Abstract
Pluripotent and post‐natal, tissue‐specific stem cells share functional features such as the capacity to differentiate into multiple lineages and to self‐renew, and are endowed with specific cell maintenance mechanism as well as transcriptional and epigenetic signatures that determine stem cell identity and distinguish them from their progeny. Calcium is a highly versatile and ubiquitous second messenger that regulates a wide variety of cellular functions. Specific roles of calcium in stem cell niches and stem cell maintenance mechanisms are only beginning to be explored, however. In this review, I discuss stem cell‐specific regulation and roles of calcium, focusing on its potential involvement in the intertwined metabolic and epigenetic regulation of stem cells.
Keywords: calcium, epigenetics, metabolism, pluripotent stem cells, post‐natal stem cells
Subject Categories: Regenerative Medicine
This review discusses how calcium regulates stem cell niches and stem cell maintenance and how it is possibly involved in the intertwined metabolic and epigenetic regulation of stem cells.

Glossary
- 5hmC
5 hydroxymethyl cytosine
- 5mC
5‐methylcytosine
- ADP
adenosine diphosphate
- AMPK
AMP‐activated protein kinase
- AP‐1
activator protein 1
- Atp2b1‐4
ATPase plasma membrane Ca2+ transporting 1‐4
- ATPA2
ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2
- ATP
adenosine triphosphate
- ATPC1
ATPase secretory pathway Ca2+ transporting 1
- BM
bone marrow
- CamK
calmodulin kinase
- CaMKII
calcium/calmodulin‐dependent kinase 2
- Cas9
CRISPR‐associated protein 9
- CaSR
calcium‐sensing receptor
- CoA
Co‐enzyme A
- CRAC
calcium release‐activated calcium
- CRISPR
clustered regularly interspaced short palindromic repeats
- Crtc
Creb‐regulated transcriptional co‐activator
- DHA
dihydroxyacetone phosphate
- ER
endoplasmic reticulum
- ESC
embryonic stem cell
- ETC
electron transport chain
- FAD
flavine adenine dinucleotide
- FGF2
fibroblast growth factor 2
- G3P
glycerol‐3‐phosphate
- GEXC
gene expression commons
- GGCX
gamma‐glutamyl carboxylase
- Gla
gamma‐carboxyglutamate
- GPCR
G protein‐coupled receptor
- GPD2
FAD‐glycerol‐3‐phosphate dehydrogenase 2
- GSK3
glycogen synthase kinase 3
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- h
human
- HIF
hypoxia‐induced factor
- HSC
hematopoietic stem cell
- IDH
isocitrate dehydrogenase
- IP3R
IP3 receptor
- iPSC
induced pluripotent stem cell
- ISC
intestinal stem cell
- JMJC
Jumanji C
- KGD
a‐ketoglutarate dehydrogenase
- LIF
leukemia inhibitory factor
- MAM
mitochondria‐associated membrane
- MAPK2
mitogen‐activated protein kinase 2
- MCU
mitochondrial calcium uniporter
- MeCP2
methyl CpG‐binding protein 2
- MEF2C
myocyte enhancer factor 2C
- MFN2
mitofusin 2
- MGP
Matrix Gla protein
- m
mouse
- mTOR
mammalian target of rapamycin
- NAADP
nicotinic acid adenine dinucleotide phosphate
- NAD
nicotine adenine dinucleotide
- NCLX
sodium calcium lithium exchanger
- NCX
sodium calcium exchanger
- NFAT
nuclear factor of activated T cells
- OAA
oxaloacetic acid
- ORAI1
ORAI calcium release‐activated calcium modulator 1
- PDH
pyruvate dehydrogenase complex
- PDK
pyruvate dehydrogenase kinase
- PDP1
pyruvate dehydrogenase phosphatase 1
- PHD
prolyl hydroxylase domain
- PIP2
phosphatidylinositol 4,5‐bisphosphate
- PLC
phospholipase C
- PMCA
plasma membrane calcium efflux pump
- POU5F
POU class 5 homeobox 1
- PSC
pluripotent stem cell
- PTH
parathyroid hormone
- RISP
Riske iron‐sulfur protein
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmatic calcium pump
- Slc8a1
solute carrier family 8 member a1
- Slc8b1
solute carrier family 8 member a1
- SOCE
store‐operated calcium entry
- STIM1
stromal interaction molecule 1
- TCA
tricarboxylic acid cycle
- Tcf7l1
transcription factor 7‐like 1
- TET
ten‐eleven translocated
- TPC
two‐pore channel
- TRPV6
transient receptor potential cation channel subfamily V member 6
- VDAC1
voltage‐dependent anion‐selective channel 1
Introduction
Because of the promise of stem cells for the treatment of a wide variety of diseases, their biology has received enormous attention and major progress was made. The term “stem cell” covers pluripotent stem cells (PSCs), which either arise in the inner cell mass of preimplantation embryos (embryonic stem cells, ESCs) or can be generated by reprogramming of somatic cells (induced pluripotent stem cells, iPSCs) and give rise to all lineages of the embryo proper, totipotent stem cells that also generate extraembryonic tissues, and post‐natal stem cells that endow specific organs and tissues in which they reside with regenerative capacity 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13. These stem cell types share functional features such as multipotentiality and the capacity to self‐renew. However, while distinct from more mature cells and progenitors, marked differences exist in the transcriptional and epigenetic regulation and the response to external cues among the different types of stem cells. More recent studies have begun to investigate the specific cell biology of stem cells, focusing on organellar functions, cellular quality control, and metabolism 14, 15, 16, 17, 18, 19, 20. Here, too, stem cells appear endowed with maintenance mechanisms, organellar physiology, and metabolic regulation that differ from those in mature cells, but also vary among types of stem cells. An understudied area is the specific role of calcium in the biology of stem cells. As calcium is a highly versatile and ubiquitous second messenger that regulates a wide variety of cellular functions, stem cell‐specific functional roles and regulation of calcium are to be anticipated.
Calcium signaling and regulation
Calcium signaling and regulation is a very extensive field of research. Here, I point out some important features (Fig 1), mostly citing appropriate reviews. Calcium concentration in steady state is low in the cytoplasm (10−7 M). Most cellular calcium is stored in the ER, lysosomes, and mitochondria, where concentrations are up to 100‐fold higher. Calcium is removed against a steep gradient (extracellular calcium is ~1–2 M−3) in an ATP‐dependent process from the cytoplasm by plasma membrane efflux pumps (PMCAs) of which there are four isoforms (PMCA1‐4, encoded by Atp2b1‐4, respectively) 21 and, predominantly in excitable cells, the Na+/Ca2+ exchanger, NCX (Slc8a1) 22. ATP‐driven SERCA pumps transport calcium into the endoplasmic reticulum (ER). Calcium entry into the intermembrane space of the mitochondria is mediated by the VDAC1 channel in the outer membrane. The mitochondrial calcium uniporter (MCU) and its multiple regulatory accessory proteins transport calcium across the inner membrane, driven by the mitochondrial membrane potential gradient 23, 24. Finally, approximately 200 calcium‐binding proteins are known that, among others, buffer calcium and therefore reduce the concentration of free calcium 25. Calcium entry into the cytoplasm from the extracellular space is initiated by voltage‐gated channels or by receptor‐operated ionotropic channels and can be amplified by store‐operated calcium entry (SOCE) 26. SOCE is also induced by any signaling pathway that leads to the generation of inositol 1,4,5‐trisphosphate (IP3). IP3 arises by the action of phospholipase C family members, which catalyze the conversion of phosphatidylinositol 4,5‐bisphosphate (PIP2) to diacylglycerol and IP3. IP3 binds IP3 receptors (IP3Rs) on the ER, triggering their opening and causing influx of calcium from the ER to the cytoplasm. The resulting ER calcium depletion is sensed by the EF‐hand domain protein, STIM1, which associates with calcium channels on the plasma membrane, ORAI1, 2 and 3 (collectively called CRAC channels). The CRAC channels subsequently open, allowing influx of calcium from the extracellular space, thus amplifying the calcium transient and refilling ER calcium stores 26. In neurons and in skeletal, heart, and smooth muscle cells, three homologous ryanodine receptors (RyRs) function as calcium‐gated calcium channels on the ER 27, 28. Both RyRs and IP3Rs are subjected to positive (calcium‐induced calcium release) or negative feedback by calcium depending on local calcium concentration, while PLC activity itself is enhanced by calcium. Calcium can also be released from the mitochondria through the NCLX (Slc8b1) Ca2+/Na+ exchanger 29. Furthermore, acidic organelles such as lysosomes contain two‐pore channels (TPC) that can release calcium in response to the second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP) 30.
Figure 1. Calcium regulation in cells.
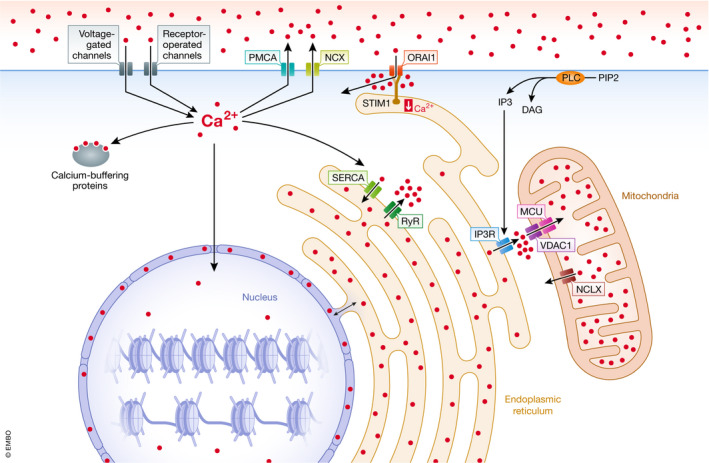
Schematic representation of cellular calcium influx, efflux, and buffering mechanisms. DAG: diacylglycerol; IP3: inositol 1,4,5‐triphosphate; MCU: mitochondrial calcium uniporter; NCLX: mitochondrial sodium/calcium exchanger; NCX: plasma membrane sodium/calcium exchanger; ORAI1: calcium release‐activated calcium channel protein 1; PIP2: phosphoinositol 4,5‐biphosphate; PLC: phospholipase C; PMCA: plasma membrane calcium efflux pump; SERCA: sarcoplasmatic calcium pump; RyR: ryanodine receptor; STIM1: stromal interaction molecule 1; VDAC1: voltage‐dependent anion‐selective channel 1.
Cytoplasmic calcium regulates an enormous variety of cellular processes, ranging from excitation–contraction coupling in muscle cells to neurotransmitter release, intracellular signaling, transcription and translation, cell motility and shape, hormone release, and metabolism, often via binding of calmodulin, which regulates calmodulin kinases (CaMKs). Calmodulin also regulates nuclear factor of activated T cells (NFAT). An increase in cytoplasmic calcium activates the serine/threonine phosphatase, calcineurin, via calcium–calmodulin. Calcineurin dephosphorylates NFAT family members, thus allowing their migration into the nucleus, where they regulate transcription of a broad array of genes in a cell‐type‐dependent fashion in conjunction with AP‐1 31.
