Abstract
Background:
Prenatal exposure to persistent organic pollutants (POPs) may affect child neurobehavior; however, exposures to mixtures of POPs have rarely been examined.
Methods:
We estimated associations of prenatal serum concentrations of 17 POPs, namely 5 polybrominated diphenyl ethers (PBDEs), 6 polychlorinated biphenyls (PCBs), dichlorodiphenyldichloroethylene, dichlorodiphenyltrichloroethane, and 4 per- and polyfluoroalkyl substances (PFAS), with Wide Range Achievement Test-4 reading composite scores at age 8 years in 161 children from a pregnancy/birth cohort (HOME Study, 2003-present) in Cincinnati, OH. We applied 6 statistical methods: least absolute shrinkage and selection operator (LASSO), elastic net (ENET), Sparse Principal Component Analysis (SPCA), Weighted Quantile Sum (WQS) regression, Bayesian Kernel Machine Regression (BKMR), and Bayesian Additive Regression Trees (BART), to estimate covariate-adjusted associations with individual and their mixtures in multi-pollutant models.
Results:
Both LASSO and ENET models indicated inverse associations with reading scores for BDE-153 and BDE-28, and positive associations for CB-118, CB-180, perfluoroctanoate (PFOA), and perfluorononanoate (PFNA). The SPCA identified inverse associations for BDE-153 and BDE-100 and positive associations for perfluorooctane sulfonate (PFOS), PFOA, and PFNA, as parts of different principal component scores. The WQS regression showed the highest weights for BDE-100 (0.35) and BDE-28 (0.16) in the inverse association model and for PFNA (0.29) and CB-180 (0.21) in the positive association model. The BKMR model identified BDE-100 and BDE-153 for inverse associations and CB-118, CB-153, CB-180, PFOA, and PFNA for positive associations. The BART method found dose-response functions similar to the BKMR model. No interactions between POPs were identified.
Conclusions:
Despite some inconsistency among biomarkers, these analyses revealed inverse associations between prenatal PBDE concentrations and children’s reading scores. Positive associations of PCB congeners and PFAS with reading skills were also found.
Keywords: Persistent organic pollutants (POPs), chemical mixtures, polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFAS), neurodevelopment
1. Introduction
Mounting evidence from epidemiological studies suggests that early life exposure to certain environmental chemicals adversely affects neurodevelopment. Persistent organic pollutants (POPs), including polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyl ethers (PCBs), per- and polyfluoroalkyl substances (PFAS), dichlorodiphenyltrichloroethane (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE), are recognized for their potential role as neurotoxicants (Goodman et al., 2010; Lam et al., 2017; Liew et al., 2018; Polanska et al., 2013; Vuong et al., 2018). POPs are resistant to environmental degradation, accumulate in human tissue, have long biological half-lives, and can cross the placenta during gestation. Perturbations of programmed neuronal development and network connectivity by exogenous chemicals during this critical period of susceptibility can be detrimental and induce changes in neurodevelopment and behavior (Gore et al., 2014). Potential mechanisms of action include thyroid hormone disruption and affecting brain cells by inducing neuronal death, interfering with neurotransmitter synthesis, disturbing neuronal connectivity, and disrupting neural cell differentiation (Costa et al., 2014; Lee and Viberg, 2013; Moser et al., 2001; Reistad et al., 2013; Seegal, 1996; Slotkin et al., 2008).
Despite the critical need to examine mixtures of developmental neurotoxicants, a staggering majority of epidemiological studies investigating chemical exposures have utilized a single-pollutant approach. These studies examine chemicals in isolation rather than taking into account the totality of chemical exposures, which upon acting together may have additive, synergistic, antagonistic, or potentiating effects (Lazarevic et al., 2019). The development of statistical methodologies for multipollutant models has gained considerable traction recently given the compelling need for novel approaches. However, very few studies investigating POPs as potential neurotoxicants have applied advanced statistical methods to examine chemical mixtures (Braun et al., 2014; Forns et al., 2016; Hamra et al., 2019; Lenters et al., 2019; Zhang et al., 2017). Further, a majority of these studies utilized only one type of multi-pollutant statistical model to examine POP mixtures, with only one that employed three mixture statistical approaches (Forns et al., 2016). In addition, studies comparing results from various mixture approaches using data in real-world scenarios are insufficient. Environmental epidemiological studies stand at the precipice of great change in the examination of chemical mixtures, the enormity of which we have yet to even realize. The development of multi-pollutant statistical models currently exceeds its actual application in neurodevelopmental studies.
To address this research gap we used data from the Health Outcomes and Measures of the Environment (HOME) Study to investigate the associations between a mixture of 17 POPs and school age children’s reading skills utilizing six developed statistical methods. While several neurodevelopmental domains are important for everyday functioning, including full scale IQ, executive function, and externalizing behaviors, fewer epidemiological studies have examined reading ability as a potential endpoint of neurotoxicity. Thus, we focused our analysis on reading abilities assessed at age 8 years, a time period when patterns of reading development are more distinctive with inter-individual variations (Verhoeven et al., 2011). No mixture statistical method is considered the gold standard. Thus, we compared six mixture statistical methods, including: 1) Least Absolute Shrinkage and Selection Operator (LASSO) (Tibshirani, 1996); 2) Elastic Net (ENET) (Zou and Hastie, 2005); 3) Sparse Principal Component Analysis (SPCA) (Zou et al., 2006); 4) Weighted Quantile Sum (WQS) regression (Carrico, 2013; Gennings et al., 2013); 5) Bayesian Kernel Machine Regression (BKMR) (Bobb et al., 2015); and 6) Bayesian Additive Regression Trees (BART) (Chipman et al., 2010). These statistical methods were selected based on their ability for chemical variable selection (LASSO, ENET, SPCA, WQS regression, BKMR, BART), analysis of the impact of a single chemical (LASSO, ENET, SPCA, WQS regression, BKMR, BART), assessment of interactions between chemicals (LASSO, ENET, BKMR, BART), assessment of joint effects (SPCA, WQS regression, BKMR, BART), and estimation of the cumulative impact of multiple chemicals on reading ability (SPCA, WQS regression, BKMR, BART) (Lazarevic et al., 2019). All selected methods can also handle highly correlated measurements of POPs to estimate associations with reading skills at age 8 years. We will compare the findings generated from the six selected mixture statistical methods in order to enhance our ability to identify the impact of a mixture of chemicals on child neurodevelopment while assessing the differences in the findings of the analyses.
2. Methods
2.1. Study population
We used data from an ongoing prospective pregnancy and birth cohort, the HOME Study (Cincinnati, Ohio, USA). Detailed recruitment, conducted from March 2003 to February 2006, and sample collection procedures were described previously by Braun et al. (2017). Of 468 pregnant women at 16±3 weeks of gestation enrolled in the study, 390 remained to deliver live singletons. We restricted our study dataset to 161 mother-child dyads who had complete information on 17 POPs: 5 major PBDE congeners, 6 major PCB congeners, 2 major organochlorine pesticides (OCPs: DDT and DDE), and 4 PFAS, potential confounding factors, as well as an assessment of reading ability at age 8 years. The study protocol was approved by the Institutional Review Board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The Centers for Disease Control and Prevention (CDC) deferred to CCHMC IRB as the IRB of record.
