Abstract
EphA10 (erythropoietin‐producing hepatocellular carcinoma receptor A10) is a catalytically defective receptor protein tyrosine kinase in the ephrin receptor family. Although EphA10 is involved in the malignancy of some types of cancer, its role as an oncogene has not been extensively studied. Here, we investigated the influence of EphA10 on the tumorigenic potential of pancreatic cancer cells. Analysis of expression profiles from The Cancer Genome Atlas confirmed that EphA10 was elevated and higher in tumor tissues than in normal tissues in some cancer types, including pancreatic cancer. EphA10 silencing reduced the proliferation, migration, and adhesion of MIA PaCa‐2 and AsPC‐1 pancreatic cancer cells. These effects were reversed by overexpression of EphA10 in MIA PaCa‐2 cells. Importantly, overexpression and silencing of EphA10 respectively increased and decreased the weight, volume, and number of Ki‐67‐positive proliferating cells in MIA PaCa‐2 xenograft tumors. Further, EphA10 expression was positively correlated with invasion and gelatin degradation in MIA PaCa‐2 cells. Moreover, overexpression of EphA10 enhanced the expression and secretion of MMP‐9 in MIA PaCa‐2 cells and increased the expression of MMP‐9 and the vascular density in xenograft tumors. Finally, expression of EphA10 increased the phosphorylation of ERK, JNK, AKT, FAK, and NF‐κB, which are important for cell proliferation, survival, adhesion, migration, and invasion. Therefore, we suggest that EphA10 plays a pivotal role in the tumorigenesis of pancreatic epithelial cells and is a novel therapeutic target for pancreatic cancer.
Keywords: EphA10, pancreatic cancer, receptor protein tyrosine kinase, tumorigenesis, xenograft
The role of a catalytically defective receptor protein tyrosine kinase EphA10 for tumorigenesis has been investigated in pancreatic cancer cells. Overexpression and knockdown of EphA10 respectively increased and decreased the proliferation, migration, adhesion, and invasion of MIA PaCa‐2 and AsPC‐1 pancreatic cancer cells as well as the weight and volume of MIA PaCa‐2 xenograft tumors. Our data suggest that EphA10 plays a pivotal role in tumorigenesis of pancreatic epithelial cells and is a novel therapeutic target for pancreatic cancer.
1. INTRODUCTION
The erythropoietin‐producing hepatocellular carcinoma (Eph) receptors are the largest family of receptor protein tyrosine kinases (RPTKs). 1 Unlike other members of RPTK families that bind to soluble ligands, Eph receptors mediate contact‐dependent cell‐cell communication through interaction with plasma membrane‐anchored ligands, called ephrins, on adjacent cells. The 14 human Eph receptors are divided into 2 classes based on their ephrin specificity. 2 Nine EphA receptors (EphA1‐A8 and EphA10) interact with 5 GPI‐linked ephrin‐A ligands (ephrinA1‐A5), and 5 EphB receptors (EphB1‐B4 and EphB6) interact with 3 transmembrane ephrin‐B ligands (ephrinB1‐B3). 3 Of note, EphA4 and EphB2 can also bind to ephrin‐B and ephrin‐A5 ligands, respectively. 3 Binding of Eph receptors to ephrins on juxtaposed cell surfaces induces forward signaling that leads to receptor oligomerization, receptor trans‐phosphorylation, activation of tyrosine kinases, and recruitment of downstream signaling proteins, such as Rho GTPases, in the Eph receptor‐expressing cells. 3 In addition to forward signaling, Eph receptors can also induce reverse signaling in the ephrin‐expressing cells by phosphorylation of ephrins and/or recruitment of signaling proteins. 3
Eph receptors and ephrins regulate adult tissue homeostasis and developmental processes. 1 , 4 Therefore, aberrant regulation of Eph receptors and ephrins has been implicated in various diseases, including cancer. Interestingly, upregulation or downregulation of specific Eph receptors can be associated with either tumor promotion or suppression, depending on the context. 5 This promiscuity occurs through varied interactions between phosphorylated receptors and intracellular proteins that are involved in cell proliferation and survival pathways.
Of the Eph receptors, EphB6 and EphA10 have alterations in essential motifs that contribute to their catalytic tyrosine kinase activity, leaving them catalytically defective. 3 EphB6 is known to play dual roles as a tumor suppressor or an oncogene, depending on the cell type and expression level. Upregulation of EphB6 in breast cancer reportedly reduces the expression of matrix metalloprotease (MMP)‐7 and MMP‐19 and increases the expression of TIMP‐2, thereby decreasing invasiveness. 6 Although EphB4 enhances invasiveness without EphB6, the combination of EphB4 and EphB6 suppresses invasiveness in breast cancer cells. 7 However, in triple‐negative breast cancer, EphB6 potentiates tumor initiation, promotes the maintenance of tumor‐initiating cell populations, and augments drug sensitivity. 8 Additionally, EphB6 overexpression, together with APC gene mutations, enhances proliferation, invasion, and metastasis in colorectal epithelial cells. 9
Unlike EphB6, EphA10 is only suggested as an oncogene. EphA10 is overexpressed in prostate and breast cancers 10 , 11 ; whereas, its expression is not detected in normal tissues, with the exception of the testis. 10 , 12 Moreover, EphA10 expression positively correlates with malignant transformation and expression of programmed death‐ligand 1 (PD‐L1) in breast tissue. 13 , 14 EphA10 is also expressed in prostate cancer cell lines, and anti‐EphA10 monoclonal antibodies are cytotoxic in prostate cancer VCaP cells. 11 Although EphA10 is known to increase the malignancy of some types of cancer, its role as an oncogene has not been extensively studied.
To identify cancer types in which EphA10 plays a role as an oncogene, we surveyed EphA10 expression using The Cancer Genome Atlas (TCGA) database. Of the cancer types with high levels of EphA10 mRNA, we chose to investigate the oncogenic potential of EphA10 in pancreatic cancer, a well‐known refractory malignancy. We analyzed oncogenic phenotypes following knockdown and overexpression of EphA10 in pancreatic cancer cell lines as well as in a xenograft mouse model. In addition, we assessed the involvement of EphA10 and the corresponding gelatinolytic enzymes in invasiveness. Finally, the effect of EphA10 expression on activation of signaling proteins involved in proliferation, survival, adhesion, and invasion was analyzed. Together, our results from these studies suggest that EphA10 is an important target for the detection and treatment of pancreatic cancer.
2. MATERIALS AND METHODS
2.1. Analysis of public dataset
A preprocessed RNA‐seq count matrix of 20 165 specimens in the TCGA database was obtained from the UCSC Xenabrowser (https://xenabrowser.net/) in the Genomic Data Commons (GDC; https://gdc.cancer.gov/). Gene expression was quantitated as fragments per kilobase of transcript per million mapped reads upper quartile (FPKM‐UQ), which is an RNA‐Seq‐based expression normalization method. 15 The EphA10 mRNA level is depicted as log2(FPKM‐UQ + 1). For certain types of cancer, a paired analysis was performed on EphA10 mRNA levels in normal and tumor tissues.
