ABSTRACT
The evolutionarily conserved lethal-7 (let-7) microRNAs (miRNAs) are well-known activators of proliferative quiescence and terminal differentiation. However, in the murine auditory organ, let-7g overexpression delays the differentiation of mechano-sensory hair cells (HCs). To address whether the role of let-7 in auditory-sensory differentiation is conserved among vertebrates, we manipulated let-7 levels within the chicken auditory organ: the basilar papilla. Using a let-7 sponge construct to sequester let-7 miRNAs, we found that endogenous let-7 miRNAs are essential for limiting the self-renewal of HC progenitor cells. Furthermore, let-7b overexpression experiments revealed that, similar to mice, higher than normal let-7 levels slow/delay HC differentiation. Finally, we identify CHD7, a chromatin remodeler, as a candidate for mediating the repressive function of let-7 in HC differentiation and inner ear morphogenesis. Our analysis uncovered an evolutionarily conserved let-7-5p-binding site within the chicken Chd7 gene and its human and murine homologs, and we show that let-7g overexpression in mice limits CHD7 expression in the developing inner ear, retina and brain. Haploinsufficiency of CHD7 in humans causes CHARGE syndrome and attenuation of let-7 function may be an effective method for treating CHD7 deficiency.
KEY WORDS: let-7 miRNA, CHD7, Hair cell, Inner ear, Auditory, Differentiation
Summary: CHD7 is an evolutionarily conserved let-7 miRNA target and its deficiency is linked to the repressive function of let-7 in auditory-sensory progenitor self-renewal and hair cell differentiation in birds and mice.
INTRODUCTION
The inner ear auditory organ contains an array of highly specialized mechano-sensory cells, termed hair cells (HCs), that are crucial for our ability to detect sound. Auditory HCs and their surrounding supporting cells (SCs) derive from a common pool of progenitor cells, referred to as pro-sensory cells (Fekete et al., 1998). The high-mobility group transcription factor SOX2 is an evolutionarily conserved master regulator of inner ear sensory development. Inner ear-specific ablation of SOX2 abolishes the formation of pro-sensory cells within the auditory and vestibular regions of the inner ear (Kiernan et al., 2005; Pan et al., 2013; Neves et al., 2011). Moreover, SOX2 is crucial for the transcriptional activation of Atoh1 in the developing inner ear (Neves et al., 2012; Ahmed et al., 2012; Kempfle et al., 2016). ATOH1, a basic helix-loop-helix transcription factor, is the earliest marker of nascent HCs and is both necessary and sufficient for the formation of both auditory and vestibular HCs (Bermingham et al., 1999; Zheng and Gao, 2000; Chen et al., 2002).
In the murine auditory organ, termed cochlea, terminal mitosis and differentiation of pro-sensory cells follow steep longitudinal gradients that initiate at opposing ends of the sensory epithelium. The timing of these seemingly uncoupled events is controlled by, among others, the let-7 family of microRNAs (miRNAs) and the RNA-binding protein (RBP) LIN28B (Golden et al., 2015). let-7 (lethal-7) and LIN-28 were initially discovered in C. elegans as members of a cascade of genes that regulate the timing of developmental events (heterochrony) (Ambros and Horvitz, 1984; Reinhart et al., 2000). Vertebrates have multiple let-7 isoforms and to date nine mature let-7 miRNA isoforms (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i and mir-98) have been identified in humans and mice (reviewed by Roush and Slack, 2008). As for most miRNAs, the primary transcript of let-7 miRNAs is processed by the RNase III enzymes Drosha and Dicer into a 22-nucleotide miRNA duplex, of which one strand, termed the lead strand (5-p), is incorporated into the miRNA-induced silencing effector complex (miRISC) (reviewed by Yates et al., 2013). The let-7a-5p sequence of UGAGGUAGUAGGUUGUAUAGUU is identical across all animal species, and other let-7-5p isoforms differ from the let-7a-5p consensus sequence by only 1-4 nucleotides. Furthermore, all let-7-5p isoforms share a highly conserved seed sequence (nucleotides 2-8) GAGGUAG. The seed sequence is used to recognize and to bind to target mRNAs, and is identical across all vertebrate species (reviewed by Lee et al., 2016). Recruitment of miRISC complex to the target mRNA results in deadenylation, leading to mRNA decay or translational repression (Wakiyama et al., 2007). let-7 miRNAs limit self-renewal of stem cells and progenitor cells, and promote their differentiation by targeting pro-growth genes such as Lin28a, Lin28b, Hmga2 and Igf2bp1 (IMP1). In turn, LIN28B and the closely related RBP LIN28A maintain stemness and proliferation by interfering in the processing of primary let-7 transcripts into functional mature miRNAs (Piskounova et al., 2008; Viswanathan et al., 2008; West et al., 2009; Nishino et al., 2013). High levels of mature let-7 miRNAs are associated with a terminal differentiated state. Consistent with such link, we previously found that in developing cochlear epithelia the expression of mature let-7 miRNAs steadily increased during terminal differentiation. However, surprisingly, even though let-7g overexpression triggered premature pro-sensory cell-cycle exit, let-7g overexpression failed to induce precocious HC differentiation in the developing murine cochlea. Instead, we found that let-7g overexpression mildly delayed HC differentiation (Golden et al., 2015).
To address whether the role of let-7 miRNAs in auditory sensory development is conserved among vertebrates, we manipulated let-7 levels in the chicken auditory organ, the basilar papilla (BP), using in ovo electroporation. We observed that attenuation of let-7 function in the developing BP prolonged pro-sensory cell proliferation and delayed terminal differentiation, indicating that endogenous let-7 miRNAs play a key role in restricting pro-sensory cell self-renewal. Moreover, we found that, while let-7b overexpression promoted the proliferative quiescence of pro-sensory cells, it also delayed the differentiation of pro-sensory cells into HCs, suggesting that the levels of mature let-7 miRNAs in pro-sensory cells have to be precisely controlled to ensure proper timing of terminal mitosis and differentiation. Finally, we identified CHD7, a key regulator of sensory-neural development, as a novel target of let-7 miRNAs. We uncovered that the chicken Chd7 gene is harboring an evolutionarily highly conserved and functional let-7 binding site within its 3′UTR sequence. Furthermore, we found that overexpression of let-7 miRNAs significantly reduced CHD7 protein expression within the murine otocyst, cochlea, retina and brain. CHD7 function is dose dependent and the here described negative regulation of CHD7 by let-7 miRNAs constitutes a novel mechanism for fine-tuning CHD7 protein levels during sensory and neural development.
RESULTS
Opposing cell-cycle exit and HC differentiation gradients in the chicken BP
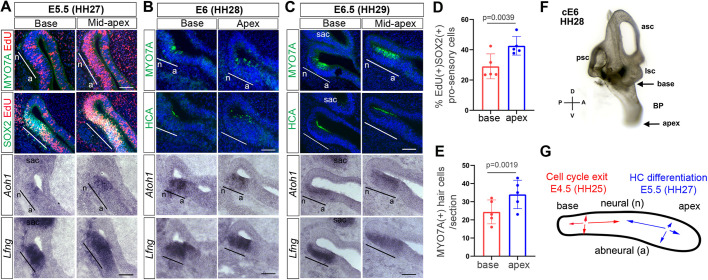
In the murine cochlea pro-sensory cell-cycle exit and differentiation occurs in opposing longitudinal gradients. Pro-sensory cells located at the apex are the first to withdraw from the cell cycle but are the last to differentiate into HCs and SCs (Chen and Segil, 1999; Ruben, 1967; Chen et al., 2002). Opposing gradients of cell-cycle exit and differentiation, however less pronounced, may also exist in the developing chicken BP. Previous studies reported that, as early as embryonic day 5.5 (E5.5) (Hamburger and Hamilton's stage 26; HH26), a narrow band of post-mitotic pro-sensory cells can be detected at the more basal (proximal) region of the developing BP (Katayama and Corwin, 1989), whereas stereocilia-bearing HCs can be detected as early as E6.5 (HH29) at the mid-apical (distal) end of the BP (Cotanche and Sulik, 1984; Goodyear and Richardson, 1997).
To further characterize the spatial and temporal pattern of pro-sensory cell-cycle exit and differentiation in the developing chicken BP, we added the thymidine analog EdU to chicken embryos at E4.5, E5.5 and E6.5, and analyzed EdU incorporation and HC differentiation 1 day later (Fig. 1A-E, and Fig. S1). Addition of EdU at E4.5 labeled more than 40% of apically located SOX2+ pro-sensory cells (Fig. 1A,D). By contrast, pro-sensory cells located at the base incorporated EdU at a 25% lower rate with 30% of SOX2+ pro-sensory cells incorporating EdU, indicating that cell-cycle exit in the developing BP occurs in a basal-to-apical gradient (Fig. 1A,D). A similar pattern of EdU incorporation was observed when EdU was added at E5.5 (Fig. S1, top panel). When EdU was added at E6.5 and analyzed at E7.5, only a few remaining EdU+ SOX2+ cells were detected at the abneural edge of the differentiated BP sensory epithelium (Fig. S1, bottom panel), suggesting that pro-sensory cells located at the abneural edge of the sensory epithelium are the last to exit the cell cycle.
Fig. 1.
Characterization of terminal mitosis and HC differentiation in the developing chicken BP. (A-C) Adjacent cross-sections through the basal (proximal) and apical (distal) segment of wild-type BP stages (E5.5-E6.5; HH27-HH29). MYO7A and HCA immunostaining (green) marks HCs, Atoh1 in situ hybridization marks HC precursors and HCs, Lfng in situ hybridization and SOX2 immunostaining mark the pro-sensory/sensory domain within the developing BP. EdU was added at E4.5 and incorporation was analyzed at E5.5. Black and white lines mark the neural (n) to abneural (a) axis of the pro-sensory domain. (A) At E5.5 MYO7A+ HCs have not yet formed, but Atoh1+ HC precursors are present within the Lfng+ pro-sensory domain, with the highest number in the mid-apex. Pro-sensory cells (SOX2+, Lfng+) located at the base incorporated EdU at a lower rate than pro-sensory cells located more apically. (B) At E6, the number of Atoh1+ HC precursors/HCs increases and the sensory epithelium contains few scattered MYO7A + and HCA+ HCs. (C) At E6.5 the number of MYO7A+ HCA+ HCs increases, the majority of Atoh1(+) cells reside within the HC layer and Lfng expression is limited to the SC layer. (D) Quantification of EdU incorporation in SOX2+ basal and apical pro-sensory cells in A. Data are mean±s.d. (n=5 animals per group). (E) Quantification of MYO7A+ HCs in the base and apex of E6.0-E6.5 BPs. Data are mean±s.d. (n=5 animals per group). Individual data points in D,E represent value per animal. P-values were calculated using paired two-tailed Student's t-test (P≤0.05 is deemed significant). (F) Paint-filled chicken inner ear at ∼stage E6.0 indicating the position and orientation of tissue sections. (G) Summary model of terminal mitosis and differentiation in the developing BP. sac, sacculus; n, neural; a, abneural; psc, posterior semicircular canal; asc, anterior semicircular canal; lsc, lateral semicircular canal. Scale bars: 100 µm.