2.2. Assessment of POP biomarkers
Women provided blood samples at 16±3 weeks of gestation via venipuncture. Serum was separated and samples were stored at −80°C until analysis. Details regarding quantification procedures and lipid standardization have been previously described elsewhere (Phillips et al., 1989). Maternal serum was used to measure PBDEs, PCBs, and OCPs by using gas chromatography/isotope dilution high-resolution mass spectrometry (Jones et al., 2012; Sjodin et al., 2004). Maternal serum was also used to measure PFAS by using high-performance liquid chromatography-isotope dilution-tandem mass spectrometry (Kato et al., 2011). Concentrations less than the limit of detection (LOD) were replaced with LOD/V2 (Hornung and Reed, 1990). Concentrations of PBDEs, PCBs, and OCPs were lipid-normalized and given in ng/g lipid. PFAS concentrations were expressed as ng/mL. PBDEs, PCBs, and OCPs were quantified using serum collected at 16 weeks gestation. For a small number of women without serum at 16 weeks, PFAS were measured using serum at 26 weeks (n=11) or at delivery (n=3). If more than one PFAS measurement was available during pregnancy (n=35), an average was used. For the current analyses, we focused on the following chemicals: 1) PBDE congeners: BDE-28, BDE-47, BDE-99, BDE-100, BDE-153; 2) PCB congeners: CB-74, CB-99, CB-118, CB-138/158, CB-153, CB-180; 3) OCPs: DDT and DDE; and 4) PFAS: perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexane sulfonate (PFHxS), and perfluorononanoate (PFNA). POP biomarkers were log10-transformed to reduce the influence of outliers and treated as continuous variables in statistical analyses.
2.3. Assessment of reading ability
We assessed children’s reading ability using the Wide Range Achievement Test 4 (WRAT-4) at age 8 years (Wilkinson and Robertson, 2006), an assessment of fundamental academic skills that has high validity and reliability (Jastak and Jastak, 1965). HOME Study staff members were trained and certified by a developmental psychologist and administered the WRAT-4, which had a duration of 15-25 minutes. For our study, we examined the Reading Composite score, which is comprised of the two subtests Word Reading (letter and word decoding) and Sentence Comprehension (ability of an individual to obtain meaning from words and to understand ideas and information in sentences).
2.4. Covariates
From a review of the literature, all models were adjusted for maternal age, race/ethnicity, education, household income at enrollment, marital status, serum cotinine, blood lead levels, fish consumption, maternal IQ, maternal depression (Beck et al., 1996), child sex, and the quality and quantity of the caregiving environment using Home Observation for Measurement of the Environment (HOME) scores at age 12 months (Caldwell and Bradley, 1984).
2.5. Statistical Analyses
First, we approached our research question using the traditional single-pollutant approach, modeling each POP separately using multiple linear regression to estimate the association between individual POP concentrations and reading ability in school age children, with adjustment for covariates. We additionally implemented generalized additive models to assess whether relationships between the individual POPs and Reading Composite score were monotonic. We subsequently implemented six different statistical methods that accounted for the complete set of complex mixtures of POPs (Table 1). These methods included LASSO, ENET, SPCA, WQS regression, BKMR, and BART. LASSO and ENET are penalized regression methods that identify ‘drivers’ versus ‘passengers’ in the POP mixture by entering all chemicals into the same regression model. These methods minimize the sum of the squared residuals plus either the product of a constant λ1 and the sum of the absolute value of regression parameter (LASSO) or the product of a constant λ2 and the sum of squared regression parameter (ridge regression) or both (ENET). In addition, LASSO and ENET can shrink regression estimates to zero and they are robust to extreme correlations between predictors. LASSO and ENET analyses were performed by R package “glmnet,” and the tuning parameter for ENET α= λ1/ (λ1+ λ2) was chosen by a cross-validation using R package “caret.” We ran the model 100 times and chose the best λ value that gave the lowest mean squared error, and used this λ value to rerun the model. For the subset of chemical identified by LASSO or ENET, we also constructed a multiple linear regression model with mutual adjustment of the chemicals to obtain estimates and 95% confidence intervals (Lenters et al., 2016).
Table 1.
Statistical mixture methods addressing research questions
| Research questions | LASSO and ENET | SPCA | WQS | BKMR | BART |
|---|---|---|---|---|---|
| Chemical variable selection | X | X (PCs) | X (Weight) | X (PIP) | X (threshold) |
| Interactions | X | X | X | ||
| Cumulative impact | X | X | X | X | |
| Nonlinear dose response | X | X | X |
Abbreviations: PCs, Principal Components; PIP, Posterior Inclusion Probability
For SPCA, the LASSO was incorporated into principal component (PC) analysis to impose a constraint on the chemicals to obtain sparse loadings. This method extracts a small number of PC scores from the higher dimensional set of POPs that explain most of the variability of the data by setting PCA loadings to zero. Identified PC scores were used as independent variables in the regression model with the reading scores. R package “elasticnet” was used for SPCA. In WQS regression, individual POPs were categorized into quartiles to obtain a weighted average of quartile scores in the regression. Weights were estimated to maximize the associations with WRAT-2’s Reading Composite score and the weighted sum. Separate models for positive and negative associations were constructed to ensure non-negative weights. WQS regression estimates were calculated from 1000 bootstraps using the R package “gWQS.” Due to small sample size, we did not split the dataset to obtain training and validation datasets.
In BKMR, a nonparametric high-dimensional exposure-response function is constructed with kernel machine regression using component-wise or hierarchical variable selection with 10,000 iterations. Each component in the high-dimensional function is assigned a posterior inclusion probability (PIP). The BKMR can examine potential interactions between two chemicals when all other chemicals are held at the median levels. R package “bkmr” was used to construct BKMR models. Lastly, BART uses a sum of trees approach, with each POP contributing to the outcome a small and distinct amount of information as a simple weak learner. We imposed a prior regularizing the fit by keeping the individual POP effects small. To fit the model, we used a tailored version of Bayesian backfitting Markov Chain Monte Carlo (MCMC) method that interactively constructed and fit successive residuals. R package “bartMachine” was used for the BART analysis. We used R version 3.6.1 (R Core Team, Vienna, Austria) for the analyses and graphing.
3. Results
3.1. Study participants
Approximately 60% of the included HOME Study mothers identified as non-Hispanic white with an average age at delivery of 29 years. Educational attainment among study participants mainly consisted of mothers with at least some college or higher degree (>70%). Median concentrations of BDE-47, BDE-153, CB-118, CB-153, DDT, and DDE were 20.1, 4.6, 4.8, 10.7, 2.6, and 70.8 ng/g lipid, respectively (Supplemental Figure S1). HOME Study children’s Reading Composite score had a mean ± standard deviation (SD) of 108±15. Median concentrations of PFOS and PFOA were 12.7 and 5.2 ng/mL, respectively. In bivariate analyses, we identified 3 clusters within the 17 POPs, specifically among the PBDE congeners (rp=0.45-0.93), PFAS (rp=0.40-0.65), and between PCBs and OCPs (rp=0.38-0.95) (Supplemental Figure S2). We also observed low to moderate correlation between chemical classes besides PCBs and OCPs. For example, between BDE-100 and CB-99 (rp=0.20) and between BDE-28 and DDT (rp=0.29).