2.2. Cell culture
Human pancreatic adenocarcinoma PANC‐1, MIA PaCa‐2, and AsPC‐1 cells; breast cancer MDA‐MB‐436 cells; and melanoma MDA‐MB‐435 cells were purchased from the Korea Cell Line Bank (Seoul, Korea). All cells were grown in Dulbecco's modified Eagle medium (DMEM; HyClone) supplemented with 10% bovine serum (BS) for HEK293T cells or 10% fetal bovine serum (FBS) for other cells, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cells were maintained at 37°C in 5% CO2 in air.
2.3. Xenografts
MIA PaCa‐2 cells expressing EphA10‐FLAG or EphA10 shRNA (EphA10‐KD‐341 and 387) (1 × 106 cells/site) were resuspended in growth‐factor‐reduced Matrigel (BD Biosciences) and injected subcutaneously on the backs of 4~5‐wk‐old male immune‐deficient athymic nude (BALB/c nu/nu) mice, which were purchased from Orient Bio Inc. The tumor volume was measured weekly using calipers (length × width × depth/2). After 6 wk, the xenograft tumors were recovered to measure tumor weight and to prepare paraffin tissue blocks.
Details of the experimental methods are provided in the Appendix S1.
3. RESULTS
3.1. EphA10 is upregulated in various cancers, including pancreatic cancer
We analyzed EphA10 mRNA levels in various cancer types using RNA‐seq data from TCGA database. Of the 34 analyzed cancer types, the median level of EphA10 mRNA was higher in 17 cancer types than the overall mean [log2(FPKM‐UQ + 1) = 12.33] of all cancer tissues (Figure 1A). Paired EphA10 mRNA levels from normal and tumor tissues were available in 9 of the 17 cancer types (Figure 1B). Of note, the median levels of EphA10 mRNA were higher in tumor tissues than in normal tissues in 7 of the 9 cancer types, including breast invasive carcinoma, cholangiocarcinoma, lung adenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, stomach adenocarcinoma, and thyroid carcinoma (Figure 1B). These data confirm that EphA10 expression is upregulated in specific cancer types.
FIGURE 1.
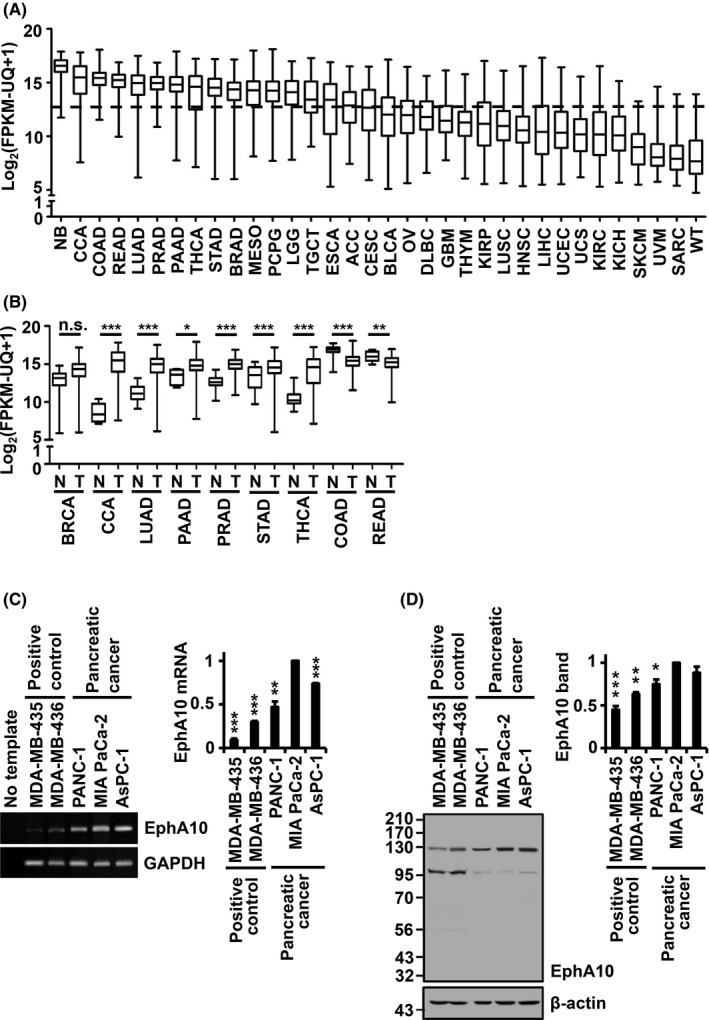
Expression level of EphA10 in various cancer tissues and pancreatic cancer cell lines. A, EphA10 mRNA levels were examined in tumor tissues of 34 cancer types in TCGA database. The horizontal dashed line indicates the overall mean value (12.33) of EphA10 mRNA levels of all cancer types tested. B, EphA10 mRNA levels between normal (N) and tumor (T) tissues were compared in 9 cancer types that demonstrated EphA10 mRNA levels above the mean EphA10 mRNA level. *P < .05, **P < .01, ***P < .001 vs normal tissue. C, D, The level of EphA10 expression was assessed in PANC‐1, MIA PaCa‐2, and AsPC‐1 pancreatic cancer cells. Melanoma MDA‐MB‐435 and breast cancer MDA‐MB‐436 cells were included as positive controls. The EphA10 mRNA level was analyzed by conventional RT‐PCR (C, left panel) and quantitative real‐time RT‐PCR of 3 independent samples (C, right panel). No template control was included as a negative control of RT‐PCR. The EphA10 polypeptide level was analyzed by western blot (D, left panel) and the 130‐kDa EphA10 band in 3 independent samples was quantified (D, right panel). GAPDH and β‐actin were used as endogenous controls for RT‐PCR and western blots, respectively. *P < .05, **P < .01, ***P < .001 vs MIA PaCa‐2 cells. TCGA abbreviations: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CCA, cholangiocarcinoma; CESC, cervical squamous cell carcinoma; COAD, colon adenocarcinoma; DLBC, lymphoid neoplasm diffuse large B cell lymphoma; ESCA, esophageal carcinoma; GBM, glioblastoma multiforme; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; NB, neuroblastoma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PCPG, pheochromocytoma and paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma; WT, high‐risk Wilms tumor
In this study, we selected pancreatic adenocarcinoma for continued investigation, because the 5‐y survival rate of pancreatic cancer patients is extremely low. 16 To begin characterizing EphA10 function in pancreatic adenocarcinoma, we assessed the expression of EphA10 in pancreatic carcinoma PANC‐1, AsPC‐1, and MIA PaCa‐2 cell lines. We found that the mRNA and protein levels of EphA10 were higher in pancreatic carcinoma cell lines compared with breast cancer MDA‐MB‐436 and melanoma MDA‐MB‐435 cell lines, which are known to express EphA10 (Figure 1C,D).
