Abstract
Vertebrates release glucocorticoids during stressful events. If stress occurs during reproduction, the resulting offspring can show altered phenotypes that are thought to arise from increased exposure to maternal glucocorticoids. Developing offspring can metabolize maternal glucocorticoids, which can alter the pattern of exposure they encounter. For egg laying vertebrates, we are just beginning to understand how embryonic steroid metabolism impacts embryonic exposure to maternal glucocorticoids. Here we injected three doses of radioactive corticosterone into Japanese quail (Coturnix japonica) eggs to determine the degree of embryonic exposure at days six and nine of development. We found that increasing injection dose increased the amount of radioactivity found in the embryo at day six but by day nine the effect of injection dose disappeared as the amount of radioactivity within the embryo dropped to equivalent levels for all three doses. Interestingly, when examined as a percentage of initial dose, there were no differences between treatment groups at any time points. Importantly, using thin-layer chromatography we characterized that some free steroid, putatively identified as corticosterone, does reach the developing embryo. Together, our data suggest that the in ovo metabolism of maternal corticosterone can eventually eliminate it from the egg, but before this happens, embryos developing in eggs with elevated amounts of maternal corticosterone are exposed to higher levels early in development. This has important implications for how we understand the developmental steroid environment and the mechanisms underlying maternal stress effects.
Keywords: Glucocorticoids, Maternal effects, Developmental endocrinology, Corticosterone, Avian, Stress
1. Introduction
All living organisms endure an ever-changing environment where competition, predation, ambient conditions, and food supply fluctuate (McEwen and Wingfield, 2003). Survival and reproductive success depends on reacting to these stressors with behavioral and physiological changes that increase the probability of survival (McEwen and Wingfield, 2003). In vertebrates, the response to stress is mediated in part by the release of glucocorticoid hormones (GCs) through activation of the hypothalamic-pituitaryadrenal (HPA) axis (Sapolsky et al., 2000, Wingfield and Kitaysky, 2002). Evidence from a variety of vertebrate taxa, ranging from humans to fish, shows that exposing females to stressful conditions during reproduction can result in long-lasting phenotypic effects to her offspring (e.g. Henriksen et al., 2011, Moisiadis and Matthews, 2014, Nesan and Vijayan, 2013). There are currently a number of hypotheses that aim to provide a framework for how maternal stress effects may or may not confer fitness advantages in a context dependent manner (Gluckman et al., 2005, Love and Williams, 2008, Romero et al., 2009). However, while these potential evolutionary consequences are interesting and deserving of study, our current understanding of the underlying mechanistic causes is still ambiguous; in other words we may be putting the evolutionary cart before the mechanistic horse.
One mechanistic hypothesis posits that maternal stress effects are the result of direct embryonic exposure to elevated levels of maternal GCs, also known as the excess GC exposure hypothesis (Seckl and Holmes, 2007). For this reason, a substantial amount of work was devoted to characterizing how maternal stress influences GC levels in the developing offspring (Saino et al., 2005, Moisiadis and Matthews, 2014). One major finding of this work is that the placenta acts as a metabolic buffer that can effectively modulate embryonic GC exposure (Jansson and Powell, 2007, Braun et al., 2013). Specifically, the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) is highly expressed in the placenta and converts active GCs such as cortisol and corticosterone to inactive forms, effectively limiting embryonic exposure to active GCs (Cottrell et al., 2014). Additional mechanistic hypotheses suggest that the effects of maternal stress are the result of changes in maternal physiology altering the amount of resources available to the developing embryo suggesting that maternal stress effects can arise independently of embryonic exposure to maternal GCs (Cottrell et al., 2012).
Unfortunately, it is difficult to disentangle how maternal physiology, placental function, and the embryonic response individually influence the effects of maternal stress, given their intimate and dynamic relationship. Manipulation of any one component typically disrupts the other components simultaneously. However, in oviparous species, the embryonic endocrine environment can be manipulated without any confounding changes in maternal physiology making them particularly valuable to explore underlying mechanisms (Groothuis and Schwabl, 2008). Experimental biologists have already taken advantage of these models to characterize some of the impacts of maternal stress effects on offspring phenotype (Henriksen et al., 2011). Furthermore, evolutionary biologists have utilized oviparous species to investigate the adaptive potential of maternal stress effects on offspring development (Love et al., 2013). The general approach of these studies, done primarily in birds, is to mimic exposure to excess maternal GCs by injecting additional GCs into the egg (Henriksen et al., 2011).
