Abstract
Approximately 7–9% of people develop posttraumatic stress disorder in their lifetime, but standard pharmacological treatment or psychotherapy shows a considerable individual variation in their effectiveness. Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) hold promise for the treatment of posttraumatic stress disorder. The objective of this meta-analysis was to summarize the existing evidence on the therapeutic effects of these brain stimulation treatments on posttraumatic core symptoms. We systematically retrieved articles published between 1st January 2000 and 1st January 2020 comparing the effects of active with sham stimulation or no intervention in posttraumatic patients from eight databases. Random-effects model was used for meta-analysis. Meta-regression and subgroup meta-analysis was performed to investigate the influence of stimulation dose and different stimulation protocols, respectively. 20 studies were included in this review, where of 11 randomized controlled trials were subjected to quantitative analysis. Active stimulation demonstrated significant reductions of core posttraumatic symptoms with a large effect size (Hedge’s g = −0.975). Subgroup analysis showed that both excitatory and inhibitory rTMS of the right dorsolateral prefrontal cortex led to symptom reductions with a large (Hedges’ g = −1.161, 95% CI, −1.823 to −0.499; p = 0.015) and medium effect size (Hedges’ g = −0.680, 95% CI: −0.139 to −0.322; p ≤ 0.001) respectively. Results further indicated significant durability of symptom-reducing effects of treatments during a two to four weeks period post stimulation (Hedges’ g = −0.909, 95% CI: −1.611 to −0.207; p = 0.011). rTMS of the right dorsolateral prefrontal cortex appears to have a positive effect in reducing core symptoms in patients with posttraumatic stress disorder.
Subject terms: Psychiatric disorders, Neuroscience, Psychiatric disorders, Neuroscience
Introduction
Posttraumatic stress disorder (PTSD) is a common psychiatric disorder that occurs after direct or indirect exposure to a traumatic event. PTSD is characterized by four core symptoms including re-experiencing, hyperarousal, avoidance of trauma-related stimuli and negative cognition and mood1. Approximately 7 to 9% of people develop PTSD in their lifetime, whereas the rate is estimated to be much higher in military veterans2,3. PTSD is frequently associated with mood dysregulation, addiction, shame, feelings of guilt, aggression, shallow sleep and poor physical health, thereby leading to occupational disability and poor quality of life4. Furthermore, over half of patients with PTSD also suffer from a major depressive disorder (MDD)5,6. However, standard pharmacological treatment or psychotherapy has only been partly successful, showing a considerable individual variation in their effectiveness7. Hence, various studies have been conducted to explore alternative treatments. Here, non-invasive brain stimulation (NIBS) including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) received much attention lately, given their ability to modulate cortical excitability. Indeed, research employing animal models and neuroimaging studies in humans suggest that altered brain excitability could be a major pathophysiological factor contributing to PTSD. A hyperactivity of the amygdala and dorsal anterior cingulate cortex, regions that are known to promote fear responses in animals and humans, has been associated with PTSD. On the other hand, hypoactivity of the ventromedial and dorsolateral prefrontal cortex (VMPFC and DLPFC, respectively) has been reported, regions that are known to be involved in the suppression of fear responses8–11. Specifically, the right hemisphere’s dominant role in stress modulation has been linked to PTSD, with studies indicating structural abnormalities especially of the right hemisphere12.
rTMS and tDCS are frequently employed as save alternative options to pharmacotherapy for the treatment of a number of psychiatric disorders. The magnetic field elicited by rTMS passes through the scalp and skull and changes cortical and subcortical activity in specific brain networks without injury. In general, high frequency (HF) stimulation (>5 Hz) increases cortical excitability, while low frequency (LF) stimulation (≤1 Hz) decreases cortical excitability13. In addition, a patterned form of rTMS called theta-burst stimulation (TBS) was established in 200514. Standard protocols of TBS consist of 50 Hz bursts of 3 pulses that are repeated at 5 Hz to reach a total number of 600 pulses. TBS can be applied continuously (cTBS) or in an intermittent form (iTBS), while the latter exhibits facilitatory, and the former inhibitory effects on neural excitability14. tDCS leads to sub-threshold shifts of resting membrane potentials by applying direct currents via scalp electrodes over targeted cortical areas15. Anodal tDCS increases the excitability of cortex whereas cathodal tDCS decreases it. Several meta-analyses on the effects of rTMS and tDCS in depression indicate that presumed brain excitability changes of these two NIBS techniques may effectively reduce depressive symptoms16,17. In addition, a number of studies also explored the potential of rTMS and tDCS in the treatment of PTSD in order to increase the inhibitory control of amygdala activity. Four reviews and meta-analyses investigating the effects of rTMS in PTSD have been published so far. One review and one meta-analysis indicated promising effects of rTMS on PTSD symptom reductions. However, the results remain preliminary, as these two studies only included three and five randomized controlled trials (RCTs), respectively18,19. A more recent meta-analysis including nine original studies (six RCTs) demonstrated positive effects of rTMS on PTSD with an effect size of −0.8820. Another meta-analysis including 11 RCTs suggested that LF rTMS could reduce overall PTSD and depression symptoms21. In addition, several studies also applied tDCS on the DLPFC in order to alleviate mood symptoms in people with PTSD with promising effects22,23. However, no meta-analysis has been conducted so far to summarize these effects. Therefore, there is still limited meta-analytic research investigating different rTMS and tDCS protocols on core symptoms of PTSD, as well as the relationship between stimulation parameters and effect sizes. Thus, the aim of the current study was to (1) summarize existing evidence on the therapeutic effects of rTMS and tDCS on core symptoms of PTSD using meta-analysis and to (2) probe the association between different stimulation parameters and effect sizes using meta-regression.
