Abstract
Our understanding of depression and its treatment has advanced with the advent of ketamine as a rapid acting antidepressant and the development and refinement of tools capable of selectively altering the activity of populations of neuronal subtypes. This work has resulted in a paradigm shift away from dysregulation of single neurotransmitter systems in depression towards circuit level abnormalities impacting function across multiple brain regions and neurotransmitter systems. Studies on the features of circuit level abnormalities demonstrate structural changes within the prefrontal cortex (PFC) and functional changes in its communication with distal brain structures. Treatments that impact the activity of brain regions, such as transcranial magnetic stimulation or rapid acting antidepressants like ketamine, appear to reverse depression associated circuit abnormalities though the mechanisms underlying the reversal, as well as development of these abnormalities remains unclear. Recently developed optogenetic and chemogenetic tools that allow high fidelity control of neuronal activity in pre-clinical models have begun to elucidate the contributions of the PFC and its circuitry to depression- and anxiety-like behavior. These tools offer unprecedented access to specific circuits and neuronal subpopulations that promise to offer a refined view of the circuit mechanisms surrounding depression and potential mechanistic targets for development and reversal of depression associated circuit abnormalities.
Depression is ranked by the World Health Organization as one of the world’s most burdensome diseases1. In the United States alone, over 15% of the population is impacted by depression2 resulting in an economic burden over $200 billion3. Current antidepressant medications are ineffective in nearly one third of patients and suffer from weeks long treatment lag4. The difficulty in finding candidate genes for depression5, 6 has led to increased efforts to understand brain circuitry underlying depression. Much of this effort has focused on the prefrontal cortex (PFC) and hippocampus as these regions display structural, as well as functional changes potentially triggered by altered glutamatergic and gamma-aminobutyric acid (GABA) transmission7, 8. Reversal of circuit abnormalities in depression by therapies that manipulate neuronal activity provides hope for those with treatment resistant depression.
For those with treatment resistant depression there are several therapies both in use and under investigation that are designed to modulate neuronal activity. These include non-invasive techniques utilizing electrical stimulation (e.g. electroconvulsive therapy (ECT), transcranial direct-current stimulation (tDCS)) or magnetic fields (e.g. repetitive transcranial magnetic stimulation (rTMS), magnetic seizure therapy (MST)) to transiently manipulate neuronal activity. Alternatively, deep brain stimulation (DBS) is an invasive therapy that produces site specific regulation of neuronal activity through implanted electrodes for continuous manipulation. Many of these therapies appear to impact PFC circuitry as part of the antidepressant response9–12. These therapies typically require numerous treatments for effective relief, though in the case of DBS relief may be almost immediate12. Notably the response to ketamine, an effective antidepressant even in treatment resistant cases13, 14, also appears to transiently elevate PFC activity indicative of immediate circuit level effects15. Importantly, ketamine dramatically reduces depressive symptoms within hours13 suggesting that an appropriate level of PFC intervention may rapidly induce antidepressant effects. However, ketamine is not without off-target effects that limit its utility as an antidepressant therapy.
The pre-clinical research community studying depression has gained insight on the effects of the aforementioned therapeutic interventions, but these approaches have significant limitations, including regional and cellular specificity. To address these issues, studies are also being conducted with sophisticated, cutting edge cell-type and circuit specific approaches, including optogenetic and chemogenetic tools. These tools offer critical insights into PFC circuitry and the role of neuronal subpopulations that may offer more specific targets for antidepressant therapies. The following examines on the role PFC circuits in depression, with particular focus on information produced by population specific neural manipulations.
PFC pathology in depression
There are numerous lines of evidence demonstrating that PFC circuitry is dysregulated in depression. These include alterations of structure, markers of glutamatergic and GABAergic neurotransmission, and connectivity with downstream structures (for a thorough review of these topics see8). Evidence for depression related structural changes in the PFC come largely from secondary measures. For instance, the volume of PFC is reduced in depressed patients and this decrease is correlated with length of illness7. Evidence of reduced synapse number is provided by a recent positron emission tomography study reporting decreased levels of ligand binding to synaptic vesicle glycoprotein 2A (SV2A) in depressed patients16. This imaging suggests that presynaptic vesicle protein SV2A is reduced across the brains of patients with severe depression, and is associated with altered PFC connectivity. Direct evidence of synaptic loss has been reported in postmortem studies of depressed subjects, including reduced synaptic markers and number of synapses in PFC17. Animal models provide further evidence for structural changes, demonstrating that chronic stress exposure, an often used model of depression, decreases spine density and dendrite complexity of medial PFC (mPFC) neurons, and that fast acting antidepressants rapidly reverse the synaptic as well as behavioral deficits caused by stress18–22. Though others have postulated that such structural changes may eventually lead to neuronal loss to date the literature does not support overt neuronal loss in depression, or models for studying depression23, 24.
Increasing evidence suggests that glutamatergic and GABAergic transmission is altered in depression8, 25 (for a thorough review of this topic see26). PFC glutamate metabolites are reduced in depression27 and postmortem studies demonstrate changes in ionotropic and metabotropic glutamate receptors28, 29. mPFC levels of the GABA synthetic enzyme glutamate decarboxylase-67 are also reduced in postmortem brains of depressed subjects30 as are markers of the somatostatin/calbindin (SST) GABAergic subtype31, 32. Cortical TMS studies utilizing motor threshold measurements report reduced cortical GABAergic tone in depressed patients33, 34. Magnetic resonance spectroscopy studies also provide evidence of reduced GABA levels in depressed individuals35, 36 and reversal after successful TMS treatment37. Together these findings indicate that neurotransmission within the PFC and communication with downstream targets is dysregulated in depression. Consistent with this, there are reports indicating that default mode network connectivity is elevated in depression, and reduced by successful TMS9, 10, and that functional connectivity between fronto-limbic and fronto-striatal targets may classify depression subytpes and inform response to TMS treatment38. Normalization of frontal activity and functional connectivity is also observed with DBS12 and ECT11. It is also notable that the glutamate burst produced by ketamine results from acute, transient pharmacological inhibition of GABAergic transmission, that results in persistent synaptic plasticity that is associated with the antidepressant response well after the acute phase of treatment8.
These findings highlight the importance of balanced PFC function to mental health. Dysregulated glutamatergic and GABAergic transmission within the PFC would be expected to negatively impact cognitive function and emotion through altered local processing of afferent information and generation of efferent activity necessary to communicate with distal structures. Importantly, these findings also point to the importance of the refined understanding of PFC network function and structure that pre-clinical research can provide for future advances in targeted neuromodulation.
Assessing PFC circuits via manipulation of selected neuronal populations
It is challenging to gain insight into the specific brain targets or, circuits, that initiate a therapeutic response following implementation of ECT or other neuromodulatory therapies because the technologies are inherently non-specific. In all electrical or magnetic stimulation techniques the brain area impacted by the manipulation is a function of “dose” (i.e. pulse width, duration, etc.). Broadly, because of the necessity of passing the electrical stimulus directly through the skull ECT may produce electric field strengths throughout the brain volume that are well above neuronal stimulation threshold (Fig. 1A)39. Similarly, though the use of magnetic stimulation bypasses skull shunting to refine the electric field, TMS still offers limited ability to target a specific brain region, especially as “dose” is increased to support neuronal stimulation at greater depth from the skull surface (Fig. 1B)40–42. Invasive DBS allows manipulation of brain activity through surgical placement of electrodes and continuous application of current12. Activity change produced by DBS is non-specific in the area of the electric field, and may also include fibers of passage. Additionally, the stimulation frequency utilized in DBS is above physiologically sustainable levels making it unclear whether the local modulation by DBS represents activation or depolarization induced-inhibition43. A greater understanding of potential targets for these circuit therapies may therefore be gained through the use of tools that allow for precise targeting of location, and defined activation or inhibition of neuronal activity and even specific cell populations.
