Abstract
Background
Cerebrolysin is a mixture of low‐molecular‐weight peptides and amino acids derived from porcine brain that has potential neuroprotective properties. It is widely used in the treatment of acute ischaemic stroke in Russia, Eastern Europe, China, and other Asian and post‐Soviet countries. This is an update of a review first published in 2010 and last updated in 2017.
Objectives
To assess the benefits and harms of Cerebrolysin for treating acute ischaemic stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register, CENTRAL, MEDLINE, Embase, Web of Science Core Collection, with Science Citation Index, LILACS, OpenGrey, and a number of Russian databases in October 2019. We also searched reference lists, ongoing trials registers, and conference proceedings.
Selection criteria
Randomised controlled trials (RCTs) comparing Cerebrolysin, started within 48 hours of stroke onset and continued for any length of time, with placebo or no treatment in people with acute ischaemic stroke.
Data collection and analysis
Two review authors independently applied the inclusion criteria, assessed trial quality and risk of bias, extracted data, and applied GRADE criteria to the evidence.
Main results
Seven RCTs (1601 participants) met the inclusion criteria of the review.
In this update we re‐evaluated risk of bias through identification, examination, and evaluation of study protocols and judged it to be low, unclear, or high across studies: unclear for all domains in one study, and unclear for selective outcome reporting across all studies; low for blinding of participants and personnel in four studies and unclear in the remaining three; low for blinding of outcome assessors in three studies and unclear in four studies. We judged risk of bias to be low in two studies and unclear in the remaining five studies for generation of allocation sequence; low in one study and unclear in six studies for allocation concealment; and low in one study, unclear in one study, and high in the remaining five studies for incomplete outcome data. The manufacturer of Cerebrolysin supported four multicentre studies, either totally, or by providing Cerebrolysin and placebo, randomisation codes, research grants, or statisticians. We judged three studies to be at high risk of other bias and the remaining four studies to be at unclear risk of other bias.
All‐cause death: we extracted data from six trials (1517 participants). Cerebrolysin probably results in little to no difference in all‐cause death: risk ratio (RR) 0.90, 95% confidence interval (CI) 0.61 to 1.32 (6 trials, 1517 participants, moderate‐quality evidence).
None of the included trials reported on poor functional outcome defined as death or dependence at the end of the follow‐up period or early death (within two weeks of stroke onset), or time to restoration of capacity for work and quality of life.
Only one trial clearly reported on the cause of death: cerebral infarct (four in the Cerebrolysin and two in the placebo group), heart failure (two in the Cerebrolysin and one in the placebo group), pulmonary embolism (two in the placebo group), and pneumonia (one in the placebo group).
Serious adverse events (SAEs): Cerebrolysin probably results in little to no difference in the total number of people with SAEs (RR 1.15, 95% CI 0.81 to 1.65, 4 RCTs, 1435 participants, moderate‐quality evidence). This comprised fatal SAEs (RR 0.90, 95% CI 0.59 to 1.38) and an increase in the total number of people with non‐fatal SAEs (RR 2.15, 95% CI 1.01 to 4.55, P = 0.047, 4 trials, 1435 participants, moderate‐quality evidence). In the subgroup of dosing schedule 30 mL for 10 days (cumulative dose 300 mL), the increase was more prominent: RR 2.86, 95% CI 1.23 to 6.66, P = 0.01 (2 trials, 1189 participants).
Total number of people with adverse events: four trials reported on this outcome. Cerebrolysin may result in little to no difference in the total number of people with adverse events: RR 0.97, 95% CI 0.85 to 1.10, P = 0.90, 4 trials, 1435 participants, low‐quality evidence.
Non‐death attrition: evidence from six trials involving 1517 participants suggests that Cerebrolysin results in little to no difference in non‐death attrition, with 96 out of 764 Cerebrolysin‐treated participants and 117 out of 753 placebo‐treated participants being lost to follow‐up for reasons other than death (very low‐quality evidence).
Authors' conclusions
Moderate‐quality evidence indicates that Cerebrolysin probably has little or no beneficial effect on preventing all‐cause death in acute ischaemic stroke, or on the total number of people with serious adverse events. Moderate‐quality evidence also indicates a potential increase in non‐fatal serious adverse events with Cerebrolysin use.
Plain language summary
Cerebrolysin for acute ischaemic stroke
What did we want to know?
In this Cochrane Review, we wanted to find out how well a medicine called Cerebrolysin works to treat a stroke.
What is a stroke?
A stroke is a sudden attack of weakness that usually affects one side of the body. It happens when the flow of blood to part of the brain is cut off, stopping the supply of oxygen and nutrients to the brain cells. If the supply of blood to the brain is stopped, brain cells begin to die. This can lead to brain injury, disability, and possibly death.
Ischaemic strokes are the most common type of stroke. An ischaemic stroke happens when the flow of blood is blocked by a blood clot or a piece of fatty material in an artery.
Why is this review important?
Strokes are a medical emergency, and urgent treatment is essential. Ischaemic strokes are usually treated with a combination of medicines to prevent and dissolve blood clots, reduce blood pressure, and lower cholesterol levels.
Cerebrolysin is a mixture of proteins purified from the brains of pigs. Some of the proteins in Cerebrolysin are found naturally in the human brain and may help to protect and repair brain cells. Cerebrolysin is commonly used in some countries as a treatment for stroke.
What did we do?
We searched for studies looking at the use of Cerebrolysin to treat acute ischaemic stroke. We searched for randomised controlled studies, in which the treatment people receive is randomly decided, because these studies give the most reliable evidence about treatments.
Search date: We included evidence published up to October 2019.
What we found
We found seven studies in 1601 people who had had an acute ischaemic stroke. The studies looked at the effect of giving Cerebrolysin alongside medicines to prevent and dissolve blood clots (standard therapy) during the first 48 hours after a stroke. The studies compared this treatment with standard therapy alone or standard therapy plus a dummy treatment (placebo).
The studies were conducted in hospitals in Austria, Croatia, the Czech Republic, Hungary, Russia, Slovakia, Slovenia, China, Hong Kong, Iran, Myanmar, and South Korea, and lasted from 28 days to 90 days.
Results of our review
Adding Cerebrolysin to standard therapy probably makes little or no difference to the risk of dying from any cause after a stroke (6 studies; 1517 people).
Cerebrolysin added to standard therapy probably made little or no difference to: • the total number of people who had serious unwanted effects (life‐threatening effects that could result in death, disability, or a longer hospital stay) (4 studies; 1435 people); • the number of serious unwanted effects that caused death (3 studies; 1335 people).
However, more people given Cerebrolysin plus standard therapy had serious unwanted effects that did not kill them than those who were given standard therapy (alone or with placebo) (4 studies; 1435 people).
Cerebrolysin may make little or no difference to the total number of people who had any less serious unwanted effects (4 studies; 1435 people).
We are uncertain whether adding Cerebrolysin to standard therapy made any difference to the numbers of people who dropped out of studies (6 studies; 1517 people).
We did not find enough evidence about how Cerebrolysin affected: • risk of dying or needing continuing care at the end of the study; • risk of dying within two weeks of having a stroke; • the time taken for people to be able to go back to work; or • people's well‐being (quality of life).
Our confidence in the results
We are moderately confident (certain) in the results of this review. However, the evidence comes from a small number of studies. Four studies involved a pharmaceutical company that makes Cerebrolysin, which may have affected how those studies were designed, carried out, and reported. Our conclusions are likely to change if results from further studies become available.
Conclusions
Adding Cerebrolysin to standard therapy after an ischaemic stroke probably: • does not reduce the risk of dying; • does not affect how many people have serious unwanted effects overall; but • increases the number of serious, non‐fatal unwanted effects.
Summary of findings
Summary of findings 1. Cerebrolysin compared to placebo for acute ischaemic stroke.
| Cerebrolysin compared to placebo for acute ischaemic stroke | ||||||
| Patient or population: people with acute ischaemic stroke Settings: inpatient health facilities in 7 European countries: Austria, Croatia, the Czech Republic, Hungary, Russia, Slovakia, Slovenia; and 5 Asian countries: China, Hong Kong, Iran, Myanmar, South Korea Intervention: Cerebrolysin added to standard therapy (in most studies aspirin; in 1 study thrombolysis) Comparison: placebo added to standard therapy (in most studies aspirin; in 1 study thrombolysis) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | ||
| Assumed risk | Corresponding risk | |||||
| Placebo | Cerebrolysin | |||||
| All‐cause death at the end of the follow‐up period | 65 per 1000 | 59 per 1000 (40 to 86) | RR 0.89 (0.60 to 1.31) | 1517 (6 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d |
|
| Total number of people with SAEs | at the end of the follow‐up period | 72 per 1000 | 83 per 1000 (59 to 119) |
RR 1.15 (0.81 to 1.65) |
1435 (4 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d |
| fatal, at the end of the follow‐up period | 63 per 1000 | 57 per 1000 (37 to 87) | RR 0.90 (0.59 to 1.38) | 1335 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d | |
| non‐fatal, at the end of the follow‐up period | 14 per 1000 |
30 per 1000 (14 to 63) |
RR 2.15 (1.01 to 4.55) |
1435 (4 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c,d | |
| non‐fatal: a subgroup by Cerebrolysindose and length of treatment, at the end of the follow‐up period | 12 per 1000 |
33 per 1000 (14 to 78) |
RR 2.86 (1.23 to 6.66) |
1189 (2 RCTs) |
⊕⊕⊕⊝ Moderateg,h,i,j | |
| Total number of people with adverse events at the end of the follow‐up period | 447 per 1000 | 452 per 1000 (402 to 501) | RR 1.01 (0.9 to 1.12) | 1435 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c,d,e | |
| Non‐death attrition | 155 per 1000 |
151 per 1000 (70 to 320) |
RR 0.97 (0.45 to 2.06) |
1517 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,d,f | |
| Death or dependence at the end of the follow‐up period | Not reported | Not reported | ‐ | 1601 (7 RCTs) |
‐ | |
| Early death (within 2 weeks of stroke onset) | Not reported | Not reported | ‐ | 1601 (7 RCTs) |
‐ | |
| Quality of life | Not reported | Not reported | ‐ | 1601 (7 RCTs) |
‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial: RR: risk ratio; SAE: serious adverse event | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aWe downgraded by one level for risk of bias because most information came from studies at low or unclear risk of bias. bNo serious inconsistency. Six trials contributed to the outcome all‐cause death; we did not detect any heterogeneity. Three eligible multicentre studies contributed to the outcomes total number of people with SAEs, total number of people with fatal SAEs, and total number of people with non‐fatal SAEs, and the newly included Gharagozli 2017 study contributed to the outcomes total number of people with SAEs and total number of people with non‐fatal SAEs. We detected no statistical heterogeneity for any of these outcomes. cNo serious imprecision: the six trials that contributed to the primary outcome all‐cause death, synthesised with a total of 1517 participants, had enough power to detect difference. There was no significant difference: 47 deaths in Cerebrolysin group (out of 764 randomised participants) and 49 deaths in placebo group (out of 753 randomised participants). Although the confidence intervals were wide, there was no heterogeneity; the four studies that contributed to the outcomes total number of people with SAEs, total number of people with non‐fatal SAEs, and total number of people with adverse events, of which three were multicentre, synthesised totalling 1435 participants, would have had enough power to detect differences. dNo serious indirectness. The studies, three of which were multicentre, were conducted in seven European countries: Austria, Croatia, the Czech Republic, Hungary, Russia, Slovakia, Slovenia; and five Asian countries: China, Hong Kong, Iran, Myanmar, South Korea. The results can be generalised to other populations and situations between 2003 and 2014. eWe downgraded by one level for inconsistency. Four trials contributed to the outcome total number of people with adverse events; we detected heterogeneity with I2 = 37% for the overall effect estimate owing to the opposite direction of effect estimate in the Ladurner 2005 study, which used the high cumulative dose of Cerebrolysin, and heterogeneity with I2 = 65% in the subgroup of two multicentre studies with the same dosing schedule (CASTA 2012; CERE‐LYSE‐1 2012). fWe downgraded once for inconsistency and once for imprecision. Six trials contributed to the outcome non‐death attrition; we detected heterogeneity with I2 = 76% for the overall effect estimate and I2 = 81% for subgroup differences owing to the opposite direction of effect estimate in the Ladurner 2005 study (the high cumulative dose of Cerebrolysin) and the Gharagozli 2017 study (the low cumulative dose of Cerebrolysin), and heterogeneity with I2 = 47% in the subgroup of two multicentre studies with the same dosing schedule (CASTA 2012; CERE‐LYSE‐1 2012). The confidence intervals were wide.
gDowngraded by one level for risk of bias. These two newer multicentre studies, which contributed to the outcome total number of people with non‐fatal SAEs, were considered across domains of unclear and high risk of bias due to high levels of exclusions from the final analyses, retrospective registration, and multiple other methodological flaws as described in Assessment of risk of bias in included studies. The manufacturer of Cerebrolysin supported CASTA 2012 and CERE‐LYSE‐1 2012 by providing services including: provision of Cerebrolysin and placebo, randomisation codes, statisticians, funding of study authors. hNo serious inconsistency. The two multicentre studies contributed to the outcome total number of people with non‐fatal SAEs. We detected no statistical heterogeneity. iNo serious imprecision. The two multicentre studies, when synthesised totalling 1189 participants, had enough power to detect the difference: 20 SAEs in the Cerebrolysin group (589 randomised participants) and seven SAEs in the placebo group (600 randomised participants); there was no heterogeneity. jNo serious indirectness. These two studies were conducted in five European countries: Austria, Croatia, the Czech Republic, Slovakia, Slovenia; and in four Asian countries: China, Hong Kong, South Korea, Myanmar. The results can be generalised to other populations and situations.
Background
Effective, simple, and reliable treatment methods are urgently needed to reduce stroke mortality and disability. Many clinical trials and Cochrane Reviews have addressed the question of benefits and risks of potential pharmacological treatment options for acute ischaemic stroke. However, strategies with proven therapeutic effects and an acceptable benefit‐to‐risk ratio are still lacking. Potential strategies can be grouped according to the existing evidence of their benefits and harms determining their role in clinical practice.
Evidence of benefit
Aspirin at a dose of 160 mg to 300 mg daily (orally or per rectum), started within 48 hours of onset of presumed ischaemic stroke, appears to be the only effective treatment for early secondary prevention, reducing the risk of early recurrent ischaemic stroke without a major risk of early haemorrhagic complications, and improving long‐term outcomes (Sandercock 2014). Despite the positive overall conclusions of a Cochrane Review, Wardlaw 2014, and individual patient data meta‐analysis, Emberson 2014, of thrombolysis in acute ischaemic stroke, there is still some debate regarding the optimal use of intravenous recombinant tissue plasminogen activators (rtPA) (Alper 2015). It is estimated that for every person with a good stroke outcome at six months, another person would have symptomatic intracranial bleeding, and for every three to four people without neurological deficits at six months, there is an excess of one death after thrombolysis (Appelros 2015; Brunström 2015). The evidence is inadequate to conclude whether lower doses of thrombolytic agents are more effective than higher doses, whether one agent is better than another, or which route of administration is the best for treatment of people who have had an acute ischaemic stroke (Wardlaw 2013), or whether percutaneous vascular interventions offer any advantages over intravenous thrombolysis in terms of patient‐oriented outcomes (Lindekleiv 2018).
Evidence of harm
Glycoprotein IIb‐IIIa inhibitors (abciximab and tirofiban) increase the risk of intracranial haemorrhage without evidence of any reduction in death or disability in stroke survivors (Ciccone 2014). These data do not support their routine use in clinical practice. Abciximab contributed 89% of the total number of participants of the Cochrane Review (Ciccone 2014). Anticoagulants (standard unfractionated heparin, low‐molecular‐weight heparins, heparinoids, oral anticoagulants, and thrombin inhibitors) as immediate therapy for acute ischaemic stroke are not associated with net short‐ or long‐term benefit. Reduced rate of recurrent stroke, deep vein thrombosis, and pulmonary embolism with anticoagulant therapy is offset by the increased risk of intracranial haemorrhage and extracranial bleeding. The data do not support the routine use of any of the currently available anticoagulants in acute ischaemic stroke (Berge 2002; Sandercock 2015; Sandercock 2017). Long‐term anticoagulant therapy in people with presumed non‐cardioembolic ischaemic stroke or transient ischaemic attack is not associated with any benefit, but there is a significant risk of bleeding (Sandercock 2009).
Tirilazad, an amino steroid inhibitor of lipid peroxidation, increases the combined endpoint of 'death or disability' in people with acute ischaemic stroke (TISC 2001). Lubeluzole, an ion channel modulator of glutamate release that has a benzothiazole structure with potential neuroprotective properties, does not reduce death or dependency in acute ischaemic stroke patients; in contrast, it increases heart‐conduction disorders (Q‐T prolongation) (Gandolfo 2002).
Lack of evidence of benefit
Several treatment options that have been tested in clinical trials have not shown any evidence of benefit. The results of these trials have been systematically reviewed: corticosteroids (Sandercock 2011), calcium antagonists (Zhang 2019), haemodilution (Chang 2014), excitatory amino acid antagonists (including ion channel modulators and N‐methyl‐D‐aspartic acid; NMDA) (Muir 2003), piracetam (Ricci 2012a), a free radical trapping agent NXY‐059 (Shuaib 2007), and Cerebrolysin (Ziganshina 2017). There is no evidence that colloids lead to lower odds of death or dependence after stroke compared with crystalloids (Visvanathan 2015).
Role in clinical practice
There is still inadequate evidence from randomised controlled trials for the following antithrombotic agents: oral antiplatelet drugs other than aspirin (clopidogrel, ticlopidine, cilostazol, satigrel, sarpolgrelate, KBT 3022, iisbogrel), Sandercock 2014, and the fibrinogen‐depleting agents ancrod and defibrase (Hao 2012).
The list of interventions of agents tested in clinical trials with subsequent Cochrane Reviews of results that document inadequate evidence to establish a role in clinical practice includes: ginkgo biloba (Zeng 2005); gamma aminobutyric acid (GABA) receptor agonists (Liu 2018); percutaneous vascular interventions, including intra‐arterial thrombolysis with urokinase and pro‐urokinase (O'Rourke 2010); sonothrombolysis (Ricci 2012b); glycerol (Righetti 2004); mannitol (Bereczki 2007); naftidrofuryl, a 5‐HT2 serotonergic antagonist (Leonardi‐Bee 2007); theophylline or methylxanthine derivatives (Bath 2004a; Bath 2004b); nitric oxide donors (Bath 2017); blood pressure‐altering interventions (Bath 2014; Geeganage 2010); prostacyclin and its analogues (Bath 2004c); buflomedil (Wu 2015); vinpocetine (Bereczki 2008); gangliosides (Candelise 2001); colony‐stimulating factors (Bath 2013); stem cells (Boncoraglio 2019); Chinese herbal medicines such as sanchi (Chen 2008), puerarin (Liu 2016), mailuoning (Yang 2015), and tongxinluo (Zhuo 2008); and the neuroprotective agent edaravone (Feng 2011).
Description of the condition
Ischaemic stroke occurs when the brain loses its blood and energy supply, resulting in damage to brain tissue; it is the brain equivalent of a heart attack. Most strokes (87%) are ischaemic (AHA 2019). Worldwide 15 million people suffer a stroke every year; five‐and‐a‐half million people die, and another five million are left permanently disabled, placing a burden on family and community (WHO 2019a). Stroke is one of the major causes of disability and mortality (AHA 2019; GBD Stroke Collaborators 2019; WHO 2019a). It is the third most common cause of death after coronary disease and cancer. In 2014 the World Health Organization (WHO) stroke statistics registered the number of deaths from stroke to be more than 200,000 in the Russian Federation, as well as in China and in India, with the highest number of 1,652,885 in China and 517,424 in Russia in 2002 (WHO 2019a). According to the Russian data, there were on average 3.52 and 3.27 cases per 1000 population registered in the Russian Federation in 2009 and 2010, respectively, and mortality was 1.19 and 0.96 per 1000 population in 2009 and 2010, with significant differences between different regions (Gusev 2013). Standardised incidence was 2.39 (3.24 in men and 2.24 in women) per 1000 population (Gusev 2013). In 2016 in Russia there were 345,861 stroke deaths (95% confidence interval (CI) 267,315 to 444,861), 676,846 incident cases (95% CI 607,894 to 746,828), and 6,082,727 disability‐adjusted life‐years (DALYs) (95% CI 4,773,920 to 7,736,480) (GBD Stroke Collaborators 2019). The case fatality rate of stroke is 40.4% (61.4% for haemorrhagic stroke and 21.8% for ischaemic stroke). The northwest regions of Russia had the highest stroke incidence of 7.43 per 1000, followed by some cities in mid areas of the country (5.37 per 1000) and the far east (4.41 per 1000) (Gusev 2003; Vilenskiĭ 2006). The rate of recurrence of stroke was 30% (Suslina 2009). Stroke survivors experience serious neurological disorders (loss of vision or speech, or both; paralysis; confusion), and in 30% to 66% of cases these are not restored six months after a stroke (French 2007). In Russia, stroke is the primary cause of death and disability in adults: 32 cases per 100,000 population. Twenty‐five per cent to 30% of stroke survivors develop dementia by the end of one year. Stroke presents a huge financial burden for the health system (Martynchik 2013). The burden of stroke is projected to rise globally to 61 million DALYs in 2020 (WHO 2019a).
Description of the intervention
Cerebrolysin is a mixture of low‐molecular‐weight peptides and amino acids derived from porcine brain, and has potential neuroprotective and neurotrophic properties. The manufacturer of Cerebrolysin promotes it for multiple neurological conditions, and it is widely used in the treatment of acute ischaemic stroke in Russia, China, and other Asian and post‐Soviet countries.
How the intervention might work
The term 'neuroprotection' is used to describe the putative effect of interventions protecting the brain from pathological damage. In ischaemic stroke, the concept of neuroprotection includes inhibition of pathological molecular events leading to calcium influx, activation of free radical reactions, and cell death. Knowledge of pathophysiology in acute ischaemic stroke stimulated the development of a number of potential neuroprotective agents. Many neuroprotective agents have proven to be efficacious in animal studies. Cerebrolysin is a mixture of low‐molecular‐weight peptides (80%) and free amino acids (20%) derived from porcine brain, with proposed neuroprotective and neurotrophic properties similar to naturally occurring growth factors such as nerve growth factor and brain‐derived neurotrophic factor (Alvarez 2000; Fragoso 2002). In a study that identified 638 unique peptides in Cerebrolysin, none appeared to be related to any known trophic factor or trophic factor precursor, and it was suggested that the active peptides belong to proteins containing hidden functional peptide sequences (Gevaert 2015).
Results of in vitro and animal studies of Cerebrolysin have traditionally been used to suggest its potential for treating acute ischaemic neuronal damage (Masliah 2012). For example, Cerebrolysin has been shown to be effective in tissue culture models of neuronal ischaemia, dose‐dependently increasing neuronal survival (Schauer 2006). In brain slices it counteracts necrotic and apoptotic cell death induced by glutamate (Riley 2006). Cerebrolysin also demonstrates neuroprotective activity in rat models of haemorrhagic stroke, Makarenko 2005, and ischaemic stroke (Zhang 2010), as well as in spinal cord trauma (Sapronov 2005). One randomised double‐blind placebo‐controlled trial showed no effect of Cerebrolysin in acute haemorrhagic stroke on chosen efficacy measures including the Barthel Index, Unified Neurological Stroke Scale, and Syndrome Short Test (Bajenaru 2010).
Why it is important to do this review
Despite the effectiveness of neuroprotective agents in animal models of stroke, the results of clinical trials of neuroprotective agents in humans have been disappointing (European Ad Hoc Consensus 1998; Ginsberg 2016; Goenka 2019). Cochrane Reviews of the effects of individual neuroprotective agents and pharmacological groups confirm this (Gandolfo 2002; Muir 2003; Ricci 2012a; TISC 2001). Yet, other means of neuroprotection are being sought. Cerebrolysin is well accepted by Russian, Eastern European, and Asian physicians, and is widely used in the treatment of acute ischaemic stroke and other neurological disorders (Chukanova 2005; Gromova 2006; Onishchenko 2006). Research data from observational studies and clinical trials of Cerebrolysin in acute stroke or head injury, most of which have been performed in Russia and China, have accumulated (Chukanova 2005; Gafurov 2004; Gromova 2006; Ladurner 2005; Skvortsova 2004; Wong 2005).
As assessed in a Cochrane Review for vascular dementia, Cerebrolysin may have positive effects on cognitive function and global function in elderly people with mild to moderate dementia, but the review authors did not recommend it for routine use in vascular dementia owing to the limitations of the studies in the resulting review, small number of included trials, wide variety of treatment durations, short‐term follow‐up, and high risk of bias of the included studies (Cui 2019). Cerebrolysin has also been proposed as a treatment for people with Alzheimer's disease (Fragoso 2002). Trials of Cerebrolysin in acute haemorrhagic stroke have been assessed in a meta‐analysis (Shu 2012), which concluded on its safety and supported implementation of new trials for definitive efficacy assessment.
Previous versions of this Cochrane Review did not find evidence of clinical benefit of Cerebrolysin for treating acute ischaemic stroke (Ziganshina 2010a; Ziganshina 2015; Ziganshina 2016), and the most recent update provided moderate‐quality evidence of an increase in non‐fatal serious adverse events with Cerebrolysin use (Ziganshina 2017). It is important to evaluate the data that have accumulated since then in order to provide better‐quality evidence.
Ziganshina 2017 created heated debate in the journal Stroke (Bereczki 2017). However, the debate did not address the challenges of dealing with potential risk of bias in clinical trials, which in our view reflects an important contribution of Cochrane Reviews.
The previous version of this review also provoked a number of published papers, particularly in Russian language academic media, in favour of using Cerebrolysin for treating acute ischaemic stroke, which we illustrate in the PRISMA flow diagram (Figure 1). Amongst the English language publications, there is a meta‐analysis of nine clinical trials (Bornstein 2018), presenting a critique of the findings of the Cochrane Review (Ziganshina 2017). We critically appraise Bornstein 2018 in the Agreements and disagreements with other studies or reviews of the Discussion section.
1.
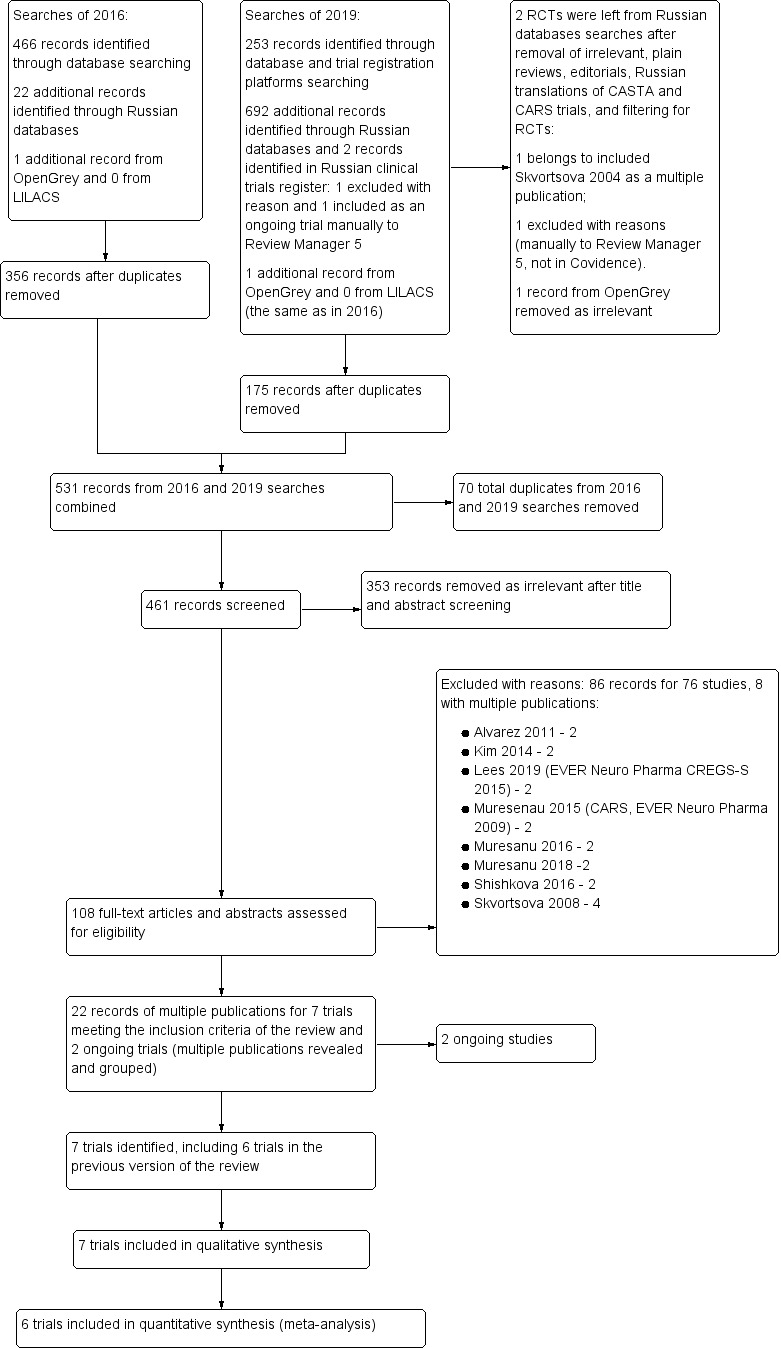
Study flow diagram.
This interest and attention to the research question of our Cochrane Review, particularly in view of the debate around reliable evidence (Horton 2019), encouraged us to update the review and revisit the question of reliability of evidence.
In this review update we prospectively refined our approach to sensitivity analyses for future updates. We added one more sensitivity analysis to explore the effects of stroke diagnosis methods (i.e. confirmation of stroke diagnosis by neuroimaging and clinical diagnosis) on reported outcomes.
Studies reporting on our outcome measures was not an inclusion criterion for this review; changes in the reporting of outcomes in our data synthesis depended on data reported by the authors of eligible included trials in their trial reports.
