Abstract
Regular exercise is a formidable regulator of insulin sensitivity and overall systemic metabolism through both acute events driven by each exercise bout and through chronic adaptations. As a result, regular exercise significantly reduces the risks for chronic metabolic disease states, including type 2 diabetes and non-alcoholic fatty liver disease. Many of the metabolic health benefits of exercise depend on skeletal muscle adaptations; however, there is plenty of evidence that exercise exerts many of its metabolic benefit through the liver, adipose tissue, vasculature and pancreas. This review will highlight how exercise reduces metabolic disease risk by activating metabolic changes in non-skeletal-muscle tissues. We provide an overview of exercise-induced adaptations within each tissue and discuss emerging work on the exercise-induced integration of inter-tissue communication by a variety of signalling molecules, hormones and cytokines collectively named ’exerkines’. Overall, the evidence clearly indicates that exercise is a robust modulator of metabolism and a powerful protective agent against metabolic disease, and this is likely to be because it robustly improves metabolic function in multiple organs.
Keywords: Adipose tissue, Endothelium, Exercise, Exerkines, Liver, Muscle, NAFLD, Pancreas, Review, Type 2 diabetes
Graphical Abstract
Introduction
Regular exercise can reduce the risks for developing obesity [1] and the metabolic complications and disease associated with obesity, including non-alcoholic fatty liver disease (NAFLD) [2] and type 2 diabetes [3]. Exercise has these powerful effects on metabolism, not only because of its well-known effects on skeletal muscle metabolism, but also as a result of the metabolic adaptations it confers in multiple other tissues. Herein we provide an overview of the evidence that exercise is a powerful tool for the prevention of metabolic disease and that it exerts its protective effects by improving the metabolic phenotypt of non-skeletal-muscle tissues, including the liver, vasculature, adipose tissue and pancreas. We also highlight that these multi-tissue adaptations occur not only through exercise activating intrinsic signalling events in each tissue but also through the unique, exercise-induced integration of inter-tissue communication by a variety of signalling molecules, hormones, cytokines, changes in substrate flux and blood flow. Rather than drilling down on specific mechanisms of action, this perspective provides a broad overview. We also seek to leverage this evidence to suggest that a certain minimum volume of exercise or physical activity is required for normal metabolic function.
Overview of metabolic disease pathology
Insulin resistance, the inability of insulin to effectively stimulate glucose uptake into metabolic tissues (skeletal muscle, adipose and liver), turn off hepatic glucose production and reduce adipose lipolysis, is a primary event underlying type 2 diabetes. Insulin resistance not only contributes to hyperglycaemia in type 2 diabetes but also putatively plays a role in the inappropriate excess storage of fat (ectopic) in the liver (hepatic steatosis) [4], In turn, higher levels of ectopic storage of lipids in muscle and the liver is also associated with insulin resistance. Causality between hepatic steatosis, intramuscular lipid storage and insulin resistance remains controversial, but evidence clearly shows that insulin resistance is often associated with greater ectopic fat storage [5]. For reasons not understood, the liver becomes selectively insulin resistant in controlling glucose output but remains highly sensitive to the lipogenic effects of insulin [6], Finally, insulin resistance also plays a fundamental role in reduced metabolic flexibility, which is defined as the capacity to switch between metabolic substrates depending on which is most readily available [7].
Inflammation, oxidative stress and endoplasmic reticulum stress have also been linked to metabolic dysfunction through both lipid- and non-lipi-mediated pathways (for an extensive review, see [8]). In general, these pathways are believed to impair molecular insulin signalling pathways in metabolic tissues, resulting in tissue-specific and systemic metabolic alterations that, when combined with chronic positive energy balance, can lead to NAFLD and type 2 diabetes. A prevailing view is that obesity precedes and causes insulin resistance, and this is followed by hyper insulin secretion by the pancreatic beta cells to compensate for impaired insulin signalling [9], However, emerging evidence indicates that hyperinsulinaemia is likely to contribute to metabolic dysfunction and the development of obesity in a fee-forward manner [10]. This effect is witnessed in humans who transition from highly active to very inactive lifestyles for 1–2 weeks, and display elevated insulin responses paired with rapid gains in adiposity [11,12].
Exercise and prevention of metabolic disease
Only recently has the biomedical scientific community fully recognised the powerful effects of exercise in the prevention and treatment of metabolic disease, even proving to be more beneficial than some low-performing pharmacological agents. Type 2 diabetes and NAFLD are two primary metabolic disease states with prevalence rates that are increasing at epidemic rates but can be prevented with regular exercise.
Prevention of type 2 diabetes
A daily threshold for a relatively small volume of physical activity (>3,500 steps/day or >20 min/day) [13, 14] has beerl shown to be protective in reducing the transition of those with impaired glucose tolerance to frank type 2 diabetes. Overall, pooled results show that 150 min/week of moderate to vigorous physical activity will reduce the risk for type 2 diabetes by 30% [15]. More information on this effect has been reviewed previously [3]. Individuals who maintain a long-term habit of moderate volume but vigorous running have a significantly reduced risk for obesity and type 2 diabetes [16] and, in a more recent report, indices of volume, distance and intensity in leisure runners were all linearly associated with reduced risk for type 2 diabetes [17]. Furthermore, studies have repeatedly shown that a threshold of measured cardiorespiratory fitness (i.e. aerobic capacity or maximum oxygen consumption [VO2max]) of ~9–10 metabolic equivalents (METs) also lowers risk for transitioning to a diagnosis of type 2 diabetes [18].
Prevention of NAFLD
Excessive intrahepatic fat storage (hepatic steatosis), is the entryway to NAFLD, an umbrella condition that also encompasses steatohepatitis, fibrosis and cirrhosis. The development of hepatic steatosis also increases the risk for type 2 diabetes. Despite being an important target for the pharmacological industry, NAFLD currently has no treatment other than lifestyle interventions, including weight loss and exercise. Overall, there are far less data available on how exercise impacts NAFLD because it is a newer problem and because NAFLD can only be evaluated with invasive liver biopsies or with technologically advanced imaging methodologies. However, higher volumes of physical activity have been shown to reduce intrahepatic lipid levels [19]. Cardiorespiratory fitness, a measurable marker of regular exercise, has been shown to independently reduce the risk of NAFLD [20, 21], an effect that is independent of, or only moderately impacted by, obesity status [19].
Exercise also induces a large number of other positive effects, including improved weight management, improved bone density, reduced frequency and severity of cardiovascular disease and hypertension and of depression and anxiety, reduced risk for specific forms of cancer, reduced dementia, and improved strength, mobility and healthspan. In fact, increased risk for 35 chronic disease conditions have been independently linked to physical inactivity [22], leading us to speculate that daily physical activity and exercise may be required for normal health and function. Thus, our drive for technological advances and ease of daily living is working in opposition to our biology [22], The excessive consumption of hyperenergetic, nutrient-poor diets further synergises with inactivity to drive epidemic rates of metabolic disease.
