Abstract
The natural history of COVID‐19 caused by SARS‐CoV‐2 is extremely variable, ranging from asymptomatic or mild infection, mainly in children, to multi‐organ failure, eventually fatal, mainly in the eldest. We propose here the first model explaining how the outcome of first, crucial 10‐15 days after infection, depends on the balance between the cumulative dose of viral exposure and the efficacy of the local innate immune response (natural IgA and IgM antibodies, mannose‐binding lectin). If SARS‐CoV‐2 runs the blockade of this innate immunity and spreads from the upper airways to the alveoli in the early phases of the infections, it can replicate with no local resistance, causing pneumonia and releasing high amounts of antigens. The delayed and strong adaptive immune response (high‐affinity IgM and IgG antibodies) that follows, causes severe inflammation and triggers mediator cascades (complement, coagulation, and cytokine storm), leading to complications often requiring intensive therapy and being, in some patients, fatal. Low‐moderate physical activity can still be recommended. However, extreme physical activity and oral breathing with hyperventilation during the incubation days and early stages of COVID‐19 facilitates re‐inhalation and early direct penetration of high numbers of own virus particles in the lower airways and the alveoli, without impacting on the airway’s mucosae covered by neutralizing antibodies ("viral auto‐inhalation" phenomenon). This allows the virus to bypass the efficient immune barrier of the upper airway mucosa in already infected, young, and otherwise healthy athletes. In conclusion, whether the virus or the adaptive immune response reaches the lungs first is a crucial factor deciding the fate of the patient. This “quantitative and time‐/sequence‐dependent” model has several implications for prevention, diagnosis, and therapy of COVID‐19 at all ages.
Keywords: antibodies, COVID‐19, glycans, immunoglobulin M, pneumonia, prediction, protection, SARS‐CoV‐2
Short abstract

Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ARDS
acute respiratory distress syndrome
- CFR
case fatality ratio
- COVID‐19
coronavirus disease
- ICU
intensive care unit
- IgA
immunoglobulin isotype A
- IgG
immunoglobulin isotype G
- IgM
immunoglobulin isotype M
- IQR
interquartile range
- mAb
monoclonal antibody
- MBL
mannose‐binding lectin
- MERS
Middle East respiratory syndrome
- NK
natural killer
- PCR
polymerase chain reaction
- POCT
point‐of‐care test
- RBD
receptor‐binding domain
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
transmembrane protease serine 2
- XLA
X‐linked agammaglobulinemia
Key Message.
This model developed in this article has several implications for Public Health intervention, diagnosis, and therapy of COVID‐19.
The quantitative balance between innate immunity (natural IgM and IgA antibodies, MBL) versus the cumulative exposure dose to SARS‐CoV‐2 is a crucial factor deciding if the virus will penetrate the lower airways and alveoli early enough, ie before an adaptative immune response is established, massively replicate and cause a severe pneumonia.
Complications may arise when high affinity antibodies are produced, if the virus has already reached the alveoli and massive viral replication has occurred. Indeed, antibodies can limit viral infectivity, but non‐neutralizing antibodies also enhance infection by activating the complement system, the coagulation system and causing a IL‐6 driven cytokine storm, leading to complications.
Exercise‐induced oral breathing during Covid‐19 incubation and early pauci‐symptomatic stages causes not only heterologous (ie from other infected athletes), but also own viral particles contained in own exhaled aerosol to be re‐inhaled and penetrate the lower airways and alveoli (viral auto‐inhalation hypothesis). This phenomenon causes early, hence severe pneumonia.
This first, holistic model of COVID‐19 has several implications for Public Health intervention, diagnosis and therapy of COVID‐19.
Foreword—This article is dedicated to the memory of Dr. Li Wenliang, who on December 2019 first recognized a new disease and alerted the World of the SARS‐CoV‐2 epidemic before dying of COVID‐19 on 7 February 2020, at the age of 33, of Dr. Carlo Urbani, who on February 2003 first recognized a new disease and alerted the World of the SARS epidemic before succumbing to it on 29 March 2003, and of all the doctors and allied health personnel who have sacrificed their own lives to save those of their patients. We wish to honor their competence, bravery, and generosity.
1. NATURAL HISTORY OF COVID‐19 AND ANTIBODY RESPONSE INDUCED BY SARS‐COV‐2
1.1. Same virus, diverging disease evolution
SARS‐CoV‐2 is a zoonotic RNA betacoronavirus, 1 , 2 similar to SARS‐CoV, 3 , 4 emerging around November 2019 in humans living in the province of Hubei, China, 5 and rapidly spreading with a pandemic trend all over the World. 6 The consequences of infection with SARS‐CoV‐2 broadly vary from benign to fatal (Figure 1). 1 While many infected individuals remain asymptomatic or experience only mild upper airway symptoms, others develop symptomatic pneumonia, which may progress to ARDS requiring intubation in intensive care unit (ICU) and may undergo complications that can be fatal. 1 Viral shedding begins 2‐3 days before symptom onset. 7 Infectivity seems to decline significantly already after 10 days from symptom onset, 8 but the virus can be detected for a median of 20 days, up to 37 days among survivors. 9
Figure 1.
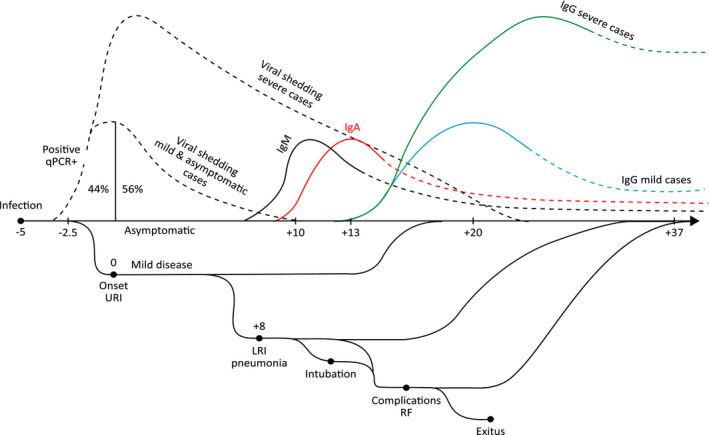
Different COVID‐19 clinical courses and trajectories of adaptive immune response and viral shedding. Quantitative polymerase chain reaction (qPCR); disseminated intravascular coagulation (DIC); upper respiratory airway infection (URI); lower respiratory airways infection (LRI); respiratory failure (RF)
The cumulative amount of virus exposure acquired by the patient at the start of infection cannot be measured. However, it may broadly range from a minimal amount, below the average number of viral particles needed to establish an infection (infectious dose), 10 to higher doses repeatedly acquired from multiple patients in hospitals or overcrowded environments. 11 This pattern of exposure has probably been common among healthcare personnel, especially in the early phases of the pandemic. 12
1.2. First stage: upper respiratory infection
Among infected humans, those developing coronavirus disease (COVID‐19) show their first symptoms on average 5‐6 days after infection 7 with a 95% confidence interval ranging from 2 to 14 days. 13 Initial symptoms are limited to upper airways (cough, sore throat) accompanied by fever, fatigue, and muscle ache, while nausea or vomiting and diarrhea are infrequent symptoms at onset. 14 At disease onset, the virus RNA is usually detected through swabs from nostrils or pharynx, and amplified and detected by PCR with rapid, qualitative POCT or classical, quantitative laboratory methods. 15 However, lower viral loads have been detected also in throat swabs from infected but asymptomatic humans. 15 , 16
1.3. Second stage: pneumonia
While most patients experience only mild fever and symptoms of the upper airways, others develop pneumonia with or without symptoms, mostly dyspnea. 1 , 17 Among 41 patients hospitalized in Wuhan, China, the median time from onset of symptoms to shortness of breath was 8.0 days (interquartile range, IQR, 5.0‐13.0). 18
1.4. Third stage: complications
Among the same 41 patients hospitalized in Wuhan, China, the median time from onset of symptoms to acute respiratory distress syndrome (ARDS) was 9.0 days (8.0‐14.0), to mechanical ventilation was 10.5 days (7.0‐14.0), and to ICU admission was 10.5 days (8.0‐17.0). 18 Dyspnea associated with decreased oxygenation index was the more frequent sign of respiratory failure (RF) or ARDS. Data from China reported that 53% of deaths were related to RF, 7% to shock (presumably from fulminant myocarditis), 33% to both, and 7% to unclear mechanisms. 19 Among 191 patients admitted in two hospitals in Wuhan, the median time from disease onset and from dyspnea to intubation was 14.5 days (12.0‐19.0) and 10 days (IQR 5.0‐12.5), respectively. 20 ARDS, acute cardiac and kidney injury, sepsis, and secondary infection were the most frequent complications. 20
1.5. Fourth stage: exitus or healing
Among patients dying for COVID‐19 in the Chinese study, death occurred 18.5 (15.0‐22.0) days after disease onset. 20 Among survivors, permanence in ICU lasted 7.0 days (2.0‐9.0); and discharge from the hospital occurred shortly thereafter. 20 Mortality is associated with older age, comorbidities (including hypertension, diabetes, cardiovascular disease, chronic lung disease, and cancer), higher severity of illness scores, worse RF, higher D‐dimer and C‐reactive protein concentrations, lower lymphocyte counts, and secondary infections (Figure 1). 21
2. MOLECULAR MECHANISMS OF SARS‐COV‐2 CELL ENTRY
2.1. Distribution of SARS‐CoV‐2
SARS‐CoV primarily infects pneumocytes and enterocytes of the small intestine. 22 SARS‐CoV‐2 has a similar tropism and also infects type II pneumocytes, enterocytes, and macrophages. 23 , 24 In addition, SARS‐CoV‐2 can infect cells expressing angiotensin‐converting enzyme 2 (ACE2) receptor and the serine protease TMPRSS2. 25 Investigations with a holistic data science platform suggested that the virus may infect many other cell types, among which are tongue keratinocytes (which may explain dysgeusia reported by some patients), airway club (Clara) cells, and ciliated cells. 26 This outcome also suggests that the exact distribution of SARS‐CoV‐2 in humans may be broader than expected from the sole distribution of membrane receptors directly docked by the virus.
