Abstract
The impact of body mass index (BMI) on cardiovascular outcomes in patients receiving intensive low-density lipoprotein cholesterol (LDL-C) lowering therapy is uncertain. We performed meta-analysis of 29 randomized controlled trials using PubMed, Embase, and CENTRAL through April 2019. Therapies were grouped as more intensive LDL-C lowering therapy (statins, ezetimibe + statin or PCSK9 inhibitors) and less intensive LDL-C lowering therapy (less potent active control or placebo). Random effects meta-regressions and meta-analyses were performed to evaluate association of BMI with cardiovascular endpoints. In 265,766 patients, for every 1 kg/m2 increase in BMI, more intensive therapy compared with less intensive therapy was associated with hazard ratio (HR) of 1.07 for cardiovascular mortality (95% confidence interval 1.02 to 1.13); HR of 1.03 for all-cause mortality (0.99 to 1.06) HR of 1.06 for myocardial infarction (1.02 to 1.09), HR of 1.08 (1.03 to 1.12) for revascularization and HR of 1.04 for MACE (1.01 to 1.07). Meta-analysis showed that patients with BMI <25 kg/m2 had the highest risk reduction in mortality and cardiovascular outcomes compared with patients with BMI ≥30 kg/m2 (p-interaction ≤0.05). In conclusion, patients with normal BMI treated with intensive LDL-C lowering regimens may derive a larger clinical benefit compared with patients with larger BMI. The results could be due to the higher mortality rate of obese patients that may artificially lower the efficacy of therapy, or due to a true therapeutic limitation in these patients.
Low density lipoprotein cholesterol (LDL-C) is a well-established target for prevention of atherosclerotic cardiovascular disease (ASCVD); intensive LDL-C reduction with statins alone or in combination with ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, decreases the risk of major adverse cardiovascular events (MACE)1. Within this framework, obesity represents an independent predictor of ASCVD.2 Body mass index (BMI) is considered a noninvasive surrogate marker of body fat and screening tool for obesity and health risks.3 A recent study reported a monotonic correlation between maximum BMI measured over 24 years of weight history and total mortality, with an incremental risk in the obese I and II groups compared with normal weight group.3 Another collaborative analysis of 57 prospective studies showed that BMI was the strongest predictor of all-cause mortality.4 Lipid disorders occur frequently in obese patients and are often associated with cardiovascular risk factors, which consequently lead to higher cardiovascular morbidity and mortality.3 Furthermore, obesity has been documented to alter lipid metabolism, drug distribution, and clearance.5 However, it remains uncertain whether and to which extent BMI affects the clinical efficacy of contemporary LDL-C lowering therapies. Herein, we performed a meta-analysis and metaregression of randomized controlled trials to investigate the impact of BMI on the efficacy of LDL-C lowering therapies.
Methods
This meta-analysis followed the Cochrane Collaboration guidelines and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).6,7 Two independent researchers (M.U.K and M.S.K) conducted the literature search using PubMED, Embase and CENTRAL databases through April 2019. Additional online resources included ClinicalTrials.gov, Clinical Trial Results (http://www.clinicaltrialresults.org), and TCTMD (https://www.tctmd.com/). A broad search strategy was applied using relevant search terms: “lipid,” “low density lipoprotein cholesterol,” “LDL-C,” “statin,” “proprotein convertase sublilisin/kexin type 9,” and “ezetimibe” (e Table 1). The search was restricted to Human and clinical trial. Duplicates were removed and 2 investigators (M.U.K and M.S.K) scrutinized the remaining articles at the title and abstract level followed by full text screening based on the predefined study selection criteria.
The predetermined inclusion criteria were1: randomized controlled trials of therapies that reduce LDL-C levels by upregulating the LDL receptors and recommended for intensive LDL-C lowering (statins and nonstatin therapies used in combination with statins [ezetimibe and PCSK9 inhibitors]),2 sample size ≥1,000 patients and follow-up duration of at least 48 weeks, and3 trials that reported baseline BMI (kg/m2) of participants and cardiovascular outcomes of interest. We excluded trials of lipid lowering therapies that do not reduce the LDL-C level by upregulation of LDL-C receptors (cholesteryl ester transfer protein inhibitors, fibrates, and niacin), as these therapies have shown inconsistent results in yielding cardiovascular benefits.8 Omega 3 fatty acids trials were excluded since they are known to increase LDL-C levels.9 Trials with competing interests, such as renal failure and heart failure were also excluded.
