Abstract
Aims:
Klinefelter (XXY) and XXYY syndromes are genetic disorders in males characterized by additional sex chromosomes compared to the typical male karyotype of 46,XY. Both conditions have been previously associated with motor delays and motor skills deficits. We aimed to describe and compare motor skills in males with XXY and XXYY syndromes, and to analyze associations with age, cognitive abilities and adaptive functioning.
Methods:
Sixty-four males with XXY and 46 males with XXYY, ages 4–20 were evaluated using the Beery Test of Visual Motor Integration and the Bruininks-Oseretsky Test of Motor Proficiency – 2nd Edition assessments, Vineland–2 adaptive scales, and cognitive testing.
Results:
Motor coordination impairments were found in 39% of the males with XXY and 73% of the males with XXYY. Both groups showed strengths in visual perceptual skills. Males with XXYY had lower mean scores compared to males with XXY across all assessments. Fine motor dexterity and coordination deficits were common. There was a positive correlation between VMI scores and adaptive functioning.
Conclusion:
Occupational and Physical therapists should be aware of the motor phenotype in XXY and XXYY both to aid in diagnosis of unidentified cases and to guide intervention.
Keywords: 47, xxy, xxyy, Klinefelter syndrome, motor skills, visual motor integration, activities of daily living, occupational therapy, physical therapy
Occupational therapists (OTs) and physical therapists (PTs) often provide services for individuals with genetic conditions. Sex chromosome aneuploidies, including XXY and XXYY syndromes, are common genetic conditions associated with increased risk for neurodevelopmental delays and adaptive functioning deficits often requiring OT and PT services (Davis et al., 2016). An understanding of the unique motor phenotype in these conditions would help guide assessment and intervention. XXY, also known as Klinefelter syndrome (KS), is characterized by the presence of an additional X chromosome and occurs in ~1/500–1000 male births (Abramsky & Chapple, 1997). XXYY syndrome, where there is an extra copy of both the X and Y chromosomes, occurs in ~1/18,000–40,000 male births (Muldal & Ockey, 1960; Sorensen, Nielsen, Jacobsen, & Rolle, 1978). Y chromosome material in both these syndromes confers the male phenotype, however, the presence of the additional genetic material leads to gene overexpression and a unique phenotype of physical features and neurodevelopmental differences including cognitive, language, and motor deficits (Ross et al., 2008; Salbenblatt, Meyers, Bender, Linden, & Robinson, 1987; Tartaglia et al., 2008).
Associated physical characteristics in both syndromes include tall stature, clinodactyly (curved 5th finger), low muscle bulk and tone, flat feet with ankle pronation, joint hyperextensibility, and tremor. Facial dysmorphic features are usually absent or very subtle. The extra X chromosome results in progressive testicular dysfunction resulting in low testosterone production, and evaluation for testosterone replacement therapy is important in adolescents and adults. While there are many similarities to XXY, males with XXYY can have additional physical features that affect motor functioning including cubitus varus (narrowed elbow carrying angle), radioulnar synostosis or congenital elbow dislocation leading to decreased range of supination / pronation, clubfoot, and scoliosis (Tartaglia et al., 2008). Males with XXYY also have more frequent congenital anomalies and seizure disorders (Tartaglia et al., 2008).
Developmental and psychological features
There is a large spectrum of severity in neurodevelopmental profiles among individuals with XXY and XXYY, with the phenotype generally being more complex in XXYY. Some individuals, particularly with XXY, proceed through childhood and into adulthood without significant developmental or educational difficulties; most individuals, however, show some neurodevelopmental features of these conditions (Tartaglia et al., 2008). Delays in both speech-language development and early motor skills are common (Garvey and Mutton, 1973: Garvey and Kellett, 1975: Fryns et al., 1995).
Language-based learning difficulties and reading disorders commonly emerge during early elementary school, and approximately 75–80% of individuals with XXY and nearly all with XXYY require additional educational supports and/or speech-language therapy (Bender, Puck, Salbenblatt, & Robinson, 1986; Ross et al., 2008). Intellectual disability occurs in less than 5% of boys with XXY and nearly half with supernumerary sex chromosome aneuploidies (Tartaglia, et al. (2011). Most studies evaluating cognitive profiles find that verbal IQ’s are lower than performance IQ in individuals with XXY and XXYY, and visual-perceptual skills are an area of relative strength (Boada, Janusz, Hutaff-Lee, & Tartaglia, 2009).
Adaptive functioning, which encompasses an individual’s ability to perform age-appropriate skills in domains important in daily living, is low, particularly for individuals with XXYY. Importantly, adaptive functioning is lower than what would be expected for their IQ, suggesting factors other than cognitive limitations are likely interfering with daily living skills and creating significant disability. (Tartaglia, Cordeiro, Howell, Wilson, & Janusz, 2010; Bishop et al.,2011). Attention difficulties, executive function impairments, anxiety, depression and social difficulties including autism spectrum disorders are also present in some individuals with XXY and XXYY (Tartaglia et al., 2010; Visootsak & Graham, 2009).
