Abstract
Purpose of review
This review discusses the mortality and morbidity of hypertensive disorders of pregnancy (HDP) and the current diagnostic thresholds. It then explores measurement of variability in blood pressure (BP) during pregnancy as an opportunity to identify women at high risk of cardiovascular disease (CVD) later in life.
Recent findings
HDP is known to be associated with increased risk of long-term CVD. Current CVD prognostic tools do not incorporate a history of HDP given a lack of improved risk discrimination. However, HDP diagnostic criteria are currently based on a binary threshold, and there is some evidence for the use of variability in BP throughout gestation as a marker of CVD risk.
Summary
HDP increases long-term risk of CVD. Future studies investigating changes in diagnostic criteria, including the use of BP variability, may improve long-term CVD risk prediction and be incorporated into future risk assessment tools.
Keywords: Hypertension, Pregnancy, Blood Pressure, Cardiovascular Disease Risk, Variability
Introduction
The World Health Organization (WHO) estimates that 10% of pregnant women worldwide are affected by hypertensive disorders of pregnancy (HDP) [1]. The classification includes gestational hypertension, chronic hypertension, pre-eclampsia (PE) and chronic hypertension with superimposed PE, all of which are associated with a range of perinatal outcomes, as well as short- and long-term, maternal morbidity and mortality rates.
Over the past 20 years, the linear association between blood pressure (BP) and cardiovascular disease (CVD) risk has become increasingly apparent, [2–5] leading to a lowering of BP targets at a population level in most treatment guidelines. However, both the diagnostic and treatment thresholds for HDP remain higher than those for hypertension in the general population, and HDP is still regarded as a dichotomous trait. Given the current approach, it is likely that women who develop HDP are identified late or not at all; therefore, opportunities to minimize the risk of HDP to both mother and baby in the short- and long-term may be missed.
This report reviews: a) the associated mortality and morbidity of HDP, including the increased risk of CVD in later life and b) the current diagnostic thresholds for HDP. We then discuss risk assessment for CVD before proposing BP variability in pregnancy as a mechanism to identify at-risk women. Quantifying BP variability throughout gestation is an approach to diagnosing HDP that may capture additional women at risk of CVD, which would facilitate earlier identification, monitoring and appropriate treatment.
Mortality and morbidity of HDP
HDP is one of the major causes of global maternal mortality, accounting for approximately 30,000 maternal deaths annually [6]. However, in high-income countries (HIC), mortality from hypertension in pregnancy has in fact been declining as a result of improved prevention, diagnosis and treatment [7].
Despite the overall decline in maternal mortality, the incidence of HDP is increasing [8] mainly due to changes in maternal health characteristics associated with the epidemiological transition seen in both developed and emerging economies. These include delayed child-bearing, an increase in pre-existing health problems such as hypertension and obesity, and a rise in assisted conception births [6, 9–11].
Maternal morbidity increases with worsening HDP, as a general rule. Based on the 14 markers (e.g. disseminated intravascular coagulation, eclampsia, acute renal failure, cardiac arrest), which the Centers for Disease Control and Prevention recommend for defining ‘severe maternal morbidity’, severe PE is associated with the highest morbidity followed by uncomplicated PE, and then chronic hypertension [12]. HDP also results in adverse perinatal outcomes, including fetal growth restriction (FGR), preterm birth and small for gestational age (SGA) newborns [13, 14]. There may also be an intergenerational effect in this last group as SGA babies are at increased risk of CVD in later life [15].
Cardiovascular Disease Risk
Women with HDP also have an increased long-term risk of non-communicable diseases (NCDs) including hypertension and CVD [16–19]. CVD is the leading cause of global mortality [20], and the leading cause of mortality for women in the United States, resulting in over 400,000 deaths per year [21].
The precise nature of the relationship between HDP and CVD has yet to be determined. The physiological stress of pregnancy may unmask a woman’s pre-existing predisposition to CVD via metabolic disturbances, inflammation, hypercoagulability and genetic factors. The relationship may also be causal: HDP or PE may induce long-lasting vascular, renal and metabolic changes that increase CVD risk. Alternatively, hypertension in pregnancy may compound the risk of future disease [22]. Regardless of the underlying pathophysiology, the association between HDP and CVD risk is clear [18, 23–27, 17, 28, 29]. This applies particularly to PE, which increases the risk of CVD by approximately two-fold compared to normotensive pregnancies, as shown in several meta-analyses [17, 23–25].
The Cardiovascular Health after Maternal Placental Syndromes (CHAMPS) cohort study, published in 2005, followed over 1 million women free of CVD prior to their first delivery, of whom 75,380 were subsequently diagnosed with a ‘maternal placental syndrome’ (including HDP). The study found women who had gestational hypertension and PE were at a significantly increased risk of long-term CVD, HR 1.8 (1.4 – 2.2) and HR 2.1 (1.8 – 2.4) respectively [27].
