Highlights
-
•
Less positive self-schemas are associated with smaller grey matter volume in youth.
-
•
More negative self-schemas are associated with smaller grey matter volume in youth.
-
•
Smaller grey matter volume may serve as a neural marker of early depression risk.
-
•
Self-schemas may mediate links between grey matter volume and depressive symptoms.
Keywords: Self-referent encoding task, Depression, Cognitive vulnerability, sMRI, Grey matter volume, Preadolescent
Abstract
Self-referential processing (i.e., self-schemas that guide processing of self-descriptive information) emerges early in youth, with deeper encoding of negative self-descriptors and/or shallower encoding of positive self-descriptors causally linked to depression. However, the relationship between depressogenic self-schemas and brain structure is unclear. We investigated associations between self-schemas and regional grey matter volume (GMV) in 84 never-depressed preadolescents oversampled for depression risk based on maternal depression history. Self-schemas were assessed using a Self-Referent Encoding Task (SRET) and regional GMV was indexed via voxel-based morphometry analysis of structural magnetic resonance imaging data. Youths’ positive self-schemas were associated with greater regional GMV within the ventrolateral prefrontal cortex (vlPFC) and posterior cingulate cortex (PCC), while negative self-schemas were associated with smaller regional GMV within vlPFC and PCC, areas important to emotion regulation and self-referential processing. These associations remained significant after controlling for youths’ concurrent depressive symptoms. Exploratory mediation analysis suggested that adolescents’ depressogenic self-schemas may mediate associations between GMV and depressive symptoms. Our findings suggest that the observed GMV variations within vlPFC and PCC may serve as neurobiological markers of depressogenic self-schemas during preadolescence.
1. Introduction
Cognitive theories of depression contend that cognitive vulnerabilities, including biased self-schemas, play a causal role in the development of depression (Beck, 2008). Self-schemas (or self-referential processing), an aspect of normative self-knowledge, are conceptualized as early emerging, latent cognitive constructs that guide the processing of positive and negative personal information as related to the self (Northoff et al., 2006). Depressogenic patterns of self-schemas (i.e., more negative and/or less positive) are associated with both concurrent and prospective depressive symptoms in clinical samples of depressed adults and adolescents (e.g., Auerbach et al., 2015, Dobson and Shaw, 1987, Kuiper and Derry, 1982, Prieto et al., 1992) and in non-clinical samples of youth (Goldstein et al., 2015, Gotlib et al., 2006, Hayden et al., 2008, Hayden et al., 2013, Hayden et al., 2014). This literature supports the validity and utility of self-schemas as an early predictor of depression and its potential as a target for early prevention.
Research on the neural correlates of self-referential processing is limited in children, although this knowledge is important for understanding the etiology of depression (Disner et al., 2011). When assessing depressogenic self-schemas, the self-referent encoding task (SRET; Derry and Kuiper, 1981, Kuiper and Derry, 1982) is one of the best-established paradigms. In SRET, participants are shown a series of words describing negative and positive traits and indicate whether they want to endorse each word as self-descriptive; next, they are unexpectedly asked to recall as many of the presented words as possible. Based on performance on SRET, positive and negative self-schemas are typically indexed by proportion of positive or negative words both endorsed and recalled, i.e., positive or negative SRET scores, with lower positive and higher negative scores considered as depressogenic. This paradigm is especially valuable for studying children, who may be limited in capacity to self-report on more complex aspects of the self. Linking SRET scores to potential neural markers of depression risk may be valuable, given that neural markers of risk during childhood may emerge earlier than depressive symptoms and show greater sensitivity than behavioral measures in tapping risk, permitting the detection of early risk processes prior to overt manifestations (Manoach and Agam, 2013). In developmental studies, it is not uncommon to identify neural markers of risk in the absence of behavioral correlates, especially in children without disorder (Fu et al., 2017, Thai et al., 2016). Understanding the neural substrates of cognitive risk can therefore potentially inform early brain-based prevention/intervention for depression.
Functional magnetic resonance imaging (fMRI) has been used to characterize the neural substrates of normative and depressive self-referential processing. Normative self-referential (versus other-referential) processing typically activates the cortical midline structures, including the medial prefrontal cortex (mPFC), cingulate cortex (CC), precuneus, as well as certain fronto-limbic regions (e.g., hippocampus) in non-depressed adults (see meta-analyses; Denny et al., 2012, Hu et al., 2016, Northoff et al., 2006) and typically developing youth (Pfeifer et al., 2007, Pfeifer et al., 2009, Pfeifer and Blakemore, 2012, Pfeifer and Peake, 2012, Romund et al., 2017). In depressed adults and adolescents, heightened activation during SRET have been reported within these regions (Bradley et al., 2016, Ramel et al., 2007; see review Nejad et al., 2013). Recently, we found similar patterns of heightened activation during SRET in never-depressed preadolescents oversampled for depression risk based on a maternal history of depression. Compared to youth without maternal depression, high-risk youth showed greater activation in mPFC and ventrolateral PFC (vlPFC) during positive self-referential processing, although no association was found between fMRI activity and performance on SRET (Liu et al., 2020). Thus, maladaptive patterns of functional brain activity related to self-referential processing may emerge prior to disorder, serving as a neurobiological predictor or mediator of depression vulnerability.
Compared to the fMRI literature, less is known about the neural structural correlates of normative or depressogenic self-schemas, particularly in youth. Neurocognitive functions are subserved by the neuroanatomical architecture of the brain (Stiles and Jernigan, 2010); structural variations in regions supporting self-referential processing may also contribute to depressogenic self-schemas in important ways. The lack of structural research is also surprising given the methodological advantages of structural MRI (sMRI) measure, which render this measure especially useful for studying youth. During task-fMRI, it may be challenging for children to remain still while performing a task; during sMRI, in contrast, children merely focus on staying still without any task, which may reduce head motion and thus increase the reliability of data (De Bie et al., 2010, Raschle et al., 2012). sMRI also avoids a potential problem of task-fMRI that a specific task may fail to elicit expected activation in all participants; this consideration is especially important in studying children given their greater inter-individual variability during the development of neural function (Church et al., 2010).
While not studies of self-schemas, sMRI has been used to study associations between grey matter volume (GMV) and depression. Overall, clinically depressed adults and youth show reduced GMV in distributed regions including PFC, anterior cingulate cortex (ACC), amygdala, and hippocampus (see meta-analyses Arnone et al., 2016, Bora et al., 2012, Lai, 2013, Peng et al., 2016, Sacher et al., 2012, Schmaal et al., 2017), although inconsistent findings have also been reported, potentially due to the heterogeneity of the depressed participants used in past work (e.g., treatment status; Amico et al., 2011, Besteher et al., 2019, Li et al., 2015, Li et al., 2017, Merz et al., 2018). In non-depressed youth, subthreshold depressive symptoms were associated with smaller GMV in vmPFC, ACC, and caudates (Boes et al., 2008, Vulser et al., 2015). Unsurprisingly, these regions, mostly within the cortical midline structures and fronto-limbic system, overlap with those subserving self-referential processing identified in fMRI studies, which demonstrate altered activation in individuals with depression or depression risk (e.g., Bradley et al., 2016, Liu et al., 2020, Nejad et al., 2013, Ramel et al., 2007). Therefore, the structural characteristics of these regions may also be associated with depressogenic self-schemas in youth.
Given this literature, we conducted the first study to examine the concurrent association between depressogenic self-schemas and regional GMV (in the same sample of preadolescents characterized in Liu et al., 2020). As described earlier, while these youth had no history of depressive disorders, we selectively oversampled youth of mothers with a lifetime history of depression, a well-established marker for offspring development of depression (Goodman et al., 2011). Indeed, previous work found that compared to their low-risk peers, never-depressed youth of parents with a lifetime history of depressive disorders showed decreased volume in amygdala (Chai et al., 2015) and hippocampus (Chen et al., 2010), as well as decreased grey matter density in hippocampus (Chen et al., 2010). While diagnosable depression is relatively rare during pre- and early adolescence, this stage is characterized by increases in depressive symptoms (Costello et al., 2003, Pine et al., 1998) and marked changes in self-knowledge and brain maturation (Arain et al., 2013); it thus represents a unique window to examine early risk processes not confounded by clinical disorder. Building on the sMRI literature on depression and fMRI studies on self-referential processing, we focused on seven a priori ROIs, including three cortical midline structures important for self-referential processing (CC, vmPFC, precuneus) and four fronto-limbic regions commonly involved in emotion processing and regulation (amygdala, hippocampus, vlPFC, and dorsolateral PFC [dlPFC]). Given that these ROIs involve both cortical and subcortical regions, we used GMV, a metric that can be used for both cortical and subcortical structures (rather than other metrics such as cortical thickness that can only be used for cortical regions). Further, GMVs of cortical regions are thought to be influenced by, hence associated with, both cortical thickness and surface area, while cortical thickness and surface area are relatively independent of each other (Winkler et al., 2010). Thus, GMV may capture structural characteristics across broader regions and dimensions compared to the other two indices.
