Abstract
NMDA-type glutamate receptors are ligand-gated ion channels that mediate a major component of excitatory neurotransmission in the central nervous system (CNS). They are widely distributed at all stages of development and are critically involved in normal brain functions, including neuronal development and synaptic plasticity. NMDA receptors are also implicated in the pathophysiology of numerous neurological and psychiatric disorders, such as ischemic stroke, traumatic brain injury, Alzheimer’s disease, epilepsy, mood disorders, and schizophrenia. For these reasons, NMDA receptors have been intensively studied in the past several decades to elucidate their physiological roles and to advance them as therapeutic targets. Seven NMDA receptor subunits exist that assemble into a diverse array of tetrameric receptor complexes, which are differently regulated, have distinct regional and developmental expression, and possess a wide range of functional and pharmacological properties. The diversity in subunit composition creates NMDA receptor subtypes with distinct physiological roles across neuronal cell types and brain regions, and enables precise tuning of synaptic transmission. Here, we will review the relationship between NMDA receptor structure and function, the diversity and significance of NMDA receptor subtypes in the CNS, as well as principles and rules by which NMDA receptors operate in the CNS under normal and pathological conditions.
Keywords: Ionotropic glutamate receptor, neurotransmitter, NMDA, ion channel, regulation, structure-function, disease, synaptic transmission
1. Introduction
Glutamatergic neurotransmission in the CNS is mediated by metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs). The iGluRs are ligand-gated ion channels permeable to cations (Na+, K+, and Ca2+) that can be divided into three functional classes, namely the α-amino-3-hydroxy-5-methyl-4-isoxasolepropionic acid (AMPA) receptors, kainate receptors, and N-methyl-D-aspartate (NMDA) receptors [1,2] (Fig. 1a,b). These functional classes were historically named on the basis of their pharmacological properties (i.e. the activating agonist), but the division was firmly established by subsequent cloning that demonstrated strong correlation between the sequence identity and the pharmacological properties of subunits in these functional classes. The δ (delta) receptors are also considered iGluRs, primarily based on sequence identity, but their function is not fully understood [3–5]. The δ (delta) receptors appear to play important roles in synapse organization and some forms of synaptic plasticity [6–8], but it is uncertain whether they are capable of forming functional ion channels [9–11]. NMDA receptors exhibit voltage-dependent Mg2+-block, high permeability to Ca2+, and require simultaneous binding of the co-agonists glycine and glutamate for activation. These hallmark features distinguish NMDA receptors from AMPA/kainate receptors (i.e. non-NMDA receptors) and have profound impact on their physiological roles in the CNS.
Figure 1. Functional classes of ionotropic glutamate receptors.
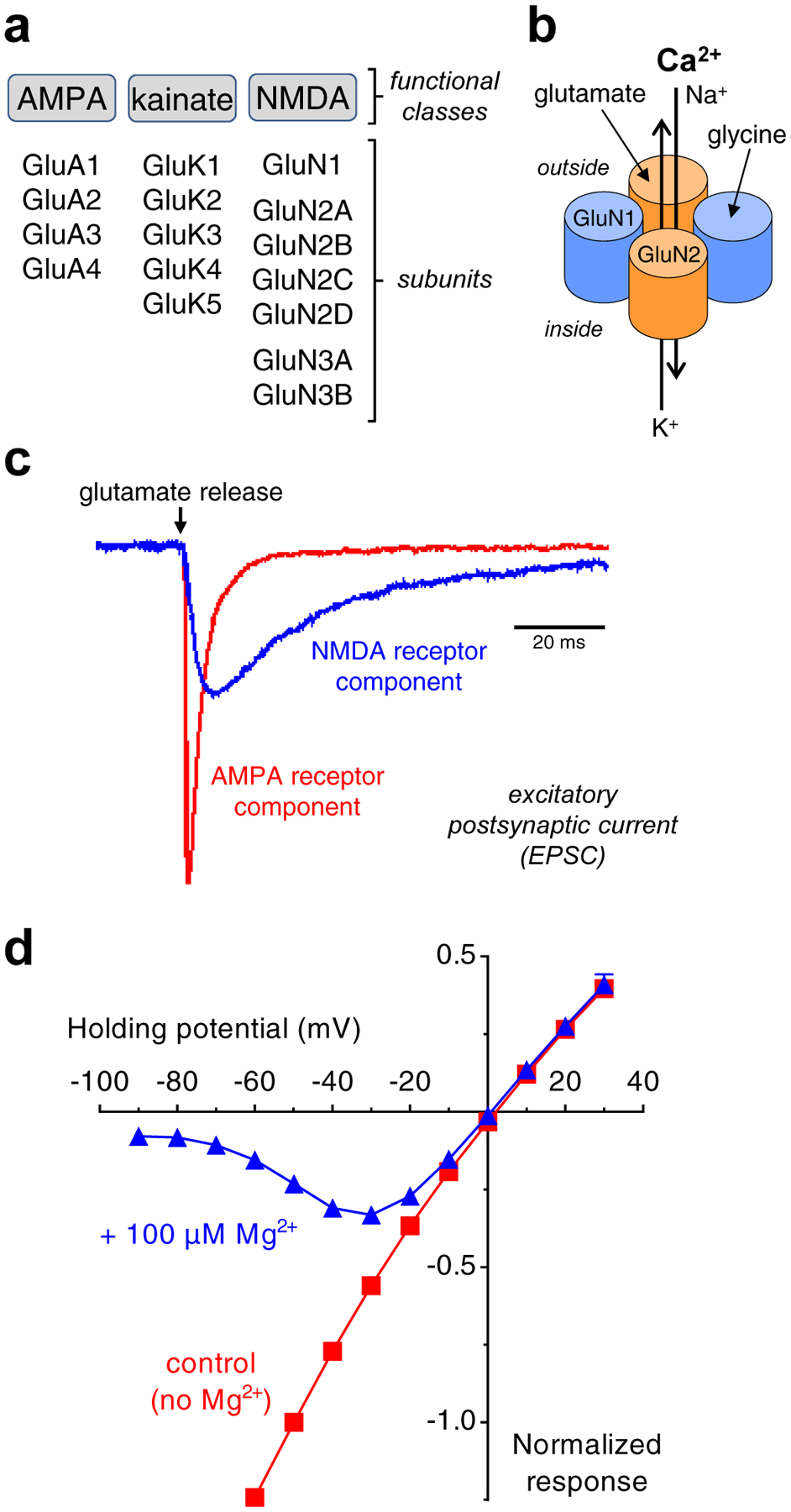
a) Ionotropic glutamate receptors are divided into three functional classes, namely AMPA, kainate, and NMDA receptors. Multiple subunits have been cloned in each of these classes. b) The majority of NMDA receptors in the CNS are composed of two glycine-binding GluN1 and two glutamate-binding GluN2 subunits, which form a central cation-permeable channel pore. c) AMPA and NMDA receptor-mediated components of the EPSC at a central synapse. The slow NMDA receptor-mediated component is isolated in the absence of Mg2+ using the AMPA receptor antagonist CNQX, whereas the fast AMPA receptor-mediated component is isolated using the NMDA receptor antagonist AP5. The figure shows unpublished data from Lonnie P. Wollmuth and is adapted with permission from Traynelis et al. [1]. d) Relationship between NMDA receptor current response and membrane potential (i.e. I/V-relationship) in the presence and absence of 100 μM extracellular Mg2+. Voltage-dependent Mg2+-block is relieved with depolarization of the membrane potential (i.e. as the membrane potential approaches 0 mV). Unpublished data from Feng Yi and Kasper B. Hansen.
In most central synapses, the release of glutamate activates excitatory postsynaptic currents (EPSCs) with a time course that can be described primarily by two exponential components corresponding to activation of AMPA and NMDA receptors. Activation of AMPA receptors mediates a fast component with rapid rise time and decay, whereas activation of NMDA receptors mediates a slower component with slower rise time and a time course lasting for tens to hundreds of milliseconds [12–15] (Fig. 1c). Activation of postsynaptic kainate receptors typically result in EPSCs with a slower time course than AMPA receptors and a comparable, but generally faster time course than NMDA receptors [16]. At resting membrane potential, the NMDA receptor ion channel is blocked by physiological levels of extracellular Mg2+, but synaptic release of glutamate and the resulting rapid activation of AMPA/kainate receptors can depolarize the membrane potential and thereby relieve voltage-dependent Mg2+-block of NMDA receptors [17,18] (Fig, 1d). Thus, NMDA receptors serve as coincidence detectors that require simultaneous presynaptic release of glutamate and postsynaptic depolarization in order to produce the slow Ca2+-permeable component of the EPSC [19,20].
The NMDA receptors can mediate substantial Ca2+-influx during the EPSC due both to their high Ca2+ permeability and prolonged time course. The resulting increase in intracellular Ca2+ can trigger multiple downstream signaling events in the postsynaptic neuron, which are central to the roles of NMDA receptors under both normal and pathophysiological conditions [21,22,2,1,23]. The rise in intracellular Ca2+ triggers both short-term and long-term effects, which are accompanied by changes in synaptic efficacy and neuronal morphology (i.e. synaptic plasticity) [24]. Robust NMDA receptor-mediated Ca2+-influx for a short duration can lead to long-term potentiation (LTP) of synaptic efficacy, whereas less pronounced Ca2+-influx for a longer duration can result in long-term depression (LTD) [25,26]. Thus, the frequency and duration of synaptic NMDA receptor activation can result in either potentiation or depression of synaptic efficacy, which is considered a cellular correlate of memory and learning [27,28].
Glutamate is sufficient for activation of AMPA and kainate receptors, whereas the NMDA receptors are unique in that they require simultaneous binding of two distinct agonists, glutamate and glycine/D-serine, for activation [29–35]. In the CNS, NMDA receptors mainly rely on synaptic release of glutamate for activation, since extracellular glycine (or D-serine) is thought to be continuously present. Whether glycine or D-serine serves as the endogenous co-agonist may depend on brain region and subcellular compartment [36–38]. For example, it has recently been suggested that D-serine is the predominant co-agonist in synapses, whereas glycine is more prevalent at extrasynaptic sites [39]; more work is needed to determine whether this is a principle that transcends anatomical regions. Furthermore, glycine and D-serine are not present at concentrations that fully saturate the NMDA receptor co-agonist binding sites, at least in some brain regions [40,41]. Thus, the co-agonist requirement enables an additional mechanism of NMDA receptor regulation, in which activation is controlled by phasic changes in glutamate concentrations (i.e. synaptic release), but the magnitude of activation can be modulated by changes in the tonic concentration of glycine/D-serine. Given the central roles of NMDA receptors in normal brain function, it is not surprising that their dysregulation has been linked to a number of pathophysiological conditions [2,1,42,23]. In acute conditions, such as ischemia, seizures, and traumatic brain injury, the increase in extracellular glutamate that follows increased release and impaired uptake can produce profound NMDA receptor-mediated Ca2+-flux into the neuron, which may promote neuronal death [43–46]. Impairment of neuronal health by glutamate-mediate signaling is often referred to as “excitotoxicity” [47]. Under chronic conditions of enhanced neuronal susceptibility, as in Parkinson’s and Alzheimer’s diseases, the NMDA receptor-mediated excitotoxicity may contribute to impairment of neuronal health over many years (e.g. see [48]). NMDA receptor antagonists have been proposed to be beneficial under such conditions involving excitotoxicity (e.g. see [49]), but side effects, such as psychosis, memory impairment, anesthesia, and neuronal cell death, can accompany strong and non-selective NMDA receptor blockade, thereby limiting the clinical usefulness of such drugs for chronic conditions [50,51].
Interestingly, the side effects of high-affinity NMDA receptor channel blockers resemble symptoms exhibited by patients suffering from schizophrenia. The observations of these “psychotomimetic” properties of the channel blockers PCP and ketamine have led to the “NMDA receptor hypofunction model of psychosis”, which proposes that multiple symptoms associated with in schizophrenia may be caused by lower than normal NMDA-receptor-mediated glutamatergic activity in key brain circuits [52,51,53]. In theory, enhancing NMDA receptor function, perhaps selectively in key brain circuits, could be beneficial for treating cognitive disorders and schizophrenia. However, NMDA receptor agonists have not been fully studied in this context due to the risk that excessive stimulation may cause excitotoxicity, and indirect methods to enhance NMDA receptor function through block of glycine uptake have been inconclusive. Moreover, only very recently have subunit-selective NMDA receptor positive allosteric modulators been identified that allow this idea to be further investigated (see below). In this regard, subunit-selective modulators of NMDA receptors may be therapeutically beneficial in some CNS disorders, since these modulators would target NMDA receptor subtypes in specific neuronal population or brain regions associated with the disease without affecting NMDA receptors in other regions [54–56].
1.1. NMDA receptor subunit diversity
The arrival of the action potential at the presynaptic terminal triggers the release of glutamate into the synaptic cleft. Termination of glutamatergic neurotransmission is mediated by diffusion and rapid removal of glutamate from synaptic and extrasynaptic sites via reuptake by excitatory amino acid transporters (EAATs; i.e. glutamate transporters) [57]. Synaptically-released glutamate reaches a very high peak concentration (~1 mM) for a brief duration (~1 ms) [58]. In this short period of time, glutamate will bind iGluRs and initiate receptor conformational changes that lead to opening of the ion channel (i.e. ion channel gating). However, the NMDA receptor-mediated component of the EPSC continues for tens to hundreds of millisecond after synaptic glutamate is removed, during which time, NMDA receptors transition between glutamate-bound open and closed conformational states until glutamate eventually unbinds and the EPSC is terminated. Thus, the time course of the EPSC is governed by glutamate binding affinity, the connectivity and lifetime of the receptor in pre-gating conformations that must be traversed before unbinding, and the rates into and out of the desensitized states following agonist binding [59–61]. For NMDA receptors, these functional properties are controlled by the subunit composition [62–64] (Fig. 2). Subunit diversity among NMDA receptors and assembly of different receptor subtypes with distinct functional properties enable precise tuning of the synaptic response and allows variation in the physiological roles of NMDA receptors at synaptic versus extrasynaptic sites, in different neuronal cell types and brain regions, and during neuronal development.
Figure 2. GluN2 subunit-specific expression and functional properties of recombinant NMDA receptor subtypes.
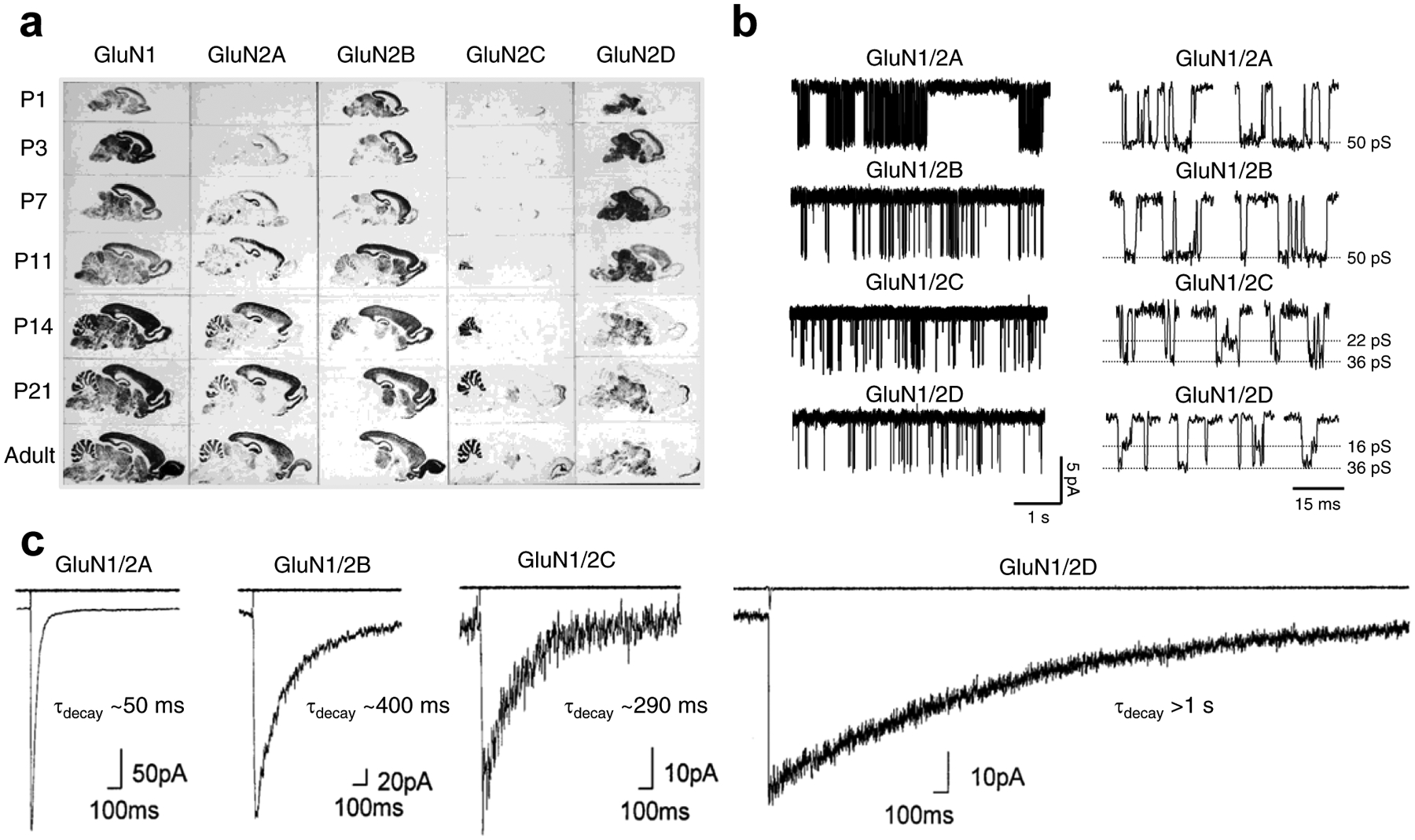
a) Regional and developmental expression of GluN2 subunits in rat brain revealed in autoradiograms using in situ hybridizations of oligonucleotide probes for the relevant mRNAs to parasagittal sections. Modified with permission from Akazawa et al. [92]. b) Single-channel recordings of currents from diheteromeric NMDA receptor subtypes expressed in HEK293 cells (outside-out membrane patches). Open probability is ~0.5 for GluN1/2A, ~0.1 for GluN1/2B, and <0.05 for GluN1/2C and GluN1/2D. Highlights of individual openings are shown on the left. GluN1/2A and GluN1/2B have higher channel conductance (~50 pS) compared to GluN1/2C (~22 and ~36 pS) and GluN1/2D (~16 and ~36 pS). Adapted with permission from Yuan et al. [524]. c) Whole-cell patch-clamp recordings of responses from brief application of glutamate (1 ms of 1 mM glutamate) to recombinant diheteromeric NMDA receptor subtypes expressed in HEK293 cells. The open tip current indicating the duration of the drug application is shown in the upper trace. Adapted with permission from Vicini et al. [62].
Seven genes that encode NMDA receptor subunits have been identified, which include GluN1, four different GluN2 (GluN2A-D), and two GluN3 subunits (GluN3A-B) [2,1] (Fig. 1a). All NMDA receptors are obligatory heteromeric assemblies of four subunits that form a central ion channel pore, and the majority of NMDA receptors in the CNS are composed of two glycine-binding GluN1 and two glutamate-binding GluN2 subunits (i.e. GluN1/2 receptors) [65–67] (Fig. 1b). However, the glycine-binding GluN3 subunits can also assemble with GluN1 and GluN2 subunits to form GluN1/2/3 receptors or with GluN1 alone to form GluN1/3 receptors [68–72].
1.2. The GluN1 subunit
The glycine/D-serine-binding GluN1 subunit is ubiquitously distributed in the brain and is an obligatory subunit in all NMDA receptor subtypes. GluN1 has eight different isoforms that arise from alternative splicing of three exons within of a single gene product [73–76] (Fig. 3a,b). Exon 5 encodes 21 highly charged amino acids in the GluN1 amino-terminal domain (ATD) named the N1 cassette, exon 21 encodes 37 amino acids in the carboxyl-terminal domain (CTD) named the C1 cassette, and exon 22 encodes 38 amino acids in the CTD named the C2 cassette. Deletion of exon 22 eliminates a stop codon and causes a reading frame shift, which results in the inclusion of 22 alternative amino acids named the C2’ cassette. Different GluN1 splice variants have distinct regional and developmental expression patterns [77–79] and display differences in NMDA receptor function and pharmacology (see below; Fig. 3b,c).
Figure 3. Expression and functional properties of GluN1 splice variants.
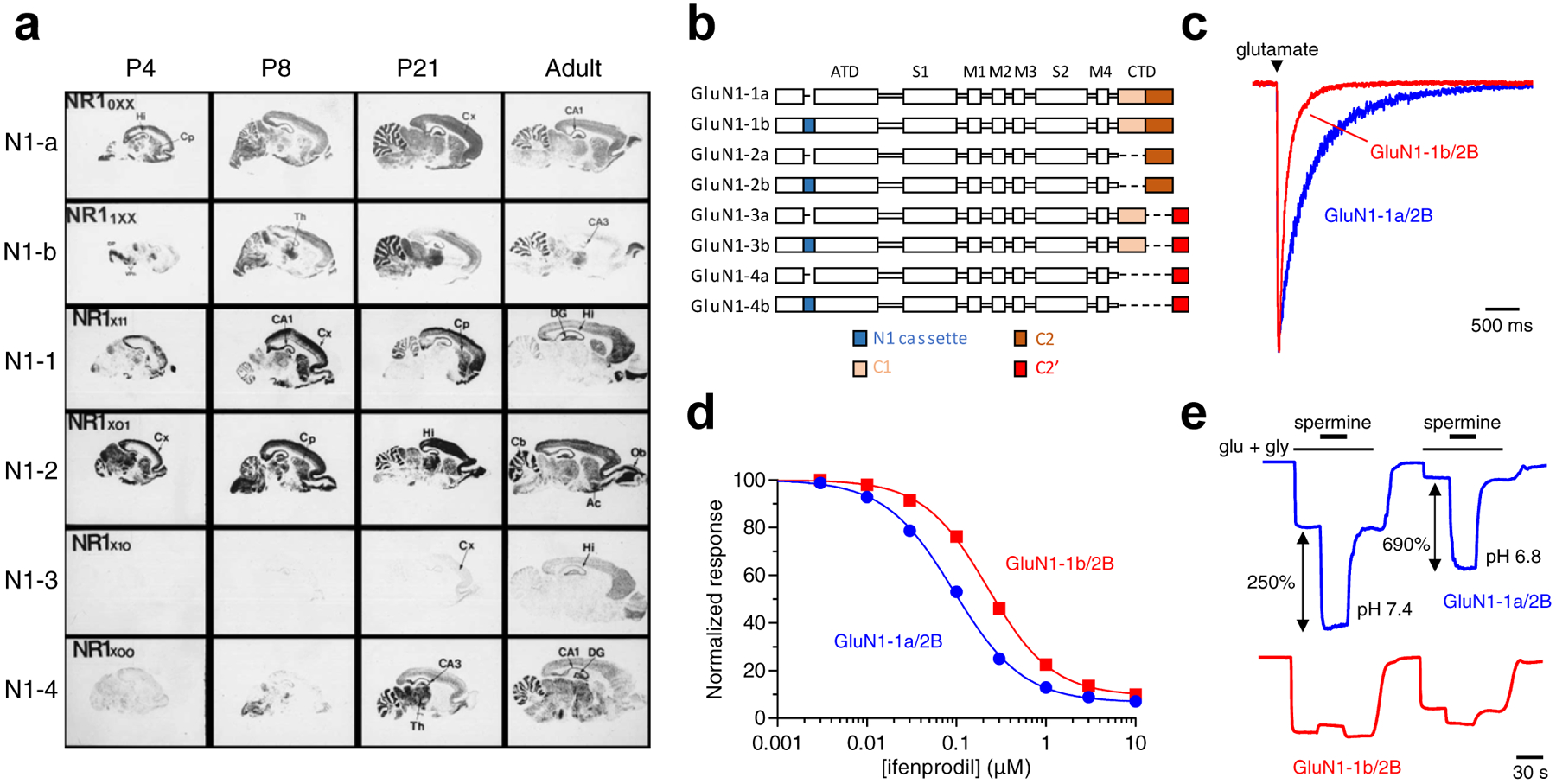
a) Regional and developmental expression of GluN1 splice variants in rat brain revealed in autoradiograms using in situ hybridizations of oligonucleotide probes for the relevant mRNAs to parasagittal sections. Ac, nucleus accumbens; Cb, cerebellum; Cp, caudate-putamen; Cx, cortex; DG, dentate gyrus; DP, dorsal pons; Hi, hippocampus; Ob, olfactory bulb; Th, thalamus; VPn, ventro-posterial thalamic nuclei. Modified with permission from Paupard et al. [78]. b) Linear representation of the GluN1 polypeptide chain for eight alternative splice variants. GluN1 subunits are composed of the amino-terminal domain (ATD), S1 and S2 segments that form the ligand binding domain (LBD), three transmembrane helices (M1, M3, and M4) and a membrane reentrant loop (M2), and the intracellular carboxyl-terminal domain (CTD). The N1 cassette (blue) is 21 amino acids in the ATD encoded by exon 5. The C1 cassette (yellow) is 37 amino acids in the CTD encoded by exon 21, while the C2 cassette (orange) is 38 amino acids in the CTD encoded by exon 22. Deletion of exon 22 creates a shift in the open reading frame, resulting in the alternate exon 22’ that encodes the C2’ cassette (red; 22 amino acids). c) Whole-cell patch-clamp recordings of responses from brief application of glutamate (1 ms of 1 mM glutamate) to recombinant GluN1–1a/2B and GluN1–1b/2B receptors expressed in HEK293 cells. NMDA receptors containing exon 5 (e.g. as in GluN1–1b) display faster deactivation time course compared to receptors lacking exon 5 (e.g. as in GluN1–1a). d) Ifenprodil concentration-inhibition relationships for recombinant GluN1–1a/2B and GluN1–1b/2B receptors expressed in Xenopus oocytes. Ifenprodil potency is lower for receptors containing exon 5. e) Representative recordings for spermine potentiation of responses from recombinant GluN1–1a/2B and GluN1–1b/2B receptors expressed in Xenopus oocytes. Spermine sensitivity is dramatically reduced for receptors containing exon 5. Data in c-e) are unpublished from Feng Yi and Kasper B. Hansen.
NMDA receptors with GluN1 subunits that contain the N1 cassette (i.e. exon 5) have reduced agonist potency (i.e. increased EC50) and are less sensitive to inhibition by protons and extracellular zinc [80,81]. Consistent with the effect on agonist potency, the presence of the N1 cassette accelerates deactivation of glutamate-activated NMDA receptor responses, which shortens the time course of EPSCs [82–84] (Fig. 3c). Furthermore, the N1 cassette has a negative impact on modulation of NMDA receptor function by GluN2B-selective antagonists, such as ifenprodil [85,86], and potentiation by extracellular polyamines, such as spermine [82,81,74,87,88] (Fig. 3d,e). Alternative splicing of exons 21 and 22 dramatically alter the length and the amino acid sequence of the intracellular GluN1 CTD, which mediates interactions with several intracellular proteins, including PSD-95, calmodulin, and the neurofilament subunit NF-L [1]. Many of these proteins are involved in surface trafficking and anchoring of receptors at synaptic sites, and alternative splicing of exons 21 and 22 can therefore mediate changes in the subcellular distribution of NMDA receptors [89–91]. Contrasting with the changes observed upon inclusion of exon 5, there has not been convincing demonstration that alternative splicing of exons 21 and 22 has strong effects on the functional and pharmacological properties of NMDA receptors.
1.3. The GluN2 subunits
The four different glutamate-binding GluN2 subunits (GluN2A-D) have pronounced differences in both developmental and regional expression levels and endow NMDA receptors with strikingly different pharmacological and functional properties [92,62,93,63,64,94,95,79] (Fig. 2). Thus, the GluN2 subunits essentially dictate the physiological roles of NMDA receptor subtypes in the CNS. Since GluN1 is an obligatory subunit in all NMDA receptors, the significant sites of structural variation among subtypes are located in the GluN2 subunits of the receptor complex. Efforts to pharmacologically manipulate specific NMDA receptor subtypes in the CNS therefore focus on the development of ligands that can distinguish NMDA receptors on the basis of GluN2 subunits [96,56,54,55,97]. In recent years, the therapeutic rationale for the development of GluN2-selective ligands has been reinforced by increasingly precise localization of GluN2 subunits in neuronal populations relevant to CNS diseases.
The GluN2 subunits impart NMDA receptor subtypes with differences in sensitivity to voltage-dependent Mg2+ block [63,98,99,64] and inhibition by endogenous modulators, such as protons and extracellular Zn2+ [80,81,100]. The potency of glutamate and glycine/D-serine, as well as other agonists, is influenced by the GluN2 subunits [101–103]. For example, the EC50 for glutamate activating NMDA receptors containing two GluN1 and two GluN2D subunits (i.e. diheteromeric GluN1/2D receptors) is 6- to 10-fold lower (i.e. glutamate is more potent) compared to that for GluN1/2A, whereas GluN1/2B and GluN1/2C receptors show intermediate EC50 values [2,1,104–106]. GluN1/2A and GluN1/2B have higher single channel conductances compared to GluN1/2C and GluN1/2D receptors [2,1,104–106]. In addition, the probability that the channel will be open when all the agonist binding sites are fully occupied by agonists (i.e. the open probability) is ~0.5 for recombinant GluN1/2A, ~0.1 for GluN1/2B, and <0.05 for GluN1/2C and GluN1/2D [2,1,104–106] (Fig. 2). Importantly, the time constants of deactivation (τdecay) are also highly dependent on the GluN2 subunits; τdecay is ~50 ms for GluN1–1a/2A, ~400 ms for GluN1–1a/2B, ~290 ms for GluN1–1a/2C, and >1 s for GluN1–1a/2D [64,62,94] (Fig. 2). Thus, the GluN2 subunits control functional NMDA receptor properties relevant to synaptic transmission. Furthermore, the amino acid sequence of the intracellular CTD displays pronounced variation among GluN2 subunits and harbor distinct interaction sites for phosphatases, kinases, and proteins responsible for surface trafficking and anchoring at synaptic sites [1]. The GluN2 subunits therefore also affect subcellular localization, cell-surface expression, and recycling/degradation of NMDA receptor subtypes.
1.4. The GluN3 subunits
GluN3A and GluN3B were cloned based on similarity to GluN1 and GluN2 subunits and were the last NMDA receptor subunits to be discovered (reviewed in [107,71,70,72,69,68]). The GluN3 subunits bind glycine and D-serine [108–110], but the functional properties and physiological roles of GluN3-containing NMDA receptors remain elusive.
Triheteromeric NMDA receptors that are assembled from a combination of GluN1, GluN2, and GluN3 subunits have been consistently reported in both the CNS and heterologous expression systems on the basis of biochemical and functional experiments [111–122]. It is therefore surprising that the subunit stoichiometry of triheteromeric GluN3-containing NMDA receptors has not been resolved; it is unknown whether GluN3 replaces GluN1, GluN2, or both GluN1 and GluN2 in these receptors. Despite this gap in our understanding, numerous studies suggest that the inclusion of GluN3 in the NMDA receptor complex reduces Mg2+-block and Ca2+-permeability as well as response amplitudes (reviewed in [107,71,70,72,69,68]). Thus, the GluN3 subunits appear to have dominant-negative effects on NMDA receptor function.
