Abstract
Background
Depression is associated with insulin resistance (IR). However, the potential beneficial effect, on antidepressant treatment response, of adjunctive therapy with insulin sensitivity-enhancing lifestyle and dietary interventions (exercise; supplementation with: vitamin D, magnesium, zinc, probiotics or omega-3 fatty acids) has not been systematically explored.
Aims
To determine the effect of the above stated adjuncts on antidepressant treatment response in clinically depressed patients via a systematic review and meta-analysis.
Methods
RCTs which assessed the effect, on antidepressant treatment response of adjunctive therapy with any of the interventions in comparison with treatment as usual were included.
Results
The interventions had a significant antidepressant effect, with SMD for follow-up (end of study) scores and change (from baseline) scores being -0.88, [95% CI: -1.19 to -0.57; P < 0.001] and -1.98 [95% CI -2.86 to -1.10; P < 0.001], respectively. The odds ratio (OR) for remission was 2.28 (95% CI 1.42 to 3.66; P < 0.001). The number-needed-to-treat (NNT) for remission was 6. Subgroup analysis of the follow-up scores revealed age effect: SMD significant in those with mean age ≤50 (-1.02 SMD; 95% CI: -1.40 to -0.64; p < 0.001) and insignificant in those with mean age >50 (-0.38 SMD (95% CI: -0.82 to 0.05; P = 0.08)). Also, the interventions were more beneficial among outpatients- SMD: -0.97 (95% CI: -1.32 to -0.62; P < 0.001) compared to inpatients- SMD: -0.34 (95% CI: -0.88 to 0.20; P = 0.22). Sensitivity analysis did not change the results.
Conclusion
The finding that antidepressant treatment response may be improved using insulin sensitivity-enhancing lifestyle and dietary adjuncts is worthy of further exploration.
Keywords: Psychiatry, Biological psychiatry, Depression, Pharmacology, Endocrinology, Endocrine system, Adjuncts, Insulin sensitivity, Lifestyle, Dietary supplements, Antidepressants, Systematic review
Psychiatry; Biological Psychiatry; Depression; Pharmacology; Endocrinology; Endocrine System; depression; Adjuncts; Insulin sensitivity; Lifestyle; Dietary Supplements; Antidepressants; systematic review.
1. Introduction
Depression remains a major contributor to global disease burden due to the relatively large proportion of people (4.4% of world's population) affected and its association with morbidity and mortality (World Health Organisation, 2017).
Currently available antidepressants (mostly monoamine-based) are ineffective in up to 30% of depressed patients (Voineskos et al., 2020). Treatment in these patients is challenging and often involves diverse augmentation strategies using pharmacological agents (e.g. atypical antipsychotics, psychostimulants) and/or non-pharmacological interventions such as psychotherapy, aerobic exercise and neurostimulation (Voineskos et al., 2020). The mechanistic basis of antidepressant treatment resistance remains unclear.
Depression is a heterogeneous disease. Its pathophysiology is linked to metabolic and other distinct factors (Dean and Keshavan, 2017). Growing evidence suggests a bidirectional relationship between depression and Insulin resistance (IR). Potential mediators underpinning this bidirectional link include glutamate, brain derived neurotrophic factor and peroxisome proliferator-activated receptor gamma (Watson et al., 2018). Co-morbid depression and IR increases depression symptoms' severity, while also decreasing the effectiveness of antidepressants (Lin et al., 2015). In fact, research has shown antidepressant effects of insulin sensitizers (Colle et al., 2017), particularly when used as add-on therapy in patients with treatment resistant depression (TRD) (Lin et al., 2015; Rasgon et al., 2010).
We hypothesize that IR, which is often undiagnosed and hence uncorrected, may undermine conventional antidepressant treatment and that antidepressant effectiveness may be enhanced through correction of any underlying IR. IR can be corrected either pharmacologically or non-pharmacologically.
Some lifestyle and dietary-related adjuncts, which are known to enhance insulin sensitivity, have also been shown to enhance antidepressant treatment effectiveness. These include exercise (Bird and Hawley, 2017); supplementation with: zinc (Cruz et al., 2017; Islam et al., 2016), vitamin D (Seyyed Abootorabi et al., 2018; Wu et al., 2017), magnesium (Morais et al., 2017), probiotics (Ruan et al., 2015) and omega-3 polyunsaturated fatty acids (PUFA) (Imamura et al., 2016). A number of systematic reviews have also shown that these interventions possess antidepressant potential when used either singly or as add-on therapy in depression (Hallahan et al., 2016; Huang et al., 2016; Lai et al., 2012; Schuch et al., 2016; Serefko et al., 2016; Spedding, 2014).
In spite of their common ability to enhance insulin sensitivity, to date, no systematic review has collectively evaluated their capacity to enhance antidepressant treatment response in clinically depressed populations. Therefore, the aim of this study was to determine the effect of the above-stated interventions (vs treatment as usual with or without placebo) on antidepressant treatment response (primary outcomes) through synthesis of the available clinical evidence (from randomized controlled trials involving patients with depression) and meta-analysis of relevant data. We also set out to assess the effect of these interventions on parameters of insulin sensitivity (secondary outcomes) in the same population as with the primary outcomes, where reported with a view to examining the association between their potential to correct insulin resistance and their antidepressant effectiveness-enhancing potential.
2. Methods
The protocol for this systematic review has already been published (Jeremiah et al., 2019).
2.1. Search strategy
The search was conducted on the following databases and trials registers: Cochrane central register of controlled trials (CENTRAL), MEDLINE PubMed, Embase, PsychINFO, ClinicalTrials.gov and EU clinical trials register. The search was first conducted in March 2019, initially targeting publication dates from January, 1990 to December, 2018. An update search was later conducted on 6th July, 2020; this was designed to capture any relevant studies with publication dates from January 2019 up to 5th July, 2020. Only studies published in English language were considered. The search was based on two main concepts: the disease in question (depression) and the different interventions of interest, as detailed in the previously published study protocol (Jeremiah et al., 2019). Reference list of selected studies were also searched to identify any other relevant articles or systematic reviews. Additionally, unpublished data were traced from relevant conference abstracts/proceedings and clinical trials registers.
2.2. Eligibility criteria
Randomised controlled trials investigating the effect of any of the following insulin sensitivity-enhancing lifestyle and dietary-related adjuncts on depression: exercise, vitamin-D, zinc, magnesium, probiotics, omega-3 polyunsaturated fatty acids (PUFA) and other hygienic dietary recommendations. To be included in this review, RCTs had to include adults (aged ≥18 years) with a diagnosis of major depressive disorder (MDD), persistent depressive disorder (PDD)/dysthymia or bipolar disorder (BD), based on standard diagnostic tools pre-specified in the protocol, and experiencing a depressive episode at baseline. Additionally, the intervention had to be used adjunctively, for at least 3 weeks, with any of the conventional antidepressants highlighted a priori: monoamine oxidase inhibitors, tricyclic antidepressants, selective serotonin reuptake inhibitors, noradrenaline reuptake inhibitors, serotonin-noradrenaline reuptake inhibitors and miscellaneous standard antidepressants, e.g. mirtazapine; any of these plus any mood stabiliser in BD. Exclusion criteria include: use of other psychotropic medications (except anxiolytics) apart from antidepressants; other neuropsychiatric comorbidity or any medical comorbidity which could impact the results. For trials which assessed the relevant outcomes at different time points, the data at the last time point was taken as the follow-up (end-of-study) data. For studies involving more than two arms, we only included the two arms relevant to our study. In other cases in which there were more than one intervention group of interest, the different intervention arms (vs control) were included in the meta-analyses as separate studies.
2.3. Outcomes
The primary outcomes were: a) Depression scores at follow-up (end of study) and change (from baseline to follow-up) scores, as measured on a continuous scale using standard depression scores; b) remission, measured as a binary outcome in terms of the number or proportion of participants who attained remission at the end of the study. The standard depression rating scales considered were: Hamilton Rating Scale for Depression (HAM-D or HRSD), Beck Depression Inventory (BDI), Inventory of Depressive Symptomatology (IDS) or Montgomery-Asberg Depression Rating Scale (MADRS) (Cusin et al., 2009). None of the included studies reported the secondary outcomes of interest (parameters of insulin sensitivity) described in the published protocol (Jeremiah et al., 2019).
2.4. Data collection
Records retrieved from electronic and other searches were managed as previously described. The data extraction form was developed to capture the following: general information (e.g. the study ID, study location, study setting, sources of funding, possible conflicts of interest, etc.), study and participant characteristics (e.g. type of study, mean age/age range, sex, primary diagnosis, diagnostic system, sample size, severity of depression at baseline, antidepressants in use, comorbidities and other psychotropics), details of intervention (type, dose/frequency and duration of intervention), attrition details (total randomized, number excluded or lost to follow-up, number analysed) and measures of outcomes (mean baseline and follow-up depression scores and/or mean change and standard deviation (SD) depression scores for continuous data or number and percentage in remission for binary outcome measure). Where depression was assessed using more than one scale, we preferably chose the Hamilton Depression Rating scale (HDRS/HAM-D) for clinician-administered scales and the Beck Depression Inventory (BDI) for self-rating questionnaires.
2.5. Quality assessment
Risk of bias assessment of the included studies was carried out using the ‘risk of bias’ assessment tool described in the Cochrane handbook for systematic reviews of interventions (Higgins and Green, 2008). Based on the adapted Cochrane ‘risk of bias’ table used, trials assessed as having low-risk of bias across all the domains were considered in the overall rating as having low-risk of bias, trials with unclear risk of bias in one or up to three of the domains were considered to be of moderate risk of bias while those with high risk of bias in one or more of the domains as well as those with unclear risk of bias in four or more of the domains were rated as having high risk of bias. The quality of evidence across the included studies was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (Ryan and Hill, 2016).
