Abstract
Background
The management of critically ill patients with coronavirus disease 2019 (COVID‐19), caused by a new human virus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is challenging. Recently, there have been several reports with inconsistent results after treatment with convalescent plasma (CP) on critically ill patients with COVID‐19, which was produced with a neutralizing antibody titer and tested in a P3 or P4 laboratory. However, due to the limitation of the conditions on mass production of plasma, most producers hardly had the capability to isolate the neutralizing antibody. Here, we report the clinical courses of three critically ill patients with COVID‐19 receiving CP treatments by total immunoglobulin G (IgG) titer collection.
Methods
Three patients with COVID‐19 in this study were laboratory confirmed to be positive for SARS‐CoV‐2, with radiographic and clinical features of pneumonia. CP was collected by total IgG titer of 160 (range, 200‐225 mL), and patients were transfused between 20 and 30 days after disease onset at the critical illness stage as a trial in addition to standard care. The clinical courses of these patients, including laboratory results and pulmonary functional and image studies after receiving convalescent plasma infusions, were reviewed.
Results
No therapeutic effect of CP was observed in any of the patients; instead, all three patients deteriorated and required extracorporeal membrane oxygenation treatment. A potential cytokine storm 4 hours after infusion of CP in Patient 2 was observed. No more patients were put on the trial of CP transfusion.
Conclusions
We recommend extreme caution in using CP in critically ill patients more than 2 weeks after the onset of COVID‐19 pneumonia.
Abbreviations
- COVID‐19
coronavirus disease 2019
- CP
convalescent plasma
- ECMO
extracorporeal membrane oxygenation
- IgG
immunoglobulin G
- IL
interleukin
- MODS
Multiple Organ Dysfunction Score
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TACO
transfusion‐associated with circulatory overload
- TNF‐α
tumor necrosis factor‐α
- TRALI
transfusion‐associated acute lung injury
1. INTRODUCTION
Since the report of coronavirus disease 2019 (COVID‐19) in Wuhan, China, in December 2019, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), in only several months (as of 28 April 2020), 3 123 357 people have been diagnosed to have confirmed disease, and over 210 000 deaths have resulted. 1 The spectrum of COVID‐19 illness can range from mild to severe, and about 6.1% of hospitalized patients require intensive care and/or use of mechanical ventilation or die from this illness. The reported death rate of COVID‐19 ranges from 1.4% to 15%. 1 , 2 Historically, when there is an emergence of a new infectious disease, passive antibody immunotherapy is always a treatment option. 3 The practice of taking blood or plasma from convalescent individuals to treat the communicable diseases including viral infections has been used for over a century. 4 However, the therapeutic efficiency of convalescent plasma (CP) in these settings is mixed. With the rapid outbreak and potential high mortality of COVID‐19, convalescent plasma infusion is thought to be beneficial and is highly recommended for severely or critically ill patients with COVID‐19 in China. 5 , 6 , 7 , 8 A report on 10 cases of CP therapy for severe COVID‐19 has shown the related positive results. The plasma in this study was collected following a titer of 1:640 for neutralizing antibody of SARS‐CoV‐2. The process of isolation and purification of neutralizing antibody for virus requires an extremely high biosafety level laboratory (P3 or P4) to verify the practical effect of antibody. However, these kinds of laboratories are limited and costly in most countries, which undoubtedly limits the production of plasma. Now the number of patients with COVID‐19 has reached over 3 million, and a mass production method is urgent. The collection of plasma followed by total immunoglobulin G (IgG) of convalescent individuals with COVID‐19 is recommended in the early compassionate use for severely or critically ill patients. 9 One publication with five patients and another with four patients who were critically ill with COVID‐19 showed the relative positive results on CP therapy. 10 , 11 However, a large randomized clinical trial showed that CP therapy led to no significant improvement for patients with severe or critical COVID‐19. 12 Here, we report on three critically ill patients with laboratory‐confirmed COVID‐19 pneumonia treated with convalescent plasma transfusion collected by total IgG titer. The patients were collected from three different hospitals in China, and all patients are male. Two of them were in Wuhan, and one was outside Hubei province. They were all diagnosed with severe or critical COVID‐19 pneumonia and received 200 to 225 mL of convalescent plasma. We observed a deteriorating clinical trend after treatment, including spiking cytokines after CP infusion. All patients were subsequently put on extracorporeal membrane oxygenation (ECMO) for life support. CP should be given to patients with COVID‐19 with extreme caution, particularly when given long after disease onset.
