Abstract
Objectives
The aetiology and burden of viral-induced acute liver failure remains unclear globally. It is important to understand the epidemiology of viral-induced ALF to plan for clinical case management and case prevention.
Participants
This systematic review was conducted to synthesize data on the relative contribution of different viruses to the aetiology of viral-induced acute liver failure in an attempt to compile evidence that is currently missing in the field. EBSCOhost, PubMed, ScienceDirect, Scopus and Web of Science were searched for relevant literature published from 2009 to 2019. The initial search was run on 9 April 2019 and updated via PubMed on 30 September 2019 with no new eligible studies to include. Twenty-five eligible studies were included in the results of this review.
Results
This systematic review estimated the burden of acute liver failure after infection with hepatitis B virus, hepatitis A virus, hepatitis C virus, hepatitis E virus, herpes simplex virus/human herpesvirus, cytomegalovirus, Epstein-Barr virus and parvovirus B19. Data were largely missing for acute liver failure after infection with varicella-zostervirus, human parainfluenza viruses, yellow fever virus, coxsackievirus and/or adenovirus. The prevalence of hepatitis A-induced acute liver failur was markedly lower in countries with routine hepatitis A immunisation versus no routine hepatitis A immunisation. Hepatitis E virus was the most common aetiological cause of viral-induced acute liver failure reported in this review. In addition, viral-induced acute liver failure had poor outcomes as indicated by high fatality rates, which appear to increase with poor economic status of the studied countries.
Conclusions
Immunisation against hepatitis A and hepatitis B should be prioritised in low-income and middle-income countries to prevent high viral-induced acute liver failure mortality rates, especially in settings where resources for managing acute liver failure are lacking. The expanded use of hepatitis E immunisation should be explored as hepatitis E virus was the most common cause of acute liver failure.
Registration
PROSPERO registration number: CRD42017079730.
Keywords: epidemiology, hepatology, virology
Strengths and limitations of this study.
Comprehensive and exhaustive search for relevant studies from several databases.
Comprehensive diagnostic inclusion criteria for acute liver failure cases according to international guideline.
Lack of language restrictions in search led to inclusion of geographically diverse data.
Findings limited by lack of data for some of the viral aetiologies of acute liver failure which may have led to an underestimation of the global burden of viral-induced acute liver failure.
Diversity of viruses attributable to acute liver failure cases and viral detection methods led to high heterogeneity and low statistical power in meta-analyses conducted.
Background
Acute liver failure (ALF) refers to the development of encephalopathy and synthetic function impairment after acute liver injury in an individual without pre-existing liver disease.1 The presence of encephalopathy is not required to define ALF in paediatric cases, but is an essential component of the definition of ALF in adults.1 Possible causes of ALF include viral infections, drugs and toxins, pregnancy-related liver diseases, vascular causes and/or malignancies. Acute viral hepatitis has been identified as the most common cause of ALF among all ages in Asia and Africa and one of the most common causes of ALF in children in Asia and South America.1 2 The incidence of viral-induced ALF has substantially declined in Europe after the introduction of universal immunisation against the hepatitis B virus (HBV), with only 19% of all ALF cases now attributable to viral infection in the European population.3 The introduction of routine immunisation against the hepatitis A virus (HAV) in Argentina has reduced the number of hepatitis A-induced ALF cases by more than 25%.3
Fatality rates associated with ALF vary between 60% and 80% depending on the disease aetiology as well as a patient’s access to care.4 5 Liver transplantation plays a central role in the management of ALF and remains the only definitive treatment for patients who fail to demonstrate spontaneous recovery.6 A large proportion of patients with ALF in both high and low resource settings, however, are deemed to have contraindications to transplantation or deteriorate beyond transplantation before a liver donor is found.7–9
The burden of viral-induced ALF around the world still remains unclear, with little to no data collected regarding the disease incidence.1 Establishing the aetiology of viral-induced ALF is important for early initiation of treatment, determining the prognosis of the liver failure and identifying potential contraindications to liver transplantation. Most importantly, understanding the epidemiology of vaccine-preventable aetiologies of ALF should be prioritised in under-resourced regions with limited access to facilities for transplantation. This review aims to synthesise data on the relative contribution of different viruses to the aetiology of viral-induced ALF in an attempt to compile evidence that is currently missing in the field.
Bernal et al. completed a review of the burden of acute liver failure based on literature published between 1997 and 2009. The review became the bases for guidelines for clinical practice.4 In this systematic review, we assess whether data have changed following the Bernal et al. publication, and whether there is evidence to warrant a review of clinical practice.
Objectives
To estimate the prevalence of hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), hepatitis E virus (HEV), Epstein-Barr virus (EBV), herpes simplex virus-1 (HSV1), herpes simplex virus-2 (HSV2), varicella-zoster virus (VZV), parvovirus B19, human parainfluenza viruses (HPIVs), yellow fever virus (YFV), human herpesvirus 6 (HHV-6), cytomegalovirus (CMV), coxsackievirus (CA16) and/or adenovirus (HAdVs) among patients with ALF.
To estimate the mortality rate for cases of ALF after infection with HAV, HBV, HCV, HDV, HEV, EBV, HSV1, HSV2, VZV, parvovirus B19, HPIVs, YFV, HHV-6, CMV, CA16 and/or HAdVs.
