Abstract
Clinical studies examining the interaction between traumatic brain injury (TBI) and stress-related disorders (e.g., post-traumatic stress disorder) are often complicated by methodological constraints, such as heterogeneity in injury type and severity, time post-trauma, and predisposing risk factors. Developing relevant animal models whereby many variables can be efficiently controlled is thus essential to understanding this elusive relationship. Here, we use our repeated unpredictable stress (RUS) paradigm, in combination with our established mouse model of repetitive mild TBI (r-mTBI), to assess the impact of repeated exposures to these paradigms on behavioral and neurobiological measures. C57BL/6J male mice were exposed to RUS and r-mTBI at 3 and 6 months of age followed by batteries of behavioral testing. Mice were euthanized 10 days and 3 months post-exposure, with brain and plasma samples collected for molecular profiling. The RUS paradigm involved exposure to a predator odor (trimethylthiazoline; TMT) while under restraint, daily unstable social housing, five inescapable footshocks on separate days, and chronic social isolation. Animals receiving r-mTBI ( × 5) and stress were exposed to a single closed-head injury 1 h after each footshock. Stress-alone mice showed significant weight loss, recall of traumatic memories, and anxiety-like and passive stress-coping behavior when compared with control mice. However, in stress+r-mTBI animals, the changes in cued fear memory, anxiety, and stress-coping tests were diminished, possibly due to TBI-induced hyperactivity. We also report complex brain molecular and neuropathological findings. Stress and r-mTBI, either individually or comorbidly, were associated with a chronic reduction in dendritic spine GluN2A/GluN2B ratio in the hippocampus. While stress augmented the r-mTBI–dependent astrogliosis in the corpus callosum, it mitigated r-mTBI–induced increases in hippocampal pro–brain-derived neurotrophic factor. We anticipate that our model will be a good platform to untangle the complex comorbid pathophysiology in stress disorders and r-mTBI.
Keywords: animal studies; behavior, locomotor function, neuroplasticity; traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the result of a forceful blow or impact to the head, and is often accompanied by both short-term and long-term neuropsychological symptoms, such as confusion, irritability, impulsiveness, and depression.1 Mild TBI (mTBI) is the most common form of TBI, representing more than 80% of all TBI cases, and often co-exists with other psychiatric conditions such as depression, post-traumatic stress disorder (PTSD), and suicidal ideation.2,3
Particularly, comorbid mTBI and PTSD has received recent attention due to the increasing number of veterans returning from Iraq and Afghanistan who have experienced one or both of these conditions. The prevalence of PTSD in U.S. soldiers returning from Operation Iraqi Freedom and Operation Enduring Freedom ranges from 4% to 17%.4,5 Further, a report from the RAND corporation in 2009 indicated that 19% of the soldiers returning from deployment in Iraq and Afghanistan were diagnosed with TBI, and 5% were diagnosed with co-morbid PTSD and TBI.6 The probability of developing PTSD is higher for soldiers in combat roles relative to those in noncombat roles.7
The interaction between TBI and stress-related disorders is also relevant to the civilian population. A study conducted on civilians showed that patients who survived a mTBI were more likely to develop PTSD than patients with no brain injury.8 In a different study, 22% of patients admitted to the hospital for mTBI develop a psychiatric disorder within a year of sustaining the injury9; these psychiatric disorders included depression, generalized anxiety disorder, and PTSD.10
Many questions concerning comorbid stress disorders such as PTSD and mTBI remain unanswered. For example, would a history of mTBI increase the likelihood of developing PTSD after exposure to a traumatic event? Does the timing of mTBI relative to the traumatic event affect stress-related outcomes? The complexity of these questions increases when considering the different severities of TBI (i.e. mild, moderate, or severe). Further, attempting to answer these questions using human studies is challenging due to the clinical heterogeneity and overlapping symptoms of these conditions. Therefore, studying the interaction between mTBI and stress in pre-clinical models is necessary in order to understand the underlying neurobiological mechanisms and establish better therapeutic strategies.
Our group has ample experience in developing single and repetitive mTBI mouse models that have been characterized from 24 h to 24 months after injury.11,12 We have mostly focused on the chronic effects of repetitive mTBI (r-mTBI), utilizing a mild injury delivered on the midline of a closed skull administered five times over a 9-day period.11 With this model, we have demonstrated that in contrast to single mTBI, repetitive mTBI was associated with “chronic” spatial memory deficits, axonal injury, and glial activation long after the last injury.11,12
We have also recently shown that mice exposed to 21 days of repeated unpredictable stress (RUS) and chronic social isolation show long-lasting abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis and the hippocampus, but only moderate chronic changes in behavior.13
Although we acknowledge that the complex phenotypes observed in human PTSD cannot be captured by a single mouse model, the demonstration of several PTSD-like neurobiological alterations, and the long-term recall of trauma-related cues in mice exposed to RUS suggested that our model may have relevance to PTSD.13
Because trauma and injury-related symptoms are persistent in humans, the presence of long-lasting behavioral alterations after exposure to stressors or mTBI in an animal model is crucial for translational relevance. To this end, herein, we explore the consequences of exposure to chronic stress and r-mTBI on long-term behavioral and neurobiological outcomes. Chronic stress procedures involved double exposures to RUS at 3 and 6 months of age, while the r-mTBI procedures involved double exposures to our r-mTBI model (five hits, 48-h intra-injury interval) at the same time-points. We hypothesized that both stress-dependent and r-mTBI–dependent phenotypes will be more prominent after double exposure to our RUS paradigm compared with a single RUS exposure. This approach was adopted because the behavioral effects observed after a single RUS paradigm were moderate at 6 months post-exposure. Hence, a secondary objective in the current study is to explore a possible dose–response relationship of RUS and r-mTBI.
The comprehensive characterization of the behavioral and neurobiological consequences of chronic stress and r-mTBI exposure that will be presented here may have relevance to active-duty soldiers receiving multiple combat deployments and victims of multiple traumatic experiences from the civilian population. We anticipate that our model will be a useful platform to explore the long-lasting outcomes of combined exposure to stress and r-mTBI.
Methods
Animals
We purchased 12-week-old C57BL/6 male mice from Jackson Laboratories (Bar Harbor, ME) and housed them in standard cages under a 12-h light/12-h dark schedule at ambient temperature. All procedures were performed in accordance with Office of Laboratory Animal Welfare guidelines under a protocol approved by the Roskamp Institute Institutional Animal Care and Use Committee. Animals were randomly assigned to four groups: control, stress, r-mTBI, or stress+r-mTBI (n = 15 per group).
The 21-day repeated unpredictable stress and repetitive mild traumatic brain injury paradigm
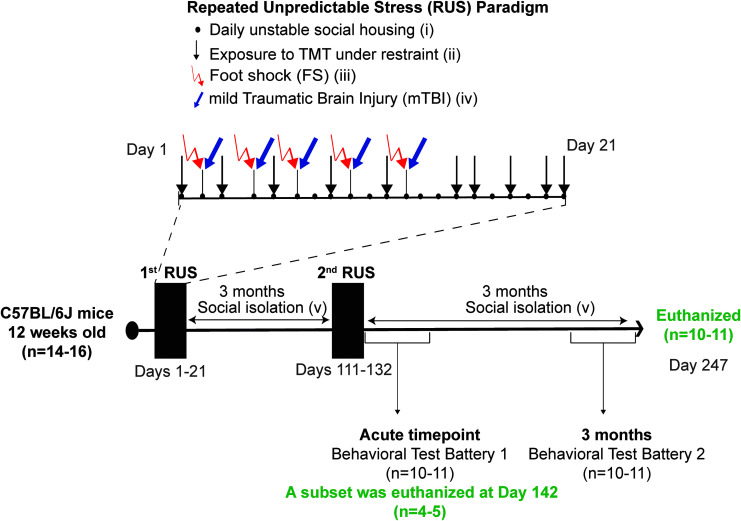
Our RUS paradigm was implemented as described previously13 and as depicted in Figure 1. In brief, 12-week-old mice received 21 days of RUS involving the following: 1) unstable social housing with a different mouse every day; 2) 30 min of unpredictable exposure to a predator odor (TMT) under restraint on 10 different days; and 3) five inescapable footshocks. Animals assigned to the stress+r-mTBI group received a mild traumatic brain injury 1 h after exposure to each footshock in addition to the RUS paradigm, while animals in the r-mTBI group received the five mTBIs only without any footshocks. The 1-h delay was introduced to allow for consolidation of fear memory.
FIG. 1.
Study timeline and experimental procedures for the stress paradigm. Twelve week old mice were exposed to the repeated unpredictable stress (RUS) paradigm at 3 months and at 6 months of age. The stress paradigm involved 21 days of daily unstable social housing (i), unpredictable repetitive exposures to predator odor (Fox urine component, TMT), while under restraint for 30 mins (ii), and five repeated inescapable footshocks (iii). Animals receiving repeated mild traumatic brain injury (r-mTBI) in addition to stress (Stress+r-mTBI group) were exposed to (iv) five closed-head injuries 1 h after exposure to inescapable footshock, for a total of five mTBIs. Closed-head injuries were conducted using our previously established model involving a 1-mm depth impact to the closed skull using a 5-mm flat tip electromagnetic impactor at a velocity of 5 m/sec with a dwell time of 200 msec. All animals in the stress groups were socially isolated (single housing) after RUS until the end of the study (v). A battery of behavioral testing was conducted at an acute time-point (Days 133–151) and again at 3 months after the second RUS (Days 224–241). Brain tissue and plasma were collected at the acute time-point (Day 142) and at the 3-month time-point (Day 247). Color image is available online.
Closed-head injuries were conducted using our previously established model involving a 1-mm depth impact delivered to the closed skull using a 5-mm flat tip electromagnetic stereotaxic impactor (Leica Biosystems, Buffalo Grove, IL) at a velocity of 5 m/sec with a dwell time of 200 msec.12 Mice were anesthetized using 1.5 L/min of oxygen and 3% isoflurane and maintained under anesthesia through a nose cone throughout the injury procedure. Footshocks and head injuries were administered at 48- to 72-h intervals to replicate our previous characterization of the r-mTBI model. Control and r-mTBI–only animals were exposed to the fear-conditioning chamber for the same frequency and duration as the stressed mice, without administration of footshocks or auditory cues, and were never restrained or exposed to TMT. Stress-only and control animals received sham anesthesia (3 min) 1 h after the footshock or contextual habituation, respectively. The same procedures were repeated 3 months after the first RUS (i.e., when animals were 27 weeks old). Animals in the stress and stress+r-mTBI groups were housed individually after the RUS procedure, whereas animals in the control and r-mTBI groups were housed in groups of 2–3 mice throughout the study.
A subset of animals from each group (n = 4–5 per group) were euthanized at the acute time-point (Day 142 of the study timeline) for molecular studies. To increase the number of animals in the anxiety tests, these animals received the Open Field test and the Elevated Plus Maze only once at the acute time-points with no additional testing. The remaining animals (n = 9–11 per group) received a behavioral testing battery beginning at Day 1 after the second RUS (Day 132 of the study timeline) and then were retested using a similar battery at 3 months after the second RUS. The exact timeline of the behavioral test batteries is shown in Supplementary Figure S1. All animals undergoing the full behavioral testing battery (n = 9–11 per group) were euthanized 1 week after the last behavioral experiment for molecular studies (around 3 months after the second RUS).
Body weight
Mice were weighed in the light cycle before receiving any stress or r-TBI manipulations in that particular day. Baseline body weights (at least the average of two independent measurements) were measured before the start of each RUS exposure. The percentage change in body weight was then calculated relative to each animal's baseline. One animal from the stress-only group and one animal from the stress+r-mTBI group died during the restraint procedures of the second RUS and were excluded from the analysis.
TMT
TMT was purchased from SRQBio (Sarasota, FL) and diluted 1:10 (v/v) in double distilled water before use. A total of 50 μL of 10% TMT was dispensed on a tissue paper next to the head of the restrained animal.
