Abstract
Aging is an inevitable process that involves changes across life in multiple neurochemical, neuroanatomical, hormonal systems, and many others. In addition, these biological modifications lead to an increase in age-related sickness such as cardiovascular diseases, osteoporosis, neurodegenerative disorders, and sleep disturbances, among others that affect activities of daily life. Demographic projections have demonstrated that aging will increase its worldwide rate in the coming years. The research on chronic diseases of the elderly is important to gain insights into this growing global burden. Novel therapeutic approaches aimed for treatment of age-related pathologies have included the endocannabinoid system as an effective tool since this biological system shows beneficial effects in preclinical models. However, and despite these advances, little has been addressed in the arena of the endocannabinoid system as an option for treating sleep disorders in aging since experimental evidence suggests that some elements of the endocannabinoid system modulate the sleep-wake cycle. This article addresses this less-studied field, focusing on the likely perspective of the implication of the endocannabinoid system in the regulation of sleep problems reported in the aged. We conclude that beneficial effects regarding the putative efficacy of the endocannabinoid system as therapeutic tools in aging is either inconclusive or still missing.
Keywords: Cannabidiol, childhood, pharmacology, sleep, rapid eye movement sleep, Aging
1. INTRODUCTION
The demographic and epidemiologic projections have indicated growth in longevity worldwide in the coming years [1-3]. A critical distinction is between 1900, when only 4.1% of the 76 million people in the United States were > 65 years old, 1950 with 8% of the total population under the same age, and 2000 with a percentage that had increased to 12.6% [4]. These numbers are enhancing worldwide since fertility and mortality rates are declining in most countries [5, 6]. Aging is also linked with the coexistence of two or more chronic medical conditions, known as multimorbidity [7-9] leading to interactions between multiple disorders as well as among treatments prescribed for different disorders [9-12]. The geriatric-related health issues comprise multiple medical conditions such as hypertension, stroke, sleep disorders, among others [6, 13-18].
2. SLEEP MEDICINE AND AGING
The sleep-wake cycle is a complex biological phenomenon that involves the interaction of multiple complex neurobiological networks, including neuroanatomical, neurochemical, and genetic factors [19-24]. Normal sleep comprises two states, Non-REM (NREM) sleep, that has three phases (N1 to N3), and Rapid Eye Movement sleep (REMS). However, during aging, sleep displays aberrant features [25-29]. The sleep disorders have been classified by the American Academy of Sleep Medicine into the “International Classification of Sleep Disorders -third edition” (ICSD-3). Amongst the most common sleep complaints in the aged population are insomnia and excessive daytime sleepiness (EDS) or hypersomnolence [30-37].
Insomnia has been classified in several subtypes by polysomnographic studies and additional criteria. Sleep onset insomnia (persistent difficulty in sleep initiation), sleep maintenance insomnia (difficulty to maintain sleep during nighttime), early morning insomnia (identified with early morning awakenings), and psychophysiological insomnia (cognitive and behavioral elements, stress, intrusive thoughts, and attention bias) are reported in aging.
Based on the duration, insomnia is categorized in transient (few insomniac episodes before or during a stressful experience), short-term (events presented along a few weeks during an extended period of stress), or chronic (sleep disturbance and associated daytime symptoms have been present for at least three months) [30, 31, 36]. Remarkably, chronic insomnia affects 57% of the elderly in the USA [37-39].
On the other side of the coin, EDS is understood as the irresistible tendency to fall asleep during daytime [25, 27, 34, 40]. The diverse causes that originate EDS include, for instance, obstructive sleep apnea (interruptions in breathing during sleep for > 10 s leading to intermittent, partial (hypopnea), and/or complete (apnea) collapse of the upper airway during nighttime). Because of obstruction, any inspiratory effort causes snoring. In turn, this issue is commonly associated with the awakening of the subject across nighttime leading to sleep fragmentation. On the next day, and as a consequence, sleep fragmentation produces EDS [41-43], which is very common in older adults [44-47].
An additional sleep disturbance in aging is the periodic limb movement disorder (PLMD), characterized by repetitive cramping or jerking of the legs during sleep. PLMD is another cause of EDS [46-50]. Patients complaining of PLMD show involuntary and stereotypical movements of their limbs while they sleep. The movement of limbs across night induces recurrent awakenings that limit sleep consolidation and eventually, causes drowsiness on the next day. The prevalence of PLMD is higher among older adults [51-53].
Lastly, the neurodegeneration of a cluster of neurons located into the lateral hypothalamus, named orexin or hypocretin, seems to be the origin of the sleep disturbance named narcolepsy [54]. The core symptoms of this disease comprise extreme sleepiness (sleep attacks) during daytime and cataplexy [28, 54-56]. Although narcolepsy has been normally found during the adolescence, recent data have suggested the putative link between hypocretin/orexin and aging [57-61].
Currently, most of the sleep disturbances reported in aged subjects are managed by pharmacological interventions. Medical prescriptions for the elderly include antidepressants, antihistamines, sedative-hypnotics, among others [41, 49, 51, 53, 54, 62, 63]. However, since the study of the properties of the endocannabinoid system in modulating wider spectrum of neurobiological phenomena, we would like to highlight the putative use of the endocannabinoid system elements in sleep disorders during aging.
3. THE ENDOCANNABINOID SYSTEM
During the 1990s, several experimental approaches were designed to describe the transmembranal proteins that pharmacologically responded to the principal compound of Cannabis sativa, the delta-9-tetrahydrocannabinol (Δ9-THC). At this date, it is widely accepted that the receptors that respond to Δ9-THC are CB1 and CB2 cannabinoid receptors [64-67]. In addition, the presence of both receptors has been mapped in different organs, including the human brain [68-71].
Next, the quest of the endogenous ligands that naturally bind to these receptors leads to the discovery of the first agonist to the cannabinoid receptors named arachidonoylethanolamine or anandamide, and in later years, the second lipid with cannabimimetic properties was described: 2-arachidonylglycerol (2-AG) [72]. Currently, the family of the endocannabinoids comprises different compounds aside anandamide and 2-AG, such as virodhamine, noladin ether, and N-arachidonyldopamine [73-77]. In addition, the endocannabinoid system is integrated by several molecular elements, including the enzymes that synthesize/degrade anandamide (fatty acid hydrolase [FAAH]) or 2-AG (monoacylglycerol lipase [MAGL]), the anandamide membrane transporter (AMT) and the receptor channel TRPV1 (Transient Receptor Potential Vanilloid 1; [78-82]). Along the decades, preclinical studies have shown that the endocannabinoid system exerts critical neurobiological functions, some of them, with clinical relevance, including the control of the sleep-wake cycle [80, 83-93].
4. THE ENDOCANNABINOID SYSTEM IN HEALTH ISSUES
Multiple experimental models strongly suggest that the endocannabinoid system plays a critical role in numerous pathologies. Converging preclinical experiments, including a wider spectrum of data, from modulating the endogenous tone of anandamide, to the treatment with a direct CB1 cannabinoid receptor agonist/antagonist, the pharmacological inhibition of the FAAH, or the genetic deletion of cannabinoid receptors, have been shown to control adverse medical conditions, such as schizophrenia, and ataxias. Amongst these pathological issues, important advances have been achieved in neurodegenerative disorders, including Parkinson´s and Alzheimer disease [94-101]. Yet the hypothesis that the endocannabinoid system might be critically involved in ongoing pathologies associated with aging, such as sleep disorders, has been dismissed. However, an accumulating body of evidence is providing pieces to the puzzle to appreciate the putative role of the endocannabinoid system in the modulation of sleep disorders in the elderly.
5. AGING AND THE ENDOCANNABINOID SYSTEM
It is often argued that aging is associated with multiple deteriorating medical conditions, such as neurodegenerative disorders, leading to the dysregulation of endogenous neurochemical compounds. In this regard, the levels of anandamide and 2-AG have been described increased in Parkinson´s disease patients compared to respective healthy subjects [102, 103]. Complementary, it is worth to emphasize that in patients with Alzheimer´s disease, the FAAH gene expression has been found higher compared to age-matched controls [104-106]. Furthermore, the lack of the CB2 cannabinoid receptors has been linked with schizophrenia-like pathologies whereas contents of anandamide are significantly enhanced in schizophrenic patients compared to respective controls [107-110]. Taken together, these data obligate us to address an important question that remains to be answered: Are the health disturbances the cause or consequence of the disruption of the endocannabinoid system functioning? While pertinent experimental approaches are aimed to addresses this concern, the body of evidence suggests that there is a neurobiological role of the endocannabinoid system in aging processes, including perhaps, sleep disturbances.
