Abstract
The pro-apoptotic Bax isoform BaxΔ2 was originally discovered in cancer patients with a microsatellite guanine deletion (G8 to G7). This deletion leads to an early stop codon; however, when combined with the alternative splicing of exon 2, the reading frame is restored allowing production of a full-length protein (BaxΔ2). Unlike the parental Baxα, BaxΔ2 triggers apoptosis through a non-mitochondrial pathway and the expression in human tissues was unknown. Here, we analyzed over 1000 tissue microarray samples from 13 different organs using immunohistochemistry. BaxΔ2-positive cells were detected in all examined organs at low rates (1–5%) and mainly scattered throughout the connective tissues. Surprisingly, over 70% of normal colon samples scored high for BaxΔ2-positive staining. Only 7% of malignant colon samples scored high, with most high-grade tumors being negative. A similar pattern was observed in most organs examined. We also showed that both Baxα and BaxΔ2 can co-exist in the same cells. Genotyping showed that the majority of BaxΔ2-positive normal tissues contain no G7 mutation, but an unexpected high rate of G9 was observed. Although the underlying mechanism remains to be explored, the inverse correlation of BaxΔ2 expression with tissue malignancy suggests that it may have a clinical implication in cancer development and treatment.
Keywords: Bax isoform, BaxΔ2, Apoptosis, Microsatellite mutation, Cancer
Introduction
BaxΔ2 is a pro-apoptotic Bax family member, first discovered in colorectal tumors with microsatellite instability (MSI) (Haferkamp et al. 2012; Zhang et al. 2015). MSI is detected in ~ 15% of all colorectal cancers and in ~ 90% of hereditary nonpolyposis colorectal cancers (HNPCC). Patients with high-MSI have a higher risk for not only colon cancer, but also other types of cancer, such as endometrial, stomach and ovarian cancer at young age (Aarnio et al. 1999; Duval and Hamelin 2002; Peltomäki 2003). The microsatellite region of the Bax gene is located in exon 3 and contains a repeat of 8 guanines (G8). The most common mutation in the Bax gene is a mononucleotide deletion, from G8 to G7. This mutation has been widely reported in many MSI tumors, with frequencies as high as 45–50%, especially in colorectal cancer (Akira and Tohoku 1998; Yagi et al. 1998; Kawaguchi et al. 2009).
When there is a G8 to G7 mutation in the Bax microsatellite, protein translation is out of frame and results in a short, non-functional truncated protein (Haferkamp et al. 2012). Tumors with such mutation are usually considered as “Bax negative” phenotype. Interestingly, alternative splicing can rescue this situation. Alternative splicing events are very common in the Bax family, giving rise to many functional isoforms (Shi et al. 1999; Schmitt et al. 2000; Jin et al. 2001; Cartron et al. 2002; Fu et al. 2009; Haferkamp et al. 2013). However, alternative splicing of exon 2 by itself leads to a reading frameshift and premature termination. Intriguingly, co-occurrence of both exon 2 alternative splicing and microsatellite G7 mutation can “resolve” the out-of-frame problem, generating a new full-length protein, BaxΔ2 (Haferkamp et al. 2012). This new isoform lacks exon 2 but contains all other Bax functional domains, including the critical BH3 killing domain (Haferkamp et al. 2012; Mañas et al. 2017). BaxΔ2 also has 10 new “frameshifted” amino acids at the N-terminus and the antibody against this region is specific for detection of BaxΔ2 and does not cross-react with Baxα (Haferkamp et al. 2012; Zhang et al. 2014; Mañas et al. 2017, 2018b).
Like parental Baxα, BaxΔ2 is pro-apoptotic and can form dimers with Baxα or Bcl-2 (Haferkamp et al. 2012). However, the pro-death potency of BaxΔ2 in cancer cells is significantly higher than that of Baxα. BaxΔ2-positive cancer cells are more sensitive to certain chemotherapeutics than either Baxα-positive or Bax-negative cells (Haferkamp et al. 2012; Zhang et al. 2014; Mañas et al. 2018a). Unlike Baxα, BaxΔ2 does not target mitochondria; instead it can form large cytosolic aggregates which serve as a platform to recruit caspase 8 through the C-terminal helical structure. Disruption of helix α9 by a single point mutation abolishes the aggregate-mediated activation of caspase 8 (Mañas et al. 2017). Intriguingly, endogenous BaxΔ2 is very unstable and susceptible to proteasomal degradation. Treatment of colon cancer cells with proteasome inhibitors, such as bortezomib and carfilzomib, promotes accumulation of BaxΔ2 and activation of caspase 8-mediated cell death (Mañas et al. 2018a).
Tissue distribution of Baxα is well documented. It is ubiquitously expressed in nearly all human tissues, both normal and malignant (Krajewski et al. 1994; Penault-Llorca et al. 1998; Navani 2011). We previously showed that, apart from colon tumors, BaxΔ2 expression could also be detected in the buccal epithelial cells of healthy individuals with a family history of microsatellite instability (Zhang et al. 2015). We also detected BaxΔ2-positive cells in cerebellar tissues from several healthy individuals. In addition to the G7 mutation, surprisingly, we detected a G9 mutation in one of the BaxΔ2-positive cerebellar samples (Mañas et al. 2018b). In an effort to determine the distribution of BaxΔ2 in human tissues, we screened over 1000 tissue samples from 13 different organs using immunohistochemistry. We analyzed the nature of BaxΔ2-positive cells and the correlation between expression and tissue malignancy. Next, we genotyped BaxΔ2-positive tissues and individual cells, and showed that microsatellite mutations are not necessary for BaxΔ2 expression. Finally, we discussed several alternative mechanisms that could lead to production of BaxΔ2, and the significance of BaxΔ2 expression in cancer development and treatment.
Materials and methods
Tissue slides
Human tissue microarray slides and other formalin-fixed paraffin-embedded (FFPE) tissue slides were obtained from US Biomax. All tissue samples were collected less than 6 h postmortem, de-identified and met the Institutional Review Board (IRB) requirements at Illinois Institute of Technology. The number of samples mentioned in the text does not represent the exact number of patients, some samples were different sections of the same patient and normal adjacent tissue (NAT) samples were usually paired with tumors samples from the same patient. The detail list of slides used is provided in the supplementary data (Suppl Table S1).