Interestingly, the Control of Hypertension in Pregnancy (CHIPs) randomized controlled trial (RCT), published in 2016, found that severe hypertension in pregnancy was associated with adverse maternal and perinatal outcomes regardless of PE occurrence [30]. Studies including women with any HDP compared to those with normotensive pregnancies have also found significant increases in CVD risk. A 2010 study of 4782 women, of whom 13.4% had a history of HDP, reported an 88% increased risk of long-term hypertension, HR 1.88 (1.49 – 2.39), and a 2.1 times increased risk of stroke, HR 2.10 (1.19 – 3.71) [16]. In a subsequent study, the same group found 1.6 greater odds of peripheral arterial disease OR 1.61 (1.04 – 2.49), when controlling for traditional risk factors including hypertension in later life [18].
A more recent Australian retrospective cohort study, published in 2016, included 31,656 women of whom 13.9% had a history of HDP: the odds of CVD mortality were increased by 56%, OR 1.56 (1.28 – 1.89) [28]. The Nord-Trøndelag Health prospective cohort study, published in 2019, further supports these findings. Amongst 23,885 participants, 9.2% had a history of HDP: their risk of CVD was increased by 57%, HR 1.57 (1.32 – 1.87) [29].
Differences in the diagnosis of hypertension and HDP
It is well established that there is generally a direct and continuous association with mean BP and CVD risk. This was initially demonstrated by the Prospective Studies Collaboration that undertook a meta-analysis of individual data including one million adults in 61 prospective studies. BP was directly related to CVD mortality without any evidence of a threshold down to at least 115/75 mmHg [2]. These findings are supported by a large, prospective, observational study undertaken by Rapsomaniki et al. [3] and the more recent SPRINT RCT, published in 2014, that confirmed lower targeted BP control reduces rates of fatal and non-fatal CVD events [4].
As a result of this body of work, there has been a recent trend towards lowering mean BP targets in treatment guidelines. The 2017 American Heart Association (AHA) guidelines reclassified elevated BP as >120/80 mmHg (previously categorized as pre-hypertension), >130–39/>80–89 mmHg as stage 1 hypertension and >140/>90 mmHg as stage II hypertension. Treatment initiation is guided by total CVD risk score assessment, which takes into account BP measures among other risk factors [5]. At present, the European Society of Cardiology (ESC) and Other Societies on CVD Prevention in Clinical Practice Guidelines and the National Institute for Health and Care Excellence (NICE) guidelines in the UK classify a BP of 140 – 159 mmHg systolic and/or 90 – 99 mmHg diastolic as grade I hypertension [31] [32].
Despite the known association between HDP and CVD risk, the threshold for diagnosing hypertension in pregnancy remains higher than that in the general population according to the current AHA guidelines. Mild to moderately elevated BP in pregnancy is defined as systolic BP >140 mmHg and <160 mmHg, or diastolic BP of >90 mmHg and <110 mmHg. Severely elevated BP is defined as >160/110 mmHg [33]. The recently released American College of Obstetricians and Gynecologists (ACOG) guidelines on chronic hypertension in pregnancy do in fact recognize those with previously diagnosed hypertension as per the new AHA criteria. However, they do not recommend making a new diagnosis of chronic hypertension in pregnancy when BP is <140/90 mmHg [31].
Current treatment of HDP
According to the 2019 ACOG guidelines, antihypertensive treatment of BP levels below 160 mmHg systolic is not routinely recommended unless other comorbidities or end-organ damage is present [34]. The 2018 ESC guidelines for the management of CVD during pregnancy recommend initiation of drug treatment for all women with BP persistently >150/95 mmHg or at >140/90 mmHg if gestational hypertension is diagnosed or end-organ damage present [35].
The fact that the American and European definitions and related treatment targets for hypertension in pregnancy have long remained higher than those for the general population is in part due to concerns about the effect of reduced BP on utero-placental blood flow. A metaregression analysis of 45 RCTs, published in 2002 and often cited, demonstrated a 145g decrease in birth weight with a 10 mmHg fall in mean arterial pressure (MAP) [36]. Unfortunately, high-quality evidence of the long-term effects of antihypertensive treatment in pregnancy is scarce: the most recent dates back to 1976 [37]. Interestingly, a 2018 Cochrane systematic review of treatment for mild to moderate hypertension in pregnancy did not find any significant effects on perinatal outcomes including SGA, preterm birth and infant mortality [38].
Although there is little evidence of the effects of drugs on utero-placental blood flow and fetal growth, there is some evidence of short-term benefits of treating mild to moderate hypertension for the mother. The same Cochrane review of hypertension in pregnancy suggested that treatment prevents progression to severe hypertension by 50%, without any effect on the incidence of PE [38]. The large, multinational, CHIPS RCT, published in 2016 by Magee et al. supported these findings [39].
ACOG suggest that the lack of short-term benefits of treatment during pregnancy is enough to justify discontinuation of any pre-pregnancy initiated treatment [34]. However, there is some evidence that stopping the antihypertensive medication of a pregnant woman who has mild or moderate chronic hypertension prior to pregnancy increases the risk of adverse maternal and perinatal outcomes [40].
Based on the summation of this evidence, the recently updated NICE guidelines now recommend initiation of treatment for women in pregnancy with a sustained systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg with a treatment target of 135/85 mmHg [41]. Furthermore, NICE recommend women continue on antihypertensive treatment unless SBP and/or DBP are sustained below <110 mmHg and <70 mmHg respectively, or the women have symptomatic hypertension. We hope these changes will be introduced into other guidelines.