The aim of this update was to establish whether the inclusion of data from newly identified trials would affect the conclusions of the former version of the review in view of a thorough reassessment of the risk of bias in included studies through identification, examination, and evaluation of study protocols.
Objectives
To assess the benefits and harms of Cerebrolysin for treating acute ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included all published randomised controlled trials (RCTs) comparing Cerebrolysin with placebo or no treatment in people with acute ischaemic stroke. We excluded uncontrolled studies, as well as quasi‐RCTs where allocation to treatment or control was not concealed (e.g. allocation by alteration, open random number list, date of birth, day of the week, or hospital number).
Types of participants
People with acute ischaemic stroke, irrespective of age, sex, or social status, whose symptom onset was less than 48 hours previously. Stroke symptoms include: sudden weakness or numbness of the face, arm, or leg, often unilateral; confusion; difficulties in speaking or seeing with one or both eyes; difficulties walking; loss of balance or co‐ordination; severe no‐cause headache; fainting or loss of consciousness. Stroke diagnosis confirmation with neuroimaging was not an inclusion criterion. For future updates stroke diagnosis confirmation by neuroimaging will be mandatory.
Types of interventions
We compared Cerebrolysin added to standard treatment against either placebo or no treatment added to standard treatment.
Standard treatment is not defined precisely and differs between studies. Study medication must have been started within 48 hours of onset of stroke and continued for any period of time. We planned to add a separate analysis for the comparison 'Cerebrolysin versus other neuroprotective agents' and to combine data for Cerebrolysin with data for newer peptide‐mixtures, which we have termed 'Cerebrolysin‐like agents', but the available studies did not permit this.
Types of outcome measures
We used one primary outcome and six secondary outcomes with special attention to adverse events and effects.
Primary outcomes
All‐cause death, to be measured as the number of people who died from the start of tested treatment to the end of the follow‐up period.
Secondary outcomes
Non‐death attrition. After identifying and evaluating available trial registration protocols, we decided to add this new outcome to the update as a measure not only of attrition per se, but also as a grey zone in the presentation of trial populations allowing us to characterise attrition and reporting bias better.
Poor functional outcome defined as death or dependence at the end of the follow‐up period: various scales, such as the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale (mRS), and the Barthel Scale/Index (BI) can be used to evaluate impairment brought about by stroke. The mRS is commonly used and is a scale from 0 to 6, with 0 being no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderate to severe disability; 5, severe disability; 6, death.
Early death (within two weeks of stroke onset).
Quality of life, if assessed in the included studies.
Time to restoration of capacity for work, either as a time‐to‐event outcome (e.g. analysed as a hazard ratio) or as a continuous outcome, depending on study data.
Cause of death: we added this new outcome in order to understand deaths of people treated with Cerebrolysin or placebo and reported narratively on the results.
Adverse events and effects
A serious adverse event (SAE), as defined according to the International Council for Harmonisation guideline, is "any untoward medical occurrence that, at any dose, results in death, is life‐threatening, requires inpatient hospitalisation or results in prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, is a congenital anomaly/birth defect, or is a medically important event or reaction" (ICH 2003). We confirmed the definition of SAE used by researchers and the numbers of people with SAEs in the CASTA 2012 trial through correspondence with the manufacturer of Cerebrolysin and the lead author of this trial, and extracted data from the CERE‐LYSE‐1 2012 trial report that used Medical Dictionary for Regulatory Activities (MedDRA) coded SOC (System Organ Class) and Preferred Term (PT) (MedDRA 2011), developed under the auspices of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH 2003).
We used the following outcomes for SAEs.
Total number of people with SAEs.
Total number of people with fatal SAEs.
Total number of people with non‐fatal SAEs.
Total number of people with adverse events.
Search methods for identification of studies
The methods for the Cochrane Stroke Group Specialised Register are shown at www.dcn.ed.ac.uk/csrg/entity/searchmethods.pdf. We attempted to identify all relevant trials regardless of language or publication status, and arranged for the translation of relevant papers where necessary.
Electronic searches
We searched the following databases:
the Cochrane Stroke Group Trials Register (last searched 24 October 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2019, Issue 9 of 12, October 2019 (last searched 24 October 2019; Appendix 1);
MEDLINE Ovid (from 1946; last searched 24 October 2019; Appendix 2);
Embase Ovid (from 1980; last searched 24 October 2019; Appendix 3);
Science Citation Index Expanded Indexes and Conference Proceedings Citation Index ‐ Science – Web of Science Core Collection (last searched 24 October 2019; Appendix 4);
LILACS (Latin American and Caribbean Health Sciences Literature database) (1982 to 24 October 2019; Appendix 2);
OpenGrey (System for Information on Grey Literature in Europe; www.opengrey.eu; 1980 to 24 October 2019; Appendix 3);
the following Russian Databases: e‐library (elibrary.ru; 1998 to 24 October 2019) and EastView (online.ebiblioteka.ru/index.jsp; 2006 to October 2019; Appendix 4).
The Cochrane Stroke Group Information Specialist developed the search strategies for CENTRAL, MEDLINE, Embase, Web of Science indexes, and trial registers. We then adapted the MEDLINE strategy for the additional Russian language databases.
Searching other resources
We also searched the following ongoing trials and research registers (24 October 2019):
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (last searched 24 October 2019; Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (last searched 24 October 2019; Appendix 6);
Russian State Register of Approved Medicines (grls.rosminzdrav.ru) (last search November 2019).
In an effort to identify further published, unpublished, and ongoing trials and to obtain additional trial information, we checked the reference lists of all trials identified by the above methods, and searched the following neurology conference proceedings held in Russia: Chelovek i Lekarstvo [Man and Medicine] (2011 to 2019), National'niy congress cardiologov [The National Congress of Cardiology] (2006 to 2016), Rossiyskiy Mezhdunarodniy Congress Cerebrovascularnaya patologiya i insult [Russian International Congress of Cerebrovascular Pathology and Stroke] (2012 to 2019).
For this update we did not contact the pharmaceutical company EVER Neuro Pharma GmbH, the manufacturer of Cerebrolysin, given that we added only one study for which all data were publicly available and clear.
We cross‐referenced all studies included in this review with the Retraction Watch (both the Retraction Watch site and the Retraction Watch Database); last searched November 2019; Appendix 7).
Data collection and analysis
Selection of studies
Two review authors (LEZ and CHVH) independently examined titles and abstracts of records from the electronic searches and excluded those studies that were obviously irrelevant. We used Covidence, which allowed for quick detection and resolution of conflicts between review authors. We obtained the full texts of the remaining papers, and the same two review authors independently selected studies for inclusion based on the predetermined inclusion criteria refined for this update. Any disagreements were resolved through discussion. We excluded studies that did not meet the inclusion criteria, providing reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (LEZ and CHVH) independently extracted data using Covidence. We extracted data on the methods of the studies, participants, interventions, and outcomes. We resolved any differences in the extracted data by referring to the original articles and through discussion. We extracted data to allow an intention‐to‐treat (ITT) analysis (including all participants in the groups to which they had been randomly allocated) and presented the data in the Characteristics of included studies table, generated by Covidence. We calculated the percentage loss to follow‐up and presented this information in the 'Risk of bias' tables.
For binary outcomes, we extracted the number of participants with the event in each group. For continuous outcomes, we planned to use arithmetic means and standard deviations for each group.
Assessment of risk of bias in included studies
Two review authors (LEZ and CHVH) independently evaluated the methodological quality of studies with regard to the generation of allocation sequence, allocation concealment, blinding, loss to follow‐up, and other risk of bias using the Cochrane 'Risk of bias' assessment tool (Higgins 2011).
We followed the guidance in the risk of bias assessment tool to assess whether adequate steps had been taken to reduce the risk of bias across seven domains: generation of allocation sequence; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome data (attrition bias); selective outcome reporting; and other sources of bias. We assigned judgements of 'low', 'high', or 'unclear' risk of bias for these domains. We considered loss to follow‐up to be acceptable (low risk of bias) if it was less than 10%.
For the assessment of other sources of bias, we evaluated how study authors described funding sources for their trials and how conflict of interest statements were presented, if presented at all. We judged the risk of bias to be high in cases of clear sponsorship by the manufacturer of Cerebrolysin, involvement of the manufacturer with trial planning and design, sequence generation, medication provision, statistical procedures, blinding of personnel and outcome assessors, and involvement in reporting, as well as in cases of declared relationship of study authors to the manufacturer of Cerebrolysin. Where there was no mention of funding sources and no conflict of interest statements, we judged the risk of bias to be unclear.
We resolved any disagreements arising at any stage by discussion.
Measures of treatment effect
We presented dichotomous data and combined them using risk ratios (RRs). We showed RRs accompanied by 95% confidence intervals (CIs). We planned to present continuous outcomes, if identified, as means accompanied by standard deviations (SD)/standardised mean difference (SMD).
Unit of analysis issues
We only included studies that randomised individual participants. We did not include cluster or cross‐over trials and did not have multiple time points.
Dealing with missing data
We undertook analysis according to the ITT principle; unless otherwise stated we used the number of initially randomised participants as a denominator. We extracted the total numbers of people who died or had serious adverse events and used them as numerators. We used the data on the number of deaths in both comparison groups to generate the primary outcome of all‐cause death, and the number of people initially randomised into each comparison group as the denominator. If there was a concern, we would conduct sensitivity analysis to explore robustness of the results.
Assessment of heterogeneity
We tested for heterogeneity of effect sizes between studies by inspecting the forest plots and using the I2 statistic (Higgins 2003), considering a value of 30% to 60% as denoting moderate levels of heterogeneity (Deeks 2011). If there was clinical heterogeneity, we would explore it in subgroup analysis if the amount of data permitted, or describe narratively rather than pooling heterogenous data.
Assessment of reporting biases
If there was a sufficient number of studies (10 or more), we would use funnel plots to examine asymmetry that may have been caused by publication bias or heterogeneity.
We compared the outcomes predefined in study protocols with those reported in the published manuscripts to detect potential selective reporting.
Data synthesis
We used the ITT principle for data synthesis. We used Review Manager 5 to analyse the data (Review Manager 2014). We used RR as a measure of effect for binary outcomes, and we used a fixed‐effect model for pooling the data in cases of no or a low level of heterogeneity.
Where we detected heterogeneity (forest plot inspection and I2 statistic > 30%), and it was still appropriate to pool the data, we used the random‐effects model.
We used and presented 95% CIs for RRs of all studied outcomes.
Subgroup analysis and investigation of heterogeneity
We investigated potential sources of heterogeneity for all outcomes using the following criteria for subgroups.
Cerebrolysin dose.
Length of treatment.
We identified the following subgroups by Cerebrolysin dose and the length of treatment.
30 mL for 10 days: cumulative dose 300 mL over 10 days.
50 mL for 21 days: cumulative dose 1050 mL over 21 days.
30 mL for seven days then 10 mL five days per week for three weeks: cumulative dose 360 mL over 28 days.
10 mL and 50 mL for 10 days: cumulative dose 100 mL and 500 mL over 10 days.
We planned to perform a sensitivity analysis to test the robustness of the results. We planned to investigate the effect of methodological study quality ('low', 'high', or 'unclear' risk of bias) using a sensitivity analysis. We planned to use funnel plots to examine asymmetry, which may be caused by publication bias or heterogeneity.
Sensitivity analysis
We planned to perform a sensitivity analysis to test the robustness of the results. We planned to investigate the effect of methodological study quality ('low', 'high', or 'unclear' risk of bias) using a sensitivity analysis. We planned to use funnel plots to examine asymmetry, which may be caused by publication bias or heterogeneity.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2011). We employed GRADEpro GDT, and imported data from Review Manager 5 to create a Table 1 for the primary outcome of all‐cause death at the end of the follow‐up period; total number of people with SAEs at the end of the follow‐up period, comprising fatal and non‐fatal SAEs, and a subgroup by Cerebrolysin dose and length of treatment, at the end of the follow‐up period; total number of people with adverse events at the end of follow‐up period; non‐death attrition; death or dependence at the end of the follow‐up period; early death (within two weeks of stroke onset); and quality of life (Review Manager 2014).
Table 1 includes information on the overall quality of the evidence from the trials and information of importance for healthcare decision making. The GRADE approach determines the quality of the evidence based on an evaluation of eight criteria (risk of bias, inconsistency, indirectness, imprecision, publication bias, effect size, presence of plausible confounding that will change effect, and dose‐response gradient). We used the criteria of risk of bias, inconsistency, indirectness, and imprecision to guide our conclusions and recommendations
Results
Description of studies
We report here on seven trials, which met inclusion criteria, and how we identified these trials.
Results of the search
In the new searches we identified:
253 records through database and trial registration platforms searches, of which 175 were left after duplicate removal;
692 records through Russian database searches, which we reduced to two records after removal of irrelevant articles, plain reviews, editorials, Russian translations of CASTA, CASTA 2012, and CARS, Guekht 2015a, trials, and manually filtering for RCTs: one, Skvortsova 2004, reported on an existing included study as a multiple publication and was already in Covidence and Review Manager 5, and one we excluded with reasons manually in Review Manager 5, not through Covidence. We also identified two records in the Russian trials register, excluding one with reasons and including one as an ongoing study manually in Review Manager 5, not uploading into Covidence owing to incompatibility of resources;
one record from OpenGrey, which was the same as one identified in the 2016 search and which we removed as irrelevant;
nothing through our search of LILACS;
nothing through Retraction Watch.
Combined with the search results of 2016 (489 records), we dealt with 1444 records in total.
We moved 175 records into Covidence which resulted in 531 records, combined with what populated Covidence since the initiation of Covidence in 2016. Whilst combining searches, Covidence removed 57 duplicates and we removed manually 13 to the total of 70 duplicates. Of the remaining 461 records we excluded 353 as irrelevant and identified 108 records for eligibility assessment as per protocol. We excluded 76 studies which were presented in 86 records due to multiple publications (two to four per study), which we grouped in Excluded studies. Reasons for the exclusion of studies are shown in Characteristics of excluded studies. The remaining 22 records were of multiple publications of seven trials we identified for inclusion, of which six were included in the previous version of the review, and two ongoing studies. For details, see Characteristics of included studies and Characteristics of ongoing studies.
The results of the search are illustrated in the study flow diagram (Figure 1).
Included studies
Seven trials met the published inclusion criteria.
Amiri Nikpour 2014 was performed in the Islamic Republic of Iran. The trial compared Cerebrolysin with placebo (normal saline) in 46 people (23 participants in each group) with acute ischaemic stroke confirmed by computed tomography (CT) scan or magnetic resonance imaging (MRI), or both. Cerebrolysin was started within 24 hours of stroke onset and continued for 10 days as a once‐daily intravenous infusion of 30 mL in addition to standard treatment of 100 mg of aspirin daily. The average age of trial participants was 60 years. There were no significant differences between the two groups in terms of baseline characteristics. The duration of follow‐up was 90 days; one participant in the Cerebrolysin group and two participants in the placebo group died within 30 days of trial initiation. The causes of death were not reported; these three people were excluded from the final analyses. The study protocol is not publicly available, and there is no mention of a study protocol in the text of the published trial report. The study authors reported the results of the trial in two publications (Amiri Nikpour 2014).
CASTA 2012 was a multicentre, placebo‐controlled trial performed in four countries: China, Hong Kong, South Korea, and Myanmar. The trial compared Cerebrolysin with placebo added to standard baseline therapy in 1070 people with acute ischaemic stroke with CT or MRI results compatible with a clinical diagnosis of acute hemispheric stroke (529 participants in the Cerebrolysin group and 541 participants in the control group). Cerebrolysin was started within 12 hours of stroke onset and continued for 10 days as a once‐daily intravenous infusion of 30 mL diluted in saline (total of 100 mL) in addition to standard treatment of 100 mg of aspirin daily. Placebo was 100 mL saline as a daily intravenous infusion for 10 days starting within 12 hours of stroke onset. The average age of the trial participants was 65 years. The duration of follow‐up was 90 days; 180 participants were lost to follow‐up (16.8%). There were differences between the two groups in terms of baseline prognostic variables having more people with chronic diseases in the placebo group than in the Cerebrolysin group, 293 versus 251 (55% versus 46% of randomised participants). There were more people with diabetes, 117 (21.7%) versus 108 (20.5%); arrhythmia, 90 (16.7%) versus 71 (13.5%); and coronary heart disease, 86 (16.0%) versus 72 (13.7%) in the placebo group compared to the Cerebrolysin group. The trial was supported by the manufacturer of Cerebrolysin, EVER Neuro Pharma GmbH. The study authors reported the results of the trial in five publications (CASTA 2012 with the protocol registered at ClinicalTrials.gov NCT00868283 and published as a separated paper (Hong 2009), both retrospectively).
CERE‐LYSE‐1 2012 was a multicentre, placebo‐controlled trial performed in five countries: Austria, Croatia, the Czech Republic, Slovakia, and Slovenia. The trial compared Cerebrolysin with placebo in 119 people (60 in the Cerebrolysin group and 59 in the control group) with acute hemispheric ischaemic stroke after exclusion of brain haemorrhage by CT. Cerebrolysin was started within two hours of stroke onset and continued for 10 consecutive days as a once‐daily intravenous infusion of 30 mL mixed with 70 mL of normal saline (total volume 100 mL over a time period of 30 minutes), starting immediately one hour after thrombolytic treatment (alteplase). The placebo consisted of 100 mL normal saline. The average age of the trial participants was 66 years. There were no significant differences between treatment groups in terms of baseline prognostic variables. The duration of follow‐up was 90 days, and 19 participants of 119 (16%) were lost to follow‐up. The study authors did not report any information on funding sources of the trial, including provision of Cerebrolysin. The statistician of the study was contracted by EVER Neuro Pharma GmbH, the manufacturer of Cerebrolysin. The study authors reported the results of the trial in one publication (CERE‐LYSE‐1 2012), with the protocol registered at ClinicalTrials.gov retrospectively (NCT00840671).
Gharagozli 2017 was a placebo‐controlled trial involving three neurological hospitals in the Islamic Republic of Iran. Cerebrolysin was compared against placebo (normal saline) in 100 people (50 in each comparison group) with clinically confirmed acute embolic or thrombotic stroke in the territory of internal carotid artery branches. Neuroimaging was not used to confirm the diagnosis. Treatment began within 18 hours of the onset of stroke and lasted for four weeks. In the initial acute phase, during the first seven days, 80 mL of the study drug (30 mL Cerebrolysin plus 50 mL saline) was administered as an intravenous infusion for 30 minutes. Subsequently, Cerebrolysin was administered as 10 mL intravenously five days per week for four weeks. The placebo (normal saline) was given in the same volume as the Cerebrolysin solutions. Standard treatment was aspirin 100 mg daily, pentoxifylline, or low‐dose heparin with Cerebrolysin or placebo given as an adjunct. Participants were aged 45 to 85 years; the average was 68 years. The authors noted significant differences in the baseline characteristics of participants. The primary efficacy analysis was based on the National Institutes of Health Stroke Scale (NIHSS). The NIHSS scores were significantly different at baseline between the two groups. The secondary efficacy analysis was based on the modified Rankin Scale (mRS). The mRS scores were significantly different at baseline between groups. There were also statistically significantly more participants with aphasia in the Cerebrolysin group than in the placebo group, 14/50 versus 10/50, respectively (P = 0.04), and significantly fewer participants with obesity in the Cerebrolysin group, 14/50 versus 28/50 (P = 0.01). The duration of follow‐up was four weeks. Seventeen participants in the Cerebrolysin group and eight participants in the placebo group did not complete the study (overall loss of participants = 25%). EVER Neuro Pharma GmbH provided the study medication. Three of the eight study authors had declared links to EVER Neuro Pharma. The study authors reported the results of the trial in one publication (Gharagozli 2017), with the trial protocol registered at the Iranian Registry of Clinical Trials retrospectively (IRCT138803272042N1).
Ladurner 2005 was a multicentre, placebo‐controlled trial conducted in Austria, the Czech Republic, and Hungary. The trial compared Cerebrolysin with placebo (100 mL normal saline) added to standard baseline therapy in 146 people with acute ischaemic stroke with clinical symptoms of the middle cerebral artery area after exclusion of brain haemorrhage by CT. Cerebrolysin (50 mL mixed with 50 mL of normal saline) and placebo were started within 24 hours of stroke onset and continued for 21 days as a once‐daily intravenous infusion over a period of 20 minutes. The same basic therapy was used in the treatment group and the control group (pentoxifylline and acetylsalicylic acid): Cerebrolysin plus basic therapy, 78 participants and placebo plus basic therapy, 68 participants. The average age of the trial participants was 65 years. The duration of follow‐up was 90 days. Twenty‐five participants (17%) were lost to follow‐up, nine in the treatment group and 16 in the control group. There were no significant differences between the two groups in terms of baseline characteristics. The trial was supported by the manufacturer of Cerebrolysin, EVER Neuro Pharma GmbH, who also provided the study centres with Cerebrolysin. The study authors reported the results of the trial in three publications (Ladurner 2005).
Skvortsova 2004 was performed in Russia. The trial compared Cerebrolysin with placebo added to standard baseline therapy in 36 people with acute ischaemic stroke in the territory of the internal carotid artery, confirmed by CT or MRI. Cerebrolysin was started within 12 hours of stroke onset and was continued for 10 days as a once‐daily intravenous infusion of either 10 mL or 50 mL. There were three groups, 12 participants in each, treated with 10 mL Cerebrolysin, 50 mL Cerebrolysin, or placebo. Standard baseline therapy consisted of aspirin 100 mg per day, haemodilution, pentoxifylline, and heparin (when needed). There were no significant differences in baseline characteristics between groups. The average age of the trial participants was 69 years. The duration of follow‐up was 30 days, and there were no losses to follow‐up. No information on funding sources for the trial and no conflict of interest statement was provided. The study authors reported the results of the trial in three publications (Skvortsova 2004).
Xue 2016 was performed in China. The trial compared Cerebrolysin with placebo and another neuroprotective agent (DL‐3‐n‐butylphthalide; NBP) in 60 people with acute ischaemic stroke, confirmed by CT or MRI (20 participants each). There were no significant differences in baseline characteristics between the Cerebrolysin and placebo groups. Cerebrolysin was administered for 10 days as a once‐daily intravenous infusion of 30 mL mixed with 70 mL of normal saline; the infusions lasted for 50 to 70 minutes. Participants in the control group received intravenous infusions of 100 mL of normal saline, whilst the Cerebrolysin group received an intravenous infusion of 100 mL of 25 mg NBP in normal saline, twice daily for 10 days starting within 12 hours after stroke onset. Standard baseline therapy consisted of antithrombotics, hypoglycaemics, antilipaemic agents, antihypertensives, and dehydration, according to local current guidelines for the management of ischaemic stroke in neurological intensive care units, and 100 mg aspirin orally. The duration of follow‐up was 90 days. The study authors reported the results of the trial in one publication (Xue 2016), with the protocol registered at ClinicalTrials.gov retrospectively (NCT02149875).
For details of the included trials, see Characteristics of included studies.
There are no trials awaiting classification.
Excluded studies
We excluded 76 studies reported in 99 publications with records identified by our searches, because of:
ineligible research question: research questions not relevant, e.g. effects of Cerebrolysin on stroke volume;
ineligible study design, including lack of randomisation or control arm;
ineligible patient population, including participants with treatment initiation exceeding the protocol‐specified 48 hours after stroke onset;
reported as an abstract only without any prior or subsequent publication of full paper.
The reasons for exclusion of these studies are detailed in the Characteristics of excluded studies table. The number of multiple publications ranged from two to four.
Risk of bias in included studies
Seven RCTs met the inclusion criteria.
Allocation
For sequence generation, we judged two trials to be at low risk of bias, Gharagozli 2017; Ladurner 2005, and five trials to be at unclear risk of bias because the study authors did not provide any information on sequence generation (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Skvortsova 2004; Xue 2016).
In Ladurner 2005, the manufacturer of Cerebrolysin, EVER Neuro Pharma GmbH, provided the randomisation method, that is a computer‐generated randomisation code, which we judged to fit the criteria of low risk of bias. However, we noted the direct involvement of EVER Neuro Pharma with regard to the randomisation codes and the unavailability of the study protocol.
In Gharagozli 2017 the study authors used a predefined randomisation plan, which we judged to be the basis for an assessment of low risk of bias. However, detailed information about the actual process of generation of randomisation sequence was not provided, either in the published trial report or in the retrospective trial registration information.
In Amiri Nikpour 2014 and Skvortsova 2004, no information was provided on sequence generation procedures, which combined with the unavailability of a study protocol resulted in a judgement of unclear risk of bias.
We carefully reviewed the published protocol of the CASTA 2012 study, which was published retrospectively to participant enrolment as Hong 2009, and did not find a description of the procedure for sequence generation, resulting in a judgement of unclear risk of bias.
In CERE‐LYSE‐1 2012 the described procedure for sequence generation did not fit the criteria for an assessment of low risk of bias. There was no information about the actual process of generation of a randomisation sequence. Adding to this was a retrospective protocol registration and a statistician contracted by the manufacturer of Cerebrolysin, EVER Neuro Pharma, resulting in a judgement of unclear risk of bias.
In Xue 2016 the sequence generation was performed with computer‐generated numbers by a third party; however, it was unclear who the third party was, and together with the retrospective nature of the trial registration, resulted in a judgement of unclear of bias.
For allocation concealment, we judged one trial to be at low risk of bias because they used identical vials (CERE‐LYSE‐1 2012), and the remaining six included trials to be at unclear risk of bias because the study authors did not provide a clear description of concealment. The exception was Ladurner 2005, in which the trial authors used sealed envelopes with information on the actual treatment dispensed, and provided these envelopes to the investigator in case of emergency. The published report described that all envelopes remained sealed throughout the study. However, as the trial authors did not describe the envelopes as opaque, and the trial protocol was unavailable, we judged Ladurner 2005 to be at unclear risk of bias for allocation concealment.
In Gharagozli 2017 the trialists provided no information about allocation concealment, and there was no central randomisation. Owing to the fact that the statistician in charge of randomisation was unblinded and also an employee of the manufacturer and supplier of Cerebrolysin, we judged this study to be at unclear risk of bias for allocation concealment.
Blinding
For blinding of participants and personnel (performance bias), we judged four trials to be of low risk of bias (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005), and the remaining three trials, which did not provide clear information on blinding, as at unclear risk of bias (Amiri Nikpour 2014; Skvortsova 2004; Xue 2016). For blinding of outcome assessors (detection bias), we judged three studies to be of low risk of bias (CASTA 2012; CERE‐LYSE‐1 2012; Ladurner 2005), and the remaining four studies to have an unclear risk of bias owing to no or insufficient information to judge low or high risk of bias (Amiri Nikpour 2014; Gharagozli 2017; Skvortsova 2004; Xue 2016).
Incomplete outcome data
Amiri Nikpour 2014 reported no loses to follow‐up and was therefore judged as having a low risk of attrition bias. Skvortsova 2004 reported no loss of participants, but presented data on death ambiguously; we therefore judged this study to have an unclear risk of bias. The five remaining studies all reported participant losses in excess of 10% (between 16% and 29%, Table 2), and were therefore judged to be at high risk of attrition bias (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005; Xue 2016).
1. Loss to follow‐up (attrition).
| Study | Number of randomised participants | Number lost to follow‐up (%) |
| Amiri Nikpour 2014 | 46 | 0 (0)* |
| CASTA 2012 | 1070 | 180 (17) |
| CERE‐LYSE‐1 2012 | 119 | 19 (16) |
| Gharagozli 2017 | 100 | 25 (25) |
| Ladurner 2005 | 146 | 25 (17) |
| Skvortsova 2004 | 24 | 0 (0)* |
| Xue 2016 | 84 | 24 (29) |
*Number lost to follow‐up not stated; we assumed the value to be '0'.
The authors of CERE‐LYSE‐1 2012 used the 'last observation carried forward' (LOCF) method for their NIHSS analysis to fill in their missing data points. There was a 16% loss of participants, but there is no indication as to when these participants were lost, nor for any of the time points is there any indication as to when or how many virtual (i.e. imputed) data were used. It is well understood that using LOCF can introduce bias that may exaggerate the effectiveness of a drug (Molnar 2008; Salim 2008): "The only condition where LOCF is unbiased is when the missing data occurs completely by chance and the data used as the basis for the LOCF imputation has exactly the same distribution as does the unknown missing data. Since it can never be proven that these distributions are exactly the same, all LOCF analyses are suspect and should be dismissed" (Lachin 2016). LOCF provides biased results and its use is to be deprecated (Lachin 2016; Molnar 2008; Salim 2008).
Gharagozli 2017 reported a substantial loss of participants to follow‐up, around 31% for the Cerebrolysin group. NIHSS scores were evaluated at five time points, but there is no information as to when the Cerebrolysin or the placebo group participants dropped out. The study authors applied LOCF analysis (see above), and with such a large amount of virtual data we judged the reporting to have a high risk of bias.
Ladurner 2005 also applied LOCF analysis, in which 146 participants were randomised, of whom 119 completed the study; 27 participants were therefore lost to follow‐up, but the study authors state that there were only 25 cases lost. Either way, this is a 17% to 18% loss, greater than the 10% which we would find acceptable. The trial authors studied six time points but are silent as to which time points include virtual data or how much virtual data, claiming a complete cohort of N = 146 (despite losing 25 or 27 participants).
Xue 2016 was the only study that compared Cerebrolysin and another neuroprotective agent (NBP). There were 84 participants at the trial initiation; however, data are presented for only 60 participants (20 participants in each of the three comparison groups) without any explanation for the loss of 24 participants (29% attrition). We could not include any data from this study in the quantitative synthesis.
Selective reporting
We judged the risk of bias for selective outcome reporting to be unclear for all seven included studies.
For three studies there were no protocols in the public domain, with no mention of protocols in the texts of the reports (Amiri Nikpour 2014; Ladurner 2005; Skvortsova 2004). This made it impossible to assess whether the study authors had reported on all of their predefined outcomes. Four studies published their protocols retrospectively (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Xue 2016).