Methods
Data source and literature search
The present review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)24. Four English bibliographic databases including PubMed, PsycINFO, Web of Science and EMBASE and four Chinese databases including the Chinese National Knowledge Infrastructure (CNKI), WeiPu, WanFang and the Chinese Biomedical Literature Database (CBM), were systematically searched for articles published from 01 January 2000 to 01 January 2020 using the key words “Repetitive Transcranial Magnetic Stimulation” OR “rTMS” OR “Theta-burst stimulation” OR “TBS” OR “Transcranial direct current stimulation” OR “tDCS”) AND (“Posttraumatic stress disorder” OR “PTSD”). In addition, we manually screened reference lists of related published reviews and meta-analyses for additional relevant studies. Two authors (RLDK and BBBZ) independently identified potential studies by reading the title and abstract, and any disagreement was settled through discussion with the third author (JJQZ).
Inclusion and exclusion criteria
We included RCTs published in English and Chinese language with patients having a diagnosis of PTSD according to standard operationalized diagnostic criteria. We included studies comparing any form of rTMS or patterned TMS or tDCS with sham stimulation or no intervention in the treatment of PTSD. We excluded studies published as conference abstracts without full text, as well as book chapters and dissertations. Poor quality RCTs (PEDro<6) were also excluded25.
Quality assessment and data extraction
After identifying potentially eligible studies, full texts were retrieved and two authors (RLDK and BBBZ) extracted the relevant information and assessed the quality of each article independently. Any disagreements were resolved by consultation with the third author (JJQZ). The Physiotherapy Evidence Database (PEDro) Scale was used to assess the quality of included RCTs. Extracted information included: the study design; diagnosis and group membership of participants; stimulation details; main outcomes and assessment time points.
Statistical analysis
All statistical analyses were conducted using the software package Comprehensive Meta-analysis version 3.0 for Windows. For articles reporting incomplete data, corresponding authors were contacted by email. The formula SD = SEM × √n (n = sample size) was used for conversion of standard errors of the mean (SEM) into standard deviations (SD). GetData Graph digitizer 2.26 (http://www.webcitation.org/77dui8IFb) was used to extract data that were reported as a graph only26.
Individual study effect estimates
PTSD symptoms in individual trials were measured using standardized rating scales, including the Clinician-Administered PTSD Scale (CAPS), an observer-rating scale, and the self-report scale PTSD Checklist (PCL)27,28. High convergent validity of the CAPS and PCL scales has been demonstrated29. The individual effect sizes were estimated using absolute change scores (i.e., post- minus pre-stimulation scores) to correct for baseline differences between groups. The standardized mean difference (Hedges’ g) and 95% confidence intervals (CIs) comparing subjects with and without NIBS was computed for each trial. Hedges’ g is a variation of Cohen’s d, which corrects for possible bias of small sample sizes30.
Summary effect estimates
Random-effects meta-analysis was performed given the clinical and methodological diversity among included trials. Heterogeneity among the included studies was assessed using Higgins’I2 statistic31,32. Meta-regression was used to test the relationship between protocol type, dose and effect size. Subgroup analysis was used to explore the effects of different rTMS protocols (i.e. targets, frequencies, TMS as monotherapy or as augmentation treatment) on PTSD symptom reductions. Sensitivity analysis was performed using the leave-one-out method in case of significant results. Publication bias was assessed by visual inspection (funnel plot) and Egger’s test in case of more than ten articles33,34. The statistical threshold was set at p < 0.05 and p < 0.1 (two-tailed) for the main tests and for the Egger’s test, respectively.
Results
Literature search results and characteristics of included studies
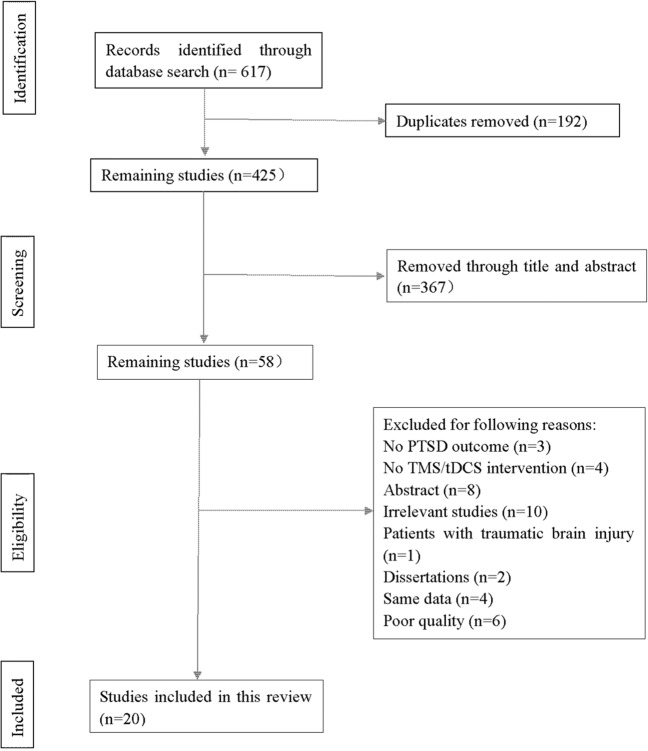
Our search strategy yielded 617 results in total. After the removal of duplicates, 425 articles were identified, whereas 58 studies remained after reading titles and abstracts. Studies were further excluded because they were irrelevant to the topic (ten studies), they were only published as abstracts (eight studies), they did not include rTMS or tDCS as intervention (four studies), they did not report PTSD symptoms as outcome measure (three studies), they included participants with traumatic brain injury (one study), they were published as dissertations (two studies), or they included the same data (four studies). Moreover, six studies were excluded given their poor quality (a PEDro score < 6). The remaining 20 studies were included in the present review. 15 of them were RCTs while five were single group studies and were therefore not included in the quantitative analysis (see study flow chart in Fig. 1).
