Abstract
To find pesticidal lead compounds with high activity, a series of novel benzamides substituted with pyridine-linked 1,2,4-oxadiazole were designed by bioisosterism, and synthesized easily via esterification, cyanation, cyclization and aminolysis reactions. The structures of the target compounds were confirmed by 1H-NMR, 13C-NMR and HRMS. The preliminary bioassay showed that most compounds had good larvicidal activities against mosquito larvae at 10 mg/L, especially compound 7a, with a larvicidal activity as high as 100%, and even at 1 mg/L was still 40%; at 50 mg/L, all the target compounds showed good fungicidal activities against the eight tested fungi. Moreover, compound 7h exhibited better inhibitory activity (90.5%) than fluxapyroxad (63.6%) against Botrytis cinereal. Therefore, this type of compound can be further studied.
Keywords: amide compound; fungicidal activity; insecticidal activity; 1,2,4-oxadiazole; synthesis
1. Introduction
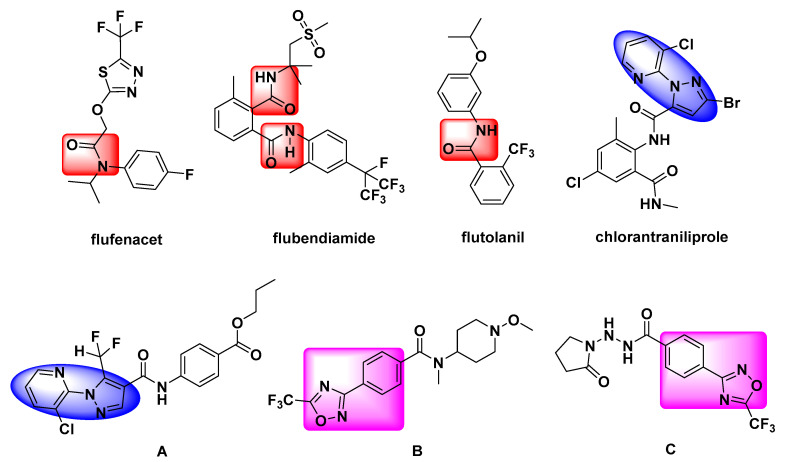
Recently, the demand for pesticides has been increasing with the improvement of people’s living standards. Many new kinds of pesticides with high efficiency, low toxicity and low residue have been developed. Heterocyclic derivatives received important attention due to their various biological activities [1,2,3,4,5,6]. Among these compounds, 1,2,4-oxadiazole heterocyclic derivatives, which contain nitrogen and oxygen atoms, displayed diverse activities, such as insecticidal activity [7,8,9], antifungal activity [10,11], herbicidal activity [12], anti-inflammatory [13], hypotensive [14] and other physiological activity. In addition, amide groups could easily generate hydrogen bonds with the activated part of the target enzymes and control the target organisms. Moreover, introducing the amide structure was beneficial for the biodegradation of pesticides. So, amide derivatives were wildly studied [15,16,17,18,19,20], such as the commercial herbicide flufenacet (Figure 1), insecticide flubendiamide (Figure 1) and fungicide flutolanil (Figure 1).
Figure 1.
Chemical structures of flufenacet, flubendiamide, flutolanil, chlorantraniliprole and compounds A, B, C.
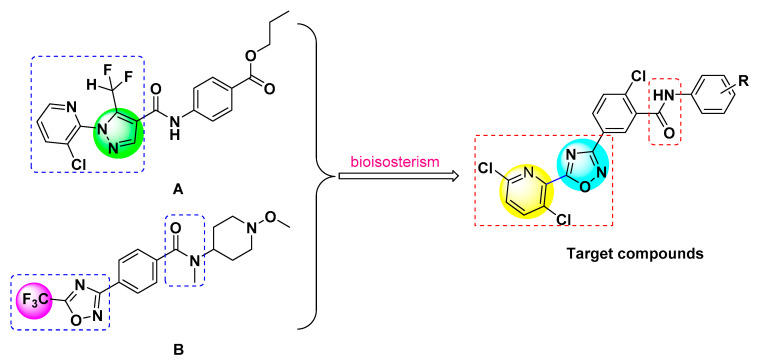
Amide compounds could be roughly classified as carboxyamides, mandelic acid amides and phenylamides according to their chemical structures. Through analyzing the reported amide compounds, it was found that when the carboxylic acid parts of the compounds include the structural units of pyridine-linked heterocycle, amide compounds usually have better insecticidal or fungicidal activities. Most of the compounds that have been reported include the structural units of pyridine-linked pyrazole, such as the commercial insecticide chlorantraniliprole (Figure 1) and the compound A (Figure 1, at 50 mg/L, its fungicidal activity against Botrytis cinerea was 80%) synthesized by Xu et al. [21]. However, there are few reports about pyridine-linked 1,2,4-oxadiazole. Moreover, Syngenta has reported a lot of 1,2,4-oxadiazole substituted benzamides such as compounds B (Figure 1) and C (Figure 1) that had good fungicidal activities [22,23]. In view of these facts mentioned above, to find pesticides with high biological activity, using A and B as lead compounds and replacing the structure of trifluoromethyl with the pyridine ring, a series of novel benzamides substituted with pyridine-linked 1,2,4-oxadiazole have been designed and synthesized according to bioisosterism (Figure 2). Target compounds were confirmed by 1H-NMR, 13C-NMR and HRMS. Their insecticidal and fungicidal activities were studied and the result showed that the target compounds had good insecticidal and fungicidal activities. The synthetic route of the target compounds is shown in Scheme 1.
Figure 2.
Design strategy of the target compounds.
