Abstract
With the increasing appreciation of mitochondria in modulating cellular homeostasis, various disease biology researchers have started exploring the detailed role of mitochondria in multiple diseases beyond neuronal and muscular diseases. In this context, emerging shreds of evidence in lung biology indicated the meticulous role of lung epithelia in provoking a plethora of lung diseases in contrast to earlier beliefs. As lung epithelia are ceaselessly exposed to the environment, they need to have multiple protective mechanisms to maintain the integrity of lung structure and function. As ciliated airway epithelium and type 2 alveolar epithelia require intense energy for executing their key functions like ciliary beating and surfactant production, it is no surprise that defects in mitochondrial function in these cells could perturb lung homeostasis and engage in the pathophysiology of lung diseases. On one hand, intracellular calcium plays the central role in executing key functions of lung epithelia, and on the other hand maintenance of intracellular calcium needs the buffering role of mitochondria. Thus, the regulation of mitochondrial calcium in lung epithelia seems to be critical in lung homeostasis and could be decisive in the pathogenesis of various lung diseases.
Keywords: Lung homeostasis, Lung epithelia, Mitochondrial Ca2+, Dysfunctional mitochondria, Mitophagy
1. Introduction
Since time immemorial, lung health has been underestimated even though the lung is a vulnerable organ as it has to face numerous environmental irritants. Though air pollution levels touched well beyond the safety limit and have silently killed so many lives hitherto, we didn’t make sufficient efforts to reduce air pollution. This is because there is no acute mortality owing to air pollution. As an outcome of this, people across the world have repeatedly ignored it. However, coronavirus pandemic taught, even an illiterate person in the world about respiratory hygiene and the importance of lung health (Christopher et al., 2020). If we maintain this respiratory hygiene forever, this would reduce the burden of our lungs as these lungs are incessantly exposed to the environment that encompasses a variety of physical, chemical, and biological irritants. So, our lungs should have sufficient protective mechanisms to maintain overall lung homeostasis and also overall body health. Among the variety of lung cells, lung epithelia act as a frontline soldier with physical and immune armamentaria. These mechanisms are mostly innate immune types and can avert the entry of various irritants like pollutants, bacteria, and viruses (Salvator et al., 2020). If these mechanisms are not adequate, acquired immune cells are recruited to eliminate these irritants. Thanks to the immune era, the innate protective mechanisms of lung epithelia have been underestimated. However, emerging evidence indicates the key role of lung epithelia in maintaining lung homeostasis (Spella et al., 2017). As a result, exploring the epithelial protective agents seems to be a novel therapeutic option in various lung diseases.
While lung (organ for whole body respiration) supplies oxygen to whole body, mitochondria (organelle for cellular respiration) use oxygen to generate energy. Both the respiratory apparatuses are crucial in survival of whole body and cell, respectively. The calcium regulation seems to be crucial in both lung and mitochondrion. While lung needs calcium for secretion of mucus, bronchoconstriction, degranulation of various immune cells (Middleton 1983), calcium is a crucial controller of mitochondrial function (Brookes et al., 2004). In this review, we describe how mitochondria and mitochondrial calcium are having key roles in lung epithelial function in the context of lung diseases.
2. Mitochondrion: A regulator of calcium signalling
Mitochondria for a long time were just considered only as a power house of the cell as it can generate adenosine triphosphate (ATP) through oxidative phosphorylation and the energy generated is utilized by the cells for their survival and functions. But now accumulating evidences suggest that it has a wide variety of roles including intracellular calcium homeostasis, regulation of thermogenesis, reactive oxygen species (ROS) generation, apoptosis, regulation of metabolism, inflammatory responses and aging (Vazquez-Calvo et al., 2020). Among all these functions, calcium flux in the mitochondria seems to be critical as it influences almost every mitochondrial function.
Though there was indirect evidence in the 1950s to demonstrate the calcium uptake by mitochondria, only in 1961, it was directly demonstrated using isolated mitochondria. Later, it was discovered that this process was energy/ATP driven and mitochondrial calcium uptake can occur even in the absence of respiration but the presence of ATP is obligatory (Carafoli 2010). However, in a living cell, mitochondrial respiration could be the main inducer for the calcium uptake process. Thus, mitochondrial respiration and its calcium uptake are exclusively interdependent. Earlier functional studies indicated that mitochondria can uptake a large amount of calcium in the presence of phosphate whereas its uptake could be limited in the absence of phosphate (Carafoli 2010). Later, a number of proteins had been discovered that regulate the influx and efflux of calcium across mitochondria in both directions (Finkel et al., 2015). The mitochondrial calcium uniporter (MCU) complex, Na+/Ca2+ exchanger, and H+/Ca2+ exchanger are the major proteins that help mitochondrial calcium migrate back and forth (Finkel et al., 2015). To enter into the mitochondria, cytosolic Ca2+ has to cross the outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) (Xu et al., 2016). Cytosolic Ca2+ entry across the membranes is regulated via certain special protein pores. The OMM has voltage-dependent anion-selective channel proteins (VDACs), which form pores in the OMM and represent the first molecular interface between mitochondria and calcium (Ca2+) stores. Once the Ca2+ reaches intermembranous space, it passes the IMM through the mitochondrial Ca2+ uniporter complex (MCUC). MCU independent pathways are also observed. (Xu et al., 2016).
MCU has two transmembrane domains and it oligomerizes with other proteins. Mitochondrial calcium uptake 1 (MICU1) oligomerizes with MCU and along with mitochondrial calcium uptake 2 (MICU2) forms the mitochondrial calcium uniporter complex MCUC. MICU1 and its homolog M1CU2 interacts with MCU and regulates the transfer of calcium ions by MCU. MCU uniporter homocomplex is regulated by the MCU regulator, MCUb. MICU1 is a single-pass transmembrane protein structure that has two calcium binding EF hands. At low calcium concentration, M1CU1 act as an inhibitor of MCU and prevents the entry of calcium. At a higher calcium level, EF hands of MICU1 and MICU2 get bounded by calcium. The inhibition of MICU2 is relaxed, MICU1 is activated which then helps MCU to facilitate the uptake of calcium (Xu et al., 2016). The main role of MICU1 and MICU2 dimers is to set a threshold of Ca2+ for MCU thereby regulating the detrimental accumulation of Ca2+ inside the matrix under basal conditions (Giorgi et al., 2018). Mitochondria are renowned to have the cellular Ca2+ levels regulated and they can drive ROS generation. Under the condition of mitochondrial Ca2+ overload and in conjugation with the pathological accumulation of ROS results in the sustained opening of the high conductance cyclosporin A-sensitive permeability transition pore. This opening lead to rapid collapse of the mitochondrial membrane potential (ΔΨm), mitochondrial swelling and ultimately release of cytochrome c from mitochondria to the cytosol to initiate cellular apoptosis (Sebag et al., 2018).
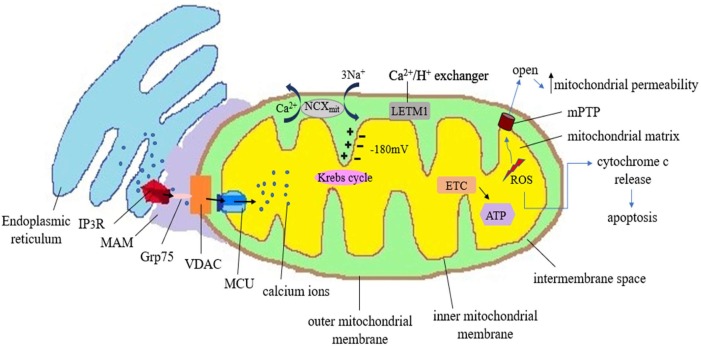
The cytosolic calcium concentration is tightly regulated in the eukaryotic cell. Calcium ions which play the crucial role of second messenger in the signalling pathways are controlled by various pumps, exchangers, channels and numerous binding proteins. Endoplasmic reticulum (ER) and golgi bodies are the reservoirs of calcium in the cells (Rizzuto et al., 2009). The movement of calcium ions from the ER signifies the increase in the level of intracellular calcium. Such movement of calcium ions from the stores occurs primarily through transmembrane protein inositol-1, 4, 5-triphosphate receptor (IP3R) (Xu et al., 2016). When agonists bind to G protein coupled receptor, phospholipase C is formed. It causes generation of inositol-1,4,5-triphosphate (IP3) which diffuses into ER and binds to IP3R (Fig. 1 ). This leads to the clustering of IP3R on the ER membranes and causes calcium wave. Another protein which mediates transmission of calcium in the ER membrane is ryanodine receptor (RyR). Ryanodine and calcium activate RyR (Rizzuto et al., 2009). IP3R causes calcium transport in a quasi-synaptic fashion. This occurs via mitochondria-associated ER membrane (MAM) that harbours both IP3R and VDAC (Fig. 1). The existing negative potential of −180 mV inside mitochondria is the major driving force that instigates calcium uptake by mitochondria (Kania et al., 2017). Voltage dependent anion channel (VDAC), abundantly present in OMM, helps in the import of calcium across the OMM. The negative potential in the transmembrane region then aids in the further transport of calcium ions across the strictly selective MCU ion channel present in IMM (Rizzuto et al., 2009) (Fig. 1). Some associated proteins help in the formation of ER-mitochondria tethering complex. Glucose regulated protein 75 (Grp75) is one such protein which forms IP3R-Grp75-VDAC molecular complex inside the MAM. This cytoplasmic Grp75 that links IP3R with VDAC also brings mitochondria and ER in close proximity with each other (Lee and Min, 2018). MAM is enriched in lipids and regulates intracellular calcium homeostasis. (Vance, 2014) There are some efflux mechanistic pathways that regulate outward movement of calcium from mitochondria. Na2+/Ca2+ exchanger (NCX) found in the mitochondrial membrane is reported to cause efflux of calcium ions (Rizzuto et al., 2009) (Fig. 1). Leucine Zipper And EF-Hand Containing Transmembrane Protein 1 (LETM1) is another eukaryotic proton-calcium antiporter protein that regulates calcium homeostasis (Austin and Nowikovsky, 2019). The uptake of calcium inside mitochondria is a key phenomenon to regulate cell life. The rate-limiting enzymes of Krebs cycle are activated by mitochondrial calcium. So, increase in matrix calcium level also increases the mitochondrial respiration and ATP synthesis (Jaña et al., 2019). However, increased calcium level beyond a threshold proves to be detrimental as it can accelerate ROS generation. Increased mitochondrial calcium level and ROS cause opening of mitochondrial permeability transition pore (mPTP) which further increases mitochondrial permeability and swelling. Calcium overload also promotes cytochrome c release and finally cell death by apoptosis (Görlach et al., 2015) (Fig. 1).