6. THE ENDOCANNABINOID SYSTEM MODULATING THE SLEEP-WAKE CYCLE
Significant achievements have been obtained by describing the modulatory role of the endocannabinoid system in the sleep-wake cycle. Pharmacological evidence from experiments on central or peripheral administrations has shown that elements of the endocannabinoid family participate in sleep control. For example, pioneer studies by the laboratory of Gerard Le Fur [111], Vincent Santucci, and coworkers demonstrated that the systemic injections of the CB1 cannabinoid receptor antagonist SR 141716A (0.1, 0.3, 1, 3 and 10 mg/Kg) increased the time spent in wakefulness (W) while decreased slow-wave sleep (SWS) and REMS whereas opposite results were described by our group in 1998 after icv injection of anandamide [112]. Remarkably, the sleep-inducing effects of anandamide were blocked by SR141716A [113].
Another element of the endocannabinoid system involved in sleep modulation includes the FAAH. This assumption is supported by studies in which administrations either icv or into the lateral hypothalamus of URB597, a relatively selective inhibitor of FAAH, enhanced W and decreased SWS during the lights-on period [114, 115]. Previous findings have indicated that injections of URB597 enhance waking and decrease sleep. An unexpected result since one might think that inhibition of FAAH should increase anandamide contents and then, facilitates sleep onset. However, data suggest that the effects on sleep caused by UR597 might be due to the accumulation of oleoylethanolamide (OEA) and/or palmitoylethanolamide (PEA) since FAAH also synthesizes both lipids in greater amounts compared to anandamide. In line with this idea, our group demonstrated that the administration of OEA or PEA increases wakefulness and reduces SWS and REM sleep mimicking URB597´s effects [114, 115]. Lastly, OEA behaves as an agonist with high-affinity to the PPAR-α [116], raising the possibility that PPAR-α could be the mechanism of action of OEA to induce waking [117, 118]. Further studies have also shown that additional system are engaged in the pharmacological effects on sleep of URB597, OEA or PEA, including the dopaminergic neurotransmission [114, 115, 117, 118].
In another set of experiments in which rats received AMT inhibitors, VDM-11 or OMDM-2, REMS was increased [119, 120]. This data suggested that REMS promotion could be as a consequence of the enhancement in the endogenous contents of anandamide.
To elucidate the role of the CB1 cannabinoid receptor, FAAH and AMT in sleep homeostasis, experimental animals were subjected to total sleep deprivation and they received before the sleep rebound period, systemic injection of either SR141716A, URB597, or VDM-11 (5, 10, 20mg/Kg; ip; separately each one). As expected, SR141716A and URB597 blocked the sleep rebound whereas VDM-11 exerted opposite effects [121].
Finally, what might be the role of the endocannabinoid system in sleep medicine? The fact that anandamide has been associated with sleep apnea [86, 122-124] points to the possibility that the CB1 cannabinoid receptor, FAAH, MAGL, or AMT may be also related to sleep disorders and they could act as criteria for diagnostic sleep disturbances.
Thus, the information available allows us to identify the elements of the endocannabinoid family as candidates for sleep control. Nevertheless, there is a lack of data demonstrating whether the endocannabinoid system may control sleep disorders in aging since most of the current data has been mostly reported in young or adult animals.
7. DOES THE ENDOCANNABINOID SYSTEM MODULATE SLEEP DISORDERS IN THE ELDERLY?
There is a significant amount of experimental and preclinical evidence demonstrating that the endocannabinoid system is linked with neurobiological processes related to aging. For example, murine models lacking the CB1 cannabinoid receptors display an early onset of learning disturbances linked with aging. These findings have contributed to the design of drugs aimed to manage age-related human pathologies via the endocannabinoid system elements, such as the CB1 cannabinoid receptors, anandamide, FAAH, AMT, amongst others [124-132]. We would like to describe a hypothetical framework regarding the likely modulatory role of the endocannabinoid system on sleep disorders in the elderly (Fig. 1).
Fig. (1).
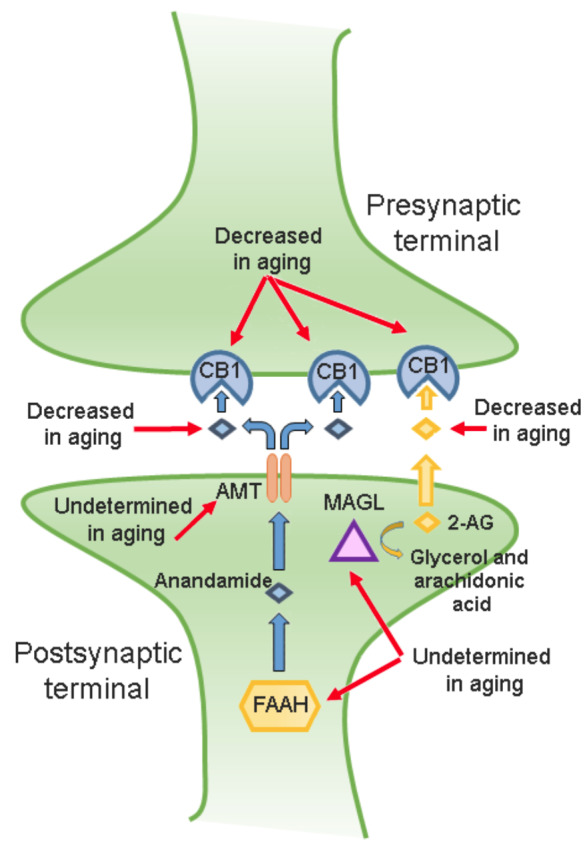
The endocannabinoid system in aging. The CB1 cannabinoid receptor, anandamide, 2-AG, monoacylglycerol lipase (MAGL), anandamide membrane transporter (AMT), and fatty acid amide hydrolase (FAAH) belong to the endocannabinoid system. Briefly, anandamide is synthesized by the activity of FAAH in the postsynaptic terminal. Next, the AMT displaces anandamide into the extracellular space to binding to the CB1 cannabinoid receptor. In addition, the 2-AG also binds to the CB1 cannabinoid receptor at the presynaptic terminal. As mentioned in the text, anandamide, 2-AG, the CB1 cannabinoid receptor, FAAH, and AMT participate in sleep modulation. As shown in the drawing, the CB1 cannabinoid receptor, anandamide, and 2-AG levels have been found decreased in aging. However, the activity of FAAH, AMT, and MAGL across aging remains to be described.
7.1. The CB1 Cannabinoid Receptor
Anandamide and 2-AG promote sleep via activation of the CB1 cannabinoid receptor [92, 112, 113]. The available literature has indicated that the CB1 cannabinoid receptor in aged rats seems to be decreased [125, 133].
7.2. The Endogenous Tone of Anandamide or 2-AG
Diurnal variation of endocannabinoids has been detected in cerebrospinal fluid, pons, hippocampus, or hypothalamus in rats [134, 135]. For example, anandamide in CSF showed an enhancement during the lights-on period whereas decreased across the lights-off period in young rats whereas anandamide and 2-AG levels are decreased in aging [126, 136, 137].
7.3. FAAH and MAGL
It is widely accepted that anandamide is synthesized on demand and regulated by the activity of the FAAH whereas 2-AG is predominantly hydrolyzed to arachidonic acid and glycerol by MAGL [138]. Thus, the activity of these enzymes in the regulation of anandamide and 2-AG contents is critical. In this regard, Pascual et al., (2014) demonstrated that FAAH activity was decreased in the frontal cortex from patients with Alzheimer's disease [139] whereas the similar findings were reported for MAGL in Alzheimer's disease experimental murine models [140-142]. In line with this data, Piyanova et al., (2015) found an elevated MAGL activity during aging [143].
7.4. AMT
As mentioned previously, the AMT behaves as a carrier-mediated transport for anandamide across the neuronal membrane. Unfortunately, limited data are available regarding the role of AMT in aging. To our understanding, the only research that has covered this issue comes from Maccarrone et al., (2001) who reported that mice lacking the CB1 cannabinoid receptor showed a significant increase in AMT with age, whereas minor changes were found in wild-type animals [142].
Since aging is characterized by the progressive impairment of diverse physiological functions leading to developing medical disturbances, including neurodegenerative diseases, then the study of the likely interaction of the endocannabinoid system with the mechanisms linked to age-related disturbances represents an interesting route for testing novel therapeutics. Here, we propose a conceptual framework linking the endocannabinoid system and the control of sleep disturbances in aging. However, our hypothetical proposal requires further evaluation by, for example, describing the distribution in the central nervous system of the CB1 cannabinoid receptor in aging and how they are altered under specific sleep disorders. Second, further evidence is required to address the modulation of the endocannabinoid system using experimental models of sleep disturbances targeting cannabinoid receptors with agonists or antagonists, increasing anandamide and 2-AG endogenous tone by the inhibition of FAAH or MAGL activity as well as the functioning of AMT. Lastly, translational studies should provide perspectives on how to bridge from basic science to human health for the developing endocannabinoid-based diagnostics and therapies for sleep disturbances in aging.
