ABSTRACT
Identification of immunogenic tumor antigens that are efficiently processed and delivered by dendritic cells to prime the immune system and to induce an appropriate immune response is a research hotspot in the field of cancer vaccine development. High biosafety is an additional demand. Tumor-derived exosomes (TEXs) are nanosized lipid bilayer encapsulated vesicles that shuttle bioactive information to the tumor microenvironment facilitating tumor progression. However, accumulating evidence points toward the capacity of TEXs to efficiently stimulate immune responses against tumors provided they are appropriately administered. After briefly describing the function of exosomes in cancer biology and their communication with immune cells, we summarize in this review in vitro and preclinical studies eliciting the potency of TEXs in inducing effective anti-tumor responses and recently modified strategies further improving TEX-vaccination efficacy. We interpret the available data as TEXs becoming a lead in cancer vaccination based on tumor antigen-selective high immunogenicity.
KEYWORDS: Tumor-derived exosome (TEX), cancer vaccine, immunotherapy, Dendritic cells (DCs)
Introduction
Chemotherapy and radiotherapy are the most frequent types of adjuvant therapies in progressed cancer, which are curative for some tumor types.1,2 Although they have been shown to have pivotal benefits in the elimination of primary tumors, the challenges imposed by tumor recurrence and metastasis remain largely unsolved. Therefore, alternative therapeutic modalities are urgently warranted. Immunotherapy is a promising pillar in cancer therapy with the advantage of having no or minor side effects. Unfortunately, despite exciting progress, therapeutic efficacy has been limited and rather sporadic; this has been ascribed to the poor or absent immunogenicity of most tumor antigens as well as immunosuppressive features of many tumor cells.3,4
Dendritic cells (DCs) are known as the most potent professional antigen-presenting cells, which activate helper T cells (Th) via presenting antigenic peptides in MHCII molecules. This finding has created new hopes for boosting cancer immunotherapy, these as activated Th cells can support maturation and activation of CD8+ cytotoxic T cells.5 DCs also could strengthen NK activity through by facilitating antibody secretion via Th or directly via B cells.6,7 Indeed, the elaboration of in vitro expansion and activation of DCs, and loading DCs with tumor antigens provided considerable advantages in tumor immunotherapy.8
However, in spite of clinical benefits of DC vaccination in cancer treatment, not all patients respond and missing responses could not consistently be coordinated with the patients’ data. Low tumor antigen immunogenicity, immune escape, and tumor-induced immunosuppression were frequently identified as responsible factors.9,10 Nonetheless, progress in chemotherapy and checkpoint inhibitors together with the increasing knowledge on the power of exosomes in intercellular communication and their mode of action are promising that these drawbacks can be overcome such that immunotherapy may become a reliable and efficient adjuvant therapy during cancer progression.
Tumor-derived exosomes (TEXs) have discrete sets of proteins such as major histocompatibility complex class I and II (MHC-I and MHC-II), phosphatidylserine, milk fat globulin-E8 (MFGE8), rab7, liposome-associated membrane protein 1 (LAMP1), CD9, CD81, Annexin II, CD54, and CD63 that facilitate exosome-binding and uptake by relative ligands on DCs.11-14 In addition, TEXs express and transfer a wide spectrum of tumor-associated antigens to DCs that can prime tumor-specific cytotoxic T lymphocytes (CTL) and induce potent antitumor immunity.15-18 Further, immunostimulatory components are enriched in exosomes as compared to cells and previous studies in mice showed that TEXs improved vaccine efficacy compared to tumor lysates.14,19,20 This relies on uptaken TEXs being preferably transferred to the MHC-II-loading compartment, which is accompanied by a minor loss due to lysosomal degradation. Finally, the peptide-loaded DCs promote CD4+ T helper cell activation.14 DCs recruit TEXs via exosomal LFA-1 and CD54 that are major ligands for exosomes.21 The majority of previous studies used DC-derived exosomes as vaccines ignoring the potential of TEXs as an independent vaccine to stimulate DCs.22-27 However, TEXs being a rich reservoir of the whole panel of tumor antigens, TEXs can stimulate a broad array of T cell clones to respond toward the multiple antigenic epitopes.28 Moreover, TEXs can easily be isolated and purified from patients’ sera and malignant effusions. Thus, TEXs are an attractive alternative source of tumor antigens for cell-free cancer vaccines in personalized tumor immunotherapy,16,29 and it is becoming increasingly appreciated that TEXs can serve as a new promising cell-free therapeutic tool in cancer immunotherapy.13,14,30-33
To our knowledge, there has been no comprehensive review on the stimulatory efficacy and the antitumor immune responses induced by TEXs. Here, we will first introduce exosomes with a particular focus on the composition and targets of TEXs and their crosstalk with the tumor and the immune system. Following that, we will focus on the TEX application to induce immune responses.
Exosomes: biogenesis, structure, composition and function
Exosomes are cell-derived nanoscale (30–140 nm in diameter) vesicles possessing a lipid bilayer. They are found in almost all biological fluids including blood, serum, urine, breast milk, amniotic fluid, nasal secretions, saliva, cerebrospinal fluid (CSF), and bile as well as cell culture supernatants.34-41 Exosomes were first identified as small vesicles involved in the maturation of sheep reticulocytes. Subsequently, these functional vesicles were named as exosomes by Johnstone in 1989.42,43
The most significant factor in exosomes discrimination from other extracellular vesicles is their mode of biogenesis, target binding, and uptake. Exosomes are formed by endocytosis of several plasma membrane microdomains and creation of early and late endosomes, which receive their selective cargo and become integrated into multivesicular bodies (MVBs). When MVBs fuse with the plasma membrane, exosomes are released into the extracellular environment through exocytosis.44-49 Exosomes are the body’s most efficient system in mediating biological data exchange.
In addition to constitutive exosome membrane and cytosolic molecules, exosomes contain a large variety of membrane proteins and soluble factors related to cell-type specific functions (e.g. integrins, selectins, Rab proteins, SNAREs, tetraspanins such as CD9, CD81, CD63, growth receptors), lipids (e.g. steroids, sphingolipids, glycerophospholipids), nucleic acids (mRNAs, miRNAs, sRNAs, DNAs), and others.40,45,50-54 According to the current version of Exocarta (http://www.exocarta.org), the largest exosome content database, 41,860 proteins, more than 7,540 RNA and 1,116 lipid molecules have been identified from more than 286 exosomal studies.55 These exosomal-shuttle molecules play key roles in exosome function. Exosomes can interact (by deliver or uptake) with their recipient cells via different mechanisms such as specific receptor binding, direct fusion with the plasma membrane, and phagocytosis.51 By their distribution throughout the body, these vesicles transfer information from host cells to target cells over long distances. Furthermore, due to the presence of exosomes in biofluids and origin-dependent content, which closely reflects various physiological and pathological conditions, they may also serve as an ideal noninvasive or minimally invasive tool for diagnosis and monitoring the efficacy of treatment regimes.
Depending on the cell or tissue origin, exosomes have diverse biological functions in both normal and pathophysiological conditions which include beside others elimination of unnecessary proteins and molecules, blood coagulation, propagation of pathogens (prions and viruses), programmed cell death, angiogenesis, inflammation, modulation and regulation of immune response, and antigen presentation, where cell-cell communication promotes signaling and transcription.56-61
Several exosome isolation methods can be applied based on the sample volume, experimental design, research main questions, and type or origin of exosomes from cell culture supernatants or biological fluids. Most commonly employed isolation methods are differential ultracentrifugation, density gradients, and commercial exosome isolation kits making use of precipitation, bead-based, or immunoaffinity-based methods.61-64 Exosomes are visualized and characterized based on size distribution, specific exosomal markers, enriched proteins and RNAs and other selective contents. Most common techniques are transmission electron microscopy (TEM), cryo-EM, flow cytometry, ELISA, western blot, RNA profile using chip-based capillary electrophoresis, RNA sequencing, RNA microarrays, polymerase chain reaction (PCR), nanoparticle tracking analysis (NTA), and Dynamic Light Scattering (DLS).62,65 Each of the aforementioned isolation and characterization methods has some potential advantages as well as limitations, which warrants careful attention to the research purposes before choosing the isolation and characterization protocols.
TEXs in tumor cell and stroma modulation
Malignant cells secrete larger amounts of exosomes than non-transformed cells. There is evidence that TEXs can be involved in all steps of cancer development including oncogenesis and tumor growth via apoptosis inhibition and promotion of drug resistance, tumor cell spreading and metastatic settlement as well as angiogenesis, tumor immune escape, and immunosuppression.64 In the following, we briefly summarize and give some examples on these multitude of activities sparing the communication with the immune system that will be discussed in detail in the following section.
