Abstract
This paper provides the first nationally representative estimates of vulnerability to severe complications from COVID-19 overall and across race-ethnicity and socioeconomic status. We use the Panel Study of Income Dynamics (PSID) to examine the prevalence of specific health conditions associated with complications from COVID-19 and to calculate, for each individual, an index of the risk of severe complications from respiratory infections developed by DeCaprio et al. (2020). We show large disparities across race-ethnicity and socioeconomic status in the prevalence of conditions, including hypertension, which are associated with the risk of severe complications from COVID-19. Moreover, we show that these disparities emerge early in life, prior to age 65, leading to higher vulnerability to such complications. Our results suggest particular attention should be paid to the risk of adverse outcomes in midlife for non-Hispanic blacks, adults with a high school degree or less, and low-income Americans.
INTRODUCTION
The health impact of COVID-19 differs substantially across demographic groups. Hospitalization rates are higher for men and for older adults (Garg et al., 2020). Blacks are overrepresented while Hispanics and whites are underrepresented relative to their population percentages (Garg et al., 2020). Differences in mortality rates by race are staggering: in Washington D.C., African Americans make up 44% of the population yet suffer 75% of deaths (CDC, 2020a).1
Behind these disparities in outcomes lie disparities in housing density, in the use of public transportation, in occupational hazards, in health care access, and in underlying health conditions (Blumenshine et al., 2008). Testing in New York City is less prevalent in poor, dense, and non-white neighborhoods, but infection rates conditional on testing are higher (Borjas, 2020). Less-educated, lower income, and less wealthy workers are more likely to hold occupations where working from home and remaining physically distant on the job are harder (Mongey, Pilosoph, & Weinberg, 2020). Race differences in hospitalization rates persist even after controlling for comorbidities, and blacks tend to be tested later in the course of the disease, suggesting unequal access to quality health care (Azar et al., 2020).
The CDC reports that over 90% of hospitalized patients have at least one underlying health condition (CDC, 2020b). Based on such underlying conditions, this paper explores differences in vulnerability to severe complications from COVID-19 across demographic and socioeconomic groups. Previous work has documented large gaps in health by socioeconomic status and race (Case & Deaton, 2020; Harris & McDade, 2018; Mead et al., 2008; NCHS, 2012) and large mortality gradients by education, race, and income, especially in midlife (Case & Deaton, 2015; Chetty et al., 2016; Currie & Schwandt, 2016). Vulnerability to severe complications from COVID-19 collides with these disparities.
While existing surveillance systems have provided data on confirmed hospitalizations and deaths from COVID-19, we know very little about how this varies in the population, aside from by race-ethnicity, and that evidence is not available nationally. The lack of such information has hindered assessment of the distributional impacts of the disease. Central to predicting the likely impact across demographic and socioeconomic groups is knowing who is most vulnerable to severe illness and hospitalization if infected. To determine as much, this paper uses a “vulnerability index” (see DeCaprio et al, 2020) based on a predictive model developed by artificial intelligence data science platform ClosedLoop. The model is trained on medical claims data to predict severe respiratory infection prior to the pandemic. We apply those model weights to the Panel Study of Income Dynamics (PSID) to form population-representative estimates of the distribution of vulnerability to severe complications from COVID-19 and how it varies by education, income, and race-ethnicity. We complement this analysis by examining the distribution of underlying health conditions reported in the PSID across these groups.
We find that disparities in underlying health conditions across race-ethnicity and socioeconomic status translate into large gaps in vulnerability. Overall, adults with a high school education or less are twice as likely as those with a college degree to face severe complications. Adults in the lowest income quartile face three times the risk compared to those in the highest quartile. These gaps are due to underlying health conditions that emerge early in life for less-educated and low-income adults. By race-ethnicity, we find large gaps in vulnerability appearing in midlife for non-Hispanic blacks, whereas Hispanics actually face lower risks based on underlying health conditions and age.
To our knowledge, we are the first to provide population-representative estimates of the risks of severe complications from COVID-19 based on underlying health conditions. In the absence of a predictive model based on actual COVID-19 outcomes, our approach provides an immediate means of translating underlying health factors into estimates of the likely impact of the pandemic across vulnerable sub-populations.
Understanding such variation in vulnerability is crucial for assessing the likely disparities in the effect of the disease. For that task, population-representative estimates are essential. Because we focus only on disparities in underlying health conditions, our work should be seen as a complement to analysis of disparities in exposure to the virus and in access to health care.
BACKGROUND
Frameworks by Blumenshine et al. (2008) and Kumar and Quinn (2012) that describe the role of socioeconomic factors and race/ethnicity, in influenza pandemics are useful for understanding COVID-19. First, socioeconomic factors influence exposure. Disadvantaged populations may be less able to socially distance, because of their occupations, living arrangements, neighborhood densities, dependence on childcare outside the home, and reliance on public transportation. Second, socioeconomic status influences susceptibility to infection and to subsequent complications. Factors influencing susceptibility include, age, smoking, preexisting medical conditions, nutrition, and stress. These factors are correlated with socioeconomic status and with race. Finally, more advantaged populations have better access to medical care, which may lead to earlier and more effective treatment.
Underlying Health Conditions and COVID-19
Our approach uses an individual’s underlying conditions to assess their risk for severe complications from COVID-19. This approach is supported by worldwide evidence that shows that severe complications are associated with underlying health conditions. In China, Italy, and the United States, most people admitted to the hospital have had at least one underlying condition (Arentz et al., 2020; Bhatraju et al., 2020; Cummings et al., 2020; Emami et al., 2020; Grasselli et al., 2020; Ma et al., 2020; Richardson et al., 2020; Scodia et al., 2020; Yang et al., 2020). Richardson et al. (2020), showed that 94% of patients hospitalized in New York City, Long Island, and Westchester County, NY, had at least one underlying condition, 88% had more than one, and the median number was between 2 and 4 (Cummings et al., 2020; Richardson et al., 2020). Among those already in severe respiratory distress when hospitalized, 82% had at least one underlying condition (Cummings et al., 2020, for 2 northern Manhattan hospitals). In a small study in Washington state, the prevalence was lower, with 33% of hospitalized patients having more than one pre-existing condition (Bhatraju et al., 2020). Differences across studies can be attributed in part to how investigators defined comorbidities, to differences in the prevalence of comorbidities across communities, and to local variation in access to hospital beds and in public health guidance.
The most common underlying health condition among patients hospitalized for COVID-19 is hypertension (Arentz et al., 2020; Bhatraju et al., 2020; Cummings et al., 2020; Emami et al., 2020; Grasselli et al., 2020; Ma et al., 2020; Richardson et al., 2020; Scodia et al., 2020; Yang et al., 2020). Other common conditions include diabetes, cardiovascular disease, kidney disease, and obesity (Emami et al., 2020; Cummings et al., 2020; Grasselli et al., 2020; Ma et al., 2020; Richardson et al., 2020; Scordia et al., 2020).
Underlying health conditions are associated with more severe outcomes – such as the development of acute respiratory distress syndrome and death—even for hospitalized patients (Cummings et al., 2020; Ma et al., 2020; Scordia et al., 2020). A meta-analysis of seven studies from China shows that severely ill patients have more comorbid conditions than hospitalized patients whose illnesses are less severe (Yang et al., 2020). Case fatality rates in China are higher among those with comorbid conditions (Wu & Googan, 2020). In Italy, patients with hypertension who are admitted to the ICU are more likely to die (Grasselli et al., 2020).
Why Our Estimates Might Understate Socioeconomic and Race-Ethnic Disparities
Although we find large differences in the risk of severe complications from COVID-19 by race-ethnicity and socioeconomic status, our estimates likely understate those disparities. Some dimensions of health that affect severe complications are unmeasured, and they are likely more common among disadvantaged populations. In addition, the DeCaprio et al. (2020) model does not interact risk factors with race-ethnicity or other socioeconomic factors, yet there is some evidence from influenza studies that the effect of preexisting conditions larger for populations from disadvantaged areas (Glezen et al., 2000). Disadvantaged populations also have higher rates of undiagnosed diseases, implying that observed disparities understate the truth (Barcellos et al., 2012; Chobanian, 2009; Pleasants et al., 2016; Smith, 2007; Zallman et al., 2013). Finally, socioeconomic status affects whether an individual is hospitalized regardless of preexisting conditions (Lowcock et al., 2012), and among those diagnosed with a disease, rates of control are lower for disadvantaged populations (Chatterji et al., 2012; Chobanian, 2009; Pleasants et al., 2016; Zallman et al., 2013).
DATA
In this section we discuss the data and methods used to analyze the underlying health conditions that the CDC and the developing literature indicate are risk factors for severe illness from COVID-19. We begin with a discussion of the Panel Study of Income Dynamics (PSID) data that we use to determine the distribution of and disparities in risk factors in the adult U.S. population. We also describe the index of relative vulnerability to severe illness from respiratory infections developed by ClosedLoop (DeCaprio et al., 2020), which is a function of the CDC risk factors, recent hospitalization, and age. We discuss the construction of the index and how we apply it to nationally representative data.
Panel Study of Income Dynamics
The PSID is a national longitudinal survey that has interviewed the original sample members and their descendants since 1968. The 2017 wave samples 26,455 individuals representative of the national population.2 We study adults ages 25 and older, a sample of 13,529 household heads and spouses (or cohabiting partners)3 who are not missing data on age, race-ethnicity, educational attainment or household income.4 Throughout our analyses, we apply the 2017 PSID cross-sectional weights.
Demographics and Socioeconomic Status
Race-ethnicity is classified as Hispanic, non-Hispanic black, non-Hispanic white, and non-Hispanic other. Because of sample size we do not report separate estimates for individuals who are non-Hispanic other race. Education is categorized as no more than 12 years (high school or less), 13–15 years (some college), and at least 16 years (BA degree or more). Income is measured at the household level. We classify each adult according to the income quartile of their household.5
The population characteristics of our sample are displayed in Table 1. We show the distributions of gender, race-ethnicity, educational attainment, and household income quartile overall and for three age groups (25–44, 45–64 and 65+). We also show the distribution of age across each race-ethnic, educational attainment, and household income category. In Appendix A, we compare the PSID data to that of the Current Population Survey (CPS) from the 2017 Annual Social and Economic Supplement (ASEC). Overall, the samples produce almost identical distributions.6 Prior work has shown that estimates of income from the PSID match well with those from the March CPS (McGonagle et al., 2012).
