Abstract
Genital herpes increases the risk of acquiring and transmitting HIV, is a source of anxiety for many about transmitting infection to intimate partners, and is life-threatening to newborns. A vaccine that prevents genital herpes infection is a high public health priority. An ideal vaccine will prevent both genital lesions and asymptomatic subclinical infection to reduce the risk of inadvertent transmission to partners, will be effective against genital herpes caused by herpes simplex virus types 1 and 2 (HSV-1, HSV-2), and will protect against neonatal herpes. Three phase 3 human trials were performed over the past 20 years that used HSV-2 glycoproteins essential for virus entry as immunogens. None achieved its primary endpoint, although each was partially successful in either delaying onset of infection or protecting a subset of female subjects that were HSV-1 and HSV-2 uninfected against HSV-1 genital infection. The success of future vaccine candidates may depend on improving the predictive value of animal models by requiring vaccines to achieve near-perfect protection in these models and by using the models to better define immune correlates of protection. Many vaccine candidates are under development, including DNA, modified mRNA, protein subunit, killed virus and attenuated live virus vaccines. Lessons learned from prior vaccine studies and select candidate vaccines are discussed, including a trivalent nucleoside-modified mRNA vaccine that our laboratory is pursuing. We are optimistic that an effective vaccine for prevention of genital herpes will emerge in this decade.
A. Need for a genital herpes vaccine.
A genital herpes vaccine is a high public health priority for the following reasons: First, genital herpes is a major risk factor for acquiring and transmitting HIV [1–4]. Estimates are that a successful vaccine will reduce the incidence of HIV by 30-40% over 20 years, and even a modestly effective vaccine will have a substantial impact on HSV-2 transmission [5–7]. Second, neonatal herpes affects 14,000 infants annually worldwide with high mortality, particularly in resource limited countries [8]. Third, genital infection caused by HSV-2 affects 500 million people worldwide, while 140 million have genital herpes caused by HSV-1 [9, 10]. Painful genital lesions develop in some individuals, recurrent episodes of meningitis develop in others, and emotional distress occurs in many because of concerns about transmitting infection to intimate partners [11–13].
A.1). Goals of a genital herpes vaccine:
A successful prophylactic genital herpes vaccine ideally will prevent both clinical disease and subclinical infection (we define the combination as sterilizing immunity). Preventing subclinical infection will reduce the risk of inadvertent transmission to non-vaccinated intimate partners [14, 15]. The target population will likely be adolescent males and females prior to sexual debut, similar to the current HPV vaccine, or perhaps young adults at the time of sexual debut. Immunity should be durable, preferably lasting multiple years between booster doses. A successful genital herpes vaccine should also protect against HSV-1 genital infection because approximately 50% of first-time genital herpes infections in resource-rich countries are caused by HSV-1 [10, 16, 17]. Results from the Herpevac Trial for Women suggested that cross-protection against genital HSV-1 can be obtained with an HSV-2 vaccine (see section B.3 below) [18]. The frequency of first-episode genital herpes caused by HSV-2 has declined over the past two decades, while rates for HSV-1 genital herpes have remained stable [19]. HSV-1 genital infections recur less frequently than HSV-2; therefore, HSV-2 contributes more than HSV-1 to the overall burden of infection [20]. Genital herpes caused by more than one HSV-2 strain is uncommon (3–7%), suggesting that the vaccine is likely to be highly effective if vaccine-induced immunity is at least as potent as immunity after natural infection [21].
Neonatal herpes is a feared sequela of genital herpes. Although uncommon, neonatal herpes causes significant morbidity and mortality, with half of infected newborns experiencing disseminated or central nervous system disease, while the remainder have disease limited to the skin, eyes, and mucous membranes [8, 22–24]. Neonatal herpes infection primarily occurs by transmission during vaginal delivery through contact with infected genital secretions, while intrauterine and postnatal transmission are less frequent [25]. Genital infection with HSV-1 or HSV-2 is often asymptomatic, and although clinically silent, such infections can result in HSV transmission. The highest risk for the newborn is when the mother acquires a first-episode genital infection late in pregnancy. The absence of pre-existing maternal antibodies increases the risk of transmission of HSV-1 or HSV-2 to the newborn, although transmission during recurrent infection is also possible [26–28]. To determine women at risk for primary genital herpes requires identifying women of reproductive age without prior HSV infection; however, the US Preventive Services Task Force guidelines do not recommend routine serologic screening of asymptomatic adolescents and adults for genital HSV infection [29]. Reasons cited include high false-positive and false-negative rates, and inability of serologic testing to discriminate between HSV-1 oral and genital infection. This recommendation includes individuals who are pregnant, and is consistent with those of other relevant groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the Centers for Disease Control. Therefore, no diagnostic techniques are currently available to identify pregnant women at risk of acquiring primary HSV infection, leaving vaccination as the best intervention to prevent neonatal herpes infections.
B. Phase 3 human trials of prophylactic genital herpes vaccines.
Three publications have reported results of large human trials for prevention of genital herpes [18, 30, 31]. Subjects in each trial were immunized with glycoproteins required for virus entry into cells with the intent of producing neutralizing antibodies that block infection. The trials were partially successful as discussed below, but failed to meet their primary endpoints (Table 1).
Table 1.
Summary of prior phase 3 human trials
| Study population | Antigen & adjuvant | Primary endpoint | Outcome | Concerns and observations |
|---|---|---|---|---|
| HSV-2 seronegative discordant couples, HSV-2 seronegatives at STD clinics [30] | gB2, gD2 & MF59 | Time to genital infection (culture or seroconversion) | 5-month delay in onset of genital infection; by 1 year, no benefit | Durability problematic; accuracy of serology in vaccinated subjects undefined |
| Discordant couples: double seronegative or any serostatus [31] | gD2 & MPL/alum | Genital disease (lesions, culture+ or PCR+, & seroconversion) | Subset analysis: Efficacious in double seronegative women | Follow up study did not reproduce results [18]; concerns whether vaccine effective in men or HSV-1 seropositive women |
| Double seronegative women [18] | gD2 & MPL/alum | Genital lesions (signs & symptoms & culture+ or seroconversion) | Efficacious against HSV-1 but not HSV-2 | Neutralizing antibody titers low and not durable; gD2 ELISA titers correlated with HSV-1 protection |
B.1). gB2/gD2 MF59:
A study sponsored by Chiron Corp. reported two randomized, placebo-controlled trials that used recombinant subunit HSV-2 glycoproteins B and D (gB2, gD2) administered with MF59 as adjuvant [30]. Vaccine recipients were either HSV-1 seropositive or seronegative and HSV-2 seronegative. One study included partners of HSV-2-infected individuals (discordant couples), while the other involved HSV-2 seronegative individuals attending STD clinics. The primary endpoint was time to acquisition of genital herpes infection as determined by HSV-2 virus culture or seroconversion. Time to acquisition of infection was reduced by 50% in vaccine recipients compared with placebo over the first five months, but by one year, no overall benefit was detected. The vaccine produced neutralizing antibody titers comparable to those in naturally infected subjects. The durability of neutralizing antibodies was not reported; however, durability was likely problematic based on a rapid decline in neutralizing antibody titers six months after the final (third) immunization in a phase1/2 human trial with the same vaccine candidate [32].
Immunized subjects had lower than expected antibody-dependent cellular cytotoxicity (ADCC) titers, suggesting the possible importance of potent ADCC titers in vaccine protection [33]. The low ADCC titers may be explained, in part, by immune evasion properties of the virus. HSV-1 and HSV-2 glycoprotein E (gE) function as IgG Fc receptors [34]. Our lab determined that the HSV-1 Fc receptor inhibits antibody functions mediated by the IgG Fc domain, including ADCC. Therefore, immune evasion mediated by gE may account for the low ADCC titers in this trial (Figure 1) [35, 36]. Our vaccine approach to prevent immune evasion by gE is discussed in section F.2.
Figure 1. Blocking immune evasion by HSV-2 gC2 and gE2.
