Abstract
Over the past few decades, obesity has become a public health issue of global concern. Even though disparities exist between human populations, e.g., the higher liver fat content of the Japanese despite a lower body mass index (BMI), studies on the genetics of obesity still largely focus on populations of European descent, leading to a dearth of genetic data on non-European populations. In this context, this study aimed to establish a broad picture of the genetic attributes of the Japanese population, by examining a representative sample of 18,889 individuals participating in the Tohoku Medical Megabank Project cohort. We applied linear mixed model methods to 17 traits related to obesity and associated diseases to estimate the heritabilities explained by common genetic variants and the genetic correlations between each pair of traits. These analyses allowed us to quantify the SNP heritability of health indicators such as BMI (0.248 ± 0.032) and HDL cholesterol (0.324 ± 0.031), and to provide one of the few estimates of the SNP heritability of cystatin C in unrelated individuals (0.260 ± 0.025). We discuss potential differences between the Japanese and people of European ancestry with respect to the genetic correlations between urinary biomarkers and adiposity traits, for which large estimates were obtained. For instance, the genetic correlations between urine potassium level and the values for weight, BMI, waist circumference, and waist-to-height ratio ranged from 0.290 to 0.559, much higher than the corresponding estimates in the UK Biobank.
Keywords: Obesity, Heritability, Genetic correlation, Japanese population, Polygenic model analysis
Historically, obesity first emerged as a public health concern in Western high-income countries (Caballero 2007). Nowadays most low- and middle-income countries are facing rapid increases in overweight and obesity prevalence (NCD Risk Factor Collaboration 2016a), and as of 2016, almost 40% of the world’s adult population is estimated to be overweight, defined as body mass index (BMI) > 25 (World Health Organization 2018). The health problems are expected to increase further given that waist circumference (WC) has been rising at each BMI level (Popkin and Slining 2013) and that increases in abdominal fat have been shown to substantially heighten the risks of health problems at a given BMI level (Després et al. 2008).
Despite this worrying global trend, there is a gap between the amount of research conducted on the genetics of obesity in populations of European descent and non-European populations (Hruby and Hu 2015; Akiyama et al. 2017; Stryjecki et al. 2018). Thorough research in all human populations is warranted given the wide range of diseases that are affected by obesity, such as hypertension, kidney disease and type 2 diabetes (Hall 2000; Gansevoort et al. 2013; NCD Risk Factor Collaboration 2016b), and because the association between obesity and comorbidities varies across genetic groups (Setiawan et al. 2016; Heymsfield et al. 2016). For instance, compared with non-Hispanic whites in the US population, Japanese men are more susceptible to fatty liver with small increases in BMI and generally have higher liver fat content despite their lower BMI (Azuma et al. 2009).
It is essential to investigate how these differences relate to genetics. Examining population-level characteristics such as heritability, which measures the proportion of the total phenotypic variation that is due to genetic variation, provides insights into this diversity between populations and key information about the genetic basis of complex traits. Given that phenotypic variation is strongly dependent on both environmental and genetic factors, changes in the environment lead to changes in heritability. Studying heritability is therefore critical not only because of the genetic diversity that exists between genetic groups, but also because of the environmental differences that can be found across the world’s populations, as well as within a given population over time.
In this study, we focused on the genetic characteristics of the Japanese population, which is largely understudied compared with populations of European descent despite obvious genetic and environmental differences. By examining 17 traits related to obesity and associated conditions in a dataset of 18,889 individuals from the Miyagi and Iwate Prefectures, in Northeast Japan, we aimed to clarify the genetic correlations between each pair of traits and the SNP heritability of these traits in the Japanese population. We discuss the similarities and differences with other populations, such as that of the UK Biobank cohort.
Materials and Methods
Study population
The 23K dataset of the larger 150K Tohoku Medical Megabank Project (TMM) Community-Based and TMM Birth and Three-Generation cohorts was used for this study (Kuriyama et al. 2016). TMM was launched in the aftermath of the Great East Japan Earthquake of March 11, 2011, and aims to contribute to the realization of personalized healthcare and medicine through the construction of an integrated biobank consisting of clinical information, genome and omics data, and biospecimens. TMM follows a prospective cohort design, targeting a total of 150,000 participants from the general population of the Miyagi and Iwate Prefectures (Hozawa et al. 2020).
Using information on the age and sex of participants, we analyzed the phenotypes of 17 traits related to obesity and associated conditions: systolic blood pressure (SBP), diastolic blood pressure (DBP), triglycerides (TG), HDL cholesterol (HDL-C), height, weight, BMI, WC, waist-to-height ratio (WHtR), urine creatinine (uCre), urine chloride (uCl), urine potassium (uK), serum cystatin C (CysC), serum creatinine (SCre), serum uric acid (SUA), blood urea nitrogen (BUN), and hemoglobin (Hb). For each trait, the number of individuals with data available is listed in Table 1. These traits were chosen because they represent a wide range of biomarkers, including cardiovascular risk factors (SBP, DBP, TG, HDL-C), adiposity traits (weight, BMI, WC, WHtR), and urinary and blood renal function biomarkers (uCre, uCl, uK, CysC, SCre, SUA, BUN); these are either used as a measure of obesity or related to diseases for which obesity status is highly relevant. In particular, renal function biomarkers are of interest given that the relationship between obesity and chronic kidney disease (CKD) is known to be complex, obesity paradoxically being both a risk factor for the onset of CKD and a predictor of greater survival in CKD patients (Rhee et al. 2016). Associations between Hb and metabolic syndrome have also been reported (Hashimoto et al. 2015).