8. CANNABINOIDS SEEM TO BE PROMISING BUT WAIT! WHAT ABOUT THE LONG-TERM EFFECTS IN THE ELDERLY DUE TO THE STIMULATION BY CANNABINOIDS DURING CHILDHOOD?
Δ9-THC and cannabidiol (CBD) are the major chemicals in Cannabis sativa. While the first compound has been related to neurobiological disturbances, the second one does not exert psychotropic effects. Thanks to the research during the decades of the 1980s and 1990s, it was possible the characterization of the membrane binding sites for Δ9-THC. Currently, it is accepted that exogenous and endogenous cannabinoids bind to the CB1 and CB2 cannabinoid receptors [68, 69, 71, 72]. Due to the description of the cannabinoid receptors in human organs, including the central nervous system, the localization of these receptors correlated the behavioral effects and the administration of Δ9-THC [144-147].
Due to CBD not inducing undesirable effects, it has been considered as a promising compound in the last decades. In line with this, CBD seems to manage several medical conditions such as psychosis, inflammation, asthma, autism, and pediatric epilepsy [148]. In recent years, it has been suggested the clinical application of CBD for managing anxiety and sleep disturbances such as posttraumatic stress disorder [149-153]. Thus, as one can suspect, the accelerating acceptance of CBD-based products aimed for treating several illnesses should raise questions whether long-uses of CBD could induce adverse effects in aging.
The importance of discussing the putative long-term effects of CBD on sleep in aging lies in the evidence that shows the interaction among this compound and some elements of the endocannabinoid system. For example, current approaches have suggested that CBD behaves as a CB1 cannabinoid receptor negative allosteric modulator [154-157]. Moreover, CBD inhibits FAAH activity and modulates anandamide and 2-AG levels [158]. Thus, it seems reasonable to suspect that long-term exposure to CBD during early years might induce sleep disturbances in aging.
To increase awareness in regards to putative effects derived from long-term uses of CBD, various reports have demonstrated that this phytocannabinoid engages multiple neurobiological systems [148], including the increase of the extracellular contents of acetylcholine, adenosine, dopamine, and serotonin [159-161]. Since it has not been described which of these neurobiological pathways could be responsible for the presumably therapeutic properties of CBD, then multiple neurobiological routes might be engaged in the long-term prescription of this compound. Due to CBD tested exerting positive outcomes in epilepsy [162-165], then this phytocannabinoid has been also evaluated as a therapeutical option for managing schizophrenia, Alzheimer disease, autism, among others pathological conditions [148-150]. Therefore, the chronic use of CBD might cause undesirable effects in aging. To sum concerns, on-line availability and accessibility to CBD to products which do not fulfill FDA standards [166], could add healthy risks due to the chronic use of CBD. If used at juvenile ages, the neuronal development might be under the influence of CBD since the brain networks are still actively developing during adolescence [167]. Hence, the long-term use of CBD might induce neuromolecular dysfunctions if treatment extends from childhood to adulthood as previously suggested (Fig. 2 [168-171]).
Fig. (2).
The likely long-term neurobiological effects in aging after chronic cannabidiol (CBD) usage during childhood. Due to the susceptibility of the brain in childhood, it is possible that long term prescription of CBD might cause effects in learning, memory or causing sleep disorders in aging.
9. THE LIMITATIONS OF CURRENT EVIDENCE
At this date, the evidence of long-term disturbances in aging by lasting uses of CBD across childhood is limited and restricts to draw solid conclusions. We have identified that limitations of available literature include the variability of the experimental designs used animal model/clinical condition (epilepsy, anxiety, depression, etc), dosage (e.g., low vs. high), route of administration (central vs. peripheral), frequency of usage (chronic vs. acute), among other conditions [145, 148, 172]. Moreover, achieving longitudinal studies that show time‐varying exposure related to neurobiological effects in chronically CBD-treated subjects will provide experimental evidence to translate to medical areas where uses of CBD seems to be common such as pediatric epilepsy.
CONCLUSIONS
The increase in the number of aged people around the world and age-related health disturbances is a worldwide challenge. To note, the costs of dementia in the USA was more than US$800 billion in 2015, corresponding to a 35% increase compared to 2010 [1-6]. Thus, aging phenomena should promote the development of new therapeutical approaches.
During aging, the sleep-wake cycle includes abnormalities classified as sleep disorders, such as insomnia, obstructive sleep apnea, and excessive somnolence, among many others. Since projective models have indicated that the number of the oldest population would be higher, then this situation will also represent a public health challenge to assist age-related health issues, including sleep disorders. Here, we integrated the current knowledge of some sleep disorders in aging. The endocannabinoid system and its relationship with aging were revised as well. Lastly, the hypothetical link between the endocannabinoid system and sleep disorders in aging were proposed.
Moving our analysis from the endocannabinoid system and the neuronal circuits engaged on the sleep-wake cycle regulation is beyond the aim of the current Review since further complexity has been recognized by the localization of the elements of the endocannabinoid system in multiple sleep-related brain areas as well as the neuronal networks that underlie the influence of the endocannabinoid system in sleep control. Since the CB1 cannabinoid receptor, anandamide, 2-AG, and FAAH have been identified in sleep brain areas such as the cerebral cortex, hypothalamus and pons [173, 174], then we can assume that the endocannabinoid system might modulate the sleep-wake cycle. However, clinical implications between the endocannabinoid system functioning and sleep disturbances in aging require further investigation. Moreover, future research should focus on identifying the mechanisms of action of the multiple elements of the endocannabinoid system in aging and sleep disturbances as well as the risks of neuromolecular durable effects in aging if the chronic prescription of CBD is provided across childhood.
Given the medical regulations in several countries allowing the medicinal uses of Cannabis sativa or compounds derived of cannabinoids, and the on-line accessibility to CBD, a diversity of uncontrolled and available CBD-containing products (e.g. oils, foods, drinks, gums, etc.) on the internet, which some of them do not meet the rigorous FDA standards [168] would increase the influx of subjects with acute illnesses searching purported medicinal products that could cause additional concerns than resolution of these. Thus, legitimate concerns are raised about the risks of neurobiological durable effects in adulthood if CBD is prescripted during juvenile ages. Moreover, there is no solid evidence in regards to each Cannabis sativa ingredient, or its mixture could exert positive outcomes for treating sleep disorders.
Although a plethora of pharmacological evidence of the endocannabinoid system have recently been suggested relevant for its likely sleep-modulatory properties in aging, the possibility that some beneficial outcomes for age-related sleep disturbances regulation could be achieved by indirect mechanism such as the control of pain, anxiety, depression, among other medical conditions [175-177] remains to be unclear at present.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by The University of California Institute for Mexico and the United States (UC MEXUS) and Consejo Nacional de Ciencia y Tecnología (CONACyT; Grant# CN-17-19) and Escuela de Medicina, Universidad Anáhuac Mayab (Grant: PresInvEMR2017) given to E M-R.
