Abstract
Osteogenesis imperfecta is a phenotypically and molecularly heterogeneous group of inherited connective tissue disorders that share similar skeletal abnormalities causing bone fragility and deformity. Previously, the disorder was thought to be an autosomal dominant bone dysplasia caused by defects in type I collagen, but in the past 10 years discoveries of novel (mainly recessive) causative genes have lent support to a predominantly collagen-related pathophysiology and have contributed to an improved understanding of normal bone development. Defects in proteins with very different functions, ranging from structural to enzymatic and from intracellular transport to chaperones, have been described in patients with osteogenesis imperfecta. Knowledge of the specific molecular basis of each form of the disorder will advance clinical diagnosis and potentially stimulate targeted therapeutic approaches. In this Seminar, together with diagnosis, management, and treatment, we describe the defects causing osteogenesis imperfecta and their mechanism and interrelations, and classify them into five groups on the basis of the metabolic pathway compromised, specifically those related to collagen synthesis, structure, and processing; post-translational modification; folding and cross-linking; mineralisation; and osteoblast differentiation.
Introduction
The identification of the first gene for recessive osteogenesis imperfecta in 20061,2 initiated a burst of exciting new information about the genetics and mechanism of this bone dysplasia.3 Osteogenesis imperfecta, or brittle bone disease, is a fairly common rare disorder (one in 15–20 000 births). This generalised connective tissue disorder has major manifestations in bone, leading to skeletal fragility and substantial growth deficiency.4 Previously, osteogenesis imperfecta was known as an autosomal dominant disorder caused by mutations in COL1A1 and COL1A2, coding for the α1(I) and α2(I) chains of type I collagen, the most abundant protein of bone, skin, and tendon extracellular matrices. Although about 85–90% of cases are caused by structural or quantitative mutations in the collagen genes themselves, the disorder is now more fully understood as a predominantly collagen-related disorder.3,5 Seven recessive forms are caused by defects in genes whose protein products interact with collagen for folding or post-translational modifications. Two other rare defects mainly affect bone mineralisation, but also decrease collagen production. Now, the most recently identified genes could open a new chapter, with primary defects in osteoblast differentiation. Each discovery revealed a protein or pathway whose crucial importance for bone development had not been appreciated (see Orgel and colleagues6 for a review on bone composition and collagen-extracellular proteins interaction). The exciting advances in rare forms of the disorder sparked renewed interest in classic osteogenesis imperfecta with collagen defects, revealing changes in bone cell metabolism and development, and initiating a new era for diagnosis and potential treatment.
The classification evolved with the new genetic discoveries. The 1979 Sillence classification7 divided osteogenesis imperfecta into four types, from mild to lethal, on the basis of clinical and radiographic features.8 Identification of collagen defects showed that mild Sillence type I was related to quantitative deficiency of structurally normal collagen, whereas lethal (type II), severe (type III), and moderate (type IV) forms had mutations altering collagen structure.4 A genetic classification incorporated a new type for each defective gene. To make this information user friendly for clinicians and patients and to establish a structure for future development, we propose to group the genetic types on the basis of altered intracellular or extracellular metabolic pathways.
Diagnosis
The initial diagnosis is largely based on clinical and radiographic findings.7,8 Fractures from mild trauma, bowing deformities of long bones, and growth deficiency are the hallmark features. Dependent on age and severity, skeletal features can include macrocephaly, flat midface and triangular facies, dentinogenesis imperfecta, chest wall deformities such as pectus excavatum or carinatum, barrel chest, and scoliosis or kyphosis.3 Skeletal radiographs reveal generalised osteopenia, and some combination of long-bone bowing, undertubulation and metaphyseal flaring, gracile ribs, narrow thoracic apex, and vertebral compressions. Osteogenesis imperfecta is a generalised connective tissue disorder, whose findings often extend to non-skeletal features, including blue sclerae, hearing loss, decreased pulmonary function, and cardiac valvular regurgitation. Notably, the most common secondary features of the disorder are absent from newly recognised recessive forms, which generally have white sclerae, and normal cranial size, teeth, and hearing.3 Mild osteogenesis imperfecta can present a diagnostic challenge to discriminate from early onset osteoporosis in adults or physical abuse in children. Dual-energy x-ray absorptiometry (DXA) bone density provides useful, if not diagnostic, information in these cases, as does consultation with experts on connective tissue disorders.
In osteogenesis imperfecta caused by a collagen defect (types I–IV), bone histomorphometry reveals low bone volume and trabecular number, with high turnover kinetics,9,10 whereas histology is distinctive in types V and VI.11,12 This invasive test has been superseded by molecular testing for all types, and measures of pigment epithelium-derived factor (PEDF) serum concentration for type VI. When possible, clinicians should do a full screen of osteogenesis imperfecta causative genes and identify carrier status or presence of second mutations to understand the complexity of the disorder rather than stopping the investigation when the first plausible mutation is identified. However, for economic reasons, a first screen for COL1 genes is recommended in families with a single proband, followed by a complete sequence of the other osteogenesis imperfecta genes in case of negative result. When more than one child in a family is affected by the disorder, investigation of the whole osteogenesis imperfecta gene panel represents a better approach than waiting to rule out COL1 genes first. A molecular diagnosis is very useful for counselling for prognosis, recurrence, and heritability, and for variable response to drugs.
Defects in collagen
Type I collagen is a heterotrimer, containing two α1(I) and one α2(I) chains. It is synthesised as a procollagen molecule, with N-terminal and C-terminal globular propeptides flanking the helical domain. The helical domains contain uninterrupted Gly-Xaa-Yaa triplets because the small glycine side chain fits in the internal helical space.
Procollagen chains assemble at their C-propeptides and fold toward the N-terminal. The unfolded chains are subjected to multiple post-translational modifications. Proline and lysine residues along the helical regions of both chains are hydroxylated by prolyl 4-hydroxylase 1 (which acts on the proline ring carbon-4) and lysyl hydroxylase 1 (LH1); hydroxylysine residues can subsequently be glycosylated.13 By contrast, prolyl 3-hydroxylase 1 (P3H1), acting as part of a complex with cartilage associated protein (CRTAP) and cyclophylin B (CyPB),14 hydroxylates the carbon-3 of discrete proline residues α1(I)Pro986 and α2(I)Pro70715 Isomerisation of peptidyl prolyl bonds, crucial for proper collagen folding, is catalysed by specific peptidyl prolyl cis-trans isomerases (PPIase).16
The most common structural defects in type I collagen causing osteogenesis imperfecta are glycine substitutions in the helical domain (figure 1). Glycine substitutions delay helical folding, prolonging access time for modifying enzymes. Each α chain has a distinct genotype-phenotype relation. In the α1(I) chain, substitutions with charged or branched side chains disrupt helix stability and are predominantly lethal. Substitutions in two major ligand binding regions near the carboxyl end of α1(I) have exclusively lethal outcomes, pointing to crucial interactions between the collagen monomer and non-collagenous matrix proteins. In the α2(I) chain, substitutions are mainly non-lethal; however, eight lethal clusters along the chain align with proteoglycan binding sites on collagen fibrils.4 Finally, less than 5% of mutations that cause classic osteogenesis imperfecta occur in the procollagen C-propeptide, impairing chain association or folding (figure 1).17
Figure 1: Mutations in specific positions along type I procollagen molecule cause distinct clinical phenotypes.
Mutations affecting the helical region of the α chains and the C-propeptide domain cause classic osteogenesis imperfecta, including the osteogenesis imperfecta/Ehlers-Danlos syndrome variant, which is caused by substitutions in the N-anchor domain. Molecular defects in the N-propeptide cleavage site cause Ehlers-Danlos syndrome VII A/B and in the C-propeptide cleavage site cause high bone mass osteogenesis imperfecta. Hexagons represent sugar molecules linked to hydroxyl lysine residues. OH-=hydroxyl group linked to proline or lysine residues.
Collagen with a primary structural defect has more severe consequences for intracellular metabolism and matrix structure than does a reduced amount of normal collagen. Combined endoplasmic reticulum stress and mutant matrix leads to impaired maturation of bone-forming osteoblasts. Also, in the Brtl mouse model for classic osteogenesis imperfecta, which is heterozygous for an α1(I) Gly349Cys substitution, mesenchymal stem cells, precursors to both osteoblasts and adipocytes, show increased plasticity to adipogenesis, although osteoblast number is not deficient.18 This evidence remains to be extended to patients.
