Abstract
BACKGROUND
In the ISCHEMIA trial, an invasive strategy with angiographic assessment and revascularization did not reduce clinical events among patients with stable ischemic heart disease and moderate or severe ischemia. A secondary objective of the trial was to assess angina-related health status among these patients.
METHODS
We assessed angina-related symptoms, function, and quality of life with the Seattle Angina Questionnaire (SAQ) at randomization, at months 1.5, 3, and 6, and every 6 months thereafter in participants who had been randomly assigned to an invasive treatment strategy (2295 participants) or a conservative strategy (2322). Mixed-effects cumulative probability models within a Bayesian framework were used to estimate differences between the treatment groups. The primary outcome of this health-status analysis was the SAQ summary score (scores range from 0 to 100, with higher scores indicating better health status). All analyses were performed in the overall population and according to baseline angina frequency.
RESULTS
At baseline, 35% of patients reported having no angina in the previous month. SAQ summary scores increased in both treatment groups, with increases at 3, 12, and 36 months that were 4.1 points (95% credible interval, 3.2 to 5.0), 4.2 points (95% credible interval, 3.3 to 5.1), and 2.9 points (95% credible interval, 2.2 to 3.7) higher with the invasive strategy than with the conservative strategy. Differences were larger among participants who had more frequent angina at baseline (8.5 vs. 0.1 points at 3 months and 5.3 vs. 1.2 points at 36 months among participants with daily or weekly angina as compared with no angina).
CONCLUSIONS
In the overall trial population with moderate or severe ischemia, which included 35% of participants without angina at baseline, patients randomly assigned to the invasive strategy had greater improvement in angina-related health status than those assigned to the conservative strategy. The modest mean differences favoring the invasive strategy in the overall group reflected minimal differences among asymptomatic patients and larger differences among patients who had had angina at baseline. (Funded by the National Heart, Lung, and Blood Institute and others; ISCHEMIA ClinicalTrials.gov number, NCT01471522.)
THE PRINCIPAL GOALS OF TREATING PAtients with stable ischemic heart disease are to prolong survival, prevent disease progression, and optimize patients’ health status: their symptoms, function, and quality of life. To prevent death and myocardial infarctions, secondary prevention with lifestyle and pharmacologic intervention is recommended for all patients with stable ischemic heart disease.1,2 Although the incremental utility of revascularization, as compared with modern guideline-based medical therapy alone, for improving prognosis in patients with stable ischemic heart disease has been unsettled, guidelines and appropriate-use criteria endorse revascularization for the relief of symptoms that are not adequately controlled with medical therapy.3,4
Previous studies of invasive strategies in which percutaneous coronary intervention was used in the management of stable ischemic heart disease have shown a transient health-status benefit as compared with a conservative strategy,5 although a more durable benefit after coronary-artery bypass grafting (CABG) has been observed.6 These studies, however, were performed in an era before drug-eluting stents and often did not include the option of CABG, and patients underwent randomization after the coronary anatomy had been defined. To formally evaluate strategies for managing substantial ischemia in high-risk patients, the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) randomly assigned patients with moderate or severe ischemia to receive treatment with either an initially invasive strategy involving angiographic assessment and revascularization (when feasible) along with guideline-based medical therapy or an initially conservative strategy of guideline-based medical therapy alone.7 The primary analysis in the main trial, which is now reported in the Journal,8 showed no benefit of an invasive strategy with respect to clinical events over a median of 3.2 years of observation. In this report, we document the health-status outcomes of these two treatment strategies in high-risk patients with stable ischemic heart disease, a key secondary outcome of the trial.
