Abstract
The vitamin A metabolite, retinoic acid, is an important signaling molecule during embryonic development serving critical roles in morphogenesis, organ patterning and skeletal and neural development. Retinoic acid is also important in postnatal life in the maintenance of tissue homeostasis, while retinoid-based therapies have long been used in the treatment of a variety of cancers and skin disorders. As the number of people living with chronic disorders continues to increase, there is great interest in extending the use of retinoid therapies in promoting the maintenance and repair of adult tissues. However, there are still many conflicting results as we struggle to understand the role of retinoic acid in the multitude of processes that contribute to tissue injury and repair. This review will assess our current knowledge of the role retinoic acid signaling in the development of fibroblasts, and their transformation to myofibroblasts, and of the potential use of retinoid therapies in the treatment of organ fibrosis.
Keywords: fibroblast, fibrosis, regeneration, retinoic acid, vitamin A
Introduction
Tissue injury triggers the process of wound repair which leads to the proliferation and activation of inflammatory and fibrogenic effector cells and the remodelling of the extracellular matrix (ECM). A successful repair process regenerates and restores the tissue to its original form and function. However, the process of wound healing often results in the deposition of excess amounts of ECM proteins, which cause scarring, increased tissue stiffness, and decreased elasticity. Increased tissue stiffness causes mechanical stress and promotes the activation of fibroblasts and myofibroblasts, thus perpetuating the process of fibrosis (Herrera, Henke, & Bitterman, 2018; Wells, 2013). Organ fibrosis is a hallmark and final outcome of many chronic disorders such as hypertrophic cardiomyopathy, heart failure, cirrhosis, and pulmonary and renal fibrosis (Ho et al., 2010; Rockey, Bell, & Hill, 2015; Wynn & Ramalingam, 2012).
Fibroblasts perform vital roles in multicellular organisms. Known for their ability to generate and remodel the ECM, fibroblasts are responsible for the stiffness and stromal architecture of tissues and organs. Fibroblasts also interact with parenchymal cells by secreting and responding to chemokines and growth factors. Tissue resident fibroblasts play important roles in the pathophysiology of wound repair and organ fibrosis. Fibroblasts can transform into myofibroblasts, which are the main fibrogenic effector cells during fibrosis. However, fibrogenic cells can also be derived via transdifferentiation of other cell types such as perivascular, epithelial, mesenchymal, and bone marrow cells. A better understanding of the cellular origins and development of fibroblasts, and of the signaling pathways that control their activity could lead to therapies to help reduce, or perhaps even reverse fibrosis.
The role of vitamin A in connective tissue function and in promoting the formation and turnover of the ECM has been appreciated since early days following its discovery (Wolbach & Howe, 1925). This wound-healing effect of vitamin A-based therapies is important in the treatment of various skin or epithelial disorders (Comptour et al., 2016; Fisher et al., 1996; Fisher & Voorhees, 1996; Griffiths et al., 1993). Vitamin A also influences fibroblast specification and differentiation and may also play a potentially important role in tissue repair and regeneration (Gudas, 2012; Maden, 2007). Alterations in vitamin A metabolism and/or signaling are frequently seen during the process of fibroblast activation in various organs. While some evidence suggests an anti-fibrotic effect of vitamin A in fibrotic conditions, there are also reports of profibrotic effects of vitamin A, as well as observations that changes in vitamin A metabolism or signaling are a consequence of fibroblast (or stellate cell) activation.
Vitamin A Uptake and Metabolism
Vitamin A is essential for a multitude of physiological processes in both fetal and postnatal life. The two main bioactive metabolites of vitamin A are all-trans-retinoic acid (atRA), which is a ligand of nuclear hormone receptors, and 11-cis-retinaldehyde, the photosensitive chromophore of rod and cone opsins required for vision (Dowling & Wald, 1960; Karrer, Morf, & Schöpp, 1931; Wald, 1933). Other pathways which will not be discussed here, lead to the formation of 13,14-dihydroretinoids or to retro-retinoids whose roles are currently less well understood (Buck et al., 1993; Derguini et al., 1995; Moise et al., 2008; Moise, Kuksa, Imanishi, & Palczewski, 2004). The term retinoid refers to either an endogenous vitamin A metabolite, or a synthetic analog that exhibits “vitamin A activity”, which initially concerned the ability to activate the retinoic acid receptor (RAR), but has more recently also included its function as a visual chromophore (Sporn, Roberts, & Goodman, 1994; Travis, Golczak, Moise, & Palczewski, 2007). AtRA is essential to ensure proper embryonic development and a multitude of physiological processes which include reproduction, nervous system function, immune response, cell proliferation, differentiation, and apoptosis (Clagett-Dame & Knutson, 2011; Cunningham & Duester, 2015; Rhinn & Dolle, 2012; Ross, 2012). To explore the role of atRA in cell differentiation, we will first review the mechanisms by which atRA signals within target cells.
The activity of atRA is mediated through two subfamilies of nuclear receptors, RAR and its heterodimeric partner retinoid X receptor (RXR) (Giguere, Ong, Segui, & Evans, 1987; Kastner et al., 1997; Kliewer, Umesono, Mangelsdorf, & Evans, 1992; Mangelsdorf, Ong, Dyck, & Evans, 1990; Petkovich, Brand, Krust, & Chambon, 1987). AtRA only binds RAR with high affinity (low nM), however, 9-cis-retinoic acid (9cRA) displays high affinity for both RXR as well as RAR (Heyman et al., 1992; A. A. Levin et al., 1992). However, atRA is present in most fetal and postnatal tissues at various stages and levels, whereas 9cRA has a much more limited presence (Jones, Pierzchalski, Yu, & Kane, 2015; Kane, 2012; Kane, Chen, Sparks, & Napoli, 2005; Kane et al., 2010; Kane, Folias, Wang, & Napoli, 2008; Kane & Napoli, 2010). RAR and RXR are each encoded by three related genes (α, β, or γ). The resulting three different subtypes of RAR, or RXR, activate both overlapping as well as distinct sets of genes, and display significant redundancy in vivo (Ghyselinck et al., 1997; Lohnes et al., 1993; Lohnes et al., 1994; Lufkin et al., 1993). Unliganded RAR/RXR heterodimers are bound to retinoic acid response elements (RARE) found within enhancer regions of target genes. Ligand binding induces specific conformational changes within RAR/RXR to allow the dissociation of repressor complexes and the association of co-activator complexes (Chandra et al., 2017). Other atRA-signaling modes which have been proposed include both non-genomic signaling of atRA-RAR, as well as atRA-signaling through alternate nuclear hormone receptors (N. Chen & Napoli, 2008; N. Chen, Onisko, & Napoli, 2008; Kruse et al., 2008; Shaw, Elholm, & Noy, 2003; X. E. Zhou et al., 2011).
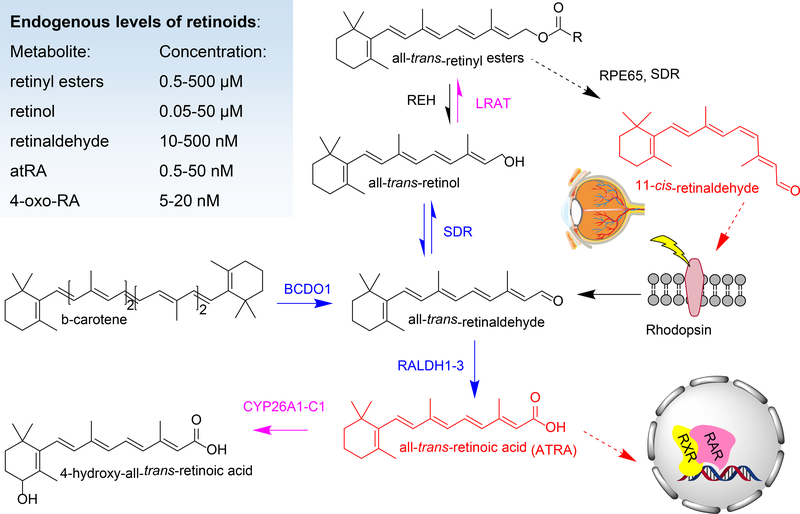
RAR/RXR signaling is governed by the availability of atRA ligand. We will briefly discuss the pathways that lead to the formation and breakdown of atRA and refer the reader to more in-depth recent reviews (Figure 1) (Napoli, 2017; Shannon, Moise, & Trainor, 2017). In brief, atRA is formed from dietary precursors consisting of preformed vitamin A (all-trans-retinol, or retinyl esters - REs) and provitamin A carotenoids defined as having at least one unsubstituted β-ionone ring. Dietary REs are hydrolyzed in the intestinal lumen and then re-esterified mainly by lecithin:retinol acyltransferase (LRAT), and to a lesser extent by enzymes with acyl CoA:retinol acyltransferase activity (Ables et al., 2012; Batten et al., 2004; L. Liu & Gudas, 2005; O’Byrne et al., 2005; Ong, Kakkad, & MacDonald, 1987; Ruiz et al., 1999; Wongsiriroj et al., 2008; Yen, Monetti, Burri, & Farese, 2005). Newly formed REs are then packaged in chylomicrons and secreted into the lymphatic system for distribution to peripheral sites such as adipose tissue where a percentage of REs are hydrolyzed and taken up via lipoprotein lipase (Blaner et al., 1994; van Bennekum et al., 1999). The bulk of REs remain associated with chylomicron remnants to be cleared by the liver and ultimately stored in hepatic stellate cells (HSCs) (Blaner et al., 2016).
Figure 1.
Schematic representation of vitamin A metabolism leading to the formation of bioactive metabolites indicated in red, namely, all-trans-retinoic acid, a ligand for RAR/RXR nuclear hormone receptors involved in gene transcription, and 11-cis-retinaldehyde, the visual chromophore which binds rod and cone opsins. The dietary sources of vitamin A are either preformed retinol and retinyl esters or provitamin A precursors, such as all-trans-β-carotene. Enzymes involved in retinoid metabolism include: beta-carotene dioxygenase 1 (BCDO1), cytochrome P450 family 26 subfamily A1, B1 or C1 (CYP26A1-C1), lecithin:retinol acyltransferase (LRAT), retinaldehyde dehydrogenases (RALDH), retinyl ester hydrolases (REH), retinal pigment epithelium-specific 65 kDa protein (RPE65), short chain dehydrogenase reductases (SDR) such as DHRS3 and RDH10. The endogenous serum or tissue levels of various retinoid metabolites are shown inset (Arnold, Amory, Walsh, & Isoherranen, 2012; Kane & Napoli, 2010).
Most of the absorbed provitamin A carotenoids are converted to retinol in the intestine and then follow the same fate as retinoids derived from preformed vitamin A. Intestinal absorption of provitamin A carotenoids requires the scavenger receptor B type 1 (SCARB1) (Kiefer, Sumser, Wernet, & Von Lintig, 2002; Toomey et al., 2017; Voolstra et al., 2006). The enzyme beta-carotene dioxygenase 1 (BCO1) coverts both β-carotene and β-apo-10′-carotenal to all-trans-retinaldehyde via oxidative cleavage (Hessel et al., 2007; Kiefer et al., 2001; Lampert et al., 2003; von Lintig, Dreher, Kiefer, Wernet, & Vogt, 2001; von Lintig & Vogt, 2000; von Lintig & Wyss, 2001). Asymmetric cleavage via beta-carotene dioxygenase 2 (BCO2) permits the utilization of asymmetric provitamin A carotenoids for atRA production (Kelly et al., 2018; Kiefer et al., 2001). Intestinal absorption and conversion of provitamin A carotenoids is under tight regulation via feedback mechanisms. AtRA produced in enterocytes activates RAR/RXR which upregulates the expression of the intestinal-specific homeobox domain transcription factor (ISX). In turn, ISX suppresses the expression of Bco1 and Scarb1, thereby restricting the absorption and conversion of provitamin carotenoids to vitamin A (Bachmann et al., 2002; Lobo et al., 2013; Lobo et al., 2010; Seino et al., 2008; M. A. Widjaja-Adhi, Lobo, Golczak, & Von Lintig, 2015; M. A. K. Widjaja-Adhi et al., 2017). A non-negligible amount of provitamin A carotenoids, however, enter the circulation and are delivered and stored in an uncleaved form in liver, fat and other organs. These carotenoids can be cleaved to retinal to sustain retinol or atRA production during both adult as well as fetal life (Y. K. Kim et al., 2011; Lampert et al., 2003; Mora et al., 2004; I. Shmarakov et al., 2010).
Uptake of retinol by intestinal and liver cells is facilitated by a cross membrane gradient established through the sequestration of retinol by intracellular retinol binding proteins and the esterification of retinol by LRAT. Intracellular retinol binding proteins also shield retinol (and retinal) from non-specific reactions (Boerman & Napoli, 1991; Lapshina, Belyaeva, Chumakova, & Kedishvili, 2003). Cellular retinol binding protein 1 (CRBP1; HUGO gene nomenclature committee (HGNC) approved symbol:RBP1) has been shown to be important in regulating the uptake, storage and metabolism of vitamin A in most tissues other than the intestine (Ghyselinck et al., 1999; W. Jiang & Napoli, 2012; Kane, Bright, & Napoli, 2011; Kane, Folias, et al., 2011; Matt et al., 2005; Pierzchalski et al., 2014; Pierzchalski, Yu, Norman, & Kane, 2013). Meanwhile, CRBP2 (HGNC:RBP2) is essential for vitamin A uptake and homeostasis in the intestine, and CRBP3 (HGNC: RBP5) has been reported to have functions in mammary metabolism of vitamin A and incorporation of REs in milk (E et al., 2002; Herr et al., 1993; Kakkad & Ong, 1988; M. S. Levin & Davis, 1997; McDonald et al., 2012; Piantedosi, Ghyselinck, Blaner, & Vogel, 2005; Vogel et al., 2001; Zizola, Schwartz, & Vogel, 2008). Unbound CRBP1 has been reported to have an additional regulatory influence on atRA homeostasis through inhibition and stimulation of specific enzymes in order to control flux through the vitamin A pathway (Boerman & Napoli, 1991; Herr & Ong, 1992; Lapshina et al., 2003). The enzymes, transporters and binding proteins responsible for the uptake, storage and conversion of retinol to atRA are regulated by vitamin A status and/or by retinoid signaling (Bouillet et al., 1995; Bouillet et al., 1997; Mangelsdorf et al., 1991; Sapin et al., 2000; Taneja et al., 1995; L. Wu & Ross, 2010; Zolfaghari & Ross, 2002).
Storage forms of vitamin A consist of retinyl esters found primarily in HSCs, and also in adipose tissue, lung, retinal pigmented epithelial cells and kidney. These stores are hydrolyzed to generate retinol to meet tissue demands. Retinol is secreted by hepatocytes bound to serum RBP4 which forms a complex with transthyretin (Kanai, Raz, & Goodman, 1968). RBP4 binds to its receptor, stimulated by retinoic acid 6 (STRA6), a membrane protein which mediates both the import and export of retinol from cells (Amengual et al., 2014; Y. Chen et al., 2016; Isken et al., 2008; Kawaguchi et al., 2007; Kawaguchi, Zhong, Kassai, Ter-Stepanian, & Sun, 2012; Muenzner et al., 2013). STRA6 is expressed in many important retinoid target tissues such as eye, choroid plexus, heart, and placenta but not by the liver which expresses an alternate RBP4-receptor (Alapatt et al., 2013).
Synthesis of atRA from retinol begins with the reversible oxidation of retinol to retinaldehyde mediated primarily by NAD(P) -dependent microsomal, short-chain retinol dehydrogenases/ reductases (SDR). Given the abundance of the NAD(P) dinucleotide cofactors under normal cellular conditions, SDR enzymes with NAD-specificity catalyze oxidation of retinol, while NADP-dependent enzymes catalyze reduction of retinaldehyde. SDR oxidoreductases are part of one of the largest superfamily of enzymes known, with over 70 members in the human genome, and are responsible for transformation of many endogenous and exogenous compounds (Persson et al., 2009). In vitro, many SDR enzymes show activity with retinoids, but relatively few have been shown to significantly affect retinoid metabolism or signaling when ablated in mice (Kedishvili, 2016; Parker & Crouch, 2010). The NAD-dependent retinol dehydrogenase 10 (RDH10) is the main enzyme responsible for the oxidation of retinol during development (Cunningham, Chatzi, Sandell, Trainor, & Duester, 2011; Rhinn, Schuhbaur, Niederreither, & Dolle, 2011; Sandell, Lynn, Inman, McDowell, & Trainor, 2012; Sandell et al., 2007). The NADP-dependent enzyme dehydrogenase/ reductase 3 is primarily responsible for the reduction of retinaldehyde to prevent excess formation of atRA (Billings et al., 2013; Feng, Hernandez, Waxman, Yelon, & Moens, 2010; Haeseleer, Huang, Lebioda, Saari, & Palczewski, 1998; Kam et al., 2013). Interestingly, RDH10 and DHRS3 form a functional heterodimer which by carrying out coupled antagonistic activities ensures atRA homeostasis (Adams, Belyaeva, Wu, & Kedishvili, 2014; Belyaeva, Adams, Wu, & Kedishvili, 2017). In addition to RDH10 and DHRS3, other SDRs acting on retinoids have been shown to affect retinoid metabolism in vivo in postnatal visual and non-visual tissues (Kedishvili, 2016; Parker & Crouch, 2010). It is also possible that other enzyme families such as medium-chain dehydrogenases and aldo-keto reductases (AKR) participate in retinol oxidation or retinal reduction, respectively, under some circumstances (Kumar, Sandell, Trainor, Koentgen, & Duester, 2012; Porte et al., 2013).
All-trans-retinaldehyde undergoes irreversible conversion to atRA by means of cytosolic retinal dehydrogenases (RALDH) enzymes, RALDH1, 2, and 3 of the aldehyde dehydrogenase 1A (ALDH1A) family. RALDH enzymes have distinct expression patterns and, consequently, have tissue specific roles in atRA production. RALDH2 (ALDH1A2) is the main enzyme responsible for atRA synthesis during early embryogenesis, in the fetal heart and liver, in the immune system and most tissues; meanwhile RALDH3 (ALDH1A3) is expressed in developing sensory systems (Dupe et al., 2003; Mic, Molotkov, Fan, Cuenca, & Duester, 2000; Niederreither, Subbarayan, Dolle, & Chambon, 1999; Paschaki et al., 2013; Romand et al., 2006). RALDH1, on the other hand, appears to play a role in testes and adipose tissue, however, the latter role may not be contingent on atRA synthesis (Arnold et al., 2015; Yang et al., 2017). Independently of ALDH1A enzymes, the cytochrome P450 enzyme CYP1B1 was also proposed to contribute to atRA production (Chambers, Wilson, Maden, & Lumsden, 2007; F. Li, Zhu, & Gonzalez, 2017; Maguire, Larsen, Foong, Tanumihardjo, & Jefcoate, 2017).
Retinol or atRA are catabolized mainly by a number of cytochrome (Cyp) P450 enzymes found in microsomal membranes (S. S. Abu-Abed et al., 1998; MacLean et al., 2001; Taimi et al., 2004; Topletz, Zhong, & Isoherranen, 2019; J. A. White et al., 1997; J. A. White et al., 1996; J. A. White et al., 2000). Within cells, atRA is found bound to cellular retinoic acid binding proteins, CRABP1 or CRABP2 (Napoli, 2017). CRABPs play important roles in modulating atRA signaling by channelling atRA towards P450 enzymes for oxidation, or towards RAR for signaling (Cai et al., 2012; Delva et al., 1999; Fiorella & Napoli, 1994; Nelson et al., 2016; Zhong, Ortiz, Zelter, Nath, & Isoherranen, 2018). Oxidized atRA metabolites, such as 4-oxo-atRA, do not appear to play a role in activating RAR during embryonic development, but it is possible that they carry out signaling roles in other settings (Niederreither et al., 2002; Pijnappel et al., 1993; Topletz et al., 2015). Through their distinct expression profiles, P450 enzymes of CYP26 family, namely CYP26A1, B1 and C1 control signaling by atRA in various tissues (S. Abu-Abed et al., 2002; Pennimpede et al., 2010; Reijntjes, Gale, & Maden, 2004; Tahayato, Dolle, & Petkovich, 2003). CYP26 enzymes also display regio- and stereospecific preference for atRA oxidation. CYP26A1 and B1 carry out initial oxidation of atRA, while CYP26C1 carries out secondary oxidation reactions (Topletz et al., 2012; Zhong et al., 2018). In vivo, ablation of CYP26A1 or CYP26B1 leads to embryonic lethality, meanwhile, CYP26C1-deficient mice are viable (S. Abu-Abed, 2001; Uehara et al., 2007; Yashiro et al., 2004). Other P450 enzymes contribute to atRA metabolism including CYP3A7 which is expressed in the fetal liver (Shimshoni et al., 2012; Topletz et al., 2019). AtRA metabolism is extensively autoregulated to ensure its homeostasis. Enzymes involved in atRA synthesis, such as RDH10 and RALDH2 are downregulated by atRA, while those whose actions result in reduction in the levels of atRA, such as CYP26A1 and DHRS3, are upregulated by atRA (Loudig et al., 2000; Sandell et al., 2012; Strate, Min, Iliev, & Pera, 2009; J. A. White et al., 1997; R. J. White, Nie, Lander, & Schilling, 2007).