Table 1. Baseline characteristics of the study population.
| Clinical trait (unit) | Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Min. | Max. | n | Mean | SD | Min. | Max. | |
| Age (years) | 6,278 | 60.87 | 12.19 | 20 | 90 | 12,611 | 54.32 | 14.67 | 17 | 88 |
| SBP (mmHg) | 4,918 | 130.16 | 17.23 | 81 | 222 | 8,309 | 126.17 | 17.61 | 77 | 216 |
| DBP (mmHg) | 4,918 | 78.65 | 10.05 | 37 | 125 | 8,309 | 74.24 | 10.41 | 36 | 132 |
| TG (mg/dl) | 4,920 | 147.18 | 104.94 | 21 | 698 | 8,308 | 118.73 | 71.45 | 18 | 540 |
| HDL-C (mg/dl) | 4,920 | 57.97 | 16.15 | 22 | 151 | 8,308 | 66.21 | 16.12 | 22 | 154 |
| ht (cm) | 4,919 | 165.22 | 6.41 | 141.8 | 189 | 8,310 | 153.18 | 5.94 | 128.2 | 177 |
| wt (kg) | 4,919 | 66.22 | 10.18 | 38.8 | 117.9 | 8,310 | 54.44 | 8.96 | 30.0 | 104.3 |
| BMI (kg/m2) | 4,919 | 24.23 | 3.21 | 16.0 | 42.3 | 8,310 | 23.21 | 3.67 | 16.0 | 45.2 |
| WC (cm) | 4,907 | 85.08 | 8.63 | 60.0 | 130.5 | 8,291 | 81.48 | 9.51 | 56.7 | 126 |
| WHtR | 4,907 | 0.515 | 0.052 | 0.376 | 0.821 | 8,291 | 0.533 | 0.066 | 0.367 | 0.840 |
| uCre (g/l) | 6,157 | 1.09 | 0.63 | 0.07 | 4.73 | 11,432 | 0.80 | 0.55 | 0.02 | 4.01 |
| uCl (g/l) | 6,157 | 5.34 | 2.08 | 0.4 | 12 | 11,432 | 4.81 | 2.19 | 0.2 | 14.1 |
| uK (g/l) | 6,157 | 1.66 | 0.95 | 0.1 | 7.1 | 11,432 | 1.58 | 1.00 | 0.0 | 7.4 |
| CysC (mg/l) | 6,160 | 0.83 | 0.24 | 0.42 | 2.16 | 11,445 | 0.72 | 0.14 | 0.37 | 1.53 |
| SCre (mg/dl) | 6,161 | 0.82 | 0.34 | 0.37 | 2.58 | 11,445 | 0.59 | 0.12 | 0.22 | 1.27 |
| SUA (mg/dl) | 6,161 | 5.86 | 1.29 | 0.6 | 12.6 | 11,445 | 4.38 | 1.06 | 0.0 | 10.0 |
| BUN (mg/dl) | 6,161 | 16.19 | 4.45 | 6 | 42 | 11,445 | 14.26 | 4.00 | 3 | 35 |
| Hb (g/dl) | 6,154 | 14.80 | 1.21 | 8.0 | 19.8 | 10,700 | 13.20 | 1.05 | 6.9 | 18.0 |
BMI, body mass index; BUN, blood urea nitrogen; CysC, cystatin C; DBP, diastolic blood pressure; Hb, Hemoglobin; HDL-C, high-density lipoprotein cholesterol; ht, height; n, number of individuals; SBP, systolic blood pressure; SCre, serum creatinine; SD, standard deviation; SUA, serum uric acid; TG, triglycerides; uCl, urine chloride; uCre, urine creatinine; uK, urine potassium; WC, waist circumference; WHtR, waist-to-height-ratio; wt, weight.
To remove extreme phenotypes, participants with a BMI lower than 16 (‘severe thinness’ according to the World Health Organization international classification of BMI (1995)) were excluded, and in each sex group, individuals whose phenotypic value lay more than six standard deviations from the mean were also excluded.
The phenotypes were standardized by regressing the values for each trait on age in each sex group and converting the resulting residuals to z-scores (Supplementary Figure 1) (Yang et al. 2013). In the univariate and bivariate linear mixed models used for estimating heritabilities and genetic correlations, covariates comprised prefecture, genotyping platform, and the first ten eigenvectors (principal components) of the genotype data from principal component analysis (PCA) to adjust for population structure.
Genotyping and quality control
The samples of the 23K dataset provided by the Tohoku Medical Megabank Organization for this study were genotyped with Japonica v1 and v2 (Toshiba, Tokyo, Japan), Human Omni 2.5 (Illumina, San Diego, CA, USA), and OmniExpressExome (Illumina) genotyping arrays (Nagasaki et al. 2015; Kawai et al. 2015), and imputed with IMPUTE2 version 2.2.2 (Howie et al. 2009) using a whole-genome reference panel of 2,049 Japanese individuals (2KJPN) (Kuriyama et al. 2016). All statistical models used took into account the effect of genotyping platform, as described below.
Only SNPs that were available on all platforms and had an info score over 0.5 in each platform were included in our analyses. The following criteria were applied in PLINK v1.90 (Purcell et al. 2007) for quality control purposes: individual call rate ≥ 98%, SNP call rate ≥ 98%, minor allele frequency ≥ 1%, and Hardy-Weinberg equilibrium (P ≥ 10−6). All individuals with a heterozygosity rate more than three standard deviations from the mean were removed.
Related individuals were removed based on identity-by-descent (pi-hat > 0.2), which was calculated after performing linkage disequilibrium pruning (-indep-pairwise 50 5 0.2); for each pair of individuals, the individual with the highest genotyping rate was kept. Pruning was performed again before calculation of the first ten principal components (-indep-pairwise 50 5 0.2) and to produce the final dataset (-indep-pairwise 50 5 0.7).
After the quality control and filtering steps, 18,889 individuals and 866,089 autosomal SNPs remained for the downstream analysis.
Statistical analysis
The following univariate linear mixed model was used to estimate the genome-wide SNP heritabilities.
with Var() = and Var() = ,
where is a vector that represents the phenotypes (i.e., the sex- and age-adjusted z-scores), is a vector of fixed effects (including the overall mean, prefecture, genotyping platform, and the first ten principal components from PCA), is a vector of random effects representing the genomic additive effect, and is a vector of residual effects. is the design matrix for the fixed effects, is the genomic relationship matrix (GRM), and is a unit matrix; and represent the genetic and residual variances, respectively.
A bivariate model was used to estimate the genetic correlations between each pair of phenotypes. The bivariate model, which is a direct extension of the above univariate model, was as follows.
With
where and are the variance-covariance matrices for the genetic effects and residual effects, respectively (e.g., is the genetic covariance between traits 1 and 2), are n × 1 vectors of observations on the i-th trait (n being the number of individuals), are p × 1 vectors of fixed effects on the i-th trait (p being the number of levels for fixed effects), are n × p design matrices relating the vectors to the observation vectors , are n × 1 vectors of random effects on the i-th trait, and refers to the vectors of random residual effects associated with each individual on the i-th trait. These methods are described in more detail elsewhere (Thompson 1973; Szwaczkowski 2003; Lee et al. 2012).
The GRMs were computed with the Genetic Complex Trait Analysis tool (GCTA) by using all SNPs that passed quality control. The following equation was used to calculate the genetic relationship between two individuals and (Yang et al. 2011):
where and are the genotypes of the j-th and k-th individuals, respectively, at the i-th SNP; is the frequency of the reference allele at the i-th SNP; and is the total number of SNPs.