3.2. Knockdown of EphA10 reduces proliferation, migration, and adhesion of pancreatic cancer cells
To examine the effect of EphA10 downregulation on the oncogenic properties of pancreatic cancer cells, EphA10 was knocked down in pancreatic cancer MIA PaCa‐2 and AsPC‐1 cells by infection with a lentivirus encoding EphA10 shRNAs (EphA10‐KD‐341 and ‐387). EphA10 knockdown with EphA10‐KD‐341 and ‐387 reduced EphA10 expression in MIA PaCa‐2 cells to 57.1 ± 6.5% and 37.8 ± 6.1%, respectively, and to 51.9 ± 2.8% and 35.8 ± 6.1% in AsPC‐1 cells, respectively (Figure 2A,B). EphA10 knockdown by EphA10‐KD‐341 and ‐387 decreased cell proliferation to 77.7 ± 7.7% and 55.5 ± 4.2% in MIA PaCa‐2 cells and to 75.7 ± 5.5% and 65.4 ± 5.3% in AsPC‐1 cells, respectively, compared with the control vector at 3 d of culture (Figure 2C,D). EphA10 knockdown by EphA10‐KD‐341 and ‐387 also reduced adhesion to a collagen‐coated surface to 78.1 ± 4.8% and 66.9 ± 5.1% % in MIA PaCa‐2 cells and to 80.1 ± 1.0% and 70.0 ± 4.5% % in AsPC‐1 cells in comparison with control cells (Figure 2E,F). In addition, EphA10 knockdown by EphA10‐KD‐341 and ‐387 reduced cell migration, was measured using a gelatin‐coated Boyden chamber, as 81.4 ± 2.3% and 69.5 ± 1.6% in MIA PaCa‐2 cells and as 81.4 ± 3.1% and 55.5 ± 2.3% in AsPC‐1 cells, respectively, compared with control cells (Figure 2G,H). Moreover, EphA10 knockdown by EphA10‐KD‐341 and ‐387 also reduced wound healing, measured 2 d after wound on a monolayer, to 64.5 ± 1.1% and 46.9 ± 5.9% in MIA PaCa‐2 cells and 40.4 ± 15.6% and 29.9 ± 10.0% in AsPC‐1 cells, respectively, compared with control cells (Figure 2I,J). Thus, EphA10 knockdown substantially reduced the tumorigenic potential of MIA PaCa‐2 and AsPC‐1 cells.
FIGURE 2.
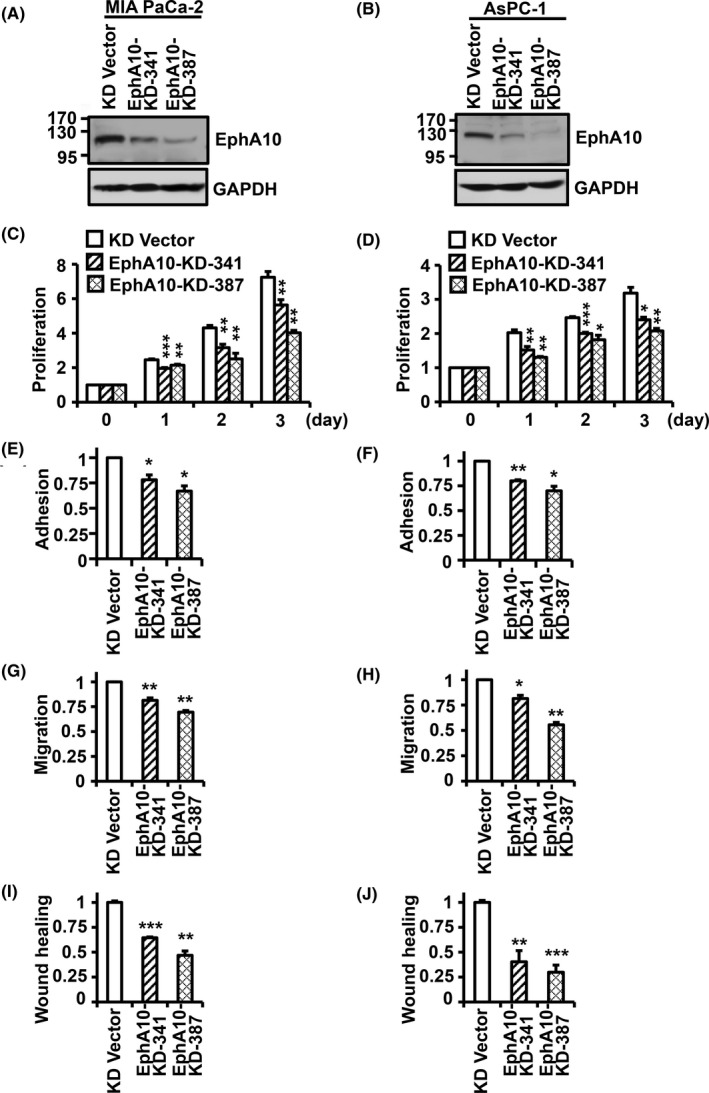
Effect of EphA10 knockdown on the oncogenic properties of pancreatic cancer MIA PaCa‐2 and AsPC‐1 cells. The level of EphA10 expression in MIA PaCa‐2 (A) and AsPC‐1 (B) cells infected with lentiviruses for control (KD Vector) and EphA10 knockdown shRNA (EphA10‐KD‐341 and EphA10‐KD‐387) was analyzed by western blotting. Proliferation of the control and EphA10‐knockdown MIA PaCa‐2 (C) and AsPC‐1 (D) cells was analyzed for 3 d. Adhesion of the control and EphA10‐knockdown MIA PaCa‐2 (E) and AsPC‐1 (F) cells was analyzed 1 h after plating. Migration of the control and EphA10‐knockdown MIA PaCa‐2 (G) and AsPC‐1 (H) cells was analyzed for 24 h. Wound healing of the control and EphA10‐knockdown MIA PaCa‐2 (I) and AsPC‐1 (J) cells was analyzed for 48 h. Each bar represents the mean ± standard deviation (SD) from 3 independent experiments. *P < .05, **P < .01, ***P < .001 vs 0 d (C, D) or control vector (E‐J)
3.3. Overexpression of EphA10 increases proliferation, migration, and adhesion in pancreatic cancer cells
Because EphA10 knockdown was found to impair the oncogenic phenotypes of pancreatic cancer cells, we next investigated the impact of EphA10 overexpression in MIA PaCa‐2 cells. Infection of an EphA10‐FLAG lentivirus into MIA PaCa‐2 cells increased the 130‐kDa EphA10 level to 194.4 ± 5.5% (Figure 3A). Overexpression of EphA10 increased cell growth to 157.8 ± 38% and 176.9 ± 15% at 1 and 2 d of culture, respectively, compared with the control vector (Figure 3B). Compared with the control vector, EphA10 overexpression also increased the adhesion, migration, and wound healing of MIA PaCa‐2 cells by 147.99 ± 2.9%, 234.5 ± 11.3% and 127.7 ± 3.0%, respectively (Figure 3C‐E). These results demonstrated that knockdown and overexpression of EphA10 had directly opposing effects on the tumorigenic potential of pancreatic cancer cells and suggested that EphA10 acts as an oncogene.
FIGURE 3.
