Abstract
Aging represents the major risk factor for cancer. Cancer and aging are characterized by a similar dysregulated metabolism consisting in upregulation of glycolysis and downmodulation of oxidative phosphorylation. In this respect, metabolic interventions can be viewed as promising strategies to promote longevity and to prevent or delay age-related disorders including cancer. In this review, we discuss the most promising metabolic approaches including chronic calorie restriction, periodic fasting/fasting-mimicking diets, and pharmacological interventions mimicking calorie restriction.
Metabolic interventions can also be viewed as adjuvant anticancer strategies to be combined to standard cancer therapy (chemotherapeutic agents, ionizing radiation, and drugs with specific molecular target), whose major limiting factors are represented by toxicity against healthy cells but also limited efficacy easily circumvented by tumor cells. In fact, conventional cancer therapy is unable to distinguish normal and cancerous cells and thus causes toxic side effects including secondary malignancies, cardiovascular and respiratory complications, endocrinopathies, and other chronic conditions, that resemble and, in some cases, accelerate the age-related disorders and profoundly affect the quality of life.
In this scenario, geroscience contributes to the understanding of the mechanisms of protection of normal cells against a cytotoxic agent and finding strategies focused on the preserving healthy cells while enhancing the efficacy of the treatment against malignant cells.
1. INTRODUCTION
Aging represents the major risk factor for cancer. In fact, the incidence of cancer increases with age due to accumulation of mutations. This association can be explained not only by the multihit or Knudson hypothesis, according to which tumor cells need to accumulate the mutations responsible for genome instability and carcinogenesis, but also by a decline of homeostasis occurring during aging (Knudson, 1971; Wu et al., 2014b). The discovery of the role of protooncogenes in accelerating aging and promoting cellular sensitization to stress has encouraged the investigation of metabolic interventions able to promote longevity but also useful in generating differential protection and sensitization of normal and cancer cells, respectively. In this review, we will discuss the most promising treatments including chronic calorie and protein restriction, fasting and fasting-mimicking diets (FMD), and pharmacological interventions mimicking calorie restriction (CR) relevant to both aging and cancer.
In addition to their antiaging effects, the above-mentioned strategies can also function as adjuvant approaches to be combined with standard cancer treatments in order to protect healthy cells and tissues and sensitize malignant cells to cytotoxic agents. In fact, one of the major limitations of cancer therapy, including chemotherapy, ionizing radiation, and target-specific drugs, is their inability or limited ability to distinguish normal and cancerous cells. This aspect is associated with the appearance of long-term and often serious side effects that not only impair the quality of life but may also accelerate the aging process. For this reason, geroscience, whose purpose is to understand the mechanisms of damage and protection during aging, could lead to novel interventions able to protect the host from the standard cancer treatment while enhancing its efficacy.
2. DYSREGULATION OF METABOLISM IN CANCER AND AGING
2.1. Mitochondrial Homeostasis in Cancer
Dysregulation of metabolism represents one of the hallmarks of tumorigenesis. In particular, cancer cells reprogram their carbon metabolism by upregulating glycolysis (Warburg effect) and glutaminolysis and by downregulating oxidative phosphorylation (OXPHOS). These events lead to the increase of biosynthetic intermediates such as nucleotides, amino acids, and lipids which are necessary for cell proliferation and survival (Vander Heiden et al., 2009). Moreover, disruption of mitochondrial homeostasis causes accumulation of reactive oxygen species (ROS) which can contribute to cancer progression (Hamanaka and Chandel, 2010).
In accordance with the normal function of their encoded proteins, oncogenes or tumor suppressors regulate cellular metabolism (Cairns et al., 2012). For example, Myc and phosphatidyl inositol 3-kinase (PI3K) are potent inducers of glutaminolysis and glucose uptake, respectively (Elstrom et al., 2004; Wise et al., 2008), while the tumor suppressor p53 inhibits glucose transporters (GLUT), glycolysis, pentose phosphate pathway, de novo fatty acid synthesis, and enhances mitochondrial OXPHOS and tricarboxylic acid (TCA) cycle rate (Wang et al., 2014). A similar effect on the regulation of metabolism is caused by the tumor suppressor liver kinase B1 (LKB1) that is frequently mutated in Peutz-Jegher’s syndrome, characterized by an increased risk of gastrointestinal cancer. LKB1 encodes for a serine-threonine kinase that activates the energetic sensor AMP-dependent kinase (AMPK) and downmodulates the regulator of growth and proliferation known as mammalian target of rapamycin (mTOR) (Shackelford and Shaw, 2009). Von Hippel Lindau disease is another example of disorders associated to cancers and caused by mutations in the Vhl gene, which inhibits the hypoxia-inducible factor α (HIF-α) (Kapitsinou and Haase, 2008; Maxwell et al., 1999). HIF-α is a transcriptional factor which suppresses oxidative metabolism and promotes glycolysis (Semenza, 2010).
In the last 10 years, several genetic defects affecting TCA enzymes associated to oncogenesis have been described. In particular, loss-of-function mutations or changes in the amino acid residues have been identified in the cytoplasmic and mitochondrial isoforms of the enzymes succinate dehydrogenase (SDH), isocitrate dehydrogenase (IDH), and fumarate hydratase (FH) (Cardaci and Ciriolo, 2012).
The SDH complex is an highly conserved heterotetrameric tumor suppressor, composed by two catalytic subunits (SDHA and SDHB) and two hydrophobic subunits (SDHC and SDHD) (Bardella et al., 2011), which have been found mutated in patients affected by different malignancies including hereditary paragangliomas, pheochromocytomas, gastrointestinal stromal, thyroid and renal tumors, neuroblastoma, and testicular seminoma (Astuti et al., 2001; Bardella et al., 2011; Baysal et al., 2000).
FH is a homotetrameric TCA cycle enzyme, which catalyzes the hydration of fumarate to L-malate. Heterozygous FH mutations predispose to multiple cutaneous and uterine leiomyomas, hereditary leiomyomatosis, renal cell cancer, breast, bladder, as well as Leydig cell tumors (Carvajal-Carmona et al., 2006; Lehtonen et al., 2006; Tomlinson et al., 2002).
IDH is a member of the β-decarboxylating dehydrogenase family, which catalyzes the oxidative decarboxylation of isocitrate to produce 2-oxoglutarate (α-KG). Three isoforms have been identified so far: the cytosolic IDH1, and the mitochondrial IDH2 and IDH3. Mutations associated to IDH1 and IDH2 have been identified in 70% of grade II–III gliomas, secondary glioblastomas, acute myeloid leukemia, angioimmunoblastic T-cell lymphomas, thyroid, colorectal, and prostate cancer (Abbas et al., 2010; Cairns et al., 2012; De Carli et al., 2009; Yen et al., 2010). As a result of gain-of-function mutations, IDH1 and IDH2 are unable to efficiently convert isocitrate into α-KG and acquire a neomorphic catalytic activity that allows a NADPH-dependent reduction of α-KG into the oncometabolite (R)-2-hydroxyglutaric acid ((R)-2HG) (Dang et al., 2010; Ward and Thompson, 2012). The mechanisms underlying tumorigenesis in cancers characterized by TCA cycle enzyme mutations involve the accumulation of metabolites (succinate, fumarate, and (R)-2-HG) that convey oncogenic signals (oncometabolites) (Yang et al., 2013). In particular, the abnormal accumulation of (R)-2HG mediates its potential tumorigenic effects via several mechanisms: (i) inhibition of ten-eleven translocation family (TET) of dioxygenases and histone lysine demethylase (KDM) which results into enhanced CpG island and histone methylation and the consequent remodeling of the cancer cell epigenome toward an undifferentiated and aggressive phenotype, (ii) inhibition of collagen prolyl and lysyl hydroxylases causing impaired collagen maturation and disrupted basement membrane formation, and (iii) inhibition of HIF-α prolyl hydroxylase (PHD) interactions causing a decrease of HIF-1α degradation and an enhancement of pseudohypoxic condition. In addition, accumulation of fumarate and succinate participates in oncogenic signaling through: (i) modification of cysteine residues in proteins which confers a state of constitutive activation of the antioxidant defense pathway mediated by NF-E2-related factor (NRF2), that generates a reductive milieu and promotes cell proliferation; (ii) inhibition of the reactions involved in arginine and purine synthesis; (iii) epigenetic alterations by inhibition of TET and KDM proteins; and (iv) accumulation of HIF-1α which in its turn promotes aerobic glycolysis and angiogenesis (Selak et al., 2005).
Furthermore, succinate has recently emerged as a key player in the promotion of inflammation which is functionally associated to cancer development and progression. In particular, proinflammatory macrophages shift their metabolism from OXPHOS to glycolysis resulting in succinate accumulation and oxidation by SDH, which drives ROS production that, finally, leads to increase of HIF-1α and proinflammatory cytokines including IL-1β (Mills et al., 2016).
A recent study highlighted the importance of the oncometabolite fumarate as a driver of tumorigenesis (Sciacovelli et al., 2016) by discovering that accumulation of fumarate, associated to FH loss, induces epithelial mesenchymal transition (EMT), a well-known process involved in cancer initiation, dissemination, and metastasis. Specifically, fumarate has been shown to inhibit TET-mediated demethylation of a regulatory region of the antimetastatic miRNA cluster mir-200ba429, leading to the expression of EMT-related transcription factors and enhanced migratory properties (Sciacovelli et al., 2016).
Beside the above-mentioned oncometabolites, ADP-ribose, which is synthesized by poly-ADP-ribose polymerases (PARPs) from NAD(+) and is responsible for protein posttranslational modifications, can be considered as an oncometabolite as well. A recent study demonstrated that nuclear pyruvate kinase M2 (PK)M2 binds directly to ADP-ribose, and this poly-ADP-ribose-binding capability is critical for its nuclear localization. Accordingly, PARP inhibition prevents nuclear retention of PKM2 and therefore suppresses cell proliferation and tumor growth (Li et al., 2016).
The general mechanism underlying tumor metabolism dysregulation is still under investigation. The prevailing point of view is that the reprogramming of tumor metabolism (Warburg-like effect) occurs after cancer cells accumulate key mutations, promoting additional genome instability (Vander Heiden et al., 2009). According to alternative hypothesis, known as geroncogenesis, during age, normal cells undergo natural alterations in oxidative metabolism, with the consequent increased generation of ROS, which promote additional mutations and tumorigenesis. The latter hypothesis may help explain why aging is the major risk factor for most tumors (Wu et al., 2014b).
2.2. Geroncogenesis and Gerometabolites: The Pseudohypoxic Aging Side of Oncometabolites
Consistent with geroncogenesis, aging in mammals is characterized by metabolic alterations similar to those associated with cancer cells, and by a reduction of OXPHOS as a result of alteration of specific electron transport chain (ETC) complexes as well as an increase in aerobic glycolysis in different tissues (Bowling et al., 1993; Hagen et al., 1997; Trounce et al., 1989). For example, in humans, age leads to a decrease in cytochrome oxidase activity in brain and heart (Muller-Hocker, 1989; Ojaimi et al., 1999). Along this line, the activity of complex V decreases in the heart of the Fischer 344 rats and is accompanied by structural changes during aging. In contrast, complexes I and III activity remain unaltered in the heart, liver, and skeletal muscle of mice during aging (Kwong and Sohal, 1998, 2000). As a result of alterations, aging is associated with a higher production of ROS due to a decreased flux through the ETC. This event reduces the activity of upstream complexes, especially complexes I and III, enhancing “electron leak” that generates ROS (Chen and Lesnefsky, 2006). The increased production of ROS by mitochondria leads to greater oxidative damage within mitochondria, including protein sulfhydryl oxidation, lipid peroxidation, and mitochondrial (mt)DNA damage (Floyd et al., 2001; Van Remmen and Richardson, 2001). In support of the importance of mitochondrial-derived ROS to the aging process, it has been shown that interventions and mutations that prolong survival tend to decrease the production of ROS from mitochondria (Chen et al., 2007; de Cabo et al., 2014; Lagouge and Larsson, 2013).
