INTRODUCTION:
The prognosis of Cronkhite–Canada syndrome (CCS) is considered poor. Despite the recent therapeutic improvements, the survival outcomes and prognostic factors have been less studied. This study aimed to investigate the long-term clinical and endoscopic outcomes of CCS.
METHODS:
Thirty-one patients diagnosed since 1999 and followed up for over 6 months were included. Data regarding survival outcomes, clinical symptoms, endoscopic findings, and treatment were collected and analyzed. R (version 3.6.1) was used to perform the survival analyses.
RESULTS:
The median (interquartile range) follow-up time was 42.5 (19.5–85.8) months. The 5-year overall survival (OS) was 87.4%. The maximum gastric polyp size over 2 cm was associated with worse OS (Hazard ratio [HR]: 18, 95% confidence interval [CI]: 1.6–210, P = 0.021). The 3-year relapse-free survival (RFS) after corticosteroid treatment was 66.8%. Age older than 60 years (HR: 7.0, 95% CI: 1.5–33.0, P = 0.015) and maximum gastric polyp size over 2 cm (HR: 6.0, 95% CI: 1.6–23.0, P = 0.009) were associated with worse RFS. Twenty-three patients received follow-up endoscopic examinations, with a median (interquartile range) follow-up time of 29.0 (14.0–53.5) months. Eight (34.8%) and 12 (52.2%) patients achieved complete remission under gastroscopy and colonoscopy, respectively. Colonic lesions showed a tendency of earlier responses compared with gastric lesions (25.0 [11.3–39.8] months vs 31.0 [21.0–39.8] months).
DISCUSSION:
Patients with CCS usually responded well to glucocorticoids with a fairly good 5-year survival rate. Large gastric polyp was associated with worse OS and RFS, whereas age older than 60 years was another predictor for worse RFS. Diffuse gastrointestinal lesions partly or completely resolved after treatment, and colonic lesions showed a better response than gastric lesions.
INTRODUCTION
Cronkhite–Canada syndrome (CCS) is a rare nonhereditary disease characterized by diffuse gastrointestinal (GI) polyposis and ectodermal abnormalities (1). Patients typically present with GI-related symptoms (e.g., refractory diarrhea, abdominal pain, and anorexia) accompanied by ectodermal changes, such as alopecia, onychodystrophy, and hyperpigmentation (2). Under endoscopy, CCS polyps typically present as multiple distinct sessile polyps with involvement of the entire GI tract excluding the esophagus (3). The diagnosis of CCS is based on a combination of characteristic clinical, endoscopic, radiologic, and histologic findings (4–6).
The etiology of CCS remains controversial, but it is generally considered a chronic inflammatory disease associated with an autoimmune mechanism (4). Such evidence includes elevated immunoglobulin G4 (IgG4) levels in circulation (7), polyp infiltration by IgG4 plasma cells (8), and generally good clinical response to immunosuppressive therapies (7,9). Whole-exome sequencing of one patient with CCS identified a rare germline mutation in the protein kinase, DNA-activated, catalytic subunit (PRKDC) gene, whose dysfunction might account for some of the manifestations, providing a possible clue for the pathogenesis (10). Treatment options include immunosuppressive therapy (corticosteroids and immunosuppressants), nutritional support, antibiotics, acid suppression, and surgical interventions (2,3,6,10,11). Corticosteroids are the mainstay in remission induction, with a proportion of patients having their GI polyps partly or completely disappeared (7,9). However, relapse is frequent during dosage tapering (7,9), which constitutes a major challenge in disease management.
CCS was traditionally recognized with a relentlessly progressive disease course and usually unfavorable prognosis. The mortality was reported to be over 50% in previous literature (2). Sustained malnutritional state, complex comorbidities, and increased incidence of GI malignancies may account for the poor outcomes (2,6,12), although convincing evidence is still needed. However, recovery from this disease and long-term remission have been reported (6,9,13–15). Diminution of polyp size and number was also observed (6,9). An improved prognosis of CCS has been proposed, but the precise survival rate and factors affecting the long-term outcomes were not specified (9). With improvements in diagnosis, changes in treatment modes, and progress in surveillance techniques during the past 2 decades, the long-term prognosis of CCS should be reexamined. Therefore, the aims of this retrospective cohort study were to explore the serial clinical and endoscopic outcomes of patients with CCS, to update the overall survival (OS) and relapse-free survival (RFS), and to identify the potential prognostic factors based on the 20-year experience from our center.
METHODS
Ethical aspects
This study was approved by the Institutional Review Board of the Peking Union Medical College Hospital (PUMCH) (No. ZS-1725).
Study population
Patients were identified through a PUMCH registry; the query dates were from January 1999 to July 2019. The diagnosis of CCS was confirmed by 2 experienced gastroenterologists (J.L. and J.Q.) based on a chart review. The diagnostic criteria (as shown in Supplementary File 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A255) included diffuse GI polyposis and ectodermal changes (alopecia, nail dystrophia, and skin pigmentation) supported by clinical, endoscopic, radiologic, and histologic findings, which were adopted in previous studies (5,6). Other polyposis syndromes, such as familial adenomatous polyposis, juvenile polyposis, Cowden syndrome, and Peutz–Jeghers syndrome, were excluded (3).
