Abstract
Purpose
Purpose The role of endothelial Yes-associated protein 1 (YAP) in the pathogenesis of retinal angiogenesis and the astrocyte network in the mouse oxygen-induced retinopathy (OIR) model is unknown.
Methods
For in vivo studies, OIR was induced in conditional endothelial YAP knockout mice and their wild-type littermates. Retinal vascularization and the astrocyte network were evaluated by whole-mount fluorescence and Western blotting. In vitro experiments were performed in astrocytes cultured with human microvascular endothelial cell-1–conditioned medium to analyze the mechanisms underlying the effect of endothelial YAP on astrocytes.
Results
Endothelial YAP deletion not only impaired retinal blood vessels, but also caused a sparse and disrupted astrocyte network in response to OIR. Levels of the immature astrocyte marker (platelet-derived growth factor A) in the retina were substantially increased owing to YAP deficiency, suggesting a possible failure in astrocyte maturation, whereas retinal expression of leukemia inhibitory factor (LIF) was decreased. In vitro studies suggested that loss or overexpression of YAP resulted in elevated or decreased LIF secretion by human microvascular endothelial cell-1, respectively. Increased LIF levels in the culture medium promoted astrocyte maturation and proliferation and rescued YAP inhibition-induced astrocyte loss. Finally, activating YAP could protect against the pathology of the astrocyte network and even suppress pathologic retinal vascularization in control OIR mice, but not in endothelial YAP-deficient OIR mice.
Conclusions
Endothelial YAP regulation of LIF secretion is required for normalized astrocyte network formation in OIR, thereby providing a novel target for protecting the astrocyte network and thus benefiting retinal blood vessels.
Keywords: endothelial YAP, LIF, astrocyte maturation, oxygen-induced retinopathy
The mouse model of oxygen-induced retinopathy (OIR) has been well established to study retinal angiogenesis in diseases including retinopathy of prematurity, AMD, and diabetic retinopathy.1–3 Over the years, the OIR model has expanded our understanding of pathologic angiogenesis and has helped develop antiangiogenic therapeutics.4,5
In this model, retinal angiogenesis mainly occurs in two stages characterized by initial hyperoxia-induced vaso-obliteration (phase 1) and subsequent hypoxia-caused neovascularization (NV) (phase 2).1,6,7 Although defective retinal angiogenesis is the major hallmark of OIR, the pathogenesis of astrocytes has gradually emerged as an important factor and a promising therapeutic target,8 whereby decreased astrocytic cell density, which occurs as early as the hyperoxia phase, is directly correlated with pathologic retinal NV,9 and protection of the retinal astrocytes is able to normalize revascularization in OIR.10 However, the relationship between the astrocyte network and angiogenesis is still largely unknown.
Yes-associated protein 1 (YAP 1) is a conserved transcriptional coactivator that is a major effector in the Hippo signaling pathway.11,12 YAP is an important regulator of a wide variety of biological processes, such as cell plasticity, organ growth, and tumor metastasis.13 Recently, studies have confirmed that YAP is essential for regulating endothelial cell behavior and retinal angiogenesis in mice.14,15 Based on these findings, we hypothesized that endothelial cells may play an important role in governing astrocyte network development and that this role is mediated through YAP.
In this study, we first found that endothelial-specific knockout of YAP not only resulted in abnormal retinal blood vessels, but also led to a hypoplastic astrocyte network, which was characterized by insufficient density of the plexus, disrupted distribution and integrity, and decrease expression of markers for mature astrocytes. Previous studies have demonstrated that leukemia inhibitory factor (LIF), which is predominantly expressed in the developing endothelium, is crucial for the maturation and differentiation of astrocytes in the development of the optic nerve, brain, and retina by functionally binding LIF receptor (LIFR) in the surrounding astrocytes.16–20 Therefore, we hypothesized that YAP may regulate LIF secretion in endothelial cells, which facilitates astrocyte network formation, and depletion of endothelial YAP would aggravate the failure in astrocyte maturation in OIR. First, we partly rescued the astrocytic pathology via pharmacologic activation of YAP by Y27632, and the retinal vascularization associated with OIR was concomitantly improved. In vitro studies using astrocytes cultured with human microvascular endothelial cell (HMEC-1)–conditioned medium showed that endothelial YAP is crucial for LIF excretion, which controls astrocyte maturation and proliferation. In conclusion, we identified a previously unrecognized pathway in which endothelial YAP mediates cytoplasmic LIF secretion, which promotes the formation and maturation of the astrocyte network; thus, our results suggest a possible strategy to protect both the astrocyte network and vascularization in OIR.
Methods
Mice
All procedures involving animals were approved by the Administration of Affairs Concerning Experimental Animals Guidelines of the Army Medical University and accordance with the ARVO Animal Statement. Mice were housed in the Animal Care Center of the Army Military Centre of PLA (Chongqing, China). C57BL/6 mice (000664), YAPf/f mice (J032192), and TEK-iCre mice (J004128) were purchased from Jackson Laboratories (Bar Harbor, ME). TEK-Cre transgenic mice were mated with YAPflox/flox mice to generate TEK-Cre–specific YAP knockout mice (TEKCre+YAPf/f). YAPf/f mice in the same litter were used as controls.
Mouse toe clips were used for genotyping with PCR. PCR primers for the mice were downloaded from Jackson Laboratories (www.jax.org/jax-mice-and-services).
OIR Model
The mouse model of OIR was established as previously described.1,21 This model is useful for investigating the dynamic pathologic process of OIR, which includes initial microvascular degeneration (P7–P12), vascular regrowth (P12–P17), and neovessel formation (P14–P17). Briefly, P7 mouse pups were kept with nursing mothers exposed to 75% oxygen until P12, followed by a return to room air (21% oxygen) to induce retinal NV until P17. Age-matched YAPf/f mice were kept in room air throughout the postnatal development to serve as normoxic controls.
