Abstract
Epidemiological studies show mixed findings for serum vitamin B12 and both cognitive and regional volume outcomes. No studies to date have comprehensively examined, in non-supplemented individuals, serum B12 level associations with neurodegeneration, hypometabolism, and cognition across the Alzheimer’s disease (AD) spectrum. Serum vitamin B12 was assayed from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). Voxel-wise analyses regressed B12 levels against regional gray matter (GM) volume and glucose metabolism (p<.05, family-wise corrected). For ADNI GM, there were 39 cognitively normal (CN), 73 mild cognitive impairment (MCI), and 31 AD participants. For AIBL GM, there were 311 CN, 59 MCI, and 31 AD participants. Covariates were age, sex, baseline diagnosis, APOE4 status, and Body Mass Index (BMI). In ADNI, higher B12 was negatively associated with GM in the right precuneus and bilateral frontal gyri. When diagnostic groups were examined separately, only participants with MCI or above an established cutoff for CSF total tau showed such associations. In AIBL, higher B12 was associated with more grey matter in the right amygdala and right superior temporal pole, which largely seemed to be driven by CN participants that constituted most of the sample. Our results suggest that B12 may show different patterns of association based on clinical status and, for ADNI, AD CSF biomarkers. Accounting for these factors may clarify the relationship between B12 with neural outcomes in late-life.
Keywords: Nutrition, MRI, Biomarkers, Neuroimaging
Introduction
Deficient levels of vitamin B12 or folate lead to increased levels of homocysteine, which is a risk factor for thrombosis, microbleeds, strokes, cognitive impairment and neuronal atrophy(1; 2). Vitamin B12, or cobalamin, normally contributes to the production of myelin in the central nervous system and fatty acid metabolism. Vitamin B12 is naturally occurring in meat, fish, milk, and eggs(3). When vitamin B12 is consumed, it first binds to haptocorrin (or transcobalamin I) to protect the B12 from stomach acid. Intrinsic factor is produced in the stomach and binds to vitamin B12 in the intestines to allow absorption into the ileum enterocytes via the membrane protein cubilin(4; 5). Importantly, vitamin B12 is transported to the liver via transcobalamin II, where it takes place in folate/B12 dependent remethylation to facilitate the conversion of homocysteine to methionine and the subsequent methylation of DNA, proteins and lipids(6).
The literature is currently mixed about the role of vitamin B12 in brain health and late-life adults with or without Alzheimer’s disease (AD) related cognitive impairment. On the one hand, vitamin B12 is significantly lower in both plasma(7) and CSF(8) of patients with AD versus normally aging controls. Further, vitamin B12 deficiency in aged, cognitively normal adults with diabetes was associated with less grey matter volume in the left middle temporal pole and the left insula(9) suggesting that supplementation may be useful. Indeed, clinical trials have found that B12 supplementation may slow brain atrophy in Mild Cognitive Impairment, or MCI, which is often a precursor state to AD(10), when Omega-3 fatty acid levels are sufficiently high, perhaps by changing homocysteine levels(11; 12). On the other hand, aged adults with mildly elevated plasma homocysteine levels showed less total brain volume after 2 years of daily supplementation with 500 μg of vitamin B12 and 400 μg of folic acid versus placebo tablet(13). This complication may in part be due to Apolipoprotein E4 (APOE4) carrier status, the strongest genetic risk factor for developing AD, which may modify associations between B12 and regional GM(14).
For cognitive function, the literature is also mixed regarding B12 supplementation efficacy or its use as a biomarker to track AD-related cognitive decline. Despite controversy(15) surrounding the meta-analysis by Clarke et al.(16) in selecting rigorous clinical trials and sensitive global cognitive measures in normal aging, meta-analyses indicate that vitamin B12 supplementation may not influence cognitive decline among cognitively normal (CN) aged adults with type 2 diabetes(16), perhaps due to the mild nature of cognitive decline in normal aging and difficulty in controlling for nutritional status(17). Vitamin B12 combined with folate does appears to have modest clinical efficacy in CN or MCI participants, however(18). Qin and colleagues found that individuals in the top quintile for B12 intake showed increased performance in working memory, but no differences in memory or executive function tests(19). Thus, clinical status and perhaps underlying features of AD, such as amyloid-beta (Aβ) and total tau, may modulate B12 supplementation. While B12 in rodent models is protective against Aβ(20) and total tau fibrillar accumulation(21), it is not clear what B12 is tracking in terms of neural or cognitive outcomes when clinically significant levels of these AD hallmarks are already present in the brain.
Thus, wide variability in vitamin B12 associations with GM atrophy or FDG metabolism may be due to vascular factors, clinical status, CSF levels of Aβ and total tau, and/or genetic methylation patterns specific to B12(21; 22; 23). Thus, beyond examining the main effects of B12 on neural outcomes of interest, we examined potential modulators such as: 1) the vascular risk marker homocystine; 2) Established cut-offs of Aβ and total tau accumulation relevant to AD(24); 3) baseline clinical status; and 4) Single Nucleotide Polymorphisms (SNPs) among four a priori selected genes involved in B12 transport, uptake, and metabolism, cubilin (CUBN), methylenetetrahydrofolate reductase (MTHFR), 5-Methyltetrahydrofolate-Homocysteine Methyltransferase Reductase (MTRR) and transcobalamin II (TCN2)(25). To further elucidate conflicting findings, analyses were separately conducted in two large cohorts spanning North America and Australia that had similar MRI and cognitive data but different proportions of adults without impairment vs. MCI or AD.
Materials and Methods
Participants
Data from aged adults were obtained from the ADNI database (http://adni.loni.usc.edu) and AIBL study group(26). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information, see http://www.adni-info.org. Written informed consent was obtained from all ADNI participants at their respective ADNI sites. The ADNI protocol was approved by site-specific institutional review boards. Data was collected in accord with the Helsinki Declaration of 1975. To eliminate the influence of B12 supplementation, especially in participants who were receiving increased care from physicians, analyses only included participants who did not report taking vitamin B12 supplements or multivitamins, excluding 299 ADNI participants and 71 AIBL participants.