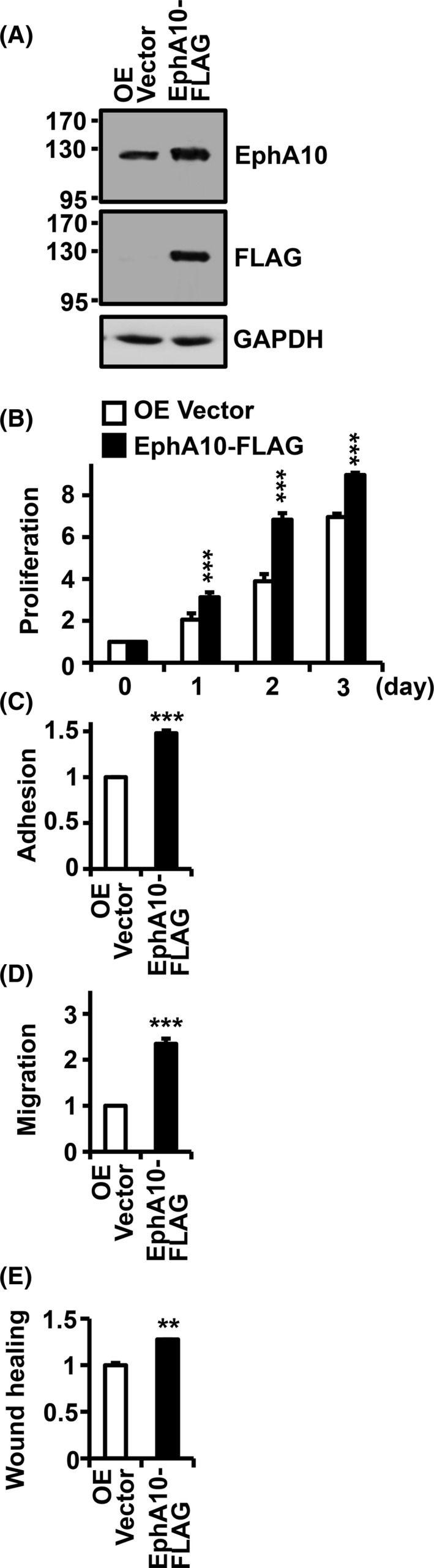
Effect of EphA10 overexpression on the oncogenic properties of MIA PaCa‐2 cells. A, The level of EphA10 expression in pancreatic cancer MIA PaCa‐2 cells infected with lentiviruses for control (OE Vector) and EphA10‐FLAG was analyzed by western blotting with EphA10 and FLAG antibodies. Proliferation (B), adhesion (C), migration (D), and wound healing (E) of the control and EphA10‐overexpressing MIA PaCa‐2 cells were analyzed using the same methods as in the knockdown cells. Each bar represents the mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001 vs 0 d (B) or control vector (C, D)
3.4. EphA10 is positively associated with tumorigenic potential of pancreatic cancer cells in vivo
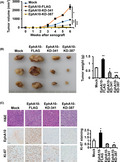
To investigate the tumorigenic ability of EphA10 in vivo, we transplanted mock, EphA10‐FLAG, EphA10‐KD‐341, or EphA10‐KD‐387 MIA PaCa‐2 cells into immune‐deficient athymic nude mice. During the xenograft period, the tumor volume increased significantly with EphA10 overexpression and decreased significantly with EphA10 knockdown compared with the mock control (Figure 4A). At 6 wk after the transplantation, animals were sacrificed, and tumor weights were recorded. The weight of the tumor mass increased to 181.6 ± 8.6% in EphA10‐overexpressing tumors and decreased to 50.7 ± 13.2% and 22.0 ± 5.4% in EphA10‐silenced tumors with EphA10‐KD‐341 and EphA10‐KD‐387, respectively, compared with the mock control (Figure 4B).
FIGURE 4.
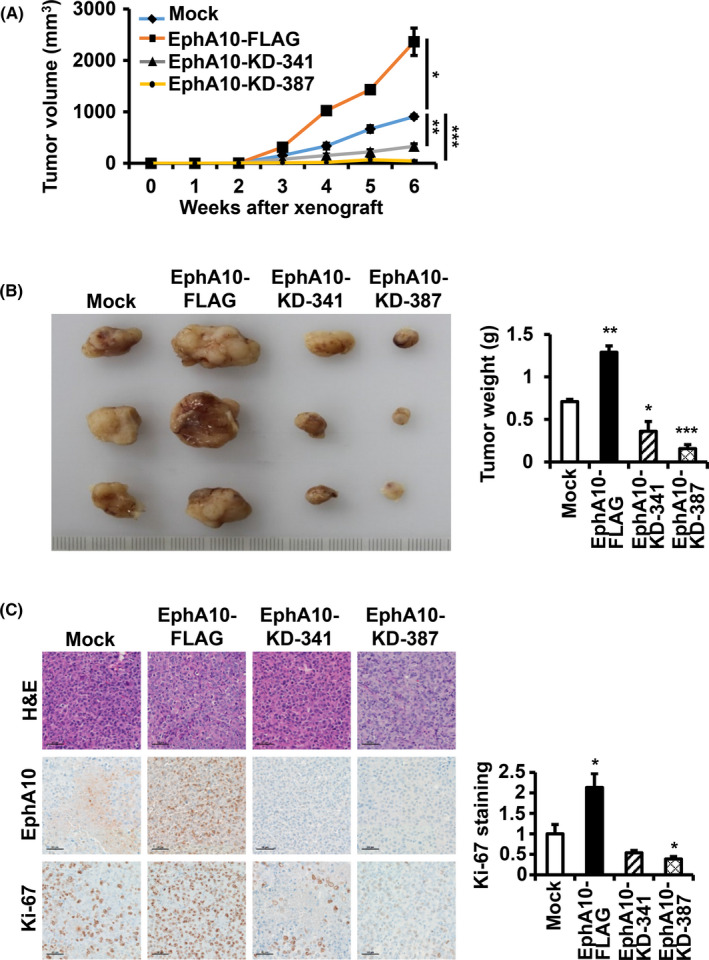
Effect of overexpression and knockdown of EphA10 on tumor growth in a xenograft model. Recipient mice were injected with MIA PaCa‐2 cells mock‐transfected, overexpressing (EphA10‐FLAG), or silencing (EphA10‐KD‐341 and ‐387) EphA10. A, Tumor volumes were recorded for the 6‐wk duration. B, Representative tumors taken from mice at 6 wk post‐injection and the corresponding weights (g) are shown. C, Representative images of H&E staining as well as IHC staining for EphA10, and Ki‐67 are shown. The scale bar represents 50 μm. The relative intensity of Ki‐67 staining is quantified. Each bar represents the mean ± SD of 3 mice. *P < .05, **P < .01, ***P < .001 vs mock‐transfected cells
To evaluate cell morphology and proliferation, we performed hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) staining for EphA10 and Ki‐67 in tumor sections. EphA10 staining confirmed that EphA10 levels were elevated in EphA10‐FLAG tumors and were reduced in EphA10‐KD‐341 and −387 tumors compared with the mock control (Figure 4C). Importantly, we found that Ki‐67 staining, a marker of proliferation, was stronger (213.2 ± 34.0%) in EphA10‐overexpressing tumors and was weaker (53.6 ± 5.9% in EphA10‐KD‐341 and 38.7 ± 6.0% in EphA10‐KD‐387) in EphA10‐knockdown tumors compared with the control tumors (Figure 4C). These results confirmed that EphA10 is an important oncogene in pancreatic cancer in vivo as well as in vitro.
