Abstract
Human exposures to environmental metals, including uranium (U) and arsenic (As) are a global public health concern. Chronic exposures to U and As are linked to many adverse health effects including, immune suppression and autoimmunity. The gastrointestinal (GI) tract is home to many immune cells vital in the maintenance of systemic immune health. However, very little is known about the immunotoxicity of U and As at this site. The present study examined the burden of U and As exposure in the GI tract as well as the resultant immunotoxicity to intraepithelial lymphocytes (IELs) and innate immune cells of the small intestine following chronic drinking water exposures of male and female mice to U (in the form of uranyl acetate, UA) and As (in the form of sodium arsenite, As3+). Exposure to U or As3+ resulted in high levels of U or As in the GI tract of male and female mice, respectively. A reduction of small intestinal CD4+ IELs (TCRαβ+, CD8αα+) was found following As3+ exposure, whereas U produced widespread suppression of CD4− IEL subsets (TCRαβ+ and TCRγδ+). Evaluation of innate immune cell subsets in the small intestinal lamina propria revealed a decrease in mature macrophages, along with a corresponding increase in immature/proinflammatory macrophages following As3+ exposures. These data show that exposures to two prevalent environmental contaminants, U and As produce significant immunotoxicity in the GI tract. Collectively, these findings provide a critical framework for understanding the underlying immune health issues reported in human populations chronically exposed to environmental metals.
Keywords: Metals, Uranium, arsenic, Arsenite (As3+), Immunotoxicity, Gut Associated Lymphoid Tissues, Small Intestine, Intraepithelial Lymphocytes, Innate Immune Cells
Introduction
Elevated metals exposures resultant from the more than 160,000 abandoned mine waste sites across the Western United States represent a significant health concern (Lewis et al., 2017). Rural communities located throughout this region are at particular risk of metals exposures, as most of these populations rely on unregulated drinking water sources (i.e. ground water wells) that often exceed the World Health Organization and United States Environmental Protection Agency maximum contaminant levels for several heavy metals, including uranium (U) (30 μg/L; ppb) and arsenic (As) (10 ppb) (US EPA, 2006; deLemos et al., 2009; Orescanin et al., 2011; WHO, 2011; US EPA, 2012a; WHO, 2012; Blake et al., 2015; Hoover et al., 2017). Chronic exposures to elevated U and As are linked to a multitude of adverse health outcomes, including cardiovascular and neurological disorders, cancer, immune suppression, and autoimmunity (Hughes, 2002; Lourenco et al., 2013; Naujokas et al., 2013; Lu-Fritts et al., 2014; Tyler and Allan, 2014; Ferrario et al., 2016; Erdei et al., 2019).
The immunotoxicity of As is well established (Kozul et al., 2009; Ferrario et al., 2016; Lauer et al., 2019; Parvez et al., 2019), but very little is known about the immune effects of U exposure. Epidemiological studies on the long-term health effects of metals exposures among Native American populations living in proximity to mine waste sites report a high incidence of immune dysregulation among these individuals, as evidenced by increased prevalence of antinuclear antibodies in blood and autoimmune disorders, such as systemic lupus erythematosus (Ong et al., 2014; Erdei et al., 2019). The impacts of U and As on the immune system of the gut represent an understudied area of immunotoxicology, which may provide critical links between metal exposure and immune dysregulation reported in epidemiological studies.
The gastrointestinal (GI) tract is a critical organ comprised of multiple lymphoid tissues (i.e., intestinal mucosa, lamina propria, and Peyer’s patches) that are home to a diversity and abundance of essential immune cells and microbiota (Mowat, 2003). Lymphocytes of the small intestine represent unique immune cell types that directly interface with the external environment and maintain a delicate balance between immune protection and symbiotic associations with commensal microorganisms (Cheroutre et al., 2011; Hooper et al., 2012; Sommer and Backhed, 2013; Peterson et al., 2015; Olivares-Villagomez and Van Kaer, 2018). The interface between the environment, immune cells, and microbiota is crucial for maintaining systemic immune health (Hooper et al., 2012; Sommer and Backhed, 2013; Peterson et al., 2015), but also represents a site of vulnerability to environmental toxicant exposures. Numerous studies have linked metals exposure to disruptions of microbiota of the GI tract (Breton et al., 2013; Chi et al., 2016; Chi et al., 2017; Tsiaoussis et al., 2019); however, to our knowledge, no previous studies have evaluated the effects of U or As on immune subsets of the small intestinal epithelial layer or lamina propria following drinking water exposures.
IELs are critical immune cell types of the GI tract and other mucosal tissues of the body and have diverse functions (Cheroutre et al., 2011; Olivares-Villagomez and Van Kaer, 2018). Although the developmental origins of IELs are not fully resolved and a topic of debate among the scientific community, current belief is that both natural and induced IEL subsets originate from T cell precursors in the thymus (Cheroutre et al., 2011; McDonald et al., 2018; Olivares-Villagomez and Van Kaer, 2018). Natural IELs initiate from T cell differentiation in the thymus, where they mature to the triple negative stage (CD3ε−, CD4−, and CD8αβ−), prior to entering an alternative differentiation pathway and homing to the gut where they give rise to CD3ε+, CD4−, TCRαβ+, CD8αα+/− or CD3ε+, CD4−, TCRγδ+, CD8αα+/− IEL subsets (Lambolez et al., 2002; Lambolez et al., 2006; Cheroutre and Lambolez, 2008; Cheroutre et al., 2011; Peaudecerf et al., 2011). In contrast, induced IEL subsets are antigen experienced T cells that differentiate to mature CD4+ or CD8αβ+ stages in the thymus prior to migration to the gut (Cheroutre and Lambolez, 2008; Cheroutre et al., 2011; Olivares-Villagomez and Van Kaer, 2018; Zhou et al., 2019). Regardless of origin and location of differentiation, IELs represent critical immune cells that have diverse functions ranging from regulatory, helper-like activities to effector and cytotoxic properties (Cheroutre et al., 2011; Olivares-Villagomez and Van Kaer, 2018).
In addition to IELs, the small intestinal lamina propria is home to many essential innate immune cells, including dendritic cells (DC), macrophages, neutrophils, and monocytes that have critical functions in neutralizing foreign antigens, interactions with commensal microbes, gut barrier maintenance, and in mediating IEL and other immune cell responses (Cheroutre et al., 2011; Gross et al., 2015; Hoytema van Konijnenburg et al., 2017; Joeris et al., 2017; Montalban-Arques et al., 2018). Innate immune cells function independently and in cooperation with IELs and microbiota through a network of complex interrelationships to maintain local and systemic immune health (Cheroutre et al., 2011; Mu et al., 2017). Disruption of this network by environmental toxicants, including heavy metals has been found to impair gut barrier function, resulting in a range of autoimmune disorders, including chronic inflammatory conditions of the GI tract, type I diabetes, and systemic lupus erythematosus (Campbell, 2014; Liu et al., 2014; Campbell, 2015; Mu et al., 2017; Sprouse et al., 2019). Additionally, perturbation of the microbiome has profound effects on the stability of IEL and innate immune cell populations, which in turn, can drive or increase severity of immunological dysfunction (Cheroutre et al., 2011; Montalban-Arques et al., 2018; Sprouse et al., 2019).
The goal of this study was to develop a foundational understanding of the immunotoxic effects of U and As on immune cells in the gut. Herein, we assessed the burden of U and As exposure in the GI tract of male and female mice following drinking water exposures to U (in the form of uranyl acetate, UA) and As (in the form of sodium arsenite, As3+). Additionally, the effects of UA and As3+ on immune cells in the small intestinal intraepithelial layer and lamina propria were evaluated. Taking into account the prominent role of the GI tract in excretion as well as being the primary site for both U and As absorption (Pomroy et al., 1980; Vahter and Norin, 1980; Harrison, 1991; Dublineau et al., 2005), we hypothesized that immune cells in the gut receive significant exposures and are at a heightened risk of associated toxicity. Findings from this study provide novel insights into the immunotoxicity of U and As, which is critical for risk assessment and understanding the underlying mechanisms of immune dysregulation reported in human populations chronically exposed to U and As containing mine wastes.
Methods
Chemicals and reagents
Depleted U, in the form of UA (>98% purity, CAS #541-09-3, Cat. No. 22400), UO2(OCOCH3)2·2H2O; U238 – 99.9%, U235 – 0.1%, was purchased from Electron Microscopy Sciences (Hatfield, PA). The radioactivity of stock UA is approximately 0.3 μCi/g. Sodium meta-As3+ (NaAsO2 >95% purity, CAS #774-46-5, Cat. No. S7400) was purchased from Sigma Aldrich (St. Louis, MO). A list of reagents utilized in this study is provided in Supplementary Materials.