ACKNOWLEDGEMENTS
All authors contributed substantially to the article.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1. World Health Organization. Global Health and Aging. National Institute on Aging National Institutes of Health U.S. Department of Health and Human Services. USA. NIH. Publication no. 11-7737. 2011 [Google Scholar]
- 2.Beard J.R., Officer A., de Carvalho I.A., Sadana R., Pot A.M., Michel J.P., Lloyd-Sherlock P., Epping-Jordan J.E., Peeters G.M.E.E.G., Mahanani W.R., Thiyagarajan J.A., Chatterji S. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Götmark F., Cafaro P., O’Sullivan J. Aging human populations: good for us, good for the earth. Trends Ecol. Evol. (Amst.) 2018;33(11):851–862. doi: 10.1016/j.tree.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L., Giallauria F., Guralnik J.M. Epidemiology of aging. Radiol. Clin. North Am. 2008;46(4):643–652. doi: 10.1016/j.rcl.2008.07.005. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohde J.E. Far more than 7 billion. Lancet Infect. Dis. 2012;12(1):12. doi: 10.1016/S1473-3099(11)70338-3. [DOI] [PubMed] [Google Scholar]
- 6.Beard J.R., Officer A., de Carvalho I.A., Sadana R., Pot A.M., Michel J.P., Lloyd-Sherlock P., Epping-Jordan J.E., Peeters G.M.E.E.G., Mahanani W.R., Thiyagarajan J.A., Chatterji S. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–2154. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tugwell P., Knottnerus J.A. Multimorbidity and Comorbidity are now separate MESH headings. J. Clin. Epidemiol. 2019;105:vi–viii. doi: 10.1016/j.jclinepi.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Mayor S. Commonly used approach for multimorbidity fails to improve quality of life. BMJ. 2018;361:k287. doi: 10.1136/bmj.k2875. [DOI] [Google Scholar]
- 9.Vetrano D.L., Palmer K., Marengoni A., Marzetti E., Lattanzio F., Roller-Wirnsberger R., Lopez Samaniego L., Rodríguez-Mañas L., Bernabei R., Onder G. Joint action advantage WP4 group. Frailty and multimorbidity: a systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74(5):659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 10.Tisminetzky M., Gurwitz J.H., Fan D., Reynolds K., Smith D.H., Magid D.J., Sung S.H., Murphy T.E., Goldberg R.J., Go A.S. Multimorbidity burden and adverse outcomes in a community-based cohort of adults with heart failure. J. Am. Geriatr. Soc. 2018;66(12):2305–2313. doi: 10.1111/jgs.15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderón-Larrañaga A., Fratiglioni L. Multimorbidity research at the crossroads: developing the scientific evidence for clinical practice and health policy. J. Intern. Med. 2019;285(3):251–254. doi: 10.1111/joim.12872. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson K., Makovski T.T., Griffith L.E., Raina P., Stranges S., van den Akker M. Multimorbidity and comorbidity revisited: refining the concepts for international health research. J. Clin. Epidemiol. 2019;105:142–146. doi: 10.1016/j.jclinepi.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung C.H., Vitiello M.V., Alessi C.A., Kuchel G.A. AGS/NIA sleep conference planning Committee and faculty. Report and research agenda of the American geriatrics society and national institute on aging bedside-to-bench conference on sleep, Circadian rhythms, and aging: New avenues for Improving brain health, physical health, and functioning. J. Am. Geriatr. Soc. 2016;64(12):e238–e247. doi: 10.1111/jgs.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner B., Kryger M.H. Sleep in the aging population. Sleep Med. Clin. 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mander B.A., Winer J.R., Walker M.P. Sleep and human aging. Neuron. 2017;94(1):19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musiek E.S., Bhimasani M., Zangrilli M.A., Morris J.C., Holtzman D.M., Ju Y.S. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75(5):582–590. doi: 10.1001/jamaneurol.2017.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong X., Milholland B., Vijg J. Evidence for a limit to human lifespan. Nature. 2016;538(7624):257–259. doi: 10.1038/nature19793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murillo-Rodriguez E., Arias-Carrion O., Zavala-Garcia A., Sarro-Ramirez A., Huitron-Resendiz S., Arankowsky-Sandoval G. Basic sleep mechanisms: an integrative review. Cent. Nerv. Syst. Agents Med. Chem. 2012;12(1):38–54. doi: 10.2174/187152412800229107. [DOI] [PubMed] [Google Scholar]
- 20.Spiegelhalder K., Riemann D. Losing sleep. Lancet Neurol. 2015;14(6):571. doi: 10.1016/S1474-4422(15)00065-4. [DOI] [PubMed] [Google Scholar]
- 21.Arnulf I. Sleep: what the day owes the night. Lancet Neurol. 2015;14(1):19–20. doi: 10.1016/S1474-4422(14)70234-0. [DOI] [PubMed] [Google Scholar]
- 22.Siclari F., Baird B., Perogamvros L., Bernardi G., LaRocque J.J., Riedner B., Boly M., Postle B.R., Tononi G. The neural correlates of dreaming. Nat. Neurosci. 2017;20(6):872–878. doi: 10.1038/nn.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill V.M., O’Connor R.M., Shirasu-Hiza M. Tired and stressed: Examining the need for sleep. Eur. J. Neurosci. 2018 doi: 10.1111/ejn.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gent T.C., Bandarabadi M., Herrera C.G., Adamantidis A.R. Thalamic dual control of sleep and wakefulness. Nat. Neurosci. 2018;21(7):974–984. doi: 10.1038/s41593-018-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sateia M.J. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 26.Morin C.M., Drake C.L., Harvey A.G., Krystal A.D., Manber R., Riemann D., Spiegelhalder K. Insomnia disorder. Nat. Rev. Dis. Primers. 2015;1:15026. doi: 10.1038/nrdp.2015.26. [DOI] [PubMed] [Google Scholar]
- 27.Ruoff C., Rye D. The ICSD-3 and DSM-5 guidelines for diagnosing narcolepsy: clinical relevance and practicality. Curr. Med. Res. Opin. 2016;32(10):1611–1622. doi: 10.1080/03007995.2016.1208643. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney C.E., Cogswell A., Koralnik I.J., Scammell T.E. The neurobiological basis of narcolepsy. Nat. Rev. Neurosci. 2019;20(2):83–93. doi: 10.1038/s41583-018-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dauvilliers Y., Schenck C.H., Postuma R.B., Iranzo A., Luppi P.H., Plazzi G., Montplaisir J., Boeve B. REM sleep behaviour disorder. Nat. Rev. Dis. Primers. 2018;4(1):19. doi: 10.1038/s41572-018-0016-5. [DOI] [PubMed] [Google Scholar]
- 30.Jaussent I., Bouyer J., Ancelin M.L., Akbaraly T., Pérès K., Ritchie K., Besset A., Dauvilliers Y. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep (Basel) 2011;34(8):1103–1110. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg A.M., Canham S.L., Smith M.T., Spira A.P. Optimizing sleep in older adults: treating insomnia. Maturitas. 2013;76(3):247–252. doi: 10.1016/j.maturitas.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbaraly T.N., Jaussent I., Besset A., Bertrand M., Barberger-Gateau P., Ritchie K., Ferrie J.E., Kivimaki M., Dauvilliers Y. Sleep complaints and metabolic syndrome in an elderly population: the Three-City Study. Am. J. Geriatr. Psychiatry. 2015;23(8):818–828. doi: 10.1016/j.jagp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Mattis J., Sehgal A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab. 2016;27(4):192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabelle A., Gutierrez L.A., Jaussent I., Navucet S., Grasselli C., Bennys K., Marelli C., David R., Andrieu S., Berr C., Vellas B., Dauvilliers Y. Excessive sleepiness and longer nighttime in bed increase the risk of cognitive decline in frail elderly subjects: The MAPT-sleep study. Front. Aging Neurosci. 2017;9:312. doi: 10.3389/fnagi.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Incze M., Redberg R.F., Gupta A. I have insomnia-what should I do? JAMA Intern. Med. 2018;178(11):1572. doi: 10.1001/jamainternmed.2018.2626. [DOI] [PubMed] [Google Scholar]
- 36.Patel D., Steinberg J., Patel P. Insomnia in the elderly: A Review. J. Clin. Sleep Med. 2018;14(6):1017–1024. doi: 10.5664/jcsm.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sindi S., Kåreholt I., Johansson L., Skoog J., Sjöberg L., Wang H.X., Johansson B., Fratiglioni L., Soininen H., Solomon A., Skoog I., Kivipelto M. Sleep disturbances and dementia risk: A multicenter study. Alzheimers Dement. 2018;14(10):1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Desaulniers J., Desjardins S., Lapierre S., Desgagné A. Sleep environment and insomnia in elderly persons living at home. J. Aging Res. 2018;2018:8053696. doi: 10.1155/2018/8053696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abad V.C., Guilleminault C. Insomnia in elderly patients: Recommendations for pharmacological management. Drugs Aging. 2018;35(9):791–817. doi: 10.1007/s40266-018-0569-8. [DOI] [PubMed] [Google Scholar]