The crosstalk of TEXs with non-transformed cells might suffice to promote oncogenesis.66 Thus, uptake of malignant breast cancer cell-derived exosomes has been shown to educate non-tumorigenic epithelial cells to generate tumors.67 Similarly, TEXs have been demonstrated to induce malignant transformation in prostate cancer.68
Furthermore, the heterogeneous tumor cell mass contains a small subpopulation so-called cancer stem cells (CSCs) with self-renewal and differentiation potential, considered to be the main cause of tumor recurrence.69-71 One of the prominent features of CSCs is their plasticity and dynamic equilibrium, where CSC-TEXs play a crucial role. Although their mechanisms of action are rarely investigated, CSC-TEXs can induce a stemness phenotype by stemness-related molecule transfer and targeting upstream or downstream genes, by recruiting and altering the phenotype and function of stromal cells, as well as by enhancing tumor aggression and metastatic features.72-74 Thus, CSC-TEXs can remodel the tumor niche through influencing resident tumor cells as well as the tumor microenvironment including fibroblasts and immune cells, which leads to local tumor progression.68 In prostate cancer, differential microRNA patterns were observed in bulk cell-TEXs and CSC-TEXs. The CSC-TEXs miRNA profile suggested an involvement in angiogenesis, proliferation, and pre-metastatic niche formation.75 Similar results were found for CD90+ liver cancer cell-derived exosomes and CD105+ CSC-TEXs have a set of pro-angiogenic mRNAs, microRNAs, and lncRNA known to contribute to the stimulation of angiogenesis via endothelial cell phenotype modulation and lung pre-metastatic niche formation.76,77 Other studies indicated TEXs being involved in oncogenic cell signaling pathways especially mediated by molecules such as p53, MAPK, and Wnt.78 For example, CD82 and CD9 tetraspanins, which are enriched in exosomes, can suppress Wnt signaling.79
Further, TEXs molecular constituents can be associated with enhanced tumor growth. For instance, in colorectal cancer, TEXs enhance the proliferation of endothelial cells and tumor growth by their enriched content of cell cycle-involved mRNAs.80 Moreover, activation of PI3K/Akt and MAPK/ERK pathways by TEXs could promote cell proliferation in gastric cancer.81 TEXs have also been shown to inhibit the differentiation of bone marrow cells and alter macrophage physiology and function in favor of tumor growth.82-85 There are similar reports on the induction of tumor growth by TEXs from melanoma, hepatoma, glioblastom, and many other cancers.86-88
TEX exchange can instigate migratory behavior and metastatic potential in recipient cells through transfer of their cytoplasmic contents such as miRNA and pre-miRNA transcripts into either tumor cells or cells in the tumor microenvironment.89,90 CD44 transfer to surrounding peritoneal mesothelial cells through ovarian TEXs can promote invasive potential of cancer cells.91 Findings also suggested that deregulation of the extracellular matrix (ECM) through proteinases, glycoproteins, and matrix metalloproteinases released from TEXs could help invadopodia maturation and fibroblast remodeling of the tumor ECM leading to tumor cell invasiveness and migration.92-95 Furthermore, circulating TEXs have been proposed to prepare a pre-metastatic niche for incoming tumor cells.96 Exosomal release of cytokines, growth factors, and microRNAs can support recruitment of bone marrow-derived cells (BMDCs) to potential metastatic sites modulating pre-metastatic organ cells.87,97 Moreover, exosomal integrin expression patterns were demonstrated dictating organ sites of forthcoming metastasis.98
TEXs may also contribute to epithelial-to-mesenchymal transition (EMT), one of the hallmarks of tumor progression and invasion, through oncogenic transmission and TGFβ upregulation.99,100 Additionally, TEXs can inhibit apoptosis in many cancer cell lines.101 For instance, bladder TEXs inhibit apoptosis through Bcl-2 and Cyclin D1 proteins upregulation and Bax and caspase-3 proteins reduction.102 Exosomal survivin released from cancer cells also mediates inhibition of apoptosis in vitro.103
Angiogenesis is one of the crucial steps in tumor growth. In various types of cancers, uptake of TEXs by endothelial cells accelerates angiogenesis.95,104-109 These pro-angiogenic activities of exosomes occur through reprogramming of endothelial cells by different molecules including exosomal miR-135b in multiple myeloma, miR-130 in gastric cancer, and miRNA-210 in leukemia and breast cancer.110-113 Hence, TEXs can affect tumor progression and metastasis through vascular remodeling.
Of special interest for immunotherapy is the capacity of TEXs to promote cancer resistance to conventional therapies. Thus, TEXs horizontally transfer exosomal miRNAs involved in target cell drug resistance.114,115 Similarly, the transfer of long non-coding RNAs via TEXs led to increased tamoxifen resistance in breast cancer cells.116 Also, high survivin level in TEXs decreased radiation efficacy.117 Moreover, exosome-mediated miR-32-5p delivery could increase drug resistance through activation of the PI3K/AKT pathway and inhibition of PTEN.118
TEXs in tumor immunology
As mentioned, TEXs are enriched in tumor antigens, but also promote immune escape.119,120 The literature abounds with evidence suggesting TEXs as mediators of immune cell-tumor cell communication, and regulators of immune responses through both immunosuppressive and immunostimulatory functions, promoting either tumor progression or regression. In the following section, the double-edged role of TEXs in suppression and activation of immune responses against cancer is summarized based on immune cell types (Figure 1).
Figure 1.
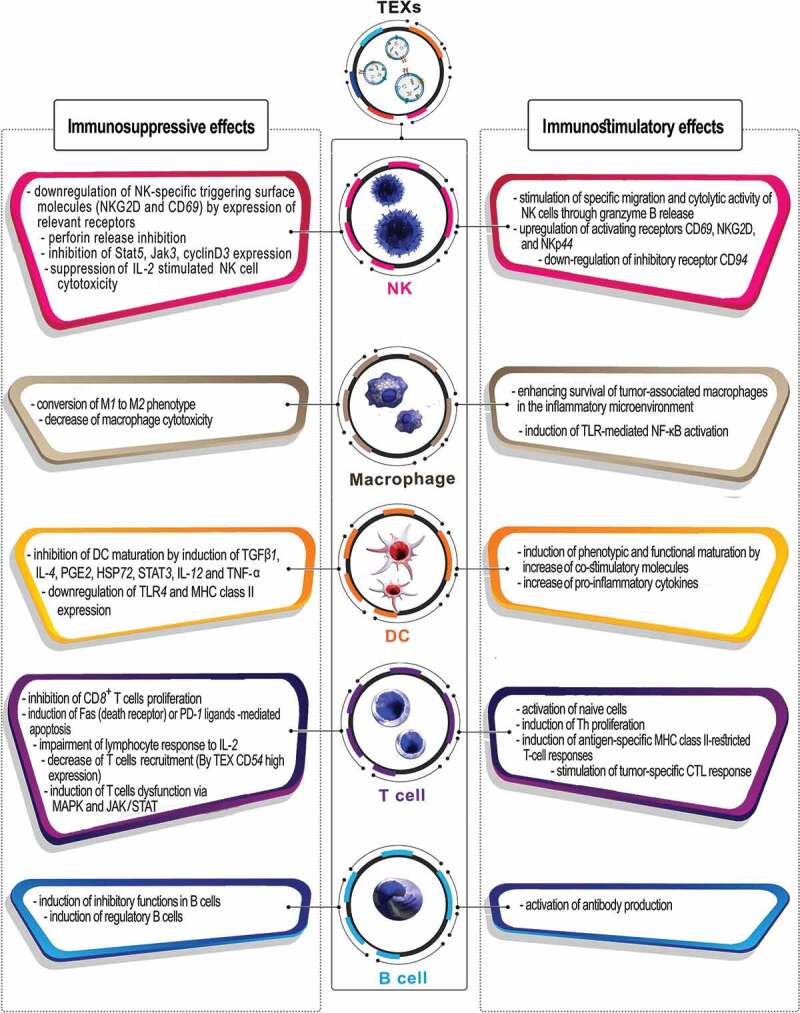
Schematic representation of dual functions of TEXs in suppression and activation of immune responses against cancer.
Natural killer (NK) cells
TEXs have been shown to inhibit tumor immune surveillance by exerting immunosuppressive impacts on NK cells as the main anti-tumor weapons of the innate immune system. Indeed, TEXs hamper NK at multiple levels by interfering with activation and, particularly, effector functions. Thus, TEXs account for significant down-regulation of NK-specific triggering surface molecules (natural killer group 2D (NKG2D) and CD69) by inhibition of Stat5, Jak3, CyclinD3 expression, increasing the number of myeloid-derived suppressor cells (MDSCs), decreasing of perforin secretion, all resulting in impaired migration and cytotoxic potential of peripheral NK cells.121-124 In addition, TEXs promote a decrease in the number of NK cells in the spleen and lungs through induction of tumor-infiltrating natural killer (TINK) through activation of the TGF-ß/SMAD pathway mediated by exosomal TGF-ß1, which also suppress IL-2 stimulated NK cell cytotoxicity.121,125-127 In contrast, some TEX constitutive markers facilitate NK activation; For instance, co-culture of NK cells with heat shock protein 70 (HSP-70) surface-positive TEXs, as an attractant and target structure, stimulates NK recruitment and cytolytic activity through granzyme B release of CD94+ NK cells.128 Moreover, the HSP-70-stimulated NK up-regulate the expression of CD69, NKG2D, and NKp44 stimulating receptors, while the CD94 inhibitory receptor becomes down-regulated.129
Macrophages
TEXs exert an important impact on macrophages by forcing them toward the immunosuppressive M2 phenotype.130 Previous studies reported that TEXs stimulate pro-inflammatory activity and TLR-mediated NF-κB activation in macrophages, resulting in increased secretion of pro-inflammatory cytokines/chemokines, such as IL-6, TNFα, GM-CSF, and CCL2; this leads to prolonged survival of tumor-associated macrophages in the inflammatory microenvironment.83,131 Indeed, TEXs mediate upregulation of Wnt 5α in macrophages; subsequently macrophage-derived exosomes transfer Wnt 5α into tumor cells which leads to enhanced tumor invasion through the activation of β-catenin-independent Wnt signaling.84
Dendritic cells (DCs)
Differentiation of monocytes toward DCs, the professional antigen-presenting cells (APCs) and the bridge between the innate and adaptive immunity, can be modulated by TEXs via expression of PGE2, HSP72, and TGF-β, that have been reported to induce the production of inhibitory cytokines, decrease the expression of co-stimulatory molecules, increase the expression of STAT3, and inhibit the maturation and T cell stimulatory capacity of DCs through induction of IL-6 phosphorylation.82,132,133 Further, under TEXs impact, DC maturation and antigen-specific responses can become impaired via downregulation of TLR4 and MHC-II expression as well as induction of IL-4, TNF-α, IL-12, and TGFβ1.132,134 Moreover, TEXs uptake by immature DCs has been demonstrated to block DC maturation by exosome-mediated mechanisms and also to induce tumor suppression via redirecting toward myeloid-derived suppressor cell (MDSCs) differentiation and proliferation.133,135
Despite these immunosuppressive effects, TEXs express tumor antigens as well as markers, which facilitate TEXs uptake by DCs and direct tumor antigens toward multivesicular bodies. After processing, peptides are loaded into newly generated MHC-II molecule grooves and are presented to Th cells promoting their activation. Several studies documented that in vitro uptake of TEXs by DC enhance the expression levels of co-stimulatory receptors (CD80, CD86), CD11c and MHC, and induce phenotypic and functional DC maturation.130,136,137
T cells
TEXs block CD8+ T cells proliferation, activation and cytotoxic activity and increase their immunosuppressive functions. They are also responsible for induction of Fas or programmed cell death-1 (PD-1) ligands-mediated apoptosis of antitumor CD8+ effector T cells in several cancers.130,138-140 The expression of CD73, CD39 immunoregulatory proteins, TGF-β1 cytokine, and galectin-1 (Gal-1) in TEXs can induce a suppressive phenotype in CD8+ and CD4+ T cells.130,141,142 In addition, TEXs impair the lymphocyte response to IL-2143 and have been shown to induce IL-8 production in epithelial cells suppressing T cell responses.144 Finally, TEXs can contribute to tumor escape via induction of CD4+ CD25+ FOXP3+ T regulatory cell (Treg) expansion, accompanied by increasing expression levels of TGF-β, IL-10, and CTLA4. They also indirectly facilitate Treg generation through inducing tolerogenic DCs.133,145
On the other hand, through expression of MHC class I and class II complexes on their surface, TEXs can directly present antigen, activate T cells, and induce antigen-specific MHC class II-restricted T-cell responses. Also, many studies unraveled TEXs expressing some markers that could facilitate CD8+ T cell activation and stimulate tumor-specific CTL responses in vivo and anti-tumor immune responses in mice.13,140,146 However, the TEXs armament may preferentially suffice for memory T cell activation, whereas activation of naïve T cells requires TEXs modulation.
