Cellular pH homeostasis is regulated through the activities of N transporters and proton pumps affecting proton production or consumption during root acquisition, short and long-distance transport, and assimilation of N.
Keywords: Ammonium, assimilation, ATPase, charge balance, cellular pH, homeostasis, nitrate, pump, transport, uptake
Abstract
The enzymatic controlled metabolic processes in cells occur at their optimized pH ranges, therefore cellular pH homeostasis is fundamental for life. In plants, the nitrogen (N) source for uptake and assimilation, mainly in the forms of nitrate (NO3–) and ammonium (NH4+) quantitatively dominates the anion and cation equilibrium and the pH balance in cells. Here we review ionic and pH homeostasis in plant cells and regulation by N source from the rhizosphere to extra- and intracellular pH regulation for short- and long-distance N distribution and during N assimilation. In the process of N transport across membranes for uptake and compartmentation, both proton pumps and proton-coupled N transporters are essential, and their proton-binding sites may sense changes of apoplastic or intracellular pH. In addition, during N assimilation, carbon skeletons are required to synthesize amino acids, thus the combination of NO3– or NH4+ transport and assimilation results in different net charge and numbers of protons in plant cells. Efficient maintenance of N-controlled cellular pH homeostasis may improve N uptake and use efficiency, as well as enhance the resistance to abiotic stresses.
Introduction
Nitrogen (N) is required for plants to complete their life cycles and is the most important nutrient acquired in greatest quantities by roots (Xu et al., 2012; Oosterhuis et al., 2014). NO3– and NH4+ are the most prominent forms of inorganic N taken up by land plant species, and their root uptake rapidly causes primary effects on ionic and pH balance in plant cells. Cellular homeostasis of ions and pH is fundamental to basic cellular processes and is needed to maintain normal plant growth and development as well as responses to stresses (Bassil and Blumwald, 2014; Reguera et al., 2015). In addition, pH varies within different intracellular compartments and the proton gradient is important for the viability of cells (Shen et al., 2013).
Within plant cells, several compartments with different pH exist in parallel. The cytosol has pH values at 7.2–7.4 to ensure proper biochemical reactions (Schumacher, 2014), while the vacuole and apoplast maintain more acidic pH levels at 5.0–5.5 (Felle, 2001; Martinière et al., 2013a; Shen et al., 2013; Schumacher, 2014). Cytoplasmic pH (pHc) homeostasis is the result of a variety of processes. First, cytoplasmic chemical buffering components, such as bicarbonate, phosphate, and protein buffers, play important roles in stabilizing pHc (Kurkdjian and Guern, 1989). Secondly, the physical pH-stat, which is proton transport across membranes, contributes to pHc homeostasis (Felle 2001; Britto and Kronzucker, 2005). The maintenance of optimal pH in plant cells has to be tightly regulated and is established by different primary active H+ pumping complexes, such as the plasma membrane (PM) or P-type H+-ATPase (PM-ATPase), vacuolar H+-ATPase (V-ATPase), and the vacuolar H+-pyrophosphatase (V-PPase) (Schumacher, 2006; Gaxiola et al., 2007; Marshansky and Futai, 2008). The P-type ATPases can be present in both the PM and vacuole (Li et al., 2016). The physical pH-stat is also determined by transport of other ions to maintain the electrochemical balance, and H+-coupled ion transporters contribute to intracellular pH homeostasis (Gerendás and Schurr, 1999; Reguera et al., 2015). Thirdly, a biochemical pH-stat participates in pHc regulation, including the metabolic processes of proton production or consumption, and organic acid production or degradation (Raven and Smith, 1976; Felle, 2001; Britto and Kronzucker, 2005). For example, the malate anion shuttle between the cytosol and vacuole is an important element of pHc regulation (Raven and Smith, 1976; Felle, 2001; Britto and Kronzucker, 2005). The primary root acquisition of NO3– and/or NH4+ dominates anion and cation balance in plant cells, with uptake and vacuolar storage driven by PM-ATPases, V-ATPases, and V-PPases, while they consume energy and are essential components of cellular pH homeostasis providing a ‘physical pH-stat’ (Serrano, 1990; Barkla and Pantoja, 1996; Sze et al., 1999; Martinoia et al., 2000; Palmgren, 2001). In addition, the processes of NO3– and NH4+ assimilation inside the cell are considered to consume or produce protons, contributing to ‘biochemical pH-stat’ (Britto and Kronzucker, 2005; Fan et al., 2016, 2017). In addition, NO3– reduction leads to biochemical pH-stat by increasing malate and other organic acid anions (van Beusichem et al., 1988; Lüttge et al., 2000; Pasqualini et al., 2001).
In this review, we summarize the general behaviours of N uptake, distribution, and assimilation inducing changes in plant cellular and rhizosphere pH. We discuss the regulatory mechanisms of the maintenance of cellular pH under altered N supplies in both physiology and molecular aspects.
Regulation of pH by N acquisition: from cell to rhizosphere
In response to the uptake of varied N forms, plants change their ionic balance, cellular transmembrane electric potentials, and proton pumping activity, resulting in altered cellular and rhizosphere pH.
N supply-controlled ionic and electronic balance in plants
Plant uptake of NH4+ or NO3– accompanies the flux of other nutrient ions including K+, Cl–, and H+ for charge balance. It is well known that an antagonism or a cooperation between NH4+ or NO3– and potassium (K+) arises from their charge and influence on the membrane potential, namely K+–NH4+ competition and K+–NO3– cooperation (Li et al., 2016; reviewed by Coskun et al., 2017). NH4+ competes with low-affinity K+ uptake and accumulation (Wang et al., 1996; Szczerba et al., 2008; Hoopen et al., 2010; Chen et al., 2015). The acquisition rates of cationic K+ and anionic NO3– are often found to be positively correlated, probably due to improved charge balance or activation of the enzymes involved in NO3– assimilation (Hagin et al., 1990; Roosta and Schjoerring, 2008; Balkos et al., 2010; Yang et al., 2014; Xia et al., 2015). NO3– is transported from root to shoot with K+ as a counter ion in the xylem; thus, limited K+ supply can result in high accumulations of NO3– in roots (Rufty et al., 1981; Förster and Jeschke, 1993). Knockout of the nitrate transporter AtNPF7.3/NRT1.5 in Arabidopsis and OsNPF2.4 in rice not only decreased NO3– loading to xylem sap, but also limited K+ content in the xylem (Lin et al., 2008; Xia et al., 2015; Li et al., 2017), indicating the interaction of NO3– and K+ in plant cells.
In the vacuole, the monovalent anions NO3–, malate, and Cl– show an interaction; for example, the Cl– concentration in leaves can be reduced by the NO3– supply (Glass and Siddiqi, 1985; Guo et al., 2017). Two maize nitrate transporters, ZmNPF6.4 and ZmNPF6.6, are permeable to both NO3– and Cl– (Wen et al., 2017), indicating that the two anions could be facilitated by the similar transport systems in plants. There are also chloride-specific MATE transporters in the vacuolar membrane (Zhang et al., 2017). Diurnal changes in vacuolar malate have been observed to compensate for NO3– and K+ fluctuations (Niedziela et al., 1993).
Instant response of cellular membrane potential and pH
The cell membrane potential (∆Ψ, negative inside the cell compared with outside the cell) can be affected by fluxes of charged ions across the PM. An immediate physiological response of root cells to NH4+ and NO3– exposure is a transient change of ∆Ψ, which is caused by NH4+ and NO3– influx carrying H+ into the cell and compensated by activation of the PM H+-ATPase to repolarize and maintain ∆Ψ (Ullrich and Novacky, 1990; Wang et al., 1994; Liu et al., 2017). However, the initial membrane depolarization was not commensurate with the increased influx of NH3/NH4+ (pKa 9.25) at pH 6.25 in the medium in roots of barley, suggesting that the increased transport of electroneutral NH3 dominates uptake (Coskun et al., 2013). NO3– is co-transported with H+ through a symporter into cells, and the stoichiometry of NO3– and H+ is ~2 (Glass et al., 1992; Miller and Smith, 1996). Root NO3– acquisition commonly leads to ∆Ψ depolarization of the cells suggesting an H+ stoichiometry >1 (Meharg and Blatt, 1995; Mistrik and Ullrich, 1996; Britto and Kronzucker, 2005).