Recent work has shown that not all maternal stress effects require embryonic exposure to maternal GCs (Cottrell et al., 2012), so it is not always appropriate to equate effects observed after GC injections to maternal stress effects. Only through further characterization of underlying mechanisms will researchers be able to verify whether phenotypic effects arise directly via increased GC exposure. Likewise, simply observing an elevation in maternal GC levels or yolk GC levels following maternal stress does not mean that embryos are being exposed to excess GCs during development. Our work in Japanese quail (Coturnix japonica) shows that, similar to placental vertebrates, birds also possess a metabolic buffer in ovo that regulates embryonic exposure to GCs (Vassallo et al., 2014). In this study, corticosterone was metabolized to a conjugated metabolite (putatively identified as a glucuronide) that accumulated in the yolk, and to a lesser extent, the embryo, throughout development. Following an injection of 0.4 ng of corticosterone in the yolk, even at their highest, levels of free steroids found within the embryo only represented 0.4% of the injected corticosterone, while most radioactivity was present as a metabolite contained within the yolk (Vassallo et al., 2014). A similar study in chickens (Gallus gallus), which limited exploration of steroid metabolism to the first six days of development, also found prolific metabolism of tritiated corticosterone in ovo (von Engelhardt et al., 2009). Since neither of these studies confirmed that the radioactivity within the embryo was corticosterone itself, and not some other ether-soluble metabolite, it remains to be demonstrated that maternal corticosterone reaches the embryo without being metabolized. Thus, in ovo metabolism of GCs may provide a mechanism through which embryos are protected from excess GC exposure, and therefore, the activity of this metabolic buffer has important implications for actual embryonic GC exposure.
Currently, it is unclear how the embryonic metabolic buffer responds to varying concentrations of maternal GCs. To address this, we performed an experiment in Japanese quail (Coturnix japonica) to examine how varying concentrations of maternal GCs are regulated during development and to determine if higher concentrations of maternal GCs are more likely to reach the embryo before being metabolized. We had three, mutually-exclusive, alternative hypotheses regarding how the embryonic metabolic buffer responds to different concentrations of maternal GCs in the yolk. First, the metabolic buffer may metabolize an absolute amount of GCs to prevent embryonic exposure. GC concentrations above this threshold would overwhelm the buffer and affect the embryo. Second, the metabolic buffer may be flexible such that increasing concentrations of GCs induce an increased rate of metabolism. In this scenario, maternal GCs may still reach the developing embryo, but we would expect the concentration of GCs reaching the embryo to be proportional to the concentration of maternal GCs initially available. Third, the capacity of the metabolic buffer may be high enough that all GCs could be metabolized across the physiological range of maternal GCs, and thus no GCs would reach the embryo. Complicating matters further, initial levels of maternal GCs could alter the timing and duration of embryonic exposure. Determining which, if any, of these hypotheses is supported is important to understand if, when, and to what degree maternal corticosterone can reach the embryo. This in turn is necessary to further our mechanistic understanding of, if, and how GC exposure underlies maternal stress effects.
2. Materials and methods
2.1. Overview
Eggs in this study were collected from a breeding population of Japanese quail (Coturnix japonica) maintained at Bucknell University. Multiple breeding quail were housed in six pens and provided food and water ad libitum. All eggs used were collected within 24 h of laying and injected immediately upon collection with the doses of 1,2,6,7-3H-corticosterone (3H-CORT; PerkinElmer) specified in Section 2.3. All doses of corticosterone were dissolved in sesame oil and a final injection volume of 5 μL was used. Eggs were then incubated at 37.8C and 50–65% humidity in a Sportsman Incubator (GQF Manufacturing Co.). At the time of sampling, eggs were removed from the incubator, wrapped in tinfoil, and placed in a −20C freezer. Eggs were then processed for analysis of steroid movement and prepared for Thin Layer Chromatography (TLC) for putative characterization of metabolites as described in greater detail in Section 2.5. All procedures were conducted with approval from the Bucknell University Institutional Animal Care and Use Committee.
2.2. Injection Validation
We did preliminary work to determine how injecting GCs in the yolk or albumen affected distribution and metabolism of GCs during days six and nine of development. Embryonic distribution of 3H-CORT did not differ by injection site, but we did observe differences in 3H-CORT levels in the yolk and albumen compartments at both time points based on injection site (Supplementary Fig. 1). Additionally, the site of injection did not impact the identity of the primary metabolites formed, as characterized by thin layer chromatography (data not shown). Because there were some differences in the distribution of 3H-CORT based upon injection site, we chose to inject into the yolk since yolks tend to contain higher concentrations of maternal GCs (Larsen et al., 2015).