Two investigators (M.U.K and S.V) independently abstracted the data on prespecified data collection forms, adjudicated the data and resolved any disagreements related to data with discussion or third-party review (S.U.K). Data were extracted on baseline characteristics of the trials and patients, crude point estimates, number of events and sample sizes, baseline and achieved LDL-C in each group, difference between the groups and percentage reduction in LDL-C values. If available, we abstracted data directly from the trial manuscript; otherwise, previous meta-analyses were examined for the required information.10,11
The between-group LDL-C difference (absolute amount of LDL-C reduction of more intensive LDL-C lowering therapy) values and percentage reduction of LDL-C in the active ann were calculated over the course of follow-up duration of each trial.12 The data acquisition was performed according to intention to treat principle. The trial level risk of bias assessment was performed on the Cochrane Risk of Bias Tool (e Table 2). Consistent with previous reports, patients were randomized to more intensive LDL-C lowering therapy (Stalin, ezetimibe + statin or PCSK9 inhibitors) or less intensive LDL-C lowering therapy groups (less potent active control or placebo).13 The primary outcome of interest was cardiovascular mortality. The secondary end points were all-cause mortality, myocardial infarction (MI), revascularization (coronary artery bypass grafting, coronary or other arterial percutaneous interventions), cerebrovascular events, and MACE. The cerebrovascular events and MACE are defined in e-Tables 3 and 4.
To account for variation in follow-up duration across trials, we calculated the hazard ratio (HR) with 95% confidence intervals (CI). The HRs with 95% CIs for each outcome was extracted for each end point from the trials when reported. For trials in which HRs was not reported, we estimated the log (HR) and its variance using a previously validated method.14 Estimates were pooled using DerSimonian and Laird random effects models. Heterogeneity was evaluated through Q statistics with I2 >75% being consistent with a high degree of heterogeneity.15
The random effects metaregression analyses were conducted to calculate change in estimates per increase in BMI (each 1 kg/m2 increase). In supplemental analyses, we included a series of sequentially adjusted multivariable regressions in the model to determine the impact of increasing BMI after accounting for covariates (baseline LDL-C, absolute reduction in LDL-C, percentage reduction in LDL-C, age, gender, and risk profile) (e Table 5). The index R2 value was used to determine the proportion of variance accounted for by increase in BMI. Additional analyses were conducted to examine association of baseline LDL-C and absolute reduction in LDL-C values with clinical end points (e Table 6).
Meta-analyses were stratified according to BMI thresholds, which were predefined according to the Centers for Disease Control and Prevention Criteria, that is, <25 (normal or healthy weight), 25.0 to 29.9 (overweight) and ≥30 (obese).16 Analyses were reported with respect to weighted mean absolute LDL-C reductions in each BMI group to show LDL-C lowering potential of more intensive LDL-C therapy per BMI category. Additional sensitivity analyses were conducted according to prespecified LDL-C groups, drug class, primary versus secondary prevention trials, and exclusion of one trial at a time and to assess potential sources of heterogeneity (e Tables 7–11). The prespecified stratification for baseline LDL-C values followed the National Cholesterol Education Program Adult Treatment Panel III, that is, <100, 100 to 129, 130 to 159, and ≥160 mg/dl17; whereas absolute LDL-C reduction was stratified as <35 mg/dl, 35-65 mg/dL and >65 mg/dl, and percent reduction in LDL-C as <25, 25 to 50 and >50.18 Publication bias was visually assessed using funnel plots. For all analyses, statistical significance threshold was 5%. Statistical analyses were conducted using the “Metafor” package version 3.30 (R Project for Statistical Computing)19 and Comprehensive Meta-Analysis Software 3.0 (Biostat, Englewood, New Jersey).
Results
Of 5,032 identified records, ultimately, 29 trials (266,148 patients) met our inclusion criteria (Figure 1). A total of 21 stalin trials, 3 trials of ezetimibe + statin and 5 trials of PCSK9 inhibitor therapy were included. The baseline characteristics of trials and participants are reported in e Table 12. The pooled mean BMI was 27.6 ± 1.64 kg/m2. The pooled mean baseline LDL-C was 129.1 ± 28.4 mg/dl and the pooled mean absolute LDL-C reduction was 40.9 ± 17.4 mg/dl. The weighted mean LDL-C difference in patients with BMI <25 was 46.6 ± 19.8 mg/dl, BMI 25 to 29.9 was 38.8 ± 15.7 mg/dl and ≥30 was 57.9 ± 8.5 mg/dl. The weighted mean follow-up duration was 3.9 ± 1.6 years.
Figure 1.
Study selection process. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow chart describing the study selection process along with the reasons for exclusion.