It is controversial whether such transport mechanisms would lead to longer term cytosol alkalinization by NH4+/NH3 uptake or acidification by NO3– uptake, but at least in the initial period after the addition of NH4+ or NO3– some pH changes are generally accepted. For NO3– uptake, only small changes in cytoplasmic pH occurred in roots of maize seedlings growing in nutrient solutions at different pH and supplemented with normal NO3– (5 mM) (Gerendás et al., 1990). It is proposed that these results are attributed to the presence of tight regulatory mechanisms for intracellular pH. An important component of NH4+/NH3 or NO3– uptake in plants is the assimilatory consumption of these ions. An initial NO3–-induced cytosolic acidification was measured in Limnobium stoloniferum root hairs (Raven, 1985, 1986; Ullrich and Novacky, 1990). NO3– assimilation, which is a proton-consuming process, might cause an increase of cytoplasmic pH and thus partially compensate for H+ influx coupled with NO3– uptake. In maize roots, the inhibition of NO3– assimilation using tungstate, an inhibitor of NO3– reductase activity, resulted in acidification of the cytosol (Espen et al., 2004). Another regulatory mechanism to prevent NO3– uptake generating acidification of the cytoplasm is an increase in PM-ATPase activity. Decreased cytoplasmic pH is a signal triggering the PM-ATPase to pump H+ out of the cytosol (Espen et al., 2004) and hyperpolarize the PM ∆Ψ (Glass et al., 1992; McClure et al., 1990a, b). In contrast to NO3–, the effect of NH4+ uptake on intracellular pH is dependent on external medium pH (Gerendás et al., 1990; Kosegarten et al., 1997; Gerendás and Ratcliffe, 2000). Maize root tip intracellular pH showed no change at external pH 6, but decreased at pH 4 and increased at pH 8 with 5 mM NH4+ supply (Gerendás et al., 1990). At high external pH, the NH4+/NH3 equilibrium shifts in favour of the NH3 molecule that readily permeates the PM through aquaporins (Kleiner, 1981; Macfarlane and Smith, 1982; Coskun et al., 2013). At external pH 9, both the cytosol and vacuole were alkalinized in 1 h with NH4+ supply from 5 mM to 20 mM (Gerendás and Ratcliffe, 2000). Both NH4+ transport and assimilation were assumed to contribute to the alkalinization of cytosolic pH (Kosegarten et al., 1997). In the external pH range from 5 to 7, the cytoplasmic buffer capacity may be able to balance the NH4+-elicited pH changes (Kosegarten et al., 1997).
Some caution is needed when evaluating the influence of other accompanying cations (e.g. K+, Mg2+, or Ca2+) and anions (e.g. Cl–) on the alteration of cellular pH grown with NO3– and NH4+ supply. For example, increased H+/K+ antiport at the PM under high K+ supply may compensate for the NO3– uptake-induced cytosolic acidification via 2H+/NO3– symport (Kurkdjian and Guern, 1989; Ullrich and Novacky, 1990; Guern et al., 1991; Briskin and Hanson, 1992; Sacchi and Cocucci, 1992; Nocito et al., 2002).
Activity of ATPase and PPase in response to alternative supplies of N
The activity of membrane ATPases, PPases, and H+-coupled transporters establishes and can regulate cytoplasmic pH homeostasis. The PM H+-ATPase plays an important physiological role in maintaining the plasma membrane electrical potential difference and generating a transmembrane H+ chemical gradient (∆H; acidic on the outside) during the uptake of nutrients (Palmgren, 2001; Falhof et al., 2016). For example, it was found that adding PM H+-ATPase inhibitors dramatically decreased root NO3– uptake (McClure et al., 1990b), and eliminated the NH4+ uptake-generated depolarization of ∆Ψ (Wang et al., 1994). In early adjustment to N uptake, the PM H+-ATPase plays an important role in maintaining cytosolic pH homeostasis. When compared with CaSO4 solution, (NH4)2SO4 induced the PM H+-ATPase activity in roots of barley seedlings (Yamashita et al., 1995). Similarly, Ca(NO3)2 treatment also induced a significantly higher transcription of PM-ATPase genes after a 3 h exposure and a significantly higher protein concentration and activity after a 6 h exposure (Santi et al., 2003). Interestingly, PM H+-ATPase activity including both hydrolytic and H+-pumping activity and its related gene expression showed no difference in rice plants grown in 2.5 mM NH4+ or NO3– solution when the solution was buffered at the same pH (Zhu et al., 2009).
NO3– transport into the vacuole from the cytosol is mediated by an H+/NO3– antiport mechanism, which is driven by P- and V-type ATPases and V-PPase activity (Granstedt and Huffaker, 1982; Blumwald and Poole, 1985; Schumaker and Sze, 1987; Glass et al., 1992; Miller and Smith, 1992; Krebs et al., 2010). High concentrations of NO3– could inhibit V-ATPase activity in isolated vacuoles (Blumwald and Poole, 1985). Inhibiting the activity of V-ATPase or V-PPase or knockout of their encoding genes significantly decreased NO3– storage and influx into vacuoles of Brassica napus plants (Han et al., 2016).
Factors dominating N supply effects on rhizosphere pH
Soil alkalinity above pH 8.0 or acidity below pH 5.5 limits plant growth and development (Schubert et al., 1990; Koyama et al., 2001; Cha-Um et al., 2009; Patil et al., 2012). Uptake of NH4+ or NO3– (i.e. transport and assimilation) results in rapid acidification or alkalinization of the apoplast (Geilfus, 2017) and rhizosphere (Taylor and Bloom 1998; Kosegarten et al., 1997; Gerendás and Ratcliffe, 2000; Ruan et al., 2000; Hinsinger et al., 2003). It has been shown that decreasing external pH to acidic levels can up-regulate the expression of 20–41% of the NH4+-responsive genes in Arabidopsis thaliana, suggesting that apoplastic acidification is a component of NH4+-induced stress (Patterson et al., 2010).
The N supply factors causing changes in rhizosphere or apoplastic pH include N concentrations and forms, balance of N with other major nutrients, and plant species. (i) High NH4+ supply induced rhizosphere acidification and high NO3– induced alkalinization (Marschner and Römheld, 1983; Römheld, 1986; Hinsinger et al., 2003) controlled by the processes of N transport (see ‘Extra- and intracellular pH regulation at short- and long-distance N distribution’) and assimilation (see ‘Cellular pH homeostasis during N assimilation’). (ii) For charge balance, NO3– may increase, while NH4+ decreases, cation uptake by root cells. The imbalanced uptake of cations and anions triggers release of H+ or OH– (or HCO3–) into the apoplast, resulting in opposing pH changes in the rhizosphere (Haynes, 1990; Marschner, 1995; Hinsinger et al., 2003). (iii) The extent of the N supply-induced pH change in the rhizosphere or apoplast is also dependent on plant species. For example, the rhizosphere of lentils and chickpea could be acidified even at relatively high NO3– supply (Römheld, 1986). The effects of N supply on rhizosphere pH can be simply shown using pH indicators in agar (see Fig. 1 for rice).
Fig. 1.
Rhizosphere pH regulated by uptake of NH4+ and NO3– in rice roots. (A) The rhizosphere pH of rice roots shown with a colour pH indicator. (B) Agar profile showing rhizosphere pH after removing the roots. Rice seedlings (Oryza sativa L ssp. japonica, Nipponbare) were grown in full nutrient solution containing 1.25 mM NH4NO3 for 4 weeks and then transferred to 2.5 mM NH4+ or 2.5 mM NO3– for 72 h. After 72 h N treatment, the plant root was washed by dipping in 0.2 mM CaSO4 for 1 min before placement on the agar. An intact plant was placed on agar (0.9 g l–1, containing the pH indicator (0.03 g l–1 bromocresol purple). The initial pH was 5.2–5.3 from 11.00 h to 11.30 h, roots were kept in darkness covered with a moist paper tissue and under a 0.5×12×12 cm3 Plexiglas plate, and the picture was taken after 2–4 h in contact with the pH indicator agar. (C) pH of the hydroponic growth medium during 2.5 mM NH4+ or 2.5 mM NO3– solution after 24, 48, and 72 h. The initial pH was 5.2–5.3.
Extra- and intracellular pH regulation at short- and long-distance N distribution
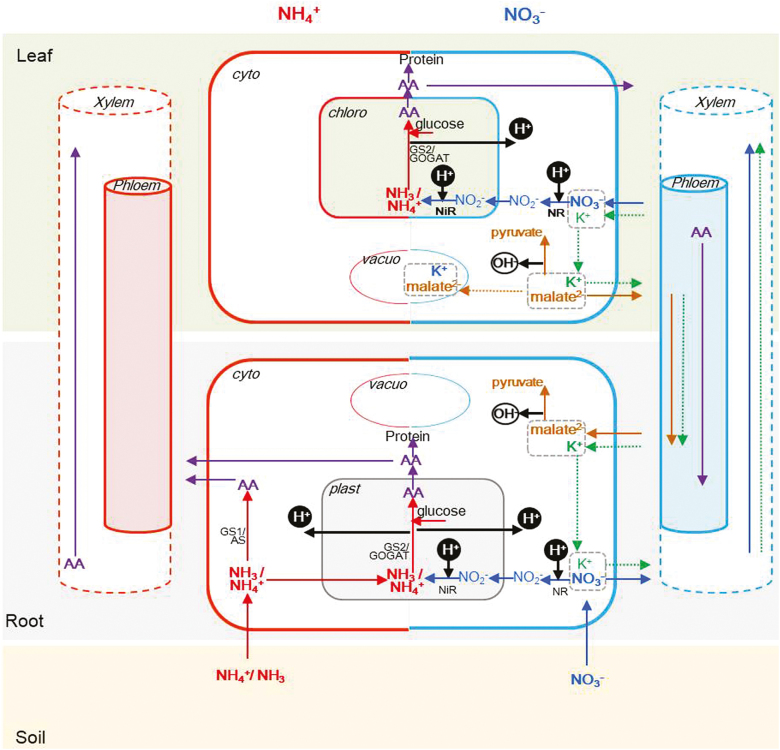
A variety of root and shoot NH4+ and NO3– transporters may be involved in cellular pH homeostasis through the processes of H+ production or consumption within cellular compartments (Fig. 2). Cellular pH homeostasis is also dependent on the activity of the proton pumps, the PM-ATPase, V-ATPase, and V-PPase (Fig. 2). NH4+ transport is controlled by NH4+ transporters (AMTs) and non-saturable low-affinity uptake systems (i.e. aquaporins TIPs or cation channels) in plants. NO3– transport is mediated by the NO3– Transporter (NRT1 and NRT2) family, and the NRT1 family is renamed the NO3– Transporter1/Peptide Transporter Family (NPF) (Léran et al., 2013). The Chloride Channel (CLC) family also function as anion/proton exchangers or anion channels (De Angeli et al., 2006), mediating NO3– transport at the vacuole or in endomembrane vesicles (Zifarelli and Pusch, 2010).