Based on previous work showing an association between reduced GMV and heightened depressive symptoms in non-depressed youth (Boes et al., 2008, Vulser et al., 2015), we hypothesized that depressogenic self-schemas (i.e., lower positive or higher negative SRET scores) would be associated with smaller regional GMV within a priori ROIs; we also expected youth with maternal depression to show smaller GMV compared to those without maternal depression. In hypothesis testing, we used voxel-based morphometry (VBM) analysis in conjunction with non-parametric permutation test constrained within a priori ROIs. While traditional volumetric analysis requires manual delineation of ROIs and indexes the global volume of gross anatomical regions, VBM provides an automated, unbiased, and more efficient approach with greater sensitivity to individual differences in regional GMV (Kurth et al., 2015). The permutation test is not constrained by assumptions of the data and has validity in broader situations (Holmes et al., 1996, Nichols and Holmes, 2002).
Additionally, toward the goal of developing a conceptual model for future study, we conducted exploratory, theory-driven mediation analyses, where GMV within a priori ROIs support the development of self-schemas, which in turn predict depressive symptoms (GMV → self-schemas → depressive symptoms; Fig. 1). Given our cross-sectional data, the goal in conducting these analyses was not to test directional or causal associations; rather, we present these analyses with the goal of informing future, longitudinal research by providing estimates of effect sizes of model paths.
Fig. 1.
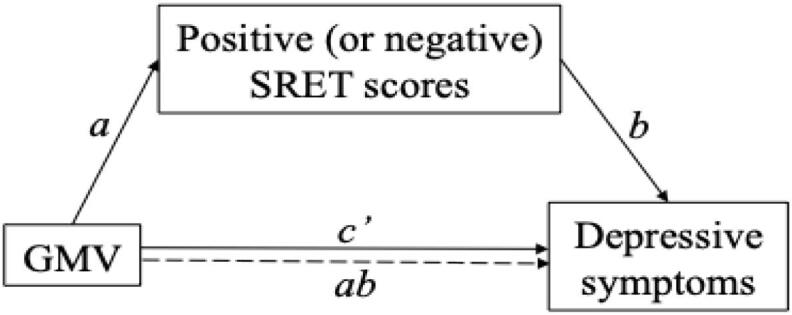
Conceptual mediation model.
2. Material and methods
2.1. Participants and procedures
Participants were recruited from a longitudinal cohort that began at age 3. At baseline, children with major medical or psychological conditions were excluded, and normative cognitive development was verified by the Peabody Picture Vocabulary Test (Dunn and Dunn, 2007; Mean = 111.94, SD = 14.32). In this study, we oversampled children with heightened depression risk based on the depression history of their mothers, who had been assessed for lifetime psychopathology using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders Non-Patient Edition (First et al., 1997; Kappa = 1.00, n = 10). Overall, 229 families were contacted, 110 were enrolled, and 87 children participated (49 boys; Mage = 11.09, SD = 0.66; 96.6% White). Of these, 29 youth (17 boys) had a maternal history of recurrent (minimum two) major depressive episodes (n = 26), or one major depressive episode and a serious anxiety disorder1 (n = 3), given that both patterns mark risk for offspring depression (Barnett et al., 1991, Goodman et al., 2011). Depressive symptoms were measured by maternal report using the withdrawn-depressed subscale (Cronbach’s α = 0.70) of Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001) and youth self-report on Children’s Depression Inventory (CDI, Kovacs and Staff, 2003; Cronbach’s α = 0.82). Youth were screened for current or lifetime depressive disorder via the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version (KSADS; Kaufman et al., 1997), conducted with both the primary caregiver and the child (100% interrater agreement, n = 11). No child had lifetime or current depressive disorder2.
sMRI data were collected at the UWO Centre for Functional and Metabolic Mapping on a Siemens Magnetom Prisma fit 3 T scanner with a 32-channel head coil. High-resolution T1-weighted structural images were acquired using a magnetization-prepared rapid gradient-echo sequence (TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, flip angle = 9°, 192 slices, FOV = 256 mm, voxel = 1 mm3). Following sMRI session, youth completed a SRET in the scanner. In this study, we used the behavioral metrics generated by this SRET as a measure of self-schemas.
Following common practice (Auerbach et al., 2016, Auerbach et al., 2015, Goldstein et al., 2015, Gotlib et al., 2006, Hayden et al., 2006, Hayden et al., 2008, Hayden et al., 2013, Hayden et al., 2014, Jacobs et al., 2008, Mackrell et al., 2013, Prieto et al., 1992, Speed et al., 2016), youth first watched an age-appropriate, 3-minute sad movie clip (The Neverending Story) to induce dysphoric mood, which is thought necessary to activate latent cognitive vulnerability (Abela and Hankin, 2008). Youth rated their mood before and after watching the clip on a 5-point scale (1 = very sad, 5 = very happy). Comparing their ratings pre- (M = 3.72, SD = 0.75) and post-induction (M = 2.18, SD = 0.76) indicated that mood induction was effective, t(83) = 15.68, p < .01. Youth with and without maternal depression did not differ in mood change from pre- to post-induction, p = .88.
Youth next completed a SRET in the scanner, which was adopted from the standard SRET commonly used in the developmental literature (Auerbach et al., 2016, Goldstein et al., 2015, Hayden et al., 2006, Hayden et al., 2008, Hayden et al., 2013, Hayden et al., 2014). To ensure that all words could be properly understood by all youth, we selected 28 trait adjectives (12 positive, e.g., smart; 12 negative, e.g., lazy; four neutral, e.g., tall) at Grade Three level or lower, with age-specific word frequency matched across valences (Warren et al., 1973). This number of stimuli is comparable to those used in previous studies with children of similar age (e.g., Goldstein et al., 2015, Hayden et al., 2013).
The 28 adjectives were organized into seven blocks, each with four words of the same valence (one neutral, three positive, and three negative blocks). The task started and ended with the neutral block to eliminate primacy and recency effects. Between neutral blocks, alternating positive and negative blocks were presented both visually and aurally in fixed order. Each word was visually presented for 4 s, followed by a 0.5 s fixation. Each block was followed by a 10 s-interval. For each word, youth pressed a button to indicate whether they endorsed this word as self-descriptive (“Is this word like or not like you?” pointer finger = yes, middle finger = no). Following the endorsement task, they were unexpectedly asked to recall as many of the presented words as possible for up to two minutes. The fMRI data collected during SRET have been reported elsewhere (Liu et al., 2020).
2.2. Calculation of SRET metrics
Following standard scoring of SRET (Auerbach et al., 2015, Auerbach et al., 2016, Derry and Kuiper, 1981, Dobson and Shaw, 1987, Goldstein et al., 2015, Gotlib et al., 2006, Hayden et al., 2006, Hayden et al., 2008, Hayden et al., 2013, Hayden et al., 2014, Jacobs et al., 2008, Kuiper and Derry, 1982, Mackrell et al., 2013, Prieto et al., 1992, Speed et al., 2016, Wisco, 2009), words both endorsed and recalled by children were used to calculate a positive SRET score (number of positive words endorsed and recalled/all words endorsed) and a negative SRET score (number of negative words endorsed and recalled/all words endorsed). As is typical in SRET studies of children (Goldstein et al., 2015, Hayden et al., 2008), 50 children did not endorse and recall any negative words, leading them to receive a ‘zero’ for their negative SRET scores; thus, non-parametric permutation tests were used to account for the non-normal distribution of the data. Seven children’s SRET scores were missing due to software error. These data were missing completely at random according to Little’s test (Little, 1988), χ2 = 29.32, df = 46, p = .97, and subjected to multiple imputation (R mice package; van Buuren and Groothuis-Oudshoorn, 2011). Variables used in Little’s test and imputation included age, sex, risk group, SRET scores, child-report and maternal-report symptoms, mood ratings before and after induction. We imputed positive and negative SRET scores by running 50 imputations with 10 iterations each and averaged data across the 50 imputed datasets for subsequent analysis3.