Similar metabolic dysregulations have been observed in type 2 diabetes: an age-related disorder characterized by accumulation of HIF-1α and a decline of OXPHOS (Petersen et al., 2004; Ptitsyn et al., 2006).
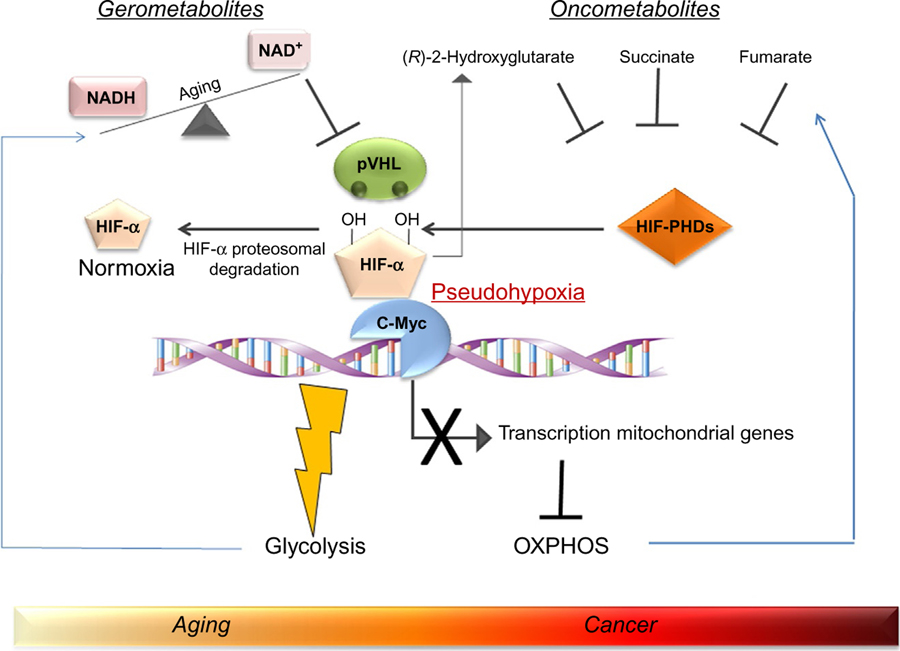
An additional possible link between aging and tumorigenesis is supported by the suggestive theory according to which the so-called “gerometabolites” (defined as small-molecule components of normal metabolism whose depletion drives aging) promote the chronic accumulation of oncometabolites which in turn are responsible for pathological metabolic reprogramming. In this scenario, nuclear NAD+, a central metabolic cofactor that decreases during aging (Verdin, 2015), plays a pivotal role. NAD+ is cosubstrate of sirtuins, a family of NAD+-dependent deacetylases that are regulators of metabolism in cancer and aging. Specifically, sirtuin 1 deacetylases HIF-1α and promotes its interaction with the ubiquitin ligase VHL which leads to the degradation of HIF-1α (Gomes et al., 2013; Haase, 2009). The decline of NAD+ during aging represents the causal inducer of the accumulation of HIF-1α which promotes a pseudohypoxic state that reduces the carboxylation of α-ketoglutarate. This event leads to the production of the oncometabolite (R)-2HG (Gomes et al., 2013), that, together with the other oncometabolites fumarate and succinate, creates a pseudohypoxic state. The latter condition promotes the activation of c-Myc which is responsible for repressing the transcription of mitochondrial genes. As a consequence, a reprogramming of metabolism characterized by promotion of glycolysis and inhibition of OXPHOS occurs. This scenario, as depicted in Fig. 1, offers the possible connection between aging and cancer where the conjunction ring is represented by pseudohypoxia that is induced by gerometabolites. Pseudohypoxia is then associated with the generation of oncometabolites which block the differentiation and promote stemness through epigenetic mechanisms (Menendez et al., 2014). In this context, several report indicate that hypoxia favors the increase in the cancer stem cell (CSC) pool through HIF-1α and HIF-2α which, in turn, amplify the CSC pool and induce additional dedifferentiation of tumor cells (Carnero and Lleonart, 2016; Keith and Simon, 2007; Li et al., 2009). In particular, hypoxia and HIFs induce stemness in differentiated progenitors and non-CSCs by inducing the expression of genes such as OCT4, SOX2, and NANOG, or the activation of the Notch signaling pathway that regulates cell self-renewal and differentiation (Bennewith and Durand, 2004). The molecular mechanisms that underlie the impact of hypoxic conditions on the CSC compartment require either HIF-1α expression or the inactivation of the hydroxylase activity of PHD domain-containing protein 3 (PHD3) whose loss prevents CSC differentiation and induces dedifferentiation of mature tumor cells (Iriondo et al., 2015). Finally, HIF-1α mediates telomerase transcription in hypoxic cancer cells, maintaining the immortal life span of the tumor mass. In fact, 90% of tumors show increased telomerase activity, suggesting that it is an important factor in the maintenance of CSC properties (Blanco et al., 2007). HIF-α and pseudohypoxia have been also implicated in aging although in Caenorhabditis elegans this issue is a matter of debate because HIF-α has been shown to function as both a positive and negative modulator of aging (Leiser and Kaeberlein, 2010). There is also evidence that HIF-α plays a relevant role in mammalian aging. In particular, activation of HIF-1α, which is accompanied by increased HIF-1α DNA binding and activation of transcription of HIF-1α-dependent genes, has been observed in aged rat liver (Kang et al., 2005). Another study examining PHD3 in rats found that PHD3 levels increase with age in liver, heart, and skeletal muscle, and this increase correlates with a decrease in HIF-1α activity (Rohrbach et al., 2008). Finally, HIF-1α seems to have a contradictory role in age-related disorders; in fact, studies examining Aβ accumulation in Alzheimer’s disease suggest that increase of HIF-1α tends to be protective in disease-free states, but that increased HIF-1α levels are also a sign of advanced disease progression (Ogunshola and Antoniou, 2009). These seemly conflicting results might be due to the fact that in stress response HIF-1α upregulation can be either protective or reactive in nature (Ogunshola and Antoniou, 2009).
Fig. 1.
The link between metabolites and oncometabolites in aging and cancer. Metabolites, such as NAD+, which decline as we age, cause the accumulation of oncometabolites through the induction of a pseudohypoxia condition (Gomes et al., 2013). This latter event occurs as a consequence of an increased HIF-1α stabilization due to the ubiquitin ligase pVHL inhibition. Oncometabolites inhibit the interaction between HIF-1α and prolyl hydroxylases (PHDs) resulting in a decreased HIF-1α degradation. As a consequence, oncometabolites generate a pseudohypoxia condition as other metabolites do. This event converges on c-Myc activation, which represses the transcription of mitochondrial genes resulting in glycolysis promotion and oxidative phosphorylation (OXPHOS) inhibition.
2.3. Sirtuins: Regulators of Metabolism of Cancer and Aging
Sirtuins are mono-ADP-ribosyltransferase and beta-nicotinamide adenine dinucleotide (NAD+)-dependent lysine deacetylase enzymes that play key roles in the regulation of metabolism, inflammation, and DNA repair and are critical regulators at the crossroads between cancer and aging (Chalkiadaki and Guarente, 2015; Saunders and Verdin, 2007). The mechanisms underlying the protective effect of sirtuins in aging and cancer include: (1) protection against DNA damage and oxidative stress and (2) protection against accumulation of mutations and genomic instability (Saunders and Verdin, 2007). In fact, the loss of sirtuin expression, activity, or regulation allows cell division to proceed without the proper repair of DNA, resulting in accumulation of mutations and genomic instability which can lead to tumor development. More recently, sirtuin proteins have also been found to finely regulate energy metabolism. In particular, sirtuin 3 facilitates TCA cycle, OXPHOS, and fatty acid metabolism (Hirschey et al., 2010). For example, deletion of Sirtuin 3 causes spontaneous formation of mammary tumors and a metabolic reprogramming characterized by increased glucose uptake and decreased ATP generation (Kim et al., 2010). This metabolic switch is accompanied by an enhanced production of ROS that stabilize HIF-1α, which in its turn promotes glycolysis and reduces OXPHOS (Bell et al., 2011; Finley et al., 2011). The role of sirtuin 3 as a tumor suppressor is however controversial since other studies indicated that sirtuin 3 is overexpressed in breast cancer compared to normal tissues (Alhazzazi et al., 2011; Ashraf et al., 2006).
Another sirtuin protein involved in tumor metabolism regulation is sirtuin 6, whose deletion is associated with an increase in HIF-1α and c-Myc, leading to activation of glycolysis (Sebastian et al., 2012; Zhong et al., 2010). Similarly, sirtuin 1, which was the first family member identified as a tumor suppressor that delays lymphoma and protects mice against carcinogen-mediated hepatocellular carcinoma (Herranz et al., 2010; Oberdoerffer et al., 2008), regulates the transcriptional activity of HIF-1α and activates LKB1 through deacetylation (Lan et al., 2008; Lim et al., 2010). Sirtuin 2 is another tumor suppressor involved in maintaining genome stability and suppressing tumorigenesis by deacetylating and activating Cadherin-1 (CDH1), a protein that limits glycolysis (Almeida et al., 2010; Kim et al., 2011). More recently, sirtuin 4 and sirtuin 7 have also been implicated in tumorigenesis with an effect of sirtuin 4 in repressing the mitochondrial glutamine metabolism, and a role of sirtuin 7 in negatively regulating HIF-1α and HIF-2α (Hubbi et al., 2013; Jeong et al., 2013).
Although all the above studies indicate that sirtuins can function as tumor suppressors, their role in tumorigenesis is still controversial since different reports indicated that sirtuins, including sirtuin 1, sirtuin 2, and sirtuin 3, also promote cancer (Bosch-Presegue and Vaquero, 2011). In this regard, sirtuin 1 has been shown to inhibit p53 and to stabilize c-Myc, which may explain why its overexpression can increase tumor growth (Menssen et al., 2012; Suh et al., 2011; Villeda et al., 2011).
