Abstract
Objective:
Iron is emerging as a key player in aging-associated diseases due to its propensity for driving free radical formation. Studies examining the role of iron in the pathogenesis of primary osteoarthritis (OA) are limited. Our objective was to establish a direct relationship between excess iron and OA by administering iron dextran to a guinea pig strain with decreased propensity for developing OA.
Design:
Twenty, 12-week-old Strain 13 guinea pigs received either iron dextran or dextran control intraperitoneally once weekly for 4 weeks; termination occurred at 16 weeks of age. Iron levels were determined systemically (serum and liver), within femoral head articular cartilage, and infrapatellar fat pads (IFPs) of knee joints. One knee was collected to score structural changes associated with OA via microcomputed tomography (microCT) and histology using published grading schemes. Articular cartilage and IFPs were harvested from contralateral knees for gene expression analyses.
Results:
Iron overload was confirmed systemically via increased serum iron and iron staining in the liver. Articular cartilage and IFPs in the iron dextran group also had higher levels of iron. Excess iron worsened knee OA using both microCT and histologic scoring systems. Gene analyses revealed that exogenous iron altered the expression of iron trafficking proteins, select cytokines, and structural components of cartilage.
Conclusion:
These results demonstrate that systemic iron overload caused cellular iron accumulation in the knee joint. This excess iron is associated with increased expression of local inflammatory mediators and early onset and progression of knee joint OA in Strain 13 animals.
Keywords: guinea pig, Strain 13, iron, aging
Introduction.
Iron is a ubiquitous element that participates in numerous physiologic processes, including hemoglobin synthesis and oxidative phosphorylation. Unfortunately, excess levels can produce reactive oxygen species via the Fenton or Haber Weiss reactions1,2. Despite this, there are no excretion mechanisms for iron beyond turnover of skin and enterocytes, and tissue iron accumulation occurs during aging3,4. Indeed, progressive iron accrual has been linked to many age-associated chronic diseases, including type II diabetes, neurodegenerative disorders, atherosclerosis, and cancer5,6. Additionally, iron overload within joint tissues has been implicated in arthropathies associated with hereditary hemochromatosis7, rheumatoid arthritis8,9, traumatic arthropathy, and hemophilic arthropathy10. In these conditions, iron can accumulate from two sources: blood that enters the joint from either trauma or inflamed synovium and/or exchange from the non-heme iron pool9,11. Several human studies have shown hemosiderin deposits in cartilage and synovium, as well as increased ferritin in the synovial fluid of affected joints8,9. In vivo work has demonstrated that iron-overloaded synoviocytes release pro-inflammatory cytokines, including interleukin-1β (IL-1β), IL-6, and tumor necrosis factor (TNF), that stimulate catabolic activity in chondrocytes10. Iron also has a direct effect on cartilage by inducing hydroxyl radical-driven chondrocyte apoptosis10,12 and the breakdown of matrix components13,14.
As age is the largest risk factor for primary/idiopathic OA15, we theorize that iron accumulation may also be associated with the development of this disease. Interestingly, its role in the pathogenesis of OA has been explored in a handful of manuscripts. One study found that increased serum ferritin was correlated with more severe knee cartilage damage in individuals with primary OA; this finding was independent of age, sex, and BMI16. Another manuscript demonstrated that synovial fluid iron concentrations were significantly higher in patients with OA than age-matched healthy subjects17. Finally, iron deposition in synovium has been reported in individuals with OA18. This evidence suggests that iron may play a role in both aging and knee OA and holds potential as a mechanistic connection between these coinciding conditions.
Because of this intriguing association, the current work was designed to demonstrate a direct relationship between excess cellular iron accumulation and OA in the absence of a genetic disorder. We hypothesized that administration of exogenous iron, resulting in moderate iron overload, would incite pathology in Strain 13 guinea pigs, a strain with decreased propensity for OA19.
Materials and Methods.
Animals.
Procedures were approved by the Institutional Animal Care and Use Committee and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Group size was determined from a pilot study. Using a within group error of 0.5 and a detectible contrast of 1.0 (based on histologic assessment of OA) in a linear regression model considering treatment and sex, power associated with a Tukey’s significant difference post-hoc analysis was calculated as 0.8 with a sample size of 4 per sex per treatment group. To ensure adequate power for all outcomes, 20 Strain 13 guinea pigs (11 males, 9 females) were purchased at 8 weeks of age from the US Army Medical Research Institute of Infectious Disease (Fort Detrick, MD). Animals were housed individually in solid bottom cages and provided standard guinea pig chow, hay cubes, and water ad libitum.
Iron dextran injections.
Injections were initiated when animals were 12 weeks of age and administered under isoflurane anesthesia. Ten guinea pigs (5 males, 5 females) were randomly assigned with respect to sex to the iron overload group; 10 animals (6 males, 4 females) were allocated to the control group. To induce a burden consistent with iron overload disorders, animals in the experimental group were administered 400 mg/kg of iron dextran solution20 intraperitoneally once weekly for 4 weeks. Animals in the control group received 400 mg/kg of dextran solution. Body weights were recorded weekly.
Specimen collection.
Termination occurred when animals were 16 weeks of age. Whole blood was collected via direct cardiac puncture under isoflurane anesthesia. Animals were then transferred to a CO2 chamber for euthanasia. Serum was separated for iron quantification using the Roche Cobas 6000 (Basel, Switzerland). Complete blood count and serum biochemistry profiles were determined (Supplemental Figure S1).
Hind limbs were removed at the coxofemoral joints. The left femurs were measured using calipers. The left limb was placed into 10% neutral buffered formalin for 48 hours and transferred to PBS for microcomputed tomography (microCT) analysis. After microCT, left limbs were transferred to a 12.5% solution of ethylenediaminetetraacetic acid (pH 7) for decalcification. The right knee was dissected for gene expression analyses: the infrapatellar fat pad (IFP) was placed into All Tissue Protect reagent (Qiagen, Hilden, Germany); articular cartilage was isolated from the articular surface of the patella and the weight-bearing regions of the femoral condyles and tibial plateaus, and stored in RNAlater (Qiagen). Right femoral heads were placed into 10% neutral buffered formalin for 48 hours for iron quantification.
Iron quantification by atomic absorption spectroscopy (AAS).
Iron quantification was performed on samples of formalin-fixed liver and femoral head articular cartilage. Briefly, dried tissue weighed, ashed, sonicated in 3.6N nitric acid, and diluted 30-fold with deionized water21. Diluted samples were analyzed using a Model 240 AA flame atomic absorption specrometer and SpectrAA software (Agilent Technologies, Santa Clara, California)22. Iron levels were reported as parts per million (ppm) dry weight (dw)23.
Enhanced iron staining of the IFP.
To identify the distribution of iron in the IFP, an enhanced Prussian Blue staining protocol24 was used on mid-sagittal sections of the knee joint. Negative control slides for each joint were made by following the protocol24 without incubation in potassium ferrocyanide solution. Nikon Elements software (Tokyo, Japan) was used to quantify the iron-positive surface area in the IFP, with respect to the negative control samples. A single photo of the IFP was taken and analyzed for each animal.
Microcomputed tomography of knee joints.
Knee joints were scanned using the Scanco microCT system (Scanco uCT80, Scanco Medical AG, Bruttisellen, Switzerland) with an isotropic voxel size of 18μm. Clinical features of OA were scored blindly in duplicate by a veterinary radiologist (AJM) using a whole joint grading scheme25, with all tissue planes assessed by serial 2D microCT image stacks. Built-in software (Scanco Medical AG IPL v4.05) was used to evaluate bone volume fraction (BV/TV), trabecular number, trabecular thickness, trabecular spacing, and mean tissue mineral density in subchondral trabecular bone for regions of interest (ROI) in the medial/lateral tibia and femur25 from each animal (Supplemental Table S1). ROIs avoided the physis and epiphyseal trabecular bone in all study animals25. Fifty image slices were used to create femoral and tibial ROIs.
Histologic grading using OARSI recommendations.
After decalcification, samples were paraffin-embedded and 5-micron sagittal sections were stained with Toluidine Blue. Medial and lateral femoral condyles and tibias were scored in a blinded fashion by two independent pathologists (LBR and KSS) using published guidelines19. Briefly, this semiquantitative histopathologic grading scheme is based on articular cartilage structure, proteoglycan content, cellularity, and tidemark integrity. One slide from the medial and lateral compartment of each knee joint was graded, for a total of 2 slides assessed per animal. Values from the 4 anatomic locations were summed to obtain a whole knee joint OA score.
Gene expression of cartilage and IFP using NanoString technology.
