Abstract
The lysosome is a central player in the cell, acting as a clearing house for macromolecular degradation, but also plays a critical role in a variety of additional metabolic and regulatory processes. The lysosome has recently attracted the attention of neurobiologists and neurologists since a number of neurological diseases involve a lysosomal component. Among these is Parkinson’s disease (PD). While heterozygous and homozygous mutations in GBA1 are the highest genetic risk factor for PD, studies performed over the past decade have suggested that lysosomal loss of function is likely involved in PD pathology, since a significant percent of PD patients have a mutation in one or more genes that cause a lysosomal storage disease (LSD). Although the mechanistic connection between the lysosome and PD remains somewhat enigmatic, significant evidence is accumulating that lysosomal dysfunction plays a central role in PD pathophysiology. Thus, lysosomal dysfunction, resulting from mutations in lysosomal genes, may enhance the accumulation of α-synuclein in the brain, which may result in the earlier development of PD.
Keywords: lysosomal storage diseases, Parkinson’s disease, Gaucher disease, α-synuclein
1. Introduction
The lysosome is a membrane-bound organelle first described by Christian de Duve in the 1950s [1,2]. In addition to its well-characterized role in macromolecular degradation [3,4], the lysosome plays pivotal roles in other aspects of cell homeostasis and is crucial to numerous physiological processes. Subsequent to the discovery of the lysosome, a family of diseases was shown to be associated with the defective activity of lysosomal proteins. These diseases, known as lysosomal storage diseases (LSDs), are typically classified according to the substrate that accumulates [5]. To date, about 70 genetically distinct conditions causing different LSDs have been described, although more are likely based on the number of known lysosomal proteins.
Mutations in one of these lysosomal genes, namely GBA1, result in Gaucher disease (GD) [6], and mutations in GBA1 are now recognized as the highest genetic risk factor for Parkinson’s disease (PD) [7]. However, the mechanistic association between GBA1 mutations and PD is unclear, with some favoring the “gain of function” hypothesis (i.e., a mutated lysosomal protein gains a new function when mutated) and others favoring the “loss of function” hypothesis (i.e., one or another lysosomal function is compromised [8]). We are of the opinion that most of the evidence [9] is consistent with the loss-of-function hypothesis, which is supported by a recent whole exome sequencing study on PD patients [10].
In this sequencing study [10], 54 genes associated with LSDs were analyzed (Table 1). Remarkably, the majority of PD patients (about 56%) displayed at least one mutation in a gene that causes an LSD, and 21% had two or more, suggesting that a combination of mutations in genes encoding proteins that cause an LSD could contribute to lysosomal dysfunction, and thereby increase PD susceptibility. The prevalence of LSD mutations in PD patients strengthens the idea that lysosomal dysfunction is a key player in PD pathogenesis and identifies a burden of LSD variants associated with PD. This study also revealed new susceptibility loci. While these results appear to be supportive of an association between lysosomal genes and PD, considerable additional work is needed to cement this relationship. For instance, the Human Lysosome Gene Database contains about 400 lysosomal proteins (http://lysosome.unipg.it) [11] and a recent proteomics study suggested 343 unique lysosomal proteins [12]. These numbers are significantly more than the roughly 50 proteins currently known to be associated with an LSD, and that were analyzed in the whole exome sequencing study [10]. Might some of these other lysosomal genes also be associated with LSDs, even though not all of the approximately 400 proteins in this database are likely to be bonafide lysosomal proteins?
Table 1.
Genes that cause lysosomal storage diseases (LSDs) and the percent of mutated variants compared to the number of tested variants. Data were obtained by whole exome sequencing of PD patients. The analysis in this study targeted several variants from each LSD gene, looking for an association with PD. Variants that were significantly associated with PD are given as the percentage of total variants examined for each gene. The study was performed under strict conditions, only using patients with a common European ancestry and quality-controlled samples. Moreover, subjects were excluded with mutations in well-established Mendelian genes known to cause PD. Adapted from [10].
| LSD | Gene | Mutated Variants (%) |
|---|---|---|
| Action mycolonus-renal failure syndrome | SCARB2 | 70.0 |
| Alpha-mannosidosis | MAN2B1 | 91.7 |
| Aspartylglucosaminuria | AGA | 33.3 |
| Beta-mannosidosis | MANBA | 83.3 |
| Cystinosis | CTNS | 92.3 |
| Danon disease | LAMP2 | 77.8 |
| Fabry disease | GLA | 77.8 |
| Farber Lipogranulomatosis | ASAH1 | 85.0 |
| Fucosidosis | FUCA1 | 80.0 |
| Galactosialidosis | CTSA | 78.6 |
| Gaucher disease | GBA | 82.1 |
| GM1-gangliosidosis/Morquio B | GLB1 | 50.0 |
| GM2-gangliosidosis | GM2A | 100.0 |
| Hunter syndrome | IDS | 88.9 |
| Hurler syndrome | IDUA | 50.0 |
| I-Cell disease | GNPTAB | 79.5 |
| Krabbe disease | GALC | 83.3 |
| Kufor-Rakeb syndrome | ATP13A2 | 75.0 |
| Maroteaux–Lamy disease | ARSB | 90.9 |
| Metachromatic leukodystrophy | ARSA | 100.0 |
| Morquio A disease | GALNS | 63.6 |
| Mucolipidosis type IV | MCOLN1 | 73.7 |
| Mucopolysaccharidosis type IX | HYAL1 | 69.2 |
| Neuronal ceroid lipofuscinosis | CLN3 | 92.3 |
| CLN6 | 70.0 | |
| CLN8 | 44.4 | |
| CTSD | 57.1 | |
| CTSF | 81.8 | |
| DNAJC5 | 100.0 | |
| GRN | 63.2 | |
| KCTD7 | 75.0 | |
| MFSD8 | 77.8 | |
| PPTI | 77.8 | |
| TPPI | 86.7 | |
| Niemann–Pick disease type A/B | SMPD1 | 84.0 |
| Niemann–Pick disease type C1 | NPC1 | 81.4 |
| Niemann–Pick disease type C2 | NPC2 | 100.0 |
| Pompe disease | GAA | 66.7 |
| Pycnodysostosis | CTSK 6 | 83.3 |
| Salla disease | SLC17A5 | 94.4 |
| Sandhoff disease | HEXB | 75.0 |
| Sanfilippo A syndrome | SGSH | 80.0 |
| Sanfilippo B syndrome | NAGLU | 90.0 |
| Sanfilippo C syndrome | HGSNAT | 83.3 |
| Sanfilippo D syndrome | GNS | 55.0 |
| Schindler disease/Kanzaki disease | NAGA | 88.9 |
| Sly disease | GUSB | 58.8 |
| Sphingolipid-activator deficiency | PSAP | 72.7 |
| Tay–Sachs disease | HEXA | 90.0 |
| Wolman disease | LIPA 14 | 71.4 |
Just under ten years ago [9], we documented the prevalence of PD features in LSD patients and in cellular and animal models, and came to the somewhat surprising conclusion, at least at that time, that there was indeed an association between LSDs and PD rather than just between GD and PD. We suggested that additional genetic, epidemiological, and clinical studies should be performed to check the precise incidence of mutations in genes encoding lysosomal proteins in patients displaying PD symptoms. In the current review, we update studies performed in the last decade or so that lend further support to the association between the lysosome and PD, and then briefly discuss progress on understanding the mechanistic relationship between the lysosome and PD.