Role of AtRA in the Development and Function of Cardiac Fibroblasts
Cardiovascular disease including ischemic (coronary) heart disease, cerebrovascular disease (stroke), and hypertension affects 11.5% of American adults and is the leading cause of death (Benjamin et al., 2018). Despite gains in the prevention and survival following a myocardial infarct in the last few decades, there is increased prevalence of chronic heart conditions such as heart failure in our aging population. This trend is projected to increase by 46% from 2012 to 2030 resulting in >8 million Americans living with heart failure (Heidenreich et al., 2013). Excess deposition of ECM by cardiac fibroblasts is a prominent feature of heart failure. Given that treatment options for heart failure are still limited, it is important to explore the factors that control the formation and function of cardiac fibroblasts as potential therapeutic targets in heart disease.
As the first organ to form, the embryonic heart is required to accommodate the increasing needs of the embryo. The cardiac fibroblast, one of the predominant cardiac cell populations, carries out essential roles in the development and function of the vertebrate heart. Cardiac fibroblasts ensure that the heart can meet these demands throughout the life of an organism by altering the cardiac ECM in response to changes in the mechanical load placed upon the heart. To further support heart function, fibroblasts promote cardiomyocyte proliferation (Ieda et al., 2009), reinforce the coronary vessels and restrict electrical conduction to allow for the sequential contraction of atria and ventricles (Gourdie, Dimmeler, & Kohl, 2016). Following injury, resident cardiac fibroblasts become activated (myofibroblasts) and protect the heart by creating a scar. However, this process can also lead to the increased stiffness, and reduced compliance associated with heart disease (Kanisicak et al., 2016; van Putten, Shafieyan, & Hinz, 2016). Despite their significant and multifaceted roles in heart biology, we still know very little regarding the mechanisms that guide the embryonic development of cardiac fibroblasts. We review recent studies that implicate atRA as an important signaling molecule in the development of cardiac fibroblasts.
The majority of adult cardiac fibroblasts and myofibroblasts are believed to be derived from the embryonic epicardium. The developmental origin of cardiac fibroblasts was demonstrated using dye- and retroviral labeling of the proepicardium in chicken embryos and further supported by the usage of lineage-tracing of epicardial-derived cells in transgenic mouse models (Mikawa & Fischman, 1992; Moore-Morris et al., 2018; Tallquist & Molkentin, 2017). The embryonic epicardium originates from the proepicardium, which forms transiently as an outgrowth of the septum transversum. Proepicardial cells migrate to envelope the heart and thereby establish the visceral pericardial layer, commonly referred to as the epicardium. Proepicardial cells also find their way into the mesenchymal subepicardial space. In response to various stimuli, epicardial cells and subepicardial cells undergo epithelial-to-mesenchymal transition (EMT), migrate and colonize the myocardium where some differentiate into interstitial fibroblasts, while others respond to endothelial-derived signals and transform into mural cells and perivascular fibroblasts (Sharma, Chang, & Red-Horse, 2017). Several factors have been identified as critical for epicardial EMT, including neurofibromin 1 (Nf1), Wilms tumour 1 (Wt1), transforming growth factor-β (TGF-β), platelet-derived growth factor receptor (PDGFR), as well as atRA (Baek & Tallquist, 2012; Mellgren et al., 2008; Smith, Baek, Sung, & Tallquist, 2011; von Gise & Pu, 2012; S. Wang, W. Huang, et al., 2018; S. Wang, J. Yu, et al., 2018).
AtRA-signaling plays critical roles during early heart development (Stefanovic & Zaffran, 2017; Xavier-Neto et al., 2015). Recently several lines of evidence suggest that atRA-signaling also plays a critical role in the regulation of epicardial development during late gestation. The embryonic epicardium expresses atRA metabolic enzymes, binding proteins and receptors such as Rbp1, Rdh10, Raldh2 (Aldh1a2), Cyp26a1, Dhrs3, Rara, Rarb, Rarg, Rxra, and Stra6, and carries out active atRA-signaling (Brade et al., 2011; Guadix et al., 2011; Moss et al., 1998; Perez-Pomares et al., 2002; S. Wang, W. Huang, et al., 2018; S. Wang, J. Yu, et al., 2018; Xavier-Neto, Shapiro, Houghton, & Rosenthal, 2000). In mouse embryos excess or deficiency of atRA during late gestation, Rxra-deficiency or the expression of dominant-negative form of RAR caused similar defects in the development of the coronary vasculature and/or the growth of the myocardial compact zone (Gruber et al., 1996; Lin et al., 2010; Merki et al., 2005; Shen et al., 2015; Sucov et al., 1994; S. Wang, W. Huang, et al., 2018). It has recently been demonstrated that epicardial atRA-signaling promotes cytoskeletal rearrangement, EMT and migration of epicardial cells and we identified the Ras homolog gene family, member A (RhoA)-signaling pathway to be required for the atRA-induced cytoskeletal remodeling of epicardial cells (Guadix et al., 2011; von Gise et al., 2011; S. Wang, W. Huang, et al., 2018; S. Wang, J. Yu, et al., 2018).
In addition to epicardial EMT, atRA also controls the differentiation of epicardial-derived precursor cells (EPDCs) into vascular smooth muscle cells and fibroblasts (Azambuja et al., 2010; Braitsch, Combs, Quaggin, & Yutzey, 2012; von Gise et al., 2011; S. Wang, W. Huang, et al., 2018; S. Wang, J. Yu, et al., 2018). Having migrated into the myocardium EPDCs differentiate primarily into coronary vascular smooth muscle cells (VSMCs) and fibroblasts with minor contributions to other lineages (Smits, Dronkers, & Goumans, 2018). The embryonic epicardium and the proepicardial organ from which it originates accommodate a heterogeneous population of progenitor cells which follow distinct signaling pathways to achieve various developmental fates (Katz et al., 2012; Plavicki et al., 2014). A subset of proepicardial, epicardial cells and EPDCs express the transcription factor 21 (Tcf21; also known as Pod1 or epicardin), a member of the basic helix-loop-helix (bHLH) family of transcription factors whose expression in the epicardium is induced by atRA (Braitsch et al., 2012; Quaggin, Vanden Heuvel, & Igarashi, 1998; Robb et al., 1998). TCF21 is important in proepicardial specification and cardiac fibroblast development (Acharya et al., 2012; Braitsch et al., 2012; Kanisicak et al., 2016; Tandon, Miteva, Kuchenbrod, Cristea, & Conlon, 2013; Xiang, Fang, & Yutzey, 2017). Like TCF21, atRA suppresses the expression of VSMC markers in cultured chick proepicardium and embryonic hearts (Azambuja et al., 2010; Braitsch et al., 2012). Therefore, atRA acting via TCF21, favors the formation of cardiac fibroblasts and thus plays an important role in the formation and fate specification of EPDCs. In addition to signaling through TCF21, atRA also interacts with fibroblast growth factor (FGF) signaling which regulates multiple aspects of cardiac development including epicardial EMT, and cardiac fibroblast development (Lavine et al., 2005; Pennisi & Mikawa, 2009; Vega-Hernandez, Kovacs, De Langhe, & Ornitz, 2011; S. Wang, W. Huang, et al., 2018).
Though, the epicardium constitutes the major source of ventricular fibroblasts, the endocardium also contributes to a significant percentage of cardiac fibroblasts (reviewed in (Y. Li, Lui, & Zhou, 2018). Similar to the epicardium, endocardial cells undergo endothelial-to-mesenchymal transition (endo-MT) and contribute to the formation of the atrioventricular valves. Using lineage tracing technique, Moore-Morris et al. reported that about 18% of fibroblasts in the myocardium originate from Tie2-positive endocardial population (Moore-Morris et al., 2014). These endocardial-derived fibroblasts primarily populate the interventricular septum. The exact influence of atRA on the formation of endocardial-derived fibroblasts has, so far, not been examined but atRA was previously reported to regulate endo-MT by regulating the expression of Tbx2 and Tgfβ2, both known to be critical morphogens for endo-MT and valve formation (Sakabe, Kokubo, Nakajima, & Saga, 2012).
Extra-cardiac sources, such as the neural crest, also contribute to a small percentage of cardiac fibroblasts. Neural crest is a transient migratory cell population whose derivatives can be found in craniofacial cartilage and bone, muscles and connective tissues of the face and neck, heart and endocrine glands, skin and peripheral nervous system (Mayor & Theveneau, 2013). Following induction in the neural plate, neural crest cells undergo EMT to migrate along specific paths to colonize target organs in response to guidance molecules and morphogens which include atRA. In the heart, cardiac neural crest cells contribute to the septation of the outflow tract, the aortic and pulmonary valves, sympathetic and parasympathetic innervation of the heart, and differentiate into VSMCs and cardiac fibroblasts found in the proximal coronary artery and outflow tract, respectively (Ali et al., 2014; Arima et al., 2012; Odelin et al., 2018). AtRA signaling plays critical roles in fate decision, migration and survival of neural crest cells (Uribe, Hong, & Bronner, 2018). Therefore, it is plausible that atRA may also regulate the formation of neural crest-derived cardiac fibroblasts in vivo.
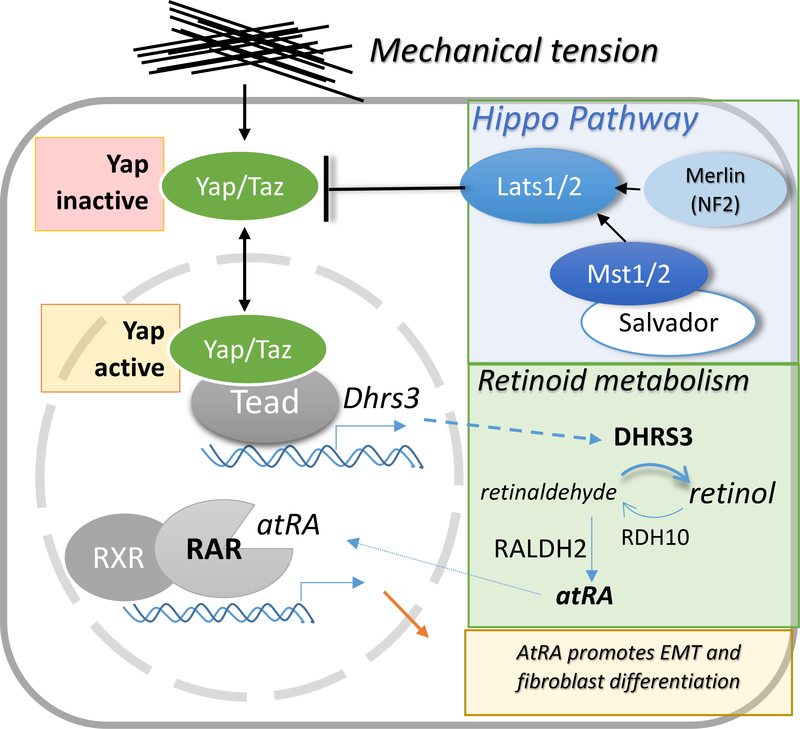
Recently, a potential interaction of atRA- and the Hippo-signaling pathways during the development of cardiac fibroblasts has been revealed in studies by Xiao et al. (Y. Xiao et al., 2018) (depicted in Figure 2).. The evolutionarily conserved Hippo-signaling pathway controls organ size and patterning and plays important roles in heart development and regeneration (J. Wang, Liu, Heallen, & Martin, 2018). When the Hippo-pathway is activated (Hippo-ON), the mammalian STE20-like kinases (Mst1/2) and large tumor suppressor kinases (Lats) 1/2 cascade cause the phosphorylation, cytoplasmic retention, and degradation of the transcriptional regulators Yes-associated protein (Yap) and transcriptional coactivator with PDZ-binding motif (Taz). When the Hippo kinases are not active (Hippo-OFF), nuclear-localized Yap/Taz respond to mechanical tension by binding the transcriptional factors TEA-domain protein (TEAD) to activate gene expression programs associated with apoptosis, growth or differentiation. Mechanical stress is a major inducer of Yap/Taz-signaling and a physiologically relevant stimulus during heart development (Dupont et al., 2011; Majkut, Dingal, & Discher, 2014). In the embryonic epicardium ablation of Yap/Taz results in impaired epicardial EMT (Singh et al., 2016), meanwhile, constitutively active Yap blocks fibroblast differentiation (Y. Xiao et al., 2018). Fibroblast precursors that accumulate in the presence of active Yap express Dhrs3, which is a direct target of TEAD. In light of evidence that atRA promotes fibroblast differentiation (Azambuja et al., 2010; Braitsch et al., 2012), and that Dhrs3 functions as a negative regulator of atRA synthesis (Billings et al., 2013; Feng et al., 2010; Kam et al., 2013), Xiao et al. proposed a model by which active Yap suppresses fibroblast differentiation of EPDCs by attenuating atRA signaling. Since Yap is activated by mechanical stress in epicardial cells (Y. Xiao et al., 2018), these results also imply that mechanical stress modulates atRA-signaling and consequently fibroblast differentiation in EPDCs (Fig. 2). This model provides a mechanistic explanation of how form follows function, whereby mechanical stress guides cell fate decisions that give rise to fibroblasts which shape the ECM of the heart. However, many of the details of the putative interaction of Yap/Taz and atRA-signaling remain to be elucidated.
Figure 2.
Schematic representation of the proposed interaction of the Yap/Taz and atRA-signaling pathways during fibroblast development (adapted based on findings presented by Xiao et al. (Y. Xiao et al., 2018). Yap/Taz transduce mechanical stress and are regulated by the Hippo kinase cascade including Merlin, Salvador, Mst1/2 and Lats1/2 and the upstream regulators Merlin (Mer, or Nf2), and Salvador. Active Yap/Taz inhibits fibroblast differentiation potentially by inducing the expression of Dhrs3, a retinaldehyde reductase that restricts atRA synthesis.
The main role of cardiac fibroblasts being to form ECM and provide structural and mechanical support to the heart, it stands to reason that their development and ECM production is influenced by mechanical tension (Herum, Lunde, McCulloch, & Christensen, 2017; van Putten et al., 2016). Transduction of mechanical signals (mechanotransduction) controls the differentiation of many cell types including VSMCs and fibroblasts (Hinz, Celetta, Tomasek, Gabbiani, & Chaponnier, 2001), and plays a crucial role in both heart development and repair (Majkut et al., 2013; Poelmann & Gittenberger-de Groot, 2018; van Putten et al., 2016). Despite the long-established role of atRA-signaling as a differentiation signal, its potential for involvement in ECM stiffness-dependent lineage specification has only recently come to light. In addition to aforementioned evidence that expression of retinaldehyde reductase Dhrs3 is induced by the mechanoregulator Yap/TEAD (Y. Xiao et al., 2018), it has been shown that mechanical stress regulates the nuclear localization of RARγ via lamin-A (Swift et al., 2013). Reciprocally, atRA regulates the cellular response to mechanical stress by controlling the expression of lamin-A. This interaction is potentially relevant in heart disease since mutations in lamin-A are often associated with dilated cardiomyopathy (Tesson et al., 2014).
The regenerative capacity of heart after injury varies between species. In mammals, the heart is one of the least regenerative organs (Bergmann et al., 2009; Senyo et al., 2013). Cardiac injury such as myocardial infarction (MI) leads to ventricular remodeling, including ventricular hypertrophy, and myofibroblast pool expansion which causes deposition of collagen and scarring, reducing compliance and negatively affecting the conductive and contractile functions of the heart (Ongstad & Gourdie, 2016). However, in some cases, a robust cardiac regenerative capacity can be found. The hearts of zebrafish, and even mouse and pig neonates can regenerate with minimal scarring (Porrello et al., 2011; Poss, Wilson, & Keating, 2002; Zhu et al., 2018) by relying on cardiomyocyte regeneration from pre-existing diploid cardiomyocytes (Gonzalez-Rosa et al., 2018; Y. Li, He, et al., 2018; Patterson et al., 2017; Senyo et al., 2013), and by being sustained through collateral artery growth (Das et al., 2019; Marin-Juez et al., 2016). Based on limited evidence, it seems that the same is true in the case of human newborn hearts which are mitotically active and can regenerate (Haubner et al., 2016; Macmahon, 1937).
The cardiac repair program relies on cell precursors and trophic factors derived from the epicardium. In the healthy adult heart, the epicardium acts as a simple mesothelial layer of the serous pericardium that protects and reduces the friction of the heart (as reviewed in (Blom & Feng, 2018; Cao & Poss, 2018; Limana, Capogrossi, & Germani, 2011; Smits et al., 2018). Heart injury induces the reactivation of epicardial EMT to give rise to VSMCs to help restore vascularization and support heart repair (Braitsch, Kanisicak, van Berlo, Molkentin, & Yutzey, 2013; Kikuchi, Holdway, et al., 2011; Lepilina et al., 2006; Marin-Juez et al., 2016; Smart et al., 2011). Adult EPDCs generated in response to injury also contribute to the formation of new fibroblasts, which can differentiate to myofibroblast and lead to cardiac fibrosis (Duan et al., 2012; Ruiz-Villalba et al., 2015). However, the preponderance of activated myofibroblasts are derived from pre-existing resident cardiac fibroblasts developed from EPDCs during embryogenesis ((Ali et al., 2014; Kanisicak et al., 2016; Moore-Morris et al., 2018; Moore-Morris et al., 2014). Despite earlier reports that the epicardium also contributes to the formation of new cardiomyocytes in the regenerating heart, more recent studies suggest that adult EPDCs are limited to non-myocardial fates (Kikuchi, Gupta, et al., 2011; Y. Li, He, et al., 2018). Injury also induces the secretion of epicardial- and fibroblast-derived mitogens such as IGF2, thymosin-β4, follistatin-like 1 (Fstl1), which promote myocardial growth, VSMCs differentiation, fibroblast migration and proliferation (Y. Huang et al., 2013; Maruyama et al., 2016; Rossdeutsch, Smart, Dube, Turner, & Riley, 2012; Smart et al., 2011; Smart et al., 2007; K. Wei et al., 2015; B. Zhou et al., 2011).
There is emerging evidence of the involvement of atRA-signaling in epicardial activation and its response to injury. Like other epicardial developmental pathways, atRA production and signaling re-emerge in the epicardium activated by heart injury or disease (Bilbija et al., 2014; Bilbija et al., 2012; Kikuchi, Holdway, et al., 2011). In mice subjected to myocardial ischemia–reperfusion injury, there were increases in both cardiac retinol levels and in the levels of expression of RAR-target genes in cardiac fibroblasts from the peri-infarct area (Bilbija et al., 2012). In vitro, atRA also suppresses cardiomyocyte hypertrophic responses (J. Wu, Garami, Cheng, & Gardner, 1996; M. D. Zhou, Sucov, Evans, & Chien, 1995). In mouse models of obesity, atRA-treatment prevented fibrosis and cardiomyocyte apoptosis (Manolescu et al., 2014). Conversely, in rodent models of myocardial infarction, vitamin A insufficiency was associated with adverse ventricular remodeling (Asson-Batres et al., 2016; Minicucci et al., 2010). These observations are also supported by evidence that mice deficient in β-carotene-15,15’-dioxygenase (BCO1), the enzyme required to convert provitamin A carotenoids to retinol, also exhibit systolic dysfunction (Hessel et al., 2007; S. A. Lee et al., 2014).
It is difficult to establish the roles of atRA in heart regeneration in adult mice since this process is not efficient in adult mammals. However, a pro-regenerative role of atRA in the heart was demonstrated in zebrafish. Transgenic expression of Cyp26a1, or of a dominant-negative RAR in zebrafish led to decreased atRA-signaling and was associated with a dramatic reduction in cardiomyocyte proliferation during regeneration (Kikuchi, Holdway, et al., 2011). Interestingly, atRA production is rapidly initiated in both endocardium and epicardium in the injured zebrafish heart. However, in the injured mouse adult heart atRA production can only be detected in the epicardium. It is not clear if the absence of endocardial expression of Raldh2 in mice has any bearing on their reduced cardiac regenerative capacity.
While epicardial reactivation contributes to heart regeneration in zebrafish, it may potentially have unfavourable consequences in other species. During both embryogenesis and epicardial reactivation, expression of Raldh2 and Wt1 is controlled by the transcription factor C/EBPβ which recruits the SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeling complex (G. N. Huang et al., 2012; Vieira et al., 2017). Interestingly, expression of a dominant negative C/ EBPβ in mouse epicardium not only abrogated the expression of Raldh2 and Wt1 but also led to increased ejection fraction and decreased fibrosis following ischemia-reperfusion (G. N. Huang et al., 2012). More recent evidence indicates that depletion of EPDCs in the adult mouse heart led to improved function and reduced fibrosis following myocardial infarction (Quijada et al., 2019). Similarly, inactivation of cardiac fibroblasts limited the extent of fibrosis following cryoinjury in zebrafish (Sánchez-Iranzo et al., 2018). Therefore, from a therapeutic standpoint, increased atRA signaling in adult epicardial cells following cardiac injury could have both positive effects by supporting neovascularization and myocardial growth, as well as negative effects in promoting cardiac fibrosis.