Table 1.
Proportions of Demographic and SES Groups by Age across Age Categories & Mean Age
| Prop. across Age Categories |
Prop. within Age Categories |
Prop., All Ages (25+) | Mean Age | Sample sizes | |||||
|---|---|---|---|---|---|---|---|---|---|
| 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ | ||||
| Overall | 0.373 | 0.383 | 0.244 | 52.0 | 13,529 | ||||
| Gender | |||||||||
| Female | 0.362 | 0.381 | 0.256 | 0.516 | 0.528 | 0.557 | 0.531 | 52.5 | 7,420 |
| Male | 0.385 | 0.385 | 0.230 | 0.484 | 0.472 | 0.443 | 0.469 | 51.5** | 6,109 |
| Race and Ethncity | |||||||||
| NH White | 0.331 | 0.387 | 0.282 | 0.632 | 0.719 | 0.825 | 0.712 | 53.7 | 7,252 |
| NH Black | 0.416 | 0.416 | 0.168 | 0.120 | 0.117 | 0.074 | 0.107 | 49.4*** | 4,274 |
| Hispanic | 0.509 | 0.360 | 0.131 | 0.180 | 0.124 | 0.071 | 0.132 | 46.7*** | 1,550 |
| NH Other | 0.523 | 0.322 | 0.154 | 0.068 | 0.041 | 0.031 | 0.049 | 47.4 | 453 |
| Education | |||||||||
| HS or less | 0.312 | 0.405 | 0.283 | 0.324 | 0.408 | 0.448 | 0.387 | 54.4*** | 5,450 |
| Some college | 0.388 | 0.385 | 0.227 | 0.245 | 0.237 | 0.219 | 0.235 | 51.2** | 3,577 |
| BA or more | 0.425 | 0.360 | 0.215 | 0.431 | 0.355 | 0.333 | 0.378 | 50.1 | 4,502 |
| Household Income | |||||||||
| Bottom Quartile | 0.307 | 0.328 | 0.365 | 0.151 | 0.157 | 0.275 | 0.183 | 56.7*** | 2,651 |
| Second Quartile | 0.393 | 0.312 | 0.295 | 0.239 | 0.185 | 0.274 | 0.227 | 52.4*** | 3,269 |
| Third Quartile | 0.409 | 0.363 | 0.228 | 0.301 | 0.261 | 0.257 | 0.275 | 50.4** | 3,757 |
| Top Quartile | 0.366 | 0.484 | 0.150 | 0.309 | 0.397 | 0.194 | 0.315 | 50.4 | 3,852 |
| Sample sizes | 6,962 | 4,552 | 2,015 | ||||||
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
Notes: Stars (*) are for tests of risk factors being of a subgroup being significantly different from base group, where:
P-value ≤ 0.10
P-value ≤ 0.05
P-value ≤ 0.01.
Tests of differences in age distributions: (a) Female vs Male: p = 0.0218; (b) NH Blacks vs NH Whites: p = 0.000; [c] Hispanics vs NH Whites: p = 0.000; (d) HS or less vs BA or more: p = 0.0000; (e) Some Coll vs BA or more: p = 0.0429; (d) Second income quartile vs Highest quartile: p = 0.0000; (f) Third income quartile vs Highest quartile: p = 0.0000.
Differences in the age distribution across demographic and socioeconomic groups matter because the risks from COVID-19 vary with age. Females are one year older, which is consistent with well-documented higher mortality rates for men. Non-Hispanic whites (NH white) makes up about 71% of the adult U.S. population, while 10.7% are Non-Hispanic blacks (NH blacks), 13.2% are Hispanics and 4.9% are other non-Hispanic groups. NH whites are the oldest on average (53.7 years old), followed by NH blacks (49.4), and Hispanics are the youngest at 46.7 years. Among NH whites, 28.2% are 65 or older compared to only 16.8% of NH blacks, and 13.1% of Hispanics. With respect to educational attainment, 38.7% of people have a high school degree or less, 23.5% have completed some college, and 37.8% have a BA degree or higher. Due to educational differences across birth cohorts, those with a high school or less are older (54.4 years old) than those with some college (51.2) or those with a BA (50.1). Finally, 18.3% of individuals in the adult population are in the lowest quartile of household income, while 22.7%, 27.5% and 31.5% are in the second, third and top quartiles, respectively. Again, we see age differences across these quartiles, with those in the lowest quartile being older (56.7 years) compared to the top two quartiles, which average 50.4 years.
Self-Reported Health Conditions (Risk Factors)
The PSID includes questions about whether a person was ever told by a health professional they have certain health conditions. Many of these conditions are listed by the CDC as risk factors for becoming severely ill from infection of COVID-19. The health conditions (risk factors) in the PSID include asthma, diabetes, heart disease, heart attack, hypertension, lung disease, neurological conditions, cancer, stroke, and kidney disease. In addition, the PSID asks respondents whether they were hospitalized in the previous year. Hospitalization is a risk factor in the DeCaprio et al. (2020) Model described below. Finally, respondents report their height and weight, which we use to compute each respondent’s BMI. We then create an indicator for severe obesity (BMI ≥ 40). See Appendix Table B-2 for the question wording for all self-reported health conditions in the PSID. One risk factor that the CDC identifies that we are not able to include is living in a nursing home, as the sample size is insufficient.
PSID staff has compared these self-reported health questions to gold-standard topical surveys to evaluate the quality of the data. The PSID estimates for nearly all measures are close to those in the National Health Interview Survey (NHIS), a personal household interview study that has been used by the National Center for Health Statistics to monitor Americans’ health since 1957.7 PSID estimates also show time trends consistent with those from the NHIS (Insolera & Freedman, 2017). There are two exceptions. Chronic kidney disease is two to three times more common in the NHIS than in the PSID, in which 0.80 percent report kidney disease.8 The PSID information about kidney disease, unlike all other conditions we examine, comes from reports about “other serious chronic conditions,” without a specific prompt for kidney-related conditions. The NHIS information is obtained from a specific question about “weak or failing kidneys,” and specific questions are more likely to elicit these reports. The other exception is the prevalence of “neurocognitive conditions,” which we indicate by whether a doctor or health professional ever told the respondent that they had “permanent loss of memory or mental ability.” However, the PSID self-reported measure has external validity as demonstrated by its consistent positive association with the Eight Item Interview to Differentiate Aging and Dementia Screen (AD8) (Freedman, McFall, & Ryan, 2019).
Relative Vulnerability Index
According to the CDC, the risk of severe complications from COVID-19 resulting in hospitalization increases with a number of preexisting health conditions, i.e., risk factors or comorbidities, and with age, but the size of the effect of each risk factor is currently unknown (CDC, 2020). To circumvent the lack of current data on these effects, DeCaprio et al. (2020) assessed the risk of hospitalization for respiratory infections available in existing medical claims data. In particular, they looked at (in-patient) hospitalizations associated with: acute respiratory distress syndrome, pneumonia (other than caused by tuberculosis), influenza, acute bronchitis and other upper respiratory infections. DeCaprio et al. (2020) used medical claims data on hospitalizations for Medicare recipients – using the Center for Medicare and Medicaid Services Limited Data Set, which contains a 5% sample of the Medicare population – and from Healthfirst, a New York (non-Medicare population) insurer. They mapped in-patient hospitalizations from these respiratory infections to individual-level data on 11 pre-existing health conditions (risk factors) coded in the Medicare and Healthfirst data, along with patients’ gender, age and whether they had been hospitalized in the last three months.
As described in DeCaprio et al. (2020), the authors estimated several alternative versions of their model. In our paper, we use their “Survey Model,” which is based on a logistic regression of incidence of hospitalizations due to the above conditions and is suitable for use with the health conditions available in survey data such as the PSID.9
The model is of the following form. Let di denote a 0/1 indicator of whether individual i has a severe respiratory infection and ends up hospitalized. Then,
| (1) |
where di is a 0/1 indicator for whether i is hospitalized and VIi denotes individual i’s Vulnerability Index score which is given by the following function
| (2) |
where Agei is i’s current age, Malei is a 0/1 indicator of whether i is a male; PHospi is a 0/1 indicator of whether i has been recently hospitalized, and Ri is a vector of 0/1 indicators for whether i has particular health conditions, or risk factors, Rki k, k = 1,..., K. Given the logistic form of (1), it follows that is just the log odds of severe illness for individual i that is a function of their age, gender, recent hospitalization and health-related risk factors. It follows that
| (3) |
is their odds ratio of risk for a severe illness or the relative vulnerability index score for individual i.
Given the expression for in (2), technically the base group for the DeCaprio et al. model is a female with no underlying risk factors who is age equal to 0. But, to provide a more meaningful base group for vulnerability score, we use the following relative vulnerability index score in all of our calculations in the paper:
| (4) |
so, the score we report, VIi, provides the odds that individual i has of a severe illness relative to that for a 30-year-old female with no risk factors. We report average VIi by age, race-ethnicity, education, and household income.
In Appendix Table B-1, we display, in the first two columns, respectively, the risk factors in Ri for the DeCaprio et al. model and estimates of the βj’s for in (2). The third column in Appendix Table B-1 indicates which health risk factors are available in the PSID. As one can see, we are missing several risk factors from the DeCaprio et al. model. In our implementation of the vulnerability index with PSID data, we do not attempt to adjust the formula for absent variables; rather, we act as if the members of the PSID sample do not have these conditions. Because of this, the values of the vulnerability index scores for our sample will be biased downwards. As discussed in Appendix B, almost all of the risk factors used in the DeCaprio et al. model that are not available in the PSID, with the exception of liver disease (cirrhosis of the liver), either have very low marginal impacts on the vulnerability index (based on the coefficient estimates for these conditions) or the incidence of the risk factor is very low in the U.S. population.