Immunohistochemistry and tissue analysis
Tissue slides were immunohistochemically stained as previously described (Mañas et al. 2018b). Monoclonal antibody against BaxΔ2 (2D4, 1:200 dilution) was previously generated and validated in both cell culture and tissue staining (Haferkamp et al. 2012; Mañas et al. 2018b, a). Antibody anti-Baxα N-20 (1:100 dilution) was purchased from Santa Cruz Biotechnology. For antibody neutralization validation assay, the BaxΔ2 antibody was incubated with the peptide containing antigen (the underlined amino acids are the epitope peptide, QPRGGGFHGSSRANGGEAP, 1:100 ratio) overnight at 4 °C before application to tissue slides. For the control, equivalent Baxα peptide (ALLLQGFIQDRA-GRMGGEAP) was used. For staining, duplicated samples on the same slide were treated with or without neutralized antibody in separated incubation chambers at the same time. After DAB (3,3′-diaminobenzidine) immunohistochemical staining (ImmPACT DAB from Vector Laboratories), the slides were scanned using a CRi Pannoramic Scan Whole Slide Scanner with a 40 × NA 0.95 LWD Zeiss objective, on high resolution (0.12 μm/pixel), at the Integrated Light Microscopy Core Facility at the University of Chicago, and visualized using Pannoramic Viewer 1.15.2. Staining was analyzed using visual analysis and software image analysis. For visual analysis, all the available samples were examined by three independent viewers and samples with debated evaluation were analyzed by a fourth individual. BaxΔ2 staining was scored according to 4 categories: “0” negative staining, “1+” weak positive staining, “2+” intermediate positive staining, and “3+” strong positive staining. For software image analysis, a selection of samples was processed using CellProfiler 2.2.0 (Carpenter et al. 2006), which can quantitate the total number of nuclei and the number of positive cells per sample. At least 80% of the surface of each sample was analyzed. Certain samples had to be excluded of the CellProfiler analysis to avoid its limitations, like excluding false positives (such as blood cells).
Immunoblotting
HCT116 colon cancer cells were transfected with either Baxα or BaxΔ2 constructs for 24 h. Equal amount of protein lysates from each sample was separated by a 12% SDS-PAGE gel and transferred onto a 0.2 mm PVDF membrane (BIO-RAD). Membranes were blocked with 5% BSA, followed by incubation with primary antibody against BaxΔ2 (1:200 dilution), Baxα (N20, 1:200) or actin (1:3000) overnight at 4 °C. For antigen peptide blocking, the BaxΔ2 antibody was incubated with the antigen peptide (QPRGGGFHGSSRANGGEAP, 1:100 ratio) overnight at 4 °C before incubation with the membranes for additional 16 h. The membranes were then incubated with the corresponding HRP-conjugated secondary antibodies for 1 h. The protein bands were visualized using X-ray films with Pierce®ECL Western Blotting Substrate developing kit (ThermoScientific).
Microdissection and genotyping
Stained slides were uncovered using xylene and rehydrated with graded ethanol solutions. Whole cores were collected under a microscope using a sterile needle. Individual BaxΔ2-positive cells were collected using a Zeiss PALM microdissection system at the Northwestern University Center for Advanced Microscopy. DNA was extracted using Arcturus PicoPure DNA Extraction Kit (ThermoFisher), purified with AMPure XP magnetic beads (Beckman Coulter), and a section of approximately 150 bp of the Bax gene containing the microsatellite was amplified by PCR with forward primer (5′ GAGTGACACCCCGTTCTGAT 3′) and reverse primer (5′ ACTCGCTCAGCTTCTTGGTG 3′). Sequencing was performed at the DNA Sequencing & Genotyping Facility at the University of Chicago Comprehensive Cancer Center.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 7.03. The visual data was analyzed using the non-parametric Mann–Whitney test and Kruskal–Wallis test. The Bonferroni correction was applied when many organs were compared. For the software analysis, one-way ANOVA and t-test were performed. p < 0.05 was considered significant.
Results
Expression and distribution of BaxΔ2 in human colon tissues
BaxΔ2 was originally discovered in colon cancer patients with the Bax microsatellite G7 mutation (Haferkamp et al. 2012; Zhang et al. 2015). The function and behavior of BaxΔ2 have been well characterized in cultured cell systems (Haferkamp et al. 2012, 2013; Zhang et al. 2014; Mañas et al. 2017, 2018a). However, the expression and distribution of BaxΔ2 in human tissues has not been examined before. To do this, we screened a panel of colon tissue microarrays (TMA) using immunohistochemical (IHC) staining with a BaxΔ2-specific antibody (Haferkamp et al. 2012; Mañas et al. 2018b). BaxΔ2-positive cells clearly stood out above the background and were scattered or clustered in the connective tissues between crypts (Fig. 1a, a’’’, b, b’’’). The majority of columnar epithelial cells and smooth muscle cells in the muscularis mucosa and the muscularis externa were negative. BaxΔ2 appeared to be distributed in the cytosol of cells and the morphology of BaxΔ2-positive cells were varied (Fig. 1a’, a’’). To confirm the specificity of BaxΔ2 positive staining, we further validated the BaxΔ2 antibody by peptide blocking. As we observed previously, BaxΔ2 antibody did not cross with Baxα (Fig. 1c) (Haferkamp et al. 2012; Zhang et al. 2014), and was blocked well by the BaxΔ2 antigen peptide (Fig. 1d). For IHC staining, we used cerebellar tissue as a positive control, in which the antibody neutralization was complete in Purkinje cells (Fig. 1d’), as we observed previously (Mañas et al. 2018b). For the colon tissue, the antibody was partially blocked with BaxΔ2 peptide, both the intensity of positive staining and number of positive cells were decreased (Fig. 1f, f’). The quantitative analysis from CellProfiler software showed that there is a significant decrease (58%) of positive staining in the presence of antigen peptide (Fig. 1f’’). These results indicate that detection of BaxΔ2 is specific, and distribution of BaxΔ2-positive cells is not ubiquitous and might involve selective cells.
Fig. 1.