2.6. Statistical analysis
Data synthesis and analysis were carried out for the primary outcomes (effect on depression symptomatology). Random-effects meta-analysis model was used to obtain the summary effect estimate, 95% confidence interval and P-value, using Review Manager (RevMan) software, version 5.3. Due to the nature of the data obtained, the continuous variable (depression scores) was assessed by carrying out separate meta-analyses for both the follow-up scores and mean change scores. Variation in the depression rating scales used in the included trials was factored into the analyses via estimation of the standardized mean difference (SMD) for each included study. In clinical studies, SMD values of 0.2, 0.5 and 0.8 are taken as small, medium and large effect sizes, respectively (Faraone, 2008). A pooled odds ratio were estimated for remission.
Heterogeneity between studies was explored by visual inspection of the forest plots and quantified using I2 statistic. For studies in which the data reported was not sufficient to enable us read or calculate the standard deviations of the follow-up and/or mean change scores, the corresponding authors were contacted. In cases where the data were presented in graphic format, approximate mean values were imputed from the graphs. For the analysis of the follow-up scores, in instances where missing data were not obtained from authors or through imputation from graphs, estimation of SDs for the affected studies was done by imputing the baseline SD of the same study (n = 1) or borrowing SDs (n = 2) from the most similar study (e.g. in terms of rating scale, duration and/or intervention type) included in the meta-analysis (Higgins and Green, 2008). A sensitivity analysis was subsequently carried out excluding studies with incomplete data. For mean change scores, only studies with complete data were included in the meta-analysis. As per our protocol, a sensitivity analysis was also carried out by excluding studies with high risk of bias. In the analysis of remission data, the effect size was further quantified by calculating the number needed to treat (NNT) using the formula: NNT = 1 ÷ (remission rate with intervention – remission rate with control). Funnel plots were used to assess the risk of publication bias. These were assessed for asymmetry by both visual inspection and Egger's regression test (Jakobsen et al., 2014), using the STATA software.
2.7. Subgroup analysis
As described a priori and subject to data availability, subgroup analyses were performed based on the duration of studies and intervention types, thus: short duration (≤10 weeks) versus long duration (>10 weeks) studies; exercise only, Omega-3 PUFA only, zinc only, probiotic only and vitamin D only subgroups. Only one study assessed magnesium supplementation, hence, subgroup meta-analysis was not possible in this case. However, for completeness, the ‘magnesium study’ was retained on the forest plot of the overall subgroup analysis of all the included studies. We also carried out some additional subgroup analyses that were not pre-specified in the protocol. These include subgroup analysis based on patient setting (outpatient vs inpatient) and age (mean age ≤50 vs mean age >50), due to potential differences across settings and age. Subgroup analysis based on medication type [SSRI(s) only vs SSRI or ≥1 more class (TCA, SNRI, NRI, NaSSA, others) vs TCA only vs Antidepressant (not specified)] was also carried out, since varied classes of antidepressant drugs were used across the included studies.
2.8. Deviations from the protocol
In our search strategy, the publication date was capped to July 2020 as opposed to November 2018 stated in the protocol. This was to allow us capture all studies published up to July 2020 and indexed in the relevant databases. Also, contrary to what we had stated in the protocol, we included a trial (Belvederi et al., 2015) which featured participants with other medical co-morbidities (stable cases of hypertension, musculoskeletal problems, eye, ear, nose and throat-related conditions) apart from metabolic disorders. This was based on the conviction that these co-morbidities are not likely to affect the effect of the intervention (exercise) as it was stated by the authors that those with severe or unstable physical illness that would prevent exercise were excluded. Trials (Belvederi et al., 2015; Carneiro et al., 2015; Danielsson et al., 2014; Lavretsky et al., 2011; Mota-Pereira et al., 2011; Ryszewska-Pokrasniewicz et al., 2018) which allowed the use of other reported psychotropics (anxiolytics/sedatives/hypnotics), apart from antidepressant, were also included. Moreover, we also included two trials (Ho et al., 2014; Shachar-Malach et al., 2015) with a study duration of 3 weeks as opposed to the minimum of 4 weeks stated in the protocol. Sensitivity analysis was carried out in each case excluding the affected studies, the results of the analysis did not change. Additionally, three subgroup analyses carried out based on a) Patient setting (outpatient vs inpatient), b) Age (mean age ≤50 vs mean age >50) and c) medication type [SSRI(s) only vs SSRI or ≥1 more class (TCA, SNRI, NRI, NaSSA, others) vs TCA only vs Antidepressant (not specified)] were not pre-specified in the protocol. This was to ensure exploration of all the possible reasons (as much as permitted by the available data) behind the observed substantial heterogeneity.
3. Results
3.1. Study identification and trial characteristics
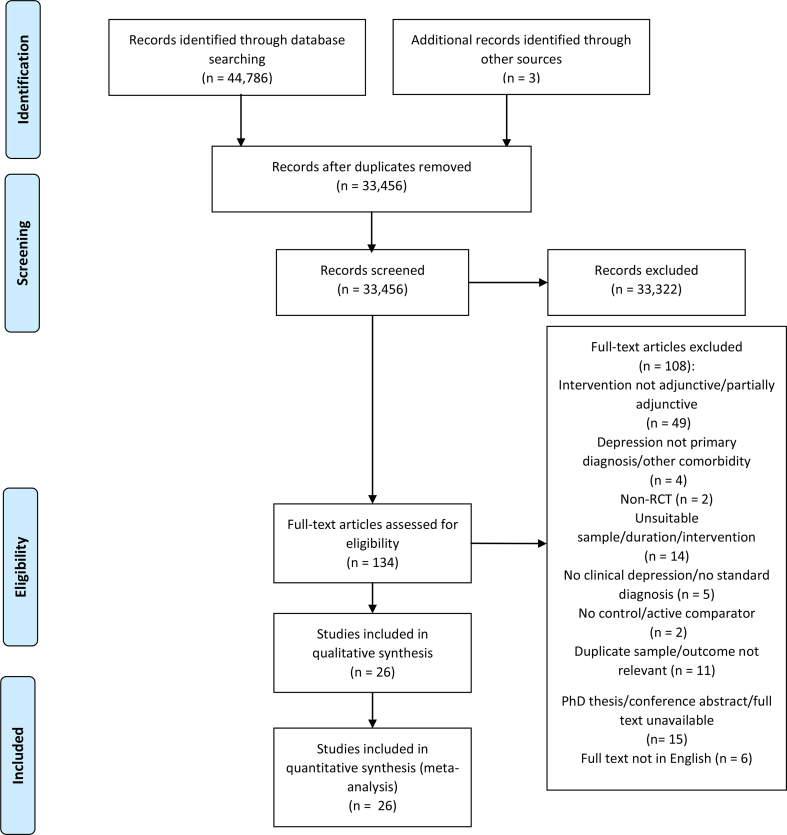
Our electronic and manual search yielded 33,456 citations (after removing duplicates) for title and abstract screening. From these, 134 full text articles were assessed for eligibility, 26 studies were considered eligible for both qualitative & quantitative analyses. A study (Nemets et al., 2002), which included patients (n = 3) with unacceptable comorbidity and reported the raw data, was only included in our analysis after excluding the data belonging to the affected participants. Details of the study selection process are presented in Figure 1, adapted from Moher et al. (2009).
Figure 1.
Flow diagram of study selection process.
The characteristics of the included studies are summarised in Table 1. All were published between 1999 and 2018. Of the twenty-six included studies, seven were conducted in Iran (Jahangard et al., 2018; Jazayeri et al., 2008; Khoraminya et al., 2013; Mozaffari-Khosravi et al., 2013; Nazarinasab et al., 2017; Ranjbar et al., 2013; Zeinab et al., 2018), four in Poland (Nowak et al., 2003; Rudzki et al., 2018; Ryszewska-Pokrasniewicz et al., 2018; Siwek et al., 2009), three in USA (Blumenthal et al., 1999; Gertsik et al., 2012; Lavretsky et al., 2011), two in Israel (Nemets et al., 2002; Shachar-Malach et al., 2015), two in Italy (Belvederi et al., 2015; Pilu et al., 2007), two in Portugal (Carneiro et al., 2015; Mota-Pereira et al., 2011), two in the UK (Mather et al., 2002; Peet and Horrobin, 2002) while the remaining four were conducted in Brazil (Siqueira et al., 2016), Hong-Kong (Ho et al., 2014), Japan (Miyaoka et al., 2018) and Sweden (Danielsson et al., 2014).
Table 1.