In addition, we also calculated the average response times (RTs) of four categories, (1) positive endorsed, (2) positive rejected, (3) negative endorsed, and (4) negative rejected, with faster RTs indicating greater ease with which participants determined whether the trait was self-descriptive (Derry and Kuiper, 1981, Kuiper and Derry, 1982). Specifically, faster RTs to positive words endorsed and negative words rejected reflect are thought to reflect lower cognitive vulnerability; faster RTs to positive words rejected and negative words endorsed reflect greater vulnerability (Derry and Kuiper, 1981, Kuiper and Derry, 1982). One child had an overall average RT > 3SD above the grand mean and had the RTs for each category replaced by 2SD + Mean. Thirty-three youth (out of the 50 with a negative SRET score of zero) did not endorse any negative words and 24 did not reject any positive words, thus having no RTs for these categories. However, given that endorsing no negative words and rejecting no positive words is likely meaningful (i.e., such patterns reflect less negative or more positive self-schemas), these RT data were not imputed. Given the reduced sample size for analyses using these variables, results should be regarded as exploratory.
2.3. sMRI processing and analysis
sMRI processing and analysis were conducted using the Computational Anatomy Toolbox (Dahnke and Gaser, 2017) of SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). T1-weighted structural images were first corrected for bias, noise, and global intensity; corrected images were then spatially normalized to the MNI152 template using the DARTEL algorithm (Ashburner, 2007). Next, normalized images were segmented into gray matter, white matter, and cerebrospinal fluid (Ashburner and Friston, 2000, Ashburner and Friston, 2005). The normalized images were modulated by multiplying the voxel values with the Jacobian determinant derived from spatial normalization, generating images that indicate the absolute amount (or volume) of the GM tissue (Good et al., 2001). Total intracranial volume (ICV) was calculated for each individual during segmentation and was used to control for head size in subsequent analysis (Greve, 2011). Finally, all scans were smoothed with a 6-mm Gaussian kernel and resampled into 1.5 mm3 voxel size. Quality assurance was conducted via visual inspection and an automated quality check protocol embedded in CAT12. Together, 84 of the 87 participants provided usable sMRI data (one had braces, one withdrew before completion, one had excessive motion).
In VBM analysis, we initially tested two multiple regression models with positive (or negative) SRET scores and maternal risk group as two predictors, with child age, sex, and ICV as covariates. Children of mothers with and without a history of depression did not differ in regional GMV within the a priori ROIs, nor did maternal depression history interact with SRET scores in predicting GMV (i.e., no cluster was formed at the p < .001 threshold or survived the cluster-wise family-wise correction of 0.05, ps > 0.11); therefore, we dropped these two predictors (‘group’ and ‘group × SRET scores interaction’) from the model to conserve power and increase parsimony. Results of the full model are presented in the Supplement, which demonstrated highly similar effects of SRET scores as those reported in the main text.
To increase sensitivity of analysis, we constrained our analysis within seven hypothesis-driven, a priori ROIs (Automated Anatomical Labeling; Tzourio-Mazoyer et al., 2002), including cortical midline (vmPFC, CC, precuneus) and fronto-limbic regions (bilateral amygdala, hippocampus, vlPFC, dlPFC). Instead of the traditional parametric analysis, we took a non-parametric statistical approach in both models by running 5000 permutations within each a priori ROI (Statistical nonParametric Mapping toolbox version 13; Nichols and Holmes, 2002). Unlike parametric test, non-parametric permutation testing is applicable to a broader range of situations with minimal assumptions of the data (Holmes et al., 1996, Nichols and Holmes, 2002), and can therefore account for the non-normal distribution of the negative SRET scores in this study. In the permutation test, clusters were first formed at a threshold of uncorrected p < .001; next, clusters that survived a family-wise error correction of 0.05 were identified as significant. For each significant cluster, indicators of GMV were extracted from each model for subsequent data plotting and post-hoc analysis (SPSS 24.0.1, IBM, Armonk, NY).
2.4. Exploratory mediation analysis
We conducted exploratory mediation analysis (PROCESS; Hayes, 2013) to examine our conceptual model (GMV → self-schemas → depressive symptoms; Fig. 1). Mediation models were run for positive and negative self-referential conditions separately, which included the regional GMV of significant clusters identified in VBM as the predictor, positive (or negative) SRET scores as the mediator, and youth symptoms (maternally reported or youth self-reported) as the outcome. An alternative model was also tested by switching the predictor and mediator in the primary model (i.e., self-schemas → GMV → depressive symptoms). The bootstrapping technique used by PROCESS accommodates the non-normal distribution of the negative SRET scores (Pek et al., 2018).
3. Results
3.1. Descriptives of, and correlations between, behavioral variables
Table 1 presents the means, SDs, and bivariate correlations for major variables for descriptive purposes. Maternally reported youth symptoms were associated with lower positive SRET scores, while youth-reported symptoms were associated with both lower positive, and higher negative, SRET scores. Youth-reported symptoms were associated with slower RTs in rejecting negative words, and marginally associated with faster RTs in rejecting positive words. Maternally reported symptoms were marginally correlated with slower RTs in endorsing positive words. These patterns indicate that the SRET metrics are tapping meaningful variation in risk for depression. Positive and negative SRET scores were negatively correlated with each other. Positive SRET scores were associated with slower RTs in rejecting positive and faster RTs in rejecting negative words.
Table 1.
Mean, SD, and bivariate correlation for major variables.
| Mean | (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Maternally reported depression | 1.3 | (−1.78) | ||||||||||||
| 2 | Self-reported depression | 5.02 | (−5.30) | 0.45** | |||||||||||
| 3 | Positive SRET scores | 0.31 | (−0.15) | −0.48** | −0.50** | ||||||||||
| 4 | Negative SRET scores | 0.04 | (−0.06) | 0.19 | 0.30** | −0.30** | |||||||||
| 5 | RT positive endorsed (ms) | 1371.8 | (−291.61) | 0.23+ | 0.04 | −0.14 | −0.19 | ||||||||
| 6 | RT positive rejected (ms) | 1954.6 | (−703.88) | −0.08 | −0.26+ | 0.30* | −0.26 | 0.34* | |||||||
| 7 | RT negative endorsed (ms) | 1692.3 | (−453.06) | −0.13 | −0.16 | 0.14 | 0.02 | 0.49** | 0.47** | ||||||
| 8 | RT negative rejected (ms) | 1323.9 | (−282.97) | 0.15 | 0.25* | −0.23* | 0.22 | 0.58** | 0.24+ | 0.35* | |||||
| 9 | Positive SRET_vlPFC | 0.54 | (0.09) | −0.21+ | −0.27* | 0.36** | −0.11 | 0.14 | 0.30* | 0.19 | 0.04 | ||||
| 10 | Positive SRET_PCC | 0.69 | (0.09) | −0.21+ | −0.21+ | 0.33** | −0.21+ | 0.01 | 0.21 | 0.20 | 0.08 | 0.42** | |||
| 11 | Negative SRET_left vlPFC | 0.66 | (0.07) | −0.26* | −0.20+ | 0.10 | −0.27* | 0.09 | 0.09 | −0.04 | 0.05 | 0.51** | 0.56** | ||
| 12 | Negative SRET_right vlPFC | 0.62 | (0.08) | −0.17 | −0.25* | 0.09 | −0.28* | 0.13 | 0.17 | −0.06 | −0.01 | 0.51** | 0.37** | 0.76** | |
| 13 | Negative SRET_PCC | 0.80 | (0.12) | −0.24* | −0.16 | 0.18 | −0.29** | 0.06 | 0.12 | 0.14 | 0.02 | 0.32** | 0.66** | 0.60** | 0.47** |
Italicized: non-parametric correlation used for negative SRET scores.
**p<.01; * p<.05; + 0.05 < p < .10.
SD: standard deviation; RT: response time; SRET: Self-Referent Encoding Task; vlPFC: ventrolateral prefrontal cortex; PCC: posterior cingulate cortex.