2. MATERIALS AND METHODS
2.1. Patients
All the clinical information of patients was collected and integrated by Wuhan Center Hospital. Written informed consents were obtained from all patients or their family members, and the study was approved by the institutional review board of the hospital. All the medical records of patients were supplied by the local hospitals, and all the patients have no smoking history.
2.2. Collection and transfusion of CP
CP was collected by a local certified blood center and distributed by the blood bank within each hospital. Donors were COVID‐19 survivors who had fully recovered and tested negative for the virus. All convalescent plasma products were manufactured based on protocols of plasma collection and production following publication of “The First Edition: The Procedure of Convalescent Plasma Treatment for New Coronavirus Patients” by the China National Health Commission. Details are described in supplemental materials. Briefly, the plasma was collected from COVID‐19 convalescent donors and frozen at −20°C rapidly or stored at 2‐6°C. Testing for common transfusion‐transmitted viral diseases such as hepatitis B and C, human immunodeficiency virus, syphilis, and alanine transaminase were also conducted. The gold immunochromatography assay or enzyme‐linked immunosorbent assay (ELISA) was recommended to select plasma products with a positive IgG antibody against SARS‐CoV‐2, with a titer of 160. The plasma transfusion was performed following the ABO blood compatibility principle and standard protocol for blood transfusion, including observing for any transfusion‐related adverse reactions.
2.3. Laboratory testing
Confirmation and negative testing of COVID‐19 by nucleic acid test was performed at a certified facility or tertiary care hospital following the standard World Health Organization protocol, as previously reported. 1 Routine laboratory tests such as complete blood count, coagulation, and chemistry were done in each hospital where patients were treated. Cytokines were tested by ELISA per standard protocol following the manufacturer's instruction. The kit was from Shanghai Jianglai Biology Inc. Samples from Patient 2 to test the cytokine level were obtained 4 hours before and after infusion of CP. Samples from Patient 3 were obtained 1 day before and 2 days after infusion.
The Multiple Organ Dysfunction Score (MODS) was calculated based on standards 13 listed in Table S1.
3. RESULTS
Patient 1 is a 42‐year‐old healthy male with no coexisting diseases, who was feeling unwell with fever of unknown etiology, dizziness, nausea, and vomiting since January 14, 2020. One week later, he was admitted to the hospital for pneumonia, and subsequently confirmed to be SARS‐CoV‐2 positive by nucleic acid test. He was given supportive care and put on mechanical ventilation as well as other standard supportive care. As his pneumonia worsened, he was intubated. He also developed azotemia, signaling further progression of the disease. The patient then received 200 mL CP on 15 February 2020 as an additional treatment option. His pulmonary condition declined sharply, as measured by oxygenation index and lung dynamic compliance (Figure 1), and so did his lymphocyte numbers (Tables 1 and 2). Worsening renal function parameters (blood urea nitrogen and creatinine) and coagulation (d‐dimer) were also noted after treatment (Table 2). Due to a deteriorating clinical course, a decision was made not to administer a second unit of CP. He remained intubated and respirator dependent and required repeated bronchoscopy for sputum suction and bronchoalveolar lavage to maintain sufficient oxygenation status. The coagulation disorders of this patient also exacerbated with notable ecchymosis of the skin (Figure S1 ). Computed tomography of his lungs showed extensive fibrosis (Figure S2). He was put on ECMO for life support on 25 February 2020, 10 days after receiving CP treatment. As of the date of our manuscript submission, this patient remained in respiratory and renal failure and still in extreme critical condition, with a MODS of 11, and on ECMO for life support.