Motor skills
A small number of studies have described motor deficits in XXY and XXYY (Bender et al. 1984; Ross et al. 2009; Salbenblatt, et. al. 1987). Slightly over half of infants with XXY identified by newborn screening in the 1960’s (n=17) exhibited hypotonia and delays in independent ambulation with a mean age of walking of14.3 months (range 10 to 17 months) (Robinson, Puck, Pennington, Borelli, & Hudson, 1979). A study using standardized motor assessments in a subset of males with XXY ages 6–19 identified by newborn screening was the first to describe deficits in fine motor coordination, speed, dexterity, motor planning, praxis, and overall gross motor skills in this group (Salbenblatt et al., 1987). More recently, Ross et al. (2008) found deficits in visual motor integration, strength, speed, and agility in 50 children with XXY compared to age-matched controls, with a mean motor composite > 1.5 standard deviations lower in XXY. Tartaglia et al. (2008) reported on early motor skills in children with XXYY. In this cohort, motor delays were present in 75%, with an average age of independent ambulation of 18 months.
Tremor has also been reported in these conditions and has significant implications for motor skills. The tremor associated with XXY has previously been described as upper extremity intention or essential tremor (Boisen & Rasmussen, 1978; Jakubowski et al., 1999). In the cohort of males with XXYY, 67% above age 10 (n=60) exhibited upper extremity tremor. Ten of these individuals completed further evaluation using a standardized tool to assess tremor, and the majority had tremor of mild severity that most often was classified as kinetic and/or postural (Tartaglia, Borodyanskaya, Hall 2009). The impact of tremor on motor skills and adaptive functioning has not been evaluated.
In summary, studies to date describe motor skill deficits in males with XXY and XXYY. No studies have used standardized motor assessments to compare the motor profiles between individuals with these genetic disorders, and it is still unclear if motor impairments contribute to limitations in functional and daily living skills. Addressing these gaps will increase awareness and allow more targeted OT and PT evaluation and treatment of motor deficits in children with XXY and XXYY syndromes. Therefore, the aims of this study were to describe the pattern of strengths and weaknesses in motor skills in males with XXY and XXYY syndromes, along with an assessment of the relationship between motor skills, cognitive functioning, and adaptive functioning.
METHODS
Design-
This was a two center, single-visit, cross-sectional study of males with XXY and XXYY syndromes. This study was approved by the local Institutional Review Board at both sites. All participants and/or a parent/legal guardian provided informed consent or assent for this study.
Participants-
A total of 100 participants (64 XXY, 46 XXYY) enrolled in the study. Inclusion criteria included male, ages 4 to 20 years with a karyotype of 47,XXY or 48,XXYY without mosaicism. Participants were recruited through national advocacy groups from 2007–2015 for a study on neurodevelopmental, motor and psychological features of XXY and XXYY. The age, Wechsler IQ scores, Vineland adaptive functioning scores, and VMI scores for the 64 males with XXY and 46 with XXYY are summarized in Table 1. There was no significant difference in age between groups, however, males with XXYY had significantly lower scores for all domains of cognition, adaptive functioning and VMI. Mean cognitive scores of males with XXY on the Wechsler scales were in the average range for verbal, performance, and full-scale IQ, however mean verbal IQ scores were lower than nonverbal IQ (92.7±17.4 vs 100.6±15.7, p<0.001). Verbal IQ scores of males with XXYY were lower than their nonverbal IQ scores (75.9±14.3 vs 87.1±12.9, p<0.001). On the Vineland adaptive functioning scale, with the exception of the Motor domain, all other domains of adaptive functioning were on average 9 points lower than full-scale IQ for both males with XXY and XXYY.
TABLE 1.
Comparison of Age, Cognitive Scores, and Beery VMI results in XXY and XXYY
| XXY N=64 | XXYY N=46 | T-test p-value | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 12.2 (3.48) | 13.3 (4.46) | 0.149 |
| Range | 4.8–21.5 | 4.4–20.2 | |
| IQ- (WASI or WISC-IV) | |||
| Verbal- Mean (SD) | 92.7 (17.37) | 75.9 (14.36) | <0.001* |
| Range | 53–119 | 53–102 | |
| Performance- Mean (SD) | 100.6 (15.74) | 87.1 (12.99) | <0.001* |
| Range | 61–129 | 63–108 | |
| Full Scale- Mean (SD) | 96.1 (17.74) | 79.9 (14.86) | <0.001* |
| Range | 46–125 | 47–121 | |
| Vineland-2 | |||
| Communication- Mean (SD) | 84.6 (17.80) | 63.8 (19.99) | <0.001* |
| Range | 36–117 | 25–112 | |
| Daily Living Skills- Mean (SD) | 89.7 (17.99) | 73.9 (14.12) | <0.001* |
| Range | 40–134 | 43–102 | |
| Socialization- Mean (SD) | 89.1 (18.70) | 75.2 (13.3) | <0.001* |
| Range | 47–135 | 46–97 | |
| Motor- Mean (SD) | 105.8 (15.35) | 96.7 (18.56) | 0.05 |
| Range | 67–121 | 70–121 | |
| Adaptive Beh. Composite- Mean (SD) | 86.9 (17.30) | 71.9 (11.51) | |
| Range | 43–135 | 46–98 | <0.001* |
| Beery Visual Motor Integration Means (SD) | |||
| Visual Motor Integration | 92.3 (14.35) | 81.5 (11.26) | <0.001* |
| Visual Perception supplementary test | 99.6 (15.14) | 88.2 (13.58) | <0.001* |
| Motor Coordination supplementary test | 87.5 (16.88) | 77.7 (15.45) | 0.003* |
p<0.05 for T-test comparing XXY to XXYY
Measures-
Beery Buktenica Test of Visual Motor Integration 5th Edition (VMI) evaluates the ability to coordinate visual perceptual and motor skills (Beery & Beery, 2006). The individual is asked to copy shapes and figures of increasing complexity. Two VMI supplementary tests help discriminate individual visual perceptual and motor components of visual-motor skills (Beery & Beery, 2006; Kulp & Sortor, 2003). The VMI motor coordination task requires the participant to keep their pencil within a path for different shapes with increasing complexity and precision, measuring the ability to plan motor movements without requiring visual perception skills. The visual perceptual supplementary test requires visual matching of figures of increasing complexity without a motor component. Mean standard score for each subtest is 100, with a standard deviation of 15. The “below average” range is defined as those more than one standard deviation below the mean. All subjects completed the VMI and complete data were available for all analyses.