A fascinating question is how one second messenger can carry such a tremendously high information content resulting in the regulation of an astounding but coordinated diversity of cellular process. Part of the answer lies in cell‐intrinsic features that allow a specific response to a calcium signal. Only cells endowed with a contractile apparatus will show shortening in response to a calcium transient, and specific calcium transients will only occur in cells expressing the required calcium channels. The other part of the answer lies in spatiotemporal decoding, which links a calcium signal with a specific output within a given cell. Calcium increases in the cytoplasm can be sustained, transient or oscillatory, with associated and sometimes reciprocal changes occurring in organelles. The biological basis of calcium oscillations is still unclear, but involves complex feedback mechanisms between cytoplasmic calcium, intracellular calcium stores, and influx and efflux mechanism at the plasma membrane 32. Frequency, duration, and amplitude of periodic variation in calcium concentration are decoded by calcium‐regulated mechanisms 33, 34, 35, 36. Although several reports suggested a major role for frequency encoding 35, 36, more recent studies suggest that width, amplitude, and degree of sustainability of the spikes as well as baseline calcium are integrated to generate a specific functional output 32. The resulting responses are determined, among others, by the binding kinetics of calcium to specific effectors and by the kinetics of the enzymatic activity of these effectors on their targets. Among these effectors, calmodulin and CaMKs are the most intensely studied. Furthermore, cytoplasmic calcium concentrations are not uniform. Calcium concentrations can locally be very high in so‐called calcium microdomains, such as those that occur near CRAC channels, ryanodine receptors, and IP3Rs (Fig 1) 37. The importance of such microdomains is illustrated by the mitochondrial calcium transporter, MCU. The affinity of the MCU for calcium is too low to be active at ambient cytosolic calcium concentrations. However, the MCU complex is located near mitochondria‐ER contacts (mitochondria‐associated membranes or MAMs) where local concentrations are much higher during calcium release from the ER 38, 39. Finally, nuclear calcium is subjected to influx originating from calcium stores in the nuclear envelope and the nucleoplasmic reticulum, which is continuous with the ER. Differential roles and partially independent regulation of nuclear and cytoplasmic calcium have been reported 40, 41. These principles are nicely illustrated by NFAT. NFAT is preferentially activated by calcium transients of specific amplitude, duration, and frequency 35, while one isoform, NFAT1, is specifically activated in microdomains near CRAC channels, whereas NFAT4 requires an increase in both cytoplasmic and nuclear calcium for activation 42.
As stem cells reside in a specific niche that orchestrates their function, the field should also consider regulation of extracellular calcium in the stem cell microenvironment. Serum calcium concentration is tightly regulated by endocrine hormones (parathyroid hormone, calcitonin, and vitamin D) which regulate intestinal absorption, renal secretion, and organismal buffering of calcium in bones 43. Tissue calcium concentrations are likely locally regulated as well, although very little information is available on this technically challenging subject 44. Given the role of the niche in the regulation of stem cell function and maintenance in various tissues 8, 45, 46, 47, this area of research would be of major relevance, however. Although little research has been done on the regulation of tissue calcium, multiple extracellular proteins bind calcium, which not only regulates their function, but may also affect extracellular free calcium concentration 48. Deletion of matrix Gla protein (MGP), for example, causes ectopic calcification of arteries and cartilage 49. MGP is part of a family of proteins with γ‐carboxyl glutamic acid (Gla) domains, which relatively avidly bind and therefore potentially buffer calcium. Gla proteins include, in addition to MGP, coagulation factors, anti‐coagulation factors (Protein S and C), the osteoblast‐specific hormone osteocalcin, and periostin 50. Gamma‐carboxylation is performed by the vitamin K‐dependent enzyme, gamma‐glutamyl carboxylase (GGCX). This likely explains why vascular calcification, tracheal calcification, and osteoporosis are calcium‐related side effects of anticoagulant treatment with warfarin, an inhibitor of the vitamin K cycle 51, 52. Both specific regulation of and signaling mediated by intracellular calcium and the availability of calcium in the niche should therefore be considered when examining the calcium physiology of stem cells.
Calcium and stem cell regulation
I focus here on data suggesting specific roles of calcium in the maintenance, self‐renewal, and fate decisions of pluripotent and post‐natal stem cells. Studies on specific roles of calcium in stem cell maintenance and tissue regeneration have been published in PSCs, the hematopoietic system, the skin, and the fly intestine.
Hematopoietic stem cells
Hematopoietic stem cells (HSCs) are perhaps the most paradigmatic type of post‐natal stem cell. HSCs reside in the bone marrow (BM), are quiescent, can self‐renew, and generate all lineages of the hematopoietic system 9. If is not fully understood how steady‐state function and homeostatic responses of HSCs are regulated, however 9, 53, 54. HSCs display clonal heterogeneity in their differentiation potential ranging from rare lymphoid‐biased HSCs to balanced myeloid/lymphoid and myeloid‐dominant HSCs with low lymphoid potential. The latter become progressively preponderant with age 55, 56, 57, 58. The first evidence that calcium affects HSC function came from studies showing that hematopoiesis‐specific deletion of mitofusin 2 (Mfn2) impaired the function of HSCs with extensive lymphoid potential 59. MFN2 is one of two homologous mitofusins that promote fusion of mitochondria. In addition, MFN2 is located at MAMs where it promotes transfer of calcium from ER to mitochondria 60, 61. Mfn2 mRNA is relatively selectively expressed in adult HSCs, which have highly fused mitochondria. In the absence of Mfn2, intracellular calcium in HSCs was elevated, leading to the nuclear translocation of NFAT. Inhibition of NFAT signaling using a peptide that inhibits calcineurin‐NFAT interaction rescued the defect 59.
Further studies then showed that overall, HSCs are endowed with low intracellular calcium compared with progenitors conveyed, among others, by elevated expression and activity of PMCA pumps compared with other hematopoietic cells (Fig 1A). Culture of HSCs in vitro in the presence of cytokines leads to a rapid loss of stem cell potential. Culture of BM HSCs in low calcium, however, increased their maintenance as determined by phenotype, function, and single cell expression signature. Although quantitative expansion was not observed, maintenance over a 2‐week culture period in the presence of cytokines was improved 20‐fold. Furthermore, in particular HSCs with serial repopulation capacity were functionally maintained. Similar data were obtained with human cord blood cells. One of the underlying mechanisms is likely inhibition of differentiation and maintenance of a HSC single cell expression signature. Corroborating these beneficial effects of a low‐calcium environment on HSC maintenance, the calcium concentration in the BM interstitial fluid was approximately fourfold lower than that of serum (Fig 2A) 62. A requirement for the calcium‐sensing receptor (CaSR), a G protein‐coupled receptor (GPCR) that responds to increased extracellular calcium concentrations, has been reported in the homing of HSCs from fetal liver, where fetal hematopoiesis takes place, to the BM around birth 63. These data would suggest that HSCs are attracted to the BM because of higher calcium concentrations. Luchsinger et al, however, could not confirm expression of CaSR on adult BM cells 64. Analysis of Casr mRNA expression in the publicly available Gene Expression Commons (GEXC) database (https://gexc.riken.jp/) 65, which contains genome‐wide microarray expression profiles of hematopoietic populations, also revealed very low expression throughout the hematopoietic system, although temporary expression of CaSR in neonatal HSCs cannot be excluded. Although around osteoporotic osteoclastic erosion sites, calcium can be 20‐fold higher than in serum 66, and bone is the prime organismal calcium buffer. Trabecular bone may therefore absorb calcium in steady state in young individuals. Intriguingly in this context, a recent report indicates that HSCs localize near areas of bone turnover, which may affect local calcium concentrations 67. While a recent report showed expansion of HSCs in vitro, among other measures by replacing albumin with polyvinyl alcohol l 68, low‐calcium cultures can open avenues for HSC manipulation based on their specific physiological features. It could, for example, be envisaged that low‐calcium culture may be used for gene transfer and gene editing and for purging of BM from malignant cells as the capability to maintain HSCs in vitro may allow elimination of contaminating tumor cells using specific drugs or antibodies prior to autologous transplantation.
Figure 2. Calcium regullation in various stem cell types and niches.

Schematic representation of calcium concentration and its effect in HSCs (A), skin (B), in vitro cultured keratinocytes (C), mESCs (D), and fly ISCs (E).
Further supporting a role for intracellular calcium concentration in HSC function, Umemoto et al showed that after induction of HSC cycling in vivo, intracellular calcium rose in association with an increase in mitochondrial membrane potential, indicating a metabolic role of calcium in HSCs 69 (Fig 2A). In disagreement with these findings, however, Fukushima et al reported that the most potent and quiescent (so‐called dormant) HSCs are in fact endowed with higher intracellular calcium compared with cycling HSCs and progenitors 70. The specific calcium indicator dye used in that study, CaSIR1, differs from more classically used dyes, such Indo1 and FluoForte, and may potentially explain some of this discrepancy. While Umemoto et al 69 found that nifedipine, an antagonist of L‐type voltage‐gated calcium channels promoted maintenance of HSCs, Fukushima et al 70 observed no effect of another L‐type channel inhibitor, verapamil. Voltage‐gated calcium channels are, however, typically expressed in excitable cells. Furthermore, examination of the GEXC database 65 indicates that expression of voltage‐gated calcium channels is very low in HSCs.
Some clinical and experimental data suggest a role for endocrine regulation of serum calcium—and potentially of calcium in the BM space—on hematopoiesis. Single daily injection of parathyroid hormone (PTH1‐34, teriparatide) or expression of a constitutively active PTH receptor in osteoblasts promotes bone anabolism 71. In mice, both interventions increased HSC number and function 72, 73. The underlying mechanism was reported to involve activation of Notch signaling 73. Although it is now accepted that not osteoblasts but rather BM sinusoids are the niche for HSCs 45, single dose PTH may affect BM calcium, thus potentially explaining its effect on HSC maintenance. Further supporting this notion is the fact that PTH has been reported to predominantly stimulate trabecular and not periosteal osteoblasts 74. Trabecular bone is where most of the hematopoietic activity resides. On the other hand, primary and secondary hyperparathyroidism, where PTH production is sustained and mobilization of calcium occurs through predominant osteoclast activation leading to hypercalcemia, has been reported to be associated with hematological abnormalities that were corrected by treatment of PTH overproduction 75, 76, 77, 78, 79, 80, 81, 82, 83. Many of these reports indicate the presence of myelofibrosis, which is also associated with myeloproliferative disease. Myeloproliferative changes were also observed in a mouse model of osteogenesis imperfecta associated with high bone turnover 84. An association between myelodysplasia and osteoporosis has been suggested 85. Osteopontin likely inhibits hydroxyapatite crystal formation 86, thus preventing deposition of calcium into the bone, and is a negative regulator of HSC function in mice 87. Finally, it was recently reported that vitamin K antagonists, of which tissue calcification and osteoporosis are clinical side effects 51, 52, change bone composition and impair HSC function in mice and that their use correlates with increased incidence of myelodysplasia, although a direct role for BM calcium was not considered 88. Taken together, published evidence, though circumstantial, supports the hypothesis that bone dynamics affect hematopoiesis and HSC function in vivo and that calcium might be involved.
