ABSTRACT
The high prevalence of obesity and its associated metabolic diseases has heightened the importance of understanding control of adipose tissue development and energy metabolism. In mammals, 3 types of adipocytes with different characteristics and origins have been identified: white, brown, and beige. Beige and brown adipocytes contain numerous mitochondria and have the capability to burn energy and counteract obesity, while white adipocytes store energy and are closely associated with metabolic disorders and obesity. Thus, regulation of the development and function of different adipocytes is important for controlling energy balance and combating obesity and related metabolic disorders. Melatonin is a neurohormone, which plays multiple roles in regulating inflammation, blood pressure, insulin actions, and energy metabolism. This article summarizes and discusses the role of melatonin in white, beige, and brown adipocytes, especially in affecting adipogenesis, inducing beige formation or white adipose tissue browning, enhancing brown adipose tissue mass and activities, improving anti-inflammatory and antioxidative effects, regulating adipokine secretion, and preventing body weight gain. Based on the current findings, melatonin is a potential therapeutic agent to control energy metabolism, adipogenesis, fat deposition, adiposity, and related metabolic diseases.
Keywords: melatonin, adipocyte, beige, adiposity, adipogenesis, metabolic disorder
Introduction
The obesity epidemic and its associated metabolic diseases has heightened the significance of understanding the mechanisms controlling adipose tissue development. In mammals, adipose tissue is generally classified as white adipose tissue (WAT) and brown adipose tissue (BAT). These 2 kinds of adipose tissues have distinct morphologies and functions. WAT, consisting of mature white adipocytes and preadipocytes, serves as the major energy storing tissue and is closely associated with obesity (1, 2). BAT, containing mature brown adipocytes with numerous mitochondria and high amounts of uncoupling protein 1 (UCP1), can dissipate extra energy as heat and mediate nonshivering thermogenesis (3, 4). Recently, a third type of adipocytes called beige or “brite” (brown in white) adipocytes has been identified in WAT after cold exposure or chemical stimulation (5–7). Beige adipocytes are a kind of brown-like cells that contain lots of mitochondria with high expression of UCP1 (5). Both brown and beige adipocytes have the capability to burn energy and counteract obesity. Interestingly, UCP1-positive brown and beige adipocytes have been observed in adult humans (8–13). Moreover, the appearance and activities of brown and beige adipocytes in humans are inversely correlated with obesity and insulin resistance (14, 15), suggesting a crucial role for beige and brown adipocytes in regulating energy balance and fat deposition. Thus, understanding the development and regulation of adipose tissues, especially the brown and beige fat cells, may provide novel strategies for combating obesity and related metabolic disorders.
A number of factors have been identified that regulate adipose development and biogenesis of brown and beige adipocytes (16–22). Gender, genetics, and cold exposure affect metabolism of different adipose tissues, beige formation, and BAT thermogenesis (16, 17, 23). Aging changes the morphology and function of BAT and decreases UCP1 expression and thermogenic capacity (16, 17). Several microRNAs, transcriptional and epigenetic factors, and signaling pathways can regulate adipose tissue development and biogenesis of brown and beige adipocytes (18–22, 24). Moreover, in rodent and cell studies, hormones, chemical factors, and dietary factors including irisin (7, 25, 26), melatonin (27–30), bone morphogenetic proteins (31), fibroblast growth factor 21 (25, 32), resveratrol (33), green tea catechins (34), capsinoids (35), and berberine (36), have been identified as key regulators of the development and function of brown and beige adipocytes. These studies suggest that the regulation of brown and beige adipocytes provides a variety of promising therapeutic targets for controlling metabolic disorders.
Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone originally isolated from bovine pineal tissue (37). It is mainly synthesized and secreted by the pineal gland during nighttime (38, 39) and is involved in control of the circadian rhythm in mammals (38, 40, 41). Melatonin is also produced in other tissues or organs including the gut, ovary, testes, and retina (42–44). In addition, melatonin is found in herbs, olive oil, vegetables, fruits, and red wine (43, 45–48). Multiple physiological functions of melatonin have been reported, including circadian and seasonal rhythms (49), antioxidant (50–52) and anti-inflammatory properties (39, 53–55), tumor growth inhibition (56–60), tissue repair and regeneration (61–63), and metabolism (43, 64–66). Evidence indicates that melatonin is involved in regulation of adipose tissue development, lipid accumulation, body weight, BAT activation, and browning of WAT (27–29, 43, 64, 65, 67). In rats and Siberian hamsters, melatonin promotes weight loss through stimulation of WAT browning (27, 68) and BAT growth and activation (28, 43, 69). In addition, melatonin also affects differentiation of preadipocytes (70–72) and mesenchymal stem cells (42, 73), and is involved in regeneration of several tissues including bone (74, 75), heart (76, 77), lung (62, 78), kidney (63, 79), liver (80), and gut (81, 82).
The roles of melatonin in regulation of energy metabolism and obesity have been reviewed recently and discussed by different laboratories (55, 64–66, 83, 84). Cardinali and Vigo addressed the role of melatonin in attenuating inflammatory responses in metabolic syndrome (65). Cipolla-Neto et al. discussed the effects of melatonin on energy metabolism, energy balance, and obesity (64). Navarro-Alarcón et al. mainly reviewed the role of melatonin in energy expenditure, body weight, oxidative stress, hyperglycemia, and postprandial dysmetabolism (66). In addition, Szewczyk-Golec et al. discussed the interrelations between melatonin and the endocrine products of adipocytes, leptin, and adiponectin, as well as their implications for obesity (84). In the current review, we describe the characteristics and origins of white, beige, and brown adipocytes. Moreover, we summarize and discuss recent findings in the regulatory roles of melatonin in adipocytes, especially in regulating adipogenesis and cell fates of brown, white, and beige adipocytes.
Characteristics of White, Beige, and Brown Adipocytes
The main characteristics and morphological differences of white, beige, and brown adipocytes are shown in Figure 1. Most mammals have 2 major types of WAT depots, subcutaneous and visceral (or intra-abdominal). White adipocytes contain few mitochondria and a single large lipid droplet (Figure 1). In addition, white adipocytes express high amounts of transcription factor 21 (Tcf21), and homeobox protein Hox-C9 (Hoxc9) (24, 85) (Figure 1).
FIGURE 1.
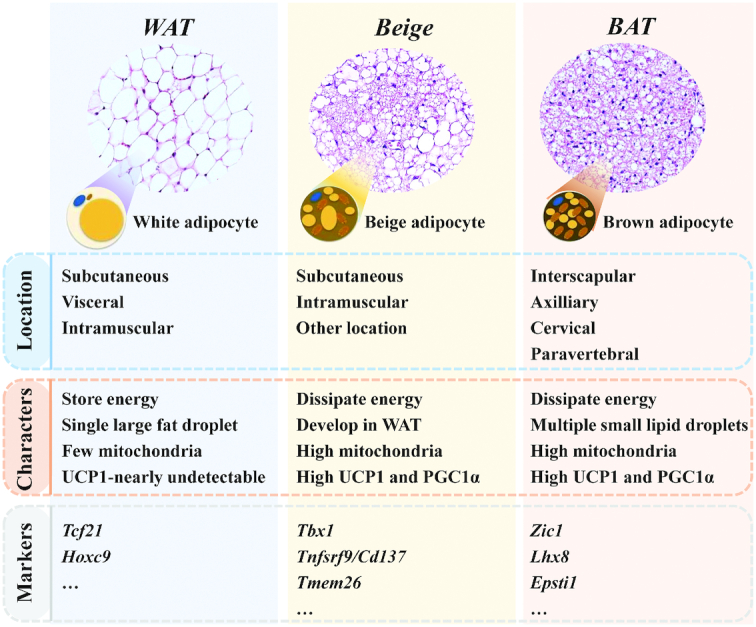
Morphology, characteristics and marker genes of white, beige, and brown adipocytes. BAT, brown adipose tissue; Characters, Characteristics; Epsti1, epithelial stromal interaction 1; Hoxc9, homeobox protein Hox-C9; Lhx8, LIM homeobox 8; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1-α; Tbx1, T-Box 1; Tcf21, transcription factor 21; Tmem26, transmembrane protein 26; Tnfsrf9/Cd137, tumor necrosis factor receptor superfamily member 9; UCP1, uncoupling protein 1; WAT, white adipose tissue; Zic1, Zic family member 1.
BAT is present and active in almost all mammals including human newborns (9, 11). Moreover, functionally active BAT was identified in adult humans challenged by external stimuli such as cold (9, 13, 86). At low ambient temperatures, BAT is primarily found in the interscapular space, axillary regions, paravertebrally, and perirenally (9, 13, 86). Brown adipocytes contain high amounts of mitochondria and multiple small lipid droplets (Figure 1). Brown adipocytes express high UCP1 and have the capacity to dissipate energy into heat. In addition, high Zic family member 1 (Zic1), LIM homeobox 8 (Lhx8), and epithelial stromal interaction 1 (Epsti1) are found in brown adipocytes (18, 24, 85) (Figure 1).
Beige adipocytes are the third type of adipocytes that have been identified within WAT after cold exposure, hormones, or chemical reagent stimulation (5–7). Beige adipocytes share many morphological and biochemical characteristics with white and brown adipocytes. Distinct to the white and brown adipocytes, beige cells express high T-Box 1 (Tbx1), tumor necrosis factor receptor superfamily member 9 (Tnfsrf9/Cd137), and transmembrane protein 26 (Tmem26) (5, 18, 24, 85, 87) (Figure 1). Beige adipocytes also contain many mitochondria and show high expression of UCP1 (5). Both beige and brown adipocytes have the capacity to burn energy into heat and express key thermogenic genes including Ucp1, Cidea, Prdm16, Pparα, and Pgc1α (24). Thus, enhancing the development and function of brown and beige adipocytes may represent a promising strategy in stimulating thermogenesis and treating obesity.
