. doi: 10.1155/2014/284517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Lubbe A., Seibert I., Klimkait T., Kooy F.V.D. Ethnopharmacology in overdrive: The remarkable anti-HIV activity of Artemisia annua. J. Ethnopharmacol. 2012;141:854–859. doi: 10.1016/j.jep.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 378.Oh C.S., Price J., Brindley M.A., Widrlechner M.P., Qu L., Coy J.-A.M., Murphy P., Hauck C., Maury W. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol. J. 2011;8:188. doi: 10.1186/1743-422X-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Said M.S., Chinchansurea A.A., Nawaleb L., Durgec A., Wadhwanic A., Kulkarnic S.S., Sarkarb D., Joshia S.P. A new butenolide cinnamate and other biological active chemical constituents from Polygonumglabrum. Nat. Prod. Res. 2015;29:2080–2086. doi: 10.1080/14786419.2015.1004674. [DOI] [PubMed] [Google Scholar]
- 380.Sakurai N., Wu J.H., Sashida Y., Mimaki Y., Nikaido T., Koike K., Itokawa H., Lee K.H. Anti-AIDS agents. Part 57: Actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorg. Med. Chem. Lett. 2004;14:1329–1332. doi: 10.1016/j.bmcl.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 381.Tai B.H., Nhut N.D., Nhiem N.X., Quang T.H., Ngan N.T.T., Luyen B.T.T., Huong T.T., Wilson J., Beutler J.A., Ban N.K., et al. An evaluation of the RNase H inhibitory effects of Vietnamese medicinal plant extracts and natural compounds. Pharmaceut. Biol. 2011;49:1046–1051. doi: 10.3109/13880209.2011.563316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wu P.L., Su G.C., Wu T.S. Constituents from the stems of Aristolochia manshuriensis. J. Nat. Prod. 2003;66:996–998. doi: 10.1021/np0301238. [DOI] [PubMed] [Google Scholar]
- 383.Wu T.S., Leu Y.L., Chan Y.Y. Constituents from the stem and root of Aristolochia kaempferi. Biol. Pharm. Bull. 2000;23:1216–1219. doi: 10.1248/bpb.23.1216. [DOI] [PubMed] [Google Scholar]
- 384.Nakanishi T., Iwasak K., Nasu M., Miura I., Yoneda K. Aristoloside, an aristolochic acid derivative from stems of Aristolochia manshuriensis. Phytochemistry. 1982;21:1759–1762. doi: 10.1016/S0031-9422(82)85055-3. [DOI] [Google Scholar]
- 385.Wu T.S., Kao M.S., Wu P.L., Lin F.W., Shi L.S., Teng C.M. The heartwood constituents of Tetradium glabrifolium. Phytochemistry. 1995;40:121–124. doi: 10.1016/0031-9422(95)00248-6. [DOI] [Google Scholar]
- 386.Wu T.S., Tsai Y.L., Wu P.L., Lin F.W., Lin J.K. Constituents from the leaves of Aristolochia elegans. J. Nat. Prod. 2000;63:692–693. doi: 10.1021/np990483o. [DOI] [PubMed] [Google Scholar]
- 387.Wu X.D., Cheng J.T., He J., Zhang X.J., Dong L.B., Gong X., Song L.D., Zheng Y.T., Peng L.Y., Zhao Q.S. Benzophenone glycosides and epicatechin derivatives from Malania oleifera. Fitoterpia. 2012;83:1068–1071. doi: 10.1016/j.fitote.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 388.Wu S.Y., Fu Y.H., Zhou Q., Bai M., Chen G.Y., Han C.R., Song X.P. Biologically active oligostilbenes from the stems of Vatica mangachapoi and chemotaxonomic significance. Nat. Prod. Res. 2019;33:2300–2307. doi: 10.1080/14786419.2018.1443091. [DOI] [PubMed] [Google Scholar]
- 389.Hakim E.H., Juliawaty L.D., Syah Y.M., Din L.B., Ghisalberti E.L., Latip J., Achmad S.A. Cytotoxic properties of oligostilbenoids from the tree barks of Hopea dryobalanoides. Z Naturforsch C. 2005;60:723–727. doi: 10.1515/znc-2005-9-1011. [DOI] [PubMed] [Google Scholar]