Fig. 1.
This is a schematic representation to illustrate the mitochondira-ER crosstalk and its implications in various cellular functions. ER gets tethered to mitochondria in mitochondrial associated membrane (MAM) regions which has a major role in the mobilisation of calcium ions. The inner negative potential inside the mitochondrial matrix is the thermodynamic motive due to which mitochondria can uptake cations like calcium ions. Calcium leaves ER through channels like IP3R proteins. There are some associated linker proteins in the MAM like cytoplasmic GRP75 (75-kDa glucose-regulated protein). Being a chaperone protein, GRP75 causes a link between IP3R and Voltage Dependent Anion Channel (VDAC) and facilitates calcium uptake. Calcium ions first cross outer mitochondrial membrane (OMM) through gatekeeper VDAC and via tremendously selective MCU (Mitochondrial calcium uniporter), these calcium ions enter into the IMM (Inner Mitochondrial Membrane). Complexes I and III of respiratory chain generate ROS as by-products in the mitochondria. When a certain threshold level is crossed, mitochondrial calcium can promote oxidative stress. As mitochondrial permeability transition pore (mPTP) opens, there is significant increase in membrane permeability which is detrimental to cell. Such direct and indirect increase in the oxidative stress promotes the release of cytochrome c to initiate the apoptosis. Mitochondria have some ion exchanger anti-porters that help to regulate the level of calcium and homeostasis. Na2+ /Ca2+ exchanger (NCX) uses the energy of electrochemical gradient of mitochondria and cause efflux of calcium ion in exchange of sodium ions. Leucine Zipper And EF-Hand Containing Transmembrane Protein 1 (LETM1), a proton calcium exchanger, also has a role in regulating the level of calcium ions inside the mitochondria.
Thus, calcium affects differently mitochondria under physiological and pathological conditions. While calcium is required for most of the mitochondrial function like oxidative phosphorylation under physiological conditions, same calcium leads to mitochondrial stress and cell death under pathological conditions (Brookes et al., 2004).
3. Mitochondrial dysfunction and mitochondrial calcium perturbation in asthma.
3.1. Role of lung epithelial mitochondria in maintaining lung homeostasis
The lung homeostasis depends on the elimination of exogenous irritants. In this defence mechanism of safeguarding, the airway epithelium is robust enough to protect the entire lung. The airway epithelium has one effective strategy, mucociliary apparatus, for efficacious protection, and this is based on the trapping of foreign particles inside the “gel” or “viscous” layer followed by continuous upward ciliary beating to propel the foreign particles out of the airways (Ridley and Thornton 2018). It seems that mitochondria have a major role in the effectiveness of mucociliary apparatus in two ways: a) by providing energy for the ciliary beating and b) by less uptake of intracellular calcium into the mitochondria. Among various regulatory mechanisms for ciliary beat frequency, the increase of intracellular calcium is crucial. More importantly, calcium is also important to coordinate the synchronization of ciliary beats of subsequent airway epithelial cells of the larger airway (Schmid and Salathe 2011). As increased intracellular calcium is a requisite for the ciliary beat, the reduction in intracellular calcium by mitochondrial uptake theoretically will reduce ciliary beating. This was demonstrated by acetylcholine that has the capacity to inhibit ciliary beating through increased mitochondrial uptake of intracellular calcium. All these indicate the feasible role of mitochondria and mitochondrial calcium towards lung homeostasis.
The densely located mitochondria in close proximity to the axoneme basal body of cilia seems to provide energy continuously for the movement of cilia. So, one can expect curtailed ciliary beating if there are dysfunctional mitochondria existing in the ciliated epithelia. Further, the oxidants generated by mitochondria may give stress to the motile cilia (Price and Sisson, 2019). Thus, robust and healthy mitochondria are required for an effective ciliary beating.
3.2. Mitochondrial calcium perturbations in asthmatic airway epithelia
In evidence to the importance of healthy mitochondria in maintaining lung homeostasis, mitochondrial damage with reduced ciliary numbers have been found in severe asthmatic patients compared to mild asthmatic patients. The mitochondrial swelling with abnormal cristae was found in the ciliated epithelia of these severe asthmatic patients (Thomas et al., 2010). It is well acclaimed that mitochondrial swelling is the end product of mitochondrial calcium overload.
Even before this study, our lab had demonstrated such mitochondrial abnormality in asthmatic mice (Mabalirajan et al., 2008). Indeed, after our demonstration, a number of reports have demonstrated the mitochondrial dysfunction in asthmatic conditions (Reddy, 2011). In addition, inducing mitochondrial dysfunction in airway epithelium aggravated the asthmatic airway inflammation (Aguilera-Aguirre et al., 2009). Further, we have demonstrated that these mitochondrial abnormalities in asthmatic conditions are dependent on 15-lipoxygenase (15-LOX) (Mabalirajan et al., 2009). Interestingly, this 15-LOX is required for the physiological disappearance of mitochondria in the process of maturation of red blood cells (RBCs) and lens fibres. Very importantly, calcium induces the binding of 15-LOX to reticulocyte mitochondria to initiate the mitochondrial degradation (Watson and Doherty, 1994) towards the RBC maturation. This indicates that 15-LOX seems to be a crucial enzyme in asthma pathogenesis in causing mitochondrial degradation in airway epithelia. We have further demonstrated that 15-LOX overexpression alone was sufficient to cause mitochondrial damage in naïve mice lungs along with the development of spontaneous asthma-like features even without allergen exposure (Mabalirajan et al., 2009).
Interleukin-4, a main pro-inflammatory T helper type 2 (Th2) cytokine, up-regulates human 15-LOX especially 15-LOX-1 through acetylation of nuclear histones and activation of signal transducer and activator of transcription-6 (Snodgrass and Brüne, 2019). In humans, 15-LOX has been sub-classified into two forms: 15-LOX-1 (reticulocyte), and 15-LOX-2 (epidermis type). 15-LOX-2 exclusively uses arachidonic acid (AA) whereas 15-LOX-1 predominantly uses linoleic acid (LA) as substrates. In mice, there are three major isoforms of 12- and 15 LOX: leukocyte (L), epidermal (E), and platelet (P). 12 & 15 lipoxygenases of humans and rabbits have been classified as 12/15-lipoxygenase (12/15-LOX) as they produce 12-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid from AA and predominantly 13-hydroxyoctadecadienoic acid (13-HODE) from LA. This occurs by incorporation of molecular oxygen in a stereospecific and regiospecific way into respective fatty acids (Archambault et al., 2018). 12/15-LOX deoxygenates mitochondrial membranes of reticulocytes in the process of their maturation towards RBCs. This process is biologically programmed in reticulocytes. However, the pathological increase of 12/15-LOX is known to cause pro-inflammatory effects in various diseases (Singh and Rao, 2019).
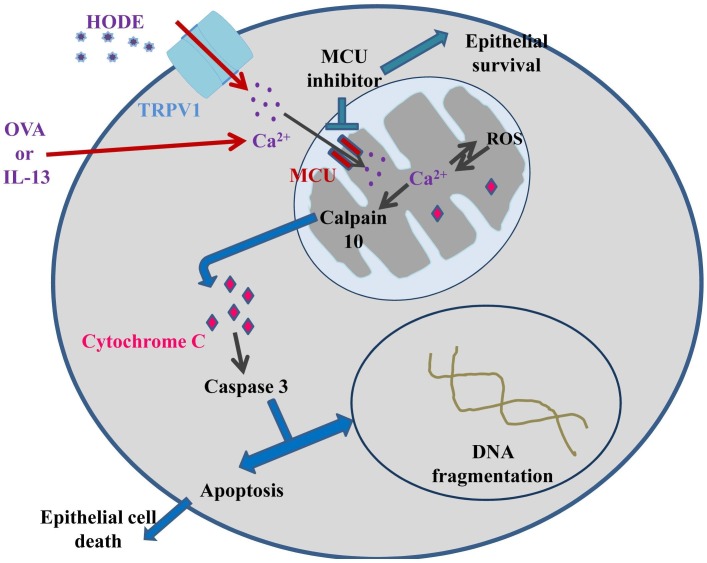
We further demonstrated the role of 13-HODE in causing severe asthma-like conditions (Fig. 2 ). In asthmatic condition, inflammatory cells including macrophages secrete 13-HODE that activates transient receptor potential cation channel subfamily V member 1 (TRPV1) which in turn disturbs calcium homeostasis in epithelial cells. Thus, it increases intra-mitochondrial calcium, and its overload is known to activate calpain 10 in mitochondria, which can cause mitochondrial fragmentation accompanied with release of cytochrome c and ultimately apoptosis of airway epithelial cell (Mabalirajan et al., 2013a, Mabalirajan et al., 2013b, Mabalirajan and Ghosh, 2013). Overall, maintenance of optimal mitochondrial calcium executes a vital role in airway epithelial cell survival.
Fig. 2.
Schematic representation illustrating the possible mechanism of involvement of intramitochondrial Ca2+ in epithelial apoptosis. 13-S HODE secreted from inflammatory cells including macrophages can activate TRPV1 (transient receptor potential cation channel subfamily V member 1) present in the epithelial membrane which perturbs the Ca2+ homeostasis, resulting in an increased intramitochondrial Ca2+, which can activate calpain 10. Calpain 10 releases cytochrome c from mitochondria into the cytosolic milieu. Cytochrome c then activates Caspase 3 which is involved in DNA fragmentation and eventually foments the apoptosis of epithelia. The allergen, chicken egg ovalbumin (OVA)/IL-13 may be involved in increasing intramitochondrial calcium via the MCU complex. Increased intramitochondrial Ca2+ is apparent in inducing ROS production and vice versa, this leads to the release of cytochrome c into the cytoplasm which further activates caspase-3. Caspase-3 can induce epithelial cell apoptosis. MCU inhibitor may inhibit epithelial apoptosis and augment the magnitude of survivability of epithelia.