Total RNA was isolated from knee articular cartilage and IFPs using the RNeasy Lipid Tissue Mini Kit (Qiagen) and sent to University of Arizona Genetics Core (University of Arizona, Tucson, AZ). A custom set of guinea pig-specific probes were designed and manufactured by NanoString Technologies (Seattle, WA) for the following genes: transferrin receptor (TFR), divalent metal transporter 1 (DMT1), zrt- irt- like protein 14 (ZIP14), cluster of differentiation protein 163 (CD163), ferritin heavy chain 1(FTH-1), ferroportin (FPN), collagen type II, aggrecan, IL-1β, TNF, IL-6, and transforming growth factor beta 1 (TGFβ1). Target sequences are presented in Supplemental Table S2. Per Qubit and Fragment Analyzer quality control subsets, the optimal amount of total RNA (150–400 ng) was hybridized with the custom code-set in an overnight incubation (17 hours) at 65°C, followed by processing on the NanoString nCounter FLEX Analysis system. Results were reported as absolute transcript counts normalized to positive controls and two housekeeping genes, β-actin and eukaryotic translation elongation factor 1 alpha 1. Any potential sample input variance was corrected by use of housekeeping genes and application of a sample-specific correction factor to all target probes. Data analysis was conducted using nSolver™ software (NanoString Technologies).
Statistical analyses.
Statistical analyses were performed with GraphPad Prism 8.3.1 (La Jolla, CA, USA). Data underwent normality and variance testing via the Shapiro-Wilk and F Test, respectively. Normally distributed data with similar variance were compared using parametric t tests†. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. Statistical tests used are noted in Figure Legends using superscripts. Statistical significance was set at P ≤ 0.05.
Results.
General animal description.
Animals in the iron dextran group exhibited skin hyperpigmentation but otherwise appeared clinically healthy; no changes in cage activity were noted. Statistical differences in total body weight were not present between the overload and dextran control groups. At the final week of the study, mean total body weight was 698.80 g in the iron overload group and 744.20 g in the dextran control group (95% confidence interval [CI] −136.50 – 45.69 g; P = 0.3089). Body weights of animals throughout the study are included in Supplemental Figure S2. Mean femur lengths between the iron overload (40.42 mm) and dextran control (40.97 mm) groups were also similar, indicating iron overload did not alter skeletal growth in these animals (95% CI −1.46 – 0.36 mm; P= 0.2203).
Liver and serum iron quantification.
Changes in systemic iron levels resulting from iron dextran injections were quantified. Liver iron content was significantly different between the two groups (P < 0.0001) (Figure 1A); this effect was also observed in serum iron concentration (P = 0.0001) (Figure 1B).
Figure 1.
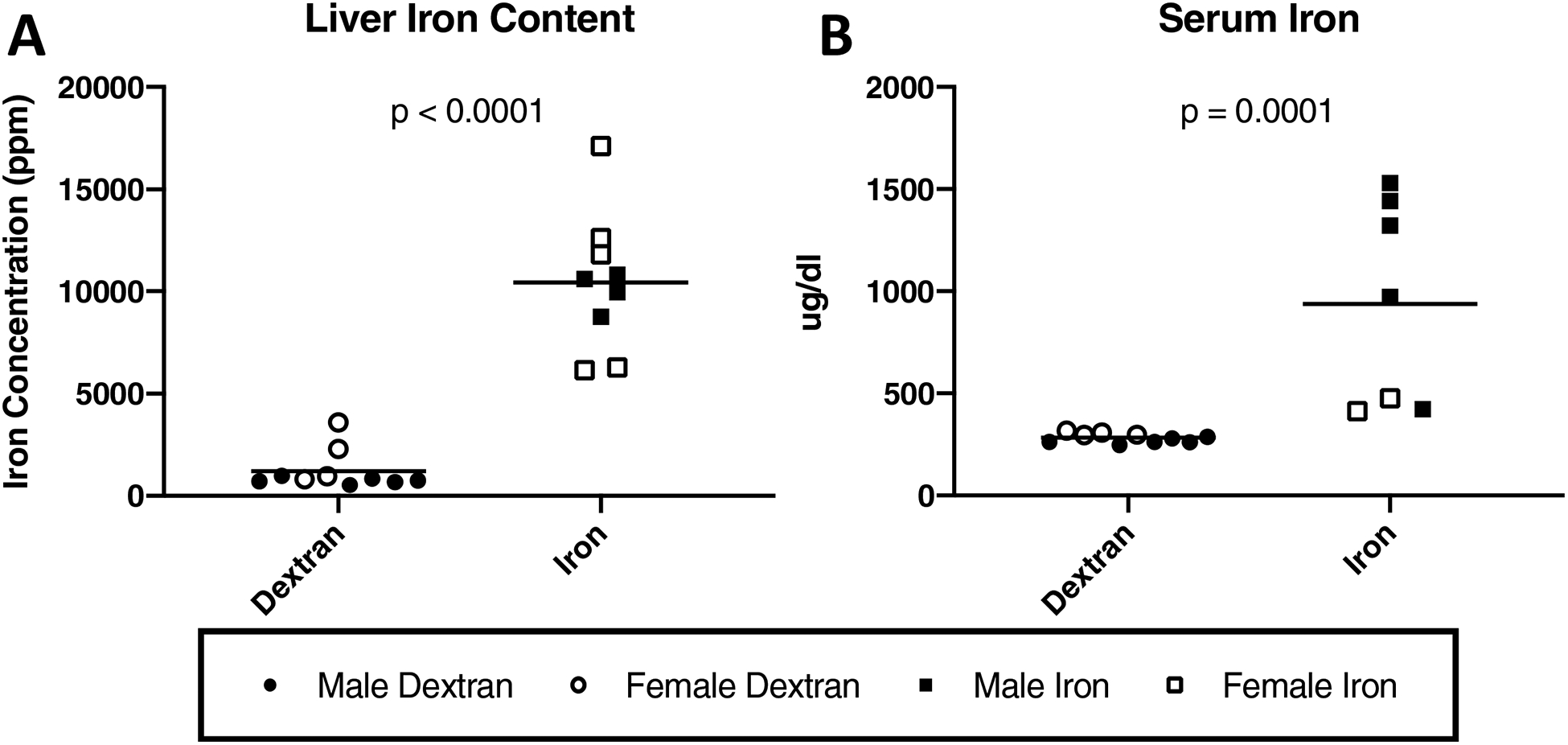
Systemic iron quantification. Black lines on graphs indicate mean values. [A] Liver iron concentrations determined by iron AAS×. Livers from animals in the iron overload group had a mean iron concentration of 10,448.00 ppm (n = 9). Livers from animals in the dextran control group had a mean iron concentration of 1,214.00 ppm (n = 10; 9,777.00 ppm difference between medians). One animal from the iron overload group was unable to be included in liver iron analysis due to lack of available tissue. [B] Serum iron concentration×. Mean serum iron concentration was 939.10 μg/dl in iron overloaded animals (n = 7) and was 281.90 μg/dl in dextran control animals (n = 10; 689.50 μg/dl difference between medians). Serum was unable to be collected from 2 animals in the iron overload group and, as such, these animals were not included in this analysis. Additionally, 1 serum sample from an iron overload animal was identified for preanalytical sample preclusion due to hemolyzed serum. As serum iron measurement can be influenced by hemolysis during blood collection, the hemolyzed sample was excluded from serum iron analysis (n = 7). Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. No significant sex differences were present for liver iron concentration or serum iron concentration.
Enhanced iron staining of the IFP.
An enhanced stain Prussian blue stain was utilized to highlight iron accumulation in the IFP (Figure 2A–B). Relative to the control group, surface area of iron staining was significantly higher in the IFPs from animals in the iron overload group (P < 0.0001) (Figure 2C).
Figure 2.