Epidermal stem cells
The upper layer of the skin (stratum corneum or cornified layer) forms a tight physical barrier that is continuously sloughed and replaced by cells differentiating from cycling epidermal stem cells in the basal layer that undergo cell division perpendicular or oblique to the underlaying basement membrane, rather than parallel as in self‐renewing divisions. These cells then stop dividing and move through successive layers and corresponding stages of differentiation (spinous layer and granular layer) before becoming cellular ghosts consisting of proteinaceous material enveloping insoluble keratin filaments and being embedded in cellular lipids. After wounding, more basal epidermal progenitors reenter the cell cycle and subsequently migrate to close the defect. Furthermore, mostly quiescent hair follicle stem cells, that in steady state are periodically activated during the hair cycle, may participate in wound healing as well 8, 12. In the intact skin, a calcium gradient exists with low concentrations in the basal layers, increasing concentrations in spinous and granular layers, and lower concentrations again in the cornified layer 89, 90, 91, 92 (Fig 2B). The contribution of intracellular and extracellular calcium to these measurements, which were performed on fixed samples and measured total and not only free calcium, is still unclear. More recent data, using two‐photon fluorescence lifetime imaging in unfixed tissues, suggest that intracellular calcium stores are the prime locale of this gradient, but that cellular calcium in the basal layer appears heterogeneous 93 (Fig 2B). This heterogeneity may be linked to heterogeneous composition of this layer in terms of proliferative potential and clone size of epidermal stem and progenitor cells 8, 12. This study also showed that the extracellular space in the skin is very small. A drawback, however, was that the dissociation constant of the dye used may have been below the range of the extracellular calcium concentration gradient, which would therefore have gone undetected.
There is a substantial amount of indirect evidence linking extracellular calcium concentration to skin homeostasis, however. The calcium gradient is rapidly dissipated in injured and diseased skin 94, 95, suggesting a role for the skin barrier in maintaining this calcium gradient and a role for calcium in maintaining and restoring skin barrier function 90. Furthermore, extracellular calcium regulates in vitro growth and differentiation of basal keratinocytes (Fig 2C). Culture protocols for keratinocyte expansion call for low (~0.05‐0.1 mM) extracellular calcium, whereas increasing extracellular calcium induces differentiation and expression of differentiation‐associated genes through both transcriptional and post‐transcriptional mechanisms 90 in human 96, 97 and in murine 98, 99 cultured keratinocytes. Furthermore, the differentiation‐inducing effect of calcium requires the CaSR in vitro 62, 100 and in vivo 101, and, as well as SOCE, at least in vitro 102. Several cation channels are expressed in a layer‐specific fashion in the skin 90. One of those, the selective calcium‐entry channel, TRPV6, increases keratinocyte intracellular calcium in high‐calcium conditions 103, thus promoting differentiation, and is itself induced by another calcium‐regulating factor, 1,25‐hydroxy‐vitamin D 104, that is also required for differentiation and acts in concert with CasR in this respect 105. Together, these findings indicate a role for regulated extracellular calcium concentrations in skin homeostasis. On the other hand, several genetic skin diseases are caused by mutation in genes encoding ATPases that pump calcium into intracellular stores, thus illustrating their importance in maintaining skin homeostasis. Mutation of the Golgi calcium ATPase, ATP2C1, in the blistering dermatosis, Hailey–Hailey disease 106, abrogates the calcium gradient, even in unaffected skin 107. Darier's disease or follicular keratosis is a dyskeratotic dermatosis caused by mutation in the SERCA pump, ATP2A2 108, and is associated with ER stress caused by chronic ER calcium depletion. Physiological ER stress, caused by activation of calcium release from the ER in keratinocytes, is associated with differentiation and skin repair, however 109. Altogether, these observations indicate a major role for both intracellular and extracellular calcium regulation in skin homeostasis.
Pluripotent stem cells
PSCs occur in two states that may be the extremes of a developmental continuum 7, 13. The first is the naïve or “ground” state, which has been best documented in mouse (m) ESCs and is still somewhat controversial in human (h) PSCs. The naïve state is characterized by a distinct colony morphology, culture requirements (typically GSK3 and MAPK2 inhibition in the presence of leukemia inhibitory factor (2i/LIF)), genome‐wide expression signature, genome‐wide hypomethylation, and by highly efficient contribution to the germline after blastocyst injection. “Primed” PSCs are, as the name implies, epigenetically primed for the expression of lineage‐specific genes, require FGF2 and activin for expansion, and do not contribute as efficiently to chimeras after blastocyst injection. They are believed to represent post‐implantation epiblast stem cells. hPSCs are most similar to the latter 7, 13.
Some early studies addressed levels and intracellular dynamics of calcium in mESCs. As these studies mostly predate a clear definition of naïve and primed mESCs, they used mESCs grown in serum+LIF, a condition where cellular potentials are heterogeneous and lineage‐specific genes are expressed. The functional machinery for calcium signaling typical of non‐excitable cells (i.e. the absence of ryanodine receptors and voltage‐gated channels) is present in mESCs 110, and signaling mediated by IP3 is required for preimplantation embryos 111, blastocyst formation 112, and mESC proliferation 111, 113. Oscillation of cytoplasmic calcium was observed that depended on release from the ER and refilling of ER stores through SOCE, occurred predominantly during the G1‐to‐S transition, and appeared to promote cell cycle progression 114. Furthermore, the TPC2 channel inhibited neural differentiation of mESC 115. Overall, however, manipulation of these mechanisms only had a limited effect on mESC self‐renewal in these conditions 116.
A critical role for calcium signaling was identified when naïve and primed mESCs were compared in defined culture conditions (Fig 2D). In a CRISPR/Cas9 screen for genes involved in the transition from the naïve to the primed state, MacDougall et al 117 found that one of the limited set of genes that appeared to be required for this transition was Atp2b1. Atp2b1 encodes the plasma membrane calcium efflux pump, PMCA1. During exit from the naïve state, intracellular calcium decreased. In the absence of Atp2b1, exit from naïve pluripotency was impaired, and baseline cytosolic calcium strongly increased. Nevertheless, changes in Atp2b1 expression were not documented. Furthermore, a proportional reduction in intracellular calcium was still observed in Atp2b1 −/− cells during exit from naïve pluripotency, suggesting that, while Atp2b1 may be responsible for relatively low‐calcium levels in mESCs overall, this gene likely does not explain the difference in intracellular calcium between the naïve and primed states. The role of other PMCAs was not explored, however. Combining deletion of both Atp2b1 and Tcf7l1, a transcriptional repressor of genes required for naïve pluripotency, allowed for the growth of mESCs in the absence of 2i/LIF for multiple passages with a gene expression signature close, but not identical to that of naïve mESC in 2i/LIF conditions. Combining GSK3 inhibition with calcitonin allowed for the maintenance of pluripotency as evidenced by the potential to generate embryoid bodies containing markers for all three germlayers, albeit that after more prolonged culture in these conditions, primitive streak and definitive endoderm markers were induced. These conditions are therefore unlikely to be useful for the long‐term maintenance of naïve mESCs. Furthermore, the most stringent test of naïve pluripotency, contribution to the germline after blastocyst injection, was not performed. Though intuitively attractive, the role of calcitonin is somewhat curious. Calcitonin is an endocrine hormone produced by parafollicular cells in the thyroid that can acutely reduce serum calcium by increasing the fractional excretion of calcium and phosphate by the kidney and by inhibiting the osteolytic activity of osteoclasts in the bone 118, 119. Its receptor is a GPCR that, similar to most GPCRs, induces IP3 production and release of calcium from the ER 118 resulting in calcium transients. While playing a role in regulating serum calcium, this receptor is not known to specifically regulate baseline intracellular calcium levels. It is therefore possible that the effect of calcitonin on the maintenance of naïve pluripotency could be recapitulated by agonism of other GPCRs. We do note, however, that calcitonin is also produced by the preimplantation endometrium in response to progesterone 120 and that calcitonin stimulates trophoblast growth prior to implantation 121. An effect on the inner cell mass, where ESCs reside, has not been demonstrated. The authors could also identify a role for calcineurin/NFAT signaling, as the calcineurin inhibitor, cyclosporine A (CsA), could partially compensate for the defect observed in Atp2b1 −/− cells and facilitate transition to a primed state. However, CsA could not maintain self‐renewal after withdrawal of LIF and the MAPK2 inhibitor 117. This finding contrasts with a previous report showing that calcineurin/NFAT signaling is required for differentiation of mESCs and that inhibiting this signaling pathway promoted maintenance of mESC in the absence of LIF 122. It is possible that differing culture conditions (e.g. the presence of serum in the report of Li et al 122) and the fact that MacDougal et al 117 examined naïve‐to‐primed transition while Li et al 122 examined differentiation after LIF withdrawal could play a role in these discrepancies. Taken together, the role of calcium in mESC biology is still unclear, and no studies have reported a role for calcium in human pluripotency.
Intestinal stem cells
In the fly midgut, absorption of l‐glutamate induced PLC activation mediated by the metabotropic l‐glutamate receptor and resulted in reduced calcium oscillation frequency but increased average cytosolic calcium (Fig 2E). Increased cytosolic calcium then activated Creb‐regulated transcriptional co‐activator (Crtc), a conserved substrate for calcineurin, thus promoting proliferation of intestinal stem cells (ISCs). Not only l‐glutamate feeding, but also a multitude of signaling pathways and injuries resulted in reduced oscillation and elevated cytosolic calcium. These observations indicate calcium as major integrator of signals inducing ISC proliferation 123. It is not known to what extent these findings hold in mammalian ISCs. It is interesting to note, however, that l‐glutamate also promoted proliferation and maintenance of the intestinal epithelium in mice 124. To what extent calcium‐induced ISC proliferation also enhanced differentiation and how this striking integration of signals impinging on cellular calcium dynamics occurs is not yet clear. It has been shown, however, that the mechanoreceptor, Piezo, expressed in a subset of unipotent precursors of enteroendocrine cells, promotes enteroendocrine differentiation by increasing intracellular calcium through ER calcium release and SOCE, leading to Notch inhibition 125.
Conclusion
Calcium appears to play a major and previously underappreciated role in the regulation of stem cell fate and maintenance. The preponderance of the evidence, at least in skin and in the hematopoietic system as well as in primed mESCs (and therefore likely also in hPSCs), suggests that low baseline calcium is associated with the stem cell state. Furthermore, in skin keratinocytes and in HSCs, a low‐calcium environment promotes maintenance and prevents differentiation.
Mechanisms of action of calcium in stem cells
Calcium is involved in a multitude of generic signaling pathways downstream of cytokines and morphogens and can affect an enormous range of cellular processes, including transcriptional activation of NFAT, all of which may be relevant to stem cells. However, many unique roles of calcium in stem cells may be explained by its effects on two closely linked, interacting processes that are regulated in a highly specific fashion in various types of stem cells and play a major role in maintaining stem cell identify and function: the epigenome and metabolism.
Calcium and epigenetic regulation
Cellular identity is determined and maintained by the epigenome, a set of covalent modifications comprising DNA methylation and hydroxymethylation 126, and now more than a dozen modifications of histones 127. These DNA and histone modifications determine accessibility to transcription factors and, therefore, gene expression. When stem cells differentiate, the chromatin landscape shifts dramatically: Genes involved in the maintenance of stem cell identity are repressed, and lineage‐specific genes are activated. Some of these lineage‐specific genes are endowed with both activating and inhibitory epigenetic marks, a configuration termed “poised”, in the stem cell state 128, 129.
