Abstract
Inflammation is emerging as a critical factor in the pathophysiology of intracranial aneurysm. Toll-like receptor 4 (TLR4) contributes not only to the innate immune responses but also to the inflammatory processes associated with vascular disease. Therefore, we examined the contribution of the TLR4 pathway to the development of the rupture of intracranial aneurysm. We utilized a mouse model of intracranial aneurysm. TLR4 inhibition significantly reduced the development of aneurysmal rupture. In addition, the rupture rate and levels of pro-inflammatory cytokines were lower in TLR4 knockout mice than the control littermates. Macrophage/monocyte-specific TLR4 knockout mice had a lower rupture rate than the control littermate mice. Moreover, the deficiency of myeloid differentiation primary-response protein 88 (MyD88), a key mediator of TLR4, reduced the rupture rate. These findings suggest that the TLR4 pathway promotes the development of intracranial aneurysmal rupture by accelerating inflammation in aneurysmal walls. Inhibition of the TLR4 pathway in inflammatory cells may be a promising approach for the prevention of aneurysmal rupture and subsequent subarachnoid hemorrhage.
Keywords: stroke, intracranial aneurysm, subarachnoid hemorrhage, intracranial hemorrhage, toll-like receptor 4
Graphical Abstract
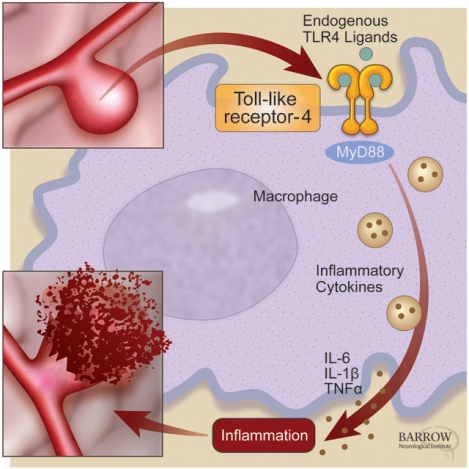
Introduction
Intracranial aneurysm rupture causes subarachnoid hemorrhage, resulting in severe mortality and morbidity.1 Established therapies for the prevention of aneurysmal rupture are limited to invasive treatments such as surgical clipping and endovascular coiling.1 Although these invasive therapies are well-established, the adverse outcome rates from these procedures are not still negligible.1 Therefore, the pharmacological prevention of aneurysmal rupture may be an attractive approach for patients with unruptured aneurysms.2–6
Recent studies have suggested inflammation as a critical factor for the development of intracranial aneurysm rupture.2, 4, 7 Thus, a better understanding of molecular pathways that regulate the inflammatory process in intracranial aneurysmal rupture may contribute to the establishment of effective medical therapies for the prevention of aneurysmal rupture and subsequent subarachnoid hemorrhage.
Toll-like receptors (TLRs) have crucial roles in activating the innate immune system, and at least ten members of the TLR family have been identified in humans.8, 9 These receptors are activated by several ligands and modulate inflammation.10 Toll-like receptor 4 (TLR4) is one of TLRs that is expressed on the cell surface of both immune and vascular cells, including endothelial cells,11 smooth muscle cells,12 and inflammatory cells.10, 13 Previous descriptive studies showed the expression of TLR4 in human intracranial aneurysms and a rat model of intracranial aneurysm.14, 15 However, the causal link between the TLR4 pathway and intracranial aneurysmal rupture has still not been studied.
TLR4 has a cytoplasmic toll/IL-1R (TIR; IL-1: interleukin-1) homologous domain that can bind to myeloid differentiation primary-response protein 88 (MyD88). The binding between TLR4 and MyD88 leads to the activation of IL-1R-associated kinases (IRAKs) and downstream expression of proinflammatory cytokines.10, 16 It has been shown that the pathway involves TLR4 and MyD88 promotes inflammation in the development of tissue inflammation and remodeling in atherosclerosis12, 17 and myocardial ischemia.18 TLR4 may contribute to the development of aneurysmal rupture through the MyD88. Therefore, in this study, we examined the potential roles of TLR4 and MyD88 in the development of aneurysmal rupture using a mouse model of intracranial aneurysm.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
All experiments were conducted following the guidelines approved by the Institutional Animal Care and Use Committee. Male C56BL/6 mice and knockout mice in the C56BL/6 background were used in all experiments. TLR4 knockout mice,19 MyD88 knockout mice,20 TLR4 flox/flox mice,21 and mice expressing Cre recombinase under the control of the myeloid-specific lysosome M (LysM) promoter22 were obtained from Jackson Laboratory (Bar Harbor, Maine). No abnormalities in the appearance, growth, or body weight were observed in these mice.
Induction of aneurysmal rupture model in mice
To induce intracranial aneurysms in mice, we combined systemic hypertension (deoxycorticosterone acetate-salt hypertension) and a single injection of elastase into the cerebrospinal fluid at the right basal cistern as previously described.3, 23 To induce systemic hypertension, we used a widely used DOCA-salt hypertension method. DOCA-salt hypertension requires the subcutaneous implantation of DOCA (deoxycorticosterone acetate) pellet, unilateral nephrectomy, and high-salt drinking water (0.9% NaCl) as previously described.3, 24 This method causes a milder increase in blood pressure compared to angiotensin II infusion.23, 24 One week after the unilateral nephrectomy, a DOCA pellet (2.4 mg/day, Innovative Research of America, Sarasota, FL, USA) was implanted, and 0.9% sodium chloride drinking water was started. Mice received a single injection of elastase (0.035 units) into the cerebrospinal fluid at the right basal cistern on the same day as DOCA pellet implantation.3, 24
To detect aneurysmal rupture, two blinded observers performed neurological examinations daily as previously described.2–4, 25, 26 Neurological symptoms were scored as follows: 0: normal function; 1: reduced eating or drinking activity demonstrated by a weight loss > 2 grams of body weight (≈ 10% weight loss) for 24 hours; 2: flexion of the torso and forelimbs on lifting the whole animal by the tail; 3: circling to one side with a normal posture at rest; 4: leaning to one side at rest; and 5: no spontaneous activity. When mice were found to show neurological symptoms associated with aneurysmal rupture (neurological score, 1 – 5), they were euthanized immediately (within 4 hours).2–4, 25, 26 Because our previous studies using this model showed that aneurysmal rupture occurs within 3 weeks of aneurysm induction,3, 26 asymptomatic mice were euthanized 21 days after aneurysm induction.2, 25 The brain samples were perfused with phosphate-buffered saline (PBS), followed by a gelatin-containing blue dye to visualize cerebral arteries. Aneurysms were defined as a localized outward bulging of the vascular wall, whose diameter was greater than the parent artery diameter.4
TLR4 inhibitor treatment
As a TLR4 inhibitor, lipopolysaccharide from the photosynthetic bacterium Rhodobacter sphaeroides (LPS-RS) was used as previously described.27, 28 Unlike other bacterial lipopolysaccharides, LPS-RS acts as a potent antagonist of TLR4.29 LPS-RS has been widely used as a TLR4 inhibitor in both in-vivo and in-vitro settings.18, 29, 30
Our previous study showed that in this model, aneurysmal formation occurs first 6 days after aneurysm induction and that aneurysmal rupture can be found 7 days after the aneurysm induction.2–4, 25 Therefore, we started intraperitoneal injection of LPS-RS (50 μg/kg, Invitrogen, San Diego, CA) in 200 μl saline twice a week for the treatment group or just 200 μl saline for the vehicle group from 6 days after aneurysm induction to 3 weeks after aneurysm induction as previously described.2–4, 25 We measured systolic blood pressure every week and body weight and general appearance daily.