To estimate the prevalence and incidence of liver transplantation for cases of ALF after infection with HAV, HBV, HCV, HDV, HEV, EBV, HSV1, HSV2, VZV, parvo-virus B19, HPIVs, YFV, HHV-6, CMV, CA16 and/or HAdVs.
Methods
This systematic review was registered with PROSPERO (registration number CRD42017079730) and the review methods have been published.10 The results of the review are reported using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines checklist.
Study eligibility criteria
Published cross-sectional, surveillance and cohort studies reporting the outcomes of interest in patients with ALF after infection with HAV, HBV, HCV, HDV, HEV, EBV, HSV1, HSV2, VZV, parvovirus B19, HPIVs, YFV, HHV-6, CMV, CA16 and/or HAdVs were eligible for inclusion in this study. Studies were eligible for inclusion if they had clearly stated case definitions of viral-induced ALF and confirmed ALF cases using both clinical and serological, molecular or culture diagnostic methods.
Search strategy
A combination of the following search terms (including the use of Medical Subject Headings (MESH)) was used and adapted for each of the relevant electronic databases: epidemiology, prevalence, incidence, burden, mortality, morbidity, fulminant hepatic failure, fulminant liver failure, acute hepatic failure, acute liver failure, hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), hepatitis E virus (HEV), Epstein Barr virus (EBV), herpes simplex virus-1 (HSV1), herpes simplex virus-2 (HSV2), varicella-zoster virus (VZV), parvo-virus B19, human parainfluenza viruses (HPIVs), yellow fever virus (YFV), human herpesvirus 6 (HHV-6), cytomegalovirus (CMV), coxsackievirus and adenovirus.
The following electronic databases were searched for relevant literature published from 2009 to 2019: EBSCOhost, PubMed, ScienceDirect, Scopus and Web of Science. The search was run on 9 April 2019 and updated via PubMed on 30 September 2019 with no new eligible studies to include.
Data extraction
Study characteristics and outcomes of interests were extracted from the included studies on a predesigned data extraction form by two independent reviewers (JP and HSH). Prior to use by the two reviewers, the reliability of the extraction form was assessed by piloting 10 randomly selected articles that met the inclusion criteria. The study team resolved any disagreements in data extraction through consensus in consultation with RM. In cases where studies were in German, HSH provided translation. In cases where studies were not available in English or German, Google translate was used to translate the article to English.11
Data synthesis and analysis
A random-effects model was fitted to the study data as it included data taken from a series of independently performed studies in different populations. We assessed heterogeneity by calculating I2 statistics (threshold I2>40%). The values of I2 were categorised for heterogeneity as follows: ‘not important’ (40%), ‘moderate’ (40% to 60%) and ‘considerable’ (60% to 80%) and ‘substantial’ (80% to 100%). Where ‘not important’ or ‘moderate’ heterogeneity existed between studies (I2≤60%), pooled outcome measures were reported with 95% CI for each respective outcome. Where ‘considerable’ or ‘substantial’ heterogeneity exists between studies (I2>60%), forest plots and prevalence ranges calculated using the random-effects model were used to narratively describe each outcome.
Risk of bias assessment
Each included study was assessed for risk of bias and quality using the Hoy et al tool for observational studies.12 13 Studies were judged as having ‘low risk’ if scored 8–10, ‘moderate risk’ if scored 5–7 and ‘high risk’ if scored 0–5. All risk of bias judgements were made by both JP and HSH. In case of disagreement in risk of bias and quality assessment, a final decision was made through consensus in consultation with RM.
Patient and public involvement
This review was developed as part of an ongoing project by the research team that aims to generate evidence to facilitate evidence-based decision-making of introducing routine hepatitis A vaccination in South Africa. The findings of this review contribute to the knowledge base that aims to enhance global vaccination strategies against viral-associated ALF. As this is a systematic review, no patient involvement was required; however, it is hoped that the findings of this review will help to highlight the burden that ALF places on populations without routine vaccination.
Results
The initial database searches yielded 6952 records, from which 3545 duplicates were removed. A further 3263 were excluded following the screening of titles and abstracts (figure 1). The full text of the remaining 144 records were screened by JP and HSH, from which 25 studies were deemed to meet the final inclusion criteria. Twenty-four (96%) of the included studies were cohort studies. As detailed in table 1, the included studies were published between 2009 and 2017. Included studies were conducted globally, with seven studies and three studies conducted in India and Pakistan, respectively. The populations represented by the included studies spanned all age groups and included participants primarily from hospital settings. As the data in this review were sourced from a variety of countries, age groups and settings, the heterogeneity was considerable and/or substantial for all results. Thus, we narratively and graphically reported estimates of combined prevalence rates and the spreads of prevalence.
Figure 1.
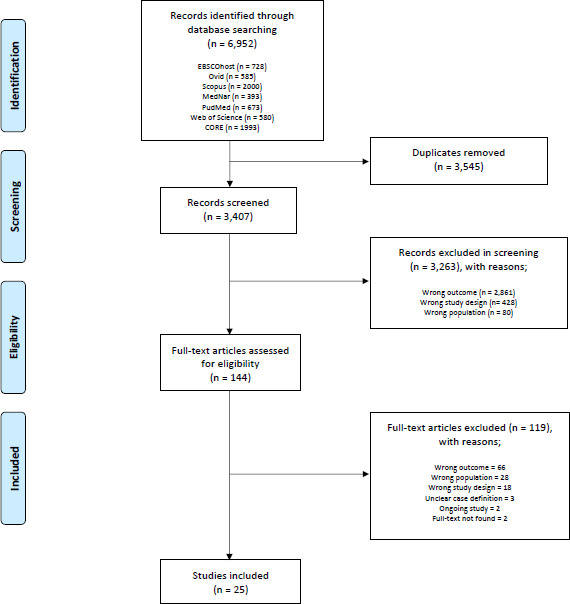
Flow diagram for selection of studies.
