Abstract
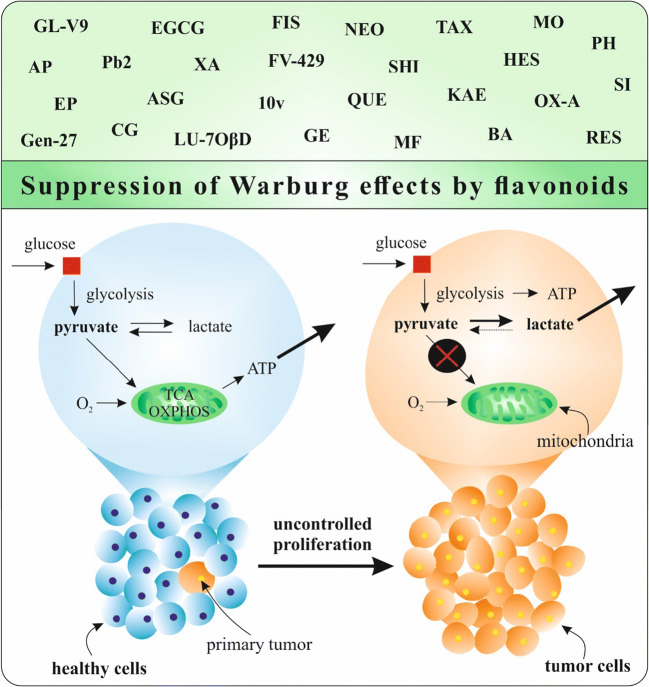
The Warburg effect is characterised by increased glucose uptake and lactate secretion in cancer cells resulting from metabolic transformation in tumour tissue. The corresponding molecular pathways switch from oxidative phosphorylation to aerobic glycolysis, due to changes in glucose degradation mechanisms known as the ‘Warburg reprogramming’ of cancer cells. Key glycolytic enzymes, glucose transporters and transcription factors involved in the Warburg transformation are frequently dysregulated during carcinogenesis considered as promising diagnostic and prognostic markers as well as treatment targets. Flavonoids are molecules with pleiotropic activities. The metabolism-regulating anticancer effects of flavonoids are broadly demonstrated in preclinical studies. Flavonoids modulate key pathways involved in the Warburg phenotype including but not limited to PKM2, HK2, GLUT1 and HIF-1. The corresponding molecular mechanisms and clinical relevance of ‘anti-Warburg’ effects of flavonoids are discussed in this review article. The most prominent examples are provided for the potential application of targeted ‘anti-Warburg’ measures in cancer management. Individualised profiling and patient stratification are presented as powerful tools for implementing targeted ‘anti-Warburg’ measures in the context of predictive, preventive and personalised medicine.
Keywords: Predictive preventive personalised medicine (PPPM / 3PM), Cancer, Warburg phenotype, Flavonoids, Anticancer effect, Cell metabolism, Co-morbidities, Malignancy, Disease manifestation, Age, Patient stratification, Aggressive metastatic disease, Multi-omics, Biomarker patterns, Liquid biopsy, Modifiable risk factors, Risk assessment, Microcirculation, Systemic hypoxia, Ischemic lesions, Prognosis, Individualised patient profiles, Treatment algorithms, Liver malignancy, Triple-negative breast cancer, Prostate cancer, Pregnancy, Chemoresistance, Radioresistance, Glucose metabolism, Oxidative phosphorylation, Proliferation, Metabolic reprogramming, Positron emission tomography, Magnetic resonance spectroscopy, Tumour imaging, FDG-PET, Glucose intake, PET-CT, Individual outcome, Palliative medicine, Polyphenols, Glycolysis, Carcinogenesis, Prognostic markers, Aerobic glycolysis, Glycolytic inhibitors, Pleiotropic activity, HIF-1
Introduction
Despite significant progress in the therapy of oncological diseases in the twenty-first century, cancer remains a leading cause of death globally [1]. The association of neoplastic transformation with alterations at the metabolic level has been studied for almost a century. Malignant cells show profound alterations in their metabolic pathways changing the intra- and extracellular biochemistry that is associated with increased proliferation and angiogenesis [2]. Metabolic reprogramming leads to the switch from oxidative phosphorylation to aerobic glycolysis that represents a well-recognised signature of cancer energy metabolism associated with diminished drug-mediated apoptosis or chemo/radio-resistance of tumour cells [3, 4]. Like an orchestra, the enzymes that contribute to glycolysis represent individual players closely cooperating in the glucose degradation cascade. Specific differences in their enzymatic activities or expression rates act as prognostic biomarkers in cancer. The multi-omics (epigenomics, proteomics, transcriptomics, metabolomics) approach introduces novel challenges to the detection of individual signatures directly connected to cancer development as a consequence of metabolic disequilibrium of tumour cells [5, 6]. Acquired data from the multi-omic technologies is applicable in the novel clinical trend focused on predictive, preventive and personalised (3P) medicine strategies considered as the medicine of the future [5, 7]. During the last decade, increasing interest is recorded in targeting of essential steps of aerobic glycolysis as a promising approach to anticancer research [8]. To this end, several enzymatic inhibitors aimed at essential components of glucose metabolism have been tested in clinical trials. However, their application in routine clinical practice remains is not yet established [4, 9]. Increased aerobic glycolysis promotes the growth of various cancers; therefore, it is necessary to develop new therapeutic approaches to prevent metabolic reprogramming in cells [10]. Since ancient times, humans have been looking for therapeutic drugs against their diseases in nature [11]. Long-lasting investigations of medicinal plants led to the discoveries of numerous bioactive compounds that are now defined as phytochemicals [12]. Naturally occurring phytochemicals are promising agents against the initiation, promotion and progression of cancer with multiple mechanisms of action [13, 14]. Flavonoids, which represent a broad class of bioactive compounds in plants, are found in various functional foods. Recent evidence suggests beneficial functions of flavonoids in human health. The regular consumption of flavonoids is associated with the prevention of numerous chronic diseases including cancer [15]. Flavonoids inhibit carcinogenesis through multiple pathways [16, 17]. Amongst these, the modulation of cellular energy metabolism represents an extraordinarily interesting topic in the field of anticancer research. As mentioned above, there is a need to identify novel therapeutic approaches, and flavonoids could act as promising metabolism-regulating agents in suppressing malignant transformations of cells.
Aim of the study
This paper focuses on the anticancer potential of flavonoids—specifically, those related to the suppression of the Warburg effect in cancer cells. Firstly, this review discusses fundamental aspects of the Warburg effect, glycolytic enzymes and molecular pathways directly regulating cancer energy metabolism. The core of our article summarises experimental studies evaluating the anticancer effects of flavonoids via modulation of essential steps and compartments of glycolysis. The beneficial role of flavonoids in cancer metabolism is well-documented in preclinical research; in this review, we emphasise the need for targeted clinical cancer research on the effects of flavonoids in cellular metabolic reprograming.
Data sources
Data were obtained from the English-language biomedicine literature by the use of ‘flavonoids’ or ‘polyphenols’ or ‘cancer’ or ‘Warburg effect’ and ‘glycolysis’ as either a keyword or medical subject heading (MeSH) term in searches of the PubMed database (years 2015–2020).
Fundamental aspects of cancer metabolism
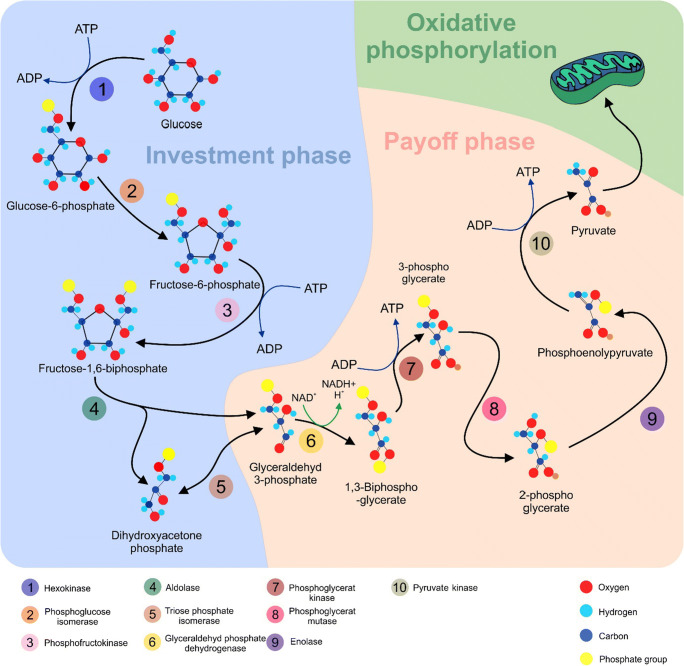
The cross-connection between cancer and altered metabolism is well characterised in cancer research. Almost 100 years ago, Otto Heinrich Warburg and colleagues observed that molecules of glucose undergo predominantly aerobic glycolysis in tumours, even under normoxic conditions, to support rapid cell division. In contrast, normal cells primarily utilise oxidative phosphorylation to generate adenosine triphosphate (ATP) in the presence of oxygen. The observation mentioned above became known as ‘the Warburg effect’ [18]. Glucose metabolism represents an enzymatic cascade consisting of two phases: the investment phase and the payoff phase, which involve several enzymes (Fig. 1).
Fig. 1.
Metabolism of glucose. The investment phase begins with the first enzyme, hexokinase (HK), which adds a phosphate group to glucose leading to the generation of glucose-6-phosphate (G6P). In the next step, phosphoglucose isomerase (PGI) modifies G6P into fructose-6-phosphate (F6P). The enzyme phosphofructokinase (PFK1) transforms F6P into fructose-1,6-bisphosphate (FBP). FBP is further lysed into dihydroxyacetone (DHAP) and glyceraldehyde 3-phosphate (G3P) by fructose-bisphosphate aldolase (aldolase). The enzyme triosephosphate isomerase (TPI) converts DHAP into G3P. The payoff phase begins with the metabolisation of G3P into 1,3-bisphosphoglycerate (1,3BGP) by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Next, phosphoglycerate kinase (PGK) converts 1,3BGP into 3-phosphoglycerate (3PG). Phosphoglycerate mutase (PGM) generates 2-phosphoglycerate (2PG) from the 3PG, and 2PG then converts enolase into phosphoenolpyruvate (PEP). Pyruvate kinase (PK) catalyses the final step, in which PEP is transformed into pyruvate [19, 20]. Subsequently, pyruvate undergoes oxidative phosphorylation in mitochondria resulting in the generation of 36 molecules of ATP from the tricarboxylic acid cycle (TCA). ATP, adenosine triphosphate; ADP, adenosine diphosphate; NAD+, nicotinamide adenine dinucleotide (oxidised form); NADH, nicotinamide adenine dinucleotide (reduced form)
The above pathway illustrates the metabolism of glucose under aerobic conditions in normal cells (Fig. 1). Conversely, in cancer cells, pyruvate is transformed into lactate by lactate dehydrogenase A (LDHA) and the yield of ATP is far lower, as only two ATP molecules are released per molecule of glucose through glycolysis [21, 22]. Although glycolysis is less efficient than oxidative phosphorylation in generating ATP, it produces energy more rapidly per unit of glucose [23]. An initial hypothesis predicted mitochondrial defects in cancer cells (i.e. lack of oxidative phosphorylation) and, thus, postulated aerobic glycolysis as the necessary way to produce ATP [24]. However, the rate of glucose uptake in cancer cells is dramatically elevated and lactate is created even in the presence of functional mitochondria [25]. Moreover, defective mitochondria are rather rare, and tumour cells maintain the ability of oxidative phosphorylation [26]. Proliferating cells are constantly supplied with glucose and various nutrients. Additionally, these cells require high ATP/ADP, NAD+/NADH and NADP+/NADPH ratios [25, 27]. Any imbalance between these molecules leads to the suppression of proliferation. Therefore, oxidative phosphorylation is not suitable for the maintenance of the above-mentioned ratios; however, for biosynthetic purposes, it produces ATP, while NAD+/NADH and NADP+/NADPH are under-dimensioned [28]. The preference of aerobic glycolysis to oxidative phosphorylation in cancer cells is linked to external conditions such as low extracellular pH and hypoxia [29]. Moreover, the abnormalities in signalling pathways as consequences of certain genetic aberrations promote cancer-associated glycolysis [30]. Thus, the determination of the cancer metabolism phenotype characterised by increased glycolytic activity is controlled by intrinsic alterations as well as external responses of the cell to the tumour microenvironment [31].
Molecular view into the Warburg effect
Recent advances in the fields of genetics and molecular biology can shed light on the principles and mechanisms of aerobic glycolysis in tumours. Disequilibrium in the PI3K signalling pathway is frequently detected in various cancers [32–35]. These aberrations of PI3K support the biological processes such as proliferation and invasion via modification of glucose metabolism. Evidence suggests that mutation in PIK3CA promotes glycolysis in cervical cancer cells [36]. Akt1, the downstream effector of PI3K, is a major player in cancer. Akt1 stimulates various intracellular events associated with elevated levels of glycolysis including translocation, stability and overexpression of glucose transporters (GLUT1, 2 and 4) [37–39], or negatively via suppression of forkhead box subfamily O1 (FOXO1) acting as a repressor of glycolysis. Akt1 activates mTOR which plays a crucial role in protein synthesis and cell proliferation. Moreover, mTOR is essential for glucose uptake, lipid biosynthesis and glycolysis [40]. In addition, mTOR induces hypoxia-inducible factor-1 (HIF-1) activity, even under normoxic conditions resulting in aerobic glycolysis [41]. HIF-1 can also be activated by the loss of von Hippel–Lindau (pVHL) regulation either as a direct or indirect consequence of mutations of succinate dehydrogenase (SDH) or fumarate hydratase (FH) [42]. The transcription factor c-Myc is an oncogene modulating various intracellular processes, including proliferation and growth. In addition, it has been suggested that c-Myc and HIF-1 cooperate in the regulation of glycolysis [43]. Oncoprotein c-Myc is associated with increased expression of GLUT genes [44] or enzymes such as LDHA [45] and PDK1 [46]. In contrast to Akt1, which is connected to enhanced proliferation and aerobic glycolysis via activation of mTOR, AMP-activated kinase (AMPK) suppresses mTOR signalling [47] that depends on the AMP/ATP ratio in the cell. When the AMP/ATP ratio is low, AMPK phosphorylates enzymes responsible for the generation of ATP via oxidative phosphorylation [48]. Activation of AMPK is mediated via liver kinase B1 (LBK1) which supports the maintenance of an intracellular energy balance and negatively regulates the Warburg effect. Dysfunction of the LBK1/AMPK pathway is defined as a hallmark of cancer progression [49].