Fear conditioning and contextual and cued fear testing
Fear conditioning was performed with five total footshocks, administered on Days 2, 4, 6, 9, and 12 of the RUS paradigm. Animals were placed in a shuttle box chamber for 3 min with a pure tone (70 dB, 2.9 kHz) introduced during the last 30 sec. At the end of the auditory cue, animals received a single uncontrollable/inescapable footshock at 1 mA for 4 sec and were left for 1 additional min in the same context before returning to their home cages. Mice in the control group were placed in the same fear-conditioning context for 3 min without introducing a tone or delivering an electric shock. To determine retention of contextual/cued fear memory, animals were tested at the acute time-point, and at 3 months after the second RUS. This involved placing animals in the fear-conditioning spatial context for 3 min and measuring the freezing response using an automated video tracking system (Ethovision; Noldus, the Netherlands). A cued fear memory test was conducted approximately 1 h after the contextual fear memory test. Animals were placed in a novel context for 3 min in the absence of any cues followed by 3 min with the tone turned on. Freezing response was measured throughout the entire 6 min period by Ethovision Software using the optimized immobility parameter (5% immobility threshold, 20 frames per sec) as previously described.14
Open Field test
All animals were acclimated to the testing room for at least 1 h before the test procedures. Animals were placed in the center of a circular maze (120 cm diameter) and left to explore it for 15 min. The time spent in a predefined center zone and the number of entries into the center zone were used to examine anxiety. The testing room was lit at 80 lux (dim light).
Elevated Plus Maze (EPM)
The maze is comprised of two open and two closed arms (elevated 70 cm from the floor) forming a plus shape. Each mouse was placed in the middle of the maze facing an open arm and allowed to freely explore it for 5 min in a darkly lit room (∼1 lux). The number of entries into the open arms and the total time spent in the open arms were calculated using Ethovision video tracking system to evaluate anxiety. Animals were retested in the same room at 3 months after the second RUS.
Forced Swim Test (FST)
The test was performed in a cylindrical container (20 cm height × 20 cm diameter) filled with water at room temperature. Animals were gently placed in the middle of the container and allowed to swim for 6 min. The time spent floating (not swimming nor climbing) was calculated using the Ethovision immobility parameter (7.5% immobility threshold, 20 frames per sec) as a measure of passive stress coping behavior.
Radial Arm Water Maze (RAWM)
Spatial learning and memory were assessed using the RAWM test as described previously.15 In brief, the maze consists of a circular water pool (120 cm diameter and 30–40 cm height), containing six swim tracks (arms) extending from an open central arena. Each arm was marked with a unique visual cue, with only one target arm containing a hidden escape platform at the end of it. Animals were trained for 12 trials per day over 5 days to locate the hidden goal arm. Each trial lasted for 60 sec. Memory errors were recorded for each entry to a wrong arm. An Ethovision video-tracking system was used to record the number of errors per trial. All animals were subjected to the test for 5 days at the acute time-point as depicted in Supplementary Figure S1. The test was repeated for 5 more training days using the same maze and visual cues at 3 months after initial training.
Social interaction and novelty recognition tests (three-chamber test)
The testing apparatus consists of a rectangular box with three chambers separated by two doors. Each of the outer chambers contains a cage enclosure. The mice were habituated to the testing box for 5 min prior to testing. Testing occurred in two sessions. In the first session, an intruder mouse (Stranger1) was placed in one cage, while the cage in the other chamber was left empty. The tested mouse was placed in the middle chamber and was allowed to explore the three chambers for 10 min. The time spent in the chamber containing Stranger1 versus the chamber with the empty cage was calculated to evaluate social interaction of the test subject. In the second session, a novel intruder mouse (Stranger2) was placed in the second cage while the more familiar intruder mouse (Stranger1) remained in the first cage, and the test subject was allowed to freely explore all three chambers for 10 min. The time spent in the chamber with Stranger2 versus the chamber with Stranger1 was used to evaluate the social memory of the tested animal.
Blood collection and ex vivo lipopolysaccharide stimulation of whole blood
To obtain blood samples, animals were anesthetized with isoflurane, and approximately 500 μL of blood were collected into heparin tubes by cardiac puncture immediately prior to euthanasia. A total of 400 μL of blood from each animal were kept aside for the ex vivo lipopolysaccharide (LPS) stimulation studies. The remaining blood was centrifuged at 3000 g for 3 min, and plasma samples (clear supernatant fraction) were flash frozen in liquid nitrogen and stored at −80°C. For the ex vivo LPS challenge, 400 μL blood samples from each animal were divided into two 200 μL samples and incubated with either Roswell Park Memorial Institute medium (RPMI; 1% glutamine, 1% pyruvate, 1% garamycin) or RPMI containing 1 μg/mL LPS at 37°C for 6 h. All blood samples were then centrifuged for 3 min at 3000 g for plasma collection.
Enzyme-linked immunosorbent assay (ELISA) and multiplex ELISA
Commercially available ELISA kits were used to measure the concentration of several plasma markers: the neuropeptide Y (NPY) and cortisol ELISA kits were purchased from Life-Span Biosciences (Seattle, WA), the norepinephrine ELISA kit was purchased from Eagle Biosciences (Amherst, NH), and the corticosterone ELISA kit was purchased from Arbor Assays (Ann Arbor, MI). Tissue brain-derived neurotrophic factor (BDNF) levels were measured using an ELISA from Boster Bio (Pleasanton, CA). All ELISA kits were used as per manufacturers' instructions. Brain and plasma cytokine levels were measured using the MSD proinflammatory Panel I for the following cytokines: interferon γ (IFN-γ), interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, and tumor necrosis factor α (TNFα; MesoScale Discovery, Gaithersburg, MD). A single plasma sample (12.5 μL) from each mouse was used for the detection of plasma cytokines, while 25 μL of hippocampal homogenate in M-PER protein extraction reagent (∼8 mg/mL total protein) was used for the measurements of hippocampal cytokine levels.
Brain tissue preparation and Western blotting
Brains from 4–6 animals per group were used for biochemical analysis while the brains from the remaining animals were used for immunohistochemical experiments. Western blot experiments were conducted as described previously.16 In brief, following transcardial perfusion by gravity drip with phosphate-buffered saline (PBS), brain tissue was collected, and the hippocampus and hypothalamus were dissected and frozen immediately in liquid nitrogen and kept at −80°C. For Western blotting analyses, the hypothalamus or hippocampi from both hemispheres were homogenized in 200 μL of M-PER protein extraction reagent containing proteinase and phosphatase inhibitors (Thermofisher) using a probe sonicator. Samples were sonicated with a 2-sec pulse followed by incubation on ice for 10 sec, and this process was repeated three times. Homogenized samples were centrifuged at 15,000 g for 10 min followed by collection of the supernatants. Diluted supernatant fractions (2 mg/mL total protein) were then mixed with Laemmli buffer (Bio-Rad) containing DDT and denatured by boiling at 99°C. Samples were subsequently resolved on 4% to 15% gradient polyacrylamide criterion gels (Bio-Rad). After electrotransferring to polyvinylidene difluoride membranes, membranes were blocked in 5% milk made in Tris-buffered saline (TBST) and subsequently incubated with different primary antibodies overnight (Supplementary Table S1). After three washing steps using TBST, membranes were probed with horseradish peroxidase-linked secondary antibodies (Supplementary Table S1). Signal intensity ratios were quantified by chemiluminescence imaging with the ChemiDocTM XRS (Bio-Rad) with Anti-GAPDH antibody used as a housekeeping protein.
Golgi-Cox staining and immunohistochemistry
A total of 5–6 animals per group were used in Golgi-Cox staining and immunohistochemistry experiments. At 3 months after the second RUS exposure, mice were anesthetized with 3% isoflurane and transcardially perfused with PBS solution. Right brain hemispheres were extracted and immediately processed according to FD Rapid Golgi stain Kit instructions (FD NeuroTechnologies, Baltimore, MD), while the other hemispheres (left side) were fixed in 4% paraformaldehyde for 24–48 h followed by embedding in paraffin for subsequent immunohistochemistry (IHC) experiments.
For the Golgi experiments, 100 μm sections of impregnated tissue were cut using a cryostat at −20°C, mounted onto positively charged glass slides (Fisher, Superfrost Plus, Pittsburgh, PA) and analyzed using an Olympus BX63 upright microscope at 100 × magnification. The following three criteria were met by a dendrite to be chosen for spine density analysis: 1) it had to be emerging either from an apical neuron in the hippocampal CA1 region or layer II/III in the frontal cortex; 2) the neuron had to be fully stained, including the cell body with no cuts in the axons; and 3) the origin of the dendrite should not be obscured by any nearby overlapping neurons.
For IHC experiments, a series of 6-μm thick sagittal sections were cut throughout the extent of the cortex and hippocampus guided by known bregma coordinates using a microtome (2030 Biocut; Reichert/Leica, Buffalo Grove, IL). Cut sections were mounted onto positively charged glass slides (Superfrost Plus; Fisher, Pittsburgh, PA). Before the IHC procedure, all sections were deparaffinized in xylene and rehydrated in a gradient of ethanol solutions of decreasing concentrations. Sections were then rinsed in distilled water and subsequently incubated at room temperature in a solution of hydrogen peroxide (3% in water) for 15 min to block endogenous peroxidase activity. Antigen retrieval was necessary for ionized calcium-binding adaptor molecule 1 (Iba1) staining and included treatment with boiling citrate buffer solution (pH 6) for 8 min in the microwave. Following antigen retrieval, sections were blocked for 30 min to 1 h in normal blocking serum, using the same serum in which the secondary antibody was raised. Next, sections were immunostained in batches with primary antibodies diluted in supersensitive wash buffer (BioGenex, Fremont, CA). The primary antibodies used are listed in Supplementary Table S2.
After overnight incubation at 4°C, sections were washed in PBS, then incubated with the appropriate secondary antibody (Vectastain Elite ABC Kit; Vector Laboratories) at room temperature for 30 min to 1 h, depending on the specific requirement of the antibody protocol. After rinsing in water, sections were incubated with avidin-biotin horseradish peroxidase or alkaline phosphatase enzyme solution (Vector Laboratories) for 30 min at room temperature. Immunoreactivity was visualized with 3,3′-diaminobenzidine (DAB) peroxidase solution (0.05% DAB - 0.015% H2O2 in 0.01 M PBS, pH 7.2; Vector Laboratories). Development with the chromogen was timed and applied consistently across batches to limit technical variability before progressing to quantitative image analysis. Reactions were terminated by rinsing sections in distilled water. Sections were counterstained with hematoxylin, dehydrated through a gradient of ethanol of increasing concentrations, cleared in xylene, and cover-slipped with permanent mounting medium. Immunoreacted sections were viewed using a motorized Olympus BX63 upright microscope and photographs were taken using the high-resolution DP72 color digital camera.
Image analysis
Immunoreactivity for glial fibrillary acidic protein (GFAP) and Iba1 was measured by quantitative image analysis (optical segmentation) by an observer blinded to experimental grouping. Multiple regions of interest were analyzed in a standardized fashion for each cell marker/antibody. A survey of immunostained tissue sections was first performed independently to verify specific immunoreactivity that was subsequently progressed to quantitative image analysis. Briefly, non-overlapping red, green, blue (RGB) images were digitally captured randomly within the defined areas from each section (comprising an average of five sections per animal for each marker), providing a systematic survey of each region of interest for each animal within a group. A minimum of 10 microscopic fields (60 × magnification) were analyzed per region per animal. Immunoreactive profiles that were optically segmented were analyzed using CellSens morphometric image analysis software (Olympus, Center Valley, Pennsylvania). A semiautomated RGB histogram-based protocol (specified in the image analysis program) was used to determine the optimal segmentation (threshold setting) for immunoreactivity for each antibody. Immunoreactive profiles discriminated in this manner were used to determine the specific immunoreactive percentage area. Data were separately plotted as the mean percentage area of immunoreactivity per field (% area) ± standard error of mean for each region and grouping.