Calcium regulates the activity of calpains, a family of 14 homologous proteolytic enzymes involved in the post‐translational regulation of multiple proteins 130. Calpains have been shown to target, among others, Ten‐eleven translocated (TET) enzymes in mESCs 131. The three TET homologues convert 5‐methylcytosine (5mC) to 5‐hydroxymethyl cytosine (5hmC) 132, 133. 5hmC is an epigenetic mark and a step in the processive demethylation of 5mC 132, 133, 134. TET enzymes play important roles in early development and differentiation of mPSCs 135. TET2 plays a major role in HSCs by restraining expression of epigenetically poised lineage‐specific genes 134. Tet2 −/− HSCs show progressive myeloid bias in vivo that ultimately leads to myeloid malignancy 136, 137, 138, 139, 140, 141. Heterozygous TET2 mutations are drivers in a large fraction of myeloid malignancies 142, 143, 144, 145 and are frequently encountered in clonal hematopoiesis of undermined significance, a condition where the contribution of one HSC clone to the blood increases with age and that is associated with elevated incidence of hematological malignancies 146, 147. Together, these findings suggest that TET2 may be an epigenetic master tumor suppressor in HSCs. TET2 is also required for the beneficial effect of low‐calcium conditions on HSC maintenance in vitro 64 (Fig 3). More specifically, calpain inhibition by small molecules or by overexpression of the ubiquitously expressed calpain inhibitor, calpastatin, mimicked the effect of a low‐calcium culture on HSC maintenance in vitro 64. Furthermore, low‐calcium culture increased TET protein expression and 5hmC deposition in the nucleus. Consistent with these findings, Tet2 −/− HSCs were impervious to the enhancing effect of low calcium on HSC maintenance. Interestingly, calcium may also regulate other tumor suppressors. There is, for example, evidence that p53 is also a target of calpains 148. Tumor suppressors in general have been suggested to be essential for steady‐state HSC maintenance 149. These findings therefore raise the hypothesis that BM might, by virtue of its low‐calcium milieu, provide a tumor suppressor environment for HSCs.
Figure 3. Calcium regulation of DNA and histone modifications.
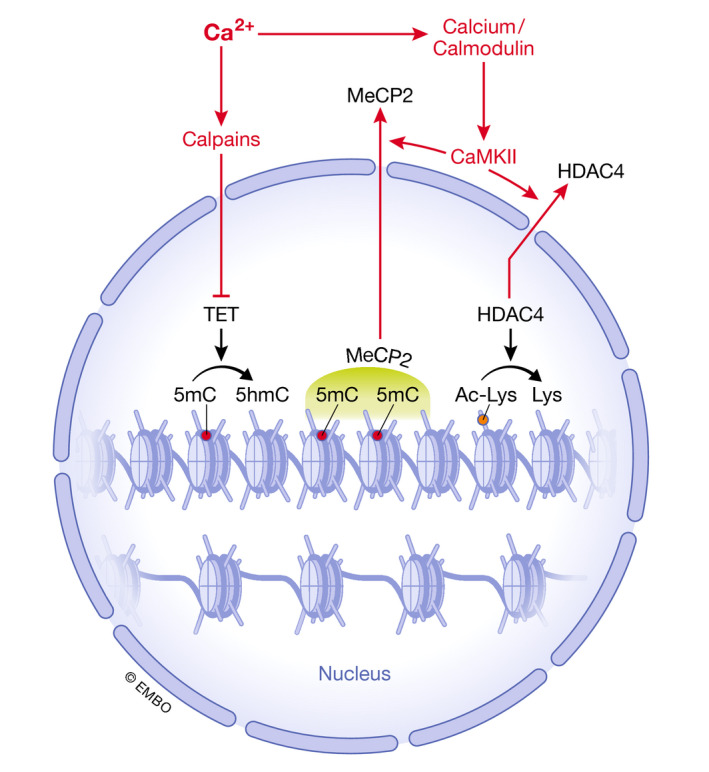
Schematic representation of potential direct regulation of covalent histone and DNA modifications by calcium. 5hmC: 5 hydroxymethyl cytosine; 5mC: 5‐methyl cytosine; CaMKII: calcium/calmodulin‐dependent kinase 2; HDAC4: histone deacetylase 4; MeCP2: methyl CpG‐binding protein 2; TET: ten‐eleven translocated.
Although a direct role of calcium on chromatin remodeling in stem cells has not been demonstrated yet, it is likely that the aforementioned role of calcium in the transition from naïve‐to‐primed mESCs 117 directly or indirectly involves epigenetic modifications, as the naïve and primed PSC epigenome and transcriptome differ 7, 13. In other cell types, calcium is known to affect histone modifications. The most studied type of post‐translational histone modification, acetylation on lysine residues, which opens chromatin, is regulated by the balanced action of several classes of histone acetyl transferases (HATs) and deacetylases (HDACs). Calcium signaling through CaMKs mediates export of several class IIa histone deacetylases from the nucleus 150. In muscle progenitors, HDAC4 negatively regulates transcription by myocyte enhancer factor 2C (MEF2C). Calcium‐activated CaMKII phosphorylates HDAC4, which forms complexes with another class IIa HDAC, HDAC5 151, promoting transport of HDAC4 to the cytoplasm (Fig 3), thus allowing MEF2C to induce muscle differentiation. A similar mechanism plays a role in cardiac hypertrophy 152, 153. HDAC4 is induced upon differentiation of mESCs and is repressed by the pluripotency factor, POU5F1, potentially suggesting a negative regulatory role of HDAC4 in the maintenance of pluripotency 154.
Further evidence for a role of calcium in epigenetic regulation comes from a screen for small molecules that derepress epigenetically silenced tumor suppressor genes in a colon cancer cell line 155. Of the 12 hits observed, 3 were known DNA methylation or HDAC inhibitors, 5 were cardiac glycosides, and 3 were other compounds, including AsO3, which is used in the treatment of acute promyelocytic leukemia. All, except for the known epigenetic modifiers, affected cellular calcium handling and increased the activity of CaMKII, leading to nuclear export of methyl CpG‐binding protein, MeCP2 (Fig 3). This resulted in derepression of silenced loci without epigenetically affecting those. The underlying mechanism is unclear, however, as cardiac glycosides reduced SOCE and ER calcium without affecting cytosolic calcium, suggesting that this effect may be mediated by (undetected) cytosolic calcium sparks. Other compounds, on the other hand, caused sustained increases in cytosolic calcium. Altogether, the data suggest that CaMKII, and therefore calcium, plays a role in epigenetic regulation by affecting the binding of epigenetic readers to CpG‐methylated DNA, a finding that may have relevance to epigenetic regulation of stem cells as well.
Calcium and metabolism in stem cells
Multiple metabolites act as substrates for or as competitive inhibitors of enzymatic reactions involved in covalent epigenetic modifications. Metabolism shapes the epigenome 156, 157, 158, 159 and therefore drives cell fate and plays a major role in the establishment and maintenance of pluripotency 15, 17. Through its profound effects on metabolism, calcium likely also indirectly affects the epigenome (Fig 4). Furthermore, metabolism may in turn regulate intracellular calcium (Fig 5).
Figure 4. Calcium regulation of cellular metabolism and its repercussion on epigenetic modifications.
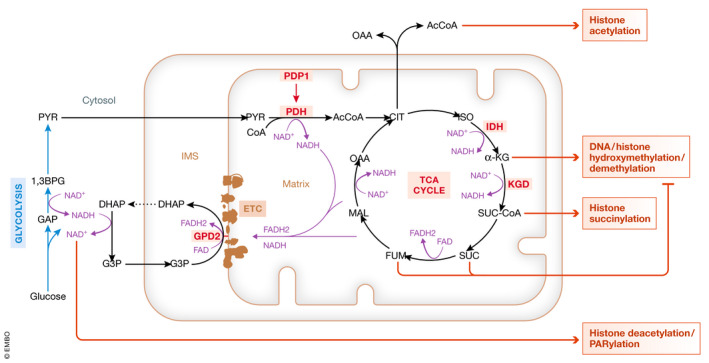
Effect of calcium on mitochondrial function and on mitochondrial metabolites involved in chromatin remodeling. Enzymes activated by calcium are marked in red. DHA: dihydroxyacetone phosphate; ETC: electron transport chain; G3P: glycerol‐3‐phosphate; GPD2: glycerol‐3‐phosphate dehydrogenase 2; IDH: isocitrate dehydrogenase; KGD: α‐ketoglutarate dehydrogenase; OAA: oxaloacetic acid; PDH: pyruvate dehydrogenase complex; PDP1: pyruvate dehydrogenase phosphatase 1.
Figure 5. Interplay between calcium, glycolysis and TCA.
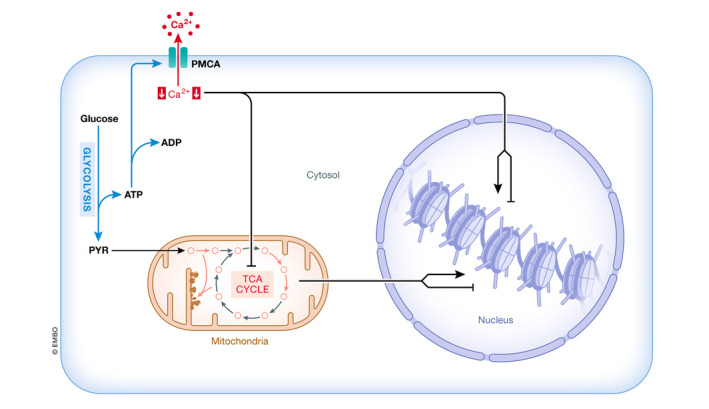
Interplay between regulation of intracellular calcium by glycolysis and its effect on the TCA and chromatin remodeling. ETC: electron transport chain; OAA: oxaloacetic acid; PMCA: plasma membrane calcium efflux pump; TCA: tricarboxylic acid cycle.
Stem cell metabolism
Most stem cells where metabolism could be assessed rely on glycolysis for ATP production to a larger extent than progenitors and mature cells 14, 160, 161, 162, 163, 164. This suggests that mitochondrial respiration is more dispensable for stem cells than it is for progenitors and mature cells, a notion supported by some experimental data in HSCs 165, 166. A similar phenomenon exists in tumor cells, where aerobic glycolysis and the pentose phosphate shunt provide anabolic building blocks to support cell proliferation and thus an increase in biomass. This effect, also called the Warburg effect 167, is unlikely to play a role in HSCs, as these are quiescent. Furthermore, both naïve and primed mESCs proliferate very rapidly yet show differential reliance on glycolysis 17, 168: Naïve mESCs are more oxidative despite having a more immature mitochondrial morphology, whereas primed mESCs are glycolytic, but possess more mature mitochondrial morphology 168. In addition, at least HSCs in fact have high mitochondrial mass 169, whereas within the HSC population, cells with high mitochondrial mass are more quiescent and possess higher repopulation capacity after transplantation 170. Taken together, the purpose of glycolytic ATP production and attenuated mitochondrial respiration in stem cells is unclear. As detailed below, however, calcium is regulated by glycolysis and in turn regulates mitochondrial activity, suggesting a link between calcium and stem cell metabolism.
Calcium and glycolysis
A prevailing idea in the field is that glycolysis and reduced mitochondrial respiration in quiescent HSCs are driven by hypoxia. HSCs are maintained more efficiently in hypoxic than in normoxic conditions in vitro, likely through reduced generation of reactive oxygen species (ROS) in hypoxic conditions 171, 172. Using oxygen‐sensitive probes and two‐photon live imaging, it was shown that BM is hypoxic particularly near vascular niches, where most HSCs reside 45, but that hypoxia may be caused by oxygen consumption in regions of high cellularity 173. HSCs showed enhanced staining in situ for the hypoxia marker, pimonidazole 174, 175, 176, 177, 178, but did so irrespective of their location, while many neighboring cells did not stain 179. Pimonidazole forms adducts with cellular constituents after reacting with electrons arising from the respiratory chain that do not find an oxygen acceptor. Pimonidazole staining therefore detects cells with low oxidative phosphorylation and not necessarily only hypoxic cells 179. Indeed, it was recently documented that HSCs do not reside in the areas with the deepest hypoxia 67. Thus, it is difficult to understand why HSCs stain relatively selectively for pimonidazole. Furthermore, even in normoxic conditions in vitro, glycolytic flux is increased and mitochondrial respiration and ATP production are reduced in HSCs compared with progenitors 164, 169. Altogether, these observations may suggest that glycolysis and attenuated respiration in HSC are not only a response to hypoxia, but may be to some extent hardwired.