The difference in global recommendations between non-pregnant and pregnant women, and relative lack of consensus, certainly warrants further attention. This is particularly so given that even slightly elevated BP during pregnancy (<120/80 mmHg) has been found to be associated with a 2.6 fold higher risk of chronic hypertension in the decade after delivery compared with women with BP <120 mmHg [42]. Furthermore, there is evidence that recurrent elevations in BP across a women’s reproductive years increases CVD risk [43].
Long-term risk assessment
HDP is recognized as an important set of disorders that can predict a woman’s future risk of CVD. Given this association and indeed the magnitude of global CVD burden, a history of HDP should be included in any medical consultation [44]. Moreover, the period between pregnancy and the development of CVD should be seen as window of opportunity for primary prevention [45]. This is in keeping with a life-course approach to women’s health and is aligned with the Global Strategy for Women’s Children’s and Adolescents’ Health (2016 – 2030) commitment to reduce premature mortality from non-communicable diseases by one third [46].
The importance of identifying women with a history of HDP is recognized by the AHA, American Stroke Association (ASA) and ESC in their recent guidelines [47, 48]. In fact, the ESC and Other Societies on CVD prevention in clinical practice guidelines recommend considering periodic screening for hypertension in women with a history of HDP [31]. The ASA guidelines for the prevention of stroke in women highlight a need for better clinician and patient education about the association between HDP and CVD, citing evidence of a lack of knowledge regarding this association [49].
Furthermore, several studies have called for incorporation of HDP as a risk factor in CVD risk assessment tools [50, 51]. There are a large number of such tools currently available, including the Framingham Risk Score (FRS) [52, 53], ATP III [54], QRISK [55, 56], Reynolds Risk Score (RRS) [57, 58], EUROSCORE [59], PROCAM [60], ASSIGN – SCORE [61], CUORE [62], Globorisk [63], and the Pooled Cohort Risk Equations (PCEs) used in the Atherosclerotic Cardiovascular Disease (ASCVD) Risk Calculator [64]. The most recent is the WHO revised models of CVD risk charts [65]. All of these tools utilize age, HDL and SBP as components of the assessment. Gender, total cholesterol, LDL, CRP, BP treatment, diabetes, HbA1c, family history, body mass index, social factors, ethnicity, deprivation and chronic disease are assessed in various combinations across the tools [66, 35]. Many of these additional variables do not seem to help discriminate when assessing 10-year risk of CVD. A meta-analysis of 86 prospective studies found no differences between SCORE, FRS, RRS, and PCE predictions when recalibrated for the populations in the study [67].
Notably, none of these risk assessment tools take into account a history of HDP, despite the known and significant association. However, research to date suggests that the inclusion of HDP does not significantly improve discrimination in 10-year CVD prediction [68–72]. The Markovitz et al. study did show small improvements in CVD prediction by including all pregnancy complications, but the differences were thought unlikely to be clinically meaningful [70]. These studies were however largely limited to older white women and relied on maternal recall of HDP. Therefore, populations who experience higher rates of pregnancy complications were underrepresented and the association between HDP and CVD risk was more likely to have been mediated by traditional CVD risk factors [73].
It is worth highlighting that the aforementioned risk assessment tools all predict 10-year risk, with the exception of the PCEs which also calculates life-long risk based on gender-specific categorization. A 10-year risk assessment likely underestimates the need for preventative strategies in women who tend to live longer and experience CVD later in life, approximately 10 years after men [74, 75]. The upper age limit, which varies between 65 to 79 years, also works against identifying women at risk and the clinical relevance of any interventions. This was highlighted by Farzadar in a recent Lancet commentary: “…specification of a 10-year risk prediction, which gives considerable weight to the age variable, does not allow the model to single out the long-term risk, specifically in younger age groups. The same problem exists for women at a smaller scale.” [76]
Furthermore, there is often a mismatch between predicted and observed risks when using risk assessment tools such as the PCEs [77]. It may be that this mismatch and our inability to use HDP or other risk factors to identify women at risk more accurately is a result of how we measure BP itself. Studies of HDP as a marker of future CVD risk or treatment effects consider it a dichotomous trait based on a single threshold value, which makes little biological sense. Hence, HDP may be a stronger discriminating risk factor if considered a continuous quantitative variable rather than a dichotomous trait based upon an arbitrary diagnostic threshold.
Variation in BP, a missed opportunity
The current binary, population-based threshold for identifying hypertension in pregnancy may not capture all women at risk of future CVD and lowering the threshold may include women who are not at risk. Instead, quantifying BP variation throughout pregnancy is an approach that may be more sensitive and specific and warrants renewed attention.
The 1993 Report of the Joint National Committee (JNC) on Detection, Evaluation, and Treatment of High Blood Pressure used change in BP as part of the diagnostic criteria for hypertension in pregnancy [78]. The diagnostic criteria required a SBP increase of 30 mmHg or more or a DBP increase of 15 mmHg or more compared with the woman’s average values before 20 weeks’ gestation. If previous BP measurements were not available, a reading of 140/90 mmHg or above was considered abnormal. Interestingly, this approach to the diagnosis of hypertension in pregnancy disappeared in the 1997 JNC report and all subsequent reports [79]. One can only hypothesize that this occurred because of difficulty in the practical application of such criteria.