2. Associations Between LSDs and PD
LSDs are monogenic diseases that are mainly inherited in a recessive manner. Individually, LSDs are rare, but collectively are estimated to occur in as many as 1:5000 live births [13]. However, due to their low individual prevalence, early age of onset and death in many cases, limited pathophysiological information as well as limited information on natural history, especially in late- onset forms, is available for many of the diseases. Thus, in some cases, it is difficult to make a compelling argument for an association with PD. Having said that, our earlier review [9] documented that PD was detected in LSD carriers and patients, as well as in relatives of LSD patients. Additionally, in some LSD animal models, PD features such as substantia nigra (SN) pathology, Lewy body formation or α-synuclein aggregation, ubiquitinated protein aggregates, and/or down-regulation of UCH-L1 were observed [9]. We will now discuss the significant progress that has been made in the last few years that supports a clear association between three of the most common LSDs and PD.
2.1. Gaucher Disease
Mutations in GBA1, which encodes acid-β-glucosidase (GCase), are the highest genetic risk factor for PD [7]. A large multi-center study showed that by the age of 80, GD patients have a 9.1% risk of developing PD, and GBA1 carriers have a risk of 7.7% [14], consistent with an earlier study [7]. Furthermore, the severity of GBA1 mutations, determined by their strong association with the presence or absence of a GD neuronopathic phenotype [15], correlates with the risk and severity of PD [16,17,18,19]. PD patients with a GBA1 mutation leading to a severe neurological form of GD displayed worse motor and non-motor symptoms than GBA1 mutations that lead to a milder form of GD in both heterozygotes and homozygotes [19]. However, results have not been consistent, with some suggesting that PD patients with or without a GBA1 mutation are clinically and cognitively heterogeneous [20], though some studies were limited by the number of available patients and the kind of mutations examined. Numerous other studies have attempted to address both the genetic and mechanistic relationship between GBA1 and PD (see, for instance, [21,22,23,24,25,26]).
2.2. Niemann–Pick Disease
Significant advances in delineating the relationship between mutations in the SMPD1 gene, which causes Niemann–Pick disease types A and B [27], and PD have been reported in the past few years, with SMPD1 repeatedly identified as a genetic risk factor for PD [28,29]. Thus, 3.1% of Jewish Ashkenazi PD patients were SMPD1 carriers [30], and an association between SMPD1 and sporadic PD was also reported in Chinese patients [31]. Similar to GD, different SMPD1 mutations may influence the risk and the course of PD; thus, patients with the L302P mutation have a greater chance of developing PD than those with the R496L mutation [29]. Although there are no documented cases of PD in Niemann–Pick disease, parkinsonian phenotypes were reported in a 9-month-old Niemann–Pick type A patient with tremors on one side of her body [32].
Niemann–Pick type C disease was originally classified as a similar disease to Niemann–Pick types A and B, although it is now known to be caused by mutations in two completely different genes, namely NPC1 and NPC2 [33]. Two adult heterozygous carriers with mutations in NPC1 have been reported with PD and a further heterozygous individual has been identified with Parkinsonism [34].
2.3. Fabry Disease
Fabry disease is an X-linked disorder caused by mutations in GLA, which encodes α-galactosidase A (α-Gal). Several studies suggesting an association between Fabry disease and PD have recently been published. In the first, α-Gal activity was about 10% lower in blood spots from PD patients [35]. This reduction was seen in all types of PD patients, including idiopathic PD and patients with LRRK2 or GBA1 mutations, suggesting that the reduced activity of α-Gal in PD patients is not affected by genetic risk factors. A second study revealed that α-Gal levels and activity were reduced in the temporal cortex in late-stage PD, which correlated with elevated α-synuclein levels [36]. A third study demonstrated reduced α-Gal activity along with the reduced activity of multiple lysosomal hydrolases in the SN of PD patients [37]. A further study revealed that 8.3% of Fabry patients over the age of 60 were diagnosed with PD and 7.4% of the families of these patients had a close relative that matched the criteria for a PD diagnosis [38]; however, the latter was an online survey with no direct clinical examination of PD. In addition, α-Gal activity was significantly lower in the serum of PD patients compared to parkinsonian syndrome [39]. Finally, reduced nigral volume (suggesting neurodegeneration in this region) correlating with increased susceptibility of this region was observed in Fabry disease patients [40].