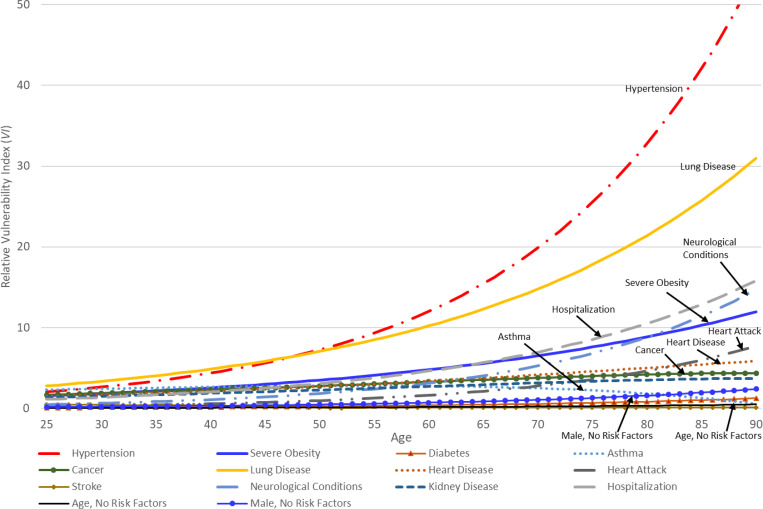
Finally, to provide a sense of how the various risk factors affect the relative vulnerability index scores, we present, in Figure 1, the age profiles of the marginal contributions of each risk factor to relative vulnerability. These marginal contribution at age, Agei, for risk factor, Rki, is given by:
| (5) |
where we evaluate all of the other risk factors, Rmi, m ≠ k at 0.
Figure 1.
Marginal Contribution of Each Risk Factor Available in PSID to Relative Vulnerablity Index (VI) by Age
In Figure 1, we draw attention to those risk factors that have the most sizeable contributions to vulnerability at various ages by labeling them in the figure. As one can see from Figure 1, having hypertension and lung disease have the largest impacts on the relative vulnerability index generated by the DeCaprio et al. model at all ages, and both effects rise rapidly with age. Severe obesity – and, to a lesser extent, having had cancer – also consistently have a large impact on the vulnerability index at all ages. In early adulthood (25–44), asthma contributes a sizeable impact on relative vulnerability, but this risk factor is less important at midlife (45–64) and older ages (65+). With age, one also sees that heart disease and heart attacks contribute to relative vulnerability in the DeCaprio et al. model. At older ages (65+), neurological conditions start to have substantial impacts on relative vulnerability. Finally, recent hospitalizations, which presumably proxy for compromised health, have larger impacts on relative vulnerability as individuals age.
All of the other risk factors individually have more modest impacts, raising the vulnerability odds of severe disease relative to a 30-year old female with no risk factors 2 to 5 times and do not vary substantially with age. Nonetheless, the DeCaprio et al. model implies that the increase in relative vulnerability to severe illness will result, in large part, from the number of conditions one has at any age.
Three final points about Figure 1. First, age, per se, has a very small impact on relative vulnerability. That is, growing older, when one has no risk factors, has virtually no impact on vulnerability. It is the accumulation of risk factors and the interactions of these risk factors with age that leads to higher vulnerability at older ages. Second, a male, who has no other health-related risk factors, is at slightly higher vulnerability to severe illness compared to females and this male-female differential in vulnerability rises modestly with age, so that, by age 72, a male’s vulnerability, relative to a 30-year old female, is 1.14 times higher. Third, the distribution of and disparities in the relative vulnerability to severe illness from COVID-19 in the U.S. adult population reported below will be driven by the marginal contributions of individual risk factors displayed in Figure 1 based on the DeCaprio et al. model and by the prevalence of these risk factors in the population.
RESULTS
In this section, we document the prevalence of health-related risk factors and VIi and examine how these risk factors are differentially distributed by age, race-ethnicity and socioeconomic status.
Prevalence of Health-Related Risk Factors in the United States and their Disparities
In Table 2, we present the prevalence of the risk factors and how they vary by age, demographic group and socioeconomic status. We test whether differences within each group are statistically significant from the base category (age 25–44, NH white, BA or more, top income quartile) with asterisks.
Table 2.
Age, Race/Ethnicity, and SES in prevalence of health-related risk factors
| Age | Race-ethnicity | Education | Household Income | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Overall | 25–44 | 45–64 | 65+ | NH White | NH Black | Hispanic | HS or less | BA or more | Bottom Quartile | Top Quartile |
| Hypertension | 0.33 | 0.14 | 0.36*** | 0.59*** | 0.34 | 0.44*** | 0.24*** | 0.39*** | 0.26 | 0.46*** | 0.25 |
| Severe obesity | 0.17 | 0.16 | 0.18* | 0.16 | 0.16 | 0.19*** | 0.20*** | 0.19*** | 0.14 | 0.18*** | 0.13 |
| Diabetes | 0.12 | 0.04 | 0.14*** | 0.23*** | 0.11 | 0.16*** | 0.14** | 0.15*** | 0.09 | 0.18*** | 0.08 |
| Asthma | 0.11 | 0.12 | 0.10** | 0.09*** | 0.11 | 0.14** | 0.09** | 0.11 | 0.10 | 0.15*** | 0.09 |
| Hospitalization | 0.10 | 0.07 | 0.09** | 0.17*** | 0.10 | 0.12* | 0.09 | 0.13*** | 0.07 | 0.18*** | 0.06 |
| Cancer | 0.09 | 0.02 | 0.07*** | 0.21*** | 0.10 | 0.06*** | 0.05*** | 0.09 | 0.09 | 0.10** | 0.08 |
| Lung disease | 0.06 | 0.03 | 0.05*** | 0.10*** | 0.06 | 0.07 | 0.03*** | 0.08*** | 0.03 | 0.12*** | 0.02 |
| Heart disease | 0.06 | 0.01 | 0.05*** | 0.14*** | 0.06 | 0.07 | 0.02*** | 0.07*** | 0.04 | 0.11*** | 0.03 |
| Heart attack | 0.04 | 0.01 | 0.04*** | 0.10*** | 0.05 | 0.05 | 0.02*** | 0.06*** | 0.02 | 0.07*** | 0.02 |
| Stroke | 0.03 | 0.01 | 0.03*** | 0.08*** | 0.03 | 0.06*** | 0.02 | 0.05*** | 0.02 | 0.07*** | 0.02 |
| Neurological conditions | 0.03 | 0.01 | 0.02*** | 0.05*** | 0.03 | 0.02** | 0.01*** | 0.04*** | 0.01 | 0.06*** | 0.00 |
| Chronic Kidney Disorder | 0.01 | 0.00 | 0.01** | 0.01*** | 0.01 | 0.01 | 0.00** | 0.01 | 0.01 | 0.01* | 0.00 |
| Sample Sizes | 13,529 | 6,962 | 4,552 | 2,015 | 7,252 | 4,274 | 1,550 | 5,450 | 4,502 | 2,651 | 3,852 |
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights. Income quartiles are determined across households using family weights.
Stars (*) are for tests of risk factors being of a subgroup being significantly different from base group, where:
P-value ≤ 0.10
P-value ≤ 0.05
P-value ≤ 0.01.
The risk factors are listed in order of their prevalence in the overall U.S. population based on the PSID data. Hypertension is the most prevalent factor, at 33%. Hypertension increases with age, with 59% of those 65 and older having been diagnosed. The next most prevalent risk factor is severe obesity, affecting 17% of adults. Unlike hypertension, the severe obesity varies little by age. Diabetes and asthma are the third and fourth most prevalent risk factors at 12% and 11%, respectively. Diabetes increases with age, with only 4% of those in early adulthood (25–44 years old) having been diagnosed while one-quarter (23%) of those over 65 have the disease. In contrast, asthma declines with age, going from 12% for the 25–34 year old group to 9% for those 65 and older. Some 9% of the adult population has had (or still has) cancer and its incidence rises with age up to 1 in 5 (21%) of those over 65. Among the remaining health-related risk factors, the prevalence ranges from 1% to 6%, all increasing with age. Finally, 10% of adults report being hospitalized in the preceding year for one night or more, including 17% of those over 65.
As the other columns in Table 2 make clear, there is an unequal distribution of these health-related risk factors by race-ethnicity, educational attainment and income. Compared to NH whites, NH black adults have higher prevalence of almost all of the risk factors for COVID-19, with most of these differences being statistically significant. For example, NH blacks are almost one third more likely to have been diagnosed with hypertension than NH whites (44% vs 34%). Cancer is the only risk factor that is more prevalent for NH whites than NH blacks (10% for NH whites vs 6% for NH blacks). On the other hand, prevalence rates for the risk factors listed in Table 2 are lower for Hispanic adults than NH whites (or NH blacks). The prevalence of hypertension is 24% for Hispanics compared to 34% for NH whites. These racial differences in prevalence are well-known and have been documented in many other studies10 (Hertz et al, 2005; Hicken et al., 2014; NCHS, 2018).
The prevalence of risk factors is also quite different by education. Adults in the United States with a high school education have higher rates for almost all risk factors compared to adults with a college degree. For example, 40% suffer from hypertension compared to one quarter (26%) of the higher educated group. Two notable exceptions to this pattern are cancer and chronic kidney disease, which do not vary by education.
We also see substantial income differences in risk factors. Adults in the bottom quartile of have higher rates for every risk factor compared to those in the top quartile. These differences are sizeable. Compared to those in the top income quartile, those in the bottom quartile are around twice as likely to have been diagnosed with hypertension (25% vs 46%), diabetes (8% vs 18%) and asthma (9% vs 15%), are around three times more likely to have had a heart attack (2% vs 7%) or a stroke (2% vs 7%) and are six times more likely to suffer from lung disease (2% vs 12%). Those in the lowest income group are three times more likely to have had an in-patient hospitalization in the past year (6% vs 18%).
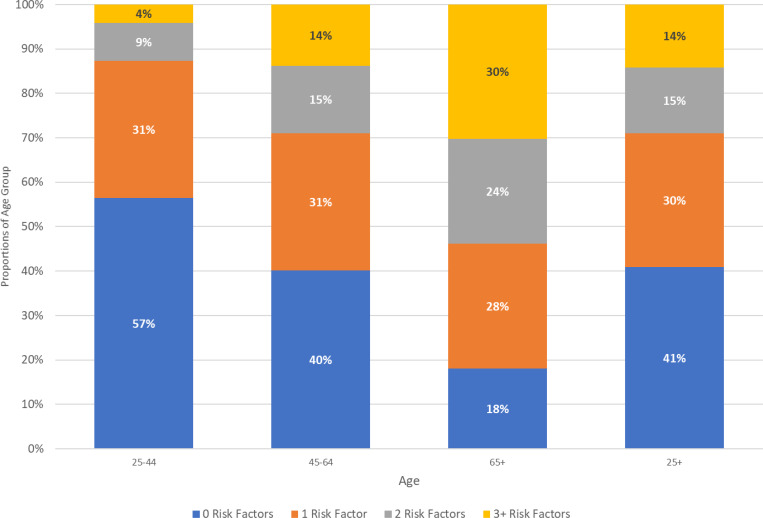
Table 2 also shows that the prevalence of each risk factor increases with age, as does the distribution of the total number of risk factors. In Figure 2, we display proportions of the population with 0, 1, 2 and 3 or more of the risk factors listed in Table 1 across three age groups. Among all adults, 41% have 0 risk factors, while almost 15% have 2 and another 14% have 3 or more, for an average of 1.14 risk factors in the U.S. adult population. The number of risk factors increases across age groups, with 57% of adults 24–44 having no risk factors while only 18% of those 65 years and older having none. Only 4% of younger adults have 3 or more risk factors, 30% of those 65 and older have 3 or more.