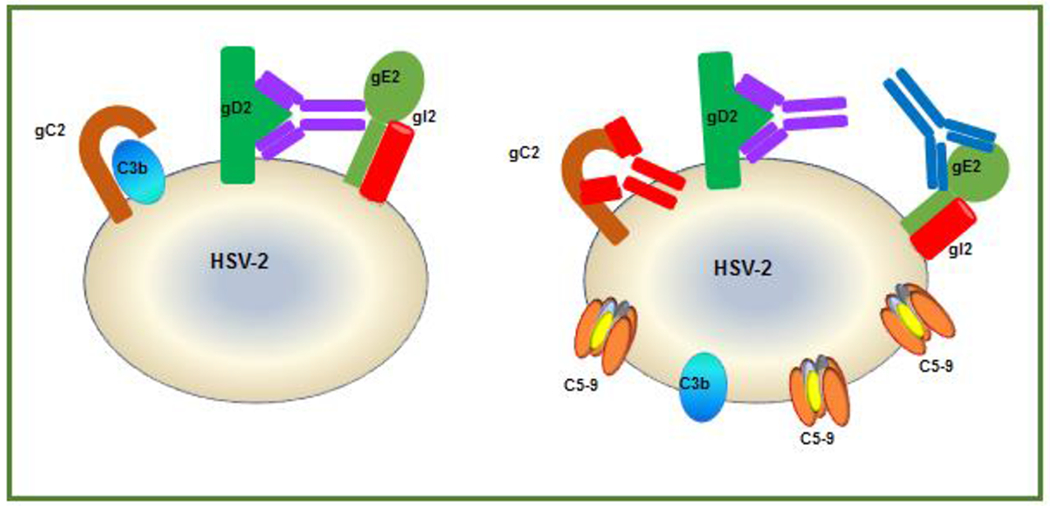
Left: gC2 on virus or infected cells (brown) binds to complement component C3b (blue) and blocks downstream complement activation to protect the virus against complement-mediated neutralization or cell lysis. Virus-specific antibody (purple), shown as anti-gD2, binds to gD2 by the F(ab’)2 domain while the Fc domain of the same antibody binds to gE2, which blocks activities mediated by the Fc domain, such as complement activation and ADCC. Right: Adding gC2 and gE2 immunogens to the gD2 vaccine produces antibodies (red and blue) that bind and block the ability of gC2 to bind C3b and gE2 to bind the Fc domain of IgG (shown as purple gD2 antibody), which results in the generation of the membrane attack complex (C5-9) and virus neutralization.
The primary study endpoint included seroconversion to HSV-2 antigens not included in the vaccine to establish a diagnosis of genital herpes infection. Serology was used to ascertain the diagnosis in 60/126 (48%) individuals that had no genital lesions, while 66/126 (52%) subjects had genital lesions. The authors did not comment on the performance characteristics of serology testing in subjects with positive HSV-2 cultures, which may have helped define the accuracy of the assay in individuals with proven genital infection. In subjects without genital lesions (asymptomatic individuals), the diagnosis of genital infection was entirely dependent on serology. In asymptomatic subjects in the general population, ELISA-based serology identifies as many false positive as true positive HSV-2 genital infections [29]. The serology test used in the human trial was Western blot, which is considered more accurate than ELISA; however, the accuracy of Western blot has not been established in vaccine recipients. A possible explanation for false positive results in vaccinated individuals is that some individuals may be exposed to HSV-2, have sufficient virus replication in genital tissues to develop HSV antibodies but not enough virus replication to develop genital lesions or establish latency. These subjects may seroconvert despite an aborted infection. Exposed but uninfected individuals develop HSV-specific T cells in the absence of HSV antibodies [37]. Perhaps similar events for antibody responses occur in exposed but uninfected individuals, particularly in vaccinated subjects. Evaluating the accuracy of serology in vaccine recipients can be addressed in animal models to determine whether some vaccine recipients that develop HSV antibodies are exposed but remain uninfected as defined by no genital lesions or latency (see section D).
B.2). gD2 MPL/alum:
A study sponsored by GlaxoSmithKline (GSK) reported results of a gD2 vaccine administered with MPL and alum as adjuvants [31]. Two separate studies were performed in HSV-2-uninfected partners of discordant couples. The first study evaluated HSV-1 and HSV-2 double seronegative subjects, while the second study enrolled subjects with any HSV serologic status. The primary endpoint of the first study was genital herpes lesions in men and women, while in the second study the primary endpoint was genital herpes lesions in HSV-2 seronegative women that were HSV-1 seropositive or seronegative. Vaccine efficacy in the first study was 38% (double seronegative men and women) (P=0.14), and in the second study 42% (HSV-2 seronegative women that were either HSV-1 seropositive or seronegative) (P=0.19). A subgroup analysis was performed to assess efficacy in double seronegative women. In study 1, efficacy was 73% (P=0.01), and study 2, 74% (P=0.02). The vaccine was not efficacious in HSV-1 seropositive women or in men of any serostatus. Neutralizing antibody titers and ELISA titers produced by the vaccine were similar in male and female subjects, although the actual titers were not reported.
The primary endpoint of the study required: i) genital signs or symptoms, ii) a positive culture for HSV or positive PCR for HSV DNA, and iii) seroconversion. Our concerns about the accuracy of seroconversion in vaccine recipients are discussed above. The observation that the vaccine was not efficacious in HSV-1 seropositive/HSV-2 seronegative women suggests that prior HSV-1 infection may interfere with vaccine protection. The incidence of HSV-2 infection was reduced in HSV-1 seropositive/HSV-2 seronegative women compared with double seronegative women, indicating that prior HSV-1 infection is somewhat protective against HSV-2 infection. The trial was not powered to detect the low numbers of genital infections in HSV-1 seropositive women; therefore, the low infection rate in HSV-1 seropositive women may account for the lack of vaccine efficacy in this group, although it is also possible that prior HSV-1 infection blunted the immune response to the HSV-2 vaccine. The vaccine failed to protect men, a result that mirrored findings in the gB2/gD2 MF59 vaccine study, raising concerns about possible sex differences in vaccine efficacy [30].
B.3). gD2 MPL/alum follow up study:
The gD2 MPL/alum result of the first GSK study suggested that the vaccine may be protective in double seronegative women. Efficacy in this group was not the primary endpoint; therefore, the FDA requested a follow up study that was called the Herpevac Trial for Women [18]. The trial was performed using gD2/MPL/alum in double seronegative women. The primary endpoint was genital herpes lesions caused by HSV-1 or HSV-2 beginning one month after the second immunization and extending to month 20. The definition of genital lesions was signs or symptoms compatible with genital lesions confirmed by viral culture and/or seroconversion within six months of lesions. Vaccine efficacy was 20% overall; however, vaccine efficacy was significant against HSV-1 genital disease after two immunizations (58%) or three (77%). The vaccine was not efficacious against HSV-2 genital disease. ELISA titers waned markedly over 20 months, while even at peak times (one month after the third dose) neutralizing antibody titers were low (1:29) and fell to undetectable levels by 16 months, raising concerns about vaccine potency and durability.
In a subset analysis, vaccine efficacy against HSV-1 was 69% after two doses and 82% after three doses if the case definition required only a positive culture (clinical and serologic criteria were excluded). This case definition eliminates our concern about inaccuracies of serologic diagnosis and represents improved vaccine efficacy of 11% after two doses and 5% after three doses. A substudy using sera from 30 vaccinated subjects showed 3.5-fold higher neutralizing antibody titers to HSV-1 than HSV-2, providing a possible explanation for protection against HSV-1 [38]. ELISA gD2 antibody titers correlated with protection against HSV-1, while T cell responses did not, suggesting antibodies are important for vaccine efficacy [39].
C. Phase 1 human trial with HSV529.
HSV529 is a live attenuated replication-defective HSV-2 vaccine sponsored by Sanofi Pasteur in which the UL5 and the UL29 genes are deleted [40]. The attenuated virus is capable of infecting cells and generating broad immune responses [41]. A phase 1 trial included healthy volunteers that were: i) double seronegative to assess immunogenicity in subjects naïve to HSV-1 and HSV-2; ii) HSV-1 seropositive or seronegative and HSV-2 seropositive to evaluate immunogenicity as a possible therapeutic vaccine in individuals previously infected with HSV-2; and iii) HSV-1 seropositive and HSV-2 seronegative to determine whether prior HSV-1 infection blunted vaccine responses to HSV-2. The vaccine or placebo was administered three times at 0, 1 and 6 months. The primary endpoint was safety.