FIGURE 1.
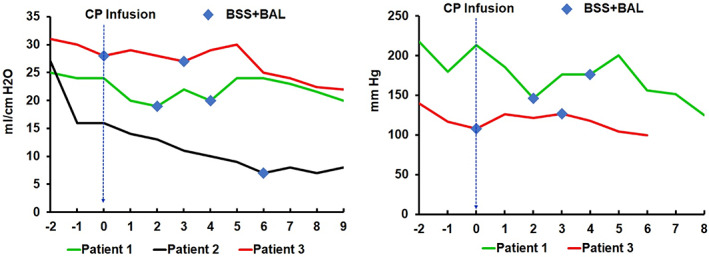
Changes in patients' pulmonary function peri‐transfusion of CP. The lung dynamic compliance (left panel) of three patients and oxygenation index of two patients (right panel) before and after convalescent plasma infusion are shown here. Day 0 indicates the day of convalescent plasma infusion; negative number denotes the day before the infusion and positive number denotes the day after the infusion. Indicates bronchoscopic sputum suction plus bronchoalveolar lavage [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Demographics and clinical characteristics
| Patient 1 | Patient 2 | Patient 3 | |||||
|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | ||||
| Age (years) | 42 | 56 | 58 | ||||
| COVID‐19 test | Nucleic acid test | Before | After | Before | After | Before | After |
| + | N/A | + | + | + | + | ||
| Characteristics | Clinical classification | Critical | Critical | Severe to critical | |||
| Coexisting chronic diseases | None | Hypertension | None | ||||
| Progression after plasma therapy | DD (+), APTT (+), OI (‐), Cdyn (‐), Ecchymosis, Anuria | DD (+), OI (‐), Cdyn (‐), Severe sepsis, BUN (+), NT‐pro‐BNP (+) | PT (+), OI (‐), Cdyn (‐), melena, GI bleeding, TB (+), PT (+), AST/ALT>1 | ||||
| Outcome | Multiple organ failure | Multiple organ failure | Multiple organ failure | ||||
| Treatment | Mechanical ventilation | + | + | + | + | + | + |
| Intubation | + | + | + | + | + | + | |
| ECMO | − | + | − | + | − | + | |
| BSS+BAL | − | + | − | + | − | + | |
| Complications | Coagulation disorders | + | + | − | DIC | − | + |
| Renal damages | Azotemia | RF | − | RF | − | − | |
| Heart damages | − | − | − | HF | − | − | |
| Liver damages | − | − | − | − | − | LF | |
| CRI | − | + | − | + | − | + | |
| Electrolytes imbalance | − | − | − | − | − | + | |
| SU | − | − | − | − | − | + | |
Note: All the results got from the tests within 2 weeks before and after infusion of CP. (+) Constant increase; (‐) Constant decrease; +, positive; ‐, negative.
Abbreviations: APTT, activated partial thromboplastin time; BSS+BAL, bronchoscope sputum suction plus bronchoalveolar lavage; BUN, blood urea nitrogen; Cdyn, lung dynamic compliance; CRI, catheter‐related bloodstream infection; DD, d‐dimer; DIC, disseminated intravascular coagulation; ECMO, extracorporeal membrane oxygenation; GI, gastrointestinal; HF, heart failure; LF, liver failure; N/A, not available; NT‐pro‐BNP, N‐terminal pro B‐type natriuretic peptide; OI, oxygenation index; PT, prothrombin time; RF, renal failure; SGU, stress ulcer; TB, total bilirubin.
TABLE 2.