- 40.Brandão G.S., Camelier F.W.R., Sampaio A.A.C., Brandão G.S., Silva A.S., Gomes G.S.B.F., Donner C.F., Oliveira L.V.F., Camelier A.A. Association of sleep quality with excessive daytime somnolence and quality of life of elderlies of community. Multidiscip. Respir. Med. 2018;13:8. doi: 10.1186/s40248-018-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garbarino S., Scoditti E., Lanteri P., Conte L., Magnavita N., Toraldo D.M. Obstructive sleep apnea with or without excessive daytime sleepiness: Clinical and experimental data-driven phenotyping. Front. Neurol. 2018;9:505. doi: 10.3389/fneur.2018.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.W., Kim T., Shin J., Choe G., Lim H.J., Rhee C.S., Lee K., Cho S.W. Prediction of obstructive sleep apnea based on respiratory sounds recorded between sleep onset and sleep offset. Clin. Exp. Otorhinolaryngol. 2019;12(1):72–78. doi: 10.21053/ceo.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carneiro-Barrera A., Díaz-Román A., Guillén-Riquelme A., Buela-Casal G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obes. Rev. 2019;20(5):750–762. doi: 10.1111/obr.12824. [DOI] [PubMed] [Google Scholar]
- 44.Zalai D., Bingeliene A., Shapiro C. Sleepiness in the elderly. Sleep Med. Clin. 2017;12(3):429–441. doi: 10.1016/j.jsmc.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Junho B.T., Kummer A., Cardoso F., Teixeira A.L., Rocha N.P. Clinical predictors of excessive daytime sleepiness in patients with Parkinson’s disease. J. Clin. Neurol. 2018;14(4):530–536. doi: 10.3988/jcn.2018.14.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maugeri A., Medina-Inojosa J.R., Kunzova S., Agodi A., Barchitta M., Sochor O., Lopez-Jimenez F., Geda Y.E., Vinciguerra M. Sleep duration and excessive daytime sleepiness are associated with obesity independent of diet and physical activity. Nutrients. 2018;10(9):E1219. doi: 10.3390/nu10091219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hombali A., Seow E., Yuan Q., Chang S.H.S., Satghare P., Kumar S., Verma S.K., Mok Y.M., Chong S.A., Subramaniam M. Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry Res. 2018;S0165-1781(18):30268–3. doi: 10.1016/j.psychres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 48.K, Pavlova M., Latreille V. Sleep disorders. Am. J. Med. 2018; S0002-9343(18):30944–6. [Google Scholar]
- 49.Gulia K.K., Kumar V.M. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. 2018;18(3):155–165. doi: 10.1111/psyg.12319. [DOI] [PubMed] [Google Scholar]
- 50.Iranzo A. Parasomnias and sleep-related movement disorders in older adults. Sleep Med. Clin. 2018;13(1):51–61. doi: 10.1016/j.jsmc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Miner B., Kryger M.H. Sleep in the aging population. Sleep Med. Clin. 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grandner M.A., Winkelman J.W. Nocturnal leg cramps: Prevalence and associations with demographics, sleep disturbance symptoms, medical conditions, and cardiometabolic risk factors. PLoS One. 2017;12(6): e0178465. doi: 10.1371/journal.pone.0178465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yaremchuk K. Sleep disorders in the elderly. Clin. Geriatr. Med. 2018;34(2):205–216. doi: 10.1016/j.cger.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Wang C., Wang Q., Ji B., Pan Y., Xu C., Cheng B., Bai B., Chen J. The orexin/Receptor dystem: Molecular mechanism and therapeutic potential for neurological diseases. Front. Mol. Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonvalet M., Ollila H.M., Ambati A., Mignot E. Autoimmunity in narcolepsy. Curr. Opin. Pulm. Med. 2017;23(6):522–529. doi: 10.1097/MCP.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takenoshita S., Sakai N., Chiba Y., Matsumura M., Yamaguchi M., Nishino S. An overview of hypocretin based therapy in narcolepsy. Expert Opin. Investig. Drugs. 2018;27(4):389–406. doi: 10.1080/13543784.2018.1459561. [DOI] [PubMed] [Google Scholar]
- 57.Fronczek R., van Geest S., Frölich M., Overeem S., Roelandse F.W., Lammers G.J., Swaab D.F. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging. 2012;33(8):1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Roh J.H., Jiang H., Finn M.B., Stewart F.R., Mahan T.E., Cirrito J.R., Heda A., Snider B.J., Li M., Yanagisawa M., de Lecea L., Holtzman D.M. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med. 2014;211(13):2487–2496. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nixon J.P., Mavanji V., Butterick T.A., Billington C.J., Kotz C.M., Teske J.A. Sleep disorders, obesity, and aging: the role of orexin. Ageing Res. Rev. 2015;20:63–73. doi: 10.1016/j.arr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovalská P., Kemlink D., Nevšímalová S., Maurovich Horvat E., Jarolímová E., Topinková E., Šonka K. Narcolepsy with cataplexy in patients aged over 60 years: a case-control study. Sleep Med. 2016;26:79–84. doi: 10.1016/j.sleep.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Gabelle A., Jaussent I., Hirtz C., Vialaret J., Navucet S., Grasselli C., Robert P., Lehmann S., Dauvilliers Y. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol. Aging. 2017;53:59–66. doi: 10.1016/j.neurobiolaging.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki K., Miyamoto M., Hirata K. Sleep disorders in the elderly: Diagnosis and management. J. Gen. Fam. Med. 2017;18(2):61–71. doi: 10.1002/jgf2.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salas-Crisostomo M., Torterolo P., Veras A.B., Rocha N.B., Machado S., Murillo-Rodríguez E. Therapeutic approaches for the management of sleep disorders in geriatric population. Curr. Med. Chem. 2019;26(25):4775–4785. doi: 10.2174/0929867325666180904113115. [DOI] [PubMed] [Google Scholar]
- 64.Augustin S.M., Lovinger D.M. Functional relevance of endocannabinoid-dependent synaptic plasticity in the central nervous system. ACS Chem. Neurosci. 2018;9(9):2146–2161. doi: 10.1021/acschemneuro.7b00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aymerich M.S., Aso E., Abellanas M.A., Tolon R.M., Ramos J.A., Ferrer I., Romero J., Fernández-Ruiz J. Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system. Biochem. Pharmacol. 2018;157:67–84. doi: 10.1016/j.bcp.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 66.Ibarra-Lecue I., Pilar-Cuéllar F., Muguruza C., Florensa-Zanuy E., Díaz Á., Urigüen L., Castro E., Pazos A., Callado L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018;157:97–107. doi: 10.1016/j.bcp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Jordan CJ., Xi Z.X. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci. Biobehav. Rev. 2019;S0149-7634(18):30829–7. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu S.S., Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handb. Exp. Pharmacol. 2015;231:59–93. doi: 10.1007/978-3-319-20825-1_3. [DOI] [PubMed] [Google Scholar]
- 69.Kendall D.A., Yudowski G.A. Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Front. Cell. Neurosci. 2017;10:294. doi: 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Navarro G., Morales P., Rodríguez-Cueto C., Fernández-Ruiz J., Jagerovic N., Franco R. Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with Focus on neurodegenerative disorders. Front. Neurosci. 2016;10:406. doi: 10.3389/fnins.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, Costello H, Ogunbiyi MO, Bossong MG, Freeman TP. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol Ther. 2018;S0163-7258(18):30190–6. doi: 10.1016/j.pharmthera.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maccarrone M., Bab I., Bíró T., Cabral G.A., Dey S.K., Di Marzo V., Konje J.C., Kunos G., Mechoulam R., Pacher P., Sharkey K.A., Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015;36(5):277–296. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deshmukh R.R., Sharma P.L. Stimulation of accumbens shell cannabinoid CB(1) receptors by noladin ether, a putative endocannabinoid, modulates food intake and dietary selection in rats. Pharmacol. Res. 2012;66(3):276–282. doi: 10.1016/j.phrs.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Pertwee R.G. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 75.Redmond W.J., Cawston E.E., Grimsey N.L., Stuart J., Edington A.R., Glass M., Connor M. Identification of N-arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors. Br. J. Pharmacol. 2016;173(1):115–127. doi: 10.1111/bph.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawton S.K., Xu F., Tran A., Wong E., Prakash A., Schumacher M., Hellman J., Wilhelmsen K. N-Arachidonoyl dopamine modulates acute systemic inflammation via nonhematopoietic TRPV1. J. Immunol. 2017;199(4):1465–1475. doi: 10.4049/jimmunol.1602151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pandey P., Chaurasiya N.D., Tekwani B.L., Doerksen R.J. Interactions of endocannabinoid virodhamine and related analogs with human monoamine oxidase-A and -B. Biochem. Pharmacol. 2018;155:82–91. doi: 10.1016/j.bcp.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chanda D., Neumann D., Glatz J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids. 2019;140:51–56. doi: 10.1016/j.plefa.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 79.Maccarrone M. Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front. Mol. Neurosci. 2017;10:166. doi: 10.3389/fnmol.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nazıroğlu M., Taner A.N., Balbay E., Çiğ B. Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model. Mol. Cell. Biochem. 2019;453(1-2):143–155. doi: 10.1007/s11010-018-3439-0. [DOI] [PubMed] [Google Scholar]