As Figure 1 shows, 36 patients with CCS were identified in the registry. Patients who were lost to follow-up after discharge (n = 3) and who were newly diagnosed in the past 6 months (n = 2) were excluded; in total, 31 patients were eligible for further analysis. Baseline characteristics were extracted from the medical records, including demographics, clinical and physical findings at the initial admission, and laboratory, endoscopy, and histopathologic findings. The treatment regimen and follow-up information were collected from later inclinic/outclinic records of all patients and were confirmed by a trained researcher (S.L.) using telephone interviews (n = 25). The clinical symptoms, medication usage, and major adverse events were documented.
Figure 1.
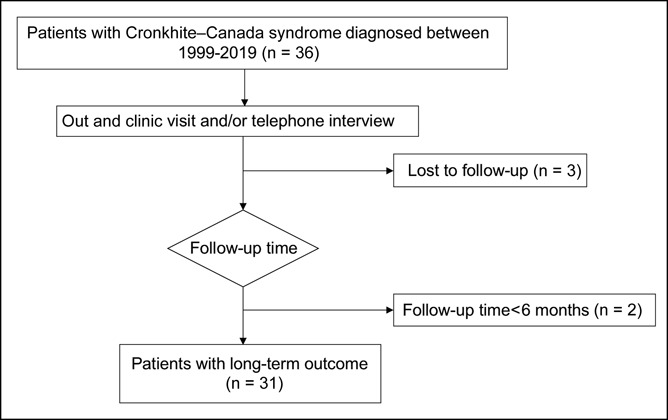
Flowchart of patient selection and follow-up.
Disease assessment
Disease assessment was performed independently by 2 gastroenterologists (J.L. and D.W.) at baseline and at each follow-up. The clinical assessments were based on the medical records and information provided by patients or caretakers during a telephone interview, whereas the endoscopic assessments were based on the imaging records retrieved from the endoscopic database of the PUMCH. The histopathologic findings of all tissue specimens were reviewed anonymously and recorded by an experienced GI pathologist (Y.Y.).
The definition of clinical outcomes was modified based on previous studies (7,9,16). Clinical complete remission (CR) was defined as formed stool with a frequency no more than 2 times per day and restoration of GI and ectodermal abnormalities. Clinical partial remission (PR) was defined as semiformed or watery stool with a frequency no more than 4 times per day or 50% of previous frequency, with alleviated GI and ectodermal symptoms. Clinical nonresponse referred to the conditions where patients did not undergo improvements after treatment.
The definition of endoscopic responses was as follows: endoscopic CR (eCR) was defined as the disappearance of polyps or less than 5 isolated polyps remaining, without congestion and edema or swelling in adjacent mucosa; endoscopic PR was defined as at least 50% reduction of involved GI segment, polyp number, or polyp size compared with baseline along with alleviation of mucosal inflammation; endoscopic nonresponse referred to the same or aggravated findings compared with baseline.
Variables related to outcomes
The primary outcome was OS, which was defined as the time interval from the diagnosis of CCS to death for any cause or the last follow-up. The secondary outcome was RFS, which was defined as the time interval from the corticosteroid treatment to the first relapse. Relapse was defined as recurrence of clinical symptoms (typically diarrhea accompanied with one or more ectodermal symptoms) along with active endoscopic findings. To explore the potential factors related to the survival and relapse of CCS, we analyzed the following variables: sex, age at diagnosis, disease duration, severe comorbidities at baseline, autoimmune diseases, maximal size of gastric and colonic polyps, detection of GI adenoma, usage of long-term corticosteroids, usage of immunosuppressants, and responses to corticosteroids.
Disease duration was defined as the time interval between the onset of indicative symptoms to the final diagnosis. Severe comorbidities referred to those diseases having extensive effects on the general health, such as dysfunction of vital organs, systemic diseases, and malignancies. Because corticosteroid was typically scheduled to be withdrawn in 3–6 months in our center, we define the use of long-term corticosteroids as taking corticosteroids for over 1 year (regardless of dosage). To differentiate patients with sustained remission and relapse propensity, we define responses to corticosteroids as follows: prolonged response (defined as sustained clinical remission after the first round of corticosteroids), corticosteroid dependency (defined as clinical remission followed by relapse during corticosteroid tapering or discontinuance), and refractoriness (defined as no obvious improvement with the standard corticosteroid regimen).
Statistical analysis
Continuous variables were expressed as either the median plus interquartile range (IQR) for nonparametric data or the mean ± SD for parametric data. Categorical data were summarized as the percentage of the total group. The difference in remission rate under gastroscopy and colonoscopy was compared by the McNemar test. The R package “survival” was used to calculate the 3-, 5-, and 10-year OS and 1-, 3-, and 5-year RFS and plot survival curves for the whole cohort and within each stratification of variables (17). The Cox proportional hazard model was used for the univariate and multivariate analyses to identify the significant or independent prognostic factors for OS and RFS. Variables with P values less than 0.2 in the univariate analysis were included in the multivariate model. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed with R (version 3.6.1).