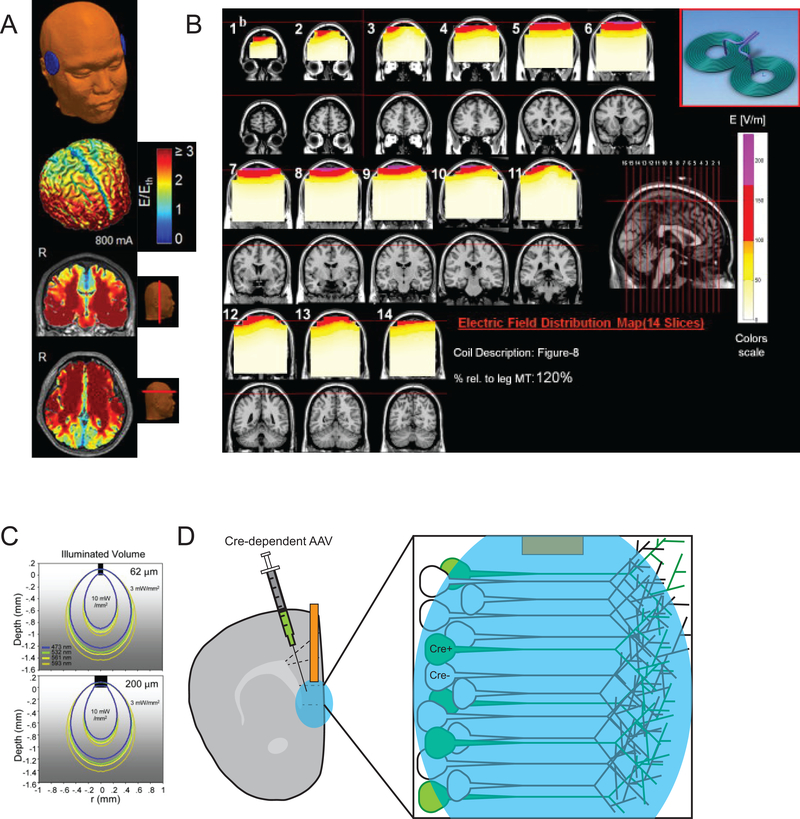
Fig. 1.
Regional specificity of modalities for transiently manipulating neuronal activity a. Model estimates of electric field strength (E) above stimulation threshold (Eth) for conventionally applied (bilateral) ECT at 800mA. Stimulation strength on the cortical surface, and representative coronal and axial slices from realistic head models are shown (adapted from39) b. Model estimates of electric field strength for 1ms TMS pulse applied at 120% of the leg motor threshold. Red and purple areas indicate power above neuronal activation threshold (adapted from42) c. Iso-contour lines depicting monte-carlo estimated light spread and intensity at 10mW output power with typically utilized cannula diameter (62μm top, 200μm bottom) and light wavelengths for optogenetic stimulation demonstrates the discrete area below the implanted fiber optic cannula expected to be directly impacted by light delivery (adapted from44) d. The effects of optogenetic stimulation may be further refined using viral vectors with population specific promoters or Cre-recombinase dependence.
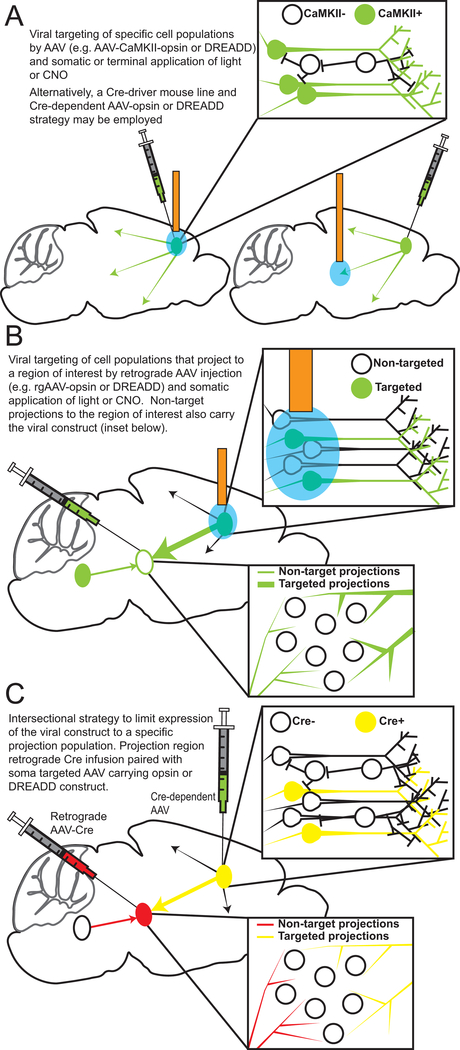
Tools available to researchers using preclinical models offer greater precision and offer avenues for understanding the impact of neurostimulation on neural circuits. In recent years a variety of tools have been developed that allow control of neuronal activity through introduction of light sensitive channels (i.e. optogenetics) or engineered receptors sensitive to exogenous ligands (i.e. chemogenetics; designer receptors exclusively activated by designer drugs (DREADDs)). The use of optogenetic or chemogenetic tools in combination with viral-vector strategies enhances the spatial selectivity of modulation when compared to clinically utilized tools for neuromodulation, particularly ECT and TMS. An adeno-associated virus (AAV) carrying an optogenetic or chemogenetic vector may be placed directly into a target region of interest producing very discrete spatial selectivity. This is particularly true with optogenetic techniques where the neuronal manipulation is limited to the area directly below a fiber optic implant (Fig. 1C)44. Further selectivity may be obtained through the use of strategies to control expression in specific cell populations (Fig. 1D, Fig. 2). AAV expression may be gated by packaging specific promotor sequences within the expression vector (Fig. 2A), for example the calmodulin-dependent protein kinase II (CaMKII) promoter allows biased targeting of excitatory neurons in a region of interest45, 46. An alternative strategy to gain population specific vector expression employs Cre-recombinase transgenic mice with viral vectors that require Cre-recombinase for expression. This strategy limits viral expression to those cells within an area of interest expressing Cre-recombinase. Injection of viral constructs with retrograde transport properties allows targeting of populations projecting to regions of interest (Fig. 2B), and intersectional strategies are capable of targeting individual projections populations (Fig. 2C). For instance, one might combine a retrograde AAV-Cre viral placement in a terminal region of interest with a Cre-dependent viral placement in the somatic area of interest to target cellular populations of a specific projection pattern (e.g., placement of retrograde AAV-Cre into the basolateral amygdala (BLA) and a Cre-dependent construct into the PFC to target PFC cells that target the BLA47 ).
Fig. 2.
Viral strategies for targeting neuronal populations. a. Targeting a population using promoter specific or Cre-dependent AAV and somatic or terminal manipulation b. Targeting a population of cells that project to a region of interest using retrograde AAV and somatic manipulation c. Intersectional AAV strategy to limit AAV vector expression to a discrete projection population.
Considerations for the use of optogenetic and chemogenetic tools
The use of optogenetic tools allows high fidelity temporally specific activation (i.e., channelrhodopsin (Chr2), light activated cation channel) or inhibition (i.e., halorhodopsin (NpHR), light active Cl- channel) of target cells with light delivery through fiber optic cannula into the region of interest48. Heating of surrounding tissue is a well documented result of light application44, 49, 50, and when sufficient may alter neuronal activity44, 50. This is dependent on the light intensity as well as duration of application. Similarly, blue light of the type used for optogenetic experiments (i.e. ~470nm), may alter transcription, even in the absence of optogenetic constructs51. These findings highlight the need to employ proper controls to account for changes that may be induced by light delivery.
Chemogenetic tools lack the temporal fidelity of optogenetic tools as G protein coupled receptors are engineered to respond to an exogenous ligand and to couple to either stimulatory (e.g., muscarinic receptor M3 to Gq) or inhibitory (e.g., M4 to Gi) signaling pathways in targeted cells52; this means that the onset and offset of actions are governed by the pharmacokinetics of the exogenous ligand, and may be better described as altering the firing threshold rather than directly impacting firing. However, because the exogenous ligand can be administered systemically, or directly applied to brain targets through local infusion, the animal does not have to be tethered, as is the case for real time behavior for optogenetic studies. Notably, the absence of light is an advantage to chemogenetic work as the above described concerns for optogenetic work are obviated. This may make chemogenetic approaches a better choice when long-duration stimulation/inhibition is necessary. However, recent evidence demonstrates that the DREADD ligand clozapine-n-oxide (CNO) may be converted to the atypical antipsychotic clozapine53. Thus, proper controls must be included in DREADD studies, such as administration of CNO to control animals for analysis of the effects of CNO metabolites on behavior to rule out any off target effects.