Heterozygous null COL1A1 alleles result in synthesis of a reduced amount (about half) of structurally normal collagen and cause the mildest form of the disorder.19 Homozygous null α2(I) mutations cause symptoms ranging from severe osteogenesis imperfecta to a mild Ehlers-Danlos syndrome-like disorder that is associated with cardiac defects,20,21 whereas heterozygotes have no apparent osteogenesis imperfecta phenotype.22
Processing defects
After secretion, procollagen molecules undergo an extracellular maturation process, in which the N-propeptides and C-propeptides are removed by specific proteases. After processing, the collagen helices are able to spontaneously assemble into fibrils in tissue, to be further stabilised by crosslinks. Mutations altering the propeptide cleavage sites cause interesting and specific variants of osteogenesis imperfecta.23
The N-propeptide cleavage site is encoded by exon 6 in both COL1A1 and COL1A2; it is cleaved by the metalloenzyme ADAMTS-2, which requires a helical substrate conformation. One cause of defective N-propeptide processing is so-called skipping of exon 6 at the RNA level—effectively removing the procollagen cleavage site—causing Ehlers-Danlos syndrome VII A if COL1A1 is affected or Ehlers-Danlos syndrome VII B if COL1A2 is affected (figure 1). Ehlers-Danlos syndrome type VII cases show tissue hyperlaxity, with severe joint hypermobility, congenital bilateral hip dislocation, ligamentous laxity causing scoliosis, and joint dislocations. It also involves mild osteopenia, resulting in fractures in some patients.24 A second cause of defective N-propeptide processing implicates dominant mutations in the first 85 residues of the helical region of α1(I) or α2(I) collagen, which unfold the contiguous N-propeptide cleavage site, causing combined osteogenesis imperfecta and Ehlers-Danlos syndrome. Mutations in this region of COL1A1 cause moderate to severe osteogenesis imperfecta, whereas the Ehlers-Danlos syndrome symptoms predominate from COL1A2 mutations. Clinically, patients with combined osteogenesis imperfecta and Ehlers-Danlos syndrome have striking laxity of large and small joints, and paraspinal ligamentous laxity causes early and aggressive scoliosis. Congenital hip dislocation is also reported in combined COL1A2 osteogenesis imperfecta and in Ehlers-Danlos syndrome. Dermal collagen fibrils are very thin, as in Ehlers-Danlos syndrome type VII.25 A third type of defective N-propeptide processing has recessive inheritance, caused by mutations affecting ADAMTS-2 activity (Ehlers-Danlos syndrome VIIC). This disorder is more severe than the other types of defective N-propeptide processing and is caused by defective processing of type II and type I collagen, characterised by extreme skin fragility, characteristic facies, joint laxity, droopy skin, umbilical hernia, and blue sclera.26 In mice lacking both procollagen N-proteinase alleles, skin collagen fibrils revealed reduced diameter with ageing and bizarre curls in transverse sections.27
Similarly, processing defects of the C-propeptide have a dominant form with substitutions in the cleavage site and a recessive form with defects in the processing enzyme.17,28–32 Quite unexpectedly and paradoxically, these defects collectively lead to so-called high-bone-mass osteogenesis imperfecta (figure 1).29 Dominant mutations in both residues (Ala-Asp) of the C-propeptide cleavage site in COL1A1 and COL1A2 have been reported,29,32 resulting in incorporation of pC-collagen into the extracellular matrix and collagen fibrils with irregular cross-sections.29 Prepubertal children with COL1A1 (Asp1219Asn) and COL1A2 (Ala1119Thr) mutations have mild osteogenesis imperfecta with few fractures, normal stature, white sclerae, normal teeth, and increased DXA Z scores of 3 and 0 respectively, compared with the negative Z score usually reported for both dominant and recessive osteogenesis imperfecta. The bone tissue of children with these mutations showed substantial increases in mineralisation, beyond the standard hypermineralisation of bone with the disorder. COL1A1 (Ala1218Thr) was reported in adults with scoliosis, normal stature, white sclerae, and raised DXA Z scores, whereas COL1A2 (Asp1120Ala) causes joint laxity, blue sclerae, and DXA Z scores of 3·5. High bone mass in these patients was hypothesised to be caused by localisation of pC-collagen on the fibril surface, serving as nucleators of mineralisation.29 Decreased type I C-propeptide trimer might also affect the phenotype, since its absence could affect bone vascularisation.33
Cleavage of the C-propeptide is more complex than that of the N-propeptide, with four different C-proteases and modulation by an enhancer protein.34–37 Mutations of the major C-protease enzyme BMP1/mTLD result in more severe osteogenesis imperfecta than do substrate defects because these enzymes also process other procollagens and activate lysyl oxidase, the initiator of crosslinks.30,31 Knockout Bmp1 mice have collagen with a barbed-wire appearance due to retention of the C-propeptide.38 A mutant zebrafish revealed that BMP1 affects mainly the ability to generate mature collagen fibrils, rather than osteoblast development.30 Notably, patients with a homozygous substitution in the BMP1 signal peptide have high bone mineral density that is similar to that in collagen substrate defects.30 However, homozygous substitution (Phe249Leu) in the BMP1 protease domain had an osteoporotic outcome. In this situation, residual BMP1 activity probably accounts for the reduction in severity.31
Defects in collagen post-translational modification and folding
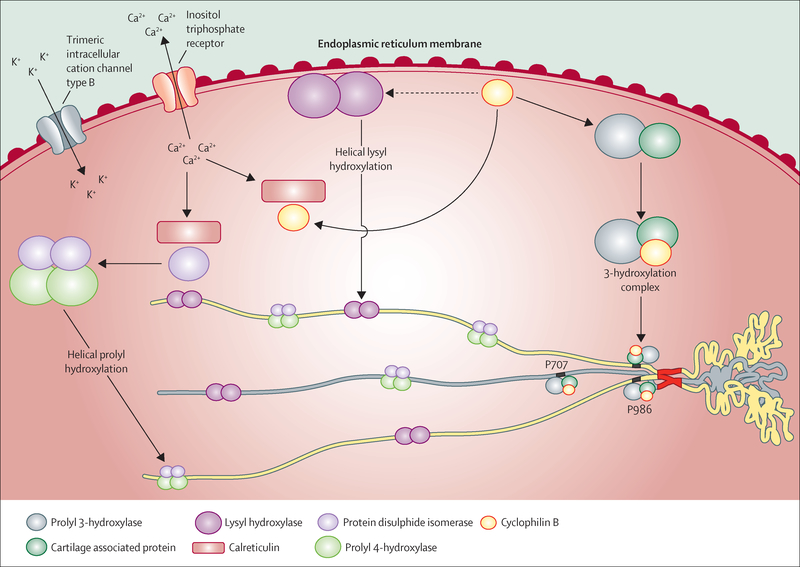
Procollagen undergoes several post-translational modifications during synthesis, which are important for procollagen folding, secretion, and extracellular matrix assembly and are cell, site, and temporally regulated. Most of these crucial processing steps occur in the endoplasmic reticulum. The identification of a multiprotein complex13 has helped researchers to further understand rare osteogenesis imperfecta forms and to identify a mechanism by which mutations in different proteins can cause clinically similar forms of the disorder.
The collagen prolyl 3-hydroxylation complex opened the floodgate to understanding rare forms of osteogenesis imperfecta. P3H1, the enzyme that causes 3-hydroxylation of α1(I)Pro986 in type I collagen, was isolated39 as part of a complex of three proteins in a 1:1:1 ratio of P3H1, CRTAP, and CyPB (figure 2).14 P3H1 is the catalytically active component, whereas CRTAP is a helper protein but does not have a catalytic domain. CRTAP is predominantly located in the endoplasmic reticulum, although a small amount is secreted.40 CyPB is a well known peptidyl-prolyl cis-trans isomerase. The trans proline configuration is required for protein folding; prolyl isomerisation is rate limiting for collagen folding.41 The precise function of collagen 3-hydroxylation is still unclear, although a role in collagen fibril formation has been proposed.42
Figure 2: Proteins involved in type I procollagen post-translational modification and folding in the endoplasmic reticulum.
Prolyl 3-hydroxylase, cartilage associated protein, and cyclophilin B act as a trimeric complex for hydroxylation of the 3-carbon position of the α1(I)Pro986 and α2(I) Pro707 proline residues. Cyclophilin B also affects the activity of lysyl hydroxylase 1, which hydroxylates lysine residues in the helical region of type I procollagen. The regulation of calcium ion concentration in the endoplasmic reticulum is relevant to procollagen post-translational modification because of its role in modulating calreticulin interaction with cyclophilin B and with protein disulphide isomerase. Protein disulphide isomerase complexes with prolyl 4-hydroxylase to hydroxylate the 4-carbon position of proline residues located in the Y-position of the repetitive collagenic triplet (Gly-X-Y).