This notion is further supported by studies on the role of hypoxia‐induced factor (HIF) in HSCs. Anaerobic glycolysis is driven by dimers of HIF1α or HIF2α and HIF1β. HIF1α and HIF2α are targeted for degradation through prolyl hydroxylation by oxygen‐sensitive dioxygenases, PHD enzymes 180. HSCs in mice with inducible deletion of HIF1α or with deletion of pyruvate dehydrogenase kinase (PDK) 2 and PDK4, both HIF targets that inhibit entry of pyruvate into the mitochondrial tricarboxylic acid (TCA) cycle thus enhancing glycolysis at the expense of respiration, were reported to lose quiescence and display defects after transplantation 161, 175. However, it was subsequently shown that deletion of HIF1α and HIF2α individually 181, 182, of HIF1β or of both HIF1α and HIF2α 183 resulted in no defects or in a subtle loss of HSC function and minimal changes in the expression of glycolytic enzymes 183. Furthermore, although, similar to Pdk2−/−Pdk4−/− mice, HSC function is impaired in Pdk1 −/− mice, these authors observed that conditional deletion of HIF1α had no effect on the expression any PDK isoforms in vivo 184. HIF1α may also not only be directly stabilized by hypoxia (except in very severe hypoxia), but also, and perhaps primarily, by mitochondrial ROS arising through as yet unclear mechanisms during hypoxia 185, 186, 187, 188. This conflicts with the notion that mitochondrial ROS are low in HSCs and are detrimental to their maintenance 189. It is therefore possible that glycolysis, while undoubtedly important for HSC maintenance as demonstrated by the consistent phenotypes of Pdk‐deleted mice 161, 184, is not primarily driven by a HIF‐mediated response to hypoxia.
A possible reason for the apparent glycolytic hardwiring of HSCs is that glycolytic ATP production is required to reduce intracellular calcium as PMCA pumps are fueled by glycolysis 190, 191, 192, 193, possibly because the glycolytic machinery is located near the plasma membrane, at least in erythrocytes 193. Indeed, inhibition of glycolysis increased intracellular calcium in HSCs 64. Furthermore, two ubiquitously expressed “housekeeping” PMCA pumps, PMCA1 and PMCA4, are expressed more highly in HSCs compared with progenitors. Pharmacological inhibition of these pumps increased cytoplasmic calcium 64, indicating their involvement in the reduced intracellular concentration in HSCs. Similarly, the transition of naïve‐to‐primed mESCs, which are more glycolytic 168, was promoted by expression of PMCA1 117. It is therefore possible that glycolysis is required for maintaining reduced intracellular calcium.
Calcium and TCA cycle
Mitochondria affect the epigenome through intermediates of the TCA cycle 157, 158, 159, 194. The importance of the TCA for epigenetic regulation is, for example, illustrated by the fact that the TCA cycle, but not the electron transport chain (ETC.) activity, regulates histone acetylation 194. Furthermore, conditional deletion of the Riske iron‐sulfur protein (RISP), a component of complex III of the electron transport chain, in the hematopoietic system severely impaired HSC function. RISP deletion, likely by increasing the NADH/NAD+ ratio, increased TCA metabolites that play a role in the epigenome, including α‐ketoglutarate, fumarate, succinate, and L(S)‐2‐hydroxyglurate, and was associated with histone hypermethylation and hypoacetylation and DNA hypermethylation in hematopoietic cells 195. At least in HSCs, therefore, attenuated production of these metabolites appears beneficial for their maintenance.
Calcium enhances mitochondrial activity to match ATP production to cellular demands. Furthermore, constitutive release of calcium from the ER to the mitochondria is required to maintain mitochondrial respiration in chicken DT40 B cells. In its absence, a bioenergetic crisis ensues that, through activation of AMPK and subsequent inhibition mTOR, leads to autophagy 196. This finding is consistent not only with low mitochondrial activity, but also with the relatively active autophagy in HSCs, which is required for their maintenance and function 19, 197. Similar to other cells, increased intracellular calcium also enhances respiration in HSCs. When cycling of HSCs was induced by the administration of 5‐fluorouracil, which eliminates cycling progenitors and forces HSCs out of quiescence to replace these, both cytoplasmic calcium and mitochondrial membrane potential increased 69. Furthermore, culture of HSCs in vitro in the presence of high‐calcium concentrations increased mitochondrial respiration and compromised their functional maintenance, while inducing increased expression of differentiation markers 64. Increased respiration has also been shown to be essential for exit from the stem cell state and differentiation of HSCs in vivo 166.
The mechanistic basis of the calcium‐mediated enhancement of mitochondrial respiration, which in most stem cells is associated with exit from the stem cell state, is most likely the well‐established fact that the activity of two enzymes of the TCA cycle, α‐ketoglutarate dehydrogenase (KDH) 198 and isocitrate dehydrogenase (IDH) 199, and of pyruvate dehydrogenase phosphatase I (PDP1) 200, 201 is increased by calcium 23 (Fig 4). It is compelling to note that most or all of the metabolites that were present in excess in RISP‐deleted mice 195 are generated or affected by these enzymes. PDP1 activates the pyruvate dehydrogenase complex. Activation of PDP1 (but not of the differentially expressed homologue PDP2) by calcium therefore promotes flux of pyruvate into the TCA cycle and leads to increased formation of acetylCoA, a co‐factor in acetylation reactions, from pyruvate. After condensation with oxaloacetate to form citrate, acetylCoa can be regenerated in the cytoplasm by ATP‐citrate lyase 202. In hPSCs, acetylCoA, generated from glycolytically derived pyruvate, delays exit from pluripotency 203. Mitochondrial IDH performs oxidative decarboxylation of isocitrate to α‐ketoglutarate, a co‐factor for dioxygenases such as TET enzymes and JMJC proteins involved in DNA and histone demethylation, respectively. KDH in turn converts α‐ketoglutarate to succinyl‐CoA. Succinate, and its downstream derivative in the TCA cycle, fumarate, both inhibit dioxygenases as competitive inhibitors of α‐ketoglutarate 204, whereas succinylation modifies histones 158. Interestingly, recent evidence indicates that some metabolic enzymes can be recruited to the nucleus, where they locally generate metabolites involved in chromatic remodeling 159. KDH is one of these enzymes, and nuclear KGH has been reported to be involved in histone succinylation 205. The role of nuclear calcium would be of major interest, but has not been addressed yet in stem cells. In addition to generating metabolites involved in epigenetic modifications, these three critical calcium‐regulated dehydrogenase complexes also generate reducing equivalents in the form of NADH. Calcium therefore is expected to reduce the mitochondrial NAD+/NADH ratio. Interestingly, calcium also increases the activity of FAD‐glycerol phosphate dehydrogenase (GPD2), which is involved in transferring reducing equivalents from glycolysis‐generated NADH to the mitochondria as FADH2 206, 207, regenerating cytoplasmic NAD+ (Fig 4). NAD+ in turn affects chromatin structure, as a class of HDACs, the sirtuins, and the histone poly(ADP)‐ribosylating enzyme, poly(ADP)ribose polymerase, are positively regulated by their co‐factor NAD+ 208.
Taken together, it appears very plausible that differential regulation of intracellular calcium and of calcium in the extracellular niche between stem cells and their progeny plays a major role in the maintenance or the dismantling of the stem cell epigenome and therefore in stem cell function, by tuning or balancing the concentrations of TCA‐generated metabolites that regulate the deposition of covalent modifications on DNA and DNA‐associated proteins.
The interplay between calcium, glycolysis and the TCA
In HSCs 64 and primed mESCs 117, intracellular calcium is reduced through the action of PMCA pumps. These are driven primarily by glycolysis‐derived ATP 190, 191, 192, 193. Furthermore, in many stem cells, mitochondrial activity is attenuated, a phenomenon that in HSCs has been shown to be associated with reduced intracellular calcium 64, 69. It is therefore plausible that calcium regulation contributes to the metabolic wiring of stem cells. More specifically, in HSCs glycolysis reduces intracellular calcium, which in turn attenuates mitochondrial activity 64, most likely by reducing the activity of the aforementioned dehydrogenases involved in the generation of substrates and/or of inhibitors of epigenetic modification (Fig 5). In HSCs, reduced calcium also stabilizes TET enzymes by inhibiting calpains 64, further reinforcing the notion that calcium regulates the epigenome. While these principles may be differentially operative in distinct types of stem cells, similar mechanisms could play a role in ESCs. In mESCs, calpains have been shown to cleave TET enzymes 131. Furthermore, naïve mESCs display higher intracellular calcium compared with primed mESCs, where intracellular calcium is reduced, although the purported role of PMCA1 in this decrease has not been convincingly demonstrated 117. Naïve mESC are also more oxidative, whereas primed mESCs are more glycolytic 168. Together, these observations might be consistent with repressed respiration in the presence of reduced cytoplasmic calcium concentrations regulated by glycolysis‐driven PMCA pumps in primed mESCs, a situation that would be remarkably similar to that observed in HSCs, but remains to be experimentally verified. Taken together, it is possible and even likely that the metabolism in stem cells, at least in part, regulates and is in turn regulated by intracellular calcium to shape the stem cell epigenome.
Box 1: In need of answers.
What are the exact calcium concentrations and dynamics and their functional significances in various stem cells in situ?
Stem cell‐specific genetically encoded calcium reporters need to be developed for in vivo imaging.
What are the calcium concentrations and how are they regulated in the stem cell microenvironment or niche?
What are the further downstream stem cell‐specific mechanisms regulated by calcium?
We need strategies to harness newly acquired insights into the calcium physiology of stem cells for applications in regenerative medicine.
Future prospects
Recent evidence begins to integrate calcium physiology and signaling into an array of mechanisms that determine stem cell identity, lineage potential, maintenance, and self‐renewal and suggests that calcium regulation, metabolism, and the transcriptional and epigenetic landscape of stem cells are closely interconnected. This area of research is still in its infancy, however. A detailed, granular understanding of how exactly metabolism regulates the epigenome is lacking, and insights into how calcium directly or indirectly through its metabolic effects regulates the epigenome are just beginning to emerge. Furthermore, we still do not know the exact calcium concentrations and dynamics of calcium oscillations as well as their significance in the cytoplasm and in intracellular calcium stores of stem cells in situ. Rather than measuring intracellular calcium using fluorescent dyes ex vivo, transgenic expression of genetically encoded calcium reporters targeted to various subcellular compartments 209 would be preferable for the determination of calcium ex vivo and, by intravital imaging, in situ, as was reported in the fly intestine 123. This will allow to not only asses baseline calcium, but also to examine calcium events, which may change coordinately with extracellular calcium and with stem cell function. Given the evidence of tissue‐specific calcium gradients in skin and bone, which affect stem and progenitor cell function, it would also be of major interest to determine extracellular calcium concentrations in stem cell niches, understand how calcium is regulated in the various stem cell niches, and how extracellular niche calcium impacts stem cell function. In the bone, for example, it is likely that proximity to either blood vessels or trabecular bone and the degree of ongoing remodeling of trabecular bone will determine local calcium concentration and affect HSC function. Despite the technical hurdles, such studies would be of major interest to the field.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by grants RO1 AG029262 and 1R01HL135039 (HWS).