Characteristics of included studies.
| Author, (year) | Country | Participants Mean age (SD)/Age range (yrs.) % female |
Severity of depression at baseline | N at baseline (N analyzed) | Conventional Antidepressant at standard dosage regimen (TAU) | Type of intervention (Adjunctive to TAU); Control | Frequency/Dose/Other details | Duration |
|---|---|---|---|---|---|---|---|---|
| Belvederi et al. (2015) | Italy | Outpatients Mean age: 75 (6) 72.6% female |
HAM-D ≥ 18 | 84 (84) | Sertraline | Supervised group progressive exercise; TAU |
Three 60-min sessions per week | 24 weeks |
| Blumenthal et al. (1999) | USA | Outpatients Mean age: 57 (6.5) 71.8% female |
HAM-D ≥ 13 | 103 (103) | Sertraline | Supervised aerobic exercise; TAU |
Three exercise sessions per week | 16 weeks |
| Carneiro et al. (2015) | Portugal | Outpatients Mean age: 50.2 (12.1) 100% female |
Mean BDI score = 45.83 | 26 (19) | SSRIs (Fluoxetine, escitalopram, sertraline, paroxetine) | Supervised aerobic exercise; TAU |
45–50 min/week, three times a week | 24 weeks |
| Danielsson et al. (2014) | Sweden | Outpatients Mean age: 45.5 76.2% female |
Mean MADRS score = 24 | 42 (42) | TCAs (clomipramine), SSRIs (sertraline, fluoxetine), SNRI (venlafaxine), others (mirtazapine, bupropion) | Person-centered aerobic exercise; TAU |
Two individual sessions (first 2 weeks), followed by 8 weeks of two group weekly sessions (1 h per session) | 10 weeks |
| Gertsik et al. (2012) | USA | Outpatients Mean age: 40.5 (10.2) No detail of % female |
HAM-D21 ≥ 17 | 42 (40): modified ITT | Citalopram | Omega-3 Polyunsaturated fatty acids (PUFA) supplementation; TAU |
2 capsules (each containing 450 mg EPA, 100 mg DHA & 50 mg other omega-3 fatty acids) twice daily. | 8 weeks |
| Ho et al. (2014) | Hong kong | Inpatients Mean age: 46.2 67.3% female |
BDI ≥9 | 52 (52) | Not specified. ‘…antidepressant’ |
Supervised aerobic exercise; TAU |
30 min per session, five times a week. | 3 weeks |
| Jahangard et al. (2018) | Iran | Outpatients Mean age: 42.46 32% female |
Mean BDI score = 35.9 | 50 (50) | Sertraline | Omega-3 PUFA; TAU |
1 capsule (1000 mg) per day. | 12 weeks |
| Jazayeri et al. (2008) | Iran | Outpatients Mean age: 34.8 65.6% female |
HAM-D ≥ 15 | 40 (32) | Fluoxetine | Omega-3 PUFA (EPA); TAU |
Two ethyl EPA soft gels (1000 mg EPA) daily. | 8 weeks |
| Khoraminya et al. (2013) | Iran | Outpatients Mean age: 38.88 85% female |
HAM-D ≥ 15 | 42 (40) | Fluoxetine | Vitamin D supplementation; Placebo + TAU |
1500 IU/day | 8 weeks |
| Lavretsky et al. (2011) | USA | Outpatients Mean age: 70.55 61.6% female |
HAM-D24 ≥ 16 | 73 (73) | Escitalopram | Tai Chi Chih (Mind body exercise); TAU | One 2-hr session per week. | 10 weeks |
| Mather et al. (2002) | Scotland | Outpatients Mean age: 64.95 68.6% female |
Mean HAM-D score = 17.05 | 86 (85) | Not specified. ‘…antidepressant therapy’ |
Weight-bearing exercises; TAU |
Two 45-min classes per week. | 10 weeks |
| Miyaoka et al. (2018) | Japan | Outpatients Mean age: 43.05 52% female |
HAM-D ≥ 16 | 40 (40) | SSRIs (fluvoxamine, paroxetine, escitalopram, sertraline, duloxetine), SNRI (milnacipran). | Probiotic-CBM588; TAU |
Week 1: 20 mg twice (40 mg) daily; Weeks 2–8: 20 mg three times (60 mg) daily. |
8 weeks |
| Mota-Pereira et al. (2011) | Portugal | Outpatients Mean age: 47.01 65.5% female |
Mean HAM-D score = 16.16 | 33 (29) | TCAs (clomipramine, maprotiline, amitryptiline), SSRIs (fluoxetine, escitalopram, paroxetine, sertraline), SNRI (venlafaxine). | Exercise; TAU |
Five (One supervised) 30-40-min daily walk per week. | 12 weeks |
| Mozaffari-Khosravi et al. (2013) | Iran | Outpatients Mean age: 35.1 61.3% female |
Mean HAM-D score = 15.7 | 81 (62): Modified ITT |
Tricyclics, Bupropion, MAOIs, SSRIs. | Omega-3 PUFA: a. EPA b. DHA; TAU |
a. EPA- 1 g/day b. DHA- 1 g/day |
12 weeks |
| Nazarinasab et al. (2017) | Iran | Inpatients Mean age: 38.54 (0.86) 63.8% female |
BDI >13 | 58 (58) | SSRIs (citalopram, sertraline). | Zinc (sulphate) supplementation; Placebo + TAU |
25 mg daily | 8 weeks |
| Nemets et al. (2002) | Isreal | Unclear whether inpatients or outpatients Mean age: 56 (10.1) 88.2% female |
HAM-D24 ≥ 18 | 17 (17) [ITT, after excluding 3 participants] | SSRIs- Paroxetine, Fluoxetine, Fluvoxamine & Citalopram; ∗NaSSA- Mirtazapine. | Omega-3 PUFA (E-EPA); TAU |
1 g twice (2 g) daily. | 4 weeks |
| Nowak et al. (2003) | Poland | Unclear whether inpatients or outpatients Mean age: 42.8 57.1% female |
Mean HAM-D score = 23.55 | 20 (14) | TCAs (clomipramine, amitriptyline), SSRIs (citalopram, fluoxetine). | Zinc supplementation; Placebo + TAU | 25 mg daily | 12 weeks |
| Peet and Horrobin (2002) | England | Outpatients Mean age: 44.75 84.3% female |
HAM-D ≥ 15 | 70 (69) | TCAs, SSRIs, Other (NRI/SNRI). | Omega-3 PUFA (pure Ethyl EPA); TAU |
Ethyl EPA a.1 g/day b. 2 g/day c. 4 g/day |
12 weeks |
| Pilu et al. (2007) | Italy | Outpatients Age range: 40 -60 100% female |
HAM-D > 13 | 30 (30) | SSRI, SNRI, NARI, TCA. | Physical activity; TAU |
Two 60-min sessions per week | 32 weeks |
| Ranjbar et al. (2013) | Iran | Outpatients Mean age: 37.25 89.5% female |
Mean BDI score = 29.15 | 44 (38) | SSRIs (citalopram, fluoxetine) | Zinc supplementation; Placebo + TAU |
25 mg daily | 12 weeks |
| Rudzki et al. (2018) | Poland | Outpatients Mean age: 39.02 71.7% female |
Mean HAM-D score = 21.77 | 79 (60) | SSRIs (Escitalopram, sertraline, paroxetine, fluoxetine); TAU |
Probiotic- Lactobacillus plantarum 299v; TAU |
1 capsule, morning and night. | 8 weeks |
| Ryszewska-Pokrasniewicz et al. (2018) | Poland | Inpatients Mean age: 48.9 56.8% female |
Mean HAM-D 21 score = 29 | 37 (32) | Fluoxetine; TAU |
Magnesium supplementation; TAU |
Mg- 40 mg three times daily. | 8 weeks |
| Shachar-Malach et al. (2015) | Israel | Inpatients Mean age: 43.34 75% female |
HAM-D21 > 14 | 12 (12) | “…antidepressant medication according to usual clinical practice…” | Aerobic exercise; Stretching exercise + TAU |
4 sessions/week of 30 min walking on a treadmill at moderate intensity. | 3 weeks |
| Siqueira et al. (2016) | Brazil | Outpatients Mean age: 38.83 (10.72) 71.9% female |
HAM-D ≥ 15 | 57 (57) | Sertraline | Aerobic exercise (individualized & supervised); TAU. |
Four (continuous & intermittent) sessions/week | 4 weeks |
| Siwek et al. (2009) | Poland | Inpatients & outpatients Mean age: 45.95 66.7% female |
HAM-D score = 22.9 (3.3) | 60 (52) | Imipramine | Zinc supplementation; Placebo + TAU |
25 mg daily | 12 weeks |
| Zeinab et al. (2018) | Iran | Outpatients Mean age: 36.3 (10.4) 100% female |
Mean BDI score = 38.9 | 26 (26) | SSRIs (Fluoxetine, Sertraline, Citalopram) | Vitamin D supplementation; TAU |
50,000 IU weekly | 8 weeks |
Key: TAU- Treatment as usual, SSRIs- Selective serotonin reuptake inhibitors, SNRI- Serotonin-norepinephrine reuptake inhibitor, NRI- Norepinephrine reuptake inhibitor, NaSSA-Noradrenergic and specific serotonergic antidepressant, TCAs- Tricyclic antidepressants, MAOIs- Monoamine oxidase inhibitors, EPA- Eicosapentaenoic acid, DHA- Docosahexaenoic acid, PUFA- Polyunsaturated fatty acids, HAM-D- Hamilton depression rating scale, BDI- Beck's depression inventory, MADRS- Montgomery-asberg depression rating scale.
