Introduction
Insertable cardiac monitors (ICMs) serve as an essential tool for the diagnosis of arrhythmias that fail to be captured through noninvasive recording methods. Similar to pacemakers and implantable cardioverter-defibrillators (ICDs), ICMs rely on software and algorithms to decipher intracardiac signals to differentiate sinus rhythm from arrhythmias as well as filter out noise and other nonphysiologic signals. Despite their sophistication, ICMs are electronic devices that are prone to sensing abnormalities and must be programmed specifically for each patient. Failure to tailor device settings to each individual patient may allow significant arrhythmias to go undetected. This case report illustrates that sole reliance on the ICM for arrhythmia detection may fail to diagnose certain arrhythmias. Health care professionals proficient in ICM electrogram (EGM) interpretation should review each episode in detail to either confirm or dispute device interpretation results.
Case report
A 52-year-old woman presented with witnessed spontaneous syncope and collapse in October 2019 while she was shopping at the grocery store. The patient had palpitations with lightheadedness just prior to the syncopal event. She was transferred via ambulance to our center for further evaluation.
This was the first syncopal event for the patient. Prior medical history consisted of bileaflet mitral valve prolapse (MVP) with mild mitral regurgitation initially diagnosed in 2017. She had no prior history of ischemic heart disease including any myocardial infarction. She previously wore a 48-hour Holter monitor in early 2019 for palpitations, which revealed ventricular ectopy of 9.3% consisting of 10 episodes of both monomorphic and polymorphic nonsustained ventricular tachycardia (VT) that were premature ventricular contraction (PVC) initiated with the fastest episode consisting of 5 beats at 145 beats per minute (bpm).
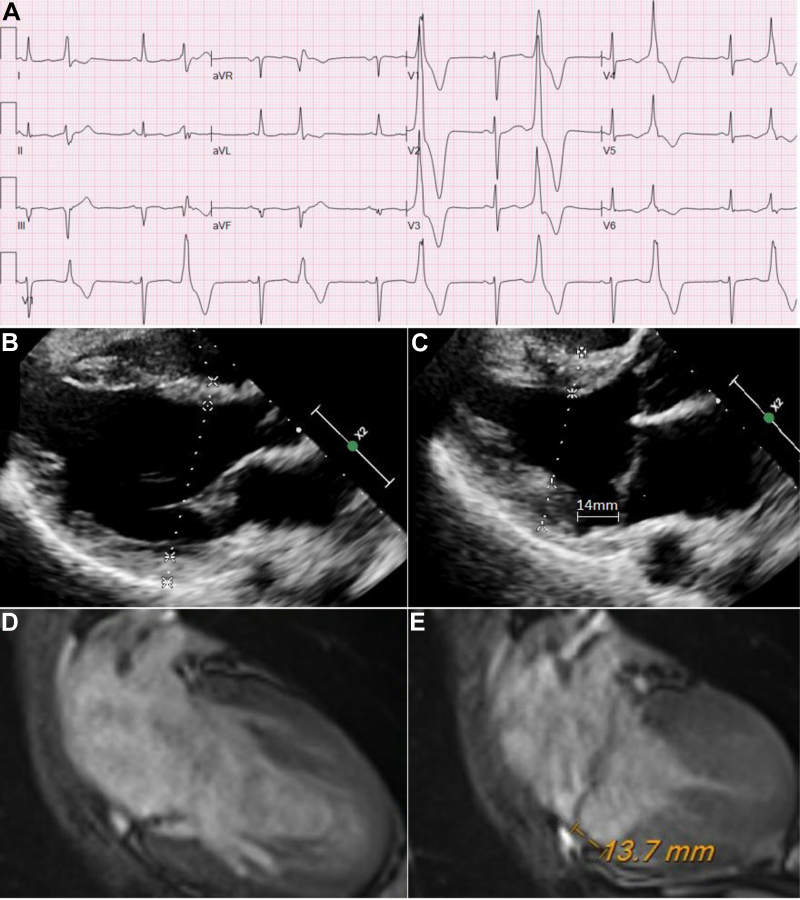
Inpatient telemetry and 12-lead electrocardiogram demonstrated frequent polymorphic PVCs with nonspecific repolarization abnormalities consisting of flattened T waves in the inferior and lateral leads along with QS waves in leads III and aVF. Two predominant PVC morphologies were localized using the 12-lead electrocardiogram to the region of the posteromedial mitral annulus (Figure 1A). Transthoracic echocardiogram demonstrated normal ejection fraction (60%), normal wall motion, and bileaflet mitral valve prolapse with mild mitral regurgitation similar to the prior echocardiogram. After careful review of the echocardiogram images, the patient was noted to have mitral annulus disjunction (MAD) of the posterior wall, most prominently recognized on the parasternal long-axis images (Figure 1B and C and Supplemental Video 1). Further review of the 2017 echocardiogram images also revealed that she had MAD at that time that went unnoticed on official echocardiogram reporting. While inpatient, she underwent cardiac magnetic resonance imaging, which demonstrated no late gadolinium enhancement, ruling out prior myocardial infarction. The magnetic resonance imaging also redemonstrated the MAD observed on the echocardiogram (Figure 1D and E and Supplemental Video 2).