RESULTS
Patient characteristics
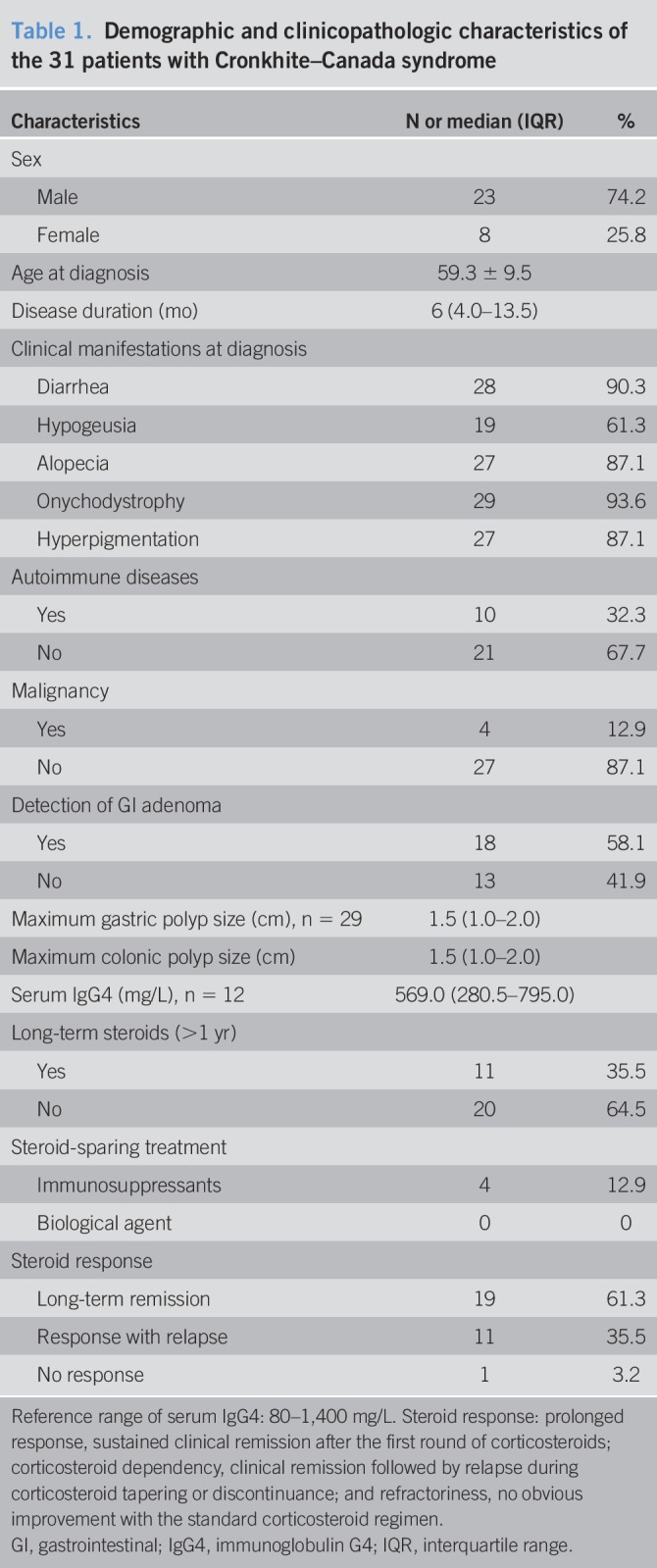
The demographic and clinical characteristics of the 31 patients with CCS are shown in Table 1, with details listed in Supplementary File 2 (see Supplementary Digital Content 2, http://links.lww.com/CTG/A256). Among these patients, 23 (74.2%) were men, generating a male-to-female ratio of 2.9:1. At the time of diagnosis, the patients were 59.3 ± 9.5 years old, with a median (IQR) disease duration of 6 (4.0–13.5) months. Twenty-two (71.0%) patients had comorbidities, including 10 with autoimmune diseases and 4 with malignant tumors (2 colorectal cancers and 2 extra-GI cancers). For autoimmune disorders, 3 patients were complicated with the Hashimoto thyroiditis, 2 with vitiligo, 2 with nephrotic syndrome, 1 with rheumatoid arthritis, 1 with adult-onset Still disease, and 1 with xerophthalmia. Regarding the clinical manifestations at onset, 28 (90.3%) patients reported diarrhea, 29 (93.5%) patients had onychodystrophy, 27 (87.1%) patients had alopecia, 27 (87.1%) patients had hyperpigmentation, and 19 (61.3%) patients had hypogeusia.
Table 1.