Origins of Brown, White, and Beige Adipocytes
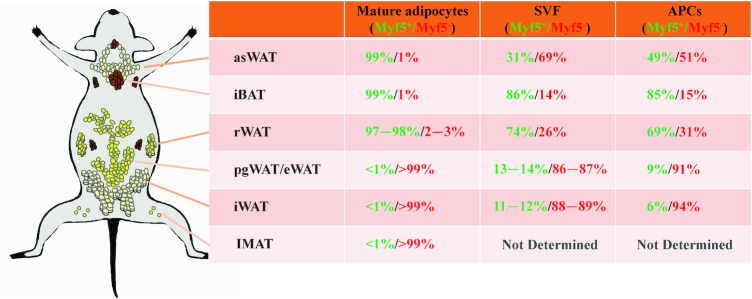
The origins of brown, white, and beige adipocytes are presented in Figure 2. Previous studies demonstrated that brown adipocytes are derived from the myogenic factor 5 (Myf5)-expressing cell lineage, whereas white adipocytes are from the non-Myf5-lineage progenitors (88). However, recent studies provide evidence that white and beige adipocytes derive from both Myf5-positive (Myf5+) and Myf5-negative (Myf5−) precursors (89–91). By lineage tracing, different groups quantified the Myf5-lineage contribution to the mature adipocytes (89–91), as well as to the adipose stromal vascular fractions and purified adipocyte precursor cells (89–91) (Figure 2). The contribution of Myf5-lineage precursors to BAT or WAT appears to vary between different adipose depots (Figure 2). Indeed, in the interscapular BAT and subscapular BAT, there are about 99% and 100% of the brown adipocytes, respectively, from Myf5-lineage cells (89). However, only 58−66% of brown adipocytes are traced with Myf5-Cre in cervical BAT (89). No Myf5+ brown adipocytes were found in perirenal BAT and periaortic brown fat (89). Interestingly, in anterior-subcutaneous WAT and retroperitoneal WAT, almost all of the mature adipocytes are from Myf5-lineage cells (89). Notably, Myf5-Cre does not label any mature adipocytes in perigonadal WAT/epididymal WAT, mesenteric WAT, and inguinal WAT (89).
FIGURE 2.
The lineage tracing results of WAT and BAT. The green numbers indicate Myf5-lineage, and the red numbers indicate non-Myf5-lineage. APC, adipocyte precursor cell; asWAT, anterior-subcutaneous white adipose tissue; BAT, brown adipose tissue; eWAT, epididymal white adipose tissue; iBAT, interscapular brown adipose tissue; IMAT, intermuscular/intramuscular adipose tissue; iWAT, inguinal white adipose tissue; pgWAT, perigonadal white adipose tissue; rWAT, retroperitoneal white adipose tissue; SVF, stromal vascular fractions; WAT, white adipose tissue.
Pax3, a paired box homeodomain transcriptional factor, is a key regulator of embryonic development (92). Pax3-positive cells give rise to brown fat, skeletal muscle, skin, and smooth muscle cells during embryonic development (92). By lineage tracing, 2 groups previously demonstrated that 99−100% of mature adipocytes in interscapular BAT are derived from Pax3-expressing cell lineage (89, 93). Similar to the Myf5-lineage pattern, almost all anterior-subcutaneous WAT and retroperitoneal WAT adipocytes are derived from Pax3-lineage, whereas no mature adipocytes are Pax3-lineage in mesenteric WAT or psWAT (89). Notably, 58% of the male perigonadal WAT adipocytes are Pax3-lineage cells (89). Likewise, the ratio of Pax3-lineage adipocyte precursor cells in most depots is nearly identical to that of Myf5-lineage (89).
Beige adipocytes have similar functions to brown adipocytes, but beige adipocytes have different origins and responses to browning reagents (5, 16, 17, 94). Interestingly, the non-Pax3-lineage cells contribute more significantly to beige cell formation than do the Pax3-lineages in the same WAT depot (90, 95). These results suggest that non-Pax3-lineage adipocytes are more responsive to browning of WAT. However, other studies demonstrate that beige adipocytes likely have different origins in different depots (91, 96).
Intermuscular/intramuscular adipose tissues (IMATs) are WAT depots that exist in skeletal muscle. Ectopic accumulation of IMAT is associated with insulin resistance and muscle wasting (93, 97). Recent advances in lineage tracing demonstrate that IMAT is exclusively derived from a non-Pax3-lineage and gives rise to white adipocytes in cultures (93). Likewise, only ∼1% of Myf5 lineage progenitors contribute to the intramuscular adipogenic precursor pool (96) (Figure 2). Moreover, the resident Sca-1+Cd34+Cd31−Cd45−fibro/adipogenic progenitors in skeletal muscle are derived from a non-Myf5-lineage (98). The ectopic fat cell formation and adipogenesis originate from platelet-derived growth factor receptor α-positive progenitors in skeletal muscle (99, 100). In addition, a subpopulation of inducible brown adipocyte progenitors has been identified to reside in skeletal muscle (101). These results suggest that IMAT is a type of WAT derived from non-Myf5 and non-Pax3-lineage cells.
Perivascular adipose tissue (PVAT) is the adipose tissue surrounding the blood vessels. PVAT not only serves as vessel-supporting connective tissue, but also acts as an endocrine organ releasing bioactive molecules to regulate vascular health (102, 103). The differences and similarities between PVAT compared with WAT, beige fat, or BAT have been reviewed (102, 103). Briefly, PVAT differs between location and species. The thoracic PVAT appears to be similar to BAT, whereas the abdominal PVAT is a WAT-like tissue (102–104). However, the origin of PVAT is distinct from either brown or white adipocytes (102, 103). PVAT originates from SM22αlineage precursor cells (102, 103, 105). However, future studies are needed to clarify the development and function of PVAT.
In addition to Myf5-Cre and Pax3-Cre, many other Cre lines have been used to trace the origins of preadipocytes and mature adipocytes, as has been well reviewed by others (96, 106). Taken together, the heterogeneous lineages and different origins could explain the metabolic and molecular differences between WAT, BAT, and beige adipocytes.
Regulatory Roles of Melatonin in White, Beige, and Brown Adipocytes
Because of the growing obesity epidemic in the world, there is great interest in development, biogenesis, and regulation of white, beige, and brown adipocytes. Promotion of BAT function or induction of beige formation in WAT may represent potential therapies to combat obesity and its associated disorders. Melatonin is one of the pineal secreted hormones that can regulate adipogenesis, fat deposition, BAT growth, beige formation, and WAT browning, and subsequently affects energy expenditure and insulin sensitivity (27–30).
Melatonin affects adipogenic differentiation
The effects of melatonin on differentiation of preadipocytes or adipose-derived stem cells have been studied by different groups and recent findings are outlined in Table 1. Notably, the regulatory roles of melatonin on adipogenesis reported by different groups are contradictory. There exist 2 different views on the effect of melatonin on adipogenesis: that melatonin promotes preadipocyte differentiation and adipogenesis (67, 71, 72); and that melatonin is a negative regulator and inhibits differentiation and adipogenesis (70,73, 75, 107). Peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) are the 2 master regulators of terminal adipogenesis (108). PPARγ controls the expression of adipogenesis related genes such as fatty acid-binding protein 4 (Fabp4; also known as aP2) and fatty acid synthase and promotes the intracytoplasmic accumulation of lipids (109). In differentiating 3T3-L1 preadipocytes, treatments with 1 mM melatonin throughout the adipogenic differentiation process could promote adipogenic differentiation and increase expression of both C/EBPα and PPARγ (71, 72). In addition, 1 mM melatonin induced 3T3-L1 preadipocyte differentiation and increased intracytoplasmic triglyceride accumulation in both differentiating and previously differentiated 3T3-L1 adipocytes (71). Consistent with these findings, melatonin (1 mM) supplementation in bovine intramuscular preadipocytes (BIPs) significantly increased PPARγ and C/EBPα expression and promoted the differentiation of BIPs into mature adipocytes with large lipid droplets and high intracytoplasmic triacylglycerol accumulation (67). Interestingly, Kato et al. demonstrated that 1 mM melatonin promoted 3T3-L1 preadipocyte differentiation into adipocytes, while the melatonin-treated differentiated 3T3-L1 adipocytes formed smaller lipid droplets (72). Moreover, they found high expression of several molecules associated with lipolysis, including hormone-sensitive lipase, adipose triglyceride lipase, perilipin, and comparative gene identification-58 (CGI-58) in melatonin-treated 3T3-L1 adipocytes (72). Despite large lipid droplets and large amounts of triglycerides, melatonin also significantly enhanced lipolysis and upregulated the expression of lipolytic genes and proteins, including hormone-sensitive lipase, adipose triglyceride lipase, and perilipin 1 in BIPs (67). Notably, 1 mM melatonin treatment also increased mitochondrial activity and content, and upregulated expression of UCP1 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) in melatonin-treated 3T3-L1 adipocytes (72). Taken together, these results suggest that melatonin promotes adipogenesis and accumulation of intracytoplasmic triglyceride during differentiation of 3T3-L1 preadipocytes and BIP through activation of C/EBPα and PPARγ. Meanwhile, melatonin treatments also increased basal lipolysis, promoted mitochondrial function and PGC1α activation, and subsequently reduced lipid droplet size and induced white to brown-like adipocyte transformation.