Bruininks-Oseretsky Test of Motor Proficiency-2nd edition (BOT-2) is a standardized assessment measuring fine and gross motor skills in individuals 4–21 years of age (Bruininks & Bruininks, 2005). The BOT-2 is divided into domains including: fine manual control (fine motor precision, fine motor integration), manual coordination (manual dexterity, upper limb coordination), body coordination (bilateral coordination, balance) and strength and agility (running speed/agility, strength). A total motor composite is comprised from 4 subdomain scores. BOT-2 subtest scores are scaled scores with a mean of 15±5, while BOT-2 composite scores are standard scores with a mean of 50±10. Scaled scores were converted to standard scores. Sixty-six percent of participants completed the BOT-2.
The Vineland Adaptive Behavior Scales-2nd Edition includes adaptive functioning domains of communication, daily living, and socialization through a semi-structured interview with the primary caregiver. Scores are reported as standard scores (mean 100±15) (Sparrow, Cicchetti, & Balla, 2005).
The Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999) or Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV, Wechsler, 2003) was used to measure intellectual ability. Verbal (VIQ), performance (PIQ), and full-scale IQ (FSIQ) scores were determined with a mean of 100 and standard deviation of 15.
Procedures
A medical and developmental history was obtained from the parents and a physician conducted a physical examination. Medical records were reviewed, including postnatal genetic testing results to confirm diagnosis of XXY or XXYY. The BOT-2 evaluations were administered by a single certified, licensed occupational therapist. The VMI was administered by one of three individuals: an occupational therapist, a developmental pediatrician, or by a trained research assistant under the supervision of a developmental pediatrician. Interrater reliability in administration and scoring to 90% was established prior to research administration. Cognitive and adaptive measures were administered by a licensed clinical psychologist and a developmental pediatrician.
Data Analysis-
Data were assessed for normality to ensure normal distributions and expected variances. Outlying values (>3 SD from the group mean) were removed prior to analysis. In the case of missing data, analyses were performed with the available data without imputations. Descriptive statistics were used to describe the sample. Scores for males with XXY and XXYY were compared using two-sided t-tests to look for significant differences in age, intelligence quotient (IQ,), adaptive functioning scores, VMI scores and BOT-2 scores.
The percentages of scores classified as below-average for the VMI and BOT-2 were compared to the normative sample using proportion tests (Inc. S. SPSS Statistics 17.0 Command Syntax Reference manual, 2007; Newcombe, 1998) and then between groups using either the chi-squared tests or Fischer’s exact tests depending on the sample sizes. Cognitive and adaptive functioning scores were then compared among boys who had below-average VMI scores versus average/above-average using two-sided t-tests. The a priori significance level was set at 0.05. Alpha values were adjusted using Bonferroni correction for multiple comparisons within specific analyses. Statistical analyses were carried out using SPSS version 24.
RESULTS
Visual Motor Integration
Mean VMI score for the males with XXY was in the average range while the mean for males with XXYY was in the below-average range as shown in Table 1. Results of the visual perception supplementary test were in the average range for both groups, while results of the motor coordination supplementary test were average for males with XXY and below average for males with XXYY. Males with XXYY scored significantly lower than males with XXY for all VMI tests.
The percentage of males with XXY with scores in the below average range was similar to the normative sample for VMI scores (21% compared to 25% expected), as shown in Figure 1. However, on the supplementary tests, there was a significantly higher proportion of individuals with XXY with below-average scores on the motor coordination subtest (39% versus 25% expected), while only 15% fell in the below-average range for visual perception. A significantly higher percentage of individuals with XXYY had scores in the below-average range for all three VMI subtests. 73% of males with XXYY had motor coordination subtest scores that were below-average.
FIGURE 1.