Statistical analysis
All data passed normality testing using Spiro-Wilk normality test and therefore were examined using appropriate parametric tests. The three-chamber test was analyzed using one-sample t-tests. Two-way analysis of variance (ANOVA) with Stress and r-mTBI as between-subjects independent variables was used to analyze total immobility time in the Forced Swim Test, RAWM last-day trials, immunohistochemical data for GFAP and Iba1 per brain region, and profiles of plasma and brain markers using ELISA and Western blotting. Body weight measurements, contextual fear memory, time-bin analysis of the cued fear memory tests and RAWM training trials were analyzed using three-way repeated measures ANOVA (RM ANOVA) with Stress and r-mTBI as two independent between-subjects variables and Time as within-subject independent variable. EPM and Open Field tests were analyzed using three-way ANOVA with Stress, r-mTBI, and Time as three independent variables because of the unequal number of animals at each time-point. Recall of cued fear memory at each time-point and the levels of plasma cytokines in the ex vivo LPS challenge were both analyzed using three-way-RM ANOVA with Stress and r-mTBI as between-subjects variables and Cue or LPS as a within-subject variable. Group differences at each time-point were analyzed using three-way ANOVA, followed by a two-way ANOVA at each repeated measure if any of the interaction terms were significant.
All ANOVA experiments were followed by pairwise comparisons with correction for multiple comparisons using the two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli (BKY) to control the false discovery rate.17 Three-way-RM ANOVA was performed using IBM SPSS Statistics software (Armonk, NY), while all other analyses were performed with Graph Pad prism version 7.0 statistical software (La Jolla, CA). Mauchley test was used to examine the assumption of sphericity before three-way-RM ANOVA analyses. If the sphericity assumption was violated, the Geisser-Greenhouse correction to the degrees of freedom and p values was reported.
Statistical outliers were identified using Grubbs' test. In the EPM, statistical outliers were removed from the stress-only and r-mTBI groups at the acute time-point and from the stress-only and stress+r-mTBI groups at the chronic time-point (one animal per group). Four animals (one per group) did not receive the RAWM training and were excluded from the analysis. The same animals were excluded from the FST analysis due to the absence of previous exposure to swim stress. In the RAWM 3-month recall trial (Fig. 4D), one statistical outlier was removed from the stress-only group. One statistical outlier (stress-only group) was removed from the plasma cytokines measurements (Table1) and another outlier (control group) was removed from the Western blot quantification of the hippocampal GluN1 levels at 3 months after the second RUS (Fig. 7E).
FIG. 4.
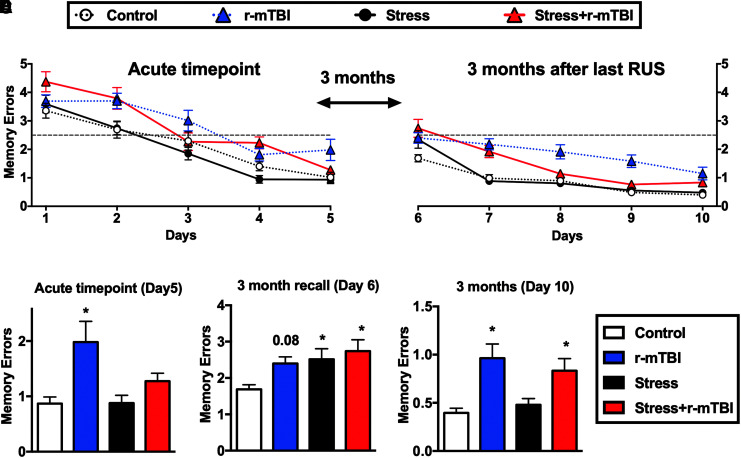
Effect of stress and repeated mild traumatic brain injury (r-mTBI) on spatial learning and memory. The performance of each group during two separate 5-day training sessions in a Radial Arm Water Maze (RAWM) is depicted in (A) and (B). In the last trial at the acute time-point (Day 5), only animals in the r-mTBI group showed spatial memory deficits as evidenced by a significant increase in the mean memory errors relative to controls (C). All treatment groups showed increased mean memory errors relative to controls in the 3-month recall of learned memories (Day 6; D). A main r-mTBI effect was observed in the last trial at the 3-month time-point (Day 10) as animals in the r-mTBI and stress+r-mTBI groups showed increased mean memory errors relative to controls (E). Data in (A) and (B) were analyzed using repeated measures three-way repeated measures analysis of variance (ANOVA), while data in (C-E) were analyzed using a two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (n = 9-11). Statistically significant discoveries versus the control group are denoted by “*”. The chance level (2.5 memory errors) is indicated by a dashed line in (A) and (B). Color image is available online.
Table 1:
Baseline Cytokine Levels (mean ± SEM) at 3 Months after the Second RUS
| Plasma (pg/mL) | Hippocampus (pg/mg protein) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | r-mTBI | Stress | Stress+ r-mTBI | Control | r-mTBI | Stress | Stress+ r-mTBI | |
| IFNγ | 1.85 ± 0.30 | 1.58 ± 0.14 | 1.30 ± 0.12 | 1.24 ± 0.09 | 1.91 ± 0.9 | 1.18 ± 0.23 | 1.554 ± 0.77 | 2.75 ± 1.37 |
| TNFα | 8.74 ± 0.82 | 7.52 ± 0.59 | 8.28 ± 1.57 | 6.4 ± 0.76 | 0.049 ± 0.09 | 0.06 ± 0.09 | 0.052 ± 0.07 | 0.053 ± 0.056 |
| IL-1β | 0.101 ± 0.03 | 0.081 ± 0.01 | 0.079 ± 0.02 | 0.072 ± 0.01 | 24.68 ± 3.57 | 19.71 ± 4.05 | 18.64 ± 1.878 | 21.65 ± 3.12 |
| IL-2* | 0.505 ± 0.09 | 0.387 ± 0.05 | 0.272 ± 0.06 | 0.248 ± 0.05 | 43.73 ± 12.2 | 21.29 ± 10.61 | 25.15 ± 5.98 | 31.2 ± 4.9 |
| IL-4 | 0.015 ± 0.008 | 0.026 ± 0.008 | 0.022 ± 0.01 | 0.04 ± 0.01 | 13.56 ± 1.93 | 15.95 ± 4.13 | 16.91 ± 1.8 | 12.92 ± 2.09 |
| IL-5* | 0.664 ± 0.04 | 0.473 ± 0.07 | 0.609 ± 0.08 | 0.507 ± 0.03 | 19.03 ± 2.27 | 19.39 ± 2.74 | 22.58 ± 1.02 | 20.47 ± 1.382 |
| IL-6 | 14.65 ± 4.3 | 25.28 ± 7.9 | 11.42 ± 1.8 | 8.05 ± 1.3 | 1.585 ± 0.35 | 1.191 ± 0.14 | 1.423 ± 0.27 | 1.04 ± 0.047 |
| IL-10 | 15.1 ± 2.2 | 11.08 ± 1.1 | 10.67 ± 0.65 | 10.8 ± 0.68 | 281.3 ± 32.05 | 346.8 ± 36.25 | 292.6 ± 27.08 | 294 ± 17.16 |
| IL-12 | 2.9 ± 1.7 | 2.7 ± 2.0 | 7.6 ± 4.5 | 4.2 ± 1.9 | 057 ± 0.33 | 0.155 ± 0.155 | 0.09 ± 0.09 | 0.71 ± 0.29 |
Asterisks denote statistical significance (p < 0.05).
Significantly different cytokine values in the stress and/or r-mTBI groups relative to the control group are bold.
SEM, standard error of the mean; RUS, repeated unpredictable stress; r-mTBI, repetitive mild traumatic brain injury; IFN, interferon; TNF, tumor necrosis factor; IL, interleukin.
FIG. 7.
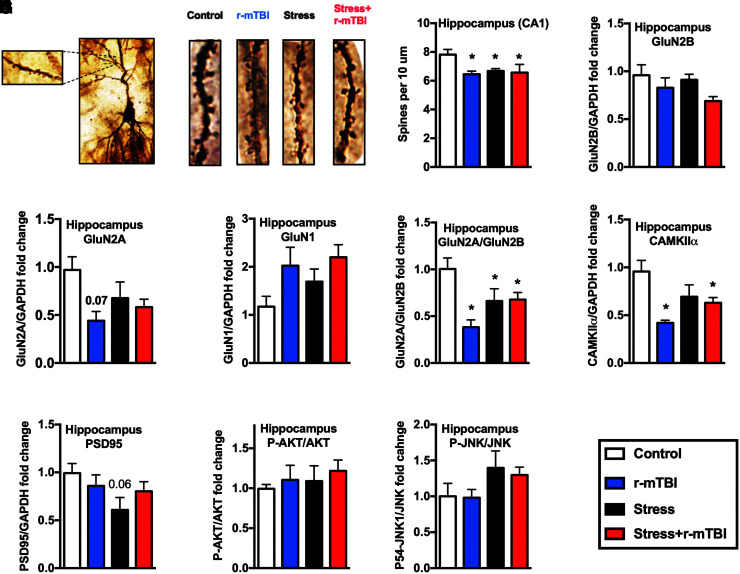
Effect of stress and repeated mild traumatic brain injury (r-mTBI) on spine density and a number of synaptic markers at 3 months after the second repeated unpredictable stress (RUS). Representative dendritic segments of the hippocampal CA1 region (A). Quantification of spine density in the CA1 of the hippocampus (B). Stress, stress+r-mTBI and r-mTBI groups showed reduced spine density only in the hippocampal CA1 region when compared with the control group. Quantification of Western blot images for GluN2B (C), GluN2A (D), GluN1 (E), GluN2A/GluN2B ratio (F), CAMKIIα (G), PSD-95 (H), the ratio of p-AKT/AKT (I), and P-JNK/JNK (J) in the hippocampus at 3 months after the second RUS. GluN2A/GluN2B ratio and CAMKIIα levels were reduced in the hippocampus in all stress and/or r-mTBI groups relative to controls. Data in (B-J) were analyzed using a two-way analysis of variance followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (n = 4–6). Statistically significant discoveries versus the control group are denoted by “*”. Color image is available online.
Results
Repeated exposure to stress and/or mTBI results in acute body weight loss
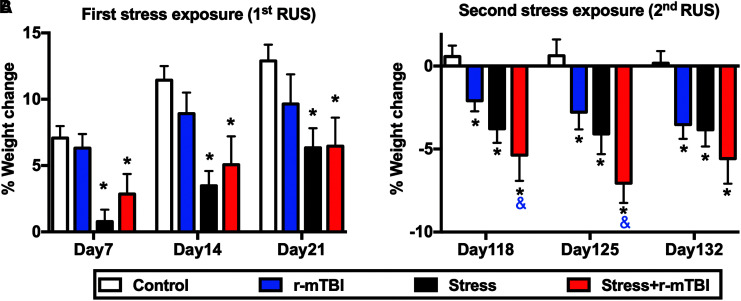
We have previously shown that a single exposure to the RUS paradigm results in a reduction in normal body weight gain in young adult mice.13 In the current study, we examined the effect of repeated exposure to stress and mTBI on body weight. To avoid bias due to differences in baseline body weight values (Supplementary Fig. S2A, S2B), we measured the percentage change in body weight relative to each animal baseline. During the first RUS exposure, body weight gain was only affected by stress and time, as evidenced by reduced weight gain in the stress and stress+r-mTBI groups in the first 3 weeks of stress relative to controls (three-way-RM ANOVA: main Time effect [F(2, 74) = 49.65, p < 0.0001], main Stress effect [F(1, 37) = 27.9, p < 0.0001], main r-mTBI effect [F(1, 37) = 0.023, p = 0.88], Stress*r-mTBI interaction [F(1, 37) = 2.63, p = 0.11], Stress*Time interaction [F(2, 74) = 1.044, p = 0.354], r-mTBI*Time interaction [F(2, 74) = 0.189, p = 0.828], and Stress*r-mTBI*Time interaction [F(2, 74) = 0.594, p = 0.555]; Fig. 2A). No significant r-mTBI effects on body weight gain were observed during the first RUS (Fig. 2A).
FIG. 2.
