Abstract
Purpose
Urinary iodine concentration (UIC (μg/ml) from spot urine samples collected from school-aged children is used to determine the iodine status of populations. Some studies further extrapolate UIC to represent daily iodine intake, based on the assumption that children pass approximately 1 L urine over 24-h, but this has never been assessed in population studies. Therefore, the present review aimed to collate and produce an estimate of the average 24-h urine volume of children and adolescents (> 1 year and < 19 years) from published studies.
Methods
EBSCOHOST and EMBASE databases were searched to identify studies which reported the mean 24-h urinary volume of healthy children (> 1 year and < 19 years). The overall mean (95% CI) estimate of 24-h urine volume was determined using a random effects model, broken down by age group.
Results
Of the 44 studies identified, a meta-analysis of 27 studies, with at least one criterion for assessing the completeness of urine collections, indicated that the mean urine volume of 2–19 year olds was 773 (654, 893) (95% CI) mL/24-h. When broken down by age group, mean (95% CI) 24-h urine volume was 531 mL/day (454, 607) for 2–5 year olds, 771 mL/day (734, 808) for 6–12 year olds, and 1067 mL/day (855, 1279) for 13–19 year olds.
Conclusions
These results demonstrate that the average urine volume of children aged 2–12 years is less than 1 L, therefore, misclassification of iodine intakes may occur when urine volumes fall below or above 1 L. Future studies utilizing spot urine samples to assess iodine status should consider this when extrapolating UIC to represent iodine intakes of a population.
Electronic supplementary material
The online version of this article (10.1007/s00394-019-02151-w) contains supplementary material, which is available to authorized users.
Keywords: Iodine, Urine, Urine volume, Children, Adolescents, Nutrition, Assessment
Introduction
Monitoring the iodine nutrition of populations and individuals is important to identify those at risk of deficiency, as deficiencies during childhood have been linked with impaired cognitive and motor functions in schoolchildren [1, 2]. Current recommendations for assessing the iodine nutrition of populations using spot urine samples from school-aged children were developed by the World Health Organization (WHO) over a number of years (1986–2007) [3–7]. Whilst the original version of these recommendations was based on the association between a daily estimated iodine intake, extrapolated from creatinine excretion, equivalent to 100 µg of iodine and reduced goiter prevalence among children and adults [8], later iterations extrapolated this value to represent a concentration, urinary iodine concentration (UIC), expressed as µg/L [6]. Whilst these recommendations were originally developed as a marker of iodine status within populations, some studies have utilized UIC from spot urine samples as an indicator of iodine intake [9–14]. In such instances, issues may arise as UIC would only be reflective of daily intake if urine volume was equivalent to 1 L. For example, in a population with a daily urinary volume of 1 L, a UIC of 100 µg/L could be extrapolated to be indicative of a daily iodine intake of 100 µg/24-h. As this value is equal to the daily intake originally associated with reduced goiter prevalence [8], this population can be classified as having a sufficient iodine intake. However, in a population of children with a daily urine volume closer to 0.5 L, a UIC of 100 µg/L may be indicative of a daily iodine intake closer to 50 µg/24-h. This could result in the misclassification of this population as iodine sufficient when their daily iodine intake may, in fact, place them at risk of developing iodine deficiency disorders.
Therefore, iodine monitoring programs which have extrapolated daily iodine intake from UIC determined from spot urine samples in populations of children and adolescents [9–14] and have not taken the lower daily urinary output into account, may be inaccurately estimating iodine intakes. As such, this may have resulted in the misclassification of populations as having sufficient iodine intakes, when their true intakes may be lower than the 100 µg/day originally associated with reduced goiter prevalence. Such misclassifications may have prevented the implementation of necessary iodine fortification programs. To date, there has been no global systematic collation of the average 24-h urine volume of children and adolescents. This information could help researchers estimate population dietary iodine intake of children and adolescents from spot urine samples. Therefore, the aim of the current study was to estimate the average 24-h urine volume measured in healthy children and adolescents, by conducting a systematic review and meta-analysis of studies which have reported the 24-h urine volume of children and adolescents aged 2–19 years.
Methods
This protocol adheres to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement [15] and was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number CRD42016033682).
Information sources and search
A search strategy was developed to identify papers published up to October 2018, which have reported the 24-h urine volume of children and adolescents (> 1 year and ≤ 19 years). An electronic literature search of EBSCOHOST (MEDLINE complete, CINAHL, Academic Search Complete and Global Health) and EMBASE databases was conducted. The search strategy was developed in consultation with a research librarian. Free text keywords were used to conduct the search. Search criteria specific to each database are outlined in Table 1. The search strategy was piloted across each database to improve the effectiveness of the final search. Only peer-reviewed original research articles published in English and conducted in humans were included. It was beyond the scope of this review to include and examine sources from ‘grey’ literature. The reference lists of included studies identified through the search were also reviewed.
Table 1.
Search criteria specifications for each database
| Database | Search options | Search terms |
|---|---|---|
| EBSCOHOST | ||
| Academic search complete |
Limiters—full text; scholarly (peer reviewed) journals; language: English Search modes—boolean/phrase |
(“24 h urin*” OR “twenty four hour urin*” OR “24 h urin*”) AND (sampl* OR collection* OR volume* OR excretion* OR output*) AND (schoolchild* OR child* OR adolescen* OR teen*) |
| CINAHL complete |
Limiters—English language; peer reviewed Search modes—boolean/phrase |
|
| Global health |
Limiters—language: English Search modes—boolean/phrase |
|
| MEDLINE complete |
Limiters—English language; human Search modes—boolean/phrase |
|
| EMBASE |
Advanced search No mapping options used No date limits specified Sources: Embase only (Medline not selected as separate search) Field labels: abstract, article title, index term and subheading Quick limits: human, only in English Publication types: article, article in press EBM, gender, age and animal advanced options left blank |
#1: ‘24 h urin*’ OR ‘twenty four hour urin*’ OR ‘24 h urin*’ OR ‘twenty four h urin*’ #2: sampl* OR collection* OR volume* OR excretion*or AND output* #3: child* OR adolescen* OR teen* #4: #1 AND #2 AND #3 |
Inclusion/exclusion criteria
Only peer-reviewed original research studies were included and any reviews, meta-analyses, editorials, case reports, conference proceedings or other grey literature identified through the search were excluded at the screening stage. As the primary focus of this review was to determine the average daily urinary output of children and adolescents, only studies which reported the 24-h urinary output of healthy children and adolescents > 1 and ≤ 19 years of age were included. Where multiple published reports were available from the same study, the most recently published and/or the study with the largest sample size was included.
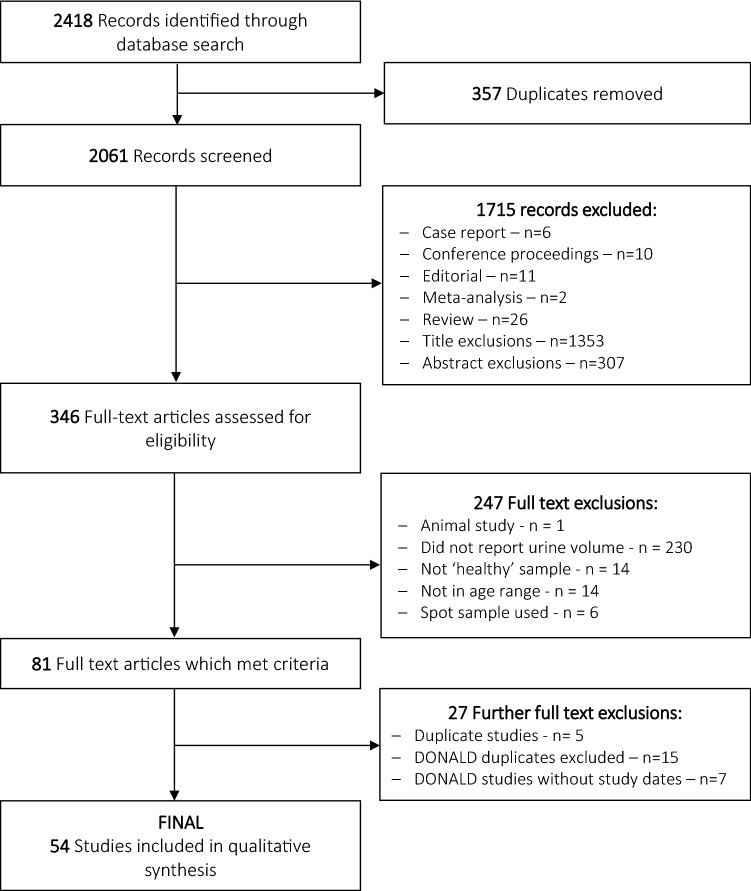
All papers identified from the initial electronic search process were imported into an Endnote library, and duplicates removed. Titles and abstracts were screened and studies included based on the eligibility criteria as outlined above. Two investigators (KB and MW) independently screened the titles and abstracts of the articles independently to assess eligibility for inclusion. If agreement was reached, articles were either excluded or moved to the next stage (full-text). If agreement was not reached, the article was moved to the full-text stage. Following this screening process, the full text of eligible studies was retrieved and studies which collected 24-h urine samples but did not report the final 24-h urine volume were excluded. At this stage, the reference lists of included studies were scanned, and the full text of any relevant studies retrieved and reviewed for inclusion. The PRISMA flow chart [16] was used to document the number of studies identified during the search process and those excluded and included according to the outlined eligibility criteria (Fig. 1).
Fig. 1.
PRISMA flowchart
As multiple studies had utilised data from the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study [17], cross-checking of study dates and participant characteristics was carried out to minimise participant overlap between the studies. Some studies (n = 7) [18–24] were excluded from the final analysis as they did not report which years of data collection had been analysed, therefore, possible participant overlap with other studies could not be determined. No additional information apart from that published was available from authors. Where participant overlap was possible, the study with the larger participant number was included in the final analysis. Of the 27 DONALD studies initially identified, six studies [25–30] were considered for the final analyses as they captured the full range of data collection years and included the largest number of participants whilst minimising possible participant overlap.
Data extraction and synthesis
Data extraction was completed using a data extraction template (Table 2). The template was initially piloted on five eligible studies and modifications made where necessary. As 24-h urinary creatinine excretion either alone, in relation to expected creatinine based on sex and/or weight, is often used as a marker for complete urine collection under the assumption that urinary creatinine excretion, as an indicator of body mass is stable within individuals from day to day [31–33], data pertaining to 24-h excretion of creatinine was also extracted where reported.
