Abstract
Objective
In this retrospective observational study, we evaluated awareness among patients using bisphosphonates (BPs) regarding the risk of developing medication-related osteonecrosis of the jaw (MRONJ) and whether they received appropriate dental screening and treatment prior to commencing medication.
Methods
Patients using BPs who attended the endodontics clinic at Jordan University Hospital in 2019 were interviewed using a pre-designed questionnaire. Data were analysed using descriptive statistics and chi-squared tests.
Results
In total, 110 patients were interviewed (84 women, 26 men; age 40–78 years). A total of 94 patients were using oral BP and 16 received intravenous (IV) BP. We found that only 12.4% of participants were aware about the risk of MRONJ following BP use, and only one third of them has received information from their prescribing physicians. In total, 5% of participants were referred to a dentist for screening prior to initiating BP treatment. Patients receiving IV BP and those with a university-level education had better awareness about the risk of MRONJ than oral BP users and those with a high school education level.
Conclusion
Patients’ awareness about MRONJ risk was low in our population. Better patient education and collaboration among physicians and dentists are needed prior to starting BP treatment.
Keywords: Medication-related osteonecrosis of the jaw, bisphosphonates, antiresorptive drugs, risk awareness, patient education, multidisciplinary team
Introduction
Bisphosphonates (BPs) are antiresorptive medications used to manage osteoclast-mediated bone loss including osteoporosis, bone malignancies, Paget disease of bone, and multiple myeloma.1 BPs have a considerably positive effect on quality of life in patients with such lesions.2 However, BPs are also associated with the development of osteonecrosis of the jaw.3
The first cases of osteonecrosis of the jaw associated with BP use were reported at the University of Miami in 2003 when Marx described 36 cases of painful bony exposures in the mandible, maxilla, or both that were unresponsive to medical or surgical treatment in patients receiving intravenous (IV) BP.4 In 2004, Ruggiero et al.5 reported the development of nonhealing extraction sockets or exposed jawbone in 63 patients with chronic use of BPs. Numerous reports followed thereafter, including addition of a warning label by the manufacturing company (Novartis) in 2004 of a “possible relationship of Aredia (pamidronate disodium) and/or Zometa (zolendronic acid) with osteonecrosis of the jaw”.2 This condition was later referred to as bisphosphonate-related osteonecrosis of the jaw (BRONJ). In 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) changed the nomenclature to medication-related osteonecrosis of the jaw (MRONJ) to accommodate cases of jaw osteonecrosis associated with other antiresorptive and antiangiogenic medications.2
MRONJ is defined as exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for more than 8 weeks in patients with current or previous treatment with antiresorptive or antiangiogenic agents and no history of radiation therapy to the jaw or obvious metastatic disease of the jaw.2
The risk of developing MRONJ depends upon several factors, including the therapeutic indication and type of medication used, duration of medication,6 oral/dental treatment received,7 anatomic factors,7 oral disease,8 other medications used,9 and genetic factors.10 However, two major risk factors must be considered when assessing the risk of MRONJ; an oral surgical procedure including tooth extraction, and IV route of administration.11 In total, 61.8% of patients report tooth extraction directly prior to developing MRONJ.12 The prevalence of MRONJ in users of oral BP for treatment of osteoporosis ranges from 0.1% to 0.21%13 and a reported 0.33% to 2.9% in patients with cancer who are treated with IV zolendronate.7,14–16
Whereas the incidence of MRONJ among BP users remains relatively low, the morbidity experienced by affected patients is considerable. In a recent qualitative study in the United Kingdom (UK), MRONJ was reported to have a significant negative impact on the quality of life of patients owing to a substantial amount of pain, the need for analgesics and antibiotics, social anxiety related to challenges with eating and drinking, psychological implications, and the need to travel long distances to attend multiple appointments in a secondary care dental hospital.17 Therefore, thorough education of all involved is essential. Patients must be educated about the risk of MRONJ before being prescribed a BP, physicians need to be educated about oral health and its influence on the risk of developing MRONJ, and dentists must be trained to recognize and manage MRONJ so as to improve its outcome and avoid litigation.7
Tanna et al. demonstrated poor awareness regarding the medications associated with MRONJ and reluctance to treat patients using BP in primary care among general dental practitioners (GDPs) in the UK.18 Only 25% of general medical practitioners (GMPs) and GMP trainees in Birmingham, UK were aware of the clinical presentation of MRONJ and only 8% were aware of any associated guidelines.19 Patients using BPs without a diagnosis of MRONJ report minimal awareness of the risk of MRONJ whereas all patients with a diagnosis of MRONJ report having received no education about the risk of MRONJ prior to commencing treatment with a BP.17
Patients scheduled for treatment with antiresorptive or antiangiogenic medications would benefit from early oral health screening and the provision of appropriate oral and dental care prior to initiating their medication,20,21 which has been shown to reduce the incidence of osteonecrosis in this group of patients by 33% to 50%.22,23
The aims of this research were to assess a) the awareness among patients using BPs in Jordan regarding their risk of developing MRONJ and b) whether patients received proper dental care prior to initiating their BP treatment.