The underlying mechanisms by which BP effects CVD prognosis are not completely understood. Although mean BP is obviously important, increased BP variation is thought to reflect a pathophysiological imbalance of cardiovascular regulation in response to internal and external hemodynamic stressors. Variation in BP has also been shown to be reproducible rather than a random occurrence that hinders the ascertainment of ‘usual BP’ measurements [80, 81]. There is growing interest and evidence from interventional and observational studies for the use of BP variability as a key measure of CVD risk [81–85]. A recent systematic review and meta-analysis concluded that long-term BP variability independent of mean BP was associated with risk of all-cause mortality, HR 1.15 (1.09 – 1.22), CVD mortality HR 1.18 (1.09 – 1.28), CVD events, HR 1.18 (1.07 – 1.30), coronary heart disease, HR 1.10 (1.04 – 1.16) and stroke, HR 1.15 (1.04 – 1.27). Increased mid-term and short-term variability in daytime SBP were also associated with all-cause mortality (1.15, 1.06 – 1.26; 1.10, 1.04 – 1.16) [83].
One population-based, cross-sectional study has reported on changes in BP among 452 women throughout the peri-partum period and subsequent CVD outcomes. A 10 mmHg increase in mean DBP between 12 and 42 weeks’ gestation was found to confer a 1.7 fold increased risk of future hypertension. However, the study did not include women who developed gestational hypertension, PE or eclampsia [86]. A further study by Jwa et al. also explored changes in BP during the first half of pregnancy [87]. They reviewed the Tokyo-Children’s Health, Illness and Development study cohort data to analyze the relationship between BP changes from before 16 weeks to 20 weeks’ gestation. They found that the risk of pregnancy-induced hypertension increased in those whose BP moved from the Japanese Society of Hypertension 2009 Hypertension Treatment Guidelines [88] Class I (‘Optimal’: SBP 120 mmHg, DBP 80 mmHg) or Class II (‘Normal’: SBP 120 – 129 mmHg and/or DBP 80 – 84 mmHg) before 16 weeks’ gestation to Class III (‘High Normal’: SBP 130 – 139 mmHg and/or DBP 85 – 89mmHg) at 20 weeks’ gestation. There was no greater risk if women moved down a class. A further small observational study investigated variability in BP throughout pregnancy among women with treated mild to moderate hypertension. Patterns were found to differ between those with PE, FGR and preterm birth [89].
Variability as a prognostic marker for inclusion in risk assessment tools
The concept of variability in BP during pregnancy has the potential to improve the identification and treatment of women at risk of future disease. It may also facilitate the meaningful integration of other risk factors such as HDP in assessment tools.
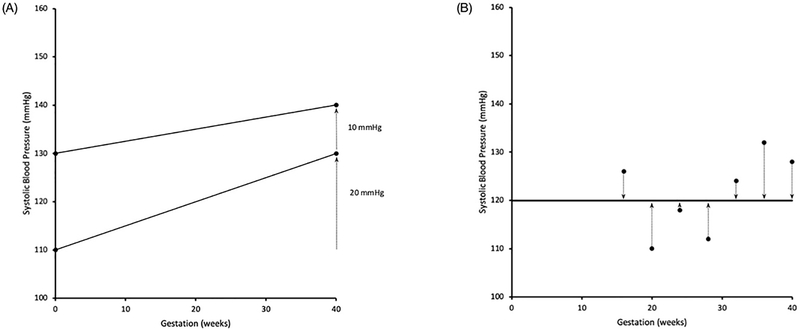
At present, there are no standardized methods or protocols for measuring BP variation and assessing its prognostic value. Broadly, there are three ways one could analyze BP changes throughout pregnancy. First, one could look at an individual’s change in SBP or DBP relative to a pre-defined baseline measurement. It may be that an individual with an SBP increase of 20 mmHg to 130 mmHg from a baseline of 110 mmHg has a higher long-term risk of CVD than another who has a smaller increase of 10 mmHg to 140 mmHg, even though only the latter would be diagnosed with mild to moderate hypertension in pregnancy (Figure 1 A). This is similar to the approach taken by the aforementioned studies by Iino et. al. and Jwa et al. [86, 87]. Examining relative change in BP allows the criteria for hypertension diagnosis to vary, either by individual and/or throughout gestation depending on the chosen reference. However, the criteria are based on a single level of BP change from baseline and still remain dichotomous.
Figure 1.
(A) Representation of an overall higher SBP trajectory compared to a SBP trajectory with lower absolute values but a greater relative increase (20 mmHg) over the gestational period. (B) Representation of variability as the standard deviation around the mean BP. Hypothetical SBP measurements are represented by dots. Mean SBP across gestation is represented by the solid line. Deviations about the mean BP are represented by the dotted lines. Conceptually t 8he standard deviation is the ‘average’ of BP deviations.