The study protocol for CASTA 2012 was available, and all of the prespecified (primary and secondary) outcomes, which were of interest to the review, were reported accordingly. However, the study authors did not describe the causes of the deaths, and the Kaplan‐Meier mortality curve presented only the subgroup of trial participants with an NIHSS score greater than 12. We judged this study to be an unclear risk of reporting bias. In their 'Analyses of Mortality', the study authors declared 28 and 32 deaths in the Cerebrolysin and placebo groups, respectively. The hazard ratio is given as 1.26 with a probability of 0.19. The study authors describe this as showing "a small superiority for the Cerebrolysin group". At this level of probability these data show nothing except that there is no significant difference between groups. Elsewhere in the study the authors claim that probabilities of 0.16 and 0.28 provide evidence in favour of Cerebrolysin in the treatment of ischaemic stroke. The study authors used NIHSS scores and stratified the participants according to scores > 12 and ≤ 12. In their > 12 group, of 252 participants, 12 and 22 Cerebrolysin‐ and placebo‐treated participants died, respectively, with a hazard ratio of 1.9661 and a probability of 0.02485 (notably quoted to five decimal places). It should be noted that in the remaining 815 participants in the ≤ 12 group, 16 and 10 participants in the Cerebrolysin and placebo groups died, respectively. The study authors do not report how many participants were treated with Cerebrolysin or placebo in either the > 12 group or the ≤ 12 group to permit calculation of a hazard ratio, but even so, in a hugely larger number of participants, there is a result that does not favour Cerebrolysin, about which the study authors are silent.
Ladurner 2005 did not report on the time when the deaths of participants in their trial occurred, and did not assess potential causality with administered medicines. Using the ITT principle, we compared the number of deaths extracted from the safety section of the trial report and presented data as all‐cause death.
Skvortsova 2004 described the causes of deaths (pulmonary embolism, pneumonia, pyelonephritis, and brainstem syndrome secondary to the brain oedema), but without a precise indication of the time when the deaths occurred and a clear indication as to which study group the participants belonged, nor the confirmed cause of death. The study authors did not report on adverse events. The timing of the outcomes presented in a table and a graph in the publication was also unclear.
Other potential sources of bias
According to publicly available information, all trials included in this meta‐analysis received either unclear or considerable support from the pharmaceutical company that manufactures Cerebrolysin. We judged three studies to be at high risk of other bias owing to the direct involvement of the manufacturer (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017).
CERE‐LYSE‐1 2012 was stopped because no significant result for the main study outcome criteria was reached. According to the study authors, there was no causal relationship with Cerebrolysin for any of the deaths observed. Neither the reasons for nor the timing of the deaths was presented; the timing of adverse events and serious adverse events was also not presented. For details, see the 'Risk of bias' section of the Characteristics of included studies table.
These judgements are illustrated in the 'Risk of bias' summary plot (Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
All‐cause death
The included studies reported on the numbers of deaths in various sections of their trial reports, including description of adverse events. We used these data on the number of deaths in the comparison groups to generate the primary outcome of all‐cause death.
We found no difference between the Cerebrolysin and placebo groups in all‐cause death: 47 deaths in the 764 Cerebrolysin‐treated participants and 49 deaths in the 753 placebo‐treated participants: risk ratio (RR) 0.90, 95% confidence interval (CI) 0.61 to 1.32 (6 trials, 1517 participants). The test for heterogeneity revealed no heterogeneity: I2 = 0% (Analysis 1.1).
1.1. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 1: All‐cause death
Secondary outcomes
None of the included trials reported on the following clinically important secondary outcomes: poor functional outcome (defined as death or dependence at the end of the follow‐up period), early death (within two weeks of stroke onset), quality of life, or the time to restoration of capacity for work.
Cause of death
Amiri Nikpour 2014: the causes of death are not described; the authors only mention that one participant in the Cerebrolysin group and two participants in the placebo group died before day 30. The study authors excluded these three participants from their final analysis; we used these data for the all‐cause death assessment.
CASTA 2012: 28/529 participants randomised to the Cerebrolysin group and 32/541 participants randomised to the placebo group died. The study authors described neither the causes of death nor the times when the deaths occurred.
CERE‐LYSE‐1 2012: four participants died in each group: 4/60 in the Cerebrolysin group and 4/59 in the placebo group. The study authors described neither the causes of death nor the times when the deaths occurred, and did not find any relationship in any of the cases to the study medication.
Ladurner 2005: 6/78 participants in the Cerebrolysin group and 6/68 participants in the placebo group died. The study authors reported on the following causes of death: cerebral infarct (four in the Cerebrolysin group and two in the placebo group), heart failure (two in the Cerebrolysin group and one in the placebo group), pulmonary embolism (two in the placebo group), and pneumonia (one in the placebo group). The trial authors did not report on the times when the deaths occurred.
Gharagozli 2017: 1/50 participants randomised to the Cerebrolysin group and 2/50 participants randomised to the placebo group died. All of the deaths occurred within the seven‐day acute‐phase post‐stroke period, owing to the severity of the stroke.
Skvortsova 2004: the study authors described the causes of death. The study authors reported the following causes of death not attributed to the stroke: pulmonary embolism, pneumonia, and pyelonephritis in three participants in the Cerebrolysin group and one in the placebo group (not clear which of these), and the causes of death associated with the stroke: brain oedema with secondary brainstem syndrome, which occurred in two participants in both the Cerebrolysin and placebo groups. The deaths occurred within 30 days after the stroke onset, but the study authors did not report precisely on the time of each death. It was unclear to which Cerebrolysin subgroup by dose these participants belonged, 10 mL or 50 mL.
Xue 2016: one death occurred in the DL‐3‐n‐butylphthalide (NBP) group.
Adverse events and effects
Serious adverse events
Four trials with a total of 1435 participants contributed to this outcome. Overall, 60 out of 717 Cerebrolysin‐treated participants and 52 out of 718 placebo‐treated participants experienced serious adverse events by the end of follow‐up (RR 1.15, 95% CI 0.81 to 1.65, Analysis 1.2). There is thus no evidence that SAEs were more or less common in participants treated with Cerebrolysin.
1.2. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 2: Total number of people with SAEs
Similarly, we found no evidence for an increase or decrease in the numbers of people treated with Cerebrolysin who experienced a fatal SAE. The total numbers of fatal SAEs were 38/667 and 42/668 in the Cerebrolysin and placebo groups, respectively (RR 0.90, 95% CI 0.59 to 1.38, 3 trials, 1335 participants, Analysis 1.3). However, we found a greater than two‐fold increase in the number of people with non‐fatal SAEs receiving Cerebrolysin treatment: 22/717 participants randomised to Cerebrolysin and 10/718 participants randomised to placebo (RR 2.15, 95% CI 1.01 to 4.55, P = 0.047, I2 = 0%, 4 trials, 1435 participants, Analysis 1.4). Examination of the resulting forest plot revealed opposite directions of effect estimates in subgroups of different Cerebrolysin dosing regimens (30 mL for 10 days and 50 mL for 21 days) despite a low level of subgroup differences in the overall data synthesis, which also accounts for the third dosing regimen of Gharagozli 2017 (30 mL for seven days then 10 mL per day five days per week) (P = 0.28, I2 = 22.1%, Analysis 1.4). In the subgroup of the dosing regimen 30 mL for 10 days we found a large difference in the total numbers of people with non‐fatal SAEs treated with Cerebrolysin: 20 out of 589 participants randomised to Cerebrolysin and 7 out of 600 participants randomised to placebo suffered a non‐fatal SAE (RR 2.86, 95% CI 1.23 to 6.66, P = 0.01, 2 trials, 1189 participants, Analysis 1.4).
1.3. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 3: Total number of people with fatal SAEs
1.4. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 4: Total number of people with non‐fatal SAEs
The authors of CASTA 2012 do not describe the nature of adverse events. In CERE‐LYSE‐1 2012 the study authors did describe SAEs. For the Cerebrolysin‐treated participants these included: acute coronary syndrome, atrial fibrillation, cardiac failure, gastric ulcer, pneumonia (three cases), rectal cancer, coma, pleural effusion, aspiration pneumonia (two cases), cerebral haematoma, and pulmonary embolism. For the placebo‐treated participants these included: cardiac arrest, cardiac failure, hepatic cirrhosis, infective arthritis, pneumonia, sepsis, renal failure, respiratory failure, cerebral haemorrhage, and haemorrhagic stroke (one case each).
Gharagozli 2017 reported four participants with SAEs, two per group, all suffering seizures. The seizures were related to the severity of the stroke, and none were attributed to the treatment by the study authors.
The Ladurner 2005 study authors reported only one serious non‐fatal adverse event in the placebo group (haematemesis).
Total number of people with adverse events
We found information on this outcome in four included studies (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005). The synthesis of the data from these studies revealed no difference between the Cerebrolysin and placebo groups, with 320 of 717 Cerebrolysin‐treated participants and 321 of 718 placebo‐treated participants suffering one or more AEs (RR 0.97, 95% CI 0.85 to 1.10, 4 trials, 1435 participants, Analysis 1.5).
1.5. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 5: Total number of people with adverse events
In CASTA 2012, the study authors reported that 242/529 participants in the Cerebrolysin group and 243/541 participants in the placebo group experienced adverse events (RR 1.02, 95% CI 0.89 to 1.16).
CERE‐LYSE‐1 2012 described the overall evaluation of safety, stating that 88% of Cerebrolysin‐treated participants and 97% of placebo‐treated participants reported at least one adverse event. We recalculated from this the outcome total number of people with adverse events: 53/60 participants in the Cerebrolysin group and 57/59 participants in the placebo group (RR 0.91, 95% CI 0.82 to 1.01).
Gharagozli 2017 reported 13 occurrences of AEs in 12 Cerebrolysin‐treated participants and 13 occurrences of AEs in 14 placebo‐treated participants. Adverse events included: nausea, vomiting, headache, confusion, insomnia, and anorexia. No AE was related to treatment. There was thus no evidence that treatment with Cerebrolysin affected the occurrence of AEs, with the RR between the two groups of 50 participants being 0.86 (95% CI 0.44 to 1.66).
In Ladurner 2005, the study authors reported the overall incidence of adverse events: 16.4% in the Cerebrolysin group and 10.3% in the placebo group. We recalculated from this the outcome total number of people with adverse events: 13/78 participants in the Cerebrolysin group and 7/68 participants in the placebo group (RR 1.62, 95% CI 0.69 to 3.82). The trial authors did not report on any adverse effects specifically associated with Cerebrolysin, for example hypersensitivity reactions.
Non‐death attrition
We included all six studies which provided numerical results for analysis of non‐death attrition (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005; Skvortsova 2004). Overall, 96 out of 764 Cerebrolysin‐treated participants and 117 out of 753 placebo‐treated participants were lost to follow‐up for reasons other than death (RR 0.97, 95% CI 0.45 to 2.06, 6 trials, 1517 participants, Analysis 1.6). Two studies reported no loss to follow‐up (no non‐death attrition) (Amiri Nikpour 2014; Skvortsova 2004). There were substantial differences amongst the trials grouped by Cerebrolysin dose regimen (subgroup differences: P = 0.006) and a significant level of heterogeneity (I2 = 76%).
1.6. Analysis.

Comparison 1: Cerebrolysin versus placebo, Outcome 6: Non‐death attrition
Two studies stand out in this analysis. In Ladurner 2005, we found a lower rate of non‐death attrition in the Cerebrolysin group, with 3 of 78 Cerebrolysin‐treated participants and 10 of 68 placebo‐treated participants being lost to follow‐up (RR 0.26, 95% CI 0.08 to 0.91, P = 0.04, Analysis 1.6). In contrast, in Gharagozli 2017 we found a higher rate of non‐death attrition in the Cerebrolysin group, with a loss of 16 out of 50 Cerebrolysin‐treated participants and 6 out of 50 placebo‐treated participants (RR 2.67, 95% CI 1.14 to 6.25, P = 0.02, Analysis 1.6).
Subgroup analysis and investigation of heterogeneity
We investigated potential sources of heterogeneity using the following subgroups of evaluated treatment regimens, which differ in Cerebrolysin dose and the length of treatment, for the outcomes all‐cause death, total number of people with adverse events, and total number of people with non‐fatal SAEs.
Cerebrolysin dose 30 mL for 10 days: cumulative dose 300 mL over 10 days (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Xue 2016). Xue 2016 did not contribute data to the quantitative analyses.
Cerebrolysin dose 50 mL for 21 days: cumulative dose 1050 mL over 21 days (Ladurner 2005).
Cerebrolysin dose 30 mL for seven days then 10 mL five days per week for three weeks: cumulative dose 360 mL over 28 days (Gharagozli 2017).
Cerebrolysin dose 10 mL or 50 mL for 10 days: cumulative dose 100 mL or 500 mL over 10 days (Skvortsova 2004).
For the outcomes all‐cause death, total number of people with SAEs, and total number of people with fatal SAEs, we found no heterogeneity between the subgroups: I2 = 0 in each case (Analysis 1.1, Analysis 1.2, Analysis 1.3).
Although there was no statistical indication of subgroup differences for the outcome total number of people with non‐fatal SAEs (test for subgroup differences: P = 0.28), we observed opposing directions of effect estimates in Subgroup 1 (Cerebrolysin dose 30 mL for 10 days: cumulative dose 300 mL over 10 days; CASTA 2012; CERE‐LYSE‐1 2012), versus Subgroup 2 (Cerebrolysin dose 50 mL for 21 days: cumulative dose 1050 mL over 21 days; Ladurner 2005), and low heterogeneity (I2 = 22.1% ) (Analysis 1.4). In Subgroup 1 (the second lowest dose amongst all tested doses in the included trials), we found a nearly threefold increase in the incidence of non‐fatal SAEs (RR 2.86, 95% CI 1.23 to 6.66, P = 0.01, I2 = 0). In Subgroup 2 (the highest dose, cumulatively more than three times that of Subgroup 1), the RR was 0.29 with the large range of the confidence intervals (95% CI 0.01 to 7.03, P = 0.45).
We found low to moderate heterogeneity (I2 = 37%) for the outcome total number of people with adverse events amongst the three subgroups of Cerebrolysin dose regimen (30 mL for 10 days; 50 mL for 21 days; 30 mL for seven days then 10 mL five days per week for three weeks). It was suggested that the highest dose (cumulatively 1050 mL of Cerebrolysin) and the 21‐day duration might be associated with a higher risk of adverse events, but with an RR of 1.62 (95% CI of 0.69 to 3.82), this did not achieve conventional levels of statistical significance (P = 0.27, Analysis 1.5). Equally, in the longer‐duration study of Gharagozli 2017 (cumulative dose of 360 mL over 28 days), we did not find evidence that Cerebrolysin affected the outcome total number of people with adverse events (RR 0.86, 95% CI 0.44 to 1.66, P = 0.65, Analysis 1.5).
Heterogeneity was high in the analysis of the outcome non‐death attrition, with I2 = 76% (Analysis 1.6). No one study affects this heterogeneity substantially, and even removing the two outermost groups from the analysis, Ladurner 2005 and Gharagozli 2017, there is little change of note in either the heterogeneity (which becomes moderate, I2 = 47%) or the effect of Cerebrolysin (which remains not statistically significant) (RR 0.78, 95% CI 0.59 to 1.02, P = 0.07, data not shown).
Sensitivity analyses
We did not perform any of our prespecified sensitivity analyses because our judgements of risk of bias both across studies and across 'Risk of bias' domains were low, unclear, and high. We were thus unable to identify studies being at overall high risk of bias.
We could not use funnel plots to examine asymmetry and small‐study effects because there were only seven included studies, with six at most contributing data to the quantitative analysis for any given outcome.
The use of either a fixed‐effect or random‐effects model made no difference to whether or not a result was statistically significant in all the analyses, although it might have made a difference to the level of probability.
Discussion
The World Health Organization (WHO) collection of National Essential Medicines List (EMLs) includes the latest acting country editions that recommend Cerebrolysin for treating various neurological conditions including acute ischaemic stroke. These include the National EMLs of the Russian Federation (GovRu 2019), Slovakia, Vietnam, and Syrian Arab Republic (WHO 2019b). However, the potential benefits of Cerebrolysin for improving clinical outcomes in people with acute ischaemic stroke and the risks of its use have not been systematically evaluated on the basis of research synthesis of RCTs of acceptable quality. In this Cochrane Review we have assessed the benefits and harms of Cerebrolysin when added to standard treatment for acute ischaemic stroke, focusing on clinically relevant and widely accepted outcomes, and specifically excluding assessment methods with numerous varying scales.
Summary of main results
Seven RCTs involving 1601 participants met our inclusion criteria. Six studies contributed to the quantitative analyses.
None of the seven included trials provided sufficient evidence of the effects of Cerebrolysin on clinically relevant outcome measures for acute ischaemic stroke such as poor functional outcome (death or dependence by the end of follow‐up period) and early death (within two weeks of stroke onset), or time to restoration of capacity for work and quality of life.
In this review update we again confirmed with moderate‐quality evidence that Cerebrolysin does not substantially alter the risk of death (Analysis 1.1).
The authors of only one of the included trials described the causes of death which could potentially be used for analysis (Ladurner 2005): cerebral infarct (four in the Cerebrolysin group and two in the placebo group), heart failure (two in the Cerebrolysin group and one in the placebo group), pulmonary embolism (two in the placebo group), and pneumonia (one in the placebo group), with 6/78 deaths in the Cerebrolysin group and 6/68 deaths in the placebo group, though not reporting the time of death. Owing to the lack of evidence from other trials we could not synthesise these data.
We confirmed with moderate‐quality evidence that Cerebrolysin probably makes little to no difference to fatal SAEs (Analysis 1.3) and total SAEs (Analysis 1.2). We also found moderate‐quality evidence that Cerebrolysin likely increases the number of participants with non‐fatal SAEs (Analysis 1.4).
By subgrouping two studies with the same dosing schedule (30 mL for 10 days), which contributed data on adverse events and were multicentre (CASTA 2012; CERE‐LYSE‐1 2012), we confirmed an almost threefold increase in the incidence of non‐fatal SAEs in participants treated with Cerebrolysin, with the resulting number needed to harm (NNH) of 45, which means that in every 45 acute ischaemic stroke patients treated with Cerebrolysin, one will experience a non‐fatal SAE (Table 1, moderate‐quality evidence).
For the total number of people with adverse events, we did not find a statistically significant difference between the Cerebrolysin and placebo groups, but identified moderate levels of heterogeneity amongst the three trials contributing to this outcome (Analysis 1.5). Likewise, for the outcome non‐death attrition, we did not find a statistically significant difference between comparison groups. However, two studies stand out in this analysis with a large discrepancy in risk ratios. In Ladurner 2005 we found a significantly lower rate of non‐death attrition in the Cerebrolysin group, but in contrast we found a significantly higher rate of non‐death attrition in the Cerebrolysin group in Gharagozli 2017 (Analysis 1.6). The main obvious difference between the two studies is that Ladurner 2005 used a much higher dose of Cerebrolysin, 50 mL for 21 days (cumulative dose 1050 mL), and Gharagozli 2017 used 30 mL for seven days and then 10 mL five days per week for three weeks (cumulative dose 360 mL).
Overall completeness and applicability of evidence
In this update we included one new study and one new secondary outcome (non‐death attrition), and followed the protocol in all respects. The new study evaluated participants with acute ischaemic stroke who had been assessed clinically, but not confirmed by neuroimaging (Gharagozli 2017).
The seven eligible studies, four of which were multicentre studies, were carried out in multiple clinical centres in Europe (seven countries): Austria, Croatia, the Czech Republic, Hungary, Russia, Slovakia, and Slovenia; and in Asia (five countries): China, Hong Kong, Iran, Myanmar, and South Korea. The participant populations were geographically diverse. The included studies were conducted in high‐, middle‐, and low‐income countries, which means the results of this Cochrane Review are likely to be applicable to settings where the burden of stroke and stroke deaths is high. Of particular importance is the fact that the results of this update are likely to be applicable to the settings of low‐income countries, where the burden of stroke deaths and disability is even higher (WHO 2019a), and poses a huge financial demand on health systems and society (Martynchik 2013), and where Cerebrolysin is in widespread use. The included studies tested various doses of Cerebrolysin (10 mL, 30 mL, and 50 mL), and treatment duration with Cerebrolysin varied from 10 days to four weeks. We did not find any clear evidence that Cerebrolysin improves clinical outcomes in acute ischaemic stroke with any of the tested treatment regimens. Treatment strategies for acute ischaemic stroke should be reviewed in light of this evidence.
Reporting of data on death and safety parameters without clarification of the time of death and the time of development of the adverse events, and the loss of data of many enrolled participants owing to attrition, hampered meaningful interpretation of these data. However, it is apparent that treatment with Cerebrolysin had no significant effect on the incidence of death, although it increased the incidence of non‐fatal SAEs.
None of the included studies reported on Cerebrolysin‐specific adverse events such as hypersensitivity or emotional disturbances, arousal and aggression or fatigue, tiredness and apathy or sleeplessness, convulsive preparedness, rise or fall in blood pressure, shortness of breath, flu‐like syndrome, or reactions on immediate intravenous administration like feelings of chills or heat, cold sweat, dizziness and tachycardia, or redness and itching at the site of administration, gastrointestinal disturbances, and others (Registry of Medicines 2019).
Quality of the evidence
We assessed the quality of the evidence using the GRADE approach (Guyatt 2008), and presented the results in Table 1. For this comparison we asked the question: should Cerebrolysin be used in acute ischaemic stroke to improve clinical outcomes?
Based on the six studies that contributed to quantitative analysis, there is no evidence that Cerebrolysin added to standard therapy reduces death in people with acute ischaemic stroke (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005; Skvortsova 2004). There is moderate‐quality evidence that Cerebrolysin performs no better or worse than placebo in preventing all‐cause death in people with acute ischaemic stroke if started within 48 hours of stroke onset and continued for 10 days to four weeks (Table 1).
The four studies that contributed to the outcomes total number of people with SAEs, total number of people with non‐fatal SAEs, and total number of people with adverse events, of which three were multicentre, which when synthesised totalled 1435 participants, would have had enough power to detect differences: 60 SAEs in the Cerebrolysin group (717 randomised participants) and 52 SAEs in the placebo group (718 randomised participants); 22 non‐fatal SAEs in the Cerebrolysin group (717 randomised participants) and 10 non‐fatal SAEs in the placebo group (718 randomised participants); 320 people with adverse events in the Cerebrolysin group (717 randomised participants) and 321 people with adverse events in the placebo group (718 randomised participants). Although the confidence intervals for Ladurner 2005 were wide and the direction of the effect was opposite, this did not result in statistical or important heterogeneity.
Four studies contributed to the outcome total number of people with non‐fatal SAEs, and there is moderate‐quality evidence that Cerebrolysin likely increases non‐fatal SAEs (but not total SAEs) in people with acute ischaemic stroke (Analysis 1.4).
Based on four studies, Cerebrolysin added to standard therapy of acute ischaemic stroke may be no different from placebo in the total number of people with adverse events (CASTA 2012; CERE‐LYSE‐1 2012; Ladurner 2005). There is low‐quality evidence that Cerebrolysin performs no better or worse than placebo in terms of the total number of people with adverse events (Table 1).
We assessed none of the trials as being at high risk of bias for all domains. For the majority of 'Risk of bias' domains, such as sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessors, we judged the risk of bias to be low or unclear. Selective outcome reporting was unclear for all seven eligible studies. Only high levels of exclusions from the final analyses caused us to assess incomplete outcome reporting as at high risk of bias in four studies (CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Ladurner 2005), all of which were multicentre studies, as described in Assessment of risk of bias in included studies. We judged that these potential limitations were unlikely to lower confidence in the estimate of effect. One of the reasons for judging incomplete outcome reporting as at high risk of bias was the high rate of attrition (Table 2); however, despite these high rates of attrition, there were large numbers of participants remaining in the trials, and there were large numbers of effects reported by the study authors, both for all‐cause death and adverse events. Furthermore, we found no difference between comparison groups in the numbers of participants lost to follow‐up for reasons other than death, which we analysed as a newly added outcome of non‐death attrition, whilst there was a significant level of heterogeneity between the subgroups (Analysis 1.6).
Potential biases in the review process
We performed the data extraction unblinded.
The included trials are published, and we obtained unpublished data on SAEs through feedback received from the manufacturer of Cerebrolysin, EVER Neuro Pharma GmbH (formerly EBEWE Pharma). We were unable to obtain sufficient data on important outcomes such as death or dependency.
Agreements and disagreements with other studies or reviews
We asked whether Cerebrolysin has a role in improving the treatment outcomes for people diagnosed with acute ischaemic stroke. The original version of this review did not provide evidence that Cerebrolysin was effective (Ziganshina 2010a), and none of the updates since then have shown that Cerebrolysin is effective (Ziganshina 2013; Ziganshina 2015; Ziganshina 2016; Ziganshina 2017).
These unfavourable results cautioned against widespread use of Cerebrolysin and its inclusion on national EMLs in Russia (GovRu 2019), Ukraine, Slovakia, Vietnam, and the Syrian Arab Republic (WHO 2019b). As new research data have accumulated, we have updated the review several times since 2010, performing new literature searches. The conclusions of the last version of this review, Ziganshina 2017, have remained largely unchanged in this update of the Cochrane Review.
In contrast to our findings is a recent meta‐analysis of nine trials, Bornstein 2018, which concluded that Cerebrolysin safety was comparable to placebo, and confirmed that Cerebrolysin has a beneficial effect on early global neurological deficits in people with acute ischaemic stroke. Six trials that Bornstein 2018 included in their meta‐analysis are included in this Cochrane Review (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Gharagozli 2017; Skvortsova 2004; Xue 2016). Of the three studies included in Bornstein 2018 that we excluded from our meta‐analysis, one was published as an abstract only (Guekht 2015b), and two did not meet our inclusion criteria (Muresanu 2016a; Shamalov 2010). The Ladurner 2005 trial, which we included in our review, was excluded from Bornstein 2018.
Commercial influences or risks of sponsored science
The most recent meta‐analysis published in Bornstein 2018 included a lengthy list of authors who have previously declared conflicts of interest relating to EVER Neuro Pharma, the manufacturer of Cerebrolysin. All of the studies included in the Bornstein 2018 meta‐analysis were supported either totally or partially by EVER Neuro Pharma, or did not provide any information on funding or disclosure. For specifics of the six overlapping trials, please see Characteristics of included studies.
Use of statistical instruments
The authors of the Bornstein 2018 meta‐analysis state: "Studies that did not provide outcome data or data usable for the meta‐analysis as well as studies that did not meet the inclusion criteria were excluded. Primary outcome measure for the meta‐analysis was the NIHSS. Efficacy was assessed at day 30 (or 21) with Last Observation Carried Forward (LOCF) replacement of missing values".
We would like to reiterate here that there is an inherent bias in the 'last observation carried forward' method, and its use is deprecated (Lachin 2016; Molnar 2008; Salim 2008), as we mentioned in Risk of bias in included studies.
The authors of the Bornstein 2018 meta‐analysis, and those of several of the studies with involvement of the same author team members, used the Wilcoxon‐Mann‐Whitney test. They state that it is the preferred analysis method if the outcome variables are not continuous or might have skewed distributions or outliers. The authors do not examine the distribution of the populations. Given the sizes of the populations under study here in the meta‐analysis, a t‐test should be preferable. It is well known that the Wilcoxon test is more powerful than a t‐test under certain conditions, but it can also yield a significant result when the t‐test does not (Lumley 2002). The author team members state in CASTA 2012: "The effect size measure associated with the Wilcoxon‐Mann‐Whitney test is the Mann Whitney statistic (MW). It defines the probability that a randomly selected patient of the treatment group is better off than a randomly chosen patient from the reference group. The following benchmark values hold for the test group under fairly general conditions: 0.50 = equality, 0.56 = small superiority, 0.64 = medium‐sized/relevant superiority, 0.71 = large superiority".
Whilst the Mann‐Whitney statistic of 0.5 represents complete overlap of the data, and values of 0 and 1 represent complete non‐overlap one way or the other, these benchmarks are arbitrary, and the authors do not define the terms "small superiority", "medium‐sized superiority", or "large superiority". The use of the word 'superiority' shows prejudice: 'difference' would be more neutral and more accurate.
In addition to contrasting with our results, the meta‐analysis of Bornstein 2018 is in contrast to another recent meta‐analysis that showed lack of benefit from Cerebrolysin treatment for ischaemic stroke compared to placebo for functional recovery at day 90 (Wang 2017). Of the six studies included in Wang 2017, four overlapped with those included in this Cochrane Review (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Ladurner 2005). Wang 2017 also included studies that we have excluded owing to dealing with different research questions and not meeting our eligibility criteria (CARS study ‐ Chang 2016; Muresanu 2016a).
Authors' conclusions
Implications for practice.
This review indicates that Cerebrolysin probably results in little to no difference in death after acute ischaemic stroke. We found moderate‐quality evidence that Cerebrolysin probably does not increase the total number of people with serious adverse events, but there was a possible increase in total number of people with non‐fatal serious adverse events with Cerebrolysin use.
Implications for research.
Future research should focus on systematic reviewing of the harms of Cerebrolysin in acute ischaemic stroke and in its potential other uses.
We advocate for no more trials with Cerebrolysin on the grounds that it would be unethical to recruit patients into a study that offers no potential benefit.
Feedback
Response from authors of the Bornstein (2018) meta‐analysis, July 2020
Summary
Dear colleagues
As the first author of a meta‐analysis that is very much in the highlight of this review (Bornstein 2018), I feel it is appropriate to offer Cochrane readership the opportunity to understand our point of view on serious matters that are being raised by Ziganshina et al (2020), on behalf of the authors of our manuscript.
1. Related citation
"The most recent meta‐analysis published in Bornstein 2018 included a lengthy list of authors who had potential conflicts of interest due to their involvement with EVER Neuro Pharma, the manufacturer of Cerebrolysin. All of the studies included in the Bornstein 2018 meta‐analysis were supported either totally or partially by EVER Neuro Pharma, or did not provide any information on funding or disclosure."