The vaccine was well tolerated. In the double seronegative group, 11/14 (78%) developed ≥4-fold rise in neutralizing antibody titers, with a peak mean titer of 1:16 [40]. The peak neutralizing titers in the double seronegative group were considerably lower than neutralizing antibody titers in HSV-1 or HSV-2 seropositive subjects prior to immunization. Although low, neutralizing titers were durable for six months after the third dose. Of concern, no vaccine recipient had ≥4-fold boost in neutralizing antibody titer in the HSV-1 or HSV-2 seropositive groups. CD4+ and CD8+ T cell responses were modest in double seronegative subjects with 5/14 (36%) demonstrating CD4+ and 2/14 (14%) CD8+ responses. In the HSV-1 or HSV-2 seropositive groups, CD4+ T cells boosted in 27%-46% and CD8+ T cell in 8%-18%. Further human phase 1/2a human trials are planned for the HSV529 vaccine candidate. HSV529 will be administered with HSV-2 subunit antigens gD2, and capsid antigens UL19 and UL25 adjuvanted with glucopyranosyl lipid A in a stable emulsion (GLA-SE) [42]. This combination will be evaluated as a therapeutic vaccine (https://clinicaltrials.gov/ct2/show/NCT04222985).
D. Animal Models for Genital HSV Vaccines
Animal models are used to evaluate most viral vaccine candidates that are capable of replicating in animals. Table 2 lists models commonly used to assess immunogenicity or efficacy of vaccines for prevention of genital herpes. Encouraging results with vaccine candidates in preclinical testing have not translated into success in human trials, raising concerns about the utility of the animal models [18, 30, 31]. We discuss advantages and disadvantages of these models, and approaches to improve their predictive value.
Table 2.
Advantages and disadvantages of animal models of HSV-2 genital infection
| Genus | Pros | Cons | References |
|---|---|---|---|
| Mus (mouse) | Different strains available including knockout and transgenic to evaluate mechanisms of protection | Low phylogeny; requires administration of progesterone agonist to ensure infection; no spontaneous recurrences or shedding after infection | [45, 51, 52, 57, 59, 60] |
| Cavia (guinea pig) | Prominent genital lesions after infection; no need for progesterone for infection; develop viral recurrences and HSV-2 DNA shedding | Low phylogeny; fewer assays to measure HSV-specific CD4+ and CD8+ T cell responses; no knockout and transgenic strains | [110–112] |
| Macaca (monkey) | Similar phylogeny as humans; useful to study immunogenicity | Rhesus macaques do not develop genital lesions | [66, 68] |
D.1). Murine model:
Female inbred mice are often used to study the efficacy of genital herpes vaccines [42–49]. These mice are readily available, easy to house in vivaria, and transgenic or knockout strains exist to evaluate mechanisms of protection [50–53]. Mice are generally pretreated with medroxyprogesterone or levonorgestrel to suppress estrus and achieve consistent results when infected intravaginally with HSV [54, 55]. Medroxyprogesterone and levonorgestrel reduce genital expression of the desmosomal cadherin desmoglein-1α, which increases mucosal epithelial permeability and susceptibility to HSV infection. Although HSV genital infection in humans is often asymptomatic or mild, infection is very different in mice. HSV-2 intravaginal infection in mice results in extensive genital disease, hindlimb paralysis, and high mortality rates [56, 57]. To improve the predictive value of the model, the endpoint for vaccine efficacy needs to be more stringent than merely preventing these severe complications.
We recently demonstrated that a genital herpes vaccine is capable of providing sterilizing immunity in mice, which we defined as no clinical disease, measured by genital lesions, weight loss, and hindlimb weakness, and no subclinical infection, determined by vaginal titers on days 2 and 4 post-infection and HSV-2 DNA in dorsal root ganglia (DRG) at early (day 4) and late (>28 days) times post-infection [58]. An important limitation of the mouse model is that infections do not spontaneously recur and yet recurrences are a hallmark of genital herpes infections in humans [59–61]. This limitation is addressed by the guinea pig genital infection model.
D.2). Guinea pig model:
The female guinea pig genital infection model more closely approximates human disease than the murine model in that both acute and recurrent genital lesions develop and animals have recurrent subclinical episodes of vaginal shedding of HSV-2 DNA [47, 58, 62, 63]. The guinea pig model uses outbred animals and does not require pretreatment with medroxyprogesterone or levonorgestrel prior to infection. The lack of synchronization of the estrus cycle mimics conditions in humans. A deficiency of the guinea pig model is the lack of reagents to evaluate T cell immune responses, although this shortfall is improving [64, 65].
D.3). Non-human primate model:
Rhesus macaques have anatomical and immunological profiles closer to humans and are commonly used in biomedical research. Some rhesus macaques develop vaginitis detected by histopathology and immunohistochemisty, acute and recurrent vaginal shedding of HSV DNA, and HSV DNA in DRG [66, 67]. However, rhesus macaques do not develop genital lesions after intravaginal HSV infection. The lack of genital lesions suggests that this model is better suited for immunogenicity than efficacy studies. A coinfection model in rhesus macaques uses HSV genital infection and a chimeric simian immunodeficiency virus expressing the HIV envelope or reverse transcriptase gene. This model may prove useful to assess mechanisms by which HSV and HIV interact [68, 69]. Some Old World non-human primates have species-specific innate mechanisms that promote HSV disease resistance that may help identify targets for novel therapies [70].
D.4). Improving the predictive value of animal models:
Several suggestions follow on approaches to improve the predictive value of murine and guinea pig models. First, use the same criteria to evaluate vaccine efficacy in animal models and humans. For example, severity of genital disease and/or days with genital disease are often used to assess vaccine efficacy in guinea pigs, while incidence of genital disease is evaluated in human trials [18, 63]. Second, seroconversion is used in human trials to confirm subclinical infection that occurs in the absence of genital lesions, although the accuracy of this test remains uncertain [29]. Animal models can determine the accuracy of seroconversion by evaluating vaginal shedding of HSV DNA or HSV DNA in DRG in animals without clinical lesions. Some studies have addressed these issues, but more are needed [43, 71]. High false positive or false negative seroconversion rates would suggest that alternate approaches are needed to confirm subclinical infection in human trials, possibly by performing self-applied genital swabs for 1-2 months to detect HSV DNA [18]. Third, durability of protection is seldom evaluated in animal models, yet this feature is crucial for vaccine success in humans. Fourth, animal models can address sex differences in vaccine protection. The best models for male genital herpes involve intrarectal infection or virus delivery by skin scarification on the medial thigh of guinea pigs [72, 73]. Fifth, animal models are useful to define immune correlates of protection, as discussed below.
E. Determining immune correlates of protection.
E.1). Immune correlates of protection:
Correlates of protection are particularly relevant when advancing from preclinical models to early phase human trials and can help determine the optimal vaccine antigen and adjuvant concentrations. Correlates of protection identified for current vaccines include antibody (ELISA, neutralizing, IgA, and ADCC) and cellular immunity (effector memory, central memory, and cytokine production) [74, 75]. More than one immune function may correlate with protection [75]. Immune correlates of protection depend on the antigens and adjuvants selected as immunogens. For example, a genital herpes vaccine consisting of a single subunit protein, such as glycoprotein D, will have different correlates of protection than a live virus vaccine that is deleted in that protein [76].
E.2). Correlates of protection for a prophylactic HSV vaccine:
HSV-1, HSV-2 and other herpesviruses have the distinct properties of causing acute (primary) infection, establishing latency and subsequently reactivating to produce recurrent disease. Herpesviruses encode many proteins that inhibit innate and acquired immunity, which likely explains why it is difficult to prevent primary infection and recurrences. Our laboratory has focused on blocking two immune evasion molecules on the virus envelope that are accessible to vaccine-induced antibodies. Our goal is to prevent the virus from establishing a primary infection or latency. Antibodies are likely important for the success of our approach and more broadly, for the success of other prophylactic genital herpes vaccines the following additional reasons: First, antibodies correlate with protection by many successful prophylactic vaccines, including influenza, polio, rabies, rotavirus, mumps, rubella, and yellow fever [74]. Second, maternal antibodies protect newborns from severe neonatal herpes [77–79]. Third, passive transfer of antibody protects in animal models [80, 81]. Fourth, antibody titers to gD2 correlated with protection against HSV-1 genital lesions in the Herpevac Trial for Women [18, 39]. Although antibodies are the focus of our vaccine efforts, other vaccine preparations may protect by different mechanisms that rely more heavily on innate or acquired cellular immune responses.
E.3). Epitope mapping to determine immune correlates of protection:
We use a high throughput biosensor-based antibody competition assay to map the epitopes recognized following infection or immunization in animal models. The assay is based on the premise that antibodies compete to bind a discrete epitope. If one antibody is already bound, a second antibody that recognizes the same or a closely overlapping epitope is blocked from binding. For example, a monoclonal antibody referred to as MC23 recognizes a crucial epitope on gD2 involved in interacting with the host-cell receptor nectin-1 [82]. When bound, MC23 prevents gD2 from interacting with nectin-1 and subsequent viral entry [82]. We can evaluate whether gD2-immunized animals generate antibody responses to this crucial epitope through competition with the MC23 monoclonal antibody (Figure 2). Incubating gD2 with IgG from an immunized animal should prevent gD2 from binding to MC23 on the chip if the animal has IgG that recognizes this crucial gD2 epitope. If the animal does not produce antibodies to a crucial epitope, gD2 will bind to the monoclonal antibody on the chip that recognizes this epitope (shown as gD2 binding to DL6 in Figure 2).