For activation of YAP in the retina, mouse pups received daily intraperitoneal injections of 3 mg/kg Y27632 (MCE, HY10583) for different periods (P7–P12, P7–P17), and the control mice were given the same dose of vehicle (dimethyl sulfoxide).
In Vitro Cell Culture Model
The HMEC-1 (American Type Culture Collection, Manassas, VA; CRL-3243) cell line was revived with MCDB131 containing 10% fetal bovine serum (FBS), 10 ng/mL epidermal growth factor, 1 µg/mL hydrocortisone, and 10 mM glutamine and cultured with Dulbecco's modified Eagle's medium (DMEM)/F12 (containing 10% FBS).
The dorsal cortex was first isolated from the dissected cerebral hemispheres and cut into pieces to obtain primary astrocytes. The tissue fragments were digested with 0.05% tryptase and then suspended in growth medium (DMEM/F12+10% FBS; Invitrogen, Carlsbad, CA). After centrifugation at 1000 r/min for 5 minutes, the cells were plated on flasks coated with poly-d-lysine in DMEM/F12 (containing 10% FBS) overnight to allow cell adhesion and cultured in DMEM (containing 10% FBS). For the separation of astrocytes and microglia, the flask was gently shaken at 225 rpm in the culture chamber overnight. The unattached microglia were discarded along with the media. The astrocytes were digested and replanted in the cell flask overnight. Then, the medium was replaced with HMEC-1–conditioned medium.
All cell measurements were routinely performed 48 hours after the intervention.
For YAP overexpression, HMEC-1 cells were transfected with LV-efla-yap1-EGFP-WPRE or LV-efla-EGFP-WPRE (control) at a concentration of 20 µm for 48 hours according to the specifications. To increase the LIF concentration in the cell medium, HMEC-1 cells were cultured with LIF protein (10 µM) for 48 hours. For YAP or LIFR knockdown experiments, HMEC-1 cells or astrocytes were transfected with 50 nM small interfering RNA (siRNA) using transfection reagent according to the protocol. For hyperoxia treatment, cells were placed in a hyperoxic (40% O2, 5% CO2) environment for 48 hours.
Staining Protocol
Retinal Whole-Mount Samples
The eyes were immediately enucleated from the euthanized mice, fixed in 4% paraformaldehyde for 30 minutes at room temperature (RT), and washed with PBS. Then, the retina cups were isolated while the debris was removed, and four incomplete radial incisions were evenly made with forceps. The retinal cups were blocked and permeabilized with goat serum that contained 0.5% Triton X-100 for 3 hours (at RT) and subsequently incubated with primary antibodies for 2 days at 4°C. After washing, the retinal cups were incubated with the appropriate fluorescence-conjugated secondary antibodies overnight at 4°C. Finally, the retinas were incubated with isolectin B4 (IB4) and glial fibrillary acidic protein (GFAP) for 1 day (at RT) if necessary and then washed and mounted on slides with slow-fade medium.
Retinal Cross-Sections
The eyeballs were embedded in ornithine carbamoyl transferase compound for cryosectioning and cut into 10-µm sections along the sagittal axis. The sections were washed, blocked, permeabilized, and incubated with primary antibodies overnight at 4°C. The sections were subjected to the appropriate secondary antibodies for 1 hour at RT. Additional nuclear staining with 4′,6-diamidino-2-phenylindole was applied.
Quantitative Analysis
Immunofluorescence images were obtained using a SP8 confocal microscope (Leica, Wetzlar, Germany). All four quarters of each whole-mount sample with eight fields randomly selected per retina were imaged and evaluated. Parameters including total vessel and astrocyte network length, end points and junctions were analyzed with AngioTool software (Informer Technologies, Inc, Los Angeles, CA).22,23 The hyperoxic avascular area on P12 and hypoxic NV area were quantified using Photoshop CS5 according to a previous study.6
Western Blot Analysis
Tissues, cells, and cell culture media were harvested and then lysed with RIPA lysis buffer (P0013E, Beyotime Biotechnology, Beijing, China) containing a protein inhibitor cocktail (5872, CST). After the samples were heated for 5 minutes at 95°C, equal amounts of protein were separated by SDS-polyacrylamide gels and then transferred to polyvinylidene fluoride membranes (88585, Thermo Scientific, Waltham, MA). After the membranes were blocked with bovine serum albumin for 1.5 hours at RT, they were incubated with specific primary antibody at 4°C overnight followed by incubation with the secondary antibodies at RT for 1.5 hours. SuperSignal West Femto Maximum Sensitivity Substrate (34096, Thermo Scientific) was used to visualize the bands. Optical density was quantified using ImageJ software (http://rsb.info.nih.gov/ij/index.html) and calculated relative to that of glyceraldehyde-3-phosphate dehydrogenase.
Statistical Methods
All results are presented as the mean ± SD. P values of less than 0.05 were defined as significant. For two-group comparisons, a two-sided Student's t-test was used. Multiple group comparisons were performed by using ANOVA followed by a post hoc Student's t-test.
Results
Knockout of YAP in Endothelial Cells Not Only Damages the Vascularization in the Retinas of OIR Mice, But Also Obstructs the Formation and Maturation of the Astrocyte Network
A mouse model of OIR was generated as illustrated in Figure 1a. Hyperoxia (75% oxygen) induced vessel regression and suspended vessel growth in the retina (P7–P12).24 Once the animals were returned to room air (P12–P17), the retina became relatively hypoxic, resulting in vascular regrowth and NV. NV peaked at P17, which was thus selected as the measurement time point for phase 2 in our study.