Figure 1.
A: Twelve-lead electrocardiogram taken during the initial hospital admission. Frequent polymorphic premature ventricular contractions in a bigeminal pattern were localized to the posteromedial mitral annulus. B: Parasternal long-axis transthoracic echocardiogram during diastole. C: Parasternal long-axis transthoracic echocardiogram during systole showing mitral annulus disjunction (MAD) of the posterior wall measuring 14 mm. D: Cardiac magnetic resonance imaging during diastole. E: Cardiac magnetic resonance imaging during systole showing MAD of the posterior wall measuring 13.7 mm.
The patient was diagnosed with probable MAD arrhythmic syndrome and an electrophysiology (EP) study was recommended for further risk stratification. The patient was, however, reluctant to proceed with any invasive procedures and rather elected to have an ICM implanted (Reveal LINQ LNQ11; Medtronic, Minneapolis, MN) to monitor for any sustained ventricular arrhythmias. ICM tachycardia detection zone was set to 176 bpm (340 ms). A beta-blocker was recommended on discharge; however, she had previous bad experiences and wanted a drug-free trial period. She was also provided with a CareLink home monitoring system (Medtronic).
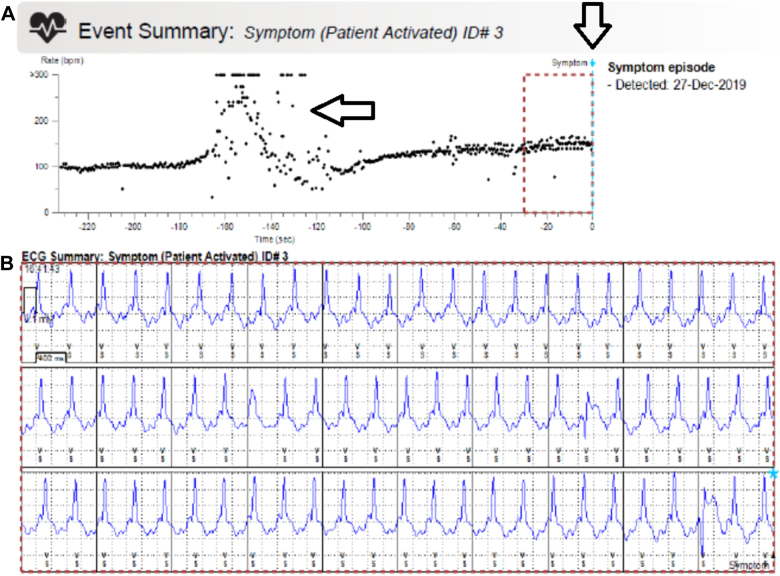
Two months after discharge, she again suffered an episode of witnessed spontaneous syncope and collapse. This episode occurred while shopping with family members out-of-state over the holiday season. Family members were aware of her medical condition and triggered the ICM to record the syncopal episode. She was taken to the nearest hospital for further management, where a CareLink transmission of the syncopal event was obtained from the emergency department. This CareLink event report contained a heart-rate plot diagram of the entire 4-minute event (Figure 2A), as well as an EGM consisting of only the final 30 seconds in which the patient was in a consistent tachycardic rhythm with positive polarity at a rate of 150 bpm (Figure 2B), for which sinus tachycardia was diagnosed by the emergency physician. A complete device interrogation was not performed in the emergency department and she was later discharged the same day.
Figure 2.