The second approach is to quantify BP variation over the course of pregnancy and utilize multiple BP measures throughout gestation to better reflect cardiovascular dysregulation. Several indices can be calculated to quantify mid and long-term BP variability. The primary summary value described is the standard deviation (SD) around an individual’s or population standard mean SBP or DBP (Figure 1 B). This method was used by Shimbo et al. in their 2012 study of the association between annual visit-to-visit BP variability and stroke in postmenopausal women. Results suggested that independent of mean BP and temporal changes over time, greater variability of SBP relative to the individual’s mean BP was associated with an increased risk of stroke, particularly in the lowest range of mean SBP [90]. Another measure often calculated in conjunction with SD, is the ‘coefficient of variation’. This is the ratio of SD to mean BP, a normalized measure of variation [80].However, mean BP is correlated with SD and it is also known to increase with age [2]. Therefore, ‘variation independent of the mean’ was formulated by Rotherwell et. al in 2010 [81]. Two further indices used to quantify BP variability are ‘successive variability’ and ‘average real variability’ which take into account the order of and variability between adjacent BP recordings [80].
Third, one could look at the rate or velocity of change in BP over time. For example, it may be that an individual with a rapid increase in BP is at higher risk than a woman with more static serial BP measures despite having higher absolute pressures. Velocity can be calculated by units of measurement per time such as mmHg/gestational week. The slope or degree of centile crossing can be measured by comparing a women’s BP trajectory with pre-defined curves, which represent specific constant velocities. This determines if BP is changing appreciable more quickly than in a woman with a baseline BP of the same centile group. This is a similar approach to that used to measure a child’s growth velocity on anthropometric charts [91]. It is worth noting that intervals of measurement need to be pre-defined for comparisons across intervals, as the shorter the interval, the greater the variability. In 2005, Zakopoulos et. al. formulated a summary value called ‘time rate BP variability’ to measure speed and steepness of BP changes over time [92]. Finally, one could look at a combination of these approaches or include one or more in a composite score or risk assessment tool.
A challenge of measuring variability in pregnancy is defining a baseline BP from which to make comparisons. Baseline can be defined as pre-pregnancy BP [93–95], although these measures are often not available in routine clinical practice. Some studies, therefore, use postnatal measures [96]. Most frequently, BP at 20 weeks’ gestation is used to differentiate between women with pre-existing chronic hypertension and those who have developed HDP. The latter is not based on strong evidence, and the recent ACOG Practice Bulletin highlights the arbitrary nature of this temporal cut-off [34].
Comparative measures are further complicated by evidence that suggests there is a physiological mid-trimester drop in BP during pregnancy. However, the Avon Longitudinal Study of Parents and Children (ALSPAC) reported women who had a normal pregnancy had drop of SBP and DBP at 20 weeks’ gestation compared to 12 weeks of approximately 1mmHg [97], which is much smaller than the commonly cited nadir of 10% [34]. Furthermore, recent results of a systematic review and meta-analysis of BP and heart rate trends in normal pregnancies by Loerup et al. suggest the assumed mid trimester drop may need to be reconsidered. Analysis was of 39 studies and included BP recordings from over 36,000 women from <16 weeks’ gestation throughout pregnancy. A substantial decrease in mid-trimester BP was not found [98]. Further work to clarify the degree of gestational age-specific BP changes in pregnancy, and indeed the creation of gestational age-specific BP centiles would allow for interpretation of subsequent BP changes from baseline measures in both research and clinical settings.
Emerging technologies may mean the ability to accurately measure individual BP variability is now possible. In the past, manual auscultation was required to measure BP in pregnancy as unvalidated automated devices tend to underestimate BP. This is highlighted in the systematic review and meta-analysis by Loreup et al. Manual compared to automated BP measurements reported higher diastolic BP values. However, only three of thirteen studies that used automated sphygmomanometers had devices validated for use in pregnancy. With the development and availability of validated ambulatory BP devices [99], women are now able to regularly measure their pressures in the home environment making an analysis of variability more reliable and readily attainable. A 2018 systematic review and meta-analysis concluded that there was no significant difference between self and clinic readings and the mean differences were between just 1.5 – 2.2 mmHg systolic and 0.7 – 1.5mmHg diastolic BP [100].
Conclusion
At present, there is a difference in diagnostic and treatment recommendations for hypertension between non-pregnant and pregnant women, which warrants further investigation. Discrepancies in the definitions of hypertension aside, HDP and its sequelae continue to contribute to the immediate and future health burden of women.
Women affected by HDP could simply be regarded as a high-risk group for CVD who need to be screened for additional risk factors and provided with preventative interventions when appropriate. However, using variability in BP rather than the traditional binary population-based thresholds for the diagnosis of HDP represents an opportunity to improve prognostic and risk assessment tools for future CVD risk. Variability may capture women at risk who currently fall outside the HDP diagnostic window and may be a more reliable indicator of the degree of risk and/or severity of future CVD. Current evidence suggests BP variability among the general population is independently associated with increased CVD risk. To determine the prognostic value of BP variability in pregnancy, longitudinal observational studies among parous women that measure BP throughout gestation are required. Creation of gestational age-specific BP centiles and associated standardized methodology for quantifying variability would assist this investigation. With access to validated home sphygmomanometers, accurate measurement of variability is now easier than when diagnostic criteria (including those that incorporated variability) were previously recommended. This is an area of research priority given the large global burden of CVD and other hypertension related disorders, particularly in women.