EMBO Reports (2020) 21: e50028
See the Glossary for abbreviations used in this article.
References
- 1. Hanna JH, Saha K, Jaenisch R (2010) Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143: 508–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- 3. Yamanaka S (2009) A fresh look at iPS cells. Cell 137: 13–17 [DOI] [PubMed] [Google Scholar]
- 4. Okita K, Yamanaka S (2011) Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci 366: 2198–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451: 141–146 [DOI] [PubMed] [Google Scholar]
- 6. Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- 7. Yilmaz A, Benvenisty N (2019) Defining human pluripotency. Cell Stem Cell 25: 9–22 [DOI] [PubMed] [Google Scholar]
- 8. Gonzales KAU, Fuchs E (2017) Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell 43: 387–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orkin SH, Zon LI (2008) Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clevers H (2013) The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284 [DOI] [PubMed] [Google Scholar]
- 11. Goodell MA, Rando TA (2015) Stem cells and healthy aging. Science 350: 1199–1204 [DOI] [PubMed] [Google Scholar]
- 12. Dekoninck S, Blanpain C (2019) Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol 21: 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberger L, Ayyash M, Novershtern N, Hanna JH (2016) Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17: 155–169 [DOI] [PubMed] [Google Scholar]
- 14. Shyh‐Chang N, Daley GQ, Cantley LC (2013) Stem cell metabolism in tissue development and aging. Development 140: 2535–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dahan P, Lu V, Nguyen RMT, Kennedy SAL, Teitell MA (2019) Metabolism in pluripotency: both driver and passenger? J Biol Chem 294: 5420–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chandel NS, Jasper H, Ho TT, Passegue E (2016) Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol 18: 823–832 [DOI] [PubMed] [Google Scholar]
- 17. Wu J, Ocampo A, Belmonte JCI (2016) Cellular metabolism and induced pluripotency. Cell 166: 1371–1385 [DOI] [PubMed] [Google Scholar]
- 18. Vilchez D, Simic MS, Dillin A (2014) Proteostasis and aging of stem cells. Trends Cell Biol 24: 161–170 [DOI] [PubMed] [Google Scholar]
- 19. Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegue E (2017) Autophagy maintains the metabolism and function of young and old stem cells. Nature 543: 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vilchez D, Boyer L, Morantte I, Lutz M, Merkwirth C, Joyce D, Spencer B, Page L, Masliah E, Berggren WT et al (2012) Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature 489: 304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruce JIE (2018) Metabolic regulation of the PMCA: Role in cell death and survival. Cell Calcium 69: 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giladi M, Tal I, Khananshvili D (2016) Structural features of ion transport and allosteric regulation in sodium‐calcium exchanger (NCX) proteins. Front Physiol 7: 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mammucari C, Raffaello A, Vecellio Reane D, Gherardi G, De Mario A, Rizzuto R (2018) Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflugers Arch 470: 1165–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578 [DOI] [PubMed] [Google Scholar]
- 25. Gilabert JA (2020) Cytoplasmic calcium buffering: an integrative crosstalk. Adv Exp Med Biol 1131: 163–182 [DOI] [PubMed] [Google Scholar]
- 26. Lewis RS (2019) Store‐operated calcium channels: from function to structure and back again. Cold Spring Harb Perspect Biol 12: a035055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meissner G (2017) The structural basis of ryanodine receptor ion channel function. J Gen Physiol 149: 1065–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kostic M, Sekler I (2019) Functional properties and mode of regulation of the mitochondrial Na(+)/Ca(2+) exchanger, NCLX. Semin Cell Dev Biol 94: 59–65 [DOI] [PubMed] [Google Scholar]
- 30. Morgan AJ (2016) Ca2+ dialogue between acidic vesicles and ER. Biochem Soc Transact 44: 546–553 [DOI] [PubMed] [Google Scholar]
- 31. Li H, Rao A, Hogan PG (2011) Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 21: 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dupont G, Combettes L (2016) Fine tuning of cytosolic Ca (2+) oscillations. F1000Res 5: F1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smedler E, Uhlen P (2014) Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840: 964–969 [DOI] [PubMed] [Google Scholar]
- 34. Uhlen P, Fritz N (2010) Biochemistry of calcium oscillations. Biochem Biophys Res Commun 396: 28–32 [DOI] [PubMed] [Google Scholar]
- 35. Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858 [DOI] [PubMed] [Google Scholar]
- 36. Dolmetsch RE, Xu K, Lewis RS (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392: 933–936 [DOI] [PubMed] [Google Scholar]
- 37. Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3‐sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747 [DOI] [PubMed] [Google Scholar]
- 38. De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011) A forty‐kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher‐Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL et al (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476: 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ljubojevic S, Bers DM (2015) Nuclear calcium in cardiac myocytes. J Cardiovasc Pharmacol 65: 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oliveira AG, Guimaraes ES, Andrade LM, Menezes GB, Fatima Leite M (2014) Decoding calcium signaling across the nucleus. Physiology (Bethesda) 29: 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kar P, Parekh AB (2015) Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms. Mol Cell 58: 232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown EM, MacLeod RJ (2001) Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297 [DOI] [PubMed] [Google Scholar]
- 44. Colella M, Gerbino A, Hofer AM, Curci S (2016) Recent advances in understanding the extracellular calcium‐sensing receptor. F1000Res 5: F1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Crane GM, Jeffery E, Morrison SJ (2017) Adult haematopoietic stem cell niches. Nat Rev Immunol 17: 573–590 [DOI] [PubMed] [Google Scholar]
- 46. Mashinchian O, Pisconti A, Le Moal E, Bentzinger CF (2018) The muscle stem cell niche in health and disease. Curr Topics Dev Biol 126: 23–65 [DOI] [PubMed] [Google Scholar]
- 47. Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10: 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elies J, Yanez M, Pereira TMC, Gil‐Longo J, MacDougall DA, Campos‐Toimil M (2020) An update to calcium binding proteins. Adv Exp Med Biol 1131: 183–213 [DOI] [PubMed] [Google Scholar]
- 49. Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G (1997) Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81 [DOI] [PubMed] [Google Scholar]
- 50. Cranenburg EC, Schurgers LJ, Vermeer C (2007) Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost 98: 120–125 [PubMed] [Google Scholar]
- 51. Poterucha TJ, Goldhaber SZ (2016) Warfarin and vascular calcification. Am J Med 129: 635 e631–635 e634 [DOI] [PubMed] [Google Scholar]
- 52. Fusaro M, Mereu MC, Aghi A, Iervasi G, Gallieni M (2017) Vitamin K and bone. Clin Cases Miner Bone Metab 14: 200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rossi L, Lin KK, Boles NC, Yang L, King KY, Jeong M, Mayle A, Goodell MA (2012) Less is more: unveiling the functional core of hematopoietic stem cells through knockout mice. Cell Stem Cell 11: 302–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL (2003) Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol 21: 759–806 [DOI] [PubMed] [Google Scholar]
- 55. Muller‐Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB (2002) Deterministic regulation of hematopoietic stem cell self‐renewal and differentiation. Blood 100: 1302–1309 [PubMed] [Google Scholar]
- 56. Sanjuan‐Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Bouriez Jones T et al (2013) Platelet‐biased stem cells reside at the apex of the haematopoietic stem‐cell hierarchy. Nature 502: 232–236 [DOI] [PubMed] [Google Scholar]
- 57. Muller‐Sieburg CE, Sieburg HB (2006) Clonal diversity of the stem cell compartment. Curr Opin Hematol 13: 243–248 [DOI] [PubMed] [Google Scholar]
- 58. Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C (2007) Long‐term propagation of distinct hematopoietic differentiation programs in vivo . Cell Stem Cell 1: 218–229 [DOI] [PubMed] [Google Scholar]
- 59. Luchsinger LL, de Almeida MJ, Corrigan DJ, Mumau M, Snoeck HW (2016) Mitofusin 2 maintains haematopoietic stem cells with extensive lymphoid potential. Nature 529: 528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Escobar‐Henriques M, Joaquim M (2019) Mitofusins: disease gatekeepers and hubs in mitochondrial quality control by E3 ligases. Front Physiol 10: 517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- 62. Tu CL, Chang W, Bikle DD (2001) The extracellular calcium‐sensing receptor is required for calcium‐induced differentiation in human keratinocytes. J Biol Chem 276: 41079–41085 [DOI] [PubMed] [Google Scholar]
- 63. Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT (2006) Stem cell engraftment at the endosteal niche is specified by the calcium‐sensing receptor. Nature 439: 599–603 [DOI] [PubMed] [Google Scholar]
- 64. Luchsinger LL, Strikoudis A, Danzl NM, Bush EC, Finlayson MO, Satwani P, Sykes M, Yazawa M, Snoeck HW (2019) Harnessing hematopoietic stem cell low intracellular calcium improves their maintenance in vitro . Cell Stem Cell 25: 225–240 e227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seita J, Sahoo D, Rossi DJ, Bhattacharya D, Serwold T, Inlay MA, Ehrlich LI, Fathman JW, Dill DL, Weissman IL (2012) Gene expression commons: an open platform for absolute gene expression profiling. PLoS ONE 7: e40321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silver IA, Murrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175: 266–276 [DOI] [PubMed] [Google Scholar]
- 67. Christodoulou C, Spencer JA, Yeh SA, Turcotte R, Kokkaliaris KD, Panero R, Ramos A, Guo G, Seyedhassantehrani N, Esipova TV et al (2020) Live‐animal imaging of native haematopoietic stem and progenitor cells. Nature 578: 278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wilkinson AC, Ishida R, Kikuchi M, Sudo K, Morita M, Crisostomo RV, Yamamoto R, Loh KM, Nakamura Y, Watanabe M et al (2019) Long‐term ex vivo haematopoietic‐stem‐cell expansion allows nonconditioned transplantation. Nature 571: 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Umemoto T, Hashimoto M, Matsumura T, Nakamura‐Ishizu A, Suda T (2018) Ca(2+)‐mitochondria axis drives cell division in hematopoietic stem cells. J Exp Med 215: 2097–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fukushima T, Tanaka Y, Hamey FK, Chang CH, Oki T, Asada S, Hayashi Y, Fujino T, Yonezawa T, Takeda R et al (2019) Discrimination of dormant and active hematopoietic stem cells by G0 marker reveals dormancy regulation by cytoplasmic calcium. Cell Rep 29: 4144–4158 e4147 [DOI] [PubMed] [Google Scholar]
- 71. Eastell R, Walsh JS (2017) Anabolic treatment for osteoporosis: teriparatide. Clin Cases Miner Bone Metab 14: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Calvi LM (2006) Osteoblastic activation in the hematopoietic stem cell niche. Anna N Y Acad Sci 1068: 477–488 [DOI] [PubMed] [Google Scholar]
- 73. Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR et al (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 [DOI] [PubMed] [Google Scholar]
- 74. Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E (2001) Activated parathyroid hormone/parathyroid hormone‐related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 107: 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Keukeleire S, Muylle K, Tsoumalis G, Vermeulen S, Vogelaers D (2017) Primary hyperparathyroidism associated to thrombocytopenia: an issue to consider? Clin Cases Miner Bone Metab 14: 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bhadada SK, Sridhar S, Ahluwalia J, Bhansali A, Malhotra P, Behera A, Mittal BR (2012) Anemia and thrombocytopenia improves after curative parathyroidectomy in a patient of primary hyperparathyroidism (PHPT). J Clin Endocrinol Metab 97: 1420–1422 [DOI] [PubMed] [Google Scholar]
