Summary
Background
Papulopustular rosacea is characterized by chronic facial erythema and inflammatory facial lesions. Minocycline has anti‐inflammatory properties which may be effective in the treatment of rosacea inflammatory lesions.
Objectives
To assess the safety and efficacy of once‐daily topical minocycline gel 1% and 3% in patients with papulopustular rosacea.
Methods
This was a prospective, 12‐week, double‐blinded study conducted at 26 sites in the United States; 270 patients with papulopustular rosacea and 12–40 inflammatory lesions were randomized to minocycline 1%, minocycline 3% or vehicle. The primary endpoint was the mean change in inflammatory lesions at week 12. Key secondary endpoints included success on an Investigator's Global Assessment (IGA).
Results
Baseline mean lesion counts were 24·6, 25·1 and 24·3 in the minocycline 1%, minocycline 3% and vehicle groups, respectively; at week 12, the counts had decreased by 12·6, 13·1 and 7·9, respectively. Minocycline significantly decreased lesions, compared with the vehicle [P = 0·01, 95% confidence interval (CI) 7·9 to 0·9, for minocycline 1%; P = 0·007, 95% CI 8·3 to 1·3, for minocycline 3%]. The proportion of patients achieving IGA success was 39% in the minocycline 1% arm [P = 0·34, odds ratio (OR) 1·396 and OR 95% CI 0·71 to 2·75 vs. vehicle], 46% in the minocycline 3% arm (P = 0·04, OR 2·03 and OR 95% CI 1·04 to 3·95 vs. vehicle) and 31% in the vehicle arm.
Conclusions
Minocycline topical gel appears to be safe and tolerable at concentrations of 1% and 3%, and both concentrations significantly decreased inflammatory lesion counts, with a significantly larger proportion of patients achieving IGA success at week 12 in the minocycline 3% arm. These findings support further evaluation of minocycline gel for treating inflammatory lesions associated with papulopustular rosacea.
Linked Comment: Hampton. Br J Dermatol 2020; 183:412–413.
Short abstract
What is already known about this topic?
Papulopustular rosacea is characterized by inflammatory facial lesions and chronic erythema of the face.
Oral minocycline has been reported to have efficacy in the treatment of inflammatory lesions of papulopustular rosacea.
What does this study add?
The study shows that a topical gel preparation of minocycline significantly decreased the number of inflammatory lesions and significantly improved the Investigator's Global Assessment score in patients with papulopustular rosacea.
This may offer a topical therapeutic alternative to oral doxycycline or oral minocycline for the treatment of inflammatory lesions in papulopustular rosacea, with potentially fewer systemic side‐effects, owing to lower systemic drug exposure.
Linked Comment: Hampton. Br J Dermatol 2020; 183:412–413.
Plain language summary available online
Rosacea is a chronic dermatological disorder that primarily affects convexities of the central face (cheeks, chin, nose and central forehead) and can have a significant impact on a patient's quality of life.1 Papulopustular rosacea is a subtype of rosacea that is characterized by central facial erythema with inflammatory papules and pustules. The pathogenesis of papulopustular rosacea is multifactorial with a significant inflammatory component.2 More recently, demodex mites have been implicated in papulopustular rosacea and may contribute to the inflammation seen in papulopustular rosacea.3
The therapeutic approach for the treatment of inflammatory lesions associated with papulopustular rosacea is dependent on the severity of the disease.4, 5, 6 Topical or oral medications are generally prescribed for mild‐to‐moderate papulopustular rosacea. These topical medications include metronidazole, azelaic acid and ivermectin.7, 8 Oral doxycycline is approved by the US Food and Drug Administration for the treatment of the inflammatory lesions of papulopustular rosacea and is typically used in more severe cases.9 However, while it is effective, a wide variety of systemic side‐effects have been reported with it, potentially limiting its use.10 It has been shown that oral minocycline is not inferior to oral doxycycline in the treatment of papulopustular rosacea, but side‐effects associated with it, some of which may be very serious, may limit its use.11 However, using a topical preparation of minocycline may result in reduced systemic (including central nervous system) exposure to minocycline and fewer serious side‐effects while maintaining high drug concentrations at the site of pathology.
Minocycline is a broad‐spectrum, semisynthetic, second‐generation tetracycline similar to doxycycline and has been reported to have both antibacterial and anti‐inflammatory properties.12 The tetracycline nucleus consists of four linear‐fused tetracycline rings, with a variety of functional groups attached at different positions; minocycline has a dimethylamino group at position 7 and no substituents at position 6.13 Minocycline, like other tetracyclines, is bacteriostatic and inhibits bacterial protein synthesis in a reversible manner by preventing the incorporation of amino acid residues into the peptide chain.14 Minocycline's anti‐inflammatory properties are due to its effects on a wide variety of immune responses. Particularly important in the pathobiology of papulopustular rosacea are: (i) the inhibition of matrix metalloproteinases in the cathelicidin pathway; (ii) the inhibition of immune cell function and infiltration; (iii) the reduction of oxidative stress; and (iv) the inhibition of nitric oxide synthase. These effects could potentially account for the clinical efficacy of tetracycline derivatives in papulopustular rosacea.