Role of AtRA in the Development and Function of Hepatic and Pancreatic Stellate Cells
The liver is the primary storage site for vitamin A in the body. REs accumulate in lipid inclusion bodies within HSCs (reviewed in (Friedman, 2008)). To meet tissue retinoid needs, REs are hydrolyzed and transferred to hepatocytes to be released in the circulation as retinol complexed with its serum binding protein (RBP4). Upon liver injury or disease, HSCs become activated and adopt a myofibroblast-phenotype (Kent et al., 1976). HSC-derived myofibroblasts are a major contributor to the process of liver scarring and fibrosis though secretion of ECM components (Iwaisako et al., 2014). Developmentally, HSCs originate from septum transversum mesenchyme (Asahina, Zhou, Pu, & Tsukamoto, 2011; Ijpenberg et al., 2007; Pérez-Pomares et al., 2004), that also gives rise to proepicardium and sinus venosus.
Vitamin A metabolism and atRA signaling play important roles in the development, function and pathology of HSCs. Coelomic epithelial progenitors lining the liver lobes, delaminate, migrate and differentiate into HSCs and liver VSMCs (Asahina et al., 2009; Pérez-Pomares et al., 2004). As in the case of epicardial development, this process requires Wt1, RXRa and atRA-signaling in both mice, and avian models (Ijpenberg et al., 2007). However, a chemical screen for disruptors of HSC differentiation identified RAR and RXR agonists as having opposite effects in preventing or promoting HSC formation in zebrafish, respectively (Yin, Evason, Maher, & Stainier, 2012). Therefore, more studies are needed to identify the exact mechanism by which atRA signaling affects the development of HSCs.
The most salient feature of quiescent HSCs is the presence of REs-containing lipid droplets recognized based on gold chloride staining and/or associated fluorescence (Nagy et al., 1997; Wake, 1971). LRAT is the main enzyme responsible for the esterification of retinol within HSCs and a specific marker of HSCs within the liver (Batten et al., 2004; Mederacke et al., 2013; O’Byrne et al., 2005). As HSCs become activated, they remodel their lipid pools of REs and triacylglycerols through cycles of hydrolysis and acylation. The net result of HSC activation is that lipid droplets become smaller, the levels of unesterified retinol increase, and the acyl composition of triacylglycerols shifts towards a higher percentage of polyunsaturated fatty acids (Ajat et al., 2017; Testerink et al., 2012; Tuohetahuntila et al., 2017). Consequently, during the progression of liver disease, hepatic retinoid stores diminish significantly (Leo & Lieber, 1982). Chronic alcohol consumption-induced loss of hepatic retinoid stores has been attributed to an initial mobilization of retinoid from hepatic towards extrahepatic storage sites followed by increased catabolism and excretion of retinoids (Clugston, Huang, & Blaner, 2015). A similar decrease in liver retinoids is associated with obesity and related conditions such as non-alcoholic fatty liver disease (NAFLD) (Trasino, Tang, Jessurun, & Gudas, 2015) reviewed in (Saeed, Dullaart, Schreuder, Blokzijl, & Faber, 2017).
It is not clear if loss of hepatic retinoids is a cause or consequence of HSC activation. While retinoid loss is invariably associated with HSC activation, a recent study suggests that loss of retinoid-laden lipid droplets occurs only after HSC activation and transdifferentiation into myofibroblasts, and more importantly, that the levels of REs within HSCs remain steady during the initial phase of HSC activation (Jophlin, Koutalos, Chen, Shah, & Rockey, 2018). It is not clear whether atRA levels or atRA-signaling change during HSC activation since atRA levels do not mirror the changes in the concentrations of hepatic REs (Blaner, 2019).
There is evidence supporting an anti-fibrotic role of hepatic atRA. Pharmacological studies have shown that atRA or RAR agonists reduced HSC proliferation and activation and led to diminished steatosis in mice (Davis, Kramer, & Davidson, 1990; Trasino, Tang, Jessurun, & Gudas, 2016). Also, expression of a dominant negative RAR in hepatocytes is associated with steatohepatitis (Yanagitani et al., 2004). Given a better knowledge of HSC specific markers, the approach of specifically targeting a dominant negative RAR in activated HSCs is now feasible (Mederacke et al., 2013). However, the role of atRA-signaling in suppressing the activation of HSCs was questioned in some studies. For example, mRNA levels of RARs were undetectable in activated HSCs, meanwhile neither retinol nor atRA could prevent HSC activation (Milliano & Luxon, 2005). Moreover, RARα protein was largely found in insoluble aggregates in activated HSCs (Mezaki et al., 2009). At the same time, absence of REs within HSCs of Lrat-deficient mice does not promote HSC activation or liver fibrosis (Kluwe et al., 2011). The issue of whether retinoids promote or counteract HSC activation is made even more complicated by the fact that high doses of dietary vitamin A and retinoid-based therapies carry a known risk of hepatotoxicity characterized by HSC activation and fibrosis (Nollevaux et al., 2006). At the same time, dietary vitamin A and hepatic retinyl ester stores are vital for liver health (Aguilar et al., 2009).
While we still have a limited understanding of the involvement of atRA in HSC activation, several studies suggest that atRA might play a role in the response of HSCs to mechanical stress. HSCs activation and transdifferentiation to myofibroblasts is influenced by matrix stiffness (Olsen et al., 2011). In turn, liver fibrosis is associated with significant increase in tissue stiffness. Similar to the effects of mechanical stress on epicardial cells, there is evidence that HSCs exposed to mechanical stress undergo changes in retinoid gene expression which are consistent with decreased atRA-signaling (Yi, Zhang, Tang, & Zhu, 2015). Reciprocally, atRA signaling downregulates the expression of myosin light chain 2 (MLC-2) and negatively regulates myosin-driven migration of HSCs towards substrates of higher stiffness (durotaxis) (Cortes, Lachowski, et al., 2019). Yap-mediated mechanotransduction plays a role in multiple liver pathologies associated with HSCs activation, liver fibrosis and tumorigenesis as well as liver regeneration (Cox et al., 2016; Lu et al., 2010; Zhubanchaliyev, Temirbekuly, Kongrtay, Wanshura, & Kunz, 2016). Given the potential crosstalk of Yap and atRA-signaling observed in epicardial cells, and of the central role for Yap in the mechano-regulation, it would be important to further explore the role of atRA in Yap-mediated activation of HSCs (Cortes, Sarper, et al., 2019; Mannaerts et al., 2015).
HSC activation and fibrosis can in many instances resolve through senescence or apoptosis of activated HSCs, or through reversion of myofibroblasts to an inactive state (Cordero-Espinoza & Huch, 2018; Kisseleva et al., 2012; Krizhanovsky et al., 2008). It was observed that hepatic retinoid stores, atRA-signaling and RXRα were positively correlated with hepatocyte proliferation and liver regeneration following hepatectomy (Imai, Jiang, Kastner, Chambon, & Metzger, 2001; H. X. Liu, Ly, Hu, & Wan, 2014; I. O. Shmarakov, Jiang, Yang, Goldberg, & Blaner, 2013). Potential roles of atRA-signaling in the resolution of fibrosis have also been proposed by others, therefore, this exciting possibility will require more studies (Panebianco, Oben, Vinciguerra, & Pazienza, 2017).
Pancreatic stellate cells (PSCs) are important in the processes of lipid metabolism and tissue repair occurring in the pancreas. The embryonic source(s) of PSCs are not clearly defined, though it appears that their origin includes the embryonic coelomic mesothelium and that their development requires Wt1 signaling similar to (pro)epicardial cells and HSCs (Ariza, Cañete, Rojas, Muñoz-Chápuli, & Carmona, 2018). Several features of adult PSCs also resemble those of HSCs. Like HSCs, quiescent PSCs store REs within lipid droplets (Apte et al., 1998; N. Kim et al., 2009) which become depleted in activated PSCs (Bachem et al., 1998). Injury causes the activation of PSCs which transdifferentiate to myofibroblasts and contribute to pancreatic fibrosis (reviewed in (Bynigeri et al., 2017; Sherman, 2018; Xue et al., 2018).
There is evidence that atRA plays an important role in the maintenance of normal function of the pancreas (reviewed in (Brun, Wongsiriroj, & Blaner, 2016)). Focusing on the role of atRA in the activation of PSCs and pancreatic fibrosis, there is evidence that PSCs express RARs and RXRs and that retinoids inhibit the proliferation and migration, and induce the quiescence of PSCs (Chronopoulos et al., 2016; Froeling et al., 2011; McCarroll et al., 2006; W. Xiao et al., 2015). The exact mechanism of atRA modulation of PSC activation is not known, but there is evidence that implicates atRA modulation of TGF-β. TGF-β is an important mediator of myofibroblast differentiation, which is released from latent ECM-associated complexes by myofibroblast-induced contractions (Wipff, Rifkin, Meister, & Hinz, 2007). AtRA-signaling promotes the quiescence of PSCs by inhibiting PSC-mechanosensing and suppressing ECM remodeling necessary to release TGF-β from its latency complex (Chronopoulos et al., 2016; Sarper, Cortes, Lieberthal, & Del Rio Hernandez, 2016). Accordingly, atRA attenuates pancreatic fibrosis in experimental models (W. Xiao et al., 2015).
Aside from manipulating atRA-signaling to reduce fibrosis, several interesting approaches rely on the retinol storage property of HSCs and PSCs to efficiently deliver antifibrotic drugs and siRNAs to HSCs and PSCs (El-Mezayen et al., 2018; Okimoto et al., 2019; Qiao et al., 2018; Sato et al., 2008; Y. Zhang et al., 2018; Z. Zhang et al., 2015). The exact mechanism responsible for the targeted delivery of retinol-conjugated drugs to HSCs is not clear. It was suggested that the uptake of retinol-coupled liposomes by HSCs is dependent on serum RBP4 (Sato et al., 2008). RBP4 is taken up by parenchymal liver cells and is also proposed to transfer retinol from hepatocytes to stellate cells (Alapatt et al., 2013; Blomhoff, Berg, & Norum, 1988; Gjoen et al., 1987; Malaba, Kindberg, Norum, Berg, & Blomhoff, 1993; Senoo et al., 1993). Nevertheless, more studies are needed to establish the factors that control the distribution and uptake of retinol-coupled drugs by the liver.
Role of AtRA in Pulmonary Fibrosis
Retinoid signaling is essential for lung development as well as maintenance of alveolar architecture and regeneration (F. Chen et al., 2010; Chytil, 1996; Gudas, 2012; Maden & Hind, 2004; D. Massaro & Massaro, 2002). During development, endogenously produced atRA controls the formation of lung primordium from the primitive foregut (F. Chen et al., 2010). AtRA plays a particularly important role in regulating alveologenesis (Hind, Corcoran, & Maden, 2002a, 2002b; Maden, 2000; Maden & Hind, 2004). AtRA synthesizing enzymes, retinoid receptors, and retinoid-binding proteins are present during development and display dynamic regulation during alveologenesis (Hind et al., 2002a, 2002b; Maden & Hind, 2004; S. E. McGowan, Harvey, & Jackson, 1995; Ong & Chytil, 1976; Whitney, Massaro, Massaro, & Clerch, 1999). Through studies in mutant mice, it has been shown that atRA, acting through specific RAR isoforms, can be both a positive and negative regulator of alveologenesis (Desai et al., 2006; G. D. Massaro, Massaro, & Chambon, 2003; G. D. Massaro et al., 2000; S. McGowan et al., 2000; Mollard, Ghyselinck, Wendling, Chambon, & Mark, 2000; Snyder et al., 2005; Wongtrakool et al., 2003). RARγ functions as a positive regulator of alveologenesis, where Rarg-mutant mice fail to form alveoli (S. McGowan et al., 2000). Meanwhile, RARα has been shown to be required for the proper number of alveoli to develop after the perinatal period (G. D. Massaro et al., 2003). In contrast, RARβ functions as a negative regulator of alveologenesis, where RARβ mutant mice form too many alveoli (G. D. Massaro et al., 2000). It is thought that reawakening of developmental pathways occurs in tissue regeneration as an evolutionary efficiency without requiring separate regenerative signaling pathways; these concepts have been reviewed in detail (Hind & Maden, 2011; Maden & Hind, 2004). Chronic vitamin A deficiency (VAD) in both humans and animal models has been associated with lung functional defects and pulmonary diseases, including pulmonary fibrosis (Baybutt, Hu, & Molteni, 2000; Baybutt & Molteni, 2007; Biesalski & Nohr, 2003; Maden & Hind, 2004; G. D. Massaro & Massaro, 2000; S. E. McGowan et al., 2002; Morabia, Menkes, Comstock, & Tockman, 1990; Sommer, Katz, & Tarwotjo, 1984). VAD has also been associated with reduced immune response and increased risk of respiratory infections, asthma, and emphysema (Arora, Kumar, & Batra, 2002; Baybutt & Molteni, 2007; F. Chen et al., 2014). VAD results in squamous cell metaplasia with a reduced proportion of ciliated and mucous-producing cells, reduced number and surface of alveoli, alveolus septum thickening (Biesalski & Nohr, 2003; H. Wei et al., 2009). Additionally, in VAD, thickening of the alveolar basement membrane (BM) is accompanied by an increase in collagen I and collagen IV and the deposition of ectopic collagen fibrils in the BM (Esteban-Pretel, Marin, Renau-Piqueras, Barber, & Timoneda, 2010).
Type 1 epithelial cells form the surface barrier in the lung where gas exchange takes place. Type II cells are epithelial cells thought to act as progenitors for Type I cells and are, thus, involved in regeneration of the alveolar epithelium after injury. Type II cells make surfactant and atRA has been shown to regulate surfactant protein synthesis in fetal lung explants (Baybutt et al., 2000). Additionally, surfactant synthesis was lower in Type II pneumocytes isolated from VAD rats (Barber, Esteban-Pretel, Marin, & Timoneda, 2014). Type II pneumocytes are in close proximity to the connective tissue layer comprised of lipofibroblasts. It is believed that lipofibroblasts may transfer neutral lipids to Type II cells to support surfactant and phospholipid synthesis (S. E. McGowan & Torday, 1997). The size of lipofibroblasts can be increased by vitamin A feeding suggesting that they are a significant storage site for retinoids in the lung (Okabe, Yorifuji, Yamada, & Takaku, 1984; Spit, 1983). Whereas α-SMA expression has been widely used to identify myofibroblasts, it has recently been shown that Tcf21 expression may identify the lipofibroblast lineage (distinct from myofibroblasts, interstitial fibroblasts, or adventitial fibroblasts) (Park et al., 2019). These lipid-laden fibroblasts participate in the synthesis of structural proteins such as collagen and elastin (Okabe et al., 1984).
Upon pulmonary injury, myofibroblast accumulation in the lung is the major contributor to the excessive collagen deposition and inflammation that causes a replacement of normal lung parenchyma with fibrotic tissue (F. Jiang, Yang, Xue, Li, & Zhang, 2017; Song et al., 2013; T. B. Zhou, Drummen, & Qin, 2012). Pulmonary fibrosis eventually yields an irreversible decrease in oxygen diffusion capacity of the lung. The pathogenesis of the progressive impairment of pulmonary function is not well understood and there are currently few therapeutic options. Fibrosis of the lung can occur without any known cause (idiopathic pulmonary fibrosis; IPF) or can be a secondary injury resulting from viral infections, sarcoidosis, environmental and occupational inhaled exposures, radiation exposure (either from radiotherapy for cancer or accidental/intentional exposures to ionizing radiation), or as a side effect of certain drugs (Timoneda et al., 2018; T. B. Zhou et al., 2012).
Lung is the second largest source of retinoids in the body where retinoids are stored as retinyl esters in intracellular lipid droplets (L. Liu & Gudas, 2005; O’Byrne et al., 2005; Okabe et al., 1984; Schmitz, Poor, Wellman, & Erdman, 1991). Storage of vitamin A in the lung is regulated by its active metabolite, where exogenous atRA can stimulate vitamin A uptake and storage in the lung (L. Wu & Ross, 2010). Retinoid metabolism has been shown to be altered in a number of lung disorders, including fibrosis. Endogenous atRA in lung was found to be reduced in several different strains of mice typically used as models of radiation-induced lung injury, in which fibrosis is a main injury endpoint. AtRA was reduced 24h after radiation exposure and the reduction in atRA persisted and continued to decline in fibrotic lung up to 180 days post-radiation (Jones et al., 2014). This observation shows that atRA is reduced by the initial insult prior to the onset of clinical symptoms. The degree of depletion of atRA at 180 days appears to be consistent with fibrosis severity, however, a systematic study to establish this relationship is needed. In addition to the observation that atRA levels are reduced after radiation exposure prior to the onset of clinical symptoms, a number of atRA-regulated proteins were observed to be changed after radiation exposure in a proteomic analysis of radiation-induced lung injury during the first week after radiation exposure, several months before clinical symptoms develop (W. Huang et al., 2019). These proteomic changes which may represent initiating molecular events towards the development of fibrosis included Rho GTPase signaling; kinase-related canonical pathways associated with cell migration, proliferation, and adhesion; and retinoid receptor dysregulation that has been reported to impact atRA biosynthetic enzyme expression (W. Huang et al., 2019). Similarly, observations have been reported where enzymes for biosynthesis and metabolism are altered after exposure to cigarette smoke before clinical symptoms of emphysema manifest (Quinn, Harvey, & Penning, 2008; R. Wang et al., 2010). Exposure to cigarette smoke or benzo[a]pyrene results in depletion of retinol in lung where the severity of emphysema was correlated with the degree of retinol deficiency in lung (Baybutt et al., 2000). The observed retinoid depletion in radiation-induced lung injury and emphysema models supports the postulate that depletion of retinoid signaling may be an initiating event in the development of pulmonary fibrosis and other lung dysfunction, which with persistence of local deficiency of endogenous atRA, presents a permissive environment for fibrotic transformation.
A common feature of pulmonary fibrotic conditions is persistent alveolitis, accumulation of myofibroblasts, and the deposition of excessive amounts of ECM (Inage et al., 2009; T. B. Zhou et al., 2012). Fibroblasts are tissue-resident mesenchymal cells that produce ECM. ECM normally functions to maintain tissue integrity and homeostasis and contributes to the regulation of alveolarization, tissue stiffness/elasticity, as well as tissue remodeling and repair (Pelosi, Rocco, Negrini, & Passi, 2007; Timoneda et al., 2018). Retinoid signaling regulates the expression of ECM proteins dysregulated in fibrosis including collagen, laminin, fibronectin, elastin, and proteoglycans (Aguilar et al., 2009; Axel et al., 2001; Barber et al., 2014; Y. S. Lee & Jeong, 2012; Matsui, 1996). Many ECM proteins are directly regulated by retinoid signaling through their gene promoters however, retinoid signaling can also regulate the expression of cell membrane ECM receptors. Chronic VAD has been reported to be associated with morphological changes to ECM in lung which was related to fibrogenic activation and deterioration of lung parenchyma (Timoneda et al., 2018). Many of the VAD-induced alterations of ECM can be reversed by atRA, yielding promise for potential therapeutic utility of retinoids against pulmonary fibrosis (Esteban-Pretel et al., 2010; Marin et al., 2005; D. Massaro & Massaro, 2006; S. E. McGowan, Holmes, & Smith, 2004; Morath et al., 2001; Takahashi & Takasu, 2011).
Myofibroblasts are a distinct cell population that expresses α-SMA and possesses contractile microfilamentous apparatus (stress fibers) (Di Carlo & Peduto, 2018). Multiple progenitor cell populations have been proposed to contribute to the formation of lung myofibroblasts including pericytes and fibroblasts, resident mesenchymal cells, bone marrow progenitors, and epithelial cells (Di Carlo & Peduto, 2018; F. Jiang et al., 2017; Song et al., 2013; T. B. Zhou et al., 2012). Polarized epithelial cells differentiate into contractile, motile mesenchymal cells via EMT. The process of EMT is critical during lung development and contributes to lung disease including fibrosis, COPD and cancer (Bartis, Mise, Mahida, Eickelberg, & Thickett, 2014; Sung, Kim, & Park, 2016). In the lung, EMT is triggered by local microenvironmental factors and signals including cytokines, growth factors, inflammation, hypoxia, disrupted cell-cell contact, and/or contact with aberrant ECM components. Particularly, TGF-β, Smad, and β-catenin signaling have been implicated as inducers of EMT in lung disease (Kage & Borok, 2012). EMT is characterized by a loss of expression of E-cadherin accompanied by increases in mesenchymal markers including vimentin, fibronectin, α-SMA, and N-cadherin (Beck, Chikwem, Solanki, & Golemis, 2014; Gonzalez & Medici, 2014; Kourtidis, Lu, Pence, & Anastasiadis, 2017). AtRA regulates a number of these markers of fibroblast activation; atRA represses TGF-β, activates E-cadherin, and regulates α-SMA, vimentin, calponin, and MMP9 (F. Chen et al., 2007; Gong et al., 2018; Hu et al., 2010; Langton & Gudas, 2008; Papi et al., 2007; Woo & Jang, 2012).