Expression levels of inflammatory cytokines
We harvested cerebral arteries and aneurysms 6 days after aneurysm induction to exclude inflammation caused by subarachnoid hemorrhage itself as previously described.4 We collected the entire cerebral arteries with or without aneurysm induction and measured gene expression of interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), monocyte chemoattractant factor-1 (MCP-1), matrix metalloproteinase (MMP)-9, and nuclear factor-kappa B (NF-κB) p65. The RNeasy Mini Kit (Qiagen, CA) was used for extraction of total RNA. The total RNAs (2μL of 4–8 ng/μl of RNA) from each sample were transcribed to cDNA with QuantiTect reverse transcription kit (Qiagen), and all resulting DNAs were used for using SYBR Green technology (Applied Biosystems, CA). Quantitative values were obtained from the threshold cycle value (CT), and the data were analyzed by the 2−ΔΔCT method. The transcript amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal RNA control. 2−ΔΔCT value of the target transcript from each mouse was normalized with the mean 2−ΔΔCT value from the wild-type mice without aneurysm induction as 1.0. Then, the normalized 2−ΔΔCT values were compared using the Mann–Whitney test.
Real-time PCR was performed using 7500 Fast Real-Time PCR System (Applied Biosystems, CA, USA). Following primer sets were used: IL-1β (forward primer: 5’-TGC GAC TTC AAC AGC AAC TC-3’; reverse primer: 5’-ATG TAG GCC ATG AGG TCC AC-3’), IL-6 (forward primer: 5’-CCG GAG AGG AGA CTT CAC AG-3’; reverse primer: 5’-GGA AAT TGG GGT AGG AAG GA-3’), TNFα (forward primer: 5’-CCA GAC CCT CAC ACT CAG ATC-3’; reverse primer: 5’-CAC TTG GTG GTT TGC TAC GAC-3’), MCP-1 (forward primer: 5’-AGG TCC CTG TCA TGC TTC TG-3’; reverse primer: 5’-GCT GCT GGT GAT CCT CTT GT-3’), MMP-9 (forward primer: 5’-AGA CCT GAA AAC CTC CAA CCT CAC-3’; reverse primer: 5’-TGT TAT GAT GGT CCC ACT TGA GGC-3’), NF-κB p65 (forward primer: 5’-ACA CCT CTG CAT ATA GCG GC-3’; reverse primer: 5’-GGT ACC CCC AGA GAC CTC AT-3’), and GAPDH (forward primer: 5’-TGC GAC TTC AAC AGC AAC TC-3’; reverse primer: 5’-ATG TAG GCC ATG AGG TCC AC-3’).
Statistical analysis
Fisher’s exact test was used to analyze the incidence of aneurysms and the rupture rate. As an exploratory analysis, the survival analysis was performed using the log-rank test. Mice that did not develop aneurysms were excluded from the survival analysis. Levels of mRNA were compared between 2 groups using the Mann–Whitney test and presented as mean ± SD. P < 0.05 was considered statistically significant (GraphPad Software, La Jolla, CA).
Results
TLR4 inhibitor prevented the development of intracranial aneurysmal rupture.
As a first step to investigate the role of TLR4 in aneurysmal rupture, we tested the effects of TLR4 inhibitor on the development of aneurysmal rupture. Figure 1 shows representative images of Circle of Willis without intracranial aneurysms (A), with an unruptured aneurysm (B), and a ruptured aneurysm (C) in the vehicle-treated mice.
Figure 1.
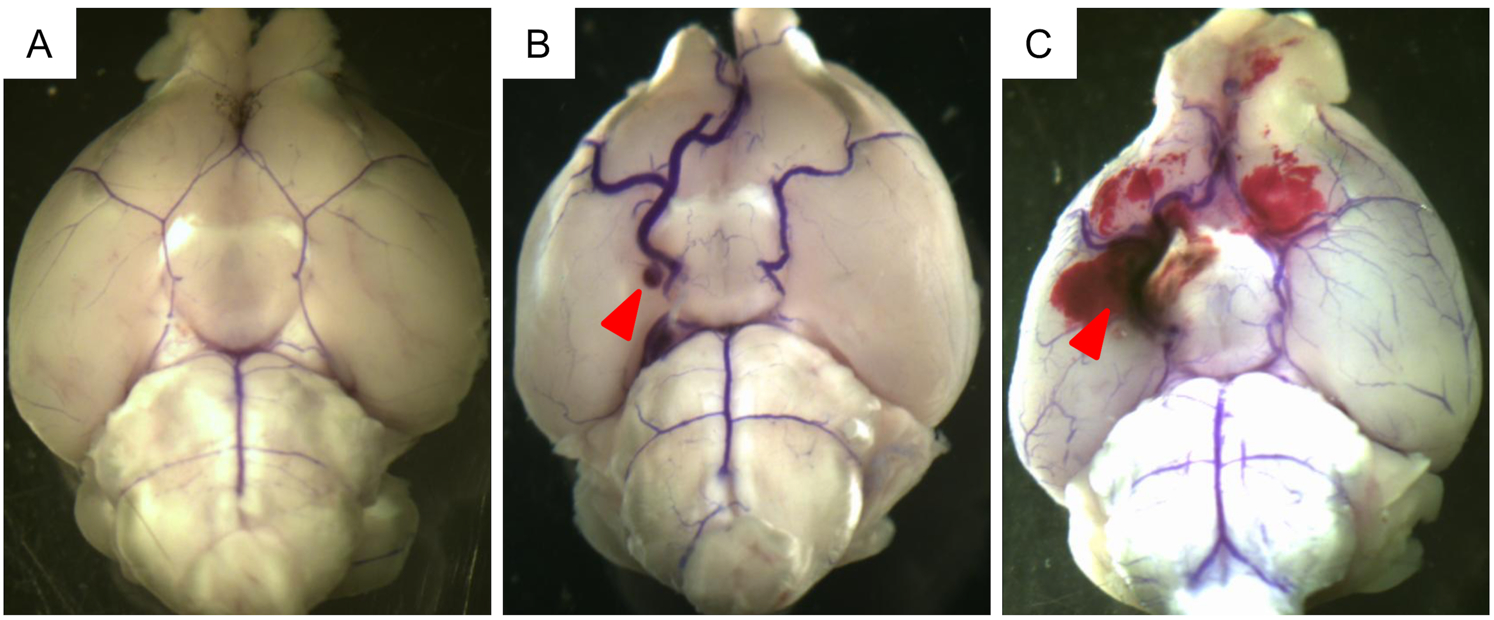
Representative images of unruptured and ruptured aneurysms. Blue dye was injected into Circle of Willis. A. no aneurysm. B. unruptured aneurysm. C. ruptured aneurysm with subarachnoid hemorrhage. Arrowheads indicate intracranial aneurysms.