Analysis of the expression of the early HC markers Atoh1 and myosin VIIa (MYO7A) (Hasson et al., 1995) revealed that HC differentiation begins just prior to E6.0 (HH28). At E5.5, no MYO7A+ cells were detected within the BP (Fig. 1A). However, Atoh1+ cells could be readily detected within the pro-sensory domain, marked by Lfng, with the highest number of Atoh1+ cells in the mid-apex (Fig. 1A). Interestingly, not all of the Atoh1+ cells were confined to the HC layer (top layer), suggesting that in the developing BP Atoh1 expression marks both HC precursors as well as nascent HCs. Furthermore, the Atoh1+ HC precursors/HCs were located close to the neural edge of the Lfng+ pro-sensory domain, suggesting that tall HCs located at the neural edge of the sensory epithelium differentiate prior to short HCs located at the abneural edge of the sensory epithelium. At E6.0 (HH28) the number of Atoh1+ cells in the base was increased compared with E5.5 (Fig. 1A,B) and we observed a few scattered MYO7A+ HCs throughout the BP. Similar results were obtained using hair cell-antigen (HCA) immunostaining, which recognizes the stereocilia-specific protein PTPRQ (Goodyear et al., 2003) (Fig. 1B). At E6.5 the number of MYO7A+ and HCA+ HCs further increased and Atoh1 and Lfng expression started to be confined to the HC layer and SC layer, respectively (Fig. 1C). Quantification of MYO7A+ HCs in the apical and basal region of BP stages E6.0 and E6.5 revealed that the number of MYO7A+ HCs was about 1.4 fold higher in apex than in the base (Fig. 1E).
Taken together, our results suggest that pro-sensory cell-cycle exit in the chicken BP starts at ∼E4.5 (HH25) within the basal region of the BP and a wave of cell-cycle exit slowly progresses towards the apex, reaching the apical region at ∼E6.5 (HH29). By contrast, induction of Atoh1 and subsequent HC differentiation starts in the mid-apical region of the BP around ∼E5.5 (HH27) and HC differentiation quickly progresses towards the base, such that within less than 12 h (E6.0, HH28) a narrow band of HCs can be detected in both the mid-apex and the base (Fig. 1F,G).
let-7 miRNAs are highly expressed in differentiating HCs
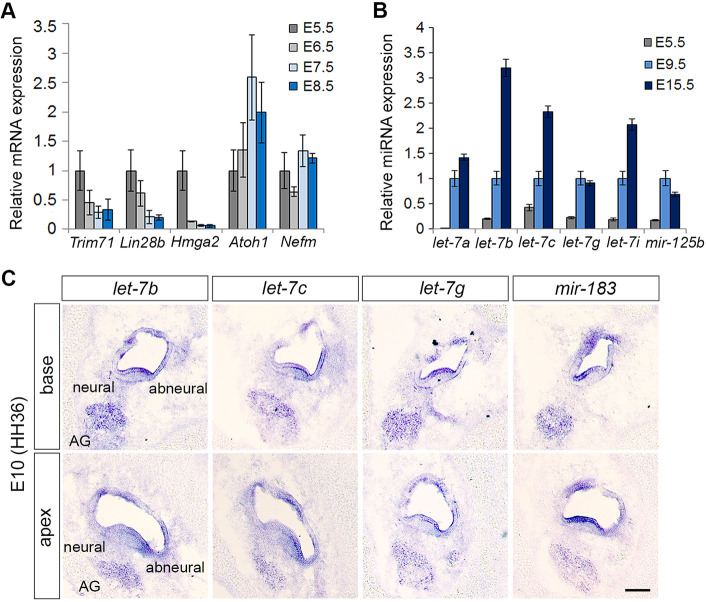
To gain insight into the role of let-7 miRNAs in avian auditory development, we analyzed the expression of let-7 target genes (Lin28b, Trim71 and Hmga2) and mature let-7 miRNAs in enzymatically purified chicken BPs stages E5.5-E15.5 using RT-qPCR. Our analysis revealed that just prior to the onset of HC differentiation (E5.5) Lin28b, Trim71 and Hmga2 were abundantly expressed, but their BP-specific expression quickly declined during the early (E6.5) and late phases (E7.5, E8.5) of HC differentiation (Fig. 2A). Conversely, the lead strands (5-p) of mature let-7 miRNA species (let-7a, let-7b, let-7c, let-7g and let-7i) and the functionally related mir-125b were two- to 20-fold higher expressed in the terminal differentiated BP (E9.5) compared with the undifferentiated BP (E5.5). The expression of mature let-7b, let-7c and let-7i miRNAs further increased during HC maturation and were two- to threefold higher expressed in the mature BPs (E15.5) compared with immature BPs (E9.5) (Fig. 2B). We also examined the expression of the let-7a and let-7c passenger strands (3-p) in developing chicken and murine auditory sensory epithelia. As expected, the passenger strands (3-p) were produced at lower frequency (∼20-100 fold less) than the corresponding lead strands (5-p) (Fig. S2).
Fig. 2.
Spatial and temporal patterns of let-7 miRNA expression in the developing chicken BP. Relative mRNA and mature miRNA expression was assayed by qPCR in enzymatically purified wild-type BP epithelia. Cell type-specific expression of mature miRNAs was analyzed using LNA miRNA in situ hybridization assays. (A) The relatively high mRNA expression of let-7 target genes Trim71, Lin28b and Hmga2 at E5.5 declines in the differentiating BP (E6.5, E7.5 and E8.5) as Atoh1 expression increases. Data are mean±s.e.m. (n=3 technical replicates; similar results were obtained in three independent experiments). (B) let-7a-5p, let-7b-5p, let-7c-5p, let-7g-5p, let-7i-5p and mir-125b-5p miRNA expression steadily increases during BP differentiation (E5.5-E9.5) and continues to increase in the post-differentiated BP at E15.5. Data are mean±s.e.m. (n=3, technical replicates; similar results were obtained in three independent experiments). (C) let-7b-5p, let-7c-5p, let-7g-5p and miR-183-5p miRNAs are expressed within the HC layer and acoustic ganglion (AG). let-7 miRNAs are expressed in a radial gradient within the auditory sensory epithelium, with higher expression in cells at the abneural side than the neural side. Scale bar: 100 µm.
Next, we used locked nucleic acid nucleotide (LNA)-based in situ hybridization assays to characterize the spatial expression pattern of mature let-7 miRNAs in chicken BPs stage E10 (HH36). We also analyzed the expression of mir-183-5p, a miRNA previously shown to be selectively expressed in HCs and auditory ganglion neurons (Zhang et al., 2015). Our analysis revealed that let-7b-5p, let-7c-5p and let-7g-5p are highest expressed in HCs and the auditory ganglion, showing partially overlapping expression with miR-183-5p (Fig. 2C). Interestingly, the expression of let-7 miRNAs in HCs differed along the basal-apical and neural-abneural axes, with most pronounced expression in abneural (short) HCs located at the basal (proximal) region of the BP. This graded pattern of expression may have functional implication for the maturation and specialization of tall (neural) and short (abneural) HCs within the BP.
Inhibition of endogenous let-7 miRNAs increases pro-sensory cell proliferation and delays HC differentiation
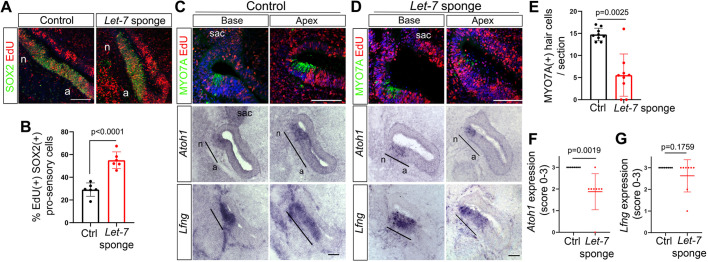
In chicken, the let-7 family of miRNAs consists of at least nine members (let-7a, let-7b, let-7c, let-7d, let-7f, let-7g, let-7i, let-7j and let-7k) (www.mirbase.org/). To disrupt the function of all let-7 miRNA family members, we used a previously described let-7 ‘sponge’ expression plasmid that produces a transcript that contains six canonical let-7-5p-binding sites (Bhattacharya et al., 2018). When expressed at sufficiently high levels, the let-7 sponge transcript functions as a decoy for all endogenous let-7-5p miRNAs, sequestering them from their endogenous targets (Ebert and Sharp, 2010). To determine successful targeting of the let-7 sponge construct, E4.5 chicken embryos were co-electroporated with let-7 sponge and GFP expression plasmids. Six to 18 h later, we used live imaging of green fluorescence (GFP) to confirm targeting of co-electroporated constructs to the ventral otocyst (Fig. S3). Only specimens with robust GFP expression in the ventral otic region were further analyzed. In a subset of experiments Gfp in situ hybridization was used to confirm targeting of Lfng+ pro-sensory cells in the presumptive BP rudiment (Fig. S4). EdU was added at time of electroporation, and EdU incorporation and HC differentiation were analyzed 44 h later in let-7 sponge-treated BPs (right ear) and corresponding untreated BPs (left ear, internal control). We found that the number of SOX2+ pro-sensory cells that incorporated EdU was significantly increased in let-7 sponge-treated BPs compared with corresponding control BPs (Fig. 3A,B), suggesting that the let-7 sponge-mediated let-7 knockdown delayed pro-sensory cell-cycle exit. Next, we analyzed the effect of let-7 sponge treatment on HC differentiation. At stage ∼E6.5 a narrow band of Atoh1+ MYO7A+ HCs can be readily detected within the Lfng+ pro-sensory/sensory domains of control BPs (Fig. 3C). However, let-7 sponge-treated BPs contained within their pro-sensory/sensory domains less than half the number of MYO7A+ HCs compared with control BPs (Fig. 3D,E). While the expression of Lfng was unchanged by let-7 sponge-treatment (Fig. 3D,G), Atoh1 expression was mildly, but significantly, reduced in let-7 sponge-treated BPs (Fig. 3D,F). In summary, these findings suggest that lower than normal levels of let-7 miRNAs result in a delay in pro-sensory cell-cycle exit and HC differentiation.
Fig. 3.
Disruption of let-7 miRNA function increases pro-sensory cell proliferation and delays HC formation in the developing chicken BP. Shown are adjacent sections of control BPs (A,C) and of corresponding let-7 sponge-treated BPs (A,D) 44 h after electroporation and EdU treatment at E4.5. Black bars indicate pro-sensory domains. (A) Mid-apical cross-sections of let-7 sponge-treated BP (A, right panel) and corresponding control BP (A, left panel) labeled for EdU (red) and the pro-sensory/sensory marker SOX2 (green). let-7 sponge treatment increased cell proliferation inside and outside the pro-sensory/sensory domain. (B) Quantification of A. Data are mean±s.d. (n=5 animals per group). (C,D) Adjacent cross-sections through the base and mid-apex of control (C) and let-7 sponge-treated (D) BPs were analyzed for MYO7A protein expression using immunostaining, and for Atoh1 and Lfng expression using RNA in situ hybridization. (E) Quantification of MYO7A+ HCs in control (C) and let-7 sponge-treated BPs (D). Individual data points represent average value per animal and treatment. Data are mean±s.d. (n=9 animals per group). (F,G) Quantification of Atoh1 (F) and Lfng (G) mRNA expression using a scoring system: 0, absent; 1, weak; 2, reduced; 3, normal expression. Four or five adjacent Atoh1- and Lfng-labeled cross-sections each representing base, mid and mid-apex were analyzed per animal and treatment. Data are mean±s.d. (n=8 animals per group). P-values were calculated using unpaired two-tailed Student's t-test (P≤0.05 is deemed significant). Ctrl, control; sac, sacculus; n, neural; a, abneural. Scale bars: 100 µm.