B cells
The effect of TEXs on B cell activation and function is not well delineated. Few studies have shown that TEXs induce differentiation of naïve B cell to a regulatory phenotype with production of inhibitory cytokines that leads to antitumor immune response inhibition.147,148 In addition, TEXs could reduce antibody-dependent cell cytotoxicity (ADCC) through interfering with tumor-reactive antibodies binding to tumor cells.149 In contrast, TEXs were also described to drive antibody production, the underlying mechanism remaining to be explored.139
TEX-based cancer vaccination
Many studies proved the feasibility and functionality of TEXs to stimulate immune responses against cancer in mouse models. For instance, a study by Bu et al., in syngeneic mice showed that vaccination with L1210 leukemia-released exosomes prevented tumor formation and elicited protection to tumor challenge.150 Lee and colleagues demonstrated that vaccination with TEXs not only elicited significant protection against tumor growth and primed Th1 immune responses to an established melanoma but also could inhibit pulmonary metastasis in metastatic melanoma mouse models.151 This capability of TEXs to trigger T cell-mediated antitumor immune responses and suppress tumor growth has been reported in other studies, as well.14,15,33,137 Furthermore, TEXs harbor tumor antigens from their donor cells. Following uptake by DCs, these tumor antigens are presented as peptides in the MHC grove and can prime naïve T cells to generate anti-tumor responses.136 However, because of TEXs-induced immunosuppression and limited TEX immunogenicity, TEXs alone application frequently resulted in unsatisfying anti-tumor immune effects in vivo. Thus, several strategies were developed to improve the efficacy of TEXs vaccination. Some of which are listed in Table 1 and will be discussed in the following sections.
Table 1.
A summary of vaccine studies utilizing tumor-derived exosomes in in vitro and in vivo preclinical animal models.
| Source of tumor exosomes | Purification method | Aim of study | Treatment regimen | Key results | Ref. |
|---|---|---|---|---|---|
| -HeLa cell line | Ultracentrifugation/sucrose gradient | In vitro investigation of HeLa-derived exosomes immunogenicity. | Capacity of HeLa-TEX for inducing immune responses was examined. |
|
135 |
| -EG7 expressing ovalbumin (OVA- transfected EL4 mouse thymoma) cell line | Ultracentrifugation | Comparison between TEX and dendritic cell-derived exosomes (DEXs) based vaccination. | Melanoma-bearing mice were immunized intravenously with OVA pulsed DC-derived exosomes (EXODC) or tumor-derived exosomes (TEXEG7) (10 µg/mouse). |
|
11 |
| -EG7 expressing ovalbumin (OVA- transfected EL4 mouse thymoma) cell line | Ultracentrifugation | Comparison of immunogenicity between OVA protein pulsed DC-derived exosomes (EXODC) and DC pulsed with TEXOVA (DCEXO). | Melanoma-bearing mice were treated intravenously with EXODC or DCEXO vaccines. |
|
144 |
| -A20 (H-2d) mouse B lymphoma/leukemia cell line (Exo) -Heat-shocked A20 (H-2d) mouse B lymphoma/leukemia cell line (HS-Exo) - murine colon cancer CT-26 cell line |
Ultracentrifugation | Evaluation of heat shock induction in improvement of exosome-based tumor vaccines. | Lymphoma/leukemia-bearing BALB/c mice were immunized subcutaneously with TEX and HS-TEX (10 µg/mouse) |
|
28 |
| - B16F1 murine melanoma cell line - CIITA gene (MHC class II transcriptional activator) transduced B16F1cell line |
Ultracentrifugation/sucrose gradient | Investigation of CIITA transduction effect in enhancing immune stimulation as compared to exosomes from parental tumor cells (TEX). | -For preventive model, C57BL/6 mice were intradermally vaccinated with PBS, TEX or CIITA-TEX (5 or 20 µg/mouse), and then challenged subcutaneously with melanoma B16F1 cells. -For therapeutic model, B16F1 melanome-bearing mice were immunized intradermally with PBS, TEX and CIITA-TEX (20 µg/mouse). |
|
29 |
| -Murine colon cancer CT-26 cell line -CIITA gene transduced CT-26 cell line |
Ultracentrifugation/sucrose gradient | Transduction of CIITA gene into CT26 cell line and evaluation of immune response induction by CT-26-CIITA TEX compared to parental CT-26 TEX. | Balb/c mice were intradermally immunized with PBS, CT-26 TEX and CT-26 -CIITA TEX (10 or 20 µg/mouse), and challenged subcutaneously with CT-26 cells. |
|
30 |
| -K562 leukemia cell line -L1210 acute lymphoblastic leukemia cell line |
Ultracentrifugation/sucrose gradient | Examination of L1210-TEX and DC- L1210 TEX ability in induction of protective antitumor immunity in leukemia mice model. | - For therapeutic model, DBA/2 mice were immunized subcutaneously with PBS, L1210-TEX (30 µg/mouse), non-pulsed DC and DC-L1210 TEX (1.0–4.0 × 106cells/mouse), then challenged with L1210 leukemia cells. - For preventive model, after challenging with L1210 leukemia cells, DBA/2 mice were immunized. |
|
31 |
| -Myeloid leukemia WEHI3B cell line -Renal cell carcinoma (RENCA) cell line |
Ultracentrifugation/sucrose gradient | Efficacy evaluation of TEX- vs. tumor lysate-loaded DC as vaccines in WEHI3 myeloid leukemia and RENCA bearing mice. | Balb/c mice were challenged intravenously or subcutaneously with WEHI3B and RENCA cells, and then were vaccinated with DC-lysate, DC-TEX and TEX alone. (200 µg/mouse) |
|
12 |
| - UNKC6141 pancreatic adenocarcinoma cell line (UNKC) | Ultracentrifugation/sucrose gradient | Strengthen of the therapeutic efficacy of DC-TEX vaccination by blocking of MDSC maturation and activation in pancreatic cancer (PaCa). | UNKC-bearing mice (subcutaneously or orthotopic) after AGS (ATRA, Gemcitabine and Sunitinib) application were immunized intravenously with TEX-loaded DC (DC-TEX). |
|
152 |
| -Murine breast cancer 4T1 cell line | Gradient ultracentrifugation |
Modification of miRNA content of TEXs for enhancement of DC maturation and effector cells activation. | TEX loaded with mimics of miRNAs (miR-155, miR-142, and let-7i) to induce potent dendritic cells (DC) and improve immune stimulation. |
|
153 |
| -Murine melanoma B16BL6 cell line | Ultracentrifugation | Co-delivery of tumor antigens and adjuvant by genetic modification of exosomes. | B16BL6 cells were transfected with immunostimulatory CpG DNA and then their derived TEXs were utilized as a vaccine in melanoma bearing mice. |
|
154 |
| -HEK293 cell line | Ultracentrifugation | Direct vaccination of murine models with antitumor let7a microRNA and GE11 binding peptide-modified TEX. | Let-7a – GE11 containing TEX were administrated in human tumor xenograft models. |
|
155 |
| -AB1 mouse mesothelioma cell line | Ultracentrifugation | Direct comparison of TEX-DC and lysate-DC vaccination in mesothelioma cancer model. | Mesothelioma bearing mice were treated with TEX-loaded DC and lysate-loaded DC vaccines. |
|
156 |
| -Autologous glioma tumor cells (from patients with glioblastoma multiform) | Ultrafiltration/centrifugation/sucrose Gradient/ultracentrifugation | Assessment of the capacity of autologous glioma tumor cells-derived exosomes in CTLs activity induction compare to tumor lysate. | DCs were pulsed with glioma cells and their derived TEXs, and in vitro CTL activity was then evaluated. |
|
157 |
| -Myeloma J558 cell line expressing P1A tumor antigen | Ultracentrifugation/sucrose gradient | The comparison of immune responses induced by EXOHSP expressing membrane-bound HSP70 (TEXHSP) or EXO expressing cytoplasmic HSP70 (TEXHS). | Myeloma J558 cell line were transfected by membrane-bound inducible HSP70 expressing vectors and then myeloma bearing mice were treated with TEXHSP or TEXHS pulsed DCs. |
|
158 |
| -MUC1-expressing CT26 cell line | Ultracentrifugation/sucrose gradient | Enhancement of the immune-stimulating activity of TEXs by heat treatment of tumor cells. | MUC1-CT26 cells were exposed to heat shock, then tumor bearing mice were vaccinated by HS-TEX or untreated TEX. |
|
159 |
| -CT26 murine colon carcinoma cell line -TA3HA murine mammary carcinoma cell line |
Ultracentrifugation/sucrose gradient | Genetic manipulation of TEXs for tumor antigens delivery. | Immunogenic potential of MUC-containing exosomes was tested in autogenic and allogeneic tumors. |
|
24 |
| -EG7 expressing ovalbumin cell line (OVA- transfected EL4 mouse thymoma cell line) | Ultracentrifugation/sucrose gradient | Protein transfer of SEA on TEXs in order to increase their immunogenicity. | C57BL/6 mice were immunized subcutaneously with TEX, SEA-TEX or mixture of TEX and SEA, and were challenged with EG7-OVA cells. |
|
160 |
| -L1210 murine B lymphocytic leukemia cell line |
Ultrafiltration Centrifugation/sucrose gradient/ultracentrifugation |
Assessment of L1210-derived TEXs impact as a prophylactic vaccine in syngeneic mice models. | DBA/2 mice were subcutaneously immunized with L1210-TEXs (2.5 or 5 µg) and then challenged with L1210 leukemia cells. |
|
149 |
| -Fon (HLA-A2+) and Mel-888 (HLA-A2−) human melanoma tumor cell lines |
Ultracentrifugation/sucrose gradient | The comparison of immune responses induced by TEXs and tumor cells lysate in prophylactic and therapeutic mice models. | Mice models were immunized by different doses of TEXs and tumor cell-derived lysate as alone or loaded on DC. |
|
13 |
| -AB1 mouse mesothelioma cell line | Ultracentrifugation | Study of the impact of antigen source in cancer immunotherapy outcome. | Mesothelioma bearing mice were treated with DCs loaded by different antigen sources (tumor cell line lysate, ex vivo tumor lysate and tumor cell line-derived TEX). |
|
161 |
| - B16-OVA and B16 melanoma cell lines | sucrose gradient/ultracentrifugation | Evaluation of the effect of VSV-G incorporation in TEXs on their immunogenicity. | Melanoma cell lines B16-OVA and B16 were transduced by VSV-G-expressing vectors and then melanoma bearing mice were treated with VSV-G containing TEXs loaded on DC. |
|
162 |
| -E.G7-OVA tumor cells expressing Ovalbumin (OVA) |
Serial centrifugation/sucrose gradients/ultracentrifugation | Further improvement of TEX-based vaccination through expression of IL-2 in them. | E.G7-OVA tumor cells were transfected with IL-2 gene and then tumor bearing mice were immunized with TEX/IL-2, TEX+IL-2 and TEX alone. |
|
163 |