Fig. 2.
Protons are involved in NH4+ and NO3– fluxes. Different transporters or channels for the fluxes of NH4+ (red arrow), NO3– (blue arrow), and H+ (black arrow). Potassium channels (AKT1), non-selective cation channels (NSCC), and aquaporins (AQP, TIP) are NH4+/NH3 channels (Hachiya and Sakakibara, 2017; Liu and von Wirén, 2017). AMT1 is an NH4+ transporter functioning as an NH4+ or NH3 channel, NH4+ uniporter, or H+/NH4+ antiporter (Giehl et al., 2017; Duan et al., 2018; reviewed by Tegeder and Masclaux-Daubresse, 2018). NPF and NRT2 are plasma membrane (PM) or tonoplast NO3– transporters functioning as an H+/ NO3– symporter or an NO3– excretion transporter (reviewed by Fan et al., 2017; Wang et al., 2018). CLCa and CLCb are tonoplast-localized chloride transporters functioning as H+/NO3– antiporters (reviewed by Zifarelli and Pusch, 2010). Intracellular pH maintenance is also established by different primary active H+ pumping complexes, such as the PM H+-ATPase (PM-ATPase), the vacuolar H+-ATPase (V-ATPase), and V-PPase (reviewed by Gaxiola et al., 2007). Cyto, cytosol. Vacuo, vacuole.
For inorganic N transporters in plants, readers are also referred to previously published reviews (Léran et al., 2013; Fan et al., 2017; Tegeder and Masclaux-Daubresse, 2018; Wang et al., 2018). Here we focus on the plant NH4+ and NO3– transporters which are involved in maintaining pH balance both in vitro and in vivo.
H+/NO3– symporters are involved in regulation of cellular pH and ion homeostasis
Both NO3– and NH4+ can be imported into root cells by H+-coupled symporters across the PM through energetically uphill processes. Most members of the nitrate transporter families NPF/NRT1 and NRT2 showed characteristics of pH-dependent NO3– transport when expressed in Xenopus laevis oocytes. After injection of the NPF/NRT1 and NRT2 genes, the oocytes showed NO3–-elicited inward current and the pH dependency (i.e. NO3–-induced current is larger at pH 5.5 than at pH 7.4) that is associated with a H+-symport mechanism (Søgaard et al., 2009; Ortiz-Ramirez et al., 2011; Fan et al., 2017; Wang et al., 2018). Many results indicate that the NPFs function as H+/NO3– co-transporters, which mediate the influx with the H+/NO3– ratio being greater than one (Zhou et al., 1998; Lin et al., 2008). AtNPF6.3/NRT1.1/CHL1 is one of the exceptions, which is identified as both a pH-dependent importer (Tsay et al., 1993; Liu et al., 1999; Wang et al., 2018) and a pH-independent exporter (Léran et al., 2013). AtNPF6.3/NRT1.1/CHL1 knockout (point mutation of P492L, chl1-9) led to impaired H+ tolerance and the disappearance of alkalinization in NO3–-sufficient growth medium (Fang et al., 2016), indicating that NRT1.1-mediated NO3– uptake contributes to plant H+ tolerance by alkalinization of the rhizosphere. However, knockout of other nitrate transporters such as AtNPF4.6/ATI1/NRT1.2, AtNRT2.1, AtNRT2.2, and AtNRT2.4 did not alter the plant H+ tolerance (Fang et al., 2016). Since NRT1.1 may contribute to root NO3– uptake by 70–80% (Huang et al., 1996; Wang et al., 1998; Orsel et al., 2004; Krouk et al., 2010; Kiba et al., 2012), it is possible that the activity of NRT1.1 masked the effect of other H+-coupled NO3– transport in the tolerance to rhizosphere acidity. Furthermore, the mechanism of H+ movement via water molecules in the peptide-binding site for some members of the NRT1/NPF/POT family of secondary active transporters was suggested to provide a mechanism enabling the proteins to transport many diverse substrates (Parker et al., 2017). Effectively, this mechanism separates substrate recognition from H+ translocation in this family of transporters.
Two members of the plant AMT family, common bean AMT1;1 and wheat AMT1;1, are characterized as H+-coupled importers. Expression of common bean PvAMT1;1 in oocytes led to NH4+-elicited inward currents and cytosolic acidification, indicating that it functions as an H+/NH4+ symporter in a 1:1 ratio (Ortiz-Ramirez et al., 2011). The activity of PvAMT1;1 was enhanced by low extracellular pH (pH 5.5), and this was demonstrated by changes in the reversal potential and by increased cytoplasm acidification measured with pH-selective microelectrodes (Ortiz-Ramirez et al., 2011). However, there was no direct evidence to show whether PvAMT1;1 was related to H+ exchange in both the cytosol and rhizosphere in vivo.
Currently, it is not clear if xylem pH is regulated by H+/NO3– co-transport. A PM NO3– transporter, AtNPF7.3/NRT1.5, which is abundantly expressed in the pericycle or xylem parenchyma cells, mediates both pH-dependent NO3– influx and efflux in oocytes, and release of NO3– from the Arabidopsis root pericycle (Lin et al., 2008). These authors proposed that there is a potential link between xylem pH and root-to-shoot NO3– transport. However, AtNPF7.3/NRT1.5 is also identified as a H+-coupled H+/K+ antiporter in Xenopus oocytes, and functions in facilitating K+ loading into the xylem (Li et al., 2017). Thus, it is unclear whether the long-distance transport of NO3– and/or K+ contributed by NPF/NRT1s such as AtNPF7.3/NRT1.5 can alter pH in the xylem.
The NRT2s are another important family of NO3– transporters, mediating uptake from the soil and transport to leaf cells and developing seeds (Xu et al., 2012; Fan et al., 2017). One of the first members of this family to be functionally characterized in oocytes was suggested not only to be an H+-coupled NO3– symporter, but also to operate in an NO3– transport mode uncoupled to H+ movement (Zhou et al., 2000). This alternative mechanism may be beneficial when external NO3– is very abundant, avoiding the pH problems that might be associated with H+ influx and cytosolic acidification. Some of the NRT2 transporters require a partner protein (NAR2) for function (Orsel et al., 2006; Feng et al., 2011; Yan et al., 2011). In both Arabidopsis and rice, it has been shown that NAR2 is required for the targeting of the NRT2 protein from internal membrane vesicles to the PM (Wirth et al., 2007; Liu et al., 2014). The accumulation of the NRT2 transporter protein may provide a mechanism for altering the pH of these endomembrane vesicles.
In the rice genome, the OsNRT2.3 gene encodes two members of a H+-coupled nitrate transporter family, OsNRT2.3a and OsNRT2.3b (Feng et al., 2011; Yan et al., 2011). OsNRT2.3a is located in root stellar cells and plays an important role in distribution of NO3– from root to shoot (Tang et al., 2012), while OsNRT2.3b is expressed in phloem and contributed to phloem pH and ion homeostasis (Fan et al., 2016). OsNRT2.3b expression in oocytes elicited a depolarized membrane potential and cytosolic acidification in response to NO3– supply (Fan et al., 2016). Notably, OsNRT2.3b functions only at a slightly alkaline cytosolic pH, and a pH-sensitive motif of OsNRT2.3b facing the cytosolic side determines its activity to acquire NO3– from the external medium (Fan et al., 2016). In rice, OsNRT2.3b overexpression decreased the phloem sap pH from 8 to 7.1 under NO3– supply, and from 7.4 to 6.8 under NH4+ supply, resulting in significantly increased grain yield and nitrogen use efficiency (NUE) at different N levels in field conditions (Fan et al., 2016). The sensing of cytosolic pH by OsNRT2.3b provides an explanation for plant adaptation to changes in the form of N supply. This finding highlights the important link between N transport, pH regulation, and NUE.