Computer software
GCTA v1.91.4 (ibid.) was used to perform most of the computations and statistical analyses: i.e., computation of GRMs, PCA, and univariate and bivariate Genomic Restricted Maximum Likelihood (GREML) analyses. The Average Information Restricted Maximum Likelihood (AI-REML) procedure was used to estimate variance components (Gilmour et al. 1995), and PLINK v1.90 (Purcell et al. 2007) was used to perform data filtering and quality control. R version 3.5.0 (Team RC 2019) was used to regress the phenotypes on age in each sex group and transform the residuals to z-scores, and the ggplot2 v3.1.0 package was used for data visualization (Wickham 2016).
Ethics statement
This project was conducted in accordance with the Japanese National Ethical Guidelines for Human Genome/Gene Analysis (Ministry of Education, Culture, Sports, Science and Technology et al. 2013) and was reviewed and approved by the ethics committee of Tohoku University. All participants gave their written consent prior to study enrollment.
Data availability
The analyses presented in this study were based on data accessed through the Tohoku Medical Megabank Organization (ToMMo) (https://www.megabank.tohoku.ac.jp/english/). To protect the privacy of the cohort participants, requests for the use of ToMMo biobank data for research projects should be made by applying directly to ToMMo, and are subject to review and approval by the Sample and Data Access Committee. Supplemental material available at figshare: https://doi.org/10.25387/g3.11295944
Results
Population characteristics
The main population characteristics are presented in Table 1. At baseline, the mean (± SD) age of the participants was 54.32 (± 14.67) for women and 60.87 (± 12.19) for men. BMI ranged from 16 to 45.2 kg/m2 (mean, 23.21 kg/m2 ± 3.67) in women and from 16 to 42.3 kg/m2 (mean, 24.23 kg/m2 ± 3.21) in men.
The participants were divided into four BMI categories: ‘thin’ (BMI < 18.5 kg/m2), ‘normal’ (18.5 ≤ BMI < 25 kg/m2), ‘overweight’ (25 ≤ BMI < 30 kg/m2), and ‘obese’ (BMI ≥ 30 kg/m2). Detailed information on the number of individuals in each BMI category is provided in Supplementary Table 1. To better visualize the profile of the target population, we also calculated the mean and standard deviation of each of the 17 traits in individuals grouped by BMI and WC category (Supplementary Tables 2 and 3). A large WC was classed as ≥85 cm in males and ≥90 cm in females, and a small WC was classed as <85 cm in males and <90 cm in females, following the clinical criteria applied for the diagnosis of metabolic syndrome in Japan (Ministry of Health, Labor and Welfare 2005).
SNP heritability estimates
For each of the traits examined, we computed the SNP heritability by performing univariate model analyses (Table 2). Given that these values only account for the additive effect of common genetic variants, they are expected to be lower than the heritability estimates typically available from twin and/or family studies. Apart from uK, all of the heritability estimates were significant (P < 0.05), indicating that a substantial share of the phenotypic variation in these complex human traits is due to common genetic variants in the Japanese population. The highest heritability estimate (± SE) was that of height (0.536 ± 0.031), with that of the other traits ranging from 0.033 (± 0.023) to 0.324 (± 0.031).
Table 2. Summary of the SNP heritabilities estimated by using univariate model analysis, for all 17 traits.
| Clinical trait | h2 | SE | P-value |
|---|---|---|---|
| SBP | 0.089 | 0.031 | 1.54E-03 |
| DBP | 0.131 | 0.031 | 6.52E-06 |
| TG | 0.196 | 0.031 | 8.98E-12 |
| HDL-C | 0.324 | 0.031 | 1.68E-28 |
| ht | 0.536 | 0.031 | 8.09E-74 |
| wt | 0.256 | 0.032 | 2.62E-17 |
| BMI | 0.248 | 0.032 | 3.33E-16 |
| WC | 0.209 | 0.032 | 9.01E-12 |
| WHtR | 0.238 | 0.032 | 6.99E-15 |
| uCre | 0.044 | 0.024 | 3.00E-02 |
| uCl | 0.060 | 0.023 | 4.29E-03 |
| uK | 0.033 | 0.023 | 7.11E-02 |
| CysC | 0.260 | 0.025 | 1.46E-29 |
| SCre | 0.252 | 0.025 | 2.01E-26 |
| SUA | 0.301 | 0.024 | 1.99E-40 |
| BUN | 0.138 | 0.024 | 3.86E-10 |
| Hb | 0.204 | 0.026 | 5.55E-17 |
BMI, body mass index; BUN, blood urea nitrogen; CysC, cystatin C; DBP, diastolic blood pressure; h2, heritability; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; ht, height; SBP, systolic blood pressure; SCre, serum creatinine; SE, standard error; SUA, serum uric acid; TG, triglycerides; uCl, urine chloride; uCre, urine creatinine; uK, urine potassium; WC, waist circumference; WHtR, waist-to-height-ratio; wt, weight.
We also computed the heritabilities by using bivariate models to compare them with the estimates of the univariate analyses. Although bivariate models are theoretically more accurate given that they take into account the information on both traits to simultaneously estimate the random effects, in practice the difference in accuracy between the two models was negligible. For instance, the SNP heritability estimates (± SE) of height calculated by using bivariate models ranged from 0.535 (± 0.031) to 0.537 (± 0.031), which was similar to 0.536 ± 0.031 in the univariate model; and those of BMI ranged from 0.245 (± 0.032) to 0.255 (± 0.032), which was comparable to 0.248 (± 0.032) in the univariate model. Given the closeness of the results, the heritability estimates calculated with the bivariate model analyses are not presented in further detail.