Table 1.
Characteristics of included studies
| Study | Study design | Aim | Country | Income level | Start of data collection | End of data collection | ALF case definition |
| Alam et al, 200916 | Prospective cohort | To evaluate the aetiology, complications and outcome of FHF | Bangladesh | Lower middle | 3 Nov | 8 May | Occurrence of hepatic encephalopathy within 8 weeks of onset of jaundice in patients with no previous liver disease and the presence of coagulopathy as proved by a PT>15 s or INR>1.5 |
| Asim et al, 200917 | Cross sectional | To analyse serum samples from patients with ALF for hepatitis A-G viral markers | India | Lower middle | 1 Jun | 4 May | Patient become deeply jaundiced and went into hepatic encephalopathy within 8 weeks of onset of the disease, with no history of chronic hepatitis |
| Bechmann et al, 201418 | Retrospective cohort | To identify currently predominant aetiologies of ALF at a transplant centre | Germany | High | 1 Jan | 12 Feb | Acute Liver Failure Study Group Germany case definition: INR>1.5 and encephalopathy of any grade. Pre-existing liver disease and systemic cause of liver failure were excluded |
| Bhatia et al, 201319 | Prospective cohort | To analyse clinical features, liver function tests, hepatitis viral markers and clinical outcomes in patients with ALF | India | Lower middle | Jun 99 | 1 Jan | Development of hepatic encephalopathy within 26 weeks of the first symptoms of acute hepatitis-like illness without any history of underlying liver disease |
| Borkakoti et al, 201320 | Prospective cohort | To determine the viral load of HEV and its association with the disease severity in patients with ALF in comparison with patients with ALF due to other hepatides | India | Lower middle | 6 Jan | 11 Dec | Development of encephalopathy within 8 weeks of the onset of jaundice without any history of chronic liver disease; diagnosed as a self-limiting disease and a serum aspartate aminotransferase elevation of at least fivefold or clinical jaundice or both |
| Bravo et al, 201221 | Prospective and retrospective cohort | To investigate the aetiology, outcomes and incidence of AHF among children 0–18 years old | Philippines | Lower middle | Jan 00 | 6 Dec | Onset of coagulopathy and/or encephalopathy ≤4 weeks after the onset of symptoms, a prothrombin time >2, an increased bilirubin and evidence for liver failure complicated by encephalopathy |
| Cervio et al, 20113 | Retrospective cohort | To investigate the impact of HAV UI on the trends in the occurrence of FHF in children | Argentina | High | Mar 93 | 5 Jul | Mieli-Vergani case definition: a multisystem disorder in which severe impairment of liver function, with or without encephalopathy, occurs in association with hepatocellular necrosis in a patient with or without recognised underlying chronic liver disease22 |
| Das et al, 201623 | Prospective cohort | To determine the profile of ALF etiologies | India | Lower middle | 7 Jan | 15 Dec | History of development of encephalopathy within 8 weeks of disease onset |
| Gupta et al, 201524 | Retrospective cohort | To determine the profile of hepatitis A, B, C and E as a cause of AHF in children in a tertiary care hospital | India | Lower middle | 11 Jan | 14 Dec | Elevated ALT levels or AST of at least fivefold with clinical jaundice and without evidence of chronic liver disease. Patients who had INR>1.5 with encephalopathy or INR>2 without encephalopathy |
| Ho et al, 201425 | Prospective cohort | To investigate the incidence, aetiology, outcomes and prognostic factors of ALF | Taiwan | High income | 05 Jan | 07 Sep | International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 570.0 |
| Latif et al, 201026 | Prospective cohort | To identify the risk factors for FHF and their relationship with the outcome in children | Pakistan | Lower middle | 6 Sep | 7 Feb | Development of encephalopathy within 8 weeks of the onset of jaundice having evidence of coagulopathy, that is, PT deranges >4 s of control and deranged liver function that is, TSB>1.5 mg/dL, AT>40 IU/L |
| Mamun et al, 200927 | Retrospective cohort | To assess the burden of HEV as a cause of ALF | Bangladesh | Lower middle | 4 Jun | 6 Dec | Previously healthy patients who presented with severe impairment of hepatocellular function, that is, encephalopathy, coagulopathy and jaundice, within 6 months of onset of symptoms |
| Manka et al, 201528 | Retrospective cohort | To investigate the causes of previously diagnosed indeterminate cases ALF | Germany | High | 6 Nov | 13 Dec | Significant liver dysfunction with pathologically increased laboratory parameters (AST, ALT, AP), an existing coagulopathy in terms of an INR>1.5, and with the concomitant presence of any degree of encephalopathy |
| Mendizabal et al, 201429 | Retrospective cohort | To determine the causes and short-term outcomes of ALF | Argentina | High | 5 Jun | 11 Dec | Presence of coagulopathy (INR> 1.5 or prothrombin index <50%)and any grade of HE within 26 weeks of the first symptoms without a known underlying liver disease |