NO3– excretion transporters may be involved in cellular pH regulation
In contrast to NO3– influx, NO3– efflux from root cells is energetically a downhill process which is also dependent on the activity of the PM H+-ATPase pump. It was shown that in isolated root PMs, NO3– efflux is tightly coupled to H+ excretion by the H+-ATPase, and that both activities of NO3– efflux and H+ excretion share similar acidic optimum pH at the cytosolic face of the PM (Vara and Serrano, 1982; De Michelis and Spanswick, 1986; Grouzis et al., 1997; Pouliquin et al., 2000). It has been shown that the Nitrate Excretion Transporter AtNPF2.7/NAXT1 mediates passive NO3– efflux across the isolated PM of plant root cells in acidic medium in vitro (Segonzac et al., 2007), suggesting that the NO3– excretion transporter can mediate both NO3– and H+ efflux in combination with PM proton pumps, thus re-balancing the acidification of cytosol to some extent.
Intracellular H+/NO3– antiporters involved in pH regulation of cellular organelles
NO3– can be stored in, and remobilized from, vacuoles. NO3– transport into vacuoles is mediated by an H+/NO3– antiporter, and the H+/NO3– symport systems also serve in NO3– efflux from the vacuole to the cytosol, which are energized by V-ATPase pumping H+ to vacuoles (De Angeli et al., 2006). Arabidopsis AtCLCa is expressed in leaf mesophyll cells; disruption of AtCLCa led to an ~50% decrease of vacuolar NO3–, suggesting an important role for AtCLCa in NO3– accumulation (Geelen et al., 2000; De Angeli et al., 2006). Measurements using the patch-clamp technique in the whole-vacuole configuration showed that AtCLCa behaves as a 1NO3–/2H+ exchanger, which transports NO3– from the cytosol to the vacuolar lumen (De Angeli et al., 2006). AtCLCa expression in oocytes indeed induced intracellular alkalinization at both pH 5.5 and pH 7.5 when oocytes were pulsed to positive voltages (Bergsdorf et al., 2009). In vitro, although transport processes such as the H+/NO3– exchanger AtCLCa play a role in alkalinization of the vacuole or acidifying the cytosol, the active accumulation of H+ in the vacuole is also accomplished by P- and V-type ATPases, which function as ‘proton pumps’. There are two ATP-binding sites, at His620 and Asp750 in the C-terminus CBS domain of AtCLCa. Adding micromolar concentrations of ATP could inhibit AtCLCa activity in isolated A. thaliana vacuoles, resulting in a decrease of NO3– influx by up to 60% (De Angeli et al., 2009). It is possible that the V-ATPases can work together with the CLC antiporter in the tonoplast to balance cytoplasmic pH during the process of vacuolar NO3– accumulation.
Currently, it is not known if there are nitrate transporters involved in NO3– flux and pH homeostasis in other cellular organelles. As members of all the NO3– transporter families (NRT1, NRT2, and CLCs) can be located in endomembrane systems, they may have important roles in the generation of compartmental pH gradients within the cell.
pH regulatory sites in N transporters
The activity of many NO3– transporters is affected by pH; however, the regulatory mechanism is not clear. Interestingly, many plant N transporters including H+/NO3– symporters, H+/NO3– antiporters, and H+/NH4+ symporters contain putative pH-sensing sites (Table 1), indicating that these transporters may sense either external (i.e. apoplast) or internal pH.
Table 1.
pH-sensing sites in plant ammonium and nitrate transporters
| Transporter | Transport mode | pH sensing site | Localization | References |
|---|---|---|---|---|
| AtNPF6.3/NRT1.1/CHL1 | 2 H+/1 NO3– symport | ExxER (E41, E44), His365 (H365) | PM | Sun et al. (2014); Parker and Newstead (2014) |
| PvAMT1;1 | 1 H+/1 NH4+ symport | His211 (H211) | PM | Ortiz-Ramirez et al. (2011) |
| OsNRT2.3b | 2 H+/1 NO3– symport | His167 (H167) | PM | Fan et al. (2016) |
| AtCLCa | 1 H+/2 NO3– antiport | Glu203 (E203), Glu270 (E270) | Tonoplast | Bergsdorf et al. (2009); Miller and Nguitragool (2009) |
At, Arabidopsis; Pv, common bean; Os, rice. PM, plasma membrane; E, glutamate; H, histidine.
Both the ExxER motif and histidine residues are essential for H+ binding in plant NPFs (Jorgensen et al., 2015; Longo et al., 2018). Removal of charged residues in the ExxER motif of AtNPF6.3/NRT1.1 abolished both H+ binding and NO3– transport activity (Sun et al. 2014). The stoichiometry of H+/NO3– transport through AtNPF6.3/NRT1.1 is at least 2H+:1NO3–, and it was proposed that the ExxER motif in TM1 binds one H+, leaving His356 on TM7 to bind another H+ and NO3– (Parker and Newstead, 2014).
It is well known that histidine residues are important H+-binding amino acids involved in the regulation or activity of pH-dependent transporters in Escherichia coli, yeast, mammals, and plants, because they can ionize within the physiological pH range (Wiebe et al., 2001; Ortiz-Ramirez et al., 2011). PvAMT1;1 is an NH4+ transporter of common bean, for which the mutation of its conserved His211 to glutamic acid (H211E) results in altering the transport mechanism to be pH independent, with its affinity for NH4+ decreasing while increasing the transport capacity (Ortiz-Ramirez et al., 2011). Exposure of PvAMT1;1 H211E-expressing oocytes to NH4+ did not affect the cytoplasmic pH but caused depolarization of the membrane potential at both pH 5.5 and pH 7.0 (Ortiz-Ramirez et al., 2011). For a rice NO3– transporter, OsNRT2.3b, His167 (H167) was located on the cytoplasmic side and has been confirmed to play a critical role in sensing cytosolic pH (Fan et al., 2016). The H167R mutation does not fully eliminate the basic activity of OsNRT2.3b as a H+-coupled NO3– transporter but results in the loss of cytosolic pH sensing (Fan et al., 2016).
Certain gating glutamate residues of some channel proteins may be involved in sensing cellular pH. Mutation of AtCLCa ‘gating glutamate Glu203 or the ‘H+ glutamate site’ Glu270 to alanine prevented its activity in generating NO3– flux-elicited currents or depolarization-induced H+ transport in oocytes (Bergsdorf et al., 2009; Miller and Nguitragool, 2009), suggesting that the two Glu sites are H+-binding sites in AtCLCa.
Cellular pH homeostasis during N assimilation
The ‘proton economy’ in N transport and assimilation
The majority of root acquired NH4+ is rapidly assimilated in roots, whereas NO3– is mainly assimilated in shoots depending on different plant species and the external N level, requiring both ATP and carbon (C) skeletons (Fig. 3; Table 2; Raven and Smith, 1976; Andrews, 1986; Bloom et al., 1992; Rachmilevitch et al., 2004; Nunes-Nesi et al., 2010; Britto and Kronzucker, 2005).
Fig. 3.
pH regulation during NH4+ and NO3– assimilation. NH4+ transport and assimilation pathways are indicated by red arrows, NO3– transport and assimilation by blue arrows, amino acid (AA) transport by purple arrows, malate transport and assimilation by brown arrows, K+ transport by green arrows, and H+ or OH– production or consumption by black arrows. NH4+ is assimilated mainly in roots, and NO3– is assimilated in both roots and shoots, which are dependent on plant species and N supply levels (Raven and Smith 1976; Andrews. 1986; Raven, 1986). The N assimilation requires ATP and carbon skeletons, glucose, malic acid (OAA), or malate. Malate accumulates in NO3–-supplied plants and can be stored in vacuoles, or transported to roots for further reactions (Raven and Smith, 1976). Malate converted to pyruvate helps overcome cytosolic acidification at low external pH (Raven and Smith, 1976). The assimilation of NH4+ produces at least one H+ per NH4+. The H+ produced are partially neutralized to counter the cytoplasmic akalinization caused by NH4+ transport in roots (Gerendás and Ratcliffe, 2000), or stored in vacuoles (Raven and Smith, 1976; Raven, 1986). NR, nitrate reductase. NiR, nitrite reductase. AS, asparagine synthetases. GS, glutamine synthetases. GOGAT, glutamine oxoglutarate aminotransferase. Cyto, cytosol. Vacuo, vacuole; chloro, chloroplast; plast, plastid.
Table 2.
Proton changes in the processes of N transport and assimilation
| N utilization processes | Equation of H+ change in cytoplasm | |
|---|---|---|
| NH4+ | NH4+ transport | NH4+(out)→NH3+H+(out) |
| NH3 protonation | NH3+1H+→NH4+ | |
| NH4+ assimilation | NH4++C6H12O6+1.5O2→C5H8NO4–+CO2+3H2O+2H+ | |
| NO3– | NO3– transport | NO3–(out)+H+(out)→ NO3–+1H+ |
| NO3– reduction | NO3–+2/3C6H12O6+ 2O2+2H+→ NH4++4CO2+3H2O | |
| NH4+ assimilation | NH4++C6H12O6+1.5O2→C5H8NO4–+CO2+3H2O+2H+ |
H+, H+ production and H+, H+ consumption in the cytoplasm. In the process of NH4+ transport, it is assumed that 1NH4+ counterbalances 1 extra H+, released to outside the cell (out). In the process of NH4+ assimilation, if the glucose is ample, 2H+ will be produced in the cytoplasm. For 1NO3–/2H+ co-transport into the cytoplasm, it is assumed that 1H+ is pumped out of the cell by the PM H+-ATPase. For NO3– reduction, 2H+ will be produced when plenty of carbon is available. Combining the NO3– transport, reduction, and assimilation, if 1NO3– is totally incorporated into 1 glutamate (Glu), it yields 1H+ in the cell, and 1H+ extra (Britto and Kronzucker, 2005). If 1NH4+ is transported and assimilated to 1Glu, it generates 1H+ in the cell, and 1H+ extra (Britto and Kronzucker, 2005).