METHODS
TRIAL POPULATION
Details of the trial and the assessments of the patients’ health status have been described previously.7,9 Details of the eligibility criteria, treatment assignments, treatment received (including anti-anginal therapy), and follow-up are reported by Maron et al.8
HEALTH-STATUS OUTCOMES
To quantify symptoms, function, and quality of life among the participants who underwent randomization, a brief symptom survey was administered before randomization, at months 1.5, 3, and 6, and every 6 months thereafter until termination of the trial. This survey included the Seattle Angina Questionnaire (SAQ), the Rose Dyspnea Scale,10 and the visual analogue scale of the European Quality of Life–5 Dimensions (EQ-5D).11 Linguistically and culturally certified translations of the SAQ (www.cvoutcomes.org) were used in each participating country. The 7-item version of the SAQ was the primary instrument used for the analyses of health status7,12 (see the Supplementary Appendix, available with the full text of this article at NEJM.org). It is a shortened version of the original 19-item SAQ and has been shown to be highly valid, reliable, and sensitive to clinical change.13–15 The SAQ captures the frequency of angina (SAQ Angina Frequency score) and the disease-specific effect of angina on patients’ physical function (SAQ Physical Limitation score) and quality of life (Quality of Life score) over the previous 4 weeks; these scores are averaged to obtain the SAQ Summary score, an overall measure of patients’ stable ischemic heart disease–specific health status. SAQ scores range from 0 to 100, with higher scores indicating less frequent angina, better function, and better quality of life. To support clinical interpretation, the SAQ Summary score, SAQ Physical Limitation score, and SAQ Quality of Life score can be categorized into ranges of 0 to 24 (very poor to poor health status), 25 to 49 (poor to fair), 50 to 74 (fair to good), and 75 to 100 (good to excellent).16,17 SAQ Angina Frequency scores of 0 to 30, 31 to 60, 61 to 99, and 100 have been shown to validly reflect angina that occurs daily, weekly, several times per month (“monthly”), and no angina, respectively, as assessed with daily diaries.18 These ranges of SAQ scores are strongly and independently correlated with the risk of subsequent death, the risk of myocardial infarction, and health care costs.16,19 The Rose Dyspnea Scale has four items indicating whether patients experience breathlessness with different activities (scores range from 0 to 4, with higher scores indicating dyspnea with milder activities). For the EQ-5D visual analogue scale, patients rate their current health along a continuum from 0, indicating the worst possible health state, to 100, indicating the best possible health state. Additional health-status domains, as described in the protocol (available at NEJM.org), were used in a subset of sites and are not reported here.
TRIAL OVERSIGHT AND ORGANIZATION
The analyses of data on health status were sponsored by the National Heart, Lung, and Blood Institute, with additional support from industry for the main trial.8 An independent data and safety monitoring board approved the trial protocol and monitored the safety of the participants. The protocol was approved by the institutional review boards of the New York University Grossman School of Medicine, Duke University, Saint Luke’s Hospital, and each participating site. All participants from the 320 sites provided written informed consent. The health-status assessments were designed by the authors and approved by the trial leadership, the National Heart, Lung, and Blood Institute, and the data and safety monitoring board. All analyses of health status were conducted at Saint Luke’s Mid America Heart Institute, which had a data confidentiality agreement with the data coordinating center. The first author vouches for the accuracy and completeness of the data on health status and for the fidelity of this analysis of health-status outcomes to the protocol.
STATISTICAL ANALYSIS
The original and final protocol and statistical analysis plan are available at NEJM.org. The statistical analysis plan was finalized in September 2019, after the feasibility of the planned analyses had been confirmed in a preliminary pooled, blinded data set that did not indicate treatment assignments. All analyses were conducted on an intention-to-treat basis.
The primary outcome was the SAQ Summary score. The prespecified plan was to provide results both for the overall population and the population stratified according to baseline angina frequency, as defined by the SAQ Angina Frequency score.12 Additional exploration of the heterogeneity of the treatment benefit according to patients’ baseline characteristics is ongoing and is not reported here. Although the analyses included all available assessments through 78 months, we present the results through the first 48 months because of substantial censoring due to participants having completed the trial. No adjustment for multiplicity of analyses was performed.
For descriptive purposes, unadjusted mean scores are reported according to treatment group at each assessment. The effect of treatment was evaluated with cumulative probability models (also called “cumulative link models”) of follow-up health-status scores, which do not impose distributional assumptions on the outcome.20 On the basis of graphical analysis, a logit link was found to provide reasonable fit and allows the effect of treatment to be expressed as an odds ratio for a higher score with the invasive strategy, and the odds ratios were found to be consistent across the range of baseline SAQ scores at 3, 12, and 36 months.
Blinded review of the trial data revealed nonlinear trajectories in health-status scores over time, with larger changes early after randomization and with substantial heterogeneity of individual participants’ trajectories. We therefore used mixed models, within the framework of a cumulative probability model, that included fixed effects for baseline score, treatment group, time since randomization, and treatment-by-time interaction, as well as patient-level random intercepts and time effects. Piecewise linear splines were used to model time trends, with knots at 3, 6, 12, 18, and 24 months for the fixed effect of time and knots at 6 and 12 months for patient-level random effects. Restricted cubic splines were used to allow for nonlinear effects of baseline scores. The mixed models allowed estimation of the effects of treatment assignment on patient-specific, in addition to population mean, health-status outcomes.