There is no doubt that skeletal muscle adaptations to exercise are important; however, physical exercise is not possible without the orchestrated cooperation of several tissues to support muscular work and maintain metabolic homoeostasis. Exercise training therefore results in adaptations to other tissues involved in these processes, including adipose tissue, liver, endothelium and pancreas.
Muscle-centric vs integrative view of the impact of exercise on metabolic health
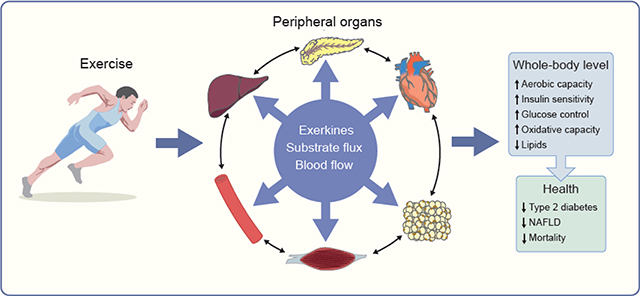
In the following sections, we discuss the acute (Fig. 1) and chronic (Fig. 2) effects of exercise on each of the peripheral organs involved in the regulation of whole-body insulin sensitivity and metabolic health, and the associated systemic health effects. Unless otherwise specified, only data from human studies are presented. Over the past years a growing body of work has implicated hundreds or even thousands of proteins secreted by the main peripheral tissues (i.e. myokines by skeletal muscle [23], hepatokines by the liver [24] and adipokines by adipose tissue [25]) involved in the regulation of energy homeostasis and whole-body insulin sensitivity. However, only a few have been characterised and had their biological function demonstrated. During exercise, some of these proteins, collectively named ’exerkines’, are secreted, and create a complex inter-organ network that contributes to the systemic metabolic health effects of exercise [26], We also posit that ’substrate flux’ between organs may work in concert with exerkines or independently to induce adaptations. Here we mention the molecular factors that have been recently identified and thought to potentially coordinate the inter-organ crosstalk in response to exercise (Fig. 3). Undoubtedly, numerous other signalling molecules will be identified in the years ahead, and future research will need to delineate the respective contribution of such molecules. In addition, because this area is still relatively new, controversies exist, and whether the findings from animals can be translated to humans is questionable.
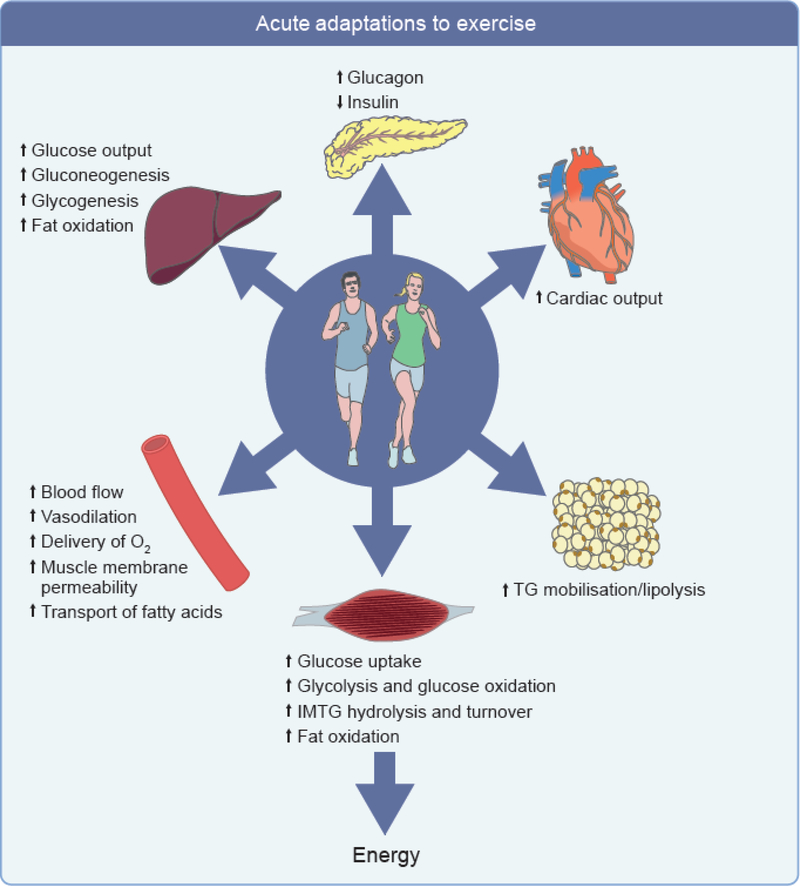
Fig. 1.
Acute metabolic effects of exercise on the key peripheral organs involved in the regulation of energy homeostasis. In response to acute exercise, muscle immediately mobilises stored glucose then fatty acids and takes up glucose and fatty acids from plasma to match energy demand. During sustained exercise, adipose tissue and the liver respectively mobilise NEFA and synthesise glucose to keep providing fuel to muscle. In the meantime, cardiac output is increased and microvascular perfusion of peripheral tissues, capillary recruitment, muscle membrane permeability and transport of substrates is increased. These changes are associated with changes in substrate fluxes and secretion of glucagon and insulin by the pancreas. IMTG, intramuscular triacylglyerols; TG, triacylglycerols.
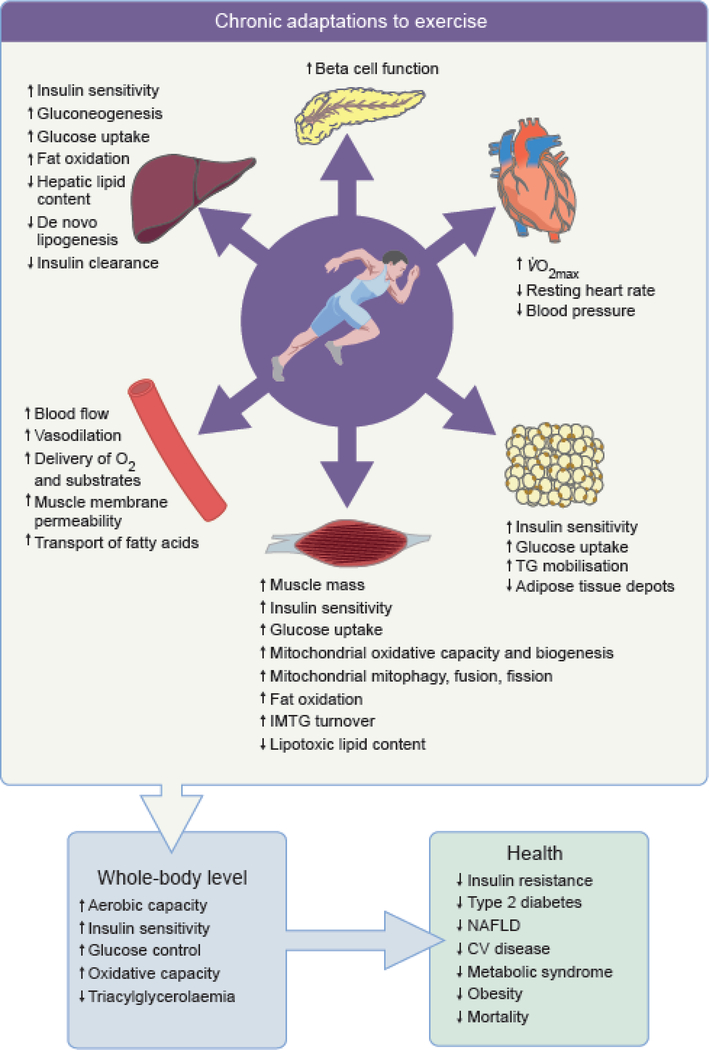
Fig. 2.