3.3. Role of mitochondrial calcium uniporter in lung epithelial survival in asthmatic condition
Interleukin-13 (IL-13), a pleiotropic Th2 cytokine, is known to promote mitochondrial ROS production, matrix Ca2+ uptake, and apoptosis in the respiratory epithelium (Sebag et al., 2018). Sebag et al. observed that MCU inhibitor is able to reduce certain pathological features like mitochondrial Ca2+ uptake, loss of mitochondrial membrane potential, ROS production and thus protect primary human respiratory epithelial cells from apoptosis with IL-13 induction. (Sebag et al., 2018). Further, decreased apoptosis of airway epithelial cells was observed upon the deficiency of MCU in the ovalbumin model of allergic inflammation (Sebag et al., 2018). In airway epithelium isolated from MCU-/- mice, the epithelial barrier was preserved upon IL-13 induction. Thus, it can be concluded that Ca2+ uptake via MCU in presence of allergen can induce excess ROS production. As a result, mitochondrial membrane potential dissipates and cytochrome c is released and it activates apoptosis in respiratory epithelium. In turn, epithelial cell death results in the loss of epithelial barrier function (Sebag et al., 2018). MCU seems to be a potential target to treat diseases like asthma which are associated with excessive ROS and compromised epithelial barrier.
3.4. Consequent biological mechanisms post-intramitochondrial calcium overload induced mitochondrial damage
Mitochondria have a sophisticated quality control mechanism by which it can regulate excessive or damaged mitochondria and eliminate it. These mitochondrial quality control (MQC) mechanisms include mitochondrial fission and fusion process and mitophagy (Garza-Lombó et al., 2020). Mitophagy has a vital role in stressed condition. Disturbance in MQC can result in cell injury/death. MCU complex regulates the uptake of cytosolic Ca2+ into mitochondria thus resists intramitochondrial Ca2+ overload. However, under stressed conditions, abnormal increase in mitochondrial Ca2+ can affect mitochondrial fusion-fission and can promote excessive mitophagy. Many studies have indicated an aberrant increase in mitochondrial Ca2+ as the main cause of mitochondrial fission (Yu et al., 2020). While the role of autophagy in asthma is controversial, the role of mitophagy in asthma pathogenesis is not yet investigated in detail.
3.5. Compartmental effects of mitochondrial calcium uptake to safeguard airway epithelia
It is well known that mitochondria act as calcium buffering tanks when there is an increase in intracellular calcium. In general, we consider that the accumulation of calcium in the mitochondria is uniform to the whole cell. But evidence indicates that mitochondria act smartly in such a way that they buffer intracellular calcium in a particular portion of the cell from where cells receive a signal through receptors. For example, when airway epithelium was induced by the purinergic receptor P2Y2 (P2Y2-R) that increases intracellular calcium, mitochondrial uptake of calcium was only observed in a cellular region where P2Y2-R is located (Ribeiro et al., 2003). So mitochondrial calcium overload seems to be restricted to a particular compartment in such a way that those mitochondria act as structural barriers to protect the airway epithelia. This smart strategy by mitochondria is very interesting in the context of multiple copies of mitochondria in a given cell and the existence of rescue mechanisms like mitophagy. Mitochondrial calcium overload is itself a menacing situation for the survival of mitochondria. But mitochondrial jeopardy should not reflect straight away to the entire cell. So, if these compartmental effects are effective, only a few copies of mitochondria might be affected and obliterated by mitophagy. Consequently, there would be a reduction in the accumulation of dysfunctional mitochondria, hence, curtailment in the generation of reactive oxygen free radicals from these dysfunctional mitochondria (Ribeiro et al., 2003). Then there is a good possibility of cell survival with the mitochondrial biogenesis, and mitophagy like rescue mechanisms. This kind of mechanism seems not only restricted to receptor-induced cell but also in response to luminal stress. For example, cough induces shear stress or luminal stress and, in this condition, the large mitochondrial barrier at the apical cellular pole acts as a buffer for intracellular calcium signals. It may serve as a functional barrier between the apical domain and the rest of the cell to prevent global Ca2+ waves, thus shielding the nuclear and basolateral cytoplasmic compartments from intracellular Ca2+modulated functions (Ribeiro et al., 2003). We all are well aware that cough is a common symptom in a number of infectious and non-infectious lung diseases. If cough leads to disturbance in the entire airway epithelial layer in the form of luminal stress, it might create havoc in lung homeostasis. Thus, the existence of such compartmental effects in airway epithelium indicates how lung epithelia that are exposed ceaselessly to the external environment manage to maintain lung homeostasis.
3.6. Asthmatic airway smooth muscle remodelling due to mitochondrial biogenesis via calcium dependent pathway
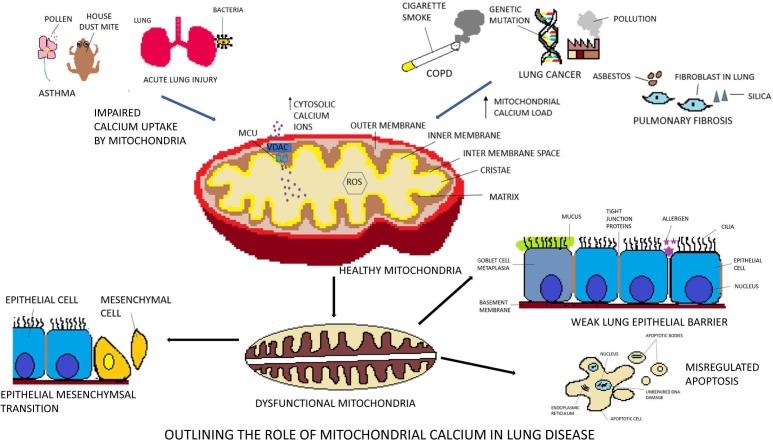
While lung epithelia had shown the mitochondrial dysfunction in various lung diseases like asthma and chronic obstructive pulmonary disease (COPD) (Fig. 3 ), mitochondrial hyperactivity with a greater number of mitochondria was observed in airway smooth muscle of severe asthmatic patients. The mitochondrial hyperactivity in airway smooth muscle of asthmatics is shown to be attributable to mitochondria biogenesis through a calcium-dependent pathway (Trian et al., 2007). Cultured human airway smooth muscle cell had shown increased cytosolic calcium with the disruption of the mitochondrial calcium flux and reduced calcium buffering of mitochondria was observed upon stimulation with tumor necrosis factor-α. Such increased cytosolic calcium owing to undermined mitochondrial calcium uptake by MCU and NCX can contribute to airway contractibility which happens in asthmatic individuals (Delmotte et al., 2012).
Fig. 3.
This is a schematic representation to underline the role of mitochondrial calcium in various lung diseases. The mitochondrial respiratory chain is the predominant source of Reactive Oxygen Species (ROS). An increase in intracellular calcium level leads to the opening of the mitochondrial membrane transition pore (mPTP). This causes depolarisation of mitochondria and ROS generation. It brings about a transmutation in the mitochondrial osmolarity and damage to the outer mitochondrial membrane (OMM). Breaches in the outer membrane cause release of proteins like cardiolipin and cytochrome c from the inner mitochondrial membrane (IMM). Dysfunctional mitochondria in the airway smooth muscle is the key player in lung disease. Asthmatic patients have impaired mitochondrial calcium buffering by MCU which cause contraction in airway smooth muscle. Such undermined calcium uptake also activates calcium dependent responses and lead to disintegration of the airway epithelial barrier in ALI. In addition, mitochondrial Ca2+ overload cause damage to the mitochondrial DNA in ALI (acute lung injury). Cigarette smoke exposure induced COPD involves enhanced ROS generation and oxidative damage to mitochondrial DNA. Thus, homeostasis in the mitochondrial calcium has a pronounced contribution in regulating the phenomenon of Epithelial Mesenchymal Transition (EMT) that eventuates both in lung cancer and pulmonary fibrosis.
4. Mitochondrial dysfunction and mitochondrial calcium perturbation in COPD
COPD is a debilitating lung condition characterized by airway inflammation (chronic bronchitis), destruction of lung tissue (emphysema) and small airway remodelling (Cloonan and Choi 2016). Although among the COPD patients, 20% are non-smokers, the majority of them have a previous history of cigarette smoking. The status of mitochondria and mitochondrial calcium in the pathogenesis of COPD have been extensively investigated.
4.1. Mitochondrial dynamics in COPD lungs
Aberrant mitochondrial morphology and dynamics have been implicated in COPD. There is increased fission as well as hyperfusion of mitochondria in COPD airways (Prakash et al., 2017). Swollen, elongated mitochondria and disrupted cristae have been found in bronchial epithelial cells of COPD patients (Lerner et al., 2016). The cigarette smoke (CS) induced increase in mitochondrial fission in human epithelial cells and airway smooth muscle is ascribed to a decrease in Mitofusin 2, a mitochondrial fusion protein and an increase in Dynamin related protein 1 (Drp1), a mitochondrial fission protein (Prakash et al., 2017). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), the primary inducer of mitochondrial biogenesis, is reduced in moderate to severe COPD patients (Lerner et al., 2016, Prakash et al., 2017). There is marked cellular hypoxic response even during normoxia in the airway epithelium of mice with COPD that leads to a decrease in mitochondrial biogenesis (Chaudhuri et al., 2019). The mitochondria damaged due to CS exposure are removed primarily by phosphatase and tensin homolog (PTEN) induced kinase 1 (PINK1)- Parkinson disease 2 (PARK2) pathway. In COPD lungs, there is a reduction in PARK2 levels, and translocation of Parkin to damaged mitochondria is hampered. This leads to impaired mitophagy resulting in the accumulation of damaged mitochondria in COPD lungs (Chaudhuri et al., 2019). In addition to the above changes in mitochondria, high levels of deoxyribonucleic acid (DNA) damage and defective DNA damage repair pathways in mitochondria of type II alveolar epithelial cells (AECs) have been found in emphysema patients (Kosmider et al., 2019).