2.4. Other LSDs
A number of other LSD genes were identified in a genome-wide association study (GWAS) of PD patients, including GALC [41] (Krabbe disease) and IDUA [42] (mucopolysaccharidoses I, also known as Hurler syndrome). Moreover, focused candidate gene studies were performed to determine the association between PD and SCARB2 (myoclonus–renal failure) with polymorphisms in the SCARB2 locus associated with the risk of developing PD [43,44], although another study suggested that SCARB2 does not confer a significant risk for PD [45]. A retrospective study demonstrated that clinical symptoms in three families with ARSA haploinsufficiency [46] (metachromatic leukodystrophy, or MLD) were similar to those described in PD patients with GBA1 mutations. Mutations in one of the genes causing neuronal ceroid lipofuscinoses, ATP13A2 (also known as PARKA), were also shown to cause a form of early-onset Parkinsonism with pyramidal degeneration and dementia [43,47].
In summary, most of the data available in 2011 [9] was based on clinical and pathological observations, while genetic associations between LSD genes and PD were limited (except for GBA1). Since then, a significant amount of genetic, clinical, and pathological data has accrued, indicating that the connection between LSDs and PD is significantly tighter than in 2011, strengthening the argument that a detailed understanding of lysosomal (dys)function in PD is of critical importance to delineating the mechanistic link between the lysosome and PD.
3. Lysosomal Dysfunction and PD
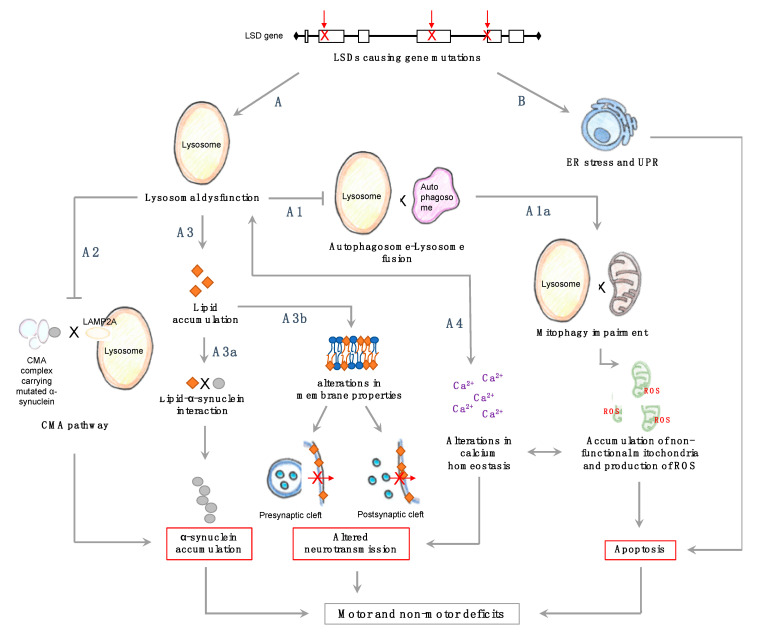
A unified hypothesis explaining the mechanistic association between mutations in lysosomal genes and PD is currently lacking. While we favor the loss-of-function paradigm, the appearance of endoplasmic reticulum stress and of the unfolded protein response in some LSDs [48,49,50,51,52] and in PD [53,54,55,56] lends some credence to the gain-of-function hypothesis (Figure 1, pathway B). In reality, both loss and gain of function probably contribute to the association between the lysosome and PD.
Figure 1.
Possible pathophysiological pathways for exacerbation of Parkinson’s disease (PD) symptoms due to mutations in LSD-causing genes. Lysosomal dysfunction may be a key player in PD pathogenesis and could be triggered or exacerbated due to LSD gene mutations. With the lysosome being involved in numerous cellular processes, lysosomal dysfunction could explain some of the symptoms observed in PD. LSDs; lysosomal storage diseases, ER; endoplasmic reticulum, UPR; unfolded protein response, ROS; reactive oxygen species. See text for more details.
Lysosomal dysfunction, although a rather loose term used mainly to describe the inappropriate execution of lysosomal function, occurs due to mutations in lysosomal proteins or to alterations in lysosomal acidification (Figure 1, pathway A). Since most LSDs are recessive and carriers do not display overt LSD symptoms, it is difficult to explain why mutations in one allele encoding for a lysosomal protein increase the risk of PD [7]. A consensus is emerging that general impairment of lysosomal function could occur over the long lifespan of an individual and may be exacerbated in the case of carriers. This is supported by data showing that GCase activity decreases with age in normal mouse brains, and that glucosylsphingosine (GlcSph) levels increase with age in normal mice [57]. Similar changes are seen in the SN and hippocampus of sporadic PD patients without GBA1 mutations [58], along with a reduction in the activity of GCase, α-mannosidase, β-mannosidase, and β-hexosaminidase in the cerebrospinal fluid of PD patients [59]. A recent study also demonstrated a reduction in GCase activity in the SN of PD patients, in addition to substrate accumulation, suggesting it is not the result of neuronal death [37].
There is only very limited information examining lipid accumulation in LSD carriers. In one study [60], the accumulation of ceramides and sphingolipids was observed in Lewy body dementia patients carrying a GBA1 mutation compared with controls, although other studies [61,62] suggested no lipid accumulation in LSD carriers. Clearly, further systematic studies on LSD carriers are required to evaluate whether substrate levels are altered. Since carriers do not present disease symptoms, it is close to impossible to obtain human brain tissue, although such tissues can be easily obtained from animal models of the relevant diseases.