| -Human RC-2 renal cancer cell line | Ultracentrifugation | Improving TEX immunogenicity by IL-12 gene modification in tumor cells. | Human renal tumor cells were genetically modified with IL-12 gene and then in vitro immune responses of TEX/IL-12 derived from these cells were examined. |
|
164 |
| -B16F1 melanoma cell line | Ultracentrifugation | In vitro assessment of immunological activity of melanoma cell-derived TEXs (mcd-TEXs). | Immunogenic potential of mcd-TEXs was tested in vitro. |
|
83 |
| - Hepa1-6 murine hepatocellular carcinoma (HCC) cell line -Human HepG2 and Hep3B HCC cell lines |
Ultracentrifugation | Human in vitro and murine in vivo study of immune efficacy of HCC cell line-derived TEXs compare to cell lysate. | -For murine in vivo study, C57BL6 mice were immunized subcutaneously with PBS, TEX-pulsed DC (30 µg/mouse), lysate-pulsed DC in therapeutic and preventive as well as ectopic and orthotopic models. -For human in vitro study, DCs were pulsed with TEXs and then in vitro CTL activity was evaluated. |
|
165 |
| -B16BL6 melanoma cell line -Murine melanoma tumor |
Homogenization/sonication/ultracentrifugation | Assessment of the therapeutic effect of autologous TEXs in melanoma tumor and metastatic mice models. | Autologous murine melanoma tumors were dissected and homogenized, and then immunogenicity of their isolated TEXs was evaluated in vitro and in vivo. |
|
139 |
| - Hepa1-6 cell line | Ultracentrifugation | In vivo evaluation of the effect of DC-TEX and sorafenib combination on treatment outcome. | Orthotopic HCC mice were vaccinated with different combinations of DC-TEX, sorafenib and PD-1 Ab, and then in vivo immune responses were evaluated. |
|
166 |
TEXs modifications to advance tumor immunotherapeutic efficacy
Tumor cells and TEXs can be modified (either genetically or non-genetically) to enrich tumor antigens, microRNAs, and immunostimulatory molecules in TEXs with the aim of enhancing tumor cell elimination directly or in concert with killing by immune cells. Heat shock proteins (HSPs), highly enriched in cancer cells and TEXs, have potent adjuvant capability. They served as one of the approaches in strengthening cancer immunotherapy. Indeed, HSP can increase immunogenicity of TEXs and improve cancer vaccine efficacy.158,159 Chen and colleagues demonstrated that heat shock treatment increases TEX proteins relevant to potentiate immune response induction and reported on efficient immunostimulatory functions of A20 lymphoma/leukemia cell line-derived heat-shock exosomes (HS-TEX) via the up-regulation of MHC, co-stimulatory molecules and cytokines, including IL-1β, IL-12p40, TNF-α, RANTES, and also MIP-1α in DC. The stronger protective antitumor immunity of HS-TEXs compared to untreated TEXs in immunized mice confirmed these results.30 Similar findings also were obtained with Hsp70-enriched TEXs isolated from heat-treated MUC1-expressing CT26 cells. These HS-TEXs showed increased expression of MHC-II and enhanced immune-stimulating activity. HS-TEXs, as an MHC-independent vaccine, stimulated strong Th1 type immune responses via the increased production of IgG2a antibody and IFN-γ, resulting in elimination of cancer cells in autologous and allogeneic tumor-bearing mice.167 This approach has also been employed by Xie and colleagues via transfection of heat-shocked J558 tumor cells with vectors expressing membrane-bound inducible HSP70 to generate TEXHSP expressing membrane-bound HSP70. They demonstrated that TEXs bearing membrane-bound HSP stimulated IL-12 secretion by TEXHSP-activated DCs and induced stronger antitumor immunity mediated by CD4+ Th1, CD8+ CTL, and NK cells than TEXs expressing cytoplasmic HSP70.160 Thus, HSP70 up-regulation in TEX membranes may be an effective strategy to enhance immune response induction.
Another modification strategy to improve TEXs immunogenic properties relies on the manipulation of cancer cells to increase the expression of tumor-specific antigens that are transferred into TEXs. Thus far, this was approached with a variety of highly immunogenic tumor antigens. For example, high expression of the MUC1 tumor antigen is related to cancer progression and poor prognosis in many types of cancer;168 In a study by Cho et al., MUC1-containing TEXs from MUC1 transduced CT26 and TA3HA cell lines induced DC maturation and IFN-γ secretion by Th1, and more efficiently inhibited autologous and allogeneic tumor growth than non-MUC1-containing control TEXs.26
TEXs-promoted immune reactions can also be strengthened by the superantigen staphylococcal enterotoxin A (SEA), which forms a complex with MHC-II on APCs. Xiu and colleagues generated TEXs containing SEA or SEA tailed with a highly hydrophobic transmembrane domain (SEA-TM) by protein transfer. Immunization of mice with SEA-membrane-anchored TEXs led to increased IL-2 and IFN-γ secretion, strong in vivo CTL responses as well as stimulation of anti-tumor effects of both CD4+ T cells and NK cells. Additionally, SEA-TEXs inhibited tumor growth and prolonged the survival of tumor-bearing mice more strongly than un-manipulated TEXs or a mixture of TEXs and SEA. These effects could possibly be ascribed to SEA facilitating TEXs binding to DC and a concomitant decrease of the immunosuppressive activity of Tregs.162 Thus, protein transfer is an appropriate method to modify TEXs toward expression of tumor antigens or other immune-enhancing proteins on their surface.
Since the recognition of MHC class II-peptide complexes by CD4+ helper T cells is required for optimal and efficient induction of anti-tumor immunity, another candidate molecule for increasing the efficacy of TEXs in cancer immunotherapy is MHC class II. Master regulatory gene MHC class II transcriptional activator (CIITA) controls expression of MHC class II molecules and introduction of CIITA gene in tumor cells can stimulate tumor-specific CD4+ and CD8+ T cells.155 To investigate the effect of CIITA expression on the immune-stimulating capability of TEXs, Lee et al., transduced melanoma B16F1 cells with the CIITA gene and assessed the impact of CIITA-enrichment in TEXs (CIITA-TEXs) on tumor regression. Compared to parental TEXs, CIITA-TEXs provoked enhanced immune responses reflected by the higher surface expression of MHC class II and CD86 and higher mRNA levels of inflammatory cytokine, TNF-α, and maturation marker CCR-7 on pulsed DCs. Notably, induction of IL-2 cytokine production by naïve splenocytes in vaccinated mice suggested the capability of CIITA-TEXs to induce CD4+ helper T cells activity. Moreover, CIITA-TEXs vaccination delayed tumor growth and improved the survival rate in both therapeutic and prophylactic immunized melanoma-bearing mice.31 Similar results were reported by Wen Fan et al., applying exosomes from the CIITA-transduced murine colon cancer CT-26 cell line. CT26-CIITA-derived TEXs increased Th1 immune responses reflected by significantly increased TNF-α, IFN-γ, and IL-12, and decreased IL-10 expression.32 Thus, MHC-II expressing TEXs are powerful in strengthening anti-tumor immune responses.
Incorporation of some viral fusion proteins in TEXs was shown to enhance their uptake by DCs and to improve immunogenicity. One of these viral fusion proteins is the G protein of vesicular stomatitis virus (VSV-G), which facilitates binding of the viral particles to the cell surface. Temchura et al. elaborated that co-expression of VSV-G and tumor antigens on TEX membranes not only accelerated internalization and presentation of TEX antigens by DC but also enhanced phenotypic and functional DC maturation. Up-regulation of CD86, CD80, CD40 co-stimulatory molecules and increased IL-12 release in DC resulted in effective and specific in vivo CTL immune responses.163 Results obtained from vaccination of mice with EGFR-expressing breast cancer xenografts, where TEXs were manipulated with GE11 peptide, which specifically binds to the EGFR, confirmed the efficacy of this approach.164
Expression of certain cytokines in tumor cells and subsequently in their TEXs might be another promising strategy to enhance TEXs immunogenicity. Interleukin 2 (IL-2), an important growth factor and vaccine adjuvant, mediates regulation and activation of immune cells including CTL, NK, B cells, and macrophages, and can generate clinically significant anti-tumor activity in cancer patients. Yang and colleagues represented a new method for improving TEXs application as a vaccine, where E.G7-OVA tumor cells were genetically engineered to express IL-2 to deliver IL-2-containing TEXs (TEX/IL-2). Immunization with TEX/IL-2 strongly enhanced T cell proliferation and affected NK and CD4+ T cells accompanied by elevated secretion of IL-2 and IFN-γ cytokines, and resulting in efficient inhibition of tumor growth.153 Similar results were obtained with IL-12-containing TEXs (TEX/IL-12), where human renal cancer cells were genetically modified with the IL-12 gene to produce TEX/IL-12, IL-12 being an essential co-stimulatory signal for cellular immune response activation. TEX/IL-12 was capable of increase in vitro CTL activity and strengthened IFN-γ release compared to non-manipulated TEXs.154
TEXs transfer diverse types of cargo including microRNAs into cancer cells. Hence, modification of TEXs to overexpress miRNAs that repress immunosuppressive targets might elevate TEX promoted immune responses. Ohno and colleagues introduced let-7a miRNA into HEK293 cells and observed that let-7a containing TEXs potently inhibited breast tumor development.164 Also, in a recent study by Taghikhani et al., TEXs modified with miR-155, miR-142, and let-7i efficiently delivered antitumor miRNAs to DCs inducing DC maturation and enhancing in vitro CTL activity.169 In another study, efficient in vitro tumor antigen presentation and higher cytokine release (TNF-α, IL-6, and IL-12p40) were observed in DCs after uptake of TEXs modified with CpG DNA, a well-known immune response modifier. Moreover, immunization with these TEXs resulted in potent cellular and humoral immunity along with upregulation of Th-1 related IgG2a as well as protective and therapeutic antitumor immunity in immunized mice.165
Taken together, all these reports indicate that appropriately modified TEXs can be a valuable tool for cancer immunotherapy.
