Abstract
Myocardial ischemia and infarction, both in the acute and chronic phases, are associated with cardiomyocyte loss and dramatic changes in the cardiac extracellular matrix (ECM). It has long been appreciated that these changes in the cardiac ECM result in altered mechanical properties of ischemic or infarcted myocardial segments. However, a growing body of evidence now clearly demonstrates that these alterations of the ECM not only affect the structural properties of the ischemic and post-infarct heart, but that they play a crucial and sometimes direct role in mediating a range of biological pathways, including the orchestration of inflammatory and reparative processes, as well as the pathogenesis of adverse remodeling. In this final part of a 4-part review series, we review the evidence on the role of the ECM in relation to the ischemic and infarcted heart, as well as its contribution to cardiac dysfunction and adverse clinical outcomes.
Keywords: Biomarker, collagen, fibroblast, heart failure, ischemic heart disease
Condensed Abstract:
Myocardial ischemia and infarction are associated with dramatic changes in the cardiac extracellular matrix (ECM). It has long been appreciated that these changes result in altered mechanical properties of affected myocardial segments. However, these changes to the ECM not only affect the structural properties of the ischemic and post-infarct heart, but they also mediate a range of important biological processes, such as inflammation and adverse remodeling. In this final of a 4-part review series, we focus on the role of the ECM in relation to the ischemic and infarcted heart, including its contribution to cardiac dysfunction and adverse clinical outcomes.
So far in this review series we have covered basic extracellular matrix (ECM) biology in Part 1, the role of ECM in vascular disease in Part 2, and the involvement of ECM in myocardial interstitial fibrosis (MIF) in non-ischemic heart disease in Part 3. Here in Part 4 we address the role of ECM in ischemic heart disease.
In the majority of patients, ischemic heart disease is caused by atherosclerosis of the epicardial coronary arteries, which leads to an imbalance between oxygen supply and demand. Typically, a coronary luminal narrowing of greater than 75% does not cause a reduction in resting blood flow, but precludes an increase in oxygen supply when myocardial demand is increased (upon exercise, during tachy-arrhythmia, etc.), resulting in ischemia. When atherosclerotic disease is complicated by superimposed thrombosis, acute coronary occlusion results in myocardial infarction (MI). Complete and prolonged interruption of blood flow to the area subserved by the vessel leads to cessation of oxidative phosphorylation, depletion of high energy phosphates, and increased accumulation of metabolites in the ischemic myocardium. After 15-20 minutes of occlusion, disruption of aerobic metabolism in cardiomyocytes results in irreversible changes that eventually lead to cardiomyocyte death. As we review in this article, both chronic ischemia and acute MI are associated with profound changes in the ECM network. Furthermore, mounting evidence suggests that in ischemic and infarcted hearts, ECM alterations not only accompany the pathologic changes in cardiomyocytes, but play a crucial role in mediating injurious pathways, orchestrating inflammatory and reparative processes, and contributing to the pathogenesis of adverse remodeling.
The ECM in the infarcted heart
In adult mammals with MI, massive loss of cardiomyocytes overwhelms the extremely limited endogenous regenerative capacity of the heart (1). Thus, the infarcted myocardium heals through replacement of dead myocardium with a matrix-based scar. The myocardial reparative response can be divided into 3 distinct, but overlapping phases: inflammatory, proliferative and maturation. As the infarcted myocardial segments heal, non-infarcted areas remodel in response to hemodynamic loading and to the effects of immune cells and fibroblasts accumulating in the infarct border zone. The dynamic changes in ECM composition not only affect the structure and mechanics of the heart, but also play a critical role in regulation of the cellular responses in the infarcted and remodeling myocardium. During the inflammatory phase, fragments of ECM generated through early activation of proteases may serve as damage-associated molecular patterns (DAMPs) that activate immune cells, triggering an inflammatory reaction. Moreover, extravasated plasma proteins enrich the ECM, generating a plastic network of provisional matrix that serves as a conduit for immune and reparative cells. Phagocytosis of dead cells and matrix debris by professional phagocytes inhibits inflammation, leading to the proliferative phase of infarct healing, characterized by activation of myofibroblasts and neovessel formation. Enrichment of the cardiac ECM through deposition of matricellular proteins plays a critical role in spatial regulation of growth factor signaling cascades, restricting the fibrotic response to areas of injury. In addition, activated fibroblasts secrete large amounts of structural ECM proteins, forming a scar. As the infarct matures, cross-linking of the ECM provides structural stability, while increasing ventricular stiffness. In viable myocardial segments, pressure and volume loads cooperate to induce chronic progressive deposition of collagen in the interstitium, thus contributing to the pathogenesis of post-infarction heart failure.
The role of ECM in the inflammatory phase of infarct healing
Activation of matrix-degrading proteases and generation of matrix fragments are the earliest ECM perturbations in infarcted hearts. In some studies, ischemic changes in the interstitial matrix have been reported to precede irreversible cardiomyocyte injury. In a porcine model, matrix metalloproteinase (MMP) activation was first detected in the cardiac interstitium after 10 minutes of coronary occlusion (2). Early MMP activation is followed by fragmentation of fibrillar collagens and other structural ECM proteins. In a pig MI model, release of type I collagen fragments in the serum was documented after 30 minutes of coronary occlusion (3). In ultrastructural studies, the earliest alterations in collagen morphology, described as “irregular arrangement” of collagen fibers, were noted after 40 min of coronary occlusion and were followed by a reduction in the density of collagen fibrils 2h after the acute event (4). Early increases in MMP activity in the ischemic myocardium reflect activation of latent stores, presumably through reactive oxygen species-mediated pathways (5). At a later stage, ischemic cardiomyocytes, fibroblasts, vascular cells and immune cells synthesize and release large amounts of proteases in the infarcted myocardium (6). Members of the collagenase family (such as MMP1), gelatinases (including MMP2 and MMP9) and cathepsins are upregulated in the infarcted heart (7,8) and also contribute to ECM fragmentation.
ECM fragments as regulators of the inflammatory and reparative response
Protease-mediated ECM fragmentation generates bioactive peptides that can modulate inflammatory, fibrogenic or angiogenic responses (9,10) (Figure 1). The terms “matrikines” and “matricryptins”, have been used to describe these biologically active matrix fragments. Although universally accepted definitions are lacking, the term “matrikine” is typically used to describe any fragment of an ECM molecule with biological properties distinct from those of the full-length molecule. On the other hand, the term “matricryptin” refers to a subtype of matrikines that require proteolytic processing to expose a functional domain and exert their biological actions (11). Although the rapid and prolonged activation of proteases may release a wide range of matrix fragments in the cardiac interstitium, their profile, time course of release, and potential involvement in regulation of myocardial inflammation and repair remain poorly understood. In the infarcted myocardium, all constituents of the cardiac ECM, (including fibrillar collagens, elastin, basement membrane proteins, fibronectin, hyaluronan, and newly-secreted matricellular macromolecules) (12-15) may be targeted by proteases to generate fragments (Table 1). Peptides derived from fibrillar collagens and elastin have been implicated in activation of immune cells in models of inflammation and tissue injury (10,16). Extensive evidence in models of pulmonary inflammation suggests that the tripeptide proline-glycine-proline (PGP) and its acetylated form ac-PGP are generated from collagen through a multistep proteolytic cascade that involves MMP8, MMP9 and prolyl endopeptidase (17), and may act as a potent neutrophil chemoattractant signaling through activation of the chemokine receptor CXCR2 (18). Although PGP generation has been demonstrated in pressure-overloaded hearts (19), evidence implicating this pro-inflammatory matrikine in post-MI inflammation is lacking. Low molecular weight hyaluronan fragments may also exert potent pro-inflammatory actions in injured tissues, by activating toll-like receptor signaling pathways (20). Interestingly, it has been suggested that impaired clearance of these fragments may prolong and accentuate pro-inflammatory signaling in leukocytes and vascular cells (21,22), thus accentuating adverse remodeling after MI. Proteolytic processing of laminins by MMP2 and MMP14 has also been demonstrated to yield neutrophil chemoattractant peptides (23), while fibronectin fragments have been identified in the infarcted heart and are suggested to reduce monocyte migratory capacity in response to chemokines (15).
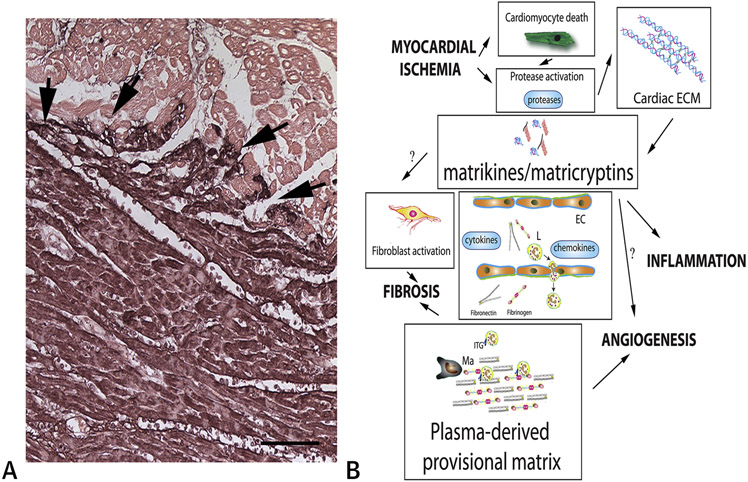
Figure 1. Generation of matrix fragments and formation of a plasma-derived provisional matrix during the inflammatory phase of cardiac repair.