Serum Biomarkers
Vitamin B12 data were downloaded at baseline for ADNI participants and obtained through a data request from AIBL. Vitamin B12 was assayed via a Siemens ADVIA Centaur XP autoanalyzer immunoassay by Quest Diagnostics as of April 16th, 2008 for ADNI participants. For the AIBL participants, vitamin B12 was assayed by the Royal Melbourne Pathology in Melbourne and PathWest Laboratory Medicine WA in Perth via ADVIA Centaur Assay - competitive immunoassay. Blood processing took place between 2007 and 2014. Homocysteine in ADNI was obtained through the ADNI Biomarker Core, using a validated enzyme immunoassay methodology(27). Homocysteine in AIBL was obtained through a data request, where all assays were conducted in two laboratories via MMULITE 2000 - competitive immunoassay. ADNI B12 levels are reported in pg/mL and AIBL levels were reported from our data request in pmol/L. All AIBL B12 values were converted to pg/mL for consistency.
APOE Genotype
The ADNI Biomarker Core at the University of Pennsylvania conducted APOE ε4 genotyping. APOE genotypic data was downloaded for AIBL participants. We characterized participants as having zero APOE4 alleles, one APOE4 allele, or two APOE ε4 alleles.
Amyloid and Tau CSF Biomarkers
CSF sample collection, processing, and quality control of p-tau, total tau, and Aβ1–42 are described in the ADNI1 protocol manual (http://adni.loni.usc.edu/) and Shaw et al (24),where CSF Aβ1–42 and total tau cut-offs were <192pg/mL and >93pg/mL respectively. Amyloid and tau markers were only available in a very small subset of AIBL participants, and so these were not assessed.
Neuropsychological Assessment
Cognitive testing was available for both ADNI and AIBL. ADNI utilizes an extensive battery of assessments to examine cognitive functioning with particular emphasis on domains relevant to AD. A full description is available at http://www.adni-info.org/Scientists/CognitiveTesting.aspx. All subjects underwent clinical and neuropsychological assessment at the time of scan acquisition. Neuropsychological assessments included: The Clinical Dementia Rating sum of boxes (CDR-sob), Mini-Mental Status Exam (MMSE), Auditory Verbal Learning Test (RAVLT), and AD Assessment Schedule - Cognition (ADAS-Cog). A composite memory score encompassing the RAVLT, ADAS-COG, MMSE, and Logical Memory assessments was also utilized(28). Additionally, a composite executive function score comprising Category Fluency—animals, Category Fluency—vegetables, Trails A and B, Digit span backwards, WAIS-R Digit Symbol Substitution, Number Cancellation and 5 Clock Drawing items was used(29). These composite scores were used in formal analyses to represent memory and executive function among subjects. Out of the cognitive tests that were available for ADNI, only Logical Memory- Immediate Recall, Logical Memory- Delayed Recall, MMSE, and Global CDR scores were available for AIBL, although the same protocols were used. An executive function composite factor was available for AIBL, although it was comprised of CDR-sob, the Stroop test, the FAS test and Category Switch Total(30).
Magnetic Resonance Imaging (MRI) Acquisition and Pre-Processing
MRI scans were available for both ADNI and AIBL. T1-weighted MRI scans were acquired within 10–14 days of the screening visit following a back-to-back 3D magnetization prepared rapid gradient echo (MP-RAGE) scanning protocol described elsewhere(31). Images were pre-processed using techniques previously described(32). Briefly, the SPM12 “New Segmentation” tool was used to normalize images and extract modulated GM and WM volume maps to Montreal Neurological Institute (MNI) space. Maps were smoothed with an 8mm Gaussian kernel and then used for voxel-wise analyses.
FDG-PET
FDG-PET images were available only for ADNI. FDG-PET acquisition and pre-processing details have been described previously(31). Briefly, 185 MBq of [18–153-F]-FDG was injected intravenously. After 30 minutes, six 5-minute frames were acquired. Frames of each baseline image series were co-registered to the first frame and combined into dynamic image sets. Each set was averaged, reoriented to a standard 160 × 160 × 96 voxel spatial matrix of resliced 1.5 mm3 voxels, normalized for intensity, and smoothed with an 8mm FWHM kernel. In order to derive the standardized uptake value ratio (SUVR), pixel intensity was normalized according to the pons since it demonstrates preserved glucose metabolism in AD(33). Normalization to the pons removed inter-individual tracer metabolism variability. The MNI template space was used to spatially normalize images using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/).)
Genomic Data Processing and Quality Control
Genomic data was only available from ADNI. Quality control of this data was conducted by analyzing Hardy-Weinberg equilibrium (HWE) accepted data for Mendelian inheritance errors. From the entire dataset, SNPs were selected for further analyses based on HWE P-value > 0.00001, MAF >0.05%, call rate 95%. Samples with greater than 5% missingness were removed. Sample genotypes were imputed using 1000Genomes data with Shapeit/Impute2 software following the protocol described previously(34). SNPs with call rates <95% or R2 ≤ 0.3 were withdrawn, leaving 2,976,223 imputed and genotyped SNPs after quality control. Subsequently, we a priori examined SNPs that comprised the CUBN, MTHFR, MTRR, and TCN2 genes.
Statistical Analysis
For voxel-wise analysis, 2nd-level linear mixed models in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) tested main effects of vitamin B12 on regional GM and WM volume as well as FDG, controlling for age, sex, BMI, baseline diagnosis and APOE4 status. Significance thresholds were set at p<.005 (uncorrected) and p<.05 (corrected) for voxels and clusters respectively. Results were considered significant at the cluster level. As described previously(35), in order to reduce type 1 error, we utilized a GM threshold of 0.2 to ensure that voxels with <20% likelihood of being GM were not analyzed. For GM and WM, Monte Carlo simulations in ClusterSim (http://afni.nimh.nih.gov/afni/doc/manual/3dClustSim) were used to estimate that 462 contiguous voxels were needed for such a cluster to occur at p < 0.05. For FDG voxel-wise analyses, Monte Carlo simulations in ClusterSim were used to estimate that 224 contiguous voxels were needed for such a cluster to occur at p < 0.05.
All genetic association analyses were conducted using PLINK v1.9 (www.cog-genomics.org/plink2). The following genes were analyzed through linear associations in White participants of European ancestry with a phenotype of the predicted GM and FDG uptake in maxima from voxel-wise analyses: CUBN, MTHFR, MTRR and TCN2. Covariates for PLINK analyses included sex, clinical diagnosis, intracranial volume, and APOE4 status. A Holm-Bonferroni threshold for significance was set of .05/4, p < 0.0125(36).