Fig. 1. PRISMA flowchart.
Process of literature search.
Table 1 shows the characteristics of included studies. Nine studies investigated PTSD symptoms in veterans23,26,35–41. Seven studies reported comorbidity with MDD in all subjects8,26,39–43, while other studies mentioned comorbid anxiety or panic in some subjects. Five studies used a minimum PCL score as inclusion criterion, ranging from 33 to 5022,35,38,41,44. Stimulation intensity of rTMS studies varied between 80% and 120% of the resting motor threshold (RMT). In addition, two studies investigated tDCS as intervention22,23.
Table 1.
Study characteristics of included studies.
| Study | Study design | Subjects | Group location | Stimulation type | Target | Total dose | Intensity | Pulse per session | Sham method | Main outcomes | Assessment time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadizadeh et al.35 | RCT | PTSD |
B-rTMS = 19 R-rTMS = 19 Sham = 20 |
rTMS/20 Hz | RDLPFC and BDLPFC |
10 sessions 24000 pulses |
100% RMT | 1200 | Sham coil | PCL-M |
Baseline Session5 Session 10 |
| Boggio et al.45 | RCT | PTSD |
RDLPFC = 10 LDLPFC = 10 Sham = 10 |
rTMS/20 Hz | RDLPFC and LDLPFC |
10 sessions 16000 pulses |
80%RMT | 1600 | Sham coil |
PCL-5 Treatment Outcome PTSD Scale HARS HDRS |
Baseline Session5 Session 10 2 weeks FU 4 weeks FU 8 weeks FU 12 weeks FU |
| Carpenter et al.42 | Open-label | PTSD and MDD | N = 35 | rTMS/5 Hz | LPFC |
40 sessions 3000 pulses |
120%RMT | 3000 | N/A |
PCL-5 IDS-SR PHQ-9 DASS PSS CGI-S PGI-S |
Pre Post |
| Cohen et al.46 | RCT | PTSD |
10Hz-rTMS = 10 1Hz-rTMS = 8 sham rTMS = 6 |
rTMS/1 Hz and 10 Hz | RDLPFC |
10 sessions 200 min |
80% RMT | / | Coil rotation (vertical to RDLPFC) |
PCL-5 Treatment Outcome PTSD Scale HARS HDRS CAPS |
Baseline 5 session 10 session 2 weeks FU 14 days |
| Fryml et al.36 | RCT | PTSD |
EG = 5 CG = 3 |
rTMS/10 Hz | RDLPFC + LDLPFC |
8 sessions 48000 pulses |
120% RMT | 6000 | Sham stimulation |
CAPS Ham-D Ham-A PTSD Checklist |
Pre post |
| Isserles et al.47 | RCT | PTSD | 9:9:8 | dTMS/20 Hz | Bilateral MPFC |
12 sessions 20160 pulses |
120% RMT | 1680 | Sham coil |
CAPS PSS-SR HDRS-24 BDI |
Pre post 2 weeks FU 2 months FU |
| Kozel et al.37 | RCT | PTSD |
EG (rTMS+CPT) = 54 CG (sham rTMS + CPT) = 49 |
rTMS/1 Hz | RDLPFC |
12 sessions 21600 pulses |
110% RMT | 1800 | Sham coil |
CAPS PCL M-PTSD QIDS-SR |
Baseline Session 5 Session 9 1 month FU 3 months FU 6 months FU |
| Kozel et al.38 | RCT | PTSD |
1Hz-rTMS =17 10Hz-rTMS = 18 |
rTMS/1 Hz and 10 Hz | RDLPFC |
36 sessions 86400 pulses |
110% RMT | 2400 | / |
PCL-5 CAPS QIDS-SR MADRS Pain Score NSI |
Pre Post 1 month FU 3 months FU |
| Nam et al.49 | RCT | PTSD |
EG = 7 CG = 9 |
rTMS/1 Hz | RDLPFC |
15 sessions 18600 pulses |
100%MT | 1200 | Coil rotation (vertical to RDLPFC) | CAPS |
Pre Session 10 1 week FU 5 weeks FU |
| Osuch et al.50 | RCT (cross over) | PTSD |
Active rTMS = 5 Sham rTMS = 5 |
rTMS/1 Hz | RDLPFC |
20 sessions 36000 pulses |
100%MT | 1800 | Coil rotation 45°to head) |
CAPS HDRS IES |
Pre Post |
| Oznur et al.39 | Open-label | PTSD | N = 20 | TMS/1 Hz | RDLPFC | 6 | 80% | 600 | / |
BDI BAI IES |
/ |
| Philip et al.43 | Open-label | PTSD and MDD | N = 10 | rTMS/5 Hz | LDLPFC |
30 + 6 sessions 10800 pulses |
120%RMT | 3000 | / |
PCL QIDS |
Pre Session 5 Session 10 Session 15 Session 20 Session 25 Post |
| Philip et al.8 | Open-label | PTSD and MDD | N = 31 | rTMS/5 Hz | LDLPFC | 40 | / | 3000-4000 | / |
PCL IDS-SR MRI |
Pre Post |
| Philip et al.40 | RCT | PTSD and MDD |
EG = 25 CG = 25 |
iTBS | RDLPFC |
10 sessions 18000 pulses |
80%AMT | 1800 | / |
CAPS Social and Occupational Functioning Assessment Scale QOL PCL IDS-SR |
Pre Post 1 month FU |
| Philip et al.41 | RCT | PTSD and MDD |
EG = 25 CG = 25 |
sTMS | / | 10 sessions | / | / | / |
PCL-5 PTSD threshold symptoms QIDS-SR |
Pre Post |
| Rosenberg et al.26 | RCT | PTSD and MDD |
10 Hz = 6 1 Hz = 6 |
TMS/1 Hz and 5 Hz | LDLPFC | 10 sessions | 90%RMT | 600 | / |
SCID-C Ham-D USC-REMT MSCS POMS |
Pre Post 1 month FU 2 months FU |
| Watts et al.44 | RCT | PTSD and MDD |
EG = 10 CG = 10 |