P53 is a well-known tumour suppressor with a crucial role in stress stimuli responses and in the initiation of cell cycle arrest, senescence or apoptosis [9, 36]. Importantly, p53 negatively regulates aerobic glycolysis by promoting TP53-induced glycolysis and apoptosis regulator (TIGAR) affecting glycolysis enzymes and synthesis of cytochrome oxidase 2 (SCO2) which participates in mitochondrial metabolism under normoxia [50, 51]. Recent evidence suggests that the transcription factor sine oculis homeobox 1 (SIX 1) contributes to the control of many glycolytic enzymes associated with tumour metabolism [52]. The transcription factor OCT1 is frequently upregulated in cancer. Recent experimental studies have demonstrated its role in the silencing of oxidative phosphorylation and switching to aerobic glycolysis [53]. Additionally, an oncogenic transcription factor, Yin Yang 1 (YY1), promotes aerobic glycolysis via upregulation of GLUT3 [54].
Pyruvate kinase plays an essential role in the transformation of normal glucose metabolism into the aerobic glycolytic phenotype responsible for transforming PEP into pyruvate and production of ATP. In mammals, four isoforms of pyruvate kinase, namely PKL, PKR, PKM1 and PKM2, exist [55]. The PKM1 isoform has greater enzymatic efficiency than the PKM2 form. Paradoxically, the less efficient M2 form is preferred in tumours due to its promotion of c-Myc [56]. Oncoprotein c-Myc affects the splicing of PK mRNA by upregulating polypyrimidine tract binding protein (PTB) and heterogeneous nuclear ribonucleoproteins (hnRNPs) A1 and A2 leading to the predominant production of PKM2 [57]. Less efficient PKM2 is advantageous for cell proliferation as it allows for entering carbohydrate metabolites of glycolysis into alternative pathways to produce the macromolecules and NADPH necessary for tumour growth [58].
Epigenetic regulation of the Warburg effect
Based on current research, epigenetic alterations such as methylation of DNA, histone modifications or miRNA expression manifest tight cross-connections that modulate cancer metabolism. All enzymes and proteins that participate in glycolysis can be post-transcriptionally regulated by miRNAs. Glucose transporters, as mentioned above, are associated with glucose uptake in cells and their upregulation is a hallmark of the Warburg phenotype in cancer cells. Many experimental studies suggest an association between miRNA deregulation and GLUT1 activity including upregulation of miR-150 [59], miR-522-3p [60] and miR-10a [61], whereas expression rates of miR-340 [62], miR-218 [63] and miR-200c [64] are reduced.
The glycolytic enzyme HK2 is upregulated in cancer. MiR-143 has been shown to repress HK2 in colon cancer cells [65]. On the other hand, overexpression of miR-155 is correlated with the upregulation of HK2 in lung cancer cells [66]. PKM2 is also overexpressed in cancer metabolism. Current evidence indicates tumour-suppressive functions of miR-148a and miR-326 in the downregulation of PKM2 in thyroid cancer cells [67]. In addition, miRNA-let-7a downregulates PKM2 in cervical cancer cells [68]. Expression of small non-coding RNAs miR-1 and miRNA-133b can suppress the expression of PKM2 via interaction with PKTB1 and can also regulate the splicing of PKM2 [69]. Interestingly, miRNAs control cancer metabolism indirectly via regulation of long non-coding RNA. MiR-586 can form a competing endogenous RNA model with LncRNA-MIF and thus suppress glycolysis via degradation of c-Myc by Fbcw7 (E3 ligase for c-Myc) [70].
Methylation, another regulatory mechanism affecting gene expression, is also involved in the Warburg effect. Intragenic methylation mediated by binding of the protein BORIS leads to an alternative splicing of PKM resulting in the predominant generation of PKM2 and consequent development of cancer-related glycolysis [71]. Furthermore, overexpression of HK2 mediated by hypomethylation occurs in glioblastoma multiforme [72].
Likewise, epigenetic changes regulated by histone modifications also participate in the interplay between metabolism and cancer. Overproduction of lactate is a fundamental characteristic of the transformation of pyruvate into lactate. An interesting way of how lactate promotes cancer phenotypes in non-metabolic roles is demonstrated by lactylation of histones in macrophage leading to the expression of genes such as Arg1, which are involved in the M2-like polarisation of tumour-associated macrophages [73]. Moreover, the monoubiquitinating of histone H2B (H2Bub1) negatively regulates the Warburg effect. Recent evidence shows a cross-connection between elevated levels of PKM2 and decreases in H2Bub1 that indicate the oncogenic function of PKM2 via its control of histone modifications [74]. Figure 2 summarises the molecular mechanisms characteristic for cancer metabolism.
Fig. 2.
Molecular mechanisms contributing to the Warburg effect in cancer cells. 6GP, glucose-6-phosphate; 6FP, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; DHAP, dihydroxyacetone; G3P, glyceraldehyde 3-phosphate; 1,3BGP, 1,3-bisphosphoglycerate; 3PG, 3-phosphoglycerate; 2PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; HK2, hexokinase 2; PGI, phosphoglucose isomerase; PFK1, phosphofructokinase; TPI, triosephosphate isomerase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; PKM2, pyruvate kinase 2; LDH, lactate dehydrogenase; TCA, tricarboxylic acid cycle
Tumour microenvironment in the regulation of aerobic glycolysis
In contrast to genetic alterations associated with the transformation of metabolic pathways and consequent aerobic glycolysis, the tumourigenic microenvironment of the Warburg phenotype gives selective advantages in the growth and proliferation of tumour cells via several distinct mechanisms. Elevated glucose intake and consequent promotion of glucose metabolism increase the acidity of the microenvironment. Accumulation of lactate as the final product of aerobic glycolysis and the need of neutral pH for cell maintenance lead to its secretion via monocarboxylate transporters (MCT) together with H+ ions into extracellular space [75]. Increased microenvironmental acidity has multiple benefits for tumour cell proliferation and invasion [76]. Extracellular lactate activates growth factors including vascular endothelial growth factor (VEGF), tumour growth factor β (TGF β) and cytokine interleukin 1 (IL-1) [77]. Moreover, acidic tumour microenvironment promotes the activation of proteinases such as matrix metalloproteinase 9 (MMP-9) and enhances the invasiveness of cancer [78]. Low pH in the tumour region affects immune cells and immune responses [79]. Lactic acid, secreted by cancer cells, contributes to M2-macrophage polarisation [80]. Tumour-derived lactate is an intrinsic inflammatory factor associated with the promotion of chronic inflammatory responses through IL-17, which is secreted by T cells and macrophages [81, 82]. Although extracellular lactate does not modify the differentiation of monocytes into dendritic cells (DCs), it alters the antigen presentation process mediated by DCs and regulates the optimal functionality of DCs [83].
Despite progress in cancer-related research, multiple challenges including the repression of aerobic glycolysis in carcinogenesis still exist. Up to now, several glycolytic inhibitors, which contribute to the blockade of essential enzymes in glucose metabolism, have been tested against tumours in preclinical and clinical trials (Table 1).
Table 1.
Selected inhibitors of glycolysis
| Target | Agent | Cancer | References |
|---|---|---|---|
| PKM2 | TLN-232/CAP-232 | Renal, melanoma | [84, 85] |
| HK2 | 2-Deoxyglucose | Prostate | [86, 87] |
| 3-Bromopyruvate | Colorectal, breast, hepatocellular | [84, 88, 89] | |
| LDHA | FX 11 | Lymphoma, pancreatic | [90] |
| Oxamate | Nasopharyngeal | [91] | |
| PDH | CPI-613 | Small cell lung, pancreatic | [92, 93] |
| PDK | Dichloroacetate | Breast | [94, 95] |
| PFKFB3 | 3PO | Bladder, tongue | [96, 97] |
| GLUT1/3 | STF-31 | Renal | [98] |
PKM2, pyruvate kinase muscle isoform 2; HK2, hexokinase 2; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3; GLUT1/3, glucose transporter 1/3
Flavonoids regulating glucose metabolism
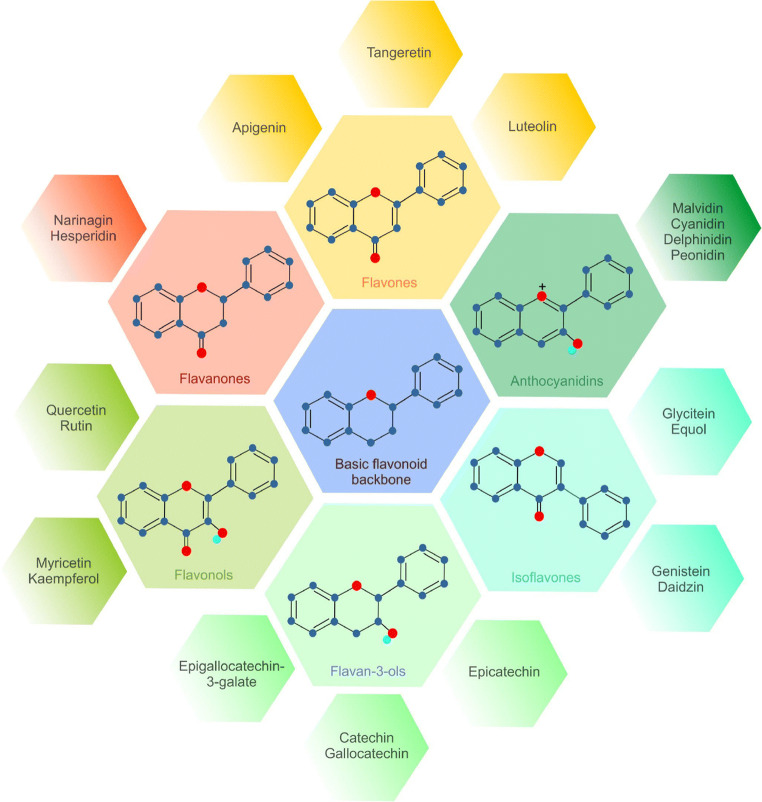
Plant-derived secondary metabolites, known as phytochemicals, exert numerous beneficial effects on human health. Many pre/clinical studies have suggested their anticancer functions at the epigenetic and proteomic levels of action [99–105]. The broad spectrum of salubrious properties and the enormous diversity within the classification predetermine phytonutrients for further analysis of their role in the regulation of tumourigenesis. Flavonoids represent a diverse group of phytochemicals (Fig. 3) that exhibit antioxidative, antiangiogenic and overall antineoplastic efficacy [14, 106]. Switching from oxidative phosphorylation to aerobic glycolysis, even under normoxic conditions, is associated with cancer transformation [107]. There is increasing evidence for the importance of flavonoids in modulating carcinogenic pathways associated with glucose metabolism. Flavonoids target the regulation of the activity of certain enzymes involved in aerobic glycolysis, expression of transporters responsible for glucose uptake, modulation of HIF-1 under normoxic conditions and many other parameters within the Warburg phenotype. Flavonoids thus represent a potential therapeutic approach for oncology-related research.
Fig. 3.
Structures of the main subgroups of flavonoids
PKM2
As described above, PKM2 supports metabolic alterations associated with the switch from benign phenotype to the malignant one [108]. Naturally occurring flavonoids are suggested as efficient regulators of aerobic glycolysis via modulation of PKM2 activity. Apigenin (AP), a common dietary flavonoid found in vegetables, exerts various benefits, including anti-inflammatory, antiviral and antioxidant effects [109]. Moreover, AP is an anticancer agent inhibiting several molecular pathways of tumour development [110]. Regarding glucose metabolism, this secondary metabolite blocked glycolysis through regulation of PKM2 activity and expression in a colon cancer cell line (HCT116). Additionally, AP is regarded as a potential allosteric inhibitor of PKM2. AP could maintain a low PKM2/PKM1 ratio as a consequence of inhibition of the β-catenin/c-Myc/PTBP1 pathway [111]. Similarly, proanthocyanidin B2 (PB2) affected the activity of PKM2 in several hepatocellular cancer cell lines (HCC-LM3, Bel-7402, SMMC-7721, Huh-7 and HepG2). Experimental data demonstrated decreased levels of PKM2 mediated by PB2 via an inhibition of nuclear translocation and expression of the analysed enzyme due to the disruption of an interaction between PKM/HSP90/Hif-1α in HCC cells [112]. Additionally, epigallocatechin-3-gallate (EGCG), a flavonoid found in green tea, demonstrated anticancer activity in many experimental studies [113–116]. Wei et al. tested the effects of EGCG (concentrations of 2, 4 and 8 μM) in a breast cancer cell line (4T1) focusing on glucose metabolism in vitro. At its highest concentration, EGCG significantly decreased the enzymatic activity and mRNA level of pyruvate kinase [117].
The inhibitory effects of several polyphenols on cancer were analysed by an evaluation of PKM2 enzymatic activity in vitro. Amongst these polyphenols, (±)-taxifolin (TAX), neoeriocitrin (NEO), fisetin (FIS), (−)-catechin gallate (CG) and (−)-epicatechin (EP) exerted the greatest inhibitory efficacy on PKM2 activity [118]. Moreover, quercetin (QUE), a plant flavonol, significantly decreased the level of glycolysis-related proteins including PKM2. Similarly, western blotting documented a decrease in PKM2 level through modulation of the Akt–mTOR pathway in vivo [119]. Additionally, QUE reduced the level of PKM2 in the colon mucosa of F344 rats in the study pointing to a chemopreventive role of the flavonoid [120]. Shikonin (SHI) is a natural flavonoid occurring in Lithospermum erythrorhizon. Chen et al. investigated the anticancer activity of SHI and its analogue alkannin against drug-sensitive and resistant cancer cells (MCF-7, MCF-7/Adr, MCF-7/Bcl-xL, MCF-7/Bcl-2 and A549) via PKM2 suppression and consequent inhibition of glycolysis. The results showed that both tested compounds repressed PKM2 activity without affecting other PKM isoforms [121]. Finally, SHI suppressed tumour growth in mice with implanted B16 melanoma cells by reducing PKM2-mediated aerobic glycolysis [122].