Chronic effects of exercise on key peripheral organs involved in the regulation of energy homeostasis and associated whole-body metabolic effects and systemic health effects.
Exercise training improves VO2max, decreases resting heart rate and blood pressure, and increases total muscle mass. Microvascular network is expanded and microvascular dilatory response is improved. Beta cell function is improved along with a greater blood glucose uptake by muscle, adipose tissue and liver and peripheral tissue insulin sensitivity is ameliorated. Capacity for mobilisation of NEFA from adipose tissue is improved along with a greater capacity of liver for glucose production and decrease in de novo lipogenesis. Capacity for oxidising fat in liver and muscle in association with greater mitochondrial oxidative capacity, biogenesis and dynamic. This results in reduced visceral adipose tissue depots and ectopic fat storage. Altogether these structural, functional and metabolic adaptations improve aerobic capacity, whole-body insulin sensitivity, glucose control, oxidative capacity and reduce triglycerolaemia and chronic inflammation. These changes reduce the risk of developing insulin resistance, type 2 diabetes, NAFL and CV diseases, the metabolic syndrome, obesity and, ultimately, early mortality.
CV, cardiovascular; IMTG, intramuscular triacylglycerols; TG, triacylglycerols.
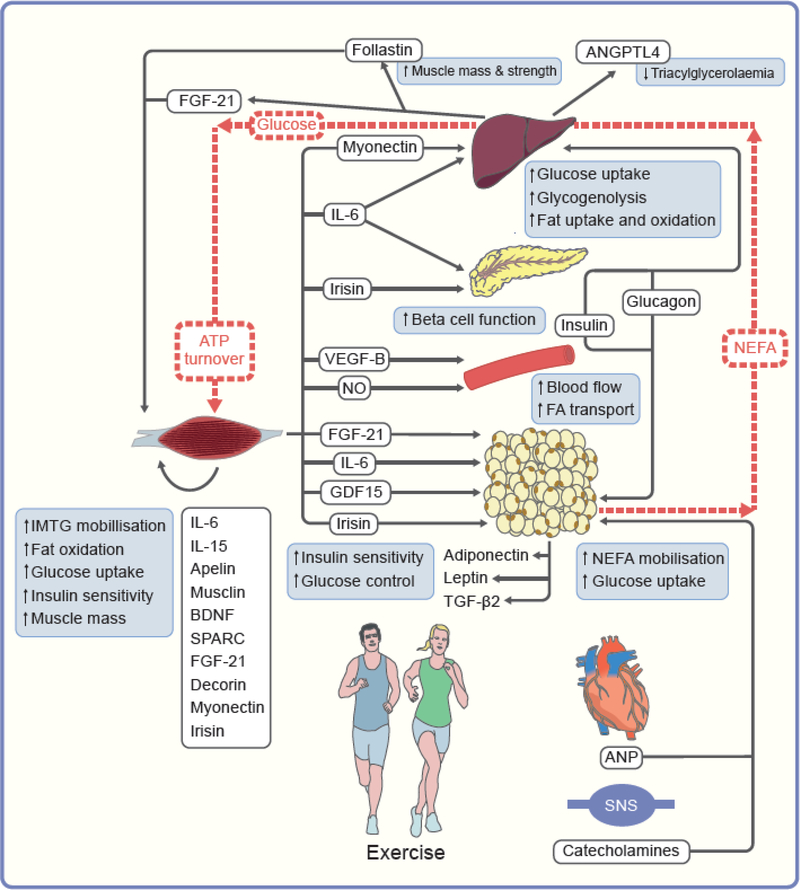
Fig. 3.
Inter-organ crosstalk and substrate fluxes during exercise. Based on recent data in animals and humans, the following cascade of events is hypothesised to occur during exercise. ATP turnover, glycogen depletion and/or muscle contraction trigger the secretion of myokines that will either act in a paracrine way and act on skeletal muscle mass and metabolism including IL-6 and IL-15, apelin, musclin and BDNF) or in an endocrine fashion on metabolism and function of liver (myonectin, IL-6), pancreas (IL-6), microvasculature (VEGF-B, NO) and adipose tissue (IL-6, FGF21, irisin, GDF15) or other tissues (SPARC and decorin). Pancreatic secretion of glucagon is increased and insulin is decreased, catecholamines are secreted by the sympathetic nervous system and ANP by the heart. These multiple actions concertedly contribute to activate skeletal muscle use of intramuscular fatty acid, fat oxidation, plasma glucose uptake and insulin sensitivity, stimulates adipose tissue NEFA mobilisation, and activates hepatic endogenous glucose production. Together these molecular factors are likely to contribute to the exercise health benefits, i.e. increase in muscle mass, reduced triacylglycerolaemia, improved insulin sensitivity and glucose control. Substrate fluxes are indicated by dotted red lines. ANGPTL4, angiopoietin-like 4;BDNF, brain-derived neurotrophic factor; FGF-21, fibroblast growth factor 21; GDF15, growth and differentiation factor 15; SNS, sympathetic nervous system; SPARC, secreted protein acidic and enriched in cysteine; TG, triacylglycerols; VEFG-B, vascular endothelial growth factor B.
Skeletal muscle adaptations
Skeletal muscle is the largest metabolic tissue in the human body and a critical site for glucose disposal both at rest and during exercise [27].During exercise, skeletal muscle uses both muscle glycogen stores and circulating plasma glucose as sources of fuel. Muscle contractions, even at low intensity and low volume [28], activate both oxidative and non-oxidative glucose disposal and glucose uptake via insulin-dependent and -independent mechanisms [29], optimising insulin action and both glucose oxidation and storage. Continuation of regular exercise further augments skeletal muscle oxidative capacity, mitochondrial biogenesis [30] and mitochondrial quality control mechanisms (mitophagy, fission, fusion), although this has been less well examined [31, 32], Endurance training promotes the trafficking of dietary fatty acids away from storage and towards oxidation via an increased capacity for myocyte fatty acid transport paired with increased fatty acid oxidation in mitochondria in both normal weight and overweight adults [33], In addition, exercise training increases intramuscular triacylglycerol turnover and reduces lipid intermediate (diacylglycerols and ceramides) content [34], Importantly, increased glucose utilisation by muscle during exercise would lead to hypoglycaemia if it was not paired with a rapid upregulation of hepatic glucose output. Thus, exercise also drives critical acute and chronic adaptations to hepatic metabolism.