The GluN3 subunits can also assemble with GluN1 in heterologous expression systems to form functional diheteromeric NMDA receptors that contain two GluN1 and two GluN3 subunits [123,65,124], but their existence in the CNS has not been firmly established. These GluN1/3 receptors have been termed “excitatory glycine receptors”, since they can be activated by glycine alone without the requirement of glutamate binding [123]. In recombinant systems, the GluN1/3 receptors are characterized by low Ca2+-permeability and relative insensitivity to extracellular Mg2+ [123,125–127]. Interestingly, agonist binding to the GluN1 subunit of GluN1/3 receptors triggers strong desensitization, whereas agonist binding to the GluN3 subunits is sufficient for activation [127–130]. Thus, in contrast to the more conventional GluN1/2 receptors [30,35], simultaneous binding of agonist to all subunits does not appear to be a requirement for activation of GluN1/3 receptors. While many aspects of the physiological roles of GluN3-containing NMDA receptors remain elusive, it is clear that GluN3 subunits endow NMDA receptors with strikingly unique functional properties.
1.5. Diheteromeric and triheteromeric NMDA receptors
The seven NMDA receptor subunits can assemble to produce receptor subtypes with distinct physiological roles across neuronal cell types and brain regions, thereby mediating changes in synaptic transmission and subcellular localization during neuronal development. At least two different GluN2 subunits are expressed in most, if not all NMDA receptor-expressing cells, and a large proportion of native NMDA receptors in the adult CNS are triheteromers that contain GluN1 and two different GluN2 subunits [131–142,84]. Examples of triheteromeric NMDA receptors identified in an increasing number of studies are the GluN1/2A/2B, GluN1/2A/2C, GluN1/2B/2D subtypes, but the existence of NMDA receptors with other compositions of GluN2 subunits have not been ruled out [140,135,137,142,143,139,136,134,144,145,138,146,147,131,133,148,132,149]. These subtypes, which are expressed in distinct neuronal populations, have been detected using co-immunoprecipitation and by intriguing functional and pharmacological observations that are not mediated by diheteromeric NMDA receptors.
Despite their prevalence in the CNS, there is a gap in our knowledge of triheteromeric NMDA receptors due to our inability to study a homogenous population of these receptors in heterologous expression systems [148,62,140,150,151]. The nature of the problem is that co-expression of GluN1 with two different GluN2 subunits (e.g. GluN2A and GluN2B) generates three populations of functional NMDA receptors, which are composed of two different diheteromeric receptors (e.g. GluN1/2A and GluN1/2B) as well as triheteromeric receptors (e.g. GluN1/2A/2B) [150,62,151]. The majority of our knowledge regarding function, pharmacology, and regulation of recombinant NMDA receptors is therefore almost exclusively derived from studies on diheteromeric receptors that are assembled from GluN1 and only one type of GluN2 (e.g. GluN1/2A). The properties of diheteromeric NMDA receptors are well-described, but little is known about how co-assembly of two different GluN2 subunits affects properties, such as deactivation time course, Mg2+-block, and the activity of subunit-selective allosteric modulators. Similarly, phosphorylation sites and trafficking properties of the intracellular GluN2 CTDs have been extensively studied, but the regulation of triheteromeric NMDA receptors that possess two distinct GluN2 CTDs is largely unknown (see [152]).
Recently, significant insight into functional and pharmacological properties of triheteromeric NMDA receptors has been gained using a method to tightly control cell surface expression of NMDA receptors with defined subunit composition [151,153]. This method has provided evidence of surprising pharmacological and functional properties of triheteromeric NMDA receptors that are distinct from the properties of the respective diheteromeric receptors [151,153–158] (Fig. 4). Furthermore, the method is enabling exciting, new opportunities to develop therapeutic agents that target disease-relevant triheteromeric NMDA receptors [159,160,156,157,155,158].
Figure 4. Functional properties of triheteromeric GluN1/2A/2B receptors.

a) Ifenprodil concentration-inhibition relationships for recombinant diheteromeric GluN1/2A and GluN1/2B receptors and triheteromeric GluN1/2A/2B receptors expressed in Xenopus oocytes using a method to control subunit composition of NMDA receptors [151]. Ifenprodil efficacy and potency are reduced for triheteromeric GluN1/2A/2B receptors that only contain one binding site for ifenprodil. b) Whole-cell patch-clamp recordings of responses from brief application of glutamate (1 ms of 1 mM glutamate) to recombinant diheteromeric GluN1/2A and GluN1/2B receptors and triheteromeric GluN1/2A/2B receptors expressed in HEK293 cells. The deactivation time course of triheteromeric GluN1/2A/2B receptors is similar to diheteromeric GluN1/2A and strikingly different from diheteromeric GluN1/2B. Data are adapted with permission from Hansen et al. [151].
2. NMDA receptor structure and function
AMPA, kainate, and NMDA receptor subunits share a common membrane topology; each subunit consists of a large extracellular ATD, a bi-lobed ligand binding domain (LBD), a transmembrane domain (TMD), and an intracellular CTD (Fig. 5a). The TMD is formed by three transmembrane helices (M1, M2, and M4) and a membrane re-entrant loop (M2). The ion channel pore of iGluRs is mainly lined with residues in the membrane re-entrant loop from all four subunits. Among NMDA receptor subtypes, the residues in the pore region, which determines basic ion permeation properties, are highly conserved. One key determinant of ion permeation, which partially defines Ca2+ permeability and Mg2+-block, resides at the apex of the membrane re-entrant loop M2 and is sometimes referred to as the Q/R/N site on the basis of amino acid residues found at this position in AMPA, kainate, and NMDA receptors. The ATD adopts a clamshell-like structure formed by the first ~350 amino acids of the subunit and plays an important role in subunit assembly and as a modulatory NMDA receptor domain. In NMDA receptors, the ATD harbors binding sites for several allosteric modulators, including extracellular zinc and polyamines, as well as GluN2B-selective antagonists (e.g. ifenprodil) (Fig. 5b). The LBD is formed by two segments of the polypeptide chain (S1 and S2), which fold into a kidney-shaped structure composed of an upper lobe (D1) and lower lobe (D2) relative to the cell membrane, and the agonist binding site is located in the cleft between the two lobes (Fig. 5c,d). The relationships between domain structures, their intra- and inter-subunit interactions, and receptor function and pharmacology have been extensively studied for more than two decades. Recently, crystallographic and cryo-EM studies have provided the first glimpses at the domain organization of NMDA receptors and mechanisms by which these domains and allosteric modulators influence receptor function [66,67,161,162].
Figure 5. NMDA receptor structure and ligand binding sites.
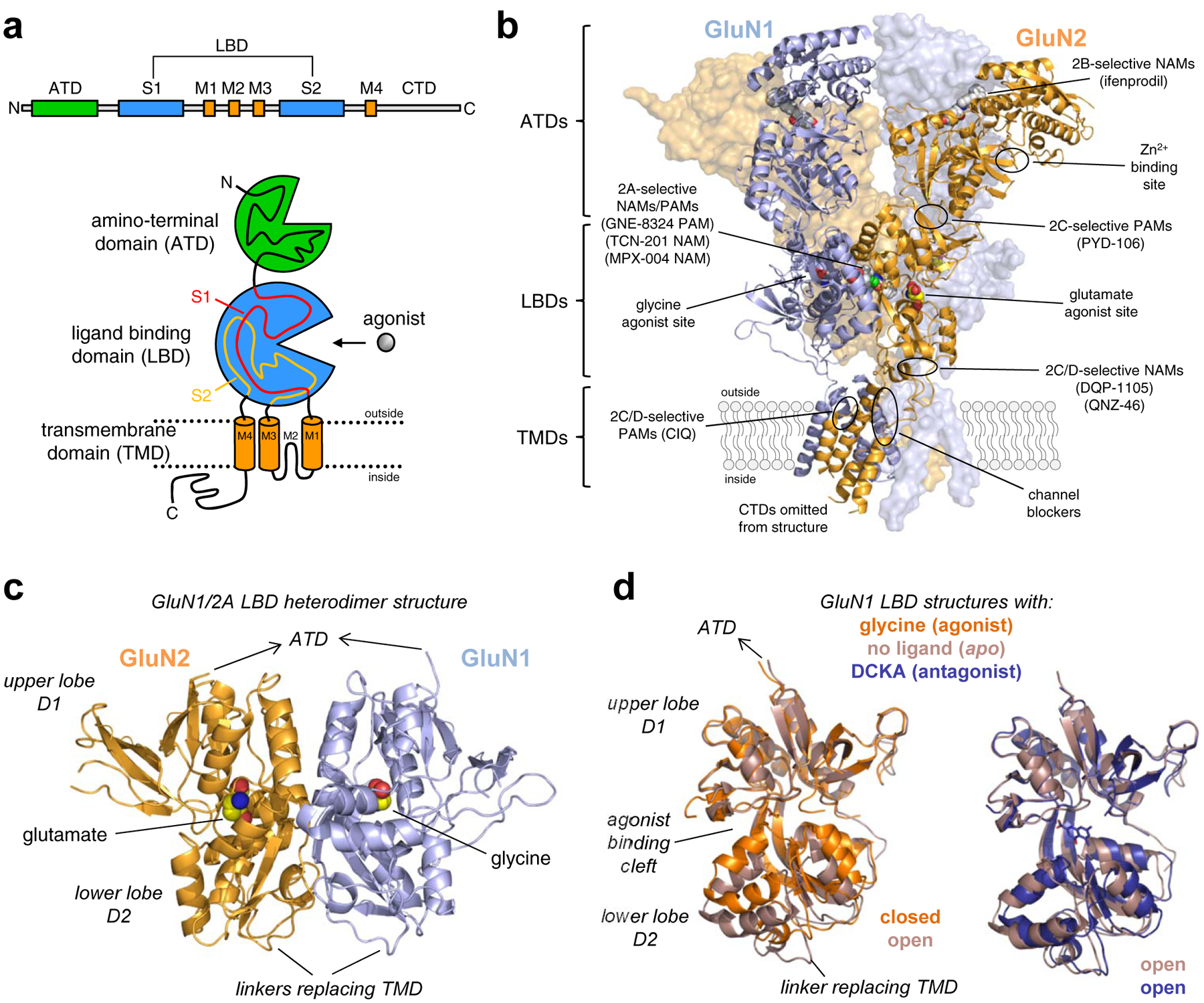
a) Linear representation and cartoon illustration of the polypeptide chain in iGluR subunits. Each subunit consists of a large extracellular amino-terminal domain (ATD), a bi-lobed ligand binding domain (LBD), a transmembrane domain (TMD), and an intracellular CTD. The TMD is formed by three transmembrane helices (M1, M2, and M4) and a membrane re-entrant loop (M2). The LBD is formed by two segments of the polypeptide chain (S1 and S2), which fold into a kidney-shaped structure composed of an upper lobe (D1) and lower lobe (D2) relative to the cell membrane, and the agonist binding site is located in the cleft between the two lobes. b) Crystal structure of the GluN1/2B NMDA receptor (PDB ID 4PE5; [67]), illustrating the subunit arrangement and the layered domain organization composed of the TMD layer and two extracellular layers formed by LBDs and ATDs. Agonist binding sites as well as known and predicted binding sites for positive and negative allosteric modulators (PAMs and NAMs) are highlighted. c) Crystal structure of the soluble GluN1/2A LBD heterodimer (PDB ID 5I57; [158]), showing the subunit interface and back-to-back dimer arrangement of the LBDs. Soluble LBD proteins composed of the S1 and S2 segments of the polypeptide chain are produced by deleting the ATD and replacing the TMD with a di-peptide linker. d) Overlay of crystal structures of the soluble GluN1 LBD in the apo-form (PDB 4KCC; [168]) or in complex with the agonist glycine (PDB ID 5I57; [158]) or competitive antagonist DCKA (PDB ID 4NF4; [166]). The upper D1 lobes are aligned to illustrate the similar conformations of antagonist-bound and apo-form structures. Agonist binding induces considerable closure of the LBD compared to the antagonist-bound and apo-form structures, and agonist-induced closure of the LBD is required for activation of NMDA receptors. Competitive antagonists bind the LBD without inducing domain closure, thereby preventing agonist binding and receptor activation.
2.1. Structure and function of GluN1 and GluN2 ligand binding domains
Recombinant proteins that comprise the NMDA receptor LBDs have been generated by combining S1 and S2 segments with a short artificial di-peptide linker [109,163,164] (Fig. 5c,d). These water-soluble LBD proteins retain ligand binding activities comparable to those in full-length NMDA receptors, indicating structural identity between the binding pockets of isolated LBDs and the corresponding intact receptor. LBD crystal structures from GluN1, GluN2, and GluN3 subunits have been solved both in complexes with agonists, antagonists, as well as allosteric modulators [165–167,157,168,108,109,169,170,163,164,155,171,158]. In addition to NMDA receptor subunits, numerous crystal structures for AMPA and kainate receptor subunits have been determined (reviewed in [172–174]). These studies have provided insight to the molecular determinants of full agonists and partial agonists, as well as the mechanism of action for competitive antagonists. Furthermore, the LBD structures have afforded new opportunities to consider the molecular determinants of subunit selectivity. The first structure of the GluN2A LBD was solved in complex with the GluN1 LBD, and provided the first view of the glutamate binding site of the NMDA receptor and the first structural information about a GluN1-GluN2 protein-protein interface within the NMDA receptor complex [164]. Glycine and glutamate bind in the cleft between the two clearly defined lobes (D1 and D2) of the GluN1 and GluN2A LBDs, respectively (Fig. 5c,d). Residues from loops within the upper lobe (D1) form most of the upper half of the binding pocket, and residues from the lower lobe (D2) form most of the lower half of the pocket. Despite being tucked away between the lobes, multiple water molecules are found in close vicinity of the agonists, some of which form hydrogen bonds with the ligand. The glycine binding pockets in GluN1 and GluN3 are smaller and more hydrophobic compared to the GluN2 glutamate binding pocket [164,163,165,168,108]. Residues lining the glutamate binding pocket are fully conserved among the GluN2 subunits, and consequently, agonists or competitive antagonists with strong selectivity among the different GluN1/2 receptor subtypes have not been identified. In order to develop subunit-selective ligands, it is presumably necessary to engage other regions of the NMDA receptor with structural variation, such as inter- or intra -subunit interfaces that are non-conserved among GluN2 subunits.
Interestingly, the heterodimer interface between GluN1 and GluN2 modulates receptor function. Three separate areas of contact between GluN1 and GluN2A are identified in the LBD heterodimer crystal structures (sites I, II, and III) [164]. The two outer regions (sites I and III) consist of hydrophobic residues from GluN1 and GluN2, and nonpolar interactions between these residues mediate heterodimerization of the soluble GluN1 and GluN2A LBDs [164]. Mutations of Y535 in GluN1 demonstrate that modification of site II, which is located at the center of the LBD heterodimer interface, results in increasing or decreasing rates of NMDA receptor deactivation [164,175]. Crystallographic studies recently showed that site II of the GluN1/2A LBD heterodimer harbor binding sites for both positive and negative allosteric modulators with strong selectivity for GluN2A-containing NMDA receptors [157,170,155,158]. These results suggest that the stability and dynamics of the LBD interface can control NMDA receptor function, similar to what has been found for AMPA and kainate receptors.
NMDA receptors are sensitive to the redox potential and reducing conditions can cause marked enhancement of NMDA receptor function [176–179]. This sensitivity is mainly mediated by a pair of cysteine residues within the GluN1 subunit (C744 and C798) that are conserved among all iGluR subunits [180,181]. These two residues form a disulfide bond (i.e. are oxidized) in the GluN1/2A LBD heterodimer structure, and relief (by reduction) of the conformational constraints imparted by this disulfide bond in GluN1, but not GluN2, enhances receptor function [180,182]. Several other disulfide bonds exist in LBD crystal structures of GluN1 and GluN2 subunits, but functional effects of their reduction or oxidation have not yet been described.
Multiple ligands have each been crystallized in complex with GluN1 and GluN2 LBDs, providing a structural basis for the effects of partial agonists, agonists, and competitive antagonists [165,169,167,163,164,166,168]. Binding of glycine and glutamate to their respective binding sites are associated with a rapid LBD rearrangement, involving closure of the angle between the two lobes D1 and D2, akin to a clamshell-like closure (Fig. 5c,d). This agonist-mediated LBD closure mediates formation of interactions between residues from the upper and lower lobes that further stabilizes the agonist-bound LBD structure [183,184]. The energy associated with agonist binding and LBD closure causes the receptor to undergo a series conformational changes that can lead to opening of the ion channel pore (i.e. channel gating). Thus, LBD closure as a result of agonist binding is the initial conformational change that triggers ion channel gating. Binding of competitive antagonists, such as the glycine site antagonist DCKA and the glutamate site antagonist D-AP5, stabilizes a more open cleft conformation of the bi-lobed LBD that is incapable of triggering ion channel gating and presumably resembles the LBD conformation in the absence of bound ligand (i.e. apo-state) [166,168,163,171]. The stabilization of the LBD in a closed conformation by agonists and an open conformation by competitive antagonists in NMDA receptors is similar to what has been found for soluble LBDs from AMPA and kainate receptor subunits (reviewed in [172–174]). However, despite this similarity, the domain closures in structures of GluN1 and GluN2 LBDs bound by partial agonists are not similar to those observed for AMPA and kainate receptor subunits. While most structures of AMPA receptor LBDs show partial domain closure that correlates with the efficacy of the partial agonist (reviewed in [172]), no such relationship is observed in GluN1 and GluN2 LBD structures. Multiple structures show that partial agonists, such as D-cycloserine, ACPC, and ACBC in GluN1 as well as NMDA and Pr-NHP5G in GluN2, bind with virtually identical degrees of domain closure in GluN1 and GluN2 LBDs compared to the complexes with full agonists glycine and glutamate, respectively [169,167,165]. However, while crystal structures capture only one low-energy conformation of the LBDs, recent single-molecule FRET and molecular dynamics studies have provided insight to the dynamic behavior of the NMDA receptor LBDs [168,185–188]. These studies suggest that the LBDs fluctuate between open and closed conformations in the absence of ligand (i.e. in the apo-state). The probability that the LBD adopts a fully closed conformation increases by binding of full agonist, whereas binding of partial agonists mainly enables the LBD to adopt conformations with intermediate domain closure. That is, a conformational selection mechanism can presumably account for partial agonism in NMDA receptors, since LBD conformations capable of triggering ion channel gating are selected with greater propensity by full agonists compared to partial agonists.
2.2. Ligand binding to GluN3 subunits
Glycine-activated diheteromeric NMDA receptors assembled from two GluN3 and two GluN1 subunits have been widely studied in heterologous expression systems (reviewed in [71,70,69,72]. However, structural and functional properties of triheteromeric GluN1/2/3 receptors are largely unresolved. For example, it is unknown how agonist or antagonist binding to the GluN3 LBD affects the function of GluN1/2/3 receptors, in terms of their activation, deactivation, and desensitization properties. However, LBD crystal structures have established that structural variation exists between the agonist binding sites of GluN1 and GluN3 subunits, even though they are both glycine-binding subunits [168,108,163,165]. Functional studies on recombinant GluN1/3 receptors suggest that these structural differences can be exploited for the development of GluN3-selective ligands targeting the agonist binding site [130,171].
As mentioned above, agonist binding to the GluN3 subunits is sufficient for activation of GluN1/3 receptors. This feature has enabled pharmacological studies on the GluN3 agonist binding site in isolation by abolishing ligand binding to GluN1 using mutagenesis [130]. This approach identified agonists and antagonists with moderate preferences between the agonist binding sites of GluN1 and GluN3 by comparing ligand activities between wild type GluN1/2 receptors and GluN1/3 receptors with mutations that render GluN1 incapable of ligand binding (hereafter denoted GluN1*/3) [130]. In addition, these studies brought to light interesting discrepancies between ligand binding to the isolated, soluble LBD proteins and full-length subunits in intact receptors. The isolated GluN1 LBD protein binds glycine with lower affinity (26 μM) compared to the isolated GluN3A LBD (0.04 μM) [109]. By contrast, the potency of glycine is higher for full-length GluN1 (e.g. in GluN1/2A the glycine EC50 is 1.2 μM) compared to for full-length GluN3A (e.g. GluN1*/3A EC50 is 57 μM) [130]. The competitive glycine site antagonist DCKA bind with higher affinity to the isolated GluN1 LBD (0.54 μM) compared to the isolated GluN3A LBD (647 μM), and the binding affinities are estimated to be 0.07 μM for GluN1 in GluN1/2A and 35 μM for GluN3A in GluN1*/3A [109,171]. Thus, in case of GluN1 and GluN3 subunits, there are marked differences in the pharmacology of isolated, soluble LBDs and full-length subunits in intact receptors. The underlying basis of these differences is poorly understood and raises caveats to evaluation of pharmacology in isolated, soluble LBDs that are excised from the intact NMDA receptor.
2.3. Structures of intact tetrameric NMDA receptors
Crystal structures of GluN1/2A LBD heterodimers and GluN1/2B ATD heterodimers have provided important insight to the overall receptor structure (reviewed in [189]). However, it is only recently that the first structures of intact GluN1/2B receptors firmly established the subunit arrangement and domain organization [67,66]. These structures show that subunits in GluN1/2B receptors are arranged in an alternating pattern (i.e. 1-2-1-2) (Fig. 6). In addition, the NMDA receptor structure shares many of the characteristics of AMPA and kainate receptors. First, the receptor seemingly adopts a layered structure composed of the TMD layer and two extracellular layers formed by LBDs and ATDs. Second, there is a symmetry mismatch between the TMDs and the extracellular portion of the receptor; the TMDs have a quasi-4-fold symmetry, whereas the extracellular portion has a 2-fold symmetry (Fig. 6). The TMDs are arranged symmetrically around the ion channel pore, but the extracellular portion of the receptor adopts a dimer-of-dimer arrangement (i.e. two GluN1/2 heterodimers). Third, there is a subunit crossover between the LBD layer and the ATD layer (Fig. 6). Furthermore, the NMDA receptor structure has several unique features compared to the structures of AMPA and kainate receptors [67,66]. First, there are extensive contacts between the two GluN1/2 LBD heterodimers in the intact NMDA receptor, which are not observed in AMPA receptor structures. Second, the NMDA receptor ATDs are arranged differently and have unique subunit interfaces compared to AMPA and kainate receptors. Third, the ATDs forms extensive contacts with the upper lobe of the LBD, giving the NMDA receptor a more compact appearance compared to AMPA and kainate receptors and creating a protein-protein interface at which modulators can bind [160]. Thus, the crystal structures of the intact NMDA receptor reveal multiple unique intra- and interdomain contacts that can provide explanations to allosteric interactions between subunits and allosteric modulation by small-molecule ligands.
Figure 6. Subunit crossover and symmetry mismatch in the NMDA receptor structure.

Side view of the GluN1/2B NMDA receptor structure (PDB ID 4PE5; [67]) and top views of the ATD, LBD, and TMD layers. The subunits in GluN1/2 receptors are arranged in an alternating pattern (i.e. 1-2-1-2) and there is a symmetry mismatch between the TMDs and the extracellular LBDs and ATDs of the receptor. The TMDs are arranged symmetrically around the ion channel pore with a quasi-4-fold symmetry, whereas the extracellular portion adopts a dimer-of-dimer arrangement (i.e. two GluN1/2 heterodimers) with a 2-fold symmetry. There is a subunit crossover between the LBD layer and the ATD layer in that the GluN1(α) ATD forms a local dimer with the GluN2B(α) ATD, whereas the GluN1(α) LBD forms a local dimer with the GluN2B(β) LBD.
Although the crystal structures of intact NMDA receptors provided major advances in our understanding of the structure-function relationship, they are limited by capturing only one low energy conformational state among many in the NMDA receptor activation cycle. In the crystal structures, agonists were bound to GluN1 and GluN2B subunits and GluN2B-selective negative allosteric modulators (NAMs) were bound at interface between GluN1 and GluN2B ATDs [67,66]. The structures therefore represented the agonist-bound inhibited state of the receptor with the ion channel in the closed conformation. However, recent breakthroughs in the cryo-EM methodology have enabled determination of structures at resolutions sufficient to assign the relative positioning of domains, thereby affording insight to agonist-bound active and inactive states as well as NMDA receptors in states inhibited by competitive antagonists or GluN2B-selective NAMs [162,161]. These cryo-EM structures provide the first dynamic pictures of conformational changes in intact NMDA receptors and provide insight the structural mechanism of ion channel gating (i.e. receptor activation) and allosteric modulation.
2.4. Channel gating in NMDA receptors
The portion of the receptor that controls whether the ion channel pore is open or closed with respect to ion permeation is often referred to as the activation gate. demonstrate that all three transmembrane helices (M1, M3, and M4) and the membrane-reentrant pore forming loop (M2) are involved the process that transitions the NMDA receptor pore to a permeant configuration, a process often referred to as gating [190–197]. Among these regions, the transmembrane helix M3 forms a helical bundle crossing that occludes the pore, and thus the M3 helices must move in order to allow permeation of ions through the channel pore [198–200] (Fig. 7). M3 contains a highly conserved nine amino acids motif (SYTANLAAF), and structural and functional studies have demonstrated that the activation gate is located within this motif [198]. Dilation of the helix bundle formed by M3 from each of the four NMDA receptor subunits is presumably the conformational change that opens the ion channel and allows ion permeation [198,201,202]. However, the structural mechanisms that control opening and closing of the NMDA receptor ion channel are not fully understood. Agonist binding induces closure of the bi-lobed NMDA receptor LBD, and this LBD closure initiates a sequence of conformational changes that result in multiple short-lived, intermediate conformations during the transition from the closed to the open state of the ion channel [203–205,60,206,207], akin to a wave of conformational changes connecting agonist binding to ion channel gating. However, there is as yet poor understanding of which elements and conformations represent rate limiting steps en route to gating. Moreover, the lifetimes of some of these intermediate conformations are too brief (i.e. they are unstable) to be observed in crystal structures or functional experiments, which has confounded attempts to link the sequence of protein conformational changes to kinetically distinct functional pre-gating steps. However, the field is poised for major advances that should occur as new, more detailed structural information emerge and efforts to conceptualize functional models take stock of structural principles. Nonetheless, the presence of intermediate states can be detected using Hidden Markov modeling of single-channel recordings, and the lifetimes of these states differ significantly among NMDA receptor subtypes in a GluN2 subunit-dependent manner [60,208,83,209,207].
Figure 7. Structural determinants in the NMDA receptor ion channel pore.
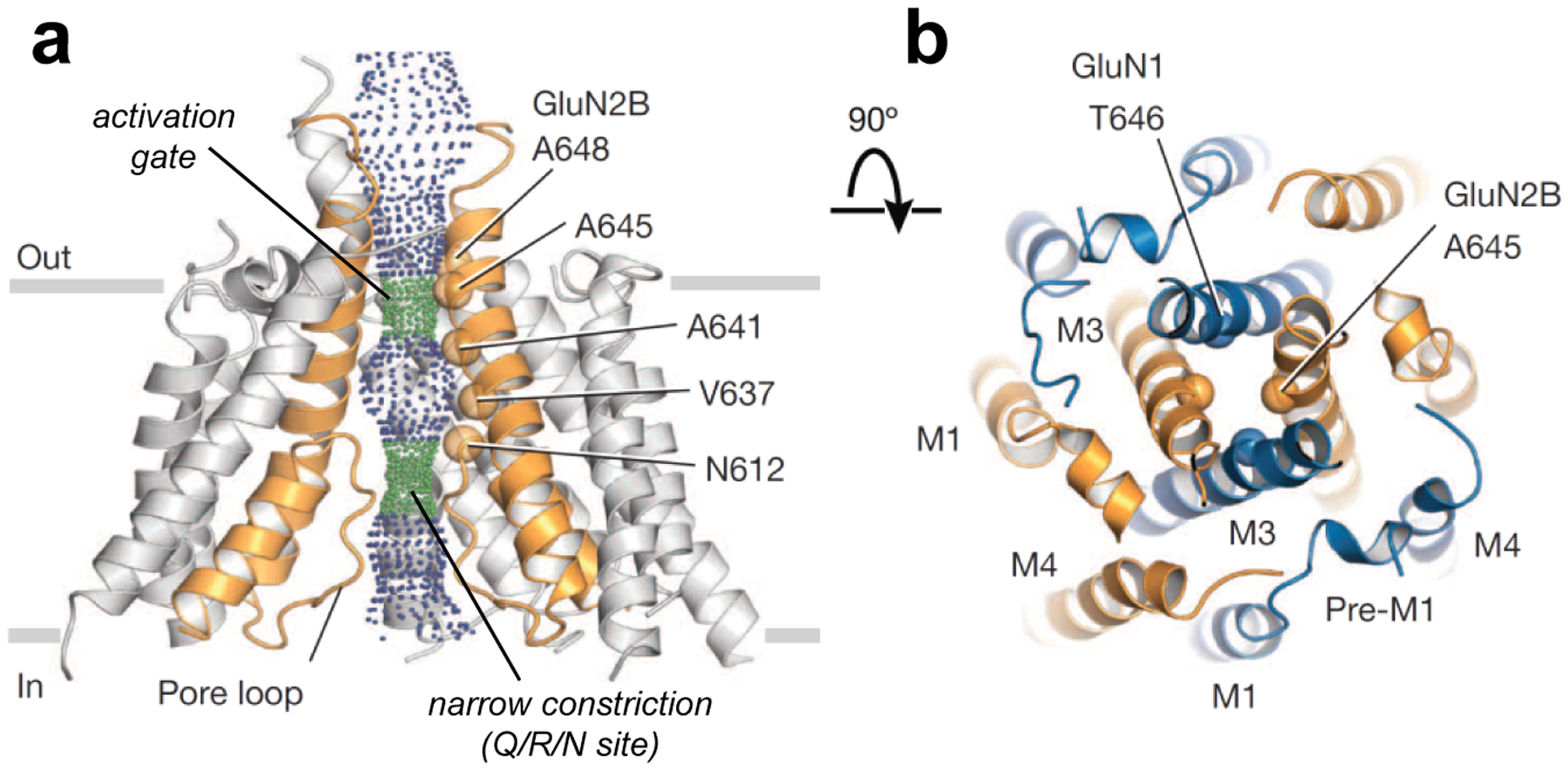
a) View parallel to the membrane of the TMDs in the GluN1/2B NMDA receptor structure (PDB ID 4TLM; [66]). The solvent accessible surface is carved along the pore axis using the computer program HOLE and shows the M3 bundle crossing near the extracellular side of the membrane, which presumably forms the activation gate, and the narrow constriction in the pore (Q/R/N site). Green dots indicate a pore radius of 1.15–2.3 Å and blue dots indicate a pore radius greater than 2.3 Å. b) View of the TMDs from the extracellular side of the membrane along the pore axis. GluN1 and GluN2B subunits are blue and orange, respectively. The α-carbon of residues T646 and A645, which appear to define the activation gate, are highlighted as spheres. Adapted with permission from Lee et al. [66].
