Abstract
The technique of CRISPR-Cas9 gene editing has been widely used to specifically delete the selected target genes through generating double strand breaks (DSBs) and inducing insertion and/or deletion (indel) of the genomic DNAs in the cells. We recently applied this technique to disrupt mineral dust-induced gene (mdig), a potential oncogene as previously reported, by single guide RNA (sgRNA) targeting the third exon of mdig gene in several cell types, including human bronchial epithelial cell line BEAS-2B, lung cancer cell line A549, and human triple negative breast cancer cell line MDA-MB-231 cells. In addition to the successful knockout of mdig gene in these cells, we unexpectedly noted generation of several alternatively spliced mdig mRNAs. Amplification of the mdig mRNAs during the screening of knockout clones by reverse transcription-polymerase chain reaction (RT-PCR) and the subsequent sanger sequencing of DNA revealed deletion and alternative splicing of mdig mRNAs induced by CRISPR-Cas9 gene editing. The most common deletions include nine and twenty-four nucleotides deletion around the DSBs. In addition, interestingly, some mdig mRNAs showed skipping of the entire exon 3, or alternative splicing between exon 2 and exon 8 using the new donor and accept splicing sites, leading to deletion of exons 3, 4, 5, 6, and 7. Accordingly, cautions should be taken when using CRISPR-Cas9 strategy to edit human genes due to the unintended alterative splicing of the target mRNAs. It is very likely that new proteins, some of which may be highly oncogenic, may be generated from CRISPR-Cas9 gene editing.
Introduction
CRISPR-Cas is a sequence array from prokaryotes, which contains CRISPR associated genes (Cas) followed by several repetitive sequences interspaced with variable sequences (spacers) [1, 2]. The application of CRISPR-Cas9 to edit genome is a great revolution in biology, life sciences and possibly, medicine [3–5]. The S. pyogenes Cas9 is guided by a co-expressed single guide RNA (sgRNA) to cleave the gene of interest, leaving a double strand breaks (DSBs), which triggers nonhomologous end-joining (NHEJ) or homology directed repair (HDR) to induce insertion/deletion (indel) or precise repair [6]. The sgRNA is a 20 nucleotides sequence that can be easily customized, and binds to genome vie complementary base pairing [6], which renders more flexibility over the DNA-protein recognition of other gene editing tools, such as zinc fingers (ZFs) [7–10] and transcription activator-like effectors (TALEs) [10–14]. However, concerns, such as the off-target effect of CRISPR-Cas9 [15–17] still remains, especially when it is used in biomedical and clinical areas [18]. Meanwhile, the outcome of CRISPR-Cas9 in transcriptional level has not been fully investigated yet.
In this report, we provide evidence showing that gene editing by CRISPR-Cas9 can induce alternative splicing of the target mRNAs. In the case of CRISPR-Cas9-based knockout of the mdig gene, in addition to cause deletion at the DSB site, several types of alternative splicing of the mdig mRNA were noted. Mdig encodes a JmjC-domain protein, which plays important role in cell proliferation, lung fibrosis, and tumor metastasis [19–24]. At the mRNA level, CRISPR-Cas9 editing causes deletion of 9–24 nucleotides at the DSB sites. These deletions resulted in a complete loss of mdig protein expression. Unexpectedly, this CRISPR-Cas9-based gene editing also induced alternative splicing of the mdig mRNAs due to exon skipping and the use of new donor and accept splicing sites. Thus, in addition to the widely concerned off-targeting, additional cautions should be taken on the alternative splicing of the target mRNAs resulted from CRISPR-Cas9 gene editing. It is very likely that some of these alternatively spliced mRNA may generate new proteins that are even more oncogenic or potentially harmful to the normal function of the cells.