Effect of double exposure to repeated unpredictable stress (RUS) on body weight gain. During the first exposure to stress and/or repeated mild traumatic brain injury (r-mTBI; 1st RUS), animals in the stress and stress+r-mTBI groups showed a significant reduction in their body weight gain throughout the 21 days of stress (A). Stress and/or r-mTBI procedures were repeated 3 months after initial stress (2nd RUS). All stress and/or r-mTBI groups showed significant weight loss during the second RUS relative to the control group (B). Animals in the stress+r-mTBI group showed a greater weight loss when compared with the r-mTBI-only group at days 118 and 125 (B). Data in (A) and (B) were analyzed using three-way repeated measures analysis of variance (ANOVA) followed by a two-way ANOVA at each day as described in the materials and methods section (n = 9-11). Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli (BKY) was used to correct for multiple comparisons. Statistically significant discoveries versus the control group are denoted by “*”, while statistically significant discoveries versus the r-mTBI group are denoted by “&”. Color image is available online.
Stress and/or r-mTBI procedures were repeated again 3 months after initial stress (second RUS), during which animals in the r-mTBI, stress and stress+r-mTBI groups showed significant weight loss compared with the control group (three-way-RM ANOVA: main Time effect [F(2, 70) = 2.02, p = 0.141], main Stress effect [F(1, 35) = 15.319, p < 0.0001], main r-mTBI effect [F(1, 35) = 7.685, p < 0.0001], Stress*r-mTBI interaction [F(1, 35) = 0.357, p = 0.554], Stress*Time interaction [F(2, 770) = 2.237, p = 0.165], r-mTBI*Time interaction [F(2, 70) = 1.193, p = 0.309], and Stress*r-mTBI*Time interaction [F(2, 70) = 0.613, p = 0.545]; Fig. 2B). The changes in body weight in all groups were driven by an intense decrease in their rate of weight gain during the first week of RUS or r-mTBI (Supplementary Fig. S2C, S2D).
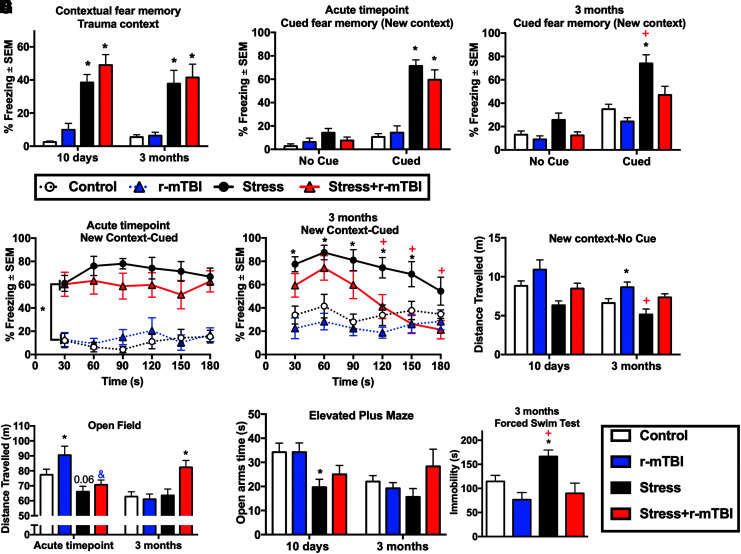
Effect of r-mTBI on overall locomotion and recall of fear memory
We next examined the effect of r-mTBI on several stress-related behaviors (Recall of fear memory, anxiety and stress-coping strategies) at the acute time-point and at 3 months after the second RUS (Fig. 3). All animals receiving RUS, independent of r-mTBI, showed a significant increase in their freezing (immobility time) when placed in the same trauma context at the acute and 3-month time-points (three-way-RM ANOVA: main Time effect [F(1,39) = 0.517, p = 0.476], main Stress effect [F(1, 39) = 68.908, p < 0.0001], main r-mTBI effect [F(1, 39) = 1.720, p < 0.197], Stress*r-mTBI interaction [F(1, 39) = 0.126, p = 0.725], Stress*Time interaction [F(1,39) = 0.392, p = 0.535], r-mTBI*Time interaction [F(1,39) = 1.177, p = 0.285], and Stress*r-mTBI*Time interaction [F(1,39) = 0.000, p = 0.988]; Fig. 3A). In the cued fear memory test at the acute time-point, stress and stress+r-mTBI groups showed similarly increased freezing responses to the cue compared with their respective control groups in the total test duration (three-way-RM ANOVA: main Cue effect [F(1,39) = 128.619, p < 0.001], main Stress effect [F(1, 39) = 59.344, p < 0.001], main r-mTBI effect [F(1, 39) = 0.546, p = 0.464], Stress*r-mTBI interaction [F(1, 39) = 2.700, p = 0.108], Stress*Cue interaction [F(1,39) = 71.646, p ≤ 0.001], r-mTBI*Cue interaction [F(1,39) = 0.221, p = 0.641], and Stress*r-mTBI*Cue interaction [F(1,39) = 0.249, p = 0.620]; Fig. 3B) and in the 30 sec time-bin analysis (three-way-RM ANOVA: main Time effect [F(3.39,132) = 0.421, p = 0.726], main Stress effect [F(1, 39) = 78.619, p ≤ 0.001], main r-mTBI effect [F(1, 39) = 0.479, p = 0.493], Stress*r-mTBI interaction [F(1, 39) = 1.666, p = 0.204], Stress*Time interaction [F(3.39,132) = 0.988, p = 0.408], r-mTBI*Time interaction [F(3.39,132) = 0.527, p = 0.687], and Stress*r-mTBI*Time interaction [F(3.39,132) = 0.817, p = 0.499]; Fig. 3D).
FIG. 3.
Effect of stress and repeated mild traumatic brain injury (r-mTBI) on fear memories, anxiety and stress-coping behavior at the acute and 3-month time-points. Stress and stress+r-mTBI groups demonstrated a similar increase in freezing time relative to controls at the acute and 3-month time-points (A). r-mTBI did not affect recall of cued fear memories in animals exposed to repeated unpredictable stress at the acute time-point, as stress and stress+r-mTBI animals showed a similar increase in the freezing time relative to control animals (B) and (D). However, at the 3-month time-point, stress+r-mTBI animals showed an attenuation in recall of cued fear memories compared with stress-only animals in the total test duration (3 min) as demonstrated by reduced freezing times relative to the stress-only group (C). In the cued fear memory test at the 3-month time-point, the stress-only group showed increased freezing time relative to controls in the first 150 sec of the test, while the stress+r-mTBI group freezing scores were higher than controls only during the first 60 sec of the test (E). At the 3-month time-point, animals receiving r-mTBI traveled longer distances in the new context (first 3 min of the cued fear memory test) relative to their respective non-injured group (F). The total distance traveled in the Open Field test was increased in the r-mTBI-only group relative to controls at the acute time-point and in the stress+r-mTBI group relative to all other groups at the 3-month time-point (G). Stress-only animals showed anxiety-like behavior at the acute time-point as demonstrated by decreased open arm time in the Elevated Plus Maze (H). Average immobility time in the last 4 min was increased in stress-only mice compared with the control, r-mTBI and stress+r-mTBI groups (I). Data in (A-H) were analyzed using three-way repeated measures analysis of variance (ANOVA; n = 9-11), while data in (I) was analyzed using two-way ANOVA. Statistically significant discoveries versus the control group are denoted by “*”, while statistically significant discoveries versus the stress+r-mTBI group are denoted by “+”. Statistically significant discoveries versus the r-mTBI group are denoted by “&”. Color image is available online.
However, at the 3-month time-point, the stress+r-mTBI group showed reduced immobility time relative to the stress-only group in the cued fear memory test (three-way-RM ANOVA: main Cue effect [F(1,39) = 113.121, p ≤ 0.001], main Stress effect [F(1, 39) = 21.011, p ≤ 0.001], main r-mTBI effect [F(1, 39) = 10.377, p = 0.003], Stress*r-mTBI interaction [F(1, 39) = 2.281, p = 0.139], Stress*Cue interaction [F(1,39) = 16.219, p ≤ 0.001], r-mTBI*Cue interaction [F(1,39) = 3.235, p ≤ 0.001], and Stress*r-mTBI*Cue interaction [F(1,39) = 0.402, p = 0.530]; Fig. 3C). Interestingly, stress+r-mTBI animals showed an increased average immobility time relative to controls in the contextual fear memory test, but not in the 3-min cued fear memory test at the 3-month time-point. A closer inspection of the freezing behavior to the cue in 30 sec time-bins (Fig. 3E) shows that stress+r-mTBI and stress-only animals exhibited a similar increase in their immobility time during the first 60 sec of the test (three-way-RM ANOVA: main Time effect [F(5,195 ) = 5.706, p ≤ 0.001], main Stress effect [F(1, 39) = 26.448, p ≤ 0.001], main r-mTBI effect [F(1, 39) = 9.753, p = 0.003], Stress*r-mTBI interaction [F(1, 39) = 1.876, p = 0.179], Stress*Time interaction [F(5,195) = 5.865, p ≤ 0.001], r-mTBI*Time interaction [F(5,195) = 0.769, p = 0.573], and Stress*r-mTBI*Time interaction [F(5,195) = 0.745, p = 0.541; Fig. 3E].
To examine the overall locomotor activity, we first checked the total distance traveled by different study groups in a safe new context and in the absence of any stress-related cues. As depicted in Figure 3F, stress+r-mTBI animals traveled longer distances in the 3-min-long test relative to and stress-only animals (three-way-RM ANOVA: main Time effect [F(1, 39) = 14.156, p = 0.001], main Stress effect [F(1, 39) = 8.736, p = 0.005], main r-mTBI effect [F(1, 39) = 10.8, p = 0.002], Stress*r-mTBI interaction [F(1, 39) = 0.263, p = 0.611], Stress*Time interaction [F(1, 39) = 1.236, p = 0.273], r-mTBI*Time interaction [F(1, 39) = 0.21, p = 0.886], and Stress*r-mTBI*Time interaction [F(1, 39) = 0.008, p = 0.929]; Fig. 3F).
The Open Field test has been widely used to evaluate overall locomotion and exploratory behavior in rodents.18 The test can also be used to examine anxiety-like behavior as long as the total ambulatory distance covered by all study groups is the same.19 At the acute time-point, the overall locomotor activity was reduced after stress and increased by r-mTBI as demonstrated by decreased distance traveled in stress-only mice and increased distance traveled in the r-mTBI-only mice relative to control mice (three-way ANOVA: main Time effect [F(1, 94) = 8.49, p = 0.004], main Stress effect [F(1, 94) = 0.57, p = 0.45], main r-mTBI effect [F(1, 94) = 8.495, p = 0.004], Stress*r-mTBI interaction [F(1, 94) = 1.01, p = 0.32], Stress*Time interaction [F(1, 94) = 19.87, p ≤ 0.001], r-mTBI*Time interaction [F(1, 94) = 0.003, p = 0.953], and Stress*r-mTBI*Time interaction [F(1, 94) = 5.96, p = 0.017]; Fig. 3G). Stress+r-mTBI mice showed a reduction in the total distance traveled in the Open Field relative to r-mTBI-only mice, but not relative to the control group, possibly due to opposing effects of stress and r-mTBI on locomotion at the acute time-point (Fig. 3G). Interestingly, at the 3-month time-point, the stress+r-mTBI group showed increased locomotion relative to controls, r-mTBI-only and stress-only groups (Fig. 3G). Examination of Open Field average velocity (Supplementary Fig. S3A) and inactivity time (Supplementary Fig. S3B) showed additional evidence of the altered ambulatory behavior after stress and/or r-mTBI. No changes in the time spent in the Open Field center zone (p > 0.05; Supplementary Fig. S3C) or center zone entries (p > 0.05; Supplementary Fig. S3D).