Non-voxel linear mixed regression was conducted using SPSS 25 (IBM Corp., Armonk, NY) to test B12 main effects, and interactions with baseline diagnosis and APOE4 status, on cognitive scores and biomarkers. Covariates included age, sex, and BMI. Years of education was also added as a covariate in models with cognitive scores. Binomial and multinomial logistic regression were used to assess the odds ratio of a given participant being diagnosed as MCI or AD versus the CN, or of diagnosis between CN vs. MCI vs. AD.
Results
Data Summary
ADNI and AIBL clinical, demographics, and biomarker data are separately presented in Table 1. A sub-sample of ADNI participants had FDG data, where sub-sample clinical and demographic data are listed in Supplementary Table 1. Three outliers of each cohort were removed for having vitamin B12 levels 3 standard deviations from the mean. Vitamin B12 values ranged from 99–1146 pg/mL for ADNI participants, and for each diagnostic group as follows: CN (157–1146 pg/mL); MCI (157–1084 pg/mL); AD (99–1121 pg/mL). Vitamin B12 values for AIBL participants ranged from 117–1149 pg/mL, and for each diagnostic group as such: CN (121–1149 pg/mL), MCI (176–1139 pg/mL); AD (117–895 pg/mL). The reference range is 200–950 pg/mL(3; 37). In the ADNI sub-sample, 11 participants had values below the reference range and 4 participants had values above the reference range. In the AIBL sub-sample, 16 participants had values below the reference range and 4 participants had values above the reference range.
Table 1.
Demographics for ADNI and AIBL participants with GM images
| ADNI | CN=39 | MCI=73 | AD=31 |
|---|---|---|---|
| Age | 74.00 ± 5.17 | 73.16 ± 7.24 | 73.58 ± 6.19 |
| Serum B12 (pg/mL) | 439.67 ± 213.00 | 382.63 ± 149.22 | 420.51 ± 238.41 |
| Serum Homocysteine (μmol/L) | 10.67 ± 2.98 | 11.59 ± 3.47 | 11.82 ± 4.46 |
| BMI | 26.61 ± 4.04 | 25.42 ± 3.68 | 25.14 ± 3.90 |
| Sex % Female | 51.3% | 41.4% | 61.3% |
| % APOE 4 carriers *** | 25.6% | 61.7% | 74.2% |
| AIBL | CN=311 | MCI=59 | AD=31 |
|
| |||
| Age *** | 71.17 ± 6.49 | 75.37 ± 7.25 | 73.23 ± 8.31 |
| Serum B12 (pg/mL) | 415.55 ± 163.63 | 429.70 ± 156.91 | 405.21 ± 181.82 |
| Serum Homocysteine (μmol/L) | 9.39 ± 3.28 | 10.41 ± 3.71 | 10.21 ± 3.15 |
| BMI | 26.29 ± 3.94 | 26.31 ± 4.54 | 25.86 ± 3.89 |
| Sex % Female ** | 56.6% | 35.6% | 45.2% |
| % APOE 4 carriers *** | 29.9% | 47.5% | 67.7% |
Values are mean ± SD.
*,**,***=p<.05, .01, .001.
Chi-square analyses were conducted to examine differences between gender and APOE4 status. ANOVA was otherwise used.
Baseline Diagnosis: Differences in Vitamin B12 Levels
Binary and multinomial logistic regression indicated that serum vitamin B12 levels did not predict a higher likelihood pf being diagnosed as MCI, AD, or cognitively impaired (MCI + AD) versus the CN reference group.
B12 and Regional Grey Matter Volume
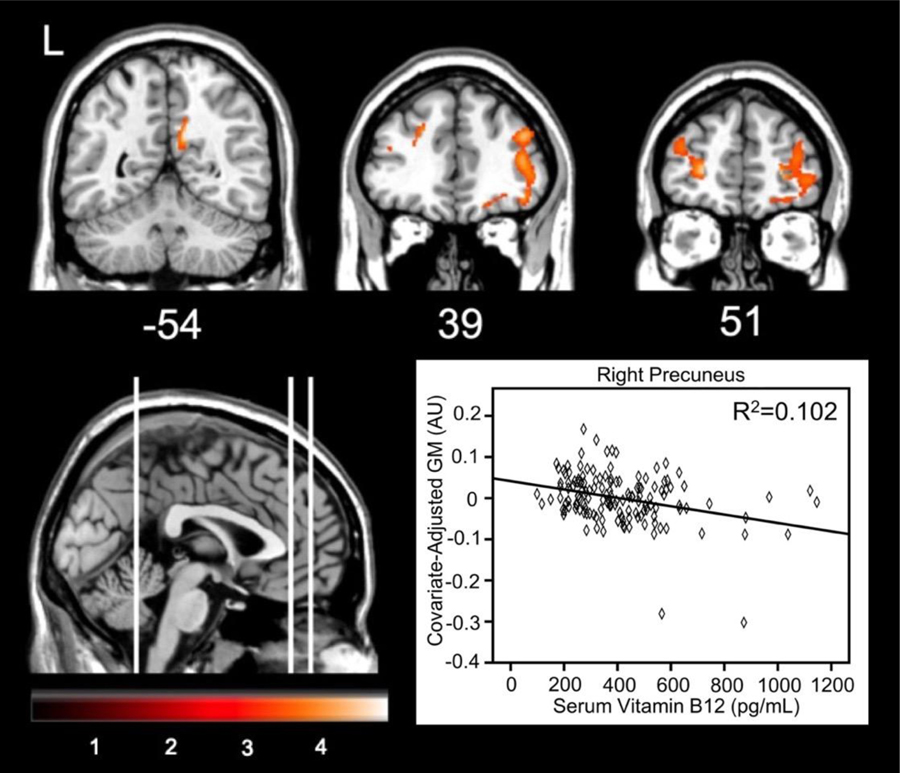
Voxel-wise analyses regressed serum vitamin B12 against regional GM at baseline separately for 144 participants from ADNI and 401 participants from AIBL. For ADNI participants, higher plasma vitamin B12 was correlated with less GM in three clusters (Table 2). The most significant cluster consisted of 507 voxels primarily in the right precuneus and posterior cingulate gyrus (Figure 1). Other clusters spanned the left middle and inferior frontal gyri. When examining each diagnostic group separately, only the MCI participants showed significant associations between B12 and GM. Specifically, higher B12 in MCI was associated with less GM in the right thalamus, precuneus, and calcarine cortex (k=1023).