The current study details the results of a multicentre, randomized, double‐masked, vehicle‐controlled study of minocycline gel in patients with papulopustular rosacea over a 12‐week treatment period.
Patients and methods
Patients
This study was registered at ClinicalTrials.gov (http://clinicaltrials.gov; NCT03263273). Institutional review board approval was obtained and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation. The study was completed at 26 sites within the United States. Patients were included in the study if they signed informed consent, were over 18 years of age and had been diagnosed with papulopustular rosacea with at least 12 and not more than 40 inflammatory facial lesions at baseline. Inflammatory lesions were defined as papules or pustules ≤ 5 mm in diameter. Nodules were counted as inflammatory lesions, but patients could not have more than five nodules at the screening or baseline visits. The patients had to have at least a grade 3 on a 5‐point Investigator's Global Assessment including erythema (IGAe), along with persistent facial erythema and facial telangiectasia.
Female patients were nonpregnant, and both male and female patients had to be willing to use appropriate contraception throughout the trial. Patients had to be willing to minimize external triggering factors such as spicy food, prolonged sun exposure and alcoholic beverages. Use of oral retinoids or therapeutic vitamin A was prohibited within 6 months of inclusion. Use of systemic or topical corticosteroids, systemic or topical antibiotics, topical retinoids, hormonal therapy, mechanical therapies or other topical therapies for rosacea were prohibited within 2 months of inclusion.
Study treatment
Of the 26 sites in the study, five conducted pharmacokinetic evaluation. Patients at clinical sites not conducting pharmacokinetic evaluations were randomized 1 : 1 : 1 to receive 1% topical minocycline gel, 3% topical minocycline gel or vehicle. At sites conducting pharmacokinetic evaluation, patients were randomized 2 : 2 : 1 to minocycline 1%, minocycline 3% or vehicle. The study product was applied to the face approximately 1 h prior to bedtime. Patients were instructed to apply a fingertip unit to each quadrant of the face and the chin and then, on the following morning, to remove the study product using POND'S® Cold Cream cleanser (Unilever PLC, London, UK). This cleanser was found to be effective at removing any residual yellow coloration caused by minocycline. The patient was then allowed to resume routine skin care, which included the use of a Cetaphil® moisturizer (Galderma, Lausanne, Switzerland), which had sunscreen included if the patient was expected to be outdoors.
Patients were randomized via an interactive web response system that provided a study kit number from a randomization list at the time of inclusion into the study. The randomization list was kept strictly confidential throughout the study and was not available to the sponsor, investigator, clinical site staff, study staff or any other personnel who could have influenced the study data. There were no unblinding events during the study. The study was double‐blinded, with both investigators and patients blinded to treatment assignment.
Assessments were conducted throughout the study period. Patients were asked to return to the clinic at weeks 1, 4, 6, 9, 12 and 16 after starting treatment. The treatment period ended at week 12, with the assessment at week 16 occurring 4 weeks after the cessation of treatment.
Efficacy endpoints
The primary outcome measure was the absolute mean reduction in inflammatory lesion count at week 12 when compared with baseline. Key secondary outcomes were the proportion of patients achieving assessments of ‘clear’ or ‘almost clear’ together with a two‐grade reduction in the Investigator's Global Assessment (IGA) score at week 12, and the proportion of patients achieving ‘clear’ or ‘almost clear’ together with a two‐grade reduction in the IGAe score at week 12 (Table S1; see Supporting Information). Independent assessments of erythema and telangiectasia on 4‐point scales were included. Investigators were trained during a live training session and provided with online resources and example booklets. Key assessments were completed by board‐certified dermatologists, and the investigator assessing the patient at baseline was asked to complete all follow‐up assessments for the patient.
Safety and tolerability endpoints
Standard safety assessments for adverse events were included at each study visit. Vital signs assessments and physical examinations were performed at each visit. Pregnancy tests were conducted on women of childbearing potential at baseline, week 6 and week 12. The investigator was asked to complete a local application site reaction scale to record application site reactions, including erythema, dryness, erosion/oedema and a skin discoloration score (on a scale of 0 to 6). At each visit, patients were asked whether they liked the product.
Pharmacokinetics
A pharmacokinetic assessment of minocycline plasma levels was completed in a subset of patients at the week 12 visit to determine the area under the curve (AUC), the maximum concentration (Cmax) and the time to maximum concentration (Tmax). The patients in the pharmacokinetic arms received one additional, in‐clinic application of the study product at the week 12 visit. Blood draws were conducted prior to in‐clinic dosing and then at 1, 2, 4, 6, 20 and 24 h after in‐clinic dosing. Levels of minocycline were analysed for each sample by Sannova Analytical Inc. (Somerset, NJ, USA).