Irrespective of the extent of substrate accumulation in LSD carriers, changes in lysosomal function could lead to inappropriate clearance of proteins, including α-synuclein (Figure 1, pathway A1), and a crosstalk between α-synuclein and lysosomal enzyme levels has been described [63,64,65]. Presumably, α-synuclein accumulation with age due to a reduction in lysosomal function may be exacerbated in LSD carriers or in individuals with multiple haploid mutations [10] due to accumulative damage, perhaps explaining the earlier onset of PD.
Evidence for lysosomal dysfunction in PD is also accumulating [66]. Thus, a reduction in some lysosomal markers in the SN was observed in some early studies in PD patients [67,68], as was the number of lysosomes [68]. Also, the selective reduction of LAMP2 and GCase in regions accumulating α-synuclein in the early stages of PD was observed, suggesting altered lysosomal function, including alterations in chaperone-mediated autophagy pathways [69]. Several familial PD-related genes are strongly linked to endo-lysosomal and autophagic pathways [70,71,72]. Treatment of neuroblastoma cells with the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) resulted in reduced lysosomal markers and accumulation of autophagosomes [68]. Impairment of lysosomal degradation might also explain why genomic variants elevate α-synuclein levels, such as mutations causing SNCA multiplication or mutations causing the enhancement of its promoter [73,74]. There is also some evidence that lysosomal integrity is altered in PD and may cause the seeding of α-synuclein aggregates [67,68,75]. Clearly, loss of lysosomal function, either in PD or as the result of an LSD, will impact a variety of up- and downstream pathways, such as autophagy, which is indeed impaired in 14 different LSDs [76], and mitophagy [77] (Figure 1, pathway A1a), which would exacerbate mitochondrial dysfunction. The mitochondrial dysfunction, observed in a number of LSDs [78], might be due to the strong reciprocal relationship between the lysosome and mitochondria [79]. Finally, different neuronal populations might be more or less sensitive to lysosomal dysfunction; thus, selective loss of GCase activity was observed in the SN and caudate, but not in the frontal cortex, hippocampus, cerebellum, or putamen of PD patients [80]. The basal activity of lysosomal enzymes (in both the control and patients) was generally higher in the SN and hippocampus than in six other brain regions [80].
As a result of lysosomal dysfunction, cellular lipid composition may also be altered. A variety of lipids, including sphingolipids, cholesterol, and fatty acids interact directly or indirectly with α-synuclein [81] (Figure 1, pathway A3a). In PD, α-synuclein is converted from a soluble protein into insoluble amyloid-like fibrils [82], which might be mediated by interactions with these lipids. Thus, when human midbrain neurons were treated with conduritol B-epoxide (CBE, a chemical inhibitor of GCase), a reversible conformational change in α-synuclein was observed [83]. Liposomes containing gangliosides GM1 and GM2 and glucosylceramide (GlcCer) induce a catalytic environment for nucleation of α-synuclein aggregation [84], and some of these gangliosides accumulate as secondary metabolites in some LSDs [85]. The effect of lipid composition is not limited to interactions with α-synuclein, since neurotransmission is also affected by membrane lipid composition (Figure 1, pathway A3b); as a result, lipid accumulation in LSD patients and carriers may alter neurotransmission and affect both motor and non-motor symptoms, such as the reduction in exocytosis observed in mucopolysaccharidosis IIIA mice [86]. Moreover, some neurotransmitters use membrane-dependent mechanisms for their uptake and thus might be dependent on membrane composition for their normal function [87,88].
Another critical pathway affected in both LSDs and PD is calcium homeostasis (Figure 1, pathway A4). Induced pluripotent stem cell (iPSC)-derived neurons from PD patients carrying a GBA1 mutation (PD-GBA) and GD patients show impaired calcium homeostasis upon stress stimulation, in addition to impaired autophagy [89]. Moreover, a reduction in lysosomal calcium store content in PD and PD-GBA fibroblasts, as well as disturbances in lysosomal morphology [90], have been observed. Altered calcium homeostasis is observed in a number of LSDs (reviewed in [91]). Since calcium is an important player in many neuronal events, it may be a critical player in the relationship between PD and LSDs.
In summary, it is likely that the lysosome acts as a central protective hub against α-synuclein toxicity, autophagy impairment, altered neurotransmission, and alterations in calcium homeostasis. Therefore, any impairment of this pathway, such as that which occurs over the long lifetime of an LSD carrier, could increase the risk of developing PD.
4. Concluding Remarks
The notion that lysosomal processes play a central role in PD pathophysiology is now gaining momentum. The lysosome is crucial for various cellular processes, and, upon disruption, lysosomal dysfunction is likely to lead to α-synuclein accumulation (Figure 1). This, together with the discovery that mutations in GBA1 are the highest genetic risk factor for PD [7], instigated the search for the mechanistic connection between PD and various LSDs [9].
The majority of clinical studies on the relationship between PD and LSDs were performed on carriers rather than LSD patients, due to the low individual prevalence of LSDs and their early age of death. In LSD carriers, lysosomal dysfunction could be exacerbated over the years which, along with a reduction in lysosomal activity [57,58], could culminate in α-synuclein accumulation (Figure 1). Assuming that α-synuclein causes PD, LSD carriers and patients are more likely to develop early onset PD, depending on whether they accumulate α-synuclein or not (and other genetic and environmental factors). We further suggest that lysosomal dysfunction could explain both motor and non-motor observations in LSD-related PD (Figure 1). Indeed, we recently documented evidence for co-existence of early PD-related non-motor symptoms in LSDs (Blumenreich et al., in press). We thus speculate that lysosomal dysfunction due to LSD mutations may enhance the aggregation and spreading of α-synuclein, and therefore trigger the PD cascade.