3.5. EphA10 increases invasion through induction of MMP‐9 secretion in MIA PaCa‐2 cells
To analyze whether EphA10 is also involved in the invasiveness of pancreatic cancer cells, a chemotactic invasion assay was performed in MIA PaCa‐2 cells with overexpressing or silencing EphA10. Overexpression of EphA10 significantly increased the invasion of MIA PaCa‐2 cells to 305.8 ± 20.3% of the control; whereas, knockdown of EphA10 with EphA10‐KD‐341 and ‐387 significantly decreased invasion to 81.2 ± 7.4% and 71.2 ± 4.4% of the control, respectively (Figure 5A). To analyze whether gelatinolytic enzymes were involved in the invasion, we performed a fluorescence gelatin degradation assay with MIA PaCa‐2 cells overexpressing or silencing EphA10. We found that EphA10 overexpression increased the degradation of FITC‐labeled gelatin to 171.7 ± 21.6% of the control, and EphA10 knockdown with EphA10‐KD‐341 and ‐387 decreased gelatin degradation to 55.3 ± 10.4% and 23.0 ± 3.5% of the control, respectively (Figures 5B and S1). To identify the gelatinase that was involved in the EphA10‐mediated gelatin degradation, conditioned medium from MIA PaCa‐2 cells overexpressing or silencing EphA10 was analyzed by gelatin zymography (Figure 5C). MMP‐9 secretion was significantly increased by EphA10 overexpression to 175.4 ± 9.0% of the control, and decreased by EphA10 knockdown with EphA10‐KD‐341 and ‐387 to 48.5 ± 4.2% and 12.8 ± 2.7% of the control, respectively. In contrast, MMP‐2 secretion was not detected in the medium of MIA PaCa‐2 cells. Moreover, the MMP‐9 mRNA level was also significantly increased by EphA10 overexpression to 206.1 ± 12.5% of the control, and decreased by EphA10 knockdown with EphA10‐KD‐341 and ‐387 to 29.8 ± 1.9% and 8.5 ± 1.5% of the control, respectively (Figure 5D). MMP‐9 knockdown did not change the expression of EphA10, but significantly reduced invasion of MIA PaCa‐2 cells to 21.6 ± 4.3% in MIA PaCa‐2 control cells and to 14.08 ± 1.44% (from 301.8 ± 11.6% to 42.5 ± 4.3%) in EphA10‐overexpressing cells (Figure S2), which indicated the importance of MMP‐9 in the invasion. These results demonstrated that EphA10 enhances invasion of MIA PaCa‐2 cells, at least in part, by inducing MMP‐9 expression.
FIGURE 5.
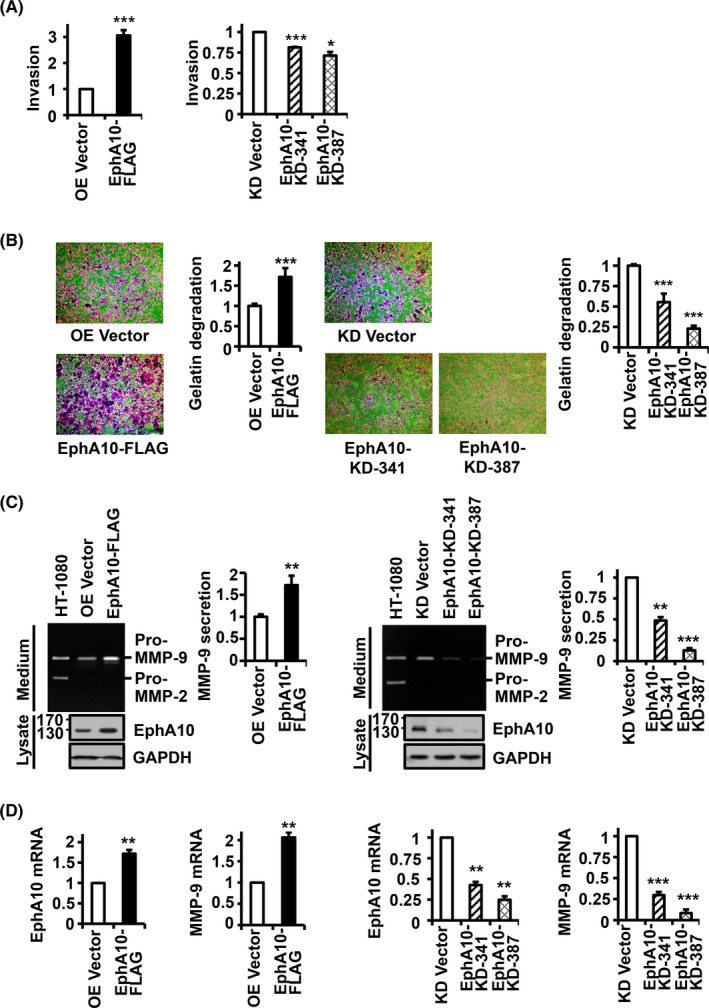
Effect of EphA10 overexpression and knockdown on invasion, gelatin degradation, and gelatinolytic MMP expression in MIA PaCa‐2 cells. MIA PaCa‐2 cells were infected with lentiviruses for control (OE Vector) and EphA10 overexpression (EphA10‐FLAG) (left panels) as well as for control (KD Vector) and EphA10 knockdown shRNA (EphA10‐KD‐341 and ‐387) (Right panels). A, Invasion of MIA PaCa‐2 cells overexpressing or silencing EphA10 after 24 h in Matrigel‐coated Boyden chambers. B, Cells were plated on FITC‐gelatin‐coated cover glasses and incubated for 48 h at 37°C. Cells were then stained with rhodamine‐phalloidin and DAPI and analyzed by fluorescence microscopy (×100). Merged images were shown here and monochrome images in Figure S1. Relative gelatin degradation was shown as FITC‐gelatin‐degraded area normalized to DAPI intensity in EphA10‐overexpressing or ‐silencing cells compared with control cells. C, Levels of secreted gelatinolytic MMPs, MMP‐2 and MMP‐9, and levels of EphA10 were analyzed by gelatin zymography and western blotting in conditioned media and cell lysates, respectively. The conditioned medium of HT‐1080 cells was used as a positive control to show the anticipated positions of MMP‐2 and MMP‐9. D, Relative mRNA levels of EphA10 and MMP‐9 were analyzed using quantitative RT‐PCR in EphA10‐overexpressing or ‐silencing cells compared with control cells. Each bar represents the mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001 vs vector control
In IHC analysis of MIA PaCa‐2 xenograft tumors, the MMP‐9 level in EphA10‐overexpressing xenograft tumors was increased to 195.8 ± 4.8% in comparison with the mock control and those in EphA10‐silenced xenograft tumors with EphA10‐KD‐341 and ‐387 were decreased to 49.3 ± 4.7 and 38.0 ± 3.9%, respectively (Figure 6A). The vascular density measured by CD31 staining was 170.3 ± 18.8% in EphA10‐overexpressing xenograft tumors, compared with the mock control, and was 70.3 ± 6.0 and 38.2 ± 6.2% in EphA10‐silenced xenograft tumors with EphA10‐KD‐341 and ‐387, respectively (Figure 6B). These results further supported the idea that EphA10 enhanced MMP‐9 induction and tumor angiogenesis for increased invasion in xenograft tumors of MIA PaCa‐2 cells.