A. Immunohistochemistry for fibrinogen/fibrin (black) shows deposition of plasma-derived proteins (including fibrin and fibronectin) that enrich the cardiac ECM during the early stages of infarct healing (arrows). Counterstained with eosin. Scalebar=120μm. Data from our own work in a canine model of reperfused MI (13). B. Schematic cartoon illustrating the dramatic changes in the interstitial ECM network during the inflammatory phase of infarct healing. Rapid activation and induction of proteases, such as MMPs and cathepsins, in the infarcted myocardium generates ECM fragments (matrikines and matricryptins) that modulate the phenotype of fibroblasts, macrophages (Ma) and endothelial cells (EC). MMPs have a wide range of substrates and process cytokines and chemokines, regulating their pro-inflammatory actions. Recruitment and migration of leukocytes (L), fibroblasts and vascular cells in the infarcted heart requires interactions between cell surface integrins (ITG) and provisional matrix proteins (such as fibrin and fibronectin). Thus, the provisional matrix plays an important role in regulation of inflammatory, fibrogenic and angiogenic responses.
Table 1.
Generation of ECM protein fragments in the infarcted heart.
| ECM protein |
Animal models | Human patients | Proposed role |
|---|---|---|---|
| Fibrillar collagens (collagen I, collagen III) | In the pig MI model, release of type I collagen fragments in the serum has been documented after 30 minutes of coronary occlusion (3). Experiments in the isolated perfused heart showed that global ischemia generates collagen I and collagen III fragments (153). Cathepsin K-specific collagen I fragments were found in infarcted mouse hearts (8). The collagen I α1 C-1158/59 fragment was identified in infarcted mouse hearts, generated through effects of MMP-2 and MMP-9 (24) | In patients with MI, an early increase in collagen degradation markers is noted (154) Plasma levels of carboxy-terminal telopeptide of type I collagen (CITP) reflect collagen I degradation, are higher in MI patients with systolic dysfunction (155) and correlate with the extent of acute injury (156). Plasma CITP levels 24h or 72h after acute MI are associated with late mortality (157) and adverse remodeling (158). A reduction in the ratio of biomarkers reflecting synthesis vs. degradation of collagen was also associated with worse post-MI remodeling (159). | Although collagen fragments have been suggested to exert pro-inflammatory actions, their role in initiation and progression of the post-MI inflammatory response has not been documented.Late generation of the collagen I α1 C-1158/59 matricryptin may promote repair by activating fibroblasts and vascular cells (4). |
| Elastin | N/A | Elastin fragments were identified in the serum of patients with MI (160). | Overexpression of elastin fragments (typically absent from myocardial infarcts) attenuated adverse remodeling in a rat MI model (161). |
| Collagen IV | Canstatin, a 24kDa polypeptide cleaved from the α2 chain of type IV collagen is found in infarcted rat hearts (30). | N/A | Canstatin may promote activation of infarct myofibroblasts (30). Tumstatin, a cleaved fragment of collagen type IV α3 chain, may generate a matricryptin that modulates fibroblast activation, angiogenesis, and cardiomyocyte survival (28,162). |
| Collagen XVIII | Endostatin, a 20kDa fragment of collagen XVIII, is upregulated in a rat MI model (163). | Serum endostatin levels were increased in patients with MI (164). | Endostatin inhibition in a rat MI model was reported to worsen outcomes (163). Endostatin may stimulate fibroblast proliferation (25), while exerting potent angiostatic actions (26). |
| Fibronectin | Fibronectin fragments were identified in the post-ischemic cardiac lymph (reflecting the cardiac extracellular environment) in a canine model of reperfused MI. | N/A | Fibronectin fragments reduced monocyte very late antigen-5 expression, attenuating the migratory capacity of monocytes in response to chemokines (15). |
It should be emphasized that the effects of matrikines and matricryptins are not limited to the inflammatory phase of infarct healing. Prolonged activation of proteases in the infarcted myocardium generates ECM fragments for several days after MI. In addition to their effects on immune cells, matrix fragments may also modulate the fibroblast and vascular cell phenotype (24). Endostatin, a 20kDa fragment of collagen XVIII, may stimulate fibroblast proliferation (25), while exerting potent angiostatic actions (26). MMP9-mediated cleavage of collagen IV generates tumstatin, a fragment with angiostatic properties (27) that can be further degraded to produce fragments that promote a proliferative and migratory phenotype in cardiac fibroblasts (28). Canstatin, another collagen IV-derived fragment, may also activate fibroblasts, stimulating their proliferation and enhancing fibroblast-derived MMP synthesis (29,30).
Broad effects of MMPs in regulation of the inflammatory response
In addition to their role in ECM remodeling, MMPs regulate inflammatory and reparative cascades through proteolytic processing of cytokines, chemokines and growth factors. Several members of the MMP family can process the cell membrane-anchored precursor of tumor necrosis factor (TNF)-α to generate the active cytokine (31). MMP3 and the gelatinases MMP2 and MMP9 can also process pro-Interleukin (IL)-1β, generating the biologically active form of the cytokine in a caspase 1-independent manner (32). Furthermore, MMPs are implicated in the complex multistep cascade that leads to generation of mature transforming growth factor (TGF)-β from secreted latent complexes (33). MMPs also process several members of the chemokine family, including CXCL12/stromal cell-derived factor (SDF)-1 and CCL2/monocyte chemoattractant protein (MCP)-1, exerting diverse and often conflicting actions that may either potentiate or inactivate the effects of the chemokine (34-38). Furthermore, MMPs may disperse chemokine gradients by degrading glycosaminoglycan binding sites that are critical for chemokine immobilization on the endothelial cell surface and subsequent interaction with activated leukocytes (39). Considering the critical involvement of chemokines and cytokines in leukocyte recruitment, repair and remodeling of the infarcted myocardium (40-42), MMP-dependent processing of inflammatory mediators may be a central regulatory mechanism in the post-infarct heart.
Discoveries suggesting subcellular localizations of some MMP family members in the cytosol, mitochondria or the nucleus have added complexity to our understanding of the role of MMPs in cardiac repair. It has been suggested that intracellular MMPs may have a wide range of substrates, including cytoskeletal proteins, signal transducers, enzymes, and transcriptional regulators (43). MMP-mediated degradation of contractile proteins, such as myosin and actinin, has been implicated in the pathogenesis of ischemic myocardial dysfunction (44,45). In vitro studies demonstrated that MMPs may also function in a non-proteolytic manner, regulating signal transduction or transcription (46). The in vivo significance of these functions remains to be fully defined.
The deposition of a plasma-derived provisional matrix regulates the post-infarction inflammatory response
Proteolytic degradation of the native myocardial ECM is accompanied by the formation of a highly plastic provisional matrix network that supports migration and differentiation of immune, vascular and reparative cells, contributing to scar formation (13,47). In models of vascular injury and of cutaneous repair, progressive changes in the composition of the provisional matrix have been described (48). Early provisional matrix is comprised predominantly of extravasated proteins (such as fibrinogen-derived fibrin and plasma fibronectin) (49). As immune cells and fibroblasts migrate into the injured tissue, the provisional matrix is enriched with a cell-derived component, comprised of cellular fibronectin and proteoglycans (50). Studies describing the cellular events in infarcted mammalian hearts suggest a similar transition ECM composition (13,51). Rapid induction and release of cytokines and growth factors, such as TNF-α and vascular endothelial growth factor (VEGF), may be responsible for increased vascular permeability (52,53), leading to extravasation of fibrinogen and fibronectin in the infarct zone (13,54). The role of the components of the provisional matrix in regulation of the inflammatory response following MI remains poorly supported by experimental in vivo evidence. In a mouse model of reperfused MI, fibrin-mediated pro-inflammatory actions were suggested to extend ischemic injury (55). Both fibrinogen and fibronectin contain integrin binding sites that may play a crucial role in adhesion and migration of leukocytes in the infarcted heart (56,57), and which may transduce signals that modulate cytokine and chemokine expression by macrophages (58-60). In addition to the direct effects of its components on inflammatory cells, the provisional matrix may also serve as a reservoir for cytokines and growth factors that mediate the transition to the proliferative phase of cardiac repair.
The role of ECM in the proliferative phase of infarct healing
The clearance of dead cells and matrix debris from the infarcted myocardium triggers a series of anti-inflammatory cascades that suppress leukocyte recruitment and generate a reparative environment. Infarct macrophages undergo phenotypic transitions, expressing and releasing mediators (like TGF-β and IL-10) that suppress inflammation, while promoting myofibroblast activation (61-63). Resident cardiac fibroblasts undergo myofibroblast conversion (64), expressing contractile proteins such as α-smooth muscle actin, and secreting collagens and other structural ECM proteins that are necessary for protection of the infarcted myocardium from catastrophic rupture (65). Although other cell types such as macrophages may produce collagen following myocardial injury (66), their relative contribution as a source of structural ECM proteins is limited, and activated myofibroblasts represent the main collagen-synthesizing cells in the healing infarct. Myofibroblast activation requires the cooperation of several different cell types, including macrophages, lymphocytes, vascular cells and border zone cardiomyocytes, which secrete growth factors and matricellular proteins, converting the ECM into a signaling hub that localizes fibroblast-activating mediators into the infarct zone.
Transition to the proliferative phase of cardiac repair is marked by dramatic changes in the composition of the provisional matrix. The fibrin-based plasma-derived provisional network is lysed by fibrinolytic enzymes (67). In vivo studies suggest that the timely clearance of the fibrin-based provisional matrix by the plasminogen/plasmin system plays a crucial role in regulation of the reparative response. Plasminogen null mice exhibit marked defects in infarct healing, associated with impaired recruitment of reparative cells and perturbed replacement of dead cardiomyocytes with granulation tissue (68,69). As the fibrin network is lysed, activated macrophages and interstitial cells generate a cell-derived ECM that contains cellular fibronectin, hyaluronan, and proteoglycans (51,70). The cell-derived provisional matrix is further enriched with a range of matricellular macromolecules (13,71) and transduces key activating signals in fibroblasts, immune cells, vascular cells, and surviving border zone cardiomyocytes (Central Illustration).