Agonist binding steps and the sequence of protein conformational changes that lead to gating can be described as reaction schemes representing agonist binding as well as the transition between different conformational states of the receptor. The first widely accepted kinetic model for the NMDA receptor gating cycle was proposed by Lester and Jahr [61]. This model was designed solely to account for the macroscopic current response waveform, and consisted of two independent, but identical glutamate binding sites, one open state, one closed state, and one desensitized state. This simple formulation adequately described key features of the macroscopic time course for NMDA responses, but was not designed to capture the complexity observed in single channel recordings. The utility of the model was further limited given the lack of glycine binding steps, which are required for receptor activation. Benveniste et al. [210] developed models that took into account glutamate and glycine binding steps, as well as allosteric interaction between the glutamate and glycine binding domains. These models captured additional features of NMDA receptor pharmacology and response time course, including an apparent glycine-dependent desensitization (see below).
Newer and more complex models, which incorporate both glutamate and glycine binding steps, have been proposed that provide a better description of single channel data by the incorporation of multiple steps between binding and gating [204,206,205,60,203]. In some studies, single-channel and macroscopic responses to full and partial agonists suggest that agonist binding to either GluN1 or GluN2 controls different steps in the receptor gating scheme [206,205,60,203]. These models can also account for some of the actions of allosteric modulators by explicitly representing the modulator bound and unbound receptor as independent states [211–213]. Additional models that exclusively enable modulators to bind to the open state have also been described for channel blockers and other use-dependent modulators [214–219].
The modular nature of the glutamate receptor structure, coupled with the established ability of AMPA receptors subunits to operate independently [220–222], raises the possibility that subunit-independent conformational changes may progress within the sequence of steps leading to channel opening. Some studies suggest that subunit-specific structural changes are required in all four subunits for channel opening, and that these conformational changes occur in any order to arrive at an intermediate state, which can then transition to the open state of the ion channel [206,205,60,223,203]. However, other models can account for single-channel and macroscopic properties by incorporating just a few sequential gating steps in a linear reaction scheme with an implicit order for fast and slow gating steps [207,204]. Importantly, all kinetic models for NMDA receptor gating, which faithfully represent both single channel data and macroscopic responses, require multiple pre-gating steps as well as multiple open states. Thus, opening of the NMDA receptor ion channel is not directly coupled to agonist-induced closure of the LBD, but rather the receptor proceed through a sequence of protein conformational changes that connects agonist binding to ion channel gating.
2.5. Structural determinants of ion permeation and channel block
In the open conformation, the NMDA receptor ion channel pore can be divided into the extracellular vestibule and the intracellular vestibule, separated by a narrow constriction. The ion permeation pathway is formed by pore-lining residues that determine ion selectivity and channel conductance. The narrow restriction, also referred to as the selectivity filter, resides at the apex of the membrane re-entrant loop M2 (i.e. the Q/R/N site), approximately halfway across the transmembrane electric field, and is a key determinant of single-channel conductance, Ca2+-permeability, and channel block by Mg2+ and organic cations (reviewed in [224,1]) (Fig. 7). In both GluN1 and GluN2 subunits, the residue at the position of the Q/R/N site is an asparagine (N), whereas this residue is glycine (G) in GluN3 subunits. Interestingly, the contribution of residues at the apex of M2 to ion permeation is asymmetric between GluN1 and GluN2 subunits [225–228]. The narrow constriction is mainly formed by the Q/R/N site asparagine in GluN1, whereas in GluN2, it is formed by the asparagine residue adjacent to the Q/R/N site (i.e. Q/R/N +1 site). Thus, the narrow constriction is formed by non-homologous residues in GluN1 and GluN2 subunits. For example, mutations at the Q/R/N site in GluN2 dramatically reduce Mg2+-block and have weak effects on Ca2+-permeability, but the same mutations at the Q/R/N site in GluN1 have the opposite effects [227,228]. In addition, substitutions of the asparagine residue at the Q/R/N +1 site in GluN2 strongly reduce Mg2+-block [228]. Thus, functional data suggest a structural asymmetry, in which the apexes of M2 in GluN1 and GluN2 are slightly staggered [225].
In terms of physiologically relevant ions, the NMDA receptor ion channel is permeable to Ca2+, Na+, and K+ ions. GluN1/2 receptors have similar permeability to K+ and Na+ ions (PK/PNa = 1.14), but are ~2–5 times more permeable to Ca2+ relative to monovalent ions (PCa/PX = 1.8–4.5), depending on the GluN2 subunit [229–233]. Interestingly, despite being highly permeable to Ca2+, NMDA receptors also exhibit voltage-dependent block by external Ca2+, which is readily observed in single-channel recordings as a reduction in channel conductance [234–236,208]. The concurrent high Ca2+-permeability and Ca2+-block of NMDA receptors are not necessarily incompatible properties, but could be expected if multiple Ca2+ binding sites exist within the ion channel pore [234,229]. One Ca2+ binding site is presumably located at the Q/R/N site in the pore, and a cluster of charged GluN1 residues, the DRPEER motif, have been suggested to form another, more external Ca2+ binding site [237,67]. The external Ca2+ binding site is located C-terminal to the transmembrane helix M3 in GluN1 at the external entrance to the ion channel. Removal of the net negative charge in DRPEER using mutagenesis (i.e. ARPAAR) reduces the fractional Ca2+ currents in NMDA receptors, consistent with an important role of this motif in mediating high Ca2+-permeability [237].
It has been suggested that diheteromeric GluN1/3 receptors form a unique narrow constriction in the extracellular vestibule of the ion channel pore [238]. This narrow constriction, which is presumably not found in GluN1/2 receptors, is proposed to be a main structural determinant for the dramatically reduced Ca2+-permeability and minimal Mg2+-block of GluN1/3 receptors [238]. Co-expression of GluN3 subunits with GluN1 and GluN2 subunits also produce NMDA receptors with reduced single-channel conductance, decreased Ca2+-permeability, and diminished Mg2+-block (reviewed in [71,70,68,72,69]). However, it is unknown whether the GluN3-specific narrow constriction is formed in the extracellular vestibule or these NMDA receptors, which are presumably triheteromeric GluN1/2/3. Furthermore, the extent and mechanisms by which GluN3 subunits influence permeation properties of triheteromeric GluN1/2/3 receptors have not been quantitatively evaluated and remains poorly understood.
NMDA receptor ion channels are blocked by divalent cations Zn2+ and Mg2+ in a membrane potential-dependent manner (i.e. voltage-dependent) (Fig. 1d). GluN1 and GluN2A mutations in the re-entrant pore loop M2 that reduce channel block by extracellular Mg2+ have similar effects on Zn2+-block, suggesting shared molecular determinants [100,239]. While Mg2+-block of NMDA receptors is centrally implicated in neuronal function, the channel block by Zn2+ is low affinity, rapidly reversing, and has far less physiological implications [240,241]. GluN1/2A and GluN1/2B are more strongly blocked by external Mg2+ compared to GluN1/2C and GluN1/2D [63,99,242,98,243]. At a holding potential of −100 mV, the IC50 values for block by Mg2+ are 2.4 μM, 2.1 μM, 14.2 μM, and 10.2 μM for GluN1/2A, GluN1/2B, GluN1/2C, and GluN1/2D, respectively [99]. The GluN2 subunit-specific effects on Mg2+-block are likely influenced by multiple structural elements, but a main determinant is a single residue, which is a serine in GluN2A/B and a leucine in GluN2C/D (i.e. the S/L-site) [243]. The S/L-site does not face the ion channel pore, but is located on the internal side of the M3 transmembrane helix, and mutagenesis data suggest that this residue interacts with tryptophan residues in the GluN1 membrane re-entrant loop M2 [243]. In addition to channel block by Mg2+, the subunit-subunit interaction between GluN1 and the GluN2 S/L site is also a main determinant of GluN2 subunit-specific variation in Ca2+-permeability and channel conductance [243]. The structural mechanism by which the GluN2 subunits control block by external Mg2+ is unknown, but it is possible that the GluN2 S/L site and other structural elements influence the binding sites for permeant ions in the channel pore, since these binding sites are different between GluN2 subunits and have been shown to profoundly modulate Mg2+-block [244–248].
Numerous organic cations with diverse chemical structures are capable of binding and blocking the NMDA receptor ion channel pore in voltage-dependent manner [219,249,250]. Most, if not all, of these compounds are positively charged at physiological pH, and almost exclusively block activated NMDA receptors with open channels. This mechanism of channel block has been termed “use-dependent” or “uncompetitive”. The open channel blockers are further classified into three categories based on their interaction with the channel: (1) “Sequential” or “foot-in-the-door” blockers, such as aminoacridine derivatives, bind to the channel only when it is open and prevent channel closure [251–254]. (2) Partial trapping blockers, such as memantine and amantadine, impede channel closure without completely preventing it [217,255,218,256–258]. (3) Trapping blockers, such as MK-801, phencyclidine (PCP) and ketamine, are trapped inside the pore as the channel returns to the closed state and agonists unbind [259]. Some channel blockers can also interact with the gate to facilitate channel closure [218,255].
Open channel blockers are generally considered non-selective among NMDA receptor subtypes [216]. However, some channel blockers, at least ketamine and memantine, may display some selectivity under physiological conditions, since 5- to 10-fold selectivity for GluN2C/D-containing receptors over GluN2A/B-containing receptors have been reported in the presence of 1 mM extracellular Mg2+ [260]. This observation may be clinically significant, since NMDA receptor channel blockers have been shown to have neuroprotective effects in animal models of CNS disorders that involve excessive stimulation of NMDA receptors, such as traumatic brain injury, epilepsy, and stroke. Unfortunately, human clinical trials have been disappointing due to patient heterogeneity, dose-limiting side effects, and a narrow temporal window for intervention, which may have confounded interpretation. High-affinity NMDA receptor channel blockers, such as phencyclidine (PCP) and ketamine, are dissociative anesthetics, but their clinical use is limited by strong psychomimetic side effects (see below). By contrast, low-affinity channel blockers, which shows fast blocking/unblocking kinetics [261], appear to have a greater therapeutic index with respect to psychomimetic effects, which may be due to less channel block under conditions of normal synaptic transmission [262]. One such low-affinity blocker, memantine, has been approved for clinical use in the treatment of moderate to severe Alzheimer’s disease. However, the mechanism by which NMDA receptor channel block by memantine may contribute to a symptomatic benefit for advanced Alzheimer’s disease patients is not well understood.
2.6. Modulation by the amino-terminal domain
Similar to the LBD, the ATD also adopts a bilobed kidney-shaped structure with upper and lower lobes termed R1 and R2, respectively [263,264]. Crystal and cryo-EM structures of intact iGluRs revealed a unique dimer-of-dimer arrangement of the NMDA receptor ATDs compared to those in AMPA and kainate receptors [67,161,265,266,66,162]. This arrangement, which is also present in crystal structures of heterodimers formed by soluble GluN1 and GluN2B ATDs, is characterized by a subunit interface formed by extensive contacts between the upper R2 lobes from GluN1 and GluN2, whereas the lower R1 lobes, which connect to the LBDs, are almost completely separated. The ATDs are resting immediately above the LBDs and strong interactions are formed between the LBD and ATD layers. By contrast, AMPA and kainate receptor ATDs associate through interactions between both upper R1 and lower R2 lobes in a back-to-back fashion and there is very little contact between the LBD and ATD layers. Numerous studies have revealed important roles of the NMDA receptor ATD as a modulatory domain that affects function and harbors modulatory binding sites for ions and small-molecule ligands (reviewed in [267,56,55,189]). Modulatory roles or ligand binding sites have not been identified for AMPA and kainate receptor ATDs, even though molecular dynamics simulations predict they should be capable of similar conformational changes as NMDA receptor ATDs [268,269]. Consistent with these differences, mutant subunits with deletion of the ATD have dramatic impact on the functional properties of NMDA receptors [94], whereas little to no changes are observed in AMPA and kainate receptors [270].
Many of the GluN2-specific differences between NMDA receptor subtypes are in large part due to variation in the weakly conserved GluN2 ATDs [271,94]. Studies with NMDA receptors containing chimeric GluN2 subunits have revealed that swapping of the ATD between GluN2A and GluN2D, which have widely different properties, shifts the open probability, deactivation time course, agonist potency in the direction of the subunit contributing the ATD [94,271]. Little is known about how the ATD controls NMDA receptor function, but the mechanism presumably involves a combination of intra- and inter-subunit allosteric interactions between the ATDs and LBDs that can affect the dynamic behavior and stability of the GluN1/GluN2 LBD heterodimer [272,273]. Functional and structural studies suggest that the ATDs adopt distinct conformations, depending on the GluN2 subunit, which may underlie some GluN2-specific functional and pharmacological NMDA receptor properties [274,151].
Extracellular Zn2+ is an endogenous modulator that inhibits NMDA receptors in a voltage-independent manner through a binding site in the GluN2A and GluN2B ATDs [275–278,80,279,280,263]. The affinity of Zn2+ to the GluN2A ATD is in the low nanomolar range, whereas the affinity to the GluN2B ATD is in the low micromolar range. Crystal structures and functional data have identified the binding site for Zn2+, which is located at the mouth of the cleft formed by the two lobes R1 and R2 [263]. Several experimental observations support a mechanism of Zn2+-modulation that involves opening and closing motions of the angle between the two lobes R1 and R2 as well as twisting motions around the hinge region of the ATD clamshell [280,263,273]. Binding of Zn2+ stabilizes a conformation of the GluN2 ATD, which presumably is accompanied by structural changes at the GluN1/2 LBD subunit interface [273].
Crystal structures of both isolated ATDs and intact NMDA receptors established that GluN2B-selective NAMs, such as ifenprodil and Ro 25–6981, bind the subunit interface between GluN1 and GluN2B ATDs [67,281,264,66]. Interestingly, only one residue in the ifenprodil binding pocket is different between GluN2A and GluN2B, but sensitivity to ifenprodil is not introduced by converting this or other residues in GluN2A to that in GluN2B [264,282]. This observation further supports that the ATD arrangement in GluN2A- and GluN2B-containing receptors is likely fundamentally different and highlights that the mechanism of subunit-selectivity for ifenprodil and its analogs remains unresolved. Recent cryo-EM structures of intact NMDA receptors, supported by functional studies and computational analyses, suggest that the mechanism of ifenprodil inhibition involves closure of the GluN2B ATD clamshell and changes in the arrangement of the GluN1/2B ATD heterodimers [161,282] (see below).
Polyamines, such as spermine and spermidine, enhance NMDA receptor function in a GluN2B-selective manner through a binding site, suggested to be located in the vicinity of clusters of negatively charged residues in the lower R2 lobes of GluN1 and GluN2B ATDs [283]. Although the precise location of this binding site for positive allosteric modulation remains to be identified, it has been shown using FRET that spermine binding opens the GluN2B ATD clamshell [284]. Furthermore, a model has been proposed where the positively charged spermine shields the negatively charged residues in GluN1 and GluN2B ATDs, thereby potentially eliminating electrostatic repulsion between the two lower R2 lobes [283]. Consistent with this model, other cations can also potentiate GluN2B-containing NMDA receptors in manner similar to spermine; for example, millimolar concentrations of extracellular Mg2+ enhance GluN1/2B responses under conditions with no channel block [285].
Functional and structural investigations appear to converge on a structural model for NMDA receptor modulation by Zn2+, ifenprodil, and spermine, in which modulator binding regulates receptor function through GluN2 ATD clamshell opening and closing motions and rearrangement of the ATD layer. It is not fully understood how these conformational changes affect other structural elements of the receptor, but several studies propose that downstream changes occur at the subunit interface of GluN1/2 LBDs. Interestingly, the activity of all three allosteric modulators (Zn2+, ifenprodil, and spermine) is reduced for NMDA receptors containing GluN1 with exon 5 (e.g. the GluN1–1b splice variant) [81,85]. Recent structures of intact NMDA receptors show that the 21 amino acids, which are encoded by exon 5, are located just above the GluN1/2 LBD heterodimer interface between the ATD and LBD layers, well-positioned to influence allosteric coupling between GluN2 ATD clamshell motions and GluN1/2 LBDs [67,66]. In addition, mutational analyses identified GluN2C residues from both the ATD and LBD that influenced the activity of PYD-106, which is a recently developed GluN2C-selective positive allosteric modulator (PAM), and molecular modeling proposed a binding site located in a pocket residing at the intra-subunit ATD/LBD interface of GluN2C [160]. Thus, the ATD is the major structural determinant of GluN2-specific variation in functional and pharmacological properties of NMDA receptors. The mechanism of allosteric modulation by the NMDA receptor ATD remains an important focus in structure-function studies, and drug discovery efforts are poised to identify novel ATD ligands with therapeutic potential. In particular, it is unknown how structure and ATD arrangement differs among the various NMDA receptor subtypes.
2.7. Control of assembly by the amino-terminal domain
The mechanism and progression of subunit assembly of two GluN1 and two GluN2 subunits in an alternating 1-2-1-2 arrangement around the central ion channel pore is not well-understood. Three main models of the steps required for NMDA receptor assembly have been proposed: 1) It has been suggested that GluN1-GluN1 and GluN2-GluN2 homodimers initially form and then associate to form the tetrameric receptor [286–289]. 2) Alternatively, two initial GluN1-GluN2 heterodimer are formed that subsequently associate to generate the tetrameric arrangement [290]. 3) Lastly, it has been suggested that a GluN1-GluN1 homodimer is initially formed to which GluN2 subunits are sequentially added to form the tetrameric NMDA receptor [291,292]. While there is some supporting experimental data for each model, this data is as yet insufficient to make a clear distinction between these models. Regardless of sequence, it appears that the NMDA receptor ATD is the main determinant of the initial subunit dimer formation [288,286,292]. This feature of the ATD in NMDA receptors appears to be shared in AMPA and kainate receptors, where the role of the ATD in subunit assembly has been extensively studied [293,294]. Interestingly, the NMDA receptor ATD may also influence receptor trafficking, since the GluN2A ATD has been shown to contain a retention signal that prevents exit from the endoplasmic reticulum unless it is masked by assembly with the GluN1 ATD [295].
3. Mechanisms of NMDA receptor regulation
Many functional and membrane trafficking properties of NMDA receptors are regulated by extracellular ions, phosphorylation, and intracellular binding proteins. Here, we will describe regulation of NMDA receptor function by extracellular ions and molecules, and highlight key phosphorylation sites and their implications for protein-protein interactions important for neuronal functions.
3.1. Desensitization of NMDA receptors
The definition of desensitization is a decrease in the receptor response in the continued presence of a stimulus. NMDA receptors exhibit several different types of desensitization with distinct mechanisms, including glycine-dependent desensitization, Zn2+-dependent desensitization, Ca2+-dependent desensitization, and glycine/Ca2+/Zn2+-independent desensitization.
Glycine-dependent NMDA receptor desensitization can be observed in the presence of subsaturating glycine concentrations, and is abolished in a saturating concentration of extracellular glycine [296]. This type of desensitization occurs due to a negative allosteric interaction between the GluN1 and GluN2 subunits such that the binding of glutamate decreases the affinity for glycine [297,210]. Thus, when glutamate binds GluN2, the affinity for the glycine binding site in GluN1 becomes lower, and in the absence of high concentrations of glycine, the current diminishes and relaxes to a new equilibrium as glycine unbinds from the receptor. The time course for the desensitization therefore reflects glycine unbinding, which is within the range of the synaptic NMDA receptor time course, suggesting glycine-dependent desensitization could impact synaptic signaling. Recent crystal and cryo-EM structures of intact NMDA receptors offer plausible structural models for the negative allosteric coupling between glutamate and glycine binding sites [66,67,161,162], but the structural mechanism of glycine-dependent desensitization is still not fully understood. Extracellular Zn2+ mediates a rapid component of desensitization that occurs by a mechanism similar to glycine-dependent desensitization [298]. It has been proposed that a positive allosteric interaction exists between the glutamate binding site in the GluN2 LBD and the Zn2+ binding site in the GluN2A ATD, which enables binding of glutamate to enhance Zn2+ binding [299,300]. Thus, glutamate binding will, in the presence of subsaturating concentrations of Zn2+, cause a relaxation of the receptor response to a new equilibrium as more Zn2+ ions bind and inhibit the receptor in a concentration-dependent fashion. The time course of Zn2+-dependent desensitization therefore reflects the time course for Zn2+ binding following a glutamate-dependent shift into a Zn2+ binding site with higher affinity.
NMDA receptors also undergo Ca2+-dependent inhibition, which is often referred to as Ca2+-dependent desensitization or inactivation, since this type of desensitization requires intracellular Ca2+ and develops slowly over seconds [301–304]. The magnitude of Ca2+-dependent desensitization varies among GluN2 subunits, and is more prominent for GluN2A-containing than for GluN2D-containing receptors and appears to be absent for GluN2B- and GluN2C-containing NMDA receptors [305,306]. It has been hypothesized that a local increase in the intracellular Ca2+ concentration occurs in the immediate vicinity of the NMDA receptor, which results in inhibition by stimulating uncoupling of the receptor from filamentous actin in a manner sensitive to second messenger systems [307]. Furthermore, calmodulin binding to the GluN1 CTD have been suggested to play an important role in this form of desensitization. Thus, Ca2+-dependent desensitization is abolished in NMDA receptors containing GluN1 splice variants in which calmodulin binding sites are absent [308,309], and mutations within calmodulin binding sites in the GluN1 CTD similarly disrupt Ca2+-dependent desensitization [310,311].
Most ligand-gated channels undergo a form of desensitization that reflects a conformational change to a relatively stable and sometimes long-lived agonist-bound receptor state with a closed ion channel. NMDA receptors can also desensitize in the continued presence of agonist by a mechanism that is independent of glycine, Zn2+, and Ca2+ (i.e. the types of desensitization discussed above). This desensitization develops with time, is sensitive to intracellular dialysis, and is thus more prominent in excised outside-out membrane patches compared to whole-cell patches [312,313]. However, desensitization can also be influenced by mutations in the conserved SYTANLAAF motif, the preM1 region, and other positions deep within the ion channel pore, the LBD, and the TMD-LBD interface [195,314,315], suggesting that this desensitization reflects a conformational change in the agonist-bound receptor.
3.2. Regulation of NMDA receptor function by protons
Extracellular protons potently (IC50 = ~50 nM) and completely inhibit NMDA receptor function [316–319]. Thus, neuronal NMDA receptors are tonically inhibited by protons at physiological pH 7.4, which corresponds to approximately the proton IC50. NMDA receptors can therefore respond to small changes in extracellular pH under physiological conditions. Moreover, extracellular pH is dynamic and changes with neuronal activity, given that synaptic vesicles are acidic and various transporters can generate proton gradients [320]. Furthermore, pathological conditions, such as seizure or ischemia, reduce extracellular pH (i.e. increase proton concentration) to levels that are sufficient to strongly inhibit NMDA receptor function [320].
As with many other NMDA receptor properties, the inhibition by extracellular protons depends on the GluN2 subunit [81]. GluN2A-, GluN2B-, and GluN2D-containing NMDA receptors are inhibited with proton IC50 values near physiological pH (7.2 –7.4), whereas GluN2C- containing receptors are much less sensitive to protons (IC50 value at pH 6.2) [81,321]. In addition, proton inhibition is reduced for NMDA receptors with the GluN1–1b isoform, which has an additional 21 amino acids inserted in the ATD [81]. Proton inhibition is voltage-independent and is also independent of actions at the agonist binding site. The location of the structural determinant for proton inhibition (i.e. the proton sensor) is unknown and it is possible that multiple sites within the NMDA receptor work in concert to mediate the actions of protons. However, residues within the ion channel gate, near the linkers that couple the TMD to the LBD, and in the GluN1-GluN2 LBD dimer interface have been shown by mutagenesis to influence pH sensitivity [321,273], suggesting that NMDA receptor gating elements are tightly coupled to the proton sensor. This idea is supported by evidence that channel blockers are sensitive to the protonation state of the receptor while entering the pore [216].
Several studies suggest that actions of ATD modulators may reflect a subtle change in the pKa of the proton sensor that either enhances or reduces tonic proton inhibition at physiological pH (see below). In this regard, both extracellular Zn2+ and ifenprodil appear to enhance proton sensitivity, which will increase tonic inhibition at physiological pH, whereas binding of extracellular polyamines, such as spermine, reduce proton sensitivity, which results in potentiation. For example, spermine potentiation of GluN1/2B strongly correlates with the degree of proton inhibition and is most robust at pH values that produce strong tonic inhibition (i.e. pH < 8). This is consistent with a mechanism in which polyamines enhance receptor function by relieving proton inhibition [81,322,323]. Similar functional evidence support a mechanism for inhibition by extracellular Zn2+ and ifenprodil in which receptor function is reduced by enhancing proton inhibition [212,85,86,324,80,275].
3.3. Regulation of NMDA receptor function by extracellular Zn2+
GluN2A-containing NMDA receptors are highly sensitive to extracellular Zn2+, and numerous studies have reported variable IC50 values in the low nanomolar range (e.g. [298,278,80,100]). A key provision in these studies was the need for a buffer system to accurately control Zn2+ concentration and unambiguously determine the IC50 value for Zn2+ inhibition, since hundreds of nanomolar Zn2+ contaminates physiological saline solutions under most experimental conditions [80,100]. Thus, in order to remove effects of extracellular Zn2+ in functional experiments, many studies include Zn2+-chelators, such as tricine or EDTA, in the extracellular recording solution. The high affinity of these chelators for Zn2+ means that 10’s of micromolar of chelator will bind virtually all of the nanomolar contaminating Zn2+ ions, but minimally alter millimolar concentrations of Ca2+ or Mg2+ (e.g. see [325]). The concentration-inhibition relationship for Zn2+ at GluN1/2A receptors is biphasic, since Zn2+ binding to the high affinity site in the ATD causes incomplete inhibition, whereas higher micromolar concentrations of Zn2+ result in voltage-dependent channel block [278]. The incomplete inhibition by high affinity Zn2+ binding is related to enhancement of proton sensitivity, since Zn2+ binding causes a leftward shift of the proton inhibition curve such that inhibition is more complete at acidic pH compared to at alkaline pH [212,80,275,277]. For example, maximal inhibition of GluN1/2A by extracllular Zn2+ is ~62% at physiological pH 7.3 compared to ~76% at pH 6.8 [277]. Interestingly, high affinity Zn2+ inhibition is maintained in triheteromeric GluN1/2A/2B receptors, albeit with less maximal inhibition and a somewhat different relationship to the extracellular pH [151,150,153].
3.4. NMDA receptor phosphorylation and membrane trafficking
The intracellular CTDs of NMDA receptor subunits contain numerous sites for posttranslational modifications (e.g. phosphorylation, nitrosylation, and palmitoylation) and for protein-protein interactions, which have important implications for receptor localization, trafficking, and signaling (reviewed in [1,326,327]). The intracellular CTDs display very little conservation among subunits, and subcellular localization and trafficking of each subunit therefore appear to be uniquely regulated. Furthermore, the intracellular CTD of GluN1 is modified by alternative RNA splicing, which removes or inserts regulatory sites with important effects on receptor trafficking.
Experimental evidence suggest that the precise subcellular localization of NMDA receptor subtypes is determined by protein-protein interactions between the extreme C-terminus of GluN2 subunits and PDZ domain-containing proteins, including the MAGUK proteins PSD-93, PSD-95, SAP97, and SAP102. Members in the MAGUK protein family have widely different subcellular localization and exhibit GluN2-specific variation in their preferential association with NMDA receptor subtypes. For example, the scaffolding protein PSD-95 is primarily expressed at the postsynaptic density (PSD), whereas SAP102 is more evenly distributed between synaptic and extrasynaptic sites. Furthermore, PSD-95 and SAP102 have been proposed to preferentially bind GluN2A and GluN2B subunits, respectively [328]. The differential interaction of GluN2A and GluN2B subunits with MAGUKs is not firmly established and has been questioned in several studies (e.g. [131]), but has been suggested to underlie differences in the subcellular localization of these subunits. For example, GluN2B-containing receptors appear to move more freely in and out of synaptic sites compared to GluN2A-containing receptors [329]. It has therefore been suggested that GluN2B can be found at both extrasynaptic and synaptic sites, whereas GluN2A is enriched at synaptic sites [329–331]. In addition to MAGUK proteins, numerous other binding partners have been implicated in the subcellular localization and membrane trafficking properties of NMDA receptors (reviewed in [1]).
The cytoplasmic CTDs of NMDA receptor subunits are differentially regulated by posttranslational modifications including phosphorylation, palmitoylation, and nitrosylation (reviewed in [1]). These modifications can affect the ability to bind intracellular proteins involved in membrane trafficking, and can therefore mediate changes in subcellular localization and surface expression. An example of the type of regulation, which has important implications on synaptic plasticity, is CaMKII phosphorylation of GluN2B subunits on Ser1303, which is located in the CaMKII binding site [332,333]. Transient NMDA receptor-mediated influx of Ca2+ induces autophosphorylation of CaMKII, which enhance its enzymatic activity and results in persistent activation of CaMKII long after cytoplasmic Ca2+ levels return to baseline [334,333]. Once activated, CaMKII rapidly and reversibly undergoes a translocation to the spine, where it binds the CTD of the GluN2B subunit [335–338]. Multiple lines of evidence show that disruption of autophosphorylation and activation of CaMKII, as well as its binding to the GluN2B subunit significantly impairs NMDA receptor-dependent LTP and affects memory in mice, consistent with a key role of CaMKII as a key mediator of some types of synaptic plasticity (reviewed in [339]). The mechanism by which the interaction between the NMDA receptor and CaMKII contributes to synaptic plasticity is largely unresolved and therefore continues to be a primary focus in studies that aim at advancing our understanding of NMDA receptor-dependent synaptic plasticity. Numerous other kinases (e.g. PKA, PKB, and PKC), many protein tyrosine kinases (e.g. Fyn and Src), and phosphatases (e.g. STEP) have been implicated in the regulation of NMDA receptors, and the consequences of modification by these proteins on neuronal function continue to be extensively studied (reviewed in [340]). In addition to CaMKII, other calcium-sensing proteins can interact with NMDA receptor subtypes to mediate downstream signaling and regulate synaptic plasticity. RAS-GRF1 and RAS-GRF2 are two such calcium sensors that selectively bind the GluN2B CTD and thereby initiate ERK- and CREB-mediated signaling pathways in response to NMDA receptor-mediated Ca2+-influx [341–343]. Thus, the implications of NMDA receptor phosphorylation and membrane trafficking on neuronal function are incredibly complex and highly dependent on NMDA receptor subunit composition.
There is a growing body of evidence for metabotropic signaling through the NMDA receptor (i.e. not mediated by ion flux), resulting from direct changes in the interaction of the receptor with other signaling complexes [344,345]. Conformational changes induced by agonist binding are required to gate the NMDA receptor ion channel, but emerging evidence suggest that the conformational changes induced by these various ligands may also have effects that are independent of ion channel flux. For example, glycine binding, but not glutamate binding, have been shown to prime NMDA receptors for internalization [346]. In this case, glycine binding to GluN1 promotes association of the NMDA receptor with clathrin-mediated endocytic machinery that is independent of glutamate binding and receptor activation. Receptor endocytosis is then triggered upon binding of both glutamate and glycine and receptor activation. More recently, evidence has emerged that metabotropic NMDA receptor signaling may play a role in synaptic depression [346]. In this case, the metabotropic effects appear dependent on glutamate binding to GluN2 [347], which results in rearrangement of the associations of protein phosphatase 1 (PP1) and CaMKII with the C-terminal tails of the NMDA receptor to modulate kinase activity [348]. It has been proposed that the Aβ peptide, a putative pathogen in Alzheimer’s disease, may cause synaptic depression and dysfunction via this mechanism [344]. At the physiological level, it may be speculated that these subunit specific metabotropic signaling mechanisms interact with ionotropic signaling mechanisms. Thus, metabotropic signaling provides another layer of signal integration by these important NMDA receptor complexes.