As the TLR4 treatment started after the formation of aneurysms, the TLR4 inhibitor did not affect the formation of intracranial aneurysms (vehicle versus TLR4 inhibitor; 89% versus 96%; n = 27 versus n = 24. Figure 2A). However, more importantly, the treatment with TLR4 inhibitor significantly decreased the rupture rate compared with the vehicle treatment (vehicle versus TLR4 inhibitor; 83% versus 52%; P < 0.05; n = 24 versus n = 23. Figure 2B). A symptom-free curve (Kaplan-Meier analysis curve) excluding mice without any aneurysms showed a significant reduction of aneurysmal rupture with TLR4 inhibitor treatment (P < 0.05. Figure 2C). TLR4 inhibitor did not affect blood pressure (Figure 2D).
Figure 2.
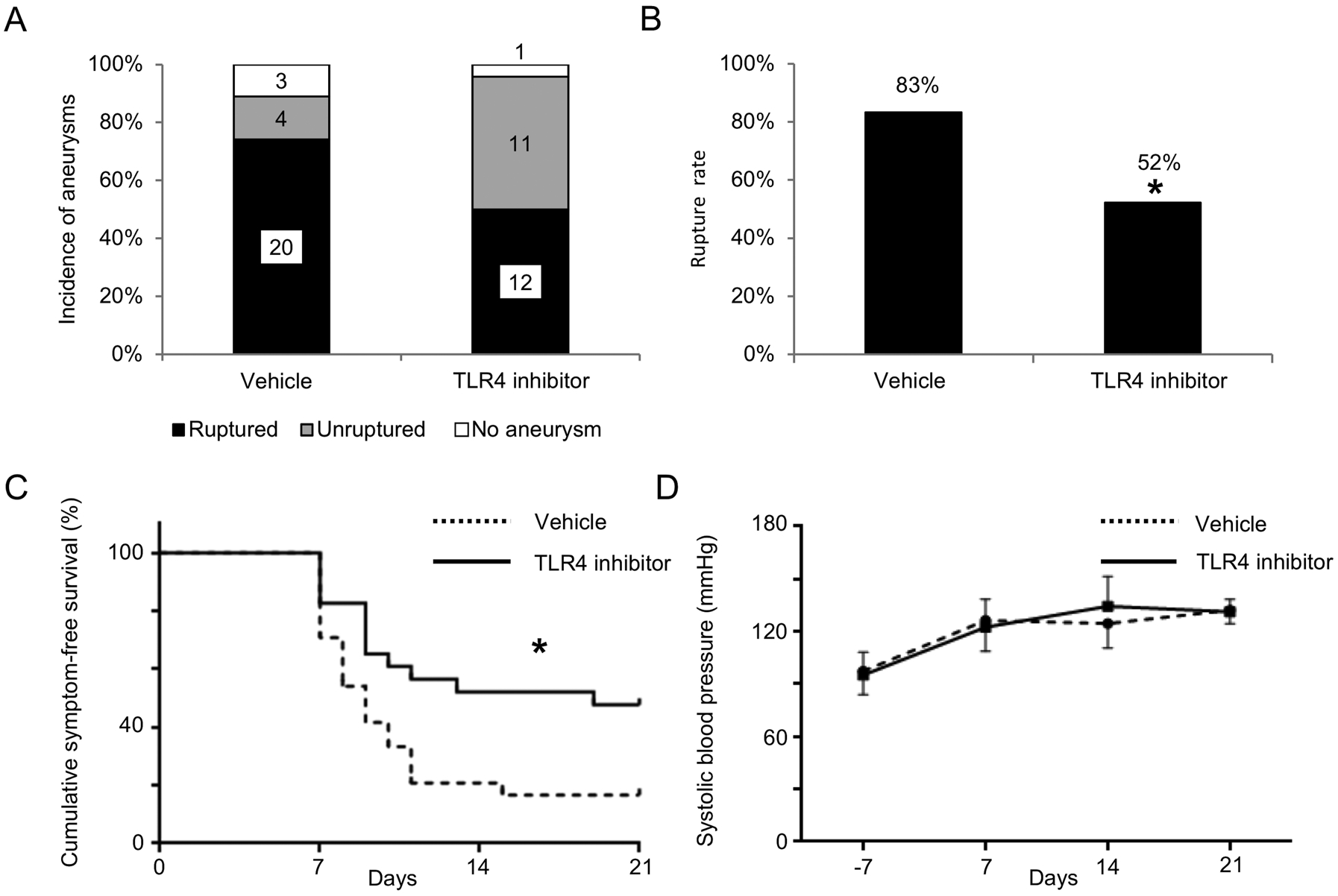
TLR4 inhibitor reduced a rupture rate but did not affect the formation of aneurysms. A. Incidence of unruptured and ruptured aneurysms. B. Rupture rate. C. Symptom-free curve (Kaplan–Meier analysis curve). Mice that did not have aneurysms were excluded from this analysis. D. Systolic blood pressure. There was no difference in blood pressure between two group at any timepoint. *P < 0.05, **P < 0.01
Lack of TLR4 reduced the development of intracranial aneurysmal rupture.
To further confirm the contribution of TLR4 to the development of aneurysmal rupture, we examined the development of aneurysmal rupture in TLR4 knockout mice. There was no significant difference in the incidence of aneurysm formation between the wild-type littermates and TLR4 knockout mice (wild-type littermates versus TLR4 knockout mice; 94% versus 86%; n = 18 versus n = 14. Figure 3A). Consistent with the TLR4 inhibitor study, TLR4 knockout mice had a lower rupture rate than wild-type littermates (wild-type littermates versus TLR4 knockout mice; 77% versus 33%; P < 0.05; n = 17 versus n = 12. Figure 3B). The survival analysis using mice that had aneurysms revealed a lower incidence of aneurysmal rupture in TLR4 knockout mice (P < 0.05. Figure 3C). TLR4 deficiency did not affect systolic blood pressure (Figure 3D).
Figure 3.