Abbreviations
| GCase | Acid-β-glucosidase |
| GD | Gaucher disease |
| LSDs | Lysosomal storage diseases |
| PD | Parkinson’s disease |
| PD-GBA | PD patient with a GBA1 mutation |
| SN | Substantia nigra |
Author Contributions
Writing—original draft preparation, S.B., O.B.B., B.J.J. and A.H.F.; writing—review and editing, S.B. and A.H.F.; visualization, S.B.; supervision, A.H.F. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the Futerman laboratory on Parkinson’s disease is supported by the Legacy Heritage Biomedical Science Partnership Program of the Israel Science Foundation (grant # 2240/17), by the Rolf Wiklund and Alice Wiklund Parkinson’s Disease Research Fund, the Children’s Gaucher Research Fund, and the Michael J. Fox Foundation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.de Duve C. Exploring cells with a centrifuge. Science. 1975;189:186–194. doi: 10.1126/science.1138375. [DOI] [PubMed] [Google Scholar]
- 2.de Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7:847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 3.Saftig P., Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 4.Settembre C., Fraldi A., Medina D.L., Ballabio A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futerman A.H., van Meer G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 6.Futerman A.H., Zimran A. In: Gaucher Diseasem. 1st ed. Futerman A., Zimran A., editors. CRC Press; Boca Raton, FL, USA: 2006. [Google Scholar]
- 7.Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R., Bar-Shira A., Berg D., Bras J., Brice A., et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Futerman A.H., Hardy J. Perspective: Finding common ground. Nature. 2016;537:160–161. doi: 10.1038/537S160a. [DOI] [PubMed] [Google Scholar]
- 9.Shachar T., Bianco L.C., Recchia A., Wiessner C., Raas-Rothschild A., Futerman A.H. Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov. Disord. 2011;26:1593–1604. doi: 10.1002/mds.23774. [DOI] [PubMed] [Google Scholar]
- 10.Robak L.A., Jansen I.E., van Rooij J., Uitterlinden A.G., Kraaij R., Jankovic J., International Parkinson’s Disease Genomics Consortium (IPDGC) Heutink P., Shulman J.M. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain. 2017;140:3191–3203. doi: 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brozzi A., Urbanelli L., Germain P.L., Magini A., Emiliani C. hLGDB: A database of human lysosomal genes and their regulation. Database (Oxford) 2013;2013:bat024. doi: 10.1093/database/bat024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyant G.A., Abu-Remaileh M., Frenkel E.M., Laqtom N.N., Dharamdasani V., Lewis C.A., Chan S.H., Heinze I., Ori A., Sabatini D.M. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science. 2018;360:751–758. doi: 10.1126/science.aar2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meikle P.J., Brooks D.A., Ravenscroft E.M., Yan M., Williams R.E., Jaunzems A.E., Chataway T.K., Karageorgos L.E., Davey R.C., Boulter C.D., et al. Diagnosis of lysosomal storage disorders: Evaluation of lysosome-associated membrane protein LAMP-1 as a diagnostic marker. Clin. Chem. 1997;43:1325–1335. doi: 10.1093/clinchem/43.8.1325. [DOI] [PubMed] [Google Scholar]
- 14.Alcalay R.N., Dinur T., Quinn T., Sakanaka K., Levy O., Waters C., Fahn S., Dorovski T., Chung W.K., Pauciulo M., et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol. 2014;71:752–757. doi: 10.1001/jamaneurol.2014.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migdalska-Richards A., Schapira A.H.V. The relationship between glucocerebrosidase mutations and Parkinson disease. J. Neurochem. 2016;139:77–90. doi: 10.1111/jnc.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan-Or Z., Amshalom I., Kilarski L.L., Bar-Shira A., Gana-Weisz M., Mirelman A., Marder K., Bressman S., Giladi N., Orr-Urtreger A. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84:880–887. doi: 10.1212/WNL.0000000000001315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cilia R., Tunesi S., Marotta G., Cereda E., Siri C., Tesei S., Zecchinelli A.L., Canesi M., Mariani C.B., Meucci N., et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 2016;80:662–673. doi: 10.1002/ana.24777. [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Boot B., Locascio J.J., Jansen I.E., Rhodes S.W., Eberly S., Elbaz A., Brice A., Ravina B., van Hilten J.J., et al. Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann. Neurol. 2016;80:674–685. doi: 10.1002/ana.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thaler A., Bregman N., Gurevich T., Shiner T., Dror Y., Zmira O., Gan-Or Z., Bar-Shira A., Gana-Weisz M., Orr-Urtreger A., et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat. Disord. 2018;55:45–49. doi: 10.1016/j.parkreldis.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Collins L.M., Williams-Gray C.H., Morris E., Deegan P., Cox T.M., Barker R.A. The motor and cognitive features of Parkinson’s disease in patients with concurrent Gaucher disease over 2 years: A case series. J. Neurol. 2018;61:1789–1794. doi: 10.1007/s00415-018-8908-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tayebi N., Parisiadou L., Berhe B., Gonzalez A.N., Serra-Vinardell J., Tamargo R.J., Maniwang E., Sorrentino Z., Fujiwara H., Grey R.J., et al. Glucocerebrosidase haploinsufficiency in A53T α-synuclein mice impacts disease onset and course. Mol. Genet. Metab. 2017;122:198–208. doi: 10.1016/j.ymgme.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taguchi Y.V., Liu J., Ruan J., Pacheco J., Zhang X., Abbasi J., Keutzer J., Mistry P.K., Chandra S.S. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017;37:9617–9631. doi: 10.1523/JNEUROSCI.1525-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruskey J.A., Zhou S., Santiago R., Franche L.-A., Alam A., Roncière L., Spiegelman D., Fon E.A., Trempe J.-F., Kalia L.V., et al. The GBA p.Trp378Gly mutation is a probable French-Canadian founder mutation causing Gaucher disease and synucleinopathies. Clin. Genet. 2018;29:1–7. doi: 10.1111/cge.13405. [DOI] [PubMed] [Google Scholar]