Expression of BaxΔ2 proteins in human colorectal tissues and antibody validation. a, b Human colorectal tissue microarray slides stained with anti-BaxΔ2 antibody using standard DAB. Images from two individuals (both normal tissues) are shown. The boxed areas are enlarged (a’–a’’’ and b’–b’’’). Arrows indicate some BaxΔ2-positive stained cells. Scale bar, 200 μm. c Immunoblotting of HCT116 colon cancer cells, transfected with either Baxα or BaxΔ2, using anti-Baxα (N-20) and anti-BaxΔ2 (2D4) antibodies. d Antibody blocking analysis by immunoblotting of transfected cells similar to c in the absence and presence of BaxΔ2 antigen peptide. e Immunohistochemical (IHC) staining of human cerebellar tissues with anti-BaxΔ2 antibody in the presence of Baxα (control, e) or BaxΔ2 (e’) antigen peptide. Scale bars, 100 μm. f IHC staining of human colorectal tissues with anti-BaxΔ2 antibody in the presence of Baxα (control, f) or BaxΔ2 (f’) antigen peptide. Scale bars, 100 μm. f’’ Quantitation of IHC staining (f, f’) on the whole slides divided into 10 sections using CellProfiler software and analyzed by non-parametric statistical analysis, ***p < 0.001
Expression of BaxΔ2 inversely correlates with malignancy in colon tissues
As we showed above, BaxΔ2-positive cells appeared scattered in the connective tissues and the overall population seemed low. To obtain a quantitative measurement, we used two methods: visual scoring and computational image analysis (see methods). The visual score system was adopted from the commonly used histological H-score system (Hirsch et al. 2003; Pirker et al. 2012), scaling from 0 to 3+. The typical appearance of these scores is shown in Fig. 2: “0” (Fig. 2a, no positive cells), “1+” (Fig. 2b 1–4 positive cells per field), “2+” (Fig. 2c 4–20 positive cells per field), and “3+” (Fig. 2d, e, more than 20 positive cells per field). Since the visual scores only gave us an overall semi-quantitative estimation, we subjected the samples to software analysis (CellProfiler). We selected a set of 136 samples to process with CellProfiler and the results were compared with the scores from the visual method (Fig. 2f). Tissues with 3+ score had an average of ~ 4% positive cells, tissues with 2+ score had an average of 2% positive cells, and tissues with score 1+ had around 0.8% positive cells. These results indicate that the visual and software analyses are consistent with each other.
Fig. 2.
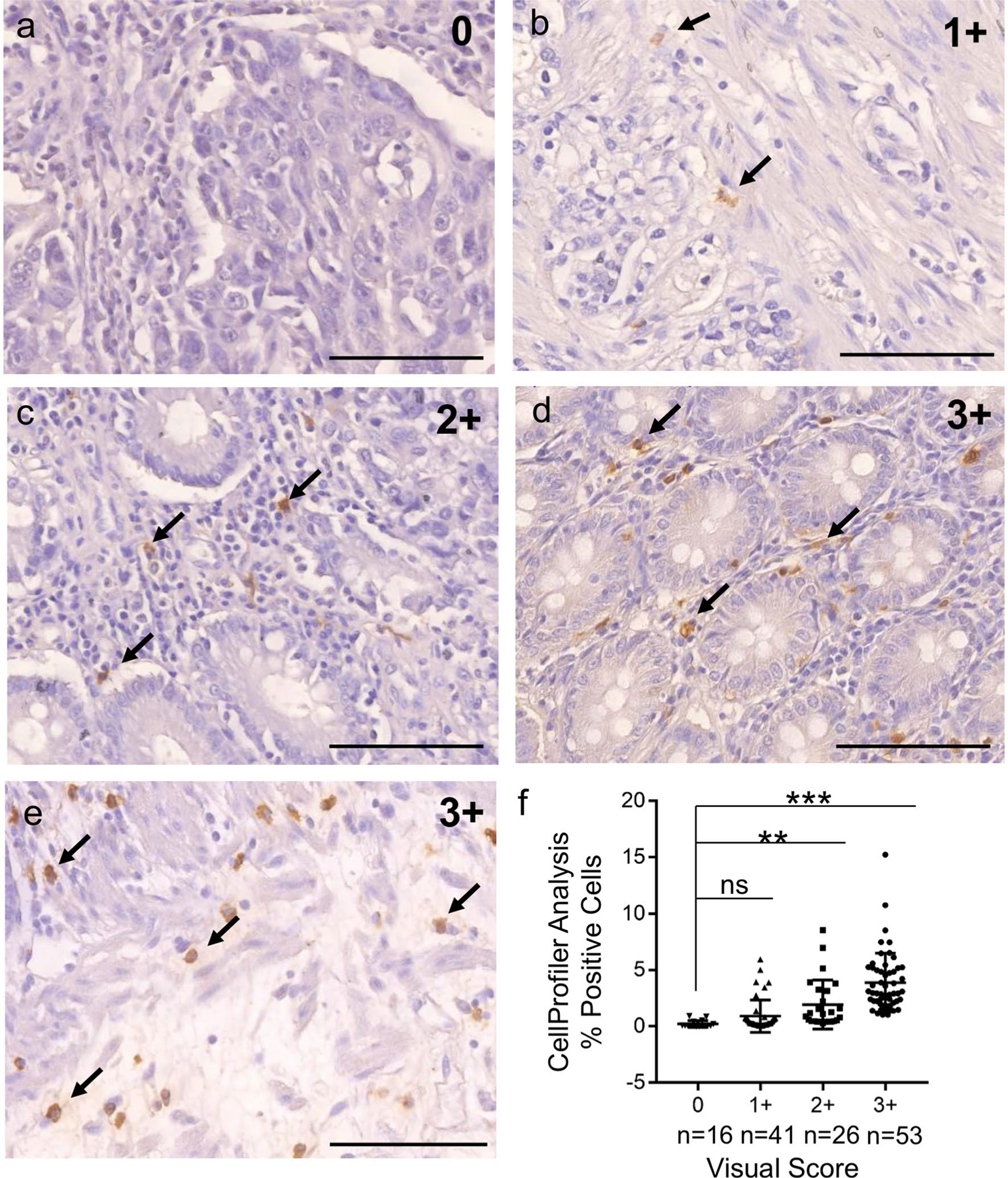
Validation of visual and computational scoring systems for quantification of BaxΔ2 expression. a–e Human colorectal tissue samples were immunohistochemically stained with anti-BaxΔ2 antibody. BaxΔ2-positive stained cells were visualized and scored by three independent viewers. Typical images from score “0” to “3+ ” are shown (a: “0”, b: 1+, c: 2+, d and e: 3+). Arrows indicate some BaxΔ2-positive cells. Scale bar, 100 μm. f Scores from visual analysis were compared with those obtained from software (CellProfiler) analysis. The results were plotted with GraphPad Prism 7. Ns not statistically significant; **p < 0.01, ***p < 0.001. Statistical significance was calculated with two-tailed Student’s t-test