3.2. Identification of POPs in single-pollutant analyses
We observed inverse associations between prenatal BDE-47 (β=−6.1, 95% CI −12.0, −0.2), BDE-100 (β=−6.1, 95% CI −11.4, −0.7), and BDE-153 (β=−5.3, 95% CI −10.3, −0.3) and Reading Composite scores at age 8 years (Table 2). In contrast, PFOA (β=12.6, 95% CI 3.0, 22.2) and PFNA (β=16.7, 95% CI 4.8, 28.6) were positively associated with Reading Composite scores. While we did not observe statistically significant associations with PCBs, there was consistent directionality, suggesting positive associations between reading ability with PCBs. For instance, prenatal CB-153 (β=9.8, 95% CI −0.6, 20.2) and CB-180 (β=8.8, 95% CI −0.8, 18.5) were associated with higher Reading Composite scores. Generalized additive models of individual POPs and Reading Composite scores with covariate adjustment mainly suggested linear relationships, with some indication of some non-monotonic associations (Supplemental Figure S3).
Table 2.
Adjusted regression coefficients and 95% CIs of POP serum concentrations modeled separately, in LASSO and Elastic Net (ENET) models, and in linear regression models using chemicals identified by LASSO and ENET models with mutual adjustment
| Chemical | Modeled separately | LASSO | Linear regression after LASSO | ENET | Linear regression after ENET |
|---|---|---|---|---|---|
| Log10 PBDEs | |||||
| BDE-28 | −5.72 (−11.95, 0.51) | −2.59 | −3.64 (−10.91, 3.63) | −2.57 | −5.03 (−16.73, 6.67) |
| BDE-47 | −6.06 (−11.96, −0.17) | ||||
| BDE-99 | −5.53 (−11.29, 0.22) | ||||
| BDE-100 | −6.05 (−11.40, −0.69) | −0.30 | 2.05 (−11.45, 15.56) | ||
| BDE-153 | −5.31 (−10.29, −0.33) | −4.70 | −6.57 (−12.35, −0.79) | −4.64 | −7.50 (−15.89, 0.90) |
| Log10 PCBs | |||||
| CB-74 | 5.22 (−6.16, 16.60) | ||||
| CB-99 | 4.43 (−5.52, 14.38) | ||||
| CB-118 | 7.80 (−0.51, 16.11) | 4.78 | 6.34 (−2.64, 15.31) | 4.88 | 6.29 (−2.72, 15.30) |
| CB-138_158 | 5.48 (−4.34, 15.30) | ||||
| CB-153 | 9.80 (−0.57, 20.16) | ||||
| CB-180 | 8.82 (−0.84, 18.48) | 1.57 | 4.66 (−5.90, 15.22) | 2.02 | 4.75 (−5.86, 15.37) |
| Log10 P,P’-DDE | 1.01 (−8.69, 10.70) | ||||
| Log10 P,P’-DDT | 2.47 (−8.01, 12.95) | ||||
| Log10 PFAS | |||||
| PFOS | 7.00 (−2.89, 16.90) | ||||
| PFOA | 12.59 (2.97, 22.21) | 7.07 | 9.88 (−1.05, 20.81) | 7.31 | 10.05 (−0.97, 21.07) |
| PFHxS | 4.46 (−3.06, 11.97) | ||||
| PFNA | 16.68 (4.77, 28.60) | 11.07 | 13.81 (0.42, 27.20) | 11.12 | 14.00 (0.51, 27.49) |
Adjusted for maternal age, race, education, household income, marital status, depression, serum cotinine, blood lead, fish consumption, IQ, and child sex and HOME score
3.3. Identification of POPs in multi-pollutant analyses
We applied LASSO to obtain a more parsimonious model that started with 17 POP biomarkers predictors of Reading Composite scores with covariate adjustment (Table 2). BDE-28 and BDE-153 showed inverse associations with reading ability. In contrast, CB-118, CB-180, PFOA, and PFNA had positive associations with the outcome. Results from ENET were similar to LASSO, with BDE-28 and BDE-153 selected as important predictors of lower reading ability, while PCBs and PFAS were associated with better reading abilities. The unpenalized regression estimates in the multiple linear models were slightly larger than LASSO or ENET estimates.
SPCA identified 6 PC scores that explained 79% of the variation in serum POP concentrations. Of the identified PCs, PC5 had a statistically significant inverse association with the Reading Composite scores (β=−2.8, 95% CI −5.3, −0.2). BDE-153 and BDE-100 loaded strongly (loading>0.22) onto PC5, indicating that these two PBDE congeners were associated with lower reading scores (Table 3). However, a significant positive association with Reading Composite score was noted for PC3 (β=2.5, 95% CI 1.0, 4.0). This PC score explained the most variation in PFAS (loading>0.41), which suggests higher reading scores with increased prenatal PFAS concentrations.
Table 3.
Sparse PCA analysis results of 17 POPs serum concentrations in relation to Reading Composite Scores at age 8 yearsa
| Chemical | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
|---|---|---|---|---|---|---|
| Log10 PBDEs | ||||||
| BDE-28 | 0.490 | |||||
| BDE-47 | 0.563 | |||||
| BDE-99 | 0.511 | |||||
| BDE-100 | 0.361 | 0.225 | ||||
| BDE-153 | 0.890 | −0.153 | ||||
| Log10 PCBs | ||||||
| CB-74 | 0.401 | −0.158 | ||||
| CB-99 | 0.424 | 0.062 | −0.015 | |||
| CB-118 | 0.354 | 0.065 | −0.226 | −0.349 | ||
| CB-138_158 | 0.441 | 0.016 | ||||
| CB-153 | 0.512 | 0.043 | ||||
| CB-180 | 0.276 | −0.196 | 0.307 | 0.358 | ||
| Log10 P,P’-DDE | 0.037 | 0.559 | 0.088 | 0.221 | ||
| Log10 P,P’-DDT | 0.829 | −0.037 | −0.099 | |||
| Log10 PFAS | ||||||
| PFOS | 0.552 | |||||
| PFOA | −0.005 | 0.500 | 0.012 | −0.079 | ||
| PFHxS | −0.066 | 0.413 | 0.062 | −0.572 | ||
| PFNA | 0.027 | 0.524 | 0.555 | |||
| % variance explained | 28.4 | 20.8 | 15.1 | 5.5 | 4.7 | 4.1 |
| Cumulative % | 28.4 | 49.2 | 64.3 | 69.8 | 74.5 | 78.6 |
| Regression estimates | 1.18 | −0.51 | 2.48 | −0.59 | −2.77 | 1.39 |
| with outcome | (−0.35, 2.71) | (−2.09, 1.07) | (0.96, 4.00) | (−3.07, 1.88) | (−5.32, −0.21) | (−1.19, 3.97) |
Blank spaces represent loadings that have been zeroed out by the SPCA model
We identified high weights for BDE-100 (35%) and BDE-28 (16%) with an estimate of - 2.6 (95% CI −5.4, 0.2) per WQS unit in the inverse association (Table 4). WQS analysis for the positive association identified high weights for PFNA (29%) and CB-180 (21%), with an estimate of 5.4 (95% CI 2.0, 8.9) per WQS unit.
Table 4.