3.2. GMV results
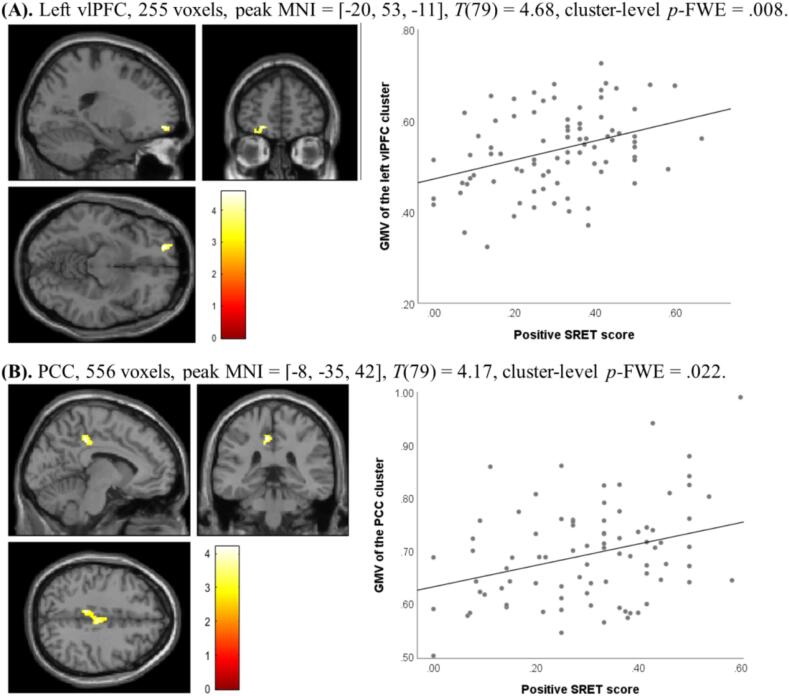
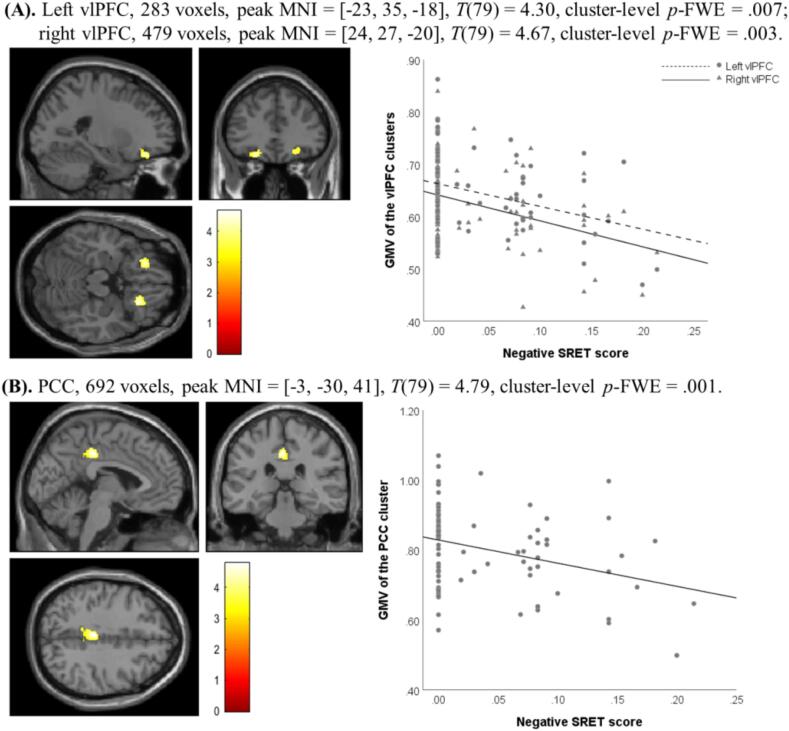
Fig. 2, Fig. 3 present the GMV results of the non-parametric permutation tests in VBM analysis. Specifically, positive SRET scores predicted the regional GMV of a cluster within left vlPFC and a cluster within PCC (Fig. 2); negative SRET scores predicted the regional GMV of two clusters within vlPFC and a cluster within PCC (Fig. 3). Indices of regional GMV were extracted from the permutation models for each significant cluster and plotted in the figures, which demonstrated that positive SRET scores were positively associated with the GMV of the vlPFC and PCC clusters (Fig. 2), and negative SRET scores were negatively associated with the GMV of the vlPFC and PCC clusters (Fig. 3).4
Fig. 2.
Significant clusters within (A) vlPFC and (B) PCC identified in non-parametric permutation test with positive SRET scores as the predictor. Brain images are displayed in accordance with the neurological convention (left is left); color map indicates T values; MNI: Montreal Neurological Institute coordinates.
Fig. 3.
Significant clusters within (A) vlPFC and (B) PCC identified in non-parametric permutation test with negative SRET scores as the predictor. Brain images are displayed in accordance with the neurological convention (left is left); color map indicates T values; MNI: Montreal Neurological Institute coordinates.
3.3. Associations between GMV, SRET performance, and symptoms
As shown in Table 1, youth depressive symptoms were generally associated with smaller regional GMV in significant clusters. For positive self-referential condition, youth self-reported symptoms were significantly associated with smaller regional GMV of the vlPFC cluster and marginally associated with lower GMV of the PCC cluster; maternally reported symptoms were marginally associated with smaller GMV of both the vlPFC and PCC clusters. For negative self-referential condition, maternally reported symptoms were significantly associated with smaller regional GMV of the left vlPFC and PCC clusters; youth self-reported symptoms were correlated with smaller GMV of the right vlPFC cluster and marginally correlated with smaller GMV of the left vlPFC cluster. GMV of the vlPFC cluster identified in the positive self-referential condition was also associated with slower RT in rejecting positive words.
3.4. Results of exploratory mediation analysis
Table 2 presents the results of exploratory mediation analysis testing the primary conceptual model (GMV → self-schemas → symptoms). For negative self-referential processing, two significant clusters were identified within vlPFC that were strongly positively correlated with each other; therefore, we computed the average GMV of these two clusters and used the mean value as the predictor in mediation testing. The results showed that overall, simple paths a (GMV → SRET scores) and b (SRET scores → symptoms) were all significant in the expected directions, except for the associations between negative SRET scores and maternally reported symptoms. The direct effects (c′) of GMV on symptoms were not significant. The mediating effect (indirect path ab) of positive SRET scores was significant for the associations between GMV of the vlPFC and PCC clusters and depressive symptoms, both maternally reported and youth self-reported. The mediating effect of negative SRET scores was significant for the links between GMV of vlPFC and PCC clusters and youth self-reported symptoms, but not maternally reported symptoms. Testing the alternative model (self-schemas → GMV → symptoms) yielded no significant mediating effect (see Supplement). This provides preliminary support for the notion that GMV alterations may contribute to the development of depressogenic self-schemas, which in turn predict depressive symptoms (cf. the alternative model).
Table 2.
Results of exploratory mediation model testing.
| Mediator | Predictor | Outcome | a | b | c' | ab | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive SRET scores |
vlPFC |
Maternal report | 0.36(0.18) | [0.27, 0.97] | −0.44(1.26) | [−7.73, −2.74] | −0.05(2.16) | [−5.37, 3.22] | −0.16(0.05) | [−0.27, −0.07] |
| Youth self-report | 0.36(0.18) | [0.27, 0.97] | −0.49(3.58) | [−24.32, −10.08] | −0.10(6.17) | [−18.33, 6.24] | −0.17(0.05) | [−0.28, −0.08] | ||
| PCC | Maternal report | 0.33(0.17) | [0.19, 0.86] | −0.44(1.24) | [−7.66, −2.74] | −0.07(1.99) | [−5.24, 2.67] | −0.14(0.06) | [−0.27, −0.04] | |
| Youth self-report | 0.33(0.17) | [0.19, 0.86] | −0.51(3.55) | [−24.97, −10.85] | −0.05(5.70) | [−14.08, 8.62] | −0.16(0.06) | [−0.30, −0.05] | ||
| Negative SRET scores | vlPFC (mean) | Maternal report | −0.36(0.08) | [−0.44, −0.12] | 0.19(3.54) | [−1.04, 13.06] | −0.15(2.74) | [−9.12, 1.79] | −0.07(0.06) | [−0.21, 0.03] |
| Youth self-report | −0.36(0.08) | [−0.44, −0.12] | 0.33(10.16) | [10.11, 50.55] | −0.12(7.86) | [−24.29, 6.98] | −0.12(0.07) | [−0.26, −0.01] | ||
| PCC | Maternal report | −0.33(0.05) | [−0.26, −0.06] | 0.19(3.49) | [−1.00, 12.87] | −0.17(1.72) | [−6.11, 0.74] | −0.06(0.06) | [−0.20, 0.03] | |
| Youth self-report | −0.33(0.05) | [−0.26, −0.06] | 0.36(10.10) | [12.99, 53.17] | −0.04(4.99) | [−11.84, 8.00] | −0.12(0.07) | [−0.27, -0.01] | ||
a, predictor to mediator; b, mediator to outcome; c′, direct effect of predictor on outcome; ab, indirect effect of predictor on outcome through mediator;
All coefficients were standardized;
Standard error of coefficients in parentheses; 95% confidence intervals in brackets;
Bold font: significant effects (confidence intervals do not include 0);
SRET: Self-Referent Encoding Task; vlPFC: ventrolateral prefrontal cortex; PCC: posterior cingulate cortex.