Methods
The protocol of this observational retrospective study received approval of the ethics review board of Jordan University Hospital on 8 January 2019 (IRB no. 10/2018/26378).
A questionnaire was designed by the authors to measure the awareness of patients receiving BPs regarding their increased risk of developing MRONJ, and to enquire whether they had received appropriate dental care prior to starting their medication. The questionnaire was piloted in five patients and modified to ensure clarity (see the appendix).
Patients using BP who attended the specialist endodontics clinic at Jordan University Hospital between January 2019 and the end of December 2019 and who provided their verbal consent to participate in the study were asked to complete the pre-designed questionnaire. The data obtained were anonymously tabulated and stored in a secure and password-protected file that was only accessible by the principal investigator (AE), thus ensuring that patients’ identities could not be ascertained.
Data were analysed using IBM SPSS version 26 (IBM Corp., Armonk, NY, USA). Descriptive statistics were performed, and the chi-squared test was used to detect any association between patients’ background characteristics and their awareness about the risk of developing MRONJ.
Results
A total of 110 patients agreed to participate in this study, including 84 women, 26 men (age 40–78 years). Patients’ demographics and level of education are summarized in Table 1. Patients’ medical conditions requiring BP prescription, route of administration, and duration of BP use are summarized in Table 2.
Table 1.
Patients’ demographic information.
| Age | <50 years | 51–70 years | >70 years |
| 8 | 65 | 37 | |
| Sex | Male | Female | |
| 26 | 84 | ||
| Education level | Up to high school | University degree | |
| 82 | 28 | ||
Table 2.
Number of patients with medical conditions requiring bisphosphonates (BPs), route of administration, and duration of use.
| Medical condition requiring BP | Osteoporosis | Osteopenia | Paget disease | Multiple myeloma | Malignancy |
| 87 | 7 | 1 | 2 | 13 | |
| Route of administration of BP | Oral | Intravenous | |||
| 94 | 16 | ||||
| Duration of BP use | <1 year | 1–3 years | >3 years | ||
| 39 | 34 | 37 | |||
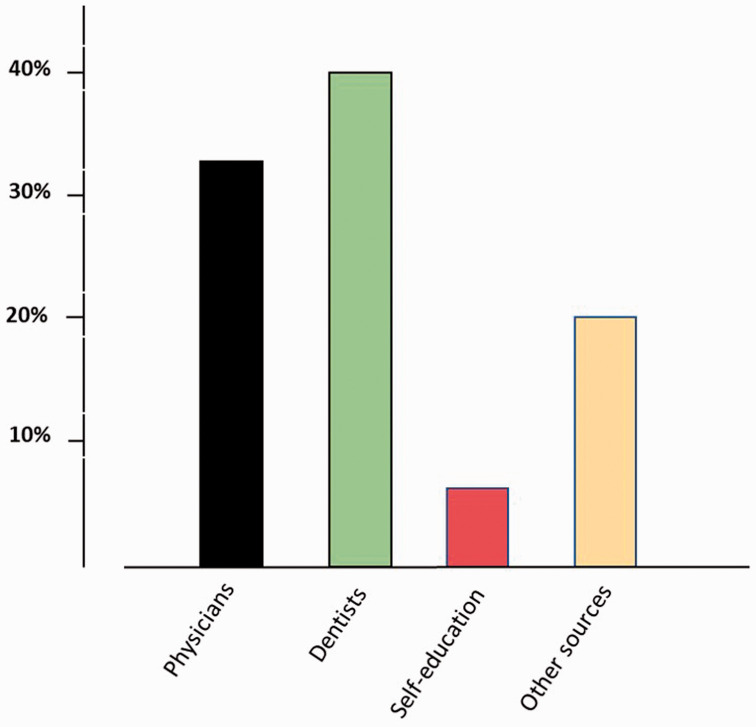
Only 12.4% (n = 15) of our study participants were aware about the risk of developing osteonecrosis of the jaw following BP use. Of those, only five (33%) had received information about the risk of MRONJ from their prescribing physician; the rest were informed by their dentist (40%, n = 6), self-educated (6.7%, n = 1), or received information from other sources (20%, n = 3) (Figure 1). Only six patients (5%) were referred to a dentist for an oral and dental health checkup prior to initiating BP treatment.