- 390.Patra A., Dey A.K., Kundu A.B., Saraswathy A., Purushothaman K.K. Shoreaphenol, a polyphenol from Shorea robusta. Phytochemistry. 1992;31:2561–2562. doi: 10.1016/0031-9422(92)83330-2. [DOI] [Google Scholar]
- 391.Yan K.X., Terashima K., Takaya Y., Niwa M. Two new stilbenetetramers from the stem of Vitis vinifera ‘Kyohou’. Tetrahedron. 2002;58:6931–6935. doi: 10.1016/S0040-4020(02)00735-4. [DOI] [Google Scholar]
- 392.Yang G.X., Zhou J.T., Li Y.Z., Hu C.Q. Anti-HIV bioactive stilbene dimers of Caragana rosea. Planta Med. 2005;71:569–571. doi: 10.1055/s-2005-864162. [DOI] [PubMed] [Google Scholar]
- 393.Zofou D., Ntie-Kang F., Sippl W., Efange S.M.N. Bioactive natural products derived from the central African flora against neglected tropical diseases and HIV. Nat. Prod. Rep. 2013;30:1098–1120. doi: 10.1039/c3np70030e. [DOI] [PubMed] [Google Scholar]
- 394.Mahwasane S.T., Middleton L., Boaduo N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S. Afr. J. Bot. 2013;88:69–75. doi: 10.1016/j.sajb.2013.05.004. [DOI] [Google Scholar]
- 395.Lagrota M.H.C., Wigg M.D., Santos M.M.G., Miranda M.M.F.S., Camara F.P., Couceiro J.N.S.S., Costa S.S. Inhibitory activity of extracts of Alternanthera brasiliana (Amaranthaceae) against the herpes simplex virus. Phytother. Res. 1994;8:358–361. doi: 10.1002/ptr.2650080609. [DOI] [Google Scholar]
- 396.Wang H.X., Ng T.B. Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus reverse transcriptase and glycohydrolases. Planta Med. 2001;67:669–672. doi: 10.1055/s-2001-17359. [DOI] [PubMed] [Google Scholar]
- 397.Thiagarajan V.R.K., Shanmugam P., Krishnan U.M., Muthuraman A., Singh N. Ameliorative potential of Butea monosperma on chronic constriction injury of sciatic nerve induced neuropathic pain in rats. An. Acad. Bras. Cienc. 2012;84 doi: 10.1590/S0001-37652012005000063. [DOI] [PubMed] [Google Scholar]
- 398.Kashiwada Y., Wang H.-K., Nagao T., Kitanaka S., Yasuda I., Fujioka T., Yamagishi T., Cosentino L.M., Kozuka M., Okabe H. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998;61:1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 399.Lam T.L., Lam M.L., Au T.K., Ip D.T., Ng T.B., Fong W.P., Wan D.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000;67:2889–2896. doi: 10.1016/S0024-3205(00)00864-X. [DOI] [PubMed] [Google Scholar]
- 400.Abdel-Malek S., Bastien J.W., Mahler W.F., Jia Q., Reinecke M.G., Robinson W.E., Shu Y.-H., Zalles-Asin J. Drug leads from the Kallawaya herbalists of Bolivia. 1. Background, rationale, protocol and anti-HIV activity. J. Ethnopharmacol. 1996;50:157–166. doi: 10.1016/0378-8741(96)01380-3. [DOI] [PubMed] [Google Scholar]
- 401.Pengsuparp T., Cai L., Constant H., Fong H.H.S., Lin L.Z., Kinghorn A.D., Pezzuto J.M., Cordell G.A., Ingolfsdóttir K., Wagner H. Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J. Nat. Prod. 1995;58:1024–1031. doi: 10.1021/np50121a006. [DOI] [PubMed] [Google Scholar]