AtRA biosynthesis and signaling has been previously shown to influence microenvironmental events such as cell fate, cell homing, cell migration, and protein secretion (Fu et al., 2010; Jones et al., 2014; Kane, Folias, et al., 2011; Kane et al., 2010; Pierzchalski et al., 2014; Pierzchalski et al., 2013; Sidell et al., 2010; Siegenthaler et al., 2009; Villablanca et al., 2011; C. Wang, Kane, & Napoli, 2011; S. Wang, J. Yu, et al., 2018; Williams et al., 2009). Endogenous atRA is depleted in pre-fibrotic and fibrotic tissue that displays characteristics typical of an activated microenvironment including increased stromal collagen as well as epithelial and stromal hypercellularity (Pierzchalski et al., 2014; Pierzchalski et al., 2013). Deposition of fibrillary collagens (especially collagen I, collagen III) occurs in pulmonary fibrosis and adds stiffness to tissues. Mechanical forces have been shown to be important to myofibroblast activation and matrix deposition in lung (Balestrini, Chaudhry, Sarrazy, Koehler, & Hinz, 2012; X. Huang et al., 2012; F. Liu et al., 2010; Wells, 2013). The resulting matrix stiffness from the deposition of fibrillary collagens increases α-SMA expression and matrix deposition. Fibronectins are mechanosensitive and among the first to be upregulated after injury (Wells, 2013). TGF-β, an important and potent pro-fibrotic factor and inducer of EMT also undergoes activation as a result of mechanical tension (Wells, 2013). However, in a bleomycin model of lung fibrosis, lineage tracing analysis of alveolar epithelial cells showed that resident mesenchymal cells, and not cell populations derived by EMT of other cell types, were the major source of matrix producing fibroblasts and myofibroblasts (Rock et al., 2011).
Treatment with atRA reversed pulmonary fibrosis in a number of models. AtRA attenuated both radiation-induced and bleomycin-induced pulmonary fibrosis in mouse lung by inhibiting proliferation and reducing collagen synthesis (Tabata, Kadokawa, et al., 2006; Tabata, Kubo, et al., 2006). In their studies, Tabata et al. demonstrated that atRA inhibited IL-6 production through a protein kinase C (PKC)-δ / NF-κB-mediated mechanism. They also reported atRA reduced radiation-induced increases in TGF-β1 production through the p38MAPK/NF-κB pathway. Based on in vitro studies, Tabata et al. also reported that atRA reduced radiation-induced production of IL-6, TGF-β1, α-SMA, collagen 1A1, and activated NF-κB p65 in lung fibroblasts (Tabata, Kadokawa, et al., 2006; Tabata, Kubo, et al., 2006). AtRA-treatment of oxygen-induced lung injury reduced the degree of fibrosis and α-SMA expression (Ozer et al., 2005). In bleomycin-induced lung injury, atRA was able to decrease the expression of IL-17A, IL-6, and TGF-β1 (Dong et al., 2012). AtRA also blocked bleomycin-induced changes in EMT by preventing the increase in α-SMA and reduction in E-cadherin (Song et al., 2013). The atRA-mediated attenuation of EMT involved inhibition of bleomycin-induced Snail and Twist expression, where Snail expression is essential for TGF-β1 –induced EMT of alveolar epithelial cells (Song et al., 2013). Administration of atRA to VAD animals restored the concentration of parenchymal elastic fibers and lung mechanical properties (S. E. McGowan & Holmes, 2007; S. E. McGowan, Takle, & Holmes, 2005). AtRA administration has also been shown to be protective towards cell injury and ECM accumulation by reducing collagen I mRNA, and inhibiting TGF-β and CTGF expression (Davis et al., 1990; Ye & Dan, 2010). Additionally, atRA treatment reduced the amount of collagen IV and downregulated inflammatory cytokine expression, including IL-1α, IL-1β, and TNFα in VAD animals (Esteban-Pretel et al., 2010).
Animal models and in vitro experiments have shown encouraging potential for retinoid-mediated attenuation of fibrosis. Additional in vivo animal experimentation and clinical trials will be needed to determine the therapeutic utility of retinoid therapy for pulmonary fibrosis. Challenges in clinical trials of retinoids in lung diseases may arise from the differing effects of retinoids on mature differentiated cells and progenitor cells in lung (Ng-Blichfeldt et al., 2018). Inhibition of retinoid signaling was needed for in vitro expansion of lung epithelial progenitor cells that are important to regeneration whereas atRA signaling promoted differentiation of alveolar and airway epithelial cells consistent with atRA’s well characterized role as an essential differentiation cue for both embryonic and adult stem/progenitor cells (Jacobs et al., 2006; Lasagni et al., 2015; Ng-Blichfeldt et al., 2018; Okada, Shimazaki, Sobue, & Okano, 2004; Peired et al., 2013; Schuldiner, Yanuka, Itskovitz-Eldor, Melton, & Benvenisty, 2000). A differing effect of retinoids in diseased lung may occur due to, in part, differing stem cell populations. Whereas cells with stem/progenitor properties have been isolated from lung, full characterization stem cell populations in the lung and elucidation of their lineages is still in need of further attention (Fujino et al., 2011; Kajstura et al., 2011; C. F. Kim et al., 2005). AtRA treatment of patients in clinical trials for emphysema demonstrate some of the potential challenges of treating with atRA (Hind & Maden, 2011; Mao et al., 2002; Roth et al., 2006). AtRA induces its own degradation, limiting the effective therapeutic drug levels that can be achieved. The oral atRA treatment in emphysema patients resulted in compensatory enzyme induction that caused a significant reduction in plasma atRA levels over time (Hind & Maden, 2011; Mao et al., 2002; Roth et al., 2006). Only the highest doses appeared to have any biological effect, where clinical improvements correlated with plasma drug levels (Mao et al., 2002; Roth et al., 2006). Increased degradation of endogenous atRA by CYP26A1 in emphysema (similar to what has been observed in some cancers) is an additional factor limiting clinical success where deficient retinoid-driven angiogenesis was reported to impair endothelial cell repair and suggested to contribute to chronic lung disease (Ng-Blichfeldt et al., 2018). RAR isoform-specific agonists have also replicated the therapeutic effects of atRA in models of alveolar regeneration (Belloni, Garvin, Mao, Bailey-Healy, & Leaffer, 2000; Garber et al., 2006; Ishizawa et al., 2004; Kaza et al., 2001; G. D. Massaro et al., 2000; Ozer et al., 2005; Perl & Gale, 2009; Tepper et al., 2000; Veness-Meehan, Bottone, & Stiles, 2000)
Conclusions and Future Directions
We summarized observations that suggest that atRA is required for the development of (pro)fibroblast cell populations in the heart, liver, pancreas and lung. The pervasive occurrence of atRA-signaling during the formation of fibroblasts hints at the existence of shared developmental pathways for fibroblasts associated with internal organs. Indeed, a growing number of studies indicate that cardiac fibroblasts, HSCs, PSCs and lipofibroblasts are derived from precursors initially found within the embryonic mesothelium covering the heart (i.e. the epicardium), lung (pleura) and GI tract (septum transversum and visceral peritoneum) (Koopmans & Rinkevich, 2018; Rinkevich et al., 2012; Wilm, Ipenberg, Hastie, Burch, & Bader, 2005). Furthermore, these mesothelial precursor cell populations employ a related set of developmental programs to give rise to fibroblasts and smooth muscle cells. For example, WT1 is expressed by EPDCs as well as by coelomic precursors that give rise to HSCs, lung fibroblasts, hepatic smooth muscle cells, and PSCs (Asahina et al., 2011; Carmona, Barrena, & Munoz-Chapuli, 2019; Ijpenberg et al., 2007; Perez-Pomares et al., 2002; Sontake et al., 2018; Takeichi, Nimura, Mori, Nakagami, & Kaneda, 2013 Ariza, 2018 #4540). In comparison to WT1, TCF21 is expressed in both overlapping as well as distinct subsets of mesothelial precursors that give rise to cardiac fibroblast and lung lipofibroblast populations (Acharya et al., 2012; Braitsch et al., 2012; Braitsch et al., 2013; Kikuchi, Gupta, et al., 2011; Park et al., 2019; Tandon et al., 2013; Vicente-Steijn et al., 2015 Kanisicak, 2016 #4022). Meanwhile, AtRA-signaling intersects with the multiple developmental programs that guide mesothelial-to-mesenchyme transitions such as WT1, TGF-β, TCF21, Rho and WNT-signaling pathways (Braitsch et al., 2012; F. Chen et al., 2010; F. Chen et al., 2007; Guadix et al., 2011; Ijpenberg et al., 2007; Lin et al., 2010; Sarper et al., 2016; von Gise et al., 2011; S. Wang, J. Yu, et al., 2018; W. Xiao et al., 2015).
Though studies highlighted in this review suggest a link between tissue fibrosis and vitamin A there is still much to be learnt about the role of atRA in various fibrotic processes before it can be realistically considered as a therapeutic target in fibrosis. While a case can be made for the importance of atRA in the developmental processes that give rise to tissue resident fibroblasts in many organs, the role of atRA in the fibroblast-myofibroblast transformation is not as clear. The most conflicting findings have been in regard to the effect of atRA in liver fibrosis, which some have argued could be a result of the dose and timing of atRA agonist employed (T. B. Zhou et al., 2012). This is certainly an important consideration given the evidence that atRA regulates its own metabolism and that a pharmacological dose of atRA can be followed by lasting atRA deficiency due to compensatory responses (L. M. Lee et al., 2012; Rydeen et al., 2015). To achieve an efficient activation of RAR in a specific tissue we need to overcome an exquisitely complex feedback regulatory system which has evolved since the beginning of the vertebrate diversification as a way to prevent the teratogenic effects caused by variations in the intake of dietary vitamin A. For this to happen, we first need to have a comprehensive understanding of factors that control the distribution and metabolism of atRA or synthetic RAR agonists. Future studies should also focus on the development of isoform-specific agonists, or RAR-agonists that resist catabolism by CYP26 enzymes and have a longer half-life; properties making them more pharmacokinetically favorable and potentially translatable.
One of the main obstacles in conclusively establishing the effect of atRA in fibrosis is that atRA influences a multitude of cellular processes such as differentiation, ECM deposition, metabolism and inflammation. It is possible that atRA may have some beneficial effect in one process but cause detrimental effects in another. One very important aspect of fibrosis is the inflammatory response and as there is considerable evidence of atRA being an important factor in immunity, it will be important to carefully assess the role of atRA in the innate immune response that promotes fibrosis (Brown & Noelle, 2015; Wynn & Ramalingam, 2012). In one such example, atRA produced by dendritic cells in the ocular mucosa promotes the activation of conjunctival fibroblasts (Ahadome, Abraham, et al., 2016; Ahadome, Mathew, et al., 2016). Therefore, in the future, it would be useful to study and refer to the effects of atRA on a specific profibrotic mechanism, as opposed to its effects on organ fibrosis in the broader sense.
Acknowledgements
This work was supported in part by the grant R01HD077260 from the National Institutes of Health (M.A.K., A.R.M.) and by Discovery Grant RGPIN-2019-04002 from the Natural Sciences and Engineering Research Council of Canada to ARM. Additional support was provided by startup funds to A.R.M. from the Northern Ontario School of Medicine. Conflicts of interest: none.
Funding Information
• NIH Grant Number: R01HD077260. Sponsor: NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development
• Discovery Grant Number RGPIN-2019-04002. Sponsor: Natural Sciences and Engineering Research Council of Canada
ABBREVIATIONS
- atRA
all-trans-retinoic acid
- CRABP
cellular retinoic acid binding protein
- CRBP
cellular retinol binding protein
- DHRS3
dehydrogenase/reductase superfamily member 3
- DGAT1
diacylglycerol O-acyltransferase 1
- EMT
epithelial-to-mesenchymal transition
- EPDCs
epicardial-derived precursor cells
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LRAT
lecithin:retinol acyltransferase
- PDGF
platelet-derived growth factor
- PDGFRA
platelet-derived growth factor receptor A
- PDGFRB
platelet-derived growth factor receptor B
- RALDH
retinaldehyde dehydrogenase
- RAR
retinoic acid receptor
- RBP
(serum) retinol binding protein 4
- RDH10
retinol dehydrogenase 10
- REs
retinyl esters
- RhoA
Ras homolog gene family, member A
- RXR
retinoid X receptor
- SDR
short-chain dehydrogenase/reductase
- SMAα
smooth muscle α-actin
- SRF
serum response factor
- TCF21
transcription factor 21
- VSMC
vascular smooth muscle cells
- WT1
Wilms-tumor 1
References
- Ables GP, Yang KJ, Vogel S, Hernandez-Ono A, Yu S, Yuen JJ, … Ginsberg HN (2012). Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J Lipid Res, 53(11), 2364–2379. doi: 10.1194/jlr.M029041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abed S (2001). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes & Development, 15(2), 226–240. doi: 10.1101/gad.855001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, & Dolle P (2002). Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech Dev, 110(1–2), 173–177. [DOI] [PubMed] [Google Scholar]
- Abu-Abed SS, Beckett BR, Chiba H, Chithalen JV, Jones G, Metzger D, … Petkovich M (1998). Mouse P450RAI (CYP26) expression and retinoic acid-inducible retinoic acid metabolism in F9 cells are regulated by retinoic acid receptor gamma and retinoid X receptor alpha. J Biol Chem, 273(4), 2409–2415. [DOI] [PubMed] [Google Scholar]
- Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, … Tallquist MD (2012). The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development, 139(12), 2139–2149. doi: 10.1242/dev.079970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MK, Belyaeva OV, Wu L, & Kedishvili NY (2014). The retinaldehyde reductase activity of DHRS3 is reciprocally activated by retinol dehydrogenase 10 to control retinoid homeostasis. J Biol Chem, 289(21), 14868–14880. doi: 10.1074/jbc.M114.552257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar RP, Genta S, Oliveros L, Anzulovich A, Gimenez MS, & Sanchez SS (2009). Vitamin A deficiency injures liver parenchyma and alters the expression of hepatic extracellular matrix. J Appl Toxicol, 29(3), 214–222. doi: 10.1002/jat.1399 [DOI] [PubMed] [Google Scholar]
- Ahadome SD, Abraham DJ, Rayapureddi S, Saw VP, Saban DR, Calder VL, … Dart JK (2016). Aldehyde dehydrogenase inhibition blocks mucosal fibrosis in human and mouse ocular scarring. JCI Insight, 1(12), e87001. doi: 10.1172/jci.insight.87001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahadome SD, Mathew R, Reyes NJ, Mettu PS, Cousins SW, Calder VL, & Saban DR (2016). Classical dendritic cells mediate fibrosis directly via the retinoic acid pathway in severe eye allergy. JCI Insight, 1(12). doi: 10.1172/jci.insight.87012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajat M, Molenaar M, Brouwers J, Vaandrager AB, Houweling M, & Helms JB (2017). Hepatic stellate cells retain the capacity to synthesize retinyl esters and to store neutral lipids in small lipid droplets in the absence of LRAT. Biochim Biophys Acta Mol Cell Biol Lipids, 1862(2), 176–187. doi: 10.1016/j.bbalip.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Alapatt P, Guo F, Komanetsky SM, Wang S, Cai J, Sargsyan A, … Graham TE (2013). Liver retinol transporter and receptor for serum retinol-binding protein (RBP4). J Biol Chem, 288(2), 1250–1265. doi: 10.1074/jbc.M112.369132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, … Ardehali R (2014). Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res, 115(7), 625–635. doi: 10.1161/CIRCRESAHA.115.303794 [DOI] [PubMed] [Google Scholar]
- Amengual J, Zhang N, Kemerer M, Maeda T, Palczewski K, & Von Lintig J (2014). STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum Mol Genet, 23(20), 5402–5417. doi: 10.1093/hmg/ddu258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, … Wilson JS (1998). Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut, 43(1), 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima Y, Miyagawa-Tomita S, Maeda K, Asai R, Seya D, Minoux M, … Kurihara H (2012). Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat Commun, 3, 1267. doi: 10.1038/ncomms2258 [DOI] [PubMed] [Google Scholar]
- Ariza L, Cañete A, Rojas A, Muñoz-Chápuli R, & Carmona R (2018). Role of the Wilms’ tumor suppressor gene Wt1 in pancreatic development. Developmental Dynamics, 247(7), 924–933. doi: 10.1002/dvdy.24636 [DOI] [PubMed] [Google Scholar]
- Arnold SL, Amory JK, Walsh TJ, & Isoherranen N (2012). A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J Lipid Res, 53(3), 587–598. doi: 10.1194/jlr.D019745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SL, Kent T, Hogarth CA, Schlatt S, Prasad B, Haenisch M, … Isoherranen N (2015). Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations. J Lipid Res, 56(2), 342–357. doi: 10.1194/jlr.M054718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P, Kumar V, & Batra S (2002). Vitamin A status in children with asthma. Pediatr Allergy Immunol, 13(3), 223–226. [DOI] [PubMed] [Google Scholar]
- Asahina K, Tsai SY, Li P, Ishii M, Maxson RE Jr., Sucov HM, & Tsukamoto H (2009). Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology, 49(3), 998–1011. doi: 10.1002/hep.22721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Zhou B, Pu WT, & Tsukamoto H (2011). Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology, 53(3), 983–995. doi: 10.1002/hep.24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asson-Batres MA, Ryzhov S, Tikhomirov O, Duarte CW, Congdon CB, Lessard CR, … Sawyer DB (2016). Effects of vitamin A deficiency in the postnatal mouse heart: role of hepatic retinoid stores. Am J Physiol Heart Circ Physiol, 310(11), H1773–1789. doi: 10.1152/ajpheart.00887.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel DI, Frigge A, Dittmann J, Runge H, Spyridopoulos I, Riessen R, … Karsch KR (2001). All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc Res, 49(4), 851–862. [DOI] [PubMed] [Google Scholar]
- Azambuja AP, Portillo-Sanchez V, Rodrigues MV, Omae SV, Schechtman D, Strauss BE, … Xavier-Neto J (2010). Retinoic acid and VEGF delay smooth muscle relative to endothelial differentiation to coordinate inner and outer coronary vessel wall morphogenesis. Circ Res, 107(2), 204–216. doi: 10.1161/CIRCRESAHA.109.214650 [DOI] [PubMed] [Google Scholar]
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, … Adler G (1998). Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology, 115(2), 421–432. [DOI] [PubMed] [Google Scholar]
- Bachmann H, Desbarats A, Pattison P, Sedgewick M, Riss G, Wyss A, … Grolier P (2002). Feedback regulation of beta,beta-carotene 15,15’-monooxygenase by retinoic acid in rats and chickens. J Nutr, 132(12), 3616–3622. [DOI] [PubMed] [Google Scholar]
- Baek ST, & Tallquist MD (2012). Nf1 limits epicardial derivative expansion by regulating epithelial to mesenchymal transition and proliferation. Development, 139(11), 2040–2049. doi: 10.1242/dev.074054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, & Hinz B (2012). The mechanical memory of lung myofibroblasts. Integr Biol (Camb), 4(4), 410–421. doi: 10.1039/c2ib00149g [DOI] [PubMed] [Google Scholar]
- Barber T, Esteban-Pretel G, Marin MP, & Timoneda J (2014). Vitamin a deficiency and alterations in the extracellular matrix. Nutrients, 6(11), 4984–5017. doi: 10.3390/nu6114984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartis D, Mise N, Mahida RY, Eickelberg O, & Thickett DR (2014). Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax, 69(8), 760–765. doi: 10.1136/thoraxjnl-2013-204608 [DOI] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, … Palczewski K (2004). Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem, 279(11), 10422–10432. doi: 10.1074/jbc.M312410200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt RC, Hu L, & Molteni A (2000). Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J Nutr, 130(5), 1159–1165. doi: 10.1093/jn/130.5.1159 [DOI] [PubMed] [Google Scholar]
- Baybutt RC, & Molteni A (2007). Vitamin A and emphysema. Vitam Horm, 75, 385–401. doi: 10.1016/S0083-6729(06)75014-2 [DOI] [PubMed] [Google Scholar]
- Beck TN, Chikwem AJ, Solanki NR, & Golemis EA (2014). Bioinformatic approaches to augment study of epithelial-to-mesenchymal transition in lung cancer. Physiol Genomics, 46(19), 699–724. doi: 10.1152/physiolgenomics.00062.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni PN, Garvin L, Mao CP, Bailey-Healy I, & Leaffer D (2000). Effects of all-trans-retinoic acid in promoting alveolar repair. Chest, 117(5 Suppl 1), 235S–241S. [DOI] [PubMed] [Google Scholar]
- Belyaeva OV, Adams MK, Wu L, & Kedishvili NY (2017). The antagonistically bifunctional retinoid oxidoreductase complex is required for maintenance of all-trans-retinoic acid homeostasis. J Biol Chem, 292(14), 5884–5897. doi: 10.1074/jbc.M117.776914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, … Stroke Statistics S (2018). Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation, 137(12), e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, … Frisén J (2009). Evidence for Cardiomyocyte Renewal in Humans. Science, 324(5923), 98. doi: 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesalski HK, & Nohr D (2003). Importance of vitamin-A for lung function and development. Mol Aspects Med, 24(6), 431–440. [DOI] [PubMed] [Google Scholar]
- Bilbija D, Elmabsout AA, Sagave J, Haugen F, Bastani N, Dahl CP, … Valen G (2014). Expression of retinoic acid target genes in coronary artery disease. Int J Mol Med, 33(3), 677–686. doi: 10.3892/ijmm.2014.1623 [DOI] [PubMed] [Google Scholar]
- Bilbija D, Haugen F, Sagave J, Baysa A, Bastani N, Levy FO, … Valen G (2012). Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. Plos One, 7(9), e44740. doi: 10.1371/journal.pone.0044740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings SE, Pierzchalski K, Butler Tjaden NE, Pang XY, Trainor PA, Kane MA, & Moise AR (2013). The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J, 27(12), 4877–4889. doi: 10.1096/fj.13-227967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner WS (2019). Hepatic Stellate Cells and Retinoids: Toward A Much More Defined Relationship. Hepatology, 69(2), 484–486. doi: 10.1002/hep.30293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner WS, Li Y, Brun PJ, Yuen JJ, Lee SA, & Clugston RD (2016). Vitamin A Absorption, Storage and Mobilization. Subcell Biochem, 81, 95–125. doi: 10.1007/978-94-024-0945-1_4 [DOI] [PubMed] [Google Scholar]