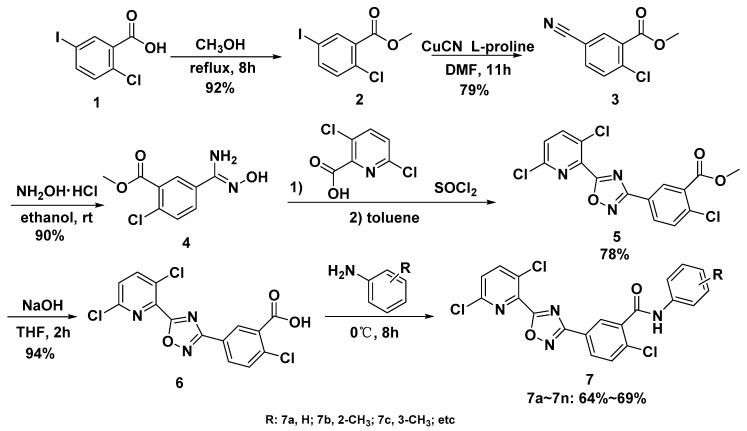
Scheme 1.
Synthetic route of target compound 7.
2. Results and Discussion
2.1. Synthesis of Target Compounds
The starting material 2-chloro-5-iodobenzoic acid 1 experienced esterification and cyanation reaction to give methyl 2-chloro-5-cyanobenzoate 3. Methyl 2-chloro-5-cyanobenzoate 3 then reacted with NH2OH•HCl under the alkaline condition to give amine oxime. This reaction could not be carried out for a long time, because amide byproducts would have formed easily. After this step, 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl) benzoate 5 was synthesized by cyclization reaction from compounds 4 with 3,6-dichloropicolinoyl chloride that had been synthesized from 3,6-dichloropicolinic acid. At last, the intermediate 5 went through hydrolysis reaction, and then reacted with substituted aniline to give the target compound 7.
In step 2, the iodine atom on the benzene ring being replaced by a cyano group is a typical nucleophilic substitution reaction. NaCN and KCN are two common nucleophilic reagents. In fact, these two reagents are highly toxic, so we chose CuCN, which has relatively low toxicity, as the cyanidation reagent to reduce the risk in the experiment and the harm to the environment. In addition, the process of our experiment was different from that reported in former papers [24,25]. Using small natural organic molecule l-proline as the catalyst, DMF as the solvent and under the condition where temperature gradually increased, the amount of by-product decreased and the yield of product 3 was the highest [26]. The influence of different experimental conditions on the yield of product 3 is shown in Table 1.
Table 1.
Effects of reaction conditions on the synthesis of the intermediate 3.
| Entry | Catalyst | Condition | Yield/% |
|---|---|---|---|
| 1 | no | 100 °C for 11 h | hardly react |
| 2 | l-proline | 100 °C for 11 h | 66 |
| 3 | l-proline | 70 °C for 2 h, further 100 °C for 9 h | 79 |
| 4 | l-proline | 70 °C for 3 h, further 100 °C for 7 h | 73 |
| 5 | l-proline | 80 °C for 2 h, further 100 °C for 9 h | 75 |
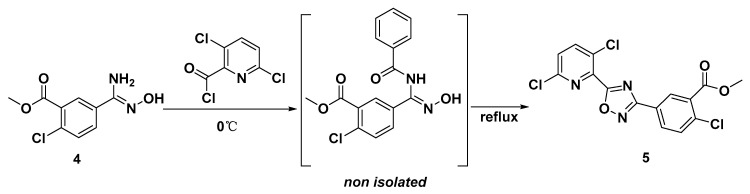
In step 4, the formation of 1,2,4-oxadiazole was achieved in a one-pot reaction. 3,6-dichloropicolinoyl chloride that was freshly prepared was dropped into the solution of methyl-2-chloro-5-(N′-hydroxycarbamimidoyl) benzoate 4 and triethylamine in toluene at 0 °C, to give the intermediate of methyl-2-chloro-5-(N-(3,6-dichloropicolinoyl)-N′-hydroxycarbamimidoyl)benzoate. Next, the intermediate was cyclized to produce 1,2,4-oxadiazole at reflux (Scheme 2). In this way, the self-cyclization of compound 4 was avoided because of the higher reactivity of acid chloride.
Scheme 2.
Reaction equation of step 4.
2.2. Spectrum Analysis of Target Compounds
The target compound 7a was taken as an example to conduct spectrum analysis. In the 1H-NMR spectra of compound 7a, the singlet at δ 10.09 ppm was the NH peak. The signals of benzene and pyridine rings were assigned at 8.37–7.04 ppm. In the 13C-NMR spectra of compound 7a, the C=O signal could be found at 171.75 ppm. The appearance of signals at 167.27 and 163.91 ppm was assigned to the carbons of the 1,2,4-oxadiazole ring. In the HRMS spectrogram, the calculated value of the ion peak of this compound was [M + Na]+ 466.9840, and the measured value was [M + Na]+ 466.9840. The absolute error was within 0.003.
2.3. Biological Activities of Target Compounds
The results of the insecticidal activity test of the target compounds are shown in Table 2 and Table 3. In Table 2, the death rates of compound 7 are all below 50% against mythimna sepatara, helicoverpa armigera and pyrausta nubilalis at 500 mg/L. Therefore, the insecticidal activities of this series of compounds were not good enough against the three targets. Nevertheless, we can see that compound 7 exhibited good larvicidal activity against mosquito larvae from Table 3. The larvicidal activities of compounds 7a and 7f were 100% at 10 mg/L. Furthermore, it was found that the larvicidal activity of compound 7a was 100% at 2 mg/L. Even at 1 mg/L, the larvicidal activity was still 40%. It exhibited better larvicidal potency than etoxazole against mosquito larvae. It can be seen from the compound 7h, 7i and 7j that the position of the substituent has little effect on the larvicidal activity of the target compound. Furthermore, as for compounds 7a to 7n, from the general trend in the larvicidal activity, it can be concluded that the less steric substitution attached to aniline may reduce the obstacles of the target compound binding to the target receptor and help to bring about an increase in activity.
Table 2.