Overexpression of let-7b reduces pro-sensory cell proliferation and interferes with auditory HC differentiation
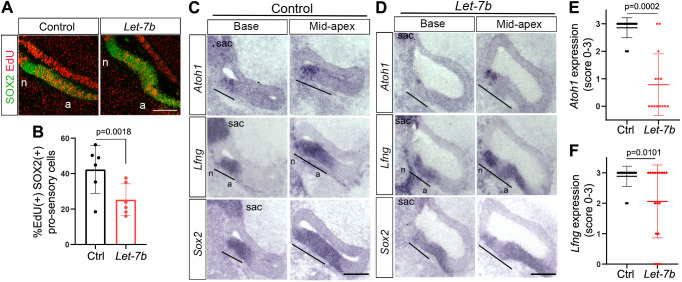
Next, we examined whether let-7 miRNA overexpression is sufficient to trigger premature HC differentiation in the developing chicken BP. To increase the abundance of mature let-7 miRNAs, we electroporated E4.5 chicken embryos with a let-7b-GFP expression plasmid (Nishino et al., 2008). Proper targeting of let-7b-GFP to the ventral otocyst was confirmed by live imaging of green fluorescence (GFP) (Fig. S3). EdU was added in ovo at the time of electroporation to characterize the rate of pro-sensory cell proliferation. let-7b-treated BPs harvested after 44 h were severely malformed and growth retarded. To minimize potentially indirect effects on HC differentiation, we modified our experimental approach and harvested electroporated embryos after 18 h at E5-E5.5 (HH26-HH27), coinciding with the onset of Atoh1 expression. Consistent with the inhibitory role of let-7 miRNAs in cell proliferation, let-7b-treated BPs showed a significant reduction in EdU incorporation in SOX2+ pro-sensory cells compared with corresponding control BPs (Fig. 4A,B). However, as observed in mice, increased let-7 miRNA expression failed to trigger precocious HC differentiation. Instead, we found that, on average, Atoh1 expression in let-7b-treated BPs was severely reduced compared with control BPs (Fig. 4C-E). The expression of Lfng was normal in the majority of let-7b-treated BPs (n=9/17). However, Lfng expression was reduced or absent (n=5/17) in about 30% of let-7b-treated BPs, compared with control (Fig. 4C,D,F), suggesting the let-7b overexpression may interfere with pro-sensory cell specification. Moreover, we noted that the size of the acoustic ganglion was severely reduced in let-7b-treated BPs compared with control BPs (Fig. 5D,E, yellow asterisks; n=5/5). To verify that electroporation by itself did not alter pro-sensory cell behavior, we analyzed pro-sensory cell proliferation and Atoh1 expression in BPs electroporated with GFP expression plasmid (GFP only) and corresponding untreated BPs (left ear, internal control). Our analysis revealed no significant differences in the rate of pro-sensory cell proliferation or in the spatial/temporal pattern of Atoh1 or Lfng expression (Fig. S5). Taken together, our findings suggest that higher than normal let-7 miRNA levels delay the induction of Atoh1 and the subsequent differentiation of pro-sensory cells into HCs.
Fig. 4.
let-7 overexpression reduces pro-sensory cell proliferation and delays Atoh1 induction in the developing chicken BP. Adjacent sections of untreated control BPs (A) and let-7b-treated BPs (A,D) 18 h after electroporation and EdU treatment at E4.5. The black bars mark pro-sensory domains. (A,B) let-7b overexpression reduces pro-sensory cell proliferation. (A) Mid-apical cross-sections of let-7b-treated BP (A, right panel) and corresponding control BP (A, left panel) labeled for EdU (red) and the pro-sensory/sensory marker SOX2 (green). let-7b overexpression reduced cell proliferation both inside and outside the pro-sensory/sensory domain. (B) Quantification of A. Data are mean±s.d. (n=6 animals per group). (C,D) Adjacent cross-sections of control (C) and let-7b-treated (D) BPs through the base and mid-apex were analyzed for Atoh1, Lfng and Sox2 expression using RNA in situ hybridization. let-7b-treated BPs are malformed. (E,F) Quantification of Atoh1 (E) and Lfng (F) mRNA expression using a scoring system: 0, absent; 1, weak; 2, reduced; 3, normal expression. Four or five adjacent Atoh1- and Lfng-labeled cross-sections each representing base, mid and mid-apex were analyzed per animal and treatment. Data are mean±s.d. (n=17 animals per group). P-values were calculated using unpaired two-tailed Student's t-test (P≤0.05 is deemed significant). Ctrl, control; sac, sacculus; n, neural; a, abneural. Scale bars: 100 µm.
Fig. 5.
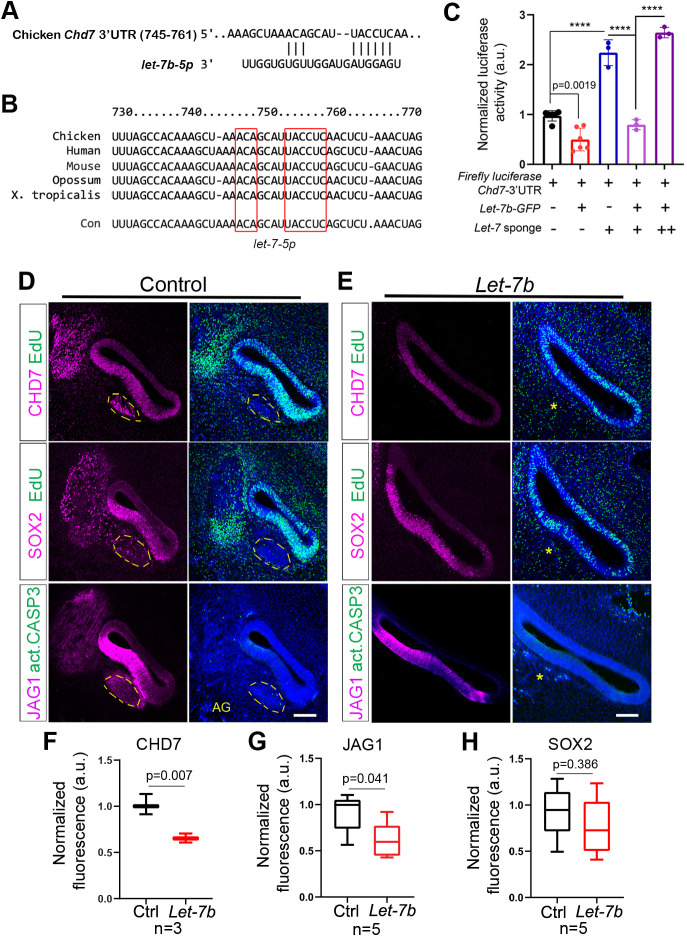
let-7 miRNAs negatively regulate CHD7 expression in the developing BP. (A,B) Chd7 is an evolutionarily conserved let-7 target gene. TargetScan Release 7.2 software was used to identify conserved let-7-5p binding sites in regulatory genes. The 3′UTR-sequence of the chicken Chd7 gene (ENST00000423902.2) contains a high probability let-7b-5p-binding site, including an exact match to positions 2-7 (seed sequence) of mature let-7 miRNAs (red boxes) (A). The let-7-5p-binding site within the Chd7 gene is highly conserved across vertebrate species (red boxes) (B). (C) let-7b represses murine Chd7 expression by targeting its 3′UTR. Equal amounts of firefly-luciferase-Chd7-3′UTR and Renilla luciferase plasmids were co-transfected into HEK 293T cells with (+) or without (−) let-7b-GFP and with (+) or without (−) let-7 sponge plasmids (++ indicates that twice the amount of let-7 sponge plasmid was used). Firefly-luciferase-reporter activity and Renilla luciferase activity was measured 48 h after transfection and Renilla luciferase activity was used to normalize firefly luciferase activity. Data are mean±s.d. Individual data points represent biological replicates. For each condition, a minimum of three independent samples were analyzed. P-values were calculated using one-way ANOVA, with Tukey's correction, ****P<0.0001 (P<0.05 is deemed not significant). (D-H) 18 h after electroporation and EdU treatment at E4.5, let-7b-treated BPs and corresponding control BPs were harvested and adjacent cross-sections were co-stained for CHD7 and EdU (top panel), SOX2 and EdU (middle panel), and JAG1 and activated caspase 3 (act-CASP3) (bottom panel). (D,E) Representative confocal images of cross-sections through the mid-apical region of control BPs (D) and let-7b-treated BPs (E). SOX2 and JAG1 immunostaining mark the pro-sensory/sensory domain. Act-CASP3 (green) immunostaining labels apoptotic cells, EdU (green) labels mitotic cells. The size of the acoustic ganglion (AG), marked by dashed yellow lines in control BPs, is severely reduced in let-7b-treated BPs (yellow asterisks). (F-H) Relative intensity of CHD7 (F), JAG1 (G) and SOX2 (H) immunofluorescence within the pro-sensory/sensory domain of control (black bars) and let-7b-treated (red bars) BPs. Box plots representing the sample median, first and third quartiles, and whiskers are drawn from minimum to maximum (n=number of animals per group, P-value calculated using unpaired two-tailed Student's t-test, P≤0.05 deemed significant). Ctrl, control. Scale bars: 100 µm.
let-7 miRNAs negatively regulate CHD7 expression in the developing chicken BP
To gain insight into how let-7 miRNAs influence HC differentiation, we screened for let-7-5p-binding sites within the Atoh1 transcript and within the transcripts of its known upstream regulators EYA1, SIX1 (Ahmed et al., 2012), SOX2 (Kiernan et al., 2005; Neves et al., 2012; Kempfle et al., 2016) and β-catenin (Ctnnb1) (Shi et al., 2010). TargetScan 7.2. (Agarwal et al., 2015) and microT-CDS (Paraskevopoulou et al., 2013), two complementary miRNA prediction software packages, failed to uncover let-7-5p target sites within the mRNA sequences of chicken Atoh1, Eya1, Six1, Sox2 and Ctnnb1 genes or their human and murine homologs. However, we identified a high probability let-7-5p binding site in the 3′ untranslated region (UTR) of the chicken Chd7 gene (Fig. 5A). Further analysis revealed that this binding site is evolutionarily conserved and is present in the 3′ UTR sequence of the human and murine Chd7 transcripts (Fig. 5B). To validate let-7 binding to the Chd7-3′UTR, we conducted luciferase-reporter assays. We subcloned the murine Chd7-3′UTR sequence downstream of the open reading frame of the firefly-luciferase gene and co-transfected the luciferase-Chd7-3′UTR reporter and a transfection control (Renilla luciferase) with or without let-7b or let-7 sponge plasmids into human HEK293T cells. As anticipated, let-7b overexpression inhibited luciferase-reporter activity, whereas let-7 sponge expression increased luciferase-reporter activity, by soaking up endogenous let-7 miRNA species expressed in HEK293T cells (Tian et al., 2012). Furthermore, as expected, co-expression of increasing amounts of let-7 sponge plasmid reversed let-7b-mediated repression of luciferase reporter activity (Fig. 5C). The vertebrate Chd7 (chromo-helicase-DNA binding protein 7) gene encodes for an ATP-dependent chromatin remodeling enzyme that, among others, is crucial for proper morphogenesis and neuro-sensory development of the mammalian inner ear (Hurd et al., 2010). Furthermore, CHD7 has been shown to cooperate with SOX2 in the transcriptional regulation of the Notch ligand JAGGED1 (JAG1) during early otic development (Engelen et al., 2011). JAG1-meditated Notch signaling is essential for the specification and maintenance of auditory and vestibular pro-sensory cells in the developing inner ear (Daudet and Lewis, 2005; Kiernan et al., 2006; Pan et al., 2010).