Mice
All experiments were performed in accordance with protocols approved by the University of New Mexico (UNM) Office of Animal Care Compliance Committee (Albuquerque, NM). Wild-type male and female C57BL/6J mice (7 weeks of age) were purchased from Jackson Laboratory (Bar Harbor, MA) and housed in the UNM Health Science Center (HSC) Animal Resource Facility. Mice were acclimated at the HSC Animal Resource Facility for 1 week prior to the onset of experiments. Throughout all experiments, mice were provided food and water ad libitum. For inductively coupled plasma-mass spectrometry (ICP-MS) experiments, mice were exposed to control (tap water from the UNM HSC, which contains ~2-9 ppb U and As) and 50 mg/L (ppm) U or control and 100 ppb As3+for 60 days (n = 5 mice/group). For gut immune cell studies, mice were exposed to control (tap water from the UNM HSC), 5, and 50 ppm U or 0, 100, and 500 ppb As3+ for 45 days (n = 6 mice/group).
U and As3+stock solutions were prepared fresh at the start of experiments and dosing water (i.e. in mouse drinking water pouches) was replaced weekly. Concentrations of U and As in dosing water were verified by ICP-MS. Water consumption was monitored weekly by weighing each water pouch before and after replacing and using the change in net weight as an estimation of water intake (1 mL = 1 g H2O).
Preparation of drinking water exposures
Stock UA solutions were prepared at concentrations 1,000 times greater than used for dosing in sterile cell culture grade water. U utilized for exposing mice was prepared by diluting stock UA solutions directly into mouse drinking water pouches to yield final exposure concentrations of 5 and 50 ppm, representing elemental U as opposed to the dihydrate compound (1.8 g UO2(OCOCH3)2·2H2O ≈ 1 g U).
Sodium As3+ stock solutions were also prepared 1,000 times more concentrated than used for dosing in sterile cell culture grade water and were diluted into mouse water pouches to yield final dosing concentrations of 100 and 500 ppb As3+, representing total elemental As, opposed to the complete sodium As3+ compound (1.7 g NaAsO2 ≈ 1 g As3+).
The aforementioned U and As3+ concentrations were selected based on relevance to environmental exposures in contaminated regions of the Western United States, such as the Navajo Nation. Levels of U measured in environmental samples throughout this region have been reported at concentrations reaching 560 ppb in ground water (~19 times greater than the current drinking water standard of 30 ppb, respectively) and at concentrations ranging from 2,200 to 6,600 ppm in soil (−700 to 2,000 times higher than the average U content of soils in non-contaminated regions of the United States) (US EPA, 2012b; ATSDR, 2013; Blake et al., 2015; Jones et al., 2020). Elevated U exposures are not confined to the Western United States, and have also been reported in unregulated ground water sources in other countries at levels up to 20 ppm (Juntunen, 1991; WHO, 2012).
In addition to U, As contamination of water sources throughout the Western United States and in many regions around the world is also a major issue of concern (Naujokas et al., 2013). In a recent study, it was reported that 15.1% of unregulated water sources in the Navajo Nation were in exceedance of the current drinking water standard of 10 ppb (Blake et al., 2015; Hoover et al., 2017), with some sources reaching levels as high as 230 ppb (Ingram et al., 2020; Jones et al., 2020). As levels >1 ppm have been documented in unregulated water sources in the United States and abroad (ATSDR, 2007; WHO, 2011; ATSDR, 2016).
Tissue collection
Following 45- or 60-day exposures to U or As3+, mice were humanely euthanized by CO2 asphyxiation followed by cardiac puncture. For ICP-MS analysis, the GI tract was harvested from 60-day U and As3+ exposed mice and sectioned into upper (i.e. stomach, small intestine, and illium) and lower (i.e. cecum, large intestine, and colon) portions. The upper and lower GI tract was weighed, snap frozen in liquid nitrogen, and stored at −80°C prior to analysis by ICP-MS. For immune cell isolation, small intestines (duodenum to ilium) were harvested from 45-day U and As3+ exposed mice, measured, and stored in mouse medium (RPMI-1640 containing 10% heat inactivated fetal bovine serum (HI FBS), 2 mM L-glutamine, and 100 mg/ml streptomycin and 100 units/mL penicillin) on ice prior to the isolation of intraepithelial and lamina propria cells.
Isolation of small intestinal intraepithelial and lamina propria cells
Immune cells of the small intestinal intraepithelial layer and lamina propria were isolated as previously described (Weigmann et al., 2007; Couter and Surana, 2016), with some modifications. Throughout the entire isolation procedure, small intestines and isolated cells were kept in mouse medium on ice. Briefly, small intestines were flushed gently with ice cold Dulbecco’s phosphate-buffered saline without calcium and magnesium (DPBS−). After flushing, Peyer’s patches were removed, and all mesenteric fat was carefully dissected from the intestine. After thorough removal of mesenteric fat, the intestine was cut longitudinally and snipped into approximately 5 cm pieces, which were transferred to a 50 mL centrifuge tube containing 15 mL cold mouse medium.
Luminal contents were further cleared by 3 sequential washes using 15 mL cold mouse medium and vortexing at high speed. After the last wash, small intestinal segments were tapped onto a paper towel to remove excess mouse medium, transferred to a 50 mL centrifuge tube containing extraction medium for the isolation of IELs (RPMI-1640 containing 2% HI FBS, 1 mM EDTA, and 0.02 % (w/v) dithiothreitol), and placed onto a rocker at 37°C for 15 mins.
Following 15 min incubation, cells liberated by the extraction process were sequentially rinsed with 25 mL mouse medium through 100 and 40 μm strainers with centrifugation at 200×g at 4°C between each rinse. Remaining tissue pieces were rinsed three times in cold RPMI-1640 containing 1% HI FBS and placed onto a dry paper towel, and gently wiped to remove residual mucous. Tissue pieces were transferred to a 1.5 mL tube containing digestion medium for the isolation of lamina propria cells (RPMI-1640 containing 1% HI FBS, 0.5 mg/mL dispase II and 1.5 mg/mL collagenase type II, Gibco) and minced into small fragments using scissors. Minced tissue pieces along with remaining medium were transferred to a 50 mL tube containing 25 mL prewarmed digestion medium and placed onto a rocker at 37°C for 40 mins. Following 40 min incubation, cells were sequentially rinsed with 25 mL mouse medium through 100 and 40 μm strainers with centrifugation at 200×g at 4°C between each rinse.
Following isolation procedures, cell viabilities and concentrations of isolated IELs and lamina propria cells were determined by acridine orange/propidium iodide (AO/PI) staining using a Nexcelom Cellometer Auto 2000.
Inductively coupled plasma-mass spectrometry
U and total As concentrations were measured using a PerkinElmer NexION 300D ICP-MS (Waltham, MA) in the upper and lower GI tract of male and female mice as previously described (Bolt et al., 2018). The upper and lower GI tract were weighed prior to acid digestion, which was performed using 70% trace metal grade nitric acid and by heating the samples to 95°C overnight. After digestion, samples were brought up to 20 mL with 18 mega-ohm water and filtered through a 0.45 μm filter prior to ICP-MS analysis. A dynamic reaction cell (DRC) was used with anhydrous ammonia gas to minimize mass interferences with U, which was analyzed in direct mode. Oxygen gas was used in the DRC to minimize mass interferences with As, which was analyzed in hydride mode. The ICP-MS was optimized using multi-element standard for instrument calibration (tuning) solution (PerkinElmer), calibrated with a blank and four calibration standards, and samples were analyzed including quality control samples for data validation and verification. In addition, each experimental sample batch included continuing quality control samples analyzed after every 20 samples to further validate the calibration and instrument stability. Data are reported as mean U or As per gram of tissue. The ICP-MS method of detection limit for U and As were 0.01 ppb and 0.05 ppb, respectively.
Flow cytometry
Approximately 1×106 intraepithelial or lamina propria cells were stained with Zombie Red Fixable Viability Stain in DPBS− at room temperature in the dark for 15 mins. Samples were washed with flow wash buffer (DPBS− with 2% FBS and 0.09% sodium azide) and resuspended in 100 μL BD Horizon Brilliant Stain Buffer (BD Biosciences) containing 0.5-1 μg of monoclonal antibodies (antibody specifics are provided in Supplementary Table S1) for surface marker staining at room temperature in the dark for 30 mins.