2.3. 3H CORT dosage
Eggs (n=48) collected from our breeding population (Section 2.1) were injected into the yolk with one of three doses of 3H-CORT to determine how increasing GC levels impacts embryonic exposure to corticosterone and to determine whether corticosterone that has not been metabolized is able to reach the developing embryo in ovo. The low dose was approximately 0.5 ng, the medium dose was approximately 2.2 ng, and the high dose was approximately 3.7 ng of [3H]-corticosterone. The low dose was chosen to be comparable to our previous work (Vassallo et al., 2014) and within the physiological range of yolk corticosterone in Japanese quail (Hayward et al., 2006). The medium dose was chosen to elevate corticosterone levels in ovo to two standard deviations above the average concentration (Hayward et al., 2006). The high dose was chosen to be pharmacological with the intention of attempting to overwhelm the metabolic buffer. Eggs from each dosing level were incubated for either six or nine days (n=8 at each day and each dose; 6 day Low dose (6dL), 6 day Medium dose (6dM), 6 day High dose (6dH), 9 day Low dose (9dL), 9 day Medium dose (9dM), 9 day High dose (9dH)). Incubation periods of six and nine days were chosen because in between these days a substantial portion of injected GCs were metabolized in both the yolk and extra-embryonic compartments (Vassallo et al., 2014). Further, day 6 was the first timepoint at which radioactivity could be detected within the embryo. Samples were prepared for ether and water-soluble metabolite tracking as described in Section 2.4.
2.4. Distribution of radioactivity
To characterize the distribution of radioactivity within each egg, eggs were thawed such that the yolk, albumen, and embryo could be separated from one another. Egg components were then weighed and thoroughly homogenized using either a metal spatula (yolk and albumen) or mechanical tissue homogenizer (embryo). Approximately 100 mg of each egg component was subjected to ether extraction as described by Vassallo et al. (2014) to separate apolar, ether-soluble metabolites and polar, water-soluble metabolites. Briefly, homogenized tissue was extracted twice in methanol and following centrifugation the supernatant was collected. Samples were dried under nitrogen gas and reconstituted in distilled water and ethyl ether and the two phases were separated by snap freezing the aqueous portion in a methanol ice bath. Aliquots of the ether and water fraction were counted in duplicate using a Tri-Carb 2910 TR Liquid Scintillation Counter (Perkin Elmer).
2.5. TLC characterization of metabolites:
TLC was used to verify whether any non-metabolized corticosterone was present within the embryo (Vassallo et al., 2014). Briefly, homogenized embryos were diluted in methanol in a 1:5 g:mL ratio. Samples were centrifuged at 950 × g for 10 min at 4 °C and two 5 mL aliquots of the resulting supernatant were each diluted in 45 mL of double distilled water. Diluted samples were run through Sep Pak solid phase extraction columns (Waters) that were prepared according to the manufacturer’s instructions in order to concentrate the metabolites. After passing the sample through the column, 5 mL of ether was run through the column to elute ether-soluble, free steroids. Ether-soluble steroids were concentrated by placing samples in a 40 °C water bath and drying under a stream of nitrogen gas. Dried samples were reconstituted in 100 μL of a steroid standard cocktail (in 100% ethanol) consisting of androstenedione, testosterone, cortisol, dihydrocorticosteorone, and corticosterone used as an internal control to assess potential metabolite formation. TLC was run in a 95:5 (v:v) Methanol:Chloroform solvent system to separate steroids. Developed plates were analyzed for the migration of radioactivity according to the methods of Paitz et al. (2012). Metabolite identity was putatively confirmed by assessing co-migration of radiolabeled peaks with non-radiolabeled steroid stan-dards.
2.6. Statistical analysis
We used general linear models in SPSS 22 to compare steroid levels (either total radioactivity level or percentage of injected radioactivity) in the yolk, albumen, and embryo homogenate for each dose and day of incubation. In all models, dose (low, medium, or high), day of incubation (day six or nine), and steroid form (free or conjugated) were included as fixed factors as well as all of the possible interactions and egg was included as a random effect. Post-hoc comparisons were carried out using Fisher’s LSD tests.
3. Results
3.1. Overall recovery
The amount of radioactivity recovered from eggs after 6 days of incubation was 0.49 ng, 2.3 ng, and 3.9 ng for the low, medium, and high doses respectively. After 9 days of incubation, 0.43 ng, 2.1 ng, and 3.9 ng of radioactivity were recovered from each respective dose.
3.2. Glucocorticoid distribution - concentration
Embryo.
Within the embryo, as the dose of corticosterone increased so did the amount of free and conjugated steroid on day six of development (F2,287=63.91, p < 0.0001), with conjugated steroid concentrations being more abundant (Fig. 1). For all doses, concentrations of both free and conjugated steroid decreased in the embryo between days six and nine of development (F1,143=32.36, p < 0.0001).
Fig. 1.