Demographic and clinicopathologic characteristics of the 31 patients with Cronkhite–Canada syndrome
Treatment procedures and clinical outcomes
All patients received corticosteroid treatment in a tapered regimen. The typical initial dosage was an equivalent of prednisone 1 mg/kg/d and tapered steadily and withdrawn in 3–6 months. If relapse occurred, the patients received an augmented dosage of corticosteroid or restarted the remission induction. The therapeutic regimen for 2 patients with nephrotic syndrome followed a slower prednisone-tapering schedule, and it involved immunosuppressants (cyclophosphamide and cyclosporin A) in the later stage. Within the time frame of this study, relapse occurred in 11 (35.5%) patients during or after the cessation of glucocorticoid use. Eleven (35.5%) patients used corticosteroid for over 1 year, among whom 5 were still on low-dose corticosteroid maintenance at the last follow-up. Four patients used immunosuppressants as steroid-sparing treatment (3 patients on azathioprine 50 mg/d and 1 patient on thalidomide 75 mg/d). No patient used biological agent. The 3-year clinical outcomes are shown in Supplementary File 3 (see Supplementary Digital Content 3, http://links.lww.com/CTG/A257). At the last follow-up, 23 (74.2%) patients achieved remission of GI symptoms, among whom 19 (61.3%) reached clinical CR. Adverse events along with corticosteroid treatment were reported in 10 of 31 (32.3%) patients, among whom the most frequent complaint was osteoporosis (4 patients).
Endoscopic assessment
All patients had undergone endoscopic evaluation either after admission into our hospital or in other hospital before admission. At the initial evaluation, the maximum gastric and colonic polyp sizes of patients with CCS were both 1.5 (1.0–2.0) cm in diameter. During the disease course, a total of 39 adenomas were detected in 18 patients, with a detection rate of 18 of 31 (58.1%). Twenty-two adenomas were resected, whereas the others were only sampled.
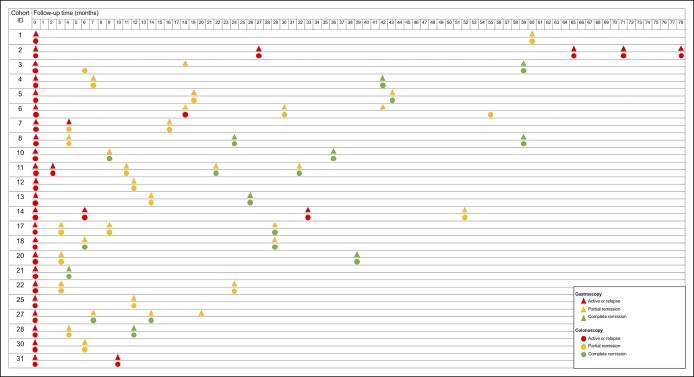
Twenty-three patients received follow-up endoscopic examinations, with a median (IQR) follow-up time of 29.0 (14.0–53.5) months. The number of patients who achieved eCR under gastroscopy and colonoscopy was 8 (34.8%) and 12 (52.2%), respectively (P = 0.37). As shown in Figure 2, all 8 patients with gastroscopic eCR also achieved eCR under colonoscopy. However, among the 12 patients who achieved colonoscopic eCR, 4 patients still had multiple polyps under gastroscopy, whereas 1 patient achieved gastroscopic CR later, which means 5 of 12 (41.7%) patients achieved CR faster under colonoscopy. The earliest eCR was observed at the fourth month in one patient, at which time both the gastric and colonic mucosa appeared normal. Supplementary File 4 (see Supplementary Digital Content 4, http://links.lww.com/CTG/A258) depicts the process in which GI polyps gradually attenuate and eventually disappear along with glucocorticoid therapy.
Figure 2.
The serial endoscopic examinations of patients with Cronkhite–Canada syndrome. Endoscopic assessments taken at a certain time point are recorded for each patient; the triangles represent gastroscopic examinations, and the circles represent colonoscopic examinations. Red, yellow, and green colors indicate active stage, partial remission, and complete remission, respectively.
OS, RFS, and prognostic factors
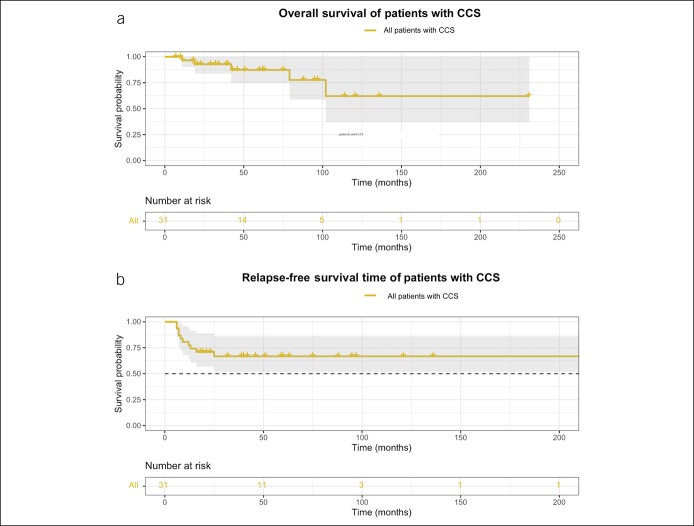
The median (IQR) follow-up time was 42.5 (19.5–85.8) months. Five patients died by the time of the last follow-up, and the causes of death included heart failure, renal failure, intussusception, rectal cancer, and cerebral hemorrhage. The 3-, 5-, and 10-year OS was 92.8% (95% confidence interval [CI]: 83.7%–100%), 87.4% (95% CI: 74.7%–100%), and 62.1% (95% CI: 36.9%–100%), respectively, as shown in Figure 3a. Univariate Cox analysis revealed that the maximum gastric polyp size >2 cm (hazard ratio [HR]: 18, 95% CI: 1.6–210, P = 0.021) was associated with worse survival outcomes (Figure 4a). Based on multivariate Cox regression analysis, a maximum gastric polyp size >2 cm was also an independent prognostic factor (HR: 30.0, 95% CI: 1.1–849.3, P = 0.003).