IEL and innate immune cell subsets were characterized based on cell surface marker phenotype as described previously (Cheroutre et al., 2011; Geem et al., 2012; Tamoutounour et al., 2012; Bain et al., 2013; Curato et al., 2016; Joeris et al., 2017; Olivares-Villagomez and Van Kaer, 2018; Na et al., 2019). Representative flow cytometry gating strategies utilized to define IEL and innate immune cell subsets are depicted in Fig. 3A and Supplementary Fig. S1, respectively. Natural IEL subsets were defined as follows: CD3ε+, CD4−, TCRαβ+, CD8αα+, CD8αβ− or CD3ε+, CD4− TCRγδ+, CD8αα+, CD8ab+/. Induced IEL subsets were defined as:CD3ε+, CD4+/−, TCRαβ+, CD8αβ+/−, CD8αα+/−.
Figure 3.
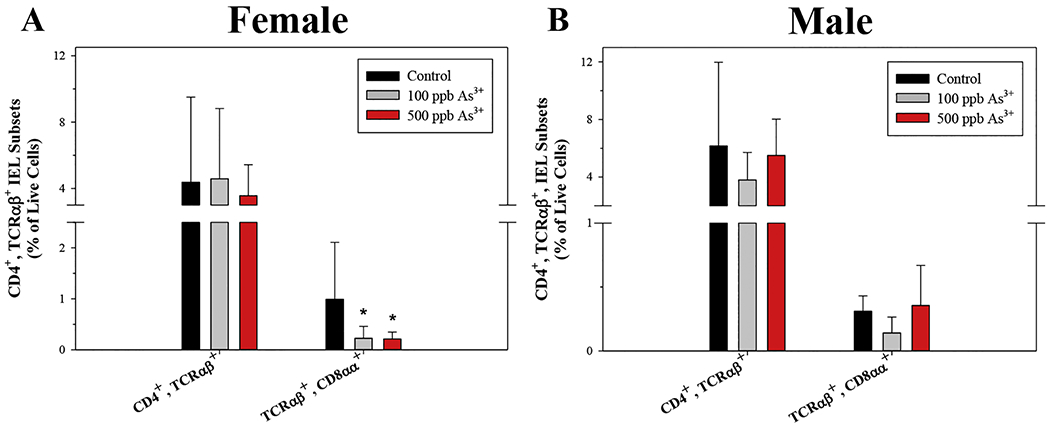
Small intestinal CD3ε+, CD4+, TCRαβ+, CD8αα+ IELs are reduced by As3+ exposure in male and female mice. Intraepithelial cells were isolated from the small intestines of male and female mice following 45-day exposure to 0, 100, and 500 ppb As3+ and IEL subsets were evaluated based on surface marker phenotype by flow cytometry. Percentages of CD3ε+, CD4+, TCRαβ+ IEL subsets in (A) female and (B) male mice. Data are expressed as mean ± SD. Male: control (n = 5), 100 ppb (n = 3), and 500 ppb As3+ (n = 5). Female: control (n = 4), 100 ppb (n = 6), and 500 ppb As3+ (n = 6). *p<0,05 in one-way ANOVA followed by a Dunnett’s t-test or Kruskal-Wallis One Way ANOVA on Ranks followed by Dunnett’s post-hoc test compared to control group.
Innate immune cells of the lamina propria were defined as follows: single positive (SP) DCs (Lineage (Lin)−(CD3ε, CD45R/B220, CD5, IgE), CD11c+, MHC II+, CD103+, CD11b−); double positive (DP) DCs (Lin−, CD11c+, MHC II+, CD103+, CD11b+); Immature/proinflammatory macrophages (Lin, CD11c+, MHC II+, CD103−, CD11b+, Ly6C+, CX3CR1+); mature macrophages (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C−, CX3CR1+); monocytes (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C+, CX3CR1−), and neutrophils (Lin−, Ly6G+, CD11b+).
Following staining, samples were washed twice and resuspended in 0.5 mL flow wash buffer prior to analysis using an Invitrogen Attune NxT Flow Cytometer (Carlsbad, CA). Gating used for the analysis of flow cytometry data was performed with the aid of fluorescence-minus-one controls. Flow cytometry data was processed using FlowJo version 10 software (FlowJo LLC, Ashland, Oregon).
Statistics
Data were analyzed using Sigma Plot version 12.5 (Systat Software). For analysis of ICP-MS results, in which only two conditions (exposed vs. unexposed) were compared, data were analyzed using a Student’s t-test. Flow cytometry data were analyzed using one-way ANOVA followed by a Dunnett’s t-test comparing each exposure group to the control (unexposed) group. In situations when ICP-MS or flow cytometry datasets did not meet conditions of normality, differences between control and exposure groups were examined using either a Mann-Whitney Rank Sum (U) Test or Kruskal-Wallis One Way ANOVA on Ranks followed by Dunnett’s post-hoc test, respectively. ANOVA results are summarized in Supplementary Table S2 and p values for post hoc analyses are indicated in the relevant text of the results section. For both ICP-MS and flow cytometry studies, n = 5 or n = 6 mice/group, respectively unless otherwise specified in figure legends. In several cases, samples were lost during cell isolation procedures. In addition, for both ICP-MS and flow cytometry datasets, statistically significant outliers were identified using Grubb’s Test (p<0.05) and were excluded prior to statistical analyses. The significance level utilized in this study to determine statistically relevant changes between control and exposure groups was p<0.05.
Results
U and As3+ do not significantly alter body weights, drinking water consumption, numbers of small intestinal Peyer’s patches, or small intestine length in male or female mice
The goal of this study was to evalaute the distribution of U and As in the GI tract of male and female mice to determine if immune cells in the gut are at an increased risk of exposure and toxicity. In this study, male and female mice were exposed to U or As3+ via their drinking water for 45 and 60 days. No evidence of overt toxicity (i.e., body weights, GI tract weights, or drinking water consumption) was found in male or female mice after 45 or 60 day exposures to U or As3+ (Table 1 and Supplementary Table S3, respectively). Additionally, no significant changes in small intestine lengths or numbers of Peyer’s patches in the small intestines were found in male or female mice exposed to U or As3+ for 45 days (Table 1).
Table 1.
Mouse drinking water consumption, body weights, small intestine length and numbers of small intestinal Peyer’s patches in male or female mice following 45-day drinking water exposure to U or As3+.a
| Sex | Metal | Dose | Water Intakeb | Body Wt. (g) | Small Intestine Length (cm) | # of Peyer’s Patches |
|---|---|---|---|---|---|---|
| Male | U | Control | 4.10 ± 0.34 | 30.68 ± 1.36 | 34.43 ± 1.82 | 7.50 ± 1.52 |
| 5 ppm | 4.39 ± 0.71 | 30.63 ± 1.93 | 34.48 ± 2.16 | 6.17 ± 0.75 | ||
| 50 ppm | 3.92 ± 0.40 | 30.49 ± 1.06 | 34.42 ± 0.97 | 6.50 ± 2.07 | ||
| As3+ | Control | 3.07 ± 0.25 | 33.36 ± 3.16 | 35.83 ± 1.44 | 5.00 ± 1.10 | |
| 100 ppb | 3.31 ± 0.31 | 31.07 ± 1.60 | 34.83 ± 1.86 | 6.17 ± 1.60 | ||
| 500 ppb | 3.24 ± 0.26 | 29.26 ± 3.26 | 35.73 ± 1.19 | 5.83 ± 0.75 | ||
| Female | U | Control | 3.22 ± 0.54 | 22.40 ± 0.80 | 32.68 ± 0.68 | 6.33 ± 1.34 |
| 5 ppm | 3.16 ± 0.27 | 23.90 ± 1.34 | 34.17 ± 1.65 | 6.17 ± 1.47 | ||
| 50 ppm | 3.17 ± 0.35 | 22.58 ± 2.15 | 32.23 ± 2.31 | 6.67 ± 0.82 | ||
| As3+ | Control | 2.72 ± 0.21 | 24.80 ± 2.85 | 35.63 ± 2.62 | 7.50 ± 0.84 | |
| 100 ppb | 2.52 ± 0.33 | 25.21 ± 1.80 | 35.70 ± 1.85 | 6.00 ± 1.55 | ||
| 500 ppb | 2.31 ± 0.20 | 23.53 ± 2.14 | 34.82 ± 1.15 | 7.17 ± 0.98 | ||
Water intake = mL of water consumed per mouse per day.