4. Pharmacological manipulation of NMDA receptor subtypes
Small-molecule modulators with selectivity for the different NMDA receptor subtypes (i.e. the GluN2 subunits) are powerful pharmacological tools to dissect the roles for NMDA receptors in neurophysiology, behavior, development, and diseases. In this regard, decades of studies aimed at developing glutamate-site agonists, competitive antagonists, and channel blockers have not identified such pharmacological tools with sufficient GluN2 subunit-selectivity; in part due to the fact that these sites are fully conserved among GluN2 subunits. However, extensive pharmacology has been developed around ifenprodil that was shown to be a GluN2B-selective NAM in 1993 [349], and until recently, ifenprodil and analogs were the only available and widely used pharmacological tool compounds with strong GluN2 subunit-selectivity. However, since approximately 2010, there has been an acceleration in the discovery of novel NMDA receptor allosteric modulators with GluN2 subunit-selectivity and multiple new binding sites on the receptor for positive and negative allosteric modulators have been identified [55,54,350,56] (see below).
4.1. GluN2A-selective allosteric modulators
NVP-AAM077 (also known as PEAQX) is a competitive antagonist that interacts with the glutamate binding site. Although it was initially reported as having a high degree of selectivity for GluN2A over GluN2B [351], subsequent evaluation of the binding affinity of NVP-AAM077 at GluN1/2A and GluN1/2B receptors found more modest selectivity (KB values were 15 nM for GluN1/2A and 78 nM for GluN1/2B) [352]. This, and other studies, suggested that the level of selectivity (5-fold) of NVP-AAM077 is insufficient for dissection of synaptic responses mediated by GluN2A- and GluN2B-containing receptors [352,353]. Many studies evaluating native receptors and excitatory synaptic transmission have been performed using NVP-AAM077, however, the results of this body of work should be carefully interpreted with regards to the experimental design, level of selectivity assumed, and conclusions drawn.
TCN-201 and TCN-213 were the first non-competitive GluN2A-selective inhibitors (or GluN2A-selective NAMs) that were identified [354]. TCN-201 has a binding affinity of 27–70 nM at GluN2A-containing receptors, with >1000-fold selectivity over other GluN2 subunits [355,356,158]. Inhibition by TCN-201 is surmounted by glycine binding, which is paradoxical since the subunit selectivity depends on the glutamate binding GluN2 subunit [356,354,355]. However, the TCN-201 binding site is located in the LBD heterodimer interface between GluN1 and GluN2A subunits, with key interacting residues around 16 Å from the glycine binding site in GluN1 [356,157,158]. Quantitative analyses show that the functionally-observed interaction between TCN-201 and glycine was best described by an allosteric model of antagonism rather than a direct competition model [356,355]. Recent analyses of crystal structures of receptor states that are activated and inhibited by the GluN2A-selective NAMs demonstrated a mechanism in which NAM binding to the modulatory site stabilizes the open conformation of the GluN1 LBD, thereby facilitating glycine unbinding and receptor inactivation [158]. Furthermore, these structures revealed that two residues in the interface between GluN1 and GluN2A LBDs play principal roles in the allosteric mechanism of GluN2A-selective NAMs by forming a molecular switch that controls the difference between low- and high-affinity NAM binding; this difference is the primary driving force for allosteric inhibition. MPX-004 and MPX-007 are newer GluN2A-selective NAMs closely related to TCN-201 that have improved potency (79 and 27 nM, respectively, determined in 3 μM glycine) compared to TCN-201 (340 nM in 3 μM glycine) [357]. The MPX compounds provide nearly complete block of GluN2A-containing NMDA receptors and have improved solubility compared to TCN-201 [357,158]. In recent years, the GluN2A-selective NAMs have been used to probe the GluN2B to GluN2A developmental switch, the expression of GluN2A in subcortical and subthalamic nuclei, as well as the role of GluN2A in nicotine reinstatement, cortical spreading depression, and hippocampal plasticity [357,355,358,359,84,360,361].
A high throughput screen performed by Genentech to identify GluN2A-selective positive allosteric modulators (PAMs) identified several structurally-related compounds, here referred to as GNE compounds. These compounds are GluN2A-selective PAMs with at least 10-fold selectivity over other GluN2 subunits that bind the LBD heterodimer interface between a GluN1 and GluN2A subunits, similar to the GluN2A-selective NAMs [157,170]. Interestingly, the GNE compounds interact with the same residue (GluN2A V783) in the GluN2A subunit that mediates the selectivity and inhibition by TCN-201, MPX-004, and MPX-007 [157,158]. This valine is non-conserved across the GluN2 subunits, and introduction of this residue into GluN2B via site-directed mutagenesis is sufficient to confer both inhibition and potentiation to GluN2B-containing NMDA receptors [157,356]. GluN1/2A LBD heterodimer crystal structures in complex with GNE compounds and the GluN2A-selective NAMs show that the binding modes of both positive and negative allosteric modulators are distinct within this pocket, a finding reinforced by the results of mutagenesis studies [158,157,170]. Interestingly, some GluN2A-selective PAMs (i.e. GNE compound analogs) also affect the function of AMPA receptors with similar potencies as for NMDA receptors [157]. The different GNE compounds display variation in the efficacy of GluN1/2A receptor potentiation (up to 6-fold potentiation of receptors activated by EC30 of glutamate) and potency (EC50 values between 0.02–60 μM) [170]. Furthermore, the series of modulators show reduced efficacy when receptors are activated by saturating concentration of agonist. For example, GNE-0723 shows ~5 fold potentiation of an EC30 response compared to ~2 fold potentiation of an EC100 response, which is presumably due to an increase in agonist potency mediated by the modulator [170,157]. A complex relationship appears to exist between GNE compound structure, efficacy, and the degree of prolongation of glutamate deactivation rate, which could reflect increased glutamate affinity and potency [170,157]. Two GNE compounds (GNE-6901 and GNE-8324) were evaluated on NMDA receptor-mediated responses in hippocampal neurons [157]. These GNE analogs differed in their ability to prolong the deactivation rate of NMDA receptors, and also showed differences in their ability to alter short- and long-term synaptic plasticity. More studies could help fully elucidate the mechanism of action of this interesting series of GluN2A-selective PAMs and demonstrate their usefulness in studies on the physiological roles of GluN2A-containing NMDA receptors. The pharmacology of GluN2A-selective modulators is summarized in Table 1.
Table 1.
Summary of GluN2A-selective modulators.
| Activity at GluN1/2X (in μM) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | 2A | 2B | 2C | 2D | |||
| NVP-AAM077 |  |
KBa | 0.015 | 0.078 | - | - | [352] |
| TCN-201 | KBa,b | 0.045 0.070 0.027 |
NE | NE | NE | [356] [355] [158] |
|
| MPX-004 | IC50c,d | 0.079c 0.198† |
NEc NE† |
-c NE† |
NEc NE† |
[357] | |
| MPX-007 | IC50c,d | 0.027c 0.143† |
NEc ND† |
-c NE† |
NEc NE† |
[357] | |
| GNE-3419 |  |
EC50c | 2.03 | NR | NR | NR | [157] |
| GNE-6901 | EC50c | 0.33 | NR | NR | NR | [157] | |
| GNE-0723 |  |
EC50c | 0.021 | ND | 7.4 | 6.2 | [170] |
| GNE-8324 |  |
EC50c | 2.43 | NR | NR | NR | [157] |
denotes not determined, NE denotes no effect at the highest concentrations evaluated, and ND indicates that the compound displayed some activity, but the affinity or potency could not be determined. NR denotes some activity, but that the numerical affinity value was not reported. Unless otherwise stated (also denoted by †), the values were determined using two-electrode voltage-clamp experiments with Xenopus oocytes.
denotes when Schild analysis was used for affinity determination.
three independent studies are published reporting the KB of TCN-201 at GluN1/2A.
denotes that potency (i.e. half maximally effective concentration) was determined using a Ca2+ imaging assay.
Experiments using MPX compounds were performed in 3 μM glycine.
4.2. GluN2B-selective allosteric modulators
Ifenprodil and its mechanistically-similar analogs have been tremendously useful tool compounds since the discovery in 1993 that they are non-competitive GluN2B-selective inhibitors (i.e. GluN2B-selective NAMs) [349]. The IC50 for ifenprodil is in the nM range with 200–400 fold selectivity for the GluN1/2B receptor over GluN1/2A [349]. The observed inhibition of GluN1/2A at high concentrations is caused by low-affinity non-selective channel block [349]. The high-affinity ifenprodil binding site is located in the interface between the GluN1 and GluN2B ATD heterodimer [362,264]. Ifenprodil inhibition is dependent on agonist concentrations; at saturating glutamate and glycine concentrations, maximally effective concentrations of ifenprodil produce incomplete inhibition, with 10–20% residual response [349,363,85,362], whereas the glycine concentration is inversely correlated with the extent of observed inhibitory effect [349]. The actions of ifenprodil at different glutamate concentrations are also complex; ifenprodil causes an increase in glutamate-site agonist affinity, which produces less inhibition with lower agonist concentrations [363]. This positive allosteric interaction between ifenprodil and glutamate binding is similar to that observed for Zn2+ acting at the GluN2A ATD and can lead to apparent potentiation at low agonist concentrations [100,300,363]. Many newer GluN2B-selective NAMs acting at the ifenprodil site have been synthesized with improved potency and selectivity (e.g. Ro 25–6981 and CP-101,606) [364,365], and additional mechanistic features such as pH-sensitivity (e.g. see [366]). Recently, crystal structures suggest that GluN2B-selective NAMs can be divided into two classes with distinct binding modes at the GluN1-GluN2B ATD heterodimer interface; one class containing ifenprodil, CP-101,606 and Ro 25–6981 and a second class typified by EVT-101 [281]. EVT-101 is and orally active compound with potent inhibition at low nanomolar concentrations [281], however, a thorough study of EVT-101 properties and mechanism of action has not been published. GluN2B-selective NAMs have been intensely studied by academic research groups and pharmaceutical companies in an effort to identify new series with therapeutic benefits as well as to expand our understanding of the role of GluN2B in normal physiology and disease, a topic thoroughly summarized in a number of excellent reviews [367–369]. GluN2B-selective inhibitors have also been evaluated in clinical trials, with mixed and complex results [370,371,369,372]. The pharmacology of GluN2B-selective modulators is summarized in Table 2.
Table 2.
Summary of GluN2B-selective modulators.
| Activity at GluN1/2X (in μM) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | 2A | 2B | 2C | 2D | |||
| Ifenprodil |  |
IC50 | 39.5 | 0.114 | 29.1 | 75.9 | [525] |
| CP-101,606 |  |
IC50 | NE | 0.039 | NE | NE | [85] |
| Ro 25–6981 | IC50 | 52 | 0.009 | - | - | [365] | |
| EVT-101 |  |
IC50 | - | 0.012 | - | - | [281] |
denotes not determined and NE denotes no effect at the highest concentrations evaluated. Unless otherwise stated (also denoted by †), the potency (i.e. half maximally inhibiting concentration) was determined using two-electrode voltage-clamp experiments with Xenopus oocytes.
4.3. GluN2C/D-selective allosteric modulators
Spurred by the description of NVP-AAM077 and other glutamate-site competitive antagonists, studies of related compounds were performed in order to find similar antagonists with variation in the selectivity at NMDA receptor subtypes [373,374]. This effort lead to the discovery of PPDA, which is similar to the earlier identified competitive antagonist PBPD [375], that had differential selectivity and showed high potency [374]. Several structural modifications were pursued in subsequent studies, yielding compounds that consistently displayed a preference for GluN2C- and GluN2D-containing NMDA receptors over GluN2A- and GluN2B-containing receptors [376]. UBP-141 was observed to have 5–7 fold selectivity for GluN1/2D over GluN1/2A and GluN1/2B, but was less potent than PPDA. Several studies have used UBP-141 to probe the role of GluN2D in certain physiological processes [143,377–380]. These studies provided important insight to the physiological roles of GluN2D in central neurons, but they also should be interpreted with the caveat of modest subunit-selectivity, which is apparently inherent to glutamate-site competitive antagonist. Further expansion and exploration of the chemical space around the compounds related to UBP-141 led to an investigation of related scaffolds, and the subsequent discovery of several mixed-action modulators, including UBP-710 and UBP-551 [381]. UBP-710 shows divergent action at concentrations of 100 μM and higher, resulting in potentiation of GluN1/2A and GluN1/2B, but inhibition of GluN1/2C and GluN1/2D [381]. UBP-551 appears to be uniquely selective for GluN2D-containing NMDA receptors and potentiates current responses with a biphasic concentration-effect relationship, with maximal potentiation of GluN1/2D observed at 30 μM, a concentration at which other NMDAR diheteromeric receptors are inhibited [381]. The mixed-action UBP compounds possess remarkably unique actions, but their utility is hampered by poor physicochemical properties of the parent scaffold and a lack of high affinity actions or high subunit-selectivity [382,381]. It will be interesting to learn more about mechanism and site of action of this class as molecules with higher potency, selectivity, and improved physicochemical properties are developed.
A series of quinazolin-4-ones (QNZ) are negative allosteric modulators of NMDA receptors that show ~50-fold selectivity for GluN2C- or GluN2D-containing NMDA receptors [383,384]. The prototypical compound QNZ-46 has an IC50 of 7.1 and 3.9 μM at GluN1/2C and GluN1/2D, respectively, and has minimal effects on AMPA and kainate receptors. QNZ-46 does not compete with glutamate or glycine binding and inhibition is voltage-independent. Interestingly, the inhibition by QNZ-46 is dependent on glutamate binding, but not glycine binding, and the potency of QNZ-46 is increased when glutamate is bound [384]. Glutamate deactivation is prolonged in the presence of QNZ-46, consistent with a mechanism in which QNZ-46 must unbind before glutamate can unbind [384]. Structural determinants of action appear to reside in the lower lobe of the GluN2D LBD, however, the precise binding site for this series remains to be determined [384].
A series of dihydroquinolone-pyrazoline (DQP) analogues are, like QNZ-46, GluN2C- and GluN2D-selective NAMs [385]. The prototypical analogue in this series, DQP-1105, is ~50-fold selective for GluN2C/D-containing receptors with IC50 values of 7.0 and 2.7 μM at recombinant GluN1/2C and GluN1/2D, respectively [385]. Inhibition by DQP-1105 is voltage-independent and is not surmounted by increased concentrations of glutamate or glycine, consistent with a non-competitive mechanism of action [385]. Inhibition by DQP-1105 is dependent on glutamate binding [385], a property it shares with the QNZ class of inhibitors. Similarly, the structural determinants of DQP-1105 action resided in the lower lobe of the GluN2D LBD and largely overlapped with those of the QNZ class of inhibitors [385]. The finding that the QNZ and DQP series share similar structural determinants on GluN2C/D-containing receptors raises the possibility their binding sites may overlap and that the binding pocket could be exploited by a wide array of ligands with distinct binding modes. Further exploration of the DQP structure-activity relationship led to the synthesis of chiral compounds with nanomolar activity at GluN2C/D-containing receptors, making the DQP series more potent and selective than the QNZ series [386]. DQP-1105 has been used in several recent studies illustrating roles for GluN2C and GluN2D in normal physiology as well as pathophysiology in various nuclei of the brain [379,84,387–389].
A series of tetrahydroisoquinoline PAMs are highly selective for GluN2C/D-containing NMDA receptors [390]. Further exploration of the structure-activity relationship for this class of PAMs resulted in a large family of stereo-selective analogues with strong selectivity for GluN2C/D-containing receptors, some of which have nanomolar EC50 values [391,392]. Separation of CIQ, an early prototype in this class of GluN2C/D-selective PAMs, into its two stereoisomers showed that (+)-CIQ contains all the activity observed for the racemic mixture [391,392] and has reduced off-target actions [393]. CIQ has similar potency and efficacy at GluN2C- and GluN2D-containing receptors, as do virtually all related analogues studied to date [391,392]. Importantly, it has been demonstrated that CIQ also potentiaties responses from triheteromeric GluN1/2A/2C and GluN1/2B/2D receptors, albeit with some reduction in efficacy [390]. CIQ has no effect on the deactivation time course for GluN1/2D, but prolong glutamate deactivation for GluN1/2C [390]. Chimeric and mutational studies suggest that the potentiation by CIQ is dependent on residues in the M1 transmembrane helix and a short preM1 helix in the GluN2 subunit [390,191]. However, whether these structural determinants correspond to the CIQ binding site remains to be determined. Racemic CIQ and (+)-CIQ have been used in several studies probing the expression and role of GluN2D in synaptic transmission in various nuclei across the brain and spinal cord [394,395,84,393,396,387,397]. One series of GluN2C-selective PAMs has been described (i.e. PYD compounds) [160,398]. The structure-activity relationship for this series revealed stereo-selective actions and additional analogues with enhanced potency [398]. To date, the PYD series is the only highly-selective positive modulator series that discriminates between GluN2C- and GluN2D-containing NMDA receptors. The prototypical analogue, PYD-106, has an EC50 of 16 μM at GluN1/2C and maximally potentiates receptor responses to 200% of control [160]. PYD-111, a closely-related analogue, is slightly more potent with an EC50 of 4 μM [398]. Interestingly, PYD-106 is highly selective for the diheteromeric GluN1/2C receptors, but has no effect on triheteromeric GluN1/2A/2C receptors [160]. PYD-106 has a weak allosteric effect on glutamate potency and modestly prolongs the glutamate deactivation time-course (in the sustained presence of glycine) [160]. Chimeric and mutational studies identified structural determinants of PYD-106 actions at a unique site residing at the interface of the GluN2C ATD and the upper lobe of the GluN2C LBD [160]. Modelling of the GluN1/2C structure on the basis of the GluN1/2B crystal structure [67] revealed that residues that affect PYD-106 actions line a large pocket, suggesting a novel modulatory site on the NMDA receptor [160]. The pharmacology of GluN2C/D-selective modulators is summarized in Table 3.
Table 3.
Summary of GluN2C/D-selective modulators.
| Activity at GluN1/2X (in μM) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | 2A | 2B | 2C | 2D | |||
| PBPD |  |
Kie | 15.8 | 5.0 | 9.0 | 4.3 | [375] |
| PPDA |  |
Kie | 0.55 | 0.31 | 0.096 | 0.125 | [374] |
| UBP141 |  |
Kie | 14.2 | 19.3 | 4.2 | 2.8 | [376] |
| QNZ-46 |  |
IC50 | 229 182 |
ND 193 |
6 7.1 |
3 3.9 |
[383] [384] |
| DQP-1105 |  |
IC50 | ND | 113 | 7.0 | 2.7 | [385] |
| CIQ, (+)-CIQ* |  |
EC50 | NE | NE | 2.7, 9.0‡ |
2.8, 8.0‡ |
[390] [391,392] |
| PYD-106 |  |
EC50 | NE | NE | 16 | NE | [160] |
NE denotes no effect at the highest concentrations evaluated, and ND indicates that the compound displayed some activity, but the affinity or potency could not be determined. Unless otherwise stated (also denoted by †), the values were determined using two-electrode voltage-clamp experiments with Xenopus oocytes.
Ki values were estimated using Cheng-Prusoff correction of the measured IC50 values.
The chiral carbon of (+)-CIQ, the active enantiomer, is denoted by the asterisk in the chemical structure.
The apparent lower potency for (+)-CIQ compared to the racemic mixture is likely due to better estimation of maximum potentiation, since the active enantiomer has increased abundance in solution at concentrations close to the solubility limit (i.e the pure enantiomers can be evaluated at higher concentrations compared racemic CIQ).
5. NMDA receptor subtypes in the CNS
As described above, the different GluN2 subunits endow the NMDA receptor subtypes with distinct functional properties, unique pharmacology, and markedly different mechanisms of regulation. This feature is a major determinant of the variation observed between distinct neuronal cell types with respect to the time course of the synaptic NMDA receptor response as well as their changes in response to neuronal activity or other stimuli. Thus, the neuronal cell types in the different brain regions or nuclei can precisely tune their functional properties by expressing different complements of GluN2 subunits. Furthermore, the expression profiles of the different NMDA receptor subtypes undergo marked changes during development to enable modifications of neuronal functions during critical neurodevelopmental periods and maturation of the CNS.
5.1. Distinct expression profiles of NMDA receptor subunits
The different GluN2 subunits have profoundly different regional and developmental expression profiles (Fig. 2a). The GluN2B subunit is widely expressed in the embryonic brain, but becomes restricted to the forebrain in the adult rodent brain [93,399,63,92,400,95]. By contrast, the expression of GluN2A subunit is ubiquitous in the CNS, initially at very low levels at birth, after which the expression increases dramatically during the second postnatal week (P7–P14). Thus, in some regions, such as the cortex and hippocampus, there is a developmental switch in the expression of GluN2B to GluN2A, and synaptic NMDA receptors change from mainly containing GluN2B early in life to also containing GluN2A (see below) [135]. In the adult brain, the GluN2A is present in virtually all regions of the CNS with particular high abundance in the cortex, hippocampus, and cerebellum [93,399,63,92,400,95]. Expression of GluN2C is undetectable at birth, but in the second postnatal week this subunits becomes highly enriched in the cerebellum and the olfactory bulb [93,63,92,400–402]. Similar to GluN2B, the GluN2D subunit is widely expressed early in development, but then expression fades in the second postnatal week. The GluN2D subunit remains expressed into adulthood with the highest abundance in the diencephalon, mesencephalon, and spinal cord [93,63,92,403].
In addition to the aforementioned overall expression profiles, the different GluN2 subunits can be found in distinct neuronal populations in some brain regions. Thus, although the overall expression of a GluN2 subunit may appear low in a specific region, the expression can still be high in a small subpopulation of neurons in that region. For example, the overall expression levels of GluN2C and GluN2D appear to be low in the cortex and hippocampus, but more precise anatomical localization of these subunits suggest that they are specifically expressed in some populations of glial cells and interneurons in these regions [63,404,401,405,393]. Similarly, GluN2B and GluN2D are highly expressed in cerebellar Golgi cells, although they are considered to have less overall abundance in the cerebellum [137,406]. In recent years, increasingly precise identification of GluN2 subunit expression and subcellular localization in distinct neuronal populations have been reported as more refined methods of detection and pharmacological tool compounds become available.
Weak expression of GluN3A can be detected in several regions of the embryonic brain, and expression increases throughout the brain during the early postnatal development [407–410,116,411]. GluN3A expression peaks around postnatal day 8 (P8) in rodents, but then diminishes with time. By adulthood, GluN3A is weakly, but widely, expressed in the CNS. By contrast, expression of GluN3B slowly increases during the late stages of postnatal development and becomes widely expressed in the adult CNS [119,412–414]. Thus, there is an apparent developmental switch from expression of GluN3A to GluN3B in the rodent brain during the first two postnatal weeks. In addition, GluN3B is also highly expressed in motoneurons in the rodent spinal cord, but here expression starts at embryonic day 16 (E16) [413]. Recent studies suggest that the GluN3 subunits also have distinct subcellular distributions with GluN3B found primarily in the postsynaptic membrane and GluN3A found mostly at extrasynaptic and presynaptic sites [415]. The contrasting expression profiles of the GluN3 subunits suggest they serve distinct physiological roles in the CNS. It should be noted, however, that the expression profiles of GluN3 subunits appears to vary markedly between brain regions [407–410,116,411,119,412–414], and also appears to be different in rodents compared to primates and humans [416,111,417,418].
Functional properties and trafficking of NMDA receptor subtypes are influenced by alternative splicing of the GluN1 mRNA. Differences between the regional and developmental distributions of GluN1 isoforms in the CNS have been described (Fig. 3a) [93,92,63,77,78]. However, the functional significance of these differences remains unclear and not as well characterized as those of GluN2 subunits. Consistent with its inclusion in all NMDA receptor subtypes, the GluN1 subunit is ubiquitously expressed in the CNS throughout development [93,92,63,77,78]. The GluN1–2 isoforms are widely distributed in the rodent brain, whereas low expression of GluN1–3 isoforms appears to be restricted to the sensorimotor cortex, the neocortex, hippocampus, and selected thalamic nuclei at later developmental stages. There is an apparent complementary distribution of GluN1–1 and GluN1–4 isoforms with GluN1–1 primarily expressed in more rostral regions, such as the cortex and hippocampus, and GluN1–4 in more caudal regions, such as the basal ganglia and cerebellum. The expression of GluN1-a and GluN1-b isoforms largely overlap, but marked variation in the relative abundance is observed between regions and even between neuronal cell types in the same region. For example, GluN1-a and GluN1-b isoforms have strikingly distinct developmental expression profiles in the hippocampus, and in the adult rodent brain, GluN1-b appears to be the major isoform in the CA3, while GluN1-a is the major isoform in the CA1 and dentate gyrus [78]. These differences in expression profiles are likely to have functional significance, since the deactivation time course of NMDA receptors containing GluN1-b (e.g. GluN1–1b) is accelerated compared to receptors containing GluN1-a (e.g. GluN1–1a) (Fig. 3c) [82,83], and GluN1–1b-containing receptor are less sensitive to endogenous negative allosteric regulators [80,81].
5.2. The GluN2B to GluN2A developmental switch
The increase in GluN2A expression during the second postnatal week in the rodent cortex and hippocampus results in a switch in the subunit composition of synaptic NMDA receptors from primarily being GluN2B-containing to also being GluN2A-containing. This switch is accompanied by the appearance of triheteromeric GluN1/2A/2B receptors, which contain two GluN1, one GluN2A, and one GluN2B subunit [132,135]. At early developmental stages, the time course of the EPSC (i.e. deactivation time constant) and the sensitivity to GluN2B-selective NAMs, such as ifenprodil, suggest that diheteromeric GluN1/2B is the primary NMDA receptor subtype in central synapses of the cortex and hippocampus [419,132,420,149,421]. However, the marked acceleration of the EPSC time course and reduced ifenprodil sensitivity observed during the second postnatal week are consistent with a switch in the synaptic content from GluN2B-containing to GluN2A-containing NMDA receptors. That is because the deactivation time constants of both diheteromeric GluN1/2A and triheteromeric GluN1/2A/2B are markedly faster than diheteromeric GluN1/2B (Fig. 4) [133,151]. Furthermore, maximal inhibition by GluN2B-selective NAMs is retained, but significantly reduced for triheteromeric GluN1/2A/2B compared to diheteromeric GluN1/2B [151,150].
The “GluN2B to GluN2A developmental switch” is evolutionarily conserved and occurs in many brain areas of frogs, birds and mammals, including cortex, hippocampus, amygdala and cerebellum. Numerous studies have reported that the timing of the switch, which varies between brain regions, is coincident with changes in specific learning abilities. The prevalent hypothesis is therefore that the GluN2B to GluN2A developmental switch is a major factor in the synaptic maturation, which is important for the refinement and fine tuning of neuronal circuits. The developmental switch in NMDA receptor subunit composition closes a critical period for the refinement of connections in the key brain regions, resulting changes in synaptic plasticity [422,423]. However, in some brain circuits, the changes in synaptic plasticity during critical developmental periods are not corresponding to the switch from GluN2B to GluN2A expression (e.g. [419]), suggesting that other NMDA receptor subunits (e.g. GluN2C/D or GluN3 subunits) may have important roles in the refinement and fine tuning of these neuronal circuits.
The switch in the GluN2 subunit composition of synaptic NMDA receptors is experience-dependent and can occur acutely following synaptic activity or sensory input. For example, the change from synaptic GluN2B- to GluN2A- containing NMDA receptors is not observed in the visual cortex of dark-reared rats until they are exposed to light [424]. Thus, the EPSCs in the visual cortex of dark-reared rats have slower time course and higher sensitivity to ifenprodil compared to light-reared rats. Remarkably, returning the animals to the dark can restore the synaptic content of GluN2B to levels observed in animals that have not been exposed to light [425]. Thus, the experience-dependent GluN2 subunit switch appears to be bi-directional, at least in some brain regions [420].
The mechanisms that mediate the exchange of synaptic GluN2B-containing NMDA receptors with GluN2A-containing receptors are not fully understood and this remains an area of intense investigation. Similarly, detailed insights to the consequences of changes in GluN2 subunit composition on the refinement of synaptic plasticity and neuronal circuits are still lacking. However, many excellent reviews discuss our accumulated understanding of these processes and highlights important studies in these areas of NMDA receptor research [426,326,23].
6. NMDA receptors in disease
NMDA receptors have been considered in the context of numerous neurological conditions, either as a potential causative feature, exacerbating component, or therapeutic target [23,1,2,427,42]. However, the interest in NMDA receptor modulators as therapeutics has grown significantly in recent years. Contributing to this interest has been the growing clinical evidence that the NMDA receptor channel blocker ketamine could act as a radically new treatment for depression. Indeed, discovery of the antidepressant activity of ketamine has been characterized as “the most important psychiatric discovery in half a century” [428,429]. Here, we will highlight two emerging mechanistic themes in this area of drug discovery. These are the growing awareness of the significance of metaplasticity in the therapeutic response to NMDA receptor modulation and the progress in linking NMDA receptor subtypes to CNS disorders.
6.1. Depression
Short intravenous infusions of the pan-NMDA receptor channel blocker ketamine (0.5 mg/kg over 40 min) has now been repeatedly demonstrated to yield a robust antidepressant response that 1) develops within hours, 2) may last for days to weeks, and 3) is effective in up to 70% of patients [430–432]. This ketamine regimen also is reported to reduce suicidal ideation [433,434] and have benefit in patients suffering bipolar depression [435], obsessive-compulsive disorder [436], and post-traumatic stress disorder [437]. The antidepressant response appears to be sustainable with repeated doses [438–440] and clinical studies are beginning to define the optimal dose and treatment chronicity [441]. Side effects include those expected for an NMDA receptor channel blocker, including cognitive disruption and neuropsychiatric symptoms; however, these appear to be mild and manageable at effective exposures [442]. Indeed, intravenous infusion may be the most significant limitation to ketamine use and clinical studies are exploring other routes of administration [443,444]. Furthermore, another NMDA receptor channel blocker, lanicemine [445,446], and GluN2B-selective negative allosteric modulators (GluN2B NAMs) [447,448] also are reported to have clinical antidepressant activity. Several detailed reviews of the rapid progress in this area have been recently published [429,449].
A remarkable aspect of the antidepressant activity of ketamine is that the clinical response develops and is sustained after the drug has been cleared from the body. In contrast, the psychotomimetic effects track closely with drug residence time and typically resolve shortly after cessation of drug infusion [430]. The antidepressant effects of other NMDA antagonists also persist beyond drug clearance from the body. In fact, it has been shown that the brief ketamine exposure is sufficient to induce a long-lasting change in human brain physiology [431]. These results may indicate that the antidepressant effects of these drugs arise from a metaplastic change in synaptic activity. Metaplasticity is ‘the plasticity of synaptic plasticity’ [450]; that is, the effect that an acute change in synaptic function has on the ability of subsequent stimuli to effect further change [451,452]. The antidepressant effects of NMDA receptor inhibitors may be interpreted as a variation on this theme. The antidepressant response is not the direct result of acute NMDA receptor inhibition, but rather a long-lasting change in synaptic function triggered by the brief inhibition. There is considerable interest in determining the nature of these long-lasting synaptic changes at the molecular level, as these findings might reveal insight into the neurobiology of depression and be applied prospectively to develop new antidepressants. There is speculation that the antidepressant effect of ketamine may be due to an effect of a metabolite that does not inhibit NMDA receptors [453]; however, this has not yet been reconciled with the clinical antidepressant effect of the other chemically and mechanistically diverse NMDA receptor modulators. Indeed, a fruitful avenue of research is through comparative analysis of these different agents to pinpoint common mechanisms that may account for the antidepressant effects.