Phenotypic and genetic correlations
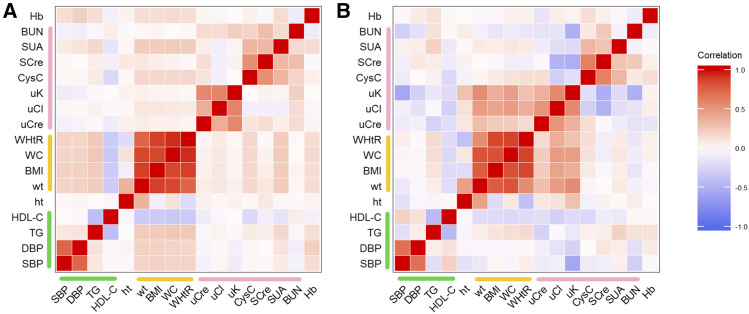
Phenotypic and genetic correlations (Figure 1, Table 3) were calculated for all pairwise combinations of traits (see Supplementary Table 4 for the covariances) by using bivariate model analysis. Unsurprisingly, large correlations were found between traits representing common health markers. For instance, the genetic correlations (± SE) between BMI and WC (0.829 ± 0.028) and between BMI and WHtR (0.874 ± 0.021), which are indirect measures of adiposity, were large and significant, as were those between SCre and CysC (0.614 ± 0.045) and between TG and HDL-C (-0.429 ± 0.072). These figures are largely comparable to the corresponding phenotypic correlations, which were computed as the Pearson correlation coefficients between the sex- and age-adjusted phenotypes: 0.874 for BMI vs. WC, 0.890 for BMI vs. WHtR, 0.543 for SCre vs. CysC, and -0.397 for TG vs. HDL-C.
Figure 1.
Heatmap of the phenotypic (A) and genetic (B) correlations between all 17 traits. The green, yellow, and pink lines correspond to cardiovascular risk factors, adiposity traits, and renal function biomarkers, respectively. BMI, body mass index; BUN, blood urea nitrogen; CysC, cystatin C; DBP, diastolic blood pressure; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; ht, height; SBP, systolic blood pressure; SCre, serum creatinine; SUA, serum uric acid; TG, triglycerides; uCl, urine chloride; uCre, urine creatinine; uK, urine potassium; WC, waist circumference; WHtR, waist-to-height-ratio; wt, weight.
Table 3.
Genetic correlations (± standard error) estimated by using bivariate model analysis (upper triangle), and phenotypic correlations (lower triangle) calculated as the Pearson correlation coefficients for the sex- and age-adjusted phenotypes, for all 17 traits.
| SBP | DBP | TG | HDL-C | ht | wt | BMI | WC | WHtR | uCre | uCl | uK | CysC | SCre | SUA | BUN | Hb | |
| SBP | 0.688 (± 0.100)** | -0.179 (± 0.175) | 0.207 (± 0.135)† | -0.174 (± 0.106)* | -0.107 (± 0.156) | 0.023 (± 0.151) | -0.044 (± 0.167) | 0.057 (± 0.152) | -0.095 (± 0.307) | -0.075 (± 0.260) | -0.555 (± 0.389)† | -0.096 (± 0.130) | -0.082 (± 0.132) | 0.051 (± 0.119) | -0.276 (± 0.176)† | -0.025 (± 0.146) | |
| DBP | 0.737 | 0.070 (± 0.136) | 0.117 (± 0.110) | -0.026 (± 0.086) | -0.026 (± 0.126) | -0.020 (± 0.128) | -0.030 (± 0.139) | -0.014 (± 0.130) | -0.069 (± 0.253) | -0.161 (± 0.220) | -0.269 (± 0.300) | 0.003 (± 0.107) | 0.100 (± 0.110) | 0.059 (± 0.100) | -0.169 (± 0.143) | 0.082 (± 0.118) | |
| TG | 0.103 | 0.108 | -0.429 (± 0.072)** | -0.042 (± 0.070) | 0.068 (± 0.100) | 0.109 (± 0.100) | 0.143 (± 0.107) | 0.161 (± 0.100)† | -0.262 (± 0.219) | -0.023 (± 0.177) | -0.117 (± 0.240) | 0.052 (± 0.087) | 0.073 (± 0.089) | 0.212 (± 0.079)** | 0.116 (± 0.119) | 0.122 (± 0.099) | |
| HDL-C | -0.011 | -0.016 | -0.397 | 0.007 (± 0.056) | -0.191 (± 0.075)** | -0.202 (± 0.076)** | -0.221 (± 0.081)** | -0.198 (± 0.077)** | -0.180 (± 0.166) | -0.270 (± 0.145)* | -0.203 (± 0.195)* | -0.215 (± 0.067)** | -0.087 (± 0.070) | -0.109 (± 0.064)* | 0.015 (± 0.093) | -0.021 (± 0.079) | |
| ht | -0.018 | 0.027 | 0.004 | -0.027 | 0.454 (± 0.053)** | -0.197 (± 0.063)** | 0.105 (± 0.068)† | -0.377 (± 0.062)** | 0.104 (± 0.131) | 0.125 (± 0.112) | 0.286 (± 0.172) | -0.056 (± 0.055) | 0.035 (± 0.056) | 0.013 (± 0.051) | 0.071 (± 0.073) | -0.002 (± 0.063) | |
| wt | 0.189 | 0.211 | 0.232 | -0.312 | 0.363 | 0.784 (± 0.029)** | 0.825 (± 0.028)** | 0.553 (± 0.058)** | 0.297 (± 0.193)† | 0.477 (± 0.169)** | 0.559 (± 0.266)** | 0.034 (± 0.078) | -0.003 (± 0.080) | 0.084 (± 0.071) | -0.070 (± 0.105) | -0.026 (± 0.090) | |
| BMI | 0.208 | 0.210 | 0.245 | -0.320 | -0.085 | 0.893 | 0.829 (± 0.028)** | 0.874 (± 0.021)** | 0.243 (± 0.193)† | 0.436 (± 0.169)** | 0.380 (± 0.239)* | 0.078 (± 0.079) | -0.021 (± 0.081) | 0.070 (± 0.072) | -0.134 (± 0.107) | -0.021 (± 0.091) | |
| WC | 0.186 | 0.193 | 0.258 | -0.329 | 0.123 | 0.870 | 0.874 | 0.881 (± 0.018)** | 0.228 (± 0.208) | 0.482 (± 0.184)** | 0.474 (± 0.269)* | 0.119 (± 0.085)† | -0.078 (± 0.089) | 0.105 (± 0.077)† | -0.093 (± 0.116) | 0.049 (± 0.097) | |
| WHtR | 0.188 | 0.179 | 0.252 | -0.315 | -0.197 | 0.739 | 0.890 | 0.947 | 0.164 (± 0.193) | 0.391 (± 0.170)** | 0.290 (± 0.233)† | 0.140 (± 0.079)* | -0.083 (± 0.083) | 0.085 (± 0.073) | -0.118 (± 0.109) | 0.051 (± 0.091) | |
| uCre | -0.062 | -0.044 | -0.020 | -0.087 | 0.046 | 0.066 | 0.049 | 0.049 | 0.034 | 0.520 (± 0.260)† | 0.374 (± 0.345) | 0.139 (± 0.160) | -0.015 (± 0.167) | -0.091 (± 0.152) | -0.152 (± 0.229) | -0.137 (± 0.192) | |
| uCl | 0.019 | 0.006 | 0.035 | -0.021 | 0.016 | 0.098 | 0.096 | 0.086 | 0.079 | 0.388 | 0.575 (± 0.245)† | -0.281 (± 0.143)* | -0.481 (± 0.156)** | -0.175 (± 0.128)† | -0.137 (± 0.193) | 0.062 (± 0.158) | |
| uK | -0.090 | -0.071 | -0.015 | -0.005 | 0.042 | 0.050 | 0.033 | 0.033 | 0.019 | 0.581 | 0.589 | -0.185 (± 0.196) | -0.510 (± 0.260)** | -0.203 (± 0.186) | -0.501 (± 0.342)* | 0.044 (± 0.210) | |
| CysC | 0.016 | 0.019 | 0.084 | -0.221 | 0.020 | 0.166 | 0.168 | 0.174 | 0.167 | 0.109 | -0.067 | -0.005 | 0.614 (± 0.045)** | 0.340 (± 0.057)** | 0.093 (± 0.090) | 0.143 (± 0.079)* | |
| SCre | -0.021 | 0.002 | 0.061 | -0.070 | 0.093 | 0.096 | 0.063 | 0.042 | 0.013 | 0.137 | -0.073 | 0.039 | 0.543 | 0.285 (± 0.059)** | 0.246 (± 0.086)** | 0.046 (± 0.080) | |
| SUA | 0.080 | 0.108 | 0.167 | -0.142 | 0.025 | 0.228 | 0.230 | 0.240 | 0.227 | 0.058 | -0.083 | 0.027 | 0.335 | 0.333 | -0.061 (± 0.088) | -0.015 (± 0.074) | |