4. Discussion
We investigated associations between depressogenic self-schemas and regional GMV in never-depressed preadolescents oversampled for depression risk based on maternal psychiatric history. Youths’ positive SRET scores were positively associated with regional GMV in clusters within vlPFC and PCC, and negative SRET scores were negatively associated with GMV within vlPFC and PCC. These patterns are consistent with a small literature indicating that non-depressed adolescents’ subthreshold depressive symptoms were associated with lower GMV in regions including PFC, CC, caudate, amygdala, and hippocampus (Boes et al., 2008, Vulser et al., 2015). We extended this literature by showing that, in never-depressed preadolescents, smaller regional GMV within cortical midline structures and prefrontal regions is associated with greater cognitive vulnerability, i.e., depressogenic self-schemas. These associations remained significant after controlling for youths’ concurrent depressive symptoms (see Supplement), indicating that the observed GMV alterations may be a unique neurobiological marker of early depressogenic cognitive vulnerability.
Our findings extend a growing literature that shows associations between GMV characteristics and cognitive phenotypes relevant to depression and other psychopathology, which has been mostly conducted in adults (Kanai and Rees, 2011). A positive association was reported between adults’ GMV in PFC and cognitive control (Chen et al., 2015, Wang et al., 2015) as well as self-esteem (Agroskin et al., 2014). Healthy adults with greater rumination, another depressogenic cognitive vulnerability, showed smaller GMV in vlPFC, ACC, and PCC (Kühn et al., 2012). Our findings are consistent with this literature and further indicate that this brain-behavior association emerges earlier in development than previously realized.
The observed associations between depressogenic self-schemas and regional GMV within vlPFC and PCC suggest that smaller GMV in these regions may reflect a less adaptive pattern of GM maturation in youth. The development of GM follows a curvilinear trajectory, roughly characterized by an early, rapid growth, followed by a plateau and subsequent decrease that reflects synaptic and neuronal pruning (Giedd, 2004, Giedd et al., 1999, Giorgio et al., 2010, Paus, 2005). However, trajectories of brain development appear to vary across brain regions; for example, compared to subcortical structures (e.g., amygdala) that tend to mature earlier, cortical regions, such as PFC and CC, typically show a prolonged, extended course of maturation that continues through early adulthood (Casey et al., 2008, Giedd, 2004, Giedd and Rapoport, 2010, Somerville et al., 2010). Therefore, in our youth sample, the regional GMV within vlPFC and PCC may be still increasing, or approaching its peak, which is followed by prolonged pruning. Thus, at this point, lower regional GMV within vlPFC and PCC may index a less adaptive pattern of GM maturation related to the neurocognitive functions subserved by these regions, including self-referential processing.
Both vlPFC and PCC are multi-functional, each underpinning a range of cognitive and affective processes. vlPFC appears important in affective processing and regulatory control (e.g., Iordan et al., 2013, Iordan and Dolcos, 2017, Wager et al., 2008). As opposed to dlPFC, a region subserving “cool” executive functions (i.e., pure logic and critical analysis), vlPFC is considered as part of the “hot” cognitive control system, activated in response to emotionally valenced, and sometimes distracting, information (Dolcos and McCarthy, 2006). Ideally, vlPFC helps downregulate amygdalar reactivity to emotionally arousing and distracting cues, thereby facilitating individuals’ concentration and performance on goal-relevant tasks (Hooker and Knight, 2010, Iordan et al., 2013, Shimamura, 2000, Wager et al., 2008). For example, heightened activation in this region was observed when optimal task performance required youth to shift attention away from threatening distractors to perform on the task (Fu et al., 2017, Telzer et al., 2008).
As a key component of the cortical midline structures, PCC is commonly involved in introspective, self-related cognitive processes such as self-referential processing and autobiographic memories (Brewer et al., 2013, Leech and Sharp, 2014). PCC also plays a role in balancing internally and externally oriented attention, showing modulated activity during attention shift between the external world and internal mentation (Brewer et al., 2013). Overall, vlPFC and PCC serve important functions for regulatory control and attention control in processing emotionally valenced, self-relevant information.
As preadolescents are experiencing rapid brain development, those with less adaptive maturation patterns in vlPFC or PCC (indicated by smaller GMV) may have suboptimal neurocognitive function for processes supported by these regions, including those described above. During SRET, for example, one speculation is that youth with lower GMV in PCC may struggle to balance attention directed toward external task versus introspective feelings or mental events. Excessive self-directed focus is implicated in depression (Klein, 2012, Moberly and Watkins, 2008), irrespective of the valence of the context (Brockmeyer et al., 2015). Further, youth with smaller GMV in vlPFC may have suboptimal regulatory control and find it more effortful to ignore or inhibit emotionally distracting, self-related feelings that are irrelevant to the task. Supporting these points, clinically depressed adolescents showed greater PCC activity during SRET, suggesting that they may need to mobilize more resources from PCC to balance self- vs. task-oriented attentional control during self-referential processing (Bradley et al., 2016). Similarly, we found that youth from the current sample with greater depression risk (i.e., maternal depression) showed heightened vlPFC activation during positive self-referential processing (Liu et al., 2020), suggesting that compared to youth without depression risk, high-risk youth might require greater vlPFC resources to inhibit the distracting, task-irrelevant feelings incurred by making positive self-judgments.
As described earlier, in our recent fMRI study of the same youth sample (Liu et al., 2020), we found that youth with maternal depression showing heightened vlPFC activity during positive self-referential processing than their low-risk peers, but no association between positive or negative SRET scores and SRET-evoked activation was significant. Interestingly, the vlPFC cluster identified in the fMRI data (MNI coordinates: −26, 50, −6) was located closely to the vlPFC cluster of sMRI data observed for positive SRET scores in this study (MNI coordinates: −20, 53, −11). These observations seem to converge across fMRI and sMRI measures in support of the role of vlPFC in self-referential processing, implying a potential association between these two neurobiological markers of cognitive risk for depression (i.e., heightened fMRI activation related to self-referential processing and reduced GMV within a priori ROIs; see details of supplemental analyses probing the sMRI-fMRI association in the Supplement). However, it should be noted that these two clusters reflected different main effects: the sMRI cluster noted a dimensional relationship with positive SRET scores, while the fMRI cluster marked group difference between youth with and without maternal depression. In other words, youth with lower GMV in vlPFC did not necessarily show heightened vlPFC activation during SRET. While these data cannot speak to the direct mapping between regional GMV and neural function of vlPFC, they imply that suboptimal GMV characteristics of a particular region may be associated with less adaptive activation patterns during cognitive processes subserved by this region.
Non-parametric tests showed that negative SRET scores were meaningfully associated with greater youth self-reported symptoms and lower GMV within vlPFC and PCC, suggesting that albeit the non-normal distribution, negative SRET scores still represented a valid risk marker for youth of this age. It is also worth mentioning that the observation that many youth did not endorse and recall any negative words is typical in SRET studies conducted in children of similar or younger ages, who typically show greater inter-individual variability in positive self-schemas than in negative ones (e.g., Hayden et al., 2006, Hayden et al., 2008, Hayden et al., 2013, Hayden et al., 2014). It is possible that prior to adolescence, positive self-schemas may be a more salient risk marker than negative ones (Felton et al., 2013, Leitenberg et al., 1986); indeed, in this study, positive SRET scores showed stronger correlations with depressive symptoms than negative scores. Future work with older samples will indicate whether negative self-schemas show greater individual variability and become more salient in the context of depressive psychopathology as youth grow older. Conducting SRET in older youth using a broader vocabulary will also permit including more nuanced negative and positive traits than those used in the current study.
Contrary to our expectation and previous findings (Chai et al., 2015, Chen et al., 2010), youth with and without a maternal history of depression did not differ in regional GMV in any a priori ROIs. While the reasons for this are unclear, it is important to note that, while maternal depression clearly marks offspring depression risk, its association with youth outcomes is probabilistic rather than deterministic. Put differently, not all children with mothers with depression evince depression vulnerability themselves and maternal depression may interact with other risks to predict youth adaptation (Goodman et al., 2011, Gotlib and Colich, 2014). Given previous findings that chronic familial adversity (e.g., inter-parental discord), but not parental psychiatric history, was related to reduced GMV in community-dwelling adolescents (Walsh et al., 2014), the lack of associations between maternal depression and children’s GMV in our study may also be related to the low-risk nature of our sample. It is also worth mentioning that there existed several methodological differences between the current study and previous research that identified altered neural structural patterns in youth with parental depression (Chai et al., 2015, Chen et al., 2010). For example, youths’ age range in the present study (9–12 years) was narrower than that in previous work (8–14 years, Chai et al., 2015; 9-15 years, Chen et al., 2010); while the current study examined both boys and girls with maternal depression, previous work focused on girls only (Chen et al., 2010) or included both maternal and paternal depression (Chai et al., 2015). In future work, longitudinal neuroimaging research is warranted to further clarify the role of maternal (or parental) depression in the development of brain structures associated with depression risks.