Both ketamine and GluN2B NAMs induce persistent increases in synaptic strength after drug washout. This is evidenced by mTOR-driven increases in synaptic protein levels in rodents [454–456], an increase in sensitivity to the induction of LTP in rodents [457], and an increase in sensory stimulus-evoked potentials in rodents [458] and in humans [431]. A working hypothesis is that such an up-regulation of synaptic strength underlies the antidepressant activity. One hypothesis for the mechanism by which ketamine induces synaptic up-regulation derives from its use-dependence for channel block, which confers selectivity for highly active NMDA receptors on PV-positive, fast spiking GABAergic interneurons [459]. It is hypothesized that inhibiting fast-spiking interneurons disinhibit cortical microcircuits, inducing gamma-band cortical activity that drives an LTP-like up-regulation of synaptic strength. While attractive, this hypothesis accounts poorly for the putative antidepressant activity of lanicemine [445] and particularly the GluN2B NAMs. These latter compounds do not induce gamma-band activity in rodents [460,458,461] or primates [462] even at high levels of receptor occupancy. This functional difference between ketamine and the GluN2B NAMs may be accounted for by differences in brain micro-circuitry modulated by these agents. GluN2B is weakly expressed in interneurons arising from medial ganglionic eminence [463] that include the ketamine-sensitive fast spiking PV- and SST-family interneurons that synapse directly with pyramidal neurons [464]. Lack of predominant GluN2B expression on these interneurons may account for the fact that GluN2B NAMs do not induce gamma-band activity. Instead, GluN2B is expressed by CCK-family interneurons that arise from the caudal ganglionic eminence [463]. These interneurons synapse with the fast-spiking interneuron classes to regulate their activity in response to long-range pyramidal neuron inputs [465]. Thus, GluN2B NAMs may be speculated to increase activity of fast-spiking interneurons by decreasing excitatory drive on CCK-family interneurons, the opposite of the putative effect of ketamine. The effects of ketamine and the GluN2B NAMs are also likely to be divergent on pyramidal neurons. Deployment of GluN2B varies across different pyramidal neuron populations [466] and in different synaptic compartments (reviewed in [467]). The deployment of GluN2B subunits is also activity-dependent and is increased at relatively inactive synapses (reviewed in [426,468]). Thus, the pan-NMDA receptor antagonist ketamine and the GluN2B NAMs likely inhibit different receptor pools on pyramidal neurons based on the subunit-selectivity of the NAMs, as well as the activity dependence of ketamine. At present there is no obvious point of convergence between these two compound classes that may account for their striking similarity in terms of functional endpoints in preclinical models and clinical antidepressant efficacy (and side effect profile, see below). However, the fact that points of convergence are apparently so few increases the power of comparative analyses to pinpoint the molecular mechanisms of their antidepressant response. This seems a promising area for continued research.
6.2. Neurodevelopmental disorders
NMDA receptor signaling plays a central role in circuit development of the central nervous system. As noted above, during development, high expression of GluN2B and GluN2D NMDA receptor subunits is superseded by expression of GluN2A [92,93,63,135,469,470]. This choreography mediates the transition from high levels of synaptic plasticity as circuits are formed and refined to the circuit stability of the adult brain. Consistent with a fundamental role in this developmental progression, variation in genetic loci encompassing GRIN2A and GRIN2B (i.e. genes encoding GluN2A and GluN2B, respectively) are identified in genome-wide association studies (GWAS) as contributing to the risk of developing the two major neurodevelopmental disorders, autism and schizophrenia [471–474]. The symptoms of autism manifest early in life, whereas those of schizophrenia do not fully manifest until late adolescence or early adulthood. Thus, these two disorders arise from derangements at different epochs of the brain’s developmental program. The association of GRIN2A and GRIN2B genetic variation in the risks for both disorders highlights a role for NMDA receptor signaling in unfolding the entire developmental program. However, there are different scenarios by which variation in NMDA receptor signaling may contribute to these disorders that are important in considering NMDA receptor modulation as a therapeutic strategy. Defective NMDA receptor signaling could impact a specific segment of the developmental program, in which case therapeutic intervention would need to occur during that developmental epoch. Alternatively, defective NMDA receptor signaling could impact a developmental trajectory and so therapeutic intervention would need to occur at some time before the symptoms begin to manifest. Finally, aberrant NMDA receptor signaling may be a factor in the expression of symptoms, in which case NMDA receptor modulation may be effective as a ‘symptomatic’ therapeutic at any time after symptoms manifest. Of these three scenarios, the most extensively studied therapeutic use of NMDA receptor modulators is as a ‘symptomatic’ approach to schizophrenia.
6.3. Schizophrenia
The association of NMDA receptor dysfunction with schizophrenia initially arose from the clinical observation that NMDA receptor inhibition in healthy individuals induces a spectrum of symptoms that are strikingly similar to those exhibited by patients suffering schizophrenia [475–477]. These “schizophrenomimetic” symptoms (e.g. see [478]) correspond closely with NMDA receptor occupancy [477,479]. This infers that symptom expression in schizophrenia patients may result from hypofunction of NMDA receptor signaling [480–482]. The NMDA receptor hypofunction hypothesis for schizophrenia has driven a great deal of research to develop drugs to potentiate NMDA receptor signaling to overcome the symptoms of this disorder.
The largest body of work aiming to overcome NMDA receptor hypofunction encompasses strategies to increase agonist occupancy of the GluN1 glycine co-agonist binding site. This has included clinical testing of the natural ligands glycine and D-serine, glycine analogs such as D-cycloserine (DCS), and inhibition of the GlyT1 transporter to increase peri-synaptic glycine levels [483,484]. Unfortunately, the effectiveness of the glycinergic approach has so far proved modest, with the most consistent effect being a reduction in negative symptoms, but with little effect on cognitive or positive symptoms [484,485]. Nonetheless, this clinical research has yielded significant insight that may be critical to further advancement of NMDA receptor potentiator strategies. It has been suggested that treatment with “glycinergics” may trigger metaplastic changes in glutamate signaling that significantly affect the drug response [486,487]. These effects on drug response include limited efficacy of continuous drug exposure and can cause complex dose responses, such as observed with the GlyT1 inhibitor bitopertin [488]. To exploit the plasticity induced by modulating NMDA receptors, Goff and colleagues have begun to explore intermittent dosing with the glycinergic DCS. In preliminary clinical studies, intermittent DCS treatment also improved negative symptoms. More significantly, intermittent DCS improved memory performance and reduced delusional severity when combined with cognitive behavioral therapy [487,489,490]. Thus, an intermittent DCS dosing regimen may be at least as efficacious as continuous treatment with regard to negative symptoms and may deliver efficacy against positive and cognitive symptoms not observed with continuous exposure regimens. This line of clinical research clearly calls for further study and begs investigation into the underlying molecular mechanisms.
Several mechanisms that may contribute to enhanced efficacy with intermittent DCS treatment. Increasing glycine-site occupancy to acutely increase NMDA receptor activity also increases NMDA receptor internalization rate, which may offset positive effects [491]. An intermittent dosing regimen may reduce drive on internalization and thereby tip the balance towards potentiation. More intriguing is the possibility that intermittent dosing enhances plasticity beyond a simple ‘drug-on’ potentiation [487]. The pharmacology of DCS is complex; the compound is a partial glycine site agonist and a single administration may therefore potentiate or inhibit NMDA receptors, and possibly both, over the exposure time course of a single dose. Furthermore, DCS is a super-agonist at GluN2C-containing receptors and will activate a larger current compared to glycine/D-serine [492,493], suggesting that at these receptors, substitution of DCS for glycine could selectively enhance synaptic NMDA receptor responses. An appropriate single dose may trigger a longer lasting metaplastic change in synaptic activity that results in sustained efficacy. Indeed, it is interesting to draw analogy between intermittent dosing with DCS in schizophrenia and intermittent dosing of NMDA antagonists in depression. In both cases, it is the metaplastic effect of the brief drug exposure, i.e., the ‘drug-off’ effects, that delivers the efficacy.
It is also of interest to understand the underlying mechanism(s) by which NMDA receptor hyopfunction may result in the expression of schizophrenia symptoms. The clinical pharmacology may be informative. First, DCS produce a maximal response that is twice as large as glycine at GluN2C-containing NMDA receptors, resulting in increased NMDA receptor signaling whenever concentrations of DCS allow it to displace glycine from its site these receptors [492,493]. GluN2C is highly expressed in cerebellum and in the thalamic reticular nucleus [63,494,495,401]. It has been speculated that the efficacy of DCS may be derived from agonist activity GluN2C-containing NMDA receptors in these brain regions, prompting an effort to develop other GluN2C-selective PAMs [390,398,160,394]. Another interesting clue to underlying mechanisms is the clinical observation that the GluN2B-selective NAM, CP-101,606, causes cognitive disruption and dissociative effects similar to those caused by ketamine [496,497,448]. Consistent with the clinical data, GluN2B NAMs and NMDA receptor channel blockers share discriminative stimulus properties in animal studies [498,499]. These findings are interesting with respect to the fact that there is little apparent overlap in the neuronal microcircuitry impacted by these two drug classes, as reviewed above. Thus, comparative analyses of the schizophrenomimetic effects of these drugs may also help pinpoint the microcircuit defects in NMDA receptor signaling relevant to the expression of schizophrenia.
6.4. Epilepsy/aphasia syndromes
GRIN1, GRIN2A, and GRIN2B have been associated with epilepsy (EpiPM consortium, “Roadmap for precision medicine in the epilepsies”, Lancet Neurology 2015 [500]). For example, a deterministic link has recently been made between genetic variation in GRIN2A and childhood epilepsy/aphasia syndromes [501–504]. The spectrum of these syndromes includes relatively benign Rolandic epilepsy, the more severe continuous spike-and-waves during slow-wave sleep syndrome (CSWSS), and Landau-Kleffner syndrome (LKS), and very severe epilepsies with significant developmental delay, intellectual disability, and dysmorphic features. Manifestation arises between ages 3–11 during the developmental epoch that is associated with language development [505]. This is also the epoch over which there is significant pruning of cortical excitatory synapses [506], in which NMDA receptor signaling is fundamentally involved. To date, more than 60 mutations in GRIN2A have been identified that appear to be causal to these developmental disorders [507]. Significantly, whereas many of these mutations result in receptor truncation or other losses of function, numerous point mutations result in a gain of function. This includes reduced Mg2+-block, enhanced agonist potency, and increased open probability and open time, at least when the receptors are expressed in heterologous expression systems [507,508]. Critical questions remain around how specific variations in GRIN2A, including both gain and loss of function, relate to the spectrum of severities in a common group of epilepsies and language disorders.
The discovery of the association of GRIN2A mutations with epilepsy/aphasia syndromes immediately suggested NMDA receptor modulators as potential therapeutics. In a first case, Pierson, Yuan, and colleagues [159,509] identified a child through the NIH Undiagnosed Diseases Program suffering early-onset epileptic encephalopathy, manifest as profound cognitive and motor development and intractable seizures resistant to standard anticonvulsant therapies, who had a point mutation in GRIN2A. Analyses in heterologous expression systems revealed that the mutation resulted in significantly increased activity of GluN2A-containing receptors, suggesting that inhibition of NMDA receptors may have a therapeutic benefit where other conventional therapeutics had failed. The treatment of this patient with the NMDA receptor antagonist memantine (approved for the treatment of Alzheimer’s disease) produced a rapid onset and persistent reduction in the number of seizures suffered by the child [509]. This suggests that the altered function of the GluN2A subunit may have contributed to seizure etiology. Unfortunately, memantine did not have an effect on the child’s cognitive or motor disability, suggesting that the GRIN2A mutation also had effects on the developmental trajectory, which were insensitive to memantine at the time treatment was initiated. It should be noted that this remains only a single case, and considerable work is needed to determine whether viable treatment options can be developed for these patients with specific mutations in NMDA receptor subunits.
6.5. Rett Syndrome
Rett Syndrome (RTT) is another severe neurodevelopmental disorder in which NMDA receptor dysfunction is implicated and NMDA receptor modulators are of therapeutic interest. RTT is a severe X-linked neurodevelopmental disorder caused by defects in transcriptional regulation by MeCP2 [510]. Although girls with RTT initially develop on a normal trajectory, developmental stasis and regression begins at 6–18 months that includes a severe reduction in the size and complexity of forebrain pyramidal neuron dendritic arbors, but without apparent reduction in neuron number [511]. Significantly, Bird and colleagues demonstrated in a mouse model that restoration of MeCP2 function in symptomatic animals reverted much of the neurological phenotype [512]. This implies that the fundamental architecture of the brain develops normally prior to the effects of MeCP2 lesion and that restoration of network function is an attainable goal. There are several emerging lines of research that suggest NMDA receptor dysfunction contributes to this network dysfunction and that modulation of these receptors may be an effective therapeutic approach [513]. Blue et al. [514] reported alteration of NMDA receptor expression in MeCP2 mutant mice. Subsequently, the Fagiolini lab reported an imbalance in GluN2A/GluN2B subunit deployment in both cortical pyramidal neurons and interneurons [515,516]. Significantly, manipulating the GluN2A/GluN2B balance through hemizygous GRIN2A knock out prevented the development of cortical dysfunction and ameliorated some of the RTT-like phenotype [515]. In another line of research, Katz and colleagues demonstrated that treatment of Mecp2 mutant mice with a low, sub-anesthetic dose of ketamine acutely reversed RTT-like phenotypes, including abnormal patterns of neuronal activation in cortical and subcortical structures as well as sensorimotor dysfunction [517]. Subsequently, Patrizi et al. [518] reported that once daily administration of this same low dose ketamine produced a sustained reduction in RTT-like symptoms and ameliorated structural circuit defects that underlie or contribute to neurological dysfunction. Significantly, in the study by Patrizi et al., neurological testing of mice occurred ~21 h after drug administrations; i.e., after ketamine had been completely eliminated. Thus, it appears that ketamine has beneficial effects in mouse RTT models during both “drug-on” [517] and “drug-off” [518] periods. The latter drug-off effects suggest a potential mechanistic parallel to the effects of ketamine in depression, particularly with respect to the possibility of durability of action beyond the acute period of NMDAR antagonism. Trials have now been initiated to test the safety and efficacy of NMDAR antagonists in RTT patients, including dextromethorphan, a weak NMDAR antagonist, and low-dose ketamine [513].
6.6. NMDA receptors as therapeutic targets
Clearly, the most significant recent advance in the area of therapeutics targeting NMDA receptors has been the emergence of ketamine as a rapidly acting antidepressant. Ketamine is now being used in clinics to treat patients for which standard of care monoaminergic reuptake inhibitors provides little relief. Ketamine is also groundbreaking from a mechanistic perspective. The therapeutic effect of ketamine is not due to an effect of the drug ‘on’ the brain per se, but to the response of the brain to the drug that manifests after the drug is gone. The concept of synaptic metaplasticity has been evolving for decades [519,451,452], and ketamine is the first example where such a metaplastic effect has been harnessed for therapeutic benefit. Indeed, this therapeutic effect may be broad, as clinical data is emerging to suggest that brief ketamine exposure may be beneficial across a range of neuropsychiatric conditions and perhaps in neurodevelopmental disorders. While the discovery of the antidepressant effect of ketamine was serendipitous [430], as were the implications of metaplasticity as its therapeutic mechanism, Goff and colleagues have been developing a parallel theme of inducing plasticity in exploring the utility of intermittent dosing of DCS in schizophrenia and anxiety disorders [487]. These research paths may be the herald of a new era in the development of CNS therapeutics in which we try and work with the brain instead of trying to overpower it.
The above notwithstanding, there is a tremendous amount of work ahead to build on the theme of harnessing the brain’s plasticity to therapeutic benefit. From a very practical perspective, the optimal duration of NMDA receptor inhibition and exposure interval remains to be determined in order to realize therapeutic benefits in depression and other conditions. For example, a single exposure to the GluN2B NAM CP-101,606, a short half-life compound, had a robust and long lasting antidepressant effect [448], whereas a long half-life GluN2B NAM, CERC 301, dosed for 28 days and likely resulting in continuous NMDA receptor occupancy, was without efficacy. Although it is not possible to draw firm conclusions from single studies, it is tempting to speculate that the difference in efficacy in these studies may be due in part to the difference in exposure duration, with the short duration exposure allowing the metaplastic mechanism to emerge.
It is also important to note the significance of back-translational research in advancing this area. Clinical data on NMDA receptor modulators provides a rich frame for preclinical studies into molecular mechanisms of disease as well as for new therapeutic approaches. As an example mentioned above, the similarity in clinical efficacy and side effect profile between ketamine and the GluN2B NAM CP-101,606, in light of the apparently scant overlap in site of action at the level of neurocircuitry, may be leveraged to gain significant insight into the role of NMDA receptor signaling in both depression and schizophrenia [520]. It also appears that the repertoire of NMDA receptor modulators is expanding rapidly [521,55,54,96]. This includes compounds with unique NMDA receptor subtype selectivity and modes of action that include both augmenting and inhibiting receptor activity. Thus, these compounds will provide new tools to interrogate the physiology of NMDA receptor signaling, which in turn may reveal new therapeutic opportunities. As insightfully pointed out by Köhr [522,523], it will be important to consider not only the locus of action of such compounds based on NMDA receptor subtype expression pattern, but also the mechanism of pharmacological action, as each may have a significant impact on the functional effects of these new compounds. Based on the new insights gained from the effects of ketamine and DCS, it will also be important to consider the metaplastic effects of these compounds in addition to their more direct effects on signaling.
7. Conclusions
Emerging information from genetic analyses linking NMDA receptors to specific disease conditions and the discovery of antidepressant effects for NMDA receptor antagonists have fortified and reinvigorated the long-standing focus on NMDA receptors as therapeutic targets. Recent year’s remarkable acceleration in the discovery of novel allosteric NMDA receptor modulators as pharmacological tools greatly facilitates studies to achieve new levels of understanding of NMDA receptor subtypes in physiology and disease. Many new modulatory binding sites in NMDA receptors have been identified along the way and combined with rapidly improving structural crystallographic and cryo-EM data, we are improving our understanding of how agonist binding is linked to channel gating and how the different subunits contribute to conformational changes during gating and allosteric modulation. These developments in the NMDA receptor field offer new perspectives and exciting opportunities to study unique roles for NMDA receptor subtypes, diheteromeric as well as triheteromeric, in distinct neuronal populations and subcellular locations. Furthermore, the converging advances in NMDA receptor pharmacology and clinical and mechanistic understanding of CNS diseases involving NMDA receptor dysfunction are poised to result in the development of new therapeutic agents.
Acknowledgements
This work was supported by grants from National Institutes of Health to S.F.T. (NS036654 and NS065371) and K.B.H. (GM103546 and NS097536).
Reference list
- 1.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62 (3):405–496. doi: 10.1124/pr.109.002451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14 (6):383–400. doi: 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- 3.Matsuda K, Yuzaki M (2012) Cbln1 and the delta2 glutamate receptor--an orphan ligand and an orphan receptor find their partners. Cerebellum 11 (1):78–84. doi: 10.1007/s12311-010-0186-5 [DOI] [PubMed] [Google Scholar]
- 4.Yuzaki M (2009) New (but old) molecules regulating synapse integrity and plasticity: Cbln1 and the delta2 glutamate receptor. Neuroscience 162 (3):633–643 [DOI] [PubMed] [Google Scholar]
- 5.Schmid SM, Hollmann M (2008) To Gate or not to Gate: Are the Delta Subunits in the Glutamate Receptor Family Functional Ion Channels? Mol Neurobiol 37 (2–3):126–141 [DOI] [PubMed] [Google Scholar]
- 6.Kakegawa W, Miyazaki T, Kohda K, Matsuda K, Emi K, Motohashi J, Watanabe M, Yuzaki M (2009) The N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivo. J Neurosci 29 (18):5738–5748. doi: 10.1523/JNEUROSCI.6013-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M (2010) Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science 328 (5976):363–368 [DOI] [PubMed] [Google Scholar]
- 8.Elegheert J, Kakegawa W, Clay JE, Shanks NF, Behiels E, Matsuda K, Kohda K, Miura E, Rossmann M, Mitakidis N, Motohashi J, Chang VT, Siebold C, Greger IH, Nakagawa T, Yuzaki M, Aricescu AR (2016) Structural basis for integration of GluD receptors within synaptic organizer complexes. Science 353 (6296):295–299. doi: 10.1126/science.aae0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakegawa W, Miyazaki T, Hirai H, Motohashi J, Mishina M, Watanabe M, Yuzaki M (2007) Ca2+ permeability of the channel pore is not essential for the delta 2 glutamate receptor to regulate synaptic plasticity and motor coordination. J Physiol-London 579 (3):729–735. doi: 10.1113/jphysiol.2006.127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid SM, Kott S, Sager C, Huelsken T, Hollmann M (2009) The glutamate receptor subunit delta2 is capable of gating its intrinsic ion channel as revealed by ligand binding domain transplantation. Proc Natl Acad Sci U S A 106 (25):10320–10325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadak S, Bouquier N, Goyet E, Fagni L, Levenes C, Perroy J (2016) mGlu1 receptor canonical signaling pathway contributes to the opening of the orphan GluD2 receptor. Neuropharmacology. doi: 10.1016/j.neuropharm.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Geiger JRP, Lubke J, Roth A, Frotscher M, Jonas P (1997) Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron 18 (6):1009–1023. doi:Doi 10.1016/S0896-6273(00)80339-6 [DOI] [PubMed] [Google Scholar]
- 13.Sah P, Hestrin S, Nicoll RA (1990) Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. J Physiol 430:605–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hestrin S, Nicoll RA, Perkel DJ, Sah P (1990) Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol 422:203–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trussell LO, Zhang S, Raman IM (1993) Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron 10 (6):1185–1196 [DOI] [PubMed] [Google Scholar]
- 16.Copits BA, Swanson GT (2012) Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci 13 (10):675–686. doi: 10.1038/nrn3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307 (5950):462–465 [DOI] [PubMed] [Google Scholar]
- 18.Mayer ML, Westbrook GL, Guthrie PB (1984) Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309 (5965):261–263 [DOI] [PubMed] [Google Scholar]
- 19.Seeburg PH, Burnashev N, Kohr G, Kuner T, Sprengel R, Monyer H (1995) The NMDA receptor channel: molecular design of a coincidence detector. Recent Prog Horm Res 50:19–34 [DOI] [PubMed] [Google Scholar]
- 20.Bourne HR, Nicoll R (1993) Molecular machines integrate coincident synaptic signals. Cell 72 Suppl:65–75 [DOI] [PubMed] [Google Scholar]
- 21.Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL (2015) Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res 1621:5–16. doi: 10.1016/j.brainres.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorumski CF, Izumi Y (2012) NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci Biobehav Rev 36 (3):989–1000. doi: 10.1016/j.neubiorev.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8 (6):413–426. doi: 10.1038/nrn2153 [DOI] [PubMed] [Google Scholar]
- 24.Holtmaat A, Svoboda K (2009) Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10 (9):647–658. doi: 10.1038/nrn2699 [DOI] [PubMed] [Google Scholar]
- 25.Granger AJ, Nicoll RA (2014) Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos Trans R Soc Lond B Biol Sci 369 (1633):20130136. doi: 10.1098/rstb.2013.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33 (1):18–41. doi: 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- 27.Morris RG (2013) NMDA receptors and memory encoding. Neuropharmacology 74:32–40. doi: 10.1016/j.neuropharm.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 28.Hunt DL, Castillo PE (2012) Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol 22 (3):496–508. doi: 10.1016/j.conb.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleckner NW, Dingledine R (1988) Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241 (4867):835–837 [DOI] [PubMed] [Google Scholar]
- 30.Benveniste M, Mayer ML (1991) Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys J 59 (3):560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clements JD, Westbrook GL (1994) Kinetics of AP5 dissociation from NMDA receptors: evidence for two identical cooperative binding sites. J Neurophysiol 71 (6):2566–2569 [DOI] [PubMed] [Google Scholar]
- 32.Anson LC, Chen PE, Wyllie DJA, Colquhoun D, Schoepfer R (1998) Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J Neurosci 18 (2):581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams K, Chao J, Kashiwagi K, Masuko T, Igarashi K (1996) Activation of N-methyl-D-aspartate receptors by glycine: role of an aspartate residue in the M3–M4 loop of the NR1 subunit. Mol Pharmacol 50 (4):701–708 [PubMed] [Google Scholar]
- 34.Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325 (6104):529–531. doi: 10.1038/325529a0 [DOI] [PubMed] [Google Scholar]
- 35.Clements JD, Westbrook GL (1991) Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 7 (4):605–613 [DOI] [PubMed] [Google Scholar]
- 36.Oliet SH, Mothet JP (2009) Regulation of N-methyl-D-aspartate receptors by astrocytic D-serine. Neuroscience 158 (1):275–283. doi: 10.1016/j.neuroscience.2008.01.071 [DOI] [PubMed] [Google Scholar]
- 37.Wolosker H (2007) NMDA receptor regulation by D-serine: new findings and perspectives. Mol Neurobiol 36 (2):152–164. doi: 10.1007/s12035-007-0038-6 [DOI] [PubMed] [Google Scholar]
- 38.Mothet JP, Le Bail M, Billard JM (2015) Time and space profiling of NMDA receptor co-agonist functions. J Neurochem 135 (2):210–225. doi: 10.1111/jnc.13204 [DOI] [PubMed] [Google Scholar]
- 39.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150 (3):633–646. doi: 10.1016/j.cell.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 40.Bergeron R, Meyer TM, Coyle JT, Greene RW (1998) Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci U S A 95 (26):15730–15734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billups D, Attwell D (2003) Active release of glycine or D-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur J Neurosci 18 (11):2975–2980 [DOI] [PubMed] [Google Scholar]
- 42.Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82 (2):279–293. doi: 10.1016/j.neuron.2014.03.030 [DOI] [PubMed] [Google Scholar]
- 43.Choi DW, Koh JY, Peters S (1988) Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci 8 (1):185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellren K, Lehmann A (1989) Calcium dependency of N-methyl-D-aspartate toxicity in slices from the immature rat hippocampus. Neuroscience 32 (2):371–379 [DOI] [PubMed] [Google Scholar]
- 45.Mody I, MacDonald JF (1995) NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci 16 (10):356–359 [DOI] [PubMed] [Google Scholar]
- 46.Wroge CM, Hogins J, Eisenman L, Mennerick S (2012) Synaptic NMDA receptors mediate hypoxic excitotoxic death. J Neurosci 32 (19):6732–6742. doi: 10.1523/JNEUROSCI.6371-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olney JW, Sharpe LG (1969) Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science 166 (3903):386–388 [DOI] [PubMed] [Google Scholar]
- 48.Surmeier DJ, Schumacker PT (2013) Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem 288 (15):10736–10741. doi: 10.1074/jbc.R112.410530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallett PJ, Standaert DG (2004) Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther 102 (2):155–174. doi: 10.1016/j.pharmthera.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 50.Low SJ, Roland CL (2004) Review of NMDA antagonist-induced neurotoxicity and implications for clinical development. Int J Clin Pharmacol Ther 42 (1):1–14 [DOI] [PubMed] [Google Scholar]
- 51.Javitt DC (2007) Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78:69–108. doi: 10.1016/S0074-7742(06)78003-5 [DOI] [PubMed] [Google Scholar]
- 52.Farber NB (2003) The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci 1003:119–130 [DOI] [PubMed] [Google Scholar]
- 53.Moghaddam B, Krystal JH (2012) Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull 38 (5):942–949. doi: 10.1093/schbul/sbs075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strong KL, Jing Y, Prosser AR, Traynelis SF, Liotta DC (2014) NMDA receptor modulators: an updated patent review (2013–2014). Expert Opin Ther Pat 24 (12):1349–1366. doi: 10.1517/13543776.2014.972938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogden KK, Traynelis SF (2011) New advances in NMDA receptor pharmacology. Trends Pharmacol Sci 32 (12):726–733. doi: 10.1016/j.tips.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu S, Paoletti P (2015) Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol 20:14–23. doi: 10.1016/j.coph.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 57.Divito CB, Underhill SM (2014) Excitatory amino acid transporters: roles in glutamatergic neurotransmission. Neurochem Int 73:172–180. doi: 10.1016/j.neuint.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL (1992) The time course of glutamate in the synaptic cleft. Science 258 (5087):1498–1501 [DOI] [PubMed] [Google Scholar]
- 59.Lester RA, Clements JD, Westbrook GL, Jahr CE (1990) Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature 346 (6284):565–567. doi: 10.1038/346565a0 [DOI] [PubMed] [Google Scholar]
- 60.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF (2005) Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol 563 (Pt 2):345–358. doi: 10.1113/jphysiol.2004.080028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lester RA, Jahr CE (1992) NMDA channel behavior depends on agonist affinity. J Neurosci 12 (2):635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JAH, Wolfe BB, Grayson DR (1998) Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol 79 (2):555–566 [DOI] [PubMed] [Google Scholar]