Next, we screened 532 colon TMA samples, which included 167 normal/NAT samples, 20 benign samples (including benign tumors and inflammatory conditions), and 345 malignant tumor samples. All tissues were immunohistochemically stained with anti-BaxΔ2 antibody and the images were subjected to both visual scoring and CellProfiler analyses. To our surprise, we found that over 90% of normal/NAT colon samples and benign samples were stained BaxΔ2-positive, and over 70% were scored 3+ (Fig. 3a). There was no significant difference between normal/NAT and benign tissues. Both visual and computational analyses showed that there was a significantly lower level of BaxΔ2-positive cells in malignant tissues compared to normal/NAT and benign tissues, and only 7% of malignant samples scored 3+ (Fig. 3a, b). Consistently, normal and benign tissues had a significantly higher number of BaxΔ2-positive cells (average 4.4%) than malignant tumors (average 0.8%) (Fig. 3b). To determine whether there was a correlation between the expression of BaxΔ2 and tumor grade, we analyzed a panel of colon tumor samples organized by grade (Fig. 3c, d). We found that there was an inverse correlation between BaxΔ2 expression and tissue malignant status: more BaxΔ2-positive cells were in lower grade and fewer in higher grade tumors, with statistical significance between grade I and grades II/III, but no differences between grades II and III (Fig. 3c, d). Both methods showed that there was no significant difference when the samples were analyzed by age and sex (Fig. 3e, h). These results suggest that the expression of BaxΔ2 proteins in human tissues is very low, but correlates with the tissue differentiation status: more BaxΔ2-positive cells in well-differentiated colon tissues and less in poorly differentiated or high-grade colon tumors.
Fig. 3.

Expression of BaxΔ2 is inversely correlated with tissue malignancy but independent of the age and sex of the patients. a, b Human colorectal tissues were subjected to immunohistochemical staining with anti-BaxΔ2 antibody and scored by both visualization (a) and CellProfiler software (b). First, the tissues were divided into three groups: normal or normal adjacent tissue (NAT), benign tumors and inflammatory conditions, and malignant tumors. The tumors in the malignant group in a were further classified into the three grade levels (when possible) and subjected to both visual (c) and Cellprofiler (d) analysis. e, h Comparison of BaxΔ2 expression based on sex (e, f) and age (g, h) by visual scoring (e, g) and computer software (f, h). n sample sizes for each group. ns not statistically significant and the p value is indicated on each graph. *p < 0.05, ***p < 0.001. Visual data waere analyzed by non-parametric Mann–Whitney test and Cellprofiler data by two-tailed Student’s t-test to calculate statistical significance
BaxΔ2 expression correlates with tissue differentiation status across different human organs
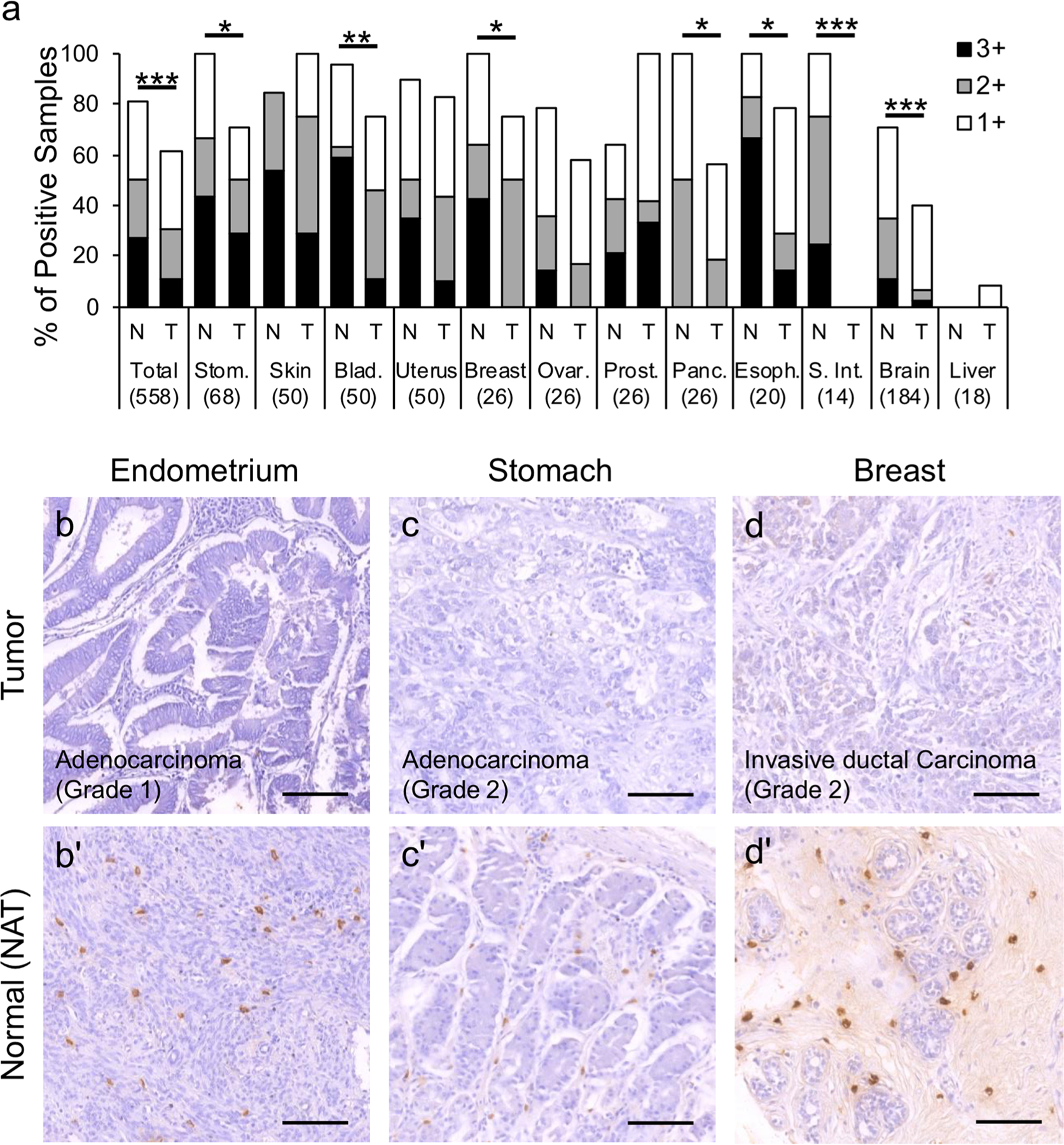
As BaxΔ2 was originally identified in colon tumors with a microsatellite mutation, we did not expect to find such a high number of BaxΔ2-positive samples, especially in normal tissues. Intrigued by this, we examined other organs to see whether this phenomenon was consistent in other organs. We immunostained 558 normal, normal adjacent (NAT), and malignant tumor samples covering 12 additional organs (Fig. 4). The samples were semi-quantitatively analyzed by visual scoring. We found BaxΔ2-positive cells in all organs examined, although expression levels varied among organs. Some organs showed strong positive staining (3+), like uterus (Fig. 4a) and breast (Fig. 4c), while others mainly showed very low levels of staining (1+ or 0), like liver (Fig. 4l). The distribution of BaxΔ2-positive cells in most organs was similar to that observed in colon, with individual positive cells