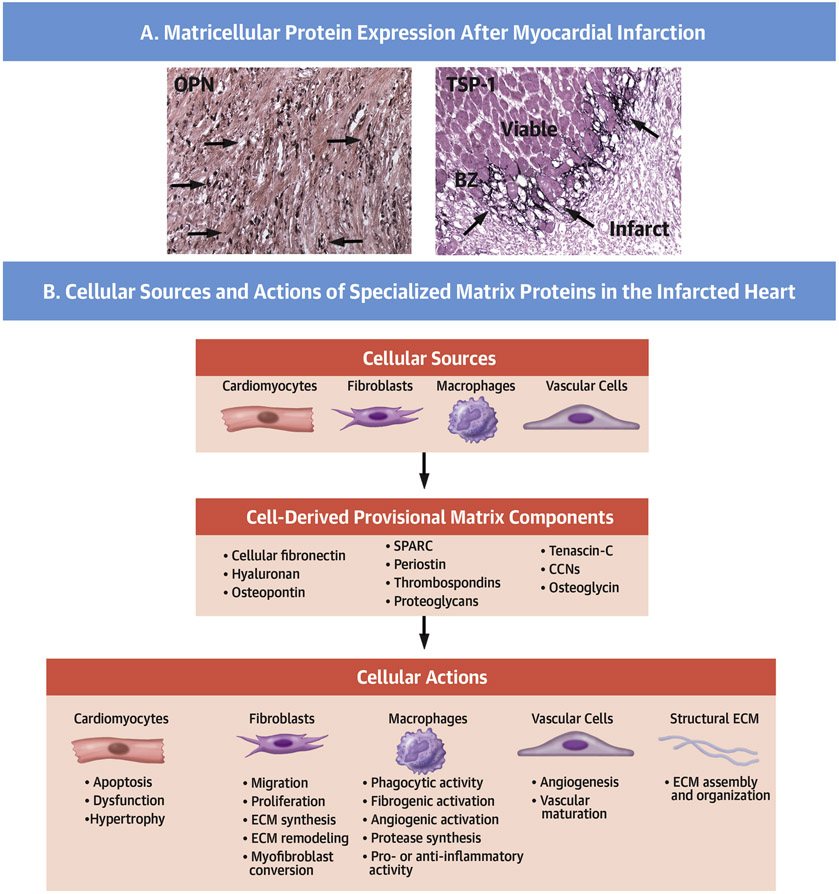
Central Illustration. Matricellular proteins regulate inflammatory, reparative, angiogenic and fibrogenic cellular responses during the proliferative phase of infarct healing.
A. Immunohistochemical staining using a peroxidase-based strategy (black) shows expression of the matricellular proteins osteopontin (OPN–top panel) and thrombospondin (TSP)-1 (lower panel) in the infarcted myocardium. Counterstained with eosin. Scalebar=100μm. Data are from our own work in a canine model of reperfused MI (13,152). Each matricellular protein shows a unique pattern of localization, likely reflecting distinct cellular sources and mechanisms of regulation. OPN is predominantly expressed by macrophages (top-arrows), whereas TSP-1 is deposited in the infarct border zone (bottom-arrows). B. Schematic cartoon illustrating the complex matrix environment in the proliferative phase of infarct healing. Cell-derived fibronectin, hyaluronan, and a wide range of matricellular proteins (OPN, TSP-1, TNC/tenascin-C, periostin, SPARC, osteoglycin and members of the CCN family) and proteoglycans enrich the infarct ECM and regulate assembly of the collagen-based structural matrix, while modulating a variety of cellular responses in cardiomyocytes, fibroblasts, macrophages and vascular cells.
The conversion of fibroblasts to activated myofibroblasts also occurs, which requires a close association with TGF-β-secreting macrophages (72) and local activation of latent TGF-β in the infarcted area. The deposition of specialized components of the matrix network plays a critical role in spatially-restricted TGF-β activation in the healing infarct. In vitro and in vivo evidence suggest that the ED-A splice variant of fibronectin mediates TGF-β1-induced myofibroblast conversion (73,74). However, the interactions between ED-A fibronectin and the TGF-β signaling cascade remain poorly understood. It has been suggested that ED-A fibronectin may act by immobilizing the large latent TGF-β complex within the ECM (75), thus localizing this growth factor in the area of fibroblast accumulation. Proteases, cell surface integrins and matricellular proteins may then cooperate to liberate the active TGF-β dimer from latent stores, triggering downstream fibrogenic signaling pathways (76). In addition to its role in stimulating TGF-β-driven fibroblast-activating responses, fibronectin may also play an important role in angiogenesis. The heparin II domain of fibronectin binds to VEGF (77) and may promote angiogenic activation of endothelial cells, thus stimulating neovessel formation in the infarct.
The modulation of interactions between growth factors and proteoglycans has profound effects on reparative, fibrogenic and angiogenic cascades. Induction of sulfatases in the infarcted heart can remove 6-O-sulfate groups from heparan sulfate in the extracellular compartment, thus perturbing interactions between VEGF-A and heparan sulfate, and increasing responsiveness to the angiogenic effects of VEGF (78). Inhibition of heparin sulfate sulfation may also affect bioactivity of several other cytokines, chemokines and growth factors, including CXCL12 and TGF-βs.
The effects of matricellular proteins
The term “matricellular protein” was coined to highlight the effects of structurally unrelated, injury-associated ECM components that regulate cellular functions by modulating signaling cascades (79,80). Prototypical matricellular proteins have a limited structural role, but are overexpressed in injured and remodeling tissues, and bind to structural matrix proteins and cell surface receptors (like integrins or syndecans), transducing cytokine and growth factor signals, or modulating the activity of proteases and other bioactive mediators (71). The family includes SPARC (Secreted protein, acidic and rich in cysteine), thrombospondins (TSP)-1, −2, and −4, tenascin-C and -X, periostin, osteopontin, and the members of the CCN (Cystein rich protein 61/ Connective tissue growth factor)/ Nephroblastoma overexpressed gene) family (81). The term is also used to describe other secreted proteins that may exhibit some matricellular functions within the broad range of their effects (including the galectins, fibulins, and small leucine rich proteoglycans such as osteoglycin). Considering the growing evidence suggesting that even prototypical matricellular proteins may act as intracellular mediators or exert cytokinelike functions, the term may be more useful to describe specific functions that govern the interactions between the ECM and cellular elements, rather than to define a group of proteins. In the infarcted heart, neurohumoral signals, cytokines and growth factors induce the expression and secretion of a wide range of matricellular proteins in fibroblasts, macrophages, cardiomyocytes and vascular cells (Central Illustration) (13,82,83). Our current understanding of the role of matricellular proteins in the infarcted heart is derived from studies using genetically targeted mice (71,84). Although each member appears to exert unique actions in vivo (Table 2), several unifying patterns have emerged on the functional properties of matricellular macromolecules. First, matricellular proteins act on many cell types, modulating fibroblast, macrophage and vascular cell phenotypes, and regulating survival, hypertrophy and dysfunction of border zone cardiomyocytes (85-87). However, the relative significance of these cell-specific actions of matricellular proteins is unclear. Second, matricellular proteins may also contribute to the cellular plasticity of interstitial cells observed in healing infarcts (88-91), both through direct actions and via modulation of growth factor-mediated pathways. Third, several members of the family exhibit a highly selective localization in the infarct border zone (92), possibly reflecting macrophage-derived secretion in this leukocyte-rich environment, or the unique interactions between surviving cardiomyocytes, macrophages and fibroblasts in this area. The localized induction of matricellular proteins in the infarct border zone highlights their role in spatial regulation of cellular responses. Large amounts of growth factors and cytokines are secreted in the infarct border zone and could diffuse into neighboring viable areas, promoting fibrogenic or inflammatory responses. The selective induction and deposition of matricellular proteins may serve to localize inflammation and fibroblast activation within the area of injury, restraining the fibrotic response and protecting from adverse remodeling. Fourth, members of the matricellular family are transiently upregulated in the healing infarct. Although the mechanisms responsible for removal of matricellular proteins from the healing area remain poorly understood, the signals involved in their clearance may protect the infarcted heart from expansion of the fibrotic response following injury.
Table 2.
Expression and role of matricellular proteins in myocardial infarction.