As previously reviewed [26, 35], the skeletal muscle secretory response, including a constellation of myokines, extracellular vesicles and their cargo, and metabolites, has recently been implicated in exercise-mediated multisystemic adaptations that improve metabolic health. They can act in a paracrine/autocrine or endocrine manner. Although numerous molecular analytes have been detected, the functions of only a few are well established. For example, levels of brain-derived neurotrophic factor (BDNF), proteins belonging to the natriuretic peptide family (e.g. B-type natriuretic peptide [BNP]), musclin, IL-15, IL-6, apelin, secreted protein acidic and rich in cysteine (SPARC), fibroblast growth factor 21 (FGF-21), decorin, myonectin and irisin have been shown to increase in the circulation in response to exercise and these molecules are likely to play a role in the control of muscle mass and skeletal muscle metabolism in an autocrine fashion, as reviewed elsewhere [36, 37], The effect of some of these molecular factors have been studied in rodents or in in vitro studies but have not been (fully) confirmed in human studies. Circulating levels of IL-6, the first myokine to be discovered [38], rapidly increase in response to an acute bout of exercise [39], Its secretion seems to be influenced by the mechanical workload of the exercise [39], skeletal muscle glycogen content [40] and, potentially, glucose ingestion and/or plasma glucose levels [41]. IL-6 may therefore act as an energy sensor. In humans, exercise-released IL-6 stimulates the mobilisation of intramuscular triacylglycerol [42], fatty acid oxidation [42], translocation of GLUT4 from the cytosol to the membrane [43] and improves skeletal muscle insulin sensitivity [43].
Liver adaptations
During short bouts of activity and high-intensity exercise, muscles rely predominantly on intramuscular stores of glucose and fat. However, when exercise is sustained, a larger supply of substrates from outside the muscle is required [44], The requirement of glucose uptake for the working muscle must be paired with increased rates of hepatic glucose output to maintain euglycaemia [44], Exercise therefore increases hepato-splanchnic glucose flux, an effect that is not seen by sampling blood glucose, but, rather, requires tracer methodology to measure glucose turnover/flux. Exercise first increases the mobilisation of hepatic glycogen (the biggest glycogen store in the body) into plasma, and thisirs followed by increased rates of gluconeogenesis during longer exercise bouts [45], To fuel these processes, exercise also increases the uptake of the gluconeogenic precursors (lactate, pyruvate, glycerol) [46], Exercis-induced changes in gluconeogenesis are dependent on the rise in glucagon and the drop in insulin that occur during exercise [44], During each bout, the exercis-induced decrease in insulin sensitises the liver to the effects of glucagon. Exercise training, in the absence of weight loss, improves the ability of insulin to suppress glucose production by the liver [45], As in skeletal muscle, exercise stimulates a reduction in lipogenic processes and a simultaneous increase in lipid oxidation [47–49], which is likely to underlie the effects of habitual exercise in the prevention of NAFLD and maintenance of reduced intrahepatic lipid storage [19].
The liver faces other challenges during exercise, such as recycling metabolites, clearing toxic compounds, and buffering the by-products of lipid oxidation released by the muscle during exercise (e.g. medium-chain acylcarnitines) [45, 46], The liver also produces ketones, which fuel neuronal tissues during exercise if there has been a prolonged period since the last meal [50], Thus, via adaptive responses in glucose and fatty acid metabolism, a well-controlled crosstalk exists between the liver and muscles to exchange substrates and maintain metabolic homeostasis during exercise.
Changes in liver metabolism during exercise are regulated, at least in part, by exercise-released myokines. IL-6 enhances hepatic fat oxidation and glucose production during exercise [51]. Myonectin also improves systemic lipid metabolism. It does so by increasing liver fatty acid uptake through upregulation of fatty acid transporter genes, at least in rodents [52], In addition, during and immediately after exercise, liver hepatokines are released, including FGF21, follastin and angiopoietin-like 4 (ANGPTL4), which are involved in the regulation of circulating triacylglycerol concentrations, skeletal muscle mass and strength, and metabolism in rodents and humans [45, 53].
Adipose tissue adaptations
Whole-body fat oxidation rates increase with prolonged exercise or physical activity, particularly in postabsorptive conditions. The energy demands of muscles and the liver are met by NEFA mobilisation from adipose tissues, the largest source of stored energy in the human body. Increased mobilisation of NEFA from adipose, paired with increased oxidation, permits sustained exercise by delaying hypoglycaemia [54], Moreover, increased NEFA oxidation in the liver during exercise is necessary to fuel the high energy costs of gluconeogenesis. Storage and mobilisation of NEFA are under the control of insulin (lipogenic and anti-lipolytic hormone) and catecholamines (epinephrine and norepinephrine, lipolytic hormones) and the atrial natriuretic peptide (ANP, lipolytic factor) [55], During exercise, levels of circulating catecholamines, via sympathetic nervous system activation, and ANP release by the heart are enhanced and the plasma insulin level is decreased [56], The combined action of these factors induces lipolysis. Even low-intensity exercise is sufficient to increase adipose tissue NEFA mobilisation [57], Exercise training improves the sensitivity of adrenergic receptors to catecholamines in adipose tissue [58], while also enhancing markers of mitochondrial biogenesis and function [59], blood flow and glucose uptake [60], However, the improvements in adipose tissue metabolism and in blood flow in response to acute exercise are less pronounced in overweight and obese adults than in their lean healthy counterparts [61]. An acute bout of exercise also increases lipoprotein lipase (LPL) activity [62], Although this may appear counterintuitive given that LPL stimulates fat storage in adipose tissue, it makes sense given the effect of an increase in systemic and muscle LPL activity [63] is the promotion of fat uptake by muscle. Importantly, because this latter effect is long lasting (12–18 h) after a single bout of activity [64], prior exercise reduces the net delivery of dietary fat to adipose tissue (arterial triacylglycerol) [64] and, potentially, fat storage. In line with this, exercise training leads to a modest reduction in adiposity [65] even in the absence of weight loss. Furthermore, it decreases two variables with negative metabolic health outcomes, namely, central adiposity (at least in men) [65] and fat cell size (at least when associated with energy deficit). Although some studies suggested a greater effect of exercise on visceral than subcutaneous adipose tissue mass, potentially because visceral adipose tissue mass is more responsive to adrenergic activation [66], meta-analysis and reviews failed to report a differential effect on fat depots [67], The existence of an independent effect of regular exercise on visceral adipose tissue is currently debated. Exercise in rodents has been shown to lower adipose inflammation, which tracks with improved whole-body insulin sensitivity [68]; however, data in humans suggest that exercise-induced changes in adipose inflammation are minimal unless paired with caloric restriction-induced weight loss [69], In summary, adipose tissue adaptations to exercise together contribute to improved systemic metabolic homeostasis and improved exercise performance.