Metaregression analysis showed that lor every 1 kg/m2 increase in BMI, more intensive therapy was associated with a HR for cardiovascular mortality of 1.07 (1.02 to 1.13) compared with less intensive therapy (Figure 2). The overall risk reduction in cardiovascular mortality with more versus less intensive LDL-C lowering was 0.85 (0.80 to 0.91) but varied by mean BMI of the patients enrolled in the trials. Patients with BMI <25 kg/m2 had the highest risk reduction in cardiovascular mortality (HR 0.57 [0.37 to 0.86]) followed by those with BMI 25 to 29.9 kg/m2 (HR 0.86 [0.80 to 0.92]). Conversely, patients with BMI ≥30 kg/m2 had a HR of 0.96 (0.68 to 1.33; p-interaction = 0.05) (Figure 3).
Figure 2.
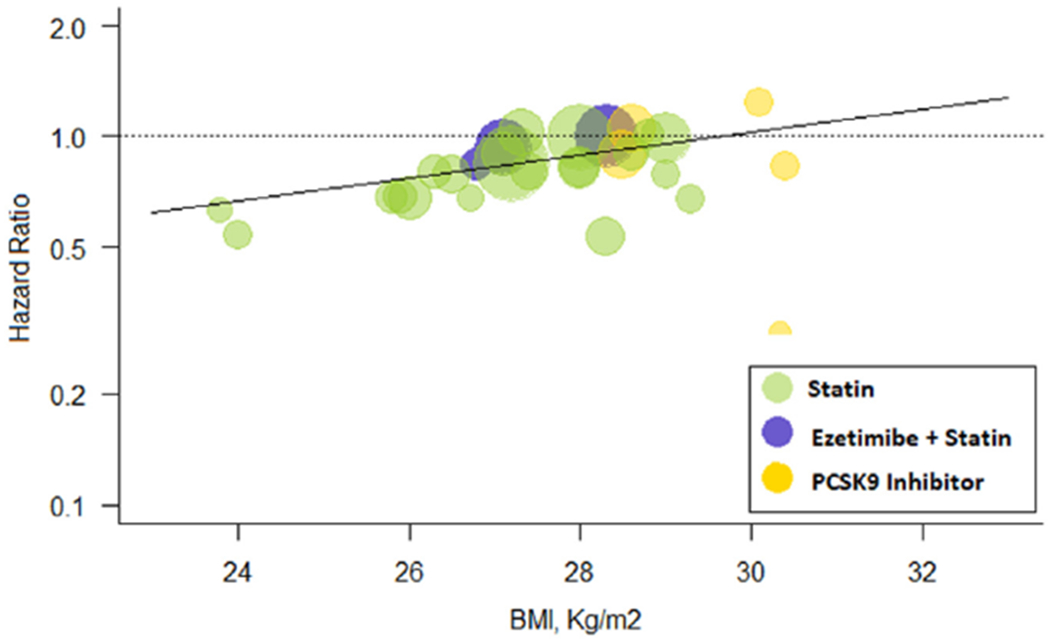
Meta-regression for BMI effect on cardiovascular mortality. Change in hazard ratios and 95% confidence intervals for cardiovascular mortality plotted against body mass index (BMI, kg/m2). Size of the data marker is proportional to the weight in the meta-regression. Data marker colors represent the classes of lipid-lowering agents used in the active treatment group as per trial randomization design. The solid line represents the meta-regression slope of the change in hazard ratio for treatment across BMI values.
Figure 3.