2.4. Nutrient-Sensing Pathways: A Common Signaling in Aging and Cancer
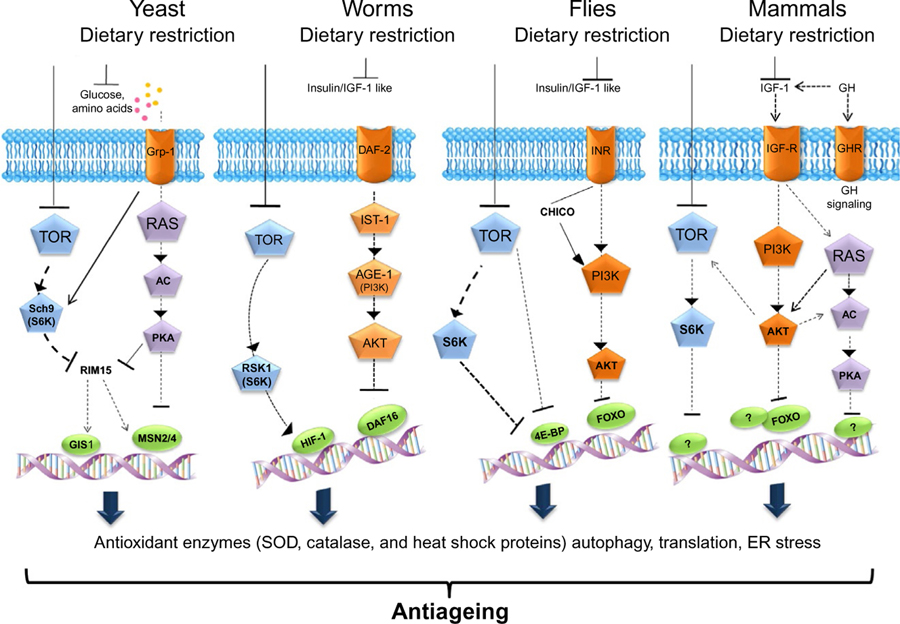
Several studies in yeast, worms, flies, and mice indicate that conserved nutrient-sensing pathways governed by insulin/insulin growth factor (IGF), glucose, and amino acids regulate aging and affect genomic integrity, DNA repair, ROS generation, and cellular apoptosis, highlighting their role in the promotion of cancer (Fontana et al., 2010; Longo and Fontana, 2010). As depicted in Fig. 2, a signaling pathway is triggered when a specific ligand binds to its cognate receptor. These ligands are mainly represented by glucose and amino acids in yeasts; Ins/IGF-1-like peptides in worms and flies; and insulin, IGF-1, and IGF-2 in mammals. In yeast, Ras is involved in the activation of adenylate cyclase (AC) which in its turn activates protein kinase A (PKA), leading to the phosphorylation and inactivation of transcription factors including MSN2/4 and GIS1. The cognate receptors include the insulin-like receptor DAF-2 in worms, InR in flies and IGF-1R, IR-A, and IR-B in mice. Upon the interaction between ligands and their receptors, the signal is transduced through adaptor proteins such as Ras in yeasts and mice, IST-1 in worms, CHICO in flies, and insulin receptor substrate 1 (IRS1–4) in mammals. These proteins are involved in the activation of PI3K which generates phosphatidyl inositol (3,4,5)-trisphosphate (PIP3). In animals, PIP3 activates AKT/PKB which phosphorylates and inactivates specific antiaging transcription factors (DAF-16 in worms, and Forkhead box protein O (FOXO) in flies and mice). This event causes the downregulation of protective stress resistance genes including superoxide dismutase (SOD), catalase, and heat shock proteins (HSPs) which contribute to the protection against oxidative damage contributing to aging as well as cancer. An additional pathway involved in the promotion of aging is controlled by mTOR and the serine kinase 6 (S6K). Dampening these nutrient-sensing pathways by specific mutations, as it occurs in the yeast sch9 null mutants, CHICO mutant flies, Ames and Snell dwarf mice lacking growth hormone (GH) and IGF-1 as well as “Laron dwarf mice” produced by targeted disruption of the GH receptor/GH-binding protein gene (GHR-KO mice), significantly increases the life span (Longo and Finch, 2003).
Fig. 2.
The nutrient-sensing pathways in aging. Certain nutrients sensing pathways are evolutionarily conserved in different organisms including yeasts, worms, flies, and mammals. These pathways are triggered by ligands (glucose and amino acids in yeast, insulin and insulin growth factor-1 like (IGF-1) in worms and flies, IGF-1 in mammals) which activate a receptor (G protein-coupled receptor 1 (Gpr1) in yeasts; DAF-2 in worms; insulin-like receptor (InR) in flies; and IGF-1R, IR-A, and IR-B in mammals). The signal is then transduced through adaptor proteins which include Ras in yeasts, IST-1 in worms, CHICO in flies, and insulin receptor substrate 1 (IRS1–4) and Ras in mice. In yeast, Ras activates adenylate cyclase and protein kinase A (PKA). In worms, flies, and mice, the adaptors proteins mediate the activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) (AGE-1 in worms) which generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 activates AKT/PKB, or PKA in yeast, phosphorylates, and inactivates specific stress resistance transcription factors (MSN2/4 and GIS1 in yeasts, DAF-16 in worms, Forkhead box protein O (FOXO) in flies and mice) involved in the upregulation of protective stress resistance genes such as superoxide dismutase (SOD), catalase, and heat shock proteins (HSPs). A parallel pathway involved in the inactivation of the above-mentioned transcription factors is governed by the target of rapamycin (TOR) and its substrate S6 kinase (S6K). The nutrient-sensing pathways can be down-modulated by diet restriction, which reduces the levels of glucose and IGF-1.
Similar effects can be obtained by strategies based on dietary restriction, which not only extend longevity but also reduce or delay age-related disorders, particularly spontaneous tumors. The effects of these mutations on aging may be explained in part by the finding that fibroblasts from long-lived adult Ames or Snell dwarf mice and GH receptor knockout mice are better protected against oxidative stress, suggesting that IGF-1 has a key role in regulating protection against toxins (Fontana et al., 2010). In agreement with the latter observation, individuals who carry mutations in the GH receptor gene that lead to severe GHR and IGF-1 deficiencies (Laron population) are protected against cancer and diabetes (Guevara-Aguirre et al., 2011). The explanation for these results is provided in part by in vitro studies in which mammary epithelial cells incubated with serum from the Laron individuals were shown to be more protected from DNA damage caused by hydrogen peroxide but are also more likely to die as a consequence of damage compared to those incubated with serum from unaffected relatives.
Nutrient signaling pathways play also a relevant role in the regulation of cell metabolism. Upon binding to its receptor, IGF-1/2 can activate Ras which, in turn, can mediate the activation of the PI3K/AKT and MAPK pathways, both of which increase glycolysis through different mechanisms. AKT increases the expression and membrane localization of the glucose transporter GLUT1, which stimulates phosphofructokinase activity and induces the translocation of hexokinases 1 and 2 into the mitochondria where they drive the first reaction of glycolysis (Barthel et al., 1999; DeBerardinis et al., 2008; Robey and Hay, 2006). Downstream of AKT, mTOR stimulates the switch to the glycolytic pathway through upregulation of HIF-1α, protein translation, and lipogenesis by inducing the SRBPE-dependent transcription of different enzymes such as ATP citrate lyase and fatty acid synthase (Krycer et al., 2010; Yang et al., 2002). Interestingly, in an HIF-1α- and Myc-dependent manner, mTOR upregulates the activity of PKM2, a crucial glycolytic enzyme involved in promoting the pro-Warburg effect (Sun et al., 2011). PKM2 interacts with HIF-1α and enhances the expression of HIF-1α target genes as well as it upregulates c-Myc transcription (Luo et al., 2011). In addition, mTOR as well as MAPK activate Myc whose overexpression causes the conversion of pyruvate into lactate thereby contributing to the Warburg effect (Dang et al., 2009). Myc is also involved in glutaminolysis induction through the upregulation of glutamate synthesis (Gao et al., 2009).
2.5. Inflammation and Cancer
One of the most typical feature and also a probable cause of aging are represented by a chronic and low level state of systemic inflammation in the absence of any infection that has been referred to as inflammaging (Franceschi and Campisi, 2014). Chronic inflammation increases cancer risk and promotes all cancer stages, including cancer initiation, progression, and metastatic diffusion (Mantovani et al., 2008). Thus, inflammation is likely to contribute to age-dependent cancer.
Inflammation can be derived from different sources including: (i) damaged macromolecules, organelles, and cells (self-debris) that accumulate with age and behave as “damage”-associated molecular targets that activate innate immunity and the Nlrp3 inflammasome; (ii) products derived from the microbial constituents of the human body, such as oral or gut microbiota, that elicit an inflammatory response (Biagi et al., 2011); (iii) senescent cells which accumulate with age and secrete proinflammatory cytokines; (iv) the coagulation system which is activated and increases during aging; (v) changes in immunosenescence; and (vi) defective or inappropriate regulation of the complement pathway that can lead to local inflammatory reactions (Franceschi and Campisi, 2014). All of these stimuli can result in the activation of nuclear transcription factor NF-κB which is considered as a hub in carcinogenesis, linking inflammation, cellular senescence, and cancer (Ostan et al., 2015).
3. METABOLIC INTERVENTIONS WITH EFFECTS ON AGING AND CANCER
Since nutrient-sensing pathways have been demonstrated to regulate longevity and to be modulated by dietary interventions, in the following sections, we will discuss different metabolic approaches demonstrated to have prolongevity effects and to prevent or delay multiple age-related diseases and improve health span. These include: (1) CR, (2) periodic fasting (PF), (3) protein restriction, and (4) pharmacological interventions mimicking CR such as inhibitors of mTOR/S6K signaling, of glycolysis, and of GH or IGF-1 signaling, activators of sirtuins, and pharmacological inhibition of inflammation (Longo et al., 2015).
3.1. Calorie Restriction
CR is defined as a 20%–40% reduction in caloric intake without causing malnutrition, and while, in most cases, maintaining meal frequency. CR represents the most potent physiological intervention able to delay and prevent aging and age-associated diseases in single-celled, invertebrate, and vertebrate animals (Fontana and Partridge, 2015). In yeast, starvation obtained by switching the cells from a medium containing nutrients to one containing only water extends the chronological life span (Wei et al., 2008). Similarly, life span extension has been also observed in worms and flies under CR (Fontana et al., 2010). More than 80 years ago, different studies demonstrated that mice and rats under CR live longer and healthier in part by delaying the occurrence of several chronic diseases such as cancer, diabetes, atherosclerosis, and cardiomyopathy (Fontana et al., 2010). In term of cancer prevention, CR has been shown to inhibit the occurrence and growth of spontaneous, chemically induced, and radiation-induced tumors in various animal models (Cheney et al., 1983; Tannenbaum and Silverstone, 1949). Moreover, CR in mice greatly increases insulin sensitivity (Patel et al., 2005). Accordingly, young adult Rhesus monkeys under CR present a significant reduction of age-related pathologies including type 2 diabetes, cardiovascular disease, sarcopenia, and cancer (Colman et al., 2014; Mattison et al., 2012). In addition, immunosenescence and atrophy of the brain’s gray matter are attenuated (Colman et al., 2014). In humans, long-term CR causes metabolic, molecular, and cellular adaptations which are responsible for its protective effect against type 2 diabetes, hypertension, cardiovascular disease, dementia, and cancer (Cava and Fontana, 2013). In particular, over-weight women and elderly subjects under 3–4 months of CR present significantly improved cognitive function (Kretsch et al., 1997). The mechanism underlying the antiaging effect of CR include three levels of adaptations: (1) metabolic adaptations associated to a decrease of insulin, IGF-1, sex hormones, oxidative stress, inflammation, and to an increase of cortisol and adiponectin; (2) molecular adaptations which result in downregulation of PI3K/AKT/S6K, mTOR, RAS/MAPK pathways and upregulate NRF2, sirtuins, AMPK, FOXO, and PTEN; and (3) cellular adaptations including a decrease in cellular proliferation, oxidative stress, and enhancement of apoptosis, autophagy, DNA repair, genome stability, and immunosurveillance (Longo and Fontana, 2010). For example, CR-mediated inhibition of AKT activates FOXOs transcription factors that, in turn, activate protective systems controlling cell proliferation, autophagy, stress resistance, and DNA repair (Webb and Brunet, 2014). Inhibition of mTORC1 increases autophagy and enhances stem cell function (Johnson et al., 2013; Kapahi et al., 2010). Moreover, genome stability and antioxidant defenses can be increased by the overexpression of certain sirtuins such as sirtuins 1, 3, and 6 and by the activation of heat shock factor 1 (HSF1) and of the NRF2 transcription factor (Akerfelt et al., 2010; Martin-Montalvo et al., 2011).