Muscle-adipose tissue crosstalk also exists. As reviewed elsewhere [26], skeletal muscle release of IL-6, FGF-21 and irisin increases in response to exercise and influence adipose tissue metabolism, oxidative capacity and glucose uptake. Following the depletion of glycogen stores, IL-6 is released by the contracting muscles during exercise. IL-6 may stimulate adipose tissue lipolysis and NEFA mobilisation during exercise, and plays a major role in the reduction of visceral adipose tissue in response to exercise training in humans [70], However, other in vivo and in vitro studies are not as conclusive, and the role of IL-6 in adipose tissue biology is still under investigation. Similarly, the effects of muscle-released FGF-21 and irisin on adipose tissue in humans are either still unknown or under debate. A novel exerkine produced by skeletal muscle contraction has recently been identified that targets human adipose tissue to promote lipolysis, namely, growth and differentiation factor 15 (GDF15) [71]. In terms of adipose tissue, adipokines modulate inflammation, lipid and glucose metabolism, blood pressure and atherosclerosis [56], Via its effect on fat mass, exercise can indirectly modulate levels of leptin and adiponectin, the two most well-studied adipokines, which are positively and negatively associated with fat mass, respectively. Although leptin and adiponectin have been associated with insulin sensitivity, the specific effect of exercise training on leptin and adiponectin is unclear [56], Recently, TGF-β2 has been shown to be secreted from adipose tissue in response to exercise and play a role in glucose homeostasis in mice [72]. Results still need to be confirmed in humans.
Pancreas adaptations
Pancreatic glucagon and insulin orchestrate the regulation of blood glucose by facilitating glucose disposal in insulin-sensitive tissues and hepatic gluconeogenesis. Insulin secretion is primarily adjusted according to the amount of glucose taken up by beta cells. Insulin secretion during a glucose challenge is dramatically altered by exercise undertaken during the previous days and hours in both healthy individuals and in those with insulin resistance and type 2 diabetes [73], Exercise cessation drives up insulin secretion, while one bout of exercise can lower insulin secretion, showing that insulin secretion is tightly regulated by exercise-driven pathways [74], The pancreas contributes to the capacity of acute exercise to increase hepatic glucose production by reducing insulin secretion (hepatic insulin clearance is also likely to be important) and increasing glucagon secretion [44], In adults with impaired glucose tolerance and type 2 diabetes, exercise improves peripheral sensitivity and pancreatic beta cell function (greater insulin secretion in response to circulating glucose) [75], The combination of enhanced insulin sensitivity and improved beta cell function is defined as the disposition index, which is the product of insulin sensitivity multiplied by the amount of insulin secreted in response to blood glucose [76], Unlike drug therapies for type 2 diabetes, which typically only influence one component of the index independently, exercise has the capacity to improve the disposition index by enhancing both components [77], Emerging evidence shows that factors secreted from contracting skeletal muscle boost beta cell insulin secretion and that the improvements in glucose homeostasis in type 2 diabetes patients in response to chronic aerobic exercise may be more closely related to improved beta cell function than insulin sensitivity [78], Although controversies still exist and mechanisms have not been fully elucidated as yet, crosstalk between skeletal muscle and pancreatic alpha and beta cells seems to exist via myokines. Two of the proteins that have been proposed to play a role in the muscle-pancreas crosstalk are muscle-released IL-6, because of its link with the protective effect of exercise against proinflammatory-induced beta cell loss [79], and the peroxisome proliferator-activated receptor γ coactivator lα (PGClα)-dependent myokine irisin (precursor protein fibronectin type III domain-containing protein 5 [FNDC5]), because of its protective effect against beta cell apoptosis induced by lipotoxic conditions [80], Other unknown exercise-induced myokines may also play a role in modulating beta cell function. Further work in this area could highlight novel therapeutic targets for type 2 diabetes.
Endothelium and cardiovascular system adaptations
The skeletal muscle microvascular ensures that delivery of oxygen and substrates (NEFA, triacylglycerols-rich lipoproteins and glucose) matches the metabolic demands of the muscle fibres (and other metabolic tissues) under resting conditions and during exercise. The microvasculature of human skeletal muscles has a complex 3D structure and is subject to a large number of complementary blood-flow regulation mechanisms, but the exact cascade of events and regulatory processes remain unknown, especially in humans [81, 82], Because of accessibility to tissues and the capacity to perform ex vivo preps, rodents have served as an important model to explore the regulation of endothelial function and blood flow in the control of skeletal muscle metabolism.
Under resting conditions, insulin increases microvascular perfusion through both vasodilatory and vasoconstrictory activities. On the one hand, insulin acts on terminal arterioles and increases nutritive blood flow to skeletal muscle [83], which can also result in increased blood flow in upstream conduit arteries. On the other hand, data from rodents shows that insulinmediated endothelin-1 (ET-1) [84] increases the vasoconstriction of arterioles that control access to the nutritive capillary beds of muscle, which receive little or no blood flow in the basal state. ET-1 in skeletal muscle arterioles is increased in individuals with obesity or type 2 diabetes compared with healthy control individuals [85], In addition, there is evidence in humans [86] and rodents [87] that increasing insulin resistance is associated with reduced capillary density. Although the vascular effects of insulin are likely to be markedly compromised in type 2 diabetes, exercise-mediated pathways are likely to be maintained [88].
During exercise, there are increases in cardiac output, via a rise in cardiac stroke and heart rate, and blood pressure. Acute exercise increases muscle insulin sensitivity by a coordinated increase in insulin-stimulated microvascular perfusion and molecular signalling that improves glucose delivery and increases muscle glucose uptake and disposal [89], This insulin-stimulated increase in microvascular perfusion is likely to be linked to the exercise-mediated increases in muscle membrane permeability to glucose and muscle blood flow [90], The increased haemodynamic forces, i.e. shear forces exerted by blood flow, are also translated by the glycocalyx layer (glycoproteins and proteoglycans) located on the luminal surface of endothelium into a vasodilatory response. This pulsatile flow-induced shear stress and release of nitric oxide induces a dynamic regulation of vascular tone via ET-1 synthesis/release [91], which could also potentiate the non-nutritive route of the blood flow. Vasodilation and additional microvascular units expand the endothelial surface area, thus enabling the delivery of nutrients to the muscle. Exercise training reduces resting blood pressure, heart rate and cardiac hypertrophy, improves the vasodilator response of the muscle microvasculature to insulin and exercise [88] and enlarges the microvascular network via angiogenesis and arteriogenesis [81]. These adaptations have been linked to a variety of changes in tissue metabolism and signalling, including the production and release of nitric oxide and prostacyclin from the vascular endothelium [82], The finding that vascular endothelial growth factor B (VEGF-B) produced by skeletal muscle links endothelial NEFA uptake to the oxidative capacity of skeletal muscle by controlling the expression of fatty acid transporter proteins in the capillary endothelium represents a major recent discovery [92], Regulation of the expression of these proteins may prevent lipotoxic NEFA accumulation, the dominant cause of insulin resistance in muscle fibres. The effects of exercise on endothelial adaptations and the underlying mechanisms continue to be explored and much is yet to be learnt, especially related to how adaptations occur in those with obesity or type 2 diabetes vs a non-diseased state [93, 94], The ability of exercise to influence endothelial transport of insulin and glucose [44] and to mediate enhanced insulin-stimulated blood flow via both the nutritive and non-nutritive routes [88] is likely to be particularly important for the prevention of type 2 diabetes. Finally, emerging data from rodents indicate that exercise also positively impacts endothelial function and vascular biology in adipose depots [95] and is likely to play a role in other key organs, such as the brain, liver and pancreas.