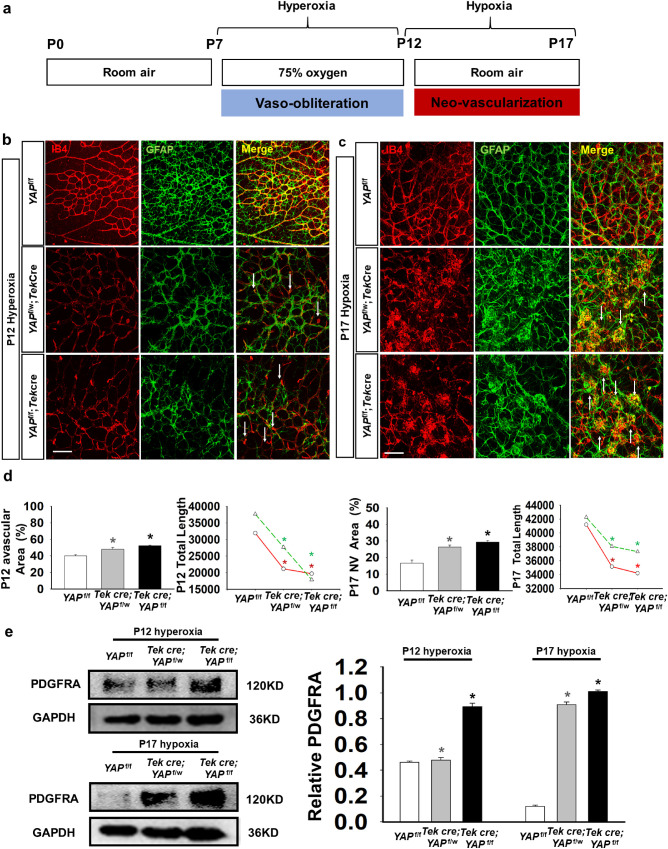
Figure 1.
YAP deletion resulted in damage to the blood vessels and the astrocytic network in the OIR model. (a) Schematic diagram of the experimental design. Seven-day-old (P7) neonatal mice with nursing mothers were exposed to hyperoxia (75% oxygen) until P12, which induced retinal vessel loss. Then, the mice were returned to room air (∼21% oxygen) to trigger maximum retinal NV at P17. (b, c) Composite immunofluorescence of whole-mount YAPf/f, YAPf/w;Tek Cre, and YAPf/f;Tek Cre retinas, which were stained for GFAP (green) and IB4 (red) to visualize the astrocytic and vascular network at P12 and P17 with OIR. The arrows in b indicate microvascular leakage and fibrous masses in c. (d) Quantification of the avascular area, NV area and total length showed a significant difference between YAP knockout mice and control littermates. (n = 3; *P < 0.05). (e) Western blot analysis showing that PDGFA expression increased in the retinas, as YAP was conditionally deleted in endothelial cells in the OIR retinas (n = 3; *P < 0.05). Bar = 50 µm.
To gain insight into the role of YAP in OIR, we first examined the localization of YAP in a mouse model. Fluorescence immunostaining demonstrated that YAP predominantly colocalized with IB4-positive blood vessels in the retina during early development (P7, P12, and P17) and in the OIR model (P12 and P17), indicating that the function of YAP may be predominantly mediated by endothelial cells (Supplementary Fig. S1).
To further study the function of endothelial YAP in the retina, we deleted YAP in endothelial cells by crossing TEK-Cre mice with YAPflox/flox mice and generating endothelial-specific YAP-deficient mice (YAPf/w; Tek Cre and YAPf/f;Tek Cre). The astrocytic and vascular networks were visualized by staining with GFAP and IB4, respectively, in retinal whole-mount samples and then quantified. At the early stage of retinal development (P7), downregulation of endothelial YAP expression led to significantly decreased vascular area and total length of blood vessels (Supplementary Fig. S2a, S2b). The junctions and end points also underwent a discernible decline in YAPf/w; Tek Cre and YAPf/f;Tek Cre mice, especially in YAPf/f;Tek Cre mice (Supplementary Fig. S2b). Notably, the GFAP-labeled skeleton network of astrocytes was altered concomitantly with IB4-positive vessels, displaying reduced GFAP-positive astrocytic area and decreased total length, junctions, and end points. In the OIR model, YAP deletion further disrupted the aberrant IB4-stained blood vessel structure (Figs. 1b–1d). On P17 after YAP deletion the retinal vessels became rarified, forming convoluted bundles and pathologic neovascular tufts (Fig. 1c). The statistics showed an expanded avascular area and decreased total length in YAP-deficient OIR mice compared with the control OIR mice at P12, as well as an increased NV area at P17 (Fig. 1d). The risk of vascular malformation was negatively associated with the YAP level, as shown by the results in the YAP complete knockout (YAPf/f;Tek Cre), partial knockout (YAPf/w;Tek Cre), and intact mice (YAPf/f). Additionally, to confirm that Tek-Cre had no effects on morbidity, we compared Tek-Cre mice with YAPf/f controls in the OIR model (Supplementary Fig. S3a, S3b). There were no differences between the Tek-Cre and YAPf/f control mice. Moreover, the astrocyte network showed severe dysplasia, with attenuation of the plexus and formation of fibrous masses (Figs. 1b, 1c). GFAP is robustly expressed in mature astrocytes but weakly expressed in immature astrocytes; thus, it is widely used for visualizing the star-like mature astrocytes and defining differentiation states.25,26 The OIR retinas with YAP deficiency showed abnormal stellate/dendritic astrocyte morphology and decreased overlapping distribution.
The total length of the GFAP-positive astrocyte network decreased sharply in YAP-deficient OIR retinas (Fig. 1d), which together with the previous findings may indicate that failure of astrocyte maturation results from the YAP deletion. To test this hypothesis, we measured the expression of platelet-derived growth factor A (PDGFRA), a typical marker of immature astrocytes,27 The data showed that PDGFRA was higher in the YAPf/w;Tek Cre and YAPf/f;Tek Cre mice than in the YAPf/f control mice after hyperoxia and hypoxia treatments, suggesting delayed astrocyte maturation in YAP-deficient mice (Fig. 1e). The degeneration of the astrocyte network together with the abnormal vascular plexus aroused our intertest. Previous studies have confirmed that astrocytes are indispensable for angiogenesis in the central nervous system, not only structurally but also functionally.28,29 Therefore, the disturbance in the astrocyte skeleton is an important pathologic change in the OIR model and may be closely associated with developmental angiogenesis in the retina.