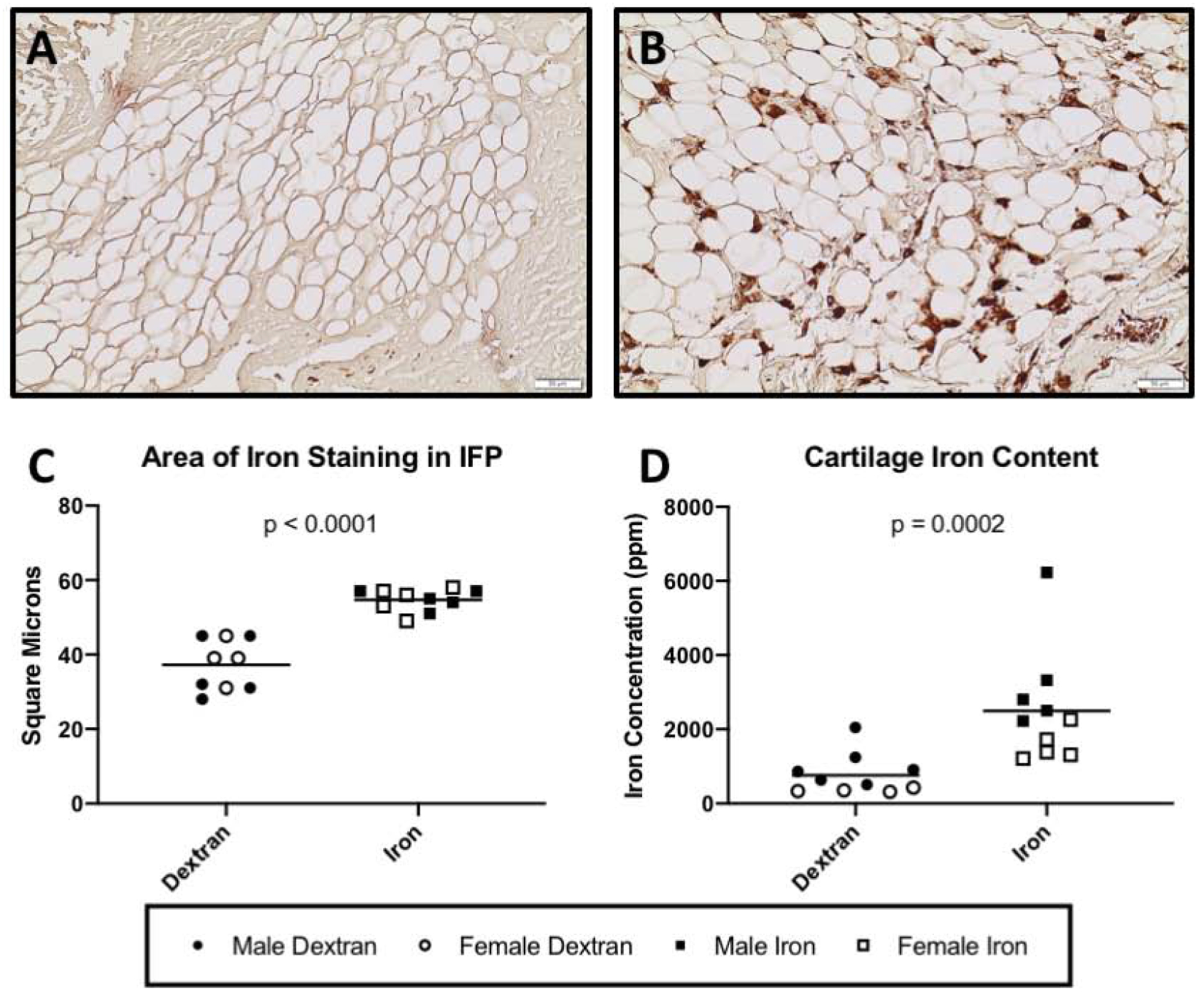
Iron content of knee joint tissue. Black lines on graph represent mean values. [A-C] Enhanced iron stain of IFP◊. Representative images of iron staining in the IFP of a control animal [A] and an iron overload animal [B]. [C] Mean surface area of iron staining was 54.70 square microns in the iron overload group (n = 10) and 37.22 square microns in the dextran control group (n = 9; 95% CI 12.01 – 22.94 square microns). One animal from the dextran control group was unable to be evaluated due to an appropriate tissue section being unavailable. [D] Iron concentration of femoral head articular cartilage by AAS×. Mean concentration of iron within articular cartilage of iron overloaded animals was 2495.00 ppm (n = 10). Mean concentration of iron within articular cartilage of dextran control animals was 765.40 ppm (n = 10; 1,667 ppm difference between medians). Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. No significant sex differences were present for surface area of iron staining in the IFP. Sex differences observed for cartilage iron concentration are presented and discussed in Supplemental Figure S4.
Cartilage iron quantification.
The allocation of knee articular cartilage to transcript expression analyses prevented iron quantification from being conducted for this tissue. Femoral head articular cartilage was submitted for iron AAS to determine if differences were present in a diarthrodial joint environment. Relative to control animals, the concentration of iron was significantly higher within femoral cartilage from iron overloaded animals (P = 0.0002) (Figure 2D).
Whole joint microCT OA scores.
Whole joint microCT OA scores provide an assessment of bony changes observed in the tibia, femur, and patella of each animal. OA scores were higher in the iron overloaded animals, indicating these animals had worsened joint disease than controls (P = 0.0405) (Figure 3A). In the iron overload group, 8 out of 10 animals had small osteophytes and/or enthesophytes on the tibia and/or femur, with 4 of these animals having osteophytes and/or enthesophytes in both locations. Whole joint microCT scores for these animals ranged from 2–8. Two animals in the iron overload group had no evidence of bony lesions and received a score of 0.
Figure 3.
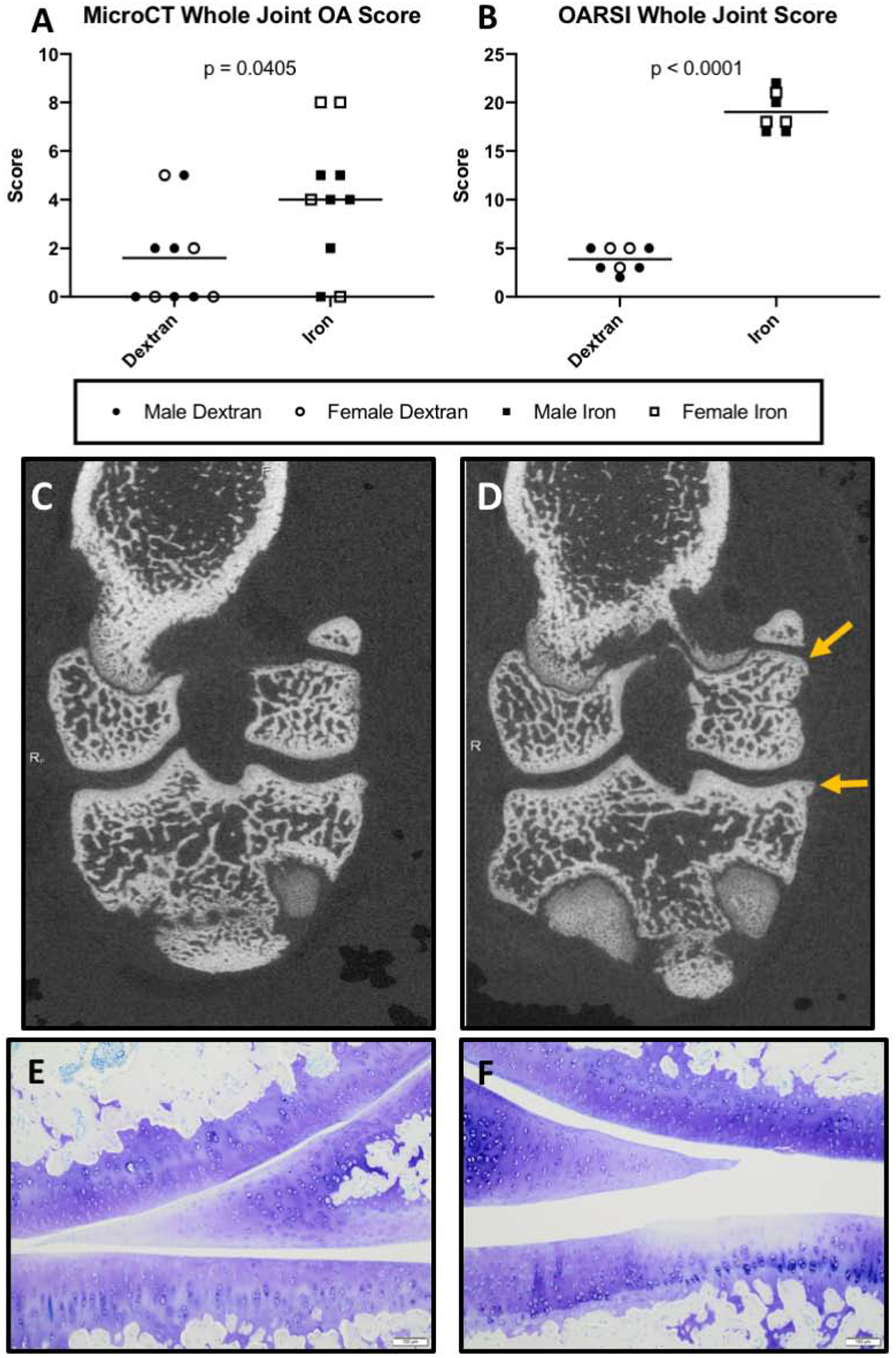
Structural analysis of knee joints. Black lines on graphs represent mean values. [A] Mean whole joint microCT score† was 4.00 in iron overloaded animals (n = 10) compared to 1.60 in dextran control animals (n = 10; 95% CI 0.12 – 4.68). Whole joint microCT score was determined by analyzing radiographic changes typically used in evaluating human OA, such as the presence and location of osteophytes, subchondral bone changes, and articular bone lysis22. [B] Mean whole joint OARSI score† was 19.00 in iron overloaded animals (n = 7) and 3.875 in dextran control animals (n = 8; 95% CI 13.29 – 16.96) (possible range of scores 0 – 84). Five animals (3 from the iron overload group and 2 from the dextran control group) were unable to be evaluated for whole joint OARSI histologic grading due to appropriate tissue sections being unavailable. [C-D] Representative microCT images of [C] a control knee joint with minimal to no radiographic evidence of OA and [D] a knee joint from the iron overload group. Arrows indicate an irregular surface with an enthesophyte forming on the femur and another, more prominent osteophyte on the tibia. [E-F] Photomicrographs of Toluidine blue stained sections from medial compartments of knee joints. [E] Representative image from the control group with a relatively smooth articular cartilage surface, minimal proteoglycan loss, and expected cellularity. [F] Representative image from the iron overload group, which displays a disrupted articular cartilage surface on the tibia and some focal loss of proteoglycans (as evidenced by lighter staining), with chondrocyte loss observed in the same area. Normally distributed data with similar variance were compared using parametric t tests†. No significant sex differences were present for total joint microCT score and total joint OARSI score.