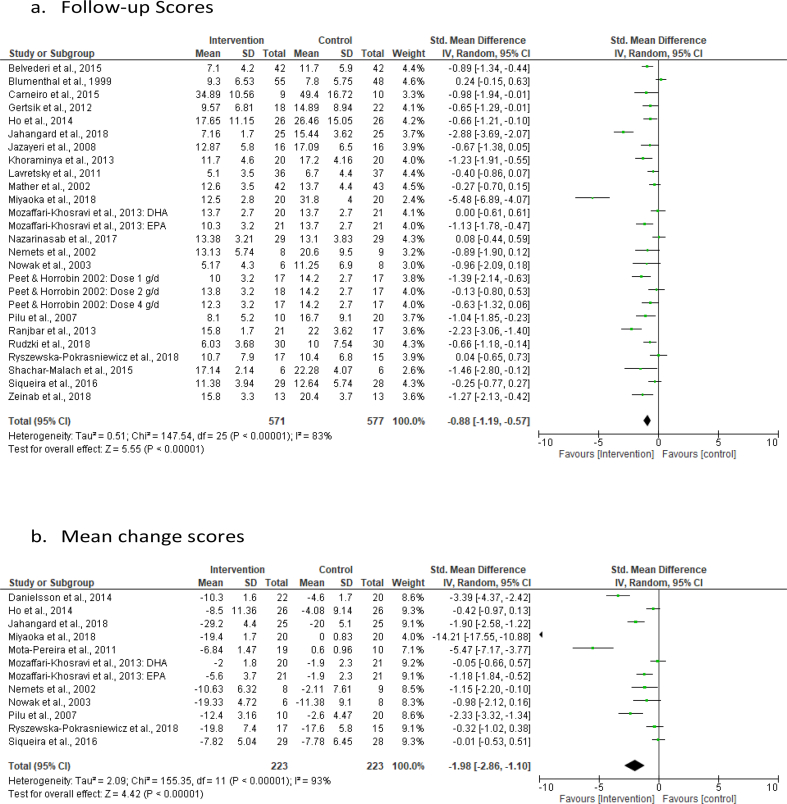
Of a total of 1,304 clinically depressed patients randomized, 1,216 (639 in intervention group, 577 in control), with mean age ranging from 34.8 to 75.0 years, were analysed for intervention effectiveness and hence included in the quantitative analysis. Twenty-three of a total of twenty-six eligible studies were included in the analysis of follow-up depression scores, two (Danielsson et al., 2014; Mota-Pereira et al., 2011) of the remaining three studies only featured in the analysis of the mean change scores while the last one (Siwek et al., 2009) was included only in the third analysis for remission data. In the first meta-analysis (using the follow-up scores), 1093 (571 in intervention group, 522 in control) participants, represented in the forest plot of results as 1148 (571 in intervention arm, 577 in control arm) (see Figure 2a), were included. The discrepancy in the actual number of participants included and that represented in the results is due to the use of repeat control (55 participants) groups for studies with more than one intervention (Mozaffari-Khosravi et al., 2013) or intervention dose (Peet and Horrobin, 2002) of interest. In the second meta-analysis (using the mean change scores), 425 (223 in intervention arm, 202 in control) represented as 446 (223 in both arms) (see Figure 2b), for same reason as above, were included. Moreover, eleven studies, with a total of 559 participants (288 in intervention arm, 271 in control) and which reported remission as a binary outcome, were included in the meta-analysis of remission data.
Figure 2.
Forest plot of the post intervention SMD of Follow-up scores (a) and Mean change scores (b) for intervention vs control. Meta-analysis for odds ratio (OR) of binary outcome (remission) data.
The main diagnosis in all the studies was MDD with one study (Miyaoka et al., 2018) reporting a diagnosis of treatment resistant depression as the specific MDD category for inclusion. The intervention in eleven (Belvederi et al., 2015; Blumenthal et al., 1999; Carneiro et al., 2015; Danielsson et al., 2014; Ho et al., 2014; Lavretsky et al., 2011; Mather et al., 2002; Mota-Pereira et al., 2011; Pilu et al., 2007; Shachar-Malach et al., 2015; Siqueira et al., 2016) of the included trials was adjunctive exercise while omega-3 PUFA was employed in six (Gertsik et al., 2012; Jahangard et al., 2018; Jazayeri et al., 2008; Mozaffari-Khosravi et al., 2013; Nemets et al., 2002; Peet and Horrobin, 2002), zinc supplementation in four (Nazarinasab et al., 2017; Nowak et al., 2003; Ranjbar et al., 2013; Siwek et al., 2009), probiotics in two (Miyaoka et al., 2018; Rudzki et al., 2018), vitamin D supplementation in two (Khoraminya et al., 2013; Zeinab et al., 2018) and magnesium supplementation in one (Ryszewska-Pokrasniewicz et al., 2018) of the trials.
3.2. Risk of bias assessment
Summary of the risk of bias assessment is presented in Table 2. In the overall classification of the studies, based on our earlier defined criteria, two studies (Mozaffari-Khosravi et al., 2013; Peet and Horrobin, 2002) were completely adequate across all the domains (low risk of bias), nine (Ho et al., 2014; Jahangard et al., 2018; Khoraminya et al., 2013; Lavretsky et al., 2011; Mather et al., 2002; Nazarinasab et al., 2017; Nemets et al., 2002; Shachar-Malach et al., 2015; Siwek et al., 2009) were classified as having moderate risk of bias and the remaining fifteen (Belvederi et al., 2015, Blumenthal et al., 1999, Carneiro et al., 2015, Danielsson et al., 2014, Gertsik et al., 2012, Jazayeri et al., 2008, Miyaoka et al., 2018, Mota-Pereira et al., 2011, Nowak et al., 2003, Pilu et al., 2007, Ranjbar et al., 2013, Rudzki et al., 2018, Ryszewska-Pokrasniewicz et al., 2018, Siqueira et al., 2016 (Zeinab et al., 2018),) as having high risk of bias. Worthy of note is the fact that almost half (seven) of the studies classified as having high risk of bias are those in which the intervention assessed was adjunctive exercise. This is unsurprising as it is difficult to blind participants and investigators to this intervention, hence the high risk of bias in this domain.
Table 2.
Summary of risk of bias assessment of included studies.
| Author (year) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessors | Incomplete outcome data | Selective reporting | Other bias | Notes on other bias |
|---|---|---|---|---|---|---|---|---|
| Belvederi et al. (2015) | Low | Low | High | Low | Low | Low | Low | |
| Blumenthal et al. (1999) | Unclear | Unclear | High | Low | Low | Low | Low | |
| Carneiro et al. (2015) | Low | Low | High | High | High | Low | Low | |
| Danielsson et al. (2014) | Low | Low | High | Low | High | Low | Low | |
| Gertsik et al. (2012) | Unclear | Unclear | High | High | Low | Low | Low | |
| Ho et al. (2014) | Low | Low | Unclear | Low | Low | Low | Low | |
| Jahangard et al. (2018) | Low | Unclear | Low | Low | Low | Low | Low | |
| Jazayeri et al. (2008) | Low | Unclear | Low | Low | High | Low | Low | |
| Khoraminya et al. (2013) | Unclear | Unclear | Low | Unclear | Low | Low | Low | |
| Lavretsky et al. (2011) | Low | Low | Unclear | Low | Low | Low | Low | |
| Mather et al. (2002) | Low | Low | Unclear | Low | Low | Low | Low | |
| Miyaoka et al. (2018) | Low | High | High | High | Low | Low | Low | |
| Mota-Pereira et al. (2011) | Low | Unclear | Unclear | Low | Unclear | Low | High | Baseline difference in depression severity |
| Mozaffari-Khosravi et al. (2013) | Low | Low | Low | Low | Low | Low | Low | |
| Nazarinasab et al. (2017) | Low | Low | Low | Unclear | Low | Low | Low | |
| Nemets et al. (2002) | Low | Unclear | Low | Low | Low | Low | Low | |
| Nowak et al. (2003) | Unclear | Unclear | Low | Unclear | High | Low | Low | |
| Peet and Horrobin (2002) | Low | Low | Low | Low | Low | Low | Low | |
| Pilu et al. (2007) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | |
| Ranjbar et al. (2013) | Unclear | Unclear | Low | Low | High | Low | Low | |
| Rudzki et al. (2018) | Low | Low | Low | Low | High | Low | Low | |
| Ryszewska-Pokrasniewicz et al. (2018) | Unclear | Unclear | Low | Low | High | Low | Low | |
| Shachar-Malach et al. (2015) | Low | Unclear | Low | Low | Low | Low | Unclear | A trend towards difference in some baseline characteristics. |
| Siqueira et al. (2016) | Low | Low | High | Low | Low | Low | Low | |
| Siwek et al. (2009) | Low | Unclear | Low | Low | Unclear | Low | Low | |
| Zeinab et al. (2018) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low |
3.3. The effect of the interventions on depression scores
Twenty-three out of the twenty-six trials were included in the meta-analysis of the follow-up depression scores to determine the effect of the interventions of interest on antidepressant treatment response. There was a significant effect of the interventions versus control, the SMD being -0.88 [95% CI -1.19 to -0.57] (Figure 2a). Eleven studies were included in the meta-analysis of the mean change depression scores and the effect estimate was also large and significant, the SMD being -1.98 [95% CI -2.86 to -1.10] (Figure 2b).
3.4. Heterogeneity; sensitivity and subgroup analyses
The heterogeneity between studies was substantial, I2 = 83%, for follow-up scores and I2 = 93%, for mean change scores. Sensitivity analysis of the follow-up scores carried out by excluding studies with high risk of bias revealed similar results (-0.78 SMD; 95% CI -1.18 to -0.39; I2 = 79%). Also, similar results (-0.72 SMD; -1.03 to -0.42; I2 = 77%) were obtained in the sensitivity analysis of the follow-up scores based on data completeness in which studies with borrowed SDs were excluded. Similarly, in the analysis of the mean change scores, the effect estimate remains significant (-0.92 SMD; -1.59 to -0.24; I2 = 79%) after excluding studies with high risk of bias.