Insecticidal activities of title compounds 7a–7n.
| Compounds | Insecticidal Activities (Death Rates %) | |||
|---|---|---|---|---|
| Concentration | Mythimna sepatara | Helicoverpa armigera | Pyrausta nubilalis | |
| (mg/L) | ||||
| 7a | 500 | 25 | 20 | 10 |
| 7b | 500 | 5 | 0 | 0 |
| 7c | 500 | 20 | 15 | 5 |
| 7d | 500 | 30 | 10 | 15 |
| 7e | 500 | 40 | 25 | 20 |
| 7f | 500 | 25 | 20 | 15 |
| 7g | 500 | 25 | 15 | 10 |
| 7h | 500 | 15 | 10 | 5 |
| 7i | 500 | 10 | 5 | 10 |
| 7j | 500 | 0 | 0 | 0 |
| 7k | 500 | 20 | 10 | 15 |
| 7l | 500 | 0 | 0 | 0 |
| 7m | 500 | 25 | 10 | 20 |
| 7n | 500 | 30 | 5 | 15 |
| Etoxazole | 500 | 100 | 100 | 100 |
Table 3.
Larvicidal activities of title compounds 7a–7n.
| Compounds | Larvicidal Activities (Death Rates %) | |
|---|---|---|
| Concentration | Mosquito Larvae | |
| (mg/L) | ||
| 7a | 10 | 100 |
| 5 | 100 | |
| 2 | 100 | |
| 1 | 40 | |
| 7b | 10 | 10 |
| 7c | 10 | 0 |
| 7d | 10 | 0 |
| 7e | 10 | 0 |
| 7f | 10 | 100 |
| 5 | 55 | |
| 7g | 10 | 5 |
| 7h | 10 | 20 |
| 7i | 10 | 15 |
| 7j | 10 | 25 |
| 7k | 10 | 30 |
| 7l | 10 | 0 |
| 7m | 10 | 45 |
| 7n | 10 | 25 |
| Etoxazole | 10 | 100 |
| 5 | 35 | |
The results of the fungicidal activities of target compounds are shown in Table 4. Overall, among the 14 new compounds, only compound 7h showed good fungidical activities (90.5%) against Botrytis cinereal, which was better than fluxapyroxad (63.6%). At the same time, it had moderate inhibitory activities against Alternaria solani (50.0%), Sclerotinia sclerotiorum (80.8%), and Thanatephorus cucumeris (84.8%). In addition, compounds 7d and 7e also showed good fungicidal activities against Botrytis cinereal (66.7%, 63.1%), which was comparable to fluxapyroxad. Most compounds had moderate inhibitory activities (40–81%) against Sclerotinia sclerotiorum, such as compounds 7b, 7e, 7h, 7i, 7j, 7l and 7n. For FusaHum graminearum, Phytophthora capsica and Fusarium oxysporum, fluxapyroxad had almost no activities, while compounds 7e, 7h, 7m had moderately strong inhibitory activities against FusaHum graminearum (40–45%), and the other two fungi had weak activity. From Table 5, we can see that compound 7h had good inhibitory activity against Sclerotinia sclerotiorum, Botrytis cinereal and Thanatephorus cucumeris, with EC50 of 11.61, 17.39 and 17.29 μg/mL, respectively. The SAR of the target compounds about fungicidal activities (Table 4) is that when the substituent of the benzene ring is 2-F, the inhibitory activities against the tested fungi are superior to other compounds.
Table 4.
Fungicidal activities of title compounds 7a–7n at 50 mg/L.
| Compounds | Fungicidal Activities (Inhibition Rate %) | |||||||
|---|---|---|---|---|---|---|---|---|
| AS | FG | CA | PC | SS | BC | TC | FO | |
| 7a | 44.4 | 36.1 | 6.7 | 8.3 | 30.8 | 33.3 | 50.0 | 4.5 |
| 7b | 16.7 | 27.8 | 6.7 | 25.0 | 46.2 | 19.0 | 30.3 | 4.5 |
| 7c | 16.7 | 27.8 | 6.7 | 25.0 | 30.8 | 33.3 | 27.3 | 4.5 |
| 7d | 27.8 | 36.1 | 13.3 | 8.3 | 30.8 | 66.7 | 40.9 | 22.7 |
| 7e | 30.8 | 40.4 | 6.7 | 28.7 | 41.4 | 63.1 | 44.5 | 31.6 |
| 7f | 11.1 | 22.2 | 18.2 | 33.3 | 15.4 | 28.6 | 15.2 | 13.6 |
| 7g | 33.3 | 25.0 | 33.3 | 8.3 | 15.4 | 23.8 | 50.0 | 9.1 |
| 7h | 50.0 | 44.4 | 40.0 | 8.3 | 80.8 | 90.5 | 84.8 | 22.7 |
| 7i | 27.8 | 36.1 | 13.3 | 16.7 | 42.3 | 47.6 | 25.8 | 18.2 |
| 7j | 27.8 | 22.2 | 6.7 | 16.7 | 73.1 | 38.1 | 54.5 | 22.7 |
| 7k | 11.1 | 30.6 | 13.3 | 8.3 | 15.4 | 23.8 | 15.2 | 18.2 |
| 7l | 16.7 | 5.6 | 13.3 | 16.7 | 46.2 | 33.3 | 22.7 | 18.2 |
| 7m | 38.9 | 41.7 | 26.7 | 25.0 | 38.5 | 38.1 | 60.6 | 13.6 |
| 7n | 22.2 | 22.2 | 6.7 | 8.3 | 46.2 | 9.5 | 22.7 | 9.1 |
| Fluxapyroxad | 88.9 | 30.3 | 100 | 38.1 | 96.4 | 63.6 | 88.4 | 44.4 |
Note: Alternaria solani (AS), FusaHum graminearum (FG), Cercospora arachidicola (CA), Phytophthora capsica (PC), Sclerotinia sclerotiorum (SS), Botrytis cinereal (BC), Thanatephorus cucumeris (TC), Fusarium oxysporum (FO). All the data were determined three times.
Table 5.