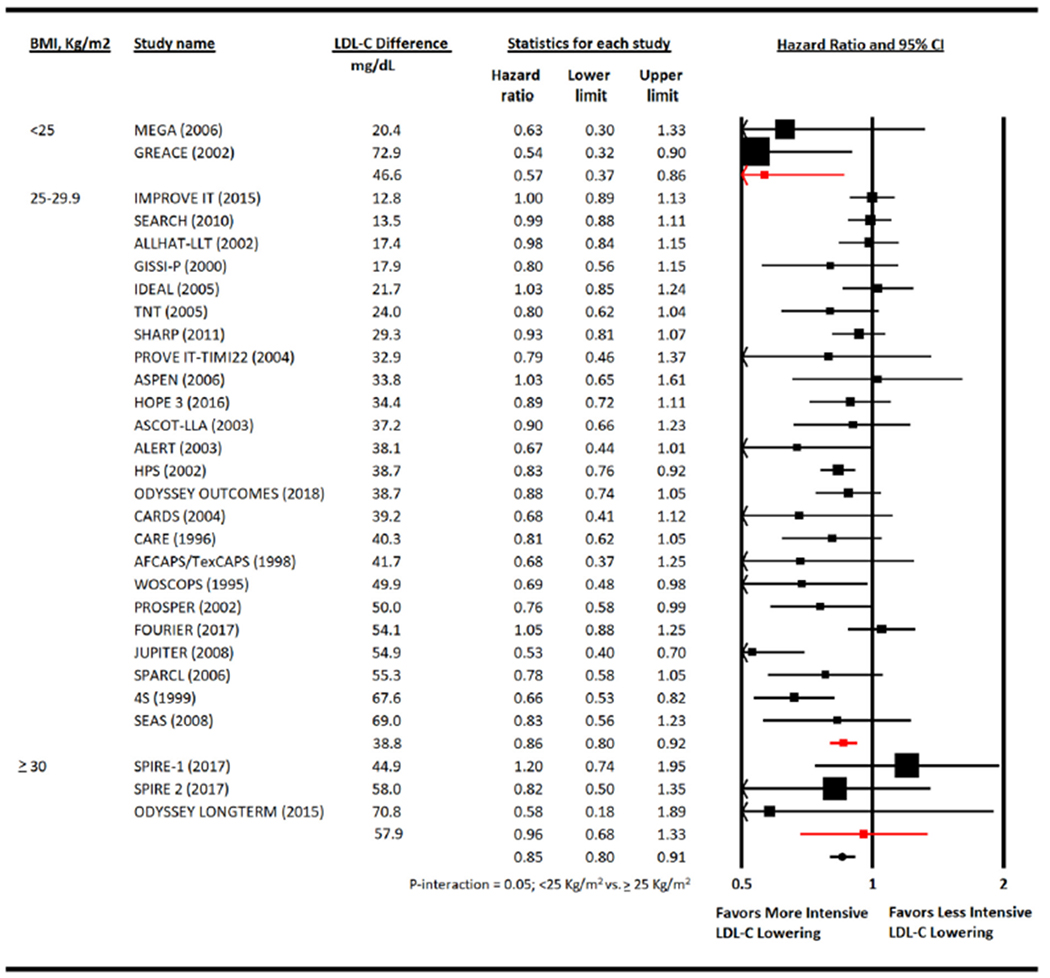
Meta-analysis for cardiovascular mortality. Individual and summary hazard ratios for myocardial infarction. 4S (SSSS) = Scandinavian Simvastatin Survival Study; ALLHAT-LLT = AFCAPS-TexCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALERT = Assessment of LEscol in Renal Transplantation Study; ASCOT-LLA = Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm; ASPEN = Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARE = Cholesterol And Recurrent Events; CARDS = Collaborative Atorvastatin Diabetes Study; FOURIER = Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk; GISSI-P = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico; HOPE-3 = Heart Outcomes Prevention Evaluation; GREACE = The GREek Atorvastatin and Coronary-heart-disease Evaluation Study; HPS = Heart Protection Study; IDEAL = Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group; IMPROVE-IT = Improved Reduction of Outcomes: Vytorin Efficacy International Trial; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin study group; LIPID = Long-term Intervention with Pravastatin in Ischaemic Disease; MEGA = Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; ODYSSEY LONG TERM = Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy; ODYSSEY Outcomes = Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab; PROVE IT-TIMI 22 = the Pravastatin or Atorvastatin Evaluation and Infection Therapy; PROSPER = PROspective Study of Pravastatin in the Elderly at Risk; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SEAS = Simvastatin and Ezetimibe in Aortic Stenosis; SHARP = Study of Heart and Renal Protection; SPARCL = The Stroke Prevention by Aggressive Reduction in Cholesterol Levels; SPIRE 1 & 2 = Studies of PCSK9 Inhibition and the Reduction of Vascular Events 1 & 2; TNT - Treating to New Targets; WOSCOPS = West of Scotland Coronary Prevention Study.
Metaregression analysis showed that for every 1 kg/m2 increase in BMI, more intensive therapy was associated with a HR of all-cause mortality of 1.03 (0.99 to 1.06) compared with less intensive therapy (Figure 4). The overall risk reduction in all-cause mortality with more versus less intensive LDL-C lowering was 0.93 (0.90 to 0.97) but varied by mean BMI of the patients enrolled. Patients with BMI <25 kg/m2 had the greatest risk reduction in all-cause mortality (HR 0.67 [0.51 to 0.89]) followed by those with BMI 25 to 29.9 kg/m2 (HR 0.94 [0.90 to 0.98]). Although patients with BMI ≥30 kg/m2 had HR of 1.00 (0.78 to 1.28; p-interaction = 0.02) (Figure 5).
Figure 4.