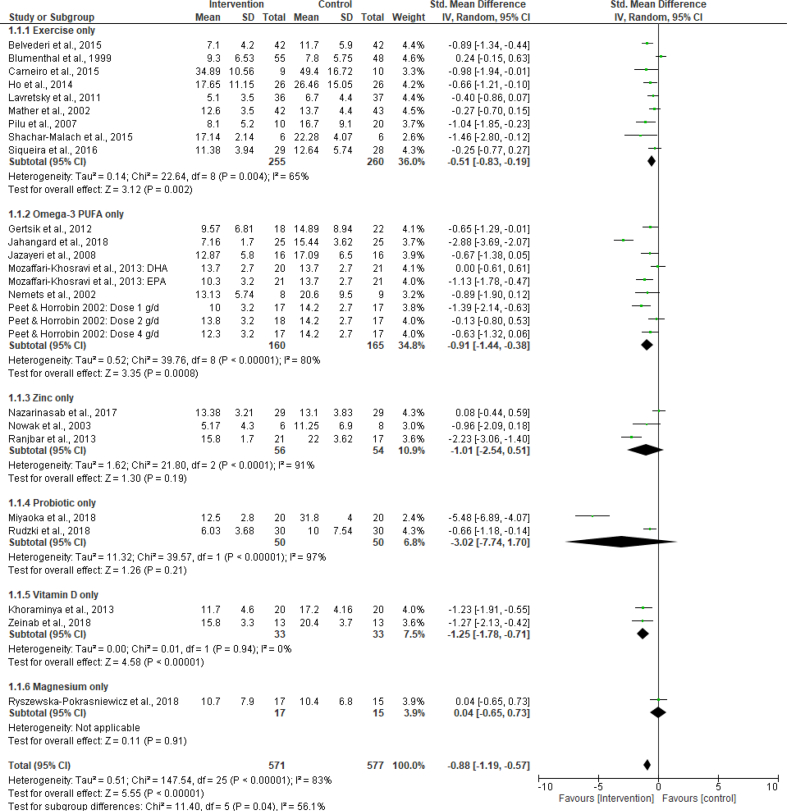
The results of the main subgroup analyses carried out are summarised in Table 3. See supplementary materials for the results of the subgroup analysis based on medication type. In the subgroup analysis of the follow-up scores based on study duration (short duration- ≤ 10 weeks vs long duration- > 10 weeks), no significant difference was found between the subgroups; test for subgroup difference: p = 0.61, I2 = 0%. The same applies to the subgroup analysis based on medication type (see supplementary material). On the other hand, the subgroup analysis, based on intervention type (exercise only vs omega-3 PUFA only vs zinc only vs probiotic only vs vitamin D only, while also retaining the ‘magnesium’ study), showed a significant difference between the subgroups; test for subgroup difference: p = 0.04, I2 = 56.1% (Figure 3). Similarly, the pooled effect estimate of trials with participants whose mean age was ≤50 years (-1.02 SMD; 95% CI -1.40 to -0.64; I2 = 83%) was found to be significantly different from that of trials with participants aged over 50 years (-0.38 SMD (95% CI -0.82 to 0.05; I2 = 74%)); test for subgroup difference-p = 0.03; I2 = 78.5%. In the subgroup analysis based on patient setting (outpatient vs inpatient), there was a significant difference (test for subgroup difference-p ≤ 0.001; I2 = 91.4 %) between outpatient and inpatient in the analysis of the mean change scores. This difference only tended towards statistical significance with follow-up scores data (test for subgroup difference-p = 0.05; I2 = 72.9%).
Table 3.
Sensitivity/Subgroup meta-analysis of continuous data (follow-up and mean change scores).
| Analysis | Number of Trials | Meta-analysis |
Heterogeneity-I2 (%) | |||
|---|---|---|---|---|---|---|
| SMD |
95% CI |
P value |
||||
| a. Follow-up scores | ||||||
| Main analysis | 26 | -0.88 | -1.19 | -0.57 | <0.001 | 83 |
|
Sensitivity Analysis | ||||||
| Analysis after excluding trials with high risk of bias | 13 | -0.78 | -1.18 | -0.39 | <0.001 | 79 |
| Test for subgroup difference | df = 1 | 0.74 | 0 | |||
| Analysis after excluding trials with borrowed SD | 19 | -0.72 | -1.03 | -0.42 | <0.001 | 77 |
| Test for subgroup difference (vs main analysis) | df = 1 | 0.48 | 0 | |||
|
Subgroup Analysis | ||||||
| Study duration | ||||||
| Short duration (≤10 weeks) | 14 | -0.80 | -1.20 | -0.40 | <0.001 | 81 |
| Long duration (>10 weeks) | 12 | -0.97 | -1.48 | -0.45 | <0.001 | 86 |
| Test for subgroup difference | df = 1 | 0.61 | 0 | |||
| Intervention type | ||||||
| Exercise | 9 | -0.51 | -0.83 | -0.19 | 0.002 | 65 |
| Omega-3 PUFA | 9 | -0.91 | -1.44 | -0.38 | ≤0.001 | 80 |
| Zinc | 3 | -1.01 | -2.54 | 0.51 | 0.19 | 91 |
| Probiotic | 2 | -3.02 | -7.74 | 1.70 | 0.21 | 97 |
| Vitamin D | 2 | -1.25 | -1.78 | -0.71 | <0.001 | 0 |
| Magnesium | 1 | 0.04 | -0.65 | 0.73 | 0.91 | - |
| Test for subgroup difference | df = 5 | ∗0.04 | 56.1 | |||
| Age (≤50 vs > 50) (yrs.) | ||||||
| ≤50 | 21 | -1.02 | -1.40 | -0.64 | <0.001 | 83 |
| >50 | 5 | -0.38 | -0.82 | 0.05 | 0.08 | 74 |
| Test for subgroup difference | df = 1 | ∗0.03 | ∗78.5 | |||
| Patient setting (Outpatients vs Inpatients) | ||||||
| Outpatients | 22 | -0.97 | -1.32 | -0.62 | <0.001 | 84 |
| Inpatients | 4 | -0.34 | -0.88 | 0.20 | 0.22 | 59 |
| Test for subgroup difference | df = 1 | 0.05 | 72.9 | |||
|
b. Mean change scores | ||||||
| Main analysis | 12 | -1.98 | -2.86 | -1.10 | <0.001 | 93 |
|
Sensitivity Analysis | ||||||
| Analysis after excluding trials with high risk of bias | 5 | -0.92 | -1.59 | -0.24 | 0.008 | 79 |
| Test for subgroup difference | df = 1 | 0.06 | 72.0 | |||
|
Subgroup Analysis | ||||||
| Patient setting (Outpatients vs Inpatients) | ||||||
| Outpatients | 10 | -2.44 | -3.55 | -1.34 | <0.001 | 94 |
| Inpatients | 2 | -0.38 | -0.82 | -0.05 | 0.08 | 0 |
| Test for subgroup difference | df = 1 | ∗≤ 0.001 | ∗91.4 | |||
Key: PUFA- Polyunsaturated fatty acids, SMD- Standardized mean difference, CI- Confidence interval, df - degree of freedom, SD- Standard deviation; ∗ indicates significant subgroup difference.
Figure 3.
Forest plot of post intervention SMD of Follow-up scores for intervention vs control: Subgroup analysis.
These results suggest that the substantial heterogeneity observed in this study may be partly due to differences in intervention type, age and patient setting.
3.5. Effect of interventions on remission
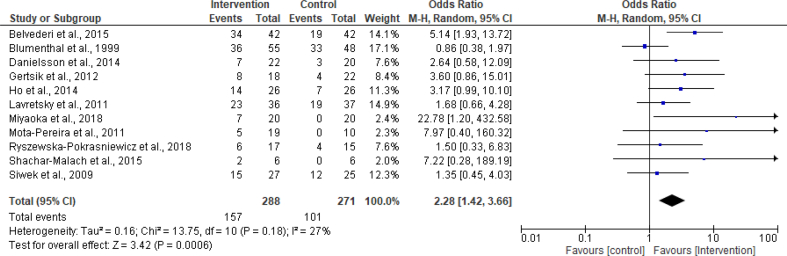
Eleven trials (n = 559) reported remission as a binary outcome. Remission at follow-up was defined in clinically similar ways but with subtle methodological variations. These include: HAM-D score ≤10 (Belvederi et al., 2015), no longer meeting DSM-IV criteria for MDD (Blumenthal et al., 1999), at least 50% improvement in the primary efficacy variable with a post-intervention MADRS score of ≤10 (Danielsson et al., 2014), MADRS score of ≤10 (Ho et al., 2014), HAM-D score ≤6 (Lavretsky et al., 2011; Ryszewska-Pokrasniewicz et al., 2018; Shachar-Malach et al., 2015), HAM-D score of ≤7 (Gertsik et al., 2012; Miyaoka et al., 2018; Mota-Pereira et al., 2011), 'very much improved' on CGI (clinical global impression) scale plus scores on MADRS ≤10 or HAM-D ≤ 7 or BDI ≤9 (Siwek et al., 2009). The random-effects meta-analysis of these trials gave an odds ratio (for remission) of 2.28 (95% CI 1.42 to 3.66; I2 = 27%), see Figure 4. The number needed to treat (NNT) was 6, meaning that 6 patients need to be treated to achieve one more remission.
Figure 4.
Forest plot of the OR of remission for intervention vs control.
Although, there was no significant heterogeneity in the ‘remission’ analysis, subgroup analyses (similar to those carried out for the continuous data) were also considered and conducted for remission. As expected, the analyses showed no evidence of age, patient setting or medication type effect (see supplementary material). Similar to the analysis of mean change scores, subgroup analysis based on intervention type was not feasible using remission data.
3.6. Publication bias
Visual inspection of the funnel plots of both the follow-up scores, mean change scores and remission data (see Supplementary Material) revealed some asymmetry and was found to be significant (p < 0.001 for both the follow-up & mean change scores; p = 0.025 for remission data) using Egger's test. This suggests a likelihood of publication bias in which small studies with small effect sizes were not published, hence, missing from this meta-analysis. It could also mean that small studies (with high level of imprecision) contributed more to the effect estimate.
3.7. Assessment of quality of evidence across studies
The quality of evidence obtained from the studies included in this systematic review was graded as moderate (effect on remission) or low (effect on follow-up and mean change scores). This is because, in the GRADE assessment, the quality of evidence was downgraded by 2 (based on inconsistency and publication bias) for the effect estimates of the follow-up and mean change scores and downgraded by 1 (publication bias) for remission. The summary of findings for all the outcomes is presented in Table 4.
Table 4.