YAP Activation in the OIR Model Alleviates Astrocyte Network Dysfunction
Because deletion of endothelial YAP damages the astrocyte network in the OIR model, we investigated whether activating YAP could reverse the astrocyte meshwork phenotype and could thus become a potential treatment strategy for diseases such as retinopathy of prematurity. Unfortunately, no specific YAP agonist has been confirmed to date. According to previous studies, the ROCK inhibitor Y27632 can decrease YAP localization to the nucleus, thus promoting retention of phosphorylated YAP in the cytoplasm where YAP is activated and interacts with multiple pathways.30–32 We applied the drug and found that treatment with Y27632 promoted YAP expression in HMEC-1 in vitro (Supplementary Fig. S4a). Intraperitoneal injection of Y27632 into the OIR mice enhanced YAP expression in the retinas (Supplementary Fig. S4a) and slightly enhanced astrocyte network formation and maturation during normal retinal development (Supplementary Fig. S4b, S4c). On P12, Y27632 treatment rescued the astrocyte network loss and alleviated the decrease in junction and end points caused by OIR. On P17, the astrocyte meshwork not only showed enhanced GFAP, but also showed structural remodeling with decreased levels of amorphous GFAP+ debris and fibrous masses and an increasingly regular appearance (Figs. 2a–2d). Moreover, developmental angiogenesis of the retina also showed certain improvements, including decreased avascular retinal areas, fewer NV areas, and an extended total length (Figs. 2c, 2d). However, the protective effects of Y27632 on the retina were decreased when YAP was deleted specifically in endothelial cells (Figs. 2a–2d). Additionally, Western blotting results showed that PDGFA expression was indeed decreased after Y27632 treatment (Fig. 2e), indicating a decrease in immature astrocytes. These data indicated that YAP in endothelial cells could be a novel and efficacious target for the astrocytic skeleton and blood vessel structure.
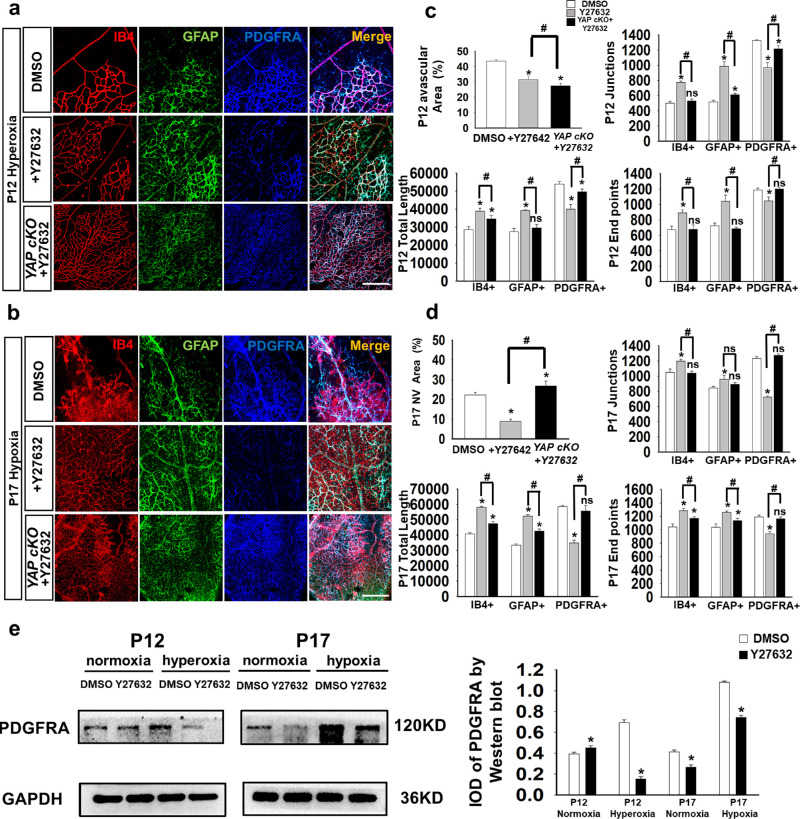
Figure 2.
The defective astrocyte network resulting from OIR was partly abrogated by YAP agonist injection. (a) Visualization of the retinal vasculature by IB4 (red) immunostaining, astrocyte network by GFAP (green), and immature astrocytes by PDGFA (blue). Compared with the mice in the dimethyl sulfoxide groups, mice injected with the YAP agonist (Y27632) showed more blood vessels and astrocytes but fewer immature astrocytes under hyperoxic conditions (P12). (b) In addition to increasing the number of mature cells, YAP agonist injection resulted in more orderly blood vessels, increased astrocyte formation and reduced levels of PDGFRA+ astrocytes in hypoxic conditions (P17). (c) Under hyperoxic conditions, YAP agonist-treated mice presented a decrease in the avascular area but an increase in total vessel length, total number of junctions and total number of endpoints in IB4+ and GFAP+ cells. PDGFRA+ cells showed a decrease in these factors (n = 3, ns, P > 0.05; *P < 0.05). (d) Under hypoxic conditions, YAP agonist-treated mice presented a decrease in the neovascular area but an increase in total vessel length, total number of junctions, and total number of endpoints in IB4+ and GFAP+ cells. PDGFRA+ cells showed a decrease in these factors (n = 3, ns, P > 0.05; *P < 0.05). (e) The expression levels of PDGFRA were measured by Western blotting; YAP injection obviously changed PDGFRA expression, which was increased at P12 under normoxic conditions but was decreased in the other groups (n = 5; *P < 0.05). Bar = 50 µm.
Endothelial Cell-Secreted LIF Regulated by YAP Promotes the Formation of the Astrocyte Meshwork in the Retina
A remaining question is how endothelial cell-specific YAP deficiency disrupts the astrocyte meshwork in the OIR model. Studies have suggested that LIFR on astrocytes interacts with endothelial cell-secreted LIF to induce the maturation and differentiation of astrocytes.17,18 Based on these findings, we propose that endothelial cell-specific YAP-induced LIF secretion promotes the formation of the astrocyte meshwork, which supports retinal vascularization.