| Matricellular protein |
Expression following MI |
Function | Possible mechanisms of action |
|---|---|---|---|
| Thrombospond in (TSP)-1 | Transient upregulation and deposition in the infarct border zone during the proliferative phase of healing in mouse, rat and canine models of MI (71,165). In human patients with end-stage heart failure, TSP-1 expression may be reduced, suggesting that injury-associated TSP-1 induction is transient (166). | Protects the infarcted heart from adverse remodeling, limiting expansion of inflammation and extension of fibrosis (71). | May activate TGF-β (167,168). May inhibit MMPs (169,170). May exert angiostatic actions (171). May modulate nitric oxide signaling and inflammation (172). May regulate ECM assembly and cross-linking (173). The relative contribution of these actions in TSP-1-mediated modulation of the reparative and remodeling responses following MI has not been studied. |
| TSP-4 | Markedly upregulated in experimental models of MI (174). | Although TSP-4 has been suggested to exert protective actions in pressure-overloaded hearts (175,176), its role in MI has not been systematically studied. | Unknown. |
| Tenascin C | Transient upregulation in infarcted area, border zone, and remote remodeling myocardium has been documented in animal models of MI (177,178) and in human patients with ischemic heart disease. | May contribute to myocardial regeneration in fish and amphibians (147,179). Promotes fibrosis and accelerates adverse post-MI remodeling in adult mammals (85,180) | May exert pro-inflammatory actions, in part through activation of Toll-like receptor signaling (181). May promote fibrogenic actions and may drive peristence of fibrotic lesions (182). May promote recruitment of myofibroblasts (85). May modulate angiogénesis(183). May regulate macrophage recruitment (184) and polarization (87). |
| SPARC | Abundant, but transient upregulation in infarcted myocardium, predominantly localized in macrophages and myofibroblasts (185,186). | Protects from cardiac rupture. May promote systolic dysfunction during the early post-ischemic phase (185,186). | May play a role in procollagen processing and collagen fibril assembly (185,187). May accentuate growth factor signaling, promoting fibroblast activation (185). May exert inotropic actions on cardiomyocytes (188). May restrain angiogenesis (189), and inhibit angiogénic actions of VEGF in endothelial cells (190). |
| Osteopontin | Markedly and transiently upregulated following MI, primarily localized in galectin-3hi/CD206+ macrophages (42,191,192). | Protects from adverse remodeling (35). | May promote proliferation and integrin-dependent activation of cardiac fibroblasts (193). May transduce survival signals (194), and activate a matrix-synthetic program in fibroblasts (195). May promote cardiomyocyte hypertrophy (196), and regulate metabolic pathways involved in diastolic dysfunction (197). May promote angiogenic responses (198). May activate macrophages to express galectin-3, promoting a fibrogenic phenotype (198). |
| Periostin | Marked, but transient upregulation in activated myofibroblasts in the infarct, border zone and remodeling area (199,200). | Protects from cardiac rupture, while promoting late fibrosis in remodeling segments (199,200). Has been suggested to promote a regenerative response in neonatal mouse myocardial injury (201) and may even activate a regenerative program in adult mice (151). | May promote migration and activate a matrix-synthetic program in cardiac fibroblasts (199,200). May regulate collagen fibrillogenesis (202). Has been suggested to promote cell cycle entry in cardiomyocyte progenitors and in cardiomyocytes (151). However, genetic manipulations of periostin in mouse models failed to confirm the significance of these actions (203). |
| CCN2 | Marked, but transient upregulation in infarcted hearts (204-206). | Cardiomyocyte-specific CCN2 overexpression studies suggested that CCN2 reduces infarct size following ischemia/reperfusion and attenuates adverse remodeling in non-reperfused infarction (204,205). In contrast, antagonism of endogenous CCN2 using a neutralizing antibody had no effects on infarct size (207). CCN2 inhibition experiments in a model of non-reperfused MI suggested that CCN2 may accentuate adverse remodeling, promoting hypertrophy and fibrosis (207). | May activate pro-survival pathways in cardiomyocytes.May attenuate inflammation and promote fibrogenic activation (207). Studies using genetic approaches in models of cardiac remodeling did not support the proposed critical role of CCN2 as a downstream effector of TGF-β (208). CCN2 and CCN5 have been suggested to exert opposing actions in remodeling and fibrosis of the pressure-overloaded heart (206); however, interactions between CCN family members in MI have not been investigated. |
Abbreviations: CCN, CYR61 (cysteine-rich angiogenic protein 61)/CTGF(connective tissue growth factor)/NOV(nephroblastoma overexpressed); SPARC, Secreted protein, acidic and rich in cysteine.
Deposition of fibrillar collagens in the infarcted heart
De novo fibrillar collagen synthesis (i.e. collagens I and III) confers mechanical strength to the healing scar, thus protecting the infarcted myocardium from rupture and attenuating dilative remodeling. In addition to their structural role, fibrillar collagens may also transduce signals to interstitial immune and vascular cells. Infarct myofibroblasts are capable of secreting large amounts of collagens in response to neurohumoral stimuli, or growth factors (such as TGF-β), and are the major collagen-synthesizing cells in the healing infarct (93). Although the functional heterogeneity of fibroblasts is increasingly appreciated (94,95), whether exaggerated matrix synthesis marks a distinct subset of myofibroblasts remains unknown.
In experimental models of MI, type III procollagen exhibits early induction, followed by the marked upregulation of procollagen I synthesis (93,96). Whether the time course of collagen isoform synthesis reflects isoform-specific effects of various fibrogenic stimuli is unknown. The relative expression of collagen I and collagen III has been suggested to regulate cardiac compliance. Collagen I forms thick and stiff fibers; in contrast, the fine reticular collagen III fibers are more compliant and may improve elasticity of the infarcted ventricle.
The synthesis of procollagens by infarct myofibroblasts is followed by secretion of soluble procollagen protein in the infarct zone (Figure 2). Subsequent processing of procollagen chains and assembly of fibrils is of critical significance for formation of an organized scar. Cleavage of the C-terminal propetide attached to procollagen involves the C proteinase bone morphogenetic protein (BMP)1 (97), which is induced in the infarcted heart (98,99). Secreted frizzle-related protein 2 (Sfrp2) is also upregulated in infarct fibroblasts and likely enhances the BMP1-procollagen interaction, promoting collagen processing (99). In addition, collagen processing may require further interactions with matricellular proteins, such as SPARC (100).
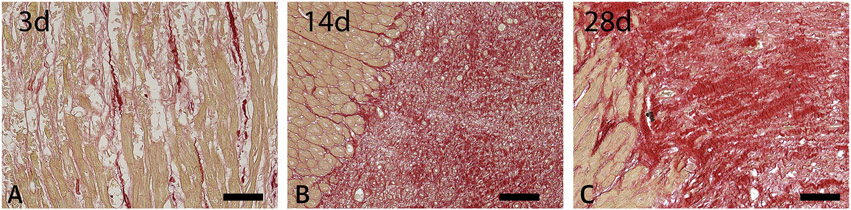
Figure 2. The time course of collagen deposition in the healing infarct.
Staining of canine infarct border zones with picrosirius red to identify collagen fibers at 3, 14 and 28 days after reperfused MI (1h of coronary occlusion followed by reperfusion). Collagen is identified by the red staining, with cardiomyocytes in light brown. Note the early disruption of the collagen network, accompanied by limited de novo synthesis at 3 days (A). After 14 days, extensive collagen deposition is noted in the healing infarct (B). The mature scar (28 days - C) exhibits a dense matrix network, typically associated with collagen cross-linking. Data from our own work in a canine model of reperfused MI (13). Scalebar=100μm.
The role of non-fibrillar collagens
Myocardial infarction is also associated with the induction of non-fibrillar collagens; a subfamily of collagens that do not form large fibrillar bundles, but which can associate with type I or III collagen fibrils to regulate anchoring, networking and organization of the ECM (101). Some non-fibrillar collagens may exert signaling functions through binding to cell surface receptors. Other members of the family may be proteolytically cleaved, generating bioactive fragments that regulate the fibroblast or vascular cell phenotype. Collagen VI, the best studied non-fibrillar collagen in MI, has been suggested to exert protective effects, reducing the size of the infarct and attenuating adverse remodeling (102). Specific actions of collagen VI on cardiomyocyte survival and activation of reparative myofibroblasts have been proposed to explain the protective effects (103,104).
The ECM in scar maturation
Maturation of the healing infarct is associated with cross-linking of the collagenous ECM and reduction of the cellular content of the scar. The molecular signals suppressing fibrogenic activation in the mature scar remain enigmatic. As the scar matures, there is a progressive reduction of the density of activated myofibroblasts in the infarct zone (105). Some descriptive studies have documented apoptosis of infarct fibroblasts, suggesting that cell-specific activation of an apoptotic program may be involved (106,107). Depletion of growth factors and clearance of matricellular proteins required for fibroblast survival may be sufficient to trigger fibroblast death or quiescence in the mature infarct (108,109). Whether active secretion of specific pro-apoptotic or de-activating signals that target infarct fibroblasts also plays a role in negative regulation of the fibrogenic response remains unknown. Recent lineage tracing approaches have suggested that fibroblasts persist in the mature infarct, but that they transition to a distinct phenotype that produces tendon genes and which may be specialized to support the scar (110). However, the mechanisms responsible for cell-specific activation of a pro-apoptotic program in the late phase of infarct healing have not been investigated, and the acquisition of a quiescent phenotype may precede apoptosis of infarct myofibroblasts. Vascular cells may also respond to the mature ECM environment by undergoing changes that profoundly affect the morphology and function of the infarct microvasculature. As the scar matures, infarct microvessels acquire a coat of mural cells through activation of platelet-derived growth factor receptor (PDGFR)-β signaling (105,111); while uncoated vessels regress. The composition of the ECM modulates interactions between pericytes and endothelial cells (112,113); however, the role of such actions in the maturation of infarct microvessels has not been investigated.
Chronic ECM expansion in the non-infarcted remodeling myocardium may play a role in heart failure progression
In the presence of a large MI, massive loss of contractile tissue results in increased ventricular filling pressures, and typically also with progressive fibrotic changes in viable myocardial segments (114). The deposition of interstitial collagen in the non-infarct zone is attributed to the pathophysiological effects of pressure and volume loads, and may be dependent on neurohumoral pathway activation (115). Moreover, in viable segments, increased wall stress may locally activate mechanosensitive cascades in macrophages and fibroblasts, triggering myocardial interstitial fibrosis (MIF; see Part 3 of this review series) and expansion of the cardiac interstitial matrix network (116,117).
The ECM in chronic ischemic cardiomyopathy
Alterations in the ECM network have been extensively documented in patients with ischemic cardiomyopathy, even in the absence of frank MI. Patients with chronic ischemic cardiomyopathy typically exhibit MIF (118), and have increased myocardial collagen levels (119) associated with accentuated MMP expression (120,121) and increased deposition of matricellular proteins such as tenascin-C (122). Considering the high prevalence of comorbidities (including hypertension, diabetes and metabolic dysfunction) that may profoundly affect ECM deposition and remodeling, the relative impact of chronic ischemia in the matrix remodeling patterns noted in ischemic cardiomyopathy patients is unclear.