Our commentary
• We kindly ask the authors of this review to consider revisiting these statements, as they imply that all authors of our cited meta‐analysis have had financial involvement with EVER Neuro Pharma, and that all studies included in the meta‐analysis have received support from this company. None of the above are true.
• None of the authors of the review group received any honoraria for their participation in this meta‐analysis. Three authors were coordinating investigators of included double‐blind randomized controlled trials. Since the meta‐analysis was based on Individual Patient Data (IPD) of these studies, which is regarded as the ‘gold standard’ for the meta‐analytic approach [1], collaboration is natural for obtaining proper access to data. We refer to the appreciation of the Cochrane Collaboration Methods Group on IPD meta‐analyses [2]: “IPD meta‐analyses can improve the quality of the data and the type of analyses that can be done and produce more reliable results. For this reason, they are considered to be a ‘gold standard’ of systematic questions, which might not have been obtained from summary data.” Similar acknowledgment is provided in the Cochrane Handbook for Systematic Reviews of Interventions [3]: “The IPD approach can bring substantial improvements to the quality of data available and offset inadequate reporting of individual studies. Risk of bias can be assessed more thoroughly and IPD enables more detailed and flexible analysis than is possible in systematic reviews of aggregate data.” We consider it problematic to conduct research in any field without collaborating with individuals with hands‐on experience with the topic at hand.
• For the methodological part of this large‐scale review, two internationally renowned biostatisticians with great methodological experience were included in the review group. Prof. Johannes C. Vester, President of the World Academy for Multidisciplinary Neurotraumatology and a highly experienced methodologist, is requested for more than three decades by multiple international organizations and regulatory authorities. Dr. Volker W. Rahlfs, the founder of IDV, the oldest German biometric institution (1967), chairman of the IDV Methodology Group, author of more than 150 methodological publications, Certificate ‘Biometry in Medicine’, Member of the Royal Statistical Society, routinely provides consultancy for numerous regulatory and academic institutions.
• Professors Volker Hömberg (Secretary General of the World Federation for Neurorehabilitation, Dafin Muresanu (President of the European Federation of Neurorehabilitaiton), and myself, are highly committed clinicians and research scientists, dedicating their lifework to progress in stroke and neurorehabilitation.
• Regarding industry involvement in clinical trials, we provide the example of the study with the strongest effect size of all included trials, performed by Xue et al. (2016). As per manuscript acknowledgments, this study was supported by the Shanghai Jiao Tong University Affiliated Sixth People's Hospital (grant nos. 1462 and 1583) and the Shanghai Science and Technology Council (grant no. 13411951401), with no contribution at all from EVER Neuro Pharma. The same applies to the study performed by Amiri‐Nikpour (2014) ‐ the study was supported by a grant from the Urmia University of Medical Sciences.
We, therefore, feel that inaccurate assertions by Ziganshina et al bring unjust prejudice to the image and impact of our research group.
References
1. Thomas D, Radji A, Benedetti, A ‐ Systematic review of methods for individual patient data meta‐analysis with binary outcomes. BMC Med Res Methodol. 2014;14:79.
2. https://methods.cochrane.org/ipdma/about‐ipd‐meta‐analyses
3. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
2. Related citation
"In addition to contrasting with our results, the meta‐analysis of Bornstein 2018 is in contrast to another recent meta‐analysis that showed lack of benefit from Cerebrolysin treatment for ischaemic stroke compared to placebo for functional recovery at day 90 (Wang 2017). Of the six studies included in Wang 2017, four overlapped with those included in this Cochrane Review (Amiri Nikpour 2014; CASTA 2012; CERE‐LYSE‐1 2012; Ladurner 2005). Wang 2017 also included studies that we have excluded owing to dealing with different research questions and not meeting our eligibility criteria (CARS study ‐ Chang 2016; Muresanu 2016a)."
Our commentary
• This paragraph is an example of a double standard logical fallacy, namely that both similarity (i.e. four overlapped studies) and difference (i.e. excluded studies) are used to offend the meta‐analysis (Bornstein, 2018). What Ziganshina et al. do not mention is that in reality, neither comparison is appropriate, owing to totally different approaches in terms of research questions, outcomes selection, and statistical analysis.
• To note that the meta‐analyses of Ziganshina (2020) evaluate beneficial effects using mortality as the primary outcome. The overall death rate in the included studies was 6%, thus any group differences are hardly expected, except with very large sample sizes. The status of the 94% survivors is completely overlooked in this review. On the contrary, our meta‐analysis focused predominantly on neurological function, thus, addressing especially beneficial effects for survivors. Two completely different approaches, which cannot be used as a scientific rationale against our meta‐analysis.
• In our work, we provide clear references to support our methodological choices. For the sake of diversity of opinion, and increased objectiveness that would be appropriate for an organization such as the Cochrane collaboration, we invite authors to also reference positive articles that have been published on the topic, such as this systematic review developed by an independent group of researchers from Australia, Canada, and Sweden: https://www.medicaljournals.se/jrm/content/html/10.2340/16501977‐2536.
We suggest that the implications and interpretation of the above‐mentioned statements are clarified, in order to enhance the quality of this material.
Conclusion
The review’s comments related to applied methodology will be addressed separately by the review group. Arguments presented by Ziganshina et al. (2020) against our meta‐analysis build a conspiratorial narrative that includes severe allegations of scientific misconduct. We hope for constructive dialogue of objective scientific matters. On subjectively approached issues, such as those related to conflict of interest, we expect authors to nuance the manuscript’s language, or to remove inappropriate passages that are not based on any evidence.
Reply
Dear Professor Bornstein
Thank you for your interest in our Cochrane Review 'Cerebrolysin for acute ischaemic stroke'.
There is no 'Disclosure' statement in the published paper Bornstein 2018, yet all the authors are known to be involved with or have been involved with EVER Neuro Pharma, the manufacturer of Cerebrolysin.
Here is the summary, which we prepared from published studies on Cerebrolysin in stroke. For direct citations and specifics of overlapping trials, please see the Characteristics of included studies table of our Cochrane Review:
Dr Bornstein has been a consultant for EVER Neuro Pharma and has received honoraria for this activity. He was also active in the speaker’s bureau of EVER Neuro Pharma;
Alla Guekht is a principal investigator of the CARS2 trial. Reports receipt of grants/research support from EVER Neuro Pharma;
Johannes C. Vester has been a senior biometric consultant of IDV (Advisory Board for EVER Neuro Pharma);
Wolf Dieter Heiss has served on the Advisory Board and Speakers bureau for EVER Neuro Pharma;
Eugene Gusev was a CARS 2 investigator;
Volker Homberg has been a member of the CAPTAIN trial scientific advisory board;
Volker Rahlfs has been an employee of IDV. Consultant for EVER Neuro Pharma and has received honoraria for this activity
Ovidiu Bajenaru was a principal investigator of the CARS trial. Reports a receipt of grants/research support from EVER Neuro Pharma;
Bogdan Popescu was a principal investigator of the CARS trial; worked for Ebewe/Ever Neuro Pharma‐clinical studies 2008–2012, received Ebewe/Ever Neuropharma–speaker fees 2008–2014;
Dafin Fior Muresanu was a coordinating investigator of the Cerebrolysin and Recovery After Stroke (CARS) trial and a member of the Cerebrolysin Asian Pacific Trial in Acute Brain Injury and Neurorecovery (CAPTAIN) trial scientific advisory board. Reports receipt of grants/research supports from EVER Neuro Pharma.
To clearer reflect this we have amended the description in the review to read:
“The most recent meta‐analysis published in Bornstein 2018 included a lengthy list of authors who have previously declared conflicts of interest relating to EVER Neuro Pharma, the manufacturer of Cerebrolysin. All of the studies included in the Bornstein 2018 meta‐analysis were supported either totally or partially by EVER Neuro Pharma, or did not provide any information on funding or disclosure. For specifics of the six overlapping trials, please see Characteristics of included studies.”
In the citation you look at, we say at the very end: “ ... or did not provide any information on funding or disclosure."
Xue 2016: no disclosure or conflict of interest statement.
Amiri‐Nikpour 2014: we judged the information on funding to be unclear. There was no information on sources of study drug or placebo. Added to the unavailability of the study protocol, we judged this information to be missing.
We support all our judgements to make the reviewing process fully transparent as per MECIR standards R52‐55 (mandatory).
We would like to draw attention to a concern about the reporting of the Bornstein 2018 paper and potential problems with study protocol registration:
The meta‐analysis does not have either a protocol, or an official registration. Instead the paper contains the section 2. Protocol and registration, reading:
"This meta‐analysis follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [3]. The nonparametric approach and the method of synthesis were operationalized under blinded conditions in the final statistical analysis plan of study CARS‐2 (2014). A separate review protocol has not been prepared for this meta‐analysis and the meta‐analysis has not been included in any study registry since the objective of this meta‐analysis was to verify the findings of the previously published meta‐analysis on early neurological benefit (CARS‐1, CARS‐2) [2], using identical methodology."
In addition to this, despite the statement in the Eligibility criteria of the Bornstein 2018 paper: "Eligible studies published as abstract only were not included in this meta‐analysis", the authors included in their meta‐analysis an abstract, Guekht 2015a, listed under Excluded studies in this Cochrane review.
Thus, we provide here clear explanations to our statements and contend that our statements and judgements are accurate and bring light to the image and impact of Cerebrolysin author team.
Contributors
Commentary submitted by Natan Bornstein, Professor of Neurology, Shaare Zedek Medical Center, Jerusalem, Israel
Response submitted by the authors of this Cochrane Review
Methodological commentary from authors of the Bornstein (2018) meta‐analysis, July 2020
Summary
Dear corresponding author
I hereby submit a commentary to the 'Cerebrolysin for acute ischaemic stroke' Cochrane Review.
Topic: Utilization of LOCF in the Bornstein (2018) meta‐analysis
Related citation from Ziganshina (2020)
“We would like to reiterate here that there is an inherent bias in the 'last observation carried forward' method, and its use is deprecated (Lachin 2016; Molnar 2008; Salim 2008), as we mentioned in Risk of bias in included studies.”
Commentary
• Ziganshina 2020 cites Lachin 20161 as reference for the LOCF criticism of Bornstein 2018, overlooking the critical warning by Lachin 2016 regarding ‘last observation carried forward’ (LOCF) method refers explicitly to a substantial fraction of missing data: “Regulatory agencies and journal editors (and reviewers) should be critical of any study with a substantial fraction of missing data” (Lachin 2016) [1]
• The rate of missing NIHSS values as compared to randomized subjects was below 10% in eight out of nine trials, thus well fulfilling the criteria for class I studies (American Academy of Neurology 2018 benchmark for class I studies [2]: <20%; to note: the 20% cutoff was suggested by David Sackett, OC, FRSC ‐ a pioneer of EBM [3]).
• In three studies, anyhow, only observed cases (OC) data were available for NIHSS evaluation[4]; the missing rates were well comparable between Cerebrolysin and placebo (7/67 vs. 8/66).
• For two[5] of the studies included in the meta‐analysis, sensitivity analysis comparing LOCF vs. observed case analysis (OC) was available on the associated primary efficacy criterion. It was shown that there was no indication for bias, results did well agree: “The OC result is well supporting the LOCF analysis (MWOC 0.62 with POC < 0.0001 vs. MWLOCF 0.62 with PLOCF < 0.0001)”[6].
• Thus, all in all, and in particular with respect to the very low dropout rate and a very low P‐value in the primary meta‐analysis (<0.0001), we do not see a rationale for rating down the level of evidence due to the non‐substantial fraction of LOCF imputations.
References
1. Lachin, JM, Fallacies of last observation carried forward analyses. SCT 2015. DOI 10.1177/1740774515602688
2. American Academy of Neurology (AAN), 2017 Edition Clinical Practice Guideline Process Manual
3. Sackett, DL, Rosenberg WMC, Muir Gray JA, Haynes RB, Richardson WS. Evidence‐based medicine. BMJ 1996;312:71
4. Skvortsova 2003, Shamalov 2010, Amiri‐Nikpour 2014
5. CARS‐1, CARS‐2
6. Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta‐analysis oft he CARS trials. Neurol Sci 2017. DOI 10.1007/s10072‐017‐3037‐z
Topic: Utilization of t‐test vs. Wilcoxon Test in the Bornstein (2018) meta‐analysis
Related citation from Ziganshina (2020)
"They state that it is the preferred analysis method if the outcome variables are not continuous or might have skewed distributions or outliers. The authors do not examine the distribution of the populations."
Commentary
• Non‐normality is not an assumption for the Wilcoxon‐test, it is the opposite: normal distribution is an assumption for the t‐test (as well as homogeneity of variances).
• Assumptions have to be checked for validity of the t‐test, not for the Wilcoxon test (which has a minimum of assumptions). To make a pre‐test on normality and then switch to the Wilcoxon test in case of non‐normality is not a recommended approach since the multiple level alpha is not preserved. The more, in the case of small sample sizes such pre‐tests are highly underpowered.
• Besides, statistically significant non‐normality of the distributions was formally demonstrated and reported as per manuscript of the included study CARS‐1 (Shapiro‐Wilk test of normality, P = 0.0137).
• See also LaVange 2005 [1] (2011‐2017 Director of the Office of Biostatistics, FDA): “methods with essentially no assumptions external to the study design are ideal. Nonparametric methods in general require minimal assumptions. In a regulator setting, the failure to meet assumption may cast doubt on the study results, even if the findings a are robust to that failure. Thus minimizing assumptions is a recommended approach.”
• Leading biostatisticians note, that rating scales or composite index values are by design ordinal scales and should be only evaluated using Wilcoxon test (see, e.g., Munzel 1998 [2]: “Hence, when analyzing data from … rating scales, statistics that are based on differences and means of scores are not appropriate”).
• The NIHSS outcome variable is not continuous (interval/ratio), it is an ordinal rating scale. Thus, also in this respect the Wilcoxon‐Mann‐Whitney test is the recommended approach, not the t‐test as recommended by Ziganshina 2020.
References
1. LaVange LM, Durham TA, Koch GG. Randomization‐based nonparametric methods for the analysis of multicentre trials. Statistical Methods in Medical Research 2005; 14: 281‐301.
2. Munzel U, Bandelow B. The use of parametric vs. nonparametric tests in the statistical evaluation of rating scales. Pharmacopsychiary 1998;31:222‐4.
Related citation from Ziganshina (2020)
"Given the size of the populations under study here in the meta‐analysis, a t‐test would be preferable."
Commentary
• This statement is highly misleading. For the inappropriateness of the t‐test see the previous comment. Else, it is a common misunderstanding that violation of assumptions can be neglected with higher sample sizes. See, e.g. LaVange (as cited above): “In a regulatory setting, the failure to meet assumption may cast doubt on the study results, even if the findings a are robust to that failure. Thus, minimizing assumptions is a recommended approach.”
• The size of the nine individual studies, on which the statistical test is applied, goes down to 16 vs 17 patients (MRI‐1). The majority of the studies has sample sizes below 50 per group. Thus, the t‐test with its various assumptions is not preferable “given the size of the populations under study”.
• In a particular meta‐analysis, there can be only one common effect size. Thus, even if one of the studies would verifiably meet the assumptions of the t‐test, the Wilcoxon test is still the preferable method for the ensemble of the trials.
Related citation from Ziganshina (2020)
"It is well known that the Wilcoxon test is more powerful than a t‐test under certain conditions, but it can also yield a significant result when the t‐test does not (Lumley 2002)."
Commentary
• This statement is correct only for special non‐normal distributions, where anyhow the t‐test is not appropriate. However, under the assumption of a normal distribution, the Wilcoxon test is not “more powerful” than a t‐test ‐ the opposite is true! The asymptotic relative efficiency (A.R.E.) of a Wilcoxon test is 3/π, which means that the Wilcoxon test has a power of 0.96 as compared to the t‐Test [1,2]. If there is no normal distribution, then the Wilcoxon test is anyhow the appropriate approach and preferable to the t‐test!
References
1. Hodges JL, Lehmann EL. The efficiency of some nonparametric competitors of the t‐test. Annals of Mathematical Statistics 1956;27:324‐35.
2. Lehmann EL. Parametric versus nonparametrics: two alternative methodologies, Journal of Nonparametric Statistics 2009;21:397‐405.
Topic: Alleged misuse of Mann‐Whitney benchmarks and interpretation in the Bornstein (2018) meta‐analysis
Related citation from Ziganshina (2020)
"The following benchmark values hold for the test group under fairly general conditions: 0.50 = equality, 0.56 = small superiority, 0.64 = medium‐sized/relevant superiority, 0.71 = large superiority". Whilst the Mann‐Whitney statistic of 0.5 represents complete overlap of the data, and values of 0 and 1 represent complete non‐overlap one way or the other, these benchmarks are arbitrary, and the authors do not define the terms "small superiority", "medium‐sized superiority", or "large superiority". The use of the word 'superiority' shows prejudice: 'difference' would be more neutral and more accurate."
Commentary
• Neither the above statement nor the citation is correct. The correct citation of the Mann‐Whitney benchmarks provided in Bornstein 2018 includes inferiority: “The traditional benchmarks for the MW effect size measure are [23, 24]: 0.29 = large inferiority, 0.36 = medium inferiority, 0.44 = small inferiority, 0.50 = equality, 0.56 = small superiority, 0.64 = medium superiority, 0.71 = large superiority.“
• The cited benchmarks are by no means “arbitrary”:
o Bornstein 2018 provides the key references for the benchmarks (Cohen 1988, Colditz 1988) [1,2].
o Under the assumption of a normal distribution the benchmarks can directly be converted to the standardized mean difference (SMD) and its associate benchmarks.
o A comprehensive overview of the transformation pathways of the Mann‐Whitney statistic to other well‐known effect size measures including the associated conversion formulas is provided by Rahlfs 2019 (Effect size measures and their benchmark values for quantifying benefit or risk of medicinal products) [3].
• The Mann‐Whitney effect size (MW) has been shown by many authors to be a gold standard for ordinal/rating scales, see, e.g., Munzel 1998 (see citation above). The use of the MW measure for obtaining a good measure of relevance in clinical research has been recommended for many years. We cite Brunner and Munzel [4], 2002, Colditz et al.2, 1988, Munzel and Hauschke [5], 2003, Newcombe [6], 2006, Wei and Lachin [7], 1984, and Wolfe and Hogg [8], 1971, among others.
• The importance of the Mann‐Whitney statistics for clinical research may be further highlighted by the fact that the leading biometric journal Statistics in Medicine dedicated a whole volume to the Mann‐Whitney Statistic on the occasion of the 25th Anniversary of the journal (d’Agostino, Campell, M., Greenhouse, J., (ed.) 2006, The Mann‐Whitney statistic: continuous use and discovery) [9]. For the use of the Mann‐Whitney approach in ordinal data analysis see also Rothmann 2012 [10] (Mark Rothmann, Director, Division of Biostatistics II, FDA).
References
1. Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Earlbaum Associates, Hilsdale.
2. Colditz GA, Miller JN, Mosteller F. Measuring gain in the evaluation of medical technology the probability of a better outcome. International Journal of Technology Assessment in Health Care 1988;4(4):637–42. https://doi. org/10.1017/S0266462300007728.
3. Rahlfs V, Zimmermann H. Effect size measures and their benchmark values for quantifying benefit or risk of medicinal products. Biometrica Journal 2019;61:973–82. https://doi.org/10.1002/bimj.201800107.
4. Brunner E, Munzel U. (2002). Nichtparametrische datenanalyse Berlin: Springer.
5. Munzel U, Hauschke D. (2003). A nonparametric test for proving noninferiority in clinical trials with ordered categorical data. Pharmaceutical Statistics 2003;2(1):31–7.
6. Newcombe RG. Confidence intervals for an effect size measure based on the Mann‐Whitney statistic. Part 1: General issues and tail‐area‐based methods. Statistics in Medicine 2006;25:543–57.
7. Wei LJ, Lachin JM. Two‐sample asymptotically distribution‐free tests for incomplete multivariate observations. Journal of the American Statistical Association 1984;79: 653–61.
8. Wolfe D, Hogg R. On constructing statistics and reporting data. The American Statistician 1971;25:27–30.
9. D'Agostino RB, Campbell M, Greenhouse J. The Mann‐Whitney statistic: Continuous use and discovery (special papers for the 25th anniversary of Statistics in Medicine). Statistics in Medicine 2006;25:541–2.
10. Rothmann MD, Wiens, BL, Chan, ISF. Design and Analysis of Non‐Inferiority Trials 1st Edition. Chapman and Hall/CRC 2011.
Conclusion
The conclusions of Ziganshina et al (2020) on Bornstein (2018) are not valid since they are based on incorrect or incomplete biometric statements and citations. We consider it mandatory to correct or remove the commented passages.
Reply
Dear Dr Rahlfs
Thank you for your interest in our Cochrane Review 'Cerebrolysin for acute ischaemic stroke'.
Re: Topic: Utilization of LOCF in the Bornstein (2018) meta‐analysis
In the Discussion section (subsection 'Agreements and disagreements with other studies or reviews'/'Use of statistical instruments') of our Cochrane review, from which you extracted our sentence, we do not rate levels of evidence in the Bornstein 2018 paper, we just very briefly describe this work and comment on the appropriateness of the use of LOCF method. In this discussion we do not go into individual trial appraisal. For this, please refer to the relevant sections of our Cochrane Review.
However, answering your query, we would like to present the full citation from Lachin 2016 and draw your attention to the last part of it, unfortunately omitted from your citation:
“Regulatory agencies and journal editors (and reviewers) should be critical of any study with a substantial fraction of missing data, and should be highly skeptical of the veracity of any results and pursuant claims based on LOCF analyses.”
Furthermore, Lachin 2016 concludes:
“In summary, the well‐known statistical properties of the mixture of two distributions are employed to demonstrate that LOCF analyses can introduce a positive or negative bias that can grossly inflate or deflate, respectively, the probability of a statistically significant test result under either the null or alternative hypothesis. Accordingly, without exception, all analyses using LOCF are suspect and should be dismissed. Statistically, last observation carried forward is specious (def: appearing to be true but actually false).”
Therefore, we confirm that all we said in our Cochrane review on the use of LOCF method was referenced to authoritative sources.
Re: Topic: Utilization of t‐test vs. Wilcoxon Test in the Bornstein (2018) meta‐analysis
Re: Topic: Alleged misuse of Mann‐Whitney benchmarks and interpretation in the Bornstein (2018) meta‐analysis
These topics and comments deal with two paragraphs of the Discussion section ('Agreements and disagreements with other studies or reviews'/'Use of statistical instruments'). Separate sentences, taken out of context, lose coherence and should be read and comprehended together. Further we provide these two paragraphs in full:
“The authors of the Bornstein 2018 meta‐analysis, and those of several of the studies with involvement of the same author team members, used the Wilcoxon‐Mann‐Whitney test. They state that it is the preferred analysis method if the outcome variables are not continuous or might have skewed distributions or outliers. The authors do not report the distribution of the populations. Given the sizes of the populations under study here in the meta‐analysis, a t‐test should be preferable. It is well known that the Wilcoxon test is more powerful than a t‐test under certain conditions, but it can also yield a significant result when the t‐test does not (Lumley 2002). The authors of the meta‐analysis state: "The effect size measure associated with the Wilcoxon‐Mann‐Whitney test is the Mann Whitney statistic (MW). It defines the probability that a randomly selected patient of the treatment group is better off than a randomly chosen patient from the reference group. The following benchmark values hold for the test group under fairly general conditions: 0.50 = equality, 0.56 = small superiority, 0.64 = medium‐sized/relevant superiority, 0.71 = large superiority".
Whilst the Mann‐Whitney statistic of 0.5 represents complete overlap of the data, and values of 0 and 1 represent complete non‐overlap one way or the other, these benchmarks are arbitrary, and the authors do not define the terms "small superiority", "medium‐sized superiority", or "large superiority". The use of the word 'superiority' suggests a degree of prejudice: 'difference' would be more neutral and more accurate.”
Here we refer to the author team both of the Bornstein 2018 meta‐analysis and of several of the included studies through the entire section.
We apologise that we did not cite the CASTA 2012 trial report, which we had intended. We lost the citation in the review drafting process.
This part of the paragraph should read:
The author team members state in (CASTA 2012):
"The effect size measure associated with the Wilcoxon‐Mann‐Whitney test is the Mann Whitney statistic (MW). It defines the probability that a randomly selected patient of the treatment group is better off than a randomly chosen patient from the reference group. The following benchmark values hold for the test group under fairly general conditions: 0.50 = equality, 0.56 = small superiority, 0.64 = medium‐sized/relevant superiority, 0.71 = large superiority". This approach is also used in the Bornstein 2018 meta‐analysis.”
However, we confirm here that despite the misplaced citation the use of the word 'superiority' indeed shows prejudice: 'difference' would be more neutral and more accurate, including from the statistical point of view.
Here, we will not go into discussion of validity of t‐test versus Wilcoxon‐Mann‐Whitney test or vice versa, we think that the following citation from Lumley 2002 speaks for itself:
“The Wilcoxon test is widely known to be more powerful than the t‐test when the distribution of data in the two groups has long tails and has the same shape in each group but has been shifted in location. Conversely, it is less powerful than the t‐test when the groups differ in the number and magnitude of extreme outlying distributions, as recognized in EPA guidelines for testing for environmental contamination in soil (33). Although its power relative to other tests depends on the details of the null and alternative hypotheses, the Wilcoxon test always has the disadvantage that it does not test for equality in any easily described summary of the data. This is illustrated by the analysis of Rascati et al. (21) in comparing overall medical costs for asthmatics prescribed steroids compared with other treatments. Although the mean cost was lower in the steroid group, a Wilcoxon test reported significantly higher costs for that group. A related disadvantage is that it is not easy to construct confidence intervals that correspond to the Wilcoxon test.
The t‐test and least‐squares linear regression do not require any assumption of Normal distribution in sufficiently large samples. Previous simulations studies show that “sufficiently large” is often under 100, and even for our extremely nonNormal medical cost data it is less than 500. This means that in public health research, where samples are often substantially larger than this, the t‐test and the linear model are useful default tools for analyzing differences and trends in many types of data, not just those with Normal distributions. Formal statistical tests for Normality are especially undesirable as they will have low power in the small samples where the distribution matters and high power only in large samples where the distribution is unimportant.”
Therefore, we believe that all our statements and citations are valid.
We have introduced one more reference to the CASTA 2012 trial report published in Stroke, to make it explicitly clear what is the source of the citation coming from the same author team members.
Contributors
Commentary submitted by Volker Rahlfs, Chairman of the IDV Methodology Group, Methodology Group, IDV Data Analysis and Study Planning.
Response submitted by the authors of this Cochrane Review.
Issues with selection bias in Ziganshina (2020), July 2020
Summary
Dear corresponding author
In this message, I want to express my perspective as researcher involved in clinical trials excluded by this review, as well as coordinator of ongoing, similar level review initiatives on the same topic. My first inquiry is related to research question selection. Authors state they "compared Cerebrolysin added to standard treatment against either placebo or no treatment added to standard treatment, while acknowledging that standard treatment is not defined precisely and differs between studies".
The review lists among exclusion criteria (ineligible research question) the CARS trial, the study of Stan (2017), as well as other studies we believe contribute to describing the effect of Cerebrolysin in the acute ischemic stroke population. The research question of the excluded CARS study is similar to the research question of included studies (randomized, placebo‐controlled, double‐blind multicenter trial to investigate the effects of Cerebrolysin after acute ischemic stroke). While the primary criterion was improved motor function in the upper extremity (ARAT), other common stroke outcomes as NIHSS or mRS, were available. It may be regarded as critical and prone to selection bias that our study (Muresanu 2016a), a class I randomized, placebo‐controlled, double‐blind multicenter trial, well demonstrating statistically significant results on the primary efficacy criterion ARAT (P < 0.0001), as well on the NIHSS (P < 0.0000) and other stroke outcomes, was excluded from the review Ziganshina (2020). For evaluation of potential selection bias, we suggest to provide further details of exclusion for all trials.
On the same topic of standard treatment definition, in addition to important above‐mentioned issues with study inclusion (selection bias), a crucial distinction must be made based on whether patients benefit or not from neurorehabilitation programs, when conducting broad‐goal meta‐analyses. Some other clinical studies were excluded due to “ineligible question: neurorehabilitation”. We kindly ask for rationale for this exclusion criterion. As rehabilitation regimens are widely accepted as effective post‐stroke interventions, we cannot say that we are comparing the same intervention in the standalone (Cerebrolysin) vs. add‐on treatment (Cerebrolysin + neurorehabilitation) paradigms. In this case, the agent’s multimodal mechanism of action further expands discrepancies between these approaches beyond the added effect of physical therapy, as the intervention work both on its own to mitigate brain damage (i.e. neuroprotection for apoptosis/inflammation), but also to pharmacologically support existing efforts, enhancing neurorecovery. Therefore, differentiation of existing literature based these criteria should at least be attempted, to ensure both internal and exteral validity of the review. Neurorehabilitation is more and more the standard of care and it is incomprehensible why beneficial effects of a pharmacological treatment should be discarded due to the additional presence of neurorehabilitation. In contrary, neurorehabilitation might open the pathway for pharmacological mode of action. Rather studies without standard neurorehabilitation could be excluded or be part of a separate review question. We see here a major limitation of the overall review conclusions. The associated limitation should at least be very clearly expressed.
In addition, one would inquire what is the rationale for restricting the initiation of treatment in the first 48 hours after stroke onset? Since this is also an exclusion criterion for the positive CARS trial, I feel this important component was left completely unreferenced in the manuscript. While the timing window for treatment initiation only partly exceeds the chosen selection benchmark (benchmark Ziganshina 2020: 48h, excluded study Muresanu 2016a: treatment initiation 24h to 72 hours after stroke onset), the exclusion of a highly positive study, based on an arbitrarily window needs to be explicitly mentioned as a limitation related to risk of selection bias. We suggest to consider an acute phase initiation window (within a week of stroke onset), based on professor Julie Bernhardt’s paper "Agreed definitions and a shared vision for new standards in stroke recovery research: The Stroke Recovery and Rehabilitation Roundtable taskforce".