Figure 2. Schema of the high throughput biosensor antibody competition assay.

Left bottom: Cartoon of the biosensor chip that has different monoclonal antibodies at each position that recognize overlapping epitopes within a group (shown as the same color) or non-overlapping epitopes (shown in different colors). Middle section: Blowup of the biosensor chip. Right side, middle of figure: Monoclonal antibody MC23 from the red group is plated on the chip. IgG purified from serum of a gD2-immunized animal is incubated with gD2 antigen and floated over the chip. gD2 does not bind to MC23 on the chip; therefore, the animal produced antibodies to the epitope recognized by MC23. Right side, bottom of figure: IgG from the same immunized animal does not contain antibodies that block gD2 binding to a blue monoclonal antibody, DL6, on the chip; therefore, the animal did not produce antibodies to the epitope recognized by the DL6 monoclonal antibody.
Combining this antibody competition assay with a biosensor capable of measuring multiple competitions simultaneously enables rapid mapping of epitopes recognized by a large number of serum samples from animals or humans. Current biosensor systems allow up to 384 monoclonal antibodies to be printed simultaneously as separate spots on a chip that then compete to bind gD2, or other antigens, premixed with serum from animals or humans. The chip is regenerated repeatedly to enable a succession of antigen and sera mixtures to be evaluated. The breadth of epitopes evaluated is dependent upon the diversity of the monoclonal antibodies printed on the chip. Therefore, we typically print large panels of monoclonal antibodies to fully cover all crucial epitopes.
E.4). Vaccine-induced antibodies to gD2 correlate with protection from genital disease in guinea pigs:
We used the biosensor-based antibody competition assay to map the epitopes recognized by guinea pigs immunized with gD2 subunit protein vaccine adjuvanted with CpG and alum [66, 80]. Guinea pigs were immunized intramuscularly three times at two-week intervals or mock-immunized with adjuvants alone and challenged intravaginally with HSV-2 [66]. The efficacy of the gD2 subunit protein vaccine compared with the mock vaccine was 80% based on days with genital lesions, but only 33% efficacious in completely preventing genital lesions [66]. That some animals developed genital lesions while others did not enabled us to evaluate correlates of protection against genital lesions in gD2-immunized animals [80].
We evaluated antibody responses using sera taken two weeks after the third (final) immunization [66, 80]. Both gD2 ELISA and neutralizing antibody titers strongly correlated with protection from genital lesions and with each other suggesting that antibodies protect and that neutralizing antibodies are important mechanistically. Functional domains on gD2 responsible for virus entry and cell-to-cell spread have been mapped to seven crucial linear and conformational epitopes [83]. Using the biosensor-based antibody competition assay, we assessed whether IgG purified from gD2-immunized guinea pig sera recognizes these crucial epitopes (Figure 2). The presence of IgG to one or more of these epitopes correlated significantly with neutralizing antibody titers and protection from genital disease [80]. Animals that produced antibodies to multiple crucial gD2 epitopes demonstrated stronger protection against genital lesions than animals that produced antibodies to only a few epitopes. These data suggest that neutralizing antibodies and antibodies to multiple gD2 epitopes involved in virus entry and cell-to-cell spread represent important immune correlates of protection.
E.5). Vaccine-induced antibodies in gD2 double seronegative vaccinated women:
We compared epitope-specific antibody responses observed in the gD2-immunized guinea pigs with those in a subset of 29 women enrolled in the Herpevac Trial for Women [80, 84]. The Herpevac trial did not protect women against genital infection caused by HSV-2; therefore, correlates of protection could not be determined [18]. However, we were able to measure epitope-specific gD2 antibody responses. The 29 women evaluated produced antibodies to significantly fewer crucial gD2 epitopes than the guinea pigs, and antibody responses to some linear epitopes involved in virus entry and cell-to-cell spread were completely missing [80]. Our study in guinea pigs suggests that the number of crucial gD2 epitopes recognized by antibodies positively correlates with protection against genital lesions [80]. We propose that future human trials containing gD2 as an immunogen will benefit by assessing antibody responses to crucial gD2 epitopes and correcting any deficiencies prior to embarking on large trials. Measuring antibody responses to epitopes involved in crucial functions mediated by gD2 and other vaccine antigens is a novel approach to evaluate vaccine immunogenicity and identify important gaps.
F. Vaccine candidates possibly headed for human trials.
Table 3 lists vaccine candidates that demonstrated promising results in preclinical testing. Two candidates are discussed in more detail below. One candidate is HSV-2 ΔgD−/+gD-1, a replication-defective strain deleted in glycoprotein D. Several replication-defective vaccine candidates have gained attention (Table 3), in part because they induce broad antibody and T cell responses. The trivalent gC2, gD2, gE2 candidate vaccine highlighted below takes a different approach, which is to stimulate very high-titered immune responses to select antigens rather than induce perhaps lower-titered immune responses to many antigens.
Table 3.
Prophylactic genital herpes vaccines in preclinical testing
| Vaccine candidates | Properties | References |
|---|---|---|
| Live attenuated virus | ||
| HSV529* | Deletion of two genes essential for replication, UL5, UL29 | [113] |
| HSV-2-ΔgD | Emphasis on inducing potent ADCC | [85] |
| HSV-1 VC2 mutations in gK and UL20 | Defective in virus entry and retrograde neuronal transport | [114] |
| HSV-2 UL24 mutant | Syncytial mutant with reduced virulence | [115] |
| HSV-2 ICP0 mutant | Replication-defective | [116] |
| HSV-2gD27 | Defective in entry in neuronal cells | [44] |
| Inactivated virus | ||
| HSV-2 with MPL/alum | Formalin-inactivated virus with adjuvant | [117] |
| Subunit protein antigens | ||
| gD2, UL19 and UL25 with GLA-SE adjuvant* | Induces potent CD4 and CD8 T cells | [42] |
| HSV-2 gB2, gD2 and UL40 with adjuvants | Stimulates potent neutralizing and CD8 T cell responses | [64] |
| gB2 and gD2 in nanoemulsion | Intranasal vaccine to block virus entry | [118] |
| Nucleoside-modified mRNA | ||
| gC2, gD2, gE2 mRNA-LNP | Induces neutralizing antibodies and antibodies that block immune evasion | [58] |
| DNA vaccine | ||
| gB2 with chemokine CCL19 | Enhanced mucosal antibody response | [119] |
HSV529 is in phase 1/2a human trials as a therapeutic vaccine combined with gD2, UL19 and UL25 and GLA-SE adjuvant (https://clinicaltrials.gov/ct2/show/NCT04222985)
F.1). HSV-2 ΔgD−/+gD-1:
HSV-2 ΔgD−/+gD-1 is a mutant virus deleted in glycoprotein D (ΔgD-2) that requires a gD1-complementing cell line for preparing virus stocks in vitro. The ΔgD-2 virus is capable of only a single cycle of replication in vivo. The rationale for developing this vaccine candidate includes: First, gD2 was a component of the prior vaccine trials that failed to meet their primary endpoints; therefore, trying a different approach is appealing [18, 30, 31, 57]. Second, the vaccine candidate produces potent ADCC antibody titers [57]. ADCC titers were low in the gB2/gD2 MF59 vaccine trial, while neutralizing antibody titers were considered satisfactory (although not durable), which stimulated the researchers to pursue a vaccine that does not rely on neutralizing antibody titers [30, 33, 85]. Third, the investigators postulated that deletion of gD2 from the virion may unmask protective antigens on other viral proteins [57].
The live attenuated vaccine protected against genital disease after intravaginal inoculation, and reduced skin lesions and inflammation after flank scarification in mice [57, 85]. The vaccine also protected male mice from lethal flank inoculation that served as a surrogate route of infection for genital herpes in males [76]. The vaccine strain when inoculated subcutaneously or intravaginally was avirulent in severe combined immunodeficiency mice [57]. The vaccine protected against multiple clinical isolates, including some from Africa, and laboratory strains of HSV-1 and HSV-2 [85]. Female mice vaccinated with ΔgD-2 and then mated passed antibodies transplacentally and through breast milk to neonates that protected the pups from lethal infection when challenged intranasally 7 or 14 days postnatal, but not on day 1 [86]. Maternal sera contained minimal neutralizing antibody activity. Protection appeared to be mediated by ADCC based on reduced potency of NK effector cells in 1-3 day-old pups compared with 7 day-old pups [86]. Protection in guinea pigs by the ΔgD-2 vaccine remains to be determined, and as discussed in section D.2, this model represents a stringent test of vaccine efficacy.