Statistical assessments
All patients in the overall population were included in the analyses. Missing data were accounted for using the last observation carried forward. Multiple imputations were used as a sensitivity analysis and confirmed results obtained with last observation carried forward. SAS software (version 9·3 or later; SAS Institute Inc., Cary, NC, USA) was used for all analyses. Sample size was calculated to give 80% power to show a difference between group mean inflammatory lesion counts of 7 lesions, with a standard deviation of 16 lesions, using a two‐sample pooled t‐test of normal mean difference with a two‐sided significance level of 0·05. The primary efficacy endpoint was defined as the change from baseline at week 12 with respect to the lesion count. The statistical analysis was not adjusted for multiplicity.
The absolute change in inflammatory lesion count from baseline at week 12 was analysed using ancova, with factors for treatment and investigational site and baseline lesion count (≥ 25 or < 25) as covariates. Change from the baseline value for each treatment group was estimated using least square means. Estimates were presented with 95% confidence intervals, and tests were two‐sided with a significance level of 0·05. For the dichotomized IGA and IGAe, score success was defined as an improvement in score to ‘clear’ (0) or ‘almost clear’ (1) and at least a two‐grade improvement from the baseline value. The dichotomized IGA and IGAe scores were analysed using logistic regression, with factors for treatment and investigational site as covariates. The percentage change in the inflammatory (papules and pustules) lesion count from baseline to week 12 was analysed using ancova, with covariates being pooled site and baseline inflammatory lesion counts of ≥ 25 or < 25.
Results
Patient demographics and baseline characteristics
Patient demographics and baseline characteristics are shown in Tables 11 and 22. Of the 270 patients enrolled in the study, 92 patients were randomized to minocycline 1%, 96 patients were randomized to minocycline 3% and 82 patients were randomized to vehicle. The overall population had a mean age of 51·1 years and was 70% female and 30% male; the demographics in the study arms were similar. At baseline, patients in the overall population had mean lesion counts of 0·2 nodules, 21·0 papules and 3·7 pustules; the counts in the study arms were similar. The mean total inflammatory lesion count (papules and pustules) for the overall population was 24·7 lesions, while those for the minocycline 1%, minocycline 3% and vehicle arms were 24·6, 25·1 and 24·3 lesions, respectively. Of the 270 patients enrolled, 219 patients completed the study. The disposition of the patients in the study is shown in Figure 11.
Table 1.
Patient demographics
| Demographic | Minocycline 1% (n = 92) | Minocycline 3% (n = 96) | Vehicle (n = 82) | Overall (n = 270) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 51·5 ± 12·5 | 50·0 ± 13·5 | 52·0 ± 14·3 | 51·1 ± 13·4 |
| Median | 53·0 | 48·5 | 54·0 | 51·0 |
| Range | 20–84 | 19–81 | 22–86 | 19–86 |
| Sex | ||||
| Female, n (%) | 62 (67) | 71 (74) | 56 (68) | 189 (70) |
| Male, n (%) | 30 (33) | 25 (26) | 26 (32) | 81 (30) |
| Race | ||||
| White, n (%) | 88 (96) | 94 (98) | 80 (98) | 262 (97) |
| Black or African American, n (%) | 2 (2) | 0 (0) | 0 (0) | 2 (1) |
| Asian, n (%) | 2 (2) | 1 (1) | 2 (2) | 5 (2) |
| Polynesian, n (%) | 0 (0) | 1 (1) | 0 (0) | 1 (0) |
| Ethnicity | ||||
| Hispanic or Latino, n (%) | 28 (30) | 27 (28) | 19 (23) | 74 (27) |
| Not Hispanic or Latino, n (%) | 64 (70) | 69 (72) | 63 (77) | 196 (73) |
| Fitzpatrick skin type | ||||
| I, n (%) | 13 (14) | 10 (10) | 16 (19) | 39 (14) |
| II, n (%) | 36 (39) | 30 (31) | 21 (26) | 87 (32) |
| III, n (%) | 30 (33) | 45 (47) | 35 (43) | 110 (41) |
| IV, n (%) | 10 (11) | 9 (9) | 10 (12) | 29 (11) |
| V, n (%) | 2 (2) | 2 (2) | 0 (0) | 4 (1) |
| VI, n (%) | 1 (1) | 0 (0) | 0 (0) | 1 (0) |
Table 2.
