Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has frequent and sometimes lethal thrombotic complications. The role that activated platelets, leukocytes, and small (0.3-micron to 1-micron) extracellular vesicles (EVs) play in ECMO thrombosis is not well understood.
Objectives
To test the effect of blood flow rate on the generation of activated platelets, leukocytes, and EVs in a simulated neonatal ECMO circuit using heparinized human whole blood.
Methods
Simulated neonatal roller pump circuits circulated whole blood at low, nominal, and high flow rates (0.3, 0.5, and 0.7 L/min) for 6 h. Coagulopathy was defined by thromboelastography (TEG), STA®-procoagulant phospholipid clot time (STA®−Procoag-PPL), and calibrated automated thrombogram. High-resolution flow cytometry measured the cellular expression of prothrombotic phospholipids and proteins on platelets, leukocytes, and EV.
Results
Despite heparinization, occlusive thrombosis halted flow in two of five circuits at 0.3 L/min and three of five circuits at 0.7 L/min. None of the five circuits at 0.5 L/min exhibited occlusive thrombosis. Phosphatidylserine (PS)-positive platelets and EVs increased at all flow rates more than blood under static conditions (P < .0002). Tissue factor (TF)-positive leukocytes and EVs increased only in low-flow and high-flow circuits (P < .0001). Tissue factor pathway inhibitor (TFPI), at 50 times more than the concentration in healthy adults, failed to suppress thrombin initiation in low-flow and high-flow circuits.
Conclusions
This in vitro study informs ECMO specialists to avoid low and high blood flow that increases TF expression on leukocytes and EVs, which likely initiate clot formation. Interventions to decrease TF generated by ECMO may be an effective approach to decrease thrombosis.
Keywords: blood cells, extracellular vesicles, extracorporeal membrane oxygenation, thrombosis, tissue factor
1 |. INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) provides lifesaving care to children and adults with organ failure and allows successful surgical repair of cardiac anatomical defects.1,2 However, overall survival for neonates supported with ECMO has decreased, as increasingly complex ECMO patients continue to experience high rates of morbidity from mechanical complications.3 Over the past 60 years the components of an ECMO circuit, which include a blood pump and an artificial lung, also known as an oxygenator, have significantly improved.1 These improvements increased the utilization of extracorporeal circuits for a wide variety of clinical situations from short-term support, such as cardiopulmonary bypass surgery, to long-term support such as left ventricle assist devices. Despite these advances, device-related thrombosis continues to result in increased mortality, neurological morbidity, and limb loss.4 One-third of neonates and children develop a thrombotic complication in the circuit, oxygenator, or patient during ECMO support.4 Current laboratory tests such as prothrombin time, partial thromboplastin time, platelet count, fibrinogen level, activated clotting time, or heparin dose are poor predictors of circuit thrombosis.5 Moreover, systemic anticoagulation and heparin-coated polymer surfaces do not decrease ECMO-related thrombotic events.4 Prolonged duration of ECMO further increases the burden of thrombosis, resolved only with an exchange of the ECMO circuit. A circuit exchange is a highrisk procedure exposing the patient to temporary oxygenation and hemodynamic failure. An increase in plasma D-dimer concentration, a product of clot degradation, is associated with the need for circuit exchange but can be unreliable as it is heavily influenced by other contributing pathologies.6 Improved understanding of the mechanisms that lead to ECMO-related thrombosis may lead to novel interventions.
During circulation, blood pumps increase the wall shear stress within the ECMO circuit causing circulating platelet and leukocyte activation.7–9 In vitro and clinical studies document that platelets, erythrocytes, and leukocytes express prothrombotic proteins and phospholipids and release prothrombotic EVs when exposed to abnormal or prolonged shear stress.10 Extracellular vesicles, also known as microparticles, are small (0.3–1 micron) cell-derived membrane vesicles11,12 that can contribute to clot formation by the initiation and propagation of clotting without exposure to an artificial surface.13 Our in vitro studies documented the release of platelet-derived EVs in a neonatal ECMO model using porcine whole blood.14,15 However, the effect of ECMO pump flow rate on the generation of prothrombotic cells, EV, and thrombin generation in human blood remains to be established.
Multidetector computer tomography and scanning electron microscopy have documented that a majority of ECMO thrombotic events occur in the hollow-fiber oxygenator.16,17 Our studies identified that ECMO can activate platelets to incorporate into clots within the membrane oxygenator.17 Wilm et al.18 further documented an increased adhesion of leukocytes into membranelike structures spanning multiple gas capillaries in oxygenators. Hollow-fiber oxygenators fail in >10% of cases because of thrombotic deposits on the gas exchange capillaries, leading to an increase in blood flow resistance and diffusion path.19 A pump-driven ECMO system will react to increased blood flow resistance with an augmentation of pump speed (revolutions/minute), which further raises the mechanical stress on cellular blood components, inducing activation of the coagulation cascade.20,21 Therefore, flow rate may have a significant effect on ECMO thrombosis that is independent of systematic heparinization or contact activation. To investigate, samples were collected from a simulated neonatal ECMO circuit at FDA approved low (i.e., minimum), nominal, and high (i.e., maximum) blood flow to determine changes in coagulopathy, thrombocytopathy, prothrombotic cells and EV, thrombin generation, and clot formation. Our overall hypothesis is that low and high ECMO blood flow rate, compared to static control and nominal flow rate, increases prothrombotic platelets, leukocytes, EVs, clot formation, and circuit failure.