- Blaner WS, Obunike JC, Kurlandsky SB, al-Haideri M, Piantedosi R, Deckelbaum RJ, & Goldberg IJ (1994). Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J Biol Chem, 269(24), 16559–16565. [PubMed] [Google Scholar]
- Blom JN, & Feng Q (2018). Cardiac repair by epicardial EMT: Current targets and a potential role for the primary cilium. Pharmacol Ther, 186, 114–129. doi: 10.1016/j.pharmthera.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Berg T, & Norum KR (1988). Transfer of retinol from parenchymal to stellate cells in liver is mediated by retinol-binding protein. Proc Natl Acad Sci U S A, 85(10), 3455–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerman MH, & Napoli JL (1991). Cholate-independent retinyl ester hydrolysis. Stimulation by Apo-cellular retinol-binding protein. J Biol Chem, 266(33), 22273–22278. [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Vicaire S, Garnier JM, Schuhbaur B, Dolle P, & Chambon P (1995). Efficient cloning of cDNAs of retinoic acid-responsive genes in P19 embryonal carcinoma cells and characterization of a novel mouse gene, Stra1 (mouse LERK-2/Eplg2). Dev Biol, 170(2), 420–433. doi: 10.1006/dbio.1995.1226 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, & Chambon P (1997). Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev, 63(2), 173–186. [DOI] [PubMed] [Google Scholar]
- Brade T, Kumar S, Cunningham TJ, Chatzi C, Zhao X, Cavallero S, … Duester G (2011). Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2. Development, 138(1), 139–148. doi: 10.1242/dev.054239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitsch CM, Combs MD, Quaggin SE, & Yutzey KE (2012). Pod1/Tcf21 is regulated by retinoic acid signaling and inhibits differentiation of epicardium-derived cells into smooth muscle in the developing heart. Dev Biol, 368(2), 345–357. doi: 10.1016/j.ydbio.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, & Yutzey KE (2013). Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol, 65, 108–119. doi: 10.1016/j.yjmcc.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, & Noelle RJ (2015). Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur J Immunol, 45(5), 1287–1295. doi: 10.1002/eji.201344398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun PJ, Wongsiriroj N, & Blaner WS (2016). Retinoids in the pancreas. Hepatobiliary Surg Nutr, 5(1), 1–14. doi: 10.3978/j.issn.2304-3881.2015.09.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, Grun F, Derguini F, Chen Y, Kimura S, Noy N, & Hammerling U (1993). Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J Exp Med, 178(2), 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynigeri RR, Jakkampudi A, Jangala R, Subramanyam C, Sasikala M, Rao GV, … Talukdar R (2017). Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J Gastroenterol, 23(3), 382–405. doi: 10.3748/wjg.v23.i3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai AQ, Radtke K, Linville A, Lander AD, Nie Q, & Schilling TF (2012). Cellular retinoic acid-binding proteins are essential for hindbrain patterning and signal robustness in zebrafish. Development, 139(12), 2150–2155. doi: 10.1242/dev.077065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, & Poss KD (2018). The epicardium as a hub for heart regeneration. Nat Rev Cardiol, 15(10), 631–647. doi: 10.1038/s41569-018-0046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona R, Barrena S, & Munoz-Chapuli R (2019). Retinoids in Stellate Cells: Development, Repair, and Regeneration. J Dev Biol, 7(2). doi: 10.3390/jdb7020010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D, Wilson L, Maden M, & Lumsden A (2007). RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development, 134(7), 1369–1383. doi: 10.1242/dev.02815 [DOI] [PubMed] [Google Scholar]
- Chandra V, Wu D, Li S, Potluri N, Kim Y, & Rastinejad F (2017). The quaternary architecture of RARbeta-RXRalpha heterodimer facilitates domain-domain signal transmission. Nat Commun, 8(1), 868. doi: 10.1038/s41467-017-00981-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Cao Y, Qian J, Shao F, Niederreither K, & Cardoso WV (2010). A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest, 120(6), 2040–2048. doi: 10.1172/JCI40253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, & Cardoso WV (2007). Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development, 134(16), 2969–2979. doi: 10.1242/dev.006221 [DOI] [PubMed] [Google Scholar]
- Chen F, Marquez H, Kim YK, Qian J, Shao F, Fine A, … Cardoso WV (2014). Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest, 124(2), 801–811. doi: 10.1172/JCI70291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, & Napoli JL (2008). All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J, 22(1), 236–245. doi: 10.1096/fj.07-8739com [DOI] [PubMed] [Google Scholar]
- Chen N, Onisko B, & Napoli JL (2008). The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem, 283(30), 20841–20847. doi: 10.1074/jbc.M802314200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Clarke OB, Kim J, Stowe S, Kim YK, Assur Z, … Mancia F (2016). Structure of the STRA6 receptor for retinol uptake. Science, 353(6302). doi: 10.1126/science.aad8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos A, Robinson B, Sarper M, Cortes E, Auernheimer V, Lachowski D, … Del Rio Hernandez A (2016). ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun, 7, 12630. doi: 10.1038/ncomms12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil F (1996). Retinoids in lung development. FASEB J, 10(9), 986–992. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, & Knutson D (2011). Vitamin A in reproduction and development. Nutrients, 3(4), 385–428. doi: 10.3390/nu3040385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugston RD, Huang LS, & Blaner WS (2015). Chronic alcohol consumption has a biphasic effect on hepatic retinoid loss. FASEB J, 29(9), 3654–3667. doi: 10.1096/fj.14-266296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comptour A, Rouzaire M, Belville C, Bonnin N, Daniel E, Chiambaretta F, … Sapin V (2016). Lysyl oxidase-like 4 involvement in retinoic acid epithelial wound healing. Sci Rep, 6, 32688. doi: 10.1038/srep32688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Espinoza L, & Huch M (2018). The balancing act of the liver: tissue regeneration versus fibrosis. J Clin Invest, 128(1), 85–96. doi: 10.1172/JCI93562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes E, Lachowski D, Rice A, Chronopoulos A, Robinson B, Thorpe S, … Del Rio Hernandez AE (2019). Retinoic Acid Receptor-beta Is Downregulated in Hepatocellular Carcinoma and Cirrhosis and Its Expression Inhibits Myosin-Driven Activation and Durotaxis in Hepatic Stellate Cells. Hepatology, 69(2), 785–802. doi: 10.1002/hep.30193 [DOI] [PubMed] [Google Scholar]
- Cortes E, Sarper M, Robinson B, Lachowski D, Chronopoulos A, Thorpe SD, … Del Rio Hernandez AE (2019). GPER is a mechanoregulator of pancreatic stellate cells and the tumor microenvironment. EMBO Rep, 20(1). doi: 10.15252/embr.201846556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, Tsomides A, … Goessling W (2016). Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol, 18(8), 886–896. doi: 10.1038/ncb3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Chatzi C, Sandell LL, Trainor PA, & Duester G (2011). Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev Dyn, 240(5), 1142–1150. doi: 10.1002/dvdy.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, & Duester G (2015). Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol, 16(2), 110–123. doi: 10.1038/nrm3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, … Red-Horse K (2019). A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell. doi: 10.1016/j.cell.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BH, Kramer RT, & Davidson NO (1990). Retinoic acid modulates rat Ito cell proliferation, collagen, and transforming growth factor beta production. J Clin Invest, 86(6), 2062–2070. doi: 10.1172/JCI114943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva L, Bastie JN, Rochette-Egly C, Kraiba R, Balitrand N, Despouy G, … Chomienne C (1999). Physical and functional interactions between cellular retinoic acid binding protein II and the retinoic acid-dependent nuclear complex. Mol Cell Biol, 19(10), 7158–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derguini F, Nakanishi K, Hammerling U, Chua R, Eppinger T, Levi E, & Buck J (1995). 13,14-Dihydroxy-retinol, a new bioactive retinol metabolite. J Biol Chem, 270(32), 18875–18880. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Chen F, Lu J, Qian J, Niederreither K, Dolle P, … Cardoso WV (2006). Distinct roles for retinoic acid receptors alpha and beta in early lung morphogenesis. Dev Biol, 291(1), 12–24. doi: 10.1016/j.ydbio.2005.10.045 [DOI] [PubMed] [Google Scholar]
- Di Carlo SE, & Peduto L (2018). The perivascular origin of pathological fibroblasts. J Clin Invest, 128(1), 54–63. doi: 10.1172/JCI93558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Tai W, Yang Y, Zhang T, Li Y, Chai Y, … Wang D (2012). The role of all-trans retinoic acid in bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res, 38(2), 82–89. doi: 10.3109/01902148.2011.646052 [DOI] [PubMed] [Google Scholar]
- Dowling JE, & Wald G (1960). The Biological Function of Vitamin a Acid. Proc Natl Acad Sci U S A, 46(5), 587–608. doi: 10.1073/pnas.46.5.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, … Deb A (2012). Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J, 31(2), 429–442. doi: 10.1038/emboj.2011.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupe V, Matt N, Garnier JM, Chambon P, Mark M, & Ghyselinck NB (2003). A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci U S A, 100(24), 14036–14041. doi: 10.1073/pnas.2336223100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, … Piccolo S (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179–183. doi: 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- E X, Zhang L, Lu J, Tso P, Blaner WS, Levin MS, & Li E (2002). Increased neonatal mortality in mice lacking cellular retinol-binding protein II. J Biol Chem, 277(39), 36617–36623. doi: 10.1074/jbc.M205519200 [DOI] [PubMed] [Google Scholar]
- El-Mezayen NS, El-Hadidy WF, El-Refaie WM, Shalaby TI, Khattab MM, & El-Khatib AS (2018). Oral vitamin-A-coupled valsartan nanomedicine: High hepatic stellate cell receptors accessibility and prolonged enterohepatic residence. J Control Release, 283, 32–44. doi: 10.1016/j.jconrel.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Esteban-Pretel G, Marin MP, Renau-Piqueras J, Barber T, & Timoneda J (2010). Vitamin A deficiency alters rat lung alveolar basement membrane: reversibility by retinoic acid. J Nutr Biochem, 21(3), 227–236. doi: 10.1016/j.jnutbio.2008.12.007 [DOI] [PubMed] [Google Scholar]
- Feng L, Hernandez RE, Waxman JS, Yelon D, & Moens CB (2010). Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev Biol, 338(1), 1–14. doi: 10.1016/j.ydbio.2009.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella PD, & Napoli JL (1994). Microsomal retinoic acid metabolism. Effects of cellular retinoic acid-binding protein (type I) and C18-hydroxylation as an initial step. J Biol Chem, 269(14), 10538–10544. [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, & Voorhees JJ (1996). Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature, 379(6563), 335–339. doi: 10.1038/379335a0 [DOI] [PubMed] [Google Scholar]
- Fisher GJ, & Voorhees JJ (1996). Molecular mechanisms of retinoid actions in skin. FASEB J, 10(9), 1002–1013. [DOI] [PubMed] [Google Scholar]
- Friedman SL (2008). Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev, 88(1), 125–172. doi: 10.1152/physrev.00013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, … Kocher HM (2011). Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology, 141(4), 1486–1497, 1497 e1481–1414. doi: 10.1053/j.gastro.2011.06.047 [DOI] [PubMed] [Google Scholar]
- Fu M, Sato Y, Lyons-Warren A, Zhang B, Kane MA, Napoli JL, & Heuckeroth RO (2010). Vitamin A facilitates enteric nervous system precursor migration by reducing Pten accumulation. Development, 137(4), 631–640. doi: 10.1242/dev.040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, … Yamaya M (2011). Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest, 91(3), 363–378. doi: 10.1038/labinvest.2010.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber SJ, Zhang H, Foley JP, Zhao H, Butler SJ, Godinez RI, … Savani RC (2006). Hormonal regulation of alveolarization: structure-function correlation. Respir Res, 7, 47. doi: 10.1186/1465-9921-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Bavik C, Sapin V, Mark M, Bonnier D, Hindelang C, … Chambon P (1999). Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO J, 18(18), 4903–4914. doi: 10.1093/emboj/18.18.4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghyselinck NB, Dupe V, Dierich A, Messaddeq N, Garnier JM, Rochette-Egly C, … Mark M (1997). Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int J Dev Biol, 41(3), 425–447. [PubMed] [Google Scholar]
- Giguere V, Ong ES, Segui P, & Evans RM (1987). Identification of a receptor for the morphogen retinoic acid. Nature, 330(6149), 624–629. doi: 10.1038/330624a0 [DOI] [PubMed] [Google Scholar]
- Gjoen T, Bjerkelund T, Blomhoff HK, Norum KR, Berg T, & Blomhoff R (1987). Liver takes up retinol-binding protein from plasma. J Biol Chem, 262(23), 10926–10930. [PubMed] [Google Scholar]
- Gong L, Jiang L, Qin Y, Jiang X, Song K, & Yu X (2018). Protective effect of retinoic acid receptor alpha on hypoxia-induced epithelial to mesenchymal transition of renal tubular epithelial cells associated with TGF-beta/MMP-9 pathway. Cell Biol Int, 42(8), 1050–1059. doi: 10.1002/cbin.10982 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, & Burns CG (2018). Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell, 44(4), 433–446 e437. doi: 10.1016/j.devcel.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DM, & Medici D (2014). Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal, 7(344), re8. doi: 10.1126/scisignal.2005189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdie RG, Dimmeler S, & Kohl P (2016). Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov, 15(9), 620–638. doi: 10.1038/nrd.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, & Voorhees JJ (1993). Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med, 329(8), 530–535. doi: 10.1056/NEJM199308193290803 [DOI] [PubMed] [Google Scholar]
- Gruber PJ, Kubalak SW, Pexieder T, Sucov HM, Evans RM, & Chien KR (1996). RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J Clin Invest, 98(6), 1332–1343. doi: 10.1172/JCI118920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadix JA, Ruiz-Villalba A, Lettice L, Velecela V, Munoz-Chapuli R, Hastie ND, … Martinez-Estrada OM (2011). Wt1 controls retinoic acid signalling in embryonic epicardium through transcriptional activation of Raldh2. Development, 138(6), 1093–1097. doi: 10.1242/dev.044594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas LJ (2012). Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta, 1821(1), 213–221. doi: 10.1016/j.bbalip.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Huang J, Lebioda L, Saari JC, & Palczewski K (1998). Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem, 273(34), 21790–21799. [DOI] [PubMed] [Google Scholar]
- Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, … Penninger JM (2016). Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ Res, 118(2), 216–221. doi: 10.1161/CIRCRESAHA.115.307017 [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, … Stroke C (2013). Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail, 6(3), 606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr FM, & Ong DE (1992). Differential interaction of lecithin-retinol acyltransferase with cellular retinol binding proteins. Biochemistry, 31(29), 6748–6755. [DOI] [PubMed] [Google Scholar]
- Herr FM, Wardlaw SA, Kakkad B, Albrecht A, Quick TC, & Ong DE (1993). Intestinal vitamin A metabolism: coordinate distribution of enzymes and CRBP(II). J Lipid Res, 34(9), 1545–1554. [PubMed] [Google Scholar]
- Herrera J, Henke CA, & Bitterman PB (2018). Extracellular matrix as a driver of progressive fibrosis. J Clin Invest, 128(1), 45–53. doi: 10.1172/JCI93557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herum KM, Lunde IG, McCulloch AD, & Christensen G (2017). The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart. J Clin Med, 6(5). doi: 10.3390/jcm6050053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, … Wyss A (2007). CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem, 282(46), 33553–33561. doi: 10.1074/jbc.M706763200 [DOI] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, & Thaller C (1992). 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell, 68(2), 397–406. [DOI] [PubMed] [Google Scholar]
- Hind M, Corcoran J, & Maden M (2002a). Alveolar proliferation, retinoid synthesizing enzymes, and endogenous retinoids in the postnatal mouse lung. Different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol, 26(1), 67–73. doi: 10.1165/ajrcmb.26.1.4575 [DOI] [PubMed] [Google Scholar]
- Hind M, Corcoran J, & Maden M (2002b). Temporal/spatial expression of retinoid binding proteins and RAR isoforms in the postnatal lung. Am J Physiol Lung Cell Mol Physiol, 282(3), L468–476. doi: 10.1152/ajplung.00196.2001 [DOI] [PubMed] [Google Scholar]
- Hind M, & Maden M (2011). Is a regenerative approach viable for the treatment of COPD? Br J Pharmacol, 163(1), 106–115. doi: 10.1111/j.1476-5381.2011.01246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, & Chaponnier C (2001). Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell, 12(9), 2730–2741. doi: 10.1091/mbc.12.9.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Lopez B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, … Seidman CE (2010). Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med, 363(6), 552–563. doi: 10.1056/NEJMoa1002659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Qin YH, Pei J, Lei FY, Hu B, & Lu L (2010). Beneficial effect of all-trans retinoic acid (ATRA) on glomerulosclerosis rats via the down-regulation of the expression of alpha-smooth muscle actin: a comparative study between ATRA and benazepril. Exp Mol Pathol, 89(1), 51–57. doi: 10.1016/j.yexmp.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, … Olson EN (2012). C/EBP transcription factors mediate epicardial activation during heart development and injury. Science, 338(6114), 1599–1603. doi: 10.1126/science.1229765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Yu J, Jones JW, Carter CL, Jackson IL, Vujaskovic Z, … Kane MA (2019). Acute Proteomic Changes in the Lung After WTLI in a Mouse Model: Identification of Potential Initiating Events for Delayed Effects of Acute Radiation Exposure. Health Phys, 116(4), 503–515. doi: 10.1097/HP.0000000000000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, … Zhou Y (2012). Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol, 47(3), 340–348. doi: 10.1165/rcmb.2012-0050OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan C, … Lien CL (2013). Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. Plos One, 8(6), e67266. doi: 10.1371/journal.pone.0067266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, & Srivastava D (2009). Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell, 16(2), 233–244. doi: 10.1016/j.devcel.2008.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijpenberg A, Perez-Pomares JM, Guadix JA, Carmona R, Portillo-Sanchez V, Macias D, … Munoz-Chapuli R (2007). Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol, 312(1), 157–170. doi: 10.1016/j.ydbio.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Imai T, Jiang M, Kastner P, Chambon P, & Metzger D (2001). Selective ablation of retinoid X receptor alpha in hepatocytes impairs their lifespan and regenerative capacity. Proc Natl Acad Sci U S A, 98(8), 4581–4586. doi: 10.1073/pnas.071056098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inage M, Nakamura H, Saito H, Abe S, Hino T, Takabatake N, … Tomoike H (2009). Vesnarinone represses the fibrotic changes in murine lung injury induced by bleomycin. Int J Biol Sci, 5(4), 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, … Sasaki H (2004). Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett, 556(1–3), 249–252. [DOI] [PubMed] [Google Scholar]
- Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, … von Lintig J (2008). RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab, 7(3), 258–268. doi: 10.1016/j.cmet.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, … Kisseleva T (2014). Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A, 111(32), E3297–3305. doi: 10.1073/pnas.1400062111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, & Evans RM (2006). Retinoic acid is required early during adult neurogenesis in the dentate gyrus. Proc Natl Acad Sci U S A, 103(10), 3902–3907. doi: 10.1073/pnas.0511294103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Yang Y, Xue L, Li B, & Zhang Z (2017). 1α,25-dihydroxyvitamin D3- Attenuates TGF-β-Induced Pro-Fibrotic Effects in Human Lung Epithelial Cells through Inhibition of Epithelial-Mesenchymal Transition. Nutrients, 9(9). doi: 10.3390/nu9090980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, & Napoli JL (2012). Reorganization of cellular retinol-binding protein type 1 and lecithin:retinol acyltransferase during retinyl ester biosynthesis. Biochim Biophys Acta, 1820(7), 859–869. doi: 10.1016/j.bbagen.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Pierzchalski K, Yu J, & Kane MA (2015). Use of fast HPLC multiple reaction monitoring cubed for endogenous retinoic acid quantification in complex matrices. Anal Chem, 87(6), 3222–3230. doi: 10.1021/ac504597q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JW, Scott AJ, Tudor G, Xu PT, Jackson IL, Vujaskovic Z, … Kane MA (2014). Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys, 106(1), 106–119. doi: 10.1097/HP.0b013e3182a4ed3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jophlin LL, Koutalos Y, Chen C, Shah V, & Rockey DC (2018). Hepatic stellate cells retain retinoid-laden lipid droplets after cellular transdifferentiation into activated myofibroblasts. Am J Physiol Gastrointest Liver Physiol, 315(5), G713–G721. doi: 10.1152/ajpgi.00251.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage H, & Borok Z (2012). EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med, 18(5), 517–523. doi: 10.1097/MCP.0b013e3283566721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D’Amario D, Sanada F, … Anversa P (2011). Evidence for human lung stem cells. N Engl J Med, 364(19), 1795–1806. doi: 10.1056/NEJMoa1101324 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kakkad BP, & Ong DE (1988). Reduction of retinaldehyde bound to cellular retinol-binding protein (type II) by microsomes from rat small intestine. J Biol Chem, 263(26), 12916–12919. [PubMed] [Google Scholar]