CareLink (Medtronic, Minneapolis, MN) event report of the syncopal episode. A: Heart-rate plot diagram with dashed box that annotates the time in which the electrogram (EGM) below corresponds. Vertical arrow corresponds to trigger activation with a retrospective 4-minute timer. Horizontal arrow corresponds to the time in which the patient had fallen to the ground with sternal rubbing and resultant noise, which was not consistent with sinus tachycardia. B: EGM consisting of the final 30 seconds of the event in which the patient was in a consistent tachycardic rhythm with positive polarity, which was interpreted as sinus tachycardia, but in actuality was unstable ventricular tachycardia.
Shortly after the patient returned home from vacation, her bedside CareLink monitor sent an automatic transmission to our clinic of the recent syncopal event (as she had traveled without her bedside monitor). This transmission contained the same information obtained by the emergency department previously (Figure 2). On closer inspection, the heart-rate plot diagram of the event was not entirely consistent with sinus tachycardia. Owing to her high-risk features of recurrent syncope with MAD, bileaflet MVP, and frequent PVCs, an urgent clinic visit was scheduled so that a complete history and thorough ICM interrogation could be performed.
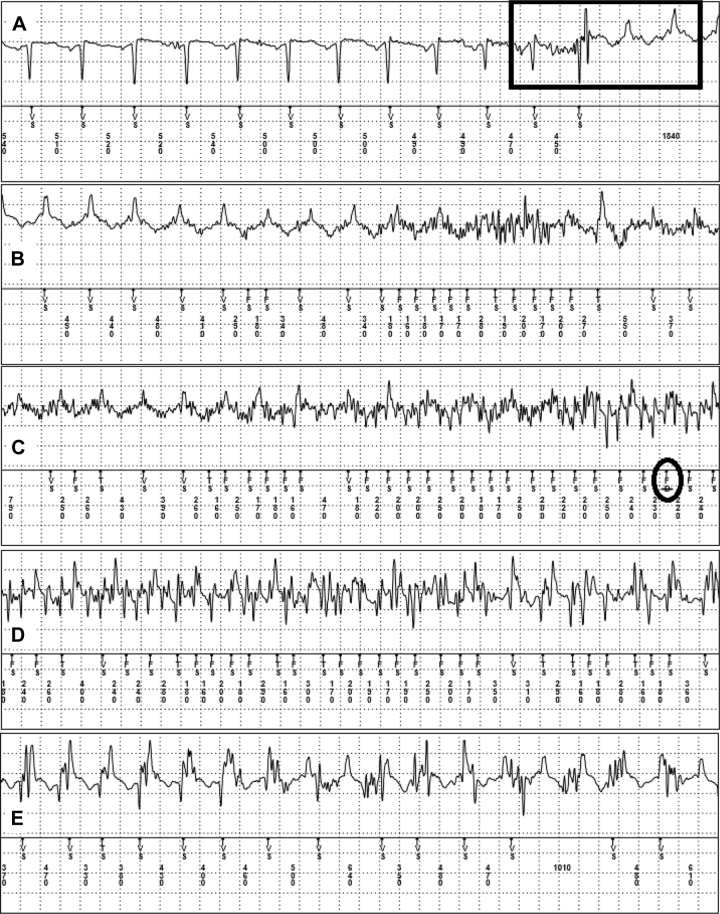
In the clinic, careful review of the entire 4-minute EGM of the syncopal episode revealed an abrupt negative-to-positive shift in QRS morphology consistent with initiation of VT at 130–150 bpm (Figure 3A), coinciding with the time of her syncopal event. This abrupt shift in QRS morphology occurred approximately 1 minute into the EGM and was thus not captured by the original CareLink event report, as that report only contained the final 30 seconds of the entire 4-minute event. Thus, the correct diagnosis of VT was never made in the emergency department owing to the absence of this shift in morphology on the EGM provided at that time.
Figure 3.
Insertable cardiac monitor electrogram of the syncopal episode proceeding from top to bottom panels in real time. A: Sinus tachycardia with negative polarity followed by abrupt shift to ventricular tachycardia (VT) with positive polarity that was initiated by a premature ventricular contraction (black rectangle). B: Continued VT with noise entering midway through the strip. C: Underlying VT with simultaneous noise detection. The black circle denotes device rejection of the ventricular fibrillation rhythm owing to noise. D: Continued underlying VT with simultaneous noise. E: Continued VT with reduced levels of noise.