Pathophysiologically-oriented studies examining the effects of brief intermittent ischemic insults, or low flow ischemia on the myocardial ECM are limited. Some animal evidence suggests that ischemia may cause significant alterations in the ECM network, even in the absence of infarction. In a pig model, reduction of coronary flow to 50% of baseline for 90 min was sufficient to activate MMP9, but had no effects on the structure of cardiac ECM (123). Whether longer intervals of chronic ischemia stimulate MMP-mediated matrix degradation remains unknown. In a mouse model, daily brief repetitive myocardial ischemia and reperfusion for 7 days was associated with interstitial collagen deposition and increased expression of the matricellular protein tenascin-C, in the absence of a completed infarction (124). The activation of oxidative stress pathways by brief repetitive ischemic insults was implicated in the observed fibrotic changes. This suggests that repetitive ischemic events of low duration or intensity may be sufficient to trigger remodeling of the cardiac ECM in the absence of cardiomyocyte necrosis (125). A small clinical study supports this notion, demonstrating that in human patients undergoing aorto-coronary bypass surgery, MMP2 and MMP9 are activated in the myocardium following reperfusion after cardioplegia (126).
Therapeutic opportunities: Targeting the ECM in ischemic heart disease
Matrix-based strategies to preserve the structure, geometry and function of the remodeling heart after MI
Because of its importance in preserving cardiac structural integrity and function (127), and its role in the regulation of cellular responses in cardiac injury, repair, and remodeling (71), the cardiac ECM may provide unique opportunities for therapeutic interventions. Following acute MI, cardiac rupture is an uncommon but dramatic complication, typically associated with accentuated matrix degradation (128), perturbed deposition of new structural matrix (129), or disorganized architecture of the scar (65). The application of patches containing ECM proteins may be sufficient to restore the structural integrity of the ventricle in acute left ventricular free wall rupture (130).
The effects of biomaterial scaffolds have been extensively studied in experimental MI models. Implantation of cellular or acellular ECM bioscaffolds, and administration of injectable materials, have been reported to exert beneficial actions on the infarcted heart (131-135), not only by improving the mechanical properties of the healing scar, but also by modulating inflammatory and reparative signals, by stimulating pro-survival pathways and promoting angiogenesis (136). Composite cell-matrix scaffolds can also be effective cell delivery platforms to enhance the reparative actions of cell therapy (137). Recently, transendocardial injection of VentriGel, a cardiac ECM hydrogel derived from decellularized porcine myocardium, was tested in 15 patients with chronic remodeling and moderate left ventricular dysfunction following MI (138). Although the study supported the safety and feasibility of the approach, it was not designed to evaluate efficacy, and large randomized clinical trials testing the efficacy of ECM hydrogels have not been performed. More importantly, despite extensive experimental work, there is currently no consensus regarding the optimal composition and method of administration of biomaterials following MI. Systematic study of the effects of various strategies in large animal models of MI is needed prior to attempts for therapeutic translation.
In chronic ischemic heart disease, there is little doubt that ECM deposition needs to be tightly regulated: excessive accumulation of cross-linked collagens may increase stiffness, promoting diastolic dysfunction, whereas overactive protease-driven matrix degradation pathways may promote dilative remodeling, causing systolic dysfunction. Established therapeutic approaches, such as angiotensin converting enzyme (ACE) inhibition, angiotensin type 1 (AT1) receptor blockade, β-adrenergic receptor antagonism, and mechanical unloading, all act to modulate the deposition and metabolism of ECM proteins (139) and may exert their protective actions (at least in part) by targeting the ECM network. However, the implementation of direct approaches to target matrix remodeling has been hampered by the broad effects of proteases in the infarcted myocardium, while attempts to inhibit MMPs in patients with MI have produced mixed results. Thus, early non-selective MMP inhibition with doxycycline attenuated dilative remodeling in patients surviving MI complicated by left ventricular systolic dysfunction (140). In contrast, administration of a selective oral MMP inhibitor in patients with MI and reduced ejection fraction showed no significant protection from adverse remodeling (141). The wide range of actions of MMPs that may affect not only ECM remodeling, but also the molecular signals involved in inflammation and repair, complicates interpretation of these findings. As also suggested in the context of MIF in the non-ischemic heart (see Part 3 of this JACC series), it is likely that successful approaches targeting the ECM may require the development and validation of biomarkers or imaging methods to identify patient subpopulations with specific patterns of ECM alterations (142). Thus, individuals with overactive matrix synthetic responses following infarction may require different approaches compared to patients with predominantly protease-driven matrix degradation.
Targeting the matricellular proteins
The critical role of the ECM in repair and remodeling of the infarcted heart suggests that therapeutic approaches modulating the biochemical composition and mechanical properties of the matrix may hold promise for patients with MI. Strategies based on matricellular proteins seem particularly attractive, as they could allow localized modulation of growth factor and cytokine signaling in the infarct area. However, despite evidence suggesting the critical actions of matricellular proteins in the infarcted heart, therapeutic translation is hampered by the complexity of their biological actions. The effects of the matricellular proteins are exerted through many cell types and may be context-dependent, depending on the local cytokine environment and the ECM composition. Moreover, matricellular proteins have multiple functional domains with different actions. Thus, successful therapeutic implementation of therapies based on matricellular proteins will likely require the identification of the functional domains responsible for their protective or detrimental actions in vivo (71,143). Considering the known limitations of in vivo models, this is a daunting task.
Matrix interventions for cardiac regeneration
ECM modulation may also hold the key to a visionary research goal: cardiac regeneration. This notion is based on several findings. First, in cardiomyocyte progenitors and neonatal cardiomyocytes, a compliant, elastin-containing ECM promotes cell cycle entry (144,145). Second, in zebrafish, fibronectin deposition has been suggested to play a critical role in myocardial regeneration (146), and in amphibians, a matrix network containing fibronectin, hyaluronan, and the matricellular protein tenascin-C has been suggested to activate a regenerative program (147). Third, in experimental cell therapy studies in mammalian models of cardiac injury, the application of matrix-based patches containing progenitor cells showed enhanced effectiveness (148,149). Whether such effects may be due to direct actions of specific matrix proteins on cardiac progenitors, or reflect matrix-dependent activation of other cell types (like immune or vascular cells) remains unknown. Fourth, mammalian models of infarction have suggested regenerative actions of certain matricellular macromolecules that may promote the proliferation of differentiated cardiomyocytes. For example, agrin, a large extracellular heparan sulfate proteoglycan, promotes cardiomyocyte cell cycle re-entry in both neonatal and adult mice (150). In addition, the matricellular protein periostin triggers cell cycle re-entry in differentiated adult cardiomyocytes through integrin-dependent mechanisms, which results in improved cardiac function when administered as an epicardial gelfoam patch following infarction (151). Nevertheless, these promising early findings should be viewed with caution. Considering that the specialized ECM proteins suggested to be critical in regeneration of fish, amphibian and neonatal mouse hearts are also induced following adult mammalian cardiac injury in the absence of regeneration, it is unlikely that simple ECM-modulatory strategies will be sufficient to remuscularize adult mammalian hearts. Myocardial regeneration may require not only a “regenerative” matrix profile, but also enhanced phenotypic plasticity of progenitor cell populations, and a favorable local cytokine and growth factor microenvironment.
Summary and conclusions
Both acute MI and chronic myocardial ischemia are associated with profound changes in the ECM network. Furthermore, these ECM changes not only alter the cardiac interstitial milieu and its functional properties, but a growing body of evidence suggests that the ECM itself plays a key role in modulating both beneficial and adverse pathways, including inflammation and post-MI remodeling, but also cardiac repair. In this context, and also the broader context of the entire cardiovascular system as reviewed in this JACC series, the therapeutic targeting of the ECM and its many players is a compelling clinical avenue of research that is certain to see intense future interest.
Highlights.
Myocardial ischemia and infarction are associated with dynamic changes in the composition of the ECM network.
In addition to their structural role, ECM proteins modulate cellular phenotype and function.
Specialized matrix proteins transduce signals that regulate inflammation, fibrosis and angiogenesis.
Targeting the ECM may attenuate remodeling and enhance repair and regeneration following infarction.
Acknowledgments:
N.G. Frangogiannis’ laboratory is supported by National Institutes of Health grants R01HL76246 and R01HL85440 and by grants PR151029, PR151134, and PR181464 from the Department of Defense Congressionally Directed Medical Research Programs. Jason Kovacic acknowledges research support from the National Institutes of Health (R01HL130423, R01HL135093).