Figure 2.
Proportions of Population with Various Numbers of Risk Factors by Age
We showed that the distribution of age varies across race-ethnicity, education, and income groups in our sample. For this reason, in Tables 3 through 5, we examine the prevalence of each of the risk factors by race-ethnicity, socioeconomic status, and age and test whether differences in the prevalence of conditions across race-ethnicity and socioeconomic status are statistically significant for each age group. In Table 3, we show that differences in the prevalence of hypertension between NH blacks and NH whites emerge early in life. The differences are statistically significant in every age group, but large gaps appear in midlife. At age 45–64, 55% of NH blacks report having been diagnosed with hypertension compared to only 34% of NH whites. Large differences in the prevalence of diabetes between NH blacks and NH whites also emerge at age 45–64. In contrast, the health advantage of Hispanics in terms of hypertension occurs mainly at younger ages and differences between NH whites and Hispanics are not statistically significant at older ages. Tables 4 and 5 show similar patterns and similarly large disparities for less-educated and low-income Americans as we see for NH blacks. Health disparities become large in midlife, especially for the most common conditions like hypertension, obesity, and diabetes. However, Tables 4 and 5 show evidence that there are statistically significant differences by education and income in the prevalence of nearly all health conditions, even among those age 25–44.
Table 3.
Prevalence of risk factors by Race and Age
| Race-ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NH White | NH Black | Hispanic | |||||||
| Risk factor | 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ |
| Hypertension | 0.14 | 0.34 | 0.56 | 0.18*** | 0.55*** | 0.78*** | 0.11* | 0.27*** | 0.63 |
| Severe obesity | 0.15 | 0.17 | 0.16 | 0.18 | 0.21** | 0.17 | 0.19** | 0.23** | 0.16 |
| Diabetes | 0.04 | 0.12 | 0.20 | 0.05 | 0.21*** | 0.32*** | 0.04 | 0.18*** | 0.42*** |
| Asthma | 0.13 | 0.11 | 0.09 | 0.15 | 0.14* | 0.09 | 0.10** | 0.07** | 0.09 |
| Hospitalization | 0.07 | 0.09 | 0.16 | 0.07 | 0.13*** | 0.22* | 0.08 | 0.07 | 0.19 |
| Cancer | 0.03 | 0.09 | 0.21 | 0.01*** | 0.04*** | 0.22 | 0.02 | 0.02*** | 0.21 |
| Lung disease | 0.03 | 0.06 | 0.11 | 0.05** | 0.07 | 0.12 | 0.02 | 0.04 | 0.05** |
| Heart disease | 0.01 | 0.05 | 0.14 | 0.03** | 0.06 | 0.18 | 0.00 | 0.02*** | 0.05*** |
| Heart attack | 0.01 | 0.04 | 0.11 | 0.01 | 0.06* | 0.12 | 0.00** | 0.03 | 0.07 |
| Stroke | 0.01 | 0.03 | 0.07 | 0.02* | 0.06*** | 0.15** | 0.00* | 0.03 | 0.11 |
| Neurological conditions | 0.01 | 0.03 | 0.05 | 0.01 | 0.02 | 0.04 | 0.00*** | 0.01*** | 0.07 |
| Chronic Kidney Disorder | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00*** |
| Sample Sizes | 3,432 | 2,419 | 1,401 | 2,318 | 1,543 | 413 | 969 | 447 | 134 |
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
Stars (*) are for tests of risk factors being of a subgroup being significantly different from base group, where:
P-value ≤ 0.05
P-value ≤ 0.01
P-value ≤ 0.001.
Table 5.
Prevalence of risk factors by Household Income and Age
| Household Income | ||||||
|---|---|---|---|---|---|---|
| Bottom Quartile | Top Quartile | |||||
| Risk factor | 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ |
| Hypertension | 0.17** | 0.49*** | 0.67*** | 0.13 | 0.29 | 0.46 |
| Severe obesity | 0.19*** | 0.18* | 0.18* | 0.11 | 0.14 | 0.13 |
| Diabetes | 0.06*** | 0.21*** | 0.27*** | 0.03 | 0.09 | 0.14 |
| Asthma | 0.16*** | 0.19*** | 0.11* | 0.10 | 0.08 | 0.08 |
| Hospitalization | 0.12*** | 0.18*** | 0.22*** | 0.05 | 0.05 | 0.11 |
| Cancer | 0.03 | 0.08 | 0.19 | 0.03 | 0.08 | 0.21 |
| Lung disease | 0.05*** | 0.16*** | 0.13*** | 0.01 | 0.02 | 0.06 |
| Heart disease | 0.03*** | 0.10*** | 0.18*** | 0.01 | 0.02 | 0.10 |
| Heart attack | 0.01 | 0.06*** | 0.13** | 0.01 | 0.02 | 0.08 |
| Stroke | 0.02** | 0.06*** | 0.11*** | 0.00 | 0.02 | 0.05 |
| Neurological conditions | 0.03*** | 0.08*** | 0.07*** | 0.00 | 0.00 | 0.01 |
| Chronic Kidney Disorder | 0.00 | 0.02** | 0.01 | 0.00 | 0.00 | 0.02 |
| Sample Sizes | 1,288 | 825 | 538 | 1,851 | 1,598 | 403 |
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights. Income quartiles are determined across households using family weights.
Stars (*) are for tests of risk factors being of a subgroup being significantly different from base group, where:
P-value ≤ 0.10
P-value ≤ 0.05
P-value ≤ 0.01.
Table 4.
Prevalence of risk factors by Educ and Age
| Education | ||||||
|---|---|---|---|---|---|---|
| HS or less | BA or more | |||||
| Risk factor | 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ |
| Hypertension | 0.17*** | 0.40*** | 0.62*** | 0.11 | 0.29 | 0.53 |
| Severe obesity | 0.19*** | 0.20*** | 0.16 | 0.13 | 0.14 | 0.13 |
| Diabetes | 0.04 | 0.17*** | 0.26*** | 0.04 | 0.10 | 0.16 |
| Asthma | 0.12 | 0.10 | 0.10* | 0.11 | 0.10 | 0.08 |
| Hospitalization | 0.09*** | 0.11*** | 0.19*** | 0.06 | 0.05 | 0.13 |
| Cancer | 0.02 | 0.06* | 0.21 | 0.03 | 0.08 | 0.22 |
| Lung disease | 0.04*** | 0.08*** | 0.13*** | 0.01 | 0.02 | 0.06 |
| Heart disease | 0.01*** | 0.06*** | 0.15* | 0.00 | 0.03 | 0.12 |
| Heart attack | 0.01*** | 0.05*** | 0.12** | 0.00 | 0.01 | 0.08 |
| Stroke | 0.01** | 0.04*** | 0.09* | 0.00 | 0.01 | 0.06 |
| Neurological conditions | 0.02*** | 0.03*** | 0.07** | 0.00 | 0.01 | 0.04 |
| Chronic Kidney Disorder | 0.00 | 0.01 | 0.01* | 0.00 | 0.01 | 0.03 |
| Sample Sizes | 2,492 | 2,017 | 941 | 2,492 | 1,378 | 632 |
Data Source: 2017 Wave, PSID. Sample: PSID heads and spouses, 25 years and older. Weights: PSID individual cross-section weights.
Stars (*) are for tests of risk factors being of a subgroup being significantly different from base group, where:
P-value ≤ 0.10
P-value ≤ 0.05
P-value ≤ 0.01.
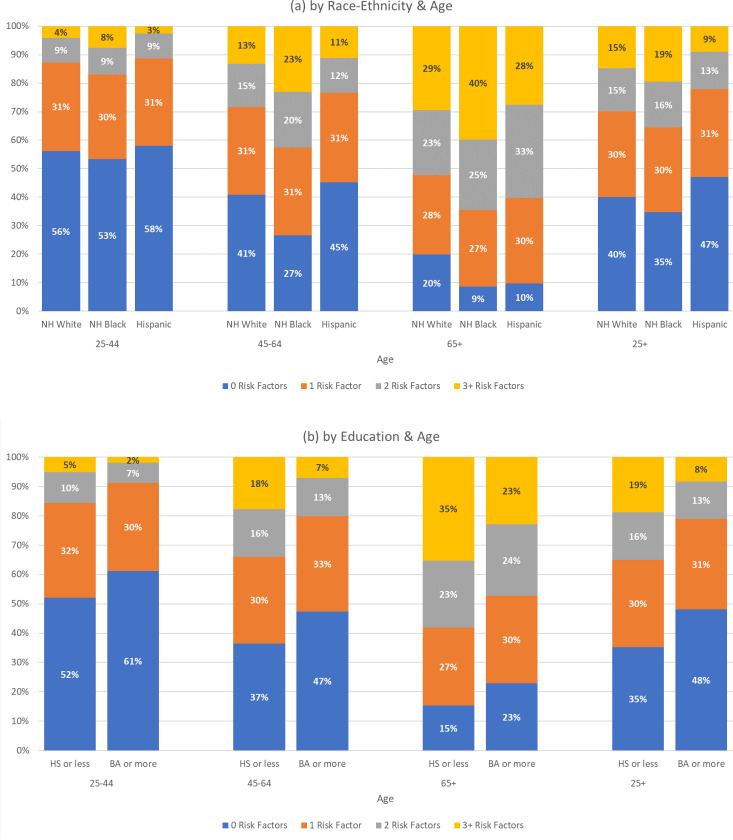
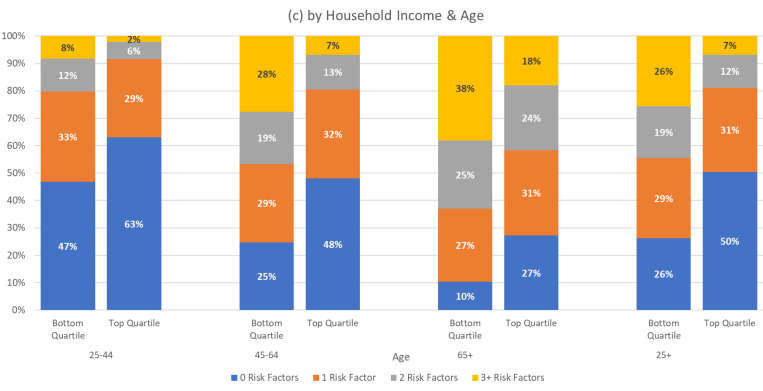
In Figure 3, we present the distribution of the number of risk factors for the overall population and by age across race-ethnic groups, educational attainment, and household income, using the same format as Figure 2. Consistent with the disparities by race and ethnicity in individual risk factors, among all adults (ages 25 and older), NH blacks are more likely to have 3 or more risk factors compared to NH whites (19% vs 15%), while only 9% of Hispanics have such a high number (Figure 3(a), 25+ age group). Among adults of all ages with 12 years of schooling or less, almost 1 in 5 (19%) have 3 or more risk factors, compared to just 8% of those with a college degree (Figure 3(b), 25+ age group). Finally, the differences in numbers of risk factors are particularly dramatic by income, with 26% of adults of all ages in the bottom quartile of household income having 3 or more risk factors compared to only 7% among those in the top income quartile (Figure 3(c), 25+ age group).