scattered in connective tissues. Some exceptions were skin, in which many positive cells were found in the basal layer of epidermis (Fig. 4i), and brain, in which a low level of positive staining was evenly distributed in neurons, with glial cells generally being negative (Fig. 4d). Out of the 12 organs, 9 showed higher expression of BaxΔ2 in normal/NAT tissues than in malignant tumor tissues, and 7 organs showed statistically significant differences. Overall, ~ 80% of all these normal/NAT samples have BaxΔ2-positive staining, consistent with the results observed in colon tissues. Further analysis of matched tumors and NAT samples from the same patients showed that there were clearly fewer BaxΔ2-positive cells in the tumor tissue (Fig. 5b–d) than in the normal adjacent regions (Fig. 5b’–d’) within the same individuals. These results confirm that BaxΔ2 detection is consistently low throughout human tissues, but its expression depends on tissue differentiation and malignancy.
Fig. 4.

BaxΔ2-positive cells can be detected across human organs. Immunohistochemical staining of tissues from different human organs (indicated on the images) with anti-BaxΔ2 antibody. Both original image (boxed) and enlarged areas are shown. All images shown are from normal/NAT samples, except liver which belongs to a grade I tumor. The arrows indicate different BaxΔ2-positive cells. Scale bars, 50 μm
Fig. 5.
The expression of BaxΔ2 correlates with the tissue differentiation status cross different human organs. a Non-parametric statistical analysis of BaxΔ2 expression using the visual score system in 12 different organs. N normal or normal adjacent, T malignant tumors. The number below indicates the sample size. Stom stomach, Ovar ovary, Prost prostate, Panc pancreas, Esoph esophagus, S. Int small intestine. *p < 0.05, **p < 0.01, ***p < 0.001. b, b’ to d, d’ Comparison of BaxΔ2 expression in matched tumor and adjacent normal tissue (NAT) samples from the same patients. Matched samples were located on the same slide and processed at the same time under the same conditions. Scale bar, 100 μm
Most BaxΔ2-positive normal cells co-express Baxα but lack the G7 mutation
The analysis of tissues from 13 organs confirmed that healthy tissues have more BaxΔ2-positive cells than malignant tumors. These results are unexpected, as the expression of BaxΔ2 requires a guanine deletion (G7) in the exon 3 microsatellite in order to restore the shifted reading frame caused by the alternative splicing of exon 2 (Haferkamp et al. 2012). Microsatellite mutations are fairly common in tumors (Akira and Tohoku 1998; Yagi et al. 1998; Grady et al. 1999; Mutter et al. 1999; Yamamoto et al. 2002; Peltomäki 2003; Kawaguchi et al. 2009). However, such mutations are usually not reported in healthy tissues, and are not expected to occur at a high level. In addition, we found that BaxΔ2 can co-exist with Baxα in the same cells, as seen in colon (Fig. 6a, a’’’) and stomach (Fig. 6b, b’’’). The antibodes used against Baxα and BaxΔ2 are highly specific against the corresponding isoform and have shown no cross-reaction (Haferkamp et al. 2012; Mañas et al. 2018b). Consistent with previous reports, the monomeric form of Baxα was found in all cells, ubiquitously distributed throughout the tissues (Fig. 6a’, b’’) (Krajewski et al. 1994; Penault-Llorca et al. 1998; Navani 2011), while BaxΔ2-positive cells were scattered in the connective tissue (Fig. 6a’, b’). This result suggests that BaxΔ2 can be co-expressed with Baxα in the same cells.
Fig. 6.