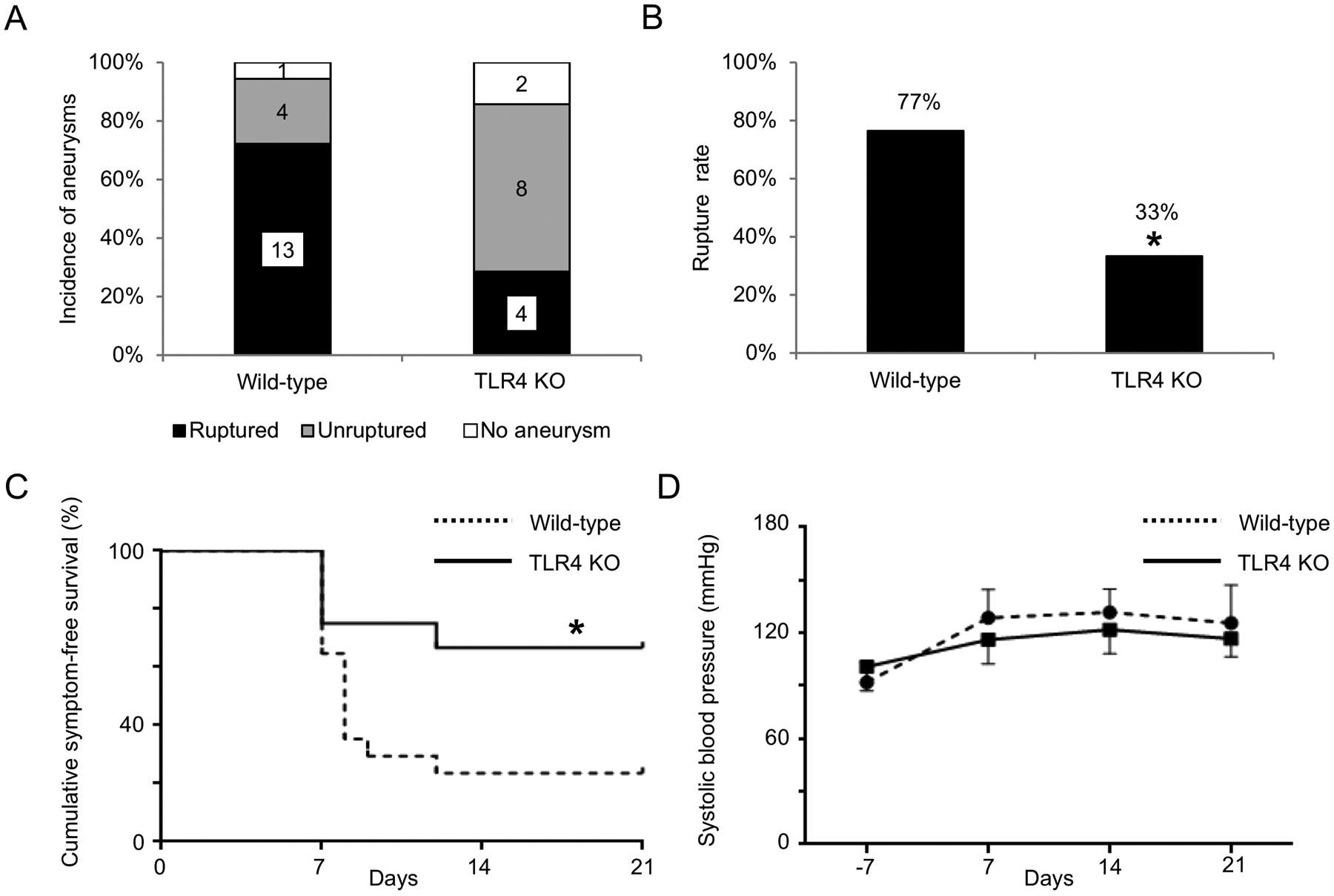
Toll-like receptor 4 (TLR4) knockout mice had a lower rupture rate than wild-type littermates. A. Incidence of unruptured and ruptured aneurysms. B. Rupture rate. C. Symptom-free curve (Kaplan–Meier analysis curve). Mice that did not have aneurysms were excluded from this analysis D. Systolic blood pressure. There was no difference in blood pressure between two group at any timepoint. *P < 0.05, **P < 0.01, TLR4 KO: toll-like receptor 4 knockout mice.
Lack of TLR4 reduced inflammatory cytokines in the cerebral arteries.
We measured the mRNA expression of inflammatory cytokines in the cerebral arteries with or without intracranial aneurysm induction in TLR4 knockout mice and control littermates. TLR4 knockout mice with aneurysm induction had the lower expression levels of IL-6, IL-1β, and TNF-α than the wild-type littermates with aneurysm induction (wild-type littermates versus TLR4 knockout mice; IL-6: 1.4 ± 0.7 versus 0.4 ± 0.3, P < 0.05; IL-1β: 0.6 ± 0.4 versus 0.2 ± 0.2, P < 0.05; TNF-α: 0.7 ± 0.3 versus 0.2 ± 0.2, P < 0.05. n = 5 in each group. Figure 4). There was no significant difference in the expression levels of inflammatory cytokines between TLR4 knockout mice without aneurysm induction and wild-type littermates without aneurysm induction (n = 5 in each group. Figure 4).
Figure 4.
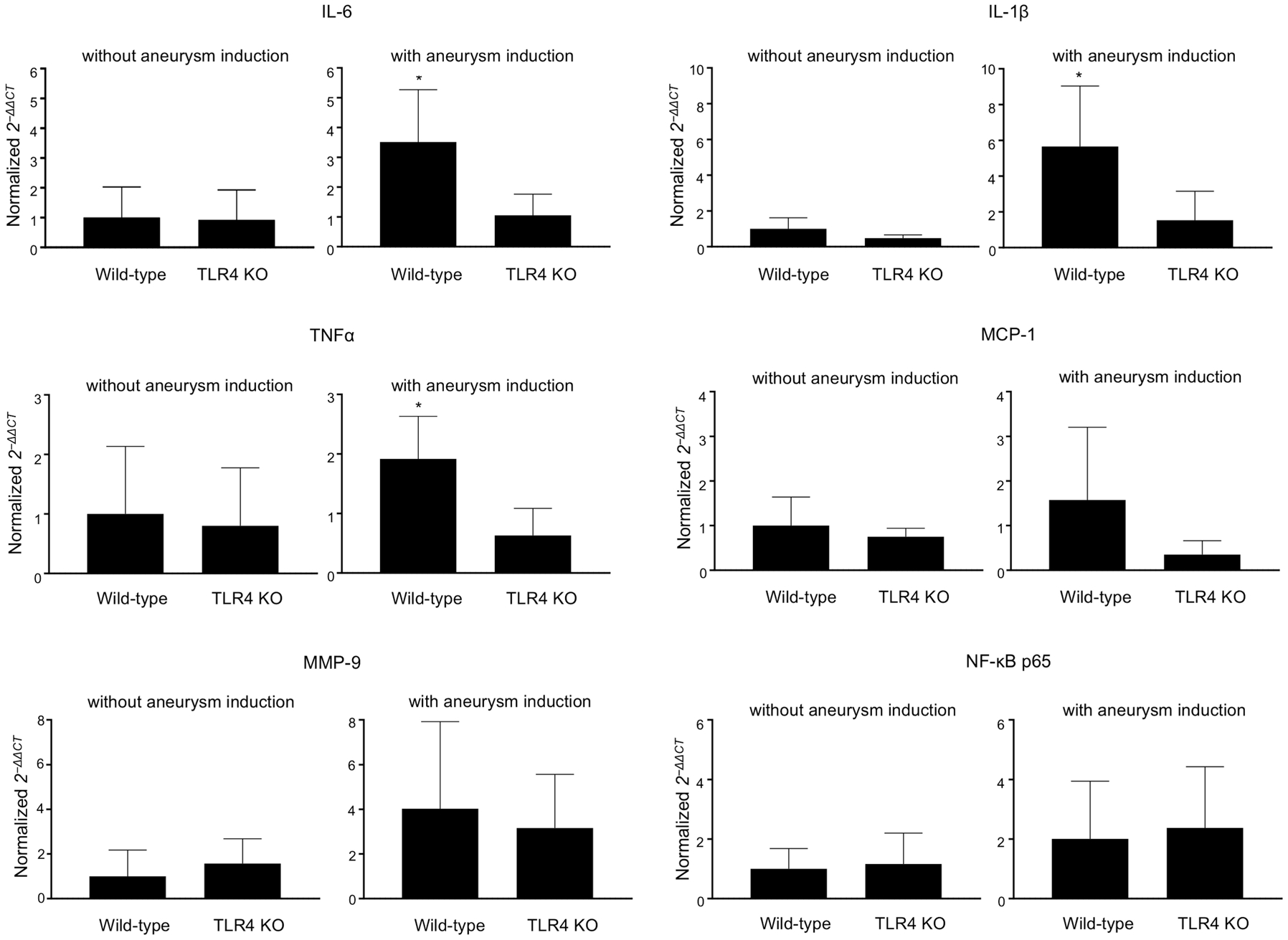
RNA expression of interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), monocyte chemoattractant factor-1 (MCP-1), matrix metalloproteinase (MMP)-9, and nuclear factor-kappa B (NF-κB) p65 in cerebral arteries from mice with or without induction of aneurysm (n = 5 each). *P < 0.05, **P < 0.01, TLR4 KO: toll-like receptor 4 knockout mice.
Lack of TLR4 on macrophage/granulocyte prevented intracranial aneurysmal rupture.