Figure 3.
Distribution of Number of Risk Factors
Figure 3 also shows the distribution of the number of risk factors by age across race-ethnicity, education, and household income. Consistent with Tables 3 through 5, Figure 3 shows that for NH blacks, those with 12 years of schooling or less, and those in the bottom quartile of income, risk factors accumulate at earlier ages. For example, by age 45–64, 43% of NH blacks have two or more risk factors compared to only 28% of NH whites. On average, adults, age 45–64, with household incomes in the top income quartile have fewer conditions than those, age 25–44, in the bottom income quartile and those in the oldest group, age 65+, have fewer conditions than those who are middle age (45–64 ) and have incomes in the bottom quartile. All differences between groups are statistically significant.
Disparities in Relative Vulnerability to Severe Illness in the U.S.
To assess how these dramatic disparities in underlying health translate to vulnerability from COVID-19, we use the DeCaprio et al. relative vulnerability index (Equation 4). We then examine how this vulnerability varies by age and across race-ethnicity and socioeconomic status to provide the first assessment of the distribution and disparities of this vulnerability at the population level.
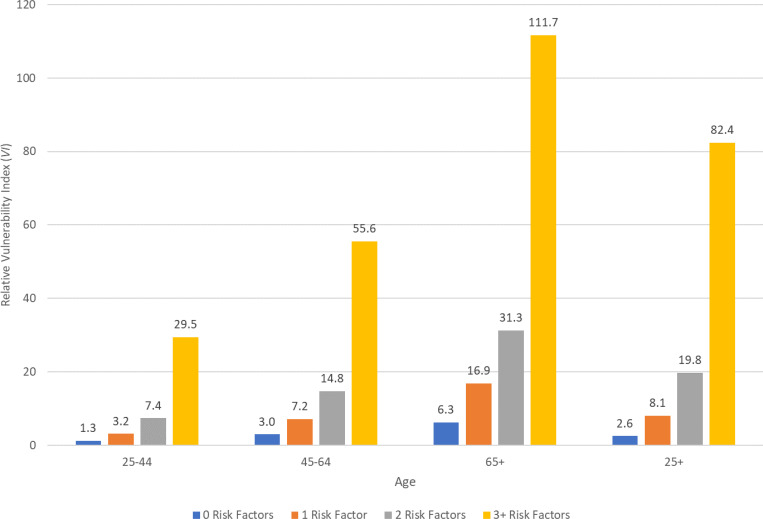
In Figure 4, we display average values of the relative vulnerability index by numbers of risk factors for the three age groups and the overall adult population. Recall that index measures the odds of having severe illness from COVID-19 relative to a 30 year-old female with no risk factors. The average value is 16.6 (not shown in Figure 4). As seen in Figure 4, relative vulnerability varies substantially by number of risk factors and by age. Among all ages (the 25+ group), those with no risk factors are 2.6 times more vulnerable compared to a 30 year-old female. As the number of risk factors increases, relative vulnerability increases, with those with 3 or more risk factors having, on average, 82.4 times greater odds of becoming severely ill. On average individuals in this high-risk group have 3.82 risk factors and 10% have 5 or more risk factors.
Figure 4.
Average Relative Vulnerability Index (VI) by Number of Risk Factors & Age
Figure 4 also makes clear that the increasing numbers of risk factors for those at older ages displayed in Figure 2, result in a progressive increase in relative vulnerability across age groups. For example, individuals who are 65 years old or older and who have 3 or more risk factors are 111.7 times more likely to become severely ill than a 30 year-old healthy (no risk factors) female. Among individuals in this high-risk group, 25% have 5 or more risk factors and half are age 75 or older. While this estimate of relative vulnerability is extraordinarily high, the relative magnitudes of the differences by age and number of risk factors clearly illustrate how the accumulation of risk factors with age makes many older Americans much more vulnerable to COVID-19.
Figure 4 also illustrates how having multiple risk factors heightens the vulnerability to severe illness from COVID-19 at any age. For example, those 65 or older with no risk factors (18.1% of that age group) have an average vulnerability that is 6.3 times that of the reference person (30 year-old female with no risk factors), which is almost the same relative vulnerability to severe illness as for 25–44 year-olds with 2 risk factors (7.4 relative vulnerability and 21.8% of the younger age group). In fact, the relative vulnerability of those 65 and older with no risk factors is more than 4 times lower than the average relative vulnerability of 25–44 year olds with 3 or more risk factors, 28.1. Those with 3 or more risk factors constitute 7% of adults ages 25–34. This comparison illustrates both the disturbing consequences of younger adults having multiple risk factors and the apparent benefits to older age adults of having fewer risk factors with respect to vulnerability of becoming severely ill from the Coronavirus.
The results in Figure 4 for relative vulnerability represent the differences in the distributions of numbers of risk factors for different age groups and the impact that particular risk factors have on relative vulnerability at different ages displayed in Figure 1. Recall for example that hypertension and lung disease consistently have the largest impacts on the relative vulnerability scores at all ages and that the impacts of both went up markedly with age. At the same time, as we noted in our discussion of Table 2, the prevalence of hypertension on the U.S. population was high (33%) and increased across age groups, while, relatively speaking, the incidence of lung disease was much lower (6%) and increased less markedly with age. Thus, the relative vulnerability index allows one to characterize the combined impact of (and differences in) prevalence of risk factors and their impact on population-level vulnerability than simply looking at the prevalence of risk factors can provide. While it would be tempting to try to isolate the contributions of particular risk factors to the vulnerability in the population, such an analysis would require modeling which is beyond the scope and focus of this paper. The primary complication to attributing the differences in vulnerability to particular risk factors is that individuals often have multiple conditions. For example, those who reported that they had been diagnosed with hypertension had, on average, 1.4 other risk factors, while those who did not have hypertension had an average of 0.5 other risk factors.
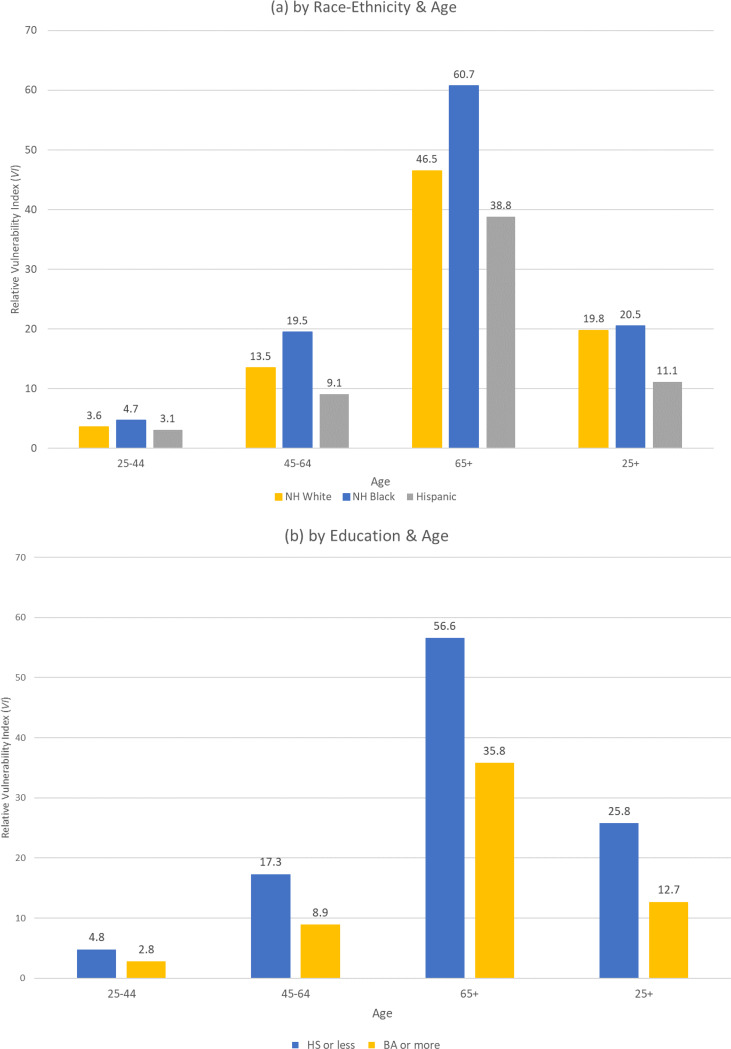
In Figure 5 we display the average relative vulnerability scores by age for race-ethnic, education, and income groups in the adult population. Recall the differences in number of risk factors among NH whites, NH blacks and Hispanics displayed in Tables 2 and 3 and Figure 3(a). We found that NH blacks had more risk factors, on average that NH whites (Table 2), that differences in the prevalence of hypertension between NH blacks and NH whites grow large by age 45–64 (Table 3), and that NH blacks had a higher number of risk factors after age 45 than NH whites (Figure 4(a)). We also found that the prevalence of risk factors was lower among Hispanics, particularly at younger ages. As shown in Figure 5(a), this same pattern across racial and ethnic groups holds for the relative vulnerability. By age 45, sizeable differences in relative vulnerability to severe illness emerge between NH blacks and NH whites and these gaps remain large at older ages. Hispanics have lower relative vulnerability at all ages. Across the whole sample (25+), the difference in relative vulnerability between NH blacks and NH whites is muted. This is due to the age distributions we show in Table 1 where those age 65 or older make up 28.2% of the population of NH whites but only 16.8% of the population of NH blacks.