2.2. Molecular mechanisms of SARS‐CoV‐2 entry
The molecular mechanisms used by SARS‐CoV‐2 to adhere and penetrate in host cells have been discovered. As SARS‐CoV, 27 SARS‐CoV‐2 uses its spike glycoprotein to bind to the ACE2 receptor. 28 The spike molecule, organized as a trimer, forms a needle whose round tip, comprised of the three S1 domains clustered together, protrudes to meet the ACE2. 28 After binding, TGRBSS2, a serine protease, cleaves the spike glycoprotein between S1 and S2, which allows the virus membrane to approach the cell membrane, fuse with it, and enter the cell. 29 The SARS‐CoV spike protein contains 22 N‐glycosylation sites. 30 The glycosylation pattern of viral particles produced in a human cell line has been already characterized in 13/22 sites. 30 Several N‐glycosylation sites, including mannose residues, surround the area of the molecule binding ACE2. This, dense glycosylation is used by the virus to mask surface peptide epitopes, which may induce and elicit neutralizing antibody responses, thus preventing docking on ACE2. 30 However, an area which remains free from glycosylation, is also suitable for ACE2 binding and potentially for other proteins, including neutralizing antibodies. 30
3. KINETICS OF THE ADAPTIVE ANTIBODY RESPONSE
3.1. New virus, no memory antibodies
SARS‐CoV‐2 is a new virus. The IgG antibodies induced by other common coronaviruses, or by SARS‐CoV and MERS, do not recognize and neutralize this new virus. 31 Accordingly, no specific IgG antibodies have been detected against the S glycoprotein in the early stages of the infection, that is, before an adaptive response has started. 31 , 32
3.2. Kinetics of the adaptive antibody response
Typical primary and secondary antibody responses to acute viral infection are efficiently induced. 32 An early Chinese study in 173 patients observed a median seroconversion time for Ab, IgM, and then IgG at day 12 and day 14, respectively. 32,32 (Figure 1) The SARS‐CoV‐2‐specific IgM antibodies appear around 8‐12 days after infection onset and vanish around the end of week 12. 33 , 34 The IgG antibody response starts appearing shortly thereafter (or simultaneously) but persist longer 31 , 32 , 33 , 34 and may be protective. 35
3.3. Persistence of the antibody levels
Most serological data currently available in the literature refer to patients examined mostly in the acute phase of the disease. Therefore, they are insufficient to exactly establish durability of the antibody titers of each isotype peak when they eventually disappear. The levels of serum IgG antibodies, however, seem to be proportional to the intensity of the viral load and to the symptom severity. 32 , 36
3.4. Effectiveness of the antibody response
The efficacy of specific Ig and their role in limiting viral spread may be indirectly assumed by observations demonstrating that plasma from subjects recovered from COVID‐19 showed a therapeutic efficacy if passively transferred to patients. 37 , 38 Similar effectiveness had been already demonstrated for plasma from patients having recovered from SARS‐CoV and MERS‐CoV. 39 , 40 Consequently, infusion of plasma from convalescent individuals to critically ill COVID‐19 patients is a therapeutic option that is being investigated. Although controlled clinical trials are not yet available, several papers report the efficacy of this treatment and the lack of serious adverse events. 41 , 42 Convalescent plasma was administered in patients with a severe disease, and it is unclear whether earlier administration might have been associated with different clinical outcomes 43 and with the prevention of respiratory distress.
4. VIRAL AND HOST FACTORS ASSOCIATED WITH SARS‐COV‐2 INFILTRATION OF THE LOWER AIRWAYS
4.1. Viral exposure dose
Only a small proportion of humans younger than 50, among those who get infected by SARS‐CoV‐2, suffer from moderate and severe COVID‐19. 44 , 45 , 46 Among them, hospital doctors frequently exposed to COVID‐19 patients are, unfortunately, highly represented. 47 Dr. Li Wenliang, the first man alerting China and the World of the new infection, died from COVID‐19 at the age of 33. 48 Similarly, Dr. Carlo Urbani, that is, the first man alerting the world of SARS‐CoV, died of SARS at the age of 46. 49 Both doctors spent weeks caring for patients with severe pneumonia with no personal protection. 48 , 49 In Italy, 114 doctors exposed to SARS‐CoV‐2 have so far (April 14, 2020) died of COVID‐19. 50 The case fatality ratio (CFR) among doctors working in hospitals and caring for patients developing severe COVID‐19 has been therefore much higher than among their age‐ and gender‐matched peers (Table 1). 50 , 51
Table 1.
COVID‐19 death rates per 1 million in Italy (April 19, 2020)
| Italy a | |||
|---|---|---|---|
| Age (years) | Deaths (n) | Population (n) | COVID‐19 deaths per 1 million |
| ≤9 | 1 | 4 994 995 | 0.2 |
| 10‐19 | 0 | 5 733 448 | 0.0 |
| 20‐29 | 7 | 6 103 436 | 1.1 |
| 30‐39 | 39 | 6 998 434 | 5.6 |
| 40‐49 | 170 | 9 022 004 | 18.8 |
| 50‐59 | 712 | 9 567 192 | 74.4 |
| 60‐69 | 2142 | 7 484 862 | 286.2 |
| 70‐79 | 5874 | 6 028 908 | 974.3 |
| 80‐89 | 7534 | 3 699 654 | 2036.4 |
| ≥90 | 2161 | 828 895 | 2607.1 |
Observations in previous viral epidemics further clarify this aspect. The reliability of high viral loads in nasopharyngeal specimens as a prognostic indicator of RF or mortality, with or without a high viral load in serum, has been previously characterized in SARS. 52 A link has been established between the initial dose and subsequent severity of the disease to the 1918‐19 Spanish Flu pandemic. It was demonstrated by simulation models that the number of simultaneous contacts a susceptible person has with infectious ones is correlated with the infectious dose; that severe cases of influenza result from higher infectious doses of the virus; and that a susceptible person can be easily exposed to very high infectious doses of influenza in overcrowded places. 53 The viral replication is more active and prolonged in patients suffering from severe influenza. Viral clearance is slow when host defenses are weakened; however, it is enhanced when antivirals start within the first 4 days of illness. 54
4.2. Age
Among over 70 thousand Chinese with COVID‐19, most were aged over 30 years (90%), while only 1% were aged 9 years or younger, 1% were aged 10‐19 years, and 8% were aged 20‐29 years. 55 Moreover, most of the relatively few pediatric cases were classified as mild (81%), only 14% severe, and 5% critical. 55 Until now (April 14, 2020), the death of only a few humans aged 18 years or less has been attributed to SARS‐CoV‐2. 44 A similar trend has been observed in the United States, where among 149 760 reported cases only 2572 (1,7%) were children aged <18 years, among which only three died of COVID‐19. 56
The reported CFR for COVID‐19 among Chinese patients increases progressively with age, being 0% below 10 years, 8% among patients aged 70‐79 years, and 14.8% among those aged 80+ years. 55 In Europe, the virus is still spreading and has already caused over 77 786 deaths (April 14, 2020) with a CFR also increasing with age. 45 Italy was the first European country facing the pandemic, with 159 516 cases of COVID‐19 diagnosed up to April 14, 2020, 46 with a CFR steadily increasing with age, with no deaths observed in patients younger than 30 years and 20.1% among those aged 80+ years. 44 (Table 1).
Among 171 Chinese children with proven SARS‐CoV‐2 infection, only three (all with severe comorbidities) required intensive care support and invasive mechanical ventilation. 57 In a larger Chinese study, over 90% of all pediatric patients had no severe disease. 58
4.3. Gender and blood group
COVID‐19 mortality has been lower among Chinese females than males. 59 In Italy, mortality and hospitalization rates have also been more frequent among males than among females. 60 Moreover, patients with blood groups 0 and A have slightly lower and a slightly higher risk, respectively, of developing COVID‐19. 61
4.4. Physical activity and COVID‐19
4.4.1. The opposite impact of low‐moderate versus strenuous exercise
Low‐moderate exercise corresponds by general consensus to <60‐minutes exercise for 3‐5 days per week and at <80% of maximum capacity. 62 This activity is beneficial for the innate immune response against respiratory infections and protects from effort‐induced inflammatory and oxidative mediators. 63
By contrast, extreme exercise performed by professional athletes (eg, bikers, rowers, marathon runners, soccer players) induces a tremendous increase of alveolar ventilation and susceptibility to URI. 62 Many professional athletes claim the occurrence of fever, dry cough, malaise, and dyspnea a few hours or days after their last performance. 64 Nearly 13% of the endurance runners reported viral URTI episodes in the week following their marathon, compared to 2.2% of control runners. 64
4.4.2. The first COVID‐19 diagnosed in Europe
The first diagnosis of COVID‐19 in Europe has been confirmed in a 38‐year‐old Italian healthy man who regularly participated in running events and soccer games. The same day when starting COVID‐19 symptoms, he had been training sport. The time‐lapse between the onset of upper airway symptoms and pneumonia was 2 days only. Only 4 days after the onset of COVID‐19, the patient was admitted to the ICU of the Policlinico San Matteo in Pavia because of RF. After weeks of intubation and supportive treatment, the patient luckily recovered and could be discharged in good conditions.