A null mutation in CRTAP causes osteogenesis imperfecta type VII, whereas a null mutation in LEPRE1 (which encodes P3H1) causes type VIII, both of which can be severe to lethal and result in overmodification of the full collagen helical region.1,2,43 Affected individuals have an osteochondrodystrophy, since the complex is also expressed in cartilage, with neonatal fractures and broad undertubulated long bones, white sclerae, and rhizomelia (shortening of the proximal segment of the limbs). Individuals who live beyond infancy have notable growth deficiency, very low bone mineral density (Z ≤−6), and bulbous metaphyses.44 About half of LEPRE1 cases have a west African founder allele found in Ghana and Nigeria and among African-Americans, which is almost uniformly lethal in homozygous form.45 CRTAP and LEPRE1 mutations always cause severely reduced to absent Pro986 3-hydroxylation, but whether the pathological defect is attributable to an absence of collagen 3-hydroxylation or an absence of the 3-hydroxylation complex is unclear.46 A null mutation in either LEPRE1 or CRTAP results in the absence of both proteins from mutant cells47 because these proteins are mutually supportive in the complex, accounting for the similar clinical outcome of these gene defects.47 CRTAP mutations can also cause renal or pulmonary abnormalities, probably because some CRTAP is secreted.47 All cases with CRTAP or P3H1 defects share the biochemical feature of overmodified collagen α chains, because of a delay in collagen folding that allows excess hydroxylation and subsequent glycosylation of helical lysine residues.3 Positive biochemical tests for overmodification are also reported in dominant osteogenesis imperfecta caused by collagen defects.4
Mutations in the third member of the complex, cyclophilin B (encoded by PPIB), are biochemically and phenotypically distinct from P3H1 and CRTAP deficiency. These cases are quite rare, with only eight reported, and have either a moderate or a lethal phenotype (type IX).48,49 The affected individuals differ from P3H1 and CRTAP cases in that they do not have rhizomelia, although they share white sclerae and broad undertubulated long bones. In the infants with the lethal form of the disorder, α1(I)Pro986 3-hydroxylation is reduced to about 30% of normal levels (but is never absent), although two children with moderate phenotypes and normal Pro986 3-hydroxylation have been independently reported.48,50 Furthermore, the amount of CRTAP and P3H1 protein is only slightly reduced in PPIB-null cells, suggesting that although CyPB is not required for 3-hydroxylation, it is needed for folding of the collagen helix through its PPIase activity.50
The distinctive phenotypic consequences of CyPB deficiency are representative of its multiple interactions with non-collagenous proteins in the endoplasmic reticulum (figure 2).49 The interaction between CyPB and LH1 was first appreciated through its effect on collagen modification in PPIB-null cells.50 LH1 hydroxylates triple helical lysine residues, which is important both for glycosylation and for extracellular collagen cross-links during fibril formation. In the American quarter horse, a CyPB missense mutation (Gly6Arg) causes Ehlers-Danlos syndrome-like dermal symptoms. Although equine collagen has normal 3Hyp content, suggesting the complex functions normally, the collagen has reduced hydroxylysine content.49 Similarly, collagen synthesised by cells from a knockout Ppib mouse shows a tissue and residue specific pattern of changes in lysine hydroxylation and glycosylation, with an important common feature of reduced hydroxylation of the helical lysine 87 residue crucial for collagen crosslinking.51 Together, the data strongly support a direct effect of CyPB on LH1 activity. Thus, CyPB affects collagen both as the major PPIase for collagen folding, and in its support of LH1 activity, affecting collagen modification and crosslinking.
Three reports have been published for recessive mutations in TMEM38B (type XIV), two in Bedouins and one in an 11-year old Albanian girl.52–54 TMEM38B encodes TRIC-B, which forms a trimeric endoplasmic reticulum-membrane cation channel synchronised with inositol trisphosphate-mediated release of calcium (figure 2).55 Since the Tmem38B knockout mouse was perinatally lethal, no skeletal phenotype was reported.55 The Bedouin mutation predicts truncation of the protein whereas the Albanian defect was a large genomic deletion that included the TMEM38B gene. Affected Bedouin individuals have generalised osteopenia with multiple long bone fractures from birth and bowing deformities but without platyspondyly. Blue sclerae but normal teeth, facies, and hearing were reported.52,53 A similar phenotype was present in the Albanian girl, with the addition of mild hearing loss. Multiple proteins in the endoplasmic reticulum involved in collagen metabolism are calcium dependent, leading to an expectation of altered collagen modification.55
Defects in collagen folding and crosslinking
Another rough endoplasmic reticulum-resident immunophilin is also crucial for normal collagen synthesis. The PPIase FKBP65 is encoded by FKBP10 (figure 3).56 As with CyPB, FKBP65 has both direct and indirect effects on procollagen through collagen modifying enzymes. Recessive defects in FKBP10 cause a continuum of three previously distinct recessive syndromes.
Figure 3: Proteins affecting procollagen intracellular trafficking and extracellular crosslinking.
Heat shock protein 47 is a specific collagen I chaperone, binding to the triple helical collagen domain in the endoplasmic reticulum, preventing aggregation, and facilitating its trafficking to the Golgi. An RDEL signal will then guide the return of heat shock protein 47 to the endoplasmic reticulum. FK506 binding protein 65 is a peptydyl prolyl cis-trans isomerase known to affect the activity of lysyl hydroxylase 2, the enzyme which hydroxylates lysine residues in the N-telopeptides and C-telopeptides that are crucial for crosslink formation in the extracellular matrix. The question marks refer to probable but not yet proven interactions.
FKBP65 deficiency was first shown to cause recessive osteogenesis imperfecta (type XI), ranging from progressive deforming, with long bone fractures, platyspondyly, and scoliosis, to moderate, with short stature and ambulation potential, all with normal sclerae and teeth.57,58 Second, FKBP10 mutations cause Bruck syndrome 1, a distinctive disorder with severe osteogenesis imperfecta and congenital contractures of large joints, short stature, pterygia, and scoliosis.59 The same FKBP10 mutations occur with or without contractures, suggesting that they are allelic. The third outcome of FKBP10 mutations, Kuskokwim Syndrome, adds a disorder with only contractures to the previous FKBP10 phenotypic range of osteogenesis imperfecta or osteogenesis imperfecta with contractures.60 Kuskokwim syndrome is a congenital contracture disorder with minor skeletal features, occurring uniquely in Yup’ik Eskimos in Alaska. Residual FKBP65 (about 5% of the amount in normal cells) in Kuskokwim syndrome is apparently sufficient to prevent substantial bone dysplasia.
Two sets of data led to the association of FKBP65 with lysyl hydroxylase 2 (LH2), the enzyme encoded by PLOD2, which hydroxylates collagen telopeptide lysines (figure 3). The first clue was biochemical, the alteration of collagen crosslinks.61 In bone, hydroxylation of telopeptide lysines is necessary to generate cross-links between collagen molecules to provide stability and tensile strength to fibrils.62 Patients with Bruck syndrome 1 with either FKBP10 or PLOD2 mutations have reduced hydroxyallysine-derived cross-links in bone collagen.59 The second clue was that cells with the FKBP10 mutation deposit a reduced amount of collagen into the extracellular matrix.58 Collagen telopeptide lysine hydroxylation in mutant cells was reduced to a minimum. These data support a direct interaction between FKBP65 and LH2, perhaps to isomerise the peptidyl-prolyl bonds in LH2. At present, no data support a direct role for the FKBP65 PPIase activity in collagen helical folding.
HSP47 is a collagen-specific chaperone that is resident in the endoplasmic reticulum and is encoded by SERPINH1. It has an RDEL recognition site to mediate shuttling of the protein between the endoplasmic reticulum and Golgi, and it binds only to triple helical collagen (figure 3).63 It functions to stabilise folded collagen and to mark it for transfer to the cis-Golgi. Serpinhl knockout mice are embryonic lethal.64 Their cells display intracellular collagen aggregation, delayed secretion, and abnormal fibrils.65 Not surprisingly, both SERPINH1 mutations reported—in dachshunds and a child with severe recessive osteogenesis imperfecta (type X)—are homozygous missense mutations, which probably have some residual activity, rather than null defects.66,67 The child lived for 3 years and had blue sclerae, dentinogenesis imperfecta, and atypical features such as skin bullae, pyloric stenosis, and renal stones. His collagen had increased sensitivity to proteases, suggesting imperfect folding.
Defects in ossification and mineralisation
Types V and VI osteogenesis imperfecta share the distinction of causing primary defects in endochondral bone ossification or mineralisation. Dominantly inherited type V and recessively inherited type VI were delineated clinically, on the basis of phenotypic, radiographic, and histological features, and normal type I collagen posttranslational modification.11,12 Whole exome sequencing revealed causative mutations in the interferon-induced transmembrane protein 5 (IFITM5) for type V68,69 and SERPINF1 genes for type VI70,71 (figure 4). Although mutations in these genes have opposite effects on mineralisation—with increased ectopic ossification in type V and reduced bone mineralisation in type VI—their metabolic pathways intersect, suggesting that the proteins have a functional connection.
Figure 4: Proteins involved in mineralisation of the collagen extracellular matrix.
The transmembrane protein-bone restricted interferon induced transmembrane protein-like—regulates the expression of pigment epithelium derived factor, an important collagen-binding protein regulating bone homoeostasis and osteoid mineralisation, which also has strong anti-angiogenic functions by an unknown mechanism. SERPINF1 is the gene for pigment epithelium derived factor. The question marks refer to the presence of other factors in this pathway, which are not yet discovered. The plus and minus signs refer to increased and decreased transcription of the SERPINF1 gene. S40L refers to the IFITM5 mutation Ser40Leu.
Unique among osteogenesis imperfecta types, all patients with type V have the same heterozygous mutation in IFITM5, a point mutation in the 5′-UTR (c.−14C→T), which generates a novel start codon and adds five residues to the N-terminal of the protein. Affected individuals have moderately severe bone dysplasia with a variable combination of distinctive features, including ossification of the forearm interosseous membrane, radial head dislocation, and a subphyseal metaphyseal radiodense band.72,73 More than half of patients with type V develop hyperplastic callus during fracture healing. Scleral hue is variable whereas teeth are normal. All patients with type V have distinctive mesh-like lamellation on bone histology. Type V has the paradoxical association of an osteoporotic phenotype from a defect in trabecular osteoblasts and exuberant bone formation in hypertrophic callus, affecting periosteal bone. These data suggest that IFITM5 functions differently in trabecular versus periosteal bone.