| Mishra et al, 201630 | Retrospective cohort | To assess the relative efficacy of HEV antigen detection by ELISA in patients with ALF | India | Lower middle | 13 Nov | 15 Jan | Any evidence of coagulation abnormality, generally INR>1.5 and any degree of mental alteration (encephalopathy) without pre-existing cirrhosis and with an illness of <4 weeks duration |
| Mumtaz et al, 200931 | Prospective cohort compared with historical control | To assess the aetiology, prothrombin time (PT), alanine aminotransferase, creatinine, albumin for non-acetaminophen-induced ALF | Pakistan | Lower middle | Jan 00 | 7 Mar | Rapid development of acute liver injury with impaired synthetic function and encephalopathy in a person who previously had a normal liver |
| Pandit et al, 201532 | Retrospective cohort | To assess the frequency of hepatotropic viruses as aetiological agents of ALF | India | Lower middle | 3 Jan | 5 Dec | Onset of encephalopathy ≤28 days after the onset of symptoms with INR>2 and increased bilirubin complicated by encephalopathy in patients without a previous history of liver disease |
| Poovorawan et al, 201333 | Prospective cohort | To determine the causes and outcomes of Thai children with AHF | Thailand | Upper middle | 2 Jan | 5 Sep | International Association for the Study of the Liver case definition: (Tandon et al, 1999)9 |
| Schwarz et al, 201434 | Retrospective cohort—Patient registry | To analyse results of viral testing among non-acetaminophen ALF study participants | USA/Canada/UK | High | Dec 99 | 12 Dec | No known evidence of chronic liver disease, with evidence of acute liver injury, and hepatic-based coagulopathy not corrected by vitamin K with the follow parameters: PT≥15 s or INR≥1.5 in the presence of clinical HE or a PT≥20 s or INR≥2.0 regardless of the presence or absence of clinical HE |
| Shalimar et al, 201735 | Retrospective cohort | To assess the differences in the course of HEV-ALF as compared with other aetiologies of ALF | India | Lower middle | Jan 86 | 15 Dec | International Association for the Study of Liver (IASL) case definition: Occurrence of encephalopathy within 4 weeks from the onset of symptoms in the absence of pre-existing liver disease |
| Silverio et al, 201536 | Retrospective cohort | To describe the clinical features of children treated for ALF | Cuba | Upper middle | 5 Jan | 11 Dec | Evidence of liver damage in the absence of prior known chronic liver disease; altered coagulation, expressed as PT>15 s with encephalopathy; or PT>20 s with or without encephalopathy—all this within 8 weeks of onset of clinical symptoms |
| Somasekar et al, 201737 | Retrospective cohort | To investigate the causes of previously diagnosed indeterminate cases ALF | USA | High | Jan 98 | 10 Dec | US Acute Liver Failure Study Group case definition |
| Uddin Jamro et al, 201338 | Retrospective cohort | To study the aetiology, outcome and risk factors for FHF in children at a tertiary care hospital | Pakistan | Lower middle | 7 Jul | 12 Jun | Presence of acute liver failure (coagulopathy PT>20 s or INR>2), HE without pre-existing liver disease, within 8 weeks of the onset of clinical liver disease |
| Tsunoda et al, 201739 | Prospective cohort | To identify the roles of CMV, EBV and HHV in immunocompetent children with ALF not resulting from hepatitis virus | Japan | High | 7 Jan | 13 Dec | Liver dysfunction with elevated AST and ALT>30 IU/L |
| Zhao et al, 201440 | Retrospective cohort | To investigate aetiologies and outcomes of children with ALF | China | Middle | 7 Jan | 12 Dec | Coagulopathy (PTA≤40% or INR≥1.5 excluding haematologic diseases) and jaundice (Tbil ≥ 171 μmol/L) within 4 weeks in a child without pre-existing liver diseases |
AHF, acute hepatic failure; ALF, acute liver failure; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ELISA, enzyme-linked immunosorbent assay; FHF, fulminant hepatic failure; HE, hepatic encephalopathy; HEV, hepatitis E virus; HHV, human herpesvirus; INR, international normalised ratio; PT, prothrombin time; PTA, plasma thromboplastin antecedent; s, second; TSB, total serum bilirubin.
Vaccine-preventable viral-induced ALF
We narratively report the prevalence of HAV-induced and HBV-induced ALF by country immunisation status. The point prevalence of HAV-induced ALF in countries with no routine HAV immunisation at the time of data collection ranged from 2% to 81% with a combined rate of 27% (95% CI 13% to 43%), while the prevalence in countries with routine HAV immunisation at the time of data collection ranged from 1% to 2% with a combined of rate of 2% (95% CI 1% to 3%) (figure 2). In Argentina, the prevalence of HAV-induced ALF prior to routine immunisation was approximately 50% (95% CI 45% to 55%), compared with approximately 1% (95% CI 0% to 5%) after immunisation was introduced. The point prevalence of HBV-induced ALF in countries without universal HBV immunisation at the time of data collection ranged from 16% to 27% with a combined rate of 22% (95% CI 16% to 30%) (figure 3). The point prevalence of HBV-induced ALF in countries with universal HBV immunisation at the time of data collection ranged from 0% to 83% with a combined rate of 20% (95% CI 8% to 35%).