2 |. MATERIALS AND METHODS
Simulated ECMO includes the Cobe CV model 43600 roller pump (Sorin), KIDS D100 oxygenator (Sorin), 400 mL Capiox venous reservoir (Terumo), and wire-wrapped arterial and venous pediatric DLP catheters, 8 Fr and 12 Fr, respectively (Medtronic). Activated clotting time (ACT) cartridges were purchased from Werfen and measured using a Haemochron Jr II Signature Elite. Blood gas, chemistry, and glucose i-STAT cartridges were purchased from Abbot Inc. Thromboelastography (TEG) kits were purchased from Haemonetics. Silica size beads were purchased from Bangs Laboratories. Accucount fluorescent particles were purchased from SPHERO™. Unfractionated heparin and dextrose were purchased from Baxter Healthcare. 4-(2-Hydroxyethyl)-1-piperazineethane-sulfonic acid buffered-saline solution with 0.05% bovine serum albumin was purchased from Sigma Aldrich Corp. and prepared by adding 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 140 mmol/L NaCl, and 0.05% bovine serum albumin to distilled water then changing the solution to a pH of 7.4 with sodium bicarbonate. Dade hepzyme (Cat# 10445730) was purchased from Siemen Industry Inc. Pacific Blue (Pac Blue) or fluorescein isothiocyanate - lactadherin antibody was purchased from Haemonetics Technology, Inc. Fluorescent conjugated anti-CD41/APC-H7 (Cat# 561422), anti-CD45/FITC or APCH7 (Cat# 347463 or 560178), anti-CD14/PerCP Cy5.5 (Cat# 550787), anti-CD142(TF)/phycoerythrin (Cat# 550312), and BD fluorescence-activated cell sorter (FACS) Lyse wash were purchased from BD Biosciences. FcR blocking reagent was purchased from Miltenyi Biotec. For immunohistochemistry staining, Prolong Golg antifade reagent with DAPI and fibrinogen-Alexa488 were purchased from Thermo Fisher Scientific (# P36931 and # PA1–85429). Additional platelet reagents for immunohistochemistry (CD61-APC) were purchased from BD Biosciences (#555752) and factor Va reagents purchased from GeneTx (# GTX21015). Drabkins reagent and hemoglobin standard for free plasma hemoglobin measurement were purchased from Fisher Scientific. Tumor necrosis factor alpha assay for the Bio-Plex® multiplex immunoassay system was purchased from BIO-RAD. For in-house EV TF activity assay the follow reagents were purchased: TF antibody from BD Biosciences (clone: HTF-1), immunoglobulin G from Sigma Aldrich, relipidated recombinant TF from Dade Innovin (Siemens), human factor VIIa (FVIIa), factor X (FX), and Pefachrome FXa 8595 from Enzyme Research Laboratories. Coagulation reagents, anti-Xa, D-dimer, and STA®-Procoag-PPL assay kits, thrombin fluorogenic substrate (Z-Gly-Gly-Arg-AMC), and calibrator (α2 macroglobulin/thrombin) reagents were purchased from STAGO. Tissue factor pathway inhibitor (TFPI) was purchased from R&D Systems (CAT# 2974-PI-010). Various blood collection tubes were purchased from Fisher Scientific.
2.1 |. Blood collection
The University of Texas Health Science Center at San Antonio and the U.S. Army Medical Research and Materiel Command Institutional Review Boards approved the study protocol (USAISR SOP L-15–003 and UTHSCSA IRB Protocol #HSC20120097H). Blood was collected into a blood transfer bag containing 1 U/mL of heparin. Preliminary studies determined that heparin was preferable to standard anticoagulant (e.g., citrate phosphate dextrose) to prevent dilution and calcium depletion. Blood samples were collected into a syringe then dispersed to blood gas cartridges, ACT cartridges, and blood collection tubes with either 3.2% sodium citrate tube, lithium-heparin, or ethylenediaminetetraacetic acid. Plasma samples were prepared from the heparinized circuit by collecting in sodium citrate to prevent clot formation after hepzyme reversal.
2.2 |. Simulated ECMO
A neonatal single-use ECMO circuit, modified with decreased volume and surface area, was constructed as in previous studies.14 The roller-pump raceway used 3/8-inch tubing14,22 and was calibrated before each experimental run as previously described..23 The pump, oxygenator, catheters, and reservoir bag were attached with sections of sterile ¼-inch × 3/32-inch′ tubing. The circuit maintained a temperature of 37 °C using a laboratory heating bath circulator and an integrated heat exchanger within the oxygenator. Within minutes of blood collection, the circuit was filled with care to remove bubbles. A static control sample was then removed from the circuit, gently mixed at regular intervals, and held in the same tubing at 37 °C for the duration of the experiment. Each single-use ECMO system circulated for 6 h with continuous pressure measurements recorded at the inlet and outlet of the hollow fiber oxygenator. Blood gas was measured every hour to keep the pH, pco2, and po2 within normal range by way of adjustments in a mixture of carbon dioxide and room air. Activated clotting time and glucose were adjusted every hour to maintain circuit values within target ranges of 180 to 220 s and 70 to 125 mg/dL, respectively; the control received matched amounts of heparin or dextrose. Blood samples were drawn from the circuit and from the control at pump start (0 h), and after 1, 2, 4, and 6 h of circulation. Flow rates were selected within the manufacturer-recommended operating range of the KIDS D100 hollow-fiber oxygenator. A low flow rate was defined as 0.3 L/min, a typical clinical flow rate was defined as 0.5 L/min (i.e., nominal), and a high flow rate was defined as 0.7 L/min. Using the same protocol and sequence of use the experiment was repeated for a total of five separate runs at 0.3, 0.5, or 0.7 L/min flow rate in a single-use neonatal ECMO system for a total of 15.
2.3 |. Hemolysis, coagulation, thrombelastography, and clot formation
Activated clotting time, blood gas, complete blood count, and thromboelastography were measured within minutes of collection of whole blood samples. The ACT and blood gas measurements were performed following manufacturer’s directions. The TEG 5000 system used heparinase and blood collected in sodium citrate to measure hemostatic characteristics. Platelet-free plasma was prepared by sequential centrifugation at 2500 × g for 15 min at room temperature × 2 and then aliquoted into 1.5-mL Nunc® Cryotubes® (Sigma) for storage at −80 °C. Using manufacturer’s directions, the STAGO STA-R Evolution® measured prothrombin time, activated partial thromboplastin time, fibrinogen, D-dimer, antithrombin III, von Willebrand antigen, anti-Xa activity, and Procoag-PPL® clot time. International Normalized Ratio (INR) was calculated by determining the ratio of sample prothrombin time to a reference prothrombin time from 30 healthy adults. For all assays except anti-Xa, samples were treated prior to analysis with 1 μL of Dade hepzyme per 100 μL sample at 37 C for 1 min to reverse residual heparin from the circuit. A previously described spectrophotometric assay24 was used to detect free plasma hemoglobin. At completion of the experiment, the oxygenators were flushed with 4% paraformaldehyde and buffered saline, then frozen at −80 °C until analysis. Using a tabletop band saw, the top and bottom of the membrane case were removed, allowing extraction of the circular hollow-fiber bundle. Sections of the membrane were then placed in Optimal Cutting Temperature Compound for cryosection at 10 microns. Histological cross-section slices of the membrane oxygenator hollow fibers were then stained with antibodies to identify platelets, leukocytes, fibrinogen, and factor Va. Preliminary images of these oxygenators were taken with a Zeiss Axio fluorescence microscope.