Comparison of laboratory values before and after the treatment of convalescent plasma
| Patient 1 | Normal range | Before | After | |
|---|---|---|---|---|
|
Chemistry |
HCRP | 0.00‐5.00 mg/L | 27.90 | 64.64 |
| Urea | 1.70‐6.30, mmol/L | 8.32 | 13.40 | |
| CREA | 44.00‐115.00 μmol/L, | 183.00 | 217.00 | |
| GGT | 10.00‐60.00 U/L | 160.00 | 132 | |
|
Coagulation |
PTT | 27.00‐45.00 S | 47.50 | 49.4 |
| FIB | 2.00‐4.00 g/L | 5.01 | 5.74 | |
| DD | 0.00‐0.50 mg/L | 11.39 | 19.47 | |
|
Blood |
WBC | 3.50‐9.50 × 109/L | 12.37 | 15.61 |
| RBC | 4.30‐5.80 × 1012/L | 2.65 | 2.83 | |
| HGB | 130.00‐175.00 g/L | 79.00 | 87.00 | |
| HCT | 40.00‐50.00% | 24.70 | 26.30 | |
| PLT | 125.00‐350.00 × 109/L | 94.00 | 114.00 | |
| NEUT | 1.80‐6.30 × 109/L | 9.16 | 13.93 | |
| LYM | 1.10‐3.20 × 109/L | 2.22 | 0.62 |
| Patient 2 | Before | After | ||
|---|---|---|---|---|
|
Chemistry |
TP | 64.00‐83.00 g/L | 76.20 | 63.30 |
| PA | 200.00‐400.00 mg/L | 91.00 | 140.00 | |
| LDH | 135.00‐225.00, U/L | 659.00 | 423.00 | |
| NA | 136.00‐145.00 mmol/L | 134.60 | 145.10 | |
| Ca | 2.15‐2.50 mmol/L | 1.98 | 2.01 | |
| Urea | 3.10‐8.00 mmol/L | 11.40 | 9.20 | |
| CREA | 59.00‐104.00 μmol/L | 65.00 | 55.00 | |
| UA | 202.30‐416.50 μmol/L | 237.00 | 87.00 | |
| CHE | 5320.00‐12920.00 U/L | 4519.00 | 4192.00 | |
|
Blood |
WBC | 3.50‐9.50 × 109/L | 23.94 | 7.16 |
| RBC | 4.30‐5.80 × 1012/L | 3.36 | 2.74 | |
| HGB | 130.00‐175.00 g/L, | 104.00 | 84.00 | |
| HCT | 40.00‐50.00% | 31.20 | 24.50 | |
| PLT | 125.00‐350.00 × 109/L | 118.00 | 96.00 | |
| NEUT | 1.80‐6.30 × 109/L | 22.94 | 6.48 | |
| LYM, | 1.10‐3.20109/L | 0.45 | 0.38 |
| Patient 3 | Before | After | ||
|---|---|---|---|---|
|
Blood |
WBC | 3.50‐9.50 × 109/L | 5.58 | 11.68 |
| RBC | 4.30‐5.80 × 1012/L | 4.70 | 3.39 | |
| HGB | 130.00‐175.00 g/L | 142.00 | 104.00 | |
| HCT, | 40.00‐50.00% | 45.60 | 32.9 | |
| NEUT | 1.80‐6.30 × 109/L | 5.23 | 10.95 | |
| LYM | 1.10‐3.20 × 109/ L | 0.19 | 0.48 | |
|
Coagulation |
PT | 9.40‐12.50 S | 12.90 | 13.10 |
| INR | 0.79‐1.15 | 1.17 | 1.19 | |
| DD | 0.00‐243.00 ng/L | 4604 | 3777 | |
| FDP | ug/mL | 30.64 | 24.33 |
Note: All the results got from the tests before and after 1 day of the infusion of CP, except cytokine results from 4 hours before and after CP transfusion.