Influence of mPFC circuit activity on depression- and anxiety-like behaviors in real-time
Correspondence of rodent mPFC and definition of areas to be discussed
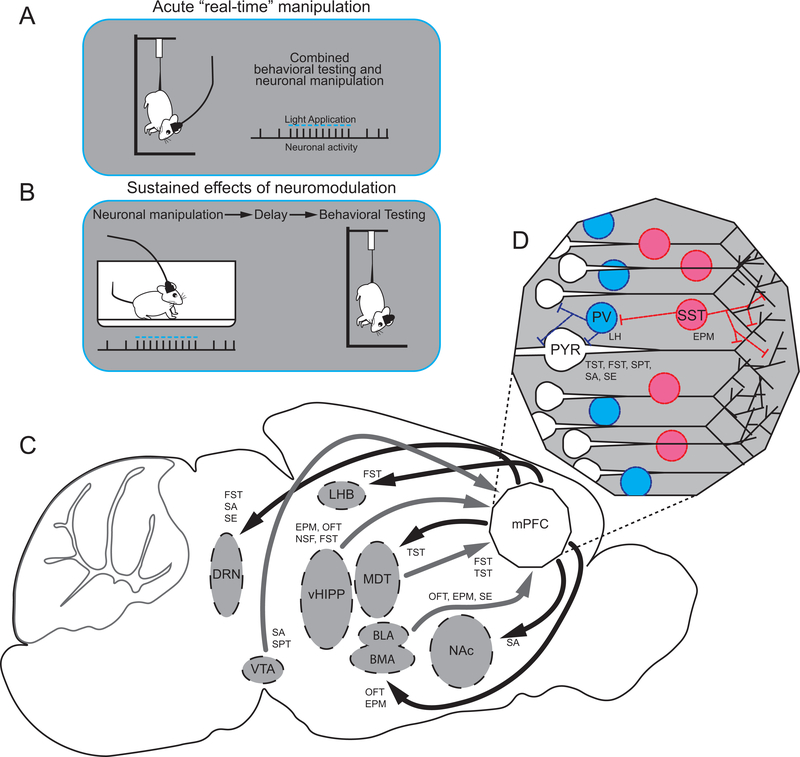
In an effort to dileneate the scope of the review it is necessary to define the regions being considered. We will focus on aspects of mood in depression, and the role of PFC in regulation of mood related behaviors. As detailed above, numerous studies have demonstrated functional and structural alterations in the PFC of depressed patients that likely play a significant role in dysfunction of mood related behaviors. There is much debate as to whether the rodent PFC is similar to PFC in non-human primates and humans54. For the purposes of this review we focus on studies of the rodent mPFC an area encompassing prelimbic (PL), infralimbic (IL), and anterior cinculate cortex (ACC). The rodent IL (also referred to herein as ventral medial mPFC (vmPFC)) appears to correspond to the subgenual cortex area (i.e. Brodmann 25) and is an important component of the striatal emotion processing network that is conserved across species55. More dorsal regions of the rodent mPFC (i.e. PL and ACC) appear to correspond to ACC though there is more debate here54–56. The mPFC plays a critical role in behavior, as a central hub that receives input from cortical, thalamic, and limbic regions and sends outputs to structures that regulate emotion, fear, and stress responses such as the amygdala, habenula, and dorsal raphe nucleus (DRN). Segregation of function between ventral and dorsal regions of mPFC is evident from work involving fear expression and extinction learning57–59 and also appears in the response to rapid-acting antidepressants described below60. Research in mPFC related to fear is often conceptualized in terms of Post Traumatic Stress Disorder and will not be covered (for review see61). Similarly, while orbital frontal cortex is likely important to depression given its role in choice based on expected outcome the current review will not discuss research focused on this brain region. Instead, we will focus on recent optogenetic and chemogenetic studies that have begun to separate the roles that specific cellular populations play within the mPFC in the regulation of depression-related behaviors in rodent models. The vast majority of these studies utilize opto- or chemogenetic manipulations at the time of testing (i.e. in real-time) and the interpretation of results are based on the acute nature of the manipulation (Fig. 3A). Previous reviews have detailed the impact of manipulating brain regions other than the mPFC on depression-like behavior62; the current review will focus on manipulations of mPFC cell populations as well as mPFC efferent and afferent fibers. Because of the high comorbidity, we include studies assessing anxiety, as well as depression-related behaviors.
Fig. 3.
Pre-clinical neuronal manipulation methods and mPFC circuitry implicated in depression like behavior. a. Optogenetic or chemogenetic manipulations may take place at the time of testing or b. may precede testing. c. mPFC afferent (grey) and efferent (black) circuitry with behaviors reported to be impacted by optogenetic and/or chemogenetic manipulations indicated. d. mPFC cellular populations with behaviors reported to be impacted by behaviors reported to be impacted by optogenetic and/or chemogenetic manipulations indicated.
Involvement of mPFC in active versus inactive coping strategies
Numerous tests of depression-like behavior incorporate a stressful challenge that provides a means to assess active and inactive behavioral periods. In the forced swim test (FST) this is achieved by placing a rodent (rat or mouse) in a container filled with water to a sufficient depth that the animals cannot support themselves and must choose between active swimming and climbing, or inactive floating. In mice, the tail suspension test (TST) also contrasts active struggling against periods of inactivity. Experiments employing learned helplessness models (rat or mouse) utilize prior uncontrollable stress to generate a behavioral state in which animals do not engage in active behavior that would allow them to escape a mild foot shock. Depression-like behavior following uncontrollable stress is also evident in reduced exploration of a novel juvenile (rat or mouse). In each of these tests the amount of inactivity has in the past been described as reflecting helplessness or despair, however more recent interpretations feature a transition between active and passive coping that is impacted by prior experience as well as learning during the test session63. The mPFC’s role in action selection, in addition to the well documented effects of stress on the mPFC21, has led to numerous investigations into the role of mPFC cellular populations in these models.
Photostimulation of glutamatergic cells within the mPFC has produced mixed results. An initial study utilizing the CamkIIa promoter to target glutamatergic neurons within the mPFC observed no effect of photostimulation during the FST64. However, photostimulation of mPFC terminals in the DRN decreased immobility, an antidepressant response, while photostimulation of mPFC terminals in the lateral habenula (LHB) increased immobility64. This finding clearly demonstrates the additional insights obtainable through sub-population specific targeting, in this case based on different mPFC projections to the DRN vs. LHB. Later studies have observed reduced immobility in the forced swim test when Thy-1 expressing neurons were targeted for photostimulation65, and when mPFC neurons that receive ventral hippocampal (vHipp) input were activated with Gq DREADDs66. Immobility was also reduced in the TST when vesicular glutamate transporter-2 (vGlut2) neurons were photostimulated67. The reason for the differences are not clear, but presumably are related to targeting different populations of excitatory neurons with the different promoters used for these studies. In addition, the stimulation parameters could account for differences in the effects observed with somatic stimulation. This is exemplified in work targeting IL mPFC projections to the medial dorsal thalamus (MDT). Photostimulation of this subpopulation with gamma bursts timed to IL oscillatory activity reduced immobility in the TST, but similar stimulation not timed to oscillatory activity did not, and more rapid stimulation produced an increase in immoblity68. The different and even opposing effects of different stimulation frequencies (Table 1) provides further insight that is especially relevant to DBS paradigms that use only very high frequency (110 Hz) stimulation.
Table 1:
Overview of studies using optogenetic/chemogenetic techniques in real-time to manipulate mPFC circuitry in studies of anxiety and depression
| Neuromodulatory approach | Target Population | Effect | Test | |
|---|---|---|---|---|
|
Soma Targeting | ||||
| Covington, 201075 | ChR2; 40ms of 100hz per 3 second period, 1–2mW | Glutamatergic and GABAergic | Antidepressant | Social avoidance, SPT |
| No effect | EPM | |||
| Lee, 201576 | ChR2; 5ms 10hz, 1mW | Glutamatergic | Antidepressant | Social avoidance |
| Warden, 201264 | ChR2; 5ms 20hz, 3mW | Glutamatergic | No effect | FST |
| Yizhar, 201178 | SSFO; light delivery produces asynchronous excitability enhancement | Glutamatergic | Pro-depressive | Social exploration |
| Parvalbumin | No effect | Social exploration | ||
| Perova, 2015102 | Gi DREADD; 10mg/kg CNO prior to training and testing | PL Parvalbumin neurons | Pro-depressive | Learned helplessness |
| Adhikari, 201598 | Chr2; 5ms 1mW, 10Hz | vmPFC Glutamatergic | No effect | OFT, EPM |
| Ferenczi, 201677 | SSFO; light delivery produces asynchronous excitability enhancement | Glutamatergic | Pro-depressive | SPT, Social exploration |
| Warthen, 201679 | Gq DREADD, 0.5–2.5mg/kg CNO | Glutamatergic | No effect | Social exploration, OFT |
| Son, 201867 | ChR2; 1 second 100hz per 4 second period | vGluT2 | Antidepressant | TST |
| Soumier, 2014101 | Gi DREADD; 5mg/kg CNO | PL Somatostatin neurons | Pro-depressive | Test battery net result |
| Kumar, 201365 – this manipulation produced a locomotor effect | ChR2; Neuron matched (~4hz), 2mW | PL mPFC Thy-1 | Antidepressant | FST |
| Carreno, 201666 | Gq DREADD; 0.5mg/kg CNO | mPFC neurons that receive vHIPP input | Antidepressant | FST |
|
Afferent Targeting | ||||
| Felix-Ortiz, 201680 | ChR2; 5ms 5mW, 20hz | BLA - mPFC | Anxiogenic, Pro-depressive | EPM, OFT, Social exploration |
| NpHR; 5mW, constant | BLA - mPFC | Anxiolytic, Antidepressant | OFT, Social exploration | |
| Chaudhury, 201382 | NpHR; 8 seconds on - 2 seconds off | VTA - mPFC | Pro-depressive, No effect | Social avoidance, SPT |
| Chr2, 0.5hz 15ms and 20hz 40ms | VTA - mPFC | No effect | Social avoidance, SPT | |
| Padilla-Coreano, 2016100 | Arch; Continuous, 10 mW, 2 minutes off – 2 minutes on | vHIPP - mPFC | Anxiolytic | EPM, OFT, NSF |
| MDT - mPFC | No effect | EPM | ||
| Carreno, 201666 – some effects observed from ketamine antidepressant baseline | ChR2; 20hz for 10 minutes, 30mW | vHIPP - mPFC | Antidepressant with DRN inactivation | FST |
| eNpHR3.0; Continuous, 10 minutes | vHIPP - mPFC | Pro-depressive | FST | |