The first Italian COVID‐19 case is world famous but, surprisingly, no official study on it has been published so far. The example of this physically active, young patient offers room for reasoning with regard to the importance of sport for virus transmission and course of disease. Indeed, other cases of COVID‐19 in (semi‐) professional athletes have been described. 65 The problem of SARS‐CoV‐2 infection is part of a more general problem, since athletes and para‐athletes have amplified susceptibility to viral respiratory tract infection and chronic respiratory diseases. 66 , 67
4.4.3. Strenuous exercise and IgA defect
Regular, moderate exercise reduces the risk of acute respiratory infections. 68 By contrast, the levels of salivary IgA decline in athletes during and after a training season. 69 This observation may explain why elite athletes are at higher risk of upper airway infections. 70 A so‐called open window of higher susceptibility to infection ranges between 3 and 72 hours after strenuous exercise. 71
4.4.4. The viral “auto‐inhalation” hypothesis
The majority of professional athletes have lungs that are usually working in near‐perfect physiological conditions, therefore are very close to those of the “ideal lung,” with an even distribution of their alveolar ventilation. 72 These pre‐existing ideal conditions together with the frequent and regular practice of extreme and long‐lasting workouts, can significantly favor the deep inhalation of several irritants, allergens, infectious agents, and also virus particles. 72 Aerosols are considered an important mode of transmission for viruses, e.g. influenza A virus. 73 During strenuous exercise, requiring up to 10 times higher respiratory flow, oronasal (combined nose and mouth) breathing dominates, with the oral component reaching up to 60% of the overall volumes. 72 High flow air and change of breathing from nose to mouth induce progressive cooling and drying of the respiratory tract mucous, decreasing movement of ciliated cells and increasing mucosal viscosity, finally impairing the filtering of microorganisms from the upper respiratory tract system. 74
The pattern of breathing during strenuous exercise changes dramatically by a tremendous increase of ventilation (i.e., inspiratory and expiratory volumes of air) and of alveolar ventilation in particular. Obviously, these changes mostly pertain to whatever kind of runners belonging to all sports disciplines, being semiprofessional and professional athletes particularly exposed (such as much more than individuals of the common population) due to their frequent practice of extreme and long‐lasting exercise. Furthermore, the majority of these athletes have lungs that usually work in perfect physiological conditions, therefore are very close to those of the “ideal lung.” Exercise‐induced oral breathing during Covid‐19 incubation stage causes own viral particles contained in the exhaled aerosol to be re‐inhaled and penetrate the lower airways and alveoli (viral auto‐inhalation hypothesis). Through this mechanism, SARS‐CoV‐2 can also spread more easily to the deepest areas of the lungs (alveolar bronchioles and alveoli) during strenuous exercise, and there start its aggressive action.
5. THE CRUCIAL FIRST 10 DAYS FROM INFECTION: INNATE IMMUNITY IS THE FIRST LINE OF DEFENSE
5.1. The facts
In COVID‐19, the occurrence of pneumonia requiring oxygen therapy is a critical event discriminating asymptomatic or mild cases, whose infection remains mostly confined to the upper airways, from those with severe disease, who experience massive viral invasion of their lower airways. 19 What makes the difference? What prevents the virus from rapidly reaching the lungs and then causing severe pneumonia? What makes COVID‐19 pneumonia a life‐threatening disease?
No efficient adaptive immune response is available at the time of infection 31 ;
Pneumonia may start before adaptive immune response develops 20 ;
Serious complications begin simultaneously with the adaptive immune response.
The first 2 weeks after infection are crucial. 18 , 20 Innate immunity is the only first‐line, early defense against the new SARS‐CoV‐2 virus. Consequently, the early confrontation between host’s innate immunity and SARS‐CoV‐2, at exposure and during the following 2 weeks, decides the natural history of the disease. This confrontation also decides whether the infection will be efficiently blocked in upper airways, or how many virus particles reach the lungs, and when. To understand which part of the innate immunity involved in early protection from SARS‐CoV‐2, we have:
examined which primary immune deficiencies are associated with pneumonia.
examined the patterns of risk factors for COVID‐19 severity: dose of exposure to SARS‐CoV‐2, age, gender, and ABO group;
identified the innate immunity components fitting the same patterns of risk factors;
examined the biologic plausibility that the candidate molecules, emerging from the previous reasoning, are really essential in limiting the consequences of SARS‐CoV‐2 infection to upper airways or to mitigate the course of pneumonia.
5.2. Lessons from patients with agammaglobulinemia
Two Italian male COVID‐19 patients with X‐linked agammaglobulinemia (XLA), males, aged 26 and 34 years under regular treatment with human gamma‐globulin have been recently reported. 75 Both patients developed pneumonia, and both recovered without any need of receiving oxygen therapy. 75 In another Italian study, the clinical course of COVID‐19 in two additional patients with agammaglobulinemia, one XLA and one autosomal recessive, was described. The clinical course resulted milder in patients with agammaglobulinemia when compared to that of other patients. 76 Agammaglobulinemic patients were receiving standard therapy with immunoglobulin preparations, which could not contain SARS‐CoV‐2‐specific antibodies, since were prepared from donors before the pandemic and are deprived by natural IgM and IgA. On the other hand, these patients have in general a natural cellular immunity compartment, including NK cells and phagocytes.
These data suggest that the lack of natural IgM and IgA in the upper respiratory airways may have contributed to the rapid viral spread to lungs, causing pneumonia. Unexpectedly in immunodeficient individuals, agammaglobulinemic patients, who are unable to develop specific SARS‐CoV‐2 Igs, did not develop severe pneumonia, suggesting that the serious complications observed in other patients may be related to the development of acquired immunity.
5.3. Summing‐up
Under the circumstances described above, innate immunity becomes an obvious candidate to act as a very first barrier protecting children, almost all adults, and most elders from SARS‐CoV‐2. Innate immunity is essential to control virus replication early enough, before a very effective adaptive immune response is generated. 77
Anti‐viral innate immunity is based on humoral elements, including components of the complement and coagulation systems, soluble proteins not specifically binding glycans (such as the mannose‐binding lectin, MBL), natural antibodies (IgM, IgA, and IgG), interferons, and other cytokines. 78
Cellular elements of the innate immunity that act as anti‐viral barrier include natural killer cells, MAIT, and γ/δ T cells that contribute to limit pathogen invasion by killing infected cells, secreting inflammatory cytokines, or promoting the adaptive immune response. 78
We focused on humoral components and, in particular, on natural antibodies and MBL, to ascertain whether these players of the innate immunity fit all the epidemiological and clinical pre‐conditions presented in the last three months by SARS‐CoV‐2.
Finally, we tentatively describe mechanisms beyond the most severe cases of pneumonia as a possible consequence of the development of adaptive immunity in individuals with an early high viral spread in lungs.
6. ANTI‐GLYCAN NATURAL IGM AND IGA ANTIBODIES
Anti‐glycan natural antibodies are detected in serum in the absence of previous immunization, are observed also in gnotobiotic animals, and belong mostly to the IgM isotype 79 but also to the IgA and IgG isotypes. 80
6.1. Natural IgM concentration mirrors the patterns of host factors associated with COVID‐19 severity
6.1.1. Natural IgM decline with age
Natural IgM is produced after birth in neonates independently on concurrent infections and rapidly reaches values comparable to adults. 81 , 82 Natural IgM broadly and nonspecifically recognizes diverse microbial determinants and autoantigens 83 including A/B blood group antigens. Median values of natural IgM anti‐A/anti‐B increased during the first years of life to reach adult values in children 5‐10 years old. 84 When examined with glycan array, IgG signals remain relatively unchanged with age. 85 By contrast, average anti‐glycan IgM signals significantly decrease with age (Figure 2) especially after the early 40s, exceeding the expected general reduction in IgM levels with increasing age. 85 This evidence may contribute to explain why severe cases of COVID‐19 start to be observed in the 4th‐5th decade of life and their prevalence increases with age. 44 , 45 , 46
Figure 2.