Weighted quantile Sum (WQS) regression analysis results for maternal POP serum concentrations and child reading skills at age 8 years
| Chemical | Inverse association with WQS | Positive association with WQS |
| WQS unit | −2.63 (−5.41, 0.15) | 5.49 (2.04, 8.94) |
| Average weight | Average weight | |
| Log10 PBDEs | ||
| BDE-28 | 0.157 | 0.000 |
| BDE-47 | 0.033 | 0.000 |
| BDE-99 | 0.078 | 0.001 |
| BDE-100 | 0.354 | 0.000 |
| BDE-153 | 0.102 | 0.003 |
| Log10 PCBs | ||
| CB-74 | 0.017 | 0.009 |
| CB-99 | 0.022 | 0.011 |
| CB-118 | 0.002 | 0.132 |
| CB-138_158 | 0.007 | 0.013 |
| CB-153 | 0.000 | 0.098 |
| CB-180 | 0.006 | 0.214 |
| Log10 P,P’-DDE | 0.039 | 0.006 |
| Log10 P,P’-DDT | 0.139 | 0.005 |
| Log10 PFAS | ||
| PFOS | 0.000 | 0.121 |
| PFOA | 0.007 | 0.035 |
| PFHxS | 0.035 | 0.062 |
| PFNA | 0.000 | 0.290 |
Adjusted for maternal age, race, education, household income, marital status, depression, serum cotinine, blood lead, fish consumption, IQ, and child sex and HOME score
In BKMR analysis with component-wise variable selection, the PIPs were high for all PBDE congeners, CB-118, CB-153, CB-180, PFOS, PFOA, and PFNA (>0.3, Table 5), with dose-response patterns shown in Supplemental Figure S4. The conditional PIP for BDE-28, BDE-47, and BDE-99 was lower than other two congeners within the PBDE group in the hierarchical variable selection, so we retained BDE-153 and BDE-100 in a reduced BKMR model. Similarly, we retained PFOA and PFNA for the PFAS group. Hence, the reduced BKMR model involved seven POPs. In this reduced model, the component-wise variable selection PIPs were highest for BDE-153 (0.80), PFNA (0.79), and PFOA (0.82) (Supplemental Table S1). The hierarchical variable selection for the reduced BKMR model had an estimated group PIP of 0.91, 0.80, and 0.94 for PBDEs, PCBs, and PFAS, respectively. BKMR model examined 42 different bivariate exposure-response functions between the seven identified chemicals, estimating a single chemical’s association while holding a second chemical fixed at the 10th, 50th, or 90th percentile (Figure 1). Inverse associations with two PBDE congeners and positive associations with PCB congeners and PFAS (PFNA and PFOA) were mainly parallel despite the increase in the concentration of the second chemical, suggesting that there is no synergistic or multiplicative interaction between the seven POPs in relation to the reading scores. Results for a change in the estimate of a single POP concentration from the 25th to the 75th percentile while holding all other POP concentrations at either the 25th, 50th or 75th percentile had similar conclusions (Supplemental Figure S5). With regard to the cumulative effect of simultaneous exposure to the seven identified POPs in the BKMR model, Figure 2 displays the overall risk summary of all seven predictors at a particular percentile compared with a reference while all predictors are at the 50th percentile. Most of the seven POPs are contributing to better Reading Composite scores in this dataset. Therefore, the estimate increases with increasing percentiles of overall exposure.
Table 5.
Bayesian Kernel Machine Regression (BKMR) variable selection results of maternal POP serum concentrations and child reading skills at age 8 years
| Chemical | Posterior Inclusion Probabilities (PIP) Component-wise variable selectiona | Posterior Inclusion Probabilities Hierarchical variable selection | |
|---|---|---|---|
| Group PIP | Conditional PIP | ||
| Log10 PBDEs | |||
| BDE-28 | 0.330 | 0.845 | 0.111 |
| BDE-47 | 0.368 | 0.845 | 0.125 |
| BDE-99 | 0.316 | 0.845 | 0.068 |
| BDE-100 | 0.360 | 0.845 | 0.212 |
| BDE-153 | 0.504 | 0.845 | 0.485 |
| Log10 PCBs | |||
| CB-74 | 0.253 | 0.668 | 0.194 |
| CB-99 | 0.253 | 0.668 | 0.092 |
| CB-118 | 0.422 | 0.668 | 0.272 |
| CB-138_158 | 0.221 | 0.668 | 0.065 |
| CB-153 | 0.375 | 0.668 | 0.207 |
| CB-180 | 0.331 | 0.668 | 0.170 |
| Log10 P,P’-DDE | 0.216 | 0.488 | 0.473 |
| Log10 P,P’-DDT | 0.255 | 0.488 | 0.527 |
| Log10 PFAS | |||
| PFOS | 0.311 | 0.929 | 0.051 |
| PFOA | 0.543 | 0.929 | 0.394 |
| PFHxS | 0.172 | 0.929 | 0.023 |
| PFNA | 0.679 | 0.929 | 0.532 |
Adjusted for maternal age, race, education, household income, marital status, depression, serum cotinine, blood lead, fish consumption, IQ, and child sex and HOME score
Shaded PIPs indicate chemicals included in the reduced BKMR model
Figure 1.
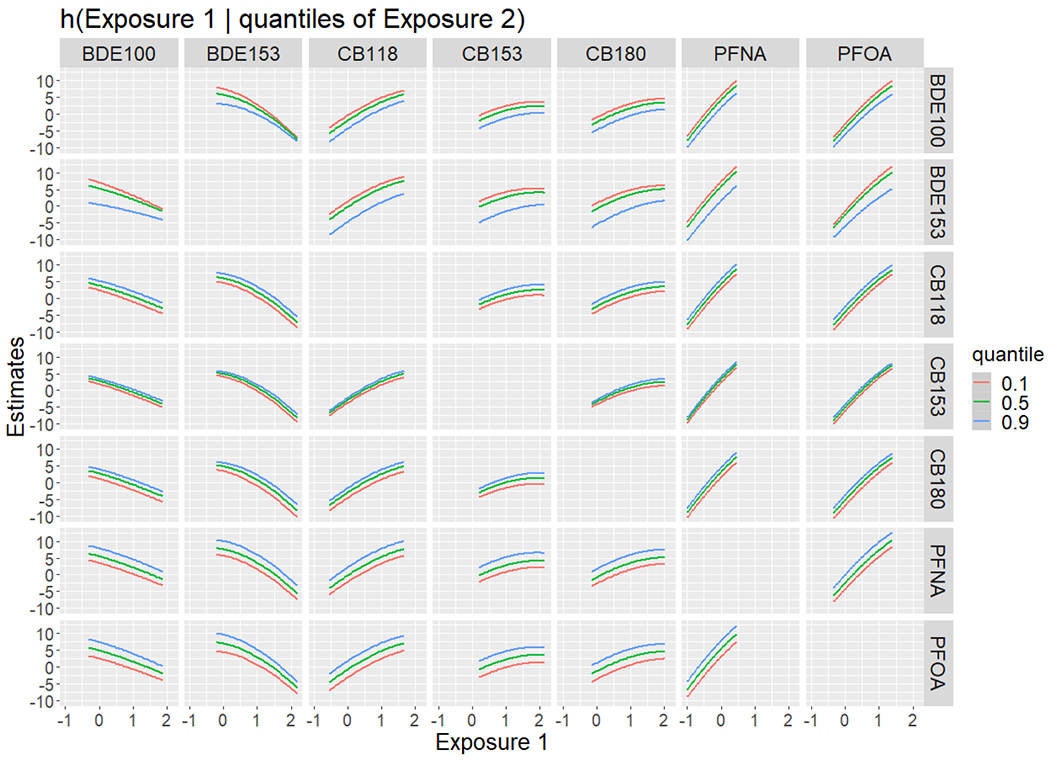
Bivariate exposure-response function showing a single predictor (Exposure 1) while the second predictor was fixed at 10th, 50th, or 90th percentile
Figure 2.