rTMS/1 Hz | RDLPFC | 10 sessions | 90%RMT | / | Sham coil |
CAPS PCL BDI STAI BNCE |
Pre Post 1 month FU 2 months FU |
| Woodside et al.48 | open-lable | PTSD and eating disorder | N = 14 | rTMS (10 Hz and 20 Hz) and iTBS | DMPFC | 20–30 sessions | 120%RMT | 3000 and 1500 | / |
PCL-C DERS |
Pre Post |
| Ahmadizadeh et al.22 | RCT | PTSD |
EG = 18 CG = 16 |
Anodal tDCS | RDLPFC and LDLPFC | 10 sessions | 2 mA | / | / |
PCL-5 BDI-II BAI |
Pre Post 1 month FU |
| Wout et al.23 | RCT | PTSD |
EG = 6 CG = 6 |
tDCS | VMPFC | 6 sessions | 2 mA | / | / |
SCR PCL-5 |
Pre Post 1 month FU |
RCT randomized controlled trial, B-rTMS bilateral repetitive transcranial magnetic stimulation, R-rTMS right repetitive transcranial magnetic stimulation, RDLPFC right dorsolateral prefrontal cortex, BDLPFC bilateral dorsolateral prefrontal cortex, RMT resting moter threshold, PCL-M PTSD checklist military version, LDLPFC left dorsolateral prefrontal cortex, PCL-5 PTSD checklist for DSM-5, HARS Hamilton Anxiety Rating Scale, HDRS Hamilton Depression Rating Scale, FU follow-up, MDD major depression disorder, LPFC left prefrontal cortex, IDS-SR Inventory of Depressive Symptomatology, PHQ-9 Patient Health Questionnaire, DASS Depression Anxiety Stress Scale, PS: Perceived Stress Scale, CGI-S global illness severity, PGI-S patient self-rated version, CAPS Clinician-Administered PTSD Scale-II, EG experiment group, CG control group, Ham-D Hamilton Rating Scale for Depression, Ham-A Hamilton Rating Scale for Anxiety, MPFC medial prefrontal cortex, PSS-SR PTSD symptom scale-self report version, HDRS-24 Hamilton Depression Rating Scale 24 items, BDI Beck Depression Inventory II, CPT cognitive processing therapy, PCL PTSD check list, M-PTSD Mississippi Scale for Combat Related PTSD, QIDS-SR quick inventory of depressive symptomatology-self report Version, MADRS Montgomery- Asberg Depression Rating Scale, NSI neurobehavioral symptom inventory, BAI The beck anxiety inventory, self-report, IES Impact of Events Scale, MRI magnetic resonance imaging, QOL quality of life, SCID-C Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version, USC-REMT University of Southern California Repeatable Episodic Memory Test, MSCS Mississippi Scale of Combat Severity, POMS Profile of Mood States Subscales, STAI The State Trait Anxiety Inventory, BNCE The Brief Neurobehavioral Cognitive Examination, DERS Difficulties in Emotional Regulation Scale, VMPFC ventromedial prefrontal cortex, BDI-II The Beck depression inventory-II, SCR skin conductance reactivity.
Quality assessment of included studies
The results of the quality assessment for the 15 RCTs are presented in the Supplementary Table S1. Eleven of them had a score of 8 on the PEDro scale, three studies had a score of 7 and one a score of 6.
Meta-analysis results of RCTs
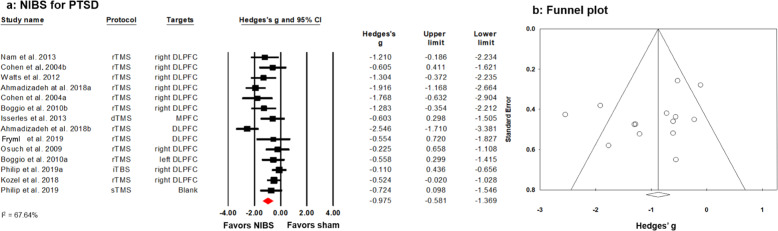
Of the 15 RCTs (out of 20 studies included in our review), three RCTs included two separate subgroups35,45,46. However, two RCTs did not include a sham stimulation condition26,38, and studies investigating tDCS for PTSD were also excluded from the meta-analysis due to their low number (two studies) and large heterogeneity of the stimulation protocol22,23. Hence, 14 separate datasets of 11 RCTs including a total of 359 patients were subjected to cumulative meta-analysis. Results revealed that rTMS is an effective treatment to reduce core symptoms of PTSD with a large effect size (Hedges’ g = −0.975) and moderate heterogeneity of individual study estimates (I2 = 67.64%) (see Fig. 2a). The Funnel plot showed no publication bias (see Fig. 2b), which was confirmed by a non-significant Egger’s test (p = 0.180. However, meta-regression did not determine a significant dose effect as tapped by the number of sessions or total pulses. Furthermore, there were no significant differences between stimulation targets or stimulation frequencies on PTSD symptom reductions (all p > 0.05). Nevertheless, given the presumed variance on neural excitability, we performed exploratory post-hoc meta-analyses separately for target sites and stimulation frequencies.