- 402.Luthra P.M., Singh R., Chandra R. Therapeutic uses of Curcuma longa (Turmeric) Indian J. Clin. Biochem. 2001;16:153–160. doi: 10.1007/BF02864854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Min B.S., Bae K.H., Kim Y.H., Miyashiro H., Hattori M., Shimotohno K. Screening of Korean plants against human immunodeficiency virus type 1 protease. Phytother. Res. 1999;13:680–682. doi: 10.1002/(SICI)1099-1573(199912)13:8<680::AID-PTR501>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 404.Ali H., Konig G.M., Khalid S.A., Wright A.D., Kaminsky R. Evaluation of selected sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for cytotoxicity. J. Ethnopharmacol. 2002;83:219–228. doi: 10.1016/S0378-8741(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 405.Wu N., Wang L., Chzn Y.K., Liao Z., Yang G.Y., Hu Q.F. Lignans from the stem of Styrax japonica. Asian J. Chem. 2011;23:931–932. [Google Scholar]
- 406.Chang Y.S., Woo E.R. Korean medicinal plants inhibiting to human immunodeficiency virus type 1 (HIV-1) fusion. Phytother. Res. 2003;17:426–429. doi: 10.1002/ptr.1155. [DOI] [PubMed] [Google Scholar]
- 407.Rukunga G.M., Kofi-Tsekpo M.W., Kurokawa M., Kageyama S., Mungai G.M., Muli J.M., Tolo F.M., Kibaya R.M., Muthaura C.N., Kanyara J.N. Evaluation of the HIV-1 reverse transcriptase inhibitory properties of extracts from some medicinal plants in Kenya. Afr. J. Health Sci. 2002;9:81–90. doi: 10.4314/ajhs.v9i1.30758. [DOI] [PubMed] [Google Scholar]
- 408.Kusumoto I.T., Nakabayashi T., Kida H., Miyashiro H., Hattori M., Namba T., Shimotohno K. Screening of various plant-extracts used in ayurvedic medicine for inhibitory effects on human-immunodeficiency-virus type-1 (HIV-1) protease. Phytother. Res. 1995;9:180–184. doi: 10.1002/ptr.2650090305. [DOI] [Google Scholar]
- 409.Sookkongwaree K., Geitmann M., Roengsumran S., Petsom A., Danielson U.H. Inhibition of viral proteases by Zingiberaceae extracts and flavones isolated from Kaempferia parviflora. Pharmazie. 2006;61:717–721. [PubMed] [Google Scholar]
- 410.Mujovo S., Hussein A., Meyer J.J.M., Fourie B., MutHIVhi T., Lall N. Bioactive compounds from Lippia javanica and Hoslundia opposita. Nat. Prod. Res. 2008;22:1047–1054. doi: 10.1080/14786410802250037. [DOI] [PubMed] [Google Scholar]
- 411.Piacente S., Pizza C., De Tommasi N., Mahmood N. Constituents of Ardisia japonica and their in vitro anti-HIV activity. J. Nat. Prod. 1996;59:565–569. doi: 10.1021/np960074h. [DOI] [PubMed] [Google Scholar]
- 412.Silprasit K., Seetaha S., Pongsanarakul P., Hannongbua S., Choowongkomon K. Anti-HIV-1 reverse transcriptase activities of hexane extracts from some Asian medicinal plants. J. Med. Plants Res. 2011;5:4194–4201. [Google Scholar]
- 413.Maroyi A. Ximenia caffra Sond. (Ximeniaceae) in sub-Saharan Africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016;184:81–100. doi: 10.1016/j.jep.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 414.Thomford N.E., Awortwe C., Dzobo K., Adu F., Chopera D., Wonkam A., Skelton M., Blackhurst D., Dandara C. Inhibition of CYP2B6 by medicinal plant extracts: Implication for use of efavirenz and nevirapine based highly active anti-retroviral therapy (HAART) in resource-limited settings. Molecules. 2016;21:211. doi: 10.3390/molecules21020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Eid A.M.M., Elmarzugi N.A., El-Enshasy H.A. A review on the phytopharmacological effect of Swietenia macrophylla. Int. J. Pharm. Pharm. Sci. 2013;5:47–53. [Google Scholar]