We ran an exploratory mediation analysis in our cross-sectional data to provide an initial examination of our conceptual model, i.e., depressogenic self-schemas may serve as an intermediate cognitive phenotype that mediates associations between GMV alterations and depressive symptoms (GMV → self-schemas → symptoms). Results were supporting of this model but not an alternative model (self-schemas → GMV → symptoms; see Supplement). For positive self-schemas, lower GMV of the vlPFC and PCC clusters predicted lower positive SRET scores (as expected, as these clusters were identified based on the significant effect of positive SRET scores in VBM analysis); lower positive scores in turn predicted heightened depressive symptoms of both maternal report and youth self-report. For negative self-schemas, lower GMV in vlPFC and PCC predicted greater negative SRET scores, which then predicted heightened self-reported, but not maternally reported, symptoms. Positive SRET scores showed greater effect sizes than negative scores as the mediator and was significant for both maternally reported and youth self-reported symptoms; this again suggests that positive self-schemas may be a more salient risk marker of depression than negative ones for youth of this age.
Additionally, there are differences in content between maternal report (CBCL) and youth self-report (CDI) of symptoms: the former covers a broader range of depression-related problems, including symptoms and more observable aspects of behaviors (e.g., withdrawal, low activity), while the latter focuses more exclusively on depressive “feelings.” It is possible that negative self-schemas are more relevant to aspects of depressive symptoms tapped by self-report but not maternal report. It is also interesting to note that, although the GMV of significant clusters showed meaningful associations with symptoms in bivariate correlations in general, the direct effects of GMV on symptoms (path c′) were no longer significant in the mediation models once SRET scores were included as a mediator. This also supports the potential role of self-schemas in linking GMV variations and depressive symptoms. Again, we acknowledge that cross-sectional data are not generally considered suitable for testing causal models via mediation. This was not the goal of these analyses, which were conducted to inform future longitudinal research on brain-self-schema development.
This study has several strengths. We examined a never-depressed preadolescent sample spanning a relatively narrow age range (9.18–12.42 years) to minimize confounding effects of clinical status and age variety. We used a multi-method approach, with self-schemas assessed by an age-appropriate experimental paradigm and symptoms assessed by child self-report and maternal report, to minimize shared method variance error. A primary limitation is the cross-sectional design; the current sample size was also suboptimal for conducting mediation analysis and may have been limited in power. For future research, we are following this cohort up into later stages of adolescence that are marked by further increases in depressive psychopathology and greater individual variation in negative self-schemas; the development of vocabulary and cognition will also allow for more refined measurements of self-schemas. By collecting longitudinal, causally informative data, we aim for a more conclusive examination of the putative mechanism that underlie the development of depressive psychopathology in relation to self-schemas and regional GMV.
5. Conclusion
In conclusion, our findings indicate that depressogenic self-schemas are associated with altered patterns of regional GMV within vlPFC and PCC. Exploratory mediation model testing suggests that self-schemas may mediate the associations between altered GMV in these regions and depressive symptoms. Future studies will benefit from longitudinal designs and experimental manipulations of youth self-schemas. Regarding the latter point, previous studies have reported that behavioral or cognitive training is associated with post-training changes in brain structure and function, in both youth (e.g., Liu et al., 2018) and adults (Lumma et al., 2018). Such work may inform the development of cognitive prevention/intervention for youth depression and contribute to further refinements of cognitive theories of depression.
Funding
This work is supported by grants from the Canadian Institutes of Health Research (MOP86458) and Ontario Mental Health Foundation to EPH. Neuroimaging costs were subsidized by a Canada First Research Excellence Fund grant to BrainsCAN. Computing infrastructure support was provided by a Nvidia Corporation grant to MFJ.
CRediT authorship contribution statement
Pan Liu: Formal analysis, Data curation, Writing - original draft, Visualization, Validation. Matthew R.J. Vandemeer: Investigation, Data curation, Writing - review & editing, Project administration. Marc F. Joanisse: . Deanna M. Barch: Writing - review & editing, Funding acquisition. David J.A. Dozois: Writing - review & editing, Funding acquisition. Elizabeth P. Hayden: Conceptualization, Methodology, Writing - review & editing, Supervision, Funding acquisition.
Acknowledgments
The authors want to thank the families who participated in our studies, and the many individuals who contributed to data collection.
Footnotes
We excluded specific phobia and social anxiety limited to public speaking given that these are less heritable, less impairing, and potentially weaker markers of children’s internalizing risk (Kendler et al., 1992).
Based on KSADS, of the 87 children that participated in this study, nine children met criteria for an anxiety disorder, eight children met criteria for attention-deficit/hyperactivity disorder, six met criteria for conduct disorder or oppositional and defiant disorder, and one for adjustment disorder. Diagnostic status was unrelated to missing MRI data (i.e., none of the three excluded children met criteria for any disorders).
The same analyses were conducted on the original, unimputed data, which yielded highly similar results, albeit with weaker effects.
We re-ran the analysis with youth depressive symptoms included as another covariate, which yielded highly similar results (see Supplement).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102422.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abela J.R.Z., Hankin B.L. Cognitive vulnerability to depression in children and adolescents: a developmental psychopathology perspective. In: Abela J.R.Z., Hankin B.L., editors. Handbook of Depression in Children and Adolescents. The Guilford Press; 2008. pp. 35–78. [Google Scholar]
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- Agroskin D., Klackl J., Jonas E. The self-liking brain: a VBM study on the structural substrate of self-esteem. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico F., Meisenzahl E., Koutsouleris N., Reiser M., Möller H.J., Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J. Psychiatry Neurosci. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arain M., Haque M., Johal L., Mathur P., Nel W., Rais A., Sandhu R., Sharma S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013;9:449–461. doi: 10.2147/NDT.S39776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D., Job D., Selvaraj S., Abe O., Amico F., Cheng Y., Colloby S.J., O’Brien J.T., Frodl T., Gotlib I.H., Ham B.-J., Kim M.J., Koolschijn P.C.M., Périco C.-A.-M., Salvadore G., Thomas A.J., Van Tol M.-J., van der Wee N.J.A., Veltman D.J., Wagner G., McIntosh A.M. Computational meta-analysis of statistical parametric maps in major depression. Hum. Brain Mapp. 2016;37:1393–1404. doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Auerbach R.P., Bondy E., Stanton C.H., Webb C.A., Shankman S.A., Pizzagalli D.A. Self-referential processing in adolescents: stability of behavioral and ERP markers. Psychophysiology. 2016;53:1398–1406. doi: 10.1111/psyp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach R.P., Stanton C.H., Proudfit G.H., Pizzagalli D.A. Self-referential processing in depressed adolescents: a high-density event-related potential study. J. Abnorm. Psychol. 2015;124:233–245. doi: 10.1037/abn0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B., Schaafsma M.F., Guzman A.-M., Parker G.B. Maternal anxiety: a 5-year review of an intervention study. J. Child Psychol. Psychiatry. 1991;32:423–438. doi: 10.1111/j.1469-7610.1991.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Beck A.T. The evolution of the cognitive model of depression and its neurobiological correlates. Am. J. Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Besteher B., Squarcina L., Spalthoff R., Bellani M., Gaser C., Brambilla P., Nenadić I. Hippocampal volume as a putative marker of resilience or compensation to minor depressive symptoms in a nonclinical sample. Front. Psychiatry. 2019;10 doi: 10.3389/fpsyt.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes A.D., McCormick L.M., Coryell W.H., Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol. Psychiatry. 2008;63:391–397. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Harrison B.J., Davey C.G., Yücel M., Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal- thalamic circuits in major depressive disorder. Psychol. Med. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Bradley K.A.L., Colcombe S., Henderson S.E., Alonso C.M., Milham M.P., Gabbay V. Neural correlates of self-perceptions in adolescents with major depressive disorder. Dev. Cogn. Neurosci. 2016;19:87–97. doi: 10.1016/j.dcn.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, J.A., Garrison, K.A., Whitfield-Gabrieli, S., 2013. What about the “self” is processed in the posterior cingulate cortex? Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed]
- Brockmeyer T., Zimmermann J., Kulessa D., Hautzinger M., Bents H., Friederich H.-C., Herzog W., Backenstrass M. Me, myself, and I: self-referent word use as an indicator of self-focused attention in relation to depression and anxiety. Front. Psychol. 2015;6:1564. doi: 10.3389/fpsyg.2015.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Hirshfeld-Becker D., Biederman J., Uchida M., Doehrmann O., Leonard J.A., Salvatore J., Kenworthy T., Brown A., Kagan E., de Los Angeles C., Whitfield-Gabrieli S., Gabrieli J.D.E. Functional and structural brain correlates of risk for major depression in children with familial depression. NeuroImage Clin. 2015;8:398–407. doi: 10.1016/j.nicl.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.C., Hamilton J.P., Gotlib I.H. Decreased hippocampal volume in healthy girls at risk of depression. Arch. Gen. Psychiatry. 2010;67:270–276. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C., Yang, J., Lai, J., Li, H., Yuan, J., Ul Hasan Abbasi, N., 2015. Correlating gray matter volume with individual difference in the Flanker interference effect. PLoS One 10. https://doi.org/10.1371/journal.pone.0136877. [DOI] [PMC free article] [PubMed]
- Church J.A., Petersen S.E., Schlaggar B.L. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum. Brain Mapp. 2010;31:852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dahnke, R., Gaser, C., 2017. Voxel-based preprocessing in CAT. In: Organization for Human Brain Mapping Annual Meeting. Geneva, Switzerland. https://doi.org/10.13140/RG.2.2.11653.70887.