Baseline characteristics
| Characteristics | Minocycline 1% (n = 92) | Minocycline 3% (n = 96) | Vehicle (n = 82) | Overall (n = 270) |
|---|---|---|---|---|
| Nodules | ||||
| Mean ± SD | 0·2 ± 0·6 | 0·2 ± 0·6 | 0·2 ± 0·5 | 0·2 ± 0·8 |
| Range | 0–4 | 0–3 | 0–3 | 0–8 |
| Papules | ||||
| Mean ± SD | 20·8 ± 7·9 | 21·5 ± 7·9 | 20·8 ± 7·9 | 21·0 ± 7·9 |
| Range | 0–39 | 6–40 | 9–40 | 0–40 |
| Pustules | ||||
| Mean ± SD | 3·8 ± 6·2 | 3·7 ± 5·1 | 3·5 ± 4·1 | 3·7 ± 5·2 |
| Range | 0–38 | 0–33 | 0–18 | 0–38 |
| Total inflammatory lesion count (papules and pustules) | ||||
| Mean ± SD | 24·6 ± 7·4 | 25·1 ± 7·9 | 24·3 ± 8·2 | 24·7 ± 7·8 |
| Range | 12–40 | 12–40 | 12–40 | 12–40 |
| Investigator's Global Assessment score | ||||
| 0 (clear), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 (almost clear), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 (mild), n (%) | 7 (8) | 3 (3) | 6 (7) | 16 (6) |
| 3 (moderate), n (%) | 75 (81) | 80 (83) | 69 (84) | 224 (83) |
| 4 (severe), n (%) | 10 (11) | 13 (13) | 7 (9) | 30 (11) |
| Investigator's Global Assessment score including erythema | ||||
| 0 (clear), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 (almost clear), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 (mild), n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 (moderate), n (%) | 85 (92) | 86 (90) | 74 (90) | 245 (91) |
| 4 (severe), n (%) | 7 (8) | 10 (10) | 8 (10) | 25 (9) |
Figure 1.
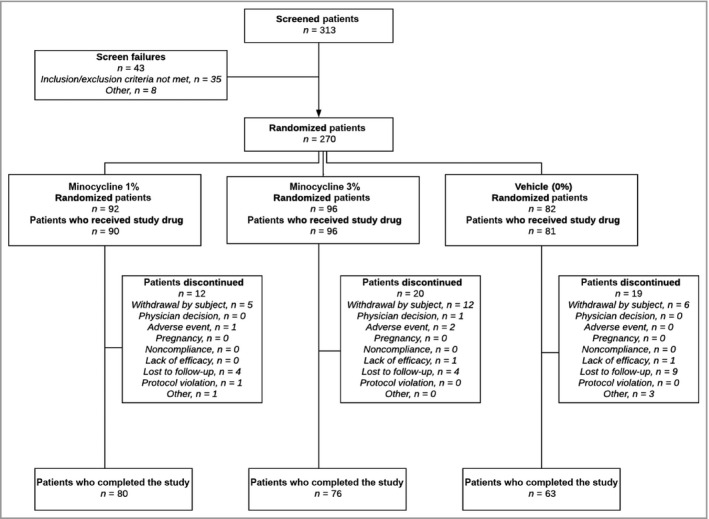
Disposition of patients. All statistical analyses were completed on the overall population, with last observation carried forward for missing data.
Inflammatory lesions
The primary outcome measure of the study was the amount of decrease in the absolute inflammatory lesion count at week 12, compared with that at baseline. In the minocycline 1% and minocycline 3% arms, the mean decrease was 12·6 lesions and 13·1 lesions, respectively. The vehicle arm had a mean decrease of 7·9 lesions. The decreases in the mean number of lesions in the minocycline 1% and minocycline 3% arms were significantly greater than that in the vehicle arm [P = 0·015, 95% confidence interval (CI) 7·9–0·9, and P = 0·0073, 95% CI 8·3–1·3, respectively].
Table 33 and Figure 22 show the decreases in lesion count for each arm at each timepoint. The minocycline 3% arm achieved a significantly greater reduction in lesions than the vehicle arm did at week 4, and this result persisted throughout the remainder of the study. Minocycline 1% achieved significance at week 9 and maintained significance, compared with vehicle, throughout the treatment period. At 4 weeks following the cessation of treatment, a significant reduction in the inflammatory lesion count was maintained in the minocycline 3% arm but not in the minocycline 1% arm, as compared with that in the vehicle arm.
Table 3.