IFITM5, also known as bone-restricted IFITM-like (BRIL), is a member of the IFITM family. It has a single transmembrane domain with an extracellular C-terminus. On the cytoplasmic side, BRIL is attached to the membrane through palmitoylation of cysteines 52 and 53 (figure 4). It shares only 30% aminoacid identity with other family members and is not induced by interferon. It is localised predominantly to the osteoblast plasma membrane and expressed throughout life, although expression decreases with age. BRIL is not detectable in either chondrocytes or osteocytes;74 low expression occurs in fibroblasts.
Neither Ifitm5 knockout mice75 nor people with deletions of one BRIL allele manifest bone dysplasia. Therefore, the likely molecular basis of osteogenesis imperfecta type V is a gain of function. Overexpression of Ifitm5 in vitro increases alkaline phosphatase expression and mineral deposition, whereas silencing has opposite effects.76 The only known BRIL binding partner in osteoblasts is FKBP11; BRIL binding disrupts the interaction of CD9 with FKBP11–CD81. Since the CD9–FKBP11 complex increases expression of interferon-induced genes, type V might have an immune component.77 Furthermore, BRIL can regulate calcium binding through C-terminal residues, which could affect both mineral formation and signal transduction pathways regulating bone development.78
Compromised mineralisation also occurs in patients with type VI osteogenesis imperfecta who have homozygous or compound heterozygous null mutations of SERPINF1, which encodes PEDF. The clinical course of these patients is distinctive, with fractures generally absent in the first year, followed by a severe progressive deforming bone dysplasia, with frequent long bone fractures, vertebral compressions, and severely reduced bone mineral density. Bone histology reveals an increased amount of unmineralised osteoid and a so-called fish-scale lamellar pattern under polarised light.70,71,79 The increased mineral lag time underlies the low response of type VI to bisphosphonates.80
PEDF belongs to the Serpin family of serine protease inhibitors but does not have protease inhibitory activity. It is a potent anti-angiogenic factor81 expressed by a wide range of cells, including chondrocytes throughout the growth plate, osteoblasts, and mesenchymal stem cells.82 In bone, PEDF functions at many levels to maintain bone homoeostasis and regulate osteoid mineralisation. A Serpinfl knockout mouse replicated the reduced bone volume and unmineralised osteoid typical of type VI.83 PEDF positively affects osteoblast development by favouring the expression of osteogenic genes and mineral deposition. It inhibits osteoclast maturation by stimulating osteoprotegerin expression.84 Thus, absence of PEDF increases osteoclast number and bone resorption by favouring RANKL binding to the osteoclast RANK receptor.85
That PEDF interacts with two sites on type I collagen is also relevant.86 One binding site overlaps the heparin and heparan sulphate proteoglycan binding site with the α1(I) C-terminal major ligand binding region. The second collagen-binding site, in the α1(I) N-terminal, overlaps integrin collagen-binding sites. Importantly, an intact collagen-binding site on PEDF is required for its anti-angiogenic activity (figure 4).
A heterozygous IFITM5 mutation that connects types V and VI has been delineated.87 The Ser40Leu substitution interferes with modification of the nearby BRIL palmitoylation sites.88 This mutation causes severe progressive deforming osteogenesis imperfecta and bone histology typical for type VI, but none of the clinical or radiographic findings of type V. Proband fibroblasts and osteoblasts do not secrete PEDF, although serum PEDF concentrations are normal. Conversely, osteoblasts with the IFITM5 mutation causing type V have increased SERPINF1 expression. These data further support the type V IFITM5 mutation as having a gain-of-function mechanism, in which the PEDF pathway is overactivated, whereas Ser40Leu probably causes loss of a BRIL function important for SERPINF1 expression (figure 4).87
Defects in osteoblast development
Three genes implicated in osteoblast differentiation have been associated with osteogenesis imperfecta phenotypes: WNT1 (type XV), CREB3L1 (type XVI), and SP7 (type XII). Defects in these genes either cause, or are expected to cause, a reduction in type I collagen expression. The reported data support early onset osteoporosis but are still accumulating for the cause of osteogenesis imperfecta. The relation of these gene defects to collagen might simply be quantitative, resulting in osteopenia that combines with other manifestations of impaired osteoblast differentiation.
Of the three genes, WNT1 has the most supportive data for osteogenesis imperfecta. Wnts are a family of secreted glycoproteins, whose binding to transmembrane receptors LRP5 and LRP6 and Frizzled initiates a complex intracellular signalling pathway. In the canonical pathway, Wnt binding enables cytosolic β-catenin to escape proteasomal destruction and be translocated into the nucleus, where it activates expression of several genes implicated in bone formation. Heterozygous WNT1 mutations were identified in patients presenting as early onset osteoporosis.89 Homozygous non-sense, missense, frameshift, or splicing mutations in WNT1 occur in patients with severe osteogenesis imperfecta, with short stature, frequent fractures, and vertebral compressions. Several patients with WNT1 defects have brain malformations. Some WNT1 mutations impair activation of the canonical pathway and mineralisation by osteoblasts in vitro.90–93 By contrast, Wnt1 knockout mice have a severe neurological defect but do not have skeletal manifestations.94 Wnt1 expression in osteoblasts, osteocytes, haemopoietic progenitor cells, and B cells support a complex regulatory role for WNT1 in bone formation.91
The second gene functioning predominantly at the osteoblast level is CREB3L1, encoding the endoplasmic reticulum-stress transducer old astrocyte specifically induced substance (OASIS). After proteolysis, the N-terminal domain of OASIS translocates into the nucleus and activates the COL1A1 promoter.95 An Oasis-null mouse has severe osteopenia with reduced bone volume, trabecular thickness, and growth retardation. Oasis−/− osteoblasts have decreased Col1a1 expression and expanded endoplasmic reticulum.95 However, in fibroblasts from siblings with lethal bone dysplasia, a large homozygous deletion including CREB3L1, did not cause qualitative or quantitative defects of type I collagen or raised endoplasmic reticulum-stress markers.96 A clear relation of CREB3L1 to the osteogenesis imperfecta phenotype awaits additional patient mutations and osteoblast investigation.
The third gene with a potential osteoblast mechanism is SP7, which encodes osterix and is a target gene of the Wnt pathways. A homozygous SP7 mutation, putatively truncating a fifth of Osterix, was reported in severe recessive osteogenesis imperfecta.97 This gene awaits functional molecular and biochemical data.
Osteogenesis imperfecta classification
Both clinical and genetic classifications have emerged to encompass the rare forms of osteogenesis imperfecta since most new genes do not have specific diagnostic features. In the clinical classification, the new forms of the disorder were merged into Sillence types I to IV by clinical severity. For example, type II osteogenesis imperfecta, the perinatal lethal form, included lethal collagen mutations and some CRTAP, LEPRE1, PPIB, SERPINH1, and SP7 mutations.98 Some clinical classifications retained types V, VI, and VII, originally delineated clinically.99 This classification results in many incongruous situations, including retyping of an individual by severity during their lifetime and siblings with different osteogenesis imperfecta types. In the same type, individuals would have dominant or recessive inheritance, confusing genetic counselling and hindering research on the disease mechanism and response to therapy.
In a genetic classification, Sillence types are used only for collagen mutations and the numeration is continued for each new gene discovery.5 This pattern eliminates the issues cited above and provides a better arrangement for research and clinical management than that of a clinical classification. However, a genetic classification has the disadvantage of an evolving list, which can be difficult for geneticists and parents to use beyond a dozen types.