Table 2.
Regional associations of higher serum vitamin B12 and less grey matter volume in ADNI
| Location | T value | X,Y,Z | Cluster size (voxels) |
|---|---|---|---|
| Precuneus (R) | 4.21 | 10, −54, 27 | 507 |
| Posterior cingulate gyrus (R) | 3.17 | 8, −46, 22 | |
| Precuneus (R) | 2.70 | 14, −62, 33 | |
| Middle frontal gyrus (R) | 3.92 | −24, 51, 6 | 754 |
| Middle frontal gyrus (L) | 3.35 | −24, 24, 39 | |
| Middle frontal gyrus (L) | 3.24 | −38, 48, 20 | |
| Middle frontal gyrus (R) | 3.85 | 26, 51, 3 | 2453 |
| Inferior frontal gyrus (R) | 3.75 | 44, 39, 27 | |
| Inferior frontal gyrus (R) | 3.62 | 51, 32, 8 |
This table depicts regions where all subjects had less gray matter volume per unit increase in vitamin B12. The highest t value for a given cluster of significant, contiguous voxels is shown. For clusters that extended over more than 15 mm, the highest t value in those areas is indicated. Coordinates are in MNI atlas space. Brains are oriented in neurological space. L and R reflect left or right hemisphere.
Figure 1.
Brain areas showing less GM corresponding to increased vitamin B12 in ADNI participants. The graph depicts the relationship at the maximum voxel in the right precuneus.
For AIBL participants, higher serum vitamin B12 was correlated with more grey matter (k=559) in the right amygdala and superior temporal pole. See Table 3 for a full listing of significant clusters. When examining each diagnostic group separately, only the cognitively normal participants showed significant associations, with more B12 associated with more grey matter in three significant clusters: one in the right superior frontal gyrus (k=1186), one in the right precuneus (k=2105), and one in the right supplementary motor area (k=496).
Table 3.
Regional associations of higher serum vitamin B12 and more grey matter volume in AIBL
| Location | T value | X,Y,Z | Cluster size (voxels) |
|---|---|---|---|
| Amygdala (R) | 3.57 | 30, 4, −24 | 559 |
| Superior temporal pole (R) | 3.13 | 45, 14, −18 |
This table depicts regions where all subjects had more predicted gray matter volume per unit increase in vitamin B12. The highest t value for a given cluster of significant, contiguous voxels is shown. For clusters that extended over more than 15 mm, the highest t value in those areas is indicated.
Coordinates are in MNI atlas space. Brains are oriented in neurological space. L and R reflect left or right hemisphere.
In ADNI where CSF was largely available, we then tested interactions with vitamin B12 and binary cut-offs for CSF AD biomarkers(38), For CSF Aβ1–42 , there was a positive interaction ((p<.05, FWE), such that higher B12 was related to more GM volume in adults with vs. without high amyloid accumulation. One cluster was present in the right middle frontal gyrus (k=522), one in the right supramarginal gyrus (k=739) and left superior frontal gyrus (k=464). This interaction was not significantly associated with less GM. For CSF total tau, there was a negative interaction (p<.05, FWE), such that B12 was related to less GM in aged adults with high tau accumulation. One cluster spanned the left superior frontal gyrus (k=604). This interaction was not significantly associated with more GM.
B12 and Regional White Matter Volume
Voxel-wise analysis was used to regress plasma vitamin B12 against regional WM at baseline for ADNI and AIBL participants, separately. In ADNI, higher vitamin B12 was associated with more WM in two small clusters spanning the cerebellum. In AIBL participants, increased vitamin B12 was associated with more WM in one small cluster spanning the left insula. For CSF Aβ1–42 , there was a negative interaction (p<.05, FWE), such that B12 was related to less WM in individuals with high amyloid accumulation. The significant cluster spanned the cerebellum (k=735). The interaction was not significantly associated with more WM. For CSF total tau, there was a negative interaction (p<.05, FEW), such that B12 was related to less WM in individuals with high tau accumulation. The significant cluster spanned the left cingulum (k=2024). The interaction was not significantly associated with more WM.
B12 and Regional FDG Metabolism
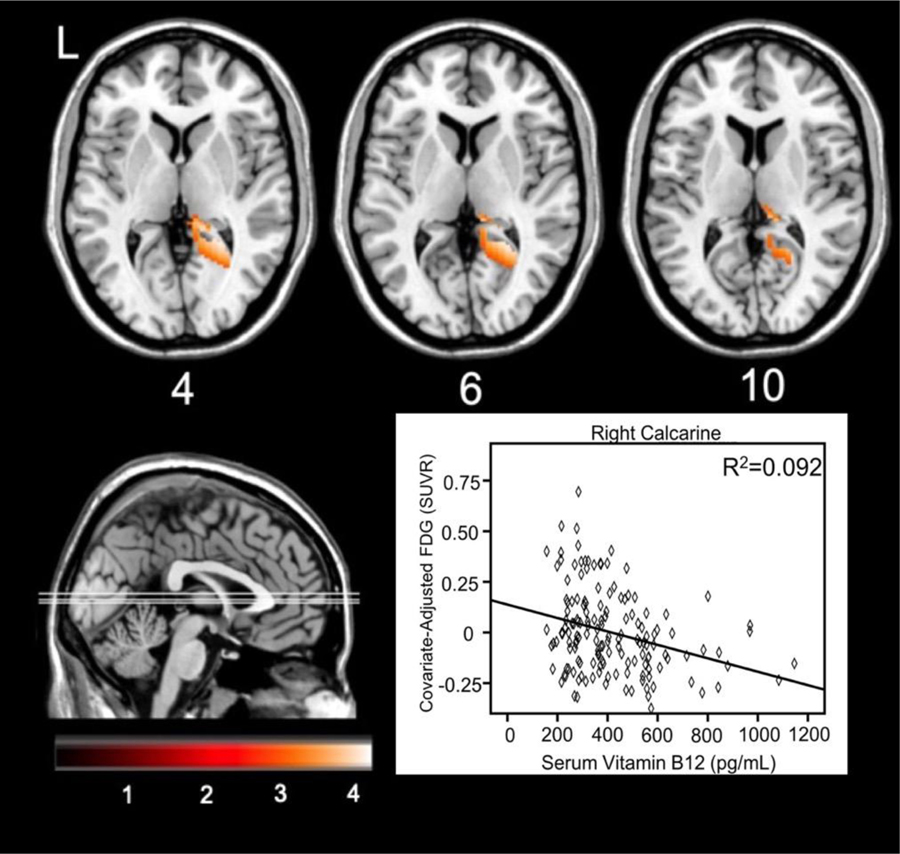
Voxel-wise analyses regressed plasma B12 concentrations against FDG uptake in 151 ADNI participants. Higher plasma vitamin B12 was correlated with less glucose metabolism in the right calcarine and precuneus, where Figure 2 illustrates the relationship at the maximum voxel in the right calcarine (24, −48, 6). See Table 4 for a full listing of significant clusters. When conducting the analyses stratified by baseline diagnosis group, no clusters survived correction for CN and AD participants. For MCI participants, higher vitamin B12 was associated with less FDG uptake in one large cluster (k=2166) spanning the right lingual gyrus, precuneus, and posterior thalamus mostly in the pulvinar nucleus.