Data expressed as mean ± SD of each group (n = 5).
Levels of U and As are elevelated in the GI tract of male and female mice following drinking water exposures
Mice were exposed to U or As3+ in their drinking water for 60 days and the levels of U and total As were measured in the upper and lower GI tract using ICP-MS. In both male and female mice, the GI tract was a site of significant U and arseinc exposure, with concentrations reaching levels greater than or equal to the administered doses of each metal (Fig. 1A–D). U levels were significantly elevated in the upper and lower GI tract of male (U = 0, p = 0.016 and U = 0, p = 0.016, respectively) and female (U = 0, p = 0.008) mice compared to untreated controls (Fig. 1A–B). The amount of total As was significantly elevated in both the upper and lower GI tract of male mice (t(8) = 7.304, p<0.001 and t(7) = −12.521, p<0.001, respectively), but only in the lower GI tract of female mice (U = 0, p = 0.008) (Fig. 1C–D). Collectively, these results suggest that immune cells in the gut receive significant U and As exposures and therefore may be at an increased risk of toxicity from these metals.
Figure 1.
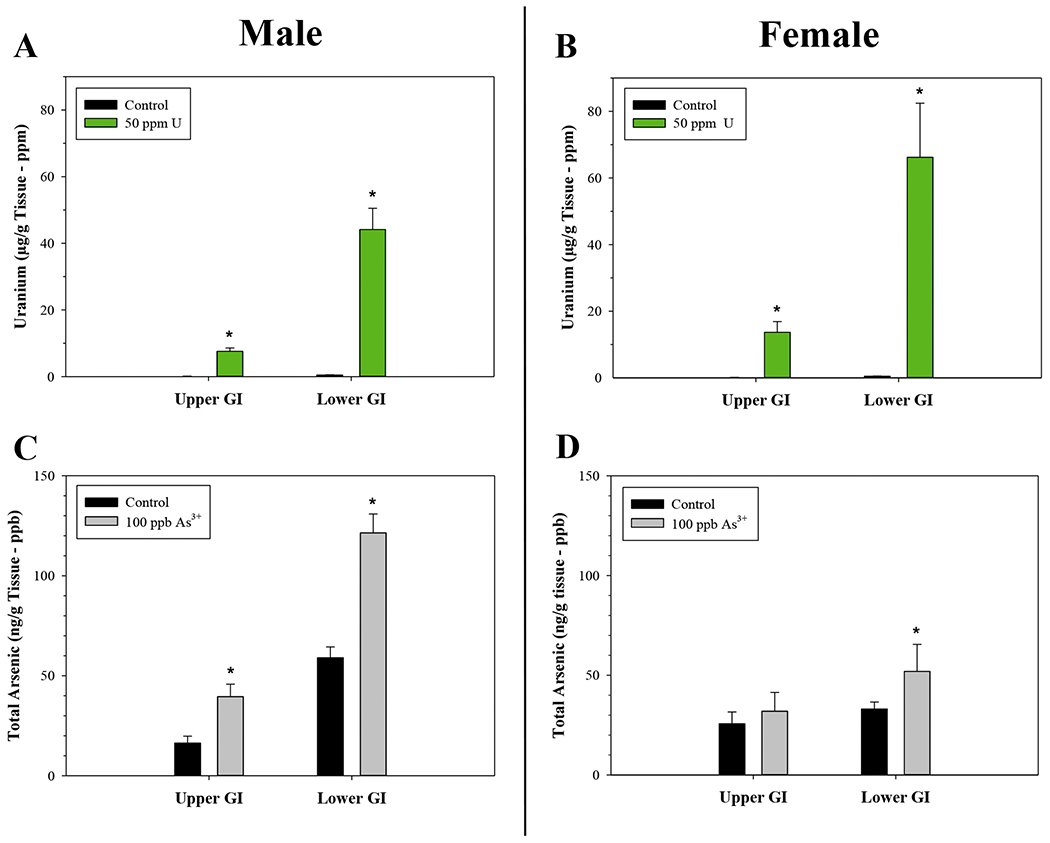
Elevated levels of U and As in the upper and lower GI tract of male and female mice following 60-day drinking water exposures to U or As3+. U and total As were measured by ICP-MS. U concentrations in the upper and lower GI tract of (A) male and (B) female mice following exposures to 0 and 50 ppm U. Total As concentrations detected in the upper and lower GI tract of (C) male and (D) female mice following exposures to 0 and 100 ppb As3+. U data (A-B) are expressed as mean μg U/g tissue (ppb) ± SD; Male: control (n = 5 for upper and lower GI tracts) and 50 ppm (n = 4 for upper and lower GI tracts). Female: control (n = 5 for upper and lower GI tracts) and 50 ppm (n = 5 for upper and lower GI tracts). As3+ data (C-D) are expressed as mean ng total As/g tissue (ppb) ± SD. Male: control (n = 5 for upper and lower GI tracts) and 100 ppb As3+ (n = 5 for upper and n = 4 for lower GI tract). Female: control (n = 5 for upper and lower GI tracts) and 100 ppb As3+ (n = 5 for upper and lower GI tracts). *p<0,05 in Student’s two-tailed t-test or Mann-Whitney Rank Sum (U) Test compared to control group.
U and As3+ significantly perturb intraepithial lymphocyte subsets in the small intestine
To determine if elevated U and As exposures in the GI tract are associated with adverse effects on immune cells in the small intestine, we evaluated IEL subsets in the small intestine of male and female mice using flow cytometry (Fig. 2A). In male mice exposed to 50 ppm U, all the CD3ε+, CD4− IEL subsets investigated were significantly reduced; i.e., CD4−, TCRαβ+ (p = 0.01), CD4−, TCRαβ+, CD8αα+, CD8αβ+ (p<0.05), and CD4−, TCRαβ+, CD8αα+, CD8αβ− (p<0.05) (Fig. 3B); and CD4−, TCRγδ+ (p<0.05), CD4−, TCRγδ+, CD8αα+, CD8αβ+(p<0.05), and CD4−, TCRγδ+, CD8αα+, CD8αβ−(p<0.05) (Fig. 2C). No statistically significant effects on CD3ε+, CD4+ IELs were found in male mice (Supplementary Fig. S2A), nor were CD4+ or CD4− IEL subsets significantly reduced in female mice with either dose of U (Fig. 2D–E and Supplementary Fig. S2B).
Figure 2.