To confirm this hypothesis, we measured the level of LIF after altering YAP expression in the OIR model. Western blot analysis showed that LIF levels decreased at P12 and P17, along with the decrease in YAP expression (Figs. 3a–3d). Intraperitoneal injection of Y27632 increased the total LIF content in the retinas on P12 and P17 under OIR as well (Figs. 3e, 3f). These data indicate that endothelial cell-specific YAP is essential for LIF expression and astrocyte maturation in OIR.
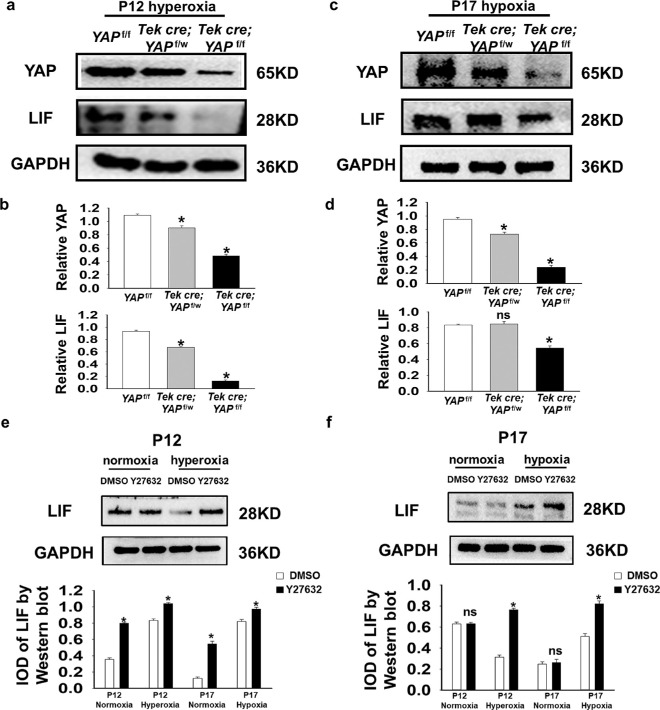
Figure 3.
Conditional knockout of YAP in endothelial cells led to a decrease in LIF expression in OIR retinas. (a–d) Western blot and quantification of total YAP and LIF levels in retinas from YAPf/f, YAPf/w;Tek Cre, and YAPf/f;Tek Cre mice at P12 (hyperoxia) and P17 (hypoxia). The LIF level decreased when YAP was knocked out. (e, f) Western blot and quantification of total LIF expression was increased in mice after Y27632 treatment at both P12 (hyperoxia) and P17 (hypoxia). Glyceraldehyde-3-phosphate dehydrogenase served as a loading control (n = 5; ns, P > 0.05; *P < 0.05).
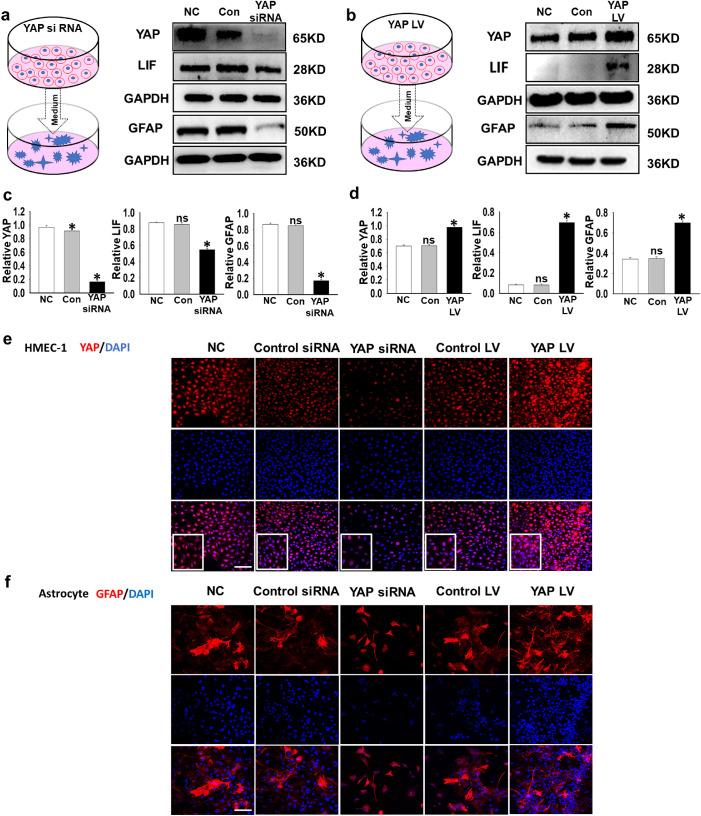
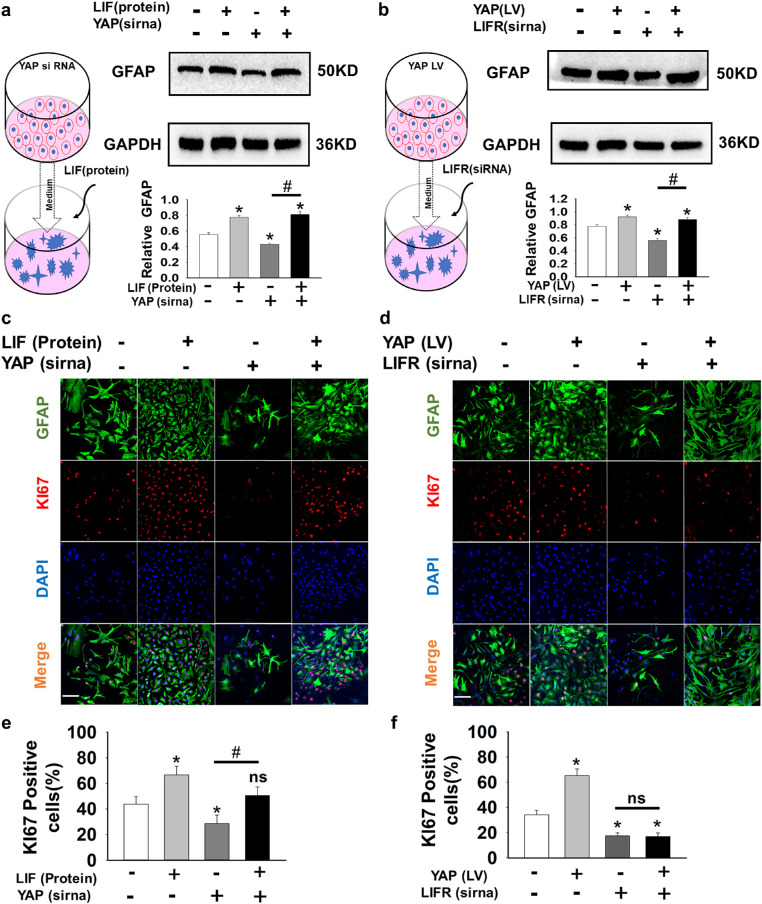
To further verify that astrocyte maturation is regulated by YAP-dominated angiogenic LIF secretion, we established an in vitro model of astrocytes cultured in HMEC-1–conditioned medium. We altered YAP expression in HMEC-1 cells with siRNA or lentiviral vector and then cultured astrocytes with HMEC-1–conditioned media. Inhibition of YAP with siRNA in HMEC-1 cells led to decreased secretion of the LIF protein into the medium, whereas overexpressing YAP with a lentiviral vector increased the LIF concentration in the medium (Figs. 4a–4d). Our immunolabeling results indicated that the number of GFAP-labeled astrocytes increased after the overexpression of YAP in HMEC-1 compared with that in the control group, whereas YAP inhibition had the opposite effects on astrocytes (Figs. 4e, 4f). Additionally, astrocytes in the YAP LV group had extensive branches that resembled those of mature astrocytes. Then, we added LIF protein to the medium of the astrocytes after YAP siRNA intervention to determine whether the phenotype could be rescued. The addition of LIF substantially increased the GFAP level with or without YAP siRNA intervention (Fig. 5a). The level of GFAP decreased when LIFR siRNA was used alone, but it showed statistically insignificant inhibitory effects in the presence of YAP LV (Fig. 5b), indicating that LIF may not be the only factor that YAP manipulates to increase GFAP. We next costained the cells with Ki-67 and GFAP to visualize the activity of the astrocytes. Ki-67 is expressed in cells at active phases of the cell cycle and is frequently used as a proliferation marker. Both LIF protein and YAP LV could increase the number of astrocytes labeled with Ki-67, and LIF siRNA or YAP siRNA were able to decrease their number (Figs. 5c–5f). While adding extra LIF protein successfully rescued the effects of YAP siRNA, YAP LV failed to restore the decrease in Ki-67–positive astrocytes caused by LIFR siRNA (Figs. 5c–5f). These data indicate that YAP promoting LIF enhances astrocyte maturation and proliferation.
Figure 4.
YAP positively regulated LIF secretion by HMEC-1 and promoted a mature phenotype in cocultured astrocytes. (a–d) The coculture system of astrocytes and endothelial cells and manipulation of YAP in HMEC-1 are shown in the schematic diagram. HMEC-1 were treated with YAP siRNA (a) or transduced with a YAP lentiviral vector (b), and the medium was then collected for astrocyte culture. Western blotting analysis of the protein levels of YAP, LIF, and GFAP (n = 5; ns, P > 0.05; *P < 0.05). (e, f) Representative immunofluorescence images showing the morphology of HMEC-1 and astrocytes after YAP intervention in HMEC-1. LV-efla-EGFP-WPRE was used in the control group, and the normal control (NC) group received no additional siRNA transfection. Bar = 100 µm.