2.4 |. Platelet and leukocyte assays
An ADVIA 120 Hematology System (Siemens Medical Solutions) used blood collected in ethylenediaminetetraacetic acid to determine platelet and leukocyte counts. Platelet and leukocyte expression of prothrombotic proteins were measured using an optimized whole blood flow protocol on a BD FACS Canto using previously described fluorescence-minus-one controls and FcR Block..25,26 A 5-μL sample of heparinized whole blood was diluted 1:20 in 4-(2-hydroxy-ethyl)-1-piperazineethanesulfonic acid buffered-saline solution and then incubated in the dark for 30 min with antibodies to CD41a, CD14, CD45, CD142, and lactadherin. Prothrombotic platelets were identified as positive for CD41a (platelet marker) and lactadherin, which binds to PS. Prothrombotic monocytes were identified, after following the manufacturer directions for BD FACS Lyse Wash, as positive for CD45 (leukocytes marker), CD14 (monocyte marker), and CD142 (tissue factor marker). Fluorescence-activated cell sorter (FACS) data were processed using FlowJo (version 7.5.5; Tree Star Inc). Concentration of TNF-α, a marker of monocyte activation, was measured following the manufacturer’s directions using the Bio-Plex® Multiplex Immunoassay system (BIO-RAD). Extracellular TF activity (pg/mL) was measured on accuSkan GO UV/Vis microplate spectrophotometer (Fischer Scientific) using an in-house assay described previously.27
2.5 |. Extracellular vesicle quantification
To detect and quantify EVs, the International Society of Thrombosis and Haemostasis (ISTH) guidelines were followed and our protocol procedures were submitted to the EV-TRACK knowledgebase (EV-TRACK ID: EV170038).28 Previous studies helped to define optimal reproducible preanalytic conditions (e.g., centrifugation and storage) for EV analysis.29,30 Separate aliquots (25 μL) of Platelet Free Plasma (PFP) were diluted with 75 μL of phosphate buffered saline containing 1% bovine serum albumin and incubated for 15 min in the dark at 37 °C. Extracellular vesicle size (300–1000 nm) was determined by flow cytometer using a representative gate derived from a standard curve established with a silica size bead mixture of 150, 300, 500, 700, 1000, and 2000 nanometers on the high-resolution forward scatter photomultiplier tube of a BD FACS Canto II. Prothrombotic platelet EVs (PEVs) were identified with antibodies for CD41a and PS. Leukocyte EVs (LEVs) expressing tissue factor were identified with CD45 and CD142.
2.6 |. Calibrated automated thrombogram
Before measuring thrombin generation in PFP samples, residual heparin effects were removed using hepzyme. Thrombin generation was analyzed in triplicate samples of PFP (80 μL) with 20 μL of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffered-saline solution/BSA (no trigger). Thrombin generation was initiated without a trigger by automatically dispensing fluorogenic substrate (Z-Gly-Gly-Arg-AMC at 16 mmol/L) and CaCl2 (416 μmol/L). Thrombin generation was calibrated against wells containing 20 μL α−2 macroglobulin/ thrombin complex and 80 μL of PFP. A thrombogram curve was analyzed using the Thrombinoscope software v3.0.0.29 (Thrombinoscope BV).12 Thrombin generation in samples from control, low, nominal, and high flow rate were then repeated with final concentrations of 200 nmol/L lactadherin or 75 nmol/l of TFPI. Lactadherin was selected as a block for PS because it does not require calcium to bind PS..31 Tissue factor pathway inhibitor was selected as a block to TF because it is naturally expressed in healthy adult plasma at a concentration of 1.6 nmol/L (68 ng/mL).32 Plasma obtained from simulated ECMO at a nominal flow (0.5 L/min) for 6 h or whole blood stimulation with lipopolysaccharide (at final concentration of 10 μg/mL)33 was used to determine final inhibitory concentrations of lactadherin and TFPI. Lactadherin and TFPI concentrations that were selected roughly decreased peak thrombin time or lag time to approximately half of pretreated value (Data S1).
2.7 |. Statistical analysis
Group results were compared by mixed-effects analysis (i.e., repeated measures analysis of variance that allows missing values) for factors of flow rate and time. If a circuit occluded, then its subsequent time points are missing values; no data were excluded. Comparisons between groups and control were completed with a Tukey’s multiple comparison test. Effects of inhibitors (e.g., lactadherin and TFPI) on thrombin initiation were compared using the Games-Howell test between groups because the samples sizes were unequal. Simple linear regression determined the best fit line and Pearson correlation coefficient determined the goodness of fit. The results for low (n = 5), nominal (n = 5), or high flow rate (n = 5) and static control (n = 15) are presented as mean ± standard error of the mean. A control was run for each flow rate and then compiled for final analysis. Significance is defined as P < .05. Figures were produced and statistical analyses performed using GraphPad Prism version 8.1.2 for Macintosh, GraphPad Software, www.graphpad.com.
3 |. RESULTS
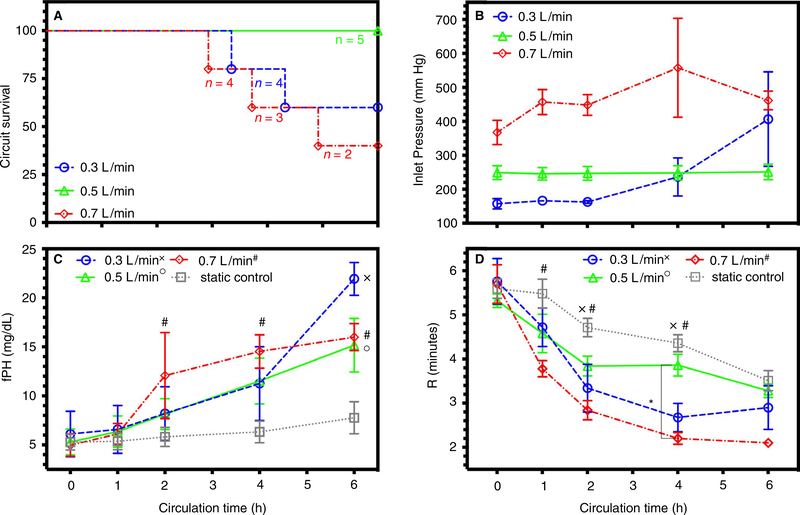
Nine men and six healthy women (aged 25–45) donated a unit (400 ± 50 mL) of whole blood. Among all groups, there were no significant differences in baseline values for age, leukocyte count, hemoglobin, platelet count, PEV, LEV, lag time, peak thrombin, or PPL clot time (Table 1). All circuits maintained a pH >7.3 for 6 h, whereas the pH in the gently mixed and sealed control significantly decreased from 7.4 ± 0.04 at pump start to 7.18 ± 0.02 at 6 h. All circuits maintained pco2 within normal range, whereas the control increased significantly from pump start at 38 ± 3.8 to 57 ± 3.7 mm Hg after 6 h. Among all groups, there were no significant changes over 6 h in the concentration of po2, sodium, potassium, chloride, or ionized calcium. Before pump start, all the groups’ mean anti-Xa activity rose above 0.2 U and then remained above 0.2 U for the duration of the experiment, a value previously documented as effective anticoagulation.34 None of the nominal-flow-rate circuits developed an occlusive thrombus compared to two of the low-flow circuits and three of the high-flow circuits (Figure 1A). Inlet pressure of the hollow-fiber oxygenator, a marker of shear stress, increased over time at low and high blood flow rates (Figure 1B). Platelet count was the only significant different baseline value in circuits that developed an occlusive thrombus at 217 ± 10 cells × 103/μL (n = 5) compared to non-occlusive circuits at 264 ± 20 cells × 103/μL (n = 10).
TABLE 1.