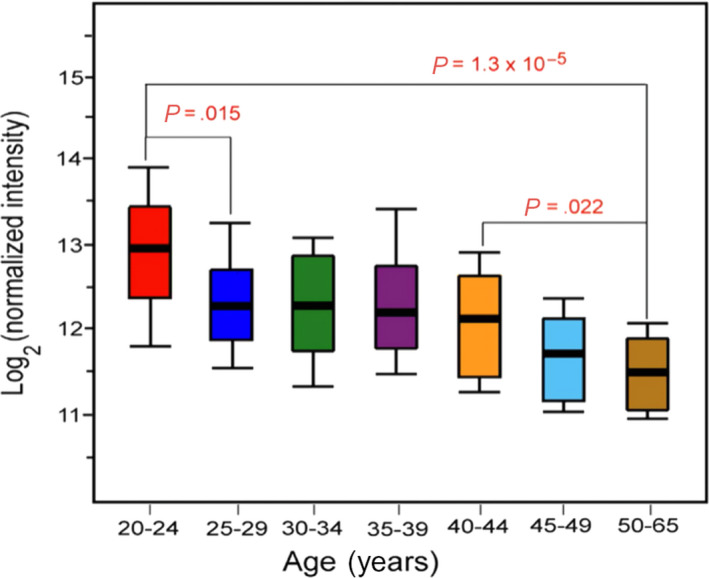
Variations in anti‐glycan IgG and IgM antibody signals with age. [Reprinted unmodified from, 85 https://www.nature.com/articles/srep19509 which is available under the Creative Commons License 4.0.]
A more recent study also found a reduced diversity in natural IgM antibodies in older donors, 86 reflecting a similar trend observed in human B‐1 B cells, the cells responsible for natural IgM production, mainly in the spleen. 87 This evidence may contribute to explain the increasing prevalence of severe cases of COVID‐19 in the eldest. 44 , 45 , 46
6.1.2. Natural IgM levels are lower in males and blood group “A” individuals
When examined with glycan array, anti‐glycan IgG was not different in females and males. 75 By contrast, anti‐glycan IgM was slightly, although not significantly, higher in females. 75 This outcome is consistent with the observation of higher total IgM levels in females. 88 It is well known that blood type has a profound influence on the repertoire of glycan‐specific IgG and especially IgM antibodies. 75 This is also why blood groups are very relevant in regulating host susceptibility to infection. 89 In a study among healthcare workers in Hong Kong, group O individuals were remarkably resistant to SARS‐CoV infection. 90 The ability to block SARS‐CoV infection in target cells was observed with high titers of human anti‐A (1:256), while low‐titer anti‐A proved to be ineffective. 91 An influence of blood group on susceptibility to severe COVID‐19 has been postulated 61 ; whether this is mediated by differences in the repertoire of glycan‐specific antibodies remains an interesting hypothesis for investigation. This evidence may contribute to explain why, among humans infected with SARS‐CoV‐2, those with a blood group “A” and males, respectively, have a slightly 61 or remarkably 59 , 60 higher risk of developing severe COVID‐19.
7. MANNOSE‐BINDING LECTIN
Mannose‐binding lectin plays a pivotal role in innate immunity, interacting with surface sugars of a wide series of microorganisms as a pattern‐recognition receptor. 92 Thus, MBL (i) activates the lectin complement pathway; (ii) promotes opsonophagocytosis 93 ; and (iii) modulates inflammation. 94
7.1. Evidence suggesting that MBL may protect in the early stages of SARS‐CoV‐2 infection
7.1.1. Serum MBL levels decline with age
Serum MBL levels are distinctly higher in children (3‐19 years) than in adults (over 20 years) and decline with age. 95 A considerable amount of serum MBL (about 1 µg/mL) is already present at birth, and this level started to increase from day 2 and attained the highest level in a human lifetime (about 2.5 micrograms/mL) within 5 days after birth. 96 The persistence of increased MBL levels at 3 months and thereafter suggests that a true post‐natal maturation takes place in the first 3 months, leading to a sustained MBL increase lasting through childhood. 97 Values of serum MBL, albumin, and the MBL/albumin ratio were significantly lower either in centenarians and in octo/nonagenarians as compared to the general population from the same geographic area (Sardinia and Campania, Italy). 98
7.1.2. MBL is polymorphic, and low levels predispose to SARS‐CoV infection
Three polymorphisms in the structural gene MBL2 and two promoter gene polymorphisms are commonly found that result in production of low serum levels of MBL. 99 Low MBL levels appear to predispose persons to bacterial infectious diseases, particularly in neonatal age and early childhood. 93 MBL gene polymorphisms were significantly associated with susceptibility to SARS‐CoV infection, possibly explained by the reduced expression of functional MBL. 100 The distribution of MBL gene polymorphisms was significantly different between patients with SARS and control subjects, with a higher frequency of haplotypes associated with low or deficient serum levels of MBL in patients with SARS than in control subjects. Serum levels of MBL were also significantly lower in patients with SARS than in control subjects. 101 MBL could bind SARS‐CoV in a dose‐ and calcium‐dependent and mannan‐inhibitable fashion in vitro, suggesting that binding is through the carbohydrate recognition domains of MBL. Furthermore, the deposition of complement C4 on SARS‐CoV was enhanced by MBL. Inhibition of the infectivity of SARS‐CoV by MBL in fetal rhesus kidney cells (FRhK‐4) was also observed. 101 These results suggested that MBL may contribute to the first‐line host defense against SARS‐CoV and that MBL deficiency is a susceptibility factor for the acquisition of SARS. 101 Mutagenesis indicated that a single N‐linked glycosylation site, N330, was critical for the specific interactions between MBL and SARS spike protein (SARS‐S).
7.1.3. MBL may interfere with the binding of SARS‐CoV to cellular receptor
The presence of glycans enriched in mannose in the S1 region next to the ACE2‐binding site (N234) 102 may lead to speculation that MBL could bind and inhibit the S1‐ACE2 interaction in SARS‐CoV‐2, as it did with SARS‐CoV. Thus, binding of MBL to SARS‐S may interfere with early pre‐ or post‐receptor‐binding events necessary for efficient viral entry. 103 Moreover, in a further study, a potential interaction of polymorphisms in both MBL and CCL2 conferring susceptibility to severe clinical symptoms provoked by SARS‐CoV was observed. 101 It is at present not known whether SARS‐CoV‐2 belongs to the category of the “evasion strong” viruses, thanks to an efficient glycan shield. 102 A pre‐print paper reinforced the hypothesis of a MBL role in SARS‐CoV‐2 infection, by showing that extracellular soluble N protein dimers interact with MASP‐2 and induce MASP‐2 auto‐activation and binding to MBL. 104
8. IMMUNOPATHOGENESIS
8.1. What kills COVID‐19 patients with severe pneumonia?
The most frequent cause of death in COVID‐19 is an ARDS with RF. Hypothesis‐driven investigations are required to adopt adequate countermeasures and eventually save lives. 105 Two major biologic cascades have been observed: the so‐called IL‐6 cytokine storm and disseminated intravascular cascade in the lung (disseminated intravascular coagulation [DIC]) (Figure 3).
Figure 3.