Figure 2.
Brain areas showing less FDG metabolism corresponding to increased vitamin B12. The graph depicts the relationship at the maximum voxel in right calcarine cortex.
Table 4.
Regional associations of higher serum vitamin B12 and less FDG glucose uptake in ADNI
| Location | T value | X,Y,Z | Cluster size (voxels) |
|---|---|---|---|
| Calcarine (R) | 3.90 | 24, −48, 6 | 2,166 |
| Precuneus (R) | 3.67 | 14, −40, 4 | |
| Thalamus (R) | 3.40 | 10, −32, 10 |
This table depicts regions where all subjects had less predicted FDG glucose uptake per unit increase in vitamin B12. The highest t value for a given cluster of significant, contiguous voxels is shown. For clusters that extended over more than 15 mm, the highest t value in those areas is indicated.
Coordinates are in MNI atlas space. Brains are oriented in neurological space. L and R reflect left or right hemisphere.
Similar to GM, interactions were then tested with vitamin B12 and cut-offs for AD biomarkers. For CSF Aβ1–42, a positive interaction ((p<.05, FWE) indicated that higher B12 was related to more GM in adults with high amyloid accumulation in one cluster spanning mid-cingulate gyrus (k=499). This interaction was not significantly associated with less FDG metabolism. For total tau, by contrast, higher B12 in adults with high tau accumulation was related to less FDG metabolism in the same mid-cingulate cluster (k=244). The interaction was not significantly associated with more FDG metabolism.
Genotype Analyses for Vitamin B12 and Predicted Differences in GM and FDG
Next, in ADNI, SNPs for genes associated with vitamin B12 uptake, transport, and metabolism were used as predictors of interest, to see if genotypes might explain the wide variance seen in B12 associations. Linear regression in PLINK tested the additive genetic model of each SNP for main effect associations with GM and FDG predicted values, from the voxel-wise analyses reported above. Nine SNPs in the CUBN gene were significantly associated with GM and passed Holm-Bonferroni correction, seven of which were detrimental for individuals who had the minor allele and two which were beneficial (Table 5). One SNP, rs7918972 in the CUBN gene, was significantly associated with FDG and passed Holm-Bonferroni correction, which was associated with less FDG uptake (β=−0.094, p=0.0065). MTHFR, MTRR, and TCN2 SNPs were not significantly associated with GM or FDG B12 predicted values.
Table 5.
Association between CUBN SNPs and predicted GM at the maximal voxel in the right precuneus
| Chromosome | SNP | BP | A1 | N | Beta | F Statistic | P value |
|---|---|---|---|---|---|---|---|
| 10 | rs10904831 | 16931344 | T | 111 | −0.08162 | −2.542 | 0.01249 |
| 10 | rs2356587 | 16979380 | C | 111 | 0.02488 | 2.594 | 0.01085 |
| 10 | rs1801234 | 16979661 | C | 111 | 0.02488 | 2.594 | 0.01085 |
| 10 | rs7072262 | 17057766 | T | 109 | −0.02607 | −2.741 | 0.007229 |
| 10 | rs4614335 | 17059826 | A | 108 | −0.0262 | −2.74 | 0.007252 |
| 10 | rs12218279 | 17060676 | A | 109 | −0.02607 | −2.741 | 0.007229 |
| 10 | rs7897550 | 17064992 | A | 111 | −0.02482 | −2.636 | 0.009651 |
| 10 | rs11254331 | 17065357 | A | 110 | −0.02465 | −2.612 | 0.01035 |
| 10 | rs17139621 | 17065761 | C | 110 | −0.02465 | −2.612 | 0.01035 |
Beta values represent the difference in the predicted value of grey matter with an increase from no risk allele to one risk allele or two risk alleles. BP= base pair location of SNP; A1= minor allele.
For vitamin B12 levels and genotype, minor allele counts of the ten significant CUBN SNPs identified in our GM and FDG analyses were not significantly associated with vitamin B12 levels.
B12 and Associations with Cognition and Biofluid Markers
Vitamin B12 was not significantly correlated with CDR Global scores, CDR-SOB 11, MMSE, composite executive factors, or the composite memory factor for ADNI or AIBL participants. There was a significant Baseline Diagnosis * B12 interaction for predicting the RAVLT learning score for ADNI participants (p=0.048). Higher B12 was associated with better scores in AD (β =0.0027±0.001, p=.042) but trending worse scores in MCI (β =−0.0022±0.001, p=.061).
For biomarkers in plasma, homocysteine was first regressed against vitamin B12. The range of homocysteine values for ADNI participants was 3.9–25.9 μmol/L, by diagnosis: CN (6.1–18.1 μmol/L); MCI (3.9–22.0 μmol/L); AD (6.1–25.9 μmol/L). The range of homocysteine for AIBL participants was 2.8–35.0 μmol/L, by diagnosis: CN (3.0–35.0 μmol/L); MCI (5.5–26.4 μmol/L); AD (2.8–18.8 μmol/L). Higher vitamin B12 was associated with lower plasma homocysteine levels in ADNI participants (β±SE=−.0034±0.001, p=0.001) and AIBL participants (β±SE=−0.0021±0.001, p<.001). For biomarkers in CSF, there were no significant associations between vitamin B12 and CSF total tau, p-tau-181 or Aβ1–42 for ADNI participants. Only a very small subset of AIBL participants had available CSF data, so these associations were not assessed.