Conversely, 5 animals in the dextran control group had scores of 0 with no radiographic evidence of OA. Remaining animals in the control group had scores ranging from 2–5. Of the 10 animals in the dextran control group, 5 had small osteophytes present on the tibia. Two of these 5 also had small osteophytes on the femur. Representative microCT images are provided (Figure 3C–D).
OARSI histology score.
Histologic OA scores were higher in the iron overload group compared to dextran controls (P < 0.0001) (Figure 3B). When medial and lateral compartments were analyzed separately, the same pattern was noted (Supplemental Figure S3A–B). Representative photomicrographs demonstrating articular cartilage in the medial compartment are depicted (Figure 3E–F). Figure 3E displays normal, healthy cartilage in a control animal. Figure 3F shows an area on the tibial surface with a mildly irregular articular surface, loss of proteoglycan content into the middle layer of cartilage, and chondrocyte loss in an iron overloaded animal. Overall, the higher histologic OA scores for iron overload animals were largely driven by irregular articular cartilage surfaces, proteoglycan loss, and changes to chondrocyte cellularity (Supplemental Figure S3C–E).
Gene expression analysis.
Iron trafficking and storage proteins.
We were curious whether cellular iron metabolism in the cartilage and IFP was affected by systemic administration of iron dextran. Iron is imported into cells by select proteins, including TFR, DMT1, ZIP14, and CD163. Within the cartilage, iron overloaded animals had decreased mRNA expression for TFR (P = 0.0007) and DMT1 (P = 0.0111) while displaying increased transcript expression for the only known cellular iron export protein, FPN (P = 0.0350) (Figure 4A–C). These effects were also observed within the IFPs of iron overloaded animals, with the exception that TFR expression did not change with iron overload in the IFP (P = 0.7529). Treatment with iron dextran did not significantly alter the gene expression of CD163 in the cartilage (P = 0.1564); however, a trend towards decreased CD163 expression was observed in the IFP (P = 0.0524) (Figure 4D). Of note, there was no change in ZIP14 gene expression in either the cartilage or IFP with respect to treatment group (P = 0.4470 and P = 0.9750, respectively) (Figure 4E).
Figure 4.
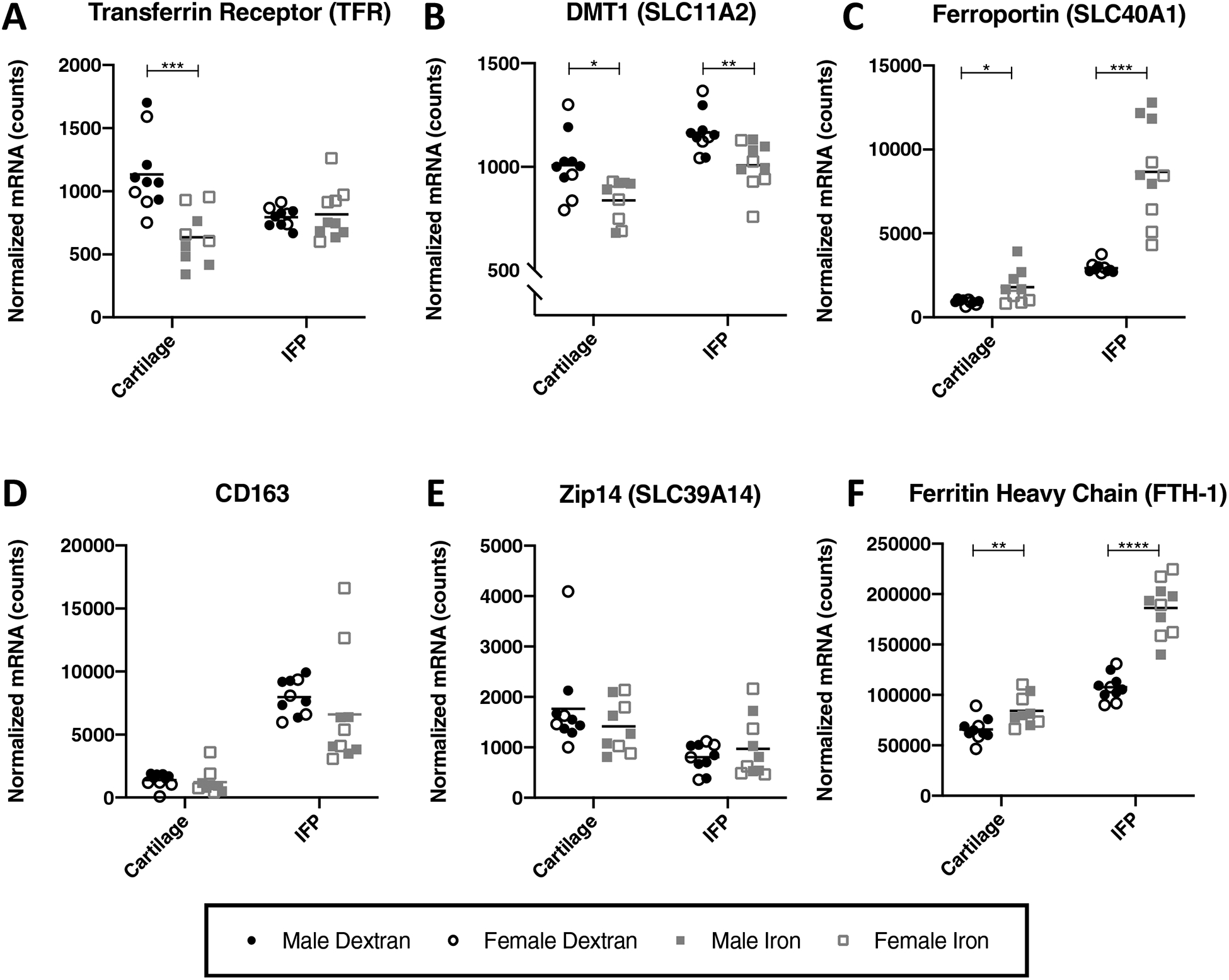
Normalized mRNA counts for iron trafficking proteins in articular cartilage and the IFP. Black lines on graphs represent mean values. [A] TFR†◊ [B] DMT1† [C] ZIP14× [D] CD163× [E] FPN× and [F] FTH-1†◊. One cartilage sample from an animal in the iron overload group did not pass initial quality control for the assay and, as a result, was not analyzed for mRNA expression. As such, the number of animals from the iron overload group for cartilage gene expression analysis was 9, while all other gene expression analyses included 10 animals per group. Normally distributed data with similar variance were compared using parametric t tests†. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. No significant sex differences were present for the genes analyzed.
Within the cell, iron can bind to the storage protein ferritin. Measuring the transcript expression of FTH-1 indicated that, relative to controls, iron overloaded animals had significantly higher mRNA expression of ferritin within the cartilage (P = 0.0082) and IFP (P < 0.0001) (Figure 4F).
Structural components of articular cartilage.
The extracellular matrix (ECM) of articular cartilage is primarily composed of type II collagen and aggrecan26. As OA is characterized by the loss of cartilage within affected joints, changes in the expression of these components can give insight to OA pathogenesis. Gene expression analysis revealed that iron overloaded animals had a lower expression of transcripts for type II collagen (P = 0.0002) and aggrecan (P = 0.0038) than control animals (Figure 5A–B). There was no significant change in the expression of type II collagen (P = 0.1431) and aggrecan (P = 0.1655) within the IFP.
Figure 5.