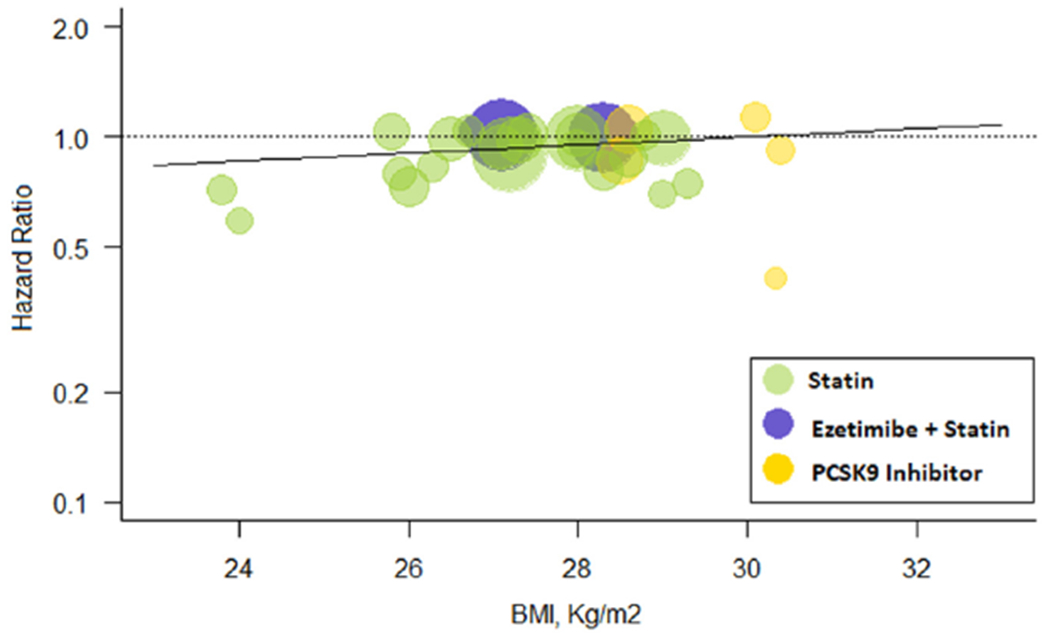
Meta-regression for BMI effect on all-cause mortality. Change in hazard ratios and 95% confidence intervals for all-cause mortality plotted against body mass index (BMI, kg/m2). Size of the data marker is proportional to the weight in the meta-regression. Data marker colors represent the classes of lipid-lowering agents used in the active treatment group as per trial randomization design. The solid line represents the meta-regression slope of the change in hazard ratio for treatment across BMI values.
Figure 5.
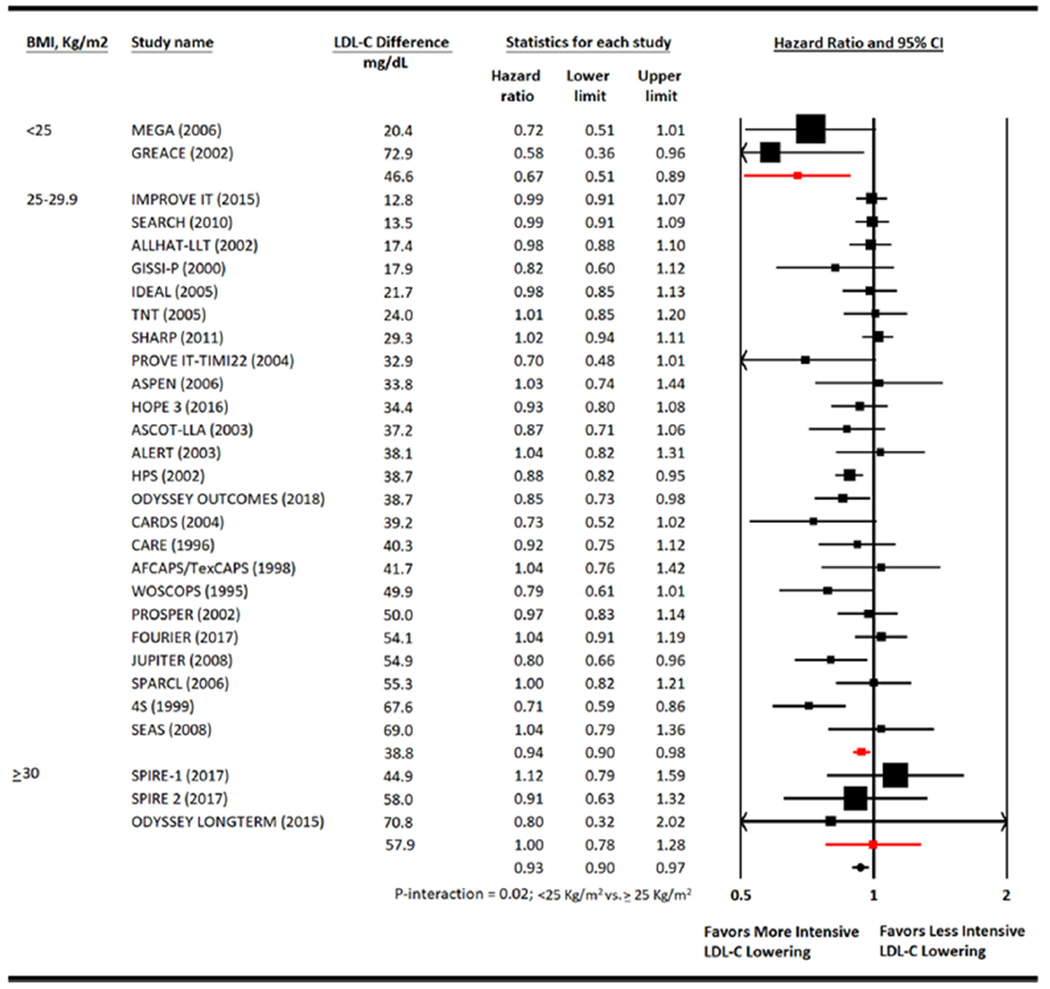
Meta-analysis for all-cause mortality. Individual and summary hazard ratios for all-cause mortality. 4S (SSSS) = Scandinavian Simvastatin Survival Study; ALLHAT-LLT = AFCAPS-TexCAPS, Air Force/Texas Coronary Atherosclerosis Prevention Study; ALERT = Assessment of LEscol in Renal Transplantation Study; ASCOT-LLA = Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm; ASPEN = Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARE = Cholesterol And Recurrent Events; CARDS = Collaborative Atorvastatin Diabetes Study; FOURIER = Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk; GISSI-P = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico; HOPE-3 = Heart Outcomes Prevention Evaluation; GREACE = The GREek Atorvastatin and Coronary-heart-disease Evaluation Study; HPS = Heart Protection Study; IDEAL = Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group; IMPROVE-IT = Improved Reduction of Outcomes: Vytorin Efficacy International Trial; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin study group; LIPID = Long-term Intervention with Pravastatin in Ischaemic Disease; MEGA = Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group; ODYSSEY LONG TERM = Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy; ODYSSEY Outcomes = Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab; PROVE IT-TIMI 22 = the Pravastatin or Atorvastatin Evaluation and Infection Therapy; PROSPER = PROspective Study of Pravastatin in