We found that in the developing chicken BP (E4.5+18 h), CHD7 protein was highly expressed in SOX2+ JAG1+ pro-sensory cells, as well as in flanking non-sensory epithelial cells. In addition, CHD7 was expressed within the developing acoustic ganglion (Fig. 5D). As observed in our earlier experiments, pro-sensory-specific SOX2 expression within the developing BP was not significantly altered by let-7b overexpression (Fig. 5D,E,H). However, consistent with being a target of let-7 regulation, we found that in response to let-7b overexpression CHD7 protein expression within the pro-sensory/sensory epithelium of the developing BP was significantly reduced (Fig. 5D-F). Moreover, let-7b overexpression led to a mild, but significant, reduction in JAG1 protein expression within the pro-sensory/sensory epithelium of the developing BP (Fig. 5D,E,G). Taken together, these findings indicate that let-7 miRNAs negatively regulate the expression of CHD7 in the developing BP, which in turn may impact the function and expression of SOX2 and JAG1, respectively.
let-7 miRNAs repress CHD7 expression in the murine brain, retina and inner ear
To establish whether the repressive function of let-7 miRNAs in Chd7 gene expression is evolutionarily conserved, we conducted let-7 overexpression experiments in mouse embryos using the well-characterized iLet-7g mouse model. In this double transgenic mouse model, a degradation-resistant form of let-7g is expressed under the control of a TRE promoter (Col1a-TRE-let-7S21L) (Zhu et al., 2011), which, in the presence of an ubiquitously expressed reverse tetracycline trans-activator transgene (R26-rtTA-M2) (Hochedlinger et al., 2005) and doxycycline (dox), allows for robust let-7g overexpression throughout the body, including the inner ear (Zhu et al., 2011; Golden et al., 2015). We administered dox to pregnant dams starting at stage E10.5 and harvested iLet-7g transgenic embryos and their single transgenic control littermates for further analysis at stage E11.5 (Fig. 6) and E13.5/E15.5 (Fig. 7).
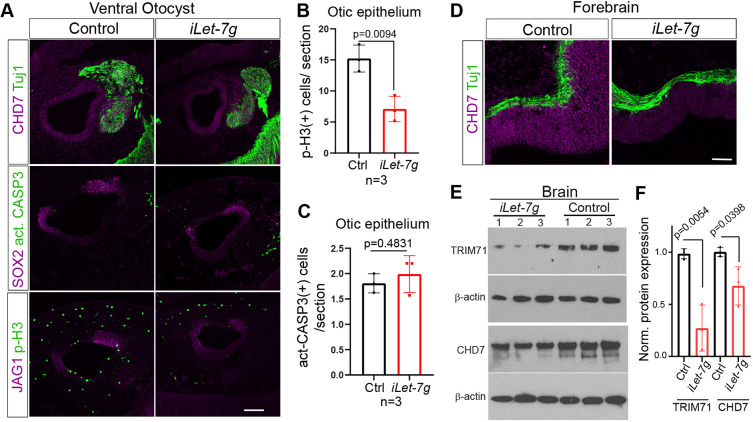
Fig. 6.
let-7 miRNAs inhibit CHD7 expression in the murine otocyst and developing brain. We administered dox to pregnant dams starting at stage E10.5 and harvested iLet-7g transgenic embryos and their single transgenic control littermates for further analysis at stage E11.5. (A-C) let-7g overexpression reduces cell proliferation and CHD7 expression in the developing otocyst. (A) Confocal images of immunostained sections through the ventral otocyst of iLet-7g embryos and control littermates. Tuj1 marks neurons, CHD7 marks otic and neuronal progenitor cells, and SOX2 and JAG1 mark pro-sensory cells. Act-CASP3 labels apoptotic cells, pH3 labels mitotic cells. (B,C) Quantification of mitotic (pH3+) cells (B) and apoptotic (act-CASP3+) cells (C) within otic epithelia of control (Ctrl, black bar) and iLet-7g transgenic mice (iLet-7g, red bar). Data are mean±s.d. (n=3 animals per group). (D-F) let-7g overexpression reduces CHD7 protein expression in the developing brain. Confocal images of CHD7 and Tuj1 immunostained sections through the forebrain of iLet-7g embryos and control littermates. (E) Western blot analysis of CHD7 and TRIM71 protein expression in control and iLet-7g transgenic brain. (F) Quantification of E. β-Actin was used to normalize TRIM71 and CHD7 protein expression in iLet-7g transgenic (red bar) and control (black bar) brain lysates. Data expressed as mean±s.d. (n=3 animals per group). P-values were calculated using unpaired two-tailed Student's t-test (P≤0.05 is deemed significant). Ctrl, control. Scale bars: 100 µm.
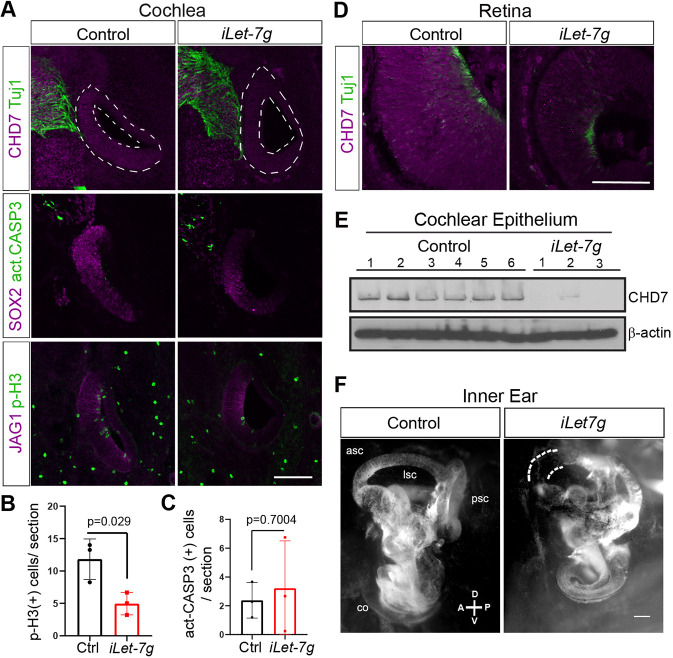
Fig. 7.
let-7g overexpression inhibits CHD7 expression in the developing murine retina and cochlea, and alters inner ear morphology. We administered dox to pregnant dams starting at stage E10.5 (A-E) or E11.5 (F) and harvested iLet-7g transgenic embryos and their single transgenic control littermates for further analysis at indicated stages. (A-C) let-7g overexpression reduces cochlear epithelial cell proliferation and cochlear pro-sensory CHD7 protein expression. (A) Confocal images of adjacent cochlear sections obtained from E13.5 iLet-7g embryos and control littermates. CHD7 (magenta) marks cochlear and neuronal progenitors; Tuj1 (green) marks neurons; SOX2 and JAG1 (magenta) mark the cochlear pro-sensory domain; act-CASP3 (green) labels apoptotic cells; pH3 (green) labels mitotic cells. Dashed white lines outline cochlear epithelia. (B,C) Quantification of the number of mitotic (pH3+) (B) and apoptotic (act-CASP3+) (C) cells in control (Ctrl, black bar) and let-7g overexpressing (iLet-7g, red bar) cochlear epithelia at stage E13.5. Data are expressed as mean±s.d. (n=3 animals per group, P-value calculated using unpaired two-tailed Student's t-test, P≤0.05 deemed significant). Scale bar: 100 µm. (D) Let-7g overexpression reduces retinal CHD7 protein expression. Confocal images of CHD7 and Tuj1 immunostained retinal sections from stage E13.5 iLet-7g embryos and control littermates. Scale bar: 100 µm. (E) let-7g overexpression reduces cochlear epithelial CHD7 protein expression. Western blot analysis of CHD7 protein expression in E15.5 control and let-7g overexpressing cochlear epithelia. β-Actin was used as loading control (n=6 animals for control and n=3 animals for iLet-7g group). (F) let-7g overexpression alters inner ear morphology. Paint-fills of E17.5 inner ears obtained from an iLet-7g transgenic embryo and a non-transgenic (control) littermate. The let-7g-overexpressing inner ear is smaller, has a shortened cochlea and malformed semicircular canals, with the anterior semicircular canal being truncated (dashed white lines) and the lateral semicircular canal being missing. asc, anterior semicircular canal; psc, posterior semicircular canal; lsc, lateral semicircular canal; co, cochlea. Scale bar: 200 µm.
At stage E11.5, CHD7 protein is highly expressed within the developing otocyst and the vestibulocochlear ganglion (Hurd et al., 2010) (Fig. 6A, control) as well as the developing brain (Gage et al., 2015) (Fig. 6D, control). By contrast, otic and brain-specific CHD7 expression was severely reduced in stage E11.5 let-7g-overexpressing embryos (iLet-7g) (Fig. 6A,D, iLet-7g). Consistent with previous reports that CHD7 positively regulates JAG1 expression in the developing otocyst (Engelen et al., 2011), the observed severe reduction in otic CHD7 expression was accompanied by a mild reduction in otic JAG1 expression (Fig. 6A). Furthermore, consistent with the repressive role of let-7 miRNAs in cell proliferation, we found that the number of mitotic cells within the otic epithelium was significantly reduced in response to let-7g overexpression, as indicated by the significantly reduced number of phospho-histone 3 (p-H3)+ cells (Fig. 6A,B). By contrast, let-7g overexpression did not significantly alter the number of apoptotic cells within the otic epithelium, as indicated by the unchanged number of cells that expressed activate caspase 3 (act-CASP3) (Fig. 6A,C). Next, we analyzed CHD7 protein expression in whole-brain lysates from E11.5 iLet-7g embryos and wild-type littermates. The expression of TRIM71, which is a well-characterized target of let-7 repression and abundantly expressed in neuronal progenitors, was analyzed as a positive control (Lin et al., 2007). As anticipated, our analysis revealed that acute let-7g overexpression significantly reduced both TRIM71 and CHD7 protein levels in the developing brain (Fig. 6E,F).
As previously reported, at stage E13.5, CHD7 protein is broadly expressed within the developing cochlear epithelial duct (Fig. 7A, control) and is highly expressed within the developing retina (Fig. 7D, control) (Hurd et al., 2007). By contrast, cochlear epithelial CHD7 expression (Fig. 7A, iLet-7g), as well as retinal CHD7 expression were severely reduced in E13.5 iLet-7g transgenic embryos (Fig. 7D, iLet-7g). To quantify the observed reduction in cochlear CHD7 expression, we conducted western blot assays using cochlear epithelia protein lysates from stage E15.5 iLet-7g transgenic embryos and control littermates. Our analysis revealed that prolonged let-7g overexpression nearly completely abolished CHD7 protein expression within cochlear epithelial cells (Fig. 7E) (Fig. S6B).
Furthermore, consistent with our previous findings, we observed that prolonged let-7g overexpression reduced the size of the cochlear duct and its SOX2+ pro-sensory domain (Fig. 7A, iLet-7g) (Fig. S6A) (Golden et al., 2015). The observed defects were unlikely due to an increase in cell death, as both control and let-7g-overexpressing cochlear epithelia contained only few scattered act-CASP3+ cells (Fig. 7A,C). However, let-7g-overexpressing cochlear epithelia contained significantly fewer p-H3+ cells compared with control (Fig. 7A,B), indicating that the cochlear defects in let-7g-overexpressing embryos were the product of reduced cochlear epithelial cell proliferation. Next, we analyzed cochlear HC differentiation in stage E15.5 let-7g-overexpressing embryos and control littermates. Our analysis revealed a mild delay in HC differentiation in response to let-7g overexpression, confirming previous findings (Fig. S6A) (Golden et al., 2015). To address the effects of let-7g overexpression on overall inner ear morphology, we paint-filled inner ears of stage E17.5 iLet-7g transgenic embryos (n=3 embryos, n=5 inner ears) and their non-transgenic littermates (n=4 embryos, 4 inner ears) that were exposed to dox starting at E11.5. All three iLet-7g transgenic embryos had smaller inner ears and their cochleae were shorter/smaller compared with control embryos. In addition, we found that all let-7g-overexpressing inner ears had malformed semicircular canals (Fig. 7F) (Table S1). Most notable, all examined let-7g-overexpressing inner ears showed defects in their lateral semicircular canals, which were either missing or smaller (Fig. 7F) (Table S1).