Results
Disruption of mdig by targeting the third exon using CRISPR-Cas9 system
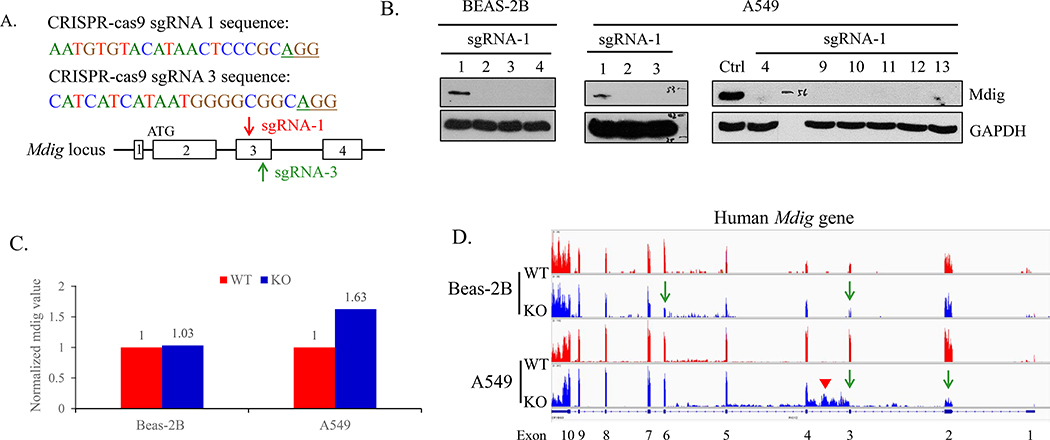
We recently depleted mdig gene by CRISPR-Cas9 in three human cell lines: bronchial epithelial cell line BEAS-2B, lung tumor cell line A549, and triple negative breast cancer cell line MDA-MB-231. The sgRNA-1 and sgRNA-3 were designed to target the third exon of mdig gene (Fig 1A). We isolated the single clones after transfection of the CRISPR-Cas9 vector, and screened for mdig protein expression by Western blotting using anti-mdig antibody purchased from Invitrogen (Cat.# 39–7300). The clones with no mdig expression were designated as knockout (KO) cells (Fig. 1B). Clones with normal mdig expression were used as wild type (WT) control (Fig 1B). In the transcription level, the normalized mdig mRNA level in KO were similar, or even slightly increased than that in WT as determined by RNA sequencing (RNA-seq) (Fig. 1C and 1D). In exon three, there were no transcript containing 5’-CCTGCGGGA-3’, reverse complementary to 5’-TCCCGCAGG-3’, around the DSBs, which confirmed mdig’s knockout by CRISPR-Cas9 (sFig. 1). In A549 cells, this deletion caused a notable retention of intron 3 in some mdig transcripts (pointed by arrow head, Fig. 1D, and sFig.1).
Fig 1.
Knockout of mdig by sgRNAs. A. Sequence and schematic of sgRNAs targeting the third exon of Mdig gene. The red arrow indicated the sgRNA-1, and the green arrow pointed sgRNA-3. B. Western blotting showing the mdig protein expression in BEAS-2B clones and A549 clones. C. Normalized mdig value from RNA sequencing (RNA-seq) of WT (red) and KO (blue) of BEAS-2B and A549 clones. D. RNA-seq showing the reads of mdig in WT (red) and KO (blue) of BEAS-2B and A549 clones.
CRISPR-Cas9 induces small deletions
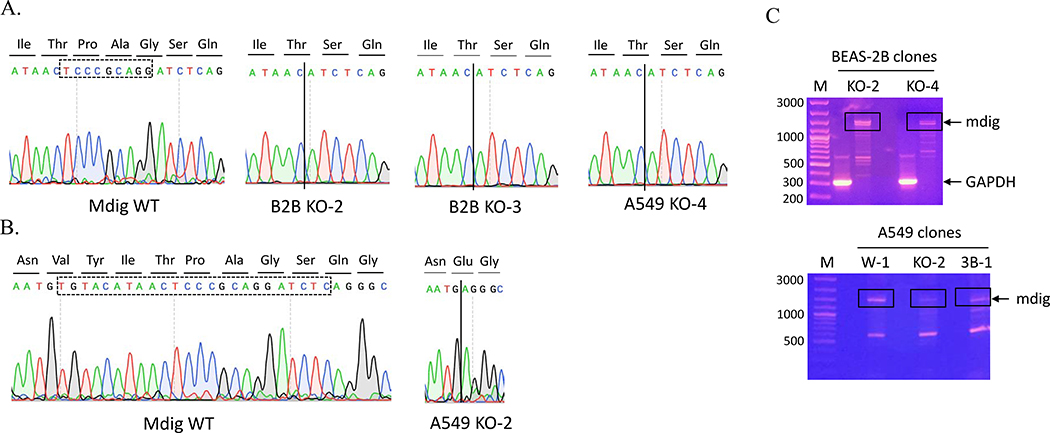
In general, sgRNA-guided Cas9 endonuclease cleavages the nucleotides before NGG, leaving a double strand breaks (DSBs) [6]. Without a repair template, some nucleotides are randomly inserted or deleted around the DSBs, which may or may not cause frameshift of the open reading frame [6]. To understand the mutations in transcriptional level, we performed RT-PCR followed by sanger sequencing on these clones. We found two forms of small deletion in mdig mRNA. The most common form is the deletion of nine nucleotides (5’-TCCCGCAGG-3’) (Fig. 2A), as confirmed by the transcripts of exon 3 from RNA-seq (Fig. 1s), which resulted in three amino acids, Pro-Ala-Gly, deletion in mdig protein. We also detected a deletion of a twenty-four nucleotides (5’-TGTACATAACTCCCGCAGGATCTC-3’) in exon three (Figs. 2A and 2B). This deletion causes omission of eight amino acids, Tyr-Ile-Thr-Pro-Ala-Gly-Ser-Gln, and Val to Glu conversion in mdig protein, if this mRNA can be translated. Fig. 2C depicts typical RT-PCR product of mdig mRNAs in different KO clones.
Fig 2.
CRISPR-Cas9 induces small deletions. A. Chromatograms showing the exon 3 sequence of mdig from sanger sequencing. B. Typical RT-PCR product of mdig in BEAS-2B and A549 clones. C. Agarose gel electrophoresis showing mdig cDNAs from RT-PCR of the BEAS-2B cells (upper panel) and A549 cells (bottom panel).