Demographics and baseline values of donors
| Category | 0.3 L/min | 0.5 L/min | 0.7 L/min |
|---|---|---|---|
| Sex (male:female) | 3M:2F | 3M:2F | 3M:2F |
| Age (years) | 34 ± 1.5 | 29 ± 2.4 | 38 ± 2.6 |
| Circulation (hours) | 5.1 ± 0.5 | 6 ± 0 | 4.7 ± 0.6 |
| Leukocytes × 103/μL | 5.4 ± 0.2 | 4.8 ± 0.37 | 6.2 ± 0.6 |
| Hemoglobin (mg/dL) | 14.8 ± 0.5 | 14.0 ± 0.4 | 13.8 ± 0.5 |
| Platelets × 103/μL | 234 ± 16 | 261 ± 28 | 251 ± 32 |
| PEV × 103/μL | 0.74 ± 0.24 | 0.75 ± 0.33 | 0.48 ± 0.26 |
| LEV × 103/μL | 0.04 ± 0.004 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| Lag time (min) | 22.5 ± 2.98 | 17.8 ± 2.53 | 19.8 ± 2.62 |
| Peak thrombin (nmol/L) | 63.3 ± 12.2 | 141.6 ± 30.6 | 64.3 ± 17.7 |
| PPL clot time (secs) | 64.6 ± 3.5 | 58.3 ± 2.9 | 71.7 ± 4.1 |
Note: There were no significant differences among all groups. Results represented as mean ± SEM for each group. There were 15 independent donors, 5 for each group.
Abbreviations: LEV, leukocyte extracellular vesicles; PEV, platelet extracellular vesicles; PPL, procoagulant phospholipid clot time; SEM, standard error of the mean.
FIGURE 1.
Simulated neonatal ECMO circuit failure, pressure, blood damage, and time to clot: Data documented as mean ± SEM. Symbol designation denoted significant difference from static control labeled as x, o, and # for low, nominal, and high flow rates, respectively. Significant differences between flow rates are denoted on the graph with a * and corresponding z-bar. A, Survival plot of each flow rate over time. B, Mean pressure in circuits measured at the inlet of the oxygenator for 0.3, 0.5, and 0.7 L/min. C, Free plasma hemoglobin (fPH) concentration measured in circuits and static control. D, Clot initiation time (R) for circuits and static control with a significant difference at low (0.3 L/min) and high (0.7 L/min) flow rates. ECMO, extracorporeal membrane oxygenation; SEM, standard error of the mean
3.1 |. Effect of flow rate on cell count, hemolysis, and thromboelastography (TEG)
Among all groups, platelet count did not significantly change over 6 h; nor were there significant differences between groups. In all circuits there was no difference in leukocyte count, but samples from control had significantly decreased leukocyte count after 6 h. All the circuits generated significant increases in free plasma hemoglobin, a sensitive marker of hemolysis and mechanical blood damage, more than control after 6 h (Figure 1C). Clot initiation time (R) significantly decreased in all groups from pump start. However, R significantly decreased in samples from low-flow and high-flow circuits more than control (Figure 1D) after 2 h. Clot strength (MA) did not change over 6 h for control-flow, nominal-flow, and high-flow circuits. MA at a low flow decreased significantly more than all groups after 6 h (n = 3). Among all groups, there was no significant change in the measurements of clot formation time (K), angle (α), clot elasticity (G), and clot lysis at 60 min during the experiment (Table S2).
3.2 |. Effect of flow rate on platelet, leukocyte, and EV expression of PS and TF
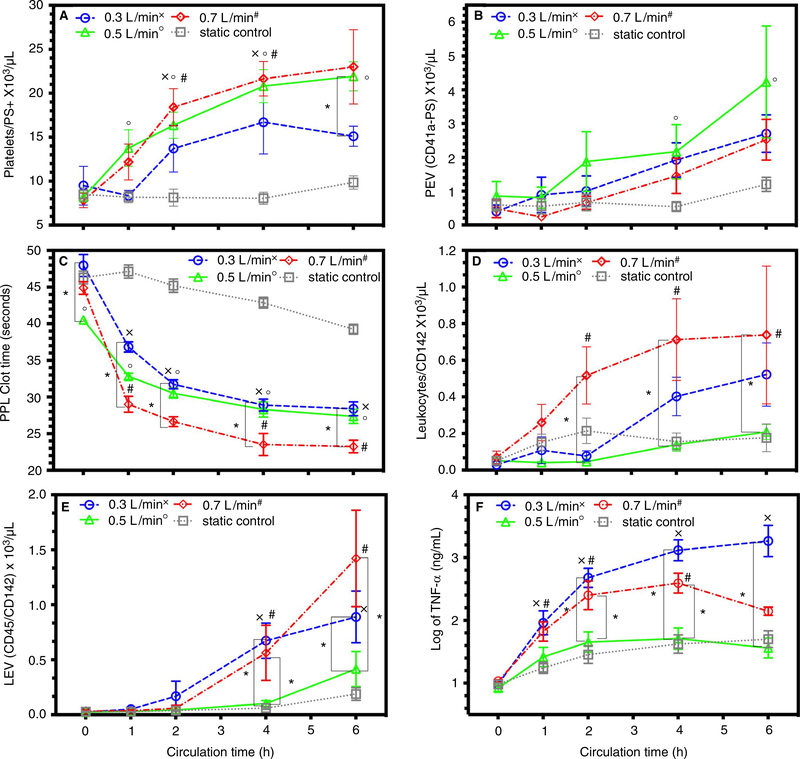
Platelet expression of PS significantly increased in samples from low blood flow, nominal blood flow, and high blood flow compared to control (Figure 2A). Prothrombotic platelet-derived EV, identified by CD41a and PS expression, increased over 6 h in all groups. However, only nominal-flow circuits increased PEV significantly greater than control after 4 h (Figure 2B). Procoagulant PPL clot time, a measurement of PS contribution to clot formation, was significantly lower in nominal circuits compared to control and low-flow circuits at pump start. Despite this initial difference, all flow rates significantly decreased PPL clot time more than control after 1 h (Figure 2C). Samples from high-flow circuits decreased PPL significantly more than low-flow circuits after 1 h and significantly more than nominal circuits after 4 h. Using simple linear regression, PPL clot time had a strong correlation to platelet expression of PS for all circulated samples with goodness of fit of R2 = .48 and significantly non-zero slope (y = −0.83x + 44.9), P < .0001. Leukocyte expression of tissue factor, a strong procoagulant protein, increased significantly in high-flow circuits compared to control (Figure 2D). After 2 h, high-flow circuits further increased leukocyte expression of tissue factor more than nominal-flow circuits. Prothrombotic LEV, identified by CD45 and TF expression, significantly increased in low-flow and high-flow circuits after 4 h compared to control or nominal circuit (Figure 2E). Log mean increases in TNF-α, a marker of monocyte activation, significantly increased in circulated blood and static control over 6 h (Figure 2F). In samples from low-flow and high-flow circuits, TNF-α increased significantly more than control after 1 h and more than nominal flow rate after 2 h.
FIGURE 2.