DISCUSSION
The highly specialized mammalian and avian auditory sensory organs have evolved independently (Fritzsch et al., 2013). Yet many of the regulatory gene networks that control patterning, specification and differentiation of the auditory sensory epithelium are conserved among birds and mammals (Munnamalai and Fekete, 2013). In mammals, cell-cycle exit and differentiation of pro-sensory cells are initiated at opposing ends of the developing auditory sensory epithelium. Pro-sensory cells located at the cochlear apex are the first to exit the cell-cycle but are the last to differentiate (Chen et al., 2002). Spatial segregation between pro-sensory cell-cycle withdrawal and differentiation, even though less pronounced, has been also reported for the developing chicken BP (Cotanche and Sulik, 1984; Katayama and Corwin, 1989). Indeed, our analysis revealed that terminal mitosis of pro-sensory cells occurs first in the proximal (basal) half of the chicken BP, whereas HC differentiation initiates in the distal (apical) half of the chicken BP. However, for reasons currently unknown, the gradients in the chicken BP run in the opposite direction to the pattern observed in the murine cochlea.
What times and coordinates these seemingly uncoupled events? We have recently shown that the LIN28B/let-7 pathway is active in the developing murine auditory sensory organ and plays a key role in timing pro-sensory cell-cycle exit and differentiation (Golden et al., 2015). Expanding on these recent findings, we now show that let-7 miRNAs are essential for the proper timing of terminal mitosis and differentiation in the developing chicken auditory organ. We demonstrate that expression of mature let-7 miRNAs sharply rises around the time of terminal mitosis and differentiation. Using a RNA sponge to sequester functional let-7 miRNAs, we show that disruption of let-7 function in pro-sensory cells delays their onset of terminal mitosis and differentiation, whereas overexpression of let-7b accelerates terminal mitosis. How do let-7 miRNAs exert control over pro-sensory cell proliferation and self-renewal? We show that the evolutionarily conserved let-7 target genes Hmga2, Lin28b and Trim71 are highly expressed in undifferentiated BP (E5.5), but are near absent in the differentiated BP. The RBPs TRIM71 and LIN28B, and the chromatin-associated protein HMGA2 are important regulators of stemness and have been shown to promote stem cell/progenitor cell proliferation in both neuronal and sensory lineages (Maller Schulman et al., 2008; Nguyen et al., 2017; Yang et al., 2015; Parameswaran et al., 2014; Nishino et al., 2008). Thus, the most likely scenario is that let-7 miRNAs accelerate the terminal mitosis of pro-sensory cells through limiting Hmga2, Lin28b and Trim71 expression. Support for such model comes from our recent study that revealed that prolonged LIN28B expression maintains auditory pro-sensory cells in a proliferative and undifferentiated state (Golden et al., 2015).
The role of let-7 miRNAs in the differentiation of neural-sensory progenitors is complex and highly dependent on the cellular context. For example, overexpression of let-7b stimulates the differentiation of cultured retinal progenitors and neuronal stem cells through targeting nuclear receptor TLX (NR2E1) (Zhao et al., 2010; Ni et al., 2014). By contrast, let-7b or let-7i overexpression in human ESC-derived neuronal progenitor cells inhibits neuronal differentiation, as it reduces the expression of the pro-neural gene ASCL1 (MASH1) (Cimadamore et al., 2013).
We have previously reported that overexpression of let-7g mildly delays auditory HC differentiation in mice. Expanding on these previous findings, we show that in the chicken BP higher as well as lower than normal levels of mature let-7 miRNAs delay auditory HC differentiation. This seemingly paradoxical finding suggests that let-7 miRNAs, in addition to targeting genes crucial for pro-sensory cell self-renewal, target genes that promote HC differentiation. Auditory HC formation depends on the function of ATOH1 (Bermingham et al., 1999), EYA1, SIX1 (Ahmed et al., 2012), SOX2 (Neves et al., 2012; Kempfle et al., 2016) and β-catenin (Shi et al., 2010). We failed to identify let-7-5p-binding sites within the mRNA sequences of these key regulatory genes.
However, we uncovered an evolutionarily conserved, canonical let-7-5p-binding site within the 3′UTR of the chicken, human and murine Chd7 gene. Moreover, using luciferase reporter assays, we experimentally validate that the 3′UTR sequence of the murine Chd7 gene is a target of let-7-mediated repression. These findings are consistent with published data obtained from genome-wide interrogation of miRNA-target RNA duplexes, which list the human CHD7 transcript as a direct binding partner of let-7-5p miRNAs (Helwak et al., 2013). Finally, consistent with being a direct target of let-7 repression, we demonstrate that let-7 overexpression significantly reduces CHD7 protein expression within less than 24 h in mice as well as in chicken.
Chd7 is abundantly expressed in auditory pro-sensory cells as well as auditory HCs (Bosman et al., 2005; Hurd et al., 2011). However, the role of CHD7 in HC differentiation/maturation has yet to be established. CHD7, an ATP-dependent chromatin remodeler, is preferentially found at active enhancer/promoter regions, and has been shown to control chromatin accessibility and transcriptional activation/repression in a cell lineage-specific manner (Schnetz et al., 2009, 2010; Bajpai et al., 2010; Feng et al., 2013; Chai et al., 2018). In Drosophila, the CHD7 orthologue Kismet is required for atonal transcription in the developing eye (Melicharek et al., 2008). To date there is no evidence that CHD7 directly activates Atoh1 transcription in vertebrates. However, there are several candidate genes through which CHD7 may exert influence over HC differentiation and HC maturation. CHD7 has been shown to physically interact with SOX2 and co-regulate the transcription of a set of common target genes (Engelen et al., 2011; Doi et al., 2017). Thus, let-7-induced reduction in CHD7 expression may compromise the proper timing of SOX2-mediated activation of Atoh1 transcription and subsequent HC differentiation (Neves et al., 2012; Kempfle et al., 2016; Ahmed et al., 2012). Furthermore, CHD7 has been found to be required for the transcriptional activation of Sox11 and Sox4 in adult neuronal stem cells (Feng et al., 2013). The transcription factors SOX11 and SOX4 are broadly expressed in cochlear and vestibular pro-sensory cells, and in their absence both auditory and vestibular HCs fail to form (Gnedeva and Hudspeth, 2015). Future investigations are warranted to address the role of CHD7 in HC development.
It is important to note that let-7 miRNAs may also influence auditory HC development through other, more indirect, mechanisms. In mammals, the acoustic ganglion is an important signaling center. It transiently produces sonic hedgehog (SHH), which maintains pro-sensory cells in an undifferentiated and proliferative state. Thus, it could be reasoned that the observed reduction in the acoustic ganglion (termed spiral ganglion in mammals) in response to let-7 overexpression may have contributed to the delay in auditory HC differentiation in chicks and mice. However, loss of SHH signaling in the murine cochlea results in premature HC differentiation (Benito-Gonzalez and Doetzlhofer, 2014; Tateya et al., 2013; Bok et al., 2013) and not in a delay of HC differentiation, as observed in response to let-7 overexpression. Moreover, the acoustic ganglion in chicks lacks such a pattern of SHH expression (Son et al., 2015). Furthermore, it could be reasoned that let-7-induced changes in cochlear morphology may negatively impact HC differentiation. However, a recent study found that auditory HCs developed relative normal in Fgf10 mutant mice, despite severe defects in cochlear morphology (Urness et al., 2015).
Similar to Chd7, let-7 miRNA expression in the inner ear is not confined to the auditory-sensory lineage. Mature let-7 miRNAs are among the highest expressed miRNAs in cochlear and vestibular epithelia of newborn mice (Rudnicki et al., 2014). In the murine inner ear, let-7 miRNAs are highly expressed in cochlear and vestibular HCs, the vestibulocochlear ganglion as well as in SCs and non-sensory epithelial cells (Golden et al., 2015). The inner ear defects observed in the iLet-7g transgenic mouse model share similarities with previously described inner ear defects in Chd7 heterozygous mouse mutants. Chd7 mutant inner ears are smaller in size and contain a smaller than normal vestibulocochlear ganglion, a shortened cochlea and malformed semicircular canals (Hurd et al., 2010). Resembling these reported inner ear abnormalities, iLet-7g transgenic inner ears were hypoplastic, with a smaller than normal vestibulocochlear ganglion, a shortened cochlea and malformed semicircular canals.
CHD7 haploinsufficiency in humans is a leading cause of CHARGE syndrome, a disorder that causes multiple birth defects, including ocular coloboma, mental retardation, ear abnormalities and deafness (Pagon et al., 1981). Our finding that CHD7 is negatively regulated by let-7 miRNAs in tissues affected by CHARGE syndrome (ear, eye and brain) may aid therapeutic interventions to correct CHD7 deficiency.
MATERIALS AND METHODS
Eggs and in ovo micro-electroporation
Fertilized chicken eggs, Gallus gallus domesticus (B&E and Charles River Laboratories) were incubated at 37°C and staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951) at HH24-HH25 (E4-E4.5). As previously described, plasmids were delivered to the right otic vesicle targeting the BP rudiment at E4-E4.5 (HH24-HH25) by electroporation with the left ears serving as untreated internal controls (Evsen and Doetzlhofer, 2016). Briefly, the right otic vesicle was filled with plasmids at a concentration of 3-5 µg/µl tinted with 0.1% Fast Green. A negative 2 mm platinum electrode was placed anterior ventral to the right otic vesicle and the positive electrode placed in parallel 1 mm apart. Four 12 V pulses with 100 ms duration and 200 ms spacing were applied using an ECM 830 square wave electroporation system (BTX Harvard Apparatus) after which the eggs were sealed and returned to the incubator. Gene targeting to the anterior-ventral BP rudiment domain was confirmed by imaging GFP signals in ovo with a Leica fluorescent microscope at 6 h and/or the time of harvest. Embryos with no GFP signal at 6 h in the targeted BP were discarded. Approximately 50% of embryos, in any given experiment, exhibited satisfactory GFP signals and were further analyzed.