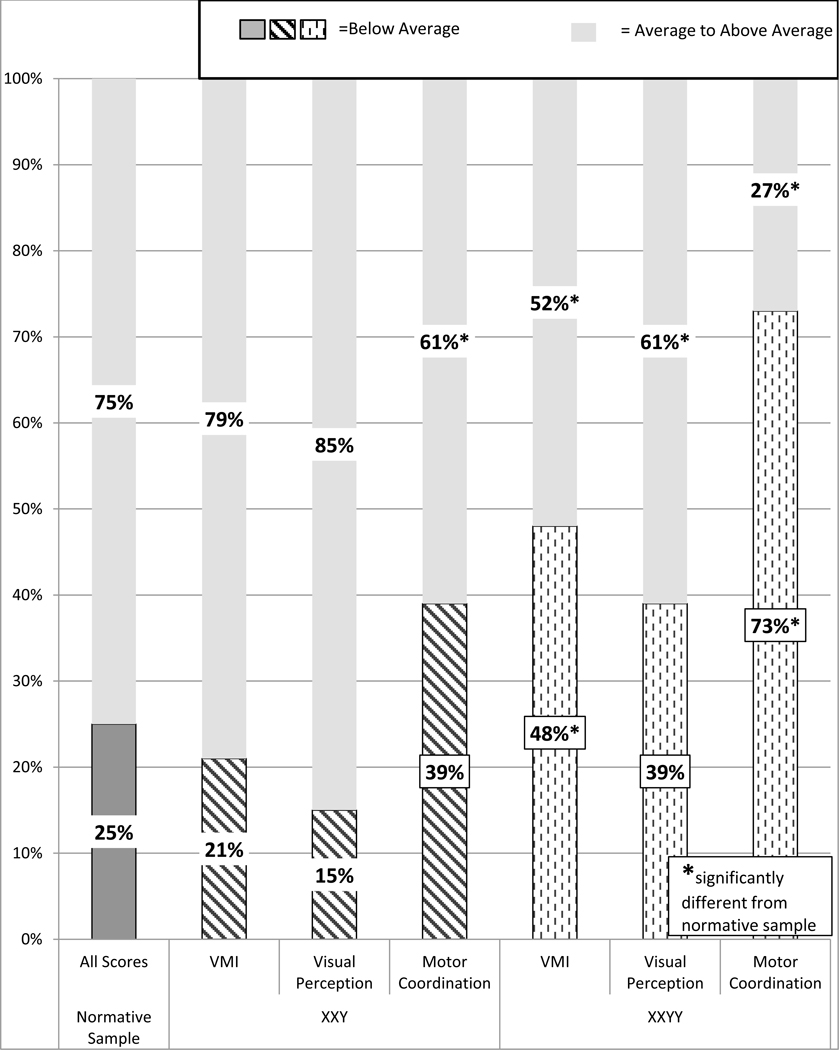
Results of Beery VMI and supplemental tests of visual perception and motor coordination in XXY and XXYY
Table 2 shows the comparisons of mean age, IQ, and adaptive functioning of males with XXY and XXYY grouped by performance on the VMI. In males with XXY, age was not significantly different between those with below-average VMI scores and average/above-average VMI scores. In males with XXYY, however, those with below-average VMI scores were older than those in the average group. The participants with XXY with average/above-average VMI scores, had higher IQ scores compared to those with below average VMI scores. However, for males with XXYY there was no difference in IQ scores between those with below-average versus average/above-average VMI scores. In the males with XXYY, the below-average VMI performers had lower mean adaptive scores for all domains. There was a significant positive correlation between VMI scores and overall Vineland-2 adaptive skills scores for both males with XXY (r=0.33) and males with XXYY (r=0.38) as shown in Figure 2. This correlation remained significant after controlling for age and FSIQ, p<0.05 for both groups.
TABLE 2.
Cognitive and adaptive functioning scores in boys with XXY and XXYY with below-average compared to average/above-average VMI scores
| VMI Below-Average (score <85) | VMI Average or Above (score 85+) | T-Test p-value | |
|---|---|---|---|
| XXY | |||
| N | 13 | 49 | |
| Age | 12.5 (2.45) | 11.99 (3.71) | 0.645 |
| IQ Mean (SD) | |||
| Verbal | 78.92 (22.2) | 95.98 (14.4) | 0.019* |
| Performance | 86.62 (15.0) | 104.70 (14.1) | <0.001** |
| Full Scale | 80.62 (20.9) | 100.44 (14.9) | <0.001** |
| Adaptive Functioning Mean (SD) | N=10 | N=45 | |
| Communication | 72.7 (19.6) | 87.7 (16.7) | 0.016* |
| Daily Living | 80.5 (22.8) | 92.1 (16.6) | 0.068 |
| Social | 82.7 (20.5) | 91.2 (18.3) | 0.201 |
| Adaptive Composite | 78.4 (19.7) | 89.2 (16.5) | 0.075 |
| XXYY | |||
| N | 22 | 24 | |
| Age | 15.0 (4.0) | 11.7(4.3) | 0.010* |
| IQ Mean (SD) | |||
| Verbal | 74.2 (14.0) | 77.5 (14.8) | 0.434 |
| Performance | 84.7 (13.9) | 89.2 (12.0) | 0.247 |
| Full Scale | 76.4 (13.2) | 83.2 (15.8) | 0.122 |
| Adaptive Functioning Mean (SD) | N=20 | N=22 | |
| Communication | 54.7 (16.8) | 72.3 (19.3) | 0.003** |
| Daily Living | 68.9 (10.5) | 78.6 (15.5) | 0.021* |
| Social | 70.8 (11.8) | 79.1 (13.5) | 0.040* |
| Adaptive Composite | 67.7 (7.27) | 75.6 (13.3) | 0.024* |
p<0.05 for T-test comparing groups with low versus average VMI scores.
p<0.007 for t-tests comparing between low versus average VMI scores (Bonferroni correction for multiple comparisons)
FIGURE 2.