Abbreviations: ALT, alanine transaminase; Ca, calcium; CHE, cholinesterase; CP, convalescent plasma; CREA, creatinine; DD, d‐dimer; FDP, fibrin degradation product; FIB, fibrinogen; GGT, gamma‐glutamyl transferase; HCRP, human C‐reactive protein; HCT, hematocrit; HGB, hemoglobin; LDH, lactate dehydrogenase; LYM, lymphocyte; NA, natrium; NEUT, neutrophil cell; PA, prealbumin; PLT, platelet; PTT, partial thromboplastin time; RBC, red blood cell; TP, total protein; UA, uric acid; WBC, white blood cell.
Patient 2 is a 56‐year‐old male with a history of hypertension who was admitted to the hospital due to fever and cough on 23 January 2020. He was diagnosed with SARS‐CoV‐2 viral pneumonia based on a positive nucleic acid test and radiographic findings of the lungs. As with Patient 1, he was given supportive care including mechanical ventilation and, with progression of his respiratory failure, intubation. Given his critically ill status, 225 mL of CP was given to this patient on 11 February 2020. There was a significant decline in the patient's pulmonary function indicated by a drop of lung dynamic compliance. Because of the unexpected deteriorating event after CP infusion, blood samples were drawn and tested for cytokines. Within 4 hours of CP infusion, a significant cytokine spike was detected, with increased levels of interleukin (IL)‐2R, IL‐6, IL‐8, IL‐10, and tumor necrosis factor‐α (TNF‐α). Among these parameters, IL‐6 increased more than 20‐fold (Table S2). The patient was put on ECMO and remained in extremely critical condition. Among the three patients, he had the worst pulmonary index based on the lung dynamic compliance (Figure 1). The application of bronchoscopic sputum suction plus bronchoalveolar lavage seemed to relieve the respiratory syndrome briefly. His progression in pulmonary decompensation was also supported by the radiographic findings (Figure S3). Since then, he has developed multiple organ failure syndromes including renal, cardiac, and hepatic failure; line infection; and disseminated intravascular coagulopathy (Tables 1 and 2), with a MODS of 14 and on ECMO for life support.
Patient 3, a 58‐year‐old previously healthy male, was diagnosed with COVID‐19 based on a positive nucleic acid test and respiratory symptoms, such as coughing, on 27 January 2020. The patient's course was marked by a gradual respiratory decline, his clinical status changed from severe to critical, and was intubated on a respirator. On 17 February, the patient was infused with 200 mL of CP. No significant changes were noted immediately after the treatment, although the patient required bronchoscope sputum suction and bronchoalveolar lavage shortly afterwards (Figure 1). Two days after the transfusion, however, the condition of patient took a sudden turn for the worse. He had a rapid decline in his pulmonary status and was put on ECMO. Additional bronchoscopic sputum suction and bronchoalveolar lavage helped to stabilize his respiratory status (Figure 1). However, he started to show signs of multiple organ failure in addition to severe respiratory fibrosis (Table S2 and Figure S4), such as coagulopathy, liver failure, electrolyte disturbances, and a stress gastric ulcer (Tables 1 and 2), with a MODS of 14. Like Patient 2, a blood sample 72 hours after CP transfusion was sent for cytokine testing, and increased IL‐8 and IL‐10 levels were observed (Table S2).
The timeline relative to symptom development, hospital admission, intubation, and decision for transfusion of CCP among the three patients is shown in Table S3.
4. DISCUSSION
It is understandable that during an acute phase of an outbreak of a highly contagious viral disease such as COVID‐19, when mortality has been reported to be up to 15% in some cases, 2 and the only standard care option is supportive care, a variety of investigational approaches will be used. Convalescent plasma, a form of passive antibody immunotherapy, was used in Ebola 14 , 15 , 16 , 17 and the H1N1 2009 virus outbreak. 18 It is therefore not surprising that convalescent plasma has drawn the attention of many people and been placed with great expectations on treating COVID‐19, and now highly recommended to treat critically ill patients with COVID‐19. To our knowledge, we are the first to report limited therapeutic effects of CP collected with a relatively low total IgG titer without P3 or P4 lab production in patients with COVID‐19 pneumonia. Not only is there no therapeutic effect on COVID‐19 pneumonia from CP treatment, but patients may have a worse outcome after the treatment (Figure S5).