Summary of findings.
| Intervention (lifestyle & dietary-related) compared with control for depression Patient/Population: depression Setting: Inpatients or Outpatients Intervention: Exercise, vitamin D supplementation, zinc supplementation, magnesium supplementation, probiotics or omega-3 fatty acids (all adjunctive) Comparison: control (treatment as usual- TAU or TAU + Placebo) | |||||
|---|---|---|---|---|---|
| Outcomes | Effects: SMD/OR (95% CI) |
No of participants (studies) | Quality of evidence (GRADE) | Comments | |
| Absolute | Relative | ||||
| Follow-up scores | SMD -0.88 (-1.19, -0.57) | 0.88 SMD lower (0.57 lower to 1.19 lower) in intervention group | 1,093 (23 RCTs) |
Low |
Lower depression score means improvement. SMD of 0.3 is deemed clinically relevant. Quality downgraded by 2 based on inconsistency (substantial heterogeneity) and publication bias (asymmetry of funnel plot & significant small study effect from Egger's test). |
| Mean change scores | SMD -1.98 (-2.86, -1.10) | 1.98 SMD lower (1.10 lower to 2.86 lower) in intervention group | 425 (11 RCTs) |
Low |
Lower depression score means improvement. SMD of 0.3 is deemed clinically relevant. Quality downgraded by 2 based on inconsistency and publication bias. |
| Remission | - | OR 2.28 (1.42, 3.66) | 559 (11 RCTs) |
Moderate |
Remission is defined (with subtle variations) as no longer meeting the criteria for depression. Quality downgraded by 1 based on publication bias. |
Meta-analysis for standardized mean difference (SMD) of continuous outcome (follow-up depression scores & mean change in depression scores) data.
| Symbol | Quality | Interpretation | GRADE ratings and their interpretation |
| High | We are very confident that the true effect lies close to that of the estimate of the effect. | ||
| Moderate | We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | ||
| Low | Our confidence in the effect estimate is limited: the true effect may be substantially different form the estimate of the effect. | ||
| Very low | We have very little confidence in the effect estimate: the true effect is likely to be substantially different form the estimate of the effect. | ||
(From the GRADE Handbook, available at http://gdt.guidelinedevelopment.org/app/handbook/handbook.html#h.9rdbelsnu4iy).
RCTs: Randomized clinical trials; SMD; Standardized mean difference; CI: Confidence interval; SD: Standard deviation.
4. Discussion
4.1. Main findings
This systematic review included 26 studies (1216 participants analysed) which assessed the effect of six different types of adjunctive insulin sensitivity-enhancing lifestyle- and dietary-related interventions in improving treatment response to conventional antidepressants. These interventions include adjunctive exercise, omega-3 polyunsaturated fatty acids (PUFA), zinc supplementation, probiotics, vitamin D supplementation and magnesium supplementation. The results showed a significant antidepressant treatment response-improving effect of the intervention, assessed as both reduction in depression scores- Figure 2 (a & b) and remission- Figure 4. From the estimated NNT, six patients need to be treated to achieve one more remission. The sensitivity analyses performed did not change the results of the study, indicating the robustness of the effect estimates. Subgroup analyses of the follow-up scores data revealed some level of intervention effect (greater effect seen with exercise, omega-3 PUFA & Vitamin D) as well as age effect, with younger adults (aged ≤50 years) having a better response. The subgroup analysis based on patient setting suggests that inpatients benefited less from the interventions than outpatients. Heterogeneity was not a concern in the meta-analysis of remission data.
4.2. The effect of the assessed interventions on depression: possible link with insulin sensitivity-enhancing potential
The results of this study is consistent with those of studies which had previously separately analyzed the antidepressant effect of exercise (Schuch et al., 2016), omega-3 PUFA (Sarris et al., 2016) and vitamin D supplementation (Spedding, 2014). It also supports the previously reported beneficial effects of magnesium (Serefko et al., 2016), zinc (Lai et al., 2012) and probiotics (Huang et al., 2016) on depression symptoms. In the subgroup analysis based on intervention type, the insignificant effect estimates from zinc and probiotic group may be due to the small number of studies that made up these two subgroups coupled with the very high level of inconsistency (heterogeneity) observed between the included studies. For the ‘magnesium subgroup’, only one study is included in this case and as such not really fit for a subgroup. Therefore, more relevant ‘magnesium studies’ will be needed in the future for a better interpretation of this particular subgroup analysis. The age effect revealed by the subgroup analysis is consistent with the findings from a study which assessed the effect of an adjunctive insulin sensitizer (pioglitazone) in patients with unremitted depression (Lin et al., 2015). Similar to our findings, the study found pioglitazone to be more beneficial in younger aged patients. This might be because older adults tend to present with a more chronic course of depression and respond less to treatment with antidepressants compared to younger population (Haigh et al., 2018). Moreover, regarding insulin sensitivity, aging-induced impairment in β-cell function and adaptation to insulin resistance (Lee and Halter, 2017) may limit the effectiveness of lifestyle interventions in correcting any underlying insulin resistance in older populations. The observed difference across patient settings (outpatients vs inpatients) may be because only few studies included inpatients, hence, the analysis of this subgroup might not be sufficiently powered to detect any significant effect. It could also be due to higher risk of placebo effect among inpatients, since inpatients generally tend to have higher level of interaction with clinicians and other relevant healthcare workers.
In this systematic review, these adjuncts were jointly assessed for antidepressant treatment response-improving effect on the basis of their previously reported insulin sensitivity-enhancing potential. This was borne out of the observed association between depression and insulin resistance (Kan et al., 2013) and the fact that insulin resistance has been tagged an ‘unmasked culprit’ in depression with the identification of molecular mediators (e.g. glutamate, BDNF) linking these two disorders (Watson et al., 2018). Moreover, depression and metabolic disorders share similar risk factors including an array of clinical, genetic, neurobiological and environmental factors and mood disorders have been proposed to exist as part of a ‘metabolic-mood syndrome’ (Mansur et al., 2015). The assessed interventions are suggested to improve insulin sensitivity via a number of mechanisms. Exercise improves insulin sensitivity via skeletal muscle contraction-induced increase in glucose uptake, resulting from exercise-induced increase in the number of glucose transporter-GLUT4 at relevant skeletal muscle sites (Bird and Hawley, 2017). It also increases glycogen synthase activity and skeletal muscle capillarisation (Bird and Hawley, 2017). Exercise and weight loss reduce C-reactive protein, adhesion molecule 1 and serum amyloid A, all of which have been linked with impaired insulin sensitivity. Zinc is an important mineral which plays crucial roles in insulin secretion (from pancreatic β-cells) and signaling pathways essential for insulin action (Cruz et al., 2018) through molecular mechanisms which are beyond the scope of this systematic review. This supports an earlier finding of a strong correlation between plasma zinc levels and insulin sensitivity in pre-diabetic subjects (Vashum et al., 2014). Similarly, magnesium acts as a cofactor for enzymes involved in carbohydrate metabolism. Importantly, it regulates electrical signaling and insulin secretion in pancreatic β-cells and plays critical role in phosphorylation of tyrosine kinase receptors, including insulin receptor (Kostov, 2019). Hence, magnesium is an insulin sensitizer which facilitates both the secretion and action of insulin. Both animal and clinical studies have also demonstrated that Vitamin D performs similar roles (involvement in insulin secretion and signaling) in the body (Al-Shoumer and Al-Essa, 2015). Indeed, vitamin D receptors are present in several body tissues and vitamin D deficiency adversely affects insulin synthesis/secretion and is linked with impaired glucose tolerance (Al-Shoumer and Al-Essa, 2015). Furthermore, mechanisms potentially underlying the insulin sensitivity-enhancing effect of probiotics include reduction of systemic inflammation, reduction of intestinal permeability and lowering of oxidative stress (Kim et al., 2018). Worthy of note is the fact that, microbial dysbiosis (with attendant increase in pro-inflammatory compounds & gut permeability) is associated with type 2 diabetes (Crommen and Simon, 2017), the precursor of which is insulin resistance. Growing evidence suggest that omega-3 polyunsaturated fatty acids directly enhance insulin secretion via regulation of membrane structure and properties, inhibition of eicosanoids (pro-inflammatory mediators), binding to G-protein-coupled receptors in β-cells, induction of insulin sensitizing adipokines and binding to peroxisome proliferator-activated receptor gamma (Wang and Chan, 2015), the nuclear receptor involved in the mechanism of action of the thiazolidinedione class of insulin sensitizers.
Of course, other mechanisms have also been suggested to underpin the antidepressant effect of these interventions. Exercise has been suggested to improve depression symptoms via its beneficial effect on hippocampal morphology as altered hippocampal morphology, especially reduction in volume, has been consistently reported in depressed patients. Exercise increases hippocampal volume and mitigates cumulative life stress on hippocampal structure, in late life (Gujral et al., 2017). A recent clinical study (Kerling et al., 2017) and systematic review (Mackay et al., 2017) have also found that exercise induces synthesis of brain derived neurotrophic factor (BDNF), a trophic factor known to promote brain plasticity, hence, associated with cognition and antidepressant effect. Also, zinc is found in all body tissues including the brain; where it is tightly regulated, predominantly found within glutamatergic neurons and with higher concentrations in the cortex, hippocampus and amygdala (Petrilli et al., 2017). Its antidepressant effect has been linked to its role in promoting BDNF synthesis and neurogenesis as well as regulation of synaptic plasticity (Petrilli et al., 2017). Zinc counteracts depression-related glutamatergic hyperactivity by modulating NMDA receptor activity (Młyniec, 2015). It also modulates monoaminergic system (Doboszewska et al., 2017). Magnesium is a natural calcium antagonist which shares similar antidepressant mechanisms as described for zinc above and promotes activation of calcium/calmodulin-dependent protein kinase II (CaMKII) (Serefko et al., 2016). Similarly, vitamin D has been suggested to improve depression symptoms by regulating expression of calcium homeostasis genes, antioxidant genes and mitochondrial proteins as well as serotonin synthesis (Berridge, 2017). It also controls inflammation and epigenetic processes (Berridge, 2017). Additionally, probiotics, via anti-inflammatory mechanisms, is thought to elicit antidepressant effect by counteracting the immune and inflammatory activation associated with depression (Park et al., 2018), while also increasing the circulation of serotonin through microbiome signaling (Wallace and Milev, 2017). The antidepressant effect of polyunsaturated fatty acids- PUFAs (especially EPA and DHA) has been proposed to be via anti-inflammatory mechanisms in neural cells and modification of neuronal signaling through modification of plasma membrane (Burhani and Rasenick, 2017).