HK2
Hexokinase (HK) is the enzyme responsible for the phosphorylation of hexoses, which is the first irreversible step of glycolysis [123]. HK (primarily HK2) is often overexpressed in many types of cancer and may represent a potential molecular target for therapy using flavonoids [124–127]. Xanthohumol (XA), a flavonoid isolated from Humulus lupulus, suppressed the growth and proliferation of many tumours in preclinical studies [128–131]. Recently, a study evaluated the impact of XA on colorectal cancer progression via the downregulation of HK2-mediated glycolysis. XA inhibited HK2 and glycolysis through the suppression of EGFR–Akt signalling colon cancer cell lines (HCT116, HT29, SW620) in vitro and in a murine xenograft model in vivo [132]. As was demonstrated earlier, QUE exerted many anticancer effects in vivo and in vitro, even in the regulation of glucose metabolism, which leads to neoplastic transformation [133]. QUE reduced the level of HK2 and suppressed Akt/mTOR signalling in hepatocellular cancer lines (SMMG-7721, BEL-7402) in vitro. Additionally, QUE significantly decreased the level of HK2 and tumour growth in vivo [134]. Moreover, EGCG inhibited several essential enzymes that contribute to cancer metabolism. Amongst them, the enzymatic activity as well as the protein level of HK2 was significantly reduced in 4T1 cell line [117].
In addition to natural flavonoids and their roles in the chemopreventive and therapeutic aspects of cancer research, synthetic metabolites with broad anticancer properties have been reported [135]. The metabolic impact and biological activity of a set of new 3′,4′,5′-trimethoxy flavonoid salicylate derivatives were observed in the colorectal cancer cell line (HCT116). A synthetic compound, 10v, demonstrated cytotoxic potency against cancer cells. Results from western blotting suggested that 10v could inhibit cancer transformation and progression via downregulation of HK2 [136]. Another synthetic flavonoid, GL-V9, was tested against breast cancer cell lines (MCF-7 and MDA-MB-231). GL-V9 inhibited the expression of HK2 in both cell lines. Moreover, it promoted the dissociation of HK2 from a voltage-dependent anion-selective channel (VDAC) in the mitochondrial outer membrane resulting in suppression of the glycolytic pathway and consequent mitochondria-mediated apoptosis [137]. Additionally, Zhou et al. studied the cytotoxic activity of the synthetic flavonoid FV-429 against MDA-MB-231 cells. In a similar fashion as GL-V9, FV-429 triggered the apoptosis and inhibited glycolysis via dissociation of HK2 from VDAC in the mitochondrial membrane [138]. Moreover, the antineoplastic effects of the synthetic flavonoid Gen-27, mediated by the suppression of HK2, were observed in the MDA-MB-231 cell line. Application of Gen-27 to breast cancer cells led to a decrease in HK2 expression and the dissociation of HK2 from VDAC [139].
The antitumour and metabolic effects of phytochemicals are also mediated by the modulation of epigenetic machinery such as miRNAs. An individual miRNA can regulate the expression of multiple genes directly linked to glycolysis. Therefore, miRNAs are possible targets for cancer prevention and therapy. The flavonoid astralangin (ASG) occurs in many traditional medicinal plants. ASG inhibited glycolysis and proliferation in hepatocellular cancer in vitro and in vivo. Experimentally, ASG reduced the expression of HK2 by upregulating miR-125b in HepG2 HCC and Huh-7 cells. In addition, in vivo experiment using Huh-7 xenografts in nude mice and H22 cells transplanted in Kunming mice demonstrated a tumour-suppressive effect of AGS. Tumour growth reduction was associated with higher expression of miR-125b which correlated with decreasing HK2 expression observed in tumour tissue [140].
Additional glycolytic enzymes
Glycolysis, as the main metabolic cascade contributing to carbohydrate degradation, involves numerous enzymes with specific roles in the metabolic pathway [141]. Alterations of the essential enzymes PKM2 and HK2 participate in the Warburg phenotype of cancer cells. Aerobic glycolysis is a complex cascade involving other enzymes; specific changes in their enzymatic activity and/or expression rate promote tumourigenesis [142]. Naturally occurring flavonoids can suppress these changes. LDH catalyses the conversion of pyruvate into lactate. An elevated level of LDH is a biomarker of poor prognosis in cancer [143]. One possible means of cancer suppression is the application of synthetic or natural inhibitors. Morin (MO), a flavonoid isolated from the Moraceae family, inhibited the enzymatic activity of LDH. MO modulated the conformation and inhibited the catalytic activity of LDH [144]. Furthermore, QUE effectively suppressed cancer growth and proliferation in MCF-7 and MDA-MB-231 cells and decreased the level of glycolysis-related enzymes including LDHA [119]. Additionally, luteolin-7-O-β-D-glucoside (LU-7OβD), a flavonoid from Phlomis curdica, inhibited an isoform of human LDH. This property could be applied to improve anticancer treatment via the inhibition of the key glycolytic enzyme by luteolin [145]. Application of the polyphenol EGCG reduced the activity of LDH and PFK in 4T1 cells [117]. Furthermore, rats fed with QUE (10 g/kg diet for 11 weeks) exhibited enhanced cellular mechanisms involved in the prevention of carcinogenesis, while a transcriptome analysis showed downregulation of the oncogenic mitogen-activated protein kinase (MAPK). QUE also downregulated glycolytic enzymes including aldolase, GAPDH and α-enolase [120].
GLUTs
Glucose transporters are essential proteins that regulate glycolytic flux in the cell. Many oncogenic pathways are connected to the regulation of GLUT [146]. Flavonoids can modulate these pathways thus influencing glucose metabolism in tumours. Genistein (GE), phloretin (PH), daidzein and AP were tested against prostate cancer androgen-sensitive (LNCaP) and androgen-insensitive (PC-3) cell lines. Glucose intake was measured using nonradiolabeled 2-deoxyglucose. GLUT1 and GLUT4 levels were evaluated by western blot and immunocytochemistry. Amongst the four flavonoids, AP and PH were the most efficient in regulating the level of GLUTs and glucose intake in prostate cancer cells [147]. Furthermore, AP has also been found to reduce glucose transport by downregulating GLUT1 at the mRNA and protein levels in the pancreatic cancer cell lines CD18 and S2-013 [148]. Hesperetin (HES) is a citrus flavonoid reported to reduce cholesterol in plasma [149, 150]. Yang et al. observed a metabolic impact of HES on breast cancer cells MDA-MB-231. Their data indicated a decrease in glucose intake due to GLUT1 downregulation. Moreover, HES reduced insulin-stimulated glucose uptake by impairing the cell membrane translocation of GLUT4 [151]. Kaempferol (KAE; tetrahydroxy flavone) is found in various plants and exerts wide anticancer properties [152]. Azavedo et al. studied alterations in the uptake of 3H-deoxy-d-glucose, a glucose analogue, in MCF-7 cells after treatment with myricetin, chrysin, KAE, resveratrol, genistein and XA. Amongst these flavonoids, KAE was found to be the most effective inhibitor of glucose analogue uptake. Long-term KAE exposure also inhibited the 3H-deoxy-d-glucose uptake via downregulation of GLUT1 mRNA level in MCF-7 cells [153]. Another study utilised 3H-deoxy-d-glucose to estimate a glucose uptake in MCF-7 and MDA-MB-231 cells treated with QUE and EGCG. Both polyphenols affected glucose intake via direct and competitive inhibition of GLUT1 and thus suppressed the glucose metabolism [154]. In addition, EGCG demonstrated anticancer efficacy against 4T1 via reduction of GLUT1 expression [117]. Furthermore, QUE blocked an intake of glucose in MCF-7 and MDA-MB-231 through inhibition of GLUT1 activity as a consequence of the regulatory effect of QUE on the protein level of the glucose transporter [119]. Silibinin (SI), a naturally occurring flavone, is a major component of silymarin isolated from Sylibum marianum. SI inhibited GLUT4 in non-tumour CHO cells. Therefore, modulation of glucose transport via SI is a potential chemopreventive approach that could be applied also in cancer cells [155].
HIF-1
HIF-1 is a transcription factor associated with the regulation of many pivotal pathways in healthy cells, and its alterations caused by genetic, epigenetic or intra/extracellular stimulation lead to cell transformation into the cancer phenotype. Elevated expression of HIF-1 promotes tumour-associated angiogenesis, proliferation and progression through the modulation of glycolytic cascades [156]. Current evidence suggests that flavonoids may play a considerable role in cancer treatment and have multiple potential targets in tumourigenesis, including HIF-1 [157]. Baicalein (BA; 5,6,7-trihydroxyflavone), a flavonoid isolated from Scutellaria baicalensis, exerts strong anticancer activity on various tumours [158–160]. A recent study evaluated the impact of BA on glycolysis-related chemoresistance to 5-fluorouracil in gastric cancer cells (AGS). BA increased the sensitivity of AGS cells to 5-fluorouracil therapy. The administration of this flavonoid led to the inhibition of hypoxia-induced Akt phosphorylation as a consequence of the improvement of PTEN accumulation associated with decreasing HIF-1α expression. Since BA suppressed glycolysis via PTEN/Akt/HIF-1α, it is a possible therapeutic sensitiser against gastric cancer [161]. Interestingly, other studies confirmed the inhibitory effects of flavonoids on HIF-1 in the regulation of glucose metabolism. For instance, methylalpinumisoflavon (MF), a flavonoid isolated from Lonchocarpus glabrescens, contributed to the regulation of glycolysis in vitro. MF demonstrated strong cytotoxic effects on T47D cells and also suppressed HIF-1 activation and its target genes including CDKN1A, VEGF and GLUT-1 in cancer cells [162]. Moreover, oroxylin A (OX-A), a bioactive compound occurring in traditional Chinese medicinal plants, was tested against MDA-MB-231 cells. OX-A administration correlated with the inhibition of cancer-related glycolysis via Sirtuin-3 mediated destabilisation of HIF-1α controlling expression of glucose degradation enzymes [163]. Furthermore, EGCG reduced the expression of HIF-1α and enzymes related to glycolysis in T47D cells [117]. Interestingly, resveratrol (RES) reduced glucose uptake and glycolysis in the Lewis lung carcinoma (LLC), breast T47D and colon HT-29 cancer cell lines. Measurements of cellular uptake of the glucose analogue 18F-fluorodeoxyglucose (18F-FDG) during RES exposure indicated that RES repressed intracellular reactive oxidative species (ROS) and thereby decreased HIF-1α accumulation, reduced GLUT-1 expression and caused glycolytic flux [164]. Table 2 summarises the results of experimental studies investigating the effects of flavonoids on the critical components of glycolysis. Figure 4 is an overview of the flavonoids used as repressors of cancer-related metabolism.
Table 2.
Flavonoids in the Warburg phenotype
| Component of glycolysis | Flavonoid | Cancer type/model | Study design | Mechanism of action | References |
|---|---|---|---|---|---|
| PKM2 | AP | CC | HCT116 | ↓ PKM2 expression | [111] |
| PB2 | HC | HCC-LM3, Bel-7402, SMMC-7721, Huh-7 and HepG2 | ↓ PKM2 expression | [112] | |
| EGCG | BC | T47D |
↓ PKM2 expression ↓ PKM2 activity |
[117] | |
| TAX, NEO, FIS, CG, EP | Only enzyme activity evaluated in vitro | ↓ PKM2 activity | [118] | ||
| QUE | BC | MCF-7, MDA-MB-231; BALB/c nude mice | ↓ PKM2 level | [119] | |
|
Colon mucosa (chemopreventive model) F344 rats |
↓ PKM2 level | [120] | |||
| SHI | BC, LC | MCF-7, MCF-7/Adr, MCF-7/Bcl-xL, MCF-7/Bcl-2, and A549 | ↓ PKM2 activity | [121] | |
| ME | B16-melanoma xenografts | ↓ PKM2 level/activity | [122] | ||
| HK2 | XA | CC | HCT116, HT29, SW620; HCT116, HT29 xenografts | ↓ HK2 expression | [132] |
| QUE | HC |
SMMG-7721, BEL-7402 SMMC-7721 xenografts |
↓ HK2 level | [134] | |
| EGCG | BC | T47D | ↓ HK2 activity | [117] | |
| 10v | CC | HCT116 | ↓ HK2 level | [136] | |
| GL-V9 | BC | MCF-7, MDA-MB-231 | ↓ HK2 expression | [137] | |
| FV-429 | BC | MDA-MB-231 | ↓ HK2 level/activity | [138] | |
| Gen-27 | BC | MDA-MB-231 | ↓ HK2 expression | [139] | |
| ASG | HC |
HepG2, Huh-7 H22, Huh-7 xenografts |
↓ HK2 expression | [140] | |
| LDH | MO | Only enzyme activity evaluated in vitro | ↓ LDH enzymatic activity | [144] | |
| QUE | BC | MCF-7, MDA-MB-231 | ↓ LDH level | [119] | |
| LU-7OβD | Only enzyme activity evaluated in vitro | ↓ LDH enzymatic activity | [145] | ||
| LDH, PFK | EGCG | BC | T47D | ↓ LDH, PFK levels/activities | [117] |
| Aldolase, GAPDH,a-enolase | QUE |
Colon mucosa (chemopreventive model) F344 rats |
↓ aldolase, GAPDH, and a-enolase levels | [120] | |
| GLUTs | AP, PH | PC | LNCaP, PC-3 | ↓ GLUT1/GLUT4 expression | [147] |
| AP | PC | CD18, S2–013 | ↓ GLUT1 level | [148] | |
| HES | BC | MDA-MB-231 |
↓ GLUT1 level ↓ GLUT4 translocation |
[151] | |
| KAE | BC | MCF-7 | ↓ GLUT1 level | [153] | |
| QUE, EGCG | BC | MCF-7, MDA-MB-231 | ↓ GLUT1 activity | [154] | |
| EGCG | BC | 4T1 | ↓ GLUT1 expression | [117] | |
| QUE | BC | MCF-7, MDA-MB-231 | ↓ GLUT1 activity | [119] | |
| SI | Non-tumour CHO | ↓ GLUT4 activity | [155] | ||
| HIF-1 | BA | GC | AGS | ↓ HIF-1 expression | [161] |
| MF | BC | T47D | ↓ HIF-1 activity | [162] | |
| OX-A | BC | MDA-MB-231 | → HIF-1 destabilisation | [163] | |
| EGCG | BC | T47D | ↓ HIF-1 expression | [117] | |
| RES | LC, BC, CC | LLC, T47D, HT-29 | ↓ HIF-1 level | [164] | |
Explanatory notes: ↓ decrease, reduction, suppression; → induction
PKM2, pyruvate kinase muscle isoform 2; HK2, hexokinase 2; LDH, lactated hydrogenase; PFK, phosphofructokinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase, GLUTs, glucose transporters; HIF-1, hypoxia-inducible factor 1; AP, apigenin; PB2, proanthocyanidin B2; EGCG, epigallocatechin-3-gallate; TAX, taxifolin; NEO, neoeriocitrin; FIS, fisetin; CG, catechin gallate; EP, epicatechin; QUE, quercetin; SHI, shikonin; XA, xantohumol; ASG, astralangin; MO, morin; LU-7OβD, luteolin-7-O-β-D-glucoside; PH, phloretin; HES, hesperitin; KAE, kaempferol; SI, silibinin; BA, baicalein; MF, methylalpinumisoflavon; OX-A, oroxylin A; RES, resveratrol; CC, colon cancer; HC, hepatocellular cancer; BC, breast cancer; LC, lung cancer; ME, melanoma; PC, prostate cancer; GC, gastric cancer
Fig. 4.