Reduction of NO3– to NH4+ is catalysed by nitrate reductase (NR) and nitrite reductases (NiRs) in the cytosol and plastids or chloroplasts, respectively, with the consumption of 2H+ (molecule) per 1NO3– in the cytosol (Fig. 3; Table 2; Lea and Miflin, 1974; Xu et al., 2012). In general, NH4+ assimilation into amino acids occurs quickly under NH4+ supply and is conducted in root plastids or shoot chloroplasts by the GS/GOGAT cycle, producing 2H+ per 1NH4+ (Fig. 3; Table 2; Masclaux-Daubresse et al., 2010). However, there is some controversy as to whether the GS/GOGAT pathway of NH4+ assimilation is net H+ consuming or producing in plants. Three conditions need to be considered for predicting the consumption or production of H+ in NH4+ assimilation. (i) If ATP and NAD(P)H for the reaction are regenerated only by other processes, the GS/GOGAT pathway is H+ consuming (Kosegarten et al., 1997). (ii) If the C skeletons can be continually provided for the regeneration of ATP and NAPD(P)H, it appears to be an H+-releasing process (Gerendás and Ratcliffe, 2000). (iii) If the C skeletons are limited, then the 2-oxoglutarate pool is replenished by re-utilization of malate (stored in the vacuole), and NH4+ assimilation may rapidly consume H+ (Gerendás and Ratcliffe, 2000). In addition, different plant species showed diverse cytoplasmic pH changes in response to NH4+; for example, rice, which has stronger GS activity than maize, showed a larger increase of cellular pH during NH4+ assimilation (Magalhaes and Huber, 1989, 1991; Kosegarten et al., 1997).
The combination of NH4+ or NO3– transport and assimilation results in different net changes of H+ numbers in plant cells (Table 2; Bloom et al., 1992; Rachmilevitch et al., 2004; Britto and Kronzucker, 2005). Incorporation of one NH4+ to glutamate produces one H+ in the cell, while assimilation of one NO3– to glutamate produces one H+. However, when NO3– or NH4+ is not immediately assimilated and presumed to accumulate, it is expected that the uptake of NO3– is a transient cytosol-acidifying process whereas that of NH4+ is a transient cytosol-alkalinizing process.
Biochemical malate pH-stat due to NO3– assimilation
In the process of NO3– reduction to NH4+, a substantial amount of the dicarboxylate malate can accumulate in the cytosol due to the anion deficit (van Beusichem et al., 1988; Lüttge et al., 2000; Pasqualini et al., 2001). Cellular malate synthesis and degradation is important for regulation of the cytosolic pH (Smith and Raven, 1979; Hurth et al., 2005). For example, knockout of the tonoplast malate transporter AttDT reduced the capacity of the mutant plant to overcome cytosolic acidification in leaf protoplasts (Hurth et al., 2005). However, these mutants did not have a strong phenotype, but the effect of changing N supply form was not tested.
pH regulation during amino acid transport
In addition to inorganic N, amino acids in the soil solution can also be directly taken up by roots (Tegeder and Masclaux-Daubresse, 2018). Inside the plant, amino acids are the major form of N for transport and re-distribution, particularly in NH4+-supplied plants (Tegeder and Hammes, 2018).
Most amino acid transporters function with a H+ co-transport mechanism and this has been shown for a broad range of amino acids, including neutral, cationic, and anionic amino acids (Tegeder and Masclaux-Daubresse, 2018; Tegeder and Hammes, 2018). The plant amino acid transporters show characteristic pH dependence in oocytes (Boorer et al., 1996; Boorer and Fischer, 1997; Hirner et al., 1998, 2006; Fischer et al., 2002). Although there is no evidence for their direct involvement in pH regulation, the root amino acid transporters can lead to a slight increase in rhizosphere pH (Näsholm et al., 2009). In sterile conditions, amino acids can be used as a positive control for experiments comparing NO3– and NH4+ as N sources.
Future perspectives
Developing the techniques to instantly monitor in real-time the dynamic changes of cellular pH by either N transport or H+ pumps in plants
For a better understanding of the underlying mechanisms of cellular pH homeostasis during N uptake and assimilation, it is essential to develop more molecular tools enabling in vivo measurements of pH in different intracellular compartments. Changes in proton concentrations are associated with both the N transporter and H+-pumping activity of ATPase (De Angeli et al., 2009; Bassil et al. 2011; Shen et al., 2013), thus both factors should be taken into account for pH regulation in plant cells. In tobacco, Martinière et al. (2013a) used a pHluorin-based pH sensor to directly measure pH of the endomembrane system, and found that luminal pH homeostasis in the trans-Golgi Network (TGN) and pre-vacuolar compartment (PVC) involved both V-ATPase-dependent acidification and H+ efflux mediated by the activity of the Arabidopsis Na+(K+)/H+ exchanger NHX5. In Arabidopsis protoplasts, Shen et al. (2013) used a modified pHluorin targeted to different organellar compartments for visualization and quantification of pH in vivo. Other pH sensors are also available for measurement of intracellular pH in plants (Martinière et al., 2013b). Some H+-coupled NO3– transporters (e.g. AtCLCa) and NH4+ transporters (e.g. PvAMT1;1) have also been identified as transporters leading to cytosolic pH changes in the oocyte system (Bergsdorf et al., 2009; Ortiz-Ramirez et al., 2011). However, there is still a lack of information about direct measurement of intercellular pH in nitrate transporter mutant plants. With the available tools for in vivo pH measurement using pH sensors (Shen et al., 2013; Reguera et al., 2015), it will now be possible to determine how they affect pH in the cytosol and endomembranes in the future.
Role of N-controlled cellular pH homeostasis in enhancing abiotic stress tolerance
In acidic media, H+ and NO3– excretion are tightly coupled. AtANPF2.7/NAXT1 mediated root NO3– excretion, and PM-ATPase stimulated H+ excretion (Segonzac et al., 2007). H+ stress enhanced NO3– uptake mediated by NRT1.1 in Arabidopsis and caused significant rhizosphere alkalinization (Fang et al., 2016), and thus decreased some heavy metal toxicity such as that of Cd and Pb (Mao et al., 2014; Zhu et al., 2019). It would be interesting to examine how much such N-controlled cellular pH homeostasis and effects on rhizosphere pH can regulate plant tolerance to other abiotic stresses, such as heavy metals, drought, or flooding and salinity.
Using natural genetic variation or point mutation of key H+-binding residues in N transporters to enhance the cellular pH homeostasis within plants for improving N uptake and utilization
It is known that intracellular pH can be a signal for modulating downstream responses (Roos, 2000; Felle, 2001; Kader and Lindberg, 2010). In rice, overexpression of OsNRT2.3b, a cellular pH-sensing nitrate transporter, could buffer N transport-induced phloem alkalinization, and thus improve NUE, phosphate and iron mobilization, C metabolism, and grain yield (Fan et al., 2016). This provides an exciting example for the possibility of utilizing pH-sensing transporters to improve plant NUE and growth. It is worth checking in other N transporters if there is a tight link between H+-binding residues and N transport activity at different medium pH. In future, utilizing the natural genetic variation among germplasm collections or making point mutations by gene-editing techniques of pH-sensing transporters may be a pathway for enhancing crop production at varied N supply levels and improving NUE.
Revealing the molecular regulatory mechanisms of synergism, antagonism, and interaction of NO3– and NH4+ on potassium and other nutrients
In plants, the transport and assimilation of NO3– and NH4+ can dominate cellular pH homeostasis, which in turn affects the availability and utilization of other nutrients. The synergism, antagonism, and interaction among N and other major nutrients, such as K+, Ca2+, Mg2+, and Cl–, are known to be physiologically relevant, while the regulatory mechanisms linking these nutrients to cellular pH homeostasis are unclear. Inactivation of some nitrate transporters, such as AtNPF7.3/NRT1.5 (Lin et al., 2008; Drechsler et al., 2015; Li et al., 2017) and OsNPF2.4 (Xia et al., 2015), affects both NO3– and K+ distribution, showing that K+/NO3– transport is tightly coordinated. However, more thorough investigation of the interactions between N and other nutrients are needed.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0100700), the Natural Science Foundation of China (31930101; 31401938), the Innovative Research Team Development Plan of the Ministry of Education of China (grant no. IRT_17R56), the 111 Project (grant no. 12009), and the PAPD of Jiangsu Higher Education Institutions project. AJM was funded by the UK Biotechnological and Biological Sciences Research Council (BBSRC) Institute Strategic Program Grants ‘Molecules from Nature’ (BB/P012523/1) and ‘Plant Health’ (BB/P012574/1), and the John Innes Foundation.