To satisfy both clinical and genetic requirements, we propose that a functional metabolic classification will have the broadest usefulness and still allow retention of genetic type numeration (table). A similar regrouping of Ehlers-Danlos syndrome by function was successful for both clinicians and families.100 In this plan, genes whose products function in the same pathway, and are likely to have shared mechanisms, are arranged in five functional groups: group A, primary defects in collagen structure or processing (COL1A1, COL1A2, and BMP1); group B, collagen modification defects (CRTAP, LEPRE1, PPIB, and TMEM38B); group C, collagen folding and cross-linking defects (SERPINH1, FKBP10, and PLOD2); group D, ossification or mineralisation defects (IFITM5 and SERPINF1); and group E, defects in osteoblast development with collagen insufficiency (WNT1, CREB3L1, and SP7). The functional genetic classification could be usefully supplemented by a parallel clinical severity score for physical rehabilitation on the basis of a proposed scheme.101
Table:
Classification of classic dominant and new recessive forms of osteogenesis imperfecta
| OMIM number | Locus | Gene symbol | Sillence type | Clinical variants | Protein | Main location | Bone deformity | Sclerae | Hearing loss | Dentinogenesis imperfecta | Other typical features | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Defects in collagen synthesis, structure, or processing (group A) | ||||||||||||
| Autosomal dominant | 166200 | 17q21.33 | COL1A1 | I | NA | Collagen type I, alpha 1 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 166210 | 17q21.33 | COL1A1 | II | NA | Collagen type I, alpha 1 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 259420 | 17q21.33 | COL1A1 | III | NA | Collagen type I, alpha 1 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 166220 | 17q21.33 | COL1A1 | IV | OI/EDS and HBM/OI | Collagen type I, alpha 1 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | Type IV OI/EDS is due to mutations at the first 85 aminoacids of α1(I); HBM/OI is caused by mutations blocking C-propeptide processing |
| Autosomal dominant | 166200 | 7q21.3 | COL1A2 | I | NA | Collagen type I, alpha 2 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 166210 | 7q21.3 | COL1A2 | II | NA | Collagen type I, alpha 2 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 259420 | 7q21.3 | COL1A2 | III | NA | Collagen type I, alpha 2 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | .. |
| Autosomal dominant | 166220 | 7q21.3 | COL1A2 | IV | OI/EDS and HBM/OI | Collagen type I, alpha 2 | Extracellular matrix | Rare to very severe | Normal, grey to dark blue | Absent to common | Absent to common | Type IV OI/EDS is due to mutations at the first 85 aminoacids of α2(I); HBM/OI is caused by mutations blocking C-propeptide processing |
| Autosomal recessive | 614856 | 8p21.3 | BMP1 | XIII | NA | Bone morphogenic protein1/procollagen C proteinase | Pericellular environment | Mild to severe | Normal | Absent | Absent | Umbelical hernia, HBM |
| Defects in collagen modification (group B) | ||||||||||||
| Autosomal recessive | 610682 | 3p22.3 | CRTAP | VII | NA | Cartilage- associated protein | Endoplasmic reticulum | Severe rhizomelia | Normal, grey | Absent | Absent | .. |
| Autosomal recessive | 610915 | 1p34.2 | LEPRE1/ P3H1 | VIII | NA | Leucine proline-enriched proteoglycan1/prolyl 3-hydroxylase 1 | Endoplasmic reticulum | Severe rhizomelia | Normal | Absent | Absent | .. |
| Autosomal recessive | 259440 | 15q22.31 | PPIB | IX | NA | Peptidylprolyl isomerase B/cyclophilin B | Endoplasmic reticulum | Severe | Grey | Absent | Absent | .. |
| Autosomal recessive | 615066 | 9q31.2 | TMEM38B | XIV | NA | Transmembrane protein 38 B | Endoplasmic reticulum membrane | Severe | Normal to blue | Absent | Absent | .. |
| Defects in collagen folding and cross-linking (group C) | ||||||||||||
| Autosomal recessive | 613848 | 11q13.5 | SERPINH1 | X | NA | Serpin peptidase inhibitor, clade H, member 1/heat shock protein 47 | Endoplasmic reticulum | Severe | Blue | Absent | Present | Skin blisters and bullae at birth, inguinal hernia |
| Autosomal recessive | 610968 | 17q21.2 | FKBP10 | XI | NA | FK506 binding protein 65 | Endoplasmic reticulum | Mild to severe | Normal, grey | Absent | Absent | Variable congenital contractures, encompasses Bruck and Kuskokwim syndromes |
| Autosomal recessive | 609220 | 3q24 | PLOD2 | NA | Procollagen- lysine, 2-oxoglutarate 5-dioxygenase 2 | Endoplasmic reticulum | Moderate to severe | .. | .. | .. | Progressive joint contractures | |
| Defects in bone mineralisation (group D) | ||||||||||||
| Autosomal dominant | 610967 | 11p15.5 | IFITM5 | V | NA | Interferon- induced transmembrane protein 5 | Plasma membra ne | Variable | Normal to blue | Infrequent | Absent | Ossification of the forearm interosseous membrane, radial head dislocation, subephyphyseal metaphyseal radiodense band |
| Autosomal recessive | 613982 | 17p13.3 | SERPINF1 | VI | NA | Pigment epithelium-derived factor | Extracellular matrix | Moderate to severe | Normal | Absent | Absent | Normal at birth, unmineralised osteoid, fish scale appearance of lamellar bone pattern, raised ALP, loss of serum PEDF |
| Defects in osteoblast development with collagen insufficiency (group E) | ||||||||||||
| Autosomal recessive | 613849 | 12q13.13 | SP7 | XII | NA | Transcription factor 7/osterix | Nucleus | Severe | Normal | Absent | Absent | Delayed tooth eruption, midface hypoplasia |
| Autosomal recessive | 615220 | 12q13.12 | WNT1 | XV | NA | Wingless-type MMTV integration site family, member 1 | Extracellular matrix | Severe | White | Absent | Absent | Possible neurological defects |
| Autosomal recessive | 616229 | 11p11.2 | CREB3L1 | XVI | NA | cAMP responsive element binding protein 3 like 1 | Endoplasmic reticulum membrane | Severe | .. | .. | .. | .. |
OMIM=Online Mendelian Inheritance in Man. NA=not applicable. OI=osteogenesis imperfecta. EDS=Ehlers-Danlos syndrome. HBM=high bone mass. ALP=alkaline phosphatase. PEDF=pigment epithelium-derived factor. MMTV=mouse mammary tumour virus.
Medical management of osteogenesis imperfecta
Osteogenesis imperfecta is best managed by a multidisciplinary team. Physical rehabilitation by a therapist with experience of the disorder is arguably the most important contribution to function. The combination of fragile bones, weak muscle, and cycles of fracture and disuse creates substantial challenges for a patient to attain and maintain gross motor skills, especially walking.102 Physiotherapy and hydrotherapy focused on muscle strength and joint range of motion are crucial to maximise an individual’s function and independence.103 Most individuals with severe osteogenesis imperfecta can attain self-care, transfer (an ability to transfer independently between their bed, toilet, and chair), and domestic skills needed for independent living, educational, and occupational success.104 Individuals with mild osteogenesis imperfecta can function at a very high level, often excluding only contact sports.105
Pulmonary function impairment is the major cause of morbidity and mortality in osteogenesis imperfecta.106 Loss of pulmonary function is related to scoliosis greater than 60°,107 and rib cage and pectal deformity, which alter respiratory muscle and chest wall action,108 contributing to respiratory infections and insufficiency. Even paediatric patients without scoliosis show progressive restrictive pulmonary disease, supporting a primary cardiopulmonary effect of abnormal collagen.109 The main cardiac manifestations are valvular, especially aortic and mitral regurgitation.110,111
Audiology examination should start in childhood, since hearing loss begins in the first or second decades in about 5% of patients.112 Hearing loss is mostly mixed sensorineural and conductive, with a few cases having pure conductive or sensory loss.113 Patients might need hearing aids or cochlear implants. Stapedectomy provides restoration of conductive loss in long-term follow-up.114
Skull base abnormalities, including platybasia, basilar invagination, and basilar impression, are prevalent in patients with height Z score less than −3.115 Basilar impression, in which the foramen magnum rim folds into the skull, is the most serious neurological complication of the disorder.116 It can lead to compression of the brainstem, hydrocephalus, and syrinx. Bisphosphonate treatment has not affected the incidence of basilar impression.117
Present and prospective drug therapies
Bisphosphonates, antiresorptive drugs, which are synthetic analogues of pyrophosphate, are widely used to treat children with osteogenesis imperfecta. Bisphosphonates are deposited on the surface of bone, where their endocytosis by precursor or mature ostoclasts induces cell death (apoptosis). Thus, treatment aims to increase bone volume by counteracting the high turnover cellular status of bone in classic osteogenesis imperfecta. The new bone would still contain defective collagen in the classic types of the disorder, but the hypothesis behind the treatment is that an increased volume of bone (even of impaired quality) would be beneficial to bone strength. Bisphosphonates have a decade long half-life in bone, and drug effects on bone density persist for years after treatment cessation.
Most children with osteogenesis imperfecta have positive vertebral effects, including increased areal bone density DXA Z score of about 1·5 SD in the first treatment year and improved vertebral compressions.118 Increased vertebral height expands thoracic volume but has negligible effects on scoliosis,119 which lends support to a pathology model in which scoliosis in osteogenesis imperfecta is mainly driven by laxity of spinal ligaments. Two studies reported that maximum bone density and histology benefits are obtained after 2–3 years of treatment.120,121 Whether treatment should be paused at 3 years with careful follow-up of bone density and individualised restarting of treatment at later intervals, or whether, after an initial 3 year regimen, children should be treated at a lower bisphosphonate dose until epiphyseal closure because anecdotal long bone fractures have been reported at the juncture of treated and untreated bone, remains an open question.122 The US National Institute of Health clinical experience has not included junctional fractures in children after bisphosphonate is discontinued.