The general impression upon reading this update is that your team went above and beyond to explore comparators, as well as a selection of absent indicators, such as quality of life, while excluding a wealth of information from studies that were not eligible for your research question. Since there a quite a few papers in this situation, I would argue that at least a subgroup/sensitivity analysis should address existing evidence.
Conclusion
Given the all the large number of restrictions this review has applied – (1) unreasonably restricted initiation window, (2) harsh exclusion of trials with slightly difference explicit, but identical implicit research questions, (3) decision not to analyze a wide range of outcome scales and existing evidence – I feel that the review’s summary translation of findings into lay language does not do justice to published literature regarding Cerebrolysin’s potential to improve outcome after acute ischemic stroke. The paper draws broad, overarching conclusions about the agent, but does not analyze all available information, nor does it suggest that an expanded approach is warranted.
Reply
Dear Dr Dafin Muresanu
Thank you for your interest in our Cochrane Review 'Cerebrolysin for acute ischaemic stroke'.
We would like to assure you that since 2008, when the protocol for this Cochrane Review had been first published, the understanding in the academic community of 'acute stroke' has not changed, nor has our eligibility criteria for participants with acute stroke:
“People with acute ischemic stroke, irrespective of age, gender, or social status, whose symptom onset was less than 48 hours previously.” (citing the Protocol of 2008, Types of participants).
“Study medication must have been started within 48 hours of stroke onset and must have been continued for at least two weeks.” (citing the Protocol of 2008, Types of interventions).
These remain the same through the last 12 years and six versions (one protocol, the first published review, and four subsequent updates):
“People with acute ischaemic stroke, irrespective of age, sex, or social status, whose symptom onset was less than 48 hours previously.” (citing the Review, latest version 2020, Types of participants).
“Study medication must have been started within 48 hours of onset of stroke and continued for any period of time.” (citing the Review, latest version 2020, Types of interventions).
The Cochrane review title is: Cerebrolysin for acute ischaemic stroke
The review objective is: “To assess the benefits and harms of Cerebrolysin for treating acute ischaemic stroke.”
The title of the paper Muresanu 2016a is: Cerebrolysin and Recovery After Stroke (CARS): a randomized, placebo‐controlled, double‐blind, multicenter trial
The Muresanu 2016a objective: “The purpose of this Cerebrolysin and Recovery After Stroke (CARS) trial was to analyze the efficacy and safety of Cerebrolysin during recovery after stroke.”
The Muresanu 2016a intervention is described as follows:
“The study medication was administered once daily for 21 days as an intravenous infusion for 20 minutes, beginning at 24 to 72 hours after stroke onset. In previous studies, drug dosages from 10 to 50 mL per day were used, and the treatment periods ranged from 10 to 30 days, with once‐daily infusions of Cerebrolysin.15–28,31Each patient included in our study participated in an accompanying standardized rehabilitation program for 21 days, beginning within 48 to 72 hours after stroke onset (5 d/wk for 2h/d). This pro‐gram included massages and passive and active movements of the upper and lower limbs.”
Hence our reason for exclusion of this trial still stands: Ineligible question and ineligible timing of cerebrolysin initiation after stroke onset
This is in full compliance with Cochrane's mandatory MECIR standards on eligibility criteria, which are described: “Predefined, unambiguous eligibility criteria are a fundamental prerequisite for a systematic review”.
MECIR stanard C2: Predefining objectives
“Define in advance the objectives of the review, including participants, interventions, comparators and outcomes (PICO).”
Thus, we regret to inform you that the study Muresanu 2016a, reported in two publications:
Muresanu D, Heiss WD, Bajenaru O, Popescu CD, Vester J, Guekht A. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo‐controlled, double‐blind, multicenter, phase II clinical study. International Journal of Stroke 2015;10 Suppl 2:92.
Muresanu DF, Heiss WD, Hoemberg V, Bajenaru O, Popescu CD, Vester JC, et al. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo‐controlled, double‐blind, multicenter trial. Stroke 2016;47(1):151‐9.
is indeed truly ineligible for this Cochrane review: Cerebrolysin for acute ischaemic stroke
Contributors
Commentary submitted by Dafin Muresanu, Chairman of the Department of Neurosciences, Iuliu Hatieganu University of Medicine and Pharmacy, Cluj‐Napoca, Romania
Response submitted by the authors of this Cochrane Review
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2020 | Feedback has been incorporated | In response to feedback we added a reference to CASTA 2012 for a citation in the 'Discussion', 'Agreements and disagreements with other studies or reviews', and 'Use of statistical instruments' sections of the review, and edited the first sentence of subsection 'Commercial influences or risks of sponsored science'. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 13 November 2019 | New search has been performed | Searches updated. Background section revised and updated and new references added. Searches updated, PRISMA diagram updated. We identified one new study; the review now has seven included studies involving 1601 participants. We edited and updated the text, 'Risk of bias' tables, and a 'Summary of findings' table. |
| 13 November 2019 | New citation required but conclusions have not changed | The conclusions have not changed. New author added. |
| 11 April 2017 | Amended | In response to feedback, we refined the outcome serious adverse events (SAEs) and replaced it with: total number of people with SAEs; total number of people with fatal SAEs; and total number of people with non‐fatal SAEs. |
| 11 April 2017 | New citation required and conclusions have changed | Conclusions changed. |
| 27 May 2016 | New citation required and conclusions have changed | The conclusions of the review have changed. |
| 27 May 2016 | New search has been performed | We refined the inclusion criteria to allow the inclusion of trials where the length of cerebrolysin use was not restricted to 14 days (any length of use). We performed a new search and included five new trials. The review now has six included studies involving 1501 participants. Ludivine Vernay joined the author team. We used Covidence for managing records, papers, and trials, to extract data and assess risk of bias, and to resolve conflicting opinions of the authors. We refined the conclusions. |
| 27 January 2015 | New citation required but conclusions have not changed | We performed a new search. The conclusions have not changed. |
| 15 July 2008 | Amended | Converted to new review format. |
Acknowledgements
This Cochrane Review was developed with support from the Cochrane Stroke Group.
We thank Peter Sandercock, Hazel Fraser, and referees from the Cochrane Stroke Group for their assistance in the development of the Cochrane Review protocol and the original Cochrane Review (Ziganshina 2010a). We thank Hazel Fraser, Daniel Bereczki, Valentina Assi, Brenda Thomas, and Peter Langhorne for their expert advice, helpful comments, suggestions, and assistance with the previous review update. We thank Brenda Thomas, the former Cochrane Stroke Group Information Specialist, for her assistance with the searches for the protocol and the earlier versions of this Cochrane Review and the first study flow diagram (Ziganshina 2015). We thank Joshua David Cheyne, the Information Specialist of the Cochrane Stroke Group, for his assistance with the searches for the last two updates, and the staff of the Scientific Library of the Kazan Federal University named after NI Lobachevsky for assistance with the Russian language databases and papers. We thank Peter Langhorne, Hazel Fraser, and Valentina Assi from Cochrane Stroke Group, and David Tovey, Toby Lasserson, and Kerry Dwan from the Cochrane Editorial Unit for their expert advice, support, and guidance through the revision of the review in response to manufacturer clarification of data on serious adverse events in 2016‐17. We thank Ludivine Vernay, who stepped down as an author. We thank the members of Cochrane Stroke Review Group Editorial Base and Board, Peter Langhorne, Hazel Fraser, Aryelly Rodriguez, Joshua Cheyne, and Daniel Bereczki for their expert advice, extremely helpful comments, suggestions, and support with the current review update. Our very special thanks go to Peter Langhorne, Co‐ordinating Editor of Cochrane Stroke and the lead editor for the current update.
Appendices
Appendix 1. CENTRAL (the Cochrane Library) search strategy
ID SearchHits #1 [mh ^"cerebrovascular disorders"] or [mh ^"basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh ^"carotid artery diseases"] or [mh ^"carotid artery thrombosis"] or [mh ^"carotid artery, internal, dissection"] or [mh ^"stroke, lacunar"] or [mh ^"intracranial arterial diseases"] or [mh ^"cerebral arterial diseases"] or [mh ^"infarction, anterior cerebral artery"] or [mh ^"infarction, middle cerebral artery"] or [mh ^"infarction, posterior cerebral artery"] or [mh "intracranial embolism and thrombosis"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vertebral artery dissection"] #2 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw #3 (isch*emi* near/6 (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva or attack*)):ti,ab,kw #4 #1 or #2 or #3 #5 (cerebrolysin* or CERE or "FPF‐1070" or FPF1070 or "FPF 1070" or "FPF 10‐70"):ti,ab,kw #6 #4 and #5
Appendix 2. LILACS search strategy
cerebrolysin or CERE or FPF‐1070 or FPF1070 or cortexin or CORT or N‐PEP‐12F
Appendix 3. OpenGrey search strategy
cerebrolysin or CERE or FPF‐1070 or FPF1070 or cortexin or CORT or N‐PEP‐12F
Appendix 4. Russian databases search strategy
#1. инсульт or цереброваск* or церебральн* or цвб*
#2. церебролизин or ЦЕРЕ or кортексин or КОРТ
#3. #1 and #2
Appendix 5. US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov
Cerebrolysin AND ( ischaemic stroke OR brain infarction OR brain ischemia OR carotid artery obstruction OR cerebral ischemia ) [DISEASE]
Appendix 6. World Health Organization International Clinical Trials Registry Platform
Trial search
Basic search: cerebrolysin Phases are: ALL
Appendix 7. Retraction Watch Database
Retraction Watch
Retraction Watch Search database
Basic search: cerebrolysin
Appendix 8. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or carotid artery diseases/ or carotid artery thrombosis/ or carotid artery, internal, dissection/ or stroke, lacunar/ or intracranial arterial diseases/ or cerebral arterial diseases/ or infarction, anterior cerebral artery/ or infarction, middle cerebral artery/ or infarction, posterior cerebral artery/ or exp "intracranial embolism and thrombosis"/ or stroke/ or exp brain infarction/ or vertebral artery dissection/
2. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
3. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw.
4. 1 or 2 or 3
5. (cerebrolysin$ or CERE or FPF‐1070 or FPF1070 or FPF 1070 or FPF 10‐70).tw.
6. 4 and 5
7. exp animals/ not humans.sh.
8. 6 not 7
Appendix 9. Embase (Ovid) search strategy
1. cerebrovascular disease/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or exp brain ischemia/ or carotid artery disease/ or exp carotid artery obstruction/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp occlusive cerebrovascular disease/ or stroke patient/ 2. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 3. (isch?emi$ adj6 (stroke$ or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or attack$)).tw. 4. 1 or 2 or 3 5. cerebrolysin/ 6. (cerebrolysin$ or CERE or FPF‐1070 or FPF1070 or FPF 1070 or FPF 10‐70).tw. 7. 5 or 6 8. 4 and 7 9. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/) 10. 8 not 9
Appendix 10. Web of Science Core Collection search strategy
#1. TOPIC: (stroke* or apoplex* or cerebral vasc* or cerebrovasc* or cva) #2. TOPIC: (cerebrolysin*) #3. #2 AND #1
Data and analyses
Comparison 1. Cerebrolysin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause death | 6 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.61, 1.32] |
| 1.1.1 Cerebrolysin dose: 30 mL for 10 days | 3 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.39] |
| 1.1.2 Cerebrolysin dose: 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.29, 2.58] |
| 1.1.3 Cerebrolysin dose: 10 mL and 50 mL for 10 days | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.37, 3.73] |
| 1.1.4 Cerebrolysin dose: 30 mL for 7 days then 10 mL 5 days a week for 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 5.34] |
| 1.2 Total number of people with SAEs | 4 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.65] |
| 1.2.1 Cerebrolysin dose: 30 mL for 10 days | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.81] |
| 1.2.2 Cerebrolysin dose: 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.26, 2.12] |
| 1.2.3 Cerebrolysin dose: 30 mL for 7 days then 10 mL 5 days a week for 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.15, 6.82] |
| 1.3 Total number of people with fatal SAEs | 3 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.38] |
| 1.3.1 Cerebrolysin dose: 30 mL for 10 days | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.57, 1.44] |
| 1.3.2 Cerebrolysin dose: 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.29, 2.58] |
| 1.4 Total number of people with non‐fatal SAEs | 4 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.01, 4.55] |
| 1.4.1 Cerebrolysin dose: 30 mL for 10 days | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.23, 6.66] |
| 1.4.2 Cerebrolysin dose: 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.01, 7.03] |
| 1.4.3 Cerebrolysin dose: 30 mL for 7 days then 10 mL 5 days a week for 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.15, 6.82] |
| 1.5 Total number of people with adverse events | 4 | 1435 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.10] |
| 1.5.1 Cerebrolysin dose: 30 mL for 10 days | 2 | 1189 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.11] |
| 1.5.2 Cerebrolysin dose 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.69, 3.82] |
| 1.5.3 Cerebrolysin dose: 30 mL for 7 days then 10 mL 5 days a week for 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.66] |
| 1.6 Non‐death attrition | 6 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.45, 2.06] |
| 1.6.1 Cerebrolysin dose: 30 mL for 10 days | 3 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.52] |
| 1.6.2 Cerebrolysin dose: 50 mL for 21 days | 1 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.08, 0.91] |
| 1.6.3 Cerebrolysin dose: 10 mL and 50 mL for 10 days | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.6.4 Cerebrolysin dose: 30 mL for 7 days then 10 mL 5 days a week for 3 weeks | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.14, 6.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amiri Nikpour 2014.
| Study characteristics | ||
| Methods | Study design: RCT Study grouping: parallel group Losses to follow‐up: none Trial protocol registration: no protocol identified |
|
| Participants | Total number of participants: 46. However, 3 participants died before day 30: 1 participant in the Cerebrolysin group and 2 participants in the placebo group. 43 participants included in the final analysis. Baseline characteristics: Cerebrolysin
Placebo
Inclusion criteria: both sexes, 18 to 85 years; focal neurological injury; ischaemic stroke within 6 to 24 hours before admission; acute focal ischaemic stroke detected by CT or MRI or both; NIHSS score of 6 to 22 at presentation Exclusion criteria: rapid improvement of signs and symptoms, or complete resolution, or both, within 24 hours; seizure upon the development of stroke; any conditions interfering with neurological examination, such as severe dementia or psychological diseases; severe heart failure; acute myocardial infarction; pregnancy or breastfeeding; significant systemic diseases associated with disability and decreased well‐being; systolic and diastolic blood pressure above 220 mmHg and 120 mmHg, respectively; CT or MRI suggesting acute or chronic haemorrhagic stroke or neoplasm, or both; hernia in the brain or increased intracranial pressure; contraindication or sensitivity to aspirin or Cerebrolysin, or both; taking other neuroprotective agents such as piracetam; and taking vasodilators such as nimodipine Pretreatment: no difference |
|
| Interventions | Cerebrolysin
Placebo
|
|
| Outcomes | We extracted data for all‐cause death (dichotomous outcome). | |
| Identification | Sponsorship source: Urmia University of Medical Sciences grant Country: Iran Setting: hospital (inpatient setting) Author: Mohammad Reza Amiri‐Nikpour Institution: Seyyed‐al‐Shohada Heart Centre Email: yousefrezaei1986@gmail.com |
|
| Notes | No protocol identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk |
Quote: "In a randomised, double‐blinded, placebo‐controlled clinical trial, patients who had signs and symptoms of acute brain stroke were assessed from March 2013 to March 2014." Comment: there was insufficient information to permit a judgement of low risk or high risk, so we opted for a judgement of unclear risk |
| Sequence Generation | Unclear risk |
Quote: "In a randomised, double‐blinded, placebo‐controlled clinical trial, patients who had signs and symptoms of acute brain stroke were assessed from March 2013 to March 2014." Comment: there was no information on allocation concealment. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Incomplete outcome data All outcomes | Low risk |
Quote: "After receiving treatments, one patient in the Cerebrolysin‐received group and two patients in the placebo‐received group died before day 30 (4.3% versus 8.7%); they were excluded from the final analysis due to lack of measuring their outcomes at 90‐day follow‐up." Comment: no losses to follow‐up. However, adverse events and causes of death were not reported, and the study protocol was not available. |
| Blinding of outcome assessors All outcomes | Unclear risk |
Quote: "In a randomised, double‐blinded, placebo‐controlled clinical trial, patients who had signs and symptoms of acute brain stroke were assessed from March 2013 to March 2014." Comment: there was no information as to whether outcome assessors were aware of the allocated interventions. No information was provided on allocation concealment. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Selective outcome reporting | Unclear risk | Comment: study protocol not available. Causes of death were not described; there was no information on clinically relevant outcomes. |
| Blinding of participants and personnel All outcomes | Unclear risk |
Quote: "All patients who met inclusion criteria were randomly assigned into two groups to receive intravenously either 30 ml of Cerebrolysin diluted in normal saline once a day for 10 days (n = 23) or normal saline, as placebo, with a prescription order similar to the main drug (n = 23)." Comment: there was no information on blinding of participants and personnel. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Other sources of bias | Unclear risk |
Quote: "We thank the vice‐chancellor of research in Urmia University of Medical Sciences for providing the grant of this study. Moreover, we would like to greatly thank all members of emergency department of Imam Khomeini Hospital, Urmia, West Azerbaijan Province, Iran, for helping us in collecting the study data." Comment: there was no clear information on funding sources, and all authors declared no conflict of interest; no protocol identified |
CASTA 2012.
| Study characteristics | ||
| Methods | Study design: phase IV clinical trial designed as a multicentre, randomised, double‐blind placebo‐controlled, parallel‐group study Study grouping: parallel group Losses to follow‐up: 180 participants (16.8%) Trial protocol registration: retrospective (3 years difference between study start date (2006) and the date registration record posted (2009), study was completed in 2011) |
|
| Participants | Total number of participants: 1070 Baseline characteristics: Cerebrolysin
Placebo
Inclusion criteria: men and women, aged 18 to 85 years with focal neurological deficit and a clinical diagnosis of acute hemispheric ischaemic stroke with CT or MRI results compatible with a clinical diagnosis of acute hemispheric stroke, NIHSS score between 6 and 22 (both inclusive), and functionally independent before stroke with a pre‐stroke Rankin Scale score of 0 or 1. Randomisation and treatment with the trial medication initiated within 12 hours after stroke onset. Signed informed consent was obtained from the participant or the participant’s legally accepted representative. Exclusion criteria: evidence on CT/MRI of intracranial haemorrhage, decreased consciousness (defined as score of ≥ 2 on NIHSS Question 1a), neurological signs and symptoms that were likely to resolve completely within 24 hours, systolic blood pressure ≥ 220 mmHg or diastolic blood pressure ≥ 120 mmHg on repeated measurement, severe congestive heart failure or presentation with acute myocardial infarction, pre‐existing systemic disease significantly limiting life expectancy, concomitant treatment with other neuroprotective or nootropic drugs, and intolerance or contraindication to aspirin or Cerebrolysin Pretreatment: more participants with diabetes (117 (21.7%) versus 108 (20.5%)); arrhythmia (90 (16.7%) versus 71 (13.5%)); and coronary heart disease (86 (16.0%) versus 72 (13.7%)) in the placebo group |
|
| Interventions | Cerebrolysin
Placebo
|
|
| Outcomes |
|
|
| Identification | Sponsorship source: EVER Neuro Pharma GmbH (Oberburgau 3, Austria) Country: China, Hong Kong, South Korea, Myanmar Setting: inpatient (hospital) Comments: all study authors were closely bound with EVER Neuro Pharma. Dr Heiss is an advisor for the company; Dr Brainin has received financial support from EVER Neuro Pharma; Dr Bornstein is a consultant for EVER Neuro Pharma; Dr Tuomilehto is active in the Speakers Bureau of EVER Neuro Pharma; and Dr Hong received a research grant from EVER Neuro Pharma. Authors: Wolf‐Dieter Heiss and Zhen Hong Institution: Max‐Planck Institut fur Neurologie and Hua Shan Hospital, Department of Neurology Email: wdh@nf.mpg.de; profzhong@sina.com |
|
| Notes | No results posted on trial registration platform. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk |
Quote: "From September 2005 to September 2009, 1070 patients were randomised. Of 1069 patients who received at least 1 infusion of study medication, 529 patients (49.5%) received Cerebrolysin and 540 patients (50.5%) placebo" Comment: there was no information on allocation concealment. We searched the published protocol, Hong 2009, for a description of allocation concealment, but this was not reported. |
| Sequence Generation | Unclear risk |
Quote: "From September 2005 to September 2009, 1070 patients were randomised. Of 1069 patients who received at least 1 infusion of study medication, 529 patients (49.5%) received Cerebrolysin and 540 patients (50.5%) placebo" Comment: there was no information on allocation concealment. We searched the published protocol, Hong 2009, for a description of allocation concealment, but this was not reported. |
| Incomplete outcome data All outcomes | High risk |
Quote: "Eighty‐nine serious adverse events occurred after start of the treatment (Cerebrolysin 50 serious adverse events, placebo 39 serious adverse events). Sixty of 1069 patients sustained fatal adverse events (Cerebrolysin 28 patients [5.3%] and placebo 32 patients [5.9%]). Of 1069 patients, 85 patients (8.0%) discontinued the study due to adverse events, 39 patients in the Cerebrolysin group" Quote: "Sixty patients died and 890 (83.2% of all randomised patients) completed the 90‐day follow‐up ..." Comment: 16.8% of participants were lost to follow‐up. The proportion of missing outcomes compared with the observed event risk was enough to induce clinically relevant bias in observed intervention effect estimate. |
| Blinding of outcome assessors All outcomes | Low risk |
Quote: "Before unblinding the study, a blind review of the data was performed. The review was within the framework of the requirements of the ICH Guideline E9. 17" Quote: "Patients and investigators remained strictly blinded to the treatment assignments, and the occurrence or nature of adverse events did not compromise the blinding either." Comment: it is impossible to assess blinding by outcome. Described in report as a randomised, double‐blind, placebo‐controlled, parallel‐group study |
| Selective outcome reporting | Unclear risk | Comment: the study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. The protocol was registered retrospectively, results not posted on trial registration platform. No causes of death were described in the trial report, Kaplan‐Meier mortality curve presented only for the subgroup of participants NIHSS > 12. |
| Blinding of participants and personnel All outcomes | Low risk |
Quote: "Patients and investigators remained strictly blinded to the treatment assignments, and the occurrence or nature of adverse events did not compromise the blinding either. Missing data were handled according to international standards or guidelines." Comment: we judged this as low risk, despite this statement about strict blinding appeared only in the discussion section of the trial report. Other details were available in the protocol published as Hong 2009, though no information on blinding was provided in the methods or results sections of the trial report. |
| Other sources of bias | High risk |
Quote: "This study was funded by EVER Neuro Pharma GmbH, Oberburgau 3, Austria. The steering committee, safety committee, and other study investigators were working independently. The sponsor assisted in the writing of the protocol, selection of study sites, data collection, and project management. The statistical data analysis was carried out by an independent statistical consultant from Idv Gauting, Germany. The interpretation of results and conclusions are those of the authors, and these and writing of the article were not influenced by the sponsor. The article was reviewed and approved by the independent steering committee and safety committee. The authors received an honorarium related to this work from the sponsor and support for travel." Quote: "Dr Heiss is an advisor for EVER Neuro Pharma and received honoraria for this activity. He is active in the speaker’s bureau of EVER Neuro Pharma and CoAxia and he receives support from the Wolf‐Dieter Heiss Foundation. Dr Brainin has received financial support for research grants from EVER Neuro Pharma and Boeh‐ ringer Ingelheim and other research support from the European Research Foundation and Life Science Krems. He is in the speaker’s bureau of Allergan, Boehringer Ingelheim, Ferrer, Pfizer, and EVER Neuro Pharma. He is active as a consultant and advisor for Allergan and EVER Neuro Pharma. Dr Bornstein is a consultant for EVER Neuro Pharma and received honoraria for this activity. He is also active in the speaker’s bureau of EVER Neuro Pharma. Dr Tuomilehto is active in the speaker’s bureau of EVER Neuro Pharma and received honoraria for this activity from EVER Neuro Pharma. Dr Hong received a research grant from EVER Neuro Pharma." |
CERE‐LYSE‐1 2012.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, placebo‐controlled, double‐blind trial Study grouping: parallel group Losses to follow‐up: 19 (16%) Trial protocol registration: retrospective (4 years difference between study start date (2005) and the date registration record posted (2009), when the trial was already completed in 2008) |
|
| Participants |
Baseline characteristics: Cerebrolysin
Placebo
Inclusion criteria: men and women, 18 to 80 years, who had a clinical diagnosis of acute ischaemic hemispheric stroke that had commenced within 3 hours prior to initiation of administration of rtPA, and had stroke symptoms being present for at least 30 minutes with no significant improvement before treatment, were eligible (further inclusion and exclusion criteria, see Table 1). All participants had to meet the admission standards of the European Medicines Agency (EMA) consensus criteria for the application of thrombolytic therapy with alteplase (rtPA): (1) clinical diagnosis of ischaemic stroke causing a measurable neurological deficit defined as impairment of language, motor function, cognition and/or gaze, vision or neglect. Ischaemic stroke is defined as an event characterised by the sudden onset of an acute focal neurologic deficit presumed to be due to cerebral ischaemia after CT scan excluded haemorrhage, (2) informed consent Exclusion criteria: evidence of intracranial haemorrhage on the CT scan; participation in another therapeutic clinical trial 3 months before baseline; people with any history of prior stroke and concomitant diabetes; prior stroke within the last 3 months; platelet count below 100 to 103/mm3; blood glucose < 50 or > 400 mg/dL (< 2.77 or > 22.15 mmol/L); known haemorrhagic diathesis; manifest or recent severe or dangerous bleeding; known bacterial endocarditis, pericarditis; acute pancreatitis; documented ulcerative gastrointestinal disease during the last 3 months, oesophageal varices, arterial‐aneurysm, arterial/venous malformation; neoplasm with increased bleeding risk; severe liver disease, including hepatic failure, cirrhosis, portal hypertension, oesophageal varices, and active hepatitis; major surgery or significant trauma in past 3 months; multiple serious drug allergies; hypersensitivity or allergy to 1 of the components of the drug; severe renal impairment; systolic blood pressure > 185 mmHg or diastolic blood pressure > 110 mmHg, or aggressive management (intravenous medication repeatedly) needed to reduce blood pressure to these limits; recent (less than 10 days) traumatic external heart massage, obstetrical delivery, recent puncture of a non‐compressible blood vessel (e.g. subclavian or jugular vein puncture); chronic intoxication or chronic substance use disorder with pharmaceuticals, drugs, alcohol, or industrial poisons; symptoms of ischaemic attack began more than 3 hours prior to start of thrombolytic therapy or if time of symptom onset is unknown; minor neurological deficit or symptoms rapidly improving before start of infusion; severe stroke as assessed clinically (e.g. NIHSS > 25) and/or by appropriate imaging techniques; epilepsy; symptoms suggestive of subarachnoid haemorrhage, even if the CT scan is normal; known history of or suspected intracranial haemorrhage; suspected subarachnoid haemorrhage or condition after subarachnoid haemorrhage from aneurysm; any history of CNS damage (i.e. neoplasm, aneurysm, intracranial, or spinal surgery); haemorrhagic retinopathy, e.g. in diabetes (vision disturbances may indicate haemorrhagic retinopathy); administration of heparin within the previous 48 h and a thromboplastin time exceeding the upper limit of normal for laboratory; people receiving oral anticoagulants, e.g. warfarin, sodium; people receiving nifedipine for acute treatment Pretreatment: the 2 groups were well balanced with respect to baseline prognostic variables, and no significant differences between treatment groups were observed |
|
| Interventions | Cerebrolysin
Placebo
|
|
| Outcomes |
|
|
| Identification | Sponsorship source: not mentioned. Only the Conflict of Interest statement: "Wilfried Lang has served as consultant for Bayer, Boehringer Ingelheim, EVER, MSD, Sanofi‐Aventis and Pfizer and has received speaking honoraria from these companies. Christian Stadler has received speaker honoraria from EVER. Zdavka Poljakovic received Principal Investigator fee for the clinical study. David Fleet is a freelance consultant statistician undertaking statistical contracts on behalf of pharmaceutical/biotechnology organizations and as such was contracted by EVER. All authors have no other financial interest in the company or its products." Country: 5 countries: Austria, Croatia, the Czech Republic, Slovakia, Slovenia Setting: inpatient (hospital) Author: Wilfried Lang Institution: Department of Neurology, Hospital St John, Austria Email: wilfried.lang@bbwien.at |
|
| Notes | No results posted on trial registration platform. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk |
Quote: "The vials containing the study drug and the placebo were visually identical." Comment: though the quote refers to potential blinding and not allocation concealment, we judged this as low risk |
| Sequence Generation | Unclear risk |
Quote: "according to a pre‐compiled 1:1 randomization schedule, stratified by centre." Comment: there was not only "insufficient information to permit judgement of low risk or high risk" as the basis for a judgement of unclear risk as per the Cochrane Handbook. The described procedure does not fit with any of the criteria for an assessment of low risk of bias, i.e. referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; or minimisation. There is no information about the process of generation of the randomisation sequence. In addition to retrospective protocol registration and statistician contracted by Cerebrolysin manufacture EVER Neuro Pharma, we judged this as unclear risk. |
| Incomplete outcome data All outcomes | High risk |
Quote: "Two patients received the incorrect study medication assignment." Quote: "Based on statistical information from the third interim analysis, it was decided to terminate the study, as no significant result for the main outcome criteria was expected to be reached." Quote: "All patients were included in the ITT population with 60 patients being assigned to Cerebrolysin and 59 assigned to placebo. In the PP population, 100 patients were included with 49 receiving Cerebrolysin and 51 receiving placebo (Fig. 1)." Comment: 19 participants of 119 (16%) were lost to follow‐up. Attrition bias. Information not available by outcome. Furthermore, the study authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of outcome assessors All outcomes | Low risk |
Quote: "All study personnel and participants were blinded to treatment assignment for the duration of the study." Comment: however, it was retrospective protocol registration, and the statistician was contracted by Cerebrolysin manufacturer EVER Neuro Pharma |
| Selective outcome reporting | Unclear risk |
Quote: "There were no obvious differences between either treatment arms. In each treatment group, four patients died, but in none of the cases was any relationship to the study medication seen. The number of patients with serious adverse events was slightly higher in the Cerebrolysin group compared to the placebo group (12 vs. 7, respectively). In total, 19 (16%) patients experienced at least one serious adverse event (Table 5). " Comment: the study was stopped because of no significant result for the main outcome criteria. According to the study authors, there was no causal relationship to the study drug for any of the deaths observed. Neither reasons for nor timing of deaths is presented. Timing of adverse events, serious adverse events not presented. Study protocol was registered retrospectively. We judged this to be unclear risk of bias. |
| Blinding of participants and personnel All outcomes | Low risk | Quote: "All study personnel and participants were blinded to treatment assignment for the duration of the study." |
| Other sources of bias | High risk |
Quote: "Ljubljana, Ljubljana/Slovenia) ClinicalTrials.gov identifier: NCT00840671 Conflicts of interest: Wilfried Lang has served as consultant for Bayer, Boehringer Ingelheim, EVER, MSD, Sanofi‐Aventis and Pfizer and has received speaking honoraria from these companies. Christian Stadler has received speaker honoraria from EVER. Zdavka Poljakovic received Principal Investigator fee for the clinical study. David Fleet is a freelance consultant statistician undertaking statistical contracts on behalf of pharmaceutical/ biotechnology organizations and as such was contracted by EVER. All authors have no other financial interest in the company or its products." Comment: no information on funding sources for the trial. Statistician was contracted by EVER, the manufacturer of Cerebrolysin. There is no information about the provider of Cerebrolysin. Retrospective protocol registration. Early stopping of the trial after an interim analysis |
Gharagozli 2017.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, placebo‐controlled, double‐blind trial Study grouping: parallel group Losses to follow‐up: 25 out of 100 (25%) Follow‐up period: 30 days Trial protocol registration: retrospective ("registered while recruiting", no actual recruitment start date provided) |
|
| Participants | Participants were between 45 and 85 years old with a clinically confirmed acute embolic or thrombotic stroke in the territory of the arterial branches of the internal carotid artery. Mean age = 68 ± 11.5 years, approximately men/women: 50/50 The first application of the study medication was within 18 hours poststroke, and the treatment lasted for 4 weeks. Inclusion criteria: people between 45 and 85 years old with a clinically confirmed acute embolic or thrombotic stroke in the territory of the arterial branches of the internal carotid artery Exclusion criteria: complete remission of symptoms within 4 hours after onset; the presence of signs and symptoms of progressive neurological deficits; signs of haemorrhagic stroke or intracranial bleeding; systolic blood pressure ≥ 200 mmHg; diastolic blood pressure ≥ 100 mmHg; signs of stupor or coma (Glasgow Coma Scale score of ≤ 6); convulsions; pupillary oedema; increased intracranial pressure; myocardial infarction; cardiac function deficit; renal or hepatic insufficiency; acute infections; pregnancy; participation in another clinical study. Patients treated with rtPA were not included in the study, and concomitant medication with piracetam or calcium channel blockers was not allowed owing to their purported neuroprotective effects. |
|
| Interventions | Acute phase during the first 7 days, the study drug was administered daily as an intravenous infusion for a period of 30 minutes (30 mL Cerebrolysin + 50 mL saline = 80 mL). Then 10 mL intravenously 5 days per week. Unclear whether the daily dose was 10 mL Cerebrolysin or 10 mL of the 30:50 Cerebrolysin Placebo: normal saline, same volume as Cerebrolysin solutions Baseline treatment: 100 mg daily aspirin, pentoxifylline, or low‐dose heparin |
|
| Outcomes |
|
|
| Identification | Sponsorship source: EVER Neuro Pharma GmbH Country: Iran Setting: inpatient (hospital) Comments: a number of study authors were closely involved with EVER Neuro Pharma Conflict of interest statement: JV is a member of the advisory board of EVER Neuro Pharma. SW is an employee of EVER Neuro Pharma. HM is a scientific consultant for EVER Neuro Pharma. The other authors declare that they have no conflict of interest. Corresponding author: Herbert Moessler Institution: COMAMO Lifesciences GmbH Mondseestrasse 34/3, 5310 Mondsee, Austria, Phone: +43 664 4633723, Email: herbert.moessler@comamo.at |
|
| Notes | No results posted on trial registration platform. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | Comment: there is no information about allocation concealment, no central randomisation |
| Sequence Generation | Low risk |
Quote: "Patients were assigned to treatment groups according to a predefined randomization plan by using a block size of 4, a ratio of 1:1, and stratified by study center." Comment: though the described procedure does not fit with any of the criteria for an assessment of low risk of bias, i.e. random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; or minimisation, we judged this as low risk because there was a predefined randomisation plan and block randomisation stratified by study centre |
| Incomplete outcome data All outcomes | High risk |
Quote: "A total of 100 patients were enrolled in this study (Cerebrolysin, n=50; placebo, N=50). All patients received at least one dose of the study medication and thus represented the safety analysis data set. One patient in the Cerebrolysin group had no efficacy baseline assessment, and another patient in the Cerebrolysin group did not provide any post‐baseline efficacy data. Thus, the mITT‐LOCF analysis consisted of 98 patients (Cerebrolysin, N=48; placebo, N=50). Of those, 23 patients discontinued participation in the study prematurely: four patients withdrew because of adverse events (AEs) (Cerebrolysin, N=2; placebo, N=2), three patients died in the acute phase due to stroke severity (Cerebrolysin, N=1; placebo, N=2) and another 16 patients were lost to follow‐up after discharge from the hospital (Cerebrolysin, N=12; placebo, N=4). Thus, a total of 75 patients (Cerebrolysin, N=33; placebo, N=42) comprised the data as the available (observed cases; OC) analysis set. Since the percentage of missing values at day 30 was comparatively high (23%), a sensitivity analysis for the primary outcome measure was also performed based on the OC population." Comment: overall attrition = 25 out of 100 ITT or 23 out of 98 who entered. However, attrition in the Cerebrolysin group was high, with only 33/50 ITT or 33/48 analysable (i.e. attrition rates of 34% or 31.25%). Furthermore, the study authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of outcome assessors All outcomes | Unclear risk |
Quote: "The statistician in charge of randomization was unblinded but was not involved in any other study‐related procedures." Comment: the statistician was not blinded |
| Selective outcome reporting | Unclear risk | Comment: overall attrition = 25 out of 100 ITT or 23 out of 98 who entered. However, attrition in the Cerebrolysin group was high, with only 33/50 ITT or 33/48 analysable (i.e. attrition rates of 34% or 31.25%). Furthermore, the study authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. The study protocol was registered retrospectively. |
| Blinding of participants and personnel All outcomes | Low risk |
Quote: "Patients, investigators and all study personnel were blinded to the treatment allocation ... EVER Neuro Pharma provided the study medication in 10‐ml ampoules labelled appropriately to maintain blinding." Comment: though there was insufficient information to permit a judgement about blinding by outcome, we judged this as low risk of bias |
| Other sources of bias | High risk |
Quote: "Overall, the safety outcome reflected the expected safety and tolerability profile of Cerebrolysin in patients after acute ischemic stroke." Quote: "JV is a member of the advisory board of EVER Neuro Pharma. SW is an employee of EVER Neuro Pharma. HM is a scientific consultant for EVER Neuro Pharma. " Quote: "Acknowledgments: EVER Neuro Pharma GmbH provided the study medication." "Conflict of Interest: JV is a member of the advisory board of EVER Neuro Pharma. SW is an employee of EVER Neuro Pharma. HM is a scientific consultant for EVER Neuro Pharma. The other authors declare that they have no conflict of interest." Quote: "Overall, patients in the placebo groups presented with somewhat milder symptoms according to the NIHSS score (9.1± 4.8; mean ± standard deviation) compared to the Cerebrolysin group (11.1 ± 5.0). Corresponding evidence for a slightly milder patient population in the placebo group was also seen in the mRS score (placebo: 3.4 ± 1.1; Cerebrolysin: 3.9 ± 1.0). Since these baseline group differences reached statistical significance for the NIHSS (MW 0.36, P=0.02) and for the mRS (MW 0.39, P=0.05), an ANCOVA sensitivity analysis was performed by using the baseline score as a covariate to confirm the robustness of the data." |
Ladurner 2005.