The role of flavonoids in the regulation of glucose metabolism in cancer. AP, apigenin; PB2, proanthocyanidin B2; EGCG, epigallocatechin-3-gallate; TAX, taxifolin; NEO, neoeriocitrin; FIS, fisetin; CG, catechin gallate; EP, epicatechin; QUE, quercetin; SHI, shikonin; XA, xantohumol; ASG, astralangin; MO, morin; LU-7OβD, luteolin-7-O-β-D-glucoside; PH, phloretin; HES, hesperitin; KAE, kaempferol; SI, silibinin; BA, baicalein; MF, methylalpinumisoflavon; OX-A, oroxylin A; RES, resveratrol; OXPHOS, oxidative phosphorylation; TCA, tricarboxylic acid cycle; ATP, adenosine triphosphate
The Warburg effect from a clinical perspective: status quo
Investigating how anticancer therapies may target the Warburg effect has been of great interest since it was first described a century ago. During the last two decades, recent advances in this field have provided valuable knowledge regarding personalised detection and treatment of cancer. An example of its utilisation in cancer detection, treatment response and surveillance is positron emission tomography (PET-CT) imaging. As described by Croteau et al. [165] by assessing oxygen consumption and mechanisms of energy substrate consumption, through 18F-FDG uptake, this imaging modality has proven its great value for comprehensive management of cancers. After introduction of PET-CT imaging, innovative treatments have been developed to deliver more precise and directed radiotherapy fields, particularly in the setting of Hodgkin lymphoma. According to Girinsky et al. [166], historic radiation fields encompassed significant portions of healthy tissue, whereas modern radiation therapy techniques are able to limit radiation fields to focus on tumours identified by pre-treatment PET-CT, decreasing toxicities without compromising oncologic outcomes. For certain cancers, PET-CT is also utilised to evaluate treatment response after administration of chemotherapy [167]. This opens a new perspective for glucose-targeted drug therapies [168].
A parasitic effect, known as the ‘reverse Warburg effect’, by which tumour cells trigger a positive growth factor feedback from surrounding stromal cells after the ‘classical’ Warburg effect, was demonstrated in triple-negative breast cancer and is related to poor prognosis [169]. Based on these findings, clinical trials to assess the benefit of FDA-approved drugs such as metformin, hydroxychloroquine and N-acetyl-cysteine have been initiated due to their postulated property of blocking this particular pathway. Blocking a Warburg effect can be traced by PET-CT to assess treatment response [170]. Although an increased risk of aggressive BC specifically for young patients is well known amongst practitioners [171], the mechanisms for this phenomenon were unclear. The discovery made by Olga Golubnitschaja and colleagues described young patients with Flammer syndrome (FS) phenotype representing a specific group of risk predisposed to aggressive metastasing breast cancer, due to systemic vasoconstriction and tissue hypoperfusion [172–174] tightly linked to the systemic hypoxic–ischemic effects and Warburg transformation.
The Warburg effect also plays a role in novel methods for detection and treatment of prostate cancer. The employment of natural 1,4-naphtoquinone and its derivate 6-O-(1,4-naphtaquinone-2-yl)-D-glucose may improve selectivity in the Warburg effect blockade [175]. Further, clinical experiences with peroxisome proliferator-activated receptor ligands (PPAR ligands), which directly block the Warburg effect, yielded discordant results in terms of PSA control in 2 consecutive phase II trials [176, 177]. Although early development of prostate cancer in younger patients is recorded [178], current standard screening measures do not include patients under 45–50 years [179]. While strategies for early screening are currently under investigation, already available information points at metabolomics and radiomics to improve early diagnosis and personalised treatment opportunities [5]. The introduction of 68Ga prostate-specific membrane antigen PET-CT (PSMA-PET) and molecule dosage as the prostate cancer antigen 3 (PCA3), both with high selectivity for diagnosis and staging, has provided new options for individualised treatment planning, based on a patient’s risk profile [180–182]. Regarding metastatic disease, Radium-223 has a demonstrated effect on bony metastasis [183]. Its high selectivity for osteoblastic regions with high metabolic activity exemplifies how ‘anti-Warburg’ measures could play an advantageous role in targeted cancer treatment.
The ‘anti-Warburg’ approach, as described in this paper, is currently considered as being synergistic to comprehensive conventional therapies [184, 185]. Contextually, combined targeted therapies are strongly recommended for the cancer treatment setting.
Individualised patient profiling as a powerful instrument for implementing targeted ‘anti-Warburg’ measures in the context of 3P medicine
Below, the most prominent examples are briefly summarised for the application of targeted ‘anti-Warburg’ measures categorised as three levels of prevention and according to patient stratification based on the individualised profiling. For more information, corresponding literature sources are provided.
Targeted ‘anti-Warburg’ measures potentially effective for primary prevention in stratified groups of individuals with modifiable risk factors and reversible damage to health
-
A.
Individuals demonstrating systemic hypoxic–ischemic lesions, due to disturbed microcirculation such as the following:
Otherwise healthy individuals with Flammer syndrome phenotype who are typically affected by primary vascular dysregulation [186]; Flammer syndrome phenotype with predisposition to related pathologies can be diagnosed applying specialised questionnaires [172, 187, 188].
Otherwise healthy individuals demonstrating disturbed microcirculation secondary to modifiable health condition such as low physical activity, due to sedentary lifestyle [189, 190], in overweight and obese individuals [191] who are per evidence at higher risk to develop cancer [192] compared to the general population, as well as in underweight individuals (malnutrition, dietary restrictions, anorexic condition), due to restricted energy resources, upregulation of HIF-1 [193] and potentially insufficient repair capacity [194].
-
B.
Excessive stress exposure and accelerated ageing processes—both linked to preventable risk factors
Excessive stress exposure at an individual level might be linked to systemic vasoconstriction with consequent hypoxic–ischemic lesions and imbalanced damage-to-repair capacity that can be detected by ‘comet assay analysis’ [195, 196] and preventable accelerated ageing processes (e.g. in smokers and ageing population) [190]—all risk factors associated with cancer development and progression involving preventable contribution of Warburg effects [197].
-
C.
Chronic inflammation and healing impairments
Chronic inflammation and healing impairments frequently resulting from disturbed microcirculation are well-acknowledged risks of cancer development and progression. Both chronic inflammation and healing impairment may occur to individuals in suboptimal health conditions [187, 194, 198]. Individualised patient profiling is the best instrument to detect cancer predisposition at a reversible stage of damage [199].
Targeted ‘anti-Warburg’ measures potentially effective for secondary prevention in stratified patient groups with non-modifiable risk factors
-
A.
Genetic predisposition to cancer
Genetic component plays a role in the Warburg effect as the central contributor to the cancer progression machinery [200]. Both inborn and acquired genetic alterations are involved in Warburg phenomenon influencing its severity [201]. Therefore, both genotyping and phenotyping are important for considering individualised ‘anti-Warburg’ treatment options.
-
B.
Mitochondrial dysfunction
Although clarity is still needed, whether dysfunctional mitochondria are primary or secondary in Warburg effect [24], there is a consensus that mitochondrial dysfunction is central for cancerous transformation. Consequently, early detection of mitochondrial impairments is highly predictive for preventive ‘anti-Warburg’ treatments.
-
C.
Benign tumours diagnosed
Benign tissue transformation is an acknowledged risk factor for a malignant development, for example, in breast cancer aetiology [202]. Considering benign tumours as potential cancer pre-stages, ‘anti-Warburg’ measures might be effective to prevent cancer development.
-
D.
Clinically manifested relevant co-morbidities
As prominent examples, cardiovascular dysfunction and metabolic syndrome both linked to impaired circulation resulting in systemic hypoxic–ischemic lesions can be considered. To this end, patients with diabetic history are of increased cancer risk with poorer outcomes compared to non-diabetic patients [203]. Contextually, ‘anti-Warburg’ treatments have a potential to significantly improve individual outcomes in diabetic patients and patients with cardiovascular pathologies [204–207].
Targeted ‘anti-Warburg’ measures potentially effective for tertiary prevention in stratified patient groups
-
A.
Clinically manifested aggressive cancer subtypes with high metastatic potential
The incidence of aggressive breast cancer sub-types such as triple-negative metastasing tumours diagnosed in young females with normal and low BMI [6] is permanently increasing worldwide presenting a significant challenge for healthcare. Although their aetiology is still poorly understood, clear indications have been provided recently for the characteristic phenotype linked to the primary vascular dysregulation and some other signs and symptoms of Flammer syndrome [173, 174, 208]. The situation is particularly dramatic in the case of aggressive metastasing breast cancer, if diagnosed during pregnancy [209] (note: the publication was selected by Springer-Nature (2018) in the category ‘Medicine and Public Health’ as an article with a potential to change the world) [210]. In this group, an effective targeted chemoprevention adapted to the clinical situation is of great importance [211].
-
B.
Palliative medical approach
Despite significant progress in early diagnostics and screening programmes during the last two decades, a large portion of malignancies are still diagnosed at advanced stages resulting in palliative approach only possible. This is particularly true for the liver malignancies, since almost all solid tumours are metastasing to the liver [212]. To this end, effective palliative approaches demonstrate a great capacity to turn liver malignancies into chronic health condition, if patient-tailored treatment algorithms are applied [213]. Contextually, ‘anti-Warburg’ measures utilising flavonoids and genoprotective strategies [196] might be helpful in stabilising health condition of patients undergoing selective internal radiation therapy (SIRT) or trans-arterial chemo-embolisation (TACE).
Conclusion and expert recommendations
The rapid rise in cancer incidence and mortality is a global challenge [214]. Oncogenesis is a complex, multistage process including initiation, promotion and progression [215], as well as metabolic changes. It is well established that the increase of glucose uptake and lactate secretion due to metabolic changes in malignant cells constitutes the Warburg effect, an essential step of carcinogenesis [2]. Alterations in glucose metabolism associated with dramatically increased proliferation activity, progression and development of chemo-/radioresistance represent an important hallmark of malignancy [216]. Due to metabolic reprogramming, cancer cells exhibit increased levels of glucose uptake. This phenomenon is widely used for diagnostic and prognostic imaging of tumours using glucose analogues [217]. For many decades, PET demonstrates a non-invasive imaging method for evaluation of metabolic or functional changes in normal tissue as well as during disease conditions [218]. 18F-fluorodeoxyglucose (18F-FDG) is a glucose analogue that is widely used in combination with PET as a diagnostic tool for various types of cancer in clinical practice [219]. Consequently, FDG-PET is a clinically used technique for tumour imaging via increasing glucose uptake [220]. Further, magnetic resonance spectroscopy (MRS) utilising hyperpolarised 13C-pyruvate demonstrates great clinical utility, e.g. for prostate cancer patients. This technique is able to precisely delineate cancer relative to surrounding healthy tissue based on metabolic alteration in the cell [221]. Continuous progress in imaging techniques associated with specific cancer metabolism provides new opportunities for improving diagnostic procedures and individual patient outcomes [222].
Therapeutic strategies focused on specific cancer metabolism linked to the Warburg effect are currently under extensive consideration [174, 175]. To this end, naturally occurring bioactive compounds of plants demonstrate clinically relevant beneficial effects against cancer development and progression [223–230]. The combination use of phytochemicals and chemotherapeutical drugs may represent an optimal modality for anticancer therapy [231]. In this regard, the application of plant-derived natural substances as anticancer agents specifically against the Warburg phenotype is a promising strategy.
Flavonoids may modulate almost all key processes involved in carcinogenesis including apoptosis [232], proliferation [233], angiogenesis and metastatic progression [234, 235]. Their anticancer effects are directly associated with modulating energy and glucose metabolism, regulating specific enzymes of aerobic glycolysis and transporters required for glucose intake, which amongst others are involved in the Warburg effects characteristic for the cancerous cell transformation [111, 112, 117–122, 132, 134, 136–140, 144, 145, 147, 148, 151, 153–155, 161–164]. Still, in-depth analysis is essential to stratify individuals and patients for targeted application of flavonoids considering ‘anti-Warburg’ effects at the level of primary, secondary and tertiary prevention. To this end, individualised patient profiling is a powerful instrument for implementing targeted ‘anti-Warburg’ measures in the context of predictive, preventive and personalised medicine, which natural substances demonstrate a great potential to contribute to [236].