All models were fit with the use of Bayesian methods. In addition to facilitating the estimation of more complex models than are produced with traditional frequentist methods, Bayesian analysis directly estimates the probability distribution of the treatment effect, which can be interpreted as the probability of different effect sizes given the observed data. Weakly informative prior distributions (e.g., heavy-tailed t distributions around 0 with standard deviations of 10) were used for all fixed and random effects, so that inference was driven predominantly by the trial data. The effect of treatment over time was estimated for a typical patient, with a baseline health-status score equal to the population mean and a random effect of 0. In addition to odds ratios being reported at each time point, effects were transformed back to the scale of the instrument scores by integrating over the predicted probabilities of each possible value for each patient. As prespecified in the protocol, expected time-averaged scores through 48 months were also calculated, with area-under-the-curve analyses used to describe the mean difference in scores over time. Results are presented as posterior means and 95% Bayesian credible intervals — specifically, highest posterior density intervals, which denote the 95% most plausible values of the parameter being estimated. (In Bayesian analysis, the posterior distribution represents the range and probabilities of possible values of the treatment effects, obtained by combining prior beliefs about the effect with the evidence provided by the data; the posterior mean is the mean of this distribution.) No P values are reported.
A key prespecified analysis was the estimation of the effect of treatment as a function of patients’ baseline angina frequency, with the a priori hypothesis being that there would be greater health-status benefits from an invasive strategy in patients with more frequent angina before randomization.12 Therefore, the above analyses were repeated with the SAQ Angina Frequency score used as a continuous variable and categorized into daily or weekly angina, monthly angina, or no angina and the differences between treatment groups calculated. To assist in the clinical interpretation of the results, we conducted analyses (not prespecified in the statistical analysis plan) estimating the probability of being angina-free as a function of baseline angina frequency. For this analysis, the model for the SAQ Angina Frequency score was augmented by inclusion of three-way interaction terms among treatment, time, and baseline score, to estimate the probability of being angina-free (SAQ Angina Frequency score, 100) at follow-up as a function of baseline score, treatment, and time. Similar responder analyses were performed to estimate the probability of having SAQ Summary and SAQ Quality of Life scores of 75 or higher, representing good to excellent disease-specific health status and quality of life, respectively. An alternative means of interpreting the clinical significance of observed changes, based on potentially clinically relevant intraparticipant changes in SAQ Summary and Angina Frequency scores, is provided in the Supplementary Appendix.
In the primary analysis of treatment effect, missing scores were assumed to be missing at random, conditional on treatment group and other available scores, because the mixed model implicitly imputes missing data through participants’ estimated health-status trajectories. However, because death may be an informative reason for missing data, we conducted a prespecified sensitivity analysis of the SAQ Summary score by fitting a joint shared-parameter model of health status and survival in which the patient-level random effects from the model described above were included as covariates in a Weibull regression model of time to death.21
The sample size was driven by the clinical power analyses and not by the health-status analyses. All analyses were conducted with SAS software, version 9.4; R software, version 3.5.3; Stan software, version 2.18.1; and R packages “rstan,” “rstanarm,” “brms,” and “tidyverse.”22–27
RESULTS
PARTICIPANTS
Among the 5179 participants who underwent randomization in the trial, we excluded those from five sites (481 participants) because of improper form completion, as well as 51 participants in the invasive-strategy group and 30 in the conservative-strategy group who were missing either a baseline or all follow-up SAQ scores. The numbers of participants included and excluded from the analysis are shown in Figure S1 in the Supplementary Appendix, and a comparison of the included and excluded participants is provided in Table S1. Table S2 shows the reasons for missing assessments, with cessation of follow-up due to trial completion being most important. An additional 5% of participants died before the 48-month assessment. The percentages of intermittent skipped assessments varied from 6 to 12% over time, with no appreciable differences between the groups with respect to how frequently data were missing or the reasons for missing data.
The baseline characteristics of the participants were balanced between the treatment groups (Table 1). The mean age was 64 years, more than three quarters of participants were male, and the majority of participants were white. Hypertension was present in 76% of participants, and 40% had diabetes. The mean (±SD) baseline SAQ Summary score was 73.4±19.1 in the invasive-strategy group and 74.8±18.8 in the conservative-strategy group. Overall, 20% of participants had daily or weekly angina, 44% had angina one to three times per month, and 35% had no angina in the month before randomization.