- 81.Storozhuk M.V., Zholos A.V. TRP Channels as Novel Targets for Endogenous Ligands: Focus on Endocannabinoids and Nociceptive Signalling. Curr. Neuropharmacol. 2018;16(2):137–150. doi: 10.2174/1570159X15666170424120802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toczek M., Malinowska B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018;204:20–45. doi: 10.1016/j.lfs.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 83.Patel S, Hill MN, Cheer JF, Wotjak CT, Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017;76(Pt A):56–66. doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burstein S.H. N-Acyl amino acids (Elmiric Acids): Endogenous signaling molecules with therapeutic potential. Mol. Pharmacol. 2018;93(3):228–238. doi: 10.1124/mol.117.110841. [DOI] [PubMed] [Google Scholar]
- 85.Morena M., Aukema R.J., Leitl K.D., Rashid A.J., Vecchiarelli H.A., Josselyn S.A., Hill M.N. Upregulation of anandamide hydrolysis in the basolateral complex of amygdala reduces fear memory expression and indices of stress and anxiety. J. Neurosci. 2018:2251–22518. doi: 10.1523/JNEUROSCI.2251-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jumpertz R., Wiesner T., Blüher M., Engeli S., Bátkai S., Wirtz H., Bosse-Henck A., Stumvoll M. Circulating endocannabinoids and N-acyl-ethanolamides in patients with sleep apnea--specific role of oleoylethanolamide. Exp. Clin. Endocrinol. Diabetes. 2010;118(9):591–595. doi: 10.1055/s-0030-1253344. [DOI] [PubMed] [Google Scholar]
- 87.Murillo-Rodríguez E., Poot-Ake A., Arias-Carrion O., Pacheco-Pantoja E. Fuente-Ortegon, A de L.; Arankowsky-Sandoval, G. The emerging role of the endocannabinoid system in the sleep-wake cycle modulation. Cent. Nerv. Syst. Agents Med. Chem. 2011;11(3):189–196. doi: 10.2174/187152411798047780. [DOI] [PubMed] [Google Scholar]
- 88.Engeli S., Blüher M., Jumpertz R., Wiesner T., Wirtz H., Bosse-Henck A., Stumvoll M., Batkai S., Pacher P., Harvey-White J., Kunos G., Jordan J. Circulating anandamide and blood pressure in patients with obstructive sleep apnea. J. Hypertens. 2012;30(12):2345–2351. doi: 10.1097/HJH.0b013e3283591595. [DOI] [PubMed] [Google Scholar]
- 89.Murillo-Rodríguez E., Palomero-Rivero M., Millán-Aldaco D., Di Marzo V. The administration of endocannabinoid uptake inhibitors OMDM-2 or VDM-11 promotes sleep and decreases extracellular levels of dopamine in rats. Physiol. Behav. 2013;109:88–95. doi: 10.1016/j.physbeh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 90.Murillo-Rodríguez E., Machado S., Rocha N.B., Budde H., Yuan T.F., Arias-Carrión O. Revealing the role of the endocannabinoid system modulators, SR141716A, URB597 and VDM-11, in sleep homeostasis. Neuroscience. 2016;339:433–449. doi: 10.1016/j.neuroscience.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Hanlon E.C., Tasali E., Leproult R., Stuhr K.L., Doncheck E., de Wit H., Hillard C.J., Van Cauter E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-Arachidonoylglycerol. Sleep (Basel) 2016;39(3):653–664. doi: 10.5665/sleep.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prospéro-García O., Amancio-Belmont O., Becerril Meléndez A.L., Ruiz-Contreras A.E., Méndez-Díaz M. Endocannabinoids and sleep. Neurosci. Biobehav. Rev. 2016;71:671–679. doi: 10.1016/j.neubiorev.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Soni N., Prabhala B.K., Mehta V., Mirza O., Kohlmeier K.A. Anandamide and 2-AG are endogenously present within the laterodorsal tegmental nucleus: Functional implications for a role of eCBs in arousal. Brain Res. 2017;1665:74–79. doi: 10.1016/j.brainres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Muguruza C., Lehtonen M., Aaltonen N., Morentin B., Meana J.J., Callado L.F. Quantification of endocannabinoids in postmortem brain of schizophrenic subjects. Schizophr. Res. 2013;148(1-3):145–150. doi: 10.1016/j.schres.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez-Cueto C., Hernández-Gálvez M., Hillard C.J., Maciel P., Valdeolivas S., Ramos J.A., Gómez-Ruiz M., Fernández-Ruiz J. Altered striatal endocannabinoid signaling in a transgenic mouse model of spinocerebellar ataxia type-3. PLoS One. 2017;12(4): e0176521. doi: 10.1371/journal.pone.0176521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Micale V., Drago F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018;834:230–239. doi: 10.1016/j.ejphar.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 97.Aso E., Andrés-Benito P., Ferrer I. Genetic deletion of CB1 cannabinoid receptors exacerbates the Alzheimer-like symptoms in a transgenic animal model. Biochem. Pharmacol. 2018;157:210–216. doi: 10.1016/j.bcp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Choi S.H., Arai A.L., Mou Y., Kang B., Yen C.C., Hallenbeck J., Silva A.C. Neuroprotective effects of MAGL (Monoacylglycerol Lipase) inhibitors in experimental ischemic stroke. Stroke. 2018;49(3):718–726. doi: 10.1161/STROKEAHA.117.019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ilyasov A.A., Milligan C.E., Pharr E.P., Howlett A.C. The endocannabinoid system and oligodendrocytes in health and disease. Front. Neurosci. 2018;12:733. doi: 10.3389/fnins.2018.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Iannotti F.A., Pagano E., Guardiola O., Adinolfi S., Saccone V., Consalvi S., Piscitelli F., Gazzerro E., Busetto G., Carrella D., Capasso R., Puri P.L., Minchiotti G., Di Marzo V. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat. Commun. 2018;9(1):3950. doi: 10.1038/s41467-018-06267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marchioni C., de Souza I.D., Acquaro V.R., de Souza Crippa J.A., Tumas V., Queiroz M.E.C. Recent advances in LC-MS/MS methods to determine endocannabinoids in biological samples: Application in neurodegenerative diseases. Anal. Chim. Acta. 2018;1044:12–28. doi: 10.1016/j.aca.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 102.Mounsey R.B., Mustafa S., Robinson L., Ross R.A., Riedel G., Pertwee R.G., Teismann P. Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Exp. Neurol. 2015;273:36–44. doi: 10.1016/j.expneurol.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Basavarajappa B.S., Shivakumar M., Joshi V., Subbanna S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017;142(5):624–648. doi: 10.1111/jnc.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.D’Addario C., Di Francesco A., Arosio B., Gussago C., Dell’Osso B., Bari M., Galimberti D., Scarpini E., Altamura A.C., Mari D., Maccarrone M. Epigenetic regulation of fatty acid amide hydrolase in Alzheimer disease. PLoS One. 2012;7(6):e39186. doi: 10.1371/journal.pone.0039186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pascual A.C., Martín-Moreno A.M., Giusto N.M., de Ceballos M.L., Pasquaré S.J. Normal aging in rats and pathological aging in human Alzheimer’s disease decrease FAAH activity: modulation by cannabinoid agonists. Exp. Gerontol. 2014;60:92–99. doi: 10.1016/j.exger.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 106.Bedse G., Romano A., Lavecchia A.M., Cassano T., Gaetani S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 2015;43(4):1115–1136. doi: 10.3233/JAD-141635. [DOI] [PubMed] [Google Scholar]
- 107.Szűcs E., Dvorácskó S., Tömböly C., Büki A., Kékesi G., Horváth G., Benyhe S. Decreased CB receptor binding and cannabinoid signaling in three brain regions of a rat model of schizophrenia. Neurosci. Lett. 2016;633:87–93. doi: 10.1016/j.neulet.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 108.Fakhoury M. Role of the endocannabinoid system in the pathophysiology of Schizophrenia. Mol. Neurobiol. 2017;54(1):768–778. doi: 10.1007/s12035-016-9697-5. [DOI] [PubMed] [Google Scholar]