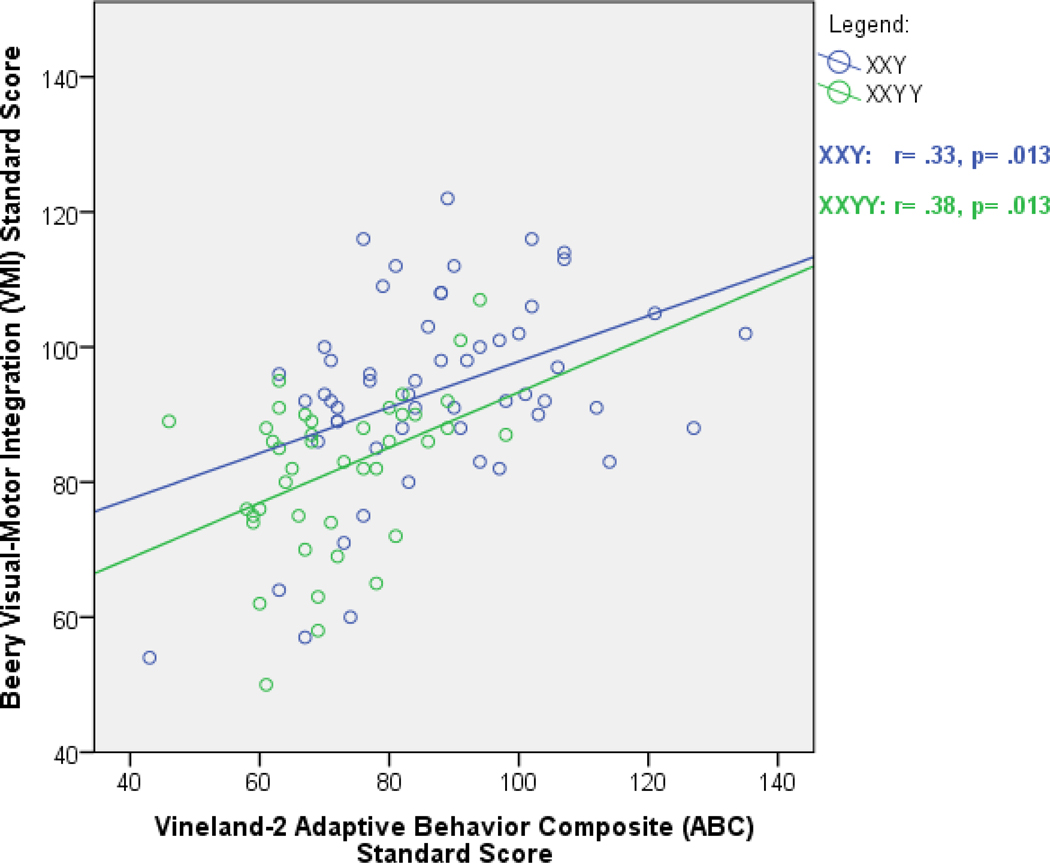
Visual Motor Integration Correlates with Adaptive Functioning in XXY and XXYY
Bruininks-Oseretsky Test of Motor Proficiency (BOT-2)
Table 3 shows BOT-2 results for each group, including mean scores in each domain as well as the proportion of the group falling in the below average range. In males with XXY, impairments were identified in fine manual control, manual coordination, manual dexterity, body coordination, and bilateral coordination compared to the normative sample. Males with XXYY showed global motor difficulties with 50% or more of participants in the below-average range across all motor domains (with the exception of bilateral coordination). Males with XXYY had lower mean scores in all BOT-2 domains compared to males with XXY, however after correcting for multiple comparisons, only the domains of manual coordination, body coordination, and strength/agility remained statistically significant.
TABLE 3.
Comparison of Motor Skills on the BOT-2 in XXY and XXYY
| BOT-2 | XXY | XXYY | T-test and Fisher’s Exact Test p-value |
|---|---|---|---|
| N | 46 | 20 | |
| Fine Manual Control Mean (SD) | 48.5 (12.6) | 41.3 (11.7) | 0.032* |
| % Below Average | 31.1%^ | 60.0%^ | 0.053 |
| Fine Motor Precision Mean (SD) | 49.1 (10.3) | 42.3 (11.0) | 0.019* |
| % Below Average | 26.1% | 60.0%^ | 0.013* |
| Fine Motor Integration Mean (SD) | 50.8 (9.7) | 46.2 (10.9) | 0.094 |
| % Below Average | 15.2% | 50.0%^ | 0.005* |
| Manual Coordination Mean (SD) | 45.00 (9.37) | 34.2 (13.8) | <0.001** |
| % Below Average | 29.5%^ | 70.0%^ | 0.006* |
| Manual Dexterity Mean (SD) | 42.4 (8.7) | 37.3 (8.6) | 0.040* |
| % Below Average | 54.3%^ | 65.0%^ | 0.589 |
| Upper Limb Coordination Mean (SD) | 50.3 (9.4) | 43.8 (9.9) | 0.013* |
| % Below Average | 15.9% | 55.0%^ | 0.002** |
| Body coordination Mean (SD) | 46.4 (9.7) | 34.9 (13.6) | <0.001** |
| % Below Average | 32.6%^ | 77.8%^ | 0.002** |
| Bilateral Coordination Mean (SD) | 46.5 (9.4) | 40.1 (8.4) | 0.013* |
| % Below Average | 32.6%^ | 47.7%^ | 0.393 |
| Balance Mean (SD) | 49.1 (10.5) | 41.3 (11.8) | 0.016* |
| % Below Average | 20.9% | 61.1%^ | 0.006* |
| Strength & Agility Mean (SD) | 46.0 (9.2) | 38.6 (5.8) | 0.003** |
| % Below Average | 19.0% | 64.7%^ | 0.002** |
| Running Speed & Agility Mean (SD) | 48.3 (8.8) | 38.5 (5.9) | <0.001** |
| % Below Average | 26.2% | 52.9%^ | 0.069 |
| Strength Mean (SD) | 46.8 (8.1) | 39.8 (7.0) | 0.002** |
| % Below Average | 23.8% | 50%^ | 0.069 |
| Total Motor Composite Mean (SD) | 45.7 (9.5) | 38.11 (7.63) | 0.005* |
| % Below Average | 26.2% | 64.7%^ | 0.008* |
p<0.05 comparing XXY or XXYY to the normative sample (where 25% are classified in the “below average” range)
p<0.05 for T-tests and Fisher’s exact tests comparing XXY to XXYY
p<0.0038 for T-tests and Fisher’s exact tests comparing XXY to XXYY (Bonferroni correction for multiple comparisons)
DISCUSSION
This study is the first to evaluate and compare motor skills in males with XXY and XXYY using standardized measures. Results confirm an increased risk for motor impairment in both populations with greater severity among males with XXYY. The domains of weakness for males with both XXY and XXYY were in the VMI motor coordination subtest and the BOT-2 manual dexterity domain, including tasks of fine motor planning, manipulation skills, praxis, bilateral integration and fine motor speed. We further found significant, positive correlations between motor skills and adaptive functioning skills, as well as a positive association with motor skills and cognitive ability in XXY. The results provide evidence of motor strengths and weaknesses in males with XXY and XXYY that have implications for clinical practice.