TABLE 1.
Effects of melatonin administration on differentiation of preadipocytes or adipose-derived stem cells, the year studied, and the reference of the original paper1
| Cell type | Treatment | Effects | Year | Reference |
|---|---|---|---|---|
| hADSCs | 0.01 M melatonin or melatonin plus vitamin D | Decreases the intracellular lipid accumulation and protein content; inhibits the expression of aP2, Pparγ, andLpl | 2017 | (107) |
| BIPs | 1 nM, 100 nM, 10 µM, or 1 mM | Promotes differentiation, lipid accumulation and lipolysis; reduces intracellular ROS; increases expression of PPARγ, C/EBPβ, and C/EBPα | 2017 | (67) |
| hMSCs | 50 nM melatonin | Inhibits adipogenic differentiation; no effect on proliferation; reduces lipid accumulation; and decreases the expression of PPARγ, C/EBPα, and C/EBPβ | 2016 | (73) |
| 3T3-L1 | 1 mM melatonin | Promotes adipogenesis, lipolysis, mitochondrial biogenesis, and adiponectin secretion; increases PPARγ, PGC1α, and UCP1 expression | 2015 | (72) |
| 3T3-L1 | Different amounts of melatonin | Increases intracytoplasmic triglyceride accumulation and differentiation; upregulates C/EBPα and PPARγ expression | 2012 | (71) |
| hMSCs | 0, 10−8,10−6, and 10−4 M melatonin | Inhibits adipogenesis and promotes osteogenesis; no effect on proliferation; downregulates mature adipocyte marker gene expression | 2010 | (75) |
| 3T3-L1 | 0.1 M melatonin | Decreases expression of Pparγ, C/ebpα, adiponectin, and aP2; reduces lipid accumulation | 2009 | (70) |
aP2, fatty acid-binding protein 4; BIP, bovine intramuscular preadipocyte; C/EBPα, CCAAT/enhancer binding protein α; C/EBPβ, CCAAT/enhancer binding protein β; hADSC, human adipose-derived stem cell; hMSC, human mesenchymal stem cell; LPL, lipoprotein lipase; PPARγ, peroxisome proliferator-activated receptor γ; ROS, reactive oxygen species; UCP1, uncoupling protein 1; 3T3-L1, 3T3-L1 preadipocytes.
In contrast to the above findings, other studies have demonstrated that melatonin inhibited adipogenic differentiation and lipogenesis (70,73, 75, 107). Using a similar strategy to the above studies in 3T3-L1 preadipocytes, Alonso-Vale et al. showed that 1 mM melatonin treatment inhibited preadipocyte differentiation, decreased lipid accumulation and expression of Pparγ, C/ebpα, adiponectin (Adipoq), and aP2 (70). They demonstrated that melatonin inhibited adipocyte differentiation through inhibiting the activity of CCAAT/enhancer-binding protein β (C/EBPβ) (70), which could regulate the initial stages of adipogenesis and expression of the terminal adipogenesis master regulators C/ebpαand Pparγ. Likewise, in human mesenchymal stem cells (hMSCs) (73, 75) and human adipose-derived stem cells (hADSCs) (107), melatonin treatment inhibited adipogenic differentiation, reduced intracellular lipid accumulation, and decreased the expression of aP2, Pparγ, and lipoprotein lipase (Lpl). In addition, 0.1 mM melatonin inhibited adipogenesis and mature adipocyte marker genes, while promoting osteogenic differentiation of hMSCs, which was the opposite of adipogenic differentiation, through enhancing Runx2 expression (75). These findings indicate that melatonin may act as a negative regulator of adipogenesis.
Based on the above reports, we conclude that the conflicting results found by different groups may be caused by differences in the cell types or dosages or time frames used by different researchers. Adipogenic differentiation is a highly orchestrated process in a time-dependent manner which can vary between different fat depots or cell types (110). In the previous studies, different cell models were used to study the effect of melatonin on adipogenesis, including 3T3-L1 preadipocytes, BIPs, hADSCs and hMSCs. The duration of adipogenic differentiation varies in different cell lines. Eight days are required for differentiation in 3T3-L1 adipocytes (71, 72), whereas hADSCs (107) and hMSCs (73) need longer times, 21 or 28 d, to format mature adipocytes. The varying durations of melatonin treatments in different cell lines might cause the varying effects on adipogenic differentiation. Notably, the doses of melatonin in hMSC experiments were 50 nM and 0.1 mM (73, 75), respectively, and in hADSC experiments was 0.01 M (107), dramatically different from that used in experiments with 3T3-L1 preadipocytes (71, 72) and BIPs (67) (1 mM) (Table1). Although 1 mM melatonin did not affect the viability of 3T3-L1 cells, this dose of melatonin was supraphysiological (72, 111). Low melatonin (10 uM, 100 nM, 10 pM) did not significantly influence the triglyceride concentrations in 3T3-L1 adipocytes (71). In addition, various strategies and concentrations of differentiation reagents (such as isobutylmethylxanthine, dexamethasone, insulin) for adipogenic differentiation were applied in previous studies (67, 70–73, 75,107). Moreover, many internal (e.g., hormones, miRNAs, cytoskeletal proteins) and external (e.g., drugs, molecules from plants) effectors could modulate preadipocyte development and adipogenesis by regulating PPAR and C/EBP transcription factors (112). During adipogenic differentiation in vitro, the various doses of isobutylmethylxanthine and dexamethasone, which could induce expression of C/EBPβ and δ might disturb the effect of melatonin on the initial stages of adipogenesis. Thus, a uniform differentiation approach should be established to confirm the effect of melatonin on adipogenic differentiation.
Similar to the phenotypes, there are different regulation mechanisms of melatonin on adipogenic differentiation (Figure 3). Some studies have demonstrated that melatonin promotes preadipocyte differentiation by increasing expression of C/EBPα and PPARγ, the key transcription factors of adipogenesis (67, 71, 72). Conversely, other studies have indicated that melatonin could directly or indirectly act through suppression of C/EBPβ to decrease expression of PPARγ and C/EBPα to inhibit adipogenesis (70, 73,75,107) (Figure 3). In mammals, numerous physiological roles of melatonin are mediated via activation of G protein-coupled receptors, in a manner dependent on melatonin concentration and exposure time, as well as cell type (67,112, 113). The effects of melatonin could be reversed by melatonin receptor antagonists such as luzindole, suggesting that melatonin can also act through an MT2 receptor to regulate expression of key transcription factors and differentiation (67,71) (Figure 3). In human PAZ6 adipocytes, the inhibitory effect of melatonin on cAMP accumulation is mediated only through MT2 receptors (114). In hMSC, melatonin inhibits phosphorylation of C/EBPβ directly via cAMP and reactive oxygen species reduction, and indirectly by blocking the extracellular regulated kinases/glycogen synthase kinase 3β (ERK/GSK-3β) site which is required for C/EBPβ phosphorylation. More research is needed to clarify the exact effect and mechanism of melatonin on adipogenesis and differentiation in the future.
FIGURE 3.
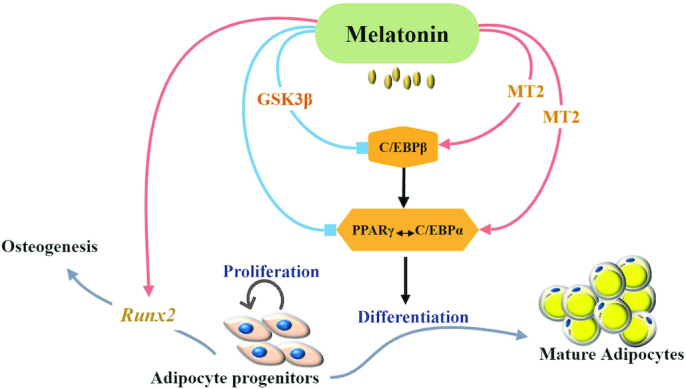
Proposed regulatory mechanism of melatonin on adipogenic differentiation. C/EBPα, CCAAT/enhancer-binding protein α; C/EBPβ, CCAAT/enhancer-binding protein β; GSK3β, glycogen synthase kinase 3 β; MT2, melatonin MT2 receptor; PPARγ, peroxisome proliferator-activated receptor γ; Runx2, Runt-related transcription factor 2.
Melatonin affects adipose inflammation
Obesity is associated with inflammation and macrophage infiltration. Melatonin has been reported to possess anti-inflammatory properties and the anti-inflammatory effects of melatonin have been reviewed recently (55,115). We will briefly discuss the more recent findings in this area. Liu et al. demonstrated that melatonin alleviates adipose inflammation by increasing cellular and exosomal α-ketoglutarate in adipose tissue (39). Moreover, melatonin increased the ratio of M2 to M1 macrophages by promoting ten-eleven translocation (Tet)-mediated DNA demethylation and transporting of exosomal αKG to macrophages (39). In addition, melatonin was shown to alleviate inflammasome-induced pyroptosis by blocking the nuclear factor κB-gasdermin D signal in adipose tissue, suggesting a novel function of melatonin on adipocyte pyroptosis (53). Based on the previous reports (39, 53, 55, 116), melatonin could be used as a potential therapy for preventing and treating obesity and other diseases caused by an inflammatory response.