Figure 5.
Average Relative Vulnerability Index (VI)
Figure 5(b) has the same format for showing the differences in average relative vulnerability between those adults with 12 or fewer years of education and those who are college educated. On average, the relative vulnerability is twice as high for those with high school or less (25.8) than for those with a college degree (12.7) and this sizeable gap by education is present for each age group, reflecting, in part, the differences in incidence of number of risk factors displayed in Figure 4(b).
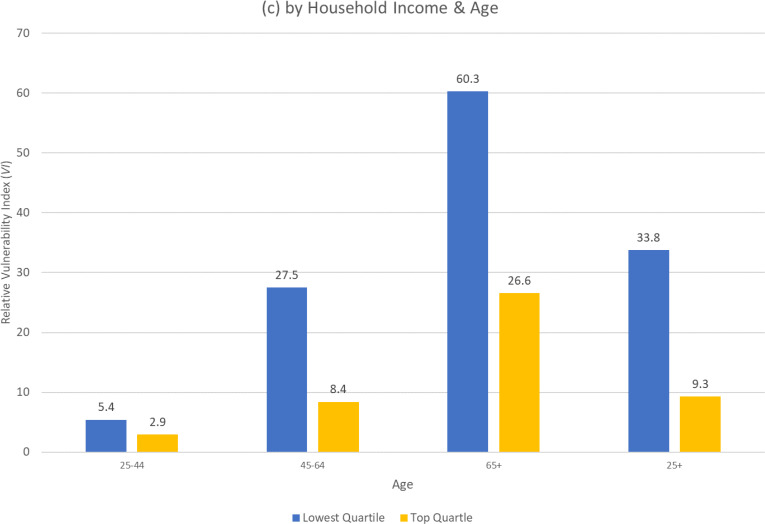
Finally, Figure 5(c) presents the differences in relative vulnerability for those in the bottom and top quartiles of household income. Among all adults (ages 25+), there is more than a 3-fold difference in the relative vulnerability, with those in the bottom quartile of income having 33.8 higher odds of severe illness compared to the reference person, while for those in the top income quartile, the odds are only 9.3 higher. This gap by income in relative vulnerability is even more pronounced for middle-age adults (27.5 vs 8.4). Again, the gap reflects the sizeable differences in prevalence of risk factors (Figure 4(c)) by income. These differences contribute significantly to the dramatic differences in the odds of severe illness from the COVID-19 pandemic in the United States.
DISCUSSION
A reasonable question to ask is whether the segment of the U.S. population that the index suggests are more vulnerable to COVID-19 resemble those who have actually been hospitalized with the virus. While information about the characteristics of those who have been hospitalized is limited, we attempt here to form a tentative answer.
In this exercise, we identify the individuals who have a high relative vulnerability score. To do so, we select those who are above the 45th percentile of the vulnerability index in our data. We chose this threshold because of the similarity between the characteristics of individuals above this threshold and the characteristics of those hospitalized from COVID-19 (CDC, 2020b) on dimensions including the rate of hypertension, the percent with zero health conditions, and the percent aged 65 and older. Using alternative thresholds produces similar conclusions.
Based on our analysis above, we know that this subsample consists of adults who tend to be older and have more risk factors than the overall population. As of this writing, the rates of hypertension, zero underlying health conditions, and the percent of people age 65 and over among those hospitalized from COVID-19 were 58%, 7.9% and 40.9%, respectively (CDC, 2020b). In our top 55% PSID subsample, the corresponding rates are 59.3%, 10.7%, and 43.6%.
Our top 55% vulnerability subsample is markedly different from the overall population. The average age is almost 10 years older (61.9 vs. 52) and there are many fewer young adults age 25–44 (12.2% vs. 37.3%). Those in the top 55% of the vulnerability index are more likely to have 3 or more risk factors (26% vs. 14.2%) and less likely to have zero health conditions (11.1% vs. 40.9%). Among younger adults (25–44) in our high-risk sample, 22.7% have 3 or more risk factors compared to only 4.1% of the overall young adult population. Finally, the average vulnerability index score for individuals in the top 55% is 30.9, which is almost double the average score of 17.8 for the overall population. Comparing this score with the scores we calculate in Figure 5, the average vulnerability score in this high-risk group is very similar to the average of adults age 45–64 from the lowest income quartile in the overall population. That is, the average vulnerability index of low-income adults in midlife is roughly the same as that of a sample of individuals whose characteristics match those hospitalized with COVID-19. The average vulnerability of NH blacks, adults with a high school degree or less, and low-income adults age 65+ is nearly double this level of risk.
While the above comparison is certainly limited in what it can say about the predictive validity of the vulnerability index for estimating hospitalization rates from COVID-19, it does suggest that the index serves as a reasonable guide. The variation in underlying health risk coupled with the disparities across race, education, and income documented here suggests that hospitalizations are likely to be unequally distributed across the U.S. population.
CONCLUSION
This paper provides the first nationally representative estimates of vulnerability to severe complications from COVID-19 overall and across race-ethnicity and socioeconomic status. We use the PSID to examine the prevalence of specific health conditions associated with complications from COVID-19 and to calculate, for each individual, an index of the risk of severe complications from respiratory infections developed by DeCaprio et al. (2020). We show large disparities across race-ethnicity and socioeconomic status in the prevalence of conditions, including hypertension, which are associated with adverse outcomes, and in the overall risk of severe complications. Moreover, we show that these disparities emerge early in life, prior to age 65.
Our results suggest particular attention should be paid to the risk of adverse outcomes in midlife for non-Hispanic blacks, adults with a high school degree or less, and low-income Americans. These results are especially important as states and municipalities start to reopen businesses and public services. Mongey, Pilosoph, & Weinberg (2020) showed that disadvantaged groups are less likely to be able to socially distance at work. The evidence that we present shows large disparities in underlying health conditions among working-age adults for these same groups. Combined, these results suggest that localities with predominantly non-white populations and those with high levels of poverty or a high concentration of less-educated adults face potentially devastating effects of the virus if there are high rates of exposure.
Several caveats are important. The first is that we are likely understating the disparities in the risk for severe complications from COVID-19 by race-ethnicity, education, and income based on underlying health conditions for the reasons we outlined in the Background section. However, even as a lower bound, the disparities in risk we find are very large. Second, we have not yet looked at the disparities in the risk of severe complications from COVID-19 across interactions of race-ethnicity and education or race-ethnicity and income. There is ample evidence of large mortality gaps across these groups (Case & Deaton, 2020), and this is an area for more extensive exploration. Finally, our analysis of disparities in the risk of severe complications from underlying health conditions does not capture the true risk of hospitalization and death from COVID-19 because it does not account for factors influencing exposure to the virus or access to high quality care. All of these factors, including the underlying health conditions we examine, are influenced by systemic inequalities in society and in our health care system. Such inequalities will make it difficult to isolate the influence of health conditions on disparities in the overall effect of COVID-19 pandemic on the U.S. population.
Funding Acknowledgement
This paper was prepared with support, in part, from the Aging Studies Institute at Syracuse University, the Duke Center for Population Health and Aging, which receives core support (P30AG034424) from the National Institute on Aging, and by the California Center for Population Research at the University of California at Los Angeles, which receives core support (P2C-HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The collection of data used in this study was partly supported by the National Institutes of Health under grant number R01 HD069609 and R01 AG040213, and the National Science Foundation under award numbers SES 1157698 and 1623684.
Appendix A: Comparison of PSID Sample with Current Population Survey (CPS)
Table A1:
Proportions of Demographic and SES Groups by Age across Age Categories & Mean Age for PSID Sample
| Prop. across Age Categories |
Prop. within Age Categories |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ | Prop., All Ages (25+) | Mean Age | Sample sizes | |
| Overall | 0.373 | 0.383 | 0.244 | 52.0 | 13,529 | ||||
| Gender | |||||||||
| Female | 0.362 | 0.381 | 0.256 | 0.516 | 0.528 | 0.557 | 0.531 | 52.5 | 7,420 |
| Male | 0.385 | 0.385 | 0.230 | 0.484 | 0.472 | 0.443 | 0.469 | 51.5 | 6,109 |
| Race and Ethncity | |||||||||
| NH White | 0.331 | 0.387 | 0.282 | 0.632 | 0.719 | 0.825 | 0.712 | 53.7 | 7,252 |
| NH Black | 0.416 | 0.416 | 0.168 | 0.120 | 0.117 | 0.074 | 0.107 | 49.4 | 4,274 |
| Hispanic | 0.509 | 0.360 | 0.131 | 0.180 | 0.124 | 0.071 | 0.132 | 46.7 | 1,550 |
| NH Other | 0.523 | 0.322 | 0.154 | 0.068 | 0.041 | 0.031 | 0.049 | 47.4 | 453 |
| Education | |||||||||
| HS or less | 0.312 | 0.405 | 0.283 | 0.324 | 0.408 | 0.448 | 0.387 | 54.4 | 5,450 |
| Some college | 0.388 | 0.385 | 0.227 | 0.245 | 0.237 | 0.219 | 0.235 | 51.2 | 3,577 |
| BA or more | 0.425 | 0.360 | 0.215 | 0.431 | 0.355 | 0.333 | 0.378 | 50.1 | 4,502 |
| Sample sizes | 6,962 | 4,552 | 2,015 | ||||||
Data Source: PSID, 2017 Wave. Sample: Heads and spouses of households, ages 25 and older. Weighted.