Table 1.
Baseline Characteristics of the Patients.*
| Patient Characteristic | Invasive Strategy (N=2295) | Conservative Strategy (N = 2322) |
|---|---|---|
| Age at randomization — yr | 64.5±9.5 | 64.3±9.6 |
| Male sex — no. (%) | 1748 (76.2) | 1797 (77.4) |
| White race — no./total no. (%)† | 1679/2276 (73.8) | 1679/2294 (73.2) |
| Hypertension — no. (%) | 1748 (76.2) | 1768 (76.1) |
| Diabetes — no. (%) | 906 (39.5) | 930 (40.1) |
| Previous myocardial infarction — no. (%) | 475 (20.7) | 476 (20.5) |
| New-onset angina within 3 mo — no./total no. (%) | 349/2165 (16.1) | 373/2200 (17.0) |
| Heart failure — no. (%) | 887 (38.6) | 899 (38.7) |
| Previous cerebrovascular disease — no. (%) | 195 (8.5) | 170 (7.3) |
| Peripheral vascular disease — no. (%) | 114 (5.0) | 85 (3.7) |
| Mean BMI‡ | 28.8±5.1 | 29.0±5.4 |
| Degree of ischemia on stress testing, as assessed by core laboratory — no./total no. (%) | ||
| None | 121/2291 (5.3) | 126/2316 (5.4) |
| Mild | 165/2291 (7.2) | 166/2316 (7.2) |
| Moderate | 829/2291 (36.2) | 808/2316 (34.9) |
| Severe | 1154/2291 (50.4) | 1194/2316 (51.6) |
| Uninterpretable | 22/2291 (1.0) | 22/2316 (0.9) |
| SAQ Summary score§ | 73.4±19.1 | 74.8±18.8 |
| SAQ Angina Frequency score§ | 80.8±20.0 | 82.1±19.3 |
| Angina frequency — % | ||
| Daily or weekly | 21.6 | 19.0 |
| Several times per month | 44.1 | 44.5 |
| None in the past 4 weeks | 34.3 | 36.6 |
| SAQ Physical Limitation score§ | 78.5±23.7 | 80.2±23.4 |
| SAQ Quality of Life score§ | 60.9±26.5 | 62.7±26.3 |
| Rose Dyspnea Scale score¶ | 1.2±1.3 | 1.2±1.3 |
| EQ-5D visual analogue scale score‖ | 68.8±16.9 | 69.2±16.7 |
Plus–minus values are means ±SD.
Race was reported by the participant.
Body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
On the Seattle Angina Questionnaire (SAQ), the summary score is obtained by averaging the SAQ Angina Frequency, SAQ Quality of Life, and SAQ Physical Limitation scores; SAQ scores range from 0 to 100, with higher scores indicating better health status. SAQ Angina Frequency scores of 0 to 30, 31 to 60, 61 to 99, and 100 have been shown to validly reflect angina that occurs daily, weekly, monthly, and no angina, respectively, as assessed with daily diaries.
On the Rose Dyspnea Scale, scores range from 0 to 4, with higher scores indicating dyspnea with milder activities.
On the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale, scores range from 0 to 100, with higher scores indicating better health status.
PRIMARY OUTCOME
Unadjusted mean health-status scores for the trial population are shown in Figure 1. Although the health status in both treatment groups improved early after randomization, this improvement was greater among participants who were treated with an invasive strategy, a small mean difference for the entire population that was sustained throughout follow-up (mean SAQ Summary scores for the invasive strategy vs. the conservative strategy were 84.7±16 vs. 81.8±17 at 3 months, 87.2±15 vs. 84.2±16 at 12 months, and 88.6±14 vs. 86.3±16 at 36 months). Similar magnitudes of mean benefit were seen for the individual SAQ subscales, as well as for the Rose Dyspnea Scale and the EQ-5D visual analogue scale. Participants in the invasive-strategy group had at least 50% higher odds (odds ratio, ≥1.5) of having a more favorable SAQ Summary score than participants in the conservative-strategy group at each time point throughout 4 years of follow-up (Table S3). Results for the individual SAQ scales, Rose Dyspnea Scale, and EQ-5D visual analogue scale were consistent with the pattern of benefit seen in the SAQ Summary score.
Figure 1. Crude Mean Health-Status Scores in the Overall Trial Population.