References
- Andrews M. 1986. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant, Cell & Environment 9, 511–519. [Google Scholar]
- Balkos KD, Britto DT, Kronzucker HJ. 2010. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant, Cell & Environment 33, 23–34. [DOI] [PubMed] [Google Scholar]
- Barkla BJ, Pantoja O. 1996. Physiology of ion transport across the tonoplast of higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47, 159–184. [DOI] [PubMed] [Google Scholar]
- Bassil E, Blumwald E. 2014. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Current Opinion in Plant Biology 22, 1–6. [DOI] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E. 2011. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. The Plant Cell 23, 3482–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsdorf EY, Zdebik AA, Jentsch TJ. 2009. Residues important for nitrate/proton coupling in plant and mammalian CLC transporters. Journal of Biological Chemistry 284, 11184–11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Sukrapanna SS, Warner RL. 1992. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiology 99, 1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ. 1985. Nitrate storage and retrieval in Beta vulgaris: effects of nitrate and chloride on proton gradients in tonoplast vesicles. Proceedings of the National Academy of Sciences, USA 82, 3683–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorer KJ, Fischer WN. 1997. Specificity and stoichiometry of the Arabidopsis H+/amino acid transporter AAP5. Journal of Biological Chemistry 272, 13040–13046. [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Frommer WB, Bush DR, Kreman M, Loo DD, Wright EM. 1996. Kinetics and specificity of a H+/amino acid transporter from Arabidopsis thaliana. Journal of Biological Chemistry 271, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Briskin DP, Hanson JB. 1992. How does the plant plasma membrane H+-ATPase pump protons? Journal of Experimental Botany 43, 269–289. [Google Scholar]
- Britto DT, Kronzucker HJ. 2005. Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: new views on old paradigms. Plant, Cell & Environment 28, 1396–1409. [Google Scholar]
- Cha-Um S, Supaibulwattana K, Kirdmanee C. 2009. Comparative effects of salt stress and extreme pH stress combined on glycinebetaine accumulation, photosynthetic abilities and growth characters of two rice genotypes. Rice Science 16, 274–282. [Google Scholar]
- Chen G, Hu Q, Luo L, Yang T, Zhang S, Hu Y, Yu L, Xu G. 2015. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant, Cell & Environment 38, 2747–2765. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Kronzucker HJ. 2017. The nitrogen–potassium intersection: membranes, metabolism, and mechanism. Plant, Cell & Environment 40, 2029–2041. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ. 2013. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiology 163, 1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H. 2006. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442, 939–942. [DOI] [PubMed] [Google Scholar]
- De Angeli A, Moran O, Wege S, Filleur S, Ephritikhine G, Thomine S, Barbier-Brygoo H, Gambale F. 2009. ATP binding to the C terminus of the Arabidopsis thaliana nitrate/proton antiporter, AtCLCa, regulates nitrate transport into plant vacuoles. Journal of Biological Chemistry 284, 26526–26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michelis MI, Spanswick RM. 1986. H-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiology 81, 542–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler N, Zheng Y, Bohner A, Nobmann B, von Wirén N, Kunze R, Rausch C. 2015. Nitrate-dependent control of shoot K homeostasis by the nitrate Transporter1/Peptide transporter family member NPF7.3/NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiology 169, 2832–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan F, Giehl RFH, Geldner N, Salt DE, von Wirén N. 2018. Root zone-specific localization of AMTs determines ammonium transport pathways and nitrogen allocation to shoots. PLoS Biology 16, e2006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espen L, Nocito FF, Cocucci M. 2004. Effect of NO3– transport and reduction on intracellular pH: an in vivo NMR study in maize roots. Journal of Experimental Botany 55, 2053–2061. [DOI] [PubMed] [Google Scholar]
- Falhof J, Pedersen JT, Fuglsang AT, Palmgren M. 2016. Plasma membrane H+-ATPase regulation in the center of plant physiology. Molecular Plant 9, 323–337. [DOI] [PubMed] [Google Scholar]
- Fan X, Naz M, Fan X, Xuan W, Miller AJ, Xu G. 2017. Plant nitrate transporters: from gene function to application. Journal of Experimental Botany 68, 2463–2475. [DOI] [PubMed] [Google Scholar]
- Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, Lian X, Shen Q, Miller AJ, Xu G. 2016. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proceedings of the National Academy of Sciences, USA 113, 7118–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang XZ, Tian WH, Liu XX, Lin XY, Jin CW, Zheng SJ. 2016. Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis. New Phytologist 211, 149–158. [DOI] [PubMed] [Google Scholar]
- Felle HH. 2001. pH: signal and messenger in plant cells. Plant Biology 3, 577–591. [Google Scholar]
- Feng H, Yan M, Fan X, Li B, Shen Q, Miller AJ, Xu G. 2011. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. Journal of Experimental Botany 62, 2319–2332. [DOI] [PubMed] [Google Scholar]
- Fischer WN, Loo DD, Koch W, Ludewig U, Boorer KJ, Tegeder M, Rentsch D, Wright EM, Frommer WB. 2002. Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. The Plant Journal 29, 717–731. [DOI] [PubMed] [Google Scholar]
- Förster JC, Jeschke WD. 1993. Effects of potassium withdrawal on nitrate transport and on the contribution of the root to nitrate reduction in the whole plant. Journal of Plant Physiology 141, 322–328. [Google Scholar]
- Gaxiola RA, Palmgren MG, Schumacher K. 2007. Plant proton pumps. FEBS Letters 581, 2204–2214. [DOI] [PubMed] [Google Scholar]
- Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelièvre F, Courtial B, Barbier-Brygoo H, Maurel C. 2000. Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. The Plant Journal 21, 259–267. [DOI] [PubMed] [Google Scholar]
- Geilfus CM. 2017. The pH of the apoplast: dynamic factor with functional impact under stress. Molecular Plant 10, 1371–1386. [DOI] [PubMed] [Google Scholar]
- Gerendás J, Ratcliffe RG. 2000. Intracellular pH regulation in maize root tips exposed to ammonium at high external pH. Journal of Experimental Botany 51, 207–219. [DOI] [PubMed] [Google Scholar]
- Gerendás J, Ratcliffe RG, Sattelmacher B. 1990. 31P nuclear magnetic resonance evidence for differences in intracellular pH in the roots of maize seedlings grown with nitrate or ammonium. Journal of Plant Physiology 137, 125–128. [Google Scholar]
- Gerendás J, Schurr U. 1999. Physicochemical aspects of ion relations and pH regulation in plants—a quantitative approach. Journal of Experimental Botany 50, 1101–1114. [Google Scholar]
- Giehl RFH, Laginha AM, Duan F, Rentsch D, Yuan L, von Wirén N. 2017. A critical role of AMT2;1 in root-to-shoot translocation of ammonium in Arabidopsis. Molecular Plant 10, 1449–1460. [DOI] [PubMed] [Google Scholar]
- Glass AD, Shaff JE, Kochian LV. 1992. Studies of the uptake of nitrate in barley: IV. Electrophysiology. Plant Physiology 99, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM, Siddiqi MY. 1985. Nitrate inhibition of chloride influx in barley: implications for a proposed chloride homeostat. Journal of Experimental Botany 36, 556–566. [Google Scholar]
- Granstedt RC, Huffaker RC. 1982. Identification of the leaf vacuole as a major nitrate storage pool. Plant Physiology 70, 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouzis JP, Pouliquin P, Rigaud J, Grignon C, Gibrat R. 1997. In vitro study of passive nitrate transport by native and reconstituted plasma membrane vesicles from corn root cells. Biochimica et Biophysica Acta 1325, 329–342. [DOI] [PubMed] [Google Scholar]
- Guern J, Felle H, Mathieu Y, Kurkdjian A. 1991. Regulation of intracellular pH in plant cells. International Review of Cytology 127, 111–173. [Google Scholar]
- Guo J, Zhou Q, Li X, Yu B, Luo Q. 2017. Enhancing NO3– supply confers NaCl tolerance by adjusting Cl– uptake and transport in G. max & G. soja. Journal of Soil Science and Plant Nutrition 17, 194–202. [Google Scholar]
- Hachiya T, Sakakibara H. 2017. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. Journal of Experimental Botany 68, 2501–2512. [DOI] [PubMed] [Google Scholar]
- Hagin J, Olsen SR, Shaviv A. 1990. Review of interaction of ammonium–nitrate and potassium nutrition of crops. Journal of Plant Nutrition 13, 1211–1226. [Google Scholar]
- Han YL, Song HX, Liao Q, et al. 2016. Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiology 170, 1684–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RJ. 1990. Active ion uptake and maintenance of cation–anion balance: a critical examination of their role in regulating rhizosphere pH. Plant and Soil 126, 247– 264. [Google Scholar]
- Hinsinger P, Plassard C, Tang C, Jaillard Bt. 2003. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil 248, 43–59. [Google Scholar]
- Hirner A, Ladwig F, Stransky H, Okumoto S, Keinath M, Harms A, Frommer WB, Koch W. 2006. Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. The Plant Cell 18, 1931–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirner B, Fischer WN, Rentsch D, Kwart M, Frommer WB. 1998. Developmental control of H+/amino acid permease gene expression during seed development of Arabidopsis. The Plant Journal 14, 535–544. [DOI] [PubMed] [Google Scholar]