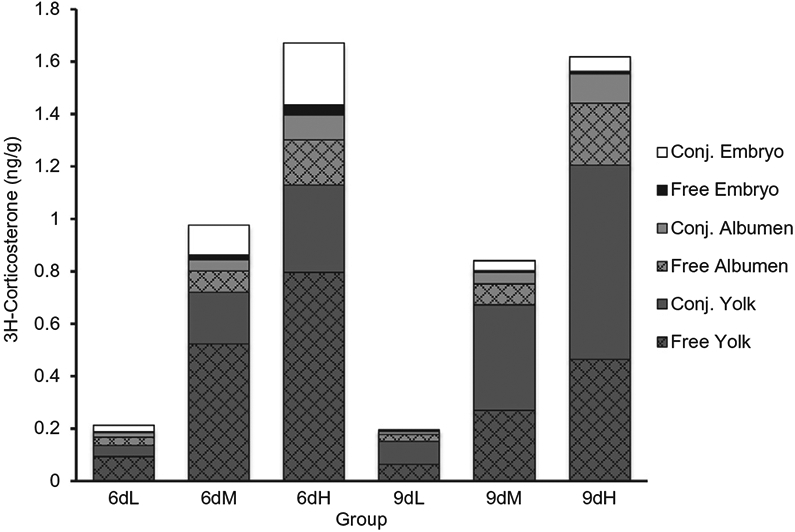
Concentration (ng/g) of recovered steroid throughout egg compartments on days six and nine of embryonic development. Polar (Conj.) and apolar (Free) steroids were extracted from the yolk, albumen, and embryo and the concentration of recovered steroid was calculated (n=8 per day per dose; 6 day Low dose(6dL), 6 day Medium dose (6dM), 6 day High dose (6dH), 9 day Low dose (9dL), 9 day Medium dose (9dM), 9 day High dose (9dH)).
Yolk.
Overall, the vast majority of recovered radioactivity was detected in the yolk on both day six and day nine of development ( ~ 80%, F2,287=163.73, p < 0.0001). Furthermore, the concentrations of conjugated steroid within the yolk increased during development at all three doses, whereas the concentration of free steroid decreased over development (F1,96=21.52, p < 0.0001).
Albumen.
Steroid concentrations within the albumen did not differ between days six and nine of development, and at both timepoints free steroid was more abundant than conjugated steroid (F1,96=12.47, p=0.0007).
3.3. Glucocorticoid distribution – percentage
Embryo.
Surprisingly, although the concentration of conjugated and free steroid that was present within the embryos on day six increased with injection dose, these concentrations represented a statistically similar percentage of the injected radioactivity (F2,96=1.68, p=0.19; Fig. 2). Regardless of dose, the proportion of total injected hormone in the embryo for both forms of steroid dropped between day 6 and 9 (F2,96=69.95, p < 0.0001), with free steroid decreasing from 2.04 ± 0.80% to 0.53 ± 0.46% and conjugated steroid decreasing from approximately 13.42 ± 3.74% to 4.00 ± 2.94.
Fig. 2.
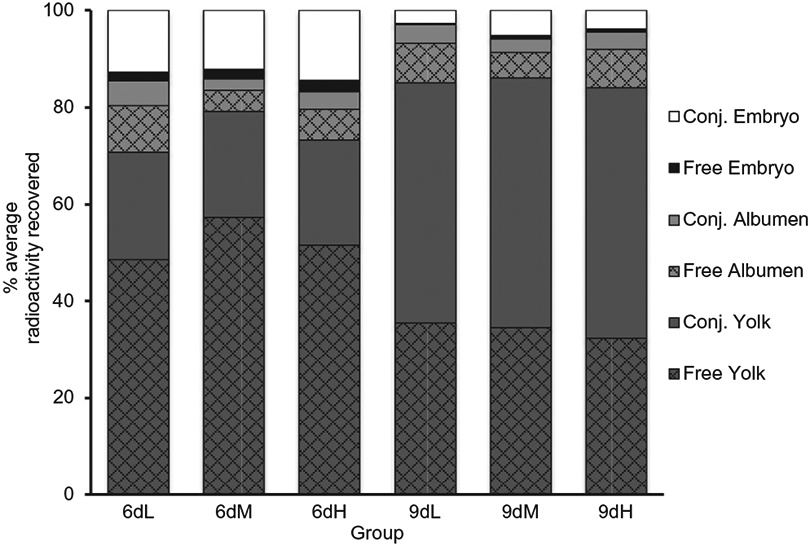
Percentage of recovered radioactivity throughout egg compartments on days six and nine of embryonic development. Polar (Conj.) and apolar (Free) steroids were extracted from the yolk, albumen, and embryo and the percentage of recovered steroid was calculated (n=8 per day per dose; 6 day Low dose (6dL), 6 day Medium dose (6dM), 6 day High dose (6dH), 9 day Low dose (9dL), 9 day Medium dose (9dM), 9 day High dose (9dH)).
Yolk and Albumen.
Furthermore, the percentage of free and conjugated steroid did not differ across the doses within both the yolk (F2,96=1.28, p=0.35) and the albumen (F2,96=0.66, p=0.52, Fig. 2). Across all three doses the percentage of conjugated steroid increased within the yolk from day 6 to day 9 (F1,48=331.85, p < 0.0001) while the percentage of free steroid decreased (F1,48=62.24, p < 0.0001). However, the percentages of free (F1,48=0.02, p=0.89) and conjugated steroid (F1,48=0.09, p=0.75) within the albumen did not differ between time points.