Platelets, leukocytes, and EV expression of PS and TF: Data documented as mean ± SEM. Symbol designation denoted significant difference from static control labeled as x, o, and # for low, nominal, and high flow rates, respectively. Significant differences between flow rates are denoted on the graph with a * and corresponding z-bar. A, Platelet expression of phosphatidylserine with significant differences between samples from circuits compared to static control. B, Platelet extracellular vesicles (PEVs) for circuits and static control. C, STA-Procoagulant Phospholipid Clot Time® (PPL) with significant differences from static control at 1, 2, and 4 h for all flow rates. D, Leukocyte expression of tissue factor with significant differences from static control and the 0.3-L/min to 0.7-L/min flow rates. E, Leukocyte EV with significant differences between 0.5-L/min flow rate and 0.3-L/min and 0.7 L/min flow rates. F, Tumor necrosis alpha (TNF-α) with significant differences after 2 h in the 0.3-L/min and 0.7-L/min flow rates and the 0.5- L/min flow rate and the static control. EV, extracellular vesicle; PS, phosphatidylserine; SEM, standard error of the mean; TF, tissue factor
3.3 |. Effect of flow rate on coagulation indices and clot formation
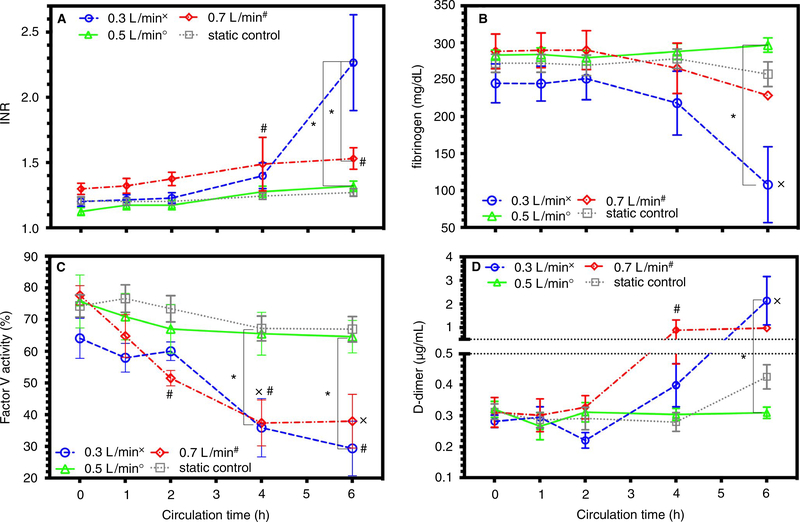
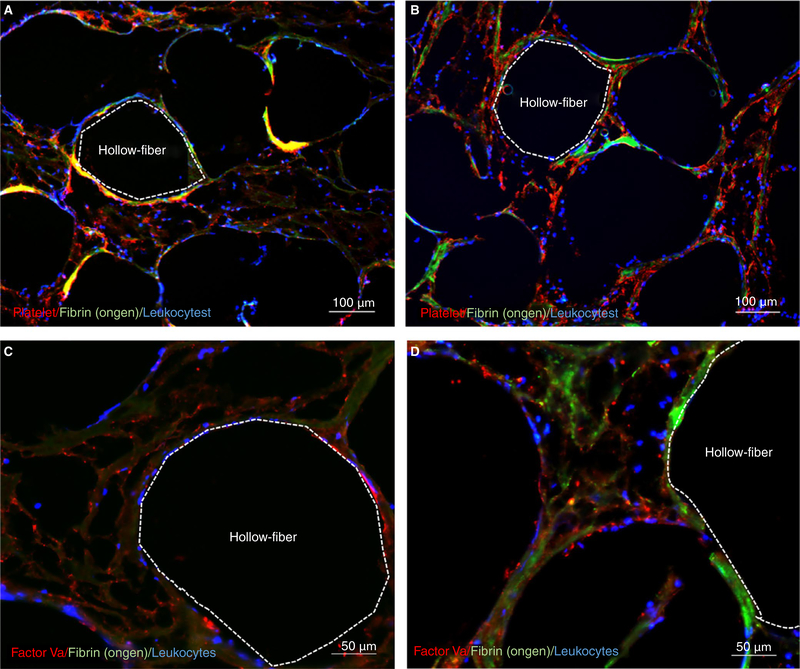
For all coagulation assays, except anti-Xa, hepzyme was used prior to analysis to inactivate residual heparin. After 2 h, INR significantly increased in high-flow circuits more than static control and start of pump. After 6 h, INR significantly increased in low-flow circuits more than control-flow rate, nominal-flow rate, or pump start (Figure 3A). Fibrinogen, a marker of clot consumption, only changed in the low-flow circuits, decreasing more than control, nominal-flow rate, or pump start after 6 h (Figure 3B). Factor V activity, a marker of tenase activity, significantly decreased in low-flow and high-flow circuits after 4 h compared to control, nominal-flow, and pump start (Figure 3C). D-dimer concentration, a clinical marker of circuit thrombosis, increased significantly in high-flow circuits after 4 h and in low-flow circuits after 6 h (Figure 3D). Among all groups, other coagulation indices of partial thromboplastin time, antithrombin III activity, or von Willebrand factor (vWF) antigen were not significantly different or changed over time (Table S3). Histological cross sections of one oxygenator from a low-flow, nominal-flow, or high-flow circuit were mounted and stained. Representative images of each of the oxygenators document an increase in platelets, leukocytes, fibrinogen, and factor Va that developed an occlusive thrombosis (0.3 and 0.7 L/min) (Figure 4). Representative images obtained from the nominal-flow circuit oxygenator documented few to no cellular deposits.
FIGURE 3.
Coagulation indices and factor V activity: Data documented as mean ± SEM. Symbol designation denoted significant difference from static control labeled as x, o, and # for low, nominal, and high flow rates, respectively. Significant differences between flow rates are denoted on the graph with a * and corresponding z-bar. A, INR measured in circuits and static control with significant differences in samples from 0.3-L/min and 0.7-L/min flow rates. B, Fibrinogen concentration (mg/dL) in circuits and static control with a significant difference at 6 h between 0.3 L/min and static control. C, Factor V activity (%) for circuits and static controls with significant differences at 4 h between 0.5-L/min flow rate and 0.3-L/min and 0.7-L/min flow rates. D, D-dimer (μg/mL) concentration for circuits and static controls with significant differences after 4 h between 0.5-L/min flow rate and 0.3-L/min and 0.7 L/min flow rates. INR, International Normalization Ratio; SEM, standard error of the mean
FIGURE 4.