Plasmids
The let-7b-GFP plasmid expresses the murine primary let-7b sequence (NR_029727.1) under the control of the mouse U6 promoter and expresses Gfp under the control of the CMV promoter (a gift from Sean Morrison, UT Southwestern and Howard Hughes Medical Institute, Dallas, TX, USA) (Addgene #25404) (Nishino et al., 2008). The pRNA-U6-let-7 sponge plasmid expresses a RNA sequence that contains six canonical let-7-5p binding sites under the control of the U6 promoter (a gift from Phillip Zamore, University of Massachusetts Medical School and Howard Hughes Medical Institute, Amherst, MA, USA (Addgene #35664). To ensure proper targeting, we co-electroporated pMES-IRES-GFP (a gift from Doris K. Wu, National Institute on Deafness and Other Communication Disorders, Bethesda, MD, USA) in which GFP expression is under the control of the chicken β-actin promoter. We also used pCMV-EGFP, in which the CMV promoter controls EGFP expression. The pCMV-EGFP plasmid was a gift from Connie Cepko (Harvard Medical School and Howard Hughes Medical Institute, Boston, MA, USA) (Addgene #11153). The pISO plasmid, a derivative of pGL3 (Promega), which expresses firefly luciferase under the control of the SV40 early enhancer/promoter, has been modified to allow to analyze the effects of 3′UTR sequences on mRNA stability. The pIS0 plasmid was a gift from David Bartel (MIT and Howard Hughes Medical Institute, Cambridge, MA, USA) (Addgene #12178) (Yekta et al., 2004). The nucleotide sequence of the 3′UTR of the murine Chd7 transcript (NM_001277149.1) (9511-10509) was purchased as a gBlocks Gene Fragment (IDT). The gBlocks Gene Fragment was PCR amplified using sequence specific primers with SacI and NheI restriction sites (Chd7-SacI-F, CTG AGC TCC AGC AGC AGT TCC ACT GAA; Chd7-NheI-R, AAG CTA GCT CTC TGC ATA TCA TGG GTC AC) and the resulting sequence was inserted into SacI and NheI sites of the pISO plasmid. The pIS2 plasmid, a derivative of pRL-SV40 (Promega), which expresses Renilla luciferase under the control of the SV40 early enhancer/promoter was used as transfection control. The pIS2 plasmid was a gift from David Bartel (Addgene #12177) (Farh et al., 2005).
Mice breeding and genotyping
All experiments and procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committees protocol, and all experiments and procedures adhered to National Institutes of Health-approved standards. The let-7g transgenic mice Col1a-TRE-let-7S21L (MGI: 5294613) (Zhu et al., 2011) were obtained from George Q. Daley (Children's Hospital and Howard Hughes Medical Institute, Boston, MA, USA). The R26-M2-rtTA mice (MGI: 3798943) (Hochedlinger et al., 2005) were purchased from Jackson Laboratories (stock number 006965). Atoh1/nEGFP transgenic mice (MGI: 3703598) (Lumpkin et al., 2003) were obtained from Jane Johnson (University of Texas, Southwestern Medical Center, USA). Mice were genotyped by PCR using the following primers: Let-7: ColfrtA/ GCA CAG CAT TGC GGA CAT GC, ColfrtB/ CCC TCC ATG TGT GAC CAA GG, ColfrtC/ GCA GAA GCG CGG CCG TCT GG; M2-rtTA: rtTAA/ GCG AAG AGT TTG TCC TCA ACC, rtTAB/ AAA GTC GCT CTG AGT TGT TAT, rtTAC/ GGA GCG GGA GAA ATG GAT ATG; Atoh1/nGFP: EGFP1/CGA AGG CTA CGT CCA GGA GCG CAC, EGFP2/GCA CGG GGC CGT CGC CGA TGG GGG TGT. Mice of both sexes were used in this study. All mouse lines were maintained on a mixed background of C57BL/6 and CD-1. Embryonic development was considered as E0.5 on the day a mating plug was observed. To induce the expression of the let-7g transgene, dox was delivered to time-mated females via ad libitum access to feed containing 2 g of doxycycline per kg of feed (Bioserv F3893), which was continued until the stage when the tissue was harvested.
Inner ear paint-fills
The inner ears were paint-filled as previously described (Bissonnette and Fekete, 1996). Briefly, chicken embryos or bisected mouse heads were fixed overnight in Bodian fixative (75% ethanol, 5% formalin and 5% glacial acetic acid), dehydrated overnight in 100% ethanol and cleared for ∼1 week in methyl salicylate. The inner ears were manually injected using glass capillary needles (Borosil 1 mm; FHC) pulled on a micro-pipet puller (Sutter Instruments) with 0.2% ‘Whiteout’ paint in methyl salicylate. All steps were conducted at room temperature. The paint-filled inner ears were imaged on black background with a stereo microscope (Leica Microsystems). The size and morphology of inner ears and their components were classified as normal (+++), reduced/smaller (++), severely reduced (+), truncated (t) or missing (−) using visual inspection.
RNA in situ hybridization, immunohistochemistry and EdU incorporation assay
Chicken and mouse embryos were staged using Hamburger Hamilton staging criteria (Hamburger and Hamilton, 1951) and EMAP eMouse Atlas Project (http://www.emouseatalas.org) Theiler staging criteria, respectively. Whole embryos or heads were briefly fixed in 4% (vol/vol) paraformaldehyde (PFA) (Electron Microscopy Sciences) in 1×PBS, cryoprotected using 30% (vol/vol) sucrose in 1×PBS overnight, and embedded in OCT (Sakura Finetek). Tissue was sectioned at a thickness of 14 µm and collected on SuperFrost Plus slides (Thermo Scientific) and stored at −80°C. In all experiments, sections for the left internal control BPs were collected on the same slides as the right experimental treated BPs. In situ hybridization assays to detect mRNA and mature miRNA transcripts were carried out as previously described (Raft et al., 2007; Golden et al., 2015). Digoxigenin (Dig)-labeled antisense RNA probes were used to detect Gfp and chicken Atoh1, Lfng and Sox2 mRNA. Dig-labeled-Locked nucleic acid (LNA) probes were used for in situ detection of mature let-7g-5p, let-7b-5p, let-7c-5p and mir-183-5p miRNAs according to the manufacturer's specifications (Exiqon, Qiagen) (Obernosterer et al., 2007). The primary antibodies used were goat polyclonal anti-GFP-(FITC) (1:400; GeneTex, GTX26662), rabbit polyclonal anti-SOX2 (1:4000 with 10 min antigen retrieval in citrate buffer; Millipore, AB5603), goat polyclonal anti-SOX2 (1:500, Santa Cruz Biotechnology, sc-17320), goat polyclonal anti-JAG1 (1:500, Santa Cruz Biotechnology, sc-6011), rabbit monoclonal anti-CHD7 (1:500; Cell Signaling, 6505), rabbit monoclonal anti-activated caspase 3 (CASP3) (1:800 with 10 min antigen retrieval in citrate buffer, Cell Signaling, 9664), mouse monoclonal anti-phospho-histone 3 (p-H3) (1:500; Millipore, 05-806), mouse monoclonal anti-Tuj1-IgG2a (1:500, BioLegend, 801202), rabbit polyclonal anti-myosin VIIa (MYO7A) (1:1000, Proteus Biosciences, 25-6790) and mouse monoclonal anti-hair cell antigen (HCA) (1:1000, gift from Guy Richardson, University of Sussex, Falmer, UK). The secondary antibodies were goat anti-rabbit Alexa Fluor 488 (1:250, Invitrogen, A11034), goat anti-mouse IgG2a 647 (1:250, Sigma, SAB4600355), donkey anti-goat Alexa Fluor 546 (1:250, Invitrogen, A11056), donkey anti-rabbit Alexa Fluor 546 (1:250, Invitrogen, A10040), donkey anti-mouse Alexa Fluor 647 (1:250, Invitrogen, A10040) and donkey anti-rabbit Alexa Fluor 647 (1:250, Invitrogen, A31573). Antibody labeling was performed according to manufacturer's recommendations. Hoechst-33258 solution (Sigma, 94403) was used to visualize cell nuclei. To analyze the rate of cell proliferation, 50 µl of 0.25 mg/ml EdU (5-ethynyl-2′-deoxyuridine) in 1×PBS sterile-filtered was added to the whole embryo in ovo as previously described (Evsen and Doetzlhofer, 2016). Click-iT AlexaFluor-488 or -546 Kit (Invitrogen A10266 and A20012) was used to detect incorporated EdU according to the manufacturer's specifications.
Western blots
Cochleae were isolated from E15.5 iLet-7g transgenic embryos and control littermates by micro-dissection and corresponding cochlear epithelia were enzymatically purified as previously described (Golden et al., 2015). Whole-brain tissue from E11.5 iLet-7g transgenic embryos and control littermates were isolated by micro-dissection. Cochlear epithelia and brains from individual animals were lyzed in RIPA lysis buffer (Sigma) supplemented with Roche Protease Inhibitor (Sigma) and Phosphatase Inhibitor Cocktail number 2 and number 3 (Sigma). Following the manufacturer's recommendations, equal amounts of cochlear epithelia or whole-brain protein extract were resolved on NuPAGE 4-12% Bis-Tris Gels (Invitrogen, NP0322BOX) or NuPAGE 3-8% Tris-Acetate Gels (Invitrogen, EA03752PK2) and transferred to Immuno-Blot PVDF membrane (Bio-Rad) by electrophoresis. Membranes were blocked in 5% non-fat dry milk in TBST and immunoblotted with rabbit monoclonal anti-CHD7 (1:2000, Cell Signaling, 6505), rabbit polyclonal anti-TRIM71 (1: 2000, gift from Gregory Wulczyn, Charité-Universitätsmedizin Berlin, Germany) and mouse anti-β-actin (1:400, Santa Cruz, no. sc-47778) used as loading control. The HRP-conjugated secondary antibodies used were goat anti-rabbit IgG (1: 5000, Jackson ImmunoResearch, 111-035-003) and sheep anti-mouse IgG (1: 5000, Jackson ImmunoResearch 515-035-003). Signal was revealed using either SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, 34577) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, 34096) according to manufacturer's instructions. To quantify protein levels, X-ray films were scanned, digital images converted to gray scale and the relative density (intensity) of bands was analyzed using the Gel Analysis plug in ImageJ. Protein levels were normalized using β-actin.
RNA extraction, RT-qPCR and TaqMan assays
Chicken auditory epithelia were isolated from wild-type chicken BPs by incubating the tissue in thermolysin (500 μg/ml in 1×PBS, Sigma) for 20 min at 37°C. Wild-type murine auditory epithelia were enzymatically isolated as previously described (Golden et al., 2015). For each experiment a minimum of four auditory epithelia were pooled per condition. At a minimum, three independent experiments were conducted. MiRNeasy Micro kit (Qiagen) was used to isolate total RNA, including miRNAs. mRNA was reverse transcribed into cDNA using iSuperscript kit (Bio-Rad) and qPCR was performed using SYBR Green kit (Life Technologies) and gene-specific primer sets on a StepOne Plus PCR Detection System (Applied Biosystems Life Technologies). Q-PCR reactions were carried out in triplicate. Relative gene expression was analyzed using the ΔΔCT method (Livak and Schmittgen, 2001). The ribosomal protein L19 gene (Rpl19) was used as endogenous reference gene. The following primers were used for qPCR in the chicken BP: Rpl19-F, CAG GAA GTT AAT TAA GGA TGG TTT GA; Rpl19-R, CAT CGT GCC CGA GAG TGAA; Atoh1-F, CAA CGA CAA GAA GCT CTC CAA GT; Atoh1-R, GGG CGC TGA TGT AGA TTT GC; Trim71-F, GGC ACT CTG GAA GCA CTT TGA; Trim71-R, AAG TGG CCC TCG TGA TTG AA; Nefm-F, TCG AAA TTG CTG CAT ACA GGA A; Nefm-R, CCA GAG AAG GCA CTG AAT CTT GT; Hmga2-F, TGG CCT CAA CAA GTG GTT CA; Hmga2-R, TCT TGT GAC GAT GTT TCT TCA GTC T; Lin28b-F, GCC TTG AAT CAA TAC GGG TAA CA; Lin28b-R, GGG TCG TCT TTC ACT TCC TAA ACA. To quantify the expression of mature let-7a-5p, let-7a-3p, let-7b-5p, let-7c-5p, let-7c-1-3p, let-7g-5p, let-7i-5p and mir-125b-5p transcripts, predesigned TaqMan Assays (Applied Biosystems, Life Technologies) were used according to the manufacturer's instructions. The snRNA U6 was used as an endogenous reference gene for TaqMan-based miRNA measurements.