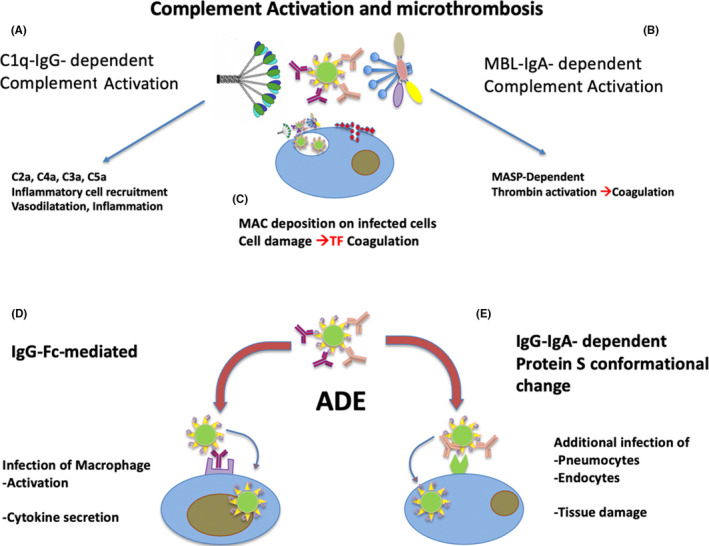
Mediator cascades causing complications during pneumonia in COVID‐19 patients. The classical pathway of complement can be activated by immunocomplexes formed by SARS‐CoV‐2 and specific IgG or IgM (A). Complement activation causes the release of pro‐inflammatory, vasoactive, and chemoattractant components that increase local inflammation. The lectin pathway of complement may be activated by virus‐IgA immunocomplexes, through MBL binding to both viral N‐glycan and IgA (B). Activation of MBL‐associated MASP may cause thrombin activation and triggering of coagulation. Both classic and lectin pathways of complement activation on the external membrane of infected cells releasing viruses may cause deposition of late complement factor and formation of the membrane attack complex (MAC) causing cell damage (C) and release of cellular components. Non‐neutralizing specific IgG and IgA binding the virus may concur to increased infection and inflammation as a consequence of antibody‐dependent enhancement (ADE) of infectivity. Ig with low affinity or non‐neutralizing effect may cause infection and activation of macrophages via Fc receptors (D). In addition, Ig binding the S protein of SARS‐CoV‐2 may cause its conformational changes making more efficacious the binding to the ACE‐2 receptor and the viral fusion with the cell membrane (D)
8.1.1. IL‐6 cytokine storm
Unexpectedly, ex vivo experiments in human explanted lungs showed that SARS‐CoV‐2 does not significantly induce types I, II, or III interferons in the infected lung tissues. 106 SARS‐CoV‐2 only upregulates IL‐6, MCP1, CXCL1, CXCL5, and CXLC10 (IP10). 106 Interestingly, ARDS and RF have been associated with increased serum levels of IL‐6. 18 Elevated serum levels of IL‐6 may be an early biomarker of a worsening clinical course. 107 Trials with tocilizumab, a monoclonal antibody recognizing IL‐6R, started after its efficacy was reported in case reports. 108
8.1.2. Intravasal coagulation
Post‐mortem analysis of lung diseases showed diffuse alveolar damage, including injury to the alveolar epithelial cells, hyaline membrane formation, fibrin deposition, and hyperplasia of type II pneumocytes. 109 Of relevance, 71.4% of fatalities, but only 0.6% of the surviving patients, met ISTH criteria for DIC, 110 a pro‐thrombotic and pulmonary congestion with microvascular thrombosis and occlusion. 111 Biomarkers for this process are elevated D‐dimer and plasma thrombomodulin and others, 112 and treatments with heparin or tissue plasminogen activator have been suggested. 111
8.2. Triggers of the cascades leading to ARDS and respiratory failure
The mechanisms triggering an IL‐6 cytokine storm or a DIC are still unclear. An intriguing observation is that ARDS symptoms start in coincidence with the onset of the SARS‐CoV‐2 antibody‐specific immune response. Interestingly, the serum levels of specific IgA, IgM, and IgG are the highest in patients with the worst clinical course. 31 , 113 We may hypothesize that in individuals in whom the virus early reaches the lung and actively replicates, a robust adaptive immune response contributes to the tissue damage and severity of the pneumonia. This hypothesis may contribute to explain why patients with agammaglobulinemia had a mild pneumonia and recovered without experiencing complications requiring oxygen therapy. 75 Antibodies may be simply a consequence of a powerful viral replication, or rather the direct trigger of a severe inflammation. This may be explained with different mechanisms:
8.2.1. The deposition of IgA immune complexes
COVID‐19 patients soon develop high titers of virus‐specific IgA antibodies. This phenomenon may lead to the formation of IgA‐virus immune complexes, which may cause inflammation and microthrombosis with a MBL‐mediated complement activation mechanism similar to that suggested in IgA nephropathy. 114 , 115
8.2.2. IgM and IgG immunocomplexes
It has been suggested that the formation of IgM and IgG immunocomplexes may contribute to inflammation 116 and to intravascular coagulation and complement activation. 117 Mannose‐binding lectin can also induce complement activation after binding the viral N‐glycans enriched in mannose. 101 , 118
8.2.3. Antibody‐dependent enhancement
Specific IgG antibodies, if generated against non‐neutralizing domains of the SARS‐CoV‐2 antigens, may have an aggressive, rather than a protective, neutralizing action. These antibodies induce conformational changes of the S protein that facilitates the fusion of viral particles with the cell membrane. 119 , 120 This “antibody‐dependent enhancement” may be a dangerous consequence of vaccine or immunotherapeutic approaches and provoke disease exacerbation.
9. A MODEL OF THE INTERACTION BETWEEN SARS‐COV‐2 AND IMMUNE SYSTEM
9.1. Introduction
The confrontation between SARS‐CoV‐2 and innate immunity, quantitative aspects, and the sequence of events is crucial. Natural antibodies and other components of the innate immunity are the first line of defense and must block the virus in the upper airways in the first 10‐12 days of infection (5‐7 from the disease onset), that is, the time required to prepare an efficient adaptive primary antibody response.
9.2. First stage (upper airways): viral clearance or pneumonia
The competition between the virus and natural antibodies may be exemplified with three major scenarios (Figures 4 and 5):
Young and healthy people—patients with efficient natural immunity, who have been exposed to relatively low doses; their natural immune response efficiently controls the infection for a couple of weeks; and the adaptive immune response will complete the clearance mission: the patient remains asymptomatic or develop a mild disease only;
Old patients—viral exposure is probably higher (the source of contagion is also an old person) but the innate immunity is much weaker; a high number of viral particles can reach the alveoli and replicate in type II pneumocytes simultaneously to or even much before the expansion of the specific immune response leading to a more severe and symptomatic pneumonia;
Young but highly exposed patients—the exposure to an excessive cumulative viral dose (i.e., unprotected healthcare personnel) will overcome their efficient innate immunity. Viral particles will reach the alveoli in early stages and cause symptomatic pneumonia;
Figure 4.
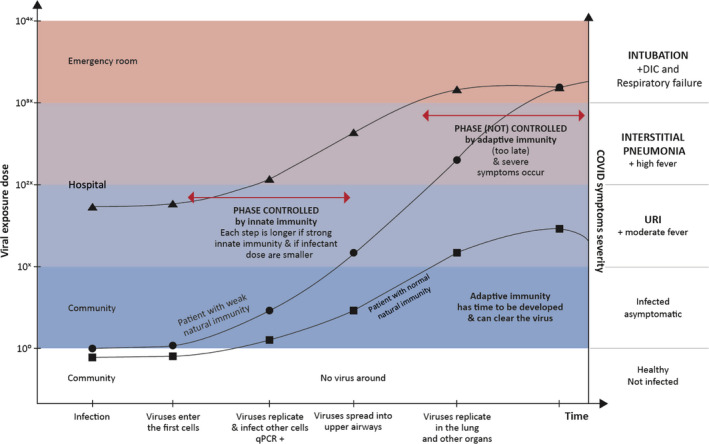
Evolution of COVID‐19 in relation to the cumulative dose of exposure and the natural immune response. Evolution of COVID‐19 in dependence of cumulative exposure dose to the virus, efficacy of natural immunity, and protective adaptive immune response. The lines represent the disease evolution of index patients, whose profile is presented in the main text; squares: young patient; circles: old patient; triangles: young doctor exposed to massive doses of virus. Quantitative polymerase chain reaction (qPCR); disseminated intravascular coagulation (DIC); upper respiratory airways infection (URI)
Figure 5.
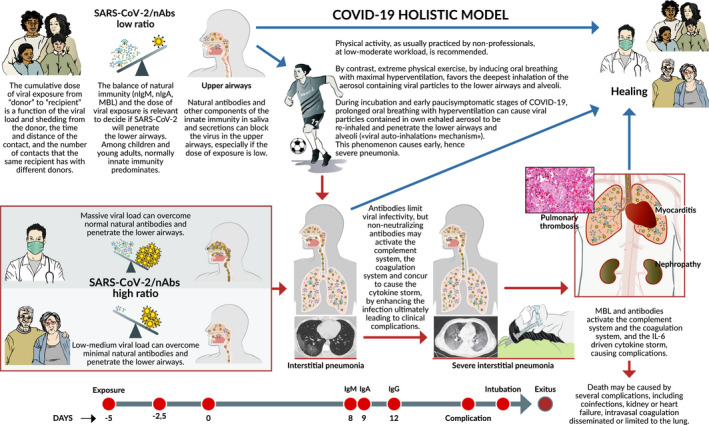
A “quantitative and time‐/sequence‐dependent” model COVID‐19.—The natural history of COVID‐19 caused by SARS‐CoV‐2 is extremely variable, ranging from asymptomatic infection, to pneumonia, and to complications eventually fatal. We propose here the first model, explaining how the outcome of first, crucial 10‐15 d after infection, hangs on the balance between the cumulative dose of viral exposure and the efficacy of the local innate immune response (natural IgA and IgM antibodies, MBL). If SARS‐CoV‐2 overcomes this first‐line immune barrier and rapidly spreads from the upper airways to the alveoli, then it can replicate with no resistance into the lungs, before a strong adaptive immune defense is established. When high‐affinity IgM and IgG antibodies are produced, the consequent severe inflammation damages the lungs and triggers mediator cascades (complement, coagulation, and cytokine storm) leading to complications that may be fatal. Strenuous exercise and high flow air in the incubation days and early stages of COVID‐19 facilitate direct penetration of the viral particles, acquired from the aerosol exhaled by other infected atletes or re‐inhaled together with the athlete's own infected aerosol (viral auto‐inhalation hypothesis), to the lower airways and the alveoli, without impacting on the airway mucosae covered by neutralizing antibodies. This allows the virus to bypass the efficient immune barriers of young and healthy athletes. In conclusion, whether the virus or the adaptive immune response reaches the lungs first is a crucial factor deciding the destiny of the patient
9.3. Second stage: recover or complications
If a relatively low number of virus particles reach the alveoli after the establishment and expansion of an efficient adaptive immune response, the patient will probably never require oxygen and will not undergo relevant complications.