Melatonin affects adipokine secretion
Adipokines, such as leptin and adiponectin, participate in regulating energy metabolism and fat deposition. Likewise, melatonin also plays an important role in regulation of whole-body energy balance. Melatonin has the capacity to influence the circulating leptin concentration (117). Notably, melatonin-supplemented mice had significantly higher plasma leptin than control mice (117). In addition, melatonin promoted the positive effects of insulin and dexamethasone on leptin expression in primary cultured adipocytes (118). However, in vitro experiments showed that melatonin incubation did not affect the amount of leptin secreted from adipose tissue fragments, suggesting that melatonin did not affect leptin secretion via mouse adipose tissue (117). Notably, acute modifications in amounts of daytime melatonin do not affect amounts of leptin in postmenopausal women (118). Melatonin combined with insulin could increase leptin expression in rat adipocytes (119). Moreover, melatonin also affects the adiponectin and other adipokine secretion in adipocytes (84, 120). Taken together, melatonin influences the expression and secretion patterns of adipokines (84), indicating that melatonin may act as a potential therapeutic agent for obesity and related disorders.
Melatonin induces WAT browning
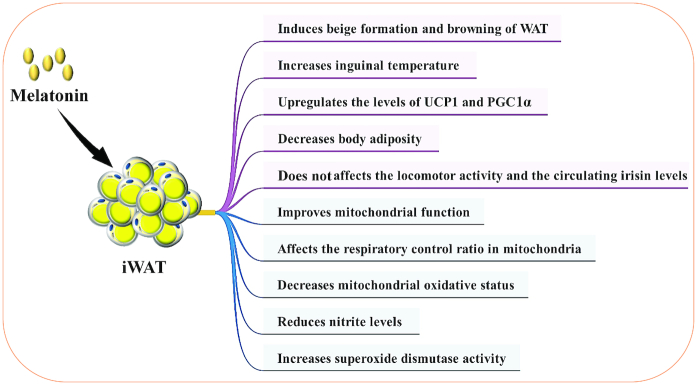
Melatonin also acts to help forming beige adipocytes or as a WAT-browning inducer in animals (27, 68) (Figure 4). Oral administration of melatonin [10 mg/(kg · d)] in drinking water for 6 wk induced beige formation and browning of inguinal WAT in Zucker diabetic fatty rats and their lean littermates (27). In addition, melatonin administration upregulated the expression of the UCP1 and increased inguinal temperature (27). Other studies indicated that oral melatonin [10 mg/(kg · d)] also improved mitochondrial function in inguinal WAT of Zucker diabetic fatty rats (68) (Figure 4). Melatonin affected the respiratory control ratio in mitochondria from beige fat and WAT (68) (Figure 4). Moreover, melatonin decreased mitochondrial oxidative status by increasing superoxide dismutase activity and by reducing nitrites (68) (Figure 4). In Siberian hamsters, subcutaneously administered melatonin induced WAT browning, promoted UCP1 expression, stimulated lipid mobilization, and subsequently decreased body adiposity (121). Hence, melatonin has the capacity to stimulate WAT browning and beige adipocyte formation and its metabolic benefits could help to prevent mitochondrial dysfunction and obesity.
FIGURE 4.
Melatonin induces beige adipocyte formation and WAT browning. iWAT, inguinal white adipose tissue; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1-α; UCP1, uncoupling protein 1; WAT, white adipose tissue..
Roles of melatonin in BAT
The regulatory role of melatonin in BAT has been well studied and is summarized and presented in Figure 5. Firstly, melatonin treatment stimulated BAT growth and increased BAT mass in adult male hamsters (29, 43). Treatment with melatonin dramatically increased the BAT mass in the Syrian hamster (122,123), Djungarian hamster (124,125), white-footed mouse (126), and 13-lined ground squirrel (43, 127). Consistently, oral melatonin treatment [10 mg/kg body weight (BW)] significantly increased BAT weight in the Zucker diabetic fatty rats (28). More recently, daily melatonin (3 mg) replacement for 3 mo increased BAT volume and activity in melatonin-deficient human patients (128). Conversely, maternal melatonin suppression during gestation decreased BAT mass of newborn lambs and increased the expression of genes related to thermogenesis and adipogenesis (30). Secondly, melatonin upregulated expression of the Ucp1 gene. In Zucker diabetic fatty rats, melatonin treatment restored expression of Ucp1 to that commonly seen in lean rats (28). Moreover, the expression of adipogenic/thermogenic genes (Ucp1, Pparγ, Pparα, Pgc1α, Cebpβ, and Perilipin) and of the clock genes (Bmal1, Clock, and Per2) were affected by melatonin in newborn sheep (30). Thirdly, melatonin affected the mitochondrial function and activities. Notably, melatonin treatment increased mitochondrial mass and activities of citrate synthase and complexes I and IV in Zucker diabetic fatty and Zucker lean rats (28). Melatonin affected mitochondrial transcript contents in isolated brown adipocytes of Siberian hamsters (69). In addition, melatonin can increase the cytochrome oxidase activity (126, 127). Fourthly, melatonin enhanced BAT function and activities. Previous studies demonstrated that melatonin treatment increased the nonshivering thermogenesis in the white-footed mouse (126), white rat (129), Djungarian hamster (124, 125), and Syrian hamster (43). Indeed, the melatonin binding site has been detected in mature brown adipocyte membranes and in the BAT of Siberian hamsters (130). Exposure of hamsters to exogenous melatonin treatment induced gonadal regression and BAT hypertrophy (123). However, the nonshivering thermogenic capacity was not improved in hamsters with increased BAT mass (123). Taken together, these findings indicate that melatonin plays a crucial role in regulation of BAT function and activation (Figure 5).
FIGURE 5.
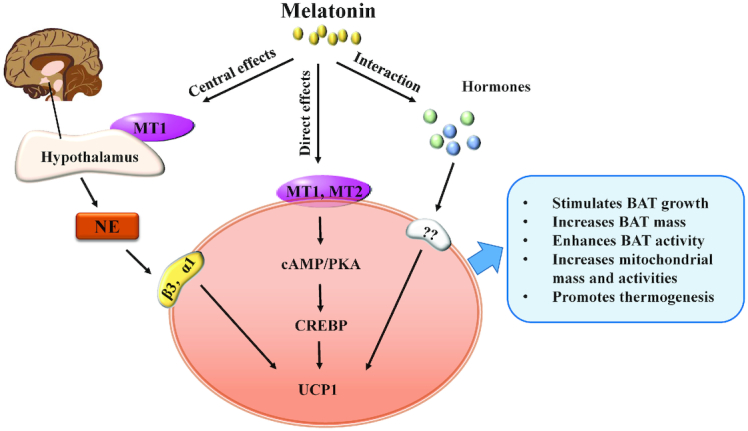
The role and regulatory mechanism of melatonin in BAT development and function. BAT, brown adipose tissue; CREBP, cAMP-response element binding protein; MT1, melatonin MT1 receptor; MT2, melatonin MT2 receptor; NE, norepinephrine; PKA, protein kinase A; UCP1, uncoupling protein 1; α1, α1-adrenergic receptor; β3, β3-adrenergic receptor.
The regulatory mechanism of melatonin on BAT may be mainly through 3 different paths including central effects, peripheral effects, and an interaction with other hormones (43) (Figure 5). Previous studies demonstrated that many of melatonin's biological effects in animals act through activation of melatonin receptors (131). In mammals, 2 melatonin receptors have been identified: the MT1 (or Mel1A or MTNR1A) and MT2 (or Mel1B or MTNR1B) (132). The MT1 subtype is expressed in the retina and the suprachiasmatic nuclei of the hypothalamus. Interestingly, previous studies demonstrated that neurons of the hypothalamus regulate BAT function and activation (43, 133). Thus, melatonin could stimulate the MT1 receptor located on neurons of the hypothalamus and act on the hypothalamus to increase UCP1 expression and promote BAT function in nonshivering thermogenesis (43) (Figure 5). In addition to these central effects, melatonin can also act directly on BAT because MT1 and MT2 receptors have been found on brown adipocytes (130). Activation of MT1 and MT2 commonly leads to a reduction of intracellular cAMP, which subsequently affects protein kinase A (PKA) activity and phosphorylation of cAMP-response element binding protein, and subsequently upregulates the expression of UCP1 (4, 43) (Figure 5). In addition to the central and peripheral effects, the interactions of melatonin with other hormones, such as thyroid hormones, leptin, and insulin, on the activation and function of BAT have been reported (43) (Figure 5). However, future studies are needed to clarify the exact regulation mechanism of melatonin on BAT function and activities.
Melatonin regulates body weight and adiposity
The increasing incidence of overweight and obesity is a severe public health threat worldwide. Recently, the possible role of melatonin in regulation of body weight and adiposity has attracted much attention, as well as its potential role in preventing obesity-related metabolic disorders (55, 64–66, 83, 84). Recent observations over the past few years show that in mice, oral melatonin supplementation (108 mg/kg BW/d for 2 wk or 50 mg/kg BW/d for 10 wk) decreased lipid accumulation, improved lipid metabolism, and reduced liver steatosis and body weight in high-fat diet (HFD)-fed mice (134, 135). Melatonin decreased the gut microbiota dysbiosis, which contributes to obesity (134, 135). Indeed, melatonin can be used to reverse HFD-induced gut microbiota dysbiosis and inhibit obesity (134, 135). Administration of melatonin prevents progression of metabolic dysfunction in circadian disruption and diet-induced obesity in rats by affecting adiposity, insulin sensitivity, and circadian activity (136). Treatment with melatonin plus insulin promoted better glycemic control and improved insulin sensitivity in WAT (137). In aging animals, melatonin supplementation improved the efficiency of energy metabolism, reduced body weight, and increased insulin sensitivity (138). Melatonin supplementation increased basal lipid metabolism and prevented further excess fat accumulation in a diet-induced obesity zebrafish model (139). Interestingly, prolonged daylight, which could decrease the production of melatonin, increased body fat and adiposity in mice (140). In addition, melatonin could improve nonalcoholic fatty liver disease by decreasing body weight and reducing inflammation through modulating the MAPK/c-Jnk NH-terminal kinase (JNK)/P38 signaling pathway in HFD-induced obese mice (141).