Figure 2.
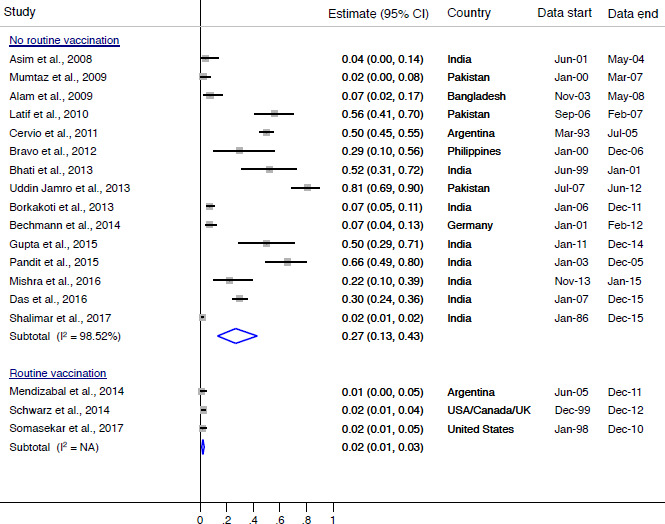
Prevalence of HAV-induced ALF by country HAV immunisation status. ALF, acute liver failure; HAV, hepatitis A virus; I2, heterogeneity statistic.
Figure 3.
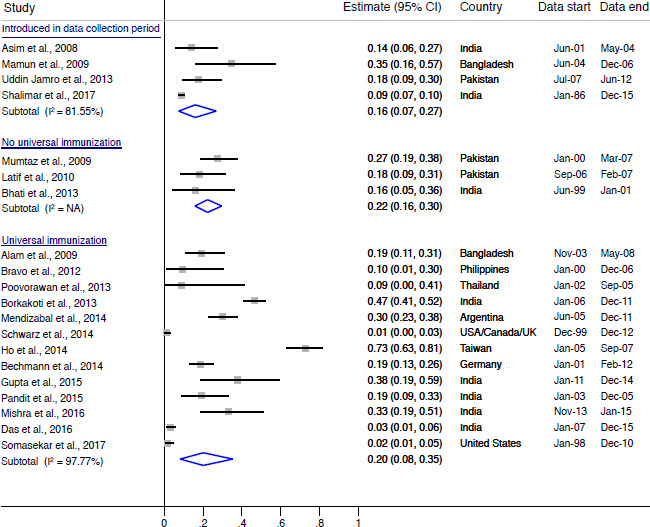
Prevalence of HBV-induced ALF by country HBV immunisation status. ALF, acute liver failure; HBV=hepatitis B virus; I2, heterogeneity statistic.
ALF attributable to non-vaccine-preventable viral infections
The point prevalence of HCV-induced ALF ranged from 2% to 25% with a combined rate of 9% (95% CI 1% to 21%) (online supplementary figure 1). The point prevalence of HEV-induced ALF ranged from 3% to 70% with a combined rate of 32% (95% CI 24% to 41%) (online supplementary figure 2). The point prevalence of HDV, HHV/HSV, CMV and EBV-induced ALF were estimated to have combined prevalences of 4% (95% CI 0% to 13%), 6% (95% CI 1% to 12%), 13% (95% CI 1% to 35%) and 6% (95% CI 0% to 24%), 10% (95% CI 2% to 22%), 2% (95% CI 0% to 5%) and 1% (95% CI 0% to 5%), respectively (online supplementary figure 3). Data were not available to estimate the burden of ALF after infection with HDV, VZV, HPIVS, YFV, CA16 and/or HAdVs as outlined per the published protocol.10
bmjopen-2020-037473supp002.pdf (74.3KB, pdf)
bmjopen-2020-037473supp003.pdf (49.7KB, pdf)
bmjopen-2020-037473supp004.pdf (63.9KB, pdf)
Outcomes of viral-induced ALF
The narratively reported outcomes of viral-induced ALF were found to be severe. The mortality rates associated with viral-induced ALF in lower middle income countries ranged from 18% to 91% with a combined mortality rate of 50% (95% CI 36% to 64%) (figure 4A). The mortality rates associated with viral-induced ALF in upper middle income countries ranged 3% to 45% with a combined mortality rate of 26% (95% CI 1% to 63%) (figure 4A). The mortality rates associated with viral-induced ALF in high-income countries ranged from 12% to 40% with a combined mortality rate of 29% (95% CI 17% to 43%) (figure 4A). The rate of encephalopathy associated with viral-induced ALF cases in children ranged from 69% to 100% with a combined rate of 89% (95% CI 79% to 97%) (figure 4B). The need for liver transplantation with viral-associated ALF ranged from 4% to 62% with a combined rate of 25% (95% CI 6% to 53%) (figure 4B). The need for renal transplant in viral-associated ALF cases ranged from 4% to 34% with a combined rate of 18% (95% CI 2% to 43%) (figure 4B).
Figure 4.