3.2. Protein Restriction
CR, describes the reduction of calories, rather than referring to both the quantity and composition of the diet, which research is now showing to be the major factor responsible of its health span effects in rodents. In fact, it has been observed that neither carbohydrate nor lipid restriction seems to have major effects on longevity alone. In contrast, several studies performed in yeast, worms, flies, and rodents point to protein restriction without a decrease of calorie as a nutritional modification able to increase the life and health span.
There is a clear evidence that the reduction of specific amino acids including serine, valine, threonine, asparagine, glutamate, or methionine extends the life span in yeast Saccharomyces cerevisiae, Drosophila melanogaster, and rodents (Ables et al., 2012; Miller et al., 2005; Richie et al., 1994; Wu et al., 2013). Particular interest has been focused on methionine restriction that exerts healthy effects by decreasing mitochondrial oxidative stress and consequently oxidative damage to mitochondrial DNA (Sanchez-Roman and Barja, 2013). The mechanisms underlying the effect of amino acids on aging are associated to their effect on the activation of mTOR and the control of the nonderepressible 2 (GCN2) gene. mTOR is modulated by different essential amino acids, mainly leucine, in a tissue-specific manner, while GCN2 is a serine/threonine-protein kinase that, once activated by amino acid deficiency, stabilizes the transcription factor ATF4 which is essential for the integrated stress response (Li et al., 2014). In yeast, methionine restriction promotes longevity in a GNC2-dependent manner (Wu et al., 2013), but other mechanisms including induction of autophagy (Ruckenstuhl et al., 2014) and mitochondrial retrograde response (Johnson and Johnson, 2014) have been proposed. The importance of other amino acids such as serine, valine, and threonine in promoting aging in yeast has been recently demonstrated (Mirisola et al., 2014). Specifically, threonine and valine promote cellular sensitization and aging primarily by activating the TOR/S6K pathway, while serine induces sensitization via phosphoinositide-dependent protein kinase 1 (PDK1) orthologs Pkh1/2. These events cause intracellular relocalization and transcriptional inhibition of the stress resistance protein kinase Rim15 (Mirisola et al., 2014).
The extension of the health span in D. melanogaster is attributable to a restriction of protein-containing yeasts or sugar (Mair et al., 2005). According to these results, in mice, a low-protein and high-carbohydrate diet exerts life-extending effects through downregulation of the hepatic mTOR pathway (Solon-Biet et al., 2014).
Protein restriction has been also associated to decrease the prevalence and the severity of age-related diseases. For example, in mice, a low-protein and high-carbohydrate diet reduces the blood pressure, low-density lipoproteins, and triglycerides; improves glucose tolerance; and increases the levels of high-density lipoprotein (Solon-Biet et al., 2014). These results are in agreement with data performed on human subjects in which high-protein and low-carbohydrate diets are associated with an increase of cardiovascular diseases (Floegel and Pischon, 2012; Lagiou et al., 2012). Thus, the balance of protein to carbohydrate, rather than energy intake, may be the driver of a healthy cardiometabolic profile.
The serum IGF-1 reduction and mTOR downregulation by an isocaloric restriction of protein has been also associated to a marked inhibition of prostate and breast cancer growth in experimental models (Fontana et al., 2013; Levine et al., 2014). In humans little is known about the effects of protein restricted diets on cancer. However, an interesting study reported that low-protein intake during middle age (between 50 and 65 years old) is associated with decreased risk of cancer and diabetes mortality as well as overall mortality. Conversely, low-protein intake is associated with increased risk of cancer and overall mortality in respondents over 65 (Levine et al., 2014). In agreement with the observation that an increased protein intake and the resulting increase in IGF-1 may prove beneficial for the skeletal muscle metabolism of older adults (Heaney et al., 1999), a very low-protein diet may be detrimental for older adults in whom weight begins to decline thus making them more susceptible to protein malnourishment (Levine et al., 2014).
3.3. Fasting and Fasting-Mimicking Diet
Fasting represents the most extreme version of CR where nutrients are totally eliminated. Two major forms of fasting can be practiced: (i) an intermittent fasting (IF) also known as alternate day fasting that involves a 24-h fast followed by a 24-h nonfasting period and (ii) prolonged and PF in which absence of food lasts two or more consecutive days every 2 or more weeks (Longo and Mattson, 2014). The first experiments performed on the yeast S. cerevisiae demonstrated that the shift from standard growth medium to only water causes a significant increase of life span as well as the resistance to multiple stress (Longo et al., 1997). This intervention is associated to downregulation of mTOR/S6K (Sch9) and RAS-AC-PKA pathways resulting in increased transcription of: (i) stress resistance genes such as SOD, catalase, and HSPs (Madia et al., 2009) and (ii) DNA repair genes including Rev1 (Wei et al., 2008) (Fig. 2). As a result, fasting exerts a protective effect against DNA damage and promotes longevity (Longo and Fontana, 2010). Similar observations have been made in the nematode C. elegans and D. melanogaster in which food deprivation increases the life span through the downmodulation of mTOR/S6K and IGF-1-like/ PI3K/AKT pathways resulting in the activation of transcription factors DAF16 and FOXO (Fontana et al., 2010; Greer et al., 2007; Piper and Partridge, 2007) (Fig. 2).
In mice, IF reduces the incidence of spontaneous tumors including lymphoma and sarcoma (Berrigan et al., 2002; Descamps et al., 2005), while PF can delay cancer progression as effectively as chemotherapy (Lee et al., 2012). In addition, IF ameliorates age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease (Halagappa et al., 2007), reduces degeneration of dopaminergic neurons in models of Parkinson’s disease (Duan and Mattson, 1999), and slows disease progression in huntingtin mutant mice by normalizing glucose metabolism and brain-derived neurotrophic factor levels (Duan et al., 2003). In agreement with the latter results, a recent study demonstrated that IF, when imposed in the middle age, delays or prevents the age-associated impairment of brain functions and promotes healthy aging by improving the motor coordination and learning response recovery (Singh et al., 2012, 2015). Furthermore, IF protects the heart against ischemic injury in myocardial infarction models (Ahmet et al., 2005) and reduces the age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-κB activation (Castello et al., 2010). IF also decreases the diabetes incidence in BB rats (Pedersen et al., 1999) and prevents/reverses different aspects associated to metabolic syndrome by increasing insulin and leptin sensitivity, suppressing inflammation, and stimulating autophagy (Wan et al., 2010). Interestingly, it has been shown that the effects of IF on life span depend on genotype and age of initiation (Goodrick et al., 1990; Kendrick, 1973).
In contrast, the use of a FMD for 4 days every 2 weeks started at middle age extends health span, reduces tumor incidence, decreases inflammation, and delays the age-dependent cognitive decline as well as bone loss decline (Brandhorst et al., 2015) In this respect, severe dietary restriction or fasting have been hypothesized to exert beneficial effects in young and middle-age mice, but to be detrimental in older animals that begin to lose weight, according to what has been observed in humans (Brandhorst et al., 2015; Levine et al., 2014).
In humans, fasting has been shown to affect certain factors implicated in aging such as insulin and IGF-1, and to cause a fivefold increase of IGF-1 binding protein 1 (IGFBP1) which sequesters IGF-1 from the circulation thus reducing its bioavailability (Fontana et al., 2010; Harvie et al., 2011; Thissen et al., 1994). Notably, the effect of fasting on IGF-1 is due to the combination of protein and CR, but protein intake represents the major regulating factor (Thissen et al., 1994). One of the most evident effects of fasting in humans has been observed in patients affected by rheumatoid arthritis who have clinically significant beneficial long-term effects in term of reduction of pain and inflammation (Muller et al., 2001). However, when normal diet is resumed, most patients relapse unless fasting is followed by a vegetarian diet (Kjeldsen-Kragh et al., 1991). The beneficial effects of fasting have also been observed in subjects affected by hypertension with an improvement of systolic blood pressure below 120 after 13 days of water-only fasting (Goldhamer et al., 2002). Interestingly, a recent case report revealed that a medically supervised 21 days of water-only fasting followed by a diet of minimally processed plant foods free of added sugar, oil, and salt causes tumor regression in a woman affected by lymphoma (Goldhamer et al., 2015). Since fasting is often challenging for individuals affected by pathology, Branhorst et al. developed a FMD to be administered periodically that is low in calories, protein, and sugar (Brandhorst et al., 2015) and which mimics the effects of fasting on markers associated with the stress resistance or longevity, including low levels of glucose and IGF-1 and high levels of ketone bodies and IGFBP-1 (Longo and Mattson, 2014). Bimonthly FMD cycles started at middle age extend the longevity of mice by lowering visceral fat, reducing cancer incidence and skin lesions, rejuvenating the immune system, and retarding bone mineral density loss. In old mice, FMD cycles promoted hippocampal neurogenesis, lowered IGF-1 levels and PKA activity, elevated NeuroD1, and improved cognitive performance. In a pilot clinical trial performed in generally healthy adults, three FMD cycles decreased risk factors/biomarkers for aging, diabetes, cardiovascular disease, and cancer without major adverse effects, providing support for the use of FMD to promote health span (Brandhorst et al., 2015). Moreover, preliminary data from a randomized pilot clinical trial conducted to assess the safety and feasibility of the FMD on patients affected by multiple sclerosis showed that the FMD is safe, feasible, and potentially effective by reducing the number of autoimmune lymphocytes (Choi et al., 2016). Among fasting mimetic compounds, polyamine spermidine deserves particular attention since it has been demonstrated to: (i) markedly extend the life span of yeast, flies and worms, and human immune cells; (ii) decrease the oxidative stress; and (iii) exert cardioprotective effects by reducing cardiac hypertrophy and by preserving diastolic function in old mice (Eisenberg et al., 2009, 2016). Interestingly, autophagy, which is able to minimize the functional decline of aged cardiomyocytes by degrading and recycling long-lived damaged proteins as well as dysfunctional mitochondria (Nakai et al., 2007; Taneike et al., 2010), has been shown to be required for spermidine-mediated cardioprotection (Eisenberg et al., 2016). In agreement with the latter results, the dietary consumption of sperimidine in humans correlates with reduced blood pressure and a lower incidence of cardiovascular disease (Eisenberg et al., 2016).
Of note, spermidine has been also implicated in cancer prevention since it is able to reduce tumor incidence through inhibition of age-associated alteration in DNA methylation status (Soda et al., 2013). Moreover, an elegant recent study demonstrated that spermidine as well as hydroxycitrate, both defined as CR mimetics, improves the inhibition of in vivo tumor growth by chemotherapy (Pietrocola et al., 2016). This effect was only observed for autophagy-competent tumors, depended on the presence of T lymphocytes, and was accompanied by the depletion of regulatory T cells from the tumor bed (Pietrocola et al., 2016).