Table 2.
Data extracted from included studies
| Study overview |
|
Aim(s) Study design Study year(s) Description of country where the study was conducted Season of the year Setting (i.e., home/school) |
| Methods |
|
Recruitment method Inclusion criteria (main study—not urine specific) Specific exclusion criteria (main study—not urine specific) Data collection overview |
| 24-h urine collection protocol |
|
24-h urine protocol description Criteria for completeness of urine samples - Time of collection (hours) - Volume of collection - Creatinine cut-off - Number of missed collections reported by child/parent Specific exclusion criteria for urine samples Adjustment for collection time/normalised? Other nutrients analysed |
| Participant characteristics |
|
Total no. participating No. exclusions No. withdrawals Final n included in analyses % male participants Total no. urine samples Age, mean and range Weight category/BMI Race/ethnicity SES (include description of SES definition) |
| Results |
|
Urine volume (L/24 h)—mean, SD/SEM, median, range Creatinine—mean, SD/SEM, median, range |
Quality assessment
The quality of the studies included in this review was assessed using a modified version of the Newcastle–Ottawa scale (NOS) for cohort studies [34], as all studies included in the final synthesis were of a cross-sectional study design. The NOS was modified to suit the context of the studies included in the review and particular consideration was made towards the 24-h urine collection methods used in each study. This scale assigns stars to indicate higher quality based on three broad criteria specific to the design of the study: (1) selection (representativeness of the study sample); (2) comparability of the findings (normalisation of the results to a 24-h period); and (3) assessment of outcome (quality of the reported 24-h urine collection methodology) (Online Supporting Material). Studies were categorised as ‘high’ ‘medium’ or ‘low’ quality according to the number of stars they received (out of a maximum of 10 stars: low: 0–3; medium: 4–7; high 8–10). As only three studies provided sufficient detail on their urine collection protocol to be classified as “high” quality [35–37], we included a second category of quality assessment, based on studies which had reported at least one criterion for the assessment of urine collection completeness.
Statistical analysis
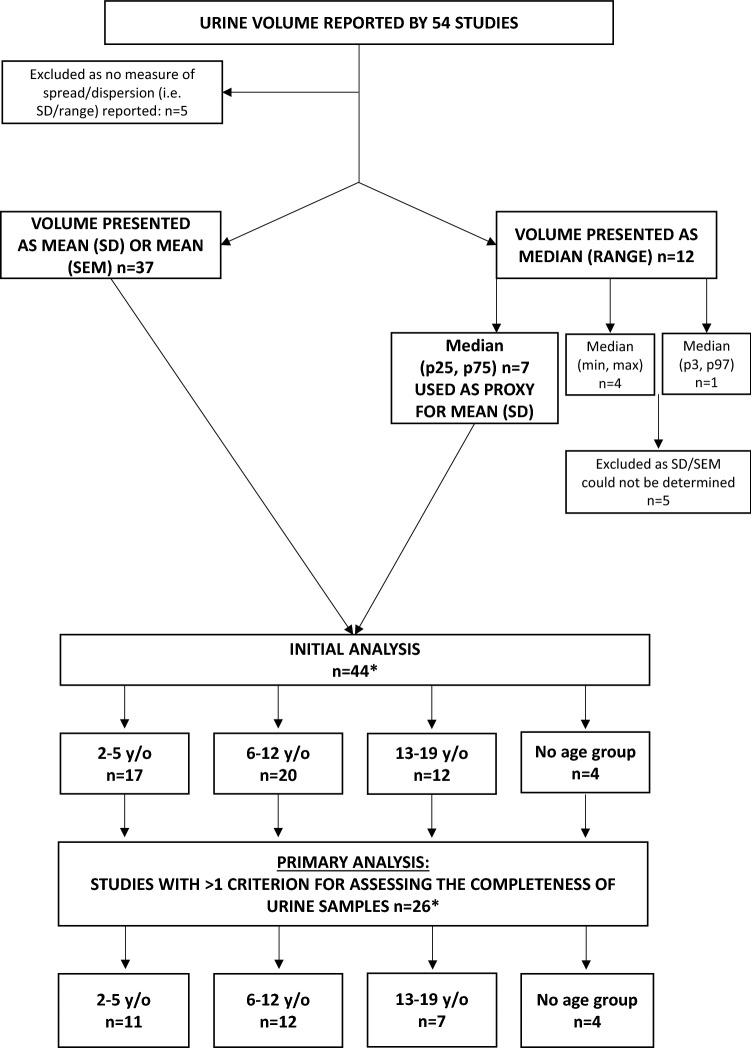
Following data extraction, data was collated and imported into STATA/SE 15.0 (StataCorp LP, College Station, TX, USA) for analysis. The main outcome variable was 24-h urine volume, presented in mL/24-h. Of the 54 studies originally included, five did not include a measure of spread/dispersion [38–42] and were subsequently excluded (Fig. 2). Most studies (n = 37) reported urine volume as mean (SD) or mean (SEM) [35–37, 43–76] (Table 3). Twelve studies reported urine volume as median, this included median (min, max) n = 4 [77–80], median (IQR) n = 7 [25, 26, 28–30, 81, 82], median (P3, P97) n = 1 [83]. For the seven studies which reported median (IQR) (Table 4), the mean (SD) was extrapolated from the median (IQR) using the median as a proxy for the mean and the IQR as a proxy for the SD (i.e., P75–P25 = SD) [84]. The calculated mean (SD) for these studies was then pooled with the results of those studies which reported urine volume as mean (SD). As such, a total of 44 studies were considered for the primary analysis.
Fig. 2.
Flow chart of studies included in analyses
Table 3.
Characteristics of studies considered for primary analysis where 24-h urine volume was presented as mean (SD/SEM), n = 37 studies
| Study | Study Year(s) | n | Male, n (%) |
Valid urine samples | Age range (years) | Age, mean (SD) years | Country | Exclusion criteria for urine samples | NOS score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Score out of 10 | Rating* | |||||||||
| Abuhaloob [43] | Not described | 216 | 104 (48%) | 216 | 3–5 | 4.1 (0.3) | Gaza strip |
(1) Urine flow rate < 140 ml in 24 h and (2) Urinary creatinine concentration < 0.1 or > 1.5 mg/ml |
5 | Medium |
| Acevedo [44] | Not described | 32 | Not described | 32 | 3–5 | 4.1 (0.8) | Caracas, Venezuela | Not described | 2 | Low |
| 31 | 31 | 3–5 | 4.3 (1.3) | San Juan de los Morros, Venezuela | ||||||
| Allevard-Burguburu [45] | Not described | 7 | Not described | 7 | 2–2.9 | Not described | Not described | Not described | 0 | Low |
| 13 | 13 | 3–4.9 | ||||||||
| 16 | 16 | 5–6.9 | ||||||||
| 16 | 16 | 7–8.9 | ||||||||
| 10 | 10 | 9–11 | ||||||||
| Aparicio [46] | 2014 | 205 | 109 (53%) | 205 | 7–11 | 8.8 (1.2) | Spain | Creatinine excretion < 0.1 mmol/kg/day | 0 | Low |
| Ballauff [47] | Not described | 21 | 10 (48%) | 21 | 6–11 | 8.8 (1.4) | Not described | Not described | 4 | Medium |
| Clark [48] | Not described | 204 | 89 (47%) | 190 | 9–10 | 9.6 (0.2) | Salisbury, United Kingdom | “Fourteen urine samples were excluded because of an incorrect collection period (n = 4), a urine volume almost 4 standard deviations below the geometric mean (n = 1), or bacterial or other contamination (n = 9)” | 4 | Medium |
| Fujishima [49] | 1980 | 84 | 84 (100%) | 76 | 15–18 | Not described | Kanagawa, Japan | Not described | 2 | Low |
| Grimes [35] | 2010 –2013 | 168 | 96 (57%) | 168 | 4–7.9 | 6.9 (0.7) | Victoria, Australia |
One or more of the following criteria: (1) Collection time < 20 or > 28 h (2) Total volume < 300 ml (3) Participant reported missing > 1 collection, or (4) Urinary creatinine excretion < 0.1 mmol/kg/day |
10 | High |
| 498 | 269 (54%) | 498 | 8–12 | 10.1 (1.2) | ||||||
| Haftenberger [50] | 1998 | 11 | 6 (55%) | 11 | 3–6 | 4.2 (1.3) | Dresden, Germany | Not described | 0 | Low |
| Haga [51] | 2005 | 29 | 13 (45%) | 29 | 3–4 | Not described | Miyagi, Japan | Not described | 2 | Low |
| 33 | 18 (55%) | 33 | 4.1–5 | |||||||
| 17 | 8 (47%) | 17 | 5.1–6 | |||||||
| He [52] | 2013 | 138 | 67 (49%) | 138 | 9–11 | 10.2 (0.5) | Changzhi, China |
(1) Urine volume < 300 ml/24-h or (2) Creatinine < 5th centile (< 2.5 mmol/24-h for girls and < 2.9 mmol/24-h for boys) |
7 | Medium |
| 141 | 67 (48%) | 141 | 9–12 | 10.0 (0.5) | ||||||
| Hesse [53] | Not described | 25 | 25 (100%) | 25 | 15–19 | Not described | Not described | Not described | 0 | Low |
| 25 | 0 (0%) | 25 | 15–19 | |||||||
| Höllriegl [54] | 2008 | 9 | Not described | 8 | 3–5 | Not described | Akure, Nigeria | Urinary creatinine values outside of the range of 0.2–3.0 g/L | 3 | Low |
| 28 | 24 | 6–10 | ||||||||
| 12 | 5 | 11–15 | ||||||||
| Juarez-Lopez [55] | Not described | 20 | 30 (54%) | 20 | 3–5 | 3.8 (0.9) | State of Mexico | Excretion rate below 9 μg F/h | 3 | Low |
| Ketley [57] | Not described | 13 | Not described | 65 | 5–6 | 5.8 (0.5) | Merseyside, England | Urinary flow rate < 9 mL/h | 3 | Low |
| Ketley [56] | 1994–1996 | 19 | Not described | 19 | 1.5–3.5 | 3.4 (0.2) | Cork, Ireland | Urinary fl̄ow rate of < 9 ml/h or > 420 ml/h | 6 | Medium |
| 18 | 18 | 1.5–3.5 | 3.1 (0.6) | Knowsley, England | ||||||
| 18 | 18 | 1.5–3.5 | 3.1 (0.4) | Oulu, Finland | ||||||
| 4 | 4 | 1.5–3.5 | 3.6 (0.3) | Reykjavik, Iceland | ||||||
| 6 | 6 | 1.5–3.5 | 3.2 (0.5) | Haarlem, the Netherlands | ||||||
| 21 | 21 | 1.5–3.5 | 3.1 (0.4) | Almada/Setubal, Portugal | ||||||
| Khandare [58] | Not described | 18 | 18 (100%) | 18 | 9–11 | 10.7 (1.4) | Andhra Pradesh, India | Not described | 0 | Low |
| Kirdpon [59] | Not described | 13 | 13 (100%) | 13 | 9–13 | 12.4 (0.3) | Not described | Not described | 0 | Low |
| Kristbjornsdottir [60] | Not described | 76 | 30 (52%) | 58 | 5–6 | 6 (0.1) | Reykjavik, Iceland | PABA recovery < 85% | 5 | Medium |