- 63.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12 (3):529–540 [DOI] [PubMed] [Google Scholar]
- 64.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256 (5060):1217–1221 [DOI] [PubMed] [Google Scholar]
- 65.Ulbrich MH, Isacoff EY (2007) Subunit counting in membrane-bound proteins. Nat Methods 4 (4):319–321. doi: 10.1038/nmeth1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X, Gouaux E (2014) NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511 (7508):191–197. doi: 10.1038/nature13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344 (6187):992–997. doi: 10.1126/science.1251915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kehoe LA, Bernardinelli Y, Muller D (2013) GluN3A: an NMDA receptor subunit with exquisite properties and functions. Neural Plast 2013:145387. doi: 10.1155/2013/145387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pachernegg S, Strutz-Seebohm N, Hollmann M (2012) GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci 35 (4):240–249. doi: 10.1016/j.tins.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 70.Henson MA, Roberts AC, Perez-Otano I, Philpot BD (2010) Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol 91 (1):23–37. doi: 10.1016/j.pneurobio.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavara NA, Hollmann M (2008) Shuffling the deck anew: how NR3 tweaks NMDA receptor function. Mol Neurobiol 38 (1):16–26. doi: 10.1007/s12035-008-8029-9 [DOI] [PubMed] [Google Scholar]
- 72.Low CM, Wee KS (2010) New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function. Mol Pharmacol 78 (1):1–11. doi: 10.1124/mol.110.064006 [DOI] [PubMed] [Google Scholar]
- 73.Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G, Heinemann S (1993) Zinc potentiates agonist-induced currents at certain splice variants of the NMDA receptor. Neuron 10 (5):943–954 [DOI] [PubMed] [Google Scholar]
- 74.Durand GM, Gregor P, Zheng X, Bennett MV, Uhl GR, Zukin RS (1992) Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci U S A 89 (19):9359–9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakanishi N, Axel R, Shneider NA (1992) Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A 89 (18):8552–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugihara H, Moriyoshi K, Ishii T, Masu M, Nakanishi S (1992) Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun 185 (3):826–832 [DOI] [PubMed] [Google Scholar]
- 77.Laurie DJ, Seeburg PH (1994) Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci 14 (5 Pt 2):3180–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paupard MC, Friedman LK, Zukin RS (1997) Developmental regulation and cell-specific expression of N-methyl-D-aspartate receptor splice variants in rat hippocampus. Neuroscience 79 (2):399–409 [DOI] [PubMed] [Google Scholar]
- 79.Zhong J, Carrozza DP, Williams K, Pritchett DB, Molinoff PB (1995) Expression of mRNAs encoding subunits of the NMDA receptor in developing rat brain. J Neurochem 64 (2):531–539 [DOI] [PubMed] [Google Scholar]
- 80.Traynelis SF, Burgess MF, Zheng F, Lyuboslavsky P, Powers JL (1998) Control of voltage-independent zinc inhibition of NMDA receptors by the NR1 subunit. J Neurosci 18 (16):6163–6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Traynelis SF, Hartley M, Heinemann SF (1995) Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268 (5212):873–876 [DOI] [PubMed] [Google Scholar]
- 82.Rumbaugh G, Prybylowski K, Wang JF, Vicini S (2000) Exon 5 and spermine regulate deactivation of NMDA receptor subtypes. J Neurophysiol 83 (3):1300–1306 [DOI] [PubMed] [Google Scholar]
- 83.Vance KM, Hansen KB, Traynelis SF (2012) GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J Physiol 590 (16):3857–3875. doi: 10.1113/jphysiol.2012.234062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y, Traynelis SF (2015) NMDA Receptors Containing the GluN2D Subunit Control Neuronal Function in the Subthalamic Nucleus. J Neurosci 35 (48):15971–15983. doi: 10.1523/JNEUROSCI.1702-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mott DD, Doherty JJ, Zhang S, Washburn MS, Fendley MJ, Lyuboslavsky P, Traynelis SF, Dingledine R (1998) Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat Neurosci 1 (8):659–667. doi: 10.1038/3661 [DOI] [PubMed] [Google Scholar]
- 86.Pahk AJ, Williams K (1997) Influence of extracellular pH on inhibition by ifenprodil at N-methyl-D-aspartate receptors in Xenopus oocytes. Neurosci Lett 225 (1):29–32 [DOI] [PubMed] [Google Scholar]
- 87.Durand GM, Bennett MV, Zukin RS (1993) Splice variants of the N-methyl-D-aspartate receptor NR1 identify domains involved in regulation by polyamines and protein kinase C. Proc Natl Acad Sci U S A 90 (14):6731–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Zheng X, Paupard MC, Wang AP, Santchi L, Friedman LK, Zukin RS, Bennett MV (1994) Spermine potentiation of recombinant N-methyl-D-aspartate receptors is affected by subunit composition. Proc Natl Acad Sci U S A 91 (23):10883–10887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD (2001) An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 21 (9):3063–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD (2003) Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron 40 (3):581–594 [DOI] [PubMed] [Google Scholar]
- 91.Scott DB, Blanpied TA, Ehlers MD (2003) Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology 45 (6):755–767 [DOI] [PubMed] [Google Scholar]
- 92.Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N (1994) Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol 347 (1):150–160. doi: 10.1002/cne.903470112 [DOI] [PubMed] [Google Scholar]
- 93.Watanabe M, Inoue Y, Sakimura K, Mishina M (1992) Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport 3 (12):1138–1140 [DOI] [PubMed] [Google Scholar]
- 94.Yuan H, Hansen KB, Vance KM, Ogden KK, Traynelis SF (2009) Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci 29 (39):12045–12058. doi: 10.1523/JNEUROSCI.1365-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, et al. (1993) Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem 268 (4):2836–2843 [PubMed] [Google Scholar]
- 96.Hackos DH, Hanson JE (2016) Diverse modes of NMDA receptor positive allosteric modulation: Mechanisms and consequences. Neuropharmacology. doi: 10.1016/j.neuropharm.2016.07.037 [DOI] [PubMed] [Google Scholar]
- 97.Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L (2014) Structure, function, and pharmacology of NMDA receptor channels. Physiol Res 63 Suppl 1:S191–203 [DOI] [PubMed] [Google Scholar]
- 98.Qian A, Buller AL, Johnson JW (2005) NR2 subunit-dependence of NMDA receptor channel block by external Mg2+. J Physiol 562 (Pt 2):319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuner T, Schoepfer R (1996) Multiple structural elements determine subunit specificity of Mg2+ block in NMDA receptor channels. J Neurosci 16 (11):3549–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paoletti P, Ascher P, Neyton J (1997) High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci 17 (15):5711–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen PE, Geballe MT, Katz E, Erreger K, Livesey MR, O’Toole KK, Le P, Lee CJ, Snyder JP, Traynelis SF, Wyllie DJ (2008) Modulation of glycine potency in rat recombinant NMDA receptors containing chimeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol 586 (1):227–245. doi: 10.1113/jphysiol.2007.143172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erreger K, Geballe MT, Kristensen A, Chen PE, Hansen KB, Lee CJ, Yuan H, Le P, Lyuboslavsky PN, Micale N, Jorgensen L, Clausen RP, Wyllie DJ, Snyder JP, Traynelis SF (2007) Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Mol Pharmacol 72 (4):907–920. doi: 10.1124/mol.107.037333 [DOI] [PubMed] [Google Scholar]
- 103.Hansen KB, Brauner-Osborne H, Egebjerg J (2008) Pharmacological characterization of ligands at recombinant NMDA receptor subtypes by electrophysiological recordings and intracellular calcium measurements. Comb Chem High Throughput Screen 11 (4):304–315. doi:Doi 10.2174/138620708784246040 [DOI] [PubMed] [Google Scholar]
- 104.Erreger K, Chen PE, Wyllie DJ, Traynelis SF (2004) Glutamate receptor gating. Crit Rev Neurobiol 16 (3):187–224 [DOI] [PubMed] [Google Scholar]
- 105.Wyllie DJ, Livesey MR, Hardingham GE (2013) Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 74:4–17. doi: 10.1016/j.neuropharm.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Glasgow NG, Siegler Retchless B, Johnson JW (2015) Molecular bases of NMDA receptor subtype-dependent properties. J Physiol 593 (1):83–95. doi: 10.1113/jphysiol.2014.273763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eriksson M, Nilsson A, Samuelsson H, Samuelsson EB, Mo L, Akesson E, Benedikz E, Sundstrom E (2007) On the role of NR3A in human NMDA receptors. Physiol Behav 92 (1–2):54–59. doi: 10.1016/j.physbeh.2007.05.026 [DOI] [PubMed] [Google Scholar]
- 108.Yao Y, Harrison CB, Freddolino PL, Schulten K, Mayer ML (2008) Molecular mechanism of ligand recognition by NR3 subtype glutamate receptors. EMBO J 27 (15):2158–2170. doi: 10.1038/emboj.2008.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yao Y, Mayer ML (2006) Characterization of a soluble ligand binding domain of the NMDA receptor regulatory subunit NR3A. J Neurosci 26 (17):4559–4566. doi: 10.1523/JNEUROSCI.0560-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nilsson A, Duan J, Mo-Boquist LL, Benedikz E, Sundstrom E (2007) Characterisation of the human NMDA receptor subunit NR3A glycine binding site. Neuropharmacology 52 (4):1151–1159. doi: 10.1016/j.neuropharm.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 111.Nilsson A, Eriksson M, Muly EC, Akesson E, Samuelsson EB, Bogdanovic N, Benedikz E, Sundstrom E (2007) Analysis of NR3A receptor subunits in human native NMDA receptors. Brain Res 1186:102–112. doi: 10.1016/j.brainres.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 112.Pilli J, Kumar SS (2012) Triheteromeric N-methyl-D-aspartate receptors differentiate synaptic inputs onto pyramidal neurons in somatosensory cortex: involvement of the GluN3A subunit. Neuroscience 222:75–88. doi: 10.1016/j.neuroscience.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 113.Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N (1998) Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393 (6683):377–381. doi: 10.1038/30748 [DOI] [PubMed] [Google Scholar]
- 114.Perez-Otano I, Schulteis CT, Contractor A, Lipton SA, Trimmer JS, Sucher NJ, Heinemann SF (2001) Assembly with the NR1 subunit is required for surface expression of NR3A-containing NMDA receptors. J Neurosci 21 (4):1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Perez-Otano I, Weinberg RJ, Philpot BD (2011) NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci 14 (3):338–344. doi: 10.1038/nn.2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Hallaq RA, Jarabek BR, Fu Z, Vicini S, Wolfe BB, Yasuda RP (2002) Association of NR3A with the N-methyl-D-aspartate receptor NR1 and NR2 subunits. Mol Pharmacol 62 (5):1119–1127 [DOI] [PubMed] [Google Scholar]
- 117.Matsuda K, Fletcher M, Kamiya Y, Yuzaki M (2003) Specific assembly with the NMDA receptor 3B subunit controls surface expression and calcium permeability of NMDA receptors. J Neurosci 23 (31):10064–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tong G, Takahashi H, Tu S, Shin Y, Talantova M, Zago W, Xia P, Nie Z, Goetz T, Zhang D, Lipton SA, Nakanishi N (2008) Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol 99 (1):122–132. doi: 10.1152/jn.01044.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsuda K, Kamiya Y, Matsuda S, Yuzaki M (2002) Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Mol Brain Res 100 (1–2):43–52. doi: 10.1016/S0169-328x(02)00173-0 [DOI] [PubMed] [Google Scholar]
- 120.Perez-Otano I, Lujan R, Tavalin SJ, Plomann M, Modregger J, Liu XB, Jones EG, Heinemann SF, Lo DC, Ehlers MD (2006) Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci 9 (5):611–621. doi: 10.1038/nn1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chowdhury D, Marco S, Brooks IM, Zandueta A, Rao Y, Haucke V, Wesseling JF, Tavalin SJ, Perez-Otano I (2013) Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J Neurosci 33 (9):4151–4164. doi: 10.1523/JNEUROSCI.2721-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan T, Mameli M, O’Connor EC, Dey PN, Verpelli C, Sala C, Perez-Otano I, Luscher C, Bellone C (2013) Expression of cocaine-evoked synaptic plasticity by GluN3A-containing NMDA receptors. Neuron 80 (4):1025–1038. doi: 10.1016/j.neuron.2013.07.050 [DOI] [PubMed] [Google Scholar]
- 123.Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D (2002) Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415 (6873):793–798. doi: 10.1038/nature715 [DOI] [PubMed] [Google Scholar]
- 124.Ulbrich MH, Isacoff EY (2008) Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci U S A 105 (37):14163–14168. doi: 10.1073/pnas.0802075105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smothers CT, Woodward JJ (2007) Pharmacological characterization of glycine-activated currents in HEK 293 cells expressing N-methyl-D-aspartate NR1 and NR3 subunits. J Pharmacol Exp Ther 322 (2):739–748. doi: 10.1124/jpet.107.123836 [DOI] [PubMed] [Google Scholar]
- 126.Sasaki YF, Rothe T, Premkumar LS, Das S, Cui J, Talantova MV, Wong HK, Gong X, Chan SF, Zhang D, Nakanishi N, Sucher NJ, Lipton SA (2002) Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cortical neurons. J Neurophysiol 87 (4):2052–2063. doi: 10.1152/jn.00531.2001 [DOI] [PubMed] [Google Scholar]
- 127.Madry C, Betz H, Geiger JR, Laube B (2010) Potentiation of Glycine-Gated NR1/NR3A NMDA Receptors Relieves Ca-Dependent Outward Rectification. Front Mol Neurosci 3:6. doi: 10.3389/fnmol.2010.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Madry C, Mesic I, Bartholomaus I, Nicke A, Betz H, Laube B (2007) Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function. Biochem Biophys Res Commun 354 (1):102–108. doi: 10.1016/j.bbrc.2006.12.153 [DOI] [PubMed] [Google Scholar]
- 129.Awobuluyi M, Yang J, Ye Y, Chatterton JE, Godzik A, Lipton SA, Zhang D (2007) Subunit-specific roles of glycine-binding domains in activation of NR1/NR3 N-methyl-D-aspartate receptors. Mol Pharmacol 71 (1):112–122. doi: 10.1124/mol.106.030700 [DOI] [PubMed] [Google Scholar]
- 130.Kvist T, Greenwood JR, Hansen KB, Traynelis SF, Brauner-Osborne H (2013) Structure-based discovery of antagonists for GluN3-containing N-methyl-D-aspartate receptors. Neuropharmacology 75:324–336. doi: 10.1016/j.neuropharm.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ (2007) NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27 (31):8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rauner C, Kohr G (2011) Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286 (9):7558–7566. doi: 10.1074/jbc.M110.182600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tovar KR, McGinley MJ, Westbrook GL (2013) Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci 33 (21):9150–9160. doi: 10.1523/JNEUROSCI.0829-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Luo JH, Wang YH, Yasuda RP, Dunah AW, Wolfe BB (1997) The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51 (1):79–86 [DOI] [PubMed] [Google Scholar]
- 135.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY (1994) Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368 (6467):144–147. doi: 10.1038/368144a0 [DOI] [PubMed] [Google Scholar]
- 136.Chazot PL, Stephenson FA (1997) Molecular dissection of native mammalian forebrain NMDA receptors containing the NR1 C2 exon: Direct demonstration of NMDA receptors comprising NR1, NR2A, and NR2B subunits within the same complex. J Neurochem 69 (5):2138–2144 [DOI] [PubMed] [Google Scholar]
- 137.Brickley SG, Misra C, Mok MH, Mishina M, Cull-Candy SG (2003) NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23 (12):4958–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pina-Crespo JC, Gibb AJ (2002) Subtypes of NMDA receptors in new-born rat hippocampal granule cells. J Physiol 541 (Pt 1):41–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones S, Gibb AJ (2005) Functional NR2B- and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J Physiol 569 (Pt 1):209–221. doi: 10.1113/jphysiol.2005.095554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chazot PL, Coleman SK, Cik M, Stephenson FA (1994) Molecular characterization of N-methyl-D-aspartate receptors expressed in mammalian cells yields evidence for the coexistence of three subunit types within a discrete receptor molecule. J Biol Chem 269 (39):24403–24409 [PubMed] [Google Scholar]
- 141.Cathala L, Misra C, Cull-Candy S (2000) Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci 20 (16):5899–5905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang Z, Gibb AJ (2014) Mg2+ block properties of triheteromeric GluN1-GluN2B-GluN2D NMDA receptors on neonatal rat substantia nigra pars compacta dopaminergic neurones. J Physiol 592 (10):2059–2078. doi: 10.1113/jphysiol.2013.267864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brothwell SL, Barber JL, Monaghan DT, Jane DE, Gibb AJ, Jones S (2008) NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol 586 (3):739–750. doi: 10.1113/jphysiol.2007.144618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sundstrom E, Whittemore S, Mo LL, Seiger A (1997) Analysis of NMDA receptors in the human spinal cord. Exp Neurol 148 (2):407–413 [DOI] [PubMed] [Google Scholar]
- 145.Dunah AW, Luo JH, Wang YH, Yasuda RP, Wolfe BB (1998) Subunit composition of N-methyl-D-aspartate receptors in the central nervous system that contain the NR2D subunit. Mol Pharmacol 53 (3):429–437 [DOI] [PubMed] [Google Scholar]
- 146.Dunah AW, Standaert DG (2003) Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem 85 (4):935–943 [DOI] [PubMed] [Google Scholar]
- 147.Lu CY, Fu ZY, Karavanov I, Yasuda RP, Wolfe BB, Buonanno A, Vicini S (2006) NMDA receptor subtypes at autaptic synapses of cerebellar granule neurons. J Neurophysiol 96 (5):2282–2294. doi: 10.1152/jn.00078.2006 [DOI] [PubMed] [Google Scholar]
- 148.Tovar KR, Westbrook GL (1999) The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19 (10):4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA (2011) Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron 71 (6):1085–1101. doi: 10.1016/j.neuron.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hatton CJ, Paoletti P (2005) Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46 (2):261–274. doi: 10.1016/j.neuron.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 151.Hansen KB, Ogden KK, Yuan H, Traynelis SF (2014) Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81 (5):1084–1096. doi: 10.1016/j.neuron.2014.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tang TT, Badger JD 2nd, Roche PA, Roche KW (2010) Novel approach to probe subunit-specific contributions to N-methyl-D-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem 285 (27):20975–20981. doi: 10.1074/jbc.M110.102210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stroebel D, Carvalho S, Grand T, Zhu S, Paoletti P (2014) Controlling NMDA receptor subunit composition using ectopic retention signals. J Neurosci 34 (50):16630–16636. doi: 10.1523/JNEUROSCI.2736-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheriyan J, Balsara RD, Hansen KB, Castellino FJ (2016) Pharmacology of triheteromeric N-Methyl-D-Aspartate Receptors. Neurosci Lett 617:240–246. doi: 10.1016/j.neulet.2016.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yi F, Mou T-C, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR, Hansen KB (2016) Structural basis for negative allosteric modulation of GluN2A-containing NMDA receptors. Neuron In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Serraz B, Grand T, Paoletti P (2016) Altered zinc sensitivity of NMDA receptors harboring clinically-relevant mutations. Neuropharmacology 109:196–204. doi: 10.1016/j.neuropharm.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 157.Hackos DH, Lupardus PJ, Grand T, Chen Y, Wang TM, Reynen P, Gustafson A, Wallweber HJ, Volgraf M, Sellers BD, Schwarz JB, Paoletti P, Sheng M, Zhou Q, Hanson JE (2016) Positive Allosteric Modulators of GluN2A-Containing NMDARs with Distinct Modes of Action and Impacts on Circuit Function. Neuron 89 (5):983–999. doi: 10.1016/j.neuron.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 158.Yi F, Mou TC, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR, Hansen KB (2016) Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors. Neuron 91 (6):1316–1329. doi: 10.1016/j.neuron.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yuan H, Hansen KB, Zhang J, Pierson TM, Markello TC, Fajardo KV, Holloman CM, Golas G, Adams DR, Boerkoel CF, Gahl WA, Traynelis SF (2014) Functional analysis of a de novo GRIN2A missense mutation associated with early-onset epileptic encephalopathy. Nat Commun 5:3251. doi: 10.1038/ncomms4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khatri A, Burger PB, Swanger SA, Hansen KB, Zimmerman S, Karakas E, Liotta DC, Furukawa H, Snyder JP, Traynelis SF (2014) Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator. Mol Pharmacol 86 (5):548–560. doi: 10.1124/mol.114.094516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tajima N, Karakas E, Grant T, Simorowski N, Diaz-Avalos R, Grigorieff N, Furukawa H (2016) Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature 534 (7605):63–68. doi: 10.1038/nature17679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu S, Stein RA, Yoshioka C, Lee CH, Goehring A, McHaourab HS, Gouaux E (2016) Mechanism of NMDA Receptor Inhibition and Activation. Cell 165 (3):704–714. doi: 10.1016/j.cell.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Furukawa H, Gouaux E (2003) Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J 22 (12):2873–2885. doi: 10.1093/emboj/cdg303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Furukawa H, Singh SK, Mancusso R, Gouaux E (2005) Subunit arrangement and function in NMDA receptors. Nature 438 (7065):185–192. doi: 10.1038/nature04089 [DOI] [PubMed] [Google Scholar]
- 165.Inanobe A, Furukawa H, Gouaux E (2005) Mechanism of partial agonist action at the NR1 subunit of NMDA receptors. Neuron 47 (1):71–84. doi: 10.1016/j.neuron.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 166.Jespersen A, Tajima N, Fernandez-Cuervo G, Garnier-Amblard EC, Furukawa H (2014) Structural insights into competitive antagonism in NMDA receptors. Neuron 81 (2):366–378. doi: 10.1016/j.neuron.2013.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hansen KB, Tajima N, Risgaard R, Perszyk RE, Jorgensen L, Vance KM, Ogden KK, Clausen RP, Furukawa H, Traynelis SF (2013) Structural determinants of agonist efficacy at the glutamate binding site of N-methyl-D-aspartate receptors. Mol Pharmacol 84 (1):114–127. doi: 10.1124/mol.113.085803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yao Y, Belcher J, Berger AJ, Mayer ML, Lau AY (2013) Conformational analysis of NMDA receptor GluN1, GluN2, and GluN3 ligand-binding domains reveals subtype-specific characteristics. Structure 21 (10):1788–1799. doi: 10.1016/j.str.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vance KM, Simorowski N, Traynelis SF, Furukawa H (2011) Ligand-specific deactivation time course of GluN1/GluN2D NMDA receptors. Nat Commun 2:294. doi: 10.1038/ncomms1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Volgraf M, Sellers BD, Jiang Y, Wu G, Ly CQ, Villemure E, Pastor RM, Yuen PW, Lu A, Luo X, Liu M, Zhang S, Sun L, Fu Y, Lupardus PJ, Wallweber HJ, Liederer BM, Deshmukh G, Plise E, Tay S, Reynen P, Herrington J, Gustafson A, Liu Y, Dirksen A, Dietz MG, Liu Y, Wang TM, Hanson JE, Hackos D, Scearce-Levie K, Schwarz JB (2016) Discovery of GluN2A-Selective NMDA Receptor Positive Allosteric Modulators (PAMs): Tuning Deactivation Kinetics via Structure-Based Design. J Med Chem 59 (6):2760–2779. doi: 10.1021/acs.jmedchem.5b02010 [DOI] [PubMed] [Google Scholar]
- 171.Kvist T, Steffensen TB, Greenwood JR, Mehrzad Tabrizi F, Hansen KB, Gajhede M, Pickering DS, Traynelis SF, Kastrup JS, Brauner-Osborne H (2013) Crystal structure and pharmacological characterization of a novel N-methyl-D-aspartate (NMDA) receptor antagonist at the GluN1 glycine binding site. J Biol Chem 288 (46):33124–33135. doi: 10.1074/jbc.M113.480210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pohlsgaard J, Frydenvang K, Madsen U, Kastrup JS (2011) Lessons from more than 80 structures of the GluA2 ligand-binding domain in complex with agonists, antagonists and allosteric modulators. Neuropharmacology 60 (1):135–150. doi: 10.1016/j.neuropharm.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 173.Kumar J, Mayer ML (2013) Functional insights from glutamate receptor ion channel structures. Annu Rev Physiol 75:313–337. doi: 10.1146/annurev-physiol-030212-183711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karakas E, Regan MC, Furukawa H (2015) Emerging structural insights into the function of ionotropic glutamate receptors. Trends Biochem Sci 40 (6):328–337. doi: 10.1016/j.tibs.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Borschel WF, Cummings KA, Tindell LK, Popescu GK (2015) Kinetic contributions to gating by interactions unique to N-methyl-D-aspartate (NMDA) receptors. J Biol Chem 290 (44):26846–26855. doi: 10.1074/jbc.M115.678656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aizenman E, Lipton SA, Loring RH (1989) Selective modulation of NMDA responses by reduction and oxidation. Neuron 2 (3):1257–1263 [DOI] [PubMed] [Google Scholar]
- 177.Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH (1994) NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron 12 (5):1031–1040 [DOI] [PubMed] [Google Scholar]
- 178.Choi YB, Lipton SA (2000) Redox modulation of the NMDA receptor. Cell Mol Life Sci 57 (11):1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang LH, Aizenman E (1993) The modulation of N-methyl-D-aspartate receptors by redox and alkylating reagents in rat cortical neurones in vitro. J Physiol 465:303–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA (1994) Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron 13 (4):929–936 [DOI] [PubMed] [Google Scholar]
- 181.Choi Y, Chen HV, Lipton SA (2001) Three pairs of cysteine residues mediate both redox and zn2+ modulation of the nmda receptor. J Neurosci 21 (2):392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Talukder I, Kazi R, Wollmuth LP (2011) GluN1-specific redox effects on the kinetic mechanism of NMDA receptor activation. Biophys J 101 (10):2389–2398. doi: 10.1016/j.bpj.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paganelli MA, Kussius CL, Popescu GK (2013) Role of cross-cleft contacts in NMDA receptor gating. PLoS ONE 8 (11):e80953. doi: 10.1371/journal.pone.0080953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kalbaugh TL, VanDongen HM, VanDongen AM (2004) Ligand-binding residues integrate affinity and efficacy in the NMDA receptor. Mol Pharmacol 66 (2):209–219. doi: 10.1124/mol.66.2.209 [DOI] [PubMed] [Google Scholar]
- 185.Dolino DM, Cooper D, Ramaswamy S, Jaurich H, Landes CF, Jayaraman V (2015) Structural dynamics of the glycine-binding domain of the N-methyl-D-aspartate receptor. J Biol Chem 290 (2):797–804. doi: 10.1074/jbc.M114.605436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dolino DM, Rezaei Adariani S, Shaikh SA, Jayaraman V, Sanabria H (2016) Conformational Selection and Submillisecond Dynamics of the Ligand-binding Domain of the N-Methyl-d-aspartate Receptor. J Biol Chem 291 (31):16175–16185. doi: 10.1074/jbc.M116.721274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dai J, Wollmuth LP, Zhou HX (2015) Mechanism-Based Mathematical Model for Gating of Ionotropic Glutamate Receptors. J Phys Chem B 119 (34):10934–10940. doi: 10.1021/acs.jpcb.5b00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dai J, Zhou HX (2015) Reduced curvature of ligand-binding domain free-energy surface underlies partial agonism at NMDA receptors. Structure 23 (1):228–236. doi: 10.1016/j.str.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Regan MC, Romero-Hernandez A, Furukawa H (2015) A structural biology perspective on NMDA receptor pharmacology and function. Curr Opin Struct Biol 33:68–75. doi: 10.1016/j.sbi.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Krupp JJ, Vissel B, Heinemann SF, Westbrook GL (1998) N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron 20 (2):317–327 [DOI] [PubMed] [Google Scholar]
- 191.Ogden KK, Traynelis SF (2013) Contribution of the M1 transmembrane helix and preM1 region to positive allosteric modulation and gating of N-methyl-D-aspartate receptors. Mol Pharmacol 83 (5):1045–1056. doi: 10.1124/mol.113.085209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Villarroel A, Regalado MP, Lerma J (1998) Glycine-independent NMDA receptor desensitization: localization of structural determinants. Neuron 20 (2):329–339 [DOI] [PubMed] [Google Scholar]
- 193.Ren H, Honse Y, Karp BJ, Lipsky RH, Peoples RW (2003) A site in the fourth membrane-associated domain of the N-methyl-D-aspartate receptor regulates desensitization and ion channel gating. J Biol Chem 278 (1):276–283 [DOI] [PubMed] [Google Scholar]
- 194.Schneggenburger R, Ascher P (1997) Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron 18 (1):167–177 [DOI] [PubMed] [Google Scholar]
- 195.Alsaloum M, Kazi R, Gan Q, Amin J, Wollmuth LP (2016) A Molecular Determinant of Subtype-Specific Desensitization in Ionotropic Glutamate Receptors. J Neurosci 36 (9):2617–2622. doi: 10.1523/JNEUROSCI.2667-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kazi R, Gan Q, Talukder I, Markowitz M, Salussolia CL, Wollmuth LP (2013) Asynchronous movements prior to pore opening in NMDA receptors. J Neurosci 33 (29):12052–12066. doi: 10.1523/JNEUROSCI.5780-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Talukder I, Borker P, Wollmuth LP (2010) Specific sites within the ligand-binding domain and ion channel linkers modulate NMDA receptor gating. J Neurosci 30 (35):11792–11804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chang HR, Kuo CC (2008) The activation gate and gating mechanism of the NMDA receptor. J Neurosci 28 (7):1546–1556. doi: 10.1523/JNEUROSCI.3485-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yuan H, Erreger K, Dravid SM, Traynelis SF (2005) Conserved structural and functional control of N-methyl-D-aspartate receptor gating by transmembrane domain M3. J Biol Chem 280 (33):29708–29716 [DOI] [PubMed] [Google Scholar]
- 200.Jones KS, VanDongen HMA, VanDongen AMJ (2002) The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci 22 (6):2044–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sobolevsky AI, Beck C, Wollmuth LP (2002) Molecular rearrangements of the extracellular vestibule in NMDAR channels during gating. Neuron 33 (1):75–85 [DOI] [PubMed] [Google Scholar]
- 202.Beck C, Wollmuth LP, Seeburg PH, Sakmann B, Kuner T (1999) NMDAR channel segments forming the extracellular vestibule inferred from the accessibility of substituted cysteines. Neuron 22 (3):559–570 [DOI] [PubMed] [Google Scholar]
- 203.Schorge S, Elenes S, Colquhoun D (2005) Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol 569 (Pt 2):395–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Popescu G, Robert A, Howe JR, Auerbach A (2004) Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature 430 (7001):790–793 [DOI] [PubMed] [Google Scholar]
- 205.Banke TG, Traynelis SF (2003) Activation of NR1/NR2B NMDA receptors. Nat Neurosci 6 (2):144–152. doi: 10.1038/nn1000 [DOI] [PubMed] [Google Scholar]
- 206.Zhou Y, Auerbach A (2005) Gating reaction mechanisms for NMDA receptor channels. J Neurosci 25 (35):7914–7923. doi: 10.1523/Jneurosci.1471-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kussius CL, Popescu GK (2009) Kinetic basis of partial agonism at NMDA receptors. Nat Neurosci 12 (9):1114–U1110. doi: 10.1038/nn.2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dravid SM, Prakash A, Traynelis SF (2008) Activation of recombinant NR1/NR2C NMDA receptors. J Physiol 586 (18):4425–4439. doi: 10.1113/jphysiol.2008.158634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Amico-Ruvio SA, Popescu GK (2010) Stationary gating of GluN1/GluN2B receptors in intact membrane patches. Biophys J 98 (7):1160–1169. doi: 10.1016/j.bpj.2009.12.4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Benveniste M, Clements J, Vyklicky L Jr., Mayer ML (1990) A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol 428:333–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Banke TG, Dravid SM, Traynelis SF (2005) Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci 25 (1):42–51. doi: 10.1523/JNEUROSCI.3154-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Erreger K, Traynelis SF (2008) Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J Physiol 586 (3):763–778. doi: 10.1113/jphysiol.2007.143941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK (2011) Zinc effects on NMDA receptor gating kinetics. Biophys J 100 (8):1910–1918. doi: 10.1016/j.bpj.2011.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kussius CL, Kaur N, Popescu GK (2009) Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J Neurosci 29 (21):6819–6827. doi: 10.1523/JNEUROSCI.0281-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Paganelli MA, Popescu GK (2015) Actions of bupivacaine, a widely used local anesthetic, on NMDA receptor responses. J Neurosci 35 (2):831–842. doi: 10.1523/JNEUROSCI.3578-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, Almonte A, Murray E, Mosely C, Barber J, French A, Balster R, Murray TF, Traynelis SF (2007) Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol 581 (Pt 1):107–128. doi: 10.1113/jphysiol.2006.124958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Blanpied TA, Boeckman FA, Aizenman E, Johnson JW (1997) Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol 77 (1):309–323 [DOI] [PubMed] [Google Scholar]