CP is a “crude” source of passive antibody or immune therapy (also known as serum therapy) and contains strain‐specific, polyclonal antibodies from survivor donors who have recovered from infections with the same disease as recipients. 3 , 4 , 19 , 20 One of the main therapeutic components within a CP product is pathogen‐specific neutralizing antibodies. One of the main reasons why CP did not seem to work or improve survival of patients infected with Ebola is the lack of measurement of Ebola virus–neutralizing antibodies within the products because of the limits of health care facilities. 18 In contrast, CP with a neutralizing antibody titer of more than 160 was shown to be effective in reducing mortality in patients with H1N1 2009 infection late after disease onset. 18 Due to the variable practice in collection and administration of CP, it is not always effective in treating infections with viral diseases. 13 , 14 , 15 , 16 , 17 In the three patients we report here, neutralizing antibody titer to SARS‐CoV‐2 was not available since the collection of COVID‐19 CP is based on total viral IgG antibody titer.
Patient 2 had a significant spike in cytokines measured 4 hours after infusion of 225 mL of CP. Due to technical limitations, viral titers from this patient before and after convalescent plasma treatment were not available. However, cytokines such as IL‐6, IL‐10, and TNF‐α have been demonstrated to correlate well with viral titers after convalescent plasma infusion in H1N1 infection, with high serum cytokine levels correlated with high viral load. 17 The spike of cytokines in Patient 2 could be due to increased viral activity. Clinically, we observed a decrease in lung dynamic compliance. Transfusion‐associated acute lung injury (TRALI) or transfusion‐associated with circulatory overload (TACO) could be other possibilities. 3 However, the posttreatment cytokine results do not support these two diagnoses. In TRALI or TACO, not all four cytokines, IL‐6, IL‐8, IL‐10, and TNF‐α, increase after plasma infusion.20
The cytokine results 72 hours after CP transfusion of Patient 3 also showed increased levels. Patient 1 was not tested for viral loads or cytokines before and after convalescent plasma transfusion. However, his oxygenation index decreased sharply after the treatment, as did his lung dynamic compliance. Further studies should be conducted to evaluate the biologic effects of antibodies isolated from CP on SARS‐COV‐2 viral function(s). Until then, our real‐world experiences based on these three patients does not support administering CP to severely or critically ill patients with COVID‐19 more than 2 weeks after the onset of disease.
Our study has limitations. Due to the urgency of the epidemic, the numbers and information of patients in this study are limited. Our report is limited by CP infusions that occurred long after the onset of disease, which is also noted in a recent randomized controlled trial with negative results on this topic as well. More rationally designed large‐scale randomized controlled trials will be essential to determine the efficacy and safety of CP in COVID‐19 in the future.
CONFLICT OF INTEREST
None of the material has been published or is under consideration for publication elsewhere. We have no conflict of interest that might influence our results or their interpretation.
AUTHOR CONTRIBUTIONS
M.L., Z.C., and M.D. designed the research, collected the information of patients, and wrote the manuscript; J.‐h.Y., X.‐b.C. participated in collecting patient information and designing the research; D.C., H.Y., X.G., Y.L., L.Y., M.‐l.Z., and X.W. participated in collection of information; J.Y. and C.G. participated in manuscript writing; D.G.T., L.C., and F.A. participated in manuscript preparation and writing. All authors participated in data interpretation and critical review of the final paper and submission.
Supporting information
Figures
Figure S1 Purpura and ecchymosis of skin observed in Patient 1 after receiving convalescent plasma transfusion.