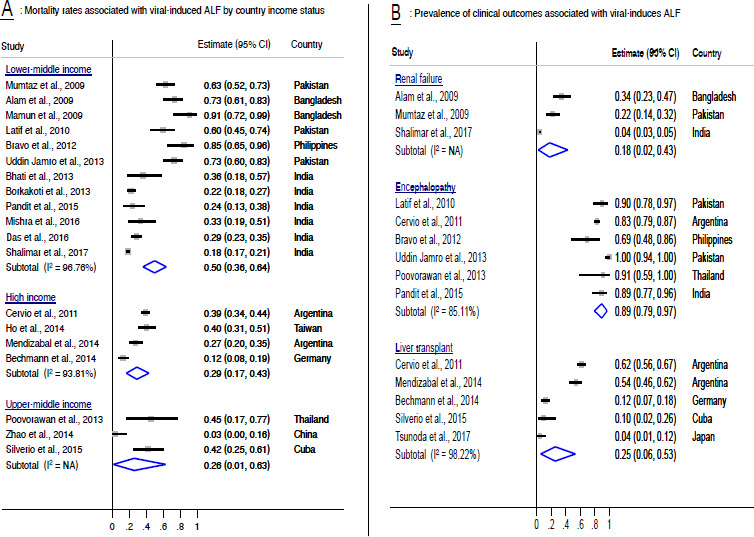
Prevalence of outcomes associated with viral-induced ALF. ALF, acute liver failure; I2, heterogeneity statistic; NA, not applicable.
Methodological quality
Risk of bias scores were assigned by two reviewers (JP and HSH) and are described in online supplementary table 1. Overall, a majority of the included studies were judged as having ‘low risk’ of bias. Only one included study was judged as having ‘moderate risk’ of bias due to lack of clarity around the representativeness of the study population to the national population, methods of participant selection and methods employed to reduce the likelihood of non-response.
bmjopen-2020-037473supp001.pdf (65KB, pdf)
Discussion
This systematic review estimated the burden of ALF after infection with HAV, HBV, HCV, HEV, HSV/HHV, CMV, EBV and parvovirus B19. The prevalence of HAV-induced ALF is markedly lower in countries with routine HAV immunisation, while HEV was the most common aetiological cause of viral-induced ALF reported in this review. In addition, viral-induced ALF had poor outcomes as indicated by high fatality rates, which seem to increase with poor economic status of the studied countries.
The estimated prevalence of HAV-induced ALF in countries with routine HAV immunisation was markedly lower than the estimated prevalence in countries without routine HAV immunisation. When looking at countries with data before and after the introduction of routine HAV immunisation, the reduction of HAV-induced ALF due to vaccination is further highlighted. The combined prevalence of HBV-induced ALF was the same in settings with or without universal HBV immunisation. Countries without universal HBV immunisation programmes are likely to have weak healthcare systems; thus, the reported prevalence of HBV-induced ALF is assumed to be an underestimate of the true burden in these populations due to weak routine testing and reporting systems. Currently, there is one HEV vaccine (Hecolin) licensed in China that has shown promise with a high degree of efficacy in preventing HEV genotype IV infection in healthy individuals 16–65 years.14 Further exploration of the efficacy of this vaccine for prevention of infection with genotypes I and II in different populations should be done to explore its application in different countries and HEV endemicity settings.15
This review estimated the mortality rate for viral-induced ALF to be approximately 50% in low-income and middle-income countries (LMICs) and less than 30% in upper-middle-income and high-income countries. Previous studies have estimated that mortality rates associated with ALF vary between 60% and 80%, depending on the disease aetiology as well as a patient’s access to care. Our review shows that viral-induced ALF still carries a significant mortality, though possibly lower than that reported for other ALF aetiologies.4 5 Mortality data largely come from hospitals with the capacity to diagnose viral-induced ALF, thus deaths outside of the hospital system or ALF deaths without virological testing may not be captured in these mortality estimates. Liver transplantation is required by approximately 25% of viral-induced ALF cases and approximately 18% of viral-induced ALF cases required renal transplantation, globally. In addition to general lack of resources for transplantation, a significant proportion of potential candidates have contraindications to transplant related to poor socioeconomic status in LMICs. The transplant data included in this review may only reflect successful and unsuccessful transplants, not those that were needed but not carried out due to resource constraints or contraindications.
This review is limited by lack of data for some of the viral aetiologies of ALF including for VZV, HPIVs, YFV, CA16 and/or HAdVs, which may have led to an underestimation of the global burden of viral-induced ALF. Additionally, we believe that our findings underestimate the global burden of viral-induced ALF as some important causes of ALF (eg, HSV/HHV) are believed to be under-recognised as they require PCR testing for diagnosis. The included studies also used varying methods of virus detection including serology and molecular tests which further added to the heterogeneity in the results of our review. This is a well-recognised limitation in studies of ALF where diagnostics are often limited by cost in under-resourced regions where viral causes of ALF are more prevalent. The limited availability of data, including lack of same country data on burden of disease before and after introduction of immunisation, hindered most of the planned subgroup analyses outlined in the study protocol. Where data were available, high heterogeneity of the data led to planned meta-analyses and meta-regression analyses not being possible. Lastly, the diversity of viruses attributable to ALF cases led to low statistical power in meta-analyses conducted.
Future research should assess the burden of viral-induced ALF after infection with HDV, VZV, HPIVS, YFV, CA16 and HAdVs. Collectively, high-quality data on all viral aetiologies of ALF would allow for better pooling of results. The review team encourages future studies to incorporate health economic estimates and mathematical modelling where data permit to assist health policy decision-makers to better design strategies for the prevention and management of viral-induced ALF. Epidemiological-economic modelling of immunisation against HAV, HBV and HEV may well show that introduction of vaccination could lead to future cost savings in the long run due to prevented medical care and liver failure.