- 77. Chow TL, Chan TT, Ho YW, Lam SH (2007) Improvement of anemia after parathyroidectomy in Chinese patients with renal failure undergoing long‐term dialysis. Arch Surg 142: 644–648 [DOI] [PubMed] [Google Scholar]
- 78. Lin CL, Hung CC, Yang CT, Huang CC (2004) Improved anemia and reduced erythropoietin need by medical or surgical intervention of secondary hyperparathyroidism in hemodialysis patients. Ren Fail 26: 289–295 [DOI] [PubMed] [Google Scholar]
- 79. Huang SC, Wu VC, Chou G, Huang TY, Lin SY, Sheu WH (2007) Benign parathyroid adenoma presenting with unusual parathyroid crisis, anemia and myelofibrosis. J Formos Med Assoc 106: S13–S16 [DOI] [PubMed] [Google Scholar]
- 80. Akyay A, Cihangiroglu G, Ozkan Y, Deveci U, Bahceci S, Cetinkaya Z (2013) Primary hyperparathyroidism as an extremely rare cause of secondary myelofibrosis in childhood. J Pediatr Endocrinol Metab 26: 1185–1188 [DOI] [PubMed] [Google Scholar]
- 81. Bellavia M, Gioviale MC, Damiano G, Palumbo VD, Cacciabaudo F, Altomare R, Buscemi G, Lo Monte AI (2011) Is secondary hyperparathyroidism‐related myelofibrosis a negative prognostic factor for kidney transplant outcome? Med Hypotheses 77: 557–559 [DOI] [PubMed] [Google Scholar]
- 82. Lim DJ, Oh EJ, Park CW, Kwon HS, Hong EJ, Yoon KH, Kang MI, Cha BY, Lee KW, Son HY et al (2007) Pancytopenia and secondary myelofibrosis could be induced by primary hyperparathyroidism. Int J Lab Hematol 29: 464–468 [DOI] [PubMed] [Google Scholar]
- 83. Kumbasar B, Taylan I, Kazancioglu R, Agan M, Yenigun M, Sar F (2004) Myelofibrosis secondary to hyperparathyroidism. Exp Clin Endocrinol Diabetes 112: 127–130 [DOI] [PubMed] [Google Scholar]
- 84. Matthews BG, Roeder E, Wang X, Aguila HL, Lee SK, Grcevic D, Kalajzic I (2017) Splenomegaly, myeloid lineage expansion and increased osteoclastogenesis in osteogenesis imperfecta murine. Bone 103: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Datzmann T, Trautmann F, Tesch F, Mies A, Hofbauer LC, Platzbecker U, Schmitt J (2018) Associations of myeloid hematological diseases of the elderly with osteoporosis: a longitudinal analysis of routine health care data. Leukemia Res 69: 81–86 [DOI] [PubMed] [Google Scholar]
- 86. Hunter GK (2013) Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 93: 348–354 [DOI] [PubMed] [Google Scholar]
- 87. Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR et al (2005) Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 201: 1781–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Verma D, Kumar R, Pereira RS, Karantanou C, Zanetti C, Minciacchi VR, Fulzele K, Kunz K, Hoelper S, Zia‐Chahabi S et al (2019) Vitamin K antagonism impairs the bone marrow microenvironment and hematopoiesis. Blood 134: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Menon GK, Grayson S, Elias PM (1985) Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion‐capture cytochemistry. J Invest Dermatol 84: 508–512 [DOI] [PubMed] [Google Scholar]
- 90. Lee SE, Lee SH (2018) Skin barrier and calcium. Ann Dermatol 30: 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Menon GK, Elias PM (1991) Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol 127: 57–63 [PubMed] [Google Scholar]
- 92. Lee SH, Elias PM, Proksch E, Menon GK, Mao‐Quiang M, Feingold KR (1992) Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest 89: 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T (2010) The epidermal Ca(2+) gradient: measurement using the phasor representation of fluorescent lifetime imaging. Biophys J 98: 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Elias P, Ahn S, Brown B, Crumrine D, Feingold KR (2002) Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol 119: 1269–1274 [DOI] [PubMed] [Google Scholar]
- 95. Menon GK, Elias PM, Lee SH, Feingold KR (1992) Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res 270: 503–512 [DOI] [PubMed] [Google Scholar]
- 96. Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M (1990) Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol 143: 294–302 [DOI] [PubMed] [Google Scholar]
- 97. Wong CW, LeGrand CF, Kinnear BF, Sobota RM, Ramalingam R, Dye DE, Raghunath M, Lane EB, Coombe DR (2019) In vitro expansion of keratinocytes on human dermal fibroblast‐derived matrix retains their stem‐like characteristics. Sci Rep 9: 18561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH (1980) Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19: 245–254 [DOI] [PubMed] [Google Scholar]
- 99. Yuspa SH, Kilkenny AE, Steinert PM, Roop DR (1989) Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro . J Cell Biol 109: 1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Oda Y, Tu CL, Pillai S, Bikle DD (1998) The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem 273: 23344–23352 [DOI] [PubMed] [Google Scholar]
- 101. Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, Elias PM, Bikle DD (2012) Ablation of the calcium‐sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol 132: 2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Numaga‐Tomita T, Putney JW (2013) Role of STIM1‐ and Orai1‐mediated Ca2+ entry in Ca2+ ‐induced epidermal keratinocyte differentiation. J Cell Sci 126: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, Prevarskaya N (2007) TRPV6 is a Ca2+ entry channel essential for Ca2+ ‐induced differentiation of human keratinocytes. J Biol Chem 282: 22582–22591. [DOI] [PubMed] [Google Scholar]
- 104. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J et al (2007) Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22: 274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ratnam AV, Bikle DD, Cho JK (1999) 1,25 dihydroxyvitamin D3 enhances the calcium response of keratinocytes. J Cell Physiol 178: 188–196 [DOI] [PubMed] [Google Scholar]
- 106. Ben LI, Ashack K, Khachemoune A (2019) Hailey‐hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol 21: 49–68 [DOI] [PubMed] [Google Scholar]
- 107. Behne MJ, Tu CL, Aronchik I, Epstein E, Bench G, Bikle DD, Pozzan T, Mauro TM (2003) Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol 121: 688–694 [DOI] [PubMed] [Google Scholar]
- 108. Schmieder SJ, Rosario‐Collazo JA (2019) Keratosis Follicularis (Darier Disease). Journal.
- 109. Celli A, Mackenzie DS, Crumrine DS, Tu CL, Hupe M, Bikle DD, Elias PM, Mauro TM (2011) Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br J Dermatol 164: 16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yanagida E, Shoji S, Hirayama Y, Yoshikawa F, Otsu K, Uematsu H, Hiraoka M, Furuichi T, Kawano S (2004) Functional expression of Ca2+ signaling pathways in mouse embryonic stem cells. Cell Calcium 36: 135–146 [DOI] [PubMed] [Google Scholar]
- 111. Gross VS, Hess M, Cooper GM (2005) Mouse embryonic stem cells and preimplantation embryos require signaling through the phosphatidylinositol 3‐kinase pathway to suppress apoptosis. Mol Reprod Dev 70: 324–332 [DOI] [PubMed] [Google Scholar]
- 112. Stachecki JJ, Armant DR (1996) Regulation of blastocoele formation by intracellular calcium release is mediated through a phospholipase C‐dependent pathway in mice. Biol Reprod 55: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 113. Quinlan LR, Faherty S, Kane MT (2003) Phospholipase C and protein kinase C involvement in mouse embryonic stem‐cell proliferation and apoptosis. Reproduction 126: 121–131 [DOI] [PubMed] [Google Scholar]
- 114. Kapur N, Mignery GA, Banach K (2007) Cell cycle‐dependent calcium oscillations in mouse embryonic stem cells. Am J Physiol Cell Physiol 292: C1510–C1518 [DOI] [PubMed] [Google Scholar]
- 115. Zhang ZH, Lu YY, Yue J (2013) Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS ONE 8: e66077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hao B, Webb SE, Miller AL, Yue J (2016) The role of Ca(2+) signaling on the self‐renewal and neural differentiation of embryonic stem cells (ESCs). Cell Calcium 59: 67–74 [DOI] [PubMed] [Google Scholar]
- 117. MacDougall MS, Clarke R, Merrill BJ (2019) Intracellular Ca(2+) homeostasis and nuclear export mediate exit from naive pluripotency. Cell Stem Cell 25: 210–224 e216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Masi L, Brandi ML (2007) Calcitonin and calcitonin receptors. Clin Cases Miner Bone Metab 4: 117–122 [PMC free article] [PubMed] [Google Scholar]
- 119. Felsenfeld AJ, Levine BS (2015) Calcitonin, the forgotten hormone: does it deserve to be forgotten? Clin Kidney J 8: 180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ding YQ, Zhu LJ, Bagchi MK, Bagchi IC (1994) Progesterone stimulates calcitonin gene expression in the uterus during implantation. Endocrinology 135: 2265–2274 [DOI] [PubMed] [Google Scholar]
- 121. Wang J, Rout UK, Bagchi IC, Armant DR (1998) Expression of calcitonin receptors in mouse preimplantation embryos and their function in the regulation of blastocyst differentiation by calcitonin. Development 125: 4293–4302 [DOI] [PubMed] [Google Scholar]
- 122. Li X, Zhu L, Yang A, Lin J, Tang F, Jin S, Wei Z, Li J, Jin Y (2011) Calcineurin‐NFAT signaling critically regulates early lineage specification in mouse embryonic stem cells and embryos. Cell Stem Cell 8: 46–58 [DOI] [PubMed] [Google Scholar]
- 123. Deng H, Gerencser AA, Jasper H (2015) Signal integration by Ca(2 + ) regulates intestinal stem‐cell activity. Nature 528: 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, Teitelbaum DH (2014) Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J 28: 2073–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. He L, Si G, Huang J, Samuel ADT, Perrimon N (2018) Mechanical regulation of stem‐cell differentiation by the stretch‐activated Piezo channel. Nature 555: 103–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bogdanovic O, Lister R (2017) DNA methylation and the preservation of cell identity. Curr Opin Genet Dev 46: 9–14 [DOI] [PubMed] [Google Scholar]
- 127. Tessarz P, Kouzarides T (2014) Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol 15: 703–708 [DOI] [PubMed] [Google Scholar]
- 128. Ferrari F, Apostolou E, Park PJ, Hochedlinger K (2014) Rearranging the chromatin for pluripotency. Cell Cycle 13: 167–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brumbaugh J, Di Stefano B, Hochedlinger K (2019) Reprogramming: identifying the mechanisms that safeguard cell identity. Development 146: dev182170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG (2011) The calpain system and cancer. Nat Rev Cancer 11: 364–374 [DOI] [PubMed] [Google Scholar]
- 131. Wang Y, Zhang Y (2014) Regulation of TET protein stability by calpains. Cell Rep 6: 278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rasmussen KD, Helin K (2016) Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30: 733–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cimmino L, Aifantis I (2017) Alternative roles for oxidized mCs and TETs. Curr Opin Genet Dev 42: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang X, Su J, Jeong M, Ko M, Huang Y, Park HJ, Guzman A, Lei Y, Huang YH, Rao A et al (2016) DNMT3A and TET2 compete and cooperate to repress lineage‐specific transcription factors in hematopoietic stem cells. Nat Genet 48: 1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ross SE, Bogdanovic O (2019) TET enzymes, DNA demethylation and pluripotency. Biochem Soc Transact 47: 875–885 [DOI] [PubMed] [Google Scholar]
- 136. Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, Rao A (2011) Ten‐Eleven‐Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci USA 108: 14566–14571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, Yang FC, Xu M (2011) Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 118: 4509–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Moran‐Crusio K, Reavie L, Shih A, Abdel‐Wahab O, Ndiaye‐Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X et al (2011) Tet2 loss leads to increased hematopoietic stem cell self‐renewal and myeloid transformation. Cancer Cell 20: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner‐Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH et al (2011) TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 20: 25–38 [DOI] [PubMed] [Google Scholar]