In humans, the effects of melatonin on fat mass have been investigated. In postmenopausal women, 1 yr of melatonin treatment reduced fat mass and increased lean mass, suggesting a possibly beneficial effect of melatonin on body composition and lipid metabolism in humans (142). Notably, melatonin had a beneficial effect on maternal obesity through improving antioxidant capacity and mitochondrial respiration (143, 144). In addition, melatonin supplementation (5 mg in the morning and 5 mg in the evening for 28 d) in overweight patients with nonalcoholic steatohepatitis resulted in an increase in plasma leptin concentrations (145). Celinski et al. found that a single dose of melatonin (10 mg) decreased the elevated plasma leptin observed in liver cirrhosis patients but increased leptin concentrations in healthy people (146). However, other researchers found that a single daytime melatonin administration (1 or 2 mg) did not affect leptin in postmenopausal women (118). It has been reported recently that oral melatonin replacement (3 mg/d for 3 mo) improved amounts of blood lipids in melatonin-deficient patients without affecting body weight and liver fat (128). Moreover, Mostafavi et al. evaluated 7 clinical trials with a total of 244 patients and did not find a significant effect of melatonin on body weight. They concluded that melatonin was more effective in children and adolescents (83,147). Based on the above controversial results, future studies need to clarify the exact effects of melatonin on body weight regulation and obesity in humans. Notably, administration of melatonin attenuated circadian disruption in obese animals (136, 148), indicating that melatonin may regulate circadian rhythm in obese animals. However, the side-effects and the disruption of circadian rhythm caused by misuse of exogenous melatonin suggest that safety of melatonin should be further studied. Moreover, the efficacy of oral melatonin supplementation should be considered. Previous studies demonstrated that efficacy of melatonin is different when administered by different routes, such as intranasally, transdermally, oral transmucosally, and subcutaneously (149). Therefore, the administration route of exogenous melatonin, as well as the dosage and timing of administration should be investigated in the future.
The regulatory mechanisms of melatonin on adiposity and body weight may involve multiple levels of action (Figure 6). Firstly, melatonin affected adipogenic differentiation and adipogenesis (70–73). Secondly, melatonin induced beige adipocyte formation and WAT browning, and subsequently increased thermogenic capacity and energy expenditure to counteract body weight gain and obesity (27, 68). Thirdly, melatonin inhibited obesity through promoting BAT growth and increasing BAT function (28, 43, 69). Indeed, melatonin treatment of obese rats increased BAT mass and recruited thermogenic function of BAT to control body weight gain and inhibit adiposity ( 28). Moreover, the prolonged day length affected the body fat mass, which decreased sympathetic input into BAT and reduced β3-adrenergic intracellular signaling (140). Fourthly, some papers reported that melatonin prevented body weight gain and adiposity, possibly through association with reprogramming gut microbiota (134, 135). They found that oral melatonin supplementation affected the diversity of intestinal microbiota, the relative abundances of Bacteroides and Alistipes, and functional profiling of microbial communities that are associated with lipid and energy metabolism (134). Moreover, microbiota transplantation experiments and antibiotic treatments further demonstrated that melatonin inhibited obesity in HFD-induced obesity through affecting gut microbiota (134). Fifthly, the antioxidant and anti-inflammatory capacity of melatonin should be considered. Melatonin reduced body weight, protected against adipose tissue dysfunction and mitochondrial dysfunction by multiple anti-inflammatory/antioxidant actions (55, 143, 144). Sixthly, melatonin supplementation restored adipokine secretion and metabolism in obese mice (84, 122), suggesting that the regulatory role of melatonin on adipokine production may also be associated with its beneficial effects on protecting against body weight gain. In addition to the aforementioned scenarios, other regulation pathways should be explored and demonstrated in the future. Taken together, despite the multitude of studies related to the associations of melatonin with body weight and obesity, the mechanisms of melatonin action are far from being fully understood. Although melatonin treatments affect adipogenesis and fat deposition, the exact role of melatonin in regulating obesity and body weight still needs to be further studied and clarified.
FIGURE 6.
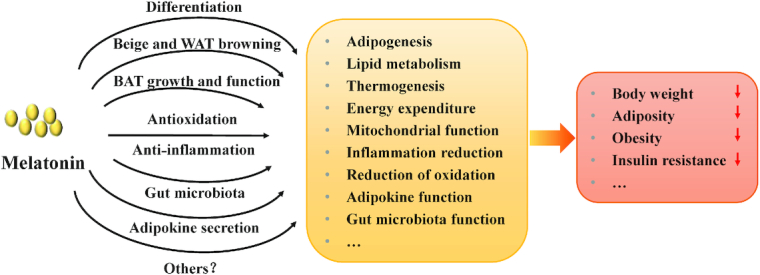
The regulatory mechanisms of melatonin on adiposity and body weight. Melatonin regulates body weight and adiposity in different ways, such as affecting adipogenic differentiation and adipogenesis, inducing beige adipocyte formation and WAT browning, promoting BAT growth and increasing BAT function, reprogramming gut microbiota, improving antioxidant and anti-inflammatory capacity, and restoring adipokine secretion and metabolism. BAT, brown adipose tissue; WAT, white adipose tissue.
Conclusions and Remarks
Based on the above data, we conclude that melatonin plays multiple crucial roles in regulating adipose tissue development and whole body metabolism, such as affecting adipogenesis, inducing WAT browning, enhancing BAT mass and activities, promoting thermogenesis and energy expenditure, decreasing insulin resistance, regulating gut microbiota and adipokine secretion, and increasing anti-inflammatory and antioxidation effects. Hence, melatonin could be investigated as a potential therapeutic candidate to control energy metabolism, fat deposition, insulin actions, adiposity, and its associated diseases. However, there are still some concerns which need to be further studied:
The exact effects of melatonin on adipocyte differentiation need to be clarified, because the current results are controversial.
Although the roles of melatonin on BAT development and function have been well studied in vivo, the effects of melatonin on brown adipocyte differentiation and function in vitro should be determined.
The characteristics and origins of white, beige, and brown adipocytes are different. Thus, it will be important to compare the regulation and mechanisms of melatonin in different types of adipocytes.
Melatonin affects adipokine secretion, metabolism, and obesity. Lipidomic and metabolomic analyses could be used to explore the effect of melatonin on adipose tissues.
Melatonin prevents obesity possibly through an association with reprogrammed gut microbiota in mice.
Whether it may work in humans in general or specifically in patients with metabolic syndrome is still unknown. Collectively, the current results and future findings could provide more evidence for the roles of melatonin in regulation of adipocyte cell fates, adipogenesis, and energy metabolism.
Acknowledgments
We thank Ruth O'Connor (Purdue University Student Health Center, West Lafayette, IN, USA) and Teresa G Valencak (Veterinary University Vienna, Vienna, Austria) for English corrections. The authors’ responsibilities were as follows—TS, ZX, and WY: designed and wrote the manuscript; JL and YW: assisted in interpretation and revising the article; and all authors: read and approved the final manuscript.
Notes
The project was partially supported by funding from the National Natural Science Foundation of China (31722053, 31672427), the Young Overseas High-Level Talents Introduction Plan funding, and the Hundred Talents Program funding from Zhejiang University to TS.
Author disclosures: ZX, WY, JL, YW, and TS, no conflicts of interest.
ZX and WY contributed equally to this work.
Abbreviations used: aP2, fatty acid-binding protein 4; BAT, brown adipose tissue; BIP, bovine intramuscular preadipocyte; BW, body weight; C/EBPα, CCAAT/enhancer binding protein α; C/EBPβ, CCAAT/enhancer binding protein β; hADSC, human adipose-derived stem cell; HFD, high-fat diet; hMSC, human mesenchymal stem cell; IMAT, intermuscular/intramuscular adipose tissue; Myf5, myogenic factor 5; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1-α; PPARγ, peroxisome proliferator-activated receptor γ; PVAT, perivascular adipose tissue; UCP1, uncoupling protein 1; WAT, white adipose tissue.