By contrast, if the virus infects the alveoli early enough (i.e., already 7 days from the infection or 2‐3 from the first symptoms), the probability of a better replication in the lung is higher. When the specific response is established, massive amounts of the virus can interact with massive amounts of antibodies with high affinity. Under these circumstances, immunopathology may contribute to tissue damage and organ failure according to the following events:
The classical pathway of complement can be activated by immunocomplexes formed by SARS‐CoV‐2 and specific IgG or IgM. Complement activation causes the release of pro‐inflammatory, vasoactive, and chemoattractant components that increase local inflammation;
the lectin pathway of complement may be activated by virus‐IgA immunocomplexes, through MBL binding to both viral N‐glycan and IgA;
activation of MBL‐associated MASP may cause thrombin activation and triggering of coagulation. Both classic and lectin pathways of complement activation on the external membrane of infected cells releasing viruses may cause deposition of late complement factors and formation of the membrane attack complex causing cell damage and release of cellular components;
non‐neutralizing specific IgG and IgA binding the virus may concur to increased infection and inflammation as a consequence of antibody‐dependent enhancement (ADE) of infectivity. Ig with low affinity or non‐neutralizing effect may cause infection and activation of macrophages via Fc receptors. In addition, Ig binding to the S protein of SARS‐CoV‐2 may cause its conformational changes that make the binding to the ACE‐2 receptor more effective for the viral fusion with the cell membrane.
The local high concentration of cytokines and chemokines that contribute to recruitment of inflammatory cells and vasodilatation permits serum natural Igs and MBL to maintain a vicious circle of inflammation with complement activation and immunocomplex deposition. In this light, it cannot be excluded that MBL‐ or IgM‐mediated immunocomplexes contribute to activation of platelets or tissue factor leading to coagulation and micro‐thrombosis that have been described in COVID‐19 patients with acute RF. In this phase of the disease, natural IgM and MBL that circulate in serum may have no protective role, but rather may contribute to tissue damage. Moreover, during this second phase of the disease, the adaptive response is also progressively on the increase. This may be one side protective against further virus spread in the lungs, but may also reinforce the immunologic and coagulation cascades provoking complications.
Mannose‐binding lectin binds to polymeric IgA and initiates complement cascade, a defense against invading pathogens in mucosal immunity. Polymeric IgA also has a role in activating lectin‐mediated complement signaling.
The complement cascade links the innate and the adaptive immune system, protecting against invading pathogens during the first phase of the diseases. In this sense, Ab‐mediated complement activation flows in parallel between MBL and C1q. Additionally, it can boost pro‐inflammatory effects of IgA deposition with the same mechanism that is supposed to occur in the glomerulus and results in renal injury.
10. IMPLICATIONS FOR DIAGNOSIS, PUBLIC HEALTH, AND CLINICAL INTERVENTION
10.1. Words of caution
The model of the interaction between the immune system and SARS‐CoV‐2 in humans is only a first attempt to produce a synthesis of what is known today. The extremely rapid acquisition of knowledge will allow correcting and improving this model very soon. However, the model can be relevant for diagnosis and intervention. The considerations listed below will require further investigation and validation and are open to evidence‐based modifications before they can be part of shared guidelines for the prevention, surveillance, and control of COVID‐19. Moreover, the implications for treatment are reported as examples of possible consequences of the model. Evidence‐based medicine should also apply for COVID‐19 patients, so that “new” or off‐label drugs or treatment regimens should only be given in a clinical study context, following approval by relevant national or international agencies. However, we believe that these points are first priorities for an intensive and focused clinically oriented research.
10.2. Prevention of severe infection
10.2.1. Identification of high virus spreaders
Symptomatic or asymptomatic high virus spreaders should be identified by quantitative PCR based on viral protein detection in saliva or nasopharyngeal swabs. Quarantine and social distancing to prevent high‐dose exposure of highly susceptible contacts should be strictly practiced by high virus spreaders. Cumulative high viral dose exposure should be prevented for everybody.
10.2.2. Identification and protection of individuals with low natural antibody levels
Glycan microarrays and other tests aimed at measuring natural antibodies, which might be protective against SARS‐CoV‐2 and other viruses, should be developed. Individuals with low natural antibody and MBL levels should be identified and specifically protected. Moreover, governments promoting herd immunity must protect individuals, even if young, who may have low levels of natural antibodies. These individuals should not be exposed to the virus, especially if shed at high doses.
10.2.3. Prevention of fast penetration of the virus in the lungs
Intensive fatiguing work, including strenuous sports requiring high respiratory volumes and flows, should be avoided during the early stage of infection when the adaptive immune response is still not initiated. Particular precautions should be given to athletes performing fatiguing sports, since a portion of sub‐micrometer size, aerosolized particles are expired by the runner or eliminated by cough or nasal secretions and may contain viruses if the athlete is an asymptomatic but SARS‐CoV‐2‐infected individual. These droplets or aerosols might be re‐inhaled and facilitate the spread of the virus from the upper to the lower airways.
10.2.4. Preventing cross‐infection of athletes in team sports or marathons
Low‐moderate physical activity is recommended. However, in sports where many athletes are in close contact, such as team sports or marathons, the same particles have high chances to be inhaled by other athletes, facilitating viral transmission. It should be emphasized that strenuous exercise induces a much more frequent spitting of secretions and this can further contribute to the environmental SARS‐CoV‐2 spreading, particularly if the distancing recommendations are not strictly followed.
10.2.5. Fostering research on sport and COVID‐19
Research on the impact of SARS‐CoV‐2 on athlete populations must be promoted, considering that “robust data need to be collected to understand the effect of general physical fitness on COVID‐19 susceptibility, disease behavior, and prognosis.” 121
10.3. Monitoring and treatment of pneumonia and its complications
10.3.1 Detection of early markers of complement activation, such as C3 and C4 consumption and C4a and C3a plasma increase, might indicate the need to investigate a specific treatment with steroids or new drugs such as eculizumab, a humanized hybrid IgG2/IgG4 monoclonal antibody directed against human C5, that prevents production of C5a and C5b‐9.
10.3.2 Detection of early markers of coagulation such as plasma thrombomodulin and D‐dimer would indicate treatment with therapeutic dosages of systemic or nebulized heparin.
10.3.3 Detection of early markers of cytokine storm by routinely measuring levels of inflammatory cytokines in addition to IL‐6 would indicate administration of other cytokine inhibitors, such as Janus kinase inhibitors.
10.3.4 Detection of non‐neutralizing antibodies by specific assays would indicate administration of hyperimmune IgG from convalescent/recovered individuals, since high dose of neutralizing Ab is described to reduce ADE, or neutralizing human or humanized monoclonal antibody, upon availability for human use. In fact, plasma administration before development of a humoral response to SARS‐CoV‐2 would be expected to be most effective in protecting patients from developing severe forms of the disease.
10.4. Population screening for public health measures and immunization
10.4.1 Tests specifically identifying natural (IgM) antibodies directed against the carbohydrate moieties flanking SARS‐CoV‐2 S1‐RBS may be useful, among elders, to identify those at higher risk of severe disease. Glycan microarrays will be instrumental for this target.
10.4.2 Studies on the prevalence of SARS‐CoV‐2 infections that include asymptomatic and paucisymptomatic individuals could be pursued by measuring SARS‐CoV‐2‐specific serum IgG and IgA that are expected to persist as a memory response to infection. Appropriate and high performing validation tests should be used to retrospectively evaluate the seroconversion status to estimate the herd immunity of a given population.
10.5. Immunization strategy: innate and adaptive immunity
10.5.1 While effective vaccines are being developed, produced, tested, and validated, a strategy to stimulate innate immunity and natural IgM antibody production, in particular, would empower the defenses of at‐risk elderly population. These measures may include influenza, pneumococcal, BCG, and other immunizations that have been proved to reinforce natural immunity in general. This can be valid especially considering that pneumococcus is also a frequent cause of co‐infection causing severe pneumonia and complications.
10.5.2 Given the relevance of the local immune response to SARS‐CoV‐2, an immunization strategy based on a mucosal vaccination would assure a higher protection.
11. CHRONOLOGY AND METHODOLOGY
The idea of elaborating a model of COVID‐19 was conceived on March 20, 2020. A systematic literature search on two open‐access platforms (medRxiv and bioRxiv) and six journals: NEJM, Lancet, JAMA, Cell, Science, and Nature & NGP group, started with the keywords COVID‐19 and/or SARS‐CoV‐2 and/or SARS‐CoV and/or MERS. Further articles were retrieved from the references of the selected papers. PMM got also first‐hand information from many clinicians (see Acknowledgments) treating COVID‐19 patients in northern Italian hospitals. The first nucleus of the model was generated on April 5 and 6 together with Roberto Nisini (Appendix S1). Further publications on the viral load, MBL, natural antibodies (IgM, IgA), B‐1 lymphocytes, primary and secondary antibody response, cytokine storm (IL‐6), complement, coagulation, pneumonia, nephropathy, myocarditis, plasmapheresis, and other treatments were updated daily on PubMed, medRxiv, and bioRxiv. The original model resisted extremely well to the new information and on April 14 was judged robust enough and almost ready for publication. However, the Italian case no. 1 still remained unexplained. On April 15, PMM elaborated the “viral auto‐inhalation hypothesis” and drafted the divulgation version of the model. On April 18, Roberto Dal Negro was contacted and independently formulated exactly the same auto‐inhalation hypothesis, thus joined PMM and RN in the final effort of manuscript preparation and submission to Pediatric Allergy and Immunology.