Improvement of TEX-based vaccination by DC loading
DCs are the professional antigen-presenting cells (APCs) of the immune system with the capability to stimulate key adaptive immune cells (naïve CD8⁺ T cells and CD4⁺ helper T-cells, B-cells). DCs loaded with different tumor-specific antigens to enhance initiation of primary and secondary immune responses, have been used as an efficacious vaccine in numerous murine cancer models as well as in clinical trials.10
In vitro activation and loading of DCs, as a potent source for initiating immune responses has several advantages compared to TEXs as vaccine. This strategy would help to overcome some limitations of using TEXs alone like the risk of eliciting immunosuppressive features by free TEXs and also inadequate induction of immune responses. It would guarantee the presentation of exclusively MHC-I or MHC-II grove fitting peptides from digested tumor antigens as well as abundant availability of co-stimulatory molecules. Indeed, several studies showed that TEXs are efficiently taken up and presented by DCs and may even support DC maturation. In vitro studies uncovered that besides stimulating peptide-specific clonal T cell expansion, TEX-loaded DCs also are able to stimulate naïve CD8+ T cells maturation toward antigen-specific CTL and induce NF-κB activation in macrophages that are involved in tumor cytotoxicity via tumor necrosis factor (TNF) release.15,85,157,160 Thus, one important advantage of TEX-loaded DC compared to free TEXs relies on tumor antigen processing and peptide loading of MHC-I molecules. Ren et al. showed that HeLa-TEXs alone were not capable of in vitro T cell activation and proliferation, whereas HeLa-TEXs loaded-DCs successfully induced T lymphocyte activation.137 In a similar study, Wolfer and colleagues observed that when TEXs were derived from the Fon melanoma cell line and loaded onto DCs, they promoted activation and IFN-γ production in CTL clones in vitro; whereas they could not raise CTL clones using free TEXs.15 In line with these studies, Yao and colleagues examined the ability of leukemia-derived exosomes (LEXs) and LEX-pulsed DCs to induce antileukemic immunity in both prophylactic and therapeutic leukemia mouse models. They demonstrated that TEX-pulsed DCs significantly enhanced the survival rate of tumor-challenged mice and more effectively induced CTL immune responses in a dose-depended manner.33 This dose-dependency was also observed in cytolysis induced by effector T cells primed with TEX-pulsed DCs in hepatocellular carcinoma (HCC) models.156 Similar results were also obtained by Gu et al., who studied the impacts of TEX-loaded DC (DC-TEX) as a vaccine in WEHI3 myeloid leukemia-bearing mice.14 Beside tumor antigens, the availability of TEX markers facilitating uptake by DC contributes to pronounced CTL activation. Bu and colleagues purified autologous TEXs from glioma cell culture supernatants from patients with glioblastoma multiform and noted that these TEXs were enriched in MHC-I, HSP70, ICAM-1, and MAGE-1 molecules, which are involved in TEX uptake and antigen presentation. The authors describe higher cytotoxic capacity of TEX-DC- than tumor lysate-DC-stimulated T cells in autologous glioma cell cultures.170 These results support the hypothesis that TEXs can be used as a promising and robust platform to improve personalized cancer immunotherapy.
Taken together, one great advantage of loading DC with TEX relies on the processing of the TEX tumor antigens and the presentation of tumor antigen peptides in the MHC groove, which strongly facilitates the capture of tumor-peptide specific CD8+ T cells driving their expansion and activation. The enrichment of tumor antigens in TEXs and the equipment of TEX with markers that facilitate the uptake by DC add to the superiority of TEXs-pulsed DC to CTL activation. Furthermore, the preferable processing of TEXs in the MHC-II-loading compartment leads to CD4+ Th activation resulting in more efficient activation of CTLs.14
Both dendritic-derived exosomes (DEXs) and TEXs have been used in tumor vaccination. Only few studies compared the efficacy of the two exosome sources as vaccine. Nevertheless, the functionality examination of DC-OVA-derived EXO (EXODC) and EG7 tumor cell line-derived EXO (TEXEG7) indicated that EXODC can more efficiently stimulate T cell proliferation and differentiation, and also promote stronger killing activities against tumor cells compared to EXOEG7 immunized mice. Similar results were observed regarding antitumor immunity and protection against lung tumor metastases. The higher immunogenicity of EXODC could be ascribed to expression of co-stimulatory molecules such as CD40, CD80 on EXODC. TEXEG7 not expressing these co-stimulatory molecules could well explain their weaker efficacy compared to EXODC.13 However, reverse results were obtained when using of TEXs for DC loading. In an early study, Hao et al. evaluated immune response induction of loading mature DC with TEX, where ovalbumin (OVA) served as tumor antigen. They observed that TEX uptake by DC is mediated by LFA-1, CD54, and CLR. Interestingly, TEX-loaded DCs expressed higher level of the co-stimulatory molecules CD40, CD80, CD54, and of MHC-II than OVA-loaded DCs. Moreover, vaccination with TEX-loaded DCs induced excessive in vitro and in vivo T cell proliferation and exerted higher protective immunity against the primary tumor and lung metastasis in tumor-bearing mice than OVA-loaded DC, exosomes derived thereof or TEX.146
We interpret these findings that the second advantage of loading DC with TEX can be ascribed to the pronounced uptake of TEX and guidance into the MHC-II processing compartment, where TEX tumor antigens are processed for loading into newly arranged MHC-II, which are transported to the DC membrane and together with the expanded repertoire of costimulatory molecules of activated DC suffice for tumor antigen-specific Th activation. This will have bearing not only on CTL activation but also on B cell activation, antibody secretion, and NK cell stimulation. These latter activities still await detailed exploration.
Before recognizing the abundant recovery of tumor antigens in TEXs, DC frequently was loaded with tumor lysates. However, a direct comparison of DC-TEXs versus DC-lysate indicated stronger anti-tumor immunity and superior therapeutic efficacy, when loading with TEXs than tumor lysate. In a comparative study, Wolfer et al. demonstrated that melanoma TEX-loaded human DCs induced in vitro IFN-γ production in CTL clones; the efficacy was comparable to that by loading with synthetic peptides and far higher than that of tumor lysate-loaded DC. Furthermore, TEX elicited more efficient protective antitumor immune responses than tumor lysate in syngeneic and allogeneic settings, even when a higher amount of lysate was applied. Interestingly, boosting with a low dose of TEXs (20 µL) protected vaccinated mice from a lethal challenge, whereas the equivalent (lysate of 2 × 104 tumor cells) was inefficient.15 The authors suggest the efficient uptake of TEXs by DCs as underlying mechanism, where the presence of CD54, CD9, and CD63 DC-ligands on TEXs facilitates uptake. Similar results were obtained by other investigators in different tumor models using, e.g. leukemia and pancreatic cancer cell-derived exosomes, where TEX-DC more efficiently than lysate-DC increased survival and suppressed tumor growth in pancreatic, renal cell carcinoma and leukemia tumor-bearing mice.14,152 The superiority of DC-TEX was due to highly efficient TEX uptake and long-lasting TEX processing in the MHC-II-loading compartment, which led to pronounced IL-12 up-regulation in DC and tumor-specific CD4+ Th and CTL activation.14 Besides activation of a wider range of T cell clones, the presence of classical DC costimulatory molecules may also contribute to TIL, MФ, and NK cells recruitment.
Notably, due to the immunotolerogenic nature of some organs and tumors deriving thereof, tumor cell lysates can be hardly immunogenic.166 To overcome this challenge, Rao et al. investigated the efficacy of HCC TEXs to stimulate immune responses in vitro and in vivo. TEX-pulsed DCs showed superiority with respect to the induction of immune response compared to cell lysates-pulsed DC in both prophylactic and therapeutic HCC mouse models. Stimulation with HCC TEXs efficiently induced the expansion of antigen-specific CTLs, provoked elevated IFN-γ levels, and decreased the release of immunosuppressive IL-10 and TGF-β, which together resulted in stronger tumor growth inhibition in both ectopic and orthotopic HCC bearing mice. Remarkably, HCC TEXs-promoted cytotoxicity exerted MHC-independent cross-protection against different HCC and pancreatic cancer cells.156 This may be due to the presence of tumor antigens in TEXs, which are shared by multiple tumors. The finding also is in line with TEX-derived tumor peptides being presented in newly generated MHC molecules of the host DC.
In brief, DC loading with TEXs rather than tumor lysate has several advantages. Although autologous TEXs and tumor lysates share avoiding allogeneic immune response, compared to tumor lysates, tumor antigens are enriched in TEXs. Furthermore and importantly, TEXs are particularly equipped for binding and uptake, and uptaken TEX are guided in DC toward the antigen processing compartment.