3.4. Thin layer chromatography
Given the relatively low concentrations of free steroid present in the embryo, TLC could only be run on ether extracts from two high dose embryo samples. TLC results suggest that two major compounds were found within the embryo. One radiolabeled compound co-migrated with the corticosterone standard (Fig. 3, sections 10/11). Another radiolabeled compound comigrated with both cortisol and dihydrocorticosterone standards (Fig. 3, sections 13/14). Since there was not enough sample available to confirm the identity of the radiolabeled compounds co-migrating with the corticosterone standards, the possibility remains that this compound is a metabolite of corticosterone and not corticosterone itself.
Fig. 3.
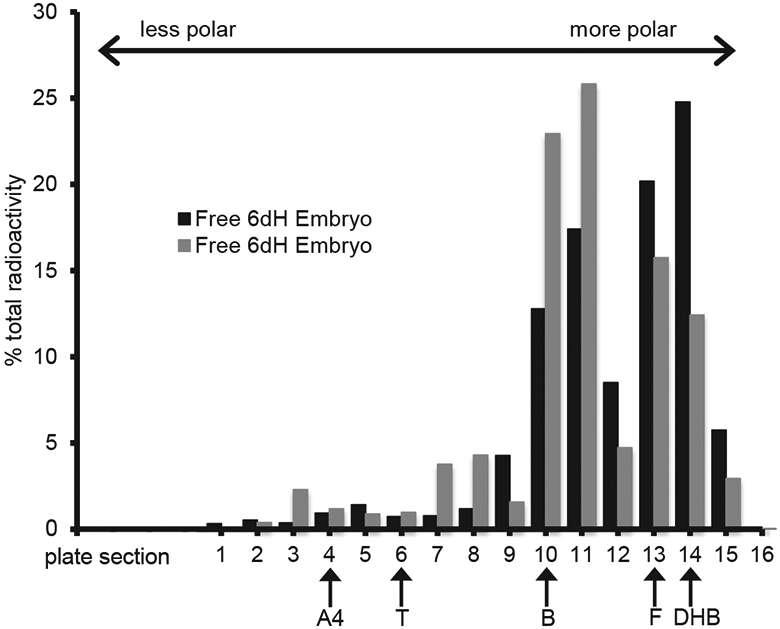
Thin layer chromatography based separation of radioactive compounds from two, day 6 high dose apolar (Free) embryo sample fractions. Samples were spotted at section 15 and were run in a solvent system in which free steroids migrate based on their polarity. Plate section 1 represents the point of greatest migration (least polar). Non radioactive steroid standards were run concurrently and their final positions are marked with arrows (A4, androstenedione; T, testosterone; B, corticosterone; F, cortisol; DHB, dihydrocorticosterone).
4. Discussion
Here we demonstrated that embryonic exposure to maternal corticosterone is significantly influenced by the concentration of GCs that is initially present in the yolk. Specifically, increasing the concentration of GC injected in the yolk, which mimics increased levels of maternally derived corticosterone, led to significant increases in embryonic exposure to free corticosterone and its metabolites (Figs. 1 and 3). Such exposure is dynamic as levels within the embryo change during the course of development (Figs. 1 and 2). With regards to corticosterone, it appears that exposure is elevated at around day six of development and then drops at day nine. Interestingly, no differences were observed between the three doses of GCs in terms of the percentage of total injected radioactivity (Fig. 2). Our findings therefore support the hypothesis that embryonic GC exposure is proportional to maternal GC concentration. The presence of free steroid in the embryo across all three doses rules out the alternative hypotheses that either the metabolic buffer has a set threshold below which everything is metabolized or that it has a capacity that exceeds the physiological range of maternal GCs. The finding that embryonic exposure was similar across all doses at day 9, despite differing just three days prior, additionally excludes the hypothesis that duration of GC exposure may increase with increasing maternal GC concentration. Overall, this would suggest that during the earlier stages of development, embryos from oviparous species are exposed to a small, but predictable, percentage of the maternal steroid present at the time of laying.
Only our highest dose of 3.7 ng resulted in enough ether soluble radioactivity within the embryo for TLC to be used to assess the identity of the recovered steroids. While other studies have demonstrated that injected radioactivity is capable of reaching the embryo in ovo (von Engelhardt et al., 2009, Vassallo et al., 2014), neither of these studies were able to confirm that the recovered embryonic radioactivity was indeed free corticosterone and not some other ether soluble steroid. Our TLC data suggest that some of the corticosterone originally injected into the yolk can reach the embryo without first being metabolized (Fig. 3). These data therefore support maternally derived corticosterone as a mediator in maternal stress effects concordant with the excess GC exposure hypothesis. Several lines of evidence confirm the critical role that active embryonic modulation of the developmental steroid environment plays in dictating embryonic exposure patterns (Reed and Clark, 2011). In European starling eggs, injected progesterone is metabolized only by eggs with a developing embryo and no metabolism is observed in eggs that were subjected to a localized freeze/thaw cycle which prevented embryonic development (Paitz and Casto, 2012). Similarly, unfertilized rock pigeon eggs do not show signs of metabolism and maternal enzymes are not present in avian eggs (Kumar et al., 2018a,b). These data implicate the embryo as the driving force behind the metabolism of maternal steroids. They additionally make our observation that the relatively small amount of ether soluble radioactivity, corresponding to active steroids that have not been metabolized, which can be found in the embryo at any one time even more striking. The highest observed amount on day six only represents about 2% (range of the three doses 1.7–2.3%) of the radioactivity within the entire egg and this drops to about 0.5% by day nine (range of the three doses 0.2–0.7%). A similar drop in the levels of water-soluble radioactivity from 14% to 4% is also observed over this timespan (Fig. 2), which demonstrates that corticosterone and its metabolites are not accumulating within the embryo early in development.