Fig. 2. Effects of NIBS in PTSD.
a Forest plot depicting studies comparing active with sham stimulation, summarizing to an effect size of −0.975. b The corresponding funnel plot comparing active with sham stimulation shows no publication bias; the Egger’s test is non-significant (p = 0.180).
Classification by protocol
Excitatory stimulation protocols
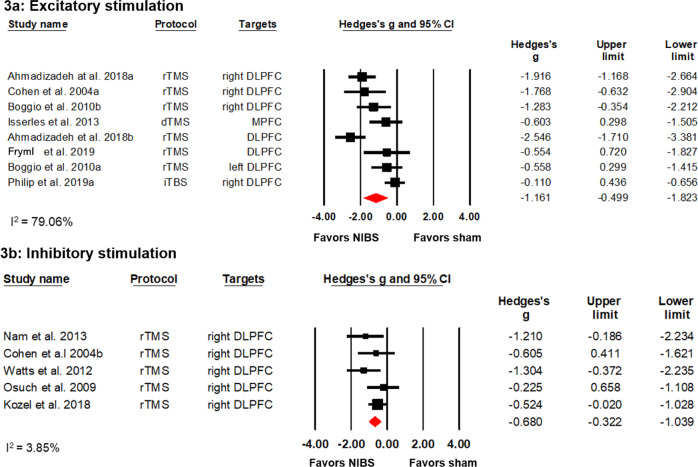
Eight datasets (six studies) investigated the effects of excitatory stimulation on PTSD symptom reductions with 107 patients in the experimental and 103 patients in the control group. Six RCTs investigated HF rTMS35,36,40,45–47, whereas two studies explored the effects of dTMS and iTBS, respectively40,47. Despite a high heterogeneity of individual study estimates (I2 = 79.06%), the meta-analysis revealed a significant symptom reducing effect with a large effect size (Hedges’ g = −1.161) (see Fig. 3a). This result was robust to leave-one-out sensitivity analysis (Hedges’ g from −1.308 to −0.528).
Fig. 3. Effects of excitatory and inhibitory stimulation protocols in PTSD.
a A forest plot showing studies that compared excitatory stimulation with sham stimulation. b A forest plot showing studies comparing inhibitory stimulation with sham stimulation.
Post-hoc analysis was conducted to investigate the effect of different stimulation targets. Four studies used HF rTMS of the right DLPFC35,40,45,46. In spite of a high heterogeneity of effect estimates (I2 = 83.32%), meta-analysis detected a significant positive effect with a large effect size (Hedges’ g = −1.225). Two studies applying high frequency stimulation on bilateral DLPFC found no significant positive effect. One study explored the effects of high frequency stimulation of the MPFC47 indicating no significant effect (p = 0.19), while one open-label study suggested that HF rTMS of the DLPFC may be effective to reduce PTSD symptoms48. Four studies explored the effects of excitatory rTMS on the left DLPFC8,42,43,45 but no meta-analysis was conducted since three of them were non-RCTs8,42,43. However, three of them showed a large8,42,43 and one a medium effect size45 in favor of active stimulation.
Inhibitory stimulation protocols
Five studies with a total of 84 patients in the experimental group and 79 patients in the sham stimulation group investigated the effects of inhibitory stimulation on PTSD symptoms and all of them applied LF rTMS on the right DLPFC37,44,46,49,50. Individual effect estimates showed low heterogeneity (I2 = 3.85%). Meta-analysis showed a significant positive effect for active compared to sham or no stimulation with a medium effect size (Hedges’ g = −0.680) see Fig. 3b. This result was robust to leave-one-out sensitivity analysis (Hedges’ g from −1.039 to −0.322). One non-RCT study on the effects of inhibitory rTMS of the right DLPFC, which was not included in our meta-analysis, indicated a positive effect of stimulation on hyperarousal39.
Studies comparing high versus low frequency stimulation protocols
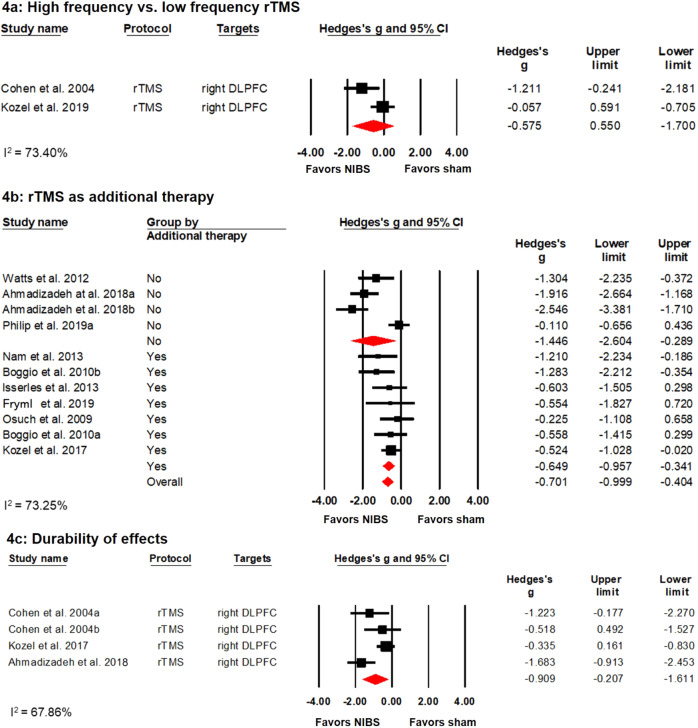
In addition, three RCTs investigated the difference between high and low frequency rTMS of the right DLPFC26,38,46 with a total of 34 patients in the high frequency stimulation group and 31 in the low frequency group. However, only two studies38,46 were suitable for meta-analysis. Studies showed that both high and low frequency rTMS led to significant symptom improvements, while no significant difference was found between the two modes of stimulation, see Fig. 4a).