Acknowledgments
Sources of support
This work was supported, in part, by NIH grant, R01 HL136348
Footnotes
Disclaimers
None
References
- 1.WHO Guidelines Approved by the Guidelines Review Committee. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Geneva: World: Health OrganizationWorld Health Organization; 2011. [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. [DOI] [PubMed] [Google Scholar]
- 3.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–911. doi: 10.1016/s0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e484–e594. doi: 10.1161/cir.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 6.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):980–1004. doi: 10.1016/s0140-6736(14)60696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstetrics and gynecology. 2017;130(2):366–73. doi: 10.1097/aog.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. American Journal of Obstetrics and Gynecology. 2012;206(2):134.e1–8. doi: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ et al. Births: final data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60(1):1–70. [PubMed] [Google Scholar]
- 10.Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. Journal of Human Hypertension. 2012;27:148. doi: 10.1038/jhh.2012.13 [DOI] [PubMed] [Google Scholar]
- 11.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Preventive Medicine. 2013;56(6):372–8. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitti J, Sienas L, Walker S, Benedetti TJ, Easterling T. Contribution of hypertension to severe maternal morbidity. American Journal of Obstetrics and Gynecology. 2018;219(4):405.e1–e7. doi: 10.1016/j.ajog.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. British Medical Journal. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panaitescu AM, Baschat AA, Akolekar R, Syngelaki A, Nicolaides KH. Association of chronic hypertension with birth of small-for-gestational-age neonate. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2017;50(3):361–6. doi: 10.1002/uog.17553. [DOI] [PubMed] [Google Scholar]
- 15.Crispi F, Miranda J, Gratacos E. Long-term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. American Journal of Obstetrics and Gynecology. 2018;218(2s):S869–s79. doi: 10.1016/j.ajog.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Garovic VD, Bailey KR, Boerwinkle E, Hunt SC, Weder AB, Curb D et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. Journal of Hypertension. 2010;28(4):826–33. doi: 10.1097/HJH.0b013e328335c29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. British Medical Journal. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissgerber TL, Turner ST, Bailey KR, Mosley TH Jr., Kardia SL, Wiste HJ et al. Hypertension in pregnancy is a risk factor for peripheral arterial disease decades after pregnancy. Atherosclerosis. 2013;229(1):212–6. doi: 10.1016/j.atherosclerosis.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black MH, Zhou H, Sacks DA, Dublin S, Lawrence JM, Harrison TN et al. Hypertensive disorders first identified in pregnancy increase risk for incident prehypertension and hypertension in the year after delivery. Journal of Hypertension. 2016;34(4):728–35. doi: 10.1097/hjh.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 20.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. doi: 10.1016/s0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/cir.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 22.Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Therapeutic Advances in Cardiovascular Disease. 2008;2(4):249–59. doi: 10.1177/1753944708094227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. European Journal of Epidemiology. 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 24.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circulation: Cardiovascular Quality and Outcomes. 2017;10(2). doi: 10.1161/circoutcomes.116.003497. [DOI] [PubMed] [Google Scholar]
- 25.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. American Heart Journal. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovascular Research. 2014;101(4):579–86. doi: 10.1093/cvr/cvu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366(9499):1797–803. doi: 10.1016/s0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 28.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Horvath J et al. Hypertension in pregnancy and long-term cardiovascular mortality: a retrospective cohort study. American Journal of Obstetrics and Gynecology. 2016;214(6):722.e1–6. doi: 10.1016/j.ajog.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 29.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H et al. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trondelag Health Study. Journal of the American Medical Association Cardiology. 2019. doi: 10.1001/jamacardio.2019.1746.* This large study provides prospective evidence of the association between HDP and increased risk of CVD later in life.
- 30.Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S et al. The CHIPS Randomized Controlled Trial (Control of Hypertension in Pregnancy Study): Is Severe Hypertension Just an Elevated Blood Pressure? Hypertension. 2016;68(5):1153–9. doi: 10.1161/hypertensionaha.116.07862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–74. doi: 10.1016/j.atherosclerosis.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Clinical guideline [CG127]. 2011. https://www.nice.org.uk/guidance/CG127/chapter/1-Guidance#diagnosing-hypertension-2. Accessed 20 July 2019.
- 33.SMFM Statement: benefit of antihypertensive therapy for mild-to-moderate chronic hypertension during pregnancy remains uncertain. American Journal of Obstetrics and Gynecology. 2015;213(1):3–4. doi: 10.1016/j.ajog.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 34.ACOG Practice Bulletin No. 203: Chronic Hypertension in Pregnancy. Obstetrics and Gynecology. 2019;133(1):e26–e50. doi: 10.1097/aog.0000000000003020.* This bulletin outlines the current ACOG position and recommendations for management of chronic hypertension in pregnancy.
- 35.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. European Heart Journal. 2018;39(34):3165–241. doi: 10.1093/eurheartj/ehy340.*This guideline outlines the current European recommendations for management of hypertension in pregnancy.
- 36.von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. Journal of obstetrics and gynaecology Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2002;24(12):941–5. [DOI] [PubMed] [Google Scholar]
- 37.Redman CW. Fetal outcome in trial of antihypertensive treatment in pregnancy. Lancet. 1976;2(7989):753–6. [DOI] [PubMed] [Google Scholar]
- 38.Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. The Cochrane Database of Systematic Reviews. 2018;10:Cd002252. doi: 10.1002/14651858.CD002252.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE et al. Less-tight versus tight control of hypertension in pregnancy. New England Journal of Medicine. 2015;372(5):407–17. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 40.Rezk M, Ellakwa H, Gamal A, Emara M. Maternal and fetal morbidity following discontinuation of antihypertensive drugs in mild to moderate chronic hypertension: A 4-year observational study. Pregnancy Hypertension. 2016;6(4):291–4. doi: 10.1016/j.preghy.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 41.National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. NICE guideline [NG133]. 2019. https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#management-of-chronic-hypertension-in-pregnancy. Accessed 29 July 2019.* This guideline outlines the current UK recommendations for management of hypertension in pregnancy.