4.2. Mitochondrial dysfunction (MD) in COPD lungs
MD is extensively associated with the pathobiology of COPD and the presence of MD in smokers with or without COPD makes them more susceptible to respiratory infections (Aghapour et al., 2020). MD has been detected in lung epithelial cells treated with smoke (Lerner et al., 2016). The effect of cigarette smoke extract (CSE) on mitochondria varies with different concentrations. At lower concentrations of CSE, mitochondria show an initial adaptive response (mitohormesis), whereas, higher concentrations or prolonged exposure leads to mitochondrial dysfunction. Mitochondrial dysfunction results in the production of huge amount of mitochondrial reactive oxygen species (mtROS), cellular stress, and cell death ushering in tissue injury. In COPD patients, tissue injury and cell death have consorted with necrosis, which leads to inflammation and further degeneration of the airways (Aghapour et al., 2020, Chaudhuri et al., 2019). Other than necrosis, apoptosis has been implicated in COPD. So, it is speculated by investigators that perhaps apoptosis occurs at lower concentrations of tobacco smoke exposure and necrosis ensues at higher concentrations or longer exposure (Vayssier et al., 1998, Ramage et al., 2006).
Damaged mitochondria can release many components into the cytosol or extracellular milieu in response to inflammation, oxidative stress or other factors called mitochondrial damage-associated molecular patterns (mtDAMPs) (Prakash et al., 2017). Cytosolic levels of mtDAMPs like mtROS, Ca2+, mitochondrial DNA (mtDNA), ATP, cardiolipin are found to be elevated in COPD lung epithelial cells. CS-induced increase in mitochondrial membrane permeability opens ion channels like mPTP that leads to iron overloading in mitochondria and cytoplasmic accumulation of mtDAMPs (Aghapour et al., 2020). CS-induced mPTP opening is associated with mtDNA release. Oxidized DNA binds to NLR Family Pyrin Domain Containing 3 (NLPR3) inflammasome, interacts with mitochondria-derived cardiolipin, and activates the inflammasome, mediating the release of inflammatory cytokines like interleukin-1β and interleukin-18 (Lerner et al., 2016). In COPD, increased levels of ATP, mtDAMP, have been found in the bronchoalveolar lavage fluid. Extracellular release of ATP from damaged cells is recognized by purinergic receptor P2X7 expressed on immune cells and lead to the release of interleukin 1β (Cloonan and Choi 2016). CS induces an increase in mtROS which has been found to dissociate tight junctions by activating epidermal growth factor receptor (EGFR) and extracellular signal-related kinase (ERK) pathway. When tight junctions are disrupted, the permeability of lung epithelial barrier increases (Aghapour et al., 2020). MtROS also activates the matrix metalloprotease-2 that results in the loss of Δψm. There is a loss of Δψm in the airway epithelial cells of COPD lungs that have been connected to COPD related inflammation (Lerner et al., 2016). Thus, lung epithelial integrity is lost in COPD patients due to mitochondria mediated oxidative stress.
4.3. Metabolic switching in mitochondria of COPD lungs
Mitochondrial metabolic switching is a contributing factor in the pathogenesis of COPD. CS exposure at toxic levels leads to diminished oxidative phosphorylation in mitochondria of lung epithelial cells. Low levels of CS exposure prompt a metabolic shift from glycolysis to β-oxidation of fatty acids (Cloonan and Choi 2016). Type II AECs produce acetyl coenzyme A in mitochondria for de novo fatty acid synthesis required for generating phospholipids for surfactant production. Altered glycolysis and increased β-oxidation of fatty acids for energy production in Type II AECs exposed to CS affects the surfactant synthesis pathway and accounts for the surfactant deficiency found in smokers and COPD patients (Cloonan and Choi, 2016, Agarwal et al., 2014).
4.4. Mitochondrial calcium perturbation in COPD lungs
Mitochondria are major regulators of second messengers like Ca2+ that control myriads of cellular processes. MD may crop up due to overload or loss of mitochondrial Ca2+ and has been linked to a plethora of lung diseases (Cloonan and Choi 2016). Mitochondrial calcium is primarily regulated by the ion channel, MCU that enhances mitochondrial Ca2+ influx and Na+/Ca2+ exchanger and Ca2+/H+ exchanger that allows Ca2+ efflux (Prakash et al., 2017). Recently, in a study by Srivastava et al. MCU was found to be highly expressed in the lungs of COPD patients. MCU silencing in CSE exposed primary bronchial epithelial cells led to loss of Δψm and mitochondrial superoxide generation. In this study, it was also found that in an in vitro model of COPD, exposure of 5% CSE for 6 h to bronchial epithelial Beas2B cell line, there was increased the mitochondrial Ca2+ loading (Srivastava et al., 2020). A rise in mitochondrial Ca2+ has been found to trigger mitophagy (Chaudhuri et al., 2019). Increased mitophagy has been found in the lungs of COPD patients (Cloonan and Choi 2016). When mitochondria become overloaded with Ca2+, the intrinsic pathway of apoptosis gets activated and the membrane potential necessary for metabolism is collapsed (Chaudhuri et al., 2019). There is evidence showing activation of the mitochondria-mediated intrinsic pathway of apoptosis, breakdown of mitochondrial membrane potential and blunted metabolism in COPD (Lerner et al., 2016, Prakash et al., 2017, Yoshida and Tuder, 2007). In a study by Barrero et al. it was found that histone 3.3 found extracellularly, binds to the cell membrane, induces Ca2+ influx, ER Ca2+ pool emptying, unfolded protein response induction, and elevation of mitochondrial Ca2+, leading to the apoptosis of structural lung cells (Barrero et al., 2013). Mitochondrial Ca2+ overload can disrupt tight junctions in airway epithelial cells (Aghapour et al., 2020). Airway epithelial barrier disruption has been widely reported in the lungs of COPD patients (Aghapour et al., 2020, Heijink et al., 2014). Thus, mitochondrial Ca2+ mediated dysfunction serves a substantial role in the pathogenesis and progression of COPD.
5. Mitochondrial dysfunction and mitochondrial calcium perturbation in other lung diseases
5.1. Mitochondrial calcium buffering role determines the alveolar endothelial barrier in acute lung injury (ALI)
While hyperpermeability of the alveolar microvascular system is a prime event in causing loss of endothelial barrier in lung injury, a recent study indicated that endothelial mitochondrial depolarization seemed to be the basis for the loss of endothelial barrier (Hough et al., 2019) Further, it has been demonstrated that the endothelial mitochondrial depolarization leads to actin depolymerization to cause failure of the endothelial barrier in the alveoli. But the crucial event that amalgamates the endothelial mitochondrial depolarization with the failure of the endothelial barrier in alveoli is the mitochondrial calcium buffering role (Hough et al., 2019). Since depolarized mitochondria lose the capability of calcium buffering, there is increased intracellular calcium that further prompts the activation of calcineurin which further dephosphorylates the calcineurin target gene, cofilin to cause actin depolymerisation. These indicate the ability of endothelial mitochondria to regulate alveolar endothelial barrier via its calcium buffering role (Hough et al., 2019). Damage to mitochondrial DNA can trigger ALI (Lee et al., 2017). The increased load of intracellular calcium proffers a burden on the mitochondria and generates an excess of scavenger molecules that cause damage to the mitochondrial DNA. A positive feedback loop occurs involving more mitochondrial DNA damage and ROS production leading to leakage in the alveoli and lung injury (Parker, 2018). Activation of Toll-like receptor 4 (TLR4) and NADPH oxidase 2 (Nox2) pathway increases ROS generation in the lipopolysaccharide (LPS) given model of lung injury. During sepsis and lung injury, high intercellular calcium oscillations occur (Parker, 2018). It causes the release of calcium from the internal store and introduction of calcium from the store-operated calcium entry (SOCE) channel located in the plasma membrane. When the internal calcium store is depleted, the amount of calcium that binds to the EF motif hand of the ER luminous domain of calcium sensor Stromal Interaction Molecule 1 (STIM1) decreases. STIM1 oligomerises and the SOCE channel located in the plasma membrane is activated. More calcium enters the cell and the calcium oscillations continue. Thus, STIM1 sense depletion of calcium and mediates endothelial cell apoptosis that occurs in LPS induced pulmonary edema. STIM1 can also play the role of an important factor in acute respiratory distress syndrome (Gandhirajan et al., 2013).
5.2. Mitochondrial calcium perturbation in idiopathic pulmonary fibrosis (IPF)
Mitochondrial calcium also plays a role in IPF, a progressive debilitating chronic lung condition. MCU gene expression is seen to be higher in IPF subjects than control and mitochondrial calcium is elevated in lung macrophages of IPF mimicking bleomycin mice model. The PGC-1α plays a major role in IPF (Caporarello et al., 2019) and it has an impact on the expression of ROS generating enzymes (Austin and St-Pierre, 2012). ROS generation is induced by the transcriptional activation of p38 mitogen-activated protein kinase and activating transcription factor 2 (ATF2) phosphorylation which cause an increase in PGC-1α gene expression. Chelating the calcium ions decreases the level of pATF2 and p38 mitogen-activated protein kinase both in the presence and absence of MCU. Thus, mitochondrial calcium influx and MCU can be a therapeutic target in IPF (Gu et al., 2019). Mitochondrial hormone Stanniocalcin 1 which regulates calcium homeostasis also has a role in the secretion of transforming growth factor beta and can be a therapeutic target in IPF (Ohkouchi et al., 2015).
5.3. Mitochondrial calcium perturbation in cystic fibrosis
The cystic fibrosis transmembrane conductance receptor (CFTR) protein is a chloride ion (Cl-) channel expressed on the apical membrane of cells that lines the airways and maintains salt and water balance on the surface of the lungs (Favia et al., 2019). It has ATPase activity and allows the exit of electrolytes such as Cl- and sodium (Na+) ions from the cells along with secretion of water. It is responsible for secretion of bicarbonate (HCO3 –) ions that is involved in the bactericidal activity of the liquid lining the airways (Favia et al., 2019). It is also involved directly in the secretion of glutathione, implicating its role in controlling oxidative stress in the airways. Over 2000 mutations of the CFTR gene has been found so far. The most frequently occurring mutations of the CFTR gene is the deletion of 3 base pairs in both the copies of the gene resulting in the loss of phenylalanine residue at position 508 of the protein (F508del) (Favia et al., 2019). The misfolded CFTR protein (F508del) is retained in the ER via interactions with many ER chaperone proteins and hence unable to reach the plasma membrane and mediate Cl- efflux (Favia and Atlante, 2016, Antigny et al., 2009).