Conclusion
Regular exercise and/or moderate to vigorous physical activity has a pronounced protective effect against metabolic disease. We posit that the multi-tissue adaptations induced by exercise underly its powerful disease-modifying impact. Detailed studies blocking individual effects in each tissue using reductionist approaches may be needed to provide greater mechanistic insight. That being said, because exercise activates so many pathways in so many tissues, it may be that target knockdown studies would not reveal that one metabolic pathway is essential, but, rather like the layers of an onion, multiple factors with built in redundancies contribute to the metabolic protection provided by regular exercise. Nevertheless, it is an exciting time to investigate and observe how exercise to mediates metabolic benefits beyond the mechanisms found in skeletal muscle.
Supplementary Material
Acknowledgments
Funding
Work in JPT’s laboratories is supported by a VA-Merit Grant 1I01BX002567, NIH R01 KD121497 and R01 AR071263. Work by AB is supported by NIH R00 DK100465.
Abbreviations
- ANP
Atrial natriuretic peptide
- FGF-21
Fibroblast growth factor 21
- LPL
Lipoprotein lipase
- NAFLD
Non-alcoholic fatty liver disease
Footnotes
Authors’ relationships and activities The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
John P. Thyfault is a Professor at the Department of Molecular and Integrative physiology, University of Kansas Medical Center, he is the Scientific Director at the Center for Children’s Healthy Lifestyle and Nutrition, Children’s Mercy Hospital, and is Senior Research Scientist at Research Service, Kansas City VA Medical Center.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK (2009) American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and science in sports and exercise 41(2): 459–471. 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- [2].Hashida R, Kawaguchi T, Bekki M, et al. (2017) Aerobic vs. resistance exercise in nonalcoholic fatty liver disease: A systematic review. J Hepatol 66(1): 142–152. 10.1016/j.jhep.2016.08.023 [DOI] [PubMed] [Google Scholar]
- [3].Katzmarzyk PT (2010) Physical activity, sedentary behavior, and health: paradigm paralysis or paradigm shift? Diabetes 59(11): 2717–2725. 10.2337/dbl0-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hodson L, Karpe F (2019) Hyperinsulinemia: does it tip the balance toward intrahepatic fat accumulation? Endocr Connect 8(10):R157–R168. 10.1530/EC-19-0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meex RCR, Blaak EE, van Loon UC (2019) Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev 20(9): 1205–1217. 10.1111/obr.l2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gl Smith, Shankaran M, Yoshino M, et al. (2019) Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 10.1172/JCI134165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Goodpaster BH, Sparks LM (2017) Metabolic flexibility in health and disease. Cell Metab 25(5): 1027–1036. 10.1016/j.cmet.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petersen MC, Shulman Gl (2018) Mechanisms of Insulin action and insulin resistance. Physiol Rev 98(4): 2133–2223. 10.1152/physrev.00063.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].lozzo P (2009) Viewpoints on the way to the consensus session: where does insulin resistance start? The adipose tissue. Diabetes Care 32 Suppl 2: S168–S173. 10.2337/dc09-S304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Templeman NM, Skovso S, Page MM, Lim GE, Johnson JD (2017) A causal role for hyperinsulinemia in obesity. J Endocrinol 232(3): R173–R183. 10.1530/JOE-16-0449 [DOI] [PubMed] [Google Scholar]
- [11].Thyfault JP, Krogh-Madsen R (2011) Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol 111(4): 1218–1224. 10.1152/japplphysiol.00478.2011 [DOI] [PubMed] [Google Scholar]
- [12].Bergouignan A, Rudwill F, Simon C, Blanc S (2011) Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. Journal of Applied Physiology 111(4): 1201–1210. 10.1152/japplphysiol.00698.2011 [DOI] [PubMed] [Google Scholar]
- [13].Laaksonen DE, Lindstrom J, Lakka TA, et al. (2005) Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes 54(1): 158–165 [DOI] [PubMed] [Google Scholar]
- [14].Fretts AM, Howard BV, McKnight B, et al. (2012) Modest levels of physical activity are associated with a lower incidence of diabetes in a population with a high rate of obesity: the strong heart family study. Diabetes Care 35(8): 1743–1745. 10.2337/dcll-2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tudor-Locke C, Schuna JM, Jr. (2012) Steps to preventing type 2 diabetes: exercise, walk more, or sit less? Front Endocrinol (Lausanne) 3:142 10.3389/fendo.2012.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williams PT (2007) Changes in vigorous physical activity and incident diabetes in male runners. Diabetes Care 30(11): 2838–2842 [DOI] [PubMed] [Google Scholar]
- [17].Wang Y, Lee DC, Brellenthin AG, et al. (2019) Leisure-time running reduces the risk of incident type 2 diabetes. Am J Med 132(10): 1225–1232. 10.1016/j.amjmed.2019.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee DC, Sui X, Church TS, Lee IM, Blair SN (2009) Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 32(2): 257–262. dc08-1377 [pii] 10.2337/dc08-1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thyfault JP, Rector RS (2020) Exercise combats hepatic steatosis: potential mechanisms and clinical implications. Diabetes 69(4): 517–524. 10.2337/dbil8-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN (2006) Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 130(7): 2023–2030 [DOI] [PubMed] [Google Scholar]
- [21].Palve KS, Pahkala K, Suomela E, et al. (2017) Cardiorespiratory fitness and risk of fatty liver: The Young Finns Study. Medicine and science in sports and exercise 49(9): 1834–1841. 10.1249/MSS.0000000000001288 [DOI] [PubMed] [Google Scholar]
- [22].Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG (2017) Role of Inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97(4): 1351–1402. 10.1152/physrev.00019.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deshmukh AS, Cox J, Jensen LJ, Meissner F, Mann M (2015) Secretome analysis of lipidinduced insulin resistance in skeletal muscle cells by a combined experimental and bioinformatics workflow. J Proteome Res 14(11): 4885–4895. 10.1021/acs.jproteome.5b00720 [DOI] [PubMed] [Google Scholar]
- [24].Meex RC, Hoy AJ, Morris A, et al. (2015) Fetuin B Is a Secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab 22(6): 1078–1089. 10.1016/j.cmet.2015.09.023 [DOI] [PubMed] [Google Scholar]
- [25].Crowe S, Wu LE, Economou C, et al. (2009) Pigment epithelium-derived factor contributes to insulin resistance in obesity. Cell Metab 10(1): 40–47. 10.1016/j.cmet.2009.06.001 [DOI] [PubMed] [Google Scholar]
- [26].Laurens C, Bergouignan A, Moro C (2020) Exercise-released myokines in the control of energy metabolism. Front Physiol 11: 91 10.3389/fphys.2020.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17(2): 162–184. 10.1016/j.cmet.2012.12.012 [DOI] [PubMed] [Google Scholar]