The decrease in the overall amount of embryonic radioactivity is accompanied by an increase in the amount of conjugated yolk radioactivity. It is tempting to speculate that free steroids enter the embryo from the yolk where they are metabolized to conjugated steroids and afterwards return to the yolk. However, more work needs to be done to further explore this hypothesis and other explanations are also feasible, especially given that the metabolites found in the yolk and embryo have not been fully identified. Additionally, the presence of free corticosterone in embryos does not necessarily mean that it is capable of inducing maternal stress effect phenotypes. The embryo may modulate the ability of free corticosterone to exert its effects in a number of non-metabolic ways. For example, levels of circulating corticosterone binding globulin impact the bioavailability of corticosterone in the bloodstream (Breuner and Orchinik, 2002). A more careful assessment of the distribution of corticosterone and its metabolites within the embryo may shed light on whether processes other than steroid metabolism are also at work.
It is currently unclear whether the small differences in embryonic steroid concentration observed between our doses may be responsible for phenotypes attributed to maternal stress effects. A recent report found that maternal glucocorticoid concentrations in ovo may be much lower than previously thought (Larsen et al., 2015). The authors used LC/MS/MS rather than enzyme- or radio-immunoassay (EIA/RIA) to measure corticosterone concentrations in lesser black-backed gull eggs in order to avoid known problems with antibody cross-reactivity. The authors determined that yolk and albumen concentrations of corticosterone were 3–6 and 5–500 times lower respectively than previously reported for other species. The injection doses used in this study were based on corticosterone concentrations measured by RIA (Hayward et al., 2006). If quail eggs are also found to have concentrations of corticosterone much lower than reported, our results warrant different interpretation. In this case, all doses used would likely be pharmacological in nature and the embryonic corticosterone we identified may be an artifact of the doses rather than reflective of the underlying physiology. Regardless, incorporation of LC/MS/MS measurements would enable detection of even extremely low concentrations of corticosterone and its metabolites in the embryo while using doses in a physiological range. Further, measurement of corticosterone concentrations with LC/MS/MS within various embryonic tissues (e.g. brain and liver) could potentially elucidate where corticosterone and its metabolites are accumulating during development and provide evidence of non-metabolic modulation strategies.
Previously we have shown that by day nine of development, the majority of the injected corticosterone has been metabolized and is present in the yolk as a conjugate where it remains until later stages of development when the embryo reabsorbs the conjugated metabolite and minute amounts of remaining free steroid (Vassallo et al., 2014). Taken together with the data presented here, these studies suggest that embryos may be exposed to free maternal steroids early in development and exposed to the conjugated metabolites later in development. Although it is important to note that based on our injections conjugated metabolites are also present early in development, and free maternal steroids are still present later in development. While the activity of metabolites formed from corticosterone is unknown, given their prevalence in the egg during development, and the precedent for other maternally derived steroid metabolites to influence embryonic development (Paitz and Bowden, 2013), corticosterone metabolite activity should be studied in the context of maternal stress effects. Furthermore, the importance of the timing of early exposure to mostly free steroids and later exposure to mostly conjugated metabolites has yet to be determined and should be an area of active focus.
Another layer of complexity is added when the origin of maternal steroids found in the embryo is considered. Although we injected only into the yolk for our dose manipulations (Figs. 1 and 2), the albumen may also represent a sizeable pool of maternal GCs given the tendency of albumen mass to be larger than yolk mass (Larsen et al., 2015). There is evidence that the timing of maternal stress may impact whether maternal GCs are increased in the yolk or albumen (Rettenbacher et al., 2005). Laying hens were injected with radioactive GCs and eggs that were further along in development, and therefore laid shortly after the injection, had radioactive GCs in the albumen and outer layers of the yolk (Rettenbacher et al., 2005). Eggs that were not as developed during the injection, and were therefore laid several days after the injection, had radioactive GCs in the inner layers of the yolk. Here we also injected a low dose into either the yolk or albumen to determine if the site of injection influenced the resulting in ovo steroid environment. Distribution of GCs differed within the yolk and albumen depending on site of injection, but we did not see differences in the distribution of steroid within the embryo after 6 or 9 days of development (Supplementary Figs. 1 and 2). We were unable to confirm that the embryonic metabolites that resulted from each site of injection were identical, leaving open the possibility that GCs may be differentially metabolized in the embryo depending on site of origin within the egg. Overall, while our data suggests the site of injection does not have major consequences for embryonic exposure patterns, future studies utilizing exogenous GCs, especially those seeking to mimic a chronically stressed mother, may consider injecting into both the yolk and albumen.