- 109.Ibarra-Lecue I., Pilar-Cuéllar F., Muguruza C., Florensa-Zanuy E., Díaz Á., Urigüen L., Castro E., Pazos A., Callado L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018;157:97–107. doi: 10.1016/j.bcp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 110.Bioque M., García-Bueno B., Macdowell K.S., Meseguer A., Saiz P.A., Parellada M., Gonzalez-Pinto A., Rodriguez-Jimenez R., Lobo A., Leza J.C., Bernardo M. FLAMM-PEPs study—Centro de Investigacio’n Biome’dica en Red de Salud mental. Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology. 2013;38(13):2568–2577. doi: 10.1038/npp.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santucci V., Storme J.J., Soubrié P., Le Fur G. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci. 1996;58(6):PL103–PL110. doi: 10.1016/0024-3205(95)02319-4. [DOI] [PubMed] [Google Scholar]
- 112.Murillo-Rodríguez E., Sánchez-Alavez M., Navarro L., Martínez-González D., Drucker-Colín R., Prospéro-García O. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812(1-2):270–274. doi: 10.1016/S0006-8993(98)00969-X. [DOI] [PubMed] [Google Scholar]
- 113.Murillo-Rodríguez E., Cabeza R., Méndez-Díaz M., Navarro L., Prospéro-García O. Anandamide-induced sleep is blocked by SR141716A, a CB1 receptor antagonist and by U73122, a phospholipase C inhibitor. Neuroreport. 2001;12(10):2131–2136. doi: 10.1097/00001756-200107200-00018. [DOI] [PubMed] [Google Scholar]
- 114.Murillo-Rodríguez E., Vázquez E., Millán-Aldaco D., Palomero-Rivero M., Drucker-Colin R. Effects of the fatty acid amide hydrolase inhibitor URB597 on the sleep-wake cycle, c-Fos expression and dopamine levels of the rat. Eur. J. Pharmacol. 2007;562(1-2):82–91. doi: 10.1016/j.ejphar.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 115.Murillo-Rodríguez E., Palomero-Rivero M., Millán-Aldaco D., Arias-Carrión O., Drucker-Colín R. Administration of URB597, oleoylethanolamide or palmitoylethanolamide increases waking and dopamine in rats. PLoS One. 2011;6(7):e20766. doi: 10.1371/journal.pone.0020766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tutunchi H., Ostadrahimi A., Saghafi-Asl M., Maleki V. The effects of oleoylethanolamide, an endogenous PPAR-α agonist, on risk factors for NAFLD: A systematic review. Obes. Rev. 2019;20(7):1057–1069. doi: 10.1111/obr.12853. [DOI] [PubMed] [Google Scholar]
- 117.Mijangos-Moreno S., Poot-Aké A., Guzmán K., Arankowsky-Sandoval G., Arias-Carrión O., Zaldívar-Rae J., Sarro-Ramírez A., Murillo-Rodríguez E. Sleep and neurochemical modulation by the nuclear peroxisome proliferator-activated receptor α (PPAR-α) in rat. Neurosci. Res. 2016;105:65–69. doi: 10.1016/j.neures.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Murillo-Rodriguez E. The Role of Nuclear Receptor PPARα in the Sleep-wake Cycle Modulation. A Tentative Approach for Treatment of Sleep Disorders. Curr. Drug Deliv. 2017;14(4):473–482. doi: 10.2174/1567201814666161109123803. [DOI] [PubMed] [Google Scholar]
- 119.Murillo-Rodríguez E., Millán-Aldaco D., Di Marzo V., Drucker-Colín R. The anandamide membrane transporter inhibitor, VDM-11, modulates sleep and c-Fos expression in the rat brain. Neuroscience. 2008;157(1):1–11. doi: 10.1016/j.neuroscience.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 120.Murillo-Rodríguez E., Palomero-Rivero M., Millán-Aldaco D., Di Marzo V. The administration of endocannabinoid uptake inhibitors OMDM-2 or VDM-11 promotes sleep and decreases extracellular levels of dopamine in rats. Physiol. Behav. 2013;109:88–95. doi: 10.1016/j.physbeh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 121.Murillo-Rodríguez E., Machado S., Rocha N.B., Budde H., Yuan T.F., Arias-Carrión O. Revealing the role of the endocannabinoid system modulators, SR141716A, URB597 and VDM-11, in sleep homeostasis. Neuroscience. 2016;339:433–449. doi: 10.1016/j.neuroscience.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 122.Engeli S., Blüher M., Jumpertz R., Wiesner T., Wirtz H., Bosse-Henck A., Stumvoll M., Batkai S., Pacher P., Harvey-White J., Kunos G., Jordan J. Circulating anandamide and blood pressure in patients with obstructive sleep apnea. J. Hypertens. 2012;30(12):2345–2351. doi: 10.1097/HJH.0b013e3283591595. [DOI] [PubMed] [Google Scholar]
- 123.Wang X., Yu Q., Yue H., Zhang J., Zeng S., Cui F. Circulating endocannabinoids and insulin resistance in patients with obstructive Sleep Apnea. BioMed Res. Int. 2016;2016:9782031. doi: 10.1155/2016/9782031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maple K.E., McDaniel K.A., Shollenbarger S.G., Lisdahl K.M. Dose-dependent cannabis use, depressive symptoms, and FAAH genotype predict sleep quality in emerging adults: a pilot study. Am. J. Drug Alcohol Abuse. 2016;42(4):431–440. doi: 10.3109/00952990.2016.1141913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3326–3341. doi: 10.1098/rstb.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Piyanova A., Lomazzo E., Bindila L., Lerner R., Albayram O., Ruhl T., Lutz B., Zimmer A., Bilkei-Gorzo A. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 2015;150:55–64. doi: 10.1016/j.mad.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 127.Bonnet A.E., Marchalant Y. Potential therapeutical contributions of the endocannabinoid system towards aging and alzheimer’s Disease. Aging Dis. 2015;6(5):400–405. doi: 10.14336/AD.2015.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vázquez C., Tolón R.M., Grande M.T., Caraza M., Moreno M., Koester E.C., Villaescusa B., Ruiz-Valdepeñas L., Fernández-Sánchez F.J., Cravatt B.F., Hillard C.J., Romero J. Endocannabinoid regulation of amyloid-induced neuroinflammation. Neurobiol. Aging. 2015;36(11):3008–3019. doi: 10.1016/j.neurobiolaging.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Sophocleous A., Marino S., Kabir D., Ralston S.H., Idris A.I. Combined deficiency of the Cnr1 and Cnr2 receptors protects against age-related bone loss by osteoclast inhibition. Aging Cell. 2017;16(5):1051–1061. doi: 10.1111/acel.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cassano T., Calcagnini S., Pace L., De Marco F., Romano A., Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative Disorders: From pathogenesis to a promising therapeutic target. Front. Neurosci. 2017;11:30. doi: 10.3389/fnins.2017.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bilkei-Gorzo A., Albayram O., Draffehn A., Michel K., Piyanova A., Oppenheimer H., Dvir-Ginzberg M., Rácz I., Ulas T., Imbeault S., Bab I., Schultze J.L., Zimmer A. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017;23(6):782–787. doi: 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- 132.Canseco-Alba A., Schanz N., Sanabria B., Zhao J., Lin Z., Liu Q.R., Onaivi E.S. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav. Brain Res. 2019;360:286–297. doi: 10.1016/j.bbr.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berrendero F., Romero J., García-Gil L., Suarez I., De la Cruz P., Ramos J.A., Fernández-Ruiz J.J. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta. 1998;1407(3):205–214. doi: 10.1016/S0925-4439(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 134.Valenti M., Viganò D., Casico M.G., Rubino T., Steardo L., Parolaro D., Di Marzo V. Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell. Mol. Life Sci. 2004;61(7-8):945–950. doi: 10.1007/s00018-003-3453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murillo-Rodriguez E., Désarnaud F., Prospéro-García O. Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life Sci. 2006;79(1):30–37. doi: 10.1016/j.lfs.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 136.Maroof N., Ravipati S., Pardon M.C., Barrett D.A., Kendall D.A. Reductions in endocannabinoid levels and enhanced coupling of cannabinoid receptors in the striatum are accompanied by cognitive impairments in the AβPPswe/PS1ΔE9 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014;42(1):227–245. doi: 10.3233/JAD-131961. [DOI] [PubMed] [Google Scholar]
- 137.Schaich C.L., Shaltout H.A., Grabenauer M., Thomas B.F., Gallagher P.E., Howlett A.C., Diz D.I. Alterations in the medullary endocannabinoid system contribute to age-related impairment of baroreflex sensitivity. J. Cardiovasc. Pharmacol. 2015;65(5):473–479. doi: 10.1097/FJC.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tuo W., Leleu-Chavain N., Spencer J., Sansook S., Millet R., Chavatte P. Therapeutic potential of fatty acid amide hydrolase, monoacylglycerol lipase, and N-Acylethanolamine acid amidase Inhibitors. J. Med. Chem. 2017;60(1):4–46. doi: 10.1021/acs.jmedchem.6b00538. [DOI] [PubMed] [Google Scholar]
- 139.Pascual A.C., Martín-Moreno A.M., Giusto N.M., de Ceballos M.L., Pasquaré S.J. Normal aging in rats and pathological aging in human Alzheimer’s disease decrease FAAH activity: modulation by cannabinoid agonists. Exp. Gerontol. 2014;60:92–99. doi: 10.1016/j.exger.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 140.Pascual A.C., Gaveglio V.L., Giusto N.M., Pasquaré S.J. Aging modifies the enzymatic activities involved in 2-arachidonoylglycerol metabolism. Biofactors. 2013;39(2):209–220. doi: 10.1002/biof.1055. [DOI] [PubMed] [Google Scholar]