Histochemistry of hollow-fiber oxygenators: Representative images from low (0.3 L/min) and high (0.7 L/min) oxygenators with orange dotted line outlying the inside of a representative gas fiber. A, Color micrograph of a cross section of a low-flow oxygenator with platelets (red), fibrinogen (green), and leukocytes (blue). B, Color micrograph of a cross section of a high-flow oxygenator with platelets (red), fibrinogen (green), and leukocytes (blue). C, Color micrograph of a cross section of a low-flow oxygenator with factor Va (red) and leukocytes (blue). D, Color micrograph of a cross section of a low-flow oxygenator with factor Va (red), fibrinogen (green), and leukocytes (blue)
3.4 |. Effect of flow rate on calibrated automated thrombogram
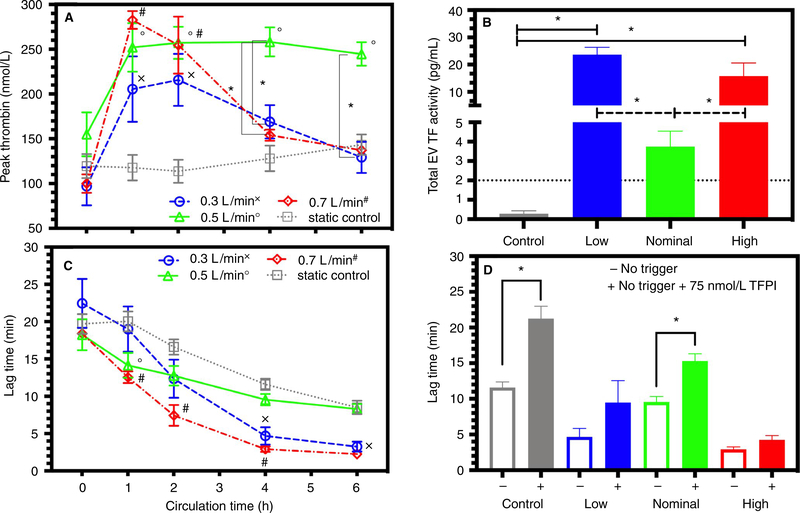
Among low-flow, nominal-flow, or high-flow circuits, CAT peak thrombin generation increased significantly more than control after 1 h (Figure 5A). Peak thrombin had a strong negative correlation to PPL for all circulated samples with goodness of fit of R2 = .31 and a significantly non-zero slope (Y = −5.741*X + 391.7), P < .0001. If we examine only the first 2 h for all circulated samples, this correlation increases with a goodness of fit of R2 = .62. After 4 h, low-flow and high-flow circuits significantly decreased peak thrombin compared to nominal flow rate. Extracellular vesicle tissue factor activity was significantly increased in the low-flow and high-flow circuits compared to nominal-flow and control conditions at the end of each pump run (Figure 5B). The CAT lag time significantly decreased in all groups. For the first hour, lag time decreased significantly more in nominal-flow-rate and high-flow-rate circuits than control samples. Low-flow and high-flow circuits significantly decreased their lag time more than control and nominal-flow rate after 4 h (Figure 5C). The TFPI treatment increased lag time for all groups. After 4 h, a nearly 50-fold increase in physiological concentration of TFPI (final concentration 75 nmol/L) was ineffective in preventing a significant increase in lag time in low-flow and high-flow circuits (Figure 5D). Lactadherin significantly suppressed lag time and peak thrombin in all circulated samples and static control (please see Figure S1).
FIGURE 5.
Thrombin generation and tissue factor activity: Data documented as mean ± SEM. Symbol designation denoted significant difference from static control labeled as x, o, and # for low, nominal, and high flow rates, respectively. Significant differences between flow rates are denoted on the graph with a * and corresponding z-bar. A, Peak thrombin measured without a trigger. B, Extracellular tissue factor activity measured after fours in all groups. C, Lag time measured without a trigger. D, Effects of TFPI (final concentration 75 nmol/L) on lag time at 4 h in control, low-flow, nominal-flow, and high-flow groups. SEM, standard error of the mean; TFPI, tissue factor pathway inhibitor
4 |. DISCUSSION
This is the first publication to document the effect of low, nominal, and high ex vivo ECMO flow on human platelets, leukocytes, and EVs. Previous laboratory studies documented that ECMO increases prothrombotic cells and EVs8 but were limited to circuits without an oxygenator, with component blood products that exclude platelets and leukocytes, or used animal blood.15,35,36 Case series documented increased concentrations of activated platelets, leukocytes, and EVs collected at the end of coronary bypass surgery or during support with a left ventricular assist device.11,37 The ECMO activated platelets and PEVs are prothrombotic through the increased surface expression of PS, a phospholipid that enhances thrombin generation by providing a surface for the assembly of the tenase and prothrombinase complexes. Because of their small size, PEVs increase 50-fold to 100-fold the expression of PS per surface area compared to expression of PS per platelet surface area.38 Circulating monocytes and LEVs under pathological conditions express TF, which is associated with an increased incidence of clinical thrombotic complications.39 Tissue factor is a transmembrane protein that functions as a high-affinity receptor for the TF-FVIIa complex, a potent initiator of thrombin generation. Therefore, this is the first report to document that blood flow generated by ECMO increases the concentration of prothrombotic phospholipids and proteins on platelet, leukocytes, and extracellular vesicles.
According to the Darcy equation of fluid dynamics, increases in fluid flow increase the change in pressure measured at the inlet and the outlet of a fixed resistance. Gu et al.9 and others have documented that pressure drop across a hollow-fiber oxygenator correlates closely to applied wall shear stress that activates platelets and leukocytes.7,40 Our study confirms and expands on this point, that shear stress accumulation (pressure drop × time) in an ECMO circuit increases surface expression of PS on platelets and PEV. For 2 h of circulation, all flow rates increase PS expression, which strongly correlates with increases in peak thrombin. After 3 h, some of the ECMO circuits set at low-flow and high-flow rates (e.g., 0.3 and 0.7 L/min) developed an occlusion within the hollow-fiber oxygenator that halted blood flow. The TEG measurements, gross observation, and immunohistochemistry of the low-flow and high-flow circuits suggest these occlusions are due to clot formation. Analysis of the plasma samples confirms clot formation with consumption of coagulation components that include decreased clot strength (MA), fibrinogen, and factor V activity. Immunofluorescent staining of thrombosis deposits in the low-flow and high-flow circuit oxygenators documented increased concentrations of factor V, platelets, and leukocytes in the clot. However, there was only a 20% decrease in platelet and leukocyte count during circulation, decreasing the likelihood that primarily cellular adhesion or cell lysis was the cause of the circuit occlusion. Furthermore, circuits that occluded began with a lower platelet count than circuits without occlusion; this confirms our previous studies14 suggesting platelet aggregation may not play a significant role in ECMO clot formation. Phosphatidylserine-positive EVs are highly prothrombotic but are not known to cause spontaneous clot formation; usually a more potent trigger is needed.41 Our data support this as the circuits that generated an occlusive thrombosis consumed their PS-positive EVs more than the circuits that did not have an occlusive thrombosis. Therefore, we surmise that ECMO circulation increases the expression of PS on platelets and EVs, which may enhance thrombin generation but do not trigger circuit thrombosis.