A previous study found that radioactive GC formed a droplet on top of the yolk following injection into chicken egg yolks and one day of incubation (von Engelhardt et al., 2009). While this droplet dissipated by day 3 of incubation, radioactive GCs were not homogenously distributed throughout the yolk or the rest of the egg. The distribution of maternal GCs throughout the egg is currently unknown. A mother deposits yolk in concentric layers (Grau, 1976), but the ability of maternal GCs to diffuse throughout various layers is untested. A systematic study comparing how maternal GCs are distributed within the egg and how exogenous GC injections are distributed would provide important insight into the physiological relevance of injection models and allow for explicit testing of how GC distribution impacts its metabolic fate and contributes to embryonic exposure.
An improved understanding of the timing and magnitude of uptake of corticosterone from the yolk, as shown here, has important implications for studies of the mechanisms underlying maternal stress in birds. Up until this point, we have focused on the fate of the maternal corticosterone already present in the egg when it is laid. During development, avian embryos also develop the capacity to produce glucocorticoids de novo (Jenkins and Porter, 2004), which means that corticosterone absorbed from the yolk will potentially be added to an endogenous pool. At this point, we have not measured endogenous levels of corticosterone within our embryos, and we don’t know how maternal corticosterone might influence the embryo’s own production of corticosterone. The fact that only about 2% of injected corticosterone was present in the embryo indicates that studies examining how injections of non-radioactive corticosterone influence embryonic exposure may not be able to detect corticosterone concentration changes within the embryo with current analytical methods. Further, it is unclear what concentration change in the embryo, and therefore what difference in initial injection dose, is required to elicit changes in offspring phenotype. From our data, we see that injecting 3.7 ng of corticosterone into the yolk resulted in an average of approximately 50 pg of corticosterone in embryos that weighed on average 2.5 g on day 6 (data not shown). Thus this increase of only 20 pg/g within the embryo may not be detectable over and above what the embryo could be producing at the time and more importantly may not actually result in changes to offspring phenotype. On the other hand, the embryo may in fact be sensitive to such minor differences early in development. Studies from domestic fowl (Gallus domesticus) suggest that embryos begin producing corticosterone potentially starting at day 8 (Jenkins and Porter, 2004). Furthermore, GC receptors are present in chicken embryos starting as early as day 3 of development (Pavlik et al., 1986). While it is difficult to account for differences in in ovo development time between species when making comparisons with our work, it is feasible that the embryonic exposure to maternal corticosterone over the first nine days of development leads to the initial setting of the HPA axis which is responsible for endogenous corticosterone production. Previous work from Zimmer and Spencer (2014) has shown that prenatal stress alters GC receptor expression in offspring at multiple sites on the HPA axis. Our results therefore provide a context for studying embryonic responses to maternal corticosterone at the molecular level. Since most corticosterone initially present in the yolk is metabolized by day nine, this suggests that embryonic responses to direct corticosterone exposure are likely to happen within this period. Understanding when quail embryos begin endogenous production of corticosterone and when and where GC receptors are expressed within quail embryos will be crucial for further mechanistic studies examining embryonic responses to maternal glucocorticoids.
Overall the work presented here offers important insight into the process by which embryonic exposure to maternal steroids occurs. Here, we have shown that injection dose influences embryonic exposure during development and that there is a possibility of active maternal corticosterone being present within the embryo. While we do not presume to have tested an exhaustive list of explanatory hypotheses, our data rule out several alternative hypotheses and support the notion that the embryonic metabolic buffer is flexible in its capacity to metabolize maternal corticosterone, based upon the initial concentration present in ovo. Importantly, although we have identified the first nine days of embryonic development as a critical period of small yet constant exposure to maternal steroids, we have yet to determine whether the metabolites formed during this period are biologically active. Future work should seek to clarify the role of these metabolites and to further elucidate the molecular mechanisms underlying maternal stress effects.
Supplementary Material
Acknowledgements
Research was supported by a National Institute of Health grant (1R15 HD083870-01A1) to M.F.H and R.T.P. We thank C. Rhone, A. Hackenberg and other members of the Bucknell Animal Care staff for their assistance with animals and V. Fasanello, R. Glynn and K. Tindley for help in the laboratory.
Footnotes
Declarations of interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygcen.2018.11.013.