EC50 of compounds 7h to Sclerotinia sclerotiorum (SS), Botrytis cinereal (BC), Thanatephorus cucumeris (TC).
| Fungus | y = a + bx | r2 | EC50/(μg·mL−1) |
|---|---|---|---|
| SS | y = 1.5805x + 3.3168 | 0.9836 | 11.61 |
| BC | y = 2.1065x + 2.3871 | 0.9758 | 17.39 |
| TC | y = 1.8992x + 2.6489 | 0.9815 | 17.29 |
3. Experimental Section
3.1. General Information
Melting points were determined using an X-4 digital microscopic melting point detector (Taike, Beijing, China) and the thermometer was uncorrected. 1H-NMR and 13C-NMR spectra were measured on NMR spectrometer (Bruker 500 MHz, Fallanden, Switzerland); High-resolution electrospray mass spectra (HR–ESI–MS) were determined using an UPLC H-CLASS/QTOF G2-XS mass spectrometer (Waters, Milford, MA, USA). All the reagents and solvents were in analytical purity. The characterisation data for all synthesised compounds are provided in the Supporting Information file.
3.2. Synthesis
3.2.1. Methyl 2-chloro-5-iodobenzoate 2
2-chloro-5-iodobenzoic acid 1 (2.8 g, 0.01 mol), methanol (50 mL) and H2SO4 (0.5 mL) were added to a three-necked flask and reacted at reflux for about 8 h. After the mixture was cooled to room temperature, methanol was removed under reduced pressure. Then, EtOAc (50 mL) was added and the pH adjusted to 7–8 by using NaHCO3. The organic layer was dried with Na2SO4. The solvent was removed to give 2.71 g creamy white solid. Yield: 92%, m.p. 40~42 °C; 1H-NMR (500 MHz, Chloroform-d) δ 8.14 (d, J = 2.0 Hz, 1H), 7.72 (dd, J = 8.5, 2.5 Hz, 1H), 7.19 (d, J = 8.0 Hz, 1H), 3.94 (s, 3H).
3.2.2. Methyl 2-chloro-5-cyanobenzoate 3
Methyl 2-chloro-5-iodobenzoate 2 (0.40 g, 1.3 mmol), CuCN (0.18 g, 2.0 mmol), l-proline (0.15 g, 1.3 mmol) and DMF (15 mL) were added to a three-necked flask. After being dissolved, the mixture was heated to 70 °C and reacted for 2 h. Then, the temperature was heated to 100 °C. The reaction was complete after 9 h. After the mixture was cooled to room temperature, it was filtered with diatomite. The filtrate was extracted by water (100 mL) and EtOAc (100 mL). The organic layer was washed with water (50 mL × 3) and then dried with MgSO4. EtOAc was removed under reduced pressure and 0.20 g yellow solid was obtained. Yield: 79%, m.p. 102–104 °C ([27], 100–103 °C).
3.2.3. Methyl 2-chloro-5-(N′-hydroxycarbamimidoyl)benzoate 4
Methyl 2-chloro-5-cyanobenzoate 3 (1.4 g, 7.2 mmol) was added to a three-necked flask and dissolved by ethanol (45 mL). Stirring was started at room temperature, and then hydroxylamine hydrochloride (0.75 g) and triethylamine (1.1 g) were gradually added. The mixture was stirred for 3 h. Then the solvent was removed under reduced pressure and the remnant was dissolved in EtOAc (50 mL) and saturated saline (50 mL). The organic layer was dried with Na2SO4 and evaporated, to give 1.5 g light yellow solid. Yield: 90%, m.p. 108–111 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.87 (s, 1H), 8.13 (d, J = 2.0 Hz, 1H), 7.85 (dd, J = 8.5, 2.0 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 5.98 (s, 2H), 3.87 (s, 3H).
3.2.4. Methyl 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzoate 5
3,6-dichloropicolinic acid (1.1 g, 5 mmol) and SOCl2 (20 mL) were added to a round bottom flask. The mixture was stirred and refluxed for 2 h. Then, SOCl2 was removed under reduced pressure to give 3,6-dichloropicolinoyl chloride.
Intermediate 4 (1.1 g, 5 mmol), triethylamine (1.2 g, 5 mmol) and dry toluene (100 mL) were added to a three-necked flask. The mixture was stirred at 0 °C for 2 h. After that, the prepared 3,6-dichloropicolinoyl chloride (dissolved by 30 mL dry toluene) was dropped into the flask. The mixture continued to be stirred for 1 h at 0 °C. Then, the temperature was increased to reflux for 2 h. When the mixture was cooled to room temperature, it was washed by saturated sodium chloride solution (150 mL × 3). The organic layer was dried by Na2SO4 and removed under reduced pressure to give 1.5 g yellow solid. Yield: 78%, m.p. 148–152 °C; 1H-NMR (500 MHz, Chloroform-d) δ 8.65 (s, 1H), 8.32–8.16 (m, 1H), 7.92 (d, J = 8.5 Hz, 1H), 7.61 (d, J = 8.5 Hz, 1H), 7.54 (d, J = 8.5 Hz, 1H), 3.98 (s, 3H).
3.2.5. 2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzoic acid 6
Intermediate 5 (0.8 g, 2.0 mmol) and THF (40 mL) were added to a three-necked flask. After being dissolved, 30% NaOH (5 mL) was also added to the flask and refluxed for 2 h. After the mixture was cooled to room temperature, the solvent was removed. Then, the pH was adjusted to 2–3 with HCl and 0.7g white solid precipitate was obtained. Yield: 94%, m.p. 203–204 °C; 1H-NMR (500 MHz, DMSO-d6) δ 13.64 (s, 1H), 8.45 (d, J = 2.1 Hz, 1H), 8.34 (s, 1H), 8.26–8.17 (m, 1H), 7.91 (d, J = 8.6 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H).