Decrease from baseline in mean inflammatory lesion count
| Week | Minocycline 3%, mean ± SD | Minocycline 1%, mean ± SD | Vehicle, mean ± SD | Minocycline 3%, LSM (95% CI) | Minocycline 1%, LSM (95% CI) | Vehicle, LSM (95% CI) |
|---|---|---|---|---|---|---|
| 1 | 6·6 ± 8·2 | 5·6 ± 8·3 | 5·1 ± 7·5 | 6·6 (8·2–5·0) | 5·7 (7·4, 4·1) | 5·0 (6·8–3·2) |
| 4 | 10·5 ± 9·5 | 8·4 ± 8·8 | 6·9 ± 7·1 | 10·5 (12·2–8·7) | 8·5 (10·2–6·7) | 7·0 (8·9–5·1) |
| 6 | 12·3 ± 9·12 | 9·7 ± 10·0 | 7·8 ± 8·6 | 12·3 (14·1–10·5) | 9·7 (11·6–7·9) | 7·8 (9·8–5·9) |
| 9 | 14·1 ± 10·1 | 11·5 ± 10·8 | 7·2 ± 10·3 | 14·0 (15·9–12·0) | 11·5 (13·5–9·5) | 7·3 (9·5–5·2) |
| 12 | 13·1 ± 13·6 | 12·6 ± 12·4 | 8·9 ± 10·8 | 12·9 (15·3–10·6) | 12·5 (14·9–10·1) | 8·1 (10·7–5·5) |
| 16 | 12·8 ± 12·1 | 11·3 ± 12·6 | 8·0 ± 11·6 | 12·6 (15·0–10·3) | 11·3 (13·6–8·9) | 8·3 (10·8–5·7) |
LSM, least squares mean; CI, confidence interval.
Figure 2.
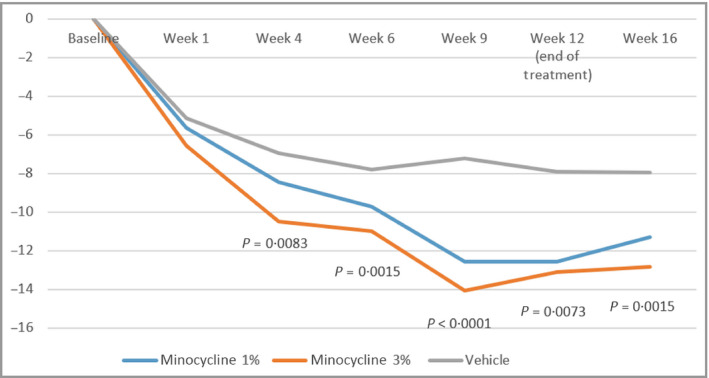
Mean absolute change from baseline in total inflammatory lesion count; P‐values are presented for minocycline 3% vs. vehicle at each timepoint following the week. Analysis performed using ancova, with treatment effect as the factor, and pooled sites and baseline lesion count (<25 or ≥25) as covariates.
Investigator's Global Assessment
Success on the IGA was achieved at week 12 in 39%, 46% and 31% of patients in the minocycline 1%, minocycline 3% and vehicles arms, respectively. Table 44 shows the proportion of patients achieving success on the IGA and the IGAe, for each treatment arm vs. the vehicle arm. Compared with the vehicle arm, the minocycline 3% arm had a significantly greater proportion of patients achieving success on the IGA [P = 0·038, odds ratio (OR) 95% CI 1·04–3·95]. The percentage of patients achieving success in each study arm is shown in Figure 33.
Table 4.
Proportion of patients achieving success on the Investigator's Global Assessmenta
| Study arm | Investigator's Global Assessment | Investigator's Global Assessment including erythema | ||
|---|---|---|---|---|
| Proportion | OR (95% CI)b | Proportion | OR (95% CI)b | |
| Minocycline 3% | 43/93 | 2·03 (1·04–3·95) | 36/93 | 1·66 (0·83–3·32) |
| Minocycline 1% | 35/90 | 1·40 (0·71–2·75) | 32/90 | 1·35 (0·67–2·73) |
| Vehicle | 24/78 | – | 22/78 | – |
OR, odds ratio; CI, confidence interval; asuccess is defined as a two‐grade reduction from baseline and assessments of ‘clear’ or ‘almost clear’ at week 12; bvs. the vehicle arm.
Figure 3.
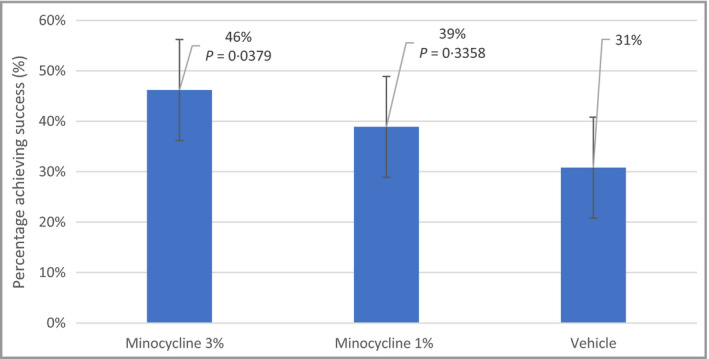
Percentage of patients achieving success (a two‐grade reduction to ‘clear’ or ‘almost clear’) at week 12 on the Investigator's Global Assessment scale. The logistic model included treatment and pooled site as independent variables.