Figure 1.
Sources of information regarding the risk of medication-related osteonecrosis of the jaw (MRONJ) among patients who were aware of this complication.
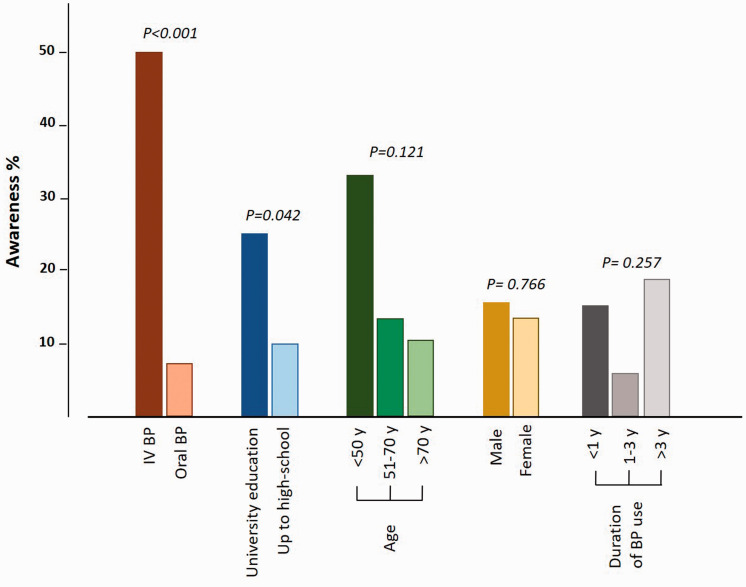
There was no association between patients’ awareness about the risk of MRONJ and their age (χ2 = 4.22), sex (χ2 = 0.09) or duration of BP use (χ2 = 2.71). However, patients receiving intravenous (IV) BP were significantly more aware of the risk of MRONJ than those receiving oral BP (50% vs. 7.4%, respectively; χ2 = 21.02, P<0.001). Furthermore, patients holding a university degree showed significantly better awareness about the risk of MRONJ than those with high school education level (25% vs. 9.8%; χ2 = 4.12, P = 0.042) (Figure 2).
Figure 2.
Clustered column chart illustrating the association between patients’ awareness about the risk of medication-related osteonecrosis of the jaw (MRONJ) and their background characteristics.
Only two patients (1.8%) had developed MRONJ; both of them were receiving IV BP.