BaxΔ2 and Baxα co-exist in the same cells and the majority of BaxΔ2-positive tissues have no G7 microsatellite mutation. Co-immunostaining of colon (a–a’’’) and stomach (b–b’’’) tissues with anti-BaxΔ2 (a’–b’, green) and anti-Baxα (a’’–b’’, red) antibodies. Nuclei were stained with DAPI (a’’’–b’’’, blue). The enlarged regions in the boxed areas are shown in the left three panels. Scale bars, 200 μm (a), 100 μm (b). c Genotypes of Bax microsatellite in correlation with BaxΔ2 expression in normal and cancer tissues. G, guanine; G7/G8 or G8/G9 indicates detection of both G7 and G8, or G8 and G9 in the same sample. d Percentage distribution of microsatellite genotypes in normal/normal adjacent (NAT) and tumor tissues in (c). e, f Genomic sequence results and chromatograms of the isolated BaxΔ2-positive cells from two tissue cores (F6 and H6) and whole core sequencing (H6), along with basic sample information. LCM stands for laser capture microdissection
To validate the microsatellite genotype of the BaxΔ2-positive samples, we isolated genomic DNA from over 100 TMA individual samples with various degrees of BaxΔ2-positive staining. The Bax microsatellite region was amplified by PCR and sequenced. Due to the limited sample size and inconsistent quality of the tissues, as well as the high GC-content of the Bax gene, the sequencing success rate was lower than 40%. Figure 6c, d shows the sequencing results obtained from 20 normal/NAT and 20 malignant samples. About 15% (3 out of 20) malignant tumor samples had a G7 mutation, which is consistent with previous reports. Only around 5% (1 out of 20) of the normal/NAT samples had a G7 mutation. Surprisingly, both tumor and normal/NAT tissues have a high rate of G9 mutations, 45% and 25%, respectively (Fig. 6c, d). The G9 mutation has been reported before (Yagi et al. 1998; Mañas et al. 2018b), but has not been extensively studied. Importantly, 19 out of 20 normal/NAT samples (95%) contain no G7 mutation, and 11 of them had strong (3+) BaxΔ2-positive staining (Fig. 6c). These results raise the possibility that BaxΔ2 might be expressed in the absence of the G7 mutation.
It is also possible that BaxΔ2-positive cells contain a heterozygous G7/G8 mutation, but it was undetected during sequencing due to the low population of BaxΔ2-positive cells in the tissues. To address this issue, we isolated individual BaxΔ2-positive cells using laser capture microdissection (LCM) microscopy (Fig. 6e, f). Colon tissues were stained using immunofluorescence and for each sample, 50–100 strong BaxΔ2-positive cells were laser captured. The genomic DNA was extracted and amplified by PCR for sequencing. From the total 12 samples attempted, we successfully obtained clear sequencing reads from two subjects: one NAT and one from low grade tumor (Fig. 6e, f). The positive cells from the NAT sample had a G8 microsatellite, and the positive cells from the grade I tumor had a G9 microsatellite. To confirm whether the G9 represented the whole sample or just the BaxΔ2-positive cells, we isolated DNA from the remaining whole core. The sequencing showed that the majority of the tissue had a G8 microsatellite, indicating that the sample had a mixed G8/G9 genotype, with the majority of cells having G8 genotype, and the BaxΔ2-positive cells G9 genotype (Fig. 6e, f, sample H6). The G9 microsatellite detected in BaxΔ2-positive cells is not surprising, as we have previously observed the same phenomenon in BaxΔ2-positive Purkinje cells in normal cerebellar samples (Mañas et al. 2018b). Although the underlying mechanism remains to be explored, these results evidenced that protein expression of the Bax isoform BaxΔ2 is possible without having the G7 microsatellite mutation at the genetic level.
Discussion
The expression and distribution in human tissues of the pro-apoptotic protein Baxα has been well studied (Krajewski et al. 1994; Penault-Llorca et al. 1998; Navani 2011), but little is known about other Bax isoforms. Here we analyzed over 1000 tissue microarray samples covering 13 different organs to survey the distribution of BaxΔ2 in human tissues. We found that BaxΔ2-positive cells were scattered mainly in the connective tissues at a low rate (1–5% in most samples). To confirm the antibody specificity for immunohistochemical staining, we further validated BaxΔ2 antibody using antigen blocking. It appears that the antibody was blocked well by BaxΔ2 antigen peptide in immunoblotting of transfected colon cancer cells and in IHC of cerebellar tissues, but partially blocked in IHC of colon tissues. One explanation for the partial blocking might be the variation of the epitope conformation, since using a longer antigencontaining peptide improved the blocking efficiency versus the short antigen peptide (data not shown). We expect that the full-length BaxΔ2 recombinant protein may have a better conformational fit for antibody neutralization.
Strikingly, BaxΔ2-positive staining was observed in ~ 80% of well-differentiated tissues including normal, NAT, benign conditions and low-grade tumors, while significantly lower levels of BaxΔ2-positive cells were found in poorly differentiated malignant tissues (Figs. 3 and 5). Consistent with other studies, we found that around 15% of tumor samples had the G7 mutation, while only 5% (1 out of 20) of normal/NAT samples had it (Fig. 6c, d). Surprisingly, 11 out of 20 samples with a wild-type G8 microsatellite had strong (3+) BaxΔ2-positive staining. This brought up two possibilities: the first is that the Bax G7 mutation exists in healthy tissues but is undetectable due to low population; the second is that BaxΔ2 can be generated without the Bax G7 mutation. Our findings from laser capture microdissection experiments showed that BaxΔ2 can be present in cells with a wild-type G8 microsatellite (Fig. 6e, f). The underlying mechanism by which BaxΔ2 is produced in wild-type cells might be through an alternative pathway that mediates BaxΔ2 expression without a genetic mutation.
The genetic level is not the only point at which a sequence change can be introduced, as these can also happen during transcription or translation (Gesteland and Atkins 1996; Namy et al. 2004; Atkins et al. 2016). Sequence changes at RNA and protein levels are much more common than DNA mutations, but they are transient and disappear as the RNA or protein is degraded. Not being permanent, like mutations in DNA, these sequence changes in mammalians have not attracted much attention until recently, but have been widely studied in virus and bacteria (Baranov et al. 2005; Dinman 2006; Ratinier et al. 2008; Atkins et al. 2016). There are several mechanisms by which changes in sequence can happen, and two of the most significant ones are transcriptional slippage and ribosomal frameshift (Atkins et al. 2016). Transcriptional slippage occurs during DNA to RNA transcription and involves “skipping” or “repeating” of one or more base pairs, with poly-adenine regions being the most prone to transcriptional slippage (Linton et al. 1997). This process has been shown to be advantageous to viruses and bacteria, as it is used to expand protein variety from a small number of genes (Baranov et al. 2005; Ratinier et al. 2008). In humans, in most cases, it produces truncated proteins that can lead to disease (Vermulst et al. 2015), but it has also been reported to work as frame-restoring mechanism that can salvage mutated genes, such as for the apolipoprotein B gene (Linton et al. 1992).