- Kam RK, Shi W, Chan SO, Chen Y, Xu G, Lau CB, … Zhao H (2013). Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning. J Biol Chem, 288(44), 31477–31487. doi: 10.1074/jbc.M113.514984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Raz A, & Goodman DS (1968). Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest, 47(9), 2025–2044. doi: 10.1172/JCI105889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA (2012). Analysis, occurrence, and function of 9-cis-retinoic acid. Biochim Biophys Acta, 1821(1), 10–20. doi: 10.1016/j.bbalip.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Kane MA, Bright FV, & Napoli JL (2011). Binding affinities of CRBPI and CRBPII for 9-cis-retinoids. Biochim Biophys Acta, 1810(5), 514–518. doi: 10.1016/j.bbagen.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Chen N, Sparks S, & Napoli JL (2005). Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J, 388(Pt 1), 363–369. doi: 10.1042/BJ20041867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Pingitore A, Perri M, Krois CR, Ryu JY, … Napoli JL (2011). CrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrations. Mol Cell Biol, 31(16), 3277–3285. doi: 10.1128/MCB.05516-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Pingitore A, Perri M, Obrochta KM, Krois CR, … Napoli JL (2010). Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A, 107(50), 21884–21889. doi: 10.1073/pnas.1008859107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, & Napoli JL (2008). Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem, 80(5), 1702–1708. doi: 10.1021/ac702030f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, & Napoli JL (2010). Quantification of endogenous retinoids. Methods Mol Biol, 652, 1–54. doi: 10.1007/978-1-60327-325-1_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, … Molkentin JD (2016). Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun, 7, 12260. doi: 10.1038/ncomms12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer P, Morf R, & Schöpp K (1931). Zur Kenntnis des Vitamins-A aus Fischtranen. Helvetica Chimica Acta, 14(5), 1036–1040. doi: 10.1002/hlca.19310140511 [DOI] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, & Chambon P (1997). Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development, 124(2), 313–326. [DOI] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, & Tabin CJ (2012). Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell, 22(3), 639–650. doi: 10.1016/j.devcel.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, … Sun H (2007). A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science, 315(5813), 820–825. doi: 10.1126/science.1136244 [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Zhong M, Kassai M, Ter-Stepanian M, & Sun H (2012). STRA6-catalyzed vitamin A influx, efflux, and exchange. J Membr Biol, 245(11), 731–745. doi: 10.1007/s00232-012-9463-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza AK, Kron IL, Kern JA, Long SM, Fiser SM, Nguyen RP, … Laubach VE (2001). Retinoic acid enhances lung growth after pneumonectomy. Ann Thorac Surg, 71(5), 1645–1650. [DOI] [PubMed] [Google Scholar]
- Kedishvili NY (2016). Retinoic Acid Synthesis and Degradation. Subcell Biochem, 81, 127–161. doi: 10.1007/978-94-024-0945-1_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ME, Ramkumar S, Sun W, Colon Ortiz C, Kiser PD, Golczak M, & von Lintig J (2018). The Biochemical Basis of Vitamin A Production from the Asymmetric Carotenoid beta-Cryptoxanthin. ACS Chem Biol, 13(8), 2121–2129. doi: 10.1021/acschembio.8b00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G, Gay S, Inouye T, Bahu R, Minick OT, & Popper H (1976). Vitamin A-containing lipocytes and formation of type III collagen in liver injury. Proc Natl Acad Sci U S A, 73(10), 3719–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, & von Lintig J (2001). Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem, 276(17), 14110–14116. doi: 10.1074/jbc.M011510200 [DOI] [PubMed] [Google Scholar]
- Kiefer C, Sumser E, Wernet MF, & Von Lintig J (2002). A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A, 99(16), 10581–10586. doi: 10.1073/pnas.162182899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, & Poss KD (2011). tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development, 138(14), 2895–2902. doi: 10.1242/dev.067041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, & Poss KD (2011). Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell, 20(3), 397–404. doi: 10.1016/j.devcel.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, … Jacks T (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell, 121(6), 823–835. doi: 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Kim N, Yoo W, Lee J, Kim H, Lee H, Kim YS, … Oh J (2009). Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut, 58(10), 1382–1390. doi: 10.1136/gut.2008.170233 [DOI] [PubMed] [Google Scholar]
- Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, & Quadro L (2011). beta-Carotene and its cleavage enzyme beta-carotene-15,15’-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J, 25(5), 1641–1652. doi: 10.1096/fj.10-175448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, … Brenner DA (2012). Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A, 109(24), 9448–9453. doi: 10.1073/pnas.1201840109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, & Evans RM (1992). Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature, 355(6359), 446–449. doi: 10.1038/355446a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, … Schwabe RF (2011). Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut, 60(9), 1260–1268. doi: 10.1136/gut.2010.209551 [DOI] [PubMed] [Google Scholar]
- Koopmans T, & Rinkevich Y (2018). Mesothelial to mesenchyme transition as a major developmental and pathological player in trunk organs and their cavities. Commun Biol, 1, 170. doi: 10.1038/s42003-018-0180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Lu R, Pence LJ, & Anastasiadis PZ (2017). A central role for cadherin signaling in cancer. Exp Cell Res, 358(1), 78–85. doi: 10.1016/j.yexcr.2017.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, … Lowe SW (2008). Senescence of activated stellate cells limits liver fibrosis. Cell, 134(4), 657–667. doi: 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse SW, Suino-Powell K, Zhou XE, Kretschman JE, Reynolds R, Vonrhein C, … Xu HE (2008). Identification of COUP-TFII orphan nuclear receptor as a retinoic acid-activated receptor. PLoS Biol, 6(9), e227. doi: 10.1371/journal.pbio.0060227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sandell LL, Trainor PA, Koentgen F, & Duester G (2012). Alcohol and aldehyde dehydrogenases: retinoid metabolic effects in mouse knockout models. Biochim Biophys Acta, 1821(1), 198–205. doi: 10.1016/j.bbalip.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert JM, Holzschuh J, Hessel S, Driever W, Vogt K, & von Lintig J (2003). Provitamin A conversion to retinal via the beta,beta-carotene-15,15’-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development, 130(10), 2173–2186. [DOI] [PubMed] [Google Scholar]
- Langton S, & Gudas LJ (2008). CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Dev Biol, 315(2), 331–354. doi: 10.1016/j.ydbio.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Lapshina EA, Belyaeva OV, Chumakova OV, & Kedishvili NY (2003). Differential recognition of the free versus bound retinol by human microsomal retinol/sterol dehydrogenases: characterization of the holo-CRBP dehydrogenase activity of RoDH-4. Biochemistry, 42(3), 776–784. doi: 10.1021/bi026836r [DOI] [PubMed] [Google Scholar]
- Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, … Romagnani P (2015). Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Remission and Can Be Pharmacologically Enhanced. Stem Cell Reports, 5(2), 248–263. doi: 10.1016/j.stemcr.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, & Ornitz DM (2005). Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell, 8(1), 85–95. doi: 10.1016/j.devcel.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Lee LM, Leung CY, Tang WW, Choi HL, Leung YC, McCaffery PJ, … Shum AS (2012). A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci U S A, 109(34), 13668–13673. doi: 10.1073/pnas.1200872109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Jiang H, Trent CM, Yuen JJ, Narayanasamy S, Curley RW Jr., … Blaner WS (2014). Cardiac dysfunction in beta-carotene-15,15’-dioxygenase-deficient mice is associated with altered retinoid and lipid metabolism. Am J Physiol Heart Circ Physiol, 307(11), H1675–1684. doi: 10.1152/ajpheart.00548.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, & Jeong WI (2012). Retinoic acids and hepatic stellate cells in liver disease. J Gastroenterol Hepatol, 27 Suppl 2, 75–79. doi: 10.1111/j.1440-1746.2011.07007.x [DOI] [PubMed] [Google Scholar]
- Leo MA, & Lieber CS (1982). Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med, 307(10), 597–601. doi: 10.1056/NEJM198209023071006 [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, & Poss KD (2006). A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell, 127(3), 607–619. doi: 10.1016/j.cell.2006.08.052 [DOI] [PubMed] [Google Scholar]
- Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, … et al. (1992). 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature, 355(6358), 359–361. doi: 10.1038/355359a0 [DOI] [PubMed] [Google Scholar]
- Levin MS, & Davis AE (1997). Retinoic acid increases cellular retinol binding protein II mRNA and retinol uptake in the human intestinal Caco-2 cell line. J Nutr, 127(1), 13–17. doi: 10.1093/jn/127.1.13 [DOI] [PubMed] [Google Scholar]
- Li F, Zhu W, & Gonzalez FJ (2017). Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol Ther, 178, 18–30. doi: 10.1016/j.pharmthera.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, … Zhou B (2018). Genetic Lineage Tracing of Nonmyocyte Population by Dual Recombinases. Circulation, 138(8), 793–805. doi: 10.1161/CIRCULATIONAHA.118.034250 [DOI] [PubMed] [Google Scholar]
- Li Y, Lui KO, & Zhou B (2018). Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat Rev Cardiol, 15(8), 445–456. doi: 10.1038/s41569-018-0023-y [DOI] [PubMed] [Google Scholar]
- Limana F, Capogrossi MC, & Germani A (2011). The epicardium in cardiac repair: from the stem cell view. Pharmacol Ther, 129(1), 82–96. doi: 10.1016/j.pharmthera.2010.09.002 [DOI] [PubMed] [Google Scholar]
- Lin SC, Dolle P, Ryckebusch L, Noseda M, Zaffran S, Schneider MD, & Niederreither K (2010). Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A, 107(20), 9234–9239. doi: 10.1073/pnas.0910430107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, & Tschumperlin DJ (2010). Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol, 190(4), 693–706. doi: 10.1083/jcb.201004082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Ly I, Hu Y, & Wan YJ (2014). Retinoic acid regulates cell cycle genes and accelerates normal mouse liver regeneration. Biochem Pharmacol, 91(2), 256–265. doi: 10.1016/j.bcp.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, & Gudas LJ (2005). Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem, 280(48), 40226–40234. doi: 10.1074/jbc.M509643200 [DOI] [PubMed] [Google Scholar]
- Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, & von Lintig J (2013). Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem, 288(13), 9017–9027. doi: 10.1074/jbc.M112.444240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, … von Lintig J (2010). ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J, 24(6), 1656–1666. doi: 10.1096/fj.09-150995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, & Chambon P (1993). Function of retinoic acid receptor gamma in the mouse. Cell, 73(4), 643–658. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, … Chambon P (1994). Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development, 120(10), 2723–2748. [DOI] [PubMed] [Google Scholar]
- Loudig O, Babichuk C, White J, Abu-Abed S, Mueller C, & Petkovich M (2000). Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol Endocrinol, 14(9), 1483–1497. doi: 10.1210/mend.14.9.0518 [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, … Johnson RL (2010). Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A, 107(4), 1437–1442. doi: 10.1073/pnas.0911427107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, … Chambon P (1993). High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A, 90(15), 7225–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean G, Abu-Abed S, Dolle P, Tahayato A, Chambon P, & Petkovich M (2001). Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev, 107(1–2), 195–201. [DOI] [PubMed] [Google Scholar]
- Macmahon HE (1937). Hyperplasia and Regeneration of the Myocardium in Infants and in Children. Am J Pathol, 13(5), 845–854 845. [PMC free article] [PubMed] [Google Scholar]
- Maden M (2000). The role of retinoic acid in embryonic and post-embryonic development. Proc Nutr Soc, 59(1), 65–73. [DOI] [PubMed] [Google Scholar]
- Maden M (2007). Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci, 8(10), 755–765. doi: 10.1038/nrn2212 [DOI] [PubMed] [Google Scholar]
- Maden M, & Hind M (2004). Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci, 359(1445), 799–808. doi: 10.1098/rstb.2004.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire M, Larsen MC, Foong YH, Tanumihardjo S, & Jefcoate CR (2017). Cyp1b1 deletion and retinol deficiency coordinately suppress mouse liver lipogenic genes and hepcidin expression during post-natal development. Mol Cell Endocrinol, 454, 50–68. doi: 10.1016/j.mce.2017.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Dingal PC, & Discher DE (2014). Stress sensitivity and mechanotransduction during heart development. Curr Biol, 24(10), R495–501. doi: 10.1016/j.cub.2014.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, & Discher DE (2013). Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol, 23(23), 2434–2439. doi: 10.1016/j.cub.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaba L, Kindberg GM, Norum KR, Berg T, & Blomhoff R (1993). Receptor-mediated endocytosis of retinol-binding protein by liver parenchymal cells: interference by radioactive iodination. Biochem J, 291 ( Pt 1), 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Ong ES, Dyck JA, & Evans RM (1990). Nuclear receptor that identifies a novel retinoic acid response pathway. Nature, 345(6272), 224–229. doi: 10.1038/345224a0 [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Umesono K, Kliewer SA, Borgmeyer U, Ong ES, & Evans RM (1991). A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell, 66(3), 555–561. [DOI] [PubMed] [Google Scholar]
- Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, … van Grunsven LA (2015). The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol, 63(3), 679–688. doi: 10.1016/j.jhep.2015.04.011 [DOI] [PubMed] [Google Scholar]
- Manolescu DC, Jankowski M, Danalache BA, Wang D, Broderick TL, Chiasson JL, & Gutkowska J (2014). All-trans retinoic acid stimulates gene expression of the cardioprotective natriuretic peptide system and prevents fibrosis and apoptosis in cardiomyocytes of obese ob/ob mice. Appl Physiol Nutr Metab, 39(10), 1127–1136. doi: 10.1139/apnm-2014-0005 [DOI] [PubMed] [Google Scholar]
- Mao JT, Goldin JG, Dermand J, Ibrahim G, Brown MS, Emerick A, … Roth MD (2002). A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med, 165(5), 718–723. doi: 10.1164/ajrccm.165.5.2106123 [DOI] [PubMed] [Google Scholar]
- Marin-Juez R, Marass M, Gauvrit S, Rossi A, Lai SL, Materna SC, … Stainier DY (2016). Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc Natl Acad Sci U S A, 113(40), 11237–11242. doi: 10.1073/pnas.1605431113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MP, Esteban-Pretel G, Alonso R, Sado Y, Barber T, Renau-Piqueras J, & Timoneda J (2005). Vitamin A deficiency alters the structure and collagen IV composition of rat renal basement membranes. J Nutr, 135(4), 695–701. doi: 10.1093/jn/135.4.695 [DOI] [PubMed] [Google Scholar]
- Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, … Walsh K (2016). Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol Med, 8(8), 949–966. doi: 10.15252/emmm.201506151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro D, & Massaro GD (2002). Invited Review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol, 282(3), L345–358. doi: 10.1152/ajplung.00374.2001 [DOI] [PubMed] [Google Scholar]
- Massaro D, & Massaro GD (2006). Toward therapeutic pulmonary alveolar regeneration in humans. Proc Am Thorac Soc, 3(8), 709–712. doi: 10.1513/pats.200605-127SF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro GD, & Massaro D (2000). Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol, 278(5), L955–960. doi: 10.1152/ajplung.2000.278.5.L955 [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D, & Chambon P (2003). Retinoic acid receptor-alpha regulates pulmonary alveolus formation in mice after, but not during, perinatal period. Am J Physiol Lung Cell Mol Physiol, 284(2), L431–433. doi: 10.1152/ajplung.00245.2002 [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, & Chandraratna RA (2000). Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics, 4(1), 51–57. doi: 10.1152/physiolgenomics.2000.4.1.51 [DOI] [PubMed] [Google Scholar]
- Matsui T (1996). Differential activation of the murine laminin B1 gene promoter by RAR alpha, ROR alpha, and AP-1. Biochem Biophys Res Commun, 220(2), 405–410. doi: 10.1006/bbrc.1996.0418 [DOI] [PubMed] [Google Scholar]
- Matt N, Schmidt CK, Dupe V, Dennefeld C, Nau H, Chambon P, … Ghyselinck NB (2005). Contribution of cellular retinol-binding protein type 1 to retinol metabolism during mouse development. Dev Dyn, 233(1), 167–176. doi: 10.1002/dvdy.20313 [DOI] [PubMed] [Google Scholar]
- Mayor R, & Theveneau E (2013). The neural crest. Development, 140(11), 2247–2251. doi: 10.1242/dev.091751 [DOI] [PubMed] [Google Scholar]
- McCarroll JA, Phillips PA, Santucci N, Pirola RC, Wilson JS, & Apte MV (2006). Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut, 55(1), 79–89. doi: 10.1136/gut.2005.064543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KG, Leach MR, Brooke KW, Wang C, Wheeler LW, Hanly EK, … Newberry RD (2012). Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids. Am J Pathol, 180(3), 984–997. doi: 10.1016/j.ajpath.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, & Snyder JM (2000). Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol, 23(2), 162–167. doi: 10.1165/ajrcmb.23.2.3904 [DOI] [PubMed] [Google Scholar]
- McGowan SE, Harvey CS, & Jackson SK (1995). Retinoids, retinoic acid receptors, and cytoplasmic retinoid binding proteins in perinatal rat lung fibroblasts. Am J Physiol, 269(4 Pt 1), L463–472. doi: 10.1152/ajplung.1995.269.4.L463 [DOI] [PubMed] [Google Scholar]
- McGowan SE, & Holmes AJ (2007). Vitamin A deficiency alters pulmonary parenchymal collagen and tissue mechanics. Respir Physiol Neurobiol, 156(3), 312–319. doi: 10.1016/j.resp.2006.11.008 [DOI] [PubMed] [Google Scholar]
- McGowan SE, Holmes AJ, & Smith J (2004). Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am J Physiol Lung Cell Mol Physiol, 286(2), L437–444. doi: 10.1152/ajplung.00158.2003 [DOI] [PubMed] [Google Scholar]
- McGowan SE, Smith J, Holmes AJ, Smith LA, Businga TR, Madsen MT, … Kline JN (2002). Vitamin A deficiency promotes bronchial hyperreactivity in rats by altering muscarinic M(2) receptor function. Am J Physiol Lung Cell Mol Physiol, 282(5), L1031–1039. doi: 10.1152/ajplung.00319.2001 [DOI] [PubMed] [Google Scholar]
- McGowan SE, Takle EJ, & Holmes AJ (2005). Vitamin A deficiency alters the pulmonary parenchymal elastic modulus and elastic fiber concentration in rats. Respir Res, 6, 77. doi: 10.1186/1465-9921-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan SE, & Torday JS (1997). The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol, 59, 43–62. doi: 10.1146/annurev.physiol.59.1.43 [DOI] [PubMed] [Google Scholar]
- Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, … Schwabe RF (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun, 4, 2823. doi: 10.1038/ncomms3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, … Tallquist MD (2008). Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circ Res, 103(12), 1393–1401. doi: 10.1161/CIRCRESAHA.108.176768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merki E, Zamora M, Raya A, Kawakami Y, Wang J, Zhang X, … Ruiz-Lozano P (2005). Epicardial retinoid X receptor alpha is required for myocardial growth and coronary artery formation. Proc Natl Acad Sci U S A, 102(51), 18455–18460. doi: 10.1073/pnas.0504343102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezaki Y, Yamaguchi N, Yoshikawa K, Miura M, Imai K, Itoh H, & Senoo H (2009). Insoluble, speckled cytosolic distribution of retinoic acid receptor alpha protein as a marker of hepatic stellate cell activation in vitro. J Histochem Cytochem, 57(7), 687–699. doi: 10.1369/jhc.2009.953208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Fan X, Cuenca AE, & Duester G (2000). RALDH3, a retinaldehyde dehydrogenase that generates retinoic acid, is expressed in the ventral retina, otic vesicle and olfactory pit during mouse development. Mech Dev, 97(1–2), 227–230. [DOI] [PubMed] [Google Scholar]
- Mikawa T, & Fischman DA (1992). Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A, 89(20), 9504–9508. doi: 10.1073/pnas.89.20.9504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milliano MT, & Luxon BA (2005). Rat hepatic stellate cells become retinoid unresponsive during activation. Hepatol Res, 33(3), 225–233. doi: 10.1016/j.hepres.2005.08.007 [DOI] [PubMed] [Google Scholar]