F.2). HSV-2 trivalent gC2, gD2, gE2 vaccine.
F.2.a). Rationale for the trivalent vaccine:
Three phase 3 trials for prevention of genital herpes targeted entry glycoproteins, either gD2 alone or gD2 and gB2, but did not meet their primary endpoints (see section B) [18, 30, 31]. HSV gD along with gB and glycoproteins H and L (gH/gL) are essential for HSV entry [87]. Antibodies to gD neutralize virus by blocking its interaction with entry receptors, HVEM (herpesvirus entry mediator) and nectin-1 and by interfering with its interaction with gH/gL [82]. We postulate that the vaccine candidates aimed at entry molecules were not successful, in part, because HSV evades host immunity through the actions of HSV-2 glycoprotein C (gC2) and glycoprotein E (gE2) [88]. HSV gC binds complement component C3b to inhibit complement activation, while gE binds the Fc domain of IgG, including the Fc domain of IgG antibodies that are targeting viral antigens, to inhibit Fc-mediated ADCC and complement activation (Figure 1, left side) [36, 47, 56]. By including gC2 and gE2 as antigens, our strategy is to produce antibodies to gC2 that block gC-mediated evasion of complement and to gE2 that block gE-mediated evasion of IgG Fc (Figure 1, right side). Additional benefits of including gC2 and gE2 in a vaccine are that antibodies to gC2 are neutralizing while antibodies to gE2 block HSV cell-to-cell spread [47, 89].
F.2.b). Trivalent protein subunit vaccine:
Subunit antigens are often weakly immunogenic and require adjuvants to stimulate potent and durable immune responses. Adjuvants reduce the concentration of antigen and the number of immunizations needed for effective immune responses [58, 90, 91]. Most adjuvants enhance immune responses by stimulating innate immunity, rather than acting directly on B or T cells [92].
Our initial studies with the trivalent genital herpes vaccine included species-specific CpG and alum as adjuvants administered with purified baculovirus proteins truncated prior to their transmembrane domains (gC2 aa 27-426, gD2 aa 26-331 and gE2 aa 24-405) [56, 66]. The vaccine was inoculated intramuscularly into mice, guinea pigs and rhesus macaques. The vaccine induced high gC2, gD2, gE2 ELISA titers, HSV-1 and HSV-2 neutralizing antibodies, and antibodies that blocked C3b binding to gC2 and IgG Fc binding to gE2 [56, 66]. In addition, antibodies were produced to crucial epitopes on gD2 (see section E.4) [80]. Vaccine efficacy was evaluated in mice by assessing acute genital disease and infection of DRG, and in guinea pigs by evaluating acute and recurrent genital lesions and asymptomatic vaginal shedding of HSV-2 DNA during the recurrent phase of infection [56, 66]. The trivalent protein vaccine prevented acute genital disease in 5/5 (100%) and 20/20 (100%) mice in two studies, while the vaccine prevented infection of DRG in 32/33 (97%) mice between days 2 and 7 or 15/20 (75%) mice between days 4 and 28 in two studies [56, 58]. The same vaccine formulation prevented acute and recurrent genital herpes lesions in 26/36 (72%) or 10/10 (100%) guinea pigs in two studies. Asymptomatic vaginal shedding of HSV-2 DNA developed in 19/36 (53%) or 5/10 (50%) guinea pigs in two studies [58, 66]. Although the results were encouraging, there is still room for improvement if we require near perfection from the animal models.
F.2.c). Trivalent nucleoside-modified mRNA vaccine:
Nucleoside-modified mRNA encapsulated in lipid nanoparticles (LNP) has the potential to revolutionize the viral vaccine field [93–99]. Potent Zika T cell and antibody responses were observed in rhesus macaques and neutralizing antibody titers were durable over one year [93]. Influenza hemagglutinin inhibition titers in mice were 25-times greater than after immunization with whole-killed virus and 4-times greater than after influenza virus infection [99]. Modified mRNA vaccines against multiple pathogens demonstrated potent and durable antibody responses in animals and humans without serious adverse events [93, 100–104]. The mechanism for these high and durable titers involves long-term expression of the immunogen resulting in prolonged loading of germinal centers and induction of abundant antigen-specific T follicular helper cells (Tfh) that stimulate germinal center formation, somatic mutation, high-affinity antibodies, and long-term memory B cells [93, 98, 103, 105–107].
Nucleoside-modified mRNA technology for vaccine delivery has advantages over DNA, protein subunit, inactivated or live-attenuated virus vaccines. First, safety: The mRNA is degraded by normal cellular processes, is non-infectious, non-integrating, and poses no risk of insertional mutagenesis. Second, efficacy: Delivery of mRNA results in transient translation that can be controlled by modifications in the untranslated regions (UTRs), cap, poly(A) tail, and coding sequences. These modifications can make the mRNA more stable and highly translatable. Efficient in vivo delivery can be achieved by formulating mRNA into carrier LNPs that promote rapid uptake and expression in the cytoplasm. Third, production: Manufacturing of conventional protein subunit, inactivated virus or replication-defective virus vaccines is time consuming and involves expensive equipment. Protein production and purification require optimization for each protein and mammalian or insect cell culture. Production carries concerns of improper folding and altered glycosylation patterns, particularly when produced in insect cells. In contrast, production of mRNA vaccines does not require cell culture for protein production and purification. Uniform optimized transcription and purification techniques can be applied to all mRNA sequences, and glycosylation patterns are similar to natural infection. These advantages coupled with the observation that the vaccines induce potent CD4+ Tfh and germinal center B cells responses led us to evaluate gC2, gD2, gE2 trivalent nucleoside-modified mRNA-LNP as a vaccine for preventing genital herpes.
We compared the immunogenicity and efficacy of the trivalent nucleoside-modified mRNA-LNP vaccine with the trivalent protein CpG/alum vaccine in mice and guinea pigs. The trivalent nucleoside-modified mRNA vaccine uses identical amino acid sequences as the trivalent protein vaccine [58, 66]. The nucleoside-modified mRNA for gC2 (corresponding amino acids 27-426), gD2 (corresponding amino acids 26-331), and gE2 (corresponding amino acids 24-405) were prepared by replacing each uridine nucleoside with 1-methylpseudouridine followed by HPLC purification to remove double stranded RNA (Figure 3).
Figure 3. Trivalent nucleoside-modified mRNA-LNP vaccine.
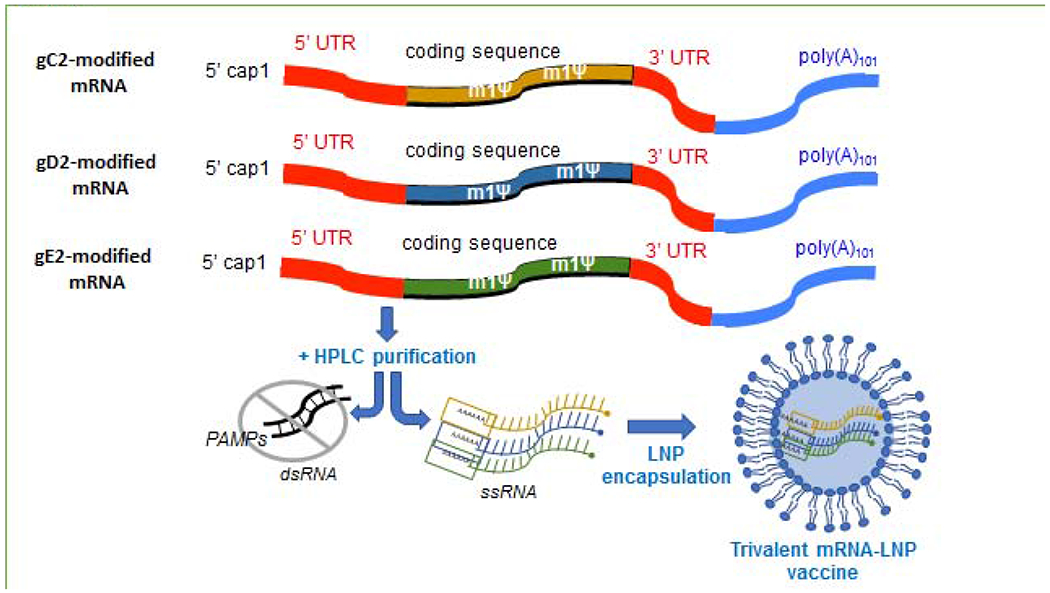
The trivalent gC2, gD2, gE2 nucleoside-modified mRNA-LNP vaccine combines the concept of blocking immune evasion with the use of nucleoside-modified mRNA for vaccine delivery. Modifications in mRNA include substitution of uridine residues with 1-methyl-pseudouridine to reduce triggering innate immune sensors that degrade mRNA, and altering the 5’ cap, 5’ and 3’ UTRs and poly(A) tail to improve mRNA stability. Modified mRNA is purified to remove double stranded RNA using HPLC followed by encapsulation in LNPs.