4.3. Strengths and limitations
The strength of this systematic review is that it was performed by a comprehensive bibliographic search, using a very broad search strategy. It is also based on a published protocol. Additionally, the inclusion of both continuous and binary outcomes enabled a robust assessment of the primary outcome (effect of the interventions in question on antidepressant treatment response). The data from the included studies was also pooled in a meta-analysis given that all the interventions are considered as lifestyle and dietary related therapies with insulin sensitivity enhancing potential. In the current systematic review, random sequence generation was adequate in 69.2% of the included trials, compared with 14.5% and 47.9% of the trials generally assessing drug and non-drug interventions, respectively (Krogh et al., 2015). Allocation concealment was adequate in 42.3%, compared with 5.5% and 15.1% of trials assessing drug and non-drug interventions, respectively. Blinding of outcome assessors was adequate in 69.2% of the included trials for this systematic review, compared with 43.8% of trials generally assessing non-drug interventions (Krogh et al., 2015).
As none of the included studies reported the secondary outcome of interest, as per our protocol (effect on parameters of insulin sensitivity) it was not possible to conduct multivariate analysis. Importantly, this identifies a research gap in this field that needs urgently to be addressed. Another limitation of this study is the non-uniform distribution of the six different interventions assessed which has the potential to limit the generalizability of the observed effect to all the intervention types. Moreover, only two of the included trials had low risk of bias across the assessed domains, with the rest classified as having either a moderate risk of bias or high risk of bias. This is partly because exercise is the assessed intervention in a relatively large proportion of the included trials, in which case, it is difficult to blind the participants and the personnel to the treatment administered.
5. Conclusions
The findings from this study suggest that antidepressant treatment response may be improved through the use of lifestyle and dietary related adjuncts which have the potential to enhance insulin sensitivity. It also indicates the likely involvement of insulin sensitivity/resistance in contributing to antidepressant effectiveness. Further clinical studies are necessary which may lead to altered treatment pathways and enhanced patient outcomes.
Declarations
Author contribution statement
O. Jeremiah and B. Ryan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
G. Cousins and B. Kirby: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
F. Boland: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
O. Jeremiah was supported by Clement Archer PhD Scholarship, Royal College of Surgeons in Ireland, RCSI, 123 St. Stephen's Green, Dublin 2, Ireland.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to acknowledge Dr. Anna Mach, MD, PhD (Medical University of Warsaw, Poland) who provided unpublished data relevant to the analyses.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary materials_Post Update
References
- Al-Shoumer K.a.S., Al-Essa T.M. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J. Diabetes. 2015;6(8):1057–1064. doi: 10.4239/wjd.v6.i8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvederi M.M., Amore M., Menchetti M., Toni G., Neviani F., Cerri M., Rocchi M.B.L., Zocchi D., Bagnoli L., Tam E., Buffa A., Ferrara S., Neri M., Alexopoulos G.S., Zanetidou S. Physical exercise for late-life major depression. Br. J. Psychiatry. 2015;207(3):235–242. doi: 10.1192/bjp.bp.114.150516. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol. Rev. 2017;69(2):80–92. doi: 10.1124/pr.116.013227. [DOI] [PubMed] [Google Scholar]
- Bird S.R., Hawley J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2017;2(1) doi: 10.1136/bmjsem-2016-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J.A., Babyak M.A., Moore K.A., Craighead W.E., Herman S., Khatri P., Waugh R., Napolitano M.A., Forman L.M., Appelbaum M., Doraiswamy P.M., Krishnan K.R. Effects of exercise training on older patients with major depression. Arch. Intern. Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Burhani M.D., Rasenick M.M. Fish oil and depression: the skinny on fats. J. Integr. Neurosci. 2017;16(s1):S115–S124. doi: 10.3233/JIN-170072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro L.S., Fonseca A.M., Vieira-Coelho M.A., Mota M.P., Vasconcelos-Raposo J. Effects of structured exercise and pharmacotherapy vs. pharmacotherapy for adults with depressive symptoms: a randomized clinical trial. J. Psychiatr. Res. 2015;71:48–55. doi: 10.1016/j.jpsychires.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Colle R., De Larminat D., Rotenberg S., Hozer F., Hardy P., Verstuyft C., Fève B., Corruble E. Pioglitazone could induce remission in major depression: a meta-analysis. Neuropsychiatric Dis. Treat. 2017;13:9–16. doi: 10.2147/NDT.S121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crommen S., Simon M.C. Microbial regulation of glucose metabolism and insulin resistance. Genes. 2017;9(1) doi: 10.3390/genes9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz K.J.C., De Oliveira A.R.S., Morais J.B.S., Severo J.S., Mendes P.M.V., De Sousa Melo S.R., De Sousa G.S., Marreiro D.D.N. Zinc and insulin resistance: biochemical and molecular aspects. Biol. Trace Elem. Res. 2018 doi: 10.1007/s12011-018-1308-z. [DOI] [PubMed] [Google Scholar]
- Cruz K.J.C., Morais J.B.S., De Oliveira A.R.S., Severo J.S., Marreiro D.D.N. The effect of zinc supplementation on insulin resistance in obese subjects: a systematic review. Biol. Trace Elem. Res. 2017;176(2):239–243. doi: 10.1007/s12011-016-0835-8. [DOI] [PubMed] [Google Scholar]
- Cusin C., Yang H., Yeung A., Fava M. Rating scales for depression. In: Baer L., Blais M.A., editors. Handbook of Clinical Rating Scales and Assessment in Psychiatry and Mental Health, Current Clinical Psychiatry. Springer Science+Business Media, LLC: Humana Press; 2009. [Google Scholar]
- Danielsson L., Papoulias I., Petersson E.L., Carlsson J., Waern M. Exercise or basic body awareness therapy as add-on treatment for major depression: a controlled study. J. Affect. Disord. 2014;168:98–106. doi: 10.1016/j.jad.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Dean J., Keshavan M. The neurobiology of depression: an integrated view. Asian J. Psychiatr. 2017;27:101–111. doi: 10.1016/j.ajp.2017.01.025. [DOI] [PubMed] [Google Scholar]
- Doboszewska U., Wlaz P., Nowak G., Radziwon-Zaleska M., Cui R. 2017. Zinc in the Monoaminergic Theory of Depression: its Relationship to Neural Plasticity; p. 3682752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone S.V. Interpreting estimates of treatment effects: implications for managed care. Pharm. Therapeut. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- Gertsik L., Poland R.E., Bresee C., Rapaport M.H. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 2012;32(1):61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral S., Aizenstein H., Reynolds C.F., Butters M.A., Erickson K.I. Exercise effects on depression: possible neural mechanisms. Gen. Hosp. Psychiatr. 2017;49:2–10. doi: 10.1016/j.genhosppsych.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh E.a.P., Bogucki O.E., Sigmon S.T., Blazer D.G. Depression among older adults: a 20-year update on five common myths and misconceptions. Am. J. Geriatr. Psychiatr. 2018;26(1):107–122. doi: 10.1016/j.jagp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Hallahan B., Ryan T., Hibbeln J.R., Murray I.T., Glynn S., Ramsden C.E., Sangiovanni J.P., Davis J.M. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br. J. Psychiatry. 2016;209(3):192–201. doi: 10.1192/bjp.bp.114.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Green S. John Wiley & Sons Ltd; Chichester, England: 2008. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- Ho C.W.H., Chan S.C., Wong J.S., Cheung W.T., Chung D.W.S., Lau T.F.O. Effect of aerobic exercise training on Chinese population with mild to moderate depression in Hong Kong. Rehabil. Res. Pract. 2014 doi: 10.1155/2014/627376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Wang K., Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):483. doi: 10.3390/nu8080483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F., Micha R., Wu J.H.Y., De Oliveira Otto M.C., Otite F.O., Abioye A.I., Mozaffarian D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7) doi: 10.1371/journal.pmed.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.R., Attia J., Ali L., Mcevoy M., Selim S., Sibbritt D., Akhter A., Akter S., Peel R., Faruque O., Mona T., Lona H., Milton A.H. Zinc supplementation for improving glucose handling in pre-diabetes: a double blind randomized placebo controlled pilot study. Diabetes Res. Clin. Pract. 2016;115(Suppl. C):39–46. doi: 10.1016/j.diabres.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Jahangard L., Sadeghi A., Ahmadpanah M., Holsboer-Trachsler E., Sadeghi Bahmani D., Haghighi M., Brand S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders - results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res. 2018;107:48–56. doi: 10.1016/j.jpsychires.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Jakobsen J.C., Wetterslev J., Winkel P., Lange T., Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med. Res. Methodol. 2014;14:120. doi: 10.1186/1471-2288-14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri S., Tehrani-Doost M., Keshavarz S.A., Hosseini M., Djazayery A., Amini H., Jalali M., Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatr. 2008;42(3):192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- Jeremiah O.J., Cousins G., Leacy F.P., Kirby B.P., Ryan B.K. Evaluation of the effect of insulin sensitivity-enhancing lifestyle- and dietary-related adjuncts on antidepressant treatment response: protocol for a systematic review and meta-analysis. Syst. Rev. 2019;8(1):62. doi: 10.1186/s13643-019-0978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan C., Silva N., Golden S.H., Rajala U., Timonen M., Stahl D., Ismail K. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care. 2013;36(2):480–489. doi: 10.2337/dc12-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerling A., Kuck M., Tegtbur U., Grams L., Weber-Spickschen S., Hanke A., Stubbs B., Kahl K.G. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. J. Affect. Disord. 2017;215:152–155. doi: 10.1016/j.jad.2017.03.034. [DOI] [PubMed] [Google Scholar]