Deletion of mdig by CRISPR-Cas9 gene editing causes alternative splicing of mdig mRNAs
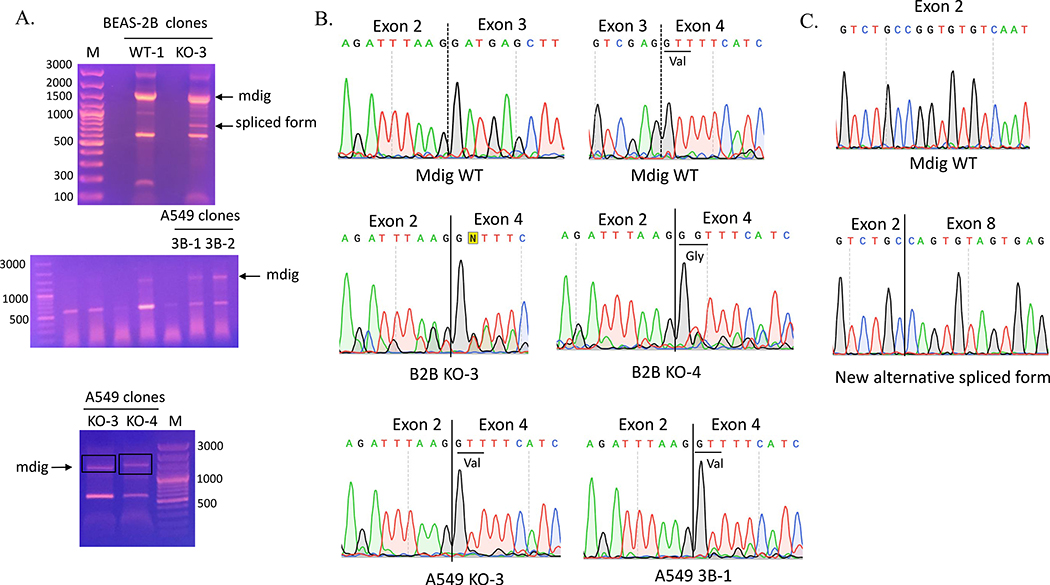
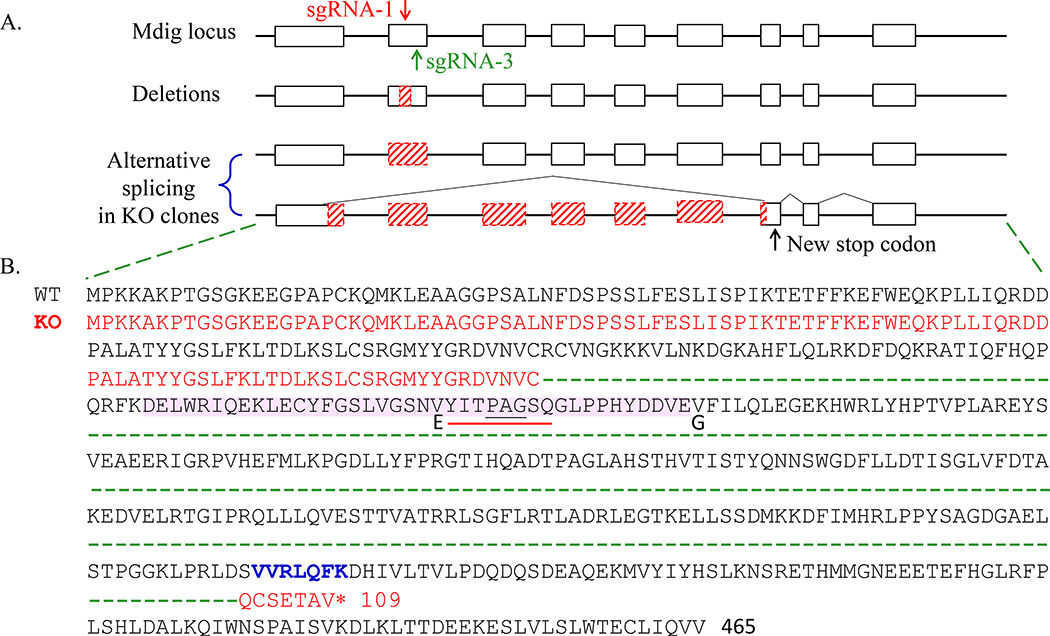
In reverse transcription of mdig mRNA, we frequently noted presence of two major cDNA fragments in KO-3 BEAS-2B clones (Fig. 3A). The density of the large size product is comparative to that in the WT cells, but the size is marginally smaller than the band in WT cells. There is a smaller size product, around 800 bp, that was detected in the KO cells only (Fig. 3A). DNA sequencing of the retrieved cDNA fragments revealed that the third exon was completed spliced out in the larger size fragment (Fig. 3B and Table 1). This fragment was also identified in other KO clones, including KO-4 BEAS-2B clone, KO-3 A549 clone, and 3B-1 A549 clone (Fig. 3B and Table 1). Interestedly, the smaller fragment contained only exon one, 5’−309nt of exon two (missing 3’−123nt), 3’−67nt of exon eight (missing 5’−22nt), exon nine, and exon ten (Fig. 3C, and Table 1), indicating that multiple exons were skipped out due to alternative splicing caused by CRISPR-Cas9 gene editing. This skipping of multiple exons caused frameshift and generated a premature stop codon in exon eight (Fig. 4 and Table 1). This unique alternative splicing uses the conserved intronic “GT” donor site and a non-canonical intronic “GT” acceptor site.