The trivalent nucleoside-modified mRNA-LNP vaccine was superior to the trivalent protein vaccine in stimulating ELISA IgG antibodies, neutralizing antibodies, antibodies that bind to crucial gD2 epitopes involved in entry and cell-to-cell spread, CD4+ T cell responses, and Tfh and germinal center B cell responses [58]. Both formulations completely prevented genital lesions in mice and guinea pigs; however, differences in efficacy emerged when evaluating subclinical infection. In mice immunized with the trivalent protein vaccine, 23 of 30 (73%) animals had no genital lesions or subclinical infection (HSV-2 DNA in DRG or positive virus cultures on day 2 or 4 post-infection) compared with 63 of 64 (98%) mice immunized with the nucleoside-modified mRNA-LNP [58]. In guinea pigs, 5/10 animals in the trivalent protein group had recurrent shedding of HSV-2 DNA on 19/210 (9%) days compared with 2/10 animals in the trivalent mRNA group that shed HSV-2 DNA on 5/210 (2%) days [58].
We compared the trivalent nucleoside-modified mRNA-LNP vaccine results to published guinea pig studies using vaccines that were later evaluated in human trials, including gD2 MPL/alum and HSV529 [58, 108, 109]. The trivalent nucleoside-modified mRNA-LNP vaccine produced superior immune responses and better protection than the gD2 MPL/alum or HSV529 vaccine in guinea pigs (Tables 4, 5). Future preclinical studies will need to address the durability of protection produced by the trivalent nucleoside-modified mRNA-LNP vaccine, cross-protection against vaginal HSV-1 infection, and efficacy against multiple HSV-2 isolates. Encouraging results will suggest that the trivalent nucleoside-modified mRNA-LNP vaccine is a promising candidate for human trials.
Table 4.
gD2/MPL/alum or trivalent nucleoside-modified mRNA-LNP vaccine in guinea pigs
| Outcome | gD2 MPL/alum [109] | Trivalent nucleoside-modified mRNA-LNP [58] |
|---|---|---|
| gD2 ELISA | 1:7200 | 1:512,000 |
| Neutralizing antibody titers | 1:813 | 1:5120 |
| Genital disease | 2/12 (17%) | 0/10 (0%) |
| Genital shedding HSV-2 DNA recurrent phase of infection | 12/12 (100%) | 2/10 (20%) |
| Means days shedding | 7.0 ± 0.6# | 0.5 ± 1.3 |
gD2 data corrected for 21 days instead of 28 for comparison with mRNA study
Table 5.
HSV529 or trivalent nucleoside-modified mRNA-LNP vaccine in guinea pigs
| Outcome | HSV529 [108] | Trivalent nucleoside-modified mRNA-LNP [58] |
|---|---|---|
| Neutralizing antibody titers | 1:400 | 1:5120 |
| Day 2 vaginal titers after infection | 2x102 | <101 |
| Day 4 vaginal titers after infection | 102 | Negative |
| Acute genital disease | + (some animals) | 0/10 (0%) |
| Recurrent genital disease | + (some animals) | 0/10 (0%) |
G. Remaining challenges.
Important obstacles to a genital herpes vaccine remain. First, major pharmaceutical companies may hesitate to pursue a prophylactic genital herpes vaccine because the trials are expensive, require enrolling thousands of subjects, and prior vaccine candidates that seemed promising in animal models did not succeed in humans [18, 30, 31]. Second, the potential lack of durable immunity remains a concern [18, 32]. Third, it is unknown whether prior infection with HSV-1 will interfere with vaccine responses to HSV-2 antigens, and whether males will be protected [30, 31, 40]. Fourth, a vaccine that targets genital HSV-2 needs to protect against genital HSV-1, which may be challenging. Fifth, a vaccine that prevents genital lesions without reducing subclinical genital shedding of virus may not modify transmission to sex partners [14, 15]. Sixth, no clear consensus exists on whether the primary endpoint of a vaccine trial should be HSV genital disease (lesions) or HSV genital infection (both genital lesions and subclinical infection). Preventing lesions and subclinical infection is preferred; however, the diagnosis of subclinical infection relies on serology, a test that has no proven accuracy in immunized individuals (section B.1). Genital shedding of HSV DNA may be a better approach than serology to diagnose subclinical infection in immunized individuals.
The criteria are not defined as to when a vaccine candidate is ready to advance to human trials. Ideally, the vaccine will induce durable immunity, be efficacious in HSV-2 seronegative recipients that are HSV-1 seropositive, protect males and females, prevent HSV-1 and HSV-2 genital infection, and greatly reduce or eliminate subclinical genital shedding of HSV DNA. We anticipate that a vaccine candidate for prevention of genital herpes that meets these criteria will be available this decade.
Acknowledgments
All authors have read the journal’s policy on disclosure of potential conflicts of interest and the journal’s authorship statement. The manuscript has been reviewed and approved by all authors. Sita Awasthi and Harvey Friedman are inventors on patents awarded to the University of Pennsylvania or patents that are under review on the use of HSV-1 or HSV-2 subunit proteins or nucleoside-modified mRNA for prevention or treatment of genital herpes. The inventors have in place a plan approved by the University of Pennsylvania for managing potential conflicts arising from licensing of the patents. None of the other authors reports any potential conflicts of interest. This work was supported by grant RO1 AI 139618 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health and by a grant from BioNTech SE (both to HMF), and by training grants T32 NS007180 from the National Institute of Neurological Disorders and Stroke (KE, post-doctoral recipient) and T32 AH 18684 from the National Institute of Allergy and Infectious Disease (AD, post-doctoral recipient).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. [DOI] [PubMed] [Google Scholar]
- [2].Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20:73–83. [DOI] [PubMed] [Google Scholar]
- [3].Keller MJ, Huber A, Espinoza L, Serrano MG, Parikh HI, Buck GA, et al. Impact of Herpes Simplex Virus Type 2 and Human Immunodeficiency Virus Dual Infection on Female Genital Tract Mucosal Immunity and the Vaginal Microbiome. J Infect Dis. 2019;220:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Looker KJ, Welton NJ, Sabin KM, Dalal S, Vickerman P, Turner KME, et al. Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data. Lancet Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwartz EJ, Blower S. Predicting the potential individual- and population-level effects of imperfect herpes simplex virus type 2 vaccines. J Infect Dis. 2005;191:1734–46. [DOI] [PubMed] [Google Scholar]
- [6].Alsallaq RA, Schiffer JT, Longini IM Jr., Wald A, Corey L, Abu-Raddad LJ. Population level impact of an imperfect prophylactic vaccine for herpes simplex virus-2. Sex Transm Dis. 2010;37:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Freeman EE, White RG, Bakker R, Orroth KK, Weiss HA, Buve A, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine. 2009;27:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Newman LM, et al. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health. 2017;5:e300–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10:e114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10:e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–72. [DOI] [PubMed] [Google Scholar]
- [12].Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–50. [DOI] [PubMed] [Google Scholar]
- [13].Logan SA, MacMahon E. Viral meningitis. BMJ. 2008;336:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. Jama. 2011;305:1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. Journal of the Royal Society, Interface / the Royal Society. 2014;11:20140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bernstein DI, Bellamy AR, Hook EW 3rd, Levin MJ, Wald A, Ewell MG, et al. Epidemiology, Clinical Presentation, and Antibody Response to Primary Infection With Herpes Simplex Virus Type 1 and Type 2 in Young Women. Clin Infect Dis. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Magdaleno-Tapial J, Hernandez-Bel P, Valenzuela-Onate C, Ortiz-Salvador JM, Garcia-Legaz-Martinez M, Martinez-Domenech A, et al. Genital Infection With Herpes Simplex Virus Type 1 and Type 2 in Valencia, Spain: A Retrospective Observational Study. Actas Dermosifiliogr. 2019. [DOI] [PubMed] [Google Scholar]
- [18].Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dabestani N, Katz Da, Dombrowski J, Magaret A, Wald A, Johnston C. Time Trends in First-Episode Genital Herpes Simplex Virus Infections in an Urban Sexually Transmitted Disease Clinic. Sex Transm Dis. 2019;46:795–800. [DOI] [PubMed] [Google Scholar]
- [20].Engelberg R, Carrell D, Krantz E, Corey L, Wald A. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis. 2003;30:174–7. [DOI] [PubMed] [Google Scholar]