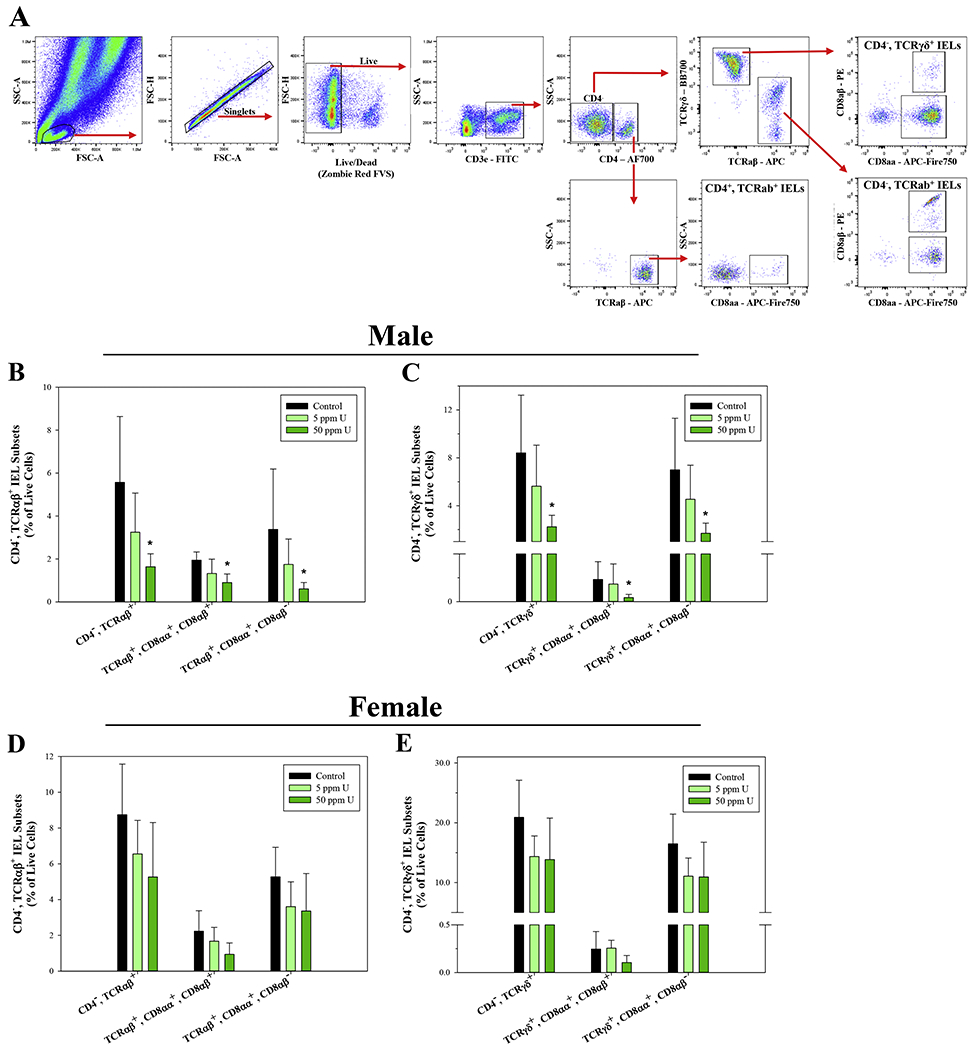
U exposure suppresses CD3ε+, CD4−, TCRαβ+ and TCRγδ+ IEL subsets in the small intestine of male mice. Small intestinal intraepithelial cells were isolated from male and female mice following 45-day exposure to 0, 5, and 50 ppm U and IEL subsets were evaluated based on surface marker phenotype by flow cytometry. (A) Representative flow cytometry plots demonstrating the gating strategy used to define small intestinal IEL subsets. Percentages of (B) CD3ε+, CD4−, TCRαβ+ and (D) CD3ε+, CD4−, TCRγδ+ IEL subsets in male mice. Percentages of (E) CD3ε+, CD4−, TCRαβ+ and (F) CD3ε+, CD4−, TCRγδ+ IEL subsets in female mice. Data are expressed as mean ± SD. Male and female: n = 6 mice/group. *p<0.05 in one-way ANOVA followed by a Dunnett’s t-test or Kruskal-Wallis One Way ANOVA on Ranks followed by Dunnett’s post-hoc test compared to control group. Abbreviations: FVS, fixable viability stain; AF700, Alexa Fluor 700.
Interestingly, whereas U primarily disrupted CD4− IEL subsets in male mice, a significant reduction of CD4+, TCRαβ+, CD8αα+, CD8αβ− IELs was found in females exposed to 100 and 500 ppb As3+ (p = 0.041 and 0.031, respectively; Fig. 3A). No statistically significant alterations in CD3ε+, CD4+ IELs were found in As3+ exposed male mice (Fig. 3B). Additionally, there were no significant changes in CD4− IELs in male or female mice with either As3+ dose (Supplementary Fig. S3A–D).
These results suggest that U and As3+ produce differential effects on IEL subsets, with U producing disruptions to both natural and induced CD4−, TCRαβ+ and TCRγδ+ IEL subsets; whereas, the effects of As3+ were limited to induced CD4+, TCRab+, CD8αα+ IELs.
Innate immune cell subsets of the lamina propria are significantly disrupted by As3+ exposure
In addition to IEL subsets, we also evaluated innate lymphoid cell subsets in the lamina propria of the small intestine by flow cytometry following U and As3+ exposures (Supplementary Fig. S1). U exposures in male and female mice did not significantly alter any of the innate immune cell subsets evaluated in this study (i.e., DP DCs, SP DCs, mature macrophages, immature/proinflammatory macrophages, monocytes, and neutrophils) (Supplementary Fig. S4A–B). However, a significant decrease in mature macrophages (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C−, CX3CR1+), along with a corresponding increase in immature/proinflammatory macrophages (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C+, CX3CR1+) was found in female mice following exposure to 500 ppb As3+ (p = 0.014 and 0.028, respectively; Fig. 4A–B). A similar, albeit non-statistically significant trend of decrease was observed in mature macrophages from As3+ exposed males, but there were no changes to immature/proinflammatory macrophages (Fig. 4C). No significant changes in DP DCs, SP DCs, monocytes, or neutrophils were found with either As3+ dose in male or female mice (Fig. 4B–C).
Figure 4.
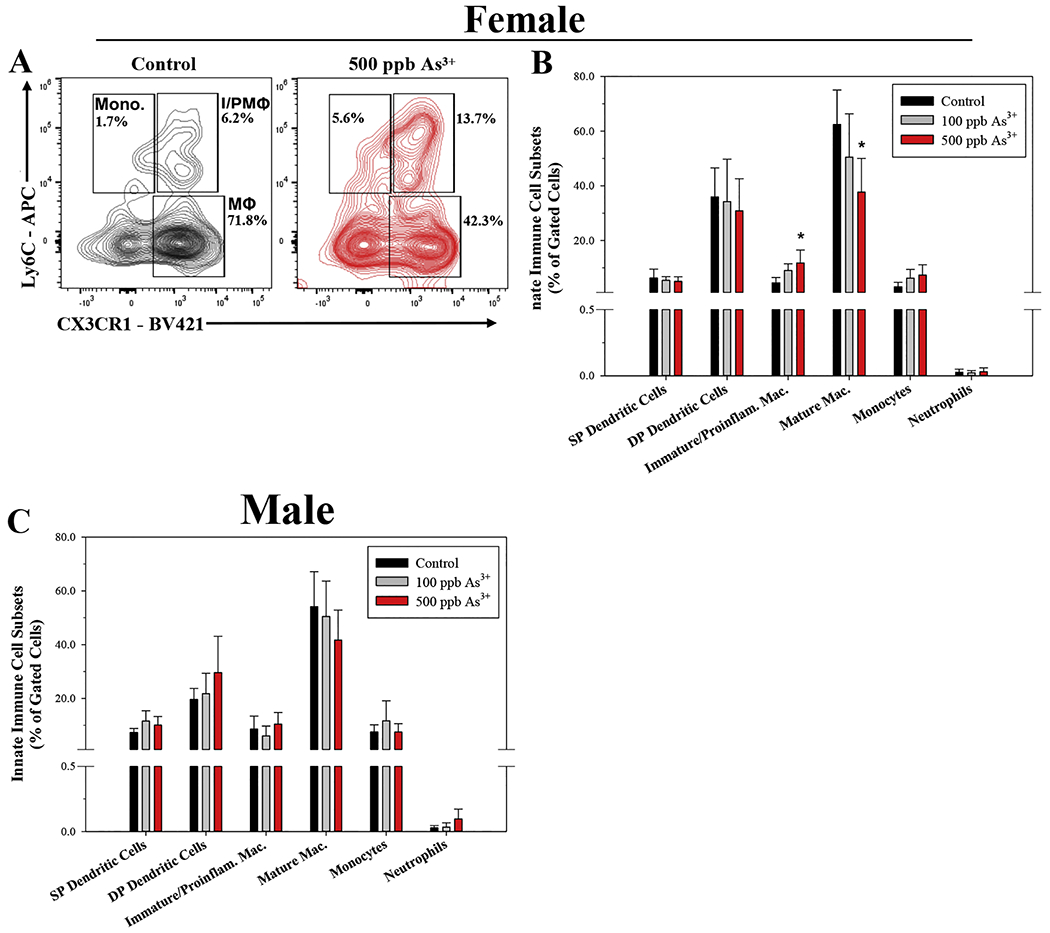
As3+-induced perturbations of innate immune cell subsets in the lamina propria of male and female mice. Small intestinal lamina propria cells were isolated from male and female mice following 45-day exposure to 0, 100, and 500 ppb As3+ and innate immune cell subsets were evaluated based on surface marker phenotype by flow cytometry. Innate immune cell subsets were defined as follows: single positive dendritic cells (SP DCs) (Lineage (Lin)− (CD3ε, CD45R/B220, CD5, IgE), CD11c+, MHC II+, CD103+, CD11b−); double positive dendritic cells (DP DCs) (Lin−, CD11c+, MHC II+, CD103+, CD11b+); Immature/proinflammatory macrophages (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C+, CX3CR1+); mature macrophages (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C−, CX3CR1+); monocytes (Lin−, CD11c+, MHC II+, CD103−, CD11b+, Ly6C+, CX3CR1−), and neutrophils (Lin−, Ly6G+, CD11b+). (A) Representative flow cytometry plot depicting effects of control (unexposed) and 500 ppb As3+ on immature/proinflammatory macrophages (I/PMΦ), mature macrophages (MΦ), and monocytes (Mono) in female mice. Percentages of innate immune cell subsets in (B) female and (C) male mice. Data are expressed as mean ± SD. Male: control (n = 4), 100 ppb (n = 5), and 500 ppb As3+ (n = 6). Female: control (n = 4), 100 ppb (n = 5), and 500 ppb As3+ (n = 6). *p<0.05 in one-way ANOVA followed by a Dunnett’s t-test or Kruskal-Wallis One Way ANOVA on Ranks followed by Dunnett’s post-hoc test compared to control group.