Finally, we want to point out that the efficacy of vaccination with TEX-pulsed DC can profit from a concomitant treatment with drugs that particularly affect immunosuppressive cells or factors. Xiao et al. focused on improving the efficacy of DC-TEX vaccination in pancreatic cancer through combination with cytotoxic drugs that attack MDSC. Akin to previous studies, they confirmed that TEX-loaded DC could activate T lymphocytes in DC-TEX vaccinated UNKC6141 PaCa-bearing mice, prolong survival, and significantly decrease the metastatic capacity of UNKC tumor cells. Interestingly, combining DC-TEX vaccination with the application of most frequent adjuvant drugs in PaCa treatment, such as Gemcitabine (GEM), ATRA, and Sunitinib (SUN), which interfere with MDSC maturation and/or persistence, resulted in higher numbers of activated T cells in the tumor tissue and significantly improved survival rates compared to only DC-TEX vaccinated mice.161 Combining DC-TEX vaccination with sorafenib, a chemotherapeutic drug for advanced HCC, as well as PD-1 antibody, promoted immune responses in orthotopic HCC vaccinated mice. The combination of DC-TEX and sorafenib significantly reduced Treg cells and increased CD8+ T cells, although in this model the combination therapy did not significantly improve the survival rate of vaccinated mice.171 Thus, drug combinations, as a coping strategy for preventing activation and recruitment of immunosuppressive cells and factors should be kept in mind as a possibly strong support in DC-TEX vaccination.
Taken together, DC vaccination keeps the lead role in cancer immunotherapy. Unexpectedly, the mostly immunosuppressive TEXs turn into the currently most efficient “immunogenic” tumor antigen source, provided they are used for DCs loading.
Conclusions, challenges and future direction
Throughout the evolution, the immune system has become a highly efficient organ that keeps the human body’s integrity without external support. With the discovery of the memory of the adaptive immune system of higher developed organisms and the notion of forcing the immunological memory by vaccination, the power of the immune system was expanded toward disease prevention. Yet, despite some progress, the success rates by forcing the immune system to cope with tumor growth and progression remained below expectations. One of the so far most promising anti-tumor vaccination strategies makes use of the professional arm of the innate immune system, DCs. Recent research in this regard center on TEXs, which are tumor cell-derived small vesicles that are well defined for not only promoting tumor progression but also for their immunosuppressive activities. Fortunately, clarifying the underlying mechanisms has not only paved the way for tumor growth prevention but also opened new doors toward using TEXs as immunotherapeutic drugs. The most important features of TEX are their preferential collection of tumor antigens (which is not fully explored yet), their distribution throughout the body, their membrane composition, which facilitates TEX binding and uptake for the good or the bad, and lastly, the efficient delivery of the function-competent TEX content. Furthermore, owing to their membrane organization, uptaken TEXs are preferentially guided toward MVB. TEXs have additional advantages for a wide range of clinical applications. They can be collected by non- or minimally invasive methods from all biological fluids as well as from tumor cell culture supernatants. In addition, long-term storage does not strongly impair TEXs functional competence. Thus far, the therapeutic application of TEXs has mostly been explored in animal studies, which can be divided into two categories: the application of modified TEXs and the use of TEXs as antigen providers for DC in vaccination.
Manipulated TEXs have either been derived from genetically modified tumor cells or have been directly transfected. The former strategy is advantageous for membrane integrated proteins that facilitate binding to ligands on selected target cells. It has the additional advantage of a persisting donor for preparation. Direct loading of TEXs will be the method of choice for transferring high quantities of densely packed therapeutic agents, which could be non-coding RNA (e.g. miRNA), cytokines, and chemokines, as well as immune response checkpoint proteins, antibodies, and cytotoxic drugs. Obviously, combining the genetic modification of the TEX owner cells with direct TEX loading will be beneficial, as TEX content delivery can be targeted toward specified cells. This option has rarely been taken into account and requires a scrutinized analysis of TEX ligands on the aimed for target cell. As far as DC-TEX vaccination is concerned, TEXs manipulation may be required for tumors that display tumor-associated antigens at a level too low for promoting sufficient MHC loading of DC. In many studies, TEXs were used as a vaccine for CD8+ T cell maturation and activation. However, one should be aware that this requires profound engineering to equip TEX with targeting units, cytokines, and sufficient amounts of tumor antigens as well as immunosuppressive molecule blockers. Using TEX after a proceeding vaccination with DC for stimulation of memory CD8+ T cells avoids these drawbacks, will be far less demanding, and may be considered as a clinically most relevant option (Figure 2a).
Figure 2.
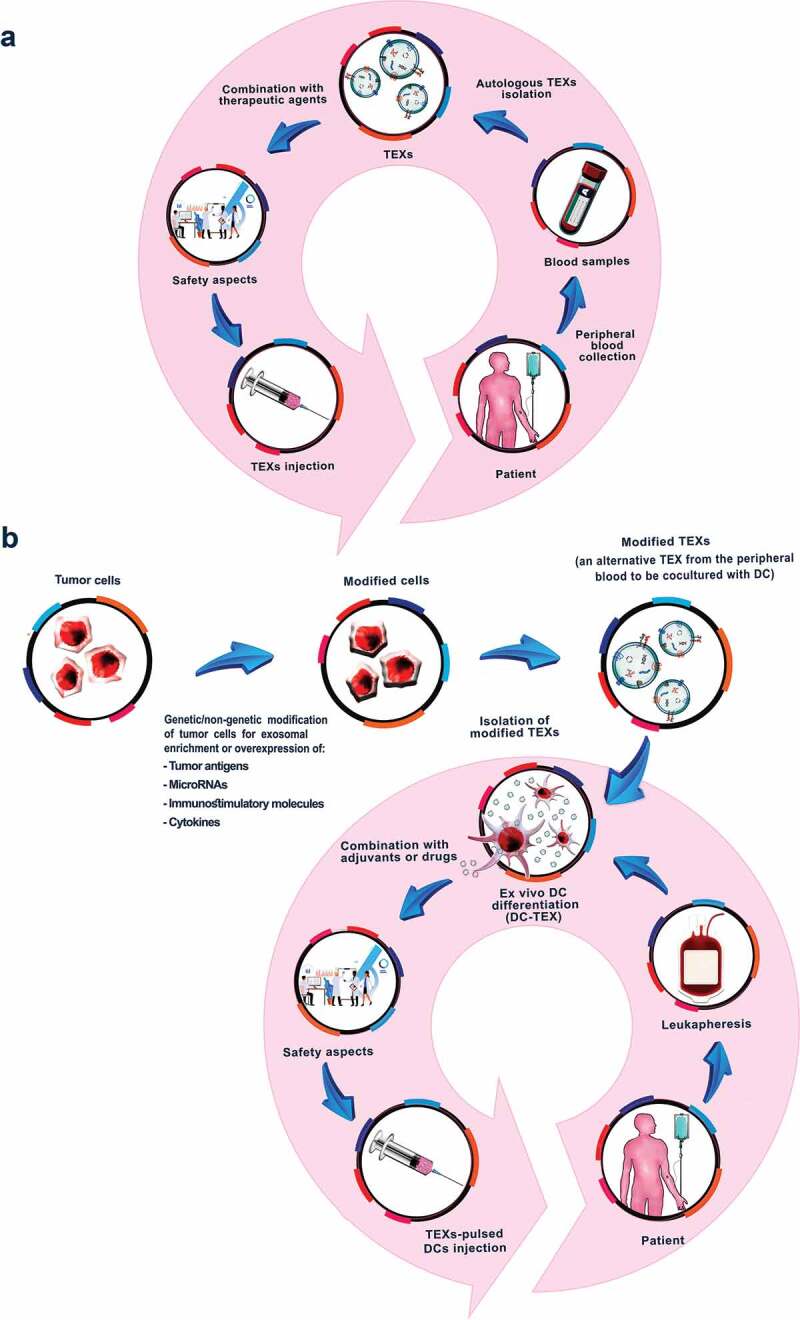
Tumor-derived exosome modulations aiming for increased efficacy of future TEX-based vaccines. (a). Autologous TEXs can be collected from the patients’ peripheral blood. They can be modified by transfection with immune response promoting agents to strengthen the efficacy as vaccine, which treatment may be combined with additional therapeutic agents, either loaded into TEX or independently applied. (b) TEXs can be directly or indirectly modified through overexpression of some tumor antigens, miRNAs, immunostimulatory molecules and cytokines that increase their immunogenic potential. Native or modified TEXs can be used for DC pulsing, the transfer of which provoking a strong immune response, at present, considered as the most effective cancer vaccines. The efficacy of DC-TEX can be supported by cytotoxic drugs, preferentially hampering immunosuppressive cells and agents.
Many studies have demonstrated the efficacy of TEX-loaded DC to induce in vitro and in vivo CTL and T helper cells, to stimulate B cells, NK cells, and macrophages. An important advantage of loading DCs with autologous TEXs is provided by the individual patient’s complete tumor-antigen repertoire being presented. An additional benefit relies on a single peripheral blood collection sufficing for DC and concomitant TEXs preparation. The loading that being performed in vitro at an appropriate stage of DC maturation, does not require support for targeting or processing, and only for TEX from very low level antigen expressing tumors it might be required to additional loading of TEX with an excess of the relevant antigens. Nonetheless, the preparation of DCs under the required safety conditions is cost and time intensive. It remains to be hoped that the pharmacy succeeds in reducing these factors, which so far hamper a desirable wide range of clinical application (Figure 2b).
The wide range clinical application of TEXs as a therapeutic drug or an antigen provider for DC vaccination remains a hopefully soon realized desire. Particularly, the broad field of potentially therapeutic TEXs requires further experimental studies to guarantee efficacy and negligible side effects. Nonetheless, TEXs as a major contributor to tumor progression obviously have a second face, which suggests them as a possibly most efficient tumor defense item. Taken together, there is some hope that TEXs may become the breakthrough in tumor immunotherapy.