Discussion
MRONJ is a severe adverse effect associated with the use of antiresorptive and antiangiogenic medication. MRONJ can be difficult to treat and can have a negative impact on patients’ quality of life. The reported prevalence has been relatively low but recent literature has shown that this morbidity may be underestimated.24 The implementation of preventative measures including dental consultation (and treatment) is recommended for optimal patient care.2
Our results showed poor awareness among participants regarding the risk of MRONJ. Even more alarming, only one-third of participants who were aware of the risk of MRONJ had received information about MRONJ from their prescribing physician. This reflects a serious issue that must be highlighted and acted upon. Physicians who prescribe BP must inform their patients of the risk of MRONJ. It then becomes the patients’ decision whether they wish to accept this risk or not. Lack of or improper patient education may result in legal liability. Clinicians are not only expected to maintain up-to-date knowledge about MRONJ and its risk factors but they are also required to convey this information to patients scheduled for BP treatment.25 The lack of patient education about possible MRONJ may also be attributed to the lack of awareness among treating physicians. Most Lebanese physicians were found to have poor understanding of MNROJ, and 37% of them were unaware of this complication.26 Similar findings were reported in a survey of Saudi physicians, where less than one-third were aware of MRONJ and its management.27 In Birmingham, UK, 11% of GMPs know about the risk of developing MRONJ upon prescribing antiresorptive medications.19
Dentists demonstrate better awareness regarding the incidence of MRONJ. Sixty percent of dentists in Ontario, Canada reported good knowledge about MRONJ prevention and management; however, half of these dentists were not comfortable treating patients with MRONJ and preferred referral to a secondary health care centre.28 In a surveyed sample of 129 British GDPs, most were aware of the risk of MRONJ in BP users but were not sufficiently confident or keen to perform dentoalveolar procedures for these patients [19]. Oral and maxillofacial surgeons in Iraq had better awareness of the risk of MRONJ than physicians and GDPs.29
Although osteonecrosis of the jaw may develop spontaneously in a small proportion of patients using antiresorptive and antiangiogenic medication, most affected patients develop osteonecrosis following dentoalveolar surgery.2 Therefore, good collaboration between the treating physician and dentist is essential, to optimize dental and oral health prior to commencing antiresorptive treatment and to avoid dentoalveolar surgery during bone-targeted treatment.2,21 Dental preventive measures have been shown to reduce the incidence of developing MRONJ among BP users by 50%.23 In a prospective study among patients with cancer undergoing intravenous zoledronate therapy, Bonacina at al. reported a 10.8% incidence of MRONJ among patients in whom oral and dental prevention strategies were not applied prior to commencing BP treatment during an 18-month observation period; none of the patients who received oral and dental screening prior to medication initiation developed MRONJ during the same observation period.30 Most of our participants were not referred for dental screening prior to BP treatment, which highlights the need for better education of treating physicians about oral health and its implication in the risk of MRONJ. Furthermore, the use of health education tools during routine dental visits is encouraged as this has been shown to improve patients’ oral health and enable them to identify early signs of jaw osteonecrosis.31
Whereas this study conveys an important message to health care professionals, the retrospective nature of the study and the relatively small sample should be noted. It is worth mentioning that the study was intended to involve patients receiving other antiresorptive medication (Denosumab) and antiangiogenics but none of the patients attended during that period of time reported use of these medications. This study may be reproduced in the future using a different sample of patients and questionnaire and statistical analysis described in the present methods section.
Conclusion
Osteonecrosis of the jaw is a significant adverse effect of antiresorptive medication and has a negative impact on patients’ quality of life. Management of MRONJ requires a multidisciplinary approach as many health care professionals have a role in prevention and patient education. Educating patients about the risks and outcomes of MRONJ, educating physicians about oral health and its importance for patients, and a multidisciplinary approach including dental screening and optimizing of oral health prior to commencing antiresorptive medication may reduce the risk of MRONJ, improve patients’ acceptance of this potential risk, and reduce medical litigation. National guidelines on the management of patients who receive BP and prevention of MRONJ are recommended, to help minimize complications.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520955066 for Awareness of the risk of developing medication-related osteonecrosis of the jaw among bisphosphonate users by Ahmad El-Ma’aita, Noor Da’as, Mais Al-Hattab, Yazan Hassona, Mohammad Al-Rabab’ah and Mohammad-Awni Al-Kayed in Journal of International Medical Research
Acknowledgements
The authors would like to thank Professor Faleh Sawair for his assistance with statistical analysis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Ahmad El-Ma’aita https://orcid.org/0000-0002-7836-0351