Fig. 4. Effects of different NIBS protocols in PTSD.
a Forest plot showing studies that compared high frequency with low frequency rTMS. b Forest plot showing studies using rTMS as an augmentation therapy or not. c Forest plot showing studies that investigated the effects of NIBS during follow-up examinations.
rTMS as an augmentation therapy
Seven datasets from six studies investigated the augmentation effects of rTMS. That is, patients in these studies were allowed to maintain their current psychopharmacological and/or psychotherapeutic treatment during the study period36,37,45,47,49,50. Conversely, four datasets from three studies investigated rTMS as monotherapy35,41,44. Meta-regression revealed no significant difference (p = 0.149) between studies investigating rTMS as mono- or as augmentation therapy. Separate meta-analysis for the two groups indicated that rTMS as well as augmentation therapy showed significant positive effects with a medium (Hedges’ g = −0.649) and large (Hedges’ g = −1.446) effect size, respectively. (see Fig. 4b).
Follow-up
Nine studies explored the durability of NIBS on PTSD, two of them investigating tDCS and the rest applying rTMS stimulation. However, only four data sets (three studies) were suitable for meta-analysis22,37,46. Follow-up assessments ranged from two to four weeks. Results indicated durability of effects with large effect size (−0.909) and moderate heterogeneity (I2 = 67.86%) (see Fig. 4c). This result was robust to leave-one-out sensitivity analysis (Hedges’ g from −1.611 to −0.207).
Discussion
This systematic review including 20 studies, of which 14 datasets were subjected to meta-analysis, revealed significant positive effects of rTMS on the reduction of core PTSD symptoms in patients with PTSD. Subgroup analysis revealed that HF as well as LF rTMS of the right DLPFC is an effective treatment for PTSD with potential durability. Moreover, rTMS seems to be effective as an augmentation treatment for military-related PTSD. However, no dose dependency was revealed in our meta-regression. No definite conclusion could be reached regarding the effects of rTMS on the left DLPFC or MPFC. This may be due to the limited sample size of included studies. The two studies investigating the effects of active compared to sham tDCS both suggested a significant reduction in PTSD symptom with the anode and cathode being placed over the left and the right DLPFC, respectively22,23. However, more studies are needed in the future for further quantitative analysis.
Previous evidence showed that activation of the right hemisphere is associated with anxious arousal and symptoms of PTSD during the processing of trauma-specific information51. For example, a study measuring regional cerebral blood flow indicated increased blood flow in the right compared to the left hemisphere upon auditory recall of the traumatic event52. Our review demonstrates that rTMS targeting the right DLPFC in people with PTSD shows positive effects, which is consistent with previous reviews18,20 and in line with a stress modulating effect of right-hemispheric DLPFC stimulation. Interestingly, however, our review indicates that both HF and LF rTMS exerts positive, PTSD symptom reducing effects. A possible reason might lie in the variety of core symptoms of PTSD. Different neural networks and their activity imbalances may underlie the four symptom clusters mentioned in the introduction21. More specifically, alterations within and between networks including the default mode network (DMN), the salience network (SN) and the central executive network (CEN), have been associated with PTSD53. Reduced functional connectivity within the DMN has been consistently observed and a disorganization between regions belonging to the DMN has been related to the consolidation of trauma-related memories and the preparation for avoidance of trauma reminders. On the other hand, functional connectivity within the SN seems to be increased and a relative SN predominance over DMN has been proposed53. Indeed, increased connectivity between DMN and regions belonging to SN and CEN, especially between amygdala and hippocampus, and a decreased connectivity between amygdala and medial prefrontal cortex, were shown to be related to memory intrusion and the re-experiencing of traumatic events53,54. The reduced functional connection between the latter two regions has also been linked to excessive fear53, whereas a hyperactivation of the right prefrontal cortex and insula, as well as a general neural sensitization has been related to hyperarousal21,55. Therefore, it is possible that by influencing different neural networks and associated symptom clusters, both the excitatory and inhibitory stimulation would result in an overall positive effect. This is in line with studies comparing excitatory and inhibitory DLPFC stimulation directly26,38,46. Although one study suggested that high-frequency stimulation is superior over low-frequency stimulation, no such effects were found when combining all three studies in our meta-analysis. However, in order to keep side effects such as headache at a minimum, which tend to be stronger for excitatory compared to inhibitory stimulation, clinicians may opt for LF rTMS of the right DLPFC for clinical practice. In any case, well-powered future studies investigating the effects of different rTMS protocols on different symptom clusters in PTSD are needed for definitive answers.