Abbreviations
- BMP
bone morphogenetic protein
- CITP
carboxy-terminal telopeptide of collagen type I
- DAMP
damage-associated molecular patterns
- ECM
extracellular matrix
- IL
interleukin
- MI
myocardial infarction
- MIF
myocardial interstitial fibrosis
- MMP
matrix metalloprotease
- PGP
proline-glycine-proline
- SPARC
secreted protein, acidic and rich in cysteine
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures:
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santini MP, Forte E, Harvey RP, Kovacic JC. Developmental origin and lineage plasticity of endogenous cardiac stem cells. Development 2016;143:1242–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etoh T, Joffs C, Deschamps AM et al. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol 2001. ;281:H987–94. [DOI] [PubMed] [Google Scholar]
- 3.Villarreal F, Omens J, Dillmann W, Risteli J, Nguyen J, Covell J. Early degradation and serum appearance of type I collagen fragments after myocardial infarction. J Mol Cell Cardiol 2004;36:597–601. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Ashraf M, Millard RW, Fujiwara H, Schwartz A. Connective tissue changes in early ischemia of porcine myocardium: an ultrastructural study. J Mol Cell Cardiol 1983;15:261–75. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res 2002;53:165–74. [DOI] [PubMed] [Google Scholar]
- 6.Alfonso-Jaume MA, Bergman MR, Mahimkar R et al. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am J Physiol Heart Circ Physiol 2006;291 :H1838–46. [DOI] [PubMed] [Google Scholar]
- 7.Danielsen CC, Wiggers H, Andersen HR. Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J Mol Cell Cardiol 1998;30:1431–42. [DOI] [PubMed] [Google Scholar]
- 8.Fang W, He A, Xiang MX et al. Cathepsin K-deficiency impairs mouse cardiac function after myocardial infarction. J Mol Cell Cardiol 2019;127:44–56. [DOI] [PubMed] [Google Scholar]
- 9.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest 2016;126:3176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol 2015;44–46:122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol 2014;23:457–63. [DOI] [PubMed] [Google Scholar]
- 12.Lauten A, Gerhard-Garcia A, Suhr F, Fischer JH, Figulla HR, Bloch W. Impact of ischemia-reperfusion on extracellular matrix processing and structure of the basement membrane of the heart. PLoS One 2014;9:e92833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res 2006;324:475–88. [DOI] [PubMed] [Google Scholar]
- 14.Huebener P, Abou-Khamis T, Zymek P et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol 2008; 180:2625–33. [DOI] [PubMed] [Google Scholar]
- 15.Trial J, Baughn RE, Wygant JN et al. Fibronectin fragments modulate monocyte VLA-5 expression and monocyte migration. J Clin Invest 1999;104:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest 1980;66:859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaggar A, Jackson PL, Noerager BD et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 2008;180:5662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weathington NM, van Houwelingen AH, Noerager BD et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 2006;12:317–23. [DOI] [PubMed] [Google Scholar]
- 19.Russo I, Cavalera M, Huang S et al. Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program. Circ Res 2019;124:1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang D, Liang J, Fan J et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–9. [DOI] [PubMed] [Google Scholar]
- 21.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004;279:17079–84. [DOI] [PubMed] [Google Scholar]
- 22.Teder P, Vandivier RW, Jiang D et al. Resolution of lung inflammation by CD44. Science 2002;296:155–8. [DOI] [PubMed] [Google Scholar]
- 23.Mydel P, Shipley JM, Adair-Kirk TL et al. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem 2008;283:9513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey ML, Iyer RP, Zamilpa R et al. A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis. J Am Coll Cardiol 2015;66:1364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada M, Oba Y, Yamawaki H. Endostatin stimulates proliferation and migration of adult rat cardiac fibroblasts through PI3K/Akt pathway. Eur J Pharmacol 2015;750:20–6. [DOI] [PubMed] [Google Scholar]
- 26.O'Reilly MS, Boehm T, Shing Y et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277–85. [DOI] [PubMed] [Google Scholar]
- 27.Hamano Y, Zeisberg M, Sugimoto H et al. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell 2003;3:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuda J, Fukui K, Okada M, Yamawaki H. T3 peptide, a fragment of tumstatin, stimulates proliferation and migration of cardiac fibroblasts through activation of Akt signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 2017;390:1135–1144. [DOI] [PubMed] [Google Scholar]
- 29.Okada M, Murata N, Yamawaki H. Canstatin stimulates migration of rat cardiac fibroblasts via secretion of matrix metalloproteinase-2. Am J Physiol Cell Physiol 2017;312:C199–C208. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama A, Okada M, Yamawaki H. Pathophysiological roles of canstatin on myofibroblasts after myocardial infarction in rats. Eur J Pharmacol 2017;807:32–43. [DOI] [PubMed] [Google Scholar]
- 31.Gearing AJ, Beckett P, Christodoulou M et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol 1995;57:774–7. [DOI] [PubMed] [Google Scholar]
- 32.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 1998;161:3340–6. [PubMed] [Google Scholar]
- 33.Dayer C, Stamenkovic I. Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-beta (TGF-beta) Activation and Fibroblast Differentiation. J Biol Chem 2015;290:13763–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol 2007;82:1375–81. [DOI] [PubMed] [Google Scholar]
- 35.Feng G, Hao D, Chai J. Processing of CXCL12 impedes the recruitment of endothelial progenitor cells in diabetic wound healing. FEBS J 2014;281:5054–62. [DOI] [PubMed] [Google Scholar]
- 36.Peng H, Wu Y, Duan Z, Ciborowski P, Zheng JC. Proteolytic processing of SDF-1alpha by matrix metalloproteinase-2 impairs CXCR4 signaling and reduces neural progenitor cell migration. Protein Cell 2012;3:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denney H, Clench MR, Woodroofe MN. Cleavage of chemokines CCL2 and CXCL10 by matrix metalloproteinases-2 and −9: implications for chemotaxis. Biochem Biophys Res Commun 2009;382:341–7. [DOI] [PubMed] [Google Scholar]
- 38.Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol 2013;190:401–10. [DOI] [PubMed] [Google Scholar]
- 39.Cox JH, Dean RA, Roberts CR, Overall CM. Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J Biol Chem 2008;283:19389–99. [DOI] [PubMed] [Google Scholar]
- 40.Bujak M, Dobaczewski M, Chatila K et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 2008;173:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujak M, Dobaczewski M, Gonzalez-Quesada C et al. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res 2009;105:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewald O, Zymek P, Winkelmann K et al. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005;96:881–9. [DOI] [PubMed] [Google Scholar]
- 43.Cauwe B, Opdenakker G. Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 2010;45:351–423. [DOI] [PubMed] [Google Scholar]
- 44.Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 2010;122:2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz R Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 2007;47:211–42. [DOI] [PubMed] [Google Scholar]
- 46.Jobin PG, Butler GS, Overall CM. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta Mol Cell Res 2017;1864:2043–2055. [DOI] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 2017;127:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker TH, Engler AJ. The provisional matrix: setting the stage for tissue repair outcomes. Matrix Biol 2017;60–61:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark RA, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol 1982;79:264–9. [DOI] [PubMed] [Google Scholar]
- 50.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 1998;18:1363–70. [DOI] [PubMed] [Google Scholar]
- 51.Ulrich MM, Janssen AM, Daemen MJ et al. Increased expression of fibronectin isoforms after myocardial infarction in rats. J Mol Cell Cardiol 1997;29:2533–43. [DOI] [PubMed] [Google Scholar]
- 52.Andersson L, Scharin Tang M, Lundqvist A et al. Rip2 modifies VEGF-induced signalling and vascular permeability in myocardial ischaemia. Cardiovasc Res 2015;107:478–86. [DOI] [PubMed] [Google Scholar]
- 53.Frangogiannis NG, Lindsey ML, Michael LH et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998;98:699–710. [DOI] [PubMed] [Google Scholar]
- 54.Brown LF, Yeo KT, Berse B et al. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 1992;176:1375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petzelbauer P, Zacharowski PA, Miyazaki Y et al. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med 2005;11:298–304. [DOI] [PubMed] [Google Scholar]
- 56.Flick MJ, Du X, Witte DP et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 2004;113:1596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva LM, Lum AG, Tran C et al. Plasmin-mediated fibrinolysis enables macrophage migration in a murine model of inflammation. Blood 2019;134:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corbett SA, Schwarzbauer JE. Fibronectin-fibrin cross-linking: a regulator of cell behavior. Trends Cardiovasc Med 1998;8:357–62. [DOI] [PubMed] [Google Scholar]
- 59.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 2001;167:2887–94. [DOI] [PubMed] [Google Scholar]
- 60.White ES, Livant DL, Markwart S, Arenberg DA. Monocyte-fibronectin interactions, via alpha(5)beta(1) integrin, induce expression of CXC chemokine-dependent angiogenic activity. J Immunol 2001;167:5362–6. [DOI] [PubMed] [Google Scholar]
- 61.Shiraishi M, Shintani Y, Shintani Y et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 2016;126:2151–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honold L, Nahrendorf M. Resident and Monocyte-Derived Macrophages in Cardiovascular Disease. Circ Res 2018;122:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen B, Huang S, Su Y et al. Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ Res 2019;125:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanisicak O, Khalil H, Ivey MJ et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016;7:12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong P, Shinde AV, Su Y et al. Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium. Circulation 2018;137:707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simoes FC, Cahill TJ, Kenyon A et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun 2020;11:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schafer BM, Maier K, Eickhoff U, Todd RF, Kramer MD. Plasminogen activation in healing human wounds. Am J Pathol 1994;144:1269–80. [PMC free article] [PubMed] [Google Scholar]
- 68.Creemers E, Cleutjens J, Smits J et al. Disruption of the plasminogen gene in mice abolishes wound healing after myocardial infarction. Am J Pathol 2000;156:1865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong Y, Zhao Y, Li Y, Fan Y, Hoover-Plow J. Plasminogen regulates cardiac repair after myocardial infarction through its noncanonical function in stem cell homing to the infarcted heart. J Am Coll Cardiol 2014;63:2862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 71.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 2012;92:635–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lodyga M, Cambridge E, Karvonen HM et al. Cadherin-11 -mediated adhesion of macrophages to myofibroblasts establishes a profibrotic niche of active TGF-beta. Sci Signal 2019;12. [DOI] [PubMed] [Google Scholar]