Although TLR4 is expressed in multiple cell types, TLR4 signaling in macrophage/monocyte is pivotal for tissue inflammation associated with pathological tissue remodeling,31–33 a biological process that plays critical roles in the rupture of intracranial aneurysms.24, 26 To test the contribution of the TLR4 on macrophage/monocyte to the development of aneurysmal rupture, we examine the development of aneurysmal rupture in mice lacking TLR4 in macrophage and monocytes, macrophage/granulocyte-specific TLR4 knockout mice (TLR4 flox/flox LysMCre +). There was no significant difference in the incidence of aneurysms between macrophage/granulocyte-specific TLR4 knockout mice and the control littermate mice (control littermate mice versus macrophage/granulocyte-specific TLR4 knockout mice; 88% versus 81%; n = 24 versus n = 16. Figure 5A). However, there was a lower rupture rate in macrophage/granulocyte-specific TLR4 knockout mice as compared to the control littermate mice (control littermate mice versus macrophage/granulocyte-specific TLR4 knockout mice; 91% versus 39%; P < 0.05; n = 21 versus n = 13. Figure 5B). The survival analysis using those mice that had aneurysms showed a lower rupture rate in macrophage/granulocyte-specific TLR4 knockout mice (P < 0.05. Figure 5C). There was no difference in blood pressure between macrophage/granulocyte-specific TLR4 knockout mice and control littermate mice (Figure 5D).
Figure 5.
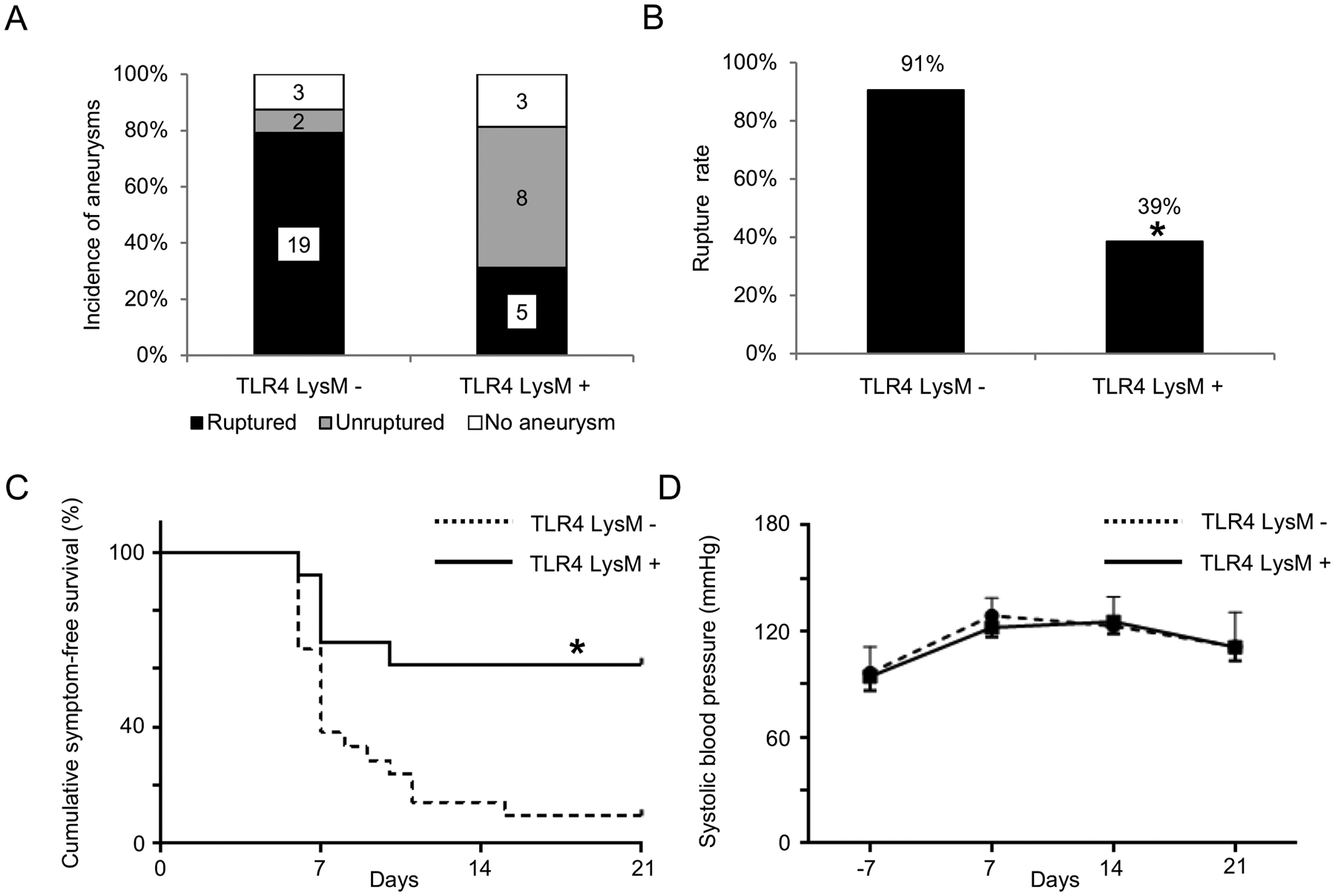
Macrophage/granulocyte-specific toll-like receptor 4 (TLR4) knockout mice (TLR4 flox/flox LysMCre +) had a lower rupture rate than control littermate mice (TLR4 flox/flox LysMCre −). A. Incidence of unruptured and ruptured aneurysms. B. Rupture rate. C. Symptom-free curve (Kaplan–Meier analysis curve). Mice that did not have aneurysms were excluded from this analysis. D. Systolic blood pressure. There was no difference in blood pressure between two group at any timepoint. *P < 0.05, **P < 0.01, TLR4 LysM - indicates toll-like receptor 4 flox/flox LysMCre - mice; TLR4 LysM +, TLR4 flox/flox LysMCre + mice.