- De Bie H.M.A., Boersma M., Wattjes M.P., Adriaanse S., Vermeulen R.J., Oostrom K.J., Huisman J., Veltman D.J., Delemarre-Van De Waal H.A. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur. J. Pediatr. 2010;169:1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J. Cogn. Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry P.A., Kuiper N.A. Schematic processing and self-reference in clinical depression. J. Abnorm. Psychol. 1981;90:286–297. doi: 10.1037/0021-843X.90.4.286. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A.P., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Dobson K.S., Shaw B.F. Specificity and stability of self-referent encoding in clinical depression. J. Abnorm. Psychol. 1987;96:34–40. doi: 10.1037/0021-843X.96.1.34. [DOI] [PubMed] [Google Scholar]
- Dolcos F., McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, L.M., Dunn, D.M., 2007. Peabody Picture Vocabulary Test. fourth ed. Summ. Shute. Inst. https://doi.org/10.1037/t15144-000.
- Felton J.W., Cole D.A., Martin N.C. Effects of rumination on child and adolescent depressive reactions to a natural disaster: the 2010 nashville flood. J. Abnorm. Psychol. 2013;122:64–73. doi: 10.1037/a0029303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M.B., Gibbon, M., Spitzer, R.L., Benjamin, L.S., Williams, J.B.W., 1997. Structured clinical interview for DSM-IV® axis ii personality disorders SCID-II. American Psychiatric Pub.
- Fu X., Taber-Thomas B.C., Pérez-Edgar K. Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biol. Psychol. 2017;122:98–109. doi: 10.1016/j.biopsycho.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study [2] Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A., Watkins K.E., Chadwick M., James S., Winmill L., Douaud G., De Stefano N., Matthews P.M., Smith S.M., Johansen-Berg H., James A.C. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Goldstein B.L., Hayden E.P., Klein D.N. Stability of self-referent encoding task performance and associations with change in depressive symptoms from early to middle childhood. Cogn. Emot. 2015;29:1445–1455. doi: 10.1080/02699931.2014.990358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodman S.H., Rouse M.H., Connell A.M., Broth M.R., Hall C.M., Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin. Child Fam. Psychol. Rev. 2011;14:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gotlib I.H., Colich N.L. Handbook of Depression. third Ed. Guilford Press; New York, NY, US: 2014. Children of parents with depression; pp. 240–258. [Google Scholar]
- Gotlib I.H., Joormann J., Minor K.L., Cooney R.E. Cognitive and biological functioning in children at risk for depression. In: Canli T., editor. Biology of Personality and Individual Differences. The Guilford Press; 2006. pp. 353–382. [Google Scholar]
- Greve, D.N., 2011. An absolute beginner’s guide to surface- and voxel-based morphometric analysis. In: Proceedings of the International Society for Magnetic Resonance in Medicine. pp. 1–7.
- Hayden E.P., Hankin B.L., Mackrell S.V.M., Sheikh H.I., Jordan P.L., Dozois D.J.A., Singh S.M., Olino T.M., Badanes L.S. Parental depression and child cognitive vulnerability predict children’s cortisol reactivity. Dev. Psychopathol. 2014;26:1445–1460. doi: 10.1017/S0954579414001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden E.P., Klein D.N., Durbin C.E., Olino T.M. Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Dev. Psychopathol. 2006;18:409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Hayden E.P., Olino T.M., Mackrell S.V.M., Jordan P.L., Desjardins J., Katsiroumbas P. Cognitive vulnerability to depression during middle childhood: stability and associations with maternal affective styles and parental depression. Pers. Individ. Dif. 2013;55:892–897. doi: 10.1016/j.paid.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden E.P., Shankman S.A., Olino T.M., Durbin C.E., Tenke C.E., Bruder G.E., Klein D.N. Cognitive and temperamental vulnerability to depression: longitudinal associations with regional cortical activity. Cogn. Emot. 2008;22:1415–1428. doi: 10.1080/02699930701801367. [DOI] [Google Scholar]
- Hayes A.F. The Guilford Press; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Holmes A.P., Blair R.C., Watson J.D.G., Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Hooker C.I., Knight R.T. The role of lateral orbitofrontal cortex in the inhibitory control of emotion. In: Zald D., Rauch S., editors. The Orbitofrontal Cortex. Oxford University Press; New York: 2010. pp. 307–324. [DOI] [Google Scholar]
- Hu C., Di X., Eickhoff S.B., Zhang M., Peng K., Guo H., Sui J. Distinct and common aspects of physical and psychological self-representation in the brain: a meta-analysis of self-bias in facial and self-referential judgements. Neurosci. Biobehav. Rev. 2016;61:197–207. doi: 10.1016/j.neubiorev.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Iordan A.D., Dolcos F. Brain activity and network interactions linked to valence-related differences in the impact of emotional distraction. Cereb. Cortex. 2017;27:731–749. doi: 10.1093/cercor/bhv242. [DOI] [PubMed] [Google Scholar]
- Iordan A.D., Dolcos S., Dolcos F. Neural signatures of the response to emotional distraction: a review of evidence from brain imaging investigations. Front. Hum. Neurosci. 2013;7:200. doi: 10.3389/fnhum.2013.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R.H., Reinecke M.A., Gollan J.K., Kane P. Empirical evidence of cognitive vulnerability for depression among children and adolescents: a cognitive science and developmental perspective. Clin. Psychol. Rev. 2008;28:759–782. doi: 10.1016/j.cpr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendler, K.S., Neale, M.C., Kessler, R.C., Heath, A.C., Eaves, L.J., 1992. The genetic epidemiology of phobias in women: the interrelationship of agoraphobia, social phobia, situational phobia, and simple phobia. Arch. Gen. Psychiatry. https://doi.org/10.1001/archpsyc.1992.01820040025003. [DOI] [PubMed]
- Klein, S.B., 2012. Self, Memory, and the Self-Reference Effect: An Examination of Conceptual and Methodological Issues. Personal. Soc. Psychol. Rev. https://doi.org/10.1177/1088868311434214. [DOI] [PubMed]
- Kovacs M., Staff M. Multi-Health Systems Inc.; North Tonawanda, NY, USA: 2003. Children’s Depression Inventory (CDI): Technical Manual Update. [Google Scholar]
- Kühn S., Vanderhasselt M.A., De Raedt R., Gallinat J. Why ruminators won’t stop: the structural and resting state correlates of rumination and its relation to depression. J. Affect. Disord. 2012 doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Kuiper N.A., Derry P.A. Depressed and nondepressed content self-reference in mild depressives. J. Pers. 1982;50:67–80. doi: 10.1111/j.1467-6494.1982.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Kurth F., Gaser C., Luders E. A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM) Nat. Protoc. 2015;10:293–304. doi: 10.1038/nprot.2015.014. [DOI] [PubMed] [Google Scholar]
- Lai C.H. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. Neuroimag. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014 doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitenberg H., Yost L.W., Carroll-Wilson M. Negative cognitive errors in children. questionnaire development, normative data, and comparisons between children with and without self-reported symptoms of depression, low self-esteem, and evaluation anxiety. J. Consult. Clin. Psychol. 1986;54:528–536. doi: 10.1037/0022-006X.54.4.528. [DOI] [PubMed] [Google Scholar]
- Li H., Wei D., Sun J., Chen Q., Zhang Q., Qiu J. Brain structural alterations associated with young women with subthreshold depression. Sci. Rep. 2015;5 doi: 10.1038/srep09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang Z., Hwang J.W., Zhao B., Yang X., Xin S., Wang Y., Jiang H., Shi P., Zhang Y., Wang X., Lang C., Park J., Bao T., Kong J. Anatomical brain difference of subthreshold depression in young and middle-aged individuals. NeuroImage Clin. 2017;14:546–551. doi: 10.1016/j.nicl.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R.J.A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988;83:1198–1202. doi: 10.1080/01621459.1988.10478722. [DOI] [Google Scholar]