In the airway epithelium, mitochondria functions like a Ca2+ buffering system and acts as a barrier conferring protection to the airway epithelial cells against non-specific activation of Ca2+ regulated functions. Increased intracellular calcium and large negative potential across the inner mitochondrial membrane produced by H+ gradient drives the intake of calcium by mitochondria mediated by the electrogenic channel, mitochondrial calcium uniporter (MCU) (Antigny et al., 2009). Even before CFTR gene cloning, Feigal et al. had observed an increased calcium uptake by mitochondria of the fibroblasts of cystic fibrosis (CF) patients and attributed it to altered oxidative phosphorylation (Valdivieso and Santa-Coloma, 2013). On the contrary, more recently, Antigny et al. reported that the ability of the mitochondria to uptake calcium is decreased in human F508del-CFTR tracheal gland cells (CF-KM4 cells) in comparison to non-CF cells (tracheal serous gland epithelial MM39 cells). In addition, F508del-CFTR cells also had shown the depolarization of mitochondrial membrane. Thus, it leads to a dysfunction of the Ca2+ uniporter and ultimately inhibition of the buffering power of mitochondria in CF cells (Antigny et al., 2009).
CF patients are prone to infection from Pseudomonas aeruginosa. Such an infection in CF patients, causes an aggravated inflammatory response in their bronchial epithelial cells. This is because bacteria-derived flagellin in those patients induces mitochondrial calcium uptake via MCU that leads to NLPR3 inflammasome activation resulting in a heightened inflammatory response (Rimessi et al., 2015).
Studies show that the impairment of autophagy in CF bronchial cells with P. aeruginosa infection is due to increase in the tethering of mitochondria and ER and alteration in the calcium exchange via MCU. MCU inhibitor KB-R7943 minimises such autophagic defects. This MCU inhibitor also helps in reducing the heightened inflammatory response that occurs CF patients having infection with P. aeruginosa (Rimessi et al., 2020).
Thus, it can be seen that calcium homeostasis in mitochondria is disturbed in CF condition. However, the reason for the differential uptake of calcium by mitochondria in different cell types in the lungs of CF patients is not clear and requires further investigation.
5.4. Mitochondrial calcium perturbation in lung cancer
Disrupted mitochondrial calcium dynamics is blameworthy in promoting lung cancer. The ability of the tumour cells to survive is connected to disbalance in mitochondrial dynamics. Calcium levels in mitochondria seem to play a role in promoting epithelial-mesenchymal transition (EMT), a major phenomenon in cancer. The major components of SOCE, STIM1 and Orai 1 (Calcium release-activated calcium channel protein 1) are upregulated and calcium is released from ER (Romero-Garcia and Prado-Garcia 2019). Calcium transfer from the ER to mitochondria demonstrates a crucial signal for apoptotic induction. Calcium load in the mitochondria causes the opening of the mPTP and depolarisation of mitochondria. This cause mitochondrial fragmentation and release of cytochrome c and death of cell by the process called apoptosis (Romero-Garcia and Prado-Garcia 2019). Myeloid cell leukemia 1 (Mcl-1), a member of anti-apoptotic B cell lymphoma 2 protein family, is seen to be overexpressed in lung cancer cell lines and non-small cell lung cancer. A study has shown that Mcl-1 has the ability to bind to VDAC-1 and can promote migration of the lung cancer cell. This promotes more calcium uptake by mitochondria and causes more ROS generation. An important tumor suppressor in lung cancer is PTEN that plays a role in influencing the calcium transfer from ER to mitochondria and its depletion disrupts the calcium release and slashes the calcium concentration inside mitochondria (Morciano et al., 2018). Investigative studies have found that the exposure to calcium channel blockers (CCB) tends to increase the risk of lung cancer (Rotshild et al., 2018).
6. Mitochondrial calcium homeostasis at the crossroad of aging and regulating the lung epithelial barrier
Aging is an inevitable process that occurs at the price of other evolutionary selected functions and represents a progressive loss of homeostasis and physiological integrity. With the expected rapid growth of the aging population worldwide, an explicit understanding of the complex process of aging is demanded to develop novel interventions that might boost up the healthy lifespan. An array of aging theories has been proposed, and the mitochondrial free radical theory of aging (MFRTA) has been in the limelight for several decades (Bratic & Larsson 2013). The role of mitochondria in aging was proposed more than 40 years ago by Denham Harman in 1956 (MacNee et al., 2014) for the very first time, suggesting that mitochondria are one of the predominant sources and targets of ROS that could function as an ‘aging clock’. The MFRTA theory is based on several observations: (a) Enhanced mitochondrial ROS generation with age because of slackening in mitochondrial function, (b) activity of several ROS-scavenging enzymes declines with age, (c) accumulation of aging-associated mutations in mtDNA (Li et al., 2020), and (d) a vicious cycle occurs because somatic mtDNA mutations impair the respiratory chain function, which in turn kindles the accumulation of molecular damage to proteins, lipids, and DNA which accounts for progressive deleterious changes described as cellular senescence (Bratic and Larsson, 2013, MacNee et al., 2014, Seo et al., 2010).
Mitochondria regulates a milieu of metabolic and signaling pathways and also plays an important role in programmed cell death. Beyond their main role in the cell to produce NADH and ATP via the process of oxidative phosphorylation, it is now well accepted that mitochondria are pivotal organelles in Ca2+ buffering. As we all are aware that oxidative phosphorylation and Ca2+ homeostasis are mutually interdependent, intricately enough, increasing mitochondrial Ca2+ concentration activates the three mitochondrial enzymes, such as pyruvate dehydrogenase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase of the Krebs cycle, thus causing increased ATP production. However, mitochondrial functions decline with aging, concomitant with the appearance of mitochondrial morphological alterations, e.g., mitochondrial DNA damage, abnormally rounded mitochondria in aged mammals, and diminished capacity to produce energy through oxidative phosphorylation due to the perturbed mitochondrial calcium homeostasis (Srivastava, 2017).
In 1980s, Khachaturian proposed the notion of “Ca2+ hypothesis of aging” including the role of abnormal Ca2+ homeostasis in neurodegeneration (Nikoletopoulou & Tavernarakis 2012). The mitochondrion, due to its multiple cellular functions, plays a critical role in the aetiology of several aging and age-related human diseases. To prove this, breaches in mPTP has been proclaimed in aged animals compared to young ones and importantly mPTP inhibition had shown to ameliorate the lifespan (Panel et al., 2018). In addition, the plasticity and motility of mitochondria have been shown to reduce with aging. Further, their capacity for mitochondrial biogenesis is reduced owing to a decline in PGC-1α activity, which might be instigated by an age-related increase in ROS production. The reduction in matrix calcium causes decrease in oxidative phosphorylation, ATP production and oxidation of NAD(P)H resulting in reduced antioxidant capacity (Finkel et al., 2015). Mitochondrial dynamics studies (Liu et al., 2020a), have revealed that functional and structural alterations in mitochondrial morphology associated with aging are the crucial factors engaged by the cell to cope with the decline in mitochondrial activity.
With the increased focus on age-dependent alterations in organ structure and function mediated by deregulated mitochondrial calcium homeostasis, there is an emerging interest in the impact of deregulated mitochondrial calcium homeostasis with aging on the lung epithelial barrier. The main physiological function of the lung is to optimize alveolar gas exchange and match ventilation with blood flow in the circulation; this requires the contraction and relaxation of airway and vascular smooth muscle cells. Successful gas exchange needs coordinative help from various lung cells and most of these lung cells are mitochondria rich. For example, ciliated airway epithelia, airway smooth muscle, demand more robust mitochondria for ciliary beating, smooth muscle contraction, respectively (Cloonan and Choi, 2016, Pan et al., 2019). Hence, lung mitochondria play a central role in the provision of energy for this function; however, apart from energy metabolism, intracellular mitochondrial signalling functions are indispensable for cell protection and disease prevention. Further, mitochondrial regulation of the epithelial barrier seems to be decisive in regulating mucociliary function.
A wealth of data suggests that increased mtROS generation and calcium overload in mitochondria damages epithelial tight junctions and thus affect the airway epithelial barrier. Ito and Barnes proposed that with increasing age the lung loses its ability to maintain its integrity (Mercado et al., 2015). The damage to the mitochondrial DNA has been shown to be associated with COPD. Mitochondrial dysfunction with aging induces oxidative stress by overproduction of mtROS (Wu et al., 2019), which increases the permeability of the epithelial barrier leading to a leaky airway which consequentially engenders various lung pathogenesis (Aghapour et al., 2020). The mtROS may dissociate the tight junctions through the activation of EGFR and ERK signalling (Aghapour et al., 2020). Age-related alterations impair the mitochondrial function particularly in cells with high energy demands, including ciliated cells of the airway epithelium, and alveolar epithelial cells. The tight junctions present in airway epithelial cells seal the intercellular regions and thus maintain the epithelial barrier integrity (Yanagi et al., 2015). Similarly, the barrier in alveolar epithelia is very crucial as its loss of integrity leads to irreversible lung scarring and fibrosis. (Yanagi et al., 2015).
Thus, aging is an important risk factor for the development of lung diseases as increasing age is strongly associated with deregulated mitochondrial calcium homeostasis along with loss of lung epithelial integrity.