References
- 1. Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302(1):E19–31. [DOI] [PubMed] [Google Scholar]
- 2. Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221(Pt Suppl 1):jeb162958. [DOI] [PubMed] [Google Scholar]
- 3. Oelkrug R, Polymeropoulos ET, Jastroch M. Brown adipose tissue: physiological function and evolutionary significance. J Comp Physiol B. 2015;185(6):587–606. [DOI] [PubMed] [Google Scholar]
- 4. Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 5. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G et al.. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown?. Gene Dev. 2013;27(3):234–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ et al.. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K et al.. High incidence of metabolically active brown adipose tissue in healthy adult humans effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y, Doria A et al.. Identification and importance of brown adipose tissue in adult humans. New Engl J Med. 2009;360(15):1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23(9):3113–20. [DOI] [PubMed] [Google Scholar]
- 11. Cypess AM, White AP, Vemochet C, Schulz TJ, Xue RD, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C et al.. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P et al.. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19(5):631–4. [DOI] [PubMed] [Google Scholar]
- 13. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. New Engl J Med. 2009;360(15):1500–8. [DOI] [PubMed] [Google Scholar]
- 14. Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, Reimold M, Haring HU, Claussen CD, Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–9. [DOI] [PubMed] [Google Scholar]
- 16. Chondronikola M, Sidossis LS. Brown and beige fat: From molecules to physiology. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(1):91–103. [DOI] [PubMed] [Google Scholar]
- 17. Carobbio S, Guenantin AC, Samuelson I, Bahri M, Vidal-Puig A. Brown and beige fat: From molecules to physiology and pathophysiology. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(1):37–50. [DOI] [PubMed] [Google Scholar]
- 18. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Pan R, Pfeifer A. Regulation of brown and beige fat by microRNAs. Pharmacol Therapeut. 2017;170:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Goody D, Pfeifer A.. MicroRNAs in brown and beige fat. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(1):29–36. [DOI] [PubMed] [Google Scholar]
- 21. Bartness TJ, Ryu V.. Neural control of white, beige and brown adipocytes. Int J Obes Suppl. 2015;5(Suppl 1):S35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Bio. 2016;17(8):480–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277(45):42958–63. [DOI] [PubMed] [Google Scholar]
- 24. Wang WS, Seale P.. Control of brown and beige fat development. Nat Rev Mol Cell Bio. 2016;17(11):691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US et al.. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Li R, Meng Y, Li SW, Donelan W, Zhao Y, Qi L, Zhang MX, Wang XL, Cui TX et al.. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514–25. [DOI] [PubMed] [Google Scholar]
- 27. Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, Reiter RJ, Agil A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res. 2013;55(4):416–23. [DOI] [PubMed] [Google Scholar]
- 28. Vazquez GF, Reiter RJ, Agil A. Melatonin increases brown adipose tissue mass and function in Zucker diabetic fatty rats: implications for obesity control. J Pineal Res. 2018;64(4):e12472. [DOI] [PubMed] [Google Scholar]
- 29. Heldmaier G, Hoffmann K.. Melatonin stimulates growth of brown adipose-tissue. Nature. 1974;247(5438):224–5. [DOI] [PubMed] [Google Scholar]
- 30. Seron-Ferre M, Reynolds H, Mendez NA, Mondaca M, Valenzuela F, Ebensperger R, Valenzuela GJ, Herrera EA, Llanos AJ, Torres-Farfan C. Impact of maternal melatonin suppression on amount and functionality of brown adipose tissue (BAT) in the newborn sheep. Front Endocrinol. 2015;5:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schulz TJ, Huang P, Huang TL, Xue RD, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signaling induces compensatory browning of white fat. Nature. 2013;495(7441):379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E et al.. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P et al.. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127(6):1109–22. [DOI] [PubMed] [Google Scholar]
- 34. Okla M, Kim J, Koehler K, Chung S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv Nutr. 2017;8(3):473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes. 2016;65(5):1410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang ZG, Zhang HZ, Li B, Meng XJ, Wang JQ, Zhang YF, Yao SS, Ma QY, Jin LN, Yang J et al.. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493. [DOI] [PubMed] [Google Scholar]
- 37. Lerner AB, Case JD, Mori W, Wright MR. Melatonin in peripheral nerve. Nature. 1959;183(4678):1821. [DOI] [PubMed] [Google Scholar]
- 38. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52(2):139–66. [DOI] [PubMed] [Google Scholar]
- 39. Liu Z, Gan L, Zhang T, Ren Q, Sun C. Melatonin alleviates adipose inflammation through elevating ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2018;64(1):e12455. [DOI] [PubMed] [Google Scholar]
- 40. de Farias TDM, de Oliveira AC, Andreotti S, do Amaral FG, Chimin P, de Proenca ARA, Leal FLT, Sertie RAL, Campana AB, Lopes AB et al.. Pinealectomy interferes with the circadian clock genes expression in white adipose tissue. J Pineal Res. 2015;58(3):251–61. [DOI] [PubMed] [Google Scholar]
- 41. Reiter RJ. Pineal melatonin—cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12(2):151–80. [DOI] [PubMed] [Google Scholar]
- 42. Luchetti F, Canonico B, Bartolini D, Arcangeletti M, Ciffolilli S, Murdolo G, Piroddi M, Papa S, Reiter RJ, Galli F. Melatonin regulates mesenchymal stem cell differentiation: a review. J Pineal Res. 2014;56(4):382–97. [DOI] [PubMed] [Google Scholar]
- 43. Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 2011;12(3):167–88. [DOI] [PubMed] [Google Scholar]
- 44. Bubenik GA. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signal Recept. 2001;10(6):350–66. [DOI] [PubMed] [Google Scholar]
- 45. Chen GF, Huo YS, Tan DX, Liang Z, Zhang WB, Zhang YK. Melatonin in Chinese medicinal herbs. Life Sci. 2003;73(1):19–26. [DOI] [PubMed] [Google Scholar]
- 46. Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtanikaneko R, Hara M, Suzuki T, Reiter RJ. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Int. 1995;35(3):627–34. [PubMed] [Google Scholar]
- 47. Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet. 1997;350(9091):1598–9. [DOI] [PubMed] [Google Scholar]
- 48. Meng JF, Shi TC, Song S, Zhang ZW, Fang YL. Melatonin in grapes and grape-related foodstuffs: A review. Food Chem. 2017;231:185–91. [DOI] [PubMed] [Google Scholar]
- 49. Jarzynka MJ, Passey DK, Johnson DA, Konduru NV, Fitz NF, Radio NM, Rasenick M, Benloucif S, Melan MA, Witt-Enderby PA. Microtubules modulate melatonin receptors involved in phase-shifting circadian activity rhythms: in vitro and in vivo evidence. J Pineal Res. 2009;46(2):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J Pineal Res. 2018;65(1):e12514. [DOI] [PubMed] [Google Scholar]
- 51. Tan SS, Han XL, Sivakumaran P, Lim SY, Morrison WA. Melatonin protects human adipose-derived stem cells from oxidative stress and cell death. Arch Plast Surg. 2016;43(3):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu P, Liu JF, Shi JX, Zhou Q, Liu J, Zhang XW, Du ZY, Liu QW, Guo YY. Melatonin protects ADSCs from ROS and enhances their therapeutic potency in a rat model of myocardial infarction. J Cell Mol Med. 2015;19(9):2232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu ZJ, Gan L, Xu YT, Luo D, Ren Q, Wu S, Sun C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-kappa B/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63(1):e12414. [DOI] [PubMed] [Google Scholar]
- 54. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, Gonzalez-Gallego J. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54(1):1–14. [DOI] [PubMed] [Google Scholar]
- 55. Prado NJ, Ferder L, Manucha W, Diez ER. Anti-Inflammatory Effects of Melatonin in Obesity and Hypertension. Curr Hypertens Rep. 2018;20(5):45. [DOI] [PubMed] [Google Scholar]
- 56. Alvarez-Garcia V, Gonzalez A, Alonso-Gonzalez C, Martinez-Campa C, Cos S. Melatonin interferes in the desmoplastic reaction in breast cancer by regulating cytokine production. J Pineal Res. 2012;52(3):282–90. [DOI] [PubMed] [Google Scholar]
- 57. Rodriguez-Garcia A, Mayo JC, Hevia D, Quiros-Gonzalez I, Navarro M, Sainz RM. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine-induced apoptosis. J Pineal Res. 2013;54(1):33–45. [DOI] [PubMed] [Google Scholar]
- 58. Asghari MH, Moloudizargari M, Ghobadi E, Fallah M, Abdollahi M. Melatonin as a multifunctional anti-cancer molecule: Implications in gastric cancer. Life Sci. 2017;185:38–45. [DOI] [PubMed] [Google Scholar]
- 59. Mayo JC, Hevia D, Quiros-Gonzalez I, Rodriguez-Garcia A, Gonzalez-Menendez P, Cepas V, Gonzalez-Pola I, Sainz RM. IGFBP3 and MAPK/ERK signaling mediates melatonin-induced antitumor activity in prostate cancer. J Pineal Res. 2017;62(1):e12373. [DOI] [PubMed] [Google Scholar]