Afterword—SARS‐CoV‐2 pandemic and COVID‐19 are challenging humanity and a quick response is urgent. According to WHO statistics, in the 30 days during which these pages have been elaborated (March 20, 2020, to April 19, 2020), many thousand people died for COVID‐19 and many countries have been locked down. Scientific and clinical efforts of great scientists and clinicians are producing a storm of knowledge only partially reproduced here. On the basis of their discoveries and observations, the Authors tried to produce a first model of COVID‐19. A scientific model is based on observations perceived by humans and on assumptions elaborated by their brains. Hence, a model is only an approximate interpretation of the reality and it is always wrong in some small or relevant elements. The destiny of the model presented here is to be rapidly improved thanks to novel knowledge coming from new observations and better assumptions. The Authors hope that many and more brilliant minds will read the present pages, will identify and highlight putative mistakes, will get inspiration for their research, and will produce better, more complete, and useful models of the interactions between our immune system and SARS‐CoV‐2. If the speculations presented here on implications for surveillance, control, and therapy of COVID‐19 will contribute, even only minimally, to save some human life and accelerate the end of the pandemic, then the Authors have accomplished their small mission. Berlin (Europe), Verona (Europe), and Rome (Europe), 22.April 2020.
CONFLICT OF INTEREST
The text contains the personal opinion of the Authors, not of their institutions: Charité Universitaetsmedizin Berlin, Germany (PMM), and Istituto Superiore di Sanità, Rome, Italy (RN). All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Paolo Maria Matricardi: Conceptualization (lead). Roberto Dal Negro: Conceptualization (supporting). Roberto Nisini: Conceptualization (lead).
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Supplementary Material
ACKNOWLEDGMENTS
A special thank you goes to Ekaterina Sergueevna Potapova, for her continuous, enthusiastic, optimistic, and patient support given to the authors. The authors are also indebted to Atanas Valev (literature search) and Dania Puggioni (infographics). Many colleagues and friends have contributed with information and advice, among them are Raffaele Badolato and Alessandro Plebani (primary immune deficiency); Antonio Pizzulli (Pediatrics); Marcello Cottini and Carlo Lombardi (natural history of COVID‐19 in children and adults); Mario Plebani and Danilo Villalta (laboratory tests); Raffaele D’Amelio and Roberto Paganelli (Clinical Immunology); and Stefano Del Giacco (sports medicine).
Matricardi PM, Dal Negro RW, Nisini R. The first, holistic immunological model of COVID‐19: Implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31:454–470. 10.1111/pai.13271
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13271
Funding information
PM Matricardi is funded by the Deutsche Forschungsgemeinschaft (DFG; grant number MA 4740/21).
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967‐1976. [DOI] [PubMed] [Google Scholar]
- 4. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. 10.1101/2020.02.07.937862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heymann D, Shindo N. COVID‐19: what is next for public health? Lancet. 2020;395:542‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO pandemic statement . http://www.euro.who.int/en/health‐topics/health‐emergencies/coronavirus‐covid‐19/news/news/2020/3/who‐announces‐covid‐19‐outbreak‐a‐pandemic. Accessed on April 8th, 2020.
- 7. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26:672‐=675 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Litvinova M, Wang W, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020. 10.1016/S1473-3099(20)30230-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woelfel R, Corman VM, Guggemos W, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel‐associated transmission cluster. medRxiv preprint 2020. 10.1101/2020.03.05.20030502 [DOI] [Google Scholar]
- 10. Yezli S, Otter JA. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ Virol. 2011;3:1‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heneghan C, Brassey J, Jefferson T. SARS‐CoV‐2 viral load and the severity of COVID‐19. https://www.cebm.net/covid‐19/sars‐cov‐2‐viral‐load‐and‐the‐severity‐of‐covid‐19/ Accessed on April 11, 2020.
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9:E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoehl S, Rabenau H, Berger A, et al. Evidence of SARS‐CoV‐2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID‐19). Radiol Cardiothorac Imaging. 2020;2:e200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID‐19: challenges and recommendations. Lancet Respir Med. 2020;8(5):506‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippi G, Mattiuzzi C, Sanchis‐Gomar F, Henry BM. Clinical and demographic characteristics of patients dying from COVID‐19 in Italy versus China. J Med Virol. 2020. 10.1002/jmv.25860. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. To KF, Tong JH, Chan PK, et al. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in‐situ hybridization study of fatal cases. J Pathol. 2004;202:157‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS‐CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS‐CoV‐2 and SARS‐CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID‐19. Clin Infect Dis. 2020;ciaa410. [e‐pub] 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS‐CoV) and its putative receptor, angiotensin‐converting enzyme 2 (ACE2). J Pathol. 2020;203:740‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Venkatakrishnan AJ, Puranik A, Anand A, et al. Knowledge synthesis from 100 million biomedical documents augments the deep expression profiling of coronavirus receptors. bioRxiv preprint 2020. [e‐pub] 10.1101/2020.03.24.005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science. 2005;309:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 28. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(2):281‐292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou D, Qi R, Zhang W. Accessible surface glycopeptide motifs on Spike glycoprotein of 2019‐nCoV: implications on vaccination and antibody therapeutics. Preprints. 2020:2020020381. 10.20944/preprints202002.0381.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7). 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV 2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo L, Ren L, Yang S, et al. Humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020:ciaa310. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan W, Lu Y, Zhang J, et al. Viral kinetics and antibody responses in patients with COVID‐19. medRxiv preprint 2020. [e‐pub] 10.1101/2020.03.24.20042382 [DOI] [Google Scholar]
- 35. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woo P, Lau SKP, Wong BHL, et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;138745. 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Casadevall A, Pirofski L. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130:1545‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li G, Chen X, Xu A. Profile of specific antibodies to the SARS‐associated coronavirus. N Engl J Med. 2003;349:508‐509. [DOI] [PubMed] [Google Scholar]
- 40. Koenig KL. Identify‐Isolate‐Inform: a modified tool for initial detection and management of Middle East Respiratory Syndrome patients in the emergency department. West J Emerg Med. 2015;16:619‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117:9490‐9496. 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roback JD, Guarner J. Convalescent plasma to treat COVID‐19: possibilities and challenges. JAMA. 2020;323(16):1561. [DOI] [PubMed] [Google Scholar]
- 44. Centre for Evidence‐Based Medicine, University of Oxford . 2020. https://www.cebm.net/covid‐19/global‐covid‐19‐case‐fatality‐rates/. Accessed on April 8, 2020.
- 45. European Centre for Disease Prevention and Control . https://qap.ecdc.europa.eu/public/extensions/COVID‐19/COVID‐19.html. Accessed on April 8, 2020.
- 46. Istituto Superiore di Sanità . 200. https://www.epicentro.iss.it/en/coronavirus/bollettino/Infografica_13aprile%20ENG.pdf. Accessed on April 14, 2020.
- 47. Belingheri M, Paladino ME, Riva MA. Beyond the assistance: additional exposure situations to COVID‐19 for healthcare workers. J Hosp Infect. 2020;S0195‐6701(20):30132‐30138. 10.1016/j.jhin.2020.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petersen E, Hui D, Hamer DH, et al. Li Wenliang, a face to the frontline healthcare worker. The first doctor to notify the emergence of the SARS‐CoV‐2, (COVID‐19), outbreak. Int J Infect Dis. 2020;93:205‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reilley B, Van Herp M, Sermand D, Dentico N. SARS and Carlo Urbani. N Engl J Med. 2003;348:1951‐1952. [DOI] [PubMed] [Google Scholar]
- 50. Data taken from the Italian National Federation of Doctors, FNOMCeO. https://portale.fnomceo.it/elenco‐dei‐medici‐caduti‐nel‐corso‐dellepidemia‐di‐covid‐19. Accessed on April 8, 2020.
- 51. Heneghan C, Brassey J, Jefferson T.SARS‐CoV‐2 viral load and the severity of COVID‐19. https://www.cebm.net/covid‐19/sars‐cov‐2‐viral‐load‐and‐the‐severity‐of‐covid‐19/ Accessed on April 11, 2020.