Luciferase reporter assay
HEK 293T (ATCC CRL-3216) cells were split into 12-well plates and equal amounts of firefly-luciferase-Chd7-3′UTR (pIS0-Chd7-3′UTR) (1 µg/µl) plasmid and Renilla luciferase (pIS2) (1 µg/µl) plasmid were co-transfected with let-7b-GFP (1 µg/µl) or let-7 sponge plasmids (1 µg/µl or otherwise stated) using Fugene-6 (Promega). 48 h after transfection, cells were washed with PBS and luciferase activities were measured with a Dual-Luciferase Assay Kit (Promega) using a TD-20/20 Luminometer (Turner Designs) following the manufacturers' recommendations. Luciferase assays in HEK 293T cells were performed at least in triplicate.
Imaging and image analysis
In situ and immuno-labeled tissue sections of experimental and control animals were processed at the same time and imaged with identical settings using upright and LSM 700 confocal microscopes (Zeiss). In all electroporation experiments, the tissue sections of the left BP (internal control) are collected on the same slides as tissue sections of the right treated BP (experimental). Images were viewed and analyzed in Photoshop CS6 (Adobe) and LSM image viewer (Zeiss). A minimum of three animals per treatment and genotype was analyzed for all experiments. For analysis of SOX2, JAG1 and CHD7 protein expression in control and let-7b electroporated BP tissue sections, ImageJ software was used to quantify fluorescent intensity in background corrected single plane confocal images, taken with identical settings. Using the shape tool, the pro-sensory domain was outlined in control and let-7b electroporated BP tissue and the fluorescent intensity within the outlined pro-sensory domain measured. Three or four sections representative of the apical, mid and basal pro-sensory domain of control and let-7b electroporated BPs were analyzed per animal and treatment, and their mean values reported. To determine whether let-7b overexpression or let-7 knockdown caused defects in HC differentiation, we conducted Atoh1 in situ hybridization experiments and scored Atoh1 mRNA expression in treated and untreated (control) BPs. To determine potential defects in pro-sensory cell specification/survival, we conducted Lfng in situ hybridization experiments and scored Lfng mRNA expression in treated and untreated control BPs. Four or five adjacent Atoh1- and Lfng-labeled cross-sections each representing base, mid and mid-apex were analyzed per animal and treatment. The following scale was used: 0, absent; 1, weak expression (more than half of sections showed reduced expression); 2, reduced expression (less than half of sections showed reduced expression); 3, normal expression.
Cell counts
Pro-sensory cell proliferation in the chick BP was quantified by counting SOX2+ pro-sensory cells and SOX2+ EdU+ pro-sensory cells in tissue sections. Four to six BP tissue sections representing apex, mid and base were analyzed per animal and treatment, and the mean percentage of SOX2+ EdU+ pro-sensory cells/section was reported. HC differentiation in stage ∼E6.5 BPs was quantified by manually counting MYO7A+ HCs in tissue section. Three or four BP tissue sections representing apex, mid and base were analyzed per animal and treatment, and the mean number of MYO7A+ cells/section was reported. Cell survival and cell proliferation in otic epithelia or at later stages in cochlear epithelia were analyzed by counting p-H3+ and activated-CASP3+ cells. Five or six sections per animal of E11.5 otic tissue spanning the dorsal-ventral extent or four to six sections of E13.5 cochlear tissue spanning the apical-basal extent were analyzed. Only p-H3+ or activated-CASP3+ cells within the otic epithelium or cochlear epithelium were analyzed. Data shown are the mean number of act-CASP3+ cells/section or the mean number p-H3+ cells/section. Cells were counted manually and a minimum of two independent experiments were conducted and a minimum of three animals were analyzed per genotype and treatment.
Statistical analyses
Data are presented as mean±standard deviation (s.d.) unless otherwise indicated, n=animals per group. All results were confirmed by at least two independent experiments. One-way ANOVA with Tukey's post hoc test or two-tailed unpaired and paired Student's t-tests were used to determine confidence interval. P≤0.05 was considered significant. P>0.05 was considered not significant.
Supplementary Material
Acknowledgments
We thank the members of A.D.’s laboratory for the help and advice provided throughout the course of this study. We thank Dr Doris K. Wu for in situ probes and Dr Georg Dailey for iLet-7 mice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.E., A.D.; Methodology: L.E., X.L., S.Z., S.R.; Validation: L.E., X.L., S.Z., S.R.; Formal analysis: L.E., X.L., A.D.; Investigation: L.E., X.L., S.Z., S.R.; Resources: A.D.; Data curation: A.D.; Writing - original draft: L.E., A.D.; Writing - review & editing: L.E., A.D.; Supervision: A.D.; Project administration: A.D.; Funding acquisition: A.D.
Funding
The work was supported by National Institute on Deafness and Other Communication Disorders (T32 DC000023 to L.E., R01 DC011571 to A.D. and P30 DC005211 to the Sensory Mechanisms Research Core Center); and by the David M. Rubenstein Fund for Hearing Research (A.D.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.183384.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.183384.reviewer-comments.pdf
References
- Agarwal V., Bell G. W., Nam J. W. and Bartel D. P. (2015). Predicting effective microRNA target sites in mammalian mRNAs. eLife 4, e05005 10.7554/eLife.05005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Wong E. Y. M., Sun J., Xu J., Wang F. and Xu P.-X. (2012). Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 22, 377-390. 10.1016/j.devcel.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambros V. and Horvitz H. R. (1984). Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409-416. 10.1126/science.6494891 [DOI] [PubMed] [Google Scholar]
- Bajpai R., Chen D. A., Rada-Iglesias A., Zhang J. M., Xiong Y. Q., Helms J., Chang C.-P., Zhao Y. M., Swigut T. and Wysocka J. (2010). CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463, U958-U135. 10.1038/nature08733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Gonzalez A. and Doetzlhofer A. (2014). Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J. Neurosci. 34, 12865-12876. 10.1523/JNEUROSCI.1494-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham N. A., Hassan B. A., Price S. D., Vollrath M. A., Ben-Arie N., Eatock R. A., Bellen H. J., Lysakowski A. and Zoghbi H. Y. (1999). Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837-1841. 10.1126/science.284.5421.1837 [DOI] [PubMed] [Google Scholar]
- Bhattacharya D., Rothstein M., Azambuja A. P. and Simoes-Costa M. (2018). Control of neural crest multipotency by Wnt signaling and the Lin28/let-7 axis. Elife 7, e40556 10.7554/eLife.40556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette J. P. and Fekete D. M. (1996). Standard atlas of the gross anatomy of the developing inner ear of the chicken. J. Comp. Neurol. 368, 620-630. [DOI] [PubMed] [Google Scholar]
- Bok J., Zenczak C., Hwang C. H. and Wu D. K. (2013). Auditory ganglion source of Sonic hedgehog regulates timing of cell cycle exit and differentiation of mammalian cochlear hair cells. Proc. Natl. Acad. Sci. USA 110, 13869-13874. 10.1073/pnas.1222341110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman E. A., Penn A. C., Ambrose J. C., Kettleborough R., Stemple D. L. and Steel K. P. (2005). Multiple mutations in mouse Chd7 provide models for CHARGE syndrome. Hum. Mol. Genet. 14, 3463-3476. 10.1093/hmg/ddi375 [DOI] [PubMed] [Google Scholar]
- Chai M., Sanosaka T., Okuno H., Zhou Z., Koya I., Banno S., Andoh-Noda T., Tabata Y., Shimamura R., Hayashi T. et al. (2018). Chromatin remodeler CHD7 regulates the stem cell identity of human neural progenitors. Genes Dev. 32, 165-180. 10.1101/gad.301887.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. and Segil N. (1999). p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581-1590. [DOI] [PubMed] [Google Scholar]
- Chen P., Johnson J. E., Zoghbi H. Y. and Segil N. (2002). The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495-2505. 10.1242/dev.00114 [DOI] [PubMed] [Google Scholar]
- Cimadamore F., Amador-Arjona A., Chen C., Huang C.-T. and Terskikh A. V. (2013). SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. USA 110, E3017-E3026. 10.1073/pnas.1220176110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche D. A. and Sulik K. K. (1984). The development of stereociliary bundles in the cochlear duct of chick embryos. Brain Res. 318, 181-193. 10.1016/0165-3806(84)90024-5 [DOI] [PubMed] [Google Scholar]
- Daudet N. and Lewis J. (2005). Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132, 541-551. 10.1242/dev.01589 [DOI] [PubMed] [Google Scholar]
- Doi T., Ogata T., Yamauchi J., Sawada Y., Tanaka S. and Nagao M. (2017). Chd7 collaborates with Sox2 to regulate activation of oligodendrocyte precursor cells after spinal cord injury. J. Neurosci. 37, 10290-10309. 10.1523/JNEUROSCI.1109-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert M. S. and Sharp P. A. (2010). MicroRNA sponges: progress and possibilities. RNA 16, 2043-2050. 10.1261/rna.2414110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen E., Akinci U., Bryne J. C., Hou J., Gontan C., Moen M., Szumska D., Kockx C., Van Ijcken W., Dekkers D. H. et al. (2011). Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 43, 607-611. 10.1038/ng.825 [DOI] [PubMed] [Google Scholar]
- Evsen L. and Doetzlhofer A. (2016). Gene transfer into the chicken auditory organ by In Ovo micro-electroporation. J. Vis. Exp. 110, e53864 10.3791/53864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farh K. K.-H., Grimson A., Jan C., Lewis B. P., Johnston W. K., Lim L. P., Burge C. B. and Bartel D. P. (2005). The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310, 1817-1821. 10.1126/science.1121158 [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Muthukumar S. and Karagogeos D. (1998). Hair cells and supporting cells share a common progenitor in the avian inner ear. J. Neurosci. 18, 7811-7821. 10.1523/JNEUROSCI.18-19-07811.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Khan M. A., Bellvis P., Zhu Z., Bernhardt O., Herold-Mende C. and Liu H. K. (2013). The chromatin remodeler CHD7 regulates adult neurogenesis via activation of SoxC transcription factors. Cell Stem Cell 13, 62-72. 10.1016/j.stem.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Fritzsch B., Pan N., Jahan I., Duncan J. S., Kopecky B. J., Elliott K. L., Kersigo J. and Yang T. (2013). Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evol. Dev. 15, 63-79. 10.1111/ede.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. J., Hurd E. A. and Martin D. M. (2015). Mouse models for the dissection of CHD7 functions in eye development and the molecular basis for ocular defects in CHARGE syndrome. Invest. Ophthalmol. Vis. Sci. 56, 7923-7930. 10.1167/iovs.15-18069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnedeva K. and Hudspeth A. J. (2015). SoxC transcription factors are essential for the development of the inner ear. Proc. Natl. Acad. Sci. USA 112, 14066-14071. 10.1073/pnas.1517371112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden E. J., Benito-Gonzalez A. and Doetzlhofer A. (2015). The RNA-binding protein LIN28B regulates developmental timing in the mammalian cochlea. Proc. Natl. Acad. Sci. USA 112, E3864-E3873. 10.1073/pnas.1501077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear R. and Richardson G. (1997). Pattern formation in the basilar papilla: evidence for cell rearrangement. J. Neurosci. 17, 6289-6301. 10.1523/JNEUROSCI.17-16-06289.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear R. J., Legan P. K., Wright M. B., Marcotti W., Oganesian A., Coats S. A., Booth C. J., Kros C. J., Seifert R. A., Bowen-Pope D. F. et al. (2003). A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J. Neurosci. 23, 9208-9219. 10.1523/JNEUROSCI.23-27-09208.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. and Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92. 10.1002/jmor.1050880104 [DOI] [PubMed] [Google Scholar]