- Huang NC, Chiang CS, Crawford NM, Tsay YF. 1996. CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. The Plant Cell 8, 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurth MA, Suh SJ, Kretzschmar T, Geis T, Bregante M, Gambale F, Martinoia E, Neuhaus HE. 2005. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiology 137, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen ME, Olsen CE, Geiger D, Mirza O, Halkier BA, Nour-Eldin HH. 2015. A functional EXXEK motif is essential for proton coupling and active glucosinolate transport by NPF2.11. Plant & Cell Physiology 56, 2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader MA, Lindberg S. 2010. Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signaling & Behavior 5, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Feria-Bourrellier AB, Lafouge F, et al. 2012. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. The Plant Cell 24, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D. 1981. The transport of NH3 and NH4+ across biological membranes. Biochimica et Biophysica Acta 639, 41–52. [DOI] [PubMed] [Google Scholar]
- Kosegarten H, Grolig F, Wieneke J, Wilson G, Hoffmann B. 1997. Differential ammonia-elicited changes of cytosolic pH in root hair cells of rice and maize as monitored by 2',7'-bis-(2-carboxyethyl)-5 (and -6)-carboxyfluorescein-fluorescence ratio. Plant Physiology 113, 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Toda T, Hara T. 2001. Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: pectin–Ca interaction may play an important role in proton rhizotoxicity. Journal of Experimental Botany 52, 361–368. [PubMed] [Google Scholar]
- Krebs M, Beyhl D, Görlich E, Al-Rasheid KA, Marten I, Stierhof YD, Hedrich R, Schumacher K. 2010. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proceedings of the National Academy of Sciences, USA 107, 3251–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937. [DOI] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J. 1989. Intracellular pH: measurement and importance in cell activity. Annual Review of Plant Physiology and Plant Molecular Biology 40, 271–303. [Google Scholar]
- Lea PJ, Miflin BJ. 1974. Alternative route for nitrogen assimilation in higher plants. Nature 251, 614–616. [DOI] [PubMed] [Google Scholar]
- Léran S, Muños S, Brachet C, Tillard P, Gojon A, Lacombe B. 2013. Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot nitrate translocation. Molecular Plant 6, 1984–1987. [DOI] [PubMed] [Google Scholar]
- Li C, Tang Z, Wei J, Qu H, Xie Y, Xu G. 2016. The OsAMT1.1 gene functions in ammonium uptake and ammonium–potassium homeostasis over low and high ammonium concentration ranges. Journal of Genetic Genomics 43, 639–649. [DOI] [PubMed] [Google Scholar]
- Li H, Yu M, Du XQ, Wang ZF, Wu WH, Quintero FJ, Jin XH, Li HD, Wang Y. 2017. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. The Plant Cell 29, 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Provenzano S, Bliek M, et al. 2016. Evolution of tonoplast P-ATPase transporters involved in vacuolar acidification. New Phytologist 211, 1092–1107. [DOI] [PubMed] [Google Scholar]
- Lin SH, Kuo HF, Canivenc G, et al. 2008. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. The Plant Cell 20, 2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Huang CY, Tsay YF. 1999. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. The Plant Cell 11, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang D, Tao J, Miller AJ, Fan X, Xu G. 2014. Identification and functional assay of the interaction motifs in the partner protein OsNAR2.1 of the two-component system for high-affinity nitrate transport. New Phytologist 204, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, von Wirén N. 2017. Ammonium as a signal for physiological and morphological responses in plants. Journal of Experimental Botany 68, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Longo A, Miles NW, Dickstein R. 2018. Genome mining of plant NPFs reveals varying conservation of signature motifs associated with the mechanism of transport. Frontiers in Plant Science 9, 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U, Pfeifer T, Fischer-Schliebs E, Ratajczak R. 2000. The role of vacuolar malate-transport capacity in crassulacean acid metabolism and nitrate nutrition. Higher malate-transport capacity in ice plant after crassulacean acid metabolism-induction and in tobacco under nitrate nutrition. Plant Physiology 124, 1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane JJ, Smith FA. 1982. Uptake of methylamine by Ulva rigida: transport of cations and diffusion of free base. Journal of Experimental Botany 33, 195–207. [Google Scholar]
- Magalhaes JR, Huber DM. 1989. Ammonium assimilation in different plant species as affected by nitrogen form and pH control in solution culture. Fertility Research 21, 1–6. [Google Scholar]
- Magalhaes JR, Huber DM. 1991. Response of ammonium assimilation enzymes to nitrogen form treatments in different plant species. Journal of Plant Nutrition 14, 175–185. [Google Scholar]
- Mao QQ, Guan MY, Lu KX, Du ST, Fan SK, Ye YQ, Lin XY, Jin CW. 2014. Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiology 166, 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd edn London: Academic Press. [Google Scholar]
- Marschner H, Römheld V. 1983. In vivo measurement of root-induced pH changes at the soil–root interface: effect of plant species and nitrogen source. Zeitschrift für Pflanzenphysiologie 111, 241–251. [Google Scholar]
- Marshansky V, Futai M. 2008. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Current Opinion in Cell Biology 20, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N. 2013a In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. The Plant Cell 25, 4028–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Desbrosses G, Sentenac H, Paris N. 2013b Development and properties of genetically encoded pH sensors in plants. Frontiers in Plant Science 4, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Massonneau A, Frangne N. 2000. Transport processes of solutes across the vacuolar membrane of higher plants. Plant & Cell Physiology 41, 1175–1186. [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. 2010. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Annals of Botany 105, 1141–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure PR, Kochian LV, Spanswick RM, Shaff JE. 1990a Evidence for cotransport of nitrate and protons in maize roots: I. Effects of nitrate on the membrane potential. Plant Physiology 93, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure PR, Kochian LV, Spanswick RM, Shaff JE. 1990b Evidence for cotransport of nitrate and protons in maize roots: II. Measurement of NO3– and H+ fluxes with ion-selective microelectrodes. Plant Physiology 93, 290–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg AA, Blatt MR. 1995. NO3– transport across the plasma membrane of Arabidopsis thaliana root hairs: kinetic control by pH and membrane voltage. Journal of Membrane Biology 145, 49–66. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Smith SJ. 1992. The mechanism of nitrate transport across the tonoplast of barley root cells. Planta 187, 554–557. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Smith SJ. 1996. Nitrate transport and compartmentation in cereal root cells. Journal of Experimental Botany 47, 843–854. [Google Scholar]
- Miller C, Nguitragool W. 2009. A provisional transport mechanism for a chloride channel-type Cl–/H+ exchanger. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrik I, Ullrich C. 1996. Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO3− and H+/H2PO4− stoichiometries. Plant Physiology & Biochemistry 34, 629–636. [Google Scholar]
- Näsholm T, Kielland K, Ganeteg U. 2009. Uptake of organic nitrogen by plants. New Phytologist 182, 31–48. [DOI] [PubMed] [Google Scholar]
- Niedziela C, Nelson P, Peet M, Jackson W. 1993. Diurnal malate and citrate fluctuations as related to nitrate and potassium concentrations in tomato leaves. Journal of Plant Nutrition 16, 165–175. [Google Scholar]
- Nocito FF, Sacchi GA, Cocucci M. 2002. Membrane depolarization induces K+ efflux from subapical maize root segments. New Phytologist 154, 45–51. [Google Scholar]
- Nunes-Nesi A, Fernie AR, Stitt M. 2010. Metabolic and signaling aspects underpinning the regulation of plant carbon–nitrogen interactions. Molecular Plant 3, 973–996. [DOI] [PubMed] [Google Scholar]
- Oosterhuis DM, Loka DA, Kawakami EM, Pettigrew WT. 2014. The physiology of potassium in crop production. Advances in Agronomy 126, 203–233. [Google Scholar]
- Orsel M, Chopin F, Leleu O, Smith SJ, Krapp A, Daniel-Vedele F, Miller AJ. 2006. Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis. Physiology and protein–protein interaction. Plant Physiology 142, 1304–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F. 2004. Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219, 714–721. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ramirez C, Mora SI, Trejo J, Pantoja O. 2011. PvAMT1;1, a highly selective ammonium transporter that functions as H+/NH4+ symporter. Journal of Biological Chemistry 286, 31113–31122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG. 2001. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annual Review of Plant Physiology and Plant Molecular Biology 52, 817–845. [DOI] [PubMed] [Google Scholar]
- Parker JL, Li C, Brinth A, et al. 2017. Proton movement and coupling in the POT family of peptide transporters. Proceedings of the National Academy of Sciences, USA 114, 13182–13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JL, Newstead S. 2014. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S, Ederli L, Piccioni C, Batini P, Bellucci M, Arcioni S, Antonielli M. 2001. Metabolic regulation and gene expression of root phosphoenolpyruvate carboxylase by different nitrogen sources. Plant, Cell & Environment 24, 439–447. [Google Scholar]
- Patil NS, Apradh VT, Karadge BA. 2012. Effects of alkali stress on seed germination and seedlings growth of Vigna aconitifolia (Jacq.) Marechal. Pharmacognosy Journal 4, 77–80. [Google Scholar]