Ribosomal frameshift is a mechanism that requires the ribosome to skip one or more nucleotides either forward (+1) or backwards (− 1) and continue reading the RNA at a different frame (Ketteler 2012; Atkins et al. 2016). This mechanism can happen by “mistake”, but it can also happen “on purpose” and has been well documented as a programmed event that is used as a source of alternative proteins by many organisms, including humans (Dinman 2006; Advani and Dinman 2016; Atkins et al. 2016). So far, five human genes have been shown to use programmed ribosomal frameshift (PRF): PEG10 (Shigemoto et al. 2001; Manktelow et al. 2005; Clark et al. 2007) and Ma3 (Wills et al. 2006), which require a − 1PRF event, and the three ornithine decarboxylase antizyme genes (Ivanov and Atkins 2007), which are not expressed unless a +1PRF event happens. The human antizyme genes have been widely studied and it is known that the +1PRF event is regulated by the specific amino acid sequence of the nascent peptide (Matsufuji et al. 1995; Ivanov and Atkins 2007; Yordanova et al. 2015).
At the DNA and RNA levels, the sequence of BaxΔ2 matches that of Baxα, since BaxΔ2 mRNA only lacks exon 2 and has no additions. However, at the protein level, BaxΔ2 contains 10 new amino acids due to a localized reading frameshift (Haferkamp et al. 2012). This 10-amino acid peptide not only serves as a unique antigen for generating the specific antibody against BaxΔ2 (Haferkamp et al. 2012; Mañas et al. 2018b), but might also play a role in regulating protein production at the translational level. We carefully analyzed this 10 amino acids, located upstream from the microsatellite, and found several amino acids that are highly prone to interact with the ribosomal exit tunnel (Elgamal et al. 2014; Starosta et al. 2014; Atkins et al. 2016). Therefore, it is possible that this unique 10-amino acid peptide contains a specific regulatory signal that triggers a ribosomal shift and restores the reading frame during translation. Further studies are needed to confirm this hypothesis.
Finally, the fact that BaxΔ2 is overwhelmingly detected in well-differentiated normal and benign tissues, but barely found in poorly differentiated malignant tumors across almost all cancer-prone organs, suggests that BaxΔ2 might serve as a possible prognostic indicator, along with other factors. Why most malignant tumors have no or low levels of BaxΔ2 still remains to be explored. Our previous studies show that endogenous BaxΔ2 is very susceptible to proteasomal degradation (Zhang et al. 2014; Mañas et al. 2018a), and treatment of cancer cells with proteasome inhibitors, such as Bortezomib, significantly blocks the degradation of BaxΔ2 and allows it to exert its cytotoxic function (Mañas et al. 2018a). This may explain why highly malignant cancer cells have lower levels of BaxΔ2; it might not be due to low expression but enhanced degradation of the protein. Further-more, BaxΔ2 induces cell death through a non-mitochondrial pathway and has the ability to sensitize cancer cells to specific chemotherapeutics, such as 5-FU and bortezomib (Zhang et al. 2014; Mañas et al. 2018a). All these results make BaxΔ2 not only distinct from other Bax family members, but also a unique pro-apoptotic factor with a potential clinical implication in cancer development and treatment.
Supplementary Material
Acknowledgements
We would like to thank Dr. John Hart from the University of Chicago (Department of Pathology) for his assistance regarding tumor pathology, and Dr. Li Ma from Mumetel LLC for providing his expertise for microsatellite genotyping. We also would like to thank our students Jodi Curtin, Irena Grauzinis and Ana Marija Fonceva, for their contribution. Imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
Funding This work was supported by the National Institutes of Health R15 CA195526.
Footnotes
Conflict of interest Dr. Xiang has a patent related to this project but has not received any financial compensation in association with the patent or this work. All authors declare no conflict of interests.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00418-020-01874-w) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aarnio M, Sankila R, Pukkala E et al. (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81:214–218 [DOI] [PubMed] [Google Scholar]
- Advani VM, Dinman JD (2016) Reprogramming the genetic code: the emerging role of ribosomal frameshifting in regulating cellular gene expression. BioEssays 38:21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira T, Tohoku M (1998) The Bax gene, the promoter of apoptosis, is mutated in genetically unstable cancers of the colorectum, stomach and endometrium. Clin Cancer Res 4:1071–1074 [PubMed] [Google Scholar]
- Atkins JF, Loughran G, Bhatt PR et al. (2016) Ribosomal frameshifting and transcriptional slippage: from genetic steganography and cryptography to adventitious use. Nucleic Acids Res 44:7007–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranov PV, Hammer AW, Zhou J et al. (2005) Transcriptional slippage in bacteria: distribution in sequenced genomes and utilization in IS element gene expression. Genome Biol 6:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AE, Jones TR, Lamprecht MR et al. (2006) Cell Profiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron P-F, Oliver L, Martin S et al. (2002) The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum Mol Genet 11:675–687 [DOI] [PubMed] [Google Scholar]
- Clark MB, Jänicke M, Gottesbühren U et al. (2007) Mammalian gene PEG10 expresses two reading frames by high efficiency-1 frameshifting in embryonic-associated tissues. J Biol Chem 282:37359–37369 [DOI] [PubMed] [Google Scholar]
- Dinman JD (2006) Programmed ribosomal frameshifting goes beyond viruses: organisms from all three kingdoms use frameshifting to regulate gene expression, perhaps signaling a paradigm shift. Microbe Wash DC: 1:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A, Hamelin R (2002) Mutations at coding repeat sequences in mismatch repair-deficient human cancers: toward a new concept of target genes for instability. Cancer Res 62:2447–2454 [PubMed] [Google Scholar]
- Elgamal S, Katz A, Hersch SJ et al. (2014) EF-P dependent pauses integrate proximal and distal signals during translation. PLoS Genet. 10.1371/journal.pgen.1004553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu NY, Sukumaran SK, Kerk SY, Yu VC (2009) Baxβ: a constitutively active human bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol Cell 33:15–29 [DOI] [PubMed] [Google Scholar]
- Gesteland RF, Atkins JF (1996) Recoding: dynamic reprogramming of translation. Annu Rev Biochem 65:741–768 [DOI] [PubMed] [Google Scholar]