Fig 3.
CRISPR-Cas9 causes alternative splicing of mdig mRNAs. A. RT-PCR product of mdig in BEAS-2B and A549 clones. B. Chromatograms showing the missing of the third exon of mdig in mdig mRNA from BEAS-2B and A549 clones. C. Chromatograms of the new alternative spliced form of mdig in KO-3 BEAS-2B clone compared to Mdig WT.
Table 1.
Summary of deleted and alternative spliced nucleotides as well as corresponding protein sequence in mdig mRNAs from KO clones of BEAS-2B, A549, and MDA-MB-231.
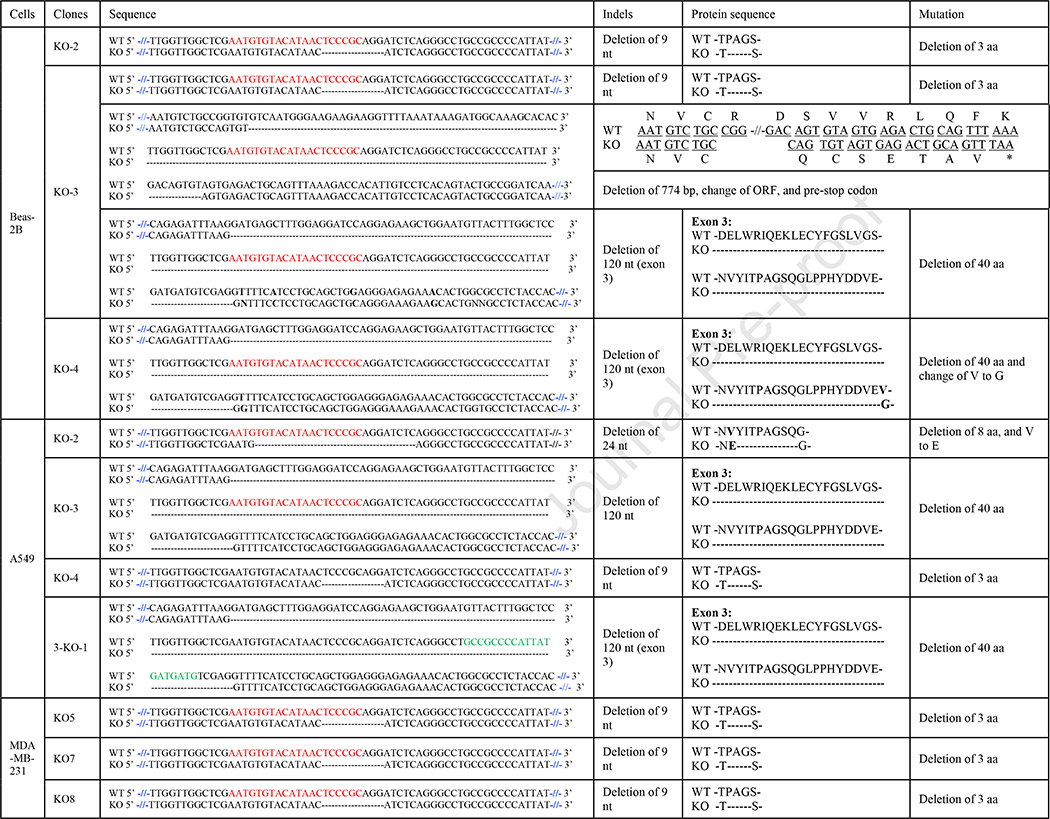 |
Fig 4.
Summary of the deletion and alternative splicing in mdig mRNA from KO clones. A. Schematic of deletions and alternative splicing in KO clones. White bars represent exons and blank lines are introns. Red slash bars represent deletion or alternative spliced nucleotides. The red and green arrow indicate where the sgRNA-1 and sgRNA-3 are targeted respectively. B. Corresponding protein sequence. Mdig protein sequence is in black. The third exon protein sequence is shaded in pink. The nine and twenty-four nucleotides are corresponding to amino acids shown in black underlines or red underlines respectively. The amino acid substitute in KO cells are marked with bold. The sequence in red is the new alternative spliced form with new stop codon (*).