Effect of r-mTBI on anxiety and stress-coping strategies
We examined the effect of r-mTBI on anxiety-like behavior and stress-coping strategies using the EPM and the FST, respectively. Animals exposed to stress-only showed anxiety-like behavior at the acute time-point as demonstrated by reduced time spent in the open arms of the EPM (three-way ANOVA: main Time effect [F(1,90) = 5.73, p = 0.02], main Stress effect [F(1, 90) = 2.037, p = 3.22], main r-mTBI effect [F(1, 90) = 1.132, p = 0.295], Stress*r-mTBI interaction [F(1, 90) = 3.19, p = 0.077], Stress*Time interaction [F(1, 90) = 5.18, p = 0.025], r-mTBI*Time interaction [F(1, 90) = 0.16, p = 0.694], and Stress*r-mTBI*Time interaction [F(1, 90) = 0.757, p = 0.386]; Fig. 3H, followed by two-way ANOVA and correction for multiple comparisons at each time-point) and reduced open arm entries in the EPM (three-way ANOVA: main Time effect [F(1, 90) = 47.5, p ≤ 0.001], main Stress effect [F(1, 90) = 6.23, p = 0.014], main r-mTBI effect [F(1, 90) = 7.44, p = 0.008], Stress*r-mTBI interaction [F(1, 90) = 0.013, p = 0.9], Stress*Time interaction [F(1, 90) = 3.82, p = 0.083], r-mTBI*Time interaction [F(1, 90) = 0.02, p = 0.968], and Stress*r-mTBI*Time interaction [F(1, 90) = 0.87, p = 0.357]; Supplementary Fig. S3E, followed by two-way ANOVA and correction for multiple comparisons at each time-point). No significant stress or r-mTBI effects were observed at the 3-month time-point in the elevated plus maze (p > 0.05; Fig. 3H and Supplementary Fig. S3E). The validity of retesting at the 3-month time-point is questionable since control animals showed a reduction in time spent in the open arms and number of entries into the open arms after retesting (Fig. 3H and Supplementary Fig. S3E).
At 3 months after the second RUS, animals in the stress-only group showed negative stress-coping behavior as demonstrated by increased immobility time relative to controls in the last 4 min of the FST (two-way ANOVA: main Stress effect [F(1, 35) = 4.19, p = 0.05], main r-mTBI effect [F(1, 35) = 13.02, p = 0.001], Stress*r-mTBI interaction [F(1, 35) = 1.48, p = 0.23]; Fig. 3I) and (three-way-RM ANOVA: main Time effect [F(3.96,1.38.9) = 58.6, p ≤ 0.0001], main Stress effect [F(1, 35) = 5.84, p = 0.021], main r-mTBI effect [F(1, 35) = 11.63, p = 0.002], Stress*r-mTBI interaction [F(1, 35) = 2.076, p = 0.159], Stress*Time interaction [F(3.96,1.38.9) = 1.6, p = 0.17], r-mTBI*Time interaction [F(3.96,1.38.9) = 3.14, p = 0.017], and Stress*r-mTBI*Time interaction [F(3.96,1.38.9) = 0.471, p = 0.8]; Supplementary Fig. S3F). We found that r-mTBI ameliorated the effects of RUS on stress-coping behavior as evidenced by decreased immobility time in the stress+r-mTBI group compared with the stress-only group (Supplementary Fig. S3F, S3I). The total distance traveled in the FST is depicted in Supplementary Figure S3G. A trend towards increased total distance traveled was observed for mice in the r-mTBI groups (two-way ANOVA: main Stress effect [F(1, 35) = 1.373, p = 0.247], main r-mTBI effect [F(1, 35) = 13.16, p = 0.0001], Stress*r-mTBI interaction [F(1, 35) = 1.37, p = 0.25; Supplementary Fig. S3G).
Stress modulates acute r-mTBI impairments in spatial learning and memory
Our r-mTBI mouse model is associated with persistent and progressive deficits in spatial memory and learning, previously evaluated by the Barnes Maze.11 Here, we used the RAWM to examine the effect of stress on spatial memory deficits induced by r-mTBI. The performance of each group during training sessions in a radial arm water maze (RAWM) is depicted in Figure 4A. Mice received two sets of acquisition training sessions (5 days each). The first set started at the acute time-point followed by a second set 3 months after. All groups demonstrated a learning curve during the two sets of training (three-way-RM ANOVA: main Time effect [F(3.2, 114) = 81.061, p ≤ 0.001], main Stress effect [F(1, 35) = 0.342, p = 0.562], main r-mTBI effect [F(1, 35) = 20.146, p ≤ 0.001], Stress*r-mTBI interaction [F(1, 35) = 0.075, p = 0.786], Stress*Time interaction [F(3.2, 114) = 3.141, p = 0.026], r-mTBI*Time interaction [F(3.2, 114) = 0.760, p = 0.525], and Stress*r-mTBI*Time interaction [F(3.2, 114) = 1.627, p = 0.184]; Fig. 4A) and (three-way-RM ANOVA: main Time effect [F(2.5,88) = 66.083, p ≤ 0.001], main Stress effect [F(1, 35) = 1.726, p = 0.197], main r-mTBI effect [F(1, 35) = 59.864, p ≤ 0.001], Stress*r-mTBI interaction [F(1, 35) = 7.076, p = 0.012], Stress*Time interaction [F(2.5, 88) = 5.375, p = 0.003], r-mTBI*Time interaction [F(2.5, 88) = 2.122, p = 0.133], and Stress*r-mTBI*Time interaction [F(2.5, 88) = 0.857, p = 0.045]; Fig. 4B).
The mean number of memory errors at training Day 5 was used to evaluate spatial memory at the acute time-point (Fig. 4C), while the mean number of memory errors at training Day 10 was used to assess spatial memory at the 3-month time-point (Fig. 4E). In addition, the first day of the second training session (Day 6) was used to evaluate the long-term recall of spatial memory (Fig. 4D). At the acute time-point, the r-mTBI-only group showed increased mean memory errors relative to the control group (two-way ANOVA: main Stress effect [F(1, 35) = 2.8, p = 0.1], main r-mTBI effect [F(1, 35) = 8.1, p = 0.007], Stress*r-mTBI interaction [F(1, 35) = 2.2, p = 0.15], with correction for multiple comparisons; Fig. 4C), while at the chronic time-point both r-mTBI-only and stress+r-mTBI groups showed increased mean memory errors when compared with control mice (two-way ANOVA: main Stress effect [F(1, 35) = 0.8, p = 0.38], main r-mTBI effect [F(1, 35) = 18.35, p = 0.0001], Stress*r-mTBI interaction [F(1, 35) = 2.38, p = 0.13], with correction for multiple comparisons; Fig. 4E). Mice in stress, r-mTBI and stress+r-mTBI groups made more memory errors during the 3-month recall when compared with those in the control group (two-way ANOVA: main Stress effect [F(1, 34) = 5.78, p = 0.022], main r-mTBI effect [F(1, 34) = 3.71, p = 0.06], Stress*r-mTBI interaction [F(1, 34) = 1.01, p = 0.322], with correction for multiple comparisons; Fig. 4D).
The three-chamber test was used to assess social interaction and social memory after RUS and r-mTBI. At the acute time-point, as expected, control mice showed increased preference for the stranger mouse over the empty cage (one-sample t-test: t(10) = 3.577, p = 0.005; Supplementary Fig. S4A), while mice receiving stress-only, r-mTBI-only, and stress+r-mTBI showed no preference for the stranger mouse over the empty cage (one-sample t-test: p > 0.05; Supplementary Fig. S4A). All the groups showed no preference for the stranger mouse over the empty cage, when retested for social interactions at the 3-month time-point (one-sample t-test: p > 0.05; Supplementary Fig. S4A). In the subsequent social memory test, no group differences were observed at the acute time-point (one-sample t-test: p > 0.05; Supplementary Fig. S4B). However, at the 3-month time-point, control animals and stress-only animals showed a preference for Stranger2 over Stranger1 (one sample t-test: t(10) = 3.4, p = 0.007 for controls, t(9) = 3.2, p = 0.011 for the stress-only group), while r-mTBI and stress+r-mTBI animals spent comparable time with both strangers (One sample t-test: p > 0.05; Supplementary Fig. S4B). The lack of preference for Stranger2 over Stranger1 in the social memory test suggests that social memory was impaired in r-mTBI and stress+r-mTBI animals (Supplementary Fig. S4B).
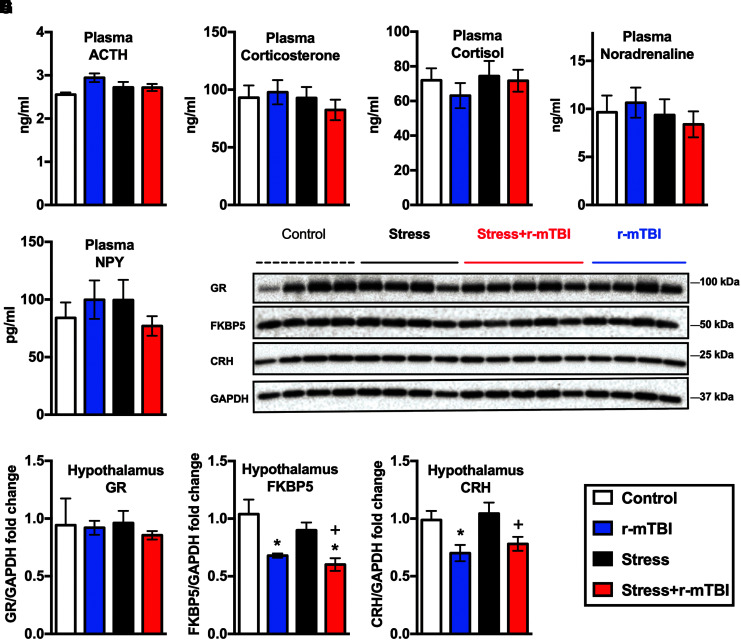
No dysregulation in baseline HPA-axis markers after repeated exposure to RUS
A single exposure to the RUS paradigm is associated with chronic HPA axis dysregulation (low baseline plasma corticosterone levels in stressed animals) as per our previous model characterization.13 To understand the effects of repeated RUS exposure on the HPA-axis, we first measured baseline plasma adrenocorticotropic hormone (ACTH) and corticosterone levels at 3 months after the second RUS. Surprisingly, no significant changes were observed in baseline plasma ACTH or corticosterone levels after repeated exposure to RUS at the chronic time-point (p > 0.05; Fig. 5A, 5B). In addition, baseline ACTH and corticosterone levels did not change after r-mTBI (p > 0.05; Fig. 5A, 5B). Similarly, baseline plasma cortisol, norepinephrine and NPY levels did not change after stress and/or r-mTBI (p > 0.05; Fig. 5C-E). No changes in the levels of corticosterone, cortisol, norepinephrine and NPY were observed in any group at the acute time-point (p > 0.0; Supplementary Fig. S5).
FIG. 5.
Effect of stress and repeated mild traumatic brain injury (r-mTBI) on hypothalamic-pituitary-adrenal (HPA) axis markers at 3 months after the second repeated unpredictable stress (RUS). No changes in plasma levels of adrenocorticotropic hormone (ACTH; A), corticosterone (B), cortisol (C), noradrenaline (D), or neuropeptide Y (E) were detected at 3 months after the second RUS. Representative Western blot images for a number of HPA axis markers in the hypothalamus (F). Quantification of Western blot images for GR (G), FKBP51 (H), and CRH (I) levels in the hypothalamus at 3 months after the second RUS. Levels of FKBP5 and CRH were reduced in r-mTBI and stress+r-mTBI groups relative to the control and stress-only groups, respectively. Data were analyzed using a two-way analysis of variance followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (n = 4–6). Statistically significant discoveries versus the control group are denoted by “*”, while statistically significant discoveries versus the stress-only group are denoted by “+”. Color image is available online.
We then examined the regulation of glucocorticoids in the brain by measuring the expression levels of glucocorticoid receptor (GR), FK506 binding protein 51 (FKBP51) and corticotropin-releasing hormone (CRH) in the hypothalamus (Fig. 5). Levels of FKBP51 and CRH were downregulated in the hypothalamus of r-mTBI animals at 3 months after the second RUS (two-way ANOVA: main Stress effect [F(1, 13) = 2.16, p = 0.17], main r-mTBI effect [F(1, 13) = 19.17, p = 0.0007], Stress*r-mTBI interaction [F(1, 13) = 0.178, p = 0.68] for Fig. 5H and two-way ANOVA: main Stress effect [F(1, 13) = 0.79, p = 0.39], main r-mTBI effect [F(1, 13) = 13.03, p = 0.0032], Stress*r-mTBI interaction [F(1, 13) = 0.029, p = 0.88] for Fig. 5I). Exposure to RUS did not affect the expression of GR, FKBP51, or CRH in the hypothalamus at the 3-month time-point (Fig. 5F-I).