3.2.6. Preparation of Target Compound 7
Intermediate 6 (4.1 g, 11 mmol), triethylamine (0.2 g), DCM (30 mL) and EDCI (0.3 g) were added to a three-necked flask. Substituted aniline (12 mmol) was stirred and dropped into the flask at 0 °C. TLC was used to track reaction progress. At last, target compound 7 was obtained by using the method of column chromatography separation.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-phenylbenzamide7a. Yellow solid, yield 64%, m.p. 234–238 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.70 (s, 1H), 8.36 (d, J = 8.5 Hz, 1H), 8.25–8.18 (m, 2H), 7.92 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 8.0 Hz, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.19–7.09 (m, 1H); 13C-NMR (500 MHz, DMSO-d6) δ 171.75, 167.27, 163.91, 148.60, 143.34, 139.71, 138.69, 137.84, 133.65, 131.28, 131.10, 129.52, 129.27, 128.88, 127.32, 124.77, 124.13, 119.78; HRMS calcd. for C20H11Cl3N4O2 [M + Na]+ 466.9840, found 466.9840.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(o-tolyl)benzamide7b. Yellow solid, yield 77%, m.p. 220–223 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.60 (s, 1H), 8.34 (d, J = 9.0 Hz, 1H), 8.21 (d, J = 7.0 Hz, 2H), 7.91 (d, J = 9.0 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.61 (d, J = 8.5Hz, 2H), 7.17 (d, J = 8.5 Hz, 2H), 2.29 (s, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.72, 167.26, 163.68, 148.59, 143.31, 139.68, 137.90, 136.19, 133.65, 133.12, 131.25, 131.07, 129.43, 129.24, 129.21, 127.31, 124.73, 119.76, 20.52; HRMS calcd. for C21H13Cl3N4O2 [M + Na]+ 480.9996, found 480.9995.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(m-tolyl)benzamide7c. Yellow solid, yield 74%, m.p. 260–261 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.69 (s, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.25–8.17 (m, 2H), 7.92 (d, J = 9.0 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.60 (s, 1H), 7.51 (d, J = 8.5 Hz, 1H), 7.25 (t, J = 8.0 Hz, 1H), 6.96 (d, J = 7.5 Hz, 1H), 2.32 (s, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.73, 167.27, 163.88, 148.60, 143.34, 139.70, 138.63, 138.10, 137.90, 133.65, 131.27, 131.08, 129.47, 129.27, 128.69, 127.30, 124.80, 124.74, 120.29, 117.01, 21.22; HRMS calcd. for C21H13Cl3N4O2 [M + Na]+ 480.9996, found 480.9998.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(p-tolyl)benzamide7d. White solid, yield 68%, m.p. 230–232 °C, 1H-NMR (500 MHz, DMSO-d6)δ 10.19 (s, 1H), 8.36 (d, J =10 Hz, 1H), 8.27 (d, J = 1.9 Hz, 1H), 8.24–8.18 (m, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 8.4 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 7.29 (d, J = 7.3 Hz, 1H), 7.25 (t, J = 7.3 Hz, 2H), 7.20–7.16 (m, 1H), 2.32 (s, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.76, 167.31, 164.23, 148.63, 143.34, 139.74, 137.98, 135.55, 133.66, 132.97, 131.28, 131.08, 129.43, 129.28, 127.40, 126.19, 126.12, 126.04, 124.74, 18.01; HRMS calcd. for C21H13Cl3N4O2 [M + Na]+ 480.9996, found 480.9995.
N-(4-(tert-butyl)phenyl)-2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide7e. Grey solid, yield 69%, m.p. 208–210 °C, 1H-NMR (500 MHz, DMSO-d6)δ 10.64 (s, 1H), 8.35 (d, J = 9.0 Hz,1H), 8.23–8.18 (m, 2H), 7.92 (d, J = 8.5 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.38 (d, J = 8.5 Hz, 2H), 1.28 (s, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.70, 167.25, 163.71, 148.58, 146.49, 143.31, 139.67, 137.91, 136.12, 133.65, 131.24, 131.05, 129.41, 129.24, 127.28, 126.44, 125.44, 124.71, 119.54, 34.09, 31.18; HRMS calcd. for C24H19Cl3N4O2 [M + Na]+ 523.0466, found 523.0466.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(3-(trifluoromethyl)phenyl)benzamide7f. White solid, yield 77%, m.p. 191–195 °C, H-NMR (500 MHz, DMSO-d6) δ 11.02 (s, 1H), 8.30 (d, J = 8.5 Hz, 1H), 8.25 (s, 1H), 8.19 (s, 2H), 7.87 (d, J = 8.5 Hz, 2H), 7.81 (d, J = 8.5 Hz, 1H), 7.58 (t, 1H), 7.45 (d, J = 7.5 Hz, 1H); 13C-NMR (500 MHz, DMSO-d6) δ 171.76, 167.24, 164.35, 148.61, 143.32, 139.67, 139.42, 137.30, 133.66, 131.27, 131.15, 130.19, 129.81, 129.26, 127.45, 124.84, 123.37, 120.48, 115.84; HRMS calcd. for C21H10Cl3F3N4O2 [M + Na]+ 534.9714, found 534.9717.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2,4-dimethylphenyl)benzamide7g. Yellow solid, yield 60%, m.p. 225–226 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.37 (d, J = 8.5 Hz, 1H), 8.25 (d, J = 2.0 Hz, 1H), 8.22 (d, J = 2.0 Hz,1H), 8.20 (d, J = 2.0 Hz, 1H), 7.94 (d, J = 8.5 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.09 (s, 1H), 7.04 (d, J = 8.0 Hz, 1H), 2.29 (s, 3H), 2.27 (s, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.76, 167.31, 164.23, 148.62, 143.34, 139.75, 138.05, 135.33, 133.65, 132.94, 132.83, 131.27, 131.07, 130.98, 129.37, 129.28, 127.38, 126.61, 125.99, 124.71, 20.56, 17.92; HRMS calcd. for C22H15Cl3N4O2 [M + Na]+ 495.0153, found 495.0154.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(2-fluorophenyl)benzamide7h. Yellow solid, yield 60%, m.p. 203–207 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.57 (s, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.27–8.19 (m, 2H), 7.92 (d, J = 8.5 Hz, 1H), 7.90–7.86 (m, 1H), 7.83 (d, J = 8.0 Hz, 1H), 7.35–7.21 (m, 3H); 13C-NMR (500 MHz, DMSO-d6) δ 171.78, 167.30, 164.41, 148.65, 143.34, 139.73, 137.35, 133.79, 131.29, 131.10, 129.65, 129.29, 127.54, 126.81 (d, J = 7.5 Hz), 125.70, 125.28, 125.19, 124.69, 124.51 (d, J = 3.0 Hz), 115.88 (d, J = 19.0 Hz); HRMS calcd. for C20H10Cl3FN4O2 [M + Na]+ 484.9746, found 484.9747.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(3-fluorophenyl)benzamide7i. Yellow solid, yield 62%, m.p. 228–230 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.35 (d, J = 9.0 Hz, 1H), 8.30–8.20 (m, 2H), 7.92 (d, J = 8.5 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.70 (d, J = 11.5 Hz, 1H), 7.53–7.36 (m, 2H), 7.03–6.92 (m, 1H); 13C-NMR (500 MHz, DMSO-d6) δ 171.76, 167.23, 164.15, 163.10, 161.18, 148.61, 143.33, 139.69, 137.46, 133.62, 131.21 (d, J = 16.0 Hz), 130.58 (d, J = 9.5 Hz), 129.72, 129.27, 127.37, 124.82, 115.55, 110.64 (d, J = 21.0 Hz), 106.68, 106.47; HRMS calcd. for C20H10Cl3FN4O2 [M + Na]+ 484.9746, found 484.9747.