Identifying single nuclear polymorphisms (SNPs) of mdig gene
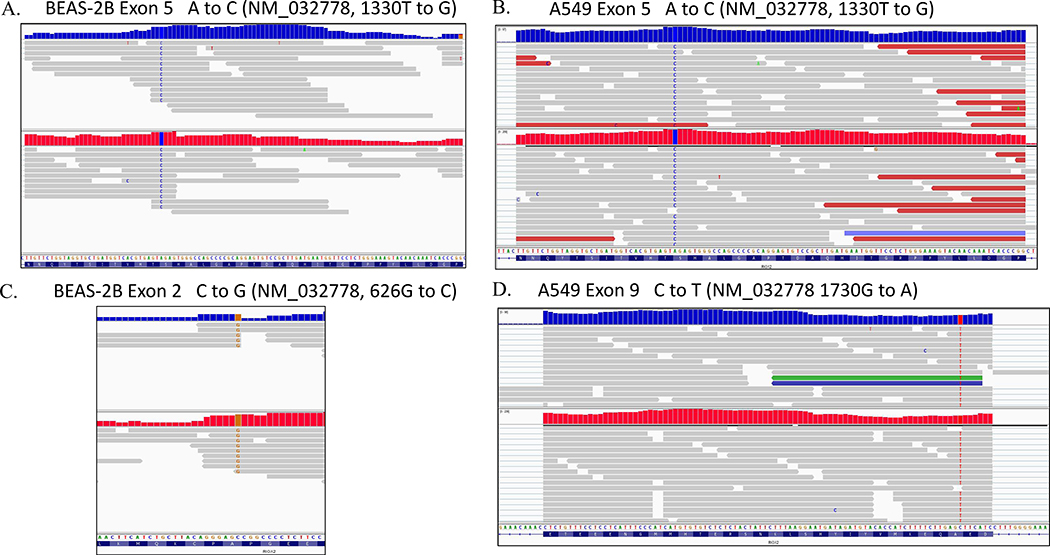
In addition to the deletion and alternative splicing of mdig mRNA that resulted from CRISPR-Cas9 gene editing, analysis of the RNA-seq data from both WT and KO cells, we also noted three previously unreported SNPs of the mdig gene in both BEAS-2B cells and A549 cells (Fig. 5). Both BEAS-2B and A549 cells exhibit 1330T to G SNP at exon 5 (reference sequence NM_032778). This SNP does not change the encoded amino acid. In BEAS-2B cells, there is a 626G to C SNP in exon 2, which results in a substitution of Ala with Pro at amino acid 17 of the mdig protein. In A549 cells, a SNP of 1730G to A in exon 9 was observed, which leads to replacement of Ala with Thr at amino acid 385 of the mdig protein. It is unknown at the present whether these amino acid substitutions due to SNPs affect the structure and function of mdig protein.
Fig 5.
Single nuclear polymorphisms (SNPs) of mdig gene identified from RNA-seq. A. and B. The SNP of A to C of the exon 5 of mdig in both WT and KO of BEAS-2B and A549 cells. This is comparable to the exchange of 1330T to G in reference sequence NM_032778. C. The substitution of C to G of the exon 2 in WT and KO BEAS-2B cells which is equal to the substitution of 626G to C in NM_032778 sequence. D. The SNP of C to T in exon 9 in WT and KO A549 cells. This is equal to the exchange of 1730G to A in NM_032778 sequence.
Discussion
In the current report, we provided evidence showing deletion and alternative splicing of mdig mRNA after CRISPR-Cas9 mediated-gene knockout for mdig. As expected, the deletion occurs at the sgRNA targeting region of exon 3 of the mdig gene. The most common deletions at the mRNA levels of mdig are 9 and 24 nucleotide deletion, respectively, in both BEAS-2B cells and A549 cells. In addition, we also revealed that gene editing by CRISPR-Cas9 approach induces alternative splicing of the mdig mRNA, including complete skipping out of entire exon 3 and alternative splicing between exon 2 and exon 8, leading to omission of exons 3, 4, 5, 6, and 7.
CRISPR-Cas9 is the most precise and simplest method used for genetic manipulation by removing, adding, or altering the genomic sequence. A tide of excitement had been achieved in the past few years because of its faster, cheaper and easy to perform in laboratories and potential applications in clinics for a number of single gene disorders, such as sickle cell disease [25–27] and cystic fibrosis [28, 29], and even some complex human diseases, including cancer [30–32], heart diseases [33, 34], or viral infection [35–38]. Some concerns had been raised in CRISPR-Cas9 technology due to possible off-target mutation that may result in unpredicted consequences. This is the main reason at the present why this technology is prohibited in germline or embryonic gene editing.
The results from the present report suggest that in addition to the uncontrollable size of indels on the genome created by CRISPR-Cas9 cleavage and the NHEJ repair, different patterns of alternative splicing of the target mRNA may be warrant for an additional layer of cautions when applying this technology for disease-related gene editing. Physiological alternative splicing is considered as an evolutional advantage allowing human cells to generate more proteins than would be expected from the given number of genes in the genome. It is estimated that more than 90% human multi-exonic genes are alternatively spliced with different degrees [39, 40]. For the mdig gene we studied in the present report, we had previously detected an alternative splicing of mdig that lacks the entire exon two (exon 3), but contains a new alternative exon between exon five and exon six in a cancer cell line [19]. However, alternative splicing induced by the non-physiological conditions, such as CRISPR-Cas9 gene editing, may be harmful due to the generation of proteins that may be pathogenic or carcinogenic. Although we did not detect new mdig proteins resulted from alternative splicing, possibly because of the limitation of the currently available antibodies that can recognize these new isoforms, our proteomic analysis of the triple negative breast cancer cell line MDA-MD-231 cells did detect some peptides derived from exon 3 and exon 7 (data not shown).