- Patterson K, Cakmak T, Cooper A, Lager I, Rasmusson AG, Escobar MA. 2010. Distinct signaling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant, Cell & Environment 33, 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliquin P, Boyer JC, Grouzis JP, Gibrat R. 2000. Passive nitrate transport by root plasma membrane vesicles exhibits an acidic optimal pH like the H+-ATPase. Plant Physiology 122, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilevitch S, Cousins AB, Bloom AJ. 2004. Nitrate assimilation in plant shoots depends on photorespiration. Proceedings of the National Academy of Sciences, USA 101, 11506–11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA. 1985. Regulation of pH and generation of osmolarity in vascular plants: a cost–benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytologist 101, 25–77. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1986. Biochemical disposal of excess H+ in growing plants? New Phytologist 104, 175–206. [Google Scholar]
- Raven JA, Smith FA. 1976. Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytologist 76, 415–431. [Google Scholar]
- Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E. 2015. pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. The Plant Cell 27, 1200–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V. 1986. pH-Veränderungen in der Rhizosphäre verschiedener Kulturpflanzenarten in Abhängigkeit vom Nährstoffangebot. Potash Review 55, 1–8. [Google Scholar]
- Roos W. 2000. Ion mapping in plant cells—methods and applications in signal transduction research. Planta 210, 347–370. [DOI] [PubMed] [Google Scholar]
- Roosta HR, Schjoerring JK. 2008. Effects of nitrate and potassium on ammonium toxicity in cucumber plants. Journal of Plant Nutrition 31, 1270–1283. [Google Scholar]
- Ruan J, Zhang F, Ming HW. 2000. Effect of nitrogen form and phosphorus source on the growth, nutrient uptake and rhizosphere soil property of Camellia sinensis L. Plant and Soil 223, 65–73. [Google Scholar]
- Rufty TW, Jackson WA, Raper CD. 1981. Nitrate reduction in roots as affected by the presence of potassium and by flux of nitrate through the roots. Plant Physiology 68, 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi GA, Cocucci M. 1992. Effects of deuterium oxide on growth, proton extrusion, potassium influx, and in vitro plasma membrane activities in maize root segments. Plant Physiology 100, 1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Locci G, Monte R, Pinton R, Varanini Z. 2003. Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. Journal of Experimental Botany 54, 1851–1864. [DOI] [PubMed] [Google Scholar]
- Schubert S, Schubert E, Mengel K. 1990. Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans (Vicia faba). Plant and Soil 124, 239–244. [Google Scholar]
- Schumacher K. 2006. Endomembrane proton pumps: connecting membrane and vesicle transport. Current Opinion in Plant Biology 9, 595–600. [DOI] [PubMed] [Google Scholar]
- Schumacher K. 2014. pH in the plant endomembrane system—an import and export business. Current Opinion in Plant Biology 22, 71–76. [DOI] [PubMed] [Google Scholar]
- Schumaker KS, Sze H. 1987. Decrease of pH gradients in tonoplast vesicles by NO3– and Cl: evidence for H+-coupled anion transport. Plant Physiology 83, 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Boyer JC, Ipotesi E, Szponarski W, Tillard P, Touraine B, Sommerer N, Rossignol M, Gibrat R. 2007. Nitrate efflux at the root plasma membrane: identification of an Arabidopsis excretion transporter. The Plant Cell 19, 3760–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. 1990. Recent molecular approaches to the physiology of the plasma-membrane proton pump. Botanica Acta 103, 230–234. [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L. 2013. Organelle pH in the Arabidopsis endomembrane system. Molecular Plant 6, 1419–1437. [DOI] [PubMed] [Google Scholar]
- Smith FA, Raven JA. 1979. Intracellular pH and its regulation. Annual Review of Plant Physiology 30, 289–311. [Google Scholar]
- Søgaard R, Alsterfjord M, Macaulay N, Zeuthen T. 2009. Ammonium ion transport by the AMT/Rh homolog TaAMT1;1 is stimulated by acidic pH. European Journal of Physiology 458, 733–743. [DOI] [PubMed] [Google Scholar]
- Sun J, Bankston JR, Payandeh J, Hinds TR, Zagotta WN, Zheng N. 2014. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 507, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ. 2008. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. Journal of Experimental Botany 59, 303–313. [DOI] [PubMed] [Google Scholar]
- Sze H, Li X, Palmgren MG. 1999. Energization of plant cell membranes by H+-pumping ATPases. Regulation and biosynthesis. The Plant Cell 11, 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Fan X, Li Q, Feng H, Miller AJ, Shen Q, Xu G. 2012. Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiology 160, 2052–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Bloom AJ. 1998. Ammonium, nitrate, and proton fluxes along the maize root. Plant, Cell & Environment 21, 1255–1263. [Google Scholar]
- Tegeder M, Hammes UZ. 2018. The way out and in: phloem loading and unloading of amino acids. Current Opinion in Plant Biology 43, 16–21. [DOI] [PubMed] [Google Scholar]
- Tegeder M, Masclaux-Daubresse C. 2018. Source and sink mechanisms of nitrogen transport and use. New Phytologist 217, 35–53. [DOI] [PubMed] [Google Scholar]
- Hoopen F, Cuin TA, Pedas P, Hegelund JN, Shabala S, Schjoerring JK, Jahn TP. 2010. Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. Journal of Experimental Botany 61, 2303–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay YF, Schroeder JI, Feldmann KA, Crawford NM. 1993. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. [DOI] [PubMed] [Google Scholar]
- Ullrich CI, Novacky AJ. 1990. Extra- and intracellular pH and membrane potential changes induced by K+, Cl–, H2PO4–, and NO3– uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiology 94, 1561–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beusichem ML, Kirkby EA, Baas R. 1988. Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in Ricinus communis. Plant Physiology 86, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara F, Serrano R. 1982. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. Journal of Biological Chemistry 257, 12826–12830. [PubMed] [Google Scholar]
- Wang MY, Glass A, Shaff JE, Kochian LV. 1994. Ammonium uptake by rice roots (III. Electrophysiology). Plant Physiology 104, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Siddiqi MY, Glass ADM. 1996. Interactions between K+ and NH4+: effects on ion uptake by rice roots. Plant, Cell & Environment 19, 1037–1046. [Google Scholar]
- Wang R, Liu D, Crawford NM. 1998. The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proceedings of the National Academy of Sciences, USA 95, 15134–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Cheng YH, Chen KE, Tsay YF. 2018. Nitrate transport, signaling, and use efficiency. Annual Review of Plant Biology 69, 85–122. [DOI] [PubMed] [Google Scholar]
- Wen Z, Tyerman SD, Dechorgnat J, Ovchinnikova E, Dhugga KS, Kaiser BN. 2017. Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. The Plant Cell 29, 2581–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe CA, Dibattista ER, Fliegel L. 2001. Functional role of polar amino acid residues in Na+/H+ exchangers. The Biochemical Journal 357, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J, Chopin F, Santoni V, Viennois G, Tillard P, Krapp A, Lejay L, Daniel-Vedele F, Gojon A. 2007. Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. Journal of Biological Chemistry 282, 23541–23552. [DOI] [PubMed] [Google Scholar]
- Xia X, Fan X, Wei J, Feng H, Qu H, Xie D, Miller AJ, Xu G. 2015. Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and long-distance transport. Journal of Experimental Botany 66, 317–331. [DOI] [PubMed] [Google Scholar]
- Xu G, Fan X, Miller AJ. 2012. Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology 63, 153–182. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Kasai M, Ezaki B, Shibasaka M, Yamamoto Y, Matsumoto H, Sasakawa H. 1995. Stimulation of H+ extrusion and plasma membrane H+-ATPase activity of barley roots by ammonium-treatment. Soil Science & Plant Nutrition 41, 133–140. [Google Scholar]
- Yan M, Fan X, Feng H, Miller AJ, Shen Q, Xu G. 2011. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant, Cell & Environment 34, 1360–1372. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang S, Hu Y, et al. 2014. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiology 166, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao FG, Tang RJ, Yu Y, Song J, Wang Y, Li L, Luan S. 2017. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proceedings of the National Academy of Sciences, USA 114, E2036–E2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Theodoulou FL, Muldin I, Ingemarsson B, Miller AJ. 1998. Cloning and functional characterization of a Brassica napus transporter that is able to transport nitrate and histidine. Journal of Biological Chemistry 273, 12017–12023. [DOI] [PubMed] [Google Scholar]
- Zhou JJ, Trueman L, Boorer KJ, Theodoulou F, Forde BG, Miller AJ. 2000. A high-affinity fungal nitrate carrier with two transport mechanisms. Journal of Biological Chemistry 275, 39894–39899. [DOI] [PubMed] [Google Scholar]
- Zhu J, Fang XZ, Dai YJ, Zhu YX, Chen HS, Lin XY, Jin CW. 2019. Nitrate transporter 1.1 alleviates lead toxicity in Arabidopsis by preventing rhizosphere acidification. Journal of Experimental Botany 70, 6363–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q. 2009. Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant, Cell & Environment 32, 1428–1440. [DOI] [PubMed] [Google Scholar]
- Zifarelli G, Pusch M. 2010. CLC transport proteins in plants. FEBS Letters 584, 2122–2127. [DOI] [PubMed] [Google Scholar]