List of abbreviations
- 1,3BGP
1,3-bisphosphoglycerate
- 18F-FDG
18F-fluorodeoxyglucose
- 2PG
2-phosphoglycerate
- 3PG
3-phosphoglycerate
- ADP
adenosine diphosphate
- AMPK
AMP-activated kinase
- AP
apigenin
- ASG
astralangin
- ATP
adenosine triphosphate
- BA
baicalein
- BC
breast cancer
- CC
colon cancer
- CG
catechin gallate
- DCs
dendritic cells
- DHAP
dihydroxyacetone
- EGCG
epigallocatechin-3-gallate
- EP
epicatechin
- F6P
fructose-6-phosphate
- FBP
fructose-1,6-bisphosphate
- FH
fumarate hydratase
- FIS
fisetin
- FOXO1
forkhead box subfamily O1
- FS
Flammer syndrome
- G3P
glyceraldehyde-3-phosphate
- G6P
glucose-6-phosphate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GC
gastric cancer
- GE
genistein
- GLUTs
glucose transporters
- H2Bub1
monoubiquitinating of histone H2B
- HC
hepatocellular cancer
- HES
hesperitin
- HIF-1
hypoxia-inducible factor-1
- HK
hexokinase
- HnRNPs
heterogeneous nuclear ribonucleoproteins
- IL-1
interleukin 1
- KAE
kaempferol
- LBK1
liver kinase B1
- LC
lung cancer
- LLC
Lewis lung carcinoma
- LDH
lactate dehydrogenase
- LDHA
lactate dehydrogenase A
- LU-7OβD
luteolin-7-O-β-D-glucoside
- MAPK
mitogen-activated protein kinase
- MCT
monocarboxylate transporters
- ME
melanoma
- MF
methylalpinumisoflavon
- MMP-9
matrix metalloproteinase 9
- MO
morin
- MRS
magnetic resonance spectroscopy
- NAD+
nicotinamide adenine dinucleotide (oxidised form)
- NADH
nicotinamide adenine dinucleotide (reduced form)
- NEO
neoeriocitrin
- OX-A
oroxylin A
- PB2
proanthocyanidin B2
- PC
prostate cancer
- PCA3
prostate cancer antigen 3
- PEP
phosphoenolpyruvate
- PET
positron emission tomography
- PFK1
phosphofructokinase
- PGI
phosphoglucose isomerase
- PGK
phosphoglycerate kinase
- PGM
phosphoglycerate mutase
- PH
phloretin
- PK
pyruvate kinase
- PKM2
pyruvate kinase muscle isoform 2
- PPAR ligands
peroxisome proliferator-activated receptor ligands
- PPPM / 3PM
predictive, preventive, personalised medicine
- PTB
polypyrimidine tract binding protein
- pVHL
von Hippel–Lindau
- QUE
quercetin
- RES
resveratrol
- ROS
reactive oxidative species
- SCO2
synthesis of cytochrome C oxidase 2
- SDH
succinate dehydrogenase
- SHI
shikonin
- SI
silibinin
- SIRT
selective internal radiation therapy
- SIX 1
sine oculis homeobox 1
- TACE
trans-arterial chemo-embolisation
- TAX
taxifolin
- TCA
tricarboxylic acid cycle
- TGF β
tumour growth factor β
- TIGAR
TP53-induced glycolysis and apoptosis regulator
- TPI
triosephosphate isomerase
- VDAC
voltage-dependent anion-selective channel
- VEGF
vascular endothelial growth factor
- XA
xanthohumol
- YY1
Yin Yang 1
Authors’ contribution
P.K. and O.G. developed concepts of the manuscript and supervised the manuscript preparation; M.S., L.K., A.L., S.M.S., K.Z., E.V., M.A., T.Q., M.K., P.K., C.B., G.R.S. and O.G. performed the literature search; L.K. and M.S. created the figures; M.S., P.K., O.G., D.B., T.K.K., J.M., A.Z., M.S. and G.R.S. contributed to the paper contents and manuscript editing; P.K., O.G., D.B., T.K.K. and P.Z. elaborated on the final version of the manuscript.
Funding information
Open Access funding provided by Projekt DEAL. This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic under Contract No. VEGA 1/0136/19 and the Slovak Research and Development Agency under Contract No. APVV-16-0021. This publication is the result of the project implementation: “Center of Excellence for Research in Personalized Therapy (CEVYPET)”, ITMS: 26220120053 supported by the Operational Programme Research and Innovation funded by the ERDF. EV, SMS and DB were supported by NPRP grant [11S-1214-170101] from the Qatar National Research Fund (a member of Qatar Foundation).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marek Samec, Email: marek.samec@gmail.com.
Alena Liskova, Email: liskova80@uniba.sk.
Lenka Koklesova, Email: koklesova.lenka@gmail.com.
Samson Mathews Samuel, Email: sms2016@qatar-med.cornell.edu.
Kevin Zhai, Email: kez4003@qatar-med.cornell.edu.
Constanze Buhrmann, Email: constanze.buhrmann@med.uni-muenchen.de.
Elizabeth Varghese, Email: elv2007@qatar-med.cornell.edu.
Mariam Abotaleb, Email: mariam.abotaleb@aucegypt.edu.
Tawar Qaradakhi, Email: tawar.qaradakhi@live.vu.edu.au.
Anthony Zulli, Email: Anthony.Zulli@vu.edu.au.
Martin Kello, Email: martin.kello@yahoo.com.
Jan Mojzis, Email: jan.mojzis@upjs.sk.
Pavol Zubor, Email: pavzub@ous-hf.no.
Taeg Kyu Kwon, Email: kwontk@dsmc.or.kr.
Mehdi Shakibaei, Email: mehdi.shakibaei@med.uni-muenchen.de.
Dietrich Büsselberg, Email: dib2015@qatar-med.cornell.edu.
Olga Golubnitschaja, Email: olga.golubnitschaja@ukbonn.de.
Peter Kubatka, Email: peter.kubatka@uniba.sk.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Sciacovelli M, Gaude E, Hilvo M, Frezza C. The metabolic alterations of cancer cells. Methods Enzymol. 2014;542:1–23. 10.1016/B978-0-12-416618-9.00001-7. [DOI] [PubMed]
- 3.Wu Z, Wu J, Zhao Q, Fu S, Jin J. Emerging roles of aerobic glycolysis in breast cancer. Clin Transl Oncol. 2020;22:631–646. doi: 10.1007/s12094-019-02187-8. [DOI] [PubMed] [Google Scholar]
- 4.Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18:494–504. doi: 10.2174/1568026618666180523111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu M, Zhan X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018;9:77–102. doi: 10.1007/s13167-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golubnitschaja O, Filep N, Yeghiazaryan K, Blom HJ, Hofmann-Apitius M, Kuhn W. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: lessons for predictive, preventive and personalised medicine. Amino Acids. 2018;50:383–395. doi: 10.1007/s00726-017-2524-0. [DOI] [PubMed] [Google Scholar]
- 7.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed]
- 8.Ganapathy-Kanniappan S, Geschwind J-FH. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Wang N, Chen J, Shen J (2020) Emerging glycolysis targeting and drug discovery from Chinese medicine in cancer therapy. Available online: https://www.hindawi.com/journals/ecam/2012/873175/ (Accessed on 18 Apr 2020) [DOI] [PMC free article] [PubMed]
- 10.Yu L, Chen X, Sun X, Wang L, Chen S. The glycolytic switch in tumors: how many players are involved? J Cancer. 2017;8:3430–3440. doi: 10.7150/jca.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddouks M, Chattopadhyay D, De Feo V, Cho WC. Medicinal plants in the prevention and treatment of chronic diseases 2013. Evid Based Complement Alternat Med. 2014;2014:180981. 10.1155/2014/180981. [DOI] [PMC free article] [PubMed]
- 12.Mahady G. Medicinal plants for the prevention and treatment of bacterial infections. CPD. 2005;11:2405–2427. doi: 10.2174/1381612054367481. [DOI] [PubMed] [Google Scholar]
- 13.Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, et al. Role of phytochemicals in cancer prevention. Int J Mol Sci. 2019;20:4981. 10.3390/ijms20204981. [DOI] [PMC free article] [PubMed]
- 14.Abotaleb M, Samuel S, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancers. 2018;11:28. 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed]
- 15.Kozłowska A, Szostak-Wegierek D. Flavonoids--food sources and health benefits. Rocz Panstw Zakl Hig. 2014;65:79–85. [PubMed] [Google Scholar]
- 16.Busch C, Burkard M, Leischner C, Lauer UM, Frank J, Venturelli S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenetics. 2015;7:64. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-García C, Sánchez-Quesada C, Gaforio JJ. Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants (Basel). 2019;8:137. 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed]
- 18.Potter M, Newport E, Morten KJ. The Warburg effect: 80 years on. Biochem Soc Trans. 2016;44:1499–1505. doi: 10.1042/BST20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaudhry R, Varacallo M. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Biochemistry, glycolysis. [PubMed] [Google Scholar]
- 20.Berg, JM, Tymoczko JL, Stryer L. Glycolysis is an energy-conversion pathway in many organisms. Biochemistry. 5th ed. New York: WH Freeman; 2002.
- 21.Kurihara-Shimomura M, Sasahira T, Nakashima C, Kuniyasu H, Shimomura H, Kirita T. The multifarious functions of pyruvate kinase M2 in oral cancer cells. Int J Mol Sci. 2018;19:2907. 10.3390/ijms19102907. [DOI] [PMC free article] [PubMed]
- 22.Lebelo MT, Joubert AM, Visagie MH. Warburg effect and its role in tumourigenesis. Arch Pharm Res. 2019;42:833–847. doi: 10.1007/s12272-019-01185-2. [DOI] [PubMed] [Google Scholar]
- 23.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 24.Senyilmaz D, Teleman AA. Chicken or the egg: Warburg effect and mitochondrial dysfunction. F1000Prime Rep. 2015;7:41. 10.12703/P7-41. [DOI] [PMC free article] [PubMed]
- 25.Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frezza C, Gottlieb E. Mitochondria in cancer: not just innocent bystanders. Semin Cancer Biol. 2009;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 27.da Veiga Moreira J, Hamraz M, Abolhassani M, Bigan E, Pérès S, Paulevé L, et al. The redox status of cancer cells supports mechanisms behind the Warburg effect. Metabolites. 2016;6:33. 10.3390/metabo6040033. [DOI] [PMC free article] [PubMed]
- 28.Ngo H, Tortorella SM, Ververis K, Karagiannis TC. The Warburg effect: molecular aspects and therapeutic possibilities. Mol Biol Rep. 2015;42:825–834. doi: 10.1007/s11033-014-3764-7. [DOI] [PubMed] [Google Scholar]
- 29.Jiang B. Aerobic glycolysis and high level of lactate in cancer metabolism and microenvironment. Genes Dis. 2017;4:25–27. doi: 10.1016/j.gendis.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing Y, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of the tumour microenvironment. FEBS J. 2015;282:3892–3898. doi: 10.1111/febs.13402. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi N, Das GM. Metabolic reprogramming in breast cancer and its therapeutic implications. Cells. 2019;8:89. doi: 10.3390/cells8020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alqahtani A, Ayesh HSK, Halawani H. PIK3CA gene mutations in solid malignancies: association with clinicopathological parameters and prognosis. Cancers. 2020;12:93. doi: 10.3390/cancers12010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada T, Nowak JA, Ogino S. PIK3CA mutation and colorectal cancer precision medicine. Oncotarget. 2017;8:22305–22306. doi: 10.18632/oncotarget.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasparri ML, Bardhi E, Ruscito I, Papadia A, Farooqi AA, Marchetti C, et al. PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe Frauenheilkd. 2017;77:1095–103. 10.1055/s-0043-118907. [DOI] [PMC free article] [PubMed]
- 36.Jiang W, He T, Liu S, Zheng Y, Xiang L, Pei X, et al. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J Hematol Oncol. 2018;11:139. 10.1186/s13045-018-0674-5. [DOI] [PMC free article] [PubMed]
- 37.Hinz N, Jücker M. Distinct functions of AKT isoforms in breast cancer: a comprehensive review. Cell Commun Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zambrano A, Molt M, Uribe E, Salas M. Glut 1 in cancer cells and the inhibitory action of resveratrol as a potential therapeutic strategy. Int J Mol Sci. 2019;20:3374. 10.3390/ijms20133374. [DOI] [PMC free article] [PubMed]
- 39.Avanzato D, Pupo E, Ducano N, Isella C, Bertalot G, Luise C, et al. High USP6NL levels in breast cancer sustain chronic AKT phosphorylation and GLUT1 stability fueling aerobic glycolysis. Cancer Res. 2018;78:3432–44. 10.1158/0008-5472.CAN-17-3018. [DOI] [PubMed]