It will be interesting to understand how gene editing by CRISPR-Cas9 causes alternative splicing of the targeting mRNA, a phenomenon that hadn’t been well documented at the present. In a study to establish cyclin B3 (Ccnb3) knockout mice through CRISPR-Cas9-based gene editing, alternative splicing of the target gene Ccnb3 was noted and believed as a result of deletion of the exon-intron boundary sequences [41]. Similarly, knockout of FLOT1 gene by CRISPR-Cas9 approach also induced alternative splicing of FLOT1 mRNA in Hela cells [42], and was speculated the indels created by CRISPR-Cas9 might disrupt the exonic regulatory element for splicing. Accordingly, despite CRISPR-Cas9 is a major breakthrough in genome editing and very likely to be applied for the treatment of certain human diseases, some risks remain and new risks, such as alternative splicing of the target genes, need to be recognized.
Material and methods
Cell culture
The human bronchial epithelial cell line BEAS-2B, lung adenocarcinoma epithelial cell line A549, and breast cancer cell line MDA-MB-231 were purchased from America Type Culture Collection (ATCC, Manassas, VA). BEAS-2B and A549 were cultured in DMEM-high glucose with 5% FBS, 1% penicillin-streptomycin (Gibco, cat.no. 15140122), and 1% L-Glutamine (Gibco, cat.no. 25030164). MDA-MB-231 cells were maintained in DMEM F-12 with 10% FBS, 1% penicillin-streptomycin, and 1% L-Glutamine.
CRISPR constructs
To generate the CRISPR-Cas9 plasmids, sgRNA-1(5’-AATGTGTACATAACTCCCGC-3’) and sgRNA-3 (5’-CATCATCATAATGGGGCGGC-3’) were selected. Single-stranded top and bottom primers for each sgRNA were annealed to be double-stranded. Double stranded sgRNAs were cloned into vector pSpCas9(BB)-2A-Puro (Addgene plasmid ID: 48139) or pSpCas9(BB)-2A-Blast as the standard procedure described [24].
Plasmid transfection and colonies selection
Cells (2.5 × 105) were transfected with Lipofectamine 2000 (Thermo fisher scientific) according to the manufacturer’s protocol. 48h after transfection, cells were split into 10 cm dish for Blasticidin or puromycin (Thermo fisher scientific) selection. BEAS-2B, A549, and MDA-MB231 cells were cultured in 2ug/ml, 4ug/ml, and 4ug/ml Blasticidin respectively for selection until single colonies were formed. Colonies were collected and subjected for western blotting to screen mdig protein expression.
Western blotting
Total protein lysis was prepared in 1x RIPA buffer (Cell signaling) supplemented with PMSF and phosphatase and protease inhibitors cocktail (Thermo Fisher Scientific). Protein concentration were measured using Micro BCA Protein Assay Reagent kit (Thermo Fisher Scientific). 30 ug of total protein were separated into 10% SDS-PAGE gels, and transferred on PVDF membranes (Millipore). Membranes were probed with1:1000 anti-mdig primary antibody (Invitrogen Cat.no. 39–7300) or 1:5000 GAPDH (Cell signaling).
RT-PCR
Total RNA was isolated by RNeasy plus mini kit (QIAGEN) according to manufacturer’s protocol. Total 1 ug RNA were converted to cDNA using High-Capacity RNA-to cDNA kit (Applied Biosystems) according to manufacturer’s protocol. PCR were performed to amplify mdig cDNA using primers as follow: forward primer, 5’-TCATGTCGGGCCTAAGAGAC-3’; and reverse primer, 5’-GGCATTTGATTCTGCAAAGG-3’. PCR products were run in 1% agarose gels. The alternative isoforms were extracted from gel and then purified by QIAquick gel extraction kit (QIAGEN). The purified PCR products were sent directly or cloned into pJET1.2/blunt cloning vector (Thermo fisher scientific) for sanger sequencing.
RNA sequencing
RNA-seq was performed as described [24]. Data was visualized by Integrative Genomics Viewer (IGV).
Supplementary Material
Fig 1s. Transcripts of mdig from BEAS-2B and A549 clones from RNA-seq. The enlarged lower panel shows transcripts of the third exon. The double black arrow indicates the deletion of sequence 5’-CCTGCGGA-3’, reverse complementary to sequence 5’-TCCCGCAGG-3’ around DSBs. The red dotted lines indicate the sgRNA-1 sequence.
Highlights.