- 42.Dunietz GL, Strutz KL, Holzman C, Tian Y, Todem D, Bullen BL et al. Moderately elevated blood pressure during pregnancy and odds of hypertension later in life: the POUCHmoms longitudinal study. British Journal of Obstetrics and Gynaecology. 2017;124(10):1606–13. doi: 10.1111/1471-0528.14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitos J, Paradis G. Recurrent pre-eclampsia and subsequent cardiovascular risk. Heart (British Cardiac Society). 2017;103(3):235–43. doi: 10.1136/heartjnl-2016-309671. [DOI] [PubMed] [Google Scholar]
- 44.Kattah AG, Garovic VD. Preeclampsia: Cardiovascular and Renal Risks During and After Pregnancy In: LaMarca B, Alexander BT, editors. Sex Differences in Cardiovascular Physiology and Pathophysiology. Academic Press; 2019. ISBN: 9780128131978 [Google Scholar]
- 45.Smith GN. Development of preeclampsia provides a window of opportunity for early cardiovascular risk screening and intervention. Expert Review of Obstetrics & Gynecology. 2009;4(4):355–7. doi: 10.1586/eog.09.16. [DOI] [Google Scholar]
- 46.Kuruvilla S, Bustreo F, Kuo T, Mishra CK, Taylor K, Fogstad H et al. The Global strategy for women’s, children’s and adolescents’ health (2016–2030): a roadmap based on evidence and country experience. Bulletin of the World Health Organization. 2016;94(5):398–400. doi: 10.2471/blt.16.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–88. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation. 2016;133(9):916–47. doi: 10.1161/cir.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 49.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertension in Pregnancy. 2012;31(1):50–8. doi: 10.3109/10641955.2010.544955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–80. doi: 10.1161/circulationaha.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermes W, Tamsma JT, Grootendorst DC, Franx A, van der Post J, van Pampus MG et al. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: a longitudinal follow-up study. BioMed Central Pregnancy and Childbirth. 2013;13:126. doi: 10.1186/1471-2393-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/circulationaha.107.699579. [DOI] [PubMed] [Google Scholar]
- 53.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 54.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 55.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. British Medical Journal. 2007;335(7611):136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. British Medical Journal. 2008;336(7659):1475–82. doi: 10.1136/bmj.39609.449676.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. Journal of the American Medical Association. 2007;297(6):611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118(22):2243–51, 4p following 51. doi: 10.1161/circulationaha.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. European Heart Journal. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 60.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Munster (PROCAM) study. Circulation. 2002;105(3):310–5. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 61.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC). Heart (British Cardiac Society). 2007;93(2):172–6. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giampaoli S CUORE: a sustainable cardiovascular disease prevention strategy. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14(2):161–2. doi: 10.1097/HJR.0b013e328157f3e5. [DOI] [PubMed] [Google Scholar]
- 63.Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. The Lancet Diabetes & Endocrinology. 2015;3(5):339–55. doi: 10.1016/s2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 65.World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Global Health. 2019;7(10):e1332–e45. doi: 10.1016/s2214-109x(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennells L, Kaptoge S, Wood A, Sweeting M, Zhao X, White I et al. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. European Heart Journal. 2019;40(7):621–31. doi: 10.1093/eurheartj/ehy653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. Journal of the American College of Cardiology. 2018;72(11):1252–63. doi: 10.1016/j.jacc.2018.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation. 2016;133(22):2149–58. doi: 10.1161/circulationaha.115.017854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. European Heart Journal. 2019;40(14):1113–20. doi: 10.1093/eurheartj/ehy863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Timpka S, Fraser A, Schyman T, Stuart JJ, Asvold BO, Mogren I et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. European Journal of Epidemiology. 2018;33(10):1003–10. doi: 10.1007/s10654-018-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gunnarsson OS, Timpka S. Pregnancy Complication History in 10-Year Cardiovascular Disease Risk Prediction: a Review of Recent Evidence. Current Epidemiology Reports. 2019. doi: 10.1007/s40471-019-00208-2.* A recent review of evidence which suggests the inclusion of HDP history does not significantly improve 10-year CVD risk prediction for women.