Collectively, these results demonstrate that U and As3+ disrupt distinct, but complementary components of the immune system in the gut. Whereas, U predominantly impacts IELs, As3+ also disrupts innate immune cells in the lamina propria. This suggests that combined U and As exposures may produce additive or synergistic immunotoxic effects to immune cells in the gut.
Discussion
Lymphocytes of the small intestine represent unique immune cell subsets that directly interface with the external environment to maintain a delicate balance between immune protection and symbiotic associations with commensal microorganisms (Cheroutre et al., 2011; Hooper et al., 2012; Sommer and Backhed, 2013; Peterson et al., 2015; Olivares-Villagomez and Van Kaer, 2018). The interface between the environment, immune cells, and microbiota is crucial for maintaining systemic immune health (Hooper et al., 2012; Sommer and Backhed, 2013; Peterson et al., 2015), but also represents a process vulnerable to environmental toxicant exposures. Numerous studies have linked metals and other toxicant exposures to disruptions of gut microbiota (Chi et al., 2016; Chi et al., 2017; Tsiaoussis et al., 2019); however, to our knowledge, no previous studies have evaluated the effects of U or As3+ on immune subsets of the small intestinal intraepithelial layer or lamina propria following drinking water exposures. In this study, we show that drinking water exposures to environmentally relevant levels of U and As3+ results in high levels of exposure in the GI tract and adversely impact two critical immune cell types in the small intestinal intraepithelial layer and lamina propria.
We found that drinking water exposures to environmentally relevant levels of U and As3+ result in significant levels of U and As exposure in the GI tract of male and female mice. Levels of U measured in the upper and lower GI were substantially higher than levels previously measured in other immune and systemic organs, including the spleen, thymus, bone marrow, and liver (Bolt et al., 2018). These findings are consistent with those reported in previous studies which established the small intestine as the major site of U and As3+ absorption (Pomroy et al., 1980; Vahter and Norin, 1980; Harrison, 1991; Dublineau et al., 2005). This is significant because immune cells and microbiota in the GI tract are in direct contact with these elevated exposures and therefore at increased risk of metal-induced toxicity.
In this study, we found a reduction of induced CD4+ IELs with As3+ exposure, which is consistent with previous findings from our lab demonstrating that T cell differentiation in the thymus is inhibited by As exposures (Xu et al., 2016a; Xu et al., 2016b; Xu et al., 2016c; Xu et al., 2016d; Xu et al., 2017a; Xu et al., 2017b). In the thymus, T cells undergo multiple stages of differentiation in which very early triple negative (i.e. CD3ε−, CD4−, CD8αβ−) cells gain expression of CD3ε, CD4, and CD8αβ prior to undergoing positive and negative selection and exiting the thymus as either mature CD4 or CD8αβ single positive cells (Burt and Verda, 2004). Loss of normal T cell development in the thymus may limit the number of mature CD4+ cells that reach the periphery and inevitably migrate to the gut.
The lack of effects on other putative thymus derived IEL subsets may be related to the differentiation stage at which the cells exit the thymus and home to the gut. In response to a variety of microenvironmental cues, early triple negative T cells are hypothesized to give rise to natural IELs, which home to the gut and complete maturation into fully functional IELs (Lambolez et al., 2002; Lambolez et al., 2006; Cheroutre and Lambolez, 2008; Cheroutre et al., 2011; Peaudecerf et al., 2011). Since our previous studies in the thymus focused on double negative (CD3ε+, CD4−, and CD8αβ−) stages of T cell differentiation and did not evaluate very early triple negative stages, the possibility that As3+ selectively impacts double negative vs. triple negative or subsequent thymic-IEL precursor subsets cannot be ruled out and requires further investigation. Another feasible explanation is that the effects on CD4+ IELs are independent of T cell suppression in the thymus and instead result from direct cytotoxicity of the metals or via indirect effects to supporting innate immune cells, epithelial cells, or gut microbiota.
U exposure produced effects on natural and induced classes of CD4−, TCRαβ+ and TCRγδ+ IELs. CD4−, TCRαβ+ and TCRγδ+ IELs not only have important regulatory roles in preventing inflammation, but also have vital roles in maintaining homeostatic interactions with gut microbiota and innate immune cells to ensure proper mucosal barrier function and neutralization of pathogenic threats from the external environment (Cheroutre et al., 2011; McDonald et al., 2018; Olivares-Villagomez and Van Kaer, 2018). Follow-up studies are required to determine the implications of reductions of CD4−, TCRαβ+ and TCRγδ+ IEL subsets, but such suppression may produce dysregulation of the gut immune system in ways that disrupt gut barrier function and enhance the likelihood of developing autoimmune disorders, such as inflammatory conditions of the GI tract.
The effects of U exposure on T cell development in the thymus have not been extensively studied, but our previous studies show very little U exposure at this site and no effects on T cell subsets following drinking water exposures in male and female mice (Bolt et al., 2018; Bolt et al., 2019). Therefore, rather than impacting T cell development in the thymus, it is more feasible that U produces effects locally in the gut either through direct toxicity to IELs or through indirect effects on innate immune cell populations or the microbiome.
In the present study, we found a disruption of macrophage subsets in the small intestinal lamina propria in a manner suggestive of inflammation among As3+ exposed mice (Na et al., 2019; Viola and Boeckxstaens, 2020). Multiple studies report that immature/proinflammatory macrophages, which produce inflammatory cytokines, including IL-12, IL-6, IL-Iβ, and TNF-α are associated with the promotion of inflammatory conditions, such as colitis and inflammatory bowel disorder (Bain et al., 2013; Zigmond and Jung, 2013; Na et al., 2019; Viola and Boeckxstaens, 2020). Our finding that As3+ modulates macrophage dynamics in a manner that may promote inflammation is consistent with previous findings showing As3+, and As3+ metabolites can drive and exacerbate inflammatory disease states mediated through macrophage activities, such as atherosclerosis (Lemaire et al., 2011; Negro Silva et al., 2017).
Although the trend of effects observed in IEL and innate cell subsets were generally consistent between U and As3+ exposed male and female mice, there were some differences between sexes that should be interpreted with caution but may have important biological consequences. Intriguingly, the major differences between female and male mice identified in this study were to immune subsets that have known roles in autoimmunity and inflammatory conditions. The reduction of CD4+ IELs, which have known regulatory functions in suppressing inflammatory immune responses (Groux et al., 1997; Reis et al., 2013; Costes et al., 2019; Zhou et al., 2019), combined with the imbalance of immature/proinflammatory and mature macrophages among females exposed to environmentally relevant levels of As3+ may be creating conditions that increase susceptibility to inflammatory and autoimmune conditions.
Consistent with this hypothesis is that the prevalence of autoimmune disorders is disproportionately higher among females than males, both naturally and in the context of metal-induced/exacerbated disease (Roitt and Rabson, 2000; Nielsen and Hultman, 2002; Lu-Fritts et al., 2014; Ortona et al., 2016). This suggests that females may have an inherent sensitivity to autoimmune disorders that is exacerbated as a result of metals exposures; however, this hypothesis requires further investigation and will be the topic of future studies. In addition, there were considerable differences between males and females in the levels of As measured in the GI tract, which may suggest sex-specific differences in accumulation or clearance between the two sexes. Future studies should be carefully designed to thoroughly evaluate sex-specific differences in the immunotoxicity of U and As3+.
Our results show distinct changes in small intestinal immune cell subsets caused by U or As3+ exposures; however, it remains unclear whether such changes are directly resultant of metal exposure or indirectly related to changes in the microbiota or other microenvironmental changes. In this study we did not measure any endpoints indicative of IEL or innate immune cell functions, so it remains to be determined if in addition to population-level changes, U and As3+ also impair functionality of IEL and/or innate cell subsets.