Figure 3.
Survival outcomes of patients with CCS. (a) Overall survival (OS) of patients with CCS. OS was defined as the time interval from the diagnosis of CCS to death for any cause or the last follow-up. (b) Relapse-free survival (RFS) of patients with CCS. RFS was defined as the time interval from the corticosteroid treatment to the first relapse. Relapse was defined as recurrence of gastrointestinal and ectodermal manifestations along with active endoscopic findings. CCS, Cronkhite–Canada syndrome.
Figure 4.
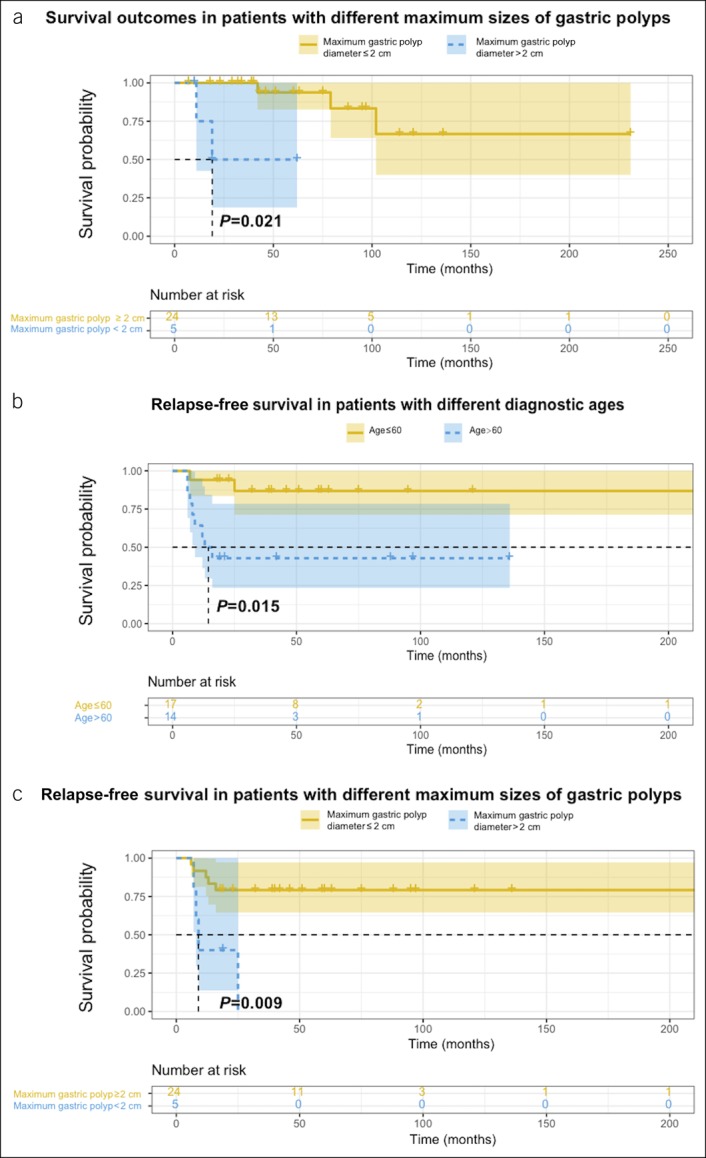
Prognostic factors of overall survival (OS) and relapse-free survival (RFS). (a) OS in patients with different maximum sizes of gastric polyps. (b) RFS in patients with different diagnostic ages. (c) RFS in patients with different maximum sizes of gastric polyps.
The 1-, 2-, and 3-year RFS was 77.4% (95% CI: 64.0%–93.6%), 71.0% (95% CI: 56.7%–88.9%), and 66.8% (95% CI: 51.8%–86.2%), respectively, as shown in Figure 3b. On univariate Cox analysis, age older than 60 years (HR: 7.0, 95% CI: 1.5–33.0, P = 0.015) and maximum gastric polyp size >2 cm (HR: 6.0, 95% CI: 1.6–23.0, P = 0.009) were significantly associated with worse RFS (Figure 4b,c). These 2 factors were also significant on multivariate Cox regression (age older than 60 years: HR: 8.0, 95% CI: 1.5–43.0, P = 0.015; maximum gastric polyp size >2 cm: HR: 7.7, 95% CI: 1.6–38.3, P = 0.012).