Moreover, several lines of evidence indicate that right stimulation is related to greater improvements in core PTSD symptoms, while left stimulation leads to improvements in mood but only to modest improvements in core trauma symptoms21,26. This is consistent with the notion that PTSD is associated with a right-sided pathology and concurs with a study by Cirillo et al., demonstrating the superiority of right prefrontal rTMS to reduce anxiety and PTSD symptoms20. The relative severity of symptoms in patients with comorbid PTSD and MDD should therefore determine the decision for applying a left or right stimulation protocol.
In addition, our sub-group analysis examining the augmentation effects of rTMS showed that both mono-, as well as augmentation therapy yielded a significant positive effect, although effect sizes were smaller for augmentation therapy when compared with a control group. This might be due to patients in the control group benefiting from psychopharmacological and/or psychotherapeutic treatment.
Our analysis of studies investigating the durability at follow-up visits indicates positive treatment effects with a durability of at least two to four weeks. This concurs with a recent study not included in our meta-analysis, which explored the long-term effects of iTBS for PTSD56. Authors found a clinically meaningful improvement of PTSD symptoms upon iTBS even after a year of treatment. Hence, brain stimulation seems to be a potential alternative approach for the treatment of PTSD, given that two-thirds of patients continue to meet full criteria of PTSD after pharmacological and psychotherapeutic interventions57.
Limitation
There are several limitations in our review. First, we used two different outcome measures in our meta-analysis, the CAPS and the PCL, which is an observer rating and a self-report scale, respectively. Although we computed the standardized mean difference for each outcome, the choice of a specific rating scale may be confounded by the application of a specific stimulation protocol. This was, however, not systematically evaluated in our study. Second, only the total score of CAPS or PCL scales was used for analysis in our review. Hence, a more detailed evaluation of the effects of NIBS on the four main symptom clusters of PTSD in relation to the function of different brain areas awaits to be determined. Third, patient-specific features such as treatment resistance to other therapies may have affected our results. For example, five studies included patients showing a lack of response to an antidepressant medication and/or trauma-focused psychotherapy22,42,44,47,50. Future reviews with a sufficient number of studies should investigate this systematically.
Conclusion
High- as well as low-frequency rTMS of the right DLPFC appears to significantly reduce core PTSD symptoms in patients with PTSD. rTMS may therefore be a promising alternative or add-on treatment for PTSD patients who show limited response to antidepressant medication and/or trauma-focused psychotherapy. More high-quality studies are necessary to explore the effects of NIBS on different symptom clusters in PTSD.
Supplementary information
Acknowledgements
This research was supported by a Grant from the Hong Kong Research Grants Council (25100219) to Georg S. Kranz.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rebecca L. D. Kan, Bella B. B. Zhang
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-0851-5).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. https://www.psychiatry.org/psychiatrists/practice/dsm.
- 2.Martin P. The epidemiology of anxiety disorders: a review. Dialogues clin. Neurosci. 2003;5:281. doi: 10.31887/DCNS.2003.5.3/pmartin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morina N, Stam K, Pollet TV, Priebe S. Prevalence of depression and posttraumatic stress disorder in adult civilian survivors of war who stay in war-afflicted regions. A systematic review and meta-analysis of epidemiological studies. J. Affect Disord. 2018;239:328–338. doi: 10.1016/j.jad.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Bryant RA, et al. Acute and chronic posttraumatic stress symptoms in the emergendce of posttraumatic stress disorder a network analysis. JAMA Psychiatry. 2017;74:135–142. doi: 10.1001/jamapsychiatry.2016.3470. [DOI] [PubMed] [Google Scholar]
- 5.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015;17:141. doi: 10.31887/DCNS.2015.17.2/jflory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichter B, Haller M, Norman S, Pietrzak RH. Risk and protective factors associated with comorbid PTSD and depression in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. J. Psychiatr. Res. 2020;121:56–61. doi: 10.1016/j.jpsychires.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee DJ, et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analysis to determine first-line treatments. Depress Anxiety. 2016;33:792–806. doi: 10.1002/da.22511. [DOI] [PubMed] [Google Scholar]
- 8.Philip N, et al. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress and major depressive disorders. Biol. Psychiatry. 2018;81:S42–S43. doi: 10.1016/j.biopsych.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quirk GJ, Garcia R, González-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf EJ, et al. Posttraumatic stress disorder as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biol. Psychiatry. 2016;80:363–371. doi: 10.1016/j.biopsych.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George MS, et al. Mechanisms and state of the art of transcranial magnetic stimulation. J. ECT. 2002;18:170–181. doi: 10.1097/00124509-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci. Biobehav Rev. 2015;57:46–62. doi: 10.1016/j.neubiorev.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Cao, X., Deng, C., Su, X. & Guo, Y. Response and remission rates following high-frequency vs. low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (mdd): a meta-analysis of randomized, double-blind trials. Front. Psychiatry10.3389/fpsyt.2018.00413 (2018). [DOI] [PMC free article] [PubMed]
- 18.Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014;7:151–157. doi: 10.1016/j.brs.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Trevizol AP, et al. Transcranial magnetic stimulation for posttraumatic stress disorder: an updated systematic review and meta-analysis. Trends Psychiatry Psychother. 2016;38:50–55. doi: 10.1590/2237-6089-2015-0072. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo P, et al. Transcranial magnetic stimulation in anxiety and trauma‐related disorders: a systematic review and meta‐analysis. Brain Behav. 2019;9:e01284. doi: 10.1002/brb3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan T, Xie Q, Zheng Z, Zou K, Wang L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): A systematic review and meta-analysis. J. Psychiatr. Res. 2017;89:125–135. doi: 10.1016/j.jpsychires.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadizadeh MJ, Rezaei M, Fitzgerald PB. Transcranial direct current stimulation (tDCS) for post-traumatic stress disorder (PTSD): A randomized, double-blinded, controlled trial. Brain Res Bull. 2019;153:273–278. doi: 10.1016/j.brainresbull.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Mascha van ‘t Wout-Frank M, Shea MT, Larson VC, Greenberg BD, Philip NS. Combined transcranial direct current stimulation with virtual reality exposure for posttraumatic stress disorder: Feasibility and pilot results. Brain Stimul. 2019;12:41–43. doi: 10.1016/j.brs.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamseer, L. et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ354, i4086 (2016). [DOI] [PubMed]
- 25.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 26.Rosenberg PB, et al. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J. Neuropsychiatry Clin. Neurosci. 2002;14:270–276. doi: 10.1176/jnp.14.3.270. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behav. Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 28.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol. Assess. 1999;11:124–133. [Google Scholar]
- 29.Weathers FW, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30:383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins, J. P. T, & Green, S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011] (Cochrane Collaboration, 2011).