| BUN | -0.064 | -0.076 | -0.033 | 0.070 | -0.014 | 0.017 | 0.026 | 0.004 | 0.009 | 0.149 | 0.151 | 0.217 | 0.190 | 0.311 | 0.156 | -0.091 (± 0.104) | |
| Hb | 0.135 | 0.219 | 0.142 | -0.069 | 0.035 | 0.143 | 0.139 | 0.161 | 0.147 | 0.033 | -0.034 | -0.001 | 0.051 | -0.015 | 0.146 | -0.092 |
BMI, body mass index; BUN, blood urea nitrogen; CysC, cystatin C; DBP, diastolic blood pressure; Hb, Hemoglobin; HDL-C, high-density lipoprotein cholesterol; ht, height; SBP, systolic blood pressure; SCre, serum creatinine; SUA, serum uric acid; TG, triglycerides; uCl, urine chloride; uCre, urine creatinine; uK, urine potassium; WC, waist circumference; WHtR, waist-to-height-ratio; wt, weight.
P < 0.1.
P < 0.05.
P < 0.01.
High genetic correlations were also observed among urinary traits, e.g., uK vs. uCl (0.575 ± 0.245). Although this is consistent with the pattern observed above, it is an interesting result given the low heritability of these traits. This finding highlights the fact that a low contribution of genetic components to phenotypic variation for a given set of traits does not necessarily imply a low genetic correlation between these traits. On the other hand, the low heritability of these traits was reflected in the higher standard error of the corresponding genetic correlation coefficients.
The phenotypic correlations were stronger between CysC and each of the adiposity traits (weight, BMI, WC, WHtR) and HDL-C than between SCre and these traits; this result supports previous research that showed that CysC is more closely associated with obesity than SCre (Ying et al. 2017). A similar finding was observed for genetic correlations: significant estimates were detected between CysC and WHtR and between CysC and HDL-C but not between SCre and these traits.
Discussion
The effect of WC on health indicators
By dividing the target population by BMI and WC group (Supplementary Tables 2 and 3), we observed that regardless of gender, in normal weight and overweight individuals, within each BMI category the subgroups with a large WC displayed significantly higher weight, WHtR, higher CysC and SUA levels, and a lower HDL-C level than those with a small WC (P < 0.01 in all subgroups, except SUA in overweight men for which P < 0.05). When examining women and men of normal weight only, the subgroups with a large WC also displayed higher DBP and higher TG and Hb levels (P < 0.01). More surprisingly, however, overweight men with a small WC displayed lower CysC levels than men with a normal BMI and a large WC (P < 0.01). These findings strongly suggest that increases in WC within each BMI category are associated with a worsening of many health risk indicators in both women and men; they are also in line with previous research indicating that abdominal fat, as measured by WC, may represent a substantial health risk factor independently of BMI, and that WC may be an effective tool for the detection of at-risk individuals, including normal weight individuals (Després et al. 2008).
Heritabilities and genetic correlations of the traits in the Japanese population
The heritability estimates calculated for the Japanese population were largely comparable to those from previous research conducted in populations of European descent. For example, in a recent phenome-wide heritability analysis of the UK Biobank carried out in British (Caucasian) individuals, the heritability estimates (± SE) were 0.685 (± 0.004) for height, 0.274 (± 0.004) for BMI, 0.277 (± 0.004) for weight, 0.155 (± 0.004) for WC, 0.156 (± 0.004) for SBP, and 0.184 (± 0.004) for DBP (Ge et al. 2017). In another study, performed in the Lifelines Cohort (Netherlands), the heritability estimates (± SE) were 0.489 (± 0.032) for height, 0.248 (± 0.032) for BMI, 0.173 (± 0.032) for SBP, 0.170 (± 0.033) for DBP, 0.186 (± 0.034) for HDL-C, 0.191 (± 0.035) for TG, 0.190 (± 0.032) for Hb, and 0.268 (± 0.032) for SCre (Nolte et al. 2017). Our findings are also in line with previous estimates of the SNP heritability of CysC (0.27; 95% CI: 0.15-0.39), SUA (0.144 ± 0.039), uCre (0.065 ± 0.003), and uK (0.042 ± 0.002) (Chen et al. 2015; Zanetti et al. 2018; Nakatochi et al. 2019). This implies that, in spite of the genetic and environmental differences that exist between European and Japanese populations, overall the contribution of common genetic variants to phenotype variation between these groups is comparable.
A major study on BMI in a large Japanese cohort reported the discovery of dozens of novel loci, highlighting the genetic diversity between Japanese and European populations (Akiyama et al. 2017). This led the authors to suggest differences in the causal variants and their effect sizes as potential explanations for their findings, and to advocate for more research in diverse ethnic groups to uncover the genetic processes underlying BMI susceptibility. Although that call is certainly warranted given the well-established genetic diversity across human populations, our study of the SNP heritability of obesity-related traits indicates that the impact of these differences on phenotypic variation between Japanese and European populations is likely to be moderate. Conversely, as this example illustrates, similarity in heritability estimates does not necessarily imply similarity in terms of genetic architecture; whether the genetic architecture of each trait is similar in Japanese and European populations is an essential research topic that requires further inquiry.