the Elderly at Risk; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SEAS = Simvastatin and Ezetimibe in Aortic Stenosis; SHARP = Study of Heart and Renal Protection; SPARCL = The Stroke Prevention by Aggressive Reduction in Cholesterol Levels; SPIRE 1 & 2 = Studies of PCSK9 Inhibition and the Reduction of Vascular Events 1 & 2; TNT = Treating to New Targets; WOSCOPS = West of Scotland Coronary Prevention Study.
Metaregression analysis showed that for every 1 kg/m2 increase in BMI, more intensive therapy was associated with HR of MACE of 1.04 (1.01 to 1.07) compared with less intensive therapy (e Figure 1). The overall risk reduction in MACE with more versus less intensive LDL-C lowering was 0.82 (0.79 to 0.86) which varied by baseline BMI of those in trials (p-interaction = 0.03) (e Figure 2).
Metaregression analysis showed that for every 1 kg/m2 increase in BMI, more intensive therapy was associated with a HR of MI of 1.06 (1.02 to 1.09) compared with less intensive therapy (e Figure 3). The overall risk reduction in MI with more versus less intensive LDL-C lowering was 0.77 (0.73 to 0.82) but varied by baseline BMI. Patients with BMI <25 kg/m2 had highest risk reduction in risk of MI (HR 0.47 [0.32 to 0.67]) followed by those with BMI 25 to 29.9 kg/m2 (HR 0.78 [0.74 to 0.82]). Although patients with BMI ≥30 kg/m2 had HR of 0.89 (0.67 to 1.19; p-interaction = 0.01) (e Figure 4).
Metaregression analysis showed that for every 1 kg/m2 increase in BMI, more intensive therapy was associated with HR of revascularization of 1.08 (1.03 to 1.12) compared with less intensive therapy (e Figure 5). The overall risk reduction in revascularization with more versus less intensive LDL-C lowering was 0.78 (0.73 to 0.83) which varied by baseline BMP Patients with BMI <25 kg/m2 had highest risk reduction in revascularization (HR 0.56 [0.41 to 0.76]) followed by those with BMI 25 to 29.9 kg/m2 (HR 0.78 [0.74 to 0.83]). Although patients with BMI ≥30 kg/m2 had HR of 1.15 (0.69 to 1.94; p-interaction = 0.02) (e Figure 6).