Conclusions
We successfully addressed the aim of the study, although data on VZV, HPIVs, YFV, CA16 and/or HAdVs were missing. Notwithstanding the noted limitations, it is clear that HAV, HBV and HEV—vaccine-preventable ALF aetiologies—account for a large proportion of ALF (approximately 21%, 20%, 32% of viral-induced ALF cases, respectively). The burden of ALF that is associated with vaccine-preventable ALF aetiologies should be used in conjunction with other available key evidence to inform practice and policies on immunisation, particularly in LMICs. A majority of LMICs have established universal vaccination against HBV. The WHO has recently recommended the introduction of an HBV birth dose which is aimed at elimination of the virus and, if successful, will subsequently reduce the burden of HBV-induced ALF. Routine HAV immunisation in LMICs, however, is lacking. More data are urgently needed to guide routine use of the vaccine in prevention of morbidity and mortality caused by the virus. Lastly, further applicability of HEV vaccines should be explored, especially in LMICs where resources for managing viral-induced ALF are glaringly lacking.
Supplementary Material
Footnotes
Contributors: JP, GDH, BMK and RM conceived this study. JP implemented the review under the supervision of RM. JP and HSH performed the study search, screening and extraction of data under the guidance of RM. GDH and BMK provided methodological expertise for this review. SS, LG, MS, and WS provided content expertise for this review and all authors will provide comments on the final manuscript before publication. JP is the guarantor of this review.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The Vaccines for Africa Initiative (VACFA) has funded the costs associated with the research and dissemination of the results, including publications.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. All data were taken from published articles available in the public domain.
References
- 1.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, Clinical practice guidelines panel, Wendon, J, et al. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047–81. 10.1016/j.jhep.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Morabito V, Adebayo D. Fulminant hepatitis: definitions, causes and management. Health 2014;06:1038–48. 10.4236/health.2014.610130 [DOI] [Google Scholar]
- 3.Cervio G, Trentadue J, D'Agostino D, et al. Decline in HAV-associated fulminant hepatic failure and liver transplant in children in Argentina after the introduction of a universal hepatitis: a vaccination program. Hepat Med 2011;3:99–106. 10.2147/HMER.S22309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet 2010;376:190–201. 10.1016/S0140-6736(10)60274-7 [DOI] [PubMed] [Google Scholar]
- 5.Wlodzimirow KA, Eslami S, Abu-Hanna A, et al. Systematic review: acute liver failure - one disease, more than 40 definitions. Aliment Pharmacol Ther 2012;35:1245–56. 10.1111/j.1365-2036.2012.05097.x [DOI] [PubMed] [Google Scholar]
- 6.Lee WM, Stravitz RT, Larson AM. Introduction to the revised American association for the study of liver diseases position paper on acute liver failure 2011. Hepatology 2012;55:965–7. 10.1002/hep.25551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spearman CWN, McCulloch M, Millar AJW, et al. Liver transplantation at red cross war Memorial children's Hospital. S Afr Med J 2006;96:960–3. [PubMed] [Google Scholar]
- 8.O'Grady JG, failure Aliver. Acute liver failure. Postgrad Med J 2005;81:148–54. 10.1136/pgmj.2004.026005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandon BN, Bernauau J, O'Grady J, et al. Recommendations of the International association for the study of the liver Subcommittee on nomenclature of acute and subacute liver failure. J Gastroenterol Hepatol 2002;14:403–4. 10.1046/j.1440-1746.1999.01905.x [DOI] [PubMed] [Google Scholar]
- 10.Patterson J, Hussey HS, Abdullahi LH, et al. The global epidemiology of viral-induced acute liver failure: a systematic review protocol. BMJ Open 2019;9:e029819. 10.1136/bmjopen-2019-029819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balk E, Ching M, Chen M, et al. Assessing the accuracy of Google translate to allow data extraction from trials published in non-English languages. Rockville, USA: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 12.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 13.Werfalli M, Musekiwa A, Engel ME, et al. The prevalence of type 2 diabetes mellitus among older people in Africa: a systematic review study protocol. BMJ Open 2014;4:e004747. 10.1136/bmjopen-2013-004747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S-W, Zhao Q, Wu T, et al. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother 2015;11:908–14. 10.1080/21645515.2015.1008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Chen P, Lin H, et al. Hepatitis E virus: current epidemiology and vaccine. Hum Vaccin Immunother 2016;12:2603–10. 10.1080/21645515.2016.1184806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam S, Azam G, Mustafa G, et al. Natural course of fulminant hepatic failure: the scenario in Bangladesh and the differences from the West. Saudi J Gastroenterol 2009;15:229–33. 10.4103/1319-3767.56094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asim M, Singla R, Gupta RK, et al. Clinical & molecular characterization of human TT virus in different liver diseases. Indian J Med Res 2010;131:545–54. [PubMed] [Google Scholar]