FIGURE 6.
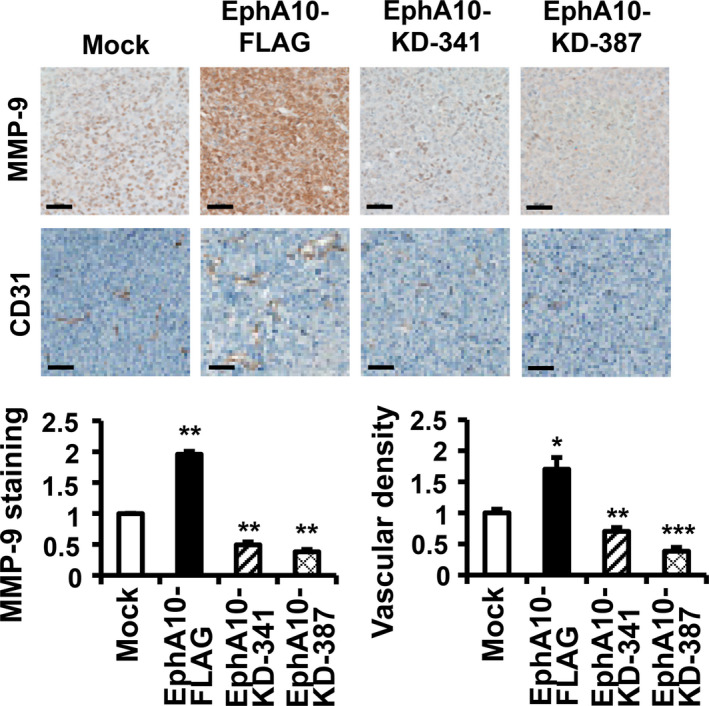
Analysis of MMP‐9 level and vascular density in the xenograft model for EphA10. Representative images of IHC staining for MMP‐9 and CD‐31 in the tumor of xenograft model using MIA PaCa‐2 cells mock‐transfected, overexpressing (EphA10‐FLAG), or silencing (EphA10‐KD‐341 and ‐387) of EphA10 are shown. The scale bars represent 50 μm. The relative intensity of the MMP‐9 and CD‐31 (vascular density) staining is quantified below the images. Each bar represents the mean ± SD of 3 mice. *P < .05, **P < .01, ***P < .001 vs mock‐transfected cells
3.6. EphA10 enhances the activation of cellular signaling proteins
Cell proliferation, survival, migration, adhesion, and invasion result from activation of various signaling pathways, including MAP kinases, PI3‐kinase/AKT, and FAK pathways. 17 , 18 , 19 In addition, transactivation of MMP9 expression involves activation of the transcription factors, NF‐κB and AP‐1, which can be readily detected by phosphorylation of NF‐κB, ERK, and JNK. 20 , 21 Therefore, we investigated phosphorylation of these signaling proteins in MIA PaCa‐2 cells overexpressing or silencing EphA10. The phosphorylation of ERK, JNK, AKT, FAK, and NF‐κB was substantially increased by EphA10 overexpression and decreased by EphA10 knockdown (Figure 7). These results indicated that EphA10 activates the signaling pathways for cell proliferation, survival, migration, adhesion, and invasion, resulting in increased tumorigenesis of pancreatic cancer cells.
FIGURE 7.
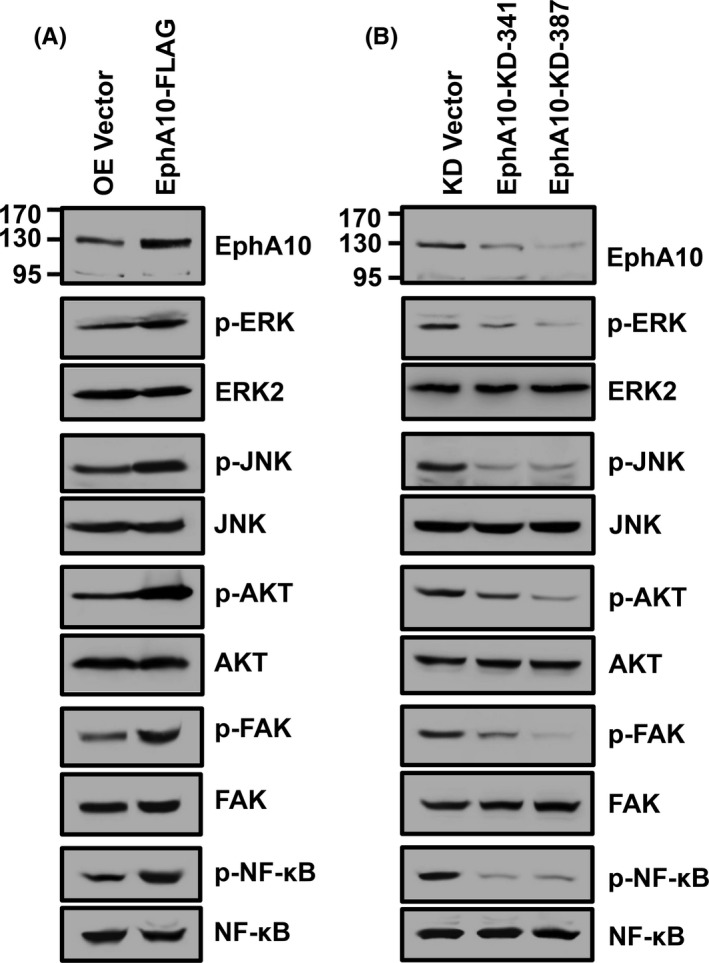
Effect of overexpression and knockdown of EphA10 on activation of signaling molecules in MIA PaCa‐2 cells. Phosphorylation of ERK, JNK, AKT, FAK, and NF‐κB was examined by western blotting in MIA PaCa‐2 cells infected with lentiviruses for control (OE Vector) and EphA10 overexpression (EphA10‐FLAG) (A) as well as for control (KD Vector) and EphA10 knockdown shRNA (EphA10‐KD‐341 and ‐387) (B)
EphA10 has been reported to interact with EphA7 in breast cancer cells. 22 We thus analyzed whether EphA10 interacts with and activates EphA7 in MIA PaCa‐2 cells. EphA7 was poorly expressed but was clearly detectable in MIA PaCa‐2 cells. In reciprocal co‐immunoprecipitation assays, EphA10 did not interact EphA7 (Figure S3A). Furthermore, overexpression of EphA10 did not change the level of EphA7 phosphorylation while EphA7 was phosphorylated in MIA PaCa‐2 cells (Figure S3B). Therefore, the oncogenic effects of EphA10 in MIA PaCa‐2 cells are unlikely to be mediated by EphA7.
4. DISCUSSION
EphA10 is poorly expressed or is not expressed in most human adult tissues. 12 Although upregulation and oncogenic role of EphA10 have been reported in prostate and breast cancers, 10 , 13 , 23 its expression and tumorigenic role in other cancers are poorly understood. Here, we examined EphA10 mRNA expression in 34 cancer types using TCGA database and found that EphA10 mRNA expression was higher in 17 cancer types, including breast, prostate, colon, gastric, pancreas, and lung cancers, compared with the overall mean EphA10 mRNA level. Importantly, EphA10 mRNA levels were higher in tumor tissues compared with normal tissues in 7 out of 9 cancer types that demonstrated higher EphA10 mRNA levels than the overall mean of all cancer patients. Among these cancer types, we investigated the role of EphA10 in pancreatic cancer because the early detection and development of therapeutic agents for pancreatic cancer are important.