- Hasson T., Heintzelman M. B., Santos-Sacchi J., Corey D. P. and Mooseker M. S. (1995). Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc. Natl. Acad. Sci. USA 92, 9815-9819. 10.1073/pnas.92.21.9815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwak A., Kudla G., Dudnakova T. and Tollervey D. (2013). Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 153, 654-665. 10.1016/j.cell.2013.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K., Yamada Y., Beard C. and Jaenisch R. (2005). Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell 121, 465-477. 10.1016/j.cell.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Hurd E. A., Capers P. L., Blauwkamp M. N., Adams M. E., Raphael Y., Poucher H. K. and Martin D. M. (2007). Loss of Chd7 function in gene-trapped reporter mice is embryonic lethal and associated with severe defects in multiple developing tissues. Mamm. Genome 18, 94-104. 10.1007/s00335-006-0107-6 [DOI] [PubMed] [Google Scholar]
- Hurd E. A., Poucher H. K., Cheng K., Raphael Y. and Martin D. M. (2010). The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development 137, 3139-3150. 10.1242/dev.047894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd E. A., Adams M. E., Layman W. S., Swiderski D. L., Beyer L. A., Halsey K. E., Benson J. M., Gong T. W., Dolan D. F., Raphael Y. et al. (2011). Mature middle and inner ears express Chd7 and exhibit distinctive pathologies in a mouse model of CHARGE syndrome. Hear. Res. 282, 184-195. 10.1016/j.heares.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A. and Corwin J. T. (1989). Cell production in the chicken cochlea. J. Comp. Neurol. 281, 129-135. 10.1002/cne.902810110 [DOI] [PubMed] [Google Scholar]
- Kempfle J. S., Turban J. L. and Edge A. S. (2016). Sox2 in the differentiation of cochlear progenitor cells. Sci. Rep. 6, 23293 10.1038/srep23293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Pelling A. L., Leung K. K., Tang A. S., Bell D. M., Tease C., Lovell-Badge R., Steel K. P. and Cheah K. S. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031-1035. 10.1038/nature03487 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J. and Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 10.1371/journal.pgen.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Han S., Kwon C. S. and Lee D. (2016). Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell 7, 100-113. 10.1007/s13238-015-0212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-C., Hsieh L.-C., Kuo M.-W., Yu J., Kuo H.-H., Lo W.-L., Lin R.-J., Yu A. L. and Li W. H. (2007). Human TRIM71 and its nematode homologue are targets of let-7 microRNA and its zebrafish orthologue is essential for development. Mol. Biol. Evol. 24, 2525-2534. 10.1093/molbev/msm195 [DOI] [PubMed] [Google Scholar]
- Livak K. J. and Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402-408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lumpkin E. A., Collisson T., Parab P., Omer-Abdalla A., Haeberle H., Chen P., Doetzlhofer A., White P., Groves A., Segil N. et al. (2003). Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr. Patterns 3, 389-395. 10.1016/S1567-133X(03)00089-9 [DOI] [PubMed] [Google Scholar]
- Maller Schulman B. R., Liang X., Stahlhut C., Delconte C., Stefani G. and Slack F. J. (2008). The let-7 microRNA target gene, Mlin41/Trim71 is required for mouse embryonic survival and neural tube closure. Cell Cycle 7, 3935-3942. 10.4161/cc.7.24.7397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melicharek D., Shah A., Distefano G., Gangemi A. J., Orapallo A., Vrailas-Mortimer A. D. and Marenda D. R. (2008). Identification of novel regulators of atonal expression in the developing Drosophila retina. Genetics 180, 2095-2110. 10.1534/genetics.108.093302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V. and Fekete D. M. (2013). Wnt signaling during cochlear development. Semin. Cell Dev. Biol. 24, 480-489. 10.1016/j.semcdb.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J., Parada C., Chamizo M. and Giraldez F. (2011). Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development 138, 735-744. 10.1242/dev.060657 [DOI] [PubMed] [Google Scholar]
- Neves J., Uchikawa M., Bigas A. and Giraldez F. (2012). The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS ONE 7, e30871 10.1371/journal.pone.0030871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D. T. T., Richter D., Michel G., Mitschka S., Kolanus W., Cuevas E. and Wulczyn F. G. (2017). The ubiquitin ligase LIN41/TRIM71 targets p53 to antagonize cell death and differentiation pathways during stem cell differentiation. Cell Death Differ. 24, 1063-1078. 10.1038/cdd.2017.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni N., Zhang D., Xie Q., Chen J., Wang Z., Deng Y., Wen X., Zhu M., Ji J., Fan X. et al. (2014). Effects of let-7b and TLX on the proliferation and differentiation of retinal progenitor cells in vitro. Sci. Rep. 4, 6671 10.1038/srep06671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J., Kim I., Chada K. and Morrison S. J. (2008). Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 135, 227-239. 10.1016/j.cell.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J., Kim S., Zhu Y., Zhu H. and Morrison S. J. (2013). A network of heterochronic genes including Imp1 regulates temporal changes in stem cell properties. Elife 2, e00924 10.7554/eLife.00924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernosterer G., Martinez J. and Alenius M. (2007). Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat. Protoc. 2, 1508-1514. 10.1038/nprot.2007.153 [DOI] [PubMed] [Google Scholar]
- Pagon R. A., Graham J. M. Jr, Zonana J. and Yong S. L. (1981). Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J. Pediatr. 99, 223-227. 10.1016/S0022-3476(81)80454-4 [DOI] [PubMed] [Google Scholar]
- Pan W., Jin Y., Stanger B. and Kiernan A. E. (2010). Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc. Natl. Acad. Sci. USA 107, 15798-15803. 10.1073/pnas.1003089107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Jin Y., Chen J., Rottier R. J., Steel K. P. and Kiernan A. E. (2013). Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J. Neurosci. 33, 16146-16157. 10.1523/JNEUROSCI.3150-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran S., Xia X., Hegde G. and Ahmad I. (2014). Hmga2 regulates self-renewal of retinal progenitors. Development 141, 4087-4097. 10.1242/dev.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou M. D., Georgakilas G., Kostoulas N., Vlachos I. S., Vergoulis T., Reczko M., Filippidis C., Dalamagas T. and Hatzigeorgiou A. G. (2013). DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 41, W169-W173. 10.1093/nar/gkt393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E., Viswanathan S. R., Janas M., LaPierre R. J., Daley G. Q., Sliz P. and Gregory R. I. (2008). Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 283, 21310-21314. 10.1074/jbc.C800108200 [DOI] [PubMed] [Google Scholar]
- Raft S., Koundakjian E. J., Quinones H., Jayasena C. S., Goodrich L. V., Johnson J. E., Segil N. and Groves A. K. (2007). Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development 134, 4405-4415. 10.1242/dev.009118 [DOI] [PubMed] [Google Scholar]
- Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., Rougvie A. E., Horvitz H. R. and Ruvkun G. (2000). The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901-906. 10.1038/35002607 [DOI] [PubMed] [Google Scholar]
- Roush S. and Slack F. J. (2008). The let-7 family of microRNAs. Trends Cell Biol. 18, 505-516. 10.1016/j.tcb.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Ruben R. J. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. Suppl. 220, 1-44. [PubMed] [Google Scholar]
- Rudnicki A., Isakov O., Ushakov K., Shivatzki S., Weiss I., Friedman L. M., Shomron N. and Avraham K. B. (2014). Next-generation sequencing of small RNAs from inner ear sensory epithelium identifies microRNAs and defines regulatory pathways. BMC Genomics 15, 484 10.1186/1471-2164-15-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz M. P., Bartels C. F., Shastri K., Balasubramanian D., Zentner G. E., Balaji R., Zhang X., Song L., Wang Z., Laframboise T. et al. (2009). Genomic distribution of CHD7 on chromatin tracks H3K4 methylation patterns. Genome Res. 19, 590-601. 10.1101/gr.086983.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz M. P., Handoko L., Akhtar-Zaidi B., Bartels C. F., Pereira C. F., Fisher A. G., Adams D. J., Flicek P., Crawford G. E., Laframboise T. et al. (2010). CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet. 6, e1001023 10.1371/journal.pgen.1001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Cheng Y. F., Wang X. L. and Edge A. S. (2010). Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J. Biol. Chem. 285, 392-400. 10.1074/jbc.M109.059055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son E. J., Ma J. H., Ankamreddy H., Shin J. O., Choi J. Y., Wu D. K. and Bok J. (2015). Conserved role of Sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc. Natl. Acad. Sci. USA 112, 3746-3751. 10.1073/pnas.1417856112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateya T., Imayoshi I., Tateya I., Hamaguchi K., Torii H., Ito J. and Kageyama R (2013). Hedgehog signaling regulates prosensory cell properties during the basal-to-apical wave of hair cell differentiation in the mammalian cochlea. Development 140, 3848-3857. 10.1242/dev.095398 [DOI] [PubMed] [Google Scholar]
- Tian W., Dong X., Liu X., Wang G., Dong Z., Shen W., Zheng G., Lu J., Chen J., Wang Y. et al. (2012). High-throughput functional microRNAs profiling by recombinant AAV-based microRNA sensor arrays. PLoS ONE 7, e29551 10.1371/journal.pone.0029551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness L. D., Wang X., Shibata S., Ohyama T. and Mansour S. L. (2015). Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev. Biol. 400, 59-71. 10.1016/j.ydbio.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S. R., Daley G. Q. and Gregory R. I. (2008). Selective blockade of microRNA processing by Lin28. Science 320, 97-100. 10.1126/science.1154040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakiyama M., Takimoto K., Ohara O. and Yokoyama S. (2007). Let-7 microRNA-mediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev. 21, 1857-1862. 10.1101/gad.1566707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. A., Viswanathan S. R., Yabuuchi A., Cunniff K., Takeuchi A., Park I. H., Sero J. E., Zhu H., Perez-Atayde A., Frazier A. L. et al. (2009). A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460, 909-913. 10.1038/nature08210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Yang S. L., Herrlinger S., Liang C., Dzieciatkowska M., Hansen K. C., Desai R., Nagy A., Niswander L., Moss E. G. et al. (2015). Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 142, 1616-1627. 10.1242/dev.120543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. A., Norbury C. J. and Gilbert R. J. (2013). The long and short of microRNA. Cell 153, 516-519. 10.1016/j.cell.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Yekta S., Shih I. H. and Bartel D. P. (2004). MicroRNA-directed cleavage of HOXB8 mRNA. Science 304, 594-596. 10.1126/science.1097434 [DOI] [PubMed] [Google Scholar]
- Zhang K. D., Stoller M. L. and Fekete D. M. (2015). Expression and misexpression of the miR-183 family in the developing hearing organ of the Chicken. PLoS ONE 10, e0132796 10.1371/journal.pone.0132796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Sun G., Li S., Lang M.-F., Yang S., Li W. and Shi Y. (2010). MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc. Natl. Acad. Sci. USA 107, 1876-1881. 10.1073/pnas.0908750107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. L. and Gao W. Q. (2000). Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580-586. 10.1038/75753 [DOI] [PubMed] [Google Scholar]
- Zhu H., Shyh-Chang N., Segre A. V., Shinoda G., Shah S. P., Einhorn W. S., Takeuchi A., Engreitz J. M., Hagan J. P., Kharas M. G. et al. (2011). The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81-94. 10.1016/j.cell.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.