The present study demonstrates for the first time that U and As3+ disrupt IEL and innate immune cell subsets in the small intestine, which may provide an underlying link between environmental exposures and systemic immune issues reported in human populations chronically exposed to mixed metal containing mine wastes. This study provides essential information on the immunotoxicity of U and As3+ exposures, which are critical for gaining a deeper understanding of the adverse health outcomes reported in human populations exposed to mine wastes.
Supplementary Material
Highlights.
Drinking water exposures result in high levels of U and As in the GI tract.
IELs and innate immune cells in the small intestine are sensitive to U and As.
U and As disrupt distinct subsets of immune cells in the small intestine.
Acknowledgements
This work was funded by the National Institute of Environmental Health Sciences, UNM METALS Superfund Research Program [grant number P42 ES025589]; National Cancer Institute, UNM Comprehensive Cancer Center [grant number P30 CA118100] and the UNM Flow Cytometry shared resource.
Abbreviations
- U
Uranium
- As
arsenic
- As3+
arsenite
- IEL
intraepithelial lymphocyte
- GI
gastrointestinal
- DC
dendritic cells
- SP DC
single positive dendritic cells
- DP DC
double positive dendritic cells
- Lin
lineage markers (CD3ε, CD45R/B220, CD5, IgE)
- ICP-MS
inductively coupled plasma-mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- U.S. Enivironmental Protection Agency (US EPA), 2006. Basic Information About Radionuclides in Drinking Water. Washington DC: https://safewater.zendesk.com/hc/en-us/sections/202346157 [Google Scholar]
- U.S. Enivironmental Protection Agency (US EPA), 2012a. 2012 edition of the drinking water standards and health advisories table. EPA 822-S-12-001. www.epa.gov/sites/production/files/2015-09/documents/dwstandards2012.pdf
- U.S. Enivironmental Protection Agency (US EPA), 2012b. Navajo Nation: Cleaning Up Abandoned Uranium Mines, Contaminated Unregulated Water Sources. https://www.epa.gov/sites/production/files/2016-06/documents/watersourcestable-with-mcls.pdf
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM, 2013. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6, 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JM, Avasarala S, Artyushkova K, Ali AM, Brearley AJ, Shuey C, Robinson WP, Nez C, Bill S, Lewis J, Hirani C, Pacheco JS, Cerrato JM, 2015. Elevated Concentrations of U and Co-occurring Metals in Abandoned Mine Wastes in a Northeastern Arizona Native American Community. Environ Sci Technol 49, 8506–8514. [DOI] [PubMed] [Google Scholar]
- Bolt AM, Medina S, Lauer FT, Liu KJ, Burchiel SW, 2019. Minimal uranium immunotoxicity following a 60-day drinking water exposure to uranyl acetate in male and female C57BL/6J mice. Toxicol Appl Pharmacol 372, 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt AM, Medina S, Lauer FT, Xu H, Ali AM, Liu KJ, Burchiel SW, 2018. Minimal uranium accumulation in lymphoid tissues following an oral 60-day uranyl acetate exposure in male and female C57BL/6J mice. PLoS One 13, e0205211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton J, Massart S, Vandamme P, De Brandt E, Pot B, Foligne B, 2013. Ecotoxicology inside the gut: impact of heavy metals on the mouse microbiome. BMC Pharmacol Toxicol 14, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt RK, Verda L, 2004. Immune Reconstitution. Elsevier Academic Press, Burlington, MA. [Google Scholar]
- Campbell AW, 2014. Autoimmunity and the gut. Autoimmune Dis 2014, 152428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AW, 2015. The gut, intestinal permeability, and autoimmunity. Altern Ther Health Med 21, 6–7. [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, 2008. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol 20, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D, 2011. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11, 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Ru H, Tu P, Lu K, 2016. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem Res Toxicol 29, 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Tu P, Ru H, Lu K, 2017. The Effects of an Environmentally Relevant Level of Arsenic on the Gut Microbiome and Its Functional Metagenome. Toxicol Sci 160, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes LMM, Lindenbergh-Kortleve DJ, van Berkel LA, Veenbergen S, Raatgeep HRC, Simons-Oosterhuis Y, van Haaften DH, Karrich JJ, Escher JC, Groeneweg M, Clausen BE, Cupedo T, Samsom JN, 2019. IL-10 signaling prevents gluten-dependent intraepithelial CD4(+) cytotoxic T lymphocyte infiltration and epithelial damage in the small intestine. Mucosal Immunol 12, 479–490. [DOI] [PubMed] [Google Scholar]
- Couter CJ, Surana NK, 2016. Isolation and Flow Cytometric Characterization of Murine Small Intestinal Lymphocytes. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curato C, Bernshtein B, Aychek T, Jung S, 2016. In Vivo Analysis of Intestinal Mononuclear Phagocytes. Methods Mol Biol 1423, 255–268. [DOI] [PubMed] [Google Scholar]
- deLemos JL, Brugge D, Cajero M, Downs M, Durant JL, George CM, Henio-Adeky S, Nez T, Manning T, Rock T, Seschillie B, Shuey C, Lewis J, 2009. Development of risk maps to minimize uranium exposures in the Navajo Churchrock mining district. Environ Health 8, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dublineau I, Grison S, Baudelin C, Dudoignon N, Souidi M, Marquette C, Paquet F, Aigueperse J, Gourmelon P, 2005. Absorption of uranium through the entire gastrointestinal tract of the rat. Int J Radiat Biol 81, 473–482. [DOI] [PubMed] [Google Scholar]
- Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, Rubin RL, 2019. Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo Nation. J Autoimmun 99, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario D, Gribaldo L, Hartung T, 2016. Arsenic Exposure and Immunotoxicity: a Review Including the Possible Influence of Age and Sex. Curr Environ Health Rep 3, 1–12. [DOI] [PubMed] [Google Scholar]
- Geem D, Medina-Contreras O, Kim W, Huang CS, Denning TL, 2012. Isolation and characterization of dendritic cells and macrophages from the mouse intestine. J Vis Exp, e4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Salame TM, Jung S, 2015. Guardians of the Gut - Murine Intestinal Macrophages and Dendritic Cells. Front Immunol 6, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG, 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742. [DOI] [PubMed] [Google Scholar]
- Harrison JD, 1991. The gastrointestinal absorption of the actinide elements. Sci Total Environ 100 Spec No, 43–60. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ, 2012. Interactions between the microbiota and the immune system. Science 336, 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J, 2017. Elevated Arsenic and Uranium Concentrations in Unregulated Water Sources on the Navajo Nation, USA. Expo Health 9, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D, 2017. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 171, 783–794 e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, 2002. Arsenic toxicity and potential mechanisms of action. Toxicol Lett 133, 1–16. [DOI] [PubMed] [Google Scholar]
- Ingram JC, Jones L, Credo J, Rock T, 2020. Uranium and arsenic unregulated water issues on Navajo lands. J Vac Sci Technol A 38, 031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joeris T, Muller-Luda K, Agace WW, Mowat AM, 2017. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol 10, 845–864. [DOI] [PubMed] [Google Scholar]
- Jones L, Credo J, Parnell R, Ingram JC,, 2020. Dissolved Uranium and Arsenic in Unregulated Groundwater Sources - Western Navajo Nation. Dissolved Uranium and Arsenic in Unregulated Groundwater Sources - Western Navajo Nation 169, 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntunen R, 1991. Uranium and radon in wells drilled into bedrock in Southern Finland. Report of Investigation, Geological Survey of Finland 98. [Google Scholar]
- Kozul CD, Ely KH, Endow RI, Hamilton JW, 2009. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect 117, 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambolez F, Arcangeli ML, Joret AM, Pasqualetto V, Cordier C, Di Santo JP, Rocha B, Ezine S, 2006. The thymus exports long-lived fully committed T cell precursors that can colonize primary lymphoid organs. Nat Immunol 7, 76–82. [DOI] [PubMed] [Google Scholar]