MyD88 knockout showed reduced rupture rate
Since the activation of TLR4 mediates tissue inflammation through MyD88,10 we assessed the formation and rupture of intracranial aneurysm in MyD88 knockout mice. There was no significant difference in the incidence of aneurysms between the wild-type littermates and MyD88 knockout mice (wild-type littermates versus MyD88 knockout mice; 100% versus 95%; n = 24 versus n = 19. Figure 6A). However, MyD88 knockout mice had a lower rupture rate compared with wild-type littermates (wild-type littermates versus MyD88 knockout mice; 88% versus 53%; P < 0.05; n = 24 versus n = 18. Figure 6B). The survival analysis revealed a lower incidence of aneurysmal rupture in MyD88 knockout mice (P < 0.05. Figure 6C). There was no difference in blood pressure between MyD88 knockout mice and the wild-type littermates (Figure 6D).
Figure 6.
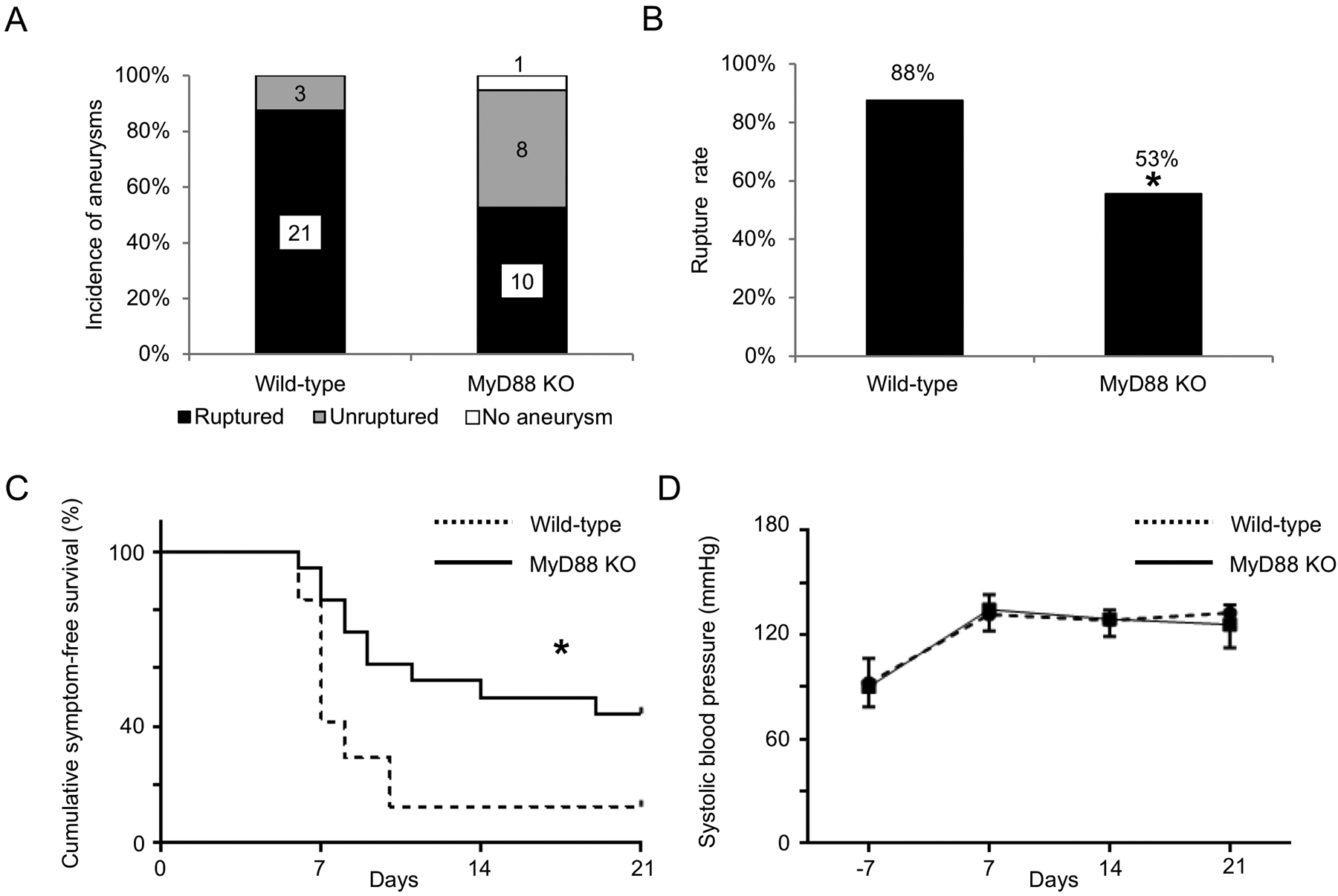
Myeloid differentiation primary-response protein 88 (MyD88) knockout mice had a lower rupture rate than wild-type littermates. A. Incidence of unruptured and ruptured aneurysms. B. Rupture rate. C. Symptom-free curve (Kaplan–Meier analysis curve). Mice that did not have aneurysms were excluded from this analysis. D. Systolic blood pressure. There was no difference in blood pressure between two group at any timepoint. *P < 0.05, **P < 0.01, MyD88 KO: myeloid differentiation primary-response protein 88 knockout mice
Discussion
In this study, we demonstrated that TLR4 contributes to the development of intracranial aneurysmal rupture by accelerating inflammation in mice. Mice treated with the TLR4 inhibitor showed a lower rupture rate than vehicle mice. We used TLR4 knockout mice to confirm the contribution of TLR4 in aneurysmal rupture. Gene expressions of inflammatory cytokines were suppressed in TLR4 knockout mice. In addition, we have shown that TLR4 on macrophages and monocytes mediates the promotion of aneurysmal rupture. Finally, the deficiency of the TLR4’s adaptor protein, MyD88, significantly reduced the rupture rate, suggesting that TLR4 may promote aneurysmal rupture through MyD88.