Discussion
We hypothesized that serum vitamin B12 levels may be a useful biomarker for AD-related brain atrophy, hypometabolism, and cognitive decline. We originally predicted that there would be an inverse correlation between higher serum vitamin B12 levels and lower levels of AD markers, due to previous work from other groups(39; 40). This hypothesis was true for AIBL participants, where higher vitamin B12 was related to more grey matter in the right amygdala and superior temporal pole, which has been shown to progressively decrease in volume across the AD spectrum(41). This leads us to the hypothesis that vitamin B12 is protective against grey matter deterioration in the cognitively unimpaired population, as the majority of AIBL participants were cognitively unimpaired. Conversely, in ADNI participants, higher serum vitamin B12 was instead related to less GM volume and FDG metabolism in precuneus, as well as middle and inferior frontal gyri, where these regions show atrophy, hypometabolism, and less default mode neural network strength in aged adults with MCI or AD(42; 43; 44). These results contrast with our AIBL findings and lead us to our second hypothesis that vitamin B12 correlates with worse neurological outcomes in the cognitively impaired population, as the majority of the ADNI participants in this cohort were cognitively impaired.
In support of our first hypothesis and the positive correlation between vitamin B12 and neurological outcomes among AIBL participants, factor analysis of dietary patterns among a group of cognitively unimpaired adults showed that diets that were especially rich in vitamin D, vitamin B12, and zinc were associated with increased grey matter volume in the temporal and frontal cortices(22). Additionally, Erickson and colleagues conducted a study with 3-day food recalls and cross-sectional MRI scans among 32 cognitively unimpaired older adults(45). The group found that the individuals with higher vitamin B12 intake had increased grey matter in both the left and right superior parietal sulcus. Lastly, in patients with their first lacunar stroke, lower serum vitamin B12 was correlated with more severe Fazekas scale graded periventricular white matter lesions(46).
Supplementation with B12 in individuals with dementia has been inconclusive. For example, there was no significant improvement in memory or cognition in individuals with dementia who had low levels of vitamin B12 and were subsequently supplemented, however the supplemented individuals showed better verbal fluency scores compared to non-supplemented controls (47). However, the VITACOG trial showed that B-vitamin supplementation was related to positive cognitive outcomes in individuals in the top tertile of baseline omega-3 fatty acids, compared to individuals with lower baseline omega-3 fatty acid levels, perhaps due to a synergistic effect on phospholipid production for the brain(48).
For further explanation of our second hypothesis, B12 levels in the context of other disease states and AD may support it being an indicator of pathological load later in a given disease process. For example, plasma vitamin B12 levels were correlated with increased all-cause mortality in women aged 85 or greater(49). In addition, Arendt and colleagues found that in a population of Danish patients who had serum vitamin B12 tested and recorded in their electronic medical record, patients with higher vitamin B12 had a higher overall cancer risk within one year of follow-up(50). The mechanism behind this relationship was not known, however the authors postulated that vitamin B12 may rise along with malignant processes(50). Also, individuals with hyperlipidemia and non insulin-dependent diabetes had significantly higher levels of vitamin B12 compared to healthy controls(51). Additionally, higher serum vitamin B12 may be a sign of its decreased cellular uptake(52). Vascular damage is a common feature of AD(53), and the vascular endothelium via the CD320 receptor may mediate the homeostasis between the serum and tissue homeostasis of vitamin B12(54) . Finally, we found that higher serum B12 was related to better memory performance and more GM in AIBL and seemed to be driven by CN participants, which make up a majority of the AIBL cohort. By contrast, higher B12 was associated with worse memory performance, less GM, and less FDG in MCI participants, which constitute the majority of the ADNI1 cohort. These differing patterns of association may be due to MCI vs. CN participants usually having more tau accumulation(55).
As an alternative or concurrent explanation for our ADNI findings, it has also been shown that vegetarians, though unlikely to be classified as clinically deficient, are more likely to have low-normal levels of vitamin B12(56). This could likely be extended to individuals who consume plants as a larger portion of their meals, compared to meat. Perhaps vitamin B12 status may act as an indicator of the ratio of meat intake to plant intake in the diet, and this may manifest in predicting AD outcomes, which have also been linked to plant-based dietary habits(57). It is still puzzling that we found a negative relationship between vitamin B12 and neural outcomes in light of the inverse correlation between serum vitamin B12 and homocysteine levels. Hooshmand and colleagues found that among 2,570 individuals 60 and older, higher serum methionine to serum total homocysteine ratios were associated with lower risk for dementia and AD, and higher serum vitamin B12 was positively correlated with methionine to homocysteine ratios. The vast majority of patients in this study were free of dementia or AD(58). Potentially in our ADNI cohort, vitamin B12 still is taken up by the liver in individuals with higher serum vitamin B12 to reduce homocysteine production from methionine, but their high B12 levels may be indicative of other tissues, such as the brain, being unable to transport B12 or utilize it properly.
Alternatively, genetic polymorphisms in B vitamin uptake, transport, and metabolism may modify how B12 is utilized and affect B12 levels themselves. We found that minor allele polymorphisms in the CUBN gene tracked the association between high B12 and less regional GM. Besides its role in receptor-mediated uptake of vitamin B12 in the ileum, cubilin is also an apolipoprotein receptor, and is involved in the absorption of high density lipoproteins in the kidney(59). Perhaps cubulin polymorphisms, some of which we have shown are associated with higher serum vitamin B12 levels, may lead to decreased apolipoprotein reuptake in the kidneys, which may increase dementia risk.
There are several limitations of this secondary data analysis study. First, only vitamin B12 was measured in the participants. Ideally, holotranscobalamin and methylmalonic acid would also have been measured, which would indicate the level of vitamin B12 available for cellular uptake, and the absence of vitamin B12 from necessary methylation reactions, respectively(60). Current practice in the healthcare field is to test serum vitamin B12 levels or holotranscobalamin first (if a patient has risk factors for vitamin B12 deficiency); a serum vitamin B12 level of less than 200 pg/mL is considered deficient(3). Subsequently, current practice is for clinicians to test an additional metabolic indicator, either methyl malonic acid or homocysteine(61). Second, it is difficult to determine the mechanisms involved that affect an individual’s vitamin B12 levels. This can be impacted by proton pump inhibitors(62), level of animal product intake(63), and genetic variability(25) as we have illustrated. Perhaps the interaction between vitamin B12 and one or more of these variables may play a role in cognitive decline. We were also unable to assess associations with physical activity, circulating vitamin B12, and neurological outcomes, as this data was not collected in ADNI or available to us through AIBL. Additionally, FDG scans, genetic, and CSF biomarker data were not available in AIBL, which limited our ability to compare ADNI and AIBL. Lastly, because the overwhelming majority of ADNI participants are of European ancestry, we were only able to reliably test the interactions between genetic data and volumetric/function brain outcomes in those individuals. It would be worthwhile to determine if similar genes are implicated in an African-American cohort, as the incidence of AD is much higher among African-Americans compared to Whites of European descent(64).