Several observations deserve to be emphasized with respect to the genetic correlations (Table 3). First, although the phenotypic and genetic correlations among the three urinary traits examined (uCre, uCl, and uK) were similar in terms of sign and magnitude, sharp differences between phenotypic and genetic correlations were observed when comparing this group of urinary traits to the group of adiposity traits (weight, BMI, WC, and WHtR), as illustrated by Figure 1: the highest phenotypic correlation between any pair of traits among these two groups was below 0.1, whereas the corresponding genetic correlations ranged from 0.164 to 0.559, the highest genetic correlation being between uK and weight (P < 0.01).
In people of European descent, a recent study in the UK Biobank has identified a relationship between these two groups of traits, yet with much lower genetic correlations (e.g., 0.104 between uK and BMI, vs. 0.380 in our study) (Zanetti et al. 2018). Given the dearth of research on the genetic architecture governing urinary biomarkers and their relationship with obesity-related traits, it is difficult to completely rule out that these discrepancies are caused by differences in methodology; however, the unmistakable differences that have been highlighted in the literature between individuals from Japan and western Europe in the relationship between adiposity, lipid biomarker data, kidney disease, and cardiovascular risk may indicate that the strength of the correlations between urinary traits and adiposity traits is specific to the Japanese (Nakamura et al. 2012; Wanner et al. 2016).
On a different note, significant genetic correlations were found between HDL-C and adiposity traits (weight, BMI, WC, WHtR), renal function biomarkers (uCl, uK, CysC, SUA), and cardiovascular risk factors (TG, SUA). This highlights the complex, intertwined relationship between these traits, as do the large negative genetic correlations between SCre and uK and between SCre and uCl (P < 0.01). Surprisingly, however, despite phenotypic correlations of over 0.2, we could not reproduce genetic correlations between BMI and SBP, DBP, TG, or SUA (Kanai et al. 2018), although we found significant genetic correlations between SUA and HDL-C and between SUA and TG.
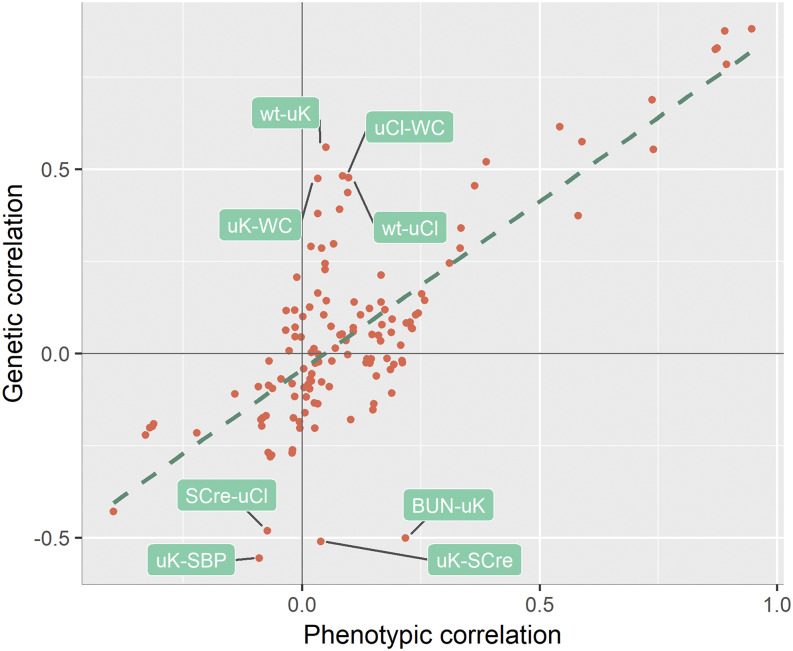
Even though the correspondence between phenotypic and genetic correlation estimates is not a systematic result (Lynch and Walsh 1998), it has been observed previously (Vattikuti et al. 2012). In this study, we found correspondence between these estimates to a certain degree. For instance, the absolute value of the maximum difference between the phenotypic and genetic correlations was below 0.3 for all traits, excluding the three traits with the lowest heritabilities: uCre, uCl, and uK. Furthermore, the Pearson correlation coefficient calculated for phenotypic correlation vs. genetic correlation estimates for the 17 traits was high (0.767). These results therefore seem to support the view that phenotypic correlations may prove valuable in the context of linear mixed model methodology, for instance in the calculation of the preliminary estimates of the parameters used for the initialization of maximum likelihood procedures.
At the same time, all three traits with low heritability, i.e., the traits for which environmental factors accounted for almost all of the variation in phenotype, displayed a difference of over 0.3 between the phenotypic and genetic correlations with at least one of the other traits. Figure 2 also indicates much variation between the phenotypic and genetic correlations for the pairs of traits whose phenotypic correlation coefficients were close to zero. Although this issue needs to be examined in more depth to draw a firmer conclusion, our observation that the differences between phenotypic and genetic correlations depend on the trait pairs analyzed may partly explain why there is no consensus on the conformity of the relationship between phenotypic and genetic correlations, and leads us to recommend exercising ample caution when using phenotypic correlations as proxies for genetic correlations.
Figure 2.
Scatter plot of the genetic and phenotypic correlations for each pair of traits and corresponding regression line. BUN, blood urea nitrogen; SBP, systolic blood pressure; SCre, serum creatinine; uCl, urine chloride; uK, urine potassium; WC, waist circumference; wt, weight.
Obesity and biomarkers for obesity-related diseases
A substantial contribution of this study is the estimation of the genetic correlations between traits associated with obesity and indicators used as health markers for obesity-related diseases. The link between obesity and obesity-related diseases is often complex, and furthering our understanding of this relationship requires undertaking a variety of analyses on a wide range of traits and in diverse populations. This complexity is well illustrated by the case of CKD: despite the known strong association between metabolic syndrome and CKD, it has been reported that traditional CKD risk factors (such as diabetes mellitus and hypertension) may be independent of kidney dysfunction in obesity, and that kidney dysfunction may appear long before these factors develop in individuals affected with metabolic syndrome (Singh and Kari 2013; Sarathy et al. 2016). The large genetic correlations that we found between adiposity traits and urinary biomarkers (e.g., 0.436 between BMI and uCl; 0.380 between BMI and uK), as well as between SBP and uK (-0.555), provide a starting point for further research into how the genetic architecture of these biomarkers fits into the broader context of disease onset and etiology.