- Grady WM, Myeroff LL, Swinler SE et al. (1999) Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 59:320–324 [PubMed] [Google Scholar]
- Haferkamp B, Zhang H, Kissinger S et al. (2013) BaxΔ2 family alternative splicing salvages Bax microsatellite-frameshift mutations. Genes and Cancer 4:501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferkamp B, Zhang H, Lin Y et al. (2012) BaxΔ2 is a novel bax isoform unique to microsatellite unstable tumors. J Biol Chem 287:34722–34729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA et al. (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21:3798–3807 [DOI] [PubMed] [Google Scholar]
- Ivanov IP, Atkins JF (2007) Ribosomal frameshifting in decoding antizyme mRNAs from yeast and protists to humans: close to 300 cases reveal remarkable diversity despite underlying conservation. Nucleic Acids Res 35:1842–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KL, Graham SH, Mao XO et al. (2001) Bax κ, a novel Bax splice variant from ischemic rat brain lacking an ART domain, promotes neuronal cell death. J Neurochem 77:1508–1519 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Banno K, Yanokura M et al. (2009) Analysis of candidate target genes for mononucleotide repeat mutation in microsatellite instability-high (MSI-H) endometrial cancer. Int J Oncol 35:977–982 [DOI] [PubMed] [Google Scholar]
- Ketteler R (2012) On programmed ribosomal frameshifting: the alternative proteomes. Front Genet 3:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Shabaik A et al. (1994) Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336 [PMC free article] [PubMed] [Google Scholar]
- Linton MF, Pierotti V, Young SG (1992) Reading-frame restoration with an apolipoprotein B gene frameshift mutation. Proc Natl Acad Sci USA 89:11431–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton MF, Raabe M, Pierotti V, Young SG (1997) Reading-frame restoration by transcriptional slippage at long stretches of adenine residues in mammalian cells. J Biol Chem 272:14127–14132 [DOI] [PubMed] [Google Scholar]
- Mañas A, Chen W, Nelson A et al. (2018a) BaxΔ2 sensitizes colorectal cancer cells to proteasome inhibitor-induced cell death. Biochem Biophys Res Commun 496:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañas A, Davis A, Lamerand S, Xiang J (2018b) Detection of pro-apoptotic BaxΔ2 proteins in the human cerebellum. Histochem Cell Biol 150:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañas A, Wang S, Nelson A et al. (2017) The functional domains for BaxΔ2 aggregate-mediated caspase 8-dependent cell death. Exp Cell Res 359:342–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manktelow E, Shigemoto K, Brierley I (2005) Characterization of the frameshift signal of Edr, a mammalian example of programmed-1 ribosomal frameshifting. Nucleic Acids Res 33:1553–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsufuji S, Matsufuji T, Miyazaki Y et al. (1995) Autoregulatory frameshifting in decoding mammalian ornithine decarboxylase antizyme. Cell 80:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter GL, Wada H, Faquin WC, Enomoto T (1999) K-ras mutations appear in the premalignant phase of both microsatellite stable and unstable endometrial carcinogenesis. J Clin Pathol 52:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namy O, Rousset JP, Napthine S, Brierley I (2004) Reprogrammed genetic decoding in cellular gene expression. Mol Cell 13:157–168 [DOI] [PubMed] [Google Scholar]
- Navani S (2011) The human protein atlas. J Obstet Gynecol India 61:27–31 [Google Scholar]
- Peltomäki P (2003) Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol 21:1174–1179 [DOI] [PubMed] [Google Scholar]
- Penault-Llorca F, Bouabdallah R, Devilard E et al. (1998) Analysis of BAX expression in human tissues using the anti-BAX, 4F11 monoclonal antibody on paraffin sections. Pathol Res Pract 194:457–464 [DOI] [PubMed] [Google Scholar]
- Pirker R, Pereira JR, Von Pawel J et al. (2012) EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 13:33–42 [DOI] [PubMed] [Google Scholar]
- Ratinier M, Boulant S, Combet C et al. (2008) Transcriptional slippage prompts recoding in alternate reading frames in the hepatitis C virus (HCV) core sequence from strain HCV-1. J Gen Virol 89:1569–1578 [DOI] [PubMed] [Google Scholar]
- Schmitt E, Paquet C, Beauchemin M et al. (2000) Characterization of Bax-σ, a cell death-inducing isoform of Bax. Biochem Biophys Res Commun 270:868–879 [DOI] [PubMed] [Google Scholar]
- Shi B, Triebe D, Kajiji S et al. (1999) Identification and characterization of Baxε, a novel Bax variant missing the BH2 and the transmembrane domains. Biochem Biophys Res Commun 254:779–785 [DOI] [PubMed] [Google Scholar]
- Shigemoto K, Brennan J, Walls E et al. (2001) Identification and characterisation of a developmentally regulated mammalian gene that utilises-1 programmed ribosomal frameshifting. Nucleic Acids Res 29:4079–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starosta AL, Lassak J, Peil L et al. (2014) Translational stalling at polyproline stretches is modulated by the sequence context upstream of the stall site. Nucleic Acids Res 42:10711–10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermulst M, Denney AS, Lang MJ et al. (2015) Transcription errors induce proteotoxic stress and shorten cellular lifespan. Nat Commun 6:8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills NM, Moore B, Hammer A et al. (2006) A functional-1 ribosomal frameshift signal in the human paraneoplastic Ma3 gene. J Biol Chem 281:7082–7088 [DOI] [PubMed] [Google Scholar]
- Yagi OK, Akiyama Y, Nomizu T et al. (1998) Proapoptotic gene BAX is frequently mutated in hereditary nonpolyposis colorectal cancers but not in adenomas. Gastroenterology 114:268–274 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Imai K, Perucho M (2002) Gastrointestinal cancer of the microsatellite mutator phenotype pathway. J Gastroenterol 37:153–163 [DOI] [PubMed] [Google Scholar]
- Yordanova MM, Wu C, Andreev DE et al. (2015) A nascent peptide signal responsive to endogenous levels of polyamines acts to stimulate regulatory frameshifting on antizyme mRNA. J Biol Chem 290:17863–17878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lin Y, Mañas A et al. (2014) BaxΔ2 promotes apoptosis through caspase-8 activation in microsatellite-unstable colon cancer. Mol Cancer Res 12:1225–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Tassone C, Lin N et al. (2015) Detection of Bax microsatellite mutations and BaxΔ2 isoform in human buccal cells. J Cell Sci Ther. 10.4172/2157-7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