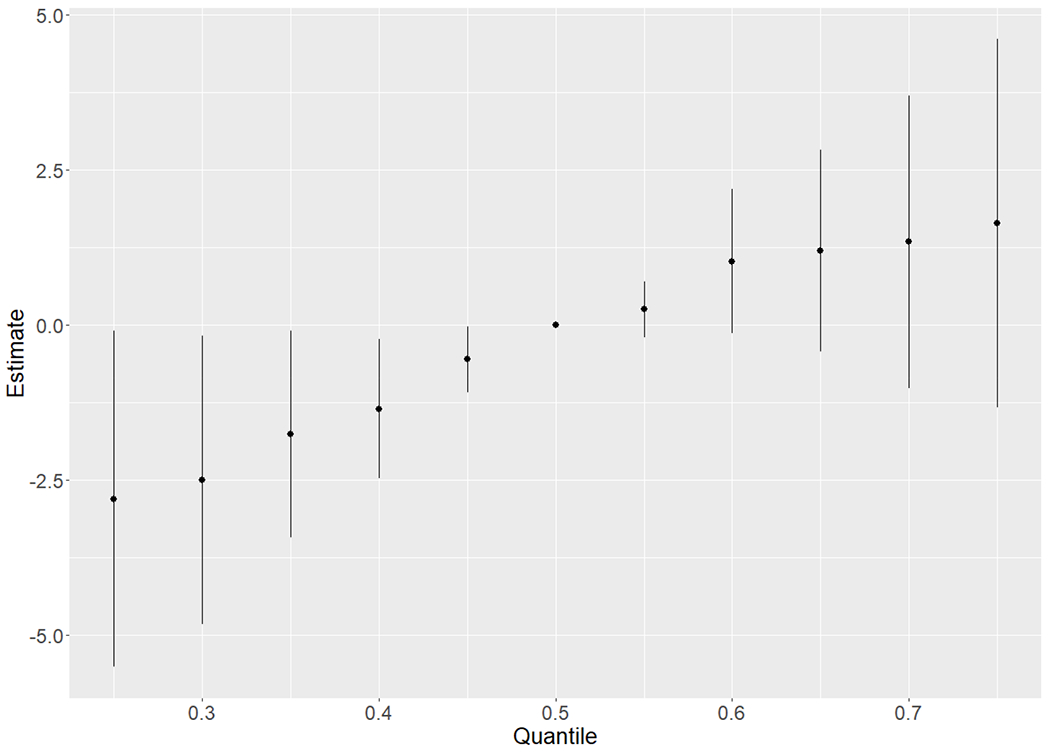
Overall risk summary for Reading Composite score of all 7 predictors identified in BKMR analysis (BDE-100, BDE-153, CB-118, CB-153, CB-180, PFOA, and PFNA) at a particular percentile (from 25th to 75th at an interval of 5th) compared with a reference while all predictors are at 50th percentile
In the BART analysis, the Partial Dependence Plots indicating dose-response pattern of POP exposures showed similar results to BKMR mixture analysis: inverse associations with PBDE congeners, and positive associations with PCB congeners and PFAS (Supplemental Figure S6). The variable selection “Local” procedure identified maternal IQ, maternal education, PFOS, CB-180, and PFOA above the individual thresholds of inclusion proportion (Supplemental Figure S7). BDE-153, CB-118, PFNA, and BDE-100 did not meet the thresholds but were among the top 10 variables with high inclusion proportion. The variable selection “Global Max” or “Global SE” procedures were more stringent, with maternal IQ meeting both thresholds and additionally maternal education and CB-180 meeting the threshold of “Global SE” procedure.
The results of the six mixture statistical methods are summarized in Table 6. Despite some inconsistency in individual chemicals, the methods identified inverse associations for BDE-28, BDE-100, and BDE-153, and positive associations for CB-118, CB-153, and CB-180. The findings of DDE and DDT were not remarkable. PFOA, PFNA, and to some extent, PFOS were positively associated with reading skills.
Table 6.
Summary of the associations identified from different methods
| Chemical | LASSO | Elastic Net | SPCA | WQS | BKMR | BART |
|---|---|---|---|---|---|---|
| Log10 PBDEs | ||||||
| BDE-28 | ↓ | ↓ | ↓ | |||
| BDE-47 | ||||||
| BDE-99 | ↓ | |||||
| BDE-100 | ↓ | ↓ | ↓ | ↓ | ||
| BDE-153 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Log10 PCBs | ||||||
| CB-74 | ||||||
| CB-99 | ||||||
| CB-118 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| CB-138_158 | ||||||
| CB-153 | ↑ | ↑ | ↑ | |||
| CB-180 | ↑ | ↑ | ↑ | ↑ | ↑ | |
| Log10 P,P’-DDE | ||||||
| Log10 P,P’-DDT | 1 | |||||
| Log10PFAS | ||||||
| PFOS | ↑ | ↑ | ↑ | |||
| PFOA | ↑ | ↑ | ↑ | ↑ | ↑ | |
| PFHxS | ||||||
| PFNA | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
4. Discussion
We used 6 statistical approaches, namely LASSO, ENET, SPCA, WQS regression, BKMR, and BART, to examine chemical mixtures of 17 POPs during pregnancy in relation to reading skills at age 8 years in a sample of 161 mother-child dyads from the HOME Study. Findings across these multi-pollutant models were relatively consistent but not interchangeable. All mixture analyses revealed inverse associations between prenatal PBDEs and childhood reading skills at school age. We also noted better Reading Composite scores on the WRAT-4 with higher concentrations of PCBs and PFAS. The six statistical approaches have their own strengths and limitations, and multiple approaches may reveal different aspects of the associations between mixture exposure and the outcome.
Previously, the HOME Study and the Norwegian Human Milk Study (HUMIS) utilized frequentist shrinkage methods to examine chemical mixtures of POPs as neurotoxicants. In the HOME Study, a mixture of PBDEs and PCBs were investigated to determine the relationship with reading skills, Full Scale IQ, and externalizing problems in children at 8 years (Zhang et al., 2017). Findings from the LASSO method were similar to the present study’s overall conclusions, with decrements in reading composite scores noted with increased prenatal PBDEs. Zhang et al. (2017) reported that higher PBDE serum concentrations were associated with lower FSIQ and more externalizing behavior problems in children. They additionally observed higher scores for WRAT-II and FSIQ with prenatal CB-118, CB-153, and CB-180. The elastic net regression models produced mixed results between studies. Lenters et al. (2019) examined a mixture of 27 POPs, including PCBs, OCPs, brominated flame retardants, and PFAS, measured in breastmilk and attention-deficit/hyperactivity disorder (ADHD) in children ages 7-14 years using elastic net penalized logistic regression models. They identified increased odds of ADHD in children with higher β-hexachlorocyclohexane (β-HCH) and PFOS and lower odds of an ADHD diagnosis with increased p,p’-DDT (Lenters et al., 2019).