- [28].Bergouignan A, Latouche C, Heywood S, et al. (2016) Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep 6: 32044 10.1038/srep32044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thyfault JP (2008) Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol 294(4): R1103–R1110.. 10.1152/ajpregu.00542.2007 [DOI] [PubMed] [Google Scholar]
- [30].Yan Z, Okutsu M, Akhtar YN, Lira VA (2011) Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol (1985) 110(1): 264–274. 10.1152/japplphysiol.00993.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim Y, Triolo M, Hood DA (2017) Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev 2017: 3165396 10.1155/2017/3165396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parousis A, Carter HN, Tran C, et al. (2018) Contractile activity attenuates autophagy suppression and reverses mitochondrial defects in skeletal muscle cells. Autophagy 14(11): 1886–1897. 10.1080/15548627.2018.1491488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lefai E, Blanc S, Momken I, et al. (2017) Exercise training improves fat metabolism independent of total energy expenditure in sedentary overweight men, but does not restore lean metabolic phenotype. Int J Obes (Lond) 41(12): 1728–1736. 10.1038/ijo.2017.151 [DOI] [PubMed] [Google Scholar]
- [34].Badin PM, Langin D, Moro C (2013) Dynamics of skeletal muscle lipid pools. Trends in endocrinology and metabolism: TEM 24(12): 607–615. 10.1016/j.tem.2013.08.001 [DOI] [PubMed] [Google Scholar]
- [35].Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8(8): 457–465. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- [36].Lee JH, Jun HS (2019) Role of myokines in regulating skeletal muscle mass and function. Front Physiol 10: 42 10.3389/fphys.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Piccirillo R (2019) Exercise-induced myokines with therapeutic potential for muscle wasting. Front Physiol 10: 287 10.3389/fphys.2019.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pedersen BK, Steensberg A, Fischer C, et al. (2003) Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24(2–3): 113–119 [DOI] [PubMed] [Google Scholar]
- [39].Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K (2002) Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8: 6–48 [PubMed] [Google Scholar]
- [40].Keller C, Steensberg A, Pilegaard H, et al. (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15(14): 2748–2750. 10.1096/fj.01-0507fje [DOI] [PubMed] [Google Scholar]
- [41].Febbraio MA, Steensberg A, Keller C, et al. (2003) Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle madmans. The Journal of physiology 549(Pt 2): 607–612. 10.1113/jphysiol.2003.042374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wolsk E, Mygind H, Grondahl TS, Pedersen BK, van Hall G (2010) IL-6 selectively stimulates fat metabolism in human skeletal Tiiuscie. Am J Physiol Endocrinol Metab 299(5): E832–E840. 10.1152/ajpendo.00328.2010 [DOI] [PubMed] [Google Scholar]
- [43].Carey AL, Steinberg GR, Macaulay SL, et al. (2006) lnterleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55(10): 2688–2697. 10.2337/db05-1404 [DOI] [PubMed] [Google Scholar]
- [44].Wasserman DH (2009) Four grams of glucose. Am J Physiol Endocrinol Metab 296(1): Ell–21. 10.1152/ajpendo.90563.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Trefts E, Williams AS, Wasserman DH (2015) Exercise and the regulation of hepatic metabolism. Prog Mol Biolfransl Sci 135: 203–225. 10.1016/bs.pmbts.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hu C, Hoene: M, Plomgaard P, et al. (2019) Muscle-liver substrate fluxes in exercising humans and potential effects on hepatic metabolism. J Clin Endocrinol Metab. 105(4): 1196–1209.. 10.1210/clinem/dgz266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rector RS, Thyfault JP, Morris RT, et al. (2008) Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. American journal of physiology Gastrointestinal and liver physiology 294(3): G619–G626 [DOI] [PubMed] [Google Scholar]
- [48].Linden MA, Fletcher JA, Morris EM, et al. (2015) Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Medicine and science in sports and exercise 47(3): 556–567. 10.1249/MSS.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rector RS, Uptergrove GM, Morris EM, et al. (2011) Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. American journal of physiology Gastrointestinal and liver physiology 300(5): G874–G883. 10.1152/ajpgi.00510.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25(2): 262–284. 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karstoft K, Pedersen BK (2016) Skeletal muscle as a gene regulatory endocrine organ. CurrOpin Clin Nutr Metab Care 19(4): 270–275. 10.1097/MC0.0000000000000283 [DOI] [PubMed] [Google Scholar]
- [52].Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW (2012) Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287(15): 11968–11980. 10.1074/jbc.Mill.336834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ingerslev B, Hansen JS, Hoffmann C, et al. (2017) Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Mol Metab 6(10): 1286–1295. 10.1016/j.molmet.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Horowitz JF (2003) Fatty acid mobilization from adipose tissue during exercise. Trends in endocrinology and metabolism: TEM 14(8): 386–392 [DOI] [PubMed] [Google Scholar]
- [55].Lafontan M, Sengenes C, Galitzky J, et al. (2000) Recent developments on lipolysis regulation in humans and discovery of a new lipolytic pathway. Int J Obes Relat Metab Disord 24 Suppl 4: S47–S52. 10.1038/sj.ijo.0801505 [DOI] [PubMed] [Google Scholar]
- [56].Kl Stanford, Goodyear U (2016) Exercise regulation of adipose tissue. Adipocyte 5(2): 153–162. 10.1080/21623945.2016.1191307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moro C, Pillard F, de Glisezinski I, et al. (2007) Sex differences in lipolysis-regulating mechanisms in overweight subjects: effect of exercise intensity. Obesity (Silver Spring) 15(9): 2245–2255. 10.1038/oby.2007.267 [DOI] [PubMed] [Google Scholar]
- [58].Richterova B, Stich V, Moro C, et al. (2004) Effect of endurance training on adrenergic control of lipolysis in adipose tissue of obese women. J Clin Endocrinol Metab 89(3): 1325–1331 10.1210/jc.2003-031001 [DOI] [PubMed] [Google Scholar]
- [59].Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC (2009) Exercise and adrenaline increase PGC-la mRNA expression in rat adipose tissue. The Journal of physiology 587(Pt 7): 1607–1617. 10.1113/jphysiol.2008.165464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Trevellin E, Scorzeto M, Olivieri M, et al. (2014) Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 63(8): 2800–2811. 10.2337/dbl3-1234 [DOI] [PubMed] [Google Scholar]
- [61].Moro C, Pillard F, de Glisezinski I, et al. (2008) Exercise-induced lipid mobilization in subcutaneous adipose tissue is mainly related to natriuretic peptides in overweight men. Am J Physiol Endocrinol Metab 295(2): E505–E513. 10.1152/ajpendo.90227.2008 [DOI] [PubMed] [Google Scholar]
- [62].Perreault L, Lavely JM, Kittelson JM, Horton TJ (2004) Gender differences in lipoprotein lipase activity after acute exercise. Obesity research 12(2): 241–249. 10.1038/oby.2004.31 [DOI] [PubMed] [Google Scholar]