- Liu P., Taber-Thomas B.C., Fu X., Pérez-Edgar K.E. Biobehavioral markers of attention bias modification in temperamental risk for anxiety: a randomized control trial. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:103–110. doi: 10.1016/j.jaac.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Vandermeer M.R.J., Joanisse M.F., Barch D.M., Dozois D.J.A., Hayden E.P. Neural activity during self-referential processing in children at risk for depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:429–437. doi: 10.1016/j.bpsc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- Lumma A.L., Valk S.L., Böckler A., Vrtička P., Singer T. Change in emotional self-concept following socio-cognitive training relates to structural plasticity of the prefrontal cortex. Brain Behav. 2018;8 doi: 10.1002/brb3.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackrell S.V.M., Johnson E.M., Dozois D.J.A., Hayden E.P. Negative life events and cognitive vulnerability to depression: Informant effects and sex differences in the prediction of depressive symptoms in middle childhood. Pers. Individ. Dif. 2013;54:463–468. doi: 10.1016/j.paid.2012.09.007. [DOI] [Google Scholar]
- Manoach D.S., Agam Y. Neural markers of errors as endophenotypes in neuropsychiatric disorders. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., He X., Noble K.G. Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clin. 2018;20:243–251. doi: 10.1016/j.nicl.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly N.J., Watkins E.R. Ruminative self-focus and negative affect: an experience sampling study. J. Abnorm. Psychol. 2008 doi: 10.1037/0021-843X.117.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front. Hum. Neurosci. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pek J., Wong O., Wong A.C.M. How to address non-normality: a taxonomy of approaches, reviewed, and illustrated. Front. Psychol. 2018;9 doi: 10.3389/fpsyg.2018.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W., Chen Z., Yin L., Jia Z., Gong Q. Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J. Affect. Disord. 2016;199:114–123. doi: 10.1016/j.jad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Blakemore S.J. Adolescent social cognitive and affective neuroscience: past, present, and future. Soc. Cogn. Affect. Neurosci. 2012;7:1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Lieberman M.D., Dapretto M. “I know you are but what am I?!”: Neural bases of self- and social knowledge retrieval in children and adults. J. Cogn. Neurosci. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Dev. Cogn. Neurosci. 2012;2:55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Cohen P., Gurley D., Brook J., Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch. Gen. Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Prieto S.L., Cole D.A., Tageson C.W. Depressive self-schemas in clinic and nonclinic children. Cognit. Ther. Res. 1992;16:521–534. doi: 10.1007/BF01175139. [DOI] [Google Scholar]
- Ramel W., Goldin P.R., Eyler L.T., Brown G.G., Gotlib I.H., McQuaid J.R. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol. Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romund L., Golde S., Lorenz R.C., Raufelder D., Pelz P., Gleich T., Heinz A., Beck A. Neural correlates of the self-concept in adolescence—a focus on the significance of friends. Hum. Brain Mapp. 2017;38:987–996. doi: 10.1002/hbm.23433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J., Neumann J., Fünfstück T., Soliman A., Villringer A., Schroeter M.L. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Sämann P.G., Hall G.B., Baune B.T., Jahanshad N., Cheung J.W., Van Erp T.G.M., Bos D., Ikram M.A., Vernooij M.W., Niessen W.J., Tiemeier H., Hofman A., Wittfeld K., Grabe H.J., Janowitz D., Bülow R., Selonke M., Völzke H., Grotegerd D., Dannlowski U., Arolt V., Opel N., Heindel W., Kugel H., Hoehn D., Czisch M., Couvy-Duchesne B., Rentería M.E., Strike L.T., Wright M.J., Mills N.T., De Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Goya-Maldonado R., Gruber O., Krämer B., Hatton S.N., Lagopoulos J., Hickie I.B., Frodl T., Carballedo A., Frey E.M., Van Velzen L.S., Penninx B.W.J.H., Van Tol M.J., Van der Wee N.J., Davey C.G., Harrison B.J., Mwangi B., Cao B., Soares J.C., Veer I.M., Walter H., Schoepf D., Zurowski B., Konrad C., Schramm E., Normann C., Schnell K., Sacchet M.D., Gotlib I.H., MacQueen G.M., Godlewska B.R., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Hall J., Sussmann J.E., Li M., Walter M., Aftanas L., Brack I., Bokhan N.A., Thompson P.M., Veltman D.J. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22:900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A.P. The role of the prefrontal cortex in dynamic filtering. Psychobiology. 2000;28:207–218. doi: 10.3758/BF03331979. [DOI] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed B.C., Nelson B.D., Auerbach R.P., Klein D.N., Hajcak G. Depression risk and electrocortical reactivity during self-referential emotional processing in 8 to 14 year-old girls. J. Abnorm. Psychol. 2016;125:607–619. doi: 10.1037/abn0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Mogg K., Bradley B.P., Mai X., Ernst M., Pine D.S., Monk C.S. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol. Psychol. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai N., Taber-Thomas B.C., Pérez-Edgar K.E. Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: an ERP study. Dev. Cogn. Neurosci. 2016;19:200–210. doi: 10.1016/j.dcn.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Buuren S., Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- Vulser H., Lemaitre H., Artiges E., Miranda R., Penttilä J., Struve M., Fadai T., Kappel V., Grimmer Y., Goodman R., Stringaris A., Poustka L., Conrod P., Frouin V., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Büchel C., Flor H., Gallinat J., Garavan H., Gowland P., Heinz A., Ittermann B., Lawrence C., Loth E., Mann K., Nees F., Paus T., Pausova Z., Rietschel M., Robbins T.W., Smolka M.N., Schumann G., Martinot J.L., Paillère-Martinot M.L., Dalley J., Subramaniam N., Theobald D., Bach C., Fauth-Bühler M., Millenet S., Spanagel R., Albrecht L., Ivanov N., Rapp M., Reuter J., Strache N., Ströhle A., Lalanne C., Poline J.B., Schwartz Y., Thyreau B., Lathrop M., Ireland J., Rogers J., Bordas N., Bricaud Z., Filippi I., Galinowski A., Gollier-Briant F., Massicotte J., Andrew C., Cattrell A., Desrivieres S., Hall D., Havatzias S., Jia T., Mallik C., Nymberg C., Reed L., Ruggeri B., Smith L., Stueber K., Topper L., Werts H., Brühl R., Ihlenfeld A., Walaszek B., Hübner T., Müller K., Ripke S., Rodehacke S., Mennigen E., Schmidt D., Vetter N., Ziesch V., Carter D., Connolly C., Nugent S., Jones J., Whelan R., Yacubian J., Schneider S., Head K., Heym N., Newman C., Tahmasebi A., Stephens D. Subthreshold depression and regional brain volumes in young community adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:832–840. doi: 10.1016/j.jaac.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh N.D., Dalgleish T., Lombardo M.V., Dunn V.J., Van Harmelen A.-L., Ban M., Goodyer I.M. General and specific effects of early-life psychosocial adversities on adolescent grey matter volume. NeuroImage Clin. 2014;4:308–318. doi: 10.1016/j.nicl.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jin C., Yuan K., Shakir T.M., Mao C., Niu X., Niu C., Guo L., Zhang M. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R.E., Carroll J.B., Davies P., Richman B. The American heritage word frequency book. Am. J. Psychol. 1973;86:207. doi: 10.2307/1421864. [DOI] [Google Scholar]
- Winkler A.M., Kochunov P., Blangero J., Almasy L., Zilles K., Fox P.T., Duggirala R., Glahn D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco B.E. Depressive cognition: self-reference and depth of processing. Clin. Psychol. Rev. 2009;29:382–392. doi: 10.1016/j.cpr.2009.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.