In the minocycline 3% arm, the percentage of patients achieving success was significantly greater than that in the vehicle arm, beginning at day 63 (week 9; P = 0·031, OR 95% CI 1·08–4·54). This significance persisted through the duration of the treatment period and persisted to the last visit, 4 weeks following the cessation of treatment (P = 0·030, OR 95% CI 1·08–4·26). Figure 44 shows a representative patient from the minocycline 3% arm, at baseline and at week 12.
Figure 4.
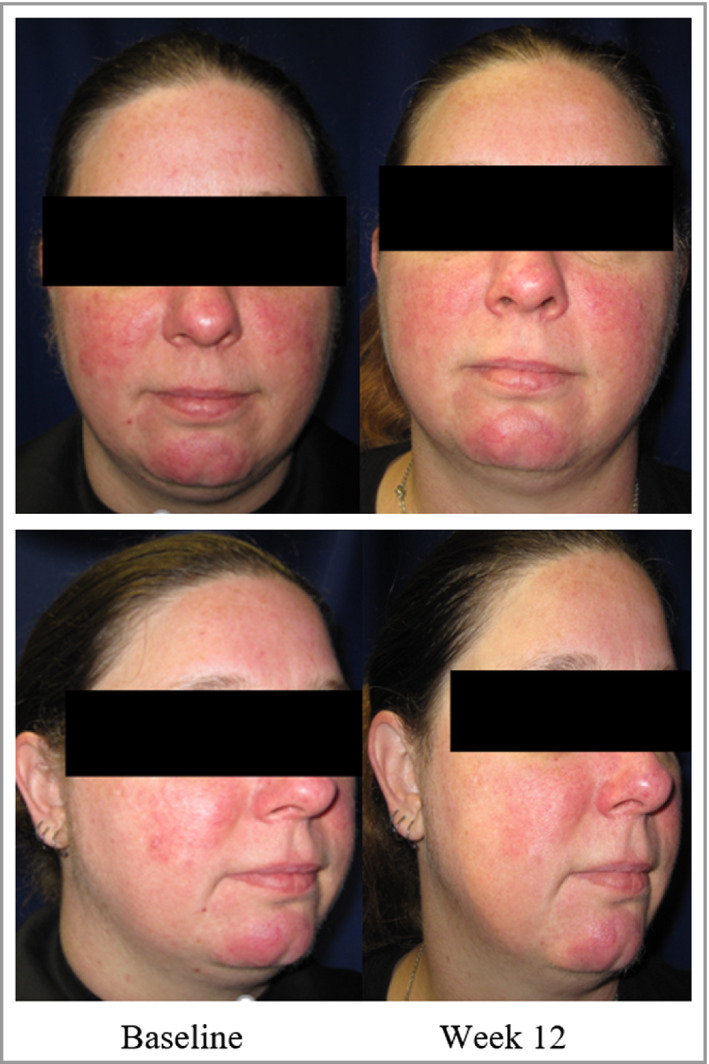
A representative patient from the minocycline 3% arm, at baseline and at week 12; the inflammatory lesion count for the patient decreased from 21 lesions at baseline to one lesion at week 12, and the Investigator's Global Assessment score increased from 3 at baseline to 1 at week 12.
Investigator's Global Assessment including erythema
When using the IGAe, there were no statistically significant differences among the treatment groups with respect to the percentage of patients achieving success at week 12. At week 12, 36%, 39% and 28% of patients achieved success in the minocycline 1%, minocycline 3% and vehicle arms, respectively (P = 0·40, OR 95% CI 0·67–2·73, and P = 0·1521, OR 95% CI 0·83–3·32, for minocycline 1% vs. vehicle and for minocycline 3% vs. vehicle, respectively).
Erythema severity score
Erythema was measured on a 4‐point scale from none to severe at each treatment visit. The decreases in erythema severity at week 12, with respect to baseline values, were 0·4, 0·6 and 0·4 for the minocycline 1%, minocycline 3% and vehicle arms, respectively. The minocycline 3% arm had a significantly greater reduction in the erythema severity score, compared with the vehicle arm (P = 0·039, 95% CI 0·4–0·0).
Patient satisfaction and safety
With respect to patient satisfaction, in response to the question ‘Do you like the product?’, 72%, 70% and 49% of patients responded ‘Yes’ at day 84 in the minocycline 1%, minocycline 3% and vehicle arms, respectively.
With respect to safety, there were three treatment‐related treatment‐emergent adverse events in the minocycline 1% arm, five in the minocycline 3% arm and one in the vehicle arm (Table 55). These adverse events were typically mild and transient.
Table 5.