- 416.Hasegawa H., Matsumiya S., Uchiyama M., Kurokawa T., Inouye Y., Kasai R., Ishibashi S., Yamasaki K. Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities. Planta Med. 1994;60:240–243. doi: 10.1055/s-2006-959467. [DOI] [PubMed] [Google Scholar]
- 417.Xu H.-X., Wan M., Loh B.-N., Kon O.-L., Chow P.-W., Sim K.-Y. Screening of traditional medicines for their inhibitory activity against HIV-1 protease. Phytother. Res. 1996;10:207–210. doi: 10.1002/(SICI)1099-1573(199605)10:3<207::AID-PTR812>3.0.CO;2-U. [DOI] [Google Scholar]
- 418.Grzybek J., Wongpanich V., Mata-Greenwood E., Angerhofer C.K., Pezzuto J.M., Cordell G.A. Biological evaluation of selected plants from Poland. Pharm. Biol. 1997;35:1–5. doi: 10.1076/phbi.35.1.1.13269. [DOI] [Google Scholar]
- 419.Bedoya L.M., Sanchez-Palomino S., Abad M.J., Bermejo P., Alcami J. Anti-HIV activity of medicinal plant extracts. J. Ethnopharmacol. 2001;77:113–116. doi: 10.1016/S0378-8741(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 420.Birt D.F., Widrlechner M.P., Hammer K.D.P., Hillwig M.L., Wei J., Kraus G.A., Murphy P.A., McCoy J.A., Wurtele E.S., Neighbors J.D. Hypericumin infection: Identification of anti-viral and anti-inflammatory constituents. Pharm. Biol. 2009;47:774–782. doi: 10.1080/13880200902988645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Zhang L., Luo R.-H., Wang F., Jiang M.-Y., Dong Z.-J., Yang L.-M., Zheng Y.-T., Liu J.-K. Highly functionalized daphnane diterpenoids from Trigonostemon thyrsoideum. Org. Lett. 2010;12:152–155. doi: 10.1021/ol9025638. [DOI] [PubMed] [Google Scholar]
- 422.Chen J.C., Zhang G.H., Zhang Z.Q., Qiu M.H., Zheng Y.T., Yang L.M., Yu K.B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008;71:153–155. doi: 10.1021/np0704396. [DOI] [PubMed] [Google Scholar]
- 423.Magadula J.J., Tewtrakul S. Anti-HIV-1 protease activities of crude extracts of some Garcinia species growing in Tanzania. Afr. J. Biotechnol. 2010;9:1848–1852. [Google Scholar]
- 424.Au T.K., Lam T.L., Ng T.B., Fong W.P., Wan D.C.C. A Comparison of HIV-1 integrase inhibition by aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2001;68:1687–1694. doi: 10.1016/S0024-3205(01)00945-6. [DOI] [PubMed] [Google Scholar]
- 425.Ngwira K.J., Maharaj V.J., Mgani Q.A. In vitro antiplasmodial and HIV-1 neutralization activities of root and leaf extracts from Berberis holstii. J. Herb. Med. 2015;5:30–35. doi: 10.1016/j.hermed.2014.12.001. [DOI] [Google Scholar]
- 426.Louvel S., Moodley N., Seibert I., Steenkamp P., Nthambeleni R., Vidal V., Maharaj V., Klimkait T. Identification of compounds from the plant species Alepidea amatymbica active against HIV. S. Afr. J. Bot. 2013;86:9–14. doi: 10.1016/j.sajb.2013.01.009. [DOI] [Google Scholar]
- 427.Woradulayapinij W., Soonthornchareonnon N., Wiwat C. In vitro HIV type 1 reverse transcriptase inhibitory activities of Thai medicinal plants and Canna indica L. rhizomes. J. Ethnopharmacol. 2005;101:84–89. doi: 10.1016/j.jep.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 428.Xiao W.L., Pu J.X., Chang Y., Li X.L., Huang S.X., Yang L.M., Li L.M., Lu Y., Zheng Y.T., Li R.T. Sphenadilactones A and B, two novel nortriterpenoids from Schisandra sphenanthera. Org. Lett. 2006;8:1475–1478. doi: 10.1021/ol060324d. [DOI] [PubMed] [Google Scholar]