- 40.Shi D, Zhao D, Niu P, Zhu Y, Zhou J, Chen H. Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells. BMC Complement Altern Med. 2018;18:317. doi: 10.1186/s12906-018-2380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo YM, Shin Y, Lee EJ, Lee S, Jeong SH, Kong HK, et al. Inhibition of aerobic glycolysis represses Akt/mTOR/HIF-1α axis and restores tamoxifen sensitivity in antiestrogen-resistant breast cancer cells. PLoS One. 2015;10:e0132285. 10.1371/journal.pone.0132285. [DOI] [PMC free article] [PubMed]
- 42.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetzman ES, Prochownik EV. The role for Myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front Endocrinol (Lausanne). 2018;9:129. 10.3389/fendo.2018.00129. [DOI] [PMC free article] [PubMed]
- 44.Broecker-Preuss M, Becher-Boveleth N, Bockisch A, Dührsen U, Müller S. Regulation of glucose uptake in lymphoma cell lines by c-MYC- and PI3K-dependent signaling pathways and impact of glycolytic pathways on cell viability. J Transl Med. 2017;15:158. doi: 10.1186/s12967-017-1258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He T-L, Zhang Y-J, Jiang H, Li X-H, Zhu H, Zheng K-L. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med Oncol. 2015;32:187. doi: 10.1007/s12032-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zawacka-Pankau J, Grinkevich VV, Hünten S, Nikulenkov F, Gluch A, Li H, et al. Inhibition of glycolytic enzymes mediated by pharmacologically activated p53 targeting Warburg effect to fight cancer. J Biol Chem. 2011;286:41600–15. 10.1074/jbc.M111.240812. [DOI] [PMC free article] [PubMed]
- 47.Kishton RJ, Barnes CE, Nichols AG, Cohen S, Gerriets VA, Siska PJ, et al. AMPK is essential to balance glycolysis and mitochondrial metabolism to control T-ALL cell stress and survival. Cell Metab. 2016;23:649–62. 10.1016/j.cmet.2016.03.008. [DOI] [PMC free article] [PubMed]
- 48.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Huang D, Lu N, Luo L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (review) Oncol Rep. 2015;34:2821–2826. doi: 10.3892/or.2015.4288. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Liu J, Wu R, Liang Y, Lin M, Liu J, et al. Tumor suppressor p53 negatively regulates glycolysis stimulated by hypoxia through its target RRAD. Oncotarget. 2014;5:5535–46. [DOI] [PMC free article] [PubMed]
- 51.Eriksson M, Ambroise G, Ouchida AT, Lima Queiroz A, Smith D, Gimenez-Cassina A, et al. Effect of mutant p53 proteins on glycolysis and mitochondrial metabolism. Mol Cell Biol. 2017;37:e00328-17. 10.1128/MCB.00328-17. [DOI] [PMC free article] [PubMed]
- 52.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33:368–385.e7. 10.1016/j.ccell.2018.01.010. [DOI] [PubMed]
- 53.Goodwin TJ, Christofidou-Solomidou M. Oxidative stress and space biology: An organ-based approach. Int J Mol Sci. 2018;19:959. 10.3390/ijms19040959. [DOI] [PMC free article] [PubMed]
- 54.Wang Y, Wu S, Huang C, Li Y, Zhao H, Kasim V. Yin Yang 1 promotes the Warburg effect and tumorigenesis via glucose transporter GLUT3. Cancer Sci. 2018;109:2423–2434. doi: 10.1111/cas.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang KM, Kim K. Protein kinase CK2 modulation of pyruvate kinase M isoforms augments the Warburg effect in cancer cells. J Cell Biochem. 2018;119:8501–8510. doi: 10.1002/jcb.27078. [DOI] [PubMed] [Google Scholar]
- 56.Iqbal MA, Gupta V, Gopinath P, Mazurek S, Bamezai RNK. Pyruvate kinase M2 and cancer: an updated assessment. FEBS Lett. 2014;588:2685–2692. doi: 10.1016/j.febslet.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Wiese EK, Hitosugi T. Tyrosine kinase signaling in cancer metabolism: PKM2 paradox in the Warburg effect. Front Cell Dev Biol. 2018;6:79. 10.3389/fcell.2018.00079. [DOI] [PMC free article] [PubMed]
- 58.Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, et al. PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol Lett. 2016;11:1980–1986. 10.3892/ol.2016.4168. [DOI] [PMC free article] [PubMed]
- 59.Yuan G, Zhao Y, Wu D, Gao C. Mir-150 up-regulates Glut1 and increases glycolysis in osteosarcoma cells. Asian Pac J Cancer Prev. 2017;18:1127–1131. doi: 10.22034/APJCP.2017.18.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen R, Lin J, Yan W, Chen D. miR-522-3p promotes osteosarcoma cell growth by regulating glucose uptake and GLUT1 expression. Onco Targets Ther. 2019;12:9053–9058. doi: 10.2147/OTT.S217324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y-H, Song Y, Yu Y-L, Cheng W, Tong X. miRNA-10a promotes cancer cell proliferation in oral squamous cell carcinoma by upregulating GLUT1 and promoting glucose metabolism. Oncol Lett. 2019;17:5441–5446. doi: 10.3892/ol.2019.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu P, Li Y, Zhang H, Li M, Zhu H. MicroRNA-340 mediates metabolic shift in oral squamous cell carcinoma by targeting glucose transporter-1. J Oral Maxillofac Surg. 2016;74:844–850. doi: 10.1016/j.joms.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 63.Xu X-J, Yuan J, Sun W-J, Chen Q-Y, Lin Y, Tang L, et al. Inhibition of microRNA-218 promotes oral squamous cell carcinoma growth by targeting GLUT1 to affect glucose metabolism. Eur Rev Med Pharmacol Sci. 2018;22:7726–34. 10.26355/eurrev_201811_16394. [DOI] [PubMed]
- 64.Yan Y, Yan F, Huang Q. MiR-200c inhibited the proliferation of oral squamous cell carcinoma cells by targeting Akt pathway and its downstream Glut1. Arch Oral Biol. 2018;96:52–57. doi: 10.1016/j.archoralbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Gregersen LH, Jacobsen A, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-143 down-regulates hexokinase 2 in colon cancer cells. BMC Cancer. 2012;12:232. doi: 10.1186/1471-2407-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv X, Yao L, Zhang J, Han P, Li C. Inhibition of microRNA-155 sensitizes lung cancer cells to irradiation via suppression of HK2-modulated glucose metabolism. Mol Med Rep. 2016;14:1332–1338. doi: 10.3892/mmr.2016.5394. [DOI] [PubMed] [Google Scholar]
- 67.Yu G, Sun W, Shen Y, Hu Y, Liu H, Li W, et al. PKM2 functions as a potential oncogene and is a crucial target of miR-148a and miR-326 in thyroid tumorigenesis. Am J Transl Res. 2018;10:1793–1801. [PMC free article] [PubMed]
- 68.Guo M, Zhao X, Yuan X, Jiang J, Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017;8:28226–28236. doi: 10.18632/oncotarget.15999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taniguchi K, Sakai M, Sugito N, Kumazaki M, Shinohara H, Yamada N, et al. PTBP1-associated microRNA-1 and -133b suppress the Warburg effect in colorectal tumors. Oncotarget. 2016;7:18940–52. 10.18632/oncotarget.8005. [DOI] [PMC free article] [PubMed]
- 70.Zhang P, Cao L, Fan P, Mei Y, Wu M. LncRNA-MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting Fbxw7-mediated c-Myc degradation. EMBO Rep. 2016;17:1204–1220. doi: 10.15252/embr.201642067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh S, Narayanan SP, Biswas K, Gupta A, Ahuja N, Yadav S, et al. Intragenic DNA methylation and BORIS-mediated cancer-specific splicing contribute to the Warburg effect. PNAS. 2017;114:11440–5. 10.1073/pnas.1708447114. [DOI] [PMC free article] [PubMed]
- 72.Blakeway D, Karakoula K, Morris M, Rowther F, Eagles L, Darling J, et al. Overexpression of hexokinase 2 is epigenetically regulated by frequent hypomethylation in glioblastoma multiforme. Neuro-Oncology. 2018;20:i12–2. 10.1093/neuonc/nox238.053.
- 73.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–80. 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed]
- 74.Jing Y-Y, Cai F-F, Zhang L, Han J, Yang L, Tang F, et al. Epigenetic regulation of the Warburg effect by H2B monoubiquitination. Cell Death Differ. 2019:1–17. 10.1038/s41418-019-0450-2. [DOI] [PMC free article] [PubMed]
- 75.Swietach P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Rev. 2019;38:5–15. doi: 10.1007/s10555-018-09778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boedtkjer E, Pedersen SF. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 77.Song J, Lee K, Park SW, Chung H, Jung D, Na YR, et al. Lactic acid upregulates VEGF expression in macrophages and facilitates choroidal neovascularization. Invest Ophthalmol Vis Sci. 2018;59:3747–54. 10.1167/iovs.18-23892. [DOI] [PubMed]
- 78.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89. 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed]
- 79.Erra Díaz F, Dantas E, Geffner J. Unravelling the interplay between extracellular acidosis and immune cells. Mediat Inflamm. 2018;2018:1218297. 10.1155/2018/1218297. [DOI] [PMC free article] [PubMed]
- 80.Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17:428–38. 10.1080/15384101.2018.1444305. [DOI] [PMC free article] [PubMed]
- 81.Shime H, Yabu M, Akazawa T, Kodama K, Matsumoto M, Seya T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–83. 10.4049/jimmunol.180.11.7175. [DOI] [PubMed]
- 82.Yabu M, Shime H, Hara H, Saito T, Matsumoto M, Seya T, et al. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. Int Immunol. 2011;23:29–41. 10.1093/intimm/dxq455. [DOI] [PubMed]
- 83.Domblides C, Lartigue L, Faustin B. Control of the antitumor immune response by cancer metabolism. Cells. 2019;8:104. 10.3390/cells8020104. [DOI] [PMC free article] [PubMed]
- 84.Seo M, Crochet RB, Lee Y-H. Chapter 14 - targeting altered metabolism—emerging cancer therapeutic strategies. In: Neidle S, editor. Cancer drug design and discovery (Second edition) San Diego: Academic; 2014. pp. 427–448. [Google Scholar]
- 85.Phase II Study of CAP-232 in patients with refractory metastatic renal cell carcinoma - full text view - ClinicalTrials.gov Available online: https://clinicaltrials.gov/ct2/show/NCT00422786 (accessed on 16 Apr 2020)
- 86.Stein M, Lin H, Jeyamohan C, Dvorzhinski D, Gounder M, Bray K, et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate. 2010;70:1388–94. 10.1002/pros.21172. [DOI] [PMC free article] [PubMed]
- 87.Dose escalation trial of 2-deoxy-D-glucose (2DG) in subjects with advanced solid tumors - full text view - ClinicalTrials.gov Available online: https://clinicaltrials.gov/ct2/show/NCT00096707 (accessed on 16 Apr 2020)
- 88.Gong L, Wei Y, Yu X, Peng J, Leng X. 3-Bromopyruvic acid, a hexokinase II inhibitor, is an effective antitumor agent on the hepatoma cells: in vitro and in vivo findings. Anti Cancer Agents Med Chem. 2014;14:771–776. doi: 10.2174/1871520614666140416105309. [DOI] [PubMed] [Google Scholar]
- 89.Sun Y, Liu Z, Zou X, Lan Y, Sun X, Wang X, et al. Mechanisms underlying 3-bromopyruvate-induced cell death in colon cancer. J Bioenerg Biomembr. 2015;47:319–29. 10.1007/s10863-015-9612-1. [DOI] [PMC free article] [PubMed]
- 90.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed]
- 91.Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30:2983–2991. doi: 10.3892/or.2013.2735. [DOI] [PubMed] [Google Scholar]
- 92.Philip PA, Buyse ME, Alistar AT, Lima CM, Luther S, Pardee TS, et al. A phase III open-label trial to evaluate efficacy and safety of CPI-613 plus modified FOLFIRINOX (mFFX) versus FOLFIRINOX (FFX) in patients with metastatic adenocarcinoma of the pancreas. Future Oncol. 2019;15:3189–96. 10.2217/fon-2019-0209. [DOI] [PMC free article] [PubMed]
- 93.Lycan TW, Pardee TS, Petty WJ, Bonomi M, Alistar A, Lamar ZS, et al. A phase II clinical trial of CPI-613 in patients with relapsed or refractory small cell lung carcinoma. PLoS One. 2016;11:e0164244. 10.1371/journal.pone.0164244. [DOI] [PMC free article] [PubMed]
- 94.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ko L, Allalunis-Turner J. Investigation on the mechanism of dichloroacetate (DCA) induced apoptosis in breast cancer. JCO. 2009;27:e14637–7. 10.1200/jco.2009.27.15_suppl.e14637.