- 218.Blanpied TA, Clarke RJ, Johnson JW (2005) Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 25 (13):3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huettner JE, Bean BP (1988) Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A 85 (4):1307–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rosenmund C, Stern-Bach Y, Stevens CF (1998) The tetrameric structure of a glutamate receptor channel. Science 280 (5369):1596–1599 [DOI] [PubMed] [Google Scholar]
- 221.Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E (2003) Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci 6 (8):803–810. doi: 10.1038/nn1091 [DOI] [PubMed] [Google Scholar]
- 222.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF (2011) Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci 14 (6):727–735. doi: 10.1038/nn.2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Erreger K, Geballe MT, Dravid SM, Snyder JP, Wyllie DJA, Traynelis SF (2005) Mechanism of partial agonism at NMDA receptors for a conformationally restricted glutamate analog. J Neurosci 25 (34):7858–7866. doi: 10.1523/Jneurosci.1613-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wollmuth LP, Sobolevsky AI (2004) Structure and gating of the glutamate receptor ion channel. Trends Neurosci 27 (6):321–328. doi: 10.1016/j.tins.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 225.Sobolevsky AI, Rooney L, Wollmuth LP (2002) Staggering of subunits in NMDAR channels. Biophys J 83 (6):3304–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wollmuth LP, Kuner T, Seeburg PH, Sakmann B (1996) Differential contribution of the NR1- and NR2A-subunits to the selectivity filter of recombinant NMDA receptor channels. J Physiol 491 (Pt 3):779–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Gunther W, Seeburg PH, Sakmann B (1992) Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science 257 (5075):1415–1419 [DOI] [PubMed] [Google Scholar]
- 228.Wollmuth LP, Kuner T, Sakmann B (1998) Adjacent asparagines in the NR2-subunit of the NMDA receptor channel control the voltage-dependent block by extracellular Mg2+. J Physiol 506 (Pt 1):13–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sharma G, Stevens CF (1996) Interactions between two divalent ion binding sites in N-methyl-D-aspartate receptor channels. Proc Natl Acad Sci U S A 93 (24):14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schneggenburger R (1996) Simultaneous measurement of Ca2+ influx and reversal potentials in recombinant N-methyl-D-aspartate receptor channels. Biophys J 70 (5):2165–2174. doi: 10.1016/S0006-3495(96)79782-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schneggenburger R (1998) Altered voltage dependence of fractional Ca2+ current in N-methyl-D-aspartate channel pore mutants with a decreased Ca2+ permeability. Biophys J 74 (4):1790–1794. doi: 10.1016/S0006-3495(98)77889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Burnashev N, Zhou Z, Neher E, Sakmann B (1995) Fractional Calcium Currents through Recombinant Glur Channels of the Nmda, Ampa and Kainate Receptor Subtypes. J Physiol-London 485 (2):403–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jatzke C, Watanabe J, Wollmuth LP (2002) Voltage and concentration dependence of Ca(2+) permeability in recombinant glutamate receptor subtypes. J Physiol 538 (Pt 1):25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Premkumar LS, Auerbach A (1996) Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron 16 (4):869–880 [DOI] [PubMed] [Google Scholar]
- 235.Premkumar LS, Qin F, Auerbach A (1997) Subconductance states of a mutant NMDA receptor channel kinetics, calcium, and voltage dependence. J Gen Physiol 109 (2):181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D (1996) Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc Biol Sci 263 (1373):1079–1086. doi: 10.1098/rspb.1996.0159 [DOI] [PubMed] [Google Scholar]
- 237.Watanabe J, Beck C, Kuner T, Premkumar LS, Wollmuth LP (2002) DRPEER: a motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J Neurosci 22 (23):10209–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wada A, Takahashi H, Lipton SA, Chen HS (2006) NR3A modulates the outer vestibule of the “NMDA” receptor channel. J Neurosci 26 (51):13156–13166. doi: 10.1523/JNEUROSCI.2552-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kawajiri S, Dingledine R (1993) Multiple structural determinants of voltage-dependent magnesium block in recombinant NMDA receptors. Neuropharmacology 32 (11):1203–1211 [DOI] [PubMed] [Google Scholar]
- 240.Vogt K, Mellor J, Tong G, Nicoll R (2000) The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26 (1):187–196 [DOI] [PubMed] [Google Scholar]
- 241.Christine CW, Choi DW (1990) Effect of zinc on NMDA receptor-mediated channel currents in cortical neurons. J Neurosci 10 (1):108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Clarke RJ, Johnson JW (2006) NMDA receptor NR2 subunit dependence of the slow component of magnesium unblock. J Neurosci 26 (21):5825–5834. doi: 10.1523/JNEUROSCI.0577-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Siegler Retchless B, Gao W, Johnson JW (2012) A single GluN2 subunit residue controls NMDA receptor channel properties via intersubunit interaction. Nat Neurosci 15 (3):406–413, S401–402. doi: 10.1038/nn.3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Qian A, Johnson JW (2006) Permeant ion effects on external Mg2+ block of NR1/2D NMDA receptors. J Neurosci 26 (42):10899–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Antonov SM, Johnson JW (1999) Permeant ion regulation of N-methyl-D-aspartate receptor channel block by Mg(2+). Proc Natl Acad Sci U S A 96 (25):14571–14576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Qian A, Antonov SM, Johnson JW (2002) Modulation by permeant ions of Mg(2+) inhibition of NMDA-activated whole-cell currents in rat cortical neurons. J Physiol 538 (Pt 1):65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhu Y, Auerbach A (2001) K(+) occupancy of the N-methyl-d-aspartate receptor channel probed by Mg(2+) block. J Gen Physiol 117 (3):287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhu Y, Auerbach A (2001) Na(+) occupancy and Mg(2+) block of the n-methyl-d-aspartate receptor channel. J Gen Physiol 117 (3):275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Brackley PT, Bell DR, Choi SK, Nakanishi K, Usherwood PN (1993) Selective antagonism of native and cloned kainate and NMDA receptors by polyamine-containing toxins. J Pharmacol Exp Ther 266 (3):1573–1580 [PubMed] [Google Scholar]
- 250.Parsons CG, Quack G, Bresink I, Baran L, Przegalinski E, Kostowski W, Krzascik P, Hartmann S, Danysz W (1995) Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 34 (10):1239–1258 [DOI] [PubMed] [Google Scholar]
- 251.Sobolevsky AI (1999) Two-component blocking kinetics of open NMDA channels by organic cations. Biochim Biophys Acta 1416 (1–2):69–91 [DOI] [PubMed] [Google Scholar]
- 252.Barygin OI, Gmiro VE, Kim K, Magazanik LG, Tikhonov DB (2009) Blockade of NMDA receptor channels by 9-aminoacridine and its derivatives. Neurosci Lett 451 (1):29–33. doi: 10.1016/j.neulet.2008.12.036 [DOI] [PubMed] [Google Scholar]
- 253.Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG (2003) Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem 87 (1):56–65 [DOI] [PubMed] [Google Scholar]
- 254.Benveniste M, Mayer ML (1995) Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol 483 (Pt 2):367–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Johnson JW, Glasgow NG, Povysheva NV (2015) Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol 20:54–63. doi: 10.1016/j.coph.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen HS, Lipton SA (1997) Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol 499 (Pt 1):27–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mealing GA, Lanthorn TH, Murray CL, Small DL, Morley P (1999) Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther 288 (1):204–210 [PubMed] [Google Scholar]
- 258.Kotermanski SE, Wood JT, Johnson JW (2009) Memantine binding to a superficial site on NMDA receptors contributes to partial trapping. J Physiol 587 (Pt 19):4589–4604. doi: 10.1113/jphysiol.2009.176297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sobolevsky AI, Yelshansky MV (2000) The trapping block of NMDA receptor channels in acutely isolated rat hippocampal neurones. J Physiol 526 Pt 3:493–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kotermanski SE, Johnson JW (2009) Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci 29 (9):2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Parsons CG, Gruner R, Rozental J, Millar J, Lodge D (1993) Patch clamp studies on the kinetics and selectivity of N-methyl-D-aspartate receptor antagonism by memantine (1-amino-3,5-dimethyladamantan). Neuropharmacology 32 (12):1337–1350 [DOI] [PubMed] [Google Scholar]
- 262.Chen HS, Lipton SA (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97 (6):1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x [DOI] [PubMed] [Google Scholar]
- 263.Karakas E, Simorowski N, Furukawa H (2009) Structure of the zinc-bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J 28 (24):3910–3920. doi: 10.1038/emboj.2009.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Karakas E, Simorowski N, Furukawa H (2011) Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature 475 (7355):249–253. doi: 10.1038/nature10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sobolevsky AI (2015) Structure and gating of tetrameric glutamate receptors. J Physiol 593 (1):29–38. doi: 10.1113/jphysiol.2013.264911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Meyerson JR, Kumar J, Chittori S, Rao P, Pierson J, Bartesaghi A, Mayer ML, Subramaniam S (2014) Structural mechanism of glutamate receptor activation and desensitization. Nature 514 (7522):328–334. doi: 10.1038/nature13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hansen KB, Furukawa H, Traynelis SF (2010) Control of assembly and function of glutamate receptors by the amino-terminal domain. Mol Pharmacol 78 (4):535–549. doi: 10.1124/mol.110.067157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Dutta A, Shrivastava IH, Sukumaran M, Greger IH, Bahar I (2012) Comparative dynamics of NMDA- and AMPA-glutamate receptor N-terminal domains. Structure 20 (11):1838–1849. doi: 10.1016/j.str.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sukumaran M, Rossmann M, Shrivastava I, Dutta A, Bahar I, Greger IH (2011) Dynamics and allosteric potential of the AMPA receptor N-terminal domain. The EMBO journal 30 (5):972–982. doi: 10.1038/emboj.2011.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Pasternack A, Coleman SK, Jouppila A, Mottershead DG, Lindfors M, Pasternack M, Keinanen K (2002) Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J Biol Chem 277 (51):49662–49667. doi: 10.1074/jbc.M208349200 [DOI] [PubMed] [Google Scholar]
- 271.Gielen M, Siegler Retchless B, Mony L, Johnson JW, Paoletti P (2009) Mechanism of differential control of NMDA receptor activity by NR2 subunits. Nature 459 (7247):703–707. doi: 10.1038/nature07993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhu S, Stroebel D, Yao CA, Taly A, Paoletti P (2013) Allosteric signaling and dynamics of the clamshell-like NMDA receptor GluN1 N-terminal domain. Nat Struct Mol Biol 20 (4):477–485. doi: 10.1038/nsmb.2522 [DOI] [PubMed] [Google Scholar]
- 273.Gielen M, Le Goff A, Stroebel D, Johnson JW, Neyton J, Paoletti P (2008) Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron 57 (1):80–93. doi: 10.1016/j.neuron.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhu S, Riou M, Yao CA, Carvalho S, Rodriguez PC, Bensaude O, Paoletti P, Ye S (2014) Genetically encoding a light switch in an ionotropic glutamate receptor reveals subunit-specific interfaces. Proc Natl Acad Sci U S A 111 (16):6081–6086. doi: 10.1073/pnas.1318808111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Choi YB, Lipton SA (1999) Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron 23 (1):171–180 [DOI] [PubMed] [Google Scholar]
- 276.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J (2000) Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron 25 (3):683–694 [DOI] [PubMed] [Google Scholar]
- 277.Low CM, Zheng F, Lyuboslavsky P, Traynelis SF (2000) Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors. Proc Natl Acad Sci U S A 97 (20):11062–11067. doi: 10.1073/pnas.180307497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Williams K (1996) Separating dual effects of zinc at recombinant N-methyl-D-aspartate receptors. Neurosci Lett 215 (1):9–12 [DOI] [PubMed] [Google Scholar]
- 279.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P (2005) The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci 25 (2):308–317. doi: 10.1523/JNEUROSCI.3967-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J (2000) Molecular organization of a zinc binding n-terminal modulatory domain in a NMDA receptor subunit. Neuron 28 (3):911–925 [DOI] [PubMed] [Google Scholar]
- 281.Stroebel D, Buhl DL, Knafels JD, Chanda PK, Green M, Sciabola S, Mony L, Paoletti P, Pandit J (2016) A novel binding mode reveals two distinct classes of NMDA receptor GluN2B-selective antagonists. Mol Pharmacol. doi: 10.1124/mol.115.103036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Burger PB, Yuan H, Karakas E, Geballe M, Furukawa H, Liotta DC, Snyder JP, Traynelis SF (2012) Mapping the binding of GluN2B-selective N-methyl-D-aspartate receptor negative allosteric modulators. Mol Pharmacol 82 (2):344–359. doi: 10.1124/mol.112.078568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mony L, Zhu S, Carvalho S, Paoletti P (2011) Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J 30 (15):3134–3146. doi: 10.1038/emboj.2011.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sirrieh RE, MacLean DM, Jayaraman V (2015) Subtype-dependent N-methyl-D-aspartate receptor amino-terminal domain conformations and modulation by spermine. J Biol Chem 290 (20):12812–12820. doi: 10.1074/jbc.M115.649723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Paoletti P, Neyton J, Ascher P (1995) Glycine-independent and subunit-specific potentiation of NMDA responses by extracellular Mg2+. Neuron 15 (5):1109–1120 [DOI] [PubMed] [Google Scholar]
- 286.Meddows E, Le Bourdelles B, Grimwood S, Wafford K, Sandhu S, Whiting P, McIlhinney RAJ (2001) Identification of molecular determinants that are important in the assembly of N-methyl-D-aspartate receptors. J Biol Chem 276 (22):18795–18803. doi:DOI 10.1074/jbc.M101382200 [DOI] [PubMed] [Google Scholar]
- 287.Schorge S, Colquhoun D (2003) Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci 23 (4):1151–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Papadakis M, Hawkins LM, Stephenson FA (2004) Appropriate NR1-NR1 disulfide-linked homodimer formation is requisite for efficient expression of functional, cell surface N-methyl-D-aspartate NR1/NR2 receptors. J Biol Chem 279 (15):14703–14712 [DOI] [PubMed] [Google Scholar]
- 289.Qiu S, Hua YL, Yang F, Chen YZ, Luo JH (2005) Subunit assembly of N-methyl-d-aspartate receptors analyzed by fluorescence resonance energy transfer. J Biol Chem 280 (26):24923–24930 [DOI] [PubMed] [Google Scholar]
- 290.Schuler T, Mesic I, Madry C, Bartholomaus I, Laube B (2008) Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly. J Biol Chem 283 (1):37–46. doi: 10.1074/jbc.M703539200 [DOI] [PubMed] [Google Scholar]
- 291.Atlason PT, Garside ML, Meddows E, Whiting P, McIlhinney RA (2007) N-Methyl-D-aspartate (NMDA) receptor subunit NR1 forms the substrate for oligomeric assembly of the NMDA receptor. J Biol Chem 282 (35):25299–25307. doi: 10.1074/jbc.M702778200 [DOI] [PubMed] [Google Scholar]
- 292.Farina AN, Blain KY, Maruo T, Kwiatkowski W, Choe S, Nakagawa T (2011) Separation of domain contacts is required for heterotetrameric assembly of functional NMDA receptors. J Neurosci 31 (10):3565–3579. doi: 10.1523/JNEUROSCI.6041-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Greger IH, Ziff EB, Penn AC (2007) Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci 30 (8):407–416. doi: 10.1016/j.tins.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 294.Sukumaran M, Penn AC, Greger IH (2012) AMPA receptor assembly: atomic determinants and built-in modulators. Adv Exp Med Biol 970:241–264. doi: 10.1007/978-3-7091-0932-8_11 [DOI] [PubMed] [Google Scholar]
- 295.Qiu S, Zhang XM, Cao JY, Yang W, Yan YG, Shan L, Zheng J, Luo JH (2009) An endoplasmic reticulum retention signal located in the extracellular amino-terminal domain of the NR2A subunit of N-Methyl-D-aspartate receptors. J BiolChem 284 (30):20285–20298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mayer ML, Vyklicky L Jr., Clements J (1989) Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature 338 (6214):425–427. doi: 10.1038/338425a0 [DOI] [PubMed] [Google Scholar]
- 297.Lester RAJ, Tong G, Jahr CE (1993) Interactions between the Glycine and Glutamate Binding-Sites of the Nmda Receptor. J Neurosci 13 (3):1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen N, Moshaver A, Raymond LA (1997) Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol 51 (6):1015–1023 [DOI] [PubMed] [Google Scholar]
- 299.Erreger K, Traynelis SF (2005) Allosteric interaction between zinc and glutamate binding domains on NR2A causes desensitization of NMDA receptors. J Physiol-London 569 (2):381–393. doi: 10.1113/jphysiol.2005.095497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zheng F, Erreger K, Low CM, Banke T, Lee CJ, Conn PJ, Traynelis SF (2001) Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci 4 (9):894–901 [DOI] [PubMed] [Google Scholar]
- 301.Clark GD, Clifford DB, Zorumski CF (1990) The effect of agonist concentration, membrane voltage and calcium on N-methyl-D-aspartate receptor desensitization. Neuroscience 39 (3):787–797 [DOI] [PubMed] [Google Scholar]
- 302.Legendre P, Rosenmund C, Westbrook GL (1993) Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci 13 (2):674–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Rosenmund C, Feltz A, Westbrook GL (1995) Calcium-dependent inactivation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol 73 (1):427–430 [DOI] [PubMed] [Google Scholar]
- 304.Vyklicky L Jr. (1993) Calcium-mediated modulation of N-methyl-D-aspartate (NMDA) responses in cultured rat hippocampal neurones. J Physiol 470:575–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Krupp JJ, Vissel B, Heinemann SF, Westbrook GL (1996) Calcium-dependent inactivation of recombinant N-methyl-D-aspartate receptors is NR2 subunit specific. Mol Pharmacol 50 (6):1680–1688 [PubMed] [Google Scholar]
- 306.Medina I, Filippova N, Charton G, Rougeole S, Ben-Ari Y, Khrestchatisky M, Bregestovski P (1995) Calcium-dependent inactivation of heteromeric NMDA receptor-channels expressed in human embryonic kidney cells. J Physiol 482 (Pt 3):567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rosenmund C, Westbrook GL (1993) Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron 10 (5):805–814 [DOI] [PubMed] [Google Scholar]
- 308.Ehlers MD, Fung ET, O’Brien RJ, Huganir RL (1998) Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci 18 (2):720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ehlers MD, Zhang S, Bernhadt JP, Huganir RL (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84 (5):745–755 [DOI] [PubMed] [Google Scholar]
- 310.Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL (1999) Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci 19 (4):1165–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL (1998) Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron 21 (2):443–453 [DOI] [PubMed] [Google Scholar]
- 312.Sather W, Dieudonne S, MacDonald JF, Ascher P (1992) Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol 450:643–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sather W, Johnson JW, Henderson G, Ascher P (1990) Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron 4 (5):725–731 [DOI] [PubMed] [Google Scholar]
- 314.Chen NS, Li B, Murphy TH, Raymond LA (2004) Site within N-methyl-D-aspartate receptor pore modulates channel Gating. Mol Pharmacol 65 (1):157–164. doi:DOI 10.1124/mol.65.1.157 [DOI] [PubMed] [Google Scholar]
- 315.Hu B, Zheng F (2005) Molecular determinants of glycine-independent desensitization of NR1/NR2A receptors. J Pharmacol Exp Ther 313 (2):563–569. doi: 10.1124/jpet.104.080168 [DOI] [PubMed] [Google Scholar]
- 316.Giffard RG, Monyer H, Christine CW, Choi DW (1990) Acidosis reduces NMDA receptor activation, glutamate neurotoxicity, and oxygen-glucose deprivation neuronal injury in cortical cultures. Brain Res 506 (2):339–342 [DOI] [PubMed] [Google Scholar]
- 317.Traynelis SF, Cull-Candy SG (1990) Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345 (6273):347–350 [DOI] [PubMed] [Google Scholar]
- 318.Traynelis SF, Cull-Candy SG (1991) Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol 433:727–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Vyklicky L Jr., Vlachova V, Krusek J (1990) The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol 430:497–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83 (4):1183–1221. doi: 10.1152/physrev.00010.2003 [DOI] [PubMed] [Google Scholar]
- 321.Low CM, Lyuboslavsky P, French A, Le P, Wyatte K, Thiel WH, Marchan EM, Igarashi K, Kashiwagi K, Gernert K, Williams K, Traynelis SF, Zheng F (2003) Molecular determinants of proton-sensitive N-methyl-D-aspartate receptor gating. Mol Pharmacol 63 (6):1212–1222. doi: 10.1124/mol.63.6.1212 [DOI] [PubMed] [Google Scholar]
- 322.Kashiwagi K, Fukuchi J, Chao J, Igarashi K, Williams K (1996) An aspartate residue in the extracellular loop of the N-methyl-D-aspartate receptor controls sensitivity to spermine and protons. Mol Pharmacol 49 (6):1131–1141 [PubMed] [Google Scholar]
- 323.Kashiwagi K, Pahk AJ, Masuko T, Igarashi K, Williams K (1997) Block and modulation of N-methyl-D-aspartate receptors by polyamines and protons: role of amino acid residues in the transmembrane and pore-forming regions of NR1 and NR2 subunits. Mol Pharmacol 52 (4):701–713 [DOI] [PubMed] [Google Scholar]
- 324.Bhatt JM, Prakash A, Suryavanshi PS, Dravid SM (2013) Effect of ifenprodil on GluN1/GluN2B N-methyl-D-aspartate receptor gating. Mol Pharmacol 83 (1):9–21. doi: 10.1124/mol.112.080952 [DOI] [PubMed] [Google Scholar]
- 325.Anderson CT, Radford RJ, Zastrow ML, Zhang DY, Apfel UP, Lippard SJ, Tzounopoulos T (2015) Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc Natl Acad Sci U S A 112 (20):E2705–2714. doi: 10.1073/pnas.1503348112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sanz-Clemente A, Nicoll RA, Roche KW (2013) Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19 (1):62–75. doi: 10.1177/1073858411435129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lussier MP, Sanz-Clemente A, Roche KW (2015) Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications. J Biol Chem 290 (48):28596–28603. doi: 10.1074/jbc.R115.652750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sans N, Petralia RS, Wang YX, Blahos J, Hell JW, Wenthold RJ (2000) A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci 20 (3):1260–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D (2006) NMDA receptor surface mobility depends on NR2A-2B subunits. Proc Natl Acad Sci U S A 103 (49):18769–18774. doi: 10.1073/pnas.0605238103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G (2000) C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci 20 (12):4573–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Martel MA, Wyllie DJ, Hardingham GE (2009) In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience 158 (1):334–343. doi: 10.1016/j.neuroscience.2008.01.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB (1996) Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 271 (49):31670–31678 [DOI] [PubMed] [Google Scholar]
- 333.Raveendran R, Devi Suma Priya S, Mayadevi M, Steephan M, Santhoshkumar TR, Cheriyan J, Sanalkumar R, Pradeep KK, James J, Omkumar RV (2009) Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J Neurochem 110 (1):92–105. doi: 10.1111/j.1471-4159.2009.06108.x [DOI] [PubMed] [Google Scholar]
- 334.Rellos P, Pike AC, Niesen FH, Salah E, Lee WH, von Delft F, Knapp S (2010) Structure of the CaMKIIdelta/calmodulin complex reveals the molecular mechanism of CaMKII kinase activation. PLoS Biol 8 (7):e1000426. doi: 10.1371/journal.pbio.1000426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Strack S, Colbran RJ (1998) Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J Biol Chem 273 (33):20689–20692 [DOI] [PubMed] [Google Scholar]
- 336.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411 (6839):801–805. doi: 10.1038/35081080 [DOI] [PubMed] [Google Scholar]
- 337.Bayer KU, LeBel E, McDonald GL, O’Leary H, Schulman H, De Koninck P (2006) Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci 26 (4):1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J (2004) Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci 24 (42):9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lisman J, Yasuda R, Raghavachari S (2012) Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13 (3):169–182. doi: 10.1038/nrn3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen BS, Roche KW (2007) Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53 (3):362–368. doi: 10.1016/j.neuropharm.2007.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Li S, Tian X, Hartley DM, Feig LA (2006) Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci 26 (6):1721–1729. doi: 10.1523/JNEUROSCI.3990-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tian X, Gotoh T, Tsuji K, Lo EH, Huang S, Feig LA (2004) Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. EMBO J 23 (7):1567–1575. doi: 10.1038/sj.emboj.7600151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40 (4):775–784 [DOI] [PubMed] [Google Scholar]
- 344.Dore K, Aow J, Malinow R (2016) The Emergence of NMDA Receptor Metabotropic Function: Insights from Imaging. Front Synaptic Neurosci 8:20. doi: 10.3389/fnsyn.2016.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Gray JA, Zito K, Hell JW (2016) Non-ionotropic signaling by the NMDA receptor: controversy and opportunity. F1000Res 5. doi: 10.12688/f1000research.8366.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW (2003) Glycine binding primes NMDA receptor internalization. Nature 422 (6929):302–307. doi: 10.1038/nature01497 [DOI] [PubMed] [Google Scholar]
- 347.Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, Malinow R (2013) Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A 110 (10):4027–4032. doi: 10.1073/pnas.1219454110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Aow J, Dore K, Malinow R (2015) Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc Natl Acad Sci U S A 112 (47):14711–14716. doi: 10.1073/pnas.1520029112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Williams K (1993) Ifenprodil Discriminates Subtypes of the N-Methyl-D-Aspartate Receptor - Selectivity and Mechanisms at Recombinant Heteromeric Receptors. Mol Pharmacol 44 (4):851–859 [PubMed] [Google Scholar]
- 350.Santangelo RM, Acker TM, Zimmerman SS, Katzman BM, Strong KL, Traynelis SF, Liotta DC (2012) Novel NMDA receptor modulators: an update. Expert Opin Ther Pat 22 (11):1337–1352. doi: 10.1517/13543776.2012.728587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M (2002) 5-phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett 12 (7):1099–1102. doi: 10.1016/S0960-894x(02)00074-4 [DOI] [PubMed] [Google Scholar]
- 352.Frizelle PA, Chen PE, Wyllie DJ (2006) Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquino xalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol 70 (3):1022–1032 [DOI] [PubMed] [Google Scholar]
- 353.Neyton J, Paoletti P (2006) Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26 (5):1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Bettini E, Sava A, Griffante C, Carignani C, Buson A, Capelli AM, Negri M, Andreetta F, Senar-Sancho SA, Guiral L, Cardullo F (2010) Identification and characterization of novel NMDA receptor antagonists selective for NR2A- over NR2B-containing receptors. J Pharmacol Exp Ther 335 (3):636–644. doi: 10.1124/jpet.110.172544 [DOI] [PubMed] [Google Scholar]
- 355.Edman S, McKay S, Macdonald LJ, Samadi M, Livesey MR, Hardingham GE, Wyllie DJ (2012) TCN 201 selectively blocks GluN2A-containing NMDARs in a GluN1 co-agonist dependent but non-competitive manner. Neuropharmacology 63 (3):441–449. doi: 10.1016/j.neuropharm.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Hansen KB, Ogden KK, Traynelis SF (2012) Subunit-selective allosteric inhibition of glycine binding to NMDA receptors. J Neurosci 32 (18):6197–6208. doi: 10.1523/JNEUROSCI.5757-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Volkmann RA, Fanger CM, Anderson DR, Sirivolu VR, Paschetto K, Gordon E, Virginio C, Gleyzes M, Buisson B, Steidl E, Mierau SB, Fagiolini M, Menniti FS (2016) MPX-004 and MPX-007: New Pharmacological Tools to Study the Physiology of NMDA Receptors Containing the GluN2A Subunit. PLoS ONE 11 (2):e0148129. doi: 10.1371/journal.pone.0148129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.McKay S, Griffiths NH, Butters PA, Thubron EB, Hardingham GE, Wyllie DJ (2012) Direct pharmacological monitoring of the developmental switch in NMDA receptor subunit composition using TCN 213, a GluN2A-selective, glycine-dependent antagonist. Br J Pharmacol 166 (3):924–937. doi: 10.1111/j.1476-5381.2011.01748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Izumi Y, Zorumski CF (2015) Sensitivity of N-methyl-D-aspartate receptor-mediated excitatory postsynaptic potentials and synaptic plasticity to TCN 201 and TCN 213 in rat hippocampal slices. J Pharmacol Exp Ther 352 (2):267–273. doi: 10.1124/jpet.114.220582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A 110 (22):9124–9129. doi: 10.1073/pnas.1220591110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Bu F, Du R, Li Y, Quinn JP, Wang M (2016) NR2A contributes to genesis and propagation of cortical spreading depression in rats. Scientific reports 6:23576. doi: 10.1038/srep23576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Masuko T, Kashiwagi K, Kuno T, Nguyen ND, Pahk AJ, Fukuchi J, Igarashi K, Williams K (1999) A regulatory domain (R1–R2) in the amino terminus of the N-methyl-D-aspartate receptor: effects of spermine, protons, and ifenprodil, and structural similarity to bacterial leucine/isoleucine/valine binding protein. Mol Pharmacol 55 (6):957–969 [DOI] [PubMed] [Google Scholar]
- 363.Kew JN, Trube G, Kemp JA (1996) A novel mechanism of activity-dependent NMDA receptor antagonism describes the effect of ifenprodil in rat cultured cortical neurones. J Physiol 497 (Pt 3):761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Chenard BL, Bordner J, Butler TW, Chambers LK, Collins MA, De Costa DL, Ducat MF, Dumont ML, Fox CB, Mena EE, et al. (1995) (1S,2S)-1-(4-hydroxyphenyl)-2-(4-hydroxy-4-phenylpiperidino)-1-propanol: a potent new neuroprotectant which blocks N-methyl-D-aspartate responses. J Med Chem 38 (16):3138–3145 [DOI] [PubMed] [Google Scholar]
- 365.Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA (1997) Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283 (3):1285–1292 [PubMed] [Google Scholar]
- 366.Yuan H, Myers SJ, Wells G, Nicholson KL, Swanger SA, Lyuboslavsky P, Tahirovic YA, Menaldino DS, Ganesh T, Wilson LJ, Liotta DC, Snyder JP, Traynelis SF (2015) Context-Dependent GluN2B-Selective Inhibitors of NMDA Receptor Function Are Neuroprotective with Minimal Side Effects. Neuron 85 (6):1305–1318. doi: 10.1016/j.neuron.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hashimoto K, Malchow B, Falkai P, Schmitt A (2013) Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 263 (5):367–377. doi: 10.1007/s00406-013-0399-y [DOI] [PubMed] [Google Scholar]
- 368.Shipton OA, Paulsen O (2014) GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos Trans R Soc Lond B Biol Sci 369 (1633):20130163. doi: 10.1098/rstb.2013.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. doi: 10.1016/j.pneurobio.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 370.Yurkewicz L, Weaver J, Bullock MR, Marshall LF (2005) The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma 22 (12):1428–1443. doi: 10.1089/neu.2005.22.1428 [DOI] [PubMed] [Google Scholar]
- 371.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, Group G-CS (2015) Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract 21 (2):140–149. doi: 10.1097/01.pra.0000462606.17725.93 [DOI] [PubMed] [Google Scholar]
- 372.Farin A, Marshall LF (2004) Lessons from epidemiologic studies in clinical trials of traumatic brain injury. Acta Neurochir Suppl 89:101–107 [DOI] [PubMed] [Google Scholar]