| Study characteristics | ||
| Methods | Study design: multicentre, randomised, double‐blind controlled trial Mean duration of follow‐up: 90 days Study grouping: parallel group Loss to follow‐up: 15 of 146 (10%) Trial protocol registration: no protocol identified |
|
| Participants |
Baseline characteristics: Cerebrolysin
Placebo
Inclusion criteria: men and women suffering from their first acute ischaemic stroke with clinical symptoms of middle cerebral artery area were enrolled. Patients were eligible if they were admitted to the hospital and received the first dose of study medication within 24 hours of the onset of the stroke and were between 45 and 85 years of age at study entry. Participants were also required to have a GCS score of greater than 10 and a CNS score between 4.5 and 8.0 at baseline. Exclusion criteria: people with haemorrhagic strokes, transient ischaemic attacks, uncontrollable hypertension, acute myocardial infarction, congestive heart failure, moderate‐severe dementia prior to the stroke, coma or stupor, other severe concomitant diseases, impaired renal function, and people with a history of prior stroke Pretreatment: no significant group differences of the demographic characteristics were observed at baseline, and the severity of the stroke at study entry was comparable between the 2 groups |
|
| Interventions | Cerebrolysin
Placebo
|
|
| Outcomes |
|
|
| Identification | Sponsorship source: EBEWE Pharma Country: Austria, the Czech Republic, Hungary Setting: inpatient (hospital) Authors: Dr G Ladurner and H Moessler Institution: Department of Neurology, Christian‐Doppler Hospital, Salzburg, Austria Email: g.ladurner@lks.at and herbert.moessler@ebewe.com |
|
| Notes | Population: concomitant use of nootropic drugs (e.g. piracetam), drugs with dilatating effects on peripheral blood vessels (naftidrofuryl, cinnarizine, flunarizine, nimodipine), as well as chronic intake of antidepressants, tranquillisers, sedatives, or CNS stimulants was prohibited throughout the study No study protocol identified. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk |
Quote: "For each patient a sealed envelope with information on the actual treatment dispensed was provided to the investigator for emergency cases. All envelopes remained sealed throughout the study." Comment: sealed envelopes were used to conceal allocation, but it was not mentioned if they were opaque. Adding to the unavailability of study protocol, we judged this as unclear risk of bias. |
| Sequence Generation | Low risk |
Quote: "Patients who met all entry criteria were assigned to the treatment groups in a 1:1 ratio, according to a randomisation code generated by a computer software (EBEWE Pharma, Unterach, Austria). The randomisation was carried out in blocks of 12 patients, stratified by study centre." Comment: the computer software used to generate the random numbers was provided by EBEWE Pharma, which is also the provider of Cerebrolysin |
| Incomplete outcome data All outcomes | High risk |
Quote: "146 patients were randomised to two treatment groups and constituted the ITT population: 78 patients to the Cerebrolysin group and 68 patients to the placebo group. Of these patients, 67 of the Cerebrolysin group and 52 of the placebo group completed the study. Reasons for the 25 cases of study discontinuation were death (6 Cerebrolysin, 6 placebo), serious adverse event (1 placebo), and consent withdrawn (3 Cerebrolysin; 9 placebo)." Comment: attrition bias, 25 out of 146 randomised participants were lost to follow‐up (17%). Information on the outcomes of interest to this review was available only for serious adverse events including death. Furthermore, the study authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of outcome assessors All outcomes | Low risk | Quote: "The investigators and all other study personnel were blind as to the random code assignment until the completion of the statistical analysis." |
| Selective outcome reporting | Unclear risk |
Quote: "Twelve patients died during the study: 6 in the Cerebrolysin group (7.69%) and 6 in placebo group (8.83%). None of the deaths was reportedly related to the study drug administration." Quote: "With the exception of one SAE (hematemesis) in the placebo group which was rated to be likely related to the study drug, there was no causal relationship to the study drug for any other of the SAEs, as per the investigator’s assessment." Comment: the trial authors did not report on the time when deaths occurred, and did not assess potential causality with administered medicines. Furthermore, the authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of participants and personnel All outcomes | Low risk |
Quote: "The investigators and all other study personnel were blind as to the random code assignment until the completion of the statistical analysis." Comment: impossible to assess blinding by outcome |
| Other sources of bias | Unclear risk |
Quote: "The participants of the Cerebrolysin study group were as follows: G. Ladurner, Christian‐Doppler Clinic, Salzburg, Austria; K. Niederkorn, University Hospital for Neurology, Graz, Austria; I. Szirmai, Semmelweis University of Medicine, Budapest, Hungaria; P. Kalvach, Charles University, FNKV, Department of Neurology, Prague; F. Stockenhuber, Landeskrankenhaus, Oberpullendorf, Austria; Z. Haffner, Petz Alada ´Megyei Koorha'z, Gyoor, Hungaria; P. Ridzon, Thomayer’s Hospital, Praha, Czech Republic; E. Diabl, Linz General Hospital, Linz, Austria." Quote: "The study medication was provided to the study centres by EBEWE Pharma in the form of a ready‐to‐use infusion solution. The active medication contained 50 ml Cerebrolysin mixed with 50 ml of normal saline." Comment: there was no information on funding sources for the trial, and no conflict of interest statement was provided. EBEWE Pharma provided the medication and randomisation codes. No study protocol publicly available |
Skvortsova 2004.
| Study characteristics | ||
| Methods | Study design: RCT Study grouping: parallel group Losses to follow‐up: none Trial protocol registration: no protocol identified |
|
| Participants | Cerebrolysin
Placebo
Inclusion criteria: people with first‐in‐lifetime ischaemic stroke in the basin of internal carotid artery, aged 45 to 85 years, admitted to the ICU within 12 hours of stroke symptoms onset Exclusion criteria: disappearance of symptoms within 4 hours from the beginning of stroke; people with haemorrhagic stroke or stroke in the vertebrobasilar system; people with blood pressure levels higher than 200/100 mmHg; people with acute myocardial infarction, with a priori severe dementia; pregnant women; and participants in other studies Pretreatment: no difference |
|
| Interventions | Cerebrolysin
Placebo
|
|
| Outcomes |
|
|
| Identification | Sponsorship source: not reported Country: Russia Setting: inpatient Author's name: Skvortsova Institution: Department of Basic and Clinical Neurology, Russian State Medical University Address: Moscow |
|
| Notes | No study protocol identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk |
Quote: "Всем пациентам рандомизированно и вслепую было назначено плацебо или церебролизин в дозе 10 либо 50 мл (по 12 человек в каждой группе)." ["Vsem patsiyentam randomizirovanno i vslepuyu bylo naznacheno platsebo ili tserebrolizin v doze 10 libo 50 ml (po 12 chelovek v kazhdoy gruppe)": "All patients were randomly and blindly assigned to placebo or Cerebrolysin at 10 or 50 mL (12 in each group)."] Comment: Insufficient information to permit a judgement of low risk or high risk. There was no mention of allocation concealment. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Sequence Generation | Unclear risk |
Quote: "Всем пациентам рандомизированно и вслепую было назначено плацебо или церебролизин в дозе 10 либо 50 мл (по 12 человек в каждой группе)." ["Vsem patsiyentam randomizirovanno i vslepuyu bylo naznacheno platsebo ili tserebrolizin v doze 10 libo 50 ml (po 12 chelovek v kazhdoy gruppe)": "All patients were randomly and blindly assigned to placebo or Cerebrolysin at 10 or 50 mL (12 in each group)."] Comment: there was no information on allocation concealment. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Incomplete outcome data All outcomes | Unclear risk |
Quote: "Анализ исходов инсульта к 30‐м суткам не обнаружил достоверных различий между группами в летальности. Причины смерти 3 из 5 больных, получавших церебролизин, а также одного пациента из группы плацебо не были связаны с инсультом (тромбоэмболия легочной артерии, пневмония, пиелонефрит). У 2 пациентов, получавших церебролизин, и 2 получавших плацебо смерть наступила вследствие отека мозга с развитием вторичного стволового синдрома." ["Analiz iskhodov insul’ta k 30‐m sutkam ne obnaruzhil dostovernykh razlichiy mezhdu gruppami v letal’nosti. Prichiny smerti 3 iz 5 bol’nykh, poluchavshikh tserebrolizin, a takzhe odnogo patsiyenta iz gruppy platsebo ne byli svyazany s insul’tom (tromboemboliya legochnoy arterii, pnevmoniya, piyelonefrit). U 2 patsiyentov, poluchavshikh tserebrolizin, i 2 poluchavshikh platsebo smert’ nastupila vsledstviye oteka mozga s razvitiyem vtorichnogo stvolovogo sindroma.": "Analysis of stroke outcomes by day‐30 did not uncover significant differences between groups in lethality. The causes of death of 3 out of 5 patients, treated with Cerebrolysin, and one patient from the placebo group were not attributed to stroke (pulmonary oedema, pneumonia, pyelonephritis). In 2 patients, treated with Cerebrolysin, and 2 treated with placebo, deaths occurred due to cerebral oedema with development of secondary brain stem syndrome."] This sentence is ambiguous. Comment: despite no losses to follow‐up, only information on death (outcome of interest) was reported, though very ambiguously. The causes of death were described, although the numbers of participants who died per cause of death were not reported. Furthermore, the specific timing of each death was not provided, and the number of deaths in each Cerebrolysin dosing group (10 mL or 50 mL) was not reported. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Blinding of outcome assessors All outcomes | Unclear risk | Comment: there was no information on blinding of outcome assessors. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Selective outcome reporting | Unclear risk |
Comment: study protocol not available, we judged this as unclear risk Quote: "Причины смерти 3 из 5 больных, получавших церебролизин, а также одного пациента из группы плацебо не были связаны с инсультом (тромбоэмболия легочной артерии, пневмония, пиелонефрит)". ["Prichiny smerti 3 iz 5 bol’nykh, poluchavshikh tserebrolizin, a takzhe odnogo patsiyenta iz gruppy platsebo ne byli svyazany s insul’tom (tromboemboliya legochnoy arterii, pnevmoniya, piyelonefrit).": "The causes of death for 3 of 5 patients who received Cerebrolysin and 1 patient in the placebo group were not associated with stroke (pulmonary embolism, pneumonia, pyelonephritis)".] Comment: the time when deaths occurred was not reported. Furthermore, the study authors considered that deaths were not drug‐related. Adverse events were not reported. The timing was not clear for outcomes presented in a table and a graph, although these outcomes were not those of interest for the review. |
| Blinding of participants and personnel All outcomes | Unclear risk |
Quote: "Всем пациентам рандомизированно и вслепую было назначено плацебо или церебролизин в дозе 10 либо 50 мл (по 12 человек в каждой группе)". ["Vsyem patziyentam randomizirovanno i vslyepooyo bilo naznachyeno platzyebo ili tzyeryebrolizin v dozye 10 libo 50 ml (po 12 chyelovyek v kaʐdoy gurooppye).": "All patients were randomly and blindly assigned to placebo or Cerebrolysin at 10 or 50 mL (12 in each group)."] Comment: there was no information on blinding of participants and personnel. Added to the unavailability of study protocol, we judged this as unclear risk. |
| Other sources of bias | Unclear risk | Comment: no information on funding sources for the trial, and no conflict of interest statement was provided. No study protocol available |
Xue 2016.
| Study characteristics | ||
| Methods | Study design: RCT Study grouping: parallel group Losses to follow‐up: 19 (16%) Trial protocol registration: retrospective (4 years difference between study start date (2010) and the date registration record posted (2014), when the trial was already completed in 2010) |
|
| Participants | Cerebrolysin
Placebo
Other neuroprotective agent
Inclusion criteria: acute ischaemic stroke for the first time < 12 h prior to entry into the study, with a score of 6 to 25 on the NIHSS. Prior to randomisation, all participants were evaluated using cranial CT or MRI scanning and were followed with serial neurological examinations to confirm acute ischaemic stroke. Exclusion criteria: people with lacunar infarction, cerebral haemorrhagic infarction, epilepsy or epileptic seizures, history of neurological diseases, myocardial infarction, renal and hepatic abnormalities, metabolic diseases, and contraindications to antiplatelet treatments Pretreatment: comparison of baseline characteristics amongst the treatment groups revealed no significant differences (P > 0.05) |
|
| Interventions | Cerebrolysin
Placebo
Other neuroprotective agent
|
|
| Outcomes |
|
|
| Identification | Sponsorship source: this study was supported by the Shanghai Jiao Tong University Affiliated Sixth People's Hospital (grant nos. 1462 and 1583) and the Shanghai Science and Technology Council (grant no. 13411951401) Country: China Setting: "from January 2010 to May 2010, a randomised, double‑blind trial was conducted, which involved patients with acute ischaemic stroke in the neurology ward of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (Shanghai, China)" Comments: there were 3 treatment groups: NBP, Cerebrolysin, or placebo. We found the numbers randomised and evaluated to be unclear, thus the numerical results were meaningless for the purposes of this review. Author's name: Dr Hao Chen Institution: Department of Neurosurgery, Shanghai Jiao Tong University, Affiliated Sixth People's Hospital Email: chenhao_316@aliyun.com |
|
| Notes | Results posted on trial registration platform. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk |
Quote: "The random numbers were placed in concealed envelopes." Comment: concealed envelopes; not clear by whom and from whom the envelopes were concealed, and who might have had access to the envelopes. Added to the retrospective nature of the trial registration, we judged this as unclear risk of bias. |
| Sequence Generation | Unclear risk |
Quote: "Patients were randomly assigned to the NBP group, Cerebrolysin group or placebo group." Quote: "Randomization was performed by means of computer‑generated numbers through software by a third party who was not involved in patient management." Comment: the investigators describe a random component (computer random number generator) in the sequence generation process. Unclear who the third party was; added to the retrospective nature of the trial registration, we judged this as unclear of bias. |
| Incomplete outcome data All outcomes | High risk |
Quote: "During the trial period, 84 patients with AIS underwent randomization. Among these, 60 patients who received study intervention were included in the efficacy analysis. The NBP group contained 9 male and 11 female patients, whose ages ranged from 53 to 79 years. The Cerebrolysin group contained 9 males and 11 females, and their ages ranged from 54 to 85 years. The placebo group contained 10 males and 10 females, whose ages were from 52 to 87 years." Comment: 84 − 60 = 24, which is 29% of randomised participants lost in the trial report, no description of why only rounded numbers 20, 20, and 20 were included in any data presentation. Furthermore, the authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of outcome assessors All outcomes | Unclear risk |
Quote: "Patients and methods: patient selection. From January 2010 to May 2010, a randomised, double‑blind trial was conducted, which involved patients with AIS in the Neurology Ward of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (Shanghai, China)." Comment: there was no information on blinding of outcome assessors. We looked for specifics on blinding in the trial registration record with results posted, but did not find the relevant information. It was not possible to assess blinding by outcome, therefore we judged this as unclear risk. |
| Selective outcome reporting | Unclear risk |
Quote: "Missing values were substituted by last observation carried forward. P < 0.05 was considered to indicate a statistically significant result." Comment: 84 − 60 = 24, which is 29% of randomised participants lost in the trial report, no description of why only rounded numbers 20, 20, and 20 were included in any data presentation. Furthermore, the authors used the 'last observation carried forward' (LOCF) method to fill in the missing data points. There is not a single peer‐reviewed statistical publication that describes general conditions under which LOCF provides a statistically unbiased result. |
| Blinding of participants and personnel All outcomes | Unclear risk |
Quote: "a randomised, double‑blind trial was conducted, ..." Comment: no description of blinding, impossible to assess blinding by outcome |
| Other sources of bias | Unclear risk | Comment: no conflict of interest statement was provided. Retrospective trial registration |
ACE: angiotensin‐converting enzyme AIS: acute ischaemic stroke ANCOVA: Analysis of covariance CNS: central nervous system CT: computed tomography GCS: Glasgow Coma Score ICU: intensive care unit ITT: intention‐to‐treat MRI: magnetic resonance imaging mRS: modified Rankin Scale MW: Mann‐Whitney NBP: DL‐3‐n‐butylphthalide NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial rtPA: recombinant tissue plasminogen activator SD: standard deviation SEM: standard error of the mean TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 2011 | Trial registration record and trial report: completed study with ineligible question and ineligible population (dementia). |
| Barolin 1996 | Ineligible study design, not an RCT |
| Bavarsad Shahripour 2011 | Reported as an abstract only; ineligible patient population: the time window not specified (review protocol specifies that symptom onset should be less than 48 hours from the onset of stroke) |
| Bayer 1980 | Ineligible study design, not an RCT |
| Capisizu 2016 | Ineligible question: poststroke rehabilitation, not acute stroke management |
| Chang 2016 | Ineligible question: poststroke rehabilitation, not acute stroke management |
| Cuparnecu 2001 | Reported as an abstract only, no further full‐text publications; no follow‐up data |
| Dobi 2010 | Reported as an abstract only; efficacy assessment with Barthel Index only; no clinically relevant information, no information on death |
| Domzal 1995 | Ineligible study design, not an RCT |
| Ershov 2009 | Ineligible study design: no randomisation |
| Ershov 2011 | Ineligible study design: no randomisation |
| Gasecki 2016 | Ineligible question and reported as an abstract only: the relationship between aortic stiffness and objective and indirect tissue distraction parameters (neuroimaging) |
| Gheorghias 2019 | Ineligible question: stroke rehabilitation |
| Guekht 2015a | Reported as an abstract only; not an RCT, a meta‐analysis of CARS1 and CARS2 |
| Guekht 2015b | Reported as an abstract only; ineligible question and population: early rehabilitation after stroke |
| Guekht 2016 | Ineligible question (stroke rehabilitation), reported as an abstract only |
| Guekht 2017 | Ineligible question and not an RCT, a review paper: meta‐analysis of CARS trials |
| Haffner 2001 | Reported as an abstract only; efficacy assessment with stroke scales; no information on death |
| Hassanein 2016 | Ineligible question and study population: infants with perinatal brain insult |
| Hong 2005 | Reported as an abstract only |
| Imahori 2016 | Ineligible study design: a case study |
| IRCT201107226907N3 | Ongoing study with ineligible question and population (cerebral palsy), and findings not reported |
| IRCT2014040312888N2 | Ongoing study with ineligible question and population (head trauma and brain injury), and findings not reported |
| Jianu 2010 | Ineligible study design: randomisation not described; therapeutic time window was 72 hours (review protocol specifies 48 hours) |
| Jianu 2015 | Ineligible study design: no randomisation, therapeutic time window was 72 hours |
| Kim 2014 | Reported as an abstract only. Not a relevant condition ‐ subacute stroke; treatment initiated after 8 days of stroke onset |
| Kim 2015 | Reported as an abstract only. Cerebrolysin given 7 days after stroke onset. |
| Kim 2016 | Ineligible question in a review article |
| Kim 2019 | Ineligible study design: observational retrospective clinical study |
| Lebedeva 2018 | Ineligible question and population: people in the pre‐ and postoperative period of coronary artery bypass graft surgery |
| Lees 2019 | Ongoing study with ineligible question and study design: registry study. Trials registration record and reported as abstract only, as final results |
| Martinez 2017 | Ineligible question and study design: stroke rehabilitation in retrospective study |
| Martinez Sanchez 2015 | Ineligible study design: not an RCT: "Open label, one arm, and dose decreasing exploratory study" |
| Melnikova 2018 | Ineligible question (poststroke depression) and reported as an abstract only |
| Moskovko 2019 | Ineligible study design: retrospective study, reported as an abstract only |
| Muresanu 2015 | Ongoing study with ineligible question: delayed recovery/motor function after stroke. Trials registration record and reported as abstract only |
| Muresanu 2016a | Ineligible question and ineligible timing of cerebrolysin initiation after stroke onset |
| Muresanu 2016b | Ineligible question: potentiation of stem cell‐induced neuroprotection, and reported as an abstract only |
| Muresanu 2018 | Ineligible question and reported as 2 abstracts only |
| Nag 2017 | Ineligible question (acute ischaemic infarct CT neuroimaging) and reported as an abstract only |
| Nasiri 2017 | Ineligible question and study population: children with cerebral palsy |
| NCT00947531 | Trial registration record: completed study with ineligible question and ineligible population (vascular dementia) |
| NCT01059461 | Trial registration record: completed study with ineligible question, findings not reported |
| NCT01388738 | Ineligible question and patient population: neuroprotective drug efficiency in people after ischaemic stroke; people from 3 to 6 months after ischaemic stroke |
| NCT01606111 | Trial registration record: study with ineligible question and ineligible population (brain injury), with unknown status, findings not reported |
| NCT01787123 | Ongoing study with ineligible question and ineligible study population (aneurysmal subarachnoid haemorrhage). Trial registration record |
| NCT01822951 | Trial registration record: study with ineligible question and population (mild to moderate dementia), withdrawn |
| NCT01996761 | Completed trial registration record with published results. Ineligible question and patient population: effects of cerebrolysin on motor recovery in people with subacute stroke; cerebrolysin started within 7 days after stroke onset |
| NCT02116348 | Trial registration record: study with ineligible question, of unknown status, findings not reported |
| NCT02581371 | Ineligible question and patient population: effect of cerebrolysin at the level of paresis; the time from the stroke onset to the introduction of the drug was 72 hours |
| NCT02768571 | Completed trial registration record without posted results. Ineligible question and patient population: effects of cerebrolysin on motor recovery in people with severe motor involvement at subacute phase of stroke; subacute stage (less than 1 week after stroke) |
| NCT03480698 | Ongoing study with ineligible study design: registry/observational study. Trial registration record |
| NCT03506841 | Ongoing study with ineligible question: trial registration record |
| Park 2018 | Ineligible question and study design: treatment of aneurysmal subarachnoid haemorrhage in adults in a retrospective chart review |
| Poljakovic 2019 | Ineligible question and reported as an abstract only |
| Pushkarev 2015 | Ineligible study design; not an RCT: "An analysis of 42 case histories of patients from the period 2000 to 2014 with the diagnosis of lacunar stroke who were hospitalised in a stroke center." |
| RDPh_12_03 | Trial registration record in Russian register: ineligible question and patient population (patients with chronic brain ischaemia and non‐dementia cognitive impairment) |
| Shamalov 2005 | Ineligible question: effects on infarct volume after acute ischaemic stroke |
| Shamalov 2010 | Ineligible question: change in stroke volume of lesion detected by MRI: "Effect of cerebrolysin at a dose of 50 mL on morphometric picture of brain damage in ischemic stroke" |
| Shishkova 2015 | Ineligible question; ineligible population: "60 patients with hand paresis and 60 with aphasia were randomly assigned to treatment with cerebrolysin (25 mL/daily) or placebo group (which received saline infusions)" |
| Shishkova 2016 | Ineligible question and population: people with poststroke aphasia and diabetes mellitus, and reported as an abstract only |
| Shul'ginova 2016 | Ineligible question: red blood cell membrane lipid spectrum in patients with chronic cerebral ischaemia on the background of hypertensive disease |
| Skvortsova 2006 | Ineligible study design: no randomisation |
| Skvortsova 2008 | Reported as an abstract only: multiple publications |
| Stan 2013 | Ineligible question: change in stroke volume |
| Stan 2017 | Ineligible question: neurorehabilitation |
| Stanescu 2017 | Ineligible question: recovery of upper limb function in subacute ischaemic stroke |
| Uivarosan 2018 | Ineligible question: poststroke patients at 6 and 12 months after stroke |
| Uivarosan 2019 | Ineligible question: rehabilitation of poststroke patients |
| Vilenskii 1999 | Ineligible study design, not an RCT |
| Vilenskii 2007 | Ineligible question: repeated cerebrolysin course in stroke survivors |
| Winkler 2018 | Ineligible question, ineligible study design: retrospective study of upper extremity motor recovery after stroke |
| Yavorskaya 2008 | Ineligible patient population, ineligible question: patients with cognitive disorders |
| Zamfirescu 2017 | Ineligible question and population: poststroke rehabilitation |
| Zhu 2003 | Ineligible question and population: cerebrolysin used in people with stroke episode duration of 28 ± 7 days; efficacy assessment with stroke scales only |
| Zimin 2019 | Ineligible question and reported as an abstract only: cerebrolysin used after thrombectomy |
CT: computed tomography MRI: magnetic resonance imaging NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CERE‐REHA‐RU/01.