- 96.Lea MA, Altayyar M, desBordes C. Inhibition of growth of bladder cancer cells by 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one in combination with other compounds affecting glucose metabolism. Anticancer Res. 2015;35:5889–5899. [PubMed] [Google Scholar]
- 97.Chen L, Zhao J, Tang Q, Li H, Zhang C, Yu R, et al. PFKFB3 control of cancer growth by responding to circadian clock outputs. Sci Rep. 2016;6:24324. 10.1038/srep24324. [DOI] [PMC free article] [PubMed]
- 98.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed]
- 99.Kubatka P, Kapinová A, Kello M, Kruzliak P, Kajo K, Výbohová D, et al. Fruit peel polyphenols demonstrate substantial anti-tumour effects in the model of breast cancer. Eur J Nutr. 2016;55:955–65. 10.1007/s00394-015-0910-5. [DOI] [PubMed]
- 100.Kubatka P, Uramova S, Kello M, Kajo K, Kruzliak P, Mojzis J, et al. Antineoplastic effects of clove buds (Syzygium aromaticum L.) in the model of breast carcinoma. J Cell Mol Med. 2017;21:2837–51. 10.1111/jcmm.13197. [DOI] [PMC free article] [PubMed]
- 101.Kubatka P, Uramova S, Kello M, Kajo K, Samec M, Jasek K, et al. Anticancer activities of Thymus vulgaris L. in experimental breast carcinoma in vivo and in vitro. IJMS. 2019;20:1749. 10.3390/ijms20071749. [DOI] [PMC free article] [PubMed]
- 102.Kubatka P, Kapinová A, Kružliak P, Kello M, Výbohová D, Kajo K, et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition. 2015;31:560–9. 10.1016/j.nut.2014.08.010. [DOI] [PubMed]
- 103.Kubatka P, Kello M, Kajo K, Samec M, Jasek K, Vybohova D, et al. Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. bark in experimental breast carcinoma: mechanistic in vivo and in vitro analyses. Molecules. 2020;25:1399. 10.3390/molecules25061399. [DOI] [PMC free article] [PubMed]
- 104.Deb G, Shankar E, Thakur VS, Ponsky LE, Bodner DR, Fu P, et al. Green tea-induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol Carcinog. 2019;58:1194–207. 10.1002/mc.23003. [DOI] [PubMed]
- 105.Atwell LL, Zhang Z, Mori M, Farris PE, Vetto JT, Naik AM, et al. Sulforaphane bioavailability and chemopreventive activity in women scheduled for breast biopsy. Cancer Prev Res. 2015;8:1184–91. 10.1158/1940-6207.CAPR-15-0119. [DOI] [PMC free article] [PubMed]
- 106.Li Y, Zhang T, Chen GY. Flavonoids and colorectal cancer prevention. Antioxidants. 2018;7:187. doi: 10.3390/antiox7120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spencer NY, Stanton RC. The Warburg effect, lactate, and nearly a century of trying to cure cancer. Semin Nephrol. 2019;39:380–393. doi: 10.1016/j.semnephrol.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Dayton TL, Jacks T, Vander Heiden MG. PKM2, cancer metabolism, and the road ahead. EMBO Rep. 2016;17:1721–1730. doi: 10.15252/embr.201643300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50. 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed]
- 110.Madunić J, Madunić IV, Gajski G, Popić J, Garaj-Vrhovac V. Apigenin: a dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018;413:11–22. doi: 10.1016/j.canlet.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 111.Shan S, Shi J, Yang P, Jia B, Wu H, Zhang X, et al. Apigenin restrains colon cancer cell proliferation via targeted blocking of pyruvate kinase M2-dependent glycolysis. J Agric Food Chem. 2017;65:8136–44. 10.1021/acs.jafc.7b02757. [DOI] [PubMed]
- 112.Feng J, Wu L, Ji J, Chen K, Yu Q, Zhang J, et al. PKM2 is the target of proanthocyanidin B2 during the inhibition of hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:204. 10.1186/s13046-019-1194-z. [DOI] [PMC free article] [PubMed]
- 113.Kwak TW, Park SB, Kim H-J, Jeong Y-I, Kang DH. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. Onco Targets Ther. 2016;10:137–144. doi: 10.2147/OTT.S112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen S-J, Yao X-D, Peng BO, Xu Y-F, Wang G-C, Huang J, et al. Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp Ther Med. 2016;11:1243–8. 10.3892/etm.2016.3050. [DOI] [PMC free article] [PubMed]
- 115.Bimonte S, Cascella M, Barbieri A, Arra C, Cuomo A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: an update of the current state of knowledge. Infect Agents Cancer. 2020;15:2. doi: 10.1186/s13027-020-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered. 2019;10:374–382. doi: 10.1080/21655979.2019.1657327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei R, Mao L, Xu P, Zheng X, Hackman RM, Mackenzie GG, et al. Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models. Food Funct. 2018;9:5682–96. 10.1039/c8fo01397g. [DOI] [PMC free article] [PubMed]
- 118.Aslan E, Guler C, Adem S. In vitro effects of some flavonoids and phenolic acids on human pyruvate kinase isoenzyme M2. J Enzyme Inhib Med Chem. 2016;31:314–317. doi: 10.3109/14756366.2015.1022173. [DOI] [PubMed] [Google Scholar]
- 119.Jia L, Huang S, Yin X, Zan Y, Guo Y, Han L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018;208:123–130. doi: 10.1016/j.lfs.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 120.Dihal AA, van der Woude H, Hendriksen PJM, Charif H, Dekker LJ, Ijsselstijn L, et al. Transcriptome and proteome profiling of colon mucosa from quercetin fed F344 rats point to tumor preventive mechanisms, increased mitochondrial fatty acid degradation and decreased glycolysis. Proteomics. 2008;8:45–61. 10.1002/pmic.200700364. [DOI] [PubMed]
- 121.Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–4306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 122.Zhao X, Zhu Y, Hu J, Jiang L, Li L, Jia S, et al. Shikonin inhibits tumor growth in mice by suppressing pyruvate kinase M2-mediated aerobic glycolysis. Sci Rep. 2018;8:1–8. 10.1038/s41598-018-31615-y. [DOI] [PMC free article] [PubMed]
- 123.Siu MKY, Jiang Y-X, Wang J-J, Leung THY, Han CY, Tsang BK, et al. Hexokinase 2 regulates ovarian cancer cell migration, invasion and stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 signaling cascades. Cancers (Basel). 2019. 10.3390/cancers11060813. [DOI] [PMC free article] [PubMed]
- 124.Yao J, Liu J, Zhao W. By blocking hexokinase-2 phosphorylation, limonin suppresses tumor glycolysis and induces cell apoptosis in hepatocellular carcinoma. Onco Targets Ther. 2018;11:3793–3803. doi: 10.2147/OTT.S165220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson M, Marayati R, Moffitt R, Yeh JJ. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget. 2017;8:56081–56094. doi: 10.18632/oncotarget.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Y, Zheng X, Lu J, Chen W, Li X, Zhao L. Ginsenoside 20(S)-Rg3 inhibits the Warburg effect via modulating DNMT3A/ MiR-532-3p/HK2 pathway in ovarian cancer cells. Cell Physiol Biochem. 2018;45:2548–2559. doi: 10.1159/000488273. [DOI] [PubMed] [Google Scholar]
- 127.Wu J, Hu L, Hu F, Zou L, He T. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget. 2017;8:32332–32344. doi: 10.18632/oncotarget.15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Logan IE, Miranda CL, Lowry MB, Maier CS, Stevens JF, Gombart AF. Antiproliferative and cytotoxic activity of xanthohumol and its non-estrogenic derivatives in colon and hepatocellular carcinoma cell lines. Int J Mol Sci. 2019;20:1203. 10.3390/ijms20051203. [DOI] [PMC free article] [PubMed]
- 129.Kunnimalaiyaan S, Sokolowski KM, Balamurugan M, Gamblin TC, Kunnimalaiyaan M. Xanthohumol inhibits Notch signaling and induces apoptosis in hepatocellular carcinoma. PLoS One. 2015;10:e0127464. doi: 10.1371/journal.pone.0127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang C-H, Sun T-L, Xiang D-X, Wei S-S, Li W-Q. Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (Humulus lupulus L.) Front Pharmacol. 2018;9:530. doi: 10.3389/fphar.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun Z, Zhou C, Liu F, Zhang W, Chen J, Pan Y, et al. Inhibition of breast cancer cell survival by xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol Lett. 2018;15:908–16. 10.3892/ol.2017.7434. [DOI] [PMC free article] [PubMed]
- 132.Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15:2497–2508. doi: 10.7150/ijbs.37481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keating E, Martel F. Antimetabolic effects of polyphenols in breast cancer cells: focus on glucose uptake and metabolism. Front Nutr. 2018;5:25. 10.3389/fnut.2018.00025. [DOI] [PMC free article] [PubMed]
- 134.Wu H, Pan L, Gao C, Xu H, Li Y, Zhang L, et al. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and AktmTOR pathway. Molecules. 2019;24:1993. 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed]
- 135.Menezes JCJMDS, Orlikova B, Morceau F, Diederich M. Natural and synthetic flavonoids: structure–activity relationship and chemotherapeutic potential for the treatment of leukemia. Crit Rev Food Sci Nutr. 2016;56:S4–S28. doi: 10.1080/10408398.2015.1074532. [DOI] [PubMed] [Google Scholar]
- 136.Deng X, Liu R, Li J, Li Z, Liu J, Xiong R, et al. Design, synthesis, and preliminary biological evaluation of 3′,4′,5′-trimethoxy flavonoid salicylate derivatives as potential anti-tumor agents. New J Chem. 2019;43:1874–84. 10.1039/C8NJ04533J.
- 137.Guo Y, Wei L, Zhou Y, Lu N, Tang X, Li Z, et al. Flavonoid GL-V9 induces apoptosis and inhibits glycolysis of breast cancer via disrupting GSK-3β-modulated mitochondrial binding of HKII. Free Radic Biol Med. 2020;146:119–29. 10.1016/j.freeradbiomed.2019.10.413. [DOI] [PubMed]
- 138.Zhou Y, Lu N, Qiao C, Ni T, Li Z, Yu B, et al. FV-429 induces apoptosis and inhibits glycolysis by inhibiting Akt-mediated phosphorylation of hexokinase II in MDA-MB-231 cells. Mol Carcinog. 2016;55:1317–28. 10.1002/mc.22374. [DOI] [PubMed]
- 139.Tao L, Wei L, Liu Y, Ding Y, Liu X, Zhang X, et al. Gen-27, a newly synthesized flavonoid, inhibits glycolysis and induces cell apoptosis via suppression of hexokinase II in human breast cancer cells. Biochem Pharmacol. 2017;125:12–25. 10.1016/j.bcp.2016.11.001. [DOI] [PubMed]
- 140.Li W, Hao J, Zhang L, Cheng Z, Deng X, Shu G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. J Agric Food Chem. 2017;65:5961–5972. doi: 10.1021/acs.jafc.7b02120. [DOI] [PubMed] [Google Scholar]
- 141.Li X, Gu J, Zhou Q. Review of aerobic glycolysis and its key enzymes – new targets for lung cancer therapy. Thorac Cancer. 2015;6:17–24. 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed]
- 142.Sreedhar A, Zhao Y. Dysregulated metabolic enzymes and metabolic reprogramming in cancer cells. Biomed Rep. 2018;8:3–10. doi: 10.3892/br.2017.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiang J, Zhou L, Zhuang Y, Zhang J, Sun Y, Li S, et al. Lactate dehydrogenase is correlated with clinical stage and grade and is downregulated by si-SAΤB1 in ovarian cancer. Oncol Rep. 2018;40:2788–97. 10.3892/or.2018.6658. [DOI] [PubMed]
- 144.Mazlaghaninia M, Atri MS, Seyedalipour B. Scopoletin and morin inhibit lactate dehydrogenase enzyme activity, which is critical for cancer metabolism. Hormozgan Med J. 2019; 10.5812/hmj.88269.
- 145.Bader A, Tuccinardi T, Granchi C, Martinelli A, Macchia M, Minutolo F, et al. Phenylpropanoids and flavonoids from Phlomis kurdica as inhibitors of human lactate dehydrogenase. Phytochemistry. 2015;116:262–8. 10.1016/j.phytochem.2015.03.007. [DOI] [PMC free article] [PubMed]
- 146.Ancey P-B, Contat C, Meylan E. Glucose transporters in cancer - from tumor cells to the tumor microenvironment. FEBS J. 2018;285:2926–2943. doi: 10.1111/febs.14577. [DOI] [PubMed] [Google Scholar]
- 147.Gonzalez-Menendez P, Hevia D, Rodriguez-Garcia A, Mayo JC, Sainz RM. Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology. 2014;155:3238–3250. doi: 10.1210/en.2014-1260. [DOI] [PubMed] [Google Scholar]
- 148.Melstrom LG, Salabat MR, Ding X-Z, Milam BM, Strouch M, Pelling JC, et al. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37:426–31. 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed]
- 149.Kim H-J, Jeon S-M, Lee M-K, Cho Y-Y, Kwon E-Y, Lee JH, et al. Comparison of hesperetin and its metabolites for cholesterol-lowering and antioxidative efficacy in hypercholesterolemic hamsters. J Med Food. 2010;13:808–14. 10.1089/jmf.2009.1320. [DOI] [PubMed]
- 150.Kim HK, Jeong T-S, Lee M-K, Park YB, Choi M-S. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin Chim Acta. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 151.Yang Y, Wolfram J, Boom K, Fang X, Shen H, Ferrari M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem Funct. 2013;31:374–9. 10.1002/cbf.2905. [DOI] [PMC free article] [PubMed]
- 152.Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24:2277. 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed]
- 153.Azevedo C, Correia-Branco A, Araújo JR, Guimarães JT, Keating E, Martel F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr Cancer. 2015;67:504–513. doi: 10.1080/01635581.2015.1002625. [DOI] [PubMed] [Google Scholar]
- 154.Moreira L, Araújo I, Costa T, Correia-Branco A, Faria A, Martel F, et al. Quercetin and epigallocatechin gallate inhibit glucose uptake and metabolism by breast cancer cells by an estrogen receptor-independent mechanism. Exp Cell Res. 2013;319:1784–95. 10.1016/j.yexcr.2013.05.001. [DOI] [PubMed]
- 155.Zhan T, Digel M, Küch E-M, Stremmel W, Füllekrug J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J Cell Biochem. 2011;112:849–859. doi: 10.1002/jcb.22984. [DOI] [PubMed] [Google Scholar]
- 156.Singh D, Arora R, Kaur P, Singh B, Mannan R, Arora S. Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci. 2017;7:62. 10.1186/s13578-017-0190-2. [DOI] [PMC free article] [PubMed]
- 157.Deng X, Peng Y, Zhao J, Lei X, Zheng X, Xie Z, Tang G (2020) Anticancer activity of natural flavonoids: inhibition of HIF-1α signaling pathway. Available online: https://www.ingentaconnect.com/contentone/ben/coc/2019/00000023/00000026/art00004 (accessed on 13 Apr 2020).