- 140. Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell‐Flack M et al (2017) Restoration of TET2 function blocks aberrant self‐renewal and leukemia progression. Cell 170: 1079–1095 e1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W et al (2017) Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549: 476–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tefferi A, Lim KH, Levine R (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 361: 1117; author reply 1117‐1118 [DOI] [PubMed] [Google Scholar]
- 143. Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A et al (2009) Mutation in TET2 in myeloid cancers. N Engl J Med 360: 2289–2301 [DOI] [PubMed] [Google Scholar]
- 144. Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens‐Linders E, van Hoogen P, van Kessel AG, Raymakers RA et al (2009) Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 41: 838–842 [DOI] [PubMed] [Google Scholar]
- 145. Kosmider O, Gelsi‐Boyer V, Ciudad M, Racoeur C, Jooste V, Vey N, Quesnel B, Fenaux P, Bastie JN, Beyne‐Rauzy O et al (2009) TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica 94: 1676–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Shlush LI (2018) Age‐related clonal hematopoiesis. Blood 131: 496–504 [DOI] [PubMed] [Google Scholar]
- 147. Bowman RL, Busque L, Levine RL (2018) Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell 22: 157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kubbutat MH, Vousden KH (1997) Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol Cell Biol 17: 460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Levi BP, Morrison SJ (2008) Stem cells use distinct self‐renewal programs at different ages. Cold Spring Harb Symp Quantit Biol 73: 539–553 [DOI] [PubMed] [Google Scholar]
- 150. Di Giorgio E, Brancolini C (2016) Regulation of class IIa HDAC activities: it is not only matter of subcellular localization. Epigenomics 8: 251–269 [DOI] [PubMed] [Google Scholar]
- 151. Backs J, Backs T, Bezprozvannaya S, McKinsey TA, Olson EN (2008) Histone deacetylase 5 acquires calcium/calmodulin‐dependent kinase II responsiveness by oligomerization with histone deacetylase 4. Mol Cell Biol 28: 3437–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN (2006) CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Little GH, Bai Y, Williams T, Poizat C (2007) Nuclear calcium/calmodulin‐dependent protein kinase IIdelta preferentially transmits signals to histone deacetylase 4 in cardiac cells. J Biol Chem 282: 7219–7231 [DOI] [PubMed] [Google Scholar]
- 154. Addis RC, Prasad MK, Yochem RL, Zhan X, Sheets TP, Axelman J, Patterson ES, Shamblott MJ (2010) OCT3/4 regulates transcription of histone deacetylase 4 (Hdac4) in mouse embryonic stem cells. J Cell Biochem 111: 391–401 [DOI] [PubMed] [Google Scholar]
- 155. Raynal NJ, Lee JT, Wang Y, Beaudry A, Madireddi P, Garriga J, Malouf GG, Dumont S, Dettman EJ, Gharibyan V et al (2016) Targeting calcium signaling induces epigenetic reactivation of tumor suppressor genes in cancer. Cancer Res 76: 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Singer BD, Chandel NS (2019) Immunometabolism of pro‐repair cells. J Clin Invest 129: 2597–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Reid MA, Dai Z, Locasale JW (2017) The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat Cell Biol 19: 1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Sabari BR, Zhang D, Allis CD, Zhao Y (2017) Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol 18: 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Li X, Egervari G, Wang Y, Berger SL, Lu Z (2018) Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol 19: 563–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Folmes CD, Nelson TJ, Dzeja PP, Terzic A (2012) Energy metabolism plasticity enables stemness programs. Anna N Y Acad Sci 1254: 82–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Takubo K, Nagamatsu G, Kobayashi CI, Nakamura‐Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T et al (2013) Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ito K, Suda T (2014) Metabolic requirements for the maintenance of self‐renewing stem cells. Nat Rev Mol Cell Biol 15: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Suda T, Takubo K, Semenza GL (2011) Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9: 298–310 [DOI] [PubMed] [Google Scholar]
- 164. Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D (2011) Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8: 499–510 [DOI] [PubMed] [Google Scholar]
- 166. Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, Finkel T, Broxmeyer HE, Qu CK (2013) Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 12: 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR (2016) Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19: 476–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. de Almeida MJ, Luchsinger LL, Corrigan DJ, Williams LJ, Snoeck HW (2017) Dye‐independent methods reveal elevated mitochondrial mass in hematopoietic stem cells. Cell Stem Cell 21: 725–729e724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Takihara Y, Nakamura‐Ishizu A, Tan DQ, Fukuda M, Matsumura T, Endoh M, Arima Y, Chin DWL, Umemoto T, Hashimoto M et al (2019) High mitochondrial mass is associated with reconstitution capacity and quiescence of hematopoietic stem cells. Blood Adv 3: 2323–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cipolleschi MG, Dello Sbarba P, Olivotto M (1993) The role of hypoxia in the maintenance of hematopoietic stem cells. Blood 82: 2031–2037 [PubMed] [Google Scholar]
- 172. Mantel CR, O'Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, Brustovetsky N, Srour EF, Lee MR, Messina‐Graham S et al (2015) Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell 161: 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R et al (2014) Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kubota Y, Takubo K, Suda T (2008) Bone marrow long label‐retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun 366: 335–339 [DOI] [PubMed] [Google Scholar]
- 175. Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M et al (2010) Regulation of the HIF‐1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402 [DOI] [PubMed] [Google Scholar]
- 176. Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, Nowlan B, Nilsson SK (2007) Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia‐inducible transcription factor‐1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells 25: 1954–1965 [DOI] [PubMed] [Google Scholar]
- 177. Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT (2014) Cell‐state‐specific metabolic dependency in hematopoiesis and leukemogenesis. Cell 158: 1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD (2007) Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104: 5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Nombela‐Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE (2013) Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ivan M, Kaelin WG Jr (2017) The EGLN‐HIF O2‐sensing system: multiple inputs and feedbacks. Mol Cell 66: 772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Vukovic M, Sepulveda C, Subramani C, Guitart AV, Mohr J, Allen L, Panagopoulou TI, Paris J, Lawson H, Villacreces A et al (2016) Adult haematopoietic stem cells lacking Hif‐1alpha self‐renew normally. Blood 127: 2841–2846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Guitart AV, Subramani C, Armesilla‐Diaz A, Smith G, Sepulveda C, Gezer D, Vukovic M, Dunn K, Pollard P, Holyoake TL et al (2013) Hif‐2alpha is not essential for cell‐autonomous hematopoietic stem cell maintenance. Blood 122: 1741–1745 [DOI] [PubMed] [Google Scholar]
- 183. Krock BL, Eisinger‐Mathason TS, Giannoukos DN, Shay JE, Gohil M, Lee DS, Nakazawa MS, Sesen J, Skuli N, Simon MC (2015) The aryl hydrocarbon receptor nuclear translocator is an essential regulator of murine hematopoietic stem cell viability. Blood 125: 3263–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Halvarsson C, Eliasson P, Jonsson JI (2017) Pyruvate dehydrogenase kinase 1 is essential for transplantable mouse bone marrow hematopoietic stem cell and progenitor function. PLoS ONE 12: e0171714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kaelin WG Jr (2005) ROS: really involved in oxygen sensing. Cell Metab 1: 357–358 [DOI] [PubMed] [Google Scholar]
- 187. Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1: 409–414 [DOI] [PubMed] [Google Scholar]
- 188. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia‐induced ROS production and cellular oxygen sensing. Cell Metab 1: 401–408 [DOI] [PubMed] [Google Scholar]
- 189. Tan DQ, Suda T (2017) Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal 29: 149–168 [DOI] [PubMed] [Google Scholar]
- 190. James AD, Chan A, Erice O, Siriwardena AK, Bruce JI (2013) Glycolytic ATP fuels the plasma membrane calcium pump critical for pancreatic cancer cell survival. J Biol Chem 288: 36007–36019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Paul RJ, Hardin CD, Raeymaekers L, Wuytack F, Casteels R (1989) Preferential support of Ca2+ uptake in smooth muscle plasma membrane vesicles by an endogenous glycolytic cascade. FASEB J 3: 2298–2301 [DOI] [PubMed] [Google Scholar]
- 192. Hardin CD, Raeymaekers L, Paul RJ (1992) Comparison of endogenous and exogenous sources of ATP in fueling Ca2+ uptake in smooth muscle plasma membrane vesicles. J Gen Physiol 99: 21–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Campanella ME, Chu H, Low PS (2005) Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci USA 102: 2402–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Martinez‐Reyes I, Diebold LP, Kong H, Schieber M, Huang H, Hensley CT, Mehta MM, Wang T, Santos JH, Woychik R et al (2016) TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol Cell 61: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Anso E, Weinberg SE, Diebold LP, Thompson BJ, Malinge S, Schumacker PT, Liu X, Zhang Y, Shao Z, Steadman M et al (2017) The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat Cell Biol 19: 614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I et al (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142: 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegue E (2013) FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature 494: 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. McCormack JG, Denton RM (1979) The effects of calcium ions and adenine nucleotides on the activity of pig heart 2‐oxoglutarate dehydrogenase complex. Biochem J 180: 533–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Denton RM, Richards DA, Chin JG (1978) Calcium ions and the regulation of NAD+‐linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J 176: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM (1998) Isoenzymes of pyruvate dehydrogenase phosphatase. DNA‐derived amino acid sequences, expression, and regulation. J Biol Chem 273: 17680–17688 [DOI] [PubMed] [Google Scholar]
- 201. Denton RM, Randle PJ, Martin BR (1972) Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J 128: 161–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009) ATP‐citrate lyase links cellular metabolism to histone acetylation. Science 324: 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen‐Orr S, Laevsky I, Amit M et al (2015) Glycolysis‐mediated changes in acetyl‐CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21: 392–402 [DOI] [PubMed] [Google Scholar]
- 204. Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y et al (2012) Inhibition of alpha‐KG‐dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev 26: 1326–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wang Y, Guo YR, Liu K, Yin Z, Liu R, Xia Y, Tan L, Yang P, Lee JH, Li XJ et al (2017) KAT2A coupled with the alpha‐KGDH complex acts as a histone H3 succinyltransferase. Nature 552: 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Hansford RG, Chappell JB (1967) The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight‐muscle mitochondria. Biochem Biophys Res Commun 27: 686–692 [DOI] [PubMed] [Google Scholar]
- 207. Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787: 1309–1316 [DOI] [PubMed] [Google Scholar]
- 208. Yaku K, Okabe K, Hikosaka K, Nakagawa T (2018) NAD metabolism in cancer therapeutics. Front Oncol 8: 622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Rose T, Goltstein PM, Portugues R, Griesbeck O (2014) Putting a finishing touch on GECIs. Front Mol Neurosci 7: 88 [DOI] [PMC free article] [PubMed] [Google Scholar]