3.4. Pharmacological Interventions Mimicking CR
3.4.1. Inhibitors of Nutrient-Sensing Pathways
Reduced nutrient-sensing pathways including mTOR/S6K, PI3K/AKT, Ras, or AC/PKA signaling, through genetic or pharmacological interventions lead to life span extension in yeast, worm, flies, and mice (Johnson et al., 2013). mTOR kinase is formed by two subunits called TORC1 and TORC2, where the former induces activation of S6K and 4E-BP1. The core components of mTORC1 consist of mTOR, mammalian lethal with sec-13 protein 8 (mLST8), and regulatory-associated protein of TOR (raptor), to which additional proteins including a DEP-domain-containing mTOR-interacting protein (DEPTOR) and proline-rich Akt substrate 40 kDa (PRAS40) are associated. The mTOR complex 2 (mTORC2) core is composed of mTOR, the rapamycin insensitive companion of mTOR (rictor), stress-activated protein kinase-interacting protein 1 (mSIN1), and mLST8. One of the best-known mTOR inhibitors is rapamycin, a pharmacological agent which was initially discovered as an antifungal metabolite and subsequently found to be immunosuppressive by inhibiting S6K1 activation (Chung et al., 1992). In the context of mTOR complex, only mTORC1, which integrates different signals governed by nutrients, growth factors, oxygen, and energy and activates anabolic processes required for cell proliferation and growth, is acutely sensitive to inhibition by rapamycin. Rapamycin binds to the intracellular protein FKBP12 to generate a complex that binds to and destabilized mTORC1 at all times after drug addition, consistent with the capacity of FKBP12-rapamycin to bind to it and weaken the raptor–mTOR interaction (Kim et al., 2002). In contrast, the modulation of mTORC2 by rapamcin is more complex and controversial. Since acute treatment with rapamycin does not perturb mTORC2 signaling and FKBP12-rapamycin cannot bind to intact mTORC2, this complex was originally thought to be rapamycin insensitive (Jacinto et al., 2004; Sarbassov et al., 2004). However, prolonged treatment with rapamycin has been shown to reduce mTORC2 signaling in some, but not all cell types and does so by suppressing mTORC2 assembly (Laplante and Sabatini, 2012; Sarbassov et al., 2006).
Rapamycin extends the life span in various model organisms including mice (Harrison et al., 2009; Miller et al., 2005). However, its serious side effects such as metabolic dysregulation (i.e., hyperglycemia, hyperinsulinemia, and insulin resistance) and blockade of hematopoietic cell lineage proliferation hampered its clinical use as an antiaging therapeutic (Soefje et al., 2011). Current clinical trials on healthy older subjects will help to understand whether inhibition of mTOR could be a safe and feasible antiaging intervention.
3.4.2. Inhibitors of Glycolysis
Consistent with the metabolic dysregulation associated with aging, i.e., reduction of OXPHOS and increase of glycolysis, there is currently great interest in applying pharmacological interventions aimed at inhibiting glucose catabolism and generation. Among these drugs, specific attention has been focused on 2-deoxy-D-glucose (2DG), a glucose analog which, once it is phosphorylated by hexokinase, cannot be further metabolized and consequently blocks glycolysis. Administration of 2DG to rats improves glucose and insulin regulation and increases recovery from stress (Minor et al., 2010). Another compound is an avocado extract called mannoheptulose that inhibits hexokinase and increases the life span in nematodes and mice (Minor et al., 2010). Acarbose, which limits glucose supply to cells by inhibiting α-glucosidase in the intestine, increases the life span of mice (Harrison et al., 2014), and exerts cardioprotective effects in patients affected by diabetes type 2 (Chiasson et al., 2002).
3.4.3. Inhibitors of the GH/IGF-1 Axis
Several reports indicate that downregulation of GH/IGF-1 pathway can extend life span in different species (Longo and Finch, 2003). For example, human IGF-1 receptor gene mutations have been found in centenarians (Suh et al., 2008) and decreased serum levels of IGF-1 predict survival in humans with exceptional longevity (Milman et al., 2014). Accordingly, GHR/IGF-1-deficient mice present a lower incidence and delayed occurrence of fatal neoplastic lesions compared with their wild-type littermates (Ikeno et al., 2009; Zhou et al., 1997). Similarly to what it was observed in (GHR/BP) knockout mice, patients affected by GHR-deficient Laron syndrome are characterized by a significant decrease of cancer and diabetes risk (Guevara-Aguirre et al., 2011; Ikeno et al., 2009; Shevah and Laron, 2007; Zhou et al., 1997).
According to these results, pharmacological interventions aimed at inhibiting GH/IGF-1 such as monoclonal antibodies and drugs directed against IGF-1R have been used in cancer patients (Carboni et al., 2009). Moreover, several classes of compounds that inhibit GH/IGF-1 axis have been used in patients affected by acromegaly (Giustina et al., 2014). Among the latter drugs, particular attention has been given to somatostatin analogs which lower serum GH and IGF-1 but, unfortunately also reduce other endocrine hormones and can cause serious side effects such as anorexia, diarrhea, and gallstones. For these reasons, the clinical antiaging use of somatostatin analogs is currently poorly understood and pursued. A GHR antagonist currently used in patients affected by acromegaly is pegvisomant which specifically binds to and inhibits GHR (Kopchick et al., 2002; Trainer et al., 2000; van der Lely et al., 2001b). Interestingly, this compound lowers serum IGF-1 levels and blocks the diabetogenic action of GH (Trainer et al., 2000; van der Lely et al., 2001a). However, the very high cost and the need for frequent injections (van der Lely et al., 2012) provide a very tall obstacle for its application as a prolongevity and health span intervention. Finally, an interesting strategy to reduce IGF-1 action is to decrease its availability. In this respect, loss of the protease pregnancy-associated plasma protein-A (PAPP-A) reduces IGF-1 signaling and extends life span in mice while also reducing age-related diseases (Conover, 2013).
3.4.4. Activators of Sirtuins
Given their beneficial effects in promoting longevity, sirtuin family proteins are a very interesting drug target. Sirtuin-activating compounds (STACs) including plant-derived metabolites and the well-known resveratrol, represent the first and most potent sirtuin activator and have been shown to extend life span in various organisms (Hubbard and Sinclair, 2014; Pearson et al., 2008). Synthetic activators such as SRT1720 and SRT2104 improve the metabolic profile and extend life span and health span of mice under a high-fat and normal diet (Mitchell et al., 2014; Minor et al., 2011). Interestingly, SRT1720 improves insulin sensitivity, lowers plasma glucose, and increases mitochondrial capacity in experimental diabetes models, thus representing a promising new therapeutic approach for treating age-related diseases such as type 2 diabetes (Milne et al., 2007). Moreover, in rhesus monkeys, under a high-fat and high-sugar diet, resveratrol exerts antiinflammatory effects in visceral white adipose tissue (Jimenez-Gomez et al., 2013). In mice and nonhuman primates fed a high-fat diet, resveratrol protects against the effects of obesity and age-related metabolic decline, increases insulin sensitivity and mitochondrial functions, and prevents liver steatosis (Baur et al., 2006; Fiori et al., 2013; Jimenez-Gomez et al., 2013). In addition, resveratrol delays the onset of neurodegeneration and improves learning and memory in aged mice (Graff et al., 2013; Zhao et al., 2013). The promising results in preclinical models led the clinicians to test resveratrol in humans where it exerts beneficial effects on elderly and obese subjects (Timmers et al., 2011). Furthermore, resveratrol provides positive effects for systolic blood pressure, hemoglobin A1c, and creatinine in patients affected by type 2 diabetes (Hausenblas et al., 2015). Synthetic STACS have also been demonstrated to exert beneficial cardiovascular effects on healthy cigarettes smokers (Venkatasubramanian et al., 2013). The mechanism underlying the beneficial effects of resveratrol is controversial since it has been proposed that the direct activation of sirtuin 1 by resveratrol is an in vitro artifact (Beher et al., 2009; Kaeberlein et al., 2005; Pacholec et al., 2010) and that resveratrol works primarily by activating AMPK (Canto et al., 2009), potentially by inhibition of phosphodiesterases (PDE). AMPK may then activate sirtuin 1 indirectly by elevating intracellular levels of its cosubstrate NAD+ (Canto et al., 2009). Alternatively, resveratrol may first activate sirtuin 1 in vivo, leading to AMPK activation via deacetylation and activation of the AMPK kinase LKB1 (Hou et al., 2008; Lan et al., 2008).
3.4.5. Activators of AMPK Pathway
AMPK is a serine/threonine kinase which, upon activation by low cellular energy levels, exerts insulin-sensitizing effects resulting in increased glucose uptake in skeletal muscle and fatty acid oxidation in several tissues as well as decreased hepatic glucose production (Ruderman et al., 2013). Different AMPK activators have been developed including biguanides, thiazolidinediones, agonists of glucagon-like peptide-1 receptor, salicylates, and resveratrol (Coughlan et al., 2014). Among biguanides, metformin is a drug used in the first line therapy for type 2 diabetes (Rena et al., 2013) that reduces the risk of cancer and overall mortality (Evans et al., 2005; Franciosi et al., 2013), cardiovascular disease, and possibly cognitive decline (Ng et al., 2014; No author, 1998; Wu et al., 2014a). However, before introducing metformin as an antiaging agent in generally healthy people, further studies to understand the mechanism of action of this compound are necessary. In fact, metformin is also able to inhibit gluconeogenesis, suggesting that, for people under diet-restricted or ketogenic diet, who depend on gluconeogenesis, it might be toxic. According to its prolongevity effects, metformin has been demonstrated to extend the life span of worms and rodents by targeting the folate cycle and methionine metabolism (Anisimov, 2010; Cabreiro et al., 2013; Martin-Montalvo et al., 2013).
3.4.6. Inhibitors of Inflammation Pathways
Strategies aimed at decreasing inflammatory pathways can be viewed as candidate target to combat and prevent aging-associated diseases. Various factors contribute to the generation of inflammatory conditions. Some of them are exogenous such as persistent cytomegalovirus infection (Sansoni et al., 2014), while some others are endogeneously produced. The latter include circulating mitochondrial DNA (mtDNA) that is a potent inflammatory stimulus increasing with aging (Pinti et al., 2014), galactosylated N-glycans and proinflammatory micro-RNA (inflammaMIR), all of them potent inflammatory stimuli which increase in circulation with age (Dall’Olio et al., 2013; Olivieri et al., 2013). Approaches targeting one of the pathways that promote age-dependent inflammation, including the de-activation of inflammasomes (Youm et al., 2013), elimination of senescent cells (Tchkonia et al., 2013), and specific dietary restriction regimens have been developed (Berendsen et al., 2013). Initial evidence for the potential of antiinflammatory drugs in retarding aging comes from the effect of nordihydroguaiaretic drugs such as aspirin on life span increase in mice (Strong et al., 2008).
4. GEROSCIENCE AS A STRATEGY TO OPTIMIZE CANCER THERAPY
Conventional cancer therapy is mainly based on the use of chemotherapeutics, ionizing radiation, and novel drugs with specific molecular targets, whose major limitation is represented by the toxicity toward healthy cells. Thus, normal cells are damaged and acquire phenotypes similar to those observed during aging. For example, chemotherapy causes mutations and DNA alterations and induces oxidative stress in agreement with what occurs during aging. Moreover, epidemiologic studies have also observed that long-term cancer survivors treated with chemotherapy or radiotherapy have good response in term of tumor regression but often suffer of delayed effects related to cancer treatment including secondary tumors, cardiac pathologies, respiratory complications, infertility, and other chronic disorders. Thus, the discovery of strategies able to generate differential stress resistance (DSR) and sensitization conditions in which tumor cells are sensitized while normal cells are protected against the cytotoxicity of a chemotherapeutic drug is of central importance, particularly as many therapies include multiple chemotherapy drugs or the combination of chemotherapy and non-chemotherapy drugs which can also have adverse effects.