Increased tissue factor expression on monocytes during cardiopulmonary bypass surgery or simulated extracorporeal circulation significantly increases thrombin generation, clot formation, and thromboembolic events.8,42 Moreover, studies suggest that aseptic tissue factor expression is driven by mechanical shear stress through the induction of the tissue factor gene.43 This would explain the lack of tissue factor expression, thrombin generation, and clot formation in the static control. In the low-flow and high-flow circuits, INR increased while partil thromboplastin time did not change, suggesting that the clot initiation was primarily a TF:FVIIa-driven tenase process44 instead of a contact activation process. Larsson et al. 45 also documented a bypass of the contact activation pathway in a previous rabbit ECMO study. Larsson et al.45 documented deposition of fibrin within the oxygenator despite heparin anticoagulation or an antibody against FXIIa (3F7), the initiator of contact activation. This suggests that neither shear accumulation nor contact activation is the sole driver of an occlusive circuit thrombus. Last, only the low-flow and high-flow circuits documented a log-fold increase in a proinflammatory cytokine. Tumor necrosis factor alpha, a specific marker of monocyte activation, is associated with a significant increase in thrombogenicity.46 The combined generation of procoagulant and proinflammatory stimuli likely contribute to ECMO-related morbidity and offer targets for therapeutic intervention.
Laboratory studies document that areas of low flow velocity increase thrombin generation.47 These areas of low flow (e.g., 0.3 L/min) require significantly less tissue factor than nominal flow to trigger clot formation..48 Histological studies of thrombosis formation further confirm that fibrin clots preferentially form in low-shear zones such as tubing connections in ECMO circuits.49 Therefore, a low shear stress can induce the expression of tissue factor and combined with increased PS this can promote clot formation. Shibeko et al.50 documented that above a certain threshold flow rate, this TF-driven process is inhibited through flow removal of activated factor Xa. This would explain the slow or absent rate of clot formation at the nominal-flow rate (e.g., 0.5 L/min). High-shear (e.g., 0.7 L/min) thrombosis occlusion is characterized by rapid accumulation of elongated vWF, adherence of platelets to vWF, release of vWF from activated platelets, and then exponential capture of platelets causing an occlusion.51 Surprisingly, samples from high-flow-rate circuits did not significantly change their total von Willebrand Factor antigen, expression of GP1B, or platelet count compared to control (data not shown). However, samples from the low-flow and high-flow circuits did increase the concentration of activated monocytes after 1 h, generated TF-positive monocytes after 2 h, and generated LEV expressing TF after 4 h. This time course suggests a rapid and possible positive feedback increase in TF expression leading to low-flow and high-flow circuit occlusion. Moreover, the concomitant generation of TNF-α may have amplified the thrombin generation even further. This large increase in TF overwhelms conventional heparin anticoagulation or TFPI at a concentration that is nearly 50 times the concentration in healthy plasma from adults. Thus, while PEVs appear to provide the “fuel” to fire thrombosis, the “spark” appears to be TF expressed on LEV and WBCs following exposure to shear stress. Recent studies documented that tissue factor activity can be suppressed with microRNAs,52 which suggests increasing TF expression is regulated by posttranscriptional modulation. As other studies support miRNAs’ ability to modulate blood coagulation, our clinically relevant model of clot formation may provide a testbed for future drug studies. Future studies are planned to confirm and extend that TF expressing leukocytes and EVs have a role in initiating thrombus in ECMO.
Our paper has several limitations, including missing values from circuits that occluded before 6 h and a low number of replicates. The simulated ECMO circuit differed from clinical practice by priming with whole blood, to prevent dilution and change in acid-base balance, instead of priming the circuit with a mixture of component blood products, albumin, calcium, crystalloid, and sodium bicarbonate. The circuits contained a polypropylene hollow-fiber oxygenator instead of a polymethylpentene hollow-fiber oxygenator, which is more common in neonatal ECMO. However, the benefits of decreased plasma leakage with polymethylpentene are not relevant during the short 6 h of circulation. Despite increased utilization of centrifugal pumps within ECMO circuits in the United States, the tested circuits contained a roller pump because that remains the most commonly used pump for neonates.53
Our high-fidelity neonatal model of simulated ECMO is the first to demonstrate circuit occlusion as observed in clinical practice. Further understanding and improvement of the simulated ECMO model will create a testbed for anticoagulation drug discovery. Our in vitro findings suggest that operating ECMO at the low-flow-rate and high-flow-rate limits, defined by the manufacturer, may increase the risk of occlusive thrombosis. Future clinical and in vitro studies are needed to define whether the blockade of tissue factor and phosphatidylserine-positive cells and EVs will decrease ECMO-induced thrombus formation and circuit occlusion.
Supplementary Material
Essentials.
Thrombotic complications are common during extracorporeal membrane oxygenation (ECMO).
Simulated ECMO models may establish biological mechanisms that promote thrombotic complications.
Increasing blood flow amplifies phospholipids on platelets and their extracellular vesicles.
Low and high flow rates increase tissue factor on leukocytes and their extracellular vesicles.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ron Bryant, Alia Elkhalili, Neslihan Cingoz, Linda McManus, PhD; Armando Rodriquez; Joshua Walker, CCP; and Kerfoot P. Walker III, who were essential in protocol development, ECMO circuit design, assay and data analysis, manuscript review, and general troubleshooting. The authors would also like to thank Robert William Travis and Xiao Wu MD, for obtaining representative fluorescent images of the oxygenator fibers.
Funding information
The NIH/National Heart Lung Blood Institute supported this project with Grant K23 HL124336. The Morrison Trust with the Blood and Coagulation Task Area, U.S. Army Institute of Surgical Research also supplied funding and support. STAGO supplied experimental reagents.