References
- 1.Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, González-Fernández Á. Assessment of the evolution of cancer treatment therapies. Cancers. 2011;3(3):3279–18. doi: 10.3390/cancers3033279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A.. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Chen J. Current status and future directions of cancer immunotherapy. J Cancer. 2018;9(10):1773. doi: 10.7150/jca.24577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Willigen WW, Bloemendal M, Gerritsen WR, Schreibelt G, de Vries IJM, Bol KF. Dendritic cell cancer therapy: vaccinating the right patient at the right time. Front Immunol. 2018 Oct 1;9: 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos PM, Butterfield LH. Dendritic cell–based cancer vaccines. J Immunol. 2018;200(2):443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed M, Bae Y-S. Dendritic cell-based therapeutic cancer vaccines: past, present and future. Clin Exp Vaccine Res. 2014;3(2):113–116. doi: 10.7774/cevr.2014.3.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized dendritic cell vaccines—recent breakthroughs and encouraging clinical results. Front Immunol. 2019;10. doi: 10.3389/fimmu.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elster JD, Krishnadas DK, Lucas KG. Dendritic cell vaccines: a review of recent developments and their potential pediatric application. Hum Vaccine Immunother. 2016;12(9):2232–2239. doi: 10.1080/21645515.2016.1179844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escola J-M, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 12.Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 13.Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–211. [PubMed] [Google Scholar]
- 14.Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136(4):E74–E84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- 15.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 16.Andre F, Schartz NEC, Chaput N, Flament C, Raposo G, Amigorena S, Angevin E, Zitvogel L. Tumor-derived exosomes: a new source of tumor rejection antigens. Vaccine. 2002;20:A28–A31. doi: 10.1016/S0264-410X(02)00384-5. [DOI] [PubMed] [Google Scholar]
- 17.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 18.Altieri SL, Khan ANH, Tomasi TB. Exosomes from plasmacytoma cells as a tumor vaccine. J Immunother. 2004;27(4):282–288. doi: 10.1097/00002371-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Hong CS, Muller L, Boyiadzis M, Whiteside TL. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9(8):e103310. doi: 10.1371/journal.pone.0103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palazzolo G, Albanese NN, DI Cara G, Gygax D, Vittorelli ML, Pucci-Minafra I. Proteomic analysis of exosome-like vesicles derived from breast cancer cells. Anticancer Res. 2012;32:847–860. [PubMed] [Google Scholar]
- 21.Xie Y, Zhang H, Li W, Deng Y, Munegowda MA, Chibbar R, Qureshi M, Xiang J. Dendritic cells recruit T cell exosomes via exosomal LFA-1 leading to Inhibition of CD8+ CTL responses through downregulation of peptide/MHC class I and Fas ligand-mediated cytotoxicity. J Immunol. 2010;185(9):5268–5278. doi: 10.4049/jimmunol.1000386. [DOI] [PubMed] [Google Scholar]
- 22.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci. 2003;100(11):6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89(2–3):125–131. doi: 10.1016/S0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 24.Hsu D-H, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, Zitvogel L, Le Pecq J-B. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26(5):440–450. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4(5):594. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 26.Cho JA, Yeo D-J, Son H-Y, Kim H-W, Jung D-S, Ko J-K, Koh JS, Kim Y-N, Kim C-W. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114(4):613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 27.Chaput N, Schartz NEC, André F, Taïeb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172(4):2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 28.Chiang C, Coukos G, Kandalaft L. Whole tumor antigen vaccines: where are we? Vaccines. 2015;3(2):344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaput N, Schartz NEC, Andre F, Zitvogel L. Exosomes for immunotherapy of cancer. In: Handbook of cancer vaccines. Springer; 2004. p. 331–340. Humana Press, Totowa, NJ. [Google Scholar]
- 30.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat‐shocked lymphoma cells. Eur J Immunol. 2006;36(6):1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- 31.Lee YS, Kim SH, Cho JA, Kim CW. Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med. 2011;43(5):281. doi: 10.3858/emm.2011.43.5.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan W, Tian X-D, Huang E, Zhang -J-J. Exosomes from CIITA-transfected CT26 cells enhance anti-tumor effects. Asian Pac J Cancer Prev. 2013;14(2):987–991. doi: 10.7314/APJCP.2013.14.2.987. [DOI] [PubMed] [Google Scholar]
- 33.Yao Y, Wang C, Wei W, Shen C, Deng X, Chen L, Ma L, Hao S. Dendritic cells pulsed with leukemia cell-derived exosomes more efficiently induce antileukemic immunities. PLoS One. 2014;9(3):e91463. doi: 10.1371/journal.pone.0091463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller S, Ridinger J, Rupp A-K, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9(1):86. doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 37.Admyre C, Johansson SM, Qazi KR, Filén -J-J, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Vidaurre S, Eldh M, Larssen P, Daham K, Martinez-Bravo M-J, Dahlén S-E, Dahlén B, van Hage M, Gabrielsson S. RNA-containing exosomes in induced sputum of asthmatic patients. J Allergy Clin Immunol. 2017. e2;140(5):1459–1461. doi: 10.1016/j.jaci.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 41.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107(2):102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 43.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartzt AL. Late endosomes derive from early endosomes by maturation. Cell. 1991;65(3):417–427. doi: 10.1016/0092-8674(91)90459-C. [DOI] [PubMed] [Google Scholar]
- 45.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 46.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 47.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20(1):4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 49.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 50.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 53.Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CIE, Lehtiö J, EL Andaloussi S. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6(1):22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2011;40(D1):D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–692. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta-Gen Subj. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, Boyiadzis M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep. 2017;7(1):14684. doi: 10.1038/s41598-017-14661-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid–binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183(7):1187–1191. doi: 10.1083/jcb.200810038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kharaziha P, Ceder S, Li Q, Panaretakis T. Tumor cell-derived exosomes: a message in a bottle. Biochim Biophys Acta-Rev Cancer. 2012;1826(1):103–111. doi: 10.1016/j.bbcan.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):19. doi: 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;30(1):3.22. 1–3.22. 29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 63.Bu H, He D, He X, Wang K. Exosomes: isolation, analysis, and applications in cancer detection and therapy. Chembiochem. 2019;20(4):451–461. doi: 10.1002/cbic.201800470. [DOI] [PubMed] [Google Scholar]
- 64.de la Torre Gomez C, Goreham RV, Bech Serra JJ, Nann T, Kussmann M. “Exosomics”—a review of biophysics, biology and biochemistry of exosomes with a focus on human breast milk. Front Genet. 2018;9:92. doi: 10.3389/fgene.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carpintero-Fernández P, Fafián-Labora J, O’Loghlen A. Technical advances to study extracellular vesicles. Front Mol Biosci. 2017;4:79. doi: 10.3389/fmolb.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tickner JA, Urquhart AJ, Stephenson S-A, Richard DJ, O’Byrne KJ. Functions and therapeutic roles of exosomes in cancer. Front Oncol. 2014;4:127. doi: 10.3389/fonc.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ciardiello C, Leone A, Budillon A. The crosstalk between cancer stem cells and microenvironment is critical for solid tumor progression: the significant contribution of extracellular vesicles. Stem Cells Int. 2018;2018:1–11. doi: 10.1155/2018/6392198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carnero A, Garcia-Mayea Y, Mir C, Lorente J, Rubio IT, LLeonart ME. The cancer stem-cell signaling network and resistance to therapy. Cancer Treat Rev. 2016;49:25–36. doi: 10.1016/j.ctrv.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS. Concise review: targeting cancer stem cells using immunologic approaches. Stem Cells. 2015;33(7):2085–2092. doi: 10.1002/stem.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madjd Z, Gheytanchi E, Erfani E, Asadi-Lari M. Application of stem cells in targeted therapy of breast cancer: a systematic review. Asian Pac J Cancer Prev. 2013;14(5):2789–2800. doi: 10.7314/APJCP.2013.14.5.2789. [DOI] [PubMed] [Google Scholar]
- 72.Sun Z, Wang L, Dong L, Wang X. Emerging role of exosome signalling in maintaining cancer stem cell dynamic equilibrium. J Cell Mol Med. 2018;22(8):3719–3728. doi: 10.1111/jcmm.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valcz G, Buzás EI, Szállási Z, Kalmár A, Krenács T, Tulassay Z, Igaz P, Molnár B. Perspective: bidirectional exosomal transport between cancer stem cells and their fibroblast-rich microenvironment during metastasis formation. NPJ Breast Cancer. 2018;4(1):18. doi: 10.1038/s41523-018-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, Liao K, Zhou W. Exosomes regulate the transformation of cancer cells in cancer stem cell homeostasis. Stem Cells Int. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, Triviño JC. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2016;7(4):3993. doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 77.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14(1):155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, Liu L. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8:1661. [PMC free article] [PubMed] [Google Scholar]
- 79.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong BS, Cho J-H, Kim H, Choi E-J, Rho S, Kim J, Kim J, Choi D-S, Kim Y-K, Hwang D, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10(1):556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu J-L, Qu X-J, Zhao M-F, Teng Y-E, Zhang Y, Hou K-Z, Jiang Y-H, Yang X-H, Liu Y-P. Gastric cancer exosomes promote tumour cell proliferation through PI3K/Akt and MAPK/ERK activation. Digest Liver Dis. 2009;41(12):875–880. doi: 10.1016/j.dld.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 82.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 83.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt 5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4(11):2057. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, Szegletes Z, Varo G, Siklos L, Katona RL. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Lett. 2012;148(1):34–38. doi: 10.1016/j.imlet.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 86.Kogure T, Lin W-L, Yan IK, Braconi C, Patel T. Intercellular nanovesicle‐mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 89.Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, Lieberman J. miR-200–containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zomer A, Maynard C, Verweij F, Kamermans A, Schäfer R, Beerling E, Schiffelers R, de Wit E, Berenguer J, Ellenbroek S. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Morishige KI, Kurachi H, et al. Exosomes promote ovarian cancer cell invasion through transfer of CD44 to peritoneal mesothelial cells. Mol Cancer Res. 2017 Jan 1;15(1):78-92. [DOI] [PubMed] [Google Scholar]
- 92.Hoshino D, Kirkbride K, Costello K, Clark E, Sinha S, Grega-Larson N, Tyska M, Weaver A. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lazar I, Clement E, Dauvillier S, Milhas D, Ducoux-Petit M, LeGonidec S, Moro C, Soldan V, Dalle S, Balor S. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 2016;76(14):4051–4057. canres. 0651.2016. doi: 10.1158/0008-5472.CAN-16-0651. [DOI] [PubMed] [Google Scholar]