- 73.Arslan F, Smeets MB, Riem Vis PW et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res 2011;108:582–92. [DOI] [PubMed] [Google Scholar]
- 74.Serini G, Bochaton-Piallat ML, Ropraz P et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 1998;142:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klingberg F, Chau G, Walraven M et al. The fibronectin ED-A domain enhances recruitment of latent TGF-beta-binding protein-1 to the fibroblast matrix. J Cell Sci 2018;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frangogiannis NG. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J Thorac Dis 2017;9:S52–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wijelath ES, Rahman S, Namekata M et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res 2006;99:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korf-Klingebiel M, Reboll MR, Grote K et al. Heparan Sulfate-Editing Extracellular Sulfatases Enhance VEGF Bioavailability for Ischemic Heart Repair. Circ Res 2019;125:787–801. [DOI] [PubMed] [Google Scholar]
- 79.Bornstein P Matricellular proteins: an overview. J Cell Commun Signal 2009;3:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 2001;107:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 2002;14:608–16. [DOI] [PubMed] [Google Scholar]
- 82.Mackie EJ, Scott-Burden T, Hahn AW et al. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol 1992;141:377–88. [PMC free article] [PubMed] [Google Scholar]
- 83.Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood 1999;94:837–44. [PubMed] [Google Scholar]
- 84.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 2004;64:24–31. [DOI] [PubMed] [Google Scholar]
- 85.Tamaoki M, Imanaka-Yoshida K, Yokoyama K et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol 2005;167:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imanaka-Yoshida K, Hiroe M, Nishikawa T et al. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest 2001;81:1015–24. [DOI] [PubMed] [Google Scholar]
- 87.Shimojo N, Hashizume R, Kanayama K et al. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin alphaVbeta3/nuclear factor-kappaB/interleukin-6 axis. Hypertension 2015;66:757–66. [DOI] [PubMed] [Google Scholar]
- 88.Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 2011;4:469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ubil E, Duan J, Pillai IC et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 2014;514:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeisberg EM, Tarnavski O, Zeisberg M et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007;13:952–61. [DOI] [PubMed] [Google Scholar]
- 91.Saxena A, Chen W, Su Y et al. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol 2013;191:4838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frangogiannis NG, Ren G, Dewald O et al. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 2005;111:2935–42. [DOI] [PubMed] [Google Scholar]
- 93.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol 1995;147:325–38. [PMC free article] [PubMed] [Google Scholar]
- 94.Frangogiannis NG. The Functional Pluralism of Fibroblasts in the Infarcted Myocardium. Circ Res 2016;119:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tallquist MD. Cardiac Fibroblast Diversity. Annu Rev Physiol 2020;82:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoue K, Kusachi S, Niiya K, Kajikawa Y, Tsuji T. Sequential changes in the distribution of type I and III collagens in the infarct zone: immunohistochemical study of experimental myocardial infarction in the rat. Coron Artery Dis 1995;6:153–8. [DOI] [PubMed] [Google Scholar]
- 97.Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol 2012;303:H234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He W, Zhang L, Ni A et al. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proc Natl Acad Sci U S A 2010;107:21110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi K, Luo M, Zhang Y et al. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 2009;11:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bradshaw AD, Baicu CF, Rentz TJ et al. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 2009;119:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shamhart PE, Meszaros JG. Non-fibrillar collagens: key mediators of post-infarction cardiac remodeling? J Mol Cell Cardiol 2010;48:530–7. [DOI] [PubMed] [Google Scholar]
- 102.Luther DJ, Thodeti CK, Shamhart PE et al. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res 2012;110:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naugle JE, Olson ER, Zhang X et al. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol 2006;290:H323–30. [DOI] [PubMed] [Google Scholar]
- 104.Skrbic B, Engebretsen KV, Strand ME et al. Lack of collagen VIII reduces fibrosis and promotes early mortality and cardiac dilatation in pressure overload in mice. Cardiovasc Res 2015;106:32–42. [DOI] [PubMed] [Google Scholar]
- 105.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 2002;50:71–9. [DOI] [PubMed] [Google Scholar]
- 106.Takemura G, Ohno M, Hayakawa Y et al. Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res 1998;82:1130–8. [DOI] [PubMed] [Google Scholar]
- 107.Zhao W, Lu L, Chen SS, Sun Y. Temporal and spatial characteristics of apoptosis in the infarcted rat heart. Biochem Biophys Res Commun 2004;325:605–11. [DOI] [PubMed] [Google Scholar]
- 108.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 2007;127:526–37. [DOI] [PubMed] [Google Scholar]
- 109.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–63. [DOI] [PubMed] [Google Scholar]
- 110.Fu X, Khalil H, Kanisicak O et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 2018;128:2127–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zymek P, Bujak M, Chatila K et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 2006;48:2315–23. [DOI] [PubMed] [Google Scholar]
- 112.Davis GE, Norden PR, Bowers SL. Molecular control of capillary morphogenesis and maturation by recognition and remodeling of the extracellular matrix: functional roles of endothelial cells and pericytes in health and disease. Connect Tissue Res 2015;56:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rivera LB, Brekken RA. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-beta1 activity. J Cell Biol 2011;193:1305–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Volders PG, Willems IE, Cleutjens JP, Arends JW, Havenith MG, Daemen MJ. Interstitial collagen is increased in the non-infarcted human myocardium after myocardial infarction. J Mol Cell Cardiol 1993;25:1317–23. [DOI] [PubMed] [Google Scholar]
- 115.van Krimpen C, Smits JF, Cleutjens JP et al. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat: effects of captopril. J Mol Cell Cardiol 1991;23:1245–53. [DOI] [PubMed] [Google Scholar]
- 116.Chen B, Frangogiannis NG. Macrophages in the Remodeling Failing Heart. Circ Res 2016;119:776–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sager HB, Hulsmans M, Lavine KJ et al. Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure. Circ Res 2016;119:853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beltrami CA, Finato N, Rocco M et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation 1994;89:151–63. [DOI] [PubMed] [Google Scholar]
- 119.Mukherjee D, Sen S. Alteration of collagen phenotypes in ischemic cardiomyopathy. J Clin Invest 1991;88:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reinhardt D, Sigusch HH, Hensse J, Tyagi SC, Korfer R, Figulla HR. Cardiac remodelling in end stage heart failure: upregulation of matrix metalloproteinase (MMP) irrespective of the underlying disease, and evidence for a direct inhibitory effect of ACE inhibitors on MMP. Heart 2002;88:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spinale FG, Coker ML, Heung LJ et al. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000;102:1944–9. [DOI] [PubMed] [Google Scholar]
- 122.Frangogiannis NG, Shimoni S, Chang SM et al. Active interstitial remodeling: an important process in the hibernating human myocardium. J Am Coll Cardiol 2002;39:1468–74. [DOI] [PubMed] [Google Scholar]
- 123.Lu L, Gunja-Smith Z, Woessner JF et al. Matrix metalloproteinases and collagen ultrastructure in moderate myocardial ischemia and reperfusion in vivo. Am J Physiol Heart Circ Physiol 2000;279:H601–9. [DOI] [PubMed] [Google Scholar]
- 124.Dewald O, Frangogiannis NG, Zoerlein M et al. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A 2003;100:2700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circ Res 2019;125:117–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lalu MM, Pasini E, Schulze CJ et al. Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur Heart J 2005;26:27–35. [DOI] [PubMed] [Google Scholar]
- 127.Clarke SA, Richardson WJ, Holmes JW. Modifying the mechanics of healing infarcts: Is better the enemy of good? J Mol Cell Cardiol 2016;93:115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Honda S, Asaumi Y, Yamane T et al. Trends in the clinical and pathological characteristics of cardiac rupture in patients with acute myocardial infarction over 35 years. J Am Heart Assoc 2014;3:e000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayashidani S, Tsutsui H, Ikeuchi M et al. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 2003;285:H1229–35. [DOI] [PubMed] [Google Scholar]
- 130.Holubec T, Caliskan E, Bettex D, Maisano F. Repair of post-infarction left ventricular free wall rupture using an extracellular matrix patch. Eur J Cardiothorac Surg 2015;48:800–3. [DOI] [PubMed] [Google Scholar]
- 131.Pattar SS, Fatehi Hassanabad A, Fedak PWM. Acellular Extracellular Matrix Bioscaffolds for Cardiac Repair and Regeneration. Front Cell Dev Biol 2019;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mewhort HEM, Svystonyuk DA, Turnbull JD et al. Bioactive Extracellular Matrix Scaffold Promotes Adaptive Cardiac Remodeling and Repair. JACC Basic Transl Sci 2017;2:450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res 2010;3:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seif-Naraghi SB, Singelyn JM, Salvatore MA et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med 2013;5:173ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McLaughlin S, McNeill B, Podrebarac J et al. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat Commun 2019;10:4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wassenaar JW, Gaetani R, Garcia JJ et al. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J Am Coll Cardiol 2016;67:1074–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Godier-Furnemont AF, Martens TP, Koeckert MS et al. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci U S A 2011;108:7974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Traverse JH, Henry TD, Dib N et al. First-in-Man Study of a Cardiac Extracellular Matrix Hydrogel in Early and Late Myocardial Infarction Patients. JACC Basic Transl Sci 2019;4:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Silvestre JS, Heymes C, Oubenaissa A et al. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation 1999;99:2694–701. [DOI] [PubMed] [Google Scholar]
- 140.Cerisano G, Buonamici P, Valenti R et al. Early short-term doxycycline therapy in patients with acute myocardial infarction and left ventricular dysfunction to prevent the ominous progression to adverse remodelling: the TIPTOP trial. Eur Heart J 2014;35:184–91. [DOI] [PubMed] [Google Scholar]
- 141.Hudson MP, Armstrong PW, Ruzyllo W et al. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol 2006;48:15–20. [DOI] [PubMed] [Google Scholar]