Profile of treatment‐emergent adverse events related to investigational products
| Event | Minocycline 1%, n (%) | Minocycline 3%, n (%) | Vehicle, n (%) |
|---|---|---|---|
| Patients having at least one event | 3 (3) | 5 (5) | 1 (1) |
| Nausea | 0 (0) | 1 (1) | 0 (0) |
| Application site dermatitis | 1 (1) | 0 (0) | 0 (0) |
| Application site erythema | 0 (0) | 1 (1) | 0 (0) |
| Application site pruritus | 1 (1) | 1 (1) | 0 (0) |
| Hypersensitivity | 0 (0) | 1 (1) | 0 (0) |
| Headache | 0 (0) | 1 (1) | 0 (0) |
| Rosacea | 0 (0) | 1 (1) | 1 (1) |
| Urticaria | 0 (0) | 1 (1) | 0 (0) |
Eighty‐seven per cent of the patients in the minocycline 1% arm, and 79% in the minocycline 3% arm, completed the study, while 76% of patients in the vehicle arm completed the study. This finding appears to be similar to those reported in the published literature for similarly sized studies. Overall, minocycline gel appeared to be well tolerated and safe.
Pharmacokinetic evaluation
At week 12, a subset of the study population, consisting of 30 patients, was selected to participate in the pharmacokinetic evaluation portion of the study. The patients had blood draws at baseline and then on day 84 (after 12 weeks of investigational product use) at 1, 2, 4, 6, 20 and 24 h following the dose. The plasma was analysed for the presence of minocycline at each timepoint on day 84. The lower limit of quantification (LLOQ) was 0·999 ng mL−1.
In the minocycline 1% arm, 13 patients completed the pharmacokinetic evaluation; of these, four patients had plasma concentrations above the LLOQ. These patients had a mean Cmax of 1·411 ± 0·170 ng mL−1 with a mean Tmax of 8·467 h and a mean AUC of 0·023 h ∙ μg mL−1. In the minocycline 3% arm, 11 patients completed the pharmacokinetic evaluation. Of these, three had plasma minocycline levels above the LLOQ. For these patients, the mean Cmax was 1·420 ± 0·178 ng mL−1, the mean Tmax was 6 h, and the mean AUC was 0·021 h ∙ μg mL−1. Plasma minocycline levels were below the LLOQ in six patients receiving vehicle, nine patients receiving minocycline 1%, and eight patients receiving minocycline 3%. Figure 55 shows the mean plasma minocycline concentration for each timepoint for day 84.
Figure 5.
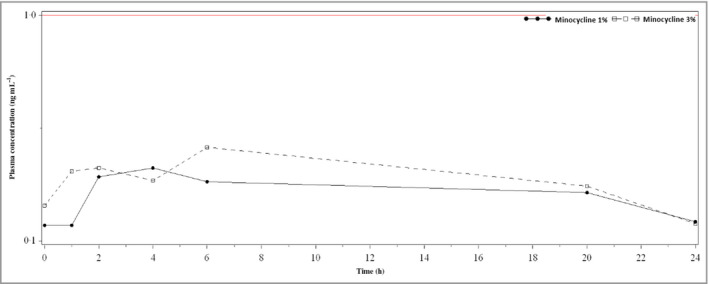
Mean plasma minocycline concentration by timepoint on day 84, linear scale; the lower limit of quantification (0·999 ng mL −1) is shown as a red line in the plot.
Discussion
The results from the current study demonstrate that a topical minocycline gel is generally safe, well tolerated and potentially efficacious in the treatment of papulopustular rosacea. Both minocycline 1% and minocycline 3% significantly decreased the number of inflammatory lesions at week 12, compared with vehicle, in a dose‐dependent manner (P = 0·014 and P = 0·007, respectively). Notably, this decrease had an early onset of action and durability of effect after cessation of treatment, with significance noted as early as week 4 and persisting through the treatment period and maintaining significance for 4 weeks following the cessation of treatment in the 3% treatment arm. In addition to achieving the primary efficacy endpoint, a significantly greater proportion of patients in the minocycline 3% arm achieved an assessment of ‘clear’ or ‘almost clear’ on the IGA (P = 0·038).
While oral therapy with minocycline is usually well tolerated, there may be systemic side‐effects.16 While such side‐effects are typically mild and transient, they may sometimes be serious. Gastrointestinal side‐effects are the most common side‐effects seen with oral minocycline, while more serious adverse events such as idiopathic intracranial hypertension, hypersensitivity reactions, serum sickness, drug‐induced lupus‐like reaction, pigmentary changes, photosensitivity, candidiasis and paediatric tooth discoloration are all reported for oral minocycline. In addition, minocycline is associated with a higher frequency of several severe reactions than doxycycline.17
One proposed advantage of topical minocycline gel over the oral delivery of the medication is the limited systemic absorption associated with topical application. The current study showed that levels of systemic minocycline were very low following the final administration at week 12 in most patients in both treatment arms. Solodyn©, an extended‐release minocycline used for acne rosacea, has a Cmax of 2·63 μg mL−1 and a mean AUC of 33·32 h ∙ μg mL−1.15 In comparison, serum exposure to minocycline in this study was much lower in both treatment arms, with the higher dose arm (minocycline 3%) presenting a Cmax of 0·001 μg mL−1 and an AUC of 0·021 h ∙ μg mL−1.