- 52. Hung IFN, Lau SKP, Woo PCY, Yuen KY. Viral loads in clinical specimens and SARS manifestations. Hong Kong Med J. 2009;15:S20‐S22. [PubMed] [Google Scholar]
- 53. Paulo AC, Correia‐Neves M, Domingos T, Murta AG, Pedrosa J. Influenza infectious dose may explain the high mortality of the second and third wave of 1918–1919 influenza pandemic. PLoS ONE. 2010;5:e11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID‐19) outbreak in China. Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 56. CDC COVID‐19 Response Team . Coronavirus disease 2019 in children – United States. February 12 – April 2, 2020. MMWR. 2020;69:422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020; [e‐pub]. 10.1542/peds.2020‐0702 31914947 [Google Scholar]
- 59. Dudley JP, Lee NT. Disparities in age‐specific morbidity and mortality from SARS‐CoV‐2 in China and the Republic of Korea. Clin Infect Dis. 2020. ciaa354. [Epub ahead of print] 10.1093/cid/ciaa354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. medRxiv PREPRINT. 2020. [e‐pub] 10.1101/2020.03.11.20031096 [DOI] [Google Scholar]
- 62. Hull JH, Loosemore M, Schwellnus M. Respiratory health in athletes: facing the COVID‐19 challenge. Lancet Respir Med. 2020;S2213‐2600(20):30175‐2. [Epub ahead of print]. 10.1016/S2213-2600(20)30175-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nieman D, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nieman DC, Johansseen LM, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30:316‐328. [PubMed] [Google Scholar]
- 65. Chan J, Yuan S, Kok KA. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hull JH, Loosemore M, Schwellnus M. Respiratory health in athletes: facing the COVID‐19 challenge. Lancet Respir Med. 2020. Apr 8. pii: S2213–2600(20)30175–2. 10.1016/S2213-2600(20)30175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahmadinejad Z, Alijani N, Mansori S, Ziaee V. Common sports‐related infections: a review on clinical pictures, management and time to return to sports. Asian J Sports Med. 2014;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grande AJ, Keogh J, Silva V, Scott AM. Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections. Cochrane Database Syst Rev. 2020;4(4): CD010596. 10.1002/14651858.CD010596.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gleeson M, McDonald WA, Pyne DB, et al. Salivary IgA levels and infectious risk in elite swimmers. Med Sci Sports Exerc. 1999;31:67‐73. [DOI] [PubMed] [Google Scholar]
- 70. Nehlsen‐Cannarella SL, Nieman DC, Fagoaga OR, et al. Saliva immunoglobulins in elite women rowers. Eur J Appl Physiol. 2000;81:222‐228. [DOI] [PubMed] [Google Scholar]
- 71. Peters E. Exercise, immunology and upper respiratory tract infections. Int J Sports Med 1997;18(S‐1):S69‐S77. [DOI] [PubMed] [Google Scholar]
- 72. Bouhuys A. The Physiology of Breathing. New York, NY: Grune & Stratton; 1977:60‐98. [Google Scholar]
- 73. Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nieman DC. Exercise, upper respiratory tract infection, and the immune system. Med Sci Sports Exerc. 1994;26:128‐139. [DOI] [PubMed] [Google Scholar]
- 75. Soresina A, Moratto D, Chiarini M, et al. Favorable outcome of COVID19 in two patients with X‐linked agammaglobulinemia. Pediatr Allergy Immunol. 2020. 10.1111/pai.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID‐19?: lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;S0091‐6749(20):30557‐1. 10.1016/j.jaci.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu JC, Khodadadi H, Malik A, et al. Innate immunity of neonates and infants. Front Immunol. 2018;9:1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis. 2004;57:236‐247. [PubMed] [Google Scholar]
- 79. New JS, King RG, Kearney JF. Manipulation of the glycan‐specific natural antibody repertoire for immunotherapy. Immunol Rev. 2016;270:32‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Muthana MS, Xia L, Campbell CT, Zhang Y, Gildersleeve JC. Competition between serum IgG, IgM, and IgA anti‐glycan antibodies. PLoS ONE. 2015;10:e0119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mouthon L, Nobrega A, Nicolas N, et al. Invariance and restriction toward a limited set of self‐antigens characterize neonatal IgM antibody repertoires and prevail in autoreactive repertoires of healthy adults. Proc Natl Acad Sci USA. 1995;92:3839‐3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812‐818. [DOI] [PubMed] [Google Scholar]
- 83. Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. 2015;194:13‐20. [DOI] [PubMed] [Google Scholar]
- 84. Auf der Maur C, Hodel M, Nydegger UE, Rieben R. Age dependency of ABO histo‐blood group antibodies: reexamination of an old dogma. Transfusion. 1993;33:915‐918. [DOI] [PubMed] [Google Scholar]
- 85. Muthana SD, Gildersleeve JC. Factors affecting anti‐glycan IgG and IgM repertoires in human serum. Sci Rep. 2016;6:19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Listi F, Candore G, Modica MA, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. 2006;1089:487‐495. [DOI] [PubMed] [Google Scholar]
- 87. Rodriguez‐Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, Hernandez AM. Human B‐1 cells and B‐1 cell antibodies change with advancing age. Front Immunol. 2019;10:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stoica GH, Samborschi C, Michiu V. Influence of sex and age on serum immunoglobulin concentrations in healthy subjects. Med Interne. 1978;16:23‐31. [PubMed] [Google Scholar]
- 89. Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28:803‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cheng Y, Cheng G, Chui CH, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450‐1451. [DOI] [PubMed] [Google Scholar]
- 91. Guillon P, Clement M, Sebille V, et al. Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology. 2008;18:1085‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dommett RM, Klein N, Turner MW. Mannose‐binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Scorza M, Liguori R, Elce A, Salvatore F, Castaldo G. Biological role of mannose binding lectin: from newborns to centenarians. Clin Chim Acta. 2015;451:78‐81. [DOI] [PubMed] [Google Scholar]
- 94. Eisen DP, Minchinton RM. Impact of mannose‐binding lectin on susceptibility to infectious diseases. Clin Infect Dis. 2003;37:1496‐1505. [DOI] [PubMed] [Google Scholar]
- 95. Terai I, Kobayashi K, Fujita T, Hagiwara K. Human serum mannose binding protein (MBP): development of an enzyme‐linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem Med Metab Biol. 1993;50(1):111‐119. [DOI] [PubMed] [Google Scholar]
- 96. Terai I, Kobayashi K. Perinatal changes in serum mannose‐binding protein (MBP)levels. Immunol Lett. 1993;38(3):185‐187. [DOI] [PubMed] [Google Scholar]
- 97. Thórarinsdóttir HK, Lúdvíksson BR, Víkingsdóttir T, et al. Childhood levels of immunoglobulins and mannan‐binding lectin in relation to infections and allergy. Scand J Immunol. 2005;61(5):466‐474. [DOI] [PubMed] [Google Scholar]
- 98. Tomaiuolo R, Ruocco A, Salapete C, et al. Activity of mannose‐binding lectin in centenarians. Aging Cell. 2012;11:394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tu X, Chong WP, Zhai Y, et al. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect. 2015;71:101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang H, Zhou G, Zhi L, et al. Association between mannose‐binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;192:1355‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ip EWK, Chan KH, Law HKW, Tso GHW, Kong EKP, Wong WHS. Mannose‐binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005;191:1697‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site‐specific analysis of the SARS‐CoV‐2 glycan shield. bioRxiv preprint. 2020;[e‐pub]. 10.1101/2020.03.26.010322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhou Y, Lu K, Pfefferle S, et al. A single asparagine‐linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose‐binding lectin through multiple mechanisms. J Virol. 2010;84:8753‐8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gao T, Hu M, Zhang X, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP‐2‐mediated complement over‐activation. medRxiv preprint 2020. 10.1101/2020.03.29.20041962 [DOI] [Google Scholar]
- 105. Ledford H. How does COVID‐19 kill? Uncertainty is hampering doctors' ability to choose treatments. Nature. 2020;580:311‐312. [DOI] [PubMed] [Google Scholar]
- 106. Chu H, Chan JF, Wang Y, et al. Comparative replication and immune activation profiles of SARS‐CoV‐2 and SARS‐CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID‐19. Clin Infect Dis. 2020;ciaa410. 10.1093/cid/ciaa410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Herold T, Jurinovic V, Arnreich C, et al.Level of IL‐6 predicts respiratory failure in hospitalized symptomatic COVID‐19 patients. medRxiv. 2020:20047381. 10.1101/2020.04.01.20047381 [DOI] [Google Scholar]
- 108. Di Giambenedetto S, Ciccullo A, Borghetti A, et al. Off‐label use of tocilizumab in patients with SARS‐CoV‐2 infection. J Med Virol. 2020. [e‐pub]: 10.1002/jmv.25897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod Pathol. 2020;1‐8. 10.1038/s41379-020-0536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020. 10.1111/jth.14828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Orwoll BE, Spicer AC, Zinter MS, et al. Elevated soluble thrombomodulin is associated with organ failure and mortality in children with acute respiratory distress syndrome (ARDS): a prospective observational cohort study. Crit Care. 2015;19:435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS‐CoV‐2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020. 10.1515/cclm-2020-0443 [DOI] [PubMed] [Google Scholar]
- 114. El Karoui K, Hill GS, Karras A, et al. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. 2012;23:137‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Roos A, Bouwman LH, van Gijlswijk‐Janssen DJ, et al. Human IgA activates the complement system via the mannan‐binding lectin pathway. J Immunol. 2001;167:2861‐2868. [DOI] [PubMed] [Google Scholar]
- 116. Monsalvo AC, Batalle JP, Lopez MF, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17:195‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chapin J, Terry HS, Kleinert D, Laurence J. The role of complement activation in thrombosis and hemolytic anemias. Transfus Apher Sci. 2016;54:191‐198. [DOI] [PubMed] [Google Scholar]
- 118. Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID‐19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wan Y, Shang J, Sun S, et al. Molecular mechanism for antibody‐dependent enhancement of coronavirus entry. J Virol. 2020;94. 10.1128/JVI.02015-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Walls AC, Xiong X, Park YJ, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176: 1026‐1039.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hull JH, Loosemore M, Schwellnus M. Respiratory health in athletes: facing the COVID‐19 challenge. Lancet Respir Med. 2020;S2213‐2600(20):30175‐2. 10.1016/S2213-2600(20)30175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Supplementary Material