- 429.Voravuthikunchai S.P., Phongpaichit S., Subhadhirasakul S. Evaluation of antibacterial activities of medicinal plants widely used among AIDS patients in Thailand. Pharmaceut. Biol. 2005;43:701–706. doi: 10.1080/13880200500385194. [DOI] [Google Scholar]
- 430.Chinsembu K.C. Ethnobotanical study of plants used in the management of HIV/AIDS-related diseases in Livingstone, Southern Province, Zambia. Evid. Based Complement. Alternat. Med. 2016;2016:4238625. doi: 10.1155/2016/4238625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Zhang H.J., Tan G.T., Hoang V.D., Hung N.V., Cuong N.M., Soejarto D.D., Pezzuto J.M., Fong H.H.S. Natural anti-HIV agents. Part 2: Litseaverticillol a, a prototypic litseane sesquiterpene from Litsea verticillata. Tetrahedron Lett. 2001;42:8587–8591. [Google Scholar]
- 432.Esposito F., Carli I., Del Vecchio C., Xu L., Corona A., Grandi N., Piano D., Maccioni E., Distinto S., Parolin C. Sennoside a, derived from the traditional Chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine. 2016;23:1383–1391. doi: 10.1016/j.phymed.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 433.Chang C.W., Lin M.T., Lee S.S., Liu K.C.S.C., Hsu F.L., Lin J.Y. Differential inhibition of reverse transcriptase and cellular DNA polymerase-α activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antivir. Res. 1995;27:367–374. doi: 10.1016/0166-3542(95)00020-M. [DOI] [PubMed] [Google Scholar]
- 434.Bessong P.O., Rojas L.B., Obi L.C., Tshisikawe P.M., Igunbor E.O. Further screening of venda medicinal plants for activity against HIV type 1 reverse transcriptase and integrase. Afr. J. Biotechnol. 2006;5:526–528. [Google Scholar]
- 435.Asres K., Bucar F., Kartnig T., Witvrouw M., Pannecouque C., De Clercq E. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother. Res. 2001;15:62–69. doi: 10.1002/1099-1573(200102)15:1<62::AID-PTR956>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 436.Shriwas P., Chen X., Kinghorn A.D., Ren Y. Plant-derived glucose transport inhibitors with potential antitumor activity. Phytother. Res. 2019 doi: 10.1002/ptr.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Cary D.C., Peterlin B.M. Natural products and HIV/AIDS. AIDS Res. Hum. Retrovir. 2018;34:31–38. doi: 10.1089/aid.2017.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Tietjen I., Ntie-Kang F., Mwimanzi P., Onguéné P.A., Scull M.A., Idowu T.O., Ogundaini A.O., Meva’a L.M., Abegaz B.M., Rice C.M., et al. Screening of the Pan-African natural product library identifies ixoratannin A-2 and boldine as novel HIV-1 inhibitors. PLoS ONE. 2015;10:e0121099. doi: 10.1371/journal.pone.0121099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Richard K., Williams D.E., de Silva E.D., Brockman M.A., Brumme Z.L., Andersen R.J., Tietjen I. Identification of novel HIV-1 latency-reversing agents from a library of marine natural products. Viruses. 2018;10:348. doi: 10.3390/v10070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Margolis D.M., Garcia J.V., Hazuda D.J., Haynes B.F. Latency reversal and viral clearance to cure HIV-1. Science. 2016;353:aaf6517. doi: 10.1126/science.aaf6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Andersen R.J., Ntie-Kang F., Tietjen I. Natural product-derived compounds in HIV suppression, remission, and eradication strategies. Antiviral. Res. 2018;158:63–77. doi: 10.1016/j.antiviral.2018.07.016. [DOI] [PubMed] [Google Scholar]