| Maguire [61] | Not described | 12 | Not described | 12 | 6–7 | 6.6 (0.3) | Northern England |
(1) Urinary flow < 5 ml/h for < 6 year olds and < 9 mL/h for children ≥ 6 years (2) Creatinine excretion < 11.3 mg/kg/day or > 21.1 mg/kg/day |
7 | Medium |
| 11 | 11 | 6–7 | 6.6 (0.5) | |||||||
| 13 | 13 | 6–7 | 7.0 (0.7) | |||||||
| Marrero [36] | 2007–2010 | 150 | 61 (52%) | 126 | 5–6 | 5.5 (0.6) | London, England |
(1) The participant admitted to have missed at least one urine collection (2) 24-h urinary creatinine of < 0.1 mmol/kg/d (3) urine output of < 0.5 ml/kg/h for 5–6 and 8–9 year olds or < 500 mL/24-h for 13-17 year olds, and/or (4) If the timing of the collection was < 20 h or > 28 h |
8 | High |
| 167 | 56 (50%) | 111 | 8–9 | 8.5 (0.5) | ||||||
| 125 | 57 (55%) | 103 | 13–17 | 14.6 (1.3) | ||||||
| Martins [62] | 2008 | 11 | 5 (45%) | 11 | 2–4 | 3.7 | Ibiá, Brazil [62] | Not described | 2 | Low |
| Maruhama [63] | Not described | 11 | 6 (55%) | 11 | 6–9.9 | 8 (1.3) | Not described | Not described | 0 | Low |
| 11 | 5 (45%) | 11 | 10–18 | 14 (1.9) | ||||||
| Matkovic [64] | Not described | 381 | 0 (0%) | 359 | 8–13 | 10.9 (7.7) | Ohio, United States | “Inappropriate urine collections identified by residual analysis after fitting a regression equation of 24-h creatinine excretion on LBM as obtained by dual-energy X-ray absorptiometry” | 5 | Medium |
| Melse-Boonstra [76] | 1996 | 9 | 7 (100%) | 7 | 6–9 | 8.1 | Guatemala, Central America | Not described | 2 | Low |
| 13 | 13 (100%) | 13 | 8–12 | 9.7 | Benin, West Africa | |||||
| Mori [65] | 2002 | 56 | 54 (46%) | 54 | 6–11 | 8.1 (1.4) | Japan | Not described | 3 | Low |
| 364 | 0 (0%) | 193 | 13–18 | 16.7 (1.3) | ||||||
| Padrao [66] | 2014 | 86 | 86 (100%) | 86 | 7–11 | 8.7 (0.8) | Portugal | Creatinine excretion < 0.1 mmol/kg/day | 6 | Medium |
| 86 | 0 (0%) | 86 | 7–11 | |||||||
| Rafie [67] | 2015 –2016 | 128 | 42 (33%) | 128 | 11–18 | 14.4 (2.0) | Isafahn, Iran |
(1) Volume < 500 mL, and/or (2) Creatinine excretion < 0.1 mmol/kg/day |
4 | Medium |
| 129 | 57 (44%) | 129 | 11–18 | 14.3 (2.2) | ||||||
| 117 | 55 (47%) | 117 | 11–18 | 14.6 (2.1) | ||||||
| Rugg-Gunn [68] | Not described | 27 | 27 (100%) | 27 | 4–6 | Not described | Dambulla, Sri Lanka | Not described | 4 | Medium |
| 26 | 0 (0%) | 26 | 4–6 | |||||||
| 20 | 20 (100%) | 20 | 4–6 | Newcastle, England | ||||||
| 24 | 0 (0%) | 24 | 4–6 | |||||||
| Saied [37] | 2015–2016 | 131 | 68 (52%) | 131 | 6–18 | 9.8 (2.4) | Rabat, Morocco | Urine collections were considered incomplete and then excluded if (1) the volume is less than 300 ml; (2) more than a few drops of urine were reported lost during urine collection; (3) the collection time was outside of < 20 h or > 28 h; and (4) urinary creatinine was < 280 mg/l or > 2590 mg/l | 9 | High |
| Sinaiko [69] | Not described | 40 | 20 (50%) | 40 | 11–14 | 13.9 (1.3) | Minnesota, United States | Not described | 1 | Low |
| 243 | 122 (50%) | 243 | 11–14 | 13.3 (1.6) | ||||||
| Staessen [70] | 1979–1981 | 82 | 82 (100%) | 82 | 10–19 | 14.3 (3.0) | Belgium | “Urinary creatinine excretion was plotted against body weight after stratification for age and sex. Subjects who fell outside the two standard deviation range of the regression lines were excluded” | 4 | Medium |
| 78 | 0 (0%) | 78 | 10–19 | 14.4 (2.6) | ||||||
| Touitou [71] | Not described | 9 | 9 (100%) | 9 | 9–11 | 10.8 (0.11) | France | Not described | 0 | Low |
| Villa [72] | Not described | 20 | 20 (100%) | 20 | 3–6 | 4.4 (0.8) | Santiago, Chile | Not described | 3 | Low |
| Zhang [73] | 2012 | 10 | 5 (50%) | 16 | 2–11 | 10.0 (3.2) | China | Urinary creatinine used—cutoff not described | 3 | Low |
| Zohouri [74] | 1995–1996 | 78 | Not described | 78 | 3–4.9 | Not described | Fars Province, Iran | Creatinine excretion < 14 mg/kg/day or > 20 mg/kg/day | 3 | Low |
| Zohouri [75] | 2002 | 7 | 5 (71%) | 7 | 1–3 | 3 (0.6) | Newcastle, England | “Validated by measuring the urinary excretion of creatinine in each 24-h urine sample and comparing this with the standard reference value for creatinine excretion” | 1 | Low |
NOS Newcastle–Ottawa Scale for cohort studies
*NOS rating based on score out of 10—low: 0–3; medium: 4–7; high 8–10
Table 4.
Characteristics of studies considered for the primary analysis where 24-h urine volume was presented as median (IQR), n = 7 studies
| Study | Study Year(s) | n | Male, n (%) |
Valid urine samples | Age range (years) | Age, mean (SD) years | Country | Exclusion criteria for urine samples | NOS score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Score out of 10 | Rating* | |||||||||
| Campanozzi [81] | Not described | 303 | 303 (100%) | 303 | 6–9 | 10.1 (2.9) | Italy | Creatinine excretion < 0.1 mmol/kg/day [81] | 6 | Medium |
| 228 | 228 (100%) | 228 | 9–11.3 | |||||||
| 235 | 235 (100%) | 235 | 11.4–18 | |||||||
| 162 | 0 (0%) | 162 | 6–9 | |||||||
| 180 | 0 (0%) | 180 | 9–11.3 | |||||||
| 316 | 0 (0%) | 316 | 11.4–18 | |||||||
| Degen [25] | 1985–1989 | 69 | 35 (51%) | 164 | 6–10 | 10.2 (*) | Dortmund, Germany |
(1) Creatinine excretion < 0.1 mmol/kg/day (2) Samples reported or found to contain incomplete micturations |
6 | Medium |
| 1990–1994 | 70 | 35 (50%) | 197 | 11–13 | 13.0 (*) | |||||
| Grases [82] | Not described | 105 | 64 (61%) | 105 | 5–17 | 12.0 (3.0) | Majorca, Spain | Not described | 1 | Low |
| Libuda [26] | 2003–2009 | 15 | 15 (100%) | 18 | 3–3.9 | 3.1 (*) | Dortmund, Germany | Creatinine excretion < 0.1 mmol/kg/day | 5 | Medium |
| 54 | 54 (100%) | 83 | 13–14.9 | 14.0 (*) | ||||||
| 66 | 66 (100%) | 145 | 15–18 | 16.2 (*) | ||||||
| 24 | 0 (0%) | 25 | 3–3.9 | 3.2 (*) | ||||||
| 56 | 0 (0%) | 84 | 13–14.9 | 14.0 (*) | ||||||
| 70 | 0 (0%) | 144 | 15–18 | 16.2 (*) | ||||||
| Montenegro-Bethancourt [28] | 2000–2010 | 146 | 146 (100%) | 269 | 4–6.9 | 5.0 (*) | Dortmund, Germany |
(1) Creatinine excretion < 0.1 mmol/kg/day (2) Collection time < 20 h |
5 | Medium |
| 147 | 0 (0%) | 264 | 4–6.9 | 5.0 (*) | ||||||
| Montenegro-Bethancourt [29] | 1996–2002 | 30 | 30 (100%) | 30 | 13–18 | 15.0 (*) | Dortmund, Germany |
(1) Creatinine excretion < 0.1 mmol/kg/day (2) No reported missed collections |
6 | Medium |
| 30 | 0 (0%) | 30 | 13–18 | 14.0 (*) | ||||||
| Montenegro-Bethancourt [30] | 1993–2010 | 265 | 265(100%) | 985 | 6–13 | 9.0 (*) | Dortmund, Germany | Creatinine excretion < 0.1 mmol/kg/day | 6 | Medium |
| 251 | 0 (0%) | 974 | 6–13 | 9.0 (*) | ||||||
NOS Newcastle-Ottawa Scale for cohort studies
*NOS rating based on score out of 10—low: 0–3; medium: 4–7; high 8–10
Due to the wide age range of participants of the included studies, studies were grouped into three groups according to the age of the participants; 2–5 years (17 studies) [26, 28, 36, 43–45, 50, 51, 54–57, 60, 62, 68, 72, 74, 75], 6–12 years (20 studies) [25, 30, 35, 36, 45–48, 52, 54, 58, 59, 61, 63–66, 71, 76, 81] and 13 to < 19 years (12 studies) [25, 26, 29, 36, 49, 53, 54, 63, 65, 67, 69, 81]. These cut-points were chosen based on the WHO criteria for assessing population iodine deficiency, which defines school-aged children as between 6 and 12 years of age [6]. As some studies presented results broken down by age group, a single study may have been assigned to multiple age groups. Studies which crossed the age group cut-offs were assigned to the age group in which the majority of the participants would fall (e.g., a study with participants 9–13 years [59] would fall into the 6–12 year age group. Four studies [37, 70, 73, 82] which encompassed very large age ranges (i.e., > 8 years) were excluded from the sub group analyses using age cut offs.