- 142.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014; 11:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sawyer AJ, Kyriakides TR. Matricellular proteins in drug delivery: Therapeutic targets, active agents, and therapeutic localization. Adv Drug Deliv Rev 2016;97:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qiu Y, Bayomy AF, Gomez MV et al. A role for matrix stiffness in the regulation of cardiac side population cell function. Am J Physiol Heart Circ Physiol 2015;308:H990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang J, Karra R, Dickson AL, Poss KD. Fibronectin is deposited by injury-activated epicardial cells and is necessary for zebrafish heart regeneration. Dev Biol 2013;382:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mercer SE, Odelberg SJ, Simon HG. A dynamic spatiotemporal extracellular matrix facilitates epicardial-mediated vertebrate heart regeneration. Dev Biol 2013;382:457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gaetani R, Feyen DA, Verhage V et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015;61:339–48. [DOI] [PubMed] [Google Scholar]
- 149.Serpooshan V, Zhao M, Metzler SA et al. Use of bio-mimetic three-dimensional technology in therapeutics for heart disease. Bioengineered 2014;5:193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bassat E, Mutlak YE, Genzelinakh A et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 2017;547:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuhn B, del Monte F, Hajjar RJ et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med 2007;13:962–9. [DOI] [PubMed] [Google Scholar]
- 152.Huebener P, Frangogiannis NG. Matricellular Proteins in Myocardial Infarction. Current Cardiology Reviews 2006;2:163–171. [Google Scholar]
- 153.Takahashi S, Geenen D, Nieves E, Iwazumi T. Collagenase degrades collagen in vivo in the ischemic heart. Biochim Biophys Acta 1999;1428:251–9. [DOI] [PubMed] [Google Scholar]
- 154.Manhenke C, Ueland T, Jugdutt BI et al. The relationship between markers of extracellular cardiac matrix turnover: infarct healing and left ventricular remodelling following primary PCI in patients with first-time STEMI. Eur Heart J 2014;35:395–402. [DOI] [PubMed] [Google Scholar]
- 155.McGavigan AD, Maxwell PR, Dunn FG. Serological evidence of altered collagen homeostasis reflects early ventricular remodeling following acute myocardial infarction. Int J Cardiol 2006;111:267–74. [DOI] [PubMed] [Google Scholar]
- 156.Murakami T, Kusachi S, Murakami M et al. Time-dependent changes of serum carboxy-terminal peptide of type I procollagen and carboxy-terminal telopeptide of type I collagen concentrations in patients with acute myocardial infarction after successful reperfusion: correlation with left ventricular volume indices. Clin Chem 1998;44:2453–61. [PubMed] [Google Scholar]
- 157.Barthelemy O, Beygui F, Vicaut E et al. Relation of high concentrations of plasma carboxy-terminal telopeptide of collagen type I with outcome in acute myocardial infarction. Am J Cardiol 2009;104:904–9. [DOI] [PubMed] [Google Scholar]
- 158.Cerisano G, Pucci PD, Sulla A et al. Relation between plasma brain natriuretic peptide, serum indexes of collagen type I turnover, and left ventricular remodeling after reperfused acute myocardial infarction. Am J Cardiol 2007;99:651–6. [DOI] [PubMed] [Google Scholar]
- 159.Eschalier R, Fertin M, Fay R et al. Extracellular matrix turnover biomarkers predict long-term left ventricular remodeling after myocardial infarction: insights from the REVE-2 study. Circ Heart Fail 2013;6:1199–205. [DOI] [PubMed] [Google Scholar]
- 160.Skjot-Arkil H, Clausen RE, Rasmussen LM et al. Acute Myocardial Infarction and Pulmonary Diseases Result in Two Different Degradation Profiles of Elastin as Quantified by Two Novel ELISAs. PLoS One 2013;8:e60936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mizuno T, Mickle DA, Kiani CG, Li RK. Overexpression of elastin fragments in infarcted myocardium attenuates scar expansion and heart dysfunction. Am J Physiol Heart Circ Physiol 2005;288:H2819–27. [DOI] [PubMed] [Google Scholar]
- 162.Yasuda J, Okada M, Yamawaki H. Protective effect of T3 peptide, an active fragment of tumstatin, against ischemia/reperfusion injury in rat heart. J Pharmacol Sci 2019;139:193–200. [DOI] [PubMed] [Google Scholar]
- 163.Isobe K, Kuba K, Maejima Y, Suzuki J, Kubota S, Isobe M. Inhibition of endostatin/collagen XVIII deteriorates left ventricular remodeling and heart failure in rat myocardial infarction model. Circ J 2010;74:109–19. [DOI] [PubMed] [Google Scholar]
- 164.Seko Y, Fukuda S, Nagai R. Serum levels of endostatin, vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in patients with acute myocardial infarction undergoing early reperfusion therapy Clin Sci (Lond: ) 2004;106:439–42. [DOI] [PubMed] [Google Scholar]
- 165.Sezaki S, Hirohata S, Iwabu A et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion Exp Biol Med (Maywood: ) 2005;230:621–30. [DOI] [PubMed] [Google Scholar]
- 166.Batlle M, Perez-Villa F, Lazaro A et al. Decreased expression of thrombospondin-1 in failing hearts may favor ventricular remodeling. Transplant Proc 2009;41:2231–3. [DOI] [PubMed] [Google Scholar]
- 167.Belmadani S, Bernal J, Wei CC et al. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol 2007;171:777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol 1993;122:923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem 2000;275:32167–73. [DOI] [PubMed] [Google Scholar]
- 170.Xia Y, Dobaczewski M, Gonzalez-Quesada C et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 2011;58:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation 1999;100:1423–31. [DOI] [PubMed] [Google Scholar]
- 172.Isenberg JS, Qin Y, Maxhimer JB et al. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 2009;28:110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosini S, Pugh N, Bonna AM, Hulmes DJS, Farndale RW, Adams JC. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci Signal 2018;11. [DOI] [PubMed] [Google Scholar]
- 174.Mustonen E, Leskinen H, Aro J et al. Metoprolol treatment lowers thrombospondin-4 expression in rats with myocardial infarction and left ventricular hypertrophy. Basic Clin Pharmacol Toxicol 2010;107:709–17. [DOI] [PubMed] [Google Scholar]
- 175.Cingolani OH, Kirk JA, Seo K et al. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res 2011;109:1410–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Frolova EG, Sopko N, Blech L et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J 2012;26:2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol 1996;179:321–5. [DOI] [PubMed] [Google Scholar]
- 178.Bujak M, Ren G, Kweon HJ et al. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation 2007;116:2127–38. [DOI] [PubMed] [Google Scholar]
- 179.Chablais F, Jazwinska A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 2012;139:1921–30. [DOI] [PubMed] [Google Scholar]
- 180.Kimura T, Tajiri K, Sato A et al. Tenascin-C accelerates adverse ventricular remodelling after myocardial infarction by modulating macrophage polarization. Cardiovasc Res 2019;115:614–624. [DOI] [PubMed] [Google Scholar]
- 181.Midwood K, Sacre S, Piccinini AM et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009;15:774–80. [DOI] [PubMed] [Google Scholar]
- 182.Bhattacharyya S, Wang W, Morales-Nebreda L et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun 2016;7:11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ballard VL, Sharma A, Duignan I et al. Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. FASEB J 2006;20:717–9. [DOI] [PubMed] [Google Scholar]
- 184.Abbadi D, Laroumanie F, Bizou M et al. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc Res 2018;114:123–137. [DOI] [PubMed] [Google Scholar]
- 185.Schellings MW, Vanhoutte D, Swinnen M et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 2009;206:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McCurdy SM, Dai Q, Zhang J et al. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 2011. ;301 :H497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol 2011;301:H841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deckx S, Johnson DM, Rienks M et al. Extracellular SPARC increases cardiomyocyte contraction during health and disease. PLoS One 2019;14:e0209534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bradshaw AD, Reed MJ, Carbon JG, Pinney E, Brekken RA, Sage EH. Increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges in SPARC-null mice. Wound Repair Regen 2001;9:522–30. [DOI] [PubMed] [Google Scholar]
- 190.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem 1998;273:29635–40. [DOI] [PubMed] [Google Scholar]
- 191.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol 1994;145:1450–62. [PMC free article] [PubMed] [Google Scholar]
- 192.Shirakawa K, Endo J, Kataoka M et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation 2018;138:2021–2035. [DOI] [PubMed] [Google Scholar]
- 193.Ashizawa N, Graf K, Do YS et al. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest 1996;98:2218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lorenzen JM, Schauerte C, Hubner A et al. Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur Heart J 2015;36:2184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sawaki D, Czibik G, Pini M et al. Visceral Adipose Tissue Drives Cardiac Aging Through Modulation of Fibroblast Senescence by Osteopontin Production. Circulation 2018;138:809–822. [DOI] [PubMed] [Google Scholar]
- 196.Trueblood NA, Xie Z, Communal C et al. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 2001;88:1080–7. [DOI] [PubMed] [Google Scholar]
- 197.Yousefi K, Irion CI, Takeuchi LM et al. Osteopontin Promotes Left Ventricular Diastolic Dysfunction Through a Mitochondrial Pathway. J Am Coll Cardiol 2019;73:2705–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao X, Johnson JN, Singh K, Singh M. Impairment of myocardial angiogenic response in the absence of osteopontin. Microcirculation 2007;14:233–40. [DOI] [PubMed] [Google Scholar]
- 199.Oka T, Xu J, Kaiser RA et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 2007;101:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shimazaki M, Nakamura K, Kii I et al. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 2008;205:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen Z, Xie J, Hao H et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3beta/cyclin D1 signalling pathway. Cardiovasc Res 2017;113:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Norris RA, Damon B, Mironov V et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 2007;101:695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res 2009;104:e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ahmed MS, Gravning J, Martinov VN et al. Mechanisms of Novel Cardioprotective Functions of CCN2/CTGF in Myocardial Ischemia/Reperfusion Injury. Am J Physiol Heart Circ Physiol 2011. [DOI] [PubMed] [Google Scholar]
- 205.Gravning J, Orn S, Kaasboll OJ et al. Myocardial connective tissue growth factor (CCN2/CTGF) attenuates left ventricular remodeling after myocardial infarction. PLoS One 2012;7:e52120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jeong D, Lee MA, Li Y et al. Matricellular Protein CCN5 Reverses Established Cardiac Fibrosis. J Am Coll Cardiol 2016;67:1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vainio LE, Szabo Z, Lin R et al. Connective Tissue Growth Factor Inhibition Enhances Cardiac Repair and Limits Fibrosis After Myocardial Infarction. JACC Basic Transl Sci 2019;4:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Accornero F, van Berlo JH, Correll RN et al. Genetic Analysis of Connective Tissue Growth Factor as an Effector of Transforming Growth Factor beta Signaling and Cardiac Remodeling. Mol Cell Biol 2015;35:2154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]