References
- Braun T, Challis JR, Newnham JP, Sloboda DM, 2013. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and longterm disease risk. Endocr. Rev 10.1210/er.2013-1012. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M, 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ, Seckl JR, 2012. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J. 26, 1866–1874. 10.1096/fj.12-203489. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR, Holmes MC, Wyrwoll CS, 2014. Foetal and placental 11B-HSD2: a hub for developmental programming. Acta Physiol, 10.1111/apha.12187. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol 20, 527–533. 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Grau CR, 1976. Ring structure of avian egg yolk. Poult. Sci 10.3382/ps.0551418. [DOI] [Google Scholar]
- Groothuis TG, Schwabl H, 2008. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philos. Trans. R. Soc. Lond. B. Biol. Sci 363, 1647–1661. 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Richardson JB, Grogan MN, Wingfield JC, 2006. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. Gen. Comp. Endocrinol 146, 144–148. 10.1016/j.ygcen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG, 2011. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL, 2007. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin. Sci 113, 1–13. 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- Jenkins SA, Porter TE, 2004. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domest. Anim. Endocrinol, 10.1016/j.domaniend.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kumar N, van Faassen M, de Vries B, Kema I, Gahr M, Groothuis TGG, 2018a. Gonadal steroid levels in rock pigeon eggs do not represent adequately maternal allocation. Sci. Rep 10.1038/s41598-018-29478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, van Faassen M, Kema I, Gahr M, Groothuis TGG, 2018b. Early embryonic modification of maternal hormones differs systematically among embryos of different laying order: a study in birds. Gen. Comp. Endocrinol, 10.1016/j.ygcen.2018.08.014. [DOI] [PubMed] [Google Scholar]
- Larsen TR, Fairhurst GD, De Baere S, Croubels S, Müller W, De Neve L, Lens L, 2015. Novel insights into relationships between egg corticosterone and timing of breeding revealed by LC-MS/MS. J. Avian Biol 46, 643–647. 10.1111/jav.00735. [DOI] [Google Scholar]
- Love OP, Williams TD, 2008. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am. Nat 172, E135–E149. 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Love OP, Mcgowan PO, Sheriff MJ, 2013. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Funct. Ecol 27, 81–92. 10.1111/j.1365-2435.2012.02040.x. [DOI] [Google Scholar]
- McEwen BS, Wingfield JC, 2003. The concept of allostasis in biology and biomedicine. Horm. Behav 10.1016/S0018-506X(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, Matthews SG, 2014. Glucocorticoids and fetal programming part 1: outcomes. Nat. Rev. Endocrinol 10, 391–402. 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- Nesan D, Vijayan MM, 2013. Role of glucocorticoid in developmental programming: evidence from zebrafish. Gen. Comp. Endocrinol 10.1016/j.ygcen.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol 895–901. 10.1093/icb/ict027. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Casto JM, 2012. The decline in yolk progesterone concentrations during incubation is dependent on embryonic development in the European starling. Gen. Comp. Endocrinol 10.1016/j.ygcen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Sawa AR, Bowden RM, 2012. Characterizing the metabolism and movement of yolk estradiol during embryonic development in the red-eared slider (Trachemys scripta). Gen. Comp. Endocrinol 176, 507–512. 10.1016/j.ygcen.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Pavlik A, Novotna B, Jelinek R, 1986. Glucocorticoid receptor-mediated teratogenesis and cell-proliferation in the limbs and face of the chick-embryo. Teratog. Carcinog. Mutagen 6, 441–450. 10.1002/tcm.1770060510. [DOI] [PubMed] [Google Scholar]
- Reed WL, Clark ME, 2011. Beyond maternal effects in birds: responses of the embryo to the environment. Integr. Comp. Biol 73–80. 10.1093/icb/icr032. [DOI] [PubMed] [Google Scholar]
- Rettenbacher S, Möstl E, Hackl R, Palme R, 2005. Corticosterone in chicken eggs. Ann. NY Acad. Sci 10.1196/annals.1343.016. [DOI] [PubMed] [Google Scholar]
- Romero LM, Dickens MJ, Cyr NE, 2009. The reactive scope model – A new model integrating homeostasis, allostasis, and stress. Horm. Behav 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP, 2005. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. Part A Comp. Exp. Biol 303, 998–1006. 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck a.U., 2000. How do glucocorticoids influence stress responses? preparative actions. Endocr. Rev 21, 55–89. 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Holmes MC, 2007. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab 3, 479–488. 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF, 2014. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol. Lett 10, 13–16. 10.1098/rsbl.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N, Henriksen R, Groothuis TGG, 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol 163, 175–183. 10.1016/j.ygcen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Kitaysky AS, 2002. Endocrine responses to unpredictable environmental events: stress or anti-stress hormones? Integr Comp Biol 42, 600–609. 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- Zimmer C, Spencer KA, 2014. Modifications of glucocorticoid receptors mRNA expression in the hypothalamic-pituitary-adrenal axis in response to early-life stress in female Japanese quail. J. Neuroendocrinol 26, 853–860. 10.1111/jne.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