- 24.Thomas R.E., Vincow E.S., Merrihew G.E., MacCoss M.J., Davis M.Y., Pallanck L.J. Glucocerebrosidase deficiency promotes protein aggregation through dysregulation of extracellular vesicles. PLoS Genet. 2018;14:e1007694. doi: 10.1371/journal.pgen.1007694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H., Ham A., Ma T.C., Kuo S.-H., Kanter E., Kim D., Ko H.S., Quan Y., Sardi S.P., Li A., et al. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy. 2018;15:113–130. doi: 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arkadir D., Dinur T., Cohen M.B., Vilk S.R., Tiomkin M., Brüggemann N., Cozma C., Rolfs A., Zimran A. Prodromal substantia nigra sonography undermines suggested association between substrate accumulation and the risk for GBA-related Parkinson’s disease. Eur. J. Neurol. 2019;26:1013–1018. doi: 10.1111/ene.13927. [DOI] [PubMed] [Google Scholar]
- 27.Schuchman E.H., Wasserstein M.P. Types A and B Niemann-Pick Disease. Pediatr. Endocrinol. Rev. 2016;13:674–681. [PubMed] [Google Scholar]
- 28.Gan-Or Z., Ozelius L.J., Bar-Shira A., Saunders-Pullman R., Mirelman A., Kornreich R., Gana-Weisz M., Raymond D., Rozenkrantz L., Deik A., et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology. 2013;80:1606–1610. doi: 10.1212/WNL.0b013e31828f180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gan-Or Z., Orr-Urtreger A., Alcalay R.N., Bressman S., Giladi N., Rouleau G.A. The emerging role of SMPD1 mutations in Parkinson’s disease: Implications for future studies. Parkinsonism Relat. Disord. 2015;21:1294–1295. doi: 10.1016/j.parkreldis.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Dagan E., Schlesinger I., Ayoub M., Mory A., Nassar M., Kurolap A., Peretz-Aharon J., Gershoni-Baruch R. The contribution of Niemann-Pick SMPD1 mutations to Parkinson disease in Ashkenazi Jews. Parkinsonism Relat. Disord. 2015;21:1067–1071. doi: 10.1016/j.parkreldis.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Mao C.-Y., Yang J., Wang H., Zhang S.-Y., Yang Z.-H., Luo H.-Y., Li F., Shi M., Liu Y.-T., Zhuang Z.-P., et al. SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Parkinsonism Relat. Disord. 2017;34:59–61. doi: 10.1016/j.parkreldis.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Vykuntaraju K.N., Lokanatha H. Shivananda Niemann-Pick disease type A presenting as unilateral tremors. Indian Pediatr. 2012;49:919–920. doi: 10.1007/s13312-012-0196-0. [DOI] [PubMed] [Google Scholar]
- 33.Evans E.L., Platt F.M. Lipids on Trial: The Search for the Offending Metabolite in Niemann-Pick type C Disease. Traffic. 2010;11:419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 34.Kluenemann H.H., Nutt J.G., Davis M.Y., Bird T.D. Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J. Neurol. Sci. 2013;335:219–220. doi: 10.1016/j.jns.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcalay R.N., Wolf P., Levy O.A., Kang U.J., Waters C., Fahn S., Ford B., Kuo S.H., Vanegas N., Shah H., et al. Alpha galactosidase A activity in Parkinson’s disease. Neurobiol. Dis. 2018;112:85–90. doi: 10.1016/j.nbd.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson M.P., Boutin M., Tse T.E., Lu H., Haley E.D., Ouyang X., Zhang J., Auray-Blais C., Shacka J.J. The lysosomal enzyme alpha-Galactosidase A is deficient in Parkinson’s disease brain in association with the pathologic accumulation of alpha-synuclein. Neurobiol. Dis. 2018;110:68–81. doi: 10.1016/j.nbd.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huebecker M., Moloney E.B., van der Spoel A.C., Priestman D.A., Isacson O., Hallett P.J., Platt F.M. Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease. Mol. Neurodegener. 2019;14:40. doi: 10.1186/s13024-019-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wise A.H., Yang A., Naik H., Stauffer C., Zeid N., Liong C., Balwani M., Desnick R.J., Alcalay R.N. Parkinson’s disease prevalence in Fabry disease: A survey study. Mol. Genet. Metab Rep. 2018;14:27–30. doi: 10.1016/j.ymgmr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niimi Y., Ito S., Mizutani Y., Murate K., Shima S., Ueda A., Satake W., Hattori N., Toda T., Mutoh T. Altered regulation of serum lysosomal acid hydrolase activities in Parkinson’s disease: A potential peripheral biomarker? Parkinsonism Relat. Disord. 2019;61:132–137. doi: 10.1016/j.parkreldis.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 40.Russo C., Pontillo G., Pisani A., Saccà F., Riccio E., Macera A., Rusconi G., Stanzione A., Borrelli P., Brescia Morra V., et al. Striatonigral involvement in Fabry Disease: A quantitative and volumetric Magnetic Resonance Imaging study. Parkinsonism Relat. Disord. 2018;57:27–32. doi: 10.1016/j.parkreldis.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Chang D., Nalls M.A., Hallgrímsdóttir I.B., Hunkapiller J., van der Brug M., Cai F., International Parkinson’s Disease Genomics Consortium. 23andMe Research Team. Kerchner G.A., Ayalon G., et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pankratz N., Wilk J.B., Latourelle J.C., DeStefano A.L., Halter C., Pugh E.W., Doheny K.F., Gusella J.F., Nichols W.C., Foroud T., et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum. Genet. 2008;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Do C.B., Tung J.Y., Dorfman E., Kiefer A.K., Drabant E.M., Francke U., Mountain J.L., Goldman S.M., Tanner C.M., Langston J.W., et al. Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson’s Disease. PLoS Genet. 2011;7:e1002141. doi: 10.1371/journal.pgen.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michelakakis H., Xiromerisiou G., Dardiotis E., Bozi M., Vassilatis D., Kountra P.-M., Patramani G., Moraitou M., Papadimitriou D., Stamboulis E., et al. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson’s disease. Mov. Disord. 2012;27:400–405. doi: 10.1002/mds.24886. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y., Yuan X., Cao B., Wei Q., Ou R., Yang J., Chen X., Zhao B., Song W., Wu Y., et al. No association of FAM47E rs6812193, SCARB2 rs6825004 and STX1B rs4889603 polymorphisms with Parkinson’s disease in a Chinese Han population. J. Neural Transm. 2015;122:1547–1552. doi: 10.1007/s00702-015-1430-4. [DOI] [PubMed] [Google Scholar]