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008; 83: 1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 2014; 72: 1938–1956. [DOI] [PubMed] [Google Scholar]
- 3.Kuroshima S, Sasaki M, Sawase T. Medication-related osteonecrosis of the jaw: A literature review. J Oral Biosci 2019; 61: 99–104. [DOI] [PubMed] [Google Scholar]
- 4.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61: 1115–1117. [DOI] [PubMed] [Google Scholar]
- 5.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62: 527–534. [DOI] [PubMed] [Google Scholar]
- 6.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 7.Saad F, Brown JE, Van Poznaket C. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012; 23: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki T, Yamori M, Ishizaki T, et al. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: a cohort study. Int J Oral Maxillofac Surg 2012; 41: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 9.Tsao C, Darby I, Ebeling PR, et al. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J Oral Maxillofac Surg 2013; 71: 1360–1366. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletti P, Cartsos VM, Palaska DK, et al. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: the role of RBMS3. Oncologist 2012; 17: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosella D, Papi P, Giardino R, et al. Medication-related osteonecrosis of the jaw: Clinical and practical guidelines. J Int Soc Prev Community Dent 2016; 6: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012; 23: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 13.Lo JC, O'Ryan FS, Gordon NP, et al. Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg 2010; 68: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauri D, Valachis A, Polyzos IP, et al. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat 2009; 116: 433–439. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti GV, Hirsh V, Siena S, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol 2012; 7: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 16.Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): Incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol 2009; 112: 605–609. [DOI] [PubMed] [Google Scholar]
- 17.Sturrock A, Preshaw PM, Hayes C, et al. Perceptions and attitudes of patients towards medication-related osteonecrosis of the jaw (MRONJ): a qualitative study in England. BMJ Open 2019; 9: e024376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanna N, Steel C, Stagnell S, et al. Awareness of medication related osteonecrosis of the jaws (MRONJ) amongst general dental practitioners. Br Dent J 2017; 222: 121–125. [DOI] [PubMed] [Google Scholar]
- 19.Rahman Z, Nayani S, Anstey H, et al. A survey evaluating the awareness of MRONJ within the Birmingham GMP community. Oral Surgery 2019; 12: 22–29. [Google Scholar]
- 20.Zurányi A, Vasziné Szabó E, Tóth Z. Risk assessment of medication-related osteonecrosis of the jaw in general dental practice. Orv Hetil 2019; 160: 243–251. [DOI] [PubMed] [Google Scholar]
- 21.Yarom N, Shapiro CL, Peterson DE, et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J Clin Oncol 2019; 37: 2270–2290. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Kastritis E, Bamia C, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 2009; 20: 117–120. [DOI] [PubMed] [Google Scholar]
- 23.Vandone AM, Donadio M, Mozzati M, et al. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience. Ann Oncol 2012; 23: 193–200. [DOI] [PubMed] [Google Scholar]
- 24.Galis B, Zajko J, Hirjak D, et al. Is the prevalence of the medication-related osteonecrosis of the jaws underestimated, evaluation in oncological and non-oncological disease. Bratisl Lek Listy 2017; 118: 724–731. [DOI] [PubMed] [Google Scholar]
- 25.Lo Russo L, Ciavarella D, Buccelli C, et al. Legal liability in bisphosphonate-related osteonecrosis of the jaw. Br Dent J 2014; 217: 273–278. [DOI] [PubMed] [Google Scholar]
- 26.El Osta L, El Osta B, Lakiss S, et al. Bisphosphonate-related osteonecrosis of the jaw: awareness and level of knowledge of Lebanese physicians. Support Care Cancer 2015; 23: 2825–2831. [DOI] [PubMed] [Google Scholar]
- 27.Al-Mohaya MA, Al-Khashan HI, Mishriky AM, et al. Physicians' awareness of bisphosphonates-related osteonecrosis of the jaw. Saudi Med J 2011; 32: 830–835. [PubMed] [Google Scholar]
- 28.Alhussain A, Peel S, Dempster L, et al. Knowledge, practices, and opinions of Ontario dentists when treating patients receiving bisphosphonates. J Oral Maxillofac Surg 2015; 73: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 29.Al-Samman AA, Al-Ani RS. Across-sectional survey on medication-related osteonecrosis of the jaws' knowledge and awareness in a sample of dental society. J Craniomaxillofac Surg 2019; 47: 926–931. [DOI] [PubMed] [Google Scholar]
- 30.Bonacina R, Mariani U, Villa F, et al. Preventive strategies and clinical implications for bisphosphonate-related osteonecrosis of the jaw: a review of 282 patients. J Can Dent Assoc 2011; 77: b147. [PubMed] [Google Scholar]
- 31.García-Martínez L, Martín-Payo R, Pelaz-García A, et al. Intervention to improve awareness of the risk factors for osteonecrosis of the jaw in patients under treatment with bisphosponates. Randomised clinical trial. Enferm Clin 2017; 27: 352–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520955066 for Awareness of the risk of developing medication-related osteonecrosis of the jaw among bisphosphonate users by Ahmad El-Ma’aita, Noor Da’as, Mais Al-Hattab, Yazan Hassona, Mohammad Al-Rabab’ah and Mohammad-Awni Al-Kayed in Journal of International Medical Research