- Khoraminya N., Tehrani-Doost M., Jazayeri S., Hosseini A., Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. N. Z. J. Psychiatr. 2013;47(3):271–275. doi: 10.1177/0004867412465022. [DOI] [PubMed] [Google Scholar]
- Kim Y.A., Keogh J.B., Clifton P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018;31(1):35–51. doi: 10.1017/S095442241700018X. [DOI] [PubMed] [Google Scholar]
- Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019;20(6):1351. doi: 10.3390/ijms20061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh J., Hjorthoj C.R., Jakobsen J.C., Lindschou J., Kessing L.V., Nordentoft M., Gluud C. DEPERROR: risks of systematic errors in drug and non-drug randomized clinical trials assessing intervention effects in patients with unipolar depression. J. Affect. Disord. 2015;179:121–127. doi: 10.1016/j.jad.2015.03.042. [DOI] [PubMed] [Google Scholar]
- Lai J., Moxey A., Nowak G., Vashum K., Bailey K., Mcevoy M. The efficacy of zinc supplementation in depression: systematic review of randomised controlled trials. J. Affect. Disord. 2012;136(1-2):e31–e39. doi: 10.1016/j.jad.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Lavretsky H., Alstein L.L., Olmstead R.E., Ercoli L.M., Riparetti-Brown M., Cyr N.S., Irwin M.R. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am. J. Geriatr. Psychiatr. 2011;19(10):839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.G., Halter J.B. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40(4):444–452. doi: 10.2337/dc16-1732. [DOI] [PubMed] [Google Scholar]
- Lin K.W., Wroolie T.E., Robakis T., Rasgon N.L. Adjuvant pioglitazone for unremitted depression: clinical correlates of treatment response. Psychiatr. Res. 2015;230(3):846–852. doi: 10.1016/j.psychres.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay C.P., Kuys S.S., Brauer S.G. The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural Plast. 2017;9 doi: 10.1155/2017/4716197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur R.B., Brietzke E., Mcintyre R.S. Is there a "metabolic-mood syndrome"? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015;52:89–104. doi: 10.1016/j.neubiorev.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Mather A.S., Rodriguez C., Guthrie M.F., Mcharg A.M., Reid I.C., Mcmurdo M.E.T. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder. Br. J. Psychiatr. 2002;180(MAY):411-415. doi: 10.1192/bjp.180.5.411. [DOI] [PubMed] [Google Scholar]
- Miyaoka T., Kanayama M., Wake R., Hashioka S., Hayashida M., Nagahama M., Okazaki S., Yamashita S., Miura S., Miki H. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin. Neuropharmacol. 2018;41(5):151-155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Młyniec K. Zinc in the glutamatergic theory of depression. Curr. Neuropharmacol. 2015;13(4):505–513. doi: 10.2174/1570159X13666150115220617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D., The P.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais J.B.S., Severo J.S., De Alencar G.R.R., De Oliveira A.R.S., Cruz K.J.C., Marreiro D.D.N., Freitas B.D.J.E.S.D.A., De Carvalho C.M.R., Martins M.D.C.D.C.E., Frota K.D.M.G. Effect of magnesium supplementation on insulin resistance in humans: a systematic review. Nutrition. 2017;38:54–60. doi: 10.1016/j.nut.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Mota-Pereira J., Silverio J., Carvalho S., Ribeiro J.C., Fonte D., Ramos J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J. Psychiatr. Res. 2011;45(8):1005–1011. doi: 10.1016/j.jpsychires.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Mozaffari-Khosravi H., Yassini-Ardakani M., Karamati M., Shariati-Bafghi S.E. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2013;23(7):636–644. doi: 10.1016/j.euroneuro.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Nazarinasab M., Behrouzian F., Salmanpour R. Evaluating the effectiveness of zinc sulfate in improving depression symptoms in patients treated with selective serotonin reuptake inhibitors in Golestan Hospital in Ahvaz, Iran. Minerva Psichiatr. 2017;58(3):156-161. [Google Scholar]
- Nemets B., Stahl Z., Belmaker R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatr. 2002;159(3):477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- Nowak G., Siwek M., Dudek D., Zieba A., Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol. J. Pharmacol. 2003;55(6):1143–1147. [PubMed] [Google Scholar]
- Park C., Brietzke E., Rosenblat J.D., Musial N., Zuckerman H., Ragguett R.M., Pan Z., Rong C., Fus D., Mcintyre R.S. Probiotics for the treatment of depressive symptoms: an anti-inflammatory mechanism? Brain Behav. Immun. 2018;73:115–124. doi: 10.1016/j.bbi.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Peet M., Horrobin D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatr. 2002;59(10):913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- Petrilli M.A., Kranz T.M., Kleinhaus K., Joe P., Getz M., Johnson P., Chao M.V., Malaspina D. The emerging role for zinc in depression and psychosis. Front. Pharmacol. 2017;8:414. doi: 10.3389/fphar.2017.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilu A., Sorba M., Hardoy M.C., Floris A.L., Mannu F., Seruis M.L., Velluti C., Carpiniello B., Salvi M., Carta M.G. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin. Pract. Epidemiol. Ment. Health. 2007;3 doi: 10.1186/1745-0179-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar E., Kasaei M.S., Mohammad-Shirazi M., Nasrollahzadeh J., Rashidkhani B., Shams J., Mostafavi S.A., Mohammadi M.R. Effects of Zinc supplementation in patients with major depression: a randomized clinical trial. Iran. J. Psychiatry. 2013;8(2):73–79. [PMC free article] [PubMed] [Google Scholar]
- Rasgon N.L., Kenna H.A., Williams K.E., Powers B., Wroolie T., Schatzberg A.F. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. Sci. World J. 2010;10:321–328. doi: 10.1100/tsw.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y., Sun J., He J., Chen F., Chen R., Chen H. Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki L., Ostrowska L., Pawlak D., Malus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2018;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Ryan R., Hill S. Cochrane Consumers and Communication Group; 2016. How to GRADE the Quality of Evidence.http://cccrg.cochrane.org/author-resources Available at. Version 3.0 December 2016. [Google Scholar]
- Ryszewska-Pokrasniewicz B., Mach A., Skalski M., Januszko P., Wawrzyniak Z.M., Poleszak E., Nowak G., Pilc A., Radziwon-Zaleska M. Effects of magnesium supplementation on unipolar depression: a placebo-controlled study and review of the importance of dosing and magnesium status in the therapeutic response. Nutrients. 2018;10(8) doi: 10.3390/nu10081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J., Murphy J., Mischoulon D., Papakostas G.I., Fava M., Berk M., Ng C.H. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am. J. Psychiatr. 2016;173(6):575–587. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Serefko A., Szopa A., Poleszak E. Magnesium and depression. Magnes. Res. 2016;29(3):112–119. doi: 10.1684/mrh.2016.0407. [DOI] [PubMed] [Google Scholar]
- Seyyed Abootorabi M., Ayremlou P., Behroozi-Lak T., Nourisaeidlou S. The effect of vitamin D supplementation on insulin resistance, visceral fat and adiponectin in vitamin D deficient women with polycystic ovary syndrome: a randomized placebo-controlled trial. Gynecol. Endocrinol. 2018;34(6):489–494. doi: 10.1080/09513590.2017.1418311. [DOI] [PubMed] [Google Scholar]
- Shachar-Malach T., Cooper Kazaz R., Constantini N., Lifschytz T., Lerer B. Effectiveness of aerobic exercise as an augmentation therapy for inpatients with major depressive disorder: a preliminary randomized controlled trial. Isr. J. Psychiatry Relat. Sci. 2015;52(3):65–70. [PubMed] [Google Scholar]
- Siqueira C.C., Valiengo L.L., Carvalho A.F., Santos-Silva P.R., Missio G., De Sousa R.T., Di Natale G., Gattaz W.F., Moreno R.A., Machado-Vieira R. Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PloS One. 2016;11(5) doi: 10.1371/journal.pone.0154195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek M., Dudek D., Paul I.A., Sowa-Kućma M., Zieba A., Popik P., Pilc A., Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J. Affect. Disord. 2009;118(1-3):187–195. doi: 10.1016/j.jad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. 2014;6(4):1501–1518. doi: 10.3390/nu6041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashum K.P., Mcevoy M., Milton A.H., Islam M.R., Hancock S., Attia J. Is serum zinc associated with pancreatic beta cell function and insulin sensitivity in pre-diabetic and normal individuals? Findings from the hunter community study. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0083944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos D., Daskalakis Z.J., Blumberger D.M. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatric Dis. Treat. 2020;16:221–234. doi: 10.2147/NDT.S198774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C.J.K., Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann. Gen. Psychiatr. 2017;16:14. doi: 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chan C.B. n-3 polyunsaturated fatty acids and insulin secretion. J. Endocrinol. 2015;224(3):R97. doi: 10.1530/JOE-14-0581. [DOI] [PubMed] [Google Scholar]
- Watson K., Nasca C., Aasly L., Mcewen B., Rasgon N. Insulin resistance, an unmasked culprit in depressive disorders: promises for interventions. Neuropharmacology. 2018;136:327–334. doi: 10.1016/j.neuropharm.2017.11.038. [DOI] [PubMed] [Google Scholar]
- World Health Organisation Depression and other common mental Health disorders: global estimates. 2017. http://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf Available:
- Wu C., Qiu S., Zhu X., Li L. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Metabolism. 2017;73:67–76. doi: 10.1016/j.metabol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Zeinab M.F., Mehdi R., Hussein Q. The effect of vitamin D3 on depression in Iranian women. J. Clin. Diagn. Res. 2018;12(8):CC13–CC16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials_Post Update