Conclusion
This study showed contrasting associations between vitamin B12 and neurological outcomes among an Australian cohort with a strong cognitively unimpaired makeup and a North American cohort made up mostly of participants with some degree of cognitive impairment. While we found that vitamin B12 was associated with positive structural associations in the brain in the Australian cohort which was mostly cognitively unimpaired, we found that vitamin B12 correlated with detrimental outcomes among the North American cohort, which consisted mainly of participants with some degree of cognitive impairment. This lends to our hypothesis that vitamin B12 may be protective of neurological decline in the healthy population, but may increase for an undetermined reason in those that are experiencing cognitive decline. Additionally, we have shown that these results may be influenced by genetic mutations related to vitamin B12 uptake, as well as CSF markers of amyloid and total tau accumulation that may reflect or work in concert with baseline clinical diagnosis. Future research should focus on the rate of uptake of vitamin B12 into healthy and diseased neuronal cells, the role of other B vitamin markers in AD onset and progression, and determine if high amyloid or tau accumulation affects the function or efficacy of vitamin B12.
Supplementary Material
Acknowledgements:
This study was funded by Iowa State University, NIH R00 AG047282, and AARGD-17–529552. Neither funding source had any involvement in the report. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Conflict of Interest/ Disclosure Statement: The authors have no conflict of interest to report.
References
- 1.Smith AD, Refsum H (2016) Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 36, 211–239. [DOI] [PubMed] [Google Scholar]
- 2.Wang B-R, Ou Z, Jiang T et al. (2016) Independent correlation of serum homocysteine with cerebral microbleeds in patients with acute ischemic stroke due to large-artery atherosclerosis. J Stroke Cerebrovasc Dis 25, 2746–2751. [DOI] [PubMed] [Google Scholar]
- 3.Hunt A, Harrington D, Robinson S (2014) Vitamin B12 deficiency. BMJ (Clinical research ed) 349, g5226. [DOI] [PubMed] [Google Scholar]
- 4.Kozyraki R, Cases O (2013) Vitamin B12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 95, 1002–1007. [DOI] [PubMed] [Google Scholar]
- 5.Alpers DH (2016) Absorption and blood/cellular transport of folate and cobalamin: pharmacokinetic and physiological considerations. Biochimie 126, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams K, Schalinske K (2010) Homocysteine metabolism and its relation to health and disease. [DOI] [PubMed]
- 7.Lopes da Silva S, Vellas B, Elemans S et al. (2014) Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement 10, 485–502. [DOI] [PubMed] [Google Scholar]
- 8.de Wilde MC, Vellas B, Girault E et al. (2017) Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimer’s & dementia (New York, N Y) 3, 416–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Y, Wang D, Wang K et al. (2017) High serum folate is associated with brain atrophy in older diabetic people with vitamin B12 deficiency. The journal of nutrition, health & aging 21, 1065–1071. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Doody R, Kurz A et al. (2001) Current concepts in mild cognitive impairment. JAMA Neurology 58, 1985–1992. [DOI] [PubMed] [Google Scholar]
- 11.Smith AD, Smith SM, de Jager CA et al. (2010) Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PloS one 5, e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douaud G, Refsum H, de Jager CA et al. (2013) Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 110, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Zwaluw NL, Brouwer-Brolsma EM, van de Rest O et al. (2017) Folate and vitamin B(12)-related biomarkers in relation to brain volumes. Nutrients 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YM, Ha JK, Park JM et al. (2016) Apolipoprotein E genotype modulates effects of vitamin B12 and homocysteine on grey matter volume in Alzheimer’s disease. Psychogeriatrics 16, 3–11. [DOI] [PubMed] [Google Scholar]
- 15.Garrard P, Jacoby R (2015) B-vitamin trials meta-analysis: less than meets the eye. Am J Clin Nutr 101, 414–415. [DOI] [PubMed] [Google Scholar]
- 16.Clarke R, Bennett D, Parish S et al. (2014) Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr 100, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC (2011) A potential design flaw of randomized trials of vitamin supplements. JAMA 305, 1348–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler M, Nelson VA, Davila H et al. (2018) Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med 168, 52–62. [DOI] [PubMed] [Google Scholar]
- 19.Qin B, Xun P, Jacobs DR Jr. et al. (2017) Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 106, 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam P, Siddiqi MK, Chaturvedi SK et al. (2017) Vitamin B12 offers neuronal cell protection by inhibiting Aβ−42 amyloid fibrillation. International journal of biological macromolecules 99, 477–482. [DOI] [PubMed] [Google Scholar]
- 21.Rafiee S, Asadollahi K, Riazi G et al. (2017) Vitamin B12 inhibits tau fibrillization via binding to cysteine residues of tau. ACS chemical neuroscience 8, 2676–2682. [DOI] [PubMed] [Google Scholar]
- 22.Berti V, Murray J, Davies M et al. (2015) Nutrient patterns and brain biomarkers of Alzheimer’s disease in cognitively normal individuals. J Nutr Health Aging 19, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosconi L, Murray J, Davies M et al. (2014) Nutrient intake and brain biomarkers of Alzheimer’s disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open 4, e004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw LM, Vanderstichele H, Knapik-Czajka M et al. (2011) Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol (Berl) 121, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surendran S, Adaikalakoteswari A, Saravanan P et al. (2018) An update on vitamin B12-related gene polymorphisms and B12 status. Genes Nutr 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis KA, Bush AI, Darby D et al. (2009) The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr 21, 672–687. [DOI] [PubMed] [Google Scholar]
- 27.Trojanowski JQ, Vandeerstichele H, Korecka M et al. (2010) Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 6, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crane PK, Carle A, Gibbons LE et al. (2012) Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6, 502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbons LE, Carle AC, Mackin RS et al. (2012) A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnham SC, Raghavan N, Wilson W et al. (2015) Novel statistically-derived composite measures for assessing the efficacy of disease-modifying therapies in prodromal Alzheimer’s disease trials: an AIBL study. J Alzheimers Dis 46, 1079–1089. [DOI] [PubMed] [Google Scholar]