In a mixture analysis of 51 endocrine-disrupting chemicals in the HOME Study, Braun et al. (2014) utilized a two-stage semi-Bayesian model to estimate associations with autistic behaviors in children ages 4 and 5 years. Findings were mixed, with more autistic behaviors reported with higher concentrations of prenatal BDE-28 and trans-nonachlor and lower autistic behaviors observed with detectable levels of CB-178, β-HCH, and BDE-85 and increasing concentrations of PFOA. A Bayesian approach was also applied in the examination of 25 endocrine disrupting chemicals measured in maternal serum at 15-20 weeks gestation with autism spectrum disorder (ASD) and intellectual disability in the Early Markers for Autism study (Hamra et al., 2019). However, results did not support an association between any individual chemical and ASD or intellectual disability in the mixture analysis.
One study used ENET, PCA, and Bayesian model averaging (BMA) models to examine POPs as potential neurotoxicants (Forns et al., 2016). They reported that only p,p’-DDT was consistently identified across all three methods, where p,p’-DDT was associated with more behavioral problems at age 12 months in HUMIS infants in a mixture analysis of 24 POPs. However, in the present study we did not observe a consistent relationship between p,p’-DDT and reading abilities in school age children despite modest contribution to the inverse association WQS model.
Nevertheless, all six mixture methods applied in the present study revealed inverse associations between prenatal PBDEs and reading skills at age 8 years. While inverse associations for individual BDE congeners were not entirely consistent among approaches, taken together the results still support PBDEs’ adverse role in the development of reading skills. Our current findings add to the growing evidence from epidemiological studies that have examined prenatal PBDEs in isolation and reported adverse neurodevelopment and behavior in children, including decrements in FSIQ, more hyperactivity and attention problems, and impairments in executive function (Lam et al., 2017; Vuong et al., 2018). Previously only the HOME Study investigated associations between PBDEs and reading ability in children, with reports of poorer reading scores with higher prenatal and postnatal concentrations (Liang et al., 2019; Zhang et al., 2017). While exact mechanisms of action for PBDE neurotoxicity remain unclear, toxicological studies have shown PBDEs disrupt thyroid hormones through competitive binding to transthyretin or directly interacting with thyroid hormone receptors, induce neuronal cell death via oxidative stress, disrupt signal transduction, interfere with calcium signaling and homeostasis, and decrease neuron and oligodendrocyte differentiation (Costa et al., 2014; Ibhazehiebo et al., 2011; Meerts et al., 2000; Richardson et al., 2008).
A small number of studies using conventional single pollutant models have examined PFAS and reading skills. While the C8 Health Project reported null associations between PFOA and reading skills at ages 6–12 years (Stein et al., 2013), we observed positive associations between prenatal and postnatal PFOA, PFOS, and PFNA concentrations and reading skills in children at ages 5 and 8 years (Zhang et al., 2018). Our study showed that after considering joint exposures to several POPs, prenatal PFAS were consistently associated with improved reading abilities in children at age 8 years across all the applied statistical approaches. Our findings are biologically plausible given PFAS’ classification as peroxisome proliferator-activated receptor-gamma (PPARγ) agonists (Vanden Heuvel et al., 2006). PFAS may have neuroprotective effects that might alleviate inflammatory responses incurred from chronic and acute neurological insults (Kapadia et al., 2008). Moreover, protective associations have been reported in other studies, including the C8 Health Project, the Danish National Birth Cohort, the Taiwan Birth Panel Study, and Taiwan Early-Life Cohort, with regard to prenatal exposures and various neurodevelopmental domains (Fei and Olsen, 2011; Lien et al., 2016; Stein et al., 2013).
We also identified PCBs to be associated with better reading abilities, with fairly consistent results among mixture methods. The HOME Study previously reported similar findings utilizing LASSO (Zhang et al., 2017). However, Stewart et al. (2003) observed a significant inverse relationship between prenatal PCBs and word knowledge scores in children at ages 38 and 54 months. While PCB neurotoxicity has been reported in several studies in the Faroe Islands, Germany, Holland, Taiwan, and the United States (Schantz et al., 2003), studies from the Netherlands reported prenatal OH-PCBs were associated with optimal mental development and neurological functioning (Berghuis et al., 2014; Ruel et al., 2019). The positive associations observed in the present study may be due to residual confounding. The primary route of human exposure to PCBs is through ingestion of contaminated fish (Braun et al., 2014). Fish consumption can greatly benefit fetal neurodevelopment as it is the primary source of n-3 long-chain polyunsaturated fatty acid (PUFA) (Conway et al., 2018). Higher maternal n-3 PUFA status is associated with better language development in offspring (Strain et al., 2012; Strain et al., 2015). Further, PCB concentrations in the HOME Study are positively correlated with fish consumption. Although we adjusted for fish consumption during pregnancy, we do not have direct measurements of PUFA from HOME Study mothers.
The inconsistencies among studies examining POP mixtures on neurodevelopment may be attributed in part to the differences in applied statistical approaches. Our study is among the most comprehensive to date, utilizing six multipollutant models, including frequentist shrinkage, variable selection, dimension reduction, and Bayesian methodologies. These models do overlap with some approaches that were previously used, such as LASSO, ENET, and a variant of SPCA, but not all. Secondly, using multi-pollutant models does not assume that humans are exposed to chemicals in isolation. However, the selection of chemicals included in mixture analyses differed among studies. We examined 17 POPs from four chemical classes, whereas the range of POPs and chemical classes that were examined in other mixture studies were between 8-52 POPs and 2-6 chemical classes. Concentrations of chemicals also varied between studies. For example, in the HOME Study, BDE-47 had a median of 20.1 ng/g lipid in serum compared to 1.0 ng/g lipid in breastmilk in HUMIS (Lenters et al., 2019). Third, while all POP mixture studies investigated the impact on neurodevelopment, the domain itself differed. Aside from Zhang et al. (2017), the neurodevelopmental outcomes studied included autistic behaviors, behavioral problems, and ADHD. Further, age of neurodevelopmental assessment between studies differed. Last, covariate adjustment varied between studies, which depended on availability of covariate measurements as well as the population characteristics.
Our study had several strengths, including the prospective design and availability of many important confounders. We were able to adjust for maternal IQ, maternal blood lead level, serum cotinine, maternal depression, and sociodemographic factors, such as education, income, and marital status. We used of six statistical methods to examine POP mixtures. Currently there is no quintessential statistical method to examine chemical mixtures, because each methodology has its unique set of advantages and disadvantages. As such, we decided to examine POP mixtures on neurodevelopment employing approaches that allowed for chemical variable selection, interactions, and cumulative effect. The LASSO and ENET had the strength of dimension reduction to selected chemicals and the SPCA reduced the dimension to a few components that can be integrated in multiple linear models. The BKMR and BART models provided non-linear exposure-response pattern visualization, and the BKMR seemed more capable for bivariate interaction analysis. Both WQS and BKMR provided an assessment of cumulative effect in a comprehensible way for interpretation. With the understanding that no model itself is deemed superior, we would rather examine whether consistency in the identification of chemicals is present in our research question. Our overall conclusions are based on findings that were consistent across all our applied statistical approaches.