- 60. Chen XR, Hao AJ, Li X, Du ZX, Li H, Wang HZ, Yang HR, Fang ZY. Melatonin inhibits tumorigenicity of glioblastoma stem-like cells via the AKT-EZH2-STAT3 signaling axis. J Pineal Res. 2016;61(2):208–17. [DOI] [PubMed] [Google Scholar]
- 61. Majidinia M, Reiter RJ, Shakouri SK, Mohebbi I, Rastegar M, Kaviani M, Darband SG, Jahanban-Esfahlan R, Nabavi SM, Yousefi B. The multiple functions of melatonin in regenerative medicine. Ageing Res Rev. 2018;45:33–52. [DOI] [PubMed] [Google Scholar]
- 62. Yip HK, Chang YC, Wallace CG, Chang LT, Tsai TH, Chen YL, Chang HW, Leu S, Zhen YY, Tsai CY et al.. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia reperfusion injury. J Pineal Res. 2013;54(2):207–21. [DOI] [PubMed] [Google Scholar]
- 63. Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, Chen YC, Sun CK, Tsai TH, Chen YL et al.. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57(1):16–32. [DOI] [PubMed] [Google Scholar]
- 64. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81. [DOI] [PubMed] [Google Scholar]
- 65. Cardinali DP, Vigo DE.. Melatonin, mitochondria, and the metabolic syndrome. Cell Mol Life Sci. 2017;74(21):3941–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Navarro-Alarcón M, Ruiz-Ojeda FJ, Blanca-Herrera RM, A-Serrano MM, Acuna-Castroviejo D, Fernandez-Vazquez G, Agil A. Melatonin and metabolic regulation: a review. Food Funct. 2014;5(11):2806–32. [DOI] [PubMed] [Google Scholar]
- 67. Yang WC, Tang KQ, Wang YN, Zhang YY, Zan LS. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci Rep. 2017;7(1):15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jimenez-Aranda A, Fernandez-Vazquez G, A-Serrano MM, Reiter RJ, Agil A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zucker diabetic fatty rats. J Pineal Res. 2014;57(1):103–9. [DOI] [PubMed] [Google Scholar]
- 69. Prunet-Marcassus B, Ambid L, Viguerie-Bascands N, Penicaud L, Casteilla L. Evidence for a direct effect of melatonin on mitochondrial genome expression of Siberian hamster brown adipocytes. J Pineal Res. 2001;30(2):108–15. [DOI] [PubMed] [Google Scholar]
- 70. Alonso-Vale MIC, Peres SB, Vernochet C, Farmer SR, Lima FB. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBP beta transcriptional activity. J Pineal Res. 2009;47(3):221–7. [DOI] [PubMed] [Google Scholar]
- 71. Gonzalez A, Alvarez-Garcia V, Martinez-Campa C, Alonso-Gonzalez C, Cos S. Melatonin promotes differentiation of 3T3-L1 fibroblasts. J Pineal Res. 2012;52(1):12–20. [DOI] [PubMed] [Google Scholar]
- 72. Kato H, Tanaka G, Masuda S, Ogasawara J, Sakurai T, Kizaki T, Ohno H, Izawa T. Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes. J Pineal Res. 2015;59(2):267–75. [DOI] [PubMed] [Google Scholar]
- 73. Rhee YH, Ahn JC.. Melatonin attenuated adipogenesis through reduction of the CCAAT/enhancer binding protein beta by regulating the glycogen synthase 3 beta in human mesenchymal stem cells. J Physiol Biochem. 2016;72(2):145–55. [DOI] [PubMed] [Google Scholar]
- 74. Sanchez-Hidalgo M, Lu Z, Tan DA, Maldonado MD, Reiter RJ, Gregerman RI. Melatonin inhibits fatty acid-induced triglyceride accumulation in ROS17/2.8 cells: implications for osteoblast differentiation and osteoporosis. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2208–15. [DOI] [PubMed] [Google Scholar]
- 75. Zhang LM, Su PQ, Xu CX, Chen CH, Liang AJ, Du KL, Peng Y, Huang DS. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPAR gamma expression and enhancing Runx2 expression. J Pineal Res. 2010;49(4):364–72. [DOI] [PubMed] [Google Scholar]
- 76. Han D, Huang W, Li X, Gao L, Su T, Li XJ, Ma S, Liu T, Li CY, Chen JW et al.. Melatonin facilitates adipose-derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J Pineal Res. 2016;60(2):178–92. [DOI] [PubMed] [Google Scholar]
- 77. Ma Q, Yang J, Huang X, Guo W, Li S, Zhou H, Li J, Cao F, Chen Y. Poly(Lactide-Co-Glycolide)-Monomethoxy-Poly-(Polyethylene Glycol) Nanoparticles Loaded with Melatonin Protect Adipose-Derived Stem Cells Transplanted in Infarcted Heart Tissue. Stem Cells. 2018;36(4):540–50. [DOI] [PubMed] [Google Scholar]
- 78. Chen HH, Chang CL, Lin KC, Sung PH, Chai HT, Zhen YY, Chen YC, Wu YC, Leu S, Tsai TH et al.. Melatonin augments apoptotic adipose-derived mesenchymal stem cell treatment against sepsis-induced acute lung injury. Am J Transl Res. 2014;6(5):439–58. [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao J, Young YK, Fradette J, Eliopoulos N. Melatonin pretreatment of human adipose tissue-derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. Am J Physiol Renal Physiol. 2015;308(12):F1474–F83. [DOI] [PubMed] [Google Scholar]
- 80. Sun CK, Chen CH, Chang CL, Chiang HJ, Sung PH, Chen KH, Chen YL, Chen SY, Kao GS, Chang HW et al.. Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am J Transl Res. 2017;9(4):1543–60. [PMC free article] [PubMed] [Google Scholar]
- 81. Chang CL, Sung PH, Sun CK, Chen CH, Chiang HJ, Huang TH, Chen YL, Zhen YY, Chai HT, Chung SY et al.. Protective effect of melatonin-supported adipose-derived mesenchymal stem cells against small bowel ischemia-reperfusion injury in rat. J Pineal Res. 2015;59(2):206–20. [DOI] [PubMed] [Google Scholar]
- 82. Chen YT, Chiang HJ, Chen CH, Sung PH, Lee FY, Tsai TH, Chang CL, Chen HH, Sun CK, Leu S et al.. Melatonin treatment further improves adipose-derived mesenchymal stem cell therapy for acute interstitial cystitis in rat. J Pineal Res. 2014;57(3):248–61. [DOI] [PubMed] [Google Scholar]
- 83. Mostafavi SA, Akhondzadeh S, Mohammadi MR, Keshtkar AA, Hosseini S, Eshraghian MR, Motlagh TA, Alipour R, Keshavarz SA. Role of melatonin in body weight: A systematic review and meta-analysis. Curr Pharm Design. 2017;23(23):3445–52. [DOI] [PubMed] [Google Scholar]
- 84. Szewczyk-Golec K, Wozniak A, Reiter RJ. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res. 2015;59(3):277–91. [DOI] [PubMed] [Google Scholar]
- 85. Cedikova M, Kripnerova M, Dvorakova J, Pitule P, Grundmanova M, Babuska V, Mullerova D, Kuncova J. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016;; 2016:6067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Admiraal WM, Verberne HJ, Karamat FA, Soeters MR, Hoekstra JB, Holleman F. Cold-induced activity of brown adipose tissue in young lean men of South-Asian and European origin. Diabetologia. 2013;56(10):2231–7. [DOI] [PubMed] [Google Scholar]
- 87. Wankhade UD, Lee JH, Dagur PK, Yadav H, Shen M, Chen WP, Kulkarni AB, Mccoy JP, Finkel T, Cypess AM et al.. TGF-beta receptor 1 regulates progenitors that promote browning of white fat. Mol Metab. 2018;16:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Seale P, Bjork B, Yang WL, Kajimura S, Chin S, Kuang SH, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H et al.. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sanchez-Gurmaches J, Guertin DA.. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shan T, Liang X, Bi P, Zhang P, Liu W, Kuang S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J Lipid Res. 2013;54(8):2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang YF, Li HW, Guertin DA. PTEN Loss in the Myf5 Lineage Redistributes Body Fat and Reveals Subsets of White Adipocytes that Arise from Myf5 Precursors. Cell Metab. 2012;16(3):348–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Buckingham M, Relaix F.. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Bi. 2007;23:645–73. [DOI] [PubMed] [Google Scholar]
- 93. Liu WY, Liu YQ, Lai XS, Kuang SH. Intramuscular adipose is derived from a non-Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol. 2012;361(1):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chu DT, Gawronska-Kozak B. Brown and brite adipocytes: Same function, but different origin and response. Biochimie. 2017;138:102–5. [DOI] [PubMed] [Google Scholar]
- 95. Liu WY, Shan TZ, Yang X, Liang S, Zhang PP, Liu YQ, Liu XQ, Kuang SH. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J Cell Sci. 2013;126(16):3527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: Tracing back the origins of fat. Biochim Biophys Acta. 2014;1842(3):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Corcoran MP, Lamon-Fava S, Fielding RA. Skeletal muscle lipid deposition and insulin resistance: effect of dietary fatty acids and exercise. Am J Clin Nutr. 2007;85(3):662–77. [DOI] [PubMed] [Google Scholar]
- 98. Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–52. [DOI] [PubMed] [Google Scholar]
- 100. Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y et al.. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(21):3654–64. [DOI] [PubMed] [Google Scholar]
- 101. Schulz TJ, Huang TL, Tran TT, Zhang HB, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T et al.. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108(1):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hildebrand S, Stumer J, Pfeifer A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Front Physiol. 2018;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34(8):1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ye M, Ruan CC, Fu M, Xu L, Chen D, Zhu M, Zhu D, Gao P. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell Mol Life Sci. 2019;76(4):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126(9):1067–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu JQ, Xu ZY, Wu WC, Wang YZ, Shan TZ. Cre Recombinase Strains Used for the Study of Adipose Tissues and Adipocyte Progenitors. J Cell Physiol. 2017;232(10):2698–703. [DOI] [PubMed] [Google Scholar]