Although previous studies indicated potential associations between TLR4 and the pathophysiology of intracranial aneurysm,15, 34 a mechanistic link between TLR4 pathway and the development of intracranial aneurysmal rupture has not been established. TLR4 appears to be up-regulated during the formation and growth of intracranial aneurysms in rats.15 A gene microarray study suggested the association between a TLR4 single nucleotide gene polymorphism and the susceptibility for intracranial aneurysms.34 Our study represents a first study to show a potential causal link between TLR4 pathway and the development of aneurysmal rupture.
TLR4 was originally recognized as a critical receptor for innate immunity activated by exogenous ligands, such as lipopolysaccharide19, 35 or viral envelope glycoproteins.36 However, it has been shown that TLR4 can be activated by endogenous molecules, including products of the degeneration of extracellular matrix,37 heat shock proteins (HSPs),38, 39 and lipids,40 and contribute to the pathophysiology of vascular diseases by modulating vascular inflammation.37, 39 For example, in a mouse model of myocardial ischemia-reperfusion injury, the mice pretreated with TLR4 antagonist had significantly reduced myocardial infarction with the reduction of inflammatory cytokines.41 In another study, TLR4 deficiency decreased macrophage infiltration and the extent of aortic atherosclerosis in apolipoprotein E-deficient mice.17 In the present study, we measured expression levels of pro-inflammatory cytokines to examine the involvement of TLR4 in the inflammatory processes associated with aneurysmal rupture. The reduction of rupture rate in TLR4 knockout mice was associated with the decreased expression of TNF-α, IL-1β, and IL-6, cytokines that were previously implicated in the aneurysmal rupture.2, 6, 26, 42, 43 Furthermore, specific knockout of TLR4 in macrophage/granulocyte reduced the rupture rate more than the global knockout of TLR4. These results suggest that TLR4 contributes to the development of aneurysmal rupture through the modulation of inflammatory cytokine production, perhaps from macrophages and monocytes.
Activated TLR4 binds to MyD88, forming the complex with IL-1R-associated kinases (IRAKs). This complex involving TLR4 and MyD88 plays a critical role in the vascular inflammation and remodeling in the animal model of atherosclerosis12, 17 and myocardial ischemia.18 The formation of the TLR4-MyD88 complex enhances the production of inflammatory cytokines, including IL-1β, IL-6, MCP-1, and C-X-C motif ligand (CXCL) 1.12, 17, 18 These inflammatory cytokines and chemokines are associated with the development of aneurysmal rupture in previous studies.2, 6, 42–44 In the present study, TLR4 and MyD88 deficiency reduced the intracranial aneurysmal rupture rate and the expression levels of these inflammatory cytokines, indicating that activation of TLR4 may lead to the development of aneurysmal rupture through the activation of MyD88 and subsequent production of proinflammatory cytokines.
One of the intriguing findings of this study is that the roles of TLR4 and MyD88 were limited to the development of aneurysmal rupture, as the genetic deletion of TLR4 or MyD88 did not affect the formation of aneurysms while it reduced the development of aneurysmal rupture. Previous studies have also demonstrated the differential roles of inflammation and tissue remodeling between the formation of aneurysms and the development of aneurysmal rupture.2, 26 These studies collectively suggest that mechanisms for the development of aneurysmal rupture may be fundamentally different from mechanisms for the formation and growth of aneurysms. Therefore, it may be crucial to directly study mechanisms for the development of aneurysmal rupture in order to develop pharmacological therapies for the prevention of aneurysmal rupture.
Both clinical and animal studies showed the roles of hypertension in the formation of aneurysm and the development of aneurysmal rupture.23, 45–47 In our model, hypertension is required for the formation and rupture of aneurysms.23–25 As either angiotensin-II-induced hypertension or DOCA-salt hypertension can be used to induce the formation and rupture of aneurysms in this model,23, 24 individual components of DOCA-salt hypertension, namely DOCA, salt loading, or nephrectomy, are not required for this model. We have shown that the normalization of blood pressure by administration of anti-hypertensive agents after aneurysm formation prevented aneurysmal rupture,25 showing the critical role of high blood pressure in the development of aneurysmal rupture. In this study, the pharmacological inhibition or the genetic deletion of TLR4 reduced rupture rates without affecting hypertension. However, it is possible that TLR4 contributed to the development of aneurysmal rupture through the modulation of vascular inflammation or remodeling induced by hypertension.
There are several limitations in this study. First, the animal model may not completely replicate biological events that lead to aneurysm formation and growth, as aneurysms were induced, but not spontaneously formed. While many studies indicated the critical roles of vascular inflammation in the pathophysiology of intracranial aneurysms, there may be significant differences in the triggering factors of vascular inflammation between human aneurysms and this model. In addition, the time course of aneurysmal formation and rupture in this model is shorter than that of the human aneurysm. However, the phenotypes of intracranial aneurysms in the model closely mimic that of intracranial aneurysms in human.3, 24 More importantly, human intracranial aneurysms and aneurysms in this model share the end phenotypes, aneurysmal rupture and associated neurological symptoms, indicating the common biological processes between human intracranial aneurysms and this mouse model of intracranial aneurysm.3, 25 Second, it may be possible that the activation of TLR4 leads to the production of inflammatory cytokines without involving MyD88. TLR4 can interact with TIR domain-containing adaptor protein–inducing interferon-β (TRIF) and promote expressions of interferon (IFN) -β and IFN-inducible products.10, 48 This pathway may contribute to TLR4’s promotion of aneurysmal rupture. Third, this study focused on the contribution of TLR4 on inflammatory cells, as TLR4’s major roles are often mediated by inflammatory cells.10, 13 However, it is possible that TLR4 on other cell-type may contribute to the development of aneurysmal rupture.
As an initial step to study the contribution of TLR4 pathway to the development of intracranial aneurysmal rupture, we used only male mice in this study. Our previous studies showed that the protective effects of estrogen against the formation and rupture of intracranial aneurysms using male, non-ovariectomized female, and ovariectomized female mice.49 However, a comparison of these three groups would make the experimental design far more complex and highly expansive. Effects of sex differences and sex steroids on the roles of TLR4 in the development of aneurysmal rupture should be carefully examined in future studies.
In summary, our findings suggest that TLR4 pathway promotes intracranial aneurysmal rupture by modulating inflammation through MyD88. Future clinical studies that investigate the relationship between aneurysmal rupture and TLR4 pathways will be needed to validate our findings. Inhibition of TLR4 pathway in inflammatory cells may be a potentially promising strategy for the prevention of aneurysmal rupture and subsequent subarachnoid hemorrhage.