- 18.Bechmann LP, Manka P, Best J, et al. [Drug-induced liver injury as predominant cause of acute liver failure in a monocenter study]. Dtsch Med Wochenschr 2014;139:878–82. 10.1055/s-0034-1369932 [DOI] [PubMed] [Google Scholar]
- 19.Bhatia V, Dhawan A, Arora NK, et al. Urinary potassium loss in children with acute liver failure and acute viral hepatitis. J Pediatr Gastroenterol Nutr 2013;57:102–8. 10.1097/MPG.0b013e31828fc8ea [DOI] [PubMed] [Google Scholar]
- 20.Borkakoti J, Hazam RK, Mohammad A, et al. Does high viral load of hepatitis E virus influence the severity and prognosis of acute liver failure during pregnancy? J Med Virol 2013;85:620–6. 10.1002/jmv.23508 [DOI] [PubMed] [Google Scholar]
- 21.Bravo LC, Gregorio GV, Shafi F, et al. Etiology, incidence and outcomes of acute hepatic failure in 0-18 year old Filipino children. Southeast Asian J Trop Med Public Health 2012;43:764–72. [PubMed] [Google Scholar]
- 22.Dhawan A, Cheeseman P, Mieli-Vergani G. Approaches to acute liver failure in children. Pediatr Transplant 2004;8:584–8. [DOI] [PubMed] [Google Scholar]
- 23.Das AK, Begum T, Kar P, et al. Profile of acute liver failure from north-east India and its differences from other parts of the country. Euroasian J Hepatogastroenterol 2016;6:111–5. 10.5005/jp-journals-10018-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta P, Mittal M, Bhat NK, et al. A hospital based retrospective study on hepatotropic viruses as a cause of acute viral hepatitis in children in Uttarakhand, India. Indian J Comm Health 2015;27:451–5. [Google Scholar]
- 25.Ho C-M, Lee C-H, Wang J-Y, et al. Nationwide longitudinal analysis of acute liver failure in Taiwan. Medicine 2014;93:e35. 10.1097/MD.0000000000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latif N, Mehmood K. Risk factors for fulminant hepatic failure and their relation with outcome in children. J Pak Med Assoc 2010;60:175–8. [PubMed] [Google Scholar]
- 27.Mamun Al M, Rahman S, Khan M, et al. HEV infection as an aetiologic factor for acute hepatitis: experience from a tertiary hospital in Bangladesh. J Health Popul Nutr 2009;27:14–19. 10.3329/jhpn.v27i1.3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manka P, Bechmann LP, Coombes JD, et al. Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin Gastroenterol Hepatol 2015;13:1836–42. 10.1016/j.cgh.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 29.Mendizabal M, Marciano S, Videla MG, et al. Changing etiologies and outcomes of acute liver failure: perspectives from 6 transplant centers in Argentina. Liver Transpl 2014;20:483–9. 10.1002/lt.23823 [DOI] [PubMed] [Google Scholar]
- 30.Mishra S, Borkakoti J, Kumar S, et al. Role of HEV antigen detection in HEV-related acute viral hepatitis and acute liver failure. J Med Virol 2016;88:2179–85. 10.1002/jmv.24567 [DOI] [PubMed] [Google Scholar]
- 31.Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int 2009;3:563–70. 10.1007/s12072-009-9151-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandit A, Mathew LG, Bavdekar A, et al. Hepatotropic viruses as etiological agents of acute liver failure and related-outcomes among children in India: a retrospective hospital-based study. BMC Res Notes 2015;8:381. 10.1186/s13104-015-1353-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poovorawan Y, Chongsrisawat V, Shafi F, et al. Acute hepatic failure among hospitalized Thai children. Southeast Asian J Trop Med Public Health 2013;44:50–3. [PubMed] [Google Scholar]
- 34.Schwarz KBO, Dell D, Lobritto SJ, et al. For the pediatric acute liver failure study, group. Analysis of viral testing in Nonacetaminophen pediatric acute liver failure. J Pediatr Gastroenterol Nutr 2014;59:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedia S, Gunjan D, Mahapatra SJ, et al. Acute liver failure due to hepatitis E virus infection is associated with better survival than other etiologies in Indian patients. Dig Dis Sci 2017;62:1058–66. 10.1007/s10620-017-4461-x [DOI] [PubMed] [Google Scholar]
- 36.Silverio CE, Smithen-Romany CY, Hondal NI, et al. Acute liver failure in Cuban children. MEDICC Rev 2015;17:48–54. [DOI] [PubMed] [Google Scholar]
- 37.Somasekar S, Lee D, Rule J, et al. Viral surveillance in serum samples from patients with acute liver failure by metagenomic next-generation sequencing. Clin Infect Dis 2017;65:1477–85. 10.1093/cid/cix596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin Jamro BMC S, Mal Makheja P, Ahmed Soomro A. Etiology, outcome and risk factors for fulminant hepatic failure in children at a tertiary care Hospital, Sukkur, Pakistan. Rawal Medical Journal 2013;38:219–22. [Google Scholar]
- 39.Tsunoda T, Inui A, Iwasawa K, et al. Acute liver dysfunction not resulting from hepatitis virus in immunocompetent children. Pediatr Int 2017;59:551–6. 10.1111/ped.13249 [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Wang C-Y, Liu W-W, et al. Acute liver failure in Chinese children: a multicenter investigation. Hepatobiliary Pancreat Dis Int 2014;13:276–80. 10.1016/S1499-3872(14)60041-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037473supp002.pdf (74.3KB, pdf)
bmjopen-2020-037473supp003.pdf (49.7KB, pdf)
bmjopen-2020-037473supp004.pdf (63.9KB, pdf)
bmjopen-2020-037473supp001.pdf (65KB, pdf)