- [21].Johnston C, Magaret A, Roychoudhury P, Greninger AL, Reeves D, Schiffer J, et al. Dual-strain genital herpes simplex virus type 2 (HSV-2) infection in the US, Peru, and 8 countries in sub-Saharan Africa: A nested cross-sectional viral genotyping study. PLoS Med. 2017;14:e1002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Corey L, Gruber WC, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108:230–8. [DOI] [PubMed] [Google Scholar]
- [23].Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. The New England journal of medicine. 1991;324:450–4. [DOI] [PubMed] [Google Scholar]
- [24].Kimberlin DW, Whitley RJ, Wan W, Powell DA, Storch G, Ahmed A, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011;365:1284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509–15. [DOI] [PubMed] [Google Scholar]
- [27].Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. Jama. 2003;289:203–9. [DOI] [PubMed] [Google Scholar]
- [28].Pinninti SG, Kimberlin DW. Neonatal herpes simplex virus infections. Semin Perinatol. 2018;42:168–75. [DOI] [PubMed] [Google Scholar]
- [29].Feltner C, Grodensky C, Ebel C, Middleton JC, Harris RP, Ashok M, et al. Serologic Screening for Genital Herpes: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama. 2016;316:2531–43. [DOI] [PubMed] [Google Scholar]
- [30].Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr., et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. Jama. 1999;282:331–40. [DOI] [PubMed] [Google Scholar]
- [31].Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. [DOI] [PubMed] [Google Scholar]
- [32].Langenberg AG, Burke RL, Adair SF, Sekulovich R, Tigges M, Dekker CL, et al. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected]. Annals of internal medicine. 1995;122:889–98. [DOI] [PubMed] [Google Scholar]
- [33].Kohl S, Charlebois ED, Sigouroudinia M, Goldbeck C, Hartog K, Sekulovich RE, et al. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. The Journal of infectious diseases. 2000;181:335–9. [DOI] [PubMed] [Google Scholar]
- [34].Para MF, Goldstein L, Spear PG. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. Journal of Virology. 1982;41:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dubin G, Socolof E, Frank I, Friedman HM. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991;65:7046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lubinski JM, Lazear HM, Awasthi S, Wang F, Friedman HM. The herpes simplex virus 1 IgG fc receptor blocks antibody-mediated complement activation and antibody-dependent cellular cytotoxicity in vivo. J Virol. 2011. ;85:3239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Posavad CM, Remington M, Mueller DE, Zhao L, Magaret AS, Wald A, et al. Detailed characterization of T cell responses to herpes simplex virus-2 in immune seronegative persons. J Immunol. 2010;184:3250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Awasthi S, Belshe RB, Friedman HM. Better Neutralization of Herpes Simplex Virus Type 1 (hSv-1) Than HSV-2 by Antibody From Recipients of GlaxoSmithKline HSV-2 Glycoprotein D2 Subunit Vaccine. J Infect Dis. 2014;210:571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis. 2014;209:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dropulic LK, Oestreich MC, Pietz HL, Laing KJ, Hunsberger S, Lumbard K, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 1 Study of a Replication-Defective Herpes Simplex Virus 2 Vaccine, HSV529, in Adults With or Without HSV Infection. J Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79:410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Odegard JM, Flynn PA, Campbell DJ, Robbins SH, Dong L, Wang K, et al. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine. 2016;34:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Diaz FM, Knipe DM. Protection from genital herpes disease, seroconversion and latent infection in a non-lethal murine genital infection model by immunization with an HSV-2 replication-defective mutant virus. Virology. 2016;488:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang K, Goodman KN, Li DY, Raffeld M, Chavez M, Cohen JI. A Herpes Simplex Virus 2 (hSv-2) gD Mutant Impaired for Neural Tropism Is Superior to an HSV-2 gD Subunit Vaccine To Protect Animals from Challenge with HSV-2. J Virol. 2016;90:562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bernard MC, Barban V, Pradezynski F, de Montfort A, Ryall R, Caillet C, et al. Immunogenicity, protective efficacy, and non-replicative status of the HSV-2 vaccine candidate HSV529 in mice and guinea pigs. PLoS One. 2015;10:e0121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dutton JL, Li B, Woo wP, Marshak JO, Xu Y, Huang ML, et al. A novel DNA vaccine technology conveying protection against a lethal herpes simplex viral challenge in mice. PLoS One. 2013;8:e76407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Awasthi S, Lubinski JM, Shaw CE, Barrett SM, Cai M, Wang F, et al. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J Virol. 2011;85:10472–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Geltz JJ, Gershburg E, Halford WP. Herpes simplex virus 2 (HSV-2) infected cell proteins are among the most dominant antigens of a live-attenuated HSV-2 vaccine. PLoS One. 2015;10:e0116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Diaz F, Gregory S, Nakashima H, Viapiano MS, Knipe DM. Intramuscular delivery of replication-defective herpes simplex virus gives antigen expression in muscle syncytia and improved protection against pathogenic HSV-2 strains. Virology. 2018;513:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wang JP, Bowen GN, Zhou S, Cerny A, Zacharia A, Knipe DM, et al. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang X, Li Y, Liu S, Yu X, Li L, Shi C, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Halford WP, Geltz J, Messer RJ, Hasenkrug KJ. Antibodies Are Required for Complete Vaccine-Induced Protection against Herpes Simplex Virus 2. PLoS One. 2015;10:e0145228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gardner JK, Swaims-Kohlmeier A, Herbst-Kralovetz MM. IL-36gamma Is a Key Regulator of Neutrophil Infiltration in the Vaginal Microenvironment and Limits Neuroinvasion in Genital HSV-2 Infection. J Immunol. 2019;203:2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Quispe Calla NE, Vicetti Miguel RD, Boyaka PN, Hall-Stoodley L, Kaur B, Trout W, et al. Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection. Mucosal immunology. 2016;9:1571–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gillgrass AE, Ashkar AA, Awasthi S, Huang J, Shaw Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Awasthi S, Huang J, Shaw C,Friedman HM. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol. 2014;88:8421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, et al. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Awasthi S, Hook LM, Pardi N, Wang F, Myles A, Cancro MP, et al. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci Immunol. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kollias CM, Huneke RB, Wigdahl B, Jennings SR. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol. 2015;21:8–23. [DOI] [PubMed] [Google Scholar]
- [60].Gebhardt BM, Halford WP. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J. 2005;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sawtell NM, Thompson RL. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC Jr., Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. The Journal of infectious diseases. 1982;146:397–404. [DOI] [PubMed] [Google Scholar]
- [63].Bernstein DI, Cardin RD, Pullum DA, Bravo FJ, Kousoulas KG, Dixon DA. Duration of protection from live attenuated vs. sub unit HSV-2 vaccines in the guinea pig model of genital herpes: Reassessing efficacy using endpoints from clinical trials. PLoS One. 2019;14:e0213401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hensel MT, Marshall JD, Dorwart MR, Heeke DS, Rao E, Tummala P, et al. Prophylactic Herpes Simplex Virus 2 (HSV-2) Vaccines Adjuvanted with Stable Emulsion and Toll-Like Receptor 9 Agonist Induce a Robust HSV-2-Specific Cell-Mediated Immune Response, Protect against Symptomatic Disease, and Reduce the Latent Viral Reservoir. J Virol. 2017;91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bernstein DI, Cardin RD, Bravo FJ, Awasthi S, Lu P, Pullum DA, et al. Successful application of prime and pull strategy for a therapeutic HSV vaccine. NPJ Vaccines. 2019;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Awasthi S, Hook LM, Shaw CE, Pahar B, Stagray JA, Liu D, et al. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog. 2017;13:e1006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lo M, Zhu J, Hansen SG, Carroll T, Farr Zuend C, Noel-Romas L, et al. Acute Infection and Subsequent Subclinical Reactivation of Herpes Simplex Virus 2 after Vaginal Inoculation of Rhesus Macaques. J Virol. 2019;93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Aravantinou M, Frank I, Arrode-Bruses G, Szpara M, Grasperge B, Blanchard J, et al. A model of genital herpes simplex virus Type 1 infection in Rhesus Macaques. J Med Primatol. 2017;46:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, et al. A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PloS one. 2009;4:e8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang J, Zhao J, Xu S, Li J, He S, Zeng Y, et al. Species-Specific Deamidation of cGAS by Herpes Simplex Virus UL37 Protein Facilitates Viral Replication. Cell host & microbe. 2018;24:234–48 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bernstein DI, Ashley RL, Stanberry LR, Myers MG. Detection of asymptomatic initial herpes simplex virus (HSV) infections in animals immunized with subunit HSV glycoprotein vaccines. J Clin Microbiol. 1990;28:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bourne N, Banasik BN, Perry CL, Miller AL, White M, Pyles RB, et al. Development of disease and immunity at the genital epithelium following intrarectal inoculation of male guinea pigs with herpes simplex virus type 2. Virology. 2018;526:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stephanopoulos DE, Myers MG, Bernstein DI. Genital infections due to herpes simplex virus type 2 in male guinea pigs. The Journal of infectious diseases. 1989;159:89–95. [DOI] [PubMed] [Google Scholar]