DISCUSSION
In this study, we explored the clinical course, endoscopic responses, OS and RFS, and prognostic factors of CCS. It is intriguing that the 5-year OS in our cohort was 87.4%, which was higher than previously reported (2). We quantified the 3-year RFS after corticosteroid treatment to be 66.8%. This study clearly showed the changes of endoscopic features along with the treatment and first observed a better and faster recovery of colonic lesions compared with gastric lesions.
For the epidemiological aspects, we found a male predominance of patients with CCS, which was in accordance with previous studies (9,18). Our cohort showed a high incidence of autoimmune comorbidities, which was consistent with the previously reported association of CCS with autoimmune diseases (3). However, a Japanese nationwide survey showed a relatively low coincidence of autoimmune disorders, with only 6 of 210 patients having immunological thyroid diseases and 1 having rheumatoid arthritis (9). In this study, we did not observe an association between autoimmune comorbidity and long-term prognosis. Besides, the elevation of IgG4 was not prominent in our cohort. Among the 12 patients with IgG4 measurements, only 2 patients had IgG4 values above the reference range. In the subset of patients with autoimmune background, 4 patients had IgG4 checked, yet none had elevated IgG4 values.
Corticosteroid is the mainstay medication of CCS. However, the long-term efficacy and side effects of low-dose corticosteroids still require observation. In this study, 61.3% of the patients achieved long-term clinical remission after corticosteroid administration, which is similar to the 61.1% long-term response reported in the Japanese survey (9). A proportion of patients were prescribed low-dose (5–10 mg/d) corticosteroids or immunosuppressants to counteract the tendency of relapse. Only 1 relapse was reported during the above treatment, and no obvious side effects were reported. Considering the frequent relapses, a maintenance regimen might be considered for better disease control. Adverse events along with corticosteroid treatment were frequently reported in the medical records, with osteoporosis being the most common. Considering that patients with CCS are mainly the elderly, osteoporosis caused or exacerbated by corticosteroid treatment deserves more attention.
Endoscopic examination is helpful for the evaluation of treatment responses and the assessment of mucosal healing, which is important in autoimmune nonspecific bowel diseases, such as inflammatory bowel disease (19,20). Indications for follow-up endoscopy vary from disease to disease, and it has not been established in CCS. Endoscopic examination can directly reflect the efficacy of treatment and distinguish relapse from other possible causes of diarrhea, thereby guiding adjustments in treatment. We matched the medical records with endoscopy findings and found that 35 person-times of follow-up endoscopies were performed in stable conditions, 10 of which were followed by adjustments in treatment. Twelve person-times of follow-up endoscopies were performed after recurrence of symptoms, 8 of which were followed by adjustments in treatment. These observations suggested that patients with CCS may benefit from follow-up endoscopy via guidance of treatment, especially when symptoms recur. Although this is insufficient to clarify the exact role of follow-up endoscopy in CCS management, we would like to do more research to explore the indications of follow-up endoscopy in the future.
Our study clarified the changes in GI polyps during the long-term follow-ups. Considering the frequency of sporadic colonic polyps in the elderly, the definition of eCR allowed for the existence of less than 5 isolated polyps on the background of normal mucosa. We observed eCR in some patients, which supported the reversal potential of CCS polyposis (13,14). One interesting tendency is that colonic lesions responded to corticosteroids better and faster than gastric lesions. This phenomenon has not been reported in other autoimmune or autoinflammatory related GI diseases. Further investigation is needed to verify and explore the underlying mechanisms of this phenomenon. Seven of 12 (58.3%) patients achieved CR under colonoscopy after 1 year, which indicates the importance of prolonged treatment and endoscopic surveillance. This finding was in accordance with the Japanese study, in which gastric polyps and colonic polyps took on average 248 days and 238 days, respectively, to show a reduction in size and number (9). Because of the retrospective nature of our study, we could only obtain the time point when recovery was first detected, which may overestimate the time needed for remission. Regular follow-ups based on a prospective design would be necessary to further specify the endoscopic remission time.
Endoscopic monitoring also acts as an important tool for cancer screening. Although CCS polyps are not considered neoplastic, CCS is considered to harbor increased risks of GI neoplasms. A total of 15%–25% of patients have been documented with colorectal or gastric cancer at diagnosis, and 31%–71% of patients have been found with adenomas and adenomatous changes (5,9). Therefore, regular endoscopy surveillance should be performed in patients with CCS. In our cohort, 18 (58.1%) patients had adenomas during the disease course, but only 2 patients developed colorectal cancer within the follow-up time frame. Considering that sustained endoscopic remission is associated with a reduction in cancer risk (9), the low incidence of malignancy in our cohort may be partly explained by the generally good response to corticosteroid treatment.