2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)-N-(4-fluorophenyl)benzamide7j. Yellow solid, yield 63%, m.p. 248–251 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.77 (s, 1H), 8.36 (d, J = 8.5 Hz, 1H), 8.26–8.19 (m, 2H), 7.93 (d, J = 9.0 Hz,1H), 7.85 (d, J = 8.5 Hz, 1H), 7.79–7.72 (m, 2H), 7.22 (t, J = 9.0 Hz, 2H); 13C-NMR (500 MHz, DMSO-d6) δ 171.79, 167.29, 163.85, 148.64, 143.36, 139.73, 137.70, 133.68, 131.30, 131.16, 129.62, 129.30, 127.37, 124.82, 121.67 (d, J = 7.9 Hz), 115.61, 115.44; HRMS calcd. for C20H10Cl3FN4O2 [M + Na]+ 484.9746, found 484.9749.
2-chloro-N-(2-chlorophenyl)-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide7k. Yellow solid, yield 65%, m.p. 195–198 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.47 (s, 1H), 8.36 (d, J =9.0 Hz, 1H), 8.30 (s, 1H), 8.23 (d, J = 8.5 Hz, 1H), 7.93 (d, J =9.0 Hz, 1H), 7.84 (d, J = 8.5 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H); 13C-NMR (500 MHz, DMSO-d6) δ 171.76, 167.27, 164.43, 148.64, 143.34, 139.73, 137.27, 134.23, 133.81, 131.27, 131.15, 129.75, 129.66, 129.28, 128.60, 127.87, 127.70, 127.61, 127.58, 124.68; HRMS calcd. for C20H10Cl4N4O2 [M + Na]+ 500.9450, found 500.9452.
2-chloro-N-(3-chlorophenyl)-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide7l. Yellow solid, yield 65%, m.p. 214–218 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.90 (s, 1H), 8.35 (d, J = 9.0Hz, 1H), 8.26 (d, J = 2.0 Hz, 1H), 8.23 (dd, J = 8.5, 2.0 Hz, 1H), 7.96–7.90 (m, 2H), 7.85 (d, J = 8.5 Hz, 1H), 7.60 (d, J = 9.0 Hz, 1H), 7.41 (t, J = 8.5 Hz, 1H), 7.22 (s, 1H); 13C-NMR (126 MHz, DMSO-d6) δ 171.79, 167.27, 164.22, 148.64, 143.36, 140.09, 139.71, 133.67, 133.23, 131.31, 131.18, 130.66, 129.78, 129.30, 127.42, 124.86, 123.91, 119.30, 118.24; HRMS calcd. for C20H10Cl4N4O2 [M + Na]+ 500.9450, found 500.9454.
2-chloro-N-(4-chlorophenyl)-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide7m. Yellow solid, yield 67%, m.p. 242–243 °C. 1H-NMR (500 MHz, DMSO-d6) δ 10.85 (s, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.28–8.18 (m, 2H), 7.92 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 9.0 Hz, 2H), 7.44 (d, J = 9.0 Hz, 2H); 13C-NMR (126 MHz, DMSO-d6) δ 171.79, 167.28, 164.03, 148.64, 143.36, 139.72, 137.64, 137.57, 133.67, 131.31, 131.17, 129.71, 129.30, 128.84, 127.80, 127.39, 124.84, 121.39; HRMS calcd. for C20H10Cl4N4O2 [M + Na]+ 500.9450, found 500.9452.
N-(4-bromophenyl)-2-chloro-5-(5-(3,6-dichloropyridin-2-yl)-1,2,4-oxadiazol-3-yl)benzamide7n. Yellow solid, yield 69%, m.p. 242–245 °C, 1H-NMR (500 MHz, DMSO-d6) δ 10.86 (s, 1H), 8.35 (d, J = 9.0 Hz, 1H), 8.28–8.19 (m, 2H), 7.92 (d, J = 9.0 Hz, 1H), 7.85 (d, J = 8.5 Hz, 1H), 7.71 (d, J = 9.0 Hz, 2H), 7.57 (d, J = 8.5 Hz, 2H); 13C-NMR (126 MHz, DMSO-d6) δ 171.72, 167.22, 163.96, 148.59, 143.29, 139.65, 138.03, 137.52, 133.62, 131.68, 131.24, 131.10, 129.64, 129.24, 127.35, 124.78, 121.67; HRMS calcd. for C20H10BrCl3N4O2 [M + Na]+ 544.8945, found 544.8942.
3.3. Biological Activity Test
The insecticidal activity was tested according to [28]. The results of the activity test are shown in Table 2 and Table 3. The fungicidal activity of all the synthetic compounds were tested in vitro against eight fungi using a mycelia growth inhibition method according to references [29,30]. Alternaria solani (AS), FusaHum graminearum (FG), Cercospora arachidicola (CA), Phytophthora capsica (PC), Sclerotinia sclerotiorum (SS), Botrytis cinereal (BC), Thanatephorus cucumeris (TC), Fusarium oxysporum (FO) were provided by the National Pesticide Engineering Research Centre, Nankai University. The results of the activity test are shown in Table 4.