| MDT - mPFC | No effect | FST | ||
| Miller, 201769 | Gq DREADD; 1mg/kg CNO | MDT - mPFC | Antidepressant | FST, TST |
|
Efferent Targeting | ||||
| Warden, 201264 | ChR2; 5ms 20hz, 10–20mW | mPFC - DRN | Antidepressant | FST |
| mPFC - LHB | Pro-depressive | FST | ||
| Adhikari, 201598 | Chr2; 5ms 10Hz, 10mW | vmPFC - BMA | Anxiolytic | OFT, EPM |
| eNpHR3.0; 10mW | vmPFC - BMA | Anxiogenic | OFT, EPM | |
| Bagot, 201587 | ChR2; 4hz, 15–20mW | mPFC - NAc | Antidepressant | Social avoidance |
| mPFC - NAc | No effect | OFT | ||
| Hultman, 201686 | Gq DREADD; 1mg/kg CNO | mPFC – amygdala | Antidepressant | Social avoidance |
| Challis, 201488 – this manipulation was performed during the social defeat sensory contact period not testing | ChR2; 25hz, 10 ms, for 10 minutes, 10mW | PL mPFC - DRN | Pro-depressive | Social avoidance |
| Arch; 20 minutes continuous, 10mW | PL mPFC - DRN | Antidepressant | Social avoidance | |
| Carlson, 201768 | ChETA; Closed loop, gamma bursts timed to IL oscillations | IL mPFC - MDT and MDT soma | Antidepressant | TST |
| ChETA; Gamma bursts not timed to IL oscillations | IL mPFC - and MDT soma | No effect | TST | |
| ChETA, 14hz | IL mPFC - and MDT soma | Pro-depressive | TST | |
| Dolzani, 201874 – effects observed from ketamine antidepressant baseline | Real-time, Gi DREADD; 3mg/kg CNO during exposure to uncontrollable stress | PL - DRN | Pro-depressive | Social exploration |
Anterior cingulate cortex (ACC), basolateral amygdala (BLA), basomedial amygdala (BMA), dorsal raphe nucleus (DRN), elevated plus maze (EPM), forced swim test (FST), infralimbic (IL), lateral habenula (LHB), medial dorsal thalamus (MDT), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), novelty suppressed feeding (NSF), prelimbic (PL), somatostatin (SST), sucrose preference test (SPT), ventral hippocampus (vHIPP), ventral medial prefrontal cortex (vmPFC), ventral tegmental area (VTA), vesicular glutamate transporter (vGlut)
There have been only a handful of studies utilizing these approaches to target mPFC afferents. In a study attempting to determine the locus of ketamine’s antidepressant effects, photostimulation of vHIPP terminals in the mPFC reduced immobility, but only when DRN was inactivated66. This study also reported that in ketamine treated rats, inhibition of the vHIPP-mPFC population increased immobility, effectively reversing the ketamine response. In contrast, inhibition of the MDT-mPFC population did not alter the response to ketamine, demonstrating circuit specificity66. However, in the absence of prior ketamine administration MDT-mPFC stimulation using a Gq DREADD has been reported to reduce immobility69. There is strong evidence that dopaminergic neuronal activity regulates the transition between active and passive strategies70, however to our knowledge this effect has not been examined at mPFC terminals from midbrain dopamine neurons.
Involvement of mPFC in social avoidance and exploration
Normal social interactions are disrupted in depressed patients71, and social avoidance or exploration are commonly used measures for studying depression in rodent models. In social defeat stress, animals are subjected to repeated defeat followed by prolonged periods of sensory contact with a dominant agressor72. During later testing animals susceptible to this paradigm demonstrate social avoidance operationalized as less time investigating a novel conspecific. Beyond social avoidance, this model also produces anhedonic behavior in susceptible animals demonstrated as reduced preference for sucrose, increased anxiety, metabolic changes, and continued corticosterone reactivity, making it an attractive model for studying depression73. Alternative models utilize uncontrollable stress exposure to produce a reduction in juvenile exploration time at testing74. Numerous studies point to a role for mPFC in the development of these depression-like behaviors. Following demonstration of reduced immediate early gene levels in the mPFC of depressed patients, an indirect marker suggesting reduced neuronal activity, Covington et al implemented a stimulation protocol that induced an increase in immediate early gene levels that were reduced by exposure to social defeat stress75. Stimulation of all neurons, glutamatergic and GABAergic, in the mPFC of mice exposed to social defeat stress reduced social avoidance, as well as anhedonia determined in the sucrose preference test, consistent with an antidepressant response75. Similarly, others have shown that unilateral left, but not right, stimulation of glutamatergic neurons in the PL mPFC reduces social avoidance76. However, utilizing step function opsins (SSFOs) that asynchronously elevate mPFC excitability, others have shown that increased mPFC activity produces deficits in juvenile exploration and reduced sucrose preference77, 78, though this was not observed when neurons were activated using DREADDs79. The latter studies were conducted in unstressed animals, and as such the differences may be due to prior stress exposure altering mPFC network dynamics and therefore the response to stimulation. Alternatively, differences in stimulation parameters or variations in the subpopulation of neurons targeted may be the cause of the contradictory outcomes (Table 1).
Studies of mPFC afferents support a role for mPFC circuitry in altered social interaction. Use of optogenetic constructs to bidirectionally control BLA neurons projecting to the mPFC in unstressed animals demonstrated that photostimulation reduced juvenile interaction, while photoinhibition had an opposite effect80. Results from other studies raise the possibility that BLA to mPFC responses are amplified by stress exposure. Exposure to chronic stress decreases mPFC apical dendritic complexity and spine number and function, while basal dendrites, which are targeted by BLA are unchanged by stress81. This may lead to an increase in BLA control after stress. Dopaminergic afferents to mPFC also appear to play a role in social avoidance. Inhibition of ventral tegmental area (VTA) projections to mPFC increased social avoidance in animals following a sub-threshold social defeat paradigm82. This is consistent with reports demonstrating reduced dopamine83, 84, and dopamine D1 receptor signaling85 in the mPFC following social defeat. Together these studies of mPFC afferents demonstrate a clear role for mPFC in social avoidance and highlight a stress reactive circuitry that may be targeted for treatment of stress related illnesses such as depression.
Studies involving mPFC efferents highlight important projection regions impacting the response to social defeat. Hultman86 and colleagues identified an mPFC-amygdala connection as critical to maintaining synchrony in an mPFC-amygdala-VTA circuit. Increasing activity in amygdala neurons that receive input form mPFC reduced social avoidance in susceptible mice. mPFC projections to the nucleus accumbens (NAc) and DRN may also impact social behavior. Photostimulation of mPFC terminals in the NAc during avoidance testing produced an antidepressant effect, increasing social interaction in animals that had undergone social defeat stress exposure87. mPFC projections to the DRN have been shown to bidirectionally modify social defeat outcomes. Challis et al stimulated or inhibited the mPFC-DRN pathway during the sensory contact period after daily defeat and observed that increasing activity in this pathway increased subsequent social avoidance, while inhibition produced the opposite antidepressant-like effect88. A similar finding is observed in the DRN, where inhibiting GABAergic interneurons, the target of mPFC projections, during the sensory contact period blocked the effect of social defeat89. Notably, manipulation of GABAergic interneurons in the DRN during testing had no effect on social avoidance after social defeat, highlighting the importance of this pathway in adaptation to continuing social defeat.
Stress associated adaptations in the mPFC-DRN pathway have also been linked to prior experience with stressor controllability where activation of this pathway during uncontrollable stress produces outcomes similar to those where stress is controllable, leading to levels of interaction with a juvenile conspecific that are similar to that observed in unstressed animals74, 90, 91. It is interesting that this differs and in fact is opposite to the effects of activiating the mPFC-DRN pathway during the sensory contact period following social defeat. This may be due to the chronicity of manipulation, multiple experiences in social defeat versus single exposure to uncontrollable stress, and/or the nature of the stressor during neural manipulation (i.e. shock or sensory contact). Together, these findings highlight important contributions of mPFC and its downstream targets in social avoidance, and demonstrate the utility of optogenetic tools for determining likely sites of adaptation to stress experience, while also demonstrating the importance of the timing of circuit manipulations to the outcomes observed during behavioral testing. Additionally, the mPFC-DRN pathway appears important in regulating the response to defeat experience in Syrian hamsters, though a thorough examination of this literature is beyond the scope of the review92–94.
Involvement of mPFC in anxiety-like behavior
Approximately half of the individuals diagnosed with depression also have a comorbid anxiety disorder95, and this combination results in reduced rates of treatment efficacy. Optogenetic and chemogenetic work in the mPFC has largely focused on approach-avoidance conflicts when investigating the role of mPFC in anxiety-like behavior. In the elevated plus maze (EPM) and open field test (OFT) typical exploration behavior is opposed by safety cues to avoid open spaces, such as the open arms of the plus maze, or against the center of the open field. Animals that spend more time exploring the open arms of the EPM or the center of the open field are considered less anxious. The use of these tests is supported by demonstrated anxiolytic effects with compounds that produce effective anxiolysis in humans, and similar results between tests96, 97.In the novelty suppressed feeding test (NSF) a food restricted animal is placed into an open field and the time to enter the center and consume a food pellet is recorded. Longer latencies to approach and eat the pellet are an indication of greater anxiety. There is very little evidence demonstrating that acute stimulation of mPFC principle neurons alters anxiety-like behavior in the EPM or OFT, using optogenetic75, 98 or chemogenetic techniques79. However, mPFC single unit recordings are known to signal anxiogenic locations on the EPM, and show coupling to vHIPP oscillatory activity99. Consistent with this, photoinhibition of vHIPP inputs to the mPFC reduces anxiety-like behavior in EPM, OFT, and NSF100. A similar manipulation of MDT-mPFC circuitry had no effect100.
Bidirectional effects of manipulating BLA projections to mPFC have also been observed. Photostimulation of BLA neurons that project to mPFC produces an anxiogenic response, while inhibition of these BLA-mPFC projection neurons is anxiolytic80. As noted above, the influence of BLA-mPFC input appears to be augmented by stress and normalized by ketamine treatment81, and the vHIPP input to mPFC has also been implicated in the antidepressant effects of ketamine66. From vmPFC, projections to the basomedial amygdala (BMA) are involved in anxiety: vmPFC-BMA stimulation produces an anxiolytic effect in the OFT and EPM, while inhibition produces an anxiogenic effect98. In contrast, stimulation of mPFC neurons projecting to the NAc had no effect on anxiety-like behavior in the OFT87, but was effective in reducing social avoidance, demonstrating differential effects of these projection neurons on social avoidance versus anxiety. Together this work demonstrates the need to target discrete neuronal populations by input/output region, as clear effects of mPFC on anxiety-like behavior are observed only when populations targeted by, or projecting to, distinct regions are targeted.
Involvement of mPFC GABAergic neurons in models of depression
Altered excitation/inhibition balance within the mPFC is emerging as a potential causative factor in depression, and restoration has emerged as a hypothesized therapeutic outcome with rapid acting antidepressants such as ketamine8, 32. However, there have been few studies directly assessing the role of GABAergic populations in depression. As described above, SST neurons are a subpopulation of GABAergic neurons that gate input activity at the level of dendrites, and are reported to be decreased in postmortem PFC of depressed subjects32. Recent efforts to understand SST regulation of anxiety- and depression-like behavior have utilized DREADD mediated inhibition of dorsal mPFC SST neurons after both acute and chronic inhibition101. Acute administration of CNO to produce DREADD mediated inhibition of SST interneurons at the time of testing increased anxiety like behavior on the EPM, and increased time to consumption in the cookie test, a measure of increased anhedonic behavior. These positive results and consistent trends in other measures such as EPM and NSF led to a significant increase in what the authors termed behavioral emotionality. In contrast, administraton of CNO twice per day for 3 weeks produced reduced behavioral emotionality. Consistent with this result, SST interneuron ablation reduced behavioral emotionality before, and after chronic unpredictable stress101.
Parvalbumin (PV) neurons gate pyramidal cell activity at the cell body, placing this GABAergic interneuron subtype in position to limit communication with targets downstream of the mPFC. There have been limited investigations targeting this population in studies of depression. However, Perova et al observed reduced excitatory drive onto PV cells following footshock training prior to learned helplessness testing102. These authors then utilized a DREADD approach to inhibit PV interneurons during training and testing and observed an increase in escape failures. These findings indicate a role for PV interneurons in gating mPFC activity during uncontrollable stress that opposes the development of helpless behavior. Given the importance of interneuron populations to regulating mPFC function deeper investigations into the role of these interneuron subtypes in models of depression and treatment eresponse are warranted.
Sustained effects of mPFC circuit manipulations
The vast majority of optogenetic work has focused on real-time modulation of neuronal activity as it relates to circuit function and behavior. This makes sense as the multitude of opsins allow high fidelity control of neuronal activity in discrete regions along user defined time-scales. However, there are an increasing number of examples where optogenetic protocols are used to induce forms of plasticity at synapses of interest that persist well after the light application (Fig. 3B). For instance 1 hz photostimulation has been used to induce synaptic long term depression (LTD) in the lateral amygdala and reduce responding to fear conditioned cues 24 hours later103. These authors also demonstrated that 100 hz photostimulation effectively produced synaptic long term potentiation and reversed the effects produced by 1 hz optogenetic LTD. Studies of depression-like behavior have also used this approach. Optogenetically induced LTD at vHIPP-NAc synapses reduces social avoidance tested 45 minutes later, an effect not observed at mPFC-NAc synapses87. Persistent effects are not limited to optogenetic techniques. Three weeks of CNO administration in mice expressing inhibitory DREADDs in mPFC SST neurons produced antidepressant effects101 outside of the drug active period, as did 5 weeks of CNO administration in mice expressing excitatory DREADDs in excitatory neurons of entorhinal cortex104.
These studies demonstrate the utility of optogenetic and chemogenetic tools to produce sustained changes in behavior when used in a way that is somewhat analogous to clinical treatments for depression, including TMS, DBS, and ketamine. While the utility of optogenetic and chemogenetic tools in patients remains to be seen, these techniques may identify specific synaptic targets that reverse depressive phenotypes in a way that is not achievable with the indiscriminate circuit modulatory approaches available for human use. Completing this type of work may then inform clinical studies using different treatment paradigms. For instance, recent pre-clinical work in the field of addiction has demonstrated that sensitization of D1 medium spiny neuron responses to cocaine may be abolished by optogenetic stimulation using a frequency (12hz) sufficient to produce LTD. This effect was not evident with DBS at the same frequency. However, co-administration of a D1 antagonist in addition to DBS produced mGluR dependent LTD, and a behavioral response similar to that generated with optogenetically applied LTD105. The authors produced a framework for causally linking plasticity produced by circuit manipulations to persistent changes in behavior106. Key to this framework is identifying the synaptic plasticity associated with the disorder of interest, in this case cocaine associated synaptic potentiation, and designing therapeutically effective protocols to reverse these changes.
There have been multiple studies in the mPFC that may be informative within a framework linking neuroplasticity produced by circuit manipulations to persistent behavioral change that may be useful in understanding depression and designing more focused and efficacious treatments. Kumar et al65 pointed to hypotheses suggesting that DBS and TMS associated activation of descending mPFC circuits drives the antidepressant response. The authors demonstrate that acute photostimulation of deep layer anterior PL projection neurons modulates limbic system oscillatory power and synchrony, and that ~4 hz stimulation, 5 minutes daily for 14 days, reduced anxiety tested 10 days after termination of the stimulation protocol, but did not impact social avoidance after social defeat stress. Interestingly, a pair of studies107, 108 using 20 hz photostimulation, 20 minutes per day over 5 days, of neurons in deep layers of the posterior ACC/PL observed pro-depressant and anxiogenic effects for up to 5 days after stimulation. The rationale and design of the latter study was based on evidence that ACC/PL neuronal activity is increased in models of chronic pain that also produce depression-like behavior. Together these studies demonstrate that there may be regional selectivity in the mPFC response to activation and highlight the need to gain a better understanding of how different photostimulation protocols of mPFC subregions influence depression-like behavioral outcomes (Table 2).
Table 2:
mPFC optogenetic/chemogenetic manipulations with sustained effects on behaviors used to study anxiety and depression
| Neuromodulatory Approach | Target Population | Effect | Test | |
|---|---|---|---|---|
| Kumar, 201365 | ChR2; Neuron matched (~4hz), 2mW, 5 minutes daily for 14 days after social defeat | PL mPFC Thy-1 | No effect anxiolytic |
Social avoidance EPM |
| Soumier, 2014101 | Gi DREADD; 0.5mg/kg CNO 2x per day for 3 weeks including test days | PL mPFC SST | Antidepressant | Test battery |
| Friedman, 2014 | Chr2; 5 20hz pulses per 10 second period, 20 minutes per day for 5 days | VTA - mPFC | Antidepressant; No effect |
Social avoidance; SPT |
| Barthas, 2015108, 2017107 | Chr2; 20hz 40ms 4–5mW for 8 seconds followed by 2 seconds off. 4 days for 30 minutes per day. | ACC mPFC Thy-1 | Pro-depressive | NSF, Marble burying, Splash test |
| Fuchikami, 201560 | Chr2; 10hz 15ms, 5mW, 1 minute on – 1 minute off for 60 minutes | IL mPFC glutamatergic | Antidepressant | FST, SPT, NSF |
| PL mPFC glutamatergic | No effect | FST, SPT, NSF | ||
| Hare, 201947 | Chr2; 10hz 15ms, 5mW, 1 minute on – 1 minute off for 60 minutes | vmPFC Drd1 neurons | Antidepressant; No effect |
FST, EPM, NSF SPT |
| vmPFC Drd2 neurons | No effect | FST, EPM, NSF | ||
| vmPFC Drd1 - BLA | Antidepressant | FST,NSF |
Anterior cingulate cortex (ACC), basolateral amygdala (BLA), elevated plus maze (EPM), forced swim test (FST), infralimbic (IL), medial prefrontal cortex (mPFC), novelty suppressed feeding (NSF), prelimbic (PL), somatostatin (SST), sucrose preference test (SPT), ventral medial prefrontal cortex (vmPFC), ventral tegmental area (VTA)
The discovery of ketamine’s antidepressant actions was a major therapeutic advance, and has had a major conceptual impact on the field by demonstrating that pharmacological agents can rapidly (within hours) alleviate the symptoms of depression. This has shifted the focus of drug discovery toward the development of glutamatergic, as well as GABAergic agents that can produce rapid effects on these neurotransmitter systems that lead to sustained synaptic and circuit level plasticity8, 25. Ketamine produces a burst of glutamate in the mPFC in rodent models15, 109, and an antidepressant response that occurs within hours and lasts for approximately one week13, 14 indicating that brief, mPFC circuit activation produces rapid and sustained therapeutic effects. A pair of studies have employed optogenetic and chemogenetic techniques to pursue the mechanism behind this effect. Fuchikami et al60 used muscimol inactivation to demonstrate the necessity of IL mPFC for ketamine’s antidepressant effects. To mimic the rapid and transient increase of extracellular glutamate caused by ketamine, they photostimulated glutamatergic neurons in IL mPFC at 10 hz for one hour (1 minute on/1 minute off). They found that photostimulation of IL, but not PL, mPFC produced antidepressant effects in the FST, SPT, and NSF tests observable 24 hours after photostimulation and still present 17 days after the manipulation. Also consistent with the synaptic effects of ketamine19, 20, 22, this photostimulation paradigm significantly increased the number and function of spine-synapses in the mPFC, demonstrating structural as well as behavioral consequences.
A second series of studies demonstrated that co-adminstration of a D1 dopamine receptor antagonist blocked the antidepressant behavioral actions of ketamine, and used a DREADD inactivation approach to demonstrate that activity of the Drd1 expressing neuronal population in the mPFC was necessary for the antidepressant response to ketamine47. In addition, the results demonstrated that stimulation of the mPFC Drd1 population was also sufficient to generate an antidepressant response, as was photostimulation of mPFC Drd1 terminals in the BLA. In contrast, inhibition of the Drd2 expressing mPFC neuronal population did not block the response to ketamine, and photostimulation of the Drd2 population did not produce antidepressant effects. The Drd1 and Drd2 neuronal populations segregate separate classes of excitatory cells that display different electrophysiological, morphological, and projection characteristics110. Repeated stress paradigms attenuate working memory via a reduction in the activity of Drd1 expressing pyramidal cells in mPFC111. In addition, repeated stress exposure alters excitability and synaptic inputs onto Drd1 and Drd2 cells112, and causes atrophy of Drd1 expressing neurons85. These studies provide key insights into a discrete population of neurons in the mPFC and the projections of these neurons to the BLA that are necessary and sufficient for the rapid antidepressant actions of ketamine. This type of cell and circuit specific information, as well as evidence for D1 receptor involvement could aid in the development of novel therapeutic interventions. Further studies on the impact of Drd1 photostimulation on stress associated synaptic and behavioral changes could provide additional key information for drug development.
Considerations for future studies
The studies detailed herein provide clear evidence that mPFC neurons and projection target regions regulate depression and anxiety related behaviors in pre-clinical models. Additionally, this work provides examples of the informative nature of pre-clinical studies to treatment modalities such as DBS or ECT that lack the selectivity of pre-clinical tools. For instance, the stimulation paradigm is critical to the behavioral outcome. Manipulation of frequency and duration of stimulation may have strikingly different effects on behavior68. Similarly, while studies report that indiscriminate acute stimulation of the mPFC during testing does not appear to impact anxiety like behavior75, 79, 98, significant effects are observed when subpopulations of mPFC neurons are targeted80, 98, 100. These results highlight the advantage offered by viral targeting of specific cellular populations, and again point to the difficutly of interpreting negative results, in this case when large populations of neurons are targeted. With this in mind, and to generate further rationale for clinical studies using tools for brain stimulation, null results in optogenetic work should be confirmed with additional experiments using alternative stimulation parameters. Due to the challenges inherent in human studies it is important that pre-clinical studies clearly delineate the neuronal population, subregion, and stimulation paradigm to provide information that can be used to design and refine clinical interventions to produce more selective circuit effects in patients. For instance, these types of studies may be informative for TMS therapies where both high and low frequency stimulations are being used with mixed results113–116, or may inform the development of targeted pharmacotherapies that are designed to impact specific aspects of the circuit response as is ongoing with the development of compounds seeking to mimic ketamine’s rapid antidepressant action while limiting off-target effects117.
Another important takeaway is that optogenetic and chemogenetic manipulations can produce persistent neuroplasticity changes that may impact behavior (Table 2)60, 118, 119, highlighting the need to control for prior exposure to these paragidms that could impact subsequent behavioral testing. For instance, studies utilizing repeated neuronal modulation through multiple separate test sessions should take care to determine if the effects observed are due to the acute manipulation rather than a sustained effect of prior manipulations. However, the ability to produce sustained synaptic and behavioral responses indicates that the clinical interventions could be designed to produce similar effects. For example, optogenetic or chemogenetic tools could be used to study population specific effects in brain regions targeted by DBS, which would provide specific frequency and duration settings for further refinement of this therapeutic intervention. Information in specific neuronal populations within these regions could also be used to identify novel therapeutic targets. Additionally, sustained behavioral effects of photostimulation are clearly different than those obtained with acute manipulations. For example, sustained anxiolytic actions are observed well after photostimulation of glutamatergic neurons in the mPFC60, 65, and require sub-population specific control in acute situations98, 100. Understanding the mechanistic drivers that produce these sustained effects could also inform the refinement of clinical targets and pharmacological treatments in the future.
A final area to consider in future efforts is in the study of circuit and cell population specific effects underlying the actions of prophylactic agents. Notably, ketamine has been demonstrated to have prophylactic effects when administered prior to uncontrollable stress exposure in male and female rats74, 120, an effect governed by mPFC to DRN circuitry. Similary, ketamine administered 1 week before social defeat, chronic corticosterone exposure, or learned helplessness training blocked the post-stress expression of depression-like behavior121. Prophylactic ketamine also limits learned fear expression when administered one week, but not one month, or one hour before training122. Interestingly, further study in this paradigm demonstrated changes in neurometabolite levels in PFC and hippocampus only in animals given ketamine prophylactically and that were fear conditioned123. These studies further demonstrate the importance of mPFC circuitry in regulation of depression- and anxiety-like behavior and highlight the utility of ketamine to studies of brain-adaptive changes associated with the antidepressant response. To our knowledge studies examining whether optogenetic/chemogenetic stimulation may produce similar prophylactic effects have not been undertaken.
Conclusions and future directions
The combination of optogenetic and chemogenetic tools with viral tools that allow cell population specific control allows powerful insight into the diverse role of mPFC neuronal populations in pre-clinical studies of depression-like behavior (Fig. 3). The results described here highlight the impact of specific neuronal populations in discrete mPFC subregions driving active responding, social avoidance, and anxiety-like behavior (Table 3). The results also demonstrate that in addition to identifying circuitry that is acutely involved in behaviors, it is possible to produce prolonged changes in synaptic function, morphology, and depression and anxiety related behaviors. Together these studies provide key information to help direct clinical interventions such as DBS and TMS, as well as in development of targeted pharmacotherapies. Key to these type of advances will be incorporation of tools to identify the physiological changes in neuronal activity after manipulation, such as in vivo imaging using two-photon microscopy and fiber photometry, as well as multielectrode electrophysiology. Combined these efforts provide an exciting opportunity to advance our knowledge of the neuronal and circuit level determinants underlying depression- and anxiety-like behaviors as well as novel therapeutic interventions.
Table 3:
mPFC circuitry impacting behaviors used to study anxiety and depression
| Behavior | Target Population | Reference |
|---|---|---|
| Forced swim - Animal is placed in an inescapable beaker of water of sufficient depth to prevent contact with the bottom of the beaker. Time immobile during a short test is measured. Increased time immobile is interpreted as a passive coping strategy. | PL mPFC Thy1 IL mPFC Glutamatergic vmPFC Drd1 vmPFC Drd1 - BLA mPFC - DRN mPFC - LHB vHIPP - mPFC MDT - mPFC |
Kumar, 201365 Fuchikami, 201560 Hare, 201947 Hare, 201947 Warden, 201264 Warden, 201264 Carreno, 201666 Miller, 201769 |
| Tail suspension - Animal is suspended by the tail. Time immobile during a short test is measured. Increased time immobile is interpreted as behavioral despair or a passive coping strategy. | mPFC vGlut2 IL mPFC - MDT MDT - mPFC |
Son, 201867 Carlson, 201768 Miller, 201769 |
| Social avoidance - Experimental mice are placed into a cage with larger, more aggressive, conspecific and allowed to interact for a short period of time. This interaction is followed by an extended period of sensory contact without physical contact. The interaction and sensory contact are repeated over multiple days. On test, time spent investigating a novel animal separated by a mesh enclosure is measured. Reduced time investigating the novel animal is interpreted as evidence of social avoidance. Time investigating the novel animal may also be contrasted against time spent investigating the mesh enclosure without a target present. | mPFC Pl mPFC Glutmatergic PL mPFC - DRN mPFC – Nac mPFC - amygdala VTA - mPFC VTA - mPFC |
Covington, 201075 Lee, 201576 Challis, 201488 Bagot, 201587 Hultman, 201686 Chaudhury, 201382 Friedman, 2014 |
| Social exploration/Juvenile exploration - Experimental mice are placed into a box with multiple chambers. In one chamber is a conspecific in a mesh enclosure. Reduced time spent investigating the conspecific is interpreted as evidence of abnormal social behavior. Investigation time may be contrasted against time spent investigating an empty enclosure. | mPFC Glutamatergic PL mPFC - DRN BLA - mPFC |
Yizhar, 201178, Ferenczi, 201677 Dolzani, 201874 Felix-Ortiz, 201680 |
| Sucrose preference/Sucrose consumption - Animals are habituated to a sucrose solution, typically 1–2%, prior to testing. On test animals are given free access to bottles containing sucrose and water. The amount of each liquid consumed are measured. Reduced sucrose consumption as a percentage of total liquid consumed is interpreted as evidence of anhedonia. | mPFC IL mPFC Glutamatergic mPFC Glutamatergic |
Covington, 201075 Fuchikami, 201560 Ferenczi, 201677 |
| Elevated plus maze - Animals are given access to a plus shaped arena elevated above the floor. Two opposing arms have walls (‘closed’), and two are ‘open”. Increased time spent in the open arms during the test is interpreted as reduced anxiety |
PL mPFC Thy-1 PL mPFC SST vmPFC Drd1 vmPFC - BMA BLA – mPFC vHIPP – mPFC |
Kumar, 201365 Soumier, 2014101 Hare, 201947 Adhikari, 201598 Felix-Ortiz, 201661 Padilla-Coreano, 2016100 |
| Open field test - Animals are given access to a square enclosure and allowed to freely explore. Time spent in the center of the field is measured. Animals that spend more time exploring the center of the field as opposed to the periphery are interpreted as being less anxious. | vMPFC - BMA BLA – mPFC vHIPP - mPFC |
Adhikari, 201598 Felix-Ortiz, 201661 Padilla-Coreano, 2016100 |
| Novelty suppressed feeding - Food deprived animals are given access to a square enclosure with a piece of food in the center. Time spent to approach the food and take a bite is measured. Animals with shorter feeding latencies are interpreted as being less anxious. |
ACC mPFC Thy-1 IL mPFC Glutamatergic vmPFC Drd1 vmPFC Drd1 - BLA vHIPP – mPFC |
Barthas, 2015108, 2017107 Fuchikami, 201560 Hare, 201947 Hare, 201947 Padilla-Coreano, 2016100 |
Anterior cingulate cortex (ACC), basolateral amygdala (BLA), basomedial amygdala (BMA), dorsal raphe nucleus (DRN), elevated plus maze (EPM), forced swim test (FST), infralimbic (IL), lateral habenula (LHB), medial dorsal thalamus (MDT), medial prefrontal cortex (mPFC), nucleus accumbens (NAc), novelty suppressed feeding (NSF), prelimbic (PL), somatostatin (SST), sucrose preference test (SPT), ventral hippocampus (vHIPP), ventral medial prefrontal cortex (vmPFC), ventral tegmental area (VTA), vesicular glutamate transporter (vGlut) Manipulations in bold demonstrate sustained effects.
Acknowledgements
This work was supported by the USA NIMH (RO1 MH105910-04 and RO1 MH093897-06A1 to R.S.D), and the USA Brain and Behavior Research Foundation (to B.D.H)
Conflict of Interest
We declare that Dr. Duman has consulted and/or received research support from Naurex, Lilly, Forest, Johnson & Johnson, Taisho, and Sunovion. The remaining authors have no competing financial interests.
References
- 1.Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Education for health (Abingdon, England) 2003; 16(2): 230. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). Jama 2003; 289(23): 3095–3105. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76(2): 155–162. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry 2006; 163(1): 28–40. [DOI] [PubMed] [Google Scholar]
- 5.Flint J, Kendler KS. The Genetics of Major Depression. Neuron 2014; 81(5): 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF et al. No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. The American journal of psychiatry 2019: appiajp201818070881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev 2009; 33(5): 699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019; 102(1): 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubin MJ, Liston C, Avissar MA, Ilieva I, Gunning FM. Network-Guided Transcranial Magnetic Stimulation for Depression. Current behavioral neuroscience reports 2017; 4(1): 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76(7): 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Kar SK. How Electroconvulsive Therapy Works?: Understanding the Neurobiological Mechanisms. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology 2017; 15(3): 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45(5): 651–660. [DOI] [PubMed] [Google Scholar]
- 13.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47(4): 351–354. [DOI] [PubMed] [Google Scholar]
- 14.Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63(8): 856–864. [DOI] [PubMed] [Google Scholar]
- 15.Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 2018; 43(10): 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N et al. Lower synaptic density is associated with depression severity and network alterations. Nature communications 2019; 10(1): 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 2012; 18(9): 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74(10): 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69(8): 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329(5994): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59(12): 1116–1127. [DOI] [PubMed] [Google Scholar]
- 22.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019; 364(6436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: possible links to neuron death in the hippocampus. Bipolar disorders 2002; 4(2): 117–128. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs E, Czeh B, Kole MH, Michaelis T, Lucassen PJ. Alterations of neuroplasticity in depression: the hippocampus and beyond. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 2004; 14 Suppl 5: S481–490. [DOI] [PubMed] [Google Scholar]
- 25.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci 2015; 38(5): 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogaca MV, Duman RS. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Frontiers in cellular neuroscience 2019; 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33(1): 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 2015; 20(9): 1057–1068. [DOI] [PubMed] [Google Scholar]
- 30.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010; 13(4): 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 2007; 32(2): 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fee C, Banasr M, Sibille E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol Psychiatry 2017; 82(8): 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 2006; 59(5): 395–400. [DOI] [PubMed] [Google Scholar]
- 34.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 2010; 67(5): 458–464. [DOI] [PubMed] [Google Scholar]
- 35.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999; 56(11): 1043–1047. [DOI] [PubMed] [Google Scholar]
- 36.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004; 61(7): 705–713. [DOI] [PubMed] [Google Scholar]
- 37.Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KA, Kang G et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. Journal of psychiatry & neuroscience : JPN 2016; 41(3): E37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23(1): 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee WH, Lisanby SH, Laine AF, Peterchev AV. Comparison of electric field strength and spatial distribution of electroconvulsive therapy and magnetic seizure therapy in a realistic human head model. European psychiatry : the journal of the Association of European Psychiatrists 2016; 36: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng ZD, Lisanby SH, Peterchev AV. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. Journal of neural engineering 2011; 8(1): 016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain stimulation 2013; 6(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth Y, Pell GS, Chistyakov AV, Sinai A, Zangen A, Zaaroor M. Motor cortex activation by H-coil and figure-8 coil at different depths. Combined motor threshold and electric field distribution study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2014; 125(2): 336–343. [DOI] [PubMed] [Google Scholar]
- 43.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 2004; 115(6): 1239–1248. [DOI] [PubMed] [Google Scholar]
- 44.Stujenske JM, Spellman T, Gordon JA. Modeling the Spatiotemporal Dynamics of Light and Heat Propagation for In Vivo Optogenetics. Cell reports 2015; 12(3): 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res 2015; 93: 144–157. [DOI] [PubMed] [Google Scholar]
- 46.Scheyltjens I, Laramee ME, Van den Haute C, Gijsbers R, Debyser Z, Baekelandt V et al. Evaluation of the expression pattern of rAAV2/1, 2/5, 2/7, 2/8, and 2/9 serotypes with different promoters in the mouse visual cortex. J Comp Neurol 2015; 523(14): 2019–2042. [DOI] [PubMed] [Google Scholar]
- 47.Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nature communications 2019; 10(1): 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamura S, Baratta MV, Pomrenze MB, Dolzani SD, Cooper DC. High fidelity optogenetic control of individual prefrontal cortical pyramidal neurons in vivo. F1000Research 2012; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arias-Gil G, Ohl FW, Takagaki K, Lippert MT. Measurement, modeling, and prediction of temperature rise due to optogenetic brain stimulation. Neurophotonics 2016; 3(4): 045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owen SF, Liu MH, Kreitzer AC. Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 2019; 22(7): 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyssowski KM, Gray JM. Blue Light Increases Neuronal Activity-Regulated Gene Expression in the Absence of Optogenetic Proteins. eNeuro 2019; 6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009; 63(1): 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017; 357(6350): 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laubach M, Amarante LM, Swanson K, White SR. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018; 5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biol Psychiatry 2016; 80(7): 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res 2008; 14(2–3): 249–262. [DOI] [PubMed] [Google Scholar]
- 57.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 2009; 29(26): 8474–8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 2014; 505(7481): 92–96. [DOI] [PubMed] [Google Scholar]
- 59.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 2008; 454(7204): 600–606. [DOI] [PubMed] [Google Scholar]
- 60.Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A 2015; 112(26): 8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in behavioral neuroscience 2015; 9: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muir J, Lopez J, Bagot RC. Wiring the depressed brain: optogenetic and chemogenetic circuit interrogation in animal models of depression. Neuropsychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Kloet ER, Molendijk ML. Coping with the Forced Swim Stressor: Towards Understanding an Adaptive Mechanism. Neural plasticity 2016; 2016: 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 2012; 492(7429): 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J et al. Cortical control of affective networks. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013; 33(3): 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A et al. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 2015. [DOI] [PubMed] [Google Scholar]
- 67.Son H, Baek JH, Go BS, Jung DH, Sontakke SB, Chung HJ et al. Glutamine has antidepressive effects through increments of glutamate and glutamine levels and glutamatergic activity in the medial prefrontal cortex. Neuropharmacology 2018; 143: 143–152. [DOI] [PubMed] [Google Scholar]
- 68.Carlson D, David LK, Gallagher NM, Vu MT, Shirley M, Hultman R et al. Dynamically Timed Stimulation of Corticolimbic Circuitry Activates a Stress-Compensatory Pathway. Biol Psychiatry 2017; 82(12): 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ. Synaptic Regulation of a Thalamocortical Circuit Controls Depression-Related Behavior. Cell reports 2017; 20(8): 1867–1880. [DOI] [PubMed] [Google Scholar]
- 70.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai H-C, Finkelstein J et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013; 493(7433): 537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hames JL, Hagan CR, Joiner TE. Interpersonal processes in depression. Annual review of clinical psychology 2013; 9: 355–377. [DOI] [PubMed] [Google Scholar]
- 72.Challis C, Berton O. Top-Down Control of Serotonin Systems by the Prefrontal Cortex: A Path toward Restored Socioemotional Function in Depression. ACS chemical neuroscience 2015; 6(7): 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc 2011; 6(8): 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sorensen AT et al. Inhibition of a Descending Prefrontal Circuit Prevents Ketamine-Induced Stress Resilience in Females. eNeuro 2018; 5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Covington HE 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010; 30(48): 16082–16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee E, Hong J, Park YG, Chae S, Kim Y, Kim D. Left brain cortical activity modulates stress effects on social behavior. Scientific reports 2015; 5: 13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 2016; 351(6268): aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477(7363): 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warthen DM, Lambeth PS, Ottolini M, Shi Y, Barker BS, Gaykema RP et al. Activation of Pyramidal Neurons in Mouse Medial Prefrontal Cortex Enhances Food-Seeking Behavior While Reducing Impulsivity in the Absence of an Effect on Food Intake. Frontiers in behavioral neuroscience 2016; 10: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 2016; 321: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu RJ, Ota KT, Dutheil S, Duman RS, Aghajanian GK. Ketamine Strengthens CRF-Activated Amygdala Inputs to Basal Dendrites in mPFC Layer V Pyramidal Cells in the Prelimbic but not Infralimbic Subregion, A Key Suppressor of Stress Responses. Neuropsychopharmacology 2015; 40(9): 2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493(7433): 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venzala E, Garcia-Garcia AL, Elizalde N, Tordera RM. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 2013; 23(7): 697–708. [DOI] [PubMed] [Google Scholar]
- 84.Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E et al. Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32(12): 4319–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinohara R, Taniguchi M, Ehrlich AT, Yokogawa K, Deguchi Y, Cherasse Y et al. Dopamine D1 receptor subtype mediates acute stress-induced dendritic growth in excitatory neurons of the medial prefrontal cortex and contributes to suppression of stress susceptibility in mice. Mol Psychiatry 2017. [DOI] [PubMed] [Google Scholar]
- 86.Hultman R, Mague SD, Li Q, Katz BM, Michel N, Lin L et al. Dysregulation of Prefrontal Cortex-Mediated Slow-Evolving Limbic Dynamics Drives Stress-Induced Emotional Pathology. Neuron 2016; 91(2): 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nature communications 2015; 6: 7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Challis C, Beck SG, Berton O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Frontiers in behavioral neuroscience 2014; 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience 2013; 33(35): 13978–13988, 13988a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature Neuroscience 2005; 8(3): 365–371. [DOI] [PubMed] [Google Scholar]
- 91.Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci 2009; 30(6): 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hammack SE, Cooper MA, Lezak KR. Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology 2012; 62(2): 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience 2012; 210: 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 1997; 17(22): 8842–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annual review of clinical psychology 2007; 3: 137–158. [DOI] [PubMed] [Google Scholar]
- 96.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods 1985; 14(3): 149–167. [DOI] [PubMed] [Google Scholar]
- 97.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007; 2(2): 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature 2015; 527(7577): 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 2011; 71(5): 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL et al. Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior. Neuron 2016; 89(4): 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 2014; 39(9): 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perova Z, Delevich K, Li B. Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015; 35(7): 3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature 2014; 511(7509): 348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yun S, Reynolds RP, Petrof I, White A, Rivera PD, Segev A et al. Stimulation of entorhinal cortex-dentate gyrus circuitry is antidepressive. Nat Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 2015; 347(6222): 659–664. [DOI] [PubMed] [Google Scholar]
- 106.Luscher C, Pascoli V, Creed M. Optogenetic dissection of neural circuitry: from synaptic causalities to blue prints for novel treatments of behavioral diseases. Current opinion in neurobiology 2015; 35: 95–100. [DOI] [PubMed] [Google Scholar]
- 107.Barthas F, Humo M, Gilsbach R, Waltisperger E, Karatas M, Leman S et al. Cingulate Overexpression of Mitogen-Activated Protein Kinase Phosphatase-1 as a Key Factor for Depression. Biol Psychiatry 2017; 82(5): 370–379. [DOI] [PubMed] [Google Scholar]
- 108.Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 2015; 77(3): 236–245. [DOI] [PubMed] [Google Scholar]
- 109.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 1997; 17(8): 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gee S, Ellwood I, Patel T, Luongo F, Deisseroth K, Sohal VS. Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32(14): 4959–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 2015; 18(10): 1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiology of Stress 2019; 10: 100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological medicine 2009; 39(1): 65–75. [DOI] [PubMed] [Google Scholar]
- 114.Aguirre I, Carretero B, Ibarra O, Kuhalainen J, Martinez J, Ferrer A et al. Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. J Affect Disord 2011; 130(3): 466–469. [DOI] [PubMed] [Google Scholar]
- 115.Pallanti S, Bernardi S, Di Rollo A, Antonini S, Quercioli L. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience 2010; 167(2): 323–328. [DOI] [PubMed] [Google Scholar]
- 116.Schutter DJ. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychological medicine 2010; 40(11): 1789–1795. [DOI] [PubMed] [Google Scholar]
- 117.Duman RS, Shinohara R, Fogaca MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar S, Hultman R, Hughes D, Michel N, Katz BM, Dzirasa K. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nature communications 2014; 5: 4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hare BD, Ghosal S, Duman RS. Rapid acting antidepressants in chronic stress models: molecular and cellular mechanisms. Chronic Stress 2017; 1: 2470547017697317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K et al. Previous Ketamine Produces an Enduring Blockade of Neurochemical and Behavioral Effects of Uncontrollable Stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016; 36(1): 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry 2016; 79(9): 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA et al. Prophylactic Ketamine Attenuates Learned Fear. Neuropsychopharmacology 2017; 42(8): 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGowan JC, Hill C, Mastrodonato A, LaGamma CT, Kitayev A, Brachman RA et al. Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 2018; 43(9): 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]