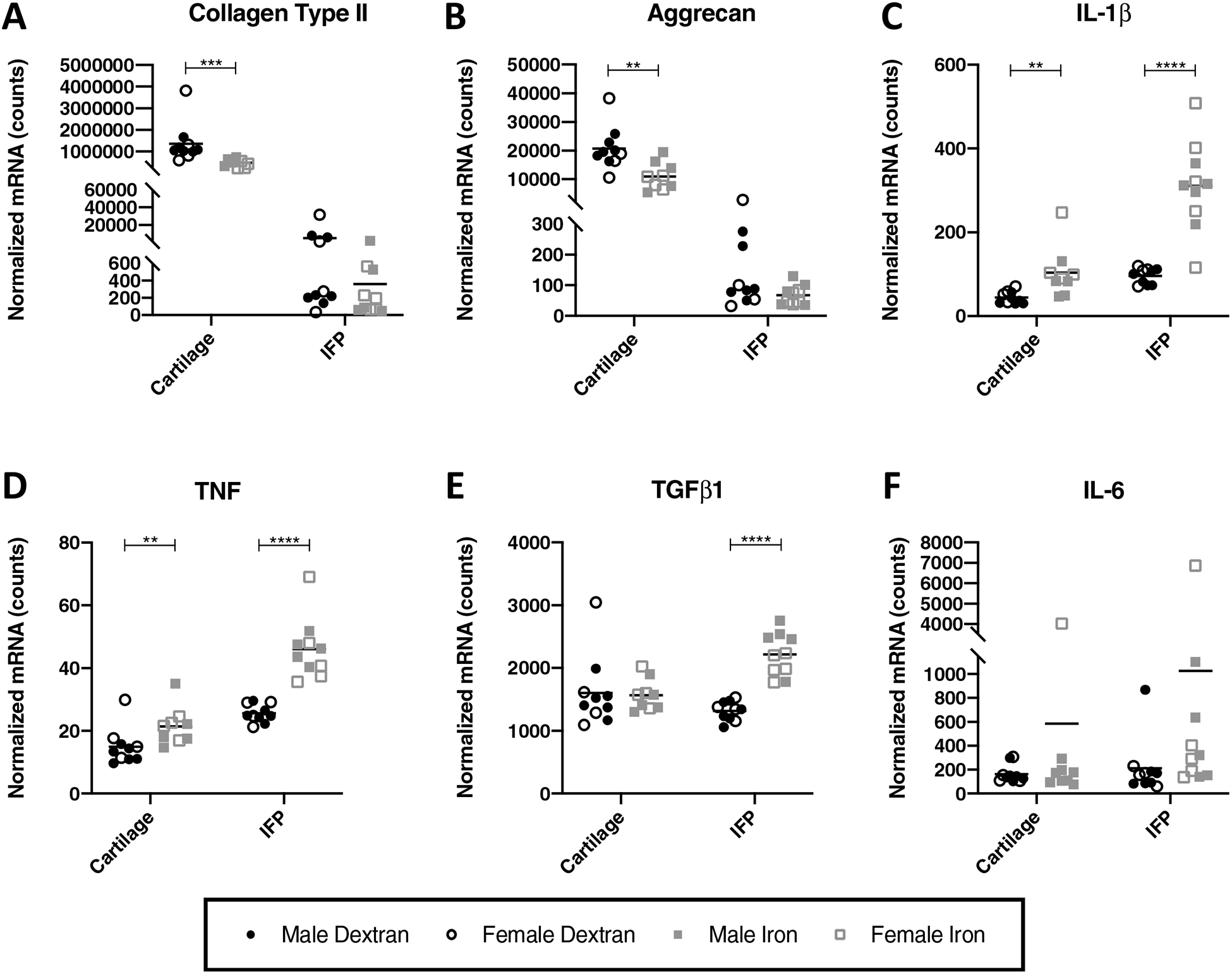
Normalized mRNA counts for select genes in articular cartilage and the IFP. Black lines on graphs represent mean values. [A] collagen type II× [B] aggrecan†× [C] IL-1β× [D] TNF× [E] IL-6× and [F] TGFß1×◊. One cartilage sample from an animal in the iron overload group did not pass initial quality control for the assay and, as a result, was not analyzed for mRNA expression. As such, the number of animals from the iron overload group for cartilage gene expression analysis was 9, while all other gene expression analyses included 10 animals per group. Normally distributed data with similar variance were compared using parametric t tests†. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. No significant sex differences were present for the genes analyzed.
Cytokines.
Administration of iron dextran altered gene expression of the proinflammatory cytokines IL-1β, TNF, and IL-6, as well as the expression of TGFβ1. Relative to control animals, mean transcript expression of IL-1β was significantly higher in the cartilage (P = 0.0015) and IFPs (P < 0.0001) from iron overloaded animals (Figure 5C). Similarly, iron overloaded animals exhibited increased gene expression of TNF in both the cartilage (P = 0.0076) and IFP (P < 0.0001) (Figure 5D). Within the IFP, transcript counts for TGFβ1 were significantly higher for iron overloaded animals (P < 0.0001), and IL-6 demonstrated a trend towards increased expression relative to the control group (P = 0.0524) (Figure 5E–F). Interestingly, treatment with iron dextran did not significantly alter the expression of IL-6 and TGFβ1 in cartilage.
Discussion.
In the current study, we demonstrated that experimental iron overload induced knee joint OA in Strain 13 guinea pigs. This work establishes that exogenous iron overload is detrimental to knee joint health and, as such, indicates that systemic and/or local iron levels may be considered a factor in the development of OA.
Systemic effects of iron dextran administration were measured and, as expected, serum iron levels were increased in the iron overload group. As this parameter often represents only a fraction of the total iron present in the body, we evaluated the extent of iron overload in a section of liver, a key iron storage organ, locally within the knee (IFP), and within a diarthrodial joint environment (femoral head articular cartilage). Results of liver AAS confirmed that we achieved an actual body burden that corresponds to a moderate degree of iron overload, consistent with what is observed in genetic iron overload disorders with associated arthropathies. Similar to our results, patients with transfusion-related iron overload from treatment of thalassemia major have been cited to have hepatic iron concentrations of 3,000.00 ppm – 16,100.00 ppm dw27, while liver iron concentrations in individuals with hereditary hemochromatosis has been measured to be 3,742.00 ppm – 41,040.00 ppm dw28.
Additionally, higher levels of iron were present in the IFPs of iron overloaded animals, which was attributed to accumulation within macrophages. The IFP has emerged as an important player in knee joint homeostasis29. As the IFP is comprised of a network of adipocytes, fibroblasts, and leukocytes30,31, it is prone to be a source of inflammatory mediators that may contribute to OA29,30; 32–34. Iron quantification of femoral head articular cartilage by AAS also revealed a significantly higher concentration of iron in overloaded animals, which was initially undetectable with the enhanced method of iron staining (data not shown). In contrast to the IFP, cartilage does not contain macrophages and is a tissue with low cellularity. Although articular cartilage is avascular, it is able to receive nutrients and other molecules by way of the synovial fluid present within the joint capsule. As synovial fluid is an ultrafiltrate from the blood supply, articular cartilage was likely exposed to systemic iron through the synovial fluid. Collectively, the increased concentration of iron within the knee IFP and femoral head articular cartilage demonstrates that circulating iron does deposit and accumulate within tissues of diarthrodial joints, and may contribute to the degradation that was noted in microCT and histologic evaluation of knee joints.
Both bony and cartilage lesions associated with OA were increased in the iron overload group. These changes are especially striking given the short study duration, young age (16 weeks), and decreased propensity for OA-development by Strain 13 guinea pigs. While Strain 13 animals are still susceptible to OA, they typically exhibit minimal to mild lesions in the medial compartment of knee joints beginning at 12 months of age, and, therefore, are often referred to as OA-resistant35. This is in contrast to the Hartley guinea pig, a strain that develops lesions starting at 3 months of age and is considered a naturally occurring model of OA35.
Using whole joint microCT scoring system, most animals in the iron overload group had more advanced OA due to increased number and/or size of peri-articular osteophytes compared to the control animals. Osteophytes are bony outgrowths arising from the periosteum at joint margins and are a common feature of degeneration in osteoarthritic joints36. These growths form early in the development of disease under the influence of TGFβ1 and may be a source of pain37. Osteophytes are thought to provide added stability to joints with OA38, although other functions may exist. Joint laxity and/or altered biomechanics are not anticipated in untreated Strain 13 animals at the age investigated; therefore, it may be possible that iron overload triggers a biochemical event that incites osteophyte formation. Of note, osteophytes are commonly present in humans with systemic iron overload due to hemochromatosis39. The link between iron overload and osteophyte formation may lie in the BMP/SMAD signaling pathways shared by TGFβ1 and the iron regulator hepcidin40,41. In the present work, this may be supported by the increase in gene expression of TGFβ1 within the IFPs of iron overloaded animals. Despite utilizing similar signaling pathways, the potential connection between iron and osteophyte formation has not been explored, and the interaction between iron overload and bony changes warrants investigation.
The increased development of OA-associated lesions in iron overloaded animals was supported by changes in tissue gene expression. In particular, cartilage degeneration in iron overloaded animals was accompanied by a relative decrease in the expression of transcripts for type II collagen and aggrecan. Previous studies have reported that chondrocyte exposure to iron in vitro decreased the synthesis of the ECM and increased the expression of matrix degrading enzymes42,43. Cartilage lesions occurring with systemic iron overload were also associated with increased transcript counts of the proinflammatory cytokines IL-1β and TNF within both the cartilage and the IFP. Elevated protein levels of IL-1β and TNF have been widely observed in patients presenting with symptomatic knee OA and are considered to be key players in OA pathogenesis44. IL-1β reduces synthesis of type II collagen and aggrecan within articular cartilage and, along with TNF, induces the production of numerous other proinflammatory mediators, such as IL-644. Joint tissues affected by OA have been demonstrated to express higher levels of IL-6 than non-OA joints45, and the IFP has been shown to be a source of IL-6 production in OA-affected knees32. In the current work, gene expression of IL-6 was relatively increased in the IFPs of iron overloaded animals. The presence of IL-6 protein regulates hepcidin, which in turn prompts the degradation of FPN and causes iron to accumulate within cells. The trend towards increased IL-6 mRNA observed within iron overloaded IFPs may have contributed to tissue iron accumulation and, therefore, the OA-associated changes to joint tissues. However, whether this increased IL-6 transcript number was driven by the iron overload or by OA remains to be determined.