Observed mean health-status scores from randomization through 48 months are shown. Shading represents the 95% confidence interval. On the Seattle Angina Questionnaire (SAQ), the SAQ Summary score is obtained by averaging the SAQ Angina Frequency, SAQ Quality of Life, and SAQ Physical Limitation scores; SAQ scores range from 0 to 100, with higher scores indicating better health status. On the Rose Dyspnea Scale, scores range from 0 to 4, with higher scores indicating dyspnea with milder activities. On the European Quality of Life–5 Dimensions (EQ-5D) visual analogue scale, scores range from 0 to 100, with higher scores indicating better health status.
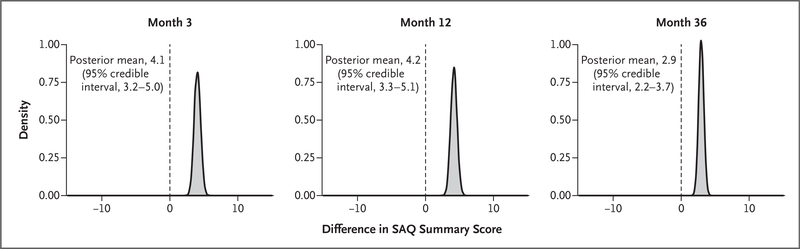
Figure 2 shows the posterior distributions for the effect of treatment on the expected SAQ Summary score of a “typical” patient (i.e., one with a baseline score equal to the population mean and a random effect of 0) in the overall population; similar distribution curves in cohorts of patients with different frequencies of angina at baseline are shown in Figure S2. The mean estimated effects of an invasive strategy on SAQ Summary scores at different times are shown in Table 2. For the overall population, the posterior mean difference in the SAQ Summary score favored the invasive strategy by 4.1 points at 3 months and by 4.2 points at 12 months; the lower limits of the 95% credible intervals were 3.2 and 3.3 points, respectively. By 36 months, the posterior mean SAQ improvement with the invasive strategy was 2.9 points, with a lower limit of the 95% credible interval of 2.2. When stratified according to baseline angina frequency, the differences were larger with the invasive strategy for patients with daily or weekly angina (8.5 points at 3 months and 5.3 points at 36 months) or monthly angina (5.5 points at 3 months and 3.1 points at 36 months), whereas patients with no angina at baseline had minimal to no incremental health-status benefit with the invasive strategy (0.1 points at 3 months and 1.2 points at 36 months). The time-averaged difference in scores through 48 months (the mean difference over time) was 3.3 points in the overall sample, 6.3 points among patients with daily or weekly angina at baseline, 3.7 points among those with monthly angina, and 1.1 points among those without angina (Table S4). The differences between the treatment groups are shown in Figure 3A for the SAQ Angina Frequency score and in Figures S3A and S4A for the SAQ Summary and SAQ Quality of Life scores. The between-group differences were attenuated when the mean baseline SAQ Angina Frequency scores were higher than 80, indicating rare or no angina at randomization. Results of the joint analysis of the SAQ Summary score and survival, accounting for potential bias due to informatively missing data associated with death, did not differ from those of the primary analysis (Table S5).
Figure 2. Distributions of the Expected Differences in SAQ Summary Scores from an Initially Invasive Strategy.
The posterior distribution of effect scores for a typical patient, with a baseline score equal to the population mean and a random effect of 0, is shown.
Table 2.
Mean Estimated Effect of an Invasive Strategy on SAQ Summary Scores.*
| Month | Overall (N = 4617) | Daily or Weekly Angina at Baseline (N = 934) | Monthly Angina at Baseline (N =2043) | No Angina at Baseline (N=1635) |
|---|---|---|---|---|
| points (95% credible interval) | ||||
| 3 | 4.1 (3.2 to 5.0) | 8.5 (5.8 to 11.1) | 5.5 (4.3 to 6.9) | 0.1 (−1.2 to 1.4) |
| 6 | 4.4 (3.5 to 5.3) | 10.5 (7.9 to 13.2) | 5.1 (3.7 to 6.4) | 0.8 (−0.4 to 2.2) |
| 12 | 4.2 (3.3 to 5.1) | 7.3 (4.8 to 9.9) | 4.8 (3.4 to 6.1) | 1.7 (0.4 to 2.9) |
| 18 | 3.3 (2.5 to 4.2) | 6.3 (3.9 to 9.0) | 3.6 (2.2 to 4.9) | 1.3 (0.1 to 2.5) |
| 24 | 2.8 (2.1 to 3.7) | 5.0 (3.0 to 7.2) | 3.5 (2.3 to 4.7) | 0.6 (−0.5 to 1.7) |
| 30 | 2.9 (2.1 to 3.6) | 5.2 (3.2 to 7.2) | 3.3 (2.2 to 4.3) | 0.9 (−0.1 to 1.9) |
| 36 | 2.9 (2.2 to 3.7) | 5.3 (3.4 to 7.5) | 3.1 (2.0 to 4.2) | 1.2 (0.2 to 2.2) |
| 42 | 3.0 (2.2 to 3.8) | 5.5 (3.3 to 7.7) | 2.9 (1.7 to 4.1) | 1.4 (0.4 to 2.5) |
| 48 | 3.1 (2.1 to 3.9) | 5.6 (3.2 to 8.0) | 2.7 (1.3 to 4.0) | 1.7 (0.5 to 2.9) |
Data are the posterior mean differences (invasive minus conservative) in SAQ Summary scores at each time point.