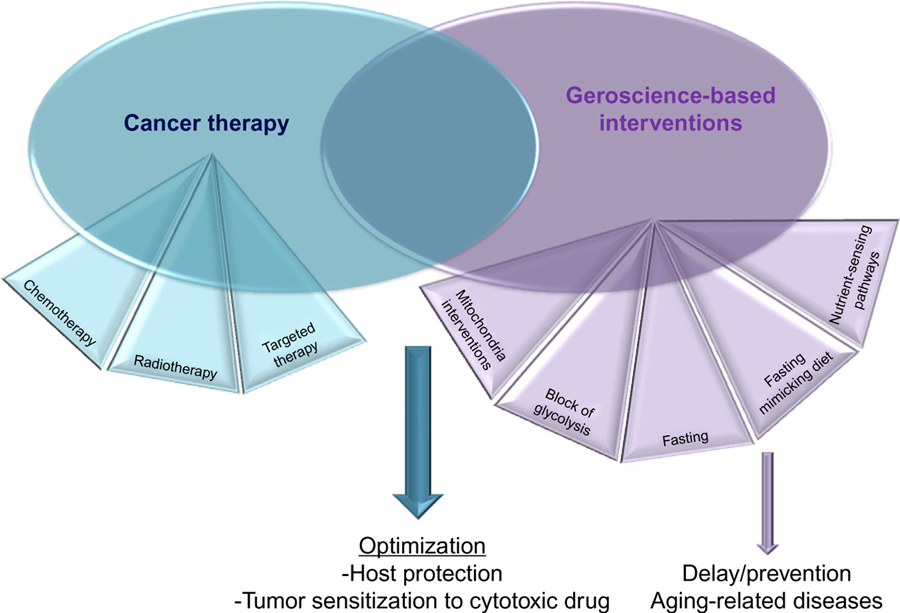
Geroscience is a multidisciplinary field whose purpose is the understanding of the association between aging and age-related diseases and the elucidation of the mechanisms underlying cellular damage, protection, and death. Thus, geroscience offers the opportunity to find strategies focused on the protection of healthy cells without interfering with the efficacy of the treatment against malignant cells. In this respect, geroscience and cancer therapy could be considered complementary to each other since the former aims to protect the patient while the second to kill tumor cells (Fig. 3). In the following sections, we will discuss some of the geroscience-based approaches used to generate DSR and stress sensitization conditions in normal and malignant cells, in order to improve the killing of cancer cells, but also to preserve the patient’s healthspan posttreatment.
Fig. 3.
Geroscience and cancer therapy. Cancer therapy is mainly based on the use of chemotherapeutics, ionizing radiation, and novel drugs with specific molecular targets, whose major limitation is represented by the toxicity toward healthy cells. This is mainly due to their inability to distinguish between healthy and cancer cells. Geroscience, defined as the science of healthy aging, can provide not only strategies able to prevent or delay aging and age-related disorders but also to optimize conventional cancer therapy by protecting the host and sensitizing the cancer cells to the cytotoxic agent. These approaches include fasting and fasting-mimicking diet, mitochondrial interventions, glycolysis blockers, and inhibitors of the nutrient-sensing pathways.
4.1. Fasting and Fasting-Mimicking Diet
Multiple cycles of fasting in tumor-bearing mice can selectively sensitize cancer cells to chemotherapy while protecting normal cells from the associated toxicity (Lee et al., 2012; Raffaghello et al., 2008). These phenomena are known as differential stress sensitization and DSR. Specifically, in healthy cells, fasting was found to activate protective metabolic pathways that confer resistance to a variety of chemotherapeutics (Raffaghello et al., 2008). In contrast, starved cancer cells were found to be unable to turn on such a protective response due to uncontrolled activation of growth promoting signaling cascades by oncogenes and to become even more sensitive to DNA damaging agents (Lee et al., 2012; Safdie et al., 2009, 2012; Shi et al., 2012). The protection of normal cells against chemotherapy-dependent damage was found to be associated to a reduction of IGF-1 and downregulation of protooncogene signals (Lee et al., 2010).
In addition to protecting hematopoietic cells from chemotoxicity, multiple cycles of fasting promote hematopoietic stem cell self-renewal to alleviate or reverse the immunosuppression or immunosenescence caused by chemotherapy treatment and aging (Cheng et al., 2014). The latter effect was associated to inhibition of IGF-1 and PKA signaling.
More recently, fasting has also been shown to enhance the therapeutic index of chemotreatments by exerting an anti-Warburg effect. In this way, cancer cells are shifted from a glycolytic mode into an uncoupled OXPHOS which promotes increased ROS generation and apoptosis (Bianchi et al., 2015). Preliminary clinical data indicate that fasting in cancer patients is not associated with major adverse effects and may reduce several of the toxic effects of chemotherapy (de Groot et al., 2015; Safdie et al., 2009) including a decreased toxicity to lymphocytes (Cheng et al., 2014). These preliminary clinical data have been collected as part of different pilot Phase I and Phase II clinical trials (NCT01304251, NCT01175837, NCT00936364, and NCT01175837) that demonstrated the safety and efficacy of fasting cycles in combination with chemotherapy in adult oncologic patients.
A recent study demonstrated that a FMD is as effective as fasting in killing different cancer cell types (Di Biase et al., 2016). In particular, the combination of chemotherapy and FMD increases the levels of lymphoid progenitor cells and cytotoxic CD8+ tumor-infiltrating lymphocytes, leading to a major delay in breast cancer and melanoma progression. In breast tumors, the mechanism underlying the latter effect involves the downregulation of the stress-protecting enzyme hemeoxygenase-1 (HO-1). These data indicate that cycles of FMD in combination with chemotherapy can enhance T cell-dependent killing of cancer cells through the stimulation of the hematopoietic system and the enhancement of CD8+-dependent tumor cytotoxicity (Di Biase et al., 2016), although it is likely that the initial damage of cancer cells caused by the FMD (Shim et al., 2015) is a key step in the activation of the T cell-dependent immune response. According to these results, back-to-back studies reported that fasting enhances chemotherapy-induced immunosurveillance (Pietrocola et al., 2016).
Fasting has also been associated to tyrosine kinase inhibitors (TKI), which represent the most broadly applied cancer therapeutics. However, a major limitation of these agents is that their efficacy is short lived since the great majority of patients unavoidably relapse (Gridelli et al., 2014a,b). Thus, strategies that help increase the efficacy of these agents making them more powerful and capable of effectively eradicating cancer cells are warranted. In this respect, fasting has been shown to potentiate the in vivo anticancer effects of various TKI including erlotinib, gefitinib, lapatinib, crizotinib, and regorafenib through inhibition of MAPK kinase signaling and E2F factor transcription (Caffa et al., 2015).
4.2. Glycolysis Blockade
As mentioned in the previous chapter, 2-DG is a glucose analog that is phosphorylated by hexokinase to 2-DG-phosphate, which causes a glycolysis blockade. While early studies demonstrated that 2-DG exerts promising anticancer effects in experimental models (Maher et al., 2004), more recent reports show that the mechanisms underlying the activity of 2-DG is heterogeneous and sometimes 2-DG was shown to activate prosurvival pathways in tumor cells (Zhong et al., 2009). Furthermore, the clinical use of 2-DG has been limited by its toxicity upon long-term application (Dwarakanath and Jain, 2009; Landau et al., 1958; Vijayaraghavan et al., 2006). A more promising approach is to combine 2-DG with cytotoxic agents such as chemotherapeutic drugs and ionizing radiation in order to enhance their efficacy (Maschek et al., 2004). In this respect, 2-DG increases the therapeutic index of chemotherapeutic agents in mice-bearing human osteosarcoma and nonsmall cell lung cancer (Maschek et al., 2004). Moreover 2-DG synergizes with etoposide through the induction of immunogenic cell death and in this way increases the life span of immunocompetent tumor-bearing mice (Beneteau et al., 2012). Clearly, the combination of 2-DG with chemotherapy will result in a even higher degree of toxicity, which may be life threatening.
4.3. Nutrient-Sensing Pathway Interventions
As described in a previous section, one of the most relevant nutrient-sensing pathway involved in longevity is governed by GH/IGF-1 and glucose which trigger the activation of downstream mTOR/S6K, PI3K/AKT, Ras, and AC/PKA axes, most of them highly conserved from yeast to humans (Fontana et al., 2010). Rapamycin and its analogs (rapalogs) including temsirolimus and everolimus are mTOR inhibitors that have been approved by the FDA for treating different cancers. In addition to their anticancer effects as single agents, rapamycin and rapalogs have been shown to potentiate the efficacy of various cytotoxic agents such chemotherapeutic drugs, ionizing radiation, and proteasome inhibitors against malignant cells through the activation of caspase-dependent apoptosis (Mondesire et al., 2004). In addition, rapamycin and rapalogs prevent epithelial stem cell senescence and protect mice from ionizing radiation-induced side effects (Iglesias-Bartolome et al., 2012). Accordingly, in vitro data indicate that also the combination of rapamacyin and metformin protects normal fibroblasts and epithelial cells against the toxicity of cell-cycle specific chemotherapeutic agents such as mitotic inhibitors (Apontes et al., 2011). However, in vivo rapamycin and other m-TOR inhibitors also cause hyperglycemia which may potentially promote the progression of certain cancers and the sensitization of normal cells to chemotherapy.
Metformin has recently emerged as an adjuvant anticancer drug to be used in combination with chemotherapy in order to increase its efficacy and lower the doses. Specifically, metformin has been shown to increase the therapeutic index of various standard chemotherapeutic agents such as paclitaxel, carboplatin, and doxorubicin. This combinatorial effect includes tumor regression and prevention of relapse (Hirsch et al., 2009; Iliopoulos et al., 2011). In agreement with these results, a retrospective analysis of esophageal adenocarcinoma patients under metformin treatment indicated a better response to chemotherapy in subjects treated with metformin compared to those treated with chemotherapy alone (Skinner et al., 2013). The proposed mechanism underlying the chemosensiting effect of metformin against tumor cells is based on downregulation of mTOR pathway and CSC genes (Honjo et al., 2014). In addition to enhancing the efficacy of chemotherapy, metformin has been shown to protect the host against the chemotoxicity by reducing the chemo-induced peripheral neuropathy and sensory deficits (Mao-Ying et al., 2014).
5. CONCLUSIONS
Cancer and aging are closely related to each other since they are both associated with damage to DNA and loss of function, but also because aging is a major risk factor for tumorigenesis. In turn, many cancer treatments are contributors to the aging process. From this perspective, geroscience-based approaches appear to be urgent since they provide a potential solution not only for preventing or delaying age-related disorders but also for the optimization and enhancement of conventional cancer treatments. In this context, there is a need to educate patients of the long-term side effects caused by standard cancer therapy and to inform them about the strategies used to prevent or reduce such toxicity. In this respect, FMD but also a few drugs including metformin targeting antiaging pathways, or the combination of dietary and pharmacological interventions represent one the most promising approaches that could be incorporated into therapeutic protocols for oncologic patients in order to ameliorate the clinical outcome for these patients with an overall impact on the costs of medical care.
ACKNOWLEDGMENTS
L.R. is funded by “Cinque per mille dell’IRPEF–Finanziamento della ricerca sanitaria,” Finanziamento Ricerca Corrente, Ministero Salute. V.L. is funded by NIH grant P01 AG034906 and by Associazione Italiana per la Ricerca sul Cancro (AIRC) 2016 (IG-17605).