Initial analysis
The overall mean [95% confidence interval (CI)] estimate of urine volume for all 44 studies was determined using a random effects model and presented for the group as a whole (i.e., 2–19 year olds), as well as broken down by age group.
Primary analysis: studies with ≥ 1 criterion for assessing the completeness of urine samples
As the inclusion of criteria for assessing the completeness of urine collections can result in the exclusion of over/under collectors, the primary analysis was limited to only those studies which reported at least one criterion for assessing the completeness of the included urine samples (n = 27, “Primary Analysis, Fig. 2). The overall mean (95% CI) estimate of daily urine volume was determined using a random effects model and displayed in forest plots, broken down by age group.
A one-way ANOVA was used to assess the differences in urine volume across the three age groups. In addition, a one-way ANOVA was used to assess differences in the urine volume between those studies which did not report any criteria for assessing the completeness of included urine samples and those which reported > 1 and > 2 criteria. Tukey’s post hoc tests were performed to determine significant differences between age subgroups. Heterogeneity was analysed using the I2 and Q statistics. The coefficient of variation (CV) of the mean volume for each age group was derived from the mean and SD random effects analysis and was calculated by dividing the SD for each age group’s urine volume by the mean and multiplying by 100. A two-sample t test used to assess the difference in volumes determined for the initial analysis compared to the primary analysis (i.e., limited to studies with > 1 criterion for assessing the completeness of the urine samples) across the three age groups. As 11 studies had presented the results broken down by gender [26, 28–30, 37, 53, 65, 66, 68, 70, 81], a two-sample t test was used to evaluate the difference in 24-h urine volume between genders.
To examine whether climate had an impact on overall urine volume, studies were classified according to climate based on their proximity to the equator. Studies which were conducted in countries which lie between the Tropic of Cancer and Tropic of Capricorn (23.5° north and south) (2 studies) [54, 55] were classified as having a “warm” climate, whereas studies which fell outside of this area were classified as having a “cold” climate (25 studies) [25, 26, 28–30, 35–37, 43, 46, 48, 52, 56, 57, 60, 61, 64, 66, 67, 70, 73–75, 81, 82]. A two sample t test was used to assess the difference in urine volume between climates.
Results
Summary of studies considered for primary analysis
The 44 studies considered for inclusion in the primary analysis were published from 1981 to 2018 (Tables 3 and 4). Of these, 14 reported findings from Europe [25, 26, 28–30, 46, 50, 56, 60, 66, 70, 71, 81, 82], seven from the United Kingdom [36, 48, 56, 57, 61, 68, 75], seven from Asia [49, 51, 52, 58, 65, 68, 73], four from North America [55, 64, 69, 76], three from South America [44, 62, 72], three from the Middle East [43, 67, 74], three from Africa [37, 54, 76], and one from Australia [35].
Twenty-five studies reported creatinine excretion [25, 29, 35–37, 43, 45, 46, 49, 52–54, 58, 61, 63, 64, 67, 69–71, 73, 75, 77, 79, 81], 17 as 24-h excretion (mmol/24 h, mg/24 h, g/24 h) [29, 35–37, 45, 46, 49, 52–54, 58, 63, 64, 69, 70, 73, 81], six as a ratio of creatinine to body weight (mmol/kg, mg/kg) [25, 61, 67, 71, 75, 77] and two as a ratio to urine volume [43, 79].
Quality of studies considered for primary analysis
The criteria used to evaluate the completeness of the urine samples was inconsistent between studies, 18 studies did not provide any information on criteria used to assess the completeness of the urine samples [44, 45, 47, 49–51, 53, 58, 59, 62, 63, 65, 68, 69, 71, 72, 76, 82]. Of the 26 studies which reported their urine assessment criteria, 21 used creatinine excretion per kilogram of bodyweight [25, 26, 28–30, 35–37, 43, 46, 52, 54, 61, 64, 66, 67, 70, 73–75, 81], based on established age and gender-specific cutoffs [85]. The remaining five studies relied solely on urine volume [56, 57], the number of reported missed collections [48], the excretion of other nutrients (i.e., fluoride) [55], and paraminobenzoic acid (PABA) recovery as a measure of completeness [60]. Across all 26 studies, a total of ten studies utilised urinary volume as an indicator of completeness [35–37, 43, 48, 52, 56, 57, 61, 67], three [35, 37, 52] used the cut-off of 300 mL/24 h based on previously published criteria [86], two studies [56, 61] utilised the WHO criteria of < 5 mL/h and < 9 mL/h for < 6 and ≥ 6 year olds, respectively, and two studies [36, 67] in older children (13–19 years) used the cutoff of < 500 mL/24 h, based on previously published criteria [86]. The cut-offs used by the remaining three studies [43, 48, 55] were based on the distribution of volume in the sample, enabling the exclusion of extreme outliers (e.g., 4SDs below the geometric mean [48]). Of the 26 studies which reported the urine exclusion criteria, 6 did not report the number of urine samples excluded from the final analysis [25, 54, 73–75, 82].
As a result of the inconsistency in the criteria used to assess the completeness of the urine samples between studies, 23 studies (54%) scored low on the NOS quality scale [44–46, 49–51, 53–55, 57–59, 62, 63, 65, 69, 71–76, 82] (Tables 3 and 4). Eighteen studies (42%) were classified as “medium” [25, 26, 28–30, 43, 47, 48, 52, 56, 60, 61, 64, 66–68, 70, 81], and only three studies provided sufficient detail regarding the 24-h urine collection procedure to be classified as “high” quality [35–37] (Tables 3 and 4).
Initial analysis
The overall mean urine volume estimate (95% CI) for all 44 studies (n = 7712, 9538 urine samples) was 722 (686, 758) mL/24-h. Eleven studies reported the results broken down by gender [26, 28–30, 37, 53, 65, 66, 68, 70, 81]. There was no difference in mean urine volume between genders (858.09 (286) mL/24-h (n = 2635, 2635 urine samples) and 818 (240) mL/24-h (n = 2504, 2504 urine samples), for males and females, respectively, P = 0.7). When broken down by age group, there were more than three times the number of urine collections for 6–12 year olds compared with 2–5 year olds and approximately half the number of samples for the 13–19 year olds. Sixteen studies reported results for 2–5 year olds (n = 1304, 1557 urine samples), 20 for 6–12 year olds (n = 3772, 5210 urine samples) and 12 for 13–19 year olds (n = 2230, 2359 urine samples). For each of the three age groups, the overall estimate (95% CI) was 461 (413, 509) mL/24-h among 2–5 year/olds (Supplemental Fig. 1), 758 (725, 791)mL/24-h for 6–12 year olds (Supplemental Fig. 2) and 1048 (973, 1123) mL/24-h for 13–19 year olds (Supplemental Fig. 3). There was a significant difference in the mean urine volume across the three age groups (P < 0.001).
Primary analysis limited to studies with > 1 urine completeness assessment criterion
Twenty-six studies reported ≥ 1 criterion for assessing the completeness of the urine samples (n = 6322, 8331 urine samples) [25, 26, 28–30, 35–37, 43, 46, 48, 55–57, 60, 61, 64, 66, 67, 70, 73–75, 81]. The overall urine volume estimate (95% CI) for these studies was 773 (654, 893) mL/24-h [median (IQR) 737 (284) mL/24-h]. When studies were assessed by climate (“warm” climate (2 studies) [54, 55] versus “cold” climate (25 studies) [25, 26, 28–30, 35–37, 43, 46, 48, 52, 56, 57, 60, 61, 64, 66, 67, 70, 73–75, 81, 82]) there was no difference in mean (95% CI) 24-h urine volume: “warm” hot 788 (244, 1332) mL/24-h vs “cold” 779 (713, 845) mL/24-h, P = 0.96.