- 158.Bie B, Sun J, Guo Y, Li J, Jiang W, Yang J, et al. Baicalein: a review of its anti-cancer effects and mechanisms in hepatocellular carcinoma. Biomed Pharmacother. 2017;93:1285–91. 10.1016/j.biopha.2017.07.068. [DOI] [PubMed]
- 159.Dou J, Wang Z, Ma L, Peng B, Mao K, Li C, et al. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget. 2018;9:20089–102. 10.18632/oncotarget.24015. [DOI] [PMC free article] [PubMed]
- 160.Wang M, Qiu S, Qin J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway. Am J Transl Res. 2019;11:3341–3352. [PMC free article] [PubMed] [Google Scholar]
- 161.Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, et al. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep. 2015;33:457–63. 10.3892/or.2014.3550. [DOI] [PubMed]
- 162.Liu Y, Veena CK, Morgan JB, Mohammed KA, Jekabsons MB, Nagle DG, et al. Methylalpinumisoflavone inhibits hypoxia-inducible factor-1 (HIF-1) activation by simultaneously targeting multiple pathways. J Biol Chem. 2009;284:5859–68. 10.1074/jbc.M806744200. [DOI] [PMC free article] [PubMed]
- 163.Wei L, Zhou Y, Qiao C, Ni T, Li Z, You Q, et al. Oroxylin a inhibits glycolysis-dependent proliferation of human breast cancer via promoting SIRT3-mediated SOD2 transcription and HIF1α destabilization. Cell Death Dis. 2015;6:e1714. 10.1038/cddis.2015.86. [DOI] [PMC free article] [PubMed]
- 164.Jung K-H, Lee JH, Quach CHT, Paik J-Y, Oh H, Park JW, et al. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species–mediated hypoxia-inducible factor-1α activation. J Nucl Med. 2013;54:2161–7. 10.2967/jnumed.112.115436. [DOI] [PubMed]
- 165.Croteau E, Renaud JM, Richard MA, Ruddy TD, Bénard F, de Kemp RA. PET metabolic biomarkers for cancer. Biomark Cancer. 2016;8:61–69. doi: 10.4137/BIC.S27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Girinsky T, van der Maazen R, Specht L, Aleman B, Poortmans P, Lievens Y, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol. 2006;79:270–7. 10.1016/j.radonc.2006.05.015. [DOI] [PubMed]
- 167.Seshachalam A, Karpurmath SV, Rathnam K, Raman SG, Janarthinakani M, Prasad K, et al. Does interim PET scan after 2 cycles of ABVD predict outcome in Hodgkin lymphoma? Real-world evidence. J Glob Oncol. 2019;5:1–13. 10.1200/JGO.19.00179. [DOI] [PMC free article] [PubMed]
- 168.Shanmugam M, McBrayer SK, Rosen ST. Targeting the Warburg effect in hematological malignancies: from PET to therapy. Curr Opin Oncol. 2009;21:531–536. doi: 10.1097/CCO.0b013e32832f57ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17. 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed]
- 170.Miao Y, Zhang L-F, Guo R, Liang S, Zhang M, Shi S, et al. (18)F-FDG PET/CT for monitoring the response of breast cancer to miR-143-based therapeutics by targeting tumor glycolysis. Mol Ther Nucleic Acids. 2016;5:e357. 10.1038/mtna.2016.72. [DOI] [PMC free article] [PubMed]
- 171.Bardia A, Hurvitz S. Targeted therapy for premenopausal women with HR+, HER2- advanced breast cancer: focus on special considerations and latest advances. Clin Cancer Res. 2018;24:5206–5218. doi: 10.1158/1078-0432.CCR-18-0162. [DOI] [PubMed] [Google Scholar]
- 172.Golubnitschaja O. Feeling cold and other underestimated symptoms in breast cancer: anecdotes or individual profiles for advanced patient stratification? EPMA J. 2017;8:17–22. doi: 10.1007/s13167-017-0086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zubor P, Gondova A, Polivka J, Kasajova P, Konieczka K, Danko J, et al. Breast cancer and Flammer syndrome: any symptoms in common for prediction, prevention and personalised medical approach? EPMA J. 2017;8:129–40. 10.1007/s13167-017-0089-3. [DOI] [PMC free article] [PubMed]
- 174.Bubnov R, Polivka J, Zubor P, Konieczka K, Golubnitschaja O. “Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer Syndrome” relevance to address the question. EPMA J. 2017;8:141–157. doi: 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dyshlovoy SA, Pelageev DN, Hauschild J, Borisova KL, Kaune M, Krisp C, et al. Successful targeting of the Warburg effect in prostate cancer by glucoseconjugated 1,4-naphthoquinones. Cancers (Basel). 2019;11:1690. 10.3390/cancers11111690. [DOI] [PMC free article] [PubMed]
- 176.Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, et al. Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A. 2000;97:10990–5. 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed]
- 177.Smith MR, Manola J, Kaufman DS, George D, Oh WK, Mueller E, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101:1569–74. 10.1002/cncr.20493. [DOI] [PubMed]
- 178.Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: an emerging young adult and older adolescent challenge. Cancer. 2020;126:46–57. doi: 10.1002/cncr.32498. [DOI] [PubMed] [Google Scholar]
- 179.Gupta S, Gupta A, Saini AK, Majumder K, Sinha K, Chahal A. Prostate cancer: how young is too young? Curr Urol. 2017;9:212–215. doi: 10.1159/000447143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chunhua L, Zhao H, Zhao H, Lu Y, Wu J, Gao Z, et al. Clinical significance of peripheral blood PCA3 gene expression in early diagnosis of prostate cancer. Transl Oncol. 2018;11:628–32. 10.1016/j.tranon.2018.02.019. [DOI] [PMC free article] [PubMed]
- 181.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. doi: 10.1186/s40644-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang F-B, Chen R, Ren S-C, Shi X-L, Zhu Y-S, Zhang W, et al. Prostate cancer antigen 3 moderately improves diagnostic accuracy in Chinese patients undergoing first prostate biopsy. Asian J Androl. 2017;19:238–43. 10.4103/1008-682X.167715. [DOI] [PMC free article] [PubMed]
- 183.McGann S, Horton ER. Radium-223 dichloride: a novel treatment option for castration-resistant prostate cancer patients with symptomatic bone metastases. Ann Pharmacother. 2015;49:469–476. doi: 10.1177/1060028014565444. [DOI] [PubMed] [Google Scholar]
- 184.Schwartz L, Supuran CT, Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anti Cancer Agents Med Chem. 2017;17:164–170. doi: 10.2174/1871520616666161031143301. [DOI] [PubMed] [Google Scholar]
- 185.Danhier P, Bański P, Payen VL, Grasso D, Ippolito L, Sonveaux P, et al. Cancer metabolism in space and time: beyond the Warburg effect. Biochim Biophys Acta Bioenerg. 2017;1858:556–72. 10.1016/j.bbabio.2017.02.001. [DOI] [PubMed]
- 186.Konieczka K, Ritch R, Traverso CE, Kim DM, Kook MS, Gallino A, et al. Flammer syndrome. EPMA J. 2014;5:11. 10.1186/1878-5085-5-11. [DOI] [PMC free article] [PubMed]
- 187.Kunin A, Polivka J, Moiseeva N, Golubnitschaja O. “Dry mouth” and “Flammer” syndromes—neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018;9:307–317. doi: 10.1007/s13167-018-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Goncharenko V, Bubnov R, Polivka J, Zubor P, Biringer K, Bielik T, et al. Vaginal dryness: individualised patient profiles, risks and mitigating measures. EPMA J. 2019;10:73–9. 10.1007/s13167-019-00164-3. [DOI] [PMC free article] [PubMed]
- 189.Robinson AT, Fancher IS, Mahmoud AM, Phillips SA. Microvascular vasodilator plasticity after acute exercise. Exerc Sport Sci Rev. 2018;46:48–55. doi: 10.1249/JES.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hurley DM, Williams ER, Cross JM, Riedinger BR, Meyer RA, Abela GS, et al. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc. 2019;51:773–81. 10.1249/MSS.0000000000001854. [DOI] [PMC free article] [PubMed]
- 191.Kadlec AO, Barnes C, Durand MJ, Gutterman DD. Microvascular adaptations to exercise: protective effect of PGC-1 alpha. Am J Hypertens. 2018;31:240–246. doi: 10.1093/ajh/hpx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gat-Yablonski G, Pando R, Phillip M. Nutritional catch-up growth. World Rev Nutr Diet. 2013;106:83–89. doi: 10.1159/000342607. [DOI] [PubMed] [Google Scholar]
- 194.Golubnitschaja O, editor. Flammer syndrome: from phenotype to associated pathologies, prediction, prevention and personalisation. In: Advances in Predictive, Preventive and Personalised Medicine. Cham: Springer International Publishing; 2019. vol. 11.
- 195.Polivka J, Polivka J, Pesta M, Rohan V, Celedova L, Mahajani S, et al. Risks associated with the stroke predisposition at young age: facts and hypotheses in light of individualized predictive and preventive approach. EPMA J. 2019;10:81–99. 10.1007/s13167-019-00162-5. [DOI] [PMC free article] [PubMed]
- 196.Koklesova L, Liskova A, Samec M, Qaradakhi T, Zulli A, Smejkal K, et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020;11:261–87. 10.1007/s13167-020-00210-5. [DOI] [PMC free article] [PubMed]
- 197.Tidwell TR, Søreide K, Hagland HR. Aging, metabolism, and cancer development: from Peto’s paradox to the Warburg effect. Aging Dis. 2017;8:662–676. doi: 10.14336/AD.2017.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017;8:23–33. doi: 10.1007/s13167-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Golubnitschaja O, Flammer J. Individualised patient profile: clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018;9:15–20. doi: 10.1007/s13167-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 201.Frezza C, Pollard PJ, Gottlieb E. Inborn and acquired metabolic defects in cancer. J Mol Med. 2011;89:213–220. doi: 10.1007/s00109-011-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Golubnitschaja O, Yeghiazaryan K, Abraham J-A, Schild HH, Costigliola V, Debald M, et al. Breast cancer risk assessment: a non-invasive multiparametric approach to stratify patients by MMP-9 serum activity and RhoA expression patterns in circulating leucocytes. Amino Acids. 2017;49:273–81. 10.1007/s00726-016-2357-2. [DOI] [PubMed]
- 203.Cebioglu M, Schild HH, Golubnitschaja O. Cancer predisposition in diabetics: risk factors considered for predictive diagnostics and targeted preventive measures. EPMA J. 2010;1:130–137. doi: 10.1007/s13167-010-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luis C, Duarte F, Faria I, Jarak I, Oliveira PF, Alves MG, et al. Warburg effect inversion: adiposity shifts central primary metabolism in MCF-7 breast cancer cells. Life Sci. 2019;223:38–46. 10.1016/j.lfs.2019.03.016. [DOI] [PubMed]
- 205.Zhang G, Darshi M, Sharma K. The Warburg effect in diabetic kidney disease. Semin Nephrol. 2018;38:111–120. doi: 10.1016/j.semnephrol.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smolders VF, Zodda E, Quax PHA, Carini M, Barberà JA, Thomson TM, et al. Metabolic alterations in cardiopulmonary vascular dysfunction. Front Mol Biosci. 2019. 10.3389/fmolb.2018.00120. [DOI] [PMC free article] [PubMed]
- 207.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Investig. 2013;43:855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kudela E, Samec M, Kubatka P, Nachajova M, Laucekova Z, Liskova A, et al. Breast cancer in young women: status quo and advanced disease management by a predictive, preventive, and personalized approach. Cancers. 2019;11:1791. 10.3390/cancers11111791. [DOI] [PMC free article] [PubMed]
- 209.Polivka J, Altun I, Golubnitschaja O. Pregnancy-associated breast cancer: the risky status quo and new concepts of predictive medicine. EPMA J. 2018;9:1–13. doi: 10.1007/s13167-018-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Change the World - One article at a time | For Researchers | Springer Nature Available online: https://www.springernature.com/gp/researchers/campaigns/change-the-world?wt_mc=SocialMedia.Twitter.10.CON417.ctw2018_tw_shared_button&utm_medium=socialmedia&utm_source=twitter&utm_content=ctw2018_tw_shared_button&utm_campaign=10_dann_ctw2018_tw_shared_button (accessed on 9 Jun 2020).
- 211.Icard P, Shulman S, Farhat D, Steyaert J-M, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11. doi: 10.1016/j.drup.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 212.Golubnitschaja O, Sridhar KC. Liver metastatic disease: new concepts and biomarker panels to improve individual outcomes. Clin Exp Metastasis. 2016;33:743–755. doi: 10.1007/s10585-016-9816-8. [DOI] [PubMed] [Google Scholar]
- 213.Golubnitschaja O, Polivka J, Yeghiazaryan K, Berliner L. Liquid biopsy and multiparametric analysis in management of liver malignancies: new concepts of the patient stratification and prognostic approach. EPMA J. 2018;9:271–285. doi: 10.1007/s13167-018-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492. [DOI] [PubMed]
- 215.Basu AK. DNA damage, mutagenesis and cancer. Int J Mol Sci. 2018;19:970. 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed]
- 216.Lin J, Xia L, Liang J, Han Y, Wang H, Oyang L, et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J Exp Clin Cancer Res. 2019;38:218. 10.1186/s13046-019-1214-z. [DOI] [PMC free article] [PubMed]
- 217.Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24:650–654. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu A, Lee D, Shim H. Metabolic PET imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55–69. doi: 10.1053/j.seminoncol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jadvar H. PET of glucose metabolism and cellular proliferation in prostate cancer. J Nucl Med. 2016;57:25S–29S. doi: 10.2967/jnumed.115.170704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van Horssen R, Freire Jorge P, van Dam GM, Nijsten MW. The Warburg effect and its role in tumour metabolism: opportunities for new cancer treatments. Ned Tijdschr Geneeskd. 2016;160:A9489. [PubMed] [Google Scholar]
- 221.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5:198ra108. 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed]
- 222.Momcilovic M, Shackelford DB. Imaging cancer metabolism. Biomol Ther (Seoul) 2018;26:81–92. doi: 10.4062/biomolther.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rais J, Jafri A, Siddiqui S, Tripathi M, Arshad M. Phytochemicals in the treatment of ovarian cancer. Front Biosci (Elite Ed) 2017;9:67–75. doi: 10.2741/e786. [DOI] [PubMed] [Google Scholar]
- 224.Hua X, Yu L, You R, Yang Y, Liao J, Chen D, et al. Association among dietary flavonoids, flavonoid subclasses and ovarian cancer risk: a meta-analysis. PLoS One. 2016;11:e0151134. 10.1371/journal.pone.0151134. [DOI] [PMC free article] [PubMed]
- 225.Crane TE, Khulpateea BR, Alberts DS, Basen-Engquist K, Thomson CA. Dietary intake and ovarian cancer risk: a systematic review. Cancer Epidemiol Biomark Prev. 2014;23:255–273. doi: 10.1158/1055-9965.EPI-13-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen C, Huang S, Chen C-L, Su S-B, Fang D-D. Isoliquiritigenin inhibits ovarian cancer metastasis by reversing epithelial-to-mesenchymal transition. Molecules. 2019;24:3725. 10.3390/molecules24203725. [DOI] [PMC free article] [PubMed]
- 227.Huang S, Xie T, Liu W. Icariin inhibits the growth of human cervical cancer cells by inducing apoptosis and autophagy by targeting mTOR/PI3K/AKT signalling pathway. J Buon. 2019;24:990–996. [PubMed] [Google Scholar]
- 228.Zhao X, Guo X, Shen J, Hua D. Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of STAT3 signaling. Mol Med Rep. 2018;18:4030–4036. doi: 10.3892/mmr.2018.9420. [DOI] [PubMed] [Google Scholar]
- 229.Xia X, Xia J, Yang H, Li Y, Liu S, Cao Y, et al. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif Cells Nanomed Biotechnol. 2019;47:2729–36. 10.1080/21691401.2019.1636055. [DOI] [PubMed]
- 230.Wang P, Zhang J, Xiong X, Yuan W, Qin S, Cao W, et al. Icariin suppresses cell cycle transition and cell migration in ovarian cancer cells. Oncol Rep. 2019;41:2321–8. 10.3892/or.2019.6986. [DOI] [PubMed]
- 231.Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2020;10:1614. 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed]
- 232.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancers (Basel). 2018;11:28. 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed]
- 233.Kikuchi H, Yuan B, Hu X, Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am J Cancer Res. 2019;9:1517–1535. [PMC free article] [PubMed] [Google Scholar]
- 234.Chae H-S, Xu R, Won J-Y, Chin Y-W, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci. 2019;20:2420. 10.3390/ijms20102420. [DOI] [PMC free article] [PubMed]
- 235.Mirossay L, Varinská L, Mojžiš J. Antiangiogenic effect of flavonoids and chalcones: an update. Int J Mol Sci. 2017;19:27. 10.3390/ijms19010027. [DOI] [PMC free article] [PubMed]
- 236.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6:9. 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed]