Autophagy is an evolutionarily conserved lysosomal-mediated degradation process that facilitates the turnover of organelles and selected long-lived proteins. Aberrant autophagy contributes to therapeutic resistance and malignant progression by generating alternative sources of metabolic fuel to maintain cell survival under stress conditions including those imposed by hypoxia, radiation, chemotherapy, and targeted agents (1). The FDA approved anti-malarial drug hydroxychloroquine (HCQ) is known to inhibit autophagy through the disruption of lysosomal function. Based on this aspect of its mechanism of action, tremendous efforts were undertaken to repurpose it for cancer therapy over the last decade. Although HCQ has been combined with an array of other anticancer regimens in clinical trials, these studies have predominantly reported modest clinical benefit characterized by partial responses and stable disease, but few sustained complete responses (2, 3). Despite the initial subdued clinical results reported from these trials, there is maintained enthusiasm for autophagy inhibition as an anticancer approach for two overarching reasons. First, it remains unclear if the maximum tolerated dose of HCQ when used in combination with other anticancer agents is sufficient to completely inhibit autophagy in tumors. The pharmacodynamic (PD) studies conducted in support of most of these early phase HCQ combination trials were not powered to test this in a definitive manner. Residual autophagic activity may have been a factor that limited efficacy in these studies. Second, despite its prevalent use as an autophagy inhibitor, the reality is that HCQ is a very old drug that was not designed to inhibit autophagy. Its continued use for this purpose is driven by the lack of better alternatives. New autophagy inhibitors with increased potency and more favorable therapeutic indices are clearly needed to definitively determine the efficacy of this approach, but none have been tested in humans to date.
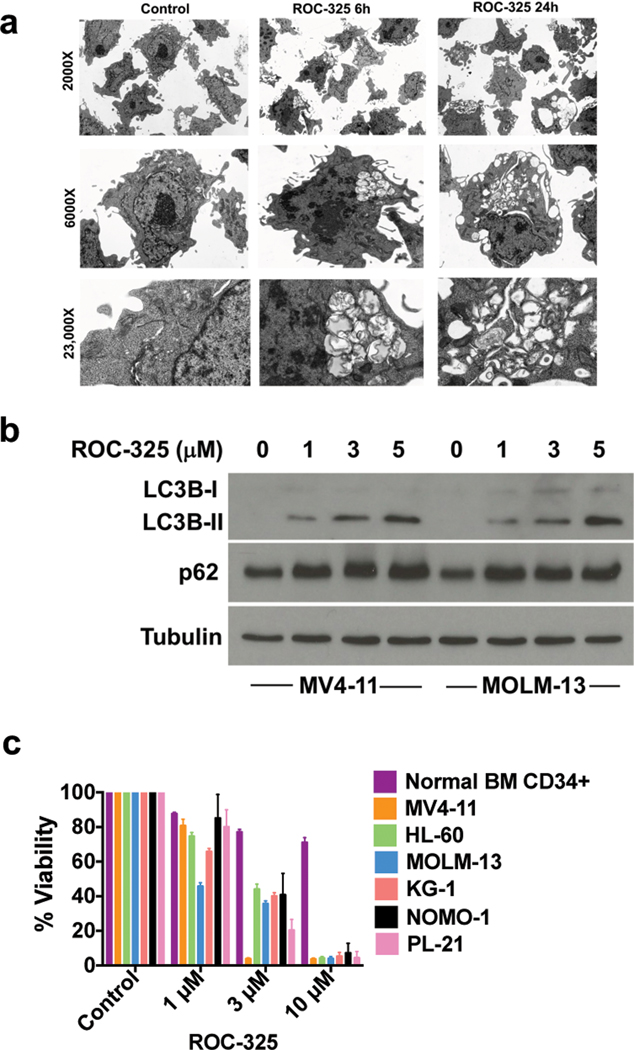
We recently generated a series of novel dimeric compounds containing modified core elements of HCQ and the anti-schistosomal drug lucanthone with the goal of developing new lysosomal autophagy inhibitors with superior potency, tolerability, and anticancer efficacy (4, 5). Initial preclinical studies identified ROC-325 as a lead agent for further investigation as it demonstrated approximately 10-fold greater potency than HCQ and is an orally bioavailable compound. We selected acute myeloid leukemia (AML) as a priority malignancy for further investigation based on the high sensitivity of AML cells to ROC-325 in early screens and the frequent aberrations in the autophagy interactome in patients with myeloid neoplasms that we recently reported (6). We first quantified the effects of ROC-325 on the hallmark features of autophagy inhibition in AML cells. Transmission electron microscopy analyses demonstrated that treatment of AML cells with ROC-325 triggered the accumulation of autophagosomes with undegraded cargo (Fig. 1a). The majority of lysosomal proteases that control the autophagy-mediated degradation of proteins and organelles require a strict acidic pH microenvironment for their enzymatic activity. Based on this, lysosomal acidity is commonly used as a marker of autophagy status. We treated AML cells with ROC-325 and used LysoSensor Green to evaluate the effects of drug treatment on lysosomal pH. Confocal microscopy and FACS-based quantification of LysoSensor Green fluorescence both showed that ROC-325 stimulated lysosomal deacidification (Suppl. Fig. 1a–b, P < 0.01). Consistent with the disruption of autophagy by ROC-325 through impairment of lysosomal function, immunoblotting showed that drug treatment led to elevated levels of the microtubule-associated protein and autophagy marker LC3B along with accumulation of p62, whose turnover is specifically mediated by autophagy (Fig. 1b).
Figure 1. ROC-325 inhibits autophagy and diminishes AML cell viability.
(a) ROC-325 induces the formation of autophagosomes with undegraded cargo. MV4–11 AML cells were treated with 1 μM ROC-325 for 6 h or 24 h as indicated and autophagosomes were visualized using transmission electron microscopy. (b) Treatment with ROC-325 increases LC3B and p62. MV4–11 and MOLM-13 cells were treated with the indicated concentrations of ROC-325 for 24 h. Protein expression was quantified by immunoblotting. (c) ROC-325 selectively antagonizes AML cell viability. Normal bone marrow CD34+ cells and a panel of AML cell lines were treated with ROC-325 as indicated for 72 h. Cell viability was quantified by MTT assay. N = 3, Mean ± SD.
An important consideration in new agent development is improved selectivity for malignant cells, which may lead to better tolerability and reduced toxicity to normal cells. Treatment of normal human CD34+ bone marrow (BM) cells with ROC-325 in parallel with a panel of established AML cell lines demonstrated strong therapeutic selectivity as evidenced by dose-dependent reduction in the viability of AML cells with limited effects on normal BM progenitors (Fig. 1c). The diminished viability caused by ROC-325 treatment was associated with dose-dependent apoptosis (Suppl. Fig. 2a–c). Additional assays conducted with primary AML blasts from patients showed that ROC-325 displayed similar potency regardless of patient risk classification (N = 5 per group, Suppl. Fig. 3). This suggests that ROC-325 may have broad applications in AML therapy and that patients that are predicted to have poor outcomes could potentially benefit from this novel agent.
We also conducted RNA sequencing and gene level analyses to further investigate the PD effects of ROC-325 in AML cells. These studies demonstrated that treatment of MV4–11 cells with ROC-325 (1 μM and 5 μM) altered the levels of autophagy-dependent degradation pathways while preserving protein synthesis through the upregulation of post-translational ribosomal, methylation, and splicing components (Suppl. Fig. 4a–c).
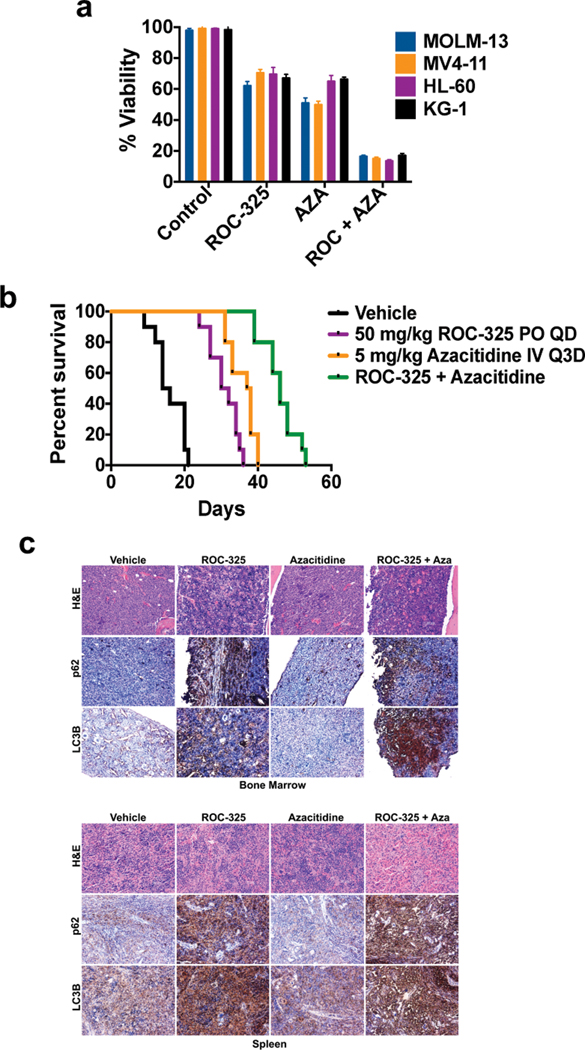
The hypomethylating agent azacitidine (AZA) is frequently used in the management of patients with myelodysplastic syndromes (MDS) and AML (7, 8). Recent reports have linked autophagy to AZA resistance and have suggested that autophagy inhibition may be an effective strategy to augment the efficacy of AZA (9, 10). To explore the potential benefit of autophagy inhibition as a new approach to increase the anti-leukemic activity of AZA, we first conducted a series of experiments to demonstrate that AZA treatment triggers autophagy in AML cells. Confocal microscopy analyses showed that AZA promoted the formation of LC3B punctae in AML cells (Suppl. Fig. 5a), which is indicative of autophagy induction. Immunoblotting demonstrated that AZA stimulated the dose-dependent accumulation of LC3B with related reductions in the levels of the autophagy controlled protein p62, further supporting that AZA triggers autophagy (Suppl. Fig. 5b). Combining ROC-325 with AZA antagonized its effects on p62, suggesting that this approach may increase its anti-leukemic activity (Suppl. Fig. 5c). To assess the impact of autophagy inhibition on AZA efficacy, we first tested the combination of ROC-325 and AZA in a panel of AML cell lines (Fig. 2a) and primary AML blasts (Suppl. Fig. 6). In both cases, the combination yielded a significantly greater than additive benefit (P < 0.01). We next conducted a xenograft study to investigate the therapeutic potential of inhibiting autophagy with ROC-325 as a strategy to increase the benefit of AZA in vivo. MV4–11 cells were injected into the tail veins of NOD/SCID mice to establish a disseminated model of leukemia. Bone marrow engraftment efficiency was assessed by CD45 immunohistochemistry (IHC, Suppl. Fig. 7). Mice (N = 10 per group) were treated with vehicle control, 50 mg/kg ROC-325, 5 mg/kg AZA, or the combination of both agents. Animal weight was measured biweekly and overall survival was used as an endpoint for the study. While both ROC-325 and AZA displayed significant anti-AML activity, combination treatment resulted in a significant further extension of overall survival compared to either single agent treatment (Fig. 2b). Importantly, combination therapy was very well tolerated and did not significantly affect animal weight (less than 5% mean transient reduction in body weight, Suppl. Fig. 8). Immunohistochemical PD analyses of BM and spleen specimens collected from animals in each group at study endpoint demonstrated that ROC-325 disrupted autophagy in vivo as shown by increased LC3B and p62 levels (Fig. 2c, Suppl. Fig. 9). These data suggest that induction of autophagy may represent a key mechanism that limits the efficacy of AZA and that approaches to abrogate this aspect of AZA’s mechanism of action could yield significant therapeutic benefit. Taken together, our findings demonstrate that inhibition of autophagy is a novel approach to augment the benefit of AZA that warrants further investigation for the treatment of MDS/AML. Our results also support continued studies specifically focused on rigorously evaluating the safety, mechanism of action, and anti-leukemic activity of the novel orally available autophagy inhibitor ROC-325.
Figure 2. ROC-325 augments the anti-leukemic activity of azacitidine to significantly extend survival.
(a) ROC-325 cooperates with azacitidine to diminish the viability of AML cells. MOLM-13, MV4–11, HL-60 and KG-1 cells were treated with 1 μM ROC-325, 1 μM azacitidine, or the combination for 72 h. Cell viability was determined by MTT assay. N = 3 ± SD. (b) ROC-325 and azacitidine significantly extend survival. MV4–11 cells (1 × 106 per mouse) were injected into the tail veins of NOD/SCID mice (N = 10 per group) to establish a disseminated xenograft model of AML. Mice were treated with vehicle control, 50 mg/kg PO QD ROC-325, 5 mg/kg azacitidine IV Q3D, or both drugs. The survival benefit of each treatment was determined by Kaplan-Meier analysis. (c) ROC-325 inhibits autophagy in vivo. Bone marrow and spleen specimens were collected from mice in each treatment group at study endpoint and levels of LC3B and p62 were measured by immunohistochemistry.
Supplementary Material
Acknowledgments
This project was supported by grants from the National Cancer Institute (R01CA172443 to JSC and R01CA190789 to STN) and the University of Arizona Cancer Center Support Grant P30CA023074.
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
Supplementary information
Supplementary methods and data are available on the Leukemia website (http://www.nature.com/leu).
References
- 1.Carew JS, Nawrocki ST. Drain the lysosome: Development of the novel orally available autophagy inhibitor ROC-325. Autophagy 2017; 13:765–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahalingam D, Mita M, Sarantopoulos J, Wood L, Amaravadi RK, Davis LE et al. Combined autophagy and HDAC inhibition: a phase I safety, tolerability, pharmacokinetic, and pharmacodynamic analysis of hydroxychloroquine in combination with the HDAC inhibitor vorinostat in patients with advanced solid tumors. Autophagy 2014; 10:1403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chude CI, Amaravadi RK. Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carew JS, Espitia CM, Esquivel JA, 2nd, Mahalingam D, Kelly KR, Reddy G, Giles FJ, Nawrocki ST. Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J Biol Chem 2011; 286:6602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carew JS, Espitia CM, Zhao W, Han Y, Visconte V, Phillips J et al. Disruption of Autophagic Degradation with ROC-325 Antagonizes Renal Cell Carcinoma Pathogenesis. Clin Cancer Res 2017; 23:2869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visconte V, Przychodzen B, Han Y, Nawrocki ST, Thota S, Kelly KR et al. Complete mutational spectrum of the autophagy interactome: a novel class of tumor suppressor genes in myeloid neoplasms. Leukemia 2017; 31:505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ornstein MC, Mukherjee S, Sekeres MA. More is better: combination therapies for myelodysplastic syndromes. Best Pract Res Clin Haematol 2015; 28:22–31. [DOI] [PubMed] [Google Scholar]
- 8.Madanat Y, Sekeres MA. Optimizing the use of hypomethylating agents in myelodysplastic syndromes: Selecting the candidate, predicting the response, and enhancing the activity. Semin Hematol 2017; 54:147–53. [DOI] [PubMed] [Google Scholar]
- 9.Romano A, Giallongo C, La Cava P, Parrinello NL, Chiechi A, Vetro C et al. Proteomic Analysis Reveals Autophagy as Pro-Survival Pathway Elicited by Long-Term Exposure with 5-Azacitidine in High-Risk Myelodysplasia. Front Pharmacol 2017; 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert G, Auberger P. Azacitidine resistance caused by LAMP2 deficiency: a therapeutic window for the use of autophagy inhibitors in MDS/AML patients? Autophagy 2019:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.