The expression of EphA10 mRNA and polypeptide was higher in PANC‐1, MIA PaCa‐2, and AsPC‐1 pancreatic cancer cells compared with breast cancer MDA‐MB‐436 cells and melanoma MDA‐MB‐435 cells, which have been reported to have high EphA10 expression. 10 There are 2 main EphA10 isoforms: EphA10 (GenBank NM_001099439.2), which encodes the full‐length molecule of 1008 amino acids, and soluble EphA10 (GenBank NM_173641.2), which encodes the first 283 amino acids and includes the ephrin‐binding domain. In our western blot analysis of pancreatic cancer cells, an antibody recognizing the N‐terminal region of EphA10 detected a strong signal at 130 kDa and a weak signal at 97 kDa (Figure 1D). Based on the knowledge that mature EphA10 lacking the signal peptide has a calculated molecular weight of 106 kDa and contains 2 potential glycosylation sites at the Asn311 and Asn486 residues, the 130‐kDa band is likely to correspond to full‐length EphA10. In fact, a recombinant full‐length EphA10 was also detected at 130 kDa. The 97‐kDa band, observed in the previous report, 13 is expected to be a stable product truncated from the full‐length EphA10. Soluble EphA10 found at approximately 40 kDa in mammary epithelial MCF‐10A cells 13 was not detected in the pancreatic cancer cells tested. We have therefore focused on the function of full‐length EphA10 in pancreatic cancer cells.
EphA10 is upregulated in prostate cancer DU‐145, LNCap, PC‐3, and VCaP cells as well as in breast cancer MDA‐MB‐231 and MDA‐MB‐436 cells compared with their respective normal epithelial cells. 10 , 11 , 13 , 24 A neutralizing monoclonal antibody to EphA10 has been shown to inhibit ephrin‐dependent proliferation in breast cancer MDA‐MB‐436 cells 10 and to cause complement‐dependent cytotoxicity in prostate cancer VCaP cells. 11 EphA10 overexpression promotes the migration of breast cancer MDA‐MB‐231 cells and the growth of MDA‐MB‐231 xenograft tumors, which are both reduced following EphA10 knockdown. 13 Similarly, we found that EphA10 knockdown in pancreatic cells reduced proliferation, migration, and adhesion, which were all enhanced by EphA10 overexpression. In addition, in vivo analysis of MIA PaCa‐2 xenograft tumors revealed that the mass, volume, and number of Ki‐67‐positive proliferating cells were higher in EphA10‐overexpressing xenograft tumors and were reduced in EphA10‐silencing xenograft tumors compared with the mock control.
Invasion and tumor metastasis are closely related and both occur in vivo in the context of the local tumor‐host extracellular matrix, which stimulates migration, proliferation, and survival of tumor cells. 25 EphA10 expression is upregulated in the more invasive prostate cancer PC‐3 ML cells compared with the less invasive PC‐3 cells. 24 EphA10 expression is also associated with tumor stage progression and lymph node metastasis in breast cancer. 23 One of the major features of pancreatic cancer is its early systemic dissemination followed by extraordinary local tumor growth. 25 Therefore, identification of target molecules involved in the invasion and metastasis of pancreatic cancer cells is clinically important. Here, we demonstrate that EphA10 overexpression increases the invasion of MIA PaCa‐2 cells; whereas, EphA10 knockdown decreases invasion. Type IV collagen is essential for the stability of the basement membrane. Loss of type IV collagen in the basement membrane is associated with increased expression of the gelatinolytic MMPs, MMP‐2 and MMP‐9, in solid human tumors. 26 , 27 In our study, EphA10 expression was associated with gelatin degradation and MMP‐9 induction in MIA PaCa‐2 cells. In addition, IHC staining of tissue sections from MIA PaCa‐2 xenograft tumors in mice showed that EphA10 overexpression and silencing respectively increased and decreased MMP‐9 expression as well as vascular density suggesting tumor angiogenesis. Our findings strongly suggest that EphA10 is an important regulator of invasion and metastasis of pancreatic cancer cells.
Activation of MAP kinases, such as ERK and JNK, and activation of AKT promotes cell proliferation, survival, and migration. 28 , 29 FAK is also involved in cell adhesion, migration, and invasion. 30 In addition, NF‐κB promotes cell proliferation, epithelial‐mesenchymal transition, and basement membrane breakdown, thus leading to invasion and metastasis. 31 Although EphA10 has no catalytic activity, we found that EphA10 enhanced the phosphorylation of signaling proteins, including ERK, JNK, AKT, FAK, and NF‐κB. These results suggested that EphA10 directly or indirectly activates catalytically active RPTK members. Catalytically inactive ERBB3 (HER3) binds to other active members of the ERBB family, such as ERBB2 (HER2), and activates downstream oncogenic signaling pathways. 32 PTK7, another catalytically defective RPTK, also binds to other catalytically active RPTKs, such as KDR and FGFR1, to enhance angiogenesis and tumorigenesis. 33 , 34 , 35 Eph receptors often form heterodimers with other members of the Eph receptor family. 5 , 36 , 37 For example, EphA10 interacts with EphA7, and the EphA10‐EphA7 complex induces expression of tumor‐promoting genes in breast cancer MDA‐MB‐231 cells. 22 However, we found that EphA10 did not activate EphA7 in MIA PaCa‐2 cells. Nevertheless, EphA10 is likely to bind catalytically active members of the Eph receptor family or different RPTK family members to enhance the phosphorylation of downstream oncogenic signaling proteins.
To our knowledge, this is the first report to demonstrate that levels of EphA10 positively correlate with the tumorigenic potential of pancreatic cancer cells both in vitro and in vivo. EphA10 has been identified as a therapeutic target in breast and prostate cancers, and neutralizing monoclonal antibodies to EphA10 as well as EphA10 antibody‐drug conjugates have been developed. 10 , 11 , 14 , 38 , 39 Our results suggested that EphA10 may be a valuable therapeutic target to treat refractory pancreatic cancer and other cancers that are positive for EphA10 expression. Counteracting EphA10 function by siRNA or with a neutralizing monoclonal antibody presents a promising strategy for treating these devastating diseases and should be pursued in future studies.
DISCLOSURE
The authors declare no conflicts of interest.
Supporting information
Figure S1
Figure S2
Figure S3
Appendix S1
ACKNOWLEDGMENTS
This work was supported by grants from NRF of Korea (2016R1A2B4007904 and 2019M3A9A8065054) and BK21 PLUS (Initiative for Biological Function and Systems, Yonsei University). WSS is a recipient of the postdoctoral trainee program (2019) and the Yonsei Frontier Laboratory Young Researcher Supporting Program (2018) of the Yonsei University Research Fund.