We updated the 5-year survival rate of CCS based on the experience of our center. Compared with the dated 5-year mortality cited as 55% (2), the current 5-year survival has improved dramatically, which is consistent with the tendency in recent studies (7,9). We believe that many factors may be associated with the improved outcome. First, because the effectiveness of immunosuppression has been proved by many studies, the widespread use of corticosteroids and immunosuppressants may contribute to the general improvement of outcomes. Second, the diagnostic delay may shorten along with improved awareness of CCS in recent years, allowing patients to receive proper treatment in the earlier stages of the disease course. Finally, therapeutic advances in supportive treatment (nutritional support, flora regulation, antibiotics, etc.) may also contribute to better prognosis.
Previous reported causes of death because of CCS include GI hemorrhage, infection, malnutrition, fluid–electrolyte imbalance, coagulation abnormalities, or congestive heart failure (3). In our study cohort, heart failure, renal failure, and cerebral hemorrhage appeared not to be directly related to CCS. It was difficult to trace back whether these events had an association with corticosteroids. Rectal cancer was diagnosed in one patient 5 years after CCS diagnosis, with the surrounding mucosa appearing normal when malignancy was detected. This patient recovered from clinical symptoms after treatment and showed PR of polyposis at 6- and 18-month follow-ups. It cannot be concluded whether the rectal cancer was related to CCS. Only intussusception, which is a major complication of CCS (2), can be attributed as a “disease-related” cause of death. Larger gastric polyps (maximum diameter >2 cm) were a predictor for worse prognosis, which may be associated with a higher risk of intussusception, GI bleeding, and malignancy (21). However, the size of colonic polyps did not show an obvious influence on prognosis. Whether this is a fortuitous finding still needs further exploration. The coexistence of colorectal adenoma and cancer was not associated with the long-term outcomes, possibly because of the low percentage of GI malignancy in the cohort. Besides, responses to corticosteroids did not show a significant influence on OS. Considering that most patients eventually reached clinical remission after various rounds of corticosteroids or with maintenance treatment, the sensitivity to corticosteroids may not be the decisive factor for survival outcomes.
We also quantified the RFS after corticosteroid treatment. RFS tended to be steady after 3 years, and most relapses occurred in the first year. In our cohort, patients who started corticosteroid treatment after the age of 60 years and who had larger gastric polyps (maximum diameter >2 cm) at baseline examination showed shorter RFS. These findings indicate that corticosteroid responsiveness may be influenced by age and polyp size, although further evidence is needed. We suggest that clinicians consider a more moderate tapering plan and maintenance regimen for patients older than 60 years and patients with larger gastric polyps.
As a retrospective single-center study, this study may have uncontrolled selection bias and incomplete records, which may lead to insignificant tendencies in statistical analysis. To overcome these limitations, we first attempted to obtain the long-term outcomes through phone interviews and outclinic visits. Since the disease was first described in 1955, only approximately 500 patients with CCS have been reported worldwide, among which over 70% were from Japan. Currently, the largest cohort was a Japanese nationwide survey that incorporated 210 cases from 139 facilities. In this study, we reported the largest single-center cohort of Chinese patients, which held valuable information considering the rarity and geographical distribution. Second, 2 experienced gastroenterologists worked separately during the study, including confirming the diagnosis, evaluating the treatment responses, and interpreting all endoscopic atlas, which helped overcome reviewer bias. In the future, further prospective studies should be performed to better identify the treatment responses and prognostic factors.
In conclusion, patients with CCS usually responded well to corticosteroids with an improved 5-year survival rate. Large gastric polyp was associated with worse OS and RFS, whereas age older than 60 years was another predictor for worse RFS. Diffuse GI polyps can partially or completely disappear after treatment, and colonic lesions showed better responses than gastric lesions. Early diagnosis, corticosteroid-centered medications, regular endoscopic monitoring, and novel treatment options might provide more insights for better prognosis in the future.
CONFLICTS OF INTEREST
Guarantor of the article: Shuang Liu, BS and Ji Li, MD.
Specific author contributions: Shuang Liu, BS and Yan You, MD, contributed equally to this work. Ji Li, MD and Jiaming Qian, MD, contributed equally to this work. S.L., Y.Y., J.L., and J.Q. conceptualized the study. G.R., D.W., X.Y., S.Z., J.L., and J.Q. acquired the clinical data. D.W., J.L., and J.Q. confirmed the disease diagnosis and performed the disease assessment. Y.Y. and W.Z. performed the histopathological assessments. S.L. and D.C. acquired the follow-up information data. S.L. organized the information and performed the statistical analysis. S.L., Y.Y., J.L., and J.Q. prepared the manuscript. All authors participated in reviewing and editing the manuscript and in the approval of the finalized manuscript.
Financial support: This work was supported by the National Key Research and Development Program of China (grant number 2016YFC0901500), the Funding for Elite Training in the Dongcheng District, Beijing (grant number 2018-34), and the Undergraduate Innovation Program (grant number 2019zlgc0651).
Potential competing interests: None to report.