- Lambolez F, Azogui O, Joret AM, Garcia C, von Boehmer H, Di Santo J, Ezine S, Rocha B, 2002. Characterization of T cell differentiation in the murine gut. J Exp Med 195, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer FT, Parvez F, Factor-Litvak P, Liu X, Santella RM, Islam T, Eunus M, Alam N, Hasan A, Rahman M, Ahsan H, Graziano J, Burchiel SW, 2019. Changes in human peripheral blood mononuclear cell (HPBMC) populations and T-cell subsets associated with arsenic and polycyclic aromatic hydrocarbon exposures in a Bangladesh cohort. PLoS One 14, e0220451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Lemarié CA, Flores Molina M, Schiffrin EL, Lehoux S, Mann KK, 2011. Exposure to Moderate Arsenic Concentrations Increases Atherosclerosis in ApoE −/− Mouse Model. Toxicological Sciences 122, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Hoover J, MacKenzie D, 2017. Mining and Environmental Health Disparities in Native American Communities. Curr Environ Health Rep 4, 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Y, Liu K, Shen J, 2014. Exposing to cadmium stress cause profound toxic effect on microbiota of the mice intestinal tract. PLoS One 9, e85323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, Pereira R, Pinto F, Caetano T, Silva A, Carvalheiro T, Guimaraes A, Goncalves F, Paiva A, Mendo S, 2013. Biomonitoring a human population inhabiting nearby a deactivated uranium mine. Toxicology 305, 89–98. [DOI] [PubMed] [Google Scholar]
- Lu-Fritts PY, Kottyan LC, James JA, Xie C, Buckholz JM, Pinney SM, Harley JB, 2014. Association of systemic lupus erythematosus with uranium exposure in a community living near a uranium-processing plant: a nested case-control study. Arthritis Rheumatol 66, 3105–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BD, Jabri B, Bendelac A, 2018. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat Rev Immunol 18, 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalban-Arques A, Chaparro M, Gisbert JP, Bernardo D, 2018. The Innate Immune System in the Gastrointestinal Tract: Role of Intraepithelial Lymphocytes and Lamina Propria Innate Lymphoid Cells in Intestinal Inflammation. Inflamm Bowel Dis 24, 1649–1659. [DOI] [PubMed] [Google Scholar]
- Mowat AM, 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3, 331–341. [DOI] [PubMed] [Google Scholar]
- Mu Q, Kirby J, Reilly CM, Luo XM, 2017. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol 8, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na YR, Stakenborg M, Seok SH, Matteoli G, 2019. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol 16, 531–543. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro Silva LF, Lemaire M, Lemarie CA, Plourde D, Bolt AM, Chiavatti C, Bohle DS, Slavkovich V, Graziano JH, Lehoux S, Mann KK, 2017. Effects of Inorganic Arsenic, Methylated Arsenicals, and Arsenobetaine on Atherosclerosis in the Mouse Model and the Role of As3mt-Mediated Methylation. Environ Health Perspect 125, 077001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Hultman P, 2002. Mercury-induced autoimmunity in mice. Environ Health Perspect 110 Suppl 5, 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagomez D, Van Kaer L, 2018. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol 39, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong J, Erdei E, Rubin RL, Miller C, Ducheneaux C, O’Leary M, Pacheco B, Mahler M, Henderson PN, Pollard KM, Lewis JL, 2014. Mercury, autoimmunity, and environmental factors on cheyenne river sioux tribal lands. Autoimmune Dis 2014, 325461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orescanin V, Kollar R, Nad K, Mikelic IL, Kollar I, 2011. Characterization and treatment of water used for human consumption from six sources located in the Cameron/Tuba City abandoned uranium mining area. J Environ Sci Health A Tox Hazard Subst Environ Eng 46, 627–635. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO), 2011. Arsenic in drinking-water. www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf
- World Health Organization (WHO), 2012. Uranium in drinking-water. Geneva Switzerland: http://www.who.int/water_sanitation_health/publications/2012/background_uranium.pdf [Google Scholar]
- Ortona E, Pierdominici M, Maselli A, Veroni C, Aloisi F, Shoenfeld Y, 2016. Sex-based differences in autoimmune diseases. Ann Ist Super Sanita 52, 205–212. [DOI] [PubMed] [Google Scholar]
- Parvez F, Lauer FT, Factor-Litvak P, Liu X, Santella RM, Islam T, Eunus M, Alam N, Sarwar G, Rahman M, Ahsan H, Graziano J, Burchiel SW, 2019. Assessment of arsenic and polycyclic aromatic hydrocarbon (PAH) exposures on immune function among males in Bangladesh. PLoS One 14, e0216662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaudecerf L, dos Santos PR, Boudil A, Ezine S, Pardigon N, Rocha B, 2011. The role of the gut as a primary lymphoid organ: CD8alphaalpha intraepithelial T lymphocytes in euthymic mice derive from very immature CD44+ thymocyte precursors. Mucosal Immunol 4, 93–101. [DOI] [PubMed] [Google Scholar]
- Peterson CT, Sharma V, Elmen L, Peterson SN, 2015. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol 179, 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomroy C, Charbonneau SM, McCullough RS, Tam GK, 1980. Human retention studies with 74As. Toxicol Appl Pharmacol 53, 550–556. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2007. Toxicological profile for Arsenic. Atlanta, GA: [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2013. Toxicological profile for uranium. Atlanta, GA: https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 2016. Addendum to the Toxicological Profile for Arsenic. Atlanta, GA: [PubMed] [Google Scholar]
- Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D, 2013. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol 14, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I, Rabson A, 2000. Autoimmune diseases In Roitt I, Rabson A, (Eds.), Really Essential Medical Immunology. Oxford: Blackwell Science, pp. 160–168. [Google Scholar]
- Sommer F, Backhed F, 2013. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol 11, 227–238. [DOI] [PubMed] [Google Scholar]
- Sprouse ML, Bates NA, Felix KM, Wu HJ, 2019. Impact of gut microbiota on gut-distal autoimmunity: a focus on T cells. Immunology 156, 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, Woltman AM, Reyal Y, Bonnet D, Sichien D, Bain CC, Mowat AM, Reis e Sousa C, Poulin LF, Malissen B, Guilliams M, 2012. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42, 3150–3166. [DOI] [PubMed] [Google Scholar]
- Tsiaoussis J, Antoniou MN, Koliarakis I, Mesnage R, Vardavas CI, Izotov BN, Psaroulaki A, Tsatsakis A, 2019. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol Lett 312, 72–97. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Allan AM, 2014. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep 1, 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Norin H, 1980. Metabolism of 74As-labeled trivalent and pentavalent inorganic arsenic in mice. Environ Res 21, 446–457. [DOI] [PubMed] [Google Scholar]
- Viola MF, Boeckxstaens G, 2020. Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol Motil, e13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF, 2007. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2, 2307–2311. [DOI] [PubMed] [Google Scholar]
- Xu H, Lauer FT, Liu KJ, Hudson LG, Burchiel SW, 2016a. Editor’s Highlight: Interactive Genotoxicity Induced by Environmentally Relevant Concentrations of Benzo(a)Pyrene Metabolites and Arsenite in Mouse Thymus Cells. Toxicol Sci 154, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Lauer FT, Liu KJ, Hudson LG, Burchiel SW, 2016b. Environmentally relevant concentrations of arsenite and monomethylarsonous acid inhibit IL-7/STAT5 cytokine signaling pathways in mouse CD3+CD4−CD8− double negative thymus cells. Toxicol Lett 247, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, McClain S, Medina S, Lauer FT, Douillet C, Liu KJ, Hudson LG, Styblo M, Burchiel SW, 2016c. Differential sensitivities of bone marrow, spleen and thymus to genotoxicity induced by environmentally relevant concentrations of arsenite. Toxicol Lett 262, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Medina S, Lauer FT, Douillet C, Liu KJ, Hudson LG, Styblo M, Aleksunes LM, Burchiel SW, 2017a. Efflux Transporters Regulate Arsenite-Induced Genotoxicity in Double Negative and Double Positive T Cells. Toxicol Sci 158, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Medina S, Lauer FT, Douillet C, Liu KJ, Styblo M, Burchiel SW, 2017b. Genotoxicity induced by monomethylarsonous acid (MMA(+3)) in mouse thymic developing T cells. Toxicol Lett 279, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhou X, Wen X, Lauer FT, Liu KJ, Hudson LG, Aleksunes LM, Burchiel SW, 2016d. Environmentally Relevant Concentrations of Arsenite Induce Dose-Dependent Differential Genotoxicity Through Poly(ADP-Ribose) Polymerase Inhibition and Oxidative Stress in Mouse Thymus Cells. Toxicol Sci 149, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Yang H, 2019. CD4CD8alphaalpha IELs: They Have Something to Say. Front Immunol 10, 2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond E, Jung S, 2013. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 34, 162–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