REFERENCES
- Abbas S, Lugthart S, Kavelaars FG, Schelen A, Koenders JE, Zeilemaker A, et al. , 2010. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood 116, 2122–2126. [DOI] [PubMed] [Google Scholar]
- Ables GP, Perrone CE, Orentreich D, Orentreich N, 2012. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 7, e51357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M, 2005. Cardioprotection by intermittent fasting in rats. Circulation 112, 3115–3121. [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L, 2010. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol 11, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL, 2011. SIRT3 and cancer: tumor promoter or suppressor? Biochim. Biophys. Acta 1816, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Bolanos JP, Moncada S, 2010. E3 ubiquitin ligase APC/C-Cdh1 accounts for the Warburg effect by linking glycolysis to cell proliferation. Proc. Natl. Acad. Sci. U.S.A 107, 738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, 2010. Metformin for aging and cancer prevention. Aging (Albany NY) 2, 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV, 2011. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne AP, George WD, et al. , 2006. Altered sirtuin expression is associated with node-positive breast cancer. Br. J. Cancer 95, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. , 2001. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet 69, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardella C, Pollard PJ, Tomlinson I, 2011. SDH mutations in cancer. Biochim. Biophys. Acta 1807, 1432–1443. [DOI] [PubMed] [Google Scholar]
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP Jr., et al. , 1999. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem 274, 20281–20286. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. , 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. , 2000. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851. [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. , 2009. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des 74, 619–624. [DOI] [PubMed] [Google Scholar]
- Bell EL, Emerling BM, Ricoult SJ, Guarente L, 2011. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30, 2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneteau M, Zunino B, Jacquin MA, Meynet O, Chiche J, Pradelli LA, et al. , 2012. Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response. Proc. Natl. Acad. Sci. U.S.A 109, 20071–20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennewith KL, Durand RE, 2004. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res 64, 6183–6189. [DOI] [PubMed] [Google Scholar]
- Berendsen A, Santoro A, Pini E, Cevenini E, Ostan R, Pietruszka B, et al. , 2013. A parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: design of the NU-AGE dietary intervention study. Mech. Ageing Dev 134, 523–530. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Perkins SN, Haines DC, Hursting SD, 2002. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 23, 817–822. [DOI] [PubMed] [Google Scholar]
- Biagi E, Candela M, Franceschi C, Brigidi P, 2011. The aging gut microbiota: new perspectives. Ageing Res. Rev 10, 428–429. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Martella R, Ravera S, Marini C, Capitanio S, Orengo A, et al. , 2015. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 6, 11806–11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Munoz P, Flores JM, Klatt P, Blasco MA, 2007. Telomerase abrogation dramatically accelerates TRF2-induced epithelial carcinogenesis. Genes Dev 21, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Presegue L, Vaquero A, 2011. The dual role of sirtuins in cancer. Genes Cancer 2, 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MF, 1993. Age-dependent impairment of mitochondrial function in primate brain. J. Neurochem 60, 1964–1967. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. , 2015. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and health span. Cell Metab 22, 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, et al. , 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffa I, D’Agostino V, Damonte P, Soncini D, Cea M, Monacelli F, et al. , 2015. Fasting potentiates the anticancer activity of tyrosine kinase inhibitors by strengthening MAPK signaling inhibition. Oncotarget 6, 11820–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Iqbal J, Lemonnier F, Kucuk C, de Leval L, Jais JP, et al. , 2012. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood 119, 1901–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. , 2009. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, et al. , 2009. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol. Cancer Ther 8, 3341–3349. [DOI] [PubMed] [Google Scholar]
- Cardaci S, Ciriolo MR, 2012. TCA cycle defects and cancer: when metabolism tunes redox state. Int. J. Cell Biol 2012, 161837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A, Lleonart M, 2016. The hypoxic microenvironment: a determinant of cancer stem cell evolution. Bioessays 38 (Suppl. 1), S65–S74. [DOI] [PubMed] [Google Scholar]
- Carvajal-Carmona LG, Alam NA, Pollard PJ, Jones AM, Barclay E, Wortham N, et al. , 2006. Adult leydig cell tumors of the testis caused by germline fumarate hydratase mutations. J. Clin. Endocrinol. Metab 91, 3071–3075. [DOI] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, et al. , 2010. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic. Biol. Med 48, 47–54. [DOI] [PubMed] [Google Scholar]
- Cava E, Fontana L, 2013. Will calorie restriction work in humans? Aging (Albany NY) 5, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L, 2015. The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 15, 608–624. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ, 2006. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic. Biol. Med 40, 976–982. [DOI] [PubMed] [Google Scholar]
- Chen JH, Hales CN, Ozanne SE, 2007. DNA damage, cellular senescence and organismal ageing: causal or correlative? Nucleic Acids Res 35, 7417–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney KE, Liu RK, Smith GS, Meredith PJ, Mickey MR, Walford RL, 1983. The effect of dietary restriction of varying duration on survival, tumor patterns, immune function, and body temperature in B10C3F1 female mice. J. Gerontol 38, 420–430. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, et al. , 2014. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, 2002. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359, 2072–2077. [DOI] [PubMed] [Google Scholar]
- Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. , 2016. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep 15, 2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J, 1992. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69, 1227–1236. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM, 2014. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat. Commun 5, 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, 2013. Role of PAPP-A in aging and age-related disease. Exp. Gerontol 48, 612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan KA, Valentine RJ, Ruderman NB, Saha AK, 2014. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes 7, 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C, 2013. N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing Res. Rev 12, 685–698. [DOI] [PubMed] [Google Scholar]
- Dang CV, Le A, Gao P, 2009. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res 15, 6479–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. , 2010. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465, 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F, 2014. The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carli E, Wang X, Puget S, 2009. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med 360, 2248, author reply 2249. [DOI] [PubMed] [Google Scholar]
- de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, et al. , 2015. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer 15, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB, 2008. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7, 11–20. [DOI] [PubMed] [Google Scholar]
- Descamps O, Riondel J, Ducros V, Roussel AM, 2005. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech. Ageing Dev 126, 1185–1191. [DOI] [PubMed] [Google Scholar]
- Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, et al. , 2016. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell 30, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mattson MP, 1999. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res 57, 195–206. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP, 2003. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. U.S.A 100, 2911–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarakanath B, Jain V, 2009. Targeting glucose metabolism with 2-deoxy-D-glucose for improving cancer therapy. Future Oncol 5, 581–585. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. , 2009. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol 11, 1305–1314. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, et al. , 2016. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med 22, 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. , 2004. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64, 3892–3899. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD, 2005. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. , 2011. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell 19, 416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, et al. , 2013. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 62, 3500–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floegel A, Pischon T, 2012. Low carbohydrate-high protein diets. BMJ 344, e3801. [DOI] [PubMed] [Google Scholar]
- Floyd RA, West M, Hensley K, 2001. Oxidative biochemical markers; clues to understanding aging in long-lived species. Exp. Gerontol 36, 619–640. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD, 2010. Extending healthy life span—from yeast to humans. Science 328, 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Adelaiye RM, Rastelli AL, Miles KM, Ciamporcero E, Longo VD, et al. , 2013. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 4, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J, 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci 69 (Suppl. 1), S4–S9. [DOI] [PubMed] [Google Scholar]
- Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A, 2013. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 8, e71583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. , 2009. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, et al. , 2014. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat. Rev. Endocrinol 10, 243–248. [DOI] [PubMed] [Google Scholar]
- Goldhamer AC, Lisle DJ, Sultana P, Anderson SV, Parpia B, Hughes B, et al. , 2002. Medically supervised water-only fasting in the treatment of borderline hypertension. J. Altern. Complement. Med 8, 643–650. [DOI] [PubMed] [Google Scholar]
- Goldhamer AC, Klaper M, Foorohar A, Myers TR, 2015. Water-only fasting and an exclusively plant foods diet in the management of stage IIIa, low-grade follicular lymphoma. BMJ Case Rep 2015. [DOI] [PMC free article] [PubMed]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, et al. , 2013. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N, 1990. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech. Ageing Dev 55, 69–87. [DOI] [PubMed] [Google Scholar]
- Graff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, et al. , 2013. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J. Neurosci 33, 8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. , 2007. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol 17, 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridelli C, de Marinis F, Cappuzzo F, Di Maio M, Hirsch FR, Mok T, et al. , 2014a. Treatment of advanced non-small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin. Lung Cancer 15, 173–181. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F, 2014b. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat. Rev 40, 300–306. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, et al. , 2011. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med 3, 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, 2009. The VHL tumor suppressor: master regulator of HIF. Curr. Pharm. Des 15, 3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, et al. , 1997. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. U.S.A 94, 3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, et al. , 2007. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis 26, 212–220. [DOI] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS, 2010. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci 35, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. , 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. , 2014. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. , 2011. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int. J. Obes. (Lond.) 35, 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenblas HA, Schoulda JA, Smoliga JM, 2015. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus—systematic review and meta-analysis. Mol. Nutr. Food Res 59, 147–159. [DOI] [PubMed] [Google Scholar]
- Heaney RP, McCarron DA, Dawson-Hughes B, Oparil S, Berga SL, Stern JS, et al. , 1999. Dietary changes favorably affect bone remodeling in older adults. J. Am. Diet. Assoc 99, 1228–1233. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, et al. , 2010. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K, 2009. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 69, 7507–7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. , 2010. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, et al. , 2014. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int. J. Oncol 45, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. , 2008. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem 283, 20015–20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Sinclair DA, 2014. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci 35, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbi ME, Hu H, Kshitiz, Gilkes DM, Semenza GL, 2013. Sirtuin-7 inhibits the activity of hypoxia-inducible factors. J. Biol. Chem 288, 20768–20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, et al. , 2012. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 11, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, et al. , 2009. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. A Biol. Sci. Med. Sci 64, 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K, 2011. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res 71, 3196–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriondo O, Rabano M, Domenici G, Carlevaris O, Lopez-Ruiz JA, Zabalza I, et al. , 2015. Distinct breast cancer stem/progenitor cell populations require either HIF1alpha or loss of PHD3 to expand under hypoxic conditions. Oncotarget 6, 31721–31739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. , 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol 6, 1122–1128. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, et al. , 2013. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23, 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, et al. , 2013. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab 18, 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Johnson FB, 2014. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS One 9, e97729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M, 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. , 2005. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem 280, 17038–17045. [DOI] [PubMed] [Google Scholar]
- Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, et al. , 2005. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology 6, 27–37. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, et al. , 2010. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 11, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitsinou PP, Haase VH, 2008. The VHL tumor suppressor and HIF: insights from genetic studies in mice. Cell Death Differ 15, 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Simon MC, 2007. Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick DC, 1973. The effects of infantile stimulation and intermittent fasting and feeding on life span in the black-hooded rat. Dev. Psychobiol 6, 225–234. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. , 2002. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. , 2010. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell 17, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, et al. , 2011. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 20, 487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, et al. , 1991. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 338, 899–902. [DOI] [PubMed] [Google Scholar]
- Knudson AG Jr., 1971. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl. Acad. Sci. U.S.A 68, 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ, 2002. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr. Rev 23, 623–646. [DOI] [PubMed] [Google Scholar]
- Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL, 1997. Cognitive effects of a long-term weight reducing diet. Int. J. Obes. Relat. Metab. Disord 21, 14–21. [DOI] [PubMed] [Google Scholar]