We propose that this lower systemic exposure may have led to a reduction in the frequency of the known systemic side‐effects of minocycline. In support of this hypothesis, we found that the number of treatment‐related adverse events was low in the current study, with only 3% and 5% of patients reporting an event in the minocycline 1% and minocycline 3% treatment arms, respectively. We thus believe that most of the adverse events encountered with oral minocycline therapy will be avoided via the minimal absorption associated with the topical preparation. However, it is important to note that the dose threshold for hypersensitivity reactions is not known and that the current study is far too small to be predictive for very uncommon events.
Minocycline has traditionally been a challenging molecule to formulate for topical use, given the hydrophobicity of the molecule. Although currently there are no approved topical formulations of minocycline for the treatment of papulopustular rosacea, there is a topical minocycline foam formulation in development. The current study shows that the efficacy and safety of the gel is similar to that reported for the foam.
There are a few differences between the foam and the gel. Firstly, the gel is formulated with the minocycline base, which is less acidic and has a higher partition coefficient than the minocycline hydrochloride used in the foam. Secondly, the hydrocarbon gel (Versagel®; CalumetTM, Indianapolis, IN, USA) used in the gel formulation enables smooth application of the product to the face. Thirdly, the gel may provide a barrier effect for areas of broken or compromised epidermis associated with rosacea, potentially contributing to the good tolerability and likeability of the formulation.
Overall, the current study demonstrates that topical minocycline gel is safe, efficacious and well tolerated in patients with papulopustular rosacea. The findings of this study support further evaluation of topical minocycline gel for the treatment of papulopustular rosacea.
Supporting information
Table S1 Scoring for the Investigator's Global Assessment, and the Investigator's Global Assessment including erythema.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Funding sources This study was sponsored and funded by Hovione Scientia Ltd. The funders had input into the design of the study. Conduct of the study, collection of data and analysis of the data were completed by a third‐party contract research organization, to minimize bias. G.N.M. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest M.S. and G.N.M. are employees of Hovione Scientia Ltd.
Plain language summary available online
References
- 1. Schaller M, Almeida LM, Bewley A et al Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus (ROSCO) 2019 panel. Br J Dermatol 2020; 182:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallo RL, Granstein RD, Kang S et al Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol 2018; 78:148–55. [DOI] [PubMed] [Google Scholar]
- 3. Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta‐analysis. J Am Acad Dermatol 2017; 77:441–7. [DOI] [PubMed] [Google Scholar]
- 4. Schaller M, Almeida LM, Bewley A et al Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol 2017; 176:465–71. [DOI] [PubMed] [Google Scholar]
- 5. Del Rosso JQ, Thiboutot D, Gallo R et al Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis 2014; 93:134–8. [PubMed] [Google Scholar]
- 6. Del Rosso JQ, Thiboutot D, Gallo R et al Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis 2014; 93:18–28. [PubMed] [Google Scholar]
- 7. Powell FC. Rosacea. N Engl J Med 2005; 352:793–803. [DOI] [PubMed] [Google Scholar]
- 8. Del Rosso JQ, Tanghetti E, Webster G et al Update on the management of rosacea from the American Acne & Rosacea Society (AARS). J Clin Aesthet Dermatol 2019; 12:17–24. [PMC free article] [PubMed] [Google Scholar]
- 9. Del Rosso JQ, Webster GF, Jackson M et al Two randomized phase III clinical trials evaluating anti‐inflammatory dose doxycycline (40‐mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol 2007; 56:791–802. [DOI] [PubMed] [Google Scholar]
- 10. Kircik LH. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol 2010; 9:1407–11. [PubMed] [Google Scholar]
- 11. van der Linden MMD, Van Ratingen AR, Van Rappard DC et al DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single‐blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol 2017; 176:1465–74. [DOI] [PubMed] [Google Scholar]
- 12. Garrido‐Mesa N, Zarzuelo A, Galvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol 2013; 169:337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhanel GG, Homenuik K, Nichol K et al The glycylcyclines: a comparative review with the tetracyclines. Drugs 2004; 64:63–88. [DOI] [PubMed] [Google Scholar]
- 14. Ochsendorf F. Minocycline in acne vulgaris: benefits and risks. Am J Clin Dermatol 2010; 11:327–41. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Food and Drug Administration, Center for Drug Evaluation and Research . SOLODYN™ In New Drug Application 50‐808. Washington, DC: Food and Drug Administration, 2006; 4–32. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/050808lbl.pdf (last accessed 21 January 2020). [Google Scholar]
- 16. Garner SE, Eady A, Bennett C et al Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev 2012; 2012:CD002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 1997; 133:1224–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Scoring for the Investigator's Global Assessment, and the Investigator's Global Assessment including erythema.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