- 141.Pascual A.C., Gaveglio V.L., Giusto N.M., Pasquaré S.J. Cannabinoid receptor-dependent metabolism of 2-arachidonoylglycerol during aging. Exp. Gerontol. 2014;55:134–142. doi: 10.1016/j.exger.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 142.Maccarrone M., Attinà M., Bari M., Cartoni A., Ledent C., Finazzi-Agrò A. Anandamide degradation and N-acylethanolamines level in wild-type and CB1 cannabinoid receptor knockout mice of different ages. J. Neurochem. 2001;78(2):339–348. doi: 10.1046/j.1471-4159.2001.00413.x. [DOI] [PubMed] [Google Scholar]
- 143.Piyanova A., Lomazzo E., Bindila L., Lerner R., Albayram O., Ruhl T., Lutz B., Zimmer A., Bilkei-Gorzo A. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech. Ageing Dev. 2015;150:55–64. doi: 10.1016/j.mad.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 144.Lu H.C., Mackie K. An Introduction to the endogenous cannabinoid system. Biol. Psychiatry. 2016;79(7):516–525. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boggs D.L., Nguyen J.D., Morgenson D., Taffe M.A., Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacology. 2018;43(1):142–154. doi: 10.1038/npp.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Russo E.B. Cannabis therapeutics and the future of neurology. Front. Integr. Nuerosci. 2018;12:51. doi: 10.3389/fnint.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schrot R.J., Hubbard J.R. Cannabinoids: Medical implications. Ann. Med. 2016;48(3):128–141. doi: 10.3109/07853890.2016.1145794. [DOI] [PubMed] [Google Scholar]
- 148.World Health Organization 2018. Cannabidiol(CBD) Critical Review Report. https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf
- 149.Ibeas Bih C., Chen T., Nunn A.V., Bazelot M., Dallas M., Whalley B.J. Molecular targets of cannabidiol in neurological Disorders. Neurotherapeutics. 2015;12(4):699–730. doi: 10.1007/s13311-015-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press.; 2017. National Academies of Sciences, engineering, and medicine. [DOI] [PubMed] [Google Scholar]
- 151.Chagas M.H., Eckeli A.L., Zuardi A.W., Pena-Pereira M.A., Sobreira-Neto M.A., Sobreira E.T., Camilo M.R., Bergamaschi M.M., Schenck C.H., Hallak J.E., Tumas V., Crippa J.A. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson’s disease patients: a case series. J. Clin. Pharm. Ther. 2014;39(5):564–566. doi: 10.1111/jcpt.12179. [DOI] [PubMed] [Google Scholar]
- 152.Shannon S., Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm. J. 2016;20(4):16–005. doi: 10.7812/TPP/16-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shannon S., Lewis N., Lee H., Hughes S. Cannabidiol in anxiety and sleep: A large case series. Perm. J. 2019;23:18–041. doi: 10.7812/TPP/18-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pertwee R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morales P., Goya P., Jagerovic N., Hernandez-Folgado L. Allosteric modulators of the CB1 cannabinoid receptor: A structural Update Review. Cannabis Cannabinoid Res. 2016;1(1):22–30. doi: 10.1089/can.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Navarro G., Reyes-Resina I., Rivas-Santisteban R., Sánchez de Medina V., Morales P., Casano S., Ferreiro-Vera C., Lillo A., Aguinaga D., Jagerovic N., Nadal X., Franco R. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. 2018;157:148–158. doi: 10.1016/j.bcp.2018.08.046. [DOI] [PubMed] [Google Scholar]
- 157.Tham M., Yilmaz O., Alaverdashvili M., Kelly M.E.M., Denovan-Wright E.M., Laprairie R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019;176(10):1455–1469. doi: 10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fogaça M.V., Campos A.C., Coelho L.D., Duman R.S., Guimarães F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology. 2018;135:22–33. doi: 10.1016/j.neuropharm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 159.Mijangos-Moreno S., Poot-Aké A., Arankowsky-Sandoval G., Murillo-Rodríguez E. Intrahypothalamic injection of cannabidiol increases the extracellular levels of adenosine in nucleus accumbens in rats. Neurosci. Res. 2014;84:60–63. doi: 10.1016/j.neures.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 160.Murillo-Rodríguez E., Di Marzo V., Machado S., Rocha N.B., Veras A.B., Neto G.A.M., Budde H., Arias-Carrión O., Arankowsky-Sandoval G. Role of N-arachidonoyl-serotonin (AA-5-HT) in sleep-wake cycle architecture, sleep homeostasis, and neurotransmitters Regulation. Front. Mol. Neurosci. 2017;10:152. doi: 10.3389/fnmol.2017.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Murillo-Rodríguez E., Arankowsky-Sandoval G., Rocha N.B., Peniche-Amante R., Veras A.B., Machado S., Budde H. Systemic injections of vannabidiol rnhance scetylcholine levels from basal forebrain in rats. Neurochem. Res. 2018;43(8):1511–1518. doi: 10.1007/s11064-018-2565-0. [DOI] [PubMed] [Google Scholar]
- 162.Slomski A. Fewer seizures with cannabidiol in vatastrophic Epilepsy. JAMA. 2017;318(4):323. doi: 10.1001/jama.2017.8846. [DOI] [PubMed] [Google Scholar]
- 163.Devinsky O., Patel A.D., Cross J.H., Villanueva V., Wirrell E.C., Privitera M., Greenwood S.M., Roberts C., Checketts D., VanLandingham K.E., Zuberi S.M. GWPCARE3 Study Group. Effect of cannabidiol on drop seizures in the Lennox-gastaut Syndrome. N. Engl. J. Med. 2018;378(20):1888–1897. doi: 10.1056/NEJMoa1714631. [DOI] [PubMed] [Google Scholar]
- 164.Rubin R. The path to the first FDA-approved cannabis-derived treatment and what comes next. JAMA. 2018;320(12):1227–1229. doi: 10.1001/jama.2018.11914. [DOI] [PubMed] [Google Scholar]
- 165.Ebbert J.O., Scharf E.L., Hurt R.T. Medical cannabis. Mayo Clin. Proc. 2018;93(12):1842–1847. doi: 10.1016/j.mayocp.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 166.Freedman D.A., Patel A.D. Inadequate regulation contributes to mislabeled online cannabidiol products. Pediatr. Neurol. Briefs. 2018;32:3. doi: 10.15844/pedneurbriefs-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Keshavan M.S., Giedd J., Lau J.Y., Lewis D.A., Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- 168.Luján M.Á., Castro-Zavala A., Alegre-Zurano L., Valverde O. repeated cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology. 2018;143:163–175. doi: 10.1016/j.neuropharm.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 169.Schiavon A.P., Bonato J.M., Milani H., Guimarães F.S., Weffort de Oliveira R.M. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:27–34. doi: 10.1016/j.pnpbp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 170.Carvalho R.K., Souza M.R., Santos M.L., Guimarães F.S., Pobbe R.L.H., Andersen M.L., Mazaro-Costa R. Chronic cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reprod. Toxicol. 2018;81:34–40. doi: 10.1016/j.reprotox.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 171.Iffland K., Grotenhermen F. An Update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139–154. doi: 10.1089/can.2016.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sands T.T., Rahdari S., Oldham M.S., Caminha N.E., Tilton N., Cilio M.R. Long-Term Safety, Tolerability, and Efficacy of cannabidiol in children with refractory Epilepsy: Results from an expanded access program in the US. CNS Drugs. 2019;33(1):47–60. doi: 10.1007/s40263-018-0589-2. [DOI] [PubMed] [Google Scholar]
- 173.Deutsch D.G., Ueda N., Yamamoto S. The fatty acid amide hydrolase (FAAH). Prostaglandins Leukot. Essent. Fatty Acids. 2002;66(2-3):201–210. doi: 10.1054/plef.2001.0358. [DOI] [PubMed] [Google Scholar]
- 174.Hu S.S., Mackie K. Distribution of the rndocannabinoid dystem in the central nervous system. Handb. Exp. Pharmacol. 2015;231:59–93. doi: 10.1007/978-3-319-20825-1_3. [DOI] [PubMed] [Google Scholar]
- 175.Shin M., Ware T.B., Lee H.C., Hsu K.L. Lipid-metabolizing serine hydrolases in the mammalian central nervous system: endocannabinoids and beyond. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;1864(6):907–921. doi: 10.1016/j.bbalip.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Donvito G., Nass S.R., Wilkerson J.L., Curry Z.A., Schurman L.D., Kinsey S.G., Lichtman A.H. The endogenous cannabinoid system: A budding source of targets for treating inflammatory and neuropathic Pain. Neuropsychopharmacology. 2018;43(1):52–79. doi: 10.1038/npp.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stampanoni Bassi M., Gilio L., Maffei P., Dolcetti E., Bruno A., Buttari F., Centonze D., Iezzi E. Exploiting the multifaceted effects of cannabinoids on mood to boost their therapeutic Use Against Anxiety and Depression. Front. Mol. Neurosci. 2018;11:424. doi: 10.3389/fnmol.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]