Gene expression analysis may suggest that both cartilage and IFPs are able to detect and respond to alterations in systemic iron status. Chondrocytes within iron overloaded cartilage exhibited decreased transcript expression for several proteins responsible for importing iron into the cell, while increasing the transcript expression for the iron export protein, FPN, and the storage protein, ferritin. These changes imply that cells within the cartilage were attempting to regain iron homeostasis, which is a well-documented response when iron stores are replete and/or overloaded46,47. A similar trend was observed in the IFP, with the exception that TFR mRNA levels did not alter relative to iron status. Although the transcripts for TFR and DMT1 both contain at least one iron responsive element and, therefore, would be expected to be similarly regulated, DMT1 transports metals other than iron and may exhibit different expression in this tissue. The observation that ZIP14 mRNA levels did not change with cellular iron status in either joint tissue evaluated is consistent with previously published findings48,49 and suggests that ZIP14 expression is regulated post-translationally49.
While this study provides data coupling iron overload to development of OA, potential limitations of the work should be discussed. It is recognized that the moderate degree of iron overload achieved in a relatively short timeframe is likely not optimal to reflect age-related iron accumulation. Second, animals included in this study were slightly younger than skeletal maturity, which is 16 weeks in guinea pigs19. This timeframe was selected to ensure a lack of OA while avoiding any additional age-related or underlying pathologies that may have confounded data interpretation. Further, while we did not note any changes in behavior or skeletal size between groups over the course of the study, animals in the iron overload group started to exhibit a trend towards decreased weight in the final week of treatment, as well as rust-colored skin hyperpigmentation. Bronzed skin is a common clinical manifestation in patients with unmanaged iron overload disorders50 and we terminated the study after 1 month to ensure proper animal welfare. Despite this, the significant difference in histologic OA scores was maintained between treatment groups after controlling for variations in body weight. Next, it should be mentioned that, except for articular cartilage iron content (Supplemental Figure S4), sex differences were largely absent in the current study. It is possible that the dose of iron utilized for this proof-of-principle study may not have allowed for sex differences to be detected for most experimental outcomes. Finally, it should be noted that changes in gene expression do not necessarily correlate to protein abundance or activity, and additional work is underway to determine the effect of systemic iron levels on protein expression for the genes presented in this work. Overall, it may be worthwhile to determine whether a decrease in iron load prevents and/or delays OA lesions. Studies examining iron trafficking pathways within joint tissues are also needed to increase understanding of how this element contributes to development of primary OA in animals and humans.
Supplementary Material
Supplemental Figure S1. Select CBC and serum biochemistry values. Notably, a significant difference in hematocrit was not present between iron overload and control animals [A]. Evidence of systemic inflammation is supported by a relative increase in platelet counts† [B] and total WBC counts† [C], as well as decreased albumin† [D] and increased globulins† [E]. A significant increase in heterophils, lymphocytes, and monocytes all contributed to the increased WBC counts (data not shown). Increased cholesterol× [F] is likely due to hepatocellular damage from iron overload, as supported by increased ALT× [G] and AST† [H]. The higher AST values may also be due to an increase in creatine kinase, which was also present in iron dextran treated animals (data not shown). Blood and serum were unable to be collected from 2 animals in the iron overload group (n = 8). Additionally, 1 serum sample from an iron overload animal was identified for preanalytical sample preclusion due to hemolyzed serum. As measurement of AST can be influenced by hemolysis during blood collection, the hemolyzed sample was excluded from AST analysis (n = 7). Normally distributed data with similar variance were compared using parametric t tests†. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×.
Supplemental Figure S2. Body weights of animals throughout study. [A] There was no significant difference in the weekly body weights of study animals at any time point throughout the study. During the first week of the study, animals within the iron overload group had a mean body weight of 683.60 g (n = 10) and animals within the dextran control group had a mean body weight of 663.40 g (n = 10; 95% CI −107.00 – 66.60 g). In the second week of the study, animals within the iron overload group had a mean body weight of 691.90 g (n = 10) while animals within the dextran control group had a mean body weight of 686.80 g (n = 10; 95% CI −98.68 – 88.48 g). By the third week of the study, animals within the iron overload group had a mean body weight of 704.40 g (n = 10) and animals within the dextran control group had a mean body weight of 720.40 g (n = 10; 95% CI −81.97 – 114.00 g). At the final week of the study, animals within the iron overload group had a mean body weight of 698.8 g (n = 10) while animals within the dextran control group had a mean body weight of 744.20 g (n = 10; 95% CI −76.23 – 167.00 g). Data was analyzed using a two-way ANOVA with Geisser-Greenhouse correction for unequal variability of differences and Sidak’s post-hoc analysis for multiple comparisons. [B] Relative to the dextran control group, iron overloaded animals gained weight at a decreased rate throughout the study. Between the first and second week of the study†, animals within the iron overload group had a mean weight gain of 8.30 g (n = 10), while dextran control animals had a mean weight gain of 23.40 g (n = 10; 95% CI −32.35 – 2.15 g). Between the second and third week of the study†, iron overloaded animals had a mean weight gain of 12.50 g (n = 10), while dextran control animals had a mean weight gain of 33.60g (n = 10; 95% CI −37.92 – 4.28 g). Between the third and fourth week of the study×, animals within the iron overload group had a mean weight loss of −5.60 g (n = 10), and the dextran control animals had a mean weight gain of 23.80 g (n = 10; −14.50g difference between medians). The study was terminated after 1 month to ensure proper animal welfare. Normally distributed data with similar variance were compared using parametric t tests†. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. Statistical modeling was conducted to confirm that the differences observed in OA development were maintained after controlling for differences in the rate of weight gain between treatment groups. A multiple linear regression model was fit using the whole joint OARSI histologic score as the response variable, treatment group as an independent variable, and the overall difference in the rate of weight gain throughout the study (week 4 – week 1) as a covariate. Based on this model, there is evidence that the significant difference in histologic OA scores between groups is maintained after controlling for differences in the rate of weight gain (estimated difference between treatment groups was 15.04 for whole joint OARSI score; p < 0.0001). As such, the differences in the rate of weight gain do not appear to have impacted the histologic OA changes observed in the present work.
Supplemental Figure S3. Contributions to whole joint OARSI score components. Black lines on graphs represent mean values. [A] Medial compartment OARSI score† (possible range of scores 0 – 42). Mean OARSI histologic score in the medial compartment of the knee was 10.88 in the iron overload group (n = 8) and 1.89 in the dextran control group (n = 9; 95% CI 6.69 – 11.28). Three animals (2 from the iron overload group and 1 from the dextran control group) were unable to be evaluated for medial compartment OARSI histologic grading due to appropriate tissues sections being unavailable. [B] Lateral compartment OARSI score◊ (possible range of scores 0 – 42). Mean OARSI histologic score in the lateral compartment of the knee was 7.88 in the iron overload group (n = 8) and 2.11 in the dextran control group (n = 9; 95% CI 2.70 – 8.83). Three animals (2 from the iron overload group and 1 from the dextran control group) were unable to be evaluated for medial compartment OARSI histologic grading due to appropriate tissues sections being unavailable. [C] Articular cartilage structure† (possible range of scores 0 – 32 for the sum of all compartments). Mean score for articular cartilage structure was 4.14 in the iron overload group (n = 7) and 1.88 in the dextran control group (n = 8; 95% CI 0.76 – 3.78). [D] Proteoglycan content◊ (possible range of scores 0 – 24 for the sum of all compartments). Mean score for proteoglycan content was 7.43 in the iron overload group (n = 7) and was 1.50 in the dextran control group (n = 8; 95% CI 3.85 – 8.00). [E] Cellularity† (possible range of scores 0 – 12 for the sum of all compartments). Mean score for cellularity was 5.57 in the iron overload group (n = 7) and was 0.38 in the dextran control group (n = 8; 95% CI 4.05 – 6.34). [F] Tidemark integrity× (possible range of scores 0 – 4 for the sum of all compartments). Mean score for tidemark integrity was 1.86 in the iron overload group (n = 7) and was 0.13 in the dextran control group (n = 8; 2.00 difference between medians). Five animals (3 from the iron overload group and 2 from the dextran control group) were unable to be evaluated for whole joint OARSI histologic grading due to appropriate tissues sections being unavailable. Normally distributed data with similar variance were compared using parametric t tests†. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×.
Supplemental Figure S4. Sex differences in iron concentration of femoral head articular cartilage. Significant sex differences were present in cartilage iron concentrations both with and without iron dextran treatment. [A] Sex differences in dextran control femoral head cartilage iron concentrations◊. Within the control group, mean cartilage iron concentration was 1035.00 ppm for males (n = 6) and was 360.30 ppm for females (n = 4; 95% CI −1,259.00 – −91.20 ppm). [B] Sex differences in iron overload group cartilage iron concentrations×. Within the iron overload group, mean cartilage iron concentration was 3,414.00 ppm in males (n = 5) and was 1,576.00 ppm in females (n = 5; 1,420.00 ppm difference between medians). This data suggests that males have more iron within articular cartilage tissue than females, even when excess iron is not present systemically. The presence of sex differences for cartilage iron concentration within both treatment groups is intriguing given that sex differences were absent for all other study outcomes, including liver iron concentration. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊.
Acknowledgements.