| Study name | CERE‐REHA‐RU |
| Methods | Double‐blind placebo‐controlled multi‐centre randomised parallel group clinical study of phase IV on effectiveness of adding Cerebrolysin to standard rehabilitation complex interventions in patients with ischaemic stroke |
| Participants | 180 ‐ target |
| Interventions | Cerebrolysin, 30 mL for 20 days, no further details provided |
| Outcomes | No details provided. |
| Starting date | 19 January 2015 |
| Contact information | Company 'Ligand Research': 3/7 Odoevskiy driveway, Moscow, Russia, 117574 |
| Notes | Stopped, reasons not provided |
IRCT201406169014N36.
| Study name | The effect of Cerebrolysin versus placebo on improvement of patients with acute ischemic stroke: a double blinded randomized clinical trial |
| Methods | Interventional, randomised, parallel group, double‐blind (clinical trial) |
| Participants | 122 participants aged 45 to 85 years with ischaemic stroke, referred to the hospital within less than 24 hours after stroke |
| Interventions | Intervention: Cerebrolysin 10 mL in 100 mL normal saline daily for 7 days added to routine therapy Control: placebo ‐ 100 mL normal saline alone daily for 7 days added to routine therapy |
| Outcomes | Primary: measuring motor function before intervention and 3 and 7 days after intervention using Canadian Stroke Scale Secondary: measuring motor function 1 month after intervention using modified Rankin Scale and Bartel Index |
| Starting date | 23 July 2013; retrospective registration; no results posted |
| Contact information | Sajedeh Nazari, Farshchian Hospital, Mirzadeh Eshghi Ave. Hamadan, Iran (Islamic Republic of); +98 81 3264 0021; sajed_nazari@yahoo.com |
| Notes | Funding: Dr Saeid Bashirian, Vice‐chancellor for Research the Technology, Hamadan University of Medical Sciences |
Differences between protocol and review
2010, Issue 4 (first review version): we followed the Cochrane protocol precisely (Ziganshina 2010a).
2015, Issue 6 (second review version): we did not incorporate changes to the structure of the previously published version of the review. We updated searches, followed the protocol precisely, and confirmed the conclusions (Ziganshina 2015).
2016, Issue 11 (third review version): we changed the inclusion criteria to allow varying durations of Cerebrolysin use and included a total of six studies with one comparison: Cerebrolysin versus placebo for acute ischaemic stroke. We restructured the outcomes: all‐cause death became the primary outcome, with the remaining outcomes listed as secondary outcomes. We changed the wording of "total number of adverse events" to "total number of people with adverse events". Ludivine Verney joined the team as a co‐author (Ziganshina 2016).
2017, Issue 4 (fourth review version): we refined the outcome serious adverse events (SAEs), replacing it with the following three outcomes: total number of people with SAEs; total number of people with fatal SAEs; and total number of people with non‐fatal SAEs (Ziganshina 2017).
2019 (fifth review version): we refined the eligibility criteria (type of participants) for future updates. In the current update we added two new secondary outcomes: non‐death attrition and cause of death.
Contributions of authors
Liliya‐Eugenevna Ziganshina (LEZ) prepared the protocol, was the author of the original review, and was responsible for this update. All authors were involved in the conception of this review update. Tatyana R Abakumova (TRA) performed literature searches of the Russian language studies. For this update, LEZ and Charles HV Hoyle (CHVH) assessed citations, abstracts, and full texts of trial reports for eligibility; LEZ and CHVH extracted data, assessed risk of bias, managed the references using Covidence, and imported data from Covidence to Review Manager 5. LEZ and CHVH drafted the updated sections of the review text.
Sources of support
Internal sources
-
Cochrane Stroke Group, UK
Editorial support and advice
-
Liverpool School of Tropical Medicine, UK
Mentoring support at the initiation stage of the title registration
External sources
-
New Source of support, UK
no external sources of support
Declarations of interest
LEZ: none known. TRA: none known. CHVH: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Amiri Nikpour 2014 {published data only}
- Amiri-Nikpour MR, Nazarbaghi S, Ahmadi-Salmasi B, Mokari T, Tahamtan U, Rezaei Y. Cerebrolysin effects on neurological outcomes and cerebral blood flow in acute ischemic stroke. Neuropsychiatric Disease and Treatment 2014;10:2299-306. [DOI: 10.2147/NDT.S75304] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarbaghi S, Amiri Nikpoor M. Acute stroke management efficiency of cerebrolysin in treatment of patients with acute ischemic stroke. International Journal of Stroke 2014;9 Suppl 3:91-2. [Google Scholar]
CASTA 2012 {published data only}
- Bornstein NM. CASTA - the background for the study and protocol design. International Journal of Stroke 2010;5 Suppl 2:50. [Google Scholar]
- Brainin M, Heiss WD, Bornstein N, Tuomilehto J, Hong Z. CASTA - results of a double-blind, placebo-controlled, randomized trial with cerebrolysin on patients with acute ischaemic stroke in Asia. Cerebrovascular Diseases 2012;33 Suppl 2:459-60. [Google Scholar]
- Heiss WD, Brainin M, Bornstein N, Zhen H. Cerebrolysin in patients with acute ischemic stroke - the CASTA study. International Journal of Stroke 2010;5 Suppl 2:71. [Google Scholar]
- Heiss WD, Brainin M, Bornstein NM, Tuomilehto J, Hong Z, Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators. Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke 2012;43(3):630-6. [DOI] [PubMed] [Google Scholar]
- Hong Z, Moessler H, Bornstein N, Brainin M, Heiss W-D, CASTA Investigators. A double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of cerebrolysin in patients with acute ischaemic stroke in Asia - CASTA. International Journal of Stroke 2009;4(5):406-12. [DOI] [PubMed] [Google Scholar]
- NCT00868283. The safety and efficacy of cerebrolysin in patients with acute ischemic stroke (CASTA). clinicaltrials.gov/show/NCT00868283 (first received 24 March 2009).
CERE‐LYSE‐1 2012 {published data only}
- Lang W, Stadler CH, Poljakovic Z, Fleet D, Lyse Study Group. A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy combined treatment with alteplase (rt-PA) and cerebrolysin in acute ischaemic hemispheric stroke. International Journal of Stroke 2012;8(2):95-104. [DOI] [PubMed] [Google Scholar]
- NCT00840671. Combined treatment with alteplase (Rt-PA) and cerebrolysin® in acute ischemic hemispheric stroke (CERE-LYSE). clinicaltrials.gov/show/NCT00840671 (first received 10 February 2009).
Gharagozli 2017 {published data only}
- Gharagozli K, Harandi AA, Houshmand S, Akbari N, Muresanu DF, Vester J, et al. Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. Journal of Medicine and Life 2017;10(3):153-60. [PMC free article] [PubMed] [Google Scholar]
- IRCT138803272042N1. The efficacy of cerebrolysin in the treatment of acute ischemic stroke. en.irct.ir/trial/1663 (first received 29 August 2009).
Ladurner 2005 {published data only}
- Ladurner G, Gmeinbauer R, Moessler H. Cerebrolysin in acute ischaemic stroke: a randomized, placebo-controlled trial with a neuroprotective agent. Cerebrovascular Diseases 2001;11(4):75. [Google Scholar]
- Ladurner G, Kalvach P, Moessler H, Cerebrolysin Study Group. Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of Neural Transmission 2005;112(3):415-28. [DOI] [PubMed] [Google Scholar]
- Ladurner G. Neuroprotection in acute ischaemic stroke. Stroke 2001;32 Suppl 1:323. [Google Scholar]
Skvortsova 2004 {published data only}
- Skvortsova VI, Shamalov NA, Stakhovskaya LV, Gubsky LV, Tikhonova IV, Smichkov AS. Cerebrolysin in acute ischaemic stroke: results of randomised, double blind, placebo-controlled study. Cerebrovascular Diseases 2005;19 Suppl 2:76. [Google Scholar]
- Skvortsova VI, Stakhovskaia LV, Gubskii LV, Shamalov NA, Tikhonova IV, Smychkov AS. A randomized, double-blind, placebo-controlled study of Cerebrolysin safety and efficacy in the treatment of acute ischemic stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2004;Suppl 11:51-5. [PubMed] [Google Scholar]
- Skvortsova VI, Stakhovskaya LV, Gubsky LV, Shamalov NA, Tikhonova IV, Smychkov AS. Evaluation of safety and efficacy of cerebrolysin for treating acute ischaemic stroke [Issledovaniye bezopasnosti i effektivnosti tserebrolizina dlya lecheniya ostrogo ishemicheskogo insul’ta]. Рецепт [Prescription] 2007;5(55):94-8. [Google Scholar]
Xue 2016 {published data only}
- NCT02149875. Dl-3-n-butylphthalide and cerebrolysin treatment in acute ischemic stroke. clinicaltrials.gov/show/NCT02149875 (first received 29 May 2014).
- Xue LX, Zhang T, Zhao YW, Geng Z, Chen JJ, Chen H. Efficacy and safety comparison of DL-3-n-butylphthalide and cerebrolysin: effects on neurological and behavioral outcomes in acute ischemic stroke. Experimental and Therapeutic Medicine 2016;11(5):2015-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 2011 {published data only}
- Alvarez XA, Cacabelos R, Sampedro C, Couceiro V, Aleixandre M, Vargas M, et al. Combination treatment in Alzheimer's disease: results of a randomized, controlled trial with cerebrolysin and donepezil. Current Alzheimer Research 2011;8(5):583-91. [DOI: 10.2174/156720511796391863] [DOI] [PubMed] [Google Scholar]
- NCT00911807. Comparative study to test safety and efficacy of neurotrophic and cholinergic treatment of Alzheimer's disease (combi). clinicaltrials.gov/ct2/show/results/NCT00911807 (first received 2 June 2009).
Barolin 1996 {published data only}
- Barolin GS, Koppi S, Kapeller E. Old and new aspects of stroke treatment with emphasis on metabolically active medication and rehabilitative outcome. EuroRehab 1996;3:135-43. [Google Scholar]
Bavarsad Shahripour 2011 {published data only}
- Bavarsad Shahripour R, Shalil Ahmadi A. The efficacy of cerebrolysin in patients with acute ischemic stroke. In: 20. European Stroke Conference. 2011 May 24-27; Hamburg, Germany. 2011.
Bayer 1980 {published data only}
- Bayer E. Therapeutic use of a brain hydrolysate. Medizinische Welt 1980;31(17):636-7. [PubMed] [Google Scholar]
Capisizu 2016 {published data only (unpublished sought but not used)}
- Capisizu A, Aurelian SM, Mirsu-Paun A, Badiu C, Dascalescu R, Nica AS, et al. A key for post stroke rehabilitation in the elderly. Acta Medica Mediterranea 2016;32(4):1003-7. [Google Scholar]
Chang 2016 {published data only}
- Chang WH, Park CH, Kim DY, Shin YI, Ko MH, Lee A, et al. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurology 2016;16:31. [DOI: 10.1186/s12883-016-0553-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuparnecu 2001 {published data only}
- Cuparnecu B. Efficacy of cerebrolysin in patients with ischaemic stroke of the middle cerebral artery. Pharmacology and Toxicology 2001;89(Suppl 1):136. [Google Scholar]
Dobi 2010 {published data only}
- Dobi D, Xhaxho S, Kapisyzi M, Papadhopulli Z. The effect of cerebrolysin in acute ischemic stroke. Revue Neurologique 2010;166(3):355. [Google Scholar]
Domzal 1995 {published data only}
- Domzal T, Zaleska B. Cerebrolysin in treatment of acute ischemic stroke. Neurologia i Neurochirurgia Polska [Polish Journal of Neurology and Neurosurgery] 1995;29(3):325-31. [PubMed] [Google Scholar]
Ershov 2009 {published data only}
- Ershov VI. Comparative characteristics of the efficacy of neuroprotectors in the treatment of ischaemic stroke [Sravnitel’naya kharakteristika effektivnosti neyroprotektorov v lechenii ishemicheskogo insul’ta]. Nevrologichesky Vestnik. Zhurnal im. VM Bekhtereva [Neurological Bulletin] 2009;41(3):65-9. [УДК: 616.831-005.1-08УДК: 616.831-005.1-08] [Google Scholar]
Ershov 2011 {published data only}
- Ershov VI. Comparative aspects of the use of neuroprotectors in the management of patients with ischemic stroke [Sravnitel’nyye aspekty primeneniya neyroprotektorov pri vedenii bol’nykh s ishemicheskim insul’tom]. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of of Neurology and Psychiatry] 2011;111(8 Pt 2):41-4. [PubMed] [Google Scholar]
Gasecki 2016 {published data only}
- Gasecki D, Kwarciany M, Kowalczyk K, Nowicki T, Szurowska E, Boutouyrie P, et al. Pulse wave velocity is associated with early cerebral lesion growth in ischemic stroke. European Stroke Journal 2016;1(1S):680. [DOI: 10.1177/2396987316642910] [AS15-006] [DOI] [Google Scholar]
Gheorghias 2019 {published data only}
- Gheorghias M, Andrei D, Poenaru DV, Banariu GM, Miclau O, Preda MA, et al. Elaborate ways for rehabilitation stroke patients using drug treatment with cerebrolysin vs. advanced physical therapy techniques. Revista de Chimie 2019;70(6):2269-72. [Google Scholar]
Guekht 2015a {published data only}
- Guekht A, Heiss WD, Bajenaru OA, Popescu C, Gusev E, Vester JC, et al. Cerebrolysin and recovery after stroke (CARS): a meta-analysis of two randomized, controlled, double-blind trials assessing the combination of Cerebrolysin and standardized rehabilitation on motor recovery. European Journal of Neurology 2015;22:598. [Google Scholar]
Guekht 2015b {published data only}
- Guekht A, Heiss D, Gusev E, Vester J, Doppler E, Muresanu D. Cerebrolysin and recovery after stroke (CARS 2): a randomized, placebo-controlled, double-blind, multicenter clinical study. Journal of the Neurological Sciences 2015;357:e103. [Google Scholar]
Guekht 2016 {published data only}
- Guekht A, Doppler E, Vester JC, Muresanu D. The impact of proper handling of ARAT zero scores on the heterogeneity of data in rehabilitation studies. European Journal of Neurology 2016;23(Suppl 2):725. [DOI: 10.1111/ene.13094] [P31245] [DOI] [Google Scholar]
Guekht 2017 {published data only}
- Guekht A, Vester J, Heiss WD, Gusev E, Hoemberg V, Rahlfs VW, et al. Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurological Sciences 2017;38(10):1761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haffner 2001 {published data only}
- Haffner Z, Gmeinbauer R, Moessler H. A randomized, double-blind, placebo-controlled trial with cerebrolysin in acute ischaemic stroke. Cerebrovascular Diseases 2001;11(Suppl 4):76. [Google Scholar]
Hassanein 2016 {published data only}
- Hassanein SMA, Deifalla SM, El-Houssinie M, Mokbel SA. Safety and efficacy of cerebrolysin in infants with communication defects due to severe perinatal brain insult: a randomized controlled clinical trial. Journal of Clinical Neurology 2016;12(1):79-84. [DOI: 10.3988/jcn.2016.12.1.79] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hong 2005 {published data only}
- Hong Z, Zhu G, Chen H. The clinical efficacy of cerebrolysin in the treatment of acute ischemic stroke. Chinese Journal of Geriatric Heart Brain and Vessel Diseases 2005;7(5):331-3. [Google Scholar]
Imahori 2016 {published data only}
- Imahori T, Fujita A, Hosoda K, Kohmura E. Acute ischemic stroke involving both anterior and posterior circulation treated by endovascular revascularization for acute basilar artery occlusion via persistent primitive trigeminal artery. Journal of the Korean Neurosurgical Society 2016;59(4):400-4. [DOI: 10.3340/jkns.2016.59.4.400] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT201107226907N3 {published data only}
- IRCT201107226907N3. The effect of Cerebrolysin in cerebral palsy [Study the effects of cerebrolysin on the improvement of spasticity and behavioral development of patients with cerebral palsy]. en.irct.ir/trial/7339 (first received 2 December 2015).
IRCT2014040312888N2 {published data only}
- IRCT2014040312888N2. The effect of cerebrolysin on patients with head trauma and brain injury [The effect of cerebrolysin on patients with head trauma and diffuse axonal injury]. en.irct.ir/trial/12894 (first received 20 May 2014).
Jianu 2010 {published data only}
- Jianu DC, Muresanu DF, Bajenaru O, Popescu BO, Deme SM, Moessler H, et al. Cerebrolysin adjuvant treatment in Broca's aphasics following first acute ischemic stroke of the left middle cerebral artery. Journal of Medicine and Life 2010;3(3):297-307. [PMC free article] [PubMed] [Google Scholar]
Jianu 2015 {published data only}
- Jianu DC, Jianu SN, Petrica L, Deme SM, Dan F, Falup Pecurariu C, et al. Cerebrolysin adjuvant treatment in Broca's aphasics following first acute ischemic stroke of the left middle cerebral artery. International Journal of Stroke 2015;10:187. [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim YH, Kim DY, Shin YI, Ko MH, Chang WH, Lee AH. Effects of cerebrolysin on motor recovery in patients with subacute stroke. International Journal of Stroke 2014;9:107-8. [Google Scholar]
- Kim YH, Park CH, Kim DY, Shin YI, Ko MH, Chang WH, et al. Effects of cerebrolysin on motor recovery of stroke patients: a diffusion tensor imaging study. International Journal of Stroke 2014;9:49. [Google Scholar]
Kim 2015 {published data only}
- Kim YH, Kim DY, Shin YI, Ko MH, Chang WH, Lee AH. Effects of cerebrolysin on motor recovery in patients with severe stroke motor impairment after stroke. International Journal of Stroke 2015;10 Suppl 2:90. [Google Scholar]
Kim 2016 {published data only}
- Kim JS, Kim BJ. Why are CASTA and CARS results different? Analysis of CASTA data from Korea and Hong Kong. Journal of Stroke 2016;18(2):233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim JY, Kim HJ, Choi HS, Park SY, Kim DY. Effects of cerebrolysin in patients with minimally conscious state after stroke: an observational retrospective clinical study. Frontiers in Neurology 2019;10:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lebedeva 2018 {published data only}
- Lebedeva EV, Gorokhov AS, Schastnyy ED, Repin AN, Simutkin GG, Shishneva EV, et al. Time course of cognitive dysfunction and biochemical marker of CNS lesions S100 beta in coronary artery bypass graft [Dinamika kognitivnoy disfunktsii i biokhimicheskogo markera povrezhdeniya TSNS S100ß pri koronarnom sh·chntirovanii]. Бюллетень сибирской медицины [Bulletin of Siberian Medicine] 2018;17(4):72-84. [DOI: ] [DOI] [Google Scholar]
Lees 2019 {published data only}
- Lees KR, Bornstein NM, Brainin M, Dawson J, Dichgans M, Heiss D, et al. Cerebrolysin registry study in stroke (CREG-S): final results. European Stroke Journal 2019;4 Suppl 1:784. [Google Scholar]
- NCT02541227. Cerebrolysin registry study in stroke (CREGS-S) [Cerebrolysin registry study in stroke a registry study to assess practices, safety and effectiveness of cerebrolysin in routine treatment of acute ischemic stroke]. clinicaltrials.gov/ct2/show/NCT02541227 (first received 4 September 2015).
Martinez 2017 {published data only}
- Martinez RM. Efficacy of Cerebrolysin in the reduction of spasticity during stroke rehabilitation. Journal of Medicine and Life 2017;10(3):161-6. [PMC free article] [PubMed] [Google Scholar]
Martinez Sanchez 2015 {published data only}
- Martinez Sanchez BR, Hernandez Hernandez JJ, Franco Del Aguila DI, Gryzbowski Gainza E, Paz Ballesteros WC, Herrera Rojas J, et al. An open label, one arm study to evaluate the efficacy and safety of cerebrolysin in patients with acute severe ischemic stroke in Mexico. Value in Health 2015;18(7):A827-8. [Google Scholar]
Melnikova 2018 {published data only}
- Melnikova E, Maltseva M, Shmonin A. Combination of cerebrolysin and occupational therapy for men with post-stroke depression. European Stroke Journal 2018;3(1S):131. [DOI: 10.1177/2396987318770127] [AS04-047] [DOI] [Google Scholar]
Moskovko 2019 {published data only}
- Moskovko S. Cerebrolysin after thrombolysis: is the positive trend sustainable? European Stroke Journal 2019;4(1S):251. [DOI: 10.1177/2396987319845581] [AS02-065] [DOI] [Google Scholar]
Muresanu 2015 {published data only}
- EUCTR2007-000870-21. Cerebrolysin And Recovery after Stroke (CARS) - a randomized, placebo-controlled, double-blind, multicenter, phase II clinical study. www.clinicaltrialsregister.eu/ctr-search/search?query=2007-000870-21 (first received 29 October 2009).
- Muresanu D, Heiss WD, Bajenaru O, Popescu CD, Vester J, Guekht A. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter, phase II clinical study. International Journal of Stroke 2015;10 Suppl 2:92. [Google Scholar]
Muresanu 2016a {published data only}
- Muresanu D, Heiss WD, Bajenaru O, Popescu CD, Vester J, Guekht A. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter, phase II clinical study. International Journal of Stroke 2015;10 Suppl 2:92. [Google Scholar]
- Muresanu DF, Heiss WD, Hoemberg V, Bajenaru O, Popescu CD, Vester JC, et al. Cerebrolysin and recovery after stroke (CARS): a randomized, placebo-controlled, double-blind, multicenter trial. Stroke 2016;47(1):151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muresanu 2016b {published data only}
- Muresanu D, Sharma A, Lafuente J, Patnaik R, Tian Z, Ozkizilcik A, et al. Nanowired cerebrolysin potentiates mesenchymal stem sells induced neuroprotection and neurorepair following heat stroke. Journal of Cerebral Blood Flow & Metabolism 2016;36:317. [Google Scholar]
Muresanu 2018 {published data only}
- Muresanu D, Johannes V, Adina S, Vitalie V. The association of stroke severity with discriminative power of selected endpoints in clinical trials. European Stroke Journal 2018;3 Suppl 1:121. [DOI: 10.1177/2396987318770127] [DOI] [Google Scholar]
- Muresanu FD, Vester JC, Stan A. The impact of stroke severity on the outcome of clinical trials. European Journal of Neurology 2018;25 Suppl 2:208. [Google Scholar]
Nag 2017 {published data only}
- Nag M, Koley S, Das D, Chakraborty C, Sadhu A, Ghosh N. Computer assisted identification of acute ischemic infarct using CT number and wavelet features from CT images. International Journal of Computer Assisted Radiology and Surgery 2017;12(Suppl 1):155-7. [DOI: 10.1007/s11548-017-1588-3] [DOI] [Google Scholar]
Nasiri 2017 {published data only}
- Nasiri J, Safavifar F. Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial. Acta Neurologica Belgica 2017;117(2):501-5. [DOI: 10.1007/s13760-016-0743-x] [DOI] [PubMed] [Google Scholar]
NCT00947531 {published data only}
- NCT00947531. A clinical trial to evaluate the safety and efficacy of 20 ml cerebrolysin in patients with vascular dementia. clinicaltrials.gov/ct2/show/NCT00947531 (first received 28 July 2009).
NCT01059461 {published data only}
- NCT01059461. Study of cerebrolysin for treatment of infants with history of neonatal hypoxic ischemic encephalopathy [Phase 2 nerve growth factor (Cerebrolysin®) for treatment of neonatal hypoxic ischemic encephalopathy]. clinicaltrials.gov/ct2/show/NCT01059461 (first received 1 February 2010).
NCT01388738 {published and unpublished data}
- NCT01388738. Navigation brain stimulation for evaluation of the neuroprotective drug efficiency in patients after ischemic stroke. clinicaltrials.gov/ct2/show/NCT01388738 (first received 7 July 2011).
NCT01606111 {published data only}
- NCT01606111. The CAPTAIN trial: cerebrolysin Asian Pacific trial in acute brain injury and neurorecovery (CAPTAIN) [A randomized, double-blind, placebo-controlled trial to investigate safety and efficacy of cerebrolysin in patients with traumatic brain injury]. clinicaltrials.gov/ct2/show/NCT01606111 (first received 25 May 2012).
NCT01787123 {published data only}
- NCT01787123. Randomized, double-blind, placebo-controlled trial to investigate safety and efficacy of Cerebrolysin™ in patients with aneurysmal subarachnoid hemorrhage (CESAR). clinicaltrials.gov/show/NCT01787123 (first received 8 February 2013).
NCT01822951 {published data only}
- NCT01822951. Cerebrolysin compared to donepezil in patients with mild to moderate dementia of Alzheimer's type (DAT) [Comparison of cerebrolysin and donepezil: a randomized, double-blind, controlled trial on efficacy and safety in patients with mild to moderate Alzheimer's disease]. clinicaltrials.gov/ct2/show/NCT01822951 (first received 4 April 2013).
NCT01996761 {published data only}
- NCT01996761. Effects of cerebrolysin on motor recovery in patients with subacute stroke (E-COMPASS). clinicaltrials.gov/ct2/show/NCT01996761 (first received 27 November 2013).
NCT02116348 {published data only}
- NCT02116348. Cerebrolysin neural repair therapy in children with traumatic brain injury and cerebral palsy [Effect of nerve growth factor (cerebrolysin) therapy on neurodevelopment, sleep pattern and quality of life in children with traumatic brain injury and cerebral palsy]. clinicaltrials.gov/ct2/show/NCT02116348 (first received 16 April 2014).
NCT02581371 {published and unpublished data}
- NCT02581371. Comprehensive reparative therapy in ischemic stroke complex repair in ischemic stroke-arm. clinicaltrials.gov/ct2/show/NCT02581371 (first received 21 October 2015).
NCT02768571 {published and unpublished data}
- NCT02768571. Effects of cerebrolysin combined with rehabilitation on motor recovery in stroke. www.clinicaltrials.gov/ct2/show/NCT02768571 (first received 11 May 2016).
NCT03480698 {published data only}
- NCT03480698. Cerebrolysin registry study in stroke - a high-quality observational study of comparative effectiveness (C-REGS2) [C-REGS2 - A registry study to observe clinical practices, safety and efficiency of routine use of cerebrolysin in the treatment of patients with moderate to severe neurological deficits after acute ischaemic stroke]. clinicaltrials.gov/show/NCT03480698 (first received 29 March 2018).
NCT03506841 {published data only}
- NCT03506841. Cerebrolysin and neurodevelopment in preterm infants [Efficacy and safety of cerebrolysin on neurodevelopmental outcome of preterm infants]. clinicaltrials.gov/ct2/show/NCT03506841 (first received 24 April 2018). [Mansoura NICU 2016]
Park 2018 {published data only}
- Park YK, Yi H-J, Choi K-S, Lee Y-J, Kim D-W, Kwon SM. Cerebrolysin for the treatment of aneurysmal subarachnoid hemorrhage in adults: a retrospective chart review. Advances in Therapy 2018;35(12):2224-35. [DOI: 10.1007/s12325-018-0832-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poljakovic 2019 {published data only}
- Poljakovic Z. Possible synergistic effect of thrombolytic therapy with alteplase and systemic cerebrolysine in the acute stroke patients. European Stroke Journal 2019;4(1S):277. [DOI: 10.1177/2396987319845581] [AS04-060] [DOI] [Google Scholar]
Pushkarev 2015 {published data only}
- Pushkarev K, Tsoy R. Neuroprotective therapy for lacunar stroke - which is better Ceraxon or Cerebrolysin? European Journal of Neurology 2015;22:775. [Google Scholar]
RDPh_12_03 {published data only}
- RDPh_12_03. Double-blind placebo-controlled parallel group randomised clinical trial of effectiveness and safety of cerebrolysin in patients with chronic brain ischaemia and non-dementia cognitive impairment [Dvoynoye slepoye platsebo-kontroliruyemoye prospektivnoye v paralell’nykh gruppakh randomizirovannoye klinicheskoye issledovaniye effektivnosti i bezopasnosti preparata TSerebrolizin®]. clinline.ru/reestr-issledovatelej/podrobno-ob-issledovatele.html?id=2668 (first received 3 November 2013).