- 46.Antelmi E., Rizzo G., Fabbri M., Capellari S., Scaglione C., Martinelli P. Arylsulphatase A activity in familial parkinsonism: A pathogenetic role? J. Neurol. 2014;261:1803–1809. doi: 10.1007/s00415-014-7425-5. [DOI] [PubMed] [Google Scholar]
- 47.Najim al-Din A.S., Wriekat A., Mubaidin A., Dasouki M., Hiari M. Pallido-pyramidal degeneration, supranuclear upgaze paresis and dementia: Kufor-Rakeb syndrome. Acta Neurol. Scand. 1994;89:347–352. doi: 10.1111/j.1600-0404.1994.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 48.Maor G., Rencus-Lazar S., Filocamo M., Steller H., Segal D., Horowitz M. Unfolded protein response in Gaucher disease: From human to Drosophila. Orphanet J. Rare Dis. 2013;8:140. doi: 10.1186/1750-1172-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tessitore A., del P Martin M., Sano R., Ma Y., Mann L., Ingrassia A., Laywell E.D., Steindler D.A., Hendershot L.M., d’Azzo A. GM1-Ganglioside-Mediated Activation of the Unfolded Protein Response Causes Neuronal Death in a Neurodegenerative Gangliosidosis. Mol. Cell. 2004;15:753–766. doi: 10.1016/j.molcel.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Johnson J.L., Napolitano G., Monfregola J., Rocca C.J., Cherqui S., Catz S.D. Upregulation of the Rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol. Cell. Biol. 2013;33:2950–2962. doi: 10.1128/MCB.00417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marotta D., Tinelli E., Mole S.E. NCLs and ER: A stressful relationship. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1273–1281. doi: 10.1016/j.bbadis.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irahara-Miyana K., Otomo T., Kondo H., Hossain M.A., Ozono K., Sakai N. Unfolded protein response is activated in Krabbe disease in a manner dependent on the mutation type. J. Hum. Genet. 2018;63:699–706. doi: 10.1038/s10038-018-0445-8. [DOI] [PubMed] [Google Scholar]
- 53.Mercado G., Castillo V., Soto P., Sidhu A. ER stress and Parkinson’s disease: Pathological inputs that converge into the secretory pathway. Brain Res. 2016;1648:626–632. doi: 10.1016/j.brainres.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 54.Hoozemans J.J.M., van Haastert E.S., Eikelenboom P., de Vos R.A.I., Rozemuller J.M., Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 55.Heman-Ackah S.M., Manzano R., Hoozemans J.J.M., Scheper W., Flynn R., Haerty W., Cowley S.A., Bassett A.R., Wood M.J.A. Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum. Mol. Genet. 2017;26:4441–4450. doi: 10.1093/hmg/ddx331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdés P., Mercado G., Vidal R.L., Molina C., Parsons G., Court F.A., Martinez A., Galleguillos D., Armentano D., Schneider B.L., et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl. Acad. Sci. USA. 2014;111:6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallett P.J., Huebecker M., Brekk O.R., Moloney E.B., Rocha E.M., Priestman D.A., Platt F.M., Isacson O. Glycosphingolipid levels and glucocerebrosidase activity are altered in normal aging of the mouse brain. Neurobiol. Aging. 2018;67:189–200. doi: 10.1016/j.neurobiolaging.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rocha E.M., Smith G.A., Park E., Cao H., Brown E., Hallett P., Isacson O. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015;2:433–438. doi: 10.1002/acn3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balducci C., Pierguidi L., Persichetti E., Parnetti L., Sbaragli M., Tassi C., Orlacchio A., Calabresi P., Beccari T., Rossi A. Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov. Disord. 2007;22:1481–1484. doi: 10.1002/mds.21399. [DOI] [PubMed] [Google Scholar]
- 60.Clark L.N., Chan R., Cheng R., Liu X., Park N., Parmalee N., Kisselev S., Cortes E., Torres P.A., Pastores G.M., et al. Gene-Wise Association of Variants in Four Lysosomal Storage Disorder Genes in Neuropathologically Confirmed Lewy Body Disease. PLoS ONE. 2015;10:e0125204. doi: 10.1371/journal.pone.0125204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gegg M.E., Sweet L., Wang B.H., Shihabuddin L.S., Sardi S.P., Schapira A.H.V. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 2015;30:1085–1089. doi: 10.1002/mds.26278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boudewyn L.C., Walkley S.U. Current concepts in the neuropathogenesis of mucolipidosis type IV. J. Neurochem. 2018;148:669–689. doi: 10.1111/jnc.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rothaug M., Zunke F., Mazzulli J.R., Schweizer M., Altmeppen H., Lüllmann-Rauch R., Kallemeijn W.W., Gaspar P., Aerts J.M., Glatzel M., et al. LIMP-2 expression is critical for β-glucocerebrosidase activity and α-synuclein clearance. Proc. Natl. Acad. Sci. USA. 2014;111:15573–15578. doi: 10.1073/pnas.1405700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sardi S.P., Clarke J., Kinnecom C., Tamsett T.J., Li L., Stanek L.M., Passini M.A., Grabowski G.A., Schlossmacher M.G., Sidman R.L., et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. USA. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mazzulli J.R., Zunke F., Tsunemi T., Toker N.J., Jeon S., Burbulla L.F., Patnaik S., Sidransky E., Marugan J.J., Sue C.M., et al. Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. J. Neurosci. 