- 31.Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res. Synth. Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JAC, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 35.Ahmadizadeh M-J, Rezaei M. Unilateral right and bilateral dorsolateral prefrontal cortex transcranial magnetic stimulation in treatment post-traumatic stress disorder: a randomized controlled study. Brain Res. Bull. 2018;140:334–340. doi: 10.1016/j.brainresbull.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Fryml L, et al. Exposure therapy and simultaneous repetitive transcranial magnetic stimulation: a controlled pilot trial for the treatment of posttraumatic stress disorder. J. ECT. 2019;35:1. doi: 10.1097/YCT.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 37.Kozel FA, et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: a randomized clinical trial. J. Affect Disord. 2018;229:506–514. doi: 10.1016/j.jad.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 38.Kozel FA, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273:153–162. doi: 10.1016/j.psychres.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Oznur T, et al. Is transcranial magnetic stimulation effective in treatment-resistant combat related posttraumatic stress disorder? Neurosciences. 2014;19:29–32. [PubMed] [Google Scholar]
- 40.Philip NS, et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am. J. Psychiatry. 2019;176:939–948. doi: 10.1176/appi.ajp.2019.18101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philip N, et al. Synchronized transcranial magnetic stimulation for posttraumatic stress disorder and comorbid major depression. Brain Stimul. 2019;12:1335–1337. doi: 10.1016/j.brs.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter LL, et al. 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J. Affect Disord. 2018;235:414–420. doi: 10.1016/j.jad.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Philip NS, Ridout SJ, Albright SE, Sanchez G, Carpenter LL. 5-Hz Transcranial magnetic stimulation for comorbid posttraumatic stress disorder and major depression. J. Trauma Stress. 2016;29:93–96. doi: 10.1002/jts.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5:38–43. doi: 10.1016/j.brs.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Boggio PS, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen H, et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am. J. Psychiatry. 2004;161:515–524. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- 47.Isserles M, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder – a pilot study. Brain Stimul. 2013;6:377–383. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Woodside D. Blake, Colton Patricia, Lam Eileen, Dunlop Katharine, Rzeszutek Julia, Downar Jonathan. Dorsomedial prefrontal cortex repetitive transcranial magnetic stimulation treatment of posttraumatic stress disorder in eating disorders: An open-label case series. International Journal of Eating Disorders. 2017;50(10):1231–1234. doi: 10.1002/eat.22764. [DOI] [PubMed] [Google Scholar]
- 49.Nam D-H, Pae C-U, Chae J-H. Low-frequency, repetitive transcranial magnetic stimulation for the treatment of patients with posttraumatic stress disorder: a double-blind, sham-controlled study. Clin. Psychopharmacol. Neurosci. 2013;11:96–102. doi: 10.9758/cpn.2013.11.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osuch EA, et al. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J. Anxiety Disord. 2009;23:54–59. doi: 10.1016/j.janxdis.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rabe S, Beauducel A, Zollner T, Maercker A, Karl A. Regional brain electrical activity in posttraumatic stress disorder after motor vehicle accident. J. Abnorm Psychol. 2006;115:12. doi: 10.1037/0021-843X.115.4.687. [DOI] [PubMed] [Google Scholar]
- 52.Pagani M, et al. Regional cerebral blood flow during auditory recall in 47 subjects exposed to assaultive and non-ssaultive trauma and developing or not posttraumatic stress disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255:359–365. doi: 10.1007/s00406-005-0559-9. [DOI] [PubMed] [Google Scholar]
- 53.Kunimatsu, A., Yasaka, K., Akai, H., Kunimatsu, N. & Abe, O. MRI findings in posttraumatic stress disorder. J. Magn. Reson. Imaging10.1002/jmri.26929 (2019). [DOI] [PubMed]
- 54.Viard A, et al. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage. Clin. 2019;22:101731. doi: 10.1016/j.nicl.2019.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 56.Petrosino Nicholas J., Wout-Frank Mascha van ’t, Aiken Emily, Swearingen Hannah R., Barredo Jennifer, Zandvakili Amin, Philip Noah S. One-year clinical outcomes following theta burst stimulation for post-traumatic stress disorder. Neuropsychopharmacology. 2019;45(6):940–946. doi: 10.1038/s41386-019-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314:489–500. doi: 10.1001/jama.2015.8370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.