The initiation of the VT episode was classified as sinus tachycardia by the ICM, as it fell below the VT zone (176 bpm). Shortly after the initiation of VT, significant noise was recorded by the ICM, consistent with collapse of the patient and sternal rubbing by family members (Figure 3). Owing to VT and concomitant noise from sternal rubbing, the ICM recorded oversensing of the true underlying rhythm with rates up to 375 bpm (160 ms). This noisy section of the EGM was classified initially as ventricular fibrillation (VF) [“FS” as noted by the ICM marker channel]; however, this VF rhythm was rejected by the ICM, as it was felt to be more consistent with noise and not that of true VF (Figure 3C).
The abrupt negative-to-positive shift in the EGM along with spontaneous syncope and collapse solidified the diagnosis of MAD arrhythmic syndrome with unstable VT. A secondary prevention dual-chamber ICD was implanted along with initiation of beta-blocker and flecainide therapy for arrhythmia suppression. She has not had further syncopal episodes thus far; however, future EP study and possible VT ablation for further arrhythmia suppression will be pursued should she have VT recurrence.
Discussion
MVP is defined as the superior displacement (≥2 mm) of any part of the mitral leaflet beyond the mitral annulus, according to the American Society of Echocardiography. The clinical profile of a patient with arrhythmic MVP is characterized by female sex, bileaflet myxomatous disease, midsystolic click, repolarization abnormalities in the inferior leads, and complex ventricular arrhythmias with polymorphic/right bundle branch block morphology, with minimal regurgitation.1 Bileaflet MVP, as our patient had, has also been linked to a higher risk for VT/VF and sudden cardiac death than those with single-leaflet MVP.1 The incidence of sudden cardiac death attributable to mitral valve prolapse has been estimated at 2%–4% in the general population.2
MAD is described as the abnormal atrial displacement of the hinge point of the mitral valve away from the ventricular myocardium, and has been closely linked to MVP.3 MVP and MAD are both independent and combined risk factors for unstable ventricular arrhythmias including sudden cardiac death.4 In 1 study, patients with MAD had an overall 22% incidence of ventricular arrhythmias with a 10% incidence of severe arrhythmic events ranging from syncope to sudden cardiac death. Physicians should consider the diagnosis of MAD and/or MVP in patients with no other apparent cause for PVCs and refer these patients for an echocardiogram. Beta-blockers are the preferred first-line medications for the management of symptomatic or asymptomatic nonsustained or sustained ventricular arrhythmias as well as the avoidance of caffeine, alcohol, or other stimulants that can increase catecholamine levels.5 Patients who experience sudden cardiac death should receive ICDs for secondary prevention. There is currently insufficient data on the use of primary-prevention ICDs in patients with MAD and/or MVP with high-risk features, although some institutions use EP studies to risk-stratify patients with inducible VT through the use of extrastimuli.4
Patients with ICMs implanted require regular clinic follow-up to obtain additional history as well as undergo complete interrogation of the ICM to ensure proper device function, including a thorough review of all stored events, especially those triggered by the patient. Regular interrogation of the ICM also allows the opportunity to make proper changes to the settings of the device. In our patient, the previous Holter monitor showed nonsustained VT at rates of 145 bpm. If this information was realized at a follow-up clinic visit, it would have allowed proper lowering of the tachycardia zone to a level that was better personalized for our patient and thus would have detected VT instead of sinus tachycardia. The tachycardia detection zone of the ICM was nominally set to 176 bpm (as this has been the standard at our institution). Given the patient’s normal ejection fraction, we did not initially believe that her syncope was due to a slow VT at approximately 150 bpm; however, a 2005 international multicenter prospective study of slow VT (<150 bpm) revealed that a subset of patients with slow VT do suffer from syncope, palpitations, and congestive heart failure, leading to a high incidence of hospitalization and even death.6 This highlights the need to consider slow VT as a cause of arrhythmogenic syncope.
It is imperative that all patient-triggered events be reviewed in their entirety to allow the identification of any arrhythmia that may correlate with patient symptoms. This case report emphasizes the need for complete and thorough EGM review of a patient-triggered event, as the miniaturized EGM in the initial CareLink report (Figure 2B) did not contain the abrupt negative-to-positive shift in QRS polarity that was necessary for the diagnosis of VT, especially since the rate was below the tachycardia detection zone. This case report also illustrates how appropriate ICM function does not substitute for an experienced individual to review EGMs to determine if any arrhythmia may have been undersensed, occurred below the VT detection zone, or coincided simultaneously with noise artifact.