- Minicucci MF, Azevedo PS, Oliveira SA Jr., Martinez PF, Chiuso-Minicucci F, Polegato BF, … Zornoff LA (2010). Tissue vitamin A insufficiency results in adverse ventricular remodeling after experimental myocardial infarction. Cell Physiol Biochem, 26(4–5), 523–530. doi: 10.1159/000322320 [DOI] [PubMed] [Google Scholar]
- Moise AR, Dominguez M, Alvarez S, Alvarez R, Schupp M, Cristancho AG, … Palczewski K (2008). Stereospecificity of retinol saturase: absolute configuration, synthesis, and biological evaluation of dihydroretinoids. J Am Chem Soc, 130(4), 1154–1155. doi: 10.1021/ja710487q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moise AR, Kuksa V, Imanishi Y, & Palczewski K (2004). Identification of all-trans-retinol:all-trans-13,14-dihydroretinol saturase. J Biol Chem, 279(48), 50230–50242. doi: 10.1074/jbc.M409130200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard R, Ghyselinck NB, Wendling O, Chambon P, & Mark M (2000). Stage-dependent responses of the developing lung to retinoic acid signaling. Int J Dev Biol, 44(5), 457–462. [PubMed] [Google Scholar]
- Moore-Morris T, Cattaneo P, Guimaraes-Camboa N, Bogomolovas J, Cedenilla M, Banerjee I, … Evans SM (2018). Infarct Fibroblasts Do Not Derive From Bone Marrow Lineages. Circ Res, 122(4), 583–590. doi: 10.1161/CIRCRESAHA.117.311490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, … Evans SM (2014). Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest, 124(7), 2921–2934. doi: 10.1172/JCI74783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora O, Kuri-Melo L, Gonzalez-Gallardo A, Melendez E, Morales A, Shimada A, & Varela-Echavarria A (2004). A potential role for beta-carotene in avian embryonic development. Int J Vitam Nutr Res, 74(2), 116–122. doi: 10.1024/0300-9831.74.2.116 [DOI] [PubMed] [Google Scholar]
- Morabia A, Menkes MJ, Comstock GW, & Tockman MS (1990). Serum retinol and airway obstruction. Am J Epidemiol, 132(1), 77–82. [DOI] [PubMed] [Google Scholar]
- Morath C, Dechow C, Lehrke I, Haxsen V, Waldherr R, Floege J, … Wagner J (2001). Effects of retinoids on the TGF-beta system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol, 12(11), 2300–2309. [DOI] [PubMed] [Google Scholar]
- Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, & Rosenthal N (1998). Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol, 199(1), 55–71. doi: 10.1006/dbio.1998.8911 [DOI] [PubMed] [Google Scholar]
- Muenzner M, Tuvia N, Deutschmann C, Witte N, Tolkachov A, Valai A, … Schupp M (2013). Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor alpha activity. Mol Cell Biol, 33(20), 4068–4082. doi: 10.1128/MCB.00221-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy NE, Holven KB, Roos N, Senoo H, Kojima N, Norum KR, & Blomhoff R (1997). Storage of vitamin A in extrahepatic stellate cells in normal rats. J Lipid Res, 38(4), 645–658. [PubMed] [Google Scholar]
- Napoli JL (2017). Cellular retinoid binding-proteins, CRBP, CRABP, FABP5: Effects on retinoid metabolism, function and related diseases. Pharmacol Ther, 173, 19–33. doi: 10.1016/j.pharmthera.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CH, Peng CC, Lutz JD, Yeung CK, Zelter A, & Isoherranen N (2016). Direct protein-protein interactions and substrate channeling between cellular retinoic acid binding proteins and CYP26B1. FEBS Lett, 590(16), 2527–2535. doi: 10.1002/1873-3468.12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng-Blichfeldt JP, Schrik A, Kortekaas RK, Noordhoek JA, Heijink IH, Hiemstra PS, … Gosens R (2018). Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine, 36, 461–474. doi: 10.1016/j.ebiom.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, & Dolle P (2002). Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat Genet, 31(1), 84–88. doi: 10.1038/ng876 [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, & Chambon P (1999). Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet, 21(4), 444–448. doi: 10.1038/7788 [DOI] [PubMed] [Google Scholar]
- Nollevaux MC, Guiot Y, Horsmans Y, Leclercq I, Rahier J, Geubel AP, & Sempoux C (2006). Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption. Liver Int, 26(2), 182–186. doi: 10.1111/j.1478-3231.2005.01207.x [DOI] [PubMed] [Google Scholar]
- O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, … Blaner WS (2005). Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J Biol Chem, 280(42), 35647–35657. doi: 10.1074/jbc.M507924200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelin G, Faure E, Coulpier F, Di Bonito M, Bajolle F, Studer M, … Zaffran S (2018). Krox20 defines a subpopulation of cardiac neural crest cells contributing to arterial valves and bicuspid aortic valve. Development, 145(1). doi: 10.1242/dev.151944 [DOI] [PubMed] [Google Scholar]
- Okabe T, Yorifuji H, Yamada E, & Takaku F (1984). Isolation and characterization of vitamin-A-storing lung cells. Exp Cell Res, 154(1), 125–135. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, & Okano H (2004). Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol, 275(1), 124–142. doi: 10.1016/j.ydbio.2004.07.038 [DOI] [PubMed] [Google Scholar]
- Okimoto S, Kuroda S, Tashiro H, Kobayashi T, Taogoshi T, Matsuo H, & Ohdan H (2019). Vitamin A-coupled liposomal Rho-kinase inhibitor ameliorates liver fibrosis without systemic adverse effects. Hepatol Res. doi: 10.1111/hepr.13317 [DOI] [PubMed] [Google Scholar]
- Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, … Wells RG (2011). Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol, 301(1), G110–118. doi: 10.1152/ajpgi.00412.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong DE, & Chytil F (1976). Changes in levels of cellular retinol- and retinoic-acid-binding proteins of liver and lung during perinatal development of rat. Proc Natl Acad Sci U S A, 73(11), 3976–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong DE, Kakkad B, & MacDonald PN (1987). Acyl-CoA-independent esterification of retinol bound to cellular retinol-binding protein (type II) by microsomes from rat small intestine. J Biol Chem, 262(6), 2729–2736. [PubMed] [Google Scholar]
- Ongstad EL, & Gourdie RG (2016). Can heart function lost to disease be regenerated by therapeutic targeting of cardiac scar tissue? Semin Cell Dev Biol, 58, 41–54. doi: 10.1016/j.semcdb.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EA, Kumral A, Ozer E, Duman N, Yilmaz O, Ozkal S, & Ozkan H (2005). Effect of retinoic acid on oxygen-induced lung injury in the newborn rat. Pediatr Pulmonol, 39(1), 35–40. doi: 10.1002/ppul.20131 [DOI] [PubMed] [Google Scholar]
- Panebianco C, Oben JA, Vinciguerra M, & Pazienza V (2017). Senescence in hepatic stellate cells as a mechanism of liver fibrosis reversal: a putative synergy between retinoic acid and PPAR-gamma signalings. Clin Exp Med, 17(3), 269–280. doi: 10.1007/s10238-016-0438-x [DOI] [PubMed] [Google Scholar]
- Papi A, Bartolini G, Ammar K, Guerra F, Ferreri AM, Rocchi P, & Orlandi M (2007). Inhibitory effects of retinoic acid and IIF on growth, migration and invasiveness in the U87MG human glioblastoma cell line. Oncol Rep, 18(4), 1015–1021. [DOI] [PubMed] [Google Scholar]
- Park J, Ivey MJ, Deana Y, Riggsbee K, Sorensen E, Schwabl V, … Tallquist MD (2019). The Tcf21 lineage constitutes the lung lipofibroblast population. Am J Physiol Lung Cell Mol Physiol. doi: 10.1152/ajplung.00254.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RO, & Crouch RK (2010). Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res, 91(6), 788–792. doi: 10.1016/j.exer.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschaki M, Cammas L, Muta Y, Matsuoka Y, Mak SS, Rataj-Baniowska M, … Ladher RK (2013). Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev, 8, 13. doi: 10.1186/1749-8104-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, … Sucov HM (2017). Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet, 49(9), 1346–1353. doi: 10.1038/ng.3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, … Romagnani P (2013). Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol, 24(11), 1756–1768. doi: 10.1681/ASN.2012090950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Rocco PR, Negrini D, & Passi A (2007). The extracellular matrix of the lung and its role in edema formation. An Acad Bras Cienc, 79(2), 285–297. [DOI] [PubMed] [Google Scholar]
- Pennimpede T, Cameron DA, MacLean GA, Li H, Abu-Abed S, & Petkovich M (2010). The role of CYP26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res A Clin Mol Teratol, 88(10), 883–894. doi: 10.1002/bdra.20709 [DOI] [PubMed] [Google Scholar]
- Pennisi DJ, & Mikawa T (2009). FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Dev Biol, 328(1), 148–159. doi: 10.1016/j.ydbio.2009.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pomares JM, Carmona R, González-Iriarte M, Macías D, Guadix JA, & Muñoz-Chápuli R (2004). Contribution of mesothelium-derived cells to liver sinusoids in avian embryos. Developmental Dynamics, 229(3), 465–474. doi: 10.1002/dvdy.10455 [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, & Wessels A (2002). Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs). Dev Biol, 247(2), 307–326. doi: 10.1006/dbio.2002.0706 [DOI] [PubMed] [Google Scholar]
- Perl AK, & Gale E (2009). FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol, 297(2), L299–308. doi: 10.1152/ajplung.00008.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, … Oppermann U (2009). The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chem Biol Interact, 178(1–3), 94–98. doi: 10.1016/j.cbi.2008.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M, Brand NJ, Krust A, & Chambon P (1987). A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature, 330(6147), 444–450. doi: 10.1038/330444a0 [DOI] [PubMed] [Google Scholar]
- Piantedosi R, Ghyselinck N, Blaner WS, & Vogel S (2005). Cellular retinol-binding protein type III is needed for retinoid incorporation into milk. J Biol Chem, 280(25), 24286–24292. doi: 10.1074/jbc.M503906200 [DOI] [PubMed] [Google Scholar]
- Pierzchalski K, Taylor RN, Nezhat C, Jones JW, Napoli JL, Yang G, … Sidell N (2014). Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod, 91(4), 84. doi: 10.1095/biolreprod.114.119677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierzchalski K, Yu J, Norman V, & Kane MA (2013). CrbpI regulates mammary retinoic acid homeostasis and the mammary microenvironment. FASEB J, 27(5), 1904–1916. doi: 10.1096/fj.12-219410 [DOI] [PubMed] [Google Scholar]
- Pijnappel WW, Hendriks HF, Folkers GE, van den Brink CE, Dekker EJ, Edelenbosch C, … Durston AJ (1993). The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature, 366(6453), 340–344. doi: 10.1038/366340a0 [DOI] [PubMed] [Google Scholar]
- Plavicki JS, Hofsteen P, Yue MS, Lanham KA, Peterson RE, & Heideman W (2014). Multiple modes of proepicardial cell migration require heartbeat. BMC Dev Biol, 14, 18. doi: 10.1186/1471-213X-14-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmann RE, & Gittenberger-de Groot AC (2018). Hemodynamics in Cardiac Development. J Cardiovasc Dev Dis, 5(4). doi: 10.3390/jcdd5040054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, & Sadek HA (2011). Transient regenerative potential of the neonatal mouse heart. Science, 331(6020), 1078–1080. doi: 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte S, Xavier Ruiz F, Gimenez J, Molist I, Alvarez S, Dominguez M, … Farres J (2013). Aldo-keto reductases in retinoid metabolism: search for substrate specificity and inhibitor selectivity. Chem Biol Interact, 202(1–3), 186–194. doi: 10.1016/j.cbi.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, & Keating MT (2002). Heart Regeneration in Zebrafish. Science, 298(5601), 2188. doi: 10.1126/science.1077857 [DOI] [PubMed] [Google Scholar]
- Qiao JB, Fan QQ, Xing L, Cui PF, He YJ, Zhu JC, … Jiang, H. L. (2018). Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. J Control Release, 283, 113–125. doi: 10.1016/j.jconrel.2018.05.032 [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Vanden Heuvel GB, & Igarashi P (1998). Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev, 71(1–2), 37–48. [DOI] [PubMed] [Google Scholar]
- Quijada P, Misra A, Velasquez LS, Burke RM, Lighthouse JK, Mickelsen DM, … Small EM (2019). Pre-existing fibroblasts of epicardial origin are the primary source of pathological fibrosis in cardiac ischemia and aging. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2019.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn AM, Harvey RG, & Penning TM (2008). Oxidation of PAH trans-dihydrodiols by human aldo-keto reductase AKR1B10. Chem Res Toxicol, 21(11), 2207–2215. doi: 10.1021/tx8002005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, & Maden M (2004). Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev Dyn, 230(3), 509–517. doi: 10.1002/dvdy.20025 [DOI] [PubMed] [Google Scholar]
- Rhinn M, & Dolle P (2012). Retinoic acid signalling during development. Development, 139(5), 843–858. doi: 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- Rhinn M, Schuhbaur B, Niederreither K, & Dolle P (2011). Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc Natl Acad Sci U S A, 108(40), 16687–16692. doi: 10.1073/pnas.1103877108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR Jr., & Weissman IL (2012). Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol, 14(12), 1251–1260. doi: 10.1038/ncb2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb L, Mifsud L, Hartley L, Biben C, Copeland NG, Gilbert DJ, … Harvey RP (1998). epicardin: A novel basic helix-loop-helix transcription factor gene expressed in epicardium, branchial arch myoblasts, and mesenchyme of developing lung, gut, kidney, and gonads. Dev Dyn, 213(1), 105–113. doi: [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, … Hogan BL (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A, 108(52), E1475–1483. doi: 10.1073/pnas.1117988108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, & Hill JA (2015). Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med, 373(1), 96. doi: 10.1056/NEJMc1504848 [DOI] [PubMed] [Google Scholar]
- Romand R, Kondo T, Fraulob V, Petkovich M, Dolle P, & Hashino E (2006). Dynamic expression of retinoic acid-synthesizing and -metabolizing enzymes in the developing mouse inner ear. J Comp Neurol, 496(5), 643–654. doi: 10.1002/cne.20936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC (2012). Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr, 96(5), 1166S–1172S. doi: 10.3945/ajcn.112.034637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossdeutsch A, Smart N, Dube KN, Turner M, & Riley PR (2012). Essential role for thymosin beta4 in regulating vascular smooth muscle cell development and vessel wall stability. Circ Res, 111(4), e89–102. doi: 10.1161/CIRCRESAHA.111.259846 [DOI] [PubMed] [Google Scholar]
- Roth MD, Connett JE, D’Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, … Investigators, F. S. (2006). Feasibility of retinoids for the treatment of emphysema study. Chest, 130(5), 1334–1345. doi: 10.1378/chest.130.5.1334 [DOI] [PubMed] [Google Scholar]
- Ruiz-Villalba A, Simon AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, … Perez-Pomares JM (2015). Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol, 65(19), 2057–2066. doi: 10.1016/j.jacc.2015.03.520 [DOI] [PubMed] [Google Scholar]
- Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, & Bok D (1999). Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem, 274(6), 3834–3841. [DOI] [PubMed] [Google Scholar]
- Rydeen A, Voisin N, D’Aniello E, Ravisankar P, Devignes CS, & Waxman JS (2015). Excessive feedback of Cyp26a1 promotes cell non-autonomous loss of retinoic acid signaling. Dev Biol, 405(1), 47–55. doi: 10.1016/j.ydbio.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A, Dullaart RPF, Schreuder T, Blokzijl H, & Faber KN (2017). Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients, 10(1). doi: 10.3390/nu10010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe M, Kokubo H, Nakajima Y, & Saga Y (2012). Ectopic retinoic acid signaling affects outflow tract cushion development through suppression of the myocardial Tbx2-Tgfbeta2 pathway. Development, 139(2), 385–395. doi: 10.1242/dev.067058 [DOI] [PubMed] [Google Scholar]
- Sánchez-Iranzo H, Galardi-Castilla M, Sanz-Morejón A, González-Rosa JM, Costa R, Ernst A, … Mercader N (2018). Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proceedings of the National Academy of Sciences, 115(16), 4188–4193. doi: 10.1073/pnas.1716713115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Lynn ML, Inman KE, McDowell W, & Trainor PA (2012). RDH10 oxidation of Vitamin A is a critical control step in synthesis of retinoic acid during mouse embryogenesis. Plos One, 7(2), e30698. doi: 10.1371/journal.pone.0030698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, … Trainor PA (2007). RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev, 21(9), 1113–1124. doi: 10.1101/gad.1533407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapin V, Bouillet P, Oulad-Abdelghani M, Dastugue B, Chambon P, & Dolle P (2000). Differential expression of retinoic acid-inducible (Stra) genes during mouse placentation. Mech Dev, 92(2), 295–299. [DOI] [PubMed] [Google Scholar]
- Sarper M, Cortes E, Lieberthal TJ, & Del Rio Hernandez A (2016). ATRA modulates mechanical activation of TGF-beta by pancreatic stellate cells. Sci Rep, 6, 27639. doi: 10.1038/srep27639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, … Niitsu Y (2008). Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol, 26(4), 431–442. doi: 10.1038/nbt1396 [DOI] [PubMed] [Google Scholar]
- Schmitz HH, Poor CL, Wellman RB, & Erdman JW Jr. (1991). Concentrations of selected carotenoids and vitamin A in human liver, kidney and lung tissue. J Nutr, 121(10), 1613–1621. doi: 10.1093/jn/121.10.1613 [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, & Benvenisty N (2000). Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A, 97(21), 11307–11312. doi: 10.1073/pnas.97.21.11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, … Seino S (2008). Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15’-monooxygenase (Bcmo1) expression. J Biol Chem, 283(8), 4905–4911. doi: 10.1074/jbc.M707928200 [DOI] [PubMed] [Google Scholar]
- Senoo H, Smeland S, Malaba L, Bjerknes T, Stang E, Roos N, … Blomhoff R (1993). Transfer of retinol-binding protein from HepG2 human hepatoma cells to cocultured rat stellate cells. Proc Natl Acad Sci U S A, 90(8), 3616–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, … Lee RT (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature, 493(7432), 433–436. doi: 10.1038/nature11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon SR, Moise AR, & Trainor PA (2017). New insights and changing paradigms in the regulation of vitamin A metabolism in development. Wiley Interdiscip Rev Dev Biol, 6(3). doi: 10.1002/wdev.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Chang A, & Red-Horse K (2017). Coronary Artery Development: Progenitor Cells and Differentiation Pathways. Annu Rev Physiol, 79, 1–19. doi: 10.1146/annurev-physiol-022516-033953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N, Elholm M, & Noy N (2003). Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem, 278(43), 41589–41592. doi: 10.1074/jbc.C300368200 [DOI] [PubMed] [Google Scholar]
- Shen H, Cavallero S, Estrada KD, Sandovici I, Kumar SR, Makita T, … Sucov HM (2015). Extracardiac control of embryonic cardiomyocyte proliferation and ventricular wall expansion. Cardiovasc Res, 105(3), 271–278. doi: 10.1093/cvr/cvu269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MH (2018). Stellate Cells in Tissue Repair, Inflammation, and Cancer. Annual Review of Cell and Developmental Biology, 34(1), 333–355. doi: 10.1146/annurev-cellbio-100617-062855 [DOI] [PubMed] [Google Scholar]
- Shimshoni JA, Roberts AG, Scian M, Topletz AR, Blankert SA, Halpert JR, … Isoherranen N (2012). Stereoselective formation and metabolism of 4-hydroxy-retinoic Acid enantiomers by cytochrome p450 enzymes. J Biol Chem, 287(50), 42223–42232. doi: 10.1074/jbc.M112.404475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmarakov I, Fleshman MK, D’Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, … Blaner WS (2010). Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys, 504(1), 3–10. doi: 10.1016/j.abb.2010.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmarakov IO, Jiang H, Yang KJ, Goldberg IJ, & Blaner WS (2013). Hepatic retinoid stores are required for normal liver regeneration. J Lipid Res, 54(4), 893–908. doi: 10.1194/jlr.M029801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, … Taylor RN (2010). Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol, 24(1), 148–160. doi: 10.1210/me.2009-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, … Pleasure SJ (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell, 139(3), 597–609. doi: 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Ramesh S, Cibi DM, Yun LS, Li J, Li L, … Singh MK (2016). Hippo Signaling Mediators Yap and Taz Are Required in the Epicardium for Coronary Vasculature Development. Cell Rep, 15(7), 1384–1393. doi: 10.1016/j.celrep.2016.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, … Lythgoe MF (2011). De novo cardiomyocytes from within the activated adult heart after injury. Nature, 474(7353), 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, & Riley PR (2007). Thymosin [bgr]4 induces adult epicardial progenitor mobilization and neovascularization. Nature, 445(7124), 177–182. doi:http://www.nature.com/nature/journal/v445/n7124/suppinfo/nature05383_S1.html [DOI] [PubMed] [Google Scholar]
- Smith CL, Baek ST, Sung CY, & Tallquist MD (2011). Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res, 108(12), e15–26. doi: 10.1161/CIRCRESAHA.110.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits AM, Dronkers E, & Goumans MJ (2018). The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol Res, 127, 129–140. doi: 10.1016/j.phrs.2017.07.020 [DOI] [PubMed] [Google Scholar]
- Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, … McGowan SE(2005). Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res, 57(3), 384–391. doi: 10.1203/01.PDR.0000151315.81106.D3 [DOI] [PubMed] [Google Scholar]
- Sommer A, Katz J, & Tarwotjo I (1984). Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr, 40(5), 1090–1095. doi: 10.1093/ajcn/40.5.1090 [DOI] [PubMed] [Google Scholar]