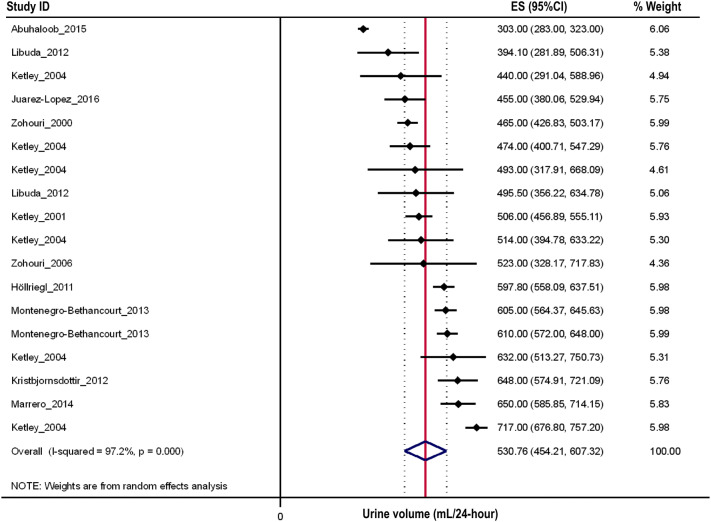
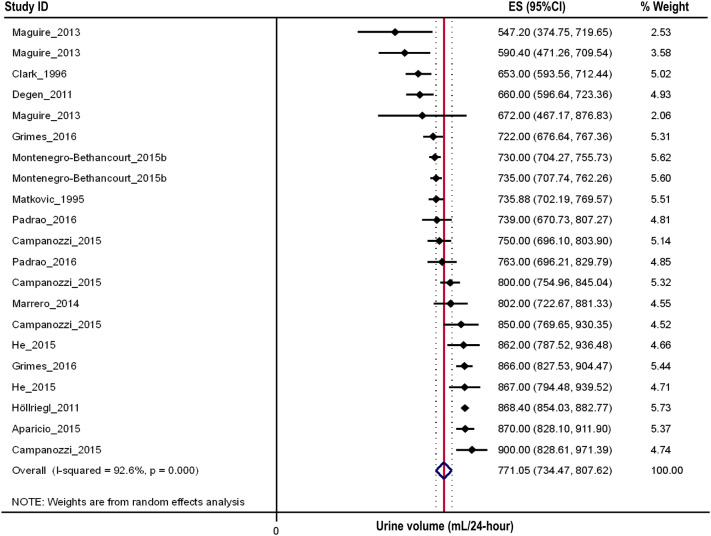
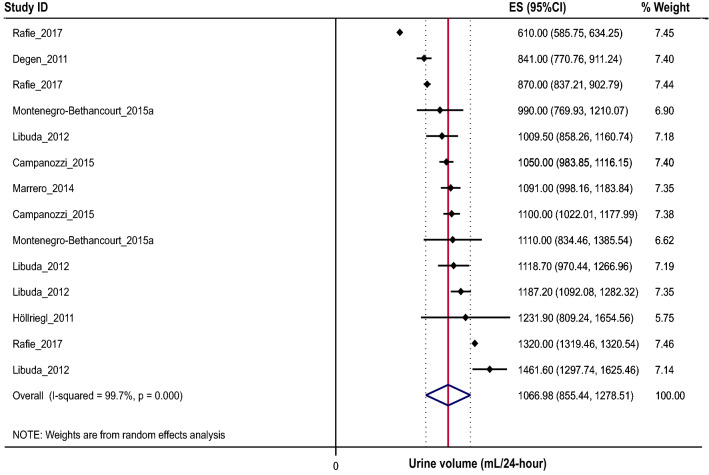
When broken down by age group, 11 studies reported results for 2–5 year olds (n = 987, 1240 urine samples) [26, 28, 36, 43, 54–57, 60, 74, 75], 12 for 6–12 year olds (n = 3596, 5038 urine samples) [25, 30, 35, 36, 46, 48, 52, 54, 61, 64, 66, 81] and seven for 13–19 year olds (n = 1438, 1746 urine samples) [25, 26, 29, 36, 54, 67, 81]. The overall estimate (95% CI) for each of the three age groups were 531 (454, 607) (Fig. 3), 771 (734, 808) (Fig. 4), and 1067 (855, 1279) (Fig. 5) mL/24-h, respectively. There was a significant difference in the mean urine volume across the three age groups (P < 0.001). Posthoc analyses revealed that children in the oldest age group had a 28% higher 24-h urine volume compared to those aged 6–12 years (1067 vs. 771 mL/24-h, P < 0.001) and approximately 50% higher urine volume compared to those aged 2–5 years (1067 vs. 531 mL/24-h, P < 0.001). Similarly, those aged 6–12 had a 31% higher volume compared to 2–5 year olds (771 vs. 531 mL/24 h, P < 0.001). There was significant between study heterogeneity across all three age groups (2–5 years/olds: I2 = 97.2%, P < 0.001, Fig. 3; 6–12 years/olds: I2 = 92.6%, P < 0.001, Fig. 4; 13–19 years/olds: I2 = 99.7%, P < 0.001, Fig. 5).
Fig. 3.
Forest plot of studies assessing 24-h urine volumes of 2–5 year olds with > 1 urine assessment criterion (n = 1084)
Fig. 4.
Forest plot of studies assessing 24-h urine volumes of 6–12 year olds with > 1 urine assessment criterion (n = 3628)
Fig. 5.
Forest plot of studies assessing 24-h urine volumes of 13–19 year olds with > 1 urine assessment criterion (n = 1438)
When comparing the mean urine volume between those studies which reported at least one criterion for assessing the completeness of urine samples and those which reported none, the only difference in mean urine volume was amongst the 2–5 year old age group. In this age group, the mean urine volume in the initial analysis (i.e., all studies were included) was 27% lower compared to the primary analysis (i.e., when the analysis was limited to only those studies with ≥ 1 criterion for assessing the completeness of urine samples (386 vs. 529 mL/24-h, P = 0.001).
Of the 26 studies with at least one criterion for assessing the completeness of the urine samples, only twelve [25, 28, 29, 35–37, 43, 48, 52, 55, 61, 64] had at least two urine criteria (n = 2867, 3191 urine samples). In these twelve studies, the mean (95% CI urine volume estimate was 742 (639, 844) mL/24-h. There was no difference in the mean (95% CI) urine volume estimate of these studies, compared to those with only one assessment criterion [n = 15, 798 (664, 932) mL/24-h, P = 0.33] or those with none [n = 18, 635 (577, 692) mL/24-hs P = 0.28].
For those studies with at least one criterion for assessing the completeness of urine collections there was less variation in daily urinary volume for those 6–12 years compared to 2–5 and 13–19 year olds. The co-efficient of variation (CV) for 6–12 year olds was 13% compared to 20% in 2–5 and 13–19 year olds. There was no difference in CVs between those studies with no reported criterion for completeness and those reporting at least one criterion (overall CV: 32% vs. 27%). In contrast, the CV for those aged between 13 and 19 years was reduced to 12% in those studies utilising least two criteria for assessing completeness of urine collection, compared with 20% for those studies utilising only one criterion for assessment for completeness.
Discussion
This is the first study to systematically review the 24-h urine volume of children and adolescents. The overall 24-h overall urine volume estimate (95% CI) of 2–19 year olds was 778 (661, 895) mL/24-h urine. As expected, older children had higher urine volumes with children in the oldest age group (13–19 years) having a 28% higher 24-h urine volume compared to those aged 6–12 years (1067 vs. 771 mL/24-h, P < 0.001) and approximately 50% higher urine volume compared to those aged 2–5 years (1067 vs. 531 mL/24-h, P < 0.001).
As approximately 90% of ingested iodine is excreted in the urine within 24–48 h [87], current recommendations for assessing the severity of iodine deficiency within a population are based on the measurement of urinary iodine concentration (UIC), expressed as µg iodine per liter of urine, in random ‘spot’ urine samples collected from school-aged children (i.e., 6–12 years [6]). Although these recommendations were originally based on the observation that goiter prevalence was < 10% in populations of children and adults where the average daily iodine intakes were > 100 µg, later iterations extrapolated this value to represent a concentration, expressed as µg/L [4]. However, results from this analysis indicate that the average 24-h urine volume of school-aged children, the group commonly recommended for use in population iodine monitoring, is not 1 L and is closer to 0.8 L. Therefore, a median spot urine concentration of 100 µg/L (extrapolated to a median iodine intake of 100 µg/day) would overestimate iodine intake by approximately 30 µg/day.
Iodine excretion from spot urine samples is most often expressed as a concentration or as a ratio to creatinine excretion (I/Cr, µg iodine/g creatinine) [29, 88, 89]. The use of I/Cr to estimate daily iodine intake is believed to provide an accurate estimate of daily iodine excretion from spot urine samples as creatinine, an endogenous indicator of lean body mass is relatively constant from day to day in healthy adult populations [32, 90]. Whilst this is true in adults, estimating expected creatinine excretion values for children can be difficult as creatinine excretion can be affected by muscle mass, age, gender, ethnicity and onset of puberty [91]. Some equations for estimating daily creatinine excretion are able to account for these factors, whilst others provide a more crude estimate of daily creatinine excretion [92].
In addition, whilst the variation in individual iodine excretion between days is largely dependant on the iodine content of the diet, iodine excretion has also been found to vary over the course of the day in individuals [29, 88, 89, 92–97]. One study conducted in 42 adults and children (aged 4–60 years) found that urinary iodine excretion varied significantly by the timing of collection (P < 0.001), with lowest levels occurring in the morning and peaks observed following meals [98]. A recent systematic review of studies comparing spot and 24-h urine samples for estimating the iodine intakes of a population concluded that there is currently not enough evidence to determine whether iodine intake determined from spot urine samples provides an accurate reflection of daily iodine intake, as measured using 24-h urine samples [99].
Furthermore, whilst the WHO recommendation for assessing the iodine status of populations are primarily meant for use in school-aged children, and were derived primarily on data based on goiter prevalence estimates in school-aged children, they have also been used to define the iodine status of adult populations [100–103]. Issues concerning different urinary volume outputs among different subsets of the population and implications for iodine nutrition assessment have been previously identified by Zimmerman and Andersson [104]. They highlighted that as the urine volume of adults is closer to 1.5 L [27, 105], the use of the median UIC determined using spot urine samples could result in the underestimation of the iodine intakes of adult individuals within the population [104]. This was demonstrated in a recent study in 301 adults (18–64 years) from New Zealand, which compared median UIC from 24-h urine samples to the WHO criteria, both with and without adjustment for total urine volume [101]. This sample of adults was classified as iodine deficient using the WHO criteria, based on a median UIC of 73 µg/L. However, the measured 24-h UIE, which accounts for urine volume and averaged 2 L was closer to 127 µg/day [101]. This value is in excess of the 100 µg/day originally associated with reduced goiter prevalence [8] and would indicate that the iodine intakes of this group of adults may be sufficient [8]. The New Zealand study demonstrates the potential impact of not accounting for the daily urine volume may have on the assessment of iodine deficiency in populations when UIC determined from spot urine samples is used as a surrogate index of iodine intake.
Strengths and limitations
We observed considerable between-study heterogeneity across all three age groups in the primary analysis limited to studies with > 1 indicator of urine completeness. For all three age groups the age range of participants included within each group varied considerably. For example, within the 2–5 year age group one study consisted of participants aged 1.5–3.5 years [56] whilst another consisted of participants aged 3–6 years [72]. Differences in both the age range of participants as well as the number of participants between studies, along with season of assessment and overall diet composition may have contributed to the observed heterogeneity.
In addition, there were considerable variations in the mean/median reported urine volumes, even within the three age groups, particularly for 2–5 year olds. This may represent the practical difficulties in obtaining accurate 24 h urine collections in young children, however, it is important to note that the included studies did not consistently report their 24-h urine collection protocol, nor the indicators used to assess the completeness of included urine samples. Of the 44 studies reviewed, 17 studies (40%) did not report at least one indicator for completeness of the 24-h urine samples. The only difference in the mean urine volume estimate between the total sample and those studies with ≥ 1 indicator for completeness of urine samples, was seen among the youngest age group (2–5 years) where average urine volume was 143 ml/24-h less in the studies which reported no criterion for assessing the completeness of included urine samples. A recent systematic review of methods for assessing the completeness of 24-h urine collections in adults and children (15–89 years) concluded that that the use of two or more indicators for assessing urine completeness increases the likelihood of detecting incomplete samples, thus increasing the validity of the results [33]. Our findings are contrary to this in that we found no difference in urine volume among those studies that had at least two urine assessment criteria, compared to those with only one. In the present review, only 12 studies utilised more than one criterion [25, 28, 29, 35–37, 43, 48, 52, 55, 61, 64], and there was no difference in mean 24-h urine volume estimated from these studies compared to the overall estimate from all 44 studies. However, there was less variation in daily urinary volume in the 6–12 year group (CV 13%). Overall there seemed to be little impact on urine volume variation of including a number of criteria for completeness of 24 h urine collection, except for the 13–19 year age group where studies that included at least two criteria for completeness appeared to have less variation (CV 16%) compared with studies with only one criterion (CV 20%).