Our patient experienced an episode of unstable VT with syncope that occurred below the VT detection zone; thus the ICM identified this as sinus tachycardia based on its timing algorithm. The ICM also appropriately rejected a VF diagnosis as it coincided with noise interference, during which time the family was sternal-rubbing the patient. The negative-to-positive shift in the QRS, however, was not identified by the ICM, as it lacks the algorithm contained in present-day ICDs whereby a change in morphology of the QRS is used to determine the likelihood of an arrhythmia. An abrupt change in morphology (as seen in Figure 3A) is a supportive criterion for initiation of an arrhythmia in an ICM. Therefore, the diagnosis of VT in this patient was facilitated by the ICM but was not made solely by the device. This emphasizes that ICMs aid in the diagnosis of arrhythmias but do not serve as a substitute for physician interpretation.
A major limitation of ICMs is their lack of modern-day tachyarrhythmia discriminators to discern a true arrhythmia from that of sinus origin. They rely heavily on physician interpretation for diagnosis of tachyarrhythmias. Using “rate” as a criterion for determination of an arrhythmia is also a major limitation of ICMs because it can lead to the false diagnosis of arrhythmias as noise and sinus tachycardia with rates in the tachycardia detection zone can be labeled as VT.7
Conclusions
Patients with bileaflet MVP and MAD are at elevated risk for sudden cardiac death owing to ventricular arrhythmias. In patients with ICMs and syncope, a careful and detailed history should be obtained, and all EGMs should be reviewed in detail by a specialist to determine if any arrhythmias were present that may have eluded detection by the device. Unstable ventricular arrhythmias in patients with bileaflet MVP and/or MAD should receive ICDs for secondary prevention. Beta-blockers are first-line agents for arrhythmia suppression; however, VT ablation may be needed for continued breakthrough episodes of ventricular arrhythmias.
Key Teaching Points.
-
•
Insertable cardiac monitors aid in the diagnosis of arrhythmias but do not serve as a substitute for physician interpretation.
-
•
Insertable cardiac monitors are prone to sensing abnormalities and complete electrogram review should be performed to determine if patient symptoms correlate with an arrhythmia.
-
•
Patients with bileaflet mitral valve prolapse and mitral annulus disjunction are at elevated risk for unstable ventricular arrhythmias.
Footnotes
The authors have no conflicts of interest to declare.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.06.015.
Appendix. Supplementary data
Echocardiogram displaying MAD at the posterior wall. The arrow points to the area of atrial and ventricular displacement during systole.
MRI Cine of MAD located at the posterior wall. The arrow points to the area of atrial and ventricular displacement during systole.
References
- 1.Basso C., Iliceto S., Thiene G. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 2019;140:952–964. doi: 10.1161/CIRCULATIONAHA.118.034075. [DOI] [PubMed] [Google Scholar]
- 2.Miller M., Dukkipati S., Turagam M. Arrhythmic mitral valve prolapse. J Am Coll Cardiol. 2018;72:2904–2914. doi: 10.1016/j.jacc.2018.09.048. [DOI] [PubMed] [Google Scholar]
- 3.Carmo P., Andrade M.J., Aguiar C., Rodrigues R., Gouveia R., Silva J.A. Mitral annular disjunctions in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53. doi: 10.1186/1476-7120-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejgaard L., Skjolsvik E., Lie O. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72:1600–1609. doi: 10.1016/j.jacc.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2018;72:e91–e220. doi: 10.1016/j.jacc.2017.10.054. Erratum in: J Am Coll Cardiol 2018;72:1760. [DOI] [PubMed] [Google Scholar]
- 6.Sadoul N., Mletzko R., Anselme F. Incidence and clinical relevance of slow ventricular tachycardia in implantable cardioverter-defibrillator recipients: An international multicenter prospective study. Circulation. 2005;112:946–953. doi: 10.1161/CIRCULATIONAHA.105.533513. [DOI] [PubMed] [Google Scholar]
- 7.De Ponti R., My I., Vilotta M. Advanced cardiac signal recording. Card Electrophysiol Clin. 2019;11:203–217. doi: 10.1016/j.ccep.2019.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiogram displaying MAD at the posterior wall. The arrow points to the area of atrial and ventricular displacement during systole.
MRI Cine of MAD located at the posterior wall. The arrow points to the area of atrial and ventricular displacement during systole.