- [63].Lithell H, Scheie R, Vessby B, Jacobs I (1984) Lipoproteins, lipoprotein lipase, and glycogen after prolonged physical activity. J Appl Physiol Respir Environ Exerc Physiol 57(3): 698–702. 10.1152/jappl.1984.57.3.698 [DOI] [PubMed] [Google Scholar]
- [64].Maikova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE (2000) Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab 279(5): E1020–E1028 [DOI] [PubMed] [Google Scholar]
- [65].Wilmore JH, Despres JP, Stanforth PR, et al. (1999) Alterations in body weight and composition consequent to 20 wk of endurance training: the HERITAGE Family Study. The American journal of clinical nutrition 70(3): 346–352. 10.1093/ajcn/70.3.346 [DOI] [PubMed] [Google Scholar]
- [66].Mauriege P, Galitzky J, Berlan M, Lafontan M (1987) Heterogeneous distribution of beta and alpha-2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. Eur J Clin Invest 17(2): 156–165. 10.1111/j.l365-2362.1987.tb02395.x [DOI] [PubMed] [Google Scholar]
- [67].Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I (2007) A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 31(12): 1786–1797. 10.1038/sj.ijo.0803683 [DOI] [PubMed] [Google Scholar]
- [68].Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA (2009) Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab 296(5): E1164–E1171. 10.1152/ajpendo.00054.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA (2011) Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring: ) 19(6): 1131–1136. 10.1038/oby.2010.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. (2019) Exercise-induced changes in visceral adipose tissue mass are regulated by il-6 signaling: a Randomized controlled trial. Cell Metab 29(4): 844–855 e843. 10.1016/j.cmet.2018.12.007 [DOI] [PubMed] [Google Scholar]
- [71].Laurens C, Parmar A, Murphy E, et al. (2020) Growth and Differentiation Factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 10.1172/jci.insight.131870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Takahashi H, Alves CRR, Stanford Kl, et al. (2019) TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabo%jffi Nat Metab 1(2): 291–303. 10.1038/S42255-018-0030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Curran M, Drayson MT, Andrews RC, et al. (2020) The benefits of physical exercise for the health of the pancreatic beta-cell: a review of the evidence. Exp Physiol 105(4):579–589. 10.1113/EP088220 [DOI] [PubMed] [Google Scholar]
- [74].Heath GW, Gavin JR 3rd, Hinderfifer JM, Hagberg JM, Bloomfield SA, Holloszy JO (1983) Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol 55(2): 512–517 [DOI] [PubMed] [Google Scholar]
- [75].Kahn SE, Prigeon RL, McCulloch DK, et al. (1993) Quantification of the relationship between insulin sensitivitVand beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42(11): 1663–1672. 10.2337/diab.42.11.1663 [DOI] [PubMed] [Google Scholar]
- [76].Bergman RN, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68(6): 1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP (2010) Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 33(7): 1561–1566. 10.2337/dc09-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Solomon TP, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP (2013) Pancreatic beta-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab 98(10): 4176–4186. 10.1210/jc.2013-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Christensen CS, Christensen DP, Lundh M, et al. (2015) Skeletal muscle to pancreatic beta-cell cross-talk: the effect of humoral mediators liberated by muscle contraction and acute exercise on beta-cell apoptosis. J Clin Endocrinol Metab 100(10): E1289–1298. 10.1210/jc.20144506 [DOI] [PubMed] [Google Scholar]
- [80].Natalicchio A, Marrano N, Biondi G, et al. (2017) The myokine irisin is released in response to saturated fatty acids and promotes pancreatic beta-cell survival and insulin secretion. Diabetes 66(11): 2849–2856. 10.2337/dbl7-0002 [DOI] [PubMed] [Google Scholar]
- [81].Wagenmakers AJ, Strauss JA, Shepherd SO, Keske MA, Cocks M (2016) Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing. J Physiol 594(8): 2207–2222. 10.1113/jphysiol.2014.284513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Olver TD, Ferguson BS, Laughlin MH (2015) Molecular mechanisms for exercise traininginduced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci 135: 227–257. 10.1016/bs.pmbts.2015.07.017 [DOI] [PubMed] [Google Scholar]
- [83].Bergman RN (2003) Insulin action and distribution of tissue blood flow. %Cfln Endocrinol Metab 88(10): 4556–4558. 10.1210/jc.2003-031431 [DOI] [PubMed] [Google Scholar]
- [84].Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P (2002) Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56(3): 464–471. S000863630200593X [pii] [DOI] [PubMed] [Google Scholar]
- [85].Reynolds U, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP (2017) Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (1985) 122(1): 38–47. 10.1152/japplphysiol.00286.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Solomon TP, Haus JM, Li Y, Kirwan JP (2011) Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endoifinol Metab 96(5): 1377–1384. 10.1210/jc.2010-2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Frisbee JC (2005) Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 289(2): R307–R316 [DOI] [PubMed] [Google Scholar]
- [88].Padilla J, Olver TD, Thyfault JP, Fadel PJ (2015) Role of habitual physical activity in modulating vascular actions of insulin. Exp Physiol 100(7): 759–771. 10.1113/EP085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sjoberg KA, Frosig C, Kjobsted R, et al. (2017) Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66(6): 1501–1510. 10.2337/dbl6-1327 [DOI] [PubMed] [Google Scholar]
- [90].McConel! GK, Sjoberg KA, Ceutz F, et al. (2020) Insulin-induced membrane permeability to glucose in human muscles at rest and following exercise. The Journal of physiology 598(2): 303–315. 10.1113/JP278600 [DOI] [PubMed] [Google Scholar]
- [91].Rapoport RM, Merkus D (2017) Endothelin-1 regulation of exercise-induced changes in flow: dynamic regulation of vascular tone. Front Pharmacol 8: 517. 10.3389/fphar.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Hagberg CE, FalkevaII A, Wang X, et al. (2010) Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 464(7290): 917–921. 10.1038/nature08945 [DOI] [PubMed] [Google Scholar]
- [93].Martin JS, Padilla J, Jenkins NT, et al. (2012) Functional adaptations in the skeletal muscle microvasculature to endurance and interval sprint training in the type 2 diabetic OLETF rat. J Appl Physiol (1985) 113(8): 1223–1232. 10.1152/japplphysiol.00823.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mikus CR, Rector RS, Arce-Esquivel AA, et al. (2010) Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol 109(4): 1203–1210. 10.1152/japplphysiol.00064.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].DeVallance E, Branyan KW, Lemaster KC, et al. (2019) Exercise training prevents the perivascular adipose tissue-induced aortic dysfunction with metabolic syndrome. Redox Biol 26: 101285 10.1016/j.redox.2019.101285 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.