More broadly, these observations underscore the fact that the relationship between obesity and obesity-related diseases is not a simple cause-and-effect relationship, and compel us to revisit and better quantify the connection between the many associated traits and biomarkers, even if they typically display a somewhat low heritability and do not necessarily constitute the deciding factor in terms of disease diagnosis.
Limitations and strengths of this study
Given that our main objective was to shed light on the genetic characteristics of the Japanese population, our findings may not be generalizable to other human populations or age groups. This is partly due to the diversity found across genetic groups, but it is also a consequence of the physiological and social differences that exist between human populations, as illustrated for instance by the differences in the WC threshold values used for the diagnosis of metabolic syndrome in Japanese and European populations. Factors such as smoking, alcohol consumption, and physical activity, as well as potential subgroup effects within the cohort, are also aspects that have not been addressed in this paper and warrant further research. When comparing our findings with the results of other studies, it is also important to keep in mind that the TMM project targets the regions affected by the Great East Japan Earthquake of March 11, 2011.
On the other hand, this study is one of only a few analyses of large-scale cohorts that focus on identifying the general genetic characteristics of the Japanese population; it includes rare and valuable information, such as one of the few estimates of the SNP heritability of cystatin C in unrelated individuals, as well as estimates of the genetic correlations between urinary biomarkers and obesity-related traits. Also, given that this study was based on high-quality clinical data, and that participants were recruited as part of the general population regardless of their medical background, it constitutes a useful point of comparison for similar studies conducted in the Japanese and other populations.
Conclusion
By quantifying the genomic parameters of a large cohort of Japanese adults, our study provides evidence that the contribution of common SNPs to phenotype variation is significant in the Japanese for traits related to obesity and obesity-related diseases, and therefore supports the assumption that common genetic variants account for a considerable share of phenotypic variation. Our study also shows that the heritability estimates for these traits are similar in magnitude to those of populations of European descent, and suggests potential differences with respect to the genetic correlations; however, further research is needed to compare the genetic architecture of each trait in these populations. The heritabilities presented in this paper are useful in that they provide information about the proportion of phenotypic variance that could be explained by common-variant GWAS of the traits studied, and the high genetic correlations estimated for several pairs of traits with low heritabilities underscore that we must be careful not to neglect research on the genetics of biomarkers with low heritabilities.
Acknowledgments
Computational resources were provided in part by the ToMMo supercomputer system (https://sc.megabank.tohoku.ac.jp/en). We are indebted to all volunteers who participated in the Tohoku Medical Megabank project. This research was supported by the Japan Society for the Promotion of Science (JSPS Kakenhi) under grants 18K14757 and 17K08682, as well as by the Japan Agency for Medical Research and Development (AMED) under grants JP19km0405205, JP18km0105001, and JP18km0105002.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25387/g3.11295944.
Communicating editor: J. Prendergast
Literature Cited
- Akiyama M., Okada Y., Kanai M., Takahashi A., Momozawa Y. et al. , 2017. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49: 1458–1467. 10.1038/ng.3951 [DOI] [PubMed] [Google Scholar]
- Azuma K., Kadowaki T., Cetinel C., Kadota A., El-Saed A. et al. , 2009. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism 58: 1200–1207. 10.1016/j.metabol.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B., 2007. The global epidemic of obesity: an overview. Epidemiol. Rev. 29: 1–5. 10.1093/epirev/mxm012 [DOI] [PubMed] [Google Scholar]
- Chen X., Kuja-Halkola R., Rahman I., Arpegård J., Viktorin A. et al. , 2015. Dominant genetic variation and missing heritability for human complex traits: insights from twin vs. genome-wide common SNP models. Am. J. Hum. Genet. 97: 708–714. 10.1016/j.ajhg.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després J. P., Lemieux I., Bergeron J., Pibarot P., Mathieu P. et al. , 2008. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28: 1039–1049. 10.1161/ATVBAHA.107.159228 [DOI] [PubMed] [Google Scholar]
- Gansevoort R. T., Correa-Rotter R., Hemmelgarn B. R., Jafar T. H., Heerspink H. J. et al. , 2013. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382: 339–352. 10.1016/S0140-6736(13)60595-4 [DOI] [PubMed] [Google Scholar]
- Ge T., Chen C. Y., Neale B. M., Sabuncu M. R., and Smoller J. W., 2017. Phenome-wide heritability analysis of the UK Biobank. PLoS Genet. 13: e1006711 10.1371/journal.pgen.1006711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour A. R., Thompson R., and Cullis B. R., 1995. Average information REML: an efficient algorithm for variance parameter estimation in linear mixed models. Biometrics 51: 1440–1450. 10.2307/2533274 [DOI] [Google Scholar]
- Hall J. E., 2000. Pathophysiology of obesity hypertension. Curr. Hypertens. Rep. 2: 139–147. 10.1007/s11906-000-0073-4 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Tanaka M., Kimura T., Kitagawa N., Hamaguchi M. et al. , 2015. Hemoglobin concentration and incident metabolic syndrome: a population-based large-scale cohort study. Endocrine 50: 390–396. 10.1007/s12020-015-0587-9 [DOI] [PubMed] [Google Scholar]
- Heymsfield S. B., Peterson C. M., Thomas D. M., Heo M., and Schuna J. M. Jr, 2016. Why are there race/ethnic differences in adult body mass index–adiposity relationships? A quantitative critical review. Obes. Rev. 17: 262–275. 10.1111/obr.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B. N., Donnelly P., and Marchini J., 2009. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5: e1000529 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozawa A., Tanno K., Nakaya N., Nakamura T., Tsuchiya N. et al. , 2020. Study profile of The Tohoku Medical Megabank Community-Based Cohort Study. J. Epidemiol. 10.2188/jea.JE20190271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby A., and Hu F. B., 2015. The epidemiology of obesity: a big picture. Pharmacoeconomics 33: 673–689. 10.1007/s40273-014-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M., Akiyama M., Takahashi A., Matoba N., Momozawa Y. et al. , 2018. Genetic analysis of quantitative traits in the Japanese population links cell types to complex human diseases. Nat. Genet. 50: 390–400. 10.1038/s41588-018-0047-6 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Mimori T., Kojima K., Nariai N., Danjoh I. et al. , 2015. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1,070 Japanese individuals. J. Hum. Genet. 60: 581–587. 10.1038/jhg.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S., Yaegashi N., Nagami F., Arai T., Kawaguchi Y. et al. , 2016. The Tohoku medical megabank project: design and mission. J. Epidemiol. 26: 493–511. 10.2188/jea.JE20150268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Yang J., Goddard M. E., Visscher P. M., and Wray N. R., 2012. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 28: 2540–2542. 10.1093/bioinformatics/bts474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., and Walsh B., 1998. pp. 629–656 in Genetics and analysis of quantitative traits, Sinauer Associates, Sunderland, MA. [Google Scholar]
- Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; and Ministry of Economy, Trade and Industry, 2013 Ethical guidelines for human genome/gene analysis research [hito genomu/idenshi kaiseki kenkyu ni kan suru rinri shishin]; available at: http://www.lifescience.mext.go.jp/files/pdf/n1115_01.pdf (in Japanese). Accessed: October 10, 2019.