- 95.Millimaggi D, Mari M, D’Ascenzo S, Carosa E, Jannini EA, Zucker S, Carta G, Pavan A, Dolo V. Tumor vesicle—associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9(4):349–357. doi: 10.1593/neo.07133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18(1):39. doi: 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):IN14–IN31. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greening DW, Gopal SK, Mathias RA, Liu L, Sheng J, Zhu H-J, Simpson RJ. Emerging roles of exosomes during epithelial–mesenchymal transition and cancer progression. Semin Cell Dev Biol. 2015;40:60–71. Elsevier. doi: 10.1016/j.semcdb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 100.Vella LJ. The emerging role of exosomes in epithelial–mesenchymal-transition in cancer. Front Oncol. 2014;4:361. doi: 10.3389/fonc.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Valenzuela MMA, Ferguson Bennit HR, Gonda A, Diaz Osterman CJ, Hibma A, Khan S, Wall NR. Exosomes secreted from human cancer cell lines contain inhibitors of apoptosis (IAP). Cancer Microenviron. 2015;8(2):65–73. doi: 10.1007/s12307-015-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang L, Wu X-H, Wang D, Luo C-L, Chen L-X. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8(4):1272–1278. doi: 10.3892/mmr.2013.1634. [DOI] [PubMed] [Google Scholar]
- 103.Khan S, Jutzy JMS, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taverna S, Flugy A, Saieva L, Kohn EC, Santoro A, Meraviglia S, De Leo G, Alessandro R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int J Cancer. 2012;130(9):2033–2043. doi: 10.1002/ijc.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J, De Veirman K, Faict S, Frassanito MA, Ribatti D, Vacca A, Menu E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J Pathol. 2016;239(2):162–173. doi: 10.1002/path.4712. [DOI] [PubMed] [Google Scholar]
- 106.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89(11):1317. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gopal SK, Greening DW, Hanssen EG, Zhu H-J, Simpson RJ, Mathias RA. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget. 2016;7(15):19709. doi: 10.18632/oncotarget.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P. Tumor‐derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. 2019;234(10):16885–16903. doi: 10.1002/jcp.28374. [DOI] [PubMed] [Google Scholar]
- 109.Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22(5):409–417. doi: 10.1080/14728222.2018.1464141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013 Apr 12;288(15):10849–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan G-C. Hypoxic exosomes promote angiogenesis. Blood. 2014;124(25):3669–3670. doi: 10.1182/blood-2014-10-607846. [DOI] [PubMed] [Google Scholar]
- 112.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013 Nov 29;288(48):34343–34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, Li S, Sun W, Deng T, Zhang L. Exosome-derived miR-130a activates angiogenesis in gastric Cancer by targeting C-MYB in vascular endothelial cells. Mol Ther. 2018;26(10):2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Chen W-X, Liu X-M, Lv -M-M, Chen L, Zhao J-H, Zhong S-L, Ji M-H, Hu Q, Luo Z, Wu J-Z, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9(4):e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao L, Liu W, Xiao J, Cao B. The role of exosomes and “exosomal shuttle microRNA” in tumorigenesis and drug resistance. Cancer Lett. 2015;356(2):339–346. doi: 10.1016/j.canlet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 116.Xu C, Yang M-F, Ren Y-Q, Wu C-H, Wang L-Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:4362–4368. [PubMed] [Google Scholar]
- 117.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, De Leon M, Langridge WHR, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37(1):52. doi: 10.1186/s13046-018-0677-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whiteside TL, Mandapathil M, Szczepanski M, Szajnik M. Mechanisms of tumor escape from the immune system: adenosine-producing Treg, exosomes and tumor-associated TLRs. Bull Cancer. 2011;98(2):E25–E31. doi: 10.1684/bdc.2010.1294. [DOI] [PubMed] [Google Scholar]
- 121.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 122.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA* 008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang H-G. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109(10):4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Katsiougiannis S, Chia D, Kim Y, Singh RP, Wong DTW. Saliva exosomes from pancreatic tumor–bearing mice modulate NK cell phenotype and antitumor cytotoxicity. Faseb J. 2016;31(3):998–1010. doi: 10.1096/fj.201600984R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-β1. Haematologica. 2011;96(9):1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia Y, Zhang Q, Zhen Q, Zhao Y, Liu N, Li T, Hao Y, Zhang Y, Luo C, Wu X. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget. 2017;8(23):37783. doi: 10.18632/oncotarget.16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang H-G, Kim H, Liu C, Yu S, Wang J, Grizzle WE, Kimberly RP, Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta Mol Cell Res. 2007;1773(7):1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lv L-H, Wan Y-L, Lin Y, Zhang W, Yang M, Li G-L, Lin H-M, Shang C-Z, Chen Y-J, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Czernek L, Düchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp (Warsz). 2017;65(4):311–323. doi: 10.1007/s00005-016-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song X, Ding Y, Liu G, Yang X, Zhao R, Zhang Y, Zhao X, Anderson GJ, Nie G. Cancer cell-derived exosomes induce mitogen-activated protein kinase-dependent monocyte survival by transport of functional receptor tyrosine kinases. J Biol Chem. 2016;291(16):8453–8464. doi: 10.1074/jbc.M116.716316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology. 2015;4(9):e1027472. doi: 10.1080/2162402X.2015.1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang C, Kim S-H, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6(8):e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiang X, Poliakov A, Liu C, Liu Y, Deng Z-B, Wang J, Cheng Z, Shah SV, Wang G-J, Zhang L. Induction of myeloid‐derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu H, Chen L, Peng Y, Yu S, Liu J, Wu L, Zhang L, Wu Q, Chang X, Yu X, et al. Dendritic cells loaded with tumor derived exosomes for cancer immunotherapy. Oncotarget. 2018;9(2):2887. doi: 10.18632/oncotarget.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ren G, Wang Y, Yuan S, Wang B. Dendritic cells loaded with HeLa-derived exosomes simulate an antitumor immune response. Oncol Lett. 2018;15(5):6636–6640. doi: 10.3892/ol.2018.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taylor DD, Gerçel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-ζ by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9:5113–5119. [PubMed] [Google Scholar]
- 139.Kunigelis K, Graner M. The dichotomy of tumor exosomes (TEX) in cancer immunity: is it all in the ConTEXt? Vaccines. 2015;3(4):1019–1051. doi: 10.3390/vaccines3041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195(10):1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maybruck BT, Pfannenstiel LW, Diaz-Montero M, Gastman BR. Tumor-derived exosomes induce CD8+ T cell suppressors. J Immunother Cancer. 2017;5(1):65. doi: 10.1186/s40425-017-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 143.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 144.Qazi KR, Torregrosa Paredes P, Dahlberg B, Grunewald J, Eklund A, Gabrielsson S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax. 2010;65(11):1016–1024. doi: 10.1136/thx.2009.132027. [DOI] [PubMed] [Google Scholar]
- 145.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory t cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T‐lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer. 2018;6(1):145. doi: 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang C, Chalasani G, Ng Y-H, Robbins PD. Exosomes released from mycoplasma infected tumor cells activate inhibitory B cells. PLoS One. 2012;7(4):e36138. doi: 10.1371/journal.pone.0036138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, Zeidler R. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–648. doi: 10.1007/s00262-011-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bu N, Li Q-L, Feng Q, Sun B-Z. Immune protection effect of exosomes against attack of L1210 tumor cells. Leuk Lymphoma. 2006;47(5):913–918. doi: 10.1080/10428190500376191. [DOI] [PubMed] [Google Scholar]
- 151.Lee E-Y, Park K-S, Yoon YJ, Lee J, Moon H-G, Jang SC, Choi K-H, Kim Y-K, Gho YS. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS One. 2012;7(3):e33330. doi: 10.1371/journal.pone.0033330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahaweni NM, Kaijen-Lambers MEH, Dekkers J, Aerts JGJV, Hegmans JPJJ. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J Extracell Vesicles. 2013;2(1):22492. doi: 10.3402/jev.v2i0.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang Y, Xiu F, Cai Z, Wang J, Wang Q, Fu Y, Cao X. Increased induction of antitumor response by exosomes derived from interleukin-2 gene-modified tumor cells. J Cancer Res Clin Oncol. 2007;133(6):389–399. doi: 10.1007/s00432-006-0184-7. [DOI] [PubMed] [Google Scholar]
- 154.Zhang Y, Luo C-L, He B-C, Zhang J-M, Cheng G, Wu X-H. Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int J Oncol. 2010;36:133–140. [PubMed] [Google Scholar]
- 155.Meazza R, Comes A, Orengo A, Ferrini S, Accolla R. Tumor rejection by gene transfer of the MHC class II transactivator in murine mammary adenocarcinoma cells. Eur J Immunol. 2003;33(5):1183–1192. doi: 10.1002/eji.200323712. [DOI] [PubMed] [Google Scholar]
- 156.Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 157.Klimp A, de Vries EGE, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44(2):143–161. doi: 10.1016/S1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- 158.Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101(11):4485–4491. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 159.Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood. 2001;97(11):3505–3512. doi: 10.1182/blood.V97.11.3505. [DOI] [PubMed] [Google Scholar]
- 160.Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y. Membrane‐bound HSP70‐engineered myeloma cell‐derived exosomes stimulate more efficient CD8+ CTL‐and NK‐mediated antitumour immunity than exosomes released from heat‐shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14(11):2655–2666. doi: 10.1111/j.1582-4934.2009.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiao L, Erb U, Zhao K, Hackert T, Zöller M. Efficacy of vaccination with tumor-exosome loaded dendritic cells combined with cytotoxic drug treatment in pancreatic cancer. Oncoimmunology. 2017;6(6):e1319044. doi: 10.1080/2162402X.2017.1319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X. Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med. 2007;85(5):511–521. doi: 10.1007/s00109-006-0154-1. [DOI] [PubMed] [Google Scholar]
- 163.Temchura VV, Tenbusch M, Nchinda G, Nabi G, Tippler B, Zelenyuk M, Wildner O, Überla K, Kuate S. Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus. Vaccine. 2008;26(29–30):3662–3672. doi: 10.1016/j.vaccine.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Morishita M, Takahashi Y, Matsumoto A, Nishikawa M, Takakura Y. Exosome-based tumor antigens–adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 166.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 167.Cho J-A, Lee Y-S, Kim S-H, Ko J-K, Kim C-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 168.Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20(6):332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taghikhani A, Hassan ZM, Ebrahimi M, Moazzeni S-M. microRNA modified tumor‐derived exosomes as novel tools for maturation of dendritic cells. J Cell Physiol. 2019;234(6):9417–9427. doi: 10.1002/jcp.27626. [DOI] [PubMed] [Google Scholar]
- 170.Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R, Zhou L. Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J Neurooncol. 2011;104(3):659–667. doi: 10.1007/s11060-011-0537-1. [DOI] [PubMed] [Google Scholar]
- 171.Shi S, Rao Q, Zhang C, Zhang X, Qin Y, Niu Z. Dendritic cells pulsed with exosomes in combination with PD-1 antibody increase the efficacy of sorafenib in hepatocellular carcinoma model. Transl Oncol. 2018;11(2):250–258. doi: 10.1016/j.tranon.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