- 107. Basoli V, Santaniello S, Cruciani S, Ginesu GC, Cossu ML, Delitala AP, Serra PA, Ventura C, Maioli M. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. Int J Mol Sci. 2017;18(5):E981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rosen ED, Macdougald OA.. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Bio. 2006;7(12):885–96. [DOI] [PubMed] [Google Scholar]
- 109. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4(4):263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14(11):1293–307. [PubMed] [Google Scholar]
- 111. Zwirska-Korczala K, Jochem J, Adamczyk-Sowa M, Sowa P, Polaniak R, Birkner E, Latocha M, Pilc K, Suchanek R. Influence of melatonin on cell proliferation, antioxidative enzyme activities and lipid peroxidation in 3T3-L1 preadipocytes-an in vitro study. J Physiol Pharmacol. 2005;56 Suppl 6:91–9. [PubMed] [Google Scholar]
- 112. Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60(7):1407–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Arash Z, Iraj Ragerdi K, Mohammad B, Azim H, Reza M, Safoura V, Mohammad Ali S. Effects of melatonin on the proliferation and differentiation of rat adipose-derived stem cells. Indian J Plast Surg. 2008;41(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology. 2001;142(10):4264. [DOI] [PubMed] [Google Scholar]
- 115. Cardinali DP, Hardeland R.. Inflammaging, metabolic syndrome and melatonin: A call for treatment studies. Neuroendocrinology. 2017;104(4):382–97. [DOI] [PubMed] [Google Scholar]
- 116. Alonso-Vale MI, Andreotti S, Borges-Silva C, Mukai PY, Cipolla-Neto J, Lima FB. Intermittent and rhythmic exposure to melatonin in primary cultured adipocytes enhances the insulin and dexamethasone effects on leptin expression. J Pineal Res. 2006;41(1):28–34. [DOI] [PubMed] [Google Scholar]
- 117. Song YM, Chen MD.. Effects of melatonin administration on plasma leptin concentration and adipose tissue leptin secretion in mice. Acta Biol Hung. 2009;60(4):399–407. [DOI] [PubMed] [Google Scholar]
- 118. Cagnacci A, Malmusi S, Zanni A, Arangino S, Cagnacci P, Volpe A. Acute modifications in the levels of daytime melatonin do not influence leptin in postmenopausal women. J Pineal Res. 2002;33(1):57–60. [DOI] [PubMed] [Google Scholar]
- 119. Alonso-Vale MIC, Andreotti S, Peres SB, Anhe GF, Borges-Silva CD, Neto JC, Lima FB. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab. 2005;288(4):E805–E12. [DOI] [PubMed] [Google Scholar]
- 120. Favero G, Stacchiotti A, Castrezzati S, Bonomini F, Albanese M, Rezzani R, Rodella LF. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr Res. 2015;35(10):891–900. [DOI] [PubMed] [Google Scholar]
- 121. Ryu V, Zarebidaki E, Albers HE, Xue BZ, Bartness TJ. Short photoperiod reverses obesity in Siberian hamsters via sympathetically induced lipolysis and browning in adipose tissue. Physiol Behav. 2018;190:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wade GN, Bartness TJ, Alexander JR. Photoperiod and body-weight in female Syrian-hamsters – skeleton photoperiods, response magnitude, and development of photorefractoriness. Physiol Behav. 1986;37(6):863–8. [PubMed] [Google Scholar]
- 123. Viswanathan M, Hissa R, George JC. Effects of short photoperiod and melatonin treatment on thermogenesis in the Syrian-hamster. J Pineal Res. 1986;3(4):311–21. [DOI] [PubMed] [Google Scholar]
- 124. Heldmaier G, Steinlechner S, Rafael J, Vsiansky P. Photoperiodic control and effects of melatonin on nonshivering thermogenesis and brown adipose-tissue. Science. 1981;212(4497):917–9. [DOI] [PubMed] [Google Scholar]
- 125. Holtorf AP, Heldmaier G, Thiele G, Steinlechner S. Diurnal changes in sensitivity to melatonin in intact and pinealectomized Djungarian hamsters – effects on thermogenesis, cold tolerance, and gonads. J Pineal Res. 1985;2(4):393–403. [DOI] [PubMed] [Google Scholar]
- 126. Lynch GR, Epstein AL.. Melatonin induced changes in gonads, pelage and thermogenic characters in white-footed mouse, Peromyscus leucopus. Comp Biochem Physiol C. 1976;53(2):67–8. [DOI] [PubMed] [Google Scholar]
- 127. Sinnamon WB, Pivorun EB.. Melatonin induces hypertrophy of brown adipose-tissue in Spermophilus tridecemlineatus. Cryobiology. 1981;18(6):603–7. [DOI] [PubMed] [Google Scholar]
- 128. Halpern B, Mancini MC, Bueno C, Barcelos IP, Edna de Melo M, Lima MS, Carneiro CG, Sapienza MT, Buchpiguel CA, Gaspar do Amaral F et al.. Melatonin increases brown adipose tissue volume and activity in melatonin deficient patients: a proof-of-concept study. Diabetes. 2019;68(5):947–52. [DOI] [PubMed] [Google Scholar]
- 129. Hagelstein KA, Folk GE.. Effects of photoperiod, cold-acclimation and melatonin on the white-rat. Comp Biochem Phys C. 1979;62(2):225–9. [DOI] [PubMed] [Google Scholar]
- 130. Le Gouic S, Atgie C, Viguerie-Bascands N, Hanoun N, Larrouy D, Ambid L, Raimbault S, Ricquier D, Delagrange P, Guardiola-Lemaitre B et al.. Characterization of a melatonin binding site in Siberian hamster brown adipose tissue. Eur J Pharmacol. 1997;339(2-3):271–8. [DOI] [PubMed] [Google Scholar]
- 131. Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26(8):412–9. [DOI] [PubMed] [Google Scholar]
- 132. Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: Cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17(3):100–2. [DOI] [PubMed] [Google Scholar]
- 133. Tanida M, Gotoh H, Taniguchi H, Otani H, Shen J, Nakamura T, Tsuruoka N, Kiso Y, Okumura N, Nagai K. Effects of central injection of L-carnosine on sympathetic nerve activity innervating brown adipose tissue and body temperature in rats. Regul Peptides. 2007;144(1-3):62–71. [DOI] [PubMed] [Google Scholar]
- 134. Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X et al.. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65(4):e12524. [DOI] [PubMed] [Google Scholar]
- 135. Xu PF, Wang JL, Hong F, Wang S, Jin X, Xue TT, Jia L, Zhai YG. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4):e12399. [DOI] [PubMed] [Google Scholar]
- 136. Thomas AP, Hoang J, Vongbunyong K, Nguyen A, Rakshit K, Matveyenko AV. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology. 2016;157(12):4720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Oliveira AC, Andreotti S, Sertie RAL, Campana AB, de Proenca ARG, Vasconcelos RP, Oliveira KA, Coelho-de-Souza AN, Donato-Junior J, Lima FB. Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 2018;199:158–66. [DOI] [PubMed] [Google Scholar]
- 138. Mendes C, Lopes AMD, do Amaral FG, Peliciari-Garcia RA, Turati AD, Hirabara SM, Falcao JHS, Cipolla-Neto J. Adaptations of the aging animal to exercise: role of daily supplementation with melatonin. J Pineal Res. 2013;55(3):229–39. [DOI] [PubMed] [Google Scholar]
- 139. Montalbano G, Mania M, Abbate F, Navarra M, Guerrera MC, Laura R, Vega JA, Levanti M, Germana A. Melatonin treatment suppresses appetite genes and improves adipose tissue plasticity in diet-induced obese zebrafish. Endocrine. 2018;62(2):381–93. [DOI] [PubMed] [Google Scholar]
- 140. Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TCM, Lucassen EA, Sips HCM, Chatzispyrou IA et al.. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci USA. 2015;112(21):6748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sun H, Wang XC, Chen JQ, Song KX, Gusdon AM, Li L, Bu L, Qu S. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Amstrup AK, Sikjaer T, Pedersen SB, Heickendorff L, Mosekilde L, Rejnmark L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: A randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2016;84(3):342–7. [DOI] [PubMed] [Google Scholar]
- 143. Ireland KE, Maloyan A, Myatt L. Melatonin improves mitochondrial respiration in syncytiotrophoblasts from placentas of obese women. Reprod Sci. 2018;25(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Han LS, Wang HC, Li L, Li XY, Ge J, Reiter RJ, Wang Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res. 2017;63(3):e12341. [DOI] [PubMed] [Google Scholar]
- 145. Gonciarz M, Bielanski W, Partyka R, Brzozowski T, Konturek PC, Eszyk J, Celinski K, Reiter RJ, Konturek SJ. Plasma insulin, leptin, adiponectin, resistin, ghrelin, and melatonin in nonalcoholic steatohepatitis patients treated with melatonin. J Pineal Res. 2013;54(2):154–61. [DOI] [PubMed] [Google Scholar]
- 146. Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Gonciarz M, Bielanski W, Reiter RJ, Konturek SJ. Altered basal and postprandial plasma melatonin, gastrin, ghrelin, leptin and insulin in patients with liver cirrhosis and portal hypertension without and with oral administration of melatonin or tryptophan. J Pineal Res. 2009;46(4):408–14. [DOI] [PubMed] [Google Scholar]
- 147. Mostafavi SA, Solhi M, Mohammadi MR, Akhondzadeh S. Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2017;27(5):440–4. [DOI] [PubMed] [Google Scholar]
- 148. Liu ZJ, Gan L, Luo D, Sun C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-myc interaction in mouse adipose tissue. J Pineal Res. 2017;27(5):e12383. [DOI] [PubMed] [Google Scholar]
- 149. Zetner D, Andersen LP, Rosenberg J. Pharmacokinetics of alternative administration routes of melatonin: a systematic review. Drug Res (Stuttg). 2016;66(4):169–73. [DOI] [PubMed] [Google Scholar]