In comparison to other published studies of visual-motor skills in XXY, Ross et al. (2008) evaluated VMI in 41 boys with XXY and reported a mean VMI score of 87, similar to the mean score of 92 in this study. Although this summary statistic is within the average range, it is important to note that in our sample, 39% of males with XXY and 73% of males with XXYY had below average VMI scores. Our study further evaluates contributors to VMI skills with the supplementary tests of visual perception and motor coordination. For both males with XXY and XXYY, the VMI pattern of strengths in visual perceptual skills and weaknesses in motor coordination suggest that motor coordination deficits are the predominant contributor to VMI deficits. This finding highlights the need for targeted interventions to address motor coordination, as well as encouragement of strengths in visual perception.
Salbenblatt et al. (1987) and Ross et al. (2008) both utilized the original edition of the BOTMP assessment in their studies in males with XXY and found the subdomains of upper limb speed/dexterity and running speed/agility to be areas of weakness. Our study utilized the updated BOT-2 that includes additional manual dexterity items (transferring pennies, pegboard, sorting cards, and a timed writing task), which was found to be an area of weakness in both males with XXY and XXYY. In contrast to previous studies, our XXY sample did not show significant impairments in running speed/agility but did have more frequent impairments in bilateral coordination with specific difficulties in praxis, sequencing, execution and timing of motor tasks.
Dyspraxia has been previously described as a feature of XXY and XXYY syndromes (Salbenblatt et al., 1987, Samango-Sprouse & Rogol, 2002, Tartaglia et al., 2008). Ayres (1972) defined dyspraxia as “a motor planning disorder,” and as “a disorder of sensory integration interfering with ability to plan and execute skilled or non-habitual motor tasks”. In our study, dyspraxia was observed on the BOT-2 testing as difficulties in the ability to initiate, sequence and execute gross and fine motor tasks (such as performing jumping jacks or other complex motor actions).
Poor fine motor coordination, dexterity, bilateral coordination, and praxis can affect the ability to perform adaptive skills important in home and academic settings, such as dressing, buttoning/fastening, brushing teeth, tying shoes, handwriting, scissors, and keyboarding. This is consistent with our results showing that lower VMI skills are correlated with lower daily living and overall adaptive skills in males with XXY and XXYY. These difficulties may subsequently affect other areas such as the ability to participate in play and sports activities, which can have important psychosocial and health implications. Although we did not explore attention, executive function or learning disabilities, these are common deficits in XXY and XXYY syndromes and may compound with the motor difficulties to affect organization and performance of adaptive skills. Given the correlation between motor skills and adaptive functioning, interventions targeting motor skills (especially fine motor coordination and dexterity), even when only mild deficits exist, may improve adaptive functioning in these populations.
Relative strengths were in nonverbal cognitive skills and VMI visual-perceptual skills, consistent with previous studies. Males with XXY and XXYY each had the highest mean BOT-2 scores in fine motor integration and upper limb coordination subtests. These areas of strength are important to note for planning successful intervention approaches and supports. It is also important to point out the large spectrum of involvement in both groups - while we focus on areas of increased risk to guide evaluation and intervention, there are many males without deficits, and Figure 1 is notable for the proportion who fall within the average range, especially for the XXY group. The reasons for the broad phenotypic variability are not fully understood, however it is felt that genetic factors, family history, environmental factors, and interventions all play a role (Boada et al., 2009; Samango-Sprouse et al., 2014; Tartaglia et al., 2010; Zitzmann, Depenbusch, Gromoll, & Neischlag, 2004).
Whether observed motor deficits in males with XXY and XXYY are secondary to the gene-dosage effects of the extra chromosomes or to testosterone deficiency is an on-going question in this field. Although this study did not assess testosterone concentration or treatment, testosterone deficiency is assumed to be similar in XXY and XXYY syndromes.
Therefore, given the greater severity of motor deficits observed in boys with XXYY compared to XXY, we would propose the gene-dosage effect is a greater contributor to the observed motor phenotype than testosterone deficiency.
Implications for Practice
Implications for practice are summarized in Table 4. Occupational and Physical therapists can utilize areas of strengths with this population when planning treatment interventions. When developing a treatment plan for children with XXY or XXYY, therapists should consider individualized therapeutic approaches that include strengths of nonverbal and visual spatial skills and weaknesses with manual dexterity, praxis and bilateral integration skills. Difficulties with daily occupations of self-care are impacted by learning new motor and adaptive skills which can be exacerbated by neuropsychological weaknesses associated with XXY or XXYY such as learning disabilities, attention deficits, slow processing speed, language disorders, executive dysfunction or anxiety/emotional symptoms. These must also be evaluated and considered when developing an individualized treatment plan.