Effect of stress and r-mTBI on the immune system
To examine the effect of repeated RUS and r-mTBI on the peripheral immune system, we measured plasma cytokine levels at baseline and after ex vivo stimulation of whole blood with lipopolysaccharide (LPS) at the chronic time-point. At baseline, IL-2 levels were reduced in the stress groups (i.e., stress and stress+r-mTBI groups) relative to the control group (two-way ANOVA: main Stress effect [F(1, 30) = 8.44, p = 0.007], main r-mTBI effect [F(1, 30) = 1.22, p = 0.28], Stress*r-mTBI interaction [F(1, 30) = 0.53, p = 0.47]; Table 1), while IL-5 levels were reduced in r-mTBI groups (i.e., r-mTBI and stress+r-mTBI groups) relative to the control group (two-way ANOVA: main Stress effect [F(1, 30) = 1.92, p = 0.176], main r-mTBI effect [F(1, 30) = 6.17, p = 0.018], Stress*r-mTBI interaction [F(1, 30) = 0.71, p = 0.41]; Table 1). Baseline levels of other cytokines were not significantly altered (Table 1). In the ex vivo whole blood challenge, IL-1β, IL-10, TNFα, IL-6, and IL-12 levels were increased in all groups after incubation with LPS for 6 h (RM-three-way ANOVA: significant main LPS effect [p < 0.05] with no significant main stress or r-mTBI effects and no significant interactions [Stress*LPS, r-TBI *LPS and Stress*r-mTBI*LPS, p > 0.05]; Supplementary Fig. S6E-I). No changes in hippocampal cytokine levels were observed at 3 months after the second RUS (p > 0.05; Table1).
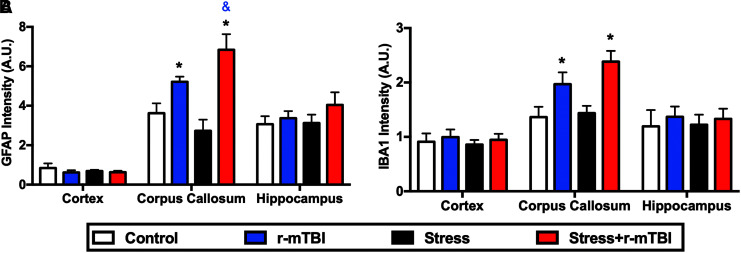
Stress augments r-mTBI-dependent astrogliosis in the corpus callosum
To assess the effect of stress on inflammatory markers associated with repetitive mTBI, brain tissue from different treatment groups was immunostained for GFAP and Iba1 at 3 months following the second RUS. GFAP levels were increased in the corpus callosum of stress+r-mTBI mice relative to r-mTBI-only mice, with both groups showing significantly higher levels than controls (two-way ANOVA: main Stress effect [F(1, 18) = 0.44, p = 0.51], main r-mTBI effect [F(1, 18) = 27.2, p < 0.0001], Stress*r-mTBI interaction [F(1, 18) = 5.28, p = 0.037]; Fig. 6A). In addition, Iba1 levels were significantly increased in the corpus callosum of all r-mTBI groups relative to controls (two-way ANOVA: main Stress effect [F(1, 18) = 0.82, p = 0.374], main r-mTBI effect [F(1, 18) = 16.82, p < 0.0001], Stress*r-mTBI interaction [F(1, 18) = 1.67, p = 0.213]; Fig. 6B). No significant changes in GFAP or Iba1 immunostaining were observed in the cortex or the hippocampus after stress or r-mTBI (Fig. 6A, 6B). Representative images for GFAP and Iba1 staining are depicted in Supplementary Figure S7.
FIG. 6.
Effect of stress on brain injury markers at 3 months after the second repeated unpredictable stress. Quantitative assessments of ionized calcium-binding adaptor molecule 1 (Iba1) and glial fibrillary acidic protein (GFAP) immunostaining in different brain regions are depicted in (A) and (B), respectively. Levels of Iba1 (A) and GFAP (B) were upregulated at 3 months after the last injury in the corpus callosum of repeated mild traumatic brain injury (r-mTBI) and stress+r-mTBI animals. Stress increases astrogliosis in injured animals as demonstrated by increased levels of GFAP immunostaining in the stress+r-mTBI group relative to the r-mTBI-only group (B). No changes in GFAP or Iba1 were observed in the cortex or the hippocampus (A, B). Data in each region in (A) and (B) were analyzed using two-way ANOVA followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (n = 5–6). Statistically significant discoveries versus the control group are denoted by “*”, while statistically significant discoveries versus the r-mTBI group are denoted by “&”. Color image is available online.
Reduced spine density and chronic alterations in synaptic plasticity markers after r-mTBI and/or stress exposures
Variations in dendritic spine density and morphology have been reported after stress. To examine the chronic effects of repeated RUS and r-mTBI on spine density, we used the Golgi-cox staining method to quantify the number of spines per 10-um dendritic length in the prefrontal cortex and in the hippocampus at 3 months after the second RUS. Figure 7A shows representative images from the Golgi-stained hippocampal CA1 neurons. Stress and/or r-mTBI did not change spine density in the prefrontal cortex (one-way ANOVA, p > 0.05; Supplementary Fig. S8A). However, stress and/or r-mTBI resulted in a reduction in spine density in hippocampal CA1 pyramidal neurons (two-way ANOVA: main Stress effect [F(1, 14) = 2.585, p = 0.132main r-mTBI effect [F(1, 14) = 5.39, p = 0.036], Stress*r-mTBI interaction [F(1, 14) = 3.9, p = 0.69]; Fig. 7B). We then examined the expression levels of notable markers/mediators of neuroplasticity using immunoblot analysis of hippocampal lysates. The ratio of GluN2A/GluN2B and the levels of with stress and/or r-mTBI relative to controls (two-way ANOVA: main Stress effect [F(1, 17) = 0.05, p = 0.82 main r-mTBI effect [F(1, 17) = 7.9, p = 0.012], Stress*r-mTBI interaction [F(1, 17) = 8.64, p = 0.009]; Fig. 7F), while CAMKIIα were downregulated in r-mTBI groups relative to controls (two-way ANOVA: main Stress effect [F(1, 17) = 0.07, p = 0.789, main r-mTBI effect [F(1, 17) = 9.6, p = 0.006], Stress*r-mTBI interaction [F(1, 17) = 5.96, p = 0.026]; Fig. 7G). GluNR1 levels were downregulated in all groups relative to controls at the acute time-point (two-way ANOVA: main Stress effect [F(1, 12) = 6.8, p = 0.023, main r-mTBI effect [F(1, 12) = 5.231, p = 0.041], Stress*r-mTBI interaction [F(1, 12) = 5.233, p = 0.04]; Supplementary Fig. S8D). At the 3-month time-point, there was a trend towards upregulation of GluNR1 in stress+r-mTBI groups relative to controls (two-way ANOVA: main Stress effect [F(1, 17) = 2.3, p = 0.15, main r-mTBI effect [F(1, 17) = 7.3, p = 0.015], Stress*r-mTBI interaction [F(1, 17) = 0.8, p = 0.38]; Fig. 7E). Levels of PSD-95 showed a trend towards reduction in the stress-only group relative to controls at the 3-month time-point (two-way ANOVA: main Stress effect [F(1, 17) = 7.23, p = 0.067 main r-mTBI effect [F(1, 17) = 0.739, p = 0.789], Stress*r-mTBI interaction [F(1, 17) = 4.65, p = 0.16]; Fig. 7H). The levels of other neuroplasticity markers such as GluN2B, P-JNK/JNK, P-CREB, and P-AKT/AKT were not significantly altered after RUS and r-mTBI (p > 0.05; Fig. 7 and Supplementary Fig. S8). Representative Western blot images for the hippocampal synaptic plasticity markers are depicted in Supplementary Figure S9.
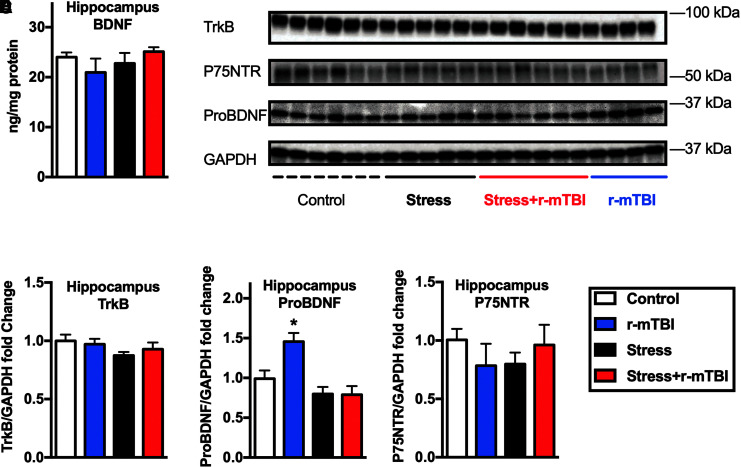
Effect of stress and r-mTBI on neurotrophic signaling markers
BDNF levels in the hippocampus were not altered at 3 months after exposure to RUS and r-mTBI (p > 0.05; Fig. 8A), while BDNF precursor (ProBDNF) levels were only increased in the r-mTBI group (two-way ANOVA: main Stress effect [F(1, 17) = 16.42, p = 0.0008 main r-mTBI effect [F(1, 17) = 4.64, p = 0.046], Stress*r-mTBI interaction [F(1, 17) = 5.05, p = 0.038]; Fig. 8D). We did not find any detectable changes in levels of BDNF receptor (TrkB) or ProBDNF receptor (P75NTR) in the hippocampus at 3 months after the second RUS (one-way ANOVA, p > 0.05; Fig. 8C, 8E). Interestingly, there was an effect for the combination of stress and r-mTBI on hippocampal P75NTR expression levels at the acute time-point (two-way ANOVA: main Stress effect [F(1, 12) = 2.02, p = 0.18, main r-mTBI effect [F(1, 12) = 5.57, p = 0.036], Stress*r-mTBI interaction [F(1, 12) = 1.5, p = 0.24]; Supplementary Fig. S8G). No changes in BDNF, ProBDNF or TrkB levels were detected at the acute time-point (p > 0.05; Supplementary Fig. S8).
FIG. 8.
Effect of repeated unpredictable stress (RUS) on hippocampal neurotrophic factors and receptors at 3 months after the second RUS. Hippocampal brain-derived neurotrophic factor (BDNF) levels were quantified using enzyme-linked immunosorbent assay (A). Representative Western blot images for TrkB, proBDNF, and P75NTR in the hippocampus (B). Quantification of hippocampal TrkB (C), ProBDNF (D), and P75NTR (E) levels using Western blotting. Hippocampal ProBDNF levels were upregulated only in the repeated mild traumatic brain injury group at 3 months after the second RUS. No changes in TrkB, BDNF, or P75NTR were observed at the 3-month time-point. Data in (A) and (C-E) were analyzed using two-way analysis of variance followed by two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli to correct for multiple comparisons (n = 4–6). Statistically significant discoveries versus the control group are denoted by “*”. Color image is available online.
Discussion
Summary
In the current study, we present a detailed characterization of the long-term consequences of recurrent and combined exposure to our well-characterized models of RUS13 and mTBI.11,12 Chronic stress or mTBI alone caused unique and distinct behavioral and neurobiological alterations long after the second exposure to mTBI or RUS. Combining both exposures resulted in complex domain-dependent outcomes with no overall additive or opposite effects. Animals receiving chronic stress alone showed acute weight loss, acute anxiety-like behavior, long-term recall of contextual and cued fear memories, and chronic passive stress-coping behavior. Moreover, chronic stress alone was associated with remodeling of dendritic spines and changes in several synaptic plasticity signaling markers in the hippocampus 3 months after the second RUS. The unique r-mTBI traits were typified by persistent spatial and social memory deficits, microgliosis, astrogliosis, and axonal damage. Combined exposure to r-mTBI and chronic stress resulted in an apparent amelioration of stress-related behaviors in the cued fear memory and forced swim tests at the 3-month time-point. However, these observations should be interpreted carefully due to the confounding effects of variable locomotion between study groups. Animals receiving combined exposure to chronic stress and r-mTBI showed additional TBI-related deficits represented by hyperactivity, and spatial and social memory deficits at the chronic time-point. Additionally, chronic stress augmented the injury dependent astrogliosis in the corpus callosum and mitigated the mTBI-dependent increase in pro-BDNF in the hippocampus.