Figure 3. Effect of Treatment as a Function of Patients’ Baseline Angina Frequency.
Panel A shows the effect of each treatment strategy on the SAQ Angina Frequency score, measured as the estimated difference (invasive minus conservative) in the mean score, as a function of patients’ baseline SAQ Angina Frequency score. Panel B shows the probability of being angina-free (SAQ Angina Frequency score, 100) at 3, 12, and 36 months if treated with an invasive strategy (red) or a conservative strategy (blue). Shading represents 95% credible intervals.
PROBABILITY OF BEING ANGINA-FREE
Figure 3B shows the probability of being angina-free as a function of the SAQ Angina Frequency score at baseline. At each time point, the difference favoring the invasive strategy in the probability of being angina-free was larger among participants who had angina at baseline but was minimal among those who were asymptomatic before randomization. For example, among patients with a baseline SAQ Angina Frequency score of 50 (weekly angina), 45% of those treated invasively would be expected to be angina-free at 3 months, as compared with 15% of those treated conservatively. Conversely, among patients with a baseline SAQ Angina Frequency score of 100 (no angina in the previous month), the majority remained asymptomatic at follow-up, with minimal differences according to treatment strategy. This pattern was also observed for having a good to excellent (score, ≥75 points) quality of life or overall disease-specific health status (Figs. S3B and S4B). Table S6 shows the proportion of patients with small (but potentially clinically relevant) and moderate-to-large changes in the SAQ Summary and SAQ Angina Frequency scores according to treatment group as a function of their baseline SAQ Angina Frequency scores.
DISCUSSION
In this large strategy trial involving high-risk patients with stable ischemic heart disease and at least moderate ischemia, participants who were randomly assigned to the invasive treatment strategy had larger improvements in disease-specific health status (including angina symptoms, physical function, and disease-specific quality of life) than did participants assigned to the conservative strategy. The modest differences favoring the invasive strategy in the overall trial population reflected differences that were confined to participants who had had angina within the 4 weeks before randomization, with minimal, if any, benefit among those who had been asymptomatic at randomization.28 The magnitude of the difference was largest among participants who had entered the trial with daily or weekly angina. Since the main trial showed no difference in mortality over a period of 3.2 years, these health-status outcome data should help inform detailed, patient-specific discussions of the risks and benefits of an invasive approach in the management of stable ischemic heart disease. Our results provide patient-specific estimates of treatment benefit that can be used as a starting point for such patient-centered decision-making discussions.
These data substantially extend the published evidence from studies of the health-status benefits of an invasive strategy for the management of stable ischemic heart disease, particularly from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial.29 In our trial, we found a more sustained health-status benefit over time than was found in the COURAGE trial.5 Whether this difference reflects the evolution of stent technology, the inclusion of patients who underwent CABG, or the greater burden of ischemia warrants further investigation. Second, in our trial, in contrast to COURAGE and other trials of strategies for the treatment of stable ischemic heart disease, patients underwent randomization before invasive angiography was performed. This approach removed the selection biases that result from patients not undergoing randomization if the treating physicians think they need intervention. The approach also supports a model of care in which patients are engaged in a shared decision-making process before angiography, a more natural breakpoint in clinical workflow than taking patients “off the table” after diagnostic angiography has been performed.5
These findings should be interpreted in the context of several potential limitations. First, there were some missing health-status assessments, although only approximately 10% of assessments were missing at any point in time, and our mixed-effects models required only 91 patients to be excluded from the entire analysis. Second, to minimize the use of cardiac catheterization in the conservative-strategy group, the trial excluded patients who had unacceptable angina despite maximal medical therapy; this resulted in a less symptomatic sample, with baseline SAQ scores that were more than 10 points higher (i.e., more favorable) than those in the COURAGE trial. Third, because the health-status benefits of an invasive strategy were present only among participants with angina and a large proportion of participants had minimal symptoms at randomization, the overall mean difference in scores for the entire trial population was much smaller than the mean differences among participants with more frequent angina. Fourth, our results apply only to patients who meet the inclusion criteria of the trial and should not be extended to patients with left main coronary artery disease, acute coronary syndromes, or depressed ejection fractions. An additional concern may be that our analyses did not adjust for multiple comparisons. Although we did declare the SAQ Summary score to be our primary outcome, we did not define a specific time point to be the focus of our conclusions, given that we wanted to assess the magnitude and durability of differences throughout follow-up. Lastly, masking patients’ treatment assignments was not possible in this trial.