4. Conclusions
Novel benzamides substituted with pyridine-linked 1,2,4-oxadiazole were designed by bioisosterism, and synthesized easily via esterification, cyanation, cyclization and aminolysis reactions. Through using CuCN as the cyanidation reagent, l-proline as the catalyst, and increasing the temperature gradually in the cyanation reaction, we got the best yield (79%) and reduced the risk of this experiment. The structures of the target compounds were confirmed by 1H-NMR, 13C-NMR and HRMS. The preliminary bioassay results showed that most compounds had good larvicidal activity against mosquito larvae at 10 mg/L, especially compound 7a with excellent larvicidal activity (100%); even at 1 mg/L, the larvicidal actiity was still 40%; at 50 mg/L, all the target compounds showed good fungicidal activity against the eight tested fungi. Compound 7h exhibited good inhibitory activity (90.5%) against Botrytis cinereal, which was better than fluxapyroxad (63.6%). In addition, it had moderate inhibitory activities against Alternaria solani (50.0%), Sclerotinia sclerotiorum (80.8%) and Thanatephorus cucumeris (84.8%). Therefore, these compounds could potentially be the lead compounds for further optimisation.
Acknowledgments
This work was supported financially by the National Key R&D Program (No. 2017YFD0200507).
Supplementary Materials
The supplementary materials are available online.
Author Contributions
S.Y., X.-Y.T., T.-Y.M., L.D., C.-L.R., J.-C.M. carried out experimental work, S.Y. prepared the manuscript, C.-X.T. designed the material and supervised the project. X.-H.L. and C.-X.T. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program, grant number 2017YFD0200507.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Liu X.H., Yu W., Min L.J., Wedge D.E., Tan C.X., Weng J.Q., Wu H.K., Cantrell C.L., Bajsa-Hischel J., Hua X.W., et al. Synthesis and pesticidal activities of new quinoxalines. J. Agric. Food Chem. 2020 doi: 10.1021/acs.jafc.0c01042. [DOI] [PubMed] [Google Scholar]
- 2.Alnufaie R., Alsup N., Whitt J., Chambers A.S., Gilmore D., Alam A.M. Synthesis and antimicrobial studies of coumarin-substituted pyrazole derivatives as potent anti-staphylococcus aureus agents. Molecules. 2020;25:2758. doi: 10.3390/molecules25122758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu J.X., Shi Y.X., Yang M.Y., Sun Z.H., Liu X.H., Li B.J., Sun N.B. Design, Synthesis, DFT Study and Antifungal Activity of Pyrazolecarboxamide Derivatives. Molecules. 2016;21:68. doi: 10.3390/molecules21010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X.H., Qiao L., Zhai Z.W., Cai P.P., Cantrell C.L., Tan C.X., Weng J.Q., Han L., Wu H.K. Novel 4-Pyrazole Carboxamide Derivatives Containing Flexible Chain Motif: Design, Synthesis and Antifungal Activity. Pest Manag. Sci. 2019;75:2892–2900. doi: 10.1002/ps.5463. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Dai F.Y., Zhu J., Dong K.K., Wang Y.L., Chen T. Synthesis and antibacterial activities of pleuromutilin derivatives with thiazole-5-carboxamide and thioether moiety. J. Chem. Res. 2011;35:313–316. doi: 10.3184/174751911X13057375208346. [DOI] [Google Scholar]
- 6.Fu Q., Cai P.P., Cheng L., Zhong L.K., Tan C.X., Shen Z.H., Han L., Liu X.H. Synthesis and herbicidal activity of novel pyrazole aromatic ketone analogs as HPPD inhibitor. Pest Manag. Sci. 2020;76:868–879. doi: 10.1002/ps.5591. [DOI] [PubMed] [Google Scholar]
- 7.King W.F., Wheeler R.E. Substituted Oxadiazoles and Their Use as Corn Root Worm Insecticides. US4237121A. U.S. Patent. 1981 Dec 2;
- 8.Haugwitz R.D., Martinez A.J., Venslavsky J., Angel R.G., Maurer B.V., Jacobs G.A., Narayanan V.L., Cruthers L.R., Szanto J. Synyhesis and anthelmintic acyivities of novel isothiocyanatophenyl-1,2,4-oxadiazoles. J. Med. Chem. 1985;28:1234–1241. doi: 10.1021/jm00147a019. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q., Zhu R., Gao S., Ma S.H., Tang H.J., Diao Y.M., Wang H.L., Zhu H.J. Structure-based bioisosterism design, synthesis, insecticidal activity and structure-activity relationship (SAR) of anthranilic diamide analogues containing 1,2,4-oxadiazole rings. Pest Manag. Sci. 2017;73:917–924. doi: 10.1002/ps.4363. [DOI] [PubMed] [Google Scholar]
- 10.Terteryan-Seiser V., Grammenos W., Wiebe C., Kretschmer M., Craig I.R., Escribano C.A., Marcus F., Tobias M., Palomar M.A.Q., Grote T., et al. Substituted Oxadiazoles for Combating Phytopathogenic Fungi. WO2017178245A1. WO Patent. 2017 Oct 19;
- 11.Iwata J., Nakamura Y., Hayashi T., Watanabe S., Sano H. Oxadiazole Compound and Fungicide for Agricultural and Horticultural Use. WO2019022061A1. WO Patent. 2019 Jan 31;
- 12.Ryu E.K., Chung K.H., Lee W.H., Kim J.N., Hong K.S. Herbicidal Quinolinyloxadiazoles. WO9404530A1. WO Patent. 1994 Mar 3;
- 13.Nosalova G., Strapkova A., Korpas J. Studies of the antitussive effect of prenoxdiazine of experimentally induced cough. Bratisl. Lek. Listy. 1982;78:47–54. [PubMed] [Google Scholar]