TABLE 4.
Implications for Occupational and Physical Therapists
| Recommend medical genetic evaluation if they recognize a phenotypic pattern consistent with XXY or XXYY syndromes: |
| ▪ Features such as tall stature, clinodactyly (curved 5th finger), hypotonia, flat feet, joint hyperextensibility, radioulnar synostosis, and/or testosterone deficiency in a child or adolescent with minimal or no facial dysmorphic features. |
| ▪ In early development, features such as hypotonia or delayed motor milestones. |
| ▪ Intention tremor in a child or adolescent. |
| ▪ Motor coordination and manual dexterity deficits, or global motor deficits, with relative strengths in visual perceptual skills. |
| ▪ The presence of comorbid conditions such as learning disabilities, language delays, sensory sensitivities, anxiety, or autism symptoms. |
| In individuals with an established diagnosis of XXY or XXYY, consider features of the syndrome and the profile of strengths and weaknesses when setting therapy goals and planning interventions: |
| ▪ Utilize areas of relative strengths with this population when planning intervention strategies, including strengths in visual perceptual and visual memory skills. |
| ▪ Individuals with XXY and XXYY with lower visual motor integration abilities have lower adaptive functioning and daily living skills, therefore therapies targeting motor skills may lead to improvement in adaptive outcomes. |
| ▪ When developing a treatment plan for XXY or XXYY, consider therapy approaches to address impairments in manual dexterity, motor praxis, and bilateral integration |
| ▪ Offer parent education and materials for implementation of home-based activities using visual strategies that capitalize on the strengths in nonverbal, visual-spatial skills |
| ▪ Testosterone deficiency often develops during adolescence in XXY and XXYY, and some deficits in motor strength and stamina may respond to testosterone therapy by an endocrinologist. |
| ▪ Difficulties with completing self-care demands and learning new motor or adaptive skills may be exacerbated by neuropsychological weaknesses associated with XXY or XXYY such as learning disabilities, attention deficits, slow processing speed, language disorders, executive dysfunction or anxiety/emotional symptoms. These must also be evaluated and considered when developing an intervention program. |
| ▪ We recommend using the International Classification of Functioning, Disability, and Health (ICF) model (WHO 2001) along with the Occupational Therapy Practice Framework (OTPF), (American Occupational Therapy Association, 2014) and American Physical Therapy Association’s guide to Physical Therapist Practice (2001) to guide evaluation and intervention goals. |
| ▪ Provide support and direction to families to promote healthy lifestyles and successful motor activities during recreation and leisure time. Children and adolescents with XXY and XXYY often do well in and enjoy individual sports such as swimming, cycling, martial arts, dance, golf, or skiing. Other activities based on interest and skill should not be excluded. |
| ▪ Address difficulties with self-regulation, coping skills, sensory sensitivities, and provide other behavioral strategies to address anxiety and adaptive skills. |
| ▪ Provide consultation to families and school teams that includes specific examples of how the impairments associated with XXY or XXYY present in academic, home, and community settings. |
Limitations and Recommendations for Further Research
Although our sample is adequate for describing a general phenotypic profile, this study could be improved by larger sample sizes followed prospectively, and comparison with an XY control group. A more comprehensive assessment of self-care and daily living skills in future studies would allow further analysis of specific domains of daily living skills to guide intervention strategies. Assessments using specific instruments designed to measure praxis are also an important future goal.
CONCLUSIONS
The results of this study further characterize the motor phenotype of males with XXY and XXYY syndromes, providing practitioners evidence of performance on standardized measures of visual motor and motor proficiency to guide clinical decision making. The most pertinent findings of this study include similar patterns of motor strengths and weaknesses in males with XXY and XXYY but greater severity in XXYY, as well as the important relationship of visual-motor abilities with higher adaptive skills. Occupational therapists and physical therapists may be the first providers to evaluate children with XXY or XXYY, and awareness of the features of these syndromes may help identify children who are undiagnosed and guide intervention planning.
REFERENCES
- Abramsky L, & Chapple J. (1997). 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenatal Diagnosis, 17(4), 363–368. [DOI] [PubMed] [Google Scholar]
- American Physical Therapy Association,. Guide to Physical Therapist Practice, 3rd ed., Physical Therapy (2001). 81:9–746. [PubMed] [Google Scholar]
- Ayres AJ (1972). Sensory integration and learning disorders. Los Angeles: Western Psychological Services. [Google Scholar]
- Beery KE, & Beery N. (2006). The Beery-Buktenica Developmental Test of Visual-motor Integration (5th ed.). [Google Scholar]
- Bender BG, Puck MH, Salbenblatt JA, & Robinson A. (1986). Dyslexia in 47,XXY boys identified at birth. Behavioral Genetics, 16(3), 343–354. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Body PA, et al. , Scerif G. (2011). Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood, 96(10), 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, & Tartaglia N. (2009). The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews, 15(4), 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen E, & Rasmussen L. (1978). Tremor in XYY and XXY men. Acta Neurologica Scandinavia, 58(1), 66–73. [DOI] [PubMed] [Google Scholar]
- Bruininks R, & Bruininks B. (2005). Bruininks–Oseretsky Test of Motor Proficiency (2nd ed.). Minneapolis, MN: Pearson Assessment. [Google Scholar]
- Davis S, Howell S, Wilson R, Tanda T, Ross J Zeitler P, Tartaglia N. (2016). Advances in the interdisplinary care of children with Klinefelter syndrome. Advances in Pediatrics, 63(1), 15–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryns JP, Kleczkowska A, Kubien E, Van den Berghe H. XXY syndrome and other Y chromosome polysomies. Mental status and psychological functioning. Genet couns. 1995; (6):197–206. [PubMed] [Google Scholar]
- Garvey M, Kellett B. Case studies of three “XXYY” children. Br J Discord Commun. 1975; (10):17–30. [DOI] [PubMed] [Google Scholar]
- Garvey M, Mutton DE. Sex chromosome aberrations and speech development. Arch Dis Child. 1973; 48:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inc. S. SPSS Statistics 17.0 Command Syntax Reference manual. (2007). Chicago, IL. [Google Scholar]
- Jakubowski L, Sabatowska M, Filipiak-Miastkowska I, Nadratowski P, Rutkowska A, Nowakowska D, & Kaluzewski B. (1999). Neurological aspects of two patients with non-mosaic and mosaic polysomy of the X and Y chromosomes. Neurologia i Neurochirurgia Polska, 33(1), 169–175. [PubMed] [Google Scholar]
- Kulp MT, & Sortor JM (2003). Clinical value of the Beery visual-motor integration supplemental tests of visual perception and motor coordination. Optometry Vision Science, 80(4), 312–315. [DOI] [PubMed] [Google Scholar]
- Muldal S, & Ockey CH (1962). Double male:New chromosome constitution in Klinefelter’s syndrome. Acta Endocrinologica, 39(2), 183–203. [DOI] [PubMed] [Google Scholar]
- Newcombe RG (1998). Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistical Medicine, 17(8), 857–872. [DOI] [PubMed] [Google Scholar]
- Robinson A, Puck M, Pennington B, Borelli J, & Hudson M. (1979). Abnormalities of the sex chromosomes: a prospective study on randomly identified newborns. Birth Defects Original Article Series, 15(1), 203–241. [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MP, Kushner H, . . . Zinn AR (2008). Cognitive and motor development during childhood in boys with Klinefelter syndrome. American Journal of Medical Genetics Part A, 146A(6), 708–719. [DOI] [PubMed] [Google Scholar]
- Ross JL, Zeger MPD, Kushner H, Zinn AR, Roeltgen DP (2009). An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 15(4): 309–317. [doi: 10.1002/ddrr.85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbenblatt J, Meyers DC, Bender B, Linden MG, & Robinson A. (1987). Gross and fine motor development in 47,XXY and 47,XYY males. Pediatrics, 80(240–244). [PubMed] [Google Scholar]
- Samango-Sprouse C and Rogol A. (2002). XXY The Hidden Disability and a Prototype for an Infantile Presentation of Developmental Dyspraxia. Infants and Young Children 15(1), 11–18. [Google Scholar]
- Samango-Sprouse CA, Stapleton EJ, Mitchell FL, Sadeghin T, Donahue TP, & Gropman AL (2014). Expanding the phenotypic profile of boys with 47, XXY: the impact of familial learning disabilities. American Journal of Medical Genetics Part A, 164A(6), 1464–1469. [DOI] [PubMed] [Google Scholar]
- Sorensen K, Nielsen J, Jacobsen P, & Rolle T. (1978). The 48,XXYY syndrome. Journal of Mental Deficiency Research, 22(3), 197–205. [PubMed] [Google Scholar]
- Sparrow S, Cicchetti D, & Balla D. (2005). Vineland Adaptive Behavior Scales - 2nd Edition. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Tartaglia N, Cordeiro L, Howell S, Wilson R, & Janusz J. (2010). The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome). Pediatric Endocrinology Reviews, 8 Supplement 1, 151–159. [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Davis S, Hench A, Nimishakavi S, Beauregard R, Reynolds A, Fenton L Albrecht L, Ross J, Visootsak J, Hansen R, Hagerman RJ (2008). A New Look at XXYY Syndrome: Medical and Psychological Features. American Journal of Medical Genetics Part A, 146A(12), 1509–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Borodyanskya M, Hall D. (2009). Tremor in 48,XXYY syndrome. Mov Disord, 24(13), 2001–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, et al. , (2011). “48 XXXY and 49,XXXXY syndromes; not just variants of Klinefelter syndrome. ActaPaediatr 100(6); 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Wilson R, Miller J, Rafalko J, Cordeiro L, Davis S, Hessl D, Ross J, (2017). Autism Spectrum Disorder in Males with Sex Chromosome Aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. Journal of Developmental Behavioral Pediatrics, 38(3), 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Howell S, Wilson R, Boada R, Janusz J, Martin S, Frazier J, Pfeiffer M, Regan K, McSwegin S, Zeitler P. (2015). The eXtraordinarY Kids Clinic: An Interdisciplinary Model of Care for Children and Adolescents with Sex Chromosome Aneuploidy. Journal of Multidiscipinary Healthcare, 8, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, & Graham JM Jr. (2009). Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Developmental Disabilities Research Reviews, 15(4), 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1999). Weschler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation. [Google Scholar]
- Wechsler D. (2003). The Wechsler Intelligence Test for Children Fourth Edition. San Antonio: The Psychological Corporation. [Google Scholar]
- Zitzmann M, Depenbusch M, Gromoll J , & Neischlag E. (2004). X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. Journal of Clinical Endocrinology & Metabolism, 89(12), 6208–6217. [DOI] [PubMed] [Google Scholar]