The possibility of finding health-status benefits as large as those in our trial should be considered in the context of the COURAGE trial, the Objective Randomised Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA), and ISCHEMIA-CKD (an ISCHEMIA companion trial, now reported in the Journal,30 involving patients with coronary and kidney disease, in which identical health-status measures and analytic techniques were used). In the COURAGE trial, which similarly tested invasive and conservative strategies, the benefits of an invasive approach dissipated after 12 months, whereas the benefits in ISCHEMIA persisted for more than 3 years. In ORBITA, a sham-controlled trial of percutaneous coronary intervention, the mean effect sizes (a difference of 4.4 points [95% confidence interval, −3.3 to 12.0] in SAQ Angina Frequency scores at 6 weeks) and the responder analyses (30% vs. 50% of participants were angina-free at 6 weeks in the ORBITA trial) were similar to what we observed.31,32 Finally, the ISCHEMIA-CKD trial did not show a significant or sustained benefit for health status with invasive treatment, and it seems unlikely that a sustained clinically relevant placebo effect would be present in the main trial but absent among patients with advanced chronic kidney disease.30
In summary, in the overall trial population of patients with stable ischemic heart disease and moderate or severe ischemia, including 35% of participants who had no angina at baseline, participants in the invasive-strategy group had larger improvements in angina-related health status than did participants in the conservative-strategy group. The modest mean benefit of the invasive strategy with respect to health status reflected minimal benefits in asymptomatic patients and larger benefits in patients who had angina at baseline.
Supplementary Material
Acknowledgments
Supported by a grant (U01HL105565) from the National Heart, Lung, and Blood Institute. Dr. Harrell is the recipient of a grant (UL1 TR002243) from the National Center for Advancing Translational Sciences, National Institutes of Health. The main ISCHEMIA trial was also supported by grants (U01HL105907, U01HL105462, and U01HL105561) from the National Heart, Lung, and Blood Institute, by Arbor Pharmaceuticals and AstraZeneca Pharmaceuticals, and by a Clinical and Translational Science Award (11UL1 TR001445) from the National Center for Advancing Translational Sciences. Devices or medications used in the trial were provided by Abbott Vascular, Medtronic, St. Jude Medical, Volcano, Amgen, Arbor Pharmaceuticals, AstraZeneca Pharmaceuticals, Espero Pharmaceuticals, Merck Sharp & Dohme, Omron Healthcare, and Sunovion Pharmaceuticals.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, the National Institutes of Health, or the Department of Health and Human Services.
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The full list of members of the ISCHEMIA Research Group is provided in the Supplementary Appendix, available at NEJM.org.
Contributor Information
John A. Spertus, Saint Luke’s Mid America Heart Institute Kansas City; University of Missouri–Kansas City, Kansas City.
Philip G. Jones, Saint Luke’s Mid America Heart Institute Kansas City University of Missouri–Kansas City, Kansas City.