- 14.Sakai K., Mizusawa H., Araki H., Higuchi M., Yoshikawa Y., Tomomatsu E., Okajima Y., Furukawa T., Sakanashi M., Atobe Y. Effects of 5[[2-(diethylamino)-ethyl]amino]-3-phenyl-1, 2, 4-oxadiazole dihydrochloride (DEPO) on cardiovascular system. Oyo Yakuri. 1979;18:667–672. [Google Scholar]
- 15.Zhao W., Shen Z.H., Xing J.H., Yang G., Xu T.M., Peng W.L., Liu X.H. Synthesis characterization nematocidal activity and docking study of novel pyrazole-4-carboxamide derivatives. Chin. J. Struct. Chem. 2017;36:423–428. [Google Scholar]
- 16.Hua X.W., Liu W.R., Su Y.Y., Liu X.H., Liu J.B., Liu N.N., Wang G.Q., Jiao X.Q., Fan X.Y., Xue C.M., et al. Studies on the Novel Pyridine Sulfide Containing SDH Based Heterocyclic Amide Fungicide. Pest Manag. Sci. 2020;76:2368–2378. doi: 10.1002/ps.5773. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W., Shen Z.H., Xing J.H., Xu T.M., Peng W.L., Liu X.H. Synthesis and nematocidal activity of novel pyrazole carboxamide derivatives against meloidogyne incognita. Lett. Drug Des. Discov. 2017;14:323–329. doi: 10.2174/1570180813666160930164327. [DOI] [Google Scholar]
- 18.Liu X.H., Zhao W., Shen Z.H., Xing J.H., Xu T.M., Peng W.L. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. Eur. J. Med. Chem. 2017;125:881–889. doi: 10.1016/j.ejmech.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Liu X.H., Wang Q., Sun Z.H., Wedge D.E., Becnel J.J., Estep A.S., Tan C.X., Weng J.Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 2017;73:953–959. doi: 10.1002/ps.4370. [DOI] [PubMed] [Google Scholar]
- 20.Vishnoi S., Agrawal V., Kasana V.K. Synthesis and structure-Activity relationships of substituted cinnamic acids and amide analogues: A new class of herbicides. J. Agric. Food Chem. 2009;57:3261–3265. doi: 10.1021/jf8034385. [DOI] [PubMed] [Google Scholar]
- 21.Ji W.J., Xu T.M., Zheng Z.W., Zhu B.C., Li J., Hu W.Q., Kong X.L. Synthesis and fungicidal activity of 1-(3-chloropyridin-2-yl)-5-difluorometyl-1H-pyrazole-4-carboxamide derivtives. Chin. J. Pestic. Sci. 2013;15:393–397. [Google Scholar]
- 22.Bou-Hamdan F., Stierli D., Jeanmart S.A.M., Godfrey C.R.A., Hoffman T.J., Beaudegnies R., Pouliot M. Oxadiazole Derivatives for Use as Pesticides and Fungicides and Their Preparation. WO2017174158A1. WO Patent. 2017 Oct 12;
- 23.Hoffman T.J., Stierli D., Beaudegnies R., Pouliot M., Pitterna T. Fungicidal Oxadiazole Derivatives. WO2018065414A1. WO Patent. 2018 Apr 12;
- 24.Schweizer E., Hoffmann-Roeder A., Olsen J.A., Seiler P., Obst-Sander U., Wagner B., Kansy M., Banner D.W., Diederich F. Multipolar interacetions in the D pocket of thrombin:large differences between tricyclic imide and lactam inhibitors. Org. Biomol. Chem. 2006;4:2364–2375. doi: 10.1039/B602585D. [DOI] [PubMed] [Google Scholar]
- 25.Hamann L.G., Manfredi M.C., Sun C.Q., Krystek S.R., Huang Y.T., Bi Y.Z., Augeri D.J., Wang T., Zou Y., Betebenner D.A., et al. Tandem optimization of target activity and elimination of mutagenic potential in a potent series of N-aryl bicyclic hydantoin-based selective androgen receptor modulators. Bioorg. Med. Chem. Lett. 2007;17:1860–1864. doi: 10.1016/j.bmcl.2007.01.076. [DOI] [PubMed] [Google Scholar]
- 26.Esvan Y.J., Zeinyeh W., Boibessot T., Nauton L., Thery V., Knapp S., Chaikuad A., Loaec N., Meijer L., Anizon F., et al. Discovery of pyrido [3,4-g]quinazoline derivatives as CMGC family protein kinase inhibitors: Design, synthesis, inhibitory potency and X-ray co-crystal structure. Eur. J. Med. Chem. 2016;118:170–177. doi: 10.1016/j.ejmech.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Patrick D.A., Bakunov S.A., Bakunova S.M., Kumar E.V.K.S., Lombardy R.J., Jones S.K., Bridges A.S., Zhirnov O., Hall J.E., Wenzler T., et al. Med. Chem. 2007;50:2468–2485. doi: 10.1021/jm0612867. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Zhu H.W., Shang J.F., Wang B.L., Li Z.M. Synthesis and Biological Activities of Novel 3-(((3-Bromo1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl)methylene)amino)-substituted-benzo [d] [1,2,3] triazin-4(3H)-ones. Chin. J. Org. Chem. 2019;39:861–866. doi: 10.6023/cjoc201808033. [DOI] [Google Scholar]
- 29.Zhai Z.W., Shi Y.X., Yang M.Y., Zhao W., Sun Z.H., Weng J.Q., Tan C.X., Liu X.H., Li B.J., Zhang Y.G. Microwave Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-triazolo [4,3-a] pyridine. Moiety. Lett. Drug Des. Discov. 2016;13:521–525. doi: 10.2174/157018081306160618181757. [DOI] [Google Scholar]
- 30.Chen W.T., Wang Q., Min L.J., Wu H.K., Weng J.Q., Tan C.X., Zhang Y.G., Hu B.Z., Liu X.H. Synthesis, Crystal Structure, Fungicidal Activity, Molecular Docking, and Density Functional Theory Study of 2-Chloro-N-(p-tolylcarbamoyl) nicotinamide. Indian J. Heterocycl. Chem. 2019;29:429–435. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.