- 73.Catov JM, Countouris M, Hauspurg A. Hypertensive Disorders of Pregnancy and CVD Prediction: Accounting for Risk Accrual During the Reproductive Years. Journal of the American College of Cardiology. 2018;72(11):1264–6. doi: 10.1016/j.jacc.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 74.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–54. doi: 10.1161/circulationaha.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. Journal of the American Medical Association. 2012;308(17):1795–801. doi: 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farzadfar F Cardiovascular disease risk prediction models: challenges and perspectives. The Lancet Global Health. 2019;7(10):e1288–e9. doi: 10.1016/S2214-109X(19)30365-1. [DOI] [PubMed] [Google Scholar]
- 77.Cook NR, Ridker PM. Calibration of the Pooled Cohort Equations for Atherosclerotic Cardiovascular Disease: An Update. Annals of internal medicine. 2016;165(11):786–94. doi: 10.7326/m16-1739. [DOI] [PubMed] [Google Scholar]
- 78.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Archives of Internal Medicine. 1993;153(2):154–83. [PubMed] [Google Scholar]
- 79.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Archives of Internal Medicine. 1997;157(21):2413–46. [DOI] [PubMed] [Google Scholar]
- 80.Muntner P, Joyce C, Levitan EB, Holt E, Shimbo D, Webber LS et al. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. Journal of Hypertension. 2011;29(12):2332–8. doi: 10.1097/HJH.0b013e32834cf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 82.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375(9718):938–48. doi: 10.1016/s0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 83.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. British Medical Journal. 2016;354:i4098. doi: 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stergiou GS, Parati G, Vlachopoulos C, Achimastos A, Andreadis E, Asmar R et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement: current status and future directions - Position statement of the European Society of Hypertension Working Group on blood pressure monitoring and cardiovascular variability. Journal of Hypertension. 2016;34(9):1665–77. doi: 10.1097/hjh.0000000000000969.** This is a position statement developed by international experts following the 2014 European Society of Hypertension/International Society of Hypertension meeting. It outlines research and practical issues regarding BP measurement, including BP variability.
- 85.Mehlum MH, Liestøl K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. European Heart Journal. 2018;39(24):2243–51. doi: 10.1093/eurheartj/ehx760. [DOI] [PubMed] [Google Scholar]
- 86.Iino K, Higuchi T, Ogawa M, Yamauchi Y, Misaki N, Tanaka K et al. Blood pressure during pregnancy is a useful predictive maker for hypertension and dyslipidemia later in life, a population-based, cross-sectional study. Maturitas. 2016;87:84–8. doi: 10.1016/j.maturitas.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 87.Jwa SC, Arata N, Sakamoto N, Watanabe N, Aoki H, Kurauchi-Mito A et al. Prediction of pregnancy-induced hypertension by a shift of blood pressure class according to the JSH 2009 guidelines. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34(11):1203–8. doi: 10.1038/hr.2011.107. [DOI] [PubMed] [Google Scholar]
- 88.Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertension research : official journal of the Japanese Society of Hypertension. 2009;32(1):3–107. [PubMed] [Google Scholar]
- 89.Morgan JL, Nelson DB, Roberts SW, Wells CE, McIntire DD, Cunningham FG. Blood Pressure Profiles Across Pregnancy in Women with Chronic Hypertension. American Journal of Perinatology. 2016;33(12):1128–32. doi: 10.1055/s-0036-1584581. [DOI] [PubMed] [Google Scholar]
- 90.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension. 2012;60(3):625–30. doi: 10.1161/hypertensionaha.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cole TJ. The development of growth references and growth charts. Annals of Human Biology. 2012;39(5):382–94. doi: 10.3109/03014460.2012.694475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zakopoulos NA, Tsivgoulis G, Barlas G, Papamichael C, Spengos K, Manios E et al. Time rate of blood pressure variation is associated with increased common carotid artery intima-media thickness. Hypertension. 2005;45(4):505–12. doi: 10.1161/01.Hyp.0000158306.87582.43. [DOI] [PubMed] [Google Scholar]
- 93.Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. Journal of Hypertension. 2014;32(4):849–56. doi: 10.1097/hjh.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 94.Shen M, Tan H, Zhou S, Smith GN, Walker MC, Wen SW. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: a functional data analysis approach. Scientific Reports. 2017;7(1):6227. doi: 10.1038/s41598-017-06606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–8. doi: 10.1161/circulationaha.114.009029. [DOI] [PubMed] [Google Scholar]
- 96.Grindheim G, Estensen ME, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. Journal of Hypertension. 2012;30(2):342–50. doi: 10.1097/HJH.0b013e32834f0b1c. [DOI] [PubMed] [Google Scholar]
- 97.Macdonald-Wallis C, Silverwood RJ, Fraser A, Nelson SM, Tilling K, Lawlor DA et al. Gestational-age-specific reference ranges for blood pressure in pregnancy: findings from a prospective cohort. Journal of Hypertension. 2015;33(1):96–105. doi: 10.1097/hjh.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S et al. Trends of blood pressure and heart rate in normal pregnancies: a systematic review and meta-analysis. BioMed Central Medicine. 2019;17(1):167. doi: 10.1186/s12916-019-1399-1.** This study describes the average BP trajectory throughout gestation. A substatial mid-trimester drop was not found.
- 99.Kitt J, Fox R, Tucker KL, McManus RJ. New Approaches in Hypertension Management: a Review of Current and Developing Technologies and Their Potential Impact on Hypertension Care. Current Hypertension Reports. 2019;21(6):44. doi: 10.1007/s11906-019-0949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tucker KL, Bankhead C, Hodgkinson J, Roberts N, Stevens R, Heneghan C et al. How Do Home and Clinic Blood Pressure Readings Compare in Pregnancy? Hypertension. 2018;72(3):686–94. doi: 10.1161/hypertensionaha.118.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]