Metaregression analysis showed that for every 1 kg/m2 increase in BMI, more intensive therapy was associated with HR of cerebrovascular events of 0.99 (0.94 to 1.03) compared with less intensive therapy (e Figure 7). The overall risk reduction in cerebrovascular events with more versus less intensive LDL-C lowering was 0.81 (0.77 to 0.86) but did not vary by baseline BMI of those in trials (p-interaction = 0.74) (e Figure 8).
Publication bias assessment for each end point is shown in funnel plots (e Figures 9–14). The association with risk reductions for each outcome was consistent for drug type, setting, LDL-C groups or after removal of a trial at a time (e Tables 5–11). BMI accounted for 54% of the variance for cardiovascular mortality, 23% for all-cause mortality, 63% for MI, 51% for revascularization, and 27% for MACE.
Discussion
The main findings of this large-scale report are as follows1: the risk reduction for mortality in patients receiving more intensive LDL-C lowering therapy versus less intensive LDL-C lowering therapy varied by the baseline BMI of the patients enrolled2; there was an inverse association between BMI and reduction of cardiovascular mortality, MI, revascularization, and MACE, but not cerebrovascular events; and3 the BMI effect on cardiovascular outcomes was independent of LDL-C lowering and baseline patient risk profile. These findings suggest that patients with normal BMI treated with intensive lipid-lowering regimens may derive a larger clinical benefit compared with patients with larger BMI values. The results could be due to the higher mortality rate of obese patients that may artificially lower the efficacy of therapy, or due to a true limitation of therapeutic efficacy in these patients.
In HPS (Heart Protection Study) trial, vascular event rates were higher in obese than nonobese patients receiving simvastatin or placebo.20 Similarly, in a landmark analysis of the WOSCOPS (West of Scotland Coronary Prevention Study), the risk of fatal coronary heart disease was significantly higher in patients with BMI between 30.0 and 39.9 after adjusting for traditional risk factors, including statin therapy.21 We speculate that there are at least a few mechanisms that may explain the association we reported. First, the hallmark of dyslipidemia in obesity is elevated total cholesterol, triglycerides (TG), and very low-density lipoprotein cholesterol with lower high density lipoprotein cholesterol (HDL-C) levels.22 However, the association of body weight with LDL-C levels has been variable.22 An analysis of the NHANES database showed that increasing BMI was associated with higher total cholesterol, TG, and non-HDL-C, but LDL-C levels that did not vary with BMI.23 Current guidelines consider elevated TG levels as a “risk enhancing factor,” and in the setting of TG >200 mg/dl, increased levels of very low-density lipoprotein cholesterol significantly enhance the risk predicted by LDL-C levels.24 Therefore, arguably non—HDL-C, instead of LDL-C might serve as a better therapeutic target in the setting of hypertriglyceridemia associated with obesity. Statin therapy is a drug of choice for reducing LDL-C and non-HDL levels.24 However, statins have a marginal impact on TG, and might not fully treat the characteristic dyslipidemia associated with obesity.22 Similarly, ezetimibe can lower LDL-C an additional ~10% to 20% on top of statin therapy, but has no effect on TG absorption.22
Second, inflammation is responsible for a substantial residual risk for ASCVD after optimal medical therapy is implemented.24 Sandfort et al showed progression of carotid atherosclerosis in obese patients having on treatment (statin) LDL-C levels of 74 mg/dl.25 In the same analysis, the C-reactive protein level was higher in obese patients compared with nonobese patients and was associated with plaque progression. In another study, plaque progression was not controlled using moderate to low intensity statin iherapy, suggesting a need for aggressive risk factor modification in addition to LDL-C lowering in obese patients. Finally, obesity has been found associated with a reduced expression of LDL receptors that in turn can modulate the efficacy of LDL-C lowering therapies.22 Ultimately, these factors might lead to greater plaque vulnerability and higher mortality and cardiovascular events in obese patients.
Several limitations of this analysis are acknowledged common to all meta-analysis, owing to lack of patient level data, this meta-analysis is limited to and by the trial level information. However, we generated various multivariate metaregression and sensitivity analyses to account for heterogeneity and results have been found consistent. The accuracy of BMI as a marker of obesity is controversial, as it reflects both lean and fat mass26; however, we used BMI to be consistent with the Centers for Disease Control report and large cohort studies of obesity.3,4,16 Furthermore, recent data have shown no difference between BMI or other anthropometric measures such as waist circumference for estimation of cardiovascular risk.4,27 Since the included trials were not powered to assess the impact of BMI on mortality and cardiovascular end points, these findings can be viewed as hypothesis generating and must be validated by high quality clinical trials.