- Krycer JR, Sharpe LJ, Luu W, Brown AJ, 2010. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol. Metab 21, 268–276. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS, 1998. Substrate and site specificity of hydrogen peroxide generation in mouse mitochondria. Arch. Biochem. Biophys 350, 118–126. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS, 2000. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys 373, 16–22. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E, 2012. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ 344, e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Larsson NG, 2013. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med 273, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y, 2008. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem 283, 27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau BR, Laszlo J, Stengle J, Burk D, 1958. Certain metabolic and pharmacologic effects in cancer patients given infusions of 2-deoxy-D-glucose. J. Natl. Cancer Inst 21, 485–494. [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, et al. , 2010. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 70, 1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. , 2012. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med 4, 124ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen HJ, Kiuru M, Ylisaukko-Oja SK, Salovaara R, Herva R, Koivisto PA, et al. , 2006. Increased risk of cancer in patients with fumarate hydratase germline mutation. J. Med. Genet 43, 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Kaeberlein M, 2010. The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging. Biol. Chem 391, 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. , 2014. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. , 2009. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li X, Miller RA, 2014. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13, 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Feng L, Liu H, Wang J, Kasembeli M, Tran MK, et al. , 2016. PARP inhibition suppresses growth of EGFR-mutant cancers by targeting nuclear PKM2. Cell Rep 15, p843–p856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW, 2010. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 38, 864–878. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE, 2003. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299, 1342–1346. [DOI] [PubMed] [Google Scholar]
- Longo VD, Fontana L, 2010. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol. Sci 31, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP, 2014. Fasting: molecular mechanisms and clinical applications. Cell Metab 19, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Ellerby LM, Bredesen DE, Valentine JS, Gralla EB, 1997. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol 137, 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, et al. , 2015. Interventions to slow aging in humans: are we ready? Aging Cell 14, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, et al. , 2011. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia F, Wei M, Yuan V, Hu J, Gattazzo C, Pham P, et al. , 2009. Oncogene homologue Sch9 promotes age-dependent mutations by a superoxide and Rev1/ Polzeta-dependent mechanism. J. Cell Biol 186, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JC, Krishan A, Lampidis TJ, 2004. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother. Pharmacol 53, 116–122. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L, 2005. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 3, e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F, 2008. Cancer-related inflammation. Nature 454, 436–444. [DOI] [PubMed] [Google Scholar]
- Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, et al. , 2014. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One 9, e100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Villalba JM, Navas P, de Cabo R, 2011. NRF2, cancer and calorie restriction. Oncogene 30, 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. , 2013. Metformin improves healthspan and lifespan in mice. Nat. Commun 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, et al. , 2004. 2-Deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64, 31–34. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. , 2012. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, et al. , 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Alarcon T, Joven J, 2014. Gerometabolites: the pseudohypoxic aging side of cancer oncometabolites. Cell Cycle 13, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, et al. , 2012. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl. Acad. Sci. U.S.A 109, E187–E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M, 2005. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Kelly B, Logan A, Costa AS, Varma M, Bryant CE, et al. , 2016. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, et al. , 2014. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 13, 769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. , 2007. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Smith DL Jr., Sossong AM, Kaushik S, Poosala S, Spangler EL, et al. , 2010. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol. Appl. Pharmacol 243, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, et al. , 2011. SRT1720 improves survival and healthspan of obese mice. Sci. Rep 1, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirisola MG, Taormina G, Fabrizio P, Wei M, Hu J, Longo VD, 2014. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet 10, e1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, et al. , 2014. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, et al. , 2004. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin. Cancer Res 10, 7031–7042. [DOI] [PubMed] [Google Scholar]
- Muller H, de Toledo FW, Resch KL, 2001. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scand. J. Rheumatol 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Muller-Hocker J, 1989. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart—an age-related phenomenon. A histochemical ultracytochemical study. Am. J. Pathol 134, 1167–1173. [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. , 2007. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med 13, 619–624. [DOI] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B, 2014. Long-term metformin usage and cognitive function among older adults with diabetes. J. Alzheimers Dis 41, 61–68. [DOI] [PubMed] [Google Scholar]
- No author, 1998. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865. [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, et al. , 2008. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135, 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunshola OO, Antoniou X, 2009. Contribution of hypoxia to Alzheimer’s disease: is HIF-1alpha a mediator of neurodegeneration? Cell. Mol. Life Sci 66, 3555–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaimi J, Masters CL, McLean C, Opeskin K, McKelvie P, Byrne E, 1999. Irregular distribution of cytochrome c oxidase protein subunits in aging and Alzheimer’s disease. Ann. Neurol 46, 656–660. [PubMed] [Google Scholar]
- Olivieri F, Rippo MR, Monsurro V, Salvioli S, Capri M, Procopio AD, et al. , 2013. MicroRNAs linking inflamm-aging, cellular senescence and cancer. Ageing Res. Rev 12, 1056–1068. [DOI] [PubMed] [Google Scholar]
- Ostan R, Lanzarini C, Pini E, Scurti M, Vianello D, Bertarelli C, et al. , 2015. Inflammaging and cancer: a challenge for the Mediterranean diet. Nutrients 7, 2589–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. , 2010. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem 285, 8340–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, et al. , 2005. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging 26, 995–1000. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. , 2008. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CR, Hagemann I, Bock T, Buschard K, 1999. Intermittent feeding and fasting reduces diabetes incidence in BB rats. Autoimmunity 30, 243–250. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI, 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med 350, 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. , 2016. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, et al. , 2014. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging” Eur. J. Immunol 44, 1552–1562. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L, 2007. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet 3, e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn A, Hulver M, Cefalu W, York D, Smith SR, 2006. Unsupervised clustering of gene expression data points at hypoxia as possible trigger for metabolic syndrome. BMC Genomics 7, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. , 2008. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. U.S.A 105, 8215–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Pearson ER, Sakamoto K, 2013. Molecular mechanism of action of metformin: old or new insights? Diabetologia 56, 1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richie JP Jr., Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA, 1994. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J 8, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N, 2006. Mitochondrial hexokinases, novel mediators of the anti-apoptotic effects of growth factors and Akt. Oncogene 25, 4683–4696. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Teichert S, Niemann B, Franke C, Katschinski DM, 2008. Caloric restriction counteracts age-dependent changes in prolyl-4-hydroxylase domain (PHD) 3 expression. Biogerontology 9, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, et al. , 2014. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet 10, e1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman NB, Carling D, Prentki M, Cacicedo JM, 2013. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest 123, 2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. , 2009. Fasting and cancer treatment in humans: a case series report. Aging (Albany NY) 1, 988–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. , 2012. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One 7, e44603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roman I, Barja G, 2013. Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction. Exp. Gerontol 48, 1030–1042. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni FF, Akbar A, Arens R, Chiu YL, et al. , 2014. New advances in CMV and immunosenescence. Exp. Gerontol 55, 54–62. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. , 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol 14, 1296–1302. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. , 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Verdin E, 2007. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504. [DOI] [PubMed] [Google Scholar]
- Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, et al. , 2016. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, et al. , 2012. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. , 2005. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7, 77–85. [DOI] [PubMed] [Google Scholar]
- Semenza GL, 2010. HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev 20, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ, 2009. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevah O, Laron Z, 2007. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm. IGF Res 17, 54–57. [DOI] [PubMed] [Google Scholar]
- Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA, 2012. Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. BMC Cancer 12, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim HS, Wei M, Brandhorst S, Longo VD, 2015. Starvation promotes REV1 SUMOylation and p53-dependent sensitization of melanoma and breast cancer cells. Cancer Res 75, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, et al. , 2012. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr.) 34, 917–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Manchanda S, Kaur T, Kumar S, Lakhanpal D, Lakhman SS, et al. , 2015. Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 16, 775–788. [DOI] [PubMed] [Google Scholar]
- Skinner HD, McCurdy MR, Echeverria AE, Lin SH, Welsh JW, O’Reilly MS, et al. , 2013. Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol 52, 1002–1009. [DOI] [PubMed] [Google Scholar]
- Soda K, Kano Y, Chiba F, Koizumi K, Miyaki Y, 2013. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS One 8, e64357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soefje SA, Karnad A, Brenner AJ, 2011. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol 6, 125–129. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, et al. , 2014. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, et al. , 2008. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, et al. , 2008. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc. Natl. Acad. Sci. U.S.A 105, 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh DH, Kim MK, No JH, Chung HH, Song YS, 2011. Metabolic approaches to overcoming chemoresistance in ovarian cancer. Ann. N. Y. Acad. Sci 1229, 53–60. [DOI] [PubMed] [Google Scholar]
- Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. , 2011. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc. Natl. Acad. Sci. U.S.A 108, 4129–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, et al. , 2010. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606. [DOI] [PubMed] [Google Scholar]
- Tannenbaum A, Silverstone H, 1949. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res 9, 724–727. [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL, 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest 123, 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE, 1994. Nutritional regulation of the insulin-like growth factors. Endocr. Rev 15, 80–101. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. , 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14, 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. , 2002. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet 30, 406–410. [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, et al. , 2000. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N. Engl. J. Med 342, 1171–1177. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Marzuki S, 1989. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet 1, 637–639. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, et al. , 2001a. Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358, 1754–1759. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Muller A, Janssen JA, Davis RJ, Zib KA, Scarlett JA, et al. , 2001b. Control of tumor size and disease activity during cotreatment with octreotide and the growth hormone receptor antagonist pegvisomant in an acromegalic patient. J. Clin. Endocrinol. Metab 86, 478–481. [DOI] [PubMed] [Google Scholar]
- van der Lely AJ, Biller BM, Brue T, Buchfelder M, Ghigo E, Gomez R, et al. , 2012. Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab 97, 1589–1597. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Richardson A, 2001. Oxidative damage to mitochondria and aging. Exp. Gerontol 36, 957–968. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB, 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, et al. , 2013. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J. Am. Heart Assoc 2, e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, 2015. NAD(+) in aging, metabolism, and neurodegeneration. Science 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R, Kumar D, Dube SN, Singh R, Pandey KS, Bag BC, et al. , 2006. Acute toxicity and cardio-respiratory effects of 2-deoxy-D-glucose: a promising radio sensitiser. Biomed. Environ. Sci 19, 96–103. [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. , 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. , 2010. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem 21, 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wang L, Li Y, Hu H, Shen L, Shen X, et al. , 2014. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin. Cancer Res 20, 4107–4114. [DOI] [PubMed] [Google Scholar]
- Ward PS, Thompson CB, 2012. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AE, Brunet A, 2014. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem. Sci 39, 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, et al. , 2008. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet 4, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. , 2008. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U.S.A 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu SQ, Huang D, 2013. Dietary restriction depends on nutrient composition to extend chronological lifespan in budding yeast Saccharomyces cerevisiae. PLoS One 8, e64448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JW, Boudreau DM, Park Y, Simonds NI, Freedman AN, 2014a. Commonly used diabetes and cardiovascular medications and cancer recurrence and cancer-specific mortality: a review of the literature. Expert Opin. Drug Saf 13, 1071–1099. [DOI] [PubMed] [Google Scholar]
- Wu LE, Gomes AP, Sinclair DA, 2014b. Geroncogenesis: metabolic changes during aging as a driver of tumorigenesis. Cancer Cell 25, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YA, Han WF, Morin PJ, Chrest FJ, Pizer ES, 2002. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res 279, 80–90. [DOI] [PubMed] [Google Scholar]
- Yang M, Soga T, Pollard PJ, 2013. Oncometabolites: linking altered metabolism with cancer. J. Clin. Invest 123, 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen KE, Bittinger MA, Su SM, Fantin VR, 2010. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene 29, 6409–6417. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, et al. , 2013. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 18, 519–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, et al. , 2013. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun 435, 597–602. [DOI] [PubMed] [Google Scholar]
- Zhong D, Xiong L, Liu T, Liu X, Liu X, Chen J, et al. , 2009. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J. Biol. Chem 284, 23225–23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. , 2010. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, et al. , 1997. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U.S.A 94, 13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]