We would like to thank Crystal Richt and the staff at the University of Arizona Genetics Core; Kevin Daniels and Zaria Torres-Poche at Colorado State University Veterinary Diagnostic Laboratories; and Lauren Culver from the Department of Microbiology, Immunology, and Pathology at Colorado State University for their assistance in generating experimental data. Additionally, we would like to thank the Laboratory Animal Resources staff at Colorado State University for their excellent commitment and care provided to the animals used in this study. Finally, we would like to acknowledge Ann Hess from the Department of Statistics at Colorado State University for consulting with the authors on statistical analyses.
Role of the Funding Source.
NIH R21 AG056807 provided funding to support the acquisition, analysis, and interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests.
No authors have any conflicts of interest to disclose for this work.
References.
- 1.Rigg T, Taylor W, Weiss J. The rate constant of the bimolecular reaction between hydrogen peroxide and ferrous ion. Experientia. 1954;10(5):202–03. doi: 10.1007/bf02159268. [DOI] [PubMed] [Google Scholar]
- 2.Walling C Fenton’s reagent revisited. Acc Chem Res. 1975;8(4):125–31. doi: 10.1021/ar50088a003. [DOI] [Google Scholar]
- 3.Killilea DW, Wong SL, Cahaya HS, Atamna H, Ames BN. Iron accumulation during cellular senescence. Ann N Y Acad Sci. 2004; 1019(1): 365–7. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]
- 4.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 140 (1): 98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Marzetti E, Seo AY, Kim JS, Prolla TA, Leeuwenburgh C. The emerging role of iron dyshomeostasis in the mitochondrial decay of aging. Mech Ageing Dev. 2010;131(7–8):487–93. doi: 10.1016/j.mad.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Sun B, Yin H, Liu S. Hepcidin: a promising therapeutic target for iron disorders. Med (United States). 2016;95(14):e3150. doi: 10.1097/MD.0000000000003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camacho A, Simão M, Ea HK, Cohen-Solal M, Richette P, Branco J, et al. Iron overload in a murine model of hereditary hemochromatosis is associated with accelerated progression of osteoarthritis under mechanical stress. Osteoarthr Cartil. 2016;24(3):494–502. doi: 10.1016/j.joca.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Biemond P, Swaak AJ, van Eijk HG, Koster JF. Intraarticular ferritin-bound iron in rheumatoid arthritis. A factor that increases oxygen free radical-induced tissue destruction. Arthritis Rheum. 1986;29(10):1187–93. doi: 10.1002/art.1780291002. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg ED. The hazards of iron loading. Metallomics. 2010;2(11):732–40. doi: 10.1039/c0mt00023j. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen L, Schutgens RE, van Asbeck BS, Wenting MJ, van Veghel K, Roosendaal G, et al. Identification and expression of iron regulators in human synovium: evidence for upregulation in haemophilic arthropathy compared to rheumatoid arthritis, osteoarthritis, and healthy controls. Haemophilia. 2013;19(4):e218–27. doi: 10.1111/hae.12208. [DOI] [PubMed] [Google Scholar]
- 11.Abbott DF, Gresham GA. Arthropathy in transfusional siderosis. Br Med J. 1972;1(5797):418–9. doi: 10.1136/bmj.1.5797.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooiveld MJ, Roosendaal G, van den Berg HM, Bijlsma JW, Lafeber FP. Haemoglobin-derived iron-dependent hydroxyl radical formation in blood-induced joint damage: an in vitro study. Rheumatology (Oxford). 2003;42(6):784–90. doi: 10.1093/rheumatology/keg220. [DOI] [PubMed] [Google Scholar]
- 13.Carroll GJ, Sharma G, Upadhyay A, Jazayeri JA. Ferritin concentrations in synovial fluid are higher in osteoarthritis patients with HFE gene mutations (C282Y or H63D). Scand J Rheumatol. 2010;39(5):413–20. doi: 10.3109/03009741003677449. [DOI] [PubMed] [Google Scholar]
- 14.Askari AD, Muir WA, Rosner IA, Moskowitz RW, McLaren GD, Braun WE. Arthritis of hemochromatosis. Clinical spectrum, relation to histocompatibility antigens, and effectiveness of early phlebotomy. Am J Med. 1983;75(6):957–65. doi: 10.1016/0002-9343(83)90875-6. [DOI] [PubMed] [Google Scholar]
- 15.Anderson AS, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24(1):15. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nugzar O, Zandman-Goddard G, Oz H, Lakstein D, Feldbrin Z, Shargorodsky M. The role of ferritin and adiponectin as predictors of cartilage damage assessed by arthroscopy in patients with symtpmatic knee osteoarthritis. Best Pract Res Clin Rheumatol. 2018;32(5):662–8. doi: 10.1016/j.berh.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Yazer M, Sarban S, Kocyigit A, Isikan UE. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol Trace Elem Res. 2005;106(2):123–32. doi: 10.1385/BTER:106:2:123. [DOI] [PubMed] [Google Scholar]
- 18.Ogilvie-Harris DJ, Fornasier VL. Synovial iron deposition in osteoarthritis and rheumatoid arthritis. J Rheumatol. 1980;7(1):30–6. [PubMed] [Google Scholar]
- 19.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18 Suppl 3(Suppl 3):S35–52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz KA, Fisher J, Adams ET. Morphologic investigations of the guinea pig model of iron overload. Toxicol Pathol. 1993;21(3):311–20. doi: 10.1177/019262339302100307. [DOI] [PubMed] [Google Scholar]
- 21.Helrich K, eds; Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. 15th ed. Gaithersburg, MD, USA; AOAC International; 1990. Official method 968.08D. [Google Scholar]
- 22.Helrich K, eds; Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. 15th ed. Gaithersburg, MD, USA; AOAC International; 1990. Official method 974.27A, B, E and F. [Google Scholar]
- 23.Helrich K, eds; Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. 15th ed. Gaithersburg, MD, USA; AOAC International; 1990. Official method 985.40D. [Google Scholar]
- 24.Sands SA, Leung-Toung R, Wang Y, Connelly J, LeVine SM. Enhanced histochemical detection of iron in paraffin sections of mouse central nervous system tissue: application in the APP/PS1 mouse model of alzheimers disease. ASN Neuro. 2016;8(5). doi: 10.1177/1759091416670978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radakovich LB, Marolf AJ, Shannon JP, Pannone SC, Sherk VD, Santangelo KS. Development of a microcomputed tomography scoring system to characterize disease progression in the Hartley guinea pig model of spontaneous osteoarthritis. Connect Tissue Res. December 2017:1–11. doi: 10.1080/03008207.2017.1409218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berdoukas V, Chouliaras G, Moraitis P, Zannikos K, Berdoussi E, Ladis V. The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: a clinical observational study. J Cardiovasc Magn Reson. 2009;11:20. doi: 10.1186/1532-429X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olynyk JK, Luxon BA, Britton RS, Bacon BR. Hepatic iron concentration in hereditary hemochromatosis does not saturate or accurately predict phlebotomy requirements. Am J Gastroenterol. 1998;93(3):346–50. doi: 10.1111/j.1572-0241.1998.00346.x. [DOI] [PubMed] [Google Scholar]
- 29.Santangelo KS, Radakovich LB, Fouts J, Foster MT. Pathophysiology of obesity on knee joint homeostasis: contributions of the infrapatellar fat pad. Horm Mol Biol Clin Investig. 2016;26(2):97–108. doi: 10.1515/hmbci-2015-0067. [DOI] [PubMed] [Google Scholar]
- 30.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthr Cartil. 2010;18(7):876–82. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Mace J, Bhatti W, Anand S. Infrapatellar fat pad syndrome: a review of anatomy, function, treatment and dynamics. Acta Orthop Belg. 2016;82(1):94–101. [PubMed] [Google Scholar]
- 32.Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15(6):225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Huebner JL, Kraus VB, Griffin TM. Effect of Aging on Adipose Tissue Inflammation in the Knee Joints of F344BN Rats. Journals Gerontol Ser A Biol Sci Med Sci. 2016;71(9):1131–40. doi: 10.1093/gerona/glv151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwata M, Ochi H, Hara Y, Tagawa M, Koga D, Okawa A, et al. Initial responses of articular tissues in a murine high-fat diet-induced osteoarthritis model: pivotal role of the IPFP as a cytokine fountain. Fritz JH, ed. PLoS One. 2013;8(4):e60706. doi: 10.1371/journal.pone.0060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthr Cartil. 2002;10(10):758–67. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- 36.Menkes CJ, Lane NE. Are osteophytes good or bad? Osteoarthr Cartil. 2004;12 Suppl A:S53–4. doi: 10.1016/j.joca.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 37.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthr Cartil. 2007;15(3):237–44. doi: 10.1016/J.JOCA.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Felson DT, Gale DR, Elon Gale M, Niu J, Hunter DJ, Goggins J, et al. Osteophytes and progression of knee osteoarthritis. Rheumatology. 2005;44(1):100–4. doi: 10.1093/rheumatology/keh411. [DOI] [PubMed] [Google Scholar]
- 39.Carroll GJ, Breidahl WH, Bulsara MK, Olynyk JK. Hereditary hemochromatosis is characterized by a clinically definable arthropathy that correlates with iron load. Arthritis Rheum. 2011;63(1):286–94. doi: 10.1002/art.30094. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Feng T, Vujić Spasić M, Altamura S, Breitkopf-Heinlein K, Altenoder J, et al. Transforming Growth Factor β1 (TGF-β1) Activates Hepcidin mRNA Expression in Hepatocytes. J Biol Chem. 2016;291(25):13160–74. doi: 10.1074/jbc.M115.691543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R-H, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Simao M, Gavaia PJ, Camacho A, Porto G, Pinto IJ, Ea HK, et al. Intracellular iron uptake is favored in Hfe-KO mouse primary chondrocytes mimicking an osteoarthritis-related phenotype. Biofactors. 2019;45(4):583–97. doi: 10.1002/biof.1520. [DOI] [PubMed] [Google Scholar]