Figure 5.
(a, b) Treatment of HMEC-1 in the coculture system. HMEC-1 were treated with YAP siRNA (a) or transduced with a YAP lentiviral vector (b), and the medium was then collected for astrocyte culture. LIF protein (a) or LIFR siRNA (b) were added to the astrocyte culture medium accordingly. Western blotting results indicated that transferring the YAP lentiviral vector into HMEC-1 or providing extra LIF protein in the cell culture increased GFAP expression in astrocytes, while silencing YAP or LIF with siRNA eliminated GFAP expression (n = 5; ns, P > 0.05; *P < 0.05). (c, d) Astrocytes cocultured with pretreated HMEC-1. Antibody labeling visualizes astrocytes expressing Ki-67 (red), GFAP (green), and DAPI (blue). (e, f) The percentage of Ki-67-positive cells among GFAP-positive cells. (n = 5; ns, P > 0.05; *P < 0.05). Bar = 100 µm.
Discussion
Astrocyte Network During Physiologic Vascularization and Pathologic Revascularization
The morbidity of astrocytes has long been discovered in the OIR model.9 However, the interactions of these cells with blood vessels and the underlying mechanisms remain to be investigated. During the development of the retina, retinal astrocytes develop from the optic nerve head and migrate in a centrifugal pattern to form an astrocytic network across the inner surface of the retina.33 In contrast, endothelial cells migrate slightly behind astrocytes and grow based on the preexisting astrocytic template to form the superficial vascular plexus.34–37 However, endothelial cells were found to modulate astrocyte functions.38–40 These effects create a reciprocal feedback loop that maintains the astrocyte network and blood vessels in the retina. YAP was found to be necessary for the vascular development of the retina.11 It determines the proliferation and migration of endothelial cells that regulate angiogenic sprouting and branching.15,41 In the OIR model, where vascular development was disrupted by factors such as oxygen, knocking out YAP in endothelial cells aggravated the injury of the dysfunctional vessels and astrocyte degeneration, which led to a sparse glial network (Fig. 1). YAP activation via Y27632 significantly attenuated the abnormalities in the astrocyte network (Fig. 5) and simultaneously improved the blood vessels. Although we could not exclude the possibility that the morbidity of retinal vasculature and astrocyte network in OIR model may be partly owing to the deficiency of YAP during early postnatal stages (Supplementary Figs. S1, S2), the current results gave prominent evidences to verify the protective role of endothelial YAP in OIR. The data also imply that YAP in endothelial cells is crucial for the structure and function of astrocytes through specific mechanisms. However, it is less clear to what degree the improvement of the astrocyte network contributes to vessel revascularization. Previous studies have suggested that hypoxia-affected astrocytes combined with an attenuated astrocytic network during the hypoxic phase lead to pathologic retinal revascularization,9 and restoring astrocytes can prevent vascular pathology.10 Based on this observation, it is fair to say that normalization of the astrocyte network through activation of YAP can promote normal vascularization in our OIR model.