Perspective
This study showed a potential role of the TLR4 pathway in inflammatory cells in the development of intracranial aneurysm rupture. As the immune system involving TLR4 is highly complex, further mechanistic and clinical studies are needed to firmly establish the contribution of TLR4 pathway to the development of aneurysmal rupture and subarachnoid hemorrhage.
Supplementary Material
Novelty and Significance.
1). What Is New?
This study provides first evidence suggesting the link between TLR4 pathway and intracranial aneurysmal rupture.
2). What Is Relevant?
This study provides a basis for future human studies to firmly establish the contribution of TLR4 pathway in inflammatory cells to intracranial aneurysmal rupture and subsequent subarachnoid hemorrhage.
3). Summary
We demonstrated that TLR4 pathway plays a pivotal role in the pathophysiology of intracranial aneurysmal rupture by modulating inflammation in mice. Inhibition of TLR4 pathway in inflammatory cells may be a promising direction for the prevention of aneurysmal rupture and subsequent subarachnoid hemorrhage.
Acknowledgments
The authors would like to thank Ms. Cindy Giljames and Ms. Chinami Michaels (Barrow Neurological Institute Neuroscience Publications) for the production of graphic abstract.
Sources of funding
The project was supported by grant number R01NS082280 (TH) R01NS109382 (TH), and R01NS109584 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), Barrow Neurological Foundation (TH), and Brain Aneurysm Foundation (TH). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
Conflicts of Disclosures
None.
Reference
- 1.Thompson BG, Brown RD Jr., Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr., Duckwiler GR, Harris CC, Howard VJ, Johnston SC, Meyers PM, Molyneux A, Ogilvy CS, Ringer AJ, Torner J, American Heart Association Stroke Council CoC, Stroke N, Council on E, Prevention, American Heart A, American Stroke A. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2368–2400. [DOI] [PubMed] [Google Scholar]
- 2.Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Protective Role of Peroxisome Proliferator-Activated Receptor-gamma in the Development of Intracranial Aneurysm Rupture. Stroke. 2015;46:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke. 2012;43:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, Lawton MT, Hashimoto T. Roles of Nicotine in the Development of Intracranial Aneurysm Rupture. Stroke. 2018;49:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasan DM, Mahaney KB, Brown RD Jr., Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC, International Study of Unruptured Intracranial Aneurysms I. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, Owens GK, Dumont AS. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflammation. 2012;9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 11.Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P. Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Invest. 2009;119:1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen H, Eguchi K, Kono N, Fujiu K, Matsumoto S, Shibata M, Oishi-Tanaka Y, Komuro I, Arai H, Nagai R, Manabe I. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arterioscler Thromb Vasc Biol. 2013;33:2596–2607. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Wang J, Wang S, Zhang D, Zhao Y, Wang R, Zhao J. DNA Methylation Regulates Gene Expression in Intracranial Aneurysms. World Neurosurg. 2017;105:28–36. [DOI] [PubMed] [Google Scholar]
- 15.Aoki T, Nishimura M, Ishibashi R, Kataoka H, Takagi Y, Hashimoto N. Toll-like receptor 4 expression during cerebral aneurysm formation. Laboratory investigation. J Neurosurg. 2010;113:851–858. [DOI] [PubMed] [Google Scholar]
- 16.Fang H, Wang PF, Zhou Y, Wang YC, Yang QW. Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflammation. 2013;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W. Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem. 2011;286:31308–31319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 20.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAlees JW, Whitehead GS, Harley IT, Cappelletti M, Rewerts CL, Holdcroft AM, Divanovic S, Wills-Karp M, Finkelman FD, Karp CL, Cook DN. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2015;8:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. [DOI] [PubMed] [Google Scholar]
- 23.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Kitazato KT, Nagahiro S, Hashimoto T. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014;45:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, Nagahiro S, Hashimoto T. Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35:1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J Neurosci. 2015;35:13487–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doring C, Regen T, Gertig U, van Rossum D, Winkler A, Saiepour N, Bruck W, Hanisch UK, Janova H. A presumed antagonistic LPS identifies distinct functional organization of TLR4 in mouse microglia. Glia. 2017;65:1176–1185. [DOI] [PubMed] [Google Scholar]
- 29.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CR. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 30.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. [DOI] [PubMed] [Google Scholar]
- 31.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52:318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. [DOI] [PubMed] [Google Scholar]
- 33.Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli FR, Amann-Vesti B, Gay S, von Eckardstein A, Luscher TF, Maier W. Cellular actors, Toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J. 2010;31:1457–1469. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Zhang Q, Xiong XY, Gong QW, Liao MF, Yang QW. TLR4 gene polymorphisms rs11536889 is associated with intracranial aneurysm susceptibility. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2018;53:165–170. [DOI] [PubMed] [Google Scholar]
- 35.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 36.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. [DOI] [PubMed] [Google Scholar]
- 37.van Keulen JK, de Kleijn DP, Nijhuis MM, Busser E, Velema E, Fijnheer R, van der Graaf Y, Moll FL, de Vries JP, Pasterkamp G. Levels of extra domain A containing fibronectin in human atherosclerotic plaques are associated with a stable plaque phenotype. Atherosclerosis. 2007;195:e83–91. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Zhang C, Wei X, Li P, Cui Y, Qin Y, Wei X, Jin M, Kohama K, Gao Y. Heat shock protein 60 stimulates the migration of vascular smooth muscle cells via Toll-like receptor 4 and ERK MAPK activation. Scientific reports. 2015;5:15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218, 221p following 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–274. [DOI] [PubMed] [Google Scholar]
- 42.Hosaka K, Rojas K, Fazal HZ, Schneider MB, Shores J, Federico V, McCord M, Lin L, Hoh B. Monocyte Chemotactic Protein-1-Interleukin-6-Osteopontin Pathway of Intra-Aneurysmal Tissue Healing. Stroke. 2017;48:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikedo T, Minami M, Kataoka H, Hayashi K, Nagata M, Fujikawa R, Higuchi S, Yasui M, Aoki T, Fukuda M, Yokode M, Miyamoto S. Dipeptidyl Peptidase-4 Inhibitor Anagliptin Prevents Intracranial Aneurysm Growth by Suppressing Macrophage Infiltration and Activation. Journal of the American Heart Association. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu Y, Wilson K, Gu H, Wegman-Points L, Dooley SA, Pierce GL, Cheng G, Pena Silva RA, Heistad DD, Hasan D. Myeloperoxidase is increased in human cerebral aneurysms and increases formation and rupture of cerebral aneurysms in mice. Stroke. 2015;46:1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juvela S Recommendations for the management of patients with unruptured intracranial aneurysms. Stroke. 2001;32:815–816. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats. Surgical neurology. 1978;10:3–8. [PubMed] [Google Scholar]
- 47.Hashimoto T, Meng H, Young WL. Intracranial aneurysms: links among inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satoh J, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T, Saito Y. TMEM119 marks a subset of microglia in the human brain. Neuropathology. 2016;36:39–49. [DOI] [PubMed] [Google Scholar]
- 49.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75:690–695; discussion 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