The major intent of administration of bisphosphonates in osteogenesis imperfecta is to decrease fractures. Controlled trials are equivocal about reduction of long bone fractures,118,123 requiring use of unspecified adjustment factors to obtain changes in relative risk. Furthermore, meta-analyses, including two Cochran reviews in 2008 (403 patients)124 and 2014 (819 patients)118 and a separate analysis of only placebo-controlled trials (424 patients total),125 did not support a statistically significant effect of bisphosphonates on fractures in osteogenesis imperfecta. Supporters of prolonged treatment have raised the question of whether the studies had adequate power to detect a fracture decrease.118,124,125 In view of the number of patients in the meta-analyses, the effects on fracture of bisphosphonate administration would not be the robust improvement that patients and their caregivers are often told. A likely explanation for the equivocal reduction of fractures, despite a clear increase in bone mineral density, is that bone quality is reduced by bisphosphonate administration, as seen in animal studies, causing increased bone brittleness.126 Long-term inhibition of osteoclasts leads to non-dynamic bone, in which microcracks are often not repaired. Microcracks and foci of retained mineralised cartilage from cyclic bisphosphonate infusions can facilitate crack propagation.127 Studies of use of risedronate in children128 or adults 129 showed a moderate improvement in fractures in children given the drug for more than 3 treatment years, but no difference in adult fracture rate. Similarly, treatment of adults with teriparatide showed benefit for only mildly affected patients, leaving adult treatment options sparse.130 Controlled trials118,123 also have not supported improved mobility or pain status with bisphosphonates; despite anecdotal reports of bone pain relief, only increased activity endurance was reported.
Drugs with anabolic action on bone formation are undergoing testing in animal models of osteogenesis imperfecta and paediatric trials are anticipated. This mechanism is supported by previous paediatric trials of recombinant human growth hormone,10,131 which showed improved bone histology and bone mineral density in linear growth responders. The novel drugs are both antibodies, one to sclerostin,132 a negative regulator of bone formation in the Wnt pathway, and one to transforming growth factor-β,133 a coordinator of bone remodelling produced by osteoblasts. Treatment of Brtl mice with Scl-AB increases bone mass and load to fracture without inhibiting bone turnover. Importantly, tissue brittleness, a hallmark feature of the disorder, is decreased.132 Transforming growth factor-β neutralising antibody increases bone mass while reducing bone turnover in Crtap knock-mice and Col1a2+/G610C dominant mice; bone load to fracture was improved but brittleness was not changed.133
Orthopaedic surgery
Placement of an intramedullary telescoping rod in a long bone can stabilise a severe fracture, provide internal support for healing after correction of bone deformity, or interrupt fracture or disuse cycles.134 The Fassier-Duval rod aims to be minimally invasive, with a single entry point, and does not require arthrotomies.135 Correction of deformity with Fassier-Duval rods is associated with improved ambulation.135 Telescoping rods have a substantial incidence of migration, perhaps due to poor bone material quality.136 Non-expanding Kirchner wires are suited to severe osteogenesis imperfecta types with slow growth.137
Ageing and walking in patients with osteogenesis imperfecta contributes to joint osteoarthritis. Hip and knee arthroplasty are increasingly frequent in adults; the best results involve custom implants based on computer-assisted design.138
Scoliosis in osteogenesis imperfecta is not amenable to bracing. Spinal fusion is generally done to stabilise and partly correct curvature greater than 50°. Standard approaches involve posterior fusion with Harrington instrumentation139 or preoperative correction with halo traction followed by instrumented fusions.140 However, laxity of spinal ligaments contributes to gradual loss of curve correction.
Conclusions
An exciting series of discoveries has rapidly advanced understanding of osteogenesis imperfecta and has identified genes whose importance for bone development was not previously appreciated. Common themes have emerged for dominant and recessive forms, including collagen-related mechanisms, abnormal mineralisation, osteoblast signalling, endoplasmic reticulum-stress, and cell-cell and cell-matrix signalling. Rare osteogenesis imperfecta types are prompting investigators to re-examine old themes, such as collagen modification, folding, and cross-linking. At present, availability of exome sequencing and advances in bone metabolism hold the prospect to identify all the remaining causes of the disorder and herald a new era in osteogenesis imperfecta diagnosis and therapeutics.
Acknowledgments
The authors thank the members of the Marini Laboratory for critical review of the manuscript, especially Wayne A Cabral and Aileen M Barnes for feedback on figures. Figures were prepared by Jeremy Swan and Erin Fincher of The Unit on Computer Support Services, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). When writing this Seminar, JCM was supported by intramural funding from the NICHD and AF was supported by the Fondazione Cariplo grant n. 2013–0612 and Telethon grant n. GGP13098. The funders did not have any role in study design, the collection, analysis, and interpretation of data, the writing of the Seminar, or the decision to submit the Seminar for publication.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Antonella Forlino, Department of Molecular Medicine, Biochemistry Unit, University of Pavia, Pavia, Italy.
Joan C Marini, Bone and Extracellular Matrix Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Morello R, Bertin TK, Chen Y, et al. CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 2006; 127: 291–304. [DOI] [PubMed] [Google Scholar]
- 2.Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 2006; 355: 2757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 2011; 7: 540–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat 2007; 28: 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marini JC, Blissett AR. New genes in bone development: whaf s new in osteogenesis imperfecta. J Clin Endocrinol Metab 2013; 98: 3095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orgel JP, San Antonio JD, Antipova O. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res 2011; 52: 2–17. [DOI] [PubMed] [Google Scholar]
- 7.Sillence DO, Rimoin DL, Danks DM. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser 1979; 15: 113–29. [PubMed] [Google Scholar]
- 8.Marini JC. Osteogenesis imperfecta In: Kliegman RM, Stanton B, St Geme J, Schor N, Behrman RE, eds. Nelson textbook of pediatrics. 19th edn. Philadelphia: Elsevier Health Sciences, 2011: 2437–40. [Google Scholar]
- 9.Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone 2000; 26: 581–89. [DOI] [PubMed] [Google Scholar]
- 10.Marini JC, Hopkins E, Glorieux FH, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res 2003; 18: 237–43. [DOI] [PubMed] [Google Scholar]
- 11.Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 2002; 17: 30–38. [DOI] [PubMed] [Google Scholar]
- 12.Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res 2000; 15: 1650–58. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa Y, Bachinger HP. A molecular ensemble in the rER for procollagen maturation. Biochim Biophys Acta 2013; 1833: 2479–91. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa Y, Wirz J, Vranka JA, Nagata K, Bächinger HP. Biochemical characterization of the prolyl 3-hydroxylase 1.cartilage-associated protein.cyclophilin B complex. J Biol Chem 2009; 284: 17641–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weis MA, Hudson DM, Kim L, Scott M, Wu JJ, Eyre DR. Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J Biol Chem 2010; 285: 2580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bächinger HP. The influence of peptidyl-prolyl cis-trans isomerase on the in vitro folding of type III collagen. J Biol Chem 1987; 262: 17144–48. [PubMed] [Google Scholar]
- 17.Pace JM, Wiese M, Drenguis AS, et al. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. J Biol Chem 2008; 283: 16061–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gioia R, Panaroni C, Besio R, et al. Impaired osteoblastogenesis in a murine model of dominant osteogenesis imperfecta: a new target for osteogenesis imperfecta pharmacological therapy. Stem Cells 2012; 30: 1465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willing MC, Deschenes SP, Slayton RL, Roberts EJ. Premature chain termination is a unifying mechanism for COL1A1 null alleles in osteogenesis imperfecta type I cell strains. Am J Hum Genet 1996; 59: 799–809. [PMC free article] [PubMed] [Google Scholar]
- 20.Deak SB, Nicholls A, Pope FM, Prockop DJ. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem 1983; 258: 15192–97 [PubMed] [Google Scholar]
- 21.Malfait F, Symoens S, Coucke P, Nunes L, De Almeida S, De Paepe A. Total absence of the alpha2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J Med Genet 2006; 43: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wet WJ, Pihlajaniemi T, Myers J, Kelly TE, Prockop DJ. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem 1983; 258 : 7721–28. [PubMed] [Google Scholar]
- 23.Malfait F, Symoens S, Goemans N, et al. Helical mutations in type I collagen that affect the processing of the amino-propeptide result in an osteogenesis imperfecta/Ehlers-Danlos syndrome overlap syndrome. Orphanet J Rare Dis 2013; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byers PH, Duvic M, Atkinson M, et al. Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen. Am J Med Genet 1997; 72: 94–105. [DOI] [PubMed] [Google Scholar]
- 25.Cabral WA, Makareeva E, Colige A, et al. Mutations near amino end of alphal(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem 2005; 280: 19259–69. [DOI] [PubMed] [Google Scholar]