Informed consent: Informed consent was obtained from all participants.
Study Highlights.
WHAT IS KNOWN
✓ The prognosis of CCS is considered poor.
✓ The long-term outcomes of CCS have been less studied despite the recent improvements in treatment.
WHAT IS NEW HERE
✓ The 5-year survival rate of CCS was 87.4% with glucocorticoid treatment.
✓ Large gastric polyp size was associated with worse OS.
✓ Large gastric polyp size and age older than 60 years were predictors for worse RFS.
✓ Colonic lesions showed a better response compared with gastric lesions.
Supplementary Material
ACKNOWLEDGMENTS
We would thank all patients for their support and Tian Du for his assistance with statistical analysis. All authors (S.L., Y.Y., G.R., L.Z., D.C., D.W., X.Y., S.Z., W.Z., J.L., and J.Q.) approved the final version of the article.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A255, http://links.lww.com/CTG/A256, http://links.lww.com/CTG/A257, and http://links.lww.com/CTG/A258
REFERENCES
- 1.Cronkhite LW, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med 1955;252:1011–5. [DOI] [PubMed] [Google Scholar]
- 2.Daniel ES, Ludwig SL, Lewin KJ, et al. The Cronkhite-Canada syndrome: An analysis of clinical and pathologic features and therapy in 55 patients. Medicine (Baltimore) 1982;61:293–309. [PubMed] [Google Scholar]
- 3.Slavik T, Montgomery EA. Cronkhite–Canada syndrome six decades on: the many faces of an enigmatic disease. J Clin Pathol 2014;67:891–7. [DOI] [PubMed] [Google Scholar]
- 4.Goto A. Cronkhite-Canada syndrome: Epidemiological study of 110 cases reported in Japan. Nihon Geka Hokan 1995;64:3–14. [PubMed] [Google Scholar]
- 5.Sweetser S, Boardman LA. Cronkhite-Canada syndrome: An acquired condition of gastrointestinal polyposis and dermatologic abnormalities. Gastroenterol Hepatol (N Y) 2012;8:201–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Goto A, Mimoto H, Shibuya C, et al. Cronkhite-Canada syndrome: An analysis of clinical features and follow-up studies of 80 cases reported in Japan. Nihon Geka Hokan 1988;57:506–26. [PubMed] [Google Scholar]
- 7.Sweetser S, Ahlquist DA, Osborn NK, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: Support for autoimmunity. Dig Dis Sci 2012;57:496–502. [DOI] [PubMed] [Google Scholar]
- 8.Riegert-Johnson DL, Osborn N, Smyrk T, et al. Cronkhite-Canada syndrome hamartomatous polyps are infiltrated with IgG4 plasma cells. Digestion 2007;75:96–7. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe C, Komoto S, Tomita K, et al. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: A Japanese nationwide survey. J Gastroenterol 2016;51:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland BS, Bagi P, Valasek MA, et al. Cronkhite-Canada syndrome: Significant response to infliximab and a possible clue to pathogenesis. Am J Gastroenterol 2016;111:746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe D, Ooi M, Hoshi N, et al. Successful treatment of Cronkhite-Canada syndrome using anti-tumor necrosis factor antibody therapy. Endoscopy 2014;46(Suppl 1 UCTN):E476–7. [DOI] [PubMed] [Google Scholar]
- 12.Chadalavada R, Brown DK, Walker AN, et al. Cronkhite-Canada syndrome: Sustained remission after corticosteroid treatment. Am J Gastroenterol 2003;98:1444–6. [DOI] [PubMed] [Google Scholar]
- 13.Marin SE, Guillen MP, Perez-Requena J, et al. Cronkhite-Canada syndrome: An acquired, potentially reversible polyposis. Gastroenterol Hepatol 2006;29:619–21. [DOI] [PubMed] [Google Scholar]
- 14.Doyle T, Jarvis R. Reversal of changes in Cronkhite-Canada syndrome. Australas Radiol 1984;28:19–22. [DOI] [PubMed] [Google Scholar]
- 15.Yu YQ, Whorwell PJ, Wang LH, et al. Cases report the Cronkhite-Canada syndrome: Improving the prognosis. Medicine (Baltimore) 2015;94:e2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon HH, Lee HJ, Jang HW, et al. Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis. World J Gastroenterol 2013;19:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer, New York, NY, 2013. [Google Scholar]
- 18.Ward EM, Wolfsen HC. Review article: The non-inherited gastrointestinal polyposis syndromes. Aliment Pharmacol Ther 2002;16:333–42. [DOI] [PubMed] [Google Scholar]
- 19.Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: A systematic review. Gut 2012;61:1619–35. [DOI] [PubMed] [Google Scholar]
- 20.Dulai PS, Levesque BG, Feagan BG, et al. Assessment of mucosal healing in inflammatory bowel disease: Review. Gastrointest Endosc 2015;82:246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shussman N, Wexner SD. Colorectal polyps and polyposis syndromes. Gastroenterol Rep (Oxf) 2014;2:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.