Nevertheless, Y27632 may have direct protective effects on blood vessels through YAP regulation or even other pathways, because Y27632 is nonspecific. For further studies, YAP-specific agonists and EC-Super-YAP mice are needed to validate our findings.
YAP–LIF Is a Novel Pathway Regulating Astrocyte Behavior
TEK-Cre;YAPloxp transgenic mice are a helpful tool to investigate the cell-specific molecular mechanisms underlying the high oxygen-induced vascular degeneration followed by pathologic vascular regrowth in the OIR model.
Together, our in vivo and in vitro data indicated that YAP expression in endothelial cells is positively associated with LIF secretion (Figs. 2, 3), and increasing the LIF content significantly promoted astrocyte maturation and proliferation (Figs. 4, 5). LIF has been reported to be associated with multiple neuroprotective effects, including stimulating the proliferation of progenitor cells and persisting as an intracellular survival factor during injury and autoimmune diseases.42,43 It also has certain anti-inflammatory properties exerted by macrophages.44 Therefore, the protective effects of LIF in the OIR model may have complex causes and deserve further investigation.
Previous studies have shown that LIF is an upstream regulator of YAP-TEAD, activating YAP to induce the differentiation of mouse embryonic stem cells.45 LIF was shown to increase YAP transcriptional activity in human pancreatic ductal adenocarcinoma cells.46 For the first time, we identified YAP as a regulator of LIF, and the YAP–LIF axis was shown to be a novel link between astrocytes and endothelial cells. However, LIF may not be the sole downstream effector that endothelial YAP manipulates to regulate the behavior of astrocytes. In Figure 5b, we show that silencing the LIFR in the presence of YAP LV seemed to decrease GFAP expression slightly but was not enough to cause a significant difference. Because YAP tends to mediate multiple pathways, the detailed mechanisms underlying this regulatory loop need to be explored further.
In addition, the OIR model mimics many aspects of proliferative and neovascular retinopathies, which present YAP–LIF as a potential target in these diseases. However, it is also noteworthy that the pathologic changes that occur upon exposure to hyperoxia in the mouse OIR model can be quite different from what happens in human retinal disease, such as retinopathy of prematurity.1 For starters, human infants possess more mature retinal vessel systems than mice because development occurs postnatally in mice and at an early stage as the fetal period in humans.3,47 Additionally, whether retinal hypoxia is sufficient to induce retinal NV is experimentally undecided.48
Angiogenesis is a delicately regulated process involving a series of events to ensure a functional and healthy vascular network. Multiple signaling pathways that control angiogenesis have been confirmed to concomitantly activate YAP to regulate key events in angiogenesis. However, no studies have related YAP to astrocyte–endothelial cell interactions. Our study not only discovered a novel pathway of endothelial cell-expressed YAP that regulates astrocyte proliferation and maturation in the OIR retina, but also identified a potential therapeutic alternative for treating injured astrocyte networks through blood vessels in the OIR model.
Supplementary Material
Acknowledgments
The authors thank Yuan-Guo Zhou from the Research Institute of Surgery & Daping Hospital and Feng Mei from the Department of Histology and Embryology, Army Medical University for data evaluation.
Supported by grants from the National Natural Science Foundation of China (No. 81570840 and No. 81371006), Basic Research and Scientific Frontier Foundation of Chongqing (cstc2019jcyj-msxmX0010) and Research Foundation of Dept. Ophthalmology in Daping Hospital, Army Medical Center of PLA (No. 9-2543).