In this analysis, only two studies collected 24-h urine samples from countries classified as having a “warm” climate”, compared to 25 studies from a “cold” climate. There is also considerable within and between person daily variability in iodine excretion [92, 95, 96, 98, 106, 107]. One study conducted in 42 adults and children (aged 4–60 years) observed that the lowest level of iodine excretion occurred in the morning with peaks observed following meals [98]. Furthermore, a study in adults noted that UIC determined from a fasting spot urine samples was 10% lower than that determined in a non-fasting spot urine sample [96]. Although this variation has yet to be assessed in children, this study indicates that the timing of a spot urine sample used to estimate the iodine intake of a population may also have a significant impact on the overall assessment of iodine nutrition. Therefore, it is clear that a number of factors need to be considered when making population estimates of iodine intake using spot urine collections across the age range from early childhood to adolescence.
Conclusion
This is the first systematic review to report the average 24-h urine volume of children and adolescents from 44 studies representing 7712 individuals with 3772 individuals within the 6–12 year old age group, which included at least one criterion for completeness of urine collection. The average urine volume in this group was 771 mL, which is less than 1 L. This has implications when extrapolating median iodine values (µg/L) from spot urine samples to daily iodine intakes of 6–12 year old children as the average 24-h urine volume is less than 1 L, potentially resulting in an overestimate of dietary iodine intake in the order of 30%. Future studies employing spot urine samples to determine the iodine status of children and adolescents should consider undertaking 24-h urine collections in a subset of participants, to determine total urine volume and iodine excretion. This will allow the assessment of the accuracy of utilizing UIC as a proxy measure of daily iodine intake and potentially prevent the misclassification of iodine intakes in the population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
CAN, LJR and SAS created the study concept and design. KB and MW performed the literature search and study selection. KB conducted data extraction and data analysis and wrote the manuscript. All of the authors were involved in and contributed to analysis and interpretation of data and the critical revision of the final manuscript. The authors declare that they have no conflict of interest.
Abbreviations
- DONALD
Dortmund Nutritional and Anthropometric Longitudinally Designed Study
- NOS
Newcastle–Ottawa scale
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- UIC
Urinary iodine concentration
- UIE
Urinary iodine excretion
- WHO
World Health Organization
Funding
At the time of this work KB was funded by an Australian Postgraduate Award Scholarship (Award ID: 0000019017).
References
- 1.Gordon RC, Rose MC, Skeaff SA, Gray AR, Morgan KM, Ruffman T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am J Clin Nutr. 2009;90(5):1264–1271. doi: 10.3945/ajcn.2009.28145. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann MB, Connolly K, Bozo M, Bridson J, Rohner F, Grimci L. Iodine supplementation improves cognition in iodine-deficient schoolchildren in Albania: a randomized, controlled, double-blind study. Am J Clin Nutr. 2006;83(1):108–114. doi: 10.1093/ajcn/83.1.108. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders . A practical guide to the correction of iodine deficiency. Wageningen: International Council for the Control of Iodine Deficiency Disorders; 1990. [Google Scholar]
- 4.World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders (1993) Indicators for assessing iodine deficiency disorders and their control programmes. In: Report of a joint WHO/UNICEF/ICCIDD consultation, Geneva, Switzerland, 3–5 November 1992. World Health Organisation, Geneva
- 5.World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders . Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 2. Geneva: World Health Organisation; 2001. [Google Scholar]
- 6.World Health Organization, United Nations Children’s Fund, International Council for the Control of Iodine Deficiency Disorders . Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3. Geneva: World Health Organisation; 2007. [Google Scholar]
- 7.World Health Organization (2013) Urinary iodine concentrations for determining iodine status in populations. World Health Organistion. http://www.who.int/vmnis/indicators/urinaryiodine/en/. Accessed 26 March 2014
- 8.Ascoli W, Arroyave G. Epidemiologia el bocio endémico en Centro América. Relación entre prevalencia y excreción urinaria de yodo [Epidemiology of endemic goiter in Central America. Association between prevalence and urinary iodine excretion] Arch Latinoam Nutr. 1970;20:309–320. [Google Scholar]
- 9.World Health Organization . Iodine status worldwide: WHO global database on iodine deficiency. Geneva: World Health Organization; 2004. [Google Scholar]
- 10.Andersson M, Takkouche B, Egli I, Allen HE, De Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull WHO. 2007;85(5):518–525. [PMC free article] [PubMed] [Google Scholar]
- 11.de Benoist B, McLean E, Andersson M, Rogers L. Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull. 2008;29(3):195–202. doi: 10.1177/156482650802900305. [DOI] [PubMed] [Google Scholar]
- 12.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142(4):744–750. doi: 10.3945/jn.111.149393. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann MB. Iodine deficiency and excess in children: worldwide status in 2013. Endocr Pract. 2013;19(5):839–846. doi: 10.4158/EP13180.RA. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: where do we stand in 2013? Thyroid. 2013;23(5):523–528. doi: 10.1089/thy.2013.0128. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroke A, Manz F, Kersting M, Remer T, Sichert-Hellert W, Alexy U, Lentze MJ. The DONALD study. History, current status and future perspectives. Eur J Nutr. 2004;43(1):45–54. doi: 10.1007/s00394-004-0445-7. [DOI] [PubMed] [Google Scholar]
- 18.Bokhof B, Günther ALB, Berg-Beckhoff G, Kroke A, Buyken AE. Validation of protein intake assessed from weighed dietary records against protein estimated from 24 h urine samples in children, adolescents and young adults participating in the Dortmund Nutritional and Longitudinally Designed (DONALD) Study. Public Health Nutr. 2010;13(6):826–834. doi: 10.1017/S136898000999317X. [DOI] [PubMed] [Google Scholar]
- 19.Dimitriou T, Huesemann S, Neubert A, Remer T. Measurements of urinary leptin and capillary leptin: alternative tools for the assessment of the leptin status? Horm Res. 2001;56(3–4):93–97. doi: 10.1159/000048098. [DOI] [PubMed] [Google Scholar]
- 20.Ebner A, Manz F. Sex difference of urinary osmolality in German children. Am J Nephrol. 2002;22(4):352–355. doi: 10.1159/000065226. [DOI] [PubMed] [Google Scholar]
- 21.Esche J, Johner S, Shi L, Schonau E, Remer T. Urinary citrate, an index of acid-base status, predicts bone strength in youths and fracture risk in adult females. J Clin Endocrinol Metab. 2016;101(12):4914–4921. doi: 10.1210/jc.2016-2677. [DOI] [PubMed] [Google Scholar]
- 22.Penczynski KJ, Krupp D, Bring A, Bolzenius K, Remer T, Buyken AE. Relative validation of 24-h urinary hippuric acid excretion as a biomarker for dietary flavonoid intake from fruit and vegetables in healthy adolescents. Eur J Nutr. 2015;56:757–766. doi: 10.1007/s00394-015-1121-9. [DOI] [PubMed] [Google Scholar]
- 23.Krupp D, Doberstein N, Shi L, Remer T. Hippuric acid in 24-h urine collections is a potential biomarker for fruit and vegetable consumption in healthy children and adolescents. J Nutr. 2012;142(7):1314–1320. doi: 10.3945/jn.112.159319. [DOI] [PubMed] [Google Scholar]
- 24.Remer T, Shi L, Buyken AE, Maser-Gluth C, Hartmann MF, Wudy SA. Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. J Clin Endocrinol Metab. 2010;95(6):3002–3009. doi: 10.1210/jc.2009-2583. [DOI] [PubMed] [Google Scholar]
- 25.Degen GH, Blaszkewicz M, Shi L, Buyken AE, Remer T. Urinary isoflavone phytoestrogens in German children and adolescents—a longitudinal examination in the DONALD cohort. Mol Nutr Food Res. 2011;55(3):359–367. doi: 10.1002/mnfr.201000325. [DOI] [PubMed] [Google Scholar]
- 26.Libuda L, Kersting M, Alexy U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr. 2012;15(3):433–441. doi: 10.1017/S1368980011002138. [DOI] [PubMed] [Google Scholar]
- 27.Manz F, Johner SA, Wentz A, Boeing H, Remer T. Water balance throughout the adult life span in a German population. Br J Nutr. 2012;107(11):1673–1681. doi: 10.1017/S0007114511004776. [DOI] [PubMed] [Google Scholar]
- 28.Montenegro-Bethancourt G, Johner SA, Remer T. Contribution of fruit and vegetable intake to hydration status in schoolchildren. Am J Clin Nutr. 2013;98(4):1103–1112. doi: 10.3945/ajcn.112.051490. [DOI] [PubMed] [Google Scholar]
- 29.Montenegro-Bethancourt G, Johner SA, Stehle P, Neubert A, Remer T. Iodine status assessment in children: spot urine iodine concentration reasonably reflects true twenty-four-hour iodine excretion only when scaled to creatinine. Thyroid. 2015;25(6):688–697. doi: 10.1089/thy.2015.0006. [DOI] [PubMed] [Google Scholar]
- 30.Montenegro-Bethancourt G, Johner SA, Stehle P, Remer T. Dietary ratio of animal:plant protein is associated with 24-h urinary iodine excretion in healthy school children. Br J Nutr. 2015;114(1):24–33. doi: 10.1017/S0007114515001567. [DOI] [PubMed] [Google Scholar]