Our study had several limitations. Residual confounding cannot be excluded as with any epidemiological study. We do not have information on other factors that may have affected our exposures and outcome, such as measured maternal PUFA levels and school settings. Second, study attrition may have resulted in selection bias. Although, for the most part we did not observe significant differences in demographics and POP serum concentrations between children followed at age 8 years and those who were not (Table 7). Significantly higher concentrations of prenatal PFHxS was noted among mother-child dyads who were excluded (2.3±3.4 ng/mL) from the analysis due to missing information compared to those included (1.7±1.4 ng/mL). Mothers excluded also had higher educational attainment and IQ compared to those included. Third, our modest sample size precluded us from examining potential interactions by sex using LASSO and ENET. Fourth, there was high correlation within PBDE congeners and within PCB congeners. Therefore, it is difficult to tease out associations of one congener from others in the same chemical group despite utilizing a mixture analysis. Fourth, when applying for these statistical methods, we were limited by the model assumptions and method constraints. LASSO and ENET might select chemicals without sufficient biological underpinnings, and SPCA was not a supervised approach and the relevance with the outcome might be compromised. These methods might be used as initial screening of chemicals in linear models, but the biological relevance and cumulative effects need to be confirmed by other models. The WQS had to split positive and negative models to assure positive weights, reducing its ability to estimate overall effect. BKMR might perform better with reduced chemical variables as constructing high-dimensional dose-response space was more complicated with a large amount of exposures. BART seemed to be stringent in selecting chemical variables and the interpretation of results was not straightforward for cumulative effects. More detailed summary of these method strengths and limitations can be found in a comprehensive review article (Lazarevic et al., 2019).
Table 7.
Comparing maternal and child characteristics of participants included (n=161) and excluded (n=229) in the examination of prenatal POPs and reading ability at age 8 years, HOME Studya
| Included | Excluded | |||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Maternal age, years | ||||||
| <25 | 41 | (25.5) | 55 | (24.1) | ||
| 25-34 | 97 | (60.3) | 134 | (58.8) | ||
| >35 | 23 | (14.3) | 39 | (17.1) | ||
| Race/ethnicity | ||||||
| Non-Hispanic White | 96 | (59.6) | 141 | (63.2) | ||
| Non-Hispanic Black and Others | 65 | (40.4) | 82 | (36.8) | ||
| Education | ||||||
| High school or less | 41 | (25.5) | 59 | (26.5) | ||
| Some college or 2 year degree | 46 | (28.6) | 47 | (21.1) | ||
| Bachelor’s | 51 | (31.7) | 59 | (26.5) | ||
| Graduate or professional | 23 | (14.3) | 58 | (26.0) | ||
| Parity | ||||||
| Nulliparous | 69 | (42.9) | 102 | (45.1) | ||
| Primiparous | 47 | (29.2) | 77 | (34.1) | ||
| Multiparous | 45 | (28.0) | 47 | (20.8) | ||
| Mode of Delivery | ||||||
| Vaginal | 121 | (75.2) | 157 | (68.9) | ||
| Cesarean | 40 | (24.8) | 71 | (31.1) | ||
| Breastfeeding current child | ||||||
| No | 29 | (18.0) | 42 | (19.8) | ||
| Yes | 132 | (82.0) | 170 | (80.2) | ||
| Maternal Vitamin Use | ||||||
| Daily | 129 | (80.1) | 165 | (74.0) | ||
| <Daily | 22 | (13.7) | 41 | (18.4) | ||
| Never | 10 | (6.2) | 17 | (7.6) | ||
| Maternal alcohol consumption | ||||||
| Never | 91 | (56.5) | 125 | (56.1) | ||
| <1 per month | 47 | (29.2) | 68 | (30.5) | ||
| >1 per month | 23 | (14.3) | 30 | (13.5) | ||
| Maternal Smoking | ||||||
| None | 140 | (87.0) | 181 | (79.4) | ||
| ETS | 12 | (7.5) | 23 | (10.1) | ||
| Active | 9 | (5.6) | 24 | (10.5) | ||
| Maternal BMI | ||||||
| Underweight/Normal | 61 | (37.9) | 104 | (45.6) | ||
| Overweight | 59 | (36.7) | 69 | (30.3) | ||
| Obese | 41 | (25.5) | 55 | (24.1) | ||
| Child Sex | ||||||
| Male | 68 | (42.2) | 114 | (49.8) | ||
| Female | 93 | (57.8) | 115 | (50.2) | ||
| Marital Status | ||||||
| Married | 119 | (73.9) | 185 | (83.0) | ||
| Not Married | 42 | (26.1) | 38 | (17.0) | ||
| Mean (SD) | Mean (SD) | |||||
| Maternal Blood | ||||||
| Leadb | 0.7 (0.3) | 0.8 (0.4) | ||||
| Maternal Serum | ||||||
| DDT | 3.1 (3.9) | 4.0 (8.1) | ||||
| DDE | 95.8 (188.3) | 118.9 (207.4) | ||||
| BDE-28 | 1.7 (2.0) | 1.7 (3.0) | ||||
| BDE-47 | 35.2 (49.3) | 38.8 (103.4) | ||||
| BDE-99 | 8.4 (11.9) | 11.3 (37.6) | ||||
| BDE-100 | 7.3 (11.6) | 8.5 (18.0) | ||||
| BDE-153 | 10.6 (19.9) | 11.4 (19.2) | ||||
| CB-74 | 3.2 (1.9) | 3.5 (2.0) | ||||
| CB-99 | 3.3 (2.3) | 3.3 (2.4) | ||||
| CB-118 | 6.0 (5.3) | 6.5 (5.9) | ||||
| CB-138/158 | 9.7 (8.2) | 10.5 (8.9) | ||||
| CB-153 | 13.5 (13.0) | 14.9 (13.6) | ||||
| CB-180 | 8.4 (9.9) | 8.9 (7.7) | ||||
| ΣPCBs | 53.4 (45.1) | 52.6 (39.4) | ||||
| PFOA | 6.1 (3.8) | 6.2 (3.7) | ||||
| PFOS | 13.9 (7.9) | 15.0 (7.4) | ||||
| PFHxS | 1.7 (1.4) | 2.3 (3.4) | ||||
| PFNA | 1.0 (0.4) | 1.0 (0.4) | ||||
| Maternal IQb | 105.1 (14.7) | 108.4 (14.3) | ||||
Units: lead (μg/dL); OCPs, PBDEs and PCBs (ng/g lipid); PFAS (ng/mL)
Percentages may not add to 100% due to rounding.
p < 0.05
5. Conclusions
Analyses from six statistical methods examining chemical mixtures consistently identified inverse associations between maternal PBDE serum concentrations and children’s reading skills in the HOME Study. In contrast, we observed positive associations between PCBs and PFAS and reading skills. Future studies examining POP mixtures on neurodevelopment should incorporate analytical approaches that are able to assess nonlinear dose response and effect measure modification by sex if they are adequately powered. Studies examining repeated postnatal exposures to POP mixtures are warranted to determine whether there are sensitive windows of susceptibility during childhood using methods like lagged kernel machine regression (LKMR) (Liu et al., 2018). For statistical method comparisons, we believe these methods are complementary rather than equal in utilization. LASSO and ENET can be used for screening chemicals from a large chemical mixture, WQS and BKMR can be used for cumulative effect estimation, and SPCA can be supplemented by a supervised approach like sparse partial least square regression method (Chun and Keles, 2010).