- 31.Jagust WJ, Bandy D, Chen K et al. (2010) The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 6, 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willette AA, Xu G, Johnson SC et al. (2013) Insulin resistance, brain atrophy, and cognitive performance in late middle–aged adults. Diabetes Care 36, 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowling NM, Hermann B, La Rue A et al. (2010) Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology 24, 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Leeuwen EM, Kanterakis A, Deelen P et al. (2015) Population-specific genotype imputations using minimac or IMPUTE2. Nat Protoc 10, 1285–1296. [DOI] [PubMed] [Google Scholar]
- 35.Willette AA, Bendlin BB, Starks EJ et al. (2015) Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol 72, 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holm S (1979) A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6, 65–70. [Google Scholar]
- 37.Andrès E, Serraj K, Zhu J et al. (2013) The pathophysiology of elevated vitamin B12 in clinical practice. QJM: An International Journal of Medicine 106, 505–515. [DOI] [PubMed] [Google Scholar]
- 38.Shaw LM, Vanderstichele H, Knapik-Czajka M et al. (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho HS, Huang LK, Lee YT et al. (2018) Suboptimal baseline serum vitamin B12 is associated with cognitive decline in people with Alzheimer’s disease undergoing cholinesterase inhibitor treatment. [DOI] [PMC free article] [PubMed]
- 40.Hooshmand B, Mangialasche F, Kalpouzos G et al. (2016) Association of vitamin b12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: A longitudinal population-based study. JAMA Psychiatry 73, 606–613. [DOI] [PubMed] [Google Scholar]
- 41.Hanggi J, Streffer J, Jancke L et al. (2011) Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer’s disease. J Alzheimers Dis 26, 719–734. [DOI] [PubMed] [Google Scholar]
- 42.Jones DT, Machulda MM, Vemuri P et al. (2011) Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 77, 1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan X, Shan B, Ma Y et al. (2010) Multi-center study on Alzheimer’s disease using FDG PET: group and individual analyses. Journal of Alzheimer’s disease : JAD 19, 927–935. [DOI] [PubMed] [Google Scholar]
- 44.Ye BS, Seo SW, Kim CH et al. (2014) Hippocampal and cortical atrophy in amyloid-negative mild cognitive impairments: comparison with amyloid-positive mild cognitive impairment. Neurobiology of aging 35, 291–300. [DOI] [PubMed] [Google Scholar]
- 45.Erickson KI, Suever BL, Prakash RS et al. (2008) Greater intake of vitamins B6 and B12 spares gray matter in healthy elderly: a voxel-based morphometry study. Brain research 1199, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pieters B, Staals J, Knottnerus I et al. (2009) Periventricular white matter lucencies relate to low vitamin B12 levels in patients with small vessel stroke. Stroke 40, 1623–1626. [DOI] [PubMed] [Google Scholar]
- 47.Eastley R, Wilcock GK, Bucks RS (2000) Vitamin B12 deficiency in dementia and cognitive impairment: the effects of treatment on neuropsychological function. Int J Geriatr Psychiatry 15, 226–233. [DOI] [PubMed] [Google Scholar]
- 48.Oulhaj A, Jernerén F, Refsum H et al. (2016) Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J Alzheimers Dis 50, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mendonça N, Jagger C, Granic A et al. (2018) Elevated total homocysteine in all participants and plasma vitamin B12 concentrations in women are associated with all-cause and cardiovascular mortality in the very old: the newcastle 85+ study. J Gerontol 73, 1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arendt JFB, Pedersen L, Nexo E et al. (2013) Elevated plasma vitamin B12 levels as a marker for cancer: a population-based cohort study. Journal of the National Cancer Institute 105, 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasilewska A, Narkiewicz M, Rutkowski B et al. (2003) Is there any relationship between lipids and vitamin B levels in persons with elevated risk of atherosclerosis? Medical science monitor : international medical journal of experimental and clinical research 9, Cr147–151. [PubMed] [Google Scholar]
- 52.Pennypacker LC, Allen RH, Kelly JP et al. (1992) High prevalence of cobalamin deficiency in elderly outpatients. Journal of the American Geriatrics Society 40, 1197–1204. [PubMed] [Google Scholar]
- 53.Kirschen GW, Kéry R, Ge S (2018) The hippocampal neuro-glio-vascular network: metabolic vulnerability and potential neurogenic regeneration in disease. Brain Plasticity 3, 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannibal L, Bolisetty K, Axhemi A et al. (2018) Transcellular transport of cobalamin in aortic endothelial cells. FASEB J 32, fj.201701141RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jack CR Jr., Knopman DS, Jagust WJ et al. (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. The Lancet Neurology 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilsing AMJ, Crowe FL, Lloyd-Wright Z et al. (2010) Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr 64, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pistollato F, Iglesias RC, Ruiz R et al. (2018) Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol Res 131, 32–43. [DOI] [PubMed] [Google Scholar]
- 58.Hooshmand B, Refsum H, Smith AD et al. (2019) Association of methionine to homocysteine status with brain magnetic resonance imaging measures and risk of dementia. JAMA Psychiatry. [DOI] [PMC free article] [PubMed]
- 59.Hammad SM, Stefansson S, Twal WO et al. (1999) Cubilin, the endocytic receptor for intrinsic factor-vitamin B(12) complex, mediates high-density lipoprotein holoparticle endocytosis. Proc Natl Acad Sci U S A 96, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harrington DJ (2017) Laboratory assessment of vitamin B12 status. J Clin Pathol 70, 168–173. [DOI] [PubMed] [Google Scholar]
- 61.Yetley EA, Pfeiffer CM, Phinney KW et al. (2011) Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 94, 313s–321s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maes ML, Fixen DR, Linnebur SA (2017) Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf 8, 273–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gille D, Schmid A (2015) Vitamin B12 in meat and dairy products. Nutr Rev 73, 106–115. [DOI] [PubMed] [Google Scholar]
- 64.Weuve J, Barnes LL, Mendes de Leon CF et al. (2018) Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 29, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.