- 31.Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr. 2015;35(349–387):333p. doi: 10.1146/annurev-nutr-071714-034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingham SA, Cummings JH. The use of creatinine output as a check on the completeness of 24-h urine collections. Hum Nutr Clin Nutr. 1985;39(5):343–353. [PubMed] [Google Scholar]
- 33.John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJM, Patel SM. Accuracy and usefulness of select methods for assessing complete collection of 24-h urine: a systematic review. J Clin Hypertens. 2016;18(5):456–467. doi: 10.1111/jch.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (1999) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2 Aug 2016
- 35.Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24-h urinary sodium excretion is associated with obesity in a cross-sectional sample of Australian schoolchildren. Br J Nutr. 2016;115(6):1071–1079. doi: 10.1017/S0007114515005243. [DOI] [PubMed] [Google Scholar]
- 36.Marrero NM, He FJ, Whincup P, Macgregor GA. Salt intake of children and adolescents in South London: consumption levels and dietary sources. Hypertension. 2014;63(5):1026–1032. doi: 10.1161/HYPERTENSIONAHA.113.02264. [DOI] [PubMed] [Google Scholar]
- 37.Saeid N, Elmzibri M, Hamrani A, Latifa Q, Belghiti H, El Berri H, Benjeddou K, Bouziani A, Benkirane H, Taboz Y, Elhamdouchi A, El Kari K, Aguenaou H. Assessment of sodium and potassium intakes in children aged 6 to 18 years by 24 h urinary excretion in city of Rabat, Morocco. J Nutr Metab. 2018;1:8. doi: 10.1155/2018/8687192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borawski KM, Sur RL, Miller OF, Pak CYC, Preminger GM, Kolon TF. Urinary reference values for stone risk factors in children. J Urol. 2008;179(1):290–294. doi: 10.1016/j.juro.2007.08.163. [DOI] [PubMed] [Google Scholar]
- 39.Ajayi OA, Adeyemi IA, Osifo BOA. The urinary excretion of riboflavin among Nigerian teenagers. Ecol Food Nutr. 1977;6(1):49–52. [Google Scholar]
- 40.M’Buyamba-Kabangu JR, Fagard R, Lijnen P, Mbuy WA, Mbuy R, Staessen J, Amery A. Blood pressure and urinary cations in urban Bantu of Zaire. Am J Epidemiol. 1986;124(6):957–968. doi: 10.1093/oxfordjournals.aje.a114485. [DOI] [PubMed] [Google Scholar]
- 41.Omid N, Maguire A, O’Hare WT, Zohoori FV. Total daily fluoride intake and fractional urinary fluoride excretion in 4- to 6-year-old children living in a fluoridated area: weekly variation? Commun Dent Oral Epidemiol. 2017;45(1):12–19. doi: 10.1111/cdoe.12254. [DOI] [PubMed] [Google Scholar]
- 42.Vanderas AP, Papagiannoulis L. Urinary catecholamine levels in children with and without a history of dentofacial injuries. Endod Dent Traumatol. 1995;11(5):205–209. doi: 10.1111/j.1600-9657.1995.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 43.Abuhaloob L, Maguire A, Moynihan P. Fractional urinary fluoride excretion (FUFE) of 3–4 year children in the gaza strip. Community Dent Health. 2015;32(1):8–15. [PubMed] [Google Scholar]
- 44.Acevedo AM, Febres-Cordero C, Feldman S, Arasme MA, Pedauga DF, Gonzalez H, Rojas-Sanchez F. Urinary fluoride excretion in children aged 3 to 5 years exposed to fluoridated salt at 60 to 90 mgF/Kg in two Venezuelan cities. A pilot study. Acta Odontol Latinoam. 2007;20(1):9–16. [PubMed] [Google Scholar]
- 45.Allevard-Burguburu AM, Geelen G, Sempore B, Louis F, Legros JJ, Gharib C. Urinary excretion of immunoreactive vasopressin in prepubertal children. Lack of correlation with urinary excretion of immunoreactive neurophysins. Eur J Pediatr. 1981;137(3):291–294. doi: 10.1007/BF00443260. [DOI] [PubMed] [Google Scholar]
- 46.Aparicio A, Rodriguez-Rodriguez E, Cuadrado-Soto E, Navia B, Lopez-Sobaler AM, Ortega RM. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7–11 years. Eur J Nutr. 2015;56(1):171–178. doi: 10.1007/s00394-015-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ballauff A, Kersting M, Manz F. Do children have an adequate fluid intake? Water balance studies carried out at home. Ann Nutr Metab. 1988;32(5–6):332–339. doi: 10.1159/000177483. [DOI] [PubMed] [Google Scholar]
- 48.Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clin Endocrinol (Oxf) 1996;45(6):721–726. doi: 10.1046/j.1365-2265.1996.8560864.x. [DOI] [PubMed] [Google Scholar]
- 49.Fujishima S, Tochikubo O, Kaneko Y. Environmental and physiological characteristics in adolescents genetically predisposed to hypertension. Jpn Circ J. 1983;47(2):276–282. doi: 10.1253/jcj.47.276. [DOI] [PubMed] [Google Scholar]
- 50.Haftenberger M, Viergutz G, Neumeister V, Hetzer G. Total fluoride intake and urinary excretion in German children aged 3–6 years. Caries Res. 2001;35(6):451–457. doi: 10.1159/000047489. [DOI] [PubMed] [Google Scholar]
- 51.Haga M, Sakata T. Daily salt intake of healthy Japanese infants of 3–5 years based on sodium excretion in 24-h urine. J Nutr Sci Vitaminol (Tokyo) 2010;56(5):305–310. doi: 10.3177/jnsv.56.305. [DOI] [PubMed] [Google Scholar]
- 52.He FJ, Wu Y, Feng XX, Ma J, Ma Y, Wang H, Zhang J, Yuan J, Lin CP, Nowson C, MacGregor GA. School based education programme to reduce salt intake in children and their families (School-EduSalt): cluster randomised controlled trial. BMJ. 2015;350:h770. doi: 10.1136/bmj.h770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hesse A, Classen A, Knoll M, Timmermann F, Vahlensieck W. Dependence of urine composition on the age and sex of healthy subjects. Clin Chim Acta. 1986;160(2):79–86. doi: 10.1016/0009-8981(86)90126-9. [DOI] [PubMed] [Google Scholar]
- 54.Hollriegl V, Arogunjo AM, Giussani A, Michalke B, Oeh U. Daily urinary excretion of uranium in members of the public of Southwest Nigeria. Sci Total Environ. 2011;412–413:344–350. doi: 10.1016/j.scitotenv.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Juárez López MLA, Hernández Guerrero JC, Adriano Anaya MP, Pruneda FM. Urinary fluoride excretion in 3–5-year-old children with and without malnutrition. Fluoride. 2016;49(2):178–184. [Google Scholar]
- 56.Ketley CE, Cochran JA, Holbrook WP, Sanches L, van Loveren C, Oila AM, O’Mullane DM. Urinary fluoride excretion by preschool children in six European countries. Community Dent Oral Epidemiol. 2004;32(Suppl 1):62–68. doi: 10.1111/j.1600-0528.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 57.Ketley CE, Lennon MA. Determination of fluoride intake from urinary fluoride excretion data in children drinking fluoridated school milk. Caries Res. 2001;35(4):252–257. doi: 10.1159/000047466. [DOI] [PubMed] [Google Scholar]
- 58.Khandare AL, Rao GS, Lakshmaiah N. Effect of tamarind ingestion on fluoride excretion in humans. Eur J Clin Nutr. 2002;56(1):82–85. doi: 10.1038/sj.ejcn.1601287. [DOI] [PubMed] [Google Scholar]
- 59.Kirdpon S, Kirdpon W, Airarat W, Thepsuthammarat K, Nanakorn S. Changes in urinary compositions among children after consuming prepared oral doses of aloe (Aloe vera Linn) J Med Assoc Thai. 2006;89(8):1199–1205. [PubMed] [Google Scholar]
- 60.Kristbjornsdottir OK, Halldorsson TI, Thorsdottir I, Gunnarsdottir I. Association between 24-h urine sodium and potassium excretion and diet quality in six-year-old children: a cross sectional study. Nutr J. 2012;11:94. doi: 10.1186/1475-2891-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maguire A, Walls R, Steen N, Teasdale L, Landes D, Omid N, Moynihan P, Zohoori FV. Urinary fluoride excretion in 6- to 7-year-olds ingesting milk containing 0.5 or 0.9 mg fluoride. Caries Res. 2013;47(4):291–298. doi: 10.1159/000346549. [DOI] [PubMed] [Google Scholar]
- 62.Martins CC, Paiva SM, Cury JA. Effect of discontinuation of fluoride intake from water and toothpaste on urinary excretion in young children. Int J Environ Res Public Health. 2011;8(6):2132–2141. doi: 10.3390/ijerph8062132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maruhama Y, Hikichi I, Hashimoto T, Saito F, Ninomiya K. Further evidence for insulin hypersecretion not as an initial event but as a consequence of body growth and maturation in juvenile-onset obesity. Diabetes Res Clin Pract. 1986;2(2):83–90. doi: 10.1016/s0168-8227(86)80064-x. [DOI] [PubMed] [Google Scholar]
- 64.Matkovic V, Ilich JZ, Andon MB, Hsieh LC, Tzagournis MA, Lagger BJ, Goel PK. Urinary calcium, sodium, and bone mass of young females. Am J Clin Nutr. 1995;62(2):417–425. doi: 10.1093/ajcn/62.2.417. [DOI] [PubMed] [Google Scholar]
- 65.Mori M, Mori H, Hamada A, Yamori Y. Taurine in morning spot urine for the useful assessment of dietary seafood intake in Japanese children and adolescents. J Biomed Sci. 2010;17(Suppl 1):S43. doi: 10.1186/1423-0127-17-S1-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padrao P, Neto M, Pinto M, Oliveira AC, Moreira A, Moreira P. Urinary hydration biomarkers and dietary intake in children. Nutr Hosp. 2016;33(Suppl 3):314. doi: 10.20960/nh.314. [DOI] [PubMed] [Google Scholar]
- 67.Rafie N, Mohammadifard N, Khosravi A, Feizi A, Safavi SM. Relationship of sodium intake with obesity among Iranian children and adolescents. ARYA Atheroscler. 2017;13(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 68.Rugg-Gunn AJ, Nunn JH, Ekanayake L, Saparamadu KD, Wright WG. Urinary fluoride excretion in 4-year-old children in Sri Lanka and England. Caries Res. 1993;27(6):478–483. doi: 10.1159/000261584. [DOI] [PubMed] [Google Scholar]
- 69.Sinaiko AR, Gomez-Marin O, Smith CL, Prineas RJ. Comparison of serum calcium levels between junior high school children with high-normal and low-normal blood pressure. The child and adolescent blood pressure program. Am J Hypertens. 1994;7(12):1045–1051. doi: 10.1093/ajh/7.12.1045. [DOI] [PubMed] [Google Scholar]
- 70.Staessen J, Bulpitt C, Fagard R, Joossens JV, Lijnen P, Amery A. Four urinary cations and blood pressure. A population study in two Belgian towns. Am J Epidemiol. 1983;117(6):676–687. doi: 10.1093/oxfordjournals.aje.a113601. [DOI] [PubMed] [Google Scholar]