7. Possible therapeutic targets of mitochondrial calcium regulators in lung diseases
7.1. Calcium channel blockers (CCBs) in asthma therapy and its relation with mitochondrial calcium perturbation
A surge in the intracellular calcium has been demonstrated to be involved in various pathophysiological features of asthma-like airway constriction, eosinophil migration into the airways, mast cell degranulation, and secretion from airway epithelia. Due to this widespread involvement of intracellular calcium, CCBs have been suggested and also demonstrated to counteract asthma (So et al., 1986). Among various calcium-mediated asthma features, airway smooth muscle contraction seems to be dominant effect of increased intracellular calcium. So calcium channel blockers that inhibit L-type calcium channel have been tried in asthma. The dihydropyridines, phenyl alkylamines, and benzothiazepines are three major CCBs (So et al., 1986). Though these CCBs might have an effect on mitochondrial calcium, detailed studies are scarce in the context of lung diseases. In the 1980 s, it has been suggested to use CCBs for asthma management at least for the patients who also have cardiovascular comorbid conditions and needed to take adrenergic blockers (So et al., 1986). In any event, CCBs had shown very weak bronchodilatory effects. This could be due to the fact that available CCBs mostly act by inhibiting voltage-operated calcium channels whereas bronchodilators need inhibition of receptor-operated calcium channels (Hendeles and Harman, 1987). As a result, CCBs were not used in asthma as a regular option. So we might need next-generation CCBs that could inhibit calcium flux through receptor-operated calcium channels and more specifically act also through mitochondrial calcium channels. More precisely, we also need mitochondria targeted drug delivery strategies like using liposomal-based carriers, MITOporter to improve the efficacy (Yamada and Harashima, 2017). However, gallopamil, a CCB, medicated to severe asthmatic patients for a year decreased proliferation of bronchial smooth muscle cells, reduced airway remodelling along with less sudden exacerbations compared to the control group (Girodet et al., 2015). And it is known that asthmatic patients undergoing airway remodelling show proliferation in the bronchial smooth muscle mass due to increased mitochondrial biogenesis. Similarly, the treatment of asthmatic patients with CCBs increased the endurance of asthmatic patients. Though ruthenium 360 (Ru360), a ruthenium red related MCU inhibitor, has been used as an in vitro tool to study the mitochondrial calcium, it has not been assessed in any in vivo models. However, MCU knockout mice had shown a beneficial effect in asthma by reducing mitochondrial calcium overload and also by maintaining epithelial barrier (Sebag et al., 2018).
7.2. Mitochondrial calcium as COPD target
On the one hand, oxidative stress is a very common feature that is linked to many of the COPD pathophysiological features, and on the other hand, mitochondrial calcium perturbation along with MD has been reported in lung epithelia of COPD. Drp1 phosphorylation happens in COPD and this plays an important role in mitochondrial fragmentation. Thus, targeting oxidative stress and mitochondrial calcium could be a potential COPD therapeutic option. A mitochondria targeted antioxidant like MitoQ and mitochondria localized antioxidant Tiron can attenuate the stress that happens upon cigarette smoke exposure (Agarwal et al., 2014). The silencing of MCU causes loss of mitochondrial membrane potential and hence mitochondrial dysfunction. Such depolarised mitochondria reduce the capacity of mucociliary clearance which is an important survival strategy against mucus hypersecretion in COPD. Thus, restoring healthy mitochondria by reducing mitochondrial calcium load can be a therapeutic strategy against COPD (Srivastava et al., 2020).
7.3. Mitochondrial calcium as a target in acute lung injury
While MCU inhibition seems to have beneficial effects in asthma and COPD; MCU overexpression had shown the attenuation of LPS induced acute lung injury (Seeley et al., 2013). This indicates the controversial role of MCU in maintaining lung epithelial homeostasis. Thus, it needs a thorough investigation to find the exact role of MCU in lung diseases. However, treatment with bistrifluoromethyl pyrazole derivative, N-{4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl] phenyl}-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2), an inhibitor of calcium release-activated calcium channel, causes a reduction in TLR4 mediated ROS generation. It also causes a reduction in calcium flux and lung injury (Seeley et al., 2013). This shows an established positive feedback loop that eventuates between mitochondrial calcium, mitochondrial DNA damage and ROS production to cause alveolar leakage in acute lung injury.
7.4. Mitochondrial calcium as a target in lung cancer
MCU also plays a critical role in regulating lung cancer. MCU knockdown reduces invasion of cells and lung metastasis while MCU overexpression encourages lung metastasis by mediating aerobic glycolysis. MicroRNA 340 reported in breast cancer was used to inhibit MCU and lung metastasis by regulating the Warburg effect (Yu et al., 2017). A novel small molecule DS16570511 inhibits the influx of calcium into mitochondria. By blocking the interaction between MCU and MICU1 it forestalls calcium load in mitochondria in the cardiac cells (Kon et al., 2017). Mitoxantrone is a recently developed molecule by a combinational approach that blocks MCU calcium uptake without influencing oxidative phosphorylation (Arduino et al., 2017). Another well-known MCU inhibitor is Ru 360, which reduces uptake of calcium, prevents dissipation of the mitochondrial membrane potential and mPTP opening. Studies have shown the use of Ru 360 in colorectal cancer (CRC) (Liu et al., 2020b). However, Ru360 has its own drawback. Recent studies developed a new modified molecule Ru265 which is a more potent inhibitor. Minimally toxic Ru265 inhibits MCU activity and is shown to be effective against hypoxia and regeneration injury (Woods et al., 2019). In any event, the effects of these small molecules in lung diseases are yet to be investigated. However, small therapeutic molecules targeting mitochondria sometimes fail to enter due to the semi-permeable double-layered outer mitochondria membrane (Wang et al., 2016).
8. Conclusions
In lung disease biology, most of the currently available therapeutics is based on the earlier conceptual changes (Chu and Drazen 2005). For example, asthma was considered a psychosomatic disorder or “asthma nervosa” in Hippocrates period and as a result “mind control” was one of the major therapeutic options for asthma in those times. This is partially true now also because the neurogenic axis causes sudden bronchospasm when the asthma patient feels either the greatest joy or sadness. Later, in the 20th century “asthma nervosa” concept was updated with “Airway smooth muscle involvement” followed with “airway hyperresponsiveness” and then with “airway inflammation”. These conceptual changes led to the introduction of anti-cholinergic bronchodilator (by smoking Asthma cigarettes that deliver Belladonna alkaloids), nonanti-cholinergic bronchodilators (epinephrine, theophylline followed by specific beta agonists like salbutamol) and anti-inflammatory agents (corticosteroids, leukotriene agonists) in asthma therapy (Chu and Drazen, 2005). As a result, currently we have a very common drug combination: corticosteroids and bronchodilators for better asthma management. Now one of the major problems in asthma research as well as for the thoracic clinicians is “airway remodeling” as there is no single drug that can reduce this remodeling, though bronchial thermoplasty (application of radiofrequency energy to heat the airway wall and this reduces the quantity of airway smooth muscle) had shown some benefits (Boulet 2018). In airway remodeling, almost each and every cell in the airways increases in number and size, hyperplasia and hypertrophy respectively. The sub-epithelial fibrosis, goblet cell metaplasia, airway smooth muscle hypertrophy and hyperplasia are major components of airway remodeling. Initially, it was believed that airway remodeling is merely a continuation of repeated airway inflammation. Now, we know that this concept is not entirely true for two major reasons: a) anti-inflammatory agents could not stop the initiation of airway remodeling, b) both airway inflammation and airway remodeling changes appear simultaneously. All these indicate that airway remodeling is a very different phenomenon and only a small part of it occurs as a resolution of inflammation (Fehrenbach et al., 2017). Now, we have a concept that airway epithelial injury can happen even without airway inflammation and this injury induces airway remodeling changes (Fehrenbach et al., 2017). On the one hand, mitochondrial calcium overload seems to be crucial in causing airway epithelial injury through mitochondrial dysfunction that could also disrupt the epithelial integrity (Mabalirajan et al., 2013a) and thus aggravate the asthma condition by more allergen entry into the airways. On the other hand, mitochondrial calcium had been shown to be important in airway smooth muscle hypertrophy in severe asthmatic patients through mitochondrial biogenesis (Trian et al., 2007). Thus, mitochondrial calcium seems to have very different roles in airway epithelium and airway smooth muscle. But both converge in airway remodeling as epithelial injury activate epithelial mesenchymal trophic unit (EMTU) to cause sub-epithelial fibrosis and mitochondrial biogenesis in airway smooth muscle to cause smooth muscle hypertrophy. In summary, mitochondrial status depends on cell type in asthmatic lung and so more careful cell specific investigations are required to understand the exact role of mitochondria and mitochondrial calcium in asthma pathogenesis. It could be applicable to other lung diseases as well, because similar airway remodeling in small airways and huge parenchymal fibrosis occurs in COPD and lung fibrosis respectively. These complex mitochondria mediated pathology needs to be thoroughly investigated to get a clear concept for the airway remodeling. This clear concept would lead to next level of therapeutics in lung diseases and it seems that structural cells of lungs like epithelium, smooth muscle etc may be focussed, in contrast to earlier concepts when it was believed that these structural cells were mere victims of inflammatory cells.
Acknowledgments
This work was supported by the projects MLP137 (MISSION LUNG) and MLP 126 at CSIR-Indian Institute Chemical Biology, Council of Scientific and Industrial Research, Govt. of India. AR, AJ, JD, and SS acknowledge Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India, for their Ph.D registrations.