David J. Maron, Department of Medicine, Stanford University School of Medicine, Stanford, CA
Sean M. O’Brien, Duke Clinical Research Institute and Duke University, Durham, NC
Harmony R. Reynolds, New York University Grossman School of Medicine
Yves Rosenberg, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Gregg W. Stone, Icahn School of Medicine at Mount Sinai, Cardiovascular Research Foundation, New York
Frank E. Harrell, Jr., Department of Biostatistics, Vanderbilt University School of Medicine, Nashville
William E. Boden, Veterans Affairs (VA) New England Healthcare System, Boston University School of Medicine, Boston
William S. Weintraub, MedStar Washington Hospital Center, Washington, DC
Khaula Baloch, Duke Clinical Research Institute and Duke University, Durham, NC
Kreton Mavromatis, Atlanta VA Healthcare System, Emory University School of Medicine, Atlanta
Ariel Diaz, Centre integre universitaire de sante et de services sociaux de la Mauricie-et-du-Centre-du-Quebec (CIUSSS MCQ), University of Montreal, Campus Mauricie, Trois-Rivieres, QC
Gilbert Gosselin, Montreal Heart Institute, Montreal-both in Canada.
Jonathan D. Newman, New York University Grossman School of Medicine
Stavroula Mavromichalis, New York University Grossman School of Medicine
Karen P. Alexander, Duke Clinical Research Institute and Duke University, Durham, NC
David J. Cohen, University of Missouri–Kansas City, Kansas City
Sripal Bangalore, New York University Grossman School of Medicine
Judith S. Hochman, New York University Grossman School of Medicine
Daniel B. Mark, Duke Clinical Research Institute and Duke University, Durham, NC
REFERENCES
- 1.Smith SC Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124:2458–73. [DOI] [PubMed] [Google Scholar]
- 2.Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006;113:2363–72. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;69:2212–41. [DOI] [PubMed] [Google Scholar]
- 4.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126(25):e354–e471. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub WS, Spertus JA, Kolm P, et al. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med 2008;359:677–87. [DOI] [PubMed] [Google Scholar]
- 6.Dagenais GR, Lu J, Faxon DP, et al. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation 2011;123:1492–500. [DOI] [PubMed] [Google Scholar]
- 7.ISCHEMIA Trial Research Group, Maron DJ, Hochman JS, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: rationale and design. Am Heart J 2018;201:124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med DOI: 10.1056/NEJMoa1915922. [DOI] [PubMed] [Google Scholar]
- 9.Hochman JS, Reynolds HR, Bangalore S, et al. Baseline characteristics and risk profiles of participants in the ISCHEMIA randomized clinical trial. JAMA Cardiol 2019;4:273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 11.EuroQol Group. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 12.Spertus J, Mark D. ISCHEMIA trial update. Am Heart J 2019;218:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle Angina Questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994;74:1240–4. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts longterm outcome in outpatients with coronary disease. Circulation 2002;106:43–9. [DOI] [PubMed] [Google Scholar]
- 17.Patel KK, Arnold SV, Chan PS, et al. Validation of the Seattle Angina Questionnaire in women with ischemic heart disease. Am Heart J 2018;201:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold SV, Kosiborod M, Li Y, et al. Comparison of the Seattle Angina Questionnaire with daily angina diary in the TERISA clinical trial. Circ Cardiovasc Qual Outcomes 2014;7:844–50. [DOI] [PubMed] [Google Scholar]
- 19.Arnold SV, Morrow DA, Lei Y, et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes 2009;2:344–53. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Shepherd BE, Li C, Harrell FE Jr. Modeling continuous response variables using ordinal regression. Stat Med 2017;36:4316–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spertus JV, Hatfield LA, Cohen DJ, et al. Integrating quality of life and survival outcomes in cardiovascular clinical trials. Circ Cardiovasc Qual Outcomes 2019;12(6):e005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 23.Carpenter B, Gelman A, Hoffman M, et al. Stan: a probabilistic programming language. J Stat Softw 2017;76(1):1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stan Development Team. RStan: the R interface to Stan. R Package Version 2182. 2018.
- 25.Burkner P BRMS: an R Package for Bayesian multilevel models using Stan. J Stat Softw 2017;80:1–28. [Google Scholar]
- 26.Goodrich B, Gabry J, Ali I, Brilleman S. RStanArm: Bayesian applied regression modeling via Stan. R Package Version 2182. 2018. [Google Scholar]
- 27.Wickham H Tidyverse: easily install and lose the ‘Tidyverse.’ R Package Version 121. 2017. [Google Scholar]
- 28.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation 2004;110:3789–94. [DOI] [PubMed] [Google Scholar]
- 29.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 30.Spertus JA, Jones PG, Maron DJ, et al. Health status after invasive or conservative care in coronary and advanced kidney disease. N Engl J Med. DOI: 10.1056/NEJMoa1916374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a doubleblind, randomised controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 32.Al-Lamee R, Howard JP, Shun-Shin MJ, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation 2018;138:1780–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.