- 26.Colige A, Sieron AL, Li SW, et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet 1999; 65: 308–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SW, Arita M, Fertala A, et al. Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem J 2001; 355: 271–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Symoens S, Hulmes DJ, Bourhis JM, Coucke PJ, De Paepe A, Malfait F. Type I procollagen C-propeptide defects: study of genotype-phenotype correlation and predictive role of crystal structure. Hum Mutat 2014; 35: 1330–41. [DOI] [PubMed] [Google Scholar]
- 29.Lindahl K, Barnes AM, Fratzl-Zelman N, et al. COL1 C-propeptide cleavage site mutations cause high bone mass osteogenesis imperfecta. Hum Mutat 2011; 32: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asharani PV, Keupp K, Semler O, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet 2012; 90: 661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Glez V, Valencia M, Caparrós-Martín JA, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat 2012; 33: 343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollitt R, McMahon R, Nunn J, et al. Mutation analysis of COL1A1 and COL1A2 in patients diagnosed with osteogenesis imperfecta type I-IV. Hum Mutat 2006; 27: 716. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri D, Astigiano S, Barbieri O, et al. Procollagen I COOH-terminal fragment induces VEGF-A and CXCR4 expression in breast carcinoma cells. Exp Cell Res 2008; 314: 2289–98. [DOI] [PubMed] [Google Scholar]
- 34.Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem 2002; 277: 49820–30. [DOI] [PubMed] [Google Scholar]
- 35.Moali C, Font B, Ruggiero F, et al. Substrate-specific modulation of a multisubstrate proteinase. C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. J Biol Chem 2005; 280: 24188–94. [DOI] [PubMed] [Google Scholar]
- 36.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 1996; 271: 360–62. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol 2007; 26: 508–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol 2003; 23: 4428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vranka JA, Sakai LY, Bächinger HP. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J Biol Chem 2004; 279: 23615–21. [DOI] [PubMed] [Google Scholar]
- 40.Morello R, Tonachini L, Monticone M, et al. cDNA cloning, characterization and chromosome mapping of Crtap encoding the mouse cartilage associated protein. Matrix Biol 1999; 18: 319–24. [DOI] [PubMed] [Google Scholar]
- 41.Steinmann B, Bruckner P, Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem 1991; 266: 1299–303. [PubMed] [Google Scholar]
- 42.Eyre DR, Weis M, Hudson DM, Wu JJ, Kim L. A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen. J Biol Chem 2011; 286: 7732–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 2007; 39: 359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle 2007; 6: 1675–81. [DOI] [PubMed] [Google Scholar]
- 45.Cabral WA, Barnes AM, Adeyemo A, et al. A founder mutation in LEPRE1 carried by 1–5% of West Africans and 0–4% of African Americans causes lethal recessive osteogenesis imperfecta. Genet Med 2012; 14: 543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res 2010; 339: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang W, Barnes AM, Cabral WA, Bodurtha JN, Marini JC. Prolyl 3-hydroxylase 1 and CRTAP are mutually stabilizing in the endoplasmic reticulum collagen prolyl 3-hydroxylation complex. Hum Mol Genet 2010; 19: 223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes AM, Carter EM, Cabral WA, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med 2010; 362: 521–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishikawa Y, Vranka JA, Boudko SP, et al. Mutation in cyclophilin B that causes hyperelastosis cutis in American Quarter Horse does not affect peptidylprolyl cis-trans isomerase activity but shows altered cyclophilin B-protein interactions and affects collagen folding. J Biol Chem 2012; 287: 22253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pyott SM, Schwarze U, Christiansen HE, et al. Mutations in PPIB (cyclophilin B) delay type I procollagen chain association and result in perinatal lethal to moderate osteogenesis imperfecta phenotypes. Hum Mol Genet 2011; 20: 1595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cabral WA, Perdivara I, Weis M, et al. Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta. PLoS Genet 2014; 10: e1004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaheen R, Alazami AM, Alshammari MJ, et al. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J Med Genet 2012; 49: 630–35. [DOI] [PubMed] [Google Scholar]
- 53.Volodarsky M, Markus B, Cohen I, et al. A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum Mutat 2013; 34: 582–86. [DOI] [PubMed] [Google Scholar]
- 54.Rubinato E, Morgan A, D’Eustacchio A, et al. A novel deletion mutation involving TMEM38B in a patient with autosomal recessive osteogenesis imperfecta. Gene 2014; 545: 290–92. [DOI] [PubMed] [Google Scholar]
- 55.Cabral WA, Makareeva E, Ishikawa M, et al. Absence of ER cation channel TMEM38B/TRIC-B causes recessive osteogenesis imperfecta by dysregulation of collagen post-translational modification. Bone Abstr 2014; 3: CC3. [Google Scholar]
- 56.Davis EC, Broekelmann TJ, Ozawa Y, Mecham RP. Identification of tropoelastin as a ligand for the 65-kD FK506-binding protein, FKBP65, in the secretory pathway. J Cell Biol 1998; 140: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2010; 86: 551–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes AM, Cabral WA, Weis M, et al. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum Mutat 2012; 33: 1589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarze U, Cundy T, Pyott SM, et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum Mol Genet 2013; 22: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes AM, Duncan G, Weis M, et al. Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum Mutat 2013; 34: 1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ha-Vinh R, Alanay Y, Bank RA, et al. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am J Med Genet A 2004; 131: 115–20. [DOI] [PubMed] [Google Scholar]
- 62.Eyre DR, Weis MA. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif Tissue Int 2013; 93: 338–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koide T, Nishikawa Y, Asada S, et al. Specific recognition of the collagen triple helix by chaperone HSP47 II. The HSP47-binding structural motif in collagens and related proteins. J Biol Chem 2006; 281: 11177–85. [DOI] [PubMed] [Google Scholar]
- 64.Nagai N, Hosokawa M, Itohara S, et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol 2000; 150: 1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishida Y, Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy 2009; 5: 1217–19. [DOI] [PubMed] [Google Scholar]
- 66.Drögemüller C, Becker D, Brunner A, et al. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet 2009; 5: e1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christiansen HE, Schwarze U, Pyott SM, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet 2010; 86: 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semler O, Garbes L, Keupp K, et al. A mutation in the 5’-UTR of IFITM5 creates an in-frame start codon and causes autosomaldominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet 2012; 91: 349–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5’-UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet 2012; 91: 343–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Homan EP, Rauch F, Grafe I, et al. Mutations in SERPINF1 cause osteogenesis imperfecta type VI. J Bone Miner Res 2011; 26: 2798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2011; 88: 362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balasubramanian M, Parker MJ, Dalton A, et al. Genotype-phenotype study in type V osteogenesis imperfecta. Clin Dysmorphol 2013; 22: 93–101. [DOI] [PubMed] [Google Scholar]
- 73.Rauch F, Moffatt P, Cheung M, et al. Osteogenesis imperfecta type V: marked phenotypic variability despite the presence of the IFITM5 c.−14C>T mutation in all patients. J Med Genet 2013; 50: 21–24. [DOI] [PubMed] [Google Scholar]
- 74.Kasaai B, Gaumond MH, Moffatt P. Regulation of the bone-restricted IFITM-like (Bril) gene transcription by Sp and Gli family members and CpG methylation. J Biol Chem 2013; 288: 13278–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanagata N, Li X, Morita H, Takemura T, Li J, Minowa T. Characterization of the osteoblast-specific transmembrane protein IFITM5 and analysis of IFITM5-deficient mice. J Bone Miner Metab 2011; 29: 279–90. [DOI] [PubMed] [Google Scholar]
- 76.Moffatt P, Gaumond MH, Salois P, et al. Bril: a novel bone-specific modulator of mineralization. J Bone Miner Res 2008; 23: 1497–508. [DOI] [PubMed] [Google Scholar]
- 77.Hanagata N, Li X. Osteoblast-enriched membrane protein IFITM5 regulates the association of CD9 with an FKBP11-CD81-FPRP complex and stimulates expression of interferon-induced genes. Biochem Biophys Res Commun 2011; 409: 378–84. [DOI] [PubMed] [Google Scholar]
- 78.Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J Interferon Cytokine Res 2011; 31: 183–97. [DOI] [PubMed] [Google Scholar]
- 79.Cho SY, Ki CS, Sohn YB, Kim SJ, Maeng SH, Jin DK. Osteogenesis imperfecta Type VI with severe bony deformities caused by novel compound heterozygous mutations in SERPINF1. J Korean Med Sci 2013; 28: 1107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Land C, Rauch F, Travers R, Glorieux FH. Osteogenesis imperfecta type VI in childhood and adolescence: effects of cyclical intravenous pamidronate treatment. Bone 2007; 40: 638–44. [DOI] [PubMed] [Google Scholar]
- 81.Becerra SP, Notario V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat Rev Cancer 2013; 13: 258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quan GM, Ojaimi J, Li Y, Kartsogiannis V, Zhou H, Choong PF. Localization of pigment epithelium-derived factor in growing mouse bone. Calcif Tissue Int 2005; 76: 146–53. [DOI] [PubMed] [Google Scholar]