2016;36:7693–7706. doi: 10.1523/JNEUROSCI.0628-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein A.D., Mazzulli J.R. Is Parkinson’s disease a lysosomal disorder? Brain. 2018;141:2255–2262. doi: 10.1093/brain/awy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chu Y., Dodiya H., Aebischer P., Olanow C.W., Kordower J.H. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: Relationship to alpha-synuclein inclusions. Neurobiol. Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 68.Dehay B., Bové J., Rodríguez-Muela N., Perier C., Recasens A., Boya P., Vila M. Pathogenic Lysosomal Depletion in Parkinson’s Disease. J. Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy K.E., Gysbers A.M., Abbott S.K., Tayebi N., Kim W.S., Sidransky E., Cooper A., Garner B., Halliday G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kett L.R., Dauer W.T. Endolysosomal dysfunction in Parkinson’s disease: Recent developments and future challenges. Mov. Disord. 2016;31:1433–1443. doi: 10.1002/mds.26797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balestrino R., Schapira A.H.V. Glucocerebrosidase and Parkinson Disease: Molecular, Clinical, and Therapeutic Implications. Neuroscientist. 2018;20:1–20. doi: 10.1177/1073858417748875. [DOI] [PubMed] [Google Scholar]
- 72.Gan-Or Z., Dion P.A., Rouleau G.A. Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy. 2015;11:1443–1457. doi: 10.1080/15548627.2015.1067364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singleton A.B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 74.Soldner F., Stelzer Y., Shivalila C.S., Abraham B.J., Latourelle J.C., Barrasa M.I., Goldmann J., Myers R.H., Young R.A., Jaenisch R. Parkinson-associated risk variant in distal enhancer of α-synuclein modulates target gene expression. Nature. 2016;533:95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang P., Gan M., Yen S.-H., McLean P.J., Dickson D.W. Impaired endo-lysosomal membrane integrity accelerates the seeding progression of α-synuclein aggregates. Sci. Rep. 2017;7:7690. doi: 10.1038/s41598-017-08149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lieberman A.P., Puertollano R., Raben N., Slaugenhaupt S., Walkley S.U., Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8:719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotegher N., Duchen M.R. Mitochondrial Dysfunction and Neurodegeneration in Lysosomal Storage Disorders. Trends Mol. Med. 2017;23:116–134. doi: 10.1016/j.molmed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Osellame L.D., Duchen M.R. Quality control gone wrong: Mitochondria, lysosomal storage disorders and neurodegeneration. Br. J. Pharmacol. 2014;171:1958–1972. doi: 10.1111/bph.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plotegher N., Duchen M.R. Crosstalk between Lysosomes and Mitochondria in Parkinson’s Disease. Front. Cell Dev. Biol. 2017;5:1–8. doi: 10.3389/fcell.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chiasserini D., Paciotti S., Eusebi P., Persichetti E., Tasegian A., Kurzawa-Akanbi M., Chinnery P.F., Morris C.M., Calabresi P., Parnetti L., et al. Selective loss of glucocerebrosidase activity in sporadic Parkinson’s disease and dementia with Lewy bodies. Mol. Neurodegener. 2015;10:1–6. doi: 10.1186/s13024-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galvagnion C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Parkinsons Dis. 2017;7:433–450. doi: 10.3233/JPD-171103. [DOI] [PubMed] [Google Scholar]
- 82.Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 83.Zunke F., Moise A.C., Belur N.R., Gelyana E., Stojkovska I., Dzaferbegovic H., Toker N.J., Jeon S., Fredriksen K., Mazzulli J.R. Reversible Conformational Conversion of α-Synuclein into Toxic Assemblies by Glucosylceramide. Neuron. 2017;97:92–107. doi: 10.1016/j.neuron.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki M., Sango K., Wada K., Nagai Y. Pathological role of lipid interaction with α-synuclein in Parkinson’s disease. Neurochem. Int. 2018;119:97–106. doi: 10.1016/j.neuint.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 85.Walkley S.U. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev. Biol. 2004;15:433–444. doi: 10.1016/j.semcdb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Keating D.J., Winter M.A., Hemsley K.M., Mackenzie K.D., Teo E.H., Hopwood J.J., Brooks D.A., Parkinson-Lawrence E.J. Exocytosis is impaired in mucopolysaccharidosis IIIA mouse chromaffin cells. Neuroscience. 2012;227:110–118. doi: 10.1016/j.neuroscience.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 87.Postila P.A., Vattulainen I., Róg T. Selective effect of cell membrane on synaptic neurotransmission. Sci. Rep. 2016;6:19345. doi: 10.1038/srep19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orłowski A., Grzybek M., Bunker A., Pasenkiewicz-Gierula M., Vattulainen I., Männistö P.T., Róg T. Strong preferences of dopamine and l-dopa towards lipid head group: Importance of lipid composition and implication for neurotransmitter metabolism. J. Neurochem. 2012;122:681–690. doi: 10.1111/j.1471-4159.2012.07813.x. [DOI] [PubMed] [Google Scholar]
- 89.Schöndorf D.C., Aureli M., McAllister F.E., Hindley C.J., Mayer F., Schmid B., Sardi S.P., Valsecchi M., Hoffmann S., Schwarz L.K., et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Comm. 2014;5:1–17. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- 90.Kilpatrick B.S., Magalhaes J., Beavan M.S., McNeill A., Gegg M.E., Cleeter M.W.J., Bloor-Young D., Churchill G.C., Duchen M.R., Schapira A.H., et al. Endoplasmic reticulum and lysosomal Ca2+ stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 2016;59:12–20. doi: 10.1016/j.ceca.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vitner E.B., Platt F.M., Futerman A.H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]