Figure S2 Comparison of pulmonary computed tomography (CT) image before (8 days) and after (7 days) convalescent infusion in Patient 1
Figure S3 Pulmonary computed tomography (CT) image and chest radiograph before (16 days, left) and after (5 days, right) convalescent plasma infusion in Patient 2
Figure S4 Comparison of pulmonary computed tomography (CT) image before (13 days, left) and after (7 days, right) convalescent plasma infusion in Patient 3
Figure S5 Multiple Organ Dysfunction Score (MODS) changes of three patients pre‐ and post‐ convalescent plasma transfusion
Table S1 Multiple organ dysfunction syndrome score system
Table S2 Computed tomography (CT) of the lungs from patient 3 before (13 days) and after (7 days) convalescent plasma transfusion
Liu M, Chen Z, Dai M‐Y, et al. Lessons learned from early compassionate use of convalescent plasma on critically ill patients with Covid‐19 . Transfusion. 2020;60:2210–2216. 10.1111/trf.15975
Funding information This study was supported Health and Family Planning Commission of Wuhan Municipality (WX18A07); by the National Institutes of Health (NIH) T32 HL066987 (M.L.); by the Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award MOH‐STaR18nov‐0002 (D.G.T.); as well as NIH/NHLBI Grant P01HL095489 and Xiu Research Fund (L.C.)
Contributor Information
Li Chai, Email: lchai@bwh.harvard.edu.
Fen Ai, Email: aifen_1022@163.com.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leider JP, Brunker PA, Ness PM. Convalescent transfusion for pandemic influenza: preparing blood banks for a new plasma product? Transfusion. 2010;50:1384–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. [DOI] [PubMed] [Google Scholar]
- 5. Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q, Wang Y, Qi C, Shen L, Li J. Clinical trial analysis of 2019‐nCoV therapy registered in China. J Med Virol. 2020;92(6):540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li XY, Du B, Wang YS, et al. [The c/1keypoints in treatment of the critical coronavirus disease 2019 patient]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E026. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Wang YM, Xu JY, Cao B. [Potential antiviral therapeutics for 2019 Novel Coronavirus]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E002. [DOI] [PubMed] [Google Scholar]
- 9. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323:1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;S0012‐3692(20):30571–30577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA. 2020;3:e2010044. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang C, Su Q, Zhang SW, Yin CH, Wang H, Wang BE. [Scoring system to measure the severity of the multiple organ dysfunction syndrome]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:497–500. [PubMed] [Google Scholar]
- 14. van Griensven J, Edwards T, Baize S, Ebola‐Tx C. Efficacy of convalescent plasma in relation to dose of ebola virus antibodies. N Engl J Med. 2016;375:2307–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Griensven J, Edwards T, Gallian P, Ebola‐Tx C. Convalescent plasma for ebola virus disease. N Engl J Med. 2016;374:2500. [DOI] [PubMed] [Google Scholar]
- 17. van Griensven J, Gallian P, de Lamballerie X. Convalescent plasma and the dose of Ebola VIRUS ANTIBODIES. N Engl J Med. 2017;376:1297. [DOI] [PubMed] [Google Scholar]
- 18. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casadevall A, Pirofski LA. New concepts in antibody‐mediated immunity. Infect Immun. 2004;72(11):6191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures
Figure S1 Purpura and ecchymosis of skin observed in Patient 1 after receiving convalescent plasma transfusion.
Figure S2 Comparison of pulmonary computed tomography (CT) image before (8 days) and after (7 days) convalescent infusion in Patient 1
Figure S3 Pulmonary computed tomography (CT) image and chest radiograph before (16 days, left) and after (5 days, right) convalescent plasma infusion in Patient 2
Figure S4 Comparison of pulmonary computed tomography (CT) image before (13 days, left) and after (7 days, right) convalescent plasma infusion in Patient 3
Figure S5 Multiple Organ Dysfunction Score (MODS) changes of three patients pre‐ and post‐ convalescent plasma transfusion
Table S1 Multiple organ dysfunction syndrome score system
Table S2 Computed tomography (CT) of the lungs from patient 3 before (13 days) and after (7 days) convalescent plasma transfusion