Shin W‐S, Park MK, Lee YH, Kim KW, Lee H, Lee S‐T. The catalytically defective receptor protein tyrosine kinase EphA10 promotes tumorigenesis in pancreatic cancer cells. Cancer Sci. 2020;111:3292–3302. 10.1111/cas.14568
Funding information
National Research Foundation of Korea, (Grant/Award Number: “2016R1A2B4007904,” “2019M3A9A8065054”).
REFERENCES
- 1. Barquilla A, Pasquale EB. Eph receptors and ephrins: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2015;55:465‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rohani N, Parmeggiani A, Winklbauer R, Fagotto F. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 2014;12:e1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liang LY, Patel O, Janes PW, Murphy JM, Lucet IS. Eph receptor signalling: from catalytic to non‐catalytic functions. Oncogene. 2019;38:6567‐6584. [DOI] [PubMed] [Google Scholar]
- 4. Darling TK, Lamb TJ. Emerging Roles for Eph Receptors and Ephrin Ligands in Immunity. Front Immunol. 2019;10:1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013;5:a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox BP, Kandpal RP. EphB6 receptor significantly alters invasiveness and other phenotypic characteristics of human breast carcinoma cells. Oncogene. 2009;28:1706‐1713. [DOI] [PubMed] [Google Scholar]
- 7. Truitt L, Freywald A. Dancing with the dead: Eph receptors and their kinase‐null partners. Biochem Cell Biol. 2011;89:115‐129. [DOI] [PubMed] [Google Scholar]
- 8. Toosi BM, El Zawily A, Truitt L, et al. EPHB6 augments both development and drug sensitivity of triple‐negative breast cancer tumours. Oncogene. 2018;37:4073‐4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu D, Yuan L, Liu X, et al. EphB6 overexpression and Apc mutation together promote colorectal cancer. Oncotarget. 2016;7:31111‐31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagano K, Maeda Y, Kanasaki S, et al. Ephrin receptor A10 is a promising drug target potentially useful for breast cancers including triple negative breast cancers. J Control Release. 2014;189:72‐79. [DOI] [PubMed] [Google Scholar]
- 11. Nagano K, Yamashita T, Inoue M, et al. Eph receptor A10 has a potential as a target for a prostate cancer therapy. Biochem Biophys Res Commun. 2014;450:545‐549. [DOI] [PubMed] [Google Scholar]
- 12. Aasheim HC, Patzke S, Hjorthaug HS, Finne EF. Characterization of a novel Eph receptor tyrosine kinase, EphA10, expressed in testis. Biochim Biophys Acta. 2005;1723:1‐7. [DOI] [PubMed] [Google Scholar]
- 13. Li Y, Jin L, Ye F, et al. Isoform expression patterns of EPHA10 protein mediate breast cancer progression by regulating the E‐Cadherin and beta‐catenin complex. Oncotarget. 2017;8:30344‐30356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang WH, Cha JH, Xia W, et al. Juxtacrine signaling inhibits antitumor immunity by upregulating PD‐L1 expression. Cancer Res. 2018;78:3761‐3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahriyari L. Effect of normalization methods on the performance of supervised learning algorithms applied to HTSeq‐FPKM‐UQ data sets: 7SK RNA expression as a predictor of survival in patients with colon adenocarcinoma. Brief Bioinform. 2019;20:985‐994. [DOI] [PubMed] [Google Scholar]
- 16. Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marampon F, Ciccarelli C, Zani BM. Biological rationale for targeting MEK/ERK pathways in anti‐cancer therapy and to potentiate tumour responses to radiation. Int J Mol Sci. 2019;20:2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanteti R, Batra SK, Lennon FE, Salgia R. FAK and paxillin, two potential targets in pancreatic cancer. Oncotarget. 2016;7:31586‐31601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase‐9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013;28:391‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shin WS, Hong Y, Lee HW, Lee ST. Catalytically defective receptor protein tyrosine kinase PTK7 enhances invasive phenotype by inducing MMP‐9 through activation of AP‐1 and NF‐kappaB in esophageal squamous cell carcinoma cells. Oncotarget. 2016;7:73242‐73256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson C, Segovia B, Kandpal RP. EPHA7 and EPHA10 physically interact and differentially co‐localize in normal breast and breast carcinoma cell lines, and the co‐localization pattern is altered in EPHB6‐expressing MDA‐MB‐231 Cells. Cancer Genomics Proteomics. 2016;13:359‐368. [PMC free article] [PubMed] [Google Scholar]
- 23. Nagano K, Kanasaki S, Yamashita T, et al. Expression of Eph receptor A10 is correlated with lymph node metastasis and stage progression in breast cancer patients. Cancer Med. 2013;2:972‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox BP, Tabone CJ, Kandpal RP. Potential clinical relevance of Eph receptors and ephrin ligands expressed in prostate carcinoma cell lines. Biochem Biophys Res Commun. 2006;342:1263‐1272. [DOI] [PubMed] [Google Scholar]
- 25. Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bauvois B. New facets of matrix metalloproteinases MMP‐2 and MMP‐9 as cell surface transducers: outside‐in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29‐36. [DOI] [PubMed] [Google Scholar]
- 27. Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP‐2 and MMP‐9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20:749‐755. [DOI] [PubMed] [Google Scholar]
- 28. Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5:a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shin WS, Shim HJ, Lee YH, et al. PTK6 localized at the plasma membrane promotes cell proliferation and MigratiOn through phosphorylation of Eps8. J Cell Biochem. 2017;118:2887‐2895. [DOI] [PubMed] [Google Scholar]
- 30. Tai YL, Chen LC, Shen TL. Emerging roles of focal adhesion kinase in cancer. Biomed Res Int. 2015;2015:690690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia Y, Shen S, Verma IM. NF‐kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism‐based cancer therapeutics. Cancer Cell. 2014;25:282‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin WS, Na HW, Lee ST. Biphasic effect of PTK7 on KDR activity in endothelial cells and angiogenesis. BBA ‐ Mol Cell Res. 2015;1853:2251‐2260. [DOI] [PubMed] [Google Scholar]
- 34. Shin WS, Lee HW, Lee ST. Catalytically inactive receptor tyrosine kinase PTK7 activates FGFR1 independent of FGF. FASEB J. 2019;33:12960‐12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shin WS, Gim J, Won S, Lee ST. Biphasic regulation of tumorigenesis by PTK7 expression level in esophageal squamous cell carcinoma. Sci Rep. 2018;8:8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fox BP, Kandpal RP. A paradigm shift in EPH receptor interaction: biological relevance of EPHB6 interaction with EPHA2 and EPHB2 in breast carcinoma cell lines. Cancer Genomics Proteomics. 2011;8:185‐193. [PubMed] [Google Scholar]
- 37. Janes PW, Griesshaber B, Atapattu L, et al. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J Cell Biol. 2011;195:1033‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taki S, Kamada H, Inoue M, et al. A novel bispecific antibody against human CD3 and ephrin receptor A10 for breast cancer therapy. PLoS One. 2015;10:e0144712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang J, Yang C, Pan S, et al. Eph A10‐modified pH‐sensitive liposomes loaded with novel triphenylphosphine‐docetaxel conjugate possess hierarchical targetability and sufficient antitumor effect both in vitro and in vivo. Drug Deliv. 2018;25:723‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Appendix S1