- Song X, Liu W, Xie S, Wang M, Cao G, Mao C, & Lv C (2013). All-transretinoic acid ameliorates bleomycin-induced lung fibrosis by downregulating the TGF-beta1/Smad3 signaling pathway in rats. Lab Invest, 93(11), 1219–1231. doi: 10.1038/labinvest.2013.108 [DOI] [PubMed] [Google Scholar]
- Sontake V, Kasam RK, Sinner D, Korfhagen TR, Reddy GB, White ES, … Madala SK (2018). Wilms’ tumor 1 drives fibroproliferation and myofibroblast transformation in severe fibrotic lung disease. JCI Insight, 3(16). doi: 10.1172/jci.insight.121252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spit BJ (1983). Induction of lipid droplets in fibroblasts of the hamster lung by a diet high in vitamin A. Exp Lung Res, 4(4), 247–257. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, & Goodman DS (1994). The Retinoids : biology, chemistry, and medicine (2nd ed.). New York: Raven Press. [Google Scholar]
- Stefanovic S, & Zaffran S (2017). Mechanisms of retinoic acid signaling during cardiogenesis. Mech Dev, 143, 9–19. doi: 10.1016/j.mod.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Strate I, Min TH, Iliev D, & Pera EM (2009). Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system. Development, 136(3), 461–472. doi: 10.1242/dev.024901 [DOI] [PubMed] [Google Scholar]
- Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, & Evans RM (1994). RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev, 8(9), 1007–1018. [DOI] [PubMed] [Google Scholar]
- Sung WJ, Kim H, & Park KK (2016). The biological role of epithelial-mesenchymal transition in lung cancer (Review). Oncol Rep, 36(3), 1199–1206. doi: 10.3892/or.2016.4964 [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, … Discher DE (2013). Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science, 341(6149), 1240104. doi: 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata C, Kadokawa Y, Tabata R, Takahashi M, Okoshi K, Sakai Y, … Kubo H (2006). All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med, 174(12), 1352–1360. doi: 10.1164/rccm.200606-862OC [DOI] [PubMed] [Google Scholar]
- Tabata C, Kubo H, Tabata R, Wada M, Sakuma K, Ichikawa M, … Mishima M (2006). All-trans retinoic acid modulates radiation-induced proliferation of lung fibroblasts via IL-6/IL-6R system. Am J Physiol Lung Cell Mol Physiol, 290(3), L597–606. doi: 10.1152/ajplung.00282.2005 [DOI] [PubMed] [Google Scholar]
- Tahayato A, Dolle P, & Petkovich M (2003). Cyp26C1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Expr Patterns, 3(4), 449–454. [DOI] [PubMed] [Google Scholar]
- Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, … Petkovich M (2004). A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem, 279(1), 77–85. doi: 10.1074/jbc.M308337200 [DOI] [PubMed] [Google Scholar]
- Takahashi N, & Takasu S (2011). A close relationship between type 1 diabetes and vitamin A-deficiency and matrix metalloproteinase and hyaluronidase activities in skin tissues. Exp Dermatol, 20(11), 899–904. doi: 10.1111/j.1600-0625.2011.01351.x [DOI] [PubMed] [Google Scholar]
- Takeichi M, Nimura K, Mori M, Nakagami H, & Kaneda Y (2013). The transcription factors Tbx18 and Wt1 control the epicardial epithelial-mesenchymal transition through bi-directional regulation of Slug in murine primary epicardial cells. Plos One, 8(2), e57829. doi: 10.1371/journal.pone.0057829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, & Molkentin JD (2017). Redefining the identity of cardiac fibroblasts. Nat Rev Cardiol, 14(8), 484–491. doi: 10.1038/nrcardio.2017.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon P, Miteva YV, Kuchenbrod LM, Cristea IM, & Conlon FL (2013). Tcf21 regulates the specification and maturation of proepicardial cells. Development, 140(11), 2409–2421. doi: 10.1242/dev.093385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, & Chambon P (1995). Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc Natl Acad Sci U S A, 92(17), 7854–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper J, Pfeiffer J, Aldrich M, Tumas D, Kern J, Hoffman E, … Hyde D (2000). Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest, 117(5 Suppl 1), 242S–244S. [DOI] [PubMed] [Google Scholar]
- Tesson F, Saj M, Uvaize MM, Nicolas H, Ploski R, & Bilinska Z (2014). Lamin A/C mutations in dilated cardiomyopathy. Cardiol J, 21(4), 331–342. doi: 10.5603/CJ.a2014.0037 [DOI] [PubMed] [Google Scholar]
- Testerink N, Ajat M, Houweling M, Brouwers JF, Pully VV, van Manen HJ, … Vaandrager AB (2012). Replacement of retinyl esters by polyunsaturated triacylglycerol species in lipid droplets of hepatic stellate cells during activation. Plos One, 7(4), e34945. doi: 10.1371/journal.pone.0034945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoneda J, Rodriguez-Fernandez L, Zaragoza R, Marin MP, Cabezuelo MT, Torres L, … Barber T (2018). Vitamin A Deficiency and the Lung. Nutrients, 10(9). doi: 10.3390/nu10091132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey MB, Lopes RJ, Araujo PM, Johnson JD, Gazda MA, Afonso S, … Carneiro M (2017). High-density lipoprotein receptor SCARB1 is required for carotenoid coloration in birds. Proc Natl Acad Sci U S A, 114(20), 5219–5224. doi: 10.1073/pnas.1700751114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topletz AR, Thatcher JE, Zelter A, Lutz JD, Tay S, Nelson WL, & Isoherranen N (2012). Comparison of the function and expression of CYP26A1 and CYP26B1, the two retinoic acid hydroxylases. Biochem Pharmacol, 83(1), 149–163. doi: 10.1016/j.bcp.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topletz AR, Tripathy S, Foti RS, Shimshoni JA, Nelson WL, & Isoherranen N (2015). Induction of CYP26A1 by metabolites of retinoic acid: evidence that CYP26A1 is an important enzyme in the elimination of active retinoids. Mol Pharmacol, 87(3), 430–441. doi: 10.1124/mol.114.096784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topletz AR, Zhong G, & Isoherranen N (2019). Scaling in vitro activity of CYP3A7 suggests human fetal livers do not clear retinoic acid entering from maternal circulation. Sci Rep, 9(1), 4620. doi: 10.1038/s41598-019-40995-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasino SE, Tang XH, Jessurun J, & Gudas LJ (2015). Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci Rep, 5, 15893. doi: 10.1038/srep15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasino SE, Tang XH, Jessurun J, & Gudas LJ (2016). A retinoic acid receptor beta2 agonist reduces hepatic stellate cell activation in nonalcoholic fatty liver disease. J Mol Med (Berl), 94(10), 1143–1151. doi: 10.1007/s00109-016-1434-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, & Palczewski K (2007). Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol, 47, 469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohetahuntila M, Molenaar MR, Spee B, Brouwers JF, Wubbolts R, Houweling M, … Helms JB (2017). Lysosome-mediated degradation of a distinct pool of lipid droplets during hepatic stellate cell activation. J Biol Chem, 292(30), 12436–12448. doi: 10.1074/jbc.M117.778472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara M, Yashiro K, Mamiya S, Nishino J, Chambon P, Dolle P, & Sakai Y (2007). CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev Biol, 302(2), 399–411. doi: 10.1016/j.ydbio.2006.09.045 [DOI] [PubMed] [Google Scholar]
- Uribe RA, Hong SS, & Bronner ME (2018). Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells. Dev Biol, 433(1), 17–32. doi: 10.1016/j.ydbio.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, & Blaner WS (1999). Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res, 40(3), 565–574. [PubMed] [Google Scholar]
- van Putten S, Shafieyan Y, & Hinz B (2016). Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol, 93, 133–142. doi: 10.1016/j.yjmcc.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Vega-Hernandez M, Kovacs A, De Langhe S, & Ornitz DM (2011). FGF10/FGFR2b signaling is essential for cardiac fibroblast development and growth of the myocardium. Development, 138(15), 3331–3340. doi: 10.1242/dev.064410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veness-Meehan KA, Bottone FG Jr., & Stiles AD (2000). Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr Res, 48(4), 434–444. doi: 10.1203/00006450-200010000-00004 [DOI] [PubMed] [Google Scholar]
- Vicente-Steijn R, Scherptong RW, Kruithof BP, Duim SN, Goumans MJ, Wisse LJ, … Jongbloed MR (2015). Regional differences in WT-1 and Tcf21 expression during ventricular development: implications for myocardial compaction. Plos One, 10(9), e0136025. doi: 10.1371/journal.pone.0136025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JM, Howard S, Villa Del Campo C, Bollini S, Dube KN, Masters M, … Riley PR (2017). BRG1-SWI/SNF-dependent regulation of the Wt1 transcriptional landscape mediates epicardial activity during heart development and disease. Nat Commun, 8, 16034. doi: 10.1038/ncomms16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca EJ, Wang S, de Calisto J, Gomes DC, Kane MA, Napoli JL, … Mora JR (2011). MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology, 141(1), 176–185. doi: 10.1053/j.gastro.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Mendelsohn CL, Mertz JR, Piantedosi R, Waldburger C, Gottesman ME, & Blaner WS (2001). Characterization of a new member of the fatty acid-binding protein family that binds all-trans-retinol. J Biol Chem, 276(2), 1353–1360. doi: 10.1074/jbc.M005118200 [DOI] [PubMed] [Google Scholar]
- von Gise A, & Pu WT (2012). Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ Res, 110(12), 1628–1645. doi: 10.1161/CIRCRESAHA.111.259960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gise A, Zhou B, Honor LB, Ma Q, Petryk A, & Pu WT (2011). WT1 regulates epicardial epithelial to mesenchymal transition through beta-catenin and retinoic acid signaling pathways. Dev Biol, 356(2), 421–431. doi: 10.1016/j.ydbio.2011.05.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, Dreher A, Kiefer C, Wernet MF, & Vogt K (2001). Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation invivo. Proc Natl Acad Sci U S A, 98(3), 1130–1135. doi: 10.1073/pnas.031576398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J, & Vogt K (2000). Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem, 275(16), 11915–11920. [DOI] [PubMed] [Google Scholar]
- von Lintig J, & Wyss A (2001). Molecular analysis of vitamin A formation: cloning and characterization of beta-carotene 15,15’-dioxygenases. Arch Biochem Biophys, 385(1), 47–52. doi: 10.1006/abbi.2000.2096 [DOI] [PubMed] [Google Scholar]
- Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, & von Lintig J (2006). The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry, 45(45), 13429–13437. doi: 10.1021/bi060701u [DOI] [PubMed] [Google Scholar]
- Wake K (1971). “Sternzellen” in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat, 132(4), 429–462. doi: 10.1002/aja.1001320404 [DOI] [PubMed] [Google Scholar]
- Wald G (1933). Vitamin A in the Retina. Nature, 132, 316. doi: 10.1038/132316a0 [DOI] [Google Scholar]
- Wang C, Kane MA, & Napoli JL (2011). Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes. J Biol Chem, 286(8), 6542–6553. doi: 10.1074/jbc.M110.198382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu S, Heallen T, & Martin JF (2018). The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. doi: 10.1038/s41569-018-0063-3 [DOI] [PubMed] [Google Scholar]
- Wang R, Wang G, Ricard MJ, Ferris B, Strulovici-Barel Y, Salit J, … Crystal RG (2010). Smoking-induced upregulation of AKR1B10 expression in the airway epithelium of healthy individuals. Chest, 138(6), 1402–1410. doi: 10.1378/chest.09-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Huang W, Castillo HA, Kane MA, Xavier-Neto J, Trainor PA, & Moise AR (2018). Alterations in retinoic acid signaling affect the development of the mouse coronary vasculature. Dev Dyn, 247(8), 976–991. doi: 10.1002/dvdy.24639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yu J, Jones JW, Pierzchalski K, Kane MA, Trainor PA, … Moise AR (2018). Retinoic acid signaling promotes the cytoskeletal rearrangement of embryonic epicardial cells. FASEB J, 32(7), 3765–3781. doi: 10.1096/fj.201701038R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Huang HM, Li TY, Qu P, Liu YX, & Chen J (2009). Marginal vitamin A deficiency affects lung maturation in rats from prenatal to adult stage. J Nutr Sci Vitaminol (Tokyo), 55(3), 208–214. [DOI] [PubMed] [Google Scholar]
- Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, … Ruiz-Lozano P (2015). Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature, 525(7570), 479–485. doi: 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG (2013). Tissue mechanics and fibrosis. Biochim Biophys Acta, 1832(7), 884–890. doi: 10.1016/j.bbadis.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, & Petkovich M (1997). cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes P450. J Biol Chem, 272(30), 18538–18541. [DOI] [PubMed] [Google Scholar]
- White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, … Petkovich M (1996). Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem, 271(47), 29922–29927. [DOI] [PubMed] [Google Scholar]
- White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, … Petkovich M (2000). Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci U S A, 97(12), 6403–6408. doi: 10.1073/pnas.120161397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Nie Q, Lander AD, & Schilling TF (2007). Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol, 5(11), e304. doi: 10.1371/journal.pbio.0050304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D, Massaro GD, Massaro D, & Clerch LB (1999). Gene expression of cellular retinoid-binding proteins: modulation by retinoic acid and dexamethasone in postnatal rat lung. Pediatr Res, 45(1), 2–7. doi: 10.1203/00006450-199901000-00002 [DOI] [PubMed] [Google Scholar]
- Widjaja-Adhi MA, Lobo GP, Golczak M, & Von Lintig J (2015). A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet, 24(11), 3206–3219. doi: 10.1093/hmg/ddv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja-Adhi MAK, Palczewski G, Dale K, Knauss EA, Kelly ME, Golczak M, … von Lintig J (2017). Transcription factor ISX mediates the cross talk between diet and immunity. Proc Natl Acad Sci U S A, 114(43), 11530–11535. doi: 10.1073/pnas.1714963114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Kondo N, Okabe T, Takeshita N, Pilchak DM, Koyama E, … Iwamoto M (2009). Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev Biol, 328(2), 315–327. doi: 10.1016/j.ydbio.2009.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilm B, Ipenberg A, Hastie ND, Burch JB, & Bader DM (2005). The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development, 132(23), 5317–5328. doi: 10.1242/dev.02141 [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, & Hinz B (2007). Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol, 179(6), 1311–1323. doi: 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbach SB, & Howe PR (1925). Tissue Changes Following Deprivation of Fat-Soluble a Vitamin. J Exp Med, 42(6), 753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, & Blaner WS (2008). The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem, 283(20), 13510–13519. doi: 10.1074/jbc.M800777200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongtrakool C, Malpel S, Gorenstein J, Sedita J, Ramirez MI, Underhill TM, & Cardoso WV (2003). Down-regulation of retinoic acid receptor alpha signaling is required for sacculation and type I cell formation in the developing lung. J Biol Chem, 278(47), 46911–46918. doi: 10.1074/jbc.M307977200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YJ, & Jang KL (2012). All-trans retinoic acid activates E-cadherin expression via promoter hypomethylation in the human colon carcinoma HCT116 cells. Biochem Biophys Res Commun, 425(4), 944–949. doi: 10.1016/j.bbrc.2012.08.038 [DOI] [PubMed] [Google Scholar]
- Wu J, Garami M, Cheng T, & Gardner DG (1996). 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest, 97(7), 1577–1588. doi: 10.1172/JCI118582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, & Ross AC (2010). Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res, 51(2), 378–387. doi: 10.1194/jlr.M001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, & Ramalingam TR (2012). Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med, 18(7), 1028–1040. doi: 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier-Neto J, Shapiro MD, Houghton L, & Rosenthal N (2000). Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev Biol, 219(1), 129–141. doi: 10.1006/dbio.1999.9588 [DOI] [PubMed] [Google Scholar]
- Xavier-Neto J, Sousa Costa AM, Figueira AC, Caiaffa CD, Amaral FN, Peres LM, … Castillo HA (2015). Signaling through retinoic acid receptors in cardiac development: Doing the right things at the right times. Biochim Biophys Acta, 1849(2), 94–111. doi: 10.1016/j.bbagrm.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang FL, Fang M, & Yutzey KE (2017). Loss of beta-catenin in resident cardiac fibroblasts attenuates fibrosis induced by pressure overload in mice. Nat Commun, 8(1), 712. doi: 10.1038/s41467-017-00840-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Jiang W, Shen J, Yin G, Fan Y, Wu D, … Wan R (2015). Retinoic Acid Ameliorates Pancreatic Fibrosis and Inhibits the Activation of Pancreatic Stellate Cells in Mice with Experimental Chronic Pancreatitis via Suppressing the Wnt/beta-Catenin Signaling Pathway. Plos One, 10(11), e0141462. doi: 10.1371/journal.pone.0141462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Hill MC, Zhang M, Martin TJ, Morikawa Y, Wang S, … Martin JF (2018). Hippo Signaling Plays an Essential Role in Cell State Transitions during Cardiac Fibroblast Development. Dev Cell, 45(2), 153–169 e156. doi: 10.1016/j.devcel.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, Jia K, Wang J, Yang L, Wang Y, Gao L, & Hao J (2018). A Rising Star in Pancreatic Diseases: Pancreatic Stellate Cells. Front Physiol, 9, 754. doi: 10.3389/fphys.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagitani A, Yamada S, Yasui S, Shimomura T, Murai R, Murawaki Y, … Shiota G (2004). Retinoic acid receptor alpha dominant negative form causes steatohepatitis and liver tumors in transgenic mice. Hepatology, 40(2), 366–375. doi: 10.1002/hep.20335 [DOI] [PubMed] [Google Scholar]
- Yang D, Krois CR, Huang P, Wang J, Min J, Yoo HS, … Napoli, J. L. (2017). Raldh1 promotes adiposity during adolescence independently of retinal signaling. Plos One, 12(11), e0187669. doi: 10.1371/journal.pone.0187669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, … Hamada H (2004). Regulation of Retinoic Acid Distribution Is Required for Proximodistal Patterning and Outgrowth of the Developing Mouse Limb. Developmental Cell, 6(3), 411–422. doi: 10.1016/s1534-5807(04)00062-0 [DOI] [PubMed] [Google Scholar]
- Ye Y, & Dan Z (2010). All-trans retinoic acid diminishes collagen production in a hepatic stellate cell line via suppression of active protein-1 and c-Jun N-terminal kinase signal. J Huazhong Univ Sci Technolog Med Sci, 30(6), 726–733. doi: 10.1007/s11596-010-0648-5 [DOI] [PubMed] [Google Scholar]
- Yen CL, Monetti M, Burri BJ, & Farese RV Jr. (2005). The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J Lipid Res, 46(7), 1502–1511. doi: 10.1194/jlr.M500036-JLR200 [DOI] [PubMed] [Google Scholar]
- Yi SH, Zhang Y, Tang D, & Zhu L (2015). Mechanical force and tensile strain activated hepatic stellate cells and inhibited retinol metabolism. Biotechnol Lett, 37(6), 1141–1152. doi: 10.1007/s10529-015-1785-5 [DOI] [PubMed] [Google Scholar]
- Yin C, Evason KJ, Maher JJ, & Stainier DY (2012). The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology, 56(5), 1958–1970. doi: 10.1002/hep.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yue D, Cheng L, Huang A, Tong N, & Cheng P (2018). Vitamin A-coupled liposomes carrying TLR4-silencing shRNA induce apoptosis of pancreatic stellate cells and resolution of pancreatic fibrosis. J Mol Med (Berl), 96(5), 445–458. doi: 10.1007/s00109-018-1629-6 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Wang C, Zha Y, Hu W, Gao Z, Zang Y, … Dong L (2015). Corona-directed nucleic acid delivery into hepatic stellate cells for liver fibrosis therapy. ACS Nano, 9(3), 2405–2419. doi: 10.1021/nn505166x [DOI] [PubMed] [Google Scholar]
- Zhong G, Ortiz D, Zelter A, Nath A, & Isoherranen N (2018). CYP26C1 Is a Hydroxylase of Multiple Active Retinoids and Interacts with Cellular Retinoic Acid Binding Proteins. Mol Pharmacol, 93(5), 489–503. doi: 10.1124/mol.117.111039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, … Pu WT (2011). Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest, 121(5), 1894–1904. doi: 10.1172/JCI45529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MD, Sucov HM, Evans RM, & Chien KR (1995). Retinoid-dependent pathways suppress myocardial cell hypertrophy. Proc Natl Acad Sci U S A, 92(16), 7391–7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou TB, Drummen GP, & Qin YH (2012). The controversial role of retinoic acid in fibrotic diseases: analysis of involved signaling pathways. Int J Mol Sci, 14(1), 226–243. doi: 10.3390/ijms14010226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XE, Suino-Powell KM, Xu Y, Chan CW, Tanabe O, Kruse SW, … Xu HE (2011). The orphan nuclear receptor TR4 is a vitamin A-activated nuclear receptor. J Biol Chem, 286(4), 2877–2885. doi: 10.1074/jbc.M110.168740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, … Zhang J (2018). Regenerative Potential of Neonatal Porcine Hearts. Circulation, 138(24), 2809–2816. doi: 10.1161/CIRCULATIONAHA.118.034886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhubanchaliyev A, Temirbekuly A, Kongrtay K, Wanshura LC, & Kunz J (2016). Targeting Mechanotransduction at the Transcriptional Level: YAP and BRD4 Are Novel Therapeutic Targets for the Reversal of Liver Fibrosis. Front Pharmacol, 7, 462. doi: 10.3389/fphar.2016.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizola CF, Schwartz GJ, & Vogel S (2008). Cellular retinol-binding protein type III is a PPARgamma target gene and plays a role in lipid metabolism. Am J Physiol Endocrinol Metab, 295(6), E1358–1368. doi: 10.1152/ajpendo.90464.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari R, & Ross AC (2002). Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr, 132(6), 1160–1164. [DOI] [PubMed] [Google Scholar]