- 71.Touitou Y, Auzeby A, Camus F, Djeridane Y. Twenty-four-hour profiles of urinary excretion of calcium, magnesium, phosphorus, urea, and creatinine in healthy prepubertal boys. Clin Biochem. 2010;43(1–2):102–105. doi: 10.1016/j.clinbiochem.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Villa A, Anabalón M, Cabezas L. The fractional urinary fluoride excretion in young children under stable fluoride intake conditions. Commun Dent Oral Epidemiol. 2000;28(5):344–355. doi: 10.1034/j.1600-0528.2000.028005344.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, Zhao F, Zhang P, Gao J, Liu C, He FJ, Lin CP. A pilot study to validate a standardized one-week salt estimation method evaluating salt intake and its sources for family members in China. Nutrients. 2015;7(2):751–763. doi: 10.3390/nu7020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zohouri FV, Rugg-Gunn AJ. Total fluoride intake and urinary excretion in 4-year-old Iranian children residing in low-fluoride areas. Br J Nutr. 2000;83(1):15–25. [PubMed] [Google Scholar]
- 75.Zohouri FV, Swinbank CM, Maguire A, Moynihan PJ. Is the fluoride/creatinine ratio of a spot urine sample indicative of 24-h urinary fluoride? Community Dent Oral Epidemiol. 2006;34(2):130–138. doi: 10.1111/j.1600-0528.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 76.Melse-Boonstra A, Rozendaal M, Rexwinkel H, Gerichhausen MJ, van den Briel T, Bulux J, Solomons NW, West CE. Determination of discretionary salt intake in rural Guatemala and Benin to determine the iodine fortification of salt required to control iodine deficiency disorders: studies using lithium-labeled salt. Am J Clin Nutr. 1998;68(3):636–641. doi: 10.1093/ajcn/68.3.636. [DOI] [PubMed] [Google Scholar]
- 77.Allison ME, Walker V. The sodium and potassium intake of 3 to 5 year olds. Arch Dis Child. 1986;61(2):159–163. doi: 10.1136/adc.61.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baker BA, Alexander BH, Mandel JS, Acquavella JF, Honeycutt R, Chapman P. Farm family exposure study: methods and recruitment practices for a biomonitoring study of pesticide exposure. J Expo Anal Environ Epidemiol. 2005;15(6):491–499. doi: 10.1038/sj.jea.7500427. [DOI] [PubMed] [Google Scholar]
- 79.Chen W, Wu Y, Lin L, Tan L, Shen J, Guo X, Wang W, Bian J, Jiang W, Zhang W. 24-h Urine samples are more reproducible than spot urine samples for evaluation of iodine status in school-age children. J Nutr. 2016;146(1):142–146. doi: 10.3945/jn.115.215806. [DOI] [PubMed] [Google Scholar]
- 80.Soto-Mendez MJ, Aguilera CM, Campana-Martin L, Martin-Laguna V, Schumann K, Solomons NW, Gil A. Variation in hydration status within the normative range is associated with urinary biomarkers of systemic oxidative stress in Guatemalan preschool children. Am J Clin Nutr. 2015;102(4):865–872. doi: 10.3945/ajcn.114.105429. [DOI] [PubMed] [Google Scholar]
- 81.Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, De Filippo G, D’Angelo G, Pensabene L, Malamisura B, Cecere G, Micillo M, Francavilla R, Tetro A, Lombardi G, Tonelli L, Castellucci G, Ferraro L, Di Biase R, Lezo A, Salvatore S, Paoletti S, Siani A, Galeone D, Strazzullo P, Grp M-GPS. High sodium and low potassium intake among italian children: relationship with age, body mass and blood pressure. PLoS One. 2015;10(4):e0121183. doi: 10.1371/journal.pone.0121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grases F, Rodriguez A, Costa-Bauza A, Saez-Torres C, Rodrigo D, Gomez C, Mir-Perello C, Frontera G. Factors associated with the lower prevalence of nephrolithiasis in children compared with adults. Urology. 2015;86(3):587–592. doi: 10.1016/j.urology.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 83.Manz F, Wentz A, Sichert-Hellert W. The most essential nutrient: defining the adequate intake of water. J Pediatr. 2002;141(4):587–592. doi: 10.1067/mpd.2002.128031. [DOI] [PubMed] [Google Scholar]
- 84.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane. http://www.training.cochrane.org/handbook [DOI] [PMC free article] [PubMed]
- 85.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75(3):561–569. doi: 10.1093/ajcn/75.3.561. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein SL. Oliguria in children. In: Vincent J-L, Hall JB, editors. Encyclopedia of intensive care medicine. Berlin: Springer; 2012. p. 1616. [Google Scholar]
- 87.Nath SK, Moinier B, Thuillier F, Rongier M, Desjeux JF. Urinary excretion of iodide and fluoride from supplemented food grade salt. Int J Vitam Nutr Res. 1992;62(1):66–72. [PubMed] [Google Scholar]
- 88.Knudsen N, Christiansen E, Brandt-Christensen M, Nygaard B, Perrild H. Age- and sex-adjusted iodine/creatinine ratio: A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur J Clin Nutr. 2000;54(4):361–363. doi: 10.1038/sj.ejcn.1600935. [DOI] [PubMed] [Google Scholar]
- 89.Konno N, Yuri K, Miura K, Kumagai M, Murakami S. Clinical evaluation of the iodide/creatinine ratio of casual urine samples as an index of daily iodide excretion in a population study. Endocr J. 1993;40(1):163–169. doi: 10.1507/endocrj.40.163. [DOI] [PubMed] [Google Scholar]
- 90.Edwards OM, Bayliss RIS, Millen S. Urinary creatinine excretion as an index of the completeness of 24-h urine collections. Lancet. 1969;294(7631):1165–1166. doi: 10.1016/s0140-6736(69)92488-x. [DOI] [PubMed] [Google Scholar]
- 91.Johner SA, Boeing H, Thamm M, Remer T. Urinary 24-h creatinine excretion in adults and its use as a simple tool for the estimation of daily urinary analyte excretion from analyte/creatinine ratios in populations. Eur J Clin Nutr. 2015;69(12):1336–1343. doi: 10.1038/ejcn.2015.121. [DOI] [PubMed] [Google Scholar]
- 92.Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, Dodd KW, Caldwell KL, Wang CY. Comparison of population iodine estimates from 24-h urine and timed-spot urine samples. Thyroid. 2014 doi: 10.1089/thy.2013.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vought RL, London WT, Lutwak L, Dublin TD. Reliability of estimates of serum inorganic iodine and daily fecal and urinary iodine excretion from single casual specimens. J Clin Endocrinol Metab. 1963;23:1218–1228. doi: 10.1210/jcem-23-12-1218. [DOI] [PubMed] [Google Scholar]
- 94.Frey HM, Rosenlund B, Torgersen JP. Value of single urine specimens in estimation of 24 hour urine iodine excretion. Acta Endocrinol (Copenh) 1973;72(2):287–292. doi: 10.1530/acta.0.0720287. [DOI] [PubMed] [Google Scholar]
- 95.Thomson CD, Smith TE, Butler KA, Packer MA. An evaluation of urinary measures of iodine and selenium status. J Trace Elem Med Biol. 1996;10(4):214–222. doi: 10.1016/S0946-672X(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 96.Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53(5):401–407. doi: 10.1038/sj.ejcn.1600762. [DOI] [PubMed] [Google Scholar]
- 97.Konig F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-h samples are needed to reliably estimate individual iodine status in women. J Nutr. 2011;141(11):2049–2054. doi: 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- 98.Als C, Helbling A, Peter K, Haldimann M, Zimmerli B, Gerber H. Urinary iodine concentration follows a circadian rhythm: a study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab. 2000;85(4):1367–1369. doi: 10.1210/jcem.85.4.6496. [DOI] [PubMed] [Google Scholar]
- 99.Ji C, Lu T, Dary O, Legetic B, Campbell NR, Cappuccio FP, PAHO-WHO Regional Expert Grp Systematic review of studies evaluating urinary iodine concentration as a predictor of 24-h urinary iodine excretion for estimating population iodine intake. Rev Panam Salud Publ. 2015;38(1):73–81. doi: 10.1016/s0022-3476(94)70025-7. [DOI] [PubMed] [Google Scholar]
- 100.Xu C, Guo X, Tang J, Guo X, Lu Z, Zhang J, Bi Z. Iodine nutritional status in the adult population of Shandong Province (China) prior to salt reduction program. Eur J Nutr. 2016;55(5):1933–1941. doi: 10.1007/s00394-015-1009-8. [DOI] [PubMed] [Google Scholar]
- 101.Edmonds JC, McLean RM, Williams SM, Skeaff SA. Urinary iodine concentration of New Zealand adults improves with mandatory fortification of bread with iodised salt but not to predicted levels. Eur J Nutr. 2016;55(3):1201–1212. doi: 10.1007/s00394-015-0933-y. [DOI] [PubMed] [Google Scholar]
- 102.Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, Tong N, Wang S, Weng J, Zhao J, Teng X, Yu X, Lai Y, Wang W, Li C, Mao J, Li Y, Fan C, Teng W. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in china: a cross-sectional study in 10 cities. Thyroid. 2016;26(8):1125–1130. doi: 10.1089/thy.2015.0613. [DOI] [PubMed] [Google Scholar]
- 103.Johner S, Thamm M, Schmitz R, Remer T. Examination of iodine status in the German population: an example for methodological pitfalls of the current approach of iodine status assessment. Eur J Nutr. 2016;55(3):1275–1282. doi: 10.1007/s00394-015-0941-y. [DOI] [PubMed] [Google Scholar]
- 104.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70(10):553–570. doi: 10.1111/j.1753-4887.2012.00528.x. [DOI] [PubMed] [Google Scholar]
- 105.Larsson G, Victor A. Micturition patterns in a healthy female population, studied with a frequency/volume chart. Scand J Urol Nephrol Suppl. 1988;114:53–57. [PubMed] [Google Scholar]
- 106.Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, Sempos CT, Gillespie CD, Burt VL. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-h calibration study. J Nutr. 2013;143(8):1276–1282. doi: 10.3945/jn.113.175927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Soldin OP. Controversies in urinary iodine determinations. Clin Biochem. 2002;35(8):575–579. doi: 10.1016/s0009-9120(02)00406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.