Supplementary Material
Acknowledgements:
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES024381, R01 ES025214, R01 ES014575, R00 ES020346, R01 ES028277, T32ES010957, P30ES006096; EPA P01 R829389).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
References
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory, 2nd ed. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Berghuis SA, Soechitram SD, Sauer PJ, Bos AF, 2014. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with neurological functioning in 3-month-old infants. Toxicol Sci 142, 455–462. [DOI] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, Hauser R, Webster GM, Chen A, Lanphear BP, 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 122, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2017. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 46, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R, 1984. Home Observation for Measurement of the Environment. University of Arkansas, Little Rock, AR. [Google Scholar]
- Carrico C, 2013. Characterization of a Weighted Quantile Score Approach for Highly Correlated Data in Risk Analsyis Scenarios. Virginia Commonwealth University. [Google Scholar]
- Chipman HA, George EI, McCulloch RE, 2010. Bart: Bayesian Additive Regression Trees. Annals of Applied Statistics 4, 266–298. [Google Scholar]
- Chun H, Keles S, 2010. Sparse partial least squares regression for simultaneous dimension reduction and variable selection. J R Stat Soc Series B Stat Methodol 72, 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MC, Mulhern MS, McSorley EM, van Wijngaarden E, Strain JJ, Myers GJ, Davidson PW, Shamlaye CF, Yeates AJ, 2018. Dietary Determinants of Polyunsaturated Fatty Acid (PUFA) Status in a High Fish-Eating Cohort during Pregnancy. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C, 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Olsen J, 2011. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect 119, 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Mandal S, Iszatt N, Polder A, Thomsen C, Lyche JL, Stigum H, Vermeulen R, Eggesbo M, 2016. Novel application of statistical methods for analysis of multiple toxicants identifies DDT as a risk factor for early child behavioral problems. Environ Res 151, 91–100. [DOI] [PubMed] [Google Scholar]
- Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA, 2013. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health 12, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Squibb K, Youngstrom E, Anthony LG, Kenworthy L, Lipkin PH, Mattison DR, Lakind JS, 2010. Using systematic reviews and meta-analyses to support regulatory decision making for neurotoxicants: lessons learned from a case study of PCBs. Environ Health Perspect 118, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D, 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev 35, 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, Lyall K, Windham GC, Calafat AM, Sjodin A, Volk H, Croen LA, 2019. Prenatal Exposure to Endocrine-disrupting Chemicals in Relation to Autism Spectrum Disorder and Intellectual Disability. Epidemiology 30, 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 5, 46–51. [Google Scholar]
- Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N, 2011. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect 119, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak JF, Jastak SR, 1965. Wide Range Achievement Test: Manual. Guidance Associates, Wilmington, DE. [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A, 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd 74, 97–98. [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R, 2008. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci 13, 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A 1218, 2133–2137. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ, 2017. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis. Environ Health Perspect 125, 086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic N, Barnett AG, Sly PD, Knibbs LD, 2019. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals: A Review of Existing Approaches and New Alternatives. Environ Health Perspect 127, 26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Viberg H, 2013. A single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology 37, 190–196. [DOI] [PubMed] [Google Scholar]
- Lenters V, Iszatt N, Forns J, Cechova E, Kocan A, Legler J, Leonards P, Stigum H, Eggesbo M, 2019. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: A multi-pollutant analysis of a Norwegian birth cohort. Environ Int 125, 33–42. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Rylander L, Vermeulen R, 2016. Prenatal Phthalate, Perfluoroalkyl Acid, and Organochlorine Exposures and Term Birth Weight in Three Birth Cohorts: Multi-Pollutant Models Based on Elastic Net Regression. Environ Health Perspect 124, 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Vuong AM, Xie C, Webster GM, Sjodin A, Yuan W, Miao M, Braun JM, Dietrich KN, Yolton K, Lanphear BP, Chen A, 2019. Childhood polybrominated diphenyl ether (PBDE) serum concentration and reading ability at ages 5 and 8years: The HOME Study. Environ Int 122, 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien GW, Huang CC, Shiu JS, Chen MH, Hsieh WS, Guo YL, Chen PC, 2016. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere 156, 118–127. [DOI] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y, 2018. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr Environ Health Rep 5, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SH, Bobb JF, Lee KH, Gennings C, Claus Henn B, Bellinger D, Austin C, Schnaas L, Tellez-Rojo MM, Hu H, Wright RO, Arora M, Coull BA, 2018. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures. Biostatistics 19, 325–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A, 2000. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci 56, 95–104. [DOI] [PubMed] [Google Scholar]
- Moser VC, Shafer TJ, Ward TR, Meacham CA, Harris MW, Chapin RE, 2001. Neurotoxicological outcomes of perinatal heptachlor exposure in the rat. Toxicol Sci 60, 315–326. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18, 495–500. [DOI] [PubMed] [Google Scholar]
- Polanska K, Jurewicz J, Hanke W, 2013. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit / hyperactivity disorder in children. Int J Occup Med Environ Health 26, 16–38. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E, 2013. Perfluoroalkylated compounds induce cell death and formation of reactive oxygen species in cultured cerebellar granule cells. Toxicol Lett 218, 56–60. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS, 2008. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol 226, 244–250. [DOI] [PubMed] [Google Scholar]
- Ruel MVM, Bos AF, Soechitram SD, Meijer L, Sauer PJJ, Berghuis SA, 2019. Prenatal exposure to organohalogen compounds and children’s mental and motor development at 18 and 30 months of age. Neurotoxicology 72, 6–14. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC, 2003. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111, 357–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, 1996. Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol 26, 709–737. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE 3rd, Patterson DG Jr., 2004. Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal Chem 76, 1921–1927. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ, 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116, 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC, 2013. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 24, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J, 2003. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol Teratol 25, 11–22. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Thurston SW, Harrington D, Mulhern MS, McAfee AJ, van Wijngaarden E, Shamlaye CF, Henderson J, Watson GE, Zareba G, Cory-Slechta DA, Lynch M, Wallace JM, McSorley EM, Bonham MP, Stokes-Riner A, Sloane-Reeves J, Janciuras J, Wong R, Clarkson TW, Myers GJ, 2012. Maternal PUFA status but not prenatal methylmercury exposure is associated with children’s language functions at age five years in the Seychelles. J Nutr 142, 1943–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JJ, Yeates AJ, van Wijngaarden E, Thurston SW, Mulhern MS, McSorley EM, Watson GE, Love TM, Smith TH, Yost K, Harrington D, Shamlaye CF, Henderson J, Myers GJ, Davidson PW, 2015. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am J Clin Nutr 101, 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R, 1996. Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society Series B-Methodological 58, 267–288. [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ, 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci 92, 476–489. [DOI] [PubMed] [Google Scholar]
- Verhoeven L, van Leeuwe J, Vermeer A, 2011. Vocabulary Growth and Reading Development across the Elementary School Years. Sci Stud Read 15, 8–25. [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A, 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm Behav 101, 94–104. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. Wide Range Achievement Test 4 professional manual. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjodin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2017. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Environ Health Perspect 125, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Ye X, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2018. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8years. Environ Int 111, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T, 2005. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society Series B-Statistical Methodology 67, 301–320. [Google Scholar]
- Zou H, Hastie T, Tibshirani R, 2006. Sparse principal component analysis. Journal of Computational and Graphical Statistics 15, 265–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