- 43.Kirkpatrick CJ, Mohr W, Haferkamp O. Alterations in chondrocytes morphology, proliferation and binding. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38(3):297–306. doi: 10.1007/bf02892825. [DOI] [PubMed] [Google Scholar]
- 44.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95–105. doi: 10.5312/wjo.v6.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson MJ, Herndler-Brandstetter D, Tariq MA, Nicholson TA, Philp AM, Smith HL, et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep. 2017;7(1)3451. doi: 10.1038/s41598-017-03759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta. 2012;1823(9):1468–83.doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–97.doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 48.Sterling J, Guttha S, Song Y, Song D, Hadziahmetovic M, Dunalef JL. Iron importers zip8 and zip14 are expressed in retina and regulated by retinal iron levels. Exp Eye Res. 2017;155:15–23.doi: 10.1016/j.exer.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao N, Zang AS, Worthen C, Knutson MD, Enns CA. An iron-regulated and glycosylation-dependent proteasomal degradation pathway for the plasma membrane metal transporter zip14. Proc Natl Acad Sci USA. 2014;111(25):9175–80. doi: 10.1073/pnas.1405355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prakash A, Aggarwal R. Thalassemia major in adults: short stature, hyperpigmentation, inadequate chelation, and transfusion-transmitted infections are key features. N Am J Med Sci. 2012;4(3):141–4. doi: 10.4103/1947-2714.93886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Select CBC and serum biochemistry values. Notably, a significant difference in hematocrit was not present between iron overload and control animals [A]. Evidence of systemic inflammation is supported by a relative increase in platelet counts† [B] and total WBC counts† [C], as well as decreased albumin† [D] and increased globulins† [E]. A significant increase in heterophils, lymphocytes, and monocytes all contributed to the increased WBC counts (data not shown). Increased cholesterol× [F] is likely due to hepatocellular damage from iron overload, as supported by increased ALT× [G] and AST† [H]. The higher AST values may also be due to an increase in creatine kinase, which was also present in iron dextran treated animals (data not shown). Blood and serum were unable to be collected from 2 animals in the iron overload group (n = 8). Additionally, 1 serum sample from an iron overload animal was identified for preanalytical sample preclusion due to hemolyzed serum. As measurement of AST can be influenced by hemolysis during blood collection, the hemolyzed sample was excluded from AST analysis (n = 7). Normally distributed data with similar variance were compared using parametric t tests†. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×.
Supplemental Figure S2. Body weights of animals throughout study. [A] There was no significant difference in the weekly body weights of study animals at any time point throughout the study. During the first week of the study, animals within the iron overload group had a mean body weight of 683.60 g (n = 10) and animals within the dextran control group had a mean body weight of 663.40 g (n = 10; 95% CI −107.00 – 66.60 g). In the second week of the study, animals within the iron overload group had a mean body weight of 691.90 g (n = 10) while animals within the dextran control group had a mean body weight of 686.80 g (n = 10; 95% CI −98.68 – 88.48 g). By the third week of the study, animals within the iron overload group had a mean body weight of 704.40 g (n = 10) and animals within the dextran control group had a mean body weight of 720.40 g (n = 10; 95% CI −81.97 – 114.00 g). At the final week of the study, animals within the iron overload group had a mean body weight of 698.8 g (n = 10) while animals within the dextran control group had a mean body weight of 744.20 g (n = 10; 95% CI −76.23 – 167.00 g). Data was analyzed using a two-way ANOVA with Geisser-Greenhouse correction for unequal variability of differences and Sidak’s post-hoc analysis for multiple comparisons. [B] Relative to the dextran control group, iron overloaded animals gained weight at a decreased rate throughout the study. Between the first and second week of the study†, animals within the iron overload group had a mean weight gain of 8.30 g (n = 10), while dextran control animals had a mean weight gain of 23.40 g (n = 10; 95% CI −32.35 – 2.15 g). Between the second and third week of the study†, iron overloaded animals had a mean weight gain of 12.50 g (n = 10), while dextran control animals had a mean weight gain of 33.60g (n = 10; 95% CI −37.92 – 4.28 g). Between the third and fourth week of the study×, animals within the iron overload group had a mean weight loss of −5.60 g (n = 10), and the dextran control animals had a mean weight gain of 23.80 g (n = 10; −14.50g difference between medians). The study was terminated after 1 month to ensure proper animal welfare. Normally distributed data with similar variance were compared using parametric t tests†. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. Statistical modeling was conducted to confirm that the differences observed in OA development were maintained after controlling for differences in the rate of weight gain between treatment groups. A multiple linear regression model was fit using the whole joint OARSI histologic score as the response variable, treatment group as an independent variable, and the overall difference in the rate of weight gain throughout the study (week 4 – week 1) as a covariate. Based on this model, there is evidence that the significant difference in histologic OA scores between groups is maintained after controlling for differences in the rate of weight gain (estimated difference between treatment groups was 15.04 for whole joint OARSI score; p < 0.0001). As such, the differences in the rate of weight gain do not appear to have impacted the histologic OA changes observed in the present work.
Supplemental Figure S3. Contributions to whole joint OARSI score components. Black lines on graphs represent mean values. [A] Medial compartment OARSI score† (possible range of scores 0 – 42). Mean OARSI histologic score in the medial compartment of the knee was 10.88 in the iron overload group (n = 8) and 1.89 in the dextran control group (n = 9; 95% CI 6.69 – 11.28). Three animals (2 from the iron overload group and 1 from the dextran control group) were unable to be evaluated for medial compartment OARSI histologic grading due to appropriate tissues sections being unavailable. [B] Lateral compartment OARSI score◊ (possible range of scores 0 – 42). Mean OARSI histologic score in the lateral compartment of the knee was 7.88 in the iron overload group (n = 8) and 2.11 in the dextran control group (n = 9; 95% CI 2.70 – 8.83). Three animals (2 from the iron overload group and 1 from the dextran control group) were unable to be evaluated for medial compartment OARSI histologic grading due to appropriate tissues sections being unavailable. [C] Articular cartilage structure† (possible range of scores 0 – 32 for the sum of all compartments). Mean score for articular cartilage structure was 4.14 in the iron overload group (n = 7) and 1.88 in the dextran control group (n = 8; 95% CI 0.76 – 3.78). [D] Proteoglycan content◊ (possible range of scores 0 – 24 for the sum of all compartments). Mean score for proteoglycan content was 7.43 in the iron overload group (n = 7) and was 1.50 in the dextran control group (n = 8; 95% CI 3.85 – 8.00). [E] Cellularity† (possible range of scores 0 – 12 for the sum of all compartments). Mean score for cellularity was 5.57 in the iron overload group (n = 7) and was 0.38 in the dextran control group (n = 8; 95% CI 4.05 – 6.34). [F] Tidemark integrity× (possible range of scores 0 – 4 for the sum of all compartments). Mean score for tidemark integrity was 1.86 in the iron overload group (n = 7) and was 0.13 in the dextran control group (n = 8; 2.00 difference between medians). Five animals (3 from the iron overload group and 2 from the dextran control group) were unable to be evaluated for whole joint OARSI histologic grading due to appropriate tissues sections being unavailable. Normally distributed data with similar variance were compared using parametric t tests†. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×.
Supplemental Figure S4. Sex differences in iron concentration of femoral head articular cartilage. Significant sex differences were present in cartilage iron concentrations both with and without iron dextran treatment. [A] Sex differences in dextran control femoral head cartilage iron concentrations◊. Within the control group, mean cartilage iron concentration was 1035.00 ppm for males (n = 6) and was 360.30 ppm for females (n = 4; 95% CI −1,259.00 – −91.20 ppm). [B] Sex differences in iron overload group cartilage iron concentrations×. Within the iron overload group, mean cartilage iron concentration was 3,414.00 ppm in males (n = 5) and was 1,576.00 ppm in females (n = 5; 1,420.00 ppm difference between medians). This data suggests that males have more iron within articular cartilage tissue than females, even when excess iron is not present systemically. The presence of sex differences for cartilage iron concentration within both treatment groups is intriguing given that sex differences were absent for all other study outcomes, including liver iron concentration. Data with non-Gaussian distribution were compared using non-parametric Mann-Whitney tests×. Normally distributed data with significant differences in variance were compared using parametric t tests with Welch’s correction◊.