Successful knockout (KO) of mdig gene in lung cells by CRISPR-Cas9 gene editing;
DNA sequencing revealed multiple alternative splicing of mdig mRNA in the KO cells;
In addition to indel, skipping of single and multiple exons were noted in KO cells.
Acknowledgement
This research project is partially supported by NIH grants R01 ES028263, R01 ES028335, and P30 ES020957 to FC.
Footnotes
Conflict of Interest
No conflict of interest is disclosed.
GenBank access IDs for the sequences described in this manuscript are: MT228915, MT228916 and MT228917.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A, Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product, Journal of bacteriology, 169 (1987) 5429–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mojica FJM, Díez-Villaseñor C, Soria E, Juez G, Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria, Molecular Microbiology, 36 (2000) 244–246. [DOI] [PubMed] [Google Scholar]
- [3].Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, Multiplex genome engineering using CRISPR/Cas systems, Science (New York, N.Y.), 339 (2013) 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM, RNA-guided human genome engineering via Cas9, Science (New York, N.Y.), 339 (2013) 823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J, RNA-programmed genome editing in human cells, eLife, 2 (2013) e00471–e00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F, Genome engineering using the CRISPR-Cas9 system, Nature protocols, 8 (2013) 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Porteus MH, Baltimore D, Chimeric Nucleases Stimulate Gene Targeting in Human Cells, Science, 300 (2003) 763. [DOI] [PubMed] [Google Scholar]
- [8].Miller JC, Holmes MC, Wang J, Guschin DY, Lee Y-L, Rupniewski I, Beausejour CM, Waite AJ, Wang NS, Kim KA, Gregory PD, Pabo CO, Rebar EJ, An improved zinc-finger nuclease architecture for highly specific genome editing, Nature Biotechnology, 25 (2007) 778–785. [DOI] [PubMed] [Google Scholar]
- [9].Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, Pierick CJ, Hoffman E, Maeder ML, Khayter C, Reyon D, Dobbs D, Langenau DM, Stupar RM, Giraldez AJ, Voytas DF, Peterson RT, Yeh J-RJ, Joung JK, Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA), Nature Methods, 8 (2011) 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wood AJ, Lo T-W, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Meyer BJ, Targeted Genome Editing Across Species Using ZFNs and TALENs, Science, 333 (2011) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moscou MJ, Bogdanove AJ, A Simple Cipher Governs DNA Recognition by TAL Effectors, Science, 326 (2009) 1501. [DOI] [PubMed] [Google Scholar]
- [12].Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF, Targeting DNA Double-Strand Breaks with TAL Effector Nucleases, Genetics, 186 (2010) 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P, Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription, Nature Biotechnology, 29 (2011) 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sanjana NE, Cong L, Zhou Y, Cunniff MM, Feng G, Zhang F, A transcription activator-like effector toolbox for genome engineering, Nature Protocols, 7 (2012) 171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F, DNA targeting specificity of RNA-guided Cas9 nucleases, Nature Biotechnology, 31 (2013) 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD, High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells, Nature Biotechnology, 31 (2013) 822–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cho SW, Kim S, Kim Y, Kweon J, Kim HS, Bae S, Kim J-S, Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases, Genome research, 24 (2014) 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang X-H, Tee LY, Wang X-G, Huang Q-S, Yang S-H, Off-target Effects in CRISPR/Cas9-mediated Genome Engineering, Molecular Therapy - Nucleic Acids, 4 (2015) e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang Y, Lu Y, Yuan B-Z, Castranova V, Shi X, Stauffer JL, Demers LM, Chen F, The Human mineral dust-induced gene, mdig, is a cell growth regulating gene associated with lung cancer, Oncogene, 24 (2005) 4873. [DOI] [PubMed] [Google Scholar]
- [20].Lu Y, Beezhold K, Chang Q, Zhang Y, Rojanasakul Y, Zhao H, Castranova V, Shi X, Chen F, Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3, Cell Cycle, 8 (2009) 2101–2109. [DOI] [PubMed] [Google Scholar]
- [21].Chen B, Yu M, Chang Q, Lu Y, Thakur C, Ma D, Yi Z, Chen F, Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin, Oncotarget, 4 (2013) 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thakur C, Wolfarth M, Sun J, Zhang Y, Lu Y, Battelli L, Porter DW, Chen F, Oncoprotein mdig contributes to silica-induced pulmonary fibrosis by altering balance between Th17 and Treg T cells, Oncotarget, 6 (2015) 3722–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thakur C, Chen B, Li L, Zhang Q, Yang Z-Q, Chen F, Loss of mdig expression enhances DNA and histone methylation and metastasis of aggressive breast cancer, Signal Transduction and Targeted Therapy, 3 (2018) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Q, Thakur C, Fu Y, Bi Z, Wadgaonkar P, Xu L, Liu Z, Liu