References
- Agarwal A.R., Yin F., Cadenas E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am. J. Respir. Cell Mol. Biol. 2014;51:284–293. doi: 10.1165/rcmb.2013-0523OC. [DOI] [PubMed] [Google Scholar]
- Aghapour M., Remels A.H.V., Pouwels S.D., Bruder D., Hiemstra P.S., Cloonan S.M., Heijink I.H. Mitochondria: at the crossroads of regulating lung epithelial cell function in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;318(1):L149–L164. doi: 10.1152/ajplung.00329.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera-Aguirre L., Bacsi A., Saavedra-Molina A., Kurosky A., Sur S., Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J. Immunol. 2009;183(8):5379–5387. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antigny F., Girardin N., Raveau D., Frieden M., Becq F., Vandebrouck C. Dysfunction of mitochondria Ca2+ uptake in cystic fibrosis airway epithelial cells. Mitochondrion. 2009;9(4):232–241. doi: 10.1016/j.mito.2009.02.003. [DOI] [PubMed] [Google Scholar]
- D.M. Arduino J. Wettmarshausen H. Vais P. Navas-Navarro Y. Cheng A. Leimpek Z. Ma A. Delrio-Lorenzo A. Giordano C. Garcia-Perez G. Médard B. Kuster J. García-Sancho D. Mokranjac J.K. Foskett M.T. Alonso F. Perocchi Systematic Identification of MCU Modulators by Orthogonal Interspecies Chemical Screening Molecular Cell 67 4 2017 711 723.e7 https://linkinghub.elsevier.com/retrieve/pii/S1097276517305373. [DOI] [PMC free article] [PubMed]
- Archambault, A. S., Turcotte, C., Martin, C., Provost, V., Larose, M. C., Laprise, C., Chakir, J., Bissonnette, É., Laviolette, M., Bossé, Y., Flamand, N., 2018. Comparison of eight 15-lipoxygenase (LO) inhibitors on the biosynthesis of 15-LO metabolites by human neutrophils and eosinophils. PloS One. 13, e020424. [DOI] [PMC free article] [PubMed]
- Austin S., St-Pierre J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Austin S., Nowikovsky K. LETM1: essential for mitochondrial biology and cation homeostasis? Trends Biochem. Sci. 2019;44(8):648–658. doi: 10.1016/j.tibs.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Barrero C.A., Perez-Leal O., Aksoy M., Moncada C., Ji R., Lopez Y., Mallilankaraman K., Madesh M., Criner G.J., Kelsen S.G., Merali S. Histone 3.3 participates in a self-sustaining cascade of apoptosis that contributes to the progression of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013;188(6):673–683. doi: 10.1164/rccm.201302-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratic A., Larsson N.-G. The role of mitochondria in aging. J. Clin. Invest. 2013;123(3):951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.-S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- L.-P. Boulet Airway remodeling in asthma: update on mechanisms and therapeutic approaches 24 1 2018 56 62 https://journals.lww.com/00063198-201801000-00010. [DOI] [PubMed]
- Caporarello N., Meridew J.A., Jones D.L., Tan Q.i., Haak A.J., Choi K.M., Manlove L.J., Prakash Y.S., Tschumperlin D.J., Ligresti G. PGC1α repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax. 2019;74(8):749–760. doi: 10.1136/thoraxjnl-2019-213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. The fateful encounter of mitochondria with calcium: How did it happen? Biochim. Biophys. Acta (BBA) – Bioenergetics. 2010;1797(6-7):595–606. doi: 10.1016/j.bbabio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- R. Chaudhuri M.A. Thompson C. Pabelick A. Agrawal Y.S. Prakash A. Richard . Johnston and Benjamin T. Suratt, Obesity, mitochondrial dysfunction, and obstructive lung disease. Chapter in a Book titled “In Mechanisms and Manifestations of Obesity in Lung Disease” 2019 Academic Press 143 167.
- Christopher DevasahayamJ, Isaac BarneyTJ, Rupali P., Thangakunam B. Health-care preparedness and health-care worker protection in COVID-19 pandemic. Lung India. 2020;37(3):238. doi: 10.4103/lungindia.lungindia_189_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E.K., Drazen J.M. Asthma: one hundred years of treatment and onward. Am. J. Respir. Crit. Care Med. 2005;171(11):1202–1208. doi: 10.1164/rccm.200502-257OE. [DOI] [PubMed] [Google Scholar]
- Cloonan, S. M., Choi, A. M., 2016. Mitochondria in lung disease. J. Clin. Invest. 126, 809-820. [DOI] [PMC free article] [PubMed]
- Delmotte P., Yang B., Thompson M.A., Pabelick C.M., Prakash Y.S., Sieck G.C. Inflammation alters regional mitochondrial Ca 2+ in human airway smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012;303(3):C244–C256. doi: 10.1152/ajpcell.00414.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia, M., Atlante, A., 2016. Mitochondria and Cystic Fibrosis Transmembrane Conductance Regulator Dialogue: Some News. J Rare Dis Res & Treatment. 1, 23-29.
- Favia M., de Bari L., Bobba A., Atlante A. An Intriguing involvement of mitochondria in cystic fibrosis. J. Clin. Med. 2019;8:1890. doi: 10.3390/jcm8111890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach H., Wagner C., Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res. 2017;367:551–569. doi: 10.1007/s00441-016-2566-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Menazza S., Holmström K.M., Parks R.J., Liu J., Sun J., Liu J., Pan X., Murphy E. The Ins and Outs of Mitochondrial Calcium. Circ Res. 2015;116(11):1810–1819. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhirajan R.K., Meng S., Chandramoorthy H.C., Mallilankaraman K., Mancarella S., Gao H., Razmpour R., Yang X.F., Houser S.R., Chen J., Koch W.J., Wang H., Soboloff J., Gill D.L., Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Lombó C., Pappa A., Panayiotidis M.I., Franco R. Redox homeostasis, oxidative stress and mitophagy. Mitochondrion. 2020;51:105–117. doi: 10.1016/j.mito.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018;19(11):713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- Girodet P.O., Dournes G., Thumerel M., Begueret H., Santos P.D., Ozier A., Dupin I., Trian T., Montaudon M., Laurent F., Marthan R., Berger P. Calcium channel blocker reduces airway remodeling in severe asthma. A proof-of-concept study. Am. J. Respir. Crit. Care Med. 2015;191:876–883. doi: 10.1164/rccm.201410-1874OC. [DOI] [PubMed] [Google Scholar]
- Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Larson Casey J.L., Andrabi S.A., Lee J.H., Meza-Perez S., Randall T.D., Carter A.B. Mitochondrial calcium uniporter regulates PGC-1α expression to mediate metabolic reprogramming in pulmonary fibrosis. Redox Biol. 2019;26:101307. doi: 10.1016/j.redox.2019.101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijink I.H., Noordhoek J.A., Timens W., van Oosterhout A.J.M., Postma D.S. Abnormalities in airway epithelial junction formation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014;189(11):1439–1442. doi: 10.1164/rccm.201311-1982LE. [DOI] [PubMed] [Google Scholar]
- HENDELES L., HARMAN E. Should we abandon the notion that calcium channel blockers are potentially useful for asthma? J. Allergy Clin. Immunol. 1987;79(6):853–856. doi: 10.1016/0091-6749(87)90231-4. [DOI] [PubMed] [Google Scholar]
- Hough, R. F., Islam, M. N., Gusarova, G. A., Jin, G., Das, S., Bhattacharya, J., 2019. Endothelial mitochondria determine rapid barrier failure in chemical lung injury. JCI. Insight. 4. [DOI] [PMC free article] [PubMed]
- Jaña F., Bustos G., Rivas J., Cruz P., Urra F., Basualto-Alarcón C., Sagredo E., Ríos M., Lovy A., Dong Z., Cerda O., Madesh M., Cárdenas C. Complex I and II are required for normal mitochondrial Ca2+ homeostasis. Mitochondrion. 2019;49:73–82. doi: 10.1016/j.mito.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania E., Roest G., Vervliet T., Parys J.B., Bultynck G. IP3 receptor-mediated calcium signaling and its role in autophagy in cancer. Front. Oncol. 2017;7:140. doi: 10.3389/fonc.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N., Murakoshi M., Isobe A., Kagechika K., Miyoshi N., Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017;3(1) doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider B., Lin C.-R., Karim L., Tomar D., Vlasenko L., Marchetti N., Bolla S., Madesh M., Criner G.J., Bahmed K. Mitochondrial dysfunction in human primary alveolar type II cells in emphysema. EBioMedicine. 2019;46:305–316. doi: 10.1016/j.ebiom.2019.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Min K.T. The interface between ER and Mitochondria: molecular compositions and functions. Mol. Cells. 2018;41(12):1000–1007. doi: 10.14348/molcells.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Y.-L. Lee B. Obiako O.M. Gorodnya M.V. Ruchko J.L. Kuck V.M. Pastukh G.L. Wilson J.D. Simmons M.N. Gillespie Mitochondrial DNA Damage Initiates Acute Lung Injury and Multi-Organ System Failure Evoked in Rats by Intra-Tracheal Pseudomonas Aeruginosa 48 1 2017 54 60 https://journals.lww.com/00024382-201707000-00008. [DOI] [PMC free article] [PubMed]
- Lerner C.A., Sundar I.K., Rahman I. Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int. J. Biochem. Cell Biol. 2016;81:294–306. doi: 10.1016/j.biocel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Slone J., Huang T. The role of mitochondrial-related nuclear genes in age-related common disease. Mitochondrion. 2020;53:38–47. doi: 10.1016/j.mito.2020.04.012. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., McIntyre R.L., Janssens G.E., Houtkooper R.H. Mitochondrial fission and fusion: a dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020;186:111212. doi: 10.1016/j.mad.2020.111212. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jin M., Wang Y., Zhu J., Tan R., Zhao J., Ji X., Jin C., Jia Y., Ren T., Xing J. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Sig. Transduct. Target Ther. 2020;5(1) doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabalirajan U., Dinda A.K., Kumar S., Roshan R., Gupta P., Sharma S.K., Ghosh B. Mitochondrial structural changes and dysfunction are associated with experimental allergic asthma. J. Immunol. 2008;181(5):3540–3548. doi: 10.4049/jimmunol.181.5.3540. [DOI] [PubMed] [Google Scholar]
- Mabalirajan U., Dinda A.K., Sharma S.K., Ghosh B. Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J. Immunol. 2009;183(3):2059–2067. doi: 10.4049/jimmunol.0900342. [DOI] [PubMed] [Google Scholar]