- 373.Feng B, Morley RM, Jane DE, Monaghan DT (2005) The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology 48 (3):354–359. doi: 10.1016/j.neuropharm.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 374.Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT (2004) Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141 (3):508–516. doi: 10.1038/sj.bjp.0705644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Buller AL, Monaghan DT (1997) Pharmacological heterogeneity of NMDA receptors: characterization of NR1a/NR2D heteromers expressed in Xenopus oocytes. Eur J Pharmacol 320 (1):87–94 [DOI] [PubMed] [Google Scholar]
- 376.Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE (2005) Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem 48 (7):2627–2637 [DOI] [PubMed] [Google Scholar]
- 377.Costa BM, Feng B, Tsintsadze TS, Morley RM, Irvine MW, Tsintsadze V, Lozovaya NA, Jane DE, Monaghan DT (2009) N-methyl-D-aspartate (NMDA) receptor NR2 subunit selectivity of a series of novel piperazine-2,3-dicarboxylate derivatives: preferential blockade of extrasynaptic NMDA receptors in the rat hippocampal CA3-CA1 synapse. J Pharmacol Exp Ther 331 (2):618–626. doi: 10.1124/jpet.109.156752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Suarez F, Zhao Q, Monaghan DT, Jane DE, Jones S, Gibb AJ (2010) Functional heterogeneity of NMDA receptors in rat substantia nigra pars compacta and reticulata neurones. The European journal of neuroscience 32 (3):359–367. doi: 10.1111/j.1460-9568.2010.07298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA (2004) Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol 558 (Pt 2):451–463. doi: 10.1113/jphysiol.2004.063792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Harney SC, Jane DE, Anwyl R (2008) Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci 28 (45):11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE, Monaghan DT (2010) A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther 335 (3):614–621. doi: 10.1124/jpet.110.174144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Laube B, Jane DE, Monaghan DT (2012) Structure-activity relationships for allosteric NMDA receptor inhibitors based on 2-naphthoic acid. Neuropharmacology 62 (4):1730–1736. doi: 10.1016/j.neuropharm.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Brauner-Osborne H, Liotta DC, Traynelis SF (2010) Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem 53 (15):5476–5490. doi: 10.1021/jm100027p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hansen KB, Traynelis SF (2011) Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J Neurosci 31 (10):3650–3661. doi: 10.1523/JNEUROSCI.5565-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC, Traynelis SF (2011) Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol 80 (5):782–795. doi: 10.1124/mol.111.073239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Acker TM, Khatri A, Vance KM, Slabber C, Bacsa J, Snyder JP, Traynelis SF, Liotta DC (2013) Structure-activity relationships and pharmacophore model of a noncompetitive pyrazoline containing class of GluN2C/GluN2D selective antagonists. J Med Chem 56 (16):6434–6456. doi: 10.1021/jm400652r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Hildebrand ME, Pitcher GM, Harding EK, Li H, Beggs S, Salter MW (2014) GluN2B and GluN2D NMDARs dominate synaptic responses in the adult spinal cord. Scientific reports 4:4094. doi: 10.1038/srep04094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Pearlstein E, Gouty-Colomer LA, Michel FJ, Cloarec R, Hammond C (2015) Glutamatergic synaptic currents of nigral dopaminergic neurons follow a postnatal developmental sequence. Frontiers in cellular neuroscience 9:210. doi: 10.3389/fncel.2015.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Ashhad S, Narayanan R (2016) Active dendrites regulate the impact of gliotransmission on rat hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 113 (23):E3280–3289. doi: 10.1073/pnas.1522180113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P, Vellano KM, Liotta DC, Traynelis SF (2010) A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors. Nat Commun 1:90. doi: 10.1038/ncomms1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Santangelo Freel RM, Ogden KK, Strong KL, Khatri A, Chepiga KM, Jensen HS, Traynelis SF, Liotta DC (2013) Synthesis and structure activity relationship of tetrahydroisoquinoline-based potentiators of GluN2C and GluN2D containing N-methyl-D-aspartate receptors. J Med Chem 56 (13):5351–5381. doi: 10.1021/jm400177t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Santangelo Freel RM, Ogden KK, Strong KL, Khatri A, Chepiga KM, Jensen HS, Traynelis SF, Liotta DC (2014) Correction to Synthesis and Structure Activity Relationship of Tetrahydroisoquinoline-Based Potentiators of GluN2C and GluN2D Containing N-Methyl-d-aspartate Receptors. J Med Chem 57 (11):4975. doi: 10.1021/jm500710w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ, Traynelis SF (2016) GluN2D-containing NMDA receptors mediate synaptic transmission in hippocampal interneurons and regulate interneuron activity. Mol Pharmacol. doi: 10.1124/mol.116.105130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ogden KK, Khatri A, Traynelis SF, Heldt SA (2014) Potentiation of GluN2C/D NMDA receptor subtypes in the amygdala facilitates the retention of fear and extinction learning in mice. Neuropsychopharmacology 39 (3):625–637. doi: 10.1038/npp.2013.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Suryavanshi PS, Ugale RR, Yilmazer-Hanke D, Stairs DJ, Dravid SM (2014) GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br J Pharmacol 171 (3):799–809. doi: 10.1111/bph.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Yamamoto H, Kamegaya E, Sawada W, Hasegawa R, Yamamoto T, Hagino Y, Takamatsu Y, Imai K, Koga H, Mishina M, Ikeda K (2013) Involvement of the N-methyl-D-aspartate receptor GluN2D subunit in phencyclidine-induced motor impairment, gene expression, and increased Fos immunoreactivity. Mol Brain 6:56. doi: 10.1186/1756-6606-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhang X, Feng ZJ, Chergui K (2014) Allosteric modulation of GluN2C/GluN2D-containing NMDA receptors bidirectionally modulates dopamine release: implication for Parkinson’s disease. Br J Pharmacol 171 (16):3938–3945. doi: 10.1111/bph.12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zimmerman SS, Khatri A, Garnier-Amblard EC, Mullasseril P, Kurtkaya NL, Gyoneva S, Hansen KB, Traynelis SF, Liotta DC (2014) Design, synthesis, and structure-activity relationship of a novel series of GluN2C-selective potentiators. J Med Chem 57 (6):2334–2356. doi: 10.1021/jm401695d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Watanabe M, Inoue Y, Sakimura K, Mishina M (1993) Distinct spatio-temporal distributions of the NMDA receptor channel subunit mRNAs in the brain. Ann N Y Acad Sci 707:463–466 [DOI] [PubMed] [Google Scholar]
- 400.Wenzel A, Fritschy JM, Mohler H, Benke D (1997) NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem 68 (2):469–478 [DOI] [PubMed] [Google Scholar]
- 401.Karavanova I, Vasudevan K, Cheng J, Buonanno A (2007) Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci 34 (3):468–480. doi: 10.1016/j.mcn.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Daggett LP, Johnson EC, Varney MA, Lin FF, Hess SD, Deal CR, Jachec C, Lu CC, Kerner JA, Landwehrmeyer GB, Standaert DG, Young AB, Harpold MM, Velicelebi G (1998) The human N-methyl-D-aspartate receptor 2C subunit: genomic analysis, distribution in human brain, and functional expression. J Neurochem 71 (5):1953–1968 [DOI] [PubMed] [Google Scholar]
- 403.Wenzel A, Villa M, Mohler H, Benke D (1996) Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem 66 (3):1240–1248 [DOI] [PubMed] [Google Scholar]
- 404.Standaert DG, Bernhard Landwehrmeyer G, Kerner JA, Penney JB, Young AB (1996) Expression of NMDAR2D glutamate receptor subunit mRNA in neurochemically identified interneurons in the rat neostriatum, neocortex and hippocampus. Mol Brain Res 42 (1):89–102. doi: 10.1016/s0169-328x(96)00117-9 [DOI] [PubMed] [Google Scholar]
- 405.Binshtok AM, Fleidervish IA, Sprengel R, Gutnick MJ (2006) NMDA receptors in layer 4 spiny stellate cells of the mouse barrel cortex contain the NR2C subunit. J Neurosci 26 (2):708–715. doi: 10.1523/JNEUROSCI.4409-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Misra C, Brickley SG, Farrant M, Cull-Candy SG (2000) Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol 524 Pt 1:147–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ciabarra AM, Sullivan JM, Gahn LG, Pecht G, Heinemann S, Sevarino KA (1995) Cloning and characterization of chi-1: a developmentally regulated member of a novel class of the ionotropic glutamate receptor family. J Neurosci 15 (10):6498–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Sucher NJ, Akbarian S, Chi CL, Leclerc CL, Awobuluyi M, Deitcher DL, Wu MK, Yuan JP, Jones EG, Lipton SA (1995) Developmental and regional expression pattern of a novel NMDA receptor-like subunit (NMDAR-L) in the rodent brain. J Neurosci 15 (10):6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Wong HK, Liu XB, Matos MF, Chan SF, Perez-Otano I, Boysen M, Cui J, Nakanishi N, Trimmer JS, Jones EG, Lipton SA, Sucher NJ (2002) Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol 450 (4):303–317. doi: 10.1002/cne.10314 [DOI] [PubMed] [Google Scholar]
- 410.Goebel DJ, Poosch MS (1999) NMDA receptor subunit gene expression in the rat brain: a quantitative analysis of endogenous mRNA levels of NR1Com, NR2A, NR2B, NR2C, NR2D and NR3A. Brain Res Mol Brain Res 69 (2):164–170 [DOI] [PubMed] [Google Scholar]
- 411.Henson MA, Roberts AC, Salimi K, Vadlamudi S, Hamer RM, Gilmore JH, Jarskog LF, Philpot BD (2008) Developmental regulation of the NMDA receptor subunits, NR3A and NR1, in human prefrontal cortex. Cereb Cortex 18 (11):2560–2573. doi: 10.1093/cercor/bhn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Fukaya M, Hayashi Y, Watanabe M (2005) NR2 to NR3B subunit switchover of NMDA receptors in early postnatal motoneurons. Eur J Neurosci 21 (5):1432–1436. doi: 10.1111/j.1460-9568.2005.03957.x [DOI] [PubMed] [Google Scholar]
- 413.Prithviraj R, Inglis FM (2008) Expression of the N-methyl-D-aspartate receptor subunit NR3B regulates dendrite morphogenesis in spinal motor neurons. Neuroscience 155 (1):145–153. doi: 10.1016/j.neuroscience.2008.03.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wee KS, Zhang Y, Khanna S, Low CM (2008) Immunolocalization of NMDA receptor subunit NR3B in selected structures in the rat forebrain, cerebellum, and lumbar spinal cord. J Comp Neurol 509 (1):118–135. doi: 10.1002/cne.21747 [DOI] [PubMed] [Google Scholar]
- 415.Wee KS, Tan FC, Cheong YP, Khanna S, Low CM (2016) Ontogenic Profile and Synaptic Distribution of GluN3 Proteins in the Rat Brain and Hippocampal Neurons. Neurochem Res 41 (1–2):290–297. doi: 10.1007/s11064-015-1794-8 [DOI] [PubMed] [Google Scholar]
- 416.Eriksson M, Nilsson A, Froelich-Fabre S, Akesson E, Dunker J, Seiger A, Folkesson R, Benedikz E, Sundstrom E (2002) Cloning and expression of the human N-methyl-D-aspartate receptor subunit NR3A. Neurosci Lett 321 (3):177–181 [DOI] [PubMed] [Google Scholar]
- 417.Mueller HT, Meador-Woodruff JH (2005) Distribution of the NMDA receptor NR3A subunit in the adult pig-tail macaque brain. J Chem Neuroanat 29 (3):157–172. doi: 10.1016/j.jchemneu.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 418.Bendel O, Meijer B, Hurd Y, von Euler G (2005) Cloning and expression of the human NMDA receptor subunit NR3B in the adult human hippocampus. Neurosci Lett 377 (1):31–36. doi: 10.1016/j.neulet.2004.11.064 [DOI] [PubMed] [Google Scholar]
- 419.Barth AL, Malenka RC (2001) NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci 4 (3):235–236. doi: 10.1038/85070 [DOI] [PubMed] [Google Scholar]
- 420.Bellone C, Nicoll RA (2007) Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55 (5):779–785. doi: 10.1016/j.neuron.2007.07.035 [DOI] [PubMed] [Google Scholar]
- 421.Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H (1997) NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17 (7):2469–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Crair MC, Malenka RC (1995) A critical period for long-term potentiation at thalamocortical synapses. Nature 375 (6529):325–328. doi: 10.1038/375325a0 [DOI] [PubMed] [Google Scholar]
- 423.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ (1999) Genetic enhancement of learning and memory in mice. Nature 401 (6748):63–69 [DOI] [PubMed] [Google Scholar]
- 424.Kirkwood A, Rioult MC, Bear MF (1996) Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381 (6582):526–528. doi: 10.1038/381526a0 [DOI] [PubMed] [Google Scholar]
- 425.Philpot BD, Sekhar AK, Shouval HZ, Bear MF (2001) Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29 (1):157–169 [DOI] [PubMed] [Google Scholar]
- 426.Yashiro K, Philpot BD (2008) Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55 (7):1081–1094. doi: 10.1016/j.neuropharm.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Zhou Q, Sheng M (2013) NMDA receptors in nervous system diseases. Neuropharmacology 74:69–75. doi: 10.1016/j.neuropharm.2013.03.030 [DOI] [PubMed] [Google Scholar]
- 428.Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338 (6103):68–72. doi: 10.1126/science.1222939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA Jr., Charney DS (2014) Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol 54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47 (4):351–354 [DOI] [PubMed] [Google Scholar]
- 431.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA Jr. (2012) Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry 72 (7):555–561. doi: 10.1016/j.biopsych.2012.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63 (8):856–864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 433.Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS (2015) Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med 45 (16):3571–3580. doi: 10.1017/S0033291715001506 [DOI] [PubMed] [Google Scholar]
- 434.Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66 (5):522–526. doi: 10.1016/j.biopsych.2009.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71 (11):939–946. doi: 10.1016/j.biopsych.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB (2013) Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 38 (12):2475–2483. doi: 10.1038/npp.2013.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS (2014) Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA psychiatry 71 (6):681–688. doi: 10.1001/jamapsychiatry.2014.62 [DOI] [PubMed] [Google Scholar]
- 438.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67 (2):139–145. doi: 10.1016/j.biopsych.2009.08.038 [DOI] [PubMed] [Google Scholar]
- 439.Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R (2014) Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 28 (6):536–544. doi: 10.1177/0269881114527361 [DOI] [PubMed] [Google Scholar]
- 440.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74 (4):250–256. doi: 10.1016/j.biopsych.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016) A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry:appiajp201616010037. doi: 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- 442.Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2015) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76 (3):247–252. doi: 10.4088/JCP.13m08852 [DOI] [PubMed] [Google Scholar]
- 443.Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76 (12):970–976. doi: 10.1016/j.biopsych.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Loo CK, Galvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, Harper S, Somogyi AA, Lai R, Weickert CS, Glue P (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134 (1):48–56. doi: 10.1111/acps.12572 [DOI] [PubMed] [Google Scholar]
- 445.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, Quirk MC (2014) Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 19 (9):978–985. doi: 10.1038/mp.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zarate CA Jr., Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA (2013) A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 74 (4):257–264. doi: 10.1016/j.biopsych.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, Potter WZ, Zarate CA Jr. (2012) A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol 32 (4):551–557. doi: 10.1097/JCP.0b013e31825d70d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28 (6):631–637. doi: 10.1097/JCP.0b013e31818a6cea [DOI] [PubMed] [Google Scholar]
- 449.Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015) Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66:509–523. doi: 10.1146/annurev-med-053013-062946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19 (4):126–130 [DOI] [PubMed] [Google Scholar]
- 451.Cooper LN, Bear MF (2012) The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci 13 (11):798–810. doi: 10.1038/nrn3353 [DOI] [PubMed] [Google Scholar]
- 452.Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135 (3):422–435. doi: 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533 (7604):481–486. doi: 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62 (1):35–41. doi: 10.1016/j.neuropharm.2011.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 (5994):959–964. doi: 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69 (8):754–761. doi: 10.1016/j.biopsych.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Graef JD, Newberry K, Newton A, Pieschl R, Shields E, Luan FN, Simmermacher J, Luchetti D, Schaeffer E, Li YW, Kiss L, Bristow LJ (2015) Effect of acute NR2B antagonist treatment on long-term potentiation in the rat hippocampus. Brain Res 1609:31–39. doi: 10.1016/j.brainres.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 458.Nagy D, Stoiljkovic M, Menniti FS, Hajos M (2016) Differential Effects of an NR2B NAM and Ketamine on Synaptic Potentiation and Gamma Synchrony: Relevance to Rapid-Onset Antidepressant Efficacy. Neuropsychopharmacology 41 (6):1486–1494. doi: 10.1038/npp.2015.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27 (43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kocsis B (2012) Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry 71 (11):987–995. doi: 10.1016/j.biopsych.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Sivarao DV, Chen P, Yang Y, Li YW, Pieschl R, Ahlijanian MK (2014) NR2B Antagonist CP-101,606 Abolishes Pitch-Mediated Deviance Detection in Awake Rats. Front Psychiatry 5:96. doi: 10.3389/fpsyt.2014.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Keavy D, Bristow LJ, Sivarao DV, Batchelder M, King D, Thangathirupathy S, Macor JE, Weed MR (2016) The qEEG Signature of Selective NMDA NR2B Negative Allosteric Modulators; A Potential Translational Biomarker for Drug Development. PLoS ONE 11 (4):e0152729. doi: 10.1371/journal.pone.0152729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ (2013) Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 16 (8):1032–1041. doi: 10.1038/nn.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Pfeffer CK, Xue MS, He M, Huang ZJ, Scanziani M (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16 (8):1068–U1130. doi: 10.1038/nn.3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Harris KD, Shepherd GM (2015) The neocortical circuit: themes and variations. Nat Neurosci 18 (2):170–181. doi: 10.1038/nn.3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF (2013) NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77 (4):736–749. doi: 10.1016/j.neuron.2012.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11 (10):682–696. doi: 10.1038/nrn2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Lee MC, Yasuda R, Ehlers MD (2010) Metaplasticity at single glutamatergic synapses. Neuron 66 (6):859–870. doi: 10.1016/j.neuron.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Chen WS, Bear MF (2007) Activity-dependent regulation of NR2B translation contributes to metaplasticity in mouse visual cortex. Neuropharmacology 52 (1):200–214. doi: 10.1016/j.neuropharm.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 470.Stocca G, Vicini S (1998) Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol 507 (Pt 1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Chiocchetti AG, Bour HS, Freitag CM (2014) Glutamatergic candidate genes in autism spectrum disorder: an overview. J Neural Transm (Vienna) 121 (9):1081–1106. doi: 10.1007/s00702-014-1161-y [DOI] [PubMed] [Google Scholar]
- 472.Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL (2011) Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry 168 (9):930–946. doi: 10.1176/appi.ajp.2011.10050723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ (2015) Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry 77 (1):52–58. doi: 10.1016/j.biopsych.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 474.Menniti FS, Lindsley CW, Conn PJ, Pandit J, Zagouras P, Volkmann RA (2013) Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Curr Top Med Chem 13 (1):26–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Domino EF, Luby ED (2012) Phencyclidine/schizophrenia: one view toward the past, the other to the future. Schizophr Bull 38 (5):914–919. doi: 10.1093/schbul/sbs011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51 (3):199–214 [DOI] [PubMed] [Google Scholar]
- 477.Xu K, Krystal JH, Ning Y, Chen da C, He H, Wang D, Ke X, Zhang X, Ding Y, Liu Y, Gueorguieva R, Wang Z, Limoncelli D, Pietrzak RH, Petrakis IL, Zhang X, Fan N (2015) Preliminary analysis of positive and negative syndrome scale in ketamine-associated psychosis in comparison with schizophrenia. J Psychiatr Res 61:64–72. doi: 10.1016/j.jpsychires.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R (1959) Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry 81 (3):363–369 [DOI] [PubMed] [Google Scholar]
- 479.Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE (2014) Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology 86:174–180. doi: 10.1016/j.neuropharm.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 480.Javitt DC, Zukin SR (1990) The role of excitatory amino acids in neuropsychiatric illness. J Neuropsychiatry Clin Neurosci 2 (1):44–52. doi: 10.1176/jnp.2.1.44 [DOI] [PubMed] [Google Scholar]
- 481.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D (2012) Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38 (5):958–966. doi: 10.1093/schbul/sbs069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R (2003) NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169 (3–4):215–233. doi: 10.1007/s00213-003-1582-z [DOI] [PubMed] [Google Scholar]
- 483.Kantrowitz ER, Javitt D (2011) Glutamate: New hope for schizophrenia therapy. Current Psychiatry 10 (4):69–74 [Google Scholar]
- 484.Tsai GE, Lin PY (2010) Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des 16 (5):522–537 [DOI] [PubMed] [Google Scholar]
- 485.Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT (2007) The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry 164 (10):1593–1602. doi: 10.1176/appi.ajp.2007.06081358 [DOI] [PubMed] [Google Scholar]
- 486.Goff DC (2012) D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull 38 (5):936–941. doi: 10.1093/schbul/sbs012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Goff DC (2016) D-cycloserine in schizophrenia: New strategies for improving clinical outcomes by enhancing plasticity. Curr Neuropharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Umbricht D, Alberati D, Martin-Facklam M, Borroni E, Youssef EA, Ostland M, Wallace TL, Knoflach F, Dorflinger E, Wettstein JG, Bausch A, Garibaldi G, Santarelli L (2014) Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: a randomized, double-blind, proof-of-concept study. JAMA psychiatry 71 (6):637–646. doi: 10.1001/jamapsychiatry.2014.163 [DOI] [PubMed] [Google Scholar]
- 489.Goff DC, Cather C, Gottlieb JD, Evins AE, Walsh J, Raeke L, Otto MW, Schoenfeld D, Green MF (2008) Once-weekly D-cycloserine effects on negative symptoms and cognition in schizophrenia: an exploratory study. Schizophr Res 106 (2–3):320–327. doi: 10.1016/j.schres.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC (2011) D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res 131 (1–3):69–74. doi: 10.1016/j.schres.2011.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R (2004) Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol 557 (Pt 2):489–500. doi: 10.1113/jphysiol.2004.063321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Sheinin A, Shavit S, Benveniste M (2001) Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 41 (2):151–158 [DOI] [PubMed] [Google Scholar]
- 493.Dravid SM, Burger PB, Prakash A, Geballe MT, Yadav R, Le P, Vellano K, Snyder JP, Traynelis SF (2010) Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J Neurosci 30 (7):2741–2754. doi: 10.1523/JNEUROSCI.5390-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Standaert DG, Testa CM, Young AB, Penney JB Jr. (1994) Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol 343 (1):1–16. doi: 10.1002/cne.903430102 [DOI] [PubMed] [Google Scholar]
- 495.Zhang Y, Buonanno A, Vertes RP, Hoover WB, Lisman JE (2012) NR2C in the thalamic reticular nucleus; effects of the NR2C knockout. PLoS ONE 7 (7):e41908. doi: 10.1371/journal.pone.0041908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Merchant RE, Bullock MR, Carmack CA, Shah AK, Wilner KD, Ko G, Williams SA (1999) A double-blind, placebo-controlled study of the safety, tolerability and pharmacokinetics of CP-101,606 in patients with a mild or moderate traumatic brain injury. Ann N Y Acad Sci 890:42–50 [DOI] [PubMed] [Google Scholar]
- 497.Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS, Landen JW (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Mov Disord 23 (13):1860–1866. doi: 10.1002/mds.22169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Chaperon F, Muller W, Auberson YP, Tricklebank MD, Neijt HC (2003) Substitution for PCP, disruption of prepulse inhibition and hyperactivity induced by N-methyl-D-aspartate receptor antagonists: preferential involvement of the NR2B rather than NR2A subunit. Behav Pharmacol 14 (5–6):477–487. doi: 10.1097/01.fbp.0000091471.79060.ed [DOI] [PubMed] [Google Scholar]
- 499.Nicholson KL, Mansbach RS, Menniti FS, Balster RL (2007) The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP-101 606 in rats and rhesus monkeys. Behav Pharmacol 18 (8):731–743. doi: 10.1097/FBP.0b013e3282f14ed6 [DOI] [PubMed] [Google Scholar]
- 500.Epi PMC (2015) A roadmap for precision medicine in the epilepsies. Lancet Neurol 14 (12):1219–1228. doi: 10.1016/S1474-4422(15)00199-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Carvill GL, Regan BM, Yendle SC, O’Roak BJ, Lozovaya N, Bruneau N, Burnashev N, Khan A, Cook J, Geraghty E, Sadleir LG, Turner SJ, Tsai MH, Webster R, Ouvrier R, Damiano JA, Berkovic SF, Shendure J, Hildebrand MS, Szepetowski P, Scheffer IE, Mefford HC (2013) GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet 45 (9):1073–1076. doi: 10.1038/ng.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, Milh M, Kortum F, Fritsch A, Pientka FK, Hellenbroich Y, Kalscheuer VM, Kohlhase J, Moog U, Rappold G, Rauch A, Ropers HH, von Spiczak S, Tonnies H, Villeneuve N, Villard L, Zabel B, Zenker M, Laube B, Reis A, Wieczorek D, Van Maldergem L, Kutsche K (2010) Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nat Genet 42 (11):1021–1026. doi: 10.1038/ng.677 [DOI] [PubMed] [Google Scholar]
- 503.Lemke JR, Lal D, Reinthaler EM, Steiner I, Nothnagel M, Alber M, Geider K, Laube B, Schwake M, Finsterwalder K, Franke A, Schilhabel M, Jahn JA, Muhle H, Boor R, Van Paesschen W, Caraballo R, Fejerman N, Weckhuysen S, De Jonghe P, Larsen J, Moller RS, Hjalgrim H, Addis L, Tang S, Hughes E, Pal DK, Veri K, Vaher U, Talvik T, Dimova P, Guerrero Lopez R, Serratosa JM, Linnankivi T, Lehesjoki AE, Ruf S, Wolff M, Buerki S, Wohlrab G, Kroell J, Datta AN, Fiedler B, Kurlemann G, Kluger G, Hahn A, Haberlandt DE, Kutzer C, Sperner J, Becker F, Weber YG, Feucht M, Steinbock H, Neophythou B, Ronen GM, Gruber-Sedlmayr U, Geldner J, Harvey RJ, Hoffmann P, Herms S, Altmuller J, Toliat MR, Thiele H, Nurnberg P, Wilhelm C, Stephani U, Helbig I, Lerche H, Zimprich F, Neubauer BA, Biskup S, von Spiczak S (2013) Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet 45 (9):1067–1072. doi: 10.1038/ng.2728 [DOI] [PubMed] [Google Scholar]
- 504.Lesca G, Rudolf G, Bruneau N, Lozovaya N, Labalme A, Boutry-Kryza N, Salmi M, Tsintsadze T, Addis L, Motte J, Wright S, Tsintsadze V, Michel A, Doummar D, Lascelles K, Strug L, Waters P, de Bellescize J, Vrielynck P, de Saint Martin A, Ville D, Ryvlin P, Arzimanoglou A, Hirsch E, Vincent A, Pal D, Burnashev N, Sanlaville D, Szepetowski P (2013) GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet 45 (9):1061–1066. doi: 10.1038/ng.2726 [DOI] [PubMed] [Google Scholar]
- 505.Strehlow V, Heyne HO, Lemke JR (2015) The Spectrum of GRIN2A-Associated Disorders. Epileptologie 32 (3):147–151 [Google Scholar]
- 506.Insel TR (2010) Rethinking schizophrenia. Nature 468 (7321):187–193. doi: 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- 507.Yuan H, Low CM, Moody OA, Jenkins A, Traynelis SF (2015) Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol Pharmacol 88 (1):203–217. doi: 10.1124/mol.115.097998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Burnashev N, Szepetowski P (2015) NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol 20:73–82. doi: 10.1016/j.coph.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 509.Pierson TM, Yuan H, Marsh ED, Fuentes-Fajardo K, Adams DR, Markello T, Golas G, Simeonov DR, Holloman C, Tankovic A, Karamchandani MM, Schreiber JM, Mullikin JC, Ph DftNCSP, Tifft CJ, Toro C, Boerkoel CF, Traynelis SF, Gahl WA (2014) GRIN2A mutation and early-onset epileptic encephalopathy: personalized therapy with memantine. Annals of clinical and translational neurology 1 (3):190–198. doi: 10.1002/acn3.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Chahrour M, Zoghbi HY (2007) The story of Rett syndrome: from clinic to neurobiology. Neuron 56 (3):422–437. doi: 10.1016/j.neuron.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 511.Robinson L, Guy J, McKay L, Brockett E, Spike RC, Selfridge J, De Sousa D, Merusi C, Riedel G, Bird A, Cobb SR (2012) Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain 135 (Pt 9):2699–2710. doi: 10.1093/brain/aws096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Guy J, Gan J, Selfridge J, Cobb S, Bird A (2007) Reversal of neurological defects in a mouse model of Rett syndrome. Science 315 (5815):1143–1147. doi: 10.1126/science.1138389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Katz DM, Bird A, Coenraads M, Gray SJ, Menon DU, Philpot BD, Tarquinio DC (2016) Rett Syndrome: Crossing the Threshold to Clinical Translation. Trends Neurosci 39 (2):100–113. doi: 10.1016/j.tins.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Blue ME, Kaufmann WE, Bressler J, Eyring C, O’Driscoll C, Naidu S, Johnston MV (2011) Temporal and regional alterations in NMDA receptor expression in Mecp2-null mice. Anat Rec (Hoboken) 294 (10):1624–1634. doi: 10.1002/ar.21380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, Carninci P, Hensch TK, Fagiolini M (2012) NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76 (6):1078–1090. doi: 10.1016/j.neuron.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Mierau SB, Patrizi A, Hensch TK, Fagiolini M (2016) Cell-Specific Regulation of N-Methyl-D-Aspartate Receptor Maturation by Mecp2 in Cortical Circuits. Biol Psychiatry 79 (9):746–754. doi: 10.1016/j.biopsych.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Kron M, Howell CJ, Adams IT, Ransbottom M, Christian D, Ogier M, Katz DM (2012) Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J Neurosci 32 (40):13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Patrizi A, Picard N, Simon AJ, Gunner G, Centofante E, Andrews NA, Fagiolini M (2016) Chronic Administration of the N-Methyl-D-Aspartate Receptor Antagonist Ketamine Improves Rett Syndrome Phenotype. Biol Psychiatry 79 (9):755–764. doi: 10.1016/j.biopsych.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Bienenstock EL, Cooper LN, Munro PW (1982) Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2 (1):32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Jones KA, Menniti FS, Sivarao DV (2015) Translational psychiatry--light at the end of the tunnel. Ann N Y Acad Sci 1344:1–11. doi: 10.1111/nyas.12725 [DOI] [PubMed] [Google Scholar]
- 521.Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE (2012) Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem Int 61 (4):581–592. doi: 10.1016/j.neuint.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Kohr G (2006) NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res 326 (2):439–446. doi: 10.1007/s00441-006-0273-6 [DOI] [PubMed] [Google Scholar]
- 523.Kohr G (2007) NMDA receptor antagonists: tools in neuroscience with promise for treating CNS pathologies. J Physiol 581 (Pt 1):1–2. doi: 10.1113/jphysiol.2007.130732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Yuan H, Geballe MT, Hansen KB, Traynelis SF (2008) Structure and function relationship of the NMDA receptor In: Hell JW, Ehlers MD (eds) Structural and Functional Organization of the Synapse. 1 edn. Springer, New York, pp 289–316 [Google Scholar]
- 525.Hess SD, Daggett LP, Crona J, Deal C, Lu CC, Urrutia A, Chavez-Noriega L, Ellis SB, Johnson EC, Velicelebi G (1996) Cloning and functional characterization of human heteromeric N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 278 (2):808–816 [PubMed] [Google Scholar]