- [74].Iwasaki A Exploiting Mucosal Immunity for Antiviral Vaccines. Annual review of immunology. 2016;34:575–608. [DOI] [PubMed] [Google Scholar]
- [75].Plotkin SA. Updates on immunologic correlates of vaccine-induced protection. Vaccine. 2019. [DOI] [PubMed] [Google Scholar]
- [76].Burn C, Ramsey N, Garforth SJ, Almo S, Jacobs WR Jr., Herold BC. An HSV-2 single-cycle candidate vaccine deleted in glycoprotein D, DeltagD-2, protects male mice from lethal skin challenge with clinical isolates of HSV-1 and HSV-2. J Infect Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sullender WM, Miller JL, Yasukawa LL, Bradley JS, Black SB, Yeager AS, et al. Humoral and cell-mediated immunity in neonates with herpes simplex virus infection. The Journal of infectious diseases. 1987;155:28–37. [DOI] [PubMed] [Google Scholar]
- [78].Prober CG, Sullender WM, Yasukawa LL, Au DS, Yeager AS, Arvin AM. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. The New England journal of medicine. 1987;316:240–4. [DOI] [PubMed] [Google Scholar]
- [79].Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis. 1989;160:770–6. [DOI] [PubMed] [Google Scholar]
- [80].Hook LM, Cairns TM, Awasthi S, Brooks BD, Ditto NT, Eisenberg RJ, et al. Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs. PLoS Pathog. 2018;14:e1007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chu CF, Meador MG, Young CG, Strasser JE, Bourne N, Milligan GN. Antibody-mediated protection against genital herpes simplex virus type 2 disease in mice by Fc gamma receptor-dependent and -independent mechanisms. J Reprod Immunol. 2008;78:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, et al. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol. 2012;86:1563–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cairns TM, Ditto NT, Lou H, Brooks BD, Atanasiu D, Eisenberg RJ, et al. Global sensing of the antigenic structure of herpes simplex virus gD using high-throughput array-based SPR imaging. PLoS Pathog. 2017;13:e1006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Whitbeck JC, Huang ZY, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, et al. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus type 2 glycoprotein D. J Virol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Petro CD, Weinrick B, Khajoueinejad N, Burn C, Sellers R, Jacobs WR, Jr., et al. HSV-2 DeltagD elicits FcgammaR-effector antibodies that protect against clinical isolates. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kao CM, Goymer J, Loh LN, Mahant A, Burn Aschner C, Herold BC. Murine Model of Maternal Immunization Demonstrates Protective Role for Antibodies that Mediate Antibody Dependent Cellular Cytotoxicity in Protecting Neonates from Herpes Simplex Virus Type 1 and Type 2. J Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. Herpes virus fusion and entry: a story with many characters. Viruses. 2012;4:800–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Friedman HM. Immunologic strategies for herpes vaccination. Jama. 2000;283:746–7. [DOI] [PubMed] [Google Scholar]
- [89].Awasthi S, Hook LM, Swaminathan G, Cairns TM, Brooks B, Smith JS, et al. Antibody responses to crucial functional epitopes as a novel approach to assess immunogenicity of vaccine adjuvants. Vaccine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wee JL, Scheerlinck JP, Snibson KJ, Edwards S, Pearse M, Quinn C, et al. Pulmonary delivery of ISCOMATRIX influenza vaccine induces both systemic and mucosal immunity with antigen dose sparing. Mucosal immunology. 2008;1:489–96. [DOI] [PubMed] [Google Scholar]
- [92].McKee AS, MacLeod MK, Kappler JW, Marrack P. Immune mechanisms of protection: can adjuvants rise to the challenge? BMC biology. 2010;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Weissman D, Kariko K. mRNA: Fulfilling the Promise of Gene Therapy. Mol Ther. 2015;23:1416–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Weissman D, Pardi N, Muramatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Methods Mol Biol. 2013;969:43–54. [DOI] [PubMed] [Google Scholar]
- [98].Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nature communications. 2018;9:3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511–20. [DOI] [PubMed] [Google Scholar]
- [101].Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol Ther. 2017;25:1316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell. 2017;170:273–83 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017. [DOI] [PubMed] [Google Scholar]
- [104].Feldman RA, Fuhr R, Smolenov I, Mick Ribeiro A, Panther L, Watson M, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326– 34. [DOI] [PubMed] [Google Scholar]
- [105].Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Crotty S A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Havenar-Daughton C, Lee JH, Crotty S. Tfh cells and HIV bnAbs, an immunodominance model of the HIV neutralizing antibody generation problem. Immunol Rev. 2017;275:49–61. [DOI] [PubMed] [Google Scholar]
- [108].Hoshino Y, Pesnicak L, Dowdell KC, Burbelo PD, Knipe DM, Straus SE, et al. Protection from herpes simplex virus (HSV)-2 infection with replication-defective HSV-2 or glycoprotein D2 vaccines in HSV-1-seropositive and HSV-1-seronegative guinea pigs. J Infect Dis. 2009;200:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of Immunization with Glycoprotein D2/AS04 on Herpes Simplex Virus Type 2 Shedding into the Genital Tract in Guinea Pigs That Become Infected. J Infect Dis. 2005;192:2117–23. [DOI] [PubMed] [Google Scholar]
- [110].Dasgupta G, Benmohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Awasthi S, Hook LM, Shaw CE, Friedman HM. A trivalent subunit antigen glycoprotein vaccine as immunotherapy for genital herpes in the guinea pig genital infection model. Hum Vaccin Immunother. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, et al. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. The Journal of infectious diseases. 2003;187:542–9. [DOI] [PubMed] [Google Scholar]
- [113].Delagrave S, Hernandez H, Zhou C, Hamberger JF, Mundle ST, Catalan J, et al. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One. 2012;7:e46714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bernstein DI, Pullum DA, Cardin RD, Bravo FJ, Dixon DA, Kousoulas KG. The HSV-1 live attenuated VC2 vaccine provides protection against HSV-2 genital infection in the guinea pig model of genital herpes. Vaccine. 2019;37:61–8. [DOI] [PubMed] [Google Scholar]
- [115].Visalli RJ, Natuk RJ, Kowalski J, Guo M, Blakeney S, Gangolli S, et al. Vaccination with a HSV-2 UL24 mutant induces a protective immune response in murine and guinea pig vaginal infection models. Vaccine. 2014;32:1398–406. [DOI] [PubMed] [Google Scholar]
- [116].Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One. 2011;6:e17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Morello CS, Kraynyak KA, Levinson MS, Chen Z, Lee KF, Spector DH. Inactivated HSV-2 in MPL/alum adjuvant provides nearly complete protection against genital infection and shedding following long term challenge and rechallenge. Vaccine. 2012;30:6541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bernstein DI, Cardin RD, Bravo FJ, Hamouda T, Pullum DA, Cohen G, et al. Intranasal nanoemulsion-adjuvanted HSV-2 subunit vaccine is effective as a prophylactic and therapeutic vaccine using the guinea pig model of genital herpes. Vaccine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yan Y, Hu K, Deng X, Guan X, Luo S, Tong L, et al. Immunization with HSV-2 gB-CCL19 Fusion Constructs Protects Mice against Lethal Vaginal Challenge. J Immunol. 2015;195:329–38. [DOI] [PubMed] [Google Scholar]