Shamalov 2005 {published data only}
- Shamalov NA, Stakhovskaya LV, Gubsky LV, Tikhonova IV, Smichkov AS, Skvortsova VI, et al. Effects of the neuroprotective drug cerebrolysin on the infarct volume after acute ischemic stroke. Cerebrovascular Diseases 2005;19(Suppl 2):107. [Google Scholar]
Shamalov 2010 {published data only}
- Shamalov NA, Stakhovskaia LV, Burenchev DV, Kichuk IV, Tvorogova TV, Botsina AI, et al. The effect of cerebrolysin in dosage 50 ml on the volume of lesion in ischemic stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2010;110(12 Pt 2):34-7. [PubMed] [Google Scholar]
Shishkova 2015 {published data only}
- Shishkova V, Zotova LI, Remennik AYU. Association between BDNF-196 G>A and BDNF-270 C>T polymorphisms, BDNF concentration, and cerebrolysin treatment outcome after ischemic stroke. International Journal of Stroke 2015;10 Suppl 2:170. [Google Scholar]
Shishkova 2016 {published data only}
- Shishkova V. An assessment of neurotrophic therapy effect on ciliary neurotrophic factor level in patients with post stroke aphasia and diabetes mellitus. European Stroke Journal 2016;1(1S):354. [DOI: 10.1177/2396987316642909] [AS03-030] [DOI] [Google Scholar]
- Shishkova V. An assessment of neurotrophic therapy effect on nerve growth factor level in patients with post stroke aphasia and diabetes mellitus. Journal of Hypertension 2016;34 Suppl 2:e227. [DOI: 10.1097/01.hjh.0000491991.88309.0e] [DOI] [Google Scholar]
Shul'ginova 2016 {published data only}
- Shul'ginova AA, Laskov VB, Konoplya AI, Karaulov AV. Pharmacological correction of red blood cell membrane lipid spectrum in patients with chronic cerebral ischemia on the background of hypertensive disease. Eksperimental'naya i Klinicheskaya Farmakologiya [Experimental and Clinical Pharmacology] 2016;79(7):3-7. [PubMed] [Google Scholar]
Skvortsova 2006 {published data only}
- Skvortsova VI, Stakhovskaia LV, Shamolov NA, Kerbikov OB. Results of the multicenter prospective study of cerebrolysin safety and efficacy in acute stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2006;Suppl 16:41-5. [PubMed] [Google Scholar]
Skvortsova 2008 {published data only}
- Jech M, Shamalov NA, Moessler H, Skvortsova VI. Safety and efficacy of cerebrolysin as adjuvant therapy in patients suffering from acute ischemic stroke. Cerebrovascular Diseases 2009;27 Suppl 6:84. [Google Scholar]
- Skvortsova VI, Shamalov NA, Moessler H, Doppler E. Safety and efficacy of cerebrolysin as adjuvant therapy in patients suffering from acute ischemic stroke. European Journal of Neurology 2008;15 Suppl 3:253. [Google Scholar]
- Skvortsova VI, Shamalov NA, Moessler H, Novak PH. Beneficial effects of the neurotrophic drug cerebrolysin on the infarct volume after acute stroke. Cerebrovascular Diseases 2008;25 Suppl 2:145. [Google Scholar]
- Skvortsova VI, Shamalov NA, Moessler H, Novak PH. Positive impacts of the neurotrophic drug cerebrolysin on the infarct volume after acute stroke. International Journal of Stroke 2008;3 Suppl 1:137. [Google Scholar]
Stan 2013 {published data only}
- Stan AD, Badisor A, Birle C, Blesneag AV, Opincariu I, Iancu M, et al. The influence of neurotrophic factors treatment on stroke volume. Romanian Journal of Neurology 2013;12(3):124-9. [Google Scholar]
Stan 2017 {published data only}
- Stan A, Birle C, Blesneag A, Iancu M. Cerebrolysin and early neurorehabilitation in patients with acute ischemic stroke: a prospective, randomized, placebo-controlled clinical study. Journal of Medicine and Life 2017;10(4):216-22. [PMC free article] [PubMed] [Google Scholar]
Stanescu 2017 {published data only}
- Stanescu I, Dogaru G, Bulboaca A, Stan A, Stanca D, Blesneag A, et al. Combined pharmacological and motor training interventions for recovery of upper limb function in subacute ischemic stroke. Balneo Research Journal 2017;8(3):114-20. [DOI: 10.12680/balneo.2017.145 ] [Google Scholar]
Uivarosan 2018 {published data only}
- Uivarosan D, Abdel-Daim MM, Endres L, Purza L, Iovan C, Bungau S, et al. Effects of a proteic swine extract associated to recovery treatment on functional independence and quality of life in patients post stroke. Farmacia 2018;66(5):826-30. [DOI: 10.31925/farmacia.2018.5.12] [DOI] [Google Scholar]
Uivarosan 2019 {published data only}
- Uivarosan D, Tit DM, Iovan C, Nistor-Cseppento DC, Endres L, Lazar L, et al. Effects of combining modern recovery techniques with neurotrophic medication and standard treatment in stroke patients. Science of the Total Environment 2019;678:80-7. [DOI] [PubMed] [Google Scholar]
Vilenskii 1999 {published data only}
- Vilenskii BS, Semenova GM, Shirokov EA. The application of cerebrolysine in ischemic stroke. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 1999;99(4):65-9. [PubMed] [Google Scholar]
Vilenskii 2007 {published data only}
- Vilenskii BC, Kuznetsov AN, Vinagradov OI. A new direction for cerebrolysin use - repeated courses to survivors of hemispheric ischaemic stroke [Новое направление применения церебролизина - повторное крусовое введение препарата больным, перенешим полушарный ишемический инсульт]. Nevrologichesky zhurnal [Neurological Journal] 2007;12(1):44-6. [Google Scholar]
Winkler 2018 {published data only}
- Winkler A, Zelenka I. Assessing the effects of a triple-combination-therapy in upper extremity motor recovery after stroke. International Journal of Stroke 2018;13(2S):18-9. [DOI: 10.1177/1747493018789543] [WSC18-0747] [DOI] [Google Scholar]
Yavorskaya 2008 {published data only}
- Yavorskaya VA, Bondar OB. Clinical features of cerebrolysin application in patients in acute period of ischemic stroke. International Journal of Stroke 2008;3 Suppl 1:141. [Google Scholar]
Zamfirescu 2017 {published data only}
- Zamfirescu A, Mirsu-Paun A, Dascalescu R, Capisizu A, Aurelian SM. Post-stroke neurorehabilitation in elderly patients. Journal of the Neurological Sciences 2017;381 Suppl 1:596-7. [DOI: 10.1016/j.jns.2017.08.1681] [DOI] [Google Scholar]
Zhu 2003 {published data only}
- Zhu GX, Hong Z, Yao JL, Yu LY. Double-blind and randomized placebo-controlled trial of cerebrolysin in improvement of nerve function and living ability in patients with ischemic stroke. Chinese Journal of Clinical Rehabilitation 2003;7(22):3084-5. [Google Scholar]
Zimin 2019 {published data only}
- Zimin I, Domashenko MA, Samorukov VYu, Arablinskiy AV, Logvinenko RL. To test whether cerebrolysin administration immediately after thrombectomy is feasible and safe in the stroke unit of Botkin Municipal Hospital, Moscow. European Stroke Journal 2019;4(1S):205. [DOI: 10.1177/2396987319845581] [AS35-093] [DOI] [Google Scholar]
References to ongoing studies
CERE‐REHA‐RU/01 {published data only}
- CERE-REHA-RU/01. Double-blind placebo-controlled multi-centre randomised parallel group clinical study of phase IV on effectiveness of adding cerebrolysin to standard rehabilitation complex interventions in patients with ischaemic stroke. grls.rosminzdrav.ru/CIPermissionMini.aspx?CIStatementGUID=70440249-c5c0-4928-979f-aac87894585a&CIPermGUID=7F9E91B4-F074-4BA4-9D79-7C730A0F32A0 (first received 19 January 2015).
IRCT201406169014N36 {published and unpublished data}
- IRCT201406169014N36. The effect of cerebrolysin versus placebo on improvement of patients with acute ischemic stroke: a double blinded randomized clinical trial. en.irct.ir/trial/9475 (first received 23 March 2014).
Additional references
AHA 2019
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke disease statistics - 2019 update: a report from the American Heart Association. Circulation 2019;139:e1-473. [DOI: 10.1161/CIR.0000000000000659] [DOI] [PubMed] [Google Scholar]
Alper 2015
- Alper BS, Malone-Moses M, McLellan JS, Prasad K, Manheimer E. Thrombolysis in acute ischaemic stroke: time for a rethink? BMJ 2015;350:h1075. [DOI: 10.1136/bmj.h1075] [DOI] [PubMed] [Google Scholar]
Alvarez 2000
- Alvarez XA, Lombardi VR, Corzo L, Pérez P, Pichel V, Laredo M, et al. Oral cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. Journal of Neural Transmission 2000;59:315-28. [DOI] [PubMed] [Google Scholar]
Appelros 2015
- Appelros P, Terént A. Thrombolysis in acute stroke. Lancet 2015;385(9976):1394. [DOI: 10.1016/S0140-6736(15)60714-0] [DOI] [PubMed] [Google Scholar]
Bajenaru 2010
- Bajenaru O, Tiu C, Moessler H, Antochi F, Muresanu D, Popescu BO, et al. Efficacy and safety of cerebrolysin in patients with hemorrhagic stroke. Journal of Medicine and Life 2010;3(2):137-43. [PMC free article] [PubMed] [Google Scholar]
Bath 2004a
- Bath PMW. Theophylline, aminophylline, caffeine and analogues for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD000211. [DOI: 10.1002/14651858.CD000211.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2004b
- Bath PMW, Bath-Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD000162. [DOI: 10.1002/14651858.CD000162.pub2] [DOI] [PubMed] [Google Scholar]
Bath 2004c
- Bath PMW. Prostacyclin and analogues for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD000177. [DOI: 10.1002/14651858.CD000177.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2013
- Bath PMW, Sprigg N, England T. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD005207. [DOI: 10.1002/14651858.CD005207.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2014
- Bath PMW, Krishnan K. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD000039. [DOI: 10.1002/14651858.CD000039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2017
- Bath PMW, Krishnan K, Appleton JP. Nitric oxide donors (nitrates), L-arginine, or nitric oxide synthase inhibitors for acute stroke. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD000398. [DOI: 10.1002/14651858.CD000398.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bereczki 2007
- Bereczki D, Fekete I, Prado GF, Liu M. Mannitol for acute stroke. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No: CD001153. [DOI: 10.1002/14651858.CD001153.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bereczki 2008
- Bereczki D, Fekete I. Vinpocetine for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD000480. [DOI: 10.1002/14651858.CD000480.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bereczki 2017
- Bereczki D. Hope dies last - evidence again fails to support a neuroprotectant cerebrolysin for acute ischemic stroke. Stroke 2017;48:2343-4. [DOI: 10.1161/STROKEAHA.117.018202] [DOI] [PubMed] [Google Scholar]
Berge 2002
- Berge E, Sandercock P. Anticoagulants versus antiplatelet agents for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD003242. [DOI: 10.1002/14651858.CD003242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boncoraglio 2019
- Boncoraglio GB, Ranieri M, Bersano A, Parati EA, Del Giovane C. Stem cell transplantation for ischemic stroke. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD007231. [DOI: 10.1002/14651858.CD007231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bornstein 2018
- Bornstein NM, Guekht A, Vester J, Heiss WD, Gusev E, Hömberg V, et al. Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurological Sciences 2018;39(4):629-40. [DOI: 10.1007/s10072-017-3214-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunström 2015
- Brunström M, Carlberg B. Thrombolysis in acute stroke. Lancet 2015;385(9976):1394–5. [DOI: 10.1016/S0140-6736(15)60715-2] [DOI] [PubMed] [Google Scholar]
Candelise 2001
- Candelise L, Ciccone A. Gangliosides for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2001, Issue 4. Art. No: CD000094. [DOI: 10.1002/14651858.CD000094] [DOI] [PubMed] [Google Scholar]
Chang 2014
- Chang TS, Jensen MB. Haemodilution for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD000103. [DOI: 10.1002/14651858.CD000103.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen X, Zhou M, Li Q, Yang J, Zhang Y, Zhang D, et al. Sanchi for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD006305. [DOI: 10.1002/14651858.CD006305.pub2] [DOI] [PubMed] [Google Scholar]
Chukanova 2005
- Chukanova EI. The effect of cerebrolysin on the clinical symptoms and the course of ischemic encephalopathy. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2005;105(1):42-5. [PubMed] [Google Scholar]
Ciccone 2014
- Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD005208. [DOI: 10.1002/14651858.CD005208.pub3] [DOI] [PubMed] [Google Scholar]
Covidence
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Cui 2019
- Cui S, Chen N, Yang M, Guo J, Zhou M, Zhu C. Cerebrolysin for vascular dementia. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD008900. [DOI: 10.1002/14651858.CD008900.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Emberson 2014
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384(9958):1929-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Ad Hoc Consensus 1998
- The European Ad Hoc Consensus Group. Neuroprotection as initial therapy in acute stroke. Third report of an ad hoc consensus group meeting. Cerebrovascular Diseases 1998;8(1):59-72. [DOI] [PubMed] [Google Scholar]
Feng 2011
- Feng S, Yang Q, Liu M, Li W, Yuan W, Zhang S, et al. Edaravone for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2011, Issue 12. Art. No: CD007230. [DOI: 10.1002/14651858.CD007230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fragoso 2002
- Fragoso Y, Dantas DC. Cerebrolysin for Alzheimer's disease. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD003801. [DOI: 10.1002/14651858.CD003801] [DOI] [Google Scholar]
French 2007
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006073. [DOI: 10.1002/14651858.CD006073] [DOI] [PubMed] [Google Scholar]
Gafurov 2004
- Gafurov BG, Alikulova NA. Clinical and pathogenetical peculiarities and treatment policy in ischemic stroke of elderly and old age. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2004;104 Suppl 11:44-6. [PubMed] [Google Scholar]
Gandolfo 2002
- Gandolfo C, Sandercock P, Conti M. Lubeluzole for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD001924. [DOI: 10.1002/14651858.CD001924] [DOI] [PubMed] [Google Scholar]
GBD Stroke Collaborators 2019
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18:439–58. [DOI: 10.1016/S1474-4422(19)30034-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geeganage 2010
- Geeganage C, Bath PMW. Vasoactive drugs for acute stroke. Cochrane Database of Systematic Reviews 2010, Issue 7. Art. No: CD002839. [DOI: 10.1002/14651858.CD002839.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gevaert 2015
- Gevaert B, D'Hondt M, Bracke N, Yao H, Wyendaele E, Vissers JPC, et al. Peptide profiling of Internet-obtained Cerebrolysin using high performance liquid chromatography – electrospray ionization ion trap and ultra high performance liquid chromatography – ion mobility – quadrupole time of flight mass spectrometry. Drug Testing and Analysis 2015;7:835-42. [DOI: 10.1002/dta.1817] [DOI] [PubMed] [Google Scholar]
Ginsberg 2016
- Ginsberg MD. Expanding the concept of neuroprotection for acute ischemic stroke: the pivotal roles of reperfusion and the collateral circulation. Progress in Neurobiology 2016;145-6:46-77. [DOI: 10.1016/j.pneurobio.2016.09.002] [DOI] [PubMed] [Google Scholar]
Goenka 2019
- Goenka L, Uppugunduri Satyanarayana CR, S SK, George M. Neuroprotective agents in acute ischemic stroke - a reality check. Biomedicine & Pharmacotherapy 2019;109:2539-47. [DOI: 10.1016/j.biopha.2018.11.041] [DOI] [PubMed] [Google Scholar]
GovRu 2019
- Government of the Russian Federation. Essential Medicines List for 2019 [Правительство российской федерации]. static.government.ru/media/files/K1fPEUszF2gmvwTkw74iPOASarj7KggI.pdf (accessed prior to 23 June 2020).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 27 June 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Gromova 2006
- Gromova OA, Tret'iakov VE, Moshkovskiĭ SA, Gusev EI, Nikonov AA, Val'kova LA, et al. An oligopeptide membrane fraction of cerebrolysin. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2006;106(7):68-70. [PubMed] [Google Scholar]
Gusev 2003
- Gusev EI, Skvortsova VI, Stakhovskaia LV. Epidemiology of stroke in Russia. Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova [Korsakov Journal of Neurology and Psychiatry] 2003;Suppl 8:4-9. [PubMed] [Google Scholar]
Gusev 2013
- Gusev EI, Martynov MYu, Kamtchatnov PR. Ischemic stroke: current status [Ishemichesky insult. Sovremennoye sostoyaniye problemy]. Nevrologiya [Neurology] 2013;83(5):2-7. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hao 2012
- Hao Z, Liu M, Counsell C, Wardlaw JM, Lin S, Zhao X. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD000091. [DOI: 10.1002/14651858.CD000091.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hong 2009
- Hong Z, Moessler H, Bornstein N, Brainin M, Heiss W-D, CASTA Investigators. A double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of cerebrolysin in patients with acute ischaemic stroke in Asia - CASTA. International Journal of Stroke 2009;4(5):406-12. [DOI] [PubMed] [Google Scholar]
Horton 2019
- Horton R. Offline: the gravy train of systematic reviews. Lancet 2019;394(16):1790. [DOI: 10.1016/S0140-6736(19)32766-7] [DOI] [PubMed] [Google Scholar]
ICH 2003
- ICH Expert Working Group. ICH Harmonised tripartite guideline. Post-approval safety data management: definitions and standards for expedited reporting E2D. database.ich.org/sites/default/files/E2D_Guideline.pdf 12 November 2003.
Lachin 2016
- Lachin JM. Fallacies of last observation carried forward. Clinical Trials 2016;13(2):161-8. [DOI: 10.1177/1740774515602688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonardi‐Bee 2007
- Leonardi-Bee J, Steiner T, Bath-Hextall F. Naftidrofuryl for acute stroke. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005478. [DOI: 10.1002/14651858.CD005478.pub2] [DOI] [PubMed] [Google Scholar]
Lindekleiv 2018
- Lindekleiv H, Berge E, Bruins Slot KMH, Wardlaw JM. Percutaneous vascular interventions versus intravenous thrombolytic treatment for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009292. [DOI: 10.1002/14651858.CD009292.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016
- Liu B, Tan Y, Wang D, Liu M. Puerarin for ischaemic stroke. Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No: CD004955. [DOI: 10.1002/14651858.CD004955.pub3] [DOI] [PubMed] [Google Scholar]
Liu 2018
- Liu J, Zhang J, Wang LN. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009622. [DOI: 10.1002/14651858.CD009622.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2002
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annual Review of Public Health 2002;23:151-69. [DOI: 10.1146/annurev.publheath.23.100901.140546] [DOI] [PubMed] [Google Scholar]
Makarenko 2005
- Makarenko AN, Kositsin NS, Nazimov IV, Svinov MM, Goloborod'ko EV, Pasikova NV. A comparative study of antistroke activity of the new drug "cerebral" and its fractions in rats [Сравнительное изучение антиинсультной активности церебрала и его отдельных субфракций в эксперименте]. Eksperimental'naia i Klinicheskaia Farmakologiia [Experimental and Clinical Pharmacology] 2005;68(2):15-20. [PubMed] [Google Scholar]
Martynchik 2013
- Martynchik SA, Sokolova OV. Medical and economic assessment and rationale for improving organization of inpatient care for cerebral stroke [Mediko-ekonomicheskaya otsenka i obosnovaniye sovershenstvovaniya organizatsionnykh form okazaniya statsionarnoy pomoshchi pri mozgovom insul’te]. Sotsial’nyye aspekty zdorov’ya naseleniya [Social Aspects of Population Health ] 2013;30(2):e-pub without numbered pages. [vestnik.mednet.ru/content/view/473/30/lang,ru/] [Google Scholar]
Masliah 2012
- Masliah E, Díez-Tejedor E. The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs of Today 2012;48 Suppl A:3-24. [DOI: 10.1358/dot.2012.48(Suppl.A).1739716] [DOI] [PubMed] [Google Scholar]
MedDRA 2011
- MedDRA Maintenance and Support Services Organization. Introductory Guide to MedDRA Version 14.0. www.meddra.org/how-to-use/support-documentation (accessed prior to 23 June 2020).
Molnar 2008
- Molnar FJ, Hutton B, Fergusson D. Does analysis using “last observation carried forward” introduce bias in dementia research? Canadian Medical Association Journal 2008;179(8):751-3. [DOI: 10.1503/cmaj.080820] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muir 2003
- Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD001244. [DOI: 10.1002/14651858.CD001244] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Rourke 2010
- O'Rourke K, Berge E, Walsh CD, Kelly PJ. Percutaneous vascular interventions for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2010, Issue 10. Art. No: CD007574. [DOI: 10.1002/14651858.CD007574.pub2] [DOI] [PubMed] [Google Scholar]
Onishchenko 2006
- Onishchenko LS, Gaikova ON, Ianishevskii SN. Changes in the focus of experimental ischemic stroke under the influence of neuroprotective drugs. Morfologiia [Morphology] 2006;130(6):40-6. [PubMed] [Google Scholar]
Registry of Medicines 2019
- Registry of Medicines. Cerebrolysin [Церебролизин® (Cerebrolysin®)]. www.rlsnet.ru/tn_index_id_3528.htm (accessed prior to 23 June 2020).
Retraction Watch
- Marcus A, Oransky I. Retraction Watch. retractionwatch.com (accessed 17 November 2019).
Retraction Watch Database
- Marcus A, Oransky I. Retraction Watch Database. retractiondatabase.org/RetractionSearch.aspx? (accessed 17 November 2019).
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci 2012a
- Ricci S, Celani MG, Cantisani TA, Righetti E. Piracetam for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD000419. [DOI: 10.1002/14651858.CD000419.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricci 2012b
- Ricci S, Dinia L, Del Sette M, Anzola P, Mazzoli T, Cenciarelli S, et al. Sonothrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD008348. [DOI: 10.1002/14651858.CD008348.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Righetti 2004
- Righetti E, Celani MG, Cantisani T, Sterzi R, Boysen G, Ricci S. Glycerol for acute stroke. Cochrane Database of Systematic Reviews 2004, Issue 2. Art. No: CD000096. [DOI: 10.1002/14651858.CD000096.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2006
- Riley C, Hutter-Paier B, Windisch M, Doppler E, Moessler H, Wronski R. A peptide preparation protects cells in organotypic brain slices against cell death after glutamate intoxication. Journal of Neural Transmission 2006;113(1):103-10. [DOI] [PubMed] [Google Scholar]
Salim 2008
- Salim A, Mackinnon A, Christensen H, Griffiths K. Comparison of data analysis strategies for intent-to-treat analysis in pre-test–post-test designs with substantial dropout rates. Psychiatry Research 2008;160(3):335-45. [DOI: 10.1016/j.psychres.2007.08.005] [DOI] [PubMed] [Google Scholar]
Sandercock 2009
- Sandercock PAG, Gibson LM, Liu M. Anticoagulants for preventing recurrence following presumed non-cardioembolic ischaemic stroke or transient ischaemic attack. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD000248. [DOI: 10.1002/14651858.CD000248.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandercock 2011
- Sandercock PAG, Soane T. Corticosteroids for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD000064. [DOI: 10.1002/14651858.CD000064.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandercock 2014
- Sandercock PAG, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD000029. [DOI: 10.1002/14651858.CD000029.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandercock 2015
- Sandercock PAG, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD000024. [DOI: 10.1002/14651858.CD000024.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandercock 2017
- Sandercock PAG, Leong TS. Low-molecular-weight heparins or heparinoids versus standard unfractionated heparin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD000119. [DOI: 10.1002/14651858.CD000119.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sapronov 2005
- Sapronov NS, Bul'on VV, Kuznetsova NN, Selina EN. The neuroprotector effect of a new taurine derivative on a model of compression spinal cord trauma in rats [Neyroprotektornyy effekt novogo proizvodnogo taurina pri kompressionnoy travme spinnogo mozga]. Eksperimental'naya i Klinicheskaya Farmakologiya [Experimental and Clinical Pharmacology] 2005;68(6):45-8. [PubMed] [Google Scholar]
Schauer 2006
- Schauer E, Wronski R, Patockova J, Moessler H, Doppler E, Hutter-Paier B, et al. Neuroprotection of Cerebrolysin in tissue culture models of brain ischemia: post lesion application indicates a wide therapeutic window. Journal of Neural Transmission 2006;113(7):855-68. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shu 2012
- Shu LLS, San Jose CZ, Pasco PP. The efficacy and safety of cerebrolysin in acute hemorrhagic stroke: a meta analysis. Cerebrovascular Diseases 2012;34 Suppl 1:36. [Google Scholar]
Shuaib 2007
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. New England Journal of Medicine 2007;357(6):562-71. [DOI] [PubMed] [Google Scholar]
Suslina 2009
- Suslina ZA, Varakin YY, Vereshchagin NV. Clinical and epidemiological research - a promising area for the study of cerebrovascular pathology (first report) [Kliniko-epidemiologicheskiye issledovaniya – perspektivnoye napravleniye izucheniya tserebrovaskulyarnoy patologii (soobshcheniye pervoye)]. Annaly klinicheskoy i eksperimental’noy nevrologii [Annals of Clinical and Experimental Neurology] 2009;3(3):4-11. [Google Scholar]
TISC 2001
- Bath PM, Iddenden R, Bath FJ, Orgogozo JM, Tirilazad International Steering Committee. Tirilazad for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2001, Issue 4. Art. No: CD002087. [DOI: 10.1002/14651858.CD002087] [DOI] [PubMed] [Google Scholar]
Vilenskiĭ 2006
- Vilenskii BS, Iakhno NN. The problem of cerebral stroke: its contemporary state. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk [Bulletin of the Russian Academy of Medical Sciences] 2006;9-10:18-24. [PubMed] [Google Scholar]
Visvanathan 2015
- Visvanathan A, Dennis M, Whiteley W. Parenteral fluid regimens for improving functional outcome in people with acute stroke. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011138. [DOI: 10.1002/14651858.CD011138.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017
- Wang Z, Shi L, Xu S, Zhang J. Cerebrolysin for functional recovery in patients with acute ischemic stroke: a meta-analysis of randomized controlled trials. Drug Design, Development and Therapy 2017;11:1273-82. [DOI: 10.2147/DDDT.S124273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wardlaw 2013
- Wardlaw JM, Koumellis P, Liu M. Thrombolysis (different doses, routes of administration and agents) for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD000514. [DOI: 10.1002/14651858.CD000514.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wardlaw 2014
- Wardlaw JM, Murray V, Berge E, Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 7. Art. No: CD000213. [DOI: 10.1002/14651858.CD000213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019a
- World Health Organization. The Atlas of Heart Disease and Stroke. www.who.int/cardiovascular_diseases/resources/atlas/en/ (accessed November 2019).
WHO 2019b
- National Essential Medicines Lists. www.who.int/selection_medicines/country_lists (accessed November 2019).
Wong 2005
- Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Acta Neurochirurgica Supplement 2005;95:59-60. [DOI: 10.1007/3-211-32318-x_13] [DOI] [PubMed] [Google Scholar]
Wu 2015
- Wu S, Zeng Q, Liu M, Yang J, He S, Lin S, et al. Buflomedil for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No: CD009570. [DOI: 10.1002/14651858.CD009570.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2015
- Yang W, Shi Z, Yang HQ, Teng J, Zhao J, Xiang G. Mailuoning for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD007028. [DOI: 10.1002/14651858.CD007028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2005
- Zeng X, Liu M, Yang Y, Li Y, Asplund K. Ginkgo biloba for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD003691. [DOI: 10.1002/14651858.CD003691.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang CL, Chopp M, Cui YS, Wang L, Zhang RL, Zhang L, et al. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of Neuroscience Research 2010;88(15):3275-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019
- Zhang J, Liu J, Li D, Zhang C, Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD001928. [DOI: 10.1002/14651858.CD001928.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhuo 2008
- Zhuo Q, Yang X, Wu T, Liu G, Zhou L. Tongxinluo capsule for acute stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD004584. [DOI: 10.1002/14651858.CD004584.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ziganshina 2010a
- Ziganshina LE, Abakumova T, Kuchaeva A. Cerebrolysin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD007026. [DOI: 10.1002/14651858.CD007026.pub2] [DOI] [PubMed] [Google Scholar]
Ziganshina 2010b
- Ziganshina LE, Abakumova T, Kuchaeva A. Cerebrolysin for acute ischaemic stroke. International Journal of Risk and Safety in Medicine 2010;22(4):179-93. [Google Scholar]
Ziganshina 2013
- Ziganshina LE, Abakumova TR. Cerebrolysin for acute ischemic stroke. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk [Bulletin of the Russian Academy of Medical Sciences] 2013;1:21-9. [DOI] [PubMed] [Google Scholar]
Ziganshina 2015
- Ziganshina LE, Abakumova T. Cerebrolysin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD007026. [DOI: 10.1002/14651858.CD007026.pub3] [DOI] [PubMed] [Google Scholar]
Ziganshina 2016
- Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD007026. [DOI: 10.1002/14651858.CD007026.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziganshina 2017
- Ziganshina LE, Abakumova T, Vernay L. Cerebrolysin for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD007026. [DOI: 10.1002/14651858.CD007026.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