Disclosure: L.-Q.-Y. Ai, None; J.-Y. Zhu, None; X. Chen, None; X. Li, None; L.-L. Luo, None; Q.-M. Hu, None; S. Lin, None; J. Ye, None
References
- 1. Kim CB, D'Amore PA, Connor KM. Revisiting the mouse model of oxygen-induced retinopathy. Eye Brain. 2016; 8: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010; 29: 500–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015; 122: 200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang H. Anti-VEGF therapy in the management of retinopathy of prematurity: what we learn from representative animal models of oxygen-induced retinopathy. Eye Brain. 2016; 8: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kermorvant-Duchemin E, Sapieha P, Sirinyan M, Beauchamp M, Checchin D, Hardy P, et al.. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. 2010; 120: 51–60. [DOI] [PubMed] [Google Scholar]
- 6. Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, et al.. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protocol. 2009; 4: 1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giacomo C, Luca F, Paola B, Giancarlo LM, Gloria C, Genny R, et al.. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014; 92: 2–20. [DOI] [PubMed] [Google Scholar]
- 8. Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. AT1 receptor inhibition prevents astrocyte degeneration and restores vascular growth in oxygen-induced retinopathy. Glia. 2008; 56: 1076–90. [DOI] [PubMed] [Google Scholar]
- 9. Bucher F, Stahl A, Agostini HT, Martin G. Hyperoxia causes reduced density of retinal astrocytes in the central avascular zone in the mouse model of oxygen-induced retinopathy. Mol Cell Neurosci. 2013; 56: 225–33. [DOI] [PubMed] [Google Scholar]
- 10. Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, et al.. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2010; 58: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu J-Y, Lin S, Ye J. YAP and TAZ, the conductors that orchestrate eye development, homeostasis, and disease. J Cell Physiol. 2019; 234: 246–58. [DOI] [PubMed] [Google Scholar]
- 12. Davis JR, Tapon N. Hippo signalling during development. Development. 2019; 146: dev167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccolo S, Dupont S, Cordenonsi M. The Biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev. 2014; 94: 1287–312. [DOI] [PubMed] [Google Scholar]
- 14. Kim J, Kim YH, Kim J, Park DK, Bae H, Lee D-H1, et al.. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. 2017; 127: 3441–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakabe M, Fan J, Odaka Y, Liu N, Hassan A, Duan X, et al.. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc Natl Acad Sci USA. 2017; 114: 10918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, Cheema SS, et al.. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci USA. 1998; 95: 3178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001; 21: 1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Susumu S, Hiroyasu K, Hisamichi N, Motohiro K, Hirokazu S, Nobuhito G, et al.. A role for endothelial cells in promoting the maturation of astrocytes through the apelin/APJ system in mice. Development. 2012; 139: 1327–35. [DOI] [PubMed] [Google Scholar]
- 19. Abbott NJ. Astrocyte–endothelial interactions and blood–brain barrier permeability. J Anat. 2002; 200: 523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan YY, Zhang JM, Wang H, Liu XY, Yang FH. Leukemia inhibitory factor inhibits the proliferation of primary rat astrocytes induced by oxygen-glucose deprivation. Acta Neurobiol Exp (Wars). 2013; 73: 485. [DOI] [PubMed] [Google Scholar]
- 21. Smith LE, Wesolowski E, Mclellan A, Kostyk SK, D'Amato R, Sullivan R, et al.. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994; 35: 101–11. [PubMed] [Google Scholar]
- 22. Klotz L, Norman S, Vieira J, Masters M, Gunadasa-Rohling M, Dube K, et al.. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015; 522: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawaguchi S, Varshney S, Owgawa M, Sakaidai Y, Yahi H, Takeshita K, et al.. O-GlcNAc on NOTCH1 EGF repeats regulates ligand-induced Notch signaling and vascular development in mammals. Elife. 2017; 6: e24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashton N. Animal experiments in retrolental fibroplasia. Trans Am Acad Ophthal Otolaryngol. 1954; 58: 51–3; discussion, 3-4. [PubMed] [Google Scholar]
- 25. Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010; 518: 2316–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee Y, Su M, Messing A, Brenner M. Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia. 2006; 53: 677–87. [DOI] [PubMed] [Google Scholar]
- 27. Mudhar HS, Pollock RA, Wang C, Stiles CD, Richardson WD. PDGF and its receptors in the developing rodent retina and optic nerve. Development. 1993; 118: 539–52. [DOI] [PubMed] [Google Scholar]
- 28. Petzold Gabor C, Murthy Venkatesh N. Role of astrocytes in neurovascular coupling. Neuron. 2011; 71: 782–97. [DOI] [PubMed] [Google Scholar]
- 29. Ma S, Kwon HJ, Huang Z. A functional requirement for astroglia in promoting blood vessel development in the early postnatal brain. PLoS One. 2012; 7: e48001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al.. Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474: 179–83. [DOI] [PubMed] [Google Scholar]
- 31. Kimura TE, Duggirala A, Smith MC, White S, Sala-Newby GB, Newby AC, et al.. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J Mol Cell Cardiol. 2016; 90: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia XF, Ye F, Wang YB, Feng DX. ROCK inhibition enhances neurite outgrowth in neural stem cells by upregulating YAP expression in vitro. Neural Regen Res. 2016; 11: 983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988; 332: 834–7. [DOI] [PubMed] [Google Scholar]
- 34. Dorrell MI, Edith A, Martin F. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002; 43: 3500–10. [PubMed] [Google Scholar]
- 35. Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987; 255: 35–49. [DOI] [PubMed] [Google Scholar]
- 36. Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007; 10: 77–88. [DOI] [PubMed] [Google Scholar]
- 37. Ling TL, Stone J. The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res. 1988; 44: 73–85. [DOI] [PubMed] [Google Scholar]
- 38. Pan Q, He C, Liu H, Lian X, Dai B, Chen Y, et al.. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol Brain. 2016; 9: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. West H, Richardson WS, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005; 132: 1855–62. [DOI] [PubMed] [Google Scholar]
- 40. Duan L-J, Pan SJ, Sato TN, Fong G-H. Retinal angiogenesis regulates astrocytic differentiation in neonatal mouse retinas by oxygen dependent mechanisms. Sci Rep. 2017; 7: 17608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi H-J, Zhang H, Park H, Choi K-S, H-w Lee, Agrawal V, et al.. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015; 6: 6943. [DOI] [PubMed] [Google Scholar]
- 42. Getchell TV, Shah DS, Partin JV, Subhedar NK, Getchell ML. Leukemia inhibitory factor mRNA expression is upregulated in macrophages and olfactory receptor neurons after target ablation. J Neurosci Res. 2002; 67: 246–54. [DOI] [PubMed] [Google Scholar]
- 43. Davis SM, Pennypacker KR. The role of the leukemia inhibitory factor receptor in neuroprotective signaling. Pharmacol Ther. 2018; 183: 50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hendriks JJA, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, et al.. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol. 2008; 204: 52–7. [DOI] [PubMed] [Google Scholar]
- 45. Tamm C, Bower N, Anneren C. Regulation of mouse embryonic stem cell self-renewal by a Yes-YAP-TEAD2 signaling pathway downstream of LIF. J Cell Sci. 2011; 124: 1136–44. [DOI] [PubMed] [Google Scholar]
- 46. Wang M-T, Fer N, Galeas J, Collisson EA, Kim SE, Sharib J, et al.. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat Commun. 2019; 10: 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, et al.. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51: 2813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang W, Ito Y, Berlin E, Roberts R, Berkowitz BA. Role of hypoxia during normal retinal vessel development and in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2003; 44: 3119–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.