W, Wang J, Kidder BL, Chen F, Mdig promotes oncogenic gene expression through antagonizing repressive histone methylation markers, Theranostics, 10 (2020) 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP, Luc S, Kurita R, Nakamura Y, Fujiwara Y, Maeda T, Yuan G-C, Zhang F, Orkin SH, Bauer DE, BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis, Nature, 527 (2015) 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoban MD, Lumaquin D, Kuo CY, Romero Z, Long J, Ho M, Young CS, Mojadidi M, Fitz-Gibbon S, Cooper AR, Lill GR, Urbinati F, Campo-Fernandez B, Bjurstrom CF, Pellegrini M, Hollis RP, Kohn DB, CRISPR/Cas9-Mediated Correction of the Sickle Mutation in Human CD34+ cells, Molecular therapy : the journal of the American Society of Gene Therapy, 24 (2016) 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grevet JD, Lan X, Hamagami N, Edwards CR, Sankaranarayanan L, Ji X, Bhardwaj SK, Face CJ, Posocco DF, Abdulmalik O, Keller CA, Giardine B, Sidoli S, Garcia BA, Chou ST, Liebhaber SA, Hardison RC, Shi J, Blobel GA, Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells, Science (New York, N.Y.), 361 (2018) 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schwank G, Koo B-K, Sasselli V, Dekkers Johanna F., Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent Cornelis K., Nieuwenhuis Edward E.S., Beekman Jeffrey M., Clevers H, Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients, Cell Stem Cell, 13 (2013) 653–658. [DOI] [PubMed] [Google Scholar]
- [29].Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM, Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs, Cell reports, 12 (2015) 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang H, Sun W, CRISPR-mediated targeting of HER2 inhibits cell proliferation through a dominant negative mutation, Cancer Letters, 385 (2017) 137–143. [DOI] [PubMed] [Google Scholar]
- [31].Song C-Q, Li Y, Mou H, Moore J, Park A, Pomyen Y, Hough S, Kennedy Z, Fischer A, Yin H, Anderson DG, Conte D, Zender L, Wang XW, Thorgeirsson S, Weng Z, Xue W, Genome-Wide CRISPR Screen Identifies Regulators of Mitogen-Activated Protein Kinase as Suppressors of Liver Tumors in Mice, Gastroenterology, 152 (2017) 1161–1173.e1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen Z-H, Yu YP, Zuo Z-H, Nelson JB, Michalopoulos GK, Monga S, Liu S, Tseng G, Luo J-H, Targeting genomic rearrangements in tumor cells through Cas9-mediated insertion of a suicide gene, Nature Biotechnology, 35 (2017) 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Long C, Li H, McAnally JR, Baskin KK, Shelton JM, Bassel-Duby R, Olson EN, CRISPR-Cpf1 correction of muscular dystrophy mutations in human cardiomyocytes and mice, Science advances, 3 (2017) e1602814–e1602814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].El Refaey M, Xu L, Gao Y, Canan BD, Adesanya TMA, Warner SC, Akagi K, Symer DE, Mohler PJ, Ma J, Janssen PML, Han R, In Vivo Genome Editing Restores Dystrophin Expression and Cardiac Function in Dystrophic Mice, Circulation research, 121 (2017) 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liao H-K, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M, Suzuki K, Xu R, Hishida T, Chang C-J, Esteban CR, Young J, Belmonte JCI, Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells, Nature Communications, 6 (2015) 6413. [DOI] [PubMed] [Google Scholar]
- [36].Kennedy EM, Kornepati AVR, Goldstein M, Bogerd HP, Poling BC, Whisnant AW, Kastan MB, Cullen BR, Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease, Journal of virology, 88 (2014) 11965–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roehm PC, Shekarabi M, Wollebo HS, Bellizzi A, He L, Salkind J, Khalili K, Inhibition of HSV-1 Replication by Gene Editing Strategy, Scientific reports, 6 (2016) 23146–23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hübner A, Petersen B, Keil GM, Niemann H, Mettenleiter TC, Fuchs W, Efficient inhibition of African swine fever virus replication by CRISPR/Cas9 targeting of the viral p30 gene (CP204L), Scientific Reports, 8 (2018) 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB, Alternative isoform regulation in human tissue transcriptomes, Nature, 456 (2008) 470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ, Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing, Nature Genetics, 40 (2008) 1413–1415. [DOI] [PubMed] [Google Scholar]
- [41].Tang J-X, Chen D, Deng S-L, Li J, Li Y, Fu Z, Wang X-X, Zhang Y, Chen S-R, Liu Y-X, CRISPR/Cas9-mediated genome editing induces gene knockdown by altering the premRNA splicing in mice, BMC Biotechnology, 18 (2018) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kapahnke M, Banning A, Tikkanen R, Random Splicing of Several Exons Caused by a Single Base Change in the Target Exon of CRISPR/Cas9 Mediated Gene Knockout, Cells, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig 1s. Transcripts of mdig from BEAS-2B and A549 clones from RNA-seq. The enlarged lower panel shows transcripts of the third exon. The double black arrow indicates the deletion of sequence 5’-CCTGCGGA-3’, reverse complementary to sequence 5’-TCCCGCAGG-3’ around DSBs. The red dotted lines indicate the sgRNA-1 sequence.