Comparison to our previous findings (single RUS model)
Previously, we showed that animals exposed to a single episode of our 21-day RUS paradigm at 3 months of age demonstrate recall of cued fear memories, passive stress-coping behavior, low baseline plasma corticosterone and ACTH levels, and reduced hippocampal BDNF levels at 6 months after the last exposure.13 Here, we hypothesized that exposure to the 21-day RUS paradigm at 3 months of age with re-exposure at 6 months of age would augment the stress-related behavioral and neurobiological phenotypes examined at a similar post-exposure time-point in our previous investigation (i.e., 3 months after the second-exposure). At the behavioral level, animals re-exposed to RUS showed the same phenotypes reported after a single exposure. However, we also observed strong recall of contextual fear memories 3 months after the second RUS and deficits in long-term recall of learned spatial memories, which was not observed in the previous single exposure paradigm.13 In contrast to our initial hypothesis, we found that re-exposure to RUS resulted in a reduction in the key neurobiological markers observed after a single RUS exposure. Stress-only animals showed no change in baseline plasma corticosterone and ACTH levels (Fig. 5) and no change in hippocampal BDNF levels relative to control animals (Fig. 8) at 3 months after the second RUS. Additional experiments measuring corticosterone levels immediately after stress or after suppression with dexamethasone are required to better understand the complexity of HPA axis involvement in the current model.
Relationship to previous pre-clinical models
Behavioral findings
A number of pre-clinical studies have suggested that mTBI is implicated in the development of PTSD-like behavior.8,20,21 Exposure to different mTBI types in rodents such as blast,21 weight drop,22 or fluid percussion23 injuries was associated with increased fear memory recall and anxiety-like behavior multiple days after the injury. In all of these studies, the injury was administered before fear conditioning and in the absence of any additional stress procedures.21–23
A study by Kwon and colleagues is among the few pre-clinical studies investigating the consequences of combined exposure to mTBI and traumatic stress in animal models. Kwon and colleagues reported acute and transient anxiety in a group receiving unpredictable stress and a single blast injury relative to animals exposed to unpredictable stress alone.24 In another study, Klemenhagen and colleagues found that repetitive concussive traumatic brain injury and post-injury footshocks had synergistic effects in tests of social recognition and stress-coping behavior with no injury effects on recall of fear memories.25
In the current study, social interaction time was decreased after chronic stress and/or r-mTBI at the acute time-point (Supplementary Fig. S5). However, these findings are possibly confounded by the presence of acute anxiety in stressed animals.26 We also found that the cued fear memory recall was diminished in animals receiving stress and r-mTBI relative to stress-only animals. Genovese and colleagues reported similar findings showing an overall decrease in the expression of conditioned fear in rats exposed to inescapable electric shocks and blast overpressure injury relative to rats receiving shocks alone.27 It is important to note that in our current study the injury was delivered 1 h after the footshocks, whereas in the study by Genovese and colleagues, the injury was delivered 22 h after fear conditioning. One explanation for the mitigation of stress-related behavior when mTBI is applied post-exposure is that the memory of the footshock is not effectively consolidated due to the impact of TBI-dependent retrograde amnesia. Nevertheless, retrograde amnesia cannot completely explain our findings because stress+r-mTBI animals showed increased freezing behavior during the first minute of the test at the 3-month time-point (Fig. 3), suggesting that the memory of the cue is present.
As demonstrated in Figure 3G, stress+r-mTBI animals traveled longer distances in the Open Field test relative to animals in other study groups. Increased locomotion in the open field has been attributed to behavioral disinhibition both in a mouse model of Alzheimer's disease (Tg2576 transgenic mice)28 and in animals receiving 7 days of restraint stress.29 Interestingly, we have previously reported disinhibition or impulsive behavior in r-mTBI animals at 12 months after the last injury as demonstrated by increased time spent in the open arms and an increased number of entries into the open arms in the EPM.11 The decreased freezing in the cued fear memory test in stress+r-mTBI animals compared with stress-only animals is most likely driven by r-mTBI-induced impulsive behavior or hyperactivity and caution should be used to avoid interpretation as strictly a deficit in cued fear memory recall. Likewise, the hyper-locomotion after r-TBI could also be responsible for the decreased immobility time in r-mTBI animals in the FST (Fig. 3H). Similar observations were reported in Tg2576 transgenic mice showing a significant decrease in immobility time in the forced swim test that was inversely correlated with locomotor activity in the Open Field.28 Our results emphasize the importance of examining the effect of study treatments on locomotion before interpreting the findings of behavioral testing in rodents.
Changes in inflammatory markers
Peripheral inflammation has been implicated in the pathogenesis of TBI, depression, and PTSD, with multiple reports of altered cytokine levels in the plasma.30–32 We reported the following changes in baseline plasma cytokine levels at the chronic time-point: IL-2 levels were reduced in all stress groups, while IL-5 levels were reduced in all r-mTBI groups relative to the control group. IL-2 possesses a number of proinflammatory biological activities such as supporting the proliferation and survival of T cells, while IL-5 triggers the growth and differentiation of B cells and eosinophils.33 Therefore, our findings may underlie an imbalance between Th1 and Th2 immune response after stress and r-mTBI. The impact of stress and mTBI on plasma cytokine levels in human patients is not clear. IL-2 levels were reduced in earthquake survivors and in middle-aged war veterans with PTSD34 relative to their age-matched healthy subjects. In contrast, veterans with PTSD had higher levels of salivary IL-2 relative to veterans without combat-related PTSD.35 Some studies reported an increase in IL-6 and TNFα production in whole blood from PTSD patients after ex vivo stimulation with LPS.36,37 We performed similar experiments on fresh blood collected from our study groups. The response to LPS stimulation appeared similar in all study treatments, although some interesting trends resembling the clinical findings were observed for TNFα and IL-6 in the stress-only group (Supplementary Fig. S6).
The lack of significant changes in inflammatory cytokines (Table 1), GFAP (Fig. 6A) and Iba1 (Fig. 6B) levels in the hippocampus is consistent with our previous findings.11,12,38 Our r-mTBI model primarily affects the white matter (corpus callosum11,12 and the optic nerve39), resulting in increased levels of inflammatory markers such as Iba1 and GFAP (Fig. 6). Interestingly, exposure to stress had a synergistic effect on the injury-related increase in GFAP levels in the corpus callosum at the 3-month time-point (Fig. 6A). It is possible that a stress-related event (e.g., the elevated corticosterone levels during the early RUS procedures) has altered astrocytes priming in injured mice resulting in more elevated GFAP levels in the stress+r-mTBI group. Nevertheless, additional experiments at earlier time-points will be needed to examine this possibility.
Changes in the hippocampal neuroplasticity markers
Spatial learning deficits after r-mTBI have been connected to changes in brain structures such as the hippocampus.40 Hippocampal and prefrontal cortex (PFC) volume reduction and synaptic loss are also implicated in stress-related disorders such as PTSD.41 Multiple pre-clinical animal models reiterate the human findings and show the deleterious effects of stress and TBI on neuronal structures in the PFC and hippocampus.29,42–44 In the current study, we focused on the hippocampus as a primary site for pathobiology since both stress and r-mTBI seem to affect hippocampal-related functions such spatial and contextual memories.
Synapses are the junctions between neurons that facilitate communication and information processing in the brain. More than 90% of excitatory synapses are localized on the dendritic spines.45 Synaptic plasticity (long-term changes in the synapses in response to increased or decreased surrounding neuronal activity) is involved in the formation and retention of memories and is often accompanied by changes in the number and shape of dendritic spines.46,47 Exposure to repeated RUS and r-mTBI alone or in combination decreased the number of dendritic spines in the CA1 of the hippocampus, but not in the PFC (Fig. 7 and Supplementary Fig. S8). Similar effects of stress and mTBI on hippocampal dendritic spines have been previously reported.48–51 A study using 10 days of immobilization stress reported dendritic atrophy and debranching in the CA3 pyramidal neurons,52 while another study using controlled cortical impact in mice showed a reduction in dendritic spines in the ipsilateral dentate gyrus at 2450 and 7251 h after injury. Remodeling of hippocampal dendritic spines has implications for acquisition, consolidation and retrieval of spatial memories.46,47,53,54 This was demonstrated in a study that found decreased spine density in the CA1 region of the hippocampus correlated with spatial memory deficits in an object placement task in aged rats.54
Synaptic plasticity in the cortex and the hippocampus is primarily mediated by N-methyl-D-aspartate (NMDA) receptor signaling, which are a class of glutamatergic excitatory receptors.45,55 NMDA receptors are tetrameric structures containing two essential GluN1 subunits and two GluN2 subunits.56 Age, brain region, and memory consolidation are known to regulate the expression of different NMDA receptor subunits.56 At early developmental stages, GluN1 and GluN2B are the main components of NMDA receptors expressed at synapses. GluN2A replaces GluN2B as the primary GluN2 subunit with aging in what is known as developmental switch.57 Evidence suggests that the ratio of GluN2A to GluN2B increases with induced synaptic plasticity or memory consolidation in adult brains.58–60 At the 3-month time-point, the ratio of hippocampal GluN2A/GluN2B was decreased in all study groups (Fig. 7H), suggesting a chronic change in the maturity of synapses after stress and/or r-mTBI. Activation of GluN2A subunits has neuroprotective and cell survival effects, while activation of GluN2B appeared to have opposite effects.61 The chronic engagement of NMDA receptors also was demonstrated at the level of downstream postsynaptic proteins CMKIIα and PSD95 (Fig. 7).
After a TBI, BDNF levels increase to lessen the impact of secondary injury, provide neuroprotection, and restore brain connectivity.62 Increased BDNF levels has been reported in the cortex and the hippocampus of injured animals63 and in the cerebrospinal fluid of TBI patients.64 In animal models of stress, prolonged exposure to glucocorticoids decrease BDNF levels in the hippocampus, causing dendritic retraction and remodeling.62 ELISA analysis of total BDNF levels in hippocampus homogenate did not show significant group differences (Fig. 8). However, the BDNF precursor, ProBDNF, was upregulated in the hippocampus of r-mTBI–only animals at the 3-month time-point. These findings support other reports showing increased BDNF signaling after mTBI. Interestingly, ProBDNF levels were not increased in the group receiving stress+r-mTBI, suggesting opposite effects of stress and r-mTBI on BDNF signaling.
Implication for comorbid PTSD and r-mTBI
The impact of mTBI on the symptom presentation in stress disorders (such as PTSD) is not fully understood, particularly in the field of clinical PTSD research. Studies examining symptom severity in co-morbid PTSD and TBI patients relative to PTSD alone have shown mixed findings. Patients with co-morbid PTSD and mild to severe TBI had fewer intrusive memories than patients with PTSD alone.65 On the contrary, Simonović and colleagues reported more detachment and hyperarousal symptoms in patients with co-morbid PTSD and mild TBI compared with those with PTSD alone.1 In our study, exposure to r-mTBI abrogated the presentation of specific behavioral phenotypes in the stress-only group, such as freezing to fear-related cues and passive stress-coping behavior, while other behavioral phenotypes, such as hyperactivity, were only present in the comorbid group (i.e., stress+r-mTBI group). As such, this model may be useful in evaluating the complex traits observed in stress disorders complicated with TBI comorbidity.
Conclusion
Our results demonstrate that animals exposed to our stress paradigm develop persisting traits that capture several aspects of stress-related disorders. Unique behavioral phenotypes were also observed with the groups exposed to repetitive mTBI and stress. The chronic time-points examined here are particularly relevant to multiple persistent symptomologies of stress-related disorders and TBI in humans. This model may serve as a useful platform to untangle the complex comorbid pathophysiology of chronic stress and repetitive mTBI.