- Mabalirajan U., Rehman R., Ahmad T., Kumar S., Singh S., Leishangthem G.D., Aich J., Kumar M., Khanna K., Singh V.P., Dinda A.K., Biswal S., Agrawal A., Ghosh B. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013;3:1349. doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabalirajan U., Rehman R., Ahmad T., Kumar S., Leishangthem G.D., Singh S., Dinda A.K., Biswal S., Agrawal A., Ghosh B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013;3:1540. doi: 10.1038/srep01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabalirajan U., Ghosh B. Mitochondrial dysfunction in metabolic syndrome and asthma. J. All. 2013;2013:1–12. doi: 10.1155/2013/340476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNee W., Rabinovich R.A., Choudhury G. Ageing and the border between health and disease. Eur. Respir. J. 2014;44(5):1332–1352. doi: 10.1183/09031936.00134014. [DOI] [PubMed] [Google Scholar]
- Mercado N., Ito K., Barnes P.J. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70(5):482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr. Role of calcium and calcium antagonists in airway function. Eur. J. Respir. Dis. Suppl. 1983;128:123–132. [PubMed] [Google Scholar]
- Morciano G., Marchi S., Morganti C., Sbano L., Bittremieux M., Kerkhofs M., Corricelli M., Danese A., Karkucinska-Wieckowska A., Wieckowski M.R., Bultynck G., Giorgi C., Pinton P. Role of Mitochondria-associated er membranes in calcium regulation in cancer-specific settings. Neoplasia. 2018;20(5):510–523. doi: 10.1016/j.neo.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V., Tavernarakis N. Calcium homeostasis in aging neurons. Front. Genet. 2012;3:200. doi: 10.3389/fgene.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkouchi S., Ono M., Kobayashi M., Hirano T., Tojo Y., Hisata S., Ichinose M., Irokawa T., Ogawa H., Kurosawa H. Myriad functions of stanniocalcin-1 (STC1) cover multiple therapeutic targets in the complicated pathogenesis of idiopathic pulmonary fibrosis (IPF) Clin. Med. Insights Circ. Respir. Pulm. Med. 2015;9(Suppl1):91–96. doi: 10.4137/CCRPM.S23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S., Conaway S., Jr., Deshpande D.A. Mitochondrial regulation of airway smooth muscle functions in health and pulmonary diseases. Arch. Biochem. Biophys. 2019;663:109–119. doi: 10.1016/j.abb.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel M., Ghaleh B., Morin D. Mitochondria and aging: A role for the mitochondrial transition pore? Aging Cell. 2018;17(4):e12793. doi: 10.1111/acel.2018.17.issue-410.1111/acel.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J. C., 2018. Mitochondrial damage pathways in ventilator induced lung injury (VILI): an update. J. Lung Health Dis. 2, 18-22. [PMC free article] [PubMed]
- Prakash Y.S., Pabelick C.M., Sieck G.C. Mitochondrial dysfunction in airway disease. Chest. 2017;152(3):618–626. doi: 10.1016/j.chest.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.E., Sisson J.H. Redox regulation of motile cilia in airway disease. Redox Biol. 2019;27:101146. doi: 10.1016/j.redox.2019.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L., Jones A.C., Whelan C.J. Induction of apoptosis with tobacco smoke and related products in A549 lung epithelial cells in vitro. J. Inflamm. (Lond) 2006;3:3. doi: 10.1186/1476-9255-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, P. H., 2011. Mitochondrial Dysfunction and Oxidative Stress in Asthma: Implications for Mitochondria-Targeted Antioxidant Therapeutics. Pharmaceuticals (Basel). 4, 429‐456. [DOI] [PMC free article] [PubMed]
- Ribeiro, C.M., Paradiso, A.M., Livraghi, A., Boucher, R.C., 2003.The Mitochondrial Barriers Segregate Agonist-induced Calcium-dependent Functions in Human Airway Epithelia. J. Gen. Physiol. 122, 377-387. [DOI] [PMC free article] [PubMed]
- Ridley C, Thornton DJ. Mucins: the frontline defence of the lung. Biochem Soc Trans. 2018;46(5):1099-1106. [DOI] [PMC free article] [PubMed]
- Rimessi, A., Bezzerri, V., Patergnani, S., Marchi, S., Cabrini, G., Pinton, P., 2015 Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat Commun. 6, 6201. [DOI] [PubMed]
- Rimessi A., Pozzato C., Carparelli L., Rossi A., Ranucci S., De Fino I., Cigana C., Talarico A., Wieckowski M.R., Ribeiro C.M.P., Trapella C., Rossi G., Cabrini G., Bragonzi A., Pinton P. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Sci. Adv. 2020;6(19):eaax9093. doi: 10.1126/sciadv.aax9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., Zecchini E., Pinton P. Ca2+ transfer from the ER to mitochondria: When, how and why. Biochim. Biophys. Acta (BBA) – Bioenerget. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Garcia S., Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer. Int. J. Oncol. 2019;54:1155–1167. doi: 10.3892/ijo.2019.4696. [DOI] [PubMed] [Google Scholar]
- Rotshild V., Azoulay L., Zarifeh M., Masarwa R., Hirsh-Raccah B., Perlman A., Muszkat M., Matok I. The risk for lung cancer incidence with calcium channel blockers: a systematic review and meta-analysis of observational studies. Drug Saf. 2018;41(6):555–564. doi: 10.1007/s40264-018-0644-4. [DOI] [PubMed] [Google Scholar]
- Salvator H., Grassin-Delyle S., Naline E., Brollo M., Fournier C., Couderc L.J., Devillier P. Contrasting effects of adipokines on the cytokine production by primary human bronchial epithelial cells: inhibitory effects of adiponectin. Front. Pharmacol. 2020;2020(11):56. doi: 10.3389/fphar.2020.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, A., Salathe, M., 2011. Ciliary beat co‐ordination by calcium. Biol. Cell. 103, 159–169. [DOI] [PubMed]
- Sebag, S.C., Koval, O.M., Paschke, J.D., Winters, C.J., Comellas, A.P., Grumbach, I.M., 2018. Inhibition of the mitochondrial calcium uniporter prevents IL-13 and allergen-mediated airway epithelial apoptosis and loss of barrier function. Exp. Cell Res. 362, 400-411. [DOI] [PMC free article] [PubMed]
- Seeley E.J., Rosenberg P., Matthay M.A. Calcium flux and endothelial dysfunction during acute lung injury: a STIMulating target for therapy. J. Clin. Invest. 2013;123(3):1015–1018. doi: 10.1172/JCI68093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo A.Y., Joseph A.-M., Dutta D., Hwang J.C.Y., Aris J.P., Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell Sci. 2010;123(15):2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.K., Rao G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019;73:28–45. doi: 10.1016/j.plipres.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass R.G., Brüne B. Regulation and functions of 15-lipoxygenases in human macrophages. Front. Pharmacol. 2019;10:719. doi: 10.3389/fphar.2019.00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So S.Y., Ip M., Lam W.K. Calcium channel blockers and asthma. Lung. 1986;164(1):1–16. doi: 10.1007/BF02713625. [DOI] [PubMed] [Google Scholar]
- Spella M., Lilis I., Stathopoulos G.T. Shared epithelial pathways to lung repair and disease. Eur. Respir. Rev. 2017;26(144):170048. doi: 10.1183/16000617.0048-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., 2017. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes. 8, 398. [DOI] [PMC free article] [PubMed]
- Srivastava S., Subramanian K., Choy K., Ledo A.M., Bonneau O., Choo-Wing R., Rowlands D. Mitochondrial dysfunction in the aged lung and COPD: A role for mitochondrial calcium? ERJ Open Res. 2020;6:36. [Google Scholar]
- Thomas B., Rutman A., Hirst R.A., Haldar P., Wardlaw A.J., Bankart J., Brightling C.E., O'Callaghan C. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J. Allergy Clin. Immunol. 2010;126(4):722–729.e2. doi: 10.1016/j.jaci.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Trian, T., Benard, G., Begueret, H., Rossignol, R., Girodet, P. O., Ghosh, D., Ousova, O., Vernejoux, J. M., Marthan, R., Tunon-de-Lara, J. M., Berger, P., 2007. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 204, 3173-3181. [DOI] [PMC free article] [PubMed]
- Valdivieso A.G., Santa-Coloma T.A. CFTR activity and mitochondrial function. Redox Biol. 2013;1(1):190–202. doi: 10.1016/j.redox.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J.E. MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta (BBA) – Mol. Cell Biol. Lipids. 2014;1841(4):595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Vayssier M., Banzet N., François D., Bellmann K., Polla B.S. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am. J. Physiol. Lung Cell. Mol. Physiol. 1998;275(4):L771–L779. doi: 10.1152/ajplung.1998.275.4.L771. [DOI] [PubMed] [Google Scholar]
- Vazquez-Calvo C., Suhm T., Büttner S., Ott M. The basic machineries for mitochondrial protein quality control. Mitochondrion. 2020;50:121–131. doi: 10.1016/j.mito.2019.10.003. [DOI] [PubMed] [Google Scholar]
- W. Wang G. Karamanlidis R. Tian Novel targets for mitochondrial medicine Sci. Transl. Med. 8 326 2016 326rv3 326rv3. [DOI] [PMC free article] [PubMed]
- Watson, A., Doherty, F. J., 1994. Calcium promotes membrane association of reticulocyte 15-lipoxygenase. Biochem. J. 298, 377-383. [DOI] [PMC free article] [PubMed]
- Woods J.J., Nemani N., Shanmughapriya S., Kumar A., Zhang M., Nathan S.R., Thomas M., Carvalho E., Ramachandran K., Srikantan S., Stathopulos P.B., Wilson J.J., Madesh M. A selective and cell-permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent. Sci. 2019;5:153–166. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen M., Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. doi: 10.1016/j.mito.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Xu Z., Zhang D., He X., Huang Y., Shao H. Transport of Calcium Ions into Mitochondria. CG. 2016;17(3):215–219. doi: 10.2174/1389202917666160202215748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Harashima H. MITO-porter for mitochondrial delivery and mitochondrial functional analysis. Handb. Exp. Pharmacol. 2017;240:457–472. doi: 10.1007/164_2016_4. [DOI] [PubMed] [Google Scholar]
- Yanagi S., Tsubouchi H., Miura A., Matsumoto N., Nakazato M. Breakdown of epithelial barrier integrity and overdrive activation of alveolar epithelial cells in the pathogenesis of acute respiratory distress syndrome and lung fibrosis. Biomed Res. Int. 2015;2015:1–12. doi: 10.1155/2015/573210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Tuder R.M. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol. Rev. 2007;87(3):1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- Yu C., Wang Y., Peng J., Shen Q., Chen M., Tang W., Li X., Cai C., Wang B., Cai S., Meng X., Zou F. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. 2017;8(48):83831–83844. doi: 10.18632/oncotarget.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Yang J., Gao X., Sun W., Liu S., Han Y., Lu X., Jin C., Wu S., Cai Y. Lanthanum chloride impairs spatial learning and memory by inducing [Ca 2+ ] m overload, mitochondrial fission–fusion disorder and excessive mitophagy in hippocampal nerve cells of rats. Metallomics. 2020;12(4):592–606. doi: 10.1039/c9mt00291j. [DOI] [PubMed] [Google Scholar]