Table A2:
Proportions of Demographic and SES Groups by Age across Age Categories & Mean Age for CPS Sample
| Prop. across Age Categories |
Prop. within Age Categories |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25–44 | 45–64 | 65+ | 25–44 | 45–64 | 65+ | Prop., All Ages (25+) | Mean Age | Sample sizes | |
| Overall | 0.348 | 0.403 | 0.249 | 52.4 | 101,462 | ||||
| Gender | |||||||||
| Female | 0.349 | 0.397 | 0.254 | 0.532 | 0.523 | 0.539 | 0.530 | 52.5 | 53,840 |
| Male | 0.346 | 0.409 | 0.245 | 0.468 | 0.477 | 0.461 | 0.470 | 52.4 | 47,622 |
| Race and Ethncity | |||||||||
| NH White | 0.303 | 0.408 | 0.289 | 0.597 | 0.695 | 0.796 | 0.686 | 54.3 | 65,151 |
| NH Black | 0.378 | 0.425 | 0.198 | 0.116 | 0.113 | 0.085 | 0.107 | 50.6 | 11,378 |
| Hispanic | 0.491 | 0.376 | 0.132 | 0.186 | 0.123 | 0.070 | 0.132 | 46.8 | 15,976 |
| NH Other | 0.466 | 0.371 | 0.163 | 0.101 | 0.069 | 0.049 | 0.075 | 48.1 | 8,957 |
| Education | |||||||||
| HS or less | 0.288 | 0.411 | 0.300 | 0.304 | 0.375 | 0.442 | 0.367 | 55.0 | 38,019 |
| Some college | 0.354 | 0.414 | 0.232 | 0.275 | 0.278 | 0.251 | 0.270 | 51.8 | 27,560 |
| BA or more | 0.403 | 0.386 | 0.211 | 0.421 | 0.348 | 0.307 | 0.363 | 50.4 | 35,883 |
| Sample sizes | 38,732 | 40,401 | 22,329 | ||||||
Data Source: CPS, 2017 Annual Social & Economic Supplement. Sample: Heads and spouses of households, ages 25 and older. Weighted.
Appendix B: Formula for the DeCaprio et al. Model and the Corresponding Risk Factors & Other Variables Available in the PSID
Table B-1 displays the variables used and coefficient estimates for the DeCaprio et al. Model11 along with the corresponding health-related risk factors that are available in the PSID. In Table B-2, we provide the variable names and question wording from the 2017 Wave of the PSID for these risk factors.
As noted in the text, the PSID does not have all of the variables that DeCaprio et al. (2020) used in the estimation of the logistic regression for this Survey Model. In our implementation of the model, we acted as if the indicators for the risk factors not collected in the PSID to a value of 0. As we note in the text, this means that our estimates of the vulnerability index scores of individuals in the PSID we understate their risk of serious disease. The extent of this understatement and how the extent of understatement will vary in the population depends on both the prevalence of the particular risk factors in the population and their marginal impacts on the vulnerability index score, which is determined by the coefficients on these variables.
We examined what is known about the prevalence of these conditions in the U.S. Here is a summary:
Sickle Cell Disease: (from CDC https://www.cdc.gov/ncbddd/sicklecell/data.html)
Sickle Cell Disease affects approximately 100,000 Americans.
SCD occurs among about 1 out of every 365 Black or African-American births.
SCD occurs among about 1 out of every 16,300 Hispanic-American births.
About 1 in 13 Black or African-American babies is born with sickle cell trait (SCT).
Hemodialysis (a form of Dialysis) (from the National Kidney Foundation https://www.kid-ney.org/news/newsroom/factsheets/KidneyDiseaseBasics)
In 2017, 746,557 Americans had kidney failure, and needed dialysis or a kidney transplant to survive.
Nearly 500,000 of these patients received dialysis at least 3 times per week to replace kidney function.
Pneumonia (from CDC https://www.cdc.gov/dotw/pneumonia/index.html)
In the United States, more than 250,000 people have to seek care in a hospital due to pneumonia each year.
Most of the people affected by pneumonia in the United States are adults.
Liver Disease (Cirrhosis of the liver) (from CDC https://www.cdc.gov/nchs/fastats/liver-dis-ease.htm)
Number of adults with diagnosed liver disease: 4.5 million
Percent of adults with diagnosed liver disease: 1.8%
Acute Rheumatic Fever and Heart Disease (from UpToDate.com https://www.up-todate.com/contents/acute-rheumatic-fever-epidemiology-and-pathogenesis)
While Rheumatic fever and heart disease remains a major cause of cardiovascular disease in developing nations, it has declined dramatically in industrialized nations like the U.S. because of access to vaccines and antibiotics to treat Group A streptococcus.
While it can occur at any age, although most cases occur in children 5 to 15 years of age.
In the U.S., the incidence of Rheumatic fever is less than 2 cases per 100,000 of school-age children.
Thus, with the exception liver disease (Cirrhosis of the liver), the risk factors in the DeCaprio et al. Model, but not in the PSID, are of very low prevalence and/or incidence. Thus, excluding them is not likely to have a significant impact on the relative vulnerability scores, VIi. The possible exception is Sickle cell anemia, which has a fairly sizeable impact in the calculation of the index for NH Blacks at ages up to around 50. However, as noted above, the prevalence of Sickle cell anemia for NH Blacks and for some of the Hispanics in our sample who reported that their “race” was Black cannot be determined for our PSID sample. Thus, the values for the vulnerability index in the PSID data will be (slightly) understated, on average, for NH blacks and some Hispanics.
Table B-1:
Risk Factors & Other Conditions from the DeCaprio et al. Model
| Variables in DeCaprio et al. Survey Model | Coefficient Estimate | Variables available in the PSID |
|---|---|---|
| intercept | −6.740 | |
| Age | 0.041 | Age |
| Male | 0.171 | Male |
| Prior Admissions | 0.682 | Hospitalized |
| Prior ER Visits | 0.413 | |
| Chronic obstructive pulmonary disease (COPD) or emphysema, cystic fibrosis, or chronic bronchitis | 1.167 | Lung Disease |
| Asthma | 1.393 | Asthma |
| Obesity (BMI ≥ 40) | 0.935 | Severe Obesity (BMI ≥ 40) |
| Diabetes (other than when you were pregnant) | 0.096 | Diabetes |
| Hypertension (or high blood pressure) | 0.832 | Hypertension |
| Congestive Heart Failure | 0.982 | Heart Disease |
| Heart attack | 0.159 | Heart Attack |
| Rheumatic heart disease | 0.788 | |
| Stroke | 0.285 | Stroke |
| Sickle cell anemia/HIV infection/Transplant | 2.582 | |
| Chronic kidney disease | 0.966 | Kidney Disease |
| Hemodialysis | 1.369 | |
| Liver disease | 0.055 | |
| Pneumonia, acute bronchitis, influenza or other acute respiratory infection | 0.696 | |
| Cancer | 1.091 | Cancer |
| Neurocognitive conditions | 0.294 | Neurological Condition |
| Pregnancy, childbirth & puerperium | 0.789 | |
| COPD × Age | −0.002 | Lung Disease × Age |
| Asthma × Age | −0.015 | Asthma × Age |
| Obesity × Age | −0.004 | Severe Obesity × Age |
| Diabetes × Age | 0.000 | Diabetes × Age |
| Hypertension × Age | 0.005 | Hypertension × Age |
| Congestive heart failure × Age | −0.007 | Heart Disease × Age |
| Myocardial infarction × Age | 0.003 | Heart Attack × Age |
| Rheumatic heart disease × Age | −0.008 | |
| Stroke × Age | −0.003 | Stroke × Age |
| Sickle cell/HIV/Transplate × Age | −0.028 | |
| Chronic kidney disease × Age | −0.008 | Kidney Disease × Age |
| Hemodialysis × Age | −0.018 | |
| Liver disease × Age | 0.001 | |
| Pneumonia, acute bronchitis, influenza or other acute respiratory infection × Age | −0.005 | |
| Cancer × Age | −0.009 | Cancer × Age |
| Neurocognitive conditions × Age | 0.004 | Neurological Condition × Age |
| Pregnancy, childbirth & puerperium × Age | −0.003 |
Table B-2:
Health-Related Risk Factors and Other Variables, Variable Names, and Question Wording from the 2017 Wave of the PSID
| Risk Factor/Variable | PSID Variable | Question Wording |
|---|---|---|
| Age | ER34504 | What is your current age? |
| Gender | ER32000 | What is your gender? |
| Past Hospitalization | ER68511, ER69638 | Were you a patient in a hospital overnight or longer at any time during 2016? |
| Lung Disease | ER68454, ER69581 | Has a doctor or other health professional ever told you that you had chronic lung disease such as bronchitis or emphysema? |
| Asthma | ER68449, ER69576 | Has a doctor or other health professional ever told you that you had asthma? |
| Severe Obesity | ER68568, ER68569, ER69695, ER69696, ER68566, ER68567 | Constructed BMI using height and weight variables and designating obese if BMI > 40 |
| Diabetes | ER68459, ER69586 | Has a doctor or other health professional ever told you that you had diabetes or high blood sugar? |
| Hypertension | ER68444, ER69571 | Has a doctor or other health professional ever told you that you had high blood pressure or hypertension? |
| Heart Disease | ER68439, ER69566 | Has a doctor or other health professional ever told you that you had coronary heart disease, angina, congestive heart failure? |
| Heart Attack | ER68433, ER69560 | Has a doctor or other health professional ever told you that you had a heart attack? |
| Stroke | ER68427, ER69554 | Has a doctor or other health professional ever told you that you had a stroke? |
| Kidney Disease | ER68498, ER69625 | Is there any other serious, chronic condition that a doctor or other health professional ever told you had? [3: Kidney disease] |
| Cancer | ER68479, ER69606 | Has a doctor or other health professional ever told you that you had cancer or a malignant tumor? |
| Neurological condition | ER68469, ER69596 | Has a doctor or other health professional ever told you that you had permanent loss of memory or mental ability? |
Footnotes
In this paper we do not have sufficient data to examine non-Hispanic American Indian or Alaska Native populations, so we do not emphasize differential mortality among these groups. However, the emerging gaps in mortality are very large. In Arizona, 22% of deaths from COVID-19 are among non-Hispanic American Indians though they make up only 2% of the state’s population (CDC, 2020).
https://psidonline.isr.umich. edu/data/Documentation/U serGuide2017.pdf
A cohabiting partner is one who has lived with the head for one year or more. Since 2017, the PSID has referred to heads as “reference person 1” and spouses and cohabiters as “reference person 2.” For ease in comparison, we continue to use “head” and “spouse.”
There are 13,822 heads and spouses age 25 and older. We dropped 293 due to missing data, producing a sample of 13,529.