Supplementary Material
Acknowledgments
This work was funded by The Roskamp Foundation. FC is a VA research career scientist. DD was supported by NIH grant R01 MH109655-0.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Simonović M.M., Radisavljević M.M., and Grbeša G.B. (2011). P02-474—clinical presentation of the posttraumatic stress disorder with and without traumatic brain injuries. Eur. Psychiatry 26, 1070 [Google Scholar]
- 2. Bigler E.D. and Tsao J.W. (2017). Mild traumatic brain injury in soldiers returning from combat. Neurology 88, 1490–1492 [DOI] [PubMed] [Google Scholar]
- 3. Lindquist L.K., Love H.C., and Elbogen E.B. (2017). Traumatic brain injury in Iraq and Afghanistan veterans: new results from a national random sample study. J. Neuropsychiatry Clin. Neurosci. 29, 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hines L.A., Sundin J., Rona R.J., Wessely S., and Fear N.T. (2014). Posttraumatic stress disorder post Iraq and Afghanistan: prevalence among military subgroups. Can. J. Psychiatry 59, 468–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 358, 453–63 [DOI] [PubMed] [Google Scholar]
- 6. Holdeman T.C. (2009). Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Psychiatr. Serv. 60, 273–273 [Google Scholar]
- 7. Kok B.C., Herrell R.K., Thomas J.L., and Hoge C.W. (2012). Posttraumatic stress disorder associated with combat service in Iraq or Afghanistan: reconciling prevalence differences between studies. J. Nerv. Ment. Dis. 200, 444–450 [DOI] [PubMed] [Google Scholar]
- 8. Bryant R.A., Creamer M., O'Donnell M., Silove D., Clark C.R., and McFarlane A.C. (2009). Post-traumatic amnesia and the nature of post-traumatic stress disorder after mild traumatic brain injury. J. Int. Neuropsychol. Soc. 15, 862–867 [DOI] [PubMed] [Google Scholar]
- 9. Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., and Silove D. (2010). The Psychiatric Sequelae of Traumatic Injury. Am. J. Psychiatry 167, 312–320 [DOI] [PubMed] [Google Scholar]
- 10. Bryant R.A., Marosszeky J.E., Crooks J., and Gurka J.A. (2000). Posttraumatic stress disorder after severe traumatic brain injury. Am. J. Psychiatry 157, 629–31 [DOI] [PubMed] [Google Scholar]
- 11. Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 12. Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., and Crawford F. (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 29, 2761–2773 [DOI] [PubMed] [Google Scholar]
- 13. Algamal M., Ojo J.O., Lungmus C.P., Muza P., Cammarata C., Owens M.J., Mouzon B.C., Diamond D.M., Mullan M., and Crawford F. (2018). Chronic hippocampal abnormalities and blunted HPA axis in an animal model of repeated unpredictable stress. Front. Behav. Neurosci. 12, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ojo J.O., Greenberg M.B., Leary P., Mouzon B., Bachmeier C., Mullan M., Diamond D.M., and Crawford F. (2014). Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front. Behav. Neurosci. 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soliman F., Glatt C.E., Bath K.G., Levita L., Jones R.M., Pattwel S.S., Jing D., Tottenham N., Amso D., Somerville L.H., Voss H.U., Glover G., Ballon D.J., Liston C., Teslovich T., Van Kempen T., Lee F.S., and Casey B.J. (2010). A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 327, 863–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ojo J.O., Mouzon B., Algamal M., Leary P., Lynch C., Abdullah L., and Crawford F. (2016). Chronic repetitive mild traumatic brain injury results in reduced cerebral blood gliosis, and tau and tau oligomers. J. Neuropathol. Exp. Neurol. 75, 636–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hochberg B. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 [Google Scholar]
- 18. Hall C. and Ballachey E.L.L. (1932). A study of the rat's behavior in a field. A contribution to method in comparative psychology. Univ. Calif. Publ. Psychol. 6, 1–12 [Google Scholar]
- 19. Seibenhener M.L. and Wooten M.C. (2015). Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. e52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy J.E. (2007). Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J. Rehabil. Res. Dev. 44, 895–920 [DOI] [PubMed] [Google Scholar]
- 21. Elder G.A., Dorr N.P., De Gasperi R., Gama Sosa M.A., Shaughness M.C., Maudlin-Jeronimo E., Hall A.A., McCarron R.M., and Ahlers S.T. (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 29, 2564–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer D.L., Davies D.R., Barr J.L., Manzerra P., and Forster G.L. (2012). Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp. Neurol. 235, 574–587 [DOI] [PubMed] [Google Scholar]
- 23. Reger M.L., Poulos A.M., Buen F., Giza C.C., Hovda D.A., and Fanselow M.S. (2012). Concussive brain injury enhances fear learning and excitatory processes in the amygdala. Biol. Psychiatry 71, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwon S.-K.C., Kovesdi E., Gyorgy A.B., Wingo D., Kamnaksh A., Walker J., Long J.B., and Agoston D. V. (2011). Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klemenhagen K.C., O'Brien S.P., and Brody D.L. (2013). Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS One 8, e74510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vukobradovic I., Woodgett J.R., Kaidanovich-Beilin O., Roder J., and Lipina T. (2011). Assessment of social interaction behaviors. J. Vis. Exp. pii, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovese R.F., Simmons L.P., Ahlers S.T., Maudlin-Jeronimo E., Dave J.R., and Boutte A.M. (2013). Effects of mild TBI from repeated blast overpressure on the expression and extinction of conditioned fear in rats. Neuroscience 254, 120–129 [DOI] [PubMed] [Google Scholar]
- 28. Gil-Bea F.J., Aisa B., Schliebs R., and Ramírez M.J. (2007). Increase of locomotor activity underlying the behavioral disinhibition in Tg2576 Mice. Behav. Neurosci. 121, 340–344 [DOI] [PubMed] [Google Scholar]
- 29. Ito H., Nagano M., Suzuki H., and Murakoshi T. (2010). Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology 58, 746–757 [DOI] [PubMed] [Google Scholar]
- 30. Gill J.M., Saligan L., Woods S., and Page G. (2009). PTSD is Associated with an excess of inflammatory immune activities. Perspect. Psychiatr. Care 45, 262–277 [DOI] [PubMed] [Google Scholar]
- 31. Acosta S.A., Diamond D.M., Wolfe S., Tajiri N., Shinozuka K., Ishikawa H., Hernandez D.G., Sanberg P.R., Kaneko Y., and Borlongan C. V. (2013). Influence of post-traumatic stress disorder on neuroinflammation and cell proliferation in a rat model of traumatic brain injury. PLoS One 8, 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Z., Caughron B., and Young M.R.I. (2017). Posttraumatic stress disorder: An immunological disorder? Front. Psychiatry 8, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kouro T. and Takatsu K. (2009). IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int. Immunol. 21, 1303–1309 [DOI] [PubMed] [Google Scholar]
- 34. Jergović M., Tomičević M., Vidović A., Bendelja K., Savić A., Vojvoda V., Rac D., Lovrić-Čavar D., Rabatić S., Jovanovic T., and Sabioncello A. (2014). Telomere shortening and immune activity in war veterans with posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 275–283 [DOI] [PubMed] [Google Scholar]
- 35. Wang Z., Mandel H., Levingston C.A., and Young M.R.I. (2016). An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of Veterans with combat-related PTSD. Hum. Immunol. 77, 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gola H., Engler H., Sommershof A., Adenauer H., Kolassa S., Schedlowski M., Groettrup M., Elbert T., and Kolassa I.T. (2013). Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gil J. (2010). Low cortisol, high DHEA, and high levels of stimulated TNFα, and IL-6 in women with PTSD. J. Trauma. Stress 21, 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mouzon B., Saltiel N., Ferguson S., Ojo J., Lungmus C., Lynch C., Algamal M., Morin A., Carper B., Bieler G., Mufson E.J., Stewart W., Mullan M., and Crawford F. (2018). Impact of age on acute post-TBI neuropathology in mice expressing humanized tau: a Chronic Effects of Neurotrauma Consortium Study. Brain Inj. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tzekov R., Dawson C., Orlando M., Mouzon B., Reed J., Evans J., Crynen G., Mullan M., and Crawford F. (2016). Sub-chronic neuropathological and biochemical changes in mouse visual system after repetitive mild traumatic brain injury. PLoS One 11, e0153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasad K.N. and Bondy S.C. (2015). Common biochemical defects linkage between post-traumatic stress disorders, mild traumatic brain injury (TBI) and penetrating TBI. Brain Res. 1599, 103–114 [DOI] [PubMed] [Google Scholar]
- 41. Sherin J.E., and Nemeroff C.B. (2011). Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 13, 263–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolf R.C., and Herringa R.J. (2016). Prefrontal–amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41, 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., and Liberzon I. (2012). Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McEwen B.S., Nasca C., and Gray J.D. (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Engert F. and Bonhoeffer T. (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 [DOI] [PubMed] [Google Scholar]
- 46. Frankfurt M. and Luine V. (2015). The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm. Behav. 74, 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li C., Brake W.G., Romeo R.D., Dunlop J.C., Gordon M., Buzescu R., Magarinos A.M., Allen P.B., Greengard P., Luine V., and McEwen B.S. (2004). Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U. S. A. 101, 2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qiao H., Li M.X., Xu C., Chen H.B., An S.C., and Ma X.M. (2016). Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016, 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shors T.J., Falduto J., and Leuner B. (2004). The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur. J. Neurosci. 19, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winston C.N., Chellappa D., Wilkins T., Barton D.J., Washington P.M., Loane D.J., Zapple D.N., and Burns M.P. (2013). Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 30, 1966–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gao X., Deng P., Xu Z.C., and Chen J. (2011). Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One 6, e24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vyas A., Mitra R., Shankaranarayana Rao B.S., and Chattarji S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 22, 6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mahmmoud R.R., Sase S., Aher Y.D., Sase A., Gröger M., Mokhtar M., Höger H., and Lubec G. (2015). Spatial and working memory is linked to spine density and mushroom spines. PLoS One 10, e0139739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luine V.N., Wallace M.E., and Frankfurt M. (2011). Age-related deficits in spatial memory and hippocampal spines in virgin, female Fischer 344 rats. Curr. Gerontol. Geriatr. Res. 2011, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ultanir S.K., Kim J.-E., Hall B.J., Deerinck T., Ellisman M., and Ghosh A. (2007). Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc. Natl. Acad. Sci. U. S. A. 104, 19553–19558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanz-Clemente A., Nicoll R.A., and Roche K.W. (2013). Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19, 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Newcomer J.W., Farber N.B., and Olney J.W. (2000). NMDA receptor function, memory, and brain aging. Dialogues Clin. Neurosci. 2, 219–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baez M.V., Cercato M.C., and Jerusalinsky D.A. (2018). NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 2018, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cui Z., Feng R., Jacobs S., Duan Y., Wang H., Cao X., and Tsien J.Z. (2013). Increased NR2A:NR2B ratio compresses long-term depression range and constrains long-term memory. Sci. Rep. 3, 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y., Wong T.P., Aarts M., Rooyakkers A., Liu L., Lai T.W., Wu D.C., Lu J., Tymianski M., Craig A.M., and Wang Y.T. (2007). NMDA Receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27, 2846–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Song Y., Zhou D., Guan Z., and Wang X. (2007). Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in Northern China. Neuroimmunomodulation 14, 248–254 [DOI] [PubMed] [Google Scholar]
- 62. Kaplan G.B., Vasterling J.J., and Vedak P.C. (2010). Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav. Pharmacol. 21, 427–437 [DOI] [PubMed] [Google Scholar]
- 63. Griesbach G.S., Hovda D.A., Molteni R., and Gomez-Pinilla F. (2002). Alterations in BDNF and synapsin I within the occipital cortex and hippocampus after mild traumatic brain injury in the developing rat: reflections of injury-induced neuroplasticity. J. Neurotrauma 19, 803–814 [DOI] [PubMed] [Google Scholar]
- 64. Failla M.D., Conley Y.P., and Wagner A.K. (2016). Brain-derived neurotrophic factor (BDNF) in traumatic brain injury–related mortality. Neurorehabil. Neural Repair 30, 83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hibbard M., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis 1 psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 13, 24–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.