In conclusion, intensive LDL-C lowering appeared to benefit patients with lower BMI more than patients with higher BMI. Although weight reduction is encouraged to improve the cardiovascular risk profile, professional guidelines focus on concrete LDL-C targets in patients with established ASCVD. With the increasing rate of obesity and its hazardous impact on cardiovascular health,2 data such the ones presented in this study should provide an enhanced rationale for public health initiatives focused on the importance of keeping a healthy body weight throughout life.
Supplementary Material
Acknowledgments
Disclosures
Dr. Navarese reports research grants from Amgen, Abbott and Medtronic, and lectures fees/honoraria from Bayer, Amgen, Sanofi and Regeneron and Pfizer, outside the submitted work.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.12.006.
References
- 1.Khan SU, Talluri S, Riaz H, Rahman H, Nasir F, Bin Riaz I, Sattur S, Ahmed H, Kaluski E, Krasuski RA. A Bayesian network meta-analysis of PCSK9 inhibitors, statins and ezetimibe with or without statins for cardiovascular outcomes. Eur J Prev Cardiol 2018;25:844–853. [DOI] [PubMed] [Google Scholar]
- 2.Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, Khan SS, Mookadam F, Krasuski RA, Ahmed H. NAssociation between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open 2018;1:e183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, Cupples LA, Stokes A, Liu CT. Association of obesity with mortality over 24 years of weight history: findings from the framing-ham heart study. JAMA Netw Open 2018;1:e184587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanley MJ, Abemethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49:71–87. [DOI] [PubMed] [Google Scholar]
- 6.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–1299. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ 2009;339. [PMC free article] [PubMed] [Google Scholar]
- 8.Riaz H, Khan SU, Rahman H, Shah NP, Kaluski E, Lincoff AM, Nissen SE. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa181279. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Bames EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering ldl-c and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 12.Khan SU, Rahman H, Okunrintemi V, Riaz H, Khan MS, Sattur S, Kaluski E, Lincoff AM, Martin SS, Blaha MJ. Association of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering therapies and risk of diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e011581 10.1161/JAHA.118.011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz FK, Weeks L. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SU, Riaz H, Khan MU, Zarak MS, Khan MZ, Khan MS, Sattur S, Desai MY, Kaluski E, Alkhouli M. Meta-analysis of temporal and surgical risk dependent associations with outcomes after transcatheter versus surgical aortic valve implantation. Am J Cardiol 2019; 124:1608–1614. 10.1016/j.amjcard.2019.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minghelli B, Nunes C, Oliveira R. Body mass index and waist circumference to define thinness, overweight and obesity in Portuguese adolescents: comparison between CDC, IOTF, WHO references. Pediatr Endocrinol Rev 2014; 12:35–41. [PubMed] [Google Scholar]
- 17.Lipsy RJ. The national cholesterol education program adult treatment panel III guidelines. J Manag Care Pharm 2003;9(1 Suppl):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarese EP, Robinson JG, Kowalewski M, Kołodziejczak M, Andreotti F, Bliden K, Tanfry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566–1579. 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 20.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF heart protection study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 21.Logue J, Murray HM, Welsh P, Shepherd J, Packard C, Macfarlane P, Cobbe S, Ford I, Sattar N. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart 2011;97:564–568. 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 22.Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013;5:1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denke MA, Sempos CT, Grundy SM. Excess body weight. An underrecognized contributor to high blood cholesterol levels in white American men. Arch Intern Med 1993;153:1093–10103. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, De Ferranti S, Faiella-Tommasino J, Forman ED, Goldberg R, Heidenreich AP, Hlatky AM, Jones DW, Jones DL. Pajares NL, Ndumele NE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani S, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation 2019;139:e1082–e1143. 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandfort V, Lai S, Ahlman MA, Mallek M, Liu S, Sibley CT, Turkbey EB, Lima JA, Bluemke DA. Obesity is associated with progression of atherosclerosis during statin treatment. J Am Heart Assoc 2016;5:pii:e003621 10.1161/JAHA.116.003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Ohes (Lond) 2008;32:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor AE, Ebrahim S, Ben-Shlomo Y, Martin RM, Whincup PH, Yarnell JW, Wannamethee SG, Lawlor DA. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all-cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr 2010;91:547–556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