Footnotes
CONFLICT OF INTEREST
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. There is an absence of any real or potential conflicts of interest for all the authors.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol. 2005;37:343–350. [PMC free article] [PubMed] [Google Scholar]
- 2.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood B, Newton D, Pallotto EK. Current trends in neonatal ECMO. Semin Perinatol. 2018;42:80–88. [DOI] [PubMed] [Google Scholar]
- 4.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med. 2015;16:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13(5):385–392. [DOI] [PubMed] [Google Scholar]
- 6.Lubnow M, Philipp A, Dornia C, et al. D-dimers as an early marker for oxygenator exchange in extracorporeal membrane oxygenation. J Crit Care 2014;29(473):e1–5. [DOI] [PubMed] [Google Scholar]
- 7.Sheriff J, Soares JS, Xenos M, Jesty J, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kappelmayer J, Bernabei A, Edmunds LH Jr, Edgington TS, Colman RW. Tissue factor is expressed on monocytes during simulated extracorporeal circulation. Circ Res. 1993;72(5):1075–1081. [DOI] [PubMed] [Google Scholar]
- 9.Gu YJ, Boonstra PW, Graaff R, Rijnsburger AA, Mungroop H, van Oeveren W. Pressure drop, shear stress, and activation of leukocytes during cardiopulmonary bypass: a comparison between hollow fiber and flat sheet membrane oxygenators. Artif Organs 2000;24:43–48. [DOI] [PubMed] [Google Scholar]
- 10.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–441. [DOI] [PubMed] [Google Scholar]
- 11.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. [DOI] [PubMed] [Google Scholar]
- 12.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–138. [PubMed] [Google Scholar]
- 14.Meyer AD, Wiles AA, Rivera O, et al. Hemolytic and thrombocy-topathic characteristics of extracorporeal membrane oxygenation systems at simulated flow rate for neonates. Pediatr Crit Care Med. 2012;13:e255–e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer AD, Gelfond JA, Wiles AA, Freishtat RJ, Rais-Bahrami K. Platelet-derived microparticles generated by neonatal extracorporeal membrane oxygenation systems. ASAIO J. 2014;61:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dornia C, Philipp A, Bauer S, et al. Visualization of thrombotic deposits in extracorporeal membrane oxygenation devices using multidetector computed tomography: a feasibility study. ASAIO J 2013;59:439–441. [DOI] [PubMed] [Google Scholar]
- 17.Beely BM, Campbell JE, Meyer A, et al. Electron microscopy as a tool for assessment of anticoagulation strategies during extracorporeal life support: the proof is on the membrane. ASAIO J. 2016;62:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilm J, Philipp A, Muller T, et al. Leukocyte adhesion as an indicator of oxygenator thrombosis during extracorporeal membrane oxygenation therapy? ASAIO J. 2018;64:24–30. [DOI] [PubMed] [Google Scholar]
- 19.Lehle K, Philipp A, Gleich O, et al. Efficiency in extracorporeal membrane oxygenation-cellular deposits on polymethylpentene membranes increase resistance to blood flow and reduce gas exchange capacity. ASAIO J. 2008;54:612–617. [DOI] [PubMed] [Google Scholar]
- 20.Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol. 1995;27:137–145. [PubMed] [Google Scholar]
- 21.Wendel HP, Philipp A, Weber N, Birnbaum DE, Ziemer G. Oxygenator thrombosis: worst case after development of an abnormal pressure gradient-incidence and pathway. Perfusion. 2001;16:271–278. [DOI] [PubMed] [Google Scholar]
- 22.Lawson DS, Ing R, Cheifetz IM, et al. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr Crit Care Med. 2005;6:573–577. [DOI] [PubMed] [Google Scholar]
- 23.Tamari Y, Lee-Sensiba K, Leonard EF, Tortolani AJ. A dynamic method for setting roller pumps nonocclusively reduces hemolysis and predicts retrograde flow. ASAIO J. 1997;43:39–52. [PubMed] [Google Scholar]
- 24.Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–1267. [DOI] [PubMed] [Google Scholar]
- 25.Hristov M, Schmitz S, Schuhmann C, et al. An optimized flow cytometry protocol for analysis of angiogenic monocytes and endothelial progenitor cells in peripheral blood. Cytometry A. 2009;75:848–853. [DOI] [PubMed] [Google Scholar]
- 26.Trummer A, De Rop C, Tiede A, Ganser A, Eisert R. Isotype controls in phenotyping and quantification of microparticles: a major source of error and how to evade it. Thromb Res. 2008;122:691–700. [DOI] [PubMed] [Google Scholar]
- 27.Hisada Y, Mackman N. Measurement of tissue factor activity in extracellular vesicles from human plasma samples. Res Pract Thromb Haemost. 2019;3:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium E-T, Van Deun J, Mestdagh P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. [DOI] [PubMed] [Google Scholar]
- 29.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2011;105:396–408. [DOI] [PubMed] [Google Scholar]
- 30.Pidcoke HF, McFaul SJ, Ramasubramanian AK, et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53(Suppl 1):137S–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou J, Fu Y, Zhou J, et al. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 2011;100:187–195. [DOI] [PubMed] [Google Scholar]
- 32.Dahm A, Van Hylckama VA, Bendz B, Rosendaal F, Bertina RM, Sandset PM. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood. 2003;101:4387–4392. [DOI] [PubMed] [Google Scholar]
- 33.Ollivier V, Wang J, Manly D et al. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res.2010;125(1):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irby K, Swearingen C, Byrnes J, Bryant J, Prodhan P, Fiser R. Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: a retrospective pediatric study. Pediatr Crit Care Med. 2014;15:e175–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–3464. [PubMed] [Google Scholar]
- 36.Kondo N, Wakayama F, Suzuki Y, Fukui K, Takaya S, Fukuda I. The state of platelets preserved in extracorporeal circulation with a glycoprotein IIb/IIIa inhibitor. Thromb Res. 2004;113(5):303–310. [DOI] [PubMed] [Google Scholar]
- 37.Diehl P, Aleker M, Helbing T, et al. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg. 2010;11(2):133–137. [DOI] [PubMed] [Google Scholar]
- 38.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- 39.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709–725. [DOI] [PubMed] [Google Scholar]
- 40.Cook KE, Maxhimer J, Leonard DJ, Mavroudis C, Backer CL, Mockros LF. Platelet and leukocyte activation and design consequences for thoracic artificial lungs. ASAIO J 2002;48:620–630. [DOI] [PubMed] [Google Scholar]
- 41.Tripisciano C, Weiss R, Eichhorn T, et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7:6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geddings JE, Mackman N. New players in haemostasis and thrombosis. Thromb Haemost. 2014;111:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin MC, Almus-Jacobs F, Chen HH, et al. Shear stress induction of the tissue factor gene. J Clin Investig. 1997;99:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackman N The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009;108:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsson M, Rayzman V, Nolte MW, et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci Transl Med. 2014;6:222ra17. [DOI] [PubMed] [Google Scholar]
- 46.Naldini A, Sower L, Bocci V, Meyers B, Carney DH. Thrombin receptor expression and responsiveness of human monocytic cells to thrombin is linked to interferon-induced cellular differentiation. J Cell Physiol. 1998;177:76–84. [DOI] [PubMed] [Google Scholar]
- 47.Funakubo A, Taga I, McGillicuddy JW, Fukui Y, Hirschl RB, Bartlett RH. Flow vectorial analysis in an artificial implantable lung. ASAIO J. 2003;49:383–387. [PubMed] [Google Scholar]
- 48.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111:3507–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastings SM, Ku DN, Wagoner S, Maher KO, Deshpande S. Sources of circuit thrombosis in pediatric extracorporeal membrane oxygenation. ASAIO J 2017;63:86–92. [DOI] [PubMed] [Google Scholar]
- 50.Shibeko AM, Lobanova ES, Panteleev MA, Ataullakhanov FI. Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa. BMC Syst Biol. 2010;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casa LDC, Ku DN. Thrombus formation at high shear rates. Annu Rev Biomed Eng. 2017;19:415–433. [DOI] [PubMed] [Google Scholar]
- 52.Eisenreich A, Leppert U. The impact of microRNAs on the regulation of tissue factor biology. Trends Cardiovasc Med. 2014;24:128–132. [DOI] [PubMed] [Google Scholar]
- 53.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal life support organization registry report 2012. ASAIO J 2013;59:202–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.