- Ministry of Health, Labour and Welfare, 2005 Diagnostic criteria for metabolic syndrome [metaborikku shindoromu no shindan kijun]; available at: https://www.e-healthnet.mhlw.go.jp/information/metabolic/m-01-003.html (in Japanese). Accessed: October 10, 2019.
- Nagasaki M., Yasuda J., Katsuoka F., Nariai N., Kojima K. et al. , 2015. Rare variant discovery by deep whole-genome sequencing of 1,070 Japanese individuals. Nat. Commun. 6: 8018 10.1038/ncomms9018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Nishida N., Kawashima M., Aiba Y., Tanaka A. et al. , 2012. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am. J. Hum. Genet. 91: 721–728. 10.1016/j.ajhg.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatochi M., Kanai M., Nakayama A., Hishida A., Kawamura Y. et al. , 2019. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun. Biol. 2: 115 10.1038/s42003-019-0339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration , 2016a Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387: 1377–1396. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration , 2016b Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387: 1513–1530. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte I. M., van der Most P. J., Alizadeh B. Z., de Bakker P. I., Boezen H. M. et al. , 2017. Missing heritability: is the gap closing? An analysis of 32 complex traits in the Lifelines Cohort Study. Eur. J. Hum. Genet. 25: 877–885. 10.1038/ejhg.2017.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B. M., and Slining M. M., 2013. New dynamics in global obesity facing low‐and middle‐income countries. Obes. Rev. 14: 11–20. 10.1111/obr.12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A. et al. , 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee C. M., and Ahmadi S. F., and Kalantar-Zadeh K.. 2016. The dual roles of obesity in chronic kidney disease: a review of the current literature. Curr. Opin. Nephrol. Hypertens. 25: 208–216. 10.1097/MNH.0000000000000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathy H., Henriquez G., Abramowitz M. K., Kramer H., Rosas S. E. et al. , 2016. Abdominal obesity, race and chronic kidney disease in young adults: results from NHANES 1999–2010. PLoS One 11: e0153588 10.1371/journal.pone.0153588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan V. W., Lim U., Lipworth L., Lu S. C., Shepherd J. et al. , 2016. Sex and ethnic differences in the association of obesity with risk of hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 14: 309–316. 10.1016/j.cgh.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K., and Kari J. A., 2013. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22: 198–203. 10.1097/MNH.0b013e32835dda78 [DOI] [PubMed] [Google Scholar]
- Stryjecki C., Alyass A., and Meyre D., 2018. Ethnic and population differences in the genetic predisposition to human obesity. Obes. Rev. 19: 62–80. 10.1111/obr.12604 [DOI] [PubMed] [Google Scholar]
- Szwaczkowski T., 2003. Use of mixed model methodology in poultry breeding: Estimation of genetic parameters, pp. 165–202 in Poultry Genetics, Breeding and Biotechnology, edited by Muir W. M., and Aggrey S. E.. CAB International, Wallingford, Oxon: 10.1079/9780851996608.0165 [DOI] [Google Scholar]
- Team, R. C., 2019 R: A language and environment for statistical computing URL: https://www.R-project.org/. Foundation for Statistical Computing: Vienna, Austria.
- Thompson R., 1973. The estimation of variance and covariance components with an application when records are subject to culling. Biometrics 29: 527–550. 10.2307/2529174 [DOI] [Google Scholar]
- Vattikuti S., Guo J., and Chow C. C., 2012. Heritability and genetic correlations explained by common SNPs for metabolic syndrome traits. PLoS Genet. 8: e1002637 10.1371/journal.pgen.1002637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner C., Amann K., and Shoji T., 2016. The heart and vascular system in dialysis. Lancet 388: 276–284. 10.1016/S0140-6736(16)30508-6 [DOI] [PubMed] [Google Scholar]
- Wickham H., 2016. ggplot2: Elegant graphics for data analysis, 2nd edn. Springer International Publishing, Switzerland. [Google Scholar]
- World Health Organization, 1995 Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. World Health Organization: Geneva. [PubMed]
- World Health Organization, 2018 Obesity and overweight; available at: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed: October 10, 2019.
- Yang J., Lee S. H., Goddard M. E., and Visscher P. M., 2011. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88: 76–82. 10.1016/j.ajhg.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Lee S. H., Goddard M. E., and Visscher P. M., 2013. Genome-wide complex trait analysis (GCTA): methods, data analyses, and interpretations, pp. 215–236 in Genome-wide association studies and genomic prediction, edited by Gondro C., van der Werf J., and Hayes B.. Humana Press, Totowa, NJ: 10.1007/978-1-62703-447-0_9 [DOI] [PubMed] [Google Scholar]
- Ying X., Jiang Y., Qin G., Qian Y., Shen X. et al. , 2017. Association of body mass index, waist circumference, and metabolic syndrome with serum cystatin C in a Chinese population. Medicine (Baltimore) 96: e6289 10.1097/MD.0000000000006289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti D., Rao A., Gustafsson S., Assimes T., Montgomery S. B. et al. , 2018. Genetic analyses in UK Biobank identifies 78 novel loci associated with urinary biomarkers providing new insights into the biology of kidney function and chronic disease. bioRxiv. (Preprint posted May 5, 2018). 10.1101/315259 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyses presented in this study were based on data accessed through the Tohoku Medical Megabank Organization (ToMMo) (https://www.megabank.tohoku.ac.jp/english/). To protect the privacy of the cohort participants, requests for the use of ToMMo biobank data for research projects should be made by applying directly to ToMMo, and are subject to review and approval by the Sample and Data Access Committee. Supplemental material available at figshare: https://doi.org/10.25387/g3.11295944