The lowest quartile consisted of those households with reported incomes between $0 and $29,096; the second quartile, $29,096 and $56,760; the third quartile, $56,760 and $101,820; and the top quartile, incomes greater than $101,820. The measure is based on reports about the income received in 2016 and is reported in 2016 dollars.
There are slight differences between the PSID and CPS samples for the distributions of educational attainment, presumably because of slightly different questions.
See https://www.cdc.gov/nchs/nhis/about_nhis.htm for more details about the NHIS. Also see https://psi-donline.isr.umich.edu/Publications/Papers/tsp/2017–01_Health_Data_update_2015.pdf for a more detailed comparison of the health conditions measures collected in the PSID with other studies, including the NHIS.
At https://closedloop.ai/c19index/ one can calculate values of what they refer to as the “C-19index” based on risk factors, hospitalization experience and age. The Survey Model that we use is similar, but not exactly the same, as the web model is trained on additional information that we do not use, e.g., zip-code.
NCHS (2018) reports that the prevalence of hypertension is higher among Hispanics than non-Hispanic whites. This is based on age-adjusted rates. Crude rates are higher for Hispanics. The crude rates that we report are nearly identical to the crude rates reported in NCHS (2018) Table 22.
The first two columns of Table B-1 are taken from Table 4 of DeCaprio et al. (2020) for their Survey Model.
REFERENCES
- Arentz M., Yim E., Klaff L., et al. (2020). Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. Published online March 19, 2020. doi: 10.1001/iama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar K. M., Zijun S., Romanelli R. J., Lockhart S. H., Smits K., Robinson S., Brown S., & Pressman A. R. (2020). Disparities In Outcomes Among COVID-19 Patients In A Large Health Care System in California. Health Affairs 39(7). 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- Barcellos S. H., Goldman D. P., & Smith J. P. (2012). Undiagnosed Disease, Especially Diabetes, Casts Doubt On Some Of Reported Health ‘Advantage’ Of Recent Mexican Immigrants. Health Affairs 31(12), 2727–37. 10.1377/hlthaff.2011.0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju P. K., Ghassemieh B. J., Nichols M., Kim R., Jerome K. R., Nalla A. K., Greninger A. L., Pipavath S., Wurfel M. M., Evans L., Kritek P. A., & West T. E. (2020). Covid-19 in Critically Ill Patients in the Seattle Region – Case Series. New England Journal of Medicine, 382, 2012–20122. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenshine P., Reingold A., Egerter S., Mockenhaupt R., Braveman P., & Marks J. (2008). Pandemic Influenza Planning in the United States from a Health Disparities Perspective. Emerging Infectious Diseases 14(5), 709–15. 10.3201/eid1405.071301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjas G. J. (2020). Demographic Determinants of Testing Incidence and COVID-19 Infections in New York City Neighborhoods. NBER Working Paper No. 26952. [Google Scholar]
- Case A., & Deaton A. (2020). Deaths of Despair and the Future of Capitalism. Princeton University Press. [Google Scholar]
- Case A., & Deaton A. (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences 112(49), 15078–15083. 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2020a, May 20). Weekly Updates by Select Demographic and Geographic Characteristics, Table 2b. https://www.cdc.gov/nchs/nvss/vsrr/covid19/index.htm.
- Center for Disease Control and Prevention. (2020b). COVID-19 Laboratory-Confirmed Hospitalization. Preliminary data as of May 16, 2020. https://gis.cdc.gov/grasp/COVID-Net/COVID19_5.html.
- Chatterji P., Joo H., & Lahiri K. (2012). Racial/Ethnic- and Education-Related Disparities in the Control of Risk Factors for Cardiovascular Disease Among Individuals With Diabetes. Diabetes Care 35(2), 305–12. 10.2337/dc11-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R., Stepner M., Abraham S., Lin S., Scuderi B., Turner N., Bergeron A., & Cutler D. (2016). The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA 315(16), 1750–1766. doi: 10.1001/iama.2016.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A. V. (2009). The Hypertension Paradox — More Uncontrolled Disease despite Improved Therapy. New England Journal of Medicine 361(9), 878–87. 10.1056/NEJMsa0903829 [DOI] [PubMed] [Google Scholar]
- Cummings M. J., Baldwin M. R., Abrams D., Jacobson S. D., Meyer B. J., Balough E. M., Aaron J. G., Claassen J., Rabbani L. E., Hastie J., Hochman B. R., Salazar-Schicchi J., Yip N. H., Brodie D., & O’Donnell M. R. (2020). Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults with COVID-19 in New York City: A Prospective Cohort Study. (2020). The Lancet, published online May 19, 2020, S0140673620311892. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie J., & Schwandt H. (2016). Inequality in mortality decreased among the young while increasing for older adults, 1990–2010. Science 352(6286), 708–712. DOI: 10.1126/science.aaf1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio D., Gartner J., McCall C. L., Burgess T., Kothari S., & Sayed S. (2020). Building a COVID-19 Vulnerability Index. medRxiv.org; 10.1101/2020.03.16.20036723. [DOI] [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., & Akbari A.. (2020). Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Archives of Academic Emergency Medicine 8(1): e35 10.22037/aaem.v8i1.600.g748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. A., McFall B. H., & Ryan L. (2019). Adding the AD8 Dementia Screen to the Panel Study of Income Dynamics PSID Technical Series Paper #19–01. University of Michigan, Ann Arbor [Google Scholar]
- Garg S, Kim L, Whitaker M, et al. (2020). Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1–30, 2020. Morbidity and Mortality Weekly Report (MMWR). 2020; 69:458–464. dx. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Greenberg S. B., Atmar R. L., Piedra P. A., & Couch R. B. (2020). Impact of Respiratory Virus Infections on Persons With Chronic Underlying Conditions. JAMA 283(4), 499–505. 10.1001/iama.283.4.499 [DOI] [PubMed] [Google Scholar]
- Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., Iotta G., Latronico N., Lorini L., Merler S., Natalini G., Piatti A., Ranieri M. V., Scandroglio A. M., Storti E., Cecconi M., & Pesenti A. (2020). Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 323(16), 1574–1581. doi: 10.1001/iama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M. & McDade T. W. (2018). The Biosocial Approach to Human Development, Behavior, and Health Across the Life Course. RSF: The Russell Sage Foundation Journal of the Social Sciences 4(4), 2–26. 10.7758/RSF.2018.4.4.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz R. P., Unger A. N., Cornell J. A., & Saunders E. (2005). Racial Disparities in Hypertension Prevalence, Awareness, and Management. Archives of Internal Medicine 165(18), 2098–2104. doi: 10.1001/archinte.165.18.2098 [DOI] [PubMed] [Google Scholar]
- Hicken M. T., Lee H., Morenoff J., House J. S., & Williams D. R. (2014). Racial/ethnic disparities in hypertension prevalence: reconsidering the role of chronic stress. American journal of Public Health, 104(1), 117–123. 10.2105/AJPH.2013.301395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insolera N. E. & Freedman V. A. (2017). Comparing Health Estimates in the PSID and NHIS, 2001–2015. Institute for Social Research, University of Michigan, Technical Series Paper #17–01. [Google Scholar]
- Kumar S., & Quinn S. C. (2012). Existing Health Inequalities in India: Informing Preparedness Planning for an Influenza Pandemic. Health Policy and Planning 27(6), 516–26. 10.1093/heapol/czr075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowcock E. C., Rosella L. C., Foisy J., McGeer A. & Crowcroft N. (2012). The Social Determinants of Health and Pandemic H1N1 2009 Influenza Severity. American Journal of Public Health 102(8), e51–58. 10.2105/AJPH.2012.300814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Gu J., Hou P., Zhang L., Bai Y., Guo Z., Wu H., Zhang B., Li P., Zhao X. (2020). Incidence, clinical characteristics an dprognostic factor of patients with COVID-19: a systematic review and meta-analysis, medRxiv.. 10.1101/2020.03.17.20037572 [DOI] [Google Scholar]
- McGonagle K., Schoeni R. F., Sastry N., & Freedman V. A. (2012) The Panel Study of Income Dynamics: Overview, recent renovations, and potential for life course research. Longitudinal and Life Course Studies 3, 268–284. dx. 10.14301/llcs.v3i2.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead H., Cartwright-Smith L., Jones K., Ramos C., Siegel B., & Woods K.. (2005). Racial and Ethnic Disparities in U.S. Health Care: A Chartbook. The Commonwealth Fund; Pub. No. 1111. https://www.commonwealthfund.org/sites/default/files/documents/_usr_doc_Mead_racialethnicdisparities_chartbook_1111.pdf [Google Scholar]
- Mongey S., Pilossoph L., & Weinberg A. (2020). Which Workers Bear the Burden of Social Distancing Policies? NBER Working Paper No. 27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. (2018). Health, United States, 2018. Hyattsville (MD): National Center for Health Statistics. [Google Scholar]
- National Center for Health Statistics. (2012). Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. Hyattsville (MD): National Center for Health Statistics (US); 2012 May. Report No.: 2012–1232. [PubMed] [Google Scholar]
- Pleasants R. A., Riley I. L., & Mannino D. M. (2016). Defining and Targeting Health Disparities in Chronic Obstructive Pulmonary Disease. International Journal of Chronic Obstructive Pulmonary Disease 11, 2475–96. 10.2147/COPD.S79077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J. S., Narasimhan M., Crawford J. M., McGinn T., Davidson K. W., and the Northwell COVID-19 Research Consortium. (2020). Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. Published online April 22, 2020. Corrected on April 24, 2020. 10.1001/iama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scodia J. A., (2020). Epidemiology and clinical features of COVID-19: A review of current literature. Journal of Clinical Virology 127 (104357). 10.1016/i.icv.2020.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. P. (2007). Nature and Causes of Trends in Male Diabetes Prevalence, Undiagnosed Diabetes, and the Socioeconomic Status Health Gradient. Proceedings of the National Academy of Sciences 104(33), 13225–31. 10.1073/pnas.0611234104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. (2020). Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. International Journal of Infectious Diseases 94, 91–95. 10.1016/i.iiid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. (2020) Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242. doi: 10.1001/iama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Zallman L., Himmelstein D. H., Woolhandler S., Bor D. H., Ayanian J. Z., Wilper A. P., & McCormick D. (2013). Undiagnosed and Uncontrolled Hypertension and Hyperlipidemia among Immigrants in the US. Journal of Immigrant and Minority Health 15(5), 858–65. 10.1007/s10903-012-9695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]