- 83.Bogan R, Riddle RC, Li Z, et al. A mouse model for human osteogenesis imperfecta type VI. J Bone Miner Res 2013; 28: 1531–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li F, Song N, Tombran-Tink J, Niyibizi C. Pigment epithelium derived factor enhances differentiation and mineral deposition of human mesenchymal stem cells. Stem Cells 2013; 31: 2714–23. [DOI] [PubMed] [Google Scholar]
- 85.Akiyama T, Dass CR, Shinoda Y, Kawano H, Tanaka S, Choong PF. PEDF regulates osteoclasts via osteoprotegerin and RANKL. Biochem Biophys Res Commun 2010; 391: 789–94. [DOI] [PubMed] [Google Scholar]
- 86.Sekiya A, Okano-Kosugi H, Yamazaki CM, Koide T. Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J Biol Chem 2011; 286: 26364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farber CR, Reich A, Barnes AM, et al. A novel IFITM5 mutation in severe atypical osteogenesis imperfecta type VI impairs osteoblast production of pigment epithelium-derived factor. J Bone Miner Res 2014; 29: 1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsukamoto T, Li X, Morita H, et al. Role of S-palmitoylation on IFITM5 for the interaction with FKBP11 in osteoblast cells. PLoS One 2013; 8: e75831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013; 19: 179–92. [DOI] [PubMed] [Google Scholar]
- 90.Faqeih E, Shaheen R, Alkuraya FS. WNT1 mutation with recessive osteogenesis imperfecta and profound neurological phenotype. J Med Genet 2013; 50: 491–92. [DOI] [PubMed] [Google Scholar]
- 91.Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N Engl J Med 2013; 368: 1809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pyott SM, Tran TT, Leistritz DF, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am J Hum Genet 2013; 92: 590–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keupp K, Beleggia F, Kayserili H, et al. Mutations in WNT1 cause different forms of bone fragility. Am J Hum Genet 2013; 92: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 1990; 346: 847–50. [DOI] [PubMed] [Google Scholar]
- 95.Murakami T, Saito A, Hino S, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 2009; 11: 1205–11. [DOI] [PubMed] [Google Scholar]
- 96.Symoens S, Malfait F, D’hondt S, et al. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J Rare Dis 2013; 8: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lapunzina P, Aglan M, Temtamy S, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet 2010; 87: 110–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rohrbach M, Giunta C. Recessive osteogenesis imperfecta: clinical, radiological, and molecular findings. Am J Med Genet C Semin Med Genet 2012; 160C: 175–89. [DOI] [PubMed] [Google Scholar]
- 99.van Dijk FS, Cobben JM, Kariminejad A, et al. Osteogenesis Imperfecta: A Review with Clinical Examples. Mol Syndromol 2011; 2: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet 2012; 82: 1–11. [DOI] [PubMed] [Google Scholar]
- 101.Aglan MS, Hosny L, El-Houssini R, et al. A scoring system for the assessment of clinical severity in osteogenesis imperfecta. J Child Orthop 2012; 6: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Engelbert RH, Uiterwaal CS, Gerver WJ, van der Net JJ, Pruijs HE, Helders PJ. Osteogenesis imperfecta in childhood: impairment and disability. A prospective study with 4-year follow-up. Arch Phys Med Rehabil 2004; 85: 772–78. [DOI] [PubMed] [Google Scholar]
- 103.Brizola E, Staub AL, Félix TM. Muscle strength, joint range of motion, and gait in children and adolescents with osteogenesis imperfecta. Pediatr Phys Ther 2014; 26: 245–52. [DOI] [PubMed] [Google Scholar]
- 104.Montpetit K, Dahan-Oliel N, Ruck-Gibis J, Fassier F, Rauch F, Glorieux F. Activities and participation in young adults with osteogenesis imperfecta. J Pediatr Rehabil Med 2011; 4: 13–22. [DOI] [PubMed] [Google Scholar]
- 105.Sousa T, Bompadre V, White KK. Musculoskeletal functional outcomes in children with osteogenesis imperfecta: associations with disease severity and pamidronate therapy. J Pediatr Orthop 2014; 34: 118–22. [DOI] [PubMed] [Google Scholar]
- 106.Singer RB, Ogston SA, Paterson CR. Mortality in various types of osteogenesis imperfecta. J Insur Med 2001; 33: 216–20. [PubMed] [Google Scholar]
- 107.Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, Schneider R. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine 1999; 24: 1673–78. [DOI] [PubMed] [Google Scholar]
- 108.LoMauro A, Pochintesta S, Romei M, et al. Rib cage deformities alter respiratory muscle action and chest wall function in patients with severe osteogenesis imperfecta. PLoS One 2012; 7: e35965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thiele F, Cohrs CM, Flor A, et al. Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum Mol Genet 2012; 21: 3535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Radunovic Z, Wekre LL, Diep LM, Steine K. Cardiovascular abnormalities in adults with osteogenesis imperfecta. Am Heart J 2011; 161: 523–29. [DOI] [PubMed] [Google Scholar]
- 111.Lamanna A, Fayers T, Clarke S, Parsonage W. Valvular and aortic diseases in osteogenesis imperfecta. Heart Lung Circ 2013; 22: 801–10. [DOI] [PubMed] [Google Scholar]
- 112.Kuurila K, Grénman R, Johansson R, Kaitila I. Hearing loss in children with osteogenesis imperfecta. Eur J Pediatr 2000; 159: 515–19. [DOI] [PubMed] [Google Scholar]
- 113.Swinnen FK, Coucke PJ, De Paepe AM, et al. Osteogenesis Imperfecta: the audiological phenotype lacks correlation with the genotype. Orphanet J Rare Dis 2011; 6: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swinnen FK, De Leenheer EM, Coucke PJ, Cremers CW, Dhooge IJ. Stapes surgery in osteogenesis imperfecta: retrospective analysis of 34 operated ears. Audiol Neurootol 2012; 17: 198–206. [DOI] [PubMed] [Google Scholar]
- 115.Charnas LR, Marini JC. Communicating hydrocephalus, basilar invagination, and other neurologic features in osteogenesis imperfecta. Neurology 1993; 43: 2603–08. [DOI] [PubMed] [Google Scholar]
- 116.Menezes AH. Specific entities affecting the craniocervical region: osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management of basilar impression. Childs Nerv Syst 2008; 24: 1169–72. [DOI] [PubMed] [Google Scholar]
- 117.Cheung MS, Arponen H, Roughley P, et al. Cranial base abnormalities in osteogenesis imperfecta: phenotypic and genotypic determinants. J Bone Miner Res 2011; 26: 405–13. [DOI] [PubMed] [Google Scholar]
- 118.Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 2014; 7: CD005088. [DOI] [PubMed] [Google Scholar]
- 119.Anissipour AK, Hammerberg KW, Caudill A, et al. Behavior of scoliosis during growth in children with osteogenesis imperfecta. J Bone Joint Surg Am 2014; 96: 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Letocha AD, Cintas HL, Troendle JF, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res 2005; 20: 977–86. [DOI] [PubMed] [Google Scholar]
- 121.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 2002; 110: 1293–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone 2007; 40: 821–27. [DOI] [PubMed] [Google Scholar]
- 123.Marini JC. Should children with osteogenesis imperfecta be treated with bisphosphonates? Nat Clin Pract Endocrinol Metab 2006; 2: 14–15. [DOI] [PubMed] [Google Scholar]
- 124.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev 2008; 8: CD005088. [DOI] [PubMed] [Google Scholar]
- 125.Hald JD, Evangelou E, Langdahl BL, Ralston SH. Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res 2015; 30: 929–33. [DOI] [PubMed] [Google Scholar]
- 126.Uveges TE, Kozloff KM, Ty JM, et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res 2009; 24: 849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone 2015; 71: 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bishop N, Adami S, Ahmed SF, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet 2013; 382: 1424–32. [DOI] [PubMed] [Google Scholar]
- 129.Bradbury LA, Barlow S, Geoghegan F, et al. Risedronate in adults with osteogenesis imperfecta type I: increased bone mineral density and decreased bone turnover, but high fracture rate persists. Osteoporos Int 2012; 23: 285–94. [DOI] [PubMed] [Google Scholar]
- 130.Orwoll ES, Shapiro J, Veith S, et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest 2014; 124: 491–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Antoniazzi F, Monti E, Venturi G, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol 2010; 163: 479–87. [DOI] [PubMed] [Google Scholar]
- 132.Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res 2013; 28: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Grafe I, Yang T, Alexander S, et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 2014; 20: 670–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Esposito P, Plotkin H. Surgical treatment of osteogenesis imperfecta: current concepts. Curr Opin Pediatr 2008; 20: 52–57. [DOI] [PubMed] [Google Scholar]
- 135.Ruck J, Dahan-Oliel N, Montpetit K, Rauch F, Fassier F. Fassier-Duval femoral rodding in children with osteogenesis imperfecta receiving bisphosphonates: functional outcomes at one year. J Child Orthop 2011; 5: 217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee K, Park MS, Yoo WJ, Chung CY, Choi IH, Cho TJ. Proximal migration of femoral telescopic rod in children with osteogenesis imperfecta. J Pediatr Orthop 2014; 35: 178–84. [DOI] [PubMed] [Google Scholar]
- 137.Imajima Y, Kitano M, Ueda T. Intramedullary fixation using Kirschner wires in children with osteogenesis imperfecta. J Pediatr Orthop 2015; 35: 431–34. [DOI] [PubMed] [Google Scholar]
- 138.Krishnan H, Patel NK, Skinner JA, et al. Primary and revision total hip arthroplasty in osteogenesis imperfecta. Hip Int 2013; 23: 303–09. [DOI] [PubMed] [Google Scholar]
- 139.Topouchian V, Finidori G, Glorion C, Padovani JP, Pouliquen JC. Posterior spinal fusion for kypho-scoliosis associated with osteogenesis imperfecta: long-term results. Rev Chir Orthop Reparatrice Appar Mot 2004; 90: 525–32. [DOI] [PubMed] [Google Scholar]
- 140.Janus GJ, Finidori G, Engelbert RH, Pouliquen M, Pruijs JE. Operative treatment of severe scoliosis in osteogenesis imperfecta: results of 20 patients after halo traction and